Abstract
The durability of textiles can be endangered in different ways. Various treatments are used to protect textiles against degradation or damage or even to restore or repair the initial properties. Other technologies are still under investigation. In this chapter an overview is presented of the work done in our laboratory in the area of smart coatings protecting textiles.
Keywords: Antimicrobial activity, Infrared protection, Responsive coatings, Self-healing, UV resistance
4.1. Introduction
The durability of textiles can be endangered in different ways. Abrasion or scratching, attack by bacteria or fungi, and the influence of UV or temperature impair the preservation of properties induced during the processing of textiles. Various textile treatments are used to protect against degradation or damage or even to restore or repair the initial properties. Besides conventional coating methodologies, increasing focus is put on smart coatings to comply with these demands.
In the following paragraphs an overview of some work done at Centexbel in the area of smart durable coatings is presented: Self-healing coatings active at room temperature were developed as a way to protect against scratches, coatings protect textiles against attack by bacteria or fungi, and UV and infrared (IR)-responsive coatings were developed enabling the detection of harmful chemicals in the environment, undesirable temperature changes and excessive UV levels.
4.2. Types and classifications of smart coatings for improving textile durability
4.2.1. Self-healing textile coatings
The lifetime of a coated textile can be prolonged if it can be healed at an early stage of damage formation (stage of microcracks). If the coating is able to self-heal and therefore able to repair deterioration of its functionality, the durability of the coated products can be extended. This self-healing process can occur autonomously or can be triggered by an external stimulus (eg, heat, radiation).
Various concepts have been developed over the past decade and are still being fine-tuned. A lot of emphasis is put on the use of self-healing concrete, composites and coatings for corrosion prevention. The use of self-healing coatings on textiles has been less investigated to date.
Both extrinsic and intrinsic systems have been developed.
Extrinsic approaches make use of a container which is filled with the healing agent. When damage occurs, the container breaks and the healing agent is released in the crack and reacts with the catalyst or curing agent present. These containers can be spherical microcapsules (Fig. 4.1 ; White et al., 2001), hollow (Fig. 4.2 ; Mauldin and Kessler, 2010), or compartmented fibres (Mookhoek et al., 2012), or a vascular system can be created (Toohey et al., 2007).
Figure 4.1.

Self-healing process based on the incorporation of microcapsules. (a) Crack propagation up to healing agent capsule, (b) monomer flow, (c) crack healing by polymerization.
Figure 4.2.
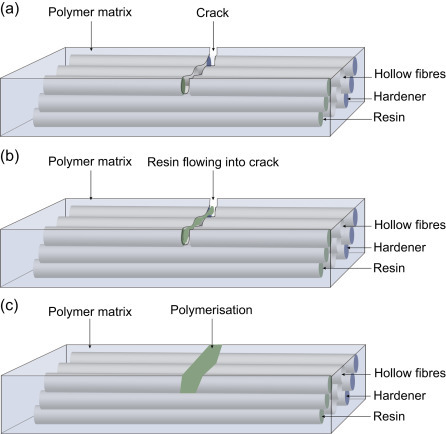
Self-healing process based on the incorporation of hollow fibres. (a) Crack propagation up to healing agent capsule, (b) monomer flow, (c) crack healing by polymerization.
In a textile coating the use of microcapsules is the most feasible extrinsic approach. Microcapsules containing a healing agent can be added to an existing coating paste and are easily applicable. A drawback of this method is the nonrecurring healing action, as damage at this particular area will be repaired only once. Also, the amount of available healing agent is limited owing to the size of the capsules.
When multiple repair actions are desired, one can use an intrinsic system based on reversible covalent chemistry, physical or supramolecular interactions (Garcia and Fischer, 2014). In this case the damaged coating is able to repair itself by means of an increase in mobility. The coating has an enhanced flow (mostly at elevated temperatures) which enables a refill of the scratch. The restored scratch gains strength because of restored bonds (chemically/physically) (García et al., 2011, Ghosh, 2009). This technology is already in use in the aerospace and automotive industry. Self-repairing car paints have been developed in which heat triggers the repair action.
Several companies have developed self-healing systems which possibly could be implemented on textiles. An overview is given in Table 4.1 .
Table 4.1.
Nonexhaustive list of suppliers of self-healing systems
| Self-healing agent | Supplier |
|---|---|
| SupraB-technologies | Suprapolix |
| Reverlink™ | Arkema |
| AMI technology | Autonomic Materials |
| Bayhydrol® U XP 2750 | Bayer MaterialScience |
Other companies such as BASF, AkzoNobel, Dow and DSM are also investigating the possibilities.
4.2.2. Antimicrobial and antifouling coatings
Because of the high surface area, roughness, and ability to absorb moisture, textiles are prone to be rapidly colonised by microorganisms. These organisms affect the textile's lifetime by destroying the fibres and discolouring the fabric and they may spread unpleasant odours (Boryo, 2013, Burgess, 1954). The latter has been an important incentive to develop antimicrobial textile treatments for garments, bedding and sportswear. In addition, textiles may act as vectors for infectious diseases or spread nosocomial bacteria throughout hospital facilities (Leung and Chan, 2006, Treakle et al., 2009, Curtis White et al., 2010).
Several methods have been devised to hamper bacterial growth (bacteriostatic) or even kill bacteria (bactericide) on textiles. The treatments include releasing toxic compounds from fabric or attack microorganisms when they approach the fibre surface. Some treatments offer broad-spectrum protection whereas others are predominantly useful against bacteria or fungi. Antimicrobial compounds include metals, organometal derivatives, phenolic compounds, nitrogen and halogen compounds and nanoparticles (Coleman, 2005, Dastjerdi and Montazer, 2010, Gouveia, 2010, Shahidi and Wiener, 2012, Varesano et al., 2011, Vaun Mcarthur et al., 2012, Lacasse and Baumann, 2004, Simoncic and Tomsic, 2010). Bio-based products have gained increasing attention (Joshi et al., 2009, Simoncic and Tomsic, 2010, Yuan and Cranston, 2008).
4.2.2.1. Metal-based antimicrobials
Metals such as silver, titanium, zinc and copper are used in finishes and coatings and during fibre extrusion. Although silver is relatively more effective against bacteria compared with copper, which is more effective towards fungi, in both cases the metal ion needs to be released from the metal core to exert its activity. The metal ion will form complexes with vital compounds of the microorganism such as the cell membrane, enzymes and DNA. As a result, the microorganism's metabolism fails in several crucial areas (Lemire et al., 2013). Therefore, resistance against these ions is unlikely to develop because the microorganism is under attack at several targets at the same time. However, as to all antimicrobial products, also for silver and copper, resistive strains have been identified but only in very specific environments such as wastewater treatment plants and mining (Silver, 2003). The active metal ions can be released by dissolving directly from the metal, or by ion exchange. The latter is often used in silver-based products in which silver ions are bound to a ceramic matrix (Lorenz et al., 2012). Although the mobile metal ion is the active compound, metal-based antimicrobials are often not considered to be leaching out. In contrast to toxic chemical compounds, silver and copper ions easily form metal complexes with any organic substance in the vicinity. Leaching test methods such as agar diffusion tests (EN 20645 and AATCC 147) will therefore show no or only limited leaching from silver-treated fabrics.
4.2.2.2. Halogen compounds
Halogen-based antimicrobials from highly reactive oxides are known for strong disinfecting properties. Chlorine- and iodine-based products are well-known cleaning and disinfectants (bleach, chlorhexidine and Betadine). Such derivatives are also used in textiles (Coleman, 2005). However, the persistence of halogens in the environment, the aggressive nature of these products (also to textile fibres) and their relation to cancer by disinfectant by-products (in particular for chlorine) limit their use in textiles. Common products are pentachlorophenol, dichlorodiphenyl methane, iodine and iodophors (Lacasse and Baumann, 2004).
4.2.2.3. Nitrogen compounds
Nitrogen compounds such as urea, amine and guanidine are used to denature proteins, hence their antimicrobial properties. One special form of nitrogen compounds for antimicrobial treatment is the so-called quats or quaternary ammonium salts (Coleman, 2005, Shahidi and Wiener, 2012, Windler et al., 2013). These molecules have a positive charge, attracting the negatively charged cell membrane of microorganisms. Mostly, the positively charged nitrogen head is followed by a long organic chain. This combination allows the molecule to puncture the cell membrane, consequently destroying the microorganisms (Curtis White et al., 2010). The advantage of the quats' molecules is their odour-free nature and the ability to add functional groups to the organic chain, which allows them to form a chemical bond to the textile fibres. In this way, leaching is limited and durability increases compared with the smaller nitrogen compounds mentioned previously.
4.2.2.4. Phenolic compounds
Phenolic compounds have a long history as disinfectants. However, the carcinogenic nature of phenols has led to the development of derivatives that are less toxic to humans. The best known is triclosan. Its relatively low toxicity to humans, effective disinfection properties and cheap production has led triclosan to become one of the highest selling disinfectants worldwide. However, its use in a wide variety of customer care products has induced the development of resistive bacteria strains (Windler et al., 2013, Lacasse and Baumann, 2004).
4.2.2.5. Aldehyde compounds
Aldehyde compounds such as formaldehyde and glutaraldehyde are commonly used for cross-linking purposes in chemistry. However, they also cross-link proteins and enzymes, rendering them useless for microorganisms (Windler et al., 2013, Lacasse and Baumann, 2004). The carcinogenic nature of these compounds has put a strict regulation on these products in textiles.
4.2.2.6. Bio-based products
One of the best known bio-based antimicrobial products is chitosan. It is a polysaccharide derived from chitin, an abundantly available waste product in shrimp farms. Although the deacetylation process to form chitosan from chitin is far from eco-friendly, it still is a renewable resource of antimicrobial products. The active groups consist of an array of amine functionalities organised on the polysaccharide chain. Its activity is therefore similar as to the nitrogen compounds described previously.
The renewed interest in bio-based products has led to the development of new antimicrobial products in textiles (De Smet et al., 2015a). Often, these products originate from food processing, and researchers try to transfer these products in useful additives for textiles. Bio-based products are mostly plant extracts such as menthol, carvacrol (Oregano sp.), thymol (Thymus sp.), eucalyptol (Eucalyptus sp.), neem extract (Azadirachta indica), aloe vera (Aloe barbadensis), prickly chaff flower (Achysanthus aspera), eugenol (Syzygium aromaticum), turmeric and cumin, to name a few (Joshi et al., 2010, De Smet et al., 2015b). The antimicrobial activity of plant extracts such as peppermint, primrose and perilla oil has been explored as well. In addition, animal extracts also has potential as antimicrobial additives, such as sericin, a macromolecular protein derived from the silkworm Bombyx mori (Joshi et al., 2009, Shahidi and Wiener, 2012).
Although a vast number of bio-based alternatives show clear potential as antimicrobial textile treatment, the durability, applicability, availability and sometimes discolouring or unpleasant smell limit their use at an industrial level in textiles.
Alternatively, and by the grace of improved biotechnology tools for peptide production, short-chain amino acids have been reported to be useful as a bio-based antimicrobial finish for textiles (Gouveia, 2010).
4.2.2.7. Antifouling
Fouling is the result of the continuous accumulation of debris at the surface of films, rigid plates, and filters. Clearly textiles are also prone to fouling, and this chapter will focus on biofouling or biofilm formation and how it can be tackled. Fouling is a result of the stagnating flow of fluids at the surface from which debris will deposit, attracted by van der Waals forces. This process occurs within minutes after submersion. In case of biofilm formation, the first molecules depositing on the surface are proteins and polysaccharides. On the one hand, these molecules cover up any surface functionality and provide a suitable ground for bacteria to attach and develop on this new extracellular matrix (ECM). Bacteria possess surface-bound adhesion proteins designed to bind to such ECM, thus mediating colonisation of the surface. Once the bacteria and other microorganisms have gathered, exertion of more and more proteins and polysaccharides form the so-called external polysaccharide matrix in which the microorganisms thrive and form a complex ecosystem (Bixler and Bhushan, 2012, Harder and Yee, 2009). Some microorganisms and even macroorganisms are expert in colonising virgin surfaces by excreting specially designed attachment proteins such as adhesins (well known in various infectious bacteria) and mussel adhesion proteins (Mytilus edulis) (Klemm and Schembri, 2000, Qin and Buehler, 2014).
To overcome fouling (and biofouling in particular), surface modifications aim to (Lejars et al., 2012):
-
•
increase shear and reduce surface energy
-
•
limit anchoring points
-
•
kill attaching organisms
-
•
periodically or gradually remove the top layer of the surface
Both biofilm formation and possible antifouling strategies are illustrated in Fig. 4.3 .
Figure 4.3.

Biofilm formation (top, left to right) and possible antifouling approaches (bottom).
Increasing the shear at the surface and limiting surface energy can be achieved by creating very sleek omniphobic surfaces (Genzer and Efimenko, 2006). Usually, water- and oil-repellent fluorine-based coatings are used to reduce surface free energy as much as possible. As a result, both the static layer of fluids near the surface and the van der Waals forces are reduced (Van Zoelen et al., 2014). However, microorganisms still manage to colonise these surfaces by secreting viscous, slime-like substances that cover the entire surface and thus cover the underneath functionalities. In contrast to creating hydrophobic and oleophobic surfaces, positive results have been obtained by creating extremely hydrophilic surfaces. The reasoning behind this is that fouling (organic) molecules are partially composed of long-chain hydrocarbon segments that are strongly repelled by the superhydrophilic surface properties (Patel et al., 2010).
Protein and polysaccharide attachment can be limited by creating a dense brush-like layer at the surface. As a result, all possible anchoring points are occupied and no new (bio)polymers can attach. It has been demonstrated that polyethylene glycol (PEG) is effective in creating such surfaces (Ding et al., 2012). Unfortunately, the rigidity of such a PEG brush is low and the layer is easily damaged. Another option is not to prevent initial protein and polysaccharide attachment but to destroy any microorganism in the vicinity of the surface. This way, only a thin fouling film is developed but biofilm formation and growth of the film is significantly hampered or blocked.
Copper and heavy metal (such as mercury and tin) based products are available in coating formulations. To be effective, however, they need to leach out toxic compounds, and thus environmental concerns rise regarding the use of such coatings (Dafforn et al., 2011).
A final option is to release a small part of the coating on the surface periodically or gradually. Any biofilm developed will be removed and a new clean surface is obtained.
4.2.3. Coatings to protect against ultraviolet and infrared radiation
4.2.3.1. Ultraviolet
Fabrics exposed to UV irradiation fade in colour, strength and durability. This so-called ageing is caused by short-wavelength light that interacts with electron-rich molecules. Such molecules will absorb energy from the light waves and subsequently break down (Rabek, 1996, Alen and Edge, 1993, Andrady et al., 1998). UV rays are predominantly found in sunlight but artificial lights such as discharge lamps (tube lights, halogen bulbs) also emit UV. The problem of UV ageing is mostly recognised for outdoor textiles, leisurewear and car interiors but also indoor textiles close to windows such as upholstery, curtains and sun blinds.
UV-protective additives in finishing, dyeing, coating and extrusion focus on blocking the highly reactive UV rays from reaching sensitive pigments, binders or fibres. Some products have a high affinity for the UV waves and are preferably attacked by UV, transforming it into harmless heat (UV absorbers). Others block the propagation of free radicals initiated by UV irradiation (radical scavengers). A third mode of action is to decompose peroxides formed by UV irradiation in combination with oxygen. These products act as antioxidants but can also improve UV durability (peroxide decomposer). A final mode of action is to quench excited states and divert the energy into lower-wavelength light (fluorescence or phosphorescence) or heat (quenchers) (Patra and Gouda, 2013, Kasza, 2013, Lacasse and Baumann, 2004). The different modes of action of UV-stabilising additives are presented in Fig. 4.4 whereas Table 4.2 summarises typical light-stabilising compounds.
Figure 4.4.

Mode of action of various UV-stabilising additives. P, polymer chain; P•, polymer with radical; CH∗, hydrocarbon chain in exited state.
Based on BASF, 2016. Light Stabilizers. Available from: http://www.dispersions-pigments.basf.com/portal/basf/ien/dt.jsp?print=center&setCursor=1_556325 (accessed 08.01.2016.); Rabek, J.F., 1996. Photodegradation of Polymers, Berlin Heidelberg, Springer-Verlag, and Alen, N.S., Edge, M., 1993. Fundamentals of Polymer Degradation and Stabilization, Springer.
Table 4.2.
Typical light-stabilising compounds and their mode of action
| Light-stabilising compound | Mode of action |
|---|---|
| Carbon black | UV-absorbent pigment |
| Titanium oxide | UV-absorbent pigment |
| Benzophenone | UV absorber |
| Benzotriazole | UV absorber |
| Halogenated benzotriazole | UV absorber |
| Triazinyl | UV absorber |
| Hydroxyphenyltriazine | UV absorber |
| Metal nanoparticles (CeO, ZnO) | UV absorber |
| Oxalic acid derivatives | Radical scavenger |
| Sterically hindered amines (HALS) | Radical scavenger |
| Sterically hindered phenols | Radical scavenger |
| Copper, manganese, sulphur complexes | Peroxide decomposer |
| Nickel compounds | UV quencher |
Although these UV stabilisers are used in numerous applications, their small molecular nature allows for easy migration throughout the polymer. Small molecule stabilisers may leach out into the environment and no longer protect the material (Scott, 1999). The current trend is to develop macromolecular UV, heat or hydrolysis stabilisers (Raching GmbH, Chemtura, Ciba and Vanderbilt). These high-molecular-weight molecules can be effective and durable and do not migrate in the polymer. However, macromolecular UV stabilisers require more synthesis, which results in more expensive additives (Kasza, 2013).
4.2.3.2. Infrared
The heat stability of coated textiles differs depending on the coating composition. Exposure of a coated textile to sunlight can have a deteriorating effect. Sunlight consists of visible light, accompanied by about 53% IR light. IR radiation with wavelengths in the range of 780 nm up to 1 mm (Fig. 4.3) is responsible for heating phenomena. Thus, coated textiles exposed to sunlight will heat up when absorbing these wavelengths. It is claimed that a lower-temperature increase in the coating will result in enhanced durability because of less polymer degradation and thermal expansion (Fang et al., 2013, Pasternack, 2013). Only limited research has been performed on the effect of reflective pigments, the related temperature rise of the coating and the durability of the coatings (Salminen, 2014).
The reflection of this IR radiation on a coated textile can be augmented by the incorporation of IR-reflective pigments. This results in a lower absorption and transmission of IR, and therefore less heating. These coatings are denominated as low-E coatings (low emissivity).
These low-E coatings are predominantly used in architectural textiles for their thermal properties (Cremers, 2010). For example, the inside temperature of a building will be lowered when such a coating is implemented on the façade of the building. This results in lower energy consumption because of reduced air conditioning. Examples of IR-reflective coatings can also be found in the ceiling of ice tracks and on roofs and window blinds.
Several types of IR-reflecting additives have been designed based on:
-
•
Pure metals (such as Al, Ag and Cu) with a specific size
-
•
Metals with surface coatings (AlO[OH] or SiO2 on Al and AgS on Ag)
-
•
Metal oxides such as titanium dioxide and zinc oxide
-
•
Multiple-layered structures: TiO2/Au/TiO2, silicon powder and metal-coated cenosphere particles
-
•
Minerals such as mica
4.3. Properties of textiles with durability-enhancing coatings
4.3.1. Scratch resistance
Research in the field of self-healing coatings focusses on corrosion protective coatings and concrete; not much research has been performed on coated textiles.
The first self-healing experiments with polyurethane-coated fabric, in which the embedded microcapsules were filled with a PU binder, show great potential but still need to be optimised. The research was executed in the framework of the FP7 Safe@Sea project, in which improved garments for fishermen were developed (Anon, 2016a).
In experiments in which heat-triggered, self-healing polymers were evaluated on textiles, the typical open textile structure proved worrisome because the elevated temperature (up to 140°C) enhanced the flow of the polymer needed to close the crack. Owing to this enhanced flowing behaviour, the coating starts to penetrate into the textile substrate, which is not advantageous for the flexibility of the coated textile (De Vilder and Vanneste, 2013). Also, introducing these elevated temperatures to the coated textiles during the lifetime of the protective garment, technical textile, and so on is not self-evident because this can alter properties such as dimensional stability.
At Centexbel, a self-healing coating was developed which acts at room temperature. An acrylate textile coating was selected which shows the desired flowing behaviour. A supramolecular additive with a self-healing property based on hydrogen bond formation was added to the coating. This additive improves the mechanical strength of the filled crack. The coating was applied on a polyester substrate via knife-over-roll (200 μm). The coating was abraded with F2 sandpaper (Martindale, EN 510, five cycles). Most of the recovery was noticeable by 2 days at room temperature. At the stage of microcracks, full recovery was observed (Fig. 4.5 ). When too much coating material is removed, the system is unable to refill the crack completely.
Figure 4.5.

Acrylic self-healing coating on textile: (left) initial damage, (right) residual damage after 2 days at room temperature.
To evaluate the effect of different formulations, a homogeneous and uniform scratch was made with a modified motorised pencil hardness tester (De Vilder and Vanneste, 2013; Fig. 4.6 ). This allowed the efficacy of the self-healing properties to be compared for different formulations.
Figure 4.6.

Acrylic self-healing coating on a polycarbonate plate: (left) initial damage, (right) residual damage after 2 days at room temperature.
Self-healing coatings can be implemented when the textile is prone to wear and tear, cuts, abrasion and so forth. From an aesthetic point of view an intact coating is desirable, but the coating should also maintain functionality as long as possible. Prolonged barrier properties such as waterproofness, electrical (Blaiszik et al., 2012) and thermal conductivity and recovered hydrophobicity and/or oleophobicity (Wang et al., 2013, Dikić et al., 2012) can be achieved.
4.3.2. Antibacterial and antifungal properties
Numerous fabrics are claimed to have antimicrobial efficacy. Table 4.3 highlights several of them.
Table 4.3.
Nonexhaustive list of products claiming antimicrobial properties
| Product | Origin | Claimed benefits |
|---|---|---|
| Agion Technologies | Zeolite agent and silver | Recognised on severe acute respiratory syndrome and H5N1 |
| AEGIS Microbe Shield® Technology | Quaternary ammonium silane | Controls bacteria and fungus that cause objectionable odours, unsightly stains and product deterioration; friendly to environment. |
| Sorbtek/Amy | Silver-based antimicrobial | Characteristics: Total moisture control combined with silver-based antimicrobial. This catch, move and release technology keeps the wearer dry, comfortable and odour free. Available in comfort and performance stretch. |
| Thermolite freshFX | Polyester insulation with antimicrobial additive spun into fibres | Characteristics: Polyester insulation with sliver-based antimicrobial additive spun into fibres to protect against odours. Provides lightweight warmth with added freshness that lasts for life of product. |
| VisaEndurance brand of fabrics | Characteristics: Performance active-wear fabrics that fights odours, wicks moisture away from skin, dries quickly and releases stains in wash. Knit, breathable, soft hand, wicks perspiration away from body, dries quickly, fights odours, releases stains. | |
| iSyS AG | Dispersed silver chloride solution | Ionic form of silver has triple effect on bacteria: destruction of cell membrane, deactivation of cell metabolism, destruction of cell division. The product is based on nanotechnological sol-gel process: iSyS AG is combined with reactive organic–inorganic binder (iSyS MTX). |
| Sanitised T27-22 | Suspension of silver chloride in water | Product has excellent wash and light fastness. Product blocks respiration and food intake of bacteria and acts on cell membrane which inhibits cell functions. |
| Sanitised TH22-27 | Dispersion of zinc pyrithione | To obtain wash-fastness the compound needs to be linked with a binder or put into wash-resistant coating. Product acts on cell membrane of microorganism. |
| Ultra-Fresh Silpure FBR-5 | Ultra-Fresh Silpure FBR-5 Activator (Part A) is an aqueous dispersion of a silver compound. Ultra-Fresh Silpure FBR-5 (Part B) is specifically formulated to combine with Part A. | Combined product is engineered for controlled release of silver ions providing control of bacterial growth. |
| Ultra-Fresh DW-56 | One of the active compounds in Ultra-Fresh DW-56 is bis(1-hydroxy-2(1H)-pyridinethionato-O,S)-(T-4) zinc. | Aqueous suspension designed to control growth of fungi, bacteria and algae in polyurethane foams, aqueous coatings and textiles. Effective in preventing infestation of house dust mites in treated articles. |
Clearly, antimicrobial effectiveness is directed not only by the product applied but also by the concentration used. Table 4.4 shows typical concentrations of antimicrobial textile additives and their limit values according to OEKO-TEX® 100 voluntary labeling (OEKO-TEX®, 2015).
Table 4.4.
Typical concentrations of antimicrobial textile additives and their limit values according to OEKO-TEX® 100 voluntary labelling
| Biocide | Typical concentration [ppm] | References | Limit value [ppm]a |
|---|---|---|---|
| Formaldehyde | <30 (clothing) | Piccinini et al., 2007 | 16–300 |
| Organometallics | 1.104–5.104 | Baumann et al., 2000 | 25.106–50.106 (copper) |
| Silver | 0.016–27.104b | Windler et al., 2013; Lorenz et al., 2012 | – |
| Triclosan | 7–195 | Windler et al., 2013 | 0.2–2 |
| Tributyltin oxide | 0.1–13.3 | Janssen et al., 2000 | 0.5–1 |
| QUAT | 2500 | Windler et al., 2013 | – |
OEKO-TEX® 100 limit values are depending on the final use of the fabric under investigation.
High levels of silver indicate the use of pure silver fibres, low values point to silver ion complexes.
Antimicrobial efficiency is expressed only as a binary annotation (growth or inhibition) or the area of inhibition for leaching substances in, for example, the agar diffusion test (eg, DIN EN ISO 20645 – 2001, AATCC 147). Quantitative test methods are also possible in which the bacterial reduction is expressed in log scale (eg, JIS L 1902, DIN EN 1276, AATCC100, ASTM E 2149-01, ASTME 2180-01). In such tests reports, negative numbers denote bacterial growth instead of a reduction of the number of bacterial colony-forming units. An overview of available test methods and their preferred use is nicely described in Pinho et al. (2011) and Llc (2010). Table 4.5 summarises some antimicrobial effectiveness results against Staphylococcus aureus obtained from various antimicrobial textile treatments.
Table 4.5.
Antimicrobial effectiveness results against Staphylococcus aureus obtained from various antimicrobial textile treatments
| Active treatment | Bacterial log reduction (hours of exposure) | Test method | References | |
|---|---|---|---|---|
| Quat | 0.8 (6) | 1.66 (24) | AATCC100 | Smith et al. (2010) |
| Triclosan | 0.68 (6) | >1.6 (24) | AATCC100 | Smith et al. (2010) |
| AgCl | >2 (3) | >2 (24) | ASTM E 2149 | Own research |
| Zn pyrithione | 0.6 (3) | 1.8 (24) | ASTM E 2149 | Own research |
| Thymol | – | Inhibition (24) | ISO 20645 | De Smet et al. (2015b) |
| Chitosan | – | Inhibition (24) | ISO 20645 | De Smet et al. (2015b) |
4.3.3. Ultraviolet resistance
From the wealth of available UV stabilisers, it may be difficult to identify the most-suited one for a specific application. The properties and action mode of a given UV stabiliser may already point towards its usefulness as a top coating (UV absorber), as a dispersed additive in a binder (quencher or peroxide decomposer), or even chemically linked to a dye if possible (sterically hindered amines). The chart in Fig. 4.7 illustrates the effect of various types of UV stabilisers in an acrylic textile coating combined with a red pigment. This chart is not intended to discriminate between good or bad UV stabilisers; nonetheless, it shows what could happen if UV stabilisers were randomly dispersed and mixed in a typical textile coating formulation.
Figure 4.7.

Effect of various UV-stabilising agents in a red-pigmented acrylic textile coating after UV exposure (according to EN 14836). Colour difference is expressed as ΔE according to ISO 105-J03 (1995), ΔE > 1 ( ) is observable by the human eye. Concentrations are advised by the TDS of the respective manufacturer. (a) and (b) denote different producers.
) is observable by the human eye. Concentrations are advised by the TDS of the respective manufacturer. (a) and (b) denote different producers.
As an example, care must be taken when mixing UV stabilisers. Hindered amine light stabilisers combined with triazine actually accelerated UV ageing. Clearly in this case, the UV absorber (triazine) should have been applied as a top coat. The same holds for metal oxides, alleged UV absorbers. However, the mixture of CeO and ZnO in this particular case shows a synergistic acceleration of ageing. Although the mode of action of the various UV stabilisers is well known, it remains sensible to test them for your specific application and challenge them with a well-chosen test method.
4.3.4. Infrared reflection
A textile can be finished or coated with IR-reflective additives added to a binder system. Depending on the additive used, up to 70% of the incident IR radiation can be reflected. This percentage will be influenced by the binder type, colour, other additives present and the coating thickness.
At Centexbel, a two-layered PVC-coated polyester fabric was made with 15 wt% of mica pigments incorporated into the top layer. After gelling, the solar reflectance was recorded according to EN 410 (Fig. 4.8 ), which showed that 62% of the solar radiation was reflected.
Figure 4.8.

Solar reflectance by a double-layered PVC coating with 15 wt% mica pigments incorporated in the top layer.
Attention should be paid to full coverage of the surface of the coating by the additives; this will block penetration of the IR light into the polymer more efficiently (Figure 4.10, Figure 4.9 ). Lower concentrations of the pigments (less than 15 wt%) resulted in incomplete coverage of the surface and therefore a lower reflection rate. Augmenting the pigment concentration even more is also not advised: Once the surface is covered, this technology shows its full potential. Inserting the pigments in both the top and base layers does not improve the rate of reflection, which again shows that the predominant part of the reflection takes place at the surface.
Figure 4.10.
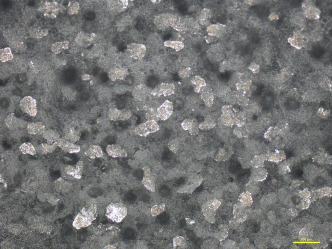
Microscopic image of IR-reflecting aluminium flakes incorporated into the top layer of a double-layered PVC coating: top view of the coating.
Figure 4.9.

Microscopic image of IR-reflecting aluminium flakes incorporated into the top layer of a double-layered PVC coating: cross-section of the coating.
4.4. Applications of smart durable and self-healing textiles
4.4.1. New generation of architectural fabrics
Developments in the building industry have shown a clear evolution of traditional construction in buildings with sensible and adaptable envelopes equipped with sensors and able to interact with the surrounding environment on the basis of inputs such as temperature, humidity and solar radiation. For structures designed for extreme applications (eg, large-span structures prone to fluttering and ponding, industrial applications characterised by high working temperatures, biogas basins with corrosive gases), there is a need for continuous monitoring to highlight anomalies and avoid progressive propagation of the initial damage.
The progressive miniaturisation of the sensor and the innovative manufacturing techniques for technical fabrics and foils resulted in new prospects for a new generation of sensible technical fabrics equipped with sensors focussing on temperature monitoring, pressure monitoring, and chemical sensing of noxious gasses.
Temperature monitoring of tensile structures is of particular interest for PVC-coated fabrics. PVC-coated fabrics for tensile structures have a glass transition temperature roughly between 70°C and 90°C (Sen, 2007, Wypych, 2008). At these temperatures, the polymer will become weaker and welded seams will slowly extend or even detach while under tension (Llorens, 2015). To signal a pending failure, temperature sensors can be mounted on the construction after erection. However, most exposed locations are often difficult to reach and attaching several sensors is laborious. In addition, the wiring compromises the aesthetics of the often admired organic shapes that can be achieved in textile architecture.
To overcome these issues, miniature temperature sensors are integrated into hybrid fabrics with integrated electric leads (Heyse et al., 2015). Electronic sensors are well known for their accuracy and ease of data acquisition, but for fabrics, connecting the monitor to the sensor fabric remains an issue of intense research (Chapman, 2012). Alternative to electronics, thermochromic pigments also respond to variations in temperature. Apart from liquid crystals, the temperature resolution of these materials is limited (Seeboth and Lötzsch, 2013). However, integrated in a coating, the spatial resolution is considerably higher than the point-wise electronic sensors. In addition, a quick visual inspection will indicate hot spots in real time. Although such thermochromic coatings may not be suitable for larger structures, they can be advantageous for quality control during welding of the seams where high enough temperatures have to be reached to ensure proper connection of the fabric parts (Fig. 4.11 ).
Figure 4.11.

Textile coating with thermochromic pigments. The coating instantaneously becomes transparent at temperatures above 70°C (right) and becomes black when cooling down (left).
Unfortunately, tensile structures are not always used for the purposes for which they are designed. In case of a defect, the user points to the manufacturer although misuse is sometimes the real cause of failure (Craig and Huntington, 2004, Seidel and Sturge, 2009). Common failure is caused by corrosive gases emitted by, for example, cattle or biological waste. For two of such gases, an irreversible indicator patch has been developed. Colour will change upon exposure to ammonia or hydrogen sulphide, indicating that the fabric properties can no longer be guaranteed (Fig. 4.12 ).
Figure 4.12.

Fabrics responsive to corrosive or toxic gases. Left: ammonia sensitive ((a) no NH3; (b) in the presence of NH3). Right: hydrogen sulphide sensitive ((c) no H2S; (d) exposed to H2S). Scale bar is 1 cm.
4.4.2. Protective clothing
Protective clothing is a comprehensive field because it contains garments for firefighters, fishermen, welders, divers, sports enthusiasts, motorcyclists, surgeons and military personnel. Specific suits have been developed for environments with chemical and/or biological danger, extreme temperatures, and so on. Each garment has specific properties adapted to the intended use, but a common demand is longevity. Besides an economical advantage, a more durable coating will result in prolonged protection, and in certain cases it can save lives.
In most cases protective clothing consists of multiple layers. The outer layer of the laminate, often coated, is most prone to wear and tear. The durability and therefore the lifetime of protective clothing can be prolonged by the use of wear- and tear-resistant coatings. Hence, the outer layer can be functionalised to improve abrasion/scratch/rub resistance and UV stability by incorporating specific additives.
Frequently used high-performance fibres such as p-aramid (PA) and m-aramid have poor resistance to abrasion. Thus they can be combined with fibres that have superior abrasion-resistant properties, protected by a PU coating (Mao, 2014). The durability of such a protective coating can be improved with additives.
The following example of a functionalised PU coating in protective clothing demonstrates the possibilities. Fishermen in the North Atlantic currently use heavyweight PVC-coated garments. Within the FP7 project Safe@Sea (Anon, 2016a) a lightweight alternative was developed based on PU-coated PA (De Vilder et al., 2012). In changing from a PVC-coated textile to a PU-coated one, an improvement in abrasion resistance was already noticeable. By incorporating specific additives into the PU coating, resistance to abrasion could be even further increased. Besides improved comfort owing to the lighter weight, prolonged durability of the garment was achieved (ie, less weight loss was observed) (Table 4.6 ).
Table 4.6.
Weight loss of coated textiles for fishermen (Martindale, EN 530-2, 10.000 cycles, F2 sandpaper)
| Fabric | Weight | Weight loss coating after Martindale (F2) |
|---|---|---|
| PVC-coated CO | 465 g/m2 | 5.9 wt% |
| Polyurethane-coated PA | 330 g/m2 | |
| Without additives | 4.0 wt% | |
| With additives | 2.2 wt% |
In many protective garments, the inner layer makes contact with the body of the person and is subjected to abrasion to a lesser extent. This fabric can be finished with additives such as sol–gel (Brzeziński et al., 2011) or silicones to diminish the impact of the abrading action.
4.4.3. Securing cargo
Although securing cargo may sound self-evident, correct loading and securing of cargo is not a trivial task (Rosi et al., 2012). Proper knowledge of the cargo mass, shape, content and expected accelerations, and appropriate use of securing tools and aids are prerequisites for the safe transportation of goods around the globe. Unfortunately, it is estimated that up to 25% of all accidents in the EU in which trucks are involved result from to inadequate securing of cargo (Copsey, 2010). Despite the lack of a fully harmonised standard and legislation in EU, the member states impose clear rules on cargo securing (Andersson et al., 2012). In addition, well-defined guidance documents are available for normal and exceptional transports explaining how and with what tools cargo should be secured safely (European Commission, 2007, European Commission, 2014). One of the most recognised tools is the use of flat fabric webbing slings and lashes that have to comply with EN 12195 standards. Off-the-shelf lashes are compliant with these strict regulations, but how do such materials behave during use and at what point is safe practise no longer guaranteed? Periodic inspection is voluntary and is based on subjective, visual inspections focusing on (ASME, 2014):
-
•
Acid or caustic burns
-
•
Melting or charring
-
•
Holes, tears, cuts or snags
-
•
Broken or worn stitching in load-bearing splices
-
•
Excessive abrasive wear
-
•
Knots in any part of the sling
-
•
Discolouration and brittle or stiff areas
-
•
Fittings that are pitted, corroded, cracked, bent, twisted, gouged or broken
-
•
Clear labels indicating lashing capacity
It is obvious that these observations can discriminate between reliable and potentially unsafe lashings but there is no clear threshold value, and if one is set, it is imposed arbitrarily. In addition, a given lashing may appear to be in good condition but there are no means to check whether it has endured excessive charging beyond the loading capacity or has elongated beyond the EN 12195 threshold of 7% at loading capacity (for flat fabric lashings). For slings, an integrated overload sensor is available via SlingMax and their patented Check-Fast® slings.
4.4.3.1. The road ahead for smart lashings
In the framework of a transnational collective research project between small and medium size enterprise associations and research organisations (Rescotex), research is focussing on identifying thresholds for UV ageing, abrasion, and repeated tensioning of flat fabric lashings for cargo securing.
This section highlights the use of smart coatings to indicate excessive UV exposure of tie-down straps.
Although the lashings are UV stabilised according to the standard, there is no indication as to what extent this stabilisation should be. Moreover, a given belt will last longer if it is used in mild UV conditions (eg, indoor, sporadically) compared with severe UV conditions (eg, southern Europe, long-distance open transport). As a result, there is no general rule of thumb to assess the safety of a used belt with respect to UV ageing. To counteract this concern, a coating has been developed using a mixture of UV stabilisers to be printed on the belt. In case of UV damage, the print will discolour and indicate the level of UV damage endured by the belt so far. If the safety limit has been exceeded, a text urging the user to replace the belt becomes visible. Fig. 4.13 exemplifies such label printed on a 35-mm flat web lashing. At UV exposures above 1000 h (EN14836, QUV accelerated ageing test), the replace text is clearly visible and indicates that the belt has endured too much UV irradiation to be used safely.
Figure 4.13.

UV-exposed label printed on 35-mm flat fabric lashing. Numbers indicate the exposure level in hours according to EN14836 (QUV accelerated ageing test).
4.5. Future trends
Because textiles are used in an increasing number of technical applications, durability is increasing in importance. Much research has been done so far and products and processes developed are commercially available. Still, new end applications are detected in which durability in various environments are a prerequisite. For instance, in the At∼Sea Project (http://www.atsea-project.eu/), coated textiles were developed for use as a substrate for growing seaweed. For this application, a unique balance between antifouling properties and seaweed anchoring points is crucial.
The concept of self-healing coating is most developed in the field of automotives, aerospace, and building (concrete). A known example is the Scratch Shield clear coat developed by Nissan and already implemented in their cars and on iPhones (Anon, 2016b). To date, no examples of commercially available self-healing textiles are known. Research in this area is limited but the outcome is promising. In the future, self-healing coatings based on the microcapsule technology or an intrinsic approach can be expected. The self-healing coating will be able to repair at an early stage of damage, enhancing the durability and prolonging the lifetime of the coated textile. All textile areas will benefit from these developments (eg, protective clothing, architectural textile, cargo securing).
4.6. Conclusions
Coated textiles are being used in a wide range of end applications in which the textile is employed as a long-lasting solution. It is clear that the durability of textile coatings is an important property. In this chapter some smart technologies were presented enabling the enhancement of the durability of coated textiles, properties that can be realised with these technologies and some (future) applications in which these durable coated textiles can be implemented.
Sources of further information
Textile chemicals (Lacasse and Baumann, 2004)
Smart coatings (Aguilar and Román, 2014)
Acknowledgments
The authors are indebted to the Flemish Agency for Innovation by Science and Technology (IWT; grant 130372) and the European Union’s Seventh Framework Programme (grant agreements nos. NMP2-SE-2009-229334, FP7-NMP-2001-SME-5-280860, FP7-SEC-2011-284931 and FP7-SME-2013–606411) for funding of their research.
References
- Aguilar M.R., Román J.S. Woodhead Publishing; 2014. Smart Polymers and Their Applications. [Google Scholar]
- Alen N.S., Edge M. Springer; 1993. Fundamentals of Polymer Degradation and Stabilization. [Google Scholar]
- Andersson P., Sökjer-Petersen S., Agelčák J. International Forum for Road Transport Technology; Stockholm, Sweden: 2012. Differences in Cargo Securing Regulations. How Could We Achieve Harmonization? International Symposium on Heavy Vehicle Transportation Technology (HVTT) [Google Scholar]
- Andrady A.L., Hamid S.H., Hu X., Torikai A. Effects of increased solar ultraviolet radiation on materials. Journal of Photochemistry and Photobiology B: Biology. 1998;46:96–103. doi: 10.1016/s1011-1344(98)00188-2. [DOI] [PubMed] [Google Scholar]
- Anon . 2016. Final Report Summary – safe@sea (protective clothing for improved safety and performance in the fisheries) CORDIS.http://cordis.europa.eu/result/rcn/57672_en.html Available from: (accessed 08.01.2016.) [Google Scholar]
- Anon . 2016. Scratch Shield.http://www.nissan-global.com/EN/TECHNOLOGY/OVERVIEW/scratch.html Available from: (accessed 08.01.2016.) [Google Scholar]
- ASME . The American Society of Mechanical Engineers; New York: 2014. Safety Standard for Cableways, Cranes, Derricks, Hoists, Hooks, Jacks, and Slings. [Google Scholar]
- BASF . 2016. Light Stabilizers.http://www.dispersions-pigments.basf.com/portal/basf/ien/dt.jsp?print=center&setCursor=1_556325 Available from: (accessed 08.01.2016.) [Google Scholar]
- Baumann W. Institute for Environmental Research; Dortmund: 2000. Gathering and Review of Environmental Emission Scenarios for Biocid. [Google Scholar]
- Bixler G.D., Bhushan B. Biofouling: lessons from nature. Philosophical Transactions. Series A Mathematical, Physical and Engineering Sciences. 2012;370:2381–2417. doi: 10.1098/rsta.2011.0502. [DOI] [PubMed] [Google Scholar]
- Blaiszik B.J., Kramer S.L.B., Grady M.E., Mcilroy D.A., Moore J.S., Sottos N.R., White S.R. Autonomic restoration of electrical conductivity. Advanced Materials. 2012;24:398–401. doi: 10.1002/adma.201102888. [DOI] [PubMed] [Google Scholar]
- Boryo D.E.A. The effect of microbes on textile material: a review on the way-out so far. The International Journal of Engineering and Science. 2013;2:09–13. [Google Scholar]
- Brzeziński S., Kowalczyk D., Borak B., Jasiorski M., Tracz A. Nanocoat finishing of polyester/cotton fabrics by the sol-gel method to improve their wear resistance. Fibres & Textiles in Eastern Europe. 2011;19:6. [Google Scholar]
- Burgess R. Micro-organisms and textiles. Journal of Applied Bacteriology. 1954;17:230–245. [Google Scholar]
- Chapman R. Elsevier Science; 2012. Smart Textiles for Protection. [Google Scholar]
- Coleman W.F. Antimicrobial agents used on textiles. Journal of Chemical Education. 2005;82:171. [Google Scholar]
- Copsey S. European Agency for Safety and Health at Work; 2010. A Review of Accidents and Injuries to Road Transport Drivers. [Google Scholar]
- Cremers J.M. 12 – Textiles for insulation systems, control of solar gains and thermal losses and solar systems. In: Pohl G., editor. Textiles, Polymers and Composites for Buildings. Woodhead Publishing; 2010. pp. 351–374. [Google Scholar]
- Curtis White W., Bellfield R., Ellis J., Vandendaele I.P. Controlling the spread of infections in hospital wards by the use of antimicrobials on medical textiles and surfaces. In: Anand S.C., Kennedy J.F., Miraftab M., Rajendran S., editors. Medical and Healthcare Textiles. Woodhead Publishing; 2010. pp. 55–75. [Google Scholar]
- Dafforn K.A., Lewis J.A., Johnston E.L. Antifouling strategies: history and regulation, ecological impacts and mitigation. Marine Pollution Bulletin. 2011;62:453–465. doi: 10.1016/j.marpolbul.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Dastjerdi R., Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids and Surfaces B: Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- De Smet D., Leppchen-Fröhlich K., Meyer M. Renewable antimicrobials for textile finishing. Melliand International. 2015:45–47. [Google Scholar]
- De Smet D., Weydts D., Myriam V. Environmental friendly fabric finishes. In: Blackburn R.S., editor. Sustainable Apparel: Production, Processing and Recycling. Woodhead Publishing; 2015. [Google Scholar]
- De Vilder I., Vanneste M. Fourth International Conference on Self-healing Materials – ICSHM2013 16/6/2013. 2013. Self-healing coatings for textile; pp. 307–311. Ghent. [Google Scholar]
- De Vilder I., Vanneste M., Peltonen C., Varheenmaa M. 5th ECPC and NOKOBETEF Congress: Future of Protective Clothing: Intelligent or Not? 2012. Safe@sea: protective clothing for improved safety and performance in the fisheries. Development of comfortable wear resistant and stain repellent coated materials. Valencia. [Google Scholar]
- Dikić T., Ming W., Van Benthem R.A.T.M., Esteves A.C.C., De With G. Self-replenishing surfaces. Advanced Materials. 2012;24:3701–3704. doi: 10.1002/adma.201200807. [DOI] [PubMed] [Google Scholar]
- Ding X., Yang C., Lim T.P., Hsu L.Y., Engler A.C., Hedrick J.L., Yang Y.-Y. Antibacterial and antifouling catheter coatings using surface grafted peg-b-cationic polycarbonate diblock copolymers. Biomaterials. 2012;33:6593–6603. doi: 10.1016/j.biomaterials.2012.06.001. [DOI] [PubMed] [Google Scholar]
- European Commission . European Commission, Directorate-General for Energy and Transport; 2007. European Best Practice Guidelines for Abnormal Road Transports. [Google Scholar]
- European Commission . Publications Office of the European Union; 2014. European Best Practices Guidelines on Cargo Securing for Road Transport. [Google Scholar]
- Fang V., Kennedy J., Futter J., Manning J. 2013. A Review of Near Infrared Reflectance Properties of Metal Oxide Nanostructures. [Google Scholar]
- Garcia S.J., Fischer H.R. 9-Self-healing polymer systems: properties, synthesis and applications. In: Aguilar M.R., Román J.S., editors. Smart Polymers and Their Applications. Woodhead Publishing; 2014. pp. 271–298. [Google Scholar]
- García S.J., Fischer H.R., Van Der Zwaag S. A critical appraisal of the potential of self healing polymeric coatings. Progress in Organic Coatings. 2011;72:211–221. [Google Scholar]
- Genzer J., Efimenko K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: a review. Biofouling. 2006;22:339–360. doi: 10.1080/08927010600980223. [DOI] [PubMed] [Google Scholar]
- Ghosh S.K. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. Self-healing Materials: Fundamentals, Design Strategies, and Applications; pp. 1–28. [Google Scholar]
- Gouveia I.C. Nanobiotechnology: a new strategy to develop non-toxic antimicrobial textiles. In: Mendez-Vilas A., editor. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center; 2010. pp. 407–414. [Google Scholar]
- Huntington C.G. ASCE Press; 2004. The Tensioned Fabric Roof. [Google Scholar]
- Harder T., Yee L.H. 5-Bacterial adhesion and marine fouling. In: Hellio C., Yebra D., editors. Advances in Marine Antifouling Coatings and Technologies. Woodhead Publishing; 2009. pp. 113–131. [Google Scholar]
- Heyse P., Buyle G., Walendy B., Beccarelli P., Loriga G., Zangani D., Tempesti A. Multitexco – high performance smart multifunctional technical textiles for the construction sector. Procedia Engineering. 2015;114:11–17. [Google Scholar]
- Janssen P.J.C.M. Health risk assessment for organotins in textiles. Dutch National Institute for Public Health and the Environment. 2000 [Google Scholar]
- Joshi M. Ecofriendly antimicrobial finishing of textiles using bioactive agents based on natural products. Indian Journal of Fibre & Textile Research. 2009;34:295–304. [Google Scholar]
- Joshi M. Antimicrobial textiles for health and hygiene applications based on eco-friendly natural products. In: Anand S.C., Kennedy J.F., Miraftab M., Rajendran S., editors. Medical and Healthcare Textiles. Woodhead Publishing; 2010. pp. 84–92. [Google Scholar]
- Kasza G. Polymer Institute of the Slovak Academy of Science; Bratislava: 2013. Thermal, Antioxidative and Photochemical Stabilization of Polymers: Low Molecular Weight versus Macromolecular Stabilizers. Polyfriend. [Google Scholar]
- Klemm P., Schembri M.A. Bacterial adhesins: function and structure. International Journal of Medical Microbiology. 2000;290:27–35. doi: 10.1016/S1438-4221(00)80102-2. [DOI] [PubMed] [Google Scholar]
- Lacasse K., Baumann W. Springer; Berlin: 2004. Textile Chemicals: Environmental Data and Facts. [Google Scholar]
- Lejars M., Margaillan A., Bressy C. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chemical Reviews. 2012;112:4347–4390. doi: 10.1021/cr200350v. [DOI] [PubMed] [Google Scholar]
- Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature Reviews Microbiology. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- Leung M., Chan A.H.S. Control and management of hospital indoor air quality. Medical Science Monitor. 2006;12:8. [PubMed] [Google Scholar]
- Llc B. Biovation; Boothbay, USA: 2010. Testing for Antimicrobial Activity in Textiles – Quick Overview.http://biovation.com/wp-content/uploads/AnOverviewofBiocideTestingsRevA11.pdf Available from: (accessed 27.06.15.) [Google Scholar]
- Llorens J. Elsevier Science; 2015. Fabric Structures in Architecture. [Google Scholar]
- Lorenz C., Windler L., Von Goetz N., Lehmann R.P., Schuppler M., Hungerbühler K., Heuberger M., Nowack B. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere. 2012;89:817–824. doi: 10.1016/j.chemosphere.2012.04.063. [DOI] [PubMed] [Google Scholar]
- Mao N. 3-High performance textiles for protective clothing. In: Lawrence C.A., editor. High Performance Textiles and Their Applications. Woodhead Publishing; 2014. pp. 91–143. [Google Scholar]
- Mauldin T.C., Kessler M.R. Self-healing polymers and composites. International Materials Reviews. 2010;55:317–346. [Google Scholar]
- Mookhoek S.D., Fischer H.R., Van Der Zwaag S. Alginate fibres containing discrete liquid filled vacuoles for controlled delivery of healing agents in fibre reinforced composites. Composites Part A: Applied Science and Manufacturing. 2012;43:2176–2182. [Google Scholar]
- OEKO-TEX® . 2015. Limit Values and Fastness.https://www.oeko-tex.com/en/manufacturers/test_criteria/limit_values/limit_values.html Available from: (accessed 29.07.15.) [Google Scholar]
- Patra J.K., Gouda S. Application of nanotechnology in textile engineering: an overview. Journal of Engineering and Technology Research. 2013;5:104–111. [Google Scholar]
- Pasternack G. May 2013. Main Factors Affecting the Coating's IR Reflectivity. SpecialChem. [Google Scholar]
- Patel P., Choi C.K., Meng D.D. Superhydrophilic surfaces for antifogging and antifouling microfluidic devices. Journal of the Association for Laboratory Automation. 2010;15:114–119. [Google Scholar]
- Piccinini P. Institute for Health and Consumer Protection; 2007. European Survey on the Release of Formaldehyde from Textiles. [Google Scholar]
- Pinho E., Magalhães L., Henriques M., Oliveira R. Antimicrobial activity assessment of textiles: standard methods comparison. Annals of Microbiology. 2011;61:493–498. [Google Scholar]
- Qin Z., Buehler M.J. Molecular mechanics of mussel adhesion proteins. Journal of the Mechanics and Physics of Solids. 2014;62:19–30. [Google Scholar]
- Rabek J.F. Berlin Heidelberg, Springer-Verlag; 1996. Photodegradation of Polymers. [Google Scholar]
- Rosi B., Cvahte T., Bálint Čeh J., Tojnko M., Lerher T., Jereb B. Load fastening and securing. JLST. 2012;3:53–57. [Google Scholar]
- Salminen L. Helsinki Metropolia University of Applied Sciences; 2014. The Influence of Solar Reflective Black Pigments on the Durability of Wood Coatings. [Google Scholar]
- Scott G. Royal Society of Chemistry; 1999. Polymers and the Environment. [Google Scholar]
- Seeboth A., Lötzsch D. Pan Stanford; 2013. Thermochromic and Thermotropic Materials. [Google Scholar]
- Seidel M., Sturge D.S. John Wiley & Sons; 2009. Tensile Surface Structures: A Practical Guide to Cable and Membrane Construction. [Google Scholar]
- Sen A.K. second ed. CRC Press; 2007. Coated Textiles: Principles and Applications. [Google Scholar]
- Shahidi S., Wiener J. Antibacterial agents in textile industry. In: Bobbarala V., editor. Antimicrobial Agents. InTech; 2012. pp. 387–406. [Google Scholar]
- Silver S. 2003. Bacterial Silver Resistance: Molecular Biology and Uses and Misuses of Silver Compounds. [DOI] [PubMed] [Google Scholar]
- Simoncic B., Tomsic B. Structures of novel antimicrobial agents for textiles – a review. Textile Research Journal. 2010;80:1721–1737. [Google Scholar]
- Smith E. Comparison of antimicrobial textile treatments. In: Anand S.C., Kennedy J.F., Miraftab M., Rajendran S., editors. Medical and Healthcare Textiles. Woodhead Publishing; 2010. pp. 38–47. [Google Scholar]
- Toohey K.S., Sottos N.R., Lewis J.A., Moore J.S., White S.R. Self-healing materials with microvascular networks. Nature Materials. 2007;6:581–585. doi: 10.1038/nmat1934. [DOI] [PubMed] [Google Scholar]
- Treakle A.M., Thom K.A., Furuno J.P., Strauss S.M., Harris A.D., Perencevich E.N. Bacterial contamination of health care workers' white coats. American Journal of Infection Control. 2009;37:101–105. doi: 10.1016/j.ajic.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesano A., Vineis C., Aluigi A., Rombaldoni F. Antimicrobial polymers for textile products. In: Mendez-Vilas A., editor. Science against Microbial Pathogens: Communicating Current Research and Technological Advances. Formatex Research Center; 2011. pp. 99–110. [Google Scholar]
- Vaun Mcarthur J., Tuckfield R.C., Baker-Austin C. Antimicrobial textiles. In: Coates A.R.M., editor. Antibiotic Resistance. Springer Berlin Heidelberg; Berlin Heidelberg: 2012. pp. 135–152. [Google Scholar]
- Wang H., Zhou H., Gestos A., Fang J., Lin T. Robust, superamphiphobic fabric with multiple self-healing ability against both physical and chemical damages. ACS Applied Materials & Interfaces. 2013;5:10221–10226. doi: 10.1021/am4029679. [DOI] [PubMed] [Google Scholar]
- White S.R., Sottos N.R., Geubelle P.H., Moore J.S., Kessler M.R., Sriram S.R., Brown E.N., Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- Windler L., Height M., Nowack B. Comparative evaluation of antimicrobials for textile applications. Environment International. 2013;53:62–73. doi: 10.1016/j.envint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Wypych G. ChemTec Publishing; 2008. PVC Degradation & Stabilization. [Google Scholar]
- Yuan G., Cranston R. Recent advances in antimicrobial treatments of textiles. Textile Research Journal. 2008;78:60–72. [Google Scholar]
- Van Zoelen W., Buss H.G., Ellebracht N.C., Lynd N.A., Fischer D.A., Finlay J., Hill S., Callow M.E., Callow J.A., Kramer E.J., Zuckermann R.N., Segalman R.A. Sequence of hydrophobic and hydrophilic residues in amphiphilic polymer coatings affects surface structure and marine antifouling/fouling release properties. ACS Macro Letters. 2014;3:364–368. doi: 10.1021/mz500090n. [DOI] [PubMed] [Google Scholar]


