Abstract
Immunoglobulins (Igs), also called antibodies, are present in milk and colostrum of all lactating species. Igs are divided into classes having different physico-chemical structures and biological activities. The major Ig classes in bovine and human milk are IgA, IgG and IgM. Bovine colostral Igs provide the newborn calf with passive immune protection against microbial infections until the calf’s own immune system matures. Colostral Ig preparations designed for farm animals have been commercially available for many years. Potential health benefits attributed to bovine colostrum have increased manufacture and marketing of colostral Ig-based dietary supplements also for human use. Furthermore, specific anti-microbial antibodies can be produced into colostrum by immunizing cows with vaccines made of pathogenic microorganisms. These antibodies can be concentrated and used to formulate so-called immune milk preparations. Such preparations have proven effective in prevention of animal and human infections caused, e.g. by rotavirus, Shigella flexneri, Escherichia coli, Clostridium difficile, Streptococcus mutans, Cryptosporidium parvum and Helicobacter pylori. Their therapeutic efficacy, however, seems limited. A few immune milk products have been commercialized and more can be expected in the future for use, e.g. as a supportive means in antibiotic treatments and for prevention of hospital infections.
Key words: immunoglobulin, antibody, colostrum, passive immunity, immune milk
10.1. Introduction
It has been recognized for more than 100 years that maternal colostrum and milk offer passive protection to a newborn infant against enteric pathogens, primarily via the transfer of immunoglobulins (Igs) and associated bioactive factors (Ehrlich, 1892). Ruminant neonates are born virtually without Igs and therefore the colostral Igs are essential for survival. Probably due to this unique function, Igs represent the major protein fraction of colostrum, accounting for 70–80% of its total protein content (Butler, 1998, Marnila and Korhonen, 2002).
The Ig fraction can be concentrated from colostrum or cheese whey and is marketed commercially as a feed supplement and replacer of colostrum, mainly for neonate calves and pigs, to prevent gastrointestinal infections (Scammell, 2001, Mehra et al., 2006).
The advent of functional foods has increased interest in the bioactive components of bovine colostrum and milk as potential ingredients for health-promoting foodstuffs and even biopharmaceuticals. To this end, scientific and commercial attention has been focused on the development of bovine-derived Ig products that contain specific antibodies targeted for prevention or treatment of microbial infections in humans (Korhonen et al., 1998, Korhonen, 2002). Such preparations have been described as ‘immune milk’. This concept dates back to the 1950s when Petersen and Campbell first suggested that orally administered bovine colostrum from hyperimmunized cows could provide passive immune protection for humans (Campbell and Petersen, 1963). Since then, a large number of animal and human studies have been carried out to demonstrate that these preparations can be effective in the prevention or treatment of human and animal diseases caused by various pathogenic microbes (for reviews see Weiner et al., 1999, Korhonen et al., 2000a, Lilius and Marnila, 2001, Hoerr and Bostwick, 2002, Korhonen and Marnila, 2006, Mehra et al., 2006). This article reviews the current state of knowledge about the properties of bovine Igs, their utilization as ingredients for immune milk preparations, and the application of these preparations for the prevention and treatment of various microbial infections in humans.
10.2. Properties of immunoglobulins
10.2.1. Structure and physicochemical properties
Igs, which carry the biological function of antibodies, are present in colostrum and milk of all lactating species. In mammals, all five known classes of Igs have been characterized: IgG, IgM, IgA, IgD and IgE. The major Ig classes in both bovine and human milk are IgA, IgG and IgM. The basic chemical structure of all Igs is similar but their biological functions differ, although in principle they all contribute to the major defence mechanism against foreign materials recognized by the body’s immune system. Igs account for up to 70–80% of the total protein content in colostrum, whereas in milk they account for only 1–2% of total protein. IgG1 is the predominant Ig class in bovine lacteal secretions as compared to IgA in human milk (Elfstrand et al., 2002, Marnila and Korhonen, 2002). Table 10.1 provides the concentrations of different Ig classes in bovine colostrum and milk.
Table 10.1.
Immunoglobulin concentrations in bovine colostrum and milk
| Immunoglobulin class | Molecular mass (kDa) | Concentration (gL− 1) |
|
|---|---|---|---|
| Milk | Colostrum* | ||
| IgG1 | 146–163 | 0.3–0.6 | 15–180 |
| IgG2 | 146–154 | 0.06–0.12 | 1–3 |
| IgG total | 0.15–0.8 | 20–200 | |
| SIgA | 385–430 | 0.05–0.1 | 1–6 |
| IgM | 900 | 0.04–0.1 | 3–9 |
Data compiled from Marnila and Korhonen (2002), Elfstrand et al. (2002) and Mehra et al. (2006).
= first milking.
Ig molecules of all classes are symmetrical, multi-chain glycoproteins composed of two identical glycosylated heavy chains and two identical non-glycosylated light chains. The basic structure of all monomeric Igs is similar (see Fig. 10.1 ). The IgG class can be considered as a general model of a monomeric Ig. The molecular weight of each light chain is around 23 kDa and of the heavy chains 53 kDa. The molecular weight of the complete Ig molecule varies around 160 kDa. Both the light and heavy chains contain domains referred as constant regions (CL, CH) and variable regions (VL, VH). The light chains are attached to the heavy chains by a disulphide bond, and also the two heavy chains are held together by two disulphide bonds near a hinge region which gives the molecule structural flexibility needed in antibody–antigen interactions (Nezlin, 1998a). The two identical antigen-binding sites needed in these interactions are formed by the N-terminal region of one heavy chain and the variable region of one light chain. The VL-region determines the immunological specificity. Antigen binding occurs by the interactions between the antigen and these regions. The Ig classes and subclasses are determined by the genes encoding the constant regions of heavy chains (Butler, 1998). The bovine IgG molecule occurs dominantly in two sub-classes: IgG1 and IgG2.
Fig. 10.1.
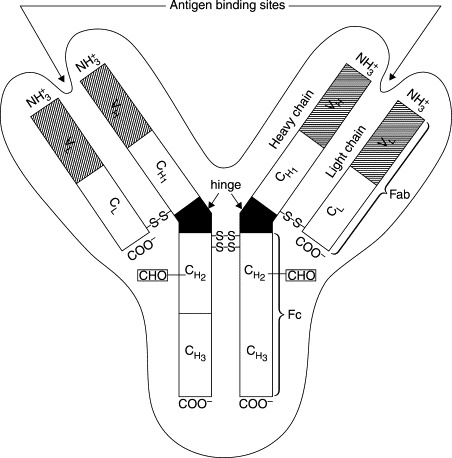
Schematic diagram of a basic immunoglobulin.
From Larson (1992).
Monomeric IgM and IgA have a similar basic structure to IgG except for the differences in heavy chain structures and the addition of a C-terminal octapeptide to the heavy chain of IgA (Butler, 1998, Nezlin, 1998b). Monomeric IgA occurs in serum, but in milk it is present as a dimer comprising two IgA molecules joined together by a polypeptide J-chain and an additional 75 kD secretory component. This secretory IgA (SIgA) has a molecular weight of about 380 kDa, and is more resistant to proteolysis and therefore more stable in the gastrointestinal tract than antibodies without the secretory component. IgM is a circular pentamer consisting of five subunits similar to those of monomeric IgA or IgG, which are linked together in a circular mode by disulphide bonds and a J chain: the molecular weight of IgM is approximately 900 kDa (see Fig. 10.2 ). The physicochemical properties of Igs are presented in Table 10.2 .
Fig. 10.2.
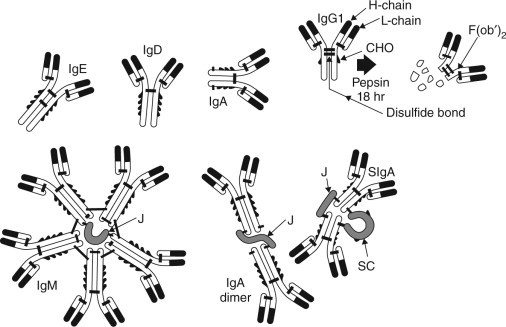
Structure of five classes of immunoglobulins.
Figure modified from Larson (1992).
Table 10.2.
Properties of bovine immunoglobulins
| IgG1 | IgG2 | IgA | SIgA | IgM | |
|---|---|---|---|---|---|
| Physico-chemical | |||||
| Molecular weight (kDa) | 146–163 | 146–154 | 160 | 385–430 | 900 |
| Heavy chain | 56–59 | 54–59 | 61–63 | 62–76 | |
| Heavy chain type | γ1 | γ2 | α | α | μ |
| Number of H- and L-chains | 2 | 2 | 2 | 4 | 10 |
| Structure | Monomer | Monomer | Monomer | Dimer | Pentamer |
| Immunological | |||||
| Opsonization | +++ | + | 0 | 0 | +++ |
| Complement fixation | ++ | + | 0 | 0 | +++ |
| Agglutination | + | + | ++ | ++ | +++ |
0 = no activity, + = low activity, ++ = moderate activity, +++ = strong activity.
Data modified from Marnila and Korhonen (2002).
Proteolysis of an Ig with papain cleaves it near the hinge region into two antigen binding-sites (Fab-fragments), which are formed by the N-terminal part of one heavy and one light chain, and to the third C-terminal fragment,termed the Fc-part. The differences between Ig classes impact upon the cleavage patterns in enzymatic proteolysis. For instance, cleavage of bovine IgG 2a(A2) by pepsin results to two Fab and one Fc parts, whereas in IgG2a(A1) the Fab parts remain together by one disulphur bridge resulting in one F(abʹ)2 and one Fc fragment which is cleaved further by pepsin (Heyermann and Butler, 1987).
10.2.2. Biological activities
Igs are produced by B lymphocytes and plasma cells diversified from B cells. The soluble Igs function as flexible adaptors between cellular and humoral immunity by binding to antigens and exhibiting one or more effector functions. The Fab parts attach the antigens while other parts (mostly the Fc region) interact with other elements of the immune system.
For many pathogenic organisms, the attachment to the epithelial lining is an obligatory first step in the establishment of colonization and infection. Many pathogens have receptors, e.g. fimbriae for epithelial surfaces. Thus, the prevention of the microbial adhesion to epithelial linings is an important mechanism of SIgA antibodies in protecting the host (Woof and Kerr, 2006). Colostrum and human milk protect the neonate intestinal mucosa against EPEC infections by inhibiting bacterial adhesion to epithelial cells (Carbonare et al., 1997). This effect has been shown to be mediated by colostrum and milk SIgA (Cámara et al., 1994; Carbonare et al., 1997, Carbonare et al., 2005, Fernandes et al., 2001, Corrêa et al., 2006), and by oligosaccharides present in human milk (Cravioto et al., 1991). M-cells in intestinal epithelia have specific receptors for SIgA, and antigen-SIgA-receptor complex can be transcytosed by M-cells into lymphoid tissue for antigen presenting and induction of mucosal immune responses (Woof and Kerr, 2006).
The ability of Igs to form cross-links between surface antigens results in a network of cells that can be removed mechanically from the body. This agglutination of microbes reduces their capability to adhere to surfaces. Agglutinated microbes are usually not able to release toxins or to colonize the host. Normal colostrum and milk are known to contain natural antibodies which can agglutinate a large number of pathogenic and non-pathogenic micro-organisms (Bostwick et al., 2000, Korhonen et al., 2000b).
Igs can bind bacterial toxins which, thereafter, can be recognized more effectively by phagocytic leukocytes. Many bacterial toxins must first be actively transported via receptors inside the host cells to cause cell death. Blocking the toxin may prevent its internalization in host cells. Also, Igs can inhibit or reduce the production of toxins and other harmful components by inhibiting bacterial metabolism and by blocking enzymes and receptors. This blocking may also reduce the ability of pathogens to produce structures needed in adherence to epithelia. Specific Igs can protect against viral infections by binding viruses and preventing the virus replication by blocking the receptor mediated internalisation of viruses in the host cells. Specific Igs augment the recognition and phagocytosis of antigens by leukocytes (opsonisation). Divalent or polyvalent binding of an Ig to antigenic structure results in conformational change of the Ig molecule, which process again enables the Fc portion to bind to the corresponding Fc receptors on the leukocyte surface. This receptor binding leads to various immune cell effector functions depending on the cell Ig and leukocyte type. Leukocytes are an integral part of normal milk and colostrum and are of vital importance in defending the mammary gland against pathogens (Korhonen et al., 2000b).
Specific Igs are rarely cidal to micro-organisms but may disturb cellular metabolism by blocking receptors and enzymes, and cause structural alterations leading to immobilization, increased membrane permeability and impaired cell growth. Igs may contribute to the killing of microbes by activating the classical pathway of complement. In blood and tissues, the activation of complement mediated bacteriolytic reactions may be the most important function of Igs, but the significance of this mechanism in milk remains obscure. Bovine and human colostrum contain an active complement system participating in the immune defense of the udder (Butler, 1998).
IgM antibodies, although produced in smaller amounts than IgG, are considerably more efficient than IgG with regard to most of the above activities, including complement fixation, opsonization and agglutination of bacteria. Bovine IgG2B is more effective than IgG2A in activating the classical pathway of complement (Bastida-Corquera, 1999). Specific IgM can prevent effectively the migration of bacteria by binding the flagellas used in the movement. Bovine IgA does not fix complement or opsonize bacteria, but it agglutinates antigens, neutralizes viruses and bacterial toxins. The SIgA dimer is found in mucosal secretions and its main function is to protect the mucosal barriers by binding microbes and preventing their attachment to the epithelium, and to contribute to the antigen presenting. The milk Igs have also been found to exert a synergistic effect on the activity of non-specific antimicrobial factors in milk, such as lactoferrin and lysozyme, as well as the lactoperoxidase-thiocyanate-hydrogen peroxide system (Loimaranta et al., 1998a, Bostwick et al., 2000).
10.3. Production and isolation of immunoglobulins
Traditionally, the globulin fraction was isolated from colostral whey by precipitation with either ammonium sulphate or ethanol. These chemical methods are, however, economically not suitable for large scale-production of Igs although they yield rather pure Ig fractions. Other challenges in isolation of active Igs from colostrum has been its complex composition and the sensitivity of Igs to heat treatments. With the rapid development of new chromatographic and membrane-separation technologies in the last thirty years, it is now possible to isolate individual milk proteins on a large-scale. Based on these techniques, a great number of pilot- or industrial-scale methods have been developed and patented for fractionation and isolation of Igs from colostral or cheese whey (for reviews see Korhonen, 2004, Korhonen and Pihlanto, 2007). With these methods, the recovery rate of Igs has varied from 40% to 70% of the level present in the starting material (Elfstrand et al., 2002). Specific chromatographic techniques, such as immobilized metal chelate chromatography, immunoaffinity chromatography and cation-exchange chromatography have been applied to improve the yield and purity of immunoglobulin preparations further (Fukumoto et al., 1994, Akita and Li-Chan, 1998). Microfiltration (MF) combined with ultrafiltration (UF) of bovine, equine and caprine colostrum has led to IgG/total solids purity of more than 90% (Piot et al., 2004). Using cheese whey, a 0.1 μm membrane and the concept of selective membrane separation through pH manipulation, Mehra and Kelly (2004) produced an Ig-rich preparation with a protein composition similar to that produced from colostral whey. Korhonen et al. (1998) used various MF methods, such as UF, MF and reverse osmosis, and a cation-exchange resin as a molecular sieve, to concentrate Igs from colostral whey. The Ig level of the final freeze-dried concentrates varied from 45% to 75%. Expanded Bed Adsorption Chromatography (EBAC) is a new technology which has been applied to isolate Igs from cheese whey using an adsorbent with tailored ligand chemistry (Nielsen et al., 2002). EBAC provides significant advantages over conventional packed bed column chromatography, and an Ig-purity from 50% to 70% can be achieved with this up-scalable method.
The technological properties of Igs have been studied extensively, as these proteins are known to affect many dairy processes (see review by Mehra et al., 2006). Igs may inhibit or retard the activity of renneting enzymes of bacterial origin. In normal milk this is not noticed due to the relatively low Ig concentration but in case of mastitic milk, or if the Ig concentration of milk is increased, e.g. by adding colostrum, renneting may be retarded. The antimicrobial properties of Igs may adversely impact upon fermentation processes. A retarded fermentation by dairy starters is noted in colostrum and mastitic milk, which contain increased amounts of Igs.Also, high Ig concentrations may adversely affect the antibiotic residual tests based on microbial growth, causing false positive results (Korhonen,2004). Igs contribute to cream formation by agglutinating fat globules,which process accelerates the ascent of cream to the surface. This phenomenon is attributed primarily to IgM, which have been termed cryoglobulins or ‘cold agglutinins’. The agglutination property of Igs can, however, be inactivated by heat treatment (pasteurization) and mechanical agitation.
Among dairy processes, the properties of Igs are most affected by the thermal treatments. In high temperature/short time (HTST) pasteurization (72 °C/15 sec) about 25–40% of the Ig activity is lost, whereas ultra high temperature (UHT) treatment (138 °C/4 sec) and evaporation processing destroy most of the specific immune activity of milk due to Ig denaturation (Li-Chan et al., 1995). In contrast, other studies (Mainer et al., 1997, Mainer et al., 1999) have reported that bovine milk Igs could resist the HTST pasteurization treatment without affecting their structure. Only 1% of IgG, 2% of IgA, and 14% of IgM concentrations were denatured in laboratory experiments. Also, the HTST pasteurization had little effect on the activity of bovine colostral IgG as the original rotavirus neutralizing activity was reduced by only 0.5%. In a recent study by McMartin et al. (2006), it was demonstrated that a rapid heat inactivation of IgG started at temperatures higher than 65°C, and at 81°C, as much as 90% of the virus neutralization activity of Igs was lost in less than two minutes. On the other hand, heating moderate or high quality colostrum at 60°C for at least 120 min had no effect on mean IgG concentration or titer of neutralizing antibodies against bovine viral diarrhea virus Type 1. In storage stability studies, it has been observed that bovine IgG added into UHT-treated milk retained its specific immune activity for over several months (Fukumoto et al., 1994). Also, Ig molecules seem to retain their specific activity well in milk powder, irrespective of the storage temperature (Mehra et al., 2006). Further, bacterial fermentation of milk by yoghurt or probiotic bacteria have not been found to reduce significantly the activity of colostral antibodies added in milk during a storage period of 50 days of at 4°C. (Wei et al., 2002).
10.4. Applications of immunoglobulins
10.4.1. Immunoglobulin and immune milk reparations
The progress in understanding the mechanisms of Ig mediated immune functions and the rapid development of industrial fractionation technologies have raised interest in developing formulations supplemented with bovine colostral or cheese whey derived Igs. Most of the current commercial Ig products are prepared from colostrum of non-immunized cows by removing the fat, followed by microfiltration or pasteurization under conditions that retain the biological activity of Igs. These products are usually in the form of spray-dried and freeze-dried powders, but some are in the form of filtered colostral whey liquids or concentrates. Dried products include whole colostrum powder, skim powders, skim colostrum protein concentrate and colostrum whey concentrates. Some of above preparations have been tested clinically for certain physiological functions or prevention/treatment of microbial infections. Accordingly, a few products boast specific health or nutrition function claims, such as boosting immunity against microbial infections or speeding recovery from physical endurance exercises. (Scammel, 2001; Kelly, 2003, Tripathi and Vashishtha, 2006). However, in most cases, the clinical evidence related to these products is very limited or not available.
The specificity of natural antibodies found in milk and colostrum of different cows reflects the wide spectrum of antigens the animals have encountered in the past in their environment and in ingested feedstuffs (Korhonen et al., 2000a, Kelly, 2003). The antibody titre against certain antigenic pathogens or structure, e.g. virulence factor, can be raised up to several hundred times by immunizing the cow before parturition with vaccines containing the antigens (Korhonen et al., 1995). The resulting immune colostra and products made thereof have fundamentally different antimicrobial properties and efficacies against pathogens than normal colostrum and these two concepts have, therefore, to be differentiated. This is emphasized by the fact that the normal colostrum and preparations made from it are, in most countries, regarded as food or dietary supplement, whereas the Ig containing preparations from immunized cows are often regarded as pharmaceuticals, e.g. in the EU and USA, or their regulatory status is not defined (Scammel, 2001; Hoerr and Bostwick, 2002, Mehra et al., 2006). Table 10.3 lists commercial Ig and immune milk preparations developed over the last decade.
Table 10.3.
Commercial colostrum and immune milk products
| Product | Company | Claimed health benefits |
|---|---|---|
| Intact™ | Numico RA (Australia) | Immune enhancing, athletic performance |
| Gastrogard-R™ (from immunized cows) | Northfield Laboratories, (Australia) | Prevents diarrhea caused by rotavirus in infants and children < 4 years |
| PRO-IMMUNE 99 | GalaGen Inc., (USA) | Prevents scours caused by E.coli in calves. |
| Proventra™ | GalaGen Inc., (USA) | Boosts immunity and enhances body’s natural resistance |
| Lactimmunoglobulin Biotest | Biotest Pharm GmbH (Germany) | Product for treatment of diarrhea in AIDS patients |
| ColostrumGold™ liquid Colostrumune™ powder |
Sterling Technology, Inc (USA) | Immune system booster |
| First DefenceR (from immunized cows) | Immucell (USA) | Reduces mortality and morbidity from scours caused by E.coli K99+ and coronavirus in calves |
| Anti-CD WPC (from immunized cows) | MucoVax Ltd (Netherlands) | Prevents relapse of C. difficilediarrhea |
| Glycomax™ Immunoglobulin | Probiotec Nutritionals Ltd (Australia) | Improves immunity against microbes and improves health and wellbeing |
| ColoPlus™ | ColoPlus Ltd. (Sweden) | Alleviates HIV-associated diarrhea |
Data modified from Mehra et al. (2006).
10.4.2. Proteolysis of immunoglobulins in the gastrointestinal tract
It is well known that the low pH of gastric acid reduces significantly the activities of ingested Igs. Furthermore, the ingested Igs are subjected to degradation by intestinal proteases. In the stomach, the enzymatic hydrolysis by pepsin, fragments the IgG molecule to F(ab′)2, Fab/c, pFc and Fv fragments (Nezlin, 1998a). In the small intestine, trypsin, chymotrypsin, carboxypeptidase and elastase initially degrade the antibodies to F(ab′)2, Fab and Fc fragments. The secretory piece of milk SIgA protects this Ig form against digestion by proteolytic enzymes. Of the SIgA present in human colostrum, 20–80% passes undegraded through the gut of the human infant. Also, bovine IgG1 makes an exception since it is rather resistant to trypsin. Bovine colostrum and mastitic milk contain a compound which inhibits trypsin activity. The resulting F(ab′)2 and Fab fragments retain at least part of the neutralizing and adhesion inhibiting activities in the intestine. Pacyna et al. (2001) supplemented whole milk with immune colostrum containing specific Igs against rotavirus. When 100 ml of this supplemented whole milk was administered to 105 children three times a day for six days, the anti-rotavirus Ig activity was detected by the ELISA method in 521 of 602 fecal specimens obtained during the study. Kelly et al. (1997) demonstrated that 10%–30% of orally administered bovine anti-Clostridium difficile Ig concentrate could be recovered from the stools of human infants and adults in the form of F(ab′)2 and Fab fragments. The survival of IgG increased remarkably by encapsulation with gelatin.
10.4.3. Efficacy of immune milk preparations against microbial pathogens
The concept of ‘immune milk’, i.e. the transfer of passive immunity by milk Igs, dates back to the 1950s. Since then, a great number of clinical studies have been published on the efficacy of various immune and non-immune milk Ig preparations in the prevention and treatment of various gastrointestinal microbial diseases. Recently, these studies have been reviewed by Mehra et al. (2006), Korhonen and Marnila (2006) and Hammarström and Weiner (2008). Thus, the protocols and results of these numerous studies are not discussed in detail in this article. Briefly, the general tendency of these studies has been that orally administered bovine milk or colostral Igs have, in most cases, proved to be effective in the prevention of orally mediated infections. Colostral or milk Igs can effectively agglutinate bacteria (Xu et al., 2006), and bovine colostrum and colostral preparations prevent effectively the attachment of a pathogen to the cells in epithelial lining. However, since bovine colostrum contain more than one adhesion inhibiting factor, the importance of specific Igs is often not known. Palmeira et al. (2001) observed that inhibition of enteropathogenic E. coli adherence to HEp-2 cultured cells by bovine colostrum was not caused by IgG fraction and was mediated by a high molecular weight fraction. However, in some studies the inhibition of pathogenic adhesion to epithelial cells has been attributed to presence of specific Igs. Casswall et al. (2002) showed that a specific bovine immune colostral preparation blocked almost 90% of Helicobacter pylori attachment to human gastric mucosal tissue in vitro and 95% of binding to Lewis b glycoconjugate, while a control colostral preparation from nonimmunized cows was inefficient. Doyle et al. (1993) reported that hyperimmune bovine colostral Ig inhibited Cryptosporidium parvum adherence to epithelial MDCK cells and infectivity in an in vitro assay, and this inhibition was correlated with the protective capacity of the bovine colostrum in vivo. No significant adhesion inhibition was observed for control preparation from colostra of sham immunized cows. Bojsen et al. (2007) studied the efficacy of bovine macromolecular whey protein fractions in prevention of infection of two human intestinal cell lines (Caco-2 and FHs 74 Int) by four different rotavirus strains (Wa, RRV, YM, RF). The major component of the protein fraction that inhibited effectively the rotavirus infectivity in vitro was shown to be bovine IgG. The same fraction was the only one affecting rotavirus shedding in an in vivo mouse model.
In the treatment of already established infections, promising therapeutic effects have been reported, mainly in such diseases where the infection is maintained through a reattachment and reinfection, e.g. inside the oral cavity or the gastrointestinal lumen, and where the secretion of toxins or other inflammatory compounds is involved, which can be neutralized by the specific colostral Igs. Diseases caused by enterotoxigenic Escherichia coli strains, Cryptosporidium parvum, Candida spp., Shigella flexneri and rotavirus are examples. As instances of clinical studies, a closer look is taken hereunder at recent progress in developing bovine immune milks targeted against Streptoccus mutans and Clostridium difficile. Table 10.4 provides data on recent clinical studies carried out with colostral Ig and immune milk preparations.
Table 10.4.
Recent in vivo studies on the efficacy of orally administered immunoglobulin preparations on health
| Antigen used in immunization | Disease or condition | Treatment regimen | Treatment effect | Reference |
|---|---|---|---|---|
| S. mutans and S. sobrinus whole cell vaccine | Dental caries | Mouth rinse 3 times daily for 3 days (human volunteers) | Higher resting pH and smaller proportion of S. mutans in dental plaque | Loimaranta et al. (1999) |
| Virulence factors of S. mutans | Dental caries | Mouth rinse twice per day for 14 days by 10 ml of immune milk (human volunteers) | Inhibited recolonization of S. mutans after antibiotic treatment | Shimazaki et al. (2001) |
| Helicobacter feliswhole cell vaccine | Gastritis | 0.2 ml of immune whey (7.5% IgG) before infection or 3 times daily for 4 weeks in treatment of infected mice | Prevented infection in non-infected mice and decreased gastric inflammation and colonization in readily infected mice | Marnila et al. (2003) |
| Helicobacter pyloriurease | Gastritis | 150 ml of yogurt with 1% avian IgY and probiotic bacteria 3 times daily for 4 weeks | Decreased values in urea breath test indicating decrease in colonization | Horie et al. (2004) |
| No immunization | EHEC-colitis | Colostral preparation IMMULACT® to mice ad libitum (around 300 mg per day) for 3 weeks | Decreased rapidly EHEC colonization and decresed attachment to cecum walls and decreased mortality in mouse | Funatogawa et al. (2002) |
| Polyvalent or monovalent E. coli vaccine | Diarrhea | IgG supplemented baby formula, daily dose 0.5 g of IgG per kg of body weight | Lower incidence of diarrhea and shorter duration of diarrhea episodes in human infants during follow-up period for 6 months | Tawfeek et al. (2003) |
| Shigella dysenteriaeantigen I | Shigellosis | 100 ml orally 3 times per day for 3 days in combination with antibiotics | No significant difference in any clinical parameter | Ashraf et al. (2001) |
| No immunization | HIV-associated diarrhea | Colostral preparation ColoPlus® to HIV-associated diarrhea patients for 7 weeks | Substantial decrease in stool frequency and in fatigue and increase in body weight | Florén et al. (2006) |
| Cocktail of 17 strains of pathogenic diarrhea bacteria | E. coli and Salmonella diarrhea | Orally 10 mg of specific Ig per day for 10 days starting 6 or 8 days before infection | Prevented enteroinvasive E. coli and Salmonella typhi diarrhea and normalized immunological parameters | Xu et al. (2006) |
| Cocktail of 17 strains of pathogenic diarrhea bacteria | E. coli, Salmonella and Shigella diarrhea | Orally once per day for 10 days starting 6 or 8 days before infection | Decreased the clinical signs of diarrhea and supported splenic NK-cell functions | Huang et al. (2008) |
| No immunization | Murine rotavirus infection in mouse | Milk IgG fraction orally at time of infection and 12 hours post infection | Decreases rotavirus shedding in stools | Bojsen et al. (2007) |
| Clostridium difficile toxin and C. difficile whole cells | C. difficile diarrhea | Orally for 2 weeks as supportive treatment after antibiotic treatment | C. difficile toxins eradicated from 15 of 16 patients and no relapses in any patient during 11-month follow-up period | Van Dissel et al. (2005) |
| No immunization | Mild hypercholesterolemia | Orally 5 g of blood derived IgG daily for 3 or 6 weeks | Both total cholesterol and LDL levels decreased from baselines | Earnest et al. (2005) |
| No immunization | Upper respiratory tract infections | 60 g of colostral protein daily for 8 weeks | Reduced significantly incidence of self-estimated symptoms of respiratory infections but no difference in duration | Brinkworth and Buckley (2003) |
| No immunization | Endotoxemia due to abdominal surgery | Colostral product Lactobin® 52 g daily in 4 doses orally for 3 days before surgery | Lower levels of endotoxin and endotoxin neutralizing capacity in blood suggesting reduced endotoxemia due to surgery | Bölke et al. (2002a) |
| No immunization | Endotoxemia due to coronary surgery | Colostral product Lactobin® 42 g daily doses orally for 2 days before surgery | Lower levels of CRP but no effect on perioperative endotoxemia | Bölke et al. (2002b) |
Parts of this table compiled and edited from Korhonen et al. (2000) and Mehra et al.. (2006).
Streptoccus mutans immune milk
The pathogenesis of dental caries caused by Streptococcus mutans involves a series of attachment and binding events that lead to accumulation of cariogenic bacteria sufficient to cause the caries. Immunization strategies against streptococcal adhesins or glucosyltransferase enzymes (GTFs) have been shown to effectively interfere with the pathogenesis of mutans streptococci (Smith et al., 2001). Blocking of the activity of S. mutans glucan binding proteins serves another effective strategy. Since humans cannot be immunized actively against caries bacteria due to the risk of side effects, bovine colostral antibodies have been studied in local passive immunization to prevent dental caries (for a review see Koga et al., 2002).
An immune milk preparation from cows immunized with a S. mutans and Streptococcus sobrinus whole cell vaccine inhibited in vitro glucosyltransferase and fructosyltransferase enzyme activities of S. mutans (Loimaranta et al., 1997), promoted aggregation of S. mutans, and inhibited adherence of the bacteria to saliva-coated hydroxyapatite particles (Loimaranta et al., 1998b). The same immune preparation resulted in a higher resting pH in dental plaque of adult volunteers as compared to control groups, when used as a mouth rinse for three days (Loimaranta et al., 1999). After the rinsing period with the immune product, the relative number of mutans streptococci had decreased significantly as compared to the controls.
Chicken egg yolk Igs obtained from immunized hens with S. mutans glucan binding protein had a clear protective effect against S. mutans infection and caries development in a rat model (Smith et al., 2001). Mitoma et al. (2002) immunized cows with a fusion protein prepared by a fusion of a saliva-binding alanine-rich region of a cell surface protein antigen (Pac) and a glucan binding domain of the glucosyltransferase-I cell surface protein from S. mutans. The immune colostrum preparation effectively prevented dental caries development in a rat model when given as concentrate once a day for 55 days, together with cariogenic diet. Shimazaki et al. (2001) examined the effect of immune colostrum containing Igs against the same fusion protein on adult subjects after an antibiotic (cetylpyridinium chloride) treatment. The immune preparation inhibited significantly recolonization of S. mutans in the saliva and plaque as compared to the control group, and the ratios of S. mutans to total streptococci in saliva and plaque were lower than in the control group. Wei et al. (2002) studied the combined effect of specific colostral Ig preparation against caries streptococci with the probiotic bacterium Lactobacillus rhamnosus GG, ATCC 53103 (LGG). LGG added in milk was earlier shown to reduce the risk of caries in day-care children (Näse et al., 2001). The LGG-bacteria and specific Igs in LGG-fermented milk synergistically inhibited adhesion of S. mutans to saliva-coated hydroxyapatite particles. The Igs remained active in UHT milk and in LGG fermented milk over the whole period of expected shelf-life of these products. Thus, the rinsing with bovine immune whey indicates favourable effects on human dental plaque by controlling S. mutans in the human oral cavity and it may be beneficial to combine specific bovine milk antibodies against mutans streptococci to probiotic LGGcontaining milk products.
Clostridium difficile immune milk
Colonization of Clostridium difficile in the intestine may cause severe infectious diarrhea and colitis. The most important virulence factors are the toxins A and B, which are associated with the development of the disease. Outbreak of C. difficile infection often results from antibiotic treatments. In animal models, bovine immune colostrum preparations have been effective in the treatment of experimental C. difficile diarrhea. Van Dissel et al. (2005) used a preparation made of milk from cows immunized against C. difficile toxins and whole cell C. difficile as a supportive treatment for two weeks after a standard antibiotic treatment in an uncontrolled cohort study. Nine of 16 patients had a history of relapsing C. difficile diarrhea. After the regimen in 15 patients, the C. difficile toxins had disappeared from feces, and during the follow-up period of 11 months, none of the patients had another episode of C. difficile diarrhea. Numan et al. (2007) assessed the efficacy of C. difficile immune milk preparation in aiding the prevention of relapses in C. difficile patients. The immune milk was administered orally 5 g/day, divided into three equal doses for 14 days starting two weeks after standard treatment with oral metronidazole or vancomycin. In 109 disease episodes, 11 (about 10%) were followed by a relapse, whereas in contemporary controls the relapse rate was 20–25%. Young et al. (2007) evaluated the safety of the same immune milk administered to 77 C. difficile patients, similarly as in the previous study. Adverse effect monitoring, physical examinations and haematological and biochemical assessments showed no adverse effects in this group. Mattila et al. (2008) immunized cows with a whole-cell vaccine made of two toxigenic C. difficile strains and the immune whey preparation was made from the colostra of these cows. In a controlled double-blind randomized study, 18 patients with C. difficile associated diarrhea received orally 1.6 g of Ig in 200 ml volume twice per day for 2 weeks, and 20 patients received 400 mg of metronidazole twice per day. A C. difficile culture and toxin test was made on days 0, 14 and 28, respectively. At day 14, 100% of the metronidazole group and 83% of the immune colostrum group, respectively, responded positively to the treatment. The authors concluded that the immune colostrum treatment is somewhat less effective than the standard treatment with antibiotics but, on the other hand, it does not cause antibiotic resistance problems and does not alter the normal colonic bacterial flora as antibiotics do. In future, by optimizing the dosage, the treatment time, and the antigens used in immunizing the cows, the efficacy of bovine milk Ig preparations could be improved and this treatment strategy could also be used as a supportive treatment together with conventional antibiotics.
10.5. Future trends
As a result of progress made in membrane separation and chromatographic techniques it is now possible to isolate Igs from bovine colostrum and cheese whey on a large scale. This progress has facilitated and boosted manufacture of Ig concentrates as dietary supplements or ingredients. Some of these preparations are being marketed with health or nutrition function claims attached, even though the clinical evidence is limited or not reported at all. More scientific research is therefore needed in this field to substantiate the claimed health benefits. As for immune milk preparations, there are already a few commercial products on the market with quite well established clinical evidence about the efficacy. The main limitation of the clinical use of bovine milk antibodies for humans is that, as proteins of another species, the Igs can be used only orally against gastrointestinal pathogens. An interesting attempt to overcome this limitation is a transchromosomic calf that has been cloned for producing humanized polyclonal Igs. As a result of five sequential genetic modifications and seven consecutive cloning events, this calf was reported to have full human IgG 10–20% of total serum IgG. After hyperimmunization of this calf with an anthrax protective antigen, both full human IgG and chimeric IgG were found to be effective in a toxin-neutralization assay (Kuroiwa et al., 2009).
The regulatory status regarding products containing specific Igs may be considered as the main reason why products (for instance those containing specific Igs against cariogenic bacteria) are not on the market in most countries. The regulatory status of immune colostral preparations was undetermined for a long time and for some years these products have been classified as pharmaceuticals in the EU and USA. In the case of diseases that can be controlled also by conventional means, e.g. dental caries, this regulatory approach has slowed down the development of immune milk preparations in many countries. However, in the case of severe diseases caused by bacterial strains resistant to antibiotics, such as C. difficile, the regulatory status of a pharmaceutical has delayed but not hampered completely the progress of such product development (Hoerr and Bostwick, 2002; Mattila et al., 2008). Currently, the US Food and Drug Administration (FDA) have accepted the safety of hyperimmune milks on the basis of clinical studies that show no adverse health effects from these products (Gingerich and Mcphillips, 2005, Krissansen, 2007).
Incidences if diarrhea and mortality rates among children in many areas of developing countries are high. The prevention of diarrhea would improve the nutritional status and health in general. The emergence of antibiotic resistant pathogen strains puts emphasis on the need to develop alternative ways to prevent and treat gastrointestinal infections. In developed countries, the control of gastrointestinal microbial flora has become an integral part of health promotion. It is concluded that synergistic effects of Igs with probiotics and other milk bioactives, such as lactoferrin, may open new prospects for developing novel means to prevent microbial diseases by nutritional intervention.
10.6 References
- Akita E.M., Li-Chan E.C.Y. Isolation of bovine immunoglobulin G subclasses from milk, colostrum, and whey using immobilised egg yolk antibodies. Journal of Dairy Science. 1998;81:54–63. doi: 10.3168/jds.S0022-0302(98)75550-X. [DOI] [PubMed] [Google Scholar]
- Ashraf H., Mahalanabis D., Mitra A.K., Tzipori S., Fuchs G.J. Hyperimmune bovine colostrum in the treatment of shigellosis in children: A double-blind, randomized, controlled trial. Acta Paediatrica. 2001;90:1373–1378. doi: 10.1080/08035250152708743. [DOI] [PubMed] [Google Scholar]
- Bastida-Corcuera F.D., Butler J.E., Yahiro S., Corbeil L.B. Differential complement activation by bovine IgG2 allotypes. Veterinary Immunololy and Immunopathology. 1999;71(2):115–123. doi: 10.1016/S0165-2427(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Bojsen A., Buesa J., Montava R., Kvistgaard A.S., Kongsbak M.B., Petersen T.E., Heegaard C.W., Rasmussen J.T. Inhibitory activities of bovine macromolecular whey proteins on rotavirus infections in vitro and in vivo. Journal of Dairy Science. 2007;90:66–74. doi: 10.3168/jds.S0022-0302(07)72609-7. [DOI] [PubMed] [Google Scholar]
- Bölke E., Jehle P.M., Hausmann F., Däubler A., Wiedeck H., Steinbach G., Storck M., Orth K. Preoperative oral application of immunoglobulin-enriched colostrums milk and mediator response during abdominal surgery. Shock. 2002;17:9–12. doi: 10.1097/00024382-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Bölke E., Orth K., Jehle P.M., Schwarz A., Steinbach G., Schleich S., Ulmer C., Storck M., Hannekum A. Enteral application of an immunoglobulin-enriched colostrum milk preparation for reducing endotoxin translocation and acute phase response in patients undergoing coronary bypass surgery – a randomized placebocontrolled pilot trial. Wiener Klinische Wochenschrift. 2002;114(21–22):923–928. [PubMed] [Google Scholar]
- Bostwick E.F., Stejins J., Braun S. Lactoglobulins. In: Naidu A.S., editor. Natural Food Antimicrobial Systems. CRC Press; Boca Raton, Florida, USA: 2000. pp. 133–158. [Google Scholar]
- Brinkworth G.D., Buckley J.D. Concentrated bovine colostrum protein supplementation reduces the incidence of self-reported symptoms of upper respiratory tract infection in adult males. European Journal of Nutrition. 2003;42(4):228–232. doi: 10.1007/s00394-003-0410-x. [DOI] [PubMed] [Google Scholar]
- Butler J.E. Immunoglobulin diversity, B-cell and antibody repertoire development in large farm animals. Revue Scientifique et Technique. 1998;17(1):43–70. doi: 10.20506/rst.17.1.1096. [DOI] [PubMed] [Google Scholar]
- Câmara L.M., Carbonare S.B., Silva M.L., Carneiro-Sampaio M.M. Inhibition of enteropathogenic Escherichia coli (EPEC) adhesion to HeLa cells by human colostrum: Detection of specific sIgA related to EPEC outer-membrane proteins. International Archives of Allergy and Immunology. 1994;103(3):307–310. doi: 10.1159/000236645. [DOI] [PubMed] [Google Scholar]
- Campbell B., Petersen W.E. Immune milk – a historical survey. Dairy Science Abstracts. 1963;25:345–358. [Google Scholar]
- Carbonare S.B., Palmeira P., Silva M.L.M., Carneiro-Sampaio M.M.S. Human colostrum IgA antibodies reacting to enteropathogenic Escherichia coli antigens and their persistence in the faeces of a breastfed infant. Journal of Diarrhoeal Diseases Research. 1997;15:53–58. [PubMed] [Google Scholar]
- Carbonare C.B., Carbonare S.B., Carneiro-Sampaio M.M. Secretory immunoglobulin A obtained from pooled human colostrum and milk for oral passive immunization. Pediatric Allergy and Immunology. 2005;16(7):574–581. doi: 10.1111/j.1399-3038.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- Casswall T.H., Nilsson H.O., Björck L., Sjöstedt S., Xu L., Nord C.K., Borén T., Wadström T., Hammarström L. Bovine anti-Helicobacter pylori antibodies for oral immunotherapy. Scandinavian Journal of Gastroenterology. 2002;37(12):1380–1385. doi: 10.1080/003655202762671242. [DOI] [PubMed] [Google Scholar]
- Corrêa S., Palmeira P., Carneiro-Sampaio M.M., Sanae Nishimura L., Guth B.E. ‘Human colostrum contains IgA antibodies reactive to colonization factors I and II of enterotoxigenic Escherichia coli. FEMS Immunology and Medical Microbiology. 2006;47(2):199–206. doi: 10.1111/j.1574-695X.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Tello A., Villafán H., Ruiz J., Vedovo S., Neeser J.R. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. The Journal of Infectious Diseases. 1991;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- Doyle P.S., Crabb J., Petersen C. Anti-Cryptosporidium parvum antibodies inhibit infectivity in vitro and in vivo. Infection and Immunity. 1993;61(10):4079–4084. doi: 10.1128/iai.61.10.4079-4084.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest C.P., Jordan A.N., Safi R.M., Weaver E., Church T.S. Cholesterollowering effects of bovine serum immunoglobulin in participants with mild hypercholesterolemia. American Journal of Clinical Nutrition. 2005;81(4):792–798. doi: 10.1093/ajcn/81.4.792. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Über Immunität durch Verebung und Zeugung. Zeitschrift für Hygiene und Infektionskrankheiten. 1892;12:183–203. [Google Scholar]
- Elfstrand L., Lindmark-Månsson H., Paulsson M., Nyberg L., Åkesson B. Immunoglobulins, growth factors and growth hormone in bovine colostrums and the effects of processing. International Dairy Journal. 2002;12:879–887. [Google Scholar]
- Fernandes R.M., Carbonare S.B., Carneiro-Sampaio M.M., Trabulsi L.R. Inhibition of enteroaggregative Escherichia coli adhesion to HEp-2 cells by secretory immunoglobulin A from human colostrum. The Pediatric Infectious Disease Journal. 2001;20(7):672–678. doi: 10.1097/00006454-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Florén C.H., Chinenye S., Elfstrand L., Hagman C., Ihse I. ColoPlus, a new product based on bovine colostrum, alleviates HIV-associated diarrhoea. Scandinavian Journal of Gastroenterology. 2006;41(6):682–686. doi: 10.1080/00365520500380817. [DOI] [PubMed] [Google Scholar]
- Fukumoto L.R., Li-Chan E., Kwan L., Nakai S. Isolation of immunoglobulins from cheese whey using ultrafiltration and immobilized metal affinity chromatography. Food Research International. 1994;27(4):335–348. [Google Scholar]
- Funatogawa K., Ide T., Kirikae F., Saruta K., Nakano M., Kirikae T. Use of immunoglobulin enriched bovine colostrum against oral challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiology and Immunology. 2002;46(11):761–766. doi: 10.1111/j.1348-0421.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- Gingerich D.A., Mcphillips C.A. Analytical approach to determination of safety of milk ingredients from hyperimmunized cows. Regulatory Toxicology and Pharmacology. 2005;41(2):102–112. doi: 10.1016/j.yrtph.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Weiner C.K. Targeted antibodies in dairy-based products. Advances In Experimental Medicine And Biology. 2008;606:321–343. doi: 10.1007/978-0-387-74087-4_13. [DOI] [PubMed] [Google Scholar]
- Heyermann H., Butler J.E. The heterogeneity of bovine IgG2-IV. Structural differencies between IgG2a molecules of the A1 and A2 allotypes. Molecular Immunology. 1987;24:1327–1334. doi: 10.1016/0161-5890(87)90128-3. [DOI] [PubMed] [Google Scholar]
- Hoerr R.A., Bostwick E.F. Commercializing colostrum based products: A case study of Galagen Inc. International Dairy Federation Bulletin. 2002;375:33–46. [Google Scholar]
- Horie K., Horie N., Abdou A.M., Yang J.O., Yun S.S., Chun H.N., Park C.K., Kim M., Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. Journal of Dairy Science. 2004;87(12):4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- Huang X.H., Chen L., Gao W., Zhang W., Chen S.J., Xu L.B., Zhang S.Q. Specific IgG activity of bovine immune milk against diarrhea bacteria and its protective effects on pathogen-infected intestinal damages. Vaccine. 2008;26:5973–5980. doi: 10.1016/j.vaccine.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Kelly C.P., Chetman S., Keates S., Bostwick E., Roush A.M., Castagliolo I., Lamont J.T., Pothoulakis C. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrobial Agents and Chemotherapy. 1997;41:236–241. doi: 10.1128/aac.41.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K.S. Bovine colostrums: A review of clinical uses. Alternative Medicine Review. 2003;8(4):378–394. [PubMed] [Google Scholar]
- Koga T., Oho T., Shimazaki Y., Nakano Y. Immunization against dental caries. Vaccine. 2002;20:2027–2044. doi: 10.1016/s0264-410x(02)00047-6. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Syväoja E.-L., Ahola-Luttila H., Sivelä S., Kopola S., Husu J., Kosunen T.U. Bactericidal effect of bovine normal and immune serum, colostrum and milk against Helicobacter pylori. Journal of Applied Bacteriology. 1995;78(6):655–662. doi: 10.1111/j.1365-2672.1995.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Syväoja E.-L., Vasara E., Kosunen T., Marnila P. Pharmaceutical composition, comprising complement proteins, for the treatment of Helicobacter infections and a method for the preparation of the composition. 1998. PCT Patent Application No. WO98/00150.
- Korhonen H., Marnila P., Gill H. Bovine milk antibodies for health: A review. British Journal of Nutrition. 2000;84(Suppl. 1):S1–S7. doi: 10.1017/s0007114500002361. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Marnila P., Gill H.S. Milk immunoglobulins and complement factors. British Journal of Nutrition. 2000;84(Suppl. 1):S75–S80. doi: 10.1017/s0007114500002282. [DOI] [PubMed] [Google Scholar]
- Korhonen H. Technology options for new nutritional concepts. International Journal of Dairy Technology. 2002;55:79–88. [Google Scholar]
- Korhonen H. Isolation of immunoglobulins from colostrum. International Dairy Federation Bulletin. 2004;389:78–81. [Google Scholar]
- Korhonen H., Marnila P. Bovine milk antibodies for protection against microbial human diseases. In: Mine Y., Shahidi F., editors. In: Nutraceutical Proteins and Peptides in Health and Disease. Taylor & Francis Group LLC; Boca Raton, USA: 2006. pp. 137–159. (Series: Nutraceutical Science and Technology 4. Series Editor Shahidi F. CRC Press). [Google Scholar]
- Korhonen H., Pihlanto A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Current Pharmaceutical Design. 2007;13(8):829–843. doi: 10.2174/138161207780363112. [DOI] [PubMed] [Google Scholar]
- Krissansen G.W. Emerging health properties of whey proteins and their clinical implications. Journal of the American College of Nutrition. 2007;26(6):713S–723S. doi: 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- Kuroiwa Y., Kasinathan P., Sathiyaseelan T., Jiao J.A., Matsushita H., Sathiyaseelan J., Wu H., Mellquist J., Hammitt M., Koster J., Kamoda S., Tachibana K., Ishida I., Robl J.M. National Biotechnology. 2009. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. 2009 Jan 18. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Larson B.L. Immunoglobulins of the mammary secretions. In: Fox P.F., editor. Advanced Dairy Chemistry 1: Proteins. Elsevier Science Publishers; London: 1992. pp. 231–254. [Google Scholar]
- Li-Chan E., Kummer A., Losso J.N., Kits D.D., Makai S. Stability of bovine immunoglobulins to thermal treatment and processing. Food Research International. 1995;28(1):9–16. [Google Scholar]
- Lilius E.-M., Marnila P. The role of colostral antibodies in prevention of microbial infections. Current Opinion in Infectious Diseases. 2001;14:295–300. doi: 10.1097/00001432-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Tenovuo J., Virtanen S., Marnila P., Syväoja E.-L., Tupasela T., Korhonen H. Generation of bovine immune colostrum against Streptococcus mutans and Streptococcus sobrinus and its effect on glucose uptake and extracellular polysaccharide formation by mutans streptococci. Vaccine. 1997;15(11):1261–1268. doi: 10.1016/s0264-410x(97)00027-3. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Tenovuo J., Korhonen H. Combined inhibitory effect of bovine immune whey and peroxidase-generated hypothiocyanite against glucose uptake by Streptococcus mutans. Oral Microbiology and Immunology. 1998;13(6):378–381. doi: 10.1111/j.1399-302x.1998.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Carlen A., Olsson J., Tenovuo J., Syväoja E.-L., Korhonen H. Concentrated bovine colostral whey proteins from Streptococcus mutans/Strep. sobrinus immunized cows inhibit the adherence of Strep. mutans and promote the aggregation of mutans streptococci. Journal of Dairy Research. 1998;65(4):599–607. doi: 10.1017/s0022029998003069. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Laine M., Söderling E., Vasara E., Rokka S., Marnila P., Korhonen H., Tossavainen O., Tenovuo J. Effects of bovine immune and non-immune whey preparations on the composition and pH response of human dental plaque. European Journal of Oral Sciences. 1999;107(4):244–250. doi: 10.1046/j.0909-8836.1999.eos107403.x. [DOI] [PubMed] [Google Scholar]
- Mainer G., Sánchez L., Ena J.M., Calvo M. Kinetic and thermodynamic parameters for heat denaturation of bovine milk IgG, IgA and IgM. Journal of Food Science. 1997;62(5):1034–1038. [Google Scholar]
- Mainer G., Dominguez E., Randrup M., Sanchez L., Calvo M. Effect of heat treatment on anti-rotavirus activity of bovine colostrum. Journal of Dairy Research. 1999;66:131–137. doi: 10.1017/s0022029998003239. [DOI] [PubMed] [Google Scholar]
- Marnila P., Korhonen H. Immunoglobulins. In: Roginski H., Fuquay J.W., Fox P.F., editors. Encyclopedia of Dairy Sciences. Academic Press; London: 2002. pp. 1950–1956. [Google Scholar]
- Marnila P., Rokka S., Rehnberg-Laiho L., Kärkkäinen P., Kosunen T.U., Rautelin H., Hänninen M.-L., Syväoja E.-L., Korhonen H. Prevention and suppression of Helicobacter felis infection in mice using colostral preparation with specific antibodies. Helicobacter. 2003;8(3):192–201. doi: 10.1046/j.1523-5378.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Mattila E., Anttila V.J., Broas M., Marttila H., Poukka P., Kuusisto K., Pusa L., Sammalkorpi K., Dabek J., Koivurova O.P., Vähätalo M., Moilanen V., Widenius T. A randomized, double-blind study comparing Clostridium difficile immune whey and metronidazole for recurrent Clostridium difficile-associated diarrhoea: Efficacy and safety data of a prematurely interrupted trial. Scandinavian Journal of Infectious Diseases. 2008;40(9):702–708. doi: 10.1080/00365540801964960. [DOI] [PubMed] [Google Scholar]
- Mcmartin S., Godden S., Metzger L., Feirtag J., Bey R., Stabel J., Goyal S., Fetrow J., Wells S., Chester-Jones H. Heat treatment of bovine colostrum. I: Effects of temperature on viscosity and immunoglobulin G level. Journal of Dairy Science. 2006;89(6):2110–2118. doi: 10.3168/jds.S0022-0302(06)72281-0. [DOI] [PubMed] [Google Scholar]
- Mehra R., Kelly P.M. Whey protein fractionation using cascade membrane filtration. International Dairy Federation Bulletin. 2004;389:40–44. [Google Scholar]
- Mehra R., Marnila P., Korhonen H. Milk immunoglobulins for health promotion: A review. International Dairy Journal. 2006;16(11):1262–1271. doi: 10.1016/j.idairyj.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma M., Oho T., Michibata N., Okano K., Nakano Y., Fukuyama M., Koga T. Passive immunization with bovine milk containing antibodies to a cell surface protein antigen-glucosyltransferase fusion protein protects rats against dental caries. Infection and Immunity. 2002;70(5):2721–2724. doi: 10.1128/IAI.70.5.2721-2724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näse L., Hatakka K., Savilahti E., Saxelin M., Pönkä A., Poussa T., Korpela R., Meurman J.H. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Research. 2001;35(6):412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- Nezlin R. Chapter 1. General characteristics of immunoglobulin molecules. In: Nezlin R., editor. The Immunoglobulins. Academic Press; New York, USA: 1998. [Google Scholar]
- Nezlin R. Chapter 2. Animal and human immunoglobulins. In: Nezlin R., editor. The Immunoglobulins. Academic Press; New York, USA: 1998. [Google Scholar]
- Nielsen W.K., Olander M.A., Lihme A. Expanding the frontiers in separation technology. Scandinavian Dairy Information. 2002;(Issue) 2. [Google Scholar]
- Numan S.C., Veldkamp P., Kuijper E.J., Van Den Berg R.J., Van Dissel J.T. Clostridium difficile-associated diarrhoea: Bovine anti-Clostridium difficile whey protein to help aid the prevention of relapses. Gut. 2007;56(6):888–889. doi: 10.1136/gut.2006.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyna J., Siwek K., Terry S.J., Roberton E.S., Johnson R.B., Davidson G.P. Survival of rotavirus antibody activity derived from bovine colostrum after passage through the human gastrointestinal tract. Journal of Pediatric Gastroenterology and Nutrition. 2001;32:162–167. doi: 10.1097/00005176-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Palmeira P., Carbonare S.B., Silva M.L., Trabulsi L.R., Carneiro-Sampaio M.M. Inhibition of enteropathogenic Escherichia coli (EPEC) adherence to HEp-2 cells by bovine colostrum and milk. Allergologia et Immunopathologia (Madr) 2001;29(6):229–237. doi: 10.1016/s0301-0546(01)79064-7. [DOI] [PubMed] [Google Scholar]
- Piot M., Fauquant J., Madec M.-N., Maubois J.-L. Preparation of serocolostrum by membrane microfiltration. Lait. 2004;84:333–341. [Google Scholar]
- Scammell A.W. Production and uses of colostrum. Australian Journal of Dairy Technology. 2001;56(2):74–82. [Google Scholar]
- Shimazaki Y., Mitoma M., Oho T., Nakano Y., Yamashita Y., Okano K., Nakano Y., Fukuyama M., Fujihara N., Nada Y., Koga T. Passive immunization with milk produced from an immunized cow prevents oral recolonization by Streptococcus mutans. Clinical and Diagnostic Laboratory Immunology. 2001;8:1136–1139. doi: 10.1128/CDLI.8.6.1136-1139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.J., King W.F., Godiska R. Passive transfer of immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infection and Immunity. 2001;69(5):3135–3142. doi: 10.1128/IAI.69.5.3135-3142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek H.I., Najim N.H., Al-Mashikhi S. Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: A field trial. International Journal of Infectious Diseases. 2003;7:120–128. doi: 10.1016/s1201-9712(03)90007-5. [DOI] [PubMed] [Google Scholar]
- Tripathi V., Vashishtha B. Bioactive compounds of colostrum and its application. Food Review International. 2006;22:225–244. [Google Scholar]
- Van Dissel J.T., De Groot N., Hensgens C.M., Numan S., Kuijper E.J., Veldkamp P., Van 't Wout J. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: Preclinical and preliminary clinical data. Journal of Medical Microbiology. 2005;54(2):197–205. doi: 10.1099/jmm.0.45773-0. [DOI] [PubMed] [Google Scholar]
- Wei H., Loimaranta V., Tenovuo J., Rokka S., Syväoja E.-L., Korhonen H., Joutsjoki V., Marnila P. Stability and activity of specific antibodies against Streptococcus mutans and Streptococcus sobrinus in bovine milk fermented with Lactobacillus rhamnosus strain GG or treated at ultra-high temperature. Oral Microbiology and Immunology. 2002;17:9–15. doi: 10.1046/j.0902-0055.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- Weiner C., Pan Q., Hurtig M., Boren T., Bostwick E., Hammarström L. Passive immunity against human pathogens using bovine antibodies. Clinical and Experimental Immunology. 1999;116:193–205. doi: 10.1046/j.1365-2249.1999.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woof J.M., Kerr M.A. The function of immunoglobulin A in immunity. Journal of Pathology. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- Xu L.B., Chen L., Gao W., Du K.H. Bovine immune colostrum against 17 strains of diarrhea bacteria and in vitro and in vivo effects of its specific IgG. Vaccine. 2006;24(12):2131–2140. doi: 10.1016/j.vaccine.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Young K.W., Munro I.C., Taylor S.L., Veldkamp P., Van Dissel J.T. The safety of whey protein concentrate derived from the milk of cows immunized against Clostridium difficile. Regulatory Toxicology and Pharmacology. 2007;47(3):317–326. doi: 10.1016/j.yrtph.2006.12.001. [DOI] [PubMed] [Google Scholar]


