(See healthmap.org for outbreaks of Diarrhea)
INTRODUCTION
There is an increasingly recognized array of bacterial, parasitic and viral organisms associated with infection of the intestinal tract which can profoundly disrupt intestinal function with or without causing acute diarrhea. Acute diarrhea is a syndrome that is frequently not differentiated clinically by specific etiologic agent. The wide spectrum of evolution varies from self-limited disease to death. Death is mainly due to dehydration and children in developing countries pay the highest tribute to acute diarrhea. This chapter examines the viral and bacterial causes of acute diarrhea, clinically defined by the emission of three or more loose or watery stools per day or a definite decrease in consistency and increase in frequency based upon an individual baseline lasting for less than 2 weeks. Parasitic infections of the gastrointestinal tract will be presented in Chapter 109 and diarrhea associated with food poisoning will be presented in Chapter 34. When diarrhea lasts for 14 days it can be considered persistent; the term chronic generally refers to diarrhea that lasts for at least 1 month (see Chapter 36).
EPIDEMIOLOGY
Prevalence
Acute diarrheal diseases ranked seventh in the causes of mortality in the low- and middle-income countries in the global disease burden series, 2001, with an estimated 1.78 million deaths (3.7%).1 Most of these deaths occur in children under the age of 5 years in developing countries and diarrhea accounted for 15% of all deaths among children younger than age 5 years, as tabulated in 2001.1 However, diarrheal diseases ranked fourth in the causes of life-years lost to disability and premature death, just after lower respiratory tract infections, HIV-related deaths and unipolar depression.2 The incidence of acute diarrhea in the general population could be estimated by prospective studies such as those organized in the Foodborne Disease Active Surveillance Network (FoodNet) in the USA. The network observed that 6% of interviewed people reported an acute diarrheal illness during the 4 weeks preceding the interview, i.e. an annualized rate of 0.72 episodes per person-year. Rates of illness were highest among children younger than 5 years (1.1 episodes per person-year) and were lowest in persons aged ≥65 years (0.32 episodes per person-year).3 A study in 2000 that estimated the economic burden of both infectious and noninfectious gastrointestinal and liver disease in the USA found that the most prevalent diseases were non-food-borne gastroenteritis (135 million cases per year) and food-borne illness (76 million cases per year).4 A report from the Centers for Disease Control and Prevention (CDC) found that food-borne diseases account for approximately 76 million illnesses, 325 000 hospitalizations and 5000 deaths each year in the USA based upon surveillance data from multiple sources.5 In the Netherlands, the incidence of gastroenteritis was 45 per 100 person-years in a prospective study.6
Surveillance
Not all enteric pathogens are notifiable, depending on the country. Moreover, report may not be timeless and a recent study in six US states indicated that multiple steps between onset of food-borne illness and its investigation by a public health agency could take up to 3 weeks.7
Sources of pathogens
Enteric pathogens are mainly food-borne and water-borne pathogens. Gastroenteritis is commonly contagious and may be transmitted by hand or saliva. While acute diarrhea occurs in most cases of food-borne illness, there are other causes of acute diarrhea that would not be captured in this type of survey. Water-borne outbreaks associated with recreational water (e.g. swimming or wading pools) are another source of acute diarrhea. The outbreaks were associated most frequently with Cryptosporidium (50%) in treated water sources and with toxigenic Escherichia coli (25%) and norovirus (25%) in freshwater sources. Swimming pools have been demonstrated as a source of norovirus gastroenteritis outbreak.8 Travel is increasingly reported as a circumstance for acute diarrhea including enterotoxigenic E. coli infection. Cruising appears to be an emerging circumstance for food-borne pathogen outbreak such as reported for norovirus.9 Aeromonas infections have been traced to aquarium water.10 As for nosocomial enteritis, patients with actual infectious diarrhea are the main source of infection and health-care workers suffering the same condition are a second source. Contaminated food is a potential source of food-borne Salmonella enteritidis outbreak and a contaminated inanimate hospital environment is a source for Clostridium difficile nosocomial acute enteritis.
PATHOGENESIS AND PATHOLOGY
Diarrhea reflects an increased water content of the stool, whether due to impaired water absorption or active water secretion by the bowel. In severe infectious diarrhea, the daily volume of stool may exceed 2 liters. Dehydration and loss of potassium (hypokalemia) are two life-threatening consequences of severe diarrhea. Water is mainly absorbed in small bowel (for about 8 liters a day in an adult) and further in the colon. By the time the initial 8 liters of fluid reaches the ileocecal valve, only about 600 ml remain, representing an efficiency of water absorption of 93%. By the time the remaining 600 ml of fluid reaches the anus, only about 100 ml of fluid remains, generally as formed feces. In the small intestine, water is absorbed by three basic mechanisms:
-
•
‘neutral’ sodium chloride (NaCl) absorption mediated by two coupled systems – one exchanges Na/H (cation exchanger) and the other exchanges Cl/HCO3 (anion exchanger);
-
•
‘electrogenic’ sodium absorption where sodium enters the cell via an electrochemical gradient (this electrogenic sodium absorption mechanism is commonly damaged during acute enteric infection); and
-
•
sodium co-transport.
Sodium absorption is coupled to the absorption of organic solutes such as glucose, many amino acids, and peptides. This co-transport mechanism remains intact during most acute diarrheal disorders. It is for this reason that oral rehydration is possible during acute diarrheal illness.
Osmotic diarrhea occurs when an absorbable solute, such as lactose, is not absorbed properly and retains water in the gut lumen. Infections that damage the intestinal epithelial cells either directly (rotavirus) or by a toxin (Shigella spp., C. difficile) can cause malabsorption and osmotic diarrhea. Secretory diarrhea results from an active, toxin-mediated secretion of water into the gut lumen. This is observed during cholera and infection by Shiga-toxin-producing E. coli and Shigella spp. Rotavirus also produces a viral enterotoxin, the nonstructural glycoprotein (NSP4).
Lastly, diarrhea can result from infection-mediated intestinal inflammation. After ingestion, an enteric organism colonizes the intestinal epithelium by adhering to an enterocyte. One of two pathways are generally followed depending upon the offending organism, either mucosal invasion or production of an enterotoxin.
ETIOLOGIES
As some enteric pathogens, such as Vibrio cholerae, are not ubiquitous, some pathogens are seasonal and some pathogens are responsible for epidemics, the prevalence of various pathogens responsible for diarrhea is variable. As for bacteria, the pathogens most frequently found are enteropathogenic clones of E. coli, Shigella spp., Salmonella enterica subsp., Campylobacter spp. and Aeromonas spp. As for viruses, the most frequent causes (outside local epidemics) include rotavirus, calicivirus (norovirus and saporovirus), astrovirus and enteric adenovirus. Less prevalent viruses include paramyxovirus, morbillivirus, rubivirus and reovirus. The prevalence of norovirus has recently been estimated to be 12% in children younger than 5 years hospitalized for severe diarrhea and 12% of mild and moderate diarrhea cases among patients of all ages.11 These authors estimated that norovirus organisms were responsible for up to 200 000 deaths of children less than 5 years of age in developing countries.11
Nosocomial enteritis is due to C. difficile,12 rotavirus and norovirus.13 Salmonella enteritidis,14 other Enterobacteriaceae pathogens and methicillin-resistant Staphylococcus aureus 15 are rare causes of nosocomial enteritis. In Canada, C. difficile enteritis has an incidence of 65 per 100 000 patient-days and a total attributable mortality of 5.7%.12 The same incidence figure has been found in children with a median age of 4 years.16
CLINICAL FEATURES
Bacterial enteritis
Aeromonas infection
Aeromonas spp. are inhabitants of aquatic environments worldwide, including rivers and lakes as well as drinking water in plants and distribution systems. Also, most pathogenic Aeromonas spp. can be found in meat and dairy products. Some Aeromonas isolates encode enterotoxins, including an alt gene-encoded heat-labile and an ast gene-encoded heat-stable enterotoxin. Aeromonas enteric infection may range from, most commonly, an acute watery diarrhea to dysenteric illness. Symptoms may include abdominal cramps (70%), nausea (40%), vomiting (40%) and fever (40%). Infection is usually self-limiting and children may be rarely hospitalized because of dehydration. Aeromonas caviae is the most prevalent species. Aeromonas veronii can be associated with rare cholera-like illness and dysenteric diarrhea resembling shigellosis with bloody and purulent stools. One-tenth of patients are co-infected with a second enteric pathogen. Intermittent and persistent diarrhea may occur for years after initial infection. Aeromonas enteritis could be complicated by the hemolytic–uremic syndrome and kidney disease.17
Campylobacter infection
(See healthmap.org for outbreaks of Campylobacter)
Campylobacter spp. comprise motile, Gram-negative, S-shaped, microaerophilic organisms responsible for zoonoses. Not only food animals such as poultry, cattle, sheep and pigs, but also domestic pets are reservoirs for worldwide human infections. Despite the incidence decreasing in the USA, Campylobacter spp. are still responsible for sporadic infections following improper manipulation of poorly cooked meat. Poultry is a major source of infection.18 Unpasteurized dairy products and water have been found to be sources of limited outbreaks. The incidence of Campylobacter spp. infection is higher in developing countries than in developed ones and travelers to developing countries are at risk of Campylobacter infection.
The pathogenesis of Campylobacter infections is poorly understood. Campylobacter jejunii and Campylobacter coli are the most frequently encountered species responsible for diarrhea. Signs and symptoms vary from asymptomatic infections and may include fever, abdominal cramps and diarrhea with or without blood and fecal white blood cells. Although generally self-limited, relapses and chronic diarrhea are possible, as well as extraintestinal infection including bacteremia. Campylobacter jejunii infection is the most frequently recognized infection preceding the development of Guillain–Barré syndrome.19 The mechanisms rely on the cross-reactivity between ganglioside-like motifs present in Campylobacter jejunii lipopolysaccharide and those of peripheral nerves. Also, this species has been associated with immunoproliferative small intestinal disease.20 Species other than Campylobacter jejunii and Campylobacter coli are increasingly isolated from the stools of patients with diarrhea, including Campylobacter fetus, mainly isolated from extraintestinal sites, and Campylobacter upsaliensis. As both species are susceptible to cephalotin, an antibiotic usually incorporated in Campylobacter jejunii-selective media, their prevalence in stools and diarrhea may be underestimated by culture methods.
Clostridium difficile infection
Clostridium difficile is a sporulated Gram-positive anaerobe of the Firmicutes phylum. Spores are highly resistant in an inanimate environment which can be a source of infection. Clostridium difficile genome analysis indicated that this extremely variable genome encodes toxin A and toxin B21 which are cytotoxic toxins implicated in the inflammatory intestinal lesions. Some C. difficile strains encode only toxin B and may be missed in laboratory tests detecting only toxin A. Also, emerging C. difficile clones have recently been identified as being responsible for both community-acquired and nosocomial severe enteritis due to the additional production of the iota toxin22 or to the hyperproduction of toxin A and toxin B due to mutation in the regulator gene.23
Clostridium difficile is indeed responsible for episodes of community-acquired and nosocomial acute enteritis, some of them associated with the previous administration of a wide variety of antibiotics, with C. difficile being responsible for about 20% of antibiotic-associated diarrhea.24 If the use of the offending antibiotic continues, C. difficile may cause severe enteritis and life-threatening pseudomembranous colitis.
Escherichia coli infection
(See healthmap.org for outbreaks of Escherichia coli)
Of the six Escherichia spp., E. coli organisms are common inhabitants of the intestinal tract of healthy people, yet a limited number of clones are responsible for acute diarrhea and extraintestinal infections. Escherichia fergusonii is frequently isolated from stools, yet its pathogenic role is unproven; Escherichia albertii is a possible agent of acute diarrhea.25 There are five groups of E. coli organisms associated with acute diarrhea:
-
•
Shiga-toxin producing E. coli (STEC), also termed enterohemorrhagic E. coli (EHEC);
-
•
enterotoxigenic E. coli (ETEC);
-
•
enteropathogenic E. coli (EPEC);
-
•
enteroaggregative E. coli (EAEC); and
-
•
enteroinvasive E. coli (EIEC).
STEC produce one or several Shiga toxins (also known as verocytotoxins) and are the most frequent E. coli organisms associated with acute diarrhea in developed countries. These organisms, comprising various E. coli O157 serotypes, are responsible for mild non-bloody and bloody acute diarrhea.26 Non-O157:H7 STEC are associated with illnesses that differ from those caused by E. coli O157:H7. Most notably, they are found later and have a lower proportion of bloody diarrhea than in patients infected with E. coli O157:H7.27 STEC are also responsible for an estimated 80% of hemolytic–uremic syndrome cases in about 4% of patients with enteric infection. Ground beef has been the major vehicle of transmission of O157 STEC, although other vehicles contaminated by bovine manure have been reported, including raw milk, sausage, apple cider, raw vegetables and nonchlorinated water supplies. Additionally, person-to-person transmission is responsible for outbreaks in communities.
ETEC produce heat-labile (LT) and heat-stable (ST) enterotoxins and are a frequent cause of acute diarrhea in developing countries, thus being a frequent cause of travelers’ diarrhea. ETEC infection manifests as relatively mild watery diarrhea and abdominal cramps, but no vomiting or fever.
EPEC comprise organisms characterized by an adherence factor plasmid and the chromosomal locus of enterocyte effacement. These organisms are responsible for severe infantile diarrhea in developing countries associated with fever, vomiting and prolonged evolution. Chronic diarrhea may follow EPEC infection and be responsible for malabsorption, weight loss and growth retardation.
EIEC are responsible for an infection mimicking shigellosis. EAEC are responsible for worldwide, mild enteric infections with non-bloody diarrhea, abdominal pain and mild fever.
Salmonella infection
(See healthmap.org for outbreaks of Salmonella)
The genus Salmonella comprises motile enteric bacteria of problematic nomenclature. Salmonella comprises two species and the species Salmonella enterica comprises five subspecies. The vast majority of human infections are due to strains of Salmonella enterica subspecies I, also isolated from warm-blooded animals, while the other Salmonella organisms are isolated from the environment and cold-blooded animals. These organisms are further serotyped and serotype generally correlates with the food source of infection. Notably, Salmonella enterica serotype Typhi is the agent of typhoid fever. Salmonella enterica serotypes Enteritidis and Typhimurium are the most commonly isolated in developed countries. Salmonellosis outcome differs significantly with serotype.28
Nontyphoidal Salmonella enterica organisms are responsible for acute diarrhea with fever and abdominal cramps lasting for an average of 1 week. Rarely, these organisms are responsible for bacteremia and extraintestinal infections in immunocompromised patients. Contacts with animals and animal foods are the sources of infection, with the Enteritidis serotype being associated with chicken and egg products. Serotype Cholerasuis is adapted to pigs and serotype Dublin is adapted to cattle. Later serotypes harbor virulence traits in common with serotype Typhi, the typhoid fever agent. Typhoid fever is a life-threatening septicemia rarely observed in developed countries but still of public health concern in developing countries. The mortality rate in patients who did not receive appropriate therapy is more than 10% whereas it is less than 1% in patients who did receive appropriate antibiotic therapy.
Clinical presentation includes fever and headache without diarrhea and healthy carriers have been observed. Humans are the only known reservoir for serotype Typhi, which can be transmitted by direct person-to-person contact and contaminated water and food. A syndrome similar to typhoid fever is due to serotypes Paratyphi A, Paratyphi B and Paratyphi C.
Shigella infection
Although from a genetic standpoint, E. coli and Shigella spp. form a single bacterial species, four subgroups of Shigella have been taken as different species, i.e. Shigella dysenteriae, Shigella flexneri, Shigella boydii and Shigella sonnei.29 Humans are the only known reservoir for Shigella spp. and transmission is by direct contact from person to person and by contaminated water and food. Sexual transmission has been observed in homosexual males. Most cases in developed countries are imported from developing countries. Shigella spp. are responsible for bacillary dysentery characterized by acute, bloody diarrhea accompanied by fever and abdominal cramps. Classic dysentery is characterized by the emission of scant stools containing mucus, pus and blood. Shigellosis is responsible for rectal and colonic ulcerations which do not develop beyond the lamina propria. Rare cases of septicemia have been observed but Shigella spp. are responsible for the hemolytic–uremic syndrome.
Vibrio infection
Vibrios are ubiquitously found in aquatic environments and are classified into more than 70 species responsible for trauma-related, extra-intestinal infections and intestinal infections with diarrhea.30 Vibrio cholerae is the etiologic agent of cholera. This is a motile, Gram-negative, facultative anaerobe bacterium which requires a small concentration of sodium for growth. Vibrio cholerae is primarily an aquatic inhabitant found in freshwater rivers and lakes as well as in estuarine and maritime environments. In these environments, V. cholerae is isolated from both inanimate environments and from plankton and various bivalves, crabs, shrimp and prawns. A viable-but-not-cultivable state has been described, the regulation of which may be phage-dependent.31
Vibrio cholerae comprises three major subgroups: V. cholerae O1, V. cholerae O139 and V. cholerae non-O1, widely distributed in tropical and subtropical areas, including the Gulf of Mexico for V. cholerae O1. The V. cholerae O1 chromosome contains a virulence cassette and pathogenicity islands, encoding virulence factors such as the pilus responsible for the attachment of V. cholerae O1 organisms to the intestinal epithelium and the cholera enterotoxin responsible for the large excretion of electrolytes and water in the intestinal lumen. Two biotypes, designated classic and El Tor, can be differentiated on the basis of simple laboratory tests including the hemolysis of sheep erythrocytes, the Voges–Proskauer test and the resistance to polymyxin, which are all positive in the El Tor biotype. The first six historical pandemics are though to be due to the classic biotype whereas the ongoing seventh pandemic (which started in 1961) is due to the El Tor biotype.
Vibrio cholerae O1 is the organism responsible for historic pandemics of cholera since 1816, including the current pandemics. Contamination may be hand transmitted through water drinking (and flies). Most patients contaminated with V. cholerae O1 have an asymptomatic or self-limited diarrhea (>75%), but massive contamination results in severe diarrhea with large volumes of ‘rice water stools’ and dehydration or infection in patients with neutral gastric pH. Clinical manifestations include loss of skin elasticity, watery eyes, painful muscle cramps and anuria. Dehydration leads to hypovolemic shock and death. Exceptional extraintestinal V. cholerae O1 bacteremia infections have been reported.
In 1992, cholera cases due to a new serogroup V. cholerae O139 (Bengal) were reported in India and Bangladesh and spread rapidly throughout Asia. The new serogroup probably resulted from the lateral gene transfer of novel capsule and somatic antigen genes to the El Tor strain. It causes disease similar to that caused by V. cholerae O1 except that adults are more frequently infected.
Some V. cholerae isolates do not agglutinate with anti-O1 and anti-O139 antisera and are therefore referred as V. cholerae non-O1 isolates. Vibrio cholerae non-O1 isolates do not produce the cholera enterotoxin and are responsible for mild watery diarrhea. Unlike V. cholerae O1 isolates, V. cholerae non-O1 isolates are responsible for extraintestinal infections, such as life-threatening septicemia, especially in patients with previous liver disease or hematologic malignancies. Vibrio cholerae non-O1 isolates have also been recovered from other anatomic sites.
Other Vibrio spp. responsible for diarrhea include V. mimicus, a species phenotypically related to V. cholerae, which is responsible for diarrhea after the consumption of raw seafoods; some strains harboring the cholera toxin can produce cholera-like symptoms. In Asia, V. parahaemolyticus is the leading cause of food-borne intestinal infections after the consumption of raw fish or shellfish. The species is responsible for watery diarrhea, rarely bloody diarrhea, and is particularly responsible for severe dehydration and death. The first pandemic clone, V. parahaemolyticus serotype O3:K6, emerged in 1996 in Taiwan and then spread throughout Asia to America, Africa and Russia. New serotypes emerged for a few years.
Vibrio vulnificus is primarily responsible for life-threatening septicemia and secondary skin infection with necrosis. It has an intestinal route of entry and is responsible for vomiting, diarrhea and abdominal cramps after the consumption of raw oysters in 95% of patients. Three biogroups have been defined in V. vulnificus, the vast majority of infections being due to biogroup 1. Vibrio fluvialis, Vibrio furnisii and Grimontia (Vibrio) hollisae cause sporadic cases of diarrhea worldwide.32, 33 Vibrio alginolyticus is seldom isolated from stools and there is little evidence for V. alginolyticus actually being responsible for intestinal infection and diarrhea.
Yersinia infection
Among the numerous members of the Yersinia genus, only Yersinia enterocolitica and Yersinia pseudotuberculosis have been associated with digestive tract infection. Yersinia enterocolitica, further divided into two subspecies enterocolitica and paleartica on the basis of 16S rDNA sequencing,34 comprises more than 70 serotypes, five of them being associated with human infection. These strains encode for an enterotoxin and some strains harbor a chromosome-borne pathogenicity island which contains the yersiniabactin gene, providing the organisms with iron. Yersinia enterocolitica is primarily an environmental organism isolated from the gastrointestinal tract of numerous animals, most commonly swine, dogs and rodents. Its distribution is mostly in Northern Europe and northern states of the USA, reflecting its increased growth at cold temperatures. This species is responsible for gastroenteritis associated with the consumption of contaminated water and food, mainly poorly cooked pork.
The disease spectrum comprises self-limited, acute diarrhea to terminal ileitis and mesenteric lymphadenitis mimicking appendicitis. Prolonged shedding has been observed. Septicemia is an uncommon complication observed in patients with an increased iron pool such as thalassaemic patients in Western countries,35 patients with liver disease or cancer and those undergoing steroid therapy. Yersinia enterocolitica is the bacterial organism most frequently associated with blood transfusion. Reactive arthritis is an uncommon sequel observed in HLA-B27 positive patients and immunocompromised patients; it is characterized by asymmetric involvement of multiple joints including the sacroiliac joint and the spine.
Yersinia pseudotuberculosis is rarely isolated as a cause of a self-limited acute diarrhea; it has been associated with outbreaks of gastroenteritis after consumption of contaminated fresh vegetables.36, 37 Yersinia pseudotuberculosis is also responsible for pseudoappendicitis, and reactive arthritis may develop after infection with Yersinia pseudotuberculosis O3.38
Miscellaneous bacteria
Arcobacter are Campylobacter-like organisms seldom isolated from the stools of patients with diarrhea, including Arcobacter butzleri 39 and Arcobacter cryaerophilus DNA group 1B.40 Particular culture conditions are required for proper isolation of these fastidious organisms, thus limiting their detection to a few studies.
Listeria monocytogenes has only recently been recognized as an agent of acute enteritis, mainly transmitted by milk, but it has now been associated with several enteritis outbreaks.41 Enteritis typically occurs after ingestion of a large inoculum and is self-limited after a few days’ evolution.42
Klebsiella oxytoca is found in the environment but its principal reservoir is the human gastrointestinal tract; it has been associated with C. difficile-negative antibiotic-associated colitis.43 Klebsiella oxytoca organisms cause experimental colitis and exhibit cytotoxicity against HEp-2 cultured cells.43 Its culture is not routinely performed and requires a specific isolation agar medium.
Laribacter hongkongensis is a facultative anaerobic Gram-negative bacillus initially reported as being responsible for acute diarrhea in Asian patients.44 A case-controlled study indicated that eating fish and travel were associated with L. hongkongensis acute diarrhea.45
Dysgonomonas capnocytophagoides (formerly CDC group DF-3)46 are Captocytophaga-like organisms isolated from the stools of immunocompromised patients.47., 48., 49.
Viral enteritis
Rotavirus infections
(See healthmap.org for outbreaks of Rotavirus)
Rotaviruses are RNA viruses presenting as 70 nm particles with a wheel-like appearance (Fig. 35.1 ). Based on group-specific antigens of the major viral structural protein VP6, rotavirus can be classified into six groups, A–G. Groups A–C infect humans; the other groups are found in animals. Human rotaviruses are responsible for severe acute diarrhea with dehydration associated with childhood death in developing countries. In Europe, children with rotavirus-positive acute gastroenteritis were more likely to have lethargy, fever, vomiting and dehydration, and, therefore, more severe disease than were children with rotavirus-negative acute gastroenteritis. Dehydration was up to 5.5 times more likely in children with rotavirus-positive acute gastroenteritis than in those with rotavirus-negative acute gastroenteritis.50 Acquisition of rotaviruses is likely from subclinical infection in parents or siblings but rotavirus infection can be a zoonosis. Rotaviruses are resistant in inanimate environments which may be implicated as a source of infection, including nosocomial outbreaks. Rotavirus infection is seasonal, with a peak incidence in winter/spring in temperate countries. Clinical symptoms include acute diarrhea for 2–3 days, fever, vomiting and anorexia.
Fig. 35.1.

Flow-chart for the direct diagnosis of acute diarrhea in the microbiology laboratory. POC, point of care.
Calicivirus infections
The family Caliciviridae comprises norovirus and saprovirus, both responsible for enteritis. These RNA viruses appear as <40 nm nonenveloped particles. Norovirus comprises five genotypes: genotypes I, II and IV are responsible for human infections, genotypes III and V are animal associated. Likewise, saprovirus comprises five genotypes; genotypes I, II, IV and V are responsible for human infections. These highly contagious human viruses reside in inanimate environments comprising contaminated surfaces, water and food. Direct evidence for animals as a reservoir for human infection is still lacking.
Noroviruses are the most prevalent cause of nonbacterial acute enteritis worldwide.51 These viruses cause large outbreaks and provoke incapacity for a few days; they have therefore been included in List B of potential bioterrorism agents by the US National Institute of Allergy and Infectious Diseases (NIAID). Outbreaks mainly occur in institutions, health-care centers and cruise ships.
Astrovirus infections
These RNA viruses, averaging 30 nm in diameter, are mainly responsible for acute diarrhea in children, although outbreaks in military troops and hospitals have also been reported. These worldwide viruses are responsible for 2–10% of pediatric cases of acute diarrhea. Clinical signs and symptoms are nonspecific.52
Enteric adenovirus infections
(See healthmap.org for outbreaks of adenovirus)
Adenoviruses are nonenveloped, 100 nm round particles containing DNA and are responsible for human infections. Adenoviruses are divided into 51 different serotypes and six subgroups, of which only two serotypes – Ad40 and Ad41 (subgroup F) – have been clearly demonstrated to be agents of acute diarrhea. Clinical characteristics include a higher prevalence in children less than 4 years of age and a mean duration of disease of 5–10 days, i.e. longer than that caused by other viruses. Prolonged diarrhea has been observed in immunocompromised patients.53
Miscellaneous viruses
A few other viruses have been associated with acute diarrhea, yet their role remains to be firmly established. These include coronavirus, definite agents of diarrhea in animals and seldom visualized by electron microscopy and isolated in culture from the stools of patients with diarrhea. Likewise, toroviruses are responsible for acute human gastroenteritis and are responsible for nosocomial cases. Aichi virus, a member of the family Picornaviridae, has been characterized by reverse transcriptase-polymerase chain reaction (RT-PCR) during an outbreak of enteritis following the consumption of oysters in Japan.54 Picobirnaviruses have been detected in stools of animals and humans; however, their significance remains to be established. Recently, cardioviruses closely related to Theiler's murine encephalomyelitis virus have been detected in stools in 1.2% patients with acute enteritis.55
Prevention
The global mortality from diarrhea declined from approximately 4.6 million annual deaths during the mid-1980s to 2.4 million deaths in 1990, and to the current estimate of 1.6–2.1 million.1, 56 The decline is generally attributed to global improvements in sanitation and the use of glucose–electrolyte oral rehydration therapy (ORT) which has dramatically reduced acute mortality from dehydration caused by diarrhea. In contrast to the fortunate decrease in mortality, morbidity remains as high as during the previous century. However, simple, cheap measures could be undertaken to make the incidence fall. A prospective study in India demonstrated that the promotion of hand washing with plain soap reduced by 53% the incidence of acute diarrhea (and of pneumonia and impetigo).57 Indeed, hand washing with soap is effective against almost all enteritis-causing pathogens, whereas efficacy of alcohol against rotavirus and calicivirus remains controversial.58 In developed countries, prevention relies on increased sanitary measures in collective sources of enteric pathogens such as swimming pools and recreational lakes for fishing and swimming, as well as better control over fresh foodstuffs. Patients should be isolated at home and children excluded from nurseries and school for the duration of the illness.
Another vaccine against ETEC, administrated as a patch, is being evaluated.59 A live oral vaccine against rotavirus has recently been licensed after a few previous attempts and its safety and preventive effects have been carefully evaluated.60 Cost-effectiveness of vaccine against rotavirus has been evaluated favorably in the Netherlands.61
As for travelers, pre-travel prophylaxis relies on vaccines. There is currently only one vaccine available that provides protection against diarrhea caused by Vibrio cholerae and by ETEC. This vaccine is licensed in only a few Western countries. Protective efficacy against cholera is 85%, while protection against the heat-labile toxin of ETEC reaches 67%. Current studies show a protective effect of up to 43%. Vaccination against cholera and ETEC should be recommended for at-risk travelers, in particular those with high exposure at their travel destination or high personal risks through fluid loss.62 Typhim Vi is a conjugate vaccine aimed at prevention of typhoid fever, and its safety and effectiveness have been favorably evaluated.63, 64 During travel, systematic administration of antibiotics including fluoroquinolones, cyclines and co-trimoxazole is controversial. Prophylaxis may rely on the basic rules of boiling fresh water or drinking bottled water, and ensuring that meat is well cooked.
DIAGNOSIS
There is no recommended serologic test for the microbiologic diagnosis of enteric pathogens and the laboratory diagnosis of diarrhea relies solely on direct diagnosis. Serologic testing is useful for epidemiologic investigation of Campylobacter spp. infections.65
Fresh stools should be collected in a clean container with a tight lid. Alternatively, a transport medium incorporating buffered glycerol in saline could be used. A rectal swab is an alternative specimen in selected situations. It is well established that hospitalized patients who did not enter the hospital with diarrhea are unlikely to develop diarrhea caused by bacterial agents other than C. difficile. As such, stool culture will not be performed in patients hospitalized for more than 72 hours (the 3-day rule) and rapid detection of C. difficile toxins will be alternatively performed.66 For routine purposes, testing a single stool specimen has acceptable sensitivity; however, testing a second specimen is mandatory when the first one was subject to a more than 2-hour delay in transport.67
Several techniques have been developed for the point-of-care diagnosis of diarrhea including the rapid (<30 minutes) agglutination-based detection of rotavirus and adenovirus as well as the detection of C. difficile toxins. The rapid detection of C. difficile toxins A and B should be routinely performed for both inpatients and outpatients. Point-of-care detection of Shiga toxin-producing E. coli in children using EIA has not been evaluated favorably.68 However, a commercially available Campylobacter antigen detection kit has been favorably evaluated,69 and a dipstick test for the rapid detection of Shigella is under appraisal.70
Further detection of the causative organism relies on stool examination in the clinical microbiology laboratory. Direct microscopic examination may yield motile bacteria such as Vibrio and Salmonella spp. and parasites. Although Gram-staining analysis of stool specimens may not be done routinely, it has demonstrated 66–94% sensitivity and >95% specificity for the rapid detection of Campylobacter species.71 We therefore recommend microscopic examination after Gram staining in all specimens in addition to Ziehl–Neelsen staining and appropriate staining for microsporidia and other parasites in immunocompromised patients. Specimens should be examined by electron microscopy for the observation of enteric viruses within 4 hours of receipt in the laboratory. Negative staining is a rapid and easy procedure. Rotavirus and adenovirus are readily detected, which may not be the case for calicivirus and astrovirus72 (Fig. 35.2 ).
Fig. 35.2.
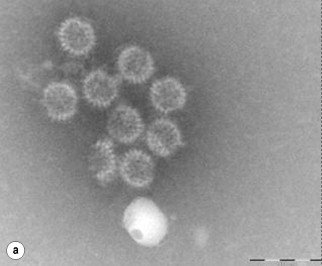

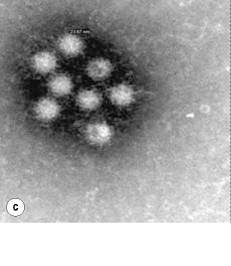

Electron microscopy examination of diarrheal stools after negative staining shows the presence of enteric viruses responsible for acute diarrhea, such as (a) rotavirus, (b), adenovirus, (c) calicivirus and (d) enterovirus.
Culture of stools will focus on frequent pathogens and the systematic search for less frequent bacterial pathogens will be guided by the local epidemiologic situation. Pathogens routinely detected by culture of diarrheal stools include E. coli O157, Shigella spp., Salmonella enterica serotypes, Campylobacter jejunii, Campylobacter coli and Aeromonas spp. Stools in O157, O111 and O26 serotypes of E. coli can be enriched by using specific, commercially available magnetic beads. Sorbitol MacConkey agar can be used for the isolation of O157 STEC as these organisms do not ferment d-sorbitol, contrary to the vast majority of E. coli strains, and several chromogenic media have been developed for the selective isolation of O157 STEC. Although many rapid methods have been proposed for confirming the identification of O157 STEC, methods for the identification of ETEC, EPEC, EAEC and EIEC are most often available in reference laboratories only.
Salmonella enterica serotypes are better isolated by using an enrichment broth before plating onto selective media. Biochemical identification of Salmonella spp. and O (somatic), H (flagellar) and Vi (capsular) antigen serotyping should be performed in order to identify Salmonella enteritis Typhi (the typhoid fever agent, being capsular antigen Vi positive) and the most prevalent non-Typhi serotypes. The Vi capsular antigen is occasionally detected in non-Typhi, Dublin and Paratyphi C serotypes. O serotype determination is carried out by agglutination using pooled antisera while further H serotype determination is performed by tube agglutination tests using broth culture and testing the two phases of the flagellar antigens. MALDI-TOF mass spectrometry rapid analysis of Salmonella enterica isolates may resolve the serotype.73
Campylobacter spp. are recovered by using the filtration method in parallel to selective, blood-containing or non-blood-containing media and a microaerophilic atmosphere. Some Campylobacter spp. require 6% hydrogen in atmosphere. Incubation at 108°F (42°C) allows the growth of Campylobacter jejuni and Campylobacter coli but not all Campylobacter and Aeromonas spp. are recovered using blood agar incorporating 20 µg/ml ampicillin and produce β-hemolytic colonies. Further identification will be needed for oxidase-positive and indole-positive colonies. Modified cefsulodin-irgasan-novobiocin agar is also suitable for the isolation of Aeromonas spp. On this medium, Aeromonas colonies are undistinguishable from Yersinia enterocolitica colonies. Molecular identification should rely not only on 16S rDNA sequencing because of intragenomic heterogeneity and further identification based on gyrB and rpoD genes is mandatory. The interpretation of recovery of Aeromonas in stools must be cautious since there is no strong evidence that all Aeromonas isolates from stools are responsible for diarrheal infection.74
Blood cultures are mandatory for the diagnosis of typhoid fever as well as bacteremia due to non-Typhi serotypes of Salmonella.
The systematic search for other enteritis pathogens will depend on local epidemiology including Yersinia enterocolitica, Vibrio spp., Klebsiella oxytoca, Listeria monocytogenes and Plesiomonas shigelloides. Growth of Yersinia enterocolitica from stools is enhanced by incubation on selective media (e.g. pectin agar) at 95°F (35°C) and the search for Listeria monocytogenes could be routinely done in certain laboratories. Vibrio cholerae will be visible as very motile, Gram-negative, slightly curved bacilli cultivated using a thiosulfate citrate bile salts sucrose (TCBS) agar after enrichment. Yellow colonies of oxidase-negative bacilli could be identified after electron microscopy observation, by 16S rDNA sequencing. In parallel with the search for pathogenic bacteria, the search for viruses should be done using electron microscope observation after negative staining as well as detection of rotavirus.
Although identification based on the observations of phenotypic traits after bacterial growth is the routine approach to the identification of bacteria responsible for diarrhea, novel techniques are warranted in order to speed the identification process in a timely fashion. Rapid and cheap identification by using mass spectrometry analysis of entire organisms has already been reported for Vibrio spp.75
MANAGEMENT
Community-acquired enteritis
Management of acute diarrhea should include the clinical evaluation of the patient, including risk factors for specific etiology and dehydration; rapid diagnosis of viral diarrhea; and treatment including rehydration, antibiotic therapy and symptomatic treatment. Rehydration is a major therapeutic measure; however, it should be borne in mind that patients on oral rehydration have a higher risk of paralytic ileus, and those on intravenous rehydration are exposed to risks of intravenous therapy. For every 25 children treated with oral rehydration one would fail and require intravenous rehydration.76
Rapid diagnosis of viral diarrhea is important in order to avoid unnecessary antibiotic treatment. Because of the absence of any antiviral drug effective against the viruses responsible for acute diarrhea, the management of viral diarrhea comprises the relief of symptoms and rehydration in cases of dehydration. Meta-analysis has confirmed that antibiotic treatment is useful to shorten the duration of signs and symptoms in travelers’ acute diarrhea,77 although most acute diarrheal episodes are self-limited and do not require antibiotic treatment. If an antibiotic is to be prescribed, fluoroquinolones are the drugs of first choice, including norfloxacin (400 mg q12h) or ciprofloxacin (500 mg q12h) and 1-day treatment is advocated except for Campylobacter and Shigella infection which should be treated for 3 days.78 In the case of patients back from countries where fluoroquinolone resistance is prevalent,79 such as Campylobacter spp. in Thailand, azithromycin (500 mg/day) could be used for 3 days.80 Antimicrobial therapy for O157 E. coli enteritis or hemolytic–uremic syndrome remains a controversial issue because some studies reported a deleterious effect on the evolution of the latter syndrome. Antibiotic treatment of other serotypes by fluoroquinolones is advocated.
The increasing resistance of Salmonella enterica serotype Typhi to antibiotics, notably ciprofloxacin, makes the choice of first-line antibiotic treatment of typhoid fever more problematic. The majority of cases of Yersinia enterocolitis gastroenteritis do not require antibiotic treatment, contrary to systemic infection which could be treated using co-trimoxazole (no resistant strain reported) or fluoroquinolones, despite the fact that a few resistant strains have been reported.
Nosocomial enteritis
The main goal is to prevent nosocomial transmission of the etiologic agent in order to prevent and limit an extensive outbreak. The first step is to avoid the admission of patients diagnosed with diarrhea but without serious signs, and patients diagnosed at point-of-care as viral diarrhea. As for hospitalized patients, cohorting was not effective in the prevention of outbreaks. Patients suspected of infectious diarrhea should be treated in a single room, using standard precautions including hand washing and barrier protection using gloves and gowns. The duration of these precautions is for the duration of the illness.
Hand washing is the single most important measure in the control of nosocomial transmission of enteric pathogens. Because of concerns regarding the low virucidal activity of ethyl and isopropyl alcohols against naked viruses (rotavirus, norovirus, saporovirus)81 and the lack of sporicidal activity (C. difficile), hand washing with soap in addition to alcohol is mandatory. The impact of improved hand hygiene using both soap and alcohol hand washing in decreasing nosocomial rotavirus infection has been proved.82 Room environments should be carefully cleansed using sporicidal biocides in cases of C. difficile diarrhea.
There is no specific treatment for nosocomial viral enteritis. Cessation of the inciting antibiotic as soon as possible is the main management measure in case of C. difficile diarrhea. Patients should benefit from oral metronidazole (500 mg q8h) or vancomycin (125 mg q6h) for 10 days. The role of immunoglobulin for the treatment of severe C. difficile infection remains controversial.83
REFERENCES
- 1.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J.L. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Hotez P.J., Molyneux D.H., Fenwick A. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Imhoff B., Morse D., Shiferaw B. Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clin Infect Dis. 2004;38(Suppl.3):S219–S226. doi: 10.1086/381590. [DOI] [PubMed] [Google Scholar]
- 4.Sandler R.S., Everhart J.E., Donowitz M. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 5.Mead P.S., Slutsker L., Dietz V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit M.A., Hoogenboom-Verdegaal A.M., Goosen E.S., Sprenger M.J., Borgdorff M.W. A population-based longitudinal study on the incidence and disease burden of gastroenteritis and Campylobacter and Salmonella infection in four regions of The Netherlands. Eur J Epidemiol. 2000;16:713–718. doi: 10.1023/a:1026754218713. [DOI] [PubMed] [Google Scholar]
- 7.Hedberg C.W., Greenblatt J.F., Matyas B.T. Enteric Disease Investigation Timeline Study Work Group. Timeliness of enteric disease surveillance in 6 US states. Emerg Infect Dis. 2008;14:311–313. doi: 10.3201/eid1402.070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podewils L.J., Zanardi Blevins L., Hagenbuch M. Outbreak of norovirus illness associated with a swimming pool. Epidemiol Infect. 2007;135:827–833. doi: 10.1017/S0950268806007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhoef L., Depoortere E., Boxman I. Food Borne Viruses in Europe Network. Emergence of new norovirus variants on spring cruise ships and prediction of winter epidemics. Emerg Infect Dis. 2008;14:238–243. doi: 10.3201/eid1402.061567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filler G., Ehrich J.H., Strauch E., Beutin L. Acute renal failure in an infant associated with cytotoxic Aeromonas sobria isolated from patient's stool and from aquarium water as suspected source of infection. J Clin Microbiol. 2000;38:469–470. doi: 10.1128/jcm.38.1.469-470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M.M., Widdowson M.A., Glass R.I., Akazawa K., Vinjé J., Parashar U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravel D., Miller M., Simor A. Canadian Nosocomial Infection Surveillance Program. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48:568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 13.Beersma M.F., Schutten M., Vennema H. Norovirus in a Dutch tertiary care hospital (2002–2007): frequent nosocomial transmission and dominance of GIIb strains in young children. J Hosp Infect. 2009;71:199–205. doi: 10.1016/j.jhin.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka D.M., Costa S.F., Mangini C. A nosocomial outbreak of Salmonella enteritidis associated with lyophilized enteral nutrition. J Hosp Infect. 2004;58:122–127. doi: 10.1016/j.jhin.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Okii K., Hiyama E., Takesue Y., Kodaira M., Sueda T., Yokoyama T. Molecular epidemiology of enteritis-causing methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2006;62:37–43. doi: 10.1016/j.jhin.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim J., Smathers S.A., Prasad P., Leckerman K.H., Coffin S., Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001–2006. Pediatrics. 2008;122:1266–1270. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanović R., Cobeljić M., Marković M. Haemolytic–uraemic syndrome associated with Aeromonas hydrophila enterocolitis. Pediatr Nephrol. 1991;5:293–335. doi: 10.1007/BF00867480. [DOI] [PubMed] [Google Scholar]
- 18.Friedman C.R., Hoekstra R.M., Samuel M. Emerging Infections Program FoodNet Working Group. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38:S285–S296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 19.Yuki N., Kuwabara S. Axonal Guillain–Barré syndrome: carbohydrate mimicry and pathophysiology. J Peripher Nerv Syst. 2007;12:238–249. doi: 10.1111/j.1529-8027.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 20.Lecuit M., Abachin E., Martin A. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239–248. doi: 10.1056/NEJMoa031887. [DOI] [PubMed] [Google Scholar]
- 21.Sebaihia M., Wren B.W., Mullany P. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 22.Voth D.E., Ballard J.D. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warny M., Pepin J., Fang A. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 25.Stock I., Rahman M., Sherwood K.J., Wiedemann B. Natural antimicrobial susceptibility patterns and biochemical identification of Escherichia albertii and Hafnia alvei strains. Diagn Microbiol Infect Dis. 2005;51:151–163. doi: 10.1016/j.diagmicrobio.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Rangel J.M., Sparling P.H., Crowe C., Griffin P.M., Swerdlow D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein E.J., Stapp J.R., Clausen C.R. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point-of-care study. J Pediatr. 2002;141:172–227. doi: 10.1067/mpd.2002.125908. [DOI] [PubMed] [Google Scholar]
- 28.Jones T.F., Ingram L.A., Cieslak P.R. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 29.Lan R., Alles M.C., Donohoe K., Martinez M.B., Reeves P.R. Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect Immun. 2004;72:5080–51518. doi: 10.1128/IAI.72.9.5080-5088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson F.L., Iida T., Swings J. Biodiversity of vibrios. Microbiol Mol Biol Rev. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam M., Sultana M., Nair G.B. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci USA. 2007;104:17801–17806. doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson F.L., Hoste B., Vandemeulebroecke K., Swings J. Reclassification of Vibrio hollisae as Grimontia hollisae gen. nov., comb. nov. Int J Syst Evol Microbiol. 2003;53:1615–1617. doi: 10.1099/ijs.0.02660-0. [DOI] [PubMed] [Google Scholar]
- 33.Hinestrosa F., Madeira R.G., Bourbeau P.P. Severe gastroenteritis and hypovolemic shock caused by Grimontia (Vibrio) hollisae infection. J Clin Microbiol. 2007;45:33462–35353. doi: 10.1128/JCM.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neubauer H., Aleksic S., Hensel A., Finke E.J., Meyer H. Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int J Med Microbiol. 2000;290:61–64. doi: 10.1016/S1438-4221(00)80107-1. [DOI] [PubMed] [Google Scholar]
- 35.Vento S., Cainelli F., Cesario F. Infections and thalassaemia. Lancet Infect Dis. 2006;6:226–233. doi: 10.1016/S1473-3099(06)70437-6. [DOI] [PubMed] [Google Scholar]
- 36.Nuorti J.P., Niskanen T., Hallanvuo S. A widespread outbreak of Yersinia pseudotuberculosis O:3 infection from iceberg lettuce. J Infect Dis. 2004;189:766–774. doi: 10.1086/381766. [DOI] [PubMed] [Google Scholar]
- 37.Jalava K., Hakkinen M., Valkonen M. An outbreak of gastrointestinal illness and erythema nodosum from grated carrots contaminated with Yersinia pseudotuberculosis. J Infect Dis. 2006;194:1209–1216. doi: 10.1086/508191. [DOI] [PubMed] [Google Scholar]
- 38.Hannu T., Mattila L., Nuorti J.P. Reactive arthritis after an outbreak of Yersinia pseudotuberculosis serotype O:3 infection. Ann Rheum Dis. 2003;62:866–869. doi: 10.1136/ard.62.9.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandenberg O., Dediste A., Houf K. Arcobacter species in humans. Emerg Infect Dis. 2004;10:1863–1867. doi: 10.3201/eid1010.040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snelling W.J., Matsuda M., Moore J.E., Dooley J.S. Under the microscope: Arcobacter. Lett Appl Microbiol. 2006;42:7–14. doi: 10.1111/j.1472-765X.2005.01841.x. [DOI] [PubMed] [Google Scholar]
- 41.Hof H. Listeria monocytogenes: a causative agent of gastroenteritis? Eur J Clin Microbiol Infect Dis. 2001;20:369–373. doi: 10.1007/pl00011277. [DOI] [PubMed] [Google Scholar]
- 42.Ooi S.T., Lorber B. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis. 2005;40:1327–1332. doi: 10.1086/429324. [DOI] [PubMed] [Google Scholar]
- 43.Hogenauer C., Langner C., Beubler E. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med. 2006;355:2418–2426. doi: 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 44.Ni X.P., Ren S.H., Sun J.R. Laribacter hongkongensis isolated from a patient with community-acquired gastroenteritis in Hangzhou City. J Clin Microbiol. 2007;45:255–256. doi: 10.1128/JCM.01400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo P.C., Lau S.K., Teng J.L. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet. 2004;363:1941–1947. doi: 10.1016/S0140-6736(04)16407-6. [DOI] [PubMed] [Google Scholar]
- 46.Hofstad T., Olsen I., Eribe E.R., Falsen E., Collins M.D., Lawson P.A. Dysgonomonas gen. nov. to accommodate Dysgonomonas gadei sp. nov., an organism isolated from a human gall bladder, and Dysgonomonas capnocytophagoides (formerly CDC group DF–3) Int J Syst Evol Microbiol. 2000;50(Pt.6):2189–2195. doi: 10.1099/00207713-50-6-2189. [DOI] [PubMed] [Google Scholar]
- 47.Heiner A.M., DiSario J.A., Carroll K., Cohen S., Evans T.G., Shigeoka A.O. Dysgonic fermenter-3: a bacterium associated with diarrhea in immunocompromised hosts. Am J Gastroenterol. 1992;87:1629–1630. [PubMed] [Google Scholar]
- 48.Martinez-Sanchez L., Vasallo E.J., Garcia-Gorrote E., Alcala L., Rodriguez-Créixems M., Bouza E. Clinical isolation of a DF-3 microorganism and review of the literature. Clin Microbiol Infect. 1998;4:344–346. [Google Scholar]
- 49.Grob R., Zbinden R., Ruef C. Septicemia caused by dysgonic fermenter 3 in a severely immunocompromised patient and isolation of the same microorganism from a stool specimen. J Clin Microbiol. 1999;37:1617–1618. doi: 10.1128/jcm.37.5.1617-1618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giaquinto C., Van Damme P., Huet F. REVEAL Study Group. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195:S26–S35. doi: 10.1086/516717. [DOI] [PubMed] [Google Scholar]
- 51.Fankhauser R.L., Monroe S.S., Noel J.S. Epidemiologic and molecular trends of ‘Norwalk-like viruses’ associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- 52.Walter J.E., Mitchell D.K. Astrovirus infection in children. Curr Opin Infect Dis. 2003;16:247–333. doi: 10.1097/00001432-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Schofield K.P., Morris D.J., Bailey A.S., de Jong J.C., Corbitt G. Gastroenteritis due to adenovirus type 41 in an adult with chronic lymphocytic leukemia. Clin Infect Dis. 1994;19:311–312. doi: 10.1093/clinids/19.2.311. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita T., Sugiyama M., Tsuzuki H., Sakae K., Suzuki Y., Miyazaki Y. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J Clin Microbiol. 2000;38:2955–2961. doi: 10.1128/jcm.38.8.2955-2961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu C.Y., Greninger A.L., Kanada K. Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc Natl Acad Sci USA. 2008;105:14124–14129. doi: 10.1073/pnas.0805968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellamy K., Alcock R., Babb J.R., Davies J.G., Ayliffe G.A. A test for the assessment of ‘hygienic’ hand disinfection using rotavirus. J Hosp Infect. 1993;24:201–210. doi: 10.1016/0195-6701(93)90049-6. [DOI] [PubMed] [Google Scholar]
- 57.Kosek M., Bern C., Guerrant R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204s. [PMC free article] [PubMed] [Google Scholar]
- 58.Luby S.P., Agboatwalla M., Feikin D.R. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–233. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 59.Frech S.A., Dupont H.L., Bourgeois A.L. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 60.Dennehy P.H. Rotavirus vaccines: an overview. Clin Microbiol Rev. 2008;21:198–208. doi: 10.1128/CMR.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goossens L.M., Standaert B., Hartwig N., Hövels A.M., Al M.J. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine. 2008;26:1118–1127. doi: 10.1016/j.vaccine.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 62.Jelinek T., Kollaritsch H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev Vaccines. 2008;7:561–567. doi: 10.1586/14760584.7.5.561. [DOI] [PubMed] [Google Scholar]
- 63.Mai N.L., Phan V.B., Vo A.H. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003;349:11390–14141. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 64.Marcus L.C., Froeschle J.E., Hill D.R. Safety of Typhim Vi vaccine in a postmarketing observational study. J Travel Med. 2007;14:386–391. doi: 10.1111/j.1708-8305.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- 65.Taylor B.V., Williamson J., Luck J., Coleman D., Jones D., McGregor A. Sensitivity and specificity of serology in determining recent acute Campylobacter infection. Intern Med J. 2004;34:250–258. doi: 10.1111/j.1444-0903.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 66.Thomson R.B., Jr . Specimen collection, transport, and processing: bacteriology. In: Murray P.R., Baron E.J., Jorgensen J.H., Landry M.L., Pfaller M.A., editors. Manual of clinical microbiology. 9th ed. American Society for Microbiology; Washington, DC: 2007. pp. 291–333. [Google Scholar]
- 67.Valenstein P., Pfaller M., Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120:206–211. [PubMed] [Google Scholar]
- 68.Klein E.J., Stapp J.R., Clausen C.R. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point-of-care study. J Pediatr. 2002;141:172–227. doi: 10.1067/mpd.2002.125908. [DOI] [PubMed] [Google Scholar]
- 69.Dediste A., Vandenberg O., Vlaes L. Evaluation of the ProSpecT Microplate Assay for detection of Campylobacter: a routine laboratory perspective. Clin Microbiol Infect. 2003;9:1085–1090. doi: 10.1046/j.1469-0691.2003.00705.x. [DOI] [PubMed] [Google Scholar]
- 70.Nato F., Phalipon A., Nguyen T.L., Diep T.T., Sansonetti P., Germani Y. Dipstick for rapid diagnosis of Shigella flexneri 2a in stool. PLoS ONE. 2007;2:e361. doi: 10.1371/journal.pone.0000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dieckmann R., Helmuth R., Erhard M., Malorny B. Rapid classification and identification of salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol. 2008;74:7767–7778. doi: 10.1128/AEM.01402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H., Murdoch D.R. Detection of Campylobacter species in faecal samples by direct Gram stain microscopy. Pathology. 2004;36:343–344. doi: 10.1080/0031302042000224575. [DOI] [PubMed] [Google Scholar]
- 73.Turgeon D.K., Fritsche T.R. Laboratory approaches to infectious diarrhea. Gastroenterol Clin North Am. 2001;30:693–707. doi: 10.1016/S0889-8553(05)70206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Von Graevenitz A. The role of Aeromonas in diarrhea: a review. Infection. 2007;35:59–64. doi: 10.1007/s15010-007-6243-4. [DOI] [PubMed] [Google Scholar]
- 75.Wilkes J.G., Rushing L.G., Gagnon J.F. Rapid phenotypic characterization of Vibrio isolates by pyrolysis metastable atom bombardment mass spectrometry. Antonie Van Leeuwenhoek. 2005;88:151–161. doi: 10.1007/s10482-005-3990-z. [DOI] [PubMed] [Google Scholar]
- 76.Hartling L., Bellemare S., Wiebe N., Russell K., Klassen T.P., Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD004390.pub2. CD004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Bruyn G., Hahn S., Borwick A. Antibiotic treatment for travellers’ diarrhoea. Cochrane Database Syst Rev. 2000;(3) doi: 10.1002/14651858.CD002242. CD002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Abri S., Beeching N., Nye F. Traveller's diarrhoea. Lancet Infect Dis. 2005;5:349–360. doi: 10.1016/S1473-3099(05)70139-0. [DOI] [PubMed] [Google Scholar]
- 79.Capilla S., Ruiz J., Goñi P. Characterization of the molecular mechanisms of quinolone resistance in Yersinia enterocolitica O:3 clinical isolates. J Antimicrob Chemother. 2004;53:1068–1071. doi: 10.1093/jac/dkh225. [DOI] [PubMed] [Google Scholar]
- 80.Tribble D.R., Sanders J.W., Pang L.W. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346. doi: 10.1086/510589. [DOI] [PubMed] [Google Scholar]
- 81.Rutala W.A. Disinfection and sterilization of patient-care items. Infect Control Hosp Epidemiol. 1996;17:377–384. doi: 10.1086/647324. [DOI] [PubMed] [Google Scholar]
- 82.Zerr D.M., Allpress A.L., Heath J., Bornemann R., Bennett E. Decreasing hospital-associated rotavirus infection: a multidisciplinary hand hygiene campaign in a children's hospital. Pediatr Infect Dis J. 2005;24:397–403. doi: 10.1097/01.inf.0000160944.14878.2b. [DOI] [PubMed] [Google Scholar]
- 83.O'Horo J., Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis. 2009;13(6):663–667. doi: 10.1016/j.ijid.2008.11.012. [DOI] [PubMed] [Google Scholar]


