Key Points
Osteonecrosis affects younger patients more often than osteoarthritis and has significantly greater long-term morbidity.
Corticosteroids constitute the most common cause of nontraumatic osteonecrosis.
The femoral head is the most common site of osteonecrosis.
Bisphosphonate use is associated with osteonecrosis of the jaw.
The final common pathway in the pathogenesis of osteonecrosis is disruption of blood supply to a segment of bone.
Abnormalities in lipid metabolism, bone homeostasis, regulation of apoptosis, coagulopathies, and oxidative stress may play a role in the pathogenesis of osteonecrosis.
Magnetic resonance imaging is currently the optimal test for early diagnosis and identification of the extent of osteonecrosis.
Nonsurgical treatment of osteonecrosis does not change the natural history of the disease.
Although there are many variations on surgical treatment of femoral head osteonecrosis, most patients eventually require total hip arthroplasty.
Knowledge of risk factors and early detection are crucial to the successful management of osteonecrosis.
Due to the lack of successful treatment options, new modes focus on prevention of osteonecrosis.
Osteonecrosis literally means “bone death” (ossis [Latin] = bone; necrosis = killing or causing to die). Other synonyms include avascular necrosis, ischemic necrosis of bone, aseptic necrosis, and subchondral avascular necrosis. The term osteonecrosis dissecans is sometimes used synonymously with osteonecrosis, although, strictly speaking, it is a consequence of osteonecrosis involving dessication of bone leading to fracturing or cracking of bone. The concept of bone death was first described by Hippocrates,1 but the first clinical description of osteonecrosis was a case of sepsis-induced bone death described by Russell in 1794.2 It was almost a century later that bone death was described to occur in the absence of infection.3 The first report of osteonecrosis in a deep sea diver appeared in 1936.4 The pathogenesis of osteonecrosis is complex, but whatever the mechanism, bone death ultimately occurs as a result of complete or partial disruption of the delivery of oxygen and/or nutrients to the bone and surrounding tissues. It is likely that multiple molecular mechanisms may be simultaneously in play in order for osteonecrosis to occur.5, 6
Epidemiology
The prevalence of osteonecrosis is unknown, but it is estimated that there are 10,000 to 20,000 new patients diagnosed per year in the United States. Osteonecrosis occurs in 15% to 80% of patients with femoral neck fractures.7 Ten percent of the 500,000 hip replacements done in the United States each year are thought to be for osteonecrosis.8 The disease primarily affects men, with a notable exception for osteonecrosis associated with systemic lupus erythematosus, which has a significant female predominance. Osteonecrosis primarily occurs in the third to fifth decade of life.9 As a result of this age distribution, long-term morbidity can be significant because most hip replacements have a finite period of viability.
Etiology
Osteonecrosis has been linked to numerous conditions (Table 103-1 ). The strength of a causal relationship varies greatly, and in some cases only case reports have been published. The most common cause of nontraumatic osteonecrosis is corticosteroid use, which was first described in 1957.10 Although other adverse effects of corticosteroids are perhaps better known, osteonecrosis of the femoral head is one of the serious complications.
Table 103-1.
Conditions Associated with Osteonecrosis
| Dietary, Drugs, and Environmental Factors |
| Musculoskeletal Conditions: Compromise in Structural Integrity |
| Metabolic Diseases: Abnormality in Fat or Other Metabolic Component |
| Hematologic Conditions: Abnormalities in Blood Components |
| Rheumatologic Conditions |
| Infectious Diseases |
| Oncologic Disorders, Transplantation, and Their Treatment |
In a 1998 study, in which the investigators reviewed associations in 2500 to 3300 cases of nontraumatic osteonecrosis, corticosteroid use was present in 34.7% of cases. Alcohol use was found in 21.7% of the cases, and the remainder was idiopathic. Although the risk of developing osteonecrosis with corticosteroid use is small, the severity of the adverse event and the high morbidity associated with osteonecrosis make this an important complication to consider when starting a patient on corticosteroids.
Studies have attempted to determine the duration of use and the dosages of corticosteroids necessary to precipitate osteonecrosis. There are several forms of corticosteroids of differing potency and half-life, and dosages and duration of use vary between studies, so any conclusions about a “safe” dose of corticosteroids are wrought with potential confounding variables and errors. In one study of 20 patients diagnosed with stage 1 osteonecrosis by magnetic resonance imaging (MRI), the interval between the use of steroids and diagnosis ranged from 1 to 16 months.11 The cumulative dose of steroids in this study ranged from 1800 to 15,505 mg (mean, 5928 mg) of prednisolone or the equivalent. In other studies cumulative doses of steroids associated with osteonecrosis ranged from 48012 to 432013 mg of dexamethasone dose equivalence. A recent paper by Powell and colleagues14 attempted to collectively analyze the available literature to derive maximum safe levels for duration, maximum daily dose, and average daily dose of corticosteroids. The study confirmed that many other confounding variables affect the development of osteonecrosis, making analysis of dose-response risk for an isolated association difficult. Nonetheless, corticosteroid-induced osteonecrosis is dependent on dosage and the risk factor is higher with the long-acting steroids and with parenteral usage.
Additional host-inherent risk factors also play a role in susceptibility. The incidence of osteonecrosis in a group of patients receiving glucocorticoid replacement therapy for primary or secondary adrenal insufficiency was 2.4%. In a study of renal transplantation patients, the 26 patients who developed osteonecrosis had a higher cumulative oral dose of prednisone after 1 and 3 months compared with 28 control transplant patients who did not develop osteonecrosis.15 A separate study estimated the incidence of osteonecrosis in renal transplant patients to be 5%.16 There is no evidence to consistently link the use of topical, inhaled, or nasal corticosteroids to osteonecrosis. The evidence for an association between osteonecrosis and intramuscular or intra-articular corticosteroids is limited to case reports.17 Parenteral use poses a higher risk because of rapid absorption and longer half-life of the drugs used.
Bisphosphonate-induced osteonecrosis of the jaw is particularly interesting because of the intended use of bisphosphonates on bone diseases.18, 19, 20 There has been a link between cigarette smoking and osteonecrosis, with smokers having a threefold higher relative risk for developing osteonecrosis, independent of all other factors.21, 22
The association between osteonecrosis and alcohol consumption was first described in 1922.23 A study of patients with idiopathic osteonecrosis revealed that the risk of osteonecrosis increased with increasing daily consumption of alcohol.21 The subjects were divided into three groups on the basis of their alcohol consumption of less than 400 mL/week, 400 to 1000 mL/week, and greater than 1000 mL/week, and the relative risk of osteonecrosis, independent of corticosteroid use or smoking, was 3-fold, 10-fold, and 18-fold, respectively, when compared with hospital controls. Liver damage was also found unnecessary for the development of osteonecrosis in alcohol-consuming patients, although elevated liver enzymes may be present.24 The incidence of osteonecrosis in patients who received treatment for alcoholism was 5.3%. The femoral head was again the most common site (82 of 92 lesions), with the other 10 sites involving the humeral head.25
Musculoskeletal conditions can lead to osteonecrosis in children. Legg-Calvé-Perthes disease was first described in children between 3 and 12 years of age in 1910.26, 27, 28 Femoral head osteonecrosis is a feature of this disease and has been linked to trauma,29, 30 congenital hip dislocation,31 and transient synovitis.32 Bilateral involvement is common, and associated clinical manifestations include abnormal growth and stature,33, 34 delayed skeletal maturation,35 disproportionate skeletal growth,33 congenital anomalies,36 and abnormal hormone levels.37, 38 Children with acute lymphoblastic leukemia can develop osteonecrosis39, 40 as well, but this may be a result of steroid use. An additional risk factor for this cohort of patients is high body mass index.41
Osteonecrosis has also been associated with metabolic disorders and in pregnancy. Diagnosis is often delayed until months after delivery. Women who develop ostenecrosis in pregnancy tended to have a small body frame and a large weight gain.42
Hematologic conditions have been associated with osteonecrosis. The long-term morbidity of osteonecrosis in patients with sickle cell anemia is dismal.43 Common deformities include decreased mobility, abnormal gait, and leg-length discrepancy.44 Osteonecrosis in hemophilia patients has been reported, but no statistically reliable causal link can be established.45, 46, 47, 48, 49, 50
Dysbaric osteonecrosis was first described in construction workers in the Elhe tunnel exposed to high-pressure environments.51 The prevalence of dysbaric osteonecrosis is 4.2% in divers and 17% in compressed air workers.52 Patients with dysbaric osteonecrosis may have more than one lesion, and common sites besides the femoral head include the tibia and the humeral head and shaft. The condition is not related to decompression sickness, and although proper decompression procedures can reduce “the bends,” they do not have any effect on the development of osteonecrosis, which can occur months or years after the last exposure to high-pressure environments.
Osteonecrosis has also been associated with a number of infectious diseases including severe acute respiratory syndrome (SARS). Many patients who contracted SARS in the early 2000s received treatment with corticosteroids, and some subsequently developed osteonecrosis.53 The incidence of osteonecrosis appears higher in this group of patients compared with patients with other conditions who were treated with corticosteroids.54 Chan and colleagues55 reported five children with SARS treated with corticosteroids who developed osteonecrosis.
Clinical Features
The primary presenting symptom in osteonecrosis is pain. In osteonecrosis of the femoral hip, the pain is located in the hip joint but may radiate to the groin, anterior thigh, or knee. The severity of the pain can vary, depending on the size of the infarct and whether the onset of disease is insidious or sudden. In trauma, where there is sudden and severe disruption of blood flow, and in Gaucher's disease, dysbarism, or hemoglobinopathy, where the infarcts are large, pain can be intense and sudden. In other conditions where the onset is more insidious, the pain can follow a gradual and slow incremental progression. The pain of osteonecrosis is usually increased with use of the joint, but in advanced disease the pain can be persistent at rest. Limitation of range of motion is progressive and is usually a late symptom, except when resulting from accompanying pain. The risk of developing osteonecrosis of the contralateral hip when one side is affected ranges from 31% to 55%.
In addition to the femoral head, osteonecrosis can affect other sites including the humeral head,56, 57, 58, 59 femoral condyles60, 61, 62, 63 and proximal tibiae,61, 64, 65, 66 wrists and ankles,67 bones of the hands and feet,68 the vertebrae,69, 70, 71 jaw,72, 73, 74, 75 and bony structures of the face.76 Osteonecrosis of the humeral head is the second most commonly seen location, and pain is usually in the shoulder and associated with reduced range of motion and weakness. Pain in the ankle is the main presenting symptom in nontraumatic osteonecrosis of the talus, and in some cases, the disease had already progressed to Ficat and Arlet stage 3 by the time of presentation of pain.67 Kienböck's disease involves osteonecrosis of the lunate. Patients present with pain in the radiolunate joint, along with weakness and limitation of motion. Keinböck's disease appears to be related to manual labor. Soccer players have been reported to develop osteonecrosis of the foot,77 and football players may be prone to developing osteonecrosis of the hip.78
The Ficat and Arlet method of staging osteonecrosis consists of four stages. Stages 1 and 2 are reversible, whereas stage 3 (subchondral collapse) and stage 4 (joint space narrowing and destruction of cartilage) are irreversible. The Marcus staging system consists of six stages, in which the first two are reversible and the subsequent four are irreversible. The modified Steinberg staging system is based on the Marcus system and also consists of six stages. Each stage is further divided into three subclasses on the basis of the extent of femoral head involvement. Subclass A involves less than 25%; B involves 26% to 50%, and C involves greater than 50%.
Table 103-2 shows the Modified Steinberg system for staging osteonecrosis. The Association of Research Circulation Osseous (ARCO) has proposed a modification to the Ficat and Arlet system, adding a stage 0 or patients with negative imaging studies but who are at risk for developing osteonecrosis. In addition, stages 1 and 3 are further stratified to take into account lesion size, location, and extent of collapse.79 In 2001 the Japanese Ministry of Health, Labor and Welfare proposed revising criteria for the diagnosis and staging of osteonecrosis of the femoral head.80 Diagnostic criteria included the following: (1) collapse of the femoral head without joint space narrowing or acetabular abnormality on plain radiograph, (2) demarcating sclerosis in the femoral head without joint space narrowing or acetabular abnormality, (3) “cold in hot” on bone scans, (4) low-intensity band on T1-weighted MRI, and (5) trabecular and marrow necrosis on histology. If a patient fulfills two of the five criteria, the diagnosis is established. The working group also proposed four types of lesions on the basis of extensiveness and defined stages of disease on the basis of diagnostic imaging.
Table 103-2.
Modified Steinberg Staging Systems for Osteonecrosis
| Stage | Radiographic Appearance | Reversible |
|---|---|---|
| I | Normal radiographs, but abnormal bone scan or magnetic resonance image | Yes |
| II | Lucent and sclerotic changes | Yes |
| III | Subchondral fracture without flattening | No |
| IV | Subchondral fracture with flattening or segmental depression of femoral head | No |
| V | Joint space narrowing or acetabular changes | No |
| VI | Advanced degenerative changes | No |
Bone Marrow Edema
Bone marrow edema is a common observation in osteonecrosis and is frequently accompanied by vascular congestion. Bone marrow edema is not specific for osteonecrosis and may be seen in many musculoskeletal disorders including osteomyelitis, osteoarthritis, occult intraosseous fracture, stress fracture, osteoporosis, and sickle cell crisis.
A specific syndrome known as bone marrow edema syndrome has been described and was initially thought to be a precursor to osteonecrosis, but it is now believed to be a separate entity. Bone marrow edema is a transitory, self-limiting condition typically seen in middle-aged men and in women in their third trimester of pregnancy. Patients complain of pain, limited range of motion, and an abnormal gait. Osteopenia is detected on conventional radiographs, and MRI confirms this with a low signal on T1-weighted images and a high signal on T2-weighted images. The three phases of bone marrow edema syndrome include an initial phase lasting about 1 month, followed by a plateau phase lasting 1 or 2 months, and finally a regression phase lasting for an additional 4 to 6 months.81 Subchondral fractures do not occur. Biopsy specimens obtained in the initial phase show diffuse interstitial edema, fragmentation of fatty marrow cells, and increased new bone formation.82
A study of 24 cases of bone marrow edema syndrome of the knee showed that although migrating bone marrow edema occurred in a third of patients at a 5-year follow-up, the patients were asymptomatic and MRI signal alterations had resolved. Biopsy specimens of affected bone were obtained using arthroscopic surgery and core decompression, and histology revealed areas of bone marrow edema and vital trabeculae covered by osteblasts and osteoid seams. None of the cases progressed to osteonecrosis.83
Bisphosphonates and Osteonecrosis of the Jaw
Bisphosphonate is a class of drug used to treat osteoporosis and diseases where bone is not formed adequately. Bisphosphonates are composed of two forms, and osteonecrosis appears to occur in association with nitrogen-containing bisphosphonates. The mechanism of action of bisphosphonate-induced osteonecrosis of the jaw appears to parallel that of glucocorticoids, with derangement in lipid metabolism, bone homeostasis, and apoptosis of bone cells. It is interesting that the jawbone seems to be the most vulnerable bone in bisphosphonate-induced disease, as opposed to the femoral head in most other associations or causes of osteonecrosis. This may be because of the high bone turnover rate in the jaw or because bisphosphonates exert their action on not only bone but also many elements of the surrounding tissue including fibroblasts and blood vessels.
Pathogenesis
Anatomic Considerations in Trauma-Related Osteonecrosis
The femoral head is the most common site of osteonecrosis. An understanding of the anatomy of the femoral head may help to explain why that is the case. Three arterial networks supply the femoral head and neck. The extracapsular arterial ring consists of the lateral femoral circumflex artery and the medial femoral circumflex artery, which arise from the profunda femoris. The medial femoral circumflex artery and its branches supply most of the blood to the head and neck of the femur. The lateral femoral artery winds anterolaterally, and the medial femoral artery winds posteromedially around the neck of the femur, ultimately anastomosing with each other at the superolateral aspect of the femoral head. The lateral femoral circumflex artery and the medial femoral circumflex artery further anastomose with the superior and inferior gluteal branches of the internal iliac artery, providing collateral circulation between the femoral artery and the internal iliac artery. Small vessels known as retinacular arteries, ascending cervical branches of the extracapsular ring, form an intra-articular ring at the level of the cartilage. Eiphyseal arterial branches arise from this ring and penetrate the head and neck of the femur including the epiphyses. The artery of the ligament of the head of the femur is a branch of the obturator artery and may be the sole supplier of blood to the proximal fragment of the head.
Some of these anatomic features may render the femoral head particularly vulnerable to ischemia. The retinacular arteries are believed to supply 80% of the femoral epiphysis. Compromising this critical vascular system may lead to osteonecrosis originating in the anterosuperior aspect of the femoral head, as indicated by angiographic studies in early osteonecrosis in which these arteries are not visualized. A schematic of the blood supply to the femoral head is shown in Figure 103-1 .
Figure 103-1.
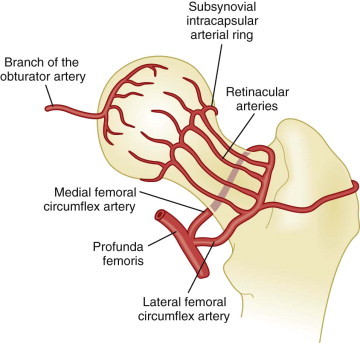
Schematic of the blood supply of the femoral head.
Histologically, after an infarct, a rim of bony thickening or sclerosis begins to form at the margins of the infarcted area. If the necrotic lesion is within the weight-bearing region of the femoral head, subchondral fractures follow. With repeated microfractures and continued weight bearing, the original fracture cannot heal completely and new fractures appear. The secondary fracture propagates along the junction between subchondral bone and the necrotic segment. As time goes on, the femoral head becomes flattened and eventually collapses. A nonspherical head articulating with the acetabulum produces friction and erosion and loss of cartilage. The cycle repeats itself, and the structure of the joint deteriorates, leading to degenerative changes and eventual total joint destruction.84
Nontraumatic Osteonecrosis
Disruption of the blood supply to the femoral head can occur through a number of different mechanisms. In traumatic osteonecrosis of the femoral head, the cause of this disruption is often viewed as completely mechanical and appears to be easily understood. But there may be an additional component to the disruption that is related to the immunologic and inflammatory changes that occur in damaged bone tissue and surrounding soft tissues.
The immunologic changes occurring in nontraumatic osteonecrosis may help explain why corticosteroids are particularly dangerous to the integrity of the blood supply of the femoral hip. Some have likened osteonecrosis to “coronary disease” of the hip85, 86 and propose that the same mechanisms that cause ischemia of the myocardium may also cause ischemia of the femoral head (Table 103-3 ).
Table 103-3.
Proposed Mechanism of Disease of Common Conditions Associated with Osteonecrosis
| Mechanism of Osteonecrosis |
||||||||
|---|---|---|---|---|---|---|---|---|
| Associated Condition | Apoptosis | Osteoblast/Osteoclast Homeostasis | Lipid Abnormalities | Coagulation Abnormalities | Oxidative Stress | Parathyroid/Calcium Imbalance | Vascular Plugging | Vasoactive Substances |
| Corticosteroids | X | X | X | X | X | X | ||
| Bisphosphonates | X | X | X | |||||
| Alcohol abuse | X | X | X | X | X | |||
| Trauma | X | X | X | |||||
| Renal transplantation | X | X | X | X | ||||
| Dialysis | X | |||||||
| Sickle cell disease | X | |||||||
Mechanical and Vascular Considerations
In Legg-Calvé-Perthes disease, obstruction to venous drainage elevates intraosseous pressure and consequently elevates intra-articular pressures. In a study of patients with Legg-Calvé-Perthes disease, bone scintigraphy using Tc99m methylene diphosphonate (Tc99m MDP) was employed to measure arterial and venous flow in the diseased hip. Although arterial flow was normal, there was significant disruption in venous drainage.87 This disturbance was reproduced in a dog model in which injection of silicone was used to obstruct venous flow distal to the hip.88 Ischemia resulted from the obstruction to venous drainage, leading to a cessation of endochondral ossification in the preosseous ephiphyseal cartilage and the physeal plate. Widening of the joint space ensued, followed by revascularization of the epiphysis and deposition of new immature bone. A weakened or unstable femoral epiphyseal plate resulted, and the subchondral bone became prone to segmental collapse and fracture.89
The pathologic mechanism of dysbaric osteonecrosis is unclear. The most intuitive explanation is that formation of gas bubbles causes arterial occlusion and ischemia. However, the true mechanism may not be quite so simple. Multiple other factors might contribute to the disease including thromboembolic events such as platelet aggregation, erythrocyte clumping, lipid coalescence, intraosseous vessel compression as a result of extravascular gas bubbles, formation of fibrin thrombi, and narrowing of arterial lumina owing to myointimal thickening caused by gas bubbles. The interaction between gas and blood can lead to the formation of vessel-occluding substances. All of these events can lead to redistribution of blood flow.
The increased vulnerability of bone to compression disorders has been explained by several factors including the relative rigidity of bone and inability to absorb increased gas pressure, inherent poor vascularization, and gas supersaturation of fatty marrow.90 A sheep model of dysbaric osteonecrosis has been developed. Exposure to compressed air at pressures of 2.6 to 2.9 atmospheres for 24 hours results in extensive bone and marrow necrosis. The authors proposed that the initial event involving elevated intramedullary pressures leads to the formation of nitrogen gas bubbles in the fatty marrow of the long bones. Radiography shows medullary opacities and endosteal thickening. Later, neovascularization of previously ischemic fatty marrow occurs, followed by new bone formation. Osteonecrosis occurs in subchondral cortical bone with marrow fibrosis and osteocyte loss.91
Changes in the vasculature, through injury or inflammation from other diseases, may in turn lead to a compromise in blood flow. Examples include structural damage to arteriolar walls, degeneration of the tunica media, smooth muscle cell necrosis, and disruption of the internal elastic lamina. These changes can lead to eventual hemorrhagic infarction, which was observed in a study of 24 core biopsy specimens from osteonecrotic femoral heads. The changes did not occur in 11 femoral heads with osteoarthrosis.92
Osteoimmunology
Although bone marrow is a critical component of the immune system, bone matrix is often perceived to be static scaffolding that functions primarily to support the musculoskeletal system. It is now known that, in fact, bone matrix is a dynamic tissue that is constantly replacing itself. It is estimated that about 10% of a person's bone is replaced every year. Diseases such as osteopetrosis and osteoporosis are a result of a dysfunction in the balance between bone deposition and bone resorption. The factors that regulate this homeostasis include cells of the bone matrix, immune cells, signaling molecules, cytokines and chemokines, and vitamins. Some of these regulatory factors may be present on both bone cells and immune cells, often serving different functions, thereby providing a link between the immune system and bone. Osteonecrosis, in fact, may be linked to such an imbalance in bone homeostasis. Immune factors may affect surrounding soft tissue as well, contributing to the development of osteonecrosis. The study of immune regulation of bone in osteonecrosis may encompasses many of the previously proposed mechanisms of osteonecrosis including apoptosis, oxidative stress, and genetic predisposition.
Immune factors involved in bone homeostasis include receptor activator of NFκB (RANK) and its ligand (RANKL), IL-1, IL-6, IL-10, TFG-β, TNF, CD80, CD86, CD40, macrophage colony-stimulating factor (M-CSF), NFATc, and vitamin D. (See Table 103-4 for roles and function.) Many of these factors can be categorized into one of two categories, those with the overall effect of inducing osteoclastogenesis and those that inhibit osteoclastogenesis. In addition, factors involved in cell survival and apoptosis such as Blimp-1 and Bcl6 may also play a role. RANKL is expressed on osteoblasts and is critical for the differentiation and proliferation of osteoclasts. Because transcription of factors involved in the regulation of bone homeostasis is often influenced by glucocorticoids, this may begin to explain why steroids may be associated with osteonecrosis.
Table 103-4.
Role and Function of Immune Factors in Osteoimmunology
| Immune Factor | Ligand | Cellular Source | Function in Bone Homeostasis | OC | Immune Function |
|---|---|---|---|---|---|
| RANK | RANKL | Osteoclasts, dendritic cells | Upon binding to RANKL, signals differentiation into osteoclast | ↑ | RANKL-RANK binding leads to dendritic cell activation |
| RANKL | RANK | Osteoblasts, T helper cells | Activation of osteoclasts. Overproduction can result in RA or PA | ↑ | Dendritic cell maturation |
| OPG | RANKL | Decoy receptor for RANKL | ↓ | ||
| M-CSF | CSF-1 receptor | Osteoblasts, macrophages, bone fibroblasts, stromal cells | Stimulates osteoclastogenesis | ↑ | Influences hematopoietic stem cells to differentiate into macrophages |
| TNF | TNF receptor | Macrophages, lymphocytes, mast cells, and many others | Stimulates osteoclastogenesis | ↑ | Influences multiple signaling pathways, including NFκB, death signaling and MAP kinase pathway |
| TGF-β | TGF-β receptor | Multiple cell lines | Induction of apoptosis | ↑ | Regulatory role, blocks activation of lymphocyte- and monocyte-derived phagocytosis |
| Blimp-1 | Bcl6 promoter | Plasmablasts, plasma cells | Binds to Bcl6 promoter, suppression expression | ↑ | Inhibits Tfh cell differentiation in mice221 |
| Bcl-6 | ? | Germinal center B cells | Inhibits osteoclastogenesis | ↓ | Stimulates Tfh cell differentiation in mice |
| IL-1 | IL-1R | Macrophages, monocytes, fibroblasts, dendritic cells | Directly activates RANK signaling to promote osteoclastogenesis222 | ↑ | Proinflammatory cytokine, endogenous pyrogen |
| IL-6 | IL-6R | Osteoblasts | Activation of osteoclastogenesis | ↑ | Proinflammatory cytokine |
| IL-10 | IL-10Rα | Monocytes, lymphocytes | Suppress bone resorption | ↓ | Anti-inflammatory cytokine, blocks NFκB activity, regulatory cytokine |
| Vitamin D | VDR | Osteoblast, monocyte/macrophage | Facilitate adhesion of osteoclast precursor to osteoblast223 | ↑ | Cell proliferation and differentiation |
| Estrogens | Estrogen receptor | Ovarian follicle cells | Reduces osteoclast IL-1 responsiveness and cell survival,224 stimulates osteoprotegrin | ↓ | Angiogenesis, endothelial healing |
| IL-17 | IL-17R | T cells | May have opposing roles of bone protection and bone loss225 | ↑↓ | Proinflammatory cytokine |
| IL-18 | IL-18R | Macrophages | Inhibits TNF-mediated osteoclastogenesis in a T cell–independent manner | ↓ | Proinflammatory cytokine, works in synergy with IL-12 |
Examples of some of the factors involved in bone metabolism. In addition to the factors listed, there are many others that play a role, either by themselves or in conjunction with other factors. The factors listed may have many other functions. Only select functions are listed.
Bcl6, B cell lymphoma 6 protein; Blimp, B lymphocyte–induced maturation protein 1; CSF-1, colony-stimulating factor 1; OC, osteoclastogenic; OPG, osteoprotegrin; PA, psoriatic arthritis; RA, rheumatoid arthritis; RANK, receptor activator for NFκB; RANKL, receptor activator for NFκB ligand; Tfh, T follicular helper cell; TGF-β, transforming growth factor beta; TNF, tumor necrosis factor; VDR, vitamin D receptor.
The action of glucocorticoids is mediated by the glucocorticoid receptor, which is present on many cell types including osteoclasts, osteoblasts, osteocytes, and cartilage. Binding of glucocorticoids to its receptor leads to the anti-inflammatory activity known to be a function of steroids. One mechanism by which this anti-inflammatory effect is mediated is by transcription of genes that inhibit the synthesis of inflammatory mediators.
Osteoblast/Osteoclast Balance
Any disturbance in the normal homeostasis between bone deposition and bone resorption can lead to bone disease. Moreover, defective bone deposition or bone resorption in which new bone is formed in an aberrant manner can lead to disease. Alcohol can affect the ability of mesenchymal stem cells to differentiate into osteogenic lineages. The bone marrow in the proximal head of femurs was isolated during hip replacement surgery from 33 patients with either femoral neck fractures or alcohol-induced osteonecrosis. The cells from femurs of patients with alcohol-induced osteonecrosis showed a reduced ability to differentiate into osteoblasts.93 A subsequent study compared the mesenchymal stem cells from patients with hip osteoarthritis, idiopathic osteonecrosis, and nontraumatic osteonecrosis associated with steroid or alcohol use. In idiopathic and alcohol-induced osteonecrosis, the ability of mesenchymal stem cells to differentiate into osteoblasts was decreased, but in steroid-induced osteonecrosis, it was elevated, although not to a statistically significant level. The adipogenic differentiation ability was similar in all four groups.94
In rats fed a diet of alcohol and glucose, lower bone mineral content and density were detected compared with controls. In hamsters, alcohol led to thinning of the trabeculae of the distal part of the femur. Cytologic effects included mitochondrial swelling in osteoblasts and osteocytes. Partial osteonecrosis of the femoral head was detected in Merino sheep that were injected with ethanol. In humans, alcohol causes increased plasma calcium levels, decreased osteocalcin and circulating parathyroid hormone levels, reduced serum calcitriol, reduced bone volume, and increased osteoclast number.
Alterations in osteoblast function may also contribute to the pathogenesis of osteonecrosis. In one study, osteoblastic cells were obtained from bone biopsy specimens from the intertrochanteric region of the femur and of the iliac crest of 13 patients with osteonecrosis and 8 patients with hip osteoarthritis. Cell replication was measured on the basis of proliferation rate in secondary culture. Levels of alkaline phosphatase activity, collagen synthesis, and the sensitivity to 1,25-dihydroxyvitamin D3 were measured. The results indicated that although differentiation was not affected, the proliferation rate of osteoblastic cells was reduced in samples obtained from the patients with osteonecrosis compared with patients with osteoarthritic hips.95
Apoptosis and Osteonecrosis
Glucocorticoids can also act via its action on apoptosis of immune and bone cells. When mice were administered prednisolone for 27 days, increased metaphyseal apoptotic activity of both osteoblasts and osteoclasts were noted.96 The result was decreased bone turnover, density, and formation; increased formation of cancellous bone; and decreased trabecular width. The decreased bone turnover can be explained by the reduced osteoclast survival, and the reduction in trabecular width can be explained by a decrease in osteoblasts. An accumulation of apoptotic elements was also found in the region of the “fracture crescent” in the femurs of glucocorticoid-treated patients. On the other hand, glucocorticoids may also increase osteoclast survival, leading to increased bone loss. Clearly, the effect of osteoclast survival on bone disease is more complicated than at first glance, and it involves the interaction of the osteoclast with the osteoblast. Because osteoblasts are also responsible for osteoclast differentiation under the right circumstances, there exists a significant feedback system that maintains bone homeostasis.
Osteocyte death is also a feature of osteonecrosis. In a rat model, ischemia caused an induction in the expression of stress proteins, oxygen-regulated protein (ORP150) and hemoxygenase 1 (HO1). Induction of ischemia in these rates caused DNA fragmentation and the presence of apoptotic bodies in chodrocytes, bone marrow cells, and osteocytes.97 Both alcohol and corticosteroids can induce osteocyte apoptosis, possibly via lipid abnormalities.
Lipids and Osteonecrosis
The bone marrow of rabbits that were fed alcohol showed fatty infiltration of the liver and adipogenesis in the bone marrow. Increases in fat cell hypertrophy and proliferation, as well as a decrease in hematopoiesis in the subchondral head, were observed. Osteocytes contained triglyceride deposits, and there was an increase in empty osteocyte lacunae. Alcohol also primarily triggered differentiation of bone marrow stromal cells into adipocytes in a dose-dependent manner. Intracellular lipid deposits led to the death of osteocytes.
In corticosteroid-induced osteonecrosis, the alteration in lipid metabolism parallels that of alcohol-induced osteonecrosis. In both cases, fatty infiltration of osteocytes has been postulated to occur.98, 99, 100 Table 103-5 lists lipid-altering effects of corticosteroids and alcohol. In addition, interosseous venous stasis affects the interosseous microcirculation, which can lead to hemodynamic and structural changes in the femoral head. The resulting decrease in blood flow leads to osteonecrosis. In chickens treated with steroids, fatty infiltration of the liver and fat cell hypertrophy and proliferation in the femoral head occurred concurrently 1 week after the initiation of steroids. As in the case of alcohol-induced osteonecrosis, adipocytes contained triglyceride vesicles. In rabbits treated with steroids, it was found that interosseous pressure was increased and the size of bone marrow fat cells was larger than in control rabbits.101 A histologic study of acetabular and proximal femoral bone in osteonecrosis of the femoral head revealed that osteonecrosis is more extensive in corticosteroid-induced compared with alcohol-induced or idiopathic osteonecrosis.102 The reason for this is unknown.
Table 103-5.
Lipid-Altering Effects of Steroids and Alcohol
|
In osteonecrosis of the jaw, bisphosphonates inhibit protein prenylation via inhibition of the enzyme farnesyl diphosphate synthase. The normal lipid metabolism of pathways that regulate cytoskeletal integrity and osteoclastogenesis such as Rho, Rac, and Ras is disrupted. This is one of the mechanisms by which bisphosphonates exert their intended action, but their ability to disrupt normal regulation of bone metabolism may instead lead to osteonecrosis.
Coagulation and Osteonecrosis
The hyperlipidemia, increased serum free fatty acids, and increased prostaglandins that are associated with alcohol-induced osteonecrosis may potentially trigger vascular inflammation and coagulation. Other triggers for intravascular coagulation include atherosclerosis and arteriolar fibroid degeneration. Jones proposed that the progression of osteonecrosis from stage 1A to 1B is linked to an inability to clear procoagulants from blood or tissue.103 He proposed that decreased clearance of procoagulants leads to persistent levels of tissue thromboplastin, leading to arteriolar thrombosis, vascular stasis, free fatty acid–induced endothelial damage, and hypercoagulability. Studies have shown that patients with osteonecrosis had a much higher frequency of having at least one and at least two abnormal coagulant levels compared with normal controls. Of patients with osteonecrosis, 82% had at least one abnormal procoagulant level, and 47% had at least two. In normal controls, only 30% had one abnormal procoagulant level and only 2.5% had two or more. The procoagulants measured included free protein S, protein C, lipoprotein A, homocysteine, plasminogen activator inhibitor, stimulated tissue plasminogen activator, anticardiolipin antibodies (IgM and IgG), and resistance to activated protein C.104
In addition, both thrombophilia and hypofibrinolysis have been associated with osteonecrosis. Hypofibrinolysis leads to an increased likelihood of clot formation, and thrombophilia results in a decreased ability to lyse clots. This is yet another mechanism by which corticosteroids lead to osteonecrosis—high-dose steroids lead to increased plasma plasminogen activator inhibitor, decreased tissue plasminogen activator activity, and inhibition of the fibrinolytic pathway, thus leading to a higher risk for clot formation. There is an early indication that coagulation abnormalities may play a significant role in corticosteroid-induced osteonecrosis in SARS patients.105, 106
Oxidative Stress and Osteonecrosis
Alcohol consumption is associated with reduced superoxide dismutase activity. Alcohol has deleterious effects on muscle including increased oxygen free radical–related damage, reduced myocardial contractility, defective mitochondrial function, and increased tissue enzymes.107 When rabbits were injected with methylprednisolone, elevations in 8-hydroxy-2′deoxyguanosine, a marker of DNA oxidative injury, were observed.108, 109, 110 This coincided with the development of osteonecrosis. A polymorphism in nitric oxide synthase, described later, was also associated with the development of osteonecrosis. This relationship between osteonecrosis and oxidative injury leads one to wonder if corticosteroid-induced osteonecrosis can be prevented or lessened in severity by simultaneous or prophylactic administration of antioxidants.
Nitric Oxide Synthase and Osteonecrosis
Glucocorticoids can cause derangements in vasacular responsiveness to vasoactive substances such as nitric oxide. Endothelial nitric oxide synthase (eNOS) stimulates the production of nitric oxide. Nitric oxide regulates vascular “tension” by acting as a vasodilator, inhibiting mononuclear adhesion to endothelial cells and preventing platelet aggregation. A defect in this activity can lead to increased vascular resistance and disruption to downstream blood flow, resulting in osteonecrosis.111
Multihit Hypothesis
Other proposed mechanisms involve endothelial cell injury,112 abnormal angiogenesis and repair mechanisms,113 the effects of vasoactive substances,114 activity of hepatic cytochrome P450 3A4,115 and intramedullary hemorrhage.116 Multiple mechanisms may be simultaneously occurring. Kenzora was the first to introduce the concept of cumulative stress.117 Corticosteroid-induced osteonecrosis seems to occur with greater frequency in patients who have significant underlying illness such as systemic lupus erythematosus118 or transplantation and less frequently or never in patients who are not chronically ill but are on steroids for an acute event such as head injury. Recent observations that corticosteroids induce osteonecrosis in SARS patients further support the notion that more than one insult to the bone or surrounding tissue may be necessary to precipitate osteonecrosis. For each of the known associations of osteonecrosis, different mechanisms may predominate such as lipid anomalies and apoptosis of osteoblasts in steroid-induced osteonecrosis, as well as elevated intraosseous pressures and coagulation abnormalities in dysbaric osteonecrosis, but additional factors may be necessary to precipitate osteonecrosis. The accumulated cell stress theory suggests that when the damaging effects of multiple events are added together, the involved bone is unable to recover from the chronic stress and osteonecrosis ensues.
Genetic Considerations
The degree to which genetics and the environment play in the pathogenesis of osteonecrosis is the subject of an ongoing investigation. Certainly, single nucleotide polymorphisms have been noted in a number of genes that may be associated with osteonecrosis. It has been argued that endothelial nitric oxide synthase is an important player in the development of osteonecrosis. Nitric oxide may have beneficial effects on three systems involved in osteonecrosis, namely skeletal, vascular, and thrombotic. Each of these may be targets for proposed mechanisms of pathogenesis of osteonecrosis. A comparative analysis of the 26-base pair repeat polymorphism in intron 4 and the Glu298Asp polymorphism in exon 7 of the eNOS gene in patients with idiopathic, steroid-induced, alcohol-induced, and normal control subjects was performed.119 The frequency of the homozygous 4a allele was found to be higher in patients with idiopathic osteonecrosis compared with control subjects. The frequency of the 4a/b allele was found to be higher in all types of osteonecrosis when compared with control subjects. The 4a allele is known to be associated with reduced synthesis of endothelial nitric oxide synthase, suggesting that nitric oxide may play a protective role against the development of osteonecrosis.
Forty-one percent of patients with osteonecrosis compared with only 20% of controls were homozygous for the 4G/4G mutation in the plasminogen activator inhibitor-1 gene.120 This mutation causes increased hypofibrinolytic plasminogen activator inhibitor activity, resulting in decreased stimulated plasminogen activator activity. This observation lends support to the theory that procoagulants may play a significant role in the pathogenesis of osteonecrosis. A polymorphism in the plasminogen activator inihibitor-1 (PAI-1) gene has also been reported to be predictive of osteonecrosis in children with acute lymphoblastic leukemia.121
Genetic variations in type and levels of lipoprotein (a) have been linked to osteonecrosis. Apo(a) is involved in lipid metabolism and the coagulation systems, and the Apo(a) low-molecular-weight phenotype is associated with an increased risk of osteonecrosis.122, 123, 124 Polymorphisms in the promoter for vascular endothelial growth factor (VEGF) and in the receptor for IL-23 were associated with osteonecrosis in the Korean population,125, 126 reflecting the significance of the association of osteonecrosis with vascular disorders and autoimmune diseases, respectively.
Diagnosis
History and Physical Examination
The diagnosis of osteonecrosis is generally made by history because many patients may not present until they develop hip pain. By the time the patient is clinically symptomatic, the disease may be quite advanced. Therefore a high index of suspicion is necessary for all patients on oral or parenteral steroids. Information that should be elicited from a good history should include any history of trauma; underlying disease; alcohol use; tobacco use; current medications; past medications; history of joint anomalies; presence of pain or limitation of motion; involvement in sports, especially high-impact sports; occupational history; gestational history; and the presence of liver disease or lipid abnormalities.
A good physical examination includes palpating the hip for tenderness, identification of limp, masses, leg-length discrepancy, the presence of masses, abnormal gait, muscle strength, and range of motion.
The Harris hip score is frequently used for evaluation of hip function and is also useful in monitoring the effectiveness of treatment (Figure 103-2 ).127, 128, 129 The Harris hip score is a multidimensional observational assessment based on eight items that address pain, walking function, daily activity, and range of motion. Scores range from 0 (maximum disability) to 100 (no disability).
Figure 103-2.
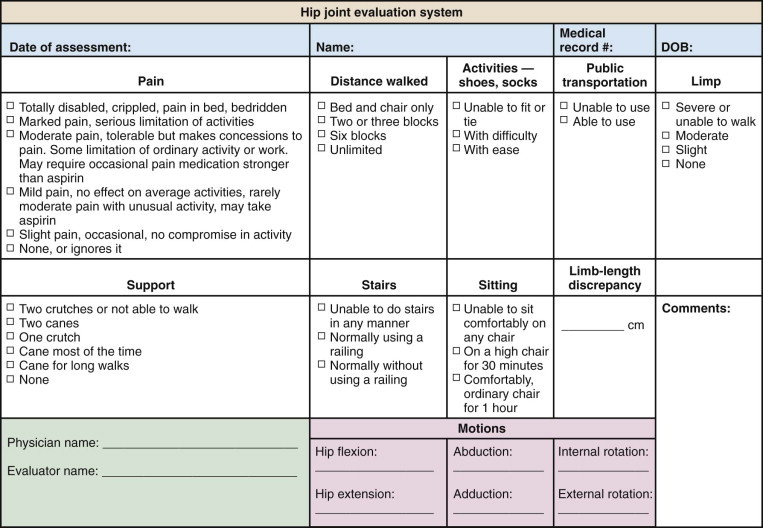
Harris hip score.
Radiologic Imaging
When the diagnosis is suspected clinically, it can be confirmed by radiologic imaging studies. Earlier employed imaging techniques such as conventional radiography were inadequate in establishing the diagnosis because in the early stages of osteonecrosis radiographs may be completely normal. The earliest radiographic sign of osteonecrosis is the presence of a radiolucent crescent-shaped rim along the contour of the femoral head (crescent sign) (Figure 103-3 ). This appearance on radiographs is the result of structural collapse of a necrotic segment of subchondral trabecular bone. At this stage, the disease is already irreversible. Later, radiographs will begin to show sclerotic changes (Figure 103-4 ). The appearance of radiographic “density” is secondary to compression of bone trabeculae after microfracture of the nonviable bone, calcification of detritic marrow, and repair of the necrotic area by deposition of new bone, the so-called creeping substitution. Flattening of the articular surface of bone is the sign of further bone collapse (Figure 103-5 ). To show best the radiographic appearance of osteonecrosis in the femoral head and better visualize the extent of the necrotic lesion, anteroposterior and frog-leg lateral films of the hip should be obtained.
Figure 103-3.
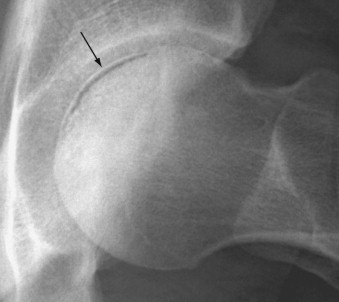
A radiolucent crescent in the subchondral region of the left femoral head (arrow) is an early radiographic sign of osteonecrosis.
Figure 103-4.
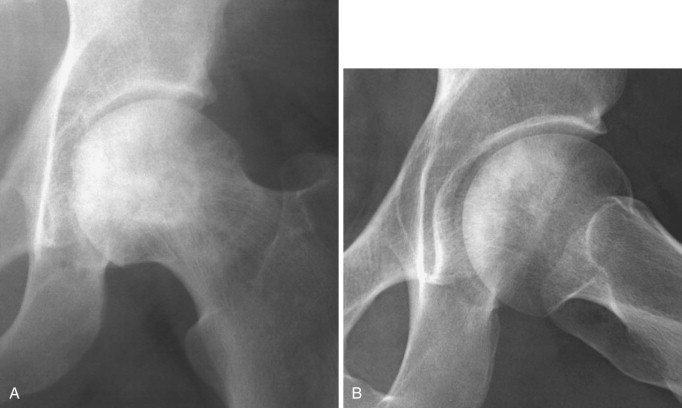
Anteroposterior (A) and frog-leg (B) views of the left hip showing sclerotic changes of the femoral head typical of advanced osteonecrosis.
Figure 103-5.
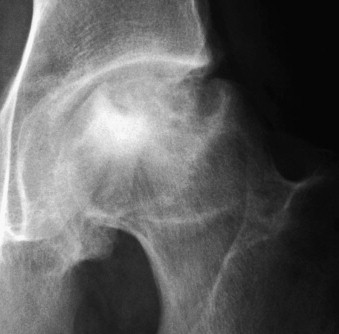
Increased density of the femoral head, loss of the normal spherical shape, and flattening of the superior aspect are characteristic radiographic features of osteonecrosis.
Skeletal scintigraphy (radionuclide bone scan) using technetium-labeled diphosphonates has also been used to diagnose osteonecrosis. The use of this technique in the early diagnosis of this condition depends on the fact that osteoblastic activity and blood flow are increased in the early stages of osteonecrosis. In an advanced stage of disease, the appearance may be one of increased activity in a subchondral distribution owing to osteoblastic activity at the reactive interface around the necrotic segment; however, the center of the osteonecrotic lesion may show much less radionuclide uptake (Figure 103-6 ) or even a complete lack of activity, reflecting decreased metabolism in the necrotic focus as a result of interruption of blood supply.6
Figure 103-6.
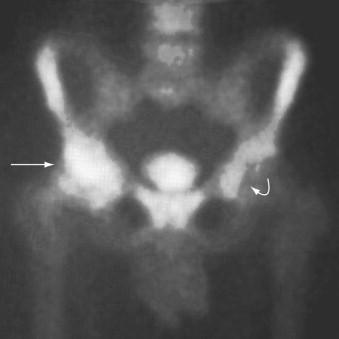
Bone scintigraphy of osteonecrosis of both femoral heads using Tc99m methylene diphosphonate showing moderate uptake of radiopharmceutical at the site of the ostenecrotic segment in the right femoral head and markedly increased uptake at the site of bone repair (straight arrow). The left femoral head (curved arrow) exhibits early-stage disease.
In addition to bone scintigraphy, single-photon emission computed tomography (SPECT) maximizes sensitivity. A study comparing conventional radiography, MRI, computed tomography (CT), and Tc99m MDP three-phase bone scan in diagnosing bisphosphonate-associated osteonecrosis of the jaw showed that CT and MRI were the best at defining the extent of the disease, but that bone scan was the best at identifying disease at an early stage. Bone scan could be an excellent screening tool for the diagnosis of osteonecrosis before further characterization of the lesions using CT or MRI.130
CT allows more detailed examination of the femoral head. A star-shaped structure, formed by weight-bearing bone trabeculae, gives the appearance of an asterisk on CT scan (the asterisk sign).131, 132, 133 This asterisk undergoes a characteristic change in ischemic bone necrosis of the femoral head, and this change was considered important for early detection of osteonecrosis. At a later stage, the collapse of necrotic bone can be well shown (Figure 103-7 ).
Figure 103-7.
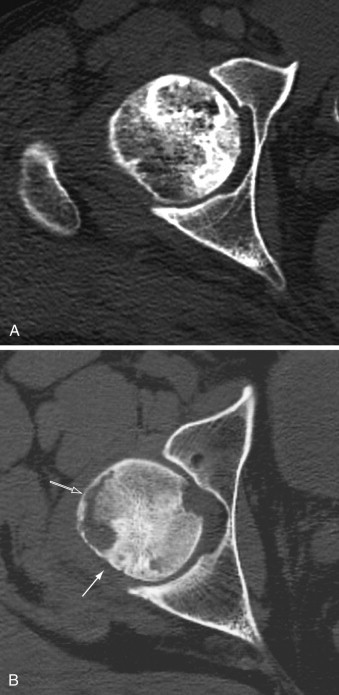
A, Computed tomography scan shows osteonecrosis of the femoral head. Although there are several sclerotic foci within the trabecular bone, the integrity of the osseous structures is preserved and the femoral head exhibits normal spherical shape. B, In more advanced stage of osteonecrosis of the femoral head, note increased sclerosis in the posterior aspect (solid arrow) and subchondral collapse of necrotic bone anterolaterally (open arrow).
Currently, MRI is the “gold standard” for imaging of osteonecrosis. Most of the staging systems for osteonecrosis are now based on MRI appearance (Table 103-6 ). MRI of osteonecrosis can show changes earlier than conventional radiography or CT. It can also detect bone marrow edema, a feature sometimes seen in the early phases of osteonecrosis that is not visible on conventional radiography or CT.
Table 103-6.
Magnetic Resonance Imaging (MRI) Changes and Their Correlation with Histology in Osteonecrosis
| Type of Appearance | Category of Observations | Histology | MRI Appearance |
|---|---|---|---|
| A | Fatlike | Premature fatty marrow development in the femoral neck or intertrochanteric region | Normal fat signal; Sclerotic margin may be seen circumscribing lesion |
| B | Bloodlike | Bone resorption; replacement by vascular granulation tissue | High signal intensity of inner border; low signal intensity of surrounding rim |
| C | Fluid-like | Bone marrow edema | Diffusely decreased signal on T1-weighted images; high signal on T2-weighted images |
| D | Fibrotic | Sclerosis owing to reinforcement of existing trabeculae at margin of live bone (repair tissue interface) | Decreased signal on T1-weighted and T2-weighted images |
The typical MRI findings in osteonecrosis are intermediate or low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Figure 103-8 ). As the disease progresses, the subchondral necrotic lesion is surrounded by a low signal line on T1-weighted images. A high signal line is seen on T2-weighted images, central to the low signal line. This produces the “double-line” sign (Figure 103-9 ). In advanced osteonecrosis, the necrotic segment exhibits low signal intensity on both T1-weighted and T2-weighted images (Figure 103-10 ). MRI is done in the sagittal, coronal, and axial planes and includes T1-weighted and T2-weighted sequences. There is excellent correlation between histologic findings and MRI appearance (see Table 103-6).
Figure 103-8.
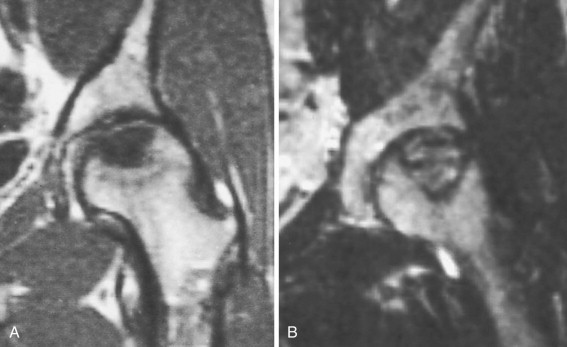
A, On T1-weighted coronal magnetic resonance image of the left hip, the osteonecrotic segment in the subchondral portion of the femoral head shows low signal intensity. B, On T2-weighted coronal image, the necrotic bone exhibits high signal intensity, surrounded by a sclerotic low-signal rim.
Figure 103-9.
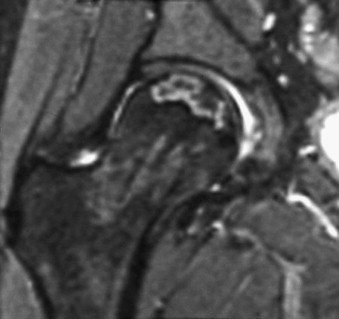
Coronal T2-weighted magnetic resonance image of the right femoral head shows the double-line sign, characteristic for osteonecrosis: low signal at periphery of the lesion and high signal band located more centrally.
Figure 103-10.
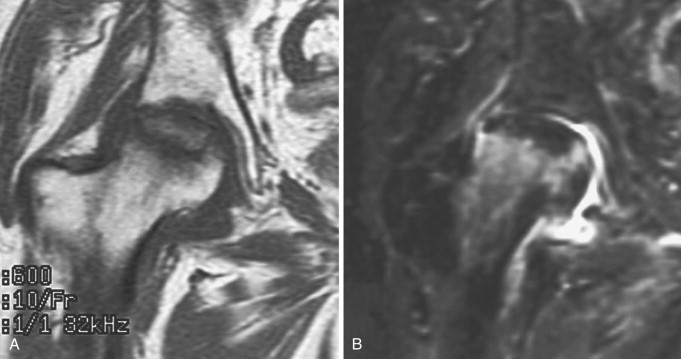
Advanced osteonecrosis of the right femoral head exhibits low signal intensity on T1-weighted (A) and T2-weighted (B) MR images.
MRI is an important tool in determining the extent of femoral head involvement in osteonecrosis. Three techniques are used to evaluate this. The first is estimating head involvement. This method was first proposed by Steinberg and colleagues134 in 1984, and it is defined by the appearance of abnormal signals on T1-weighted images. The degree of head involvement was classified into three categories: less than 15%, 15% to 30%, and greater than 30%. The second method used to evaluate extent is the index of necrotic extent, which is determined by measuring the angle created by the extent of subchondral involvement. Lesion size was estimated using a “necrotic arc angle,” defined by the angle of the arc of the necrotic segment from the center of the femoral head. Two angles are obtained: “A,” representing the necrotic arc seen on midcoronal images, and “B,” representing the necrotic arc angle seen on midsagittal images. The index is a compilation of these two angles. The third method is a variation of the second, in which the angle is identified not on midcoronal or midsagittal images but on the image that shows the maximum lesion size in the sagittal and coronal planes. It is thought that this method would correct for the underestimation that may be inherent in the second method.
Table 103-7 shows a comparison of various imaging techniques used in the diagnosis and staging of osteonecrosis. Hip arthroscopy is also used in the staging of osteonecrosis. In a study comparing radiography, MRI, and arthroscopy, there was only moderate correlation among the three methods. Arthroscopy was able to detect osteochondral degeneration, not detected by radiography or MRI in 36% of collapsed heads. Figure 103-11 is an algorithm for the diagnosis of osteonecrosis.
Table 103-7.
Comparative Sensitivity and Specificity of Diagnostic Radiologic Imaging Modalities in Osteonecrosis
| Radiologic Imaging | Earliest Sign Seen | Histologic Correlation | Stage | Degree of Specificity |
|---|---|---|---|---|
| Conventional radiograph | Crescent sign | Sclerotic rim of reactive bone | 2 | High |
| Computed tomography scan | Asterisk sign | Sclerotic rim surrounding a mottled area of osteolysis and sclerosis | 2 | High |
| Magnetic resonance image | Low signal intensity on T1-weighted images; high signal intensity on T2-weighted images | Bone marrow edema | 1 | High |
| Skeletal scintigraphy | Decreased uptake in subchondral distribution, “cold” spot | Osteonecrosis | 1 | Low |
| Increased uptake in subchondral distribution, “hot spot” | “Creeping substitution” | 2 | Low |
Figure 103-11.
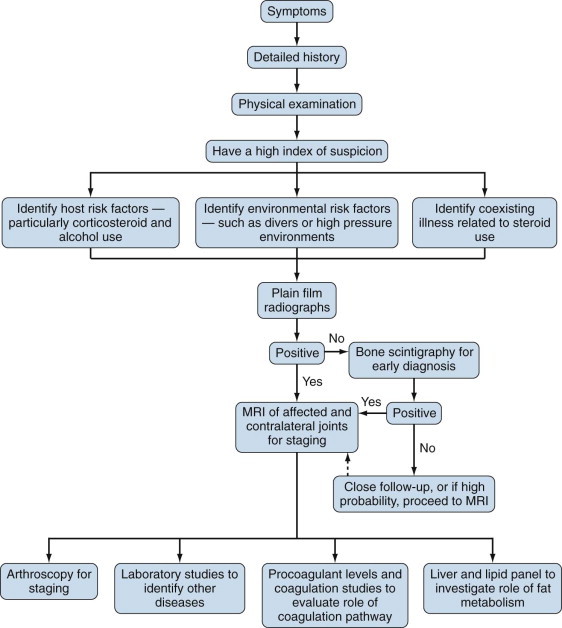
Diagnostic algorithm for osteonecrosis. MRI, magnetic resonance image.
Markers of Disease
The ability to find consistent and reliable markers of disease is always a welcome tool, for diagnosis, determination of extent of the disease, or even determination of risk of acquiring the disease. The measurement of serum and urine carboxy-terminal cross-linking telopeptide of type I collagen (CTX-1), a marker of bone resorption, has been proposed as a method of evaluating the risk of osteonecrosis of the jaw secondary to bisphosphonate usage. Serum osteocalcin is another marker for bisphosphonate-related osteonecrosis of the jaw that has been suggested as a risk predictor because levels were significantly lower in the osteonecrosis group compared with a control group.135
Treatment
Surgical Treatment
Most cases of osteonecrosis ultimately require surgical intervention. There are various surgical techniques ranging from core decompression to total hip replacement. Sometimes surgical procedures can be used in conjunction with nonsurgical approaches, as discussed later. The more advanced the disease, the more extensive the surgery.
The various surgical procedures used in the treatment of osteonecrosis include core decompression, structural bone grafting, vascularized fibula grafting, osteotomy, resurfacing arthroplasty, hemiarthroplasty, and total hip replacement. Table 103-8 shows the typical success rates for each of these procedures.
Table 103-8.
Surgical Treatment of Osteonecrosis
| Surgical Procedure | Rationale | Stages of Osteonecrosis | Outcome | Comments |
|---|---|---|---|---|
| Core decompression | Reduction of intraosseous and intramedullary pressure | Early stages | 37% radiographic success, 48% clinical success | Success rate depends on disease stage |
| Structural bone grafting | Provide support to overlying subchondral bone | 1 or 2 | Poor in advanced disease | 100% failure rate in stages 3 and 4 |
| Vascularized fibula grafting | Increase blood flow to graft | 2 to 4 | 96% success in stage 2, 90% in stage 3, and 57% in stage 4 | |
| Osteotomy | Shifting position of osteonecrotic segment out of weight-bearing region | 2 and 3 | Not available | |
| Resurfacing arthroplasty | Preservation of bone and joint mechanics with metallic or ceramic shell over femoral head | Later stages | Mean 7-year success rate is 90% | An alternative to total hip arthroscopy in later stages of disease |
| Hemiarthroplasty | Replacement of femoral head, preservation of anatomic acetabulum | Later stages | Failure rate for unilateral hemiarthroplasties is 50%-60% at 3 years, for bilateral hemarthroplasties is 44% | Various techniques available, some with better outcome |
| Total hip replacement | Complete replacement of the hip joint | Late stages | 17.4% required revision after 10 years | Eventually most patients will require multiple hip replacements |
Arthroscopy is a valuable tool used in the treatment of osteonecrosis. It has been used to determine the position of the core decompression tract to the necrotic part of the femoral head, and arthroscopic débridement has been used in the treatment of osteonecrosis of the capitellum of the humerus in adolescents, Kienböck's disease, and osteonecrosis of the scaphoid.
Core decompression, which involves the removal of a core of bone from the femoral neck and head, is indicated in less advanced stages of osteonecrosis. The core acts as a vent to reduce intraosseous pressure and intramedullary pressure, reversing ischemia and improving symptoms. Other benefits of core decompression include stimulation of angiogenesis, which leads to improved vascularization during the repair process. The effectiveness of core decompression in the treatment of nontraumatic osteonecrosis was illustrated in 34 patients with 54 affected hips. Mean age at presentation was 38 years. The patients were monitored for a mean duration of 120 months postsurgery. Success was defined as absence of symptoms, no further progression of disease, and no further surgery. Clinical success was established in 26 hips (48%), and radiographic success was established in 20 hips (37%).
Computer-assisted core decompression has been used to provide greater precision in directing the core into the ischemic area and to minimize the duration of radiation exposure to patients.136 Because early diagnosis improves outcome and there is a high incidence of developing osteonecrosis in a contralateral hip, core decompression is frequently done on both hips simultaneously. This approach adds little risk over unilateral core decompression with the benefit of better outcomes secondary to early surgical treatment of the contralateral hip.137
In structural bone grafting, or bone impaction grafting, the bone graft is inserted into the necrotic segment through the core tract. The bone graft acts in similar fashion to a stent, providing support to overlying subchondral bone. The goal is to prevent collapse. This combination of procedures is frequently used in treating stage 1 or 2 osteonecrotic femoral heads. Allogeneic and autologous bone grafts, mostly harvested from the tibia or fibula, are used. When this technique was attempted in patients with stages 3 and 4 lesions, the outcome was generally poor (100% failure after 2 to 4 years), with progression to collapse and further surgical procedures.138
Vascularized structural bone grafting also uses the core tract to insert a corticocancellous bone graft into the femoral neck and head along with its vascular pedicle. The vascular pedicle is anastomosed to a nearby vessel, adding a source of blood to the graft. The results of vascularized fibular grafting in the treatment of hips with osteonecrosis showed a survival of 61% of hips at 5-year follow-up and 42% at a median time of 8 years.139 In another study, 197 patients with 226 osteonecrotic hips were treated with a combination of autologous cancellous bone impaction and pedicled iliac bone block transfer. The anastomosis was to the ascending branch of the lateral femoral circumflex artery. Fourteen hips required conversion to total hip arthroplasty because of collapse, severe pain, or both. Of the remaining 212 hips, 92% were considered a clinical success and 76% were considered radiographically successful. The success rate declined from stage 2 to stage 4 hips (96% for stage 2 hips, 90% for stage 3 hips, and 57% for stage 4 hips).140 Free vascularized fibula grafting has been compared favorably with other modes of surgical treatment.141
Osteotomy of the femur involves shifting the position of the osteonecrotic segment by making a cut in the proximal femur so that the osteonecrotic segment is rotated or flexed out of the weight-bearing region of the acetabulum and replacing the weight-bearing region with viable bone. Healing of the necrotic region can proceed without the stress of weight bearing. Several different osteotomy techniques have been attempted to salvage hips in stage 2 or 3 osteonecrosis.
Resurfacing arthroplasty uses a metallic or ceramic shell placed over a femoral head that has been débrided of the necrotic area. The potential advantages of resurfacing arthroplasty include preservation of joint mechanics, bone conservation,142 more physiologic loading of the bone, a lower incidence of perioperative complications, and easier conversion to total hip arthroplasty in case of failure.143 Complications of this procedure include femoral neck fractures, a secondary osteonecrosis when the procedure is done for other reasons,144 and increased metal ion levels.145 Resurfacing arthroplasty has been recommended for patients with later-stage osteonecrosis including those with femoral head collapse.146 A retrospective study compared the results of limited femoral head resurfacing and total hip arthroplasty in 30 consecutive patients with Steinberg stage 3 or 4 disease. The survival rate at a 7-year mean follow-up period for the resurfacing group was 90%, whereas the survival rate at a mean 8-year follow-up for the total hip arthroplasty group was 93%.147 A recent level 3 therapeutic study showed that hip resurfacing success rates at a 5-year follow-up were comparable with those of total hip arthroplasty in osteonecrosis patients younger than 25 years of age.148
In hemiarthroplasty, only part of the hip joint is replaced. The original acetabulum is preserved, but the femoral head is replaced with a prosthesis. Two kinds of prostheses are used—a unipolar prosthesis and a bipolar prosthesis. In a unipolar prosthesis, the articulation is between the artificial femoral head and the acetabulum. In the bipolar prosthesis, presently the most frequently used, the articulation is within the prosthesis itself. Failure rates for hemiarthroplasties in osteonecrosis are 50% to 60% at 3 years for unipolar prostheses and 44% for bipolar prostheses. Another study evaluated the success rate of Charnley/Bicentric hemiarthroplasty in the treatment of Ficat and Arlet stage 3 osteonecrosis of the femoral head. Failures include three hips that needed to be revised to cementless total hip replacement, two hips with radiographic changes of loosening and imminent failure, and one hip with progressive loss of joint space and secondary degenerative changes. The success rate was 84.2% after a mean of 56 months.
Total hip arthroplasty is complete replacement of the hip joint with a prosthesis including the femoral head and the acetabulum. In a study of 55 consecutive hip arthroplasty procedures, cementless total hip arthroplasty was shown to provide favorable results in advanced-stage osteonecrosis of the femoral head. Although 10 of the 48 hips available for follow-up after a minimum of 5 years required revision, all of these patients had Ficat and Arlet stage 3 or 4 disease. A study of 53 hips in 41 patients treated with cemented total hip replacement showed that at a minimum of 10 years of follow-up, 17.4% required revision. Compared with cemented total hip replacements done for other conditions, osteonecrosis had a greater risk for loosening of acetabular and femoral components. A survivorship analysis of cemented total hip replacements in renal transplant patients with osteonecrosis of the femoral head showed that there was excellent survival after 10 years (98.8%). After 20 years, the survival rate decreased to 63.8%.
In osteonecrosis of the jaw, the most common surgical procedure is resection of the affected bone.149 Conservative treatment has also been used but carries a higher recurrence rate. A larger extent of surgical excision and a higher number of surgical débridements were associated with a lower recurrence rate. Other modes of surgical therapy for osteonecrosis of the jaw include bone-contouring procedures; fluorescence-guided bone-contouring procedures150; and segmental osteotomies, but these are generally reserved for more severe cases. Nonsurgical treatment including hyperbaric oxygen therapy151 and low-intensity laser therapy are controversial but have been used to treat osteonecrosis of the jaw.
Nonsurgical Approaches
The key to the successful treatment of osteonecrosis is early detection. The choice of conservative nonsurgical versus more aggressive surgical options depends on the clinical and pathologic staging of the disease. Figure 103-12 is an algorithm for the treatment of osteonecrosis.
Figure 103-12.
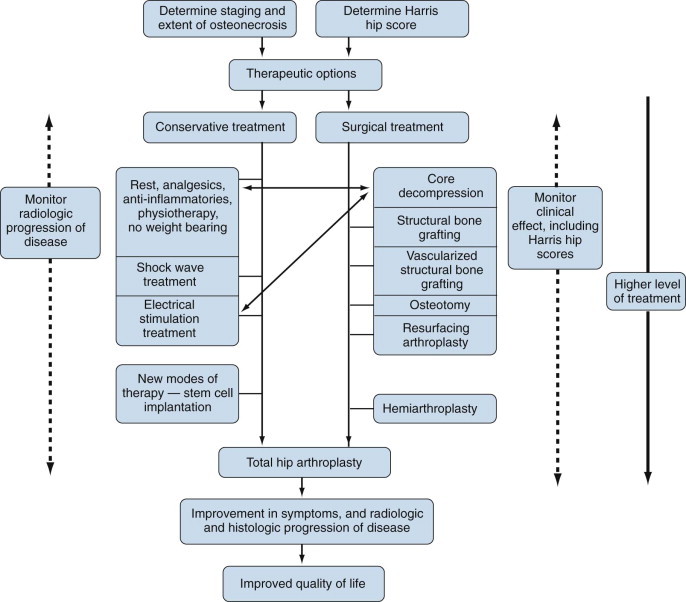
Treatment algorithm for osteonecrosis.
Nonsurgical treatment of osteonecrosis of the femoral head includes refraining from weight bearing on the affected joint, analgesic and anti-inflammatory medications, and physiotherapy. Conservative medical treatment is effective only in the early stages for symptomatic relief. Nonsurgical management does not seem to alter the natural course of the disease. Electrical stimulation has been used in the treatment of osteonecrosis, in conjunction with core decompression. Electrical stimulation enhances osteogenesis and neovascularization. It also alters the balance between osteoblast and osteoclast activity, resulting in increased bone deposition and decreased bone resorption. Delivery of electrical stimulation can be done by direct current (DC), pulsed electromagnetic field, and capacitance coupling. The success of electrical stimulation in the treatment of osteonecrosis has been rather mediocre. Eleven hips in eight patients with Ficat stage 2 osteonecrosis who underwent core decompression and placement of an electric stimulating coil within the core in the anterosuperior segment of the femoral head were studied. Of these, five hips required reoperation and six hips had progressive deterioration 13 months after initial placement of the coil. In addition, there was little histologic evidence that the coil did indeed generate new bone deposition around itself.
On the other hand, a study compared the effectiveness of conservative nonsurgical treatment with core decompression with or without direct current electrical stimulation. The clinical symptom scores and the rate of progression to arthroplasty were best in the group with core decompression and DC electrical stimulation and worst in the nonoperative group. Capacitive coupling can be done with or without core decompression and grafting. Core decompression and grafting were done on 40 patients with stage 1 to 3 osteonecrosis; half of the patients wore active capacitive coupling units with electrodes over the femoral head for 6 months. The control group was 55 patients with osteonecrosis who were treated conservatively. Two- and 4-year follow-up showed that core decompression with or without capacitive coupling provided better clinical and radiologic outcome than conservative treatment. Capacitive coupling did not improve the results further when used with core decompression and grafting.
Extracorporeal shock wave therapy has been used in the treatment of osteonecrosis of the femoral head. A study of 48 patients and 57 hips compared extracorporeal shock wave therapy with core decompression and bone grafting. Twenty-three patients with 29 affected hips were assigned to the shock wave group, and the remaining patients and hips received surgical treatment. The patients in the shock wave group were given treatment of 6000 pulses of shock waves at 28 kV to the affected hip. The patients were evaluated radiographically and by their reports of symptoms (pain), Harris hip scores, and quality of life (daily work activity assessment). Shock wave therapy produced better results than the nonvascularized bone grafting procedure, with comparatively less progression of disease. In 35 patients with 47 osteonecrotic hips, the use of shock wave therapy led to improvements in serum nitric oxide levels, angiogenic factors such as VEGF, and osteogenic factors such as bone morphogenetic protein-2 (BMP-2) and osteocalcin. Levels of inflammatory markers were reduced. It is interesting to note that although these changes did not persist beyond several months, the clinical and radiographic improvement, present in 83% of hips, was present after 12 months.152
Conservative treatment of osteonecrosis of the talus is not promising, and the affected ankles generally continue to progress, requiring either core decompression or arthrodesis. Conservative treatment of bisphosphonate-induced osteonecrosis of the jaw includes cessation of bisphosphonate usage or surgical débridement. Good oral hygiene, regular dental assessment, and avoidance of dental procedures during bisphosphonate usage can prevent onset of osteonecrosis.
Recent Developments
Prevention versus Treatment
A recent study evaluated the role of antioxidants in the treatment of osteonecrosis. Japanese white rabbits were divided into two groups and fed either a normal diet or a normal diet supplemented with α-tocopherol. Osteonecrosis developed in 14 of 20 rabbits in the control group but only in 5 of 21 rabbits in the experimental group. This suggests that oxidative stress may play a role in the pathogenesis of osteonecrosis and that there may potentially be a role for antioxidants such as vitamin E.153
A group of researchers studied the use of adrenocorticotropic hormone (ACTH) in rabbits to prevent corticosteroid-induced osteonecrosis and found that if ACTH is administered along with depot methylprednisolone acetate (DepoMedrol), osteonecrosis is reduced. The authors of this study believe that ACTH enhances osteoblast support and stimulates the production of vascular endothelial growth factor (VEGF), which stimulates the generation of new blood vessels. The result is an increase in blood flow to the vulnerable areas of bone, preventing cell death and reducing the likelihood of osteonecrosis.154
Mesenchymal Stem Cells
Corticosteroids interfere with the balance of adipogenesis and osteogenesis in the differentiation of mesenchymal stem cells. Corticosteroids shunt uncommitted osteoprogenitor cells in the bone marrow into the adipocytic pathway, leading to reduced osteoblast formation. Corticosteroids have also been shown to reduce vascular endothelial growth factor, which leads to a reduction in new blood vessel formation and potentially can lead to bone death. Alcohol has a similar effect on the differentiation of progenitor cells.
The balance between adipogenesis and osteogenesis has been targeted as a potential site for the treatment of osteonecrosis. Multipotential mesenchymal stem cells from femoral bone marrow near osteonecrosis sites are able to express messenger RNA aggrecan and type II collagen. Both are deposited into the bone matrix. These features are characteristic of chondrogenic differentiation. The mesenchymal stem cells can be differentiated into osteocytic lineage in vitro.
A pilot study evaluating the effectiveness of implantation of autologous bone marrow cells in the treatment of osteonecrosis used core decompression to implant stem cells into the necrotic lesions of the femoral head. The patients were divided into two groups—one that received core decompression alone as treatment for osteonecrosis (the control group) and one that received autologous bone marrow cell implantation along with core decompression (the treatment group). The patients were followed for 24 months, and at that time, 5 of 8 hips in the control group, but only 1 of 10 in the treatment group, advanced to stage 3 osteonecrosis. In addition, there was greater improvement in pain and joint symptoms in the treatment group and the treatment seemed to be safe. Because of the small number of patients involved, further studies are necessary to confirm these results.
Twenty-eight patients with 44 necrotic hips were treated with percutaneous decompression and autologous bone marrow mononuclear cell infusion. Patients were followed for a minimum of 2 years and evaluated for clinical and radiographic progression of the disease. There seemed to be overall slowing in the progression of the disease stage. The mean Harris hip score improved from 58 to 86.
Outcome
The natural history of osteonecrosis depends on the size of the infarcted segment, the site of occurrence, and the clinical and radiologic staging of the disease. At the onset of the disease, range of motion may be well preserved but gradually deteriorates over time. In the early stages of the disease, when it is still reversible, patients may be asymptomatic. Many patients therefore present with advanced disease. Although spontaneous resolution of femoral head osteonecrosis can occur, it is rare and occurs only when lesion size is small. A study of the prognosis of osteonecrosis of the femoral head as a function of symptoms (pain) and radiographic findings showed that in patients who were asymptomatic and had normal radiographs, progression of the disease was slow, with only 1 of 23 hips progressing to pain and radiographic changes after 5 years. If radiographic changes are already present, disease progresses to pain in 14 of 19 patients after 5 years. In a study of stage 1 osteonecrotic lesions of the hip diagnosed with MRI, 40 patients were followed for an average of 11 years. All patients had stage 1 lesions on the contralateral hip. Overall, 35 of the 40 stage 1 hips became symptomatic and 29 hips showed collapse. The mean interval between diagnosis and collapse was 92 months, whereas the mean interval between symptoms and diagnosis was 80 months. Most stage 1 hips eventually progress to a more advanced stage, requiring surgery, so these hips should be monitored closely.
Summary
Osteonecrosis is a potentially debilitating condition with significant morbidity despite medical interventions or surgery. Corticosteroids are the most common cause of osteonecrosis, and corticosteroid-induced osteonecrosis can be reproduced in animal models. The pathogenesis of osteonecrosis is multifaceted and still not completely understood. Why is it that corticosteroid-induced osteonecrosis is more common in patients with certain underlying diseases and not in others? Is there a genetic basis for osteonecrosis? Common pathogenic mechanisms known to be involved in osteonecrosis include osteoblast/osteoclast survival and apoptosis, lipid metabolism, and coagulation abnormalities. However, it is still unclear how these mechanisms inter-relate with each other. In order to better appreciate the risk factors involved in osteonecrosis, a more complete understanding of the pathogenesis is necessary. Until then, the physician should always maintain a high index of suspicion for osteonecrosis whenever known risk factors are present, especially use of corticosteroids and alcohol.
Selected References
- 1.McCarthy EF. Aseptic necrosis of bone. An historic perspective. Clin Orthop Relat Res. 1982;168:216–221. [PubMed] [Google Scholar]
- 2.Nixon JE. Avascular necrosis of bone: a review. J R Soc Med. 1983;76:681–692. doi: 10.1177/014107688307600810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axhausen G. Uber anamische Infarkte am Knochensystem und ihre Bedeutung fur die Lehre von den primaren Epiphyseonkrosen. Arch Klin Chir. 1928;151:72–98. [Google Scholar]
- 4.Hutter CD. Dysbaric osteonecrosis: a reassessment and hypothesis. Med Hypotheses. 2000;54:585–590. doi: 10.1054/mehy.1999.0901. [DOI] [PubMed] [Google Scholar]
- 5.Assouline-Dayan Y, Chang C, Greenspan A. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 6.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: current perspectives on pathogenesis and treatment. Semin Arthritis Rheum. 1993;23:47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 7.Sevitt S. Avascular necrosis and revascularisation of the femoral head after intracapsular fractures; a combined arteriographic and histological necropsy study. J Bone Joint Surg Br. 1964;46:270–296. [PubMed] [Google Scholar]
- 8.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 9.D’Aubigne RM, Frain PG. Theory of osteotomies. Rev Chir Orthop Reparatrice Appar Mot. 1972;58:159–167. [PubMed] [Google Scholar]
- 10.Peitrogrande V, Mastromarino R. Osteopatia de prolongato trattamento cortisono. Ortop Traumatol. 1957;25:793. [Google Scholar]
- 11.Koo KH, Kim R, Kim YS. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21:299–303. doi: 10.1007/s100670200078. [DOI] [PubMed] [Google Scholar]
- 12.Hurel SJ, Kendall-Taylor P. Avascular necrosis secondary to postoperative steroid therapy. Br J Neurosurg. 1997;11:356–358. doi: 10.1080/02688699746186. [DOI] [PubMed] [Google Scholar]
- 13.Gogas H, Fennelly D. Avascular necrosis following extensive chemotherapy and dexamethasone treatment in a patient with advanced ovarian cancer: case report and review of the literature. Gynecol Oncol. 1996;63:379–381. doi: 10.1006/gyno.1996.0339. [DOI] [PubMed] [Google Scholar]
- 14.Powell C, Chang C, Naguwa SM. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9:721–743. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vreden SG, Hermus AR, van Liessum PA. Aseptic bone necrosis in patients on glucocorticoid replacement therapy. Neth J Med. 1991;39:153–157. [PubMed] [Google Scholar]
- 16.Haajanen J, Saarinen O, Laasonen L. Steroid treatment and aseptic necrosis of the femoral head in renal transplant recipients. Transplant Proc. 1984;16:1316–1319. [PubMed] [Google Scholar]
- 17.Chandler GN, Jones DT, Wright V, Hartfall SJ. Charcot's arthropathy following intra-articular hydrocortisone. Br Med J. 1959;1:952–953. doi: 10.1136/bmj.1.5127.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Hirohata T, Sugioka Y. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988;234:115–123. [PubMed] [Google Scholar]
- 23.Axhausen G. Die Nekrose des proximalen Bruckstuckes beim Schenkelhals bruck und ihre Bedeutung fur das Huftgelenk. Arch Klin Chir. 1922;120:325–346. [Google Scholar]
- 24.Antti-Poika I, Karaharju E, Vankka E, Paavilainen T. Alcohol-associated femoral head necrosis. Ann Chir Gynaecol. 1987;76:318–322. [PubMed] [Google Scholar]
- 25.Orlic D, Jovanovic S, Anticevic D, Zecevic J. Frequency of idiopathic aseptic necrosis in medically treated alcoholics. Int Orthop. 1990;14:383–386. doi: 10.1007/BF00182650. [DOI] [PubMed] [Google Scholar]
- 26.Calve J. Sur une forme particuliere de pseudocoxalgie greffee sur des deformations caracteristiques de l’extremite superieure du femur. Rev Chir. 1910;30:48–54. [Google Scholar]
- 29.Bentzon P. Experimental studies on the pathogenesis of coxa plana (Calve-Legg-Perthes-Waldenstrom's disease) and other manfiestations of “local dyschondroplasia”. Acad Radiol. 1926;6:155–172. [Google Scholar]
- 31.Goff CW. Legg-Calve-Perthes syndrome (LCPS). An up-to-date critical review. Clin Orthop. 1962;22:93–107. [PubMed] [Google Scholar]
- 32.Landin LA, Danielsson LG, Wattsgard C. Transient synovitis of the hip. Its incidence, epidemiology and relation to Perthes’ disease. J Bone Joint Surg Br. 1987;69:238–242. doi: 10.1302/0301-620X.69B2.3818754. [DOI] [PubMed] [Google Scholar]
- 33.Burwell RG, Dangerfield PH, Hall DJ. Perthes’ disease. An anthropometric study revealing impaired and disproportionate growth. J Bone Joint Surg Br. 1978;60-B:461–477. doi: 10.1302/0301-620X.60B4.711791. [DOI] [PubMed] [Google Scholar]
- 35.Kristmundsdottir F, Burwell RG, Harrison MH. Delayed skeletal maturation in Perthes’ disease. Acta Orthop Scand. 1987;58:277–279. doi: 10.3109/17453678709146484. [DOI] [PubMed] [Google Scholar]
- 36.Hall DJ, Harrison MH, Burwell RG. Congenital abnormalities and Perthes’ disease. Clinical evidence that children with Perthes’ disease may have a major congenital defect. J Bone Joint Surg Br. 1979;61:18–25. doi: 10.1302/0301-620X.61B1.33996. [DOI] [PubMed] [Google Scholar]
- 37.Burwell RG, Vernon CL, Dangerfield PH. Raised somatomedin activity in the serum of young boys with Perthes’ disease revealed by bioassay. A disease of growth transition? Clin Orthop Relat Res. 1986;209:129–138. [PubMed] [Google Scholar]
- 38.Rayner PH, Schwalbe SL, Hall DJ. An assessment of endocrine function in boys with Perthes’ disease. Clin Orthop Relat Res. 1986;209:124–128. [PubMed] [Google Scholar]
- 39.Barr RD, Sala A. Osteonecrosis in children and adolescents with cancer. Pediatr Blood Cancer. 2008;50:483–485. doi: 10.1002/pbc.21405. discussion 6. [DOI] [PubMed] [Google Scholar]
- 41.Niinimaki RA, Harila-Saari AH, Jartti AE. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25:1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 42.Montella BJ, Nunley JA, Urbaniak JR. Osteonecrosis of the femoral head associated with pregnancy. A preliminary report. J Bone Joint Surg Am. 1999;81:790–798. doi: 10.2106/00004623-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Hernigou P, Allain J, Bachir D, Galacteros F. Abnormalities of the adult shoulder due to sickle cell osteonecrosis during childhood. Rev Rhum Engl Ed. 1998;65:27–32. [PubMed] [Google Scholar]
- 44.Hernigou P, Galacteros F, Bachir D, Goutallier D. Deformities of the hip in adults who have sickle-cell disease and had avascular necrosis in childhood. A natural history of fifty-two patients. J Bone Joint Surg Am. 1991;73:81–92. [PubMed] [Google Scholar]
- 45.Kandzierski G, Gregosiewicz A, Malek U. Femur head necrosis in haemophilia and after prolonged steroid therapy–description of two cases. Chir Narzadow Ruchu Ortop Pol. 2004;69:269–271. [PubMed] [Google Scholar]
- 51.Twynham G. A case of Caisson disease. BMJ. 1888;1:190–191. [Google Scholar]
- 52.Davidson JK. Dysbaric disorders: aseptic bone necrosis in tunnel workers and divers. Baillieres Clin Rheumatol. 1989;3:1–23. doi: 10.1016/s0950-3579(89)80033-0. [DOI] [PubMed] [Google Scholar]
- 53.Chan MH, Chan PK, Griffith JF. Steroid-induced osteonecrosis in severe acute respiratory syndrome: a retrospective analysis of biochemical markers of bone metabolism and corticosteroid therapy. Pathology. 2006;38:229–235. doi: 10.1080/00313020600696231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv H, de Vlas SJ, Liu W. Avascular osteonecrosis after treatment of SARS: a 3-year longitudinal study. Trop Med Int Health. 2009;14(Suppl 1):79–84. doi: 10.1111/j.1365-3156.2008.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CW, Chiu WK, Chan CC. Osteonecrosis in children with severe acute respiratory syndrome. Pediatr Infect Dis J. 2004;23:888–890. doi: 10.1097/01.inf.0000137570.37856.ea. [DOI] [PubMed] [Google Scholar]
- 56.Cushner MA, Friedman RJ. Osteonecrosis of the humeral head. J Am Acad Orthop Surg. 1997;5:339–346. doi: 10.5435/00124635-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Baumgarten KM, Mont MA, Rifai A, Hungerford DS. Atraumatic osteonecrosis of the patella. Clin Orthop Relat Res. 2001;383:191–196. doi: 10.1097/00003086-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 61.Berger CE, Kroner A, Kristen KH. Spontaneous osteonecrosis of the knee: biochemical markers of bone turnover and pathohistology. Osteoarthritis Cartil. 2005;13:716–721. doi: 10.1016/j.joca.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28:145–150. [PubMed] [Google Scholar]
- 67.Delanois RE, Mont MA, Yoon TR. Atraumatic osteonecrosis of the talus. J Bone Joint Surg Am. 1998;80:529–536. doi: 10.2106/00004623-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Hirohata S, Ito K. Aseptic necrosis of unilateral scaphoid bone in systemic lupus erythematosus. Intern Med. 1992;31:794–797. doi: 10.2169/internalmedicine.31.794. [DOI] [PubMed] [Google Scholar]
- 71.Sigmundsson FG, Andersen PB, Schroeder HD, Thomsen K. Vertebral osteonecrosis associated with pancreatitis in a woman with pancreas divisum. A case report. J Bone Joint Surg Am. 2004;86-A:2504–2508. doi: 10.2106/00004623-200411000-00024. [DOI] [PubMed] [Google Scholar]
- 75.Van Poznak C. Osteonecrosis of the jaw. J Oncol Pract. 2006;2:3–4. doi: 10.1200/jop.2006.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pathak I, Bryce G. Temporal bone necrosis: diagnosis, classification, and management. Otolaryngol Head Neck Surg. 2000;123:252–257. doi: 10.1067/mhn.2000.107459. [DOI] [PubMed] [Google Scholar]
- 77.Stavinoha RR, Scott W. Osteonecrosis of the tarsal navicular in two adolescent soccer players. Clin J Sport Med. 1998;8:136–138. doi: 10.1097/00042752-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Moorman CT, 3rd, Warren RF, Hershman EB. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A:1190–1196. doi: 10.2106/00004623-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Gardeniers J. ARCO international classification of osteonecrosis. ARCO Newsletter. 1993;5:79. [Google Scholar]
- 80.Sugano N, Atsumi T, Ohzono K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 81.Schapira D. Transient osteoporosis of the hip. Semin Arthritis Rheum. 1992;22:98–105. doi: 10.1016/0049-0172(92)90003-v. [DOI] [PubMed] [Google Scholar]
- 82.Plenk H, Jr, Hofmann S, Eschberger J. Histomorphology and bone morphometry of the bone marrow edema syndrome of the hip. Clin Orthop Relat Res. 1997;334:73–84. [PubMed] [Google Scholar]
- 83.Berger CE, Kroner AH, Kristen KH. Transient bone marrow edema syndrome of the knee: clinical and magnetic resonance imaging results at 5 years after core decompression. Arthroscopy. 2006;22:866–871. doi: 10.1016/j.arthro.2006.04.095. [DOI] [PubMed] [Google Scholar]
- 84.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;140:273–312. [PubMed] [Google Scholar]
- 85.Chandler FA. Coronary disease of the hip. J Int Coll Surg. 1948;11:34–36. [PubMed] [Google Scholar]
- 87.Heikkinen ES, Puranen J, Suramo I. The effect of intertrochanteric osteotomy on the venous drainage of the femoral neck in Perthes’ disease. Acta Orthop Scand. 1976;47:89–95. doi: 10.3109/17453677608998978. [DOI] [PubMed] [Google Scholar]
- 88.Liu SL, Ho TC. The role of venous hypertension in the pathogenesis of Legg-Perthes disease. A clinical and experimental study. J Bone Joint Surg Am. 1991;73:194–200. [PubMed] [Google Scholar]
- 89.Thompson GH, Salter RB. Legg-Calve-Perthes disease. Current concepts and controversies. Orthop Clin North Am. 1987;18:617–635. [PubMed] [Google Scholar]
- 90.Chryssanthou CP. Dysbaric osteonecrosis. Etiological and pathogenetic concepts. Clin Orthop Relat Res. 1978;130:94–106. [PubMed] [Google Scholar]
- 91.Lehner CE, Adams WM, Dubielzig RR. Dysbaric osteonecrosis in divers and caisson workers. An animal model. Clin Orthop Relat Res. 1997;344:320–332. [PubMed] [Google Scholar]
- 92.Ohzono K, Takaoka K, Saito S. Intraosseous arterial architecture in nontraumatic avascular necrosis of the femoral head. Microangiographic and histologic study. Clin Orthop Relat Res. 1992;277:79–88. [PubMed] [Google Scholar]
- 93.Suh KT, Kim SW, Roh HL. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;431:220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 94.Lee JS, Roh HL, Kim CH. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: comparative analysis according to the risk factor. J Orthop Res. 2006;24:604–609. doi: 10.1002/jor.20078. [DOI] [PubMed] [Google Scholar]
- 95.Gangji V, Hauzeur JP, Schoutens A. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003;30:348–351. [PubMed] [Google Scholar]
- 96.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 97.Sato M, Sugano N, Ohzono K. Apoptosis and expression of stress protein (ORP150, HO1) during development of ischaemic osteonecrosis in the rat. J Bone Joint Surg Br. 2001;83:751–759. doi: 10.1302/0301-620x.83b5.10801. [DOI] [PubMed] [Google Scholar]
- 98.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. doi: 10.2106/00004623-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 100.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–588. [PubMed] [Google Scholar]
- 101.Miyanishi K, Yamamoto T, Irisa T. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–190. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 102.Kim YH, Kim JS. Histologic analysis of acetabular and proximal femoral bone in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86-A:2471–2474. doi: 10.2106/00004623-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 103.Jones JP., Jr Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed] [Google Scholar]
- 104.Jones LC, Mont MA, Le TB. Procoagulants and osteonecrosis. J Rheumatol. 2003;30:783–791. [PubMed] [Google Scholar]
- 105.Sun W, Wang BL, Liu BL. Osteonecrosis in patients after severe acute respiratory syndrome (SARS): possible role of anticardiolipin antibodies. J Clin Rheumatol. 2010;16:61–63. doi: 10.1097/RHU.0b013e3181cf3464. [DOI] [PubMed] [Google Scholar]
- 107.Preedy VR, Patel VB, Reilly ME. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999;4:e58–66. doi: 10.2741/A480. [DOI] [PubMed] [Google Scholar]
- 108.Ichiseki T, Matsumoto T. Oxidative stress may underlie the sex differences seen in steroid-induced osteonecrosis models. Med Hypotheses. 2006;66:1256. doi: 10.1016/j.mehy.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerachian MA, Harvey EJ, Cournoyer D. Avascular necrosis of the femoral head: vascular hypotheses. Endothelium. 2006;13:237–244. doi: 10.1080/10623320600904211. [DOI] [PubMed] [Google Scholar]
- 113.Feng Y, Yang SH, Xiao BJ. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2009;46:32–40. doi: 10.1016/j.bone.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Jones JP, Jr, Ramirez S, Doty SB. The pathophysiologic role of fat in dysbaric osteonecrosis. Clin Orthop Relat Res. 1993;296:256–264. [PubMed] [Google Scholar]
- 115.Tokuhara Y, Wakitani S, Oda Y. Low levels of steroid-metabolizing hepatic enzyme (cytochrome P450 3A) activity may elevate responsiveness to steroids and may increase risk of steroid-induced osteonecrosis even with low glucocorticoid dose. J Orthop Sci. 2009;14:794–800. doi: 10.1007/s00776-009-1400-5. [DOI] [PubMed] [Google Scholar]
- 116.Saito S, Ohzono K, Ono K. Early arteriopathy and postulated pathogenesis of osteonecrosis of the femoral head. The intracapital arterioles. Clin Orthop Relat Res. 1992;277:98–110. [PubMed] [Google Scholar]
- 117.Kenzora JE, Glimcher MJ. Accumulative cell stress: the multifactorial etiology of idiopathic osteonecrosis. Orthop Clin North Am. 1985;16:669–679. [PubMed] [Google Scholar]
- 118.Zizic TM, Marcoux C, Hungerford DS. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am J Med. 1985;79:596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 119.Koo KH, Lee JS, Lee YJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res. 2006;24:1722–1728. doi: 10.1002/jor.20164. [DOI] [PubMed] [Google Scholar]
- 120.Glueck CJ, Fontaine RN, Gruppo R. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res. 1999;366:133–146. doi: 10.1097/00003086-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 121.French D, Hamilton LH, Mattano LA., Jr A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111:4496–4499. doi: 10.1182/blood-2007-11-123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirata T, Fujioka M, Takahashi KA. ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J Orthop Sci. 2007;12:199–206. doi: 10.1007/s00776-007-1110-9. [DOI] [PubMed] [Google Scholar]
- 123.Hirata T, Fujioka M, Takahashi KA. Low molecular weight phenotype of Apo(a) is a risk factor of corticosteroid-induced osteonecrosis of the femoral head after renal transplant. J Rheumatol. 2007;34:516–522. [PubMed] [Google Scholar]
- 124.Wang XY, Niu XH, Chen WH. [Effects of apolipoprotein A1 and B gene polymorphism on avascular necrosis of the femoral head in Chinese population] Zhongguo Gu Shang. 2008;21:99–102. [PubMed] [Google Scholar]
- 125.Kim TH, Hong JM, Lee JY. Promoter polymorphisms of the vascular endothelial growth factor gene is associated with an osteonecrosis of the femoral head in the Korean population. Osteoarthritis Cartil. 2008;16:287–291. doi: 10.1016/j.joca.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 129.Soderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 130.Chiandussi S, Biasotto M, Dore F. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol. 2006;35:236–243. doi: 10.1259/dmfr/27458726. [DOI] [PubMed] [Google Scholar]
- 131.Dihlmann W. CT analysis of the upper end of the femur: the asterisk sign and ischaemic bone necrosis of the femoral head. Skeletal Radiol. 1982;8:251–258. doi: 10.1007/BF02219619. [DOI] [PubMed] [Google Scholar]
- 134.Steinberg ME, Hayken GD, Steinberg DR. A new method for evaluaton and staging of avascular necrosis of the femoral head. In: Arlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Williams & Wilkins; Baltimore: 1984. pp. 398–403. [Google Scholar]
- 135.Kwon YD, Ohe JY, Kim DY. Retrospective study of two biochemical markers for the risk assessment of oral bisphosphonate-related osteonecrosis of the jaws: can they be utilized as risk markers? Clin Oral Implants Res. 2011;22:100–105. doi: 10.1111/j.1600-0501.2010.01965.x. [DOI] [PubMed] [Google Scholar]
- 136.Beckmann J, Goetz J, Baethis H. Precision of computer-assisted core decompression drilling of the femoral head. Arch Orthop Trauma Surg. 2006;126:374–379. doi: 10.1007/s00402-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 137.Israelite C, Nelson CL, Ziarani CF. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–290. doi: 10.1097/01.blo.0000192365.58958.84. [DOI] [PubMed] [Google Scholar]
- 138.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973;55:1351–1366. [PubMed] [Google Scholar]
- 139.Marciniak D, Furey C, Shaffer JW. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am. 2005;87:742–747. doi: 10.2106/JBJS.D.02004. [DOI] [PubMed] [Google Scholar]
- 140.Zhao D, Xu D, Wang W, Cui X. Iliac graft vascularization for femoral head osteonecrosis. Clin Orthop Relat Res. 2006;442:171–179. doi: 10.1097/01.blo.0000181490.31424.96. [DOI] [PubMed] [Google Scholar]
- 141.Korompilias AV, Beris AE, Lykissas MG. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2010;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 142.Vendittoli PA, Lavigne M, Girard J, Roy AG. A randomised study comparing resection of acetabular bone at resurfacing and total hip replacement. J Bone Joint Surg Br. 2006;88:997–1002. doi: 10.1302/0301-620X.88B8.17615. [DOI] [PubMed] [Google Scholar]
- 143.Grecula MJ. Resurfacing arthroplasty in osteonecrosis of the hip. Orthop Clin North Am. 2005;36:231–242. doi: 10.1016/j.ocl.2005.02.005. x. [DOI] [PubMed] [Google Scholar]
- 144.Little CP, Ruiz AL, Harding IJ. Osteonecrosis in retrieved femoral heads after failed resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2005;87:320–323. doi: 10.1302/0301-620x.87b3.15330. [DOI] [PubMed] [Google Scholar]
- 145.Shimmin AJ, Bare J, Back DL. Complications associated with hip resurfacing arthroplasty. Orthop Clin North Am. 2005;36:187–193. doi: 10.1016/j.ocl.2005.01.002. ix. [DOI] [PubMed] [Google Scholar]
- 146.Hungerford MW, Mont MA, Scott R. Surface replacement hemiarthroplasty for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 1998;80:1656–1664. doi: 10.2106/00004623-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 147.Mont MA, Rajadhyaksha AD, Hungerford DS. Outcomes of limited femoral resurfacing arthroplasty compared with total hip arthroplasty for osteonecrosis of the femoral head. J Arthroplasty. 2001;16:134–139. doi: 10.1054/arth.2001.28722. [DOI] [PubMed] [Google Scholar]
- 148.Sayeed SA, Johnson AJ, Stroh DA. Hip resurfacing in patients who have osteonecrosis and are 25 years or under. Clin Orthop Relat Res. 2011;469:1582–1588. doi: 10.1007/s11999-010-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 150.Pautke C, Bauer F, Tischer T. Fluorescence-guided bone resection in bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:471–476. doi: 10.1016/j.joms.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 151.Freiberger JJ, Padilla-Burgos R, Chhoeu AH. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg. 2007;65:1321–1327. doi: 10.1016/j.joms.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 152.Wang CJ, Yang YJ, Huang CC. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol Int. 2011;31:871–877. doi: 10.1007/s00296-010-1384-7. [DOI] [PubMed] [Google Scholar]
- 153.Kuribayashi M, Fujioka M, Takahashi KA. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81:154–160. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zaidi M, Sun L, Robinson LJ. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.McCarthy EF. Aseptic necrosis of bone. An historic perspective. Clin Orthop Relat Res. 1982;168:216–221. [PubMed] [Google Scholar]
- 2.Nixon JE. Avascular necrosis of bone: a review. J R Soc Med. 1983;76:681–692. doi: 10.1177/014107688307600810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axhausen G. Uber anamische Infarkte am Knochensystem und ihre Bedeutung fur die Lehr von den primaren Epiphyseonkrosen. Arch Klin Chir. 1928;151:72–98. [Google Scholar]
- 4.Hutter CD. Dysbaric osteonecrosis: a reassessment and hypothesis. Med Hypotheses. 2000;54:585–590. doi: 10.1054/mehy.1999.0901. [DOI] [PubMed] [Google Scholar]
- 5.Assouline-Dayan Y, Chang C, Greenspan A. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 6.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: current perspectives on pathogenesis and treatment. Semin Arthritis Rheum. 1993;23:47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 7.Sevitt S. Avascular necrosis and revascularisation of the femoral head after intracapsular fractures; a combined arteriographic and histological necropsy study. J Bone Joint Surg Br. 1964;46:270–296. [PubMed] [Google Scholar]
- 8.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 9.D’Aubigne RM, Frain PG. Theory of osteotomies. Rev Chir Orthop Reparatrice Appar Mot. 1972;58:159–167. [PubMed] [Google Scholar]
- 10.Peitrogrande V, Mastromarino R. Osteopatia de prolongato trattamento cortisono. Ortop Traumatol. 1957;25:793. [Google Scholar]
- 11.Koo KH, Kim R, Kim YS. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21:299–303. doi: 10.1007/s100670200078. [DOI] [PubMed] [Google Scholar]
- 12.Hurel SJ, Kendall-Taylor P. Avascular necrosis secondary to postoperative steroid therapy. Br J Neurosurg. 1997;11:356–358. doi: 10.1080/02688699746186. [DOI] [PubMed] [Google Scholar]
- 13.Gogas H, Fennelly D. Avascular necrosis following extensive chemotherapy and dexamethasone treatment in a patient with advanced ovarian cancer: case report and review of the literature. Gynecol Oncol. 1996;63:379–381. doi: 10.1006/gyno.1996.0339. [DOI] [PubMed] [Google Scholar]
- 14.Powell C, Chang C, Naguwa SM. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9:721–743. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vreden SG, Hermus AR, van Liessum PA. Aseptic bone necrosis in patients on glucocorticoid replacement therapy. Neth J Med. 1991;39:153–157. [PubMed] [Google Scholar]
- 16.Haajanen J, Saarinen O, Laasonen L. Steroid treatment and aseptic necrosis of the femoral head in renal transplant recipients. Transplant Proc. 1984;16:1316–1319. [PubMed] [Google Scholar]
- 17.Chandler GN, Jones DT, Wright V, Hartfall SJ. Charcot's arthropathy following intra-articular hydrocortisone. Br Med J. 1959;1:952–953. doi: 10.1136/bmj.1.5127.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 19.Otto S, Pautke C, Opelz C. Osteonecrosis of the jaw: effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J Oral Maxillofac Surg. 2010;68:2837–2845. doi: 10.1016/j.joms.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Otto S, Abu-Id MH, Fedele S. Osteoporosis and bisphosphonates-related osteonecrosis of the jaw: not just a sporadic coincidence—a multi-centre study. J Craniomaxillofac Surg. 2011;39:272–277. doi: 10.1016/j.jcms.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Hirohata T, Sugioka Y. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988;234:115–123. [PubMed] [Google Scholar]
- 22.Hirota Y, Hirohata T, Fukuda K. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- 23.Axhausen G. Die Nekrose des proximalen Bruckstuckes beim Schenkelhals bruck und ihre Bedeutung fur das Huftgelenk. Arch Klin Chir. 1922;120:325–346. [Google Scholar]
- 24.Antti-Poika I, Karaharju E, Vankka E, Paavilainen T. Alcohol-associated femoral head necrosis. Ann Chir Gynaecol. 1987;76:318–322. [PubMed] [Google Scholar]
- 25.Orlic D, Jovanovic S, Anticevic D, Zecevic J. Frequency of idiopathic aseptic necrosis in medically treated alcoholics. Int Orthop. 1990;14:383–386. doi: 10.1007/BF00182650. [DOI] [PubMed] [Google Scholar]
- 26.Calve J. Sur une forme particuliere de pseudocoxalgie greffee sur des deformations caracteristiques de l’extremite superieure du femur. Rev Chir. 1910;30:48–54. [Google Scholar]
- 27.Legg A. An obscure affection of the hip joint. Boston Med Surg J. 1910;162:202–204. [Google Scholar]
- 28.Perthes G. Ueber Arthritis deformans juvenilis. Deutsche Zeitschr Chir. 2010;107:111–159. [Google Scholar]
- 29.Bentzon P. Experimental studies on the pathogenesis of coxa plana (Calve-Legg-Perthes-Waldenstrom's disease) and other manfiestations of “local dyschondroplasia”. Acad Radiol. 1926;6:155–172. [Google Scholar]
- 30.Ryder CT, Lebouvier JD, Kane R. Coxa plana. Pediatrics. 1957;19:979–992. [PubMed] [Google Scholar]
- 31.Goff CW. Legg-Calve-Perthes syndrome (LCPS). An up-to-date critical review. Clin Orthop. 1962;22:93–107. [PubMed] [Google Scholar]
- 32.Landin LA, Danielsson LG, Wattsgard C. Transient synovitis of the hip. Its incidence, epidemiology and relation to Perthes’ disease. J Bone Joint Surg Br. 1987;69:238–242. doi: 10.1302/0301-620X.69B2.3818754. [DOI] [PubMed] [Google Scholar]
- 33.Burwell RG, Dangerfield PH, Hall DJ. Perthes’ disease. An anthropometric study revealing impaired and disproportionate growth. J Bone Joint Surg Br. 1978;60-B:461–477. doi: 10.1302/0301-620X.60B4.711791. [DOI] [PubMed] [Google Scholar]
- 34.Burwell RG. Perthes’ disease: growth and aetiology. Arch Dis Child. 1988;63:1408–1412. doi: 10.1136/adc.63.11.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristmundsdottir F, Burwell RG, Harrison MH. Delayed skeletal maturation in Perthes’ disease. Acta Orthop Scand. 1987;58:277–279. doi: 10.3109/17453678709146484. [DOI] [PubMed] [Google Scholar]
- 36.Hall DJ, Harrison MH, Burwell RG. Congenital abnormalities and Perthes’ disease. Clinical evidence that children with Perthes’ disease may have a major congenital defect. J Bone Joint Surg Br. 1979;61:18–25. doi: 10.1302/0301-620X.61B1.33996. [DOI] [PubMed] [Google Scholar]
- 37.Burwell RG, Vernon CL, Dangerfield PH. Raised somatomedin activity in the serum of young boys with Perthes’ disease revealed by bioassay. A disease of growth transition? Clin Orthop Relat Res. 1986;209:129–138. [PubMed] [Google Scholar]
- 38.Rayner PH, Schwalbe SL, Hall DJ. An assessment of endocrine function in boys with Perthes’ disease. Clin Orthop Relat Res. 1986;209:124–128. [PubMed] [Google Scholar]
- 39.Barr RD, Sala A. Osteonecrosis in children and adolescents with cancer. Pediatr Blood Cancer. 2008;50:483–485. doi: 10.1002/pbc.21405. discussion 6. [DOI] [PubMed] [Google Scholar]
- 40.Karimova EJ, Rai SN, Howard SC. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 41.Niinimaki RA, Harila-Saari AH, Jartti AE. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25:1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 42.Montella BJ, Nunley JA, Urbaniak JR. Osteonecrosis of the femoral head associated with pregnancy. A preliminary report. J Bone Joint Surg Am. 1999;81:790–798. doi: 10.2106/00004623-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Hernigou P, Allain J, Bachir D, Galacteros F. Abnormalities of the adult shoulder due to sickle cell osteonecrosis during childhood. Rev Rhum Engl Ed. 1998;65:27–32. [PubMed] [Google Scholar]
- 44.Hernigou P, Galacteros F, Bachir D, Goutallier D. Deformities of the hip in adults who have sickle-cell disease and had avascular necrosis in childhood. A natural history of fifty-two patients. J Bone Joint Surg Am. 1991;73:81–92. [PubMed] [Google Scholar]
- 45.Kandzierski G, Gregosiewicz A, Malek U. Femur head necrosis in haemophilia and after prolonged steroid therapy–description of two cases. Chir Narzadow Ruchu Ortop Pol. 2004;69:269–271. [PubMed] [Google Scholar]
- 46.Kemnitz S, Moens P, Peerlinck K, Fabry G. Avascular necrosis of the talus in children with haemophilia. J Pediatr Orthop B. 2002;11:73–78. doi: 10.1097/00009957-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Kilcoyne RF, Nuss R. Femoral head osteonecrosis in a child with hemophilia. Arthritis Rheum. 1999;42:1550–1551. doi: 10.1002/1529-0131(199907)42:7<1550::AID-ANR31>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 48.Paton RW, Evans DI. Silent avascular necrosis of the femoral head in haemophilia. J Bone Joint Surg Br. 1988;70:737–739. doi: 10.1302/0301-620X.70B5.3192571. [DOI] [PubMed] [Google Scholar]
- 49.MacNicol MF, Ludlam CA. Does avascular necrosis cause collapse of the dome of the talus in severe haemophilia? Haemophilia. 1999;5:139–142. [PubMed] [Google Scholar]
- 50.Perri G, Giordano V. Aseptic necrosis of the femur head in hemophiliacs. Radiol Med. 1982;68:137–140. [PubMed] [Google Scholar]
- 51.Twynham G. A case of caisson disease. BMJ. 1888;1:190–191. [Google Scholar]
- 52.Davidson JK. Dysbaric disorders: aseptic bone necrosis in tunnel workers and divers. Baillieres Clin Rheumatol. 1989;3:1–23. doi: 10.1016/s0950-3579(89)80033-0. [DOI] [PubMed] [Google Scholar]
- 53.Chan MH, Chan PK, Griffith JF. Steroid-induced osteonecrosis in severe acute respiratory syndrome: a retrospective analysis of biochemical markers of bone metabolism and corticosteroid therapy. Pathology. 2006;38:229–235. doi: 10.1080/00313020600696231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv H, de Vlas SJ, Liu W. Avascular osteonecrosis after treatment of SARS: a 3-year longitudinal study. Trop Med Int Health. 2009;14(Suppl 1):79–84. doi: 10.1111/j.1365-3156.2008.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CW, Chiu WK, Chan CC. Osteonecrosis in children with severe acute respiratory syndrome. Pediatr Infect Dis J. 2004;23:888–890. doi: 10.1097/01.inf.0000137570.37856.ea. [DOI] [PubMed] [Google Scholar]
- 56.Cushner MA, Friedman RJ. Osteonecrosis of the humeral head. J Am Acad Orthop Surg. 1997;5:339–346. doi: 10.5435/00124635-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Hasan SS, Romeo AA. Nontraumatic osteonecrosis of the humeral head. J Shoulder Elbow Surg. 2002;11:281–298. doi: 10.1067/mse.2002.124347. [DOI] [PubMed] [Google Scholar]
- 58.Hattrup SJ, Cofield RH. Osteonecrosis of the humeral head: relationship of disease stage, extent, and cause to natural history. J Shoulder Elbow Surg. 1999;8:559–564. doi: 10.1016/s1058-2746(99)90089-7. [DOI] [PubMed] [Google Scholar]
- 59.L’Insalata JC, Pagnani MJ, Warren RF, Dines DM. Humeral head osteonecrosis: clinical course and radiographic predictors of outcome. J Shoulder Elbow Surg. 1996;5:355–361. doi: 10.1016/s1058-2746(96)80066-8. [DOI] [PubMed] [Google Scholar]
- 60.Baumgarten KM, Mont MA, Rifai A, Hungerford DS. Atraumatic osteonecrosis of the patella. Clin Orthop Relat Res. 2001;383:191–196. doi: 10.1097/00003086-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 61.Berger CE, Kroner A, Kristen KH. Spontaneous osteonecrosis of the knee: biochemical markers of bone turnover and pathohistology. Osteoarthritis Cartil. 2005;13:716–721. doi: 10.1016/j.joca.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28:145–150. [PubMed] [Google Scholar]
- 63.Murakami H, Soejima T, Inoue T. A long-term follow-up study of four cases who underwent curettage and autogenous bone grafting for steroid-related osteonecrosis of the femoral condyle. Kurume Med J. 2004;51:277–281. doi: 10.2739/kurumemedj.51.277. [DOI] [PubMed] [Google Scholar]
- 64.Barnes R, Brown JT, Garden RS, Nicoll EA. Subcapital fractures of the femur. A prospective review. J Bone Joint Surg Br. 1976;58:2–24. doi: 10.1302/0301-620X.58B1.1270491. [DOI] [PubMed] [Google Scholar]
- 65.Muscolo DL, Costa-Paz M, Ayerza M, Makino A. Medial meniscal tears and spontaneous osteonecrosis of the knee. Arthroscopy. 2006;22:457–460. doi: 10.1016/j.arthro.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Radke S, Wollmerstedt N, Bischoff A, Eulert J. Knee arthroplasty for spontaneous osteonecrosis of the knee: unicompartimental vs bicompartimental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2005;13:158–162. doi: 10.1007/s00167-004-0551-3. [DOI] [PubMed] [Google Scholar]
- 67.Delanois RE, Mont MA, Yoon TR. Atraumatic osteonecrosis of the talus. J Bone Joint Surg Am. 1998;80:529–536. doi: 10.2106/00004623-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Hirohata S, Ito K. Aseptic necrosis of unilateral scaphoid bone in systemic lupus erythematosus. Intern Med. 1992;31:794–797. doi: 10.2169/internalmedicine.31.794. [DOI] [PubMed] [Google Scholar]
- 69.Allen BL, Jr, Jinkins WJ., 3rd Vertebral osteonecrosis associated with pancreatitis in a child. A case report. J Bone Joint Surg Am. 1978;60:985–987. [PubMed] [Google Scholar]
- 70.Ito M, Motomiya M, Abumi K. Vertebral osteonecrosis associated with sarcoidosis. Case report. J Neurosurg Spine. 2005;2:222–225. doi: 10.3171/spi.2005.2.2.0222. [DOI] [PubMed] [Google Scholar]
- 71.Sigmundsson FG, Andersen PB, Schroeder HD, Thomsen K. Vertebral osteonecrosis associated with pancreatitis in a woman with pancreas divisum. A case report. J Bone Joint Surg Am. 2004;86-A:2504–2508. doi: 10.2106/00004623-200411000-00024. [DOI] [PubMed] [Google Scholar]
- 72.Chowdhury S, Pickering LM, Ellis PA. Adjuvant aromatase inhibitors and bone health. J Br Menopause Soc. 2006;12:97–103. doi: 10.1258/136218006778234020. [DOI] [PubMed] [Google Scholar]
- 73.Van Poznak C, Estilo C. Osteonecrosis of the jaw in cancer patients receiving IV bisphosphonates. Oncology (Williston Park) 2006;20:1053–1062. discussion 65-66. [PubMed] [Google Scholar]
- 74.Van Poznak C. The phenomenon of osteonecrosis of the jaw in patients with metastatic breast cancer. Cancer Invest. 2006;24:110–112. doi: 10.1080/07357900500449652. [DOI] [PubMed] [Google Scholar]
- 75.Van Poznak C. Osteonecrosis of the jaw. J Oncol Pract. 2006;2:3–4. doi: 10.1200/jop.2006.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pathak I, Bryce G. Temporal bone necrosis: diagnosis, classification, and management. Otolaryngol Head Neck Surg. 2000;123:252–257. doi: 10.1067/mhn.2000.107459. [DOI] [PubMed] [Google Scholar]
- 77.Stavinoha RR, Scott W. Osteonecrosis of the tarsal navicular in two adolescent soccer players. Clin J Sport Med. 1998;8:136–138. doi: 10.1097/00042752-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Moorman CT, 3rd, Warren RF, Hershman EB. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A:1190–1196. doi: 10.2106/00004623-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Gardeniers J. ARCO international classification of osteonecrosis. ARCO Newsletter. 1993;5:79. [Google Scholar]
- 80.Sugano N, Atsumi T, Ohzono K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 81.Schapira D. Transient osteoporosis of the hip. Semin Arthritis Rheum. 1992;22:98–105. doi: 10.1016/0049-0172(92)90003-v. [DOI] [PubMed] [Google Scholar]
- 82.Plenk H, Jr, Hofmann S, Eschberger J. Histomorphology and bone morphometry of the bone marrow edema syndrome of the hip. Clin Orthop Relat Res. 1997;334:73–84. [PubMed] [Google Scholar]
- 83.Berger CE, Kroner AH, Kristen KH. Transient bone marrow edema syndrome of the knee: clinical and magnetic resonance imaging results at 5 years after core decompression. Arthroscopy. 2006;22:866–871. doi: 10.1016/j.arthro.2006.04.095. [DOI] [PubMed] [Google Scholar]
- 84.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;140:273–312. [PubMed] [Google Scholar]
- 85.Chandler FA. Coronary disease of the hip. J Int Coll Surg. 1948;11:34–36. [PubMed] [Google Scholar]
- 86.Chandler FA. Coronary disease of the hip. 1949. Clin Orthop Relat Res. 2001;386:7–10. doi: 10.1097/00003086-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Heikkinen ES, Puranen J, Suramo I. The effect of intertrochanteric osteotomy on the venous drainage of the femoral neck in Perthes’ disease. Acta Orthop Scand. 1976;47:89–95. doi: 10.3109/17453677608998978. [DOI] [PubMed] [Google Scholar]
- 88.Liu SL, Ho TC. The role of venous hypertension in the pathogenesis of Legg-Perthes disease. A clinical and experimental study. J Bone Joint Surg Am. 1991;73:194–200. [PubMed] [Google Scholar]
- 89.Thompson GH, Salter RB. Legg-Calve-Perthes disease. Current concepts and controversies. Orthop Clin North Am. 1987;18:617–635. [PubMed] [Google Scholar]
- 90.Chryssanthou CP. Dysbaric osteonecrosis. Etiological and pathogenetic concepts. Clin Orthop Relat Res. 1978;130:94–196. [PubMed] [Google Scholar]
- 91.Lehner CE, Adams WM, Dubielzig RR. Dysbaric osteonecrosis in divers and caisson workers. An animal model. Clin Orthop Relat Res. 1997;344:320–332. [PubMed] [Google Scholar]
- 92.Ohzono K, Takaoka K, Saito S. Intraosseous arterial architecture in nontraumatic avascular necrosis of the femoral head. Microangiographic and histologic study. Clin Orthop Relat Res. 1992;277:79–88. [PubMed] [Google Scholar]
- 93.Suh KT, Kim SW, Roh HL. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;431:220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 94.Lee JS, Roh HL, Kim CH. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: comparative analysis according to the risk factor. J Orthop Res. 2006;24:604–609. doi: 10.1002/jor.20078. [DOI] [PubMed] [Google Scholar]
- 95.Gangji V, Hauzeur JP, Schoutens A. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003;30:348–351. [PubMed] [Google Scholar]
- 96.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 97.Sato M, Sugano N, Ohzono K. Apoptosis and expression of stress protein (ORP150, HO1) during development of ischaemic osteonecrosis in the rat. J Bone Joint Surg Br. 2001;83:751–759. doi: 10.1302/0301-620x.83b5.10801. [DOI] [PubMed] [Google Scholar]
- 98.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. doi: 10.2106/00004623-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 100.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–588. [PubMed] [Google Scholar]
- 101.Miyanishi K, Yamamoto T, Irisa T. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–190. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 102.Kim YH, Kim JS. Histologic analysis of acetabular and proximal femoral bone in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86-A:2471–2474. doi: 10.2106/00004623-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 103.Jones JP., Jr Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed] [Google Scholar]
- 104.Jones LC, Mont MA, Le TB. Procoagulants and osteonecrosis. J Rheumatol. 2003;30:783–791. [PubMed] [Google Scholar]
- 105.Sun W, Wang BL, Liu BL. Osteonecrosis in patients after severe acute respiratory syndrome (SARS): possible role of anticardiolipin antibodies. J Clin Rheumatol. 2010;16:61–63. doi: 10.1097/RHU.0b013e3181cf3464. [DOI] [PubMed] [Google Scholar]
- 106.Sun W, Li ZR, Shi ZC. Changes in coagulation and fibrinolysis of post-SARS osteonecrosis in a Chinese population. Int Orthop. 2006;30:143–146. doi: 10.1007/s00264-005-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Preedy VR, Patel VB, Reilly ME. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999;4:e58–66. doi: 10.2741/A480. [DOI] [PubMed] [Google Scholar]
- 108.Ichiseki T, Matsumoto T. Oxidative stress may underlie the sex differences seen in steroid-induced osteonecrosis models. Med Hypotheses. 2006;66:1256. doi: 10.1016/j.mehy.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Ichiseki T, Ueda Y, Katsuda S. Oxidative stress by glutathione depletion induces osteonecrosis in rats. Rheumatology (Oxford) 2006;45:287–290. doi: 10.1093/rheumatology/kei149. [DOI] [PubMed] [Google Scholar]
- 110.Ichiseki T, Kaneuji A, Katsuda S. DNA oxidation injury in bone early after steroid administration is involved in the pathogenesis of steroid-induced osteonecrosis. Rheumatology (Oxford) 2005;44:456–460. doi: 10.1093/rheumatology/keh518. [DOI] [PubMed] [Google Scholar]
- 111.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerachian MA, Harvey EJ, Cournoyer D. Avascular necrosis of the femoral head: vascular hypotheses. Endothelium. 2006;13:237–244. doi: 10.1080/10623320600904211. [DOI] [PubMed] [Google Scholar]
- 113.Feng Y, Yang SH, Xiao BJ. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2009;46:32–40. doi: 10.1016/j.bone.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Jones JP, Jr, Ramirez S, Doty SB. The pathophysiologic role of fat in dysbaric osteonecrosis. Clin Orthop Relat Res. 1993;296:256–264. [PubMed] [Google Scholar]
- 115.Tokuhara Y, Wakitani S, Oda Y. Low levels of steroid-metabolizing hepatic enzyme (cytochrome P450 3A) activity may elevate responsiveness to steroids and may increase risk of steroid-induced osteonecrosis even with low glucocorticoid dose. J Orthop Sci. 2009;14:794–800. doi: 10.1007/s00776-009-1400-5. [DOI] [PubMed] [Google Scholar]
- 116.Saito S, Ohzono K, Ono K. Early arteriopathy and postulated pathogenesis of osteonecrosis of the femoral head. The intracapital arterioles. Clin Orthop Relat Res. 1992;277:98–110. [PubMed] [Google Scholar]
- 117.Kenzora JE, Glimcher MJ. Accumulative cell stress: the multifactorial etiology of idiopathic osteonecrosis. Orthop Clin North Am. 1985;16:669–679. [PubMed] [Google Scholar]
- 118.Zizic TM, Marcoux C, Hungerford DS. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am J Med. 1985;79:596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 119.Koo KH, Lee JS, Lee YJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res. 2006;24:1722–1728. doi: 10.1002/jor.20164. [DOI] [PubMed] [Google Scholar]
- 120.Glueck CJ, Fontaine RN, Gruppo R. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res. 1999;366:133–146. doi: 10.1097/00003086-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 121.French D, Hamilton LH, Mattano LA., Jr A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111:4496–4499. doi: 10.1182/blood-2007-11-123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirata T, Fujioka M, Takahashi KA. ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J Orthop Sci. 2007;12:199–206. doi: 10.1007/s00776-007-1110-9. [DOI] [PubMed] [Google Scholar]
- 123.Hirata T, Fujioka M, Takahashi KA. Low molecular weight phenotype of Apo(a) is a risk factor of corticosteroid-induced osteonecrosis of the femoral head after renal transplant. J Rheumatol. 2007;34:516–522. [PubMed] [Google Scholar]
- 124.Wang XY, Niu XH, Chen WH. [Effects of apolipoprotein A1 and B gene polymorphism on avascular necrosis of the femoral head in Chinese population] Zhongguo Gu Shang. 2008;21:99–102. [PubMed] [Google Scholar]
- 125.Kim TH, Hong JM, Lee JY. Promoter polymorphisms of the vascular endothelial growth factor gene is associated with an osteonecrosis of the femoral head in the Korean population. Osteoarthritis Cartil. 2008;16:287–291. doi: 10.1016/j.joca.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 126.Kim TH, Hong JM, Oh B. Association of polymorphisms in the Interleukin 23 receptor gene with osteonecrosis of femoral head in Korean population. Exp Mol Med. 2008;40:418–426. doi: 10.3858/emm.2008.40.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi HY, Mau LW, Chang JK. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18:1053–1060. doi: 10.1007/s11136-009-9512-0. [DOI] [PubMed] [Google Scholar]
- 128.Mahomed NN, Arndt DC, McGrory BJ, Harris WH. The Harris hip score: comparison of patient self-report with surgeon assessment. J Arthroplasty. 2001;16:575–580. doi: 10.1054/arth.2001.23716. [DOI] [PubMed] [Google Scholar]
- 129.Soderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 130.Chiandussi S, Biasotto M, Dore F. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol. 2006;35:236–243. doi: 10.1259/dmfr/27458726. [DOI] [PubMed] [Google Scholar]
- 131.Dihlmann W. CT analysis of the upper end of the femur: the asterisk sign and ischaemic bone necrosis of the femoral head. Skeletal Radiol. 1982;8:251–258. doi: 10.1007/BF02219619. [DOI] [PubMed] [Google Scholar]
- 132.Dihlmann W, Heller M. The asterisk sign and adult ischemic femur head necrosis. Rofo. 1985;142:430–435. doi: 10.1055/s-2008-1052680. [DOI] [PubMed] [Google Scholar]
- 133.Specchiulli F, Mele M, Capocasale N, Laforgia R. The early diagnosis of idiopathic femoral osteonecrosis. Ital J Orthop Traumatol. 1988;14:519–526. [PubMed] [Google Scholar]
- 134.Steinberg ME, Hayken GD, Steinberg DR. A new method for evaluaton and staging of avascular necrosis of the femoral head. In: Arlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Williams & Wilkins; Baltimore: 1984. pp. 398–403. [Google Scholar]
- 135.Kwon YD, Ohe JY, Kim DY. Retrospective study of two biochemical markers for the risk assessment of oral bisphosphonate-related osteonecrosis of the jaws: can they be utilized as risk markers? Clin Oral Implants Res. 2011;22:100–105. doi: 10.1111/j.1600-0501.2010.01965.x. [DOI] [PubMed] [Google Scholar]
- 136.Beckmann J, Goetz J, Baethis H. Precision of computer-assisted core decompression drilling of the femoral head. Arch Orthop Trauma Surg. 2006;126:374–379. doi: 10.1007/s00402-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 137.Israelite C, Nelson CL, Ziarani CF. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–290. doi: 10.1097/01.blo.0000192365.58958.84. [DOI] [PubMed] [Google Scholar]
- 138.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973;55:1351–1366. [PubMed] [Google Scholar]
- 139.Marciniak D, Furey C, Shaffer JW. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am. 2005;87:742–747. doi: 10.2106/JBJS.D.02004. [DOI] [PubMed] [Google Scholar]
- 140.Zhao D, Xu D, Wang W, Cui X. Iliac graft vascularization for femoral head osteonecrosis. Clin Orthop Relat Res. 2006;442:171–179. doi: 10.1097/01.blo.0000181490.31424.96. [DOI] [PubMed] [Google Scholar]
- 141.Korompilias AV, Beris AE, Lykissas MG. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2010;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 142.Vendittoli PA, Lavigne M, Girard J, Roy AG. A randomised study comparing resection of acetabular bone at resurfacing and total hip replacement. J Bone Joint Surg Br. 2006;88:997–1002. doi: 10.1302/0301-620X.88B8.17615. [DOI] [PubMed] [Google Scholar]
- 143.Grecula MJ. Resurfacing arthroplasty in osteonecrosis of the hip. Orthop Clin North Am. 2005;36:231–242. doi: 10.1016/j.ocl.2005.02.005. x. [DOI] [PubMed] [Google Scholar]
- 144.Little CP, Ruiz AL, Harding IJ. Osteonecrosis in retrieved femoral heads after failed resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2005;87:320–323. doi: 10.1302/0301-620x.87b3.15330. [DOI] [PubMed] [Google Scholar]
- 145.Shimmin AJ, Bare J, Back DL. Complications associated with hip resurfacing arthroplasty. Orthop Clin North Am. 2005;36:187–193. doi: 10.1016/j.ocl.2005.01.002. ix. [DOI] [PubMed] [Google Scholar]
- 146.Hungerford MW, Mont MA, Scott R. Surface replacement hemiarthroplasty for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 1998;80:1656–1664. doi: 10.2106/00004623-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 147.Mont MA, Rajadhyaksha AD, Hungerford DS. Outcomes of limited femoral resurfacing arthroplasty compared with total hip arthroplasty for osteonecrosis of the femoral head. J Arthroplasty. 2001;16:134–139. doi: 10.1054/arth.2001.28722. [DOI] [PubMed] [Google Scholar]
- 148.Sayeed SA, Johnson AJ, Stroh DA. Hip resurfacing in patients who have osteonecrosis and are 25 years or under. Clin Orthop Relat Res. 2011;469:1582–1588. doi: 10.1007/s11999-010-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 150.Pautke C, Bauer F, Tischer T. Fluorescence-guided bone resection in bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:471–476. doi: 10.1016/j.joms.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 151.Freiberger JJ, Padilla-Burgos R, Chhoeu AH. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg. 2007;65:1321–1327. doi: 10.1016/j.joms.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 152.Wang CJ, Yang YJ, Huang CC. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol Int. 2011;31:871–877. doi: 10.1007/s00296-010-1384-7. [DOI] [PubMed] [Google Scholar]
- 153.Kuribayashi M, Fujioka M, Takahashi KA. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81:154–160. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zaidi M, Sun L, Robinson LJ. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cruess RL. Steroid-induced avascular necrosis of the head of the humerus. Natural history and management. J Bone Joint Surg Br. 1976;58:313–317. doi: 10.1302/0301-620X.58B3.956247. [DOI] [PubMed] [Google Scholar]
- 156.Cruess RL. Steroid-induced osteonecrosis: a review. Can J Surg. 1981;24:567–571. [PubMed] [Google Scholar]
- 157.Cruess RL. Steroid-induced osteonecrosis. J R Coll Surg Edinb. 1981;26:69–77. [PubMed] [Google Scholar]
- 158.Graziani F, Cei S, La Ferla F. Association between osteonecrosis of the jaws and chronic high-dosage intravenous bisphosphonates therapy. J Craniofac Surg. 2006;17:876–879. doi: 10.1097/01.scs.0000221517.80361.6f. [DOI] [PubMed] [Google Scholar]
- 159.Biasotto M, Chiandussi S, Dore F. Clinical aspects and management of bisphosphonates-associated osteonecrosis of the jaws. Acta Odontol Scand. 2006;64:348–354. doi: 10.1080/00016350600844360. [DOI] [PubMed] [Google Scholar]
- 160.Hungerford DS, Zizic TM. Alcoholism associated ischemic necrosis of the femoral head. Early diagnosis and treatment. Clin Orthop Relat Res. 1978;130:144–153. [PubMed] [Google Scholar]
- 161.Scotter E, Moody A. Dysbaric osteonecrosis (caisson disease) Radiogr Today. 1988;54:41–43. [PubMed] [Google Scholar]
- 162.Abylaev Zh A, Bukhman AI. [Possible mechanisms of the development of osteopaties in patients with chronic lead poisoning] Gig Tr Prof Zabol. 1990;2:31–35. [PubMed] [Google Scholar]
- 163.Kazakos K, Chatzipapas C, Xarchas KC. Knee osteonecrosis due to lead poisoning: case report and review of the literature. Med Sci Monit. 2006;12:CS85–CS89. [PubMed] [Google Scholar]
- 164.Vanderstraeten L, Binns M. Osteonecrosis of the femoral head following an electrical injury to the leg. J Bone Joint Surg Br. 2008;90:1101–1104. doi: 10.1302/0301-620X.90B8.19971. [DOI] [PubMed] [Google Scholar]
- 165.Govoni M, Orzincolo C, Bigoni M. Humeral head osteonecrosis caused by electrical injury: a case report. J Emerg Med. 1993;11:17–21. doi: 10.1016/0736-4679(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 166.Rubinstein RA, Jr, Beals RK. The results of treatment of posttraumatic avascular necrosis of the femoral head in young adults: report of 31 patients. Contemp Orthop. 1993;27:527–532. [PubMed] [Google Scholar]
- 167.Burwell RG. Perthes’ disease. J Bone Joint Surg Br. 1978;60:1–3. doi: 10.1302/0301-620X.60B1.627568. [DOI] [PubMed] [Google Scholar]
- 168.Herold HZ. Avascular necrosis of the femoral head in children under the age of three. Clin Orthop Relat Res. 1977;126:193–195. [PubMed] [Google Scholar]
- 169.Morcuende JA, Meyer MD, Dolan LA, Weinstein SL. Long-term outcome after open reduction through an anteromedial approach for congenital dislocation of the hip. J Bone Joint Surg Am. 1997;79:810–817. doi: 10.2106/00004623-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 170.Narayanan UG. Reduction increasing osteonecrosis risk in slipped capital femoral epiphysis. J Bone Joint Surg Am. 2004;86-A:437. doi: 10.2106/00004623-200402000-00049. [DOI] [PubMed] [Google Scholar]
- 171.Yamasaki T, Yasunaga Y, Hisatome T. Bone remodeling of a femoral head after transtrochanteric rotational osteotomy for osteonecrosis associated with slipped capital femoral epiphysis: a case report. Arch Orthop Trauma Surg. 2005;125:486–489. doi: 10.1007/s00402-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 172.Irisa T, Yamamoto T, Miyanishi K. Osteonecrosis induced by a single administration of low-dose lipopolysaccharide in rabbits. Bone. 2001;28:641–649. doi: 10.1016/s8756-3282(01)00460-4. [DOI] [PubMed] [Google Scholar]
- 173.Jones JP., Jr Fat embolism and osteonecrosis. Orthop Clin North Am. 1985;16:595–633. [PubMed] [Google Scholar]
- 174.Barbezat GO, Miles T, Bank S, Terblanche J. Letter: necrosis of the femoral head in a black patient with pancreatitis. S Afr Med J. 1976;50:160. [PubMed] [Google Scholar]
- 175.Chao YC, Wang SJ, Chu HC. Investigation of alcohol metabolizing enzyme genes in Chinese alcoholics with avascular necrosis of hip joint, pancreatitis and cirrhosis of the liver. Alcohol Alcohol. 2003;38:431–436. doi: 10.1093/alcalc/agg106. [DOI] [PubMed] [Google Scholar]
- 176.Pais MJ. Disease states affecting both liver and bone. Radiol Clin North Am. 1980;18:253–267. [PubMed] [Google Scholar]
- 177.Watson RM, Roach NA, Dalinka MK. Avascular necrosis and bone marrow edema syndrome. Radiol Clin North Am. 2004;42:207–219. doi: 10.1016/S0033-8389(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 178.Horiuchi H, Saito N, Kobayashi S. Avascular necrosis of the femoral head in a patient with Fabry's disease: identification of ceramide trihexoside in the bone by delayed-extraction matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Arthritis Rheum. 2002;46:1922–1925. doi: 10.1002/art.10391. [DOI] [PubMed] [Google Scholar]
- 179.Ross G, Kuwamura F, Goral A. Association of Fabry's disease with femoral head avascular necrosis. Orthopedics. 1993;16:471–473. doi: 10.3928/0147-7447-19930401-12. [DOI] [PubMed] [Google Scholar]
- 180.Sellman DC, Froimson AI. Long-term follow-up of a total articular resurfacing arthroplasty and a cup arthroplasty in Gaucher's disease. Orthop Rev. 1992;21:1099–1101. 104, 107. [PubMed] [Google Scholar]
- 181.Mielants H, Veys EM, DeBussere A, van der Jeught J. Avascular necrosis and its relation to lipid and purine metabolism. J Rheumatol. 1975;2:430–436. [PubMed] [Google Scholar]
- 182.Heaf JG. Bone disease after renal transplantation. Transplantation. 2003;75:315–325. doi: 10.1097/01.TP.0000043926.74349.6D. [DOI] [PubMed] [Google Scholar]
- 183.Kjaergaard GH, Laursen JO. Osteonecrosis of a knee joint in a young man with IDDM. Ugeskr Laeger. 2000;162:4663–4664. [PubMed] [Google Scholar]
- 184.Hernigou P, Bachir D, Galacteros F. The natural history of symptomatic osteonecrosis in adults with sickle-cell disease. J Bone Joint Surg Am. 2003;85-A:500–504. doi: 10.2106/00004623-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 185.Onuba O. Bone disorders in sickle-cell disease. Int Orthop. 1993;17:397–399. doi: 10.1007/BF00180461. [DOI] [PubMed] [Google Scholar]
- 186.Mahachoklertwattana P. Zoledronic acid for the treatment of thalassemia-induced osteonecrosis. Haematologica. 2006;91:1155A. [PubMed] [Google Scholar]
- 187.Campbell WN, Joshi M, Sileo D. Osteonecrosis following meningococcemia and disseminated intravascular coagulation in an adult: case report and review. Clin Infect Dis. 1997;24:452–455. doi: 10.1093/clinids/24.3.452. [DOI] [PubMed] [Google Scholar]
- 188.Jones JP., Jr Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993;292:294–308. [PubMed] [Google Scholar]
- 189.Jones JP., Jr Coagulopathies and osteonecrosis. Acta Orthop Belg. 1999;65(Suppl 1):5–8. [PubMed] [Google Scholar]
- 190.Glueck CJ, Freiberg RA, Fontaine RN. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 191.Glueck CJ, Freiberg R, Glueck HI. Hypofibrinolysis: a common, major cause of osteonecrosis. Am J Hematol. 1994;45:156–166. doi: 10.1002/ajh.2830450212. [DOI] [PubMed] [Google Scholar]
- 192.Strau G, Kainz L, Kienzer H. Spontaneous osteonecrosis of the knee joint in clinically suspected thrombosis of the leg veins. Rontgenblatter. 1988;41:122–124. [PubMed] [Google Scholar]
- 193.Tektonidou MG, Malagari K, Vlachoyiannopoulos PG. Asymptomatic avascular necrosis in patients with primary antiphospholipid syndrome in the absence of corticosteroid use: a prospective study by magnetic resonance imaging. Arthritis Rheum. 2003;48:732–736. doi: 10.1002/art.10835. [DOI] [PubMed] [Google Scholar]
- 194.Neidel J, Boehnke M, Kuster RM. The efficacy and safety of intraarticular corticosteroid therapy for coxitis in juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1620–1628. doi: 10.1002/art.10313. [DOI] [PubMed] [Google Scholar]
- 195.Freeman HJ, Freeman KJ. Prevalence rates and an evaluation of reported risk factors for osteonecrosis (avascular necrosis) in Crohn's disease. Can J Gastroenterol. 2000;14:138–143. doi: 10.1155/2000/958086. [DOI] [PubMed] [Google Scholar]
- 196.Stiles RG, Carpenter WA, Tigges S. Osteonecrosis of the femoral heads in inflammatory bowel disease. N Engl J Med. 1994;330:791. doi: 10.1056/NEJM199403173301116. author reply 2. [DOI] [PubMed] [Google Scholar]
- 197.Wang TY, Avlonitis EG, Relkin R. Systemic necrotizing vasculitis causing bone necrosis. Am J Med. 1988;84:1085–1086. doi: 10.1016/0002-9343(88)90319-1. [DOI] [PubMed] [Google Scholar]
- 198.Yanagitani Y, Fujita M. Avascular necrosis of the femoral head associated with mucocutaneous lymph node syndrome. J Pediatr Orthop. 1986;6:107–109. doi: 10.1097/01241398-198601000-00022. [DOI] [PubMed] [Google Scholar]
- 199.Clarke AE, Bloch DA, Medsger TA, Jr, Oddis CV. A longitudinal study of functional disability in a national cohort of patients with polymyositis/dermatomyositis. Arthritis Rheum. 1995;38:1218–1224. doi: 10.1002/art.1780380907. [DOI] [PubMed] [Google Scholar]
- 200.Scribner AN, Troia-Cancio PV, Cox BA. Osteonecrosis in HIV: a case-control study. J Acquir Immune Defic Syndr. 2000;25:19–25. doi: 10.1097/00042560-200009010-00003. [DOI] [PubMed] [Google Scholar]
- 201.Gutierrez F, Padilla S, Masia M. Osteonecrosis in patients infected with HIV: clinical epidemiology and natural history in a large case series from Spain. J Acquir Immune Defic Syndr. 2006;42:286–292. doi: 10.1097/01.qai.0000225012.53568.20. [DOI] [PubMed] [Google Scholar]
- 202.Chaudhuri R, McKeown B, Harrington D. Mucormycosis osteomyelitis causing avascular necrosis of the cuboid bone: MR imaging findings. AJR Am J Roentgenol. 1992;159:1035–1037. doi: 10.2214/ajr.159.5.1414771. [DOI] [PubMed] [Google Scholar]
- 203.Seipolt B, Dinger J, Rupprecht E. Osteonecrosis after meningococcemia and disseminated intravascular coagulation. Pediatr Infect Dis J. 2003;22:1021–1022. doi: 10.1097/01.inf.0000095432.69985.f7. [DOI] [PubMed] [Google Scholar]
- 204.Appel M, Pauleto AC, Cunha LA. Osteochondral sequelae of meningococcemia: radiographic aspects. J Pediatr Orthop. 2002;22:511–516. [PubMed] [Google Scholar]
- 205.Sun W, Wang BL, Liu BL. Osteonecrosis in patients after severe acute respiratory syndrome (SARS): possible role of anticardiolipin antibodies. J Clin Rheumatol. 2010;16:61–63. doi: 10.1097/RHU.0b013e3181cf3464. [DOI] [PubMed] [Google Scholar]
- 206.Bradford DS, Szalapski EW, Jr, Sutherland DE. Osteonecrosis in the transplant recipient. Surg Gynecol Obstet. 1984;159:328–334. [PubMed] [Google Scholar]
- 207.Danzig LA, Coutis RD, Resnick D. Avascular necrosis of the femoral head following cardiac transplantation: report of a case. Clin Orthop Relat Res. 1976;117:217–220. [PubMed] [Google Scholar]
- 208.Fink JC, Leisenring WM, Sullivan KM. Avascular necrosis following bone marrow transplantation: a case-control study. Bone. 1998;22:67–71. doi: 10.1016/s8756-3282(97)00219-6. [DOI] [PubMed] [Google Scholar]
- 209.Helenius I, Jalanko H, Remes V. Avascular bone necrosis of the hip joint after solid organ transplantation in childhood: a clinical and MRI analysis. Transplantation. 2006;81:1621–1627. doi: 10.1097/01.tp.0000226062.36325.4b. [DOI] [PubMed] [Google Scholar]
- 210.Lieberman JR, Scaduto AA, Wellmeyer E. Symptomatic osteonecrosis of the hip after orthotopic liver transplantation. J Arthroplasty. 2000;15:767–771. doi: 10.1054/arth.2000.6635. [DOI] [PubMed] [Google Scholar]
- 211.Marston SB, Gillingham K, Bailey RF, Cheng EY. Osteonecrosis of the femoral head after solid organ transplantation: a prospective study. J Bone Joint Surg Am. 2002;84-A:2145–2151. doi: 10.2106/00004623-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 212.Dzik-Jurasz AS, Brooker S, Husband JE, Tait D. What is the prevalence of symptomatic or asymptomatic femoral head osteonecrosis in patients previously treated with chemoradiation? A magnetic resonance study of anal cancer patients. Clin Oncol (R Coll Radiol) 2001;13:130–134. doi: 10.1053/clon.2001.9236. [DOI] [PubMed] [Google Scholar]
- 213.Curtis MA, Tung GA, Dawamneh MF. Radiation osteonecrosis of the clavicle. Acad Radiol. 1996;3:971–974. doi: 10.1016/s1076-6332(96)80314-x. [DOI] [PubMed] [Google Scholar]
- 214.Epstein J, van der Meij E, McKenzie M. Postradiation osteonecrosis of the mandible: a long-term follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:657–662. doi: 10.1016/s1079-2104(97)90314-0. [DOI] [PubMed] [Google Scholar]
- 215.Holler U, Petersein A, Golder W. Radiation-induced osteonecrosis of the pelvic bones vs. bone metastases—a difficult differential diagnosis. Aktuelle Radiol. 1998;8:196–197. [PubMed] [Google Scholar]
- 216.Niewald M, Barbie O, Schnabel K. Risk factors and dose-effect relationship for osteoradionecrosis after hyperfractionated and conventionally fractionated radiotherapy for oral cancer. Br J Radiol. 1996;69:847–851. doi: 10.1259/0007-1285-69-825-847. [DOI] [PubMed] [Google Scholar]
- 217.Stebbings JH. Dose-response analyses of osteonecrosis in New Jersey radium workers point to roles for other alpha emitters. Health Phys. 1998;74:602–607. doi: 10.1097/00004032-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 218.Balzer S, Schneider DT, Bernbeck MB. Avascular osteonecrosis after hyperthermia in children and adolescents with pelvic malignancies: a retrospective analysis of potential risk factors. Int J Hyperthermia. 2006;22:451–461. doi: 10.1080/02656730600893619. [DOI] [PubMed] [Google Scholar]
- 219.Gurkan E, Yildiz I, Ocal F. Avascular necrosis of the femoral head as the first manifestation of acute lymphoblastic leukemia. Leuk Lymphoma. 2006;47:365–367. doi: 10.1080/10428190500282001. [DOI] [PubMed] [Google Scholar]
- 220.Wei SY, Esmail AN, Bunin N, Dormans JP. Avascular necrosis in children with acute lymphoblastic leukemia. J Pediatr Orthop. 2000;20:331–335. [PubMed] [Google Scholar]
- 221.Johnston RJ, Poholek AC, DiToro D. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee YM, Fujikado N, Manaka H. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010;22:805–816. doi: 10.1093/intimm/dxq431. [DOI] [PubMed] [Google Scholar]
- 223.Kogawa M, Findlay DM, Anderson PH. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–4625. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 224.Sunyer T, Lewis J, Collin-Osdoby P, Osdoby P. Estrogen's bone-protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J Clin Invest. 1999;103:1409–1418. doi: 10.1172/JCI4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gaffen SL. Biology of recently discovered cytokines: interleukin-17—a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6:240–247. doi: 10.1186/ar1444. [DOI] [PMC free article] [PubMed] [Google Scholar]


