Overview of Feline Parvoviral Enteritis.
First Described: 1928, Verge and Christoforoni, France1
Cause: Feline panleukopenia virus (FPV); also canine parvovirus (CPV)-2a, CPV-2b, and CPV-2c (Family Parvoviridae, subfamily Parvovirinae, genus Parvovirus)
Affected Hosts: Domestic and wild cats; foxes, mink, and raccoons
Geographic Distribution: Worldwide
Mode of Transmission: Direct contact with virus in feces and vomitus and contaminated fomites.
Major Clinical Signs: Fever, lethargy, inappetence, vomiting, diarrhea, dehydration, sudden death. Neurologic signs (primarily cerebellar signs) may also occur.
Differential Diagnoses: Other feline viral enteritides, toxins, gastrointestinal foreign body, enteric parasitic infections such as giardiasis and nematode infections, enteric bacterial infections such as salmonellosis, pancreatitis, or inflammatory bowel disease. Congenital central nervous system defects should be considered in cats with neurologic signs.
Human Health Significance: FPV is not known to infect humans, but was isolated from a monkey.
Etiology and Epidemiology
Feline panleukopenia virus (FPV) is a parvovirus that causes enteritis and panleukopenia in domestic and wild cat species worldwide.2 It has also been associated with disease in raccoons, mink, foxes, and a monkey, and can replicate in ferrets without causing disease. Feline panleukopenia is sometimes confusingly referred to as “cat plague” and “feline distemper.” FPV is a small, single-stranded non-enveloped DNA virus that is closely related to CPV-2, but in contrast to CPV-2, which emerged in the late 1970s, the existence of FPV has been known since the 1920s.1 FPV has the same ability as CPV-2 to survive long periods in the environment and resist disinfection, and has the same preference for replication in rapidly dividing cells (see Chapter 14).
Feline panleukopenia is most likely to occur in cats younger than 1 year of age, but it can occur in unvaccinated or improperly vaccinated cats of all ages. The median age of affected cats in one study was 4 months, and when disease occurred in vaccinated cats, it occurred only in cats that had not received a booster vaccine after 12 weeks of age.3 However, kitten deaths have been reported in households of fully vaccinated kittens, possibly because of exposure to large amounts of virus in the environment.4 Outbreaks of panleukopenia in cats correlate seasonally with increases in susceptible newborn kitten numbers. Panleukopenia occurs most commonly in multicat households, and especially in enclosed, shelter environments. It can also occur in cats with outdoor exposure, such as barn, feral, and stray cats. In one study, the prevalence of protective antibody titers to FPV in feral cats in Florida was only 33%, which suggested a low rate of exposure to the virus.5 In some North American shelters, devastating outbreaks of panleukopenia have led to euthanasia of large numbers of cats. Contact with other cats may not be present in the history, because fomite transmission is so effective.3 FPV replicates to a limited extent in dogs, without disease or virus shedding. Some feline panleukopenia results from infection of cats by the related mink enteritis virus, CPV-2a, CPV-2b, or CPV-2c.6., 7., 8. Mixed infections with FPV and CPV-2 variants have been detected in cats, and there is evidence for recombination between FPV and CPV-2 variants.7., 9. Infection of cats with CPV-2 variants is uncommon in Europe but predominated among cats with panleukopenia in Asia.10
Other viral pathogens that have been associated with gastroenteritis in cats include feline enteric coronavirus (see Chapter 20), FeLV, rotaviruses, caliciviruses, reoviruses, and astroviruses. A torovirus-like agent has been associated with a syndrome of diarrhea and protrusion of the nictating membranes in cats.11 Togavirus-like and picornavirus-like particles have been identified in the feces of Australian cats, but their significance is uncertain.12
Clinical Features
Signs and Their Pathogenesis
The pathogenesis of FPV infection is similar to that of CPV infection (see Chapter 14). Transmission is by the fecal-oral route, and indirect transmission through contaminated fomites represents the most important means of infection. Like CPV, FPV enters cells using transferrin receptors13 and replicates in cells that are in the S-phase of the mitotic cycle. Initially, the virus replicates in oropharyngeal lymphoid tissue, after which it disseminates in blood to all tissues. Infection of lymphoid tissues leads to lymphoid tissue necrosis. Infection of the marrow is associated with leukopenia, which is compounded by neutrophil sequestration in damaged gastrointestinal tissue. The virus replicates in the intestinal crypt epithelial cells, with shortening of villi, increased intestinal permeability, and malabsorption.
Subclinical infection is probably widespread, especially in young adult or adult, immune-competent cats. Disease severity depends on factors such as age, immune status, and concurrent infections with other bacterial or viral pathogens, which increase the turnover rate of intestinal epithelial cells and enhance viral replication and cellular destruction. Co-infections can occur with feline enteric coronavirus, Clostridium piliforme, Salmonella spp., FeLV, or astroviruses.14., 15., 16., 17. Disease generally occurs after an incubation period of 2 to 10 days. The peracute form of disease involves death without apparent premonitory signs. Infection of kittens or adult cats results in clinical signs of fever, lethargy, vocalization, weakness, and inappetence, which may progress to profound dehydration, vomiting, sometimes watery to hemorrhagic diarrhea, and rapid loss of weight. Some cats develop only anorexia and lethargy, in the absence of vomiting, diarrhea, or leukopenia.3 Secondary bacterial infections appear to be essential for signs of disease to occur. Death usually results from complications relating to dehydration, electrolyte imbalances, hypoglycemia, hemorrhage, or bacteremia and endotoxemia.
In the developing fetus or neonate, FPV replicates in a variety of tissues. Abortion, congenital abnormalities, or infertility can result from infection early in pregnancy, although the queen is generally otherwise unaffected. Later in pregnancy or in neonates up to approximately 1 week of age, viral destruction of Purkinje cells and granule precursor cells located in the cerebellar external granular layer leads to cerebellar hypoplasia (Figure 19-1 ). The severity of infection can vary between kittens in a litter. Sometimes a portion of the litter fails to survive and the remainder develop neurologic signs.18 Signs of cerebellar ataxia are nonprogressive and are most apparent when kittens begin to walk at about 2 to 3 weeks of age, although mentation and appetite are otherwise normal, and these kittens can sometimes make acceptable pets. Other developmental central nervous system (CNS) abnormalities have been reported less commonly, and include hydrocephalus, porencephaly (cystic lesions within the cerebral hemispheres), or hydranencephaly (complete replacement of the cerebral hemispheres with cystic lesions). These abnormalities may be accompanied by signs of forebrain damage, such as seizures and behavioral changes. Unlike canine parvoviruses, FPV appears to be able to infect neurons other than the cerebellar Purkinje cells, which are terminally differentiated cells.19 Ocular lesions can also develop and include retinal folding, dysplasia and degeneration, and optic nerve hypoplasia.
FIGURE 19-1.
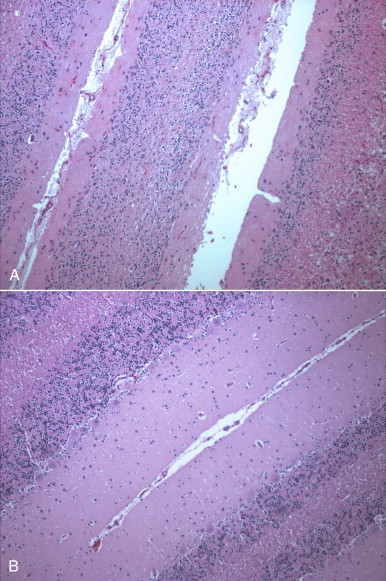
A, Severe cerebellar hypoplasia in a 1-year-old male neutered domestic shorthair that was euthanized for cerebellar signs and seizures. The neurologic signs had been present since the cat was found at approximately 4 months of age. There is a paucity of neurons and glial cells in all layers. B, Normal feline cerebellum. Note the dramatically increased width of the molecular layer when compared to (A).
The DNA of FPV has been detected in the myocardium of cats with hypertrophic, dilated, and restrictive cardiomyopathy, but was not detected in a subset of healthy control cats.20 Additional studies to assess the role of FPV in feline myocarditis and cardiomyopathy are needed.
Fecal shedding of virus usually lasts for several days, and in some cats, it may persist for up to 6 weeks. Kittens infected in utero have been reported to develop immune tolerance to the virus, with viral persistence in the kidneys and lungs for up to 1 year in the absence of shedding.
Physical Examination Findings
The most common physical examination findings are weakness, lethargy, and dehydration. Fever (103°F to 107°F or 39.5°C to 42.5°C) may be present early in the course of illness. Pain may be noted on palpation of the abdomen, or a hunched posture may be present. The perianal region may be contaminated with feces. Oral ulceration and mucosal pallor may be present in severely affected cats, and rarely, bacteremia may be accompanied by icterus. Terminally, affected cats may be hypothermic, bradycardic, and comatose.
Kittens with cerebellar signs are generally bright and alert but exhibit intention tremors, incoordination, ataxia, hypermetria, a broad-based stance, decreased postural reactions, a truncal sway, and absence of a menace response. Cats with forebrain disease may show abnormal behavior, such as aggression or decreased mentation. Examination of the ocular fundus may reveal folding of the retina, evidence of retinal degeneration with discrete gray spots, and optic nerve hypoplasia. Retinal lesions may be an incidental finding in older, recovered cats, which includes surviving cats with cerebellar hypoplasia.
Diagnosis
Laboratory Abnormalities
Complete Blood Count
The most common abnormality on the CBC in feline panleukopenia is leukopenia, which is due to a neutropenia and lymphopenia (Box 19-1).3 Total leukocyte counts may be as low as 50 cells/µL, and toxic band neutrophils may be present. In one study, only 65% of 187 cats with panleukopenia were leukopenic, so an absence of leukopenia does not rule out FPV infection. Recovery may be associated with lymphocytosis and leukocytosis. Thrombocytopenia and mild anemia are also common. Thrombocytopenia may result from damage to the marrow or, possibly, disseminated intravascular coagulation (DIC).
BOX 19-1. Prevalence of Laboratory Abnormalities in Cats with Panleukopenia.
Leukopenia: 122/187 (65%)
Thrombocytopenia: 83/153 (54%)
Anemia: 91/187 (48%)
Neutropenia: 64/137 (47%)
Lymphopenia: 53/137 (39%)
Hypoalbuminemia: 45/101 (45%)
Hypochloremia: 40/112 (36%)
Hyponatremia: 41/127 (32%)
Hypoproteinemia: 46/153 (30%)
Hyperglycemia: 48/168 (29%)
Increased AST activity: 26/98 (27%)
Hyperkalemia: 30/132 (23%)
Increased BUN: 36/168 (21%)
Hyperbilirubinemia: 19/134 (14%)
Increased ALT activity: 18/135 (13%)
Increased creatinine: 12/155 (8%)
Hypokalemia: 9/132 (7%)
Hypernatremia: 8/127 (6%)
Hypoglycemia: 10/168 (6%)
Modified from Kruse BD, Unterer S, Horlacher K, et al. Prognostic factors in cats with feline panleukopenia. J Vet Intern Med 2010;24:1271-1276.
Serum Biochemical Tests
Serum biochemistry analysis may show hypoalbuminemia, hypoglobulinemia, and/or hypocholesterolemia; electrolyte abnormalities such as hyponatremia or hypernatremia, hypochloremia, hyperkalemia, or, less commonly hypokalemia; and acid-base abnormalities (see Box 19-1 ). In severely affected cats, azotemia, increased serum activities of AST or ALT, or hyperbilirubinemia may be present. Hyperglycemia or hypoglycemia may also be identified.
Diagnostic Imaging
Plain Radiography
As in dogs with parvoviral enteritis, abdominal radiography in cats with panleukopenia may show evidence of poor serosal detail and a fluid- and gas-filled gastrointestinal tract.
MRI Findings
MRI of cats with neurologic signs due to FPV may reveal evidence of cerebellar agenesis or hypoplasia. Rarely, hydrocephalus, porencephaly, or hydranencephaly can be detected.18
Microbiologic Tests
Diagnostic assays available for feline panleukopenia are listed in Table 19-1 .
TABLE 19-1.
Diagnostic Assays Available for Feline Panleukopenia
| Assay | Specimen Type | Target | Performance |
|---|---|---|---|
| Canine parvovirus fecal antigen ELISA | Feces | Parvoviral antigen | Sensitivity varies with the assay used and the timing of specimen collection. False negatives are common, but a positive result generally indicates infection. |
| Histopathology | Usually necropsy specimens, especially gastrointestinal tissues | Crypt necrosis with intranuclear inclusions; FPV antigen with IHC or IFA | Can be used for necropsy diagnosis. |
| Polymerase chain reaction (PCR) | Feces, tissue samples | FPV DNA | Sensitivity and specificity varies depending on assay design. The extent to which attenuated live vaccine virus can be detected after vaccination is not well understood. Because of the high sensitivity of some assays, the significance of a positive result may be difficult to interpret. False-negative results may occur as a result of inhibition of PCR by components of feces. |
| Fecal electron microscopy | Feces | Virus particles | Not widely available, turnaround time can be slow, and may be expensive. Requires the presence of large amounts of virus. |
| Virus isolation | Feces, tissues | FPV | Difficult, not widely available. Used primarily as a research tool. |
FPV, feline panleukopenia virus; IFA, immunofluorescent antibody; IHC, immunohistochemistry.
Serologic Diagnosis
Use of serology for diagnosis of feline panleukopenia is complicated by widespread exposure or immunization, so serologic assays that detect antibody against FPV are generally used to assess the need for vaccination rather than for diagnosis. They can also be used in outbreak situations in order to determine which cats are at risk for development of disease and virus shedding, and which cats are protected and therefore at low risk. The gold standard method for FPV serology is hemagglutination inhibition, which measures the ability of serum to prevent agglutination of erythrocytes by the virus (see Chapter 2). Serum neutralization assays may also be used. Point-of-care assays designed to detect antibody titers to CPV have a low sensitivity (28%) for detection of antibodies to FPV in cats.21 A point-of-care assay designed to detect feline antibodies (ImmunoComb Feline VacciCheck Test Kit, Biogal, Galed Labs, Israel) also had a low sensitivity (49%), although specificity was high.21 The use of this test for risk analysis in shelter situations may lead to inappropriate removal or isolation of cats with false-negative results for protective antibody titers, which might waste time, space, and financial resources.21 However, positive results should reliably indicate protection. The test can be performed quickly (30 minutes) and requires as little as 5 µL of serum or plasma, so provided it is understood that a negative result means either a protected or susceptible status, and a positive result equates to protection, the test still has the potential to provide useful information.
Antigen Detection Enzyme Linked Immunosorbent Assay
FPV can be detected in feces or rectal swabs using antigen assays designed to detect CPV.22., 23. The sensitivity and specificity of these assays varies from one assay to another and with the stage of infection, because virus shedding may be transient. In general, false-negative results are common with these assays, but false positives are uncommon, so a positive test result in a cat with consistent clinical signs suggests a diagnosis of feline panleukopenia. The specificity of one point-of-care device (SNAP Parvo, IDEXX Laboratories, Westbrook, ME) was high; 54 of 55 positive assay results were confirmed with a PCR assay. Another study of 52 cats with diarrhea and 148 healthy cats showed variability in the sensitivity and specificity of five different test kits when compared with fecal electron microscopy.23 Sensitivity ranged from 50% to 80%, and specificity ranged from 94% to 100%. This study included only 10 cats with FPV as determined with electron microscopy. Additional studies are warranted that evaluate the sensitivity and specificity of these assays in larger numbers of cats with FPV infection when both real-time PCR and electron microscopy are used as the gold standard. False-positive fecal antigen assay results after vaccination with attenuated live viral vaccines appear to be uncommon, but again vary with the test used.24 Of the SNAP Parvo (IDEXX Laboratories), AGEN CPV (AGEN Biomedical Ltd., Brisbane, Australia), and the Witness CPV (Synbiotics Corp, San Diego, CA), the SNAP Parvo was least likely to yield positive results after vaccination.
Fecal Electron Microscopy
Fecal electron microscopy is still offered by some institutions for diagnosis of viral enteritis. It may also facilitate diagnosis of other infections such as rotavirus, astrovirus, torovirus, and coronavirus infections. Turnaround time may be slow. Generally speaking, large amounts of virus must be present for results to be positive, and technical expertise is required to accurately identify virus in the stool.
Virus Isolation
FPV can be isolated in feline cells, but as with CPV, isolation can difficult, and the virus shows minimal cytopathic effects. As a result, isolation of FPV is a specialized procedure that is uncommonly used for diagnosis.
Molecular Diagnosis Using the Polymerase Chain Reaction
Specific real-time PCR assays have been developed for detection of FPV and differentiation of FPV from CPV-2 variants and are offered by veterinary diagnostic laboratories. Clinicians should contact their laboratory to determine the specificity of the assay offered.25 These assays can be used on whole blood or feces. The extent to which these assays detect attenuated live vaccine virus after vaccination requires further study. Assays have also been developed that differentiate between field and vaccine strains of FPV.26
Pathologic Findings
Gross Pathologic Findings
Gross pathologic findings in feline panleukopenia include thymic involution; thickening, distention, and discoloration of the intestinal wall with serosal hemorrhage (Figure 19-2 ); and enlarged, edematous mesenteric lymph nodes. The intestine may contain bloody liquid contents, and mucosal hemorrhage may be identified. Hemorrhages may be visible on the surface of other organs as well. In some cats, mild pleural or peritoneal effusion is present. Cats infected prenatally may have cerebellar aplasia, or more commonly a small cerebellum (often half to three-quarters normal size).27 Rarely other developmental CNS abnormalities such as hydrocephalus, hydranencephaly, or porencephaly are observed.18
FIGURE 19-2.
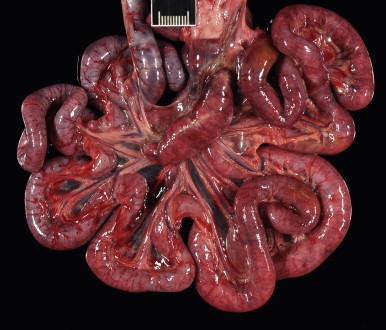
Intestinal tract of a 2-year-old intact female domestic longhair cat with severe feline panleukopenia. The intestinal loops are dilated and flaccid and discolored red to purple. Ruler = 1 cm.
Histopathologic Findings
Histopathologic findings in the intestinal tract are similar to those described for CPV-2 infection, with crypt dilation and necrosis of crypt epithelial cells, accumulation of cellular debris, neutrophil infiltration, loss of villi, and submucosal edema throughout the small and large intestines; the jejunum and ileum are usually most severely affected (Figure 19-3 , A). Acutely affected cats show widespread lymphoid depletion and there may be hyperplasia of mononuclear phagocytes. Intranuclear inclusions are found in some cats (Figure 19-3, B). Examination of the bone marrow may reveal bone marrow hypoplasia. Examination of the cerebellum shows cellular depletion; reactive astrocytosis may be present. Immunohistochemistry or immunofluorescent antibody may be used to document the presence of the virus within tissues (see Figure 19-3, C).
FIGURE 19-3.
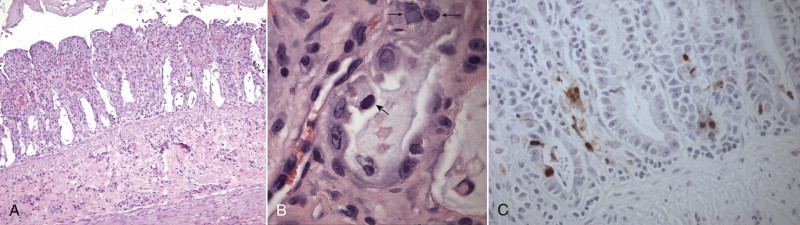
A, Histopathology of the jejunum from a kitten with panleukopenia. Villi are rounded and blunted with nearly complete epithelial loss and crypt dilation. H&E stain. B, Histopathology of the jejunum from a young barn cat that died after several days of profound weakness and neurologic signs. Six other cats in the barn died with the same signs. Mucosal crypts are dilated and contain debris, and intranuclear inclusions are present (small arrows). Another cell (large arrow) is foamy and degenerate and has a hyperchromatic nucleus. H&E stain. C, Same cat as in B. The presence of FPV in the intestinal tract is confirmed with immunohistochemistry (brown stain).
(Courtesy Dr. Patricia Pesavento, University of California, Davis Veterinary Anatomic Pathology Service.)
Treatment and Prognosis
Antimicrobial Treatment and Supportive Care
Treatment of cats with feline panleukopenia is with supportive care, especially intravenous crystalloids and parenteral antimicrobial drug treatment as for CPV-2 infections (see Chapter 14). Dextrose supplementation of the fluids may be required, and blood glucose concentration should be monitored. Oral intake of food and water should be withheld until vomiting has ceased. Experience with early enteral nutrition has not been reported in cats, and extreme care is warranted to prevent aspiration pneumonia. Antiemetics such as metoclopramide or ondansetron may be effective. In contrast to canine parvoviral enteritis, treatment with rfIFN-ω has not been beneficial for treatment of feline panleukopenia, although increased antibody production and a reduced acute inflammatory response was observed.28
Prognosis
Cats with panleukopenia that survive the first 5 days of treatment usually recover, although recovery is often more prolonged than it is for dogs with parvoviral enteritis. In 244 cats with feline panleukopenia from Europe, the survival rate was 51.1%.3 Nonsurvivors had lower leukocyte and platelet counts than survivors, and cats with white cell counts below 1000/µL were almost twice as likely to die than those with white cell counts above 2500/µL. Only total leukopenia, and not lymphopenia, was correlated with mortality. Hypoalbuminemia and hypokalemia were also associated with an increased risk of mortality. In contrast to dogs with parvoviral enteritis, mortality in cats does not appear to be correlated with age.
Cerebellar signs in kittens with cerebellar hypoplasia typically do not progress and may improve slightly as a result of compensatory responses from other senses such as vision.29
Immunity and Vaccination
Recovery from feline panleukopenia is thought to confer lifelong immunity. Effective vaccines are widely available, and include both parenteral inactivated and attenuated live viral vaccines. An intranasal FPV, FHV-1 and feline calicivirus vaccine is available; its use has been controversial, because panleukopenia is a systemic disease. An outbreak of salmonellosis and panleukopenia occurred in one cattery that used an intranasal FPV vaccine.4 No difference was noted in seroconversion rates between five cats vaccinated with the intranasal vaccine and five cats vaccinated with a parenteral attenuated live vaccine, but the number of cats in this study was small, and a larger number of cats that were vaccinated with the parenteral vaccine had protective antibody titers on day 7.30
Both inactivated and attenuated live vaccine types induce protective antibody titers after vaccination in a high proportion of cats,5 although the attenuated live vaccine may be more likely to induce protective titers than the inactivated vaccine.24 Provided maternally-derived antibody (MDA) is absent, immunity occurs within 1 week after a single vaccination with an attenuated live viral vaccine31 and lasts for at least 3 years, and possibly for life.32 Nevertheless, two doses, 3 to 4 weeks apart, have been recommended for initial vaccination with attenuated live vaccines in the absence of MDA. Two injections are always required for inactivated vaccines, and maximal immunity does not occur until 1 week after the second dose. However, even with an inactivated vaccine, challenge 7.5 years after vaccination was associated with protection.33 The use of inactivated vaccines should be reserved for immunosuppressed cats or colostrum-deprived neonates that are less than 4 weeks of age, or when there is a need to vaccinate pregnant cats. In shelter situations, the use of attenuated live vaccines is always recommended because of the slow onset of immunity with inactivated vaccines.34 FPV vaccines protect cats against challenge with CPV-2b strains.35., 36. However, cross-reactivity of antibodies induced by FPV vaccination to CPV-2 strains was lower than that to FPV as determined using hemagglutination inhibition.37 The extent to which FPV vaccines protect against infection with other CPV-2 variants requires further investigation.38
The most common reason for vaccine failure is interference by MDA. Maternal antibody persists until at least 12 weeks, and possibly longer in some cats. Virus neutralization titers above 1:10 are likely to interfere with vaccination, and kittens with titers below 1:40 are generally considered to be susceptible to infection by FPV. Kittens should be vaccinated every 3 to 4 weeks from 6 to 8 weeks of age, and it is recommended that the last vaccine in the kitten series be given no earlier than 14 to 16 weeks of age. When there is a history of an outbreak situation, the final booster could be given no earlier than 18 to 20 weeks of age.39 In all situations, a booster should be administered at 1 year, and every 3 years thereafter.
Vaccination of pregnant queens with attenuated live viral vaccines can cause cerebellar hypoplasia or fetal losses. The frequency with which this occurs is unknown. As a result, it has been suggested that pregnant queens only be vaccinated with attenuated live FPV vaccines if they are being introduced into a shelter and quarantine while immunization with inactivated vaccines is performed is not possible. Alternatively, assessment for protective antibody titers with an in-house test kit (where available) could be performed.
Prevention
New kittens should not be introduced into households that previously contained cats infected with FPV unless they are fully vaccinated. In the face of an outbreak, exposed and susceptible kittens may be effectively protected for 2 to 4 weeks through subcutaneous or intraperitoneal administration of 2 mL of type-matched serum from cats with a high antibody titer.39., 40. However, this is only effective when administered before the onset of clinical signs, and it can interfere with subsequent vaccination. For these kittens, it has been recommended that vaccination be withheld for 3 weeks after the serum has been administered.39 Passive immunization may be useful when cats are introduced into a shelter situation where a known problem exists. Repeated treatment with serum should be avoided because hypersensitivity reactions may occur. Prevention of feline panleukopenia should also include proper disinfection with disinfectants that are effective against parvoviruses, such as bleach, accelerated hydrogen peroxide, or potassium peroxymonosulfate (see Chapter 11) and, in shelter situations, isolation or removal of cats that develop gastrointestinal illness, and separate housing for healthy kittens.
Public Health Aspects
Although FPV is not known to infect humans, a unique strain of FPV was recently isolated from a diarrheic monkey in China.41 This strain was shown to cause panleukopenia in inoculated cats.
CASE EXAMPLE.
Signalment
“Callie”, a 2 year-old, female intact domestic longhair from Woodland, CA
History
Callie was brought to an emergency clinic for acute onset of collapse and severe illness. The current owner had fostered the cat for 1 week after she was found as a stray. The cat had been nursing a litter of kittens. The kittens were 4 weeks old, being weaned and apparently healthy. Since being fostered, the cat had exhibited a progressive decrease in appetite and thirst, and her feces had become soft and pasty. The night before she was brought to the emergency clinic, she had been bathed, and afterwards she vomited bile-stained fluid twice and was placed on a heating pad. The following morning she was found laterally recumbent and minimally responsive.
Physical Examination
Body Weight
2.3 kg
General
Stuporous mentation, estimated to be 8% to 10% dehydrated, T < 92°F (<33°C), HR = 132 beats/min, RR = 32 breaths/min, mucous membranes pale and tacky, unable to assess CRT. Fecal and urinary stains were present around the perineum and on the caudal aspect of the pelvic limbs.
Musculoskeletal
Body condition score 2/9. Diffuse muscle wasting was present. The cat was laterally recumbent and nonambulatory.
Cardiovascular
Weak femoral pulses. No murmurs or arrhythmias were detected.
Gastrointestinal and Urogenital
The abdomen was soft and nonpainful on palpation. Fluid-filled intestinal loops were palpated, and the urinary bladder was small (<5 cm) and soft.
All Other Systems
No abnormalities were detected.
Laboratory Findings
CBC
HCT 46% (30-50%)
MCV 49.8 fL (42-53 fL)
MCHC 30.4 g/dL (30-33.5 g/dL)
WBC 150 cells/µL (4500-14,000 cells/µL)
Neutrophils 0 cells/µL (2000-9000 cells/µL)
Lymphocytes 141 cells/µL (1000-7000 cells/µL)
Highly reactive lymphocytes 6 cells/µL
Monocytes 3 cells/µL (50-600 cells/µL)
Platelets 32,000 platelets/µL (180,000-500,000 platelets/µL).
Serum Chemistry Profile
Sodium 141 mmol/L (151-158 mmol/L)
Potassium 4.0 mmol/L (3.6-4.9 mmol/L)
Chloride 111 mmol/L (117-126 mmol/L)
Bicarbonate 23 mmol/L (15-21 mmol/L)
Phosphorus 6.0 mg/dL (3.2-6.3 mg/dL)
Calcium 7.4 mg/dL (9.0-10.9 mg/dl)
BUN 24 mg/dL (18-33 mg/dL)
Creatinine 0.5 mg/dL (1.1-2.2 mg/dL)
Glucose 68 mg/dL (63-118 mg/dL)
Total protein 3.2 g/dL (6.6-8.4 g/dL)
Albumin 1.6 g/dL (2.2-4.6 g/dL)
Globulin 1.6 g/dL (2.8-5.4 g/dL)
ALT 125 U/L (27-101 U/L)
AST 143 U/L (17-58 U/L)
ALP 4 U/L (14-71 U/L)
Creatine kinase 2409 U/L (73-260 U/L)
Gamma GT <3 U/L (0-4 U/L)
Cholesterol 68 mg/dL (89-258 mg/dL)
Total bilirubin 0.2 mg/dL (0-0.2 mg/dL)
Magnesium 2.5 mg/dL (1.5-2.5 mg/dL).
Imaging
An abdominal ultrasound showed marked fluid distention of the small intestines.
Microbiologic Testing
In-clinic ELISA serology for FeLV antigen and FIV antibody: negative.
Treatment and Outcome
Callie was treated with active warming, and a central venous access line was placed. Intravenous crystalloids (four warmed 60-mL boluses of lactated Ringer’s solution [LRS], each given over 20 minutes) were administered, after which an venous acid-base panel showed a pH of 7.267 (7.31-7.46), bicarbonate of 17.5 mmol/L (14-22 mmol/L), base excess of −8.1 mmol/L (−4 to +2 mmol/L), pCO2 of 39.7 mm Hg (25-37 mmHg), lactate of 4.5 mEq/L (<2 mEq/L), glucose of 52 mg/dL, potassium of 3.1 mEq/L, sodium of 141 mEq/L, and ionized calcium of 1.17 (1.1-1.4 mmol/L). A dextrose bolus and ticarcillin-clavulanic acid (22 mg/kg, q6h, IV) were administered. The cat repeatedly vomited blood-tinged fluid, and so treatment with metoclopramide (0.02 mg/kg/hr) and famotidine (0.5 mg/kg IV q12h) was initiated. Aggressive crystalloid fluid therapy was continued (LRS supplemented with 30 mEq/L KCl and 2.5% dextrose), and treatment with hetastarch was also initiated. Systolic blood pressure (Doppler) was 60 to 90 mm Hg, heart rate increased to 170 beats/min, and rectal temperature increased to 100°F. When the CBC results were available, the cat was placed in the isolation ward.
Pasty diarrhea that contained sloughed mucosa occurred every 1 to 2 hours, which transitioned to liquid red feces over 24 hours. A CBC again showed absolute neutropenia and 15,000 platelets/µL. After another 24 hours, the cat’s condition deteriorated despite aggressive treatment and monitoring. Partial parenteral nutrition was initiated. That evening, pyrexia developed (104.6°F) as well as tachypnea and increased respiratory effort. The owner elected euthanasia. The cat regurgitated approximately 200 mL of dark brown fluid at the time of euthanasia.
Gross Necropsy Findings
Ten mL of semiopaque red fluid was present in the peritoneal cavity. Streaks of hemorrhage were noted throughout the skeletal muscle. Between the pylorus and the ileocecocolic junction, the serosa of the small intestinal tract was dark red to purple to black (see Figure 19-2). The entire small and large intestinal tract was dilated and flaccid. The small intestinal mucosa was dark red to purple and contained a small amount of dark red mucoid material. The mesenteric lymph nodes were prominent, and their cut surface was discolored red to dark pink. The liver was pale yellow and extended beyond the costal arches. Numerous petechial hemorrhages were present on the serosal surface of the urinary bladder.
Histopathologic Findings
Within the duodenum, jejunum, and ileum there was severe, subacute, diffuse necrotizing and fibrinohemorrhagic enteritis. The villi were necrotic, fused, and markedly blunted with a minimal inflammatory response. Necrosis extended into the crypts, and there was some evidence of a regenerative response that included enterocyte hypertrophy, karyomegaly, and rare mitotic figures. Mixed bacteria, which included small gram-negative rods and large numbers of gram-positive cocci, lined and effaced the denuded villi. There was diffuse lymphoid depletion in lymph nodes, as well as the mucosa-associated lymphoid tissue in the cecum and colon. Marked depletion of erythroid and myeloid precursors was present in the bone marrow. There were multifocal areas of hemorrhage in the skeletal and cardiac muscle, intestinal tract, and lungs, as well as fibrin thrombi in the lungs. FPV antigen was identified in intestinal epithelial cells using immunohistochemistry. Culture of the jejunum for Salmonella spp. was negative.
Diagnosis
Feline panleukopenia
Comments
The cat in this report was a stray with an unknown vaccination history, which demonstrates that severe disease can occur even in adult cats. Antemortem diagnostic testing for FPV with a fecal antigen ELISA assay or PCR assay was discussed with the owner, but was declined because of the high degree of suspicion for the disease and the lack of impact that a positive or negative result would have had on the treatment plan. Secondary bacterial sepsis was suspected. Additional co-infections with other viruses, such as feline coronavirus, or enteropathogenic bacteria could not be ruled out. During the cat’s treatment, one of the kittens in the litter died suddenly and the other developed signs of illness.
Suggested Readings
- Kruse B.D., Unterer S., Horlacher K. Prognostic factors in cats with feline panleukopenia. J Vet Intern Med. 2010;24:1271–1276. doi: 10.1111/j.1939-1676.2010.0604.x. [DOI] [PubMed] [Google Scholar]
- Neuerer F.F., Horlacher K., Truyen U. Comparison of different in-house test systems to detect parvovirus in faeces of cats. J Feline Med Surg. 2008;10:247–251. doi: 10.1016/j.jfms.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen U., Addie D., Belak S. Feline panleukopenia. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:538–546. doi: 10.1016/j.jfms.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Verge J., Christoforoni N. La gastroenterite infectieuse des chats; est-elle due a un virus filtrable. C R Seances Soc Biol Fil. 1928;99:312. [Google Scholar]
- 2.Steinel A., Parrish C.R., Bloom M.E. Parvovirus infections in wild carnivores. J Wildl Dis. 2001;37:594–607. doi: 10.7589/0090-3558-37.3.594. [DOI] [PubMed] [Google Scholar]
- 3.Kruse B.D., Unterer S., Horlacher K. Prognostic factors in cats with feline panleukopenia. J Vet Intern Med. 2010;24:1271–1276. doi: 10.1111/j.1939-1676.2010.0604.x. [DOI] [PubMed] [Google Scholar]
- 4.Addie D.D., Toth S., Thompson H. Detection of feline parvovirus in dying pedigree kittens. Vet Rec. 1998;142:353–356. doi: 10.1136/vr.142.14.353. [DOI] [PubMed] [Google Scholar]
- 5.Fischer S.M., Quest C.M., Dubovi E.J. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc. 2007;230:52–58. doi: 10.2460/javma.230.1.52. [DOI] [PubMed] [Google Scholar]
- 6.Decaro N., Desario C., Miccolupo A. Genetic analysis of feline panleukopenia viruses from cats with gastroenteritis. J Gen Virol. 2008;89:2290–2298. doi: 10.1099/vir.0.2008/001503-0. [DOI] [PubMed] [Google Scholar]
- 7.Battilani M., Balboni A., Ustulin M. Genetic complexity and multiple infections with more Parvovirus species in naturally infected cats. Vet Res. 2011;42:43. doi: 10.1186/1297-9716-42-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K., Sakamoto M., Ikeda Y. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clin Diagn Lab Immunol. 2001;8:663–668. doi: 10.1128/CDLI.8.3.663-668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohshima T., Mochizuki M. Evidence for recombination between feline panleukopenia virus and canine parvovirus type 2. J Vet Med Sci. 2009;71:403–408. doi: 10.1292/jvms.71.403. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda Y., Mochizuki M., Naito R. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology. 2000;278:13–19. doi: 10.1006/viro.2000.0653. [DOI] [PubMed] [Google Scholar]
- 11.Muir P., Harbour D.A., Gruffydd-Jones T.J. A clinical and microbiological study of cats with protruding nictitating membranes and diarrhoea: isolation of a novel virus. Vet Rec. 1990;127:324–330. [PubMed] [Google Scholar]
- 12.Marshall J.A., Kennett M.L., Rodger S.M. Virus and virus-like particles in the faeces of cats with and without diarrhoea. Aust Vet J. 1987;64:100–105. doi: 10.1111/j.1751-0813.1987.tb09638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman L.B., Lyi S.M., Johnson N.C. Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection. J Virol. 2010;84:4969–4978. doi: 10.1128/JVI.02623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moschidou P., Martella V., Lorusso E. Mixed infection by feline astrovirus and feline panleukopenia virus in a domestic cat with gastroenteritis and panleukopenia. J Vet Diagn Invest. 2011;23:581–584. doi: 10.1177/1040638711404149. [DOI] [PubMed] [Google Scholar]
- 15.Lutz H., Castelli I., Ehrensperger F. Panleukopenia-like syndrome of FeLV caused by co-infection with FeLV and feline panleukopenia virus. Vet Immunol Immunopathol. 1995;46:21–33. doi: 10.1016/0165-2427(94)07003-p. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami T., Shirota K., Goto K. Enterocolitis associated with dual infection by Clostridium piliforme and feline panleukopenia virus in three kittens. Vet Pathol. 1999;36:613–615. doi: 10.1354/vp.36-6-613. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki M., Osawa N., Ishida T. Feline coronavirus participation in diarrhea of cats. J Vet Med Sci. 1999;61:1071–1073. doi: 10.1292/jvms.61.1071. [DOI] [PubMed] [Google Scholar]
- 18.Sharp N.J., Davis B.J., Guy J.S. Hydranencephaly and cerebellar hypoplasia in two kittens attributed to intrauterine parvovirus infection. J Comp Pathol. 1999;121:39–53. doi: 10.1053/jcpa.1998.0298. [DOI] [PubMed] [Google Scholar]
- 19.Url A., Truyen U., Rebel-Bauder B. Evidence of parvovirus replication in cerebral neurons of cats. J Clin Microbiol. 2003;41:3801–3805. doi: 10.1128/JCM.41.8.3801-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meurs K.M., Fox P.R., Magnon A.L. Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc Pathol. 2000;9:119–126. doi: 10.1016/S1054-8807(00)00031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Digangi B.A., Gray L.K., Levy J.K. Detection of protective antibody titers against feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus in shelter cats using a point-of-care ELISA. J Feline Med Surg. 2011;13:912–918. doi: 10.1016/j.jfms.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd-Eldaim M., Beall M., Kennedy M. Detection of feline panleukopenia virus using a commercial ELISA for canine parvovirus. Vet Ther. 2009;10:E1–E6. [PubMed] [Google Scholar]
- 23.Neuerer F.F., Horlacher K., Truyen U. Comparison of different in-house test systems to detect parvovirus in faeces of cats. J Feline Med Surg. 2008;10:247–251. doi: 10.1016/j.jfms.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson E.V., Reese M.J., Tucker S.J. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359–363. doi: 10.2460/javma.230.3.359. [DOI] [PubMed] [Google Scholar]
- 25.Decaro N., Desario C., Lucente M.S. Specific identification of feline panleukopenia virus and its rapid differentiation from canine parvoviruses using minor groove binder probes. J Virol Methods. 2008;147:67–71. doi: 10.1016/j.jviromet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi M., Yuri K., Soma T. Differentiation of vaccine virus from field isolates of feline panleukopenia virus by polymerase chain reaction and restriction fragment length polymorphism analysis. Vet Microbiol. 1996;53:283–293. doi: 10.1016/s0378-1135(96)01225-4. [DOI] [PubMed] [Google Scholar]
- 27.De Lahunta A. Comments on cerebellar ataxia and its congenital transmission in cats by feline panleukopenia virus. J Am Vet Med Assoc. 1971;158(Suppl 2):901–906. [PubMed] [Google Scholar]
- 28.Paltrinieri S., Crippa A., Comerio T. Evaluation of inflammation and immunity in cats with spontaneous parvovirus infection: consequences of recombinant feline interferon-omega administration. Vet Immunol Immunopathol. 2007;118:68–74. doi: 10.1016/j.vetimm.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penderis J. The wobbly cat. Diagnostic and therapeutic approach to generalised ataxia. J Feline Med Surg. 2009;11:349–359. doi: 10.1016/j.jfms.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lappin M.R., Veir J., Hawley J. Feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus antibody responses in seronegative specific pathogen-free cats after a single administration of two different modified live FVRCP vaccines. J Feline Med Surg. 2009;11:159–162. doi: 10.1016/j.jfms.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jas D., Aeberle C., Lacombe V. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet J. 2009;182:86–93. doi: 10.1016/j.tvjl.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Gore T.C., Lakshmanan N., Williams J.R. Three-year duration of immunity in cats following vaccination against feline rhinotracheitis virus, feline calicivirus, and feline panleukopenia virus. Vet Ther. 2006;7:213–222. [PubMed] [Google Scholar]
- 33.Scott F.W., Geissinger C.M. Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. Am J Vet Res. 1999;60:652–658. [PubMed] [Google Scholar]
- 34.Day M.J., Horzinek M.C., Schultz R.D. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract. 2010;51:1–32. doi: 10.1111/j.1748-5827.2010.00959a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamoh K., Senda M., Inoue Y. Efficacy of an inactivated feline panleucopenia virus vaccine against a canine parvovirus isolated from a domestic cat. Vet Rec. 2005;157:285–287. doi: 10.1136/vr.157.10.285. [DOI] [PubMed] [Google Scholar]
- 36.Chalmers W.S., Truyen U., Greenwood N.M. Efficacy of feline panleucopenia vaccine to prevent infection with an isolate of CPV2b obtained from a cat. Vet Microbiol. 1999;69:41–45. doi: 10.1016/s0378-1135(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K., Ikeda Y., Miyazawa T. Characterisation of cross-reactivity of virus neutralising antibodies induced by feline panleukopenia virus and canine parvoviruses. Res Vet Sci. 2001;71:219–222. doi: 10.1053/rvsc.2001.0492. [DOI] [PubMed] [Google Scholar]
- 38.Decaro N., Buonavoglia D., Desario C. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Res Vet Sci. 2010;89:275–278. doi: 10.1016/j.rvsc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truyen U., Addie D., Belak S. Feline panleukopenia. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:538–546. doi: 10.1016/j.jfms.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy J.K., Crawford P.C., Collante W.R. Use of adult cat serum to correct failure of passive transfer in kittens. J Am Vet Med Assoc. 2001;219:1401–1405. doi: 10.2460/javma.2001.219.1401. [DOI] [PubMed] [Google Scholar]
- 41.Yang S., Wang S., Feng H. Isolation and characterization of feline panleukopenia virus from a diarrheic monkey. Vet Microbiol. 2010;143:155–159. doi: 10.1016/j.vetmic.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


