Structure and Function
Edward J. Hall
The small intestine (SI) is, in essence, an interface between the external environment and the body, and is both an absorptive surface and a barrier; it must digest and absorb nutrients while excluding antigens and microbes and eliminating fecal waste. It faces a frequently changing dietary and bacterial intake, and yet has to maintain a dynamic but balanced microflora within its lumen while being intermittently exposed to pathogens. All of its functions—mixing and propulsion, secretion, digestion, absorption, regulation of blood flow, immunologic reaction and tolerance, and elimination—are fully integrated through both local and remote neuroendocrine and immunologic mechanisms (see Chapter 1). It thus has a complex task and requires specialized anatomic arrangements to perform them.
Gross Structure
Anatomic Regions
The SI is basically a tube, beginning at the pylorus of the stomach and ending at the ileocolic valve. However, this tube is ultimately in continuity with the external environment, proximally from the mouth via the esophagus and stomach, and distally to the anus via the large intestine (Figure 57-1, A ).1, 2, 3 It is relatively short, reflecting the typical dietary intake of cats and dogs. It is approximately 1 to 1.5 meters long in adult cats and ranges from 1 to 5 meters in adult dogs, in proportion to the size of the individual. It is divided arbitrarily into three anatomic segments: the duodenum proximally, then the jejunum, and finally the ileum distally (see Figure 57-1, A).
Figure 57-1.
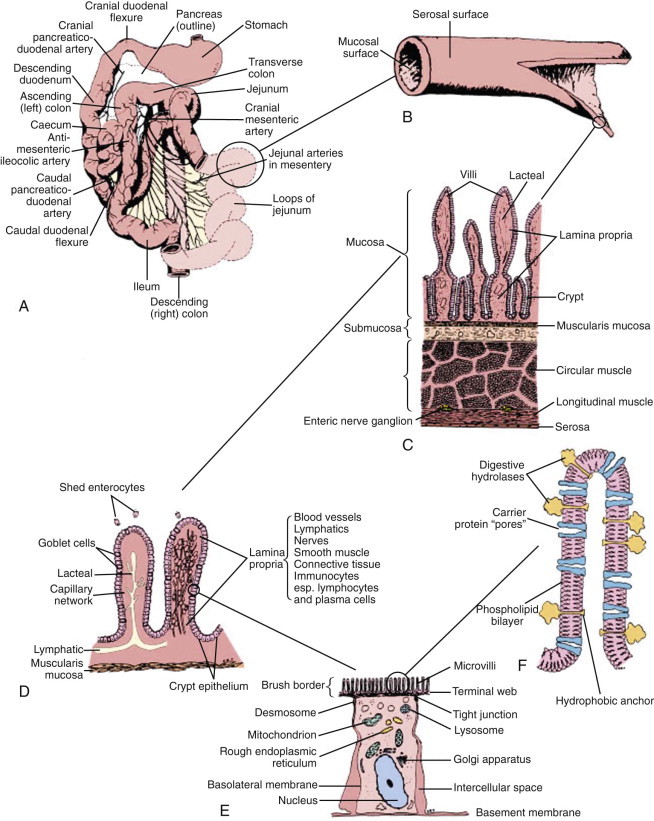
Functional anatomy of the small intestine.
A, Anatomic arrangement of the small intestine. B, The small intestine is basically a tube with a serosal surface covered by visceral peritoneum and an inner absorptive and digestive surface, the mucosa. C, Beneath the outer serosa, longitudinal and circular muscle layers produce peristaltic and segmental contractions for propelling and mixing the luminal contents. The submucosa is rich in blood and lymphatic vessels. The mucosa comprises the thin muscularis mucosa, the lamina propria, and the columnar epithelium; it is thrown into folds and is covered by finger-like villi to increase the digestive and absorptive surface area. D, Enterocytes, which are shed from the villus tip but are continually replaced through division of crypt cells, are the site of nutrient digestion and absorption. Goblet cells secrete protective mucus. Water-soluble nutrients pass into the rich capillary network of the lamina propria, and fat is passed as chylomicrons into the lacteals. Immunocytes in the lamina propria are involved in maintaining tolerance to luminal antigens. E, The luminal membrane of the enterocyte is thrown into processes called microvilli, which increase the luminal surface area. Tight junctions between enterocytes maintain epithelial integrity. Absorbed nutrients are passed from the enterocyte into the intercellular space for distribution to the body. F, Schematic of a microvillus showing digestive hydrolases anchored in the phospholipid cell membrane and protruding into the intestinal lumen. Carrier proteins in the membrane are believed to act as “pores,” shuttling nutrients across the membrane by means of conformational changes in their structure often induced by sodium influx at the expense of energy utilization through Na/K-adenosine triphosphatase (ATPase) on the basolateral membrane.
(From Hall EJ: Small intestinal disease. In: Gorman NT, editor: Canine Medicine and Therapeutics, ed 4, Oxford, UK, 1998, Blackwell Science, p 488.)
Duodenum
The first part of the SI, the duodenum, comprises approximately 10% of its total length. It passes from the pylorus dorsally and to the right, at the level of the ninth intercostal space, and is immobilized by the hepatoduodenal ligament. It then turns caudally into the descending duodenum in contact with the right flank, turning again at the caudal flexure near the pelvic brim. It is in close association with the common bile duct and the head and right limb of the pancreas, which lie in its mesentery.
The common bile duct and one pancreatic duct enter the duodenum via the major papilla. In dogs an accessory pancreatic duct often enters at a minor papilla more distally and slightly more ventrally (Figure 57-2, A ), but there is a range of variations in the actual number of ducts and their drainage pattern from the pancreas (see Chapter 60). The papillae are notable endoscopic landmarks in dogs, but may not be obvious in cats.
Figure 57-2.
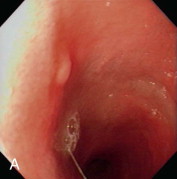
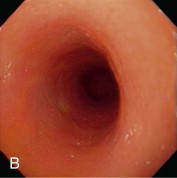
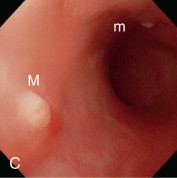
Videoendoscopic appearance of the normal upper small intestine.
A, The major duodenal papilla (M) in the duodenum of the dog is the site of entry of the common bile duct and major pancreatic duct. The minor duodenal papilla (m) is seen in some but not all dogs distal to the major papilla and approximately 100 degrees clockwise from it. B, Normal descending duodenum in a dog; the distal flexure is visible in the distance. C, Peyer patches (lymphoid aggregates) in the duodenum appear as pale oval depressions along the antimesenteric border of the descending duodenum.
(Reprinted with permission from Lhermette P, Sobel D: BSAVA Manual of Canine and Feline Endoscopy and Endosurgery. Gloucester, UK, 2008, BSAVA Publications.)
The distal duodenal flexure, where the duodenum courses to the left side of the abdomen (see Figure 57-2, B) is often at the limit of the reach of a standard 1-meter gastroscope, except in cats and small dogs. In dogs the antimesenteric side of the duodenum is marked by a line of whitish, mucosal depressions signifying the presence of specialized lymphoid areas, the Peyer patches (see Figure 57-2, C). Secretory Brunner glands and annular mucosal folds are features of the human proximal duodenum, but are not present in dogs and cats. After the distal duodenal flexure, the ascending limb of the duodenum crosses the midline and ends at the level of L6 close to the root of the mesentery near the left kidney, with a mesenteric attachment to the colon, the duodenocolic ligament.
Jejunum
The middle part of the SI, the jejunum, arises as an indistinct structural and functional transition from the duodenum and forms the majority of the SI. The jejunum is loosely suspended in the middle of the peritoneal cavity in a dorsal mesentery, forming mobile loops, and is potentially palpable throughout its length in cooperative and nonobese patients. The mesentery is normally a continuous sheet that is folded to allow the SI to loop within the peritoneal cavity, unlike in humans where segments of the duodenum (and colon) are retroperitoneal. The mesentery carries the vascular, lymphatic, and nervous connections between the SI and the rest of the body.
Defects in the mesentery, most often traumatic in origin, can allow internal hernia formation and small intestinal incarceration. An outpouching of the dorsal mesentery of the stomach forms the greater omentum. This structure functions as a protective, immunologic organ, having the ability to migrate to sites of intraperitoneal inflammation and potentially prevent leakage from an intestinal perforation and seal off pockets of infection.
Ileum
Approximately the last 30 cm of the SI comprises the ileum. The transition from jejunum to ileum in humans is based on changes in diameter, color, and the presence of Peyer patches; in dogs and cats the distinction has been arbitrarily based on the extent of attachment of the ileocolic ligament. In fact the basic structure of the ileum is no different from the rest of the SI and it is not clearly demarcated microscopically from the jejunum. However, it does have some unique functional characteristics, such as the absorption of bile salts and cobalamin. It is also a site of dense lymphoid follicle expression. Meckel diverticulum, a remnant of the embryonic omphalomesenteric duct, found in the ileum of approximately 2% of people and a potential source of bleeding, obstruction, intussusception, and volvulus, is not reported in dogs and cats. The ileum ends at the ileocolic valve in close association with the cecocolic junction.
Blood Supply, Lymphatic Drainage, and Innervation
The blood supply to the proximal duodenum is from the celiac artery. Its cranio-pancreatico-duodenal branch anastomoses with the caudo-pancreatico-duodenal branch of the cranial mesenteric artery. The latter is the major blood supply to the remainder of the SI and proximal colon, anastomosing distally with the caudal mesenteric artery. It forms an arcade along the mesenteric border of the jejunum and ileum, with a short antimesenteric ileal branch. Its branching nature is an important consideration when assessing the viability of lengths of SI during surgical resection and end-to-end anastomosis.
The venous drainage of the whole SI is ultimately to the liver via the hepatic portal vein. Multiple embryonic vessels linking portal venous drainage and the systemic venous system (i.e., via ovarian veins, caudal vena cava, and esophageal veins) exist but only become functional shunting vessels if there is chronic portal hypertension as a consequence of liver disease.
Lacteals in the villi drain via intestinal lymphatics in the mesentery to the mesenteric lymph nodes and then the cisterna chyli and on to the thoracic duct. Vagal and sympathetic innervation coordinate with the intrinsic enteric nervous system and enteric hormones to regulate SI motility and function.
Intestinal Compartments
Microflora
The microflora of the SI is an integral part of its structure and function. There is a gradual increase in bacterial numbers and a shift from aerobic to anaerobic organisms progressing distally down the SI. Chapter 2 provides a more detailed description of the composition of the microflora and its interaction with the mucosa.
Mucosa
The SI mucosa performs the intestinal barrier and absorptive functions, and is comprised of an intestinal epithelium covering the lamina propria that hosts the local mucosal immune system, and is surrounded by the submucosa and the outer muscle layers.
One of the most important structural modifications of the mucosa is a vast increase in its surface area relative to the size of the animal, with an almost 600-fold increase compared with the basic tubular structure of the intestine. The surface area of the human intestine has been estimated at 175 m2, and although the adult human intestine is longer than in even the largest dog, the villi in cats and dogs are almost twice as long (approximately 1 mm) compared with those of humans. The increase in surface area is created by folds in the mucosal wall (tripling the surface area), villus projections into the intestinal lumen (providing an approximate 10-fold increase), and microvilli on the surface of each epithelial cell (providing a further 20-fold increase in area) (see Figure 57-1, C). Diseases causing villus atrophy or even just microvillus damage are likely to produce profound malabsorption and diarrhea.
Gut-Associated Lymphoid Tissue
The GI tract is the largest immunologic organ in the body, and the SI comprises a large component of the mucosal immune system. Within the SI, the Peyer patches (see Figure 57-2, C) act as inductive sites and are covered with a specialized epithelium containing microfold (M) cells, which sample luminal antigens. Activated lymphocytes migrate via mesenteric lymph nodes to the circulation, from where they home to their effector sites, the lamina propria and epithelium. Chapter 3 details the structure and role of the gut-associated lymphoid tissue.
Microstructure
An identical, basic, tubular, cross-sectional structure is present throughout the length of the SI (see Figure 57-1, C): the external serosa surrounds the muscularis, submucosal and mucosal layers which are present throughout, and can be detected ultrasonographically (Figure 57-3 ).4, 5, 6, 7, 8, 9, 10 A very narrow hyperechoic interface between the lumen and mucosal surface is usually visible above the four true layers: (a) a slightly hypoechoic mucosa, (b) hyperechoic submucosa, (c) hypoechoic muscularis, and (d) brightly hyperechoic serosa.
Figure 57-3.
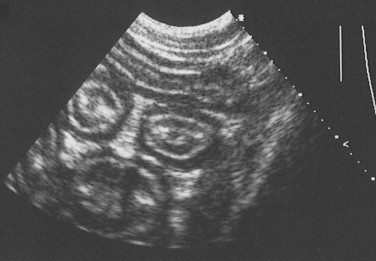
Ultrasound Image of the Small Intestine.
Abdominal ultrasound image showing transverse image of three loops of bowel in a dog, with normal layering of the small intestinal wall.
(From Ettinger SJ and Feldman EC, editors: Textbook of Veterinary Internal Medicine, ed 7, Philadelphia. 2010, Saunders, p 1541.)
Regional variations in the relative proportions of each layer reflect differences in the functions of the proximal, middle, and distal regions. The mucosa is thickest in the duodenum (normal dog ≤6 mm) and thinnest in the ileum (normal dog ≤4.7 mm). Variations in the microstructure also occur with species and age and within individual animals depending on their dietary intake, as well as disease. Submucosal Brunner glands are found in the human duodenum but not in dogs and cats. Loss of normal ultrasonographic layering is suggestive of neoplastic infiltration, and echogenic mucosal striations may indicate lymphatic dilation.
The mucosal layer is responsible for secretion and absorption as well as being a barrier to the luminal environment. The submucosa, between the muscularis mucosa and muscularis, provides connective tissue support and delivers blood vessels, nerves, and lymphatics. Within the muscularis, the outer longitudinal and inner circular muscular layers provide propulsive and segmental peristaltic contractions that mix chyme and ultimately propel it aborally. Neural plexuses are found between the muscle layers (the myenteric or Auerbach plexus) and in the submucosa (Meissner plexus), and communicate with all layers of the intestinal wall. They help coordinate intestinal motility and secretory activity, and even mucosal immune responses (see Chapters 1 and 3Chapter 1Chapter 3).
Mucosa
This is the most important layer of the intestine clinically. It is comprised of the epithelium and lamina propria overlying the muscularis mucosa, and is modified by gross folds and the villi (see Figure 57-1, C). The muscularis mucosa is a thin sheet of smooth muscle, from three to 10 cells thick, separating the mucosa from the submucosa. Smooth muscle branches within the villus lamina propria enable shortening and lengthening movements of the villi.
The lamina propria is a continuous connective tissue space bounded by the muscularis mucosa below and the epithelium above, and contains aggregates of lymphoid tissue, and nonaggregated immunocytes (see Chapter 3), enteric neurons, and blood and lymphatic vessels. A central lymphatic vessel (lacteal) within each villus drains chylomicrons into intestinal lymphatics and ultimately to the cisterna chyli.
Blood flow to a villus is provided by an arteriole that passes to the tip of the villus where it arborizes and forms a subepithelial capillary network that drains into veins. Crypts are supplied by separate arterioles and blood flow in the two regions can be controlled independently. Mucosal capillaries are fenestrated, and in conjunction with the lacteal, carry away protein-rich tissue fluid. Loss of epithelial integrity permits leakage of the protein-rich fluid and the development of a protein-losing enteropathy (PLE).
Crypt-Villus Unit
A group of crypts and their associated villus comprise the functional unit of the SI (see Figure 57-1, D).11 Crypts are continually replenished by cell division, producing undifferentiated epithelial cells. It is estimated that there are between four and 40 stem cells per crypt in the adult intestine, with further division of daughter cells occurring as the cells pass up the crypt. As the crypt cells pass through a maturation zone they undergo a final division and differentiate into immature epithelial cells. The predominant epithelial cell type is the enterocyte, but as a number of crypts may supply the enterocytes to one villus, each villus epithelium may consequently represent a polyclonal cell population.
Mucosal Epithelium
The intestinal surface is covered by a monolayer of polarized epithelial cells; their luminal surface is structurally and functionally distinct from their basolateral membrane.12, 13, 14, 15, 16, 17, 18 The epithelial basement membrane is readily permeable to nutrients, but has an important role as the structural matrix on which the epithelium grows. It expresses glycoproteins, called laminins, that interact with integrins, transmembrane recognition molecules expressed by epithelial cells. These interactions promote cell adhesion, growth, polarization, and differentiation. Enterocyte differentiation during migration up the villus may be programmed, but is likely also to be modulated by the expression of different integrins at different sites on the crypt–villus axis. Communication between epithelial cells is mediated by E-cadherin, a transmembrane molecule, linked to intracellular catenins, proteins that transmit signals to the actin cytoskeleton and to intracellular growth control pathways.
A mucosal barrier is formed by the intestinal epithelium (Box 57-1 ). This barrier depends on intercellular tight junctions between enterocytes, encircling their lateral aspects and excluding antigens and bacteria. Effete enterocytes are shed from the villus tip by a mechanism that maintains the mucosal barrier (see Figure 57-1, D). Studies in rodents suggest intercellular bridges develop between neighboring enterocytes below the effete cell before it is shed, thus maintaining mucosal integrity. However, epithelial integrity is likely to be altered in some intestinal diseases, and the integrity of the tight junctions is actually least in the crypts, where fluid secretion occurs. There is an association of cryptal lesions with the development of PLEs.
Box 57-1. Components of the Intestinal Mucosal Barrier.
Protein denaturation by gastric acid
Protein degradation by proteolytic enzymes and bacteria
Clearance of waste by peristalsis
Unstirred water layer
Surface mucus layer
Secretory immunoglobulin A
Enterocyte microvillus membrane
Epithelial tight junctions
Mucosa-associated lymphoid tissue
Crypt cells have a potent secretory capacity and the crypts are the site of most mucosal fluid secretion. As enterocytes migrate to the villus tip, maturation involves loss of secretory activity and the expression of digestive and absorptive molecules in the apical (luminal) cell membrane. Some enterocytes undergo stochastic (random) cell death, but the majority undergoes apoptosis via a caspase-dependent process, and exfoliates at the tips of the villi. The duration of migration from crypt to villus tip is believed to be 3 to 5 days in dogs and cats. More rapid transit may occur in diseases where cells are lost and compensatory crypt activity occurs, but the new enterocytes tend to be functionally immature.
Enterocytes predominate in the epithelium, representing approximately 80% of all cells, with interspersed mucus-secreting goblet cells. Goblet cell density in the SI mucosa varies, being highest in the ileum. These cells secrete protective mucus and some novel clover leaf–shaped peptides (trefoil peptides) that act as growth factors. Paneth cells, a population of cells found in some species below the proliferation zone in crypts and that secrete antibacterial peptides, are not recognized in dogs or cats. Endocrine- and paracrine-secreting cells (synonyms: enteroendocrine, enterochromaffin, argentaffin, argyrophil cells) are also present in the mucosal surface layer, and have important trophic and functional activities.
In addition to locally produced growth factors, a variety of luminal and humoral factors act as physiologically active growth regulators. Receptors for epidermal growth factor (EGF) are found on the luminal and basolateral surfaces of enterocytes, suggesting that they may respond to bloodborne EGF and to EGF secreted into the lumen by salivary and pancreatic tissue or delivered in milk. Transforming growth factor (TGF)-α, a polypeptide related to EGF and expressed throughout the mucosa, has growth regulatory properties. However, EGF and TGF-α are also probably important in repair of damaged epithelium as they stimulate repair without fibroblast activity, unlike TGF-β, which inhibits epithelialization and stimulates fibroplasia, and is important in deeper wound repair.
In the Peyer patches, enterocytes overlying lymphoid aggregates are modified into follicle epithelium and M cells, probably in response to signals from underlying lymphoid cells. The M cells sample the luminal contents and help present antigen to the mucosal immune system (see Chapter 3).
Enterocytes
Enterocytes contain the intracellular organelles, such as mitochondria, lysosomes, and endoplasmic reticulum, common to all cells, and which support normal cellular functions. However, enterocytes also perform specific digestive and absorptive functions.19, 20 Enzymes expressed on the surface of enterocytes perform terminal digestion of polysaccharides and peptides in conjunction with luminal hydrolysis of food polymers by pancreatic enzymes. The enterocytes then absorb the simple nutrients. These functions depend on the polarity of the enterocyte, involving a specialized portion of the cell membrane on the luminal surface, the microvillar membrane (MVM). The microscopic appearance of the MVM is the basis of its alternative name, the “brush-border” (see Figure 57-1, E and F). It consists of thousands of parallel cylindrical processes (microvilli) bearing the digestive enzymes and specific carrier proteins.
The MVM is a phospholipid bilayer that has specific proteins inserted into it. Enzymes responsible for the terminal stages of carbohydrate and protein digestion are usually anchored in the MVM by a small hydrophobic terminal and have an active site exposed to the intestinal lumen (see Figure 57-1, F). Specific carrier proteins traverse the MVM or basolateral membrane and, through conformational changes, shuttle nutrients into and out of the enterocyte across the cell membrane. The maximal brush-border enzyme and transport activities are expressed in the mid-villus region. Diseases damaging enterocytes often accelerate cell production and the more immature enterocytes are not as effective functionally.
Brush-border enzyme activities are highest in the proximal SI and decline in an aborad gradient. Digestive enzymes, especially disaccharidases and transport proteins, may be inducible in response to changes in the composition of the diet. This has been shown in dogs but not in cats, perhaps reflecting the obligate carnivore status of cats. Indeed dogs, which are omnivorous, show increased sucrase and maltase in response to increased dietary carbohydrate and, conversely, when fed a cereal-free diet develop reduced activities of brush-border sucrase and maltase but not lactase. Thus a sudden change in diet in dogs may cause diarrhea through transient intolerance until either existing enterocytes upregulate expression of specific enzymes and carriers, or new enterocytes expressing induced proteins differentiate, thus rendering the diarrhea self-limiting.
Enterocyte metabolism is geared toward the production of brush-border proteins and the transfer of nutrients and water from the lumen to the blood. Basolateral cell membranes export sodium from the cell via an energy-dependent N+-K+-ATPase. Water can follow osmotically, or compensatory sodium influx at the luminal surface can drive carrier-mediated nutrient absorption. Natural inhibition of glycolysis through expression of an alternate phosphofructokinase isoenzyme in enterocytes facilitates the transfer of glucose from the lumen to the blood. Gluconeogenesis is also inhibited, and so enterocytes can utilize ketone bodies. However their major energy source is actually glutamine (Figure 57-4 ). A surge in enterocyte glutamine metabolism during digestion is probably partly responsible for the postprandial rise in blood ammonia seen in some patients with hepatic dysfunction.
Figure 57-4.
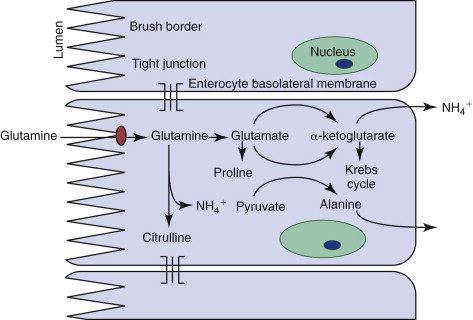
Metabolism of glutamine by enterocytes, and one potential mechanism for postprandial increases in endogenous ammonia.
Both major nutrients for enterocytes (glutamine and ketone bodies) are largely derived from the lumen, which helps explain the decline in villus structure, epithelial integrity, immune function, and absorptive function in starvation and anorexia. Consequently, attempting to maintain enteral nutrition, often by using glutamine-containing products, may be of clinical benefit.
Digestive and carrier proteins are synthesized by enterocytes and inserted in the MVM. This mechanism has been demonstrated for the synthesis of the enzyme complex of sucrase–isomaltase in pigs, but a similar process is likely to occur for this and other enzymes in dogs and cats. The sucrase–isomaltase complex is synthesized as a single polypeptide by ribosomal translation of its messenger RNA (mRNA). A terminal signal peptide extension that is ultimately cleaved, directs the intracellular trafficking of the protein from the ribosome to the endoplasmic reticulum and Golgi apparatus, where glycosylation of the protein backbone occurs. The glycosylated polypeptide is directed to the brush-border where it is inserted. It then “flips” across the apical membrane so that the active sites are on the luminal surface, and the polypeptide is anchored in the membrane by an N-terminal hydrophobic chain. The parts of the exteriorized polypeptide containing the sucrase and isomaltase activity are cleaved by pancreatic proteases but remain in close association, and form a dimer with another sucrose–isomaltase molecule.
As enterocytes migrate to the villus tip, enzymes are cleaved from the brush-border by bacterial and pancreatic proteases and are released into the lumen where they comprise solubilized enzyme activities, commonly called digestive juice. However, this liquid, the succus entericus, is not a true intestinal secretion.
Submucosa
Beneath the muscularis mucosa, the submucosa contains a heterogeneous population of cells: lymphocytes, plasma cells, macrophages, eosinophils, fibroblasts, and mast cells within a connective tissue matrix. An intricate network of blood vessels, nerve fibers, ganglia and interstitial cells of Cajal (Meissner plexus), and lymphatics supply the mucosa and muscularis.
Muscularis
Two muscle layers, the outer longitudinal and inner circular layers, encircle the submucosa. The intermuscular plane is a connective tissue layer bearing the myenteric (Auerbach) neural plexus. Ring contractions by the circular muscle and sleeve contractions by the longitudinal muscle may be tonic or rhythmic, and intestinal movements may be standing or migrating, allowing mixing and propulsion. Contraction of the muscle layers is coordinated by the enteric nervous system to produce peristaltic and segmental movements, with interstitial cells of Cajal acting as pacemakers. Chapter 1 details how intestinal motility is integrated with other functions of the SI.
Serosa
This is a single layer of mesothelial cells surrounding the intestine, and forms the visceral peritoneum.
Small Intestinal Function
The basic functions of the SI, that is digestion, absorption, and elimination, occur as a result of complex intercellular interactions between epithelial cells, immune cells, mesenchymal and neuronal cells and with luminal nutrients and microbes.21 The SI is also the largest immunologic organ in the body, interacting with the intestinal microbial flora and a diverse range of food antigens (see Chapters 2 and 3Chapter 2Chapter 3, respectively).
Digestion
To be transported across the MVM, major dietary constituents must be hydrolyzed from their initial polymeric structure into monomers. This digestive process is achieved within the SI lumen by mechanical disruption (in conjunction with bile salt emulsification of fats) that allows enzymatic hydrolysis of polysaccharides, proteins, and triglycerides.22
The SI provides the optimum environment in terms of solute, temperature, pH, and mixing for the actions of bile salts and digestive enzymes, but most enzymes are secreted by the pancreas, and exocrine pancreatic insufficiency (EPI) is a major cause of malabsorption. The brush-border peptidase enterokinase (enteropeptidase) is important in the initial activation of pancreatic trypsin from trypsinogen by cleaving a terminal octapeptide, trypsinogen activation peptide, from the native protein.
Only terminal digestion of oligomers need normally be performed by brush-border enzymes. However, brush-border activities can partially compensate for the lack of secreted proteases and amylase in EPI, with at least 40% of dietary protein still being hydrolyzed, although severe fat maldigestion persists. Even with significant diffuse SI mucosal disease there is usually sufficient reserve capacity to enable adequate digestion of starch. However, early estimates of a 10-fold reserve capacity of digestive and absorptive activity have been refuted. The “reserve capacity” that is called into action after intestinal resection probably represents not only increases of brush-border protein expression, but also compensatory hypertrophy of the remaining tissue, as it is known to take months to reach maximal effect (see “Short Bowel Syndrome” section). Capacity appears to be regulated according to physiologic demand, and probably does not normally exceed twofold, but it is relevant that it can be modified in response to dietary change.
Carbohydrate
Starch and glycogen are the major carbohydrates in the diet and must be hydrolyzed completely to glucose for absorption (Figure 57-5, A ). There is no salivary amylase activity in dogs and cats, and these complex carbohydrates are hydrolyzed by pancreatic α-amylase. Straight-chain starch molecules (amylose) are split to maltose, maltotriose, and some glucose. Branched-chain starch molecules (amylopectin) and glycogen are also hydrolyzed to the same products, except that the branched parts of their molecules remain as α-limit dextrins as their 1,6-glycosidic bond cannot be hydrolyzed by α-amylase. The digestion products of α-amylase are subsequently hydrolyzed, particularly by brush-border maltase (glucoamylase) and isomaltase (α-dextrinase). The brush-border enzyme trehalase hydrolyzes the 1,1 link in the fungal sugar trehalose, but is not expressed in cats.
Figure 57-5.
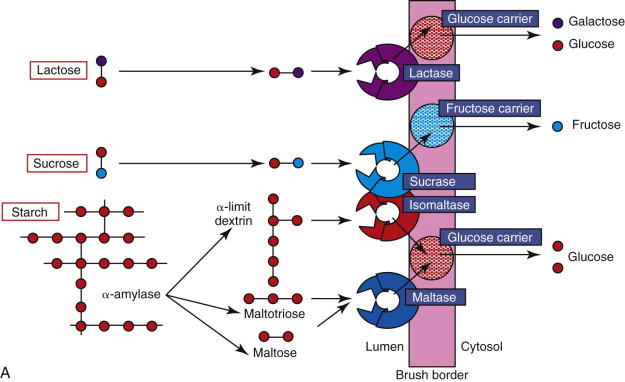
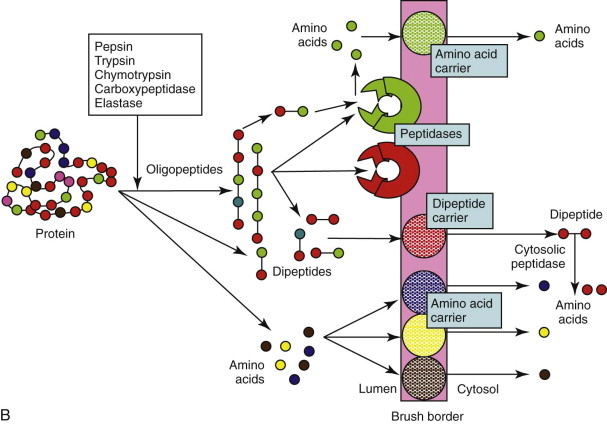
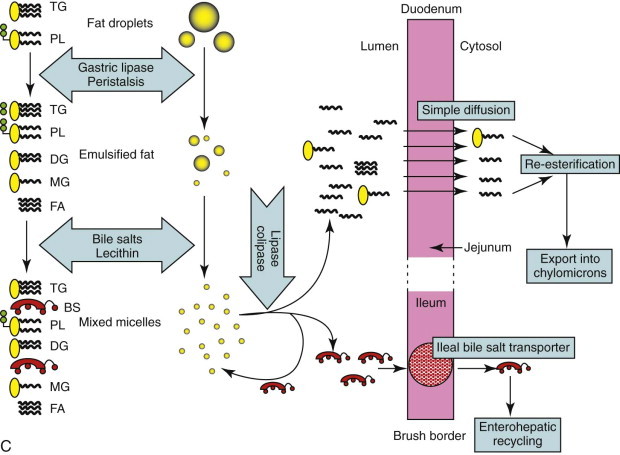
Diagram of the digestion and absorption of (A) carbohydrate, (B) protein, and (C) fat.
BS, bile salts; DG, diglyceride; FA, fatty acids; MG, monoglyceride; PL, phospholipids; TG, triglyceride.
(Adapted from Batt RM: The molecular basis of malabsorption. J Small Anim Pract 21:555, 1980.)
Sucrose is an unusual constituent of canine and feline diets unless semimoist pet foods or human foods are fed. It is hydrolyzed directly at the brush-border to glucose by sucrase, part of the sucrase–isomaltase brush-border enzyme complex. Congenital sucrase–isomaltase deficiency is a rare genetic defect of man, but has not been recorded in small animals. Sucrase activity in cats is lower than dogs (Table 57-1 ), probably reflecting the average composition of their diets.
Table 57-1.
Jejunal Brush-Border Disaccharidase Activities (U/mg Protein) Reported in Dogs and Cats
| Reference | Sucrase | Maltase | Lactase |
|---|---|---|---|
| Cats | |||
| Hore, Messer 196837 | 2.8 | 20 | 0.6 to 1.2 (U/mg wet weight) |
| Kienzle 199338 | 17 ± 23 | 102 ± 58 | 7 ± 8 |
| Dogs | |||
| Hore, Messer 196837 | 0.87 | 4.2 | 0.33 (U/mg wet weight) |
| Levanti et al. 197839 | ~80 | ~400 | ~30 |
| Noon et al. 197740 | 57 ± 2 | 240 ± 13 | 26 ± 1 |
| Kienzle 198841 | 33 | ||
| Batt et al. 198442 | 67 ± 5 | 329 ± 25 | 33.5 ± 4 |
| Hall, Batt 199043 | |||
| normal diet | 78 ± 7 | 398 ± 26 | 23 ± 1 |
| cereal-free diet | 50 ± 8 | 252 ± 32 | 20 ± 2 |
Lactose is found almost exclusively in dairy products and its hydrolysis to glucose and galactose by brush-border lactase is nutritionally most important in the nursing animal. At weaning, activities of lactase begin to decline, especially in cats, and adult animals may exhibit lactose intolerance if fed excess milk. This mirrors the age-related downregulation of lactase expression seen in certain human races. If animals have underlying SI disease, dairy products should be avoided as marginal lactase activities will be reduced even further. Absolute congenital lactase deficiency, as seen rarely in humans, has not been demonstrated in cats or dogs.
Protein
Digestion of proteins follows a similar overall pattern to carbohydrate digestion (see Figure 57-5, B), and the amounts of pancreatic enzyme secreted and mucosal peptidases expressed are influenced by the protein content of the diet. Digestion is initiated by acidic denaturation and the proteolytic activity of pepsin in the stomach. Luminal digestion under a more neutral pH is continued in the SI by pancreatic proteases (trypsin, chymotrypsin, elastase, and carboxypeptidase), which are initially secreted as inactive proforms, and are subsequently activated by enterokinase and trypsin. Luminal proteolysis results in a mixture of oligo-, tri-, and dipeptides as well as free amino acids. Oligopeptides are subsequently hydrolyzed by brush-border peptidases, which have some selectivity for particular amino acid residues. However, there is considerable overlap in specificity, and a selective deficiency of aminopeptidase N reported in dogs is of no clinical consequence. Furthermore, any tri- and dipeptides can still be absorbed on a brush-border carrier. Theoretically a deficiency of enterokinase could cause protein malabsorption through failure of trypsin activation, but this has never been documented in dogs and cats, and trypsin autoactivation would probably still occur.
Lipid
Fat digestion is completed entirely within the GI lumen by secreted enzymes and bile salts. Partial digestion is begun in the stomach by the action of gastric lipase secreted by gastric epithelial mucus cells. Subsequent mixing of the fat emulsifies it into small droplets. Further mixing with bile and pancreatic juice results in the formation of mixed micelles, which are approximately 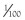 the size of the smallest fat droplet and solubilize approximately 1000 times more fatty acids. At the surface of mixed micelles, triglyceride is hydrolyzed by pancreatic lipase to di- and monoglycerides and free fatty acids (Figure 57-6
). Maximal lipase activity in the gut lumen is dependent on a protein cofactor, colipase, which is secreted by the pancreas as inactive procolipase. There is some reserve capacity for fat digestion if pancreatic function is normal, and a fat-rich diet, especially one rich in unsaturated fatty acids, also stimulates increased pancreatic lipase secretion. However, neuroendocrine mechanisms initiated by the presence of fat in the duodenum and ileum, control the rate of gastric emptying and hence the rate of fat delivery. Thus a fat-rich diet or intestinal fat malabsorption delays gastric emptying.
the size of the smallest fat droplet and solubilize approximately 1000 times more fatty acids. At the surface of mixed micelles, triglyceride is hydrolyzed by pancreatic lipase to di- and monoglycerides and free fatty acids (Figure 57-6
). Maximal lipase activity in the gut lumen is dependent on a protein cofactor, colipase, which is secreted by the pancreas as inactive procolipase. There is some reserve capacity for fat digestion if pancreatic function is normal, and a fat-rich diet, especially one rich in unsaturated fatty acids, also stimulates increased pancreatic lipase secretion. However, neuroendocrine mechanisms initiated by the presence of fat in the duodenum and ileum, control the rate of gastric emptying and hence the rate of fat delivery. Thus a fat-rich diet or intestinal fat malabsorption delays gastric emptying.
Figure 57-6.
Mechanisms of diffusion.
Pancreatic phospholipase A2 is secreted in an inactive form, and once activated in the intestinal lumen hydrolyzes phospholipid to lysophospholipids. Finally, pancreatic cholesterol esterase deesterifies cholesterol. After fat absorption, the bile salts may form further mixed micelles, but ultimately are reabsorbed by a specific sodium-linked cotransporter in the ileum and recycled from the portal blood back into bile.
Nucleotides
Little is known of the digestion of dietary nucleotides and hydrolysis of nucleic acids released from exfoliated enterocytes. Ribonuclease and deoxyribonuclease are present in pancreatic secretions, and there is a common sodium-dependent brush-border carrier for the uptake of purines and pyrimidines.
Absorption
Digested Nutrients
Simple sugars, amino acids and oligopeptides, and fatty acids and other lipids are delivered to the body across the mucosal barrier and then via the lymphatics or bloodstream.23, 24 Uptake occurs by passive diffusion or by active or facilitated carrier-mediated transport mechanisms (see Figure 57-6). Endocytosis of small, antigenic peptides is of no nutritional significance, but is involved in the neonatal absorption of colostral antibodies, and is crucial to the mucosal immune response.
The products of fat digestion are absorbed by passive diffusion from mixed micelles across the MVM and ultimately lipoproteins are passed into lacteals. The limiting factors, assuming normal pancreatic function, are the intestinal surface area and lymphatic functionality, and so villus atrophy and lymphangiectasia are likely to cause malabsorption of fat.
Mechanisms of Absorption
Passive Diffusion
Lipid-soluble products of digestion do not need a specific carrier mechanism to be absorbed across the mucosal barrier, and can bypass passive diffusion by “dissolving” in the brush-border membrane. This absorptive process obeys the Graham Law of Diffusion, being proportional to the concentration gradient across the membrane, nonsaturable, and limited only by the surface area of the membrane. Diffusion back across the brush-border is prevented by reesterification of free fatty acids within the enterocyte.
Carrier-Mediated Transport
Some small, nonpolar, water-soluble molecules (and perhaps water molecules) also appear to be absorbed by passive diffusion through “pores” in the mucosal membrane. The structure of these hypothetical pores is likened to that of ion channels in other membranes (i.e., small fixed channels through the membrane that function without a conformational change). Molecules with a molecular radius greater than 0.4 nm appear to be excluded, but larger, although numerically fewer pores may traverse tight junctions. Debate remains as to whether the predominant route is via an intracellular or paracellular route through tight junctions.
Water-soluble nutrient molecules that are too large to diffuse via the “pore route” and are insoluble in the lipid MVM must cross the brush-border on specific carrier proteins that bridge the membrane. Conformational changes in the carriers are thought to shuttle the substrate molecule across. The products of carbohydrate and protein digestion enter by this process. Transport proteins are usually highly specific and may only carry the l or d isomer of a molecule, but competitive inhibition with related solutes may occur. The number of carrier molecules in the mucosa is finite, so that the process is saturable, and although the expression of the carriers may be inducible, dietary overload is likely to cause transient intolerance and diarrhea.
Active transport of substrates across the MVM into the enterocyte is usually against a concentration gradient and energy must be expended to drive the process (see Figure 57-6). Usually the uptake of the nutrient is linked to the entry of sodium down its electrochemical gradient, with energy expenditure by a N+-K+-ATPase on the basolateral membrane of the enterocyte pumping sodium back out of the cell.
Facilitated transport is the carriage of substrates by a transport protein across the MVM, down a concentration gradient without energy expenditure (see Figure 57-6). Some sugars, oligopeptides, and folate are absorbed by this process. The number of carriers is finite, and the process saturable and subject to competitive inhibition.
Endocytosis
Small antigenic peptides may be engulfed nonspecifically within endocytotic vesicles of epithelial cells. The amounts absorbed by this route are negligible from a nutritional standpoint, but this sampling of luminal contents is crucial to the mucosal immune response (see Chapter 3). Receptor-mediated endocytosis enables the uptake of small amounts of a specific intact nutrient and is the mechanism of cobalamin absorption.
Nutrient Absorption
Carbohydrate
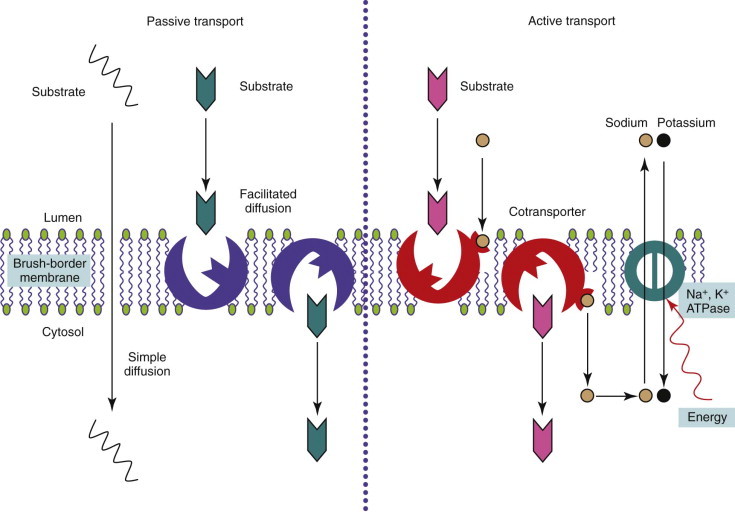
The main product of carbohydrate digestion, glucose, is absorbed by active transport on a stereo-specific carrier that recognizes a d-pyranose structure with a C-2 hydroxyl group. Glucose is cotransported with sodium; the energy required for the coupling is provided through the entry of sodium down a concentration gradient but with the basolateral N+-K+-ATPase reexporting sodium against the concentration gradient. The carrier molecule in the brush-border has been identified in many species, including dogs and cats, as the sodium-glucose cotransporter protein (SGLT1; Figure 57-7 ). This molecule has the highest affinity for glucose, but it is also the carrier for galactose. Indirect evidence for this is shown by the inability to absorb either sugar in people with glucose–galactose malabsorption in whom a single amino acid mutation (Asp28 → Asn28) in the SGLT1 protein has been identified. Glucose and galactose thus may exhibit competitive inhibition, but glucose is the major substrate. There is circumstantial evidence for another aldohexose carrier in cats.
Figure 57-7.
Diagram of the absorption of monosaccharides by enterocytes. GLUT, Glucose transporter; SGLT, sodium-glucose co-transporter protein.
Facilitated transport of glucose across mammalian cell membranes is performed by a family of facilitated glucose transporters (GLUTs) with different isoforms found in different tissues. One member of this family, GLUT2, is found on the basolateral membrane of enterocytes, where it shuttles glucose, galactose, and fructose out of the enterocyte by facilitated diffusion (see Figure 57-7). GLUT2 is absent from the brush-border, and so a mechanism exists for active transport of glucose across the MVM into the enterocyte by SGLT1 and facilitated transport into the body by GLUT2. Most of the glucose is not used within the enterocyte, because of expression of a phosphofructokinase isomer that directs metabolism away from glycolysis.
Another member of the facilitated glucose transporter family, GLUT5, is found on the brush-border. It shares homology with other family members but actually allows facilitated diffusion of fructose; it is not even competitively inhibited by glucose. In humans, GLUT5 is also probably the site of d-xylose absorption, as both fructose and xylose absorption are unaffected in glucose–galactose malabsorption. However, the mechanism of d-xylose uptake is species dependent, and evidence for facilitated diffusion in dogs and cats has largely been extrapolated from humans. Nevertheless, fructose uptake in cats is low, and d-xylose absorption is equally low. This may be one reason why the xylose absorption test is unhelpful in cats. However, potential fructose malabsorption has little clinical relevance in cats as the feline diet likely contains little fructose.
Although dietary carbohydrates must be hydrolyzed to monosaccharides to be absorbed and be nutritionally useful, a small but measurable amount of disaccharide can cross the brush-border, probably through leaky tight junctions. This is of no nutritional significance, but increased uptake and subsequent urinary excretion of disaccharides can be a marker of increased intestinal permeability when damaged.
Protein
The products of protein digestion are absorbed on carriers that are stereo-specific for l-amino acids (Figure 57-8 ; also see Figure 57-5, B).25 Sodium-linked active transport is responsible for free amino acid uptake via one of four different carriers that have a variable degree of selectivity for neutral (Gly, Ala), acidic (Asp, Glu), basic (Arg, Lys), and imino (Pro, HO-Pro) amino acids. The cat has the highest rate of uptake of basic amino acids perhaps because it has an essential requirement for arginine.
Figure 57-8.
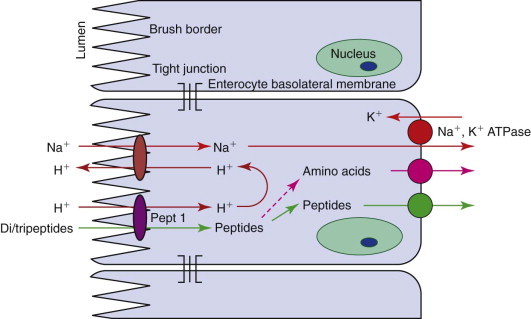
Diagram of the absorption of di- and tripeptides by enterocytes. Pept 1, a peptide carrier.
Traditionally, peptide uptake has been considered to be facilitated diffusion, with the concentration gradient being maintained by intracellular peptide hydrolysis, and only free amino acids being exported from enterocytes into the portal blood (see Figure 57-8). A single carrier for di- and tripeptides with no selectivity for their amino acid content has been demonstrated. However, in people this peptide carrier, Pept-1, is involved in the active influx of peptides, being linked to the influx of H+ down an electrochemical gradient. The protons are exchanged across the MVM with sodium, which is pumped out by the basolateral N+, K+ ATPase. A mixture of peptides and free amino acids is exported to the blood, but it appears that peptides are absorbed more readily than free amino acids. This has clinical significance as the inclusion of dipeptides in elemental diets has a theoretical advantage over simple amino acid solutions. This transport protein is also the carrier for peptidomimetic drugs such as β-lactams and angiotensin-converting enzyme inhibitors.
Lipid
The products of fat digestion are absorbed by passive diffusion from mixed micelles into lacteals (see Figure 57-5, C). The limiting factors, assuming normal pancreatic function, are the intestinal surface area and lymphatic functionality, and so villus atrophy and lymphangiectasia are likely to cause malabsorption of fat.
Generally, the products of fat digestion are reassembled within enterocytes to prevent rediffusion back out of the enterocyte. They are combined with synthesized lipoproteins for passage into the lymphatics as chylomicrons. However, medium-chain triglycerides (length = 8-12 carbon atoms) can be absorbed directly into the portal blood, and provide an alternative route for fat uptake when lymphatic flow is impaired, as in lymphangiectasia. However, evidence exists that a proportion of medium-chain triglycerides are absorbed into the lacteals as they can be found within the thoracic duct.
Fat-Soluble Vitamins
Dietary fat-soluble vitamins A, D, E, and K are solubilized in mixed micelles before passive diffusion across the brush-border. Fat malabsorption associated with inadequate amounts of bile salts (e.g., bile duct obstruction), lymphangiectasia, or severe villus atrophy is also likely to result in vitamin deficiency. This is clinically most relevant for vitamin K as its body stores are not large and, particularly in cats, can lead to vitamin K–dependent coagulation factor deficiencies.
Vitamin A (retinol) is ingested either as a dimer (beta-carotene) or as an ester that must be hydrolyzed by pancreatic esterases. Beta-carotene is absorbed directly from micelles, but retinol is insoluble and must be anchored by a binding protein before absorption. Subnormal serum vitamin A concentrations have been observed in dogs with EPI but no associated signs of deficiency have been reported. However, vitamin A supplementation has been recommended following surgery in animals subsequently treated with corticosteroids to aid wound healing, and would be particularly relevant in animals that have had surgical intestinal biopsies.
Vitamin D is absorbed from mixed micelles. It is important for calcium homeostasis, controlling calcium absorption from the gut, as well as renal excretion. Vitamin D malabsorption may be (partly) responsible for the reductions in serum ionized calcium and magnesium that are reported in protein-losing enteropathies, which cannot be due to reduced protein binding because of hypoalbuminemia.
Vitamin E (α-tocopherol) is absorbed from mixed micelles by passive diffusion, and passes into the lymphatics unchanged. Vitamin E deficiency has been reported in Beagles with severe malabsorption, and is associated with EPI and bacterial overgrowth in German Shepherd dogs.
Vitamin K is derived from dietary sources (K1) and synthesis by the enteric flora (K2). Vitamin K2 is probably absorbed in the ileum and colon. As well as bile salt deficiency, prolonged antibiotic usage may result in vitamin K deficiency.
Water-Soluble Vitamins
Water-soluble vitamins B and C are absorbed by a mixture of passive diffusion (e.g., pyridoxine [B6] and C), saturable facilitated transport (e.g., riboflavin [B2]), or active and facilitated transport (e.g., thiamine [B1]) in other species, but the mechanisms in cats and dogs are uncertain. The absorptive mechanisms for folic acid and vitamin B12 are more complex and important clinically as they may be helpful in determining the site and nature of intestinal disease.
Folic acid is present in adequate amounts in most commercial foods, but is also produced by the enteric flora. It is usually conjugated in a poorly absorbable polyglutamate form and must be hydrolyzed by folate deconjugase, a brush-border enzyme, before absorption. Folate (pteroyl monoglutamate) is absorbed by a carrier-mediated process at low luminal concentrations and by passive diffusion at high concentrations (Figure 57-9 ). After absorption folate is methylated in the cell to form methyltetrahydrofolate.
Figure 57-9.
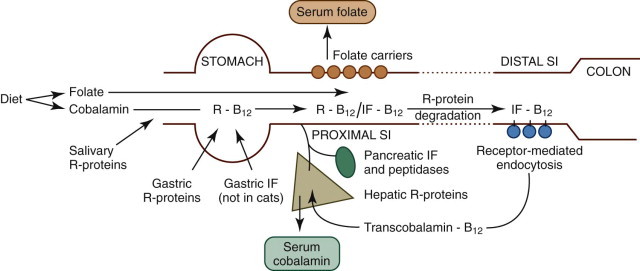
Diagram of the absorption of folate and cobalamin.
Folate is absorbed in the proximal SI by means of carrier-mediated diffusion. Dietary cobalamin is initially protected from digestion by R proteins, and is then absorbed in the ileum through receptor-mediated endocytosis bound to intrinsic factor (IF).
(From Ettinger SJ, Feldman EC, editors: Textbook of Veterinary Internal Medicine, ed 7, Philadelphia, 2010, Saunders, p 1528, Figure 270-1A.)
Vitamin B12 (cobalamin) is absorbed by receptor-mediated endocytosis in the ileum (see Figure 57-9), but the process is complex so that intact cobalamin is absorbed and potentially harmful analogues are excluded. Following ingestion, cobalamin is released from food in the stomach and then bound by R proteins (haptocorrins), which are nonspecific binding proteins of salivary and gastric origin. At acidic pH, cobalamin has high affinity for R proteins, but on entering the more alkaline environment of the SI, R proteins bind cobalamin less avidly and undergo proteolysis. Thus cobalamin is transferred to another binding protein, intrinsic factor, which promotes cobalamin absorption in the ileum. The source of intrinsic factor is the stomach and pancreas in dogs and solely the pancreas in cats. Intrinsic factor–bound cobalamin complexes pass to the ileum until they bind specific receptors and are endocytosed. Cobalamin is passed into the portal blood where it is bound to a protein, transcobalamin 2, enabling it to enter tissues and to be reexcreted in bile. Inherited abnormalities of the cobalamin–intrinsic factor receptor in breeds such as the Giant Schnauzer and Border Collie cause selective cobalamin deficiency.
Minerals
Zinc and copper are absorbed via divalent cation transporters. There is competition for binding, while intracellular binding of copper by metallothionein is also part of the normal homeostatic mechanism, as the copper is trapped within effete enterocytes and shed.
Iron is absorbed by the duodenum and proximal jejunum both as heme and nonheme iron. Heme, found largely in meat, is better absorbed as it is unaffected by other dietary constituents or intraluminal factors. Gastric acid and chelation with mucopolysaccharide, ascorbate, and citrate help maintain iron in solution for absorption, and ferrous forms of nonheme iron are better absorbed than ferric forms. Ferrous iron is absorbed by an energy-dependent carrier mechanism, and is carried out of the enterocyte bound to transferrin. However, absorption is regulated, and if the body is iron-replete, crypt cells synthesize apoferritin, which traps iron in enterocytes as ferritin. When the enterocyte is exfoliated at the villus tip, the trapped iron is excreted back into the lumen.
Calcium absorption is complex and is modulated by systemic control mechanisms. In particular, vitamin D stimulates the activity of calcium-binding protein within enterocytes. However, uptake of calcium at the brush-border is an active process and is markedly influenced by the intraluminal pH and other substances such as organic and inorganic phosphates.
Motility
Slow wave, segmental, and peristaltic contractions of the SI are generated by the coordinated contraction of smooth muscle in response to spontaneous electrical activity.26, 27, 28, 29, 30, 31, 32, 33, 34 Interstitial cells of Cajal are considered coordinating/pacemaker cells and smooth muscle contraction is also modulated by coordinated neurohumoral and neurochemical molecule release. Many of these molecules are also involved in the regulation of intestinal secretion and absorption and the mucosal immune response, producing a complex coordinated process for the digestion of food.
Intestinal motility in the fasted state in dogs is characterized by three phases. A cycle comprising a quiescent phase (lasting approximately 1 hour), phase two comprising minor contractile activity (15 to 40 minutes), and then migrating myoelectric (motor) complexes (MMCs) (4 to 8 minutes), and is repeated approximately every 3 hours. The short MMC phase is a period of intense contractile activity that sweeps undigested food, secretions, desquamated cells, and bacteria down the intestine. This process is known as the intestinal housekeeper wave and is induced by motilin secretion. Erythromycin can stimulate motilin receptors and at low doses it can be prokinetic mimicking the MMC; higher doses overstimulate and may cause emesis. The pattern of intestinal motility in cats is somewhat different, but a migrating spike complex correlates with the MMC.
In the fed state, the pattern of motility is most similar to phase two fasting motility. Its duration is determined by the nature of the diet, with fats and fiber prolonging it. The presence of unabsorbed fat in the distal SI reflexly inhibits gastric emptying by neurohormonal mechanisms. This “ileal brake” mechanism may be the cause of the delayed gastric emptying that is typically seen in malabsorption. Feeding a patient with SI disease more than four times a day is unlikely to be helpful as the stomach will be trickle-feeding it anyway.
Segmental contractions slow intestinal transit and ensure mixing and digestion of nutrients, until peristalsis propels the ingesta onwards. Reduced segmental motility may lead to rapid transit, and decreased peristalsis delays transit, conditions manifesting clinically as diarrhea and ileus, respectively.
Secretion and Absorption of Water and Electrolytes
Intestinal secretion, a function of villus crypt cells, is believed to occur by passive flux of water osmotically following active transcellular chloride secretion into the intestinal lumen. Bacterial toxins can cause hypersecretion.
The ability of the intestine to absorb fluid and electrolytes varies according to the site, with water absorption becoming increasingly efficient distally. The net amount of fluid and electrolytes in the GI tract reflects a balance between absorption and secretion, with net absorption in health. However the daily fluxes are massive (approximately 2.7 L/day in a 20-kg dog) and the consequences of net loss is not only diarrhea but also rapid dehydration.
The absorption of water is passive and follows transport of solutes across the GI epithelium by one of three processes: passive absorption, active absorption, or solvent drag. The jejunum absorbs approximately 50%, the ileum approximately 75%, and the colon approximately 90% of the fluid volume presented to it, leaving approximately 2% in feces. This gradient in absorptive ability is a function of enterocyte pore size, membrane potential difference, and the type of transport processes associated with each intestinal segment. The site of the enterocyte on the villus is also important; villus enterocytes absorb, whereas crypt cells secrete.
Colonic absorption is important in SI disease because it helps to compensate for fluid losses and diarrhea will only occur if the colonic reserve capacity is overwhelmed. SI dysfunction may then present with signs of dysfunction of the large intestine because products from the SI, such as hydroxylated fatty acids and deconjugated bile acids, stimulate colonic secretion.
Control of Fluid Balance
Intestinal fluid balance is regulated by the neurocrine systems in the submucosal plexus as a largely autonomous process.35, 36 Acetylcholine and vasoactive intestinal polypeptide are major mediators of secretion, increasing intracellular calcium and cyclic adenosine monophosphate (cAMP), inhibiting neutral sodium and chloride absorption, and facilitating transcellular chloride efflux. Many bacterial agents exert their diarrheagenic effects by increasing cAMP in enterocytes. The principal regulators of absorption—noradrenaline, somatostatin, and opioids—lower intracellular cAMP and calcium concentrations and stimulate neutral NaCl absorption and thereby can have therapeutic antidiarrheal effects.
Mucosal Immunity
The mucosal immune system is a large and complex organ and is critical to the health of not only the intestine but the whole animal. Chapter 3 describes its structure and function in detail.
Diagnostic Evaluation
Edward J. Hall
General Approach
As most cases of small intestinal disease are acute, self-limiting, and nonfatal, they require only symptomatic support and not necessarily a definitive diagnosis. Medical investigation is more necessary if acute diarrhea is hemorrhagic, accompanied by systemic signs, and unresponsive to symptomatic treatment, although the extent of the medical workup may still be a balance between the severity of the illness and the cost of investigation. By definition, chronic diarrhea is not self-limiting, and an etiologic or histopathologic diagnosis is usually required to allow specific treatment.
The history and physical examination are crucial steps toward reaching a diagnosis and in some cases may be all that is required. Preliminary laboratory investigation may include collection of baseline data (e.g., hematology, serum biochemistry, urinalysis, and fecal examination), and are performed before more specific laboratory tests, imaging, and biopsy with histopathologic investigations are undertaken.
History
Background
Information about the recent activity of a patient is helpful in acute small intestinal disease as it may follow an episode of dietary indiscretion, particularly if the animal is allowed to roam or has contact with other animals with infectious gastroenteritis. A full dietary history is helpful when investigating chronic disease, especially when trying to formulate an exclusion diet. Travel information is important as regional infectious diseases, such as histoplasmosis, may move from one geographic site to another.
Clinical Signs
The presence of dehydration should be ascertained by clinical signs (e.g., skin tenting, tachycardia, dry mucous membranes, and depression) and addressed therapeutically, as dehydration can rapidly become life-threatening through profuse diarrhea. Metabolic acidosis and hypokalemia are common acid–base and electrolyte abnormalities, and a balanced electrolyte solution containing potassium can be administered while the medical investigation is being pursued.
The cardinal sign of small intestinal disease is diarrhea, but other signs (Box 57-2 ) may occur in the absence of diarrhea. Vomiting may be stimulated by intestinal distention or inflammation, and, indeed, vomiting is the most common manifestation of inflammatory bowel disease (IBD) in cats. Vomiting of blood may indicate gastric and/or upper GI bleeding, and copious volumes of bilious vomit are suggestive of upper GI obstruction. More distal obstructions of the SI may cause infrequent vomiting of a fecal-like material.
Box 57-2. Clinical Signs of Small Intestinal Disease.
Cardinal sign
-
•
Diarrhea
-
•
Increase in frequency, volume, and consistency of stool
Other signs
-
•
Vomiting
-
•
Weight loss and/or failure to thrive
-
•
Hematemesis
-
•
Melena
-
•
Altered appetite
-
•
Inappetence/dysorexia
-
•
Anorexia
-
•
Polyphagia
-
•
Coprophagia
-
•
Pica
-
•
Abdominal discomfort, pain
-
•
Abdominal distention
-
•
Borborygmi and flatus
-
•
Halitosis
-
•
Dehydration
-
•
Polydipsia (compensatory)
-
•
Ascites and edema
-
•
Shock
Anorexia can be a feature of intestinal disease, especially if there is sepsis, severe inflammatory, or extensive neoplastic disease. Some weight loss is expected in anorexic patients, but losses in the face of an increased appetite (or very rapid losses) are often an indication of malabsorption, and/or PLE. Severe SI disease is sometimes observed despite the absence of any diarrhea, which is testament to the colon's reserve capacity for the absorption of water (see Chapter 1).
Diarrhea caused by SI disease is usually of large volume and watery, but passed only a few times each day. Urgency and tenesmus are rare findings unless there is colonic involvement in a more diffuse disease process, or if colitis has developed secondary to the chronic passage of undigested food. Diarrhea associated with SI disease may contain undigested food, especially fat (steatorrhea), and may be malodorous; patient breath may have a characteristic odor, as well. The diarrhea can be a bizarre color, such as yellow or green, indicative of incomplete intestinal bacterial metabolism of excreted bile pigments that normally impart a brown color to the feces.
If there is bleeding, the blood is usually partially digested, and if in sufficient volume, will be recognized as melena; at least 1 mL blood/kg/day must be lost before it becomes grossly visible.
Physical Examination
Nonspecific signs of SI disease, such as dehydration and weight loss, are readily apparent, and fever is sometimes present with infectious enteritis. Weight and body condition score should be recorded in all patients.
Direct, noninvasive examination of the SI is impossible, but examination of the mouth for a linear foreign body and rectal examination should be performed. Abdominal palpation is best performed with gradual, gentle, manual pressure on the abdomen. In larger dogs pressure is applied between the hands placed on either flank, but in cats and small dogs the thumb and fingers of one hand may be used. Elevating the cranial abdomen may allow masses normally within the rib cage to fall back to where they can be detected. Masses, foreign bodies, or distended or thickened loops of bowel may be readily palpated. However, the success of palpation depends not only on the skill and patience of the examiner, but also on the body condition and patient compliance. Abdominal palpation should be repeated at least daily in hospitalized patients, and the opportunity should be taken to repeat palpation if the patient is sedated or anesthetized when the abdominal wall will be relaxed.
Free fluid within the peritoneal cavity should be detected by ballottement; tapping on one side of the abdomen allows detection of a fluid wave on the opposite flank. Detection of abdominal pain may be more difficult, depending on how the patient responds. Specific localization is rarely possible as abdominal visceral sensory output is not segmental and decussates within the spinal cord. Localized peritonitis may be detected as the parietal peritoneal sensory output is segmental.
Minimum Database
Results of the hemogram, serum biochemistry, and urinalysis are rarely diagnostic of any specific SI disease. Indeed they are often more reflective of hydration status, and are largely undertaken to rule out diseases in other organ systems that may manifest with SI signs. Some changes can help assess dehydration (e.g., packed cell volume, total solids/proteins, azotemia) and secondary electrolyte abnormalities, but changes seen in SI disease are often quite nonspecific.
Hemogram
Elevation of the packed cell volume can be indicative of dehydration, whereas extreme erythrocytosis is a hallmark of hemorrhagic gastroenteritis or paraneoplastic syndrome. Paraneoplastic production of erythropoietin by intestinal stromal cell tumors causing erythrocytosis has been reported in rare cases.
Anemia can reflect chronic illness or intestinal blood loss. Mild normocytic normochromic anemia is the most common abnormality, but a regenerative anemia may be seen if there is blood loss. Hypochromic, microcytic anemia (and thrombocytosis) is indicative of iron deficiency, which can occur through chronic SI blood loss.
A stress leukogram is often associated with significant SI disease, but an inflammatory leukogram is unusual, even in the presence of marked intestinal inflammation. Neutrophilia, left shift, and sometimes toxic neutrophils can indicate incipient sepsis or SI perforation and peritonitis. Eosinophilia can be a result of parasitism, but is an unreliable marker of intestinal parasites and eosinophilic enteritis. Marked eosinophilia may be seen as a paraneoplastic effect in lymphoma and mastocytosis.
Serum Biochemistry
Total serum proteins will be increased if there is dehydration and decreased by chronic blood loss. If a PLE exists, panhypoproteinemia typically develops, as both albumin and globulin are lost through the leaky gut wall. This can usually be differentiated from protein-losing nephropathy (low albumin, normal globulin, proteinuria) and hepatic failure (low albumin, raised globulin, and hyperbilirubinemia). Occasionally hyperglobulinemia is found in severe IBD, and a monoclonal gammopathy is seen rarely in alimentary lymphoma and plasmacytoma.
Liver enzymes may be elevated secondarily in SI disease because of portal venous transport of toxins and/or bacteria from a compromised SI, but overall liver function (as assessed by serum bile acids) will usually be normal. Hypocholesterolemia is a crude indicator of malabsorption.
Hypoglycemia is a complication found in perinatal patients that have reduced nutritional intake during SI disease. Paraneoplastic hypoglycemia is occasionally found with SI stromal cell tumors that produce insulin-like factors.
Prerenal azotemia (i.e., increased urea and creatinine) will develop if the patient is dehydrated, but an increased urea-to-creatinine ratio in a fasted animal is suggestive of GI bleeding, with conversion of blood proteins to ammonia by intestinal bacteria, and hence urea formation by the liver.
Hypokalemia is common in SI disease as a result of decreased intake and intestinal losses. The finding of an abnormal sodium-to-potassium ratio may identify cases of hypoadrenocorticism, but is sometimes seen in primary SI disease, notably salmonellosis and whipworm infection,1 or if ascites and third-space effects are associated with PLE.
Fecal Examination
Fecal examinations2 are an important component in the investigation of SI disease. Tests such as quantification of fecal fat excretion are unsuitable for most practice settings, and bacteriologic culture is sometimes of questionable value, but identification of endoparasites is important.
Direct Smear
Fecal smears can be stained for undigested starch granules (Lugol iodine solution), fat globules (Sudan stain), and muscle fibers (Wright or Diff-Quik stain). Positive findings may indicate maldigestion and malabsorption but are generally unreliable and completely nonspecific. The presence of fungal elements is of uncertain significance, but rectal cytology may be useful, with fecal leukocytes being suggestive of intestinal inflammation.
Unstained wet mounts may be used to identify protozoal trophozoites of Giardia (dogs and cats) or Tritrichomonas (cats). Clostridial endospores and fungal elements (Histoplasma, Aspergillus, Pythium, and Candida spp.) may be identified. Enterotoxin production by Clostridium perfringens is a potential cause of diarrhea. The presence of a large number of clostridial endospores (more than 5 per oil field) on Diff-Quik–stained smears may be significant, but a positive fecal enterotoxin assay (enzyme-linked immunosorbent assay [ELISA] or reverse passive latex agglutination) is likely more significant. However the correlation of sporulation, toxin production, and diarrhea is unclear.3
Rectal Cytology
Although probably more relevant for large intestinal disease, the rectal wall can be very mildly abraded at the end of a rectal examination, and the gloved finger rolled on a microscope slide for special staining. Cytologic examination is often negative, showing only bacteria and fecal debris, but when positive it can provide some useful information. Although the test is fast and simple, in all cases confirmatory tests are indicated. An increased number of neutrophils may be suggestive of a bacterial problem, indicating the need for fecal culture, and malignant lymphocytes may exfoliate if lymphoma is present. Histoplasma and Prototheca organisms may be visualized.
Fecal Concentration Techniques
For detection of most parasites, fecal concentration techniques are more rewarding. Examination of three fecal samples by zinc sulfate flotation is recommended to detect Giardia oocysts. A direct smear, sedimentation, or the Baermann technique can identify larvae of Strongyloides spp.
Bacteriologic Examination
Routine Culture
The culture of all bacteria from a fecal sample in vitro is of little value, but targeted evaluation for potential pathogens may be helpful, although molecular techniques may be needed to identify pathogenic strains. For example Escherichia coli can be cultured from most fecal samples, but only certain strains are pathogenic, and polymerase chain reaction (PCR) probes are needed to detect genetic pathogenicity markers.
Culture of feces is indicated in animals with acute hemorrhagic diarrhea, with fever, and an inflammatory leukogram, and/or with neutrophils on rectal cytology. Identification of Salmonella spp., Campylobacter jejuni, and Clostridium difficile may be helpful, although the significance of a positive isolate should be interpreted in the light of the clinical history, because these organisms can be present in the feces of clinically healthy animals. Furthermore, the fecal flora does not necessarily reflect the SI flora and cannot be used to diagnose small intestinal bacterial overgrowth, but may be representative of colonic bacterial populations.
The significance of a positive result needs further evaluation, because potential pathogens can be isolated from feces from both healthy and ill dogs. Failure to speciate Campylobacter isolates may lead to erroneous conclusions, as the relatively nonpathogenic, and potentially commensal, Campylobacter upsaliensis is a more common isolate from dog feces than the pathogenic C. jejuni. Testing by PCR may aid speciation, but still does not overcome the fact that isolation does not necessarily indicate the cause of the diarrhea. Feces can be cultured for fungi, such as Histoplasma capsulatum, but isolation is difficult and slow.
Molecular Fingerprinting4
Many intestinal bacteria cannot be cultured in vitro. Identification can be performed by comparative gene sequencing of the bacterial 16S ribosomal RNA (rRNA) derived from mucosal brushings or fecal samples. This method can be used to identify a single species using degrading gradient gel electrophoresis or to look at the pattern of the flora in both mucosal brushings and feces by the high-throughput pyrosequencing metagenomic approach (see Chapters 2 and “Infection” section in this chapter).
Virologic Examination5
Viral diarrhea is usually acute and self-limiting and does not require a positive diagnosis. Electron microscopy can be used to identify the characteristic viral particles of rotavirus, coronavirus, and parvovirus. Fecal ELISA tests for parvovirus are also available.
Examination for Protozoa
Coccidia
Oocysts are best detected by fecal flotation methods.
Giardia
Zinc sulfate flotation in the hands of an experienced technician remains the diagnostic method of choice. A commercially available ELISA can be used to detect Giardia antigen in feces and may be more sensitive. PCR is also likely to be more sensitive.
Tritrichomonas fetus6
Infection with this organism, which can be an important pathogen in young cats,6 and may complicate canine diarrhea, can be diagnosed by direct evaluation of a fresh fecal smear, although experience is needed to distinguish it from Giardia. In vitro culture in pouches, developed to identify cattle infections, can be used but needs to be examined every 2 days for the presence of the organism. A fecal PCR is available, but can be problematic because of inhibitors of the PCR reaction in feces, and the intermittent excretion of the organism; false negatives are less likely if diarrheic feces or colonic washings are used.
Occult Blood
At least 1 mL of blood per kilogram of patient body weight is needed to recognize overt melena.7 The occult blood test is used to test for intestinal bleeding from ulcerated mucosa and benign or malignant tumors before melena is observed grossly. Unfortunately, it is very sensitive and tests nonspecifically for hemoglobin from any mammalian species, thus reacting with any dietary meat as well as patient blood. Consequently the patient must be fed a meat-free diet for at least 72 hours for a positive result to have any significance.
α1-Protease Inhibitor8
α1-Protease inhibitor (α1-PI; synonym: α1-antitrypsin) is a naturally occurring endogenous serum antiprotease. If lost into the intestinal lumen because of PLE, it can be found in feces as it resists bacterial degradation. The α1-PI test originally assayed the presence of α1-PI in feces by ELISA, but has been replaced by a validated radioimmunoassay.
To improve the diagnostic accuracy of the test, three fresh fecal samples should be sampled. The assay is only valid when used on fecal samples collected following spontaneous defecation, as abrasion of the colonic wall during digital evacuation is enough to elevate α1-PI concentrations. The test is less useful in patients with GI blood loss. The test appears to be of value for the diagnosis of PLE, correlating well with historical testing by fecal radioactive 51chromium-labeled albumin excretion. Fecal α1-PI may prove to be more sensitive than the finding of reduced serum albumin for the detection of early disease.
Fecal Calprotectin
Calprotectin has been characterized as a marker of neutrophil elastase activity.9 Assay of fecal calprotectin is a useful marker of inflammation in human IBD, and a dog-specific assay has been developed, but the clinical utility, sensitivity, and specificity are unknown.
Special Tests
In cases of malabsorption, intestinal biopsy is usually necessary to obtain a definitive diagnosis. However, exocrine pancreatic insufficiency should be ruled out before biopsy, because signs of malabsorption are nonspecific and not easily differentiated from maldigestive disorders.10, 11 Thus serum trypsin-like immunoreactivity (TLI) measurement must be performed in all cases (see Chapters 25 and 60Chapter 25Chapter 60). It is also well-recognized that biopsies from up to 50% of malabsorption patients are considered normal by light microscopy. Therefore before biopsy, a number of indirect tests are performed to assess for intestinal damage, altered permeability, and dysfunction. Indeed, empirical treatments, such as administration of fenbendazole or an exclusion diet trial, may be indicated before biopsy.
Serum Folate and Cobalamin Concentrations
The assay of serum folate and cobalamin concentrations12 can be performed on the same serum sample taken for the TLI test (see Chapter 25). This assay has limited value in the diagnosis of specific SI diseases and is not recommended for the diagnosis of canine small intestinal bacterial overgrowth (SIBO). However, subnormal folate and cobalamin concentrations are markers of GI disease as well as indicators for the need for vitamin supplementation (see Figure 57-9, Figure 57-10 ).
Figure 57-10.
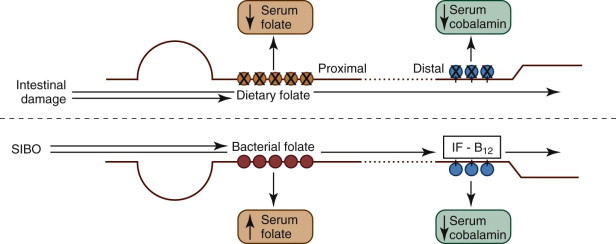
Diagram of the absorption of folate and cobalamin.
In diseased intestine, proximal and distal mucosal damage causes folate and cobalamin malabsorption, respectively. Reduced serum folate and/or cobalamin are markers for proximal and/or distal SI damage. Classically, small intestinal bacterial overgrowth (SIBO) causes increased folate uptake because of bacterial folate synthesis and decreased cobalamin uptake because of bacterial incorporation. However, these changes are poorly sensitive, and cannot be used to reliably diagnose SIBO: they do not correlate with antibiotic responsiveness. IF, Intrinsic factor.
(From Ettinger SJ, Feldman EC, editors: Textbook of Veterinary Internal Medicine, ed 7, Philadelphia, 2010, Saunders, p 1528.)
Tests of Intestinal Absorption
Attempts to assess intestinal function by measuring the absorption of numerous substrates (e.g., lactose, glucose, starch, triglyceride, and vitamin A) are no longer performed because of a lack of sensitivity and specificity. Even the d-xylose test has been abandoned because it is insensitive in dogs and nondiscriminatory in cats. GLUT5 on the MVM allows facilitated diffusion of both fructose and d-xylose, and as fructose uptake in cats is low, the xylose absorption test is particularly unhelpful in this species. The differential absorption of two sugars (xylose and 3-O-methyl-d-glucose) eliminates the nonmucosal effects that blight the xylose test, and initial results suggest that the test may be of more value in dogs and cats.13
Intestinal Permeability
Intestinal permeability13, 14, 15 is an index of mucosal integrity and is assessed by measuring noncarrier mediated uptake of nondigestible probe markers. These tests use a nonmetabolizable probe that is measurable in plasma and/or excreted in the urine. The permeability probe 51chromium-labeled ethylenediaminetetraacetic acid (51Cr-EDTA) was used in original studies, but the need for a γ-emitter limited its safe use.
Errors related to nonmucosal factors (including the gastric emptying rate, intestinal transit time, and completeness of urine collection) can be eliminated by concurrently measuring the absorption of two probes with different pathways of absorption (Figure 57-11 ). Calculation of their excretion ratio eliminates errors from extramucosal factors because both probes should be affected equally. The ratio, which is altered by villus atrophy or epithelial damage or both, offers a simple, sensitive diagnostic test.
Figure 57-11.
Principle of differential permeability testing.
Simultaneous administration of two probes selected to respond identically to each variable except mucosal permeability. The Y-to-X ratio provides a specific index of mucosal permeability.
(From Hall EJ: Small intestinal disease: is endoscopic biopsy the answer? J Small Anim Pract 35:408, 1994.)
A 5-hour urine collection is performed after oral administration of two sugars. A number of candidates can be used for the probe molecules, and a mixture of one large simple sugar (e.g., lactulose, cellobiose, raffinose) and one small one (e.g., rhamnose, arabinose, mannitol) can be chosen. The cellobiose-to-mannitol excretion ratio and lactulose/mannitol ratio have been used in companion animals, but with advances in the high-performance liquid chromatography assay of these sugars in blood and urine, the lactulose/rhamnose ratio test has become the standard test of SI permeability.
Tests for Protein-Losing Enteropathy
Historically, intestinal protein loss has been detected by measuring the fecal loss of radiolabeled molecules such as 51Cr-labeled albumin and 67Cu-labeled ceruloplasmin. These tests are difficult to perform, potentially hazardous, and have largely been discarded, although they remain the standard by which other tests, such as the assay of fecal α1-PI (see previous), are judged.
Breath Tests
Breath tests12, 16 have been used to assess bacterial metabolism in the GI tract. Intestinal bacteria synthesize hydrogen and volatile gases (e.g., methane), which are partially absorbed and excreted in breath where they can be measured. Breath hydrogen tests have been used most extensively because mammalian cells cannot produce hydrogen, and therefore any H2 gas that is measured must be of bacterial origin. Such tests can assess carbohydrate malabsorption and orocecal transit time, when most of the hydrogen is produced by colonic bacteria. Theoretically breath hydrogen can detect increased bacterial colonization of the SI, that is, bacterial overgrowth, but as colonic bacterial numbers are massive, the test is unlikely to be discriminatory.
A variety of protocols has been used, including giving xylose to assess malabsorption, lactulose to assess orocecal transit, and a test meal to assess SI bacterial fermentation. A number of studies have attempted to standardize the techniques for companion animals, and use of substrates labeled with stable isotopes has increased. However, these techniques are not widely used, even in referral centers, because of technical difficulties, and lack of specificity.
Unconjugated Bile Salts
The hypothesis behind the test to measure serum unconjugated bile acids (SUBAs) is that conjugated bile acids are excreted into the intestine via the biliary tract where some SI bacterial species carry out deconjugation reactions.17 Unconjugated bile acids are then absorbed passively by the SI, poorly cleared from portal blood, and measured in peripheral blood. Therefore, in theory, increases in SI bacterial numbers might result in an increase in SUBAs. Preliminary work suggesting that the test was sensitive and specific for canine SIBO has since been contradicted. Its utility has been questioned as there is a marked postprandial rise in healthy animals, deconjugation is partly a function of Lactobacillus activity, and results do not correlate with the diagnosis.
Miscellaneous Tests
A number of tests for intestinal bacterial metabolites have been devised to detect bacterial metabolic activity or SIBO, or to assess orocecal transit time. These include the nitrosonaphthol test, urinary indican excretion, bacterial release of sulfapyridine from sulfasalazine, and bacterial release of paraaminobenzoic acid (PABA) from a bile salt conjugate (PABA-UDCA [ursodeoxycholic acid]).18 However, none of these tests are widely used in companion animals. Evaluation of the volatile gases emitted by feces can give a profile that is characteristic of specific infections, but has not been evaluated in small animals.
Assessment of Intestinal Motility
Methods to measure intestinal transit time include barium studies with and without food, ultrasonography (including pulsed Doppler measurements), breath hydrogen following carbohydrate administration, and using visual (e.g., carmine red dye, chromic oxide) or chemical markers that are excreted in the urine after absorption (e.g., sulfasalazine, acetaminophen, nitrofurantoin, PABA-UDCA).19 Results are quite variable and often the methodologies do not correlate well as results are variable with both the composition of the test diet and stress affecting transit rates as much as disease. Intestinal transit is best studied with wireless motility capsule system and scintigraphy, but again the diet composition and stress affect the result. Measurement of myoelectrical activity either in vivo or in vitro is impractical in clinical practice.
Imaging
With the development of ultrasound, advanced imaging techniques, and endoscopy, imaging of the intestinal tract is no longer limited to plain and contrast radiography. Scintigraphy, computed tomography (CT), and magnetic resonance imaging are now being adopted, and “virtual endoscopy” by helical CT is becoming available.
Plain Radiography
Survey, plain radiographs20 are most useful in the investigation of diarrhea associated with vomiting, abdominal pain, and palpable abnormalities. The diagnostic yield is enhanced if orthogonal views are taken, although a single lateral radiograph may be adequate if combined with ultrasonography. The utility of plain radiographs in malabsorption is minimal, especially if ascites is present, as detail is obscured by fluid and lack of fat contrast, respectively. Generally the aim of plain, survey radiographs is the detection of (acute) surgical disease (e.g., foreign bodies, free gas, displacement, masses, obstructions), decreased serosal detail suggestive of effusion, and ileus, an abnormal dilation of an immotile segment of intestine. The differential diagnosis of ileus depends on whether it is localized or generalized and whether an accumulation of gas or fluid is present (Box 57-3 ). Interpretation should be cautious if the patient has been treated with drugs that affect the GI tract.
Box 57-3. Differential Diagnosis of Ileus.
-
•Gas ileus
-
•Generalized
-
•Aerophagia
-
•Smooth muscle paralyzing drugs
-
•Generalized peritonitis
-
•Enteritis
-
•Localized
-
•Localized peritonitis (e.g., pancreatitis)
-
•Early stage bowel obstruction
-
•Disruption of mesenteric arterial supply
-
•
-
•Fluid ileus
-
•Generalized
-
•Enteritis
-
•Diffuse intestinal neoplasia
-
•Localized
-
•Foreign body
-
•Tumor causing obstruction
-
•Intussusception or other mechanical obstruction, e.g., incarceration
-
•
Contrast Radiography
Since the introduction of alternative imaging techniques, especially endoscopy, contrast radiographic studies are of limited value in the assessment of SI disease.
Follow-through Examinations
Studies using microfine barium suspensions can identify ulcers and irregular mucosal detail. Although they may confirm the presence of radiolucent foreign bodies, they are of limited use in identifying mural masses and partial obstructions, and rarely provide more information than good quality survey radiographs.
Although contrast studies theoretically can be used to assess the rate of intestinal transit, results do not correlate closely to movement of ingesta as assessed by scintigraphy. Furthermore, dysmotility may occur secondary to other causes and studies provide limited etiologic information. Administration of barium may delay endoscopy for at least 24 hours. If perforation is suspected an iodine-based contrast is used if confirmation is required, although the presence of free abdominal gas on survey films is adequate for a diagnosis in most cases.
Barium-Impregnated Polyethylene Spheres
These are solid-phase radiopaque markers that provide information on gastric emptying, intestinal transit, and obstructive disorders. Given that the transit time of barium-impregnated polyethylene spheres (BIPS)21 is highly variable, their use for transit studies is limited. They may be most helpful in the detection of partial obstructions, as the larger BIPS are held up by partial obstructions that are seen clinically significant, but may not be identified by barium suspension transit.
Ultrasonography
Transabdominal ultrasound examination is now a routine part of the investigation of SI disease.22, 23 In the future, endoscopic ultrasound will allow the mucosal wall and adjacent viscera (e.g., pancreas) to be examined in more detail.
A conventional examination can detect layering of the wall, peristalsis, ileus, and luminal contents, and can measure SI wall thickness. It has excellent sensitivity for the detection of lesions such as intussusceptions, masses, radiolucent foreign bodies, and intestinal wall thickening and lymphadenopathy in chronic inflammatory, lymphatic, and neoplastic enteropathies. Intussusceptions are usually recognized in the transverse plane as multiple concentric rings and longitudinally as a thick, multilayered segment.
Values for normal SI wall thickness have been reported for dogs and cats; thickness decreases from proximal (5 to 6 mm) to distal (4 to 5 mm), but depends on body size, with the thickest seen in the large- and giant-breed dogs. Disruption of the normal five-layered sonographic appearance (mucosal surface–mucosa–submucosa–muscularis–serosa) is typical of neoplasia, whereas wall thickening can result from other infiltrative disorders and edema, as well as neoplasia. Ultrasound-guided fine-needle aspiration for cytologic examination is possible.
Duodenal Fluid Examination
Duodenal fluid17, 24, 25, 26 can be collected either by needle aspiration through the intestinal wall at laparotomy, or during duodenoscopy through a sterile polyethylene tube passed down the biopsy channel. Collection of sufficient sample without blood and tissue contamination can be difficult.
The sample can be examined for motile Giardia trophozoites, although this has not proved reliable for diagnosis. Quantitative and qualitative aerobic and anaerobic cultures can be performed. This is considered the gold standard for diagnosis of SIBO, although there are major problems in interpretation and routine diagnostic use of duodenal fluid bacterial culture is not recommended.
Intestinal Biopsy
In most cases of acute diarrhea, a histologic diagnosis is not needed, and intestinal biopsy is very rarely performed. However, in chronic diarrhea a definitive diagnosis often depends on histologic examination of intestinal tissue, although this has major limitations. Biopsy specimens are collected either endoscopically or surgically.
Best practice is to perform endoscopic biopsy first unless there is evidence that the disease is beyond the reach of the endoscopy; the surgical option is preferred if there is any possibility of extraintestinal disease or focal intestinal pathology, or if endoscopic biopsy has failed to reveal a diagnosis. Thus the client should always be made aware that surgical biopsies may ultimately be required.
Endoscopic biopsies should always be taken, even in the absence of gross abnormalities, because microscopic changes may be present but only the duodenum (and proximal jejunum, if accessible) are biopsied routinely via gastroscopy, while ileal biopsies may be obtained via colonoscopy. Multiple specimens (a minimum of six from each area) should be collected, because small size, artifacts, and fragmentation can make interpretation difficult. The size and quality of endoscopic biopsies depends not just on the equipment available, but also on the pressure exerted by the forceps, which is in part dependent on the operator's experience. At laparotomy, full-thickness biopsies are usually taken from at least three sites, the duodenum, the jejunum, and the ileum. Intestinal biopsies can also be obtained laparoscopically, but evidence of a greater diagnostic utility than endoscopy or greater safety than surgery has yet to be proven.
Tissue handling and processing also affect the diagnostic quality of the sample. Careful orientation of the sample so that the tissue is flattened with the mucosa uppermost theoretically improves the likelihood of optimal sectioning, but must be weighed against the increased time needed to lay out the tissue and the attendant development of artifacts through handling and delayed fixation.
Examination of Biopsies
Histopathology
Although histopathologic assessment of intestinal biopsies remains the gold standard for diagnosis of intestinal disease, it too has limitations.27, 28, 29, 30, 31, 32, 33 Biopsies may be normal by light microscopy, which suggests that many diseases have a functional rather than a morphologic abnormality or that sampling or interpretation problems have occurred. Histopathology may be satisfactory when there are pathognomonic changes, for example, neoplasia, but it has become evident that it may be difficult to diagnose intestinal inflammatory diseases reliably because of a lack of standardized sample preparation and staining practices and agreed upon histologic criteria.
Agreement between histopathologists often is poor, especially when examining endoscopic biopsies and a standardized approach is required. Histopathologic scoring schemes and standardized criteria have been suggested by the World Small Animal Veterinary Association (WSAVA) GI Standardization Group as a means of improving agreement. However, Group members also have shown that the experience of the endoscopist, as well as simply the quality and numbers of biopsies, can influence the reliability of the histologic interpretation. Furthermore there is emerging evidence that ileal biopsies are more likely to be diagnostic than duodenal. As expected, fewer biopsies are needed to reliably detect architectural changes the better their quality (i.e., better size, depth, and integrity) and more specimens are needed the deeper the lesion. Therefore the clinician should always interpret endoscopic biopsy results cautiously; results should be questioned if the tissue diagnosis does not fit the clinical picture, or if the response to apparently appropriate therapy is poor. In some cases, repeat biopsy (e.g., by exploratory laparotomy) may be required. Cytologic examination of endoscopic biopsy squash preparations or mucosal brushings are only an adjunct to histopathologic examination.
Even if intestinal inflammation can be diagnosed reliably, there remains the difficulty that the histologic pattern in intestinal inflammation seen is likely a common final pathway caused by a number of potential causes. Thus unless an etiologic agent is evident on microscopic (e.g., visible protozoa) biopsy alone, it may not be possible to determine the cause of any intestinal inflammation. Although different histologic patterns, for example, eosinophilic and lymphoplasmacytic, are recognized, their specificity in indicating the etiology is poor.
Alternative Examinations
A number of research tools have been applied to the investigation of intestinal biopsies. However they are largely research tools, limited by availability and/or cost.34, 35, 36 Box 57-4 outlines more specific examinations.
Box 57-4. Research Methods beyond Routine Histopathology Used for Examining Intestinal Biopsies.
-
•
Electron microscopy
-
•
Biochemical assay of brush-border enzymes
-
•
Immunocytochemical characterization of B cells, T cells, and their subsets (e.g., CD4 and CD8 cells) and major histocompatibility complex expression by immunohistochemistry and flow cytometry
-
•
PCR for cytokine and receptor (nucleotide-binding oligomerization domain, Toll-like receptors) mRNA expression
-
•
Assessment of T-cell clonality—PCR for antigen receptor rearrangement (PARR)
-
•
Fluorescence in-situ hybridization to demonstrate bacteria in biopsies
Empirical Treatment
If a specific diagnosis is made, specific treatment(s) can be given. Yet often the diagnosis is not obvious, usually because of a lack of specific or marked histopathologic changes, and it may be appropriate to perform empirical treatment(s). It is logical and safest to do this sequentially, starting with the treatment least likely to do harm. Therefore parasiticides, followed by an exclusion diet trial, and then an antibacterial trial should be considered before finally attempting immunosuppression. This empirical approach may identify occult parasitism, diet-responsive conditions, and antibiotic-responsive diarrhea, respectively. However, because treatments lack specificity, caution should be exercised in using such trials to make a diagnosis without investigation.
Inflammation
Alexander J. German
In companion animal gastroenterology, IBD is the term used to describe patients affected by persistent or recurrent GI signs, and that have histopathologic evidence of inflammation in intestinal tissues.1 It is only appropriate to use the term idiopathic IBD if no underlying cause for the inflammation can be found. Although there are recent studies into pathogenesis, diagnosis, and treatment, much controversy remains. To make a diagnosis of IBD, detailed investigations must be undertaken to exclude other potential causes of intestinal inflammation; nevertheless, the likelihood is that IBD represents a syndrome comprising a group of disorders with similar characteristics, rather than a single disease entity. It should be noted that canine and feline IBD bear little resemblance histologically or clinically to either of the main IBD variants in humans (i.e., Crohn disease and ulcerative colitis).
Companion animal IBD is further subdivided based on the predominant inflammatory cell type present as judged by histopathologic examination of intestinal biopsy samples (Table 57-2 ). However, such classification is often arbitrary, and depends upon the opinion of the pathologist concerned. Indeed, many cases have a generalized increase in several cell subsets and cannot easily be classified. The WSAVA GI Standardization Group has undertaken the task of reviewing both collection of biopsy material (endoscopy) and subsequent histopathologic interpretation.2 It is hoped that such standardization will improve the reliability and diagnostic yield from this procedure.
Table 57-2.
Histopathologic Classification of Idiopathic Inflammatory Bowel Disease
| Histopathologic Description | Comment |
|---|---|
| Lymphocytic-plasmacytic enteritis (LPE) | Most common |
| Basenji enteropathy | A variant of LPE? |
| Familial PLE and protein-losing nephropathy in soft-coated Wheaten Terriers | A variant of LPE? |
| Eosinophilic enteritis | Second most common form; marked increase in eosinophils |
| Granulomatous enteritis | Rare; due to feline infectious peritonitis in cats |
| Regional enteritis | Rare, a variant of granulomatous enteritis? |
| Neutrophilic enteritis | Rare in dogs, uncommon in cats |
Types of Small Intestinal Inflammatory Bowel Disease
Forms of small intestinal IBD include lymphocytic-plasmacytic enteritis (LPE), eosinophilic enteritis (EE), granulomatous enteritis, regional enteritis, and neutrophilic enteritis (see Table 57-2). Furthermore, certain breed-specific patterns of disease are recognized, including Basenji enteropathy and the PLE/protein-losing nephropathy (PLN) syndrome of soft-coated Wheaten Terriers. Although the histopathologic findings of these disorders may differ, the etiopathogenesis is thought to be broadly similar.
Lymphocytic-Plasmacytic Enteritis
LPE is the most common histopathological form of SI IBD, and is characterized by mucosal infiltration of lymphocytes and plasma cells (Figure 57-12 ). LPE can be associated with lymphocytic-plasmacytic inflammation in other regions of the GI tract (e.g., lymphocytic-plasmacytic gastritis [see Chapter 56 ] and lymphocytic-plasmacytic colitis [see Chapter 58]). LPE in cats can be associated with inflammatory disease in the pancreas (see Chapter 10) and/or liver (see Chapter 61) as part of the “triaditis” syndrome.
Figure 57-12.
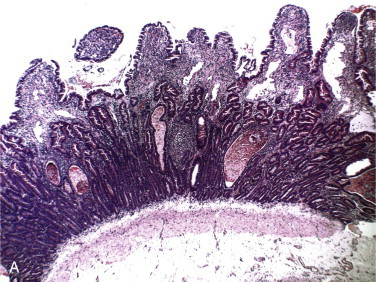
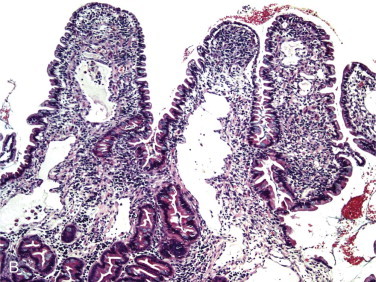
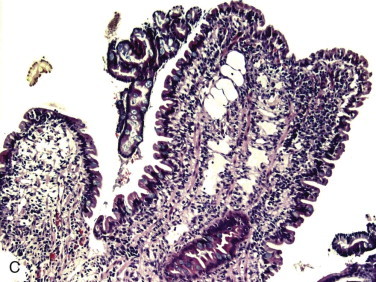
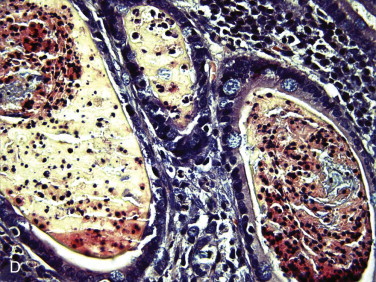
A, Low-power photomicrograph of the histologic appearance of a duodenal biopsy specimen taken from a dog with lymphocytic-plasmacytic enteritis. Hematoxylin and eosin stain, original magnification ×10. B to D, Higher-power views showing architectural characteristics of IBD, including villus stunting (B), villous fusion (C), and crypt distention (D). Hematoxylin and eosin stain, original magnifications ×40.
Clinical signs of LPE are similar to other forms of IBD and are not pathognomonic. Severe LPE is reportedly prevalent in German Shepherd dogs, Shar-Peis, and pure-bred cats. The approach to diagnosing LPE is the same as for any other form of IBD. However, in both cats and dogs, it can be difficult to differentiate severe LPE from alimentary lymphoma. Exploratory celiotomy may be a preferable means of collecting biopsy material in cats, given both concerns over differentiation of LPE from GI lymphoma on endoscopic biopsy, and the concurrence of pathologic change in other organs. Given these diagnostic dilemmas, clonality studies assessing T-cell receptor rearrangements may assist in identifying low-grade lymphoma.3
Eosinophilic Enteritis
EE is reportedly the second most common form of IBD and can be associated with disease elsewhere in the GI tract. On histopathologic examination, mucosal architectural disturbances (e.g., villus atrophy) are present in conjunction with a mixed infiltrate of inflammatory cells with eosinophils predominating (Figure 57-13 ). The diagnosis has been problematic in the past because diagnostic criteria (e.g., degree of eosinophil infiltration) varied amongst pathologists. However, the WSAVA standards suggest clear diagnostic criteria (e.g., mild 5 to 10 eosinophils per ×40 field; moderate 10 to 20 per ×40 field; marked eosinophils dominate the tissue population).2 A diagnosis of EE should only be made once other causes of eosinophilic infiltration (e.g., endoparasitism and hypersensitivity disorders) have been eliminated. EE also may be associated with systemic eosinophilic disorders (e.g., hypereosinophilic syndrome) in both cats and dogs.
Figure 57-13.
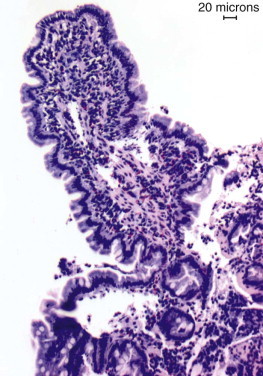
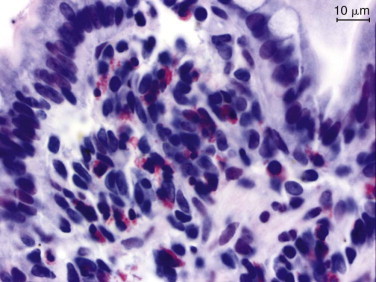
Histologic appearance of duodenal biopsy specimens taken from a dog with eosinophilic enteritis. Sirius red stain.
The condition can be seen in dogs and cats of any breed and age, although it is more common in younger adult animals. Boxers, German Shepherds, and Dobermans may be predisposed. The clinical signs reported are similar to other forms of IBD, although mucosal erosion and/or ulceration may occur more frequently and lead to hematemesis and melena. Concurrent PLE is also recognized, and severe eosinophilic gastroenteritis is also associated with spontaneous perforation of the GI tract.4
Basenji Enteropathy
Basenji enteropathy is a severe, hereditary form of LPE that has been well characterized in Basenjis, although the mode of inheritance remains unclear. Vomiting and small intestinal diarrhea are the main clinical signs. A progressive PLE is often noted, and some severe cases develop spontaneous intestinal perforation. Intestinal lesions in Basenjis are characterized by increases in CD4+ and CD8+ T cells.5, 6 In addition to lymphocytic-plasmacytic gastritis, mucosal hyperplasia occur, and this is thought to be secondary to hypergastrinemia. Treatment is usually unrewarding, with progressive clinical signs and dogs dying within months of diagnosis. However, early aggressive combination treatment with glucocorticoids, antibacterials, and dietary modification may achieve remission in some cases.
Familial Protein-Losing Enteropathy and Protein-Losing Nephropathy In Soft-Coated Wheaten Terriers
A unique clinical syndrome has been reported in soft-coated Wheaten Terriers.7 Affected dogs may present with signs of PLE, PLN, or both. A genetic basis is likely and, although the mode of inheritance is not yet clear, a common male ancestor has been identified. An immune-mediated pathogenesis is likely and dietary hypersensitivity might be involved, as suggested by alterations in antigen-specific fecal immunoglobulin (Ig) E concentrations.8, 9 Signs of PLE tend to develop at a younger age than PLN, and clinical signs include vomiting, diarrhea, weight loss, and pleural and peritoneal effusions. Affected dogs are at risk of thromboembolic disease.10 Treatment is similar to that described for general IBD.
Granulomatous Enteritis
This rare form of IBD is characterized by the development of granulomas and mucosal infiltration with macrophages. This condition is likely to be the same as regional enteritis where ileal granulomas are reported.11, 12 Potential causes of granulomatous inflammation include Yersinia and mycobacterial infections, foreign-body reactions, and fungal diseases. In cats a pyogranulomatous transmural inflammation has been associated with feline infectious peritonitis (FIP) virus infection. Although there are similarities between this condition and human Crohn disease, intestinal obstruction and fistula formation are not observed. Conventional therapy for IBD is usually not effective and the prognosis is guarded, although a combination of surgical resection and antiinflammatory treatment was reported to be successful in one case.
Neutrophilic Enteritis
Some inflammatory diseases may be characterized by infiltrates of neutrophils or by granulomatous inflammation, although these patterns are rare. If neutrophils are evident, an underlying bacterial infection should be considered. Alternatively, the neutrophilic infiltrate may have arisen from bacterial invasion secondary to mucosal barrier disruption from erosive or ulcerative lesions. Glucocorticoids are generally not recommended for such cases, unless they fail to respond to all other therapeutic modalities.
Proliferative Enteritis
Proliferative enteritis is characterized by segmental mucosal hypertrophy of the intestine. It is most common in pigs, but a similar although very rare condition has been reported in dogs.13 There may be an underlying infectious etiology and Lawsonia intracellularis infection has been implicated but not yet been proven. Other potential infectious causes include Campylobacter spp. and Chlamydia.
Etiology and Pathogenesis
Reportedly, IBD has an immune-mediated etiology and thus the GI associated lymphoid tissue likely plays a critical part in pathogenesis.14 Full details of the mucosal immune system are found in Chapter 3, while intestinal inflammation is reviewed in Chapter 4. Briefly, the intestinal mucosa has a barrier function (“immune exclusion”), and controls exposure of antigens to the gut-associated lymphoid tissue, which must generate protective immune responses against pathogens while remaining “tolerant” of harmless environmental antigens such as commensal bacteria and food. IBD develops when the normal decision-making process breaks down, leading to inappropriate immune responses and uncontrolled inflammation. Critical to the development of inflammation is a breakdown in tolerance to normal luminal antigens (particularly endogenous bacterial species). This loss of tolerance may result from disruption of the mucosal barrier leading to excessive antigen exposure to the underlying immune system, from dysregulation of normal mucosal immune system function, or from a combination of these processes. The end effect is uncontrolled inflammation, which is the result of activation of the many effector pathways. The inflammation can then lead to architectural disruption, resulting in adverse effects on function, which depend upon the part of the bowel affected.
Unfortunately, data that directly assess the pathogenesis of canine small intestinal IBD are limited, and many gaps in our understanding remain. Many studies have used histochemical and immunohistochemical techniques to quantify immune cell populations within the intestinal mucosa with variable results.14 For canine IBD, recent studies have suggested an increase in cells expressing Toll-like receptors-2, -4, and -9.15 Other studies have shown a decrease in certain lymphocyte populations (total T cells and IgG+ plasma cells), while others have shown increases (αβ T cells, CD4+ T cells, IgG+ plasma cells) as well as increased macrophage and granulocyte numbers.14 The confusion is compounded by the fact that in feline IBD, a disease with similar histopathologic changes to the canine form, the only reported difference from control samples was an increase in cells expressing major histocompatibility complex class II.16
Inconsistent results have also been seen with the studies conducted to date on soluble immunologic factors. Increased concentrations of acute phase proteins (e.g., C-reactive protein) have been documented in canine IBD in some,17 but not all studies.18, 19 Recent studies suggest decreased acute-phase proteins in feline IBD.20 Initial semiquantitative reverse transcriptase PCR (RT-PCR) studies suggested increased cytokine gene expression in canine chronic enteropathies,21 and this has been confirmed in one,22 but not all,23 more recent studies that have used real-time PCR methodology. Again, these results differ from feline studies, where histopathologic evidence on mucosal inflammation correlated with increases in a range of cytokines.24 Unfortunately, in all of these studies, gene expression alone was assessed, and not the functional protein. However, in a recent study, tumor necrosis factor (TNF)-γ protein was not detected in the serum of 15 dogs with IBD.16
The reasons for such discrepancies are not known but may relate to the fact that the chosen gold standard throughout was histopathology, which itself is variable and lacks consensus among pathologists.2 An alternative possibility is that the many studies have assessed patients in various stages of disease, and it may be that immunologic responses differ, and this lead some researchers to suggest the alternate name of “chronic enteropathy.”18
Clinical Presentation
Historical Findings
Many clinicians consider small intestinal IBD to be the most common cause of chronic vomiting and diarrhea in dogs and cats; however, given that large scale epidemiologic studies have hitherto not been conducted, the true prevalence of the condition is unknown. In reality, the condition may be overdiagnosed as a result of the ease with which endoscopic biopsy samples of the intestine can be collected, the current difficulties in interpretation of histopathologic specimens, and because alternative reasons for the clinical signs are inadequately eliminated during the diagnostic workup.
The studies that have been published to date suggest that canine SI IBD is most common in middle-aged animals and is uncommon in dogs younger than 12 months of age. Young and growing animals are most likely to suffer from either infectious causes of chronic diarrhea or adverse reactions to food components. No apparent gender predisposition has been reported. IBD can potentially occur in any breed of dog, although predispositions are reported for certain breeds (discussed previously). IBD can affect cats of any age or gender, although middle-aged animals are most commonly affected. Some pure breeds (e.g., Siamese) are said to be predisposed to IBD; the pattern of disease also differs in that concurrent histopathologic changes can be seen the intestine, pancreas, and liver (often termed triaditis) (Figure 57-14 ). For instance, there may be concurrent LPE, lymphocytic cholangitis, and chronic lymphocytic pancreatitis.2 Interestingly, concurrence of IBD and pancreatitis has been reported in dogs,25 suggesting that the distinction between species may be less clear than previously thought.
Figure 57-14.
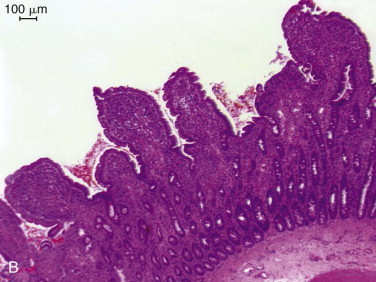
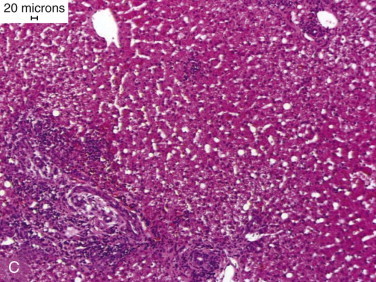
A, Eight-year-old, neutered female, domestic shorthair cat with severe cachexia, diarrhea, and Malassezia dermatitis. B, Histologic appearance of a jejunal biopsy specimen taken from the cat in (A), showing evidence of LPE. Hematoxylin and eosin stain; bar, 100 µm. C, Histologic appearance of a liver biopsy specimen taken from the cat in (A), showing evidence of mild lymphocytic-cholangitis. Hematoxylin and eosin stain; bar, 20 µm.
A range of possible clinical signs is associated with both canine and feline SI IBD, but none are pathognomonic for the condition. Not surprisingly, a “small intestinal pattern” diarrhea is most commonly seen (e.g., increased volume, watery, altered color). However, mixed-pattern diarrhea can be seen if the IBD also involves the large intestine and, in occasional cases, a large intestinal pattern diarrhea occurs, most likely the result of prolonged SI diarrhea or the presence of agents that stimulate colonic secretion (e.g., bacteria, bacterial toxins, deconjugated bile acids, or hydroxylated fatty acids).
In cats, vomiting is often the predominant clinical sign of small intestinal IBD, and diarrhea may be only occasional or absent. This may, in part, be related to the fact that some cats do not use a litter tray and thus owners may be unaware of toileting habits. Vomiting is also seen in canine SI IBD although, in the author's experience, almost invariably accompanies, and is less severe than, diarrhea. Hematemesis or melena is usually associated with more severe disease, which has caused mucosal ulceration or erosion; although it can occur in any form of IBD, it appears to occur more often with EE.
Appetite changes can be variable in SI with some cases demonstrating polyphagia, others show differing severities of anorexia, or there may be no appetite change observed. If marked inflammation is present within the SI, significant malabsorption may result, and this can lead to weight loss. Such cases may also develop panhypoproteinemia, and two studies demonstrated that hypoalbuminemia is a poor prognostic indicator in IBD.18, 26 If marked hypoalbuminemia is present (e.g., serum albumin concentrations below approximately 1.5 g/dL), associated signs such as ascites and subcutaneous edema may develop. Thromboembolism and remote organ failure is seen in some patients with PLE. Other systemic consequences of IBD include thrombocytopenia and arthropathies and typical signs may be noted. However, such reports are rare,27 and in my opinion these are uncommon findings in both canine and feline IBD. Progression of disease is variable and, in some cases, signs may wax and wane.
Physical Examination
General physical examination findings may include dehydration, alterations in demeanor, poor body condition, and signs of anemia if associated blood loss is severe. Abdominal palpation is an important component of the examination, and associated findings include (mild) abdominal discomfort, thickened intestines, turgid or thickened intestinal loops, and ascites (fluid thrill). Although rectal examination does not directly investigate the SI, it may reveal evidence of changes in fecal characteristics (e.g., melena).
Determining Clinical Severity
In humans, activity indices are used to quantify disease severity in IBD, aiding the assessment of the response to treatment and allowing comparisons between published studies in the literature. Recently, an activity index was suggested for canine IBD (canine IBD activity index [CIBDAI]; Table 57-3 ),17 and its use in the clinical setting provides a more objective measure of therapeutic response.18, 28 In one study, CIBDAI correlated with serum acute phase protein concentrations,17 although this was not confirmed by recent work.19 Clinicians must understand that increases in CIBDAI simply suggest an increase in severity of GI signs, and that high values do not confirm the diagnosis of IBD. For example, increased CIBDAI has been seen in dogs with food-responsive conditions, and values decreased on successful treatment.29 More recently, a variation of CIBDAI, the canine chronic enteropathy activity index (CCEAI; see Table 57-3) was proposed.18 All of the same signs are scored as for the CIBDAI, but additional characteristics are also assessed (e.g., presence of ascites and/or peripheral edema, pruritus, and serum protein concentrations). A recent study shows that this correlates better with prognosis than the CIBDAI18; however, the advantage of improved performance may be offset by the requirement for blood sampling and serum albumin measurement. Time will tell which system is preferred by clinicians and researchers. Finally, an activity index for feline IBD (FIBDAI) was proposed, which makes use of histology, GI signs, serum total protein and phosphorous concentrations, serum alkaline phosphatase concentration, and endoscopic lesions.30
Table 57-3.
Criteria for Assessment of Severity of Canine Inflammatory Bowel Disease
| Characteristic | CIBDAI | CCEAI |
|---|---|---|
| Attitude/activity | 0. Normal | 0. Normal |
| 1. Slight decrease | 1. Slight decrease | |
| 2. Moderate decrease | 2. Moderate decrease | |
| 3. Severe decrease | 3. Severe decrease | |
| Appetite | 0. Normal | 0. Normal |
| 1. Slight decrease | 1. Slight decrease | |
| 2. Moderate decrease | 2. Moderate decrease | |
| 3. Severe decrease | 3. Severe decrease | |
| Vomiting | 0. None | 0. None |
| 1. Mild (once/wk) | 1. Mild (once/wk) | |
| 2. Moderate (2 to 3/wk) | 2. Moderate (2 to 3/wk) | |
| 3. Severe (>3/wk) | 3. Severe (>3/wk) | |
| Stool consistency | 0. Normal | 0. Normal |
| 1. Slightly soft feces, fecal blood mucus, or both | 1. Slightly soft feces, fecal blood mucus, or both | |
| 2. Very soft feces | 2. Very soft feces | |
| 3. Watery diarrhea | 3. Watery diarrhea | |
| Stool frequency | 0. Normal | 0. Normal |
| 1. Mild increase (2 to 3/day) | 1. Mild increase (2 to 3/day) | |
| 2. Moderate increase (4 to 5/day) | 2. Moderate increase (4 to 5/day) | |
| 3. Severe increase (>5/day) | 3. Severe increase (>5/day) | |
| Weight loss | 0. None | 0. None |
| 1. Mild (<5%) | 1. Mild (<5%) | |
| 2. Moderate (5% to 10%) | 2. Moderate (5% to 10%) | |
| 3. Severe (>10%) | 3. Severe (>10%) | |
| Serum albumin | 0. Albumin >2.0 g/dL | |
| 1. Albumin 1.5 to 1.99 g/dL | ||
| 2. Albumin 1.2 to 1.49 g/dL | ||
| 3. Albumin <1.2 g/dL | ||
| Ascites and peripheral edema | 0. None | |
| 1. Mild ascites or peripheral edema | ||
| 2. Moderate ascites/peripheral edema | ||
| 3. Severe ascites/pleural effusion and peripheral edema | ||
| Pruritus | 0. No pruritus | |
| 1. Occasional episodes of itching | ||
| 2. Regular episodes, but stops when asleep | ||
| 3. Dog regularly wakes up due to itching | ||
| Final score | 0 to 3. Clinically insignificant disease | 0 to 3. Clinically insignificant disease |
| 4 to 5. Mild IBD | 4 to 5. Mild IBD | |
| 6 to 8. Moderate IBD | 6 to 8. Moderate IBD | |
| ≥9. Severe IBD | ≥9. Severe IBD |
CIBDAI, Canine inflammatory bowel disease activity index; CCEAI, canine chronic enteropathy activity index.
Approach to Diagnosis
Given that none of the clinical signs and physical examination findings that are seen with IBD are pathognomonic, further investigations are essential in order to make a diagnosis. Because the term idiopathic IBD should be restricted to use in cases in which intestinal inflammation is found without an obvious underlying cause, all other etiologies must first be excluded. Therefore detailed preliminary diagnostic investigations must be performed, prior to acquisition of GI biopsy samples, to ensure that other etiologies are excluded. Investigations include fecal analyses, routine hematologic analysis, clinical chemistry, urinalysis, assay of serum TLI, pancreatic lipase immunoreactivity, and diagnostic imaging. Although none of these tests is diagnostic for IBD, they help to eliminate the possibility of extraintestinal disease (e.g., pancreatitis, hypoadrenocorticism, renal failure, and hepatic failure), anatomic intestinal disease (e.g., tumor or intussusception), and other known causes of intestinal inflammation. Diagnostic imaging in particular allows the clinician to determine whether focal or diffuse intestinal disease is present, allowing selection of the most appropriate method of intestinal biopsy. In many cases, standardized therapeutic trials can help to confirm the diagnosis, by eliminating other possible causes of intestinal inflammation.
Hematology
In companion animals with SI IBD, hematologic examination is frequently unremarkable. Changes in white blood cells that are occasionally observed include mature neutrophilia, neutrophilia and left shift, and eosinophilia, but none are pathognomonic. Reactive “atypical” lymphocytes may be seen in patients with LPE, and lymphopenia can occur if lymphangiectasia is present. If anemia is present, it may be a reflection of either chronic inflammation or chronic blood loss. Iron-deficiency anemia, with a microcytic hypochromic pattern, also has been reported in IBD,31 and thrombocytosis also may be seen.31
Clinical Biochemistry
In many patients with SI IBD, clinical biochemistry is unremarkable. If PLE is present, serum concentrations of both albumin and globulin can be decreased. Confirmation of PLE requires the absence of significant liver changes (e.g., marked enzyme elevations, low urea, low glucose) on clinical chemistry, or the absence of anemia on complete blood cell count, and of proteinuria on urinalysis. However, fecal α1-PI measurement may help. Hypocholesterolemia may suggest malabsorption, but this finding is not pathognomonic. Ionized hypocalcemia and hypomagnesemia are also reported.32, 33 Intestinal inflammation in dogs may cause a reactive hepatopathy with mild to moderate (two- to fourfold increases) in liver enzyme (i.e., alanine transaminase [ALT] and alkaline phosphatase) activities. In contrast, as a consequence of their shorter half-lives, liver enzyme increases are less common in feline IBD, and marked elevations in ALT more commonly occur secondary to alimentary lymphoma than feline IBD.34
Fecal Examination
Fecal flotation is very important in eliminating parasitic causes of mucosal inflammation. In most cases of SI IBD, these tests yield negative results. When occasional positives do occur, determining the significance can be problematic, as these organisms can be found in the stool of healthy animals. Although trial therapy may be considered, clinicians should exercise caution given concerns over the development of therapeutic resistance.
Serum Folate and Cobalamin
Measurement of serum folate and cobalamin is available for both dogs and cats, and deficiency of these substances is associated with IBD. Recent work has highlighted the importance of hypocobalaminemia in cats,35 and hypocobalaminemia is also a negative prognostic indicator in chronic enteropathy in dogs18 as well as other alimentary tract diseases such as EPI.36 Although such alterations are not pathognomonic, deficiencies in IBD suggest the need for more aggressive therapy against the primary disease, and also the need for parenteral supplementation. This is particularly important, because cobalamin deficiency may itself have systemic metabolic consequences and cause intestinal dysfunction,37 and anecdotal evidence suggests that the response to immunosuppressive therapy for IBD may be suboptimal until cobalamin deficiency is resolved.
Diagnostic Imaging
Radiographic and ultrasonographic studies are most commonly used to eliminate other possible diseases rather than to make a diagnosis of SI IBD. However, when used in conjunction with specific clinical signs and laboratory testing, the information from imaging studies enables an appropriate choice of a biopsy method (e.g., upper or lower GI endoscopy, or exploratory celiotomy). Ultrasonography in IBD patients can help to document mesenteric lymphadenopathy.38, 39 Although intestinal wall thickness can be measured, one study suggests that this measure is of limited value in the diagnosis of SI IBD.40 In fact, the only occasions when the bowel wall was notably thickened was when edema was present secondary to marked hypoproteinemia. A recent study suggests that different ultrasonographic patterns can help to differentiate chronic enteropathies with different etiologies.41 Loss of normal intestinal layering is more commonly seen with neoplasia than IBD.
Intestinal Biopsy
Intestinal biopsy is essential to prove the presence of intestinal inflammation and confirm a diagnosis of SI IBD; either endoscopy or exploratory celiotomy can be used. During endoscopy, the gross appearance of the intestinal mucosa can also be observed. Intestinal inflammation may be indicated by findings such as increased granularity, irregularity, and friability with the presence of erosions, ulceration, and spontaneous hemorrhage. However, these findings are not pathognomonic, and correlation between gross inspection and histopathology is poor.18, 28 Limitations of endoscopy include small sample size, superficial and often fragmented samples, and the fact that tissue can only be collected from proximal regions and (occasionally) the distal ileum. Exploratory celiotomy and full-thickness biopsy may be necessary, although this is more invasive and wound healing can be problematic if severe hypoproteinemia is present.42 Nonetheless, the approach may be more suitable for cats, given the tendency for concurrent hepatic and pancreatic involvement,43 the difficulties in differentiating IBD from small-cell lymphoma in endoscopic duodenal biopsies,34 and the reliability of the small size of endoscopic biopsies that are often collected in this species.
Histopathologic Assessment of Biopsy Samples
The pattern of histopathologic changes in biopsy specimens depends upon the type of IBD. Histopathologic assessment of intestinal biopsies remains the gold standard for diagnosis of many intestinal diseases, but has marked limitations, most notably variable quality of tissue specimens obtained endoscopically44 and poor agreement between histopathologists.45 The latter may be a result of the subjective nature of interpretation of the degree of inflammation, the patchiness of inflammation, or the presence of edema (caused by hypoproteinemia) leading to difficulties in assessing cell density.
It can be difficult to distinguish severe LPE from lymphoma, particularly when endoscopic biopsy samples are examined. This may be a result of the fact that infiltration of malignant lymphocytes is patchy, that inflammatory change may accompany alimentary lymphoma (and predominate in some areas), or that lymphocytic infiltration is deep to the area sampled. Cases of feline alimentary lymphoma can be missed if duodenal endoscopic biopsy samples are used in histopathologic assessment, rather than full-thickness specimens.34 Hence, it may be preferable to collect surgical specimens in this species, and this approach gives the added advantage that other organs can be sampled (e.g., liver and pancreas) when checking for “triaditis.”
The standards published by the WSAVA GI Standardization Group2 hopefully will improve the reliability of IBD diagnosis.18, 28 Ultimately, the primary clinician should interpret histopathology results cautiously and try to relate them to the clinical presentation. Results should be questioned if the histopathologic diagnosis does not fit the clinical picture or the response to apparently appropriate therapy is poor. In some cases repeat biopsy (e.g., by exploratory celiotomy) may be required.
Diagnostic Horizons
Many research techniques have been developed to assess alterations of the immune system that occur in IBD. Examples include immunohistochemical characterization of immune cell populations,46, 47, 48, 49 measurement of gene expression for cytokines by RT-PCR,21, 22, 23 assessment of T-cell clonality,3 measurement of mucosal perinuclear antineutrophilic antibody (pANCA),50 and measurement of mucosal P-selectin expression.51 Although these techniques have not been widely adopted for clinical diagnosis, there may be potential for future application. In particular, assessment of T-cell clonality may prove to be a useful tool to differentiate LPE from low-grade lymphoma.3 In addition, there may be benefit in development of mucosal pANCA and P-glycoprotein expression for helping to predict response to therapy.50, 51
Treatment as a Diagnostic Tool
In many cases, clinicians can use an organized therapeutic plan to help confirm the diagnosis, and determine the optimal therapy for a particular case. Unless the animal is debilitated, single therapeutic modalities are instigated sequentially, and the owner is asked to record precisely in an event diary the frequency and nature of clinical signs. The clinician can then review the diary and calculate disease severity using one of the recognized scoring schemes (see Table 57-3). Although response to treatment can inevitably be judged more objectively, clinicians should still be cautious that therapy might have only invoked a placebo effect. My favored order of treatment trials is anthelmintic/antiparasitic medication, dietary modification, antibacterial trials, and, finally, trial immunosuppressive therapy.
Approach to Therapy
Regardless of the histologic type of IBD, treatment usually involves a combination of dietary modification, antibacterials, and immunosuppressive therapy. Most recommendations are based upon individual experience because objective information of efficacy is generally lacking, and no randomized controlled clinical trials have been conducted. A staged approach to therapy is recommended whenever possible, but may not be appropriate in seriously ill patients (e.g., those with severe hypoproteinemia) where immediate intervention with combination therapy may be essential. Where sequential therapeutic trials are used, initial treatment should be with antiparasitic agents to eliminate occult endoparasite infection. I most commonly use fenbendazole at 50 mg/kg q24h PO for 3 to 5 days. However, not all parasites are sensitive to this drug (e.g., Trichomonas in cats) and resistance may be present in other organisms (e.g., Giardia). Subsequently, an exclusion diet and antibacterials should also be employed, before the use of immunosuppressive medication is considered. Some authors no longer bother with antibacterial medication, as one study suggested that it is not beneficial in canine IBD.52
As mentioned above, a diary record can be maintained by the owner, and used to determine success of each therapy. Where partial responses are noted to single agents, multimodality therapy can be justified. The treatment trial approach is labor intensive, but is the best way of achieving successful resolution of the clinical signs. Clients appreciate the interest shown by the clinician, and are more accepting of the advice, than when communication is poor subsequent to diagnosis.
Therapeutic Options
Intravenous Fluid Therapy
Crystalloid therapy is usually only necessary if the patient is dehydrated, but is not required for most patients. If hypoproteinemia is present, intravenous colloid therapy may be required, and options include synthetic colloids (e.g., hydroxyl-ethyl starch), and plasma transfusion. Infusions of human albumin can be considered, but this approach is controversial. First, the half-life is shorter than for synthetic colloids such as hydroxyl-ethyl starch so that any benefit may be short-lived; second, recent work has demonstrated rapid development of immune responses to the human albumin molecule, which can lead to anaphylactic reactions53; importantly, some dogs developed anaphylaxis even on first administration of albumin, perhaps because prior exposure had occurred (e.g., during vaccination or intradermal skin testing). Finally, because the human albumin molecule will crossreact in the serum albumin assay, this parameter cannot be used as a means of monitoring response to therapy.
Dietary Management
Most clinicians agree that dietary management is a key component in the successful treatment of IBD, and in support of this, dietary modification was recently shown to play a critical part in long-term therapy for cats with chronic GI disease,54 and dogs with chronic enteropathy.50 Two main approaches exist for dietary management of SI IBD: switching to a highly digestible diet or to an exclusion diet. In reality, these strategies need not be mutually exclusive because most commercial exclusion diets are also formulated to be highly digestible.
High digestibility ensures that components can be readily assimilated in the face of suboptimal digestive function. Protein should be of high biologic value. Gluten is perceived to be a common food allergen, largely because of its known association with gluten-sensitive enteropathy in Irish Setters.55, 56 As a result, most formulated diets avoid the use of gluten. However, although undoubtedly responsible for some adverse reactions to food, there is no evidence that gluten is any more antigenic to other commonly fed proteins. Fat restriction was traditionally recommended because of concerns over malabsorption, meaning that unassimilated fatty acids could be available for hydroxylation, thus stimulating electrolyte secretion. However, the need for fat restriction has recently been challenged as most cases can tolerate a higher dietary fat content, and fat restriction may exacerbate existing weight loss. Modification of the n3:n6 fatty acid ratio may also modulate the inflammatory response and have some benefit in treatment and maintenance of remission.57, 58 However, there have been no studies done to prove a benefit of such modification in canine IBD.
An exclusion diet trial should be considered in all cases of unexplained intestinal inflammation to exclude the possibility of an adverse food reaction. Most clients are willing to try this first, given concerns over the side effects of immunosuppressive drugs, but this may not be feasible in severely ill animals. The choice of diet depends upon prior dietary exposure, and the preference of both owner and clinician, and options include home-prepared recipes or commercial single-source protein diets. Although, no GI-specific data exist to recommend one approach over the other, recent work in canine atopic dermatitis demonstrates improved efficacy of commercial rations over home-cooked rations.59
The main recent advance in this field has been the availability of hydrolyzed protein diets, where a native chicken or soy protein has undergone chemical or enzymatic treatment, producing low-molecular-weight protein derivatives. In theory, such diets should be less antigenic; this supposition is supported by recent work, in an experimental model of canine food-allergic skin disease, demonstrating reduced in vitro antigenicity compared with the native molecule.59 The other major advantage of utilizing protein hydrolysates is the improved digestibility of the protein components, which may be superior to traditional single-source protein exclusion diets. Thus they are now the exclusion diet of choice for many clinicians, and a recent clinical trial was encouraging.60
Whichever type of diet is chosen, it must be palatable and should be introduced in gradually increasing amounts over 4 to 7 days. It is best to feed the chosen diet exclusively, and in small, frequent meals (e.g., 4 to 5 per day). Finally, parenteral nutrition is occasionally required for cases of severe, debilitating IBD.
Vitamin Supplementation
Cobalamin malabsorption is relatively common in SI IBD, especially if distal regions are involved, and this can have significant metabolic consequences, including ill-thrift and poor appetite. As mentioned previously, cobalamin deficiency is a negative prognostic sign in canine chronic enteropathy18 and is associated with more severe feline alimentary tract disorders.35 When hypocobalaminemia is identified, therapy is recommended. Oral administration of cobalamin is ineffective and it must be given by subcutaneous injection (e.g., 20 µg/kg q7days for 4 weeks and then the same dose q28days for a further 3 months). Serum cobalamin concentration should be rechecked a month after the last dose and should be supranormal at that time, indicating that cobalamin supplementation can be discontinued. Less commonly, folate malabsorption may accompany severe and prolonged SI IBD, and oral supplementation is easily achieved with administration of approximately 1 mg of folic acid per day. Although such therapy is well-tolerated, no published studies are available to support the therapeutic benefit of such an approach.
Antibacterial Therapy
The use of antimicrobials in IBD can partly be justified by the potential to treat any undiagnosed enteropathogens, and partly by the fact that it is bacterial antigens which are thought to drive the pathogenetic pathways. However, this approach is not universally accepted and a recent study suggests that antibacterial therapy is of limited benefit in canine IBD.52 Metronidazole remains the preferred antibacterial for SI IBD, and it has long been suggested to have immunomodulatory properties in addition to an antimicrobial action. Tylosin also may have immunomodulatory effects and may have some efficacy in canine IBD. Although there are few studies demonstrating the efficacy of these drugs in companion animal IBD, a recent study in a rat model of IBD (colitis induced by 2,4,6-trinitrobenzene sulfonic acid) suggests that tylosin is effective at decreasing inflammation.61 This work is particularly interesting in light of the recent report of a series of dogs with diarrhea that responded to tylosin therapy.62 However, the relationship between this condition and the IBD syndrome is not well understood.
Immunosuppressive Drugs
Undoubtedly, the most important therapy for idiopathic IBD is immunosuppression, although this should only be considered a last resort. In human IBD, glucocorticoids and thiopurines (e.g., azathioprine, 6-mercaptopurine) are the most widely used.63
In dogs and cats, glucocorticoids are most frequently used for immunosuppressive therapy in SI IBD, and prednisolone (or prednisone) is the drug of choice. The standard initial dosage in dogs is 1 mg/kg PO q12h, given for 2 to 4 weeks, and then tapered slowly over the subsequent weeks to months. In most cases therapy can be only be reduced to a low maintenance dose given q48h, and in the minority of cases can be completely withdrawn. Cats are usually treated with higher doses, typically twice those of dogs (2 mg/kg q12h PO initially, then tapering).
If severe malabsorption is present, prednisolone can be administered parenterally and oral therapy instigated once signs have improved. In some cases an initial response to steroids is followed by a relapse and lack of further response even when dosages are increased. This may be either a result of transformation to lymphoma or an incorrect initial diagnosis. However, it is feasible that resistance to steroids may have developed, because of induction of the multiple-drug resistance gene and expression of P-glycoprotein.51 Despite the widespread use of these drugs in companion animal gastroenterology, controlled clinical trials demonstrating efficacy are lacking. A recent study in dogs suggests that CIBDAI decreases upon successful treatment with steroids,29 although neither mucosal permeability nor histopathologic abnormalities change significantly. This further supports the supposition that the condition is controlled rather than cured with such therapy.
In some dogs, high doses of conventional glucocorticoid therapy are poorly tolerated. In this circumstance, options include adding a drug to provide a “steroid-sparing” effect, or using a novel steroid with fewer side effects. Budesonide is a glucocorticoid with high first-pass metabolism, and an enteric-coated formulation of this drug has been successful in maintaining remission in human IBD. The use of this drug has been reported for canine IBD,13 although there is no evidence to suggest it is more efficacious than prednisolone. Interestingly, both suppression of the hypothalamic–pituitary–adrenal axis and development of glucocorticoid hepatopathy have been demonstrated in dogs, but minimal systemic effects are noted.13, 64 The optimal dose has not yet been determined, although, anecdotally, a dose of 1 to 3 mg/m2 q24h PO has been used. A delayed-release formulation is most often used, but the main concern in SI IBD is that the drug may not become active until after it reaches the large intestine. Further work is required before clinicians can be confident in the use of this drug.
Azathioprine at 2 mg/kg PO q24h is commonly used in dogs in combination with prednisolone/prednisone when the initial response to steroid therapy is poor or side effects are marked. However, its activity may show a delayed onset (up to 3 weeks) and, given its myelosuppressive potential, regular hematologic monitoring is necessary. Azathioprine is not recommended for cats, largely because cats have very low activities of thiopurine methyltransferase (TPMT), the major enzyme involved in the degradation of 6-mercaptopurine (6-MP), the active metabolite of azathioprine. Chlorambucil (2 to 6 mg/m2 PO q24h until remission, then tapering) is a better choice of a cytotoxic immunosuppressive agent in cats. A combination of prednisolone and chlorambucil can also be effective for alimentary lymphoma in cats, and is particularly suitable for use when differentiation of severe IBD from lymphoma has been problematic.
Cyclosporine binds to the cytosolic protein cyclophilin (immunophilin) of immunocompetent lymphocytes (especially T lymphocytes); the resulting complex then inhibits calcineurin, which itself is responsible for activating interleukin-2 transcription, thereby leading to a reduced function of effector T cells. As a result, it has been proven to be useful as an immunomodulator in human gastroenterology.65 Efficacy has been demonstrated in immune-mediated conditions such as anal furunculosis and atopic dermatitis. A recent uncontrolled study also shows that cyclosporine (5 mg/kg q24h PO) may be effective in IBD, which is refractory to steroid therapy.66 Most dogs achieved either complete or partial remission, and response correlated with reductions in both CIBDAI and mucosal T-lymphocyte numbers; however, no change in histopathology score was noted. The favorable response came at a cost, with side effects (including vomiting, gingival ulceration, alopecia) occurring in almost half of the dogs.
This drug is most commonly used in refractory cases of IBD, when other immunosuppressive therapy has failed, but widespread use may be limited by cost of therapy. Furthermore, given the marked immunosuppressive effect, it is vital that all possible infectious etiologies have first been eliminated; thus the drug should be used with caution in areas where fungal infections are endemic, and it may be prudent to screen for occult infectious diseases (e.g., Toxoplasma, feline leukemia virus [FeLV], and feline immunodeficiency virus in cats), prior to instigating therapy.
Other immunosuppressive cytotoxic drugs include methotrexate and cyclophosphamide. Methotrexate is effective in the treatment of human Crohn disease,67 but it is not widely used in companion animals. A single case report has reported a response of severe IBD, with concurrent hypoproteinemia and lymphangiectasia, to methotrexate after a combination of prednisolone and cyclosporine were ineffective.68 This observation should be confirmed with larger case series and, preferably, evidence-based medicine before the routine use of this drug is recommended. Cyclophosphamide has few advantages over azathioprine and is rarely used.
Mycophenolate mofetil has been used to treat human IBD, although its efficacy is variable.69 Its use is reported for the treatment of canine myasthenia gravis,70 but to my knowledge there are no published studies on its use for SI IBD in dogs or cats. Drugs that target TNF-α (e.g., thalidomide, oxpentifylline) are used in human IBD, but have not been used in dogs. Anti–TNF-α monoclonal antibody therapy is also beneficial in human IBD, but will only be suitable for canine and feline IBD if species-specific monoclonal antibodies are developed for therapeutic use.71, 72, 73
Prebiotics and Probiotics
Modulation of the enteric flora with probiotics or prebiotics included in the food may have benefits in IBD. Nondigestible carbohydrates, such as lactulose, inulin, fructooligosaccharides, and mannanoligosaccharides are the most frequently used prebiotics. However, there is little evidence that they modify the bacterial flora of the SI.73 These agents are frequently incorporated in diets formulated for therapy of SI IBD. Probiotics can directly antagonize pathogenic bacteria, but they also modulate mucosal immune responses. Probiotics are suggested to be beneficial for human IBD,74 although no truly objective data (e.g., double-blind placebo-controlled trials) exist, despite promising initial reports. More work is required before probiotic use becomes commonplace in companion animal IBD therapy.
Miscellaneous Therapies
Diuretics may reduce ascites: spironolactone at 1 to 2 mg/kg PO BID may be more effective than furosemide for treating ascites. Thromboembolism is a feature of some patients with PLE, and prophylactic low-dose aspirin at 0.5 mg/kg BID has been advocated in PLE. Some dogs with SI IBD present with severe microcytic anemia31 and these may require intravenous blood replacement (whole blood or packed red cells). Furthermore, oral ferrous sulfate (at 200 mg/dog) may be required for a prolonged period (often months).
Predicting Response to Therapy
Some studies have examined ways of predicting response to future therapy. For example, high mucosal pANCA expression prior to treatment correlates with response to dietary therapy, while those that did not respond (and required glucocorticoids) had lower levels of expression.50 P-glycoprotein is a transmembrane protein that functions as a drug-efflux pump in the intestinal epithelium. In human IBD, high lymphocyte P-glycoprotein expression is seen in patients who fail to respond to treatment with steroids. Recent work in canine IBD demonstrates that low pretreatment mucosal lymphocyte P-glycoprotein expression correlates with a favorable response to treatment.51 The lowest levels of expression were found in dogs that responded to a dietary trial, moderate levels in steroid-responders, and the highest levels in those that responded neither to steroid nor diet. However, the main limitation of this assay is the necessity for repeat endoscopy to monitor cases, given that many owners may be reluctant to allow their pet to undergo such procedures.
Prognosis and Prognostic Indicators
A recent study examining prognosis in canine IBD suggests that success of therapy is variable.26 Although many cases initially responded, only a quarter achieved complete remission; intermittent signs remained in a further half of the dogs, while response was poor in the remaining cases and many were euthanized. One negative prognostic indicator was hypoalbuminemia, a finding recently confirmed in another study, which also identified hypocobalaminemia as a negative risk factor.18 A high disease activity index (e.g., CIBDAI, CCEAI) and a high endoscopic score were also identified as risk factors for a poor outcome in this study.
Other potential markers for IBD prognosis include serum acute-phase proteins, such as C-reactive protein; in one study, C-reactive protein was found to decline upon successful therapy17; however, this acute-phase protein did not correlate with outcome in two more recent studies.18, 19 Furthermore, histopathologic scoring of biopsies, prior to and after therapy, does not correlate with outcome,18, 28 although this study was conducted prior to the release of the histologic scoring scheme.2 Mucosal pANCA expression recently was shown to be increased (prior to therapy) in cases that ultimately respond to dietary management, and expression of this marker increases posttherapy in steroid-responsive cases. Finally, low pretreatment mucosal lymphocyte P-glycoprotein expression was recently shown to predict a favorable response to therapy, and could also be of use in determining which treatments (diet or steroids were most suitable).51
Malabsorption
Michael D. Willard
Etiology
Malabsorption
Malabsorption generally connotes chronic small intestinal disease that may have one or more underlying pathophysiologic mechanisms.1 Malabsorption is usually, but not always, associated with diarrhea. Common causes of malabsorption include dietary-responsive disease (e.g., allergy and intolerance; see Chapter 31), antibiotic-responsive diarrhea (also referred to as SIBO and “dysbiosis”; see other sections in this chapter, Infection, Neoplasia, and IBD).
Protein-Losing Enteropathy
PLE is associated most importantly with lymphangiectasia (dogs only), lymphoma, parasites, fungal infections, ulceration/erosion, intussusception, and IBD,2 but dietary-responsive disease and antibiotic-responsive disease may also be responsible.
Short Bowel Syndrome
Short bowel syndrome (SBS) occurs when a patient has a large resection of the SI.3 It is almost always an iatrogenic phenomenon, and is typically caused by overly aggressive small intestinal resection as a result of diffuse malignancy, linear foreign bodies, multiple perforations, and adhesions caused by peritonitis.
Pathophysiology
Malabsorption
Malabsorption may result from reductions in absorptive surface area (e.g., villus atrophy and villus fusion), damage to enterocytes (e.g., bacterial infection), and intestinal mucosal infiltration (e.g., inflammatory or neoplastic cells).1 Multiple pathophysiologic mechanisms may be operative in an individual patient. Villus atrophy is caused by loss of enterocytes, decreased precursors in intestinal crypts, infiltrative disease causing villus fusion, and mechanical destruction of the absorptive surface area (e.g., massive gastric acid emptying into the intestine with gastrinoma). Enterocytes can be damaged by bacteria (e.g., damage to the microvillar membrane) or can be immature and poorly functional as a result of accelerated turnover. Infiltrates of the mucosa can affect mucosal permeability, villus structure and function, and lymphatic flow; therefore the host's immune system may be integral to the ultimate severity of the intestinal lesion. Intestinal pathology from any number of causes might allow otherwise normal luminal bacteria to proliferate or persist, in turn causing worsening of enterocyte damage and/or more mucosal inflammation. Finally, animals severely malnourished and protein-deficient from the intestinal disease may have a more difficult time repairing intestinal damage. In this way malabsorptive disease may become self-perpetuating.
Dietary antigens can cause immune (types I and IV hypersensitivity) as well as non–immune-mediated (i.e., intolerance) reactions in the intestinal mucosa (see Chapter 31).
Intraluminal bacteria (discussed in more detail in Chapters 2 and in other sections of this chapter, e.g., “Infection”) can elicit inflammatory responses in the intestinal mucosa, and bacterial toxins and metabolic by-products can damage enterocytes through various mechanisms such as deconjugated bile acids, alcohols, and hydroxylated fatty acids. Antibiotic-responsive disease is generally caused by nonpathogenic bacteria, hence specific toxins (as are seen with certain E. coli or Campylobacter infection) are not usually considered important.
Parasites (e.g., Giardia) can have direct cidal effects on enterocytes.
IBD is a syndrome in which intestinal mucosal inflammation becomes self-sustaining and recurrent (see Chapters 3, 56, and 59Chapter 3Chapter 56Chapter 59). Dietary and bacterial antigens play an important role in disease pathogenesis. The mechanisms are speculative, but it is believed that these antigens gain access to the mucosa, perhaps as a result of increased mucosal permeability, and then either an aberrant immune response or a constant ingress of antigens maintains the inflammatory response. Cats might have a greater incidence of IBD, which might be partially explained by their strong inflammatory response to exogenous antigens.
Protein-Losing Enteropathy
PLE occurs when serum proteins are lost into the GI tract. There are three basic mechanisms for protein loss: lymphatic obstruction or rupture, increased mucosal permeability because of mucosal infiltrates (e.g., inflammatory and neoplastic), and mechanical causes (e.g., ulcers, erosions, and congestion).2, 4
Lymphangiectasia primarily affects dogs and is usually caused by lymphatic obstruction, either anatomic or physiologic.5 Lacteals in the villi dilate as they absorb lipid and lipoprotein until they finally rupture, releasing protein and lipid back into the intestinal lumen. The severity of PLE depends mostly upon the distribution of this lesion. If only a small portion of the intestine is affected, protein loss may be offset by the ability of the healthy intestine to digest and absorb proteins and lipids, as well as the liver's ability to produce more albumin. Some PLE patients may have normal serum albumin concentrations if the obstructive process is of short duration and limited distribution but, PLE is seldom diagnosed until the discovery of hypoalbuminemia causes the clinician to look for a reason.
Lipogranulomas in the intestinal wall or mesentery (Figure 57-15 ) develop as a consequence of lymphatic leakage and further complicate the disorder. As lipogranulomas enlarge and coalesce, they alter tissue compliance and contribute to the obstructive lymphatic process. Some dogs with lymphangiectasia and severe hypoalbuminemia have minimal mucosal inflammation except perhaps for a few inflammatory cells around areas of lymphatic leakage or some macrophages attempting to eliminate the chyle.2
Figure 57-15.
A, A gross picture taken at surgery of a dog with lymphangiectasia that had severe lipogranulomas (arrows) at the mesenteric border. B, A photomicrograph of an intestinal biopsy from the tissues seen in (A). Note the large lipogranuloma in the tunica muscularis (arrow).
Inflammatory conditions (e.g., IBD, histoplasmosis) and some neoplasia (e.g., lymphoma) seemingly cause PLE because inflammatory and neoplastic cell infiltrates alter vascular and mucosal permeability, thereby permitting serum proteins and red blood cells to leak from the mucosa. Parasites (hookworms, whipworms) and ulcers/erosions cause mucosal bleeding that produces the PLE. Congestion in the tip of an intussusceptum can cause serum exudation and hypoalbuminemia.
Short Bowel Syndrome
Massive small bowel resection, nutrient malabsorption, and protein-calorie malnutrition define SBS.3 There is no precision in the amount of small bowel resection that must take place before the development of SBS. Some patients tolerate large resections with minimal complication, whereas others have significant complications following lesser amounts of resection.6 Development of SBS is influenced by the patient's preoperative nutritional status as well as postoperative pathophysiology. For example, following resection, gastric acid may cause chemical injury to the remaining unbuffered SI. Moreover, the remaining small bowel often assumes a bacterial flora more typical of the large bowel. This problem is further exacerbated with resections of the ileocolic sphincter.
Clinical Examination
Malabsorption
Weight loss or loss of body condition, small bowel-type diarrhea, and polyphagia are the primary clinical signs of malabsorptive disease, although this triad is not uniformly present in all SBS patients. Diarrhea is defined as an increase in the liquidity, frequency, or volume of feces. The colon has an abundant reserve capacity to absorb water (see Chapter 1); therefore the feces may appear “normal” to the pet owner despite severe small bowel disease. Instead these patients will have a voluminous fecal output as a result of intestinal malabsorption. If accelerated transit and hypersecretion make the patient nauseous, it may have a poor appetite instead of polyphagia.1 The vestigial SI may seem normal or thickened on abdominal palpation. Some patients with dietary allergies also will have cutaneous manifestations consistent with allergy.
Clinical pathology testing is often not very informative, except for hypoalbuminemia, hypocholesterolemia, and hypocobalaminemia. Changes in cobalamin (vitamin B12) metabolism are very specific for distal small intestinal disease, but it is a fairly insensitive biomarker; many animals with severe small intestinal disease have normal serum cobalamin concentrations.7 Imaging of the gut is also insensitive. Ultrasonographic determination of small intestinal thickness has not been shown to clearly correlate with disease8; however, changes in small intestinal layering may reflect infiltrative disease. Mild to moderate mesenteric lymphadenopathy may be seen in patients with nonneoplastic disease.
Protein-Losing Enteropathy
Diarrhea as a clinical sign is inconsistently reported in many PLE patients. Intestinal lymphangiectasia patients in particular often have normal-appearing feces.2 Ascites (low-protein transudate) and/or peripheral edema may be the only historic or physical examination findings, especially in lymphangiectasia patients. Weight loss is common but may be “hidden” by the ascites, unappreciated until palpation reveals boney prominences across the body. Two breeds at risk for PLE are the soft-coated Wheaton Terrier9 (Wheaton Terriers may have concurrent PLN) and the Norwegian Lundehund.10 Yorkshire Terriers have an increased risk for PLE, based upon data reported from one institution.11, 12 Chinese Shar-Pei and Basenji dogs are prone to severe IBD with concurrent PLE.2
Hypoalbuminemia is the key clinicopathologic finding in PLE. Panhypoproteinemia is “classic” but not invariable.2 Patients who hyperglobulinemic prior to developing PLE (e.g., chronic inflammation caused by rickettsial, fungal, cutaneous, or heartworm infection) may lose most of their serum proteins but still have normal serum globulin concentrations. Lymphopenia sometimes occurs if the intestinal lymphatics are involved in the disease process. Hypocholesterolemia is common and expected, but is also seen in hepatic insufficiency. Hypocholesterolemia is not typically seen in PLNs (which sometimes helps distinguish between these causes). Hypocalcemia is common finding,5 and would appear to be secondary to hypoalbuminemia in many patients, but suppression of parathyroid function11, 12 and/or decreased vitamin D13 may be responsible in others. The hypocalcemia is seldom associated with tetany. It is more clinically useful to measure ionized serum calcium as opposed to total serum calcium. Hypomagnesemia often occurs in severe PLE and may contribute to hypocalcemia by affecting parathyroid function.11, 12 Fecal α1-PI concentrations are generally increased in this patient population.14 Ultrasound sometimes reveals “streaking” within the mucosa, presumably associated with the dilated lymphatics in lymphangiectasia.15
A thickened segment of bowel is palpated in many patients with GI intussusception, although this can be easily missed if the intussusception takes place at the root of the mesentery. Young dogs with a recent history of enteritis (e.g., parvoviral, parasitic) from which they should have recovered, but which continue to have diarrhea, is suggestive of the disorder. Finding hypoalbuminemia in these same patients strongly suggests parasites or intussusception. Abdominal ultrasound is the best way to noninvasively detect intussusceptions.
Short Bowel Syndrome
These patients have had a recent, massive, intestinal resection and are typically losing weight (or are already emaciated) with severe, profuse diarrhea.3
Diagnosis
Malabsorption
Malabsorptive disease is presumptively diagnosed from history, physical examination findings, clinical pathology data, and by eliminating other causes of disease (e.g., feline hyperthyroidism, hepatic insufficiency).1 Finding small intestinal histopathology with an appropriate history and physical examination findings is confirmatory, but not all small intestinal malabsorptive diseases have concurrent histologic changes. In particular, dogs with antibiotic-responsive enteropathy or dietary-responsive disease may have minimal to no discernible histologic change in the SI. Therapeutic trials are often the best way to diagnose these last two diseases. For most other disorders (e.g., IBD, intestinal lymphoma), biopsy is necessary.
Protein-Losing Enteropathy
PLE is often a diagnosis of exclusion.2 Patients typically have serum albumin ≤2.0 g/dL, and other causes of hypoalbuminemia must be eliminated. PLN and hepatic insufficiency are the two major differentials for this finding. Anorexia and weight loss are inadequate explanations for a serum albumin this low. Urinalysis/urine protein-to-creatinine ratio and hepatic function tests (e.g., serum bile acid concentrations) are typically needed to eliminate these syndrome. Serum blood urea nitrogen and creatinine concentrations, ALT activity, and bilirubin concentrations will be inadequate for this purpose. It should be reemphasized that some dogs with PLE will not have diarrhea, at least as defined by an increase in the liquidity of feces.
It should also be recognized that patients with PLN or hepatic insufficiency can have concurrent PLE. If a patient with modest urinary protein loss has concurrent diarrhea and a serum albumin of 1.5 g/dL, the urinary protein is likely inadequate to explain the magnitude of the hypoalbuminemia. Such patients may require measurement of fecal α1-PI concentrations for a definitive diagnosis.14 This test is available through the GI laboratory at Texas A&M University. The test has many difficulties in the collection and storage of feces, making it critical to consult the laboratory before beginning the study.
In parasite-rich environments, one cannot eliminate parasitism because of one negative fecal examination. Adult animals can die of prepatent hookworm infections, and whipworm ova are often difficult to detect. Ultrasonographic imaging may be indispensable in the detection of neoplastic masses, inflammatory infiltrates (which might permit diagnosis by fine-needle aspiration), and intussusceptions. Ultrasound findings also may aid the clinician in deciding whether to do endoscopic or full-thickness intestinal biopsies. Intestinal biopsy is often crucial because there are numerous causes of PLE, and histopathology is often the only way to distinguish between the various causes.
There is considerable debate about endoscopic versus full-thickness biopsy (see Chapter 27). Advantages of endoscopy include its ease, noninvasive nature, ability to detect mucosal lesions allowing one to direct the biopsy to those areas, and ability to take multiple samples from one area.16 The major disadvantages of endoscopy are that it does not allow access to the mid-jejunum, and nondiagnostic tissue samples may be obtained if the operator was not trained properly. Superficial samples (e.g., villus tips only) or compressed lacteals (Figure 57-16 ) may be obtained with all too vigorous handling of the endoscopic biopsy forceps. Surgery and laparoscopy provide ready access to the mid-jejunum (which endoscopy seldom can reach) and facilitate tissue sampling that will detect submucosal lesions. Surgery also permits biopsy of mesenteric lymph nodes. Surgical and laparoscopic biopsy are more invasive, have greater risk for morbidity and mortality from dehiscence,17 are more expensive, do not reliably detect mucosal lesions (which means you may have biopsied the wrong site), do not guarantee a diagnosis, and generally require removal of ascites. The latter intervention has the possibility of lowering body albumin content further still, making it all the easier for ascites to quickly reform. Furthermore, untrained individuals can easily take nondiagnostic full-thickness biopsies.
Figure 57-16.
A photomicrograph of a villi with lymphangiectasia. Note how the lacteal has engorged so much that now only the epithelium (arrow) is holding the chyle in the lacteal. This lacteal is about to rupture and release its contents into the intestinal lumen. It would be very easy to rupture this relatively fragile “balloon” if it were compressed, as might likely happen when using endoscopic biopsy forceps.
It is important to be able to reliably diagnose lymphangiectasia. It may be more common than generally realized and yet can be particularly difficult to diagnose. Endoscopy allows strong presumptive diagnosis when erratic, grossly enlarged lacteals are observed (Figure 57-17 ).2, 4 I typically feed a small amount of a very-high-fat diet to the patient the night before the procedure to help ensure that lacteals will be filled, hopefully augmenting diagnosis.18 Lymphangiectasia can affect the entirety of the GI tract, but more often than not is localized to one segment of the SI (which can sometimes be ascertained by ultrasound). Sometimes an obvious white fluid is released as biopsies are taken, which is chyle being released from lacteals that are ruptured during the biopsy process.2 There are at least two significant problems with histologic diagnosis of lymphangiectasia. First, the lesions may be localized to one section of the gut (e.g., lymphangiectasia is found in the ileum but not the duodenum). Second, lesions may be relatively localized to the deeper layers of the intestine (e.g., the border between the mucosa and the submucosa) and can only be found with full-thickness biopsies. The extent of these problems is unknown.
Figure 57-17.
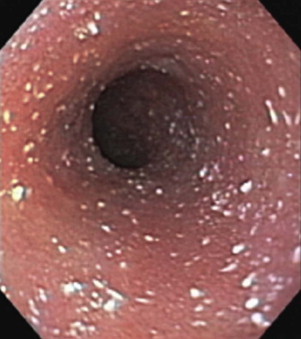
An endoscopic view of the duodenum of a dog with lymphangiectasia. The large, white spots are dilated lacteals. This dog has lymphangiectasia. Note that if a full-thickness biopsy was obtained, the likelihood of diagnosis could depend upon where the biopsy was performed, as the lesions are not uniform throughout the mucosa.
Intestinal crypt lesions have been reported in association with PLE (Figure 57-18 ).19, 20 It is not known whether this lesion is disease specific or associated with several disease entities. The need to identify lesions such as these illustrates the importance of obtaining the entire thickness of intestinal mucosa and not just the villus tips.
Figure 57-18.
A photomicrograph of intestines from a dog with PLE. Note the crypts that are engorged and dilated with pink, proteinaceous material (arrows).
Some patients with severe PLE do not have discernible histologic lesions nor do they have strongly suggestive ultrasonographic or endoscopic abnormalities. In these situations, there appear to be at least two main possibilities. First, the patient may have lymphangiectasia at a site not biopsied or observed. Second, the patient may have PLE as a consequence of a disease that does not always cause severe histologic mucosal changes, such as antibiotic-responsive enteropathy or food-responsive diarrhea.
In certain cases (e.g., client monetary constraints, inability to find a histologic lesion), a rapid increase in the serum albumin concentration after the initiation of an ultralow-fat diet is suggestive of lymphangiectasia. Likewise, an increase in serum albumin after initiating antibiotic therapy or dietary therapy suggests antibiotic-responsive disease or dietary-responsive disease, respectively. It is generally preferable to do intestinal biopsy in patients with severe PLE as opposed to observing the response to therapeutic trial; however therapeutic trials are clearly necessary in some patients.
Extra-GI disease (e.g., hypoadrenocorticism, cardiac disease,21 pulmonary disease22) may be associated with PLE. Hypoadrenocorticism may be suspected because of the classic electrolyte changes or lack of a stress leukogram, or because all other causes have seemingly been eliminated.
Short Bowel Syndrome
SBS is diagnosed based upon history and physical examination, as defined for example.
Treatment
Malabsorption
Successful treatment of malabsorptive disease requires that the underlying cause be determined. The reader is referred to other sections of this chapter, e.g., “Antibiotic-Responsive Diarrhea,” “Dietary Responsive Disease,” “IBD,” “Parasitism,” and “Neoplasia.”
Protein-Losing Enteropathy
Treatment of PLE requires that the underlying cause (see appropriate sections in this chapter, e.g., “IBD,” “Lymphoma,” “Parasites,” “Ulcers,” “Fungal Infections,” “Antibiotic Responsive Enteritis,” and “Dietary Responsive Diarrhea”) be resolved. Intussusception is treated by resection and anastomosis.
In general, patients with PLE do not benefit substantially from IV administration of plasma unless very large amounts are given. Even then, the subsequent rise in serum albumin tends to be transient because the administered albumin is all too quickly lost from the body. Generally ascites should not be removed unless it is causing a substantial problem (e.g., diaphragmatic compression and dyspnea). Removing ascites removes albumin as well as fluid, which ultimately lowers the total body albumin concentration, making it easier for ascites to reform. To lessen ascites, one may administer hetastarch IV (10 to 20 mL/kg), which hopefully stays in the intravascular compartment longer than albumin because of its size, thereby drawing third space fluid into the intravascular compartment. Although one may administer furosemide (2 to 4 mg/kg BID-TID) to lessen ascites, it is best to administer moderate doses. Massive doses of diuretics can deplete the intravascular compartment and cause hypovolemia. In emergency situations (e.g., severe dyspnea as a consequence of pressure on the diaphragm), an abdominocentesis can be performed but only to remove sufficient fluid to alleviate the dyspnea. Patients should be monitored for hypomagnesemia, which can complicate cases and worsen hypocalcemia. If magnesium supplementation is necessary, a continuous rate infusion of MgSO4 in 5% dextrose in water (35 to 50 mg/kg/day) is preferred. Orally administered magnesium tends to act as a laxative.
Lymphangiectasia is primarily treated by feeding an ultra-low-fat diet to minimize lacteal dilation and rupture.2 Historically, medium-chain triglyceride oil was supplemented to the diet to provide calories without causing lacteal dilation. Most ultra-low-fat diets were high in fiber and lacking in calories; hence, the supplementation. High fiber concentrations are not optimal because the patient often needs caloric support. If an ultra-low-fat diet without a lot of fiber is fed, medium-chain triglyceride oil is unnecessary (which is fortunate because the oils are expensive and not very palatable).
Antiinflammatory and/or cytotoxic drugs help some patients, possibly because they minimize or eliminate intestinal and mesenteric lipogranulomas. Glucocorticoids (at a dose of 1 to 2 mg prednisolone/kg/day) can help but have several important side effects, including fluid retention. Azathioprine (2.2 mg/kg once daily or every other day) or cyclosporine (3 to 5 mg/kg BID; adjust dose based upon therapeutic drug monitoring) appears to be useful in some cases. Monitoring the serum albumin concentration and body weight are probably the best way to monitoring the patient's response to therapy. Substantial improvement of the serum albumin concentration implies that therapeutic progress is occurring, even if diarrhea persists.
Short Bowel Syndrome
SBS is best treated by preventing it. Aggressive intestinal resections should be avoided, even if it requires second exploratory laparotomies a few days later to ensure that additional resection is unnecessary. Many intestinal resections resulting in SBS are unnecessarily aggressive. Once SBS has occurred, it is important to aggressively treat the animal with total parenteral and enteral nutrition (e.g., elemental diets). Decreasing gastric hyperacidity with H2-receptor antagonists (famotidine 0.5 mg/kg) or proton pump inhibitors (omeprazole 0.7 to 1.5 mg/kg daily) and suppressing the proliferation of the intestinal microflora (oral broad-spectrum antibiotics) are important components of therapy.3 The patient should receive parenteral nutrition until it is able to maintain itself on oral nutrition, which usually means multiple, small feedings of highly digestible diets, sometimes including monomeric or polymeric elemental diets. Some SBS patients may need supplementation with vitamin B12 (cobalamin) and the fat-soluble vitamins (A, D, E, and K). Treatment with ursodeoxycholate improves the nutritional status of dogs with experimentally induced SBS.23
Prognosis
The prognosis for the various nonneoplastic causes of malabsorption and PLE is usually good, assuming that the underlying cause is accurately diagnosed, the patient is seen before the disease is too far advanced, and the client can afford treatment. There are some exceptions. Intestinal lymphangiectasia that has marked lipogranuloma development within the walls of the intestines appears to be more difficult to treat. Pythiosis has a very poor prognosis if it cannot be surgically excised. Histoplasmosis usually responds to antifungal therapy, but very advanced cases may be difficult to salvage because of the marked fungal burden in the body. Diffuse intestinal lymphoma in dogs is more difficult to manage than multicentric lymphoma. Feline intestinal lymphoma may respond well to treatment, if it is well differentiated.
Infection
Michael R. Lappin
Helminths
Hookworms
Etiology
Ancylostoma caninum, Ancylostoma braziliense, and Uncinaria stenocephala are common hookworm infections in dogs. Cats are commonly infected by Ancylostoma tubaeforme and A. braziliense, but rarely by U. stenocephala. A. caninum and A. tubaeforme are found most frequently in tropical and subtropical areas; A. braziliense in warm coastal areas, Central and South America; and U. stenocephala in cooler areas like the northern United States, Canada, and Europe. Prevalence rates of hookworm infection have changed over the years.1, 2, 3, 4, 5 In one large study of 1,213,061 dogs examined at 547 private veterinary hospitals in 44 states of the United States, 4.5% of samples contained eggs of Ancylostoma spp.1 In high-risk areas and animals, infection rates can be much higher. For example, in one study in Florida A. tubaeforme and A. braziliense were found in feces of 75% and 33% of tested cats, respectively.5
Pathophysiology
Adult hookworms live in the SI and discharge eggs into the environment in the feces. The eggs develop into infective third-stage larvae in approximately 2 to 9 days depending upon environmental conditions. Dogs are infected by ingestion of larvae in the environment, skin penetration, ingestion of larvae during nursing, or ingestion of infected paratenic hosts (usually rodents). Cats are infected similarly but do not have transmammary infection. After transmammary infection in dogs, cutaneous infection, and ingestion of larvae, some larvae migrate to the lungs via the systemic circulation and molt to fourth-stage larvae in the bronchi and trachea. The larvae are then coughed up, swallowed, and develop into adults in the SI. Some larvae invade muscle tissue where they undergo arrested development (hypobiosis) and can be maintained for months to years. Upon reactivation, during stressful events like pregnancy, these larvae resume migration and reenter the intestine or concentrate in the mammary tissues of dogs and infect subsequent litters. Ingestion of infected paratenic hosts leads only to intestinal infections in dogs or cats. Puppies infected with A. caninum by nursing can shed eggs as soon as 10 days after birth. The prepatent periods for A. braziliense, and U. stenocephala are 13 to 27 days. The prepatent period for A. tubaeforme is approximately 3 weeks after ingestion and 3 to 4 weeks after transcutaneous infection.
The primary pathogenic mechanism of disease associated with hookworms is blood loss, which can start approximately 8 days postinfection, prior to shedding of eggs. To potentiate blood ingestion, hookworms release enzymes that cause local tissue necrosis as well as anticoagulants and hookworm platelet inhibitor.6 Hookworm feeding and reattachment causes small ulcerative areas in the intestine. Blunting of the microvillar membrane and eosinophilic infiltrates may result in malabsorption and diarrhea. Heavily infected dogs and cats can develop cough and pneumonia from lung migration, and transcutaneous infection can result in local skin disease.
Clinical Examination
Heavy infections can result in life-threatening blood loss with the clinical findings of pale mucous membranes, weakness, lethargy, and elevated heart and respiratory rates, particularly if concurrent Ctenocephalides spp. infestation is present. Chronic infection can lead to iron-deficiency anemia in puppies. Other clinical signs observed in young animals include small bowel diarrhea with melena and failure to thrive. Hookworm infections may induce weight loss, poor hair coat, loss of appetite, and pica. Adult dogs and cats are less likely to have clinical signs of disease; however, hookworm infection may result in eosinophilic IBD or potentiate other intestinal diseases. Cough and dyspnea can occur from heavy pulmonary infections. When skin lesions occur, they are most common in the interdigital spaces of affected animals and are characterized by pruritus, erythema, and papules.
Diagnosis
The diagnosis of hookworm infection is confirmed by microscopic visualization of eggs in feces after fecal flotation. Centrifugation techniques are more sensitive than passive flotation.7 Hookworms can produce significant intestinal pathology prior to the shedding of eggs and so fecal flotation can be falsely negative. Adult worms can be visualized in feces and in the intestinal lumen.
Treatment
Anthelmintic drugs to treat the intestinal stages and supportive care as needed are used in the management of hookworm infected dogs or cats (Table 57-4 ; see Chapter 37). There are no drugs available that can eliminate larvae from the tissues however. Some heavily infected dogs and cats may require blood transfusion and iron supplementation is needed in some cases with chronic iron deficiency.
Table 57-4.
Drugs Approved for the Treatment of Hookworm Infections of Dogs and Cats
| Animal | Approved Drugs |
|---|---|
| Dogs | |
| Adult Ancylostoma caninum | Fenbendazole, milbemycin oxime, moxidectin, pyrantel pamoate |
| Adult Uncinaria stenocephala | Fenbendazole, pyrantel pamoate, moxidectin |
| Adult Ancylostoma braziliense | Pyrantel pamoate |
| Fourth-stage and young adult A. caninum and U. stenocephala | Moxidectin |
| Cats | |
| Adult Ancylostoma tubaeforme | Emodepside, ivermectin, milbemycin oxime, moxidectin, pyrantel, selamectin |
| Fourth-stage and immature adult A. tubaeforme | Emodepside, moxidectin |
Prevention
If the risk of hookworm infection is high, all puppies and kittens (and their mothers) should be treated with appropriate anthelmintics at 2, 4, 6, and 8 weeks of age (see Chapter 37). In addition, the Companion Animal Parasite Council (CAPC) recommends that all puppies and kittens should be prescribed monthly preventives as soon as label recommendations allow and that administration be continued year round (www.capcvet.org). If puppies and kittens are not evaluated until 6 to 8 weeks of age or later, CAPC recommends the administration of a monthly preventive according to label recommendations from that point forward with administration of an anthelmintic 2 weeks later. If heavy hookworm infections have occurred in dogs, fenbendazole can be administered to pregnant bitches from the fortieth day of gestation through the fourteenth day of lactation. Kennel floors and runs should consist of tarmac or concrete, be free of crevices, and be kept as clean and dry as possible. Bedding in kennels and free feces in the environment should be removed daily to lessen larval contamination. CAPC recommends fecal examinations two to four times in the first year and one to two times per year thereafter, depending on the age of the animal and its prior history of infection to assess efficacy of the initial treatments, efficacy of the monthly control product, and client compliance.
Public Health Considerations
Cutaneous larva migrans is the most common syndrome in people associated with dog and cat hookworms.8 This syndrome results when hookworm larvae penetrate human skin at the point of contact and migrate just beneath the skin. Self-limited, serpentine lesions that are very pruritic can occur; these lesions are usually more severe following infection by A. braziliense. Deeper tissue penetration can occur in some people leading to muscle pain, lung disease, abdominal pain syndrome, and eosinophilic enteritis.9, 10
Prognosis
The prognosis for hookworm infections in dogs and cats is good to excellent.
Roundworms
Etiology
The significant roundworms (ascarids) of dogs or cats include Baylisascaris procyonis (raccoons and occasionally dogs), Toxascaris leonina (dogs or cats), Toxocara canis (dogs), and Toxocara cati. B. procyonis resides in the SI of the raccoon in North America and Europe, with higher prevalence in northeast, midwest, and west coast U.S. states. Prevalence of B. procyonis can be very high; the organism was detected in 12.7% of 188 raccoons in a recent study in Tennessee.11 Dogs occasionally are infected by B. procyonis and the life cycle can be completed in the canine host. Toxocara spp. and T. leonina have worldwide distribution. Prevalence rates vary by the study, region, and age of animals tested with puppies and kittens more likely to have patent infections. For example, the overall prevalence rate for ascarid infections in cats was 2.92% in one study.2 In other studies, T. cati was detected in 33% of kittens,12 21% of feral cats, and 18% of pet cats.13 In one large study of 1,213,061 dogs examined at 547 private veterinary hospitals in 44 states of the United States, 5.04% of samples contained eggs of T. canis.1
Pathophysiology
B. procyonis, Toxocara spp., and T. leonina eggs are passed in feces. Infective larvae then develop in the environment after varying time periods (B. procyonis, 2 to 4 weeks; T. leonina, 1 week; Toxocara spp., 2 to 4 weeks) and can survive in the environment for months to years depending upon environmental conditions. Once larvation has occurred, B. procyonis, T. leonina, and Toxocara spp. eggs are infectious for a variety of hosts, including people (B. procyonis and Toxocara spp.). All three genera are transmitted by ingestion of embryonated eggs or by ingestion of tissues from other infected vertebrate hosts. Embryonated Toxocara spp. eggs have been transmitted by earthworms, flies, and cockroaches, and have been found on the fur of pets.14, 15 Raccoons tend to use favored defecation sites called latrines that often include rooftops, woodpiles, decks, base of trees, barns, and outbuildings, and these sites can become heavily infected by B. procyonis. After ingestion of larvated eggs, the prepatent periods for B. procyonis, T. leonina, T. canis, and T. cati are 7 to 10 weeks, 8 to 10 weeks, 4 weeks, and 8 weeks, respectively. The prepatent period for T. canis can be as short as 2 weeks if infection is acquired by ingestion of another infected vertebrate host.
In the definitive host, T. leonina remains in the intestinal tract. After ingestion of infective Toxocara spp. eggs, the larvae are released, penetrate the bowel wall, migrate to the liver via the systemic circulation, and then migrate to the lungs of infected dogs and cats. The migrating larvae induce inflammation while migrating through the liver and lungs of puppies and kittens. Hepatic migration rarely results in measureable clinical disease, but pulmonary migration can cause extensive damage, resulting in clinical signs of disease and occasionally death. After reaching the lungs, Toxocara spp. larvae either undergo tracheal or somatic migration. After somatic migration, the larvae encyst in tissues. For T. canis and possibly B. procyonis, the larvae can be reactivated by a triggering mechanism like pregnancy and migrate to the placenta, resulting in infection of the puppies. Transmammary transmission of T. canis can occur but is not as important for ascarids as for the hookworms. In young puppies or kittens, tracheal migration is more likely to occur leading to patent infections intestinal infections. As the puppies or kittens become adults, somatic migration is more likely, which explains the lower prevalence of infections in older animals. Toxocara spp. infections acquired by ingestion of infected vertebrate hosts result only in intestinal infections.
Clinical Examination
In puppies and kittens, intestinal ascarids infection is often associated with vomiting, small bowel diarrhea, potbellied appearance, and general ill-thrift. Heavy infections can induce coughing, increased respiratory rate, and death during pulmonary migration. Animals with large ascarid worm burdens have developed intestinal obstruction, intussusceptions, and intestinal rupture. Neurologic disease has been recognized in some dogs infected with B. procyonis. In adult dogs and cats, ascarid infections are often subclinical.
Diagnosis
The diagnosis of ascarid infection is confirmed by microscopic visualization of eggs in feces after fecal flotation. Centrifugation techniques are more sensitive than passive flotation.7 Adult worms can sometimes be found in the vomitus of infected animals.
Treatment
Anthelmintic drugs to treat the intestinal stages and supportive care as needed are used in the management of ascarid infected dogs or cats (Table 57-5 ; see Chapter 37). There are no drugs available that can eliminate larvae from tissues.
Table 57-5.
Drugs Approved for the Treatment of Roundworm Infections in Dogs and Cats
| Animal | Approved Drugs |
|---|---|
| Dogs | |
| Toxocara canis, Toxascaris leonina | Fenbendazole, milbemycin oxime, moxidectin, pyrantel pamoate, piperazine |
| T. canis, T. leonina | Pyrantel with ivermectin or pyrantel with ivermectin and praziquantel |
| T. canis, T. leonina | Febantel with pyrantel and praziquantel |
| Cats | |
| Toxocara cati, T. leonina | Fenbendazole, milbemycin oxime, moxidectin, pyrantel pamoate, selamectin, piperazine |
| T. cati | Pyrantel, febantel, emodepside |
Prevention
In people, prevention of infection revolves around avoiding the ingestion of embryonated eggs in the environment, particularly those frequented by dogs, cats, or raccoons. In pets, hunting should be discouraged and areas frequented by large numbers of untreated dogs and cats should be avoided. Feces should be removed from the yard and litterboxes with some regularity. All puppies and kittens should be dewormed; if the potential for concurrent hookworm infection exists, deworming can begin at 2 weeks of age. CAPC recommends that all puppies and kittens should be prescribed monthly preventives that control ascarids as soon as label recommendations allow and that administration be continued year round (www.capcvet.org). Raccoons should not be kept as pets and should be discouraged from defecating in areas frequented by people or dogs. Transplacental transmission by infected pregnant bitches can be lessened by administering fenbendazole as described for hookworms. CAPC recommends performing a fecal examination two to four times in the first year and one to two times per year thereafter, depending on the age of the animal and its prior history of infection to assess efficacy of the initial treatments, the efficacy of the monthly control product, and client compliance.
Public Health Considerations
T. leonina has no known public health risks. Toxocara spp. infections of people are associated with visceral larva migrans (pulmonary disease, hepatomegaly, and eosinophilia), ocular larva migrans (unilateral granulomatous retinitis), neural larva migrans, and nonspecific clinical signs including abdominal pain. Although these syndromes are relatively rare, many people are infected by Toxocara spp. Sera collected between 1988 and 1994 from 30,930 people 6 years of age or older in the United States had an age adjusted Toxocara seroprevalence rate of 13.9%.16 In general, prevalence rates are greater in children because of potential for geophagia and otherwise poor hygienic practices. B. procyonis–associated neural larva migrans can be severe as the larvae grow and migrate through the tissues of the body, reaching sizes up to 1500 to 2000 µm. Because of this large size, as few as one to three larvae in the brain can be fatal. The severity of clinical disease depends on the number of eggs ingested, the number of larvae entering the brain, and the location and extent of migration damage. Because no effective treatment exists for larva migrans, prevention of these infections is paramount.
Prognosis
The prognosis for ascarid infections in dogs and cats is generally excellent.
Cestodes
The most common tapeworms infecting the SI of dogs or cats are Dipylidium caninum, Taenia spp., Echinococcus multilocularis, and Echinococcus granulosa. Cats or dogs are infected with D. caninum by ingesting infected Ctenocephalides felis. Dogs or cats are infected with Taenia spp. or Echinococcus spp. by ingesting other infected vertebrate hosts. The adult tapeworms live within the SI but usually are not associated with clinical signs of disease. Proglottids of Taenia spp. or D. caninum are often noted around the perineal area of infected animals. Eggs (Taenia spp. or Echinococcus spp.) or egg packets (D. caninum) can be detected on microscopic examination of feces after flotation procedures. Praziquantel (all three genera), epiquantel (Taenia spp. and D. caninum), and fenbendazole (Taenia spp.) are commonly prescribed drugs (see Chapter 37). Infections are prevented by controlling fleas (D. caninum) and preventing dogs or cats from hunting or scavenging (Taenia spp. and Echinococcus). Echinococcus spp. (ingestion of eggs) and D. caninum (ingestion of infected fleas) are zoonotic to people. The prognosis for these tapeworm infections in dogs and cats is generally excellent.
Protozoans
Cryptosporidium Spp
Etiology
Cryptosporidium spp. are coccidians that reside in the SI and are occasionally associated with disease in some, but not all, infected hosts. In the past, most of the cases of mammalian cryptosporidiosis were attributed to infection with Cryptosporidium parvum. However, molecular studies demonstrate that cats are usually infected with the host-specific C. felis and dogs are usually infected with Cryptosporidium canis.17, 18, 19, 20, 21 Infections of dogs and cats can be quite common with prevalence rates of 2% to 5% of animals with or without diarrhea, depending on the method of diagnostic testing.12, 13, 21, 22, 23, 24, 25 In one study of samples collected from centers around the United States, Cryptosporidium spp. DNA was amplified from feces of 29.4% of cats and 16.1% of dogs with diarrhea.22
Pathophysiology
C. felis and C. canis are transmitted among cats and dogs and by the ingestion of oocysts in feces from mutual grooming, shared litterboxes, ingestion of contaminated food or water, and ingestion of infected prey species. Approximately 20% of the oocysts produced in the intestine are “thin-walled” oocysts that fail to form an oocyst wall. These oocysts rupture within the intestines and when the sporozoites are released, autoinfection occurs, which allows for rapid amplification of infection. In one study of cats inoculated with C. parvum, C. parvum DNA was detected by day 2 after inoculation, but oocysts weren't detected until day 7.26 Thick-walled oocysts are passed in the feces, are environmentally resistant, are infective when passed, and are the likely source of new infections.
Although infection of dogs and cats with these agents is common, most infected animals do have clinical signs. Diarrhea is generally more common in young animals.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 Coinfection with other protozoans like Giardia spp. (dogs and cats) or Tritrichomonas foetus (cats) may be associated with more significant illness than with single infections.42, 43 The presence of immunosuppressive disorders like lymphoma, feline leukemia virus infection, and canine distemper virus can potentiate the development of clinical signs of disease. When it occurs, Cryptosporidium spp. diarrhea is associated with impaired intestinal absorption and enhanced secretion. Histopathologically, infected animals show loss of intestinal microvilli, degeneration of host epithelial cells, villus atrophy, and lymphocytic-plasmacytic infiltration.37, 44 It is possible that susceptibility to cryptosporidiosis in animals could have a genetic component as suggested for humans.45, 46
Clinical Examination
Most dogs or cats with Cryptosporidium spp. infection are clinically normal. When diarrhea occurs, it is usually watery, without mucous, blood, melena, or straining, and therefore is classified as small bowel-type diarrhea. On abdominal palpation, the SI may feel slightly thickened. Some of the infected dogs or cats with Cryptosporidium spp. infection and diarrhea have had underlying diseases like IBD, lymphoma, T. foetus, canine distemper virus, and/or feline leukemia virus, and so may have physical examination findings consistent with these conditions. Clinical signs of disease appear to be more likely in cats infected with C. felis than dogs infected with C. canis.
Diagnosis
Infectious causes of small diarrhea are common in dogs and cats and so the combination of wet mount examination and fecal flotation are usually performed as part of the initial diagnostic workup.47 However, Cryptosporidium spp. oocysts are frequently missed because of the small size (approximately 4×6 µm) and low numbers in infected dog or cat feces (often <500 oocysts/g feces). Modified acid-fast staining of a thin fecal smear can be performed to aid in the diagnosis of cryptosporidiosis. Cryptosporidium spp. are generally the only enteric organism of the appropriate size that stains pink to red with acid-fast stain. However, acid-fast staining only detects approximately 70% of Cryptosporidium spp.–infected animals when a single sample is tested.48 Fecal antigen tests for Cryptosporidium spp. are available for use with human feces, but results of these assays have been variable when applied to feces from infected animals.48, 49, 50 Fecal antigen assays are based on antibodies against C. parvum and high false-negative rates may reflect antigenic differences between C. parvum, C. felis, and C. canis. Fluorescein-labeled monoclonal antibodies react with Cryptosporidium spp. oocysts and Giardia spp. cysts (Merifluor IFA, Meridian Biosciences, Cincinnati, OH). In limited studies, this assay appears to detect both Giardia spp. and Cryptosporidium spp. isolates from dogs and cats.22, 26, 48 PCR is currently available to detect Cryptosporidium spp. DNA in canine or feline feces and is more sensitive than immunofluorescence assay (IFA) in feline studies.22, 26, 51 PCR products can be evaluated by genetic sequencing to further determine what Cryptosporidium spp. is associated with the infection (Veterinary Diagnostic Laboratory, Colorado State University, Ft. Collins; http://dlab.colostate.edu/). Cryptosporidium spp. oocysts or DNA can be detected in normal dogs and cats, consequently positive test results do not prove a disease association.
Treatment
Highly digestible diets used for small bowel diarrhea might resolve some of the clinical signs. More than 100 compounds have been used in attempts to treat Cryptosporidium spp. infections in mammals and no compound is consistently effective.52, 53, 54 There have been essentially no controlled treatment studies in dogs or cats and all protocols should be considered empirical. Cryptosporidium spp.–associated diarrhea in pets sometimes resolves after administration of tylosin (10 to 15 mg/kg PO q12h), azithromycin (10 mg/kg PO daily), paromomycin (150 mg/kg PO q12-24h), or nitazoxanide (25 mg/kg PO q12-24h). It is unlikely that tylosin has anti-Cryptosporidium effects and so cases with apparent responses may relate to the antibiotic or antiinflammatory effects of the drug. Tylosin and nitazoxanide are GI irritants. Paromomycin can be nephrotoxic in cats if absorbed and has had variable results in humans with cryptosporidiosis.55, 56, 57 If the cat or dog shows clinical improvement within the first 7 days of therapy and toxicity has not been noted, treatment should be continued for 1 week past clinical resolution of diarrhea. Some cats with Cryptosporidium spp. infection, with or without Giardia coinfection, have required several weeks of treatment prior to resolution of diarrhea. The role of fiber, silymarin, or probiotics in addition to antimicrobial therapy is unknown at this time. Some cats and dogs with resistant cryptosporidiosis have underlying diseases (e.g., IBD, T. foetus, and immunodeficiency syndromes) and the diagnostic workup should be continued if therapeutic failures occur. No drug treatment has been shown to consistently eliminate Cryptosporidium spp. infections. Thus the primary goal for the treatment of dogs or cats with cryptosporidiosis is to eliminate diarrhea. As infection is unlikely to be eliminated, following results of Cryptosporidium spp. diagnostic tests in normal animals seems to have little clinical utility.
Prevention
Cryptosporidium oocysts are environmentally resistant but can be ruptured by steam cleaning. Avoidance of contaminated food and water or potential paratenic hosts is the primary means of prevention.
Public Health Considerations
DNA of C. felis or C. canis have been amplified from the feces of some immunosuppressed people suggesting that zoonotic transfer of these agents can occur.19 However, the number of proven cross-infections is small and it appears unlikely that healthy or immunocompromised people acquire Cryptosporidium spp. infection from healthy cats or dogs.58 Thus healthy pets are not considered significant human health risks for HIV-infected people by the Centers for Disease Control and Prevention (www.cdc.gov/hiv/pubs/brochure/oi_pets.htm).
Prognosis
Cryptosporidium spp. diarrhea can be difficult to treat in dogs or cats with concurrent diseases. However, in normal animals, diarrhea generally resolves with or without treatment.
Giardia Spp
Etiology
The genus Giardia contains multiple species of flagellated protozoans that are indistinguishable morphologically. Host specificity was thought to be minimal for Giardia spp., but not all small animal isolates cause disease in human beings. There have been varying results concerning cross-infection potential of Giardia spp. Recent genetic analysis has revealed two major genotype assemblages in people.18, 19, 20 Assemblage A (Giardia duodenalis) has been found in infected humans and many other mammals including dogs and cats. Assemblage B (Giardia enterica) has been found in infected humans and dogs, but not cats. There are specific genotypes of Giardia that commonly infect dogs (Giardia canis; Assemblages C and D) and cats (Giardia felis; Assemblage F) but are uncommonly identified in people. Prevalence rates for Giardia infection vary depending on the area studied, the diagnostic method used, and the health status of the animal, however, prevalence rates are commonly 5% to 10% in healthy or clinically ill dogs or cats.3, 12, 13, 15, 24, 25, 59
Pathophysiology
Giardia spp. are found on the surface of enterocytes, with the highest concentrations of the organisms being found in the duodenum of dogs and the ileum in cats. As the organisms are on the surface, pathogenesis is unlikely to be secondary to direct cell damage. Some of the pathogenic mechanisms proposed for Giardia spp. infections include production of toxins, disruption of normal flora, induction of IBD, inhibition of normal enterocyte enzymatic function, blunting of microvilli, and dysmotility.60 There are many subclinically infected dogs and cats, and so Giardia is not always an effective primary pathogen. It has proven difficult to induce clinical signs of diseases in otherwise normal experimentally infected animals. For example, in one study, administration of 105 cysts induced infection in only 17 of 26 kittens of which diarrhea was only detected in 1 kitten for 1 day.61 There may be strains than vary in their pathogenicity or other host factors may play an important role in determining whether disease will develop. The presence of immunosuppressive diseases or coinfections may potentiate the development of clinical signs of disease as discussed for cryptosporidiosis.43, 62
Clinical Findings
Many dogs and cats have subclinical infection. When clinical disease occurs, the diarrhea is soft to watery and frequently has adherent mucus. Chronic malabsorption occurs in some animals and weight loss may be readily detected. On physical examination the SI may be slightly thickened and the animal can appear unthrifty. There may also be physical examination findings of coexisting syndromes that may potentiate giardiasis.
Diagnosis
The primary diagnostic tests for Giardia spp. infections are examination of direct fecal smear, direct saline preparation, passive fecal flotation, centrifugal fecal flotation (zinc sulfate and sugar are used most frequently), fecal IFA, fecal antigen ELISA, and fecal PCR assay. These tests can be used alone or in combination.
The direct smear and direct saline preparations can be used in the clinic for the presence of trophozoites of Giardia spp. (small bowel diarrhea), T. foetus (large bowel diarrhea), and Pentatrichomonas hominis (large bowel diarrhea). For the direct saline preparation, a 2-mm × 2-mm × 2-mm quantity of fresh feces is mixed thoroughly with 1 drop of body temperature 0.9% NaCl or water. The surface of the feces or mucus coating the feces should be used as the trophozoites are most common in these areas. After application of a coverslip, the smear is evaluated for motile organisms by examining it under ×100 magnification. Culture (T. foetus), antigen testing (Giardia), or PCR (T. foetus or Giardia) can be used to distinguish between specific organisms if the morphology is unclear.
Fecal flotation with the zinc sulfate or the sugar centrifugation technique (specific gravity 1.18 to 1.20) is optimal for the demonstration of Giardia spp. cysts and is more sensitive for detection of Giardia spp. cysts than passive flotation (www.capcvet.org).7, 63 Although sensitivity is less than 100% when a single sample is evaluated, fecal flotation remains the primary Giardia diagnostic test because of the ability of these assays to identify many other potential coinfections. Cysts are shed intermittently and their presence does not correlate very well with clinical signs of disease. Combination of fecal flotation with wet mount examination in cases with diarrhea or with a fecal antigen assay will increase sensitivity. In addition, sensitivity of fecal flotation increases to greater than 90% if at least three stool specimens are examined within 5 days.
Multiple ELISAs for detection of Giardia antigens in feces are available. In experiments performed in my laboratory, one assay labeled for veterinary use (SNAP Giardia, IDEXX Laboratories, Portland, ME) detected G. canis and G. felis.64 False-positive and false-negative rates are estimated to be approximately 2% to 5%. Although it is unknown why false-positive reactions occur, it is likely that other fecal antigens are nonspecifically binding to the reagents. False-negative results likely relate to the sensitivity cutoffs of the individual assays. In one study, combination of fecal flotation with one commercially available Giardia spp. antigen assay had a combined sensitivity of 97.8%.50
Fluorescein-labeled monoclonal antibodies that react with Cryptosporidium spp. oocysts and Giardia spp. cysts (Merifluor IFA, Meridian Biosciences, Cincinnati, OH) are available in most veterinary diagnostic laboratories. In limited studies, this assay appears to detect both Giardia spp. and Cryptosporidium spp. isolates from dogs and cats.65, 66, 67 Compared to Giardia antigen assays, this assay has the advantage of detecting a common coinfection and as the feces are examined microscopically, false-positive reactions are uncommon as the organism morphology can be assessed. The primary disadvantages include the need for a fluorescence microscope and additional technician time when compared with Giardia antigen assays.
A number of genes have been assessed for the amplification of Giardia DNA by PCR.68 Results for assemblage determination can vary based on the gene chosen and it is possible that some dog or cat isolates could be genotyped as “potentially zoonotic” by one gene but as “host specific with another.”30 In experiments in our laboratory, Giardia PCR fails to amplify DNA from approximately 20% of samples that are positive for Giardia cysts or antigens. At this time, PCR testing is only recommended for assessment of the G. duodenalis assemblage in cats and dogs, and the use of multilocus genotyping is recommended.69 This service is available at the Veterinary Diagnostic Laboratory at Colorado State University (http://dlab.colostate.edu/).
Treatment
Use of many drugs for the treatment of canine or feline giardiasis was extrapolated from use in humans.70 Because dog and cat Giardia spp. have been difficult to grow in culture, there are minimal data on antimicrobial sensitivities. It is likely that sensitivities vary amongst different isolates and it is currently impossible to predict which anti-Giardia drug will be effective in an individual case. Although there have been multiple drugs used for the treatment of giardiasis in dogs and cats, there are few studies that utilized dose titrations and evaluation of drugs in experimentally infected animals. In most studies, fecal samples were only assessed for short periods of time after treatment and neither was immune suppression induced nor necropsy performed to evaluate whether infection was eliminated or merely suppressed. Infection with Giardia does not appear to cause permanent immunity and so reinfection can occur, a finding that also hampers assessment of treatment studies.
The primary goal of Giardia treatment is to stop diarrhea. Because healthy pets are not considered significant health risks to immunocompetent people, elimination of infection (which is difficult) is a secondary goal. Treatment options currently available or used historically include metronidazole, tinidazole, ipronidazole, ronidazole, fenbendazole, albendazole, pyrantel/praziquantel/febantel, quinacrine, furazolidone, and nitazoxanide (Table 57-6 ).70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83
Table 57-6.
Drugs Used for the Treatment of Giardia Spp. Infections
| Drug | Species | Dose |
|---|---|---|
| Metronidazole | B | 15 to 25 mg/kg PO q12-24h for 5 to 7 days |
| Ronidazole | F | 20 mg/kg, PO q12h for 14 days (primarily used for T. foetus; neurotoxicity common) |
| Tinidazole | C | 44 mg/kg PO q24h for 3 days |
| Ipronidazole | C | 126 mg/L of water PO ad libitum for 7 days |
| Fenbendazole | B | 50 mg/kg PO daily for 3 to 5 days |
| B | 15 mg/kg PO q12h for 2 days (less commonly used because of bone marrow toxicity) | |
| Pyrantel, praziquantel, febantel | C | Label dose for 3 days |
| F | Feline dose: 56 mg/kg (based on the febantel component) PO daily for 5 days | |
| Quinacrine | C | 9 mg/kg PO q24h for 6 days |
| F | 11 mg/kg PO q24h for 12 days | |
| Furazolidone | F | 4 mg/kg PO q12h for 7 to 10 days |
B, Canine and feline; C, canine; F, feline.
Care should be taken when using metronidazole or ronidazole as central nervous system (CNS) toxicity can occur.84, 85, 86 Because albendazole is associated with bone marrow suppression, many clinicians use fenbendazole or febantel when that class of drugs is chosen.87
If clinical findings suggest concurrent C. perfringens overgrowth, use of metronidazole may be indicated as this drug is an antibiotic with activity against Clostridium spp. If there are clinical findings that suggest concurrent infection with a nematode, fenbendazole or febantel are indicated. Many clinicians currently use fenbendazole once daily for 3 to 5 days as initial therapy. Some clinicians currently recommend the combination of metronidazole and fenbendazole (www.capcvet.org). Others only resort to combination therapy if there is evidence of a persistent infection that is not cleared by monotherapy. If the first drug fails to control diarrhea and the organism is still detected in feces, a second drug from an alternate class is indicated. The addition of fiber to the diet may help control clinical signs of giardiasis in some animals by helping to suppress bacterial overgrowth or by inhibiting organismal attachment to intestinal microvilli. Immunotherapy with the Giardia vaccine has aided in eliminating cyst shedding and diarrhea in some infected dogs.88 However, in a controlled study in 16 experimentally infected cats, vaccination as immunotherapy was ineffective with one strain of Giardia.89 In addition, both commercial products have been discontinued. Probiotic administration has been promoted as potentially beneficial in the control of giardiasis. In one study, dogs treated with silymarin and metronidazole had superior clinical responses to dogs treated with metronidazole alone.90 However, in another study, administration of a commercially available probiotic (Forta-Flora, Nestle Purina PetCare, St. Louis, MO) did not affect the outcome of Giardia infection.91 In one study, bathing the dog on the last day of treatment was a beneficial adjunct therapy.77 In dogs and cats with persistent diarrhea and Giardia spp. infection, a more extensive workup to attempt to diagnose other underlying diseases is indicated if several therapeutic trials fail. Common underlying disorders include cryptosporidiosis, T. foetus in cats, IBD, bacterial overgrowth, exocrine pancreatic insufficiency, and immunodeficiencies.
Public Health Considerations
Healthy pets are not considered significant human health risks by the Centers for Disease Control (www.cdc.gov/hiv/pubs/brochure/oi_pets.htm) and there is no current recommendation to test healthy dogs or cats for Giardia spp. infection. However, some dogs and cats are infected with the zoonotic assemblages and the same assemblage has been detected in dogs and people in the same family.92, 93, 94, 95 All healthy dogs and cats should be screened for hookworm and roundworm infection once or twice yearly as previously discussed. Thus healthy dogs and cats that are harboring Giardia cysts will be detected. As some Giardia spp. may be zoonotic, treatment of healthy infected animals should be considered with each owner. Treatment of healthy animals is controversial because all of the drugs can potentially cause side effects, animals with normal stools are not considered human health risks, treatment is unlikely to eliminate infection, and reinfection can occur within days. For example, in a study of naturally infected dogs, approximately 15% of treated dogs were still Giardia infected when rechecked 9 or 16 days after treatment.76 In another study, 50% of the healthy, Giardia-positive dogs had adverse reactions to fenbendazole or nitazoxanide and 62.5% of those that successfully completed therapy were positive for Giardia cyst or antigen on day 34.64 It is unknown whether these infections were not eliminated or if the dogs were reinfected. If treatment is deemed appropriate by the clinician and pet owner, many clinicians currently recommend administration of a 5-day course of fenbendazole in apparently healthy dogs and cats that test positive for Giardia. The American Association of Feline Practitioners (AAFP) Advisory Panel on Zoonoses recommends attempting to remove the source of infection during the treatment period and performing a fecal centrifugal flotation after Giardia treatment one time, within 2 to 4 weeks after the end of the treatment period (www.aafponline.org). If the animal is healthy and negative for cysts, retesting is not indicated again until the next scheduled fecal flotation. Currently it is not recommended that the IFA or fecal PCR assays be used as a recheck test for any of the Giardia antigen assays. It is currently unknown how long Giardia antigens will persist in feces after successful treatment.
Occasionally, animals will be Giardia cyst-negative but Giardia antigen-positive. These animals either have a low-grade infection or a low percentage of animals (approximately 2% to 5%) have false-positive antigen test results. To further evaluate for cyst shedding, the veterinarian can perform an IFA test or two additional fecal flotations (three negative centrifugal flotation assays run within 5 days is considered adequate to ruleout a Giardia infection in both animals and humans); if these other test results are negative, the antigen test was likely falsely positive.
Prevention
Prevention of giardiasis involves boiling or filtering of water collected from the environment prior to drinking and disinfection of premises contaminated with infected feces with steam cleaning or quaternary ammonium compounds (1 minute contact time). Paratenic hosts should be controlled and treatment and bathing of all animals in the environment should be considered. Feces from infected animals should be removed from the environment promptly. The previously licensed Giardia spp. vaccines for dogs and cats were classified by American Animal Hospital Association (AAHA) and AAFP as generally not recommended as preventatives. Both products have been discontinued by their manufacturers.
Prognosis
Most dogs and cats with clinical giardiasis ultimately will have clinical signs of disease resolve with treatment and so the prognosis in otherwise healthy animals is good.
Isospora Spp
Etiology
Dogs are the definitive hosts for Isospora canis, Isospora ohioensis, Isospora neorivolta, and Isospora burrowsi and cats are the definitive hosts for Isospora felis and Isospora rivolta.96 These protozoans are host specific, have worldwide distribution, and infections are very common, particularly in young animals. In one Austrian study, 8.7% of dogs younger than 2 years of age were infected; 78% of the positive samples were in puppies younger than 4 months of age.97 In the United States, CAPC reports prevalence rates for Isospora spp. infection from 3% to greater than 30% (www.capcvet.org).
Pathophysiology
Infection by Isospora spp. in dogs or cats is initiated by ingestion of sporulated oocysts in the environment or by ingesting tissues of other infected vertebrate intermediate hosts.96, 97, 98 Infection may also occur if dogs or cats ingests sporulated oocysts carried by paratenic hosts like flies, cockroaches, or dung beetles.99 The enteroepithelial phase occurs in the SI of infected animals which culminates in the passage of unsporulated oocysts in feces. The prepatent and patent periods vary slightly by species. In one study of dogs experimentally infected with I. canis, the mean prepatent period was 9.8 days (range: 9 to 11 days, n = 22 dogs), the patent period was 8.9 days (range: 7 to 18 days, n = 20 dogs), and all of the puppies developed diarrhea, suggesting the organism can be a primary pathogen.98 In contrast, the prepatent period for I. ohioensis in one study was 6 to 7 days and diarrhea was variable.97 Numbers of oocysts shed in infected animals can vary dramatically.97, 98 Depending on the environmental conditions, sporulation can occur in as little as 12 hours. Clinical disease is most common in young, debilitated, and immunocompromised animals. All of the Isospora spp. replicate in the SI but the regions with the heaviest infection varies by the species. Microscopic lesions observed in some infected animals includes villus atrophy, dilation of lacteals, and hyperplasia of lymph nodes in Peyer patches.
Clinical Findings
Isospora spp. infections are generally only associated with disease in puppies and kittens. Clinically ill puppies and kittens can exhibit vomiting, abdominal discomfort, inappetence, and watery diarrhea that sometime contains blood. Depending on the age of the animal and the parasite burden, severe dehydration and death can occur. Puppies and kittens with subclinical infection can repeat shedding and clinical signs of disease during stressful periods.
Diagnosis
Isospora spp. oocysts are large, occur in large numbers, and are generally easy to identify on microscopic examination of feces after fecal flotation. However, normal animals also pass Isospora spp. oocysts and so positive test results do not always prove a disease association. False-negative fecal flotation results are uncommon in clinically infected animals but occasionally clinical signs precede oocyst shedding and a second fecal flotation may be needed to prove infection in some cases.
Treatment
Coccidiosis is generally self-limited and most healthy puppies and kittens will resolve clinically without therapy. However, administration of treatment can speed resolution of disease and may lessen environmental contamination and the potential for infecting other in contact animals. The only approved treatment for coccidiosis in the United States is sulfadimethoxine administered at a dose of 50 to 60 mg/kg daily for 5 to 20 days (dogs and cats). Other drug regimens have been used with some success, including trimethoprim-sulfa (30 to 60 mg/kg of trimethoprim daily for 6 days in animals weighing more than 4 kg or 15 to 30 mg/kg trimethoprim daily for 6 days in animals weighing less than 4 kg) and a variety of protocols using amprolium alone or in combination with sulfadimethoxine. However, ponazuril and toltrazuril are coccidioidal and therefore are superior for the treatment for coccidiosis.100, 101 Ponazuril has been used most frequently in the United States. The drug can be administered off label at 20 mg/kg PO twice, 1 to 7 days apart or at 50 mg/kg PO once. Many compounding pharmacies in the United States will appropriately formulate the drug by prescription.
Prognosis
Most Isospora spp.–infected puppies and kittens will survive infection making the prognosis good to excellent.
Prevention and Public Health Considerations
Isospora spp. oocysts are very resistant to environmental conditions and disinfectants. The key to control is to provide good sanitation including prompt removal of feces prior to oocyst sporulation. Steam cleaning can be used to destroy oocysts that contaminate surfaces. Treatment of dams and queens with anticoccidial agents prior to parturition can lessen the occurrence of coccidiosis in young animals. In environments with heavy infections, treatment of all in contact animals, particularly puppies and kittens, could be considered. Isospora spp. of dogs and cats do not infect people. Ponazuril administered to all at-risk puppies and kittens on intake to shelters may aid in the control of coccidiosis.101
Other Protozoans
While Sarcocystis spp., Besnoitia spp., Hammondia spp., Toxoplasma gondii, and Neospora caninum complete the sexual phase of the life cycle in the intestinal tract of dogs or cats, this phase of replication is rarely linked to GI clinical signs of disease. Clinical illness associated with T. gondii, N. caninum, and Sarcocystis neurona generally results from the tissue phase of the infections. However, IBD was linked to T. gondii infection in two cats.102
Fungal, Oomycetes, and Algae Infection
The fungal, oomycetes, and algae infections may diffusely involve the whole GI tract, and are discussed in detail in “Infection of the Large Intestine” in Chapter 58.
Bacteria
A number of bacteria are associated with GI signs of disease and may colonize or infect the SI. Campylobacter spp., Clostridium spp., E. coli, and Salmonella spp. are discussed in this chapter. Yersinia spp., and other mixed enterocolitic infections are discussed in Chapter 58 The syndrome of small intestinal bacterial overgrowth (or dysbiosis) is discussed in another section of this chapter.
Campylobacter Spp
Etiology
C. jejuni, Campylobacter coli, C. upsaliensis, and Campylobacter helveticus are commensal organisms found in the GI tract of healthy dogs and cats throughout the world.103, 104, 105 The high prevalence of these organisms in healthy nondiarrheic dogs (C. jejuni, 49%; C. coli, 5%; C. upsaliensis, 19%) and cats (C. jejuni, 46%; C. coli, 1%; C. upsaliensis, 5%; C. helveticus, 22%) complicates diagnosis.106, 107, 108, 109 Under certain conditions, however, Campylobacter can induce significant GI tract pathology. Young age, immunoincompetence, concurrent GI infections, prior therapeutic interventions (e.g., antibiotics), and poor hygienic conditions appear to be the greatest risk factors for the development of infection.110
Pathophysiology
At some point, Campylobacter organisms become enteroinvasive and induce the host inflammatory response. C. jejuni localizes in mucus-filled crypts of the intestine and colon where it induces a superficial erosive enterocolitis.110, 111 Colonic epithelial glands undergo hyperplasia and thickening with exfoliation of the brush-border and goblet cells. The colonic epithelium becomes cuboidal, crypt height is reduced, and crypt abscess are present. Shallow crypts and blunt irregular villi are features of the ileum response to infection.
Clinical Examination
Clinical signs are watery diarrhea, often containing mucous and blood pigments, tenesmus, anorexia, fever, and vomiting.111, 112 Concurrent infections with Salmonella, Giardia, or parvovirus cause more severe disease.
Diagnosis
Direct examination of a fresh fecal sample is the method of diagnosis in many instances. Large numbers of curved, highly motile bacteria along with increased numbers of leukocytes is presumptive evidence of Campylobacter infection. With Gram staining, large numbers of faintly staining Gram-negative, slender, curved (gull-wing shaped) rods are evident. Fecal cultures or PCR are the most conclusive ways to determine the presence of Campylobacter.106, 107, 108 C. jejuni is best cultured microaerophilically at 42°C (107.6°F) for 48 hours on special Campylobacter blood agar plates.
Treatment
Erythromycin is the treatment of choice, although tetracyclines, aminoglycosides, clindamycin, and quinolones are also effective. Posttreatment cultures should be performed to confirm eradication. Pet owners should be advised about the importance of proper hygiene.
Prognosis
The prognosis for recovery and cure are generally excellent unless an underlying immunosuppressive condition has increased the susceptibility to infection.
Clostridium Perfringens
Etiology
C. perfringens is a Gram-positive, spore-forming, obligate anaerobic rod-shaped bacterium that contributes to the microbial ecology and nutrition of the colon in healthy dogs and cats.113 Under certain conditions, proliferation and sporulation of C. perfringens permits enterotoxin A (or CPE) production, which may then induce mucosal damage, fluid secretion, and large bowel-type diarrhea. Evidence for and against a role for C. perfringens in the pathogenesis of large bowel diarrhea has been put forward. Enterotoxigenic C. perfringens is associated with canine nosocomial diarrhea,114 hemorrhagic enteritis,103 and acute and chronic large bowel diarrhea.115, 116 On the other hand, many dogs harbor C. perfringens and CPE in the GI tract without developing clinical signs.104, 105, 110 Until more definitive evidence is obtained, including the fulfillment of the Koch postulates, C. perfringens should probably be considered as a suspected pathogen in large bowel diarrhea.
Pathophysiology
The presumed pathogenicity of C. perfringens requires an anaerobic environment, sporulation, and enterotoxin production. There are problems with this hypothesis however; enterotoxin may be demonstrated in the feces without sporulation, and enterotoxin may be found in the feces of healthy dogs. C. perfringens isolates are classified as one of five toxigenic types (A to E) based on the production of one or more of four major (α, β, ε, ι) and seven minor (δ, θ, κ, λ, µ, ν, and sialidase) toxins.117 Although all five types of C. perfringens are capable of producing CPE, the majority is produced by type A strains. As with enterotoxigenic E. coli (ETEC) strains, CPE is believed to induce crypt epithelial cell secretion.
Clinical Examination
C. perfringens–associated colitis is believed to be a major cause of acute, nosocomial, as well as chronic, large bowel diarrhea. Acute nosocomial diarrhea often begins within 1 to 5 days of boarding or kenneling. Affected dogs develop diarrhea, often with blood pigments, mucous, and tenesmus. These diarrheas are usually self-limiting and may resolve with supportive care alone. Chronic large bowel diarrheas associated with C. perfringens are similar to other large bowel-type diarrheas, that is, chronic, intermittent, and recurring signs of colitis.
Diagnosis
There is no gold standard for the diagnosis of C. perfringens–associated diarrhea. Ideally, the diagnosis would be made on the basis of positive test results with Gram staining, fecal culture, ELISA enterotoxin (CPE) assay, PCR enterotoxin (cpe) genotyping, and ruleout of other colonic diseases on colonoscopy and biopsy.118, 119, 120, 121 Compared to normal dogs (without diarrhea), diarrheic dogs are more often CPE ELISA- and cpe PCR-positive, but many normal dogs are positive on both assays.
Treatment
Recent in vitro antimicrobial susceptibility testing suggests that C. perfringens should be susceptible to ampicillin, erythromycin, metronidazole, and tylosin.122 These antibiotics have also been used in vivo with good success. It should be emphasized that many of the same patients respond to supportive care, including intravenous fluids, intestinal protectants, and bland or fiber-supplemented diets.
Prognosis
Affected animals usually respond to appropriate therapy within a matter of days. The prognosis for recovery is excellent.
Clostridium Difficile
C. difficile is believed to share many ecological factors with C. perfringens,123, 124 but the role of this organism as a pathogen in dogs and cats has not been firmly established. Compared with normal dogs (without diarrhea), diarrheic dogs are more often toxin A ELISA-positive even though they may be toxin A PCR-negative.121 As with C. perfringens, many healthy dogs and cats carry C. difficile without developing clinical signs. In one recent study it was difficult to experimentally infect dogs with this organism, and those that were infected did not develop clinical signs.125 Antibiotic-associated diarrheas develop in dogs and cats but they may have a pathogenesis other than C. difficile.
Escherichia Coli
Etiology
Most strains of E. coli are true commensal organisms that are not associated with clinical signs. Strains of E. coli that cause diarrhea in animals can be grouped into five main categories: ETEC, enteroinvasive (EIEC), enteropathogenic (EPEC), enterohemorrhagic (EHEC), and enteroadherent (EAEC) organisms.126 Identification of pathogenic strains requires modern molecular technology such as bioassays, DNA hybridization, and PCR amplification.
Pathophysiology
Infection may result in enteritis, colitis, or both.127 ETEC strains adhere to the surface of epithelial cells and produce heat-labile and/or heat-stable toxins that induce crypt epithelial cell secretion. EIEC strains invade, replicate in, and destroy epithelial cells. EPEC strains are neither enterotoxigenic nor enteroinvasive, but they do attach to and efface the brush-border of the enterocytes. EHEC strains produce verocytotoxins that induce hemorrhagic ileitis and colitis. EAEC strains also induce enterocyte pathology, but their mechanism of action is poorly understood. ETEC, EPEC, and EHEC have all been isolated from dogs and cats with diarrhea. E. coli endotoxin colonic absorption of water and sodium and contributes to the diarrhea seen during and after episodes of sepsis.128
Clinical Examination
Affected animals typically have diarrhea and hematochezia with clinical signs relevant to the SI, colon, or both.
Diagnosis
E. coli can be grown from the feces of healthy dogs and cats, so a positive culture does not necessarily reveal the identity of an underlying pathogen. In addition to positive culture, diagnosis may require enterotoxin assays, and DNA hybridization and PCR amplification.129, 130, 131, 132, 133
Treatment
Antibiotics should be used only in those cases in which there is firm evidence of bacterial infection. Fluoroquinolones appear to be a very effective classification for the treatment of enteric E. coli infections.
Prognosis
The prognosis is generally good for recovery and cure if infection is recognized early in the clinical course.
Salmonella
Etiology
Salmonella spp. are predominantly motile, Gram-negative facultative anaerobic rod-shaped bacteria found in the feces of normal and diarrheic animals.104, 105 As with many other commensal organisms of the GI tract, the high prevalence of these organisms complicates diagnosis. From 1% to 30% of the fecal samples or rectal swabs taken from healthy domestic pet dogs, 16.7% of dogs boarded in kennels, and 21.5% of hospitalized dogs were found to be positive on bacteriologic culture for Salmonella. From 1% to 18% of healthy cats and 10.6% of random source research colony cats were also culture-positive for Salmonella (summarized in reference 122). Despite these findings, several species of Salmonella have been impugned in the pathogenesis of acute enterocolitis in dogs and cats. Salmonella typhimurium is the species most commonly isolated from diarrheic feces of dogs and cats, although other species have been identified.134, 135, 136
Pathophysiology
Those most at risk for Salmonella infection are young and immunoincompetent animals, those with concurrent GI infections (e.g., parvoviral or parasitic infections), and those animals who have had prior therapeutic interventions (e.g., antibiotics or glucocorticoids).110 Salmonella is an enteroinvasive organism that induces an acute inflammatory response resulting in enterocolitis, mucosal sloughing, and secretory diarrhea. Most Salmonella infections are resolved via the local immune response, but bacterial translocation and septicemia may evolve into systemic inflammatory response and multiple organ dysfunction syndromes in some patients. Early recognition is important in preventing this sequela.
Clinical Examination
The main clinical signs of Salmonella enterocolitis are anorexia, lethargy, fever, vomiting, diarrhea with mucous and blood pigments, dehydration, abdominal pain, and tenesmus. With bacterial translocation and septicemia, affected animals may have evidence of pale mucous membranes, weakness, tachycardia, tachypnea, and vascular collapse.
Diagnosis
Culture, serotyping, and PCR are the best methods of diagnosing Salmonella infections.134
Treatment
Treatment varies according to the severity of the clinical signs. Mild, self-limiting forms of enterocolitis may in fact resolve with little more than supportive therapy. Antibiotic therapy in such cases may prolong fecal shedding and encourage development of the carrier state. In animals with severe hemorrhagic diarrhea, history of immunosuppression, suspected or documented septicemia, and/or evidence of systemic inflammatory response syndrome, parenteral antibiotics should definitely be used. If culture results are unavailable, therapy should include enrofloxacin, amoxicillin, or trimethoprim-sulfa, all of which are effective against Salmonella. Posttreatment cultures should be performed to confirm eradication, and pet owners should be advised of the public health importance of the disease.
Prognosis
The prognosis for recovery in nonsepticemic patients is generally good, although some animals may remain chronic carriers with recrudescence during periods of stress or unrelated disease. The prognosis for the septicemic patient is more guarded.
Rickettsia
Neorickettsia Helminthoeca
Etiology
Neorickettsia helminthoeca is a gram-negative organism in the family Anaplasmataceae that induces a clinical syndrome called salmon poisoning disease in dogs.137 The syndrome is currently recognized in the Pacific Northwest of the United States, British Columbia in Canada, and an area in southern Brazil.137, 138, 139, 140 A similar clinical syndrome in dogs was called Elokomin fluke fever in Washington but now appears likely to have been caused by a strain of N. helminthoeca.
Pathophysiology
Nanophyetus salmincola is the trematode vector of N. helminthoeca and requires three hosts for the completion of its lifecycle.137 A river snail, Oxytrema silicula, is the first intermediate host and is infected by rediae and cercariae of N. salmincola. The snail releases free-living cercariae that penetrate the skin of salmon, lose their tails, and become metacercariae that develop in a number of tissues of the salmon with heavy concentrations in the kidneys, liver, heart, and tail. The life cycle is completed when the adult trematode develops in the intestine of mammals or birds that eat fish. The syndrome has also been induced in dogs that have been fed infected snails. N. helminthoeca survives through all stages of the trematode. The adult flukes develop deep within the intestinal tissues causing local edema and inflammation in 5 to 6 days. The fluke releases N. helminthoeca, which infects intestinal histiocytes and disseminates in blood and lymph to lymphoid tissues. Mesenteric lymph nodes enlarge greatly from edema and an influx of inflammatory cells. Diarrhea results from the inflammation of intestinal lymphoid tissues and can be hemorrhagic. The typical incubation period is 5 to 7 days.
Clinical Examination
Clinical signs of disease in dogs starts with fever, anorexia, and vomiting. Affected dogs can also develop periocular swelling, ocular discharge and nasal discharge which have been confused with canine distemper virus infection. Local and generalized lymphadenopathy can be detected in most cases when clinical signs are first recognized. Diarrhea is generally small bowel-type in character, but can become bloody and be confused with canine parvovirus infection. Rapid, marked weight loss, and extreme polydipsia have been reported in some dogs and death is common.
Diagnosis
History, clinical signs, detection of N. salmincola eggs after microscopic examination of feces after fecal sedimentation, and response to therapy can be used to make a presumptive diagnosis. Clinical pathology abnormalities can include thrombocytopenia, lymphopenia, eosinophilia, increased alkaline phosphatase activity, and marked hypoalbuminemia.137 Cytologic examination of enlarged lymph nodes after staining with Giemsa can reveal intracytoplasmic neorickettsial bodies within reticuloendothelial cells and lead to a definitive diagnosis. Current infection can be documented by demonstrating rising antibody titers, culture, immunohistochemical staining of tissues, or PCR assay.137
Treatment
Supportive care for dehydration is key to the management of dogs with salmon poisoning disease. N. helminthoeca is susceptible to tetracycline, doxycycline, and oxytetracycline. Parenteral treatment is indicated in dogs with concurrent vomiting. N. salmincola is susceptible to praziquantel.141
Prognosis
Without treatment, up to 90% of clinically affected dogs die within 6 to 10 days of developing clinical signs. Permanent immunity occurs in dogs that survive infection.
Prevention and Public Health Considerations
Dogs should not be allowed to feed on raw, uncooked, or smoked salmon in endemic areas. Freezing fish at −20°C (−4°F) for at least 24 hours or thoroughly cooking the tissues kills both the fluke and N. helminthoeca. N. salmincola infection can result in GI disease in people,142 but it is unclear whether N. helminthoeca is pathogenic in people.137
Viral
Canine Enteric Coronavirus
Etiology
Canine enteric coronavirus (CCV) is a single-stranded enveloped RNA virus that replicates in the cytoplasm of small intestinal epithelial cells.143 There are several genetic variants that occur in different parts of the world and prevalence varies by genotype and country.144, 145, 146, 147, 148 CCV RNA has been amplified from the feces of more than 40% of dogs with gastroenteritis in some countries,145 but CCV RNA can also be amplified from the feces of normal dogs. Canine respiratory coronavirus is genetically distinct from canine enteric coronavirus.149
Pathophysiology
CCV is transmitted by fecal–oral route via a contaminated environment and is highly contagious especially in neonatal puppies. Older dogs can be infected but seem to be less likely to develop clinical signs of disease. The inoculation period is approximately 1 to 4 days. CCV replicates intracellularly in the intestinal microvilli which become short and blunted. Necrosis and hemorrhage are rare in contrast to canine parvovirus infections. There are CCV variants that are more pathogenic than others and disease appears to be unusual in puppies greater than 6 weeks of age in the United States.150, 151 Co-infection with other infectious agents like canine parvovirus was thought to potentiate CCV-associated illness in some studies.152
Clinical Examination
Susceptibility to CCV associated gastroenteritis does appear to be overrepresented in any individual breed or sex. The primary clinical sign is small bowel–type diarrhea that may be preceded by vomiting. Secondary findings include fever, lethargy, anorexia, dehydration, and death in some affected puppies.
Diagnosis
Amplification of CCV RNA from feces by RT-PCR assay is currently the most frequently used diagnostic procedure in the United States. However, as normal dogs can be positive for CCV RNA, the positive predictive value of these assays is less than 100%.148 Depending on the timing of tested, assay results can be falsely negative.
Treatment
Fluid therapy and other supportive care is used for the treatment of CCV-associated GI disease in puppies.
Prognosis
With appropriate supportive care, the prognosis for puppies with CCV infection is generally good.
Prevention and Public Health Considerations
CCV is rapidly inactivated by many detergents and disinfectants. Multiple CCV-containing vaccines are available in the United States. However, as clinical illness is usually only detected in very young dogs in this country, the AAHA Vaccine Panel does not generally recommend this vaccine antigen.153 There are no proven human health risks with CCV.
Feline Coronaviruses
Etiology
Feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV) infections can cause GI disease in some cats.154, 155 Although enteric infection generally results in mild GI signs, systemic infection can induce a clinical syndrome with diverse manifestations commonly referred to as FIP.155 There are multiple field strains of FECV and FIPV with varying degrees of virulence. FIPV capable of inducing FIP develop in the GI tract of some infected cats as mutations or recombinant strains of endemic FECV. FECV are commonly shed in feces, rarely in saliva, and are very contagious but minimally pathogenic.156
Pathophysiology
Coronaviruses can be detected in feces by use of RT-PCR as early as 3 days postinfection.156 In studies of FECV-infected, closed cat colonies, almost every cat becomes infected, with some cats shedding continuously. Viral RNA has been detected in the ileum, colon, and rectum of cats with persistent shedding. FIPV-infected monocytes can disseminate throughout the body, potentially resulting in FIP, which has diverse manifestations related to the development of vasculitis or pyogranulomatous disease of multiple organs.155 GI disease associated with FIP can result from focal obstruction.157
Clinical Examination
Enteric replication of feline coronaviruses commonly results in fever, vomiting, and mucoid diarrhea. GI disease from FECV is most common in kittens and is generally self-limiting or responsive to supportive care within days. Fever and weight loss are common with both the effusive and noneffusive forms of FIP, and small bowel diarrhea can occur chronically in some cats. A solitary ileocecocolic or colonic mass resulting in obstruction with vomiting and diarrhea occurs in some cats.157 The other polysystemic manifestations of FIP are reviewed elsewhere.154, 155
Diagnosis
There are no specific clinical or routine laboratory findings leading to a definitive diagnosis of FECV- or FIPV-associated GI tract disease. Because virus isolation and electron microscopy are not practical clinically, RT-PCR is used most frequently to amplify coronavirus RNA in feces. However, positive test results do not prove that diarrhea is caused by a coronavirus as normal cats can also be positive. Definitive diagnosis of FIP-associated GI obstruction is based on detection of characteristic histopathologic findings, virus isolation, demonstration of the virus in tissue by use of immunocytochemical or immunohistochemical staining, or by RT-PCR demonstration of viral RNA in tissues.
Therapy
FECV-associated GI signs of disease are treated with supportive care and are generally self-limiting. GI obstruction from focal FIP is generally treated surgically.
Prognosis
Most kittens with FECV-associated GI disease respond rapidly to therapy. Cats with focal GI obstruction from FIP may progress to systemic disease, which has a grave prognosis.
Prevention and Public Health Considerations
Prevention of coronavirus infection is best accomplished by avoiding viral exposure. Although viral particles of FECV and FIPV can survive in dried secretions for up to 7 weeks, routine disinfectants inactivate the virus. An intranasally administered, mutant strain of coronavirus that induces mucosal immune response but minimal systemic immune response is available (Primucell FIP, Pfizer Animal Health, Exton, PA). Whether the vaccine protects against FECV or all field strains, mutations, or recombinants of FIPV is unknown. It is unlikely the vaccine is effective in cats that have previously been infected by a coronavirus and is considered generally not recommended by the AAFP.158 There is no known zoonotic transfer of FIP coronavirus or enteric coronavirus to humans.
Canine Distemper Virus
Etiology
Canine distemper virus (CDV) induces disease predominantly in terrestrial carnivores, but many other species, including seals, ferrets, skunks, badgers, porpoises, and exotic Felidae, have been infected by either CDV or related viruses.154, 159
Pathophysiology
CDV replicates in lymphoid, nervous, and epithelial tissues and is shed in respiratory exudates, feces, saliva, urine, and conjunctival exudates for up to 90 days after natural infection. Replication in small intestinal epithelial cells results in the GI signs seen in acute CDV infection. Clinical signs of disease generally develop approximately 8 to 9 days after infection, with the severity of illness dependent on the strain of CDV and the immune status of the host when primary infection occurs.154, 155 If poor immune response exists, massive replication of the virus in the epithelial cells of the respiratory tract, GI system, and genitourinary system usually results in death from polysystemic disease. Dogs with moderate immune responses by days 9 to 14 postinfection, usually only have mild respiratory or GI clinical signs related to replication in epithelial tissues. Dogs with good cell-mediated responses and virus-neutralizing antibody titers by day 14 postinfection clear the virus from most tissues and may not be clinically affected. Most infected dogs develop CNS infection, but clinical signs of CNS disease occur only in dogs with low or no antibody response.
Clinical Examination
Many clinically affected dogs are unvaccinated, inappropriately vaccinated, failed to receive colostrum from an immune bitch, or are otherwise immunosuppressed. Dogs with vomiting or small bowel–type diarrhea from CDV infection generally also have evidence of depression, malaise, oculonasal discharge, or cough. CNS signs may or may not be present. Tonsillar enlargement, fever, and mucopurulent ocular discharge are common physical examination findings. Increased bronchial sounds, crackles, and wheezes are usually auscultated in dogs with bronchopneumonia. When CNS disease occurs, it is usually characterized by hyperesthesia, seizures, cerebellar or vestibular disease, paresis, and chorea myoclonus that generally develop within 21 days of recovery from systemic disease. Ocular abnormalities associated with CDV infection include anterior uveitis, optic neuritis with resultant blindness and dilated pupils, keratoconjunctivitis sicca, and retinochoroiditis (medallion lesions).
Diagnosis
Lymphopenia and mild thrombocytopenia are consistent hematologic abnormalities in dogs with GI signs of CDV infection. Interstitial and alveolar pulmonary infiltrates are common radiographic findings in dogs with concurrent respiratory disease. Documentation of a fourfold increase in the CDV serum IgG titer over a 2- to 3-week period or detection of IgM antibodies in serum is consistent with recent infection or recent vaccination, but does not prove clinical disease. Definitive diagnosis of CDV infection requires demonstration of viral inclusions by cytologic examination, direct fluorescent antibody staining of cytologic or histopathologic specimens, histopathologic evaluation, virus isolation, or RT-PCR documentation of CDV RNA in peripheral blood, cerebrospinal fluid, urine, or conjunctival scrapings.154, 159, 160, 161, 162, 163 Viral inclusions can rarely be found in erythrocytes, leukocytes, and leukocyte precursors of infected dogs. Inclusions are generally present for only 2 to 9 days following infection and therefore often are not present when clinical signs occur. Recent administration of modified live CDV-containing vaccines can lead to positive results in direct fluorescent antibody assays and RT-PCR assays, making the positive predictive value of these tests less than 100% in recently vaccinated puppies. False-positive results have been detected occasionally in direct fluorescent antibody assays performed on conjunctival cells from specific pathogen-free puppies and results of these tests should be interpreted cautiously.162
Treatment
Therapy for the GI signs of CDV infection is nonspecific and supportive. Secondary bacterial infections of the GI tract are common and, if indicated, should be treated appropriately.
Prognosis
The prognosis for dogs with GI signs of CDV infection is generally good. However, in dogs with poor immune responses during primary infection, signs of CNS disease may develop several weeks after resolution of the GI tract signs. The prognosis for dogs with CNS disease resulting from CDV infection is poor.
Prevention and Public Health Considerations
CDV survives in exudates only for hours at room temperature and is susceptible to most routine hospital disinfectants. Dogs with GI or respiratory signs of disease should be housed in isolation so as to avoid aerosolization to susceptible populations and care should be taken to avoid transmission by contaminated fomites. Multiple effective CDV vaccines are available and when administered appropriately to immunocompetent puppies, can result in a sterilizing immunity that persists for years.153, 164 There is no proven public health risk associated with CDV.
Canine Parvoviruses
Etiology
Canine parvoviruses (CPVs) are nonenveloped DNA viruses that replicate in rapidly dividing cells.143 These viruses emerged in the late 1970s, arose from the feline panleukopenia virus, and now have worldwide distribution. Currently, CPV-2b and CPV-2c are the predominant genotypes in most countries studied, including the United States.165, 166, 167, 168
Pathophysiology
After a susceptible dog has oronasal exposure to secretions containing a CPV-2 virus, the organism infects lymphoid tissue and induces viremia for 1 to 5 days. CPV-2 preferentially infects rapidly dividing cells of multiple tissues, including the crypt epithelial cells of the intestine. Villus blunting, decreased absorption, inflammation, and necrosis are responsible for the classic signs of vomiting and diarrhea, the latter of which frequently contains blood. The severe inflammation and necrosis allow translocation of enteric flora that is commonly associated with sepsis. CPV-2 are shed for approximately 3 to 14 days after infection and shedding can begin prior to clinical signs. CPV-2 are environmentally resistant.
Clinical Examination
Dogs with partial or sterilizing immunity to CPV-2 frequently develop subclinical infection. Clinical signs are most likely to develop in puppies younger than 12 weeks of age that have no prior immunity. Inappetence is often the first clinical manifestation and most clinically affected puppies develop foul-smelling bloody diarrhea. Concurrent problems frequently include vomiting, leukopenia, fever, and secondary bacteremia, sepsis, and disseminated intravascular coagulation. Other clinical findings associated with CNS or cardiac inflammation may occur in some puppies.
Diagnosis
Presence of characteristic clinical and laboratory findings frequently lead to a presumptive diagnosis of CPV-2–associated disease. Infection can be documented by demonstrating the viruses in feces by electron microscopy, virus isolation, fecal antigen tests, or PCR assay of feces or blood.143, 169, 170 Fecal antigen assays and PCR assays are used most frequently in clinical practice. Recent administration of modified live vaccines containing CPV-2 can lead to transient positive results in both types of assays.169 Severe necrosis can lead to false-negative results in fecal antigen tests. Whether this occurs with PCR assay results has not been proven. It was recently shown that one currently available fecal antigen ELISA detects both CPV-2b and CPV-2c.170
Treatment
Clinical disease for CPV-2 infection is primarily supportive with administration of replacement fluids and electrolytes being paramount. Antiemetics are often indicated and antibiotics with a Gram-negative and anaerobic spectrum are usually administered to puppies with clinical evidence of bacteremia or sepsis. Other therapies, like interferons, passive immunotherapy, colony-stimulating factors, and oseltamivir, are administered by some clinicians and in some small studies with inconclusive results.171, 172 Other GI supportive therapies, like bland diets and probiotics, are often prescribed during the recovery period.
Prognosis
The prognosis with CPV-2–associated GI disease can be poor. However, with rapid and appropriate supportive care many puppies will survive.
Prevention and Public Health Considerations
Avoiding exposure to CPV-2 is the best form of prevention until puppies have been fully vaccinated. However, the organisms are ubiquitous in areas frequented by dogs and exposure is common. Inactivated and attenuated live CPV-2 containing vaccines are available and appropriate vaccination results in sterilizing immunity in normal dogs and may be persistent for life. The AAHA supports the use of attenuated live, high-antigen mass vaccines in most situations.153 There has been concern that the CPV-2b–containing vaccines do not cross protect against the more newly emergent CPV-2c.173 However, recent studies show that cross protection induced by CPV-2b–containing vaccines is likely.174, 175 To date, there is no evidence of zoonotic transmission of CPVs to humans.
Feline Panleukopenia Virus
Etiology
Feline panleukopenia virus (FPV) is a nonenveloped DNA virus with worldwide distribution that potentially induces severe clinical signs of GI disease in susceptible cats.176 Recently, it was shown that cats also can be infected with CPV-2b and CPV-2c.177, 178 Although many veterinarians in developed countries rarely diagnose FPV infection in client-owned cats, infection is still widespread in feral cats. In one study 33% of feral cats that presumably had not been vaccinated had FPV antibody titers.179
Pathophysiology
Previously exposed or vaccinated cats generally limit FPV replication and remain clinically normal. After a susceptible cat has oronasal exposure to secretions containing FPV, viremia occurs with an incubation period of approximately 2 to 7 days prior to development of clinical signs of disease. As for CPV in dogs, FPV invades and destroys actively dividing cells including those of the bone marrow, lymphoid tissues, intestinal epithelium, cerebellum of young animals, retina, embryonic, and fetal cells. In the GI tract, the resultant cellular destruction results in dilated intestinal crypts, degeneration of villi, edema, and necrosis, which are responsible for the clinical signs of disease. High numbers of viral particles are shed during the acute phase of infection and may be detected in feces for weeks following clinical recovery. FPV can survive for longer than a year in a suitable external environment.
Clinical Examination
The primary clinical signs of FPV infection include fever, depression, anorexia, vomiting, diarrhea, and acute death. Some cats with FPV have vomiting without diarrhea. On physical examination, dehydration, abdominal discomfort, thickened SI, and enlarged mesenteric lymph nodes may be detected. Less information is available concerning clinical signs in cats infected with CPVs, but these infections seem to be less severe than FPV infections.
Diagnosis
Presence of appropriate clinical signs and the presence of panleukopenia in a kitten that is FeLV antigen-negative strongly suggests FPV infection. As with dogs, feline parvovirus infections can be documented by demonstrating the agents in feces by electron microscopy, virus isolation, fecal antigen tests, or PCR assay of feces or blood.176, 180, 181, 182 Antigen assays developed for CPV-2 also detect FPV.180, 181 Recent administration of modified live vaccines containing FPV can give transient positive results in both canine antigen assays and parvovirus PCR assays so vaccination history should be considered in the interpretation of parvovirus test results in cats.181, 182
Treatment
Clinical illness from parvovirus infections in cats is primarily supportive with administration of replacement fluids and electrolytes. Antiemetics are often used for persistent vomiting and antibiotic therapy for secondary bacteremia or sepsis may be indicated. Other therapies, like interferons, passive immunotherapy, and antiviral drugs, have been attempted by some but controlled data supporting use of these treatments is not available. GI supportive therapies like bland diets and probiotics are often prescribed during the recovery period.
Prognosis
Mortality rates for FPV infection in susceptible kittens can be very high even with administration of appropriate supportive care.176 Clinical illness associated with CPV-2 infections in cats appears to be less severe and may have a better prognosis.
Prevention and Public Health Considerations
Immunization with FPV-containing inactivated or attenuated live vaccinations provides long-lasting sterilizing immunity. It is possible that some FPV-containing vaccines induce protection against canine parvovirus strains that may result in clinical illness.183 The AAFP recommends use of attenuated live vaccines in high-risk kittens in an attempt to rapidly induce primary immune responses in the presence of potential maternal immunity.158 There are no proven public health risk associated with parvovirus infections of cats.
Other Viruses
There are other viral infections of dogs and cats that may occasionally result in GI signs of disease in dogs or cats. For example, rotaviruses (dogs and cats), reoviruses (cats), and astroviruses (dogs and cats) have been detected in the feces of some animals with diarrhea.143, 184 FeLV infection is associated with a panleukopenia-like syndrome.185 Both FeLV and feline immunodeficiency virus infections are associated with intestinal lymphoma and both organisms can induce immune deficiency during the late stages of infection, which can promote GI disease induced by opportunistic infections. Lastly, feline immunodeficiency virus infection can induce a distinct enteropathy that is associated with diarrhea.154
Bacterial Overgrowth (Intestinal Dysbiosis)
Alexander J. German
Bacterial Flora
The normal small intestinal flora is a diverse mixture of aerobic, anaerobic, and facultative anaerobic bacteria, full details of which are covered in Chapter 2. The size of the bacterial microflora increases from the duodenum to the colon, and is regulated by various factors, including intestinal motility, substrate availability, cidal/bacteriostatic secretions (e.g., gastric, biliary, and pancreatic), and the presence of a functional ileocolic sphincter. Disruption of any of these factors may lead to qualitative or quantitative bacterial flora abnormalities.
There is much debate about what constitutes a normal bacterial population in dogs and cats. When luminal contents are sampled and conventional bacterial culture techniques are used, common species include Staphylococcus sp., Streptococcus sp., Enterobacteriaceae, E. coli, Clostridium sp., and Bacteroides sp., with a greater proportion of obligate anaerobic bacteria being reported in cats than in dogs. However, greater diversity can be seen when mucosal populations are assessed in addition to luminal contents.1 Recent studies employing newer techniques (e.g., sequencing of the 16S rRNA gene, fluorescence in-situ hybridization), demonstrate that conventional techniques underestimate the true complexity of the bacterial flora because a significant number of bacterial species do not grow.2, 3 By conventional techniques, the total upper small intestinal bacterial counts of healthy cats range from 102 to 108 colony-forming units (CFU)/mL, and are higher than those reported in humans (<103, 4, 5 CFU/mL).4 However, there is no clear consensus as to what constitutes a “normal” SI population in healthy dogs, and some studies suggest that healthy dogs can harbor up to 109 CFU/mL bacteria in the proximal SI.5, 6 Therefore, when using conventional culture techniques, the “cutoff” for normal flora in dogs and cats cannot be extrapolated from humans. Furthermore, clinicians must realize that numbers are likely to be greater, when taking into account the species that cannot be cultured by conventional means. The SI flora is relatively resistant to dietary changes; studies using different diets, or the addition of fructooligosaccharides to the diet have failed to demonstrate significant effects on the number or type of bacteria in the proximal SI of dogs and cat,7, 8 although some effects have been demonstrated on the colonic flora in cats.9
The resident bacterial flora is an integral part of the healthy SI; organisms are broadly divided into those with health positive effects, those that are health neutral, and, finally, those species that may have health-negative effects (Table 57-7 ; Figure 57-19 ). Beneficial effects include inhibition of the growth of bacterial pathogens (by producing antimicrobial factors, occupying receptor sites, and competing for nutrients), facilitating digestion and absorption of various nutrients, synthesizing vitamins, and stimulating immune function.10 Health-negative effects include intestinal putrefaction, production of carcinogens, hepatic damage, and clinical signs such as diarrhea and constipation. In addition, studies implicate the intestinal flora in the development of obesity in humans.11 Finally, loss of immunologic tolerance to the normal bacterial flora has been implicated in the development of chronic intestinal inflammation, abnormal intestinal function, and perhaps even neoplasia. An inability to tolerate a normal bacterial flora may explain the antibiotic-responsive enteropathy in German Shepherd dogs.12
Table 57-7.
Comparison of Enteric Flora and Effects on Health in Various Species
| Humans | Dogs | Cats | |
|---|---|---|---|
| Health positive | Bifidobacteria Lactobacilli |
Bifidobacteria Lactobacilli |
Lactobacilli |
| Health neutral | Enterococci E. coli Streptococci Bacteroides |
Enterococci Eubacteria Streptococci Fusobacteria Bacteroides |
Corynebacteria Enterobacteria Streptococci Clostridia Lc −ve Bacteroides |
| Health negative |
Pseudomonas aeruginosa Proteus sp. Staphylococci Clostridia Veillonella |
Clostridia | Clostridia Lc +ve |
LC +ve: expresses the zinc-dependent proteolytic light chain (LC) portion of the clostridial enterotoxin. LC -ve: does not express the zinc-dependent proteolytic light chain (LC) portion of the clostridial enterotoxin.
From Rastall RA: Bacteria in the gut: friends and foes and how to alter the balance. J Nutr 134(8 Suppl):2022S, 2004.
Figure 57-19.
Comparison of enteric flora and effects on health in various species.
(Redrawn from Rastall RA: Bacteria in the gut: friends and foes and how to alter the balance. J Nutr 134:2022S, 2004.)
Clinical Syndromes
Small Intestinal Bacterial Overgrowth (Or Intestinal Dysbiosis)
Genuine bacterial overgrowth is defined by an increase in the absolute number of bacteria in the upper SI during the fasting state (i.e., the number of “colony-forming units” cultured per milliliter of duodenal juice [CFU/mL]). In humans, SIBO arises secondary to a number of underlying disorders that interfere with the control mechanisms (as described previously). Although the existence of this condition in humans is not disputed, its existence in canine patients has been a subject of much controversy. By conventional methods of microbial culture in humans, SIBO is diagnosed when upper small intestinal bacterial numbers exceed 105 or 104 CFU/mL of intestinal juice for total bacteria and obligate anaerobes, respectively. Although these values were initially adopted for dogs, a number of studies suggest a larger microbial population (e.g., 107 CFU/mL or greater) in asymptomatic dogs.6 Small intestinal bacterial numbers are also greater in cats. Therefore the current diagnostic criteria for SIBO in companion animals are inappropriate, and may lead to misdiagnosis in many cases. Nonetheless, a genuine bacterial overgrowth may exist in conditions equivalent to those in humans, and it is appropriate to use the term SIBO in such circumstances.
Potential associations have been reported for SIBO (Box 57-5 ). In all such conditions it is logical to assume that a genuine increase in bacterial numbers occurs, although few studies have documented its magnitude or whether the overgrowth is actually responsible for the clinical signs. Furthermore, although diagnostic investigations may identify the presence of secondary SIBO, in practice it is better to concentrate the diagnostic effort on identifying the underlying process. As in humans, therefore, canine SIBO is best viewed as a clinical sign or pathogenetic mechanism rather than a diagnosis in its own right. The consequences of a secondary SIBO include interference with absorption of nutrients (including cobalamin and possibly taurine) and fluid, because of microvillar enzyme dysfunction, altered mucosal permeability, deconjugation of bile acids, and stimulation of colonocyte secretion.13, 14
Box 57-5. Diseases Causing Secondary Small Intestinal Bacterial Overgrowth (Intestinal Dysbiosis).
Decreased gastric acid production (achlorhydria)
-
•
Spontaneous (e.g., atrophic gastritis)
-
•
Iatrogenic (e.g., acid-blocking drugs, surgical resection)
Increased small intestinal substrates
-
•
Exocrine pancreatic insufficiency
-
•
Malabsorptive disorders?
Partial obstructive disorders
-
•
Chronic intussusceptions
-
•
Stricture
-
•
Neoplasia
Anatomic disorders
-
•
Surgical resection of ileocolic valve
-
•
Blind loop (“self-filling type”)
Motility disorders
-
•
Primary/idiopathic
-
•
Secondary
Hypothyroidism
Electrolyte disorders
Intestinal surgery
Sepsis
Peritonitis
Idiopathic Antibiotic-Responsive Diarrhea
Historically, the term idiopathic SIBO was used to describe an antibiotic-responsive condition of large-breed (especially German Shepherd) dogs,13 in which no underlying cause could be recognized. In fact the most consistent sign, as suggested by the name, is a predictable response to and remission with antibacterial therapy. Given that recent studies question whether a genuine increase in bacterial numbers occurs, these cases have been renamed idiopathic antibiotic-responsive diarrhea (ARD).12 Although cats might feasibly suffer from secondary SIBO, an idiopathic antibiotic-responsive condition similar to that in German Shepherd dogs has not been documented in this species.15
Most cases of idiopathic ARD have been seen in young German Shepherd dogs, although other breeds may be affected. There may also be similarities with the recently reported “tylosin-responsive diarrhea” (see later).16 Recent hypotheses on pathogenesis suggest that host–bacterial interactions may be important. In this respect, ARD may develop secondary to defects in the mucosal barrier, aberrant mucosal immune responses, qualitative changes in the enteric bacterial flora, or a triangulation of these mechanisms. Defects in the mucosal barrier are supported by studies documenting abnormal permeability and the presence of brush-border enzyme defects.13, 14 A further extension of the hypothesis of defective mucosal barrier is the suggested association with IgA deficiency or dysregulation.17 Some, but not all, reports suggest that German Shepherd dogs with intestinal disease may have defective small intestinal IgA production, although mucosal IgA+ plasma cell numbers in affected dogs are either normal or increased.18 However, IgA deficiency has not consistently been identified in German Shepherd dogs,19, 20 and the pathogenesis may be more complex. Recent studies suggest differences in relative expression of allelic variants of the IgA heavy gene in various dog breeds including German Shepherd dogs.21, 22 However, there is no clear association between the different allelic variants and the manifestation of GI disease.23
A further suggestion is that ARD arises secondary to a loss of tolerance toward endogenous bacterial antigen. Indirect evidence for this hypothesis comes from the findings that dogs with ARD have increased lamina propria CD4+ T cells and increased expression of certain cytokines.18, 24 Such a hypothesis is supported by the fact that antibacterial agents lead to resolution of clinical signs, and decreased cytokine expression, but not a decline in bacterial numbers.24 That the most effective antibacterials are those with immune-modulating properties (e.g., oxytetracycline, metronidazole, tylosin) may support this hypothesis. A final possibility is that the condition arises from defective acquired immune responses to an occult infectious agent (e.g., enteropathogenic E. coli or Clostridium species). Therefore the predisposition of German Shepherd dogs to this syndrome could be explained by genetic susceptibility to infection as a result of major histocompatibility complex class II antigen expression. In this respect, there could be similarities in pathogenesis with histiocytic ulcerative colitis in Boxer dogs.25
At the current time, there are many suggestions as to the pathogenesis of idiopathic ARD. Unfortunately, there is currently no direct evidence to support any particular hypothesis and further work is required.
Tylosin-Responsive Diarrhea
A condition has been described in dogs from Finland that has many similarities with idiopathic ARD.16 Detailed investigations suggest that no underlying cause for signs could be determined; furthermore, bacterial numbers were normal, there were no characteristic changes on folate or cobalamin assay, and serum unconjugated bile acid concentrations were unhelpful. In addition, there was complete resolution of signs while on tylosin and, in many cases, relapse occurred upon discontinuation of therapy. The main difference from the classical description of idiopathic ARD was that a range of ages of dog was represented, and a mixed-pattern diarrhea noted. However, its true idiopathic nature was not demonstrated, as detailed diagnostics were lacking in many cases. Despite this limitation, the similarities with idiopathic ARD are striking; although the authors of the paper favored the term tylosin-responsive diarrhea, the likelihood is that this is a form of idiopathic ARD.
Clinical Findings
The most common signs for secondary SIBO and idiopathic ARD are chronic small intestinal diarrhea and weight loss or failure to thrive. Other signs include vomiting, appetite alterations (anorexia, polyphagia, scavenging, and coprophagia), excessive borborygmi, and abdominal discomfort. In addition, signs of large bowel diarrhea are sometimes noted. Signs are similar for tylosin-responsive diarrhea aside from the fact that a mixed-pattern diarrhea is seen.16 A thorough history is important, as this may demonstrate an underlying cause (e.g., previous GI surgery) in cases of secondary SIBO. If a partial obstruction is the cause of a secondary SIBO, the history often involves relapsing small intestinal diarrhea, weight loss, and a favorable response to antibacterial therapy; the intermittent clinical signs in such cases are thought to be the result of the recurrent diarrhea temporarily flushing out the overgrowth. For cases of idiopathic ARD, deterioration on glucocorticoid therapy may sometimes be noted in the history. Abdominal palpation may demonstrate a structural cause of secondary SIBO, for example, partial intestinal obstruction, although it is unremarkable in cases of idiopathic ARD.
Diagnosis
Secondary SIBO can be detected by a number of tests, but it is essential to detect the underlying cause. A complete investigation is recommended, for example, routine hematology, serum biochemical analysis, urinalysis, fecal bacteriology and parasitology, diagnostic imaging, and gastroduodenoscopy. EPI can be diagnosed by measuring serum TLI concentration, partial obstructions can be detected with diagnostic imaging, and an enteric pathogen might be detected on fecal analysis. However, findings are usually unremarkable or nonspecific in cases with idiopathic ARD. At this stage, a treatment trial with antibacterial agents (see Chapter 39) should be contemplated, because response to empirical therapy is currently the best means of diagnosing idiopathic ARD. True idiopathic ARD can be diagnosed if the following criteria are established:
-
•
No other etiologic cause is identified with detailed preliminary testing and/or histopathologic assessment of small intestinal biopsies.
-
•
There is a positive response to an antibiotic trial (e.g., resolution of clinical signs including weight gain).
-
•
Relapse of clinical signs occurs upon withdrawal of treatment, and remission is achieved when antibiotic therapy is recommenced.
However, although response to antibacterials is critical for the diagnosis, a thorough diagnostic evaluation should ideally be performed to make certain that other reasons for a response to antibacterials (especially the causes of SIBO) have first been eliminated. Given current concerns over the development of antibiotic resistance by various bacterial flora, indiscriminate use of antibiotics in dogs and cats with diarrhea or gastroenteritis is ill-advised. Although the consequences of inappropriate antibiotic therapy are often mild and self-limiting, postantibiotic salmonellosis has had fatal consequences in cats.26
Diagnostic Tests for Small Intestinal Bacterial Overgrowth
Both direct and indirect tests are available, but none have been properly validated for companion animals, and widely accepted reference ranges have not been properly established. Therefore the results of these tests must be interpreted with caution. The main direct test is quantitative bacterial culture of duodenal juice. Indirect tests include hydrogen breath tests and serum biochemical analyses.
The current diagnostic gold standard for SIBO is duodenal juice culture, and the most commonly quoted figure for the upper limit for small intestinal bacterial numbers is 105 CFU/mL. However, the validity of this cutoff is questionable because quantitatively larger numbers (approximately 107 CFU/mL in some studies) have been documented in healthy dogs, while numbers as high as 109 CFU/mL have been found occasionally in cats and asymptomatic dogs.4, 5, 8, 9 Some of the discrepancies may reflect difficulties and differences in the methodology, as numbers vary widely when individual animals are repeatedly sampled.1
Use of an inappropriately low cutoff value will lead to the overdiagnosis of SIBO, probably explaining why it has been reported to be present in 50% of dogs with chronic intestinal disease.27 In reality true secondary SIBO is rare, with the exception of SIBO secondary to EPI. An increase in small intestinal bacterial numbers has been documented in experimentally induced EPI, although bacterial numbers decrease upon treatment of the EPI with enzyme replacement.28 Therefore in many cases the SIBO itself is of no significance. However, a proportion of naturally occurring EPI cases respond suboptimally to pancreatic enzyme supplementation alone, and may require concurrent antibiotic therapy. Given that the majority of dogs affected with EPI are German Shepherd dogs, it is not clear whether this is the result of secondary SIBO, or of a concurrent idiopathic ARD. Furthermore, duodenal juice collection is technically demanding, expensive, and rarely performed routinely. Attempts at improving diagnostic accuracy have included measuring bacteria in mucosal biopsies samples, but this has not been shown to be of added benefit.1 Finally, quantitative PCR techniques may result in improved reliability by producing a more reliable bacterial yield2, 3; however, they have not yet been used as a means of attempting to diagnose possible SIBO.
Given the limitations of the diagnostic gold standard, indirect tests have been adapted from human methodology for use in the clinical setting. The most commonly used diagnostic tests in dogs are the measurement of serum folate and cobalamin concentrations. The utility of these assays is based on the theory that many bacterial species synthesize folate, while others can bind cobalamin; therefore increased numbers of small intestinal bacteria may elevate serum folate concentrations, decrease serum cobalamin concentrations, or both. However, measurements of these parameters have poor sensitivity and specificity for canine SIBO, and cannot differentiate dogs with ARD from those with other etiologies.12 Consequently the use of folate and cobalamin measurements for the diagnosis of SIBO, and especially ARD, is questionable. However, they may still be of value in detecting vitamin malabsorption (see following discussion).
An alternative indirect means of detecting SIBO was the measurement of hydrogen concentrations in exhaled breath, either in a fasted state or after administration of a test meal.29, 30, 31, 32 However, protocols have not been universally accepted and this remains a research technique. Canine assays for SUBA were also recommended at one time.33 These tests relied on the principle that conjugated bile acids that are secreted into the intestines can be deconjugated by some bacterial species (e.g., Clostridia, Bacteroides), the so-called unconjugated bile acids, which then are absorbed passively and can be measured in blood. Although early work suggested that these assays might be promising,33 more recent work suggests that they are of limited use in the diagnosis of idiopathic ARD.12 To my knowledge, these assays are no longer available.
In summary, none of the diagnostic tests currently available are recommended for diagnosis of either secondary SIBO or idiopathic ARD. Where secondary SIBO is suspected, it is preferable to look for the underlying cause. Given that neither quantitative bacterial culture nor indirect tests reliably identify cases that respond to antibacterials, and given that correlation between all methods is poor, their use in the diagnosis of idiopathic ARD is not recommended.
Treatment
Secondary Small Intestinal Bacterial Overgrowth
Although antibacterial therapy will improve clinical signs, appropriate treatment for the underlying condition is preferable. For EPI, pancreatic enzyme supplementation can reduce bacterial numbers because exogenous proteases have antibacterial properties. Experimental studies show that bacterial numbers in dogs with EPI decline with pancreatic enzyme supplementation alone (probably because enzymes are bactericidal and available substrate is reduced), suggesting that the problem will resolve of its own accord. However, in some clinical cases concurrent antibacterial therapy is necessary.
Idiopathic Antibiotic-Responsive Diarrhea and Tylosin-Responsive Diarrhea
For idiopathic ARD, an appropriate antibacterial should be administered for an initial period of 4 weeks. If signs relapse, a longer course may be required, and many cases require long-term (or lifelong) therapy to maintain remission of signs. The choice of antibacterial is controversial; most cases of idiopathic ARD respond well to oxytetracycline at 10 to 20 mg/kg TID PO and for long-term therapy, low doses can often maintain clinical remission (10 mg/kg SID PO). However, it should not be used before permanent tooth eruption because of staining of tooth enamel. Other suitable drugs include tylosin at 10 to 15 mg/kg BID, which, unsurprisingly, is the drug most commonly used for tylosin-responsive diarrhea in Finland. A final option is metronidazole, given at 10 mg/kg TID PO.
The mechanism of action is not currently known. Interestingly, when oxytetracycline is administered bacterial numbers do not decline significantly and resistance soon develops, despite resolution of clinical signs. Hypotheses include the possibility that these drugs are exerting a selection pressure on the intestinal microflora in the same way as a prebiotic. Alternatively, immunomodulatory effects, as reported for some of the tetracyclines, are possible.
Currently, oxytetracycline remains the first choice for idiopathic ARD in the United Kingdom, but its use for secondary SIBO is controversial and other drugs may be more appropriate, for example, tylosin or metronidazole, as their spectrum of activity is better for the organisms that are likely to be present in secondary SIBO. Furthermore, some authors question whether oxytetracycline should be used at all because it is associated with rapid development of plasmid-mediated antibiotic resistance.34 However, given that long-term efficacy is maintained in most cases, oxytetracycline may not be acting through its antibacterial properties as it does not significantly reduce SI bacterial numbers.24 Instead, it may either provide a selective pressure on the intestinal flora encouraging the establishment of less harmful bacteria or utilize immunomodulatory effects, which this antibiotic group possesses. Immunomodulatory activities also have been suggested for other antibacterials, namely metronidazole and tylosin, that are commonly used to treat ARD. Finally, the drug is well-tolerated and there is no evidence that adverse effects (e.g., antibacterial-associated diarrhea) are a common effect of long-term oxytetracycline use.
Whichever antibacterial is chosen, a 4- to 6-week course is appropriate initially, although the antibiotic should be changed after 2 weeks if response has been suboptimal. In some cases, premature cessation of treatment can lead to relapse and prolonged therapy is often necessary. In some animals, a delayed relapse occurs several months after cessation of antibiotics, and such cases either require repeated courses or indefinite therapy. Efficacy is often maintained despite reducing the dosage from thrice to even once daily. Dogs may also “outgrow” the problem with age, either as a result of a decrease in caloric intake, or because of developing maturity to the mucosal immune system. It also has been suggested that idiopathic ARD in German Shepherd dogs may predispose individuals to IBD in later life, but there is currently no direct evidence to support this supposition.
Adjunctive therapy may be helpful in cases of both secondary SIBO and idiopathic ARD. This involves the feeding of a highly digestible diet. The need for fat restriction is logical for secondary SIBO, given that hydroxylation of fatty acids is implicated in the disease pathogenesis. However, whether this mechanism is involved in the pathogenesis of idiopathic ARD (and tylosin-responsive diarrhea) is not currently known. In addition, restricting fat may make it difficult for a cachectic patient to gain body weight and condition. Other suggestions include adding prebiotics (e.g., fructooligosaccharides) to the diet. Although these can modulate colonic microflora,9 the effect on small intestinal bacteria is questionable8 and there is limited current evidence for efficacy in clinical cases. Similarly, there is no published evidence supporting the use of probiotics for either secondary SIBO or idiopathic ARD. Finally, if low cobalamin concentrations are documented, parenteral cobalamin therapy is warranted.
Prognosis
The prognosis for secondary SIBO depends upon the nature of the underlying cause, and success of therapy for the particular condition. The prognosis for idiopathic ARD is guarded; many cases relapse after therapy is discontinued, and then require prolonged or even lifelong treatment. Other cases, however, require only occasional short courses of antibacterials to maintain clinical remission. Some cases may improve spontaneously as the animal enters adulthood.
Obstruction
Nick Cave
Definition
Obstruction of the SI is a common problem in companion animal practice. Severity of intestinal obstruction is classified according to a number of parameters including clinical signs, site, and patency. If ingested nutrients are unable to pass beyond the point of obstruction, it is termed complete obstruction. If some nutrients pass through the point of obstruction, even if only the liquid phase, it is termed partial obstruction. Physical or mechanical obstructions can result from intraluminal foreign bodies, intramural masses, and extramural compression. Functional obstructions result from generalized hypomotility or spasticity of a bowel segment. Functional obstructions are discussed in the “Dysmotility” section, and the mechanical obstructions of the intestine are discussed in this section.
Foreign Bodies
Luminal foreign bodies are the most common cause of acute intestinal obstruction. In a series of 174 cases in dogs in the United Kingdom, latex nipples were the most common foreign bodies, followed by plastic or rubber balls, stones, and strings.1 In the same study, linear foreign bodies accounted for 44% of feline cases. In a survey of working farm dogs in New Zealand, plastic ear tags and bones constituted 66% of the intestinal foreign bodies.2 Thus the nature of intestinal foreign bodies tends to reflect local practices and customs. In cats, trichobezoars (hairball obstructions) can cause partial or complete intestinal obstruction, often at a site of preexisting infiltrative intestinal disease.3 Obstructive trichobezoars are rarely diagnosed in dogs and when they are seen, it is usually at a site of preexisting intestinal narrowing.4 Foreign bodies can lodge anywhere along the small and large intestine, and there does not appear to be a predilection site, except that the jejunum, because of its length, is the most commonly affected region.1, 5 Linear foreign bodies may anchor around the base of the tongue, pylorus, or at more distal sites.1, 6 Most linear foreign bodies do not themselves obstruct the intestine, but gathering and pleating of the intestine around the foreign object causes partial to complete obstruction.
Intussusception
Intussusception represents an uncommon form of bowel obstruction, defined as the telescoping of a (usually) proximal segment of the GI tract, called the intussusceptum, into the lumen of the adjacent distal segment of the GI tract, called intussuscipiens. The most common sites in the dog are the ileocolic junction or jejunojejunum, whereas in cats, jejunojejunal is the most common type.7, 8, 9 In general, an intussusception occurs when a migrating peristaltic wave reaches a fixed or noncontractile segment (labeled A in Figure 57-20 ). If the fixed or noncontractile segment is of sufficient diameter, and of sufficient length, then the proximal contracted segment will fold into the outer intussuscipiens (Figure 57-21 ). The majority of intussusceptions develops aborally in the direction of normal peristalsis, but can develop in an orad, or retrograde, direction (Figure 57-22 ). Alternatively, an intraluminal, or extraluminal linear fixed linkage such as a short linear foreign body, can result in formation of a kink in the wall, which progresses to a fold and then intussusception.10
Figure 57-20.
An annular bowel lesion or region of hypomotility (shaded area) can lead to intussusception when the adjacent contracted segment produces a kink at the border between normal and abnormal tissue (labeled A). The advancing wave of peristalsis drives the proximal segment into the lesional area creating an intussusception.
Figure 57-21.
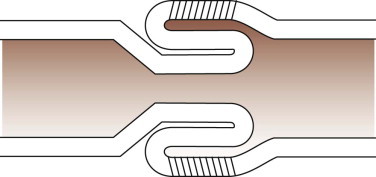
An aborad or direct intussusception is the most common form seen in dogs and cats. The internal section is termed the intussuscipiens, and the external (shaded) section is termed the intussusceptum.
Figure 57-22.
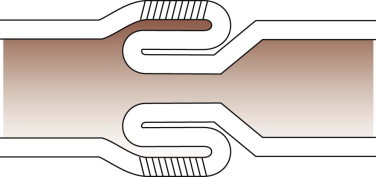
An orad or retrograde intussusception.
Intussusceptions are generally more common in younger animals, many younger than 1 year of age, although the age distribution in cats appears to be bimodal.7, 8, 11 Intussusception in young cats is most likely to be idiopathic, whereas in older cats it is more likely to be secondary to infiltrative disease such as lymphosarcoma or IBD. Intussusception has also been recorded in queens during the immediate postpartum period.12 Most intussusceptions in dogs are idiopathic, although any disease that can disturb intestinal motility, such as viral enteritis, neoplasia, foreign bodies, and prior abdominal surgery, can lead to intussusception.8, 9, 11, 13, 14 In dogs treated for severe viral enteritis (e.g., parvoviral or coronavirus), intussusception should be suspected if a sudden unexplained deterioration occurs.13, 15 Likewise, a newly diagnosed intussusception should not be assumed to be of idiopathic origin. Diagnosis always requires consideration and appropriate testing for an underlying condition.
Pathophysiology
Motility
Motility at the site of the obstruction is altered through the multiple pathogenetic effects of ischemia, inflammation, bacterial toxin absorption, and direct mechanical stimulation. In acute experimental jejunal occlusion, intestinal motor activity is almost immediately altered.16 Initially, there is increased motor activity proximal to the obstruction, and reduced motor activity distal to it (the intestinointestinal reflex, see Chapter 1). Within 2 to 3 hours, however, hypermotility extends proximally to the duodenum, and hypomotility extends distally from the obstruction to the terminal ileum. Following occlusion of the small intestinal lumen, continued peristaltic activity proximally leads to transient increases in intraluminal pressure. Normal intraluminal pressure rarely exceeds 4 mm Hg, whereas after experimental occlusion in dogs or cats, proximal intraluminal pressure is sustained between 5 and 10 mm Hg, and peaks at 20 mm Hg during intense contractions.17, 18
In chronic partial obstructions, there is an extensive remodeling of the muscularis layer proximal to the obstruction leading to smooth muscle hyperplasia and hypertrophy, neuronal degeneration, neoangiogenesis, and fibrosis.19, 20, 21 (Muscularis remodeling does not occur distally despite the local mucosal atrophy that develops acutely.) The net effect of these changes is an increase in wall stiffness, loss of slow-wave activity, reduced neural responses, and reduced contractile capacity. In chronic partial obstruction, wall stress and strain rise significantly proximal to the obstruction as a bolus is advanced by peristalsis. Mechanoreceptor stimulation triggers further increases in pressure to force the chyme through the obstruction. This may lead to localized luminal bulging. Most of this remodeling and degeneration is reversible over time once the obstruction has been removed, although neuronal degeneration may be irreversible and hypomotility may persist in some patients, particularly in more chronic cases.22
Mucosa
The mucosa proximal to the occlusion is stimulated to secrete water and electrolytes into the intestinal lumen. Distal to the obstruction, villus atrophy of the mucosa and reduced brush-border enzyme expression develop rapidly.18 Although physical disruption to local vascular supply occurs, increased vascular pressure is not the mechanism for fluid hypersecretion. When the jejunum is experimentally occluded surgically in gnotobiotic dogs, proximal segment hypersecretion does not take place,23 illustrating the role of the enteric microflora in the pathogenesis of the continued fluid secretion proximal to the obstruction. Vomiting of this hypersecreted fluid contributes to the dehydration, acid–base, and electrolyte disturbances of the disorder. Although there is a relative hypersecretion, the actual volume of fluid that accumulates proximal to the acute obstruction is surprisingly small and of low protein content.24
Intussusceptions commonly lead to more significant vascular compromise, and both lymphatic and venous vessels are occluded resulting in edema and hemorrhage, both intramurally, and into the intestinal lumen. Intramural and serosal hemorrhage can lead to fibrinous adhesions that prevent manual reduction of the intussusception.
Bacteria
Experimental occlusion of the SI leads to rapid death in conventional raised dogs. However, gnotobiotic dogs can survive for prolonged periods despite complete intestinal obstruction.25, 26 When a mixed intestinal microflora is reintroduced into an occluded intestine, it too leads to rapid death. However, common intestinal bacteria vary greatly in their pathogenicity after intestinal obstruction. C. perfringens mono-inoculation results in rapid death, Bacteroides fragilis mono-inoculation results in death after several days, whereas E. coli mono-inoculation inconsistently results in death. This emphasizes the importance of treating cases of intestinal obstruction with anaerobicidal antimicrobial agents.
The clearing function of the peristaltic waves is perhaps the most important factor that limits microbial numbers in the SI. It has long been known that a reduction in MMC activity leads to an increase in intraluminal bacteria, and a qualitative shift in the population distribution toward Gram-negative and anaerobic bacterial species.27 In the segment proximal to an acute intestinal obstruction, there is a marked proliferation of both aerobic and anaerobic enteric microflora, but most markedly anaerobic and Gram-negative facultative aerobes.28, 29 Overgrowth of bacteria is required for induction of proximal enteritis and disturbance in mucosal secretory function. At the same time, bacterial translocation into the systemic and portal circulation is dramatically increased and leads to colonization of the hepatic parenchyma.28 In experimental jejunal obstruction in rats, technetium-labeled E. coli readily translocate within 4 hours of the obstructing event.30 Bacteria migrate from the strangulated segment into the peritoneal cavity, and are then disseminated systemically. Significant translocation of bacteria is observed in the heart, liver, and kidney. Higher numbers of bacteria migrate from the strangulated section, consistent with the importance of vascular integrity for maintaining normal barrier function.
Pain
Intestinal obstruction is a painful condition, although some affected animals do not demonstrate much discomfort. Several factors contribute to pain generation, including intestinal dilation, mechanical trauma, ischemia, and bacterial toxin absorption. Sensory nerve fibers innervating the mucosa, submucosa, muscle, myenteric plexus, and serosa, respond to mechanical distortion of the gut wall, particularly distention, but also contraction of smooth muscle, and changes in the chemical environment of the intestinal lumen.31 Distention triggers release of substance P, which stimulates the secretomotor neurones via neurokinin (NK)1 and NK3 receptors, resulting in chloride and water secretion.32 Through mediators such as substance P, nitric oxide, and neurokinin A, motor activity can be stimulated or inhibited, and alterations in electrolyte, mucus and fluid secretion, arteriolar dilation, vascular permeability, mast cell degranulation, and activation of immune cells can be induced.31 Thus there is a bidirectional interaction in pain sensation and neurogenic inflammation during intestinal obstruction.
Intestinal Wall Integrity
As pressure at the site of obstruction progressively increases, lymphatic and then venous drainage is impaired while arterial perfusion is maintained, resulting in intestinal wall edema. Local endothelial barrier leakage and arteriovenous shunting causes intestinal ischemia and reperfusion injury with concomitant barrier dysfunction. Full-thickness wall necrosis may occur, leading to gross contamination of the peritoneum and septic peritonitis.
Differential Diagnoses
Patients with intestinal obstruction can present with acute clinical signs, as is often the case with complete obstruction, or with chronic clinical signs, which is more consistent with a partial obstruction. The differentials for patients with an acute onset of signs are those common to any patient with acute-onset vomiting, although acute intestinal obstruction is usually distinguished from self-limiting causes on the basis of the severity, history, and physical exam findings. The key differential diagnoses for acute onset of vomiting are discussed more fully in Chapter 23. Key differentials for abdominal pain are discussed in Chapter 6. Chronic partial intestinal obstruction should be considered for any patient with chronic vomiting, small intestinal-type diarrhea (Chapter 11), weight loss (Chapter 24), and evidence of PLE.
Evaluation of the Patient
History
Although any dog or cat of any age or breed can present with an intestinal foreign-body obstruction, young dogs and cats, especially large-breed dog conformations, are overrepresented in most case series.1, 33 Intussusceptions are more common in young dogs and cats, although they may be secondary to underlying disease (e.g., lymphoma) in older animals. The clinical signs of intestinal obstruction vary with the site and completeness of obstruction, degree of distention, presence of peritonitis, and whether there is concurrent systemic sepsis. The most obvious clinical signs are anorexia, abdominal pain, depression, and vomiting. In experimental intestinal obstruction, these signs are followed by profound dehydration, shivering and ataxia, and eventually collapse, coma, and death.34 Chronic partial foreign-body obstructions and chronic patent intussusceptions are generally less severe in their clinical signs, for example, vomiting and abdominal pain, and consequently nonspecific lethargy, inappetence, intermittent vomiting, and weight loss may be the only presenting clinical complaints. In acute cases, owners will frequently be aware of the possibility of foreign-body ingestion, and they should be questioned closely to ascertain the possibility.
In experimental complete occlusion of the duodenum, vomiting occurs within hours, and in almost all cases.34 In surgically induced distal jejunal occlusion in dogs, only four of 38 dogs vomited during a 4-day postoperation period, and the volume of vomitus was small.24 Thus the frequency and severity of vomiting may more importantly reflect the site of occlusion and factors other than simple mechanical occlusion. Nonetheless, vomiting is the most common presenting sign in both dogs and cats. When absorptive capacity is preserved oral to the site of the obstruction, fluid losses in vomitus may not be as severe. In general, it appears that more distal obstructions (e.g., jejunal) are associated with a lower frequency of vomiting than are seen with proximal obstructions (e.g., duodenal).24 Proximal obstructions generally result in the loss of large amounts of ingested and secreted intestinal fluid. The duration of vomiting prior to presentation for a complete obstruction is usually 2 to 3 days, but partial obstructions can cause chronic intermittent vomiting for many weeks or months.1, 33, 35 Thus intestinal foreign bodies are an important differential for patients presenting with a history of acute or chronic vomiting.
Diarrhea is less frequently reported in patients with acute complete intestinal obstruction, but it may be an important clinical sign with chronic partial obstruction. Patency of the lumen of an intussusceptum is often maintained with partial obstruction, such that intermittent vomiting and chronic diarrhea are frequently reported. Diarrhea associated with chronic partial obstruction is usually described as small bowel in character, and can be hemorrhagic in acute cases.1 Ileocolic intussusceptions particularly are associated with hematochezia. Chronic weight loss and evidence of PLE are also seen in partial obstructions, especially intussusceptions.36
Physical Examination
Thorough physical examination is imperative for the differentiation of self-limiting causes of acute vomiting from intestinal obstruction and other life-threatening conditions. A diagnosis of intestinal foreign-body obstruction or intussusception can frequently be made on the basis of history and physical examination alone. Animals will be variably dehydrated, and in severe cases, physical parameters indicative of poor perfusion and shock may be present. Normothermia, fever, and occasionally hypothermia may be present according to disease duration, severity of necrosis and inflammation, presence of systemic sepsis, and cardiovascular status. Abdominal pain may be manifested by hunched stance, praying position, or a reluctance to stand and move.
Abdominal palpation may permit localization of pain to a specific region of intestine, or it may be more generalized, especially when peritonitis is present. Contraction of the abdominal musculature, vocalization, and aggression may indicate abdominal pain. Cats will frequently slump into lateral recumbency when abdominal pain is elicited. In many cases, a foreign body can be directly palpated.1 Plication or bunching of the intestines may be suggestive of a linear foreign body. Gas-filled loops of bowel often are painful on palpation though minimally dilated. Intussusceptions frequently can be palpated as a firm, often painful tubular structure that is well demarcated, and cannot be indented with digital pressure, thus differentiating it from feces. The most common location for an intussusception is the cranioventral region. In some cases with severe abdominal pain, palpation may not be possible until heavy sedation or anesthesia is induced. If sedation is required for further investigation, careful palpation should be repeated.
Linear foreign bodies can have a proximal anchor point anywhere along the intestinal tract. Anchoring around the base of the tongue is sufficiently common as to make careful examination of that area imperative in a vomiting patient.1, 33
Laboratory Evaluation
The severity of hematologic and serum biochemical changes depends upon the severity and duration of the obstruction, and presence of complicating factors such as peritonitis, systemic inflammatory response, and hypovolemia. In experimental mechanical occlusion of the jejunum, hemoconcentration, leukocytosis, mature neutrophilia, and progressive hypoglycemia are common.34 A degenerative left shift in the white blood cell count is more likely when concurrent peritonitis is present.37, 38 Extravasation and hemorrhage may lead to hypoproteinemia, and variable degrees of azotemia may be seen consistent with reduced renal perfusion and/or blood loss.34 Although important in the staging of disease, it is important to note that the degree of abdominal cytopathology, hematology, and serum biochemical changes does not predict survival following surgery for septic peritonitis.37, 38
Patients with intestinal obstruction develop progressive dehydration, electrolyte imbalance, and acid–base disturbances. In a study of 138 dogs with intestinal foreign bodies, the most common electrolyte and acid–base abnormalities were hypochloremia (51.2%), metabolic alkalosis (45.2%), hyperlactatemia (40.5%), hypokalemia (25%), and hyponatremia (20.5%).33
It has been previously suggested that obstructions of the proximal SI are more likely to result in the loss of gastric chloride in excess of sodium. This would lead to an increased strong ion difference and a metabolic alkalosis, whereas more distal small intestinal obstructions would more likely result in metabolic acidosis.39 Evidence-based data studies by Boag et al. have instead shown that there is no association between the site of obstruction (proximal vs. distal) and biochemical abnormalities including venous pH, serum lactate, potassium, and chloride.33 Hypochloremic, hypokalemic metabolic alkalosis was reported in 12% and 13% of dogs with proximal and distal obstructions, respectively.
Chronic intussusception in dogs is commonly associated with hypoproteinemia, and varying degrees of hyponatremia, hypokalemia, and hypochloremia.36 Because of the strong association between preexisting enteric disease and the development of intussusception, it is likely that biochemical abnormalities are influenced by the underlying disease, rather than the effect of the intussusception.
Diagnostic Imaging
History and physical examination findings, including direct palpation of a foreign body or intussusception, are often sufficient to justify preparation for exploratory laparotomy, but direct imaging should be performed to confirm the diagnosis prior to laparotomy. Chapter 26 discusses diagnostic imaging of the intestinal tract more fully.
Plain Radiography
Radiopaque foreign bodies are easily identifiable on plain radiography (Figure 57-23 ), but even when present, it is imperative to determine whether there is evidence of obstruction that would warrant immediate surgical intervention, or if it is reasonable to delay surgery to determine whether the object will pass from the GI tract. In other cases where there is intussusception or a radiolucent foreign body, the radiographic evaluation is focused on the detection of dilated intestinal loops, “gravel” appearance of intestinal contents, free abdominal gas and loss of abdominal detail, and intestinal mass effect.
Figure 57-23.
A, Lateral abdominal radiograph of a dog with a radiopaque foreign body. Notice the fluid-distended loop of small intestine cranial to the object. Radiopaque sand-like material can be seen in the stomach. B, Ventrodorsal radiograph of the same dog. In the left caudal abdomen there is a focal region of increased soft tissue/mild mineral opacity within the intestinal lumen associated with fluid distention on one side, and gas distention (with fluid bubbles) on the other side.
Dilated Intestinal Loops
Intestinal obstruction leads to the accumulation of variable amounts of gas and/or fluid in the proximal segment. Several different parameters for assessing intestinal width have been suggested, but the best validated parameter is serosal-to-serosal width exceeding 1.6 times the height of the fifth lumbar vertebral body at its narrowest point.40 Other suggested parameters include serosal-to-serosal width less than 1.2 cm in the cat, and less than twice the width of the twelfth rib.41 With changes in patient positioning, gas and fluid will redistribute along the normal intestine, thus orthogonal views are important to increase sensitivity and specificity. Complete obstruction will usually lead to dilation of more than one loop proximally, and chronic obstruction can lead to extensive dilation. As the bowel becomes progressively distended, affected loops may lie closely adjacent to one another, creating a “stacked” appearance effect. In contrast, partial or very acute obstructions may not be associated with significant dilation at all. Thus the absence of intestinal dilation should not be used to exclude the possibility of intestinal obstruction.
Linear foreign bodies characteristically produce a bunched or pleated appearance on plain or contrast radiography.41 In addition to intestinal pleating, tapering, enteric gas bubbles, intestinal needles, and evidence of bowel obstruction are common.6 Linear foreign bodies are more likely to be associated with peritonitis from perforation of affected loops, which can produce a “ground-glass” appearance and loss of serosal detail, as well as potentially leading to the accumulation of free abdominal gas.
“Graveling” of Intestinal Contents Proximally
Chronic partial obstructions cause sedimentation and accumulation of insoluble, granular, slightly opaque material proximal to the site of obstruction. The appearance of this material has been described as “graveling” and “fecal-like,” and its presence in the SI, rather than colon, is indicative of obstruction.
Free Abdominal Gas and Loss of Serosal Detail
The presence of free abdominal gas and loss of serosal detail on plain radiography is strongly associated with GI perforation and peritonitis, and is an indicator for rapid surgical intervention. Peritoneal gas frequently accumulates dorsally on the lateral radiograph and can be seen delineating the diaphragm dorsally and cranially to the liver margin. On the ventrodorsal image, gas may again be seen between the diaphragm and liver margins.
Intestinal Mass Effect
The differentiation between radiolucent foreign body and intussusception on plain radiography is often difficult, and a simple characterization of ileus with suspicion of obstruction may be the limit of confidence. In some cases, an intussusception may create a soft-tissue density or mass effect. Ileocolic intussusceptions are characteristically apparent in the ventrodorsal view caudal to the stomach, causing displacement of the unaffected SI caudally and to the right.
Contrast Radiography
Barium-contrast radiography is indicated if the history and physical examination is equivocal, plain radiography is nondiagnostic, and ultrasonography is not available. The recommended dose is 10 to 15 mL/kg of a 30% wet weight suspension of barium sulfate. Films should be taken at 0, 15, 30, and 60 minutes following barium administration and hourly thereafter until a diagnosis is made or the barium has been transported to the colon. In health, the barium should arrive in the ileum within 60 minutes and the ileocecal sphincter after 2 hours. Filling defects and transit time are the key radiographic features for obstruction. The pattern of barium at the site of obstruction varies greatly. Contrast may accumulate proximal to the obstruction, precisely delineating the shape of the foreign body, or revealing luminal filling defects. In the case of intussusception, patency of the lumen of the intussuscepted segment determines if barium will pass, which can be seen as a thin stream of barium within the narrowed lumen. In some cases, barium will flow into the surrounding enveloping intussuscipiens producing a tube-within-a-tube effect.
Contrast radiography can also be performed utilizing BIPSs. BIPSs are a convenient method to detect partial intestinal obstructions.42 The principal advantage of BIPS over liquid barium is the ease of interpretation, especially in cases where a partial obstruction can be excluded by unimpeded transit into the colon. BIPSs are administered as capsules or mixed with food, thus avoiding the difficulties of administration of liquid barium. Unlike liquid barium, BIPS transit more closely approximates the transit of food in the GI tract. In cats and dogs that present for chronic vomiting or diarrhea, one protocol is to (a) administer the BIPS with food, (b) take radiographs 2 hours later to rule out gastric dumping, and (c) take radiographs at 8 hours to detect delayed gastric emptying. If the radiographs taken at 8 hours do not reveal some large spheres in the colon, a third set of radiographs later that same day or early the next morning should be taken to rule out partial bowel obstructions. Persistent bunching of the spheres in the SI is highly suggestive of physical obstruction of the small bowel, particularly if the markers have bunched in a dilated loop of SI or if “graveling” is apparent. If the small bowel loop in which the bunching occurs is not dilated or graveling is not apparent, a repeat radiograph should be taken 1 or 2 hours later to ensure persistence of the bunching.
Fasting and enema administration improve the diagnostic accuracy of liquid contrast techniques, and a full study may require 12 to 24 hours to definitively establish transit into the colon. In acute presentations, and especially when the patient is compromised or septic, these require a delay in intervention that may not be optimal. For these reasons, and for reasons of diagnostic accuracy, contrast radiography has largely been supplanted by ultrasonography as a means of detecting mechanical intestinal obstruction.
Ultrasonography
Intestinal dilation caused by mechanical obstruction is readily detected with ultrasonography. In addition, intestinal motility can be subjectively assessed in the proximal and distal segments. It has been reported that in chronic obstruction, generalized decreased intestinal motility is seen, whereas increased motility is more commonly associated with acute obstruction.43 Such a description is consistent with changes reported with experimental obstruction.16
The ultrasonographic appearance of intussusception is best characterized by a transverse view of the lesion, depicting the wall layers as a multilayered series of concentric rings, or a target-like lesion (Figure 57-24 ).9, 14, 44 The rings have a hyperechoic or anechoic center surrounded by multiple hyperechoic and hypoechoic concentric rings. When viewed in a longitudinal direction, the intestinal layers appear as multiple hyperechoic and hypoechoic parallel lines. Other possible findings include invagination with mesenteric fat, concurrent inflammatory pseudocysts, mesenteric lymphadenopathy, and mass-like, or even a kidney-like appearing soft tissue.9, 14, 44
Figure 57-24.
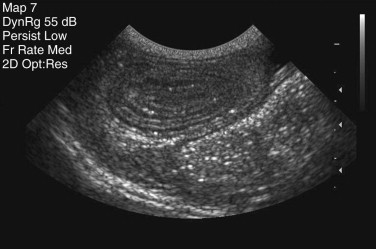
Ultrasonogram of an intussusception in transverse section showing the multilayered appearance.
Ultrasonographic evaluation has the added benefit of enabling guided fine-needle aspiration of abnormal bowel at the site of obstruction or intussusception. This would be indicated when an underlying intestinal mass, or diffuse loss of layering consistent with lymphosarcoma is suspected. A cytologic diagnosis prior to laparotomy may be invaluable for treatment planning, or even in a decision not to treat.
Treatment and Management
General Principles
Some intestinal foreign bodies will successfully pass into the colon despite clinical signs consistent with complete obstruction. In a patient with mild acute clinical signs, repeated imaging with careful monitoring is appropriate and necessary. Failure of movement of a foreign body within 8 hours, or failure of the object to pass into the colon within 36 hours, are indications for surgery.45 It should also be noted that spontaneous resolution of an intussusception has been reported in the dog.46 Therefore any delay between diagnosis and intervention should be followed by palpation and/or repeated imaging to confirm the persistence of the obstruction immediately prior to laparotomy.
Medical Preoperative Management
The primary therapeutic aim is to surgically relieve the intestinal obstruction. Rapid intervention improves the prognosis, although many patients do require preoperative stabilization. Establishment of adequate tissue perfusion by correcting dehydration is essential and of paramount concern. Volume resuscitation should continue until there is evidence of normal tissue perfusion, stabilization of central venous and arterial blood pressure, and urine production. In most cases, this can be adequately achieved using routine crystalloid replacement principles and therapy. Normal saline (0.9%) is reserved for cases of known hypochloridemic alkalemia because of its acidifying effects when administered in large volumes. The value of colloid solutions is still unsettled. In an experimental model of acute intestinal obstruction in dogs, the administration of synthetic colloid reduced loss of fluid into the intestinal lumen when compared with crystalloids.47 This effect was consistent with a transient increase in plasma oncotic pressure. Nonetheless, evidence of improved outcome has not yet been provided in clinical patients, and the choice of crystalloids or colloids should be individualized according to evidence of low oncotic pressure and cost. A suggested approach is to start with crystalloid therapy, and administer a colloid solution if adequate perfusion and normotension cannot be achieved or maintained. In the face of severe hypotension (e.g., <60 mm Hg) or hypoalbuminemia, crystalloids, colloids, and plasma, may have to be administered concurrently.
Electrolyte derangements should be managed according to proven severity because predictions cannot be made as to the direction or severity of electrolyte and acid–base derangements in intestinal obstruction. Hypokalemia is especially problematic because of its deleterious effect on intestinal motility and systemic arterial pressure.48, 49 Thus a full preoperative evaluation would include a minimum database of packed cell volume, total plasma solids, serum electrolytes, and arterial or otherwise venous pH. If this information is not available, a conservative and sensible empirical fluid choice for rehydration and support is a high, strong ion gap electrolyte solution such as lactated Ringer solution, with potassium added to approximately 24 mmol/L.39 Care should be taken not to exceed a potassium infusion rate of 0.5 mmol/kg body weight/h during initial resuscitation.
Antibiotic therapy is essential because of the likelihood of systemic sepsis, and the risk of contamination during surgery. Antibiotic therapy should be commenced preoperatively, and the duration of antibiosis should be determined following surgical correction. The vast spectrum of potential pathogens dictates broad-spectrum antibiotic coverage for Gram-negative and Gram-positive aerobes and anaerobes. Gram-positive efficacy can be achieved with a β-lactam such as amoxicillin with or without a β-lactamase inhibitor, or a first-generation cephalosporin. Efficacy against Gram-negative bacteria can be achieved with an aminoglycoside, providing renal perfusion is adequate, or a fluoroquinolone, if azotemia is present. Anaerobic coverage may be adequate with the β-lactam alone, but the addition of metronidazole or clindamycin is warranted. Administration should be parenteral during the perioperative period. It is not known whether changing to oral administration postoperatively yields any added benefit. The disturbance in the microflora that ensues could contribute to delayed recovery. In the absence of evidence, continued parenteral administration is recommended as long as is practical.
Surgical Management
Although endoscopic retrieval may be successful in some cases of gastric foreign bodies, it is impossible to ascertain foreign material in the more distal GI tract. Small intestinal obstructions are best removed via laparotomy and enterotomy. Following careful inspection for integrity and adequate perfusion, intussusception should be reduced with or without resection. Chronic or nonreducible intussusceptions should be resected. The involved segment should always be carefully inspected for the possibility of local underlying disease such as neoplasia, and intestinal histopathology is indicated if any suspicious lesions or irregularities on palpation are detected. In all cases of intestinal obstruction, devitalized intestine should be resected, and appropriate decontamination of the peritoneal cavity through copious warmed sterile lavage.
Postoperative Care
Intravenous fluid therapy should be continued as required, and electrolyte and acid–base status should be monitored as needed. Empirical analgesia is indicated, regardless of the appearance of pain preoperatively. Reduced intestinal motility or complete paralytic ileus can complicate and prolong recovery. Neuronal pathology, enteritis, surgical manipulation, and persistent electrolyte derangements may all contribute to postoperative intestinal dysmotility. Insufficient evidence is available for firm recommendations in dogs and cats, but human experience suggests analgesia is important.50
Experimental studies show that early postoperative enteral feeding after intestinal anastomosis reduces intestinal inflammation, and increases the strength of the anastomotic site when compared with oral fasting for 48 hours.51 Furthermore, the wound strength of the abdominal incision is increased when complex diets are fed enterally, compared with oral dextrose solution for 72 hours.52 In a randomized trial of human patients treated surgically for intestinal perforation, early enteric feeding accelerates the recovery of normal intestinal motility, improves nitrogen balance, reduces weight loss, and reduces the risk of sepsis.53, 54 Interestingly, the risk of dehiscence is not necessarily reduced with the early introduction of enteral nutrition in clinical cases either in humans or in dogs.54, 55 The optimal nutritional formulas following enterotomy have not yet been established.
Regardless of the etiology, there is decreased motility with delayed gastric emptying and reduced segmental contractions in almost all cases.56, 57 Feeding decreases the development and duration of ileus in most intestinal pathologies. A recent metaanalysis on the recovery of human patients following a wide spectrum of abdominal surgical procedures demonstrated that early introduction of feeding resulted in shorter time to the presence of bowel sound and a trend toward shorter hospital stays.58 At worst, continuing oral feeding will have no detrimental effect on motility, and at best it will promote normal motility and prevent ileus.
Multiple factors, including luminal nutrients, pancreaticobiliary secretions, and humoral agents are implicated in controlling the intestinal adaptive response after intestinal injury. Despite the multifactorial regulation of intestinal adaptation, luminal nutrients are fundamental to the adaptive response such that recovery is minimized or prevented in the absence of luminal nutrients. This conclusion is largely based on studies that show significant adaptive intestinal regrowth in rats and dogs fed orally compared with those fed parenterally following an intestinal resection. Indeed even in the absence of intestinal injury, total parenteral nutrition causes dramatic intestinal atrophy in dogs, cats, rats, and humans.59, 60, 61 This fasting-induced atrophy is accompanied by inflammatory cell infiltrates in the lamina propria, increased intestinal permeability, and increased bacterial translocation.
Consequently, the early implementation of enteral nutrition with a complex diet is recommended in all patients following enterotomy or anastomosis unless there are specific contraindications. However, it is unlikely that attempting to feed the daily maintenance energy requirements is a sensible approach in the short-term postoperative period, and certainly not in cases of continued vomiting. Therefore it is recommended that only 25% of the animals resting energy requirements be fed as a highly digestible, low-fat diet, whereby intestinal recovery may be optimized, and exacerbation of diarrhea and vomiting is minimized. This can be offered orally, syringe fed, or administered as a liquid enteral diet via nasoesophageal tube or other feeding tube.
Relief of an intestinal obstruction does not result in immediate normalization of intestinal motility, and the persistence of hypomotility or ileus is more likely the longer the obstruction is present.22 It is unknown if prokinetic drug therapy following relief of acute or chronic obstructions is helpful in dogs or cats. However, treatment with metoclopramide is effective in reducing the postoperative ileus in dogs that is experimentally induced by abrasion of the intestinal serosa.62
The prognosis for survival following enterotomy is generally very good, with reported survival rates of between 83% and 99%.15, 33 Studies to date have not specifically evaluated different preoperative, surgical, or postoperative protocols to establish best practice standards; however the prognosis for recovery from acute obstruction is improved with prompt treatment, which emphasizes the need for rapid diagnosis. The presence of intestinal perforation or leakage and septic peritonitis is proposed to be a negative prognostic indicator, but that has not been conclusively proved. Dehiscence of enterotomy sites is a major postoperative complication, and is more common following foreign-body removal than other reasons for enterotomy.55 Reported dehiscence rates following foreign-body surgery range from 2.9% to 27.7%.5, 33, 55, 63 The risk of dehiscence following enterotomy is significantly increased if there is preoperative peritonitis, more than 15% loss of body weight prior to surgery, hypoalbuminemia, and leukocyte left shift.55, 63
It is unknown whether the biomechanical remodeling that occurs proximal to a chronic partial obstruction could influence tissue healing and risk of dehiscence following enterotomy. Future research is likely to elucidate if surgical margins that are extend beyond the simple points of adequate blood supply to regions devoid of architectural change, might improve the prognosis.64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80
Dysmotility
Robert J. Washabau
Intestinal Motility Patterns
Contractions in the SI serve three general functions: mixing of the ingesta with digestive enzymes and other secretions, circulation of the intestinal contents to facilitate contact with the intestinal mucosa, and net propulsion of the intestinal contents in an aborad direction. Intestinal contractions are governed by four motility patterns: segmentation, peristalsis, intestinointestinal inhibition, and the migrating motility complex (see Chapter 1).1
Segmentation
If a contraction is not coordinated with activity above and below, intestinal contents are displaced both proximally and distally during the contraction and may, in fact, propagate orad during the period of relaxation. Such contractions appear to divide the bowel into segments, which accounts for the term segmentation given to the process. Segmentation serves to mix and locally circulate the intestinal contents. Segmentation primarily involves circular smooth muscle contraction (Figure 57-25 ).
Figure 57-25.
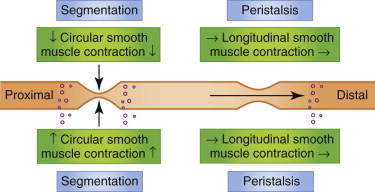
Segmentation- and peristaltic-type contractions in the small intestine.
Peristalsis
The SI is capable of eliciting a highly coordinated contractile response that is propulsive in nature. When the bowel is distended by a bolus of food the bowel responds with contraction orad and relaxation aborad to the point of distention. The neurotransmitters involved in the orad contraction are acetylcholine and substance P, and the neurotransmitters involved in the caudad relaxation are vasoactive intestinal peptide and nitric oxide. These events tend to move the material in an aborad direction. Short-segment peristalsis of the bowel is the norm in dogs and cats (see Figure 57-27). If short-segment peristalses occur sequentially they can propel a bolus the entire length of the gut in a short period of time. This peristaltic response, first characterized by Bayliss and Starling, is referred to as the “Law of the Intestine,” and is less frequent than short-segment peristalses.
Figure 57-27.
Survey lateral radiograph of a 4-month-old mixed-breed puppy with severe intestinal distention and chronic intestinal pseudoobstruction.
Intestinointestinal Inhibition
If an area of the bowel is grossly distended, contractile activity in the rest of the bowel is inhibited. This reflex prevents the movement of ingesta into more distal segments of intestine that have been severely distended or obstructed. This reflex is mediated by the extrinsic (autonomic) nervous system.2
Migrating Motility Complex
The migrating motor complex propagates indigestible materials, mucus, and secretions from the stomach to the colon during the fasting state. The enteric nervous system regulates the periodicity and migration of the migrating motility complex, but the GI hormone motilin reinforces the migrating motility complex activity. Cats do not have migrating motility complexes, and instead have a migrating spike complex that is less vigorous than the canine migrating motility complex.2, 3
Breed Differences in Gastrointestinal Transit Time
Significant differences in physiology and pharmacology have been found in dog breeds. There are more than 400 breeds of dogs recognized worldwide and 156 breeds recognized by the American Kennel Club. Among these various dog breeds, several important differences in metabolism have been noted, for example, P-glycoprotein–mediated metabolism, copper storage, and growth rates.4, 5 Differences in GI transit characteristics have also been noted. The GI tract of large-breed dogs (e.g., those weighing 60 kg) comprises 2.8% of their total body weight. In contrast, it comprises 7% of the total body weight of small-breed dogs (e.g., those weighing 5 kg). Breed-related differences in fecal water content could reflect differences in GI transit time, intestinal fermentation, diet, metabolism, and drug absorption. Using radiopaque markers (1.5-mm diameter administered in food), 12-week-old large-breed puppies (e.g., Great Danes) exhibited a significantly longer orocecal transit time (3.4 hours) as compared with small-breed puppies (e.g., Miniature Poodle, 2.5 hours). The longer transit time appears to reflect both a longer gastric emptying time and a longer small intestinal transit time among breeds. There also appear to be differences in intestinal permeability between dog breeds. The lactulose/rhamnose ratio reflects the relative absorption across the intestinal tight junction (transcellular absorption) versus the intestinal surface area (paracellular absorption, which occurs across the cell membrane of the enterocyte). The lactulose/rhamnose ratio is substantially greater in the Greyhound breed than in the Golden Retriever breed. Breed and age characteristics must be taken into account when differentiating normal and abnormal transit times. Chapter 26 outlines transit study techniques and times in more detail.
Definition of Ileus
Ileus has been defined as the functional inhibition of propulsive bowel activity, irrespective of pathogenetic mechanism.6, 7 There are several underlying causes of ileus, including dysautonomia, postoperative ileus, opioid-induced bowel dysfunction, muscular dystrophy, visceral myopathy, viral enteritis, radiation enteritis, idiopathic pseudoobstruction, and hypothyroidism. Some ileus disorders are more readily treated than others.
Dysautonomia
Etiology
Dysautonomia is a generalized autonomic neuropathy that was originally reported in cats in the United Kingdom, but that has now been documented in dogs and cats throughout Western Europe and the United States.8, 9, 10, 11, 12, 13, 14, 15, 16 The clinical signs reflect a generalized autonomic dysfunction but megaesophagus, esophageal hypomotility, gastric and small bowel distention and hypomotility, and urinary bladder distention are fairly consistent findings.8, 10, 16 Aspiration pneumonia and megacolon are seen less frequently.
Pathophysiology
Degenerative lesions are found in autonomic ganglia, intermediate gray columns of the spinal cord, and some sympathetic axons.10, 12, 13 Despite an intensive search for genetic, toxic, nutritional, and infectious etiologic agents, no definitive etiology has ever been established.
Clinical Signs
Vomiting, diarrhea, anorexia, lethargy, weight loss, dysuria, and inspiratory dyspnea are the most frequent clinical signs reported in dogs. In cats, dilated pupils, esophageal dysfunction, dry nose, reduced lacrimal secretions, prolapse of the third eyelid, regurgitation, and constipation are the most frequent clinical signs.12
Diagnosis
A clinical diagnosis is made in most cases based on historical and physical examination findings. Additional findings consistent with the diagnosis include (a) esophageal dilation and hypomotility on survey or barium contrast radiographs; (b) delayed gastric emptying on barium-contrast radiographs; (c) reduced tear production in Schirmer tear tests; (d) atropine-insensitive bradycardia; and (e) bladder and colonic distention on survey radiographs. There are few differential diagnoses to consider in a dog or cat presenting with the myriad manifestations of the syndrome. Early in the course of the illness, however, other differential diagnoses to consider are colonic or intestinal obstruction, other causes of megaesophagus, and lower urinary tract disease.
Treatment
Supportive care (e.g., artificial tears, elevated feedings, expressing the urinary bladder, antibiotics, etc.) is still the basis of therapy in this disorder, although some dogs and cats are reported to show mild improvement with parasympathomimetic drugs (e.g., bethanechol or metoclopramide). Gastrostomy tube feedings or total parenteral nutrition may sustain some animals until they regain neurologic function.
Prognosis
In general, dysautonomia carries a guarded to poor prognosis for long-term survival in both the dog and the cat. Twenty percent to 40% of affected cats are likely to recover, although cats may take 2 to 12 months to do so.12, 13, 14, 15, 16 Recovery rates are lower still in the dog.10, 12 Complete recovery is uncommon and many cats and dogs are left with residual impairment, for example, intermittent regurgitation, dilated pupils, and fecal or urinary incontinence.
Postoperative Ileus
Etiology
Postoperative ileus has been defined as “ileus that develops following abdominal surgery, resolving spontaneously with 2 to 3 days.”17 It may be exacerbated by opioid administration during and following surgery. Multiple mechanisms have been proposed for the etiopathogenesis of postoperative ileus.17
Postoperative ileus is a significant problem in human medicine and constitutes the most important reason for delayed discharge from the hospital after abdominal surgery. The economic impact of postoperative ileus has been estimated to be $750 million to $1 billion in the United States. Similar data are not yet available in veterinary medicine.
Pathophysiology
Laparotomy and manipulation of the viscera are the main mechanisms underlying postoperative ileus, but other factors such as anesthetic agents and postoperative pain medication contribute to the delay in recovery of normal transit. The effect of general anesthetic agents is short lasting and therefore of only minor importance. The use of opioids to control postoperative pain has a much greater impact on postoperative motility. Postoperative opioids have significantly improved patient comfort in the early postoperative phase, but these drugs potently inhibit GI transit. Efforts to reduce the dose of opioids or to antagonize their effects with peripherally acting opioid µ-antagonists such as methylnaltrexone or alvimopan are important to minimize the detrimental effect of opioids on GI motility.
The main cause of postoperative ileus relates to the surgical procedure itself.17, 18 The first (or neurogenic) phase is neurally mediated and involves neural reflexes activated during and immediately following surgery. The second (or inflammatory) phase is triggered by the influx of leukocytes in manipulated intestinal segments and is responsible for the sustained inhibition of GI motility (Figure 57-26 ).17
Figure 57-26.

Schematic of the two phases involved in postoperative ileus. The first phase starts during abdominal surgery and ends soon after it. The second inflammatory phase starts approximately 3 to 4 hours after surgery, lasts much longer, and is therefore clinically more relevant.
Rights were not granted to include this figure in electronic media. Please refer to the printed book.
(Reproduced with permission from Boeckxstaens GE, de Jonge WJ: Neuroimmune mechanisms in postoperative ileus. Gut 58:1300, 2009.)
© 2013 Gut
Clinical Examination
Nausea, vomiting, intestinal distention, and abdominal pain are the most important clinical signs of postoperative ileus in dogs and cats. Fever and leukocytosis also may be found depending upon the type and severity of abdominal surgery.
Diagnosis
The diagnosis of postoperative ileus is usually straightforward, and an exclusion of other known causes of ileus (see Box 57-6 ). Laboratory testing (complete blood count, serum chemistry, urinalysis) are sometimes performed to rule out metabolic disorders such as liver disease and renal failure. Abdominal imaging (e.g., survey radiography and ultrasonography) should be performed to exclude other causes of ileus and their complications, for example, mechanical obstruction, peritoneal free air or fluid accumulation, and pancreatitis.
Box 57-6. Causes of Intestinal Dysmotility in Dogs and Cats.
-
•
Dysautonomia
-
•
Postoperative ileus
-
•
Opioid-induced bowel dysfunction
-
•
Muscular dystrophy
-
•
Visceral myopathy
-
•
Viral enteritis
-
•
Radiation enteritis
-
•
Idiopathic pseudoobstruction
-
•
Hypothyroidism
Treatment
Orogastric Intubation
Intermittent orogastric or nasogastric intubation may be of benefit particularly in those patients with gaseous GI distention.
Early Postoperative Feeding
Early postoperative feeding has been recommended as a means of decreasing the duration of postoperative ileus. Feeding may stimulate a reflex that coordinates propulsive activity and elicits the secretion of GI hormones, causing an overall positive effect on bowel motility.
Laparoscopic Procedures
Laparoscopic procedures offer the theoretical advantage of decreased tissue trauma compared with open abdominal procedures. This decrease in tissue trauma may lead to faster recovery of postoperative bowel function. Animal studies have found significant decreases in the duration of postoperative ileus after laparoscopic versus open abdominal procedures.18
Prokinetic Agents
GI prokinetic agents have a clear place in the management of postoperative ileus. Chapter 52 discusses these drugs and their clinical usage in greater detail.
Cyclooxygenase-2 Inhibitors
Mechanical stretch in intestinal obstruction induces marked expression of cyclooxygenase (COX)-2 in intestinal smooth muscle cells, and stretch-induced COX-2 plays a critical role in the suppression of smooth muscle contractility in bowel obstruction.19 Therefore COX-2 inhibitors may have therapeutic potential in stretch-related disorders of the gut.
Opioid µ-Antagonists
Opioid µ-antagonists like alvimopan and methylnaltrexone may be useful in antagonizing the effects of morphine-like opioid agonists if that is part of the underlying pathogenesis of postoperative ileus.20, 21
Electrical Stimulation
Although not yet clinically applicable, GI pacing is achievable in the canine stomach and SI (but not the colon).22, 23, 24, 25, 26 The maximal entrainable frequency of the gastric and small intestinal slow waves is approximately 20% higher than the intrinsic frequency. In the future, stimulation parameters may be identified that will entrain slow waves, thereby normalizing gastric and intestinal dysrhythmias.22, 23, 24, 25, 26
Prognosis
The prognosis for short-term postoperative ileus is generally good to excellent. In animals with complicated, refractory postoperative ileus, the prognosis is less clear. More aggressive therapies may be needed in this patient population. In such cases, intestinal failure may result culminating in intestinal transplantation as a last resort.27, 28, 29
Opioid-Induced Bowel Dysfunction
Etiology
Opioid-induced bowel dysfunction may be part of a postoperative ileus syndrome, or it may relate solely to the use of opioid µ,δ-agonists as part of an analgesic therapeutic regimen. Opioids are a mainstay in the treatment of acute and chronic pain. Although opioids are very effective for pain relief from cancer and other nonmalignant diseases, their use is often limited by side effects. The most common adverse side effects are constipation and vomiting, but they also alter small bowel function causing opioid-induced bowel dysfunction. Opioid-induced bowel dysfunction can occur immediately after the first dose and persist for the duration of therapy. The peripherally acting µ-receptor antagonists methylnaltrexone and alvimopan are a new class of agents designed to reverse opioid-induced side effects on the GI tract without compromising pain relief.30, 31, 32, 33, 34, 35, 36
Pathophysiology
Endogenous opioids include endorphins, enkephalins, and dynorphins. They act selectively at opioid receptors composed of the µ, δ, and κ subtypes. Opioid µ receptors are present in the central and peripheral nervous system, as well as the GI tract. There are many species and site differences, but µ receptors have been reported on the interstitial cells of Cajal, smooth muscle, and epithelial cells. The predominant opioid effect appears to be at the local level and includes stimulation of absorption (villus epithelial cells), inhibition of secretion (crypt epithelial cells), increased segmentation (circular smooth muscle), and reduced peristalsis (longitudinal smooth muscle; reviewed in Chapter 1 in greater detail). Exogenously administered opioids have the same overall effect of opioid inhibition of peristalsis and secretion leading to the syndrome of opioid-induced bowel dysfunction.31, 32
Clinical Examination
Constipation and vomiting are the primary clinical signs of opioid-induced bowel dysfunction. Left untreated, constipation can progress to fecal impaction and mechanical obstruction.
Diagnosis
The patient usually has a well-documented history of opioid µ-agonist therapy, for example, morphine, in the management of a pain syndrome. Still, it would be important to rule out other causes of ileus, metabolic disorders, and mechanical obstruction. Therefore the minimum database should include laboratory data (complete blood count, serum chemistry, urinalysis) and imaging (survey abdominal radiography or ultrasonography).
Treatment
In most instances, discontinuation of the opioid µ-agonist is sufficient to ameliorate clinical signs. With persistence of clinical signs after drug withdrawal, laxative (reviewed in Chapter 50) and other therapies may be used to treat constipation, although it should be emphasized that a definitive role in the treatment of opioid-induced bowel dysfunction has not yet been proven. Any of the laxative agents (bulk, lubricant, osmotic, stimulant, emollient; see Chapter 50) could be used to attenuate the constipating effect of the opioid µ-agonist. Misoprostol, a synthetic prostaglandin E analogue also could be used to improve intestinal and colonic transit times.36
If the central analgesic effect of the opioid µ-agonist is paramount, the patient could be treated concurrently with an opioid µ-antagonist, methylnaltrexone or alvimopan, both of which will improve GI transit without inhibiting the central analgesic effect of the opioid µ-agonist. In dogs, methylnaltrexone at a dose range of 1 to 5 mg/kg subcutaneously abolishes the effect of morphine on GI transit without interfering with the central analgesic effect.32 Safe and effective doses of alvimopan have not yet been reported in the dog.
If morphine must be used preoperatively, the epidural route (vs. continuous low-dose infusion) facilitates the time of appearance of the first gastric interdigestive migrating complex (the migrating motility complex) in dogs with paralytic ileus after open abdominal surgery.37
Prognosis
The prognosis for acute opioid-induced bowel dysfunction is generally good to excellent. Because chronic opioid-induced bowel dysfunction may persist it has a more guarded prognosis.
Muscular Dystrophy
Etiology
Duchenne-type muscular dystrophy in the Golden Retriever dog is an X-linked genetic disorder that is characterized primarily by progressive muscular weakness. Involvement of the GI tract is frequent and may occur at any level from stomach to intestine and colon.38 The disorder is caused by mutations in the dystrophin gene responsible for production of the dystrophin membrane protein.39 The absence of dystrophin is accompanied by alteration of the dystrophin–glycoprotein complex and results in progressive degeneration of the heart, skeletal, and smooth muscle with subsequent replacement by fibrosis and fatty infiltration.
Pathophysiology
The gastroenterologic clinical signs have been attributed to motility disorders caused by smooth muscle damage, but histologic evidence of alterations has not been a consistent finding. In a more recent report, Golden Retriever dogs affected with Duchenne-type muscular dystrophy had marked degenerative lesions in the smooth musculature of the GI tract, urinary, and reproductive systems. GI smooth muscle lesions were associated with the clinical findings of gastroparesis, gastric dilation, and intestinal pseudoobstruction.38
Clinical Signs
Dysphagia, regurgitation, gastroparesis, abdominal pain, and intestinal distention have been reported in affected animals. Gastroenterologic clinical signs may be the first sign of dystrophic disease and may precede the appendicular musculoskeletal features. The impairment of GI function may be gradual and undetected by the pet owner, breeder, or veterinarian.38
Diagnosis
Definitive diagnosis may be confirmed on the basis of history and physical examination findings, serum creatine kinase activity, genomic DNA analysis, muscle electrophysiology, gross morphology, and histologic features.39 Ileus may be difficult to detect in the whole animal and may require the use of endoscopy, ultrasonography, and scintigraphy.
Treatment
Despite major advances in our understanding of the pathophysiology of the disease, therapy is still largely supportive and symptomatic. Gene replacement therapy has not yet succeeded in restoring muscle function or in prolonging life.
Prognosis
At the present time, the prognosis for cure is poor. With supportive and symptomatic therapy, some affected animals have survived for as long as 51 months.38
Visceral Myopathy
One case of visceral myopathy in a 6-month-old domestic short-hair cat has been reported in the veterinary literature.40 The kitten had a 6-day history of anorexia, intermittent vomiting, and diarrhea, and severely dilated loops of hypomotile intestine were found on survey abdominal radiography and ultrasonography. Intestinal dilation was confirmed at the time of surgery, and a 20-cm section of jejunum was resected. In the proximal jejunum, there was marked atrophy of the longitudinal muscle of the muscularis externa layer and diffuse severe degenerative vacuolar change within the myocytes and endomysial cells. The circular muscle layer was of normal thickness and morphology. Villus stunting and fusion were evident in the mucosa. In the distal jejunum, the mucosa and submucosa were normal, but the longitudinal muscle layer was markedly atrophic with focal degeneration, calcification, loss of myocytes, and replacement by proliferating fibroblasts. Based upon descriptions of human visceral myopathy, the findings were thought to be consistent with a diagnosis of visceral myopathy causing chronic intestinal pseudoobstruction.40 The cat was alive and doing well 20 months after surgery.
Parvoviral Enteritis
Ileus is a frequent finding in puppies affected with parvoviral enteritis.41 The ultrasonographic appearance of the GI tract was characterized in 40 puppies with confirmed canine parvoviral enteritis.41 Sonographic findings included fluid-filled SIs in 92.5% of the cases (Figure 57-27 ), and of the stomach and colon in 80% and 62.5% of the cases, respectively. Generalized atony was present in 75% of the cases, and weak peristaltic contractions indicative of functional ileus were observed in the remaining 25% of cases. The duodenal and jejunal mucosal layer thicknesses were significantly reduced when compared with normal puppies with mean duodenal mucosal layer measuring 1.7 mm and jejunal mucosal layer 1.0 mm. A mucosal layer with diffuse hyperechoic speckles was seen in the duodenum (15%) and the jejunum (50%). The luminal surface of the duodenal mucosa was irregular in 22.5% and the jejunal mucosa in 42.5%. Changes were accompanied by generalized indistinct wall layering in all animals. A mortality rate of 30% was found in this patient population.41
Radiation Enteritis
Radiation produces a variety of changes in GI tract motility and ileus is a common clinical finding.42 Most of the changes observed with radiation enteritis occur in other pathologic states. These include delayed gastric emptying, retrograde giant contractions and vomiting, giant migrating contractions, and abdominal cramping and diarrhea.43 The threshold for these contractile events to occur and their control mechanisms are incompletely understood. Many studies suggest that treatment (i.e., 5-HT3 antagonists) prior to exposure may be the best method to prevent the contractions from occurring. The role of dose rate is unclear. Within hours of a significant exposure to radiation, these contractions begin to occur and contribute significantly to the early stages of radiation illness.
Idiopathic Intestinal Pseudoobstruction
Etiology
Chronic intestinal pseudoobstruction is defined by the presence of chronic intestinal dilation and dysmotility in the absence of mechanical obstruction.44 In humans, chronic intestinal pseudoobstruction has many causes that have been simplified into abnormalities of enteric smooth muscle (myopathies) and the enteric nervous system (neuropathies).40 Such visceral myopathies and neuropathies are primary causes of chronic intestinal pseudoobstruction, and myopathies are either familial, or sporadic and idiopathic. Pseudoobstruction can also arise secondary to other underlying disorders, such as progressive systemic sclerosis, amyloidosis, muscular dystrophy, generalized neuromuscular diseases, endocrinopathies, infectious disease, and drug toxicity.40
Pathophysiology
Only 11 cases of chronic intestinal pseudoobstruction have been reported in companion animals (nine dogs and two cats).44, 45, 46, 47, 48, 49 Four dogs had atrophy, fibrosis, and mononuclear cell infiltration of the muscularis externa similar to what is observed in progressive systemic sclerosis in humans. Two dogs had atrophy of the muscularis externa but not fibrosis, either with or without mononuclear cell infiltration, whereas one dog had hyperplasia of the circular muscle without atrophy, fibrosis, or inflammation. In only two reported dogs with pseudoobstruction was the pathology described as primarily affecting the circular or longitudinal smooth muscle. Only two reports mention myocyte vacuolar degeneration, but it was not a prominent feature and marked myenteric plexus vacuolar degeneration was present, suggesting an underlying primary neuropathy. One of the cats reported with pseudoobstruction actually had diffuse intestinal lymphosarcoma with no additional histopathologic details.
Clinical Signs
As with visceral myopathy, the primary clinical signs seen with chronic intestinal pseudoobstruction are anorexia, intermittent vomiting, and diarrhea. Signs may be referable to one segment of the gut, but the disease is usually diffuse.
Diagnosis
Abdominal imaging showing intestinal dilation with no evidence of mechanical obstruction is the hallmark of the pseudoobstruction. Full-thickness intestinal histology is required to identify underlying causes of chronic intestinal pseudoobstruction.
Treatment
Because of the diffuse nature of the disease, surgical resection of diseased intestine is not generally recommended. Resection only benefits selected patients with localized disease. Medical therapy should otherwise be aimed at correcting electrolyte and acid–base disturbances, treating infection or sepsis, supporting nutritional needs, suppressing the inflammatory or immune response, and instituting prokinetic therapy (see Chapter 52).44, 45, 46, 47, 48, 49
Prognosis
Aside from the apparent recovery reported in one cat with visceral myopathy, most of the cases of chronic intestinal pseudoobstruction had a poor outcome.
Hypothyroidism
Untreated or poorly regulated hypothyroidism is associated with important changes in GI motility. Compared with euthyroid dogs, thyroidectomized dogs have decreased frequency of electrical control activity of the stomach and jejunum, decreased occurrence of electrical response activity (spike potentials) following stimulation, and decreased mechanical response to feeding.50
Neoplasia
Philip J. Bergman
Incidence and Risk Factors
The first publication of a canine intestinal tumor was by Schlotthauer and Grindlay in 1951.1 Since then a variety of investigations have increased our understanding of the biology of intestinal tumors. In most studies the incidence of intestinal tumors is reported to be less than 10% of all tumors,2, 3, 4, 5, 6, 7 and intestinal tumors represent approximately one-fifth (dogs) to one-third (cats) of all alimentary tumors.8 The most common intestinal tumor in most studies is lymphoma, comprising one-third of all feline tumors compared with approximately 6% in dogs.2, 4
Small intestinal neoplasia occurs typically in older dogs (mean age: 6 to 10 years) and cats (mean age: 10 to 13 years).2, 9, 10, 11, 12, 13, 14, 15, 16, 17 The mean age for feline alimentary lymphoma was younger when FeLV was more prevalent.18, 19, 20 Although not significant, there is a slight male predisposition for canine intestinal tumors,13, 15 but this is less clear in cats.9, 21, 22, 23 Approximately 90% of dogs with GI lymphoma and 80% with leiomyoma/leiomyosarcoma are male.16, 24, 25
Large breeds of dogs (e.g., German Shepherds, Collies) appear to be most at risk for small intestinal neoplasia, specifically adenocarcinoma,14, 26 but leiomyosarcomas were reported to be rare in working military German Shepherd and Belgian Malinois dogs.27 Siamese cats are consistently reported to be predisposed to intestinal lymphoma and adenocarcinoma, with one study suggesting an eightfold increased risk.2, 9, 21, 28
There are no known chemical agents or microorganisms that are reliably associated with increased risk for intestinal neoplasia in dogs. The same is true for cats except for the clear associations with FeLV and feline immunodeficiency virus.29, 30, 31, 32 Most cats with intestinal lymphoma are older and FeLV-negative by serology, but a significant portion of these cats are positive for FeLV by immunohistochemistry or PCR studies of the tumor tissue.33 Of further note is the change in presentation of feline lymphoma that occurred in recent decades. When the prevalence of FeLV was high, the most common presentation of feline lymphoma was of cranial mediastinal and multicentric disease in young, FeLV-positive cats, whereas now the most common presentation is of an older, serologically FeLV-negative cat with alimentary lymphoma.18, 34
The risk of gastric cancer is greatly increased in people with Helicobacter pylori infection,35, 36 but this association has not been confirmed in dogs or cats to date. One domestic cat and a cougar were found to have concurrent Helicobacter infection and intestinal neoplasia (lymphoma in the domestic cat and intestinal adenocarcinoma in the cougar), but these studies do not allow for delineation of causation.37, 38 Based on the lack of a large number of reported concurrent cases of Helicobacter and intestinal neoplasia in cats, in addition to the fact that some cats normally shed Helicobacter sp., this agent at present appears to play minimal to no role in the induction of feline intestinal neoplasia and may be part of the normal feline GI flora.39, 40
Pathology and Biologic Behavior
A large number of different types of neoplasia can be found in the intestine, including epithelial (e.g., carcinoma or adenocarcinoma), neuroendocrine, mesenchymal (e.g., sarcoma) or discrete/round cell neoplasia.17 Most small intestinal neoplasia in dogs and cats is malignant, whereas more distal areas of the GI tract tend to have more benign disease. Lymphoma is the most common intestinal tumor with a variety of reported subtypes including lymphoblastic, epitheliotropic, lymphocytic, and large granular lymphocytic (also called granulated cell tumor or globule leukocyte lymphoma).14, 20, 23, 24, 41, 42, 43, 44, 45, 46, 47, 48, 49 The predominant immunophenotype in feline intestinal lymphoma has historically been considered to be tumors of B-cell origin arising from germinal centers and Peyer patches; however, more recent literature suggests that B-cell predominance is no longer the case.23, 50, 51 The presence of FeLV in the tumor is not now associated with immunophenotype, contrary to previous reports of younger, serologically positive cats with primarily T-cell immunophenotypes.18, 50 Approximately 80% of cats and 25% of dogs will have distant spread of alimentary lymphoma at presentation or surgery.21, 24 Although lymphoma is often thought to be a systemic disease in dogs, additional study is necessary to better delineate the metastatic propensity of solitary canine GI lymphoma, and therefore the potential need for chemotherapy in addition to local treatment options.
Most cats with epitheliotropic or large granular lymphocytic intestinal lymphoma are serologically FeLV-negative. Large granular lymphocytic intestinal lymphomas are generally very aggressive tumors, typically have heterochromatic granules, and are perforin-positive on immunohistochemistry.46, 52, 53, 54
The second most common intestinal tumors are those of epithelial origin including adenocarcinoma (glandular), solid or undifferentiated carcinoma (no glandular formation), mucinous carcinoma (>50% mucinous), and signet ring carcinoma (>50% of the cells producing copious mucin which thereby gives a signet ring cytologic phenotype).2, 9, 14 The most common sites of metastasis of an intestinal carcinoma are the lymph node, liver, lung, omentum, mesentery, spleen, bone, kidney, peritoneum (e.g., carcinomatosis), skin, and testes.12, 55, 56, 57, 58
The third most commonly reported intestinal neoplasm in dogs is of smooth muscle lineage (leiomyoma and leiomyosarcoma).15, 16, 17, 59, 60, 61 A more recently reported variant of leiomyosarcoma and occasionally leiomyoma in dogs is the GI stromal tumor (GIST).25, 62, 63 GISTs are generally more commonly noted in the large intestine, but are reported arising in the SI and stomach. On immunohistochemistry, GISTs are typically vimentin-positive, cytokeratin-negative, and CD117 (c-kit, a transmembrane tyrosine kinase)-positive, and have minimal expression of smooth muscle actin. CD117-positive tumors (e.g., GISTs) may have a lower metastatic rate than CD117-negative leiomyosarcomas.25, 64
Less common tumors of the canine SI are carcinoid, mast cell tumor (the third most common intestinal tumor in the cat), extraskeletal osteosarcoma, ganglioneuroma, hemangiosarcoma, and extramedullary plasmacytoma. Carcinoids are of neuroendocrine origin and contain secretory granules comprising a variety of substances including gastrin, secretin, serotonin, and somatostatin.10, 17, 65 Primary intestinal carcinoids are locally aggressive and commonly metastasize to the liver.10, 66 Primary intestinal mast cell tumors are relatively common in cats, but rare in dogs.67, 68, 69, 70, 71
History and Clinical Signs
Clinical signs in dogs and cats with SI neoplasia include diarrhea, vomiting, weight loss, anorexia, melena, and possibly signs associated with nephrogenic diabetes insipidus (associated with smooth muscle tumors) or anemia. Clinical signs associated with obstruction, perforation, and/or peritonitis also are possible in severe cases. Clinical signs associated with paraneoplastic syndromes from small intestinal neoplasia include cutaneous, hyperviscosity, biochemical, and hematologic syndromes.72, 73, 74 GI leiomyosarcoma is reported to be associated with nephrogenic diabetes insipidus.75 The duration of clinical signs prior to presentation can be variable and range from 1 to 2 days to months, with an average of 4 to 8 weeks.12, 24, 75
Diagnosis
Physical Examination
Small intestinal neoplastic masses may be palpable in approximately 20% to 50% of dogs and 50% to 85% of cats.9, 12, 13, 23, 24, 45, 55, 76 Additional physical examination findings reported in dogs and cats with small intestinal neoplasia include dehydration, pain, and/or fever.9, 24, 55
Clinical Pathology
The most common finding on clinical pathology screening is anemia. This may reflect anemia of chronic disease, but is more commonly due to blood loss into the GI tract, which may then cause melena and possibly elevation in blood urea nitrogen.12, 13, 15, 16, 23, 45, 55 Other changes include leukocytosis, monocytosis, and/or a left shift.9, 12, 15, 23, 55 Serum biochemical changes include hypoproteinemia consistent with blood loss, elevations in alkaline phosphatase, and hypercalcemia (most common with lymphoma but reported across a variety of tumor types).
Diagnostic Imaging
Plain abdominal radiographs are reported to be diagnostic for an abdominal mass in approximately 40% to 50% of dogs and cats with small intestinal neoplasia.9, 12, 15, 24, 45, 55 Although difficult to offer precision across studies because of variability, a theme emerges whereby the percentage of cases with an abdominal mass on plain films is higher in dogs and cats with solid intestinal neoplasia and lower with lymphoma. This reduced ability to delineate an abdominal mass on plain films in patients with lymphoma is likely caused by numerous factors including the potential for diffuse lesions and/or the presence of peritoneal effusion and/or other organ involvement. The frequency of obstructive patterns noted on plain films in dogs and cats with intestinal neoplasia varies between studies. Because of the advent of, and widespread use of, abdominal ultrasound, use of contrast radiography has waned in the last 5 to 10 years, but this technique will find filling defects in approximately 50% to 90% of dogs or cats with intestinal neoplasia.9, 24 Three-view thoracic radiographs should be performed as part of routine staging in any case with an abdominal mass or strong suspicion of an abdominal mass. As many intestinal tumors often do not metastasize to the lungs, this is typically a low-yield procedure when staging for small intestinal neoplasia. Nevertheless it should be performed as the presence of metastasis would signal a very significant change in prognosis and therapy.
The use of abdominal ultrasound has revolutionized the diagnosis of intestinal neoplasia. Abdominal ultrasound is a much more sensitive diagnostic tool than radiography for the identification of an intestinal mass12, 13, 16, 26, 77; however, abdominal ultrasound alone is not diagnostic. The most frequent abdominal ultrasound changes noted in dogs and cats with intestinal neoplasia are loss of normal bowel wall layering and increased bowel wall thickness. Ninety-nine percent of dogs with intestinal neoplasia have loss of bowel wall layering, while 88% of dogs without intestinal neoplasia retain normal bowel wall layering. In addition, dogs with focal abdominal ultrasound lesions are approximately 20 times more likely to have neoplasia, and dogs with bowel thickness greater than 1 cm are at four times greater risk of neoplasia.78 The advantages of abdominal ultrasound over plain and/or contrast radiography include (a) evaluation of other sites within the abdomen, (b) delineation of the presence or absence of carcinomatosis, (c) less time-consuming than contrast radiography, and (d) additional diagnostic utility gained through abdominal ultrasound-guided fine-needle aspirate or needle-core biopsy.13, 26, 79 Other findings commonly reported with abdominal ultrasound in patients with small intestinal neoplasia include mixed echogenicity asymmetric lesions, diffusely thickened bowel loops, anechoic to hypoechoic mass lesions, and in patients with carcinomatosis, the presence of masses in the “double-sheet” region of the peritoneum where the parietal and visceral portions meet, and the presence of free fluid.79
Endoscopy
The use of endoscopy for the diagnosis of small intestinal neoplasia has become more commonplace over the last 10 to 20 years. Unfortunately, endoscopy has potentially significant limitations depending on the anatomic site of the tumor and the ability of the endoscope to reach the affected area. The gross appearance of neoplasia on endoscopy can range from a mass effect, to reduced dispensability of an otherwise phenotypically normal area, to a “cobblestone” and/or focal erythremic effect.76 An additional limitation to endoscopy is the size of the biopsy sample obtained, which can lead to significant interobserver variation in histopathologic interpretation.80 One study of feline lymphoma compared endoscopic samples with full-thickness biopsy samples collected at laparotomy. In that investigation, lymphoma was diagnosed in 10 cats from full-thickness samples, but in only three cats with endoscopic samples.81 For those cats with gastric lymphoma, endoscopic sampling diagnosed three of four cases, whereas in cats with intestinal lymphoma none of the six cats were diagnosed by endoscopic sampling. A similar study in dogs comparing endoscopic versus full-thickness sampling had comparable outcome.24
Laparoscopy
The use of laparoscopy to diagnose and/or treat intestinal neoplasia has increased in the last 5 years because of increased operator proficiency and more widespread availability of the equipment.82, 83 The morbidity and surgical procedure time are greatly reduced when using laparoscopy compared to laparotomy; however, the lack of intraluminal viewing can be a significant limitation of the technique when the lesions are not producing a mass and/or full-thickness effect.
Laparotomy
Exploratory laparotomy is considered the gold standard when minimally invasive techniques are unable to provide a diagnosis of intestinal neoplasia in patients with signs of persistent and/or resistant intestinal disease. There are numerous advantages to laparotomy compared with other techniques including the ability to take full-thickness biopsies, the direct visualization of the entire abdomen, and the ability to perform therapeutic resection and anastomosis and/or placement of feeding tubes.
Treatment
Surgery
The gold standard of treatment for most intestinal neoplasia, except for lymphoma, is surgical excision. This means of gross local tumor control is possible when there is no evidence of lymph node metastasis, carcinomatosis, other distant metastasis and/or adhesions or serosal-specific abnormalities that would prevent full extirpation. In patients with lymphoma causing intestinal obstruction and/or perforation, or when diagnosis is not possible through other less-invasive means, surgery may be used for diagnosis and/or treatment.
Perioperative mortality rates are high and average 30% to 50% in patients undergoing surgery for intestinal neoplasia. The causes of such mortality include peritonitis, sepsis, and euthanasia as a consequence of the presence of gross metastasis and/or lack of resectability.12, 16
Chemotherapy
The use of adjuvant chemotherapy after surgical resection of an intestinal epithelial tumor is relatively controversial because of the paucity of publications. Survival greater than 1.5 years was reported in two dogs with intestinal adenocarcinoma after surgery and adjuvant chemotherapy.13 A retrospective evaluation of adjuvant doxorubicin in cats with colonic adenocarcinoma reported a median survival time of approximately 9 months with doxorubicin versus 2 months without it.11 It is unknown if similar results would be afforded in larger scale prospective trials of dogs or cats with small intestinal epithelial malignancies. Another study reported the use of chemotherapy in dogs with leiomyosarcoma to have variable outcome, with some dogs having long survival without chemotherapy after surgery.16 Additional research is required to determine the usefulness of adjuvant chemotherapy in small intestinal nonlymphoid malignancies.
The use of chemotherapy in the treatment of intestinal lymphoma is less controversial; however, outcomes can be variable depending on species, anatomic site, histopathologic subtype, etc.20, 45, 84, 85 For example, in cats with a diagnosis of intestinal IBD or low-grade lymphocytic lymphoma (which can be difficult to distinguish histopathologically), cats treated with pulse-dose Leukeran and corticosteroid had a median survival approaching 2 years, whereas cats with intestinal lymphoblastic lymphoma treated with the same protocol had a median survival of only 3 months.85 Few publications report outcomes of chemotherapy in dogs with intestinal lymphoma. In one case series, all eight dogs with intestinal lymphoma treated with chemotherapy were euthanized within 14 weeks.24 Anecdotally, I believe that dogs with intestinal lymphoma often have very short survival.14
Radiation Therapy
Radiation therapy is rarely used in the treatment of intestinal neoplasia. There are significant toxicity concerns with the use of radiation therapy on the intestine and surrounding viscera, and it would be difficult to localize the same area of treatment for daily fractions in typical radiation therapy prescriptions because of intestinal motility.
Prognosis and Prognostic Factors
Canine Carcinoma and Adenocarcinoma
The prognosis for dogs with intestinal carcinoma or adenocarcinoma is guarded to poor. Investigators have found a 12-day survival time in dogs not treated with surgery compared with survival ranging from 4 to 10 months (40% 1-year survival) when treated surgically.12, 13, 55 Unfortunately, these poor survival times are most likely a result of a high metastatic rate. In dogs with intestinal epithelial malignancies, 40% to 50% metastasize to local lymph nodes, 30% to 40% metastasize to the liver, and approximately 10% to 20% metastasize to distant sites.10, 12, 55 The importance of the presence of lymph node metastasis is further highlighted by a report documenting a median survival time of dogs treated with surgery of 15 months (67% survival at 1 year) compared with only 3 months (20% survival at 1 year) when lymph node metastases were present.12 Similarly, male dogs with intestinal epithelial malignancies were reported to have prolonged survival time compared with females.13
Differential expression levels of tenascin-C and vesicant are reported to occur between benign and malignant lesions in dogs, but these studies have not documented prognostic import within malignancies.86, 87 p53 is the most commonly mutated tumor-suppressor gene in human oncology and overexpression is commonly associated with immunohistochemical overexpression. Overexpression of p53 is uncommon in canine small intestinal epithelial malignancies with 15% to 23% expression noted across two studies.88, 89 In addition, p53 expression did not appear to correlate with the degree of malignancy of the tumors. COX-2 is commonly overexpressed in canine and feline small intestinal epithelial tumors and therefore the use of nonsteroidal antiinflammatory agents may represent a rational treatment approach in the primary setting with nonresectable disease or alternatively in an adjuvant setting.90, 91 To date, the only nonsteroidal antiinflammatory drug (NSAID) with a documented in vivo antitumor effect is piroxicam.92, 93 I believe that from an evidence-based medicine perspective the lead NSAID for antitumor use should continue to be piroxicam until further in vivo studies with NSAIDs of improved safety profile become available.
Canine Lymphoma
There are few publications examining outcomes for dogs with intestinal lymphoma, but as discussed previously all eight dogs with intestinal lymphoma treated with chemotherapy were euthanized within 14 weeks.24
Other Canine Tumors
The prognosis for dogs with leiomyosarcoma remains guarded with median survival times after surgery of 7.5 to 24 months.15, 16, 64 Dogs with visceral metastasis from a leiomyosarcoma that underwent surgical removal of the primary tumor had a median survival time of almost 2 years, suggesting that metastasis may not be a strong prognostic factor.16 When dogs with a GIST had surgical removal of the lesion, their overall median survival time was only 1 year, whereas if the large percentage of dogs with perioperative deaths were censored, the median survival time increased to approximately 3 years.64 Maas and colleagues reported 62.6% and 52.3% 1- and 2-year survival rates, respectively, for dogs with intestinal GIST undergoing surgery.63 Intestinal perforation was not a negative prognostic factor.16
Feline Carcinoma and Adenocarcinoma
The prognosis for cats with intestinal epithelial malignancies is guarded to poor. The median survival time of cats with intestinal epithelial malignancies not treated with surgery is only approximately 2 weeks,9, 55 whereas those cats taken to surgery can have high perioperative mortality rates.56 For cats surviving the perioperative period, the median survival times are 5 to 15 months.9, 28 This prognosis is likely a result of the increased metastatic rate of epithelial malignancies in this anatomic location as approximately 50% metastasize to local lymph nodes, approximately 30% cause carcinomatosis, and approximately 20% have distant metastasis.2, 55, 56 Cats with carcinomatosis may benefit from removal of the primary tumor, but this likely requires additional study because of the very small number of cats reported (n = 2).9 The use of intracavitary chemotherapy for carcinomatosis in cats has not been examined to date; however, promising preliminary results have been noted in dogs.94
Feline Lymphoma
The prognosis for cats with intestinal lymphoma is variable because of a wide variety of factors. The combination of surgery and chemotherapy has no benefit over chemotherapy alone,51 but there were only 21 cats in this study and it was not a randomized trial. I believe that for cats with a solitary intestinal lymphoma lesion, surgery should be seriously contemplated as it is one of the quickest and least-expensive mechanisms of tumor cytoreduction, especially in light of the relative chemotherapy resistance of feline lymphoma. FeLV status was highly prognostic for feline intestinal lymphoma with FeLV-positive cats treated with chemotherapy having a median survival time of 3 months versus 17 months if the cats were FeLV-negative (but this was only true in cats with early stage lymphoma and not significant in cats with later-stage neoplasia).19 Another study reported the primary prognostic factors to be FeLV status, substage, and response to therapy,34 but in other studies FeLV status was not prognostic.51, 84 Immunophenotype is not of prognostic benefit in dogs or cats with intestinal lymphoma, but this may be a result of poor statistical power from small numbers of cases reported to date.51 Labeling of argyrophilic nucleolar organizer regions is negatively correlated to clinical outcome in a variety of malignancies across species, but for feline alimentary lymphoma argyrophilic nucleolar organizer regions were not associated with duration of remission, percentage of remission, or survival time.95 Median remission for cats with intestinal large-cell lymphoma is 5 to 7 months, but subsets that are poorly understood to date can have extended survival times.45, 84, 85
Other Feline Tumors
Cats with duodenal polyps have an excellent prognosis. One previous investigation determined that cats with surgically removed duodenal polyps were cured of their disease.22
Comparative Features
Small intestinal neoplasia is uncommon in humans, where there is a much higher incidence of large intestinal cancer. The reverse holds in small animal medicine where small intestinal tumors are much more common than those of the large intestine. The reasons for this difference are unknown but likely to involve differences across the species related to diet, genetics, toxin exposures, and/or physiology. Risk factors noted to date for small intestinal epithelial neoplasia in humans includes increased intake of fatty foods, red meats, high-temperature grilled meats, and salt-cured food products.96 Risk factors noted to date for small intestinal lymphoma include celiac disease which may progress from IBD to overt lymphoma.97
Chronic use of COX-2 inhibitors reduces intestinal neoplasia in a variety of studies by approximately 50%.98 Significant similarities across species are likely to exist in the potential use of COX-2 inhibitors for small intestinal epithelial tumors. Similarly, the use of tyrosine kinase inhibitors for GISTs across species is likely a result of the aforementioned commonly noted mutations in c-kit.99
The principles of diagnosis and treatment are similar across species. Minor differences exist in the more common use of CT scanning for the diagnosis of small intestinal neoplasia in people compared with its current use in veterinary medicine.100 The use of tyrosine kinase inhibitors in unresectable and/or metastatic GISTs in people is commonplace, suggesting that the use of tyrosine kinase inhibitors in the same setting in dogs or cats may have potential utility.99 There is a similar dearth of literature concerning the effectiveness of chemotherapy in the adjuvant treatment setting for people with small intestinal epithelial malignancies.101
References
Structure and Function
- 1.Buddington RK. Structure and functions of the dog and cat intestine. Recent advances in canine and feline nutritional research. Proceedings of the 1996 Iams International Nutrition Symposium 61, Wilmington, OH, 1996.
- 2.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 3.Snipes RL, Snipes H. Quantitative investigation of the intestines in eight species of domestic mammals. Mamm Biol. 1997;62:359. [Google Scholar]
- 4.Baum B, Meneses F, Kleinschmidt S. Age-related histomorphologic changes in the canine gastrointestinal tract: a histologic and immunohistologic study. World J Gastroenterol. 2007;13:152. doi: 10.3748/wjg.v13.i1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JF, Fossum TW, Willard MD. Changes in the intestinal mucosal cell populations of German shepherd dogs fed diets containing different protein sources. Am J Vet Res. 1995;56:340. [PubMed] [Google Scholar]
- 6.Field CJ, McBurney MI, Massimino S. The fermentable fiber content of the diet alters the function and composition of canine gut associated lymphoid tissue. Vet Immunol Immunopathol. 1999;72:325. doi: 10.1016/s0165-2427(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 7.Kuzmuk KN, Swanson KS, Schook LB. Diet and age affect canine small intestinal and colonic gut morphology. J Anim Sci. 2004;82:244. [Google Scholar]
- 8.Paulsen DB, Buddington KK, Buddington RK. Dimensions and histologic characteristics of the small intestine of dogs during postnatal development. Am J Vet Res. 2003;64:618. doi: 10.2460/ajvr.2003.64.618. [DOI] [PubMed] [Google Scholar]
- 9.Rudorf H, van Schaik G, O’Brien RT. Ultrasonographic evaluation of the thickness of the small intestinal wall in dogs with inflammatory bowel disease. J Small Anim Pract. 2005;46:322. doi: 10.1111/j.1748-5827.2005.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 10.Kull PA, Hess RS, Craig LE. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996–1998) J Am Vet Med Assoc. 2001;219:197. doi: 10.2460/javma.2001.219.197. [DOI] [PubMed] [Google Scholar]
- 11.Pageot LP, Perreault N, Basora N. Human cell models to study small intestinal functions: Recapitulation of the crypt-villus axis. Microsc Res Tech. 2000;49:394. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Jawhari A, Farthing M, Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue? Gut. 1997;41:581. doi: 10.1136/gut.41.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konturek PC, Brzozowski T, Konturek SJ. Expression of epidermal growth factor and transforming growth factor alpha during ulcer healing—time sequence study. Scand J Gastroenterol. 1997;32:6. doi: 10.3109/00365529709025056. [DOI] [PubMed] [Google Scholar]
- 14.Otto W, Thim L. Trefoil factor family-interacting proteins. Cell Mol Life Sci. 2005;62:2939. doi: 10.1007/s00018-005-5482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponce-Macotela M, Gonzalez-Maciel A, Reynoso-Robles R. Goblet cells: are they an unspecific barrier against Giardia intestinalis or a gate? Parasitol Res. 2008;102:509. doi: 10.1007/s00436-007-0790-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang YH, Srinivasan K, Siddiqui MR. A novel role for villin in intestinal epithelial cell survival and homeostasis. J Biol Chem. 2008;283:9454. doi: 10.1074/jbc.M707962200. [DOI] [PubMed] [Google Scholar]
- 17.Willard MD, Zenger E, Mansell JL. Protein-losing enteropathy associated with cystic mucoid changes in the intestinal crypts of two dogs. J Am Anim Hosp Assoc. 2003;39:187. doi: 10.5326/0390187. [DOI] [PubMed] [Google Scholar]
- 18.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium—physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 19.Labow BI, Souba WW. Glutamine. World J Surg. 2000;24:1503. doi: 10.1007/s002680010269. [DOI] [PubMed] [Google Scholar]
- 20.Marks SL, Cook AK, Reader R. Effects of glutamine supplementation of an amino acid-based purified diet on intestinal mucosal integrity in cats with methotrexate-induced enteritis. Am J Vet Res. 1999;60:755. [PubMed] [Google Scholar]
- 21.Malo C, Buddington RK, Lepine A. Postnatal development of hydrolytic and transport functions in dog small intestine. FASEB J. 1999;13:A1010. [Google Scholar]
- 22.Buddington RK, Elnif J, Malo C. Activities of gastric, pancreatic, and intestinal brush-border membrane enzymes during postnatal development of dogs. Am J Vet Res. 2003;64:627. doi: 10.2460/ajvr.2003.64.627. [DOI] [PubMed] [Google Scholar]
- 23.Fyfe JC, Madsen M, Hojrup P. The functional cobalamin (vitamin B-12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama BA, Loo DDF, Diez-Sampedro A. Sodium-dependent reorganization of the sugar-binding site of SGLT1. Biochemistry. 2007;46:13391. doi: 10.1021/bi701562k. [DOI] [PubMed] [Google Scholar]
- 25.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779. doi: 10.1152/ajpgi.00056.2003. [DOI] [PubMed] [Google Scholar]
- 26.Chiba T, Thomforde GM, Kost LJ. Motilides accelerate regional gastrointestinal transit in the dog. Aliment Pharmacol Ther. 2000;14:955. doi: 10.1046/j.1365-2036.2000.00793.x. [DOI] [PubMed] [Google Scholar]
- 27.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 28.Lammers W, Stephen B. Origin and propagation of individual slow waves along the intact feline small intestine. Exp Physiol. 2008;93:334. doi: 10.1113/expphysiol.2007.039180. [DOI] [PubMed] [Google Scholar]
- 29.Lin HC, Chen JH. Slowing of intestinal transit by fat depends on an ondansetron-sensitive, efferent serotonergic pathway. Neurogastroenterol Motil. 2003;15:317. doi: 10.1046/j.1365-2982.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohshiro H, Nonaka M, Ichikawa K. Molecular identification and characterization of the dog motilin receptor. Regul Pept. 2008;146:80. doi: 10.1016/j.regpep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Olsson C, Holmgren S. The control of gut motility. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:481. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- 32.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008;294:G372. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 33.Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Wen J, Luque-De Leon E, Kost LJ. Duodenal motility in fasting dogs: humoral and neural pathways mediating the colonic brake. Am J Physiol Gastrointest Liver Physiol. 1998;274:G192. doi: 10.1152/ajpgi.1998.274.1.G192. [DOI] [PubMed] [Google Scholar]
- 35.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 36.Sellin JH. The pathophysiology of diarrhea. Clin Transplant. 2001;15:2. doi: 10.1111/j.1399-0012.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 37.Hore P, Messer M. Studies on disaccharidase activities of the small intestine of the domestic cat and other carnivorous mammals. Comp Biochem Physiol. 1968;24:717. doi: 10.1016/0010-406x(68)90785-8. [DOI] [PubMed] [Google Scholar]
- 38.Kienzle E. Carbohydrate metabolism of the cat – part I. J Anim Physiol Anim Nutr (Berl) 1993;69:92–101. [Google Scholar]
- 39.Levanti G, Fehlmann M, Starita-Geribaldi M. Distribution of enzyme along the brush border of the canine intestine. Ann Biol Anim Biochim Biophys. 1978;18:1155–1159. [Google Scholar]
- 40.Noon KF, Rogul M, Brendle JJ, Keefe TJ. Detection and definition of canine intestinal carbohydrases, using a standardized method. Am J Vet Res. 1977;38:1063. [PubMed] [Google Scholar]
- 41.Kienzle E. Carbohydrate metabolism in the cat – part IV. J Anim Physiol Anim Nutr (Berl) 1993;70:89–96. [Google Scholar]
- 42.Batt RM, Carter MW, Peters TJ. Biochemical changes in the jejunal mucosa of dogs with a naturally occurring enteropathy associated with bacterial overgrowth. Gut. 1984;25:816. doi: 10.1136/gut.25.8.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall EJ, Batt RM. Development of wheat-sensitive enteropathy in Irish Setters: biochemical changes. Am J Vet Res. 1990;51:983. [PubMed] [Google Scholar]
Diagnostic Evaluation
- 1.Ruckstuhl N, Hoerauf A, Tomsa K. Pseudohypoadrenocorticism in two Siberian Huskies with intestinal parasitism. Schweiz Arch Tierheilkd. 2002;144:75. doi: 10.1024/0036-7281.144.2.75. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous Fecal examination procedures. The scoop on poop: a fecal wet lab demonstration video. http://www.capcvet.org/?p=Links&h=0&s=1#
- 3.Marks SL, Kather EJ. Bacterial-associated diarrhea in the dog: a critical appraisal. Vet Clin North Am Small Anim Pract. 2003;33:1029. doi: 10.1016/s0195-5616(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 4.Suchodolski JS, Ruaux CG, Steiner JM. Application of molecular fingerprinting for qualitative assessment of small-intestinal bacterial diversity in dogs. J Clin Microbiol. 2004;42:4702. doi: 10.1128/JCM.42.10.4702-4708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desario C, Decaro N, Campolo M. Canine parvovirus infection: Which diagnostic test for virus? J Virol Methods. 2005;126:179. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Gookin JL, Stauffer SH, Coccaro MR. Optimization of a species-specific polymerase chain reaction assay for identification of Pentatrichomonas hominis in canine fecal specimens. Am J Vet Res. 2007;68:783. doi: 10.2460/ajvr.68.7.783. [DOI] [PubMed] [Google Scholar]
- 7.Tuffli SP, Gaschen F, Neiger R. Effect of dietary factors on the detection of fecal occult blood in cats. J Vet Diagn Invest. 2001;13:177. doi: 10.1177/104063870101300218. [DOI] [PubMed] [Google Scholar]
- 8.Fetz K, Steiner JM, Broussard JD. Evaluation of fecal alpha(1)-proteinase inhibitor concentrations in cats with inflammatory bowel disease and cats with gastrointestinal neoplasia. J Vet Intern Med. 2005;19:439. doi: 10.1016/j.tvjl.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann RM, Suchodolski JS, Steiner JM. Development and analytic validation of a radioimmunoassay for the quantification of canine calprotectin in serum and feces from dogs. Am J Vet Res. 2008;69:845. doi: 10.2460/ajvr.69.7.845. [DOI] [PubMed] [Google Scholar]
- 10.Allenspach K. Tests to investigate gastrointestinal diseases in dogs—which markers are actually useful for the practitioner? J Small Anim Pract. 2007;48:607. doi: 10.1111/j.1748-5827.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 11.Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Neiger R, Simpson JW. Accuracy of folate, cobalamin and the hydrogen breath test to diagnose small intestinal bacterial overgrowth in dogs. J Vet Intern Med. 2000;14:376. [Google Scholar]
- 13.Johnston KL, Ballevre OP, Batt RM. Use of an orally administered combined sugar solution to evaluate intestinal absorption and permeability in cats. Am J Vet Res. 2001;62:111. doi: 10.2460/ajvr.2001.62.111. [DOI] [PubMed] [Google Scholar]
- 14.Frias R, Sankari S, Westermarck E. Cr-51-EDTA absorption blood test: An easy method for assessing small intestinal permeability in dogs. J Vet Intern Med. 2004;18:156. doi: 10.1892/0891-6640(2004)18<156:cabtae>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Randell SC, Hill RC, Scott KC. Intestinal permeability testing using lactulose and rhamnose: a comparison between clinically normal cats and dogs and between dogs of different breeds. Res Vet Sci. 2001;71:45. doi: 10.1053/rvsc.2001.0483. [DOI] [PubMed] [Google Scholar]
- 16.Bissett SA, Guilford WG, Spohr A. Breath hydrogen testing in small animal practice. Compend Contin Educ Vet. 1997;19:916. [Google Scholar]
- 17.German AJ, Day MJ, Ruaux CG. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33. doi: 10.1892/0891-6640(2003)017<0033:codait>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Suchodolski JS, Ruaux CG, Steiner JM. Development of a C-13-glycocholic acid blood test to assess bacterial metabolic activity of the small intestine in canines. Can J Vet Res. 2005;69:313. [PMC free article] [PubMed] [Google Scholar]
- 19.Washabau RJ, Hall JA. Diagnosis and management of gastrointestinal motility disorders in dogs and cats. Compend Contin Educ Vet. 1997;19:721. [Google Scholar]
- 20.Zatloukal J, Crha M, Lorenzova J. The comparative advantage of plain radiography in diagnosis of obstruction of the small intestine in dogs. Acta Vet Brno. 2004;73:365. [Google Scholar]
- 21.Weber M, Stambouli F, Martin L. Gastrointestinal transit of solid radiopaque markers in large and giant breed growing dogs. J Anim Physiol Anim Nutr (Berl) 2001;85:242. doi: 10.1046/j.1439-0396.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaschen L, Kircher P, Stussi A. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathies. Vet Radiol Ultrasound. 2008;49:56. doi: 10.1111/j.1740-8261.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 23.Barberet V, Schreurs E, Rademacher N. Quantification of the effect of various patient and image factors on ultrasonographic detection of select canine abdominal organs. Vet Radiol Ultrasound. 2008;49:273. doi: 10.1111/j.1740-8261.2008.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Lynch TM, Morris TH, Dix J. Bacterial counts in canine duodenal fluid after exposure to saline, sodium bicarbonate and hypertonic dextrose solutions used to maintain patency of chronically implanted catheters. Lab Anim. 1999;33:143. doi: 10.1258/002367799780578336. [DOI] [PubMed] [Google Scholar]
- 25.Johnston KL, Lamport A, Ballevre O. A comparison of endoscopic and surgical collection procedures for the analysis of the bacterial flora in duodenal fluid from cats. Vet J. 1999;157:85. doi: 10.1053/tvjl.1998.0294. [DOI] [PubMed] [Google Scholar]
- 26.Leib MS, Dalton MN, King SE. Endoscopic aspiration of intestinal contents in dogs and cats: 394 cases. J Vet Intern Med. 1999;13:191. doi: 10.1892/0891-6640(1999)013<0191:eaoici>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Jergens AE, Andreasen CB, Hagemoser WA. Cytologic examination of exfoliative specimens obtained during endoscopy for diagnosis of gastrointestinal tract disease in dogs and cats. J Am Vet Med Assoc. 1998;213:1755. [PubMed] [Google Scholar]
- 28.Willard MD, Jergens AE, Duncan RB. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc. 2002;220:1177. doi: 10.2460/javma.2002.220.1177. [DOI] [PubMed] [Google Scholar]
- 29.Willard MD, Lovering SL, Cohen ND. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474. doi: 10.2460/javma.2001.219.474. [DOI] [PubMed] [Google Scholar]
- 30.Day MJ, Bilzer T, Mansell J. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the world small animal veterinary association gastrointestinal standardization group. J Comp Pathol. 2008;138:S1. doi: 10.1016/j.jcpa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Casamian-Sorrosal D, Willard MD, Murray JK. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med. 2010;24:80. doi: 10.1111/j.1939-1676.2009.0427.x. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh SN, Dunning MD, McKinley TJ. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. J Am Vet Med Assoc. 2010;236:1215. doi: 10.2460/javma.236.11.1215. [DOI] [PubMed] [Google Scholar]
- 33.Willard MD, Moore GE, Denton BD. Effect of tissue processing on assessment of endoscopic intestinal biopsies in dogs and cats. J Vet Intern Med. 2010;24:84. doi: 10.1111/j.1939-1676.2009.0432.x. [DOI] [PubMed] [Google Scholar]
- 34.Luckschander N, Corazza N, Burgener I. Isolation and characterization of canine intestinal lymphocyte subsets. J Vet Intern Med. 2007;21:651. [Google Scholar]
- 35.Waly N, Gruffydd-Jones TJ, Stokes CR. The distribution of leucocyte subsets in the small intestine of healthy cats. J Comp Pathol. 2001;124:172. doi: 10.1053/jcpa.2000.0450. [DOI] [PubMed] [Google Scholar]
- 36.German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol. 1999;120:129. doi: 10.1053/jcpa.1998.0262. [DOI] [PubMed] [Google Scholar]
Inflammation
- 1.Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. ed 6. Saunders; Philadelphia: 2005. p. 1332. [Google Scholar]
- 2.Day MJ, Bilzer T, Wilcock B. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138:S1. doi: 10.1016/j.jcpa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Moore PF, Woo JC, Vernau W. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol. 2005;106:167. doi: 10.1016/j.vetimm.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Van der Gaag I, Happé RP, Wolvekamp WTC. Eosinophilic enteritis complicated by partial ruptures and a perforation of the small intestine in a dog. J Small Anim Pract. 1983;24:575. [Google Scholar]
- 5.Breitschwerdt EB, Halliwell WH, Foley CW. A hereditary diarrhetic syndrome in the Basenji characterized by malabsorption, protein losing enteropathy and hypergammaglobulinemia. J Am Anim Hosp Assoc. 1980;16:551. [Google Scholar]
- 6.Lothrop CD: Immunological characterization of intestinal lesions in Basenji dogs with inflammatory bowel disease. Proceedings of the 15th ACVIM Forum, Lake Buena Vista, FL, p 662, May 22–25, 1997.
- 7.Littman MP, Giger U. Familial protein losing enteropathy (PLE) and/ or protein losing nephropathy (PLN) in soft coated Wheaten Terriers (SCWT); 222 cases (1983–1997) J Vet Intern Med. 2000;14:68. doi: 10.1892/0891-6640(2000)014<0068:fpleap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Vaden SL, Sellon RK, Melgarejo LT. Evaluation of intestinal permeability and gluten sensitivity in soft-coated Wheaten Terriers with familial protein-losing enteropathy, protein-losing nephropathy, or both. Am J Vet Res. 2000;61:518. doi: 10.2460/ajvr.2000.61.518. [DOI] [PubMed] [Google Scholar]
- 9.Vaden SL, Hammerberg B, Davenport DJ. Food hypersensitivity reactions in soft coated wheaten terriers with protein-losing enteropathy or protein-losing nephropathy or both: gastroscopic food sensitivity testing, dietary provocation, and fecal immunoglobulin E. J Vet Intern Med. 2000;14:60. doi: 10.1892/0891-6640(2000)014<0060:fhrisc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Kovacevic A, Lang J, Lombardi CW. Thrombosis of the pulmonary trunk in a soft coated wheaten terrier as a complication of a protein-losing nephropathy; a case report. Kleintierpraxis. 2002;47:549. [Google Scholar]
- 11.Bright RM, Jenkins C, DeNovo R. Chronic diarrhea in a dog with regional granulomatous enteritis. J Small Anim Pract. 1994;35:423. [Google Scholar]
- 12.Lewis DC. Successful treatment of regional enteritis in a dog. J Am Anim Hosp Assoc. 1995;31:170. doi: 10.5326/15473317-31-2-170. [DOI] [PubMed] [Google Scholar]
- 13.Tumulty JW, Broussard JD, Steiner JM. Clinical effects of short-term oral budesonide on the hypothalamic-pituitary-adrenal axis in dogs with inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:120. doi: 10.5326/0400120. [DOI] [PubMed] [Google Scholar]
- 14.German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8. doi: 10.1892/0891-6640(2003)017<0008:ciiaid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Burgener IA, König A, Allenspach K. Upregulation of toll-like receptors in chronic enteropathies in dogs. J Vet Intern Med. 2008;22:553. doi: 10.1111/j.1939-1676.2008.0093.x. [DOI] [PubMed] [Google Scholar]
- 16.Waly N, Stokes CR, Gruffydd-Jones TJ. Immune cell populations in the duodenal mucosa of cats with inflammatory bowel disease. J Vet Intern Med. 2004;18:816. doi: 10.1892/0891-6640(2004)18<816:icpitd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Jergens AE, Schreiner CA, Frank DE. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 18.Allenspach K, Wieland B, Gröne A. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700. doi: 10.1892/0891-6640(2007)21[700:ceideo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.McCann TM, Ridyard AE, Else RW. Evaluation of disease activity markers in dogs with idiopathic inflammatory bowel disease. J Small Anim Pract. 2007;48:620. doi: 10.1111/j.1748-5827.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Jergens AE, Crandell JM, Morrison JA. Serum acute phase proteins in feline inflammatory bowel disease. J Vet Intern Med. 2007;21:612. [Google Scholar]
- 21.German AJ, Helps CR, Hall EJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 2000;45:7. doi: 10.1023/a:1005436721798. [DOI] [PubMed] [Google Scholar]
- 22.Sauter SN, Allenspach K, Blum JW. Cytokine mRNA abundance in intestinal biopsies from dogs with chronic diarrhea. Vet Med (Praha) 2007;52:353. [Google Scholar]
- 23.Peters IR, Helps CR, Calvert EL. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real-time reverse transcriptase polymerase chain reaction. J Vet Intern Med. 2005;19:644. doi: 10.1892/0891-6640(2005)19[644:cmqidm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Van N, Taglinger K, Helps CR. Measurement of cytokine mRNA expression in intestinal biopsies of cats with inflammatory enteropathy using quantitative real-time RT-PCR. Vet Immunol Immunopathol. 2006;113:404. doi: 10.1016/j.vetimm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Kathrani A, Steiner JM, Eastwood J. Chronic pancreatitis in dogs with inflammatory bowel disease (IBD) is associated with a negative outcome. J Vet Intern Med. 2007;21:612. [Google Scholar]
- 26.Craven M, Simpson JW, Ridyard AE. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995–2002) J Small Anim Pract. 2004;45:336. doi: 10.1111/j.1748-5827.2004.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 27.Jergens AE. Inflammatory bowel disease—current perspectives. Vet Clin North Am Small Anim Pract. 1999;29:501. [PubMed] [Google Scholar]
- 28.Garcia-Sancho M, Rodrguez-Franco F, Sainz C. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic-plasmacytic enteritis. J Vet Intern Med. 2007;21:11. doi: 10.1892/0891-6640(2007)21[11:eocmah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Spichiger AC, Allenspach K, Ontsouka E. Abundance of mRNA of growth hormone receptor and insulin-like growth factors-1 and -2 in duodenal and colonic biopsies of dogs with chronic enteropathies. J Vet Med A Physiol Pathol Clin Med. 2005;52:491. doi: 10.1111/j.1439-0442.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Crandell JM, Jergens AE, Morrison JA. Development of a clinical scoring index for disease activity in feline inflammatory bowel disease. J Vet Intern Med. 2006;20:788. [Google Scholar]
- 31.Ristic JME, Stidworthy MF. Two cases of severe iron-deficiency anaemia due to inflammatory bowel disease in the dog. J Small Anim Pract. 2002;43:80. doi: 10.1111/j.1748-5827.2002.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 32.Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire terriers: five cases (1992–1998) J Am Vet Med Assoc. 2000;217:703. doi: 10.2460/javma.2000.217.703. [DOI] [PubMed] [Google Scholar]
- 33.Bush WW, Kimmel SE, Wosar MA. Secondary hypoparathyroidism attributed to hypomagnesemia in a dog with protein-losing enteropathy. J Am Vet Med Assoc. 2001;219:1732. doi: 10.2460/javma.2001.219.1732. [DOI] [PubMed] [Google Scholar]
- 34.Evans SE, Bonczynski JJ, Broussard JD. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447. doi: 10.2460/javma.229.9.1447. [DOI] [PubMed] [Google Scholar]
- 35.Simpson KW, Fyfe J, Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Batchelor DJ, Noble PJM, Cripps PJ. Prognostic factors in canine exocrine pancreatic insufficiency: prolonged survival is likely if clinical remission is achieved. J Vet Intern Med. 2007;21:54. doi: 10.1892/0891-6640(2007)21[54:pficep]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Morgan LW, McConnell J. Cobalamin deficiency associated with erythroblastic anemia and methylmalonic aciduria in a border collie. J Am Anim Hosp Assoc. 1999;35:392. doi: 10.5326/15473317-35-5-392. [DOI] [PubMed] [Google Scholar]
- 38.Baez JL, Hendrick MJ, Walker LM. Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990–1997) J Am Vet Med Assoc. 1999;215:349. [PubMed] [Google Scholar]
- 39.Goggin JM, Biller DS, Debey BM. Ultrasonographic measurement of gastrointestinal wall thickness and the ultrasonographic appearance of the ileocolic region in healthy cats. J Am Anim Hosp Assoc. 2000;36:224. doi: 10.5326/15473317-36-3-224. [DOI] [PubMed] [Google Scholar]
- 40.Rudorf H, O’Brien R, Barr FJ. Ultrasonographic evaluation of the small intestinal wall thickness in dogs with inflammatory bowel disease from the UK. J Small Anim Pract. 2005;46:322. doi: 10.1111/j.1748-5827.2005.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 41.Gaschen L, Kircher P, Stussi A. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathy. Vet Radiol Ultrasound. 2008;49:56. doi: 10.1111/j.1740-8261.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 42.Shales CJ, Warren J, Anderson DM. Complications following full-thickness small intestinal biopsy in 66 dogs: a retrospective study. J Small Anim Pract. 2005;46:317. doi: 10.1111/j.1748-5827.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 43.Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc. 1996;209:1114. [PubMed] [Google Scholar]
- 44.Willard MD, Lovering SL, Cohen ND. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474. doi: 10.2460/javma.2001.219.474. [DOI] [PubMed] [Google Scholar]
- 45.Willard MD, Jergens AE, Duncan RB. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc. 2002;220:1177. doi: 10.2460/javma.2002.220.1177. [DOI] [PubMed] [Google Scholar]
- 46.German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8. doi: 10.1892/0891-6640(2003)017<0008:ciiaid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 47.German AJ, Hall EJ, Moore PF. Analysis of the distribution of lymphocytes expressing αβ and γδ T cell receptors, and the expression of mucosal addressin cell adhesion molecule-1 in the canine intestine. J Comp Pathol. 1999;121:249. doi: 10.1053/jcpa.1999.0328. [DOI] [PubMed] [Google Scholar]
- 48.German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol. 1999;120:129. doi: 10.1053/jcpa.1998.0262. [DOI] [PubMed] [Google Scholar]
- 49.Elwood CM, Hamblin AS, Batt RM. Quantitative and qualitative immunohistochemistry of T cell subsets and MHC class II expression in the canine intestine. Vet Immunol Immunopathol. 1997;58:195. doi: 10.1016/s0165-2427(97)00037-8. [DOI] [PubMed] [Google Scholar]
- 50.Luckschander N, Allenspach K, Hall J. Perinuclear antineutrophilic cytoplasmic antibody and response to treatment in diarrheic dogs with food responsive disease or inflammatory bowel disease. J Vet Intern Med. 2006;20:221. doi: 10.1892/0891-6640(2006)20[221:pacaar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Allenspach K, Bergman PJ, Sauter S. P-glycoprotein expression in lamina propria lymphocytes of duodenal biopsy samples in dogs with chronic idiopathic enteropathies. J Comp Pathol. 2006;134:1. doi: 10.1016/j.jcpa.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Munster M, Horauf A, Bilzer T. Assessment of disease severity and outcome of dietary, antibiotic, and immunosuppressive interventions by use of the canine IBD activity index in 21 dogs with inflammatory bowel disease. Berl Munch Tierarztl Wochenschr. 2006;119:493. [PubMed] [Google Scholar]
- 53.Martin LG, Luther TY, Alerin DC. Serum antibodies against human albumin in critically ill and healthy dogs. J Am Vet Med Assoc. 2008;232:1004. doi: 10.2460/javma.232.7.1004. [DOI] [PubMed] [Google Scholar]
- 54.Guilford WG, Jones BR, Markwell PJ. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med. 2001;15:7. doi: 10.1892/0891-6640(2001)015<0007:fsicwc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Hall EJ, Batt RM. Development of a wheat-sensitive enteropathy in Irish Setters: biochemical changes. Am J Vet Res. 1990;51:983. [PubMed] [Google Scholar]
- 56.Hall EJ, Batt RM. Development of wheat-sensitive enteropathy in Irish Setters: morphological changes. Am J Vet Res. 1990;51:978. [PubMed] [Google Scholar]
- 57.Belluzzi A, Brignola C, Campieri M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- 58.Bensignor E, Morgan DM, Nuttall T. Efficacy of an essential fatty acid-enriched diet in managing canine atopic dermatitis: a randomized, single-blinded, cross-over study. Vet Dermatol. 2008;19:156. doi: 10.1111/j.1365-3164.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 59.Puigdemont A, Brazis P, Serra M. Immunologic responses against hydrolyzed soy protein in dogs with experimentally induced soy hypersensitivity. Am J Vet Res. 2006;67:484. doi: 10.2460/ajvr.67.3.484. [DOI] [PubMed] [Google Scholar]
- 60.Dossin O: Soy hydrolysate in the management of canine IBD: preliminary study. Proceedings of the 12th European Society of Veterinary Internal Medicine Congress, Munich, Germany, p 167, 2002.
- 61.Menozzi A, Pozzoli C, Poli E. Effect of the macrolide antibacterial drug, tylosin, on TNBS-induced colitis in the rat. Pharmacology. 2005;74:135. doi: 10.1159/000084324. [DOI] [PubMed] [Google Scholar]
- 62.Westermarck E, Skrzypczak T, Steiner JM. Tylosin-responsive chronic diarrhea in dogs. J Vet Intern Med. 2005;19:177. doi: 10.1892/0891-6640(2005)19<177:tcdid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Travis S. Recent advances in immunomodulation in the treatment of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2003;15:215. doi: 10.1097/00042737-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Stroup ST, Behrend EN, Kemppainen RJ. Effects of oral administration of controlled-ileal-release budesonide and assessment of pituitary-adrenocortical axis suppression in clinically normal dogs. Am J Vet Res. 2006;67:1173. doi: 10.2460/ajvr.67.7.1173. [DOI] [PubMed] [Google Scholar]
- 65.Sandborn WJ, Tremaine WJ. Cyclosporin treatment of inflammatory bowel disease. A critical review of cyclosporin therapy in inflammatory bowel disease. Mayo Clin Proc. 1992;67:981. doi: 10.1016/s0025-6196(12)60930-6. [DOI] [PubMed] [Google Scholar]
- 66.Allenspach K, Rufenacht S, Sauter S. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239. doi: 10.1892/0891-6640(2006)20[239:paceoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 67.Fraser AG. Methotrexate: first-line or second-line immunomodulator? Eur J Gastroenterol Hepatol. 2003;15:225. doi: 10.1097/01.meg.0000049994.68425.dd. [DOI] [PubMed] [Google Scholar]
- 68.Yuki M, Sugimoto N, Takahashi K. A case of protein-losing enteropathy treated with methotrexate in a dog. J Vet Med Sci. 2006;68:397. doi: 10.1292/jvms.68.397. [DOI] [PubMed] [Google Scholar]
- 69.Neurath MF, Wanitschke R, Peters M. Randomised trial of mycophenolate mofetil versus azathioprine for treatment of chronic active Crohn's disease. Gut. 1999;44:625. doi: 10.1136/gut.44.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bexfield NH, Watson PJ, Herrtage MW. Mycophenolate and MG in dogs. Management of myasthenia gravis using cyclosporine in two dogs. J Vet Intern Med. 2006;20:1487. doi: 10.1892/0891-6640(2006)20[1487:momguc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 71.Bauditz J, Haemling J, Ortner M. Treatment with tumour necrosis factor inhibitor oxpentifylline does not improve corticosteroid dependent chronic active Crohn's disease. Gut. 1997;40:470. doi: 10.1136/gut.40.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Haens G, Van Deventer S, Van Hogezand R. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 73.Sparkes AH, Papasouliotis K, Sunvold G. Bacterial flora in the duodenum of healthy cats, and effect of dietary supplementation with fructooligosaccharides. Am J Vet Res. 1998;59:431. [PubMed] [Google Scholar]
- 74.Campieri M, Gianchetti P. Probiotics in inflammatory bowel disease: new insight to pathogenesis or possible therapeutic alternative. Gastroenterology. 1998;116:1246. doi: 10.1016/s0016-5085(99)70029-6. [DOI] [PubMed] [Google Scholar]
Malabsorption
- 1.Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. ed 6. Saunders; St. Louis: 2005. p. 1332. [Google Scholar]
- 2.Peterson PB, Willard MD. Protein-losing enteropathies. Vet Clin North Am Small Anim Pract. 2003;33:1061. doi: 10.1016/s0195-5616(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 3.Yanoff SR, Willard MD, Boothe HW. Short bowel syndrome in four dogs. Vet Surg. 1992;21:217. doi: 10.1111/j.1532-950x.1992.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 4.Spillmann T, Hewicker-Trautwein M. Protein-losing enteropathy. Waltham Focus. 2005;15:20. [Google Scholar]
- 5.Kull PA, Hess RS, Craig LE. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996–1998) J Am Vet Med Assoc. 2001;219:197. doi: 10.2460/javma.2001.219.197. [DOI] [PubMed] [Google Scholar]
- 6.Gorman S, Freeman L, Mitchell S. Extensive small bowel resection in dogs and cats: 20 cases (1998–2004) Am J Vet Res. 2006;228:403. doi: 10.2460/javma.228.3.403. [DOI] [PubMed] [Google Scholar]
- 7.German AJ, Day MJ, Ruaux CG. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33. doi: 10.1892/0891-6640(2003)017<0033:codait>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Rudorf H, van Schaik G, O’Brien R. Ultrasonographic evaluation of the thickness of the small intestinal wall in dogs with inflammatory bowel disease. J Small Anim Pract. 2005;46:322. doi: 10.1111/j.1748-5827.2005.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 9.Littman MP, Dambach DM, Vaden SL. Familial protein-losing enteropathy and protein-losing nephropathy in soft coated Wheaten Terriers: 222 cases (1983–1997) J Vet Intern Med. 2000;14:68. doi: 10.1892/0891-6640(2000)014<0068:fpleap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Kolbjornsen O, Press CMC, Landsverk T. Gastropathies in the Lundehund: Gastritis and gastric neoplasia associated with intestinal lymphangiectasia. APMIS. 1994;102:647. [PubMed] [Google Scholar]
- 11.Bush WW, Kimmel SE, Wosar MA. Secondary hypoparathyroidism attributed to hypomagnesemia in a dog with protein-losing enteropathy. J Am Vet Med Assoc. 2001;219:1732. doi: 10.2460/javma.2001.219.1732. [DOI] [PubMed] [Google Scholar]
- 12.Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire Terriers: five cases (1992–1998) J Am Vet Med Assoc. 2000;217:703. doi: 10.2460/javma.2000.217.703. [DOI] [PubMed] [Google Scholar]
- 13.Mellanby RJ, Mellor PJ, Roulois A. Hypocalcemia associated with low serum vitamin D metabolite concentrations in two dogs with protein-losing enteropathies. J Small Anim Pract. 2005;46:345. doi: 10.1111/j.1748-5827.2005.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy KF, German AJ, Ruaux CG. Fecal alpha-1 protease inhibitor concentration in dogs with chronic gastrointestinal disease. Vet Clin Pathol. 2003;32:67. doi: 10.1111/j.1939-165x.2003.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland-Smith J, Penninck DG, Keating JH. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilatation. Vet Radiol Ultrasound. 2007;48:51. doi: 10.1111/j.1740-8261.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Mansell J, Willard MD. Biopsy of the gastrointestinal tract. Vet Clin North Am Small Anim Pract. 2003;33:1099. doi: 10.1016/s0195-5616(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 17.Shales C, Warren J, Anderson D. Complications following full-thickness small intestinal biopsy in 66 dogs: a retrospective study. J Small Anim Pract. 2005;46:317. doi: 10.1111/j.1748-5827.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 18.Veldhuyzen Van Zanten SJO, Bartelsman JFWM, Tytgat GNJ. Endoscopic diagnosis of primary intestinal lymphangiectasia using a high fat meal. Endoscopy. 1986;18:108. doi: 10.1055/s-2007-1018344. [DOI] [PubMed] [Google Scholar]
- 19.Willard MD, Zenger E, Mansell JL. Protein-losing enteropathy associated with cystic mucoid changes in the intestinal crypts of two dogs. J Am Anim Hosp Assoc. 2003;39:187. doi: 10.5326/0390187. [DOI] [PubMed] [Google Scholar]
- 20.Willard MD, Helman G, Fradkin JM. Intestinal crypt lesions associated with protein-losing enteropathy in the dog. J Vet Intern Med. 2000;14:298. doi: 10.1892/0891-6640(2000)014<0298:iclawp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Meijers BKI, Schalla S, Eerens F. Protein-losing enteropathy in association with constrictive pericarditis. Int J Cardiovasc Imaging. 2006;22:389. doi: 10.1007/s10554-005-9067-2. [DOI] [PubMed] [Google Scholar]
- 22.Klar A, Shoseyov D, Berkun Y. Intestinal protein loss and hypoalbuminemia in children with pneumonia. J Pediatr Gastroenterol Nutr. 2003;37:120. doi: 10.1097/00005176-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Imamura M, Yamauchi H. Effects of massive small bowel resection on metabolism of bile acids and vitamin D3 and gastrin release in dogs. Tohoku J Exp Med. 1992;168:515. doi: 10.1620/tjem.168.515. [DOI] [PubMed] [Google Scholar]
Infection
- 1.Mohamed AS, Moore GE, Glickman LT. Prevalence of intestinal nematode parasitism among pet dogs in the United States (2003–2006) J Am Vet Med Assoc. 2009;234:631. doi: 10.2460/javma.234.5.631. [DOI] [PubMed] [Google Scholar]
- 2.De Santis AC, Raghavan M, Caldanaro RJ. Estimated prevalence of nematode parasitism among pet cats in the United States. J Am Vet Med Assoc. 2006;228:885. doi: 10.2460/javma.228.6.885. [DOI] [PubMed] [Google Scholar]
- 3.Little SE, Johnson EM, Lewis D. Prevalence of intestinal parasites in pet dogs in the United States. Vet Parasitol. 2009;166:144. doi: 10.1016/j.vetpar.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Jordan HE, Mullins ST, Stebbins ME. Endoparasitism in dogs: 21,583 cases (1981–1990) J Am Vet Med Assoc. 1993;203:547. [PubMed] [Google Scholar]
- 5.Anderson TC, Foster GW, Forrester DJ. Hookworms of feral cats in Florida. Vet Parasitol. 2003;115:19. doi: 10.1016/s0304-4017(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 6.Del Valle A, Jones BF, Harrison LM. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol Biochem Parasitol. 2003;129:167. doi: 10.1016/s0166-6851(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 7.Gates MC, Nolan TJ. Comparison of passive fecal flotation run by veterinary students to zinc-sulfate centrifugation flotation run in a diagnostic parasitology laboratory. J Parasitol. 2009;95:1213. doi: 10.1645/GE-2058.1. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Outbreak of cutaneous larva migrans at a children's camp—Miami, Florida, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1285. [PubMed] [Google Scholar]
- 9.Landmann JK, Prociv P. Experimental human infection with the dog hookworm, Ancylostoma caninum. Med J Aust. 2003;178:69. doi: 10.5694/j.1326-5377.2003.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 10.Croese J, Loukas A, Opdebeeck J. Human enteric infection with canine hookworms. Ann Intern Med. 1994;120:369. doi: 10.7326/0003-4819-120-5-199403010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Souza MJ, Ramsay EC, Patton S, New JC. Baylisascaris procyonis in raccoons (Procyon lotor) in eastern Tennessee. J Wildl Dis. 2009;45:1231. doi: 10.7589/0090-3558-45.4.1231. [DOI] [PubMed] [Google Scholar]
- 12.Spain CV, Scarlett JM, Wade SE, McDonough P. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med. 2001;15:33. doi: 10.1892/0891-6640(2001)015<0033:poezai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Nutter FB, Dubey JP, Levine JF. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp, Giardia spp, and Toxocara cati in feral and pet domestic cats. J Am Vet Med Assoc. 2004;225:1394. doi: 10.2460/javma.2004.225.1394. [DOI] [PubMed] [Google Scholar]
- 14.Sasmal NK, Pahari TK, Laha R. Experimental infection of the cockroach Periplaneta americana with Toxocara canis and the establishment of patent infections in pups. J Helminthol. 2008;82:97. doi: 10.1017/S0022149X07875936. [DOI] [PubMed] [Google Scholar]
- 15.Overgaauw PA, van Zutphen L, Hoek D. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol. 2009;163:115. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Won KY, Kruszon-Moran D, Schantz PM, Jones JL. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Am J Trop Med Hyg. 2008;79(4):552. [PubMed] [Google Scholar]
- 17.Morgan UM, Constantine CC, Forbes DA. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J Parasitol. 1997;83:825. [PubMed] [Google Scholar]
- 18.Monis PT, Thompson RCA. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol. 2003;3:233. doi: 10.1016/j.meegid.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Bowman DD, Lucio-Forster A. Cryptosporidiosis and giardiasis in dogs and cats: Veterinary and public health importance. Exp Parasitol. 2010;124:121. doi: 10.1016/j.exppara.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RCA, Palmer CS, O’Handley R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet J. 2008;177:18. doi: 10.1016/j.tvjl.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn-Moore D, Scorza V, Wilmot A, Lappin MR. Cryptosporidium felis in feces from cats in the United Kingdom. Proceedings of the ACVIM Forum, Seattle, June 7, 2007.
- 22.Scorza V, Lappin MR. Detection of Cryptosporidium spp. in feces of dogs and cats in the United States by PCR assay and IFA. J Vet Intern Med. 2005;19:437. [Google Scholar]
- 23.Hill S, Lappin MR, Cheney J. Prevalence of enteric zoonotic agents in cats. J Am Vet Med Assoc. 2000;216:687. doi: 10.2460/javma.2000.216.687. [DOI] [PubMed] [Google Scholar]
- 24.Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-central Colorado. J Am Anim Hosp Assoc. 2003;39(1):52. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- 25.Tzannes S, Batchelor DJ, Graham PA. Prevalence of Cryptosporidium, Giardia and Isospora species infections in pet cats with clinical signs of gastrointestinal disease. J Feline Med Surg. 2008;10:1. doi: 10.1016/j.jfms.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scorza AV, Brewer MM, Lappin MR. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces. J Parasitol. 2003;89:423. doi: 10.1645/0022-3395(2003)089[0423:PCRFTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RB, Holscher MA, Lyle SJ. Cryptosporidiosis in a pup. J Am Vet Med Assoc. 1983;183:1005. [PubMed] [Google Scholar]
- 28.Sisk DB, Gosser HS, Styer EL. Intestinal cryptosporidiosis in two pups. J Am Vet Med Assoc. 1984;184:835. [PubMed] [Google Scholar]
- 29.Miller DL, Liggett A, Radi ZA. Gastrointestinal cryptosporidiosis in a puppy. Vet Parasitol. 2003;115:199. doi: 10.1016/s0304-4017(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 30.Turnwald GH, Barta O, Taylor HW. Cryptosporidiosis associated with immunosuppression attributable to distemper in a pup. J Am Vet Med Assoc. 1988;192:79. [PubMed] [Google Scholar]
- 31.Fukushima K, Helman RG. Cryptosporidiosis in a pup with distemper. Vet Pathol. 1984;21:247. doi: 10.1177/030098588402100218. [DOI] [PubMed] [Google Scholar]
- 32.Willard MD, Bouly D. Cryptosporidiosis, coccidiosis and total colonic mucosal collapse in an immunosuppressed puppy. J Am Anim Hosp Assoc. 1999;35:405. doi: 10.5326/15473317-35-5-405. [DOI] [PubMed] [Google Scholar]
- 33.Denholm KM, Haitjema H, Gwynne BJ. Concurrent Cryptosporidium and parvovirus infections in a puppy. Aust Vet J. 2001;79:98. doi: 10.1111/j.1751-0813.2001.tb10708.x. [DOI] [PubMed] [Google Scholar]
- 34.Aydin Y, Guvenc T, Beyaz L. Intestinal cryptosporidiosis associated with distemper in a dog. Veteriner Fakültesi Dergisi. 2004;51:233. (abstract) [Google Scholar]
- 35.Greene CE, Jacobs GJ, Prickett D. Intestinal malabsorption and cryptosporidiosis in an adult dog. J Am Vet Med Assoc. 1990;197:365. [PubMed] [Google Scholar]
- 36.Poonacha KB, Pippin C. Intestinal cryptosporidiosis in a cat. Vet Pathol. 1982;19:708. doi: 10.1177/030098588201900616. [DOI] [PubMed] [Google Scholar]
- 37.Lappin MR, Dowers K, Edsell D. Cryptosporidiosis and inflammatory bowel disease in a cat. Feline Pract. 1997;25:10. [Google Scholar]
- 38.Goodwin MA, Barsanti JA. Intractable diarrhea associated with intestinal cryptosporidiosis in a domestic cat also infected with feline leukemia virus. J Am Anim Hosp Assoc. 1990;26:365. [Google Scholar]
- 39.Brizee-Buxton BL, Crystal MA. Coincident enteric cryptosporidiosis (correspondence) J Am Anim Hosp Assoc. 1994;30:307. [Google Scholar]
- 40.Monticello TM, Levy MG, Bunch SE. Cryptosporidiosis in a feline leukemia virus-positive cat. J Am Vet Med Assoc. 1987;191:705. [PubMed] [Google Scholar]
- 41.Lent SF, Burkhardt JE, Bolka D. Coincident enteric cryptosporidiosis and lymphosarcoma in a cat with diarrhea. J Am Anim Hosp Assoc. 1993;29:492. [Google Scholar]
- 42.Gookin JL, Levy MG, Law JM. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690. doi: 10.2460/ajvr.2001.62.1690. [DOI] [PubMed] [Google Scholar]
- 43.Scorza AV, Lappin MR. Diagnosis and Treatment of Cryptosporidiosis and Giardiasis in Cats and Dogs in the United States. Colorado State University; 2007. Co-infection of Cryptosporidium and Giardia in naturally infected cats. PhD Dissertation. [Google Scholar]
- 44.Buret AG. Pathogenic mechanisms in giardiasis and cryptosporidiosis. In: Ortega P, editor. Giardia and Cryptosporidium: from molecules to disease. CAB International; Wallingford, UK: 2009. p. 438. [Google Scholar]
- 45.Pierce KK, Kirkpatrick BD. Update on human infections caused by intestinal protozoa. Curr Opin Gastroenterol. 2009;25:12. doi: 10.1097/mog.0b013e32831da7dd. [DOI] [PubMed] [Google Scholar]
- 46.Flores J, Okhuysen PC. Genetics of susceptibility to infection with enteric pathogens. Curr Opin Infect Dis. 2009;22:471. doi: 10.1097/QCO.0b013e3283304eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown RR, Elston TH, Evans L. Feline zoonoses guidelines from the American Association of Feline Practitioners. Compend Contin Educ Pract Vet. 2003;25:936. doi: 10.1016/j.jfms.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marks SL, Hanson TE, Melli AC. Comparison of direct immunofluorescence modified acid fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp. in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549. doi: 10.2460/javma.2004.225.1549. [DOI] [PubMed] [Google Scholar]
- 49.Bachman D, Scorza AV, Brewer M, et al. Evaluation of chromatographic immunoassays for the diagnosis of Giardia spp. and Cryptosporidium spp. of cats and dogs. Proceedings of the 25th ACVIM Forum, Seattle: (abstract), 2007.
- 50.Mekaru SR, Marks SL, Felley AJ, Chouicha N, Kass PH. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J Vet Intern Med. 2007;21:959. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Morgan UM. The development of diagnostic PCR primers for Cryptosporidium using RAPD-PCR. Mol Biochem Parasitol. 1996;77:103. doi: 10.1016/0166-6851(96)02577-7. [DOI] [PubMed] [Google Scholar]
- 52.Gargala G. Drug treatment and novel drug target against Cryptosporidium. Parasite. 2008;15:275. doi: 10.1051/parasite/2008153275. [DOI] [PubMed] [Google Scholar]
- 53.Smith HV, Corcoran GD. New drugs and treatment for cryptosporidiosis. Curr Opin Infect Dis. 2004;17:667. doi: 10.1097/00001432-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Hemphill A, Mueller J, Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin Pharmacother. 2006;7:953. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- 55.Barr SC, Jamrosz GJ, Hornbuckle WE. Use of paromomycin for treatment of cryptosporidiosis in a cat. J Am Vet Med Assoc. 1994;205:1742. [PubMed] [Google Scholar]
- 56.Gookin JL, Riviere JE, Gilger BC. Acute renal failure in four cats treated with paromomycin. J Am Vet Med Assoc. 1999;215:1821. [PubMed] [Google Scholar]
- 57.Hewitt RG, Yiannoutsos CT, Higgs ES. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advance human immunodeficiency virus infection. Clin Infect Dis. 2000;31:1084. doi: 10.1086/318155. [DOI] [PubMed] [Google Scholar]
- 58.Glaser CA, Safrin S, Reingold A. Association between Cryptosporidium infection and animal exposure in HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:79. doi: 10.1097/00042560-199801010-00012. [DOI] [PubMed] [Google Scholar]
- 59.Bowman Carlin EP, Bowman DD, Scarlett JM. Prevalence of Giardia in symptomatic dogs and cats throughout the United States as determined by the IDEXX SNAP Giardia test. Vet Ther. 2006;7:199. [PubMed] [Google Scholar]
- 60.Buret AG. Pathophysiology of enteric infections with Giardia duodenalis. Parasite. 2008;15:261. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- 61.Scorza AV, Radecki SV, Lappin MR. Efficacy of a combination of febantel, pyrantel, and praziquantel for the treatment of feline giardiasis. J Feline Med Surg. 2006;8:7. doi: 10.1016/j.jfms.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gookin JL, Stebbins ME, Hunt E. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol. 2004;42:2707. doi: 10.1128/JCM.42.6.2707-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dryden MW, Payne PA, Smith V. Accurate diagnosis of Giardia spp. and proper fecal examination procedures. Vet Ther. 2006;7:4. [PubMed] [Google Scholar]
- 64.Clark M, Scorza AV, Lappin MR: A commercially available Giardia spp. antigen assay detects the assemblages isolated from dogs. Proceedings of the American College of Veterinary Internal Medicine Forum (abstract), Denver, 2008.
- 65.Sokolow SH, Rand C, Marks SL. Epidemiological evaluation of diarrhea dogs in an animal shelter. Am J Vet Res. 2005;66:1018. doi: 10.2460/ajvr.2005.66.1018. [DOI] [PubMed] [Google Scholar]
- 66.Lappin MR, Jensen WA, Taton-Allen G: Comparison of Znso4 centrifugation, a fecal antigen assay, and an immunofluorescent antibody assay for diagnosis of giardiasis in cats. Proceedings of the American College of Veterinary Internal Medicine Forum (abstract), Dallas, 2002.
- 67.Bachman D, Scorza AV, Brewer M, et al: Evaluation of chromatographic immunoassays for the diagnosis of Giardia spp. and Cryptosporidium spp. of cats and dogs. Proceedings of the American College of Veterinary Internal Medicine Forum (abstract), Seattle, 2007.
- 68.Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. 2008;160:75. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Scorza AV, Lappin MR, Ballweber LR: Genotyping of Giardia duodenalis isolates of mammals (dogs, cats, bobcats and cattle) by the β-giardin, glutamate dehydrogenase and triose phosphate isomerase genes 3rd International Giardia and Cryptosporidium Conference (abstract), Oct 11–15, 2009, Orvieto, Italy.
- 70.Rossignol JF. Cryptosporidium and Giardia: Treatment options and prospects for new drugs. Exp Parasitol. 2010;124:45. doi: 10.1016/j.exppara.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Keith CL, Radecki SV, Lappin MR. Evaluation of fenbendazole for treatment of Giardia infection in cats concurrently infected with Cryptosporidium parvum. Am J Vet Res. 2003;64:1027. doi: 10.2460/ajvr.2003.64.1027. [DOI] [PubMed] [Google Scholar]
- 72.Scorza V, Lappin MR. Metronidazole for treatment of giardiasis in cats. J Feline Med Surg. 2004;6:157. doi: 10.1016/j.jfms.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zimmer JF. Treatment of feline giardiasis with metronidazole. Cornell Vet. 1987;77:383. [PubMed] [Google Scholar]
- 74.Scorza AV, Radecki SV, Lappin MR. Efficacy of a combination of febantel, pyrantel, and praziquantel for the treatment of kittens experimentally infected with Giardia species. J Feline Med Surg. 2005;8:7. doi: 10.1016/j.jfms.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lappin MR, Clark M, Scorza AV. Treatment of healthy Giardia spp. positive dogs with fenbendazole or nitazoxanide. Proceedings of the ACVIM Forum, San Antonio, TX, June 4, 2008.
- 76.Miro G, Mateo M, Montoya A. Survey of intestinal parasites in stray dogs in the Madrid area and comparison of the efficacy of three anthelmintics in naturally infected dogs. Parasitol Res. 2007;100:317. doi: 10.1007/s00436-006-0258-0. [DOI] [PubMed] [Google Scholar]
- 77.Payne PA, Ridley RK, Dryden MW. Efficacy of a combination febantel-praziquantel-pyrantel product, with or without vaccination with a commercial Giardia vaccine, for treatment of dogs with naturally occurring giardiasis. J Am Vet Med Assoc. 2002;220:330. doi: 10.2460/javma.2002.220.330. [DOI] [PubMed] [Google Scholar]
- 78.Bowman DD, Liotta JL, Ulrich M. Treatment of naturally occurring, asymptomatic Giardia sp. in dogs with Drontal Plus flavour tablets. Parasitol Res. 2009;105(Suppl 1):S125. doi: 10.1007/s00436-009-1503-0. [DOI] [PubMed] [Google Scholar]
- 79.Barr SC, Bowman DD, Frongillo MF. Efficacy of a drug combination of praziquantel, pyrantel pamoate, and febantel against giardiasis in dogs. Am J Vet Res. 1998;59:1134. [PubMed] [Google Scholar]
- 80.Giangaspero A, Traldi G, Paoletti B. Efficacy of pyrantel embonate, febantel and praziquantel against Giardia species in naturally infected adult dogs. Vet Rec. 2002;150:184. doi: 10.1136/vr.150.6.184. [DOI] [PubMed] [Google Scholar]
- 81.Montoya A, Dado D, Mateo M. Efficacy of Drontal Flavour Plus (50 mg praziquantel, 144 mg pyrantel embonate, 150 mg febantel per tablet) against Giardia sp in naturally infected dogs. Parasitol Res. 2008;103:1141. doi: 10.1007/s00436-008-1107-0. [DOI] [PubMed] [Google Scholar]
- 82.Barr SC, Bowman DD, Heller RL, Erb HN. Efficacy of albendazole against giardiasis in dogs. Am J Vet Res. 1993;54:926. [PubMed] [Google Scholar]
- 83.Abbitt B, Huey RL, Eugster AK, Syler J. Treatment of giardiasis in adult Greyhounds, using ipronidazole-medicated water. J Am Vet Med Assoc. 1986;188(1):67. [PubMed] [Google Scholar]
- 84.Dow SW, LeCouteur RA, Poss ML. Central nervous system toxicosis associated with metronidazole treatment of dogs: five cases (1984–1987) J Am Vet Med Assoc. 1989;195:365. [PubMed] [Google Scholar]
- 85.Caylor KB, Cassimatis MK. Metronidazole neurotoxicosis in two cats. J Am Anim Hosp Assoc. 2001;37:258. doi: 10.5326/15473317-37-3-258. [DOI] [PubMed] [Google Scholar]
- 86.Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med. 2007;21:328. doi: 10.1111/j.1939-1676.2007.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stokol T, Randolph JF, Nachbar S. Development of bone marrow toxicosis after albendazole administration in a dog and cat. J Am Vet Med Assoc. 1997;210:1753. [PubMed] [Google Scholar]
- 88.Stein JE, Radecki SV, Lappin MR. Efficacy of Giardia vaccination in the treatment of giardiasis in cats. J Am Vet Med Assoc. 2003;222:1548. doi: 10.2460/javma.2003.222.1548. [DOI] [PubMed] [Google Scholar]
- 89.Olson ME, Ceri H, Morck DW. Giardia vaccination. Parasitol Today. 2000;16:213. doi: 10.1016/s0169-4758(99)01623-3. [DOI] [PubMed] [Google Scholar]
- 90.Chon SK, Kim NS. Evaluation of silymarin in the treatment on asymptomatic Giardia infections in dogs. Parasitol Res. 2005;97:445. doi: 10.1007/s00436-005-1462-z. [DOI] [PubMed] [Google Scholar]
- 91.Simpson KW, Rishniw M, Bellosa M. Influence of Enterococcus faecium SF68 probiotic on giardiasis in dogs. J Vet Intern Med. 2009;23:476. doi: 10.1111/j.1939-1676.2009.0283.x. [DOI] [PubMed] [Google Scholar]
- 92.Vasilopulos RJ, Rickard LG, Mackin AJ. Genotypic analysis of Giardia duodenalis in domestic cats. J Vet Intern Med. 2007;21:352. doi: 10.1892/0891-6640(2007)21[352:gaogdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 93.Zygner W, Jaros D, Skowrońska M. Prevalence of Giardia intestinalis in domestic dogs in Warsaw. Wiad Parazytol. 2006;52:311. [PubMed] [Google Scholar]
- 94.Lalle M, Pozio E, Capelli G. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 95.Traub RJ, Monis PT, Robertson I. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology. 2004;128:253. doi: 10.1017/s0031182003004505. [DOI] [PubMed] [Google Scholar]
- 96.Dubey JP. The evolution of the knowledge of cat and dog coccidia. Parasitology. 2009;136:1469. doi: 10.1017/S003118200900585X. [DOI] [PubMed] [Google Scholar]
- 97.Buehl IE, Prosl H, Mundt HC. Canine isosporosis—epidemiology of field and experimental infections. J Vet Med B Infect Dis Vet Public Health. 2006;53:482. doi: 10.1111/j.1439-0450.2006.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell SM, Zajac AM, Charles S. Cystoisospora canis Nemeséri, 1959 (syn. Isospora canis), infections in dogs: clinical signs, pathogenesis, and reproducible clinical disease in beagle dogs fed oocysts. J Parasitol. 2007;93:345. doi: 10.1645/GE-1024R.1. [DOI] [PubMed] [Google Scholar]
- 99.Saitoh Y, Itagaki H. Dung beetles, Onthophagus spp., as potential transport hosts of feline coccidia. Nihon Juigaku Zasshi. 1990;52:293. doi: 10.1292/jvms1939.52.293. [DOI] [PubMed] [Google Scholar]
- 100.Lloyd S. Activity of toltrazuril and diclazuril against Isospora species in kittens and puppies. Vet Rec. 2001;148:500. doi: 10.1136/vr.148.16.509. [DOI] [PubMed] [Google Scholar]
- 101.Daugschies A, Mundt HC, Letkova V. Toltrazuril treatment of cystoisosporosis in dogs under experimental and field conditions. Parasitol Res. 2000;86:797. doi: 10.1007/s004360000217. [DOI] [PubMed] [Google Scholar]
- 102.Peterson JL, Willard MD, Lees GE. Toxoplasmosis in two cats with inflammatory intestinal disease. J Am Vet Med Assoc. 1991;199:473. [PubMed] [Google Scholar]
- 103.Cave NJ, Marks SL, Kass PH. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc. 2002;221:52. doi: 10.2460/javma.2002.221.52. [DOI] [PubMed] [Google Scholar]
- 104.Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-central Colorado. J Am Anim Hosp Assoc. 2003;39:52. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- 105.Hill SL, Cheney JM, Taton-Allen G. Prevalence of enteric zoonotic organisms in cats. J Am Vet Med Assoc. 2000;216:687. doi: 10.2460/javma.2000.216.687. [DOI] [PubMed] [Google Scholar]
- 106.Moser I, Rieksneuwohner B, Lentasch P. Genomic heterogeneity and O-antigenic diversity of Campylobacter upsaliensis and Campylobacter helveticus strains isolated from dogs and cats in Germany. J Clin Microbiol. 2001;39:2548. doi: 10.1128/JCM.39.7.2548-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sandberg M, Bergsjo B, Hofshagen M. Risk factors for Campylobacter infection in Norwegian cats and dogs. Prev Vet Med. 2002;55:241. doi: 10.1016/s0167-5877(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 108.Steinhauserova I, Fojtikova K, Klimes J. The incidence and PCR detection of Campylobacter upsaliensis in dogs and cats. Lett Appl Microbiol. 2000;31:209. doi: 10.1046/j.1365-2672.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 109.Hald B, Madsen M. Healthy puppies and kittens as carriers of Campylobacter spp., with special reference to Campylobacter upsaliensis. J Clin Microbiol. 1997;35:3351. doi: 10.1128/jcm.35.12.3351-3352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDonough PL, Simpson KW. Diagnosing emerging bacterial infections: salmonellosis, campylobacteriosis, clostridial toxicosis, and helicobacteriosis. Semin Vet Med Surg (Small Anim) 1996;11:187. doi: 10.1016/s1096-2867(96)80032-6. [DOI] [PubMed] [Google Scholar]
- 111.Brown C, Martin V, Chitwood S. An outbreak of enterocolitis due to Campylobacter spp. in a beagle colony. J Vet Diagn Invest. 1999;11:374. doi: 10.1177/104063879901100416. [DOI] [PubMed] [Google Scholar]
- 112.Prescott JF, Barker IK, Manninen KI. Campylobacter jejuni colitis in gnotobiotic dogs. Can J Comp Med. 1981;45:377. [PMC free article] [PubMed] [Google Scholar]
- 113.Buddington RK. Postnatal changes in bacterial populations in the gastrointestinal tract of dogs. Am J Vet Res. 2003;64:646. doi: 10.2460/ajvr.2003.64.646. [DOI] [PubMed] [Google Scholar]
- 114.Kruth SA, Prescott JF, Welch K. Nosocomial diarrhea associated with enterotoxigenic Clostridium perfringens infection in dogs. J Am Vet Med Assoc. 1989;195:331. [PubMed] [Google Scholar]
- 115.Foley J, Hirsh DC, Pedersen N. An outbreak of Clostridium perfringens enteritis in a cattery of Bengal cats and experimental transmission to specific pathogen free cats. Feline Pract. 1996;24:31. [Google Scholar]
- 116.Cassutto BH, Cook LC. An epidemiological survey of Clostridium perfringens-associated enterotoxemia at an army veterinary treatment facility. Mil Med. 2002;167:219. [PubMed] [Google Scholar]
- 117.Daube G, Simon P, Limbourg B. Hybridization of 2,659 Clostridium perfringens isolates with gene probes for seven toxins (α, β, ε, ι, δ, θ, µ and enterotoxin) and for sialidase. Am J Vet Res. 1996;57:496. [PubMed] [Google Scholar]
- 118.Marks SL, Melli A, Kass PH. Evaluation of methods to diagnose Clostridium perfringens-associated diarrhea in dogs. J Am Vet Med Assoc. 1999;214:357. [PubMed] [Google Scholar]
- 119.Marks SL, Melli A, Kass PH. Influence of storage and temperature on endospore and enterotoxin production by Clostridium perfringens in dogs. J Vet Diagn Invest. 2000;12:63. doi: 10.1177/104063870001200112. [DOI] [PubMed] [Google Scholar]
- 120.Weese JS, Staempfli HR, Prescott JF. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med. 2001;15:374. [PubMed] [Google Scholar]
- 121.Marks SL, Kather EJ, Kass PH. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med. 2002;16:533. doi: 10.1892/0891-6640(2002)016<0533:gapcop>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 122.Marks SL, Kather EJ. Antimicrobial susceptibilities of canine Clostridium difficile and Clostridium perfringens isolates to commonly utilized antimicrobial drugs. Vet Microbiol. 2003;94:39. doi: 10.1016/s0378-1135(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 123.Struble AL, Yajarayma JT, Kass PH. Fecal shedding of Clostridium difficile in dogs: a period prevalence survey in a veterinary medical teaching hospital. J Vet Diagn Invest. 1994;6:342. doi: 10.1177/104063879400600310. [DOI] [PubMed] [Google Scholar]
- 124.Weese JS, Staempfli HR, Prescott JF. Isolation of environmental Clostridium difficile from a veterinary teaching hospital. J Vet Diagn Invest. 2000;12:449. doi: 10.1177/104063870001200510. [DOI] [PubMed] [Google Scholar]
- 125.Clooten J, Weese JS, Kruth S. Clostridium difficile inoculation in 6 healthy dogs. J Vet Intern Med. 2003;17:412. doi: 10.1111/j.1939-1676.2003.tb02421.x. (abstract) [DOI] [PubMed] [Google Scholar]
- 126.Levine MM. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteradherent. J Infect Dis. 1987;155:377. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 127.Drolet R, Fairbrother JM, Harel J. Attaching and effacing and enterotoxigenic Escherichia coli associated with enteric colibacillosis in the dog. Can J Vet Res. 1994;58:87. [PMC free article] [PubMed] [Google Scholar]
- 128.Cullen JJ, Spates ST, Ephgrave KS. Endotoxin temporarily impairs canine colonic absorption of water and sodium. J Surg Res. 1998;74:34. doi: 10.1006/jsre.1997.5218. [DOI] [PubMed] [Google Scholar]
- 129.Pass MA, Odedra R, Batt RM. Multiplex PCRs for identification of Escherichia coli virulence genes. J Clin Microbiol. 2000;38:2001. doi: 10.1128/jcm.38.5.2001-2004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith KA, Kruth S, Hammermueller J. A case-control study of verocytotoxigenic Escherichia coli infection in cats with diarrhea. Can J Vet Res. 1998;62:87. [PMC free article] [PubMed] [Google Scholar]
- 131.Turk J, Maddox C, Fales W. Examination for heat-labile, heat-stable, and Shiga-like toxins and for the eaeA gene in Escherichia coli isolates obtained from dogs dying with diarrhea. J Am Vet Med Assoc. 1998;212:1735. [PubMed] [Google Scholar]
- 132.Beaudry M, Zhu C, Fairbrother JM. Genotypic and phenotypic characterization of Escherichia coli isolates from dogs manifesting attaching and effacing lesions. J Clin Microbiol. 1996;34:144. doi: 10.1128/jcm.34.1.144-148.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hammermueller J, Kruth S, Prescott J. Detection of toxin genes in Escherichia coli isolated from normal dogs and dogs with diarrhea. Can J Vet Res. 1995;59:265. [PMC free article] [PubMed] [Google Scholar]
- 134.Stone GG, Oberst RD, Hays MP. Detection of Salmonella typhimurium from rectal swabs of experimentally infected beagles by short cultivation and PCR-hybridization. J Clin Microbiol. 1995;33:1292. doi: 10.1128/jcm.33.5.1292-1295.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cantor GH, Nelson S, Vanek JA. Salmonella shedding in racing sled dogs. J Vet Diagn Invest. 1997;9:447. doi: 10.1177/104063879700900424. [DOI] [PubMed] [Google Scholar]
- 136.Ikeda JS, Hirsh DC, Jang SS, Biberstein EL. Characteristics of Salmonella isolated from animals at a veterinary medical teaching hospital. Am J Vet Res. 1986;47:232. [PubMed] [Google Scholar]
- 137.Headley SA, Scorpio DG, Vidotto O, Stephen Dumler J. Neorickettsia helminthoeca and salmon poisoning disease: A review. Vet J. 2011;187:165. doi: 10.1016/j.tvjl.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 138.Headley SA, Kano FS, Scorpio DG. Neorickettsia helminthoeca in Brazilian dogs: a cytopathological, histopathological and immunohistochemical study. Clin Microbiol Infect. 2009;15(Suppl 2):21. doi: 10.1111/j.1469-0691.2008.02137.x. [DOI] [PubMed] [Google Scholar]
- 139.Headley SA, Barat N, Scorpio D. Neorickettsia helminthoeca in dogs in Brazil. Emerg Infect Dis. 2006;12:1303. doi: 10.3201/eid1208.060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Booth AJ, Stogdale L, Grigor JA. Salmon poisoning disease in dogs in southern Vancouver Island. Can Vet J. 1984;25:2. [PMC free article] [PubMed] [Google Scholar]
- 141.Foreyt WJ, Gorham JR. Evaluation of praziquantel against induced Nanophyetus salmincola infections in coyotes and dogs. Am J Vet Res. 1988;49:563. [PubMed] [Google Scholar]
- 142.Eastburn RL, Fritsche TF, Terhune CA. Human intestinal infection with Nanophyetus salmincola from salmonid fishes. Am J Trop Med Hyg. 1987;36:586. doi: 10.4269/ajtmh.1987.36.586. [DOI] [PubMed] [Google Scholar]
- 143.McCaw D, Hoskins JD. Canine viral enteritis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders; St. Louis: 2006. p. 63. [Google Scholar]
- 144.Decaro N, Desario C, Billi M. Western European epidemiological survey for parvovirus and coronavirus infections in dogs. Vet J. 2011;187:195. doi: 10.1016/j.tvjl.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Decaro N, Mari V, Elia G. Recombinant canine coronaviruses in dogs, Europe. Emerg Infect Dis. 2010;16:41. doi: 10.3201/eid1601.090726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Godsall SA, Clegg SR, Stavisky JH. Epidemiology of canine parvovirus and coronavirus in dogs presented with severe diarrhoea to PDSA PetAid hospitals. Vet Rec. 2010;167:196. doi: 10.1136/vr.c3095. [DOI] [PubMed] [Google Scholar]
- 147.Sokolow SH, Ran DC, Marks SL. Epidemiologic evaluation of diarrhea in dogs in an animal shelter. Am J Vet Res. 2005;66:1018. doi: 10.2460/ajvr.2005.66.1018. [DOI] [PubMed] [Google Scholar]
- 148.Stavisky J, Pinchbeck GL, German AJ. Prevalence of canine enteric coronavirus in a cross-sectional survey of dogs presenting at veterinary practices. Vet Microbiol. 2010;140:18. doi: 10.1016/j.vetmic.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Erles K, Brownlie J. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet Clin North Am Small Anim Pract. 2008;38:815. doi: 10.1016/j.cvsm.2008.02.008. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Evermann JF, Abbott JR, Han S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J Vet Diagn Invest. 2005;17:610. doi: 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- 151.Buonavoglia C, Decaro N, Martella V. Canine coronavirus highly pathogenic for dogs. Emerg Infect Dis. 2006;12:492. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pratelli A, Tempesta M, Roperto FP. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J Vet Diagn Invest. 1999;11:550. doi: 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
- 153.Paul MA, Carmichael LE, Childers H. American Animal Hospital Association (AAHA) Canine Vaccine Task Force, 2006 AAHA canine vaccine guidelines. J Am Anim Hosp Assoc. 2006;42:80. doi: 10.5326/0420080. [DOI] [PubMed] [Google Scholar]
- 154.Lappin MR. Polysystemic viral diseases. In: Nelson R, Couto G, editors. Small Animal Internal Medicine. ed 4. Mosby/Elsevier; St. Louis: 2009. p. 1336. [Google Scholar]
- 155.Pedersen NC. A review of feline infectious peritonitis virus infection. J Feline Med Surg. 2009;11:1963–2008. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Addie DD, Jarrett O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet Rec. 2001;148:649. doi: 10.1136/vr.148.21.649. [DOI] [PubMed] [Google Scholar]
- 157.Harvey CJ, Lopez JW, Hendrick MJ. An uncommon intestinal manifestation of feline infectious peritonitis: 26 cases (1986–1993) J Am Vet Med Assoc. 1996;209:1117. [PubMed] [Google Scholar]
- 158.Richards JR, Elston TH, Ford RB. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report. J Am Vet Med Assoc. 2006;229:1405. doi: 10.2460/javma.229.9.1405. [DOI] [PubMed] [Google Scholar]
- 159.Greene CE. Canine distemper. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders; Philadelphia: 2006. p. 25. [Google Scholar]
- 160.Kubo T, Kagawa Y, Taniyama H, Hasegawa A. Distribution of inclusion bodies in tissues from 100 dogs infected with canine distemper virus. J Vet Med Sci. 2007;69:527. doi: 10.1292/jvms.69.527. [DOI] [PubMed] [Google Scholar]
- 161.Elia G, Decaro N, Martella V. Detection of canine distemper virus in dogs by real-time RT-PCR. J Virol Methods. 2006;136:171. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 162.Burton JH, Veir JK, Pearce L, et al. Detection of canine distemper virus RNA from blood and conjunctival swabs collected from healthy puppies after administration of a modified live vaccine. ACVIM San Antonio, TX, June 4–7, 2008 (abstract).
- 163.Saito TB, Alfieri AA, Wosiacki SR. Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Res Vet Sci. 2006;80:116. doi: 10.1016/j.rvsc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 164.Larson LJ, Schultz RD. Effect of vaccination with recombinant canine distemper virus vaccine immediately before exposure under shelter-like conditions. Vet Ther. 2006;7:113. [PubMed] [Google Scholar]
- 165.Buonavoglia C, Martella V, Pratelli A. Evidence for evolution of canine parvovirus type-2 in Italy. J Gen Virol. 2001;82:1555. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- 166.Hong C, Decaro N, Desario C. Occurrence of canine parvovirus type 2c in the United States. J Vet Diagn Invest. 2007;19:535. doi: 10.1177/104063870701900512. [DOI] [PubMed] [Google Scholar]
- 167.Kapil S, Cooper E, Lamm C. Canine parvovirus types 2c and 2b circulating in North American dogs in 2006 and 2007. J Clin Microbiol. 2007;45:4044. doi: 10.1128/JCM.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cavalli A, Martella V, Desario C. Evaluation of the antigenic relationships among canine parvovirus type 2 variants. Clin Vaccine Immunol. 2008;15:534. doi: 10.1128/CVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burton JH, Veir JK, Morris AK, et al. Detection of canine parvovirus DNA from blood and feces collected from healthy puppies after administration of a modified live vaccine. Proceedings of the American Association of Veterinary Internal Medicine, San Antonio, TX, June 4–7, 2008 (abstract).
- 170.Decaro N, Desario C, Beall MJ. Detection of canine parvovirus type 2c by a commercially available in-house rapid test. Vet J. 2010;184:373. doi: 10.1016/j.tvjl.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 171.Duffy A, Dow S, Ogilvie G, Rao S, Hackett T. Hematologic improvement in dogs with parvovirus infection treated with recombinant canine granulocyte-colony stimulating factor. J Vet Pharmacol Ther. 2010;33:352. doi: 10.1111/j.1365-2885.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 172.Savigny MR, Macintire DK. Use of oseltamivir in the treatment of canine parvoviral enteritis. J Vet Emerg Crit Care (San Antonio) 2010;20:132. doi: 10.1111/j.1476-4431.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 173.Decaro N, Desario C, Elia G. Evidence for immunisation failure in vaccinated adult dogs infected with canine parvovirus type 2c. New Microbiol. 2008;31:125. [PubMed] [Google Scholar]
- 174.Larson LJ, Schultz RD. Do two current canine parvovirus type 2 and 2b vaccines provide protection against the new type 2c variant? Vet Ther. 2008;9:94. [PubMed] [Google Scholar]
- 175.Spibey N, Greenwood NM, Sutton D. Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet Microbiol. 2008;128:48. doi: 10.1016/j.vetmic.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 176.Greene CE, Addie D. Feline parvovirus infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders; Philadelphia: 2006. p. 78. [Google Scholar]
- 177.Decaro N, Buonavoglia D, Desario C. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Res Vet Sci. 2010;89:275. doi: 10.1016/j.rvsc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakamura K, Sakamoto M, Ikeda Y. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clin Diagn Lab Immunol. 2001;8:663. doi: 10.1128/CDLI.8.3.663-668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fischer SM, Quest CM, Dubovi EJ. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc. 2007;230:52. doi: 10.2460/javma.230.1.52. [DOI] [PubMed] [Google Scholar]
- 180.Abd-Eldaim M, Beall M, Kennedy M. Detection of feline panleukopenia virus using a commercial ELISA for canine parvovirus. Vet Ther. 2009;10:1. [PubMed] [Google Scholar]
- 181.Patterson EV, Reese MJ, Tucker SJ. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359. doi: 10.2460/javma.230.3.359. [DOI] [PubMed] [Google Scholar]
- 182.Gingrich EN, Scorza AV, Leutenegger CM, et al. Common enteric pathogens in cats before and after placement in an animal shelter. Proceedings of the American College of Veterinary Internal Medicine Forum (abstract), Anaheim, CA, 2010.
- 183.Gamoh K, Senda M, Inoue Y, Itoh O. Efficacy of an inactivated feline panleucopenia virus vaccine against a canine parvovirus isolated from a domestic cat. Vet Rec. 2005;157:285. doi: 10.1136/vr.157.10.285. [DOI] [PubMed] [Google Scholar]
- 184.Greene CE. Feline enteric viral infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders/Elsevier; Philadelphia: 2006. p. 103. [Google Scholar]
- 185.Lutz H, Castelli I, Ehrensperger F. Panleukopenia-like syndrome of FeLV caused by co-infection with FeLV and feline panleukopenia virus. Vet Immunol Immunopathol. 1995;46:21–33. doi: 10.1016/0165-2427(94)07003-p. [DOI] [PubMed] [Google Scholar]
Bacterial Overgrowth
- 1.Delles EK, Willard MD, Simpson RB. Comparison of species and numbers of bacteria in concurrently cultured samples of proximal small intestinal fluid and endoscopically obtained duodenal mucosa in dogs with intestinal bacterial overgrowth. Am J Vet Res. 1994;55:957–964. [PubMed] [Google Scholar]
- 2.Suchodolski JS, Ruaux CG, Steiner JM. Application of molecular fingerprinting for qualitative assessment of small-intestinal bacterial diversity in dogs. J Clin Microbiol. 2004;42:4702–4708. doi: 10.1128/JCM.42.10.4702-4708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suchodolski JS, Ruaux CG, Steiner JM. Assessment of the qualitative variation in bacterial microflora among compartments of the intestinal tract of dogs by use of a molecular fingerprinting technique. Am J Vet Res. 2005;66:1556–1562. doi: 10.2460/ajvr.2005.66.1556. [DOI] [PubMed] [Google Scholar]
- 4.Johnston KL, Swift NC, Forster-van Hijfte M. Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc. 2001;218:48–51. doi: 10.2460/javma.2001.218.48. [DOI] [PubMed] [Google Scholar]
- 5.Johnston KL, Lamport A, Batt RM. An unexpected bacterial flora in the proximal small intestine of normal cats. Vet Rec. 1993;132:362–363. doi: 10.1136/vr.132.14.362. [DOI] [PubMed] [Google Scholar]
- 6.Johnston KL. Small intestinal bacterial overgrowth. Vet Clin North Am Small Anim Pract. 1999;29:523–550. [PubMed] [Google Scholar]
- 7.Willard MD, Simpson RB, Delles EK. Effect of dietary supplementation of fructo-oligosaccharides on small intestinal bacterial overgrowth in dogs. Am J Vet Res. 1994;55:654–659. [PubMed] [Google Scholar]
- 8.Sparkes AH, Papasouliotis K, Sunvold G. Bacterial flora in the duodenum of healthy cats, and effect of dietary supplementation with fructooligosaccharides. Am J Vet Res. 1998;59:431–435. [PubMed] [Google Scholar]
- 9.Sparkes AH, Papasouliotis K, Sunvold G. Effect of dietary supplementation with fructooligosaccharides on fecal flora of healthy cats. Am J Vet Res. 1998;59:436–440. [PubMed] [Google Scholar]
- 10.Rastall RA. Bacteria in the gut: friends and foes and how to alter the balance. J Nutr. 2004;134:2022S–2026S. doi: 10.1093/jn/134.8.2022S. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Mahowald MA. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.German AJ, Day MJ, Ruaux CG. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33–43. doi: 10.1892/0891-6640(2003)017<0033:codait>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Batt RM, McLean L. Comparison of the biochemical changes in the jejunal mucosa of dogs with aerobic and anaerobic bacterial overgrowth. Gastroenterology. 1987;93:986–993. doi: 10.1016/0016-5085(87)90560-9. [DOI] [PubMed] [Google Scholar]
- 14.Batt RM, McLean L, Riley JE. Response of the jejunal mucosa of dogs with aerobic and anaerobic bacterial overgrowth to antibiotic therapy. Gut. 1988;29:473–482. doi: 10.1136/gut.29.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papasouliotis K, Sparkes AH, Werret G. Assessment of the bacterial flora of the proximal part of the small intestine in healthy cats, and the effect of sample collection method. Am J Vet Res. 1998;59:48–51. [PubMed] [Google Scholar]
- 16.Westermarck E, Skrzypczak T, Steiner JM. Tylosin-responsive chronic diarrhea in dogs. J Vet Intern Med. 2005;19:177–186. doi: 10.1892/0891-6640(2005)19<177:tcdid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8–20. doi: 10.1892/0891-6640(2003)017<0008:ciiaid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 18.German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 2001;15:14–25. doi: 10.1892/0891-6640(2001)015<0014:icpwtd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 19.German AJ, Hall EJ, Day MJ. Relative deficiency in IgA production by duodenal explants from German shepherd dogs with small intestinal disease. Vet Immunol Immunopathol. 2000;31:25–43. doi: 10.1016/s0165-2427(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 20.Peters IR, Calvert EL, Hall EJ. Measurement of immunoglobulin concentrations in the feces of healthy dogs. Clin Diagn Lab Immunol. 2004;11:841–848. doi: 10.1128/CDLI.11.5.841-848.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters IR, Helps CR, Batt RM. Quantitative real-time RT-PCR measurement of mRNA encoding alpha-chain, pIgR and J-chain from canine duodenal mucosa. J Immunol Methods. 2003;275:213–222. doi: 10.1016/s0022-1759(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 22.Peters IR, Helps CR, Batt RM. Quantitative real-time RT-PCR measurement of mRNA encoding α-chain, pIgR and J-chain from canine duodenal mucosa. J Immunol Methods. 2003;275:213–222. doi: 10.1016/s0022-1759(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 23.Peters IR, Helps CR, Calvert EL. Measurement of messenger RNA encoding the alpha-chain, polymeric immunoglobulin receptor, and J-chain in duodenal mucosa from dogs with and without chronic diarrhea by use of quantitative real-time reverse transcription-polymerase chain reaction assays. Am J Vet Res. 2005;66:11–16. doi: 10.2460/ajvr.2005.66.11. [DOI] [PubMed] [Google Scholar]
- 24.German AJ, Helps CR, Hall EJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 2000;45:7–17. doi: 10.1023/a:1005436721798. [DOI] [PubMed] [Google Scholar]
- 25.Simpson KW, Dogan B, Rishniw M. Adherent and invasive Escherichia coli is associated with granulomatous colitis in Boxer dogs. Infect Immun. 2006;74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonough PL, Simpson KW. Diagnosing emerging bacterial infections: salmonellosis, campylobacteriosis, clostridial toxicosis and helicobacteriosis. Semin Vet Med Surg (Small Anim) 1996;11:187–197. doi: 10.1016/s1096-2867(96)80032-6. [DOI] [PubMed] [Google Scholar]
- 27.Rutgers HC, Batt RM, Elwood CM. Small intestinal bacterial overgrowth in dogs with chronic intestinal disease. J Am Vet Med Assoc. 1995;206:187–193. [PubMed] [Google Scholar]
- 28.Simpson KW, Batt RM, Jones D. Pancreatic function following pancreatectomy and anastomosis of the pancreatic duct to the stomach or duodenum of dogs. Am J Vet Res. 1990;59:203–206. [Google Scholar]
- 29.Papasouliotis K, Sparkes AH, Gruffydd-Jones TJ. Use of the breath hydrogen test to assess the effect of age on orocecal transit time and carbohydrate assimilation in cats. Am J Vet Res. 1998;59:1299–1302. [PubMed] [Google Scholar]
- 30.Bissett SA, Spohr A, Guilford WG. Reproducibility of breath hydrogen concentration measurements in dogs after change of diet. Am J Vet Res. 1998;59:1523–1525. [PubMed] [Google Scholar]
- 31.Bissett SA, Guilford WG, Haslett SJ. Effect of five percent dehydration on breath hydrogen concentrations in dogs. Am J Vet Res. 1998;59:245–249. [PubMed] [Google Scholar]
- 32.Spohr A, Guilford WG, Haslett SJ. Use of breath hydrogen testing to detect experimentally-induced disaccharide malabsorption in healthy adult dogs. Am J Vet Res. 1999;60:836–840. [PubMed] [Google Scholar]
- 33.Melgarejo T, Williams DA, O’Connell NC. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci. 2000;45:407–414. doi: 10.1023/a:1005493416946. [DOI] [PubMed] [Google Scholar]
- 34.Marks SL. Editorial: Small intestinal bacterial overgrowth in dogs—less common than you think? J Vet Intern Med. 2003;17:5–7. [PubMed] [Google Scholar]
Obstruction
- 1.Hayes G. Gastrointestinal foreign bodies in dogs and cats: a retrospective study of 208 cases. J Small Anim Pract. 2009;50:576–583. doi: 10.1111/j.1748-5827.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 2.Cave NJ, Bridges JP, Cogger N. A survey of diseases of working farm dogs in New Zealand. N Z Vet J. 2009;57:305–312. doi: 10.1080/00480169.2009.60926. [DOI] [PubMed] [Google Scholar]
- 3.Barrs VR, Beatty JA, Tisdall PL. Intestinal obstruction by trichobezoars in five cats. J Feline Med Surg. 1999;1:199–207. doi: 10.1053/jfms.1999.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carobbi B, Foale RD, White RA. Trichobezoar obstruction after stapled jejunal anastomosis in a dog. Vet Surg. 2009;38:417–420. doi: 10.1111/j.1532-950X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 5.Capak D, Simpraga M, Maticic D. Incidence of foreign-body-induced ileus in dogs. Berl Munch Tierarztl Wochenschr. 2001;114:290–296. [PubMed] [Google Scholar]
- 6.Felts JF, Fox PR, Burk RL. Thread and sewing needles as gastrointestinal foreign bodies in the cat: a review of 64 cases. J Am Vet Med Assoc. 1984;184:56–59. [PubMed] [Google Scholar]
- 7.Burkitt JM, Drobatz KJ, Saunders HM. Signalment, history, and outcome of cats with gastrointestinal tract intussusception: 20 cases (1986–2000) J Am Vet Med Assoc. 2009;234:771–776. doi: 10.2460/javma.234.6.771. [DOI] [PubMed] [Google Scholar]
- 8.Wilson GP, Burt JK. Intussusception in the dog and cat: a review of 45 cases. J Am Vet Med Assoc. 1974;164:515–518. [PubMed] [Google Scholar]
- 9.Lamb CR, Mantis P. Ultrasonographic features of intestinal intussusception in 10 dogs. J Small Anim Pract. 1998;39:437–441. doi: 10.1111/j.1748-5827.1998.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 10.Reymond RD. The mechanism of intussusception: a theoretical analysis of the phenomenon. Br J Radiol. 1972;45:1–7. doi: 10.1259/0007-1285-45-529-1. [DOI] [PubMed] [Google Scholar]
- 11.Weaver AD. Canine intestinal intussusception. Vet Rec. 1977;100:524–527. doi: 10.1136/vr.100.25.524. [DOI] [PubMed] [Google Scholar]
- 12.Doherty D, Welsh EM, Kirby BM. Intestinal intussusception in five postparturient queens. Vet Rec. 2000;146:614–616. doi: 10.1136/vr.146.21.614. [DOI] [PubMed] [Google Scholar]
- 13.Evermann JF, Abbott JR, Han S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J Vet Diagn Invest. 2005;17:610–614. doi: 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- 14.Patsikas MN, Jakovljevic S, Moustardas N. Ultrasonographic signs of intestinal intussusception associated with acute enteritis or gastroenteritis in 19 young dogs. J Am Anim Hosp Assoc. 2003;39:57–66. doi: 10.5326/0390057. [DOI] [PubMed] [Google Scholar]
- 15.Rallis TS, Papazoglou LG, Adamama-Moraitou KK. Acute enteritis or gastroenteritis in young dogs as a predisposing factor for intestinal intussusception: a retrospective study. J Vet Med A Physiol Pathol Clin Med. 2000;47:507–511. doi: 10.1046/j.1439-0442.2000.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prihoda M, Flatt A, Summers RW. Mechanisms of motility changes during acute intestinal obstruction in the dog. Am J Physiol. 1984;247:G37–G42. doi: 10.1152/ajpgi.1984.247.1.G37. [DOI] [PubMed] [Google Scholar]
- 17.Ohman U. Studies on small intestinal obstruction. I. Intraluminal pressure in experimental low small bowel obstruction in the cat. Acta Chir Scand. 1975;141:413–416. [PubMed] [Google Scholar]
- 18.Mirkovitch V, Cobo F, Robinson JW. Morphology and function of the dog ileum after mechanical occlusion. Clin Sci Mol Med. 1976;50:123–130. doi: 10.1042/cs0500123. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald JA. Smooth muscle phenotypic plasticity in mechanical obstruction of the small intestine. Neurogastroenterol Motil. 2008;20:737–740. doi: 10.1111/j.1365-2982.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 20.Storkholm JH, Zhao J, Villadsen GE. Biomechanical remodeling of the chronically obstructed Guinea pig small intestine. Dig Dis Sci. 2007;52:336–346. doi: 10.1007/s10620-006-9431-7. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira-Barros LM, Costa-Casagrande TA, Cogliati B. Histologic and immunohistochemical evaluation of intestinal innervation in dogs with and without intussusception. Am J Vet Res. 2010;71:636–642. doi: 10.2460/ajvr.71.6.636. [DOI] [PubMed] [Google Scholar]
- 22.Brolin RE, Reddell MT. Gastrointestinal myoelectric activity in mechanical intestinal obstruction. J Surg Res. 1985;38:515–523. doi: 10.1016/0022-4804(85)90070-8. [DOI] [PubMed] [Google Scholar]
- 23.Heneghan JB, Robinson JW, Menge H. Intestinal obstruction in germ-free dogs. Eur J Clin Invest. 1981;11:285–290. doi: 10.1111/j.1365-2362.1981.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 24.Mishra NK, Appert HE, Howard JM. The effects of distention and obstruction on the accumulation of fluid in the lumen of small bowel of dogs. Ann Surg. 1974;180:791–795. doi: 10.1097/00000658-197411000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yale CE, Balish E. Intestinal strangulation in germfree and monocontaminated dogs. Arch Surg. 1979;114:445–448. doi: 10.1001/archsurg.1979.01370280099014. [DOI] [PubMed] [Google Scholar]
- 26.Yale CE, Balish E. The relative lethality of intestinal bacteria for gnotobiotic rats with experimental intestinal strangulation. J Med. 1992;23:265–277. [PubMed] [Google Scholar]
- 27.Summers RW, Kent TH. Effects of altered propulsion on rat small intestinal flora. Gastroenterology. 1970;59:740–744. [PubMed] [Google Scholar]
- 28.El-Awady SI, El-Nagar M, El-Dakar M. Bacterial translocation in an experimental intestinal obstruction model. C-reactive protein reliability? Acta Cir Bras. 2009;24:98–106. doi: 10.1590/s0102-86502009000200005. [DOI] [PubMed] [Google Scholar]
- 29.Sykes PA, Boulter KH, Schofield PF. Alterations in small-bowel microflora in acute intestinal obstruction. J Med Microbiol. 1976;9:13–22. doi: 10.1099/00222615-9-1-13. [DOI] [PubMed] [Google Scholar]
- 30.Galeev YM, Lishmanov YB, Grigorev EG. Scintigraphic visualization of bacterial translocation in experimental strangulated intestinal obstruction. Eur J Nucl Med Mol Imaging. 2009;36:1822–1828. doi: 10.1007/s00259-009-1146-5. [DOI] [PubMed] [Google Scholar]
- 31.Holzer P. Sensory neurone responses to mucosal noxae in the upper gut: relevance to mucosal integrity and gastrointestinal pain. Neurogastroenterol Motil. 2002;14:459–475. doi: 10.1046/j.1365-2982.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 32.Weber E, Neunlist M, Schemann M. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol. 2001;536:741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boag AK, Coe RJ, Martinez TA. Acid-base and electrolyte abnormalities in dogs with gastrointestinal foreign bodies. J Vet Intern Med. 2005;19:816–821. doi: 10.1892/0891-6640(2005)19[816:aaeaid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Mottelib AA, Misk NA. Clinical, blood picture and pathomorphological studies on experimental intestinal obstruction in dogs. Zentralbl Veterinarmed A. 1976;23:600–608. doi: 10.1111/j.1439-0442.1976.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 35.Yuki M, Sugimoto N, Takahashi K. Enterolithiasis in a cat. J Feline Med Surg. 2006;8:349–352. doi: 10.1016/j.jfms.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson PB, Willard MD. Protein-losing enteropathies. Vet Clin North Am Small Anim Pract. 2003;33:1061–1082. doi: 10.1016/s0195-5616(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 37.Lanz OI, Ellison GW, Bellah JR. Surgical treatment of septic peritonitis without abdominal drainage in 28 dogs. J Am Anim Hosp Assoc. 2001;37:87–92. doi: 10.5326/15473317-37-1-87. [DOI] [PubMed] [Google Scholar]
- 38.Staatz AJ, Monnet E, Seim HB., 3rd Open peritoneal drainage versus primary closure for the treatment of septic peritonitis in dogs and cats: 42 cases (1993–1999) Vet Surg. 2002;31:174–180. doi: 10.1053/jvet.2002.31043. [DOI] [PubMed] [Google Scholar]
- 39.Guilford WG, Strombeck DR. Intestinal obstruction, pseudo-obstruction, and foreign bodies. In: Guilford WG, Center SA, Strombeck DR, editors. Strombek's Small Animal Gastroenterology. WB Saunders; Philadelphia: 1996. pp. 487–502. [Google Scholar]
- 40.Graham JP, Lord PF, Harrison JM. Quantitative estimation of intestinal dilation as a predictor of obstruction in the dog. J Small Anim Pract. 1998;39:521–524. doi: 10.1111/j.1748-5827.1998.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 41.Bradley K. The small intestine. In: O’Brien R, Barr FJ, editors. BSAVA Manual of Canine and Feline Abdominal Imaging. British Small Animal Veterinary Association; Quedgeley, UK: 2009. p. 110. [Google Scholar]
- 42.Robertson ID, Burbidge HM. Pros and cons of barium-impregnated polyethylene spheres in gastrointestinal disease. Vet Clin North Am Small Anim Pract. 2000;30:449–465. viii. [PubMed] [Google Scholar]
- 43.Penninck DG. Gastrointestinal tract. In: Nyland TG, Maltoon JS, editors. Small Animal Diagnostic Ultrasound. Saunders; Philadelphia: 1995. pp. 207–230. [Google Scholar]
- 44.Patsikas MN, Papazoglou LG, Papaioannou NG. Ultrasonographic findings of intestinal intussusception in seven cats. J Feline Med Surg. 2003;5:335–343. doi: 10.1016/S1098-612X(03)00066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fossum TW. Soft Tissue surgery. In: Fossum TW, editor. Small Animal Surgery. ed 3. Mosby; St Louis: 2007. pp. 604–629. [Google Scholar]
- 46.Patsikas MN, Papazoglou LG, Adamama-Moraitou KK. Spontaneous reduction of intestinal intussusception in five young dogs. J Am Anim Hosp Assoc. 2008;44:41–47. doi: 10.5326/0440041. [DOI] [PubMed] [Google Scholar]
- 47.Allen D, Jr, Kvietys PR, Granger DN. Crystalloids versus colloids: implications in fluid therapy of dogs with intestinal obstruction. Am J Vet Res. 1986;47:1751–1755. [PubMed] [Google Scholar]
- 48.Haddy FJ, Scott JB, Emerson TE., Jr Effects of generalized changes in plasma electrolyte concentration and osmolarity on blood pressure in the anesthetized dog. Circ Res. 1969;24(Suppl):59–74. [PubMed] [Google Scholar]
- 49.Chen JZ, Deng AW, Xu JF. Electroenterogram manifestations and significance in hypokalemia. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:7–9. [PubMed] [Google Scholar]
- 50.Carroll J, Alavi K. Pathogenesis and management of postoperative ileus. Clin Colon Rectal Surg. 2009;22:47–50. doi: 10.1055/s-0029-1202886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalili TM, Navarro RA, Middleton Y. Early postoperative enteral feeding increases anastomotic strength in a peritonitis model. Am J Surg. 2001;182:621–624. doi: 10.1016/s0002-9610(01)00818-2. [DOI] [PubMed] [Google Scholar]
- 52.Zaloga GP, Bortenschlager L, Black KW. Immediate postoperative enteral feeding decreases weight loss and improves wound healing after abdominal surgery in rats. Crit Care Med. 1992;20:115–118. doi: 10.1097/00003246-199201000-00023. [DOI] [PubMed] [Google Scholar]
- 53.Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. 2005;29:1023–1027. doi: 10.1007/s00268-005-7491-z. discussion 1027–1028. [DOI] [PubMed] [Google Scholar]
- 54.Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. J Am Coll Surg. 1998;187:142–146. doi: 10.1016/s1072-7515(98)00154-9. [DOI] [PubMed] [Google Scholar]
- 55.Ralphs SC, Jessen CR, Lipowitz AJ. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991–2000) J Am Vet Med Assoc. 2003;223:73–77. doi: 10.2460/javma.2003.223.73. [DOI] [PubMed] [Google Scholar]
- 56.Shi XZ, Sarna SK. G protein-mediated dysfunction of excitation-contraction coupling in ileal inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G899–G905. doi: 10.1152/ajpgi.00408.2003. [DOI] [PubMed] [Google Scholar]
- 57.Hotokezaka M, Combs MJ, Mentis EP. Recovery of fasted and fed gastrointestinal motility after open versus laparoscopic cholecystectomy in dogs. Ann Surg. 1996;223:413–419. doi: 10.1097/00000658-199604000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed (traditional) oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004508.pub3. CD004508. [DOI] [PubMed] [Google Scholar]
- 59.Lippert AC, Faulkner JE, Evans AT. Total parenteral nutrition in clinically normal cats. J Am Vet Med Assoc. 1989;194:669–676. [PubMed] [Google Scholar]
- 60.Renegar KB, Johnson CD, Dewitt RC. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166:819–825. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 61.Thor PJ, Copeland EM, Dudrick SJ. Effect of long-term parenteral feeding on gastric secretion in dogs. Am J Physiol. 1977;232:E39–E43. doi: 10.1152/ajpendo.1977.232.1.E39. [DOI] [PubMed] [Google Scholar]
- 62.Graves GM, Becht JL, Rawlings CA. Metoclopramide reversal of decreased gastrointestinal myoelectric and contractile activity in a model of canine postoperative ileus. Vet Surg. 1989;18:27–33. doi: 10.1111/j.1532-950x.1989.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 63.Allen DA, Smeak DD, Schertel ER. Prevalence of small intestinal dehiscence and associated clinical factors: a retrospective study of 121 dogs. J Am Anim Hosp Assoc. 1992;28:70–76. [Google Scholar]
- 64.Bedford PN. Partial intestinal obstruction due to colonic adenocarcinoma in a cat. Can Vet J. 1998;39:769–771. [PMC free article] [PubMed] [Google Scholar]
- 65.Cairo J, Font J, Gorraiz J. Intestinal volvulus in dogs: a study of four clinical cases. J Small Anim Pract. 1999;40:136–140. doi: 10.1111/j.1748-5827.1999.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 66.Coolman BR, Marretta SM, Dudley MB. Partial colonic obstruction following ovariohysterectomy: a report of three cases. J Am Anim Hosp Assoc. 1999;35:169–172. doi: 10.5326/15473317-35-2-169. [DOI] [PubMed] [Google Scholar]
- 67.Dvir E, Leisewitz AL, Van der Lugt JJ. Chronic idiopathic intestinal pseudo-obstruction in an English bulldog. J Small Anim Pract. 2001;42:243–247. doi: 10.1111/j.1748-5827.2001.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 68.Gaskell CJ, Pass MA, Biery DN. Intestinal obstruction in a dog due to incarceration of small intestine in a coccygeal fracture. J Small Anim Pract. 1973;14:101–105. doi: 10.1111/j.1748-5827.1973.tb06899.x. [DOI] [PubMed] [Google Scholar]
- 69.Hassinger KA. Intestinal entrapment and strangulation caused by rupture of the duodenocolic ligament in four dogs. Vet Surg. 1997;26:275–280. doi: 10.1111/j.1532-950x.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 70.Johnson CS, Fales-Williams AJ, Reimer SB. Fibrosing gastrointestinal leiomyositis as a cause of chronic intestinal pseudo-obstruction in an 8-month-old dog. Vet Pathol. 2007;44:106–109. doi: 10.1354/vp.44-1-106. [DOI] [PubMed] [Google Scholar]
- 71.Johnson R. Intestinal atresia and stenosis: a review comparing its etiopathogenesis. Vet Res Commun. 1986;10:95–104. doi: 10.1007/BF02213972. [DOI] [PubMed] [Google Scholar]
- 72.Kammori M, Mafune K, Hirashima T. Forty-three cases of obturator hernia. Am J Surg. 2004;187:549–552. doi: 10.1016/j.amjsurg.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 73.Kuan SY, Ticehurst K, Hoffmann KL. Intestinal strangulation after elective ovariohysterectomy. J Feline Med Surg. 2010;12:325–329. doi: 10.1016/j.jfms.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liljebjelke KA, Abramson C, Brockus C. Duodenal obstruction caused by infection with Pythium insidiosum in a 12-week-old puppy. J Am Vet Med Assoc. 2002;220:1188–1191. doi: 10.2460/javma.2002.220.1188. 1162. [DOI] [PubMed] [Google Scholar]
- 75.Moore R, Carpenter J. Intramural intestinal hematoma causing obstruction in three dogs. J Am Vet Med Assoc. 1984;184:186–188. [PubMed] [Google Scholar]
- 76.Papazoglou LG, Tontis D, Loukopoulos P. Foreign body-associated intestinal pyogranuloma resulting in intestinal obstruction in four dogs. Vet Rec. 2009;166:494–497. doi: 10.1136/vr.b4809. [DOI] [PubMed] [Google Scholar]
- 77.Parry-Smith P, Czerwinska M, Krudewig C. Duodenal duplication cyst in a young cat. Vet Rec. 2008;162:826–827. doi: 10.1136/vr.162.25.826. [DOI] [PubMed] [Google Scholar]
- 78.Rakich PM, Grooters AM, Tang KN. Gastrointestinal pythiosis in two cats. J Vet Diagn Invest. 2005;17:262–269. doi: 10.1177/104063870501700310. [DOI] [PubMed] [Google Scholar]
- 79.Shealy PM, Henderson RA. Canine intestinal volvulus. A report of nine new cases. Vet Surg. 1992;21:15–19. doi: 10.1111/j.1532-950x.1992.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 80.Wilcox RS, Bowman DD, Barr SC. Intestinal obstruction caused by Taenia taeniaeformis infection in a cat. J Am Anim Hosp Assoc. 2009;45:93–96. doi: 10.5326/0450093. [DOI] [PubMed] [Google Scholar]
Dysmotility
- 1.Weisbrodt NW. Motility of the small intestine. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. ed 2. Raven Press; New York: 1987. pp. 631–664. [Google Scholar]
- 2.Mishra NK, Appert HE, Howard JM. The effects of distention and obstruction on the accumulation of fluid in the lumen of small bowel of dogs. Ann Surg. 1974;180:791–795. doi: 10.1097/00000658-197411000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vos WC. Migrating spike complex in the intestine of the fasting cat. Am J Physiol. 1993;265:G619–G627. doi: 10.1152/ajpgi.1993.265.4.G619. [DOI] [PubMed] [Google Scholar]
- 4.de Vos WC. Role of the enteric nervous system in the control of migrating spike complex in the feline intestine. Am J Physiol. 1993;265:G628–G637. doi: 10.1152/ajpgi.1993.265.4.G628. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer S, Sharkey M, Mealey K. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008;10:110–119. doi: 10.1208/s12248-008-9011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Bueno L, de Ponti F. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology. 2006;130:1321–1334. doi: 10.1053/j.gastro.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313–1332. doi: 10.3748/wjg.v13.i9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berghaus RD, O’Brien DP, Johnson GC. Risk factors for development of dysautonomia in dogs. J Am Vet Med Assoc. 2001;218:1285–1290. doi: 10.2460/javma.2001.218.1285. [DOI] [PubMed] [Google Scholar]
- 9.Detweiler DA, Biller DS, Hoskinson JJ. Radiographic findings of canine dysautonomia in twenty-four dogs. Vet Radiol Ultrasound. 2001;42:108–112. doi: 10.1111/j.1740-8261.2001.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 10.Harkin KR, Andrews GA, Nietfeld JC. Dysautonomia in dogs: 65 cases (1993–2000) J Am Vet Med Assoc. 2002;220:633–639. doi: 10.2460/javma.2002.220.633. [DOI] [PubMed] [Google Scholar]
- 11.Harkin KR, Nietfeld J, Fischer JR. Dysautonomia in a family of German Shorthaired Pointers. J Am Anim Hosp Assoc. 2002;38:55–59. doi: 10.5326/0380055. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien DP, Johnson GC. Dysautonomia and autonomic neuropathies. Vet Clin North Am Small Anim Pract. 2002;32:251–265. doi: 10.1016/s0195-5616(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 13.Sharp NJH. Feline dysautonomia. Semin Vet Med Surg (Small Anim) 1990;5:67–71. [PubMed] [Google Scholar]
- 14.Kidder AC, Johannes C, O’Brien DP. Feline dysautonomia in the Midwestern United States: a retrospective study of nine cases. J Feline Med Surg. 2007;10:130–136. doi: 10.1016/j.jfms.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cave TA, Knottenbelt C, Mellor DJ. Outbreak of dysautonomia in a closed colony of pet cats. Vet Rec. 2003;153:387–392. [PubMed] [Google Scholar]
- 16.Novellas R, Simpson KE, Gunn-Moore DA. Imaging findings in 11 cats with dysautonomia. J Feline Med Surg. 2010;12:584–591. doi: 10.1016/j.jfms.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 18.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138:206–214. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 19.Shi XZ, Lin SY-M, Powell DW. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G99–G108. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeHaven-Hudkins DL, DeHaven R, Little PJ. The involvement of the µ-opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008;117:162–187. doi: 10.1016/j.pharmthera.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Becker G, Bluim HE. Novel opioid antagonists for opioid-induced bowel dysfunction and post-operative ileus. Lancet. 2009;373:1198–1206. doi: 10.1016/S0140-6736(09)60139-2. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Song GQ, Yin J. Effects and mechanisms of gastrointestinal electrical stimulation on slow waves: a systematic canine study. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1392–R1399. doi: 10.1152/ajpregu.00006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huibin Q, Chen JDZ. Effects of intestinal electrical stimulation on postprandial small-bowel motility and transit in dogs. Am J Surg. 2006;192:e55–e60. doi: 10.1016/j.amjsurg.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Yin J, Chen JDZ. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1190–G1195. doi: 10.1152/ajpgi.00092.2007. [DOI] [PubMed] [Google Scholar]
- 25.Yin J, Chen JDZ. Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci. 2010;55:1208–1220. doi: 10.1007/s10620-009-0884-3. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Lei Y, Chen JDZ. Effects and mechanisms of electrical stimulation of the stomach, duodenum, ileum, and colon on gastric tone in dogs. Dig Dis Sci. 2010;55:895–901. doi: 10.1007/s10620-009-0830-4. [DOI] [PubMed] [Google Scholar]
- 27.Todo S, Tzakis A, Abu-Elmagd K. Current status of intestinal transplantation. Adv Surg. 1994;27:295–316. [PMC free article] [PubMed] [Google Scholar]
- 28.Bines JE. Intestinal failure: a new era in clinical management. J Gastroenterol Hepatol. 2009;24:S86–S92. doi: 10.1111/j.1440-1746.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- 29.Belkind-Gerson J, Graeme-Cook F, Winter H. Enteric nervous system disease and recovery, plasticity, and regeneration. J Pediatr Gastroenterol Nutr. 2006;42:343–350. doi: 10.1097/01.mpg.0000218696.58487.5b. [DOI] [PubMed] [Google Scholar]
- 30.Thomas J. Opioid-induced bowel dysfunction. J Pain Symptom Manage. 2008;35:103–113. doi: 10.1016/j.jpainsymman.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Becker G, Blum HE. Novel opioid antagonists for opioid-induced bowel dysfunction and post-operative ileus. Lancet. 2009;373:1198–1206. doi: 10.1016/S0140-6736(09)60139-2. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda H, Suenaga K, Tsuchida D. The selective mu opioid receptor antagonist, alvimopan, improves delayed GI transit of postoperative ileus in rats. Brain Res. 2006 doi: 10.1016/j.brainres.2006.02.092. [DOI] [PubMed] [Google Scholar]
- 33.Yuan C-S. Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects. Ann Pharmacother. 2007;41:984–993. doi: 10.1345/aph.1K009. [DOI] [PubMed] [Google Scholar]
- 34.Viscusi ER, Gan TJ, Leslie JB. Peripherally acting mu-opioid receptor antagonists and postoperative ileus: mechanisms of action and clinical applicability. Anesth Analg. 2009;108:1811–1822. doi: 10.1213/ane.0b013e31819e0d3a. [DOI] [PubMed] [Google Scholar]
- 35.Leslie JB. Alvimopan for the management of postoperative ileus. Ann Pharmacother. 2005;39:1502–1510. doi: 10.1345/aph.1E615. [DOI] [PubMed] [Google Scholar]
- 36.Washabau RJ. Gastrointestinal motility disorders and GI prokinetic therapy. In: Willard MD, editor. Veterinary Clinics of North America. Saunders; Philadelphia: 2003. pp. 1007–1028. [DOI] [PubMed] [Google Scholar]
- 37.Nakayoshi T, Kawasaki N, Suzuki Y. Epidural administration of morphine facilitates time of appearance of first gastric interdigestive migrating complex in dogs with paralytic ileus after open abdominal surgery. J Gastrointest Surg. 2007;11:648–654. doi: 10.1007/s11605-006-0065-z. [DOI] [PubMed] [Google Scholar]
- 38.Iyazato LG, Beretta DC, Engracia-Filho JR. Involvement of organic systems in Golden Retriever X-linked muscular dystrophy. Braz J Vet Path. 2011;4:87–94. [Google Scholar]
- 39.Bellini M, Biagi SG, Stasi C. Gastrointestinal manifestations in myotonic muscular dystrophy. World J Gastroenterol. 2006;12:1821–1828. doi: 10.3748/wjg.v12.i12.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettini G, Muracchini M, Della Salda L. Hypertrophy of intestinal smooth muscle in cats. Res Vet Sci. 2003;75:43–53. doi: 10.1016/s0034-5288(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 41.Stander N, Wagner WM, Goddard A. Normal canine pediatric gastrointestinal ultriasonography. Vet Radiol Ultrasound. 2010;51:69–74. doi: 10.1111/j.1740-8261.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- 42.Summers RW, Glenn CE, Flatt AJ. Does irradiation produce irreversible changes in canine jejunal myoelectric activity? Dig Dis Sci. 1992;27:716–722. doi: 10.1007/BF01296428. [DOI] [PubMed] [Google Scholar]
- 43.Otterson MF. Effects of radiation upon gastrointestinal motility. World J Gastroenterol. 2007;13:2684–2692. doi: 10.3748/wjg.v13.i19.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey AM, Hall EJ, Day MJ. Chronic intestinal pseudo-obstruction in a cat caused by visceral myopathy. J Vet Intern Med. 2005;19:111–114. doi: 10.1892/0891-6640(2005)19<111:cipiac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Couraud L, Jermyn K, Yam PS. Intestinal pseudo-obstruction, lymphocytic leiomyositis and atrophy of the muscularis externa in a dog. Vet Rec. 2006;159:86–87. doi: 10.1136/vr.159.3.86. [DOI] [PubMed] [Google Scholar]
- 46.Johnson CS, Fales-Williams AJ, Reimer SB. Fibrosing gastrointestinal leiomyositis as a cause of chronic intestinal pseudo-obstruction in an 8-month old dog. Vet Pathol. 2007;44:106–109. doi: 10.1354/vp.44-1-106. [DOI] [PubMed] [Google Scholar]
- 47.Eastwood JM, McInnes EF, White RN. Caecal impaction and chronic intestinal pseudo-obstruction in a dog. J Vet Med A Physiol Pathol Clin Med. 2005;52:43–44. doi: 10.1111/j.1439-0442.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- 48.Lamb WA, France MP. Chronic intestinal pseudo-obstruction in a dog. Aust Vet J. 1994;71:84–86. doi: 10.1111/j.1751-0813.1994.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 49.Dvir E, Leisewitz AL, Van Der Lugt JJ. Chronic idiopathic intestinal pseudo-obstruction in an English bulldog. J Soc Adm Pharm. 2001;42:243–252. doi: 10.1111/j.1748-5827.2001.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 50.Daher R, Yazbeck T, Jaoude JB. Consequences of dysthyroidism on the digestive tract and viscera. World J Gastroenterol. 2009;21:2834–2838. doi: 10.3748/wjg.15.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neoplasia
- 1.Schlotthauer CF, Grindlay JH. Carcinoma of rectum of a dog treated by surgical removal of rectum. North Am Vet. 1951;32:171. [PubMed] [Google Scholar]
- 2.Patnaik AK, Liu SK, Johnson GF. Feline intestinal adenocarcinoma. A clinicopathologic study of 22 cases. Vet Pathol. 1976;13:1. doi: 10.1177/030098587601300101. [DOI] [PubMed] [Google Scholar]
- 3.Engle GG, Brodey RS. A retrospective study of 395 feline neoplasms. J Am Anim Hosp Assoc. 1969;5:21. [Google Scholar]
- 4.Dorn CR, Taylor DO, Schneider R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307. [PubMed] [Google Scholar]
- 5.Bastianello SS. A survey on neoplasia in domestic species over a 40-year period from 1935 to 1974 in the Republic of South Africa. VI. Tumours occurring in dogs. Onderstepoort J Vet Res. 1983;50:199. [PubMed] [Google Scholar]
- 6.Bastianello SS. A survey of neoplasia in domestic species over a 40-year period from 1935 to 1974 in the Republic of South Africa. V. Tumours occurring in the cat. Onderstepoort J Vet Res. 1983;50:105. [PubMed] [Google Scholar]
- 7.Dobson JM, Samuel S, Milstein H. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Cotchin E. Some tumours of dogs and cats of comparative veterinary and human interest. Vet Rec. 1959;71:1040. [Google Scholar]
- 9.Kosovsky JE, Matthiesen DT, Patnaik AK. Small intestinal adenocarcinoma in cats: 32 cases (1978–1985) J Am Vet Med Assoc. 1988;192:233. [PubMed] [Google Scholar]
- 10.Patnaik AK, Hurvitz AI, Johnson GF. Canine intestinal adenocarcinoma and carcinoid. Vet Pathol. 1980;17:149. doi: 10.1177/030098588001700204. [DOI] [PubMed] [Google Scholar]
- 11.Slawienski MJ, Mauldin GE, Mauldin GN. Malignant colonic neoplasia in cats: 46 cases (1990–1996) J Am Vet Med Assoc. 1997;211:878. [PubMed] [Google Scholar]
- 12.Crawshaw J, Berg J, Sardinas JC. Prognosis for dogs with nonlymphomatous, small intestinal tumors treated by surgical excision. J Am Anim Hosp Assoc. 1998;34:45. doi: 10.5326/15473317-34-6-451. [DOI] [PubMed] [Google Scholar]
- 13.Paoloni MC, Penninck DG, Moore AS. Ultrasonographic and clinicopathologic findings in 21 dogs with intestinal adenocarcinoma. Vet Radiol Ultrasound. 2002;43:562. doi: 10.1111/j.1740-8261.2002.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik AK, Hurvitz AI, Johnson GF. Canine gastrointestinal neoplasms. Vet Pathol. 1977;14:547. doi: 10.1177/030098587701400602. [DOI] [PubMed] [Google Scholar]
- 15.Kapatkin AS, Mullen HS, Matthiesen DT. Leiomyosarcoma in dogs: 44 cases (1983–1988) J Am Vet Med Assoc. 1992;201:1077. [PubMed] [Google Scholar]
- 16.Cohen M, Post GS, Wright JC. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107. doi: 10.1892/0891-6640(2003)017<0107:glid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Head KW, Cullen JM, Dubielzig RR. Histological classification of tumors of the intestines in domestic animals. In: Head KW, Cullen JM, Dubielzig RR, editors. Histological Classifications of Tumors of the Alimentary System of Domestic Animals. ed 2. Armed Forces Institute of Pathology; Washington, DC: 2003. p. 87. [Google Scholar]
- 18.Louwerens M, London CA, Pedersen NC. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Mooney SC, Hayes AA, MacEwen EG. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981) J Am Vet Med Assoc. 1989;194:696. [PubMed] [Google Scholar]
- 20.Richter KP. Feline gastrointestinal lymphoma. Vet Clin North Am Small Anim Pract. 2003;33:1083. doi: 10.1016/s0195-5616(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 21.Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J. 1998;76:725. doi: 10.1111/j.1751-0813.1998.tb12300.x. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald JM, Mullen HS, Moroff SD. Adenomatous polyps of the duodenum in cats: 18 cases (1985–1990) J Am Vet Med Assoc. 1993;202:647. [PubMed] [Google Scholar]
- 23.Carreras JK, Goldschmidt M, Lamb M. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000) J Vet Intern Med. 2003;17:326. doi: 10.1111/j.1939-1676.2003.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 24.Couto CG, Rutgers HC, Sherding RG. Gastrointestinal lymphoma in 20 dogs. A retrospective study. J Vet Intern Med. 1989;3:73. doi: 10.1111/j.1939-1676.1989.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 25.Frost D, Lasota J, Miettinen M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42. doi: 10.1354/vp.40-1-42. [DOI] [PubMed] [Google Scholar]
- 26.Penninck DG. Characterization of gastrointestinal tumors. Vet Clin North Am Small Anim Pract. 1998;28:777. doi: 10.1016/s0195-5616(98)50078-2. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MR, Frommelt RA, Dunn DG. A study of the lifetime occurrence of neoplasia and breed differences in a cohort of German shepherd dogs and Belgian Malinois military working dogs that died in 1992. J Vet Intern Med. 2000;14:140. doi: 10.1892/0891-6640(2000)014<0140:asotlo>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Turk MA, Gallina AM, Russell TS. Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol. 1981;18:614. doi: 10.1177/030098588101800506. [DOI] [PubMed] [Google Scholar]
- 29.Gabor LJ, Love DN, Malik R. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Aust Vet J. 2001;79:540. doi: 10.1111/j.1751-0813.2001.tb10742.x. [DOI] [PubMed] [Google Scholar]
- 30.Gabor LJ, Jackson ML, Trask B. Feline leukaemia virus status of Australian cats with lymphosarcoma. Aust Vet J. 2001;79:476. doi: 10.1111/j.1751-0813.2001.tb13017.x. [DOI] [PubMed] [Google Scholar]
- 31.Levy LS, Starkey CR, Prabhu S. Cooperating events in lymphomagenesis mediated by feline leukemia virus. Leukemia. 1997;11(Suppl 3):239. [PubMed] [Google Scholar]
- 32.Hardy WD, Jr, McClelland AJ. Feline leukemia virus. Its related diseases and control. Vet Clin North Am. 1977;7:93. doi: 10.1016/s0091-0279(77)50008-1. [DOI] [PubMed] [Google Scholar]
- 33.Jackson ML, Haines DM, Meric SM. Feline leukemia virus detection by immunohistochemistry and polymerase chain reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma. Can J Vet Res. 1993;57:269. [PMC free article] [PubMed] [Google Scholar]
- 34.Vail DM, Moore AS, Ogilvie GK. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med. 1998;12:349. doi: 10.1111/j.1939-1676.1998.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 35.Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:281. doi: 10.1016/j.bpg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lee DS, Moss SF. Targeting Helicobacter pylori in gastric carcinogenesis. Expert Opin Ther Targets. 2007;11:757. doi: 10.1517/14728222.11.6.757. [DOI] [PubMed] [Google Scholar]
- 37.Fry DR, Slocombe RF, Beck C. Gastric lymphoma associated with the presence of Helicobacter in a cat. Aust Vet Pract. 2003;33:126. [Google Scholar]
- 38.Yamazaki Y, Aono I, Ohya T. Gastroduodenal adenocarcinomas and rectal adenoma in a cougar (Felis concolor) infected with Helicobacter-like organisms and spirochetes. J Vet Med Sci. 2002;64:149. doi: 10.1292/jvms.64.149. [DOI] [PubMed] [Google Scholar]
- 39.Perkins SE, Yan LL, Shen Z. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy. Antimicrob Agents Chemother. 1996;40:1486. doi: 10.1128/aac.40.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox JG, Shen Z, Xu S. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J Clin Microbiol. 2002;40:2513. doi: 10.1128/JCM.40.7.2513-2519.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coyle KA, Steinberg H. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet Pathol. 2004;41:141. doi: 10.1354/vp.41-2-141. [DOI] [PubMed] [Google Scholar]
- 42.French RA, Seitz SE, Valli VE. Primary epitheliotropic alimentary T-cell lymphoma with hepatic involvement in a dog. Vet Pathol. 1996;33:349. doi: 10.1177/030098589603300315. [DOI] [PubMed] [Google Scholar]
- 43.Gabor LJ, Canfield PJ, Malik R. Immunophenotypic and histological characterisation of 109 cases of feline lymphosarcoma. Aust Vet J. 1999;77:436. doi: 10.1111/j.1751-0813.1999.tb12085.x. [DOI] [PubMed] [Google Scholar]
- 44.Krecic MR, Black SS. Epitheliotropic T-cell gastrointestinal tract lymphosarcoma with metastases to lung and skeletal muscle in a cat. J Am Vet Med Assoc. 2000;216:524. doi: 10.2460/javma.2000.216.524. [DOI] [PubMed] [Google Scholar]
- 45.Mahony OM, Moore AS, Cotter SM. Alimentary lymphoma in cats: 28 cases (1988–1993) J Am Vet Med Assoc. 1995;207:1593. [PubMed] [Google Scholar]
- 46.McEntee MF, Horton S, Blue J. Granulated round cell tumor of cats. Vet Pathol. 1993;30:195. doi: 10.1177/030098589303000213. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki K, Yamagami T, Nomura K. T-cell lymphoma with eosinophilic infiltration involving the intestinal tract in 11 dogs. Vet Pathol. 2006;43:339. doi: 10.1354/vp.43-3-339. [DOI] [PubMed] [Google Scholar]
- 48.Roccabianca P, Vernau W, Caniatti M. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8(alpha)(alpha) phenotype. Vet Pathol. 2006;43:15. doi: 10.1354/vp.43-1-15. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg H, Dubielzig RR, Thomson J. Primary gastrointestinal lymphosarcoma with epitheliotropism in three Shar-Pei and one Boxer dog. Vet Pathol. 1995;32:423. doi: 10.1177/030098589503200413. [DOI] [PubMed] [Google Scholar]
- 50.Jackson ML, Wood SL, Misra V. Immunohistochemical identification of B and T lymphocytes in formalin-fixed, paraffin-embedded feline lymphosarcomas: relation to feline leukemia virus status, tumor site, and patient age. Can J Vet Res. 1996;60:199. [PMC free article] [PubMed] [Google Scholar]
- 51.Zwahlen CH, Lucroy MD, Kraegel SA. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997) J Am Vet Med Assoc. 1998;213:1144. [PubMed] [Google Scholar]
- 52.McPherron MA, Chavkin MJ, Powers BE. Globule leukocyte tumor involving the small intestine in a cat. J Am Vet Med Assoc. 1994;204:241. [PubMed] [Google Scholar]
- 53.Kariya K, Konno A, Ishida T. Perforin-like immunoreactivity in four cases of lymphoma of large granular lymphocytes in the cat. Vet Pathol. 1997;34:156. doi: 10.1177/030098589703400210. [DOI] [PubMed] [Google Scholar]
- 54.Wellman ML, Hammer AS, DiBartola SP. Lymphoma involving large granular lymphocytes in cats: 11 cases (1982–1991) J Am Vet Med Assoc. 1992;201:1265. [PubMed] [Google Scholar]
- 55.Birchard SJ, Couto CG, Johnson S. Nonlymphoid intestinal neoplasia in 32 dogs and 14 cats. J Am Anim Hosp Assoc. 1986;22:533. [Google Scholar]
- 56.Cribb AE. Feline gastrointestinal adenocarcinoma: a review and retrospective study. Can Vet J. 1988;29:709. [PMC free article] [PubMed] [Google Scholar]
- 57.Esplin DG, Wilson SR. Gastrointestinal adenocarcinomas metastatic to the testes and associated structures in three dogs. J Am Anim Hosp Assoc. 1998;34:287. doi: 10.5326/15473317-34-4-287. [DOI] [PubMed] [Google Scholar]
- 58.Juopperi TA, Cesta M, Tomlinson L. Extensive cutaneous metastases in a dog with duodenal adenocarcinoma. Vet Clin Pathol. 2003;32:88. doi: 10.1111/j.1939-165x.2003.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 59.Eckerlin RH. Ileal polypoid leiomyoma in a dog. J Am Vet Med Assoc. 1975;167:70. [PubMed] [Google Scholar]
- 60.Watson JG. Surgical removal of leiomyoma of the small intestine of the dog. Vet Rec. 1970;86:125. doi: 10.1136/vr.86.5.125. [DOI] [PubMed] [Google Scholar]
- 61.Weller RE, O’Brien E. Intestinal leiomyosarcoma in a dog. Mod Vet Pract. 1979;60:621. [PubMed] [Google Scholar]
- 62.LaRock RG, Ginn PE. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol. 1997;34:303. doi: 10.1177/030098589703400406. [DOI] [PubMed] [Google Scholar]
- 63.Maas CP, ter Haar G, van der Gaag I, Kirpensteijn J. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation. Vet Surg. 2007;36:302. doi: 10.1111/j.1532-950X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 64.Russell KN, Mehler SJ, Skorupski KA. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990–2003) J Am Vet Med Assoc. 2007;230:1329. doi: 10.2460/javma.230.9.1329. [DOI] [PubMed] [Google Scholar]
- 65.Sykes GP, Cooper BJ. Canine intestinal carcinoids. Vet Pathol. 1982;19:120. doi: 10.1177/030098588201900203. [DOI] [PubMed] [Google Scholar]
- 66.Sako T, Uchida E, Okamoto M. Immunohistochemical evaluation of a malignant intestinal carcinoid in a dog. Vet Pathol. 2003;40:212. doi: 10.1354/vp.40-2-212. [DOI] [PubMed] [Google Scholar]
- 67.Howl JH, Petersen MG. Intestinal mast cell tumor in a cat: presentation as eosinophilic enteritis. J Am Anim Hosp Assoc. 1995;31:457. doi: 10.5326/15473317-31-6-457. [DOI] [PubMed] [Google Scholar]
- 68.Iwata N, Ochiai K, Kadosawa T. Canine extracutaneous mast-cell tumours consisting of connective tissue mast cells. J Comp Pathol. 2000;123:306. doi: 10.1053/jcpa.2000.0420. [DOI] [PubMed] [Google Scholar]
- 69.Patnaik AK, Twedt DC, Marretta SM. Intestinal mast cell tumour in a dog. J Small Anim Pract. 1980;21:207. doi: 10.1111/j.1748-5827.1980.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi T, Kadosawa T, Nagase M. Visceral mast cell tumors in dogs: 10 cases (1982–1997) J Am Vet Med Assoc. 2000;216:222. doi: 10.2460/javma.2000.216.222. [DOI] [PubMed] [Google Scholar]
- 71.Alroy J, Leav I, DeLellis RA. Distinctive intestinal mast cell neoplasms of domestic cats. Lab Invest. 1975;33:159. [PubMed] [Google Scholar]
- 72.Barrs VR, Beatty JA, McCandlish IA. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J Small Anim Pract. 2002;43:401. doi: 10.1111/j.1748-5827.2002.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson MW, Helfand SC, Smedes SL. Primary IgG secreting plasma cell tumor in the gastrointestinal tract of a dog. J Am Vet Med Assoc. 1994;204:404. [PubMed] [Google Scholar]
- 74.Bergman PJ. Paraneoplastic syndromes. In: Withrow SJ, Vail DM, editors. Small Animal Clinical Oncology. ed 4. Elsevier Saunders; St Louis: 2007. p. 77. [Google Scholar]
- 75.Cohen M, Post GS. Nephrogenic diabetes insipidus in a dog with intestinal leiomyosarcoma. J Am Vet Med Assoc. 1999;215:1818. [PubMed] [Google Scholar]
- 76.Miura T, Maruyama H, Sakai M. Endoscopic findings on alimentary lymphoma in 7 dogs. J Vet Med Sci. 2004;66:577. doi: 10.1292/jvms.66.577. [DOI] [PubMed] [Google Scholar]
- 77.Rivers BJ, Walter PA, Feeney DA. Ultrasonographic features of intestinal adenocarcinoma in five cats. Vet Radiol Ultrasound. 1997;38:300. doi: 10.1111/j.1740-8261.1997.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 78.Penninck D, Smyers B, Webster CR. Diagnostic value of ultrasonography in differentiating enteritis from intestinal neoplasia in dogs. Vet Radiol Ultrasound. 2003;44:570. doi: 10.1111/j.1740-8261.2003.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 79.Monteiro CB, O’Brien RT. A retrospective study on the sonographic findings of abdominal carcinomatosis in 14 cats. Vet Radiol Ultrasound. 2004;45:559. doi: 10.1111/j.1740-8261.2004.04096.x. [DOI] [PubMed] [Google Scholar]
- 80.Willard MD, Jergens AE, Duncan RB. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc. 2002;220:1177. doi: 10.2460/javma.2002.220.1177. [DOI] [PubMed] [Google Scholar]
- 81.Evans SE, Bonczynski JJ, Broussard JD. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447. doi: 10.2460/javma.229.9.1447. [DOI] [PubMed] [Google Scholar]
- 82.Kovak JR, Ludwig LL, Bergman PJ. Use of thoracoscopy to determine the etiology of pleural effusion in dogs and cats: 18 cases (1998–2001) J Am Vet Med Assoc. 2002;221:990. doi: 10.2460/javma.2002.221.990. [DOI] [PubMed] [Google Scholar]
- 83.Johnson GF, Twedt DC. Endoscopy and laparoscopy in the diagnosis and management of neoplasia in small animals. Vet Clin North Am. 1977;7:77. doi: 10.1016/s0091-0279(77)50007-x. [DOI] [PubMed] [Google Scholar]
- 84.Jeglum KA, Whereat A, Young K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc. 1987;190:174. [PubMed] [Google Scholar]
- 85.Fondacaro JV, Richter KP, Carpenter JL. Feline gastrointestinal lymphoma: 67 cases (1988–1996) Eur J Comp Gastroenterol. 1999;4:5. [Google Scholar]
- 86.Mukaratirwa S, de WE, van Ederen AM. Tenascin expression in relation to stromal tumour cells in canine gastrointestinal epithelial tumours. J Comp Pathol. 2003;129:137. doi: 10.1016/s0021-9975(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 87.Mukaratirwa S, Gruys E, Nederbragt H. Relationship between cell proliferation and tenascin-C expression in canine gastrointestinal tumours and normal mucosa. Res Vet Sci. 2004;76:133. doi: 10.1016/j.rvsc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 88.McEntee MF, Brenneman KA. Dysregulation of beta-catenin is common in canine sporadic colorectal tumors. Vet Pathol. 1999;36:228. doi: 10.1354/vp.36-3-228. [DOI] [PubMed] [Google Scholar]
- 89.Gamblin RM, Sagartz JE, Couto CG. Overexpression of p53 tumor suppressor protein in spontaneously arising neoplasms of dogs. Am J Vet Res. 1997;58:857. [PubMed] [Google Scholar]
- 90.Beam SL, Rassnick KM, Moore AS. An immunohistochemical study of cyclooxygenase-2 expression in various feline neoplasms. Vet Pathol. 2003;40:496. doi: 10.1354/vp.40-5-496. [DOI] [PubMed] [Google Scholar]
- 91.McEntee MF, Cates JM, Neilsen N. Cyclooxygenase-2 expression in spontaneous intestinal neoplasia of domestic dogs. Vet Pathol. 2002;39:428. doi: 10.1354/vp.39-4-428. [DOI] [PubMed] [Google Scholar]
- 92.Knapp DW, Glickman NW, Mohammed SI. Antitumor effects of piroxicam in spontaneous canine invasive urinary bladder cancer, a relevant model of human invasive bladder cancer. Adv Exp Med Biol. 2002;507:377. doi: 10.1007/978-1-4615-0193-0_58. [DOI] [PubMed] [Google Scholar]
- 93.Henry CJ, McCaw DL, Turnquist SE. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin Cancer Res. 2003;9:906. [PubMed] [Google Scholar]
- 94.Charney SC, Bergman PJ, McKnight JA. Evaluation of intracavitary mitoxantrone and carboplatin for treatment of carcinomatosis, sarcomatosis, and mesothelioma, with or without malignant effusions: A retrospective analysis of 12 cases (1997–2002) Vet Comp Oncol. 2005;3:171. doi: 10.1111/j.1476-5810.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 95.Rassnick KM, Mauldin GN, Moroff SD. Prognostic value of argyrophilic nucleolar organizer region (AgNOR) staining in feline intestinal lymphoma. J Vet Intern Med. 1999;13:187. doi: 10.1892/0891-6640(1999)013<0187:pvoano>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Delaunoit T, Neczyporenko F, Limburg PJ. Pathogenesis and risk factors of small bowel adenocarcinoma: a colorectal cancer sibling? Am J Gastroenterol. 2005;100:703. doi: 10.1111/j.1572-0241.2005.40605.x. [DOI] [PubMed] [Google Scholar]
- 97.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128(Suppl 1):S79. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 98.Fujimura T, Ohta T, Oyama K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J Gastroenterol. 2006;12:1336. doi: 10.3748/wjg.v12.i9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siehl J, Thiel E. C-kit, GIST, and imatinib. Recent Results Cancer Res. 2007;176:145. doi: 10.1007/978-3-540-46091-6_12. [DOI] [PubMed] [Google Scholar]
- 100.Gore RM, Mehta UK, Berlin JW. Diagnosis and staging of small bowel tumours. Cancer Imaging. 2006;6:209. doi: 10.1102/1470-7330.2006.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Czaykowski P, Hui D. Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin Oncol (R Coll Radiol) 2007;19:143. doi: 10.1016/j.clon.2006.12.001. [DOI] [PubMed] [Google Scholar]


