Historical Background
Kawasaki disease (KD) is one of the most common vasculitides of childhood. It has the potential to cause severe complications, significant morbidity, and even mortality. Expeditious treatment can largely prevent these complications, underscoring the importance of early and accurate diagnosis. The diagnosis is based on clinical criteria (Table 33–1 ), and correct identification of KD can still be as exacting a challenge today as it has been for more than 40 years.
Table 33–1.
Criteria for the diagnosis of Kawasaki disease
Fever for more than five days (four days if treatment with intravenous immunoglobulin eradicates fever) plus at least four of the following clinical signs not explained by another disease process:
|
Numbers in parentheses indicate the approximate percentage of children with Kawasaki disease who demonstrate the criterion.
∗ Modified from Centers for Disease Control: Revised diagnostic criteria for Kawasaki disease, MMWR Morb Mortal Wkly Rep 39:27-28, 1990.
This vasculitis bears the eponym Kawasaki disease because of the painstaking description of this illness in 50 children by Tomisaku Kawasaki in 1967.1 Scattered case reports of young children who died of ruptured or thrombosed coronary artery aneurysms or infantile polyarteritis nodosa2., 3. have appeared in the medical literature since 1871. A clinical syndrome comprising most of the components of what is today recognized as KD was described by Munro-Faure in 19594 and by Itoga in 1960.5
Definition and Diagnostic Criteria
KD is a self-limited vasculitis of unknown origin, characterized by fever, rash, conjunctivitis, changes in the oral mucosa, changes in the extremities, cervical lymphadenopathy, and in a proportion of cases, dilatation or aneurysms of the coronary and other arteries.
The 1967 guidelines for the diagnosis of KD are shown in Table 33–1. Slightly modified criteria were commonly applied in studies from the U.S., in which fever was a required criterion. Four of the other five criteria were also needed for diagnosis. Recently proposed criteria require fever as mandatory, include perineal rash with changes in the extremities, and recognize that, in the presence of fever and coronary artery changes demonstrated by echocardiography, fewer than four criteria are required to make the diagnosis of KD.6 Muta and colleagues7 showed that fever for four or fewer days in the presence of four other criteria increased the sensitivity of the criteria. There have been no studies comparing the sensitivity and specificity of these varying formulations.
None of these guidelines has 100% sensitivity and specificity for the diagnosis of KD. If a child has the characteristic clinical features and develops coronary artery aneurysms, the diagnosis is certain. Children who do not meet the criteria may have an incomplete or atypical form of KD (discussed later). Alternatively, some patients who fulfill all criteria may have other conditions. In a study of patients referred because of possible KD, Burns and colleagues8 found that the standard clinical diagnostic criteria for KD were fulfilled in 18 (46%) of 39 patients in whom other diagnoses were established. Up to one-third of children with KD also have an identifiable infection,9 including Group A streptococcal tonsillitis, viral illnesses, pneumonia, and gastroenteritis.
More concerning from the perspective of trying to prevent disease sequelae is that many children who develop coronary artery aneurysms never meet criteria for KD.10 A review of 127 patients treated for KD found that 36% did not meet criteria. Further, the proportion developing aneurysms was higher among this group than among those who had the full clinical syndrome.11
The youngest patients are most likely to have atypical features and to develop aneurysms—up to 60% of children younger than 12 months old developed aneurysms in one series.12 The diagnosis of KD should be considered for any infant with prolonged, unexplained fever. Treating KD is seldom an emergency, especially when patients present after only five or six days of fever. Observation of children who do not fulfill criteria may be the best course of action. The mean duration of fever in children with untreated KD is 12 days,13 much longer than typical viral illnesses, so persistence of fever or development of additional signs of KD favors treatment for KD.
Epidemiology
Although worldwide in distribution, the incidence of KD is highest in Japan, where, by 2002, more than 186,000 cases of KD had been registered since 1967.14 The incidence has been steadily increasing in Japan,15 reaching 212 per 100,000 males and 163 per 100,000 females 0-4 years old in 2006. Children of Japanese descent who reside outside Japan also face a higher risk of KD than do Caucasian children.16 Rates in Korea, Taiwan, and China are also high.17 As assessed by hospital admissions, in the U.S., children of Asian or Pacific Island ancestry have the highest incidence (39/100,000 < 18 years). The incidence was intermediate for African-Americans (19.7) or children of Hispanic origin (13.6), and lowest for whites (11.4).18 In one large area of Great Britain, the annual incidence rate was 5.5 cases per 100,000 for children younger than 5 years old; the incidence for children of Asian ancestry was more than double that for Caucasian and African or Afro-Caribbean children.19
KD is an illness of early childhood; 85% of affected patients are younger than 5 years old, with an average age of approximately 2 years old,18 although there are reports of KD occurring in older children20 and adults.21 KD is more common in boys than in girls (male:female ratio of 1.36:1 to 1.62:1).22., 23.
In Japan, the highest incidence occurs between 9 and 11 months old in boys, and between 3 and 8 months old in girls.23 In North America, the peak age at onset of KD is between 2 and 3 years old. In an Australian study, only 20% of children were younger than 1 year old at diagnosis, and 25% were older than 5 years old. The reasons for the geographic differences in age at onset are unclear.24
Several reports document a seasonal incidence of KD.24., 25. In Japan, the disease occurs most frequently in January, June, and July, with a nadir in October. This pattern was observed every year for the 14 years of the study in Japan.25 This bimodal distribution was not noted in Taiwan, where the highest incidence was in the summer.17 In North America, cases have tended to occur between November and May.26 Clustering of cases in time and geographic area further suggests an unrecognized vector. Kao and colleagues27 demonstrated a temporal and spatial clustering of disease in San Diego County, California, U.S.A. Although epidemics of KD were documented in Japan up to 1987, none has occurred since then.28
In Japan, siblings of affected children have a risk of contracting KD that is approximately 10 times higher than the risk in the general population,29 but cases among children sharing the same home in other countries are uncommon.25 Dergun and colleagues30 reported 18 families in the U.S. with 24 affected members, including nine sibling pairs. Second and even third attacks have been reported in from 1.5% to 3% of cases.17
Etiology and Pathogenesis
The cause of KD remains unknown. Many of its epidemiological and clinical manifestations suggest an infectious origin. If an infectious agent does indeed cause KD, the putative organism would appear to be of very low communicability, or predominantly responsible for subclinical infections. Repeated attempts to identify a particular infectious trigger have been unsuccessful.31 It is possible that vasculitis in KD is caused by either conventional antigens or by superantigens that trigger an immune response to endothelial cells, rather than by direct infection of the vessels.32 Superantigens are produced by several bacteria, notably certain strains of Staphylococcus and Streptococcus, and are capable of stimulating large numbers of T cells in an antigen-nonspecific manner by interaction with the β chain of the T cell receptor. Overrepresentation of T cells bearing Vβ2 among lymphocytes in coronary artery aneurysms, intestinal mucosa,33., 34. and peripheral blood35 from patients with KD supports the hypothesized role of superantigens in the pathogenesis. A variety of additional circumstantial evidence30., 31., 32., 36., 37., 38. and a murine model of Lactobacillus casei-induced vasculitis lend credence to this theory. Further, children with KD have unique reactions to mycobacterial antigens,39., 40., 41. which may also function as superantigens, including recall reactions at the site of a previous bacillus Calmette-Guérin immunization.39 Nonetheless, the only human illness definitively ascribed to superantigens is toxic shock syndrome, and other researchers have failed to identify evidence of a role for superantigens in the pathogenesis of KD.42 Thus, whether these findings represent a specific response to superantigens or crossreactivity with other antigens is not clear.
A predominance of immunoglobulin A (IgA)-secreting plasma cells in the blood vessel walls of children with fatal KD has suggested to Rowley and colleagues that an organism that gained entry through mucosal surfaces underlies the disease.43 No single pathogen is regularly demonstrable, although associations with Epstein-Barr virus,43 rotavirus,44 and other viruses45., 46., 47. and with bacteria48., 49. have been reported. An association with a coronavirus50 has not been confirmed.51 It is nonetheless a possibility that the vascular injury in KD may be the result of a direct cell-mediated attack on endothelial cells that are infected with an unidentified pathogen.52
Additional clues to the cause of KD come from the humoral factors, including antiendothelial cell antibodies, circulating immune complexes, and antineutrophil cytoplasm antibodies (ANCAs) that are demonstrated in a large proportion of patients.53 Serum levels of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6),54 and growth factors, such as vascular endothelial growth factor,55 are elevated, generally in proportion to the severity of the illness. In the absence of confirmed evidence of a single etiological agent, a reasonable working hypothesis is that KD represents a stereotyped, pathological immune response to one or a variety of environmental or infectious triggers. Presumably, certain individuals are predisposed by virtue of their genetic constitution. The predilection for childhood onset may reflect the presence of developmental antigens that are targets for the inflammatory response only early in life, subtle maturational defects in immune responsiveness,56 or the timing of exposure to environmental triggers.
Genetic Background
In Japan, approximately 1% of patients with KD have a history of an affected sibling,57 and concordance for KD was 13.3% in dizygotic twins and 14.1% in monozygotic twins.58 Similarly, in Japan, there is a significantly increased frequency of a history of KD in the parents of children with the disease.59 These observations indicate that there is a genetic predisposition to this disease, although the fact that affected twin pairs became ill within two weeks of each other also suggests an important role for (an) environmental agent(s).
The exact genetic factors that may underlie the disorder are unknown. Reported genetic associations have been reviewed by Hata and Onouchi.60 Candidate genes include those at the histocompatibility locus and those for other proteins involved in immunoregulation. Human leukocyte antigen (HLA) genes for B5, B44, Bw51, DR3, and DRB3∗0301 have been associated with KD in Caucasians; Bw54, Bw15, and Bw35 in Japanese; and Bw51 in Israelis.61 However, Onouchi and colleagues62 concluded that HLA polymorphisms contributed little to the pathogenesis of KD. There has been no reported association of any HLA antigen with the risk of coronary artery disease.63
Polymorphisms of the TNF-α gene (TNF), 64 the IL 18 gene,65 the HLA E gene,66 and the gene for angiotensin converting enzyme67., 68. have been associated with KD, but their pathogenic significance is disputed. A recent report implicated polymorphisms of the mannose binding lectin (MBL) in the pathogenesis of KD.69 MBL binds to n-acetyl glucosamine and mannose present on the surface of many microbes. This interaction results in activation of complement (C3) independent of antibody. Levels of MBL are determined by polymorphisms of the MBL 2 gene and its promoters. Higher expression of MBL is associated with lower incidence of coronary artery lesions in patients under 1 year old, but it has the opposite effect in older patients. This apparent paradox is congruent with the belief that MBL is important in protecting the very young child from infectious diseases.69
The gene controlling expression of inositol 1,4,5-triphosphate 3-kinase (ITPKC) has recently been identified as a susceptibility gene not only for KD, but also for coronary artery disease.70 This enzyme is strongly expressed by peripheral blood mononuclear cells and has an important role in inflammation by decreasing IL-2 expression. The functional polymorphism ITPKC 3 is significantly increased in patients with KD and coronary artery disease in Japanese and American populations.71 Neither studies of individual genes nor genome-wide association studies72 have revealed consistent markers of disease susceptibility. Thus, to the extent that certain children are predisposed to developing KD, the effect is likely due to small contributions from multiple genetic loci.
Clinical Manifestations
Disease Course
The course of untreated KD may be divided into three phases (Fig. 33–1 ): An acute, febrile period lasting for 10-14 days is followed by a subacute phase of approximately two to four weeks. This ends with a return to normal of the platelet count and erythrocyte sedimentation rate (ESR). The subsequent convalescent or recovery period lasts months to years, during which time vessels undergo healing, remodeling, and scarring.
FIGURE 33–1.

Kawasaki disease can be viewed as an illness with acute, subacute, and recovery phases. The temporal characteristics outlined here are typical of the course of the disease.
(Adapted from ref. 1.)
Acute Febrile Phase
The onset of fever in KD is characteristically abrupt, often preceded by symptoms of an upper respiratory or gastrointestinal illness. Baker and colleagues72 studied the symptoms in the 10 days prior to diagnosis of KD in 198 patients, and reported that irritability occurred in 50%, vomiting in 44%, decreased food intake in 37%, diarrhea in 26%, and abdominal pain in 18%. Cough was reported in 28%, and 19% had rhinorrhea. In addition, 19% reported weakness, and 15% reported arthralgia or arthritis. Over the next three to four days, cervical adenitis, conjunctivitis, changes in the buccal and oral mucosa, a pleomorphic rash, and erythema and edema in the hands and feet develop (in no particular order). Perineal desquamation may be another early sign of KD.73 Untreated, these manifestations subside after an average of 12 days. If carditis occurs, it often does so early and may be manifested by tachycardia, an S3 gallop, and subtle or occasionally marked signs of congestive heart failure.74 Pericarditis, abdominal pain, ascites, and hydrops of the gallbladder may occur at this time.
Subacute Phase
After the acute phase, the child may be entirely asymptomatic if given intravenous immunoglobulin (IVIG). Untreated, fever, mucositis, and conjunctivitis usually resolve entirely by the third or fourth week. During this period, desquamation of the skin of the digits75 may be the only clinically apparent residual feature. Up to one in 13 children develops arthritis of one or several joints during the late acute and subacute phases.76 Coronary artery aneurysms most commonly first develop during the subacute phase, occasionally earlier, but rarely later in children treated with IVIG.
Convalescent Phase
Most children are asymptomatic during the convalescent phase. The acute phase response has usually returned to normal, unless there are complications. Horizontal ridging of the nails (Beau lines), characteristic of many acute inflammatory conditions, may appear during this period.
Clinical Characteristics of the Classification Criteria
Fever
Fever, often exceeding 40°C, is the most consistent manifestation of KD. The fever is typically persistent and minimally responsive to antipyretic agents, tending to remain above 38.5° C during most of the acute phase of the illness. It reflects elevated levels of TNF-α and IL-1, which are thought to mediate the underlying vascular inflammation.77 The diagnosis must be suspect in the absence of fever.
Conjunctivitis
Bilateral, nonexudative bulbar conjunctivitis occurs in more than 85% of patients with KD. Conjunctival injection typically spares the limbus, which is the zone immediately around the cornea. Inflammation of the palpebral conjunctiva is not prominent. Purulent discharge is especially unusual78 and suggests an alternative diagnosis.
Other ocular abnormalities may also occur, although they are not part of the diagnostic criteria (Table 33–2 ). During the first week of illness, about three-fourths of children are photophobic, an effect of anterior uveitis,79 which peaks between five and eight days of illness and is more common in children over 2 years old. Ocular inflammation usually resolves without specific therapy or sequelae. Exceptionally, there may be posterior synechiae, conjunctival scarring,80 changes in the retina and vitreous,81 or blindness.82
Table 33–2.
Frequency of ocular signs and symptoms in Kawasaki disease
| Ocular Sign or Symptom | Frequency (%) |
|---|---|
| Injection of bulbar conjunctivae | 89 |
| Nongranulomatous iridocyclitis | 78 |
| Superficial punctate keratitis | 22 |
| Vitreous opacities | 12 |
| Papilledema | 11 |
| Subconjunctival hemorrhage | 3 |
Data from Kumagai N, Ohno S: Kawasaki disease. In Pepose JS, Holland GM, Wilhelmus KR, editors: Ocular immunity and infection, St. Louis, 1996, Mosby.
Changes in the Lips and Oral Mucosa
Swollen, vertically cracked red lips and a strawberry tongue are characteristic; the latter is caused by sloughing of filiform papillae and prominence of the large hyperemic fungiform papillae (Fig. 33–2 ). Vesicles, ulcers, or tonsillar exudate suggest a viral or bacterial infection rather than KD.
FIGURE 33–2.
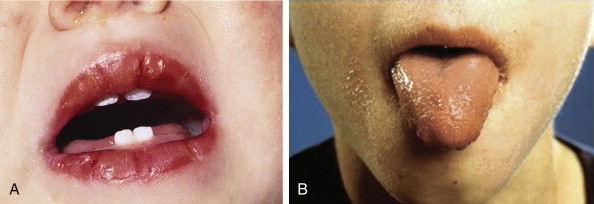
A, The intense reddening, swelling, and vertical cracking of the lips are characteristic of Kawasaki disease (KD). B, The strawberry tongue of acute KD with hypertrophied papillae on an erythematous base and the peeling of the facial skin.
Exanthem
The cutaneous manifestations of KD are protean. Although the rash usually begins on the trunk, there is often a perineal confluence during the first days of the illness, followed by desquamation in the diaper area by day six in most cases.73 Macular, morbilliform, or targetoid lesions of the trunk and extremities are most characteristic. The rash is seldom pruritic, and vesicular or bullous lesions are rare (Fig. 33–3 ). Psoriasis has been reported in several children with KD.83
FIGURE 33–3.

The nonspecific polymorphous rash is seen on the face arms and chest of this 2-year-old boy with acute KD.
Lymphadenopathy
Anterior cervical lymphadenopathy occurs during the acute phase of the disease, is usually unilateral, and may appear to involve only a single node. However, ultrasound or computed tomographic imaging of the neck typically reveals grapelike clusters of enlarged nodes similar to those seen in Epstein-Barr virus infections rather than the isolated adenopathy typical of bacterial adenitis.84 Occasionally, a node enlarges rapidly and may be mistaken for bacterial infection. After three or four days, it usually shrinks with or without specific therapy. Diffuse lymphadenopathy and splenomegaly are not typical of KD and should raise suspicions of a viral illness.
Extremity Changes
Indurated edema of the dorsum of the hands and feet and a diffuse red-purple erythema of the palms and soles occur early and last for one to three days. Sheet-like desquamation typically occurs 10 days or more after the start of the fever. It characteristically begins at the tips of the fingers (less commonly the toes), just below the distal edge of the nails (Fig. 33–4 ). Flaky desquamation may occur elsewhere, but peripheral skin peeling usually occurs late in the course of KD and may be absent or inapparent.75 Consequently, it is useful more for retrospective confirmation of the diagnosis than for making therapeutic decisions.
FIGURE 33–4.

A, Desquamation of the skin of the tips of the thumb and finger seen during the subacute phase of Kawasaki disease. B, Desquamation of the skin of the hand occurs later in the subacute and early recovery phase of the disease. In many children, the degree of desquamation is much less than is depicted here.
Incomplete Kawasaki Disease
Signs, symptoms, and outcome in children who do not meet criteria for KD tend to parallel those of children who fulfill the diagnostic criteria. In a Japanese study, 25 of 242 patients hospitalized for KD failed to meet diagnostic criteria.85 Only one patient ultimately developed transient dilatation of a coronary artery. A particularly high level of suspicion is needed in infants younger than 1 year old. In a retrospective review of 45 cases of KD, 5 (45%) of 11 infants had incomplete disease, compared with 4 (12%) of 33 older children.86 Unfortunately, infants are the group at the highest risk for developing coronary artery aneurysms, and in this study, coronary artery complications occurred in seven infants (64%), compared with three older children (9%), including all five infants with incomplete disease.86
In view of these data, the American Heart Association (AHA) has suggested additional markers for identification of children not meeting classical criteria for KD who might nonetheless be at increased risk of developing coronary artery aneurysms.87 Early reports suggest that the algorithm recommended by the AHA committee performs well in decreasing the number of children who are not treated for KD and yet ultimately develop aneurysms. 88
Other Clinical Manifestations of Kawasaki Disease (Table 33–3)
Table 33–3.
Manifestations of Kawasaki Disease
| Organ System | Common | Uncommon | Finding Suggests Alternate Diagnosis |
|---|---|---|---|
| Skin | Targetoid, urticarial, morbilliform rashes, livedo reticularis | Psoriasiform rash | Pustular, vesicular rashes |
| Lungs | Pleural effusion | Nodules, interstitial infiltrates | |
| Urinary tract | Urethritis, pyuria | Hematuria, proteinuria, orchitis | |
| Nervous system | Irritability, lethargy, anterior uveitis, sensorineural hearing loss | Seizure, stroke, cranial nerve palsy | |
| Gastrointestinal system | Diarrhea, vomiting, hydrops of gallbladder, hepatomegaly | Intestinal hemorrhage, ruptured viscus | |
| Hematological system | Anemia, thrombocytosis, leukocytosis | Thrombocytopenia, consumptive coagulopathy, hemophagocytic syndrome | Lymphocytosis∗ |
| Reticuloendothelial system | Anterior cervical lymphadenopathy | Posterior cervical, axillary lymphadenopathy | Diffuse lymphadenopathy, splenomegaly |
| Mucosa | Mucositis, glossitis, conjunctivitis | Discrete oral lesions, exudative conjunctivitis | |
| Musculoskeletal system | Extremity edema, arthritis | Raynaud Phenomenon | |
| Cardiac system | Tachycardia, gallop rhythm, myocarditis, pericarditis | Coronary artery aneurysm, aortic root dilatation, valvulitis |
Except during the convalescent phase.
Cardiovascular Disease
At onset, there is usually a tachycardia commensurate with the degree of fever. “Early myocarditis occurs in at least one-third89 to one-half90 of patients, and pericarditis also may occur. Myocardial involvement often leads to decreased contractility, commonly manifested by an S3 gallop that may become more prominent with hydration. Such children may be misdiagnosed with viral myocarditis. In more severe cases, myocardial involvement may progress to dysrhythmias and signs of congestive heart failure.91 Children with prominent KD-associated myocarditis tend to respond less briskly to treatment with IVIG, but long-term abnormalities of cardiac contractility are nonetheless very uncommon in children treated appropriately during the acute phase of KD.92
The most significant and characteristic complication of KD, the development of coronary artery aneurysms in up to 25% of untreated patients, makes KD the leading cause of acquired heart disease among children in the developed world (FIGURE 33–5, FIGURE 33–6 ). Treatment with IVIG decreases the incidence of giant aneurysms (internal diameter >8 mm) by more than 95% and the overall incidence of aneurysms by 85%. Despite treatment, however, one retrospective survey reported that 8.5% of patients younger than 12 months old developed coronary artery abnormalities, compared with 1.8% of those 12 months old or older.93 Overall, the odds ratio for development of cardiac sequelae in infants younger than 1 year old was 1.54.94
FIGURE 33–5.

Echocardiographic demonstration of aneurysms of three coronary arteries in a child with Kawasaki disease. A, aneurysms; CIRC, circumflex; LAD, left anterior descending coronary artery; RVOT, right ventricular outflow tract.
(Courtesy of Dr. Dennis Crowley.)
FIGURE 33–6.

A, Angiography of the coronary vessels in a 7-month-old boy with Kawasaki disease shows a huge aneurysmal dilatation of the right coronary artery (arrow)B, Aneurysm of the left coronary artery in a 3-year-old girl with Kawasaki disease (arrow).
(A and B, Courtesy of Dr. Zuidi Lababidi.)
Coronary aneurysms may cause morbidity early in the course due to rupture or thrombosis, resulting in sudden death or myocardial infarction.95 Development of de novo coronary artery abnormalities more than two weeks after the end of the acute illness is unusual, although ongoing scarring of existing vascular lesions may result in progressive coronary insufficiency. Approximately one-half of coronary artery aneurysms demonstrated by echocardiogram ultimately resolve, usually those smaller than 6 mm in diameter,96 but persistent vasodilatory abnormalities have been observed in arteries where aneurysms have resolved.97 Giant coronary artery aneurysms, with an internal diameter larger than 8 mm, are associated with the highest risk of morbidity and mortality. Up to one-third of such aneurysms become obstructed, leading to myocardial infarction, dysrhythmias, or sudden death.98
Although involvement of the coronary arteries is the most characteristic manifestation of the vasculitis of KD, other medium-sized muscular arteries also may be involved. Aneurysms of brachial and femoral arteries may be palpable clinically or demonstrable angiographically (Fig. 33–7 ). In severe cases, peripheral arterial obstruction may lead to ischemia and gangrene. Visceral arteries are usually spared, although there are reports of gastrointestinal obstruction99 and acute abdominal catastrophe100 occurring because of vasculitis. Such complications generally arise in children with other signs of severe vasculitis, including aneurysms in coronary and peripheral arteries.
FIGURE 33–7.

Angiographic study of a 2-year-old boy with severe Kawasaki disease resulting in multiple aneurysms of the coronary, axillary, iliac, and femoral arteries. The study revealed large aneurysms of the aorta and iliac (A) and femoral (B) arteries (arrows). Aneurysms that were palpable in the axilla and groin in this patient later resolved.
(A and B, Courtesy of Dr. G. Culham.)
Central Nervous System Complications
One of the most consistent clinical observations of children with KD, particularly infants and very young children, is their extreme irritability. This probably represents the effect of aseptic meningitis and associated headache.101 Cerebrovascular accident102 and facial nerve paralysis103 also have been reported.
Musculoskeletal Disease
Arthritis was observed by Gong and colleagues76 in 7.5% of 414 children with KD. Arthritis was oligoarticular in 55% and polyarticular in 45%. Joints most commonly affected were (in order of decreasing frequency) knee, ankle, wrist, elbow, and hip. Joint pain was often severe, but responded to IVIG and high dose aspirin in most instances. It may occur at any time during the disease course but has been described most commonly during the recovery phase. Arthritis in KD ultimately resolves, leaving no residua.
Respiratory Tract Disease
Cough, coryza, or hoarseness, and otitis media frequently occur early in the course of the disease and suggest a viral upper respiratory tract infection. Approximately one-third of children have some degree of sensorineural hearing loss when tested within 30 days of fever onset. Salicylate toxicity may be responsible for transient cases, but sensorineural hearing loss of unclear etiology may persist.104., 105.
Gastrointestinal Tract Disease and Other Abnormalities
Abdominal pain is common, and approximately one-fourth of children with KD have profuse, watery diarrhea during the acute febrile period. Abdominal distention may mimic mesenteric vasculitis or intussusception. Segmental bowel wall thickening has been described in children with KD and abdominal pain, presumably reflecting visceral arteritis.106 The relatively common occurrence of hydrops of the gallbladder demonstrated by ultrasonography107 may aid in the diagnosis of incomplete or atypical KD. Occasionally, the gallbladder becomes large enough to be seen as a bulge in the anterior abdominal wall. The specificity of gallbladder distension is limited, however, and a dilated, engorged gallbladder may be seen in cases of streptococcal and staphylococcal infections, among other mimics of KD. Hepatosplenomegaly may occur in the absence of heart disease or may reflect cardiac failure.
Genitourinary Tract Involvement
A study of 50 children with KD from Taiwan108 revealed hematuria (>5 RBC/HPF) in six patients, proteinuria (>100 mg/dL) in five, and leukocyturia (>10 white blood cells per high power field) in 19. Renal ultrasonography was abnormal in five patients, and DMSA SPECT revealed inflammatory lesions in 26 children. Although renal function remained normal, scarring was demonstrated in 46% on repeated DMSA SPECT.
Scrotal pain and swelling due to testicular inflammation are characteristic of pediatric vasculitides, including Henoch-Schönlein purpura, polyarteritis nodosa, and KD. Meatitis and dysuria also occur frequently during the acute phase of KD, and priapism has been described.109 Hemolytic-uremic syndrome, immune complex-mediated glomerulonephritis, and acute interstitial nephritis have each been reported in a few cases.110., 111. Acute renal failure is a rare complication most commonly ascribed to complications of treatment with certain preparations of IVIG.112
Differential Diagnosis
KD is often difficult to differentiate from viral exanthems of childhood, particularly early in the disease course or in children with incomplete KD (Table 33–4 ). The differential diagnosis includes poststreptococcal scarlet fever, toxic shock syndrome, drug reactions, and systemic-onset juvenile idiopathic arthritis. Viral illnesses such as measles (especially when atypical or occurring after vaccination), Epstein-Barr virus, and adenovirus infections share many of the signs of mucocutaneous involvement, but they typically have less evidence of systemic inflammation and generally lack the extremity changes of KD. Toxin-mediated illnesses, especially scarlet fever and toxic shock syndrome, lack the ocular and articular involvement typical of KD. Drug reactions, such as those in Stevens-Johnson syndrome or serum sickness, may mimic KD but have subtle differences in the ocular and mucosal manifestations. In particularly severe or prolonged KD, the possibility of a chronic vasculitis such as polyarteritis nodosa must be considered carefully.
Table 33–4.
Differential diagnosis of Kawasaki Disease
| Infectious Conditions |
| Adenovirus |
| Measles |
| Parvovirus |
| Human herpesviruses (HHV) (e.g., herpes simplex virus, cytomegalovirus, HHV-6, HHV-7) |
| Rocky Mountain spotted fever |
| Leptospirosis |
| Streptococci |
| Staphylococci |
| Immune Reactions |
| Stevens-Johnson syndrome |
| Serum sickness |
| Rheumatic Diseases |
| Systemic-onset juvenile idiopathic arthritis |
| Polyarteritis nodosa |
Pathology
The signs and symptoms of KD are due to a systemic necrotizing vasculitis with fibrinoid necrosis of the medium-sized muscular arteries; the coronary arteries are the predominant sites of involvement.113 Disruption of the lamina elastica is characteristic of the aneurysms. An early neutrophilic infiltrate occurs in all layers of the heart, including the valves. Inflammation begins in the microvasculature (i.e., arterioles, capillaries, vasa vasorum, and venules) and subsequently spreads to larger vessels, especially the coronary arteries.114 In these lesions, infiltrating cells are mostly macrophages and IgA-secreting plasma cells,42 findings that may be unique to KD.115 Endothelial cells express a variety of markers of activation, presumably as a result of the high levels of proinflammatory cytokines that characterize the acute phase of disease.116 Some children have a lymphocytic myocarditis, with endomyocardial biopsy demonstrating cellular infiltrates or myofibrosis that may persist for years in untreated cases.117
Evolution of the cardiac lesions is detailed in the study of Fujiwara and Hamashima.114 Coronary artery vasculitis predominated early in the disease but was absent in those who died after 28 days of illness. Aneurysms, thrombosis, and stenosis did not appear until 12 days of disease or later. Pericarditis, myocarditis, and endocarditis were universal findings early in the disease, but diminished as fibrosis of the myocardium became the predominant lesion in children whose death occurred 40 days or more after onset. In a study of 262 children, Suzuki and colleagues118 documented an equal frequency of aneurysms in right and left coronary arteries, but a higher propensity for development of segmental stenosis and occlusions in the right coronary artery.
Laboratory Examination
There are no specific diagnostic tests for KD, but at onset, evidence of inflammation is manifested by elevation of C-reactive protein (CRP) and ESR, leukocytosis, and a left shift in the white blood cell (WBC) differential count. Toxic granulation of neutrophils is more frequent in children with KD than in those with other febrile illnesses.119 Occasionally, significant neutropenia occurs early120; this may be a marker for particularly severe disease. Thrombocytopenia and anemia may herald the onset of macrophage activation syndrome (see Chapter 45).121 Although platelet counts may be abnormally low at disease onset, by the second week of illness they characteristically rise and may reach 1,000,000/mm3 (reactive thrombocytosis) in the most severe cases. Children with KD often present with a normocytic, normochromic anemia; hemoglobin concentrations greater than two standard deviations below the mean for age are found in one half of patients within the first two weeks of illness.8
Sterile pyuria is of urethral origin and therefore is missed on urinalyses obtained by bladder aspiration or catheterization. The WBCs are mononuclear and are not detected by dipstick tests for leukocyte esterase. Measurement of liver enzymes often reveals elevated transaminase levels or mild hyperbilirubinemia due to intrahepatic congestion. A few children develop obstructive jaundice from hydrops of the gallbladder or hepatic vasculitis.
Cerebrospinal fluid (CSF) analysis typically displays a mononuclear pleocytosis with normal glucose and protein. In a chart review of 46 children with KD, 39% were documented to have elevated CSF WBC counts.101 The median count was 22.5 cells/mm3 with 6% neutrophils and 91.5% mononuclear cells, although cell counts as high as 320/mm3 with up to 79% neutrophils were reported. Arthrocentesis of involved joints typically demonstrates synovial fluid WBC counts of 50 to 300,000 WBC/mm,3 consisting primarily of neutrophils.
Children with KD develop significant perturbations in serum lipid profiles beginning during the subacute phase of illness. These abnormalities include elevated concentrations of triglycerides and low-density lipoproteins and depressed levels of high-density lipoproteins.122 They are most likely caused by widespread endothelial injury. As with other sequelae of KD, normalization may take years in untreated children but typically occurs within weeks or months after IVIG therapy.
ANCAs123 and antibodies to endothelial cells124 may be present late but not early in the disease.125 Consequently, they have unclear pathological significance and are of little diagnostic value. Other autoantibodies are usually absent. Elevated levels of von Willebrand factor antigen indicate the presence of damaged endothelium.126 Activation products of C3 and C4 have been demonstrated on erythrocytes (C3g) and in the plasma (C4d),127 suggesting the participation of complement in at least some of the manifestations of the disease.
Treatment
General Approach
The child with suspected or definite KD should be admitted to the hospital for observation, monitoring of cardiac status, and management of systemic manifestations (Table 33–5 ). Initial evaluation of the heart should include an electrocardiogram to identify dysrhythmias, signs of ischemia, or myocarditis; and a baseline echocardiogram to detect coronary artery vasculitis, ectasia, or aneurysms. If the diagnosis is relatively certain (even if diagnostic criteria are not met), and other diagnoses have been considered and excluded, treatment should be initiated with aspirin and IVIG without further delay.
Table 33–5.
Initial Evaluation and Management of Kawasaki Disease
| Evaluate | Treat |
|---|---|
| General physical exam | |
| Cardiac status (ECHO, ECG) CNS status Hematologic and inflammatory parameters (CBC, differential, platelet count, ESR, CRP) Fluid and electrolyte status (AST, ALT, bilirubin, electrolytes, BUN, creatinine) Urinalysis Ophthalmologic status |
Aspirin If patient is febrile: 80–100 mg/kg/day in 4 doses If patient is afebrile: 3–5 mg/kg/day in 1 to 4 doses IVIG: 2 g/kg |
| Monitor cardiac status Monitor CRP (ESR) and platelet count at 2-week intervals until stable, then 1-month intervals until normal |
Keep in hospital until afebrile for 24 hr or if there are complications. If fever persists, repeat IVIG once. If no clinical response, consider intravenous methylprednisolone: 30 mg/kg |
| Repeat echocardiogram at 6-8 weeks | Maintain low-dose aspirin until ESR and platelet count are normal if there have been no coronary artery abnormalities; for 2 years if coronary abnormalities have resolved; “forever” if coronary artery disease persists. |
CNS, central nervous system; ECG, electrocardiogram; ECHO, echocardiogram; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin, CRP, c-reactive protein; AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen.
Goals of Therapy
In addition to control of the acute inflammation and its symptoms, the goal of therapy is to prevent long-term sequelae and, most importantly, coronary artery abnormalities. The consequences of failure to treat a child appropriately with KD are so important that, within reason, after very careful evaluation, error on the side of premature or unnecessary therapy is preferable to delayed or missed therapy for a child for whom the diagnosis is uncertain. The American Academy of Pediatrics and the AHA recommend that children with KD should be treated with aspirin and IVIG during the first 10 days of the illness.87., 128. Subsequent management remains controversial, however, and depends on the presence or absence of coronary artery abnormalities.
The Japanese Ministry of Health criteria129., 130. use angiography or echocardiography to define coronary arteries as abnormal if the internal lumen diameter is greater than 3 mm in children younger than 5 years old or greater than 4 mm in children at least 5 years old. In addition, vessels are considered aneurysmal if the internal diameter of a segment measures at least 1.5 times that of an adjacent segment or the coronary artery lumen is clearly irregular. Although coronary artery dimensions in normal children have been shown to increase linearly with body surface area (BSA) or length,131 these criteria are not based on body size. Evaluation of coronary arteries in KD using age-, size-, and sex-adjusted indices suggests that the incidence of abnormalities is higher than was generally recognized.132 Among patients classified as having normal coronary arteries by the Japanese Ministry of Health criteria, 27% had at least one BSA-adjusted coronary artery dimension more than two standard deviations above the mean. Even children whose vessel dimensions are within the “normal” range may demonstrate a decrease in coronary artery diameter as they convalesce from KD.133
Treatment strategies also depend on the implications of coronary artery dilatation. Long-term outcome studies are somewhat reassuring. Fifty percent of coronary artery aneurysms regress angiographically, and among such children, at least one longitudinal study demonstrated no increase in morbidity or mortality rates, even after more than two decades.98 Nonetheless, abnormalities of more subtle markers of endothelial health are a matter of concern. Vessels show histological134 and functional135 abnormalities at the sites of healed aneurysms, and vascular reactivity to endogenous vasodilators is abnormal in children who have had KD, regardless of whether they have detectable coronary artery abnormalities.136 This lends credence to the report of an increased long-term standardized mortality ratio of 2.35 among male patients with cardiac sequelae.137 Acute phase reactants and platelet counts do not return to normal for up to 2 months after apparently successful treatment, suggesting that vasculitis and endothelial inflammation may not fully resolve, even when fever is controlled. It is reasonable to ask whether persistent KD requires additional therapy and whether initial treatment should be more robust than IVIG alone, at least for some children, aiming for anatomically and functionally normal vessels in everyone.
Aspirin
Aspirin was the first medication to be used for treatment of KD because of its anti-inflammatory and antithrombotic effects.138 Anti-inflammatory regimens using high-dose (>80 mg/kg/day) or lower-dose (30 mg/kg/day) aspirin have been recommended during the acute phase of the illness. After the fever resolves, the dose is usually reduced to an antiplatelet range of 3 to 5 mg/kg/day. These doses, well below the anti-inflammatory level, have the effect of inhibiting platelet adhesion to endothelium by curtailing platelet release of thromboxane A2 without suppressing prostacyclin production by endothelial cells.139 This effect is believed to be beneficial in preventing thrombosis when platelet counts are elevated, although no studies have demonstrated such a benefit clinically, and aspirin does not appear to reduce the frequency of coronary artery abnormalities.140 In the event of aspirin sensitivity, another antiplatelet agent, such as dipyridamole, should be considered. Unless coronary artery abnormalities are detected by echocardiogram, aspirin is discontinued after results of laboratory studies return to normal, usually within 2 months of disease onset.
There have been no published comparisons of aspirin with other anti-inflammatory agents, and it is unclear whether salicylates are uniquely efficacious in this condition. A metaanalysis found that high-dose and lower-dose aspirin regimens were associated with a similar incidence of coronary artery abnormalities at 30 and 60 days after disease onset.140 Although the necessity of using high-dose aspirin might be questioned because of the rapid response to IVIG, all of the trials showing the benefit of IVIG were conducted with children who also were receiving anti-inflammatory doses of aspirin. For other effects, such as treatment of prolonged arthritis, alternative anti-inflammatory agents may be used. The AHA warns against prescribing ibuprofen because it antagonizes the antiplatelet effects of low dose aspirin.87., 141.
The risks of aspirin appear to be similar to those reported in other settings: chemical hepatitis, transient hearing loss, and, rarely, Reye syndrome.142 These risks may be increased in KD. Aspirin binding studies have suggested that the hypoalbuminemia of children with KD predisposes them to toxic levels of free salicylate, despite measured (bound) values within the therapeutic range.143
Intravenous Immunoglobulin
Furusho and coworkers144 first reported that high-dose IVIG appeared to decrease the incidence of coronary artery abnormalities. Newburger and colleagues13 verified these findings in a 19-month-long, randomized, controlled clinical trial in 168 children with KD. One-half received IVIG (400 mg/kg/day on four consecutive days) plus high-dose aspirin (100 mg/kg/day), and one-half received aspirin alone. IVIG reduced the incidence of coronary artery abnormalities by 78%, and no child suffered serious adverse effects from the therapy, confirming the remarkable therapeutic potential of IVIG.
The initial IVIG treatment regimen was based on then-current protocols for treating immune thrombocytopenic purpura. The question of whether this protocol was optimal for KD was addressed in 1991.145 Children were randomized to receive the traditional four-dose regimen or a single dose of 2.0 g/kg of IVIG infused over 8 to 12 hours. Children receiving the larger, single dose fared better. Meta-analyses have documented a dose-response benefit of IVIG therapy in the range between 200 mg/kg and 2 g/kg.146
Although standard therapy with IVIG and aspirin given within the first 10 days of illness greatly reduces the risk of coronary artery involvement, approximately 5% of children still develop coronary artery aneurysms, according to Japanese Ministry of Health criteria, and a larger number demonstrate coronary artery ectasia. In general, younger patients, especially infants younger than 6 months old, are at higher risk.147 Stockheim and colleagues148 reported that older children also were at additional risk. In their retrospective series, 21% of patients over 8 years old had coronary artery abnormalities. They attributed this increased incidence to a delay in diagnosis and treatment among older children in whom KD is rare and is therefore often not considered. A change in the biology of KD with age (analogous to the increased risk of renal involvement in adults with HSP) cannot be excluded.
In a retrospective series from Japan, Fukunishi and colleagues149 found higher serum levels of CRP, lactate dehydrogenase, and bilirubin to be predictive of failure to respond to IVIG. More recently, Kobayashi and colleagues reported that hyponatremia and high levels of hepatic transaminase at presentation were associated with decreased responsiveness to IVIG.150 In a Canadian study, Han and colleagues151 could not identify any difference in laboratory parameters between responders and non-responders. Confirming the importance of controlling inflammation in KD, Mori and coworkers152 reported that a rise in the WBC count and CRP level after IVIG infusion are independent predictors of coronary artery abnormalities.
IVIG is most effective in reducing the risk of coronary artery disease when administered within 10 days of the onset of fever. Unfortunately, the diagnosis may remain in doubt as this deadline approaches. In ambiguous cases, the physician may be guided by the epidemiology of the disease. More than 50% of infants with KD present atypically (i.e., do not fulfill diagnostic criteria), and they have a very high incidence of aneurysms. Thus, empiric treatment in very young children is worthy of consideration.
The mechanism of action of IVIG is uncertain, with recent studies adding induction of neutrophil apoptosis153 and reversal of inhibited lymphocyte apoptosis154 to a long list of immunomodulatory effects of IVIG (Table 33–6 ). The response is generally prompt, and temperature returns to normal in most children even before the end of the IVIG infusion, with rapid clearing of the rash, mucositis, and conjunctivitis. Irritability and emotional lability, however, may persist for up to several weeks before resolving.
Table 33–6.
Potential effects of Intravenous Immunoglobulin in Kawasaki Disease
| Specific Effects |
|
| Nonspecific Effects |
|
The greatest long-term concern about IVIG use is potential transmission of blood-borne pathogens. Technical deficiencies in production led to more than 100 cases of hepatitis C in recipients of a single brand of IVIG in 1994, although none was a child with KD.155 No cases of IVIG-transmitted infections have been reported since the institution of current purification and processing practices in 1995, and no cases of IVIG-transmitted HIV have ever been reported. Overall, cost-benefit analysis documents that IVIG treatment of KD is one of the most cost-effective medical therapies available, leading to impressive short- and long-term savings. 156
Infusion reactions (fever, rash, nausea, and hypotension) occasionally accompany IVIG administration and are best managed by slowing the rate of infusion and treating with diphenhydramine. With no viable alternative therapies, aggressive premedication with corticosteroids, or even use of a different brand of IVIG, is preferable to foregoing immunoglobulin. Rarely, a child might develop congestive heart failure during or after infusion of the IVIG because of the high solute load and subsequent increase in intravascular volume. Slowing the infusion rate and administration of furosemide are usually the only treatments required. Ultimately the improvement in myocardial contractility associated with IVIG treatment is almost invariably adequate therapy.87 Hemolysis is uncommon, but occasionally it may be severe, requiring transfusion. Headache up to 72 hours after the infusion is common, especially in older patients. Such children may require low-dose opiates for relief.157
Virtually all data concerning the role of IVIG are limited to treatment during the first 10 days of illness. This is not to say that treatment after 10 days of illness is ineffective or contraindicated; it is merely inadequately studied. In a report of 16 children with coronary artery aneurysms treated a mean of 17 days after onset of fever, there was a trend toward increased resolution of abnormalities by echocardiogram.158 The American Academy of Pediatrics cautiously recommends IVIG for children beyond the 10th day of illness with “manifestations of continuing inflammation,” and such an approach appears prudent.128 Questions have arisen concerning the efficacy of very early treatment of KD. Tse and colleagues,159 on the other hand, reported that IVIG given on or before the fifth day of illness resulted in fewer coronary artery abnormalities at the 1-year follow-up assessment. Thus, decisions about the optimal date for treating with IVIG are best made based on a patient’s clinical status and the certainty of the diagnosis of KD.
Glucocorticoids
Glucocorticoids, the preferred initial treatment for other forms of vasculitis, had been considered unsafe in KD. This is based primarily on a study,160 which demonstrated an extraordinarily high incidence of coronary artery aneurysms (11 of 17 patients) in a group that received oral prednisolone at a dose of 2 to 3 mg/kg/day for at least two weeks, followed by 1.5 mg/kg/day for an additional two weeks. Interestingly, seven patients in the same study received prednisolone plus aspirin, and none developed aneurysms. In fact, no subsequent study has indicated that corticosteroids are harmful when used either with IVIG or as an alternative to IVIG therapy. If they are not contraindicated, then what, if any role might steroids have for treating KD?
Potential benefits of corticosteroids are supported most convincingly as “rescue therapy.” Initially, two retrospective analyses supported the use of corticosteroids in children who were unresponsive to two doses of IVIG or who relapsed after such therapy.161., 162. Hashino and colleagues163 also found a beneficial effect of glucocorticoids in KD in a prospective trial. Children who had failed to respond to two doses of IVIG were randomized to receive a third dose of IVIG or pulse-dose methylprednisolone. Patients who received methylprednisolone had a significantly shorter duration of fever, and although transient coronary artery dilatation was associated with glucocorticoid therapy, there was no overall difference in the incidence of coronary artery abnormalities between groups. Based on these studies, IVMP has become the mainstay of “rescue” therapy in children who are unresponsive to IVIG.93
Might steroids also have a role to play earlier in the course of KD? Shinohara and colleagues164 retrospectively reviewed the results in almost 300 patients with acute KD seen between 1982 and 1998 who were treated before the 10th day of illness. All patients received aspirin, dipyrimidole, and propranolol. The addition of prednisolone therapy, either alone or with IVIG, was associated with a significantly shorter duration of fever and a lower prevalence of coronary artery aneurysms. No adverse reactions were recorded for any therapy. A prospective study was suggestive of benefit as well; Inoue165 reported that the frequency of coronary artery abnormalities in children treated with IVIG plus prednisolone 2 mg/kg/d was lower than in those treated with IVIG alone. Three other studies161., 166., 167. have shown that children treated with IVMP (or dexamethasone) plus IVIG had faster resolution of fever, more rapid improvement in markers of inflammation, and shorter length of hospitalization than those receiving IVIG alone. Two of these studies had insufficient statistical power to detect a potential benefit of glucocorticoid therapy on coronary artery outcomes. The third trial, by Newburger and colleagues, found no significant difference in the frequency or severity of coronary artery lesions between treatment groups at one week or five week followup. Interestingly, however, post hoc analysis suggested that children who ultimately failed to respond to an initial dose of IVIG were less likely to develop coronary artery aneurysms if their initial therapy had included IVMP.
Following up on this finding, the Osaka Kawasaki Disease Study Group168 conducted a comparative trial of IVIG vs. IVIG plus IVMP in children with KD regarded as being at high risk for nonresponse to IVIG.169 High risk was defined as the presence of at least two of the following: CRP ≥7 mg, total bilirubin ≥0.9 mg, or aspartate transaminase ≥200 I.U./L. Patients were given heparin (10 U/kg/hr) for 48 hours beginning two hours before receiving IVMP (30 mg/kg), followed by IVIG (2 g/kg). Aspirin (30 mg/kg/d) was started at the end of the heparin infusion and reduced to 10 mg/kg/d after resolution of fever. Therapy was effective in 44% of those given IVIG alone compared to 66% of those receiving both IVIG and IVMP. The disease was refractory in 56% of those receiving IVIG alone and 11% of those receiving IVIG and IVMP, although a rebound fever was observed in 23% of those treated with IVIG and IVMP, but not in the group receiving IVIG alone. Coronary artery abnormalities, including aneurysms, were significantly less frequent in the IVIG plus IVMP group (24%) compared to the IVIG alone group (46%). In a meta-analysis of eight studies, Wooditch and Aronson concluded that the incidence of coronary artery aneurysms was reduced by the addition of corticosteroids to aspirin-containing therapeutic regimens.162
While it is clear that corticosteroids are not a substitute for IVIG in the treatment of KD, a case may be made for the inclusion of corticosteroids in the initial management of children with KD who are high risk of being refractory to IVIG. Studies to date have shown a decrease in the frequency of coronary artery abnormalities in some children who received IVIG plus corticosteroids as initial therapy, in comparison to those who received IVIG alone. The toxicity associated with combination therapy appears to be higher than that seen with IVIG alone, although reactions are generally not severe. Further, with the addition of pulsed dose methylprednisolone, the systemic manifestations of KD (fever, rash, etc.) appear to resolve more quickly, and the duration of hospitalization is, on average, shortened. Clinical and biological markers to identify such high-risk children are improving170 and ongoing trials are examining the effects of other adjunct therapies for treatment with IVIG. These studies should refine attempts to optimize the treatment of KD.
Anti-TNF Agents
Levels of TNFα are markedly increased in children with KD, especially in those who develop coronary artery lesions.171., 172. The use of monoclonal antibodies to TNFα inhibits actions of this cytokine. An anecdotal series reported the results of the use of infliximab, an anti TNFα monoclonal antibody, in 17 children with KD who had either persistent arthritis or persistent or recurrent fever 48 hours or more after treatment with IVIG (2 g/kg) and high dose aspirin.173 All had received at least two doses of IVIG, six had received three or more doses of IVIG, and eight had received one to three doses of intravenous methylprednisolone (30 mg/kg/dose). One patient had persistent fever, 15 had persistent fever and arthritis, and one had persistent severe arthritis; symptoms lasted for from eight to 53 days before infliximab was administered. Coronary artery abnormalities were present in 12 patients (including three with aneurysms and five with ectasia). A single infusion of infliximab (5 mg/kg) was given to 15 patients; two patients received 10 mg/kg. The fever responded promptly in 14 of 16 febrile patients, and levels of CRP fell. The effect on coronary artery abnormalities was not described in detail.
A prospective randomized multicenter comparison of the effectiveness of IVIG and infliximab in children who had not responded to an initial infusion of IVIG174 showed that both agents were equally effective and well-tolerated. Hirono and colleagues175 also found that infliximab was effective in controlling fever, but did not completely prevent coronary artery changes, although single case reports document resolution of aneurysms following infliximab therapy in some patients.176., 177. A retrospective comparison of patients at two institutions treated with either methylprednisolone or infliximab for KD resistant to initial treatment with IVIG found no differences in responses.178 The role of infliximab in treatment of KD, either as initial therapy or as treatment for resistant disease, remains uncertain.
Other Therapeutic Approaches
Therapies that are effective in other forms of vasculitis have been used in KD. Pentoxifylline was alleged to be effective in preventing coronary artery aneurysms,179 but demonstration of flaws in the analysis of the data in this study180 lead to the conclusion that it is ineffective. Similarly, the human trypsin inhibitor, Ulinastatin, has been the subject of studies from Japan. Its efficacy in preventing coronary artery disease in KD is not convincing.181
A dramatic response to plasmapheresis has been reported,182 but the technical limitations and potential hazards of this therapy are considerable. It should be reserved for children with active inflammation who have failed all available medical interventions, including multiple doses of IVIG, intravenous methylprednisolone, and TNF inhibition. There have been conflicting reports of the efficacy of abciximab, a monoclonal antibody that inhibits platelet glycoprotein IIb/IIIa receptor. In one study,183 there was an increased resolution of aneurysms in patients with KD who received abciximab compared to those who received conventional treatment. However, a second study184 could not duplicate these findings.
The role for immunosuppressive agents in KD, such as cyclophosphamide185 or cyclosporine,186 is extremely limited, but they may have a role in cases with persistent active disease unresponsive to conventional therapy. In addition, alternative diagnoses including chronic vasculitides, such as PAN, should be considered.
Treatment of Relapses
Fever returns within 48 hours of treatment with IVIG in 10% to 20% of children, indicating failure to suppress the underlying inflammatory process. Because prolonged fever is an independent risk factor for the development of coronary artery aneurysms, these children should be retreated with a second dose of IVIG (2 g/kg). Those who fail to respond to a second dose—up to one-third of patients in some studies93—are at extremely increased risk of developing coronary artery aneurysms. They should be treated in rapid succession with intravenous methylprednisolone (30 mg/kg/day for one to three days)151 or with infliximab (5 mg/kg). Treatment should continue until fever resolves and the CRP is normal, and frequent evaluations of the coronary arteries should be pursued until children have fully recovered.
Prevention and Management of Thromboses
The risk of thrombosis of coronary or other arteries depends on the degree of vascular damage. In all patients with KD, irrespective of the demonstration of coronary artery abnormalities, low-dose (3 to 5 mg/kg/day) aspirin should be continued until the ESR and platelet counts have normalized. Children with coronary artery abnormalities demonstrated by echocardiography are often treated with antithrombotic agents, such as low-dose aspirin, for as long as the abnormalities persist (Table 33–7 ). Children with large aneurysms are anticoagulated with warfarin. Trials with low-molecular-weight heparin are ongoing.
Table 33–7.
Recommendations for Long-term Follow-up
| Risk Level | Pharmacological Therapy | Physical Activity | Follow-Up and Diagnostic Testing | Invasive Testing |
|---|---|---|---|---|
| I (no coronary artery changes at any stage of illness) | None beyond 1st 6-8 weeks | No restrictions beyond 1st 6-8 weeks | Cardiovascular risk assessment counseling at 5 yr intervals | None recommended |
| II (transient coronary artery ectasia disappears within 1st 6-8 weeks) | None beyond 1st 6-8 weeks | No restrictions beyond 1st 6-8 weeks | Cardiovascular risk assessment counseling at 3 to 5 yr intervals | None recommended |
| III (1 small-medium coronary artery aneurysm/major coronary artery) | Low-dose aspirin (3-5 mg/kg aspirin/day), at least until aneurysm regression documented | For patients <11 yrs old, no restriction beyond 1st 6-8 weeks; patients 11-20 yrs old, physical activity guided by biennial stress test, evaluation of myocardial perfusion scan; contact or high-impact sports discouraged for patients taking antiplatelet agents | Annual cardiology follow-up with echocardiogram + ECG, combined with cardiovascular risk assessment, counseling; biennial stress test/evaluation of myocardial perfusion scan | Angiography, if noninvasive test suggests ischemia |
| IV (≥1 large or giant coronary artery aneurysm, or multiple or complex aneurysms in same coronary artery, without obstruction) | Long-term antiplatelet therapy and warfarin (target International normalized ratio 2.0-2.5) or low-molecular-weight heparin (target: antifactor Xa level 0.5-1.0 U/mL) should be combined in giant aneurysms | Contact or high-impact sports should be avoided because of risk of bleeding; other physical activity recommendations guided by stress test/evaluation of myocardial perfusion scan outcome | Biannual follow-up with echocardiogram + ECG; annual stress test/evaluation of myocardial perfusion scan | 1st angiography at 6-12 mo or sooner if clinically indicated; repeated angiography if noninvasive test, clinical, or laboratory findings suggest ischemia; elective repeat angiography under some circumstances |
| V (coronary artery obstruction) | Long-term low-dose aspirin; warfarin or low-molecular- weight heparin if giant aneurysm persists; consider use of β-blockers to reduce myocardial O2 consumption | Contact or high-impact sports should be avoided because of risk of bleeding; other physical activity recommendations guided by stress test/myocardial perfusion scan outcome | Biannual follow-up with echocardiogram and ECG; annual stress test/evaluation of myocardial perfusion scan | Angiography recommended to address therapeutic options |
From Newburger JW, Takahashi M, Gerber MA et al: Diagnosis, treatment and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease. Council on Cardiovascular Disease in the Young: American Heart Association, Pediatrics 114:1708-1733, 2004.
When injured coronary arteries become obstructed (risk level V) in addition to anticoagulation, various therapies have been attempted to restore circulation. Control of vascular inflammation with sufficient IVIG and other agents is an essential prerequisite to arterial reperfusion. Thereafter, treatments may include thrombolytic therapy for arterial thrombosis or vasodilators if tissue viability is primarily threatened by vasospasm. Urokinase, streptokinase, and tissue-type plasminogen have all been used for the lysis of coronary artery thromboses. Similarly, peripheral arterial obstruction may be corrected by thrombolysis, after which perfusion is maintained with heparin followed by a chronic oral anticoagulant regimen. If these treatments fail, a variety of invasive approaches have been suggested, including percutaneous transluminal coronary angioplasty187 and coronary artery bypass grafting.188 A small number of children with particularly severe coronary artery disease due to KD have required cardiac transplantation.189
Monitoring Cardiac Status
There is no universal agreement about the timing and frequency of echocardiographic monitoring of patients with KD. Most protocols take into account the development of coronary artery aneurysms, which occur most frequently between the second and the eighth weeks after the onset of fever. It is recommended190 that the initial echocardiogram should be obtained at the time a diagnosis of KD is suspected and that each child with KD should have a second echocardiogram obtained six to eight weeks after onset of the disease. Patients should also have repeated clinical examinations during the first 2 months to detect dysrhythmias, congestive heart failure, valvular insufficiency, or myocarditis.191 Further follow-up is individualized, with more frequent studies performed in children with demonstrated coronary artery abnormalities (see Table 33–7).
Children whose coronary arteries have always been normal (risk level I) or are normal by echocardiographic criteria one to two months after the acute illness (risk level II) are regarded as healthy, and no further intervention is recommended after the eight-week follow-up assessment. In view of the chronic abnormalities in endothelial function, however, many physicians consider a history of KD to be a risk factor for the development of coronary artery disease later in life.192 They counsel modification of other risk factors and continue to monitor children once every 5 years.
Single small- to medium-sized aneurysms (risk level III) usually resolve as determined by echocardiographic criteria, although this is not always the case. Healing occurs by fibrointimal proliferation, often accompanied by calcification, and vascular reactivity does not return to normal despite a grossly normal appearance.136 This point is highlighted by a report of sudden death in a 3.5-year-old child three months after dilated coronary arteries had regained a normal echocardiographic appearance.194 Autopsy revealed obliteration of the lumen of the left anterior descending coronary artery due to fibrosis, with evidence of ongoing active inflammation in the epicardial arteries. Such reports emphasize the need for confirmation of complete response to therapy in children who have had KD.
Giant aneurysms with an internal diameter of at least 8 mm represent a significant risk for morbidity and mortality, including a 35% chance of infarction (risk level IV).98 These children are followed more closely and are treated with more aggressive antithrombotic and anticoagulation regimens.
Disease Course and Prognosis
Although standard therapy with IVIG and aspirin given within the first 10 days of illness greatly improves outcomes, approximately 5% of children still develop coronary artery aneurysms, and more children demonstrate coronary artery ectasia.105 The mortality rate has dropped steadily as the diagnosis and treatment have improved. Currently the rate is about 0.1% in the U.S. and Japan.194., 195.
Recurrent disease after full recovery from a first episode of KD is rare but does occur. In Japan, the recurrence rate is 2.9%, with a higher incidence of cardiac complications during the second episode.165 In the U.S., the rate of recurrence is lower.
Entire reference list is available online at www.expertconsult.com
REFERENCES
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 6.Ozen S., Ruperto N., Dillon M.J. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns J.C., Mason W.H., Glode M. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. United States Multicenter Kawasaki Disease Study Group. J. Pediatr. 1991;118:680–686. doi: 10.1016/s0022-3476(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 9.Benseler S.M., McCrindle B.W., Silverman E.D. Infections and Kawasaki disease: implications for coronary artery outcome. Arthritis Rheum. 2003;48:S516. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld E.A., Corydon K.E., Shulman S.T. Kawasaki disease in infants less than one year of age. J. Pediatr. 1995;126:524–529. doi: 10.1016/s0022-3476(95)70344-6. [DOI] [PubMed] [Google Scholar]
- 13.Newburger J.W., Takahashi M., Burns J.C. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 26.Burns J.C., Cayan D.R., Tong G. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dergun M., Kao A., Hauger S.B. Familial occurrence of Kawasaki syndrome in North America. Arch. Pediatr. Adolesc. Med. 2005;159:876–881. doi: 10.1001/archpedi.159.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brogan P.A., Shah V., Klein N. V beta-restricted T-cell adherence to endothelial cells: A mechanism for superantigen-dependent vascular injury. Arthritis Rheum. 2007;50:589–597. doi: 10.1002/art.20021. [DOI] [PubMed] [Google Scholar]
- 38.Duong T.T., Silverman E.D., Bissessar M.V. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int. Immunol. 2003;15:79–89. doi: 10.1093/intimm/dxg007. [DOI] [PubMed] [Google Scholar]
- 43.Rowley A.H., Shulman S.T., Spike B.T. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J. Immunol. 2001;166:1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 53.Savage C.O., Tizard J., Jayne D. Antineutrophil cytoplasm antibodies in Kawasaki disease. Arch. Dis. Child. 1989;64:360–363. doi: 10.1136/adc.64.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biezefeld M., Geissler J., Weverling G. Polymorphisms in the mannose binding lectin of determinants of age-defined risk of coronary aneurysms artery lesions in Kawasaki disease. Arthritis Rheum. 2006;54:369–376. doi: 10.1002/art.21529. [DOI] [PubMed] [Google Scholar]
- 70.Ounouchi Y., Gunji T., Burns J.C. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary aneurysms. Nat. Gen. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgner D., Davila S., Breunis W.B. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker A.L., Lu M., Minich L. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J. Pediatr. 2009;154:592–595. doi: 10.1016/j.jpeds.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong G.W.K., McCrindle B., Ching J.C. Arthritis presenting during the acute phase of Kawasaki disease. J. Pediatr. 2006;148:800–805. doi: 10.1016/j.jpeds.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 85.Fukushige J., Takahashi N., Ueda Y. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994;83:1057–1060. doi: 10.1111/j.1651-2227.1994.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 87.Newburger J.W., Takahashi M., Gerber M.A. Diagnosis, treatment and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young. American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 88.Yellen E.S., Gauvreau K., Takahashi M. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010 Feb;125(2):e234–e241. doi: 10.1542/peds.2009-0606. Epub 2010 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newburger J.W., Sanders S.P., Burns J.C. Left ventricular contractility and function in Kawasaki syndrome. Effect of intravenous gamma-globulin. Circulation. 1989;79:1237–1246. doi: 10.1161/01.cir.79.6.1237. [DOI] [PubMed] [Google Scholar]
- 92.Moran A., Newburger J.W., Sanders S.P. Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma globulin. Am. Heart J. 2000;139:217–223. doi: 10.1067/mhj.2000.101221. [DOI] [PubMed] [Google Scholar]
- 93.Burns J.C., Capparelli E.V., Brown J.A. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr. Infect. Dis. J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Kato H., Ichinose E., Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J. Pediatr. 1986;108:923–927. doi: 10.1016/s0022-3476(86)80928-3. [DOI] [PubMed] [Google Scholar]
- 102.Tabarki B., Mahdhaoui A., Selmi H. Kawasaki disease with predominant central nervous system involvement. Pediatr. Neurol. 2001;25:239–241. doi: 10.1016/s0887-8994(01)00290-9. [DOI] [PubMed] [Google Scholar]
- 104.Sundel R.P., Cleveland S.S., Beiser A.S. Audiologic profiles of children with Kawasaki disease. Am. J. Otol. 1992;13:512–515. [PubMed] [Google Scholar]
- 107.Suddleson E.A., Reid B., Woolley M.M., Takahashi M. Hydrops of the gallbladder associated with Kawasaki syndrome. J. Pediatr. Surg. 1987;22:956–959. doi: 10.1016/s0022-3468(87)80600-0. [DOI] [PubMed] [Google Scholar]
- 108.Wang J.N., Chiou Y.Y., Chiu N.T. Renal scarring sequelae in childhood Kawasaki disease. Pediatr. Nephrol. 2007;22:684–689. doi: 10.1007/s00467-006-0385-y. [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara H., Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61:100–107. [PubMed] [Google Scholar]
- 128.Pickering L.K., Baker C.J., Long S.S., McMillan J.A., editors. Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. American Academy of Pediatrics; Elk Grove, Village, IL: 2006. American Academy of Pediatrics. Kawasaki disease; p. 414. [Google Scholar]
- 130.Ministry of Health and Welfare; Tokyo, Japan: 1984. Report of the Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. [Google Scholar]
- 133.Crystal M.A., Manlhiot C., Yeung R.S. Coronary artery dilation after Kawasaki disease for children within the normal range. Int. J. Cardiol. 2009;136:27–32. doi: 10.1016/j.ijcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 136.Dhillon R., Clarkson P., Donald A.E. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–2106. doi: 10.1161/01.cir.94.9.2103. [DOI] [PubMed] [Google Scholar]
- 139.Akagi T., Kato H., Inoue O. Salicylate treatment in Kawasaki disease: high dose or low dose? Eur. J. Pediatr. 1991;150:642–646. doi: 10.1007/BF02072625. [DOI] [PubMed] [Google Scholar]
- 141.Catella-Lawson F., Reilly M.P., Kapoor S.C. Cyclooxygenase inhibitors and the anti-platelet effects of aspirin. New. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 145.Newburger J.W., Takahashi M., Beiser A.S. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N. Engl. J. Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 149.Fukunishi M., Kikkawa M., Hamana K. Prediction of non-responsiveness to intravenous high dose gamma-globulin therapy in patients with Kawasaki disease at onset. J. Pediatr. 2000;137:172–176. doi: 10.1067/mpd.2000.104815. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi T., Inoue Y., Takeuchi K. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 151.Han R.K., Silverman E.D., Newman A. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch. Pediatr. Adolesc. Med. 2000;154:694–699. doi: 10.1001/archpedi.154.7.694. [DOI] [PubMed] [Google Scholar]
- 152.Mori M., Imagawa T., Yasui K. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J. Pediatr. 2000;137:177–180. doi: 10.1067/mpd.2000.107890. [DOI] [PubMed] [Google Scholar]
- 155.Bresee J.S., Mast E.E., Coleman P.J. Hepatitis C virus infection associated with administration of intravenous immune globulin. A cohort study. JAMA. 1996;276:1563–1567. [PubMed] [Google Scholar]
- 157.Sherer Y., Levy Y., Langevitz P. Adverse effects of intravenous immunoglobulin therapy in 56 patients with autoimmune diseases. Pharmacology. 2001;62:133–137. doi: 10.1159/000056085. [DOI] [PubMed] [Google Scholar]
- 158.Marasini M., Pongiglione G., Gazolo D. Late intravenous gamma globulin treatment in infants and children with Kawasaki disease and coronary artery abnormalities. Am. J. Cardiol. 1991;68:796–797. doi: 10.1016/0002-9149(91)90658-8. [DOI] [PubMed] [Google Scholar]
- 159.Tse S.M., Silverman E.D., McCrindle B.W. Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J. Pediatr. 2003;140:450–455. doi: 10.1067/mpd.2002.122469. [DOI] [PubMed] [Google Scholar]
- 161.Sundel R.P., Baker A.L., Fulton D.R. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J. Pediatr. 2003;142:611–616. doi: 10.1067/mpd.2003.191. [DOI] [PubMed] [Google Scholar]
- 162.Wooditch A.C., Aronoff S.C. Effect of initial corticosteroid therapy on coronary artery aneurysm formation in Kawasaki disease: A meta-analysis of 862 children. Pediatrics. 2005;116:989–995. doi: 10.1542/peds.2005-0504. [DOI] [PubMed] [Google Scholar]
- 163.Hashino K., Ishii M., Iemura M. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr. Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 165.Inoue Y., Okada Y., Shinohara M. A multicenter randomized trial of corticosteroids in primary therapy for Kawasaki disease: Clinical course and coronary artery outcome. J. Pediatr. 2006;149:336–341. doi: 10.1016/j.jpeds.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 167.Newburger J.W., Sleeper L.A., McCrindle B.W. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. New Engl. J. Med. 2007;356:664–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 169.Sano T., Kurotobi S., Matsuzaki K. Prediction of non-responsiveness to standard high-dose gamma globulin-therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Ped. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 170.Ogata S., Ogihara Y., Nomoto K. Clinical score and transcript abundance patterns identify Kawasaki disease patients who may benefit from addition of methylprednisolone. Pediatr. Res. 2009;66:577–584. doi: 10.1203/PDR.0b013e3181baa3c2. [DOI] [PubMed] [Google Scholar]
- 173.Burns J.C., Mason W.H., Hauger S.B. Infliximab treatment for refractory Kawasaki syndrome. J. Pediatr. 2005;146:662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 174.Burns J.C., Best B.M., Mejias A. Infliximab treatment of intravenous gamma globulin-resistant Kawasaki disease. J. Pediatr. 2008;153:833–838. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirono K., Kemmotsu Y., Wittkowski H. Infliximab reduces the cytokine-mediated inflammation but does not suppress the cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr. Res. 2009;65:696–701. doi: 10.1203/PDR.0b013e31819ed68d. [DOI] [PubMed] [Google Scholar]
- 178.Son M.B., Gauvreau K., Ma L., Baker A.L. Treatment of Kawasaki disease: analysis of 27th US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 188.Gotteiner N., Mavroudis C.V., Backer C.L. Coronary artery bypass grafting for Kawasaki disease. Pediatr. Cardiol. 2002;23:62–67. doi: 10.1007/s00246-001-0015-1. [DOI] [PubMed] [Google Scholar]
- 189.Checchia P.A., Pahl E., Shaddy R.E. Cardiac transplantation for Kawasaki disease. Pediatrics. 1997;100:695–699. doi: 10.1542/peds.100.4.695. [DOI] [PubMed] [Google Scholar]
- 190.Dajani A.S., Taubert K.A., Takahashi M. Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1994;89:916–922. doi: 10.1161/01.cir.89.2.916. [DOI] [PubMed] [Google Scholar]
- 192.Cheung Y.F., Yung T.C., Tam S.C. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J. Am. Coll. Cardiol. 2004;43:120–124. doi: 10.1016/j.jacc.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 193.McConnell M.E., Hannon D.W., Steed R.D. Fatal obliterative coronary vasculitis in Kawasaki disease. J. Pediatr. 1998;133:259–261. doi: 10.1016/s0022-3476(98)70230-6. [DOI] [PubMed] [Google Scholar]
- 195.Nakamura Y., Yanagawa H., Ojima T. Cardiac sequelae of Kawasaki disease among recurrent cases. Arch. Dis. Child. 1998;78:163–165. doi: 10.1136/adc.78.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Shulman S.T. The first reported case of Kawasaki disease? J. Pediatr. 1999;135:532. doi: 10.1016/s0022-3476(99)70187-3. [DOI] [PubMed] [Google Scholar]
- 3.Matteson E.L. Notes on the history of eponymic idiopathic vasculitis: the diseases of Henoch and Schonlein, Wegener, Churg and Strauss, Horton, Takayasu, Behçet, and Kawasaki. Arthritis Care. Res. 2000;13:237–245. doi: 10.1002/1529-0131(200008)13:4<237::aid-anr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Munro-Faure H. Necrotizing arteritis of the coronary vessels in infancy. Pediatrics. 1959;23:914–926. [PubMed] [Google Scholar]
- 5.Itoga S., Yamagishi M. Steroid treatment for mucocutaneous ocular syndrome in childhood. Chiryo. (Japan) 1960;42:1174–1179. [Google Scholar]
- 6.Ozen S., Ruperto N., Dillon M.J. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muta H., Ishii M., Iemura M. Effect of revision of Japanese diagnostic criterion for fever in Kawasaki disease on treatment and cardiovascular outcome. Circ. J. 2007;71:1791–1793. doi: 10.1253/circj.71.1791. [DOI] [PubMed] [Google Scholar]
- 8.Burns J.C., Mason W.H., Glode M. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. United States Multicenter Kawasaki Disease Study Group. J. Pediatr. 1991;118:680–686. doi: 10.1016/s0022-3476(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 9.Benseler S.M., McCrindle B.W., Silverman E.D. Infections and Kawasaki disease: implications for coronary artery outcome. Arthritis Rheum. 2003;48:S516. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh Y.C., Wu M.H., Wang J.K. Clinical features of atypical Kawasaki disease. J. Microbiol. Immunol. Infect. 2002;35:57–60. [PubMed] [Google Scholar]
- 11.Witt M.T., Minich L.L., Bohnsack J.F. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999;104:e10. doi: 10.1542/peds.104.1.e10. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld E.A., Corydon K.E., Shulman S.T. Kawasaki disease in infants less than one year of age. J. Pediatr. 1995;126:524–529. doi: 10.1016/s0022-3476(95)70344-6. [DOI] [PubMed] [Google Scholar]
- 13.Newburger J.W., Takahashi M., Burns J.C. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa H., Nakamura Y., Yashiro M. Incidence of Kawasaki disease in Japan: the nationwide surveys of 1999-2002. Ped. International. 2006;48:356–361. doi: 10.1111/j.1442-200X.2006.02221.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y., Yashiro, Uehara R. Epidemiologic features of Kawasaki disease in Japan: results from the nationwide survey in 2005-6. J. Epidemiol. 2008;18:167–172. doi: 10.2188/jea.JE2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman S.T., McAuley J.B., Pachman L.M. Risk of coronary abnormalities due to Kawasaki disease in urban area with small Asian population. Am. J. Dis. Child. 1987;141:420–425. doi: 10.1001/archpedi.1987.04460040078020. [DOI] [PubMed] [Google Scholar]
- 17.Huang W.C., Huang L.M., Chang I.S. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123:e401–e405. doi: 10.1542/peds.2008-2187. [DOI] [PubMed] [Google Scholar]
- 18.Holman R.C., Curns A.T., Belay E.D. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112:495–501. doi: 10.1542/peds.112.3.495. [DOI] [PubMed] [Google Scholar]
- 19.Gardner-Medwin J.M., Dolezalova P., Cummins C. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–1202. doi: 10.1016/S0140-6736(02)11279-7. [DOI] [PubMed] [Google Scholar]
- 20.Stockheim J.A., Innocentini N., Shulman S.T. Kawasaki disease in older children and adolescents. J. Pediatr. 2000;137:250–252. doi: 10.1067/mpd.2000.105150. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama J., Hasegawa Y., Kumagai Y. Acute febrile mucocutaneous lymph node syndrome (Kawasaki disease) in adults: case report and review of the literature. Jpn. J. Med. 1991;30:285–289. doi: 10.2169/internalmedicine1962.30.285. [DOI] [PubMed] [Google Scholar]
- 22.Chang R.K. The incidence of Kawasaki disease in the United States did not increase between 1988 and 1997. Pediatrics. 2003;111:1124–1125. doi: 10.1542/peds.111.5.1124. [DOI] [PubMed] [Google Scholar]
- 23.Yanagawa H., Yashiro M., Nakamura Y. Epidemiologic pictures of Kawasaki disease in Japan: from the nationwide incidence survey in 1991 and 1992. Pediatrics. 1995;95 475–449. [PubMed] [Google Scholar]
- 24.Royle J.A., Williams K., Elliott E. Kawasaki disease in Australia, 1993-95. Arch. Dis. Child. 1998;78:33–39. doi: 10.1136/adc.78.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell D.M., Morens D.M., Holman R.C. Kawasaki syndrome in the United States 1976 to 1980. Am. J. Dis. Child. 1983;137:211–214. doi: 10.1001/archpedi.1983.02140290003001. [DOI] [PubMed] [Google Scholar]
- 26.Burns J.C., Cayan D.R., Tong G. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao A.S., Getis A., Brodine S. Spatial and temporal clustering of Kawasaki syndrome cases. Ped. Inf. Dis. J. 2008;27:881–885. doi: 10.1097/INF.0b013e31817acf4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagawa H., Nakamura Y., Ojima T. Changes in epidemic patterns of Kawasaki disease in Japan. Pediatr. Infect. Dis. J. 1999;18:64–66. doi: 10.1097/00006454-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Fujita Y., Nakamura Y., Sakata K. Kawasaki disease in families. Pediatrics. 1989;84:666–669. [PubMed] [Google Scholar]
- 30.Dergun M., Kao A., Hauger S.B. Familial occurrence of Kawasaki syndrome in North America. Arch. Pediatr. Adolesc. Med. 2005;159:876–881. doi: 10.1001/archpedi.159.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowley A.H., Wolinsky S.M., Relman D.A. Search for highly conserved viral and bacterial nucleic acid sequences corresponding to an etiologic agent of Kawasaki disease. Pediatr. Res. 1994;36:567–571. doi: 10.1203/00006450-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rowley A.H. The etiology of Kawasaki disease: superantigen or conventional antigen? Pediatr. Infect. Dis. J. 1999;18:69–70. doi: 10.1097/00006454-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Leung D.Y., Giorno R.C., Kazemi L.V. Evidence for superantigen involvement in cardiovascular injury due to Kawasaki syndrome. J. Immunol. 1995;155:5018–5021. [PubMed] [Google Scholar]
- 34.Yamashiro Y., Nagata S., Oguchi S. Selective increase of VB2+ T-cells in the small intestinal mucosa in Kawasaki disease. Pediatr. Res. 1996;39:264–266. doi: 10.1203/00006450-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Brogan P.A., Shah V., Clarke L.A. T cell activation profiles in Kawasaki syndrome. Clin. Exp. Immunol. 2007;151:267–274. doi: 10.1111/j.1365-2249.2007.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brogan P.A., Shah V., Klein N. V beta-restricted T-cell adherence to endothelial cells: A mechanism for superantigen-dependent vascular injury. Arthritis Rheum. 2007;50:589–597. doi: 10.1002/art.20021. [DOI] [PubMed] [Google Scholar]
- 37.Nomura Y., Yoshinaga M., Masuda K. Maternal antibody against toxic shock syndrome toxin-1 may protect infants younger than 6 months of age from developing Kawasaki syndrome. J. Infect. Dis. 2002;185:1677–1680. doi: 10.1086/340513. [DOI] [PubMed] [Google Scholar]
- 38.Duong T.T., Silverman E.D., Bissessar M.V. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int. Immunol. 2003;15:79–89. doi: 10.1093/intimm/dxg007. [DOI] [PubMed] [Google Scholar]
- 39.Hsu Y.H., Wang Y.H., Hsu W.Y. Kawasaki disease characterized by erythema and induration at the Bacillus Calmette-Guérin and purified protein derivative inoculation sites. Pediatr. Infect. Dis. J. 1987;6:576–578. [PubMed] [Google Scholar]
- 40.Bertotto A., Spinozzi F., Vagliasindi C. Tuberculin skin test reactivity in Kawasaki disease. Pediatr. Res. 1997;41:560–562. doi: 10.1203/00006450-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Yokota S., Tsubaki K., Kuriyama T. Presence in Kawasaki disease of antibodies to mycobacterial heat-shock protein HSP65 and autoantibodies to epitopes of human HSP65 cognate antigen. Clin. Immunol. Immunopathol. 1993;67:163–170. doi: 10.1006/clin.1993.1060. [DOI] [PubMed] [Google Scholar]
- 42.Terai M., Miwa K., Williams T. The absence of evidence of staphylococcal toxin involvement in the pathogenesis of Kawasaki disease. J. Infect. Dis. 1995;172:558–561. doi: 10.1093/infdis/172.2.558. [DOI] [PubMed] [Google Scholar]
- 43.Rowley A.H., Shulman S.T., Spike B.T. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J. Immunol. 2001;166:1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 44.Kikuta H., Sakiyama Y., Matsumoto S. Detection of Epstein-Barr virus DNA in cardiac and aortic tissues from chronic, active Epstein-Barr virus infection associated with Kawasaki disease-like coronary artery aneurysms. J. Pediatr. 1993;123:90–92. doi: 10.1016/s0022-3476(05)81546-x. [DOI] [PubMed] [Google Scholar]
- 45.Matsuno M., Utagawa E., Sugiura A. Association of rotavirus infections with Kawasaki syndrome. J. Infect. Dis. 1983;148:177. doi: 10.1093/infdis/148.1.177. [DOI] [PubMed] [Google Scholar]
- 46.Okano M., Thiele G.M., Sakiyama Y. Adenovirus infection in patients with Kawasaki disease. J. Med. Virol. 1990;32:53–57. doi: 10.1002/jmv.1890320109. [DOI] [PubMed] [Google Scholar]
- 47.Holm J.M., Hansen L.K., Oxhøj H. Kawasaki disease associated with parvovirus B19 infection. Eur. J. Pediatr. 1995;154:633–634. doi: 10.1007/BF02079066. [DOI] [PubMed] [Google Scholar]
- 48.Burns J.C., Geha R.S., Schneeberger E.E. Polymerase activity in lymphocyte culture supernatants from patients with Kawasaki disease. Nature. 1986;323:814–816. doi: 10.1038/323814a0. [DOI] [PubMed] [Google Scholar]
- 49.Normann E., Nääs J., Gnarpe J. Demonstration of Chlamydia pneumoniae in cardiovascular tissues from children with Kawasaki disease. Pediatr. Infect. Dis. J. 1999;18:72–73. doi: 10.1097/00006454-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Esper F., Shapiro E.D., Weibel C. Association between a novel human coronavirus and Kawasaki disease. J. Inf. Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann C., Klar, Lindner J. Kawasaki disease lacks association with human coronavirus NL53 and human bocavirus. Ped. Inf. Dis. J. 2009;28:553–554. doi: 10.1097/inf.0b013e31819f41b6. [DOI] [PubMed] [Google Scholar]
- 52.Nonoyama S. Immunological abnormalities and endothelial cell injury in Kawasaki disease. Acta. Paediatr. Jpn. 1991;33:752–755. doi: 10.1111/j.1442-200x.1991.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 53.Savage C.O., Tizard J., Jayne D. Antineutrophil cytoplasm antibodies in Kawasaki disease. Arch. Dis. Child. 1989;64:360–363. doi: 10.1136/adc.64.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C.Y., Lin C.C., Hwang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J. Pediatr. 1992;121:924–926. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 55.Yasukawa K., Terai M., Shulman S.T. Systemic production of vascular endothelial growth factor and fms-like tyrosine kinase-1 receptor in acute Kawasaki disease. Circulation. 2002;105:766–769. doi: 10.1161/hc0602.103396. [DOI] [PubMed] [Google Scholar]
- 56.Kuijpers T., Wiegman A., van Lier R.A. Kawasaki disease: a maturational defect in immune responsiveness. J. Infect. Dis. 1999;180:1869–1877. doi: 10.1086/315111. [DOI] [PubMed] [Google Scholar]
- 57.Yanagawa H., Yashiro M., Nakamura Y. Nationwide surveillance of Kawasaki disease in Japan, 1984 to 1993. Pediatr. Infect. Dis. J. 1995;14:69–71. doi: 10.1097/00006454-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Harada F., Sada M., Kamiya T. Genetic analysis of Kawasaki syndrome. Am. J. Hum. Genet. 1986;39:537–539. [PMC free article] [PubMed] [Google Scholar]
- 59.Uehara R., Yoshiro M., Nakamura Y. Kawasaki disease in parents and children. Acta. Paediatr. 2003;92:694–697. doi: 10.1080/08035320310002768. [DOI] [PubMed] [Google Scholar]
- 60.Hata A., Onouchi Y. Susceptibility genes for Kawasaki Disease: Toward implementation of personalized medicine. J. Hum. Gen. 2009;54:67–73. doi: 10.1038/jhg.2008.9. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y., Lee Y.J., Chen M.R. Polymorphism of transmembrane region of MICA gene and Kawasaki disease. Exp. Clin. Immunogenet. 2000;17:130–137. doi: 10.1159/000019132. [DOI] [PubMed] [Google Scholar]
- 62.Onouchi Y., Tamari M., Takahashi A. A genomewide linkage analysis of Kawasaki disease: evidence for linkage to chromosome 12. J. Hum. Gen. 2007;12:179–190. doi: 10.1007/s10038-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 63.Barron K.S., Silverman E.D., Gonzales J. Major histocompatibility complex class II alleles in Kawasaki syndrome—lack of consistent correlation with disease or cardiac involvement. J. Rheumatol. 1992;19:1790–1793. [PubMed] [Google Scholar]
- 64.Quasney M.W., Bronstein D.E., Cantor R.M. Increased frequency of alleles associated with elevated tumor necrosis factor-alpha levels in children with Kawasaki disease. Pediatr. Res. 2001;49:686–690. doi: 10.1203/00006450-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Chen S.Y., Wan L., Huang Y.C. Interleukin 18 gene 105 A/C genetic polymorphisms associated with susceptibility to Kawasaki Disease. J. Clin. Lab. Analysis. 2009;23:71–76. doi: 10.1002/jcla.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin Y.J., Wan L., Wu J. HLA-E gene polymorphism associated with susceptibility to Kawasaki disease and formation of coronary artery aneurysms. Arthritis Rheum. 2009;60:604–610. doi: 10.1002/art.24261. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi K., Yamamoto K.L., Kataoka S. High incidence of angiotensin I converting enzyme II in Kawasaki disease patients with coronary aneurysms. Eur. J. Pediatr. 1997;156:266–268. doi: 10.1007/s004310050597. [DOI] [PubMed] [Google Scholar]
- 68.Shim Y.H., Kim H.S., Hong Y.M. Insertion/deletion polymorphisms of angiotensin converting enzyme gene in Kawasaki disease. J. Korean. Med. Sci. 2000;21:208–211. doi: 10.3346/jkms.2006.21.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biezefeld M., Geissler J., Weverling G. Polymorphisms in the mannose binding lectin of determinants of age-defined risk of coronary aneurysms artery lesions in Kawasaki disease. Arthritis Rheum. 2006;54:369–376. doi: 10.1002/art.21529. [DOI] [PubMed] [Google Scholar]
- 70.Ounouchi Y., Gunji T., Burns J.C. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary aneurysms. Nat. Gen. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgner D., Davila S., Breunis W.B. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS. Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker A.L., Lu M., Minich L. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J. Pediatr. 2009;154:592–595. doi: 10.1016/j.jpeds.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chao S.M., Phua K.B. Perineal eruption as an early sign of Kawasaki disease. Ann. Acad. Med. Sing. 1991;20:244–247. [PubMed] [Google Scholar]
- 74.Yoshikawa H., Nomura Y., Masuda K. Four cases of Kawasaki syndrome complicated with myocarditis. Circ. J. 2006;70:202–205. doi: 10.1253/circj.70.202. [DOI] [PubMed] [Google Scholar]
- 75.Wang S., Best B.M., Burns J.C. Periungual desquamation in patients with Kawasaki disease. Pediatr. Infect. Dis. J. 2009;28:538–539. doi: 10.1097/INF.0b013e3181945984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong G.W.K., McCrindle B., Ching J.C. Arthritis presenting during the acute phase of Kawasaki disease. J. Pediatr. 2006;148:800–805. doi: 10.1016/j.jpeds.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 77.Barron K.S. Kawasaki disease: etiology, pathogenesis, and treatment. Cleve. Clin. J. Med. 2002;69:SII69–SII78. doi: 10.3949/ccjm.69.suppl_2.sii69. [DOI] [PubMed] [Google Scholar]
- 78.Ammerman S.D., Rao M.S., Shope T.C. Diagnostic uncertainty in atypical Kawasaki disease, and a new finding: exudative conjunctivitis. Pediatr. Infect. Dis. J. 1985;4:210–211. doi: 10.1097/00006454-198503000-00023. [DOI] [PubMed] [Google Scholar]
- 79.Smith L.B., Newburger J.W., Burns J.C. Kawasaki syndrome and the eye. Pediatr. Infect. Dis. J. 1989;8:116–118. [PubMed] [Google Scholar]
- 80.Ryan E.H., Walton D.S. Conjunctival scarring in Kawasaki disease: a new finding? J. Pediatr. Ophthalmol. Strabismus. 1983;20:106–108. doi: 10.3928/0191-3913-19830501-04. [DOI] [PubMed] [Google Scholar]
- 81.Jacob J.L., Polomeno R.C., Chad Z. Ocular manifestations of Kawasaki disease (mucocutaneous lymph node syndrome) Can. J. Ophthalmol. 1982;17:199–202. [PubMed] [Google Scholar]
- 82.Farvardin M., Kashef S., Aleyasin S. Sudden unilateral blindness in a girl with Kawasaki disease. Ped. Ophth. Strab. 2007;44:303–304. doi: 10.3928/01913913-20070901-06. [DOI] [PubMed] [Google Scholar]
- 83.Eberhard B.A., Sundel R.P., Newburger J.W. Psoriatic eruption in Kawasaki disease. J. Pediatr. 2000;137:578–580. doi: 10.1067/mpd.2000.107840. [DOI] [PubMed] [Google Scholar]
- 84.Tashiro N., Matsubara T., Uchida M. Ultrasonographic evaluation of cervical lymph nodes in Kawasaki disease. Pediatrics. 2002;109:E77–E87. doi: 10.1542/peds.109.5.e77. [DOI] [PubMed] [Google Scholar]
- 85.Fukushige J., Takahashi N., Ueda Y. Incidence and clinical features of incomplete Kawasaki disease. Acta. Paediatr. 1994;83:1057–1060. doi: 10.1111/j.1651-2227.1994.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 86.Joffe A., Kabani A., Jadavji T. Atypical and complicated Kawasaki disease in infants: Do we need criteria? West. J. Med. 1995;162:322–327. [PMC free article] [PubMed] [Google Scholar]
- 87.Newburger J.W., Takahashi M., Gerber M.A. Diagnosis, treatment and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young. American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 88.E.S Yellen, K. Gauvreau, M. Takahashi, et al., Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease, Pediatrics 125 (2) (2010 Feb) e234–e241. Epub Jan 25 2010. [DOI] [PMC free article] [PubMed]
- 89.Kato H., Takahashi K. Kawasaki’s disease. In: Ball G.V., Bridges S.L. Jr., editors. Vasculitis. second ed. Oxford University Press; Oxford: 2008. [Google Scholar]
- 90.Matsuura H., Ishikita T., Yamamoto S. Gallium-67 myocardial imaging for the detection of myocarditis in the acute phase of Kawasaki disease (mucocutaneous lymph node syndrome): the usefulness of single photon emission computed tomography. Br. Heart. J. 1987;58:385–392. doi: 10.1136/hrt.58.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newburger J.W., Sanders S.P., Burns J.C. Left ventricular contractility and function in Kawasaki syndrome: effect of intravenous gamma-globulin. Circulation. 1989;79:1237–1246. doi: 10.1161/01.cir.79.6.1237. [DOI] [PubMed] [Google Scholar]
- 92.Moran A., Newburger J.W., Sanders S.P. Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma globulin. Am. Heart. J. 2000;139:217–223. doi: 10.1067/mhj.2000.101221. [DOI] [PubMed] [Google Scholar]
- 93.Burns J.C., Capparelli E.V., Brown J.A. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr. Infect. Dis. J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 94.Yanagawa H., Tuohong Z., Oki I. Effects of gamma-globulin on the cardiac sequelae of Kawasaki disease. Pediatr. Cardiol. 1999;20:248–251. doi: 10.1007/s002469900458. [DOI] [PubMed] [Google Scholar]
- 95.Kato H., Ichinose E., Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J. Pediatr. 1986;108:923–927. doi: 10.1016/s0022-3476(86)80928-3. [DOI] [PubMed] [Google Scholar]
- 96.Kato H., Ichinose E., Yoshioka F. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am. J. Cardiol. 1982;49:1758–1766. doi: 10.1016/0002-9149(82)90256-9. [DOI] [PubMed] [Google Scholar]
- 97.Iemura M., Ishii M., Sugimura T. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato H., Sugimura T., Akagi T. Long-term consequences of Kawasaki disease: a 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 99.Mele T., Evans M. Intestinal obstruction as a complication of Kawasaki disease. J. Pediatr. Surg. 1996;31:985–986. doi: 10.1016/s0022-3468(96)90430-3. [DOI] [PubMed] [Google Scholar]
- 100.Zulian F., Falcini F., Zancan L. Acute surgical abdomen as presenting manifestation of Kawasaki disease. J. Pediatr. 2003;142:731–735. doi: 10.1067/mpd.2003.232. [DOI] [PubMed] [Google Scholar]
- 101.Dengler L.D., Capparelli E.V., Bastian J.F. Cerebrospinal fluid profile in patients with acute Kawasaki disease. Pediatr. Infect. Dis. J. 1998;17:478–481. doi: 10.1097/00006454-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Tabarki B., Mahdhaoui A., Selmi H. Kawasaki disease with predominant central nervous system involvement. Pediatr. Neurol. 2001;25:239–241. doi: 10.1016/s0887-8994(01)00290-9. [DOI] [PubMed] [Google Scholar]
- 103.Larralde M., Santos-Munoz A., Rutiman R. Kawasaki disease with facial nerve paralysis. Pediatr. Dermatol. 2003;20:511–513. doi: 10.1111/j.1525-1470.2003.20612.x. [DOI] [PubMed] [Google Scholar]
- 104.Sundel R.P., Cleveland S.S., Beiser A.S. Audiologic profiles of children with Kawasaki disease. Am. J. Otol. 1992;13:512–515. [PubMed] [Google Scholar]
- 105.Knott P.D., Orloff L.A., Harris J.P. Sensorineural hearing loss and Kawasaki disease: a prospective study. Am. J. Otolaryngol. 2001;22:343–348. doi: 10.1053/ajot.2001.26495. [DOI] [PubMed] [Google Scholar]
- 106.Maurer K., Unsinn K., Waltner-Romen M. Segmental bowel-wall thickening on abdominal ultrasonography: an additional diagnostic sign in Kawasaki disease. Pediatr. Radiol. 2008;38:1013–1016. doi: 10.1007/s00247-008-0900-3. [DOI] [PubMed] [Google Scholar]
- 107.Suddleson E.A., Reid B., Woolley M.M., Takahashi M. Hydrops of the gallbladder associated with Kawasaki syndrome. J. Pediatr. Surg. 1987;22:956–959. doi: 10.1016/s0022-3468(87)80600-0. [DOI] [PubMed] [Google Scholar]
- 108.Wang J.N., Chiou Y.Y., Chiu N.T. Renal scarring sequelae in childhood Kawasaki disease. Pediatr. Nephrol. 2007;22:684–689. doi: 10.1007/s00467-006-0385-y. [DOI] [PubMed] [Google Scholar]
- 109.Waring N.P., Ortenberg J., Galen W.K. Priapism in Kawasaki disease. JAMA. 1989;261:1730–1731. [PubMed] [Google Scholar]
- 110.Ferreiro D.M., Wolsdorf J.I. Hemolytic uremic syndrome associated with Kawasaki disease. Pediatrics. 1981;68:405–406. [PubMed] [Google Scholar]
- 111.Salcedo J.R., Greenberg L., Kapur S. Renal histology of mucocutaneous lymph node syndrome (Kawasaki disease) Clin. Nephrol. 1988;29:47–51. [PubMed] [Google Scholar]
- 112.Lande M.B., Gleeson J.G., Sundel R.P. Kawasaki disease and acute renal failure. Pediatr. Nephrol. 1993;7:593. doi: 10.1007/BF00852560. [DOI] [PubMed] [Google Scholar]
- 113.Naoe S., Takahashi K., Masuda H. Kawasaki disease: with particular emphasis on arterial lesions. Acta. Pathol. Jpn. 1991;41:785–797. doi: 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara H., Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61:100–107. [PubMed] [Google Scholar]
- 115.Jennette J.C. Implications for pathogenesis of patterns of injury in small- and medium-sized vessel vasculitis. Cleve. Clin. J. Med. 2002;69:SII33–SII38. doi: 10.3949/ccjm.69.suppl_2.sii33. [DOI] [PubMed] [Google Scholar]
- 116.Leung D.Y., Cotran R.S., Kurt-Jones E. Endothelial activation in the pathogenesis of Kawasaki disease. Trans. Assoc. Am. Physicians. 1989;102:131–138. [PubMed] [Google Scholar]
- 117.Yonesaka S., Nakada T., Sunagawa Y. Endomyocardial biopsy in children with Kawasaki disease. Acta. Paediatr. Jpn. 1989;31:706–711. doi: 10.1111/j.1442-200x.1989.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 118.Suzuki A., Kamiya T., Kuwahara N. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr. Cardiol. 1986;7:3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 119.Rowe P.C., Quinlan A., Luke B.K. Value of degenerative change in neutrophils as a diagnostic test for Kawasaki syndrome. J. Pediatr. 1991;119:370–374. doi: 10.1016/s0022-3476(05)82047-5. [DOI] [PubMed] [Google Scholar]
- 120.Hara T., Mizuno Y., Ueda K. Neutropenia in Kawasaki disease. Eur. J. Pediatr. 1989;148:580. doi: 10.1007/BF00441566. [DOI] [PubMed] [Google Scholar]
- 121.Cummings C., McCarthy P., van Hoff J. Kawasaki disease associated with reactive hemophagocytic lymphohistiocytosis. Pediatr. Infect. Dis. J. 2008;27:1116–1118. doi: 10.1097/INF.0b013e31817ecb6d. [DOI] [PubMed] [Google Scholar]
- 122.Salo E., Pesonen E., Viikari J. Serum cholesterol levels during and after Kawasaki disease. J. Pediatr. 1991;119:557–561. doi: 10.1016/s0022-3476(05)82404-7. [DOI] [PubMed] [Google Scholar]
- 123.Soppi E., Salo E., Pelkonen P. Antibodies against neutrophil cytoplasmic components in Kawasaki disease. Acta. Path. Microbiol. Immunol. Scand. 1992;100:269–272. doi: 10.1111/j.1699-0463.1992.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 124.Grunebaum E., Blank M., Cohen S. The role of anti-endothelial cell antibodies in Kawasaki disease—in vitro and in vivo studies. Clin. Exp. Immunol. 2002;130:233–240. doi: 10.1046/j.1365-2249.2002.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guzman J., Fung M., Petty R.E. Diagnostic value of anti-neutrophil cytoplasmic and anti-endothelial cell antibodies in early Kawasaki disease. J. Pediatr. 1994;124:917–920. doi: 10.1016/s0022-3476(05)83180-4. [DOI] [PubMed] [Google Scholar]
- 126.Irazuzta J.E., Elbl F., Rees A.R. Factor VIII related antigen (von Willebrand’s factor) in Kawasaki disease. Clin. Pediatr. (Phila.) 1990;29:347–348. doi: 10.1177/000992289002900613. [DOI] [PubMed] [Google Scholar]
- 127.Laxer R.M., Schaffer F.M., Myones B.L. Lymphocyte abnormalities and complement activation in Kawasaki disease. Prog. Clin. Biol. Res. 1987;250:175–184. [PubMed] [Google Scholar]
- 128.American Academy of Pediatrics. Kawasaki disease, in: L.K. Pickering, C.J. Baker, S.S Long, J.A. McMillan (Eds.), Red Book: 2006 Report of the Committee on Infectious Diseases, 27th ed., American Academy of Pediatrics, Elk Grove, Village, IL , 2006, pp. 414 .
- 129.Ishihara H., Watanabe S., Izumida N. Detection of coronary arterial lesions by two dimensional echocardiography in patients with Kawasaki disease. Bull. Tokyo. Med. Dent. Univ. 1984;31:139–143. [PubMed] [Google Scholar]
- 130.Ministry of Health and Welfare; Tokyo, Japan: 1984. Report of the Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. [Google Scholar]
- 131.Oberhoffer R., Lang D., Feilen K. The diameter of coronary arteries in infants and children without heart disease. Eur. J. Pediatr. 1989;148:389–392. doi: 10.1007/BF00595893. [DOI] [PubMed] [Google Scholar]
- 132.de Zorzi A., Colan S.D., Gauvreau K. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J. Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 133.Crystal M.A., Manlhiot C., Yeung R.S. Coronary artery dilation after Kawasaki disease for children within the normal range. Int. J. Cardiol. 2009;136:27–32. doi: 10.1016/j.ijcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 134.Tanaka N., Naoe S., Masuda H. Pathological study of sequelae of Kawasaki disease (MCLS) with special reference to the heart and coronary arterial lesions. Acta. Pathol. Jpn. 1986;36:1513–1527. doi: 10.1111/j.1440-1827.1986.tb02823.x. [DOI] [PubMed] [Google Scholar]
- 135.Furuyama H., Odagawa Y., Katoh C. Assessment of coronary function in children with a history of Kawasaki disease using (15)O-water positron emission tomography. Circulation. 2002;105:2878–2884. doi: 10.1161/01.cir.0000018652.59840.57. [DOI] [PubMed] [Google Scholar]
- 136.Dhillon R., Clarkson P., Donald A.E. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–2106. doi: 10.1161/01.cir.94.9.2103. [DOI] [PubMed] [Google Scholar]
- 137.Nakamura Y., Yanagawa H., Harada K. Mortality among persons with a history of Kawasaki disease in Japan: the fifth look. Arch. Pediatr. Adolesc. Med. 2002;156:162–165. doi: 10.1001/archpedi.156.2.162. [DOI] [PubMed] [Google Scholar]
- 138.Kusakawa S., Tatara K. Efficacies and risks of aspirin in the treatment of the Kawasaki disease. Prog. Clin. Biol. Res. 1987;250:401–413. [PubMed] [Google Scholar]
- 139.Akagi T., Kato H., Inoue O. Salicylate treatment in Kawasaki disease: high dose or low dose? Eur. J. Pediatr. 1991;150:642–646. doi: 10.1007/BF02072625. [DOI] [PubMed] [Google Scholar]
- 140.Durongpisitkul K., Gururaj V.J., Partin J.M. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–1061. [PubMed] [Google Scholar]
- 141.Catella-Lawson F., Reilly M.P., Kapoor S.C. Cyclooxygenase inhibitors and the anti-platelet effects of aspirin. New. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 142.Wei C.M., Chen H.L., Lee O.I. Reye’s syndrome developing in an infant on treatment of Kawasaki syndrome. J. Paediatr. Child. Health. 2005;41:303–304. doi: 10.1111/j.1440-1754.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 143.Koren G., Silverman E., Sundel R. Decreased protein binding of salicylates in Kawasaki disease. J. Pediatr. 1991;118:456–459. doi: 10.1016/s0022-3476(05)82168-7. [DOI] [PubMed] [Google Scholar]
- 144.Furusho K., Kamiya T., Nakano H. High-dose intravenous gamma-globulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 145.Newburger J.W., Takahashi M., Beiser A.S. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N. Engl. J. Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 146.Terai M., Shulman S.T. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J. Pediatr. 1997;131:888–893. doi: 10.1016/s0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 147.Newburger J. Kawasaki disease: who is at risk? J. Pediatr. 2000;137:149–152. doi: 10.1067/mpd.2000.109025. [DOI] [PubMed] [Google Scholar]
- 148.Stockheim A., Innocentini N., Shulman S. Kawasaki disease in older children and adolescents. J. Pediatr. 2000;137:250–252. doi: 10.1067/mpd.2000.105150. [DOI] [PubMed] [Google Scholar]
- 149.Fukunishi M., Kikkawa M., Hamana K. Prediction of non-responsiveness to intravenous high dose gamma-globulin therapy in patients with Kawasaki disease at onset. J. Pediatr. 2000;137:172–176. doi: 10.1067/mpd.2000.104815. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi T., Inoue Y., Takeuchi K. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 151.Han R.K., Silverman E.D., Newman A. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch. Pediatr. Adolesc. Med. 2000;154:694–699. doi: 10.1001/archpedi.154.7.694. [DOI] [PubMed] [Google Scholar]
- 152.Mori M., Imagawa T., Yasui K. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J. Pediatr. 2000;137:177–180. doi: 10.1067/mpd.2000.107890. [DOI] [PubMed] [Google Scholar]
- 153.Tsujimoto H., Takeshita S., Nakatani K. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin. Immunol. 2002;103:161–168. doi: 10.1006/clim.2002.5209. [DOI] [PubMed] [Google Scholar]
- 154.Yi Q.J., Li C.R., Yang X.Q. Effect of intravenous immunoglobulin on inhibiting peripheral blood lymphocyte apoptosis in acute Kawasaki disease. Acta. Paediatr. 2001;90:623–627. [PubMed] [Google Scholar]
- 155.Bresee J.S., Mast E.E., Coleman P.J. Hepatitis C virus infection associated with administration of intravenous immune globulin. A cohort study. JAMA. 1996;276:1563–1567. [PubMed] [Google Scholar]
- 156.Klassen T.P., Rowe P.C., Gafni A. Economic evaluation of intravenous immune globulin therapy for Kawasaki syndrome. J. Pediatr. 1993;122:538–542. doi: 10.1016/s0022-3476(05)83532-2. [DOI] [PubMed] [Google Scholar]
- 157.Sherer Y., Levy Y., Langevitz P. Adverse effects of intravenous immunoglobulin therapy in 56 patients with autoimmune diseases. Pharmacology. 2001;62:133–137. doi: 10.1159/000056085. [DOI] [PubMed] [Google Scholar]
- 158.Marasini M., Pongiglione G., Gazolo D. Late intravenous gamma globulin treatment in infants and children with Kawasaki disease and coronary artery abnormalities. Am. J. Cardiol. 1991;68:796–797. doi: 10.1016/0002-9149(91)90658-8. [DOI] [PubMed] [Google Scholar]
- 159.Tse S.M., Silverman E.D., McCrindle B.W. Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J. Pediatr. 2003;140:450–455. doi: 10.1067/mpd.2002.122469. [DOI] [PubMed] [Google Scholar]
- 160.Kato H., Koike S., Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63:175–179. [PubMed] [Google Scholar]
- 161.Sundel R.P., Baker A.L., Fulton D.R. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J. Pediatr. 2003;142:611–616. doi: 10.1067/mpd.2003.191. [DOI] [PubMed] [Google Scholar]
- 162.Wooditch A.C., Aronoff S.C. Effect of initial corticosteroid therapy on coronary artery aneurysm formation in Kawasaki disease: A meta-analysis of 862 children. Pediatrics. 2005;116:989–995. doi: 10.1542/peds.2005-0504. [DOI] [PubMed] [Google Scholar]
- 163.Hashino K., Ishii M., Iemura M. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr. Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 164.Shinohara M., Sone K., Tomomasa T. Corticosteroids in the treatment of the acute phase of Kawasaki disease. J. Pediatr. 1999;135:465–469. doi: 10.1016/s0022-3476(99)70169-1. [DOI] [PubMed] [Google Scholar]
- 165.Inoue Y., Okada Y., Shinohara M. A multicenter randomized trial of corticosteroids in primary therapy for Kawasaki disease: Clinical course and coronary artery outcome. J. Pediatr. 2006;149:336–341. doi: 10.1016/j.jpeds.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 166.Jibiki T., Terai M., Kurosaki T. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur. J. Pediatr. 2004;163:229–233. doi: 10.1007/s00431-003-1386-5. [DOI] [PubMed] [Google Scholar]
- 167.Newburger J.W., Sleeper L.A., McCrindle B.W. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N. Engl. J. Med. 2007;356:664–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 168.Okada K., Hara J., Maki I. Pulse methylprednisolone with gammaglobulin as an initial treatment for acute Kawasaki disease. Eur. J. Ped. 2009;168:181–185. doi: 10.1007/s00431-008-0727-9. [DOI] [PubMed] [Google Scholar]
- 169.Sano T., Kurotobi S., Matsuzaki K. Prediction of non-responsiveness to standard high-dose gamma globulin-therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Ped. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 170.Ogata S., Ogihara Y., Nomoto K. Clinical score and transcript abundance patterns identify Kawasaki disease patients who may benefit from addition of methylprednisolone. Pediatr. Res. 2009;66:577–584. doi: 10.1203/PDR.0b013e3181baa3c2. [DOI] [PubMed] [Google Scholar]
- 171.Eberhard B.A., Andersson U., Laxer R.M. Evaluation of the cytokine response in Kawasaki disease. Ped. Inf. Dis. J. 1995;14:199–203. doi: 10.1097/00006454-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 172.Matsubara T., Furukawa S., Yabuta K. Serum levels of tumor necrosis factor, interleukin-2 receptor and interferon gamma in Kawasaki disease involved coronary artery lesions. Clin. Immunol. Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 173.Burns J.C., Mason W.H., Hauger S.B. Infliximab treatment for refractory Kawasaki syndrome. J. Pediatr. 2005;146:662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 174.Burns J.C., Best B.M., Mejias A. Infliximab treatment of intravenous gamma globulin-resistant Kawasaki disease. J. Pediatr. 2008;153:833–838. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirono K., Kemmotsu Y., Wittkowski H. Infliximab reduces the cytokine-mediated inflammation but does not suppress the cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr. Res. 2009;65:696–701. doi: 10.1203/PDR.0b013e31819ed68d. [DOI] [PubMed] [Google Scholar]
- 176.Brogan R.J., Eleftheriou D., Gnanapragasam J. Infliximab for the treatment of intravenous immunoglobulin resistant Kawasaki disease complicated by coronary artery aneurysms: a case report. Ped. Rheumatol. Online J. 2009;7:3. doi: 10.1186/1546-0096-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oishi T., Fujieda M., Shiraishi T. Infliximab treatment for refractory Kawasaki disease with coronary artery aneurysm. Circ. J. 2008;72:850–852. doi: 10.1253/circj.72.850. [DOI] [PubMed] [Google Scholar]
- 178.Son M.B., Gauvreau K., Ma L., Baker A.L. Treatment of Kawasaki disease: analysis of 27th US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 179.Furukawa S., Matsubara T., Umezawa Y. Pentoxifylline and intravenous gamma globulin combination therapy for acute Kawasaki disease. Eur. J. Pediatr. 1994;153:663–667. doi: 10.1007/BF02190688. [DOI] [PubMed] [Google Scholar]
- 180.Nash M.C., Wade A.M. No evidence for use of pentoxifylline in acute Kawasaki disease. Eur. J. Ped. 1996;95:258. doi: 10.1007/BF01953955. [DOI] [PubMed] [Google Scholar]
- 181.Iwashima S., Seguchi M., Matubayashi M. Ulinastatin therapy in Kawasaki disease. Clin. Drug. Invest. 2007;27:691–696. doi: 10.2165/00044011-200727100-00004. [DOI] [PubMed] [Google Scholar]
- 182.Mori M., Tomono N., Yokota S. Coronary arteritis of Kawasaki disease unresponsive to high-dose intravenous gamma-globulin treatment successfully treated with plasmapheresis. Nihon. Rinsho. Meneki. Gakkai. Kaishi. 1995;18:282–288. doi: 10.2177/jsci.18.282. [DOI] [PubMed] [Google Scholar]
- 183.Williams R.V., Wilke V.M., Tani L.Y. Does abciximab enhance regression of coronary aneurysms resulting from Kawasaki disease? Pediatrics. 2002;109:E4. doi: 10.1542/peds.109.1.e4. [DOI] [PubMed] [Google Scholar]
- 184.Takahashi M., Weinter K. Presented at the Seventh International Kawasaki Disease Symposium; Hakone, Japan: 2001. Changes in coronary artery aneurysms following treatment with abciximab. [Google Scholar]
- 185.Wallace C.A., French J.W., Kahn S.J. Initial intravenous gamma-globulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 186.Raman V., Kim J., Sharkey A. Response of refractory Kawasaki disease to pulse steroid and cyclosporin A therapy. Pediatr. Infect. Dis. J. 2001;20:635–637. doi: 10.1097/00006454-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 187.Kuramochi Y., Ohkubo T., Takechi N. Feasibility of percutaneous transluminal coronary angioplasty to patients with Kawasaki disease as an early management strategy. Pediatr. Cardiol. 2001;22:183–187. doi: 10.1007/s002460010199. [DOI] [PubMed] [Google Scholar]
- 188.Gotteiner N., Mavroudis C.V., Backer C.L. Coronary artery bypass grafting for Kawasaki disease. Pediatr. Cardiol. 2002;23:62–67. doi: 10.1007/s00246-001-0015-1. [DOI] [PubMed] [Google Scholar]
- 189.Checchia P.A., Pahl E., Shaddy R.E. Cardiac transplantation for Kawasaki disease. Pediatrics. 1997;100:695–699. doi: 10.1542/peds.100.4.695. [DOI] [PubMed] [Google Scholar]
- 190.Dajani A.S., Taubert K.A., Takahashi M. Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1994;89:916–922. doi: 10.1161/01.cir.89.2.916. [DOI] [PubMed] [Google Scholar]
- 191.Fulton D.R., Newburger J.W. Long-term cardiac sequelae of Kawasaki disease. Curr. Rheumatol. Rep. 2000;2:324–329. doi: 10.1007/s11926-000-0070-2. [DOI] [PubMed] [Google Scholar]
- 192.Cheung Y.F., Yung T.C., Tam S.C. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J. Am. Coll. Cardiol. 2004;43:120–124. doi: 10.1016/j.jacc.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 193.McConnell M.E., Hannon D.W., Steed R.D. Fatal obliterative coronary vasculitis in Kawasaki disease. J. Pediatr. 1998;133:259–261. doi: 10.1016/s0022-3476(98)70230-6. [DOI] [PubMed] [Google Scholar]
- 194.Chang R.K. Hospitalizations for Kawasaki disease among children in the United States, 1988-1997. Pediatrics. 2002;109:e87. doi: 10.1542/peds.109.6.e87. [DOI] [PubMed] [Google Scholar]
- 195.Nakamura Y., Yanagawa H., Ojima T. Cardiac sequelae of Kawasaki disease among recurrent cases. Arch. Dis. Child. 1998;78:163–165. doi: 10.1136/adc.78.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]


