Almost 5 million children in the United States have asthma,1 and it is the most common reason for admission to pediatric hospitals.2 Each year, asthma results in 10 million school absences,2 5500 deaths,3 and 500,000 hospitalizations.4, 5 Appropriate asthma treatment prevents hospital admissions and emergency room visits, reduces the risk for death, and improves the quality of life for children with asthma.4, 6, 7 The hospitalist is ideally situated to have a major impact on asthma by treating its acute manifestations, by implementing effective long-term therapy when indicated, and by diagnosing and managing any comorbidity that accompanies or exacerbates asthma (or both).
Asthma results from airway inflammation and smooth muscle dysfunction. It is defined by the National Heart, Lung, and Blood Institute (NHLBI) and World Health Organization as follows:
“A chronic inflammatory disorder of the airways in which many cells play a role, in particular, mast cells, eosinophils, and T lymphocytes. In susceptible individuals this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and cough, particularly at night and in the early morning. These symptoms are usually associated with widespread but variable airway obstruction that is often reversible either spontaneously or with treatment. The inflammation also causes an associated increase in the existing bronchial hyperresponsiveness to a variety of stimuli.”4
PATHOPHYSIOLOGY OF ASTHMA
The underlying cause of asthma is unknown, and the course of pediatric asthma is dynamic. Early in the course of the disease, airway inflammation, bronchial hyperreactivity, and loss of lung function are evident. Atopy and a family history of asthma are strongly correlated with asthma in childhood. Exposure to allergens activates mast cells and promotes inflammation and infiltration of the airway with neutrophils, eosinophils, and lymphocytes.8 Whatever the cause, the inflammation results in airway hyperresponsiveness, which causes bronchoconstriction, edema, and mucous plugging, all of which contribute to bronchial obstruction. Chronically, collagen deposition below the epithelial basement membrane results in narrowing of the airway secondary to remodeling
ASTHMA EXACERBATIONS
An asthma exacerbation refers to an increase in a patient's respiratory symptoms above baseline as a result of increased airway obstruction. Status asthmaticus is continued or progressive airway obstruction despite bronchodilator therapy that results in sustained or worsening respiratory distress.4
Acute asthma exacerbations can be triggered by infectious respiratory illness, exposure to environmental allergens or irritants, exercise, cold air, or a combination of these factors. An asthma exacerbation involves either a slow onset of symptoms or a rapid decline in respiratory status. Persistent or acute allergen or irritant exposure promotes inflammation, bronchoconstriction, and airway hyperresponsiveness on an ongoing basis. Allergen exposure triggers a biphasic response. The “early response” occurs within minutes of allergen exposure and results in rhinorrhea, sneezing, itching of the eyes and nose, and bronchospasm secondary to release of histamine and other preformed mediators of inflammation. The “late-phase response” peaks 6 to 8 hours after allergen exposure with the development of eosinophilic inflammation and T-cell infiltration of the airway. During an exacerbation of asthma as a result of allergen exposure, both phases must be treated with medications to treat the symptoms of the early phase, as well as the subsequent inflammation of the late phase.9
Viral infections cause asthma symptoms by promoting eosinophilic or neutrophilic airway inflammation.4 Viral-induced asthma exacerbations are common in children, and in fact a majority of acute asthma admissions are associated with viral infections in children and adults.10, 11 The risk for exacerbation of asthma can be modified by a patient's underlying inflammatory state and level of airway hyperreactivity. A patient with reduced airway inflammation because of adequate controller therapy is less likely to have a severe asthma flare when exposed to offending agents.
CLINICAL PRESENTATION
The presentation of acute asthma may vary, but all patients experience worsening airflow obstruction associated with respiratory distress. Patients often complain of shortness of breath, chest tightness, and wheezing. Some patients describe chest pain, cough, or fatigue. Caregivers may report observations of breathlessness, trouble speaking, decreased activity, retractions, rapid breathing, wheezing noises, or relentless cough.12
On physical examination, tachypnea is present, often accompanied by tachycardia. Pulse oximetry may reveal decreased oxygen saturation. There is evidence of increased respiratory effort, such as intercostal, supraclavicular, or subcostal retractions. Infants and young children may demonstrate nasal flaring or head bobbing. Paradoxical motion of the thoracoabdominal wall (i.e., expansion of abdominal girth with inspiration) is another useful sign of increased work of breathing. Auscultation of the chest frequently reveals wheezing and a prolonged expiratory phase. Rales or crackles are often heard and may shift in location over a period of minutes to hours (“migratory atelectasis”). Assessment of air movement is determined by the loudness of breath sounds in various areas of the chest and may also vary over time. Patients with poor air movement may have minimal wheezing because the passage of air through the airway is what generates wheezing sounds. As air exchange improves, wheezing may become more pronounced. Conversely, patients with a deteriorating clinical course may have diminishing wheezing indicative of worsening air movement and perhaps respiratory insufficiency. Agitation and somnolence are worrisome signs and may indicate hypoxemia or hypercapnia with impending respiratory failure.
Some patients present without significant wheezing but with prominent cough as their manifestation of asthma, often referred to as “cough-variant” asthma. It is believed that the pathophysiology and response to treatment are similar to that for classic asthma.
DIFFERENTIAL DIAGNOSIS
Many conditions result in acute or chronic respiratory symptoms that mimic an asthma syndrome. Some of these conditions are discussed in the following text.
Anatomic abnormalities should be considered in young children with frequent episodes of cough or wheezing. Inhaled foreign bodies are most common in toddler-aged children (Chapter 79). These problems may manifest as cough, stridor, or wheezing. In all age groups, gastroesophageal reflux can mimic or contribute to underlying asthma (Chapter 101).13, 14 Cystic fibrosis is a genetic disorder that can also present with chronic cough or recurrent episodes of wheezing (Chapter 78).
Viral infections often cause wheezing in childhood as well. Respiratory syncytial virus is the most common cause of infantile bronchiolitis, but other respiratory viruses such as rhinovirus, parainfluenza virus, coronavirus, adenovirus, and influenza viruses are also common infectious agents.15 Viral bronchiolitis is associated with edema, bronchospasm, and increased mucus production of the smaller airways, features that overlap with asthma (Chapter 66). Because these respiratory viral infections are known to precipitate asthma exacerbations, it may be difficult to determine whether the wheezing represents an isolated episode of bronchiolitis or an asthma exacerbation triggered by the respiratory virus.
Atypical respiratory infections with agents such as Mycoplasma, Chlamydia pneumoniae, and Bordetella pertussis or parapertussis can present with chronic cough. Coughing associated with these infections can persist for several months.
Functional disorders can coexist with or mimic asthma and include vocal cord dysfunction (VCD) and psychogenic cough. VCD usually presents in adolescence with upper airway (laryngeal) inspiratory or expiratory stridor, or both, which may be difficult to distinguish from lower airway wheezing. The diagnosis of VCD is confirmed by laryngoscopy demonstrating paradoxical adduction of the vocal cords during inspiration.16, 17, 18, 19 Psychogenic cough is a habitual cough that can also persist for months, and it often occurs after an acute respiratory illness.20 Habitual cough has a characteristic sound described as barky or honking. The cough is exaggerated by stress or attention to the cough and disappears with sleep.20 These features help distinguish this entity from cough-variant asthma. A key feature of VCD and psychogenic cough is lack of response to asthma therapy.16, 17, 18, 19, 20 In addition, they are not associated with hypoxia.
EVALUATION
The initial evaluation of a patient presenting with an asthma exacerbation should include assessment of the acute respiratory symptoms, signs or symptoms of coexisting or precipitating conditions, and treatments initiated before presentation. A history should be obtained of the characteristics of the patient's asthma symptoms, the pattern and frequency of the symptoms, and any precipitating or aggravating factors, as well as features indicative of the severity and level of control of the asthma (Table 75-1, Table 75-2 ).4
Table 75-1.
Questions to Ask Patients Who Present with Wheezing
|
Types of Symptoms Cough Wheeze Shortness of breath Chest tightness Sputum production |
|
Frequency of Symptoms Daily, weekly, none Perennial, seasonally Do they have a night cough? Do they cough with activity? How often do they use their albuterol? |
|
Severity of Symptoms How often do they have flares of their asthma? How many times in the last year? How many times have they used oral steroids? How many times in the last year? How many emergency room visits? How many visits to the hospital? Have they ever been in the intensive care unit? |
Table 75-2.
Features That Place Patient at Risk for Severe Asthma
| History of respiratory failure with asthma |
| Recent or multiple emergency department visits or hospitalizations (<6 months) |
| Daily oral steroid use at the time of exacerbations |
| Comorbid psychosocial conditions that interfere with administration of medications |
The physical examination provides clues to the severity of the current illness, as well as the presence of comorbid conditions. Important physical parameters include the respiratory rate, work of breathing, air entry, wheezing, and oxygen saturation. Work of breathing refers to the use of accessory muscles of respiration and involves nasal flaring, abdominal retractions, and depth of respiration.
During an exacerbation of asthma, physical findings may vary and evolve with treatment or progression of the acute condition. A quiet or silent chest is a worrisome sign because poor movement of air can be associated with respiratory insufficiency or failure. Asymmetry of auscultatory findings may indicate other conditions. Unequal breath sounds can be found with pneumonia, pleural effusion (especially in dependent regions of the lung), or atelectasis. Unilateral breath sounds may indicate an aspirated foreign body or pneumothorax on the side with diminished breath sounds4 and may be accompanied by hyperresonance on that side, especially if significant air trapping is present.
Chest radiographs are not typically needed for patients with known asthma and a straightforward asthma exacerbation.21 Typical radiographic findings include hyperinflation, peribronchial thickening, and atelectasis (Fig. 75-1 ). Chest radiographs may be helpful when there is concern for pneumonia, pleural effusion, pneumothorax, pneumomediastinum, or foreign body aspiration.
Figure 75-1.
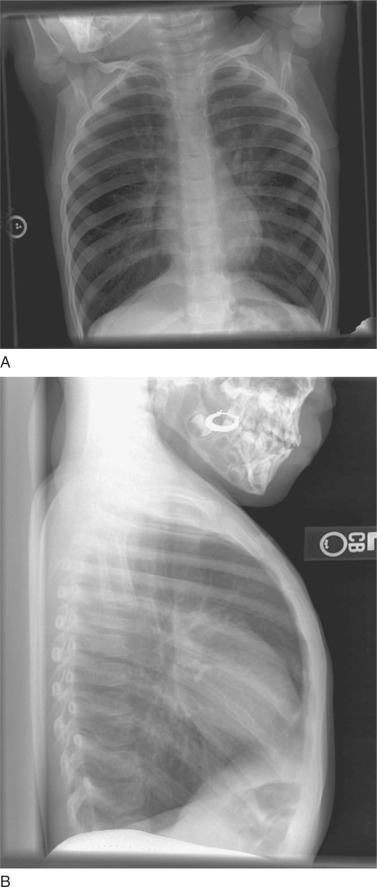
Typical radiographic findings of hyperinflation and peribronchial thickening in a patient with an acute asthma exacerbation. Flattening of the diaphragms is prominent in both the anteroposterior (A) and lateral (B) view. The lateral view demonstrates a widened anteroposterior diameter and increased prominence of the retrocardiac space.
A classification system for determining the severity of an asthma exacerbation in children 5 years of age or older is provided in Table 75-3 . Patients in mild distress typically have slightly increased respiratory rates, may not use accessory muscles of respiration, and have end-expiratory wheezes with good air entry. Patients in severe distress are working hard to breathe, with inspiratory and expiratory wheezing, and are often hypoxic. Signs of impending respiratory failure are provided in Table 75-4 . For infants and children younger than 5 years of age, clues to breathlessness include difficulty or reluctance to feed and changes in crying pattern (e.g., softer or shorter). Changes in vital signs in these younger patients must be interpreted in the context of normal values for the age range. Interestingly, paradoxical thoracoabdominal movement, a sign associated with severe respiratory distress in older children, may be seen in young children and infants, even in states of mild or moderate respiratory distress.
Table 75-3.
Clinical Classification of Severity for Asthma Exacerbation
|
Severity of Exacerbation |
||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Impending Respiratory Failure | |
| Symptoms | ||||
| Breathlessness | While walking | While talking (infants: softer, shorter cry; difficulty feeding) | While at rest (infants: stop feeding) | |
| Positioning | Can lie down | Prefers sitting | Sits upright | |
| Speaks in | Sentences | Phrases | Words | |
| Alertness | May be agitated | Usually agitated | Usually agitated | Drowsy or confused |
| Signs | ||||
| Respiratory rate | Increased | Increased | Often >30/min | |
| Use of accessory muscles, suprasternal retractions | Usually not | Commonly | Usually | Paradoxical thoracoabdominal movement |
| Wheezing | Moderate, often only end expiratory | Loud, throughout exhalation | Usually loud, throughout inhalation and exhalation | Absence of wheezing |
| Pulse/min | <100 | 100-120 | >120 | Tachycardia or bradycardia |
| Pulsus paradoxus | Absent (<10 mm Hg) | May be present (10-25 mm Hg) | Often present (>25 mm Hg for an adult, 20-40 mm Hg for a child) | Absence suggests respiratory muscle fatigue |
| Functional Assessment | ||||
| PEF, % predicted or % personal best | 80% | ∼50%-80% | <50% of predicted or personal best | |
| PaO2 (on room air) | Normal (test not usually necessary) | >60 mm Hg (test not usually necessary) | <60 mm Hg, possible cyanosis | |
| And/or PaCO2 | <42 mm Hg | <42 mm Hg | >42 mm Hg, possible respiratory failure | |
| SaO2 (on room air) at sea level | >95% | 91%-95% | <91 % | |
Asthma exacerbation usually includes several parameters, but not necessarily all. These parameters serve only as general guidelines because many have not been systemically studied.
Adapted from Moss MH, Gern JE, Lemanske RF Jr: Asthma in infancy and childhood. In Adkinson NF Jr, Yunginger JW, Busse WW, et al (eds): Middleton's Allergy Principles and Practice, 6th ed. Philadelphia, CV Mosby, 2003. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.p107.
Table 75-4.
Indicators of Impending Respiratory Failure
| Poor air movement or silent chest in combination with increased respiratory effort, decreased respiratory rate, or disorganized breathing pattern |
| Inability to speak |
| Inability to lie supine |
| Deteriorating mental status, lethargy, or agitation |
| Diaphoresis |
| Respiratory or cardiac arrest |
Objective measures for evaluation of acute asthma include pulmonary function testing, pulse oximetry, and arterial blood gases. Patients with exacerbations of asthma are at risk for hypoxemia. As a result, patients require frequent monitoring to ensure adequate oxygenation. Continuous pulse oximetry is recommended during a severe exacerbation, whereas intermittent oximetry may be acceptable as the clinical course improves.
Arterial blood gas parameters are typically obtained in critically ill patients and those with clinical deterioration or signs of respiratory insufficiency or failure. Arterial blood gases may reveal hypoxemia from ventilation-perfusion mismatch and respiratory alkalosis with hypocapnia secondary to hyperventilation. A normal or elevated partial pressure of carbon dioxide (Paco2) may be the harbinger of respiratory failure22 and may be associated with decreased blood pH because of respiratory acidosis.
Pulmonary function tests can be used to assess lung function even during an asthma exacerbation. Spirometric indices such as forced expiratory volume in 1 second (FEV1) or the peak expiratory flow rate (PEFR) are most useful to assess the severity of asthma. However, because spirometry is often not readily available in the acute care setting, PEFR can be used instead. The hand-held peak flowmeter measures PEFR, and normal values have been established according to age, gender, and height23 (Table 75-5 ). PEFR provides a measure of large-airway flow by measuring the rate of airflow in liters per minute. As a flare or asthma exacerbation worsens, PEFR typically becomes lower than baseline and may reflect the severity of the exacerbation. In patients presenting to an emergency room with an asthma exacerbation, FEV1 is typically 30% to 35% of normal24 and PEFR is less than 50% of normal. Monitoring PEFR can also assist in tapering medication during the recovery phase of an acute hospitalization. PEFR is effort and technique dependent, and therefore reliability remains a concern. It should be used in conjunction with other parameters of severity for assessment of patients (see Chapter 80).
Table 75-5.
Predicted Average Peak Expiratory Flow (L/min): Normal Children and Adolescents
|
Height |
Height |
Height |
||||||
|---|---|---|---|---|---|---|---|---|
| in | cm | Males and Females | in | cm | Males and Females | in | cm | Males and Females |
| 43 | 109 | 147 | 51 | 130 | 254 | 59 | 150 | 360 |
| 44 | 112 | 160 | 52 | 132 | 267 | 60 | 152 | 373 |
| 45 | 114 | 173 | 53 | 135 | 280 | 61 | 155 | 387 |
| 46 | 117 | 187 | 54 | 137 | 293 | 62 | 157 | 400 |
| 47 | 119 | 200 | 55 | 140 | 307 | 63 | 160 | 413 |
| 48 | 122 | 214 | 56 | 142 | 320 | 64 | 162 | 427 |
| 49 | 124 | 227 | 57 | 145 | 334 | 65 | 165 | 440 |
| 50 | 127 | 240 | 58 | 147 | 347 | 66 | 168 | 454 |
This table is a guideline. National Heart, Lung, and Blood Institute guidelines suggest using a personal best as baseline values.
From Polgar G, Promahcat V: Pulmonary Function Testing in Children. Techniques and Standards. Philadelphia, WB Saunders, 1971.
TREATMENT
Exacerbations of asthma are treated with a combination of supportive therapy and pharmacologic interventions. Treatment is tailored to the severity of symptoms and adjusted according to the patient's response to therapy. Adequate hydration should be established and maintained either orally or with intravenous fluids. Physiologic monitoring should include vital signs and pulse oximetry. Oxygen supplementation is provided to maintain oxygen saturation in a safe range. This range is widely debated, but most agree that levels greater than 91% are needed, and many target levels to greater than 93% to 95%.
Adrenergic Agonists
This class of medications works by stimulating the β2-adrenergic receptor and causing activation of adenyl cyclase, which increases the production of cyclic 3′,5-adenosine monophosphate (cAMP). This increase in cAMP, depending on the site of stimulation, results in relaxation of bronchial smooth muscle, stimulation of skeletal and cardiac muscle, and inhibition of the release of inflammatory mediators through stabilization of the mast cell membrane. Albuterol is one of the short-acting β2-adrenergic agents used as first-line therapy for an acute asthma exacerbation because of its ability to rapidly open the airways. Albuterol can be administered by nebulizer, either continuously or intermittently, or by metered-dose inhaler (MDI) with a spacer device. Studies have compared the amount of medication delivered to the lungs when given by MDI with spacer versus nebulizer.25, 26, 27 The two modes are considered equivalent if the patient can use proper technique with the MDI-spacer method of delivery. Dosing information is provided in Table 75-6 .
Table 75-6.
Dosages of Bronchodilators Commonly Used for Asthma Exacerbations
| Medications | Adult Dose | Child Dose | Onset of Action | Duration | Comments |
|---|---|---|---|---|---|
| Inhaled Short-Acting β2-Agonists | |||||
| Albuterol nebulizer 5.0 mg/mL 2.5 mg/3 mL 1.25 mg/3 mL 0.63 mg/3 mL |
2.5-5.0 mg every 20 minutes for 3 doses, then 2.5-10 mg every 1-4 hours as needed or 10-15 mg/hr continuously | 0.15 mg/kg (minimum dose, 2.5 mg) every 20 minutes for 3 doses, then 0.15-0.3 mg/kg up to 10 mg every 1-4 hours as needed or 0.5 mg/kg/hr by continuous nebulization | 15 minutes | 3-4 hours | Only selective β2-agonists are recommended. For optimal delivery, dilute aerosols to minimum of 4 mL at gas flow rates of 6-8 L/min |
| Albuterol via MDI 90 μg/puff |
2-8 puffs every 20 minutes up to 4 hours, then every 1-4 hours as needed | 2-8 puffs every 20 minutes for 3 doses, then every 1-4 hours inhalation maneuver. A spacer or holding chamber should be used | 15 minutes | 3-4 hours | As effective as nebulized therapy if patient is able to coordinate |
| Levalbuterol via nebulizer 0.31 mg/3 mL 0.63 mg/3 mL 1.25 mg/3 mL |
Adults: 0.63 mg 3 times/day, may be increased to 1.25 mg | Children 6-11 years: 0.31 mg 3 times/day every 6-8 hours Children >12 years: 0.63 mg 3 times/day, may be increased to 1.25 mg |
15 minutes | 5-6 hours | 0.63 mg of levalbuterol is equivalent to 1.25 mg of racemic albuterol in both efficacy and side effects |
| Levalbuterol via MDI | 1-2 puffs every 4-6 hours as needed | 1-2 puffs every 4-6 hours as needed | 5-10 minutes | 3-6 hours | Children 2-11 years: in a randomized, double-blind, single-dose, crossover study, doses ranging from 0.16 to 1.25 mg were used safely with clinically significant improvements in pulmonary function test values |
| Anticholinergics | |||||
| Ipratropium bromide Nebulizer solution (0.25 mg/mL) |
0.5 mg every 30 minutes for 3 doses, then every 2-4 hours as needed | 0.25 mg every 20 minutes for 3 doses, then every 2-4 hours | 1-3 minutes | 3-6 hours | May mix in same nebulizer with albuterol. Should not be used as first-line therapy. Should be added to β2-agonist therapy |
| MDI (18 μg/puff) | 2-8 puffs as needed | 4-8 puffs as needed | 1-3 minutes | 3-6 hours | Dose in MDI is low and has not been studied in asthma exacerbations |
MDI, metered-dose inhaler.
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 96-4051). Bethesda, MD, U.S. Department of Health and Human Services, National Institutes of Health, 1997, 2002.
Paradoxical and transient worsening of hypoxia because of increased ventilation-perfusion mismatching can be seen with the administration of albuterol. The medication causes increased cardiac output, which leads to increased perfusion of unventilated lung.28 Other side effects include sinus tachycardia, tremor, palpitations, headache, agitation, and ventricular irritability (e.g., ventricular premature contractions, ventricular tachycardia). In addition, because frequent or continuous dosing with adrenergic agents can lead to hypokalemia, patients receiving such treatment should have serum potassium levels checked periodically. Nonselective adrenergic agents (e.g., epinephrine) can also cause transient hyperglycemia and elevations in the neutrophil count as a result of demargination.
Albuterol is actually a racemic mixture of R-albuterol and S-albuterol, with a 50:50 ratio of these two stereoisomers. Levalbuterol (Xopenex) is made up of the R-isomer, which is thought to be the active component of the racemic product. However, S-albuterol has been found to have some bronchoconstrictive activity in select studies, but not in others, and demonstrates activation of eosinophils in vitro. In addition, S-albuterol is cleared much less rapidly, which can cause buildup of this isomer in vivo as opposed to the L-isomer. However, the vast majority of clinical studies and in vitro pharmacology data have shown no significant differences in cardiopulmonary side effects and tremor when comparing racemic with R-isomer albuterol.29, 30, 31 One study found decreased rates of admission from an emergency department with the use of levalbuterol versus racemic albuterol.32 Another study showed improved bronchodilation,33 but these findings have not been confirmed in other studies.29, 30, 34
Terbutaline, a selective β2-adrenergic agonist, and epinephrine, a nonselective adrenergic agonist, are used in asthmatics not responding to albuterol and corticosteroids or those who are deteriorating. These medications are given by subcutaneous injection or intravenous infusion. Bronchodilation is seen within 5 minutes of administration and can persist for 3 to 4 hours.35, 36 Terbutaline can also be given via continuous intravenous infusion by starting with a bolus and titrating the dose to the desired effect.
Dosing of β2-adrenergic agonists and other bronchodilators is shown in Table 75-6, Table 75-7 .
Table 75-7.
Systemic (Injected) Bronchodilators for Acute Asthma Exacerbations
| Medications | Adult Dose | Child Dose | Onset of Action | Duration | Comments |
|---|---|---|---|---|---|
| ß2-Agonists | |||||
| Epinephrine 1 : 1000 (1 mg/mL) by IV infusion | 0.3-0.5 mg every 20 minutes for 3 doses SC Loading dose: 2-10 μg/kg, followed by continuous infusion of 0.08-0.4 μg/kg/ min; titrate dose by clinical response up to 6 μg/kg/min |
0.01 mg/kg up to 0.3-0.5 mg every 20 minutes for 3 doses SC | 1-3 minutes | 30 minutes | No proven advantage of systemic therapy over aerosol |
| Terbutaline (1 mg/mL) by IV infusion | 0.25 mg every 20 minutes for 3 doses SC Loading dose: 2-10 μg/kg followed by continuous infusion of 0.08-0.4 μg/kg/min; titrate dose by clinical response up to 6 μg/kg/min |
0.01 mg/kg every 20 minutes for 3 doses, then every 2-6 hours as needed SC Loading dose: 2-10 μg/kg followed by continuous infusion of 0.08-0.4 μg/kg/min; titrate dose by clinical response up to 6 μg/kg/min |
SC: 6-15 minutes | SC: 1.5-4 hours | |
Corticosteroids
Corticosteroids are indicated for the initial treatment of status asthmaticus. They are potent anti-inflammatory medications that have been shown to hasten recovery, prevent recurrence,37, 38, 39, 40, 41 and prevent hospitalizations.42 Because of their mechanism of action, the effect of corticosteroids is not immediate. Steroids bind to the intracytoplasmic glucocorticoid receptor and translocate to the nucleus, where they effect RNA transcription in both positive and negative fashion through the transcription factors NF-κB and AP-1. In general, steroids lead to down-regulation of inflammatory cytokines. Corticosteroids also activate histone deacetylase, which inhibits DNA transcription.43 This change in transcription leads to increased expression of the β2-adrenergic receptor and decreases in airway inflammation and mucus secretion. It can take several hours to reverse airway inflammation, and benefits are typically seen within 4 hours after the administration of corticosteroids.38, 39, 44 Studies comparing oral and intravenous corticosteroids have found no significant differences in efficacy.45, 46 Oral steroids are typically preferred because intravenous access is not required.45, 47 Suggested dosing of corticosteroids is provided in Table 75-8 .
Table 75-8.
Systemic Corticosteroids in the Setting of Asthma Exacerbations
| Medication | Adult Dose | Child Dose (≤ 12 Years Old) | Onset of Action | Duration | Comments |
|---|---|---|---|---|---|
| Oral—prednisone/ prednisolone IV— methylprednisolone |
120-180 mg/day in 3-4 divided doses for 48 hours, then 60-80 mg/day until PEF reaches 70% of predicted or personal best | 1 mg/kg every 6 hours for 48 hours, then 1-2 mg/kg/day (maximum, 60 mg/ day) in 2 divided doses until PEF is 70% of predicted or personal best | 1-4 hours (variable) 1-4 hours (variable) |
12-36 hours | For outpatient burst for 3-10 days:
|
| IM methylprednisolone 40 mg/mL 80 mg/mL | 240 mg IM once | 7.5 mg/kg IM once | 1-4 hours (variable) | 36-72 hours | IM should be used in place of a short burst of oral steroids in patients who are vomiting or if adherence is a problem |
PEF, peak expiratory flow.
Adapted From National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 96-4051). Bethesda, MD, U.S. Department of Health and Human Services, National Institutes of Health, 1997. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthmafullrpt.pdf.
Inhaled Anticholinergic Agents
Anticholinergic agents work by competitively inhibiting acetylcholine at the muscarinic junction to relieve the cholinergic-mediated bronchoconstriction. Nebulized atropine is associated with significant systemic absorption, but anticholinergic medications such as ipratropium bromide have fewer side effects and less systemic absorption.48
The use of inhaled ipratropium in the initial phase of treatment has been shown to be effective in reducing the need for hospitalization. A few studies have found no benefit in comparison to β2-agonists alone, whereas others have shown a slight advantage of one to three doses in the initial phase of an acute asthma exacerbation.49 The role of ipratropium in hospitalized patients is less clear, but an initial study did not show clinical benefit.50
Combined administration of anticholinergic and β2-agonist medications increases bronchodilation, although some controversy about this effect persists.49, 50 Nonetheless, many institutions use a combination of β2-agonists and anticholinergic medications during the initial phase of acute asthma exacerbations. Studies examining the use of anticholinergic medications as monotherapy have also been controversial. Dosing is listed in Table 75-6.
Nonstandard Therapies
If initiation of the aforementioned standard therapies does not improve the level of respiratory distress or if symptoms progress, additional interventions may be necessary. The clinical experience and expertise available at the particular institution should be considered in such decisions. Safe transfer to a facility able to provide critical care management should be anticipated, and arrangements should be expedited.
Magnesium Sulfate
Magnesium sulfate has been studied as a bronchodilator in severe asthma, with conflicting results.51, 52, 53, 54 Magnesium is thought to inhibit mast cell degranulation and increase bronchial dilation because of a decrease in calcium uptake by bronchial smooth muscle.55 Its use is considered when a patient fails to improve or worsens despite treatment with continuous inhaled β2-agonists, systemic corticosteroids, and inhaled anticholinergic agents.
Methylxanthines
Intravenous methylxanthines, such as aminophylline, were commonly used in the past to manage asthma exacerbations because of their ability to act directly on β-adrenergic receptors and relax bronchial smooth muscle. Concern regarding the toxicity and efficacy of this class of medication and the availability of newer agents have limited its use. Methylxanthines may help prevent acute airway hyperresponsiveness but do not appear to produce these effects chronically.56, 57, 58 However, life-threatening events such as cardiac arrhythmia and seizures are associated with toxic levels of theophylline (>30 μg/mL). As a result, methylxanthines are recommended only as adjunctive therapy with close monitoring of serum concentrations and cardiac monitoring.
Studies examining the use of intravenous methylxanthines in children and adults with severe asthma have shown mixed benefit.59, 60, 61, 62, 63, 64 A recent Cochrane review found that theophylline in addition to β2-agonists and glucocorticoids (with or without anticholinergics) improves lung function within 6 hours of treatment. However, there is no apparent reduction in symptoms, number of nebulized treatments, and length of hospital stay.65
Aminophylline requires a loading dose followed by a continuous infusion to reach and maintain a therapeutic level (see Table 75-9 ). Dosing is titrated according to serum level, clinical efficacy, and side effects.
Table 75-9.

Nonstandard Therapies for Exacerbations of Asthma
Rights were not granted to include this table in electronic media. Please refer to the printed book.
Heliox
Heliox is a mixture of helium and oxygen used for inhalation. This agent is thought to improve airflow by creating gas with similar viscosity to air but with lower density, which in turn can increase ventilation and decrease work of breathing.66, 67, 68 Heliox is indicated in patients with a refractory exacerbation of asthma in whom respiratory failure is impending. Patients with high oxygen requirements may not be able to tolerate heliox because they need a higher Fio2 than a helium-oxygen mixture can provide. Heliox can also lower body temperature because of the high thermal conductivity of the mixture. Therefore, patients need to have their temperature monitored closely.
Dosing of nonstandard therapies is shown in Table 75-9, and adverse effects of medication are listed in Table 75-10 .
Table 75-10.
Common Side Effects of Pharmacologic Therapies
| Side Effect | Possible Causative Agent | Comment |
|---|---|---|
| Hypokalemia | Adrenergic agonists | Patients using prolonged hourly or continuous inhaled therapy or intravenous therapy should have serum potassium levels monitored |
| Tremor/agitation | Adrenergic agonists | Dose dependent |
| Hypertension | Corticosteroids, adrenergic agonists | May require reduction of dose, discontinuation of therapy, or addition of antihypertensive medication |
| Tachycardia, palpitations, ventricular premature contractions | Adrenergic agonists, aminophylline, theophylline | Usually dose dependent. Serum levels of methylxanthines should be monitored. The risk is increased with hypoxemia or acidemia |
| Hyperglycemia/glucosuria | Corticosteroids, adrenergic agonists | Resolves with completion or discontinuation of therapy |
| Emotional lability | Corticosteroids | Resolves with completion or discontinuation of therapy |
| Hyperphagia | Corticosteroids | Resolves with completion or discontinuation of therapy |
| Seizure | Theophylline, aminophylline | Serum levels of methylxanthines should be monitored. Risk is increased in the presence of acidosis |
| Elevated peripheral neutrophil count | Corticosteroids, adrenergic agonists (in particular, epinephrine) | May interfere with utility of the white blood count in assessing for infection |
Initial Treatment
The initial therapy for status asthmaticus has been outlined by the NHLBI guidelines (Fig. 75-2 ). In brief, patients are first treated with inhaled β2-adrenergic agonists (e.g., inhaled albuterol), corticosteroids either orally or intravenously, and if needed, oxygen. Inhaled anticholinergics, such as ipratropium bromide, may be added for patients who do not demonstrate prompt improvement. Patients with significant improvement after these initial interventions may not require hospitalization.
Figure 75-2.

NHLBI hospital assessment and management of acute exacerbation. PEF, peak expiratory flow; FEV1, forced expiratory volume in 1 second.
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma [NIH Publication No. 96-4051]. Bethesda, MD, U.S. Department of Health and Human Services, National Institutes of Health, 1997.
© 2007
Hospitalization is recommended for patients who continue to have moderately severe or severe symptoms after initial intervention. Treatment with inhaled albuterol (either every 1 to 2 hours by nebulizer or MDI with spacer or delivered continuously by nebulizer) and corticosteroid therapy (orally or, if not tolerated, intravenously) should be continued. Continuation of inhaled anticholinergic agents may be considered, although their benefit remains unproven.
If patients continue to deteriorate, they must be monitored for respiratory insufficiency and failure. An arterial blood gas measurement can be used to confirm the condition and should reveal decreased pH, elevated partial pressure of carbon dioxide (Paco2), and an increased alveolar-arterial oxygen gradient. Pulse oximetry remains a poor monitoring device for early detection of respiratory failure. Oxygen saturation is initially maintained despite a significant degree of hypoventilation, and the addition of supplemental oxygen would further obscure evidence of respiratory failure from this device (Chapter 66). Patients with impending respiratory failure often need mechanical ventilatory support.
Tapering Hospital Therapy
After patients are stabilized and demonstrate improvement, therapies can be gradually reduced and withdrawn. Ongoing assessment of clinical parameters is performed, including the respiratory rate, work of breathing, auscultatory findings, and requirement for supplemental oxygen. If the patient remains comfortable with minimal signs of respiratory distress, the dosing of inhaled β2-agonists is decreased. For patients receiving continuous inhaled β2-agonist therapy, the dose may be reduced and then subsequently transitioned to intermittent treatments, usually every 2 hours. As the patient continues to improve, the interval between treatments can be extended. Similarly, the amount of supplemental oxygen is titrated to maintain oxygen saturation above the desired level and eventually discontinued. Systemic corticosteroids are continued throughout the exacerbation and maintained for several days after discharge from the hospital. If inhaled anticholinergic agents have been instituted, they are usually discontinued when albuterol begins to be tapered.
Many hospitals use clinical pathways, which are tools that detail a sequence of assessments and treatments for patients with various conditions.69 Studies have shown that asthma clinical pathways shorten hospitalization and decrease the need for readmission for up to 2 weeks after discharge.70, 71 An asthma clinical pathway allows multiple caregivers, including nurses, respiratory therapists, and doctors, to modify treatment based on structured assessments. The NHLBI guidelines (see Fig. 75-2) outline specific criteria that can be used to determine a patient's severity and frequency of therapy. It also provides criteria to assist in weaning treatments.
PEFR measurements may be useful to determine readiness for reduction in medication. If PEFR is at least 70% of baseline before a bronchodilator treatment (see Table 75-5), it is appropriate to space the frequency of the β2-adrenergic agonist treatments. Technique and effort will affect measurement of PEFR; therefore, it should be used in conjunction with other clinical indicators of improvement.
Therapy after Discharge Home
Patients should be sent home on a regimen of oral corticosteroids, the duration of which depends on the length and severity of illness and the patient's frequency of exacerbations. In general, an isolated exacerbation is treated with oral corticosteroids for 5 days. However, if a patient was admitted to the hospital for an extended period, a prolonged course of corticosteroids will be required, followed by tapering doses. A taper is prescribed to prevent relapse of symptoms, as well as to prevent an addisonian crisis from adrenal suppression. The risk for an addisonian crisis is hypothetical and has not been demonstrated in any study.72, 73, 74 In addition, patients receiving their second course of steroids in a month should undergo prolonged tapering as well. A typical taper involves keeping the patient at a full daily dose of corticosteroids until stable clinical status is achieved and then decreasing the dose by 30% to 50% daily. Patients who required admission to the hospital may not have been on an adequate treatment plan. Thus, hospitalization offers an opportunity to assess the overall treatment regimen. Patients should be evaluated according to the NHLBI guidelines shown in Table 75-11A, Table 75-11B and need their outpatient preventive treatment stepped up. Specific drug choices are outlined in Table 75-12, Table 75-13, Table 75-14 .
Table 75-11A.
Stepwise Approach for Managing Infants and Young Children (5 Years and Younger) with Acute or Chronic Asthma
 |
DPI, dry powder inhaler.
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program: Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics (NIH Publication No. 02-5075). Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, 2002. Available at http://www.nhlbi.nih.gov/guidelines/asthma/execsumm.pdf.
© 2007
Table 75-11B.
Stepwise Approach for Managing Asthma in Adults and Children Older Than 5 Years: Treatment
 |
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program: Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics (NIH Publication No. 02-5075). Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, 2002. Available at http://www.nhlbi.nih.gov/guidelines/asthma/execsumm.pdf.
© 2007
Table 75-12.
Preferred Treatment of Asthma According to Severity
| Severity | Preferred Treatment | Alternative Treatments | Comments |
|---|---|---|---|
| Intermittent | No daily medication needed | Severe exacerbations may occur, separated by long periods of no symptoms. Treat with a course of systemic corticosteroids | |
| Mild persistent | Low-dose inhaled corticosteroid | Cromolyn Leukotriene modifier Nedocromil Sustained-release theophylline (level, 5-15 μg/mL) |
|
| Moderate persistent | Low- to medium-dose inhaled corticosteroid and long-acting inhaled ß2-agonist | Increased inhaled corticosteroid within medium-dose range or low- to medium-dose inhaled corticosteroid and either a leukotriene modifier or theophylline | If exacerbations occur despite daily medication, increase the inhaled corticosteroid and add a long-acting inhaled ß2-agonist |
| Severe persistent | High-dose inhaled corticosteroids and long-acting inhaled ß2-agonists | ||
Adapted from Bacharier LB, Strunk RC: Asthma in older children. In Leung DYM, Sampson HA, Gehr RS, Szefler SJ (eds): Pediatric Allergy: Principles and Practice. Philadelphia, CV Mosby, 2003, p 416.
Table 75-13.
Usual Dosages for Long-Term Control Medications
| Medication | Dosage Form | Adult Dose | Child Dose |
|---|---|---|---|
|
Inhaled Corticosteroids (see estimated daily dosages for inhaled corticosteroids) Systemic Corticosteroids | |||
| Methylprednisolone Prednisolone |
2-, 4-, 8-, 6-, 32-mg tablets 5-mg tablets 5 mg/5 mL, 15 mg/5 mL |
7.5-60 mg daily in a single dose in AM or qod as needed for control | 0.25-2 mg/kg daily as single dose in AM or qod as needed for control |
| Prednisone | 1-, 2.5-, 5-, 10-, 20-, 50-mg tablets 5 mg/mL, 5 mg/5 mL |
Short-course “burst” to achieve control: 40-60 mg/day as single or 2 divided doses for 3-10 days | Short-course “burst”: 1-2 mg/kg/day, maximum of 60 mg/day, for 3-10 days |
| Long-Acting Inhaled ß2-Agonists (should not be used for symptom relief or for exacerbation; use with inhaled corticosteroids) | |||
| Salmeterol Formoterol |
MDI: 21 μg/puff DPI: 50 μg/blister DPI: 12 μg/single-use capsule |
2 puffs q12h 1 blister q12h 1 capsule q12h |
1-2 puffs q12h 1 blister q12h 1 capsule q12h |
| Combined Medication | |||
| Fluticasone/salmeterol | DPI: 100, 250, or 500 μg/50 μg | 1 inhalation bid; dose depends on severity of asthma | 1 inhalation bid; dose depends on severity of asthma |
| Cromolyn and Nedocromil | |||
| Cromolyn Nedocromil |
MDI: 1 mg/puff Nebulizer: 20 mg/ampule MDI: 1.75 mg/puff |
2-4 puffs tid-qid 1 ampule tid-qid 2-4 puffs bid-qid |
1-2 puffs tid-qid 1 ampule tid-qid 1-2 puffs bid-tid |
| Leukotriene Modifiers | |||
| Montelukast Zafirlukast Zileuton |
4- or 5-mg chewable tablet 10-mg tablet 10- or 20-mg tablet 300- or 600-mg tablet |
10 mg qhs 40 mg daily (20-mg tablet bid) 2400 mg daily (give tablets qid) |
4 mg qhs (2-5 yr) 5 mg qhs (6-14 yr) 10 mg qhs (>14yr) 20 mg daily (7-11 yr) (10-mg tablet bid) |
| Methylxanthines (serum monitoring is important [serum concentration of 5-15 μ g/mL at steady state]) | |||
| Theophylline | Liquids, sustained-release tablets, and capsules | Starting dose, 10 mg/kg/day up to 300 mg max; usual max, 800 mg/day | Starting dose, 10 mg/kg/day; usual usual max: <1 yr of age: 0.2 (age in wk) + 5 = mg/kg/day ≥1 yr of age: 16 mg/kg/day |
DPI, dry powder inhaler; MDI, metered-dose inhaler.
Modified from National Heart, Lung, and Blood Institute, Executive Summary of NAEPP Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics (NIH Publication No. 02-5075). Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, 2002.
Table 75-14.
Estimated Comparative Daily Dosages for Inhaled Corticosteroids
| Low Daily Dose | Medium Daily Dose | High Daily Dose | ||||
|---|---|---|---|---|---|---|
| Drug | Adult | Child* | Adult | Child* | Adult | Child* |
| Beclomethasone HFA 40 or 80 μg/puff | 80-240 μg | 80-160 μg | 240-480 μg | 160-320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200-600 μg | 200-400 μg | 600-1200 μg | 400-800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5 mg | 1.0 mg | 2.0 mg | |||
| Flunisolide 250μg/puff | 500-1000 μg | 500-750 μg | 1000-2000 μg | 1000-1250 μg | >2000 μg | >1250 μg |
| Fluticasone MDI: 44, 110, or 220 μg/puff DPI: 50, 100, or 250 μg/inhalation | 88-264 μg 100-300 μg | 88-176 μg 100-200 μg | 264-660 μg 300-600 μg | 176-440 μg 200-400 μg | >660 μg >600 μg | >440 μg >400 μg |
| Trlamcinolone acetonide 100 μg/puff | 400-1000 μg | 400-800 μg | 1000-2000 μg | 800-1200 μg | >2000 μg | >1200 μg |
DPI, dry powder inhaler.
Children ≤12 years of age.
Modified from National Heart, Lung, and Blood Institute: Executive Summary of NAEPP Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics (NIH Publication No. 02-5075). Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, 2002.
CONSULTATION
Outpatient referral to an asthma specialist (e.g., pulmonologist, allergist) is associated with reduced rates of emergency department visits75 and is recommended for patients with the following scenarios76, 77, 78:
-
•
Life-threatening asthma requiring admission to intensive care or a step-down unit
-
•
Severe persistent asthma in a patient who has required a prolonged course of steroids or multiple courses of corticosteroids in 1 year
-
•
Not meeting the goals of asthma therapy or not responding to treatment so that additional testing can be performed if necessary
Asthma specialists may be available to assist in the management of an acute asthma exacerbation as needed. Involving the specialist during a hospitalization may assist in transition after discharge. Critical care physicians should be contacted for all patients who may need management in an intensive care setting.
ADMISSION CRITERIA
Admission to the hospital is individualized and based on many factors. Hospitalization should be considered in patients with the following:
-
•
Poor response to initial treatment
-
•
Oxygen saturation less than 92% on room air
-
•
Severe asthma with a relapsing course despite prolonged corticosteroid therapy
-
•
Previous emergency visits during the current period of exacerbation
-
•
Concern for noncompliance
Admission to an intensive care unit would be appropriate for patients with the following:
-
•
Life-threatening or severe asthma that is unresponsive to initial therapy
-
•
Inability to maintain oxygen saturation greater than 92% with supplemental oxygen
-
•
Evidence of impending respiratory failure
-
•
Inability to provide adequate monitoring outside an intensive care setting79
DISCHARGE CRITERIA
Patients are ready to go home when they have been successfully weaned to albuterol treatments every 4 to 6 hours. Before receiving a treatment, they should be able to breathe comfortably during ambulation or speaking. Wheezing may persist on examination, but it should not be audible without a stethoscope. A plan of care should include ongoing management of the current acute exacerbation and transition to maintenance therapy. Additionally, patients should leave with a plan of action for management of subsequent asthma exacerbations. Efforts should be coordinated with the primary care clinician and, if involved, the asthma specialist.
Before discharge, it is important to consider the environment to which the patient will return. Preventive management plans should be reviewed with the family, including identification of any potential comorbid conditions and triggers present in the environment. Table 75-15 highlights a discharge checklist that was created by the NHLBI panel.
Table 75-15.
Hospital Discharge Checklist for Patients with Asthma Exacerbations
| Intervention | Dose/Timing | Education/Advice | MD/RN Initials |
|---|---|---|---|
| Inhaled medications (MDI + spacer/holding chamber) Beta2-agonist Corticosteroids |
Select agent, dose, and frequency (e.g., albuterol) 2-6 puffs q 3-4 hr prn Medium dose |
Teach purpose Teach technique Emphasize need for spacer/holding chamber Check patient technique |
|
| Oral medications | Select agent, dose, and frequency (e.g., prednisone 20 mg bid for 3-10 days) | Teach purpose Teach side effects |
|
| Peak flow meter | Measure AM and PM. PFF and record best of three tries each time | Teach purpose Teach technique Distribute peak flow diary |
|
| Follow-up visit | Make appointment for follow-up care with primary clinician or asthma specialist | Advise patient (or caregiver) of date, time, and location of appointment within 7 days of hospital discharge | |
| Action plan | Before or at discharge | Instruct patient (or caregiver) on simple plan for actions to be taken when symptoms, signs, and PEF values suggest recurrent airflow obstruction | |
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 96-4051). Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, 1997.
PREVENTION
Educating the family about the pathogenesis of asthma, triggers, and medications is crucial for preventing exacerbations and admissions to the hospital. A patient who requires frequent admissions needs to have the treatment plan reassessed and be evaluated for comorbid conditions. Assessment of adherence to therapy and review of relevant drug delivery systems are important as well. For children experiencing symptoms on a daily basis, there are certain controllable environmental factors, such as exposure to allergens and cigarette smoke, that can cause symptoms and contribute to asthma exacerbations.80 In addition, other factors, including gastroesophageal reflux, sinusitis, and others that might exacerbate asthma, should be explored and eliminated if possible (Table 75-16 ). If an allergic component is being considered, further evaluation can be arranged and environmental control measures recommended. Both passive and active cigarette smoking significantly increases the risk for asthma and worsens asthma symptoms.81, 82, 83, 84, 85, 86, 87, 88 As a result, no smoking should be permitted around asthmatics or in their home or family car. Physicians should provide assistance for caregivers to quit smoking.
Table 75-16.
Exacerbating Factors for Asthma and Control Measures
| Factors That Worsen Asthma Severity | Control Measures |
|---|---|
| Animal dander | Remove the animal from the environment At a minimum, remove the pet from the bedroom |
| House dust mites | Encase mattress and pillows in an allergen-impermeable cover Wash bedding in hot water weekly at >130° F Remove carpets from the bedroom |
| Cockroaches | Exterminate! Do not leave garbage and food exposed |
| Pollen | During pollen season, stay indoors with windows closed, especially in the afternoon |
| Mold | Fix leaks, eliminate water sources Clean moldy surfaces |
| Cigarette/tobacco smoke | Encourage family members and caregivers to smoke outside and cease smoking |
| Sinusitis | Promote sinus drainage Antibiotic therapy when appropriate |
| Gastroesophageal reflux | No eating 3 hr before bedtime Elevate head of bed 6-8 inches Appropriate medications: H2 receptor antagonist |
| Medications | No beta-blockers Aspirin and NSAIDs in combination with severe persistent asthma, nasal polyps, and aspirin sensitivity increase the risk for a reaction |
| Viral infections | Annual influenza vaccination |
| Irritants | Decrease exposure to wood-burning stoves, fireplaces, unvented stoves or heaters, perfumes, cleaning agents, sprays |
NSAIDs, nonsteroidal anti-inflammatory medications.
Adapted from National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program Expert Panel II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 96-4051). Bethesda, MD, US Department of Health and Human Services, National Institute of Health, 1997.
Appropriate preventive medications based on daily symptoms and frequency of exacerbations should be maintained on a daily basis. These medications are essential for the prevention of asthma flares. Multiple studies have shown a strong negative correlation between hospital admission for asthma and the use of daily inhaled corticosteroids. Suggested doses and regimens have been established through national and worldwide collaboration between primary physicians, allergists, pulmonologists, and others. An example is seen in Table 75-12, Table 75-13, Table 75-14.
Peak flowmeters can be helpful in managing asthma, if used appropriately.89 However, studies comparing peak flow monitoring with symptom recognition show no benefit of peak flow–based action plans over those based on symptoms alone.89, 90, 91 Peak flowmeters have numeric indicators that allow the creation of green, yellow, and red zones (see Table 75-5). Green represents the “good” or “all clear” zone and is 80% to 100% of a child's personal best. The yellow zone is 50% to 80% of the personal best and indicates a time when the family should be “cautious” because the child may be having or is at risk for asthma symptoms. Asthma reliever medications should be started and contact with the physician considered. The red zone is indicated by a PEFR less than 50% of normal; it is cause for concern and requires a visit to the emergency room or a call to the doctor.4 Peak flow monitoring is typically helpful for children who are not good at recognizing symptoms of asthma. Current NHLBI guidelines recommend either a symptom-based or peak flow–based management plan.
Additional sources of support for families can include the Asthma and Allergy Foundation of America and Mothers of Asthmatics.
IN A NUTSHELL.
-
•
Asthma is a chronic disorder that results in airway inflammation and smooth muscle dysfunction and is manifested as recurrent episodes of wheezing, breathlessness, and chest tightness.
-
•
Exacerbations can be triggered by a variety of stimuli, including respiratory infections, exposure to allergens or irritants, exercise, and cold air.
-
•
Treatment of flares must be directed at decreasing airway inflammation and relieving bronchospasm while providing supportive care.
-
•
The mainstay of pharmacologic therapy includes inhaled short-acting β2-adrenergic agonist therapy and systemic corticosteroids. Supportive care includes supplemental oxygen if needed and maintenance of hydration.
-
•
Many of the pharmacologic agents have significant side effects, and therefore appropriate monitoring is required.
-
•
Patients with severe symptoms or those with moderately severe symptoms that fail to improve after initial therapy are candidates for admission to an intensive care setting.
-
•
At the time of discharge, patients should have a clear plan for ongoing treatment of the acute exacerbation and transition to maintenance therapy. In addition, an action plan for subsequent exacerbations should be in place.
ON THE HORIZON.
-
•
Asthma is a chronic inflammatory disease that affects many Americans, and researchers are actively investigating new drugs and therapies to improve the quality of life of asthmatics. Drugs that modify the immune response are currently under active investigation. An example of one of these drugs is omalizumab, a recombinant humanized anti-IgE antibody. This drug binds circulating free IgE and consequently reduces the level of free IgE in the bloodstream and prevents it from binding to mast cell membrane receptors, thus curtailing the early and late asthmatic responses. Omalizumab has been found to reduce symptoms, exacerbations,92 and the use of corticosteroids.
-
•
New NHLBI Expert Panel guidelines on the diagnosis and treatment of asthma are expected in late 2006 or early 2007. The new guidelines are expected to emphasize daily symptom control in addition to preventing exacerbations in the management of asthma.
SUGGESTED READING
- American Academy of Allergy, Asthma, and Immunology, Inc Pediatric Asthma: Promoting Best Practice. American Academy of Allergy, Asthma, and Immunology, Milwaukee. 1999 [Google Scholar]
- Lemanske RF, Busse WW. Asthma. J Allergy Clin Immunol. 2005;111:S502–S519. doi: 10.1067/mai.2003.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss MH, Gern JE, Lemanske RF., Jr . Asthma in infancy and childhood. In: Adkinson NF, Bochner BS, Yunginger JW, editors. Middleton's Allergy: Principles and Practice. 6th ed. CV Mosby; St Louis: 2003. [Google Scholar]
- National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program . Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 96-405) US Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 1997. [Google Scholar]
- National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program . Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 97-4051) US Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 1997. [Google Scholar]
- National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program . Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics (NIH Publication No. 02-5075) US Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2002. [Google Scholar]
REFERENCES
- 1.American Lung Association Trends in asthma morbidity and mortality. 2001 [Google Scholar]
- 2.National Heart, Lung, and Blood Institute, National Institutes of Health, National Asthma Education and Prevention Program, 2003.
- 3.U.S. Department of Health and Human Services (USDHHS), Centers for Disease Control and Prevention, National Center for Health Statistics: Compressed Mortality File, 2005.
- 4.National Heart, Lung, and Blood Institute . Guidelines for the Diagnosis and Management of Asthma: Expert Panel 2. National Institutes of Health; Bethesda, MD: 1997. [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute Data Fact Sheet. Asthma Statistics. 1999 [Google Scholar]
- 6.Hartert TV, Windom HH, Peebles RS., Jr Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386–394. doi: 10.1016/s0002-9343(97)89513-7. [DOI] [PubMed] [Google Scholar]
- 7.Pappas G, Hadden WC, Kozak LJ, Fisher GF. Potentially avoidable hospitalizations: Inequalities in rates between US socioeconomic groups. Am J Public Health. 1997;87:811–816. doi: 10.2105/ajph.87.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djukanovic R, Roche WR, Wilson JW. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;142:434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- 9.Murray CS, Simpson A, Custovic A. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1:99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 10.Johnston S, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9- to 11-year-old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SL, Pattemore PK, Sanderson G. The relationship be-tween upper respiratory infections and hospital admissions for asthma: A time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 12.Shim CS, Williams MH., Jr Evaluation of the severity of asthma: Patients versus physicians. Am J Med. 1980;68:11–13. doi: 10.1016/0002-9343(80)90155-2. [DOI] [PubMed] [Google Scholar]
- 13.Stein MR. Possible mechanisms of influence of esophageal acid on airway hyperresponsiveness. Am J Med. 2003;115(Suppl 3A):55S–59S. doi: 10.1016/s0002-9343(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 14.Nelson HS. Gastroesophageal reflux and pulmonary disease. J Allergy Clin Immunol. 1984;73:547–556. doi: 10.1016/0091-6749(84)90509-8. [DOI] [PubMed] [Google Scholar]
- 15.Busse WW. The role of respiratory infections in airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1994;150:S77–S79. doi: 10.1164/ajrccm/150.5_Pt_2.S77. [DOI] [PubMed] [Google Scholar]
- 16.Newman KB, Mason UG, 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152:1382–1386. doi: 10.1164/ajrccm.152.4.7551399. [DOI] [PubMed] [Google Scholar]
- 17.Christopher K, Wood RP, 2nd, Eckert RC. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308:1566–1570. doi: 10.1056/NEJM198306303082605. [DOI] [PubMed] [Google Scholar]
- 18.Tilles S. Vocal cord dysfunction in children and adolescents. Curr Allergy Asthma Rep. 2003;3:467–472. doi: 10.1007/s11882-003-0056-z. [DOI] [PubMed] [Google Scholar]
- 19.Wood R, Milgrom H. Vocal cord dysfunction. J Allergy Clin Immunol. 1996;98:481–485. doi: 10.1016/s0091-6749(96)70079-9. [DOI] [PubMed] [Google Scholar]
- 20.Irwin RS, Glomb WB, Chang AB. Habit cough, tic cough, and psychogenic cough in adult and pediatric populations: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):174S–179S. doi: 10.1378/chest.129.1_suppl.174S. [DOI] [PubMed] [Google Scholar]
- 21.Brooks LJ, Cloutier MM, Afshani E. Significance of roentgenographic abnormalities in children hospitalized for asthma. Chest. 1982;82:315–318. doi: 10.1378/chest.82.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Weiss EB, Faling LJ. Clinical significance of PaCO2 during status asthma: The cross-over point. Ann Allergy. 1968;26:545–551. [PubMed] [Google Scholar]
- 23.Polgar G, Promahcat V. Techniques and Standards. WB Saunders; Philadelphia: 1971. Pulmonary Function Testing in Children. [Google Scholar]
- 24.McFadden ER., Jr Clinical physiologic correlates in asthma. J Allergy Clin Immunol. 1986;77:1–5. [PubMed] [Google Scholar]
- 25.Idris AH, McDermott MF, Raucci JC. Emergency department treatment of severe asthma. Metered-dose inhaler plus holding chamber is equivalent in effectiveness to nebulizer. Chest. 1993;103:665–672. doi: 10.1378/chest.103.3.665. [DOI] [PubMed] [Google Scholar]
- 26.Kerem E, Levinson H, Schuh S. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr. 1993;123:313–317. doi: 10.1016/s0022-3476(05)81710-x. [DOI] [PubMed] [Google Scholar]
- 27.Colacone A, Afilalo M, Wolkove N, Kreisman H. A comparison of albuterol administered by metered dose inhaler (and holding chamber) or wet nebulizer in acute asthma. Chest. 1993;104:835–841. doi: 10.1378/chest.104.3.835. [DOI] [PubMed] [Google Scholar]
- 28.Rodriques RR. Gas exchange abnormalities in asthma. Lung. 1990;168:s599–s605. doi: 10.1007/BF02718183. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi F, Zaritisky A, Welch C. Clinical efficacy of racemic albuterol versus levalbuterol for the treatment of acute pediatric asthma. Ann Emerg Med. 2005;46:29–36. doi: 10.1016/j.annemergmed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Hardasmalani MD, DeBari V, Bithoney WJ, Gold N. Levalbuterol versus racemic albuterol in the treatment of acute exacerbation of asthma in children. Pediatr Emerg Care. 2005;21:415–419. doi: 10.1097/01.pec.0000169433.91196.6a. [DOI] [PubMed] [Google Scholar]
- 31.Asmus MJ, Hendeles L. Levalbuterol nebulizer solution: Is it worth five times the cost of albuterol? Pharmacotherapy. 2000;20:123–129. doi: 10.1592/phco.20.3.123.34776. [DOI] [PubMed] [Google Scholar]
- 32.Schreck DM, Babin S. Comparison of racemic albuterol and levalbuterol in the treatment of acute asthma in the ED. Am J Emerg Med. 2005;23:842–847. doi: 10.1016/j.ajem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Nelson HS, Bensch G, Pleskow WW. Improved bronchodilation with levalbuterol compared with racemic albuterol in patients with asthma. J Allergy Clin Immunol. 1998;102:943–952. doi: 10.1016/s0091-6749(98)70332-x. [DOI] [PubMed] [Google Scholar]
- 34.Ralston ME, Euwema MS, Knecht KR. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: A randomized controlled trial. J Emerg Med. 2005;29:29–35. doi: 10.1016/j.jemermed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Dulfano MJ, Glass P. The bronchodilator effects of terbutaline: Route of administration and patterns of response. Ann Allergy. 1976;37:357–366. [PubMed] [Google Scholar]
- 36.Nou E. A clinical comparison of subcutaneous doses of terbutaline and adrenaline in bronchial asthma. Scand J Respir Dis. 1971;52:192–198. [PubMed] [Google Scholar]
- 37.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518. [PubMed] [Google Scholar]
- 38.Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: A meta-analysis. Am J Emerg Med. 1992;10:301–310. doi: 10.1016/0735-6757(92)90007-k. [DOI] [PubMed] [Google Scholar]
- 39.Chapman KR, Verbeek PR, White JG, Rebuck AS. Effect of a short course of prednisone in the prevention of early relapse after the emergency room treatment of acute asthma. N Engl J Med. 1991;324:788–794. doi: 10.1056/NEJM199103213241202. [DOI] [PubMed] [Google Scholar]
- 40.Fanta CH, Rossing TH, McFadden ER., Jr Glucocorticoids in acute asthma. A critical controlled trial. Am J Med. 1983;74:845–851. doi: 10.1016/0002-9343(83)91076-8. [DOI] [PubMed] [Google Scholar]
- 41.Harris JB, Weinberger MM, Nassif E. Early intervention with short courses of prednisone to prevent progression of asthma in ambulatory patients incompletely responsive to bronchodilators. J Pediatr. 1987;110:627–633. doi: 10.1016/s0022-3476(87)80567-x. [DOI] [PubMed] [Google Scholar]
- 42.Littenberg B, Gluck EH. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. N Engl J Med. 1986;314:150–152. doi: 10.1056/NEJM198601163140304. [DOI] [PubMed] [Google Scholar]
- 43.Didonato JA, Saatcioglu F, Karin M. Molecular mechanisms of immunosuppression and anti-inflammatory activities by glucocorticoids. Am J Respir Crit Care Med. 1996;154:S11–S15. doi: 10.1164/ajrccm/154.2_Pt_2.S11. [DOI] [PubMed] [Google Scholar]
- 44.Connett GJ, Warde C, Wooler E, Lenney W. Prednisolone and salbutamol in the hospital treatment of acute asthma. Arch Dis Child. 1994;70:170–173. doi: 10.1136/adc.70.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratto D, Alfaro C, Sipsey J. Are intravenous corticosteroids required in status asthmaticus? JAMA. 1988;260:527–529. [PubMed] [Google Scholar]
- 46.Szefler SJ. Glucocorticoid therapy for asthma: Clinical pharmacology. J Allergy Clin Immunol. 1991;88:147–165. doi: 10.1016/0091-6749(91)90323-g. [DOI] [PubMed] [Google Scholar]
- 47.Harrison BD, Stokes TC, Hart GJ. Need for intravenous hydrocortisone in addition to oral prednisolone in patients admitted to hospital with severe asthma without ventilatory failure. Lancet. 1986;1:181–184. doi: 10.1016/s0140-6736(86)90654-9. [DOI] [PubMed] [Google Scholar]
- 48.Weber RW. Role of anticholinergics in asthma. Ann Allergy. 1990;65:348–350. [PubMed] [Google Scholar]
- 49.Schuh S, Johnson DW, Calahan S. Efficacy of frequent nebulized ipratropium bromide added to frequent high dose albuterol therapy in severe childhood asthma. J Pediatr. 1995;126:639–645. doi: 10.1016/s0022-3476(95)70368-3. [DOI] [PubMed] [Google Scholar]
- 50.Karpel JP, Schacter EN, Fanta C. A comparison of ipratropium and albuterol vs albuterol alone for the treatment of acute asthma. Chest. 1996;110:611–616. doi: 10.1378/chest.110.3.611. [DOI] [PubMed] [Google Scholar]
- 51.Tiffany BR, Berk WA, Todd IK, White SR. Magnesium bolus or infusion fails to improve expiratory flow in acute asthma exacerbations. Chest. 1993;104:831–834. doi: 10.1378/chest.104.3.831. [DOI] [PubMed] [Google Scholar]
- 52.Green SM, Rothrock SG. Intravenous magnesium for acute asthma: Failure to decrease emergency treatment duration or need for hospitalization. Ann Emerg Med. 1992;21:260–265. doi: 10.1016/s0196-0644(05)80885-6. [DOI] [PubMed] [Google Scholar]
- 53.Skorodin MS, Tenholder MF, Yettor B. Magnesium sulfate in exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 1995;155:496–500. [PubMed] [Google Scholar]
- 54.Kuitert LM, Kletchko SL. Intravenous magnesium sulfate in acute, life-threatening asthma. Ann Emerg Med. 1991;20:1243–1245. doi: 10.1016/s0196-0644(05)81481-7. [DOI] [PubMed] [Google Scholar]
- 55.Skobeloff EM. An ion for the lungs. Acad Emerg Med. 1996;3:1082–1084. doi: 10.1111/j.1553-2712.1996.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 56.Hendeles L, Weinberger M, Szefler S, Ellis E. Safety and efficacy of theophylline in children with asthma. J Pediatr. 1992;120:177–183. doi: 10.1016/s0022-3476(05)80423-8. [DOI] [PubMed] [Google Scholar]
- 57.Crescioli S, Spinazzi A, Plebani M. Theophylline inhibits early and late asthmatic reactions induced by allergens in asthmatic subjects. Ann Allergy. 1991;66:245–251. [PubMed] [Google Scholar]
- 58.Dutoit JI, Salome CM, Woolcock AJ. Inhaled corticosteroids reduce the severity of bronchial hyperresponsiveness in asthma but oral theophylline does not. Am Rev Respir Dis. 1987;136:1174–1178. doi: 10.1164/ajrccm/136.5.1174. [DOI] [PubMed] [Google Scholar]
- 59.Mitra A. The current role of intravenous aminophylline in acute paediatric asthma. Minerva Pediatr. 2003;55:369–375. [PubMed] [Google Scholar]
- 60.Yamauchi K, Kobayashi H, Tanifuji Y. Efficacy and safety of intravenous theophylline administration for treatment of mild acute exacerbation of bronchial asthma. Respirology. 2005;10:491–496. doi: 10.1111/j.1440-1843.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 61.Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79:405–410. doi: 10.1136/adc.79.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang D, O'Brien RG, Harman E. Does aminophylline benefit adults admitted to the hospital for an acute exacerbation of asthma? Ann Intern Med. 1993;119:1155–1160. doi: 10.7326/0003-4819-119-12-199312150-00001. [DOI] [PubMed] [Google Scholar]
- 63.Self TH, Abou-Shala N, Burns R. Inhaled albuterol and oral prednisone therapy in hospitalized adult asthmatics. Does aminophylline add any benefit? Chest. 1990;98:1317–1321. doi: 10.1378/chest.98.6.1317. [DOI] [PubMed] [Google Scholar]
- 64.Strauss RE, Wertheim DL, Bonaquera VR, Volacer DJ. Aminophylline therapy does not improve outcome and increases adverse effects in children hospitalized with acute asthmatic exacerbations. Pediatrics. 1994;93:205–210. [PubMed] [Google Scholar]
- 65.Mitra A, Bassler D, Goodman K. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;2:CD001276. doi: 10.1002/14651858.CD001276.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gluck EH, Onorato DJ, Castriotta R. Helium-oxygen mixtures in intubated patients with status asthmaticus and respiratory acidosis. Chest. 1990;98:693–698. doi: 10.1378/chest.98.3.693. [DOI] [PubMed] [Google Scholar]
- 67.Manthous CA, Hall JB, Caputo MA. Heliox improves pulsus paradoxus and peak expiratory flow in nonintubated patients with severe asthma. Am J Respir Crit Care Med. 1995;151:310–314. doi: 10.1164/ajrccm.151.2.7842183. [DOI] [PubMed] [Google Scholar]
- 68.Rivera ML, Kim TY, Stewart GM. Albuterol nebulized in heliox in the initial ED treatment of pediatric asthma: A blinded, randomized controlled trial. Am J Emerg Med. 2006;24:38–42. doi: 10.1016/j.ajem.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: Toward what end? Pediatrics. 2001;107:590–592. doi: 10.1542/peds.107.3.590. [DOI] [PubMed] [Google Scholar]
- 70.Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106:1006–1012. [PubMed] [Google Scholar]
- 71.Wazeka A, Valacer DJ, Cooper M. Impact of a pediatric asthma clinical pathway on hospital cost and length of stay. Pediatr Pulmonol. 2001;32:211–216. doi: 10.1002/ppul.1110. [DOI] [PubMed] [Google Scholar]
- 72.Cydulka RK, Emerman CL. A pilot study of steroid therapy after emergency department treatment of acute asthma: Is a taper needed? J Emerg Med. 1998;16:15–19. doi: 10.1016/s0736-4679(97)00227-8. [DOI] [PubMed] [Google Scholar]
- 73.Karan RS, Pandhi P, Behera D. A comparison of non-tapering vs. tapering prednisolone in acute exacerbation of asthma involving use of the low-dose ACTH test. Int J Clin Pharmacol Ther. 2002;40:256–262. doi: 10.5414/cpp40256. [DOI] [PubMed] [Google Scholar]
- 74.O'Driscoll BR, Kalra S, Wilson M. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993;341:324–327. doi: 10.1016/0140-6736(93)90134-3. [DOI] [PubMed] [Google Scholar]
- 75.Zeiger RS, Heller S, Mellon MH. Facilitated referral to asthma specialist reduces relapses in asthma emergency room visits. J Allergy Clin Immunol. 1991;87:1160–1168. doi: 10.1016/0091-6749(91)92162-t. [DOI] [PubMed] [Google Scholar]
- 76.Shuttari MF. Asthma: Diagnosis and management. Am Fam Physician. 1995;52:2225–2235. [PubMed] [Google Scholar]
- 77.Mayo PH, Richman J, Harris HW. Results of a program to reduce admissions for adult asthma. Ann Intern Med. 1990;112:864–871. doi: 10.7326/0003-4819-112-11-864. [DOI] [PubMed] [Google Scholar]
- 78.Joint Task force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology: Practice parameters for the diagnosis and treatment of asthma. J Allergy Clin Immunol. 1995;96:707–870. [PubMed] [Google Scholar]
- 79.Jarjour NN. Asthma in adults: Evaluation and management. In: Adkinson NF Jr, Yunginger JW, Busse WW, editors. Middleton's Allergy: Principles and Practice. 6th ed. CV Mosby; Philadelphia: 2003. p. 1269. [Google Scholar]
- 80.Eggleston P. Improving indoor environments: Reducing allergen exposures. J Allergy Clin Immunol. 2005;116:122–126. doi: 10.1016/j.jaci.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Gortmaker S, Walker DK, Jacobs FH, Ruch-Ross H. Parental smoking and the risk of childhood asthma. Am J Public Health. 1982;72:574–579. doi: 10.2105/ajph.72.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agudo A, Bardaqi S, Romero PV, Gonzalez CA. Exercise-induced airways narrowing and exposure to environmental tobacco smoke in schoolchildren. Am J Epidemiol. 1994;140:409–417. doi: 10.1093/oxfordjournals.aje.a117263. [DOI] [PubMed] [Google Scholar]
- 83.Frischer T, Kuehr J, Meinert R. Maternal smoking in early childhood: A risk factor for bronchial responsiveness to exercise in primary-school children. J Pediatr. 1992;121:17–22. doi: 10.1016/s0022-3476(05)82534-x. [DOI] [PubMed] [Google Scholar]
- 84.Arshad SH, Hide DW. Effect of environmental factors on the development of allergic disorders in infancy. J Allergy Clin Immunol. 1992;90:235–241. doi: 10.1016/0091-6749(92)90077-f. [DOI] [PubMed] [Google Scholar]
- 85.Leuenberger P, Schwartz J, Ackermann-Liebrich U. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am J Respir Crit Care Med. 1994;150:1222–1228. doi: 10.1164/ajrccm.150.5.7952544. [DOI] [PubMed] [Google Scholar]
- 86.Greer JR, Abbey DE, Burchette RJ. Asthma related to occupational and ambient air pollutants in nonsmokers. J Occup Med. 1993;35:909–915. doi: 10.1097/00043764-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 87.Abbey DE, Petersen F, Mills PK, Beeson WL. Long-term ambient concentrations of total suspended particulates, ozone, and sulfur dioxide and respiratory symptoms in a nonsmoking population. Arch Environ Health. 1993;48:33–46. doi: 10.1080/00039896.1993.9938391. [DOI] [PubMed] [Google Scholar]
- 88.Marquette CH, Salnier F, Leroy O. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am Rev Respir Dis. 1992;146:76–81. doi: 10.1164/ajrccm/146.1.76. [DOI] [PubMed] [Google Scholar]
- 89.Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care (GRASSIC) BMJ. 1994;308:564–567. [PMC free article] [PubMed] [Google Scholar]
- 90.Jones KP, Mullee MA, Middleton M. Peak flow based asthma self-management: A randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax. 1995;50:851–857. doi: 10.1136/thx.50.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gibson PG, Powell H, Coughlin J. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;1:CD001117. doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- 92.Soler M, Matz J, Townley R. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]


