Abstract
Transient receptor potential melastatin 2 (TRPM2) is a non-selective cation channel that allows Ca2+ influx across the plasma membrane and efflux from lysosomes upon opening. TRPM2 is best known as a biosensor of reactive oxygen species (ROS), which mediates some of the body’s responses to oxidative stress. As such, TRPM2 is involved in a plethora of biological processes including immune response, insulin secretion, body temperature control and neuronal cell death, and represents an emerging therapeutic target for many human diseases, from diabetes to inflammatory and neurodegenerative diseases. A direct ligand of TRPM2 is ADP-ribose (ADPR), which accumulates in cells at high levels of ROS, and activates TRPM2 synergistically with intracellular calcium (Ca2+). Here, we describe recent cryo-electron microscopy (cryo-EM) structures of TRPM2 and summarize the insights they provided into the gating mechanism of the channel.
Keywords: ADPR, calcium, cryo-EM, gating, ion channel, structure, TRP channel, TRPM2
Graphical Abstract
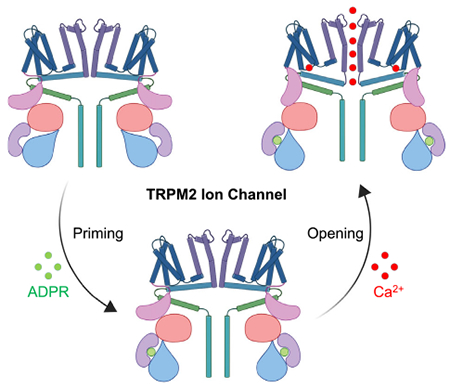
Transient receptor potential melastatin 2 (TRPM2) is a non-selective cation channel gated by ADP-ribose (ADPR) and calcium (Ca2+). A recent structural study revealed the gating mechanism of human TRPM2, with priming by ADPR as the first step followed by Ca2+-induced channel opening. The human TRPM2 structures, together with the structures of its zebrafish and sea anemone homologs, provided an in-depth mechanistic picture of TRPM2 channel gating across species.
Introduction
The transient receptor potential melastatin (TRPM) channels belong to the TRP channel superfamily of non-selective cation channels. TRP channels carry out diverse physiological functions and are known to respond to noxious stimuli [1,2]. For instance, TRPV1 responds to capsaicin and TRPM8 senses cold. Our knowledge of the gating mechanisms of TRP channels has been greatly advanced by recent structural studies, especially on TRPV channels [3,4]. Compared to the extensively studied TRPV channels, TRPMs have larger molecular weights and more complex folds, which pose greater challenges to their structural investigations. In human, there are eight TRPMs, among which TRPM2, TRPM6, and TRPM7 contain additional C-terminal enzyme or enzyme-like domains that regulate channel opening. More specifically, TRPM6 and TRPM7 have a kinase domain, and TRPM2 contains a NUDT9 homology (NUDT9H) domain [5–8].
Transient receptor potential melastatin 2 is widely expressed in the nervous and immune system and plays vital roles in numerous physiological responses to reactive oxygen species (ROS), including insulin secretion, inflammatory cytokine production, and body temperature maintenance [9–11]. Dysregulation in TRPM2 activation has been associated with many diseases such as diabetes, cancer, inflammatory and neurodegenerative diseases, and cardiac or renal ischemia–reperfusion injury [12–16]. Oxidative stress results in an increased cellular level of ADP-ribose (ADPR), which, together with intracellular calcium (Ca2+), can synergistically activate the channel. How TRPM2 is gated by ADPR and Ca2+ at the molecular level has been a long-standing question in the field.
In the past few years, cryo-electron microscopy (cryo-EM) structures of TRPM4 and TRPM8 shed light on the general architecture of the TRPM family [17–20]. However, the structures of these channels were obtained in closed, inactive states, leaving their gating mechanisms unclear. Recently, structures of human (hs), zebrafish (dr), and sea anemone (nv) TRPM2 in different conformational states were solved, from which gating mechanisms of TRPM2 have been proposed [21–24]. Together, these TRPM2 structures not only provide an understanding on species-specific contributions of the NUDT9H domain to channel activation, but also highlight an allosteric propagation mechanism that integrates cytoplasmic signals and local conformational changes at the transmembrane (TM) region to enlarge the ion permeation pore.
Overall architecture of human TRPM2
The assembled human TRPM2 (hsTRPM2) channel is a tetramer of the multi-domain subunit with dimensions of approximately 100 Å by 100 Å by 150 Å (Fig. 1A,B) [21]. Each monomer consists of an N-terminal TRPM-homology region (MHR) arm formed by MHR1/2, MHR3, and MHR4 domains, followed by a transmembrane (TM) region composed of the S1-S4 voltage sensor-like domain (VSLD) and the S5–S6 pore domain. Immediately after the TM region are the TRP helices H1 and H2, a rib helix, and a pole helix. These are then followed by the unique NUDT9H domain at the very C-terminus (Fig. 1A,C). The whole structure features a three-tier domain organization, with MHR1/2/3, the pole helix, and NUDT9H forming the bottom or most intracellular tier. The middle tier contains MHR4 and the rib helix. The top tier consists of pre-S1, the TM domain, and the TRP helices (Fig. 1A,B).
Fig. 1.
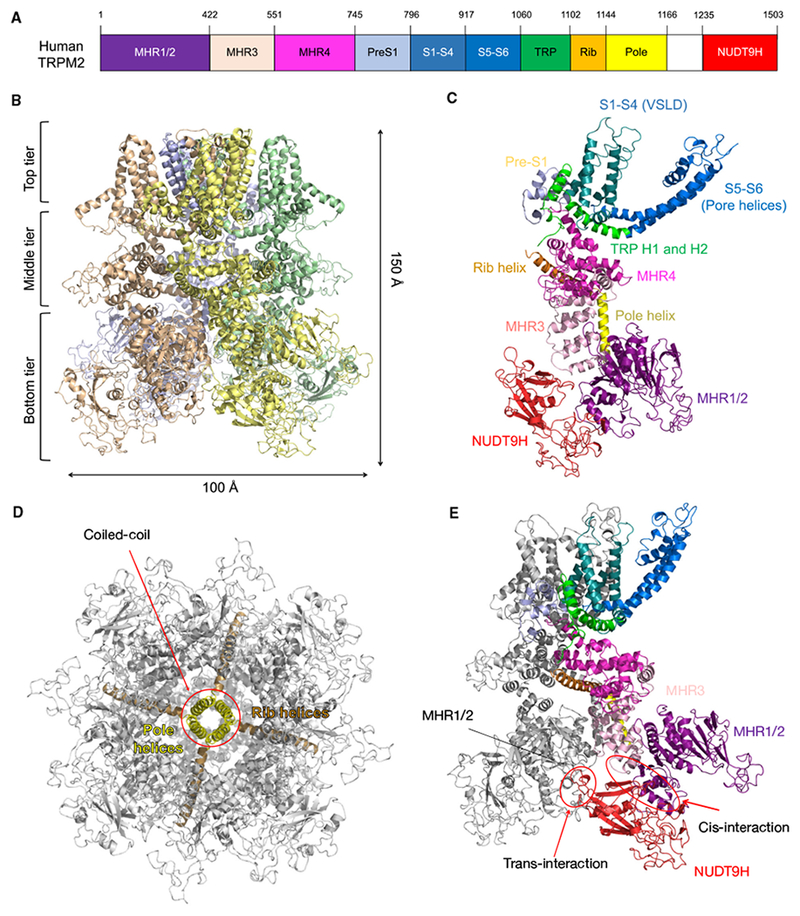
Apo-state structure of human TRPM2. (A) Domain arrangement of human TRPM2. (B) Ribbon diagram, dimensions, and three-tier architecture of human TRPM2. The four subunits are colored differently. (C) One subunit from the apo-state TRPM2 with domains colored individually. (D) The rib and pole helices form a central scaffold to support the TRPM2 channel. (E) The NUDT9H domain mediates extensive interactions with MHR domains both in cis and in trans.
Characteristic of the TM region of TRP channels, the VSLD of one subunit interacts with the S5–S6 pore domain of a neighboring subunit in a domain-swapped manner. The rib and pole helices form a scaffold that supports the channel, with the pole helices intertwined into a coiled-coil that resembles a central spine (Fig. 1D). Besides these conserved features, a notable characteristic of apo hsTRPM2 is that the C-terminal NUDT9H domain, rather than hanging flexibly at the bottom tier, forms extensive interactions with the N-terminal MHR arm both of its own subunit (in cis) and of a neighboring subunit (in trans) (Fig. 1E). The trans-interaction, mediated by the P-loop of NUDT9H and MHR1/2 of the neighboring subunit, appears to lock the channel in an inactive state by restraining subunit movements in the absence of ligands.
Molecular mechanism of human TRPM2 opening
Although NUDT9H directly binds ADPR and is required for human TRPM2 co-activation by ADPR and Ca2+, it is the S5–S6 pore domain that controls the pore size and the ion permeation pathway. Considering the spatial separation between NUDT9H and the pore domain, there likely exists an allosteric signal propagation mechanism. To uncover the gating mechanism of hsTRPM2, Wang et al. obtained the cryo-EM structures of TRPM2 in complex with ADPR (primed state) and with both ADPR and Ca2+ (open state) in addition to the apo-state structure [21]. Viewing from the intracellular side, MHR1/2 and NUDT9H undergo a dramatic 27° counterclockwise rotation from the apo state to the primed state (Fig. 2). A consequence of this large rotation at the bottom tier is that the trans-interaction mediated by the P-loop of NUDT9H is abolished to allow further conformational changes upon Ca2+ binding, hence the name ‘primed state’.
Fig. 2.
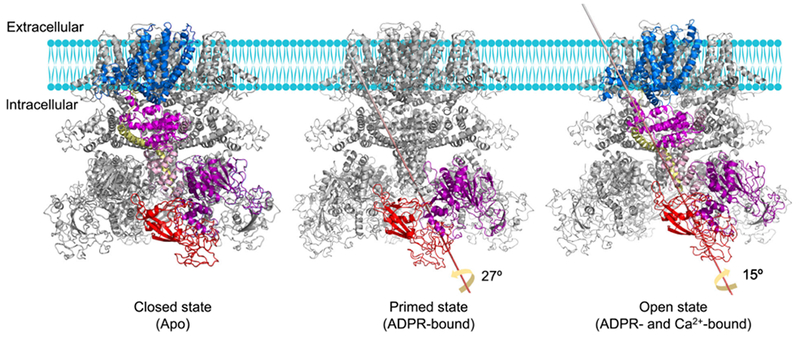
Molecular mechanism of human TRPM2 gating. In the apo state, NUDT9H mediates trans-interactions with the MHR1/2 domain, which may lock the channel in a closed conformation by limiting the rotation of the MHR arm. Upon ADPR engagement, MHR1/2 and NUDT9H undergo a large rotation, thereby disrupting the trans-interaction to prime the channel for opening. When both Ca2+ and ADPR bind, rotation of the cytosolic domains and movement of the TM region are integrated by TRP helix and then propagated to the S6 helix, leading to the enlargement of lower gate to increase the opening probability of the channel.
With ADPR binding to prime the channel, the Ca2+ ion bound at the top tier of the channel is not only coordinated by S2 and S3 of VSLD, but also by TRP helix H1, leading to a tilt at H1 in comparison to the apo and primed states. TRP H1 was here defined as an allosteric center due to its interaction with VSLD, close association with MHR4, and direct connection to the pore gating helix S6 via a bent junction. This strategic location of TRP H1 links the cytosolic and TM domains of the channel and propagates conformational changes initiated at the Ca2+-binding site. Therefore, in addition to inducing a tilt at TRP H1 to drag S6 to enlarge the pore for ion flux, Ca2+ binding allosterically elicits a 15° clockwise rotation in the cytosolic region by TRP H1-MHR coupling (Fig. 2).
In summary, the conformational changes that enable the hsTRPM2 channel opening likely take place by allosteric propagation, from ADPR-triggered disruption of the trans-interaction mediated by NUDT9H, to the Ca2+-induced tilt at TRP H1, and finally to the movement of the S6 pore gating helix. It is worth noting that although this mechanistic model depicts ADPR and Ca2+ binding as sequential events for simplicity, they may occur simultaneously and prime each other to share the energetic cost required for the large conformational changes during channel opening.
Structures and gating mechanisms of TRPM2 in other species
Other than hsTRPM2, structures of zebrafish TRPM2 (drTRPM2) and sea anemone TRPM2 (nvTRPM2) have been solved, revealing a gating mechanism similar to hsTRPM2 with some important differences [22–24]. The structure of nvTRPM2 was determined in complex with Ca2+, while the structures of drTRPM2 contain states – the apo state, in complex with Ca2+, and in complex with Ca2+ and ADPR. Many mechanistic details of channel opening are shared between hsTRPM2 and drTRPM2, including the rotation of the cytosolic MHR arm, the movement of the TM region, and Ca2+ coordination by the TRP helix H1. On the other hand, comparison of these TRPM2 structures reveals several species-specific features.
Calcium-binding sites
Structures of drTRPM2 and nvTRPM2 in complex with Ca2+ show that when ADPR is absent, the channels are in closed conformations. In both structures, Ca2+ is coordinated by residues in VSLD helices S2 and S3 (Fig. 3A) [23,24]. However, symmetry differs between the two Ca2+-bound structures, with nvTRPM2 adopting a fourfold symmetric architecture whereas drTRPM2 a twofold symmetric intermediate state obvious in the MHR1/2 and MHR3 domains. Strikingly, upon the engagement of ADPR, the Ca2+-binding site moves toward the TRP helix H1, shown by the open-state structures of hsTRPM2 and drTRPM2 (Fig. 3B) [21,22]. The consequence is that Ca2+ is not only coordinated by the S2 and S3 helices, but also by the TRP H1. This interaction with TRP H1 turns out to be a critical step toward channel opening.
Fig. 3.
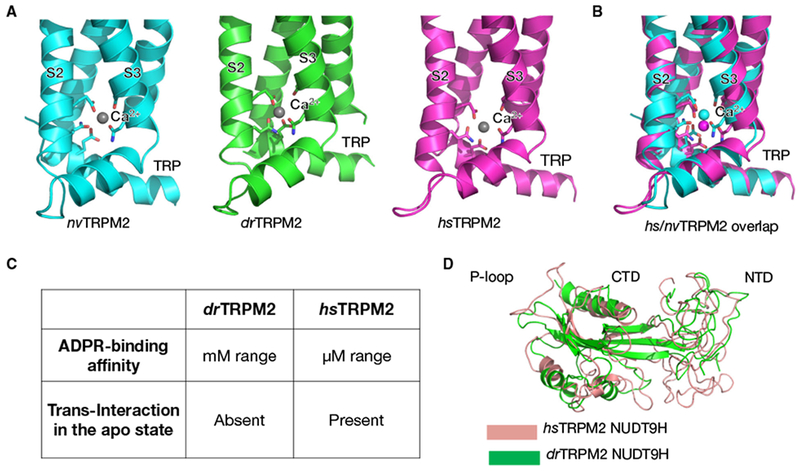
Comparison of TRPM2 gating across species. (A) Ca2+-binding sites of sea anemone (nv), zebrafish (dr), and human (hs) TRPM2. (B) Overlay of the Ca2+-binding sites of hsTRPM2 and nvTRPM2. (C) Comparison of the NUDT9H domains of drTRPM2 and hsTRPM2. (D) Overlap of the NUDT9H domains of drTRPM2 and hsTRPM2.
ADPR-binding sites
The relatively low-resolution structures of hsTRPM2 in primed and open states did not resolve the bound ADPR. However, binding measurements confirmed that the NUDT9H domain in hsTRPM2 directly associates with ADPR with an affinity of around 15 μM, and the putative binding site locates at the cleft between the N-terminal domain (NTD) and C-terminal domain (CTD) of NUDT9H (Fig. 3C) [21]. In contrast, the MHR1/2 domain, not the NUDT9H domain, was shown to bind ADPR in drTRPM2 [22]. Two conserved Arg residues were identified to be critical for ADPR engagement and channel gating in zebrafish TRPM2. However, mutation of hsTRPM2 residues equivalent to the ADPR-binding residues in drTRPM2 did not substantially compromise channel opening, suggesting a notable difference in ADPR sensing between the two species [21,22].
Comparison of NUDT9H
The positioning of and interactions formed by NUDT9H also exhibit evolutionary divergence. In hsTRPM2, the P-loop of NUDT9H is responsible for contacting MHR1/2 in trans to lock adjacent subunits in place and restrict rotational movement in the absence of ADPR and Ca2+. Comparatively, the P-loop is deleted in drTRPM2 NUDT9H, hence the lack of trans-interaction in the apo-state structure (Fig. 3C, D). In addition, it has been shown that the enzymatic activity of NUDT9H in TRPM2 varies across species [25,26]. While the NUDT9H domain of nvTRPM2 degrades ADPR, hsTRPM2 NUDT9H binds ADPR but lacks the hydrolase activity, putatively due to the loss of two glutamate residues that catalyze the reaction. While the physiological relevance of these species-specific NUDT9H features is unclear, they reflect mechanistic complexity in TRPM2 gating and merit further study.
Concluding remarks and future perspectives
Recent cryo-EM structures of TRPM2 provide insights into the gating mechanism of the channel. First, in hsTRPM2, the unique C-terminal NUDT9H domain mediates trans-interaction with a neighboring subunit to lock the channel in a closed conformation in the absence of ADPR and Ca2+. Second, the binding of ADPR and Ca2+ triggers the dramatic conformational changes that lead to channel opening, including large rotations at the MHR arm and local movements in the TM region. Third, the TRP H1 helix serves as an allosteric center responsible for integrating structural signals in the TM region and the cytosolic domains. The hsTRPM2 structures, together with the structures of TRPM2 in other species, have broadened our understanding of this functionally important channel and allow us to make new hypotheses for how TRPM2 is gated by other factors. For example, the opening of TRPM2 is also regulated by PIP2, temperature, and calmodulin [7,10,27]. We speculate that these factors may also modulate the opening of TRPM2 via conformational changes that are similar to what we have observed during the channel opening by ADPR and Ca2+. Further study to address these questions will surely advance our understanding on the gating mechanisms of TRP channels.
Acknowledgements
We thank H. Wei at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, and K. Song and C. Xu at the University of Massachusetts Cryo-EM Core Facility for their technical help and discussions, and BioRender for figure design.
Abbreviations
- ADPR
ADP-ribose
- Ca2+
calcium
- cryo-EM
cryo-electron microscopy
- CTD
C-terminal domain
- dr
zebrafish
- hs
human
- NTD
N-terminal domain
- NUDT9H
NUDT9 homology
- nv
sea anemone
- ROS
reactive oxygen species
- TRPM
transient receptor potential melastatin
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Clapham DE (2003) TRP channels as cellular sensors. Nature 426, 517–524. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey IS, Delling M & Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68, 619–647. [DOI] [PubMed] [Google Scholar]
- 3.Cao E, Liao M, Cheng Y & Julius D (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao M, Cao E, Julius D & Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, Abiria SA, Krapivinsky G, Zhang J & Clapham DE (2018) Structure of the mammalian TRPM7, amagnesium channel required during embryonic development. Proc Natl Acad Sci USA 115, E8201–E8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Jiang J & Yue L (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattkowski E, Johnsen A, Bauche A, Mockl F, Kulow F, Garcia Alai M, Rutherford TJ, Fliegert R & Tidow H (2018) Novel CaM-binding motif in its NudT9H domain contributes to temperature sensitivity of TRPM2. Biochim Biophys Acta Mol Cell Res 1866, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG & Perraud AL (2005) The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem 280, 37763–37771. [DOI] [PubMed] [Google Scholar]
- 9.Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J et al. (2005) Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280, 6138–6148. [DOI] [PubMed] [Google Scholar]
- 10.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y & Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25, 1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS & Qiao L (2013) TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 4, 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T et al. (2008) TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida K & Tominaga M (2014) The role of TRPM2 in pancreatic beta-cells and the development of diabetes. Cell Calcium 56, 332–339. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Sikka SC, Huang L, Sun C, Xu C, Jia D, Abdel-Mageed AB, Pottle JE, Taylor JT & Li M (2010) Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis 13, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi N, Kozai D, Kobayashi R, Ebert M & Mori Y (2011) Roles of TRPM2 in oxidative stress. Cell Calcium 50, 279–287. [DOI] [PubMed] [Google Scholar]
- 16.Miller BA & Cheung JY (2016) TRPM2 protects against tissue damage following oxidative stress and ischaemia-reperfusion. J Physiol 594, 4181–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autzen HE, Myasnikov AG, Campbell MG, Asarnow D, Julius D & Cheng Y (2018) Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan J, Li Z, Li J, Santa-Cruz A, Sanchez-Martinez S, Zhang J & Clapham DE (2018) Structure of full-length human TRPM4. Proc Natl Acad Sci USA 115, 2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler PA, Huang Y, Sun W, Du J & Lu W (2017) Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552, 200–204. [DOI] [PubMed] [Google Scholar]
- 20.Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC & Lee SY (2018) Structure of the cold- and mentholsensing ion channel TRPM8. Science 359, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Fu TM, Zhou Y, Xia S, Greka A & Wu H (2018) Structures and gating mechanism of human TRPM2. Science 362, eaav4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Winkler PA, Sun W, Lu W & Du J (2018) Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Toth B, Szollosi A, Chen J & Csanady L (2018) Structure of a TRPM2 channel in complex with Ca(2+) explains unique gating regulation. Elife. 7, e36409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Y, Wu M, Hsu A, Borschel W, Borgnia M, Lander G & Lee SY (2019) Visualizing structural transitions of ligand-dependent gating of the TRPM2 channel. bioRxiv, 516468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perraud AL, Shen B, Dunn CA, Rippe K, Smith MK, Bessman MJ, Stoddard BL & Scharenberg AM (2003)NUDT9, a member of the Nudix hydrolase family, is an evolutionarily conserved mitochondrial ADP-ribose pyrophosphatase. J Biol Chem 278, 1794–1801. [DOI] [PubMed] [Google Scholar]
- 26.Shen BW, Perraud AL, Scharenberg A & Stoddard BL (2003) The crystal structure and mutational analysis of human NUDT9. J Mol Biol 332, 385–398. [DOI] [PubMed] [Google Scholar]
- 27.Toth B & Csanady L (2012) Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents. Proc Natl Acad Sci USA 109, 13440–13445. [DOI] [PMC free article] [PubMed] [Google Scholar]


