INTRODUCTION
Humans and microbes have engaged in an epic struggle for survival since human life began. Rapid microbial evolution and adaptation allow bacteria, viruses and parasites to overcome human defenses (e.g. physiologic mechanisms and manmade drugs) and exploit human behaviors (e.g. sexual practices and methods of food production and preparation). Some zoonotic microbes have ‘jumped’ from animals to humans to become major human pathogens. The ancestors of smallpox virus and malaria parasites, for example, probably became human pathogens 10 000 years ago when humans began to domesticate animals in settlements that were large enough to sustain human-to-human transmission. In recent times the AIDS virus, one of the most destructive pathogens in human history, evolved from a virus carried by a nonhuman primate.1
Human evolution, though far slower and more difficult to observe, has in turn been influenced by microbes. Some pathogens have apparently been sufficiently virulent and widespread over the course of human history to affect the make-up of the human genome. The best evidence for this concerns malaria parasites, which are less likely to kill individuals who have globin gene alleles that make their red blood cells poor hosts for intracellular parasites (e.g. sickle hemoglobin and α- and β-thalassemias).2 Moreover, most or all components of the human immune system probably evolved to prevent disease agents from co-opting human cellular machinery for microbial gene expression and reproduction. In addition, the human body benefits from the presence of nonpathogenic, symbiotic microbes, such as bacteria in the human gut that synthesize components of the vitamin B complex (folic acid and biotin).
Today, the increasing complexity of human behavior coupled with our ability to change our natural environment has hastened the pace of disease emergence and re-emergence (Fig. 4.1 ). Many modern human activities facilitate microbial transmission (e.g. air travel and the globalization of the food supply; see Table 4.1 ),3–5 while others have decreased it (e.g. improvements in sanitation, antimicrobial drugs, vaccines and disease control and eradication programs). Looking ahead, the most significant risk factor for disease emergence in the 21st century is likely to be population growth and urbanization, leading to the creation of megacities (a city with 10 million inhabitants)6 throughout the developing world. According to a recent United Nations report (http://www.unfpa.org/swp/2007/english/introduction.html), more than half the world's population will be living in urban areas by the end of 2008, and by 2025 there are likely to be eight new megacities, most of them in the developing world, bringing the total to 28. By 2050, 6.4 billion people will be living in urban areas, out of a total projected population of 9.2 billion. These population increases will be associated with further stresses on resources (e.g. clean air, water and food) and natural habitats (e.g. higher temperatures caused by global warming that allow disease-carrying mosquitoes to move into new areas). Moreover, as human cities expand into forested areas, increased contact between humans and wildlife may lead to human infection with previously unknown animal pathogens that can cause human disease. Intensified livestock production – both traditional and modern – to meet increasing demand may also facilitate the spread of new and re-emerging zoonotic diseases (http://www.ifpri.org/2020/dp/dp28.pdf).
Fig. 4.1.

Interactions among humans, disease vectors and the environment that contribute to disease emergence.
Source: Institute of Medicine. Emerging infections: microbial threats to health in the United States. Washington, DC: National Academy Press; 1992.3 (In March 2003, the Institute of Medicine published a reassessment: Microbial threats to health: emergence, detection, and response. Washington DC: National Academy Press; 2003.4)
Table 4.1.
Factors in the emergence of infectious diseases
Modern demographic, environmental, and behavioral factors that favor the spread of infectious diseases include:
|
New and re-emerging infectious diseases
Over the past 40 years, more than 40 new human pathogens have been identified and characterized, often by using serologic and molecular methods (Table 4.2 ). Some of these microbes cause diseases of global importance (e.g. HIV/AIDS, hepatitis C, rotavirus diarrheal disease; and the (H1N1) 2009 influenza pandemic). Others appear to be limited (thus far) to particular countries or continents (e.g. Ebola hemorrhagic fever in central Africa (although simian Ebola has also been identified in the Philippines); Argentinian, Bolivian, Venezuelan and Sabia-associated hemorrhagic fevers in South America; hantavirus pulmonary syndrome in the Americas; and new variant Creutzfeldt–Jakob disease in Europe, Japan, Canada, and Saudi Arabia). A recent example is the 2003 outbreak of severe acute respiratory syndrome (SARS), caused by a novel coronavirus, which spread overnight from Hong Kong to Canada and several other countries by airplane (Fig. 4.2 ; see also below). In addition to these newly identified diseases, several ‘old’ threats have re-emerged in new or drug-resistant forms (Table 4.3 ). These include highly virulent epidemic strains of Mycobacterium tuberculosis (see below) and Clostridium difficile.10, 11
Table 4.2.
Newly identified microbial pathogens
| Agent | Type | Disease | |
|---|---|---|---|
| 1972 | Calicivirus (Norwalk agent) | Virus | Acute gastroenteritis |
| 1973 | Rotavirus | Virus | Major cause of infantile diarrhea worldwide |
| 1975 | Parvovirus B19 | Virus | Aplastic crisis in chronic hemolytic anemia |
| 1976 | Cryptosporidiumparvum | Parasite | Acute and chronic diarrhea |
| 1977 | Ebola virus | Virus | Ebola hemorrhagic fever |
| 1977 | Hantaan virus | Virus | Hemorrhagic fever with renal syndrome (HFRS) |
| 1977 | Legionella pneumophila | Bacterium | Legionnaires’ disease |
| 1977 | Campylobacterjejuni | Bacterium | Enteric pathogens distributed globally |
| 1980 | Human T-lymphotropic virus type 1 (HTLV-1) | Virus | T-cell lymphoma-leukemia |
| 1981 | Toxin producing strains of Staphylococcus aureus | Bacterium | Toxic shock syndrome |
| 1982 | Escherichia coli O157:H7 | Bacterium | Hemorrhagic colitis; hemolytic uremic syndrome |
| 1982 | Human T-lymphotropic virus type 2 (HTLV-2) | Virus | Hairy cell leukemia |
| 1982 | Borrelia burgdorferi | Bacterium | Lyme disease |
| 1983 | Human immunodeficiency virus (HIV) | Virus | Acquired immunodeficiency syndrome (AIDS) |
| 1985 | Helicobacter pylori | Bacterium | Peptic ulcer disease |
| 1985 | Enterocytozoon bieneusi | Parasite | Persistent diarrhea |
| 1986 | Cyclospora cayetanensis | Parasite | Persistent diarrhea |
| 1988 | Human herpesvirus 6 (HHV-6) | Virus | Exanthema subitum |
| 1988 | Hepatitis E | Virus | Enterically transmitted non-A, non-B hepatitis |
| 1989 | Ehrlichia chafeensis | Bacterium | Human ehrlichiosis |
| 1989 | Hepatitis C | Virus | Parenterally transmitted non-A, non-B hepatitis |
| 1991 | Guanarito virus | Virus | Venezuelan hemorrhagic fever |
| 1991 | Encephalitozoon hellem | Parasite | Conjunctivitis, disseminated disease |
| 1991 | New species of Babesia | Parasite | Atypical babesiosis |
| 1992 | Bartonella henselae | Bacterium | Cat-scratch disease; bacillary angiomatosis |
| 1992 | Tropheryma whipplei | Bacterium | Impaired absorption of nutrients, weight loss, joint pain, and anemia |
| 1992 | Vibrio cholerae O139 | Bacterium | Cholera with wide-spectrum drug resistance |
| 1993 | Sin Nombre virus | Virus | Hantavirus pulmonary syndrome (HPS) |
| 1993 | Encephalitozoon cuniculi | Parasite | Disseminated disease |
| 1994 | Sabia virus | Virus | Brazilian hemorrhagic fever |
| 1994 | Henipaviruses (Hendra) | Virus | Encephalitic disease carried by fruit bats that can be transmitted from horses to humans (Hendra) |
| 1995 | Human herpesvirus 8 (HHV-8) | Virus | Associated with Kaposi's sarcoma in AIDS patients |
| 1996 | New variant Creutzfeldt–Jakob disease agent | Prion | Progressive degenerative neurologic disease |
| 1997 | H5N1 strain of avian influenza | Virus | Influenza transmitted from chickens to humans; often fatal |
| 1999 | Henipaviruses (Nipah) | Virus | Encephalitic disease carried by fruit bats that can be transmitted from pigs to humans |
| 2001 | Human metapneumovirus | Virus | Acute respiratory infections |
| 2002 | Vancomycin-resistant Staphylococcus aureus | Bacterium | First vancomycin-resistant S. aureus strain identified in the USA |
| 2003 | SARS coronavirus | Virus | Severe acute respiratory syndrome (SARS) |
| 2003 | Clostridium difficile, strain NAPI/027 | Bacteria | Pseudomembranous colitis |
| 2005 | Bocavirus | Virus | Associated with lower respiratory tract infections in children; discovered through molecular screening of respiratory tract samples |
| 2007 | New strain of Ebola (the fifth one identified) | Virus | Hemorrhagic fever |
| 2008 | Transplant-associated arenavirus related to lymphocytic choriomeningitis virus | Virus | Severe febrile illness |
| 2008 | Plasmodium knowlesi as a human pathogen | Parasite | Previously classified as a cause of simian malaria, this parasite is now known to be a cause of human malaria in Malaysia, sometimes misdiagnosed as Plasmodium malariae, a milder form of malaria |
| 2009 | Pandemic (H1N1) 2009 virus | Virus | Pandemic influenza (see Chapter 161) |
Updated from World Health Organization.7
Fig. 4.2.

Chain of transmission among guests at Hotel M – Hong Kong, 2003.
Adapted from Centers for Disease Control and Prevention.8
Table 4.3.
Resurging diseases
| Disease or agent | Factors in re-emergence |
|---|---|
| Viral | |
| Rabies | Breakdown in public health measures; changes in land use; travel |
| Dengue and dengue hemorrhagic fever | Transportation; travel and migration; urbanization. Many countries reported high numbers of dengue infections in 2007; this trend continued in 2008, with a large outbreak of 120 570 cases reported in Brazil, including 75 399 cases in Rio de Janeiro |
| Yellow fever | Favorable conditions for growth of the mosquito vector. In June 2008, Paraguay reported the first identified Yellow fever cases in more than 30 years, some of them near the capital city, Asuncion. The apparent re-emergence of urban Yellow fever, which can spread rapidly through susceptible populations, has raised public health concerns in other countries in the region |
| Rift Valley fever | Infected humans, animals or mosquitoes that have traveled across East Africa to Saudi Arabia and Yemen |
| Polio | Cessation in vaccination campaigns or decline in average vaccine coverage |
| Chikungunya fever | A strain of chikungunya virus isolated during a 2005–2006 outbreak on Reunion Island (an island in the Pacific Ocean) has a mutation1,2 that may facilitate transmission by Aedes albopictus (the Tiger mosquito), which is native to South East Asia, but is now present on all continents except Antarctica. Disease spread via travelers who visited chikungunya-affected areas in India, Mauritius or other islands in the Pacific Ocean apparently led to the first cases of local transmission in Italy (in 2007) and in Australia, Singapore and Malaysia (in 2008) |
| Parasitic | |
| Malaria | Drug and insecticide resistance; civil strife; lack of economic resources |
| Schistosomiasis | Dam construction, improved irrigation and ecologic changes favorable to the snail host |
| Neurocysticercosis | Immigration; agricultural practices |
| Acanthamebiasis | Introduction of soft contact lenses |
| Visceral leishmaniasis | War; population displacement; immigrations; habitat changes favorable to insect vector and increase in immunocompromised human hosts |
| Toxoplasmosis | Increase in immunocompromised human hosts |
| Giardiasis | Increased use of child-care facilities |
| Echinococcosis | Ecologic changes that affect the habitats of the immediate (animal) hosts |
| Trypanosomiasis (African sleeping sickness) | Breakdown in public health infrastructure |
| Bacterial | |
| Tuberculosis | Human demographics and behavior; international commerce and travel; breakdown of public health measures; microbial adaptation (i.e. multidrug-resistant tuberculosis [MDR-TB] and extensively drug-resistant tuberculosis [XDR-TB]); the HIV/AIDS pandemic |
| Trench fever | Breakdown of public health measures |
| Plague | Economic development; land use |
| Diphtheria | Interruption of immunization programs due to political changes |
| Pertussis | Refusal to vaccinate in some countries because of belief that pertussis vaccines are not safe |
| Salmonella | Industry and technology; human demographics and behavior; microbial adaptation; changes in food production |
| Pneumococcus | Human demographics; microbial adaptation; international travel and commerce; misuse and overuse of antibiotics |
| Cholera | International travel; long-term increases in sea surface temperatures and sea levels that may lead to higher concentrations of Vibrio cholerae, which grow on zooplankton that flourish in warm water |
Martin E. Epidemiology: tropical disease follows mosquitoes to Europe. Science 2007; 317(5844):1485.
Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 2007;3(12):e201.
Adapted and updated from Lorber.9
Selected examples of major outbreaks since 1993 are listed in Table 4.4 .
Table 4.4.
Selected infectious disease challenges, 1993–2008
| 1993 | Hantavirus pulmonary syndrome (USA) |
| 1994 | Plague (India) |
| 1995 | Ebola fever (Zaire) |
| 1996 | New variant Creutzfeldt–Jakob disease (UK) |
| 1997 | H5N1 influenza (Hong Kong); vancomycin-intermediate resistant Staphylococcus aureus (Japan, USA) |
| 1998 | Nipah virus encephalitis (Malaysia, Singapore) |
| 1999 | West Nile encephalitis (Russia, USA) |
| 2000 | Rift Valley fever (Kenya, Saudi Arabia, Yemen); Ebola fever (Uganda) |
| 2001 | Anthrax (USA); foot and mouth disease (UK) |
| 2002 | Vancomycin-resistant Staphylococcus aureus (USA) |
| 2003 | Severe acute respiratory syndrome (SARS) |
| 2003 | Re-emergence of H5N1 influenza (beginning in South East Asia and spreading internationally) |
| 2003 | Monkeypox (USA) |
| 2003 | Highly virulent strain of Clostridium difficile (Canada, the Netherlands, UK, USA) |
| 2005–2006 | Multistate outbreak of mumps in the USA, involving 11 states (The widespread use of a second dose of mumps vaccine among US schoolchildren beginning in 1990 was followed by historically low reports of mumps cases, and it was hoped that the disease might be eliminated by 2010. However, a multistate outbreak of mumps – the largest US outbreak in two decades – occurred in 2006) |
| 2004 | Chikungunya fever outbreak associated with high fever and severe protracted joint pain in Lamu Island, Kenya |
| 2007 | New strain of Ebola (the fifth one identified), responsible for large outbreaks in Uganda and the Congo |
| 2008 | Measles outbreaks in Europe (Switzerland, Austria, Ireland and Britain), Israel and the USA (San Diego, California), associated with declining rates of measles vaccination |
| 2008 | Outbreak of Zika fever (a mosquito-borne illness similar to dengue) in the Yap Islands, Federated States of Micronesia |
| 2009 | Pandemic (H1N1) influenza. See Chapter 161. |
The essential role of the clinician in disease detection and surveillance
Physicians are in the best possible position to observe and report unusual illnesses, syndromes and disease risk factors. In 1993, for example, an Indian Health Service physician reported a cluster of fatal cases of unexplained respiratory disease in the southwestern United States that proved to be caused by a previously unrecognized hantavirus. In 1999, the first US outbreak of West Nile encephalitis was identified when an infectious disease physician reported unusual neurologic disease in three elderly people who lived in the same area (Fig. 4.3 ).12 West Nile encephalitis – whose causative agent is carried by migratory birds in Africa, the Middle East, and Europe and transmitted to humans by mosquito bite – had never before been reported in the Western hemisphere.13 In 2000, physicians in New York, London and Toronto helped identify an international outbreak of leptospirosis among athletes returning home from a competition in Malaysian Borneo. These physicians reported their findings to Geosentinel, the surveillance network of the International Society of Travel Medicine (http://www.istm.org/geosentinel/main.html), which alerted travel clinics in 11 countries. An astute clinician was also responsible for reporting the first cases of a multistate outbreak of Cyclospora infection that was associated with imported raspberries.14 Later cases were detected in 20 states, the District of Columbia and two Canadian provinces.
Fig. 4.3.

Spread of West Nile virus in the United States.In the 8 years since West Nile virus was first reported in the USA (in New York City), human infections have been reported in 47 states. West Nile activity from January 1, 2007, to February 5, 2008, is indicated above.
Source: Centers for Disease Control and Prevention. Adapted from: http://www.cdc.gov/ncidod/dvbid/westnile/Mapsactivity/surv&control107Maps.htm
The US anthrax attacks in autumn 2001 also highlighted the crucial role of clinicians in monitoring unusual and dangerous diseases. The first case of anthrax (an inhalational case) was recognized in a hospitalized patient by an infectious disease physician in Palm Beach County, Florida, and six of eight anthrax cases detected in New York City (one inhalational and seven cutaneous) were reported to city health authorities by alert physicians. Three were diagnosed by infectious disease physicians (including the inhalational case), one by a dermatologist, one by a public health physician and one by an emergency room physician. Physicians also reported anthrax cases in Washington DC and New Jersey.
As these examples illustrate, the vigilance of physicians and other health-care providers remains the most important factor in infectious disease surveillance and control and in recognition of new and emerging infectious diseases.
Linkages between animal and human disease surveillance
Between 60% and 75% of all emerging and many re-emerging infectious diseases of human health importance are zoonotic.15 The intimate relationship between animal and human health is illustrated by the emergence of the pandemic (H1N1) 2009 influenza virus, which includes gene segments from humans, birds, and pigs. Other examples include the 1999 outbreak of West Nile encephalitis, which was preceded by die-offs of crows; the 1999 Nipah encephalitis outbreak, which was preceded by illness in pigs; and the 1996 outbreak of new variant Creutzfeldt–Jakob disease, which was preceded by the recognition of an epidemic of bovine spongiform encephalopathy (BSE) among cows.
Formal mechanisms have been developed to link public health disease monitoring with surveillance for diseases in agricultural animals, migrating birds and wild animals. They include the US-government-sponsored Global Avian Influenza Network for Surveillance (http://www.gains.org), which was established in 2006 in co-operation with the Wildlife Conservation Society (WCS) to monitor high pathogenicity avian influenza (HPAI) in wild birds. Within the USA, ArboNet tracks nationally notifiable arboviral diseases (including West Nile fever; see Fig. 4.3), and the National Antimicrobial Resistance Monitoring System (NARMS; http://www.cdc.gov/narms) continues to monitor the emergence of antimicrobial resistance in zoonotic bacteria (e.g. Salmonella and Campylobacter) that infect livestock. In addition, the federal, state and local public health laboratories are also linked with agricultural, wildlife and military reference laboratories through the Laboratory Response Network (LRN), which was founded in 1998 by the Centers for Disease Control and Prevention (CDC) in partnership with the Association of Public Health Laboratories and the Federal Bureau of Investigation (FBI).
In addition to protecting human health, rapid detection and control of animal diseases will benefit agriculture, help preserve wildlife species and avert economic losses due to expensive disease control measures (Box 4.1 ). The special challenges of understanding the ecologic factors that favor the emergence and spread of zoonotic diseases are discussed below.
Box 4.1. Agricultural costs of controlling diseases carried by food animals.
When a dangerous animal-borne disease threatens human health or food safety, a government may be forced to slaughter large numbers of food animals as a control measure, despite considerable economic costs. Recent examples include two zoonotic diseases (Nipah encephalitis and H5N1 influenza) and two veterinary diseases (foot and mouth disease and bovine spongiform encephalopathy (BSE; also called mad cow disease)). Ingestion of beef containing the causative agent of BSE (a prion) may result in the development of a fatal human neurodegenerative illness (new variant Creutzfeldt–Jakob disease) many years later.
Between 1997 and 2007, the culling of animals to control outbreaks of Nipah encephalitis, avian influenza, foot and mouth disease, and BSE cost the agricultural sector billions of dollars.
-
•
Nipah encephalitis. In 1999 Malaysian health authorities were faced with an outbreak of encephalitis among farm workers, which had a nearly 50% mortality rate. The cause was a previously unknown paramyxovirus called the Nipah virus, which is carried by bats. To control the outbreak, approximately 1.1 million pigs were culled within a few weeks, severely harming the Malaysian meat industry.
-
•
Avian influenza (strain H5N1). In 1997, a similar precautionary measure was taken by the government of Hong Kong, which arranged the culling of all 1.6 million chickens on Hong Kong Island and the New Territories to prevent chicken-to-human transmission of a virulent avian form of influenza.
The 2003 re-emergence of avian influenza A[H5N1] in South East Asia – and its consequent spread to Central Asia, Africa, Europe and the Middle East – has also had a significant economic impact. In South East Asia, for example, bird deaths due to disease and to bird cullings have caused severe losses to farmers in Vietnam and Thailand (whose poultry stocks declined by 15–20%), as well as to farmers in Indonesia, China, Cambodia and Laos. According to the World Bank, economic impacts in these countries have also affected poultry traders, feed mills and breeding farms (http://web.worldbank.org/WBSITE/EXTERNAL/COUNTRIES/EASTASIAPACIFICEXT/EXTEAPHALFYEARLYUPDATE/0,contentMDK:20708543∼menuPK:550232∼page PK:64168445∼piPK:64168309∼theSitePK:550226,00.html).
-
•
Bovine spongiform encephalopathy. In 2006, the European Union lifted a 10-year ban on the export of live cattle and cattle products (other than milk) from the UK. Control measures, including the slaughter of affected cows, cost the UK government an estimated £3.5 billion sterling (about US$5 billion). The export ban cost the UK agricultural sector about £675 million a year.
-
•
Foot and mouth disease. The costs of controlling an outbreak of foot and mouth disease in the UK and continental Europe in 2001 – estimated at £8.5 billion – dwarfed those of the BSE outbreak and devastated the centuries-old British livestock industry. Foot and mouth disease does not infect humans but can be spread by travelers who have contaminated soil on their shoes or clothing or who carry contaminated food products. The St Patrick's Day parade in Ireland was cancelled due to concerns about spreading the virus, and the British army was mobilized to help bury the carcasses of 7 million animals slaughtered because of potential exposure to the disease.
In August 2007, the European Union imposed a temporary ban on export of livestock, meat and milk from the UK, and the UK halted the internal movement of farm animals nationwide, to minimize the risk of spreading foot and mouth disease after cases were detected in Surrey. The outbreak, which affected about eight farms, was traced to an accidental release of an experimental vaccine strain from a high-security animal laboratory in rural England.
Molecular diagnostics and outbreak detection
To ensure optimal medical care and disease control strategies, clinical diagnosis of unusual diseases must be confirmed by laboratory tests. Today's advances in microbial genomics, molecular immunology and bioinformatics are facilitating the development of accurate, rapid and sensitive diagnostics based on detection of microbe-specific or strain-specific nucleic acids or proteins. Molecular testing that involves amplification of genetic sequences using the polymerase chain reaction (PCR) technique is especially useful when unidentified pathogens are difficult to grow in the laboratory, making other types of testing difficult or impossible. In the future, advances in human genomics may also allow the identification of individuals with increased genetic susceptibility to certain diseases or to severe manifestations of those diseases (Table 4.5 ).16, 17
Table 4.5.
Examples of genetic factors that influence susceptibility to disease or disease progression
|
Reiche EMV, Bonametti AM, Voltarelli JC, Morimoto HK, Watanabe MAE. Genetic polymorphisms in the chemokine and chemokine receptors: impact on clinical course and therapy of the human immunodeficiency virus type 1 infection (HIV-1). Curr Med Chem 2008;14(12):1325–34.
Weatherall DJ. Thalassaemia and malaria, revisited. Ann Trop Med Parasitol 1997;91(7):885–90.
Langhi DM Jr, Bordin JO. Duffy blood group and malaria. Hematology 2006;11(5):389–98.
Roth DE, Soto G, Arenas F, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis 2004;190(5):920–7.
Harris JB, Khan AI, LaRocque RC, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun 2005;73(11):7422–7.
Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 1994;266(5182):107–9.
Nikolich-Zugich J, Fremont DH, Miley MJ, Messaoudi I. The role of mhc polymorphism in anti-microbial resistance. Microbes Infect 2004;6(5):501–12.
Mira MT, Alcaïs A, Nguyen VT, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 2004;427(6975):636–40.
Riemenschneider M, Klopp N, Xiang W, et al. Prion protein codon 129 polymorphism and risk of Alzheimer disease. Neurology 2004;63(2):364–6.
Oriá RB, Patrick PD, Blackman JA, Lima AA, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses 2007;68(5):1099–107.
Molecular testing has the potential not only to confirm clinical diagnoses but also to facilitate the detection of outbreaks. This function may be especially important when individual cases are not clustered geographically or by other easily identifiable risk factors. Examples include:
-
•
food-borne pathogens transmitted simultaneously to many localities when a contaminated product is shipped to supermarkets or restaurants in several states or countries;
-
•
disease agents dispersed by international travelers, such as the 2000 leptospirosis outbreak in Malaysian Borneo and the 2003 outbreak of SARS; and
-
•
an unannounced and undetected (covert) release of a bioterrorist agent.
Public health tools are already in place in Europe and the USA to identify and trace the source of geographically dispersed food-borne outbreaks by comparing molecular fingerprinting data from clinical and public health laboratories. This is the operating principle behind PulseNet,18 a molecular subtyping network that has used the pulse-field gel electrophoresis (PFGE) technique to detect several major multistate food-borne outbreaks. For example, PulseNet was used to detect an outbreak of Escherichia coli O157:H7 transmitted by fresh spinach sold in 26 states (in 2006) and an outbreak of Salmonella St Paul associated with jalapeno peppers sold in more than 40 states (in 2008). This technology is in use in many countries all over the world (http://www.pulsenetinternational.org). In the future, advances in genome sequencing and bioinformatics may lead to the development of increasingly sophisticated typing methods that will facilitate discovery of novel disease risk factors for hundreds of pathogens and help health authorities identify linkages among geographically dispersed cases of disease. For example, the CDC has proposed the development of an international database called Microbe. Net that could potentially match a pathogen isolated from an ill person in one country with an environmental microbe isolated in another.
Being prepared for the unexpected
Infectious pathogens are extraordinarily resilient and have a remarkable ability to evolve, adapt and develop resistance to drugs in an unpredictable and dynamic fashion. Because we do not know what new diseases will arise, we must always be prepared for the unexpected (see Table 4.4). In 2002, the first vancomycin-resistant Staphylococcus aureus strain was identified in the USA.19 In 2003, SARS emerged from an animal reservoir in Guangdong province in China and quickly spread via travelers through South-East Asia and then to Toronto, Canada. Later in the same year, more than 30 people in the midwestern United States contracted monkeypox – a disease that resembles a mild case of smallpox – from rodents imported as pets that carried an orthopox virus known to infect people who live in remote villages near tropical rainforests in Central and Western Africa (http://www.cdc.gov/MMWR/preview/mmwrhtml/mm5226a5.htm). In 2009, a new strain of pandemic influenza – the pandemic (H1N1) 2009 virus – emerged in North America and spread rapidly around the world (see later).
The unexpected may include intentional as well as naturally occurring outbreaks. In 2001, the USA experienced a multistate outbreak of anthrax that necessitated antibiotic prophylaxis for more than 30 000 people. Rapid medical, public health and law enforcement action limited the outbreak to 11 inhalational cases, 11 cutaneous cases and five deaths.
These examples of unforeseen outbreaks underscore the need to maintain a strong public health system that is supported by a well-informed and vigilant medical community.
Shifts in perspectives on infectious disease in the past 50 years
Over the past 50 years, there have been significant shifts in how infectious diseases are viewed by the medical and scientific world and by the public. In the years following the Second World War, it was widely believed that humans were winning the war against infectious microbes. Vaccines and antibiotics, coupled with earlier improvements in sanitation and water quality, had dramatically lowered the incidence of infectious diseases. Therefore, it became possible to imagine a world in which infectious pathogens would no longer prey upon humanity. In 1962, the Australian Nobel prize winner Frank Macfarlane Burnet stated:20
One can think of the middle of the twentieth century as the end of one of the most important social revolutions in history, the virtual elimination of the infectious disease as an important factor in social life.
Five years later, Surgeon General William H Stewart expressed the views of many US doctors and health experts when he stated that it was time to ‘close the book on infectious illnesses’, as long as we continue to prevent disease through vaccination’.21 He encouraged the public health community to turn its attention to chronic diseases. Over the following years, biomedical research in the USA became increasingly focused on heart disease, stroke and the ‘war on cancer’, which was declared by President Richard Nixon in 1971. Local and federal programs aimed at monitoring and studying infectious diseases were greatly reduced or abolished.
New diseases continue to emerge and re-emerge
In spite of optimistic predictions, infectious diseases continued to cause serious problems. As early as the 1940s and 1950s, certain bacteria, such as Staphylococcus aureus, began to develop resistance to penicillin. In 1957 and 1968, new strains of influenza emerged in China and Hong Kong, respectively, and spread rapidly around the globe. In the 1970s there was a resurgence of sexually transmitted diseases in the USA (perhaps due in part to changes in human sexual behaviors and to the importation of antibiotic-resistant strains of gonorrhea by infected soldiers returning from Vietnam). The final blows to our complacent attitude toward infectious diseases came in the 1980s, with the appearance of AIDS and the re-emergence of tuberculosis, including multidrug-resistant strains.
By the early 1990s, many health experts no longer believed that the threat of infectious diseases was receding in the developed world. Growing concern about the threat of emerging infectious diseases was cogently expressed in a 1992 report issued by the Institute of Medicine (IOM) of the National Academies. The report, Emerging Infections: Microbial Threats to Health in the United States, emphasized the intimate links between US health and international health.3 It described the major factors that contribute to disease emergence, including societal changes and microbial evolution (see Table 4.1). The report concluded that emerging infectious diseases are a major threat to US health, and it challenged the US government to take action.
In 1994, the CDC answered the challenge by launching a national effort to revitalize the US capacity to protect the public from infectious diseases. This ongoing effort is described in Addressing Emerging Infectious Disease Threats: A Prevention Strategy for the United States (http://www.cdc.gov/mmwr/PDF/rr/rr4305.pdf) and an updated version published in 1998 (http://www.cdc.gov/mmwr/preview/mmwrhtml/00054779.htm). In view of the public health importance of vector-borne and zoonotic diseases – which account for more than half of all emerging diseases15 – CDC is working with medical partners (e.g. the American Medical Association [AMA]) and animal health partners (e.g. the World Organization for Animal Health [OIE], the American Veterinary Medical Association [AVMA] and the Wildlife Conservation Society [WCS]); see below to study ecologic aspects of disease emergence and develop prevention strategies that take into account human, veterinary, and environmental factors (see Fig. 4.1).
Looking back to the 1950s and 1960s it is useful to remember that very little was then known about how microbes evolve or develop drug resistance. In the 1970s and 1980s, with the development of molecular biology, biologists learned how resistance genes are carried on plasmids and transmitted from one bacterium to another. They also learned how quickly viruses can evolve by generating mutations with each round of replication, as well as by reassorting gene segments or jumping species barriers. As molecular tools became available, many new viruses (pathogenic and nonpathogenic) were discovered in humans, animals and plants.
CATEGORIES OF EMERGING AND RE-EMERGING INFECTIOUS DISEASES
For the purposes of this discussion, emerging and re-emerging threats may be grouped into five (sometimes overlapping) categories:
-
•
drug-resistant diseases;
-
•
food-borne and water-borne diseases;
-
•
zoonotic and vector-borne diseases;
-
•
diseases transmitted through blood transfusions or blood products; and
-
•
chronic diseases caused by infectious agents.
Drug-resistant diseases
Drug-resistant pathogens are a growing menace to all people, regardless of age, sex or socioeconomic background. They endanger people in affluent, industrial societies such as the USA, as well as in less developed nations. Examples of clinically important microbes that are rapidly developing resistance to available antimicrobial agents include bacteria that cause pneumonia, ear infections and meningitis (e.g. Streptococcus pneumoniae), skin, bone and bloodstream infections (e.g. Staphylococcus aureus), urinary tract infections (e.g. E. coli), food-borne infections (e.g. Salmonella) and infections transmitted in health-care settings (e.g. enterococci) (Table 4.6 ).
Table 4.6.
Examples of emerging resistance in bacterial pathogens
| Gram-positive cocci | Methicillin-resistant and vancomycin-resistant Staphylococcus aureus Coagulase-negative staphylococci Penicillin-resistant pneumococci Macrolide-resistant streptococci Vancomycin-resistant enterococci |
| Gram-negative cocci | Penicillin-resistant meningococci Quinolone-resistant gonococci |
| Gram-negative bacilli |
Enterobacter spp. and other Enterobacteriaceae with chromosomal β-lactamases Multidrug-resistant Pseudomonas aeruginosa Stenotrophomonas maltophila Acinetobacter spp. with novel β-lactamases, aminoglycoside-modifying enzymes and other resistance mechanisms Enterobacteriaceae with extended-spectrum β-lactamases Multidrug-resistant diarrheal pathogens (Shigella spp., Salmonella spp., Escherichia coli, Campylobacter spp.) |
| Acid-fast bacilli | Multidrug-resistant Mycobacterium tuberculosis (MDR-TB) Extensively drug-resistant Mycobacterium tuberculosis (XDR-TB) Multidrug-resistant Mycobacterium avium complex |
Special concerns include health-care-associated and community-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA and CA-MRSA) and extensively drug-resistant tuberculosis (XDR-TB; see below). A large study led by the CDC estimates that HA-MRSA was responsible for 94 360 serious infections and associated with 18 650 hospital stay-related deaths in the USA in 2005, making it the cause of more deaths in the USA each year than HIV/AIDS.22
Until recently, most MRSA cases were seen in hospitals. However, clusters of CA-MRSA skin infections are increasingly reported among athletes, military recruits, prisoners and groups of people who live in crowded conditions. Athletes at highest risk include those who participate in contact sports such as wrestling, football and rugby, probably because most MRSA infections begin with a cut, scratch or bruise.23 However, MRSA infections have also been diagnosed among athletes in other sports such as soccer, basketball, field hockey, volleyball, rowing, martial arts, fencing and baseball. Risk factors for these athletes may include contact with contaminated objects such as towels and sports equipment. It is also possible that other strains could emerge in farms where animals are fed antibiotics as growth promoters.24
Many other pathogens – including the bacteria that cause gonorrhea; the virus that causes AIDS; the fungi that cause Candida infections; and the parasites that cause malaria – are also becoming resistant to standard therapies. In the absence of effective action to address the problem of antimicrobial resistance, drug choices for the treatment of common infections will become increasingly limited, expensive and, in some cases, unavailable.
Reasons for the rapid development of antibiotic resistance include the ability of organisms to mutate and share genetic material. However, this process has been facilitated by:
-
•
inappropriate prescription practices by physicians and veterinarians;
-
•
administration of antibiotics to agricultural animals as growth promoters;
-
•
unrealistic patient expectations that lead to requests for antibiotic treatment of nonbacterial infections;
-
•
the economics of pharmaceutical sales; and
-
•
increased use of sophisticated medical interventions that result in the administration of large quantities of antibiotics (e.g. transplant surgery and immunosuppressive and cytotoxic drug therapy).
Growing antibiotic resistance poses a substantial threat to modern gains in infectious disease control. About 70% of bacteria that cause infections in hospitals in many countries, including the US, are resistant to at least one of the drugs most commonly used to treat infections. The medical community must therefore join with patients and members of the agricultural and pharmaceutical industries in a common effort to promote appropriate use of antibiotics to safeguard their effectiveness for future generations. Steps to be taken by the European Union and the US government to facilitate this process are outlined in the Copenhagen Recommendations (http://www.im.dk/ publikationer/micro98/recommen.htm) and A Public Health Action Plan to Combat Antimicrobial Resistance, respectively (http://www.cdc.gov/ drugresistance/actionplan). In addition, the Infectious Diseases Society of America (IDSA) has issued Bad Bugs, No Drugs (http://www.idsociety.org/Content.aspx?id=5558) to call attention to the lack of new antibiotics in the pharmaceutical development pipeline.
Food-borne and water-borne diseases
(See healthmap.org for outbreaks of Food-borne)
Twentieth-century improvements in sanitation, food sterilization and processing, and water treatment have greatly reduced the burden of food-borne and water-borne illnesses in developed countries, nearly eliminating many diseases that remain major killers in the developing world (e.g. typhoid fever, cholera and dysentery). Now, in the 21st century, however, there is growing evidence that modern factors such as centralized food processing may pose challenges to food safety and water quality.
Food-borne diseases
Food-borne diseases cause an estimated 76 million illnesses, 325 000 hospitalizations and 5000 deaths in the USA each year.25 As noted above, food-borne pathogens such as Salmonella, Shigella, Cyclospora, Campylobacter and E. coli O157:H7 can be transmitted via commercial products that are processed in large quantities and shipped to different states or nations. Although food processing techniques are very advanced, when contamination does occur it can affect many people in many different localities (see Table 4.5). A recent example is an outbreak of E. coli O157:H7 (transmitted via frozen hamburger patties) that affected people in eight US states and led to the recall of 21.7 million pounds of ground beef products. Six days after the recall, the company that marketed the beef went out of business (http://www.cdc.gov/ecoli/2007/october/100207.html).
Water-borne diseases
Microbial contamination of water can occur when animal or human sewage contaminates source water that is not adequately treated by filtration, chlorination or other methods. In addition, some parasites are resistant to routine water treatment methods. These include Cryptosporidium, the causative agent of a major disease outbreak in the Milwaukee drinking water system in 1993 that affected more than 400 000 people.26 In 2006, series of articles reviewing current research on water-borne diseases was published in a special issue of the Journal of Water and Health (http://www.epa.gov/nheerl/articles/2006/waterborne_disease.html). This research was mandated by the Safe Drinking Water Act Amendments of 1996 and supported by the US Environmental Protection Agency (EPA) and the CDC. A workshop of experts from academia, the EPA and the CDC made recommendations for additional health studies related to microbial exposures in drinking water. Although associations between illness and water are often difficult to evaluate – since most people drink water every day, often from more than one source – the EPA estimates that there may be as many as 16.4 million cases of water-borne illness in the USA each year (http://www.epa.gov/nheerl/articles/2006/waterborne_disease/national_estimate.pdf).
Worldwide, approximately 1 billion people lack access to safe water and 2.6 billion lack access to basic sanitation. Water-borne diarrheal diseases cause 1.8 million deaths every year, most of them in children in developing countries with unsafe water supplies. In 2007, the World Health Organization (WHO) issued Combating Waterborne Disease at the Household Level (http://www.who.int/household_water/advocacy/combating_disease/en/index.html), which advocates the use of inexpensive point-of-use water quality interventions that can be used in the home in areas that lack access to safe drinking water.
Zoonotic diseases
Many of the novel human pathogens identified over the past decade are carried by animals. For example, Sin Nombre virus, which is carried by the deer mouse, was identified in 1993 in the USA as the cause of hantavirus pulmonary syndrome, and Hendra virus, carried by fruit bats, was identified in Australia in 1994 as a cause of encephalitis in humans and horses.27 Nipah virus, which is also carried by fruit bats, was identified in 1999 in Malaysia as a cause of encephalitis in humans and swine and has since been reported in Bangladesh and India.28., 29., 30. Like Sin Nombre virus, the Nipah and Hendra viruses (henipaviruses) are highly virulent, and there are as yet no drugs or vaccines for their treatment or prevention. Other examples include Marburg hemorrhagic fever virus, which is apparently maintained in cave-dwelling African fruit bats,31, 32 and the SARS coronavirus, which is associated with Chinese horseshoe bats.33
Zoonotic agents can become established in any geographic area that has a suitable animal reservoir. The arenavirus that causes lymphocytic choriomeningitis (first isolated in 1933) was probably introduced into the New World at the same time as its vector, Mus musculus, the common house mouse. Plague (which is both rodent-borne and vector-borne) was introduced into the USA in the early 1900s, via infected rats and fleas in ships that arrived at port cities. It quickly became established in the North American prairie ecosystem, infecting a wide range of animals, including native rodents and their fleas, which have been the most frequent sources of human infection. Like plague, newly emergent disease agents that are able to infect many animal species (e.g. Nipah and Hendra viruses and monkeypox virus) have the potential to spread worldwide.
Disease dispersion via insect vectors
Diseases that are carried by insect vectors (i.e. mosquitoes, fleas, ticks and other blood-sucking arthropods) can also spread into new geographic areas and infect new human populations. In 1999, for example, mosquito-borne transmission of three nonendemic diseases – malaria, dengue and West Nile fever – was reported in the USA. West Nile encephalitis may have entered New York City via an imported or migrating bird or a mosquito that ‘hitch-hiked’ on an airplane or (less likely) an infected traveler; dengue fever most likely arrived in Texas via both people and mosquitoes.
Asian tiger mosquitoes (Aedes albopictus) that can transmit dengue and Chikungunya fever arrived in the USA in Houston in 1987 in imported used-tire casings34 and appeared more recently in California in commercial shipments of a Chinese ornamental indoor plant called ‘lucky bamboo’.35 During the 1990s, A. albopictus became established in Italy after mosquito eggs traveled to Italy in imported tires.36 In 2007, when a traveler who had contracted Chikungunya in Kerala, India, fell ill after arriving in Ravenna, Italy, his disease was transmitted to others via the now-indigenous Italian A. albopictus mosquitoes, causing an outbreak that affected at least 204 people.37
While many cases of ‘airport malaria’ have been reported in Europe and North America (presumably involving small numbers of traveling mosquitoes), locally acquired malaria cases have also been identified that apparently involved at least one cycle of human-to-mosquito transmission. For example, a cluster of non-airport malaria cases was detected in Virginia in 2002 in a community that included immigrants from malarious countries who might have had asymptomatic malaria infections.38 Another cluster was detected in 2003 in Palm Beach County, Florida.39
Another example of vector-borne disease spread is the recent identification of Rift Valley fever (previously seen only in Africa) in the Middle East.40 Within a few years, Rift Valley fever (RVF) virus traveled from sub-Saharan Africa to northern Africa (Egypt and the Sudan) and then to Saudi Arabia and Yemen. Competent mosquito vectors for RVF, a febrile hepatitis associated with encephalitis, retinitis and hemorrhagic fever (among both humans and animals), are present throughout the world. Importation of RVF into the USA would constitute a major threat to the US livestock industry and to human public health.
Diseases transmitted through blood transfusions, blood products, or transplanted organs or tissues
Improvements in donor screening, serologic testing and transfusion practices have made the US blood supply one of the safest in the world, despite its size and complexity. However, because blood is a human tissue, it is a natural vehicle for transmission of infectious agents. During the 1980s, HIV was transmitted through clotting factor and blood transfusions, and during the 1990s, hepatitis C virus was transmitted by intravenous immunoglobulin. More recently, transmission of Chagas disease trypanosomes,41 West Nile virus,42 rabies virus,43 Creutzfeldt–Jakob disease prions,44 tuberculosis,45 a new arenavirus related to lymphocytic choriomeningitis virus46 and HIV virus (for the first time in the USA since 198647) has been reported in patients who received organ transplants, grafts or blood from infected persons.
In recent years, there has been renewed interest in xenotransplantation – the transplantation of animal organs and tissues to humans – because of the shortage of human organs and tissues. The US Food and Drug Administration (FDA) is currently developing a comprehensive approach to the regulation of xenotransplantation to address public health concerns about potential infection of recipients with recognized or unknown zoonotic pathogens and possible subsequent transmission of these pathogens to close contacts and into the general population (http://www.fda.gov/cber/xap/xap.htm).
Infectious diseases also have implications for the availability of blood and blood products, because people who have traveled in countries where they may have been exposed to blood-borne diseases are often excluded as blood donors. Examples include individuals who have recently visited malaria-endemic countries and people who have stayed for 6 months or more in the UK, where they might have ingested beef from cows with BSE and acquired the prion that causes new variant Creutzfeldt–Jakob disease.
Chronic diseases caused or exacerbated by infectious agents
Several chronic diseases once attributed to lifestyle or environmental factors (such as some forms of cancer, diabetes, heart disease, arthritis and ulcers) are actually caused by or exacerbated by an infectious agent.48., 49., 50. Three of the six major causes of cancer death in the world are caused by infectious agents: hepatocellular carcinoma by hepatitis B and C viruses, cervical cancer by human papillomavirus and gastric cancer by Helicobacter pylori bacteria (see below). Hepatitis B and C are also major causes of cirrhosis and end-stage liver disease, while H. pylori causes peptic ulcer disease in addition to stomach cancer. Moreover, Epstein–Barr virus is associated with nasopharyngeal carcinoma, Burkitt's lymphoma, B-cell lymphoma and post-transplant lymphoproliferative disease.
Current research suggests that some chronic cardiovascular, intestinal and pulmonary diseases may also have an infectious etiology. For example, Whipple's disease is caused by a bacterial infection (Tropheryma whipplei)51, 52 and tropical spastic paraparesis is caused by a viral infection (HTLV-1).53 Potential associations are being investigated for many other illnesses and syndromes, including coronary artery disease (Chlamydia infection),54 Paget's disease (paramyxoviridae infection),55, 56 Crohn's disease (mycobacterial infection)57 and bronchiectasis (respiratory infections during early childhood).58 There are also preliminary data suggesting that Wegener's granulomatosis responds to antibiotic therapy. These findings raise the possibility that some chronic conditions, including asthma, arthritis and heart disease, may someday be treated with antimicrobial drugs or prevented by vaccines.
INFECTIOUS DISEASES AND THE GLOBAL VILLAGE
As stressed in the Institute of Medicine reports in 1992 and 2003, US health and global health are inextricably linked.3, 4 Modern factors that connect us culturally, commercially and physically such as air travel and the globalization of the food supply (see Table 4.5) put us at risk of exposure to microbes that are endemic in other countries, whether we live in large cities or small rural hamlets. As the HIV/AIDS epidemic has illustrated, a disease that emerges or re-emerges anywhere in the world can spread far and wide. This concern is reflected in the most recent (2005) version of the WHO International Health Regulations (http://www.who.int/csr/ihr/en), which require reporting not only of some specific diseases, as in the 1969 version, but also of ‘all events that may constitute public health emergencies of international concern’ – including outbreaks caused by previously unidentified microbes, by microbes that appear in new, drug-resistant forms, or by bioterrorism. The list of specific notifiable diseases (which used to consist of cholera, plague and yellow fever) now includes a single case of smallpox, polio due to wild-type poliovirus, human influenza due to a new subtype and SARS.
Several diseases of global public health importance – including epidemic-prone diseases, newly emerging or re-emerging diseases, vaccine-preventable diseases and diseases slated for regional elimination or worldwide eradication (e.g. polio) – have resurged in recent years, spreading across countries and (sometimes) continents (see Table 4.3). HIV/AIDS, tuberculosis, malaria and measles continue to be major infectious causes of death worldwide, along with acute lower respiratory infections and diarrheal diseases (Fig. 4.4 ). Globally important diseases that continue to be of special domestic concern to the USA and other developed countries include HIV/AIDS, tuberculosis and pandemic influenza.
Fig. 4.4.
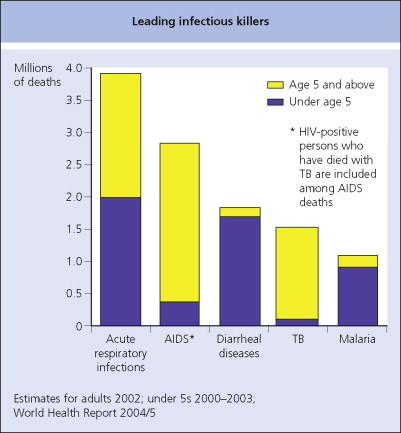
Leading infectious killers.
HIV/AIDS
(See healthmap.org for outbreaks of HIV/AIDS)
HIV/AIDS incidence in the USA increased rapidly through the 1980s and early 1990s, with the peak of new diagnoses occurring after the expansion of the AIDS surveillance case definition in 1993.59 After 1996, with the introduction of effective combination antiretroviral therapies, sharp declines were reported in AIDS incidence and deaths, while AIDS prevalence continued to increase. Currently, US disease prevention efforts are targeted primarily to young men who have sex with men, the group that currently exhibits the highest incidence nationally.60, 61
Worldwide, an estimated 33.2 million people are living with HIV/AIDS (Fig. 4.5 ). In 2007, there were 2.1 million HIV-related deaths and approximately 2.5 million new infections.
Fig. 4.5.

Estimated number of adults and children living with HIV/AIDS, by region, 1990–2007.
Malaria
(See healthmap.org for outbreaks of Malaria)
Forty-one percent of the world's population lives in areas where malaria is transmitted (e.g. parts of Africa, Asia, the Middle East, Central and South America, Hispaniola and Oceania) and 350–500 million cases occur annually. More than one million people die of malaria each year, most of them young children in sub-Saharan Africa.
Malaria prevention efforts include treatment with artemisinin-containing drugs, mosquito control (insecticide-treated bed nets and/or indoor residual spraying) and prophylactic treatment of vulnerable populations such as pregnant women and children.
Tuberculosis
(See healthmap.org for outbreaks of Tuberculosis)
Tuberculosis is the attributable cause of one-third of all adult deaths in developing nations.62 Once thought to be controlled in Western countries, tuberculosis has re-emerged in Europe and the USA, where multidrug-resistant Mycobacterium tuberculosis (MDR-TB) has been reported in 45 of the 50 states,63 and extensively drug-resistant Mycobacterium tuberculosis (XDR-TB) is present in 4% of cases. MDR-TB bacteria are defined as resistant at least to the two first-line drugs against TB (isoniazid and rifampin (rifampicin)), while XDR-TB bacteria are defined as resistant not only to isoniazid and rifampin but also to any fluoroquinolone and at least one of three injectable second-line drugs (capreomycin, kanamycin and amikacin).64 Since 1998, the percentage of US-born patients with MDR-TB has remained at less than 0.7%. However, the frequency of resistant infections in foreign-born persons increased from 25% (103 of 407) in 1993 to 80% (73 of 91) in 2006 (http://www.cdc.gov/tb/statistics/reports/2007/default.htm).
HIV infection confers the greatest known risk for the development of TB, stimulating both the activation of latent infection and rapid progression to primary disease. UNAIDS estimates that approximately 30% of all AIDS deaths result directly from tuberculosis.65
Special challenges
Climate change and infectious diseases
Understanding the effects of climate change on specific diseases – especially those of major concern to developing countries, such as malaria, dengue and cholera – is currently an active area of research, which involves separating weather effects from other environmental factors that influence disease spread, such as land use, water management, urbanization and human behavior. From a public health point of view, the major challenge is to enhance surveillance for disease vectors and reservoirs, as well as for the diseases they carry, and to address all the factors that facilitate disease transmission. The potential introduction of disease vectors and reservoirs into new areas as weather patterns change is another reason why we need to be prepared for the unexpected. See Practice Point 1 for a further discussion on the relationship between health and climate change.
An ecologic approach to prevention and control of zoonotic and vector-borne diseases
The development of public health strategies to prevent and control zoonotic and vector-borne diseases – which include the great majority of new and re-emerging diseases15 – requires an ecologic approach that takes into account the human, animal, microbial and environmental facts that influence disease emergence (see Fig. 4.1). In recognition of this fact, the Wildlife Conservation Society (WCS) has organized a series of One World – One Health symposia in New York, Thailand, China and Brazil to focus world attention on the ‘essential link between human, domestic animal, and wildlife health’ and the threat that animal diseases pose to people, food supplies and national economies.
A comprehensive approach to addressing these diseases would involve:
-
•
new partnerships among experts in animal and human health, conservation biology, law and public policy;
-
•
expansion of disease surveillance systems that integrate animal and public health data;
-
•
routine availability of veterinary and entomologic expertise at public health departments; and
-
•
development of an interdisciplinary research agenda to identify ecologic factors and interactions among animals, humans, and insects and their environments that influence disease emergence.
A better understanding of the ecologic factors that influence disease emergence will facilitate the development of innovative strategies for detection, control and prevention of zoonotic and vector-borne diseases such as the One Health Initiative (http://www.onehealthinitiative.com), as well as the creation of predictive models that indicate when outbreaks are likely to occur.
Infectious diseases as national security threat
From the end of the Cold War until 2001, US concerns about the impact of health issues on national security were focused primarily on events overseas67 (Table 4.7 ). During the 1990s, security experts expressed particular concern about the destabilizing effects of HIV/AIDS in poor countries where high death rates among young adults have damaged economic, social, political, military and educational infrastructures and created vast numbers of orphans. In July 2000, the Group of Eight Industrialized Nations pledged to reduce deaths from HIV/AIDS, malaria, tuberculosis and vaccine-preventable diseases by supporting global health initiatives launched by the World Health Organization and other groups.69 Combating HIV/AIDS and malaria was adopted by 189 nations as a Millennium Development Goal in 2000 (http://www.un.org/millenniumgoals), and the Global Fund to Fight AIDS, Tuberculosis and Malaria was established by the G8 in 2001 (http://www.theglobalfund.org/en). These ongoing international efforts reflect a growing consensus that global health security must be a shared responsibility.
Table 4.7.
Biologic national security issues
|
Adapted from Goldberg.68
Since the autumn of 2001, domestic security concerns have taken center stage, as they did in the 1950s, when the Epidemic Intelligence Service (EIS), the US training program for epidemiologists, was established in response to the Cold War threat of biologic warfare.70 The anthraxl attacks illustrated that the USA is vulnerable to bioterrorism, stimulating renewed bioterrorism preparedness and response efforts. Of the 15 National Planning Scenarios developed by the White House Homeland Security Council to guide emergency preparedness exercises, five involve infectious disease threats (anthrax, pandemic influenza, plague, food contamination and disease of agricultural animals).
Physicians are one of the three cornerstones of the ‘golden triangle’ for bioterrorism preparedness, along with the health-care delivery system and public health officials.71 All clinicians, regardless of their specialty, must have basic information about the clinical manifestations of such potential bioterrorist agents as variola virus (smallpox), Yersinia pestis (plague) and Bacillus anthracis (anthrax). They must have a high index of suspicion and know how to recognize unusual diseases and report them to local public health and law enforcement officials. The expertise and full engagement of the medical community are essential to the national effort to preserve health security. For further discussion of issues related to bioterrorism, see Chapter 71.
Pandemic influenza
New strains of influenza viruses can emerge unpredictably from animal reservoirs and spread rapidly and pervasively through susceptible populations, sometimes causing worldwide epidemics. This is due in large part to two features of the influenza virus: its ability to exchange genetic information between strains and its ability to occasionally ‘jump’ species barriers between avian and porcine (or other mammalian) hosts. Influenza pandemics typically cause major morbidity and mortality, with significant societal and economic impacts.46
The sudden and unpredictable emergence of a potential pandemic strain of influenza was illustrated in 1997 by an outbreak of avian influenza A (H5N1) in Hong Kong, which raised the specter of a worldwide epidemic similar to the one that killed 20–50 million people (including 500 000 Americans) in 1918. Epidemiologic studies suggested that the H5N1 virus was transmitted from chickens to people and only poorly (if at all) from person to person. Nevertheless, it was feared that the virus might reassort with a human influenza virus during the winter influenza season, creating a virulent strain capable of air-borne human-to-human transmission. To ensure that the H5N1 virus would have no opportunity to evolve, the Hong Kong government authorized the culling of all 1.6 million chickens in Hong Kong (see Box 4.1). Six years later (in 2003) influenza A (H5N1) re-merged in poultry in South East Asia and then spread to Central Asia, Africa, Europe and the Middle East, leading to a worldwide pandemic alert (http://www.who.int/csr/disease/avian_influenza/phase/en/index.html).
Unexpectedly, however, a different virus – unrelated to avian influenza A (H5N1) – has emerged as the cause of a new pandemic officially declared by WHO in June 2009. First detected in the United States and Mexico, the pandemic (H1N1) 2009 virus was originally referred to as ‘swine flu’ virus because laboratory tests indicated that it contained genes similar to those of influenza viruses in North American pigs. However, further study has shown that this new virus is actually a ‘quadruple reassortant’ virus, which also contains gene segments from human, avian and Eurasian swine influenza viruses.
As of August 2009, the pandemic (H1N1) 2009 virus was the predominant influenza virus in circulation worldwide. Disease severity appeared to be generally similar to the severity of disease during recent influenza seasons, although different age groups have been predominantly affected, with most cases and most severe cases occurring in older children and adults less than 65 years of age (http://www.cdc.gov/h1n1flu/).
CONCLUSION
Now more than ever, global health and well-being depend on the vigilance of concerned and well-informed clinicians. Each and every clinician plays a critical role in the public health early warning system for unusual infectious diseases, whether they are new, rare, zoonotic, vector-borne, drug-resistant or intentionally caused. These efforts are essential to maintaining a strong and effective public health system.
REFERENCES
- 1.Gao F., Bailes E., Robertson D.L. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 2.Clegg J.B., Weatherall D.J. Thalassemia and malaria: new insights into an old problem. Proc Assoc Am Physicians. 1999;111(4):278–282. doi: 10.1046/j.1525-1381.1999.99235.x. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Emerging infections: microbial threats to health in the United States. National Academy Press; Washington, DC: 1992. [PubMed] [Google Scholar]
- 4.Institute of Medicine . Microbial threats to health: emergence, detection, and response. National Academy Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Preventing emerging infectious diseases: a strategy for the 21st century. CDC; Atlanta: 1998. http://www.cdc.gov/mmwr/preview/mmwrhtml/00054779.htm Online. Available: [Google Scholar]
- 6.United National Population Division . World urbanization prospects: the 1999 revision. United Nations Population Division; New York: 2000. [Google Scholar]
- 7.World Health Organization . World Health Report, 1996. Fighting disease: fostering development. WHO; Geneva: 1996. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Update: Outbreak of severe acute respiratory syndrome – worldwide, 2003. MMWR Morb Mortal Wkly Rep. 2003;2(12):241–248. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5212a1.htm Online. Available: [PubMed] [Google Scholar]
- 9.Lorber B. Are all diseases infectious? Ann Intern Med. 1996;125:844–851. doi: 10.7326/0003-4819-125-10-199611150-00010. [DOI] [PubMed] [Google Scholar]
- 10.McDonald L. Clostridium difficile: responding to a new threat from an old enemy. Infect Control Hosp Epidemiol. 2005;6(8):672–675. doi: 10.1086/502600. [DOI] [PubMed] [Google Scholar]
- 11.McDonald L.C., Killgore G.E., Thompson A. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 12.Fine A., Layton M. Lessons from the West Nile viral encephalitis outbreak in New York City, 1999: implications for bioterrorism preparedness. Clin Infect Dis. 2000;32(2):277–282. doi: 10.1086/318469. [DOI] [PubMed] [Google Scholar]
- 13.Asnis D.S., Conetta R., Teixeira A.A., Waldman G., Sampson B.A. The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis. 2000;30(3):413–418. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- 14.Herwaldt B.L., Ackers M.L. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N Engl J Med. 1997;336(22):1548–1556. doi: 10.1056/NEJM199705293362202. [DOI] [PubMed] [Google Scholar]
- 15.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNicholl J.M., Hughes J.M. Human genetics and emerging infectious diseases: issues and prevention opportunities. US Med. 1988;34(6):4. [Google Scholar]
- 17.McNicholl J.M., Downer M., Udhayakumar V., Swerdlow D., Alper C. Host pathogen interactions in emerging and re-emerging infectious disease: a genomic perspective of TB, malaria, HIV, hepatitis B and cholera. Ann Rev Publ Health. 2000;21:15–46. doi: 10.1146/annurev.publhealth.21.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Swaminathan B., Barrett T.J., Hunter S.B., Tauxe R.V. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. http://www.cdc.gov/pulsenet PulseNet Homepage: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Staphylococcus aureus resistant to vancomycin – United States, 2002. JAMA. 2002;288(7):824–825. [PubMed] [Google Scholar]
- 20.Burnet M., White D.O. Natural history of infectious disease. Cambridge University Press; London: 1972. [Google Scholar]
- 21.Stewart W.H. A mandate for state action. Presented at the Association of State and Territorial Health Officers, Washington, DC, Dec. 4, 1967. In: Garrett L., editor. Vol. 33. Penguin; New York: 1994. (The coming plague: newly emerging diseases in a world out of balance). footnote 9. [Google Scholar]
- 22.Klevens R.M., Morrison M.A., Nadle J. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Methicillin-resistant Staphylococcus aureus infections among competitive sports participants – Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52(33):793–885. [PubMed] [Google Scholar]
- 24.van Loo I., Huijsdens X., Tiemersma E. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13(12):1834–1839. doi: 10.3201/eid1312.070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mead P.S., Slutsker L., Dietz V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. http://www.cdc.gov/ncidod/eid/vol5no5/mead.htm Online. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKenzie W.R., Hoxie N.J., Proctor M.E. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 27.Murray K., Rogers R., Selvey L. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg Infect Dis. 1995;1(1):31–33. doi: 10.3201/eid0101.950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chua K.B., Bellini W.J., Rota P.A. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288(5470):1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 29.Luby S.P., Rahman M., Hossain M.J. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12(12):1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chadha M.S., Comer J.A., Lowe L. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12(2):235–240. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leroy E.M., Kumulungui B., Pourrut X. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–666. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 32.Towner J.S., Pourrut X., Albariño C.G. Marburg virus infection detected in a common African bat. PLoS ONE. 2007;2(1):e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau S.K., Woo P.C., Li K.S. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102(39):14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francy D.B., Moore C.G., Eliason D.A. Past, present and future of Aedes albopictus in the United States. J Am Mosq Control Assoc. 1990;6(1):127–132. [PubMed] [Google Scholar]
- 35.Madon M.B., Mulla M.S., Shaw M.W., Kluh S., Hazelrigg J.E. Introduction of Aedes albopictus (Skuse) in Southern California and potential for its establishment. J Vector Ecology. 2002;27(1):149–154. [PubMed] [Google Scholar]
- 36.Knudsen A.B., Romi R., Majori G. Occurrence and spread in Italy of Aedes albopictus, with implications for its introduction into other parts of Europe. J Am Mosq Control Assoc. 1996;12(2 Pt 1):177–183. [PubMed] [Google Scholar]
- 37.Townson H., Nathan M.B. Resurgence of chikungunya. Trans R Soc Trop Med Hyg. 2008;102(4):308–309. doi: 10.1016/j.trstmh.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Local transmission of Plasmodium vivax malaria – Virginia 2002. MMWR Morb Mortal Wkly Rep. 2002;51(41):921–923. [PubMed] [Google Scholar]
- 39.Centers for Diseases Control and Prevention Local transmission of Plasmodium vivax malaria – Palm Beach County, Florida, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(38):908–911. [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention Outbreak of Rift Valley fever – Yemen, August–October 2000. MMWR Morb Mortal Wkly Rep. 2000;49(47):1065–11116. [PubMed] [Google Scholar]; Centers for Disease Control and Prevention Update: outbreak of Rift Valley Fever – Saudi Arabia, August–November 2000. MMWR Morb Mortal Wkly Rep. 2000;49(43):982–1105. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Chagas disease after organ transplantation – United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(10):210–212. [PubMed] [Google Scholar]
- 42.Public Health Dispatch Investigation of blood transfusion recipients with West Nile virus infections. MMWR Morb Mortal Wkly Rep. 2002;51(36):823. [PubMed] [Google Scholar]
- 43.Srinivasan A., Burton E.C., Kuehnert M.J. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005;352(11):1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki Y., Mimuro M., Yoshida M., Hashizume Y., Kitamoto T., Sobue G. Clinicopathologic characteristics of five autopsied cases of dura mater-associated Creutzfeldt–Jakob disease. Neuropathology. 2008;28(1):51–61. doi: 10.1111/j.1440-1789.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention Transplantation-transmitted tuberculosis – Oklahoma and Texas, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(13):333–336. [PubMed] [Google Scholar]
- 46.Palacios G., Druce J., Du L. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358:991–1108. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 47.New York Times Four transplant recipients contract HIV. Nov 13, 2007. http://www.nytimes.com/2007/11/13/health/13cnd-organ.html?em Online. Available:
- 48.O'Connor S.M., Taylor C.E., Hughes J.M. Emerging infectious determinants of chronic diseases. Emerg Infect Dis. 2006;12(7):1051–1057. doi: 10.3201/eid1207.060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis R. The infection-chronic disease link strengthens. Scientist. 2000;14(17):1. [Google Scholar]
- 50.Cassell G.H. Infectious causes of chronic inflammatory diseases and cancer. Emerg Infect Dis. 1998;4(3):475–487. doi: 10.3201/eid0403.980339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Relman D., Schmidt T., MacDermott R., Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327(5):293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 52.James D.G., Lipman M.C. Whipple's disease: a granulomatous masquerader. Clin Chest Med. 2002;23(2):513–519. doi: 10.1016/s0272-5231(02)00005-9. xi–xii. [DOI] [PubMed] [Google Scholar]
- 53.De-The G., Giordano C., Gessain A. Human retroviruses HTLV-1, HIV-1, and HIV-2 and neurological diseases in some equatorial areas of Africa. J Acquir Immune Defic Syndr. 1989;2(6):550–556. [PubMed] [Google Scholar]
- 54.Smieja M., Mahony J.B., Petrich A., Boman J., Chernesky M. Association of circulating Chlamydia pneumoniae DNA with cardiovascular disease: a systematic review. BMC Infect Dis. 2002;2(1):21. doi: 10.1186/1471-2334-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mee A.P. Paramyxoviruses and Paget's disease: the affirmative view. Bone. 1999;24(5 Suppl.):19S–21S. doi: 10.1016/s8756-3282(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 56.Ralston S.H., Helfrich M.H. Are paramyxoviruses involved in Paget's disease? A negative view. Bone. 1999;24(5 Suppl.):17S–18S. doi: 10.1016/s8756-3282(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 57.Hubbard J., Surawicz C.M. Etiological role of Mycobacterium in Crohn's disease: an assessment of the literature. Dig Dis. 1999;17(1):6–13. doi: 10.1159/000016898. [DOI] [PubMed] [Google Scholar]
- 58.Redding G., Singleton R., Lewis T. Early radiographic and clinical features associated with bronchiectasis in children. Pediatr Pulmonol. 2004;37(4):297–304. doi: 10.1002/ppul.10427. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention . HIV/AIDS surveillance report. US Department of Health and Human Services; Atlanta, GA: 2000. [Google Scholar]
- 60.Centers for Disease Control and Prevention . Taking action to combat increases in STDs and HIV risk among men who have sex with men. US Department of Health and Human Services; Atlanta, GA: 2001. [Google Scholar]
- 61.Centers for Disease Control and Prevention Trends in HIV/AIDS diagnoses among men who have sex with men – 33 states, 2001–2006. MMWR Morb Mortal Wkly Rep. 2008;57(25):681–776. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5725a2.htm Online. Available: [PubMed] [Google Scholar]
- 62.Bloom B.R. Tuberculosis – the global view. N Engl J Med. 2002;346(19):1434–1435. doi: 10.1056/NEJM200205093461902. [DOI] [PubMed] [Google Scholar]
- 63.Seaworth B.J. Multidrug-resistant tuberculosis. Infect Dis Clin North Am. 2002;16(1):73–105. doi: 10.1016/s0891-5520(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs – worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55(11):301–305. [PubMed] [Google Scholar]
- 65.UNAIDS calls for progress against TB/HIV epidemic. AIDS Alert. 1998;13(6) Suppl 3. [PubMed] [Google Scholar]
- 66.Meltzer M.I., Cox N.J., Fukuda K. The economic impact of pandemic influenza in the United States: implications for setting priorities for interventions. Emerg Infect Dis. 1999;5:659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Intelligence Council The global infectious disease threat and its implications for the United States. National Intelligence Estimate (NIE 99–17D) January 2000 http://www.fas.org/irp/threat/nie99-17d.htm Online. Available: [PubMed] [Google Scholar]
- 68.Goldberg J. Our Africa problem. New York Times. March 2, 1997 [Google Scholar]
- 69.Cohen J. The new world of global health. Science. 2006;311(5758):162–167. doi: 10.1126/science.311.5758.162. http://www.sciencemag.org/cgi/content/full/311/5758/162 Online. Available: [DOI] [PubMed] [Google Scholar]
- 70.Langmuir A.D., Andrews J.M. Biological warfare defense. 2. The Epidemic Intelligence Service of the Communicable Disease Center. Am J Public Health. 1952;42:235–238. doi: 10.2105/ajph.42.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes J.M., Gerberding J.L. Anthrax bioterrorism: lessons learned and future directions. Emerg Infect Dis. 2002;8(10) doi: 10.3201/eid0810.020466. http://www.cdc.gov/ncidod/eid/vol8no10/02-0466.htm Online. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]


