One use of cytology is to classify lesions so as to assist with the diagnosis, prognosis, and management of a case. Cytologic interpretations are generally classified into one of five cytodiagnostic groups (Box 2-1 ). A sixth category can be used for nondiagnostic interpretations. Nondiagnostic samples usually result from insufficient cellular material or excessive blood contamination.
BOX 2-1. General Categories of Cytologic Interpretation.
Normal or hyperplastic tissue
Cystic mass
Inflammation or cellular infiltrate
Response to tissue injury
Neoplasia
Nondiagnostic sample
KEY POINT.
Interpretation of cytologic material may include more than one category, such as inflammation along with a response to tissue injury or neoplasia with inflammation.
NORMAL OR HYPERPLASTIC TISSUE
Normal and hyperplastic tissues are both composed primarily of mature cell types. Normal cells display uniformity in cellular, nuclear, and nucleolar size and shape. Cytoplasmic volume is usually high relative to the nucleus (Figs. 2-1 and 2-2 ). Hyperplasia is a nonneoplastic enlargement of tissue that can occur in response to hormonal disturbances or tissue injury. Hyperplastic tissue has a tendency to enlarge symmetrically in comparison to neoplasia. Cytologically, hyperplastic cells have a higher nuclear-to-cytoplasmic ratio than normal cells. Examples of hyperplastic responses include nodular proliferations within the parenchyma of the prostate (Fig. 2-3 ), liver, and pancreas (Fig. 2-4 ).
FIGURE 2-1.

Normal skeletal muscle. Tissue aspirate. Dog. Numerous threadlike myofibrils compose each cell whose nucleus is small, condensed, and oval. Cross-striations, characteristic of skeletal muscle, are barely visible against the dark blue cytoplasm but seen in the magnified view (inset). (Wright-Giemsa; IP.)
FIGURE 2-2.
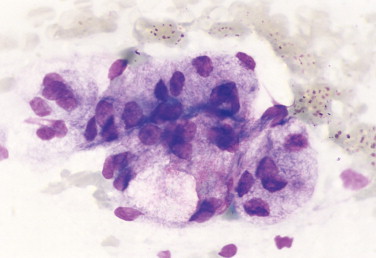
Normal salivary gland. Tissue aspirate. Dog. The gland has uniform features of nuclear size, nuclearto-cytoplasmic ratio, and cytoplasmic content. (Wright-Giemsa; HP oil.)
FIGURE 2-3.
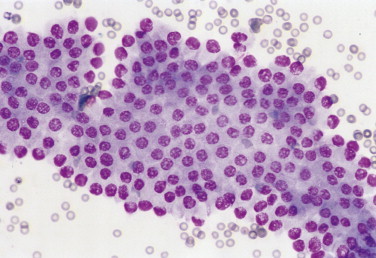
Canine prostatic hyperplasia. Tissue aspirate. Dog. The presenting clinical sign in this case involves blood dripping from the prepuce. Cytologically, the nuclear size is uniform; however, the nuclear-to-cytoplasmic ratio is increased as indicated by the close proximity of nuclei to each other. (Wright-Giemsa; HP oil.)
FIGURE 2-4.
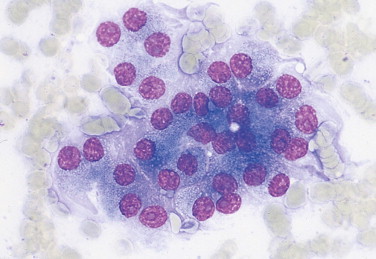
Nodular hyperplasia of the pancreas. Tissue aspirate. Dog. Ultrasound examination reveals a hypoechoic mass in the area of the pancreas. Cytologically, hyperplastic parenchymal organs commonly display binucleation. (Wright-Giemsa; HP oil.)
CYSTIC MASS
Cystic lesions contain liquid or semisolid material. The low-protein liquid usually contains a small number of cells. These benign lesions may result from proliferation of lining cells or tissue injury. Examples include seroma (Fig. 2-5 ), salivary mucocele, apocrine sweat gland cyst, epidermal/follicular cyst, and cysts associated with noncutaneous glands such as the mammary gland or prostate (Fig. 2-6 ).
FIGURE 2-5.

Seroma. Tissue aspirate. Dog. Blood-tinged fluid is removed from a swelling on the neck. Cytologically, low cellularity and low protein content is visible in the background. Large mononuclear cells with fine cytoplasmic granularity predominate along with low numbers of erythrocytes. (Wright-Giemsa; HP oil.)
FIGURE 2-6.

Prostatic cyst. Histopathology. Dog. Cuboidal epithelial cells line large cystic spaces that represent dilated ducts. (H&E; LP.)
INFLAMMATION OR CELLULAR INFILTRATE
Inflammatory conditions are classified cytologically by the predominance of the cell type involved. Recognition of the inflammatory cell type often suggests an etiologic condition.
Purulent or suppurative lesions contain greater than 85% neutrophils; they are then classified by the presence or absence of degeneration affecting the neutrophil. Nondegenerate neutrophils are morphologically normal and predominate in relatively nontoxic environments such as immune-mediated conditions, neoplastic lesions (Fig. 2-7 ), and sterile lesions caused by irritants such as urine and bile. Degenerate neutrophils display nuclear swelling and decreased stain intensity termed karyolysis (Fig. 2-8 ), indicating rapid cell death in a toxic environment (Perman et al., 1979). Increased nuclear staining with coalescence of the nucleus into a single round mass and an intact cellular membrane characterizes pyknosis (Fig. 2-9 ), a slow, progressive change often within a relatively nontoxic environment. An end stage of cell death, termed karyorrhexis, may be seen cytologically as the result of pyknosis of hypersegmented nuclei (Fig. 2-10 ). Degenerate neutrophils predominate in bacterial infections, particularly gram-negative types. Under septic conditions, bacteria may be found intracellularly (Fig. 2-11 ).
FIGURE 2-7.
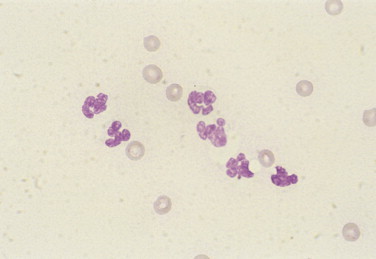
Nondegenerate neutrophils. Synovial fluid. Dog. Nonseptic inflammation with well-segmented neutrophils appears secondary to adjacent neoplasia of the bone. (Wright-Giemsa; HP oil.)
FIGURE 2-8.
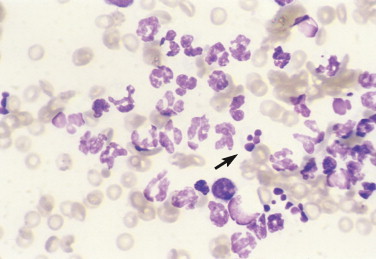
Karyolysis, karyorrhexis. Tissue aspirate. Dog. Mild to moderate karyolysis of neutrophils is evident by the decreased nuclear stain intensity and swollen nuclear lobes. Pyknosis of multiple nuclear segments appear as dark, dense, round structures, termed karyorrhexis (arrow), in this case of bacterial dermatitis. (Wright-Giemsa; HP oil.)
FIGURE 2-9.

Pyknosis. Chylous effusion. Dog. Chronic inflammation of this fluid produces neutrophil nuclei that have condensed into a large, often single, dark, round structure related to the slow progression of cellular change in a nonseptic environment. The pyknotic cell in this case also contains a second, smaller round nuclear fragment. (Wright; HP oil.)
FIGURE 2-10.

Karyorrhexis. Tissue aspirate. Inflammatory response with evidence of multiple pyknotic nuclear segments in the center cell. (Wright-Giemsa; HP oil.)
FIGURE 2-11.

Bacterial sepsis. Tissue aspirate. Dog. Markedly karyolytic neutrophils are present with intracellular and extracellular coccoid bacteria. Karyolysis is so severe that the cells are barely recognizable as neutrophils. (Wright; HP oil.)
Histiocytic or macrophagic lesions contain a predominance of macrophages, suggesting chronic inflammation (Fig. 2-12 ). In granulomas, activated macrophages that morphologically resemble epithelial cells are termed epithelioid macrophages. These cells may merge to form giant multinucleated forms (Fig. 2-13 ). The granulomatous lesions are often associated with foreign body reactions and mycobacterial infection.
FIGURE 2-12.

Macrophagic inflammation. Tissue imprint. Dog. Nodular lung disease with numerous large mononuclear cells having abundant gray cytoplasm and many cells with multiple cytoplasmic vacuoles. (Wright-Giemsa; HP oil.)
FIGURE 2-13.

Multinucleate giant cell. Tissue aspirate. Cat. Skin lesion with pyogranulomatous inflammation, including many giant cells related to the presence of fungal hyphae. Pictured is a cell with seven distinct nuclei and abundant granular blue-gray cytoplasm. (Wright-Giemsa; HP oil.)
Pyogranulomatous or mixed cell inflammatory lesions contain a mixture of neutrophils and macrophages (Fig. 2-14 ) that may include increased numbers of lymphocytes or plasma cells. This type of inflammation is often associated with foreign body reactions, fungal infections, mycobacterial infections, panniculitis, lick granulomas, and other chronic tissue injuries.
FIGURE 2-14.

Pyogranulomatous or mixed cell inflammation. Chylous effusion. Dog. Chronic chylous effusion contains a variety of cell types, including nondegenerate neutrophils, vacuolated macrophages, small to medium lymphocytes, and two mature plasma cells. (Wright; HP oil.)
Eosinophilic lesions contain greater than 10% eosinophils in addition to other inflammatory cell types (Fig. 2-15 ). They are seen with or without mast cell involvement. This inflammatory response is associated with eosinophilic granuloma, hypersensitivity or allergic conditions, parasitic migrations, fungal infections, mast cell tumors, and other neoplastic conditions that induce eosinophilopoiesis.
FIGURE 2-15.

Eosinophilic inflammation. Transtracheal wash. Cat. Clinical presentation of a chronic cough in this cat with suspected pulmonary allergy. Fluid contains 95% eosinophils. Pictured are several eosinophils that stain pink to blue-green and adhere to pink mucous material. (Wright-Giemsa; HP oil.)
Lymphocytic or plasmacytic infiltration is often associated with allergic or immune reactions, early viral infections, and chronic inflammation. The lymphoid population is heterogeneous, with small or intermediate-sized lymphocytes and plasma cells mixed with other inflammatory cells (see Fig. 2-14). A monomorphic population of lymphoid cells without other inflammatory cells present suggests lymphoid neoplasia.
RESPONSE TO TISSUE INJURY
Cytologic samples often contain evidence of tissue injury in addition to cyst formation, inflammation, or neoplasia. These changes include hemorrhage, proteinaceous debris, cholesterol crystals, necrosis, and fibrosis.
Hemorrhage that is pathologic can be distinguished from blood contamination encountered during the cytologic collection. Blood contamination is associated with the presence of numerous erythrocytes and platelets. Acute hemorrhage is associated with engulfment of erythrocytes by macrophages termed erythrophagocytosis (Fig. 2-16 ). Chronic hemorrhage is associated with active macrophages containing degraded blood pigment within their cytoplasm—for example, blue-green to black hemosiderin granules (Figs. 2-17 and 2-18 ) or yellow rhomboid hematoidin crystals (Fig. 2-18). Hemosiderin represents an excess aggregation of ferritin molecules or micelles. This form of iron storage becomes visible by light microscopy and stains blue with the Prussian blue reaction. Hematoidin crystals do not contain iron although they are formed during anaerobic breakdown of hemoglobin such as may occur within tissues or cavities. Hematomas often contain phagocytized erythrocytes if the lesion is acute, or hemosiderin-laden macrophages if the lesion is chronic.
FIGURE 2-16.

Erythrophagocytosis. Cerebrospinal fluid. Cat. Many erythrocytes are in the background along with one large macrophage that engulfed numerous intact red cells. The cat had confirmed infection with feline coronavirus (feline infectious peritonitis). (Wright; HP oil.)
FIGURE 2-17.

Chronic hemorrhage. Tissue aspirate. Dog. Several activated macrophages are present in this follicular cyst lesion. The macrophage directly below the cholesterol crystal contains blue-green granular material in the cytoplasm consistent with hemosiderin, a breakdown product of erythrocytes. On the left edge is a macrophage with large black granules suggestive of hemosiderin. (Wright; HP oil.)
FIGURE 2-18.
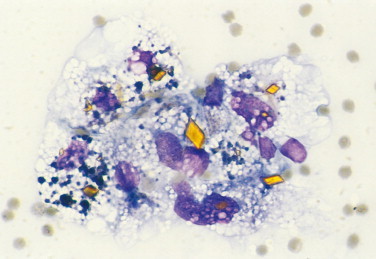
Hematoidin crystals. Pericardial fluid. Dog. Activated macrophages with bright yellow rhomboid crystals of variable size appear in this hemorrhagic fluid related to hemoglobin breakdown in an anaerobic environment. Several macrophages also contain black granular material consistent with hemosiderin. (Wright-Giemsa; HP oil.)
Proteinaceous debris may be seen within the background of the preparation. Mucus stains lightly basophilic and appears amorphous (Fig. 2-19 ). Lymphoglandular bodies (Fig. 2-20 ) are cytoplasmic fragments from fragile cells, usually lymphocytes, that are discrete, round, lightly basophilic structures (Flanders et al., 1993). Nuclear streaming refers to linear pink to purple strands of nuclear remnants (Fig. 2-21 ) produced by excessive tissue handling during cytologic preparation or with necrotic material when sampled. Clear to light-pink amorphous strands representing collagen (Fig. 2-22A ) may be admixed with spindle cells and endothelium into a fibrovascular stroma. However, when these collagen fibers undergo damage (as in the collagenolysis associated with mast cell tumor), degranulating eosinophils release collagenase that produces dense, hyalinized pink collagen bands (Fig. 2-22B). Amyloid is an uncommon pathologic protein found between cells. It appears amorphous, eosinophilic, and hyaline and may be associated with chronic inflammation.
FIGURE 2-19.

Mucus. Salivary mucocele. Dog. The background contains pale pink-blue amorphous material representative of mucus. Numerous activated macrophages or mucinophages compose the predominant population. (Wright; HP oil.)
FIGURE 2-20.

Lymphoglandular bodies. Tissue aspirate. Dog. The background of this lymph node preparation contains numerous small, blue-gray cytoplasmic fragments called lymphoglandular bodies that are related to the rupture of the fragile neoplastic lymphocytes. An activated macrophage has phagocytized cellular debris appearing as large blue-black particles. (Wright; HP oil.)
FIGURE 2-21.
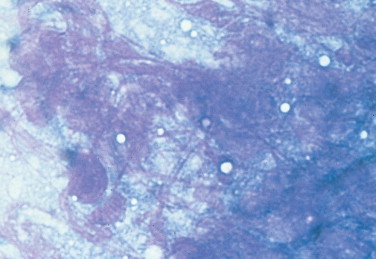
Nuclear streaming. Tissue aspirate. Purple strands of nuclear material are formed from ruptured cells either as an artifact of slide preparation or from fragile cells that are frequently neoplastic. (Wright-Giemsa; HP oil.)
(Courtesy of Denny Meyer, University of Florida).
FIGURE 2-22.

A, Collagenous fibers. Tissue aspirate. Dog. Clear to light pink strands of intact fibrous connective tissue may resemble fungal hyphae. Collagenous fibers will have poorly defined margins and a variable diameter, unlike hyphae, which have uniform width and distinct borders. (Wright-Giemsa, HP oil.) B, Collagenolysis. Tissue aspirate. Dog. Haphazard bands of collagen appear bright pink and hyalinized owing to the breakdown of the fibers through release of collagenase by degranulating eosinophils. This type of connective tissue damage occurs commonly in canine mast cell tumors. Interspersed among tumor cells are eosinophils and their granules. (Wright; IP.)
Cholesterol crystals represent evidence of cell membrane damage that is found in the background of some cytologic preparations. These rectangular, plated crystals are transparent unless background staining is enhanced as, for example, with new methylene blue stain (Fig. 2-23 ). The crystals are most often associated with epidermal/follicular cysts.
FIGURE 2-23.

Cholesterol crystal. Tissue aspirate. Clear rectangular plates with notched corners are characteristic of cholesterol. This is often associated with degenerate squamous epithelium, as in follicular cysts. Crystals may be highlighted with background cellular debris or stain. (New methylene blue; HP oil.)
(Courtesy of Denny Meyer, University of Florida.)
Necrosis and fibrosis may occur together or separately in some cytologic preparations. The death of cells is represented by fuzzy, indistinct cell outlines and definition of cell type (Fig. 2-24 ). A reparative response accompanying tissue injury involves increased fibroblastic activity. It is common to see very reactive fibrocytes (Fig. 2-25 ) along with severe inflammation. One must be careful not to overinterpret this reactivity as a neoplastic condition.
FIGURE 2-24.

Necrosis. Tissue aspirate. Dog. Prominent nucleoli remain visible while other tissue has degenerated into dark blue-gray amorphous debris representative of necrotic material. The sample was taken from a case of prostatic carcinoma in which the necrotic site was focal. (Wright-Giemsa; HP oil.)
FIGURE 2-25.

Reactive fibroplasia. Tissue scraping. Cat. Oral mass with associated septic inflammation. Pictured are several plump mesenchymal cells with a stellate to spindle appearance and prominent nucleoli along with suppurative inflammation. The severity of the inflammatory response warrants caution in suggesting a malignant mesenchymal mass or sarcoma. Note the nuclear streaming appears as purple strands. (Aqueous-based Wright; HP oil.)
NEOPLASIA
General Features
Neoplasia is initially diagnosed when a monomorphic cell population is present and significant inflammation is lacking. Further division into benign and malignant types is based on cytomorphologic characteristics. Benign cells display uniformity in size, nuclear-tocytoplasmic ratio, and other nuclear features. Malignant cells often display three or more criteria (Box 2-2 and FIGURE 2-26, FIGURE 2-27, FIGURE 2-28, FIGURE 2-29, FIGURE 2-30, FIGURE 2-31, FIGURE 2-32 ) of cellular immaturity or atypia, which should be identified before a diagnosis of malignancy is made. In cases of an equivocal diagnosis or severe inflammation, histopathologic examination is recommended.
BOX 2-2. Cytologic Criteria Used to Identify Malignant Cells.
Pleomorphism of cell size, shape, or maturation state between cells of similar origin (see Fig. 2-26)
High or variable nuclear-to-cytoplasmic ratio (see Fig. 2-27)
Variation in nuclear size, termed anisokaryosis (see Fig. 2-27)
Coarse nuclear chromatin clumping (see Fig. 2-28)
Enlarged, multiple, or variably shaped nucleoli (see Fig. 2-29)
Nuclear molding related to the rapid growth of cells (see Fig. 2-30)
Multinucleation (see Fig. 2-31)
Abnormal mitotic figures evident as uneven divisions and isolated or lag chromatin (see Fig. 2-32)
FIGURE 2-26.

Pleomorphism. Tissue aspirate. Dog. Transitional cell carcinoma cells display variability in size and shape supportive of malignancy. (Wright-Giemsa; HP oil.)
FIGURE 2-27.
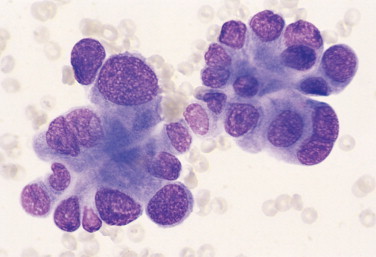
Anisocytosis, anisokaryosis. Tissue aspirate. Dog. Lung adenocarcinoma specimen has several features of malignancy. These features include high and variable nuclearto-cytoplasmic ratio, anisokaryosis, binucleation, and coarse nuclear chromatin. (Wright-Giemsa; HP oil.)
FIGURE 2-28.
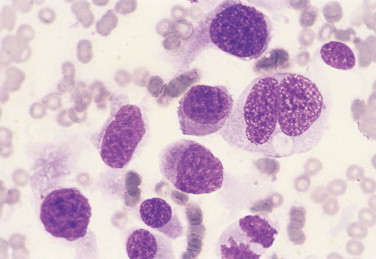
Coarse chromatin. Tissue aspirate. Dog. Same case as FIGURE 2-26. The nuclear material is mottled with light and dark spaces clearly evident. This appearance is often associated with neoplastic transitional epithelium but may be seen with other tissues. Binucleation is seen in one cell and a mitotic figure is present on the bottom edge. (Wright-Giemsa; HP oil.)
FIGURE 2-29.
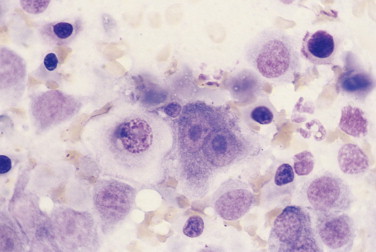
Prominent nucleoli. Tissue aspirate. Dog. Same case as FIGURE 2-24. A binucleate cell with very large single nucleoli in each nucleus is present. A prominent nucleolus is noted in the adjacent cell, which also displays coarse chromatin or chromatin clumping. (Wright-Giemsa; HP oil.)
FIGURE 2-30.
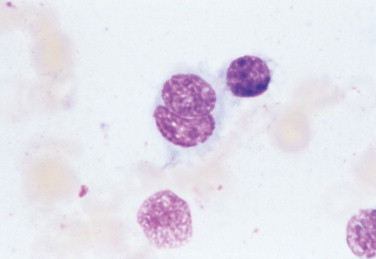
Nuclear molding. Tissue aspirate. Dog. Nasal chondrosarcoma pictured with a binucleate cell in which one nucleus is wrapped around the other. This feature is present in malignant tissues and is related to the lack of normal inhibition of cell growth. (Wright-Giemsa; HP oil.)
FIGURE 2-31.

Multinucleation. Tissue imprint. Dog. Pheochromocytoma with two multinucleate cells, one in the lower left side with three nuclei and the other to the right of center with an irregularly shaped nuclear region. Multinucleation may be found also in epithelial, mesenchymal, and round cell neoplasms. (Wright-Giemsa; HP oil.)
FIGURE 2-32.
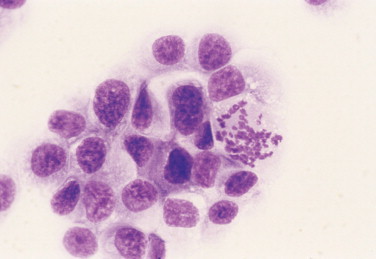
Abnormal mitosis. Tissue aspirate. Dog. Same case as Figure 2-26. Chromosomal fragments are dispersed irregularly with some isolated from the rest, termed lag chromatin. Increased mitotic activity may be suggestive of malignancy but abnormal division is diagnostic for malignancy. (Wright-Giemsa; HP oil.)
Cytomorphologic Categories
Neoplasms may be divided into four general categories to assist in making the cytologic interpretation by restricting the list of differential diagnoses (Perman et al., 1979, Alleman and Bain, 2000). The categories listed in Table 2-1 are based not on cell origin or function but rather on their general cytomorphologic characteristics. The first two terms are taken from embryology (Noden and de Lahunta, 1985).
TABLE 2-1.
Cytomorphologic Categories of Neoplasia
| Category | General Features | Examples |
|---|---|---|
| Epithelial | Clustered, tight arrangement of cells | Transitional cell carcinoma, lung tumors |
| Mesenchymal | Individualized, spindle to oval cells | Hemangiosarcoma, osteosarcoma |
| Round cell | Individualized, round, discrete cells | Lymphoma, transmissible venereal tumor |
| Naked nuclei | Loosely adherent cells with free round nuclei | Thyroid tumors, paragangliomas |
Epithelial Neoplasms
This type of neoplasm is associated with a clustered arrangement of cells into ball shapes or monolayer sheets. Cell origin of these neoplasms often involves glandular or parenchymal tissue and lining surfaces. Examples of epithelial neoplasms include lung adenocarcinoma (Fig. 2-33 ), perianal adenoma (hepatoid tumor), basal cell tumor, sebaceous adenoma, transitional cell carcinoma (Fig. 2-34 ), and mesothelioma. Specific cytologic features of epithelial neoplasms include the following characteristics:
-
•
Cells exfoliate in tight clumps or sheets
-
•
Cells adhere to each other and may display distinct tight junctions, termed desmosomes (Fig. 2-35 )
-
•
Cells are large and round to polygonal with distinct, intact cytoplasmic borders
-
•
Nuclei are round to oval
FIGURE 2-33.
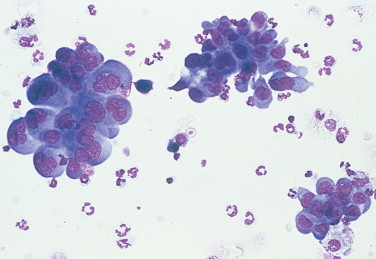
Epithelial neoplasm. Lung lavage. Dog. Large clusters of cohesive cells having distinct cell borders from a case of lung adenocarcinoma. (Wright-Giemsa; IP.)
(Courtesy of Robert King, Gainesville, FL.)
FIGURE 2-34.

Epithelial neoplasm. Tissue aspirate. Dog. Same case as Figure 2-26. Cells are formed into tight balls or as sheets. Nuclei are round to oval and cells are large, round to polygonal with distinct cytoplasmic borders. (Wright-Giemsa; HP oil.)
FIGURE 2-35.

Desmosomes. Tissue aspirate. Dog. Same case as Figure 2-24. A sheet of carcinoma cells with prominent desmosomes. These clear lines (arrow) between adjacent cells represent tight junctions that are characteristic of epithelial cells. (Wright-Giemsa; HP oil.)
Mesenchymal Neoplasms
Neoplasms with a mesenchymal appearance resemble the embryonic connective tissue, mesenchyme. This tissue is loosely arranged with usually abundant extracellular matrix (Noden and de Lahunta, 1985) and individualized spindle or stellate cells (Bacha 2000). Benign and malignant mesenchymal neoplasms often originate from connective tissue elements, such as fibroblasts, osteoblasts, adipocytes, myocytes, and vascular lining cells. Examples of mesenchymal neoplasms include hemangiosarcoma (Fig. 2-36 ), osteosarcoma (Fig. 2-37 ), hemangiopericytoma, and amelanotic melanoma (Fig. 2-38 ). Specific cytologic features of mesenchymal neoplasms include the following:
-
•
Cells usually exfoliate individually (however, clumps of cells are seen occasionally)
-
•
Cells are oval, stellate, or fusiform with often indistinct cytoplasmic borders
-
•
Samples are often poorly cellular
-
•
Cells are usually smaller compared with epithelial cells
-
•
Nuclei are round to elliptical
FIGURE 2-36.

Mesenchymal neoplasm. Tissue imprint. Dog. Neoplastic cells exfoliate individually and appear oval, spindle, or fusiform. This bone lesion was confirmed as hemangiosarcoma on histologic examination. Characteristic of hemangiosarcoma cytology is a poorly cellular sample with plump mesenchymal cells that contain numerous small punctate cytoplasmic vacuoles. (Wright-Giemsa; HP oil.)
FIGURE 2-37.

Mesenchymal neoplasm. Tissue aspirate. Dog. Individualized pleomorphic cells with abundant extracellular eosinophilic osteoid material is consistent with osteosarcoma. Binucleate and multinucleate forms are common and seen in this sample. (Wright-Giemsa; HP oil.)
FIGURE 2-38.

Mesenchymal neoplasm. Tissue imprint. Dog. Round to oval nuclei, anisokaryosis, high nuclear-to-cytoplasmic ratio, prominent and variably shaped nucleoli, and individualized cells with poorly distinct cytoplasmic borders suggest a malignant mesenchymal neoplasm. This lesion is from a gum mass with a histologically confirmed diagnosis of amelanotic melanoma. One cell in the center contains small amounts of melanin pigment granules. (Aqueous-based Wright; HP oil.)
Round Cell Neoplasms
These neoplasms have discrete, round cellular shapes and are often associated with hematopoietic cells. Examples of round cell neoplasms include mast cell tumor, histiocytoma, lymphoma (Fig. 2-39 ), plasmacytoma, and transmissible venereal tumor (Fig. 2-40 ). Specific cytologic characteristics of round cell neoplasms include the following:
-
•
Cells exfoliate individually, having distinct cytoplasmic borders
-
•
Cell shape is generally round
-
•
Samples are moderately cellular
-
•
Cells are usually smaller compared with epithelial cells
-
•
Nuclei are round to indented
FIGURE 2-39.
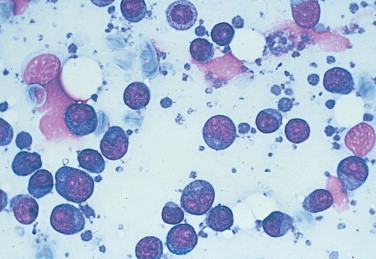
Round (discrete) cell neoplasm. Tissue aspirate. Dog. Discrete cells with a round shape, distinct cytoplasmic borders, and a very high nuclear-to-cytoplasmic ratio are characteristic of lymphoid cells. This sample is taken from a lymph node effaced by lymphoma cells. (Wright-Giemsa; HP oil.)
FIGURE 2-40.
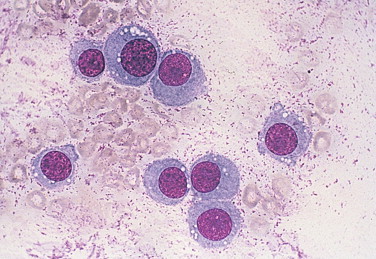
Round (discrete) cell neoplasm. Tissue aspirate. Dog. This fleshy vulvar mass is composed of round cells bearing a single prominent nucleolus and moderately abundant cytoplasm with frequent punctate cytoplasmic vacuolation. The cytologic diagnosis is transmissible venereal tumor. (Wright-Giemsa; HP oil.)
Naked Nuclei Neoplasms
Naked nuclei neoplasms have a loosely adherent cellular arrangement with free nuclei. This cytologic appearance is an artifact related to the fragile nature of these cells. These neoplasms are usually associated with endocrine and neuroendocrine tumors. Examples include thyroid tumors (Fig. 2-41 ), islet cell tumors, and paragangliomas (Fig. 2-42 ). Specific cytologic features of naked nuclei neoplasms include the following:
-
•
Cells exfoliate in loosely attached sheets with many free nuclei present, often having indistinct cytoplasmic borders
-
•
Occasional cell clusters may be present with distinct cell outlines
-
•
Cell shape is generally round to polygonal
-
•
Samples are highly cellular
-
•
Nuclei are round to indented
FIGURE 2-41.
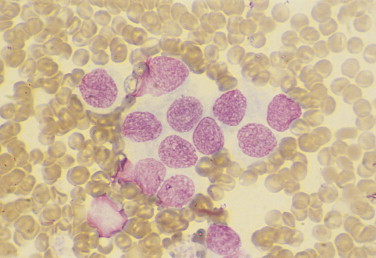
Naked nuclei neoplasm. Tissue aspirate. Dog. Cervical mass in the area of the thyroid from an animal with a honking cough. Cytologically the sample presents as a syncytium of round nuclei with relatively uniform features. This is characteristic of an endocrine mass. Typically the distinction between hyperplasia, adenoma, and carcinoma is difficult cytologically and sometimes histologically. (Wright-Giemsa; HP oil.)
FIGURE 2-42.

Naked nuclei neoplasm. Tissue imprint. Dog. Clinical signs include a head tilt and temporal muscle atrophy. Magnetic resonance imaging suggested a mass involving the osseous bulla. Surgery found a mass at the bifurcation of the common carotid artery. Cytologically, the preparation contains mostly loose or free round nuclei against a finely granular eosinophilic background. Few intact cells remain with pale cytoplasm at the edges and center. Adjacent to the center intact cell is a nucleated red cell (arrow) suggestive of extramedullary hematopoiesis. The histologic diagnosis is paraganglioma, specifically a malignant chemodectoma in this case, since it metastasized and was thought to involve the chemoreceptor organ in that site. (Wright-Giemsa; HP oil.)
KEY POINT.
The use of these four cytomorphologic categories may help to classify neoplastic lesions by their general cellular appearance and suggest specific tumor types. Remember that these categories may not fit well for some neoplasms, especially for poorly differentiated tumors. It is recommended that biopsy specimens for histopathologic examination be taken to determine the specific tumor type and extent of the lesion.
REFERENCES
- Alleman AR, Bain PJ. Diagnosing neoplasia: the cytologic criteria for malignancy. Vet Med. 2000;95:204–223. [Google Scholar]
- Bacha WJ, Bacha LM. Color atlas of veterinary histology. ed 2. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 13–15. [Google Scholar]
- Flanders E, Kornstein MJ, Wakely PE. Lymphoglandular bodies in fine-needle aspiration cytology smearsm. Am J Clin Pathol. 1993;99:566–569. doi: 10.1093/ajcp/99.5.566. [DOI] [PubMed] [Google Scholar]
- Noden DM, de Lahunta A. The embryology of domestic animals. Williams & Wilkins; Baltimore: 1985. pp. 10–11. [Google Scholar]
- Perman V, Alsaker RD, Riis RC. Cytology of the dog and cat. American Animal Hospital Association; South Bend, IN: 1979. pp. 4–7. [Google Scholar]


