IMMUNOHISTOCHEMISTRY AS A LABORATORY TEST
Although they do not publicize it, pathologists have long recognized their fallibility.1 As a result, more objective means of validating morphologic judgments have been sought. Stains using histochemical methods are of value in accentuating morphologic features but do not provide objective evidence of the lineage or biologic potential of a cell. The objective of immunohistochemistry is to use antibodies to identify antigens, increasing the specificity of the stain for the tissue with which it reacts. In doing so, immunohistology has transformed surgical pathology from a highly subjective discipline into a much more objective science, while still taking advantage of the light microscope and standard morphologic practices.
Immunohistochemistry, as the name implies, is the combination of histology and immunology. The resulting technique is a powerful tool that not only enables pathologists to detect whether particular antigens are present within a given cell but also allows the identification of the microanatomic (cellular) location of the antigen. These abilities permit the lineage of cell populations to be identified, an important consideration when confronted with a poorly differentiated neoplasm of undetermined origin. The technique is also useful in defining distinct populations of cells within the same lineage and defining functional differences. In addition, this technique preserves the histologic architecture and enables the pathologist to confirm that the positive cells are the cells in question. This confirmation is not possible with molecular methods, such as reverse transcriptase polymerase chain reaction or standard flow cytometry methods.
Immunohistochemistry is used by a variety of disciplines to study a wide range of questions. This chapter discusses the application of this technology in surgical pathology, in which immunohistochemistry has had a profound and fundamental impact on the practice of pathology.1., 2.
Technical Considerations
Immunohistochemistry has the potential to transform surgical pathology from a subjective art to an objective science, based on the recognition of cells by microscopic methods. Although this potential has resulted in its almost universal use, immunohistochemistry has not produced uniformly high standards of practice.3., 4. Therefore, certain technical considerations must be borne in mind to ensure the accuracy of results. It is self-evident that the quality of an immunohistochemical stain depends on the integrity of an antibody-antigen interaction and on the extent to which the relevant antigen has been preserved during tissue fixation and processing.1 There is a high degree of variability in the way tissues are initially prepared. These variations include differences in the fixative used; the amount, age, and pH of the fixative; how long the tissue sat unfixed before being placed in fixative; the thickness of the tissue when first placed in fixative; and the time the tissue is left in fixative. All these variables, which may not alter the results of routine hematoxylin-eosin (H&E) staining to a significant degree, can lead to widely discrepant results when it comes to immunohistochemistry. These variables cannot always be predicted or remedied, but they can be mitigated to a great extent by the advent of successful and relatively simple antigen retrieval methods (discussed later).5., 6.
In addition to the variables in tissue fixation, as in all other laboratory tests, the reagents and techniques used must be optimized and thoroughly validated to ensure consistent, reliable, and clinically meaningful results. When developing an immunohistochemical protocol, it is important for each laboratory performing a test to validate every reagent used. This validation includes a determination of the specificity and the optimal working dilution of each primary antibody, secondary antibody, linking antibody, labeling reagent, and substrate. Repeat validation is required for each new lot of reagents because of variations in origin, composition, concentration, and specificity that can occur among different lots even when supplied by the same company.1 Also, reputable manufacturers should be used. The higher standards and more rigorous quality control of products from the better manufacturers have been counterbalanced by the concurrent proliferation of smaller manufacturers that are able to produce or otherwise acquire and market large numbers of different antibodies through monoclonal antibody technology, molecular engineering, and the like. In addition to the need for high-quality antibodies and reagents, proper incubation times and ideal temperatures for each antibody must be determined. The optimal buffering agent must be determined, as well as the need for any predigestion techniques or antigen enhancement procedures.
Premanufactured Kits
Premanufactured, all-inclusive kits have been marketed in an attempt to simplify the performance of immunohistochemistry; for example, these kits obviate the need for each individual clinical laboratory to validate each reagent because preoptimized working dilutions and recommended working protocols are provided. Nevertheless, there are some pitfalls that should be kept in mind when working with premanufactured kits. The protocols and reagents have been formulated to work on the prototype tissue used by the manufacturer and may not be as effective on the actual tissues tested because of laboratories’ different fixation and processing protocols, all of which may have adverse effects on the results. Adjustments in the recommended protocol are necessary to optimize the kits in each individual laboratory, which effectively means that the kits must be customized, and any changes made in the manufacturer's protocol require that the entire staining procedure be revalidated by the performing laboratory.1 This procedure may be complicated by the fact that many of the working dilutions of the reagents supplied are already at the critical level of sensitivity.
Automated Staining
Another method that can potentially enhance consistency and reproducibility is automation. A variety of automated immunostaining systems are now commercially available. The theoretical advantages of these systems over manual staining techniques include improved reproducibility, facilitation of interlaboratory comparisons, reduced reagent expenses, and increased technician productivity. Automation does not mitigate the need to thoroughly validate each step of the staining procedure or the need to evaluate every reagent used to ensure high-quality, consistent results. The same quality control issues that apply to manual staining apply to automated systems. As with manual staining, it is important that a complete reevaluation be performed if there is any departure from the validated protocol.1 Automation does not guarantee an optimal result. Finally, automation cannot replace the pathologist, who must choose the appropriate antibodies and then interpret the final result.
Automated Image Analysis
There is a growing need to be able to quantify immunohistochemical staining results, which is probably best accomplished by automated image analysis. Current systems, including the Automated Cellular Imaging System II (ACIS II Clarient, Aliso Viejo, Calif), have the ability to assess marker positivity in terms of both percentage positive and intensity of staining. The ability to quantify markers more precisely is especially important in the identification of targets of therapy. One example of this is Her-2/neu. When Her-2/neu protein expression by immunohistochemistry was compared with gene amplification by fluorescence in situ hybridization using routine manual methods and the assistance of a digital microscope, both accuracy and reliability were improved when the digital microscope was used.7., 7a. Precise quantification of hormone receptors may also be important because there is evidence that in patients with high levels of hormone receptors, the addition of cytotoxic chemotherapy has a deleterious effect on outcome.8., 9. Another exciting development in the field of automated cellular imaging is spectral imaging. This technology allows multiple markers to be assessed on the same slide—even on a single cell. Computer software can isolate a single chromogen from other chromogens present based on its emission spectrum. Automated cellular imaging provides greater objectivity and reproducibility and thus minimizes interobserver discrepancies.
Positive and Negative Controls
Immunohistochemical tests performed and interpreted in the absence of the appropriate controls are valueless and even dangerous. Minimal controls should include a tissue known to express the particular antigen of interest, processed in a manner analogous to that of the unknown tissue (the positive control), and a second section of the test specimen in which the primary antibody is replaced either by diluent or, better, by an irrelevant antibody of the same isotype, from the same species, and at the same concentration (the negative control). In the positive control, only cells expected to express the antigen should show positivity; all other cells and structural elements should be negative. In the negative control, there should be no specific staining. The “sausage” technique, in which samples of multiple tissues are gathered into a single tissue block, is a useful control method.10
Controls are performed for a variety of purposes; in addition to indicating whether a reaction occurred (or not), they are essential for judging the nature of the reaction. A vast array of immunohistochemical tests are judged not by a positive or negative result but by the intensity and localization of the result (a good example is Her-2/neu and hormone receptor analysis in breast cancer). Immunohistochemistry results should never be interpreted in the absence of the known positive results because the assessment of quality and quantity of the reaction is essential.
Results and Reporting
Interpretation of the results of immunohistochemical stains is the province of the surgical pathologist and is best accomplished by pathologists who have the appropriate level of experience not only in the morphologic aspects of diagnosis but also with regard to immunohistochemical findings. As in any other area of pathology, experience matters: A pathologist with little experience with immunohistochemistry, who runs a few different tests each week or month, will obtain very different results from a pathologist who performs and interprets immunohistochemical tests on a daily basis. As the impact of immunohistochemistry on surgical pathology increases, these differences will become more profound.
As described previously, many factors influence the results. All these factors must be considered by the pathologist in interpreting the findings. Negative, weak, or uninterpretable results should lead to a repetition of the test after the use of antigen retrieval.11., 12. One measure of antigen preservation is to test for expression of the intermediate filament vimentin, a fixation-sensitive protein that is typically expressed by vascular or connective components; this technique often serves as an internal indicator of the conservation or loss of antigenicity.11 Test results may also be affected by technical artifacts and by the nature of the tissue under study. For instance, if tumor cells are crushed, false-positive or nonspecific staining may be encountered. Nonviable areas of tissue from a necrotic tumor may also be a source of false-positive results, attributable in part to leakage of serum proteins (e.g., immunoglobulins). The subcellular distribution of immunoreactivity is critical to the interpretation of immunohistochemical results. For example, Her-2/neu shows membranous staining, whereas antibodies to estrogen and progesterone receptors produce nuclear staining. When unexpected staining patterns are observed with an antibody, the results should be discounted.1 To interpret the results effectively, the pathologist must have extensive knowledge of the staining patterns of the primary antibodies under consideration, including a detailed knowledge of tissue specificity and subcellular localization of the antigen, and an awareness of technical variables. Each laboratory performing immunohistochemical staining should have established written criteria for determining and reporting positive and negative findings for each immunohistochemical stain, with particular reference to stains that are expected to produce cell surface membrane, cytoplasmic nuclear, or extracellular staining. Although it seems obvious, it often is overlooked that staining should be recorded as positive only if it occurs in the expected cellular or tissue location.
Validation and Proficiency Testing
The Food and Drug Administration's increased attention to the reagents used in immunohistochemistry has undoubtedly contributed to an improvement in their quality.13 It is highly recommended that all laboratories performing diagnostic immunohistochemistry participate in the College of American Pathologists’ certification program, which includes a checklist of the essential elements required for a successful immunostaining program.7a With regard to staff qualifications, the National Society of Histotechnologists has focused its efforts on continuing education and certification programs for technologists performing immunohistochemical staining.
Federal law requires a high degree of testing and validation. In the United States, laboratories performing immunohistochemistry are required under the Clinical Laboratory Improvement Amendments of 1988 to validate the performance of their test reagents for accuracy, specificity, sensitivity, and precision.14 First, the testing procedure is optimized (as described earlier), and performance expectations are established. During the validation process of each analyte, multiple slides with known pathology (generally 20 representative cases) are evaluated with the optimized procedure to assess the accuracy of diagnostic staining, sensitivity of signal, and reproducibility. Validations that meet specifications must be signed by qualified individuals, and the documents are maintained in the laboratory. Quality control and proficiency testing must be performed to monitor performance.
Limitations
Although immunohistochemistry is an extremely valuable technique in experienced hands, its limitations must be recognized for it to be used to its maximum potential.
Experience
Although immunohistochemistry is more objective than routine morphologic examination, the experience of the pathologist assessing the slides is critical. A firm understanding of the principles of immunohistochemical staining is necessary because the reporting pathologist must be equipped to deal with the unexpected and conflicting results that inevitably occur. To evaluate the immunohistochemical slides properly, the pathologist must have a firm understanding of the limitations of antibodies in terms of their technical aspects as well as their inherent specificity, sensitivity, and expected subcellular location.
Availability of Antibodies
The advent and refinement of the hybridoma technique for the production of monoclonal antibodies have produced a large number of available antibodies. Often a newly developed antibody is hailed as exquisitely specific. In time, however, most are found to be considerably less specific than initially hoped, generally because the antigen the antibody detects has a wider distribution than expected. This fact does not negate the usefulness of the antibody in question, but it may mean that panels of antibodies must be used in conjunction with standard morphologic features and clinical history.
Loss of Antigenicity in Stored Cut Paraffin Sections
Many studies have shown that a loss of antigenicity can occur on cut paraffin sections that have been stored for varying lengths of time.15., 16., 17. Among the antibodies studied, those most adversely affected by storage include p53,15., 17. MIB1,16., 17. factor VIII–related antigen,15 estrogen receptor,15 bcl-2,15 p27kip1,16 CD-44s,16 and androgen receptor.16 In many cases, the use of carefully selected and tested antigen retrieval techniques can compensate for this loss.17
Antigen Retrieval
Formalin is the most widely used fixative in surgical pathology. Cross-linking of proteins is the essential feature of formalin fixation. This cross-linking interferes with the antigen's ability to react with the primary antibody. In 1991 the antigen retrieval technique was developed.12., 18. This technique is a heat-induced modification of the protein conformation that allows the antigen to be accessible again for chemical reactions, in this case, antibody binding. Hydrolysis of cross-linking resulting from formalin fixation probably plays a major role in this modification process.19., 20., 21., 22. The application of antigen retrieval to sections derived from formalin-fixed, paraffin-embedded blocks produces consistent results of acceptable quality,6 although a few antigens remain undetectable even after antigen retrieval has been performed. Antigen retrieval methods have revolutionized immunohistochemistry and have become a standard part of diagnostic immunohistochemistry in surgical pathology. These methods result in higher sensitivity and more consistent antibody reactivity. Antigen retrieval technology has led to a proliferation of protocols that may produce different results in different laboratories. The successful application of these methods allows the detection of some antigens that were previously undetectable in paraffin sections, rendering much of the early literature (prior to 1993) obsolete. This fact continues to escape the notice of some practicing pathologists, leading to errors of interpretation (Fig. 5-1 ).
Figure 5–1 ▪.

Section of lymphoid tissue stained with antibody against lambda light chain without antigen retrieval, showing no positive cells (A), and with antigen retrieval, showing scattered positive cells (B).
It should be recognized that two major factors influence the effectiveness of antigen retrieval: the conditions under which heating takes place, and the pH value of the buffer solution used during the heating process.5., 11., 23. The most critical factor is the combination of the temperature and the duration of heating, which have a reverse correlation. Based on these two factors (heating conditions and buffer pH), a test battery approach has been developed to establish optimal antigen retrieval protocols for immunostaining on archival paraffin-embedded tissue sections.1., 6., 23. A typical test battery consists of nine serial sections of a specimen known to express the antigen under study. The sections are evaluated with buffer at three different pH values (e.g., pH 1 to 2, 7 to 8, and 10 to 11) and three heating conditions (e.g., 90°C, 100°C, and 120°C) for various lengths of time (or some other comparable heating versus time schedule). The best result is selected as the optimal retrieval condition for that antigen. In the event that a satisfactory result is not obtained, other variations may be explored, including different buffer solutions and more or less vigorous heating methods.19., 23. Protocols for antigen retrieval differ in their effectiveness for retrieving certain antigens, and a single universally effective retrieval method does not exist. Many laboratories use more than one method for different antibody and antigen combinations. Overall, citrate buffer at pH 6.0 has the broadest applicability for the widest range of antigens, although several studies have demonstrated that the use of higher pH retrieval solutions yields satisfactory results.21., 24. Retrieval solutions with lower pH values, TRIS (tromethamine) buffer at pH 8.0, and EDTA (ethylenediaminetetraacetic acid)-NaOH solution (pH 8.0), are effective in certain special situations.25., 26., 27. The selection of heating method (water bath, steamer, microwave, pressure cooker, or autoclave) is influenced by custom and availability.
CURRENT APPLICATIONS OF IMMUNOHISTOCHEMISTRY
Diagnostic Tool for Tumors of Unknown Origin
Immunohistochemistry has become an integral and essential part of surgical pathology. It is applied to define tumor origin, establish prognosis, and determine treatment response. In this textbook, the role of immunohistochemistry in defining the origin, prognosis, and treatment response of tumors is discussed in the chapters devoted to the specific organ systems; therefore, a full discussion is not provided here. Because the evaluation of tumors of unknown origin does not fall under any particular organ system, it is discussed in this chapter.
Tumors are classified most often by their tissue of origin (e.g., breast, colon, prostate) or histogenetically (e.g., tissue of epithelial, mesenchymal, or neural origin). A tumor cannot be staged accurately, and proper therapy cannot be administered, without such classification. Although accepted and fairly reproducible criteria exist for the morphologic diagnosis of most tumors, there is inherent subjectivity in any morphologic evaluation. It is well recognized that morphologic features often overlap among different entities and that one disease can present with myriad histologic pictures. Most tumors can be classified correctly by routine histologic techniques when the clinical situation is clear (e.g., a breast mass); however, an important subset of tumors defies morphologic interpretation. The magnitude of this problem is substantial. The diagnosis of “metastatic cancer of unknown primary site” is the eighth most common cancer diagnosis and may represent up to 15% of cancers at large hospitals.28 Much more common is the diagnosis of “tumor of uncertain origin.” This occurs when (1) the tumor is first identified in a metastatic site, and the primary site is not apparent; (2) the tumor is so poorly differentiated that no specific morphologic features can be identified; (3) the morphologic appearance of the tumor is compatible with more than one distinct tissue (e.g., epithelial versus lymphoid origin); and (4) the histogenesis of a tumor is clear (e.g., adenocarcinoma), but the primary site is in question. This distinction has important consequences to the patient.
Test Selection
Immunohistochemical tests should be performed with a defined objective in mind. The results of a single immunohistochemical procedure can be misleading not only because of variables in the staining procedure but also because of unanticipated patterns of reactivity of certain antibodies.1 Although myriad antibodies are available, the choice in a particular case should be judicious and designed to address the diagnostic possibilities. The use of too few antibodies rarely provides sufficient information to support a specific diagnosis and can produce misleading information. Antibodies should be selected on the basis of their ability to affirm or exclude considerations in the differential diagnosis. This so-called problem-oriented approach is based on the selection of appropriate panels of antibodies. When selecting antibodies, factors that should be considered include the clinical history, morphologic features of the tumor, and results of other tests that may have been performed, including serologic and radiographic tests. Pathologists can find guidance in the literature and in a few specialized textbooks that address the use of immunohistochemistry,2., 29. but this is a rapidly evolving field. The limited antibody panels of a few years ago are inadequate to deal with tumors of unknown or uncertain primary sites today. With this in mind, the panels presented here must be considered elementary guides.
Panel Approach: Basic Principles
When evaluating tumors of uncertain origin by immunohistochemistry, certain basic guidelines of interpretation must be kept in mind. A positive staining reaction is generally more helpful than a negative one because a lack of immunoreactivity may represent a technical problem with the tissue or the way it was fixed, as discussed earlier. The more poorly differentiated a tumor is, the less likely it is to express tissue differentiation antigens. There is often staining variation within a tumor; by extension, variations in staining patterns may be seen between the primary tumor and the metastatic focus. Most important, the final diagnosis should never depend on immunohistochemistry alone; it must be made using all the clinical, serologic, radiographic, morphologic, and epidemiologic data available. Other techniques, such as the assessment of specific DNA alterations, are becoming increasingly important adjuncts to the pathologic evaluation. Although immunohistochemical evaluation is essential, it is only one of many tools that must be used in the evaluation of pathologic processes.
The application of a primary panel of antibodies to characterize tumor histogenesis (epithelial, mesenchymal, neural, or hematopoietic) is often the first step. When this panel has been established, additional antibodies can be used to identify the tumor type more specifically. Included in the first tier would be antibodies against pan-keratin, vimentin, S-100 protein, neuron-specific enolase (NSE) and CD45 (common leukocyte antigen) to differentiate epithelial, mesenchymal, melanomatous, neural, and lymphoid malignancies (Table 5-1 ; Fig. 5-2 ).
TABLE 5–1.
Screening Immunophenotypes of Undifferentiated Neoplasms
| AE1/AE3 | Vimentin | CD45 | S-100 | Tumor Type |
|---|---|---|---|---|
| + | –/+ | – | R | Carcinoma |
| R | + | – | –/+ | Sarcoma |
| – | – | + | – | Lymphoma |
| – | + | – | + | Melanoma |
+, always positive; –, negative; –/+, mostly negative; R, rare positive cells.
Figure 5–2 ▪.
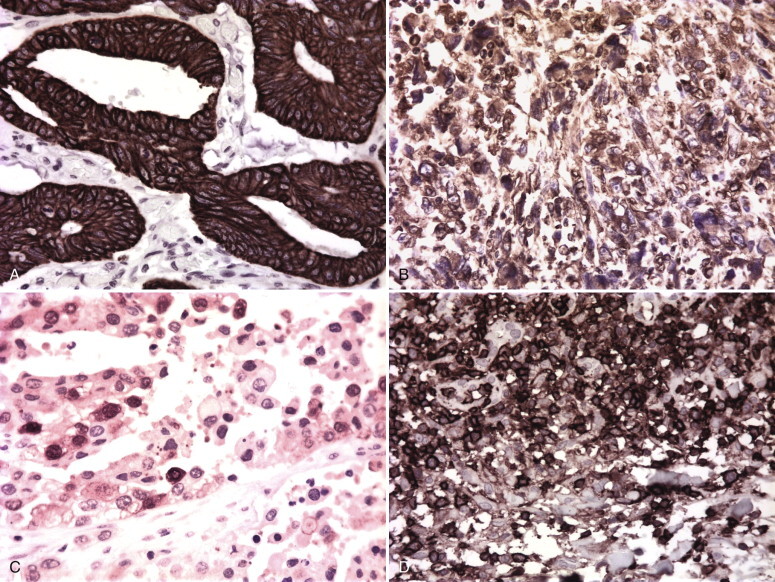
A, Colon carcinoma showing keratin positivity, which is typically seen in carcinomas. B, Malignant fibrous histiocytoma showing vimentin positivity, which is typically seen in sarcomas. Note the fine reticular pattern of the intermediate filaments in the giant cells. C, S-100–positive melanoma. Note the nuclear reactivity, characteristic of melanoma. D, CD45 (common leukocyte antigen)–positive lymphoma.
Intermediate Filaments
The expression of intermediate filament proteins, which function as the supporting cytoskeleton in normal and neoplastic cells, is extremely useful in the initial assessment of tumors of unknown primary origin.1 There are five major classes of intermediate filaments, based on protein composition and cellular distribution: cytokeratin, vimentin, desmin, neurofilament, and glial fibrillary acidic protein (GFAP).30 Most neoplasms show the predominant expression of one or more of these intermediate filaments. Carcinomas usually express cytokeratin; sarcomas, melanomas, and lymphomas are generally vimentin positive; myogenic tumors are characteristically positive for desmin or muscle actins and vimentin; and glial tumors are predominantly positive for GFAP.1 Some tumors characteristically coexpress more than one intermediate filament (e.g., renal and thyroid carcinomas contain keratin and often vimentin), whereas others show aberrant or no intermediate filament expression. Immunohistochemical markers for intermediate filaments on tumors of uncertain origin are one of the most useful and productive ways to begin classifying the lesion. Anaplastic neoplasms can be characterized as keratin positive (carcinomas, mesotheliomas), vimentin positive (sarcomas, lymphomas, melanomas), or neurofilament and GFAP positive (neuroendocrine, neural, and astrocytic tumors).1
Keratin-Positive Tumors
Cytokeratins are present in almost all epithelial cells and are highly sensitive markers for carcinomas. In a generic sense, malignant cells expressing keratin positivity indicate an epithelial origin. Antibodies against keratin are also extremely useful as markers for occult metastases (micrometastases) in the peripheral blood, bone marrow, and lymph nodes (discussed later).
There are more than 20 different subtypes of cytokeratin found in human epithelial cells. These subtypes are distinguishable by their molecular weight and isoelectric pH.31 Monoclonal antibodies specific for many of these subtypes have been developed. Carcinomas of different types tend to express characteristic keratin profiles.32 There is a general correlation between the complexity of the epithelium from which the tumor is derived and the complexity of the keratin subunits expressed. Low-molecular-weight or nonsquamous keratins appear early in development and predominate in tumors derived from simple, nonstratified epithelium (e.g., ductal carcinoma of the breast, gastrointestinal adenocarcinoma). High-molecular-weight or squamous keratins appear in more complex stratified epithelium and predominate in tumors derived from stratified epithelium (e.g., squamous cell carcinoma). Some tumors, such as those derived from pseudostratified columnar epithelium, contain a mixture of high- and low-molecular-weight keratins, with a predominance of the latter. In some instances, especially in extremely poorly differentiated tumors, as few as 5% of tumor cells may express keratin reactivity.1
When a tumor of uncertain primary site has been defined as epithelial by either immunohistochemistry or morphology, it is important to attempt to define its specific origin. This presents a problem when, for example, a patient with a prior history of breast carcinoma presents with a lung mass that, on biopsy, is adenocarcinoma. Determining whether the lung mass represents a primary pulmonary tumor or metastasis from the breast has enormous consequences in terms of patient outcome and choice of specific treatment. Although the immunohistochemical evaluation of primary epithelial tumors is problematic, advances have been made. Monoclonal antibodies against keratin subtypes may help determine the origin of certain poorly differentiated neoplasms. Hepatocellular carcinoma (positive for AE3 and CAM 5.2 but negative for AE1) can be distinguished from bile duct carcinoma and adenocarcinoma metastatic to the liver (positive for AE1).33 In particular, the differential expression of cytokeratins 7 and 20 (CK7, CK20) is extremely useful in the characterization of epithelial neoplasms (Fig. 5-3 ).32., 34., 35., 36. These patterns are not absolute, but they can be useful guides in establishing origin.
Figure 5–3 ▪.
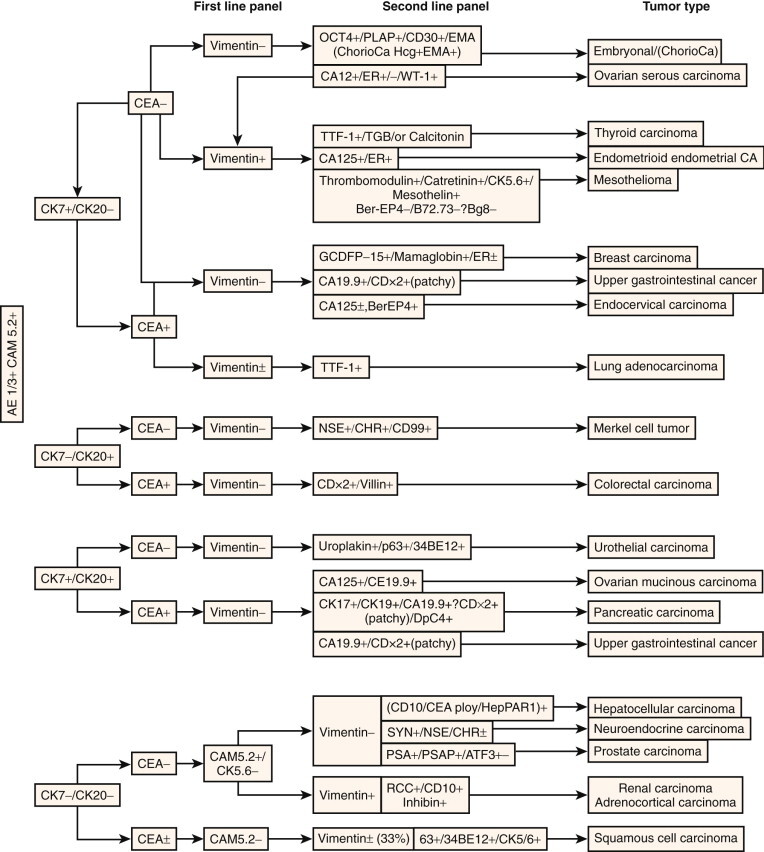
Algorithm for carcinoma of unknown primary site.
Cytokeratin 5/6 (CK5/6) has received considerable attention recently. CK5/6 has been found to be positive in the majority of squamous cell carcinomas, basal cell carcinomas, thymomas, salivary gland tumors, and biphasic malignant mesotheliomas and in a subset of endometrial adenocarcinomas, transitional cell carcinomas, and pancreatic adenocarcinomas.37 CK5/6 is rarely positive in adenocarcinoma of the lung and has therefore been used to distinguish malignant mesothelioma from pulmonary adenocarcinoma.37., 38. In addition, p63 is frequently seen in squamous cell carcinoma and transitional cell carcinoma, whereas mesothelioma is uniformly negative for p63. Therefore, positive immunostaining for both p63 and CK5/6 is highly predictive of a primary tumor of squamous epithelial origin.39., 40. Because p63 is also known to immunoreact on the basal cell nuclei in benign prostate glands, this marker can be used to distinguish prostate cancer from benign mimics.41
There is an increasing array of tissue-specific markers, such as prostate-specific antigen (PSA) and thyroglobulin, as well as tissue-associated markers, such as GCDFP-15 and mammaglobin for breast epithelium,42., 43. OC-125 for ovary,44 uroplakins for urothelium,45., 46. and synaptophysin for neuroendocrine lesions (Fig. 5-4 ).47 Table 5-2 summarizes many of these tissue-associated antibodies. It is important to keep in mind patterns of antigenic coexpression, which can lead to erroneous assessments. Also, as mentioned earlier, the more poorly differentiated the neoplasm, the less likely it is to express tissue-specific or -associated antigens. Nevertheless, the specific origin of an epithelial neoplasm of uncertain primary site can be elucidated in an increasing proportion of cases through careful evaluation of morphology, clinical data, and antigen expression.
Figure 5-4 ▪.

A, Carcinoma of the colon showing CEA positivity. B, Ovarian carcinoma showing OC-125 positivity.
TABLE 5–2.
Antibodies Useful in Determining the Origin of Undifferentiated Tumors and Tumors of Uncertain Primary Site
| Panel | Antibodies |
|---|---|
| Undifferentiated tumors |
|
| Carcinoma panel |
|
| Sarcoma panel |
|
| Breast panel |
|
| Prognosis (breast carcinoma) |
|
| Lung panel |
|
| Prostate panel |
|
| Gastrointestinal panel |
|
| Kidney/bladder panel |
|
| Ovary panel |
|
| Liver panel |
|
| Mesothelioma panel |
|
| Melanoma panel |
|
| Central nervous system/neural panel |
|
| Neuroendocrine panel |
|
| Pituitary hormone panel |
|
| Pancreatic panel |
|
| Urothelial panel |
|
CEA, carcinoembryonic antigen; CLA, common leukocyte antigen; COTA, colonic ovarian tumor antigen; HMW, high molecular weight; LMW, low molecular weight; NSE, neuron-specific enolase; PNET, primitive neuroectodermal tumor.
Keratin-Negative Tumors
LYMPHOMAS
Lymphomas often present morphologically as undifferentiated malignant neoplasms. A large cell lymphoma may be difficult to differentiate from carcinoma or melanoma. Anaplastic large cell lymphomas occasionally react with antibodies against keratin or epithelial membrane antigen. Similarly, small cell lymphomas often resemble other tumors, such as small cell undifferentiated carcinoma. Immunohistochemistry can be invaluable in classifying a tumor as lymphoid in origin. Immunohistochemistry is also used widely to aid in the subclassification of non-Hodgkin's lymphoma (discussed in detail in Chapter 41). Another important application of immunohistochemistry is the phenotyping of a lesion to determine the immunoglobulin light-chain expression. One can distinguish between malignant and benign lymphoid proliferation by demonstration of light-chain (or heavy-chain) restriction.
CD45 is an excellent screening marker to determine whether a tumor is lymphoid in origin. Staining is characteristically membranous. Some neoplasms of lymphoid origin may not express CD45, and rare nonlymphoid neoplasms may show cytoplasmic staining for CD45. More specific lymphoid markers such as CD3, which marks T cells, and CD20, which marks B cells, may further delineate a lesion. Immunohistochemistry can also be used in cases of Hodgkin's disease, in which markers useful in identifying Reed-Sternberg cells include CD15, CD30, and BLA-36.48., 49. Figure 5-5 illustrates an algorithmic approach to the immunohistochemical diagnosis of malignant lymphomas.
Figure 5-5 ▪.

Diagnostic algorithm for hematolymphoid neoplasms.
MELANOMAS
Melanomas are typically (but not always) negative for cytokeratin50 and positive for vimentin. S-100 protein is a sensitive marker for melanoma and occurs, in almost all cases, in a nuclear pattern (see Fig. 5-2C). S-100 is not specific for melanoma, however, and is seen in a variety of lesions, including Langerhans histiocytes, many sarcomas, and certain carcinomas.51., 52. Positive immunoreactivity for HMB-45, Melan A, or tyrosinase, which are much more specific markers for melanoma and melanocytes, can confirm the diagnosis.53 This topic is discussed in detail in Chapter 49.
SARCOMAS AND SOFT TISSUE TUMORS
A diagnosis of sarcoma is worth considering when a spindle cell neoplasm expresses vimentin but not keratin, CD45, or HMB-45. Immunohistochemical analysis can delineate a specific type of sarcoma such as myogenic and fibrohistiocytic tumors, tumors with neural differentiation, and vascular sarcomas. The differentiation of soft tissue tumors by immunohistochemistry is discussed in Chapter 46. The major subtypes are described briefly here.
Myogenic sarcomas react with antibodies to muscle-specific actin and desmin. Antibodies against myoD-1 smooth muscle actin show preferential reactivity with leiomyosarcomas.54 Alveolar soft part sarcomas typically coexpress vimentin and desmin.55 Tumors derived from skeletal muscle often contain myoglobin; positive immunoreactivity for muscle-specific actin, desmin, and myoglobin indicates that the tumor in question may be a rhabdomyosarcoma.
Fibrohistiocytic tumors, including malignant fibrous histiocytoma, are the most common form of soft tissue sarcoma in adults. Malignant fibrous histiocytoma reacts with vimentin and lacks significant reactivity for keratin. These lesions may also coexpress α1-antitrypsin, α1-antichymotrypsin, HAM-56, and CD68. α1-Antichymotrypsin is not specific for malignant fibrous histiocytoma and can be seen in other sarcomas, some carcinomas, and melanomas.56., 57., 58.
Neurogenic tumors, including malignant peripheral nerve sheath tumors and schwannomas, are positive for antibody against S-100 protein, myelin basic protein, and Leu-7.59., 60. Although most benign neurogenic tumors (schwannomas and neurofibromas) contain S-100, less than half of malignant peripheral nerve sheath tumors contain detectable S-100 protein.61
Normal and neoplastic vessels can be identified with endothelial markers such as factor VIII—related antigen and Ulex europaeus lectin. Antibodies to factor VIII–related antigen react only with endothelial cells and megakaryocytes and are more specific than Ulex europaeus. Other markers of endothelial cells include CD34 and CD31; these are more sensitive but less specific than factor VIII.
Other tumors to be considered in the differential diagnosis of anaplastic spindle cell tumors include liposarcomas, chondrosarcomas, osteogenic sarcomas, fibrosarcomas, and synovial sarcomas. Although certain variants of liposarcoma may be diagnosed by histologic criteria alone, the diagnosis of pleomorphic liposarcoma is aided by immunohistochemistry. Most liposarcomas react with vimentin and S-100 but are nonreactive for HMB-45, in contrast to most melanomas. The same pattern is seen for chondrosarcomas.62 Osteosarcomas react with vimentin. Antibodies to the so-called osteonectin or osteosarcoma antigens also may assist in the diagnosis.62 Fibrosarcomas are rare neoplasms that express only vimentin. Figure 5-6 provides the immunostaining patterns characteristic of anaplastic spindle cell tumors.
Figure 5-6 ▪.

Diagnostic algorithm for spindle cell tumors.
Cytogenetic profiling of sarcomas of unknown origin is becoming common. Tumors with known abnormalities include endometrial sarcoma, myeloid sarcoma, synovial sarcoma, Ewing's sarcoma, and visceral clear cell sarcoma, among others.63., 64., 65., 66., 67., 68., 69., 70. This topic is discussed in more detail in Chapter 46.
NEURAL AND NEUROENDOCRINE TUMORS
Neural and neuroendocrine tumors may be classified as neural tumors, neuroepithelial tumors, or neural neoplasms of mesenchymal origin. These three categories are based on the different predominant intermediate filaments found in the cytoplasm of these lesions. Neural tumors usually express neurofilament, NSE, chromogranin, and synaptophysin.71., 72., 73. Examples include neuroblastomas, paragangliomas, and pheochromocytomas. Neuroepithelial tumors coexpress keratin and neuroendocrine markers. These tumors include carcinoids, Merkel cell carcinomas, and small cell carcinomas. Neural neoplasms of mesenchymal origin, which consist of primitive neuroectodermal tumors, Ewing's sarcomas, and medulloblastomas, are positive for vimentin and may express NSE and Leu-7.74 A good marker for small cell tumors such as Ewing's sarcoma, primitive neuroectodermal tumor, and peripheral neuroepithelial tumor is O13, which identifies the CD99 (p30/32, mic2, HBA71) antigen.75
NSE, although a sensitive marker for neuroendocrine tumors, lacks specificity and can be seen in a wide variety of tumor types. Chromogranin and synaptophysin are more specific than NSE but lack sensitivity. Chromogranin tends to be positive in better-differentiated neuroendocrine tumors but is less often positive in the more poorly differentiated tumors, such as small cell carcinoma.72., 76., 77., 78. Table 5-3 provides immunostaining patterns of endocrine tumors, and Figure 5-7 illustrates an algorithm used to distinguish small round cell tumors.
TABLE 5–3.
Dominant Immunophenotypes of Endocrine Neoplasms
| CAM 5.2 | CEA | Chromogranin | Serotonin | Synaptophysin | TTF-1 | Vimentin | Additional Markers | Tumor Type |
|---|---|---|---|---|---|---|---|---|
| + | – | +/– | –/+ | + | – | – | Calcitonin –/+; Somatostatin –/+ Gastrin –/+ |
Carcinoid |
| + | –/+ | –/+ | – | + | –/+ | –/+ | Neuroendocrine | |
| + | – | –/+ | – | + | – | – | Insulin + | Insulinoma |
| + | – | + | – | + | – | – | Glucagon + | Glucagonoma |
| + | – | –/+ | – | + | – | – | Somatostatin + | Somatostatinoma |
| + | – | + | – | + | – | – | Gastrin + | Gastrinoma |
| + | + | + | – | + | + | + | Calcitonin + | Medullary, thyroid |
| + | – | + | – | + | – | – | PTH + | Parathyroid |
| + | – | +/– | – | + | – | – | CK20 + | Merkel cell |
| –/+ | – | +/– | – | + | – | + | S-100 +/– | Paraganglioma, pheochromocytoma |
| –/+ | – | – | – | + | – | + | Melan A +; inhibin + | Adrenal cortical |
CEA, carcinoembryonic antigen; PTH, parathyroid hormone.
Figure 5-7 ▪.

Diagnostic algorithm for small round cell tumors.
GFAP-POSITIVE TUMORS
GFAP is expressed by glial cells and is seen in astrocytomas, ependymomas, medulloblastomas, some oligodendrogliomas, and choroid plexus tumors.79., 80. It has also been reported in a few extracerebral tumors, including pleomorphic adenomas of the salivary gland,81 neurofibromas, and schwannomas.82 Intracerebral tumors in which GFAP is not expected to be positive include meningiomas, lymphomas, and metastatic carcinomas. GFAP is discussed in more detail in Chapter 51.
Molecular and Genetic Markers of Tumor Origin
Cancer results from defects in gene structure, expression, or both. Alterations in chromosome and DNA structure—including cytogenetic changes, point mutations, deletions, amplifications, translocations, and DNA methylation—are being identified at an increasing rate. Characterization of these defects is becoming an important component of tumor evaluation, particularly in terms of prognosis and response to treatment. In addition, genetic defects highly characteristic of specific tumor types are being identified. The identification of DNA alterations is also becoming an increasingly important component in the evaluation of tumors of uncertain primary site. Molecular and genetic evaluation is particularly useful in the evaluation of hematopoietic and soft tissue tumors.83., 84. Recently, a highly sophisticated evaluation of hundreds of genes using so-called microarray chip technology was used to identify gene expression differences in several tumor types84., 85., 86. and will no doubt become an important component of tumor evaluation in the future.
TRANSCRIPTION FACTORS
Transcription factors are proteins involved in the regulation of gene expression that bind to the promoter elements upstream of genes and either facilitate or inhibit transcription. They may be tissue specific, or they may be present in more than one tissue type. Even the so-called tissue-specific transcription factors are, however, usually not restricted to a single tumor type. Examples include thyroid transcription factor-1 (TTF-1), which is found in the thyroid and lung, and the pituitary transcription factor Pit-1, which is found in the placenta as well as the pituitary gland. Nevertheless, they can be useful in determining the primary site of tumors of unknown origin (see Table 5-2).
TTF-1
TTF-1 belongs to a family of homeodomain transcription factors and plays a role in regulating genes expressed within the thyroid, lung, and diencephalons. TTF-1 is considered a reliable marker for distinguishing primary tumors of the lung, including adenocarcinoma (75%), non–small cell carcinoma (63%), neuroendocrine and small cell carcinoma (>90%), and squamous cell carcinoma (10%),87., 88., 89., 90. and thyroid from other tumor types. However, primary adenocarcinoma of the colon is positive in some cases.36., 91., 92. The pattern of reactivity is nuclear. Hepatocytes and hepatocellular carcinoma reportedly show cytoplasmic positivity.93
CDX-2
CDX-2 is a homeobox gene that encodes a transcription factor involved in the development of intestinal epithelium. It is expressed in normal colonic epithelium and in most colorectal adenocarcinomas and is a useful marker to identify colorectal metastases.94., 95., 96. CDX-2 is also useful in extramammary Paget's disease; the endodermal subtype is positive for CDX-2, whereas the cutaneous subtype is negative.97
ATF3
Activating transcription factor 3 (ATF3) is a member of the basic leucine zipper/cyclic adenosine monophosphate–responsive element binding protein family of transcription factors. Studies indicate that ATF3 is an androgen-regulated gene that stimulates cell proliferation. ATF3 protein detected by immunohistochemistry is present in prostate tumors; it has increased expression in high-Gleason-score disease and in tumors refractive to therapy.98
Prognostic Markers in Cancer
One of the outstanding achievements of modern medicine is the ability to predict the behavior of tumors based on specific clinical and pathologic criteria. Tumor stage and grade provide only general estimates of outcome for a particular patient, however. Current clinical and pathologic staging parameters cannot identify those patients who are destined to experience relapse or those whose disease will be cured by local therapy alone. These considerations have obvious consequences for the patient and enormous economic implications.
Efforts are under way to identify enzymes, oncogenes, or tumor suppressor genes whose presence or absence may predict more accurately the biologic behavior of tumors. Such studies represent a fundamental shift in the means by which tumor behavior is defined—a change from an outcome-based empirical analysis (i.e., prediction of what a tumor will do based on what it has done) to one focused on tumor biology (i.e., predictions of behavior based on specific genetic alterations). The immunohistochemical analysis of tumors is also undergoing a profound shift in emphasis. Although initial studies focused on defining tumor histogenesis, the goal of much current research is to reveal the biologic potential of tumors, providing a more scientific basis for patient management.
The use of advanced technologies to define tumor prognosis is described in detail throughout this book. Presented here are some general principles that pertain to the prognostic evaluation of all tumors.
Occult Metastases
The most important factor affecting the outcome of patients with invasive cancers is whether the tumor has spread either regionally (to regional lymph nodes) or systemically. A proportion of patients with no evidence of systemic dissemination as evaluated by routine methods (careful pathologic, clinical, biochemical, and radiologic evaluation) develop recurrent disease. In addition, the success of adjuvant therapy is assumed to stem from its ability to eradicate occult metastases before they become clinically evident.99 Immunohistochemistry is commonly used to identify occult metastatic cancer cells in the bone marrow, peripheral blood, and lymph nodes of patients with cancer. Although many of the initial studies focused on breast cancer,100., 101., 102. tumors from other organs such as the stomach,103., 104. colon,105., 106. prostate,107., 108. lung,109., 110. nervous system,111 and skin112 have been investigated. Immunohistochemical methods are based on the ability of monoclonal antibodies to distinguish between cells of different histogenesis (e.g., epithelial cancer cells versus the hematopoietic and stromal cells of the bone marrow and lymph nodes). The results indicate that it is possible to identify occult metastatic cancer cells in lymph nodes and bone marrow before their detection by other methods and that the presence of these cells may be an important risk factor for disease recurrence (Fig. 5-8 ).
Figure 5–8 ▪.
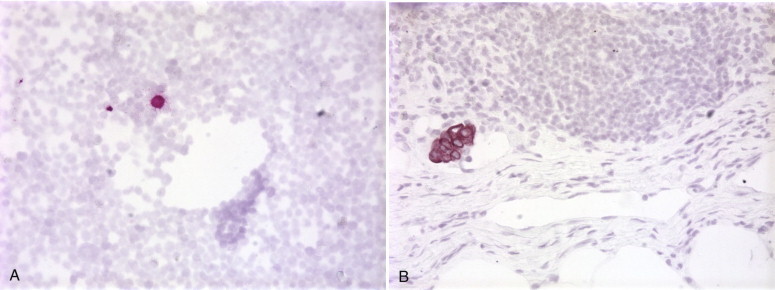
A, Bone marrow aspirate with a single keratin-positive cell from a patient with lung cancer. B, Section of lymph node shows a small focus of early metastatic breast cancer.
The most widely used monoclonal antibodies to detect occult metastatic carcinoma cells are directed toward epithelium-specific antigens. These antibodies do not react with normal hematopoietic or stromal cells present in the bone marrow or lymph nodes. None of the antibodies used in any study is specific for cancer; all react with normal and malignant epithelial cells. They are useful because they can identify an extrinsic population of epithelial cells in bone marrow or lymph nodes, where there are normally no epithelial elements. The reported sensitivity of immunohistochemistry ranges from the detection of 1 epithelial cell in 10,000 to 2 to 5 epithelial cells in 1 million hematopoietic cells.100., 113.
A potentially more sensitive approach for the detection of occult metastasis is the reverse transcriptase polymerase chain reaction (RT-PCR) technique, which has been applied to several malignancies using a variety of marker transcripts as targets. Since the first study by Smith and colleagues in 1991,114 many authors have reported molecular diagnoses in the lymph nodes, blood, and bone marrow in cancer patients.42., 107., 115., 116., 117., 118., 119. Application of RT-PCR in regional and sentinel lymph nodes has been described for a number of cancers, including melanoma, colorectal cancer, and cancers of the prostate, breast, and lung.118., 120., 121., 122., 123., 124. Many of these compare immunocytochemical-based detection with RT-PCR for sensitivity and conclude that RT-PCR may achieve enhanced detection, provided the target markers are sufficiently specific. Various formats of RT-PCR assays125., 126., 127. have also been used to detect disseminated tumor cells in the bone marrow of patients with cancers of the breast, colon, and lung, among others. With the exception of some organ-specific markers such as maspin or mammaglobin for breast cancer116., 128. or uroplakins for urothelial tumors,129 most of the molecular targets used in these RT-PCR assays lack the requisite specificity owing to illegitimate expression in nontarget hematopoietic cells.130., 131., 132. Unlike immunohistochemistry, morphologic confirmation of the cells in question to verify tumor origin is not possible with RT-PCR. RT-PCR has also been used to enhance the sensitivity of the detection of tumor cells in the peripheral blood in a variety of cancers, including prostate, breast, gastrointestinal tract, colorectal, and head and neck cancers and melanoma.117., 133., 134., 135. Concerns about nonspecificity owing to illegitimate transcription of target genes in the nontarget hematopoietic cells also apply to the blood, which has hampered the use of these assays in routine clinical diagnosis.
Bone Marrow and Peripheral Blood
In breast cancer, the bone marrow is the single most common site of metastasis, and 80% of patients with recurrent tumors develop bone marrow metastases at some point during the evolution of their disease.136 Immunohistochemistry can show the presence of occult metastases in the bone marrow in approximately 10% to 45% of patients with low-stage disease.102., 127., 137., 138., 139., 140., 141., 142., 143. Several studies have addressed the clinical significance of these early metastatic cells in the bone marrow, including a pooled analysis of more than 4700 patients.143 They have found that the presence of such cells is an independent prognostic indicator of disease-free survival and overall survival.144., 145., 146. Occult metastases in the bone marrow are also prognostically important in other malignancies, including primary non–small cell lung cancer,109., 110., 147., 148. esophageal and gastric cancers,103 colorectal cancer,149 and neuroblastoma.111 The finding of positive cells in the bone marrow of patients with colorectal cancer—a tumor that rarely shows overt metastasis to the bone—indicates that this may be a general indicator of tumor dissemination. In addition, the prognostic significance of occult metastatic cells in the blood is under investigation.
Peripheral blood has the advantage of being easier to access than bone marrow. However, detection rates are considerably lower than with bone marrow, a fact that has hampered studies to date.
In the detection of tumor cells in the bone marrow and blood, epithelial cell adhesion molecule in conjunction with immunomagnetic enrichment has been used to detect circulating tumor cells in breast cancer patients. Circulating tumor cells in the blood have been used to monitor response to therapy in patients with metastatic cancer.150., 151., 151a. The presence of epithelial adhesion molecule–positive cells before and after the initiation of therapy was found to be an independent prognostic factor. Other markers used in patients with breast cancer include mammaglobin, epidermal growth factor receptor, and carcinoembryonic antigen.116., 152., 153. Although none of these markers is entirely specific for the detection of metastatic breast cancer, and although the sensitivity of peripheral blood is less than that of bone marrow, there is growing evidence that the detection of occult metastatic cells in the peripheral blood has a negative impact on prognosis.154 A recent study found that the presence of five or more tumor cells in the peripheral blood from patients with breast cancer examined upon the initiation of therapy was important in predicting outcome.151
Peripheral blood from patients with colorectal,106 stomach,104 prostate,155 and skin 155., 156. cancer have also been studied. Markers that have been studied in colorectal cancer include cytokeratins,106 carcinoembryonic antigen,157., 158. apolipoprotein,159 and CD44v6.160 PSA messenger RNA (mRNA) is the most commonly used marker in patients with prostate cancer.155., 161. Tyrosinase mRNA is the marker of choice for detecting circulating tumor cells in patients with melanoma.161., 162.
Lymph Nodes
Studies undertaken to detect occult lymph node metastases by routine histologic methods have generally been performed by cutting serial sections from all paraffin blocks containing lymph nodes, followed by routine staining and microscopic review.163 Several studies simply reviewed the original histologic slides. Newer studies involve cytokeratin immunohistochemistry on one or more lymph node sections. PSA immunohistochemistry has also been used to confirm the prostatic origin of cytokeratin-positive cells in the lymph nodes of patients with prostate cancer.108 All these studies have shown that deposits of tumor can be detected using these methods. In previously determined node-negative cases of breast cancer, 7% to 33% convert to node-positive status after review. Neville and colleagues164 found the mean conversion rate to be approximately 13%. Although virtually all studies have shown that lymph node metastases can be overlooked, there has been surprising disagreement about the prognostic importance of these occult tumor deposits.165., 166., 167. However, it is now widely accepted that the detection of occult lymph node metastases is an important predictor of outcome in patients with histologically node-negative cancer.168., 169., 170. In a key study (Ludwig Trial V), occult breast cancer metastases were detected by immunohistochemistry in 20% of patients and were associated with significantly poor disease-free and overall survival in postmenopausal patients but not in premenopausal patients.168 Additional studies in patients with breast cancer found occult lymph node metastases to be predictive of a poorer outcome.169., 170. Studies in patients with lung,171., 172. prostate,107., 108. and colorectal 173., 174. cancer suggest that occult metastases in the lymph nodes in these patients may also predict a worse prognosis.
The finding that occult bone marrow and lymph node metastases are prognostically important has motivated several major clinical trials, notably by the American College of Surgeons Oncology Group, in breast cancer (Z0010) and lung cancer (Z0040). The advent of the use of sentinel lymph node biopsy in tumor surgery (for breast cancer and melanoma) has caused physicians to examine these lymph nodes by more sensitive techniques, owing to the limited material available for histologic review.175 It is likely that the detection of occult metastases will soon be the general standard of care; this is true at many institutions that treat large numbers of patients with cancer.
Oncogenes, Growth Factors, and Receptors
HER-2/NEU
Her-2/neu (or c-erb B-2) is a proto-oncogene. The gene encodes for a protein (185 kD) that shows homology with epidermal growth factor and displays tyrosine activity. Amplification of the gene coding for Her-2/neu has been described in breast, ovarian, prostate, gastric, salivary gland, lung, colon, and squamous cell carcinoma.176., 177., 178., 179., 180., 181., 182., 183., 184., 185., 186. When overexpressed, the protein accumulates at the cell membrane and is seen as a crisp membrane stain; a cytoplasmic staining pattern is not associated with protein or gene overexpression.176., 183.
Although Her-2/neu overexpression and amplification have been described in several tumor systems, it has been studied most extensively in the breast. Her-2/neu overexpression occurs in 10% to 34% of primary breast carcinomas187 and is restricted to cancer cells. There is an inverse association between Her-2/neu amplification and the expression of estrogen and progesterone receptors. Her-2/neu overexpression is also associated with high-grade tumors176., 188. and is considered an adverse prognostic indictor in patients with breast cancer.187 The presence of Her-2/neu overexpression is associated with resistance to tamoxifen therapy189., 190., 191. and to CMF (cyclophosphamide, methotrexate, 5-fluorouracil) adjuvant chemotherapy but is associated with an increased response to regimens that use high-dose doxorubicin.192., 193., 194., 195. Recent studies have linked amplification of the Her-2/neu and topoisomerase IIα genes to the effects of anthracyclines. Preliminary data suggest that coamplification of these two genes may identify a subgroup of high-risk breast cancer patients who might benefit from individually tailored and dose-escalated adjuvant anthracyclines.196., 197. Her-2/neu can also be assessed through amplification of the gene by fluorescence in situ hybridization.
EPIDERMAL GROWTH FACTOR RECEPTOR
Epidermal growth factor receptor (EGFR) belongs to a family of growth factor receptors involved in normal growth. The gene is located on chromosome 7p12. It is the receptor for epidermal growth factor and is a member of the receptor tyrosine kinase family. It is closely related to Her-2/neu, Her-3, and Her-4. EGFR is known to be involved in carcinogenic processes such as cell proliferation, apoptosis, angiogenesis, cell motility, and metastasis. The expression of EGFR has been examined in a wide variety of tissues, and in many cases, increased expression of EGFR is predictive of tumor progression (e.g., cancer of the breast, esophagus, adrenals, lung, bladder, thyroid, and gastrointestinal tract and glioblastoma multiforme).198., 199., 200., 201., 202., 203., 204., 205. In addition to immunohistochemical methods, fluorescence in situ hybridization has been used successfully to identify EGFR mutation or deletions on formalin-fixed, paraffin-embedded tissue.206
EGFR is also showing promise as a therapeutic target. Studies are under way in lung and colorectal cancer to determine the usefulness of targeting EGFR for anticancer therapy.207., 208.
Tumor Suppressor Genes and Gene Products
The primary characteristics of tumor suppressor genes are that they encode normal cellular products involved in growth control, and both alleles must be inactivated for loss of function (i.e., loss of tumor suppression) to occur. The most well known are retinoblastoma (Rb) protein, p53, p27, p21, and p16. The two best characterized are the Rb and p53 genes. Both are thought to be involved in growth control through the regulation of transcription.
RETINOBLASTOMA GENE
The Rb gene is located on chromosome 13q14 and is dysfunctional in a number of types of cancer. Its normal function is to prevent the replication of damaged DNA; it does so by preventing cell replication by binding and inhibiting the transcription factor E2F.209., 210. The retinoblastoma protein (pRb) is activated when it is dephosphorylated and inactivated when it is phosphorylated. Alterations in this gene have been described in many human tumors, including retinoblastoma, osteosarcoma, other sarcomas, leukemias, lymphomas, and certain carcinomas, including breast, lung, prostate, bladder, kidney, and testicular carcinoma.29., 211., 212. Gene alterations are associated with advanced tumor grade and stage in a variety of tumors.211., 213. Alterations in the Rb gene correlate with loss of expression of pRb as determined by immunohistochemistry.214 Assessment of Rb gene loss by immunohistochemistry is based on the loss of detectable nuclear staining for pRb. There is growing evidence that gene alterations may identify tumors that have a higher risk of developing metastases.215 Loss of heterozygosity, mutations, or deletions of the Rb gene usually result in the loss of pRb expression, which has been regarded as an indicator of loss of pRb function in human tumors. In addition to loss of pRb expression, aberrantly high pRb expression indicates a loss of pRb function in bladder tumors compared with moderate pRb expression.210., 215. It has been shown that tumors with pRb overexpression demonstrate pRb hyperphosphorylation, mediated in part by the loss of p16 expression or overexpression of cyclin D1.210
P53
The p53 gene is located on chromosome 17p13.1. The p53 protein is expressed by all normal cells, but the half-life of the normal protein is so short (6 to 30 minutes) that it does not accumulate in levels high enough to be detected by standard immunohistochemical techniques. Mutant p53 protein, by contrast, has an extended half-life, accumulates, and is readily detectable in the cell nucleus; mutation is indicated by positive staining. Alterations of the p53 gene are extremely common in human cancer and have been described in bladder, colon, lung, breast, and other carcinomas; astrocytomas; leukemias; sarcomas; and mesotheliomas.1., 29., 213., 216., 217. Because of the importance of p53 alterations in human cancer and the ease of detecting p53 mutations by molecular or immunohistochemical methods, p53 alterations have been the focus of intense examination. As with Rb alterations, p53 alterations are associated with tumors of high histologic grade and a high proliferative index. There is growing evidence that, at least for some types of tumors, p53 alterations identify patients with shorter disease-free intervals and poorer overall survival.217., 218.
CYCLIN-DEPENDENT KINASE INHIBITORS
The cyclin-dependent kinase inhibitors are a family of cell cycle regulators. Their primary function seems to be the formation of stable complexes with cyclin-dependent kinase proteins and the subsequent inhibition of the cell cycle. These complexes inactivate the catalytically operative units. Among the most well known and clinically relevant are p21, p27, and p16.
p21
A member of the WAF/CIP/KIP family of cyclin-dependent kinase inhibitors, p21 is probably the best characterized. It acts as a regulator of epithelial carcinogenesis and differentiation and is thought to play an important role in tumor suppression by regulating cell cycle progression, DNA replication, and DNA repair.29., 219. The protein expression of p21 has been studied in a variety of tumor types, including breast,220 gastric,221 ovary,222 colorectal,223 and bladder213., 224. carcinomas. The alteration of protein expression assessed by immunohistochemical methods has been associated with higher tumor grade and worse prognosis in patients with bladder cancer.123., 213.
p27
The p27 inhibitor is involved in the regulation of the cell cycle at the G1-S transition, ultimately through the inhibition of pRb phosphorylation.225 Mutations in the human p27 gene appear to be rare.226 Loss of p27 expression is associated with colon, breast, prostate, and gastric cancer progression.227., 228., 229., 230., 231.
p16
Also known as p16INK4 and CDKN2A, p16 is a tumor suppressor protein encoded on the INK4a/ARF locus of chromosome 9p21, which is one of the most frequent sites of genetic loss in human cancer.232 Numerous studies have found abnormal p16 protein in a variety of tumor types, including melanomas; gliomas; esophageal, pancreatic, lung, and bladder carcinomas; and certain types of lymphomas.232., 233., 234., 235., 236., 237., 238., 239., 240. In addition, p16 is known to regulate Rb, and immunohistochemical expression of pRb and p16 is inversely correlated in a variety of tumors.241., 242.
COMBINED EFFECTS OF P53, P21, AND PRB
It is known that, individually, p53, p21, and pRb are independent predictors of time to recurrence and overall survival in patients with bladder cancer.215., 217., 224. Efforts have therefore been made to examine these determinants in combination.123., 213. In one study, patients were analyzed according to whether none, one, two, or all three markers were positive. The 5-year survival rates were 70%, 58%, 33%, and 8%, respectively. These data suggest that alterations in p53, p21, and pRb act in cooperative or synergistic ways to promote bladder cancer progression.
TUMOR ONCOGENE CYCLIN D1
Cyclin D1 plays a key role in the regulation of the G1-S transition phase of the cell cycle. It has been linked to a number of different cancers, including colorectal, esophageal, gastric, lung, head and neck, and pancreatic cancer.243
Predicting Response to Therapy
Although a major purpose of the molecular assessment of cancer is to better understand the risk for disease progression, advanced technologies are also being used to understand the specific patterns of response and resistance to therapeutic regimens. The traditional means of determining appropriate systemic treatment generally involved histogenic classification. It has long been recognized, however, that response to hormonal therapy can be predicted specifically by molecular determinants (e.g., the expression of estrogen and progesterone receptors in breast and other cancers of reproductive organs).244
Tumors arising from the breast, prostate, endometrium, and ovary are known to be regulated by steroid sex hormones (estrogens, androgens). It was discovered that removing the source of hormones that control tumor growth (by oophorectomy, orchiectomy, or chemical methods) sometimes resulted in dramatic tumor remission.244 Growth regulation was found to be associated with the amount of specific hormone receptors: Tumors that expressed high levels of these receptors tended to respond well to hormone ablation, whereas those with few or no receptors tended not to respond to this type of therapy. Accurate methods for determining the presence or absence of hormone receptors are essential for determining the best method of treatment.
The availability of monoclonal antibodies to estrogen, progesterone, and androgen receptors has made immunohistochemical detection of hormone receptor status the current method of choice. These immunohistochemical methods can be performed on formalin-fixed, paraffin-embedded tissue and on cytology specimens. Immunohistochemical antireceptor assays allow one to predict breast cancer's response to hormonal treatment.245., 246. Tumors that do not express estrogen or progesterone receptors have a low probability of responding to hormonal manipulation, whereas estrogen receptor– and progesterone receptor–positive tumors have a high probability of responding to such treatment. Many practitioners believe that the only relevant result for hormone receptors in breast cancer is “positive” or “negative.” However, some investigators have shown that the level of hormone receptor is important as well.8., 9. Although a proportion of patients with low levels of hormone receptor will respond to hormone therapy, most benefit from the addition of systemic cytotoxic chemotherapy. In contrast, in patients with high levels of hormone receptor, the addition of cytotoxic chemotherapy has a deleterious effect on outcome.8., 9.
Recently, attention has focused on expression of the estrogen receptor subtypes α and β and on various isoforms of the β subtype. It has been found that estrogen receptor α–negative tumors express significant levels of estrogen receptor β1 and β5 and that their expression levels are no different from levels in estrogen receptor α–positive tumors.246 Therefore, these two estrogen receptor isoforms may be potential molecular targets for designing chemopreventive drugs to treat estrogen receptor α–negative breast cancers.
P-glycoprotein is a transmembrane protein of 170 kD that has been associated with intrinsic and acquired resistance to certain chemotherapeutic agents, particularly anthracyclines and vinca alkaloids. P-glycoprotein also may play a role in tumor progression and has been associated with blood vessel invasion and lymph node metastases.2 Some tumors inherently express P-glycoprotein, whereas other tumors acquire expression only after exposure to certain chemotherapeutic agents.2 Overexpression is associated with failure of chemotherapy.2., 247.
Other predictors of response to specific forms of chemotherapy are being explored. The prevailing view has been that p53 alterations should result in a chemoresistant phenotype. This view is based on a body of evidence showing that wild-type p53 is required for entrance into the apoptotic pathway at the G1- to S-phase transition.248., 249. Because chemotherapy works through the induction of apoptosis, p53 alterations may result in resistance to such agents. We are conducting a clinical trial concerning the role of p53 in predicting progression and response in patients with bladder cancer.250 It is also possible that p53 may promote chemoresistance by other mechanisms, such as through induction of the multidrug resistance (MDR-1) gene.249., 251. In tumors in which p53 alterations confer increased (selective) chemosensitivity, combining agents that have different actions (e.g., DNA damage versus inhibition of the G2M checkpoint) may have synergistic effects on tumor cell killing, a finding that has important implications in the design of new combination chemotherapy regimens.252
The expression of thymidylate synthase in colorectal tumors predicts resistance to the most common type of systemic chemotherapy used in that disease, 5-fluorouracil.253., 254. As mentioned earlier, Her-2/neu overexpression in breast cancer predicts resistance to hormone therapy in estrogen receptor–positive tumors189., 190., 255. and resistance to some types of chemotherapy, but increased sensitivity to doxorubicin-based regimens.193., 194. Her-2/neu and EGFR are specific targets of antibody-directed therapy. In the case of Her 2/neu, it seems that only those tumors that overexpress the target are likely to respond to such therapies.256., 257., 258. In the case of colorectal cancer, therapy directed against EGFR appears to work only in tumors with wild-type KRAS; tumors with mutant KRAS do not respond to EGFR therapy.258a Similar findings are seen in lung cancer.258b
The ability to predict the specific response of individual tumors to chemotherapeutic agents can have a profound effect on treatment decisions for patients with cancer. It is not difficult to envision the day when drug selection is based on the resistance patterns of individual tumors to specific agents. Treatment decisions will become less organ based and will better reflect the biology of the tumors.
Infections
Traditionally, the stains available to surgical pathologists to identify infectious organisms in tissue sections consisted of Gram stain, variations of the acid-fast stain, periodic acid–Schiff, and silver stains. There is now a wide range of immunohistochemical or in situ hybridization techniques available for the detection of specific types of organisms within fixed paraffin sections. Although culturing techniques remain the most important method for diagnosing most infections, immunohistochemical methods are as effective as, or even superior to, culture and routine H&E methods for the detection of certain infectious organisms such as cytomegalovirus (Fig. 5-9 ), mycobacteria, Toxoplasma, Pneumocystis carinii, Histoplasma capsulatum, Helicobacter pylori, and human papillomavirus.259., 260., 261., 262., 263., 264., 265., 266., 267., 268., 269. Table 5-4 summarizes some of the infectious agents that can be identified by immunohistochemistry.
Figure 5–9 ▪.

Placenta showing infection with cytomegalovirus by immunohistochemistry.
TABLE 5–4.
Infectious Agents for Which Antibodies Are Available for Use on Paraffin Sections
Adenovirus
|
Baboon endogenous virus
|
Buffalo pox virus
|
Coronavirus
|
| Cytomegalovirus |
Distemper virus
|
Epstein-Barr virus
|
Friend's virus
|
| Hepatitis A virus |
| Hepatitis B core antigen |
| Hepatitis B surface antigen |
| Hepatitis C virus |
Herpes simplex virus 1 and 2
|
| Human immunodeficiency virus (HIV-1) |
| Human papillomavirus |
Influenza
|
| Lymphocytic choriomeningitis virus |
| Measles antigen |
| Moloney virus |
| Mouse mammary tumor virus antigen |
Mycobacteria
|
| Polio |
Polyomavirus
|
| Rabies virus |
| Respiratory syncytial virus |
| Rotavirus |
Rubella
|
Shope's fibroma virus
|
SV40 virus
|
Varicella-zoster virus
|
REFERENCES
- 1.Taylor CR, Cote RJ. 3rd ed. WB Saunders; Philadelphia: 2006. Immunomicroscopy: A Diagnostic Tool for the Surgical Pathologist. [Google Scholar]
- 2.Cote RJ, Taylor CR. Immunohistochemistry and related marking techniques. In: Damjanov I, Linder J, editors. Anderson's Pathology. 10th ed. Mosby; St Louis: 1996. pp. 136–175. [Google Scholar]
- 3.Taylor CR. The current role of immunohistochemistry in diagnostic pathology. Adv Pathol Lab Med. 1994;7:59–105. [Google Scholar]
- 4.Wick MR. Quality assurance in immunohistochemistry: A discipline coming of age [editorial] Am J Clin Pathol. 1989;92:844. doi: 10.1093/ajcp/92.6.844. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CR, Shi S-R, Cote RJ. Antigen retrieval for immunohistochemistry status and need for greater standardization. Appl Immunohistochem. 1996;4:144–166. [Google Scholar]
- 6.Shi S-R, Cote RJ, Chaiwun B. Standardization of immunohistochemistry based on antigen retrieval technique for routine formalin-fixed tissue sections. Appl Immunohistochem. 1998;6:89–96. [Google Scholar]
- 7.Bloom K, Harrington D. Enhanced accuracy and reliability of Her-2/neu immunohistochemical scoring using digital microscopy. Am J Clin Pathol. 2004;121:620–630. doi: 10.1309/Y73U-8X72-B68T-MGH5. [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 8.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 9.Berry DA, Cirrincione C, Henderson IC. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battifora H, Mehta P. The checkerboard tissue block: An improved multitissue control block. Lab Invest. 1990;63:722. [PubMed] [Google Scholar]
- 11.Shi S-R, Imam SA, Young LL. Antigen retrieval immunohistochemstry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 1995;43:193–201. doi: 10.1177/43.2.7822775. [DOI] [PubMed] [Google Scholar]
- 12.Shi S-R, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin embedded tissues: An enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 13.Taylor CR. FDA issues final rule for classification/reclassification of immunochemistry reagents and kits. Appl Immunohistochem. 1998;6:115–117. doi: 10.1093/ajcp/111.4.443. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health and Human Services, Health Care Financing Administration, et al: Clinical Laboratory Improvement Amendments of 1988. In Public Health Service 42 CFR part 405, final rule, Feb 28, 1992, pp 7002-7134.
- 15.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88:1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 16.Vis AN, Kranse R, Nigg AL. van der Kwast: Quantitative analysis of the decay of immunoreactivity in stored prostate needle biopsy sections. Am J Clin Pathol. 2000;113:369–373. doi: 10.1309/CQWY-E3F6-9KDN-YV36. [DOI] [PubMed] [Google Scholar]
- 17.Wester K, Wahland E, Sundström C. Paraffin section storage and immunohistochemistry: Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8:61–70. [PubMed] [Google Scholar]
- 18.Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissue. Cancer Lett. 1991;59:251–256. doi: 10.1016/0304-3835(91)90149-c. [DOI] [PubMed] [Google Scholar]
- 19.Shi S-R, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry: Past, present, and future. J Histochem Cytochem. 1997;45:327–343. doi: 10.1177/002215549704500301. [DOI] [PubMed] [Google Scholar]
- 20.Raite VK, O’Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic ribonuclease A:I—structural and functional alterations. Lab Invest. 2004;84:292–299. doi: 10.1038/labinvest.3700045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita S, Okada Y. Mechanisms of heat-induced antigen retrieval: Analysis in vitro employing SDS-PAGE and immunohistochemistry. J Histochem Cytochem. 2005;53:13–21. doi: 10.1177/002215540505300103. [DOI] [PubMed] [Google Scholar]
- 22.Sompuram SR, Vani K, Messana E, Bogen SA. A molecular mechanism of formalin fixation and antigen retrieval. Am J Clin Pathol. 2004;121:190–199. doi: 10.1309/BRN7-CTX1-E84N-WWPL. [DOI] [PubMed] [Google Scholar]
- 23.Shi S-R, Cote RJ, Young LL. Antigen retrieval immunohistochemistry: Practice and development. J Histotechnol. 1997;20:145–152. [Google Scholar]
- 24.Morgan JM, Navabi H, Jasani B. Role of calcium chelation in high-temperature antigen retrieval at different pH values. J Pathol. 1997;182:233–237. doi: 10.1002/(SICI)1096-9896(199706)182:2<233::AID-PATH827>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Evers P, Uylings HBM. Microwave-stimulated antigen retrieval is pH and temperature dependent. J Histochem Cytochem. 1994;42:1555–1563. doi: 10.1177/42.12.7983356. [DOI] [PubMed] [Google Scholar]
- 26.Pileri SA, Roncador G, Ceccareli C. Antigen retrieval techniques in immunohistochemistry: Comparison of different methods. J Pathol. 1997;183:116–123. doi: 10.1002/(SICI)1096-9896(199709)183:1<116::AID-PATH1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Namimatsu S, Ghazizadeh M, Sugisake Y. Reversing the effects of formalin fixation with citraconic anhydride and heat: A universal antigen retrieval method. J Histochem Cytochem. 2005;53:3–11. doi: 10.1177/002215540505300102. [DOI] [PubMed] [Google Scholar]
- 28.Altman E, Cadman E. An analysis of 1539 patients with cancer of unknown primary site. Cancer. 1986;57:120–124. doi: 10.1002/1097-0142(19860101)57:1<120::aid-cncr2820570124>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Hawes D. Immunohistochemical detection of tumor suppressor genes using antigen retrieval technique. In: Shi S-R, Gu J, Taylor CR, editors. Antigen Retrieval Techniques: Immunohistochemistry and Molecular Morphology. Mass, Eaton; Natick: 2000. pp. 207–216. [Google Scholar]
- 30.Ross MH, Romrell LJ, Kaye GI. 3rd ed. Williams & Wilkins; Baltimore: 1995. Histology: A Text and Atlas. [Google Scholar]
- 31.Moll R, Franke WW. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang NP. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99–107. [Google Scholar]
- 33.Johnson DE, Herndier BG, Medeiros LJ. The diagnostic utility of keratin profiles of hepatocellular carcinoma and cholangiocarcinoma. Am J Surg Pathol. 1988;12:187–197. doi: 10.1097/00000478-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Stopyra GA, Warhol MJ, Multhaupt HP. Utility of CK7 and CK20 immunohistochemistry in the detection of synchronous breast and colon carcinoma in a pleural effusion: A case report and supporting survey of archival material. Diagn Cytopathol. 2001;25:54–58. doi: 10.1002/dc.2002. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Kim HS, Hong EK. Expression of cytokeratin 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metasatatic carcinomas to the ovary. Hum Pathol. 2002;33:1078–1085. doi: 10.1053/hupa.2002.129422. [DOI] [PubMed] [Google Scholar]
- 36.Su YC, Hsu YC, Chai CY. Role of TTF-1, CK20, and CK7 immunohistochemistry for diagnosis of primary and secondary lung adenocarcinoma. Kaohsiung J Med Sci. 2006;22:14–19. doi: 10.1016/S1607-551X(09)70214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: An immunohistochemical study of 509 cases. Mod Pathol. 2002;15:6–10. doi: 10.1038/modpathol.3880483. [DOI] [PubMed] [Google Scholar]
- 38.Ordonez NG. Value of cytokeratin 5/6 immunostaining in distinguishing epithelial mesothelioma of the pleura from lung adenocarcinoma. Am J Surg Pathol. 1998;22:1215–1221. doi: 10.1097/00000478-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann O, Fietze E, Mengs J. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001;116:823–830. doi: 10.1309/21TW-2NDG-JRK4-PFJX. [DOI] [PubMed] [Google Scholar]
- 40.Reis-Filho JS, Simpson PT, Martins A. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003;443:122–132. doi: 10.1007/s00428-003-0859-2. [DOI] [PubMed] [Google Scholar]
- 41.Varma M, Jasani B. Diagnostic utility of immunohistochemistry in morphologically difficult prostate cancer: Review of current literature. Histopathology. 2005;47:1–16. doi: 10.1111/j.1365-2559.2005.02188.x. [DOI] [PubMed] [Google Scholar]
- 42.Zehentner BK, Carter D. Mammaglobin: A candidate diagnostic marker for breast cancer. Clin Biochem. 2004;37:249–257. doi: 10.1016/j.clinbiochem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 43.O’Brian NA, O’Donovan N, Ryan B. Mammaglobin A in breast cancer: Existence of multiple molecular forms. Int J Cancer. 2005;114:623–627. doi: 10.1002/ijc.20780. [DOI] [PubMed] [Google Scholar]
- 44.Kabawat SE, Bast RCJ. Tissue distribution of a coelomic epithelium-related antigen recognized by monoclonal antibody OC125. Int J Gynecol Pathol. 1983;2:275. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Lobban ED, Smith BA, Hall GD. Uroplakin gene expression by normal and neoplastic human urothelium. Am J Pathol. 1998;153:1957–1967. doi: 10.1016/S0002-9440(10)65709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.JJ Lu, Kakehi Y, Takahashi T. Detection of circulating cancer cells by reverse transcription-polymerase chain reaction for uroplakin II in peripheral blood of patients with urothelial cancer. Clin Cancer Res. 2000;6:3166–3171. [PubMed] [Google Scholar]
- 47.Buffa R. Synaptophysin immunoreactivity and small clear vesicles in neurendocrine cells and related tumours. Mol Cell Probes. 1987;1:367–381. doi: 10.1016/0890-8508(87)90018-1. [DOI] [PubMed] [Google Scholar]
- 48.Ferry JA, Harris NL. Atlas of lymphoid hyperplasia and lymphoma. In: Bordin GM, editor. Atlases in Diagnostic Surgical Pathology. WB Saunders; Philadelphia: 1997. p. 194. [Google Scholar]
- 49.Della Croce DR. Anti-BLA.36 monoclonal antibody shows reactivity with Hodgkin's cells and B lymphocytes in frozen and paraffin-embedded tissues. Hematol Oncol. 1991;9:103–114. doi: 10.1002/hon.2900090206. [DOI] [PubMed] [Google Scholar]
- 50.Gatter KC. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumors. J Clin Pathol. 1985;38:1353–1357. doi: 10.1136/jcp.38.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dwarakanath S. S100 protein positivity in breast carcinomas: A potential pitfall in diagnostic immunohistochemistry. Hum Pathol. 1987;18:1144. doi: 10.1016/s0046-8177(87)80382-9. [DOI] [PubMed] [Google Scholar]
- 52.Drier JK. S100 protein immunoreactivity in poorly differentiated carcinomas: Immunohistochemical comparisons with malignant melanoma. Arch Pathol Lab Med. 1987;111:447. [PubMed] [Google Scholar]
- 53.Banks PM. Incorporation of immunostaining data in anatomic pathology reports. Am J Surg Pathol. 1992;16:808. doi: 10.1097/00000478-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Skalli O. A monoclonal antibody against a smooth muscle actin: A new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukai K. Histiogenesis of alveolar soft part sarcoma: An immunohistochemical and biochemical study. Am J Surg Pathol. 1986;10:212. doi: 10.1097/00000478-198603000-00008. [DOI] [PubMed] [Google Scholar]
- 56.DuBoulary CEH. Demonstration of alpha-1-antitrypsin and alpha-1-antichymotrypsin in fibrous histiocytomas using the immunoperoxidase technique. Am J Surg Pathol. 1982;6:559. doi: 10.1097/00000478-198209000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Fisher C. The value of electron microscopy and immunohistochemistry in the diagnosis of soft tissue sarcoma: A study of 200 cases. Histopathology. 1990;16:441. doi: 10.1111/j.1365-2559.1990.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 58.Leader M, Collins PM, Henry KA. Antichymotrypsin staining of 194 sarcomas, 38 carcinomas, and 17 malignant melanomas: Its lack of specificity as a tumor marker. Am J Surg Pathol. 1987;11:133. doi: 10.1097/00000478-198702000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Gould VE. Synaptophysin: A novel marker for neurons, certain neuroendocrine cells and their neoplasms. Hum Pathol. 1986;17:979. doi: 10.1016/s0046-8177(86)80080-6. [DOI] [PubMed] [Google Scholar]
- 60.Wick MR. Malignant peripheral nerve sheath tumor, an immunohistochemical study of 62 cases. Am J Clin Pathol. 1987;87:425. doi: 10.1093/ajcp/87.4.425. [DOI] [PubMed] [Google Scholar]
- 61.Weiss SW, Langloss JM, Enzinjger FM. Value of S100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant schwann cell tumors. Lab Invest. 1983;49:299. [PubMed] [Google Scholar]
- 62.Tetu B. Chondrosarcoma with additional mesenchymal component (dedifferentiated chondrosarcoma) II: An immunohistochemical and electron microscopic study. Cancer. 1986;58:287. doi: 10.1002/1097-0142(19860715)58:2<287::aid-cncr2820580214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 63.Pantou D. Cytogenetic profile of unknown primary tumors: Clues for their pathogenesis and clinical management. Neoplasia. 2003;5:23–31. doi: 10.1016/s1476-5586(03)80014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Micci F. Cytogenetic and molecular genetic analyses of endometrial stromal sarcomas: Nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17) Cancer Genet Cytogenet. 2003;144:119–124. doi: 10.1016/s0165-4608(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 65.Douet-Guilbert N. Rearrangement of MLL in a patient with congenital acute monoblastic leukemia and granulocytic sarcoma associated with t(1;11)(p36;q23) translocation. Leuk Lymphoma. 2005;46:143–146. doi: 10.1080/104281904000010783. [DOI] [PubMed] [Google Scholar]
- 66.Gopal S. Primary myeloid sarcoma of the testicle with t(15;17) Cancer Genet Cytogenet. 2005;157:148–150. doi: 10.1016/j.cancergencyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Taylor CA, Barnhart A, Pettanati MJ, Geisinger KR. Primary pleuropulmonary synovial sarcoma diagnosed by fine needle aspiration with cytogenetic confirmation: A case report. Acta Cytol. 2005;46:673–676. doi: 10.1159/000326260. [DOI] [PubMed] [Google Scholar]
- 68.Deeb G. Genomic profiling of myeloid sarcoma by array comparative genomic hybridization. Genes Chromosomes Cancer. 2005;44:373–383. doi: 10.1002/gcc.20239. [DOI] [PubMed] [Google Scholar]
- 69.Granville L. Visceral clear cell sarcoma of soft tissue with confirmation by EWS-ATF1 fusion detection. Ultrastruct Pathol. 2006;30:111–118. doi: 10.1080/01913120500406400. [DOI] [PubMed] [Google Scholar]
- 70.Konoplev S, Bueso-Ramos CE. Advances in the pathologic diagnosis and biology of acute myeloid leukemia. Ann Diagn Pathol. 2006;10:39–65. doi: 10.1016/j.anndiagpath.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Ishiguro Y, Kato K, Ito T. Enolase isoenzymes as markers for differential diagnosis of neuroblastoma, rhabdomyosarcoma, and Wilm's tumor. Gann. 1984;75:53. [PubMed] [Google Scholar]
- 72.Wilson BS, Lloyd RV. Detection of chromogranin in neuroendocrine cells with monoclonal antibody. Am J Pathol. 1984;115:458. [PMC free article] [PubMed] [Google Scholar]
- 73.Caillaud JM. HNK-I-defined antigen detected in paraffin-embedded neuroectoderm tumors and those derived from cells of the amine precursor uptake and decarboxylation system. Cancer Res. 1984;44:498. [PubMed] [Google Scholar]
- 74.Gould VE. Primitive neuroectodermal tumors of the central nervous system: Patterns of expression of neuroendocrine markers, and all classes of intermediate filament proteins. Lab Invest. 1990;62:498. [PubMed] [Google Scholar]
- 75.Fellinger EJ. Immunohistochemical analysis of Ewing's sarcoma cell surface antigen p30/32MIC2. Am J Pathol. 1991;139:317–325. [PMC free article] [PubMed] [Google Scholar]
- 76.Miettinen M. Immunohistochemical spectrum of rhabdomyosarcoma and rhabdomyosarcoma-like tumors: Expression of cytokeratin and the 68 kD neurofilament protein. Am J Surg Pathol. 1989;12:120. doi: 10.1097/00000478-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Bunn PAJ. Small cell lung cancer, endocrine cells of the fetal bronchus, and other neuroendocrine cells express the Leu-7 antigenic determinant present on natural killer cells. Blood. 1985;65:764. [PubMed] [Google Scholar]
- 78.Said JW. Immunoreactive neuron-specific enolase, bombesin, and chromogranin as markers for neuroendocrine lung tumors. Hum Pathol. 1985;16:236. doi: 10.1016/s0046-8177(85)80008-3. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, Zhang Y. Use of monoclonal antibodies to glial fibrillary acidic protein in the cytologic diagnosis of brain tumors. Acta Cytol. 1989;33:922. [PubMed] [Google Scholar]
- 80.Perentes E, Rubinstein LJ. Recent applications of immunoperoxidase histochemistry in human neuro-oncology: An update. Arch Pathol Med. 1987;111:796. [PubMed] [Google Scholar]
- 81.Achtstaetter T. Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratin in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation. 1986;31:206. doi: 10.1111/j.1432-0436.1986.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 82.Kawahara E. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors: A comparative study of immunoreactivity of GFAP, vimentin, S100 protein and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol. 1988;12:115. doi: 10.1097/00000478-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Hua C. Mechanisms of bcl-2 activation in human follicular lymphoma. Oncogene. 1990;5:233–235. [PubMed] [Google Scholar]
- 84.Ladanyi M, Bridge J. Contribution of molecular genetic data to the classification of sarcomas. Hum Pathol. 2000;31:832–838. doi: 10.1053/hp.2000.6706. [DOI] [PubMed] [Google Scholar]
- 85.Lasota J. Detection of the SYT-SSX fusion transcripts in formaldehyde-fixed, paraffin-embedded tissue: A reverse transcription polymerase chain reaction amplification assay useful in the diagnosis of synovial sarcoma. Mod Pathol. 1998;11:626–633. [PubMed] [Google Scholar]
- 86.Mentzel T, Fletcher CDM. Recent advances in soft tissue tumor diagnosis. Am J Clin Pathol. 1998;110:660–670. doi: 10.1093/ajcp/110.5.660. [DOI] [PubMed] [Google Scholar]
- 87.DiLoreto C. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50:30–37. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiLoreto C, Pglisi F, DiLauro V. TTF-1 protein expression in pleural malignant mesotheliomas and adenocarcinomas of the lung. Cancer Lett. 1998;127:73–78. doi: 10.1016/s0304-3835(97)00466-7. [DOI] [PubMed] [Google Scholar]
- 89.Fabbro D. TTF-1 gene expression and human lung cancer tumors. Eur J Cancer. 1996;32A:512–517. doi: 10.1016/0959-8049(95)00560-9. [DOI] [PubMed] [Google Scholar]
- 90.Bejarno PA. Surfactant proteins and thyroid transcription factor 1 in pulmonary and breast carcinomas. Mod Pathol. 1996;9:445–452. [PubMed] [Google Scholar]
- 91.Choi YL. Immunoexpression of HBME-1, high molecular weight cytokeratin, cytokeratin 19, thyroid transcription factor-1, and E-cadherin in thyroid carcinomas. J Korean Med Sci. 2005;20:853–859. doi: 10.3346/jkms.2005.20.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Comperat E. Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol. 2005;18:1371–1376. doi: 10.1038/modpathol.3800422. [DOI] [PubMed] [Google Scholar]
- 93.Gokden M, Shinde A. Recent immunohistochemical markers in the differential diagnosis of primary and metastatic carcinomas of the liver. Diagn Cytopathol. 2005;33:166–172. doi: 10.1002/dc.20345. [DOI] [PubMed] [Google Scholar]
- 94.Barbareschi M. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol. 2003;27:141–149. doi: 10.1097/00000478-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Pomjanski N. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagn Cytopathol. 2005;33:309–315. doi: 10.1002/dc.20393. [DOI] [PubMed] [Google Scholar]
- 96.Levine PH. CDX-2 expression in pulmonary fine-needle aspiration specimens: A useful adjunct for the diagnosis of metastatic colorectal adenocarcinoma. Diagn Cytopathol. 2006;34:191–195. doi: 10.1002/dc.20403. [DOI] [PubMed] [Google Scholar]
- 97.Zeng HA, Cartun RW, Ricci A. Potential diagnostic utility of CDX-2 immunophenotyping in extramammary Paget's disease. Appl Immunohisotchem Mol Morphol. 2005;13:342–346. doi: 10.1097/01.pai.0000163989.12896.d2. [DOI] [PubMed] [Google Scholar]
- 98.Pelzer AE. The expression of transcription factor activating transcription factor 3 in the human prostate and its regulation by androgen in prostate cancer. J Urol. 2006;175:1517–1522. doi: 10.1016/S0022-5347(05)00651-8. [DOI] [PubMed] [Google Scholar]
- 99.Schabel FM. Rationale for adjuvant chemotherapy. Cancer. 1977;39:2875–2882. doi: 10.1002/1097-0142(197706)39:6<2875::aid-cncr2820390675>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 100.Osborne MP. Sensitivity of immunocytochemical detection of breast cancer cells in human bone marrow. Cancer Res. 1991;51:2706. [PubMed] [Google Scholar]
- 101.Ellis G, Fergusson M, Yamanaka E. Monoclonal antibodies for detection of occult carcinoma cells in bone marrow of breast cancer patients. Cancer. 1989;63:2509–2514. doi: 10.1002/1097-0142(19890615)63:12<2509::aid-cncr2820631225>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 102.Cote RJ. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow metastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 103.Macadam R. Bone marrow micrometastases predict early post-operative recurrence following surgical resection of oesophageal and gastric carcinoma. Eur J Surg Oncol. 2003;29:450–454. doi: 10.1016/s0748-7983(03)00029-5. [DOI] [PubMed] [Google Scholar]
- 104.Chen X-M. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804–808. doi: 10.3748/wjg.v10.i6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlimok G. Epithelial tumor cells in bone marrow of patients with colorectal cancer: Immunocytochemical detection, phenotypic characterization, and prognostic significance. J Clin Oncol. 1990;8:831–837. doi: 10.1200/JCO.1990.8.5.831. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X. Detection of micrometastasis in peripheral blood by multi-sampling in patients with colorectal cancer. World J Gastroenterol. 2005;11:436–438. doi: 10.3748/wjg.v11.i3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shariat SF. Comparison of immunohistochemistry with reverse transcriptase-PCR for the detection of micrometastatic prostate cancer in lymph nodes. Cancer Res. 2003;63:4662–4670. [PubMed] [Google Scholar]
- 108.Pagliarulo V. Detection of occult lymph node metastases in locally advanced (pT3) node-negative prostate cancer. J Clin Oncol. 2006;24:18. doi: 10.1200/JCO.2005.05.4767. [DOI] [PubMed] [Google Scholar]
- 109.Cote RJ. Detection of occult bone marrow metastases in patients with operable lung carcinoma. Ann Surg. 1995;222:415–425. doi: 10.1097/00000658-199522240-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coello MC, Luketich JD, Godfrey TE. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 111.Tsang KS. Detection of micrometastasis of neuroblastoma to bone marrow and tumor dissemination to hematopoietic autographs using flow cytometry and reverse transcriptase-polymerase chain reaction. Cancer. 2003;97:2887–2897. doi: 10.1002/cncr.11389. [DOI] [PubMed] [Google Scholar]
- 112.Li W. Clinical relevance of molecular staging for melanoma: Comparison of RT-PCR and immunohistochemistry staining in sentinel lymph nodes of patients with melanoma. Ann Surg. 2000;231:795–803. doi: 10.1097/00000658-200006000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaiwun B. Immunohistochemical detection of occult carcinoma in bone marrow and blood. Diagn Oncol. 1992;2:267. [Google Scholar]
- 114.Smith B. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 115.Bostick PJ, Hoon DS, Cote RJ. Detection of carcinoembryonic antigen messenger RNA in lymph nodes from patients with colorectal cancer. N Engl J Med. 1998;339:1643–1644. [PubMed] [Google Scholar]
- 116.Zach O. Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via nested reverse transcriptase polymerase chain reaction assay for mammaglobin mRNA. J Clin Oncol. 1999;17:2015–2019. doi: 10.1200/JCO.1999.17.7.2015. [DOI] [PubMed] [Google Scholar]
- 117.Yokoyama S, Yamaue H. Prediction of distant metastasis by using reverse transcriptase-polymerase chain reaction for epithelial and variant CD44 mRNA in the peripheral blood of patients with colorectal cancer. Arch Surg. 2002;137:1069–1073. doi: 10.1001/archsurg.137.9.1069. [DOI] [PubMed] [Google Scholar]
- 118.Wallace MB. Detection of telomerase expression in mediastinal lymph nodes of patients with lung cancer. Am J Respir Crit Care Med. 2003;167:1670–1675. doi: 10.1164/rccm.200211-1297OC. [DOI] [PubMed] [Google Scholar]
- 119.Pierga JY. Prognostic value of cytokeratin 19 fragment (CYFRA21-1) and cytokeratin positive cells in bone marrow samples of breast cancer patients. Int J Biol Markers. 2004;19:23–31. doi: 10.5301/jbm.2008.649. [DOI] [PubMed] [Google Scholar]
- 120.Shariat SF. Correlation of cyclooxygenase-2 expression with molecular markers, pathological features and clinical outcome of transitional cell carcinoma of the bladder. J Urol. 2003;170:985–986. doi: 10.1097/01.ju.0000080401.85145.ee. [DOI] [PubMed] [Google Scholar]
- 121.Sakaguchi M. Clinical relevance of reverse transcriptase-polymerase chain reaction for the detection of axillary lymph node metastases in breast cancer. Ann Surg Oncol. 2003;10:117–125. doi: 10.1245/aso.2003.01.010. [DOI] [PubMed] [Google Scholar]
- 122.Blaheta HJ. Examination of regional lymph nodes by sentinel node biopsy and molecular analysis provides new staging facilities in primary cutaneous melanoma. J Invest Dermatol. 2000;14:637–642. doi: 10.1046/j.1523-1747.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 123.Shariat SF. p53, p21, pRb, and p16 Expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22:975–977. doi: 10.1200/JCO.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 124.Corradini P. Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol. 2001;12:1693–1698. doi: 10.1023/a:1013573108945. [DOI] [PubMed] [Google Scholar]
- 125.Burchill SA. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;71:278–281. doi: 10.1038/bjc.1995.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aquino A. A novel method for monitoring response to chemotherapy based on the detection of circulating cancer cells: A case report. J Chemother. 2002;14:412–416. doi: 10.1179/joc.2002.14.4.412. [DOI] [PubMed] [Google Scholar]
- 127.Wiedswang G. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–3478. doi: 10.1200/JCO.2003.02.009. [DOI] [PubMed] [Google Scholar]
- 128.Grunewald K. Mammaglobin expression in gynecologic malignancies and malignant effusions detected by nested reverse transcriptase polymerase chain reaction. Lab Invest. 2002;82:1147–1153. doi: 10.1097/01.lab.0000027840.16064.8a. [DOI] [PubMed] [Google Scholar]
- 129.Osman I. Detection of circulating cancer cells expressing uroplakins and epidermal growth factor receptor in bladder cancer patients. Int J Cancer. 2004;111:934–939. doi: 10.1002/ijc.20366. [DOI] [PubMed] [Google Scholar]
- 130.Pelkey TJ, Frierson HF, Bruns DE. Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin Chem. 1996;42:1369–1381. [PubMed] [Google Scholar]
- 131.Raj GV, Moreno JG, Gomella LG. Utilization of polymerase chain reaction technology in the detection of solid tumors. Cancer. 1998;82:1419–1442. doi: 10.1002/(sici)1097-0142(19980415)82:8<1419::aid-cncr1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 132.Ghossein RA, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res. 1999;5:1950–1960. [PubMed] [Google Scholar]
- 133.Partridge M. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin Cancer Res. 2003;9:5287–5294. [PubMed] [Google Scholar]
- 134.Palmieri G. Prognostic value of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction. J Clin Oncol. 2003;21:767–773. doi: 10.1200/JCO.2003.01.128. [DOI] [PubMed] [Google Scholar]
- 135.SM Li. Detection of circulating uroplakin positive cells in patients with transitional cell carcinoma of the bladder. J Urol. 1999;162:931–935. doi: 10.1097/00005392-199909010-00093. [DOI] [PubMed] [Google Scholar]
- 136.Body JJ. Metastatic bone disease: Clinical and therapeutic aspects. Bone. 1992;13(Suppl 1):S57–S62. doi: 10.1016/s8756-3282(09)80011-2. [DOI] [PubMed] [Google Scholar]
- 137.Osborne MP, Rosen PP. Detection and management of bone marrow micrometastases in breast cancer. Oncology (Huntingt) 1994;8:25–31. [PubMed] [Google Scholar]
- 138.Gerber B. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol. 2001;19:960–971. doi: 10.1200/JCO.2001.19.4.960. [DOI] [PubMed] [Google Scholar]
- 139.Gebauer G. Epithelial cells in bone marrow of breast cancer patients at the time of primary surgery: Clinical outcome during long-term follow-up. J Clin Oncol. 2001;19:3669–3674. doi: 10.1200/JCO.2001.19.16.3669. [DOI] [PubMed] [Google Scholar]
- 140.Janni W. The fate and prognostic value of occult metastatic cells in the bone marrow of patients with breast carcinoma between primary treatment and recurrence. Cancer. 2001;92:46–53. doi: 10.1002/1097-0142(20010701)92:1<46::aid-cncr1290>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 141.Naume B. The prognostic value of isolated tumor cells in bone marrow in breast cancer patients: Evaluation of morphological categories and the number of clinically significant cells. Clin Cancer Res. 2004;10:3091–3097. doi: 10.1158/1078-0432.ccr-03-0373. [DOI] [PubMed] [Google Scholar]
- 142.Wiedswang G. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342–5348. doi: 10.1158/1078-0432.CCR-04-0245. [DOI] [PubMed] [Google Scholar]
- 143.Braun S. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 144.Braun S. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 145.Diel IJ. Micrometastatic breast cancer cells in bone marrow at primary surgery: Prognostic value in comparison with nodal status. J Natl Cancer Inst. 1996;88:1652–1658. doi: 10.1093/jnci/88.22.1652. [DOI] [PubMed] [Google Scholar]
- 146.Mansi JL. Outcome of primary-breast-cancer patients with micrometastases: A long-term follow-up study. Lancet. 1999;354:197–202. doi: 10.1016/s0140-6736(98)10175-7. [DOI] [PubMed] [Google Scholar]
- 147.Pantel K. Frequency and prognostic significance of isolated tumor cells in bone marrow of patients with non-small cell lung cancer without overt metastases. Lancet. 1996;347:649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 148.Passlick B. Isolated tumor cells in bone marrow predict reduced survival in node-negative non-small cell lung cancer. Ann Thorac Surg. 1999;68:2053–2058. doi: 10.1016/s0003-4975(99)01125-x. [DOI] [PubMed] [Google Scholar]
- 149.Lindemann F. Prognostic significance of micrometastatic tumor cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- 150.Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 151.Cristofanilli M. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Attard G, Adjei A. Potential applications for circulating tumor cells expressing the insulin-like growth factor-1 receptor. Clin Cancer Res. 2007;13:3611–3616. doi: 10.1158/1078-0432.CCR-07-0268. [DOI] [PubMed] [Google Scholar]
- 152.Grunewald K. Mammaglobin gene expression: A superior marker of breast cancer cells in peripheral blood in comparison to epidermal-growth-factor receptor and cytokeratin-19. Lab Invest. 2000;80:1071–1077. doi: 10.1038/labinvest.3780112. [DOI] [PubMed] [Google Scholar]
- 153.XY An. Clinical significance of circulating cancer cells in peripheral blood detected by reverse transcriptase-polymerase chain reaction in patients with breast cancer. Tohoku J Exp Med. 2001;193:153–162. doi: 10.1620/tjem.193.153. [DOI] [PubMed] [Google Scholar]
- 154.Stathopoulou A. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: Evaluation of their prognostic significance. J Clin Oncol. 2002;20:3404–3412. doi: 10.1200/JCO.2002.08.135. [DOI] [PubMed] [Google Scholar]
- 155.Sardi I. The role of the detection of hematogenous micrometastasis in prostate adenocarcinoma and malignant melanoma by RT-PCR. Int J Mol Med. 1999;3:417–419. doi: 10.3892/ijmm.3.4.417. [DOI] [PubMed] [Google Scholar]
- 156.Faye RS. Immunomagnetic detection and clinical significance of micrometastatic tumor cells in malignant melanoma patients. Clin Cancer Res. 2004;10:4134–4139. doi: 10.1158/1078-0432.CCR-03-0408. [DOI] [PubMed] [Google Scholar]
- 157.Miura M. Real-time PCR (TaqMan PCR) quantification of carcinoembryonic antigen (CEA) mRNA in the peripheral blood of colorectal cancer patients. Anticancer Res. 2003;23:1271–1276. [PubMed] [Google Scholar]
- 158.Silva JM. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut. 2002;50:530–534. doi: 10.1136/gut.50.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Normanno N. Apolipoprotein A-I reverse transcriptase-polymerase chain reaction analysis for detection of hematogenous colon cancer dissemination. Int J Oncol. 1998;13:443–447. doi: 10.3892/ijo.13.3.443. [DOI] [PubMed] [Google Scholar]
- 160.Masson D, Denis MG, Lustenberger P. Limitations of CD44v6 amplification for the detection of tumour cells in the blood of colorectal cancer patients. Br J Cancer. 2000;82:1283–1289. doi: 10.1054/bjoc.1999.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghossein RA, Bhattacharya S, Coit DG. Reverse transcriptase polymerase chain reaction (RT-PCR) detection of melanoma related transcripts in peripheral blood and bone marrow of patients with malignant melanoma. Recent Results Cancer Res. 2001;158:63–77. doi: 10.1007/978-3-642-59537-0_7. [DOI] [PubMed] [Google Scholar]
- 162.Buzaid AC, Balch CM. Polymerase chain reaction for selection of melanoma in peripheral blood: Too early to assess clinical value. J Natl Cancer Inst. 1996;88:569–570. doi: 10.1093/jnci/88.9.569. [DOI] [PubMed] [Google Scholar]
- 163.Apostolikas N, Patraki C, Agnantis NJ. The reliability of histologically negative axillary lymph nodes in breast cancer. Pathol Res Pract. 1989;184:35–38. doi: 10.1016/S0344-0338(88)80188-2. [DOI] [PubMed] [Google Scholar]
- 164.Neville AM. Axillary lymph node micrometastases and breast cancer. Lancet. 1991;337:110. doi: 10.1016/0140-6736(91)91768-p. [DOI] [PubMed] [Google Scholar]
- 165.Elson CE, Kufe D, Johnson WW. Immunohistochemical detection and significance of axillary lymph node micrometastases in breast cancer—a study of 97 cases. Anal Quant Cytol Histol. 1993;15:171–178. [PubMed] [Google Scholar]
- 166.Byrne J. The use of monoclonal antibodies for the histologic detectioon of mammary axillary micrometastases. Eur J Surg Oncol. 1987;13:409. [PubMed] [Google Scholar]
- 167.Bussolati G. The immmunohistochemical detection of lymph node micrometastases from infiltrating lobular carcinoma of the breast. Br J Cancer. 1986;54:631–636. doi: 10.1038/bjc.1986.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cote RJ. Role of immunohistochemistry detection of lymph-node metastases in management of breast cancer. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 169.Kuijt GP. The prognostic significance of axillary lymph-node micrometastases in breast cancer patient. Eur J Surg Oncol. 2005;31:500–505. doi: 10.1016/j.ejso.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 170.Mullenix PS. The association of cytokeratin-only-positive sentinel lymph nodes and subsequent metastases in breast cancer. Am J Surg. 2005;189:606–609. doi: 10.1016/j.amjsurg.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 171.Passlick B. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small cell lung cancer. J Clin Oncol. 1994;12:1827–1832. doi: 10.1200/JCO.1994.12.9.1827. [DOI] [PubMed] [Google Scholar]
- 172.Kawano R. Micrometastasis to lymph nodes in stage I left lung cancer patients. Ann Thorac Surg. 2002;73:1558–1562. doi: 10.1016/s0003-4975(02)03398-2. [DOI] [PubMed] [Google Scholar]
- 173.Greenson JK. Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratins and CC49. Cancer. 1994;73:183–187. doi: 10.1002/1097-0142(19940201)73:3<563::aid-cncr2820730311>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 174.Palma RT. Micrometastasis in regional lymph nodes of extirpated colorectal carcinoma: Immunohistochemical study using anti-cytokreratin antibodies AE1/AE3. Colorectal Dig. 2003;5:164–168. doi: 10.1046/j.1463-1318.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- 175.Giuliano AR. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 176.Slamon DJ. Studies of the Her-2/neu protooncogene in human breast and ovarian cancer. Science. 1989;244:707. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 177.Riviere A, Becker J, Loening T. Comparative investigation of c-erbB2/neu expression in head and neck and mammary cancer. Cancer. 1991;67:142. doi: 10.1002/1097-0142(19910415)67:8<2142::aid-cncr2820670823>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 178.Stenman G. Expression of the erbB2 protein in benign and malignant salivary gland tumors. Genes Chromosomes Cancer. 1991;3:128. doi: 10.1002/gcc.2870030208. [DOI] [PubMed] [Google Scholar]
- 179.Ware JL. Immunohistochemical detection of c-erbB-2 protein in human benign and neoplastic prostate. Hum Pathol. 1991;22:254. doi: 10.1016/0046-8177(91)90159-m. [DOI] [PubMed] [Google Scholar]
- 180.McCann A. c-erb B-2 Oncoprotein expression in primary human tumors. Cancer. 1990;65:88. doi: 10.1002/1097-0142(19900101)65:1<88::aid-cncr2820650119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 181.Yu D. Enhanced c-erbB-2neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by EIA. Cancer Res. 1993;53:891. [PubMed] [Google Scholar]
- 182.Sasano H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24:584. doi: 10.1016/0046-8177(93)90236-a. [DOI] [PubMed] [Google Scholar]
- 183.Press MF. Her-2/neu expression in node-negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960. [PubMed] [Google Scholar]
- 184.Schneider PM. Differential expression of the c-erbB-2 gene in human small cell lung cancer. Cancer Res. 1989;49:4968. [PubMed] [Google Scholar]
- 185.Weiner DB. Expression of the neu gene-encoded protein (p185neu) in human non-small cell carcinoma of the lung. Cancer Res. 1990;50:421. [PubMed] [Google Scholar]
- 186.D’Emilia J. Expression of the c-erbB-2 gene product (p185) at different stages of neoplastic progression in the colon. Oncogene. 1989;4:1233. [PubMed] [Google Scholar]
- 187.Ross JS, Fletcher JA. Her-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999;112(Suppl 1):S53–S67. [PubMed] [Google Scholar]
- 188.Wright C. Expression of c-erbB-2 oncoprotein: A prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087. [PubMed] [Google Scholar]
- 189.Sjogren S. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 190.Burke HB. Predicting response to adjuvant and radiation therapy in patients with early stage breast carcinoma. Cancer. 1998;82:874–877. doi: 10.1002/(sici)1097-0142(19980301)82:5<874::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 191.Menard S. Role of Her2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004;61:2965–2978. doi: 10.1007/s00018-004-4277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giai M. Prognostic and predictive relevance of C-erb-B2 and ras expression in node positive and negative breast cancer. Anticancer Res. 1994;14:1441–1450. [PubMed] [Google Scholar]
- 193.Leitzel K. c-erbB2 Antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995;13:1129–1135. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 194.Berns EM. Oncogene amplification and prognosis in breast cancer: Relationship with systemic treatment. Gene. 1995;159:11–18. doi: 10.1016/0378-1119(94)00534-y. [DOI] [PubMed] [Google Scholar]
- 195.Muss HB. c-erbB-2 Expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 196.Bhargava R, Lal P, Chen B. Her-2/neu and topoisomerase IIa gene amplification and protein expression in invasive breast carcinomas. Am J Clin Pathol. 2005;123:889–895. doi: 10.1309/PCFK-8YTQ-PYWD-534F. [DOI] [PubMed] [Google Scholar]
- 197.Tanner M. Topoisomerase IIa gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in Her-2/neu-amplified breast cancer: Results from the randomized Scandinavian breast group trial 9401. J Clin Oncol. 2006;24:2428–2436. doi: 10.1200/JCO.2005.02.9264. [DOI] [PubMed] [Google Scholar]
- 198.Nicholson S, Sainsbury RJC. Epidermal growth factor receptor (EGFr); results of a 6 year follow-up study in operable breast cancer with emphasis on the node negative subgroup. Br J Cancer. 1991;63:146. doi: 10.1038/bjc.1991.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yano H. Immunohistologic detection of the epidermal growth factor receptor in human esophageal squamous cell carcinoma. Cancer. 1991;67:91. doi: 10.1002/1097-0142(19910101)67:1<91::aid-cncr2820670118>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 200.Kamio T. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum Pathol. 1990;21:277. doi: 10.1016/0046-8177(90)90227-v. [DOI] [PubMed] [Google Scholar]
- 201.Neal DE. Epidermal growth factor receptors in human bladder cancer: Comparison of invasive and superficial tumours. Lancet. 1985;1:366. doi: 10.1016/s0140-6736(85)91386-8. [DOI] [PubMed] [Google Scholar]
- 202.Kanamori A. Epidermal growth factor receptors in plasma membranes of normal and diseased human thyroid glands. J Clin Endocrinol Metab. 1989;68:899. doi: 10.1210/jcem-68-5-899. [DOI] [PubMed] [Google Scholar]
- 203.Colquhoun AJ, Mellon JK. Epidermal growth factor receptor and bladder cancer. Postgrad Med J. 2002;78:584–589. doi: 10.1136/pmj.78.924.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gamboa-Dominguez A. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: A multivariate analysis using a standardized immunohisotchemical detection system. Mod Pathol. 2004;17:579–587. doi: 10.1038/modpathol.3800085. [DOI] [PubMed] [Google Scholar]
- 205.Chakravarti A. Prognostic and pathologic significance of quantitative protein expression profiling in human gliomas. Clin Cancer Res. 2001;7:2387–2395. [PubMed] [Google Scholar]
- 206.Sauer T. Demonstration of EGFR gene copy loss in colorectal carcinomas by fluorescence in situ hybridization (FISH): A surrogate marker for sensitivity to specific anti-EGFR therapy? Histopathology. 2005;47:560–564. doi: 10.1111/j.1365-2559.2005.02252.x. [DOI] [PubMed] [Google Scholar]
- 207.Nguyen DM, Schrump DS. Growth factor receptors as targets for lung cancer therapy. Semin Thorac Cardiovasc Surg. 2004;16:3–12. doi: 10.1053/j.semtcvs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 208.Vallbohmer D, Lenz HJ. Epidermal growth factor receptor as a target for chemotherapy. Clin Colorectal Cancer. 2005;5(Suppl 1):S1–S27. [PubMed] [Google Scholar]
- 209.Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005;15:520–527. doi: 10.1016/j.gde.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 210.Chatterjee SJ. Hyperphosphorylation of pRb: A mechanism for RB tumour suppressor pathway inactivation on bladder cancer. J Pathol. 2004;203:762–770. doi: 10.1002/path.1567. [DOI] [PubMed] [Google Scholar]
- 211.Cordon-Cardo C. Altered expression of the retinoblastoma gene product: Prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992;84:1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- 212.Reissmann PT. Studies of retinoblastoma gene in sarcomas. Oncogene. 1989;4:839–843. [PubMed] [Google Scholar]
- 213.Chatterjee SJ. Combined effect of p53, p21 and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–1013. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 214.H-J Xu. Absence of retinoblastoma protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991;51:2735–2739. [PubMed] [Google Scholar]
- 215.Cote RJ. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58:1090–1094. [PubMed] [Google Scholar]
- 216.Cote RJ. Genetic alterations of the p53 gene are a feature of malignant mesotheliomas. Cancer Res. 1991;51:5410–5416. erratum in Cancer Res 51:6399, 1991. [PubMed] [Google Scholar]
- 217.Esrig D. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–1264. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- 218.Hamada H. Immunohistochemical study of p53 expression in cancer tissues from patients undergoing radiation therapy. Histol Histopathol. 1995;10:611–617. [PubMed] [Google Scholar]
- 219.Funk JO. p21CIP1 Acts as a positive regulator of cyclin B through carboxy-terminal association with novel protein, CARB. J Invest Dermatol. 1999;113:431. [Google Scholar]
- 220.Rey MJ. p21WAFa/Cip1 Is associated with cyclin D1CCND1 expression and tubular differentiation but is independent of p53 overexpression in human breast carcinoma. J Pathol. 1998;184:265–271. doi: 10.1002/(SICI)1096-9896(199803)184:3<265::AID-PATH8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 221.Ikeguchi M. Expression of p53 and p21 are independent prognostic factors in patients with serosal invasion by gastric carcinoma. Dig Dis Sci. 1998;43:964–970. doi: 10.1023/a:1018862214081. [DOI] [PubMed] [Google Scholar]
- 222.Barboule N. Increased level of p21 in human ovarian tumors is associated with increased expression of cdk2, cyclin A and PCNA. Int J Cancer. 1998;76:891–896. doi: 10.1002/(sici)1097-0215(19980610)76:6<891::aid-ijc20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 223.Sinicrope FA. Loss of p21/WAF1/Cip1 protein expression accompanies progression of sporadic colorectal neoplasms but not hereditary nonpolyposis colorectal cancers. Clin Cancer Res. 1998;45:1251–1261. [PubMed] [Google Scholar]
- 224.Stein JP. Effect of p21WAF/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998;90:1072–1079. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 225.Cordon-Cardo C. Mutation of cell-cycle regulators: Biological and clinical implications for human neoplasia. Am J Pathol. 1995;147:545–560. [PMC free article] [PubMed] [Google Scholar]
- 226.Ferrando AA. Mutational analysis of human cyclin-dependent kinase inhibitor p27/kip1 in primary breast carcinomas. Hum Genet. 1996;97:91–94. doi: 10.1007/BF00218840. [DOI] [PubMed] [Google Scholar]
- 227.Ponce-Castaneda MV. p27Kip1: Chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211–1214. [PubMed] [Google Scholar]
- 228.Cote RJ. Association of p27Kip1 levels with recurrence and survival in patients with stage C prostate carcinoma. J Natl Cancer Inst. 1998;90:916–920. doi: 10.1093/jnci/90.12.916. [DOI] [PubMed] [Google Scholar]
- 229.Loda M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 230.Porter P. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone or in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 231.JB So. Expression of cell-cycle regulators p27 and cyclin E correlates with survival in gastric carcinoma patients. J Surg Res. 2000;94:56–60. doi: 10.1006/jsre.2000.5998. [DOI] [PubMed] [Google Scholar]
- 232.Sharpless NE. INK4a/ARF: A multifunctional tumor suppressor locus. Mutat Res Fund Mol Mech Mutagen. 2004;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 233.Caldas C. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27–32. doi: 10.1038/ng0994-27. erratum in Nat Genet 8:410, 1994. [DOI] [PubMed] [Google Scholar]
- 234.Hebert J. Candidate tumor-suppressor genes MTS1 (p16INK4A) and MTS2 (p15INK4B) display frequent homozygous deletions in primary cells from T- but not from B-cell lineage acute lymphoblastic leukemias. Blood. 1994;84:4038–4044. [PubMed] [Google Scholar]
- 235.Jen J. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994;54:6353–6358. [PubMed] [Google Scholar]
- 236.Mori T. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994;54:3396–3397. [PubMed] [Google Scholar]
- 237.Schmidt EE. CDKN2(p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994;54:6321–6324. [PubMed] [Google Scholar]
- 238.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 239.Li L, Yang T, Lian X. Effects of exogenous wild-type p16 gene transfection on the expression of cell cycle-related proteins in bladder cancer cell lines. Cancer Invest. 2005;23:309–315. doi: 10.1081/cnv-58815. [DOI] [PubMed] [Google Scholar]
- 240.Mihic-Probst D. p16 Expression in primary malignant melanoma is associated with prognosis and lymph node status. Int J Cancer. 2006;118:2262–2268. doi: 10.1002/ijc.21608. [DOI] [PubMed] [Google Scholar]
- 241.Gerdts J. Correlation of abnormal Rb, p16ink4a, and p53 expression with 3p loss of heterozygosity, other genetic abnormalities, and clinical features in 103 primary non-small cell lung cancer. Clin Cancer Res. 1999;5:791–800. [PubMed] [Google Scholar]
- 242.Sartor M. Role of p16/MTS1, cyclin D1 and Rb in primary oral cancer and oral cancer cell lines. Br J Cancer. 1999;80:79–86. doi: 10.1038/sj.bjc.6690505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Garcea G. Molecular prognostic markers in pancreatic cancer: A systematic review. Eur J Cancer. 2005;41:2213–2236. doi: 10.1016/j.ejca.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 244.Jensen EV. Receptors reconsidered: A 20 year perspective. Rec Progr Horm Res. 1982;38:1. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- 245.Coombes RC. Prediction of endocrine response in breast cancer by immunocytochemical detection of oestrogen receptor in fine-needle aspirates. Lancet. 1987;2:701–703. doi: 10.1016/s0140-6736(87)91071-3. [DOI] [PubMed] [Google Scholar]
- 246.Anderson J. The prognostic value of immunohistochemical estrogen receptor analysis in paraffin-embedded and frozen sections versus that of steroid binding assays. Eur J Cancer. 1990;26:442. doi: 10.1016/0277-5379(90)90013-j. [DOI] [PubMed] [Google Scholar]
- 247.Germann UA. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing multidrug resistance-associated protein MRP. Anticancer Drugs. 1997;8:141–155. doi: 10.1097/00001813-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 248.Lowe SW. p53-Dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 249.Cote RJ, Chatterjee SJ. Molecular determinants of outcome in bladder cancer. Cancer J. 1999;5:2–15. [PubMed] [Google Scholar]
- 250.Cote RJ. p53 and Treatment of bladder cancer. Nature. 1997;385:123–125. doi: 10.1038/385123b0. [DOI] [PubMed] [Google Scholar]
- 251.Chin KV. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992;255:459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- 252.Wang Q, Fan S, Eastman A. UCN-01: A potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 253.Lenz H-J. p53 and Thymidylate synthase expression in untreated stage II colon cancer: Association with recurrence, survival and site. Clin Cancer Res. 1998;4:1227–1234. [PubMed] [Google Scholar]
- 254.Lenz H-J. p53 Point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: An analysis of response and survival. Clin Cancer Res. 1998;4:1243–1250. [PubMed] [Google Scholar]
- 255.Carlomagno C. cerb-B2 Overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J Clin Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 256.Wolff AC. Current status of taxanes as adjuvant therapy for early-stage breast cancer. Int J Fertil Womens Med. 2005;50:227–229. [PubMed] [Google Scholar]
- 257.Carlson RW. NCCN task force report: Adjuvant therapy for breast cancer. J Natl Comp Cancer. Network. 2006;4(Suppl 1):S1–S26. [PubMed] [Google Scholar]
- 258.Dent R, Clemons M. Trastumab after primary treatment for early stage Her2-positive breast cancer reduces recurrence. Cancer Treat Rev. 2006;32:144–148. doi: 10.1016/j.ctrv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Amado RG, Wolf M, Peeters M. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- Zhu CQ, da Cunha Santos G, Dmg K. Role of KRAS and EGFR as biomarkers of response erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 259.Klatt EC, Cosgrove M, Meyer PR. Rapid diagnosis of disseminated histoplasmosis in tissues. Arch Pathol Lab Med. 1986;110:1173. [PubMed] [Google Scholar]
- 260.Barbolini G. Immunohistologic analysis of myobacterial antigens by monoclonal antibodies in tuberculosis and mycobacteriosis. Hum Pathol. 1989;20:1078. doi: 10.1016/0046-8177(89)90226-8. [DOI] [PubMed] [Google Scholar]
- 261.Cartun RW. Identification of CMV in formalin-fixed, paraffin-embedded tissues: Comparison of immunocytochemistry and in situ DNA hybridization. Mod Pathol. 1989;2:15A. [Google Scholar]
- 262.Jiwa M. Three sensitive methods for the detection of cytomegalovirus in lung cancer tissue of patients with interstitial pneumonitis. Am J Clin Pathol. 1990;93:491. doi: 10.1093/ajcp/93.4.491. [DOI] [PubMed] [Google Scholar]
- 263.Cote RJ. Disseminated Pneumocystis carinii infection causing extrapulmonary organ failure: Clinical, pathologic and immunohistochemical analysis. Mod Pathol. 1990;3:25–30. [PubMed] [Google Scholar]
- 264.Burmer GC. Comparative analysis of human papillomavirus detection of polymerase chain reaction and Virapap/Viratype kits. Am J Clin Pathol. 1990;94:554. doi: 10.1093/ajcp/94.5.554. [DOI] [PubMed] [Google Scholar]
- 265.Cartun RW. Immunocytochemical identification of Helicobacter pylori in formalin-fixed gastric biopsies. Mod Pathol. 1991;4:498. [PubMed] [Google Scholar]
- 266.Felix JC, Wright TC. Analysis of lower genital tract and lesions suspicious for condyloma by in situ hybridization and consensus sequence PCR for the detection of HPV. Arch Pathol Lab Med. 1994;118:39. [PubMed] [Google Scholar]
- 267.Chehter EZ. Involvement of the pancreas in AIDS: A prospective study of 109 post-mortems. AIDS. 2000;14:1879–1886. doi: 10.1097/00002030-200009080-00001. [DOI] [PubMed] [Google Scholar]
- 268.Tamiokis D. Human decidual cells activity in women with spontaneous abortions of probable CMV aetiology during the first trimester of gestation: An immunohistochemical study with CMV-associated antigen. Acta Medica. 2004;47:195–199. [PubMed] [Google Scholar]
- 269.Morinaka S, Tominaga M, Nakamura H. Detection of Helicobacter pylori in the middle ear fluid of patients with otitis media with effusion. Otolaryngol Head Neck Surg. 2005;133:791–794. doi: 10.1016/j.otohns.2005.05.050. [DOI] [PubMed] [Google Scholar]


