![]()
Definitions
Normal stool frequency ranges from three times a week to three times a day. As a symptom, diarrhea can be described as a decrease in stool consistency (increased fluidity), stools that cause urgency or abdominal discomfort, or an increase in the frequency of stool. Consistency is defined as the ratio of fecal water to the water-holding capacity of fecal insoluble solids, which are composed of bacterial mass and dietary fiber. Because it is difficult to measure stool consistency and because stool is predominantly (60 to 85%) water, stool weight becomes a reasonable surrogate of consistency.
As a sign, diarrhea is defined by the weight or volume of stool measured over a 24- to 72-hour period. Daily stool weights of children and adults are less than 200 g, and greater stool weights are an objective definition of diarrhea; however, this definition misses 20% of diarrheal symptoms in patients who have loose stools that are less than this daily weight.
Acute diarrheas persist for less than 2 to 3 weeks or, rarely, 6 to 8 weeks. The most common cause of acute diarrhea is infection. Chronic diarrheal conditions persist for at least 4 weeks and, more typically, 6 to 8 weeks or longer. There are four mechanisms of diarrhea: osmotic, secretory, exudative, and altered motility. Because many diarrheal diseases are due to more than one of these mechanisms, it is clinically useful to categorize diarrhea as malabsorptive (fatty), watery, and inflammatory.
Epidemiology
Diarrhea is the second leading cause of mortality worldwide and is particularly problematic for elderly people and for children younger than 5 years of age in developing nations. Infectious diarrheal conditions cause 1.8 million childhood deaths annually, despite the improved use of oral rehydration solutions, zinc, and vitamin A supplements. About one third of these deaths have been due to rotavirus infection (Chapter 388), but the recently introduced oral monovalent rotavirus vaccination has diminished its incidence and mortality in developing and developed nations.
In the United States, about 48 million people suffer from food-borne illness each year, with 128,000 hospitalizations and 3000 deaths, most in elderly people. The complaint of diarrhea accounts for more than 7 million outpatient visits per year. Total costs for diarrheal diseases are about $1.2 billion in direct (health care) costs and $5.4 billion in indirect costs (days lost from work).
Pathobiology
Abnormalities of Fluid and Electrolyte Transport
To understand the osmotic, secretory, and inflammatory mechanisms of diarrhea, it is necessary to understand how the normal intestine handles fluid and solutes in health and disease. Whether a hypotonic meal, such as a steak and water, or a hypertonic meal, such as milk and a doughnut, is consumed, the volume of the meal is augmented by gastric, pancreatic, biliary, and duodenal secretions. The permeable duodenum then renders the meal approximately isotonic with an electrolyte content similar to that of plasma by the time it reaches the proximal jejunum. As the intestinal slurry moves toward the colon, the Na+ concentration in the luminal fluid remains constant, but Cl− is reduced to 60 to 70 mmol/L, and bicarbonate (HCO3 −) is increased to a similar concentration as the result of Cl− and HCO3 − transport mechanisms in the enterocyte and HCO3 − secretion in the ileum (Fig. 142-1B and C ). In the colon, K++ is secreted, and the Na+ transport mechanism of the colonocyte, together with the low epithelial permeability, extracts Na+ and fluid from the stool. As a result, the Na+ content of stool decreases to 30 to 40 mmol/L; K++ increases from 5 to 10 mmol/L in the small bowel to 75 to 90 mmol/L; and poorly absorbed divalent cations, such as Mg2++ and Ca2++, are concentrated in stool to values of 5 to 100 mmol/L. The anion concentrations in the colon change drastically because bacterial degradation of carbohydrate (i.e., unabsorbed starches, sugars, and fiber) creates short-chain fatty acids that attain concentrations of 80 to 180 mmol/L; at colonic pH, organic anions, such as acetate, propionate, and butyrate, are present. In the setting of carbohydrate malabsorption, the generation of high concentrations of these short-chain fatty acids may decrease stool pH to 4 or lower. The osmolality of the stool is approximately that of plasma (280 to 300 mOsm/kg H2O) when it is passed.
FIGURE 142-1.
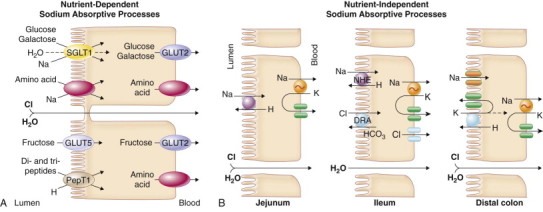
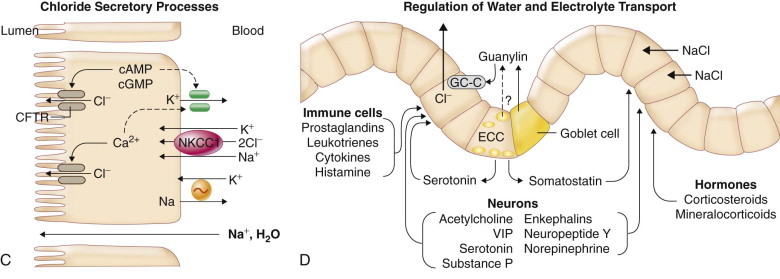
Mechanisms of intestinal transport of water and electrolytes.
A, Intestinal sodium absorption. Sodium is actively absorbed in villus cells of the small intestine and surface cells of the colon. The sodium-potassium adenosine triphosphatase (Na++,K++-ATPase) present on the cell basolateral membrane maintains a low intracellular Na+++ concentration and an electronegative cell interior favoring Na+++ movement across the apical membrane from lumen into cell. In the small intestine, glucose and galactose are taken up with sodium and water at the apical membrane by the sodium-glucose ligand transporter (SGLT1). Several different sodium-dependent amino acid carriers, some with overlapping substrate specificities, transport cationic, anionic, and neutral amino acids into villus cells. Dipeptides and tripeptides are transported by a hydrogen-coupled oligopeptide carrier, PepT1, that is driven by luminal hydrogen ions generated by the epithelial Na+/H++ exchanger. Fructose is taken up by the facilitative glucose transporter (GLUT5). B, Sodium also is absorbed by nutrient-independent transport processes in the small intestine and colon. The Na++/H++ (NHE) and Cl−/HCO3− (DRA) exchangers are inhibited by agents that elevate intracellular cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), or calcium. C, Chloride secretion by intestinal crypt cells. Chloride can be secreted actively throughout the small intestine and colon. Intracellular mediators of secretion (cAMP, cGMP, Ca2++) open apical Cl− channels (cystic fibrosis transmembrane conductance regulator [CFTR], calcium-activated chloride channel [TMEM16]) and basolateral K++ channels. Chloride moves from crypt cells into the intestinal lumen, favoring movement of Cl− from the blood into cells by the Na++/K++/2Cl− cotransporter (NKCC1). Bicarbonate (HCO3−) also may be secreted via the CFTR channel. D, Regulation of intestinal water and electrolyte transport. Normally, the intestine is in a net absorptive state under the control of extrinsic adrenergic nerves from the sympathetic nervous system. Guanylin, the natural ligand for the Escherichia coli stable-toxin receptor (membrane-bound guanylyl cyclase [GC-C]), may be important in regulating local chloride secretion. The normal tone of the intestine is modified by the enteric nervous system, endocrine and inflammatory cells in the intestinal mucosa, and circulating hormones. The enteric nervous system releases a variety of neurotransmitters, some that stimulate chloride secretion (e.g., vasoactive intestinal peptide [VIP], acetylcholine) and others that promote sodium absorption (e.g., enkephalins, neuropeptide Y). Hormones produced locally from enterochromaffin cells (ECC) in the intestinal epithelium and inflammatory mediators released from immune cells directly affect enterocytes and nearby nerves. Circulating hormones (e.g., aldosterone, glucocorticoids) enhance sodium absorption in the intestine. Glucocorticoids also inhibit release of arachidonic acid and production of prostaglandin by inflammatory cells.
At the cellular level Na++ transport by the epithelium from lumen to blood (by Na++-coupled sugar and amino acid transport in the small intestine, by Na++/H++ exchange proteins in the small intestine and proximal colon, and by aldosterone-regulated + Na++ channels in the distal colon) creates a favorable osmotic gradient for absorption (see Fig. 142-1A and B). Chloride transport by the epithelium from blood to lumen (by cystic fibrosis transmembrane conductance regulator [CFTR] and the calcium-activated chloride channel in the small intestine and colon) creates an osmotic gradient for secretion (see Fig. 142-1C). Normally, the intestine is in a net absorptive state, regulated by extrinsic adrenergic nerves and proabsorptive neuropeptides and hormones (see Fig. 142-1D). Stimulation of secretion by neurotransmitters, hormones, and inflammatory mediators (Table 142-1 ; see Fig. 142-1D) can offset this balance.
TABLE 142-1.
Stimuli of Intestinal Secretion
| AGENT | INTRACELLULAR MEDIATOR | RELATED DIARRHEAL ILLNESS |
|---|---|---|
| Enterotoxins | ||
| Cholera, E. coli heat labile toxin, Salmonella, Yersinia } | cAMP } | Travelers, endemic |
| E. coli heat stable toxin | cGMP | |
| Rotatoxin (NSP4) | ? | Viral gastroenteritis |
| Serotonin, PAF | Ca | Inflammatory, allergic |
| PG, leukotrienes | cAMP, Ca | Invasive enteric bacteria* |
| PG | cAMP | Villous adenoma |
| Histamine | Ca | Intestinal allergies, mastocytosis, scombroid poisoning |
| VIP | cAMP | VIPoma, ganglioneuromas |
| 5-HT, substance P, bradykinin | Ca | Malignant carcinoid |
| Calcitonin | ? | Medullary carcinoma thyroid |
| Acetylcholine | Ca | Insecticides, nerve gas poisoning, cholinergic drugs |
| Ricinoleic acid | cAMP, Ca | Laxative abuse† |
| Caffeine | cAMP | Coffee, sodas, tea |
5-HT = 5-hydroxytryptamine; Ca = calcium; cAMP = cyclic adenosine monophosphate; cGMP = cyclic guanosine monophosphate; PAF - platelet-activating factor; PG = prostaglandin; VIP = vasoactive intestinal peptide.
Shigella species, Clostridium difficile, enteroinvasive E. coli, Vibrio parahaemolyticus, Clostridium perfringens.
Also phenolphthalein, anthraquinones, bisacodyl, dioctyl sodium sulfosuccinate, and senna.
Diarrhea is due primarily to alterations of intestinal fluid and electrolyte transport and less to smooth muscle function. Each 24 hours, 8 to 10 L of fluid enters the duodenum. The diet supplies 2 L of this fluid; the remainder comes from salivary, gastric, hepatic, pancreatic, and intestinal secretions. The small intestine normally absorbs 8 to 9 L (80%) of this fluid and presents 1.5 L to the colon for absorption. Of the remaining fluid, the colon absorbs all but approximately 100 mL. Diarrhea can result from increased secretion by the small intestine or the colon if the maximal daily absorptive capacity of the colon (4 L) is exceeded. Alternatively, if the colon is diseased so that it cannot absorb even the 1.5 L normally presented to it by the small intestine, diarrhea results.
Watery diarrheas may be due to osmotic, secretory, or inflammatory mechanisms. With ingestion of a poorly absorbed (e.g., Mg2++) or unabsorbable (polyethylene glycol, lactulose or, in lactase-deficient individuals, lactose) solute, the osmotic force of the solute pulls water and secondarily sodium and chloride ions into the intestinal lumen. A considerable proportion of the osmolality of stool results from the nonabsorbed solute. This gap between stool osmolality and the sum of the electrolytes in the stool causes osmotic diarrhea.
Active chloride secretion or inhibited sodium absorption, which also create an osmotic gradient favorable for the movement of fluids from blood to lumen, explains the pathophysiology of the secretory diarrheas. Agents that increase enterocyte cyclic adenosine monophosphate (cAMP) (e.g., cholera toxin, prostaglandins), cyclic guanosine monophosphate (cGMP) (e.g., Escherichia coli stable toxin), or intracellular ionized calcium (Ca2++) (e.g., acetylcholine) (see Table 142-1) inhibit non-nutrient Na++ absorption and stimulate Cl− secretion (see Table 142-1 and Fig. 142-1C and D).
Inflammatory diarrheas, which may be watery or bloody, are characterized by enterocyte damage, villus atrophy, and crypt hyperplasia. The damaged enterocyte membrane of the small intestine has decreased disaccharidase and peptide hydrolase activity, reduced or absent Na++-coupled sugar or amino acid transport mechanisms, and reduced or absent sodium chloride absorptive transporters. Conversely, the hyperplastic crypt cells maintain their ability to secrete – Cl– (and perhaps HCO3 −). If the inflammation is severe, immune-mediated vascular damage or ulceration allows blood, pus, and protein to leak (exudate) from capillaries and lymphatics and contribute to the diarrhea. Activation of lymphocytes, phagocytes, and fibroblasts releases various inflammatory mediators that induce intestinal chloride secretion (see Fig. 142-1D). Interleukin-1 (IL-1) and tumor necrosis factor, which also are released into the blood, cause fever, anorexia, and malaise.
Acute Diarrhea
Clinical Manifestations
Approximately 80% of acute diarrheas are due to infections with viruses, bacteria, and parasites. The remainder is due to medications that have an osmotic force, stimulate intestinal fluid secretion, or contain poorly or nonabsorbable sugars (e.g., sorbitol), or less commonly to fecal impaction, pelvic inflammation (e.g., acute appendicitis [Chapter 144]), or intestinal ischemia (Chapter 145).
Food-Borne and Water-Borne Infectious Diarrhea
Most infectious diarrheas are acquired through fecal-oral transmission from water, food, or person-to-person contact (Table 142-2 ). Patients with infectious diarrhea often complain of nausea, vomiting, and abdominal cramps that are associated with watery, malabsorptive, or bloody diarrhea and fever (dysentery) (Chapters 310 through 320, 344, 345, 358 to 360, 364, 365, 387, and 388Chapter 310Chapter 311Chapter 312Chapter 313Chapter 314Chapter 315Chapter 316Chapter 317Chapter 318Chapter 319Chapter 320Chapter 344Chapter 345Chapter 358Chapter 359Chapter 360Chapter 364Chapter 365Chapter 387Chapter 388). As documented using polymerase chain reaction methods of diagnosis, most outbreaks of nonbacterial acute gastroenteritis in the United States and other countries are caused by noroviruses (Norwalk agent; Chapter 388). Rotavirus (Chapter 388) predominantly causes diarrhea in infants, usually in the winter months, but also may cause nonseasonal acute diarrhea in adults, particularly in elderly people. Mechanisms for diarrhea include decreased fluid absorption due to destruction of villus enterocytes and stimulation of fluid secretion by NSP4 rotatoxin and viral activation of the enteric nervous system.
TABLE 142-2.
Epidemiology of Acute Infectious Diarrhea and Infectious Food-Borne Illness
| VEHICLE | CLASSIC PATHOGENS |
|---|---|
| Water (including foods washed in such water) | Vibrio cholerae, caliciviruses (Norwalk agent), Giardia, Cryptosporidium |
| Food | |
| Poultry | Salmonella, Campylobacter, Shigella species |
| Beef, unpasteurized fruit juice | Enterohemorrhagic Escherichia coli |
| Pork | Tapeworm |
| Seafood and shellfish (including raw sushi and gefilte fish) | V. cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus; Salmonella and Shigella species; hepatitis A and B viruses; tapeworm; anisakiasis |
| Cheese, milk | Listeria species |
| Eggs | Salmonella species |
| Mayonnaise-containing foods and cream pies | Staphylococcal and clostridial food poisonings |
| Fried rice | Bacillus cereus |
| Fresh berries | Cyclospora species |
| Canned vegetables or fruits | Clostridium species |
| Sprouts | Enterohemorrhagic E. coli, Salmonella species |
| Animal-to-person (pets and livestock) contact | Salmonella, Campylobacter, Cryptosporidium, enterohemorrhagic E. coli, and Giardia species |
| Person-to-person (including sexual) contact | All enteric bacteria, viruses, and parasites |
| Daycare center | Shigella, Campylobacter, Cryptosporidium, and Giardia species; viruses; Clostridium difficile |
| Hospitalization, antibiotics, or chemotherapy | C. difficile |
| Swimming pool | Giardia and Cryptosporidium species |
| Foreign travel | E. coli of various types; Salmonella, Shigella, Campylobacter, Giardia, and Cryptosporidium species; Entamoeba histolytica |
Adapted from Powell DW. Approach to the patient with diarrhea. In: Yamada T, Alpers DH, Owyang C, et al, eds. Textbook of Gastroenterology, 3rd ed. Philadelphia: Lippincott-Raven; 1999.
Food-borne bacterial diseases in the United States are primarily due to Salmonella (Chapter 316), Campylobacter jejuni (Chapter 311), and E. coli O157:H7 (Chapter 312), and less commonly Shigella (Chapter 317). Outbreaks of E. coli O157:H7 have been associated with petting zoos, uncooked ground beef, and green leafy vegetables. These bacteria most often invade the distal small bowel and colon, where they multiply intracellularly and damage the epithelium. Diarrhea is due to the stimulation of intestinal secretion by inflammatory mediators, decreased absorption across the damaged epithelium, and exudation of protein into the lumen. Shigella species and enterohemorrhagic E. coli produce a similar toxin, the “Shiga toxin,” which is cytotoxic to intestinal epithelial cells and causes inflammation, cell damage, and diarrhea with blood and pus.
Outbreaks of Cryptosporidium (Chapter 358) have been reported in water parks. This parasite causes diarrhea by adhering and fusing to the epithelial cell membrane in the small bowel, thereby causing cell damage. Organisms that are specific for seafood include Vibrio parahaemolyticus (Chapter 310), which causes either watery or bloody diarrhea, and Vibrio vulnificus, which causes watery diarrhea and, especially in patients with liver disease, a fatal septicemia. Ingestion of meat contaminated by anthrax (Chapter 302) causes fever, diffuse abdominal pain, and bloody stool or vomitus. Anthrax invades the intestinal mucosa; the organism, or anthrax toxin, causes inflammation, ulceration, and necrosis.
In addition to enteric infections, certain systemic infections (e.g., viral hepatitis [Chapter 150], listeriosis [Chapter 301], legionellosis [Chapter 322]) and emerging infections (e.g., Hanta virus [Chapter 389], severe acute respiratory syndrome [SARS, Chapter 374], avian influenza [Chapter 372]) may cause or manifest with substantial diarrhea.
Environmental and Food Poisonings
Food poisoning refers to the accumulation of toxin in food owing to the growth of toxin-producing organisms, most commonly Staphylococcus aureus (Chapter 296), Bacillus cereus, Clostridium perfringens (Chapter 304), and Clostridium botulinum (Chapter 304). Diarrhea is usually of rapid onset, as early as 4 hours after ingestion, and is often associated with vomiting. Natural toxins also are responsible for mushroom (Amanita) poisoning (Chapter 110), which can also cause acute liver and kidney failure.
Environmental poisonings may be caused by heavy metals (arsenic from rat poison, gold, lead, mercury) that impair cell energy production. Arsenic (Chapter 21) also induces cardiovascular collapse at high doses. Insecticide (organophosphates and carbamates) poisoning occurs most commonly in field workers or from the ingestion of contaminated herbs or teas (Chapter 110); diarrhea, excessive saliva, and pulmonary secretions are caused by acetylcholine-stimulated chloride secretion in intestine and other epithelia. Patients often have associated vomiting and abdominal cramps.
Seafood is a common source of food poisoning, particularly fin fish and bivalve shellfish. Most of these toxins cause varying combinations of gastrointestinal (nausea, vomiting, diarrhea) and neurologic symptoms (tingling and burning around the mouth, facial flushing, sweating, headache, palpitations, and dizziness) within hours of seafood ingestion (Chapter 114). Similar symptoms are reported in patients with scombroid poisoning, which is caused by ingestion of decaying flesh of blood fish (tuna, mahi-mahi, marlin, or mackerel) that release large amounts of histamine (Chapter 114).
Marine dinoflagellates (algae) produce toxins that can cause paralytic shellfish poisoning, diarrhetic shellfish poisoning, and ciguatera (Chapter 114). Sporadic outbreaks of diarrhetic shellfish poisoning “red tides” occur when bivalve mollusks ingest dinoflagellates that produce saxitoxins (voltage-sensitive sodium-channel blocker) and okadaic acid (a lipid-soluble toxin that inhibits serine and threonine protein phosphatases 1 and 2A). Ingestion of contaminated mollusks by humans results in diarrhea and neurologic symptoms. Saxitoxins cause predominantly neurologic symptoms (paralytic, neurotoxic, or amnestic shellfish poisonings) and okadaic acid gastrointestinal symptoms (diarrhetic shellfish poisoning).
Food-chain passage of another dinoflagellate species (Gambierdiscus toxicus) to fin fish (mackerel, amberjack, snapper, grouper, or barracuda) results in the accumulation of ciguatoxin (Chapter 114) that causes a seafood poisoning called ciguatera. Ciguatoxin activates voltage-sensitive sodium channels and causes neurologic and gastrointestinal symptoms. Fish from the Albemarle-Pamlico estuary (eastern United States) ingest toxic dinoflagellates that cause Pfiesteria piscicida poisoning. The dinoflagellate toxins cause nausea, vomiting, abdominal pain, diarrhea, and neurologic symptoms such as fatigue, myalgias, pruritus, circumoral paresthesias, reversal of hot and cold sensation, psychiatric abnormalities, and memory loss. The neurologic symptoms may persist for months to years. Puffer fish poisoning by tetrodotoxin, a voltage-sensitive sodium-channel blocker produced by the fish, causes neurologic symptoms, respiratory paralysis, and death.
Traveler's Diarrhea
North American travelers to developing countries and travelers on airplanes and cruise ships are at high risk for acute infectious diarrhea. Most traveler's diarrhea (85%) is due to enterotoxic E. coli. E. coli heat-stable toxin binds to guanylate cyclase in the enterocyte brush-border membrane, where it results in elevation of intracellular cGMP. E. coli heat-labile toxin, similar to cholera toxin, binds to the monosialoganglioside GM1 in the brush-border membrane, thereby resulting in the activation of adenylate cyclase and the elevation of intracellular cAMP. Cyclic AMP and cGMP stimulate intestinal chloride secretion (see Fig. 142-1C) and inhibit the nutrient-independent absorption of sodium and chloride (see Fig. 142-1B). Sodium-glucose absorption is not affected, hence the basis for oral rehydration therapy. Cholera toxin permanently binds to adenylate cyclase until the natural turnover of the intestinal epithelium in 5 to 7 days, thereby resulting in persistent secretion and severe diarrhea. Of the 10 to 15 cases of cholera reported in the United States each year, about 60% are travel associated.
Antibiotic-Associated Diarrheas
Antibiotics are a common cause of hospital-acquired diarrheas that occur in about 20% of patients receiving broad-spectrum antibiotics; about 30% of these diarrheas are due to Clostridium difficile (Chapter 304). Hypervirulent, fluoroquinolone-resistant strains that produce increased levels of toxins A and B and a binary toxin have emerged. These strains are associated with an increase in the incidence and severity of C. difficile infections, including fulminant C. difficile colitis that can lead to colectomy or even death. The A and B toxins produced by C. difficile can cause diarrhea. In animal models, IL-8, substance P, and leukotriene B4 were found to mediate toxin A–stimulated intestinal fluid secretion. C. difficile can cause severe diarrhea, pseudomembranous colitis, or toxic megacolon. Patients may have a relapsing course after seemingly successful therapy with metronidazole or vancomycin.
Nosocomial Hospital Diarrhea
Diarrhea is the most common nosocomial illness among hospitalized patients and residents in long-term care facilities. Common causes include antibiotic-associated diarrhea, C. difficile infection, medications, fecal impaction, tube feeding, and underlying illness. Magnesium-containing laxatives, antacids, and lactulose cause osmotic diarrheas. Bisacodyl laxatives cause secretory diarrhea. Colchicine, neomycin, methotrexate, and para-aminosalicylic acid damage the enterocyte membrane. Cholestyramine, colestipol, and colesevelam bind bile salts and can result in malabsorption. Gold therapy causes intestinal inflammation and diarrhea. Liquid formulations of medications cause diarrhea (elixir diarrhea) because of the high content of sorbitol or other nonabsorbable sugars (e.g., mannitol) used to sweeten the elixir; patients prescribed liquid medications through feeding tubes may receive more than 20 g of sorbitol daily. An important but poorly understood cause of diarrhea is enteral (tube) feeding (Chapter 223), particularly in critically ill patients, who often develop diarrhea. Dysmotility, increased intestinal permeability, and low sodium content in enteral formulas may be contributing factors.
Patients in mental health institutions and nursing homes have a high incidence of nosocomial infectious diarrhea (e.g., C. difficile and less commonly Shigella, Salmonella, hemorrhagic E. coli, Giardia, Entamoeba histolytica). Infectious diarrhea, 50% or more of which is caused by C. difficile, is also common in acute-care hospitals. Severe C. difficile infection has also been reported among peripartum women. If outside foods are not brought to hospitalized patients, the likelihood of a nosocomial infection caused by Salmonella or Shigella is extremely rare. Immunosuppressed patients are also susceptible to nosocomial viral infections (rotavirus, norovirus, adenovirus, and coxsackievirus).
Cancer Treatment–Related Diarrhea
Abdominal or whole body radiation virtually always causes an increased frequency of bowel movements that are often watery. Cancer chemotherapy with amsacrine, azacitidine, cytarabine, dactinomycin, daunorubicin, doxorubicin, floxuridine, 5-fluorouracil, 6-mercaptopurine, methotrexate, plicamycin, IL-2, and resveratrol may cause mild to moderate diarrhea. Irinotecan (CPT-11) and the combination of 5-fluorouracil plus leucovorin are frequent causes of severe watery diarrhea.
Daycare Diarrhea
More than 7 million children in the United States attend daycare, where diarrhea is extremely common, and secondary infection of family members occurs in 10 to 20% of cases. Most outbreaks of diarrhea are due to rotavirus or norovirus; less common causes are Shigella (Chapter 317), Giardia (Chapter 359), and Cryptosporidium (Chapter 358).
Runner's Diarrhea
Diarrhea occurs in 10 to 25% of individuals who exercise vigorously, especially women marathon runners and triathletes. Some athletes have associated abdominal cramps, urgency, nausea, or vomiting. The pathophysiology of runner's diarrhea is unknown. Release of intestinal secretogogues, especially prostaglandins, hormones, or ischemia, may be involved.
Diagnosis
Acute watery diarrhea may be due to infections, food toxins, or medications, or the acute diarrhea may signal the onset of a chronic disease (Fig. 142-2 ; see TABLE 142-1, TABLE 142-2) (Chapters 310 through 320, 344, 345, 359, 360, 364, 365, 387, and 388Chapter 310Chapter 311Chapter 312Chapter 313Chapter 314Chapter 315Chapter 316Chapter 317Chapter 318Chapter 319Chapter 320Chapter 344Chapter 345Chapter 359Chapter 360Chapter 364Chapter 365Chapter 387Chapter 388). The diagnostic approach in patients with fever and watery or bloody diarrhea should focus on stool cultures for Campylobacter, Salmonella, and Shigella species. Routine stool culture is not indicated when diarrhea occurs after 3 to 5 days of hospitalization, except in patients with neutropenia, human immunodeficiency virus infection, or signs of enteric infection. In patients with a history of recent antibiotic use, hospitalization, or peripartum, stools for C. difficile toxin should be obtained. Organisms that cause diarrhea but are not routinely tested by clinical microbiology laboratories include Yersinia, Plesiomonas, enterohemorrhagic E. coli serotype O157:H7, Aeromonas, Cyclospora, microsporidia, and noncholera Vibrio. Parasites such as Giardia, Cryptosporidium, and Strongyloides can be difficult to detect in stool but may be diagnosed by stool antigen testing or intestinal biopsy. Despite all testing techniques available, 20 to 40% of acute infectious diarrheas remain undiagnosed.
Treatment  .
.
Goals for the treatment of diarrhea include fluid replacement, antidiarrheal agents, nutritional support, and antimicrobial therapy when indicated. Because death in patients with acute diarrhea is caused by dehydration, the first task is to assess the degree of dehydration and to replace fluid and electrolyte deficits.
Fluid Replacement
Severely dehydrated patients should be treated with intravenous Ringer's lactate or saline solution, with additional potassium and bicarbonate as needed. Oral rehydration solutions, which are used extensively to replace diarrheal fluid and electrolyte losses, are effective because they contain sodium, sugars, and, often, amino acids that use nutrient-dependent sodium uptake transporters. In alert patients with mild to moderate dehydration, oral rehydration solution is equally effective as intravenous hydration in repairing fluid and electrolyte losses. Oral rehydration solutions can be given to infants and children in volumes of 50 to 100 mL/kg over 4 to 6 hours; adults may need to drink 1000 mL/hr. Reduced-osmolarity solutions (Na++ 75 mmol/L, osmolarity 245 mmol/L versus Na++ 90 mmol/L, osmolarity 311 mmol/L in standard solutions) are better tolerated and effective in noncholera diarrhea but may cause hyponatremia in patients with high-volume diarrhea, particularly children. Glucose-based solutions, although effective in rehydrating the patient, may worsen the diarrhea. In contrast to glucose-based solutions, polymeric rice-based solutions decrease diarrhea in cholera victims; rice is digested to many glucose monomers that aid in the absorption of intestinal secretions. These solutions may not decrease stool output in acute diarrhea, but they will effectively rehydrate the patient despite continued diarrhea. After rehydration has been accomplished, oral rehydration solutions are given at rates equaling stool loss plus insensible losses until the diarrhea ceases.
Reducing Diarrhea
Bismuth subsalicylate (Pepto-Bismol, 525 mg orally every 30 minutes to 1 hour for five doses, may repeat on day 2) is safe and efficacious in bacterial infectious diarrheas. Opiates and anticholinergic drugs are not recommended for invasive bacterial infectious diarrheas because these drugs paralyze intestinal motility and predispose to increased colonization, invasion, and prolonged excretion of infectious organisms. The opiate loperamide is safe in acute or traveler's diarrhea, provided that it is not given to patients with dysentery (high fever, with blood or pus in the stool), and especially when administered concomitantly with effective antibiotics. A combination of loperamide (2 mg orally four times a day) plus simethicone (125 mg orally four times a day) may reduce the abdominal cramps and duration of traveler's diarrhea. Racecadotril (100 mg orally three times a day in adults, 1.5 mg/kg of body weight orally three times a day in children), an intestinal enkephalinase inhibitor that is antisecretory but does not paralyze intestinal motility, is effective in the treatment of acute diarrhea in children and adults. The diarrhea associated with enteral nutrition (Chapter 223) often can be managed with pectin (4 g/kg body weight daily) or, if there are no contraindications, with loperamide (2 mg orally four times a day for 3 to 7 days, maximal dose 16 mg daily), and diarrhea is not a reason to stop tube feeding unless stool volumes exceed 1 L/day.
Anxiolytics (e.g., diazepam 2 mg orally two to four times daily) and antiemetics (e.g., promethazine 12.5 to 25 mg orally once or twice daily) that decrease sensory perception may make symptoms more tolerable and are safe. Some foods or food-derived substances (green bananas, pectins [amylase-resistant starch], zinc) lessen the amount or duration of diarrhea in children. Unabsorbed amylase-resistant starches are metabolized in the colon to short-chain fatty acids that enhance fluid absorption. Zinc supplementation (20 mg of elemental zinc orally once a day) is effective in preventing recurrences of diarrhea in malnourished children; copper deficiency is a potential complication of prolonged zinc therapy.
Probiotics may be of benefit in children with acute diarrhea, predominantly that due to rotavirus infection. Lactobacillus GG (1010 colony-forming units [CFU]/250 mL daily until diarrhea stops) added to an oral rehydration solution decreases the duration of diarrhea.
Antibiotics
While the clinician is awaiting stool culture results to guide specific therapy (Chapter 295), the fluoroquinolones (e.g., ciprofloxacin, 500 mg orally twice a day for 1 to 3 days, or levofloxacin, 500 mg orally daily for 1 to 3 days) are the treatment of choice when antibiotics are indicated (see Fig. 142-2). Trimethoprim-sulfamethoxazole (one double-strength tablet orally twice a day for 5 days or two single-strength tablets orally twice a day for 5 days) is second-line therapy. If the symptom complex suggests Campylobacter infection, azithromycin (500 mg orally once a day for 3 days) should be added. Regardless of the cause of infectious diarrhea, patients should be treated with antibiotics if they are immunosuppressed; have valvular, vascular, or orthopedic prostheses; have congenital hemolytic anemias (especially if salmonellosis is involved); or are extremely young or old.
Certain infectious diarrheas should be treated with antibiotics, including those associated with shigellosis (Chapter 317), cholera (Chapter 310), pseudomembranous enterocolitis (Chapter 304), parasitic infestations (Chapters 358 to 360 and 365Chapter 358Chapter 359Chapter 360Chapter 365), and sexually transmitted diseases (Chapter 293). Treatment of E. coli serotype O157:H7 infection is not recommended at present because current antibiotics do not appear to be helpful and the incidence of complications (hemolytic-uremic syndrome) may be greater after antibiotic therapy. Antibiotics are not effective for viral diarrhea or cryptosporidiosis.
For traveler's diarrhea, ciprofloxacin (500 mg orally two times a day for 3 days) is an effective treatment. The nonabsorbable antibiotic rifaximin (200 mg taken orally three times a day or 400 mg two times a day for 3 days) is safe and effective for treatment of traveler's diarrhea in Mexico, but it may not be effective against Campylobacter and Shigella infections.
Fluoroquinolone-resistant and trimethoprim-sulfamethoxazole-resistant strains of Shigella, E. coli, Salmonella, Campylobacter, and C. difficile have emerged. Azithromycin, 500 mg orally on day 1 and 250 mg orally once a day for 4 days, may be an effective alternative treatment for resistant strains of Shigella and Campylobacter and for traveler's diarrhea acquired in Mexico.
If C. difficile is suspected on an epidemiologic basis, metronidazole (250 mg orally four times a day or 500 mg orally three times a day for 10 days) or oral vancomycin (125 to 250 mg orally four times a day for 10 days) should be prescribed. In patients with recurrent C. difficile infection that is associated with low serum antibody titers to toxin A, immunotherapy with monoclonal antibodies against toxin A and B1 or fecal bacteriotherapy may decrease recurrence rates. Non–C. difficile antibiotic-induced diarrhea is generally mild and self-limited, and it usually clears spontaneously or in response to cholestyramine therapy (4 g orally four times a day for 2 weeks).
Treatment for chemotherapy-induced and radiation-induced mild to moderate diarrhea includes loperamide (2 mg orally four times a day) and nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., naproxen, 250 to 500 mg orally twice daily). Octreotide may be an effective treatment in those with severe diarrhea in doses up to 700 µg administered subcutaneously daily.
FIGURE 142-2.
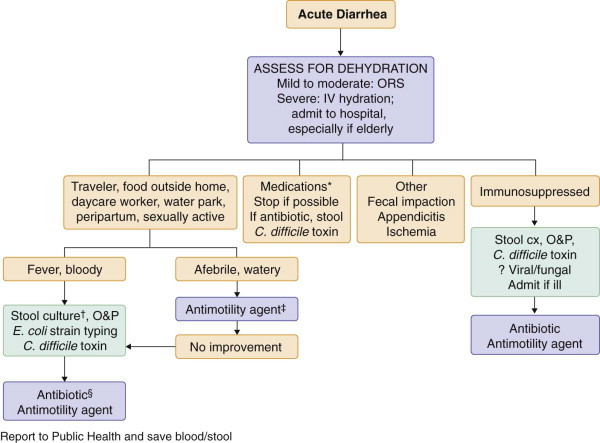
Approach to the diagnosis of acute diarrhea.
*More than 700 medications cause diarrhea, including furosemide, caffeine, protease inhibitors, thyroid preparations, metformin, mycophenolate mofetil, sirolimus, cholinergic drugs, colchicine, theophylline, selective serotonin reuptake inhibitors, proton pump inhibitors, histamine-2 blockers, 5-ASA derivatives, angiotensin-converting enzyme inhibitors, bisacodyl, senna, aloe, anthraquinones, and magnesium- or phosphorus-containing medications. †Specifically request culture for Yersinia, Plesiomonas, enterohemorrhagic Escherichia coli serotype O157:H7, and Aeromonas if suspected. ‡If high suspicion for Clostridium difficile or invasive bacterial infection, wait for stool culture and toxin studies before starting. Racecadotril has antisecretory effects without paralyzing intestinal motility and can be used if available. §Not recommended for patients with bloody diarrhea due to E. coli O157:H7. CX = culture; IV therapy = intravenous rehydration; O&P = ova and parasites; ORS = oral rehydration solution.
Prevention
Rotavirus vaccination (Chapter 388) reduces the risk of infection and generally results in milder symptoms among those infected.2, 3 Travelers to high-risk countries (Central America and parts of Latin America, Africa, Asia, the Middle East) should avoid ingestion of tap water and ice and of raw meat, raw seafood, and raw vegetables. An oral cholera vaccine against recombinant toxin B subunit and killed whole-cell (rBS-WC) is effective in preventing infection from the O1 El Tor strain and partially effective against enterotoxigenic E. coli strains.4 Cholera vaccination is recommended for relief workers and health professionals who work in endemic countries and for individuals who are immunocompromised or have chronic illnesses or hypochlorhydria. Rifaximin (200 mg orally per day for 2 weeks) is safe and effective for preventing traveler's diarrhea in Mexico,5 and the combination of rifaximin plus loperamide is better than either one alone. Bismuth subsalicylate (525 mg orally four times a day for up to 3 weeks) is also effective. Loperamide and NSAIDs are taken prophylactically by many runners who are susceptible to runner's diarrhea, but it is not clear whether they are effective.
Chronic Diarrhea
An estimated 5% of the U.S. population suffers from chronic diarrhea, and about 40% of these individuals are older than 60 years of age. In 25 to 50% of cases, expert history and physical examination may be sufficient to make a definitive diagnosis (Fig. 142-3 ). The addition of stool culture and examination for ova and parasites, determination of stool fat, and flexible sigmoidoscopy or colonoscopy with biopsy raises the diagnostic rate to about 75%. The remaining 25% of patients with chronic diarrhea may need extensive testing and perhaps hospitalization to make a diagnosis.
FIGURE 142-3.
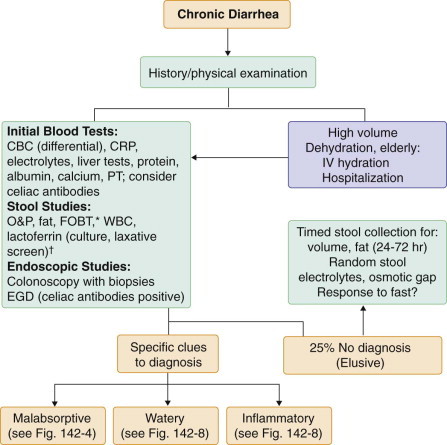
Initial approach to chronic diarrhea.
*Fecal occult blood testing (FOBT) is a sensitive test for underlying bowel inflammation. †Perform stool culture in those who are immunosuppressed; perform laxative screen if laxative abuse is suspected. CBC = complete blood count; CRP = C-reactive protein; EGD = esophagogastroduodenoscopy; IV = intravenous; O&P = ova and parasites; PT = prothrombin time; WBC = white blood cells.
Prolonged, Persistent Infectious Diarrheas
Prolonged infectious diarrheas (>2 weeks) may be due to persistent or recurrent infections. These diarrheas occur most commonly in children exposed to unsafe drinking water in developing countries, patients who have acquired immunodeficiency syndrome (AIDS) or are immunosuppressed for other reasons, and recent travelers. The most common causes in children in developing countries are enteropathogenic and enteroadherent E. coli infections (Chapter 312). Other common organisms include Giardia (Chapter 359), Cryptosporidium (Chapter 358), Entamoeba (Chapter 360), Isospora (Chapter 361), and microsporidia (Chapter 358). Recurrent or prolonged infectious diarrhea may lead to severe malnutrition and death (mortality rate, 50%). Treatment includes nutrition support with supplemental vitamin A (200,000 IU twice yearly) and zinc (20 mg elemental daily for 14 days). Severe disease may require total parenteral nutrition.
In AIDS patients, protracted diarrhea may be caused by treatable agents such as Entamoeba histolytica, Giardia, or Strongyloides or by organisms such as Cryptosporidium, Isospora belli, and microsporidia that are difficult to treat or untreatable. The most effective treatment is retroviral therapy to improve the immune system (Chapter 396).
Up to 10% of travelers returning from developing countries have infectious diarrhea that persists for longer than 3 to 4 weeks. Stool should be examined for culture and for ova and parasites; in patients with a recent history of antibiotic use, stool should also be sent for C. difficile toxin. Any specific organisms that are identified should be treated. If treatment with trimethoprim-sulfamethoxazole or a fluoroquinolone has been unsuccessful, tetracycline (250 mg orally four times a day for 7 to 10 days) or metronidazole (250 mg orally three times a day for 7 to 10 days) can be tried. After documented infectious diarrhea, 25% of patients experience pain, bloating, urgency, a sense of incomplete evacuation, and loose stools for 6 months or longer; some of these patients have celiac disease, so screening (see later) is warranted in this setting. When no other cause is found, these patients are deemed to have postinfectious irritable bowel syndrome (Chapter 139).
Sporadic outbreaks of severe, prolonged diarrhea, often greater than 1 year in duration, occasionally have been reported. This form of prolonged diarrhea is called Brainerd's diarrhea. The organism has yet to be identified. The diarrhea is difficult to treat; cholestyramine (4 g orally three times a day) may be helpful.
Malabsorptive Syndromes
Malabsorption is caused by many different diseases, drugs (e.g., the lipase inhibitor orlistat; Chapter 227), and nutritional products (the nonabsorbable fat olestra) that impair intraluminal digestion, mucosal absorption, or delivery of the nutrient to the systemic circulation (Fig. 142-5; Table 142-3 ). Dietary fat is the nutrient most difficult to absorb. Fatty stools (steatorrhea) are the hallmark of malabsorption; a stool test for fat is the best screening test. Malabsorption does not always cause diarrhea. Clinical signs of vitamin or mineral deficiencies may occur in the absence of diarrhea. A careful history is crucial in guiding further testing to confirm the suspicion of malabsorption and to make a specific diagnosis (see Fig. 142-4 ). The goals of treatment are to correct or treat the underlying disease and to replenish losses of water, electrolytes, and nutrients.
FIGURE 142-5.
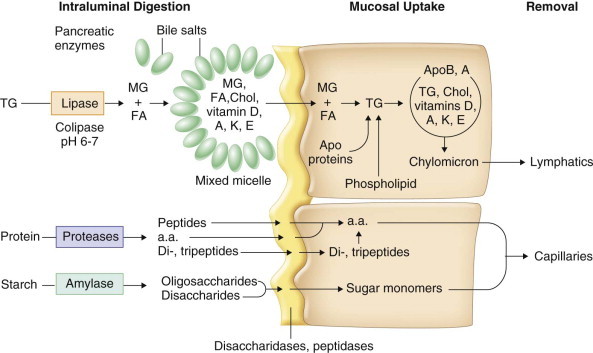
Phases of intestinal digestion and absorption of dietary fat, protein, and carbohydrate.
a.a. = Amino acids; ApoB, A = apolipoproteins B and A; Chol = cholesterol; FA = fatty acids; MG = monoglycerides; TG = triglycerides.
TABLE 142-3.
Causes of Malabsorption
| MECHANISM OF MALABSORPTION | CONDITIONS |
|---|---|
| Impaired mixing | Partial/total gastrectomy |
| Gastric bypass surgery | |
| Impaired lipolysis | Chronic pancreatitis |
| Pancreatic cancer | |
| Congenital pancreatic insufficiency | |
| Congenital colipase deficiency | |
| Gastrinoma | |
| Impaired micelle formation | Severe chronic liver disease |
| Cholestatic liver disease | |
| Bacterial overgrowth | |
| Crohn's disease | |
| Ileal resection | |
| Gastrinoma | |
| Impaired mucosal absorption | Lactase deficiency |
| Congenital enterokinase deficiency | |
| Abetalipoproteinemia | |
| Giardiasis | |
| Celiac disease | |
| Tropical sprue | |
| Agammaglobulinemia | |
| Amyloidosis | |
| AIDS-related (infections, enteropathy) | |
| Radiation enteritis | |
| Graft-versus-host disease | |
| Whipple's disease | |
| Eosinophilic gastroenteritis | |
| Megaloblastic gut | |
| Collagenous sprue | |
| Ulcerative jejunitis | |
| Lymphoma | |
| Bacterial overgrowth | |
| Short-bowel syndrome | |
| Mastocytosis | |
| Impaired nutrient delivery | Congenital lymphangiectasia |
| Lymphoma | |
| Tuberculosis | |
| Constrictive pericarditis | |
| Severe congestive heart failure | |
| Unknown | Hypoparathyroidism |
| Adrenal insufficiency | |
| Hyperthyroidism | |
| Carcinoid syndrome |
AIDS = acquired immunodeficiency syndrome.
FIGURE 142-4.
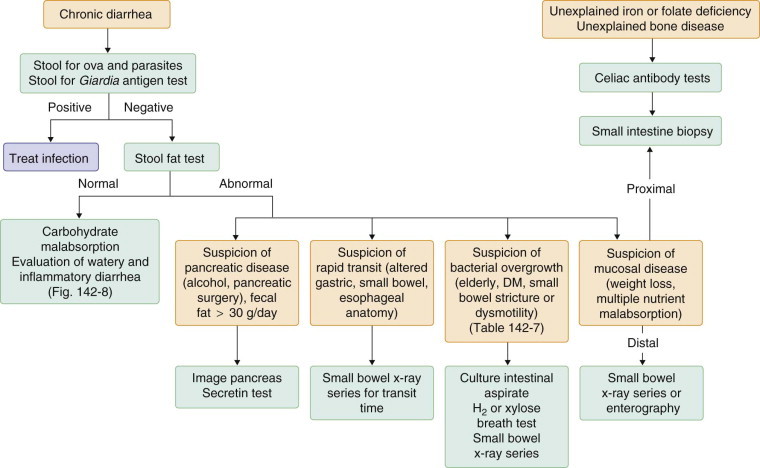
Approach to the diagnosis of malabsorption.
DM = diabetes mellitus.
Conditions That Impair Intraluminal Digestion
Most digestion and absorption of nutrients occur in the small intestine (Fig. 142-5 ). Carbohydrates and most dietary proteins are water soluble and readily digested by pancreatic enzymes. Pancreatic proteases (trypsinogen, chymotrypsinogen, procarboxypeptidases) are secreted from acinar cells in inactive forms. The cleavage of trypsinogen to trypsin by the duodenal brush-border peptidase enteropeptidase (enterokinase) allows trypsin to cleave the remaining trypsinogen and other proteases to their active form.
Most dietary lipids (long-chain triglycerides, cholesterol, and fat-soluble vitamins) are water insoluble and must undergo lipolysis and incorporation into mixed micelles before they can be absorbed across the intestinal mucosa. Pancreatic lipase, in the presence of its cofactor, colipase, cleaves long-chain triglycerides into fatty acids and monoglycerides. The products of lipolysis interact with bile salts and phospholipids to form mixed micelles, which also incorporate cholesterol and fat-soluble vitamins (D, A, K, and E) in their hydrophobic centers. Bicarbonate secreted from pancreatic duct cells is physiologically important because pancreatic enzyme activity and bile salt micelle formation are optimum at a luminal pH of 6 to 8.
Impaired Mixing
Surgical alterations, such as partial gastrectomy with gastrojejunostomy (Billroth II anastomosis) or gastrointestinal bypass surgeries for obesity, result in the release of biliary and pancreatic secretions into the intestine at a site remote from the site of entry of gastric contents. This imbalance can result in impaired lipolysis and impaired micelle formation, with subsequent fat malabsorption. Bypass of the duodenum also impairs absorption of iron, folate, and calcium. Rapid transit through the jejunum contributes to the malabsorption of nutrients. Individuals with these conditions also have surgical anastomoses that predispose to bacterial overgrowth.
Dumping Syndrome
After esophageal (distal esophagectomy, myomectomy for achalasia), gastric (Nissan wrap, hiatal hernia repair, gastrojejunostomy), and bariatric (Roux-en-Y and duodenal switch gastric bypass) surgeries, the unregulated delivery of concentrated sugars and food into the duodenum and jejunum results in altered insulin regulation, maldigestion, osmotic movement of fluid into the intestinal lumen, and rapid transit such that intestinal contact time is insufficient for absorption of nutrients.
Impaired Lipolysis
A deficiency in pancreatic lipase may be caused by the congenital absence of pancreatic lipase or by destruction of the pancreatic gland due to alcohol-related pancreatitis, cystic fibrosis, or pancreatic cancer. Pancreatic lipase also can be denatured by excess secretion of gastric acid (e.g., Zollinger-Ellison syndrome; Chapter 201).
Chronic Pancreatitis
Chronic pancreatitis (Chapter 146) is the most common cause of pancreatic insufficiency and impaired lipolysis. In the United States, chronic pancreatitis most commonly results from alcohol abuse; in contrast, tropical (nutritional) pancreatitis is most common worldwide. Malabsorption of fat does not occur until more than 90% of the pancreas is destroyed.
Clinical Manifestations
Individuals with pancreatic causes of malabsorption typically present with bulky, fat-laden stools (usually >30 g of fat per day), abdominal pain, and diabetes, although some present with diabetes in the absence of gastrointestinal symptoms. Stools usually are not watery because undigested triglycerides form large emulsion droplets with little osmotic force and, in contrast to fatty acids, do not stimulate water and electrolyte secretion in the colon. Deficiency of fat-soluble vitamins is seen only rarely, presumably because gastric and residual pancreatic lipase generates enough fatty acids for some micelle formation. In severe disease, subclinical protein malabsorption, manifested by the presence of undigested meat fibers in the stool, and subclinical carbohydrate malabsorption, manifested by gas-filled, floating stools, can occur. Weight loss, when it occurs, is most often caused by decreased oral intake to avoid abdominal pain or diarrhea and less commonly by malabsorption.
In the dumping syndrome, patients may present with severe diarrhea, malabsorption, abdominal cramping, gas, and weight loss. Some have associated sweatiness, dizziness, and altered cognition due to postprandial hypoglycemia.
Diagnosis
Between 30 and 40% of individuals with alcohol-related chronic pancreatitis have calcifications on abdominal radiographs. A qualitative or quantitative test for fecal fat is positive in individuals whose pancreas is more than 90% destroyed. Noninvasive tests of pancreatic function are not sensitive enough to detect mild to moderate insufficiency, so the secretin stimulation test is preferred (Table 142-4 ) if it can be obtained. A modified oral glucose tolerance test that shows late (120 to 180 minutes) hypoglycemia and an early (30 minutes) rise in hematocrit with an increased pulse rate suggests the dumping syndrome in patients with consistent symptoms. Small bowel barium study to assess transit time may be helpful in the diagnosis.
Treatment  .
.
Pancreatic enzyme replacement and analgesics are the mainstays of treatment for chronic pancreatitis (Chapter 146). It is difficult to correct fat malabsorption completely with exogenous pancreatic enzymes because of their inactivation by acid and pepsin in the stomach. Normally, 28,000 U of lipase is present in the duodenal lumen with each meal. A high lipase-containing pancreatic enzyme preparation (25,000 to 40,000 U of lipase in the form of uncoated enzymes or enteric-coated, pH-sensitive microspheres) should be prescribed with each meal. Mini-microsphere preparations (e.g., 20,000 U of lipase taken orally with each meal) may be best tolerated owing to their small capsule size. Pancreatic proteases present in enzyme preparations may reduce abdominal pain by inactivating cholecystokinin-releasing factor in the duodenum. Uncoated preparations may be more effective in pain relief because coated preparations release enzymes predominantly distal to the duodenum. A histamine-2 receptor antagonist (e.g., ranitidine, 150 mg orally taken two times a day) or a proton pump inhibitor (e.g., lansoprazole, 15 to 30 mg orally once a day) should be added to uncoated pancreatic enzyme replacement therapy in patients with a poor response.
In the dumping syndrome, treatment is with a diet that is low in concentrated sugars divided into six small meals. Administration of pectin (15 g with each meal) may slow gastric emptying. In patients who are refractory to dietary measures, a short-acting somatostatin analogue (e.g., octreotide, 25 to 200 µg subcutaneously three times a day) or the better tolerated long-acting octreotide preparation (10 to 20 mg subcutaneously monthly) improves dumping symptoms. In patients with predominant reactive hypoglycemia 1 to 3 hours after a meal (late dumping), an α-glycosidase hydrolase inhibitor (e.g., acarbose, 50 to 100 mg orally three times daily) that blocks carbohydrate absorption in the small bowel may be beneficial. Continuous tube feeding is also effective.
TABLE 142-4.
Tests for the Evaluation of Malabsorption*
| TEST | COMMENTS |
|---|---|
| GENERAL TESTS OF ABSORPTION | |
| Quantitative stool fat test | Gold standard test of fat malabsorption, with which all other tests are compared. Requires ingestion of a high-fat diet (100 g) for 2 days before and during the collection. Stool is collected for 3 days. Normally, <7 g/24 hr is excreted on a high-fat diet. Borderline abnormalities of 8-14 g/24 hr may be seen in secretory or osmotic diarrheas that are not caused by malabsorption. There are false-negative findings if fat intake is inadequate. False-positive results can occur if mineral oil laxatives or rectal suppositories (e.g., cocoa butter) are given to the patient before stool collection. |
| Qualitative stool fat test | Sudan stain of a stool sample for fat. Many fat droplets per medium-power field (×40) constitute a positive test result. The nuclear magnetic resonance method determines the percentage of fat in the stool (normal, <20%). The test depends on an adequate fat intake (100 g/day). There is high sensitivity (90%) and specificity (90%) with fat malabsorption of >10 g/24 hr. Sensitivity drops with stool fat in the range of 6-10 g/24 hr. |
| d-Xylose test | A test of small intestinal mucosal absorption, used to distinguish mucosal malabsorption from malabsorption due to pancreatic insufficiency. An oral dose of d-xylose (25 g/500 mL water) is administered, and d-xylose excretion is measured in a 5-hr urine collection. Normally, >4 g of d-xylose is excreted in the urine over 5 hr. The test also may be positive in bacterial overgrowth owing to metabolism of d-xylose by bacteria in the intestinal lumen. False-positive test results occur with renal failure, ascites, and an incomplete urine collection. Blood levels at 1 and 3 hr improve sensitivity. May be normal with mild or limited mucosal disease. |
| Hydrogen breath test | Most useful in the diagnosis of lactase deficiency. An oral dose of lactose (1 g/kg body weight) is administered after measurement of basal breath H2 levels. The sole source of H2 in the mammal is bacterial fermentation; unabsorbed lactose makes its way to colonic bacteria, resulting in excess breath H2. A late peak (within 3-6 hr) of >20 ppm of exhaled H2 after lactose ingestion suggests lactose malabsorption. Absorption of other carbohydrates (e.g., sucrose, glucose, fructose) also can be tested. |
| SPECIFIC TESTS FOR MALABSORPTION | |
| Tests for Pancreatic Function | |
| Secretin stimulation test | The gold standard test of pancreatic function. Requires duodenal test intubation with a double-lumen tube and collection of pancreatic juice in response to IV secretin. Allows measurement of bicarbonate (HCO3−) and pancreatic enzymes. A sensitive test of pancreatic function, but labor intensive and invasive. |
| Fecal elastase-1 test | Stool test for pancreatic function. Equal sensitivity to the secretin stimulation test for the diagnosis of moderate-to-severe pancreatic insufficiency. More specific than the fecal chymotrypsin test. Unreliable with mild insufficiency. False-positive results occur with increased stool volume and intestinal mucosal diseases. |
| Tests for Bacterial Overgrowth | |
| Quantitative culture of small intestinal aspirate | Gold standard test for bacterial overgrowth. Greater than 105 colony-forming units (CFU)/mL in the jejunum suggests bacterial overgrowth. Requires special anaerobic sample collection, rapid anaerobic and aerobic plating, and care to avoid oropharyngeal contamination. False-negative results occur with focal jejunal diverticula and when overgrowth is distal to the site aspirated. |
| Hydrogen breath test | The 50-g glucose breath test has a sensitivity of 90% for growth of 105 colonic-type bacteria in the small intestine. If bacterial overgrowth is present, increased H2 is excreted in the breath. A hydrogen level (within 2 hr) of >20 ppm suggests bacterial overgrowth. False-negative results occur with non-hydrogen-producing organisms. |
| 14C-d-xylose breath test | This test uses 1 g of carbon 14–labeled d-xylose. It has a sensitivity and specificity >90% for growth of 105 test colonic-type bacteria in the small intestine. Bacteria metabolize d-xylose with release of 14CO2, which is absorbed and exhaled. Non-degraded d-xylose is absorbed in the small bowel and does not reach the colon, yielding a greater specificity than the lactulose H2 breath test. A nonradioactive 13C-d-xylose breath test is suitable for children and pregnant women. |
| Tests for Mucosal Disease | |
| Small bowel biopsy | Obtained for a specific diagnosis when there is a high index of suspicion for small intestinal disease. Several biopsy specimens (4-5) must be obtained to maximize the diagnostic yield. Distal duodenal biopsy specimens are usually adequate for diagnosis, but occasionally enteroscopy with jejunal biopsy specimens is necessary. Small intestinal biopsy provides a specific diagnosis in some diseases (e.g., intestinal infection, Whipple's disease, abetalipoproteinemia, agammaglobulinemia, lymphangiectasia, lymphoma, amyloidosis). In other conditions, such as celiac disease and tropical sprue, the biopsy specimens show characteristic findings, but the diagnosis is made on improvement after treatment. |
| Tests of Ileal Function | |
| Schilling test | A test of vitamin B12 absorption (see Table 167-1 in Chapter 167). |
| 75SeHCAT test | This is a test of bile acid absorption. Seven days after ingestion of radiolabeled synthetic selenium-homocholic acid conjugated with taurine (75SeHCAT), whole body retention is measured by a gamma-counting device. The result is expressed as a fraction of baseline ingestion. Retention values of less than 10% are abnormal and indicate bile acid malabsorption with a sensitivity of 80-90% and specificity of 70-100%. The radiation dose is equivalent to a plain chest x-ray. Liver disease and bacterial overgrowth may give false results. Not approved for use in the United States. |
Not all these tests are readily available. A strong suspicion for any disease may warrant foregoing an extensive work-up and obtaining the test with highest diagnostic yield. In some cases, empirical treatment, such as removing lactose from the diet of an otherwise healthy individual with lactose intolerance, is warranted without any testing.
Impaired Micelle Formation
Pathobiology
Bile salt concentrations in the intestinal lumen can fall to less than the critical concentration (2 to 3 mmol/L) needed for micelle formation because of decreased bile salt synthesis (severe liver disease), decreased bile salt delivery (cholestasis), or removal of luminal bile salts (bacterial overgrowth, terminal ileal disease or resection, cholestyramine therapy, acid hypersecretion). Fat malabsorption resulting from impaired micelle formation is generally not as severe as malabsorption resulting from pancreatic lipase deficiency, presumably because fatty acids and monoglycerides can form lamellar structures, which to a certain extent can be absorbed. Malabsorption of fat-soluble vitamins (D, A, K, and E) may be marked, however, because micelle formation is required for their absorption.
Decreased Bile Salt Synthesis and Delivery
Malabsorption can occur in individuals with cholestatic liver disease or bile duct obstruction. The clinical consequences of malabsorption are seen most often in women with primary biliary cirrhosis because of the prolonged nature of the illness. Although these individuals can present with steatorrhea, osteoporosis or, less commonly, osteomalacia is the most common presentation. The cause of bone disease in these patients is poorly understood and often is not related to vitamin D deficiency. Bone disease is treated with calcium supplements (and vitamin D if a deficiency is documented), weight-bearing exercise, and a bisphosphonate (e.g., alendronate, 10 mg orally once daily or 70 mg orally once weekly).
Intestinal Bacterial Overgrowth
In health, only small numbers of lactobacilli, enterococci, gram-positive aerobes, or facultative anaerobes can be cultured from the upper small bowel lumen. Motility and acid are the most important factors in keeping the number of bacteria in the upper small bowel low. Any condition that produces local stasis or recirculation of colonic luminal contents allows development of a predominantly “colonic” flora (coliforms and anaerobes, such as Bacteroides and Clostridium) in the small intestine (see Table 142-4). Anaerobic bacteria cause impaired micelle formation by releasing cholylamidases, which deconjugate bile salts. The unconjugated bile salts, with their higher pKa, are more likely to be in the protonated form at the normal upper small intestinal pH of 6 to 7 and can be absorbed passively. As a result, the concentration of bile salts decreases in the intestinal lumen and can fall to less than the critical micellar concentration, causing malabsorption of fats and fat-soluble vitamins. Vitamin B12 deficiency and carbohydrate malabsorption also can occur with generalized bacterial overgrowth. Anaerobic bacteria ingest vitamin B12 and release proteases that degrade brush-border disaccharidases. Although anaerobic bacteria use vitamin B12, they synthesize folate. Individuals with bacterial overgrowth usually have low serum vitamin B12 levels but normal or high folate levels; this helps distinguish bacterial overgrowth from tropical sprue, in which vitamin B12 and folate levels are usually low because of decreased mucosal uptake.
Clinical Manifestations
Individuals with bacterial overgrowth can present with diarrhea, abdominal cramps, gas and bloating, weight loss, and signs and symptoms of vitamin B12 and fat-soluble vitamin deficiency. Watery diarrhea occurs because of the osmotic load of unabsorbed carbohydrates and stimulation of colonic secretion by unabsorbed fatty acids.
Diagnosis
The diagnosis of bacterial overgrowth should be considered in elderly people and in individuals with predisposing underlying disorders (see Table 142-4). Bacterial overgrowth may be associated with the irritable bowel syndrome (Chapter 139). The identification of greater than 105 CFU/mL in a culture of small intestinal aspirate is the “gold standard” in diagnosis but is not readily available. The noninvasive tests with a sensitivity and specificity comparable to intestinal culture are the glucose hydrogen breath test and the 14C- or 13C-d-xylose breath test; in individuals with low vitamin B12 levels, a Schilling test before and after antibiotic therapy can be diagnostic (Chapter 167).
Treatment  .
.
The goals of treatment are to correct the structural or motility defect, if possible; to eradicate offending bacteria; and to provide nutritional support. Acid-reducing agents should be stopped, if possible. Treatment with antibiotics should be based on culture results whenever possible; otherwise, empirical treatment is given. Rifaximin (400 mg orally three times a day) is effective,6 but less so in individuals with an excluded (blind) intestinal loop. Tetracycline (250 to 500 mg orally four times a day) or a broad-spectrum antibiotic against aerobes and enteric anaerobes (ciprofloxacin, 500 mg orally twice a day; amoxicillin–clavulanic acid, 250 to 500 mg orally three times a day; cephalexin, 250 mg orally four times a day with metronidazole, 250 mg three times a day) should be given for 14 days. Prokinetic agents such as metoclopramide (10 mg orally four times a day) or erythromycin (250 to 500 mg orally four times a day) can be tried to treat small bowel motility disorders, but often they are not efficacious. Octreotide (50 µg subcutaneously every day) may improve motility and reduce bacterial overgrowth in individuals with scleroderma. If the structural abnormality or motility disturbance cannot be corrected, the patient is at risk for malnutrition and deficiencies of vitamin B12 and fat-soluble vitamins. Cyclic treatment (1 to 3 weeks out of every 4 to 6 weeks) with rotating antibiotics may be required in these patients to prevent recurrent bouts of bacterial overgrowth. If supplemental calories are needed, medium-chain triglycerides should be given because they are not dependent on micelle formation for their absorption. Monthly treatment with vitamin B12 should be considered, along with supplemental vitamins D, A, K, and E and calcium.
Ileal Disease or Resection
Disease of the terminal ileum is most commonly due to Crohn's disease (Chapter 143), which also may lead to ileal resection, but it also can be caused by radiation enteritis, tropical sprue, tuberculosis, Yersinia infection, or idiopathic bile salt malabsorption. These diseases cause bile salt wasting in the colon.
The clinical consequences of bile salt malabsorption are related directly to the length of the diseased or resected terminal ileum. In an adult, if less than 100 cm of ileum is diseased or resected, watery diarrhea results because of stimulation of colonic fluid secretion by unabsorbed bile salts. Bile acid diarrhea responds to cholestyramine (2 to 4 g taken at breakfast, lunch, and dinner). If more than 100 cm of ileum is diseased or resected, bile salt losses (>3 g/day) in the colon exceed the capacity for increased bile salt synthesis in the liver, the bile salt pool shrinks, and micelle formation is impaired. As a result, steatorrhea ensues, and fatty acid–induced intestinal secretion synergizes with the bile acid–induced secretion to cause diarrhea. Treatment is with a low-fat diet, vitamin B12 (300 to 1000 µg subcutaneously once every month or 2 mg orally once a day), dietary supplements of calcium (500 mg orally two to three times a day, monitor 24-hour urine calcium for adequacy of dose), and a multiple vitamin and mineral supplement. An antimotility agent should be given for diarrhea. Bile salt binders may worsen diarrhea. Screening for fat-soluble vitamin deficiencies (vitamins A and E, 25-OH vitamin D, and prothrombin time) and bone disease (bone densitometry, serum calcium, intact parathyroid hormone, 24-hour urine for calcium) should be done.
Three long-term complications of chronic bile salt wasting and fat malabsorption are renal stones, bone disease (osteoporosis and osteomalacia), and gallstones. Oxalate renal stones occur as a consequence of excess free oxalate absorption in the colon. Free oxalate is generated when unabsorbed fatty acids bind luminal calcium, which is then unavailable for binding oxalate. Renal oxalate stones sometimes can be avoided with a low-fat, low-oxalate diet and calcium supplements. Bone disease is caused by impaired micelle formation with a resulting decrease in absorption of vitamin D; year-round sun exposure reduces this complication. Vitamin D (50,000 U orally one to three times a week) and calcium supplements (500 mg orally two to three times a day) should be given to susceptible individuals, but vitamin D levels and serum and urinary calcium must be monitored for response to treatment because excess vitamin D can be toxic. The mechanism of gallstone formation in these individuals is unclear; pigmented gallstones are most common.
Conditions that Impair Mucosal Absorption
Pathobiology
Nutrients are absorbed along the entire length of the small intestine, with the exception of iron and folate, which are absorbed in the duodenum and proximal jejunum, and bile salts and cobalamin, which are absorbed in the distal ileum. The efficiency of nutrient uptake at the mucosa is influenced by the number of villus absorptive cells, the presence of functional hydrolases and specific nutrient transport proteins on the brush-border membrane, and transit time. Transit time determines the contact time of luminal contents with the brush-border membrane and influences the efficiency of nutrient uptake across the mucosa.
Mucosal malabsorption can be caused by specific (usually congenital) brush-border enzyme or nutrient transporter deficiencies or by generalized diseases that damage the small intestinal mucosa or result in surgical resection or bypass of small intestine. The nutrients malabsorbed in these general malabsorptive diseases depend on the site of intestinal injury (proximal, distal, or diffuse) and the severity of damage. The main mechanism of malabsorption in these conditions is a decrease in surface area available for absorption. Some conditions (infection, celiac disease, tropical sprue, food allergies, and graft-versus-host disease) are characterized by intestinal inflammation and villus flattening; others are characterized by ulceration (ulcerative jejunitis, NSAIDs, Crohn's disease), infiltration (amyloidosis), or ischemia (radiation enteritis, mesenteric ischemia).
Long-chain fatty acids are transported across the microvillus membrane of villus epithelial cells by the fatty acid transport protein FATP4. The bile salts from mixed micelles remain in the intestinal lumen and are absorbed in the distal ileum by sodium-dependent cotransport. Oligosaccharides and larger oligopeptides (products of pancreatic enzyme digestion), sucrose, and lactose are hydrolyzed further by enzymes present in the brush-border membrane of villus epithelial cells before they are absorbed. Although only sugar monomers (glucose, galactose, fructose) can be taken up at the apical epithelial cell membrane, dipeptides and tripeptides are readily taken into the cell.
Water-soluble vitamins are readily absorbed throughout the small intestine. Fat-soluble vitamins, minerals, and cobalamin are more difficult to absorb because of the requirement for micelle formation (vitamins D, A, K, and E), a divalent charge (magnesium, calcium, iron), or selected sites of uptake in the intestine (iron, cobalamin). Calcium is absorbed best in the proximal small intestine by a vitamin D–dependent calcium channel. Magnesium is absorbed by the small intestine (throughout its length) by a poorly understood mechanism. Ferrous iron is transported into intestinal epithelial cells by a proton-coupled metal-ion transporter (Nramp2) that has specificity for Fe2++ and other divalent cations (Zn2++, Mn2++, Co2++, Cd2++, Cu2++, Ni2++, and Pb2++). The absorption of calcium and nonheme iron is enhanced by solubilization with hydrochloric acid. Intraluminal compounds such as oxalate, phytate, and long-chain fatty acids bind to calcium and magnesium, decreasing their absorption. Individuals with severe mucosal disease or short-bowel syndrome with high fecal fluid outputs lose magnesium and zinc from endogenous secretions.
Folates (Chapters 167 and 225Chapter 167Chapter 225) are both taken in the diet and produced by bacteria in the colon. Dietary folates are absorbed in the proximal small intestine through a reduced folate carrier (RFC1). Deficiency can be caused by poor intake or malabsorption secondary to intestinal disease or drugs. The cobalamins (Chapters 167 and 225Chapter 167Chapter 225) are abundant in foods containing animal proteins (e.g., meat, seafood, eggs, milk). Cobalamin deficiency in industrialized countries is rarely due to poor dietary intake but rather reflects the inability to absorb cobalamin. This inability may be caused by a lack of intrinsic factor, consumption of cobalamin by overgrowth of anaerobic bacteria in the small bowel lumen, ileal disease or resection, or defective transcobalamin II. Large amounts of cobalamin are present in the liver (2 to 5 mg), and cobalamin is reabsorbed from bile through the enterohepatic circulation, thereby limiting daily losses to less than 1 µg. It usually takes 10 to 12 years for cobalamin deficiency to develop after it is eliminated from the diet, but deficiency can occur more rapidly (2 to 5 years) with malabsorptive syndromes. If lack of gastric acid causes food-cobalamin malabsorption, treatment with oral cyanocobalamin supplementation (Chapter 167) is curative.
Lactase Deficiency
Epidemiology
Acquired lactase deficiency is the most common cause of selective carbohydrate malabsorption. Most individuals, except those of northern European descent, begin to lose lactase activity by the age of 2 years. The prevalence of lactase deficiency is highest (85 to 100%) in persons of Asian, African, and Native-American descent.
Pathobiology
The persistence or nonpersistence of lactase activity is associated with a single nucleotide polymorphism C/T−13910 that is found upstream of the lactase gene on chromosome 2q21-22. Hypolactasia is associated with the C/C−13910 genotype in diverse ethnic groups. The mechanism by which this variant downregulates the lactase gene is not known, but functional studies suggest genotype-dependent alterations in levels of messenger RNA.
Clinical Manifestations
Adults with lactase deficiency typically complain of gas, bloating, and diarrhea after the ingestion of milk or dairy products but do not lose weight. Unabsorbed lactose is osmotically active, drawing water followed by ions into the intestinal lumen. On reaching the colon, bacteria metabolize lactose to short-chain fatty acids, carbon dioxide, and hydrogen gas. Short-chain fatty acids are transported with sodium into colonic epithelial cells, facilitating the reabsorption of fluid in the colon. If the colonic capacity for the reabsorption of short-chain fatty acids is exceeded, an osmotic diarrhea results (see later discussion of carbohydrate malabsorption in watery diarrheas).
Diagnosis
The diagnosis of acquired lactase deficiency can be made by empirical treatment with a lactose-free diet, which results in resolution of symptoms; by the hydrogen breath test after oral administration of lactose; or by genetic testing. Many intestinal diseases cause secondary reversible lactase deficiency, including viral gastroenteritis, celiac disease, giardiasis, and bacterial overgrowth.
Congenital Enteropeptidase (Enterokinase) Deficiency
Enteropeptidase is a brush-border protease that cleaves trypsinogen to trypsin, triggering the cascade of pancreatic protease activation in the intestinal lumen. The rare congenital deficiency of enteropeptidase results in inability to activate all pancreatic proteases and leads to severe protein malabsorption. It manifests in infancy as diarrhea, growth retardation, and hypoproteinemic edema.
Abetalipoproteinemia
Formation and exocytosis of chylomicrons at the basolateral membrane of intestinal epithelial cells are necessary for the delivery of lipids to the systemic circulation. One of the proteins required for assembly and secretion of chylomicrons is the microsomal triglyceride transfer protein, which is mutated in individuals with abetalipoproteinemia. Children with this disorder have fat malabsorption and the consequences of vitamin E deficiency (retinopathy and spinocerebellar degeneration). Biochemical tests show low plasma levels of apoprotein B, triglyceride, and cholesterol. Membrane lipid abnormalities result in red blood cell acanthosis (burr cells). Intestinal biopsy is diagnostic; the tissue is characterized by engorgement of epithelial cells with lipid droplets. Calories are provided by treatment with a low-fat diet containing medium-chain triglycerides. Poor absorption of long-chain fatty acids sometimes can result in essential fatty acid deficiency. High doses of fat-soluble vitamins, especially vitamin E, often are needed. Mutations in the apolipoprotein B gene (hypobetalipoproteinemia) and intracellular retention of chylomicrons (Anderson's disease) cause a similar although less severe clinical syndrome.
Celiac Disease
Definition and Epidemiology
Celiac disease is an inflammatory condition of the small intestine precipitated by the ingestion of wheat, rye, and barley in individuals with certain genetic predispositions. Screening studies for the antiendomysial (EMA) and anti–tissue transglutaminase (anti-tTG) antibodies that are associated with celiac disease suggest a prevalence in white populations of about 1%. High-risk groups for celiac disease include first-degree relatives and individuals with type 1 diabetes mellitus, autoimmune thyroid disease, primary biliary cirrhosis, Turner's syndrome, or Down syndrome. About 20% of patients diagnosed with irritable bowel syndrome or with microscopic (lymphocytic) colitis have celiac disease.
Pathobiology
Environmental and genetic factors are important in the development of celiac disease. The alcohol-soluble protein fraction of wheat gluten, the gliadins, and similar prolamins in rye and barley trigger intestinal inflammation in susceptible individuals. Oat grains, which have prolamins rich in glutamine but not proline, are rarely toxic. Gliadins and similar prolamins with high proline content are relatively resistant to digestion by human proteases. A 33-mer peptide that is a natural digestion product of α2-gliadin may be important in the pathogenesis of celiac disease. This peptide resists terminal digestion by intestinal brush-border proteases and contains three previously identified antigenic epitopes. It also reacts with tissue transglutaminase and stimulates human leukocyte antigen (HLA)-DQ2-restricted intestinal T-cell clones from individuals with celiac disease.
Approximately 15% of first-degree relatives of affected individuals are found to have celiac disease. Predisposition has been mapped to the HLA-D region on chromosome 6. More than 90% of northern Europeans with celiac disease have the DQ2 heterodimer encoded by alleles DQA1*0501 and DQB1*0201, compared with 20 to 30% of controls. A smaller celiac group carries HLA DQ8. The strongest candidate non-HLA alleles identified in genome-wide association studies are 4q27 and 3q28. The DQ2 protein expressed on antigen-presenting cells has positively charged binding pockets; tTG (the autoantigen recognized by EMA) may enhance intestinal inflammation by deamidation of select glutamine residues in gliadin to negatively charged glutamic acid. In the deamidated form, most gliadin peptides have a higher binding affinity for DQ2 and are more potent stimulants of gluten-sensitized T cells. Villous atrophy may be caused by inflammation that is triggered by γ-interferon released from DQ2- or DQ8-restricted CD4 T cells in the lamina propria. Alternatively, intraepithelial lymphocytes may directly kill intestinal epithelial cells under the influence of IL-15 released from stressed enterocytes.
Clinical Manifestations
Celiac disease usually manifests early in life, at about 2 years of age (after wheat has been introduced into the diet), or later in the second to fourth decades of life, but it can occur at any age. It may first manifest clinically after abdominal surgery or an episode of infectious diarrhea.
Breast-feeding and the time of introduction of wheat in the diet may lessen the risk or delay the onset of celiac disease in children at risk. Adults with celiac disease in the United States often present with anemia or osteoporosis without diarrhea or other gastrointestinal symptoms. These individuals most likely have proximal disease that impairs iron, folate, and calcium absorption but an adequate surface area in the remaining intestine for absorption of other nutrients. Other extraintestinal manifestations of celiac disease include rash (dermatitis herpetiformis), neurologic disorders (peripheral neuropathy, ataxia, epilepsy), psychiatric disorders (depression, paranoia), reproductive disorders (infertility, spontaneous abortion), short stature, dental enamel hypoplasia, chronic hepatitis, or cardiomyopathy.
Individuals with significant mucosal involvement present with watery diarrhea, weight loss or growth retardation, and the clinical manifestations of vitamin and mineral deficiencies. Cobalamin deficiency is more common (10% of patients) than previously thought and usually corrects itself on a gluten-free diet. Symptomatic individuals require supplementation of vitamin B12. Diarrhea is caused by many mechanisms, including a decreased surface area for water and electrolyte absorption, the osmotic effect of unabsorbed luminal nutrients, an increased surface area for chloride secretion (crypt hyperplasia), and the stimulation of intestinal fluid secretion by inflammatory mediators and unabsorbed fatty acids. Some individuals have impaired pancreatic enzyme secretion caused by decreased mucosal cholecystokinin release or bacterial overgrowth that may contribute to diarrhea.
Diagnosis
The diagnosis of celiac disease is made by characteristic changes found on a small intestinal biopsy specimen and improvement when a gluten-free diet is instituted (FIGURE 142-6, FIGURE 142-7 ). Mucosal flattening may be observed endoscopically as scalloped or reduced duodenal folds. Characteristic features found on intestinal biopsy include blunted or absent villi, crypt hyperplasia, increased intraepithelial lymphocytes, and infiltration of the lamina propria with plasma cells and lymphocytes. In some individuals, the only abnormal biopsy finding is increased intraepithelial lymphocytes. A hypoplastic mucosa indicates irreversible (end-stage) intestinal disease.
FIGURE 142-6.
Intestinal biopsy appearance of flattened villi, hyperplastic crypts, and increased intraepithelial lymphocytes.
(Courtesy of John Hart, MD.)
FIGURE 142-7.
Regeneration of villi after initiation of a gluten-free diet.
(Courtesy of John Hart, MD.)
Serologic markers for celiac disease are useful in supporting the diagnosis, in screening first-degree relatives, and in monitoring the response to a gluten-free diet. EMA immunoglobulin A (IgA) antibodies, detected by indirect immunofluorescence, are highly sensitive (90%) and specific (90 to 100%) for active celiac disease in skilled laboratory testing. An enzyme-linked immunosorbent assay (ELISA) test to detect antibodies against tTG has equal sensitivity to the EMA test but is less specific. The newer anti-deamidated gliadin (a biotinylated synthetic γ-gliadin peptide with glutamic acid substituted for glutamine) IgA and IgG antibody immunofluorometric assay has a sensitivity and specificity that approaches that of EMA. The anti-tTG IgA antibody test, when obtained with a serum IgA level, is a cost-effective strategy for screening high-risk groups; very high titers of the anti-tTG IgA and EMA antibodies are virtually diagnostic of celiac disease. Patients with mild disease may have negative antibody studies. Anti-tTG, gliadin peptide, and EMA IgA antibodies tests are negative in individuals with selective IgA deficiency (present in up to 2.6% of individuals with celiac disease). In these patients, anti-tTG or gliadin peptide IgG antibodies may be helpful in diagnosis. In equivocal cases (negative serology and equivocal biopsy or positive serology and normal biopsy), HLA genotyping is useful to exclude the diagnosis of celiac disease in persons who lack the DQ2 or DQ8 gene.
Treatment  .
.
Treatment consists of a lifelong gluten-free diet. Wheat, rye, and barley grains should be excluded from the diet. Rice and corn grains are tolerated. Oats (if not contaminated by wheat grain) are tolerated by most. Early referral to a reputable celiac support group or website is often helpful in maintaining dietary compliance. Owing to secondary lactase deficiency, a lactose-free diet should be recommended until symptoms improve. Bone densitometry should be performed on all individuals with celiac disease because up to 70% have osteopenia or osteoporosis. Patients with diarrhea and weight loss should be screened for vitamin and mineral deficiencies. Documented deficiencies of vitamins and minerals should be replenished (see Table 142-4), and women of childbearing age should take folic acid supplements. Bone mass often improves on a gluten-free diet alone. Patients with vitamin D or calcium deficiency should receive supplements (Chapter 225), with the dose monitored by 25-OH vitamin D levels and a 24-hour urine test for calcium.
Prognosis
Of patients with celiac disease treated with a gluten-free diet, 90% experience symptomatic improvement within 2 weeks. The most common cause of a poor dietary response is continued ingestion of gluten. Other possibilities include a missed intestinal infection (see later), an alternative diagnosis (e.g., agammaglobulinemia [diagnosed by hypogammaglobulinemia and lack of plasma cells on small bowel biopsy], autoimmune enteritis [diagnosed by a positive antienterocyte antibody and crypt apoptosis or loss of goblet cells on small bowel biopsy]), bacterial overgrowth, pancreatic insufficiency, microscopic colitis, or other food allergies (cow's milk, soy protein).
In a small percentage of patients, symptoms and enteropathy persist despite a strict gluten-free diet. In such patients, repeat intestinal biopsy is indicated. Some patients will have collagen deposition beneath the surface epithelium (collagenous sprue). Others will have ulcerative jejunitis or a monoclonal population of intraepithelial T cells with an aberrant phenotype or clonal T-cell receptor-γ gene rearrangements, which are predictive of enteropathy-associated T-cell lymphoma (Chapter 191) that portends a poor prognosis. Video capsule endoscopy and balloon-assisted enteroscopy may be helpful in establishing these diagnoses. Patients with collagenous sprue, autoimmune enteritis, or refractory sprue with a polyclonal population of intraepithelial T lymphocytes often respond to prednisone (20 to 40 mg orally daily) or budesonide (3 mg orally three times a day).
Individuals with celiac disease are at increased risk for B-cell lymphoma (Chapter 191), gastrointestinal tract carcinomas (esophageal, small bowel, and colonic adenocarcinomas), and increased mortality; a strict gluten-free diet for life may lessen these risks. Intestinal T-cell lymphoma is rare and should be suspected in individuals who have abdominal pain, recurrence of symptoms after initial response to a gluten-free diet, or refractory celiac disease.
Tropical Sprue
Tropical sprue is an inflammatory disease of the small intestine associated with the overgrowth of predominantly coliform bacteria. It occurs in residents or travelers to the tropics, especially India and Southeast Asia. Individuals classically present with diarrhea and megaloblastic anemia secondary to vitamin B12 and folate deficiency, but some have anemia only. Intestinal biopsy characteristically shows subtotal and patchy villous atrophy in the proximal and distal small intestine, which may be caused by the effect of bacterial toxins on gut structure or by the secondary effects of vitamin B12 deficiency on the gut (megaloblastic gut). Diagnosis is based on history, documentation of vitamin B12 or folate deficiency, and the presence of an abnormal small intestinal biopsy report. Treatment is a prolonged course of broad-spectrum antibiotics, oral folate, and vitamin B12 injections until symptoms resolve. Relapses occur mainly in natives of the tropics.
Infection
Giardia lamblia
Giardia lamblia (Chapter 359) infection, the most common protozoal infection in the United States, can cause malabsorption in individuals infected with many trophozoites, especially the immunocompromised or IgA-deficient hosts. Malabsorption occurs when many organisms cover the epithelium and cause mucosal inflammation, which results in villous flattening and a decrease in absorptive surface area. Stool for ova and parasites at this stage of infection is often negative because of the attachment of organisms in the proximal small intestine. Diagnosis can be made by a stool antigen-capture ELISA test but may require duodenal aspiration and biopsies.
Human Immunodeficiency Virus
Diarrhea, malabsorption, and wasting are common in individuals with AIDS but are seen less frequently with improved antiretroviral therapy (Chapter 397). In patients who are receiving highly active antiretroviral therapy, diarrhea is more likely to be due to protease inhibitors than to enteric infection.
Malabsorption is usually due to infection with cryptosporidia, Mycobacterium avium-intracellulare complex, I. belli, or microsporidia. An organism can be identified by stool examination or intestinal biopsy about 50% of the time. AIDS enteropathy (a term used if no organism is identified) also can cause malabsorption. Mechanisms of malabsorption and diarrhea include villous atrophy, increased intestinal permeability, rapid small bowel transit (in patients with protozoal infection), and ultrastructural damage of enterocytes (in AIDS enteropathy). Among individuals with AIDS and diarrhea, results of fecal fat and d-xylose absorption are frequently abnormal. Serum albumin, vitamin B12, and zinc levels are often low. Vitamin B12 deficiency is caused mainly by ileal disease, but low intrinsic factor and decreased transcobalamin II may be contributing factors. Management of malabsorption should focus on restoring the immune system by treating the underlying HIV infection with antiviral therapy. If possible, the offending organism should be treated with antibiotics. If the organism cannot be eradicated, chronic diarrhea and malabsorption result; treatment in these cases consists of antimotility agents and a lactose-free, low-fat diet. Pancreatic enzyme replacement therapy can be tried in HIV-infected individuals who are taking highly active antiretroviral therapy or nucleoside analogues and who have fat malabsorption of obscure origin. If supplemental calories are needed, liquid oral supplements that are predigested and high in medium-chain triglycerides (semi-elemental) are tolerated best. Vitamin and mineral deficiencies should be screened for and treated.
Whipple's Disease
Whipple's disease (Chapters 144 and 283Chapter 144Chapter 283), a rare cause of malabsorption, manifests with gastrointestinal complaints in association with systemic symptoms, such as fever, joint pain, or neurologic manifestations. About one third of patients have cardiac involvement, most commonly culture-negative endocarditis. Occasionally, individuals present with ocular or neurologic disease without gastrointestinal symptoms. Men are affected more commonly than women, particularly white men. The organism responsible for causing Whipple's disease is a gram-positive actinomycete, Tropheryma whippelii. The epidemiology and pathogenesis of Whipple's disease are poorly understood. The prevalence of the disease is higher in farmers compared with other workers, which suggests that the organism lives in the soil. Using the polymerase chain reaction, T. whippelii has been detected in sewage and in duodenal biopsy specimens, gastric juice, saliva, and stool of individuals without clinical disease. Whether the latter represents a carrier state or the presence of nonpathogenic organisms is not known. Immunologic defects and an association with the HLA-B27 gene may be disease factors. Small intestinal biopsy shows villous blunting and infiltration of the lamina propria with large macrophages that stain positive with the periodic acid–Schiff method and are filled with the organism. It is important to distinguish these macrophages from macrophages infected with M. avium-intracellulare complex, which stain positive on acid-fast staining and are found in individuals with AIDS. Treatment is with a prolonged course of broad-spectrum antibiotics (e.g., 10 to 14 days of parenteral penicillin G, 1.2 million U every day and streptomycin, 1 g every day; ceftriaxone, 2 g intravenously daily, or meropenem, 1 g intravenously three times daily; then, 160 mg of trimethoprim and 800 mg of sulfamethoxazole orally two times a day for 1 year or 160 mg of trimethoprim and 800 mg of sulfamethoxazole orally two times a day for 1 year). Relapses occur, but initial treatment with parenteral ceftriaxone or meropenem appears to be associated with a low relapse rate.
Graft-versus-Host Disease
Diarrhea occurs frequently after allogeneic bone marrow or stem cell transplantation (Chapter 181). Immediately after transplantation, diarrhea is caused by the toxic effects of cytoreductive therapy on the intestinal epithelium. Twenty to 100 days after transplantation, diarrhea is usually due to graft-versus-host disease (GVHD) or infection. Patients with GVHD present clinically with a skin rash, hepatic cholestasis, buccal mucositis, anorexia, nausea, vomiting, abdominal cramps, and diarrhea. The diagnosis of GVHD in the gastrointestinal tract can be made on biopsy of the stomach, small intestine, or colon. In mild cases, the mucosa appears normal on inspection at endoscopy, but apoptosis of gastric gland or crypt cells can be found on biopsy. In severe cases, denudation of the intestinal epithelium results in diarrhea and malabsorption and often requires parenteral nutritional support. Octreotide (50 to 250 µg subcutaneously three times a day) may be helpful in controlling voluminous diarrhea. Treatment of GVHD is with steroids and antithymocyte globulin combined with parenteral nutritional support until intestinal function returns.
Short-Bowel Syndrome
Malabsorption caused by small bowel resection or surgical bypass is called the short-bowel syndrome. The most common causes in the United States are massive resection due to adhesions, volvulus, ischemia (mesenteric or after intra-abdominal surgery), and gastric bypass surgery. Short-bowel syndrome due to Crohn's disease and radiation enteritis now is less common because of improved medical and radiation therapies. The severity of malabsorption depends on the site and extent of resection; the capacity for hyperplasia, dilation, and elongation; and the function of the residual bowel. Mechanisms of malabsorption after small bowel resection include a decreased absorptive surface area, decreased luminal bile salt concentration, rapid transit, and bacterial overgrowth. Limited jejunal resection usually is tolerated best because bile salt and vitamin B12 absorption remain normal. Ileal resection is less well tolerated because of the consequences of bile salt wasting and the limited capacity of the jejunum to undergo adaptive hyperplasia.
When less than 100 cm of jejunum remains, the colon takes on an important role in caloric salvage and fluid reabsorption. Malabsorbed carbohydrates are digested by colonic bacteria to short-chain fatty acids, which are absorbed in the colon.
Treatment  .
.
Parenteral nutrition may be avoided by a diet rich in complex carbohydrates, oral rehydration solutions, and an antimotility agent. In comparison, individuals with fewer than 100 cm of jejunum and no colon have high jejunostomy outputs and often require intravenous fluids or parenteral nutrition to survive. These individuals waste sodium, chloride, bicarbonate, magnesium, zinc, and water in their ostomy effluent. Dietary modifications should include a high-salt, nutrient-rich diet given in small meals. An oral rehydration solution with a sodium concentration greater than 90 mmol/L is absorbed best. Oral vitamin and mineral doses higher than the usual U.S. recommended daily allowances are required (Table 142-5 ). Vitamin B12 should be given parenterally (500 to 1000 µg subcutaneously every month). Magnesium deficiencies are often difficult to replenish with oral magnesium because of its osmotic effect in the intestinal lumen. A liquid magnesium preparation added to an oral rehydration solution and sipped throughout the day may minimize magnesium-induced fluid losses. Potent antimotility agents, such as tincture of opium (0.5 to 1 mL orally four times a day), often are needed to slow transit and maximize contact time for nutrient absorption. High-volume jejunostomy outputs can be lessened by inhibiting endogenous secretions with a proton pump inhibitor (e.g., omeprazole, 40 mg orally one to two times a day, or lansoprazole, 30 mg orally one to two times a day) and, in severe cases, octreotide (100 to 250 µg subcutaneously three times a day; if effective, convert to an equivalent long-acting monthly dosage). The benefit of octreotide may be offset by its potential to inhibit intestinal adaptation and impair pancreatic enzyme secretion with doses greater than 300 µg/day.
TABLE 142-5.
Vitamin and Mineral Doses Used in the Treatment of Malabsorption
| VITAMIN | ORAL DOSE | PARENTERAL DOSE |
|---|---|---|
| Vitamin A* | Water-soluble A, 25,000 U/day† | |
| Vitamin E | Water-soluble E, 400-800 U/day† | |
| Vitamin D‡ | 25,000-50,000 U/day | |
| Vitamin K | 5 mg/day | |
| Folic acid | 1 mg/day | |
| Calcium§ | 1500-2000 mg elemental calcium/day | |
| Calcium citrate, 500 mg calcium/tablet† | ||
| Calcium carbonate, 500 mg calcium/tablet† | ||
| Magnesium | Liquid magnesium gluconate† | 2 mL of a 50% solution (8 mEq) both buttocks IM |
| 1-3 tbsp (12-36 mEq magnesium) in 1-2 L of ORS or sports drink sipped throughout the day | ||
| Magnesium chloride hexahydrate† 100-600 mg elemental magnesium/day | ||
| Zinc | Zinc gluconate† | |
| 20-50 mg elemental zinc/day‖ | ||
| Iron | 150-300 mg elemental iron/day | Iron sucrose¶ |
| Polysaccharide-iron complex† | Sodium ferric gluconate complex¶ | |
| Iron sulfate or gluconate | Iron dextran (as calculated for anemia) (IV or IM¶; Chapter 162) | |
| B-complex vitamins | 1 megadose tablet/day | |
| Vitamin B12 | 2 mg/day | 1 mg IM or SC/mo** |
ORS = oral rehydration solution.
Monitor serum vitamin A level to avoid toxicity, especially in patients with hypertriglyceridemia.
Form best absorbed or with least side effects.
Monitor serum calcium and 25-OH vitamin D levels to avoid toxicity.
Monitor 24-hr urine calcium to assess adequacy of dose.
If intestinal output is high, additional zinc should be given. Monitor for copper deficiency with high doses.
Parenteral therapy should be given in a supervised outpatient setting because of the risk of fatal reactions. Decreased risk of fatal reactions when compared with iron dextran.
For vitamin B12 deficiency, 1 mg IM or SC twice a week for 4 wk, then once a month.
In the most severe cases, supplemental calories must be provided by nocturnal tube feeding or parenteral nutrition. Treatment with growth hormone (0.1 mg/kg/day subcutaneously) with or without glutamine (30 g once a day orally) for 4 weeks may reduce parenteral nutrition requirements in patients who have had massive intestinal resections.7 Teduglutide (0.05 mg/kg/day subcutaneously), a glucagon-like peptide-2 analogue that stimulates adaptive hyperplasia in remnant intestine after resection, reduces parenteral nutrition requirements.8
Prognosis
Long-term complications include bone disease, renal stones (oxalate stones if the colon is present, urate stones with a jejunostomy), gallstones, bacterial overgrowth, fat-soluble vitamin deficiencies, essential fatty acid deficiency, and d-lactic acidosis. Small bowel transplantation should be considered for individuals who require parenteral nutrition to survive and then develop liver disease or venous access problems.
Conditions That Impair Nutrient Delivery to the Systemic Circulation
Insoluble lipids (present in chylomicrons) are exocytosed across the basolateral membrane of epithelial cells into the intestinal lymphatics. From there, they enter the mesenteric lymphatics and the general circulation through the thoracic duct. Sugar monomers, amino acids, and medium-chain fatty acids are transported across the basolateral membrane of intestinal epithelial cells into capillaries and into the portal circulation. Sugar monomers are transported across the basolateral membrane by the facilitative glucose transporter isoform (GLUT2) and amino acids by facilitative amino acid carriers (see Fig. 142-1A).
Impaired Lymphatic Drainage
Diseases that cause intestinal lymphatic obstruction, such as primary congenital lymphangiectasia (malunion of intestinal lymphatics), and diseases that result in secondary lymphangiectasia (lymphoma, tuberculosis, Kaposi's sarcoma, retroperitoneal fibrosis, constrictive pericarditis, severe heart failure) result in fat malabsorption. The increased pressure in the intestinal lymphatics leads to leakage and sometimes rupture of lymph into the intestinal lumen, with the loss of lipids, γ-globulins, albumin, and lymphocytes. The diagnosis of lymphangiectasia can be made by intestinal biopsy, but the specific cause may be more difficult to identify. Individuals with lymphangiectasia malabsorb fat and fat-soluble vitamins and have protein loss into the intestinal lumen. The most common presentation is hypoproteinemic edema. Nutritional management includes a low-fat diet and supplementation with medium-chain triglycerides, which are absorbed directly into the portal circulation. Fat-soluble vitamins should be given if deficiencies develop.
Watery Diarrhea
Watery diarrhea may be due to osmotic, secretory, inflammatory, or often combined mechanisms (Fig. 142-8 ).
FIGURE 142-8.
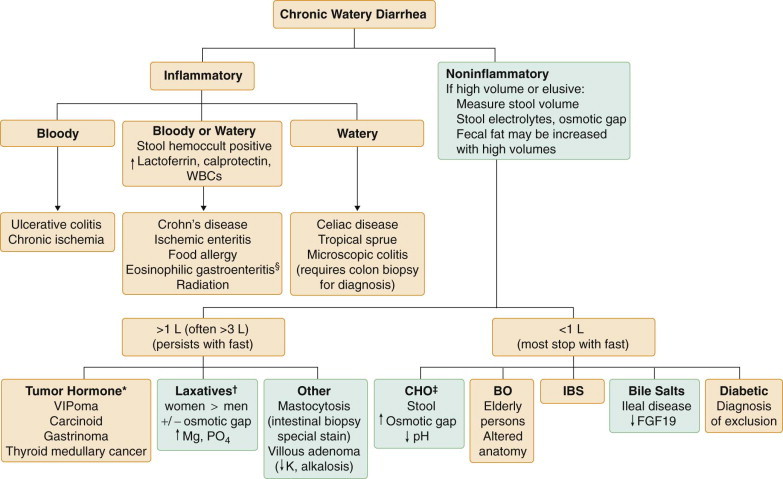
Approach to the evaluation of watery diarrheas.
Many diarrheas have more than one mechanism (i.e., osmotic, secretory, inflammatory). Other causes include medications, postsurgical (vagotomy, Nissan wrap, cholecystectomy), hyperthyroidism, and alcohol. *VIPoma: >3 L output daily “pancreatic cholera,” elevated VIP level. Carcinoid: elevated urine 5-hydroxyindole acetic acid, positive OctreoScan. Gastrinoma (Zollinger-Ellison syndrome): elevated gastrin level, positive secretin stimulation test, diarrhea due to high volume of acid secretion. Thyroid medullary cancer: elevated calcitonin level. †May be high or low volume depending on dose ingested, may respond to fast. ‡Carbohydrate malabsorption (CHO) may be due to lactase deficiency, dietary fructose, sorbitol in diabetic candies or liquid medications. §Full-thickness biopsy may be needed for diagnosis. BO = bacterial overgrowth; FGF = fibroblast growth factor; IBS = irritable bowel syndrome; WBCs = white blood cells.
Ingestion of Nonabsorbable or Poorly Absorbable Solutes
Magnesium and Sodium Phosphate and Sulfate Diarrheas
Magnesium, phosphate, and sulfate are poorly absorbed minerals. Individuals who ingest significant amounts of magnesium-based antacids or high-potency multimineral and multivitamin supplements or those who surreptitiously ingest magnesium-containing laxatives or nonabsorbable anion laxatives, such as Na2PO4 (neutral phosphate) or Na2SO4 (Glauber's or Carlsbad salt) may develop osmotically induced, watery diarrhea that may be high volume.
Sorbitol and Fructose Diarrhea
Dietetic food, chewing gum, candies, and medication elixirs that are sweetened with sorbitol, which is an unabsorbable carbohydrate, can cause diarrhea. Excessive consumption of pears, prunes, peaches, and apple juice, which also contain sorbitol and fructose, a poorly absorbable sugar, can result in diarrhea. Most soft drinks are now sweetened with fructose-containing corn syrup and may be a cause of diarrhea when ingested in high concentrations.
Glucose-Galactose Malabsorption and Disaccharidase Deficiencies
Primary and secondary lactase deficiency is the most common cause of disaccharidase deficiency (see malabsorption). Congenital lactase deficiency causes diarrhea at birth with the first breast feed. Congenital sucrose-isomaltose deficiency presents in infancy when table sugar is introduced into the diet. Glucose-galactose malabsorption is due to mutations in the SGLT1 gene and causes diarrhea at birth. The mechanism of diarrhea in these disorders is osmotic. Stools are acidic owing to conversion of unabsorbed sugars to short-chain fatty acids in the colon. Treatment is the substitution of fructose for other sugars in the diet. Patients who develop gas, bloating, or diarrhea after the ingestion of mushrooms may have a deficiency in the disaccharidase trehalase.
Rapid Intestinal Transit
A small amount of carbohydrate in the diet is unabsorbed by the normal small intestine. Diets that are high in carbohydrate and low in fat may allow rapid gastric emptying and rapid small intestinal motility, thereby leading to carbohydrate malabsorption and osmotic diarrhea. Rapid transit time also occurs in thyrotoxicosis (Chapter 233). Because of the production of H2 and CO2 gas by colonic bacteria, abdominal gas and cramping may be the predominant symptoms.
Bile Acid Malabsorption
Ileal malabsorption of bile salts results in the stimulation of colonic fluid secretion and watery diarrhea. Three types of bile acid malabsorption induce diarrhea. Type 1 results when severe disease (e.g., Crohn's disease), resection, or bypass of the distal ileum allows bile salts to escape absorption (see earlier). Type 2 may rarely be congenital, owing to a defect in the apical sodium bile acid transporter, or may more commonly be idiopathic. The idiopathic type has been associated with decreased levels of FGF19, an intestinal fibroblast growth factor that normally downregulates bile salt synthesis in the liver. The result is increased bile salt production that overwhelms reabsorption in the ileum. Type 3 is caused by various conditions, including prior cholecystectomy, celiac disease, pancreatic insufficiency, microscopic colitis, bacterial overgrowth, gastric surgery, or vagotomy. Postulated mechanisms include a bile salt storage problem, increased production, decreased recycling, or saturation of absorption.
Treatment  .
.
Diarrhea due to types 1 and 2 often responds to cholestyramine, 2 to 4 g given orally two to four times daily, or the more potent and better tolerated bile salt binder, colesevelam, 625 mg tablet orally two to six times daily. Fat-soluble vitamin deficiency is a potential risk with chronic use of bile salt binders.
Although many patients with type 3 respond to cholestyramine, some do not. In these patients, motility-altering drugs such as opiates (e.g., loperamide, 2 to 4 mg orally two to four times daily) and anticholinergics (e.g., hyoscyamine sulfate, 0.125 to 0.250 mg orally two to four times daily) may be of benefit.
Functional Watery Diarrhea (Irritable Bowel Syndrome)
See Chapter 139.
True Secretory Diarrheas
Endocrine diseases that can cause secretory diarrheas (see Fig. 142-8) include carcinoid tumors (Chapter 240), gastrinomas (Chapter 201), VIPomas of the pancreas (Chapter 201), and medullary carcinoma of the thyroid (Chapter 254). Diarrhea is also seen in 60 to 80% of patients with systemic mastocytosis (Chapter 263). Diarrhea due to gastrinoma is distinct in that it is caused by high volumes of hydrochloric acid secretion that overwhelm the reabsorptive capacity of the colon and by maldigestion of fat owing to pH inactivation of pancreatic lipase and precipitation of bile salts.
Villous Adenomas
Large (4 to 18 cm) villous adenomas (Chapter 199), particularly in the rectum or occasionally the sigmoid colon, may cause secretory diarrhea of 500 to 3000 mL/24 hours characterized by hypokalemia, chloride-rich stool, and metabolic alkalosis. Increased numbers of goblet cells and increased prostaglandin E2 are responsible for the diarrhea. Chloride wasting in the stool and metabolic alkalosis are also found in congenital chloridorrhea, which is caused by a defect in the intestinal Cl−/HCO3 − transporter. The metabolic alkalosis distinguishes these two diarrheas from most other diarrheas that cause metabolic acidosis. A villous adenoma is usually diagnosed by colonoscopy. The prostaglandin antagonist indomethacin (25 to 100 mg orally daily) reduces the diarrhea in some patients; resection is curative.
Diabetes Mellitus–Related Diarrhea
Constipation is more common than diarrhea in patients with diabetes. High-volume, watery diarrhea, often with nocturnal incontinence, occurs in 20% of poorly controlled type 1 diabetic patients. These patients usually have concomitant neuropathy, nephropathy, and retinopathy. The diarrhea may be due to several causes, including celiac disease, anal incontinence, bacterial overgrowth related to dysmotility, medications (metformin, acarbose), and autonomic neuropathy. If no specific cause is found, clonidine (initial dose 0.1 mg orally twice daily and titrated slowly to a maximal dose of 0.5 to 0.6 mg orally twice daily) may be helpful. Patients with neuropathy frequently have impaired anal sphincter function, and high-dose loperamide (4 mg orally four times a day) may improve the incontinence.
Alcoholic Diarrhea
Diarrhea related to alcohol ingestion (Chapter 32) may be due to rapid intestinal transit, decreased bile and pancreatic secretion, nutritional deficiencies such as folate or vitamin B12, or alcohol-related enteric neuropathy. Diarrhea may be acute with binge drinking, or it may be chronic and watery and persist for days or weeks. The diarrhea slowly resolves with abstinence from alcohol, proper nutrition, and the repletion of vitamin deficiencies.
Factitious Diarrhea
Approximately 30% of patients referred to tertiary centers have chronic diarrhea due to laxative abuse. The diarrhea is usually severe and watery, often with nocturnal symptoms. Some patients may have abdominal pain, weight loss, nausea, vomiting, hypokalemic myopathy, and acidosis. Stool volumes range from 300 to 3000 mL a day depending on the dose of laxative ingested. In the United States, bisacodyl is the most common cause. Other culprits include anthraquinone (senna, cascara, aloe, rhubarb) or osmotic laxatives (neutral phosphate, Epsom salts, and magnesium citrate). Some patients abuse other agents that cause diarrhea, such as the diuretics furosemide and ethacrynic acid.
More than 90% of laxative abusers are women who have underlying eating disorders such as anorexia nervosa or bulimia (Chapter 226) or middle-aged women who have complicated medical histories and who often work in health care. In patients with unexplained diarrhea, laxative screening of stool and urine (see later) should be performed to exclude this syndrome before an extensive medical evaluation is performed for other causes of chronic diarrhea.
Chronic Idiopathic Secretory Diarrhea
In a small subset of patients with secretory diarrhea, no cause is found despite an extensive evaluation. These cases are labeled as chronic idiopathic secretory diarrhea. In most patients, the diarrhea resolves within 6 to 24 months, which suggests a possible postinfectious or Brainerd's diarrhea. If no diagnosis is found after thorough testing and a search for surreptitious laxative abuse, a therapeutic trial with bile salt–binding drugs (e.g., cholestyramine, 4 g orally before meals three times a day, or the more potent colesevelam, 625-mg tablet two to six times daily) or opiates (e.g., loperamide, 2 mg orally four times a day, maximal dose 16 mg a day) is warranted.
Inflammatory Diarrheas
Diarrhea due to inflammation is characterized by watery or bloody stools, fecal leukocytes, and loss of protein in the stool (see Fig. 142-8).
Inflammatory Bowel Disease
See Chapter 143.
Eosinophilic Gastroenteritis
Eosinophilic gastroenteritis is an increasingly recognized condition of unknown etiology characterized by infiltration of eosinophils in the mucosa, muscle, or serosal layers of the gastrointestinal tract. Approximately 50% of patients have atopic histories. Infestation with nematodes (Chapter 365) must be excluded before this diagnosis is made. Diarrhea occurs in 30 to 60% of patients with mucosal disease. Patients with involvement of the muscle layer often present with abdominal pain, nausea, and vomiting indicative of gastric outlet or intestinal obstruction. Peripheral eosinophilia is present in most patients. The disease may involve the entire gastrointestinal tract from esophagus to anus, or it may be isolated to a segment. With diffuse involvement, patients may have steatorrhea, protein-losing enteropathy, and blood loss.
Collagenous Colitis and Lymphocytic Colitis
These two conditions, collectively known as microscopic colitis, may or may not be the same disease or variants of the same disease. Lymphocytic colitis is equally prevalent in men and women, whereas collagenous colitis occurs ten times more often in middle-aged or elderly women. These conditions may be associated with autoimmune disease or with NSAID use. There is an increased prevalence (15%) of microscopic colitis among individuals with celiac sprue. These diseases may be categorized as either inflammatory or secretory diarrheas. An epidemiologic relationship to medications such as NSAIDs, H2-receptor blockers, proton pump inhibitors, selective serotonin reuptake inhibitors, and others has been reported, and increased luminal prostaglandin levels may cause the diarrhea. Enteric infections, food hypersensitivity, or intraluminal bile has been proposed as a trigger for prostaglandin release from lymphocytes. The disease disappears with fecal stream diversion. Antidiarrheal agents such as loperamide (2 mg orally four times a day) are the mainstay of therapy. Budesonide (9 mg orally once a day),9, 10 bismuth subsalicylate therapy (eight chewable 262-mg tablets orally once a day), and 5-aminosalicylates (e.g., mesalamine, 400 to 800 mg orally three times daily) may be useful. Those with refractory disease may require corticosteroids (e.g., prednisone, 40 mg orally once a day).
Food Allergy
Food allergies or sensitivities, especially to cow's milk and soy protein, are a well established cause of enterocolitis in children, with an estimated frequency of 5%. Symptoms of abdominal cramps, diarrhea, and sometimes vomiting occur shortly after ingestion of the allergen (Chapter 261). The role of food allergy in causing diarrhea in adults is less clear owing to the lack of a reliable diagnostic test. Allergy testing correlates poorly with intestinal allergy. The most common food allergens are milk, soy, eggs, seafood, nuts, and wheat. Sequential elimination diets can be diagnostic and therapeutic.
Protein-Losing Enteropathy
Severe protein loss through the gastrointestinal tract can be caused by mucosal diseases such as lymphangiectasia, lymphatic obstruction, bacterial or parasitic infection, gastritis (Chapter 141), gastric cancer, collagenous colitis, inflammatory bowel disease (Chapter 143), celiac disease, sarcoidosis (Chapter 95), lymphoma (Chapter 191), tuberculosis (Chapter 332), Ménétrier's disease (Chapter 198), eosinophilic gastroenteritis, and food allergies. A variety of extraintestinal diseases, including systemic lupus erythematosus (Chapter 274), heart failure (Chapter 58), and constrictive pericarditis (Chapter 77), also can be causative. Patients with systemic lupus erythematosus (Chapter 274) may present with protein-losing enteropathy as the only manifestation of their disease. Treatment focuses on the underlying disease.
Radiation Enteritis
Patients who receive pelvic radiation for malignancies of the female urogenital tract or the male prostate may develop chronic radiation enterocolitis 6 to 12 months after total doses of radiation greater than 40 to 60 Gy (Chapters 19 and 144Chapter 19Chapter 144). Symptoms can develop 20 years after treatment, however. Early abnormalities include an increase in inflammatory mediators, an increase in cholinergic stimulation of intestinal tissue, and endothelial cell apoptosis that precedes epithelial cell apoptosis. The last finding suggests that vascular injury is the primary event. Vascular endothelial growth factor, basic fibroblast growth factor, and IL-11 protect animal intestine from experimental radiation damage. Diarrhea may be caused by bile acid malabsorption if the ileum is damaged, by bacterial overgrowth if radiation causes small intestinal strictures or bypass, or by radiation-induced chronic inflammation of the small intestine and colon. Rapid transit also may contribute to malabsorption and diarrhea.
Treatment  .
.
Treatment is often unsatisfactory. Anti-inflammatory drugs (sulfasalazine, corticosteroids) and antibiotics have been tried with little success. Cholestyramine (4 g orally three times a day) and NSAIDs (e.g., naproxen, 250 to 500 mg orally twice daily) may help, as may opiates (loperamide, 2 mg orally four times a day, or loperamide-N-oxide, 3 mg orally two times a day).
Miscellaneous Diseases
Although acute mesenteric arterial or venous thrombosis manifests as an acute bloody diarrhea, chronic mesenteric vascular ischemia (Chapter 145) may manifest as watery diarrhea. Gastrointestinal tuberculosis (Chapter 332) and histoplasmosis (Chapter 340) manifest as diarrhea that may be either bloody or watery, as do certain immunologic diseases, such as Behçet's syndrome or Churg-Strauss syndrome. All of these diseases may be misdiagnosed as inflammatory bowel disease (Chapter 143). Neutropenic enterocolitis, an ileocolitis that occurs in neutropenic leukemic patients, sometimes is caused by C. difficile infection.
Clinical Manifestations of Chronic Diarrhea
Patients with malabsorption can present with a variety of gastrointestinal or extraintestinal manifestations (Table 142-6 ). Significant malabsorption of fat and carbohydrate usually causes chronic diarrhea, abdominal cramps, gas, bloating, and weight loss. Steatorrhea (fat in the stool) manifests as oily, foul-smelling stools that are difficult to flush down the toilet. Stools may be large and bulky (e.g., pancreatic insufficiency) or watery (e.g., bacterial overgrowth, mucosal diseases). Individuals with malabsorption also can present with manifestations of vitamin and mineral deficiencies. Dyspnea can be caused by anemia from iron, folate, or vitamin B12 deficiency. Manifestations of calcium, magnesium, or vitamin D malabsorption include paresthesias and tetany due to hypocalcemia or hypomagnesemia and bone pain due to osteomalacia or osteoporosis-related fractures. Paresthesias and ataxia are manifestations of cobalamin and vitamin E deficiency. Dermatitis herpetiformis is a blistering, burning, itchy rash on the extensor surfaces and buttocks that is associated with celiac disease.
TABLE 142-6.
Clinical Consequences of Malabsorption of Nutrients, Water, and Electrolytes
| NUTRIENT MALABSORBED | CLINICAL MANIFESTATION |
|---|---|
| Protein | Wasting, edema |
| Carbohydrate and fat | Diarrhea, abdominal cramps and bloating, weight loss and growth retardation |
| Fluid and electrolytes | Diarrhea, dehydration |
| Iron | Anemia, cheilosis, angular stomatitis |
| Calcium and vitamin D | Bone pain, fractures, tetany |
| Magnesium | Paresthesias, tetany |
| Vitamin B12 and folate | Anemia, glossitis, cheilosis, paresthesias, ataxia (vitamin B12 only) |
| Vitamin E | Paresthesias, ataxia, retinopathy |
| Vitamin A | Night blindness, xerophthalmia, hyperkeratosis, diarrhea |
| Vitamin K | Ecchymoses |
| Riboflavin | Angular stomatitis, cheilosis |
| Zinc | Dermatitis, hypogeusia, diarrhea |
| Selenium | Cardiomyopathy |
| Essential fatty acids | Dermatitis |
Inflammatory diarrheas may present with fever and abdominal pain or with edema to suggest chronic protein loss. Patients may have multiple, low-volume, bloody stools with tenesmus to suggest proctitis, or have severe diarrhea due to GVHD or celiac disease. Systemic manifestations of inflammatory bowel disease include polymigratory arthritis, sacroiliitis, erythema nodosum, pyoderma gangrenosum, leukocytoclastic angiitis, uveitis, and oral aphthous ulcers.
Diagnosis of Chronic Diarrhea
A detailed history, physical examination, and certain screening tests lead to a diagnosis in 75 to 80% of patients with chronic diarrheas (see Table 142-1 and Fig. 142-3). A history of 10 to 20 daily bowel movements that do not respond to fasting suggests secretory diarrhea (see Fig. 142-8). A history of peptic ulcer should suggest gastrinoma (Chapter 201) or systemic mastocytosis (Chapter 263). Physical examination is helpful only if the thyromegaly of medullary carcinoma (Chapter 254), the cutaneous flushing of the neuroendocrine tumors and systemic mastocytosis, the dermatographism of systemic mastocytosis, or the migratory necrolytic erythema of glucagonoma (Chapter 201) is evident. Autonomic dysfunction (e.g., postural hypotension, impotence, gustatory sweating) is almost invariably present in diabetic diarrhea.
Evaluation for malabsorption begins with a careful elicitation of bowel habits, a description of the stool, weight loss, travel, food or milk tolerance, underlying gastrointestinal or liver diseases, abdominal surgery, radiation or chemotherapy treatments, family history, and drug and alcohol use.
Blood Tests
Blood measurements (see Fig. 142-3) of iron, folate, vitamin B12, vitamin D, or prothrombin time (vitamin K) help evaluate malabsorption. Peripheral blood findings of leukocytosis, eosinophilia, elevated erythrocyte sedimentation rate, hypoalbuminemia, or low total serum protein suggests an inflammatory diarrhea, whose hallmark is the presence of blood, either gross or occult, and leukocytes in the stool. There are no bedside screening tests to establish the diagnosis in watery diarrheas.
Imaging
Malabsorption may be suggested if a flat plate radiograph of the abdomen shows pancreatic calcification (Chapter 135). Some diseases (e.g., previous gastric surgery, gastrocolic fistulas, blind loops from previous intestinal anastomoses, small intestine strictures, multiple jejunal diverticula, abnormal intestinal motility that could lead to bacterial overgrowth) may be shown by computed tomography or magnetic resonance imaging of the abdomen after administration of oral contrast agents or by a traditional upper gastrointestinal radiographic series with small intestine follow-through. A small bowel barium study may show thickening of the intestinal folds (e.g., amyloidosis, lymphoma or Whipple's disease), uniform or patchy abnormalities (e.g., lymphoma or lymphangiectasia), or flocculation of barium and ilealization of jejunum to suggest celiac disease. Routine contrast radiographs of the gastrointestinal tract usually are not helpful in the diagnosis of watery diarrheas, unless they show extensive small bowel resection, the presence of a tumor (carcinoid or villous adenoma), or a bowel filled with fluid (endocrine tumor). Abdominal contrast imaging may show diagnostic evidence of advanced inflammatory bowel disease or changes suggestive of eosinophilic gastroenteritis or radiation enterocolitis. Somatostatin receptor scintigraphy with indium-111-labeled octreotide can be useful in localizing gastrinomas, pancreatic endocrine tumors, and carcinoid tumors.
Endoscopy and Biopsy
Upper endoscopy with distal duodenal biopsy should be undertaken if serologic tests for celiac disease are positive or diagnostic clues suggest small bowel mucosal malabsorption (Chapter 136). Small bowel biopsy is virtually always abnormal when the tTG IgA antibody level is very high (more than five-fold the normal range), and EMA is positive. A biopsy may be avoided in this setting. Some patients may have patchy mucosal disease and require enteroscopy with jejunal biopsies for diagnosis. Wireless video capsule endoscopy (Chapter 136) and balloon-assisted enteroscopy are increasingly used to diagnose diseases that reside deep in the small bowel. Patients with severe watery or elusive diarrhea should have a colonoscopy to assess for villous adenomas, microscopic colitis, mastocytosis, or early inflammatory bowel disease. Colonoscopy may also show brown pigmentation suggestive of melanosis coli due to chronic use of anthracene laxatives. Terminal ileal biopsy may indicate infectious or inflammatory bowel disease.
Other Laboratory Tests
Malabsorption
If chronic diarrhea is the presenting symptom, a stool examination for ova and parasites and a stool antigen-capture ELISA test for Giardia should be obtained. A stool test for fat on a high-fat diet (70 to 100 g/day) is the best available screening test for malabsorption (see Table 142-6). If the fecal fat test result is negative, selective carbohydrate malabsorption or other causes of watery diarrhea should be considered. If the fecal fat test result is positive, further testing should be based on clinical suspicion for particular diseases. If pancreatic insufficiency is suspected, imaging studies of the pancreas should be performed. If proximal mucosal damage is suspected, multiple small intestinal biopsy specimens should be obtained. If there are no clues to the cause of malabsorption, a d-xylose test may help to distinguish mucosal disease from pancreatic insufficiency. However, the d-xylose test result also can be abnormal in individuals with bacterial overgrowth; if this condition is suspected, culture of an intestinal aspirate or a breath test should be obtained (see Table 142-6). Small bowel contrast imaging is useful in detecting ileal disease and structural abnormalities that predispose to bacterial overgrowth (Table 142-7 ). Some individuals with celiac disease present with selective nutrient deficiencies without diarrhea. In these cases, tTG antibody tests and intestinal biopsy should be performed. In patients hospitalized for severe diarrhea or malnutrition, a more streamlined evaluation usually includes a stool for culture, ova and parasites, and fat; an abdominal imaging study; and a biopsy of the small intestine and colon.
TABLE 142-7.
Abnormalities Conducive to Bacterial Overgrowth
| STRUCTURAL |
|
| MOTOR |
|
| HYPOCHLORHYDRIA |
|
| MISCELLANEOUS |
|
Watery Diarrhea
Breath tests to measure the respiratory excretion of labeled CO2 after oral administration and metabolism of radioactive carbon-labeled substrates, or of H2 after administration of carbohydrates, can assess carbohydrate and bile salt malabsorption or bacterial overgrowth (see Table 142-6).
The diagnosis of endocrine tumors, such as carcinoids, gastrinoma, VIPoma, medullary carcinoma of the thyroid, glucagonoma, somatostatinoma, and systemic mastocytosis, is made by showing elevated blood levels of serotonin or urinary 5-hydroxyindoleacetic acid and serum levels for gastrin, vasoactive intestinal peptide, calcitonin, glucagon, somatostatin, histamine, or prostaglandins (Chapter 201). Somatostatin receptor scintigraphy has proved to be sensitive and useful in the diagnosis and evaluation of Zollinger-Ellison syndrome (Chapter 201).
Inflammatory Diarrhea
Stool occult blood, white blood cells, or lactoferrin and calprotectin (components of leukocytes) are helpful tests for bowel inflammation. Video capsule endoscopy (Chapter 136) of the small bowel may detect ulcerations deep in the small bowel not reachable by standard upper or lower endoscopy and not detected with conventional barium contrast radiography. However, the risk of capsule retention in the small bowel is high in patients with Crohn's disease or NSAID use, particularly when there is a history of obstructive symptoms. The most sensitive test for protein-losing enteropathy is measurement of intestinal protein loss by 24-hour stool excretion or clearance of α1-antitrypsin.
Stool Examination in Elusive Diarrhea
An important adjunct to diagnosing the cause of diarrhea is to examine the stool. The greasy, bulky stool of steatorrhea and the bloody stool of gut inflammation are distinctive. Stool collections (see Table 142-6) can be analyzed for weight, volume, fat, electrolytes (+Na++, K++, Cl−), osmolality, pH, and a laxative screen (SO4 2−, PO4 2−, Mg2+). Stool or urine can be analyzed for emetine (a component of ipecac), bisacodyl, castor oil, or anthraquinone.
Carbohydrate malabsorption lowers stool pH because of colonic fermentation of carbohydrate to short-chain fatty acids. Stool pH less than 5.3 usually means pure carbohydrate malabsorption, whereas in the generalized malabsorptive diseases, stool pH is greater than 5.6 and usually greater than 6.0.
The normal stool osmotic gap, which is the difference between stool osmolality (or 290 mOsm) and twice the stool Na++ and K++ concentrations, is 50 to 125. In secretory diarrheas, the colon's capacity for adjusting electrolyte concentrations is overwhelmed, the stool osmotic gap is less than 50, and stool electrolytes more nearly resemble plasma electrolytes (+Na++ concentrations are usually >90 mmol/L, K+ concentrations usually <10 mmol/L), except for higher HCO3 − concentrations (usually >50 mmol/L). In osmotic diarrhea, the presence of uncharged solute or unmeasured cation in the colonic lumen draws in water, depresses stool Na++ (usually <60 mmol/L) and K+ concentrations, and results in a stool osmotic gap >125. Stools with Na++ concentrations between 60 and 90 mmol/L and calculated osmotic gaps between 50 and 100 can result from either secretory or malabsorptive abnormalities. Patients with Mg2++-induced diarrhea may be diagnosed by fecal Mg2+ values of more than 50 mmol/L. Sodium anion–induced diarrheas (Na2SO4, Na2PO4) mimic secretory diarrhea because the stool Na++ content is high (>90 mmol/L), and there is no osmotic gap; this diarrhea may be diagnosed by determining stool Cl− concentration because these anions displace stool Cl− resulting in a depressed stool Cl− value (usually <20 mmol/L).
Treatment of Chronic Diarrhea  .
.
Antidiarrheal Therapy
Antidiarrheal agents are of two types: those used for mild to moderate diarrheas and those used for severe secretory diarrheas. A major shortcoming of opiates, the most commonly prescribed antidiarrheal agents, is that they have no antisecretory effect. Rather, they act by decreasing intestinal motility, thereby allowing longer contact time with the mucosa for improved fluid absorption. The exception is racecadotril, an enkephalinase inhibitor, that blocks intestinal fluid secretion without affecting motility.
Bulk-forming agents (psyllium [7 g in 8 ounces water orally up to five times a day] and methylcellulose [three to six tablets twice a day with 300 mL of water]) act by binding water and increasing the consistency of stool. Pectin has been shown to have proabsorptive activity. These agents may be useful in patients with fecal incontinence. Bismuth subsalicylates (524 mg orally every hour up to eight doses a day) have mild antisecretory and antimotility effects and are effective and safe in mild diarrheas. Agents that bind bile salts (e.g., cholestyramine, 2 to 4 g orally two to four times a day) are effective in the treatment of bile acid diarrheas but may worsen diarrhea in patients with ileal resection or disease of more than 100 cm of ileum.
The opiates may be symptomatically useful in mild to moderate diarrheas. Paregoric, deodorized tincture of opium, codeine, and diphenoxylate with atropine largely have been supplanted by loperamide. Loperamide does not pass the blood-brain barrier and has a high first-pass metabolism in the liver; it has a high therapeutic-to-toxic ratio and is essentially devoid of addiction potential. It is safe in adults, even in total doses of 24 mg/day. The usual dose is 2 to 4 mg two to four times daily. Opiates may be harmful in patients with severe diarrheas because large volumes of fluid may pool in the intestinal lumen (third space), and stool output is no longer a reliable gauge for replacing fluid losses. The antimotility effects are a problem in infectious diarrheas because stasis may enhance bacterial invasion and delay clearance of microorganisms from the bowel. Opiates and anticholinergics also are dangerous in severe inflammatory bowel disease or severe C. difficile infection, where they may precipitate megacolon.
Antidiarrhea agents that are used for the treatment of severe secretory and inflammatory diarrheas generally have profiles with more serious side effects. The somatostatin analogue octreotide (initial dose, 100 to 600 µg subcutaneously in two to four divided doses daily; maximal dose, 1500 µg daily) lessens diarrhea in the carcinoid syndrome and in neuroendocrine tumors because it inhibits hormone secretion by the tumor. It is also effective in the treatment of dumping syndrome and chemotherapy-related diarrheas. Long-acting subcutaneous octreotide preparations (20 to 30 mg intramuscularly intragluteally every month) are now available for once-a-month dosing. Octreotide can suppress pancreatic enzyme secretion and make diarrhea worse; it also may be of only limited usefulness in short-bowel syndrome and AIDS diarrhea. Agents such as phenothiazine and calcium-channel blockers have mild antisecretory effects, but side effects limit their use. Clonidine (initial dose, 0.1 mg orally twice daily, titrated slowly to a maximal dose of 0.5 to 0.6 mg twice daily) is most useful in opiate withdrawal diarrhea and is sometimes useful in diabetic diarrhea; postural hypotension may limit its use, particularly in patients with diabetes. Alosetron (0.5 mg orally twice daily for four weeks, maximal dose 1 mg orally twice daily) may be justified for severe diarrhea-predominant irritable bowel syndrome; associations with ischemic colitis and severe constipation have limited its use. Indomethacin (250 to 500 mg orally twice daily), a cyclooxygenase blocker that inhibits prostaglandin production, is useful in the treatment of diarrheas caused by acute radiation, AIDS, or villous adenomas of the rectum or colon; occasionally, it may be useful in neuroendocrine tumors and food allergy. For eosinophilic gastroenteritis, corticosteroids (prednisone, 20 to 40 mg orally once a day for 7 to 10 days) are the mainstay of therapy, but disodium cromoglycate (200 mg orally four times daily) also may be useful; food elimination diets are not usually effective. Treatment of inflammatory bowel disease is described in Chapter 143.
Grade A
- 1.Lowy I, Molrine DC, Leav BA. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 2.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 3.Madhi SA, Cunliffe NA, Steele D. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 4.Sur D, Lopez AL, Kanungo S. Efficacy and safety of modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 5.Dupont HL, Jiang ZD, Okhuysen PC. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805–812. doi: 10.7326/0003-4819-142-10-200505170-00005. [DOI] [PubMed] [Google Scholar]
- 6.Lauritano EC, Gabrielli M, Scarpellini E. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13:111–116. [PubMed] [Google Scholar]
- 7.Byrne TA, Wilmore DW, Iyer K. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655–661. doi: 10.1097/01.sla.0000186479.53295.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeppesen PB, Gilroy R, Pertkiewicz M. Randomized placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011 doi: 10.1136/gut.2010.218271. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miehlke S, Madisch A, Bethke B. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510–1516. doi: 10.1053/j.gastro.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 10.Miehlke S, Madisch A, Karimi D. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009;136:2092–2100. doi: 10.1053/j.gastro.2009.02.078. [DOI] [PubMed] [Google Scholar]
Suggested Readings
- Feurle GE, Junga NS, Marth T. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple's disease. Gastroenterology. 2010;138:478–486. doi: 10.1053/j.gastro.2009.10.041. Either antibiotic is an effective initial therapy. [DOI] [PubMed] [Google Scholar]
- Lopman BA, Hall AJ, Curns AT. Increasing rates of gastroenteritis hospital discharges in U.S. adults and the contribution of norovirus, 1996-2007. Clin Infect Dis. 2011;52:466–474. doi: 10.1093/cid/ciq163. Norovirus, which causes 10% of cause-unspecified and 7% of all-cause admissions for gastroenteritis, should be routinely considered in hospitalized patients. [DOI] [PubMed] [Google Scholar]
- van der Windt DA, Jellema P, Mulder CJ. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010;303:1738–1746. doi: 10.1001/jama.2010.549. IgA anti-tissue transglutaminase antibodies and IgA antiendomysial antibodies have a high sensitivity and specificity for diagnosing celiac disease among patients with abdominal symptoms. [DOI] [PubMed] [Google Scholar]
- Williams JJ, Beck PL, Andrews CN. Microscopic colitis: a common cause of diarrhea in older adults. Age Aging. 2010;39:162–168. doi: 10.1093/ageing/afp243. About 10 to 30% of older patients with diarrhea and normal colonoscopy have microscopic colitis. [DOI] [PubMed] [Google Scholar]
Additional Suggested Readings
- Antwan NA, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596–2604. doi: 10.1038/ajg.2009.329. Comprehensive update on mechanism and modifications of oral rehydration solutions. [DOI] [PubMed] [Google Scholar]
- Dubois PC, Trynka G, Franke L. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. GWAS reveals multiple common gene variants, especially those related to immune function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Sellin JH. Review article: small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhea. Aliment Pharmacol Ther. 2009;29:1069–1077. doi: 10.1111/j.1365-2036.2009.03970.x. A practical review. [DOI] [PubMed] [Google Scholar]
- Feurle GE, Moos V, Schinnerling K. The immune reconstitution inflammatory syndrome in Whipple disease: a cohort study. Ann Intern Med. 2010;153:710–717. doi: 10.7326/0003-4819-153-11-201012070-00004. After antibiotic therapy, 10% of patients with Whipple's disease develop the immune reconstitution inflammatory syndrome, occasionally with fatal complications. [DOI] [PubMed] [Google Scholar]
- Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361:998–1008. doi: 10.1056/NEJMra0804605. Review. [DOI] [PubMed] [Google Scholar]
- Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–49. doi: 10.1172/JCI30253. A scientific overview. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucendo AJ. Eosinophilic diseases of the gastrointestinal tract. Scand J Gastroenterol. 2010;45:1013–1021. doi: 10.3109/00365521003690251. Review. [DOI] [PubMed] [Google Scholar]
- Marth T. New insights into Whipple's disease: a rare intestinal inflammatory disorder. Dig Dis. 2009;27:494–501. doi: 10.1159/000233288. Review. [DOI] [PubMed] [Google Scholar]
- Misiakos EP, Macheras A, Kapetanakis T. Short bowel syndrome: current medical and surgical trends. J Clin Gastroenterol. 2007;41:5–18. doi: 10.1097/01.mcg.0000212617.74337.e9. Clinical overview. [DOI] [PubMed] [Google Scholar]
- Ojeda E, Cosme A, Lapaza J. Whipple's disease in Spain: a clinical review of 91 patients diagnosed between 1947 and 2001. Rev Esp Enferm Dig. 2010;102:108–123. doi: 10.4321/s1130-01082010000200006. Review emphasizing PCR diagnosis and antibiotic therapy with cotrimoxazole or ceftriaxone. [DOI] [PubMed] [Google Scholar]
- Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. Strategies for the diagnosis and management of diarrheal diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis GA, Hill C, Guerrant RL. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology. 2011;140:8–14. doi: 10.1053/j.gastro.2010.11.010. Review and perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. An updated, comprehensive review on celiac sprue. [DOI] [PubMed] [Google Scholar]
- Shaukat A, Levitt MD, Taylor BC. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152:797–803. doi: 10.7326/0003-4819-152-12-201006150-00241. Review. [DOI] [PubMed] [Google Scholar]
- Suchy FJ, Brannon PM, Carpenter TO. National Institutes of Health Consensus Development Conference: lactose intolerance and health. Ann Intern Med. 2010;152:792–796. doi: 10.7326/0003-4819-152-12-201006150-00248. Review. [DOI] [PubMed] [Google Scholar]
- Thielman NM, Guerrant RL. Acute infectious diarrhea. N Engl J Med. 2004;350:38–47. doi: 10.1056/NEJMcp031534. A summary of the diagnostic approach to and antimicrobial therapy for acute infectious diarrhea. [DOI] [PubMed] [Google Scholar]
- Walters JR, Tasleem AM, Omer OS. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acids biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1151–1154. doi: 10.1016/j.cgh.2009.04.024. A breakthrough human study that proposes a mechanism for the pathogenesis of bile salt diarrhea. [DOI] [PubMed] [Google Scholar]


