Abstract
Monoclonal antibodies are routinely used in several fields but the great challenge has been their use as therapeutic agents for the treatment of diseases, such as breast cancer, leukemia, asthma, macular degeneration, arthritis, Crohn’s disease, and transplants, among others.
Monoclonal antibodies are protein molecules made in the laboratory from hybridoma cells by recombinant DNA technology.
Important advances have been made over the past decade to improve some critical points, such as safety and efficacy of the first generation of therapeutic antibodies.
This type of molecules presents a significant challenge from the pharmaceutical point of view due to their characteristics, such as molecular size, stability, and solubility. In this chapter we have attempted to identify the major issues associated with therapeutic approaches, formulating drawbacks and delivering antibody drugs, particularly focused on the challenges and opportunities that these present for the future.
Keywords: antibodies, nanoparticles, diagnosis, treatment, cancer, microbial infection
1. General Introduction
Monoclonal antibodies are protein molecules made from hybridoma cells by recombinant DNA technology. When applied as a therapeutic, a particular monoclonal antibody is referred to as Mab. The Mabs are defined as “monospecific antibodies that are made by identical immune cells that are all clones of a unique parent cell.” Mabs are far superior to polyclonal antibodies with respect to their controlled manufacturing procedures and their reproducible affinity for specific target antigens.
Mabs are indispensable not only to health, but also to prevent food poisoning, and are used to investigate environmental pollution. However, despite their widespread distribution and significance, most people have never heard of Mabs or how they have both transformed healthcare and spawned an entire new industry. Produced in the laboratory, Mabs are derived from the billions of tiny antibodies made every day by our immune systems to combat substances, known as antigens, which are regarded as foreign or potentially dangerous. Millions of different types of antibodies can be found in the blood of humans and other mammals. Made by white blood cells known as B lymphocytes, each antibody is highly specific—that is, it has the ability to bind to only one particular antigen, which may be derived from bacteria, viruses, fungi, parasites, pollen, or nonliving substances, such as toxins, chemicals, drugs, or foreign particles considered alien to the body. Once antibodies have marked their antigen, they and other types of cells produced by the immune system can attack it. The field of genetically engineered therapeutic Mabs has relied on many inventions during decades of research, but two key discoveries in the mid-1970s stand out as seminal events that laid the groundwork for this field to exist as we know it today. Although Mabs were first described in 1975 (Kohler and Milstein, 1975), only when the original rodents forms were replaced by their human equivalents did their potential as therapeutic agents began to be properly appreciated (Lonberg, 2005, Winter et al., 1994). The reasons for this are complex, but related to a combination of perceptions including patentability, immunogenicity, effector function, and a wish to avoid undesirable side effects.
The increasing scientific interest on Mabs can be clearly seen in Fig. 25.1 , which shows the rising number of published articles using the keyword monoclonal antibodies in different databases (Pubmed and Scopus). Athough fewer published articles, the number of publications is constant over the years to Science Direct and Wiley.
Figure 25.1.
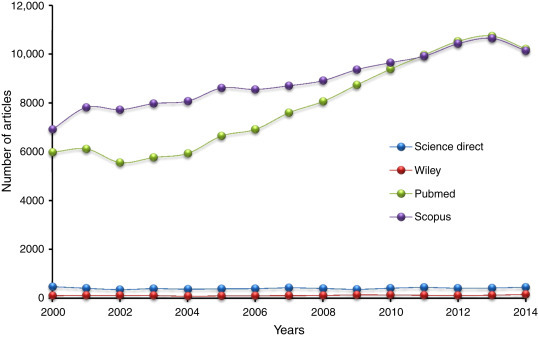
Number of Publications by Year/Database
Mabs are used not only as drugs for treating various diseases, but are also used as powerful tools for a wide range of medical applications. They are routinely used in hospitals for blood type and tissue, a vital process to ensure safe blood transfusion and organ transplantation. In other cases, they are employed as research probes to determine the pathological pathway and the cause of diseases, such as cancer, autoimmune diseases, and neurological disorders. On the diagnostic front, monoclonal antibodies are intrinsic components of test kits for the detection of ovulation, pregnancy, or menopause. They are also used for analyzing body fluids for medical diagnosis, and to determine whether there has been a heart attack.
Unlike polyclonal antibodies, Mabs are identical antibodies because they are produced by one type of immune cell. Using current hybridoma (mouse/human hybrid cells) technology originally developed by Georges Kohler, Cesar Milstein, and Neils Jerne, Mabs can be produced to bind tightly to virtually any material or antigen, which is defined as a substance that prompts the generation of antibodies that specifically bind to it. Antigens typically consist of proteins or polysaccharides. Epitopes, also known as antigenic determinants, are the part of the antigens through which actual binding to antibodies occurs.
The Mabs technology allowed scientists to produce huge quantities of pure antibodies aimed at specific selected targets, leading to the design of new diagnostic tests and therapeutics. By injecting a payload of Mabs into the bloodstream, the antibodies were headed straight to their disease target.
Today, the growth and profitability of Mabs are outstripping those of earlier types of biotechnology drugs and more traditional pharmaceutical ones. Indeed, their expansion is among the fastest in the global pharmaceutical world. In part, this reflects the sector’s embrace of Mabs as an answer to dwindling drugs in the pipeline and reduced revenue streams in the face of the expiration of key patents and the growth of generic medicines.
Drugs based on Mabs technology have names ending in “mab.” The first drug that received Food and Drug Administration (FDA) approval was Rituxan (rituximab) (Fig. 25.2 ) for the treatment of non-Hodgkin’s lymphoma, in 1998. It was developed by IDEC, and it has since merged with Biogen. Rituximab is a Mab against the protein CD20, which is primarily found on the surface of immune system B cells. Rituximab destroys B cells and is therefore used to treat diseases that are characterized by excessive numbers of B cells, overactive B cells, or dysfunctional B cells. This includes many lymphomas, leukemias, transplant rejection, and autoimmune disorders. IDEC teamed up with Genentech to get FDA approval and to comarket Rituxan. In parallel, Genentech developed Herceptin (Trastuzumab) for the treatment of breast cancer associated with the HER2/neu receptor. Herceptin was approved by the FDA in 1998 and is the first important example of personalized medicine requiring a diagnostic test to ensure efficacy, since only patients who express the Her2 gene in their breast cancer tissue have a chance to benefit from treatment with this Mab.
Figure 25.2.
Monoclonal Antibody Rituximab
Today, six therapeutic Mabs are among the 10 best-selling drugs in the world. In 2013 it was estimated that the global sales of the 10 more successful Mabs were worth more than $58.1 billion. Among these, adalimumab (Humira) is used for the treatment of rheumatoid arthritis and other autoimmune diseases and was at the top of the selling drug list across the world in 2012, reaching an annual income of $9.3 million. Thus, Mab sales are expected to be higher than those for Lipitor, used for cholesterol reduction, which has been the largest prescribed drug historically (King, 2013).
2. Mabs Production
Since the 1970s, the methodologies available for generating Mabs have dramatically evolved from the hybridoma technology used to produce murine Mabs (Kohler and Milstein, 1975) to sophisticated recombinant engineering technologies (Brüggemann et al., 1989a, Brüggemann et al., 1989b; Steinitz et al., 1977) appropriate to design specific Mabs of choice, thus producing humanized, chimeric, and completely (fully) human Mabs (Fig. 25.3 ) (Brüggemann et al., 1989a, Brüggemann et al., 1989b, Jones et al., 1986, Lonberg, 2005, Riechmann et al., 1998).
Figure 25.3.
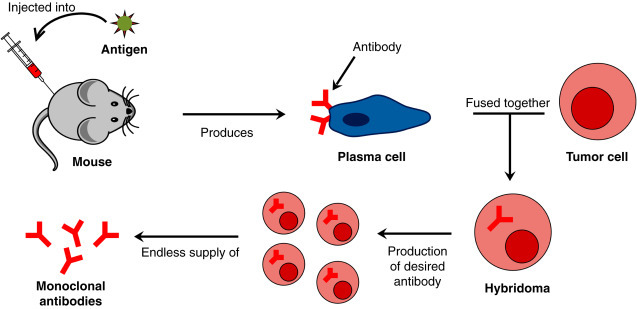
Schematic Representation of the Production of Monoclonal Antibodies
These novel methods have had major consequences in the development of clinically applicable Mabs against disease targets. The immunogenicity and safety of Mabs, a substantial limitation of antibodies produced by the hybridoma technology, could be overcome, and Mabs against any antigen specificity and with desirable physicochemical properties could be developed. High-scale production of Mabs at the industrial level could further be achieved. This has sparked a race for the development of antibody-mediated therapeutics in the clinic with the investment of large amounts of money from the biopharmaceutical sector toward that end, which is already paying off. The major success of human Mabs in immune and inflammatory conditions, such as rheumatoid arthritis (RA), Crohn’s disease, ulcerative colitis, spondyloarthropathies, juvenile arthritis, psoriasis, psoriatic arthritis, and others (Andreakos et al., 2002), marks merely the beginning of a rapidly evolving field with more than 150 diverse Mabs currently being evaluated in clinical trials or being candidates for approval by the FDA in the USA.
Scaling up Mabs production for therapeutics posed several challenges. The major issue was how to produce high quantities of drugs at a reasonable cost. Most cell lines in the 1980s yielded only half a gram of Mabs per liter, so production was time consuming and expensive. The ideal was to develop a hybrid cell line that could produce between 5 and 10 g of Mabs per liter. This demanded several steps, and even more, each requiring skill and patience. First was the creation of a hybrid cell, after which a clone secreting Mabs in high concentrations had to be selected. Then a culture medium had to be developed to encourage the optimal growth of the hybridoma. Scaling up such media was not easy in terms of quality control because they contained fifty or more ingredients, and it was important to determine how many nutrients to add. Hybridomas stop secreting Mabs, for example, if given too much glucose.
3. Antibody–Drug Conjugates
Antibody–drug conjugates (ADCs) are Mabs bearing cytotoxic drugs covalently bound via a chemical linker and can be defined as prodrugs. Prodrugs are inactive or less active derivatives of drug molecules and undergo enzymatic or chemical transformation to regenerate the active forms. ADCs are designed to be superior to either antibody therapeutics or chemotherapy alone by overcoming their limitations while preserving the merits from both. The antibody connected to the cytotoxic warhead (drug) via a linker serves as targeted delivery system to the tumor expressing the antigen/target recognized by the antibody. Ideally, in blood, after systemic administration, this prodrug is nontoxic. Upon binding of the antibody to the targeted tumor antigen and internalization of the complex into the cancer cell, the drug is then released in its active form and in sufficient quantity to kill the cell. On top of the careful choice of a target/antigen expressed in specific tumor indication, it requires finding the best combination between the antibody, the linker, and the drug, which, besides its own characteristics and constraints, are linked and impact each other (Bander, 2013).
The combination of nanoparticles and antibodies can offer versatility together with specificity (Fig. 25.4 ), allowing a huge potential market.
Figure 25.4.
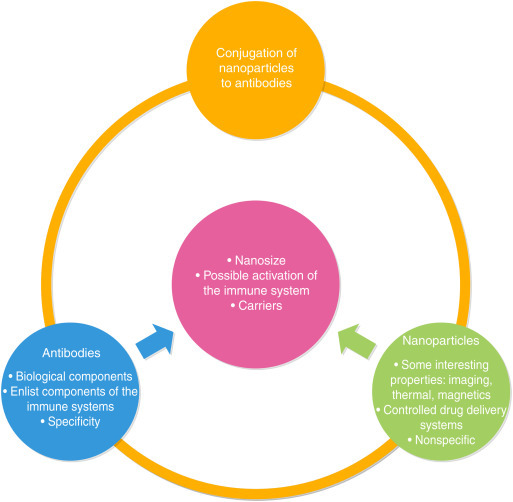
Conjugation of Nanoparticles to Antibodies and Their Advantages
The target/antigen is the starting point to build an ADC. It first determines which tumor indication will be targeted by the ADC and potentially impacts the choice of the conjugated drug. In addition, the target will also drive the criteria that will be defined for the selection of the targeted patient population within the tumor indication.
3.1. Physical Stability of Antibody–Drug Conjugates
Mabs are typically susceptible to noncovalent aggregation upon storage. Aggregation is driven by the minimization of free energy achieved when antibodies come into close contact—for instance, at relatively hydrophobic sites on the molecules. Dimerization may be thought of as the first step in aggregation, and sometimes the process proceeds no further. Dimers maybe reversible (Moore et al., 1999) or not, but growth can lead to larger and larger oligomers and even potentially insoluble particulates, as time progresses (Wang et al., 2006). Because ADCs are decorated with one or more small molecule drug moieties, they have a different set of biophysical properties compared with the unconjugated antibody, leading to new or altered intermolecular interactions. An antibody that exhibits acceptable aggregation behavior in its unconjugated form may have different physical behavior in its conjugated form, either because of changes in surface properties, such as hydrophobicity due to drug attachment or because the drugs have altered the higher-order structure of the antibody such that new modes of antibody–antibody interactions are possible. These factors could contribute to a substantially different propensity for aggregation.
This effect is most readily observed in a formulation screen designed to test both conjugated and unconjugated forms in parallel and subjected to the same assays. Such a study does not automatically reveal the exact cause of the effect, only that it is related to the conjugated form or conjugation process. The degree to which the molecule has been physically altered by conjugation may be assessed by techniques such as differential scanning calorimetry (DSC) (Wakankar et al., 2010), with physical perturbations manifested as changes (likely decreases) in the onset of melting or melting temperature, Tm, of the conjugated Mab as compared to the unconjugated antibody. This decrease in thermodynamic stability may translate to a decreased colloidal stability of the conjugate compared to an unconjugated antibody.
3.2. Chemical Stability of Antibody–Drug Conjugates
Part of ADC chemical stability is inherited from the unconjugated antibody. For instance, if the unconjugated antibody is susceptible to deamidation or isomerization with known pH dependence, this degradation mode is also likely to be present in the ADC. These types of degradations may impact the product potency, especially if the affected residues are found in the complementarity-determining region. Much has been learned through years of experience with unconjugated antibodies about the susceptibility of various amino acid residues to chemical degradations, allowing substantial insights to be gained from examination of the protein primary sequence alone, coupled to available information about local solvent exposure and flexibility (Kosky et al., 2009, Robinson and Robinson, 2001). It may be expected that most chemical modifications to the primary sequence of the unconjugated antibody will also occur in the ADC form. Fragmentation is another possible chemical degradation pathway for ADCs, driven by the breakage of covalent bonds between chains or within the peptide backbone. The altered physical state of the molecule following addition of the small molecule drug may result in some differences in the susceptibility of the ADC to fragmentation, but the fundamental susceptibility of the ADC to fragmentation is likely derived mostly from the Mab. A common unconjugated antibody degradation mode involves breaking the heavy chain peptide near the hinge region, leading to free Fab and Fab + Fc products (Cordoba et al., 2005). This is still a feasible degradation pathway for ADCs as well. Comparison of fragmentation rates between conjugated and unconjugated antibodies can reveal whether the ADC form has different stability toward fragmentation.
The covalent bonds linking drugs to antibodies may be broken by a variety of mechanisms over the shelf life of the product and under stressed storage conditions.
3.3. Formulation Development of Antibody–Drug Conjugates
Formulation development of ADCs aims to ensure that stable, high-quality products are dosed to patients. Though this goal is common to all pharmaceutical formulations, ADCs present a unique set of physicochemical properties that can impact safety, quality, and efficacy as compared to traditional therapeutic proteins. Although there is a body of literature that outlines which product attributes affect the safety, quality, and efficacy for conventional, unconjugated Mabs (Goetze et al., 2010), an understanding of attributes important to ADCs is only now emerging.
The choice of formulation has the potential to affect all categories of ADCs quality attributes. Because conjugation of an ADC requires an unconjugated antibody as an intermediate, the formulation development process for a conjugate necessarily involves formulating a Mab at some stage, likely as an intermediate for manufacturing of the final ADC form. The formulation optimization for the antibody portion of the ADC follows a similar course to that of an unconjugated antibody and, therefore, can leverage the biotechnology industry’s considerable experience developing this class of molecule. Many examples of successful commercial antibody formulations, both liquid and lyophilized, have been reported (Wang et al., 2006). However, the complexity of the formulation-development process potentially increases when quality attributes of the small molecule drug and those of the conjugated form are taken into account.
4. Nanotechnology’s Potential Impact on Mabs
The antibody-conjugated nanoparticles can be used principally in two biomedical applications: diagnosis and therapy.
In therapy, the development of targeted drug delivery represents, together with tissue repair, the main applications of antibody-conjugated nanoparticles. In diagnosis, the applications can be divided into those using in vivo and those using in vitro experimentation and include contrast agents for magnetic resonance imaging (MRI), sensing, cell sorting, bioseparation, enzyme immobilization, immunoassays, transfection (gene delivery), and purification.
Today, one of the most interesting challenges posed by drug-delivery systems is the development of smart vectors, which are required to be safe, easily administered, and economic, and which allow simultaneous diagnosis and treatment. Moreover, it is intended that the delivery is precisely controlled in term of dose and site of action to reduce adverse side effects. In this regard, there are many important site-selective drugs, such as highly toxic antitumor molecules, that must reach targeted cells or tissues without being released before.
Nanoscience and nanotechnology have found their way into the fields of biotechnology and medicine. Nanoscience is the study of structures and materials on an atomic and molecular scale, in a level that ranges usually below 100 nm.
Nanoparticles by themselves offer specific physicochemical properties that they do not exhibit in bulk form, where materials show constant physical properties regardless of size. Antibodies are nanosize biological products that are part of the specific immune system. In addition to their own properties as pathogens or toxin neutralizers, as well as in the recruitment of immune elements (complement, improving phagocytosis, cytotoxicity antibody dependent by natural killer cells, etc.), they could carry several elements (toxins, drugs, fluorochroms, or even nanoparticles, etc.) and be used in several diagnostic procedures, or even in therapy to destroy a specific target. The conjugation of antibodies to nanoparticles can generate a product that combines the properties of both.
Since the properties of materials differ significantly between atomic or subatomic level and larger scales, nanomaterials have attracted the attention of researchers for pharmaceutical application, especially in the area of drug delivery. This technology overcomes some of the drawbacks of large-size materials, such as poor bioavailability, in vivo stability, solubility, intestinal absorption, sustained and targeted delivery to site of action, and generalized side effects, among others. In this regard, nanostructures have been reported to protect drugs from the degradation in the gastrointestinal tract, and even more, to provide means of bypassing the liver, preventing the first pass metabolism. This technology also has the ability to target delivery of drugs to various areas of the body, and enables the delivery of drugs that are poorly water-soluble. Moreover, nanotechnology improves drugs bioavailability and allows them to remain circulating in the blood for a long time, controlling their release. Nanostructures can also be able to penetrate tissues and be easily taken up by cells, allowing for efficient delivery of drugs to target sites of action. Nanostructures were reported to be taken up by the cells at rates 15–250 times greater than microparticles. Nanotechnology improves performance and acceptability of dosage forms and even may enhance the performance of drugs that fail in clinical trial phases. Nanotechnology will definitely revolutionize the science of drug delivery and help overcome the major challenges of conventional drugs used for the treatment and management of chronic diseases such as, cancer, asthma, hypertension, HIV, and diabetes.
In this context, nanomedicine can be defined as the medical application of nanotechnology and involves research, and diagnostic and therapeutic applications related with nanotechnology. Nanotechnology has been applied in medicine to develop strategies to manage, treat, or even cure virtually every type of human disease.
Mabs are not only successful drugs but also powerful tools for a wide range of medical applications. In this regard, over the last 10 years several nanotechnological platforms for medical applications have converged upon a specialization toward diagnosis, therapy or even both, as in the case of magnetic nanoparticles.
The study of nanoscience and the optimization of technologies, such as ADCs and nanocarriers have driven the design of methodologies that are tailored to address specific diseases and medical anomalies. ADCs, for example, represent excellent nanoscale-based drug delivery vehicles and thus tend to be focused on delivery to certain drugs, as in the treatment of cancer.
The conjugation of different moieties to the nanoparticles widens their application fields and provides them with new or enhanced properties. A range of biomoieties can be conjugated to the nanoparticles including low molecular weight ligands (folic acid, thiamine, dimercaptosuccinic acid), peptides (RGD, LHRD, antigenic peptides, internalization peptides), proteins (BSA, transferrin, antibodies, lectins, cytokines, fibrinogen, thrombin), polysaccharides (hyaluronic acid, chitosan, dextran, oligosaccharides, heparin), polyunsaturated fatty acids (palmitic acid, phospholipids), DNA, and plasmids.
Nanotemplates provide unique access to extremely high-throughput capabilities for rapidly defining the presence of a biomarker and thus lend themselves as excellent platforms for diagnostics applications. Rapid progress is being made to drive these advances in nanomedicine, especially in the area of cancer treatment. Although still in its infancy when compared to other medical technologies, nanomedicine is undoubtedly here to stay and will have an enormous impact on the future health and well-being of man.
4.1. Methods for Conjugating Antibodies to Nanocarriers
Nanocarriers are nanometer-sized materials that have the capacity to deliver therapeutic agents at the disease site (Arias, 2011, Schroeder et al., 2012). They are designed to possess unique physicochemical properties, aiming to improve the pharmacokinetic and biodistribution of a drug molecule (Li and Huang, 2008) and to deliver a significant amount of drug molecules. Examples of some therapeutic nanocarriers are lipid-based particles (Al-Jamal and Kostarelos, 2011), micelles (Kedar et al., 2010), nanoparticles (Shi et al., 2010), dendrimers (Svenson, 2009), and polymersomes (Levine et al., 2008). Some of these have been proposed for the treatment of various diseases including cancer (Dhar et al., 2011), coronary artery diseases (Chan et al., 2011), and rheumatoid arthritis (Thomas et al., 2011). In the particular case of cancer, the unique anatomy—that is, the leakiness of the tumoral vasculatures—concedes a passive transport of the nanocarriers by enhanced permeability and retention (EPR) effect (Maeda et al., 2003). However, the porosity of the tumor blood vessels may vary with the tumor type (Bae, 2009). Even with a successful delivery by the EPR effect, the nanocarriers must be able to internalize into the cancer cells (Gradishar et al., 2005). A new paradigm in drug delivery embroils a combination of active and passive targeting. Targeting ligands, such as antibodies (Mamot et al., 2005), peptides (Srinivasan et al., 2009), small molecules (Kukowska-Latallo et al., 2005), or aptamers (Farokhzad et al., 2006) can be attached onto the surface of a nanocarrier. The carriers recognize and bind to the cell-surface receptors and are subsequently taken by the cells via receptor-mediated endocytosis for releasing the therapeutic payloads (Shi et al., 2011). The binding affinity of targeted nanocarriers can also be increased several orders of magnitude by the multivalent effect (Wang et al., 2010).
Among all the targeting ligands, antibodies are well known for their high binding affinity, specificity, and availability for a number of disease biomarkers (Manjappa et al., 2011). An antibody can be simply absorbed on the surface of a nanocarrier via hydrophobic and/or electrostatic interaction (Sokolov et al., 2003). However, using this approach, the absorbed antibody may orient randomly on the surface and result in losing the binding affinity. Furthermore, the antibody may exchange with other endogenous protein in vivo (Nobs et al., 2004). Therefore, antibodies are generally preferred to attach to the nanocarriers covalently (Arruebo et al., 2009). An antibody consists of a number of functional groups that provides many options for bioconjugation (Manjappa et al., 2011). Bioconjugation can take place by means of adsorption (at the isoelectrical point of the antibody via electrostatic interaction), by direct covalent linkage between the surface of the nanoparticle and the antibody, or by using adapter molecules.
The use of adapter molecules generally involves streptavidin and biotin for the formation of the complex. Biotin-labeled polyclonal goat anti-Escherichia coli antibodies have been attached to streptavidin-coated magnetic nanoparticles, and used for the separation and selective quantification of Escherichia coli O157:H7 in ground beef in the presence of other bacteria (Varshney et al., 2005).
5. Therapeutic Applications of Monoclonal Antibodies
Mabs are capable of specifically recognizing and binding to many molecules, mainly those of protein nature. This capability have been widely used in the field of diagnosis for the detection of hormones, vitamins, cytokines, allergens, numerous tumor markers, and a wide range of markers associated with many diseases, including microbial infections. For these properties, the Mabs are the substances most commonly used in the field of clinical diagnosis and in identifying markers and therapeutic targets. Besides applications in disease diagnosis and in drug discovery, therapeutic applications include Mabs, such as vehicles or carriers of other drugs.
Current major therapeutic applications of Mabs include cancer, chronic inflammatory disease, and infection, and they constitute the largest and fastest growing sector of the biological pharmaceutical industry.
In addition to offering a host of new drugs to fight disease, Mabs have provided the means to monitor a patient’s response to therapy and helped lead the way in personalized medicine.
Mabs are now marketed not only for cancer and autoimmune disorders, but also for a range of other diseases, including allergic conditions, such as asthma, age-related macular degeneration (an eye disorder), multiple sclerosis (a neurological disorder), and osteoporosis (brittle bones). They are also being investigated for central nervous system disorders, such as Alzheimer’s disease (a degenerative brain disease), metabolic diseases such as diabetes, and the prevention of migraines.
Mabs used for therapeutic purposes may bind to a variety of antigens present on the surface of tumor and cancer cells. They may also be utilized to deliver a number of different types of payloads to destroy the targeted cells in question including radioactive ligands, cytokines, toxins, liposomes containing drugs, and specific killer cell types. These immunoconjugates, which are defined as antibodies linked to a second molecule, such as a toxin, radioisotope, or label may act at the cell surface as killing agents or be internalized to release payloads intracellularly.
5.1. Diagnosis and Therapeutic Applications of Antibody-Conjugated Nanoparticles
Despite their high sensitivity and reproducibility, conventional diagnostic methods for a microbial infection require cumbersome sample preparation and long readout times (Kaittanis et al., 2009). Unique electrical, magnetic, luminescent, and catalytic properties of nanomaterials enable fast, sensitive, and cost-effective diagnosis, as well as rapid determination of the susceptibility and resistance of antibacterial drugs (Bruchez et al., 1998, Edgar et al., 2006).
Antibody-conjugated nanoparticles amplify the signals for bioanalysis and enumeration of highly pathogenic bacteria, such as E. coli O157:H7, resulting in highly selective, convenient, and rapid detection of single bacterium within 20 min (Look et al., 2010).
The rapid and sensitive determination of pathogenic bacteria is extremely important in biotechnology and medical diagnosis. Current methods either lack ultrasensitivity or take a long time for analysis. The use of magnetic nanoparticles also could be a very sensitive and rapid strategy to detect microbial infection. Magnetic nanoparticles, in particular superparamagnetic iron oxide nanoparticles (SPION) with appropriate surface chemistry have been widely used experimentally for numerous in vivo applications, such as magnetic resonance imaging contrast enhancement, tissue repair, immunoassay, detoxification of biological fluids, hyperthermia, drug delivery, in cell separation, and so forth (Neuberger et al., 2005).
Dextrancoated supermagnetic iron oxide nanoparticles were clustered by Con-A treatment, or equipped with Con A-conjugated nanosensors (Zhao et al., 2004).
Supermagnetic iron oxide nanoprobes greatly assisted the identification of Mycobacterium avium spp. paratuberculosis, as well as the quick quantification of these bacteria in milk and blood with high sensitivity (Basu et al., 2004).
A quick method for detecting infections in the urinary tract has also been developed using gold nano-wire arrays in conjunction with a linker arm attached to specific E. coli antibodies (de la Escosura-Muñiz and Merkoçi, 2011).
Li and Huang (2008) have shown that nanoparticles with specific Raman spectroscopic fingerprints can distinguish antibiotic resistant bacteria, by detecting single-nucleotide polymorphisms in microarray-based systems (Li et al., 2009).
A bioassay based on bioconjugated nanoparticle was developed by Zhao et al. (2004) for in situ pathogen quantification. The detection is carried out by a high fluorescent signal provided by the nanoparticle, which is easily attached to an antibody or other biorecognition molecule. These conjugated nanoparticles can be used in the specific identification of a wide range of bacteria, such as E. coli O157:H7, through antibody antigen interaction.
These nanoparticles were able to assess the microbial metabolic activity and determine antimicrobial susceptibility in blood, by rapidly quantifying polysaccharides.
The broad absorption spectra of quantum dots (QDs) can be exploited to simultaneously excite QDs emitting different colors using a single wavelength (Cao et al., 2002). These characters suggest that QDs are a promising modality for the analysis of complex samples for histology, pathology and cytology, and can facilitate double or even triple immunostaining of bacterial cells (Kaittanis et al., 2008). Studies have demonstrated that the use of nanotechnology feasibility of achieving fast and reliable pharmaceutical assays for microbial infections in opaque media (e.g., whole blood and milk), without any sample preparations (Alper et al., 2004, Tully et al., 2006).
The ability to track the distribution and differentiation of progenitor and stem cells by high resolution in vivo imaging techniques would have significant clinical and research implications. Lewin et al. (2000) developed a cell labeling approach using short HIV-Tat peptides to derivatize superparamagnetic nanoparticles. These particles were efficiently internalized into hematopoietic and neural progenitor cells in quantities up to 10–30 pg of superparamagnetic iron per cell. Iron incorporation did not affect cell viability, differentiation, or proliferation of CD34+ cells. Following intravenous injection into immunodeficient mice, 4% of magnetically CD34+ cells homed to bone marrow per gram of tissue, and single cells could be detected by magnetic resonance imaging in tissue samples. In addition, magnetically labeled cells that had homed to bone marrow could be recovered by magnetic separation columns. Localization and retrieval of cell populations in vivo enable detailed analysis of specific stem cell and organ interactions critical for advancing the therapeutic use of stem cells (Lewin et al., 2000).
Nanomaterials with fluorescent properties or nanoparticles labeled/encapsulated with fluorescent dyes have also been applied for microbial detection. Using antibody-conjugated silica nanoparticles, these materials can be used as a superior signaling element, to detect cells, proteins and bacteria in an immunoassay (Grifantini et al., 2002, Tan et al., 2004).
Ultrasensitive methods for bioassays have been developed using fluorescent bioconjugated silica nanoparticles (Santra et al., 2001, Zhao et al., 2004). Thousands of fluorescent dye molecules are encapsulated in a protective silica matrix, resulting in a nanoparticle with an amplified and reproducible signal for fluorescence-based bioanalysis. Whereas in conventional immunoassays only one or a few dye molecules are linked to an antibody molecule and then used to signal an antibody-antigen interaction event, the bioconjugated nanoparticles, which are attached to the antibody molecule, are enabled to carry many dye molecules inside, allowing a significant amplification of the analytical signal. Furthermore, bacteria present many surface antigens available for antibody recognition, and, therefore, a greatly amplified signal can be achieved since thousands of nanoparticles can attach to each bacterium. These bioconjugated nanoparticles allow the detection of a bacterium cell per given sample just in 20 min with a spectrofluorometer.
Santra and coworkers used covalent method to attach surface-modified, rubpy-doped silica nanoparticles to mouse antihuman CD10 antibody. This complex was then incubated with mononuclear lymphoid target cells. After washing away the unbound nanoparticles, target leukemia cells could be clearly detected. In comparison to control group, results of this experiment have shown that this technique was very effective to detect leukemia cells selectively (Santra et al., 2001).
Tan and coworkers developed an assay tool for in situ detection of single bacterium cells in less than 20 min and developed multicolored FRET (fluorescence resonance energy transfer) silica nanoparticles by coencapsulating three tandem dyes that emit unique colors upon excitation with a single wavelength (Tan et al., 2004).
Among therapeutic applications of antibody-conjugated nanoparticles, the use of gold nanoparticles was tested in killing Staphyloccocus aureus (Zharov et al., 2006). The bacteria were killed by light-adsorbing gold nanoparticles conjugated to antiprotein A antibodies, using laser irradiation at 532 nm. Protein A was chosen because it interacts specifically with the Fc fragment of the antibody. According to the authors, killing efficiency depends on the local overheating effects accompanied by bubble-formation phenomena. Direct irradiation of bacteria with the laser did not damage the bacteria, because of low absorption by natural endogenous cytochromes.
5.1.1. Cancer
The diagnosis of cancer at early stages of growth is a critical factor for obtaining optimal results in therapy and for improving the chances of survival.
Several imaging techniques collaborate with physicians in the diagnosis, including magnetic resonance imaging (MRI), positron emission tomography (PET), computed tomography (CT), ultrasound, radiography, photoacoustic imaging, fluoroscopy, and so forth. In some of these techniques, antibody-conjugated nanoparticles may offer increased selectivity and sensitivity.
Fluorescent semiconductor nanocrystal QDs are a novel class of multifunctional inorganic fluorophores that are promising in biological imaging, including immunofluorescence imaging (Wu, 2003), and are useful for labeling molecules (Lidke et al., 2004, Wu et al., 2003) and cells in various materials (Gao et al., 2004, Jaiswal et al., 2003, Kim et al., 2004), even clinical human samples (Tholouli et al., 2006).
The first generation of ADCs presented limitations in relation to the linker instability because the early linkers were either too stable, resulting in low potency and reduced efficacy, or too unstable, resulting in poor targeting and high systemic toxicity. Furthermore, cytotoxics without sufficient potency, inefficient internalization, limited expression of the target antigen and immunogenicity of Mabs (Alley et al., 2009, Chari, 2008).
Second-generation ADCs were designed to deliver potent anticancer agents to tumors in a targeted manner to limit systemic exposure. The success of targeted ADCs depends on antibody selection, potency of the cytotoxin and the method selected to link the antibody to the cytotoxin.
Nanotechnology is a disruptive technology that drives a new generation of cancer preventive diagnosis, and therapeutic products, resulting in dramatically improved cancer outcomes. Nanotechnology in the field of cancer has the potential to improve the monitoring of therapeutic efficacy, provide novel methods for the detection and profiling of cancers at early stages, and allow surgeons to delineate tumor margins and sentinel lymph nodes. Nanomaterials have unique features that are attractive and can be applied to biosensing. The development of various nanomaterials and nanotechnology has enabled detection of cancer biomarkers with great precision and sensitivity that could not be achieved before. Many studies are being conducted on developing sensing mechanisms that will push down the detection limit as far down as possible (Anajwala et al., 2010). Ligand-free nano-formulations generally possess passive targeting property with little tissue specificity. To address these limitations, research has been continued to advance active or specific targeting to enhance the efficacy of anticancer therapeutic agents, as well as to reduce the toxicity to nontargeted healthy tissues. Nanoparticles containing the chemotherapeutic agents are specifically designed to target the cancerous cells either by ligand receptor interaction or antibody-antigen recognition. Highly specific Mabs are used to strengthen the immune response and to intensify the immune system’s antitumor capacity. Moreover, Mabs are highly specific when attached to nanoparticles to aid in targeted delivery of various antitumor cytotoxic agents or function themselves as effective therapeutic agents (Sutradhar and Amin, 2014). There are different ways of destroying cancer cells with Mabs: directly by inducing apoptosis, blocking growth factor receptors or inducing the formation of antiidiotype, and indirectly by activating complement-mediated cellular cytotoxicity and antibody dependent cell-mediated cytotoxicity (Praetorius and Mandal, 2007).
The paramagnetic properties of iron-oxide nanoparticles have been harnessed for therapeutic and imaging applications (McCarthy et al., 2007). Chen et al. (2006) have reported that dextran-coated iron-oxide nanoparticles conjugated to radiolabeled (Iodine isotope 131,131I) anti-VEGF Mab significantly increased imaging resolution, as well as destruction of liver cancer in mice (Chen et al., 2006).
Srinivasan, Lakshmikuttyamma, and Shoyele (Srinivasan et al., 2013) studied selective targeting of Mabs to oncoproteins in cancer cells while avoiding their accumulation in normal cells. Results of fluorescence microscopy, TEM, and flow cytometry revealed that bevacizumab nanoparticles were internalized by A549 cells 3 times more than by MRC-5 cells. Macropinocytosis and energy-dependent pathways were elucidated to be involved in their uptake by A549 cells. This study presents the first evidence that uncoated Mab nanoparticles can be selectively delivered to cancer cells while avoiding normal cells.
Mabs have been also employed as an effective active targeted therapy since these antibodies can specifically recognize the over-expressed HER2 positive tumor cells and internalize through receptor mediated endocytosis.
To treat HER2 positive breast cancer, anti-HER2 humanized Mabs are commonly used, although advances can be made in targeted cellular localization via conjugation strategy through a nano-particulate system focusing on surface modified ligand/receptor-mediated nano-therapy to target the tumor cell at the molecular level.
Various nanoparticle systems, such as liposomes, micelles, and dendrimers, have been studied not only in vitro but also in vivo, concluding that the presence of receptor targeting antibody on the nano-carriers demonstrated a higher toxicity than the nano-carriers without an antibody after time dependent incubation (Hamidreza Montazeri et al., 2008, Matsumura, 2008, Yokoyama, 2005).
Antibodies against cell surface biomarkers are the commonly used ligands for the development of targeted nanoparticles. Although mouse Mabs have been used for making targeted nanoparticles, strong cross-species immune responses limit their potential for future clinical translation. Currently only a few types of humanized Mabs, such as HER-2 antibody (Herceptin), are available for the production of targeted nanoparticles (Slamon and Pegram, 2001). Alternatively, high affinity recombinant antibody fragments have been developed as targeting ligands (Yang et al., 2015). For example, a human single chain antibody against the epidermal growth factor receptor (ScFvEGFR) that is highly expressed in the majority of epithelial tumors was conjugated to different types of nanoparticles. Specificity of tumor imaging and targeted therapeutic effects of these nanoparticles have been demonstrated in several animal tumor models (Yang et al., 2015). The major advantages of using natural ligands for tumor targeting are their high binding affinity, specificity, and, most importantly, low immunogenicity.
Owen et al. (2013) reported higher antibody-conjugated lysosomal P (LA-co-TMCC)-g-PEG-furan micellar concentration in HER2 positive cell lines with significant cellular distribution resulting in two- to threefold increase in lysosomal accumulation compared to using the antibody alone (Owen et al., 2013). In addition, Nobs et al. (2004) reported that thiol functionalized anti-HER2 Mab can be conjugated with the maleimide containing liposomes. Internalization of these antibody coupled immunoliposomes by HER2 positive breast cancer cell line (SK-BR-3) was observed with enhanced effects compared to the control liposomes (Nobs et al., 2004). Many authors observed that the use of lisposomes was more effective in vitro than in vivo. To prove targeted drug-loaded liposome as an effective and safe treatment option for HER2 positive breast cancer, more in vivo studies need to be conducted.
When used, anti-HER2 Mab conjugated with dendrimers resulted in the effective delivery to target the overexpressed HER2 receptors of tumor cells in animal models. More recently, Miyano et al. (2010) developed biocompatible sixth generation (G6) anionic lysine (amino acid) dendrimer, on which the surface was modified with glutamate and, thereafter the glutamate modified G6 lysine dendrimer was coupled with trastuzumab Mab and a fluorescent label. Following this, the whole conjugated formulation was evaluated in both HER2 positive and negative cell lines to assess the targeting efficiency and cellular internalization compared to the free antibody application. The results of this study revealed high binding affinity with low cytotoxicity; and cellular internalization or lysosomal trafficking in HER2 positive cells with a dose-dependent profile compared to the HER2 negative cells (Miyano et al., 2010).
In subsequent studies by Chen et al. (2008) and Gao et al. (2009), a model protein toxin (PE38KDEL) was used to develop anti-HER2 antibody functionalized PE38KDEL loaded PLGA nanoparticles utilizing a two phase carbodiimide process. Modified encapsulated nanoparticles showed higher in vitro cytotoxicity and more protective antitumor activity compared to the controls in HER2 overexpressing cells and tumor-bearing mice, respectively. In addition, they reported well tolerability for maximum tolerated dose, as well as reduced systemic toxicity when modified nanoparticles were administered to mice model compared to control (Chen et al., 2008, Gao et al., 2009).
Nanotechnology has promoted greater opportunities for higher specific drug delivery with minimum side effects. Bio-conjugation strategies of therapeutic agents loaded nanoparticles with Mabs have exhibited a targeted drug delivery approach both in vitro and in vivo.
Mabs can act as highly specific probes when they are attached to nanoparticles to aid in targeted delivery of various antitumor cytotoxic agents (Sutradhar and Amin, 2014). The combination therapy of nanopartilces of Mabs and other chemotherapeutic agents, such as doxorubicin, 5-Fluorouracil, paclitaxel, docetaxel, campothecin, and topotecan, could bring about synergistic tumor growth inhibition. Some nanoparticulate systems combining antibody with these chemotherapeutic agents are listed in Table 25.1 .
Table 25.1.
Nanoparticulate Systems Combining Antibody With Chemotherapeutic Agents
| System | Antibody | Loaded Drug | Pathology/Target | Reference |
|---|---|---|---|---|
| PEG liposomes | Anti-MT1-MP(Fab′) | Doxorubicin | — | Hatakeyama et al. (2007) |
| Fe3O4/Au magnetic/gold nanoparticles | Bevacizumab anti-VEGF antibody | Doxorubicin | — | Ramos-Tejada et al. (2015) |
| Liposomes | Anti-HER2 monoclonal antibodies (trastuzumab) | Doxorubicin | Tumor xenograft nude mouse models | Banerjee et al. (2011); Park et al. (2007) |
| Chitosan nanoparticles | Anti-HER2 monoclonal antibodies | Doxorubicin | Cancer therapy (breast, ovary) | Yousefpour et al. (2011) |
| Human albumin serum (HAS) protein-based nanoparticle | Trastuzumab | Doxorubicin | Breast cancer therapy | Anhorn et al. (2008) |
| Chitosan scaffold-PLGA nanoparticles | Bevacizumab anti-VEGF antibody | 5-Fluorouracil | Brain cancer | Kutlu et al. (2014) |
| Poly(propylene imine) dendrimer | Primary antibody (mAbK1) | Paclitaxel | Ovarian cancer therapy | Jain et al. (2015) |
| PLGA nanoparticles | Cetuximab | Paclitaxel palmitate | Lung cancer therapy | Karra et al. (2013) |
| Poly(d,l-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles | Trastuzumab | Paclitaxel | Breast cancer therapy | Sun et al. (2008) |
| Micellar nanoparticles (vitamin E TPGS and TPGS-siRNA) | AntiHER2 antibody | Docetaxel | Breast cancer therapy | Zhao et al. (2013); Mi et al. (2013) |
| Polylactide (PLA)-based nanoparticles | AntiHER2 antibody | Docetaxel | Breast cancer therapy | Zhao et al. (2012) |
| Poly(lactide-co-glycolide) (PLGA) nanoparticles | IgG Isotype control antibody anti-Fas mAb | Camptothecin | Colorectal cancer therapy | McCarron et al. (2008) |
| Liposomes | Antibody fragments scFv antibodies | Topotecan | Prostate cancer therapy | Roth et al. (2007) |
5.1.2. Eye diseases
Neovascularization is a major cause of visual loss in a number of ophthalmic diseases. It consists of new blood vessel growth from preexisting vascular structures. This process occurs in pathologies of the anterior and posterior segments of the eye, such as corneal neovascularization, age-related macular degeneration (AMD), diabetic macular edema, viral retinitis, proliferative vitreoretinopathy, posterior uveitis, retinal vascular occlusions, choroid neovascularization (CNV), and diabetic retinopathy, to name a few.
Anti-VEGF agents have demonstrated efficacy in reducing neovascularization in both animal models and clinical trials. Specifically, anti-VEGF antibodies have shown initial therapeutic success. Bevacizumab is a full-length, humanized murine Mab that recognizes all isoforms of VEGF. Bevacizumab was initially approved by the U.S. Food and Drug Administration (FDA) to treat metastatic colon cancer, but it has also shown efficacy in the treatment of various neovascular ocular diseases and is used off-label to treat neovascular age-related macular degeneration.
Topical delivery is a relatively easy and a less risky method of drug administration. However, delivery to the posterior segment via this route is considered inefficient and unsuccessful, since less than 5% of the topically applied dose enters the eye and an even smaller fraction of it (0.001%) is expected to reach the posterior segment. Intravitreal administration involves the direct administration of drug solution/suspension into vitreous humor via pars plana using a 30-G needle. In contrast to the topical and systemic routes, intravitreal injection makes high concentrations of drug locally available to the internal eye tissue, including the choroid and the retina. Similarly, the intravitreal administration of Macugen (pegaptanib sodium; Pfizer) and Lucentis (ranibizumab; Genentech/Novartis), vascular endothelial growth factor (VEGF) inhibitors, is highly successful for the control of AMD. However, agents with molecular weight less than 500 Da when applied intravitreally tend to be drained off from the site of application with a half-life of less than 3 days, indicating a need for repetitive injections. Anyway, the period requiring a repeat dose may extend from a few days to several months for macromolecular antibodies. For example, three is the mean number of injections of bevacizumab (Avastin; Roche) required to be administered per year for the treatment of AMD. On the other hand, the recommended dosing frequency of ranibizumab (Lucentis) is once a month (0.5 mg; 50 μL) for at least 9 months (Rosenfeld et al., 2006), whereas pegaptanib sodium (Macugen) needs to be injected intravitreally at 6-week intervals for 1 year (Kitagawa and Yuzawa, 2013).
Nevertheless, repetitive intravitreal injections, even if spaced widely, are invariably associated with complications, such as vitreous hemorrhage, retinal detachment, cataract, and endophthalmitis. The rate of endophthalmitis and retinal detachment being observed with intravitreal injection is 0.2 and 0.05%, respectively (Edelhauser et al., 2010). Moreover, patient compliance is lower with such regimens because of the painful and invasive procedures requiring hospitalization and specially trained physician for administration, in addition to the high cost of the medicine per se (Kaur and Kakkar, 2014).
Biodegradable polymeric nanoparticles offer properties that make them suitable candidates to overcome these administration issues. In this regard, Abrishami et al. (2009) studied encapsulated bevacizumab into liposomesand and found that the intravitreal injection of liposomes was well tolerated through 42 days in rabbits. The clearance of this drug in vitreous from liposomal formulations was slower than the soluble form. The concentration level of bevacizumab after intravitreal injection suggested that intravitreal injection of drug by these carriers provide sufficient concentration of therapeutic drug for 6 weeks for diabetic neovascularization and probably for other neovascular eye diseases (Abrishami et al., 2009).
Other authors developed nanoparticles of PLGA loaded with bevacizumab and stabilized whit albumin. They found that the vitreous concentration of bevacizumab was sustained for about 8 weeks at values greater than 500 ng mL−1 and that the nanoparticles presented a drug vitreous medium retention time (MRT) 3.3 times higher than the control. In addition, it was confirmed the nanoparticles persistence in ocular tissues for 56 days (Varshochian et al., 2013, Varshochian et al., 2015).
Recently Lu et al. (2014) studied the effects of intravitreal injection of bevacizumab-chitosan nanoparticles on pathological morphology of retina and the expression of vascular endothelial growth factor (VEGF) protein and VEGF mRNA in the retina of diabetic rats. The results have offered a new approach for inhibiting angiogenesis of diabetic retinopathy and indicated that the intravitreal injection of bevacizumab inhibited VEGF expression in retina. In addition, bevacizumab-chitosan nanoparticles presented a longer period of action (Zhou et al., 2015).
5.1.3. Respiratory diseases
Chronic respiratory diseases (CRD) are chronic diseases of the airways and other structures of the lung. Among the most common, asthma, chronic obstructive pulmonary disease (COPD), and respiratory allergies can be mentioned.
The airway inflammation in these diseases cannot always be controlled with conventional therapies. The critical role of the combination therapy of an inhaled corticosteroid (ICS) and a long-acting β-adrenoceptor agonist (LABA) in the treatment of patients also suffering from severe asthma and chronic obstructive pulmonary disease (COPD) explains why there is a strong interest within the pharmaceutical industry in developing new pharmaceutical alternatives.
Biological therapies represented in particular by Mabs against selective targets, could improve the outcome of these diseases.
Many current publications have shown the important role of nanomedicine in the treatment of respiratory diseases (Card et al., 2008, Mansour et al., 2009, Smola et al., 2008). Systems releasing nanoparticles have several possibilities to improve treatment of upper respiratory tract since they can protect drugs from degradation by enzymes present in the epithelial lining fluid, the particle size may permit circumvent pass macrophages, and crossing the endothelium will allow systemic treatment of nanoparticles and pulmonary drug intravenously administration.
Identifying asthma phenotypes has given impetus to the search for biomarkers to help classify patients, pointing to new therapies and predicting different pathological mechanisms of disease progression with strong benefits for affected patients (Moore et al., 2007).
An ideal biomarker is easy to detect and measure, noninvasive, and inexpensive, and it can be used to identify phenotypes either for clinical response or treatment, assessing changes in disease activity, or confirming a diagnosis.
COPD is currently the fourth leading cause of death in the world, and if current trends continue, it is likely to rank as the third leading cause of death by 2030 (World Health Organization, 2008).
Both cytokines and chemokines play a fundamental role in the organization of COPD, so the use of Mabs in the treatment options is very valuable to provide more targeted therapies (Li et al., 2002, Yamagata and Ichinose, 2006).
Numerous antibodies directed to cytokines, chemokines, growth factors, and their receptors are considered for the treatment of COPD and some have already been studied in clinical trials in patients with COPD.
The macrophage imaging using MRI, together with the use of magnetic nanoparticles of iron oxide, has recently emerged as a promising noninvasive technique for preclinical and clinical studies of inflammatory diseases.
However, limited research on inflammation and passage of macrophages in the lung, using imaging technology has been performed due to difficulties in imaging the body (i.e., the signal loss due to the pulsations and heart breathing, low proton density, and susceptibility artifacts). Novel perspectives for imaging, diagnosis, and treatment of respiratory diseases, such as COPD can appear due to technical improvements and the development of techniques of magnetic resonance pulse sequence detection.
Studies reported the possibility of noninvasive monitoring of subsets of macrophages in an inflammatory model using high resolution MRI after intravenous administration. The possibility of noninvasive monitoring of subsets of macrophages was reported in an inflammatory model using high resolution MRI after intravenous administration. Furthermore, other studies have proven that polarization and proliferation was not affected using iron oxide nanoparticles with ex vivo labeled subpopulation of macrophages. The coupling of a specific antibody with the iron oxide nanoparticles directed to a particular subpopulation of macrophages may provide a promising strategy for early diagnosis and improved various inflammatory diseases noninvasively using MRI.
Al Faraj et al. (2014) evaluated the in vivo effect of intrapulmonary administration of SPION on the profiles of alveolar macrophage polarization in a model of COPD and developed a protocol MRI noninvasive to specifically target and monitor a subpopulation of macrophages using specific antibody-conjugated SPION.
An interesting approach was performed to target a subpopulation of macrophages in the lung of a COPD animal model using biocompatible antibody-conjugated iron oxide nanoparticles. This conjugation allowed noninvasive tracking using a free-breathing MRI protocol.
Antibodies to IL-8 Abgenix improved dyspnea but not lung function. This limited clinical benefit is attributed to suboptimal dosing into the airways because of intravenous administration (Mahler et al., 2004). Therefore, the use of nanoparticles may allow administration by inhalation for local concentrations and avoiding side effects. So far, clinical trials with anti-TNFalpha antibody infliximab have been carried out, but no effects in patients with COPD were observed (Dentener et al., 2008, Rennard et al., 2007, Van der Vaart et al., 2005).
Therefore, the release of cetuximab (currently undergoing clinical trials for cancer therapy) could be a valuable option for the treatment of COPD.
Molecularly targeted nanoparticles were chosen as the best strategy for imaging inflammation to make progress in health care (McCarthy and Weissleder, 2008, Weissleder, 2006).
Magnetic nanoparticle’s versatility makes them well suited for applications to enable early detection and prevention, and so improving diagnosis, treatment, and follow-up of diseases (Oghabian and Farahbakhsh, 2010).
Al Faraj et al. (2014) developed an assay to quantify the antibodies CD86 or CD206 conjugated to SPION, which involves the reduction of Cu2+ to Cu+ by proteins and the appearance of a purple-blue copper-protein complex in alkaline medium.
6. Future Trends of Monoclonal Antibodies
Some significant trends that appear to be developing include bispecific antibodies, ADCs, and companion diagnostics, which should help to identify the most appropriate patient populations for treatment.
Even though the health benefits of Mab therapeutics are proven, controversies will continue about their economic viability because of their expensive price. High prices, however, are generally associated with early innovative treatments, and Mabs are not the only drugs to have staggering prices. The cost of cancer treatments, for example, has more than doubled in the past two decades, leading to an outcry by many European and American cancer specialists. Another reason why Mabs are so expensive is the fact that many of them are still protected by patents. A drug’s patent life is 20 years from the date of filing, in order to help developers recover some of the research and development costs involved in getting a drug to market. Some of the patent life is in fact reduced because some of that time, an average of 8 years, is taken up by clinical trials and regulatory approval (Siddiqui and Rajkumar, 2012).
The slow progress of Mab therapeutics for infectious diseases can be attributed in part to the large arsenal of other antiinfective drugs, such as vaccines and antibiotics. Because they are specific to a single pathogen, Mab drugs are also commercially less attractive than traditional drugs because they cover a narrower spectrum of patients. In addition, Mabs need to be administered by intravenous or subcutaneous injection, unlike other antiinfectives that can be taken orally, so they are unsuitable for patients in developing countries who have limited access to healthcare. Mabs are also more effective at preventing infection rather than treating established ones, and unlike vaccines provide only short-term prophylaxis. And finally, the high development and manufacturing costs associated with Mabs and the poor record in winning approval for treating infectious diseases with Mabs lessen their commercial appeal (Reichert, 2006).
More promising are recent developments to enhance the potency and efficacy of Mabs, so as to make it possible to prescribe lower doses and potentially reduce costs. A number of approaches have been adopted to augment the efficacy of Mabs. One of the most encouraging is the use of genetic engineering to remove glycosylation sites from the variable domain of the antibody. This enhances the effector function of Mabs, such as antibody-dependent cell-mediated cytotoxicity (ADCC), which activates the patient’s innate immune cells to kill a target cell like cancer.
Although the new generation of Mabs may greatly improve the treatment of cancer and autoimmune disorders, which are well-established disease targets for Mab therapeutics, it remains to be seen whether Mab therapeutics will be effective in other areas. Nowhere is this question more urgent than in the case of infectious diseases.
The fact that Mab therapeutics is pathogen-specific could hinder its use for treating mixed infections. One solution might lie in the use of a cocktail of Mabs to target the diverse range of antigens that viruses carry. Such a strategy would effectively mimic the natural immune response: once infected, the body tends to develop several antibodies in response to the antigens presented by a virus, each of which attaches to one of the different antigens. It is this diversity of antibodies that helps the immune system in fighting the invader. The use of a cocktail of Mabs is already being investigated for the treatment for rabies
Recent advances in Mab engineering have opened up new opportunities for serum therapy. Importantly, Mabs offer the means to prepare standardized agents, which, when combined in a cocktail, can yield a product that is more precise and more potent than traditional serum therapy. To date, the development of antiinfective Mab products has attracted little commercial investment. In part this is because infectious diseases are short-lived and therefore have a limited market. This is in contrast to chronic conditions like cancer and autoimmune diseases, which require regular treatment and therefore have greater profit potential. Nonetheless, the pharmaceutical climate is changing, fueled by concern over the rise in new pathogens (such as West Nile and corona viruses); the reemergence of old pathogens (like tuberculosis), increasing antibiotic resistance among microorganisms; and the rise of superbugs like MRSA, as well as the growing epidemic of patients who are immunocompromised as a result of HIV infection, organ transplantation, chronic degenerative diseases, and improvements in cancer care.
Likewise, Mabs have been used to investigate and treat cancer, providing powerful tools for identifying and targeting different antigens on tumors. Although these applications did not quickly translate into successful Mab therapeutics for cancer, in more recent years Mabs therapeutics have offered alternatives to drugs with a broad spectrum and high toxicity. This has transformed the care of cancer patients, who no longer face the prospect of losing hair and other serious side effects associated with other cytotoxic drugs. The advantage of Mabs is that they can be given as maintenance therapies. This is reshaping our perceptions of some cancers from what was once seen as inevitably fatal to a chronic condition. Mabs have also enabled the prescription of specific therapeutics for particular tumor antigens in individual patients. This allows a greater degree of personalization in the management of cancer than in the past. Indeed, Mabs are expected to be an increasingly important component in personalized cancer therapies.
In a world of antibiotic-resistant superbugs and an aging population grappling with autoimmune disorders and cancer, Mabs offer the potential for new, targeted treatments and drugs that can offer personalized care—and a window into the complex, overlapping conditions that underlie human disease.
References
- Abrishami M., Ganavati S.Z., Soroush D., Rouhbakhsh M., Jaafari R., Malaekeh Nikouei P.B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (Avastin) for intravitreal administration. Retina. 2009;29(5):699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- Al Faraj A., Shaik A.S., Afzal S., Al Sayed B., Halwani R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int. J. Nanomed. 2014;24(9):1491–1503. doi: 10.2147/IJN.S59394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jamal W.T., Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- Alley S.C., Zhang X., Okeley N.M., Anderson M., Law C.L., Senter P.D., Benjamin D.R. The pharmacologic basis for antibody-auristatin conjugate activity. J. Pharmacol. Exp. Ther. 2009;330(3):932–938. doi: 10.1124/jpet.109.155549. [DOI] [PubMed] [Google Scholar]
- Alper M.D., Bertozzi C.R., Clarke J. Detection of bacteria in suspension by using a superconducting quantum interference device. Proc. Natl. Acad. Sci. USA. 2004;101(1):129–134. doi: 10.1073/pnas.0307128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anajwala C.C., Girish K.J., Vijayendra Swamy S.M. Current trends of nanotechnology for cancer therapy. Int. J. Pharm. Sci. Nanotechnol. 2010;3(3):1043–1056. [Google Scholar]
- Andreakos E., Taylor P.C., Feldmann M. Monoclonal antibodies in immune and inflammatory diseases. Curr. Opin. Biotechnol. 2002;13:615–620. doi: 10.1016/s0958-1669(02)00355-5. [DOI] [PubMed] [Google Scholar]
- Anhorn M.G., Wagner S., Kreuter J., Langer K., von Briesen H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albuminnanoparticles. Bioconjug. Chem. 2008;19(12):2321–2331. doi: 10.1021/bc8002452. [DOI] [PubMed] [Google Scholar]
- Arias J.L. Advanced methodologies to formulate nanotheragnostic agents for combined drug delivery and imaging. Expert Opin. Drug Deliv. 2011;8:1589–1608. doi: 10.1517/17425247.2012.634794. [DOI] [PubMed] [Google Scholar]
- Arruebo M., Valladares M., González-Fernández A. Antibody-conjugated nanoparticles for biomedical applications. J. Nanomater. 2009:1–24. [Google Scholar]
- Bae Y.H. Drug targeting and tumor heterogeneity. J. Control. Release. 2009;133(1):2–3. doi: 10.1016/j.jconrel.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bander N.H. Antibody–drug conjugate target selection: critical factors. In: Ducry L., editor. Antibody-Drug Conjugates. Humana Press; Switzerland: 2013. pp. 29–40. (Chapter 2) [DOI] [PubMed] [Google Scholar]
- Banerjee D., Harfouche R., Sengupta S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc. Cell. 2011;3(3):1–13. doi: 10.1186/2045-824X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M., Seggerson S., Henshaw J., Jiang J., Cordna R., Lefave C., Boyle P.J., Miller A., Pugia M., Basu S. Nano-biosensor development for bacterial detection during human kidney infection: use of glycoconjugate-specific antibody-bound gold nanowire arrays (GNWA) Glycoconj. J. 2004;21:487–496. doi: 10.1007/s10719-004-5539-1. [DOI] [PubMed] [Google Scholar]
- Bruchez M., Jr., Moronne M., Gin P., Weiss S., Alivisatos A.P. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Winter G., Waldmann H., Neuberger M.S. The immunogenicity of chimeric antibodies. J. Exp. Med. 1989;170:2153–2157. doi: 10.1084/jem.170.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Caskey H.M., Teale C., Waldmann H., Williams G.T., Surani M.A., Neuberger M.S. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc. Natl. Acad. Sci. USA. 1989;86(17):6709–6713. doi: 10.1073/pnas.86.17.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.C., Jin R., Mirkin C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 2002;297:1536–1540. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- Card J.W., Zeldin D.C., Bonner J.C., Nestmann E.R. Pulmonary applications and toxicity of engineered nanoparticles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295(3):L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.M., Rhee J.W., Drum C.L., Bronson R.T., Golomb G., Langer R., Farokhzad O.C. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipidpolymeric nanoparticles. Proc. Natl. Acad. Sci. USA. 2011;108:19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari R.V. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008;41(1):98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- Chen H., Gao J., Lu Y., Kou G., Zhang H., Fan L., Sun Z., Guo Y., Zhong Y. Preparation and characterization of PE38KDEL-loaded anti-HER2 nanoparticles for targeted cancer therapy. J. Control. Release. 2008;128(3):209–216. doi: 10.1016/j.jconrel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Chen J., Wu H., Han D., Xie C. Using anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancer. Cancer Lett. 2006;231:169–175. doi: 10.1016/j.canlet.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cordoba A., Shyong B., Breen D., Harris R. Non-enzymatic hinge region fragmentation of antibodies in solution. J Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2005;818(2):115–121. doi: 10.1016/j.jchromb.2004.12.033. [DOI] [PubMed] [Google Scholar]
- de la Escosura-Muñiz A., Merkoçi A. A nanochannel/nanoparticle-based filtering and sensing platform for direct detection of a cancer biomarker in blood. Small. 2011;7(5):675–682. doi: 10.1002/smll.201002349. [DOI] [PubMed] [Google Scholar]
- Dentener M.A., Creutzberg E.C., Pennings H.J., Rijkers G.T., Mercken E., Wouters E.F. Effect of infliximab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study. Respiration. 2008;76(3):275–282. doi: 10.1159/000117386. [DOI] [PubMed] [Google Scholar]
- Dhar S., Kolishetti N., Lippard S.J., Farokhzad O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhauser H.F., Rowe-Rendleman C.L., Robinson M.R., Dawson D.G., Chader G.J., Grossniklaus H.E., Rittenhouse K.D., Wilson C.G., Weber D.A., Kuppermann B.D., Csaky K.G., Olsen T.W., Kompella U.B., Holers M.V., Hageman G.S., Gilger B.C., Campochiaro P.A., Whitcup S.M., Wong W.T. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest. Ophthalmol. Vis. Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R., McKinstry M., Hwang J., Oppenheim A.B., Fekete R.A., Giulian G., Merril C., Nagashima K., Adhya S. High-sensitivity bacterial detection using biotin-tagged phage and quantum-dot nanocomplexes. Proc. Natl. Acad. Sci. USA. 2006;103:4841–4845. doi: 10.1073/pnas.0601211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad O.C., Cheng J., Teply B.A., Sherifi I., Jon S., Kantoff P.W., Richie J.P., Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Kou G., Wang H., Chen H., Li B., Lu Y., Zhang D., Wang S., Hou S., Qian W., Dai J., Zhao J., Zhong Y., Guo Y. PE38KDEL-loaded anti-HER2 nanoparticles inhibit breast tumor progression with reduced toxicity and immunogenicity. Breast Cancer Res. Treat. 2009;115(1):29–41. doi: 10.1007/s10549-008-0043-0. [DOI] [PubMed] [Google Scholar]
- Gao X., Cui Y., Levenson R.M., Chung L.W., Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Goetze A.M., Schenauer M.R., Flynn G.C. Assessing monoclonal antibody product quality attribute criticality through clinical studies. Mabs. 2010;2(5):500–507. doi: 10.4161/mabs.2.5.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradishar W.J., Tjulandin S., Davidson N., Shaw H., Desai N., Bhar P., Hawkins M., O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- Grifantini R., Bartolini E., Muzzi A., Draghi M., Frigimelica E., Berger J., Ratti G., Petracca R., Galli G., Agnusdei M., Giuliani M.M., Santini L., Brunelli B., Tettelin H., Rappuoli R., Randazzo F., Grandi G. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. Nat. Biotechnol. 2002;20(9):914–921. doi: 10.1038/nbt728. [DOI] [PubMed] [Google Scholar]
- Hamidreza Montazeri A., Shahin M., Brocks D.R., Lavasanifar A. Disposition of drugs in block copolymer micelle delivery systems: from discovery to recovery. Clin. Pharmacokinet. 2008;47:619–634. doi: 10.2165/00003088-200847100-00001. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H., Akita H., Ishida E., Hashimoto K., Kobayashi H., Aoki T., Yasuda J., Obata K., Kikuchi H., Ishida T., Kiwada H., Harashima H. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int. J. Pharm. 2007;342:194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Jain N.K., Tare M.S., Mishra V., Tripathi P.K. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed. Nanotech. Biol. Med. 2015;11:207–218. doi: 10.1016/j.nano.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Jaiswal J.K., Mattoussi H., Mauro J.M., Simon S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- Jones P.T., Dear P.H., Foote J., Neuberger M.S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kaittanis C., Nath S., Perez J.M. Rapid nanoparticle-mediated monitoring of bacterial metabolic activity and assessment of antimicrobial susceptibility in blood with magnetic relaxation. PLoS One. 2008;3(9):e3253. doi: 10.1371/journal.pone.0003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaittanis C., Santra S., Perez J.M. Role of nanoparticle valency in the nondestructive magnetic-relaxation-mediated detection and magnetic isolation of cells in complex media. J. Am. Chem. Soc. 2009;131(35):12780–12791. doi: 10.1021/ja9041077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra N., Nassar T., Ripin A.N., Schwob O., Borlak J., Benita S. Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: efficacy and biofate in a lung cancer mouse model. Small. 2013;9(24):4221–4236. doi: 10.1002/smll.201301417. [DOI] [PubMed] [Google Scholar]
- Kaur I.P., Kakkar S. Nanotherapy for posterior eye diseases. J. Control. Release. 2014;193:100–112. doi: 10.1016/j.jconrel.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Kedar U., Phutane P., Shidhaye S., Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kim S., Lim Y.T., Soltesz E.G., De Grand A.M., Lee J., Nakayama A., Parker J.A., Mihaljevic T., Laurence R.G., Dor D.M., Cohn L.H., Bawendi M.G., Frangioni J.V. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004;22(1):93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S., 2013. The best-selling drugs of all time: Humira joins the elite. Forbes. Available from: http://www.forbes.com/sites/simonking/2013/01/28/the-best-selling-drugs-of-all-time-humira-joins-the-elite/#21b9bed86193.
- Kitagawa T., Yuzawa M. Intravitreal pegaptanib sodium for myopic choroidal neovascularization: 1 year results of a prospective pilot study. Nihon. Ganka. Gakkai. Zasshi. 2013;117:344–350. [PubMed] [Google Scholar]
- Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kosky A.A., Dharmavaram V., Ratnaswamy G., Manning M.C. Multivariate analysis of the sequence dependence of asparagine deamidation rates in peptides. Pharm. Res. 2009;26(11):2417–2428. doi: 10.1007/s11095-009-9953-8. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo J.F., Candido K.A., Cao Z., Nigavekar S.S., Majoros I.J., Thomas T.P., Balogh L.P., Khan M.K., Baker J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- Kutlu C., Cakamak A.S., Gumusdereliglu M. Double-effective chitosan scaffold-PLGA nanoparticle system for brain tumour therapy: in vitro study. J. Microencapsul. 2014;31(7):700–707. doi: 10.3109/02652048.2014.913727. [DOI] [PubMed] [Google Scholar]
- Levine D.H., Ghoroghchian P.P., Freudenberg J., Zhang G., Therien M.J., Greene M.I., Hammer D.A., Murali R. Polymersomes: a new multi-functional tool for cancer diagnosis and therapy. Methods. 2008;46:25–32. doi: 10.1016/j.ymeth.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M., Carlesso N., Tung C.H., Tang X.W., Cory D., Scadden D.T., Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- Li L., Das A.M., Torphy T.J., Griswold D.E. What’s in the pipeline? Prospects for monoclonal antibodies (mAbs) as therapies for lung diseases. Pulm. Pharmacol. Ther. 2002;15(5):409–416. doi: 10.1006/pupt.2002.0371. [DOI] [PubMed] [Google Scholar]
- Li M., Hu B., Li J., Chen R., Zhang X., Chen H. Extractive electrospray ionization mass spectrometry toward in situ analysis without sample pretreatment. Anal. Chem. 2009;81(18):7724–7731. doi: 10.1021/ac901199w. [DOI] [PubMed] [Google Scholar]
- Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- Lidke D.S., Nagy P., Heintzmann R., Arndt-Jovin D.J., Post J.N., Grecco H.E., Jares-Erijman E.A., Jovin T.M. Quantum dot ligands provide new insights into erbB/HER receptor–mediated signal transduction. Nat. Biotechnol. 2004;22(2):198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- Look M., Bandyopadhyay A., Blum J.S., Fahmy T.M. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv. Drug Deliv. Rev. 2010;62(4–5):378–393. doi: 10.1016/j.addr.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhou N., Huang X., Cheng J.W., Li F.Q., Wei R.L., Cai J.P. Effect of intravitreal injection of bevacizumab-chitosan nanoparticles on retina of diabetic rats. Int. J. Ophthalmol. 2014;7(1):1–7. doi: 10.3980/j.issn.2222-3959.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Fang J., Inutsuka T., Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- Mahler D.A., Huang S., Tabrizi M., Bell G.M. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126(3):926–934. doi: 10.1378/chest.126.3.926. [DOI] [PubMed] [Google Scholar]
- Mamot C., Drummond D.C., Noble C.O., Kallab V., Guo Z., Hong K., Kirpotin D.B., Park J.W. Epider mal growth factor receptortargeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- Manjappa A.S., Chaudhari K.R., Venkataraju M.P., Dantuluri P., Nanda B., Sidda C., Sawant K.K., Murthy R.S. Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release. 2011 doi: 10.1016/j.jconrel.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Mansour H.M., Rhee Y.S., Wu X. Nanomedicine in pulmonary delivery. Int. J. Nanomedicine. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y. Polymeric micellar delivery systems in oncology. J. Clin. Oncol. 2008;38(12):793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]
- McCarron P.A., Marouf W.M., Quinn D.J., Fay F., Burden R.E., Olwill S.A., Scott C.J. Antibody targeting of camptothecin-loaded PLGA nanoparticles to tumor cells. Bioconjug. Chem. 2008;19:1561–1569. doi: 10.1021/bc800057g. [DOI] [PubMed] [Google Scholar]
- McCarthy J.R., Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008;60(11):1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J.R., Kelly K.A., Sun E.Y., Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomedicine. 2007;2:153–167. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- Mi Y., Zhao J., Feng S.S. Targeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. J. Control. Release. 2013;169(3):185–192. doi: 10.1016/j.jconrel.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Miyano T., Wijagkanalan W., Kawakami S., Yamashita F., Hashida M. Anionic amino acid dendrimer-trastuzumab conjugates for specific internalization in HER2-positive cancer cells. Mol. Pharm. 2010;7(4):1318–1327. doi: 10.1021/mp100105c. [DOI] [PubMed] [Google Scholar]
- Moore W.C., Bleecker E.R., Curran-Everett D., Erzurum S.C., Ameredes B.T., Bacharier L. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J., Patapoff T., Cromwell M. Kinetics and thermodynamics of dimer formation and dissociation for a recombinant humanized monoclonal antibody to vascular endothelial growth factor. Biochemistry. 1999;38(42):13960–13967. doi: 10.1021/bi9905516. [DOI] [PubMed] [Google Scholar]
- Neuberger T., Schöpf B., Hofmann H., Hofmann M., von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005;293(1):483–496. [Google Scholar]
- Nobs L., Buchegger F., Gurny R., Allemann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004;93(8):1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- Oghabian M.A., Farahbakhsh N.M. Potential use of nanoparticle based contrast agents in MRI: a molecular imaging perspective. J. Biomed. Nanotechnol. 2010;6(3):203–213. doi: 10.1166/jbn.2010.1119. [DOI] [PubMed] [Google Scholar]
- Owen S.C., Patel N., Logie J., Pan G., Persson H., Moffat J., Sidhu S.S., Shoichet M.S. Targeting HER2+ breast cancer cells: lysosomal accumulation of anti-HER2 antibodies is influenced by antibody binding site and conjugation to polymeric nanoparticles. J. Control. Release. 2013;172:395–404. doi: 10.1016/j.jconrel.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Park J.W., Hong K., Kirpotin D.B., Colbern G., Shalaby R., Baselga J., Shao Y., Nielsen U.B., Marks J.D., Moore D., Praetorius N.P., Mandal T.K. Engineered nanoparticles in cancer therapy. Recent Pat. Drug Deliv. Formul. 2007;1(1):37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- Praetorius N.P., Mandal T.K. Engineered nanoparticles in cancer therapy. Recent Pat. Drug Deliv. Formul. 2007;1(1):37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- Ramos-Tejada M.M., Viota J.L., Rudzka K., Delgado A.V. Preparation of multi-functionalized Fe3O4/Au nanoparticles for medical purposes. Colloids Surf. B Biointerfaces. 2015;1(128):1–7. doi: 10.1016/j.colsurfb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Reichert J.M. Anti-infective monoclonal antibodies: perils and promise of development. Nat. Rev. Drug Discov. 2006;5:191–195. doi: 10.1038/nrd1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S.I., Fogarty C., Kelsen S., Long W., Ramsdell J., Allison J., Mahler D., Saadeh C., Siler T., Snell P., Korenblat P., Smith W., Kaye M., Mandel M., Andrews C., Prabhu R., Donohue J.F., Watt R., Lo K.H., Schlenker-Herceg R., Barnathan E.S., Murray J. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175(9):926–934. doi: 10.1164/rccm.200607-995OC. 1. [DOI] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1998;332:323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Robinson N.E., Robinson A.B. Molecular clocks. Proc. Natl. Acad. Sci. USA. 2001;98(3):944–949. doi: 10.1073/pnas.98.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINA Study Group. Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Roth A., Drummond D.C., Conrad F., Hayes M.E., Kirpotin D.B., Benz C.C., Marks J.D., Liu B. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol. Cancer Ther. 2007;6(10):2737–2746. doi: 10.1158/1535-7163.MCT-07-0140. [DOI] [PubMed] [Google Scholar]
- Santra S., Zhang P., Wang K., Tapec R., Tan W. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal. Chem. 2001;73(20):4988–4993. doi: 10.1021/ac010406+. [DOI] [PubMed] [Google Scholar]
- Schroeder A., Heller D.A., Winslow M.M., Dahlman J.E., Pratt G.W., Langer R., Jacks T., Anderson D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer. 2012;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- Shi J., Votruba A.R., Farokhzad O.C., Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Xiao Z., Kamaly N., Farokhzad O.C. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc. Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- Siddiqui M., Rajkumar S.C. The high costs of cancer drugs and what we can do about it. Mayo Clin. Proc. 2012;87(10):935–943. doi: 10.1016/j.mayocp.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D., Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol. 2001;28:13–19. doi: 10.1016/s0093-7754(01)90188-5. [DOI] [PubMed] [Google Scholar]
- Smola M., Vandamme T., Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int. J. Nanomedicine. 2008;3(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Sokolov K., Follen M., Aaron J., Pavlova I., Malpica A., Lotan R., Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epider mal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- Srinivasan A.R., Lakshmikuttyamma A., Shoyele S.A. Investigation of stability and cellular uptake of self associated monoclonal antibody (MAb) nanoparticles by non-small lung cancer cells. Mol. Pharm. 2013;10(9):3275–3284. doi: 10.1021/mp3005935. 3. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Marchant R.E., Gupta A.S. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J. Biomed. Mater. Res. 2009;93:1004–1015. doi: 10.1002/jbm.a.32549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977;269:420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Sun B., Ranganathan B., Feng S.S. Multifunctional poly (D,L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29(4):475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Sutradhar K.B., Amin Md.L. Nanotechnology in cancer drug delivery and selective targeting review. ISRN Nanotechnol. 2014:1–12. [Google Scholar]
- Svenson S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009;71:445–462. doi: 10.1016/j.ejpb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Tan M., Wang G., Hai X., Ye Z., Yuan J. Development of functionalized fluorescent europium nanoparticles for biolabeling and time-resolved fluorometric applications. J. Mater. Chem. 2004;14(19):2896–2901. [Google Scholar]
- Tholouli E., Hoyland J.A., Di Vizio D., O’Connell F., Macdermott S.A., Twomey D., Levenson R., Yin J.A., Golub T.R., Loda M., Byers R. Imaging of multiple mRNA targets using quantum dot based in situ hybridization and spectral deconvolution in clinical biopsies. Biochem. Biophys. Res. Commun. 2006;348(2):628–636. doi: 10.1016/j.bbrc.2006.07.122. [DOI] [PubMed] [Google Scholar]
- Thomas T.P., Goonewardena S.N., Majoros I.J., Kotlyar A., Cao Z., Leroueil P.R., Baker J.R. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully E., Hearty S., Leonard P., O’Kennedy R. The development of rapid fluorescence-based immunoassays, using quantum dot-labelled antibodies for the detection of Listeria monocytogenes cell surface proteins. Int. J. Biol. Macromol. 2006;39(1–3):127–134. doi: 10.1016/j.ijbiomac.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Van der Vaart H., Koëter G.H., Postma D.S., Kauffman H.F., ten Hacken N.H. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;172(4):465–469. doi: 10.1164/rccm.200501-147OC. 15. [DOI] [PubMed] [Google Scholar]
- Varshney M., Yang L., Su X.L., Li Y. Magnetic nanoparticle-antibody conjugates for the separation of Escherichia coli O157:H7 in ground beef. J. Food Prot. 2005;68(9):1804–1811. doi: 10.4315/0362-028x-68.9.1804. [DOI] [PubMed] [Google Scholar]
- Varshochian R., Jeddi-Tehrani M., Khoshayand M.R., Dinarvand R. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur. J. Pharm. Sci. 2013;50(3-4):341–352. doi: 10.1016/j.ejps.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Varshochian R., Riazi-Esfahani M., Jeddi-Tehrani M., Mahmoudi A.R., Aghazadeh S., Mahbod M., Movassat M., Atyabi F., Sabzevari A., Dinarvand R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. A. 2015;103(10):3148–3156. doi: 10.1002/jbm.a.35446. [DOI] [PubMed] [Google Scholar]
- Wakankar A.A., Feeney M.B., Rivera J., Chen Y., Kim M., Sharma V.K., Wang Y.J. Physicochemical stability of the antibody–drug conjugate Trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjug. Chem. 2010;21(9):1588–1595. doi: 10.1021/bc900434c. [DOI] [PubMed] [Google Scholar]
- Wang W., Singh S., Zeng D.L., King K., Nema S. Antibody structure, instability, and formulation. J. Pharm. Sci. 2006;96(1):1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- Wang J., Tian S., Petros R.A., Napier M.E., Desimone J.M. The complex role of multivalency in nanoparticles targeting the transfer rin receptor for cancer therapies. J. Am. Chem. Soc. 2010;132:11306–11313. doi: 10.1021/ja1043177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R. Molecular imaging in cancer. Science. 2006;31(5777):1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- Winter G., Griffiths A.D., Hawkins R.E., Hoogenboom H.R. Making antibodies by phage display technology. Annu. Rev. Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2008. World Health Statistics.
- Wu X., Liu H., Liu J., Haley K.N., Treadway J.A., Larson J.P., Ge N., Peale F., Bruchez M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003;21(1):41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Ichinose M. Agents against cytokine synthesis or receptors. Eur. J. Pharmacol. 2006;533(1–3):289–301. doi: 10.1016/j.ejphar.2005.12.046. 8. [DOI] [PubMed] [Google Scholar]
- Yang E., Qian W., Cao Z., Wang L., Bozeman E.N., Ward C., Yang B., Selvaraj P., Lipowska M., Wang Y.A., Mao H., Yang L. Theranostic nanoparticles carrying doxorubicin attenuate targeting ligand specific antibody responses following systemic delivery. Theranostics. 2015;5(1):43–61. doi: 10.7150/thno.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. Drug targeting with nano-sized carrier systems. J. Artif. Organs. 2005;8(2):77–84. doi: 10.1007/s10047-005-0285-0. [DOI] [PubMed] [Google Scholar]
- Yousefpour P., Atyabi F., Vasheghani-Farahani E., Movahedi A.A., Dinarvand R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011;6:1977–1990. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Hilliard L.R., Mechery S.J., Wang Y., Bagwe R.P., Jin S., Tan W. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc. Natl. Acad. Sci. USA. 2004;101(42):15027–15032. doi: 10.1073/pnas.0404806101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Mi Y., Feng S.S. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immuno-micelles. Biomaterials. 2013;34(13):3411–3421. doi: 10.1016/j.biomaterials.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Zhao J., Mi Y., Liu Y., Feng S.S. Quantitative control of targeting effect of anticancer drugs formulated by ligand-conjugated nanoparticles of biodegradable copolymer blend. Biomaterials. 2012;33(6):1948–1958. doi: 10.1016/j.biomaterials.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Zharov V.P., Mercer K.E., Galitovskaya E.N., Smeltzer M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006;90(2):619–627. doi: 10.1529/biophysj.105.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Wu Y.L., Chen G., Liu X., Zhu Y., Lu S., Feng J., He J., Han B., Wang J., Jiang G., Hu C., Zhang H., Cheng G., Song X., Lu Y., Pan H., Zheng W., Yin A.Y. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2015;33(19):2197–2204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
Further Reading
- Daugherty A.L., Mrsny R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006;58(5-6):686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kirpotin D., Park J.W., Hong K., Zalipsky S., Li W.L., Carter P., Benz C.C., Papahadjopoulos D. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36(1):66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Benz C.C. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin. Cancer Res. 2002;8(4):1172–1181. [PubMed] [Google Scholar]


