Abstract
This article on poultry and avian diseases assembles a brief description of the current state of the poultry industry and the economical and public health impact of different diseases on these poultry production systems. Besides, a short explanation about the sustainable methods of production has been included in this article. Additionally, a review of the most important diseases that can affect poultry and wild avian species was performed, along with a summary of preventive and control measurements directed to reduce their economic impact. For all diseases, the etiology, clinical signs, and main lesions were reviewed.
Keywords: Bacterial, Biosecurity, Clinical signs, Diagnosis, Gross lesions, Nutritional imbalances, Parasitic and fungal diseases, Poultry, Serological techniques, Sustainable and conventional production systems, Toxicoses, Viral
Poultry Production: Economical Perspective and Public Health Impact of Diseases
The demand of poultry products has increased parallel to the continual rise in the human population. This can possibly be explained as poultry meat and eggs represent an efficient source of protein and have low cost of production in comparison with other sources of protein, such as pork and beef (Service, 2013). Nowadays almost every world region (Africa, North and Central America, South America, Asia, Europe, and Oceania) has to improve their production systems to accomplish the global demand of richer and more nutritional diets. Besides, global trading of poultry products has also increased, especially between the major producers (i.e., the United States, Brazil, and China) and the rest of the world (Stenhouse, 2008).
All infectious agents, toxics, and nutritional imbalances have an impact on the performance of the farm and consequently on the local poultry industry. Additionally, some diseases of birds, such as influenza virus, imply a potential zoonotic risk to the population and the notification of some strains related to high virulence involves commercial restrictions (ban on poultry imports/exports) and therefore reduce profitability (Yin et al., 2013). Therefore, the goals of any production system (conventional or sustainable) will always be to prevent the risk and consequences of diseases.
Sustainable Production Systems in Poultry
Sustainable or organic production systems consist of a series of strategies developed to decrease the environmental footprint to a minimum in the process of production. In poultry farms, the environmental pollution is generated during the process of growing animals, producing feed, generating manure, and obtaining housing accessories (litter materials and heating devices). Therefore, these systems have to implement measurements at the level of housing, lighting, fencing, and adequate feeder and waterer designs as well as management of litter, composting, and land (Xin et al., 2011).
These organic farming systems consist of several types of production methods that invariably allow access to pastures and involve less density of birds per square meter (Castellini et al., 2006), implying improvements that maximize animal welfare (Beaumont et al., 2010). Examples of these types of sustainable production systems include the following: free range, pastured poultry, semi-intensive, and others (Fanatico, 2007). An important point for the promoters of these systems is the search of alternatives to medication, and especially to antibiotics, which could reduce the emergence of antibiotic-resistant strains of bacteria in the birds themselves or/and the presence of residues in meat and eggs that induce resistance to infected humans (Fanatico, 2008).
Although sustainable and conventional production systems require completely different management strategies, their success depends on the establishment of adequate preventive and control measures to reduce the detrimental effects of diseases in the flock.
Basic Understanding of Disease, Prevention, and Control
Disease may be defined as an impairment of normal physiological function that reflects some structural or functional alteration in the cells or organisms (Slauson and Cooper, 2002). These may be caused by a single factor or more commonly by a combination of different factors. Multifactorial diseases are more difficult to diagnose and sometimes require an epidemiological approach. This approach allows assessing all possible etiological agents and associated risk factors, selecting the most adequate laboratory tests to determine the cause of the disease. It is also important to take the intrinsic factors (such as species, breed or strain, immune status, and age of bird) into consideration that can determine the presence of a disease (Sjaak de Wit, 2008).
Prevention and control of disease require a careful evaluation of the entire farm and establishment of a series of biosecurity measures that allow assessing the possible challenges and their impact on the production system. Once established, all factors determining disease can be better recognized and corrected. In general, the recommended approach is to perform a risk assessment and establish the Hazard analysis and critical control point (HACCP) principles on the poultry farm, determining the points where potential hazards could occur and biosecurity measures have to be implemented (Collett, 2013). Biosecurity involves measures at the level of environmental control and management, including elaboration of vaccination and medication programs and also application of effective cleansers, sanitizer, and disinfectants. These biosecurity measures are essential to control the diseases and reduce their economic and public health significance (Lister, 2008).
In summary, prevention and control of diseases depend in part on the ability to recognize the clinical signs, lesions, and all possible etiological agents related to them. For this reason, in the following section on poultry and avian diseases, emphasis was given to the identification of the agent, mechanism of disease, and lesions observed in the affected birds.
Viral Diseases
Influenza Virus
Influenza viruses are segmented negative-stranded ribonucleic acid (RNA) viruses belonging to the family Orthomyxoviridae. Avian influenza viruses are divided according to their virulence in highly pathogenic avian influenza virus (HPAIV) and low-pathogenic avian influenza virus (LPAIV). Influenza viruses are also classified on the basis of the nucleocapsid and matrix proteins into three types (A, B, and C), but especially type A genera cause disease in birds. In addition, influenza A viruses (IAVs) may further be classified into subtypes according to 16 hemagglutinin (H) and 9 neuraminidase (N) antigens. Only the IAVs belonging to the H5 and H7 subtypes are notifiable to health authorities, because they are frequently involved in HPAIV outbreaks and almost all zoonotic viruses have been from these subtypes (OIE, 2013a).
The most susceptible species to infection are gallinaceous species, such as chicken and turkeys (Perkins and Swayne, 2003). Wild birds are the main reservoir of IAVs and disseminated the virus directly to the poultry farms. Birds are infected mainly through the fecal–oral route, although the virus can also be transported mechanically (Prosser et al., 2013).
Clinical forms depend on the virulence of each IAV. LPAIVs are associated with suppurative pneumonia, sinusitis, kidney lesions, and occasionally pancreatitis. However, the HPAIVs cause hemorrhagic and necrotic lesions in different visceral organs, such as the heart, pancreas, and adrenal gland (Figure 1 ; Swayne et al., 2013).
Figure 1.
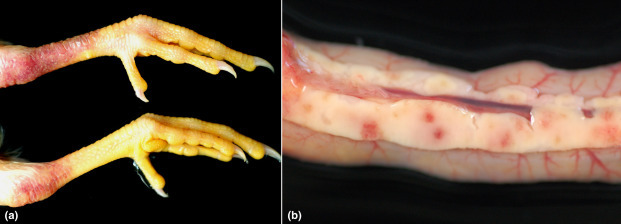
Gross lesions caused by HPAIV H7N1 in specific pathogen free (SPF) chickens. (a) Severe subcutaneous hemorrhages around the hock joint and shank of birds. (b) Multifocal petechial hemorrhages and necrosis in the pancreas of chickens intranasally inoculated with HPAIV A/chicken/5093/99 (H7N1), 5 days postinfection.
Photos provided by Natàlia Majó i Masferrer, SDPV (Servicio de Diagnóstico de Patología Veterinaria)-CReSA (Centre de Reserca en Sanitat Animal, Barcelona).
Newcastle and Other Paramyxoviruses
Newcastle disease virus (NDV) is a nonsegmented, negative sense, single-stranded RNA virus of the family Paramixoviridae and genus Avulavirus. Specifically, Newcastle disease is caused by serotype 1 viruses (APMV-1) (King et al., 2012c).
NDV induces different clinical conditions with variable severities or pathotypes. These pathotypes include the following: viscerotropic velogenic NDVs (induce hemorrhagic lesions in the intestinal tract), neurotropic velogenic NDVs (produce high mortality and respiratory and nervous signs), mesogenic NDVs (induce respiratory signs with low mortality), lentogenic respiratory NDVs (cause slight respiratory signs), and asymptomatic enteric NDVs (induce an unapparent enteric infection). In the field, these are usually overlapped between pathotypes and exacerbation by secondary pathogens (Alexander, 2008).
Approximately 250 species of birds can be infected by NDV, through fecal-oral or respiratory route of infection, according to the clinical presentation. Wild birds are the main reservoir of NDVs. As mentioned previously, the lesions are variable; viruses causing respiratory disease produce hemorrhages in the trachea, whereas the enteric viruses cause hemorrhages in the intestinal tract, particularly in the proventriculus (Miller and Koch, 2013).
NDV is zoonotic and has been reported in people with eye infections who are involved in activities related to NDV diagnosis and in poultry production (Conan et al., 2012).
Poxvirus
Fowlpox virus (FWPV) is caused by an Avipoxvirus that contains a double-stranded DNA genome and replicates in the cytoplasm of the cells. Poxvirus infections have been recognized in 230 species of birds (Gyuranecz et al., 2013). Avipoxvirus is a large virus that contrary to most of the virus is not filterable and consequently the viral particles could be visualized by light microscopy (inclusion bodies containing elementary particles). Avipoxvirus is highly host specific; therefore, infection with one type of poxvirus does not protect against others. In fact, poxviruses are named according to the susceptible host species (i.e., Fowlpox virus, Canarypox virus, Pigeonpox virus, Psittacinepox virus, Turkeypox virus, etc.) (Tripathy and Reed, 2013).
Avipoxvirus is transmitted by means of desquamation of skin lesions or by the biting of insects that sting the unfeathered skin areas, producing the characteristic papular, vesicular, and crusty lesions visible in the nares, eyelids, and legs. Birds can also ingest or inhale viral particles, producing diphtheritic lesions in the oral cavity, pharynx, larynx, and trachea (Tripathy and Reed, 2013).
Avian Encephalomyelitis Virus
Avian encephalomyelitis is produced by a nonenveloped, icosahedral, single-stranded RNA virus of the family Picornaviridae (Todd et al., 1999). The disease affects mainly chickens and occasionally turkeys, pheasants, Japanese quail, and pigeons. Clinical signs include reduction in hatchability and nervous signs in young birds, whereas adult birds show enteric signs. Gross lesions are frequently unapparent. Microscopic changes are considered pathognomonic and include diffuse gliosis and central neuronal chromatolysis in the brainstem and midbrain, as well as presence of gliosis in the molecular layer of the cerebellum. Additionally, lymphoid perivascular aggregates are seen through the central nervous system as well as in the dorsal root ganglia, proventriculus, gizzard, pancreas, and other viscera.
Other Picornaviridae family viruses causing serious diseases in birds include the following: Duck hepatitis virus (causes hemorrhagic lesions and necrosis in the liver of duck) and Turkey viral hepatitis (induce similar lesions in turkeys) (Suarez, 2013).
Infectious Bursal Disease
Infectious bursal disease (IBD) virus is a pathology to chickens and is caused by a nonenveloped, double-stranded RNA virus, that is member of the Birnaviridae family (Muller et al., 1979). The viruses causing IBD are divided into two serotypes (serotypes 1 and 2), but only viruses of the serotype 1 infect B cells. The high mutational rate of IBD viruses induces high genetic diversity and continual emergence of more virulent forms, which are able to overcome the previous acquired immunity and persists in the population, such as the European very virulent viruses (vvIBDV) (Maas et al., 2001).
The main target organ of IBDV is the bursa of Fabricius, where the virus causes lymphocytolisis and persists within the macrophages. Chickens with a well-developed bursa of Fabricius (between 3 and 6 weeks of age) are most susceptible. Gross lesions consist of petechial hemorrhages in the leg and thigh muscles, swelling of the bursa of Fabricius and petechiae, and sometimes petechiae in the mucosa of the proventriculus. Besides, there may be splenomegaly, swelling of the liver, and nephrosis (Eterradossi and Saif, 2013).
Reovirus
Reovirus corresponds to a nonenveloped virus that contains ten segment of double-stranded RNA. Reovirus causes arthritis and tenosynovitis in domestic fowl (De Gussem et al., 2010), but it also has been associated with a multisystemic disease in African gray parrots (Sánchez-Cordón et al., 2002). In addition, Reoviruses have been detected in budgerigars, black-tailed gulls, pheasants, bobwhite quail, pigeons, and mallard duck; however, only in a few cases they have been related with disease (Jones, 2013). Lesions commonly found (especially in broiler chickens) consist of tenosynotivis and arthritis, affecting the hock joints and rupture of the gastrocnemius tendon in chronic cases. Reovirus has been also related with ulcerative enteritis, acute and chronic respiratory disease, pericarditis, hydropericardium, anemia, acute hepatitis, inclusion body hepatitis, malabsorption syndrome in broilers, and poult enteritis and mortality syndrome (PEMS) in turkeys (Jones, 2013).
Rotavirus
Rotavirus also belongs to the family Reoviridae, and its genome contains 11 segments of double-stranded RNA. Rotaviruses have been detected in chickens, turkeys, pheasant, partridges, ducks, guinea fowl, ratites, pigeons, and parrots. In these species, Rotavirus may cause subclinical infections (predominantly in chickens and turkeys) and malabsorption and secretory diarrhea of different severity. The target cells of Rotavirus are the mature villous epithelial cells of the small intestine, causing villous atrophy (Day, 2013).
Circovirus
The family Circoviridae contains nonenveloped, icosahedral viruses with single-stranded circular DNA genomes. This family includes the genus Gyrovirus, which is represented by Chicken anemia virus (CAV), and the genus Circovirus, which contains Psittacine beak and feather disease virus (BFDV), Pigeon circovirus (PiCV), Canary circovirus (CaCV), and Duck circovirus (King et al., 2012b).
CAV infects only domestic fowl and does not have zoonotic potential. Clinical signs are observed in the progeny of breeder flocks that are infected for the first time (vertical transmission) and that are susceptible (i.e., chickens without maternal antibodies). In these birds, CAV causes anemia, aplasia of the bone marrow, and atrophy of the thymus, spleen, and bursa of Fabricius (Santen et al., 2004).
Adenovirus
Adenoviruses are nonenveloped double-stranded DNA viruses that replicate in the nucleus of the cell, producing inclusion bodies. The family Adenoviridae includes four genera, but only three of them (Avidenovirus, Siadenovirus, and Atadenovirus) are related to diseases in birds (King et al., 2012a).
The Aviadenovirus of domestic fowl has been associated with several conditions that include the following: inclusion body hepatitis (IBH), hydropericardium syndrome, respiratory disease, tenosynovitis, diarrhea, drop in egg production, and poor performance (Hess, 2013).
The Siadenoviruses are represented by the Turkey Adenovirus A, which causes sudden death consequence of severe hemorrhagic enteritis (Pierson and Fitzgerald, 2013).
Egg drop syndrome is induced by an Atadenovirus that replicates primarily in lymphoid tissues and massively in the pouch shell gland region of the oviduct, producing eggshell changes (loss of shell pigmentation, thin and soft shells, and shell-less eggs) (Smyth, 2013).
Parvovirus
Parvovirus is a single-stranded DNA virus. In birds there are two important diseases caused by viruses of this genus: Derzsy's disease cause by Goose parvovirus (GPV) and Muscovy duck parvovirus. GPV affects young gosling and Muscovy ducks and is also known as gosling plague, goose hepatitis, enteritis, or infectious myocarditis. GPV causes a multisystemic disease, which is characterized by the induction of Cowdry type A intranuclear inclusions in myocardial cells, enterocytes, and smooth muscle cells (Glávits et al., 2005).
Infectious Bronchitis Virus
The avian infectious bronchitis virus is a positive-sense, linear, single-stranded RNA virus of the family Coronaviridae and genera Coronavirus (Jackwood, 2012). Avian coronavirus causes respiratory signs and renal damage in broilers as well as drop in egg production in laying hens. Gross lesions consist of catarrhal exudate in the nasal cavity, sinuses, and trachea, sometimes affecting the low respiratory tract. Some IBV strains cause interstitial nephritis and visceral gout. Recently, there have been reports of IBV strains that affect the development of the reproductive tract, inducing the ‘blind or false layers,’ consequence of agenesia or hypoplasia of the oviduct (Cook et al., 2012).
Infectious Laryngotracheitis Virus
Infectious laryngotracheitis is a respiratory disease of domestic fowl that is caused by Gallid herpesvirus 1 (Iltovirus: ILT-like virus) (King et al., 2012a). The disease is observed mainly in young male birds of heavy breeds between 3 and 9 months of age. The virus can remain in the trigeminal ganglia of birds latently infected, which begin to intermittently reexcrete the virus on stress conditions (Williams et al., 1992).
The clinical presentation is restricted to the upper respiratory tract and can be peracute, acute, mild, or asymptomatic. In the peracute form, birds are found dead without prodromal signs due the induction of necrosis and hemorrhagic tracheitis. Acutely affected birds show caseous diphtheric exudates and hemorrhages in the trachea, which obstruct the respiratory pathways. In mild forms, there are mucous and caseous exudates in the trachea, and histologically it is possible to see pathognomonic Cowdry type A intranuclear inclusion bodies and multinucleate cells (syncytia) in respiratory and conjunctival epithelial cells.
Other important member within the Herpesviridae family is the Anatid herpesvirus 1 that causes Duck enteritis virus or Duck Plague (García et al., 2013).
Arhtropod-Borne Viruses
Within this group are included the arboviruses (arthropod borne), which are viruses transmitted by hematophagus arthropods to vertebrate hosts. This group includes 535 viruses. Among these, six families of viruses have been isolated in birds and ornithophilic arthropods: Togaviridae, Arenaviridae, Flaviviridae, Bunyaviridae, Reoviridae, and Rhabdoviridae (Capua, 2008).
Highlands J virus
Highlands J virus (HJV) causes disease in Chukar Partridges and turkeys. HJV corresponds to an Alphavirus of the Togaviridae family. HJV causes ovarian atrophy and mortality in young poults (Ficken et al., 1993) and encephalitis and myocarditis in Chukar Partridges (Eleazer and Hill, 1994).
Easter equine encephalitis virus
Easter equine encephalitis virus (EEEV) infects birds and humans and has been reported in North and South America. EEEV is transmitted by a highly ornithophilic mosquito (Culiseta melanura), which feeds mainly from passerine birds (Hassan et al., 2003) EEEV causes encephalitis and occasionally myocarditis in pheasants, turkeys, ducks, Chukar Partridges, and chickens (Guy, 2013).
Western equine encephalitis virus
Western equine encephalitis virus (WEEV) also infects humans and other mammalian host, besides birds. WEEV is found in the Americas and is transmitted by ornithophilic mosquitoes of the Culex genus. WEEV has been isolated from pheasants, turkeys, and Chukar Partridges. In Chukar Partridges, WEEV has been related to high mortality (Guy, 2013).
Venezuela equine encephalitis virus
Venezuela equine encephalitis virus infects more than 100 species of birds and is transmitted among them by mosquitoes belonging to the genus Culex (Capua, 2008).
West Nile virus
West Nile virus (WNV) belongs to the Flaviviridae family and its presence has been described in Africa, Asia, Europe, and North America. The virus can be transmitted by at least 43 mosquito species from 11 genera. WNV infects birds, reptiles, amphibians, mammals, mosquitoes, and ticks; however, birds are the main reservoir. Infection has been detected in geese, ducks, Chukar Partridges, pheasants, and a wide variety of feral and captive birds (Himsworth et al., 2009). Some WNV strains present in North America and the Middle East have evolved to become more virulent (Isr98), causing meningoencephalitis, myocarditis, and splenomegaly in humans (Guy, 2013).
Crimean–Congo hemorrhagic fever virus
Crimean–Congo hemorrhagic fever virus (CCHFV) is an Arbovirus transmitted by ticks that infects birds and causes disease in humans. CCHFV is found in Asia, Africa, the Middle East, and Eastern Europe and in the European Union. CCHFV have been reported in ostriches, chickens, and guinea fowl, but it does not cause clinical signs (Bente et al., 2013).
Marek’s Disease
Marek's disease (MD) is a lymphoproliferative and neuropathic disease of chickens, and occasionally turkeys, quails, and geese. This disease is caused by a cell-associated lymphotropic herpesvirus of the genus Mardivirus. The genus Mardivirus contains three serotypes, but MD is induced only by viruses of serotype 1. Viruses within this serotype show variable pathogenicity, which are subdivided into different groups: mild (mMDV), virulent (vMDV), very virulent (vvMDV), and very virulent+ (vv+MDV) (Witter, 1998).
Transmission occurs through direct and indirect routes (airborne route). The virus replicates in keratinizing epithelia cells of the feather follicle, so the desquamated cells and feathers serve as a source of infection (Jarosinski, 2012).
There are different clinical forms of the diseases that include the following: the classical form, acute form, acute cytolytic form, and transient paralysis syndrome. The classical form involves neural lesions that cause partial or complete paralysis of the legs and wings, torticollis, and paralysis. The acute form is characterized by the induction of lymphomas in visceral organs. The acute cytolitic form is caused by vvMDV strains, which produce severe atrophy of the lymphoid organs and high mortality in birds between 10 and 14 weeks of age. Transient paralysis occurs in birds between 5 and 18 weeks of age and consists of vasculitis and edema that induce flaccid paralysis (Schat and Nair, 2013).
Avian Leukosis, Reticuloendotheliosis, and Other Lymphoproliferative Diseases
The retroviruses are RNA viruses that cause neoplastic cells transformation and tumor development. Retroviruses affecting birds include the following: Avian leukosis/sarcoma group viruses (ALSV), reticuloendotheliosis viruses (REV), and lymphoproliferative disease virus of turkeys (LPDV) (King et al., 2012d).
ALSV belongs to the genus Alpharetrovirus, which is able to induce erythroid, lymphoid, and myeloid leukoses and other kind of tumors in chickens and other species of birds. Lymphoid neoplasms (B cell origin) are usually induced by ALVs subgroups A and B. The erythroid leukosis originates from bone marrow cells and is induced by subgroup J ALVs. Myeloid leukosis derives from myeloid (granulocytic) cells of the bone marrow and produce myeloblastosis and myeolocytomatosis (Venugopal et al., 2000). Additionally, ALSVs and specially ALV-J are associated with the induction of other tumors, such as fibrosarcomas, chondroma, hystiocytic sarcoma, endothelioma, hemangioma, nephroblastoma, and hepatocarcinoma. Furthermore, these viruses are able to induce osteopetrosis (Nair and Fadly, 2013).
REV includes several viruses isolated from game birds (order Galliformes) and waterfowl (order Anseriformes). REVs induce an acute multisystemic and neoplastic disease in chickens and turkeys, or in chronic cases, formation of B-cell or T-cell lymphomas (Nair et al., 2013).
Bacterial Diseases
Avian Cholera
Fowl cholera is caused by Pasteurella multocida, which is a Gram-negative, nonmotile, non-spore-forming, rod-shaped bacterium. The species P. multocida includes the subspecies multocida, septica, and gallicida. Additionally, P. multocida is divided into 16 somatic serovars (1–16) and five capsular serovars (A, B, C, D, and E). All capsular and somatic serovars (with exception of 8 and 13) have been isolated from birds. However, subspecies multocida serovar A is the most frequently isolated one in cases of fowl cholera. All types of birds are susceptible to the infection, but turkeys, partridges, and pheasants are highly susceptible.
Pasteurella multocida first colonizes the upper respiratory tract and lung and later disseminates, causing two clinical presentations. The peracute/acute form is related to septicemia, inducing petechial to ecchymotic hemorrhages in the heart, the mucous membranes of the gizzard, and in the abdominal fat. In chronic cases, lesions involve the respiratory tract (fibrinonecrotic pneumonia and fibrinopurulent pleuritis), the conjunctiva, infraorbital sinuses, and the reproductive tract. Pasteurella multocida is also related to fibrinonecrotic dermatitis affecting the dorsum, abdomen, and breast (Glisson et al., 2013).
Pasteurella multocida infections are notifiable due to their zoonotic potential (Wilson and Ho, 2013).
Mycoplasmosis
Mycoplasmas are assigned to the class mollicutes, which comprises the smallest known prokaryotes able to replicate in cell-free medium. There are 23 recognized avian mycoplasma species, but only four species are important in poultry.
Mycoplasma gallisepticum
Mycoplasma gallisepticum infects chickens, turkeys, pheasants, partridges, duck, geese, guinea fowl, quail, peafowl, racing pigeons, and other species of free-living birds. Mycoplasma gallisepticum causes catarrhal rhinitis, tracheitis, pneumonia, edema of the air sac walls, and sinusitis (Gharaibeh, 2011).
Mycoplasma synoviae
Mycoplasma synoviae strains vary in their virulence and tropism. Mycoplasma synoviae affects primarily chickens and turkeys, and less frequently guinea fowl, ducks, geese, pigeons, quails, pheasants, Red-legged Partridges, and house sparrows. Mycoplasma synoviae may cause respiratory lesions or suppurative and caseous arthritis of the foot and hock joints (Osorio et al., 2007).
Mycoplasma meleagridis
Mycoplasma meleagridis is a specific pathogen of turkeys that causes airsacculitis, osteodystrophy, and low hatchability in mature birds (Chin, 2013).
Mycoplasma iowae
Mycoplasma iowae contains six serovars, which vary in their virulence. Mycoplasma iowae causes embryo mortality in turkey, poor-quality poults, and chondrodystrophy (Bradbury and Raviv, 2013).
Chlamydiosis – Chlamydophilosis
Avian chlamydophilosis is produced by bacteria belonging to the family Chlamydiacea, which is divided in two genera: Chlamydia and Chlamydophyla. The species that affect birds belong to the genus Chlamydophyla and include the following: Chlamydophyla pecorum and Chlamydophyla psittaci. These are Gram-negative, coccoid bacteria that depend on the intracellular environment for their multiplication. (Vanrompay, 2013).
Chlamydophyla psittaci infects parrots and other psittacine birds as well as domestic poultry and free-living birds. Infected birds can transmit the infection to mammals (Beeckman and Vanrompay, 2009).
Severity of the disease varies according to the strain of C. psittacine. High-virulent strains are related to acute epidemics and high mortality, whereas low-virulent strains spread slowly and cause slightest lesions. Birds affected acutely show fibrinous peritonitis, pericarditis, myocarditis, pneumonia, and enlargement of the liver and spleen, which also show multifocal necrosis. Chronically infected birds show hepatomegaly and splenomegaly, which may lead to rupture of these organs (Vanrompay, 2013).
Ornothobacterium and Riemerella
Ornothobacterium and Riemerella genera belong to the family Flavobacteriaceae.
Ornithobacterium rhinotracheale
Ornithobacterium rhinotracheale is a slow-growing, Gram-negative, pleomorphic or rod-shaped bacterium that has been isolated from chicken, Chukar Partridge, duck, goose, guinea fowl, gull, pheasant, ostrich, partridge, pigeon, quail, rook, and turkey. Infection in broiler chickens induces purulent airsacculitis, pleuritis, and pneumonia (Chin et al., 2013). Young birds can also have purulent inflammation of the brain and osteomyelitis of the skull bones (Moreno et al., 2009).
Riemerella anatipestifer
Riemerella anatipestifer is a Gram-negative, nonsporulated bacterium that causes disease not only in young ducks but also in galliform birds, such as turkeys, pheasants, and partridges. Lesions produced by R. anatipestifer include fibrinous airsacculitis, polyserositis, sinusitis, pneumonia, meningitis, mucopurulent, or caseous salpingitis (Ruiz and Sandhu, 2013).
Enterobacteriaceae
The family Enterobacteriaceae consists of rod-shaped, Gram-negative aerobic or facultative anaerobic bacterium. This family includes bacteria within the genera Salmonella, Escherichia, Shigella, Citrobacter, Klebsiella, Proteus, and Yersenia (Lister et al., 2008).
Salmonella
This genus consists of two species: Salmonella enterica and Salmonella bongori. The former contains 6 subspecies that are also classified in serovars according to the antigenic specificity. In the veterinary field, the most important serovars are: Salmonella pullorum (pullorum disease), Salmonella gallinarum (fowl typhoid), S. enterica subsp. arizonae (Arizonosis), and other salmonella infection-causing serovars (Salmonellosis, paratyphoid infection) (Lister et al., 2008).
-
•
Pullorum disease: Almost all species of birds can get infected with S. pullorum, but only chickens, turkeys, guinea fowl, quail, and pheasant show clinical disease. Clinical signs are usually observed in birds less than 3 weeks of age, which are found dead in shell or die quickly after hatching, due to peritonitis and septicemia (Shivaprasad, 2013b).
-
•
Fowl typhoid (Salmonella gallinarum infection): S. gallinarum causes disease mainly in adult or growing chickens and turkeys but also affects ducks, pheasants, guinea fowl, peafowl, grouse, and quail. Acute cases of infection are related to septicemia. In subacute outbreaks, there are dead-in-shell embryos, or dead chicks on the hatching trays. Chronically affected birds show anemia and focal necrosis in the liver (Figure 2 ), heart, intestines, and pancreas (Lister et al., 2008).
-
•
Salmonellosis, paratyphoid infection: Within this group are included Salmonella serovars associated with foodborne human disease. This group includes Salmonella enteritidis and Salmonella typhimurium. Members of this group are found in a wide range of species (birds, mammals, reptiles, fish, and insects). Paratyphoid S. enterica produces typhlitis in young birds with dissemination toward the spleen, lungs, liver, spleen, and kidneys (Gast, 2013).
-
•
Arizonosis (S. enterica subsp. arizonae): Arizonae infection affects avian, mammal, and reptilian species. Avian arizonosis is recognized mainly in turkeys but also is found in chickens and ducks. Clinical signs are similar to the observed in cases of salmonellosis, but additionally the arizonosis is characterized by the induction retinitis and encephalitis (Shivaprasad, 2013a).
Figure 2.
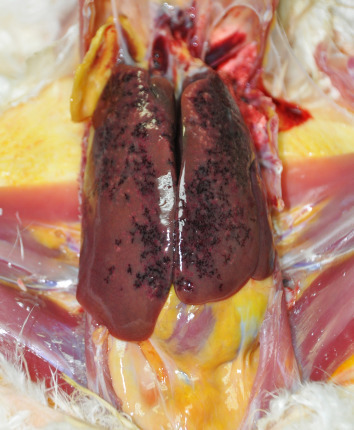
Liver from a bird naturally infected with Pasteurella, showing hepatomegaly and multifocal-coalescing pale foci of necrosis and hemorrhages.
Photos provided by Alejandro Alfaro, Universidad Nacional de Costa Rica.
Collibacillosis
Escherichia coli (E. coli)species are Gram-negative bacteria, flagellated rods that are normal inhabitants of the digestive tract in birds and mammals. Collibacillosis entitle all disease conditions that are caused by the pathogenic serovars of E. coli, including Colisepticaemia, egg peritonitis, and yolk sac infection or omphalitis, coligranuloma, swollen head syndrome, and others syndromes (Lister et al., 2008).
-
•
Colisepticemia is observed in chickens, turkeys, ducks, and pheasants. Birds affected show pericarditis, perihepatitis, airsacculitis, and peritonitis.
-
•
Egg peritonitis is observed in chickens, turkeys, ducks, and geese. Gross findings include peritonitis, salpingitis, and impaction of the oviduct.
-
•
Yolk sac infection occurs by contamination of the unhealed navel or by contact of the hatching egg with the contaminated shell. Chickens affected show septicemia and yolk sack inflammation.
-
•
Coligranuloma consists of several hard, yellow, nodular granulomas that are found in the mesenteries and wall of the intestines, especially in the cecum.
-
•
Swollen head syndrome (SHS) is observed in chickens, turkeys, and guinea fowl and consists of an acute to subacute cellulitis affecting the periorbital tissues (Nolan et al., 2013).
Clostridia
Clostridia are large Gram-positive, rod-shaped, toxin-producing bacteria that generate endospores to survive. Clostridia are ubiquitous worldwide and are found in the soil, dust, animals, and insect larvae. They are considered normal inhabitants of the intestinal tract (Kaldhusdal et al., 2008).
Clostridium perfringes (necrotic enteritis-NE and hepatic disease)
Clostridium perfringes causes two types of diseases: necrotic enteritis (NE) and colangiohepatitis. NE is caused by C. perfringes toxin types A and C and is observed mainly in chickens and turkeys but also found in ostriches, wild geese, wild crows, domestic geese, and ducks. NE occurs when predisposing factors (such as infection with Eimeria) induce overgrowth of C. perfringes, which through its toxins destroys the cell membranes, causing pseudomembranous enteritis (Figure 2) and endotoxemia. Cholangiohepatitis is related to C. perfringes toxin type A producers that arrive to the liver via the portal blood or bile, causing cholangitis, and occasionally focal necrosis, granulomas, and rarely massive liver necrosis (Opengart and Glenn Songer, 2013).
Clostridium colinum (ulcerative enteritis)
Clostridium colinum affects mainly quails but also infects chickens, turkeys, pheasants, partridges, grouse, pigeons, robins, and lories. Clostridium colinum induces ulcerative, pseudomembranous enteritis that affects the small intestine, ceca, and upper large intestine. The ulcers may become perforated, generating peritonitis (Songer and Uzal, 2013).
Clostridium botulinum (botulism and limberneck)
Botulism is a paralytic disease caused by toxins produced by C. botulinum. There are seven toxins (A–G); Toxin type C is the most important and common one. Presence of type C botulism has been described in chickens, turkeys, ducks, gulls, pheasants, and ostriches (Skarin et al., 2013).
Gangrenous dermatitis (malignant edema and cellulitis)
Gangrenous dermatitis is observed in chickens and turkeys. The disease is caused by Clostridium septicum and C. perfringes type A, and occasionally Staphylococci aureus (Opengart, 2013).
Campylobacter
The genus Campylobacter consists of curved-rod, Gram-negative, flagellate bacteria, which grow at high temperatures (42–43 °C). These organisms are found normally in the intestinal tract of a wide variety of animals, including birds (Whiley et al., 2013). Their significance lies in the ability to cause disease in humans, producing catarrhal diarrhea and self-limiting fever or in severe cases nervous system manifestations. In birds there are three main species: Campylobacter jejuni, Campylobacter coli, and Campylobacter lari. Campylobacter jejuni is usually found in poultry but can also be isolated from game birds, pigeons, and several wild bird species. Besides, poultry farm workers act as carriers and can spread the infection between broiler houses and farms. Therefore, all biosecurity measures have to be directed to reduce the horizontal transmission of the bacteria (Hermans, 2012).
Tuberculosis
Avian tuberculosis occurs in companion, captive exotic, and wild and domestic birds throughout the world. Mycobacterium avium consists of a complex group of mycobacterial organism, which is divided into four subtypes: M. avium subsp. avium, M. avium subsp. hominissuis, M. avium subsp. silvaticum, which are pathogens of birds, and M. avium subsp. paratuberculosis, which is a pathogen of mammals (Dhama, 2011).
Mycobacterium avium infects all species of birds, among which the most susceptible ones are the domestic species (such as chickens, ducks, and geese), game birds (pheasants), and birds kept in zoos (Moreno et al., 2011). In poultry, M. avium subsp. avium serotypes (1, 2, and 3) are the most virulent ones and are most frequently found. Mycobacterium avium subsp. avium produce tubercular granulomas that may be found in the intestines, liver, spleen, bone marrow, and other tissues (Fulton and Sanchez, 2013).
Other Bacterial Diseases
Yersinia pseudotuberculosis (Yersiniosis)
Yersinia pseudotuberculosis (Yersiniosis) is a Gram-negative coccobacillus that causes severe enteritis, splenomegaly, hepatomegaly, and lung lesions in young turkeys. In chronic cases it is possible to observe caseous tubercle-like lesions in the liver, spleen, and lungs (Lister et al., 2008).
Avibacterium paragallinarum
Avibacterium paragallinarum is a Gram-negative, non-spore-forming, capsulated rod-shaped bacterium that causes coryza in chickens. The lesions consist of catarrhal and fibrinopurulent inflammation of the nasal passages, infraorbital sinuses, and conjunctivae in old birds (Blackall, 1999).
Gallibacterium anatis
Gallibacterium anatis is a Gram-negative, rod-shaped, or pleomorphic bacterium that is a normal inhabitant of the upper respiratory tract and the lower genital tract. Gallibacterium anatis produces purulent salpingitis and oophoritis in chickens, turkeys, geese, ducks, pheasants, partridges, cage bird, and wild birds. In chronic cases purulent peritonitis may be found (Paudel et al., 2013).
Bordetella avium
Bordetella avium is a Gram-negative, rod-shaped bacterium. Bordetella avium causes turkey coryza, which is an acute respiratory tract disease in young turkeys. Lesions consist of mucopurulent pneumonia and respiratory stress syndrome. Bordetella avium also infects young Muscovy ducks, quail chicks, and cockatiels (Nymphicus hollandicus) (Jackwood and Saif, 2013).
Erysipelothrix rhusiopathiae
Erysipelothrix rhusiopathiae is a Gram-positive, rod-shaped bacterium. There are 26 serovars of E. rhusopathiae, but only the serovars 1, 2, and 5 produce outbreaks. Erysipelothrix rhusiopathiae is found in pigs, rodents, fish, and birds, and rarely in sheep. In birds, E. rhusopathiae infects turkeys, chickens, ducks, geese, emus, pigeons, and game birds. Birds acutely affected show lesions indicative of septicemia, such as petechial hemorrhages in the myocardium, epicardium, gizzard serosa, mesentery, liver, and pleura. In chronic cases, there is vegetative endocarditis and fibrinopurulent arthritis (Bricker and Saif, 2013). Erysipelothrix rhusiopathiae in humans may cause erysipeloid (localized infection of the fingers and hands), generalized cutaneous infections and septicemias (Wang et al., 2010).
Staphylococci, Streptococci, and enterococci
-
•
Staphylococci consist of Gram-positive cocci belonging to the family Micrococcaceae and genus Staphylococcus. The genus includes 40 species, of which Staphylococcus aureus is the most important and common one in poultry. Staphylococcus aureus may cause septicemia, fibrinous arthritis and tenosinovitis, chondronecrosis, and osteomyelitis (also called femoral head necrosis) in chickens, turkeys, ducks, and geese (Andreasen, 2013).
-
•
Streptococci and Enterococci of importance in poultry include Streptococcus gallinaceus, S. gallolyticus, S. equi subsp. zooepidemicus, Enterococcus hirae, E. durans, and E. faecalis. Lesions associated with infections by Streptococci and Enterococci consist of septicemia (common in chickens, ducks, and pigeons), caseous cellulitis (caused by S. dysgalactia in chickens and turkeys), encephalomalacia (associated with E. durans and E. hirae), vegetative endocarditis (caused by E. hirae in young birds and E. faecalis in older birds), and amyloid arthropathy (observed in chickens infected with E. faecalis) (Thayer and Waltman, 2013).
Fungal Diseases
Fungi are eukaryotes that nourish through absorption and can exist as single-celled or multicelled organisms. They are opportunistic pathogens of all species of birds and cause disease by tissue invasion or by means of the production of toxins (Dykstra et al., 2013)
Aspergillosis (Brooder Pneumonia)
Species of Aspergillus associated with disease include the following: Aspergillus fumigatus, A. flavus, A. niger, A. glaucus, and A. terreus. Aspergillus species are soil saprophytes. Birds are infected by inhalation of conidia, which may germinate and produce granulomatous lesions in the respiratory tract and air sacs epithelium (Figure 3 ). Besides, conidia may invade the blood vessels and spread to other tissues, such as the brain, pericardium, bone marrow, kidneys, and other soft tissues. Keratoconjunctivitis or panophthalmitis can also be observed (Dykstra et al., 2013).
Figure 3.
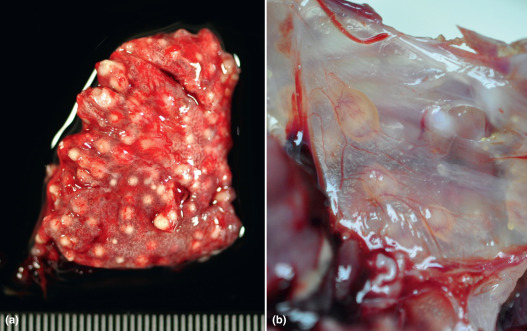
(a) Lung and air sacs. (b) Lung from naturally infected chickens showing numerous caseous nodules characteristic of respiratory asperigllosis.
Photo (a) was provided by Natàlia Majó i Masferrer, SDPV (Servicio de Diagnóstico de Patología Veterinaria)-CReSA (Centre de Reserca en Sanitat Animal, Barcelona) and Photo (b) was provided by Alejandro Alfaro, Universidad Nacional de Costa Rica.
Other Fungal Diseases
Ochroconosis
Ochroconiosis (formely Dactylariosis) is a rare neurological disease of chickens and turkeys poults that is caused by Ochroconis gallopava.
Candidiasis (crop mycosis, thrush)
Candida albicans yeast produces pseudomembranes and diphtheric membranes in the oral cavity, esophagus, and crop of immunosuppressed birds. In rare cases, it is associated with neurological, renal and intestinal disease.
Favus (white comb, ringworn)
Favus is a skin disease caused by Microsporum gallinae (formely Trichophyton gallinae) that affects mainly the unfeathered skin (comb, wattle, and shanks) and occasionally the feathered skin (Dykstra et al., 2013).
Mycotoxicosis
Mycotoxins are chemical by-products produced by fungi that are toxic to animals (mycotoxins) and/or to bacteria (antibiotics) or both. They are produced when the fungi invade the grain or feed, and there are adequate temperate, moisture, and grain substrate to growth. The most frequent mycotoxicosis in birds include the following: aflatoxicosis, ochratoxicosis, and tricothecene mycotoxicosis (Hoerr, 2013).
Aflatoxicosis
Aflatoxins are toxins produce by Aspergillus flavus, A. parasiticus, and Penicillium puberulum, which are found in corn, millet, sorghum, and other feed grains. Fungi growth is favored by warm and high humidity conditions. Aflatoxins consist of several mycotoxins (B1, B2, G1, and G2), of which the most toxic and usual one is Aflatoxin B1. This aflatoxin is biotransformed in a highly reactive metabolite that binds to the DNA and RNA, impairing the protein synthesis and immunity. Aflatoxins cause liver enlargement due to lipidosis and hyperplasia of the biliary ducts. Blood clotting factor synthesis and capillary stability are altered; as a result birds may show petechial hemorrhages or bruises.
Ochratoxicosis
Ochratoxin is a dihydroisocoumarin derivative produced mainly by Aspergillus ochracues and Penicillium veridicatum. Ochratoxin A is the most common and toxic one. Ochratoxin A inhibits proteins synthesis, producing anemia, immunosuppression, and decrease in egg production and hatchability. It also causes acute necrosis in the kidneys and visceral gout.
Trichothecene mycotoxicosis
Trichothecene mycotoxins are found in wheat, corn, and other grains contaminated with species of Fusarium, Stachybotrys, and other genera of fungi that prefer cold and moist conditions for growth. Trichothecene mycotoxins cause lesions through two mechanisms: direct epithelial necrosis or radiomimetic effects. For example, T-2 toxin and DAS cause necrosis of the epithelial cells of the commissures of the mouth, hard palate, the dorsal surface of the tongue, and rostral part of the oropharynx. Mycotoxins inducing radiomimetic effects inhibit the synthesis of proteins and reduce cellular life span and therefore affect the hematopoiesis, lymphopoiesis, immune response, and the replication of feather follicle epithelium and enterocytes (Hoerr, 2013).
Other Micotoxicosis
Fusarium fungi – Moliniformin
Moliniformin is produced by Fusarium verticillioides (formerly F. moliniforme) and other Fusarium spp. This toxin causes reduced performance and diarrhea. Experimentally, it induces acute myocardial necrosis, nephrosis, and death.
Fumonisins
Fumonisins are also produced by Fusarium verticillioides and cause catarrhal enteritis, liver necrosis, villous atrophy in the intestines, lymphoid depletion, and rickets.
Fusarochromanone (TDP-1)
TDP-1 is produced by Fusarium spp. and is related to leg deformities and tibial dyschondroplasia in chickens.
Oosporein
Oosporein is produced by Chaetomium spp and induces acute proximal tubular nephrosis and gout.
Ergotism
Ergotism is an acute disease caused by alkaloid toxins produced by Claviseps species, particularly Claviseps purpurea. Ergotism causes vascular, neurological, and endocrine disorders. Birds affected may show reduced growth and egg production, diarrhea, and necrosis as well as ulcers affecting the unfeathered skin of the comb, wattles, beak, and feet (Hoerr, 2013).
Parasitic Diseases
Coccidiosis
Coccidiosis is one of the most important diseases of poultry caused by parasites worldwide. Coccidia are intracellular protozoa parasites that belong to the phylum Apicomplexa. In poultry, most coccidia are classified within the genus Eimeria, which are highly host specific.
Chicken coccidiosis may be attributed to seven species of Eimeria, which are: E. brunetti, E. necatrix, E. tenella, E. acervulina, E. maxima, E. mitis, and E. precox. Eimeria tenella and E. necatrix are the most pathogenic species and are associated with necrohemorrhagic typhlitis. Eimeria. acervulina and E. maxima are the most prevalent ones and cause lesions in the small intestine. Turkeys are infected by coccidian of the following species: E. adenoeides, E. meleagridis, E. gallopavonis, and E. meleagrimitis. Intestinal coccidiosis can also be seen in geese, ducks, game birds, and pigeons. Infected birds may show diarrhea as a consequence of catarrhal to necrohemorrhagic enteritis. In general, infection with one species of Eimeria does not ensure protection against other species, neither does maternal antibodies; however, repetitive exposure to low numbers of oocyst does give protection (McDougald and Fitz-Coy, 2013).
Cryptosporidiosis
Cryptosporidia are apicomplexan protozoa parasites that affect mammals and birds. Cryptosporidium baileyi causes respiratory disease and C. meleagridis produces enteric infections. Cryptosporidia parasitize the margin of the epithelial cells, where it is enclosed by the cell membrane. Infection and disease have been reported in chickens, turkeys, ducks, pheasant, quail, peafowl, and other birds. In the respiratory form, cryptosporidia causes sinusitis, conjunctivitis, pneumonia, and airsacculitis. The enteric infection is associated with poor performance (McDougald, 2013a).
Histomoniasis (Blackhead)
Histomoniasis is an enterohepatitis caused by the protozoan Histomonas meleagridis. It is observed mainly in turkey poults but game birds, chickens, and guinea fowl may also be affected. Birds get infected after ingestion of eggs of the cecal nematode Heterakis gallinarum female parasites (intermediate host), which are transported by earthworms or remain alive in the soil (Armstrong and McDougald, 2011). Histomonas meleagridis causes ulcerative or hemorrhagic typhlitis and multifocal zones of necrosis in the liver. Liver lesions, which appear 10–12 days postinfection, are pathognomonic (Hess and McDougald, 2013).
Other Protozoa Parasites
Spironucleosis-Hexamitiasis
Spironucleus meleagridis causes slight catarrhal enteritis in turkey poults and young game birds.
Trichomonas gallinae
This pathogen produces necrotic and caseous lesions in the mouth, crop, and esophagus of pigeons and squabs and rarely in turkeys and chickens. Occasionally, there are systemic lesions involving the liver.
Leucocytozoonosis
Leucocytozoon spp. infects blood and tissue cells and is described in ducks and geese (L. simondi), turkeys (L. smithi), and chickens (L. caulleryi, L sabrasezi, and L. choutedeni). Within its life cycle, schizogony is found in the liver, lungs, heart, brain, and spleen, whereas gamonts are contained within blood cells. Infected birds show weakness, anemia, and mortality (Hess and McDougald, 2013).
Nematode Parasites
In birds there are a great number of nematode parasites; however, their prevalence in poultry birds is low, because half of them require an intermediate host. Therefore, they are more frequent in free-range, backyard, and wild birds. Most nematodes affect the gastrointestinal tract, such as Capillaria, which causes enteritis (C. obsignata, C. annulata, etc.), Heterakis (important in the life cycle of Histomonas), and Ascaridia (Ascaridia galli that affects performance in heavy infestations). In addition, there are nematodes that have affinity for the respiratory tract, such as Syngamus trachea and Cyathostoma bronchialis. The first one affects wild and domestic birds and causes blockage of the trachea, whereas C. bronchialis is a parasite of geese (McDougald, 2013b).
Cestodes and Trematodes
Cestodes and trematodes require intermediate hosts in their life cycles. Cestodes employ arthropods, whereas trematodes need molluscan. Therefore, their importance as pathogens of modern poultry production systems is insignificant. However, they are still relevant in free-range rearing, game, and wild birds, causing digestive and reproductive tract lesions (McDougald, 2013b).
Ectoparasites
Ectopasites of birds include species of lice, such as Menacanthus stramineus, Cuclotogaster heterographa, and Menopon gallinae, which feed on feathers and skin scales causing irritation.
Moreover, birds may be seriously infested by mites that cause anemia due to their blood-sucking habits. The most important mites in poultry are Dermanyssus gallinae (red mite) and Ornothonyssus sylvarum. Dermanyssus gallinae infects chickens and other species of birds.
Fleas may also cause irritation and blood loss. Fleas infest the host and persist in the environment; some of them, such as Echidnophaga gallinacea, can be attached to the host for months. Finally, ticks may cause poor food conversion, depressed egg output, and anemia (Hinkle and Corrigan, 2013).
Nutritional Disorders
Nutritional imbalances may arise from deficiency in the diet, destruction or inactivation during feed processing and manufacture, antagonism between nutrients, or impaired absorption and metabolism. Each of the 36 essential nutrients of poultry has specific functions that account for the distinctive diseases associated with its deficiency (summarized below). However, in the field, only rarely these changes are related to deficiency of one specific element (mineral, vitamins, etc.), making the clinical diagnosis a difficult task (Klasing, 2013).
Vitamin A (Retinol, Retinoic Acid, and Retinyl Ester)
Vitamin A is necessary to maintain the epithelial function and structure and also for a normal egg production. Consequently, its deficiency is related to epithelial and mucous metaplasia of the respiratory, intestinal, and urinary tracts. Pathognomonic hyperkeratotic lesions are observed in the cornea and the skin of the mouth and esophagus. The excess of this vitamin induces deficiency of vitamin E and D3 (Klasing, 2013).
Vitamin D (Cholecalciferol-Vitamin D3)
Vitamin D acts as a hormone to regulate calcium and phosphorus metabolism. Deficiency of vitamin D causes growth retardation, rachitic deformities, osteomalacia, osteoporosis, tibial dyschondroplasia, and egg abnormalities in layers. Excess of vitamin D is toxic, as a consequence of the deposition of calcium in several soft tissues (Klasing, 2013).
Vitamin E (α-Tocopherol)
Vitamin E is an antioxidant that has a major role in the protection of cellular membranes. Clinical conditions associated with its deficiency include the following: embryonic mortality in breeders, encephalomalacia, exudative diathesis, and nutritional muscular dystrophy in young birds (Figure 4 ). The security index of vitamin E is very high and therefore toxicity is improbable (Klasing, 2013).
Figure 4.
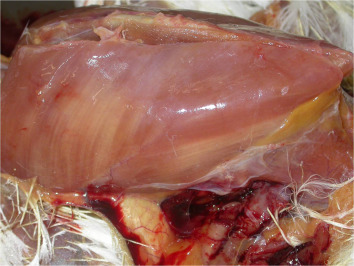
Pectoral muscles of a chicken affected with vitamin E and selenium deficiency, showing severe locally extensive areas of degeneration and necrosis, which is noted by the presence of light colored streaks of affected muscle.
Photos provided by Natàlia Majó i Masferrer, SDPV (Servicio de Diagnóstico de Patología Veterinaria)-CReSA (Centre de Reserca en Sanitat Animal, Barcelona).
Vitamin K (Vitamin K3-Menadione)
Vitamin K participates in the formation of prothrombin and so in blood coagulation. It also contributes in calcium metabolism. Deficiency causes delayed blood clotting (Klasing, 2013).
Vitamin B1 (Thiamin)
Vitamin B1 participates in the metabolism of carbohydrates. Deficiency of this vitamin is rarely seen in the field, unless feed that contains thiaminase (such as fishmeal) or treatments with the anticoccidial amprolium have been used. Deficiency affects the overall performance (Klasing, 2013).
Vitamin B2 (Riboflavin)
Riboflavin acts as coenzymes in several enzyme systems; many of them are related with oxidation–reduction reactions in the process of cellular respiration. Deficiency causes reduced egg production. Hatched chickens may be dwarfed, show depigmentation, and ‘curled toe paralysis’ due to the presence of degenerative changes in nerve trunks (Cai et al., 2009).
Vitamin B6 (Pyridoxine)
Vitamin B6 participates in the metabolism of amino acids. As a consequence, there is no specific list of signs related to its deficiency. Birds showing deficiency may have anemia, demyelinization, weakness, paresis, and chondrodysplasia (Klasing, 2013).
Niacin (Nicotinic Acid and Nicotinamide – Vitamin B3)
Niacin is part of the coenzymes involved in metabolism of proteins, carbohydrates, and fats. Deficiency in birds causes poor growth, feather disorders, inflammation of the mucous membranes of the alimentary tract, nervous degeneration, and chondrodystrophy in young birds (Klasing, 2013).
Pantothenic Acid (Vitamin B5)
Pantothenic acid is required to synthesize coenzyme A (CoA), which has a role in energy and fatty acid metabolism, citric acid cycle, neural function, antibody formation, and endocrine function (steroid hormones). Deficiency causes dermatitis, drop in egg production, embryo death, and stunted and weak chickens (Klasing, 2013).
Biotin (Vitamin H)
Biotin is necessary for the production of fatty acids, carbohydrates, and proteins; therefore, it is essential for growth, epidermal and tissue maintenance, bone development, and reproduction. Deficiency induces reduced growth and hatchability, deformed embryos, dermatitis (footpad, toes, and periocular), and beak deformities. Occasionally, there is chondrodystrophy and fatty liver and kidney syndrome (FLKS) in young broilers and layer pullets (Klasing, 2013).
Folic Acid (Folacin)
This vitamin acts as a coenzyme in the synthesis of specific amino acids (e.g., purines, choline, threonine, and histidine). Consequently, it is indispensable for nucleic acid synthesis and cell mitosis. Folic acid deficiency causes macrocytic megaloblastic anemia, defective feather development and pigmentation, chondrodystrophy, and cervical paralysis in young birds. Breeding birds show reduced hatchability and presence of embryo deformities (Klasing, 2013).
Vitamin B12
Vitamin B12 is involved in metabolism of proteins, carbohydrates, and fats. Deficiency produces embryonic death and lowered hatchability. Chick embryos affected show malposition, myopathy, and chondrodystrophy (Klasing, 2013).
Choline
Choline is part of various phospholipids (e.g., lecitin) and is necessary to maintain the cell structure (especially of cartilage) and fat metabolism. Choline also participates in nerve transmission (forms the acetylcholine) and protein synthesis as methyl group donor. Deficiency causes reduced growth rate and hatchability, chondrodystrophy, and fatty liver (Klasing, 2013).
Calcium and Phosphorus
Calcium and phosphorus are part of body fluid electrolytes. Calcium participates in nerve cell excitation, neuromuscular transmission, muscular contraction, and blood clotting. Phosphate is necessary to maintain the acid–base balance and protein and carbohydrate metabolism. Calcium and phosphorus imbalance may cause rickets in growing birds and osteomalacia or osteoporosis in mature animals. Affected laying hens produce eggs with thin or soft shell and may show paralysis, fractures, and have skeletal deformities. However, excess of calcium causes nephropathy with renal fibrosis, atrophy, and visceral gout. An excess of phosphorus decreases the quality of the eggshell and increases bone fragility in layer birds (Klasing, 2013).
Sodium Chloride
Sodium chloride is a component of the intra- and extracellular fluids; as a result, it is important to maintain the acid–base and water balance as well as for transfer of metabolites across membranes and nerve impulse transmission. Birds fed with low levels of sodium show severe drop in egg production, pecking behavior, and cannibalism. An excess of sodium produces diarrhea, excessive thirst, progressive muscular weakness, convulsions, and death. Young birds show severe kidney damage with presence of visceral gout (Klasing, 2013).
Manganese
Manganese is necessary, along with magnesium, in the activation of several enzymatic systems and formation of DNA complex. Deficiency in young birds produces growth retardation and bone deformities (e.g., chondrodystrophy). Laying or breeding birds may show decreased egg production and hatchability, in addition to embryo mortality, ataxia, and tetanic spasms in newly hatched chicks (Klasing, 2013).
Zinc
Zinc participates in almost every metabolic function in the organism; therefore, its deficiency has a great impact on the development of the skeleton and in the reproductive system. Growing birds exhibit poor feathering and scaly skin, in addition to chondrodystrophy. Laying birds show decreased egg production and hatchability. Embryo mortality with presence of deformities is observed around mid-incubation (Klasing, 2013).
Selenium
Selenium forms part of the cell enzyme glutathione peroxidase and acts in accordance with vitamin E as antioxidants. Deficiency causes muscular dystrophy (Figure 4), exudative diathesis, and encephalomalacia (Klasing, 2013).
Toxicants
Toxicity is almost always determined by the dose, because it is possible to find cases of toxicoses related to the use of apparently innocuous substances, such as feed constituents or therapeutic agents. Many of these toxicoses cause unspecific clinical signs that could be confused with infectious and metabolic diseases. Diagnosis depends on the compilation of a good history, field visits, detection and recognition of the clinical sings, necropsy findings, and examination of the samples collected in specialized laboratories (Fulton, 2013).
Toxics Found in Feed and Water
Mycotoxins
Mycotoxins were reviewed in the section of fungal diseases.
Biogenic amines and plant toxins
Biogenic amines, such as gizzerosine, are derived from overheated fish meal. Gizzerosine causes gizzard erosion syndrome. An example of a plant toxin is found from the unselected strains of rapeseed that contains erucic acid, which causes anemia and cardiomyopathy.
Nutrient additives
Some examples of poisoning caused by an excess of vitamins and elements (such as iron, zinc, calcium, sodium, etc.) were briefly described in the section of nutritional disorders.
Coccidiostats and other therapeutic drugs
Toxic coccidiostats in poultry include polyether ionophores, such as monensin, lasalosid, salinomycin, narasin, and maduramicin. These ionophores are characterized by the induction of myonecrosis in the skeletal muscles and occasionally the myocardium.
Sulfonamides are used to treat several infectious diseases; however, their therapeutic indices are very narrow, making likely an overdose. Sulfonamides induce vitamin K deficiency and consequently produce anemia, multifocal hemorrhages, jaundice, and bone marrow depression (Fulton, 2013).
Pesticide Toxicoses
Insecticides
Chlorinated hydrocarbon insecticides, such as DDT, chlrodane, heptachlor epoxide, etc., are not found in farms nowadays as they are banned due to their prolong persistence, high toxicity, and carcinogenicity in animals and humans. Organophosphorus (OPs) insecticides have a cholinesterase-inhibiting action and some of them are specifically toxic to birds. OPs compounds, such as diazinon, fenthion, and parathion, cause locomotor and respiratory muscular weakness, dyspnea, and paralysis. Carbamate anticholinesterase insecticides (bofuran, methomyl, and bendiocarb) produce similar clinical signs to the OPs, but it is more acute with marked tremors and convulsions. Finally, another insecticide widely used is the pyrethroid (permethrin, cypermethrin, and deltamethrin); however, they are very secure and only rarely cause toxicity. Clinical signs of hyperactivity, tremor, and salivation are observed in the affected birds.
Rodenticides
Rodenticides used in poultry buildings comprise first-generation anticoagulants and the most widely used second-generation anticoagulants as well as other less commonly used rodenticides (sodium strychnine, cholecalciferol, zinc phosphide, and sodium fluoroacetate). First-generation anticoagulants include warfarin, coumatetralyl, chlorphacinone, and diphacinone that can induce a subacute hemorrhagic syndrome in birds, whereas second-generation anticoagulants (brodifacoum, bromadiolone, difenacoum, difethialone, and flocoumafen) are highly toxic and are related to mortality in raptors and owls.
Toxicoses from the air or litter
Ammonia toxicity is common in poultry exposed to wet litter. Ammonia causes poor food conversion and eye and lung lesions. However, an example of air pollutant that causes toxicoses in birds is carbon monoxide, which induces ataxia and dyspnea and bright red blood (Fulton, 2013).
Diagnosis
Each laboratory test has been developed to accomplish an objective, which may be to confirm or exclude a presumptive clinical diagnosis, to perform an epidemiological study, to certificate a health status, or to monitor responses to vaccination. All pathogens have their mechanism of induction of lesions and disease; therefore, this knowledge is valuable to adequately select which samples have to be chosen and which diagnostic methods can give you better information to your purposes. In this section were summarized the diagnostic methods used to diagnose all notifiable diseases of poultry (Table 1, Table 2 ).
Table 1.
List of diagnostic methods and sample selection for the most important – notifiable viral diseases of poultry
| Methods of diagnosis for viral-origin poultry and avian diseases | |||
|---|---|---|---|
| Infectious agent | Samples of choice | Serological techniques – Immune status | Etiological diagnosis – Antigen detection |
| Avian influenza virus |
|
|
|
| Newcastle virus |
|
|
|
| Infectious bronchitis virus |
|
|
|
| Infectious Laryngotracheitis virus |
|
|
|
| Poxvirus |
|
|
|
| Infectious bursal disease |
|
|
|
| Marek's disease |
|
|
|
Abbreviations: AGID, agar gel immunodiffusion; HA, hemagglutination; HI, hemagglutination inhibition; ELISA, enzyme-linked immunosorbent assay; SPF, specific pathogen free; AC, antigen capture; RT-PCR, reverse transcription polymerase chain reaction; rRT-PCR, real time RT-PCR technique; VN, virus neutralization; TOC, tracheal organ culture; and RT-PCR-RFLP, RT-PCR-restriction fragment length polymorphism.
Source: OIE, 2013a. Manual of diagnostic tests and vaccines for terrestrial animals: Avian influenza. In: Terrestrial Manual. International Standard Setting, seventh ed. World Organization for Animal Health, pp. 436–454 (Chapter 2.3.4) and OIE, 2013b. Chapter 2.3.4. Avian influenza. In: I. S. S. T. Manual (Ed.), World Organisation for Animal Health. Paris, France: World Organisation for Animal Health, Section 2.3 (Chapters: 2.3.1., 2.3.2., 2.3.3., 2.3.4., 2.3.5., 2.3.6., 2.3.9., 2.3.10., 2.3.11., 2.3.12., 2.3.13., 2.3.14).
Table 2.
List of diagnostic methods and sample selection for the most important notifiable bacterial diseases of poultry
| Methods of diagnosis for bacterial-origin poultry and avian diseases | |||
|---|---|---|---|
| Infectious agent | Samples of choice | Serological techniques – Immune status | Etiological diagnosis – Identification of the agent |
| Fowl typhoid and pullorum disease: Salmonella |
|
|
|
| Fowl cholera: Pasteurella |
|
|
|
| Chlamydiosis |
|
|
|
| Mycoplasma |
|
|
|
| Tuberculosis |
|
|
|
Abbreviations: AGID, agar gel immunodiffusion; API, analytical profile index; CF, complement fixation; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; HPLC, high-performance liquid chromatography; PCR, polymerase chain reaction; PCR-RFLP, PCR-restriction fragment length polymorphism; REA, restriction endonuclease analysis; and RSA, rapid serum agglutination.
Source: OIE, 2013a. Manual of diagnostic tests and vaccines for terrestrial animals: Avian influenza. In: Terrestrial Manual. International Standard Setting, seventh ed. World Organization for Animal Health, pp. 436–454 (Chapter 2.3.4) and OIE, 2013b. Chapter 2.3.4. Avian influenza. In: I. S. S. T. Manual (Ed.), World Organisation for Animal Health. Paris, France: World Organisation for Animal Health, Section 2.3 (Chapters: 2.3.1., 2.3.2., 2.3.3., 2.3.4., 2.3.5., 2.3.6., 2.3.9., 2.3.10., 2.3.11., 2.3.12., 2.3.13., 2.3.14).
See also
Animal Health: Global Antibiotic Issues. Animal Health: Mycotoxins. Climate Change: Animal Systems. Marek’s Disease and Differential Diagnosis with Other Tumor Viral Diseases of Poultry. Organic Livestock Production. Vaccines and Vaccination Practices: Key to Sustainable Animal Production
Glossary
- Broiler birds
Birds bred and raised for meat production.
- Gamonts
A developmental stage within the life cycle of Eimerian species parasites. Specifically, gamonts are the parasitic forms that occur during the sexual phase of the life cycle, which lead to the formation of oocysts that are spread throughout the feces.
- Hazard analysis and critical control point (HACCP)
A management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement, and handling to manufacturing, distribution, and consumption of the finished product.
- Impaction
Accumulation of eggs and egg material within the oviduct and is usually a consequence of dystocia, metritis, or salpingitis.
- Layer birds
Birds bred and raised to lay eggs.
- Necrosis
Refers to the process when a living cell dies due to irreversible injuries.
- Notifiable diseases
Diseases that must be immediately reported to health and agriculture authorities.
- Oophoritis
An inflammation of the ovaries that is mainly caused by bacterial agents, such as Escherichia coli (E. coli).
- Pathognomonic
A term used to denominate the characteristic clinical signs or lesions of a particular disease or condition.
- Poultry
Birds used for the purpose of production, which include mainly chickens and turkeys; however, according to the region, ducks, geese, partridges, quails, and ostriches are also used.
References
- Alexander D.E. Paramyxoviridae. In: Pattison M., Mcmullin P., Bradbury J.M., Alexander D., editors. Poultry Diseases. Saunders Elseveir; Philadelphia: 2008. pp. 294–299. [Google Scholar]
- Andreasen C.B. Staphylococcosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 971–976. [Google Scholar]
- Armstrong P.L., McDougald L.R. The infection of turkey poults with Histomonas meleagridis by contact with infected birds or contaminated cages. Avian Diseases Digest. 2011;6(1):e41–e42. doi: 10.1637/9568-100710-Reg.1. [DOI] [PubMed] [Google Scholar]
- Beaumont C., Lebihan-Duval E., Mignon-Grasteau S., Leterrier C. The European experience in poultry welfare: A decade ahead. Poultry Science. 2010;89(4):825–831. doi: 10.3382/ps.2009-00359. [DOI] [PubMed] [Google Scholar]
- Beeckman D.S.A., Vanrompay D.C.G. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clinical Microbiology and Infection. 2009;15(1):11–17. doi: 10.1111/j.1469-0691.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- Bente D.A. Crimean–Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Research. 2013;100(1):159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Blackall P.J. Infectious coryza: Overview of the disease and new diagnostic options. Clinical Microbiology Reviews. 1999;12(4):627–632. doi: 10.1128/cmr.12.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J.M., Raviv Z. Mycoplasma iowae infection. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 908–910. [Google Scholar]
- Bricker J.M., Saif Y.M. Erysipelas. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 986–994. [Google Scholar]
- Cai Z., Blumbergs P.C., Finnie J.W., Manavis J., Thompson P.D. Selective vulnerability of peripheral nerves in avian riboflavin deficiency demyelinating polyneuropathy. Veterinary Pathology Online. 2009;46(1):88–96. doi: 10.1354/vp.46-1-88. [DOI] [PubMed] [Google Scholar]
- Capua I. Arthropod-borne viruses. In: Pattison M., Mcmullin P., Bradbury J.M., Alexander D., editors. Poultry Diseases. Saunders-Elsevier; Philadelphia: 2008. pp. 415–416. [Google Scholar]
- Castellini C., Bastianoni S., Granai C., Bosco A.D., Brunetti M. Sustainability of poultry production using the emergy approach: Comparison of conventional and organic rearing systems. Agriculture, Ecosystems & Environment. 2006;114(2–4):343–350. [Google Scholar]
- Collett S.R. Principles of disease prevention, diagnosis, and control. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 4–11. [Google Scholar]
- Conan A., Goutard F.L., Sorn S., Vong S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Veterinary Research. 2012;8(1):240–250. doi: 10.1186/1746-6148-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K.A., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathology. 2012;41(3):239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Chin R.P. Mycoplasma meleagridis infection. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 893–898. [Google Scholar]
- Chin R.P., van Empel P.C.M., Hafez H.M. Ornithobacterium rhinotrcheale infection. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 828–832. [Google Scholar]
- Day J.M. Rotavirus infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 385–390. [Google Scholar]
- De Gussem J., Swam H., Lievens K., De Herdt P. Reovirus tenosynovitis in a flock of layer breeders. Avian Pathology. 2010;39(3):169–170. doi: 10.1080/03079451003717597. [DOI] [PubMed] [Google Scholar]
- Dhama K.M., Tiwari M., Singh R. Tuberculosis in birds: Insights into the Mycobacterium avium infections. Veterinary Medicine International. 2011;4:1–14. doi: 10.4061/2011/712369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra M.J. Fungal infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1077–1096. [Google Scholar]
- Eleazer T.H., Hill J.E. Highlands J virus-associated mortality in chukar partridges. Journal of Veterinary Diagnostic Investigation. 1994;6(1):98–99. doi: 10.1177/104063879400600118. [DOI] [PubMed] [Google Scholar]
- Eterradossi N., Saif Y.M. Infectious bursal disease. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 219–231. [Google Scholar]
- Fanatico, A., 2007. Poultry House Management for Alternative Production. Available at: http://ucanr.org/sites/placernevadasmallfarms/files/102338.pdf (accessed 15.01.14).
- Fanatico, A., 2008. Organic Poultry Production in the United States. Available at: http://sd.appstate.edu/sites/sd.appstate.edu/files/organicpoultry.pdf (accessed 15.01.14).
- Ficken M.D., Wages D.P., Guy J.S., Quinn J.A., Emory W.H. High mortality of domestic turkeys associated with Highlands J virus and Eastern equine encephalitis virus infections. Avian Diseases. 1993;37(2):585–590. [PubMed] [Google Scholar]
- Fulton R.F., Sanchez S. Tuberculosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1008–1016. [Google Scholar]
- Fulton R.M. Toxins and poisons. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1287–1315. [Google Scholar]
- García M., Spatz S., Guy J.S. Infectious laryngotracheitis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 167–171. [Google Scholar]
- Gast R.K. Paratyphoid infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 693–701. [Google Scholar]
- Gharaibeh S.H.A. Mycoplasma gallisepticum experimental infection and tissue distribution in chickens, sparrows and pigeons. Avian Pathology. 2011;40(4):349–354. doi: 10.1080/03079457.2011.582480. [DOI] [PubMed] [Google Scholar]
- Glávits R. Comparative pathological studies on domestic geese (Anser anser domestica); and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin. Acta Veterinaria Hungarica. 2005;53(1):73–89. doi: 10.1556/AVet.53.2005.1.8. [DOI] [PubMed] [Google Scholar]
- Glisson J.R., Hofacre C.L., Christensen J.P. Fowl cholera. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 807–822. [Google Scholar]
- Guy J.S. Arbovirus infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 474–481. [Google Scholar]
- Gyuranecz M. Worldwide phylogenetic relationship of avian poxviruses. Journal of Virology. 2013;87(9):4938–4951. doi: 10.1128/JVI.03183-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.K. Avian host preference by vectors of Eastern equine encephalitis virus. American Journal of Tropical Medicine and Hygiene. 2003;69(6):641–647. [PubMed] [Google Scholar]
- Hermans D., Pasmans F., Messens W. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne and Zoonotic Diseases. 2012;12(2):89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- Hess M. Aviadenovirus infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 294–299. [Google Scholar]
- Hess M., McDougald L.R. Histomoniasis (blackhead) and other protozoan diseases of the intestinal tract. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1172–1195. [Google Scholar]
- Himsworth C.G., Gurney K.E.B., Neimanis A.S., Wobeser G.A., Leighton F.A. An outbreak of West Nile virus infection in captive lesser scaup (Aythya affinis) ducklings. Avian Diseases. 2009;53(1):129–134. doi: 10.1637/8387-063008-Case.1. [DOI] [PubMed] [Google Scholar]
- Hinkle N.C., Corrigan R.M. External parasites and poultry pests. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1099–1115. [Google Scholar]
- Hoerr F.J. Mycotoxicoses. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1271–1281. [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Diseases. 2012;56(4):634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Saif Y.M. Bordetellosis (Turkey Coryza) In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 834–844. [Google Scholar]
- Jarosinski K.W. Marek's disease virus late protein expression in feather follicle epithelial cells as early as 8 days postinfection. Avian Diseases Digest. 2012;7(4):e7–e8. doi: 10.1637/10252-052212-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.C. Reovirus infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 351–373. [Google Scholar]
- Kaldhusdal M. Clostridia. In: Pattison M., McMullin P.F., Bradbury J.M., Alexander D.J., editors. Poultry Diseases. W.B. Saunders; Edinburgh: 2008. pp. 200–214. (Chapter 18) [Google Scholar]
- King A.M.Q. Family – Adenoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Elsevier; San Diego: 2012. pp. 125–141. [Google Scholar]
- King A.M.Q. Family – Circoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Elsevier; San Diego: 2012. pp. 343–349. [Google Scholar]
- King A.M.Q. Family – Paramyxoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Elsevier; San Diego: 2012. pp. 672–685. [Google Scholar]
- King A.M.Q. Family – Retroviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Elsevier; San Diego: 2012. pp. 477–495. [Google Scholar]
- Klasing K.C. Nutritional diseases. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1205–1232. [Google Scholar]
- Lister S.A. Biosecurity in poultry management. In: Pattison M., Mcmullin P., Bradbury J.M., Alexander D., editors. Poultry Diseases. Saunders Elsevier; Philadelphia: 2008. pp. 48–65. [Google Scholar]
- Lister S.A. Enterobacteriaceae. In: Pattison M., McMullin P.F., Bradbury J.M., Alexander D.J., editors. Poultry Diseases. W.B. Saunders; Edinburgh: 2008. pp. 110–145. (Chapter 8) [Google Scholar]
- Maas R.A. Efficacy of inactivated infectious bursal disease (IBD) vaccines: Comparison of serology with protection of progeny chickens against IBD virus strains of varying virulence. Avian Pathology. 2001;30(4):345–354. doi: 10.1080/03079450120066359. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Cryptosporidiosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1167–1171. [Google Scholar]
- McDougald L.R. Internal parasites. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1117–1146. [Google Scholar]
- McDougald L.R., Fitz-Coy S.H. Coccidiosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1148–1166. [Google Scholar]
- Miller P.J., Koch G. Newcastle disease. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 98–104. [Google Scholar]
- Moreno B. Nervous signs associated with otitis and cranial osteomyelitis and with Ornithobacterium rhinotracheale infection in red-legged partridges (Alectoris rufa) Avian Pathology. 2009;38(5):341–347. doi: 10.1080/03079450903183686. [DOI] [PubMed] [Google Scholar]
- Moreno B. Outbreak of tuberculosis in farmed red-legged partridges due to Mycobacterium avium subspecies avium. Veterinary Record. 2011;168(11):304–305. doi: 10.1136/vr.c5261. [DOI] [PubMed] [Google Scholar]
- Muller H., Scholtissek C., Becht H. The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. Journal of Virology. 1979;31(3):584–589. doi: 10.1128/jvi.31.3.584-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V., Fadly A.M. Leukosis/sarcoma group. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 553–585. [Google Scholar]
- Nair V., Zavala G., Fadly A.M. Reticuloendotheliosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 593–604. [Google Scholar]
- Nolan L.K., Barnes H.J., Vaillancourt J.-P., Abdul-Aziz T., Logue C.M. Colibacillosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 765–780. [Google Scholar]
- OIE . Terrestrial Manual. International Standard Setting, seventh ed. World Organisation for Animal Health; Paris, France: 2013. Manual of diagnostic tests and vaccines for terrestrial animals: Avian influenza. pp. 436–454 (Chapter 2.3.4) [Google Scholar]
- Opengart K. Gangrenous dermatitis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 957–960. [Google Scholar]
- Opengart K., Glenn Songer J.G. Necrotic enteritis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 949–953. [Google Scholar]
- Osorio C. Pneumonia of turkey breeder hens associated with Mycoplasma synoviae. Avian Diseases. 2007;51(3):791–796. doi: 10.1637/0005-2086(2007)51[791:POTBHA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Paudel S., Alispahic M., Liebhart D., Hess M., Hess C. Assessing pathogenicity of Gallibacterium anatis in a natural infection model: The respiratory and reproductive tracts of chickens are targets for bacterial colonization. Avian Pathology. 2013;42(6):527–535. doi: 10.1080/03079457.2013.843160. [DOI] [PubMed] [Google Scholar]
- Perkins L.E.L., Swayne D.E. Comparative susceptibility of selected avian and mammalian species to a Hong Kong origin H5N1 high-pathogenicity avian influenza virus. Avian Diseases. 2003;47(s3):956–967. doi: 10.1637/0005-2086-47.s3.956. [DOI] [PubMed] [Google Scholar]
- Pierson F.W., Fitzgerald S.D. Hemorrhagic enteritis and related infections. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 311–315. [Google Scholar]
- Prosser D. Mapping avian influenza transmission risk at the interface of domestic poultry and wild birds. Frontiers in Public Health. 2013;1:1–11. doi: 10.3389/fpubh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J.A., Sandhu T.S. Riemerella anapestifer infection. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 823–827. [Google Scholar]
- Sánchez-Cordón P.J. Reovirus infection in psittacine birds (Psittacus erithacus): Morphologic and immunohistochemical study. Avian Diseases. 2002;46(2):485–492. doi: 10.1637/0005-2086(2002)046[0485:RIIPBP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Santen V.L.v. Pathogenesis of chicken anemia virus: Comparison of the oral and the intramuscular routes of infection. Avian Diseases. 2004;48(3):494–504. doi: 10.1637/7155-010904R. [DOI] [PubMed] [Google Scholar]
- Schat K.A., Nair V. Marek's disease. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 530–543. [Google Scholar]
- Service F.A. World Production, Markets, and Trade Reports. Foreign Agricultural Service, United States Department of Agriculture; Washington, DC: 2013. Livestock and poultry. [Google Scholar]
- Shivaprasad H.L. Arizonosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 708–711. [Google Scholar]
- Shivaprasad H.L., Barrow P.A. Pullorum disease and fowl typhoid. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 683–689. [Google Scholar]
- Sjaak de Wit J.J. Practical epidemiology of poultry disease and multifactorial conditions. In: Pattison M., Mcmullin P., Bradbury J.M., Alexander D., editors. Poultry Diseases. Saunders Elseveir; Philadelphia: 2008. pp. 492–494. [Google Scholar]
- Skarin H., Blomqvist G., Baverud V. Botulism. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 953–956. [Google Scholar]
- Slauson D.O., Cooper B.J. Pathology – The study of disease. In: Slaudon D.O., Cooper B.J., editors. Mechanisms of Disease: A Textbook of Comparative General Pathology. Mosby, Inc; Missouri: 2002. second and third ed. [Google Scholar]
- Smyth J.A. Atadenovirus (egg drop syndrome and related infections) In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 301–307. [Google Scholar]
- Songer J.G., Uzal F. Ulcerative enteritis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 944–948. [Google Scholar]
- Stenhouse S. The poultry industry. In: Pattison M., editor. Poultry Diseases. Saunders Elseiver; Philadelphia: 2008. pp. 2–13. [Google Scholar]
- Suarez D.L. Avian encephalomyelitis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 487–492. [Google Scholar]
- Swayne D.E., Suarez D.E., Sims L.D. Influenza. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 181–218. [Google Scholar]
- Thayer S.G., Waltman W.D. Streptococcus and Enterococcus. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 977–985. [Google Scholar]
- Todd D., Weston J.H., Mawhinney K.A., Laird C. Characterization of the genome of avian encephalomyelitis virus with cloned cDNA fragments. Avian Diseases. 1999;43(2):219–226. [PubMed] [Google Scholar]
- Tripathy D.N., Reed W.M. Pox. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 333–342. [Google Scholar]
- Vanrompay D. Avian chlamydiosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley and Sons, Inc; Iowa, IA: 2013. pp. 1055–1073. [Google Scholar]
- Venugopal K., Howes K., Flannery D.M.J., Payne L.N. Isolation of acutely transforming subgroup J avian leukosis viruses that induce erythroblastosis and myelocytomatosis. Avian Pathology. 2000;29(5):497–503. doi: 10.1080/030794500750047252. [DOI] [PubMed] [Google Scholar]
- Wang Q., Chang B.J., Riley T.V. Erysipelothrix rhusiopathiae. Veterinary Microbiology. 2010;140(3–4):405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Whiley H., van den Akker B., Giglio S., Bentham R. The role of environmental reservoirs in human campylobacteriosis. International Journal of Environmental Research and Public Health. 2013;10(11):5886–5907. doi: 10.3390/ijerph10115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.A., Ho M. Pasteurella multocida: From zoonosis to cellular microbiology. Clinical Microbiology Reviews. 2013;26(3):631–655. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.A. Pathogenicity of latent infectious laryngotracheitis virus in chickens. Avian Pathology. 1992;21(2):287–294. doi: 10.1080/03079459208418843. [DOI] [PubMed] [Google Scholar]
- Witter R.L. The changing landscape of Marek's disease. Avian Pathology. 1998;27(Suppl. 1):S46–S53. [Google Scholar]
- Xin H. Environmental impacts and sustainability of egg production systems. Poultry Science. 2011;90(1):263–277. doi: 10.3382/ps.2010-00877. [DOI] [PubMed] [Google Scholar]
- Yin J., Liu S., Zhu Y. An overview of the highly pathogenic H5N1 influenza virus. Virologica Sinica. 2013;28(1):3–15. doi: 10.1007/s12250-013-3294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Relevant Websites
- http://partnersah.vet.cornell.edu/avian-atlas/ – Cornell University of Veterinary Medicine.
- http://www.thepoultrysite.com/ – The Poultry Site.
- http://www.oie.int/en/ – World Organization for Animal Health (OIE)


