Abstract
The immune system is highly receptive to endocrine signals due to the expression of hormone receptors on immune cells. The impact of this immune–endocrine cross talk and related immune responses becomes clearly evident when assessing immunity from a sex-specific perspective. We here describe the effect of hormones, primarily sex- and stress-related steroid hormones, on cells of the innate and adaptive immune system in men and women. We specify how these effects are operational throughout the life span and also during periods of dramatic hormonal changes, such as pregnancy.
Keywords: Aging, Endocrine–immune interaction, Estrogens, Glucocorticoid, Immune privilege, Immunity, Menstrual cycle, Pregnancy, Progesterone, Sex disparity, Steroid hormone, Steroids, Testosterone
Glossary
- Immune privilege
Sites of tolerance in the body where no immune response is elicited to otherwise highly immunogenic antigens, for example, expressed by placental tissue–male germ cells.
- Immunity
The capacity to elicit, regulate, and terminate an immune response to eliminate foreign antigens, pathogens, or inflammatory cells, while at the same time maintaining tolerance to self-antigens.
- Immunocompetence
The ability of an individual to produce immune responses which is determined by age, sex, gender, disease, reproductive state, genetic predisposition, and environmental influences.
- Maternal immune adaptation to pregnancy
Alterations of the maternal immune response that establish and maintain tolerance to the semiallogeneic offspring, thereby ensuring fetal growth and progression of pregnancy.
- Sex disparity of immunity
Differences in immunity between female and male sex based on endocrine, genetic, or environmental predisposition.
Introduction
The immune system actively maintains immune homeostasis by preventing immune reactions against self-antigens and eliminating pathogens. Interestingly, the immune system is also highly receptive to signals from other systems of the body, such as the endocrine system. This immune–endocrine cross talk is possible due to the expression of hormone receptors on a wealth of immune cells. Vice versa, endocrine tissues have been shown to be responsive to immune mediators, such as cytokines.
The significant impact of hormones on the immune system becomes evident in the differential susceptibility to and progression of diseases that can be observed in men and women (Giefing-Kroll et al., 2015; Gubbels Bupp, 2015; Klein et al., 2010a, Klein et al., 2012), mirrored by, for example, a higher incidence of autoimmune diseases (Ackerman, 2006; Fairweather et al., 2008) and sexually transmitted infections (STIs) in females compared to males (Wira et al., 2015). These epidemiological observations suggest that respective sex-specific hormone concentrations account for the sex-specificity of immunity especially in adult humans (Figure 1 ).
Figure 1.
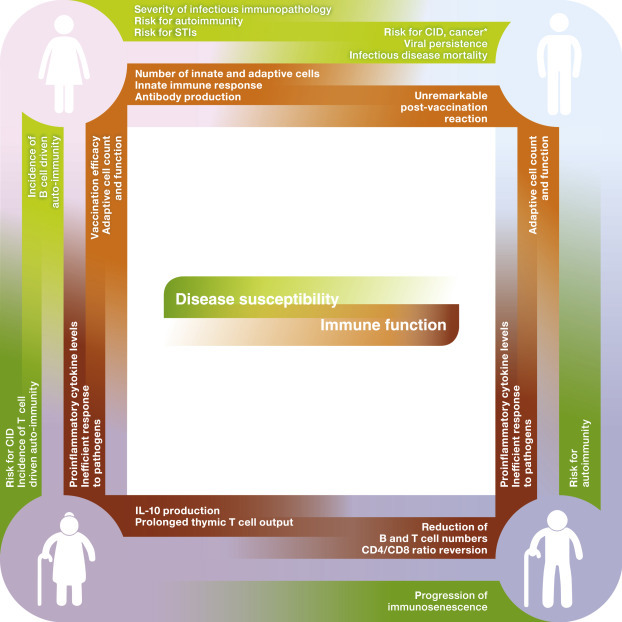
Sex disparity of immunity in adult and aged humans. Disease susceptibility and immune function in adult (30–59 years of age) and elderly (≥60 years old) men and women.∗, excluding sex-specific cancers; CID, chronic immune diseases, such as type 2 diabetes and atherosclerosis; STI, sexually transmitted infections.
Further, senescence of the endocrine system in the aging individual is paralleled by changes of the susceptibility to infectious diseases and, again, occurs differently in females and males (Giefing-Kroll et al., 2015; Gubbels Bupp, 2015). During their waning years, men show, for example, an increased mortality related to infections with human immunodeficiency virus (HIV), toxoplasmosis, and measles (Giefing-Kroll et al., 2015), while women experience a higher susceptibility and mortality to, for example, hepatitis, tetanus, leptospirosis, meningococcal, and pneumococcal infections, especially after menopause (Guerra-Silveira and Abad-Franch, 2013; Lozano et al., 2012).
Endocrine modulation of immunocompetence is not only apparent between the sexes. Intrafemale hormone levels vary tremendously throughout the reproductive cycle to ensure ovulation, fertilization, and implantation, while endocrine adaptations during pregnancy are necessary to promote successful growth of the fetus and dampen the risk of rejecting the semiallogeneic fetus. Hormonal changes occurring during the stages of the menstrual cycle as well as during pregnancy are associated with alterations in female immunocompetence.
In this article, the sex- and age-specific effects of endocrine–immune cross talk on innate, antigen-presenting, and adaptive immune cells will be highlighted. A focus is given on steroid hormones (estrogens, progesterone, testosterone, glucocorticoids (GCs)), as the effect of these hormones is best understood to date. It should be noted that immune modulation by hormones responsible for growth and metabolism (e.g., insulin (Shu et al., 2012), leptin (Procaccini et al., 2015), thyroid hormones (De Vito et al., 2011)), fluid and electrolyte balance (e.g., vasopressin and the steroid hormone aldosterone (Lastra and Sowers, 2013)), as well as norepinephrine (noradrenaline), and epinephrine (adrenaline) (Marino and Cosentino, 2013) can also affect immunity. The interested reader is referred to cited articles or related chapters in the present book.
Endocrine Modulation of Immunocompetence
Release of sex and stress steroid hormones from endocrine glands is controlled by pathways, so called axes, which originate in the brain and signal to peripheral endocrine organs of the body (Figure 2 ).
Figure 2.
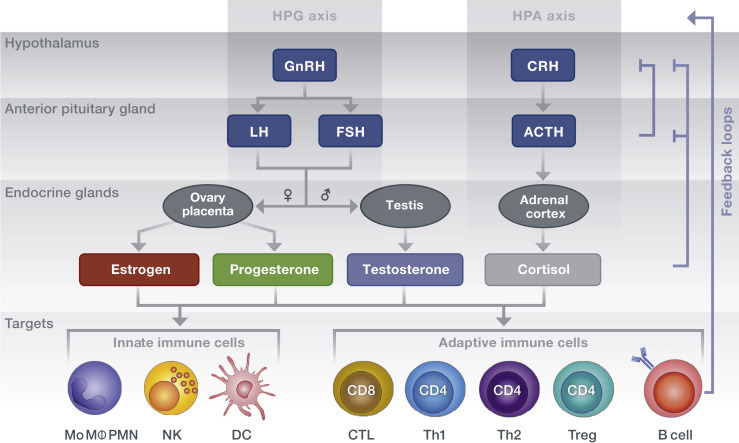
Steroid hormone secretion along the hypothalamic – anterior pituitary axes. The hypothalamus secretes releasing hormones that stimulate endocrine cells of the anterior pituitary gland to secrete trophic hormones, which in turn induce peripheral glands to secrete the sex and stress steroid hormones. Via binding to receptors expressed on immune cells, steroid hormones result in cell type–specific functional adaptations. Negative-feedback loops inhibit upstream signaling of the axes. Immune cells themselves can modulate axis activity via cytokine-mediated actions on brain glandular regions. Arrows indicate an influencing effect on downstream mediators. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; CTL, cytotoxic T lymphocyte; DC, dendritic cell; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HPA, hypothalamic–pituitary–adrenal; HPG, hypothalamic–pituitary–gonadal; LH, luteinizing hormone; Mo MΦ, monocytes/macrophages; NK, natural killer cell; PMN, polymorphonuclear leukocyte; Th, T helper cell; Treg, regulatory T cell.
Reproductive functions in females are regulated by key female sex hormones, such as estrogens and progesterone. The key male sex hormone is testosterone, which belongs to the group of androgens. In both sexes, cortisol, a GC, is the major hormone secreted in response to stress signals. The levels of these hormones are altered significantly over a human life span and differ between females and males in relation to age (Figure 3 ).
Figure 3.
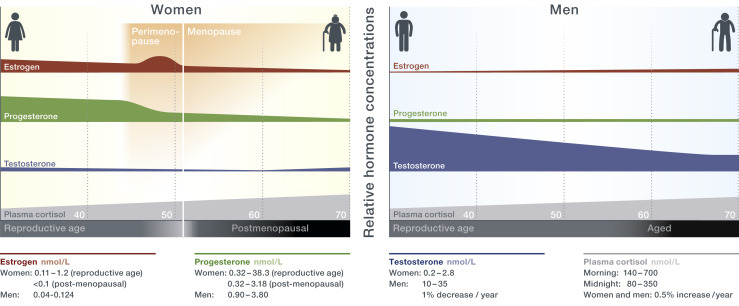
Sex-specific hormone levels during reproductive age and in aged individuals. With aging, women experience an abrupt reduction in estrogen and progesterone levels as ovaries cease to produce these steroids at menopause around their 45–55th year of age (Davison et al., 2005; Laughlin et al., 2000; Santoro et al., 1996). In males, the decline in total testosterone levels occurs progressively, with no distinct time-point of andropause (Bhasin et al., 2011; Jasuja et al., 2013; Tancredi et al., 2005). Mean glucocorticoid levels of both men and women increase linear (Van Cauter et al., 1996), while morning cortisol levels are higher in aged women than in age-matched men.
Steroid hormones bind to specific hormone receptors (which are either nuclear or membrane bound) and induce both genomic and nongenomic actions in resident and circulating immune cells (Bellavance and Rivest, 2014; Gilliver, 2010; Kovats et al., 2010). A summary of these effects on distinct cell subsets of both the innate and adaptive immune response, is depicted in Figure 4 and detailed below.
Figure 4.
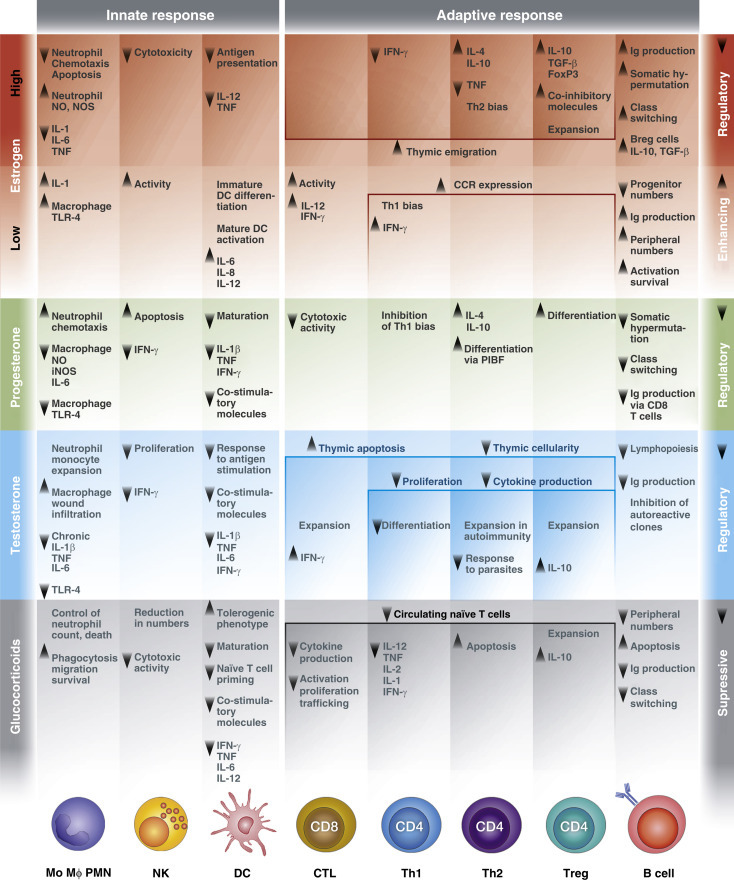
Effect of steroid hormones on cells of the adaptive and innate immune response. Arrows indicate stimulation or repression of cytokine secretion and immune cell function as well as increase or decrease of population size. CCR, CC chemokine receptor; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IFN, interferon; Ig, immunoglobulin; IL, interleukin; Mo MΦ, monocytes/macrophages; NK, natural killer cell; NO, nitric oxide; NOS, nitric oxide synthase; PIBF, progesterone-induced binding factor; PMN, polymorphonuclear leukocyte; TGF, transforming growth factor; Th, T helper cell; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cell.
Estrogens
The effect of estrogens on immune cells depends on their concentrations. Estrogen levels are considerably low during the early secretory and early postovulatory phase of the menstrual cycle as well as after menopause, whereas estrogen levels elevate to high levels during the late follicular phase prior to ovulation and further upsurge during pregnancy.
Low estrogen levels stimulate cells of the innate immune response (Keller et al., 2001; Rettew et al., 2008; Straub, 2007). Conversely, high concentrations of estrogens contribute to the repression of proinflammatory innate functions (Garcia-Duran et al., 1999; Hao et al., 2007; Harkonen and Vaananen, 2006; Miyagi et al., 1992; Molloy et al., 2003) and suppress efficient dendritic cell (DC) antigen presentation (Beagley and Gockel, 2003; Liu et al., 2002).
Estrogens also affect the adaptive immune response, for example, at the primary site of T cell generation in the thymus. Here, estrogens can induce thymic atrophy (Staples et al., 1999), increase the generation of mature CD4+ T cells (Tanriverdi et al., 2003), and their emigration (Pido-Lopez et al., 2001). One striking estrogen-mediated feature is the concentration-dependent effect on CD4+ T helper (Th) cell polarization: low concentrations of estrogens are paralleled by Th1 cell proliferation, cell-mediated immunity, and interferon (IFN)-γ as well as antibody production (Pennell et al., 2012; Pernis, 2007). This response is of rather proinflammatory nature and accompanied by increased cytotoxic CD8+ T lymphocyte (CTL) activity (White et al., 1997). Conversely, high-estrogen concentrations increase interleukin (IL)-4 production by Th2 cells, which induce humoral immunity and have an anti-inflammatory effect (Karpuzoglu and Zouali, 2011; Pennell et al., 2012; Pernis, 2007; Straub, 2007). High levels furthermore expand regulatory T (Treg) cells (Arruvito et al., 2007; Fish, 2008; Polanczyk et al., 2004), accompanied by an increased expression of IL-10 and transforming growth factor β (Luo et al., 2011), inhibitory costimulatory molecule programmed cell death protein (PD)-1 (Wang et al., 2009), and cell death–mediating perforin (Valor et al., 2011).
Although estrogens decrease B cell progenitors in the bone marrow (Masuzawa et al., 1994; Smithson et al., 1995), they increase the number of peripheral B cells (Hill et al., 2011), their activation, maturation, and survival (Grimaldi et al., 2002; Sakiani et al., 2013; Subramanian et al., 2011; Venkatesh et al., 2006), and production of antigen-specific antibodies (Fairweather et al., 2008).
Overall, the effects on estrogens on the immune system can be summarized as immunoenhancing due to the stimulation of innate immune cell function, CTL activity, and Th1 responses as well as antibody production and B cell maturation. This effect is considered to contribute to the relative immunological advantage of females in mounting an immune response toward pathogens.
Progesterone
Progesterone downregulates innate immune responses exerted by macrophages (Su et al., 2009), natural killer (NK) cells (Arruvito et al., 2008), or DCs (Butts et al., 2007; Hughes et al., 2008). Further, progesterone antagonizes the effect of estrogens on T cell cycle progression and apoptosis (McMurray et al., 2001) and inhibits proliferation of T cells after DC stimulation (Butts et al., 2007) and their differentiation into proinflammatory Th17 cells (Lee et al., 2011). Most importantly, the high concentrations of progesterone present during pregnancy inhibit a Th1 bias of the immune response by stimulating the generation of Treg cells (Lee et al., 2011) and anti-inflammatory, IL-4- and IL-10-secreting CD4+ Th2 cells (Correale et al., 1998; Miyaura and Iwata, 2002; Szekeres-Bartho et al., 1996). Moreover, progesterone decreases immunoglobulin-secreting B cell numbers (Lu et al., 2002) and suppresses their maturation (Sakiani et al., 2013).
Hence, the overall effects of progesterone can be summarized as immunosuppressive.
Testosterone
Testosterone supports the innate immune response by enhancing neutrophils (Chuang et al., 2009). While testosterone supports the production and function of actively patrolling monocytes/macrophages (Chang et al., 2013; Corrales et al., 2014), it suppresses macrophage function in chronic inflammation (Corcoran et al., 2010), dampens their sensitivity to recognize pathogenic patterns (Rettew et al., 2008), and suppresses NK cell proliferation (Page et al., 2006). DCs are suppressed by testosterone via inhibition of costimulatory molecule expression (Koh et al., 2009), a reduced proinflammatory cytokine production (Corrales et al., 2009; Meier et al., 2009) and a diminished antigen-responsive capacity (Corrales et al., 2012).
The adaptive immune response is also suppressed by testosterone, mirrored by a reduced T cell proliferation and cytokine production (Olsen and Kovacs, 1996), a lower proliferation of immature thymocytes, and accelerated thymic apoptosis (McMurray et al., 2001), thereby inducing thymic atrophy (Chang et al., 2013; Olsen et al., 1991). Testosterone inhibits Th1 differentiation (Kissick et al., 2014), IFN-γ secretion in NKT cells (Lotter et al., 2013), and parasite-induced Th2 responses (Hepworth et al., 2010). In turn, it enhances Th2 immunity to counteract overshooting Th1 responses in autoimmunity (Dalal et al., 1997) and induces expansion of suppressor CD8+ cells (Olsen and Kovacs, 1996; Tanriverdi et al., 2003) and Tregs (Fijak et al., 2011; Page et al., 2006). B cell proliferation and immunoglobulin production are reduced in response to androgens (Chang et al., 2013; Ellis et al., 2001; Smithson et al., 1998).
Overall, testosterone exerts immune-suppressive effects on the immune response, which has been suggested to protect males from autoimmunity.
Glucocorticoids
Cortisol, along with the other endogenous GCs, have a dichotomous effect on the innate immune response, as they stimulate macrophages (Ehrchen et al., 2007; Franchimont, 2004; Giles et al., 2001; Liu et al., 1999), but repress NK cells (De Lorenzo et al., 2015). Also, GCs inhibit DC maturation and antigen-presenting function (Elftman et al., 2007; Franchimont, 2004; Hunzeker et al., 2011), thereby locking DCs in a tolerogenic state (Bros et al., 2007; Luther et al., 2009). With regard to the adaptive immune response, GCs reduce the levels of circulating naïve T cells (Besedovsky et al., 2014; Fischer et al., 2013) and trigger T cell apoptosis (Purton et al., 2004). They suppress CD8 T cell activation, proliferation, and trafficking (Hunzeker et al., 2011) and reduce NKT cell number and cytotoxic activity (De Lorenzo et al., 2015). GCs suppress cellular (Th1) immunity by repressing production and release of Th1 and Th17 proinflammatory cytokines (Franchimont, 2004) and favor humoral (Th2) immunity (Miyaura and Iwata, 2002; Van Den Brandt et al., 2007). In turn, the differentiation of IL-10-producing Tregs is promoted (Chen et al., 2006). B cells are repressed by GCs (Youinou and Pers, 2010; Zen et al., 2011).
To summarize, GCs are rather strong immune-suppressors, an effect which is therapeutically utilized to dampen inflammatory symptoms in many diseases by exogenous supplementation of synthetic GCs.
Noteworthy, besides the direct effects of hormones on the immune system discussed so far, steroid hormones can also indirectly affect immunity. For example, estrogens contribute to recruitment of inflammatory cells via the upregulation of adhesion molecules on endothelial cells (Murphy et al., 2004). Testosterone strengthens tight junctions of the blood–testis barrier (Meng et al., 2005), thereby supporting the immune privilege of the testis.
We here refrain from discussing reciprocal effects of the immune system on the endocrine system, such as the effect of cytokines on key endocrine tissues, like the hypothalamus, pituitary gland, or thyroid (Boutzios and Kaltsas, 2000).
Consequences of Endocrine–Immune Cross Talk
Sex Disparity of Adult Immune Responses
Most of the described immune–endocrine effects described above and depicted in Figure 4 are compiled from observations in different species, cell subsets from different organs, from distinct disease models, or from in vitro experiments. Hence, it is difficult to appreciate the biological consequences of these cross talks between hormones and immune cells. Epidemiological observations greatly facilitate understanding of the implications of immune–endocrine interaction for health and disease (Figures 1 and 3). Here, the more readily initiated innate immune response to pathogens and a more efficient antigen presentation seen in females could be attributed to the predominance of estrogens (Gubbels Bupp, 2015; Klein et al., 2010a), which promotes the response to infections, vaccination, and trauma with a stronger adaptive response of Th1 cell–mediated immunity, along with Th2-triggered humoral immune responses (Gubbels Bupp, 2015; Klein et al., 2010a). Females also exhibit higher immunoglobulin levels both at baseline and following infection or immunization, which promotes the elimination of viral pathogens (Ackerman, 2006; Furman et al., 2014). This health advantage related to immunity has significant collateral disadvantages, mirrored by the increased risk for autoimmunity in women. Here, the estrogen-induced promotion of T cell and B cell lymphopoiesis has been proposed to promote the escape from negative selection, hereby leading to the presence of autoreactive cells (Fish, 2008; Jeganathan et al., 2014; Okuyama et al., 1992). The autoreactive cells can subsequently contribute to the etiology of rheumatoid arthritis (RA) (Cutolo et al., 2003), systemic lupus erythematosus (SLE) (Costenbader et al., 2007), Sjögren's syndrome (Fish, 2008), or multiple sclerosis (MS) (Voskuhl and Gold, 2012).
Conversely, the immunosuppressive effects of testosterone have been proposed to be advantageous in protecting males from autoimmunity. Indeed, testosterone has been shown to decrease the number of autoantibodies (Tanriverdi et al., 2003) by inhibiting the expansion of autoreactive B cell clones (Altuwaijri et al., 2009). In accordance with this, declining levels of testosterone increase the risk for autoimmune diseases in males. Conversely, the testosterone-mediated immunosuppression also explains the increased viral persistence seen in men (Klein et al., 2012). Chronic immune diseases including atherosclerosis and metabolic diseases, such as type 2 diabetes, are also more prevalent among men. This health disadvantage has been attributed to the lack of estrogens, which can protect against vascular injury and atherosclerosis and regulate metabolic pathways (Gubbels Bupp, 2015). Another potential disadvantage related to immune–endocrine interaction is the overall higher risk of adult men to develop cancer, which has been linked to the testosterone-related dampened immune responses to neoplasms (Trigunaite et al., 2015).
Sex-Based Senescence of the Immune Response
Besides sex hormone–related differences in immunity, the age-associated decrease of estrogens and progesterone in women and testosterone in men can result in altered immune responses and risk for diseases (Figures 1 and 3). Indeed, female- and male-aged individuals show higher-serum levels of a proinflammatory cytokines, which has been termed inflammaging (Bartlett et al., 2012; Vural et al., 2006; Yasui et al., 2007). Despite this inflammatory status, aging is characterized by a less-efficient innate defense against pathogens (Shaw et al., 2013; Solana et al., 2012) and a decreased functionality of the adaptive immune response (Hirokawa et al., 2013; Sasaki et al., 2011; Schenkein et al., 2008; Strindhall et al., 2013). Treg cells and differentiated T cells with memory and effector functions accumulate in the periphery (Haynes and Maue, 2009; Yan et al., 2010), which has been proposed to render the aged individual less flexible to fight novel pathogens and could explain the reduced vaccination efficacy in the aged of both sexes (Giefing-Kroll et al., 2015). Studies on sex-specific differences in immunosenescence are sparse, but the reduced levels of testosterone could allow for the increased occurrence of autoimmune diseases seen in aging males, while the decrease in estrogens reduces autoantibody-driven autoimmunity in females, while at the same time enhancing T cell–mediated autoimmune diseases (Straub, 2007). Future studies are needed to elucidate immune–endocrine mechanisms modulating the course of infectious diseases and vaccination efficacy in the elderly.
Steroid Hormones Affecting Immunity in Female Reproduction
Immunocompetence of the Cycling Female and the Window of Vulnerability
Besides the immune–endocrine interactions discussed so far with regard to systemic immunity, fluctuations in hormone concentrations that occur over the relative short-time period of the menstrual cycle are associated with marked changes in the local immune response in the reproductive tract (Figure 5 ; Hafner et al., 2013; Wira et al., 2015).
Figure 5.
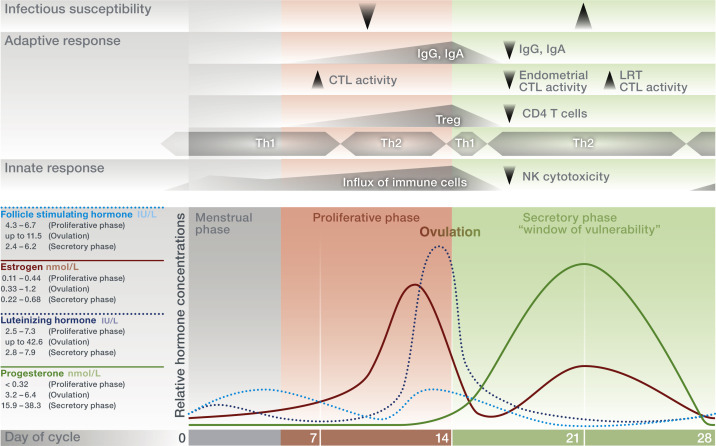
The female menstrual cycle influences local immunity. Alternating hormone levels regulate the functions of the female reproductive tract to allow fertilization and implantation. In the proliferative phase, during which the endometrial lining is reconstituted, estrogens are predominant and their levels rise, resulting in ovulation and the onset of progesterone synthesis. After ovulation, the corpus luteum produces both progesterone and estrogens, both of which control local processes in preparation for a potential pregnancy. In the absence of fertilization, corpus luteum degrades, inducing inflammatory degradation of the endometrium and the onset of menses. Local immunity changes with fluctuating hormone levels, resulting in an increased susceptibility to viral and bacterial infectious diseases during the secretory phase. LRT, lower reproductive tract; CTL, cytotoxic T lymphocyte; Ig, immunoglobulin; NK, natural killer cell; Th, T helper 1 or 2–mediated immune response; Treg, regulatory T cell.
Commencing in the late-secretory phase, immune cells in the uterine endometrium begin to increase and surge during the estrogen-rich proliferative phase. This cellular influx is increasingly facilitated with progressive formation of new vasculature and accumulation of chemokines and peaks at the time of ovulation, possibly to provide a receptive environment for blastocyst implantation (Wira et al., 2015). During ovulation, estrogens induce immunoglobulin secretion to facilitate removal of potential pathogens (Kutteh et al., 1996; Wira et al., 2005; Beagley and Gockel, 2003). Moreover, the increased number of Treg cells in the endometrium throughout the proliferative phase until ovulation points toward their possible role in promoting local immune tolerance to facilitate blastocyst implantation (Arruvito et al., 2007; Berbic et al., 2010).
During the secretory phase, both progesterone and estrogen levels are high, which dampens immunosurveillance and thereby minimizes the risk of an immune response against allogeneic sperm antigens and – if implantation occurs – the semiallogeneic blastocyst. This is accompanied by low-antibody levels in cervical secretions (Schumacher, 1973). At the same time, the endometrium prepares for a possible blastocyst implantation via an increase of uterine NK cells. These NK cells show a unique phenotype, as they exhibit low-cytotoxic activity (Evans and Salamonsen, 2012). Additionally, endometrial CD8+ cytotoxic T lymphocyte activity is suppressed (Laskarin et al., 1999). Surprisingly, after peaking around ovulation, Treg cell numbers decrease at the beginning of the secretory phase, which challenges their central role in contributing to persisting immune tolerance during very early pregnancy (Arruvito et al., 2007; Berbic et al., 2010).
The fluctuating levels of estrogens and progesterone during the menstrual cycle and the associated changes in immune function have been proposed to influence the response to mucosal pathogens. Studies performed in mice reveal a reduced protection from bacterial infection in the genital tract in response to progesterone (Kaushic et al., 1998). Also, viral infection appeared to be enhanced in dioestrus (corresponding to the secretory phase with high progesterone levels in humans), while viral replication was reduced during the estrogen-dominated preovulatory phase (Gallichan and Rosenthal, 1996). Hence, a window for increased susceptibility to STIs, for example, HIV, has been proposed for the secretory phase of the menstrual cycle due to the suppression of innate, humoral, and cell-mediated immunity by progesterone and high estrogens (Saba et al., 2013; Wira and Fahey, 2008; Hafner et al., 2013). In conclusion, immunity in the female reproductive tract faces a contradictory demand by suppressing an immune response against allogeneic sperm antigens and promoting the implantation of a semiallogeneic blastocyst. This is at the expense of immunity against local pathogen challenges.
Immune Privilege of the Pregnant Uterus: Adaptations of Maternal Immunocompetence
Besides the endocrine changes occurring over the menstrual cycle, pregnancy is accompanied by soaring hormone levels (Figure 6 ). These pregnancy-related hormonal changes skew the innate and adaptive immune response to contribute to a tolerogenic immune environment at the fetomaternal interface in which the fetus develops (Szekeres-Bartho et al., 2005; Blois et al., 2007; Arck and Hecher, 2013).
Figure 6.
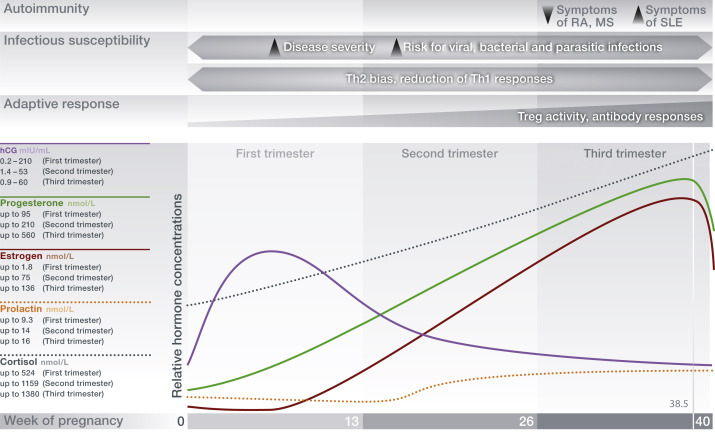
Pregnancy-associated hormone levels establish maternal immune adaptation. Concentrations of estrogens and progesterone are substantially higher during pregnancy than during the nonpregnant female menstrual cycle due to their placental secretion and stimulation of production via the HPG-axis. Pituitary-derived prolactin and placenta-derived human chorionic gonadotropin (hCG) as well as GCs sustain pregnancy. Alterations of the maternal immune response toward a Th2-biased phenotype results in an increased susceptibility to infections. Autoimmune disease progression is either ameliorated or exacerbated depending on the underlying effector mechanisms involved in autoimmune reactions (B- or Th1-mediated autoimmunity). hCG human chorionic gonadotropin; MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; Th, T helper 1 or 2–mediated immune response.
Human chorionic gonadotropin drives the corpus luteum secretion of progesterone during early pregnancy and acts as a chemoattractant for decidual tolerogenic Tregs (Schumacher et al., 2009). The pregnancy-associated activation of the hypothalamic–pituitary–adrenal (HPA) axis increases immune-suppressive GC secretion, which is even further enhanced by placenta-derived corticotropin-releasing hormone, resulting in a protective hyporesponsiveness to stress at term pregnancy due to massive negative feedback (Duthie and Reynolds, 2013; Lindsay and Nieman, 2005). Levels of estrogens are high, exerting immune-regulatory functions (Figure 4). The essential role of progesterone in maintaining a successful pregnancy becomes evident in observations in humans and mice that its reduction or blockage can result in fetal loss (Arck et al., 2008; Blois et al., 2004).
While the maternal immune changes establish an immune privilege of the pregnant uterus to allow for fetal survival, the occurring systemic alterations have been shown to impair maternal immunity against pathogens and alter the activity of autoimmune diseases. The constantly rising levels of progesterone and the enhanced GC production in response to HPA-axis activation could increase the susceptibility to or severity of infectious diseases due to the suppression of a proper immune response necessary to control and eliminate pathogens (Tait et al., 2008). In pregnant mice, infection with Leishmania major, Salmonella enterica, and Toxoplasma gondii is more severe due to the suppressed Th1 immunity that would usually mount an antiparasitic response (Krishnan et al., 2013). Pregnant women are at an increased risk to suffer from acute and chronic viral infections such as rhinovirus, severe acute respiratory syndrome, coronavirus, varicella zoster, hepatitis E/B, HIV, and cytomegalovirus (Harger et al., 2002; McDonagh et al., 2004; Wong et al., 2003). Moreover, pregnant women are highly susceptible to influenza A virus infection, mirrored by the high incidence, morbidity, and mortality, as last observed by during the 2009 pandemic (Jamieson et al., 2009; Klein et al., 2010b). This has been described to result from the contradictory demand for the immune system to mount a virus-specific immune response to clear the influenza infection, while simultaneously maintaining adaptation to pregnancy (Gabriel and Arck, 2014). Furthermore, antibody-mediated disorders such as SLE (Smyth et al., 2010), sclerodermia (Artlett et al., 1998), and thyroiditis (Klintschar et al., 2001) are exacerbated during pregnancy, possibly due to enhanced antibody production in response to the high levels of estrogens.
In contrast to these adverse effects of pregnancy on immunity, the immune–endocrine interaction during pregnancy can also be beneficial for maternal health. In female patients with MS or RA, both T cell–mediated inflammatory diseases, clinical symptoms have been reported to improve during pregnancy. This is followed by vigorous disease relapses shortly after parturition, when hormonal levels and immune adaptation are being restored to nonpregnancy levels (Confavreux et al., 1998; De Man et al., 2008; Hughes and Choubey, 2014). Strikingly, the amelioration of MS disease activity has been shown to exceed the effect size of currently available therapeutic strategies (Vukusic et al., 2004). Hence, a better understanding of underlying immune–endocrine pathways may provide insights for developing new treatments for these conditions (Patas et al., 2013).
Genetic Influence of Sex Chromosomes on Immune Response
Noteworthy, the sex-specific endocrine response does not suffice to fully explain sex-specific immune responses (Giefing-Kroll et al., 2015). Investigations also aim to understand the genetic influence of the sex chromosomes on immune differences between the sexes. It has been proposed that the escape from X chromosome silencing and X inactivation skewing may reactivate parts of the second X chromosome in females, which could promote female immune function due to enhanced expression of immune-regulatory genes. Males, in contrast, have only one copy of the X chromosome which encodes for many genes involved in immunity (Libert et al., 2010). Also gender-related lifestyle factors may contribute to differential immunity and disease susceptibility (Oertelt-Prigione, 2012).
Conclusion
In this article, interactions of sex and stress steroid hormones with the innate and adaptive immune response have been discussed from a sex- and age-specific perspective and in certain settings, such as pregnancy. Additional evidence for the impact of hormones on the immune system can be observed during endocrine therapeutic interventions, which is listed in Table 1 . We highlight the biological consequences resulting from the interaction between hormones and immune cells in the context of health and diseases, as the current knowledge opens avenues for therapeutic interventions targeting pathways involved in the immune–endocrine cross talk, for example, during pregnancy complications or autoimmunity.
Table 1.
Endocrine interventions and immunity
| Intervention | Aim | (Side-)effect on immunity | References |
|---|---|---|---|
| Oral contraceptives | Prevent ovulation | Increased risk for genital tract infections No effect on risk for MS, RA, SLE |
Fichorova et al. (2015), Mohllajee et al. (2006), Murphy et al. (2014), and Straub (2007) |
| In vitro fertilization | Induce ovulation, maintain early pregnancy | Unknown | Huang and Rosenwaks (2012) and Yanushpolsky (2015) |
| Estrogen replacement therapy | Alleviate peri- and postmenopausal symptoms | Partial restoration of immunological function seen during reproductive age Increased symptoms of SLE |
Holroyd and Edwards (2009), Giefing-Kroll et al. (2015), and Straub (2007) |
| Aromatase inhibitors | Deprive ER+ breast cancer from estrogens | Clinical signs of RA (e.g., joint pain) | Zhang et al. (2010) |
| Androgen replacement therapy | Reduction of inflammation in hypogonadism | Suppression of cellular and humoral immune responses | Cutolo et al. (2002), Kocar et al. (2000), Musabak et al. (2003), Ackerman (2006), Malkin et al. (2004), and Fijak et al. (2011) |
| CRH antagonists | Reduction of excessive glucocorticoid release | Reduction of downstream immune-suppression | Elenkov et al. (1999) and Zoumakis et al. (2006) |
MS, multiple sclerosis; ER; estrogen receptor; RA; rheumatoid arthritis; SLE, systemic lupus erythematosus; CRH, corticotropin-releasing hormone.
See also
PHYSIOLOGY OF THE IMMUNE SYSTEM | Effect of Sex on Cellular Immunity; PHYSIOLOGY OF THE IMMUNE SYSTEM | Effect of Sex on Humoral and Innate Immunity; PHYSIOLOGY OF THE IMMUNE SYSTEM | Immunity and Aging; PHYSIOLOGY OF THE IMMUNE SYSTEM | Immunology of the Female Reproductive Mucosa; PHYSIOLOGY OF THE IMMUNE SYSTEM | Immunology of the Testis and Privileged Sites.
References
- Ackerman L.S. Sex hormones and the genesis of autoimmunity. Arch. Dermatol. 2006;142:371–376. doi: 10.1001/archderm.142.3.371. [DOI] [PubMed] [Google Scholar]
- Altuwaijri S., Chuang K.H., Lai K.P., Lai J.J., Lin H.Y., Young F.M., Bottaro A., Tsai M.Y., Zeng W.P., Chang H.C., Yeh S., Chang C. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol. Endocrinol. 2009;23:444–453. doi: 10.1210/me.2008-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck P.C., Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- Arck P.C., Rucke M., Rose M., Szekeres-Bartho J., Douglas A.J., Pritsch M., Blois S.M., Pincus M.K., Barenstrauch N., Dudenhausen J.W., Nakamura K., Sheps S., Klapp B.F. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod. Biomed. Online. 2008;17:101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- Arruvito L., Giulianelli S., Flores A.C., Paladino N., Barboza M., Lanari C., Fainboim L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- Arruvito L., Sanz M., Banham A.H., Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J. Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- Artlett C.M., Smith J.B., Jimenez S.A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N. Engl. J. Med. 1998;338:1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- Bartlett D.B., Firth C.M., Phillips A.C., Moss P., Baylis D., Syddall H., Sayer A.A., Cooper C., Lord J.M. The age-related increase in low-grade systemic inflammation (inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11:912–915. doi: 10.1111/j.1474-9726.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- Beagley K.W., Gockel C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Bellavance M.A., Rivest S. The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbic M., Hey-Cunningham A.J., Ng C., Tokushige N., Ganewatta S., Markham R., Russell P., Fraser I.S. The role of Foxp3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum. Reprod. 2010;25:900–907. doi: 10.1093/humrep/deq020. [DOI] [PubMed] [Google Scholar]
- Besedovsky L., Born J., Lange T. Endogenous glucocorticoid receptor signaling drives rhythmic changes in human T-cell subset numbers and the expression of the chemokine receptor CXCR4. FASEB J. 2014;28:67–75. doi: 10.1096/fj.13-237958. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Pencina M., Jasuja G.K., Travison T.G., Coviello A., Orwoll E., Wang P.Y., Nielson C., Wu F., Tajar A., Labrie F., Vesper H., Zhang A., Ulloor J., Singh R., D'agostino R., Vasan R.S. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois S.M., Ilarregui J.M., Tometten M., Garcia M., Orsal A.S., Cordo-Russo R., Toscano M.A., Bianco G.A., Kobelt P., Handjiski B., Tirado I., Markert U.R., Klapp B.F., Poirier F., Szekeres-Bartho J., Rabinovich G.A., Arck P.C. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Blois S.M., Joachim R., Kandil J., Margni R., Tometten M., Klapp B.F., Arck P.C. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J. Immunol. 2004;172:5893–5899. doi: 10.4049/jimmunol.172.10.5893. [DOI] [PubMed] [Google Scholar]
- Boutzios G., Kaltsas G. Immune system effects on the endocrine system. In: De Groot L.J., Beck-Peccoz P., Chrousos G., Dungan K., Grossman A., Hershman J.M., Koch C., Mclachlan R., New M., Rebar R., Singer F., Vinik A., Weickert M.O., editors. Endotext. 2000. South Dartmouth (MA) [Google Scholar]
- Bros M., Jahrling F., Renzing A., Wiechmann N., Dang N.A., Sutter A., Ross R., Knop J., Sudowe S., Reske-Kunz A.B. A newly established murine immature dendritic cell line can be differentiated into a mature state, but exerts tolerogenic function upon maturation in the presence of glucocorticoid. Blood. 2007;109:3820–3829. doi: 10.1182/blood-2006-07-035576. [DOI] [PubMed] [Google Scholar]
- Butts C.L., Shukair S.A., Duncan K.M., Bowers E., Horn C., Belyavskaya E., Tonelli L., Sternberg E.M. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int. Immunol. 2007;19:287–296. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- Chang C., Yeh S., Lee S.O., Chang T.M. Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl. Recept. Signal. 2013;11:e001. doi: 10.1621/nrs.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Oppenheim J.J., Winkler-Pickett R.T., Ortaldo J.R., Howard O.M. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- Chuang K.H., Altuwaijri S., Li G., Lai J.J., Chu C.Y., Lai K.P., Lin H.Y., Hsu J.W., Keng P., Wu M.C., Chang C. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J. Exp. Med. 2009;206:1181–1199. doi: 10.1084/jem.20082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C., Hutchinson M., Hours M.M., Cortinovis-Tourniaire P., Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in multiple sclerosis group. N. Engl. J. Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Corcoran M.P., Meydani M., Lichtenstein A.H., Schaefer E.J., Dillard A., Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J. Endocrinol. 2010;206:217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J.J., Almeida M., Cordero M., Martin-Martin L., Mendez C., Miralles J.M., Orfao A. Enhanced immunological response by dendritic cells in male hypogonadism. Eur. J. Clin. Invest. 2012;42:1205–1212. doi: 10.1111/j.1365-2362.2012.02712.x. [DOI] [PubMed] [Google Scholar]
- Corrales J.J., Almeida M., Martin-Martin L., Miralles J.M., Orfao A. Testosterone replacement therapy in hypogonadal men is associated with increased expression of LAMP-2 (CD107b) by circulating monocytes and dendritic cells. Clin. Endocrinol. (Oxf.) 2014;80:577–584. doi: 10.1111/cen.12338. [DOI] [PubMed] [Google Scholar]
- Corrales J.J., Almeida M., Miralles J.M., Orfao A. Persistence of androgenic effects on the production of proinflammatory cytokines by circulating antigen-presenting cells after withdrawal of testosterone treatment in aging type 2 diabetic men with partial androgen deficiency. Fertil. Steril. 2009;92:311–319. doi: 10.1016/j.fertnstert.2008.05.040. [DOI] [PubMed] [Google Scholar]
- Correale J., Arias M., Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- Costenbader K.H., Feskanich D., Stampfer M.J., Karlson E.W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Capellino S., Montagna P., Villaggio B., Sulli A., Seriolo B., Straub R.H. New roles for estrogens in rheumatoid arthritis. Clin. Exp. Rheumatol. 2003;21:687–690. [PubMed] [Google Scholar]
- Cutolo M., Seriolo B., Villaggio B., Pizzorni C., Craviotto C., Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann. N.Y. Acad. Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- Dalal M., Kim S., Voskuhl R.R. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- Davison S.L., Bell R., Donath S., Montalto J.G., Davis S.R. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- De Lorenzo B.H., De Oliveira Marchioro L., Greco C.R., Suchecki D. Sleep-deprivation reduces NK cell number and function mediated by beta-adrenergic signalling. Psychoneuroendocrinology. 2015;57:134–143. doi: 10.1016/j.psyneuen.2015.04.006. [DOI] [PubMed] [Google Scholar]
- De Man Y.A., Dolhain R.J., Van De Geijn F.E., Willemsen S.P., Hazes J.M. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- De Vito P., Incerpi S., Pedersen J.Z., Luly P., Davis F.B., Davis P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- Duthie L., Reynolds R.M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98:106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- Ehrchen J., Steinmuller L., Barczyk K., Tenbrock K., Nacken W., Eisenacher M., Nordhues U., Sorg C., Sunderkotter C., Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–1274. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Webster E.L., Torpy D.J., Chrousos G.P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N.Y. Acad. Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. discussion 11–3. [DOI] [PubMed] [Google Scholar]
- Elftman M.D., Norbury C.C., Bonneau R.H., Truckenmiller M.E. Corticosterone impairs dendritic cell maturation and function. Immunology. 2007;122:279–290. doi: 10.1111/j.1365-2567.2007.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T.M., Moser M.T., Le P.T., Flanigan R.C., Kwon E.D. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int. Immunol. 2001;13:553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- Evans J., Salamonsen L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012;13:277–288. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- Fairweather D., Frisancho-Kiss S., Rose N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova R.N., Chen P.L., Morrison C.S., Doncel G.F., Mendonca K., Kwok C., Chipato T., Salata R., Mauck C. The Contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6 doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M., Schneider E., Klug J., Bhushan S., Hackstein H., Schuler G., Wygrecka M., Gromoll J., Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- Fischer H.J., Schweingruber N., Luhder F., Reichardt H.M. The potential role of T cell migration and chemotaxis as targets of glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Mol. Cell. Endocrinol. 2013;380:99–107. doi: 10.1016/j.mce.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N.Y. Acad. Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- Furman D., Hejblum B.P., Simon N., Jojic V., Dekker C.L., Thiebaut R., Tibshirani R.J., Davis M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. U.S.A. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel G., Arck P.C. Sex, immunity and influenza. J. Infect. Dis. 2014;209(Suppl. 3):S93–S99. doi: 10.1093/infdis/jiu020. [DOI] [PubMed] [Google Scholar]
- Gallichan W.S., Rosenthal K.L. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224:487–497. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- Garcia-Duran M., De Frutos T., Diaz-Recasens J., Garcia-Galvez G., Jimenez A., Monton M., Farre J., Sanchez De Miguel L., Gonzalez-Fernandez F., Arriero M.D., Rico L., Garcia R., Casado S., Lopez-Farre A. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circ. Res. 1999;85:1020–1026. doi: 10.1161/01.res.85.11.1020. [DOI] [PubMed] [Google Scholar]
- Giefing-Kroll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles K.M., Ross K., Rossi A.G., Hotchin N.A., Haslett C., Dransfield I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J. Immunol. 2001;167:976–986. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- Gilliver S.C. Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 2010;120:105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Grimaldi C.M., Cleary J., Dagtas A.S., Moussai D., Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp M.R. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015;294:102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Guerra-Silveira F., Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8:e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner L.M., Cunningham K., Beagley K.W. Ovarian steroid hormones: effects on immune responses and Chlamydia trachomatis infections of the female genital tract. Mucosal Immunol. 2013;6:859–875. doi: 10.1038/mi.2013.46. [DOI] [PubMed] [Google Scholar]
- Hao S., Zhao J., Zhou J., Zhao S., Hu Y., Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol. 2007;7:1765–1775. doi: 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Harger J.H., Ernest J.M., Thurnau G.R., Moawad A., Momirova V., Landon M.B., Paul R., Miodovnik M., Dombrowski M., Sibai B., Van Dorsten P., National Institute of Child, Health and Human Development, Network of Maternal-Fetal Medicine Units Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J. Infect. Dis. 2002;185:422–427. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- Harkonen P.L., Vaananen H.K. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann. N.Y. Acad. Sci. 2006;1089:218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- Haynes L., Maue A.C. Effects of aging on T cell function. Curr. Opin. Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth M.R., Hardman M.J., Grencis R.K. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur. J. Immunol. 2010;40:406–416. doi: 10.1002/eji.200939589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L., Jeganathan V., Chinnasamy P., Grimaldi C., Diamond B. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Mol. Med. 2011;17:211–220. doi: 10.2119/molmed.2010.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Hayashi Y., Kitagawa M., Makinodan T., Fulop T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing. 2013;10:19. doi: 10.1186/1742-4933-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.R., Edwards C.J. The effects of hormone replacement therapy on autoimmune disease: rheumatoid arthritis and systemic lupus erythematosus. Climacteric. 2009;12:378–386. doi: 10.1080/13697130903025449. [DOI] [PubMed] [Google Scholar]
- Huang J.Y., Rosenwaks Z. In vitro fertilisation treatment and factors affecting success. Best Pract. Res. Clin. Obstet. Gynaecol. 2012;26:777–788. doi: 10.1016/j.bpobgyn.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Hughes G.C., Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- Hughes G.C., Thomas S., Li C., Kaja M.K., Clark E.A. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J. Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- Hunzeker J.T., Elftman M.D., Mellinger J.C., Princiotta M.F., Bonneau R.H., Truckenmiller M.E., Norbury C.C. A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells. J. Immunol. 2011;186:183–194. doi: 10.4049/jimmunol.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S., Lindstrom S., Louie J.K., Christ C.M., Bohm S.R., Fonseca V.P., Ritger K.A., Kuhles D.J., Eggers P., Bruce H., Davidson H.A., Lutterloh E., Harris M.L., Burke C., Cocoros N., Finelli L., Macfarlane K.F., Shu B., Olsen S.J., Novel Influenza A Pregnancy Working Group H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- Jasuja G.K., Travison T.G., Davda M., Murabito J.M., Basaria S., Zhang A., Kushnir M.M., Rockwood A.L., Meikle W., Pencina M.J., Coviello A., Rose A.J., D'agostino R., Vasan R.S., Bhasin S. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:733–740. doi: 10.1093/gerona/gls216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan V., Peeva E., Diamond B. Hormonal milieu at time of B cell activation controls duration of autoantibody response. J. Autoimmun. 2014;53:46–54. doi: 10.1016/j.jaut.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuzoglu E., Zouali M. The multi-faceted influences of estrogen on lymphocytes: toward novel immuno-interventions strategies for autoimmunity management. Clin. Rev. Allergy Immunol. 2011;40:16–26. doi: 10.1007/s12016-009-8188-0. [DOI] [PubMed] [Google Scholar]
- Kaushic C., Murdin A.D., Underdown B.J., Wira C.R. Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect. Immun. 1998;66:893–898. doi: 10.1128/iai.66.3.893-898.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E.T., Zhang J., Yao Z., Qi Y. The impact of chronic estrogen deprivation on immunologic parameters in the ovariectomized rhesus monkey (Macaca mulatta) model of menopause. J. Reprod. Immunol. 2001;50:41–55. doi: 10.1016/s0165-0378(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Kissick H.T., Sanda M.G., Dunn L.K., Pellegrini K.L., On S.T., Noel J.K., Arredouani M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Hodgson A., Robinson D.P. Mechanisms of sex disparities in influenza pathogenesis. J. Leukoc. Biol. 2012;92:67–73. doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Passaretti C., Anker M., Olukoya P., Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol. Sex Differ. 2010;1:5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintschar M., Schwaiger P., Mannweiler S., Regauer S., Kleiber M. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J. Clin. Endocrinol. Metab. 2001;86:2494–2498. doi: 10.1210/jcem.86.6.7540. [DOI] [PubMed] [Google Scholar]
- Kocar I.H., Yesilova Z., Ozata M., Turan M., Sengul A., Ozdemir I. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter's syndrome. Clin. Exp. Immunol. 2000;121:448–452. doi: 10.1046/j.1365-2249.2000.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y.T., Gray A., Higgins S.A., Hubby B., Kast W.M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S., Carreras E., Agrawal H. Sex steroid receptors in immune cells. In: Klein S.L., Roberts C.W., editors. Sex Hormones and Immunity to Infection. Springer-Verlag; Berlin: 2010. [Google Scholar]
- Krishnan L., Nguyen T., Mccomb S. From mice to women: the conundrum of immunity to infection during pregnancy. J. Reprod. Immunol. 2013;97:62–73. doi: 10.1016/j.jri.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh W.H., Prince S.J., Hammond K.R., Kutteh C.C., Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin. Exp. Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskarin G., Strbo N., Sotosek V., Rukavina D., Faust Z., Szekeres-Bartho J., Podack E.R. Progesterone directly and indirectly affects perforin expression in cytolytic cells. Am. J. Reprod. Immunol. 1999;42:312–320. doi: 10.1111/j.1600-0897.1999.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Lastra G., Sowers J.R. Obesity and cardiovascular disease: role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm. Mol. Biol. Clin. Invest. 2013;15:49–57. doi: 10.1515/hmbci-2013-0025. [DOI] [PubMed] [Google Scholar]
- Laughlin G.A., Barrett-Connor E., Kritz-Silverstein D., Von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Ulrich B., Cho J., Park J., Kim C.H. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J. Immunol. 2011;187:1778–1787. doi: 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C., Dejager L., Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Lindsay J.R., Nieman L.K. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr. Rev. 2005;26:775–799. doi: 10.1210/er.2004-0025. [DOI] [PubMed] [Google Scholar]
- Liu H.Y., Buenafe A.C., Matejuk A., Ito A., Zamora A., Dwyer J., Vandenbark A.A., Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cousin J.M., Hughes J., Van Damme J., Seckl J.R., Haslett C., Dransfield I., Savill J., Rossi A.G. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- Lotter H., Helk E., Bernin H., Jacobs T., Prehn C., Adamski J., Gonzalez-Roldan N., Holst O., Tannich E. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNgamma secretion in natural killer T cells. PLoS One. 2013;8:e55694. doi: 10.1371/journal.pone.0055694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin Abdulhak A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., De Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F.X., Abel K., Ma Z., Rourke T., Lu D., Torten J., Mcchesney M., Miller C.J. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin. Exp. Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C.Y., Wang L., Sun C., Li D.J. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell. Mol. Immunol. 2011;8:50–58. doi: 10.1038/cmi.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther C., Adamopoulou E., Stoeckle C., Brucklacher-Waldert V., Rosenkranz D., Stoltze L., Lauer S., Poeschel S., Melms A., Tolosa E. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J. Immunol. 2009;183:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- Malkin C.J., Pugh P.J., Jones R.D., Kapoor D., Channer K.S., Jones T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Marino F., Cosentino M. Adrenergic modulation of immune cells: an update. Amino Acids. 2013;45:55–71. doi: 10.1007/s00726-011-1186-6. [DOI] [PubMed] [Google Scholar]
- Masuzawa T., Miyaura C., Onoe Y., Kusano K., Ohta H., Nozawa S., Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J. Clin. Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonagh S., Maidji E., Ma W., Chang H.T., Fisher S., Pereira L. Viral and bacterial pathogens at the maternal-fetal interface. J. Infect. Dis. 2004;190:826–834. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- McMurray R.W., Suwannaroj S., Ndebele K., Jenkins J.K. Differential effects of sex steroids on T and B cells: modulation of cell cycle phase distribution, apoptosis and bcl-2 protein levels. Pathobiology. 2001;69:44–58. doi: 10.1159/000048757. [DOI] [PubMed] [Google Scholar]
- Meier A., Chang J.J., Chan E.S., Pollard R.B., Sidhu H.K., Kulkarni S., Wen T.F., Lindsay R.J., Orellana L., Mildvan D., Bazner S., Streeck H., Alter G., Lifson J.D., Carrington M., Bosch R.J., Robbins G.K., Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Holdcraft R.W., Shima J.E., Griswold M.D., Braun R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi M., Aoyama H., Morishita M., Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J. Periodontol. 1992;63:28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- Miyaura H., Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J. Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- Mohllajee A.P., Curtis K.M., Martins S.L., Peterson H.B. Hormonal contraceptive use and risk of sexually transmitted infections: a systematic review. Contraception. 2006;73:154–165. doi: 10.1016/j.contraception.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Molloy E.J., O'neill A.J., Grantham J.J., Sheridan-Pereira M., Fitzpatrick J.M., Webb D.W., Watson R.W. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. 2003;102:2653–2659. doi: 10.1182/blood-2003-02-0649. [DOI] [PubMed] [Google Scholar]
- Murphy H.S., Sun Q., Murphy B.A., Mo R., Huo J., Chen J., Chensue S.W., Adams M., Richardson B.C., Yung R. Tissue-specific effect of estradiol on endothelial cell-dependent lymphocyte recruitment. Microvasc. Res. 2004;68:273–285. doi: 10.1016/j.mvr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Murphy K., Irvin S.C., Herold B.C. Research gaps in defining the biological link between HIV risk and hormonal contraception. Am. J. Reprod. Immunol. 2014;72:228–235. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musabak U., Bolu E., Ozata M., Oktenli C., Sengul A., Inal A., Yesilova Z., Kilciler G., Ozdemir I.C., Kocar I.H. Gonadotropin treatment restores in vitro interleukin-1beta and tumour necrosis factor-alpha production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin. Exp. Immunol. 2003;132:265–270. doi: 10.1046/j.1365-2249.2003.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun. Rev. 2012;11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Okuyama R., Abo T., Seki S., Ohteki T., Sugiura K., Kusumi A., Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J. Exp. Med. 1992;175:661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N.J., Kovacs W.J. Gonadal steroids and immunity. Endocr. Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Olsen N.J., Watson M.B., Henderson G.S., Kovacs W.J. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129:2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- Page S.T., Plymate S.R., Bremner W.J., Matsumoto A.M., Hess D.L., Lin D.W., Amory J.K., Nelson P.S., Wu J.D. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am. J. Physiol. Endocrinol. Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- Patas K., Engler J.B., Friese M.A., Gold S.M. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J. Reprod. Immunol. 2013;97:140–146. doi: 10.1016/j.jri.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Pennell L.M., Galligan C.L., Fish E.N. Sex affects immunity. J. Autoimmun. 2012;38:J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Pernis A.B. Estrogen and CD4+ T cells. Curr. Opin. Rheumatol. 2007;19:414–420. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- Pido-Lopez J., Imami N., Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin. Exp. Immunol. 2001;125:409–413. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M.J., Carson B.D., Subramanian S., Afentoulis M., Vandenbark A.A., Ziegler S.F., Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Procaccini C., Pucino V., Mantzoros C.S., Matarese G. Leptin in autoimmune diseases. Metabolism. 2015;64:92–104. doi: 10.1016/j.metabol.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Purton J.F., Monk J.A., Liddicoat D.R., Kyparissoudis K., Sakkal S., Richardson S.J., Godfrey D.I., Cole T.J. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J. Immunol. 2004;173:3816–3824. doi: 10.4049/jimmunol.173.6.3816. [DOI] [PubMed] [Google Scholar]
- Rettew J.A., Huet-Hudson Y.M., Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- Saba E., Origoni M., Taccagni G., Ferrari D., Doglioni C., Nava A., Lisco A., Grivel J.C., Margolis L., Poli G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013;6:1081–1090. doi: 10.1038/mi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiani S., Olsen N.J., Kovacs W.J. Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- Santoro N., Brown J.R., Adel T., Skurnick J.H. Characterization of reproductive hormonal dynamics in the perimenopause. J. Clin. Endocrinol. Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Sullivan M., Narvaez C.F., Holmes T.H., Furman D., Zheng N.Y., Nishtala M., Wrammert J., Smith K., James J.A., Dekker C.L., Davis M.M., Wilson P.C., Greenberg H.B., He X.S. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein J.G., Park S., Nahm M.H. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine. 2008;26:5521–5526. doi: 10.1016/j.vaccine.2008.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher G.F.B. In: The Biology of the Cervix. Blandau R.J., Moghissi K., editors. The University of Chicago Press; 1973. [Google Scholar]
- Schumacher A., Brachwitz N., Sohr S., Engeland K., Langwisch S., Dolaptchieva M., Alexander T., Taran A., Malfertheiner S.F., Costa S.D., Zimmermann G., Nitschke C., Volk H.D., Alexander H., Gunzer M., Zenclussen A.C. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu C.J., Benoist C., Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin. Immunol. 2012;24:436–442. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson G., Couse J.F., Lubahn D.B., Korach K.S., Kincade P.W. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J. Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- Smithson G., Medina K., Ponting I., Kincade P.W. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J. Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- Smyth A., Oliveira G.H., Lahr B.D., Bailey K.R., Norby S.M., Garovic V.D. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin. J. Am. Soc. Nephrol. 2010;5:2060–2068. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana R., Tarazona R., Gayoso I., Lesur O., Dupuis G., Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Staples J.E., Gasiewicz T.A., Fiore N.C., Lubahn D.B., Korach K.S., Silverstone A.E. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J. Immunol. 1999;163:4168–4174. [PubMed] [Google Scholar]
- Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Strindhall J., Skog M., Ernerudh J., Bengner M., Lofgren S., Matussek A., Nilsson B.O., Wikby A. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr.) 2013;35:985–991. doi: 10.1007/s11357-012-9400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Sun Y., Ma F., Lu P., Huang H., Zhou J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol. Lett. 2009;125:151–155. doi: 10.1016/j.imlet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Yates M., Vandenbark A.A., Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–347. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J., Faust Z., Varga P., Szereday L., Kelemen K. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. Am. J. Reprod. Immunol. 1996;35:348–351. doi: 10.1111/j.1600-0897.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J., Polgar B., Kozma N., Miko E., Par G., Szereday L., Barakonyi A., Palkovics T., Papp O., Varga P. Progesterone-dependent immunomodulation. Chem. Immunol. Allergy. 2005;89:118–125. doi: 10.1159/000087953. [DOI] [PubMed] [Google Scholar]
- Tait A.S., Butts C.L., Sternberg E.M. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J. Leukoc. Biol. 2008;84:924–931. doi: 10.1189/jlb.0208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi A., Reginster J.Y., Luyckx F., Legros J.J. No major month to month variation in free testosterone levels in aging males. Minor impact on the biological diagnosis of ‘andropause’. Psychoneuroendocrinology. 2005;30:638–646. doi: 10.1016/j.psyneuen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F., Silveira L.F., Maccoll G.S., Bouloux P.M. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J. Endocrinol. 2003;176:293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- Trigunaite A., Dimo J., Jorgensen T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Valor L., Teijeiro R., Aristimuno C., Faure F., Alonso B., De Andres C., Tejera M., Lopez-Lazareno N., Fernandez-Cruz E., Sanchez-Ramon S. Estradiol-dependent perforin expression by human regulatory T-cells. Eur. J. Clin. Invest. 2011;41:357–364. doi: 10.1111/j.1365-2362.2010.02414.x. [DOI] [PubMed] [Google Scholar]
- Van Cauter E., Leproult R., Kupfer D.J. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J. Clin. Endocrinol. Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- Van Den Brandt J., Luhder F., Mcpherson K.G., De Graaf K.L., Tischner D., Wiehr S., Herrmann T., Weissert R., Gold R., Reichardt H.M. Enhanced glucocorticoid receptor signaling in T cells impacts thymocyte apoptosis and adaptive immune responses. Am. J. Pathol. 2007;170:1041–1053. doi: 10.2353/ajpath.2007.060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh J., Peeva E., Xu X., Diamond B. Cutting edge: hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J. Immunol. 2006;176:3311–3314. doi: 10.4049/jimmunol.176.6.3311. [DOI] [PubMed] [Google Scholar]
- Voskuhl R.R., Gold S.M. Sex-related factors in multiple sclerosis susceptibility and progression. Nat. Rev. Neurol. 2012;8:255–263. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic S., Hutchinson M., Hours M., Moreau T., Cortinovis-Tourniaire P., Adeleine P., Confavreux C., The Pregnancy In Multiple Sclerosis Group, Pregnancy In Multiple Sclerosis Group Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127:1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- Vural P., Akgul C., Canbaz M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol. Res. 2006;54:298–302. doi: 10.1016/j.phrs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Wang C., Dehghani B., Li Y., Kaler L.J., Vandenbark A.A., Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H.D., Crassi K.M., Givan A.L., Stern J.E., Gonzalez J.L., Memoli V.A., Green W.R., Wira C.R. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J. Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- Wira C.R., Crane-Godreau M.A., Grant-Tschudy K.S. In: Mucosal Immunology. Mestecky J., editor. Academic Press; 2005. [Google Scholar]
- Wira C.R., Fahey J.V. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira C.R., Rodriguez-Garcia M., Patel M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.F., Chow K.M., De Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG. 2003;110:641–642. doi: 10.1046/j.1471-0528.2003.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Greer J.M., Hull R., O'sullivan J.D., Henderson R.D., Read S.J., Mccombe P.A. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun. Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanushpolsky E.H. Luteal phase support in in vitro fertilization. Semin. Reprod. Med. 2015;33:118–127. doi: 10.1055/s-0035-1545363. [DOI] [PubMed] [Google Scholar]
- Yasui T., Maegawa M., Tomita J., Miyatani Y., Yamada M., Uemura H., Matsuzaki T., Kuwahara A., Kamada M., Tsuchiya N., Yuzurihara M., Takeda S., Irahara M. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Youinou P., Pers J.O. The late news on baff in autoimmune diseases. Autoimmun. Rev. 2010;9:804–806. doi: 10.1016/j.autrev.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Zen M., Canova M., Campana C., Bettio S., Nalotto L., Rampudda M., Ramonda R., Iaccarino L., Doria A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011;10:305–310. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tang D., Zhao H. Immunological therapies can relieve aromatase inhibitor-related joint symptoms in breast cancer survivors. Am. J. Clin. Oncol. 2010;33:557–560. doi: 10.1097/COC.0b013e3181cae782. [DOI] [PubMed] [Google Scholar]
- Zoumakis E., Rice K.C., Gold P.W., Chrousos G.P. Potential uses of corticotropin-releasing hormone antagonists. Ann. N.Y. Acad. Sci. 2006;1083:239–251. doi: 10.1196/annals.1367.021. [DOI] [PubMed] [Google Scholar]


