Abstract
Conventional disinfection methods are limited by reliance on the operator to ensure appropriate selection, formulation, distribution and contact time of the agent. ‘No-touch’ automated room disinfection (NTD) systems remove or reduce reliance on operators and so they have the potential to improve the efficacy of terminal disinfection. The most commonly used systems are hydrogen peroxide vapour (H2O2 vapour), aerosolised hydrogen peroxide (aHP) and ultraviolet (UV) radiation. These systems have important differences in their active agent, delivery mechanism, efficacy, process time and ease of use. The choice of NTD system should be influenced by the intended application, the evidence base for effectiveness, practicalities of implementation and cost constraints.
Key words: aerosolised hydrogen peroxide, hydrogen peroxide vapour, ultraviolet light, disinfection, gaseous decontamination
17.1. Introduction
As the role of contaminated surfaces in the transmission of nosocomial pathogens is increasingly recognised, there has been renewed emphasis on the importance of effective cleaning and disinfection (Dancer, 2009, Otter et al., 2011). This chapter considers the rationale for ‘no-touch’ automated room disinfection (NTD) systems, which offer the potential to improve the efficacy and reliability of hospital disinfection. An assessment of the level of surface contamination that is a risk for transmission and understanding the limitations of conventional cleaning and disinfection methods is important to appreciate the potential of NTD systems. This chapter provide a detailed overview of the four classes of NTD system that are most commonly used in healthcare settings: aerosolised hydrogen peroxide (aHP), (H2O2) vapour, ultraviolet C radiation (UVC) and pulsed-xenon UV (PX-UV). The differences between these systems in terms of their technological aspects, microbiological efficacy, evidence of clinical impact and practicalities are described, along with a brief overview of other NTD systems and a consideration of their comparative effectiveness and cost. Based on these differences, the scenarios in which various NTD systems may be indicated are discussed in detail. Finally, future trends are also considered.
17.2. Reasons to consider a no-touch automated room disinfection (NTD) system
At one time, contaminated surfaces were thought to contribute negligibly to endemic transmission of pathogens in hospitals (Maki et al., 1982, McGowan, 1981). However, recent data indicate that contaminated surfaces make an important contribution to the endemic transmission of certain nosocomial pathogens (Maki et al., 1982, Otter et al., 2011, Weber et al., 2010). The most convincing evidence comes from studies showing that admission to a room previously occupied by a patient colonised or infected with certain pathogens increases the risk of subsequent occupants acquiring these pathogens by a factor of at least two (Datta et al., 2011, Drees et al., 2008, Huang et al., 2006, Nseir et al., 2011, Otter et al., 2011, Shaughnessy et al., 2011). This association has been demonstrated for Clostridium difficile, vancomycin-resistant enterococci (VRE), meticillin-resistant Staphylococcus aureus (MRSA), Acinetobacter baumannii and Pseudomonas aeruginosa (Drees et al., 2008, Huang et al., 2006, Nseir et al., 2011, Otter et al., 2011, Shaughnessy et al., 2011). The epidemiological association is strengthened by the finding that improving terminal room disinfection reduces or eliminates this increased risk (Datta et al., 2011, Passaretti et al., 2013). Thus, current terminal cleaning and disinfection following the discharge of patients with these pathogens is inadequate and needs to be improved. The increasing age and susceptibility of hospitalised patients, combined with the emergence of more virulent and epidemic strains of C. difficile such as 027/NAP1 and potentially untreatable multidrug-resistant Gram-negative bacteria such as pan-drug resistant Acinetobacter baumannii, carbapenemase producing organisms and certain viruses (for example the SARS Coronavirus), are further reasons to improve environmental decontamination (Dubberke et al., 2007, Peleg and Hooper, 2010).
The effectiveness of conventional cleaning and disinfection can be limited by several factors, including those associated with the products used and the procedure adopted. The key limitation is the reliance on a human operator to correctly select and formulate an appropriate agent and then to distribute it to all target surfaces for the necessary contact time. Improvement of these conventional methods requires modification of human behaviour, which is difficult to achieve and sustain. The use of NTD systems provides an adjunctive approach, which removes or reduces reliance on the operator (Byrns and Fuller, 2011, Davies et al., 2011, Falagas et al., 2011, Rutala and Weber, 2011).
Automated systems have been adopted widely in other areas of healthcare to reduce reliance on operators and mitigate the potential for human error. Examples include robotic surgery and many aspects of critical care such as ventilators (Bryant, 1967, Howe and Matsuoka, 1999). Indeed, commenting on the future of infection control in the late 1990s, Dr Robert Weinstein wrote: ‘Given the choice of improving technology or improving human behavior, technology is the better choice’ (Weinstein, 1998). In recognition of these potential benefits, publications about NTD systems have increased sharply in the last two years (Fig. 17.1 ).
17.1.

Pubmed referenced publications relating to healthcare applications of NTD systems since 2004.
2004 (French et al., 2004)
2005 (Jeanes et al., 2005, Bates and Pearse, 2005, Taneja et al., 2005)
2006 (Otter et al., 2006, Clark et al., 2006, McDonnell, 2006)
2007 (Hardy et al., 2007, Hall et al., 2007, Otter et al., 2007, Boyce, 2007)
2008 (Dryden et al., 2008, Boyce et al., 2008, Hall et al., 2008, Shapey et al., 2008, Bartels et al., 2008, Grare et al., 2008, Sharma and Hudson, 2008, Orlando et al., 2008)
2009 (Otter and French, 2009, Otter et al., 2009a, Otter et al., 2009b, Boyce, 2009, Barbut et al., 2009, Moat et al., 2009)
2010 (Otter et al., 2010a, Otter et al., 2010b, Otter et al., 2010c, Pottage et al., 2010, Bergman et al., 2010, Lawley et al., 2010, Po and Carling, 2010, Otter and Yezli, 2010, Andersen et al., 2010, Rutala et al., 2010, Nerandzic et al., 2010, Ray et al., 2010, Callahan et al., 2010)
2011 (Cooper et al., 2011, Berrie et al., 2011, Holmdahl et al., 2011, Manian et al., 2011, Rutala and Weber, 2011, Falagas et al., 2011, Davies et al., 2011, Byrns and Fuller, 2011, Otter and Yezli, 2011, Andersen, 2011, Piskin et al., 2011, Boyce et al., 2011, Stibich et al., 2011, Zoutman et al., 2011, Taneja et al., 2011, Chan et al., 2011, De Lorenzi et al., 2011)
2012 (Pottage et al., 2012, Galvin et al., 2012, Tuladhar et al., 2012, Bentley et al., 2012, Beswick et al., 2011)
Despite this recent interest, the concept of NTD is not new. Even before germ theory was formulated, ‘fumigation’ was performed through burning sulphur and other chemical mixtures (Blancou, 1995). A paper published in 1901 provided a step-by-step guide on how to disinfect a ‘sick-room’ through gaseous formaldehyde (Riddle, 1901). In the 1960s, formaldehyde was replaced by aerosolised chemicals such as quaternary ammonium compounds and phenolics due to concerns over toxicity and provided promising data on effectiveness (Friedman et al., 1968, Munster and Ostrander, 1974, Ostrander and Griffith, 1964). However, concerns over efficacy and safety led to advice from the US Centers for Disease Control and Prevention (CDC) since the 1970s that disinfectant fogging should not be performed routinely in patient-care areas (Munster and Ostrander, 1974, Rutala et al., 2008). The increasing recognition of the importance of environmental contamination in transmission has prompted the development of several new NTD systems based on either hydrogen peroxide or ultraviolet radiation. The improved efficacy and safety of these systems compared with the disinfectant aerosolisers of the 1960s and 1970s has prompted a re-evaluation of the CDC recommendation (Rutala and Weber, 2011).
This review considers the rationale for using NTD systems when conventional cleaning and disinfection requires improvement, compares the use of the key NTD systems in different scenarios and discusses the role of regulators and professional societies in providing evidence-based adoption.
17.3. What level of surface contamination is a risk for transmission?
The relationship between the level of residual surface contamination after disinfection and the risk of transmission has not been studied in detail. The risk of transmission from an environmental surface depends on various factors, including the characteristics of the organism involved, patient susceptibility and staff compliance with universal precautions and infection control policies (for example hand hygiene following contact with surfaces) (Hayden et al., 2008, Kramer et al., 2006, Stiefel et al., 2011). The fact that subsequent occupants of a room vacated by a previously colonised or infected patient are at increased risk of infection indicates that conventional terminal cleaning and disinfection do not reduce contamination sufficiently to prevent transmission in these cases (Drees et al., 2008, Huang et al., 2006, Nseir et al., 2011, Otter et al., 2011, Shaughnessy et al., 2011).
There is some limited evidence that the risk of transmission is proportional to the level of surface contamination. Lawley et al. (2010) developed a murine model and showed a dose–response relationship between the level of contamination in the cages and the proportion of healthy mice that developed C. difficile infection (CDI). All mice became infected when exposed for one hour to 100 spores/cm2 and 50% became infected when exposed to 5 spores/cm2. The concentration at which none of the mice became infected was less than one spore per cm2. In the healthcare environment, room exposure times are usually measured in days and so the estimates by Lawley et al. are likely to be conservative. Lawley et al. then examined which disinfectants were able to interrupt the transmission of C. difficile and established a relationship between the level of inactivation of C. difficile spores in vitro and the degree to which transmission was interrupted (Fig. 17.2 ). Although data from animal studies should be interpreted with caution, these studies suggest that a low level of contamination can transmit spores to a susceptible host, and that there is a proportional relationship between the level of surface contamination and the degree of transmission.
17.2.
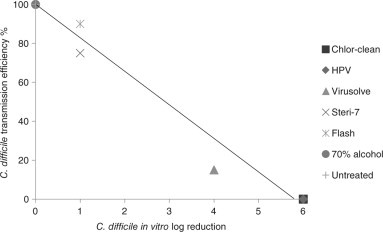
Correlation between in vitro log reduction and interruption of transmission of C. difficile spores in a murine model (data from Lawley et al. (2010)). HPV = hydrogen peroxide vapour.
The amount of shedding and the infective dose can be used to guide appropriate hospital cleaning and disinfection. Certain pathogens such as C. difficile and norovirus can be shed into the environment in high numbers and have a low infectious dose (Larson and Borriello, 1990, Otter et al., 2011, Yezli and Otter, 2011). For example, the infectious dose for norovirus is 1–100 viral particles (Yezli and Otter, 2011) while stool concentrations can reach more than 1012 particles per gram (Otter et al., 2011) and up to 105 norovirus particles per 30 cm2 have been identified on hospital surfaces (Morter et al., 2011). Therefore, the presence of a pathogen on a surface at any concentration may be a risk for transmission. This is reflected in proposed guidelines for microbiological hygiene standards (Dancer, 2004) and recent discussion surrounding the intended target for hospital disinfection (Roberts, 2012, Walder and Holmdahl, 2012).
However, in practice, a risk-based approach must be used when setting a target for an acceptable level of residual contamination, balancing patient safety with practicality and cost, as is the case when selecting liquid disinfectants. More stringent targets should be set when the risk and/or consequences of infection are high, for example, for virulent, resistant and/or highly infectious pathogens, especially in high-risk settings with immunocompromised patients whereas a lower standard may be acceptable in lower-risk settings (Dancer, 2004, Roberts, 2012, Walder and Holmdahl, 2012).
17.4. Limitations of conventional cleaning and disinfection
Conventional cleaning and disinfection is performed by a human operator with liquid detergents or disinfectants. Microbiological studies indicate that conventional cleaning and disinfection without programmes of targeted improvement rarely eliminate pathogens from surfaces (Byers et al., 1998, French et al., 2004, Manian et al., 2011, Wilcox et al., 2003). For example, MRSA was identified on 66% of surfaces in patient rooms following terminal cleaning in one study (French et al., 2004) and C. difficile spores persisted despite bleach disinfection in another (Boyce et al., 2008, Kaatz et al., 1988, Verity et al., 2001).
Problems associated with both ‘product’ and ‘procedure’ contribute to the failures of conventional cleaning and disinfection. These include the ineffectiveness of some agents against some pathogens, for example, many common hospital disinfectants are not effective against C. difficile spores (Fraise, 2011, Humphreys, 2011) and norovirus (Dettenkofer and Block, 2005); toxicity to staff or the environment; damage to materials and equipment resulting in restrictions on usage (Dettenkofer and Block, 2005, Mirabelli et al., 2007); certain agents are inhibited by organic matter on surfaces (Humphreys, 2011); and there is a potential for biocide/antibiotic cross-resistance for some agents (Meyer and Cookson, 2010).
The key problem associated with the cleaning and disinfection procedure is the reliance on the operator to repeatedly ensure adequate selection, formulation, distribution and contact time of the agent (Carling et al., 2008, Fraise, 2011). For example, a large assessment of conventional cleaning in 36 acute hospitals using fluorescent markers revealed that less than 50% of high-risk objects in hospital rooms were cleaned at patient discharge (Carling et al., 2008). Distribution of the active agent is difficult in the complex and intricate healthcare environment (Carling et al., 2008). Ensuring the correct contact time to attain the microbial reduction achieved in vitro is particularly problematic because the disinfectant will evaporate from the surface (Fraise, 2011). Other problems include the delegation of responsibility for cleaning, which can fall between staff groups such as nurses and domestic housekeepers particularly in the case of complex portable medical equipment (Havill et al., 2011); difficulties in measuring the effectiveness of cleaning and disinfection (Rutala and Weber, 2011) and achieving compliance with protocols/policies from an (often) poorly paid, poorly motivated workforce who may have limited spoken or written local language skills (Dancer, 1999); inadequate training and education of personnel (Dancer, 1999); inadequate time given to do the job properly (Dancer, 1999); insufficient (or non-existent) cleaning prior to disinfection (Humphreys, 2011); incorrect formulation of the disinfectant (Meyer and Cookson, 2010, Weber et al., 2007); and contamination of cleaning solutionsmaterials (Weber et al., 2007, Werry et al., 1988).
Modifying human behaviour is difficult but several different approaches can be taken, including routine microbiological analysis of surface hygiene, the use of fluorescent markers or ATP assays to assess the thoroughness of cleaning, feedback of cleaning performance, and education to enhance knowledge about the importance of the process (Boyce et al., 2009, Carling et al., 2008, Dancer, 2004, Datta et al., 2011, Mulvey et al., 2011, Rutala and Weber, 2011). The development of improved protocols and structured career progression for cleaning staff should be considered in addition to monitoring and feedback. This can improve the frequency of surfaces that are cleaned (Carling et al., 2006, Carling et al., 2008) and reduce the level of environmental contamination (Eckstein et al., 2007, Goodman et al., 2008). There is some evidence that improving the efficacy of conventional cleaning/disinfection can reduce the acquisition of pathogens (Dancer et al., 2009, Datta et al., 2011, Hayden et al., 2006). For example, Hayden et al. (2006) performed a 9-month prospective before and after study of educational improvements of cleaning and hand hygiene: the proportion of surfaces contaminated with VRE was reduced from 24% to 12% and patient acquisition of VRE was reduced from 33 to 17 acquisitions per 1000 patient-days. Few studies have evaluated the sustainability of such systematic improvements. One study showed that cleaning performance measured by the removal of a fluorescent marker increased from a baseline of 52% to 80–85% through training and monthly feedback (Fitzgerald et al., 2012). However, compliance soon returned towards baseline (57–66%) when the monthly feedback ceased. Similarly, recent evidence indicates that altering the location of fluorescent dye spots reduced the proportion of objects that were cleaned from 90% to approximately 60% (Rutala and Weber, 2011).
In situations where the elimination of pathogens is required, even systematic improvement of conventional cleaning and disinfection may not be sufficient. Multiple rounds of disinfection with sodium hypochlorite (bleach), taking many hours (Jeanes et al., 2005, Manian et al., 2011), risking damage (corrosion) to materials (Dettenkofer and Block, 2005, McGowan et al., 1988) and presenting health risks for operators (Mirabelli et al., 2007), can have limited success in removing environmental reservoirs of pathogens (Byers et al., 1998, Manian et al., 2011, Morter et al., 2011). For example, an average of 2.8 rounds of quaternary ammonium compound disinfections were required to eradicate VRE from a room in one study (Byers et al., 1998) and A. baumannii or MRSA were cultured from 27% of rooms sampled after four rounds of cleaning and bleach disinfection (Manian et al., 2011). NTD systems offer the potential to overcome some of these problems (Byrns and Fuller, 2011, Davies et al., 2011, Falagas et al., 2011).
17.5. Overview of NTD systems
The most commonly used NTD systems in healthcare are aHP systems (such as ASP Glosair – previously Sterinis, Steris Biogienie and Oxypharm Nocospray), H2O2 vapour systems (such as the Bioquell and Steris systems) and UVC systems (such as Lumalier Tru-D) (Boyce, 2009, Davies et al., 2011, Falagas et al., 2011, Orlando et al., 2008, Rutala and Weber, 2011). A fourth class of NTD system based on PX-UV radiation has been introduced recently and has been the subject of few limited studies so far (Stibich et al., 2011).
Considering what would make an ‘ideal’ NTD system is useful in comparing the features of the various systems available (Table 17.1 ). The ‘ideal’ system would have a short cycle time; a high efficacy to eliminate pathogens from surfaces; homogeneous distribution of the active agent; the system should be easy to operate, fully automated, require minimal safety measures, allow instant access to the room and have no environmental impact; finally, the system should have published evidence of clinical impact and the necessary regulatory approvals. Clearly, no single system meets all of these requirements and the importance of each feature will depend on the application.
Table 17.1.
An overview of ‘no-touch’ automated room disinfection systems
| The ‘ideal’ NTD system | Aerosolised hydrogen peroxide (aHP) | H2O2 vapour | UVC | Pulsed xenon UV (PX-UV) |
|---|---|---|---|---|
| Short cycle time (< 1 h) | ✗ | ✗ | ✗/✓ | ✓ |
| High level of microbial efficacy (6-log sporicidal reduction) | ✗/✓ | ✓ | ✗ | ✗ |
| Pathogens not culturable from surfaces after the cycle | ✗ | ✓ | ✗ | ✗ |
| Easy to operate | ✓ | ✗ | ✓ | ✓ |
| Fully automated operation | ✓ | ✓ | ✗/✓ | ✗ |
| Immediate room entry availablea | ✗ | ✗ | ✓ | ✓ |
| No requirement of room sealing | ✗ | ✗ | ✓ | ✓ |
| Homogeneous distribution | ✗ | ✓ | ✗ | ✗ |
| US EPA registered | ✗ | ✓ (Sterilant) | ✗ | ✗ |
| UK Rapid Review Panel recommendationb | 3 | 1 (HPV); 2 (VHP) | ✗ | ✗ |
| Evidence of clinical impact | ✗ | ✓ | ✗ | ✗ |
✓ = does meet the characteristic of the ‘ideal’ NTD system.
✗ = does not meet the characteristic of the ‘ideal’ NTD system.
✗/✓= it is not clear whether or not the characteristic of the ‘ideal’ NTD system is met.
Immediate room entry may be advantageous in the event of an emergency.
-
1.Basic research and development, validation and recent in use evaluations have shown benefits that should be available to NHS bodies to include as appropriate in their cleaning, hygiene or infection control protocols.
-
2.Basic research and development has been completed and the product may have potential value; in use evaluations/trials are now needed in an NHS clinical setting.
-
3.A potentially useful new concept but insufficiently validated; more research and development is required before it is ready for evaluation in practice.
17.5.1. Commonly used systems
aHP
aHP systems deliver a pressure-generated aerosol. The systems used most commonly in healthcare use a solution containing 5–6% hydrogen peroxide and < 50 ppm silver (Fig. 17.3 ) (Chan et al., 2011, Orlando et al., 2008, Otter and Yezli, 2011, Otter et al., 2010b, Shapey et al., 2008). These systems are sometimes known as ‘dry-mist hydrogen peroxide’, though this term is a poor reflection of their properties (Andersen et al., 2010, Barbut et al., 2009). Aerosolised droplets are introduced into an enclosure via a unidirectional nozzle (Boyce, 2009, Rutala and Weber, 2011). One manufacturer (ASP Glosair) states a particle size of 8–10 μm (Fu et al., 2012, Holmdahl et al., 2011) whereas another (Oxypharm Nocospray) states a smaller particle size of 0.5 μm (Orlando et al., 2008). The dose typically recommended for hospital rooms is 6 mL per m3, although multiple cycles of this dose have been used in several studies (Andersen et al., 2006, Holmdahl et al., 2011). Following exposure, the aerosol is usually left to decompose naturally, without any active aeration.
17.3.

Aerosolised hydrogen peroxide (aHP) systems: (a) ASP Glosair; (b) Steris Biogienie.
H2O2 vapour
H2O2 vapour systems deliver a heat-generated vapour of 30–35% w/w aqueous hydrogen peroxide through a high-velocity air stream to achieve homogeneous distribution throughout an enclosed area (enclosure) (Fig. 17.4 ) (Boyce, 2009, Otter and Yezli, 2011). Two systems using H2O2 vapour are available commercially – Bioquell and Steris. Bioquell systems are usually termed hydrogen peroxide vapour (HPV) and Steris systems vaporized hydrogen peroxide (VHP). Bioquell HPV includes a generator to produce HPV, a module to measure the concentration of HPV, temperature and relative humidity in the enclosure and an aeration unit to catalyse the breakdown of HPV to oxygen and water vapour after HPV exposure. A control pedestal is situated outside the enclosure to provide remote control. Bioquell HPV is delivered until the air in the enclosure becomes saturated and H2O2 begins to condense on surfaces (Hall et al., 2007, Ray et al., 2010). Steris VHP systems have a generator inside the room with an integral aeration unit and dehumidifier designed to achieve a set humidity level prior to the start of the cycle. The system is controlled remotely from outside the enclosure. Steris VHP systems deliver ‘non-condensing’ VHP by drying the vapour stream as it is returned to the generator. Bioquell systems do not control the H2O2 air concentration while the Steris systems hold a steady H2O2 air concentration throughout the exposure period.
17.4.

H2O2 vapour systems: (a) Bioquell hydrogen peroxide vapour (HPV); (b) Steris vaporized hydrogen peroxide (VHP).
UVC
UVC systems for room decontamination deliver specific doses (for example, 12 000 μWs/cm2 for vegetative bacteria and 22000–36000 μWs/cm2 for spores) of UVC (254 nm range) to surfaces (Fig. 17.5 ) (Boyce et al., 2011, Nerandzic et al., 2010, Rutala et al., 2010). The device is placed in the centre of the room and commonly touched mobile items are arranged close to the device for optimal exposure. UVC travels in straight lines and is less effective out of direct line of sight from the device. Some manufacturers therefore recommend multiple cycles from different locations (Boyce et al., 2011). Some UVC systems contain sensors to measure the amount of UVC light reflected back to the device to confirm the delivery of a specified dose to all parts of the room.
17.5.

Ultraviolet radiation systems: (a) UVC: Lumalier Tru-D; (b) pulsed xenon UV (PX-UV): Xenex.
PX-UV
PX-UV systems emit broad spectrum UV in short pulses (Fig. 17.5) (Stibich et al., 2011). They are placed at multiple room locations and have a relatively short cycle time.
17.5.2. Microbiological efficacy
Studies evaluating the in vitro and in situ efficacy of NTD systems are summarised in Tables 17.2 and 17.3 , respectively. One aHP system (ASP Glosair) achieves a ~4-log reduction on C. difficile spores in vitro (Barbut et al., 2009) and has limited capacity to inactivate commercially produced 6-log spore biological indicators (BIs) (Andersen et al., 2006, Holmdahl et al., 2011). Catalase-positive bacteria are considerably less susceptible to the low concentration of H2O2 used by aHP systems than catalase-negative bacteria or metabolically inert spores (Otter and French, 2009, Pottage et al., 2012). The efficacy of aHP systems against catalase-positive bacteria remains to be firmly established, with conflicting published data on the level of inactivation of MRSA and A. baumannii (Fu et al., 2012, Piskin et al., 2011) and tuberculocidal activity (Andersen, 2010; Andersen et al., 2010, Beswick et al., 2011, Grare et al., 2008). aHP systems have been shown to reduce contamination of C. difficile and MRSA on hospital surfaces (Bartels et al., 2008, Barbut et al., 2009, Chan et al., 2011, Orlando et al., 2008, Shapey et al., 2008), but have not been shown to eliminate pathogens in clinical practice. For example, one or more positive C. difficile cultures were collected from 20% of 15 (Barbut et al., 2009) and 50% of 10 (Shapey et al., 2008) rooms studied after an aHP process.
Table 17.2.
Studies evaluating the in vitro efficacy of ‘no-touch’ automated room disinfection systems
| Author | Year | Setting | Design | Results |
|---|---|---|---|---|
| H2O2 vapour | ||||
| Barbut et al. (2012) | 2012 | Plastic or laminate carriers with 5–6 log of C. difficile spores exposed to HPV in unfurnished, unoccupied 33–45 m3 rooms. | C. difficile was completely eradicated from the exposed carriers regardless of the C. difficile strain or surface used. | |
| Otter et al. (2012) | 2012 | A 100 m3 test room | MRSA carriers containing 6.1–7.3 log of MRSA suspended in distilled water, 0.3%, 3% or 10% BSA, TSB or 0.9% saline and dried on stainless steel discs were exposed to HPV. | The effectiveness of HPV was reduced in a step-wise manner as type and concentration of simulated soiling increased. No MRSA was recovered from any of the carriers after 60 min exposure to HPV. |
| Havill et al. (2012) | 2012 | 15 patient rooms with bathrooms (46–86 m3) | Carrier disks with ∼ 106C. difficile spores and BIs with 104 and 106 G. stearothermophilus spores were placed in 5 sites (3 sites were not in direct line of sight from the HPV generator). | HPV achieved > 6-log reduction on C. difficile in all 5 sites. HPV inactivated 99% (74/75) of 6-log BIs and 100% (75/75) of 4-log BIs. |
| Fu et al. (2012) | 2012 | Two rooms to simulate a patient room (50.1 m3) and an en-suite bathroom (13.2 m3) | Pouched and unpouched 4- and 6-log G. stearothermophilus BIs and in-house prepared test discs containing ∼ 106 MRSA, C. difficile spores and A. baumannii were placed at 11 locations in the test area. | HPV inactivated 91% (40/44) of the pouched 6-log BIs and 95% (42/44) of the pouched 4-log BIs. The HPV system completely inactivated (> 6-log reduction) MRSA dried in water from all replicates in 9/11 locations, A. baumannii dried in water from all replicates in 6/11 locations, and C. difficile from all replicates in all locations. |
| Bentley et al. (2012) | 2012 | A class II safety cabinet | FCV virus was dried on 1 cm2 carriers of stainless steel, glass, vinyl flooring, ceramic tile or PVC. | > 4-log reduction was achieved on all surfaces after HPV. |
| Holmdahl et al. (2011) | 2011 | A purpose-built 136 m3 test room | 6-log Tyvek-pouched G. stearothermophilus BIs were placed at 20 locations in the first test and 14 locations in another 2 tests. | HPV inactivated 100% (48/48) of 6-log BIs. |
| Berrie et al. (2011) | 2011 | A microbiology safety cabinet | Recombinant adenovirus (Ad5GFP) was dried on 10 mm diameter stainless steel discs at concentrations of 7.6–9.4 log TCID50/disc. | HPV achieved a > 8-log TCID50 reduction in virus titre. |
| Pottage et al. (2012) | 2011 | A test chamber (20.7 m3) | Stainless steel indicators of ∼ 106 MRSA or ∼ 106 commercially available G. stearothermophilus BIs were exposed to Steris VHP in a test chamber. BIs were removed and enumerated at timed intervals. | After 30 min exposure to VHP there was ∼ 3-log reduction in MRSA and ∼ 5-log reduction G. stearothermophilus spores, indicating that the catalase-positive MRSA are less susceptible to VHP than the metabolically inert spores |
| Pottage et al. (2010) | 2010 | A class III safety cabinet | MS2 bacteriophage was dried on 10 mm diameter stainless steel discs at concentrations of 7–9-log pfu/carrier. MS2 phage was also dried in 10% or 50% horse blood. Inoculated carriers were exposed to either VHP (Steris) or HPV (Bioquell). | HPV caused > 6-log reduction on the phage; VHP caused a 5–6 log reduction on the phage. Reductions for HPV were 5.8 and 2.7 when the virus was dried in 10% and 50% horse blood, respectively. Reductions for VHP were > 9 and 3.5 when the virus was dried in 10% and 50% horse blood, respectively. |
| Otter and French (2009) | 2009 | A 100 m3 test room | Five strains of MRSA and three stains of VRE, Acinetobacter spp., K. pneumoniae and C. difficile spores were dried on stainless steel discs at concentrations of 5–7-log cfu/carrier either in water or BSA to simulate soiling. | All carriers were inactivated after exposure to HPV when dried from water or 0.3% BSA. |
| Hall et al. (2007) | 2007 | A biological safety cabinet and a BSL III laboratory room (37 m3) | ∼ 3-log M. tuberculosis dried on stainless steel carriers were exposed to HPV in a biological safety cabinet and at 10 locations in a BSL III laboratory room. 6-log G. stearothermophilus BIs were also exposed to HPV in the room experiment. | No M. tuberculosis BIs grew after 30 min exposure to HPV in the safety cabinet. In the room experiment, all M. tuberculosis and G. stearothermophilus BIs were inactivated at all 10 locations following exposure to HPV for 90 min. |
| Johnston et al. (2005) | 2005 | A 0.4 m3 glovebox enclosure | > 6-log of two strains of C. botulinum spores dried on stainless steel discs and 6-log G. stearothermophilus BIs were exposed to HPV. | After 7 min exposure to HPV, all C. botulinum spores were inactivated. No viable G. stearothermophilus spores were recovered after 6 min exposure to HPV. |
| Kahnert et al. (2005) | 2005 | A 64.5 m3 laboratory room | 8 × 104 – 2.3 × 106 of two strains of M. tuberculosis were dried on tissue culture plates, placed in steam-permeable Tyvek pouches, distributed at 4 locations in the test room and exposed to Steris VHP. | No viable M. tuberculosis was recovered at any of the locations after exposure to VHP. |
| Aerosolised hydrogen peroxide (aHP) | ||||
| Fu et al. (2012) | 2012 | Two rooms to simulate a patient room (50.1 m3) and an en-suite bathroom (13.2 m3) | Tyvek-pouched and unpouched 4- and 6-log G. stearothermophilus BIs and in-house prepared test discs containing ∼ 106 MRSA, C. difficile spores and A. baumannii were placed at 11 locations in the test area. | aHP inactivated 13.6% (6/44) of the unpouched 6-log BIs, and 36.4% of the unpouched 4-log BIs. aHP generally achieved a < 4-log reduction on MRSA, A. baumannii and C. difficile spores. The level of inactivation varied considerably by room location. |
| Holmdahl et al. (2011) | 2011 | A purpose-built 136 m3 test room | 6-log Tyvek-pouched G. stearothermophilus BIs were placed at 20 locations in the first test and 14 locations in another two tests. Three back-to-back aHP cycles using 2 aHP machines was run. | aHP inactivated 50% (24/48) of BIs; 10% (2/20) of BIs in the first test and 79% (22/28) of BIs in the other two tests were inactivted. |
| Piskin et al. (2011) | 2011 | A single hospital isolation room (53 m3) | Stainless steel discs carriers inoculated with ∼ 4.5-log MRSA or A. baumannii dried from water or 5% sterile serum were placed at various locations in the test room. | ∼ 4 log reduction achieved for MRSA and A. baumannii. aHP was less effective for the bacteria dried in serum and in closed or semi-closed locations (e.g. inside a drawer). |
| Koburger et al. (2011) | 2011 | 37 m3 test room | Carriers inoculated with 4.3, 5.5, and 6.5-log of Aspergillus brasiliensis. | aHP achieved 0.4, 1.3 and 4.3-log reductions respectively at the initial fungal loads of 6.5, 5.5, and 4.3-log. |
| Andersen et al. (2010) | 2010 | TB laboratory (BSL3) | Plastic plates inoculated with ∼ 3 × 104 M. tuberculosis and placed in an open box (lid off) on an open bench. This room was treated with 3 or 6 aHP cycles. | M. tuberculosis growth was observed in all TB broth media (20/20) after 10–21 days’ incubation. |
| Grare et al. (2008) | 2008 | 80 m3 BSL3 laboratory | Cotton tissues inoculated with 105−106 dried M. tuberculosis were placed in various room locations. | aHP achieved > 5-log reduction on M. tuberculosis in all room locations. |
| Bartels et al. (2008) | 2008 | Hospital room | 5 different locations (20–100 cm2) in the room were inoculated with 100 cfu/cm2 (3–4-log) of MRSA cultures diluted in urine. One or three aHP decontamination cycles were run. | All samples were negative after one or three aHP cycles. |
| Andersen et al. (2006) | 2006 | Hospital rooms (4–58 m3) and garages (120–200 m3) | 6-log B. atrophaeus spore BIs were used. BIs were placed at various locations in rooms, ambulances parked in garages, and on the outside and inside of medical equipment. | One or two aHP cycles had no effect on BIs. Three aHP cycles inactivated 87% (127/146) of BIs in two test rooms, 62% (137/220) of BIs on or in medical equipment and all BIs (60/60) in the ambulances. |
| Ultraviolet-C radiation (UVC) | ||||
| Havill et al. (2012) | 2012 | 15 patients rooms (with bathrooms) (46–86 m3) | Carrier disks with ∼ 106C. difficile spores and BIs with 104 and 106 G. stearothermophilus spores were placed in 5 sites (3 sites were not in direct line of sight from the UVC unit) and exposed to 22 000 μWs/cm2. | UVC achieved a mean of 2.2 log reduction on C. difficile (range 1.7–3 log reduction). UVC inactivated 29% (22/75) of 4-log BIs (range 7–53%) and 0% (0/75) of 6-log BIs. UVC was significantly less effective out of direct line of sight. |
| Boyce et al. (2011) | 2011 | 25 patients rooms (with bathrooms) (46–86 m3) | Carrier disks with ∼ 105C. difficile spores were placed in 5 sites (3 sites were not in direct line of sight from the devices) using a 1-(22 000 μ Ws/cm2) or 2-stage procedure. | 1-stage procedure: 68 min median cycle time and mean of 2.2 log reduction (range 1.7–2.9 log reduction). 2-stage procedure: 84 min median cycle time and mean of 2.3 log reduction (range 1.4–3.2 log reduction). UVC was significantly less effective out of direct line of sight. |
| (Nerandzic et al., 2010) | 2010 | Laboratory bench top | C. difficile spores, MRSA and VRE suspended in PBS or 10 mg/ml BSA were dried on bench tops (1 cm2) at 3–5 log. Inactivation of pathogens was assessed at reflected doses ranging from 5 000 to 22 000 μWs/cm2. | Sporicidal cycle (22 000 μWs/cm2) achieved reductions of > 2–4 for MRSA, C. difficile and VRE. Increasing the dose from 5 000 to 20 000 μWs/cm2 increased efficacy for C. difficile spores (from 1.1 to 2.7 log) but not for VRE or MRSA. Suspending medium or room location did not affect log reductions significantly. |
| Hospital rooms | Plastic carriers with ∼ 105C. difficile spores were placed around the room and exposed to 22 000 μWs/cm2 (sporicidal cycle). | UVC achieved a 2.6-log reduction on carriers in direct line of sight and 1-log reduction on carriers out of direct line of site. | ||
| Staphylococcus warneri was dried on 1 cm2 areas on 26 frequently touched sites and on 20 portable equipment sites at 4–5 log and exposed to 12 000 μW/cm2 (vegetative cycle). | UVC achieved a ∼ 3.5-log reduction on the 26 environmental sites and a 2-log reduction on equipment. | |||
| Rutala et al. (2010) | 2010 | Patient rooms with bathroom | MRSA, VRE, A. baumannii or C. difficile spores were dried on Formica sheets (64 cm2) at ∼ 104–105 cfu, placedat various room locations and exposed to 36 000 μWs/cm2 for C. difficile (sporicidal cycle) or 12 000 μWs/cm2 (vegetative cycle) for the other organisms. | UVC achieved mean log reduction of 2.79 for C. difficile, 3.88 for A. baumannii, 3.46 for VRE and 3.94 for MRSA. UVC was less effective for sites that are out of line of sight. |
Table 17.3.
Studies evaluating the in situ efficacy of ‘no-touch’ automated room disinfection systems
| Author | Year | Setting | Design | Samples contaminated |
|---|---|---|---|---|
| HPV | ||||
| French et al. (2004) | 2004 | A 1200-bed London teaching hospital | Environmental sampling for MRSA was conducted in MRSA-patient side rooms and bathrooms before and after HPV decontamination. | Before decontamination, 61 (72%) of 85 sites were positive for MRSA; 72% by direct plating. After HPV, one (1.2%) of the 85 sites (a floor corner in one of the rooms) yielded MRSA, by selective broth enrichment. Rooms were not cleaned prior to HPV decontamination. |
| Jeanes et al. (2005) | 2005 | A UK hospital surgery ward | Environmental sampling for MRSA was conducted before and after HPV decontamination. | Before decontamination, eight (16.0%) of 50 swabs taken were positive for MRSA. After HPV, none (0%) of the 50 swabs yielded MRSA. |
| Bates and Pearse (2005) | 2005 | A UK hospital NICU | Environmental sampling of the NICU was conducted before and after HPV decontamination | Before decontamination, 2 (4.8%) and 4 (9.5%) of the 42 sites samples were positive for Serratia and MSSA respectively. After HPV, none (0%) of the 25 sites samples yielded Serratia or MSSA. |
| Boyce et al. (2006) | 2006 | A 500-bed university hospital | Surfaces in 4 wards and 3 patient rooms were sampled using moistened swabs before and after HPV decontamination. | Before decontamination, 8 (4.8%), 9 (5.5%) and 23 (13.9%) of the 165 sites samples were positive for C. difficile, MRSA and VRE respectively. After HPV, none (0%) of the 155 sites samples yielded C. difficile, MRSA or VRE. |
| Hardy et al. (2007) | 2007 | A 9-beded open plan ICU | Environmental sampling for MRSA in the ICU was conducted using cotton swabs before and after HPV decontamination. | Before decontamination, 5 (17.2%) of 29 sites sampled were positive for MRSA. After HPV, none (0%) of the 25 sites sampled yielded MRSA. |
| Otter et al. (2007) | 2007 | A 500-bed hospital | Standardised sites in a single-occupancy ward side-room with an en-suite bathroom were sampled for MRSA, GNR and VRE using cotton swabs before and after HPV decontamination. | MRSA was isolated from 12 (40.0%) and one (3.3%) of the 30 sites sampled before and after HPV respectively. GNR were isolated from 3 (10.0%) and none (0%) of the 30 sites sampled before and after HPV, respectively. VRE was isolated from one (6.7%) and none (0%) of the 15 sites sampled before and after HPV, respectively. |
| Boyce et al. (2008) | 2008 | A 500-bed university hospital | Surfaces in patient rooms, bathrooms and open ward areas were sampled for C. difficile using sponges before and after HPV decontamination. | Before decontamination, 11 (25.6%) of the 43 sites samples were positive for C. difficile. After HPV, none (0%) of the 37 sites samples yielded C. difficile. |
| Dryden et al. (2008) | 2008 | A 28-bed surgical ward | Moistened swabs were used to sample multiple surfaces for MRSA before and after HPV decontamination. | Before decontamination 8 (27.6%) of 29 sites sampled were positive for MRSA. After HPV, 1 (3.4%) of the 29 sites (a composite swab from six bed-rails) yielded MRSA. |
| Otter et al. (2008) | 2008 | A 39-bed neonatal unit (NNU) | Environmental sampling for the outbreak strain of S. aureus was conducted in the NNU before and after HPV. | Before decontamination 3 (4.0%) of 74 sites sampled were positive for S. aureus. After HPV, none (0%) of the 64 sites sampled yielded S. aureus. |
| Otter et al. (2010c) | 2010 | A 12-bed ICU | Environmental sampling was conducted in the ICU using moistened cotton swabs before and after HPV. | Before decontamination 10 (47.6%) of 21 sites sampled were positive for GNRs including MDR E. cloacae. After HPV, none (0%) of the 63 sites sampled yielded GNRs. |
| iRay et al. (2010) | 2010 | A 54-bed long-term acute care hospital | Environmental sampling for A. baumannii was conducted in the wards using moistened cotton swabs before and after VHP. | Before decontamination 8 (8.6%) of 93 sites sampled were positive for A. baumannii including MDR A. baumannii. None of the sites sampled after VHP yielded A. baumannii. |
| Manian et al. (2011) | 2011 | A 900-bed tertiary care hospital | Moistened culture swabs were used to sample rooms for MRSA and A. baumannii complex (ABC) before and after HPV. | Before decontamination, 6 (0.8%) of 740 sites were positive for MRSA and 6 (0.8%) of 740 sites were positive for ABC. After HPV, none (0%) of the 740 sites samples grew fMRSA or ABC. |
| Barbut et al. (2013) | 2012 | A burn unit | Environmental sampling of surfaces in individual patient ’s rooms before and after HPV. | Before decontamination, 6% (6/102) of surface samples grew Acinetobacter, 4% (4/102) grew S. aureus and 2% (2/102) grew E. coli. No pathogens were isolated from surfaces after HPV. |
| Environmental sampling of surfaces in individual patient’s rooms before and after HPV | Before decontamination, 4% (3/66) and 7% (1/14) of the fungal surface and air samples, respectively, grew Aspergillus spp., while 1% (1/92) of the bacterial surface samples yielded S. aureus. No pathogens were isolated from surfaces or the air after HPV. | |||
| Aerosolised hydrogen peroxide (aHP) | ||||
| Shapey et al. (2008) | 2008 | A UK hospital | Environmental sampling for C. difficile of clinical areas was performed using moistened cotton swabs before and after aHP. | C. difficile was isolated from 48 (23.6%) of 203 swabs taken before aHP and from 7 (3.4%) of 203 of the swabs taken after aHP. |
| Bartels et al. (2008) | 2008 | A Danish hospital | 14 upholstered chairs involved in an MRSA outbreak were sampled before and after decontamination with aHP. | Before decontamination, 4 (28.6%) of 14 chairs were positive for MRSA. After aHP, 1 (7.1%) of 14 chairs yielded MRSA. |
| Barbut et al. (2012) | 2009 | A French hospital | Environmental surfaces from rooms of patients with CDI were sampled for C. difficile using moistened swabs before and after aHP disinfection. | Before decontamination 34 (18.9%) of 180 sites sampled were positive for C. difficile. After aHP, 4 (2.2%) of 180 sites yielded C. difficile. |
| Ultraviolet-C radiation (UVC) | ||||
| Nerandzic et al. (2010) | 2010 | A 202-bed acute care hospital | Motioned swabs were used to sample four sites for MRSA, VRE and C. difficile from rooms of 66 discharged patients before and after a sporocidal UVC treatment (22 000 μWs/cm2). | Before decontamination, MRSA, C. difficile and VRE were isolated from 28 (10.7%), 9 (3.4%) and 7 (2.7%) of the 261 sites sampled, respectively. After UVC, MRSA, C. difficile and VRE were respectively isolated from 2 (0.8%), 1 (0.4%) and 1 (0.4%) of the 261 sites sampled, respectively. Rooms were not cleaned prior to UVC treatment. |
| Rutala et al. (2010) | 2010 | An acute care tertiary hospital | Sites in rooms that had housed patients with MRSA or VRE were sampled using Rodac plates before and after a vegetative UVC cycle (12 000 μWs/cm2). | Before decontamination, 81 (20.3%) of the 400 sites sampled were positive for MRSA. After UVC, 2 (0.5%) of the 400 sites sampled yielded MRSA. Rooms were not cleaned prior to UVC treatment. |
| Pulsed-xenon UV (PX-UV) | ||||
| Stibich et al. (2011) | 2011 | A cancer centre | Surfaces were sampled in rooms that had housed VRE patients using moistened swabs before and after PX-UV exposure. | Before decontamination, 4 (4.4%) of the 91 sites sampled were positive for VRE. After UV treatment, none of the 75 sites sampled yielded VRE. |
This study relates to the Steris VHP system; all other HPV studies relate to the Bioquell HPV system.
Both Bioquell HPV and Steris VHP systems are Environmental Protection Agency (EPA)-registered sterilants, which means they have passed the AOAC sporicide test on porous and non-porous surfaces (Humphreys, 2011). Both systems are associated with the elimination of pathogens from surfaces in situ (Bates and Pearse, 2005, Boyce et al., 2008, Hardy et al., 2007, Jeanes et al., 2005, Manian et al., 2011, Otter et al., 2008, Otter et al., 2010c, Ray et al., 2010) and cycles are validated by a > 6-log reduction of Geobacillus stearothermophilus BI spores (Bates and Pearse, 2005, Boyce et al., 2008, Hardy et al., 2007, Jeanes et al., 2005). HPV and VHP are sporicidal, bactericidal, mycobactericidal and virucidal, achieving a > 6-log reduction against a wide range of nosocomial pathogens including C. difficile spores, MRSA, VRE, A. baumannii and norovirus surrogates (Barbut et al., 2012, Bentley et al., 2012, Berrie et al., 2011, Goyal et al., 2011, Hall et al., 2007. Otter and French, 2009, Pottage et al., 2010), though efficacy may be reduced by high microbial loading and the presence of organic soil (Fu et al., 2012, Otter and French, 2009, Otter et al., 2012, Pottage et al., 2010).
UVC produces a dose-dependent 2 to 4-log reduction of nosocomial pathogens experimentally dried onto surfaces but the microbiological reduction is significantly lower out of direct line of sight of the device (Boyce et al., 2011, Nerandzic et al., 2010, Rutala et al., 2010). For example, in one study, a 1-log reduction was achieved on C. difficile spores inoculated on plastic carriers placed 10 feet (3 m) away from the device out of direct line of sight, compared with 2.6-log in direct line of sight (Nerandzic et al., 2010). Several studies of one UVC system (Lumalier Tru-D) indicated a significant reduction of surface contamination measured by total aerobic count or sampling for specific pathogens; however, there was incomplete inactivation of C. difficile, VRE, Acinetobacter or MRSA on hospital surfaces (Boyce et al., 2011, Nerandzic et al., 2010, Rutala et al., 2010).
A PX-UV system (Xenex) achieved a significant reduction in VRE contamination in a room in a 12 minute cycle (Stibich et al., 2011). Further efficacy data are awaited.
17.5.3. Clinical impact
HPV has been used to remove environmental reservoirs during outbreaks of C. difficile (Cooper et al., 2011), MRSA and meticillin-susceptible S. aureus (MSSA) (Dryden et al., 2008, Jeanes et al., 2005, Otter et al., 2008) multidrug-resistant Gram-negative bacteria (Bates and Pearse, 2005, Kaiser et al., 2011, Otter et al., 2010c) and other pathogens (Otter et al., 2010a). VHP has been used for tackling environmental reservoirs during outbreaks of A. baumannii in two reports (Chmielarczyk et al., 2012, Ray et al., 2010). The clinical impact of VHP aside from outbreak settings is not reported. On the other hand, three studies have assessed the impact of HPV in the setting of endemic infections. A prospective cohort study by Passaretti et al. demonstrated that patients admitted to rooms vacated by patients with multidrug resistant organisms (MDROs) and disinfected using HPV were 64% less likely to acquire MDROs than patients admitted to such rooms disinfected using standard methods (Passaretti et al., 2013). Thus, HPV decontamination successfully mitigated the risk from the prior room occupant.
Two pre-post studies have evaluated the clinical impact of HPV (Boyce et al., 2008, Manian et al., 2013). Boyce et al. (2008) performed a before and after study showing that HPV decontamination of rooms vacated by patients with C. difficile infection (CDI) significantly reduced the incidence of CDI both on targeted wards and hospital-wide when the analysis was restricted to the months when the epidemic strain was known to be present. Manian et al. (2011) performed a before and after study showing that HPV decontamination of rooms vacated by patients with CDI (or quadruple bleach disinfection where HPV was not available) significantly reduced the hospital-wide incidence of CDI by 37%. Whilst it was not possible to differentiate the impact of introducing HPV and quadruple bleach disinfection, a previous study by the same group showed that quadruple bleach disinfection was necessary to eliminate A. baumannii and MRSA from surfaces, and that HPV was microbiologically superior to quadruple bleach disinfection. Thus, since HPV has time and efficiency savings compared with quadruple bleach disinfection, it was cost effective to use HPV in this setting (Manian et al., 2013).
Currently, there is no published evidence that disinfection with aHP, UVC or PX-UV systems reduce epidemic or endemic infection rates.
17.5.4. Practical considerations
aHP systems
aHP is straightforward to use and relatively inexpensive compared with H2O2 vapour and UVC systems. The capacity of single units to decontaminate areas larger than single rooms is limited so multiple generators may be necessary (Holmdahl et al., 2011). Doors and air vents should be sealed and hand-held health and safety monitors are required to ensure that no leakage occurs during cycles and to verify that the concentration of hydrogen peroxide inside the enclosure is below health and safety exposure limits (Fu et al., 2012). Reported cycle times are 3–4 hours for multiple cycles (Bartels et al., 2008, Holmdahl et al., 2011) and 2 hours for single cycles (Shapey et al., 2008). However, cycle times for single rooms may be considerably longer when hand-held sensors are used to ensure the hydrogen peroxide concentrations are below health and safety limits prior to room re-entry (Fu et al., 2012). Several studies suggest that homogeneous distribution of the active agent is not achieved (Fu et al., 2012, Holmdahl et al., 2011, Shapey et al., 2008), perhaps because aHP is introduced via a unidirectional nozzle and the particles are affected by gravity. Sub-lethal exposure to hydrogen peroxide or silver could result in the development of tolerance or resistance (Chopra, 2007, McDonnell and Russell, 1999, Meyer and Cookson, 2010). The potential for transferable resistance to silver is greater than for hydrogen peroxide due to plasmid-mediated silver resistance genes (Chopra, 2007, McDonnell and Russell, 1999). Data are required confirming the compatibility of aHP systems with common hospital materials, including sensitive electronics. Finally, several studies have noted equipment reliability problems (Beswick et al., 2011, Fu et al., 2012, Shapey et al., 2008), which was a feature of older foggers (Munster and Ostrander, 1974).
H2O2 vapour systems
H2O2 vapour systems have been used to decontaminate rooms (Boyce et al., 2008, French et al., 2004), multi-bedded bays (Bates and Pearse, 2005, Boyce et al., 2008, Dryden et al., 2008) and entire units (Boyce et al., 2008, Jeanes et al., 2005, Otter et al., 2010c). However, H2O2 vapour systems are less straightforward than UV and aHP systems because they require two units (a generator and aeration unit) for a single room. Door and air vents need to be sealed. As with aHP, hand-held health and safety monitors are required to ensure that no leakage occurs during cycles and that the concentration of H2O2 inside the enclosure is below health and safety exposure limits (1 ppm) before re-entry. Thus, staff training requirements for using H2O2 systems are higher than for UV systems. The potential for selection of less susceptible strains is lower than for aHP or UV systems because the high-concentration H2O2 vapour systems ensures that few microorganisms undergo sub-lethal exposure. Reported cycle times are currently 1.5–2.5 hours for a single room for HPV (Department of Health, 2009, Otter and Yezli, 2010) and 8 hours for VHP (Ray et al., 2010). The compatibility of HPV with hospital materials, including sensitive electronics, is well established (EPA, 2010a). A recent study reported incompatibility of HPV with one brand of paint that was rectified by replacing the paint with a different brand (Passaretti et al., 2013).
UV systems
UVC is easy to use, does not require sealing of door or air vents and has a relatively short cycle time. Many high-touch sites may be out of line of sight; some manufacturers recommended multiple cycles in different parts of the room to overcome this problem but this places reliance on the operator to choose appropriate equipment locations, has implications for cycle times and requires more hands-on operator time. A recent study indicates that a UVC spore cycle in rooms ranging from 46–86 m3 took a median of 84 min (range 72–146 min) for a two-stage procedure (where the UVC unit is positioned at two locations during the cycle) and a median of 68 min (range 34–100 min) for a one-stage procedure (Boyce et al., 2011). Since some UVC systems rely on measurement of reflected dose to determine the cycle, the presence of surfaces that do not reflect UVC, or reflect it inefficiently (such as glass), variations in temperature and humidity and the age of the bulbs will affect the reflected dose and may increase the cycle times (Memarzadeh et al., 2010, Reeda, 2010). The intensity of the UV light dissipates with the square of the distance from the source, which limits the capacity of single UVC devices to disinfect areas larger than single patient rooms (Harrington and Valigosky, 2007). The long-term impact of UVC on hospital materials has not been described (Tyan et al., 2002). UVC is relatively expensive compared with other NTD systems (ECRI, 2011). Finally, UV radiation is a known mutagen (Anderson, 1995); since UVC systems do not inactivate all microbes in the room, a proportion of those that have received a sublethal dose may undergo mutation.
PX-UV systems have similar practical considerations to UVC systems, including the need to use multiple room locations to address line of sight issues, the age of the bulbs affecting intensity of the pulse, limited capacity to decontaminate areas larger than singe rooms and the potential for mutagenesis. Also, the system operates using a series of bright ‘camera flashes’, which may be disruptive to patients and staff outside the room. However, given the short cycles associated with PX-UV, it should be prioritised for further evaluation.
17.5.5. Other systems
Gaseous ozone for room disinfection has also been evaluated (Moat et al., 2009, Sharma and Hudson, 2008). Two studies of different ozone generators were performed in test chambers of 30–35 m3. which used a concentration of ozone gas peaking at 20–25 ppm. These studies indicated a 3–4 log reduction on vegetative bacteria, a < 3-log reduction on mycobacteria and a dose-dependent < 3-log reduction on bacterial endospores in one study (Moat et al., 2009) but a > 4-log spore reduction in the other (Sharma and Hudson, 2008). Both evaluations tested the systems at high humidity, one at 80–90% (Moat et al., 2009) and one at > 95% (Sharma and Hudson, 2008). Another system used a high concentration of gaseous ozone (80 ppm) and up to 3% aHP combined with high humidity (80%) to achieve a > 6-log reduction of various hospital pathogens in vitro (Zoutman et al., 2011). Substantially lower reductions were achieved at lower relative humidity (Zoutman et al., 2011). The requirement for high humidity is a major practical limitation for ozone-based systems (Li and Wang, 2003). Furthermore, ozone is toxic to humans, with a safe exposure level in the UK and USA of < 0.1 ppm (compared with 1 ppm for H2O2), so effective containment of the gas, monitoring for leakage and measurements to assure that the room is safe to enter are necessary for these systems in the healthcare setting (OSHA, 2005). Data on the compatibility of this process with hospital materials are needed, given ozone’s known corrosive properties (Davies et al., 2011).
Chlorine dioxide has a high level of efficacy against a range of pathogens (Beswick et al., 2011). However, concerns about safety and material compatibility mean that it is unlikely to be used in healthcare settings (Beswick et al., 2011, EPA, 2010a).
‘Fogging’ with various chemicals, including super-oxidised water (Clark et al., 2006, Galvin et al., 2012), solutions of H2O2 (Taneja et al., 2011, Tuladhar et al., 2012) and other chemicals (Callahan et al., 2010, De Lorenzi et al., 2011, Friedman et al., 1968, Ostrander and Griffith, 1964), have been evaluated. These systems are limited by directional introduction of the active agent and consequent non-homogeneous distribution, and the potential for the accumulation of large volumes of chemicals that require post-process removal (Taneja et al., 2011), with associated risks to operators. Data on compatibility with hospital materials are awaited.
17.5.6. Comparing systems
The performance of different systems can be evaluated by several measures, including compliance with testing standards (such as EN or ASTM standards), in vitro log reduction of bacterial loads, measurement of microbial surface contamination before and after treatment or by the use of BIs with a known concentration of a microbe, typically a bacterial endospore. BIs can be produced in-house or, more reliably, can be purchased commercially (typically containing G. stearothermophilus bacterial endospores). Most NTD systems produce a more significant reduction of bacterial contamination than conventional disinfection (Barbut et al., 2009, Boyce et al., 2011, French et al., 2004, Manian et al., 2011, Nerandzic et al., 2010, Shapey et al., 2008). However, comparison of the relative effectiveness of different NTD systems is difficult because of variations in sample sites (especially orientation and proximity to the NTD device), patient infection or colonisation status, the organism, the microbiological testing methods and the type of pre-cleaning. Thus, the best way to compare different systems is through controlled head-to-head studies (Boyce, 2009), ideally using outcomes related to reduced transmission in clinical settings. However, there have been few studies comparing these outcomes, so it is not possible to evaluate the relative clinical impact of NTD systems using current data. Thus, the available head-to-head studies are currently the most useful way to compare NTD systems.
A recently published study comparing HPV (Bioquell) with an aHP system (ASP Glosair) was performed by St George’s Hospital, London (Fu et al., 2012). Testing was performed in a 50 m3 room with a 13 m3 anteroom, selected to represent a single occupancy room with a bathroom. Safety was evaluated using a hand-held hydrogen peroxide sensor. The workplace exposure limits (WEL) for H2O2 are 1 ppm as an 8-hour time weighted average, or 2 ppm for 15 minutes as a short-term exposure limit (STEL) (Health and Safety Executive, 2005). The HPV manufacturer mandates re-entering the room only after the measurable concentration of H2O2 is < 1 ppm; the aHP manufacturer recommended room re-entry 2 hours after the start of the cycle. Thus, in this study the mean concentration of H2O2 in the room was measured 2 hours after the cycle started for both systems. The mean H2O2 concentration in the room 2 hours after the cycle started was 2.8 ± 0.8 ppm for aHP, with a maximum reading of 4.5 ppm and no readings < 2 ppm, and 1.3 ± 0.4 ppm for HPV, with no readings > 2 ppm. Thus, for both systems room re-entry must be controlled by measurements of H2O2 concentrations rather than assuming safe levels at the end of the process. A ‘controlled leakage’ experiment was performed in the St George’s study to determine whether H2O2 leaked from an unsealed room door. This was only done for the aHP system because the user manuals recommend door and air vent sealing with adhesive tape for the HPV system but not for the aHP. More than 20 ppm H2O2 was detected outside an unsealed door, indicating that doors must be sealed during cycles. These findings also imply that air vents should be sealed during room disinfection with H2O2 systems.
Microbiological efficacy was assessed by using commercially available 6-log G. stearothermophilus BIs and in-house prepared test discs inoculated with MRSA, A. baumannii and C. difficile (spores) placed at 11 locations around the room (Fu et al., 2012). In addition, in-house prepared test discs dried in 3% or 10% bovine serum albumin (BSA) to simulate dirty conditions were tested in two further room locations. There are no standard testing methods for NTD systems, so the in-house test discs were used to measure log reductions of the common nosocomial pathogens and 6-log and 4-log G. stearothermophilus BIs were used two provide two levels of challenge. HPV inactivated 91% (40/44) of the 6-log and 95% (42/44) of the 4-log G. stearothermophilus BIs. HPV generally achieved a 6-log reduction of the MRSA, A. baumannii and C. difficile BIs regardless of room location. aHP inactivated 13.6% (6/44) of the 6-log BIs, and 36.4% of the 4-log BIs. aHP achieved a < 4-log reduction at 2/11 room locations for MRSA, 7/11 for A. baumannii and 2/11 for C. difficile spores. The aHP system had reduced efficacy against the catalase-positive A. baumannii with a < 2-log reductions at 6/11 of room locations. HPV achieved a > 5-log reduction at 11/12 locations with MRSA, A. baumannii or C. difficile dried in 3% or 10% BSA compared with 3/12 locations for aHP. This suggests that HPV is more able to penetrate increasing levels of soil, which may be important with sub-optimal pre-cleaning. The log reduction of the in-house prepared test discs varied considerably by room location for aHP but not for HPV, indicating a more uneven distribution of the active agent for aHP.
Another recent head-to-head study was performed in Malmo, Sweden, and compared the same HPV and aHP systems. Testing was performed in a 136 m3 room selected to represent a dual occupancy room. An HPV cycle from a single unit inactivated all 48 6-log G. stearothermophilus BIs distributed around the test room (Holmdahl et al., 2011). After three back-to-back cycles using two units, 50% of 48 BIs were inactivated by the aHP system. Ninety percent of BIs yielded bacterial growth after the first aHP cycle compared with 21% after both cycles two and three, suggesting poor repeatability. BIs grew in different locations in repeat experiments with the aHP system, suggesting variable distribution. The HPV system was faster than the aHP system, as in the St George’s study (Fu et al., 2012).
The UK Health and Safety Laboratory performed a detailed head-to-head study of six room decontamination technologies including HPV and aHP systems (Beswick et al., 2011). The microbial challenges (including C. difficile spores) were designed to simulate ‘worst-case’ contamination encountered in laboratories. Organisms were dried onto stainless steel discs and exposed to the decontamination processes in a 35 m3 room and 105 m3 laboratory. HPV achieved a 5 to 6-log reduction of C. difficile spores in all locations apart from in a wet spillage. aHP achieved a < 1-log reduction for C. difficile spores in all room locations. Both systems were less effective than in other studies, probably because the discs were prepared using growth media that provides an additional level of protection for the microorganisms. These authors recommended that ‘All systems should be sold with a device for monitoring fumigant levels at the end of a cycle.’
These results indicate that HPV is faster and more effective for biological inactivation than aHP (Beswick et al., 2011, Fu et al., 2012, Holmdahl et al., 2011). However, the studies reported above were not performed in a clinical setting and did not evaluate surface decontamination directly or the impact on pathogen transmission.
A head-to-head study performed at a US hospital compared HPV (Bioquell) with a UVC system (Tru-D, Lumalier) (Havill et al., 2012). In-house prepared carrier discs inoculated with ~ 106 C. difficile spores and BIs with 104 and 106 G. stearothermophilus spores were placed in five sites (three sites were not in the direct line of sight of the device). UVC achieved a mean of 2.2 log reduction for C. difficile (range 1.7–3 log reduction) and inactivated 29% (22/75) of 4-log BIs (range 7–53%) and 0% (0/75) of 6-log BIs. UVC was significantly less effective out of direct line of sight: it inactivated 42% of 4-log G. stearothermophilus BIs in direct line of sight but only 7% of 4-log BIs out of direct line of sight. HPV achieved a > 6-log reduction for C. difficile in all five sites and inactivated 99% (74/75) of 6-log BIs and 100% (75/75) of 4-log BIs. UVC was faster but less effective than HPV for the inactivation of BIs and microbes on surfaces.
No head-to-head studies comparing aHP and UVC have been published. More head-to-head evaluations of all NTD systems are required, including assessment of clinically relavent outcomes.
17.5.7. Cost
NTD systems can be purchased, rented or introduced as part of a service contract. These deployment models have different costs, depending on the package and the frequency of use (Department of Health, 2009).
Several factors must be taken into account when considering the cost of NTD systems. For hospitals that purchase their own NTD system, upfront costs include the equipment itself, staff training (and possibly recruitment) and possibly costs associated with equipment storage. Ongoing costs include personnel costs, consumables (such as H2O2 and replacement UV bulbs), depreciation, maintenance and power. For hospitals that choose to purchase a service or other model, manufacturers should be contacted to discuss available options.
Few studies disclose the cost of currently available NTD systems. The Emergency Care Research Institute (ECRI) reports the list price for the Lumalier Tru-D UVC device as £77 190 (US$124 500), the Bioquell HPV system as £27 280 (US$44 000), and the Xenex PX-UV system as £1862 (US$3 000) per month over a 36 month lease (ECRI, 2011). Thus, the relative purchase cost of equipment is likely to be UVC > PX-UV > H2O2 vapour systems > aHP (ECRI, 2011). Consumables’ costs for the hydrogen peroxide systems are likely to be greater than the cost of bulb replacement for the UV systems. Manufacturers should be contacted to provide current prices and purchasing options.
No studies of the cost-effectiveness of NTD systems have been published. Performing a cost-effectiveness study on the use of an NTD system should consider the direct and indirect costs associated with the system, any impact on rates of infection with their associated costs and other factors (Perencevich et al., 2007).
17.6. When to consider an NTD system
Current CDC guidelines recommend against routine ‘disinfectant fogging’ in patient-care areas (Rutala et al., 2008). This recommendation is currently being re-evaluated by the CDC based on data that have emerged since the guidelines were published and suggest NTD systems may be warranted in some circumstances. The strongest reason for considering an NTD system is to break the chain of transmission by improving terminal disinfection of clinical areas after patients infected or colonised with certain pathogens have been discharged (Otter et al., 2011, Rutala and Weber, 2011). Key pathogens associated with contamination of the environment include C. difficile, VRE, MRSA, A. baumannii, P. aeruginosa and norovirus (Otter et al., 2011).
Because of practicality, cost and resource constraints, NTD systems are not suitable for performing disinfection of general clinical areas or daily disinfection of rooms before patients are discharged because of the need for temporary patient relocation. One study evaluated the use of HPV to disinfect the room of a patient colonised with multiple MDROs (Otter et al., 2007). The patient was temporarily relocated and his room decontaminated. Decontamination was effective, but the room was recontaminated shortly after the patient returned. Such recontamination was also seen after HPV decontamination of an intensive care unit (ICU) (Hardy et al., 2007). NTD systems have been used to control endemic infection (Boyce et al., 2008, Otter et al., 2011, Rutala and Weber, 2011) and outbreaks (Bates and Pearse, 2005, Cooper et al., 2011, Dryden et al., 2008, Jeanes et al., 2005, Kaiser et al., 2011, Otter et al., 2010c, Ray et al., 2010). Whilst disinfection of single rooms is more common, NTD systems have been used to disinfect multi-occupancy areas, particularly to remove environmental reservoirs during outbreaks (Bates and Pearse, 2005, Boyce et al., 2008, Dryden et al., 2008, Jeanes et al., 2005, Otter et al., 2010c) and whole wards have been disinfected in some studies (Boyce et al., 2008, Jeanes et al., 2005, Otter et al., 2010c). The different indications for the use of NTD systems are outlined in the following scenarios.
17.6.1. Scenarios when the use of an NTD system may be indicated
The choice of whether to rely on current cleaning and disinfection methods, enhanced conventional methods or an NTD system will be determined by the clinical scenario. The key factors are whether the area to be disinfected is a single room or a multi-occupancy area, whether the clinical setting is high risk for infection acquisition (e.g. an ICU) or low risk (e.g. a general ward), and the target organism (Barbut et al., 2009, Boyce et al., 2008, Otter et al., 2010c, Passaretti et al., 2013, Shapey et al., 2008). The risk associated with individual pathogens in the context of disinfection will depend on a number of factors, including the importance of environmental contamination in transmission, clinical implications, local epidemiology and financial outcomes. For example, a multidrug-resistant Gram-negative rod or C. difficile causing an outbreak would be considered a ‘high-risk’ pathogen, whereas VRE colonisation would be considered lower risk. Further issues that may need to be considered are the clinical, financial and reputational effects of environmental infections, especially during on-going outbreaks requiring ward closures. Closures may have particular adverse impacts when they involve specialist regional units such as those for neonatal, paediatric or adult intensive care.
The disinfection of multi-occupancy bays using NTD systems is constrained by the need to accommodate patients elsewhere during the disinfection process (Otter et al., 2009b). However, this may be necessary and justified to bring a serious outbreak of high-risk pathogens in high-risk patients under control. It may be practical to use UV systems for the disinfection of single rooms used by patients with low-risk pathogens in low-risk settings (Nerandzic et al., 2010, Rutala et al., 2010) but practical constraints limit the use of hydrogen peroxide NTD systems in this situation. Conversely, H2O2 vapour systems would be appropriate for dealing with high-risk pathogens in high-risk units because of their high levels of efficacy, homogeneous distribution and disinfection assurance (Boyce et al., 2008, Manian et al., 2011, Otter et al., 2010a). Examples include on-going outbreaks in intensive care units with NAP1/027C. difficile or a multidrug-resistant Gram-negative pathogen. UV and hydrogen peroxide systems may be suitable for disinfection of single rooms in low-risk settings with high-risk pathogens or in high-risk settings with low-risk pathogens (Bartels et al., 2008, Boyce et al., 2008, French et al., 2004, Nerandzic et al., 2010, Shapey et al., 2008). Enhanced conventional disinfection methods should also be employed in these scenarios (Dancer et al., 2009, Datta et al., 2011, Hayden et al., 2006, Mahamat et al., 2007, Mayfield et al., 2000, Wilcox et al., 2003), with the possible exception of high-risk pathogens occurring in high-risk settings where even enhanced conventional disinfection has been shown to leave residual contamination (Datta et al., 2011, Manian et al., 2011, Morter et al., 2011, Wilcox et al., 2003).
Other potential applications of NTD systems include: the removal of environmental pathogens disturbed during building works such as Aspergillus fumigatus (Vonberg and Gastmeier, 2006); as part of emergency preparedness planning (Otter et al., 2010a); the disinfection of mobile medical equipment in a dedicated facility; and decontamination of emergency vehicles or operating theatres (van’t Veen et al., 2005). The widespread need for decontamination of complex mobile medical equipment and furniture, such as blood pressure cuffs, ventilator tubing, wheelchairs, commodes, computers and other electronics (Boyce et al., 1997, Dryden et al., 2008, Dumford et al., 2009) means that dedicated disinfection rooms incorporating NTD systems are becoming recognised as very useful hospital facilities.
17.7. Using, validating and regulating NTD systems
17.7.1. The need for pre-cleaning
As with all forms of decontamination, cleaning is required prior to NTD disinfection system use in order to remove organic matter that reduces the effectiveness of NTD systems (Fu et al., 2012, Kac et al., 2010, Otter and French, 2009, Otter et al., 2012, Pottage et al., 2010. Sweeney and Dancer, 2009). The impact of organic matter has been demonstrated by several in vitro studies. For example, Otter et al. evaluated the efficacy of HPV for the inactivation of MRSA dried on stainless steel discs in suspending media containing 0.3%, 3% and 10% BSA (Otter et al., 2012). The effectiveness of HPV was reduced as the concentration of BSA increased. There is evidence that some NTD systems are more susceptible to organic soiling than others. For example, the study by Fu et al. showed that aHP is more susceptible to simulated soiling by BSA than HPV (Fu et al., 2012).
Nevertheless, several studies demonstrate that NTD systems can produce significant reductions in environmental contamination even without pre-cleaning (French et al., 2004, Nerandzic et al., 2010, Rutala et al., 2010). For example, in one study, 1 site out of 85 sampled yielded MRSA after HPV without pre-cleaning compared with 61 (72%) of 85 matched sites before HPV (French et al., 2004). In this instance, MRSA was identified by broth enrichment, indicating a low level of contamination, and was cultured from a floor corner that was visibly dirty.
17.7.2. Validation
One of the problems with conventional cleaning and disinfection is the difficulty in validating the processes. The major advantage of NTD systems is the reduction or removal of reliance on the operator to assure adequate distribution and contact time of a disinfectant. It follows that NTD systems should be validated to ensure that their automated processes are effective and repeatable.
NTD systems could be validated by routine microbiological sampling using conventional standards (Dancer, 2004), but this is time consuming, costly and requires microbiological expertise. Another option is the use of BIs, which provide a semi-quantitative measure of microbiological efficacy and repeatability (Boyce et al., 2008, Holmdahl et al., 2011). The question remains as to whether 6-log BIs are an appropriate test for validating NTD systems, given that the concentration of contamination on hospital surfaces is usually in the 2-log range (Holmdahl et al., 2011, Otter and Yezli, 2012, Walder and Holmdahl, 2012). Walder and Holmdahl (2012) argue that soiling and biofilms (Smith and Hunter, 2008, Vickery et al., 2012), occasional higher levels of contamination (Morter et al., 2011), the occurrence of pathogens with reduced susceptibility to certain agents (Pottage et al., 2012) and the potential for incomplete distribution (Fu et al., 2012, Havill et al., 2012, Holmdahl et al., 2011) mean that 6-log BIs are an appropriate target for NTD systems. Recent evidence published by Pottage et al. (2012) and others (Beswick et al., 2011, Fu et al., 2012, Otter and French, 2009) indicating that catalase-positive bacteria are less susceptible to hydrogen peroxide-based NTD systems than bacterial endospores provides a further reason to use stringent challenges for these systems (Otter and Yezli, 2012).
The US EPA requires a hospital disinfectant to achieve a > 6-log reduction of certain vegetative bacteria in vitro (EPA, 2010b). This is higher than the concentration typically found on hospital surfaces, presumably to provide assurance that the disinfectant will be effective in the ‘real world’.
The inactivation of 6-log BIs correlates well with the elimination of pathogens from surfaces and can be used as a test standard for NTD systems when the elimination of pathogens is required (Otter and Yezli, 2012, Walder and Holmdahl, 2012). H2O2 vapour systems can eliminate pathogens from surfaces, produce a > 6-log reduction of a range of pathogens in vitro and can inactivate 6-log BIs (Boyce et al., 2008, French et al., 2004, Otter and French, 2009. Otter et al., 2010c). aHP, UVC and PX-UV are much less effective in these tests (Andersen et al., 2006, Barbut et al., 2009, Boyce et al., 2011, Fu et al., 2012, Holmdahl et al., 2011, Nerandzic et al., 2010, Rutala et al., 2010, Shapey et al., 2008). However, further studies are necessary to determine the level of pathogen reduction required to interrupt transmission and set the appropriate clinical decontamination standard for NTD systems.
17.7.3. Regulation
Given the relatively recent introduction of NTD systems into the marketplace, regulatory standards have not been established. In Europe, the regulation of disinfectants is in flux because of the phased introduction of the biocidal products directive (BPD) (Low, 2011). Testing standards are generally not specified for NTD systems, although a French standard for testing NTD systems, NF72-281, is currently under evaluation for adoption as a European standard. Currently, it is not clear how the BPD will influence NTD systems, although the NTDs will need to be assessed and registered as with any other disinfectant.
In the UK, the Health and Safety Executive, Department of Health (DH), Health Protection Agency (HPA) and various professional societies have a role to play in the regulation of NTD systems. In England, the DH and HPA have established a expert group called the Rapid Review Panel (RRP) to evaluate products claiming to be useful in healthcare applications (Department of Health, 2009). The RRP has issued several recommendations on NTD systems. These provide independent, evidence-based recommendations that can guide decision making about such products. The RRP has issued the following recommendations about NTD systems (which are available at http://www.hpa.org.uk/ProductsServicesMicrobiologyPathology/RapidReviewPanel):
-
•
Bioquell HPV (recommendation 1): Basic research and development, validation and recent in use evaluations have shown benefits that should be available to NHS bodies to include as appropriate in their cleaning, hygiene or infection control protocols.
-
•
Steris VHP (recommendation 2): Basic research and development has been completed and the product may have potential value; in use evaluations/trials are now needed in an NHS clinical setting.
-
•
aHP system; Sterinis, now ASP Glosair (recommendation 3): A potentially useful new concept but insufficiently validated; more research and development is required before it is ready for evaluation in practice.
In the US, the EPA regulates disinfectant use and will likely regulate NTD systems. The EPA issued an order to stop a US hospital using a disinfectant fogger in ambulances on safety grounds (EPA, 2011). The EPA sought information from some US professional societies and the published open correspondence between the US EPA, Society for Healthcare Epidemiology of America (SHEA), Association for Professionals in Infection Control and Epidemiology (APIC) and Association for the Healthcare Environment (AHE) (SHEA/APIC/AHA, 2011) illustrates that healthcare regulators and professional societies are beginning to take an interest in NTD systems. Similarly, ANSM (previously AFSSAPS), the French regulatory body, has withdrawn several NTD systems, including an aHP system, from the French market due to a lack of efficacy data (AFSSAPS, 2011).
Regulators and professional societies will be required to make recommendations on issues such as nomenclature of NTD systems, acceptability of testing standards and guidance on safe and effective applications. Nomenclature is already confused. For example, the Oxypharm Nocospray aHP system has been incorrectly referred to (Otter and Yezli, 2011, Otter et al., 2010b) as using ‘hydrogen peroxide vapour’ (Chan et al., 2011) and correctly as using an aerosol of hydrogen peroxide (Orlando et al., 2008). Such confusion in describing the various different NTD systems is also evident in several review papers (Davies et al., 2011, Falagas et al., 2011).
17.8. Sources of further information and advice
It is likely that the literature evaluating the role of contaminated surfaces in the transmission of nosocomial pathogens will continue to expand. A better understanding of the role of contaminated surfaces in transmission will help to target interventions aimed at improving cleaning and disinfection most effectively. However, at the current time, we do not know the relationship between the level of residual contamination and infection. Ideally, the target should be zero contamination; however, practicalities require a risk-based approach.
Initial studies of systematic improvement of enhanced conventional cleaning methods can reduce transmission to some degree but they often continue to fail because they are dependent on human skill and performance, which cannot be guaranteed. Automated NTD systems are potentially an effective and efficient adjunct to decontaminating complex environmental surfaces. As the evidence base grows, the indications and cost-effectiveness of NTD systems will become clearer. At present, there is good evidence that terminal disinfection of clinical areas used by patients colonised or infected with pathogens associated with environmental contamination can reduce or eliminate the risk of onward transmission to others, and it is in this situation where NTD systems can be most useful.
The choice of system will depend on practicalities and cost effectiveness. However, more head-to-head comparisons are needed to compare both microbiological and clinical outcomes to allow better evidence-based decisions.
Technological developments mean that existing NTD systems will become more refined. For example, improvement in aeration capacity has reduced HPV process times from > 4 hours (French et al., 2004) to < 2 hours for a single room (Barbut et al., 2012, Otter and Yezli, 2010). Also, the use of UV reflective paint could reduce the impact of line-of-sight issues for UV systems (Rutala, 2012). In addition, there seems to be a natural synergy between the use of NTD systems and antimicrobial surfaces or air disinfection systems, which can help to reduce the extent of surface contamination during the stay of a patient (Sexton et al., 2006, Weber and Rutala, 2012). The emergence of novel NTD systems, combining rapid cycle times with high-level efficacy, would broaden the potential application of NTD systems.
17.9. Conclusion
There is now evidence that existing NTD systems are an effective adjunct to conventional methods of terminal disinfection, and that H2O2 vapour systems reduce transmission in endemic and epidemic settings. Further evidence on the optimal application and cost effectiveness of NTD systems in healthcare is required, but NTD systems are already beginning to be integrated into hospital disinfection policies (Boyce et al., 2008, Passaretti et al., 2013). Regulators and professional bodies should decide on the terminology and insist on standardisation for these systems and, as adoption and the evidence-base grows, the role of regulators and professional societies will become increasingly important in the provision of advice and guidelines to ensure the safe and effective use of NTD systems in healthcare settings (Department of Health and Health Protection Agency, 2009, Rutala et al., 2008).
Current data on NTD systems in the academic literature is limited but increasing (Fig. 17.1). Academic reviews of NTD systems can provide useful background data, for example, those by Otter et al. (2013), Davies et al. (2011) and Falagas et al. (2011). In addition, publications in the non-peer reviewed literature and by research institutes can provide useful background information. For example, the ECRI Institute (2011) and Infection Control Today (Pyrek, 2011) have published useful guidance documents. In addition, several studies of NTD systems with clinical outcomes are currently in progress, including a CDC-funded assessment of a UVC system.b These studies will be important in determining the appropriate use of the various NTD systems. Finally, NTD systems are increasingly included in certain guidelines as an adjunct to traditional cleaning and disinfection (APIC, 2010, Department of Health and Health Protection Agency, 2009).
Footnotes
17.10 References
- AFSSAPS Appareils de désinfection des surfaces par voie aerienne. 2011. http://www.afssaps.fr/Activites/Biocides-Appareils-de-desinfection-par-voie-aerienne/ Procedes-et-appareils-de-desinfection-des-surfaces-par-voie-aerienne/(offset)/1#paragraph_35103.
- Andersen B.M. Does ‘airborne’ hydrogen peroxide kill Mycobacterium tuberculosis? J Hosp Infect. 2011;77:81–83. doi: 10.1016/j.jhin.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Andersen B.M., Rasch M., Hochlin K., Jensen F.H., Wismar P., Fredriksen J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. 2006;62:149–155. doi: 10.1016/j.jhin.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B.M., Syversen G., Thoresen H., Rasch M., Hochlin K., Seljordslia B., Snevold I., Berg E. Failure of dry mist of hydrogen peroxide 5% to kill Mycobacterium tuberculosis. J Hosp Infect. 2010;76:80–83. doi: 10.1016/j.jhin.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Anderson P. Mutagenesis. Methods Cell Biol. 1995;48:31–58. [PubMed] [Google Scholar]
- APIC . APIC; Washington DC: 2010. Guide to the Elimination of Multidrug-resistant Acinetobacter baumannii Transmission in Healthcare Settings. [Google Scholar]
- Barbut F., Menuet D., Verachten M., Girou E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol. 2009;30:515–517. doi: 10.1086/597232. [DOI] [PubMed] [Google Scholar]
- Barbut F., Yezli S., Otter J.A. Activity in vitro of hydrogen peroxide vapour against Clostridium difficile spores. J Hosp Infect. 2012;80:85–87. doi: 10.1016/j.jhin.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Barbut F., Yezli S., Mimoun M., Pham J., Chaouat M., Otter J.A. Reducing the spread of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus on a burns unit through the intervention of an infection control bundle. Burns. 2013;39:395–403. doi: 10.1016/j.burns.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Bartels M.D., Kristoffersen K., Slotsbjerg T., Rohde S.M., Lundgren B., Westh H. Environmental meticillin-resistant Staphylococcus aureus (MRSA) disinfection using dry-mist-generated hydrogen peroxide. J Hosp Infect. 2008;70:35–41. doi: 10.1016/j.jhin.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Bates C.J., Pearse R. Use of hydrogen peroxide vapour for environmental control during a Serratia outbreak in a neonatal intensive care unit. J Hosp Infect. 2005;61:364–366. doi: 10.1016/j.jhin.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Bentley K., Dove B.K., Parks S.R., Walker J.T., Bennett A.M. Hydrogen peroxide vapour decontamination of surfaces artificially contaminated with norovirus surrogate feline calicivirus. J Hosp Infect. 2012;80:116–121. doi: 10.1016/j.jhin.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R., Shaffer R.E. Evaluation of Multiple (3-Cycle) Decontamination processing for filtering facepiece respirators. J Engineered Fibers Fabrics. 2010;5:33–41. [Google Scholar]
- Berrie E., Andrews L., Yezli S., Otter J.A. Hydrogen peroxide vapour (HPV) inactivation of adenovirus. Lett Appl Microbiol. 2011;52:555–558. doi: 10.1111/j.1472-765X.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- Beswick A.J., Farrant J., Makison C., Gawn J., Frost G., Crook B., Pride J. Comparision of multiple systems for laboratory whole room fumigation. Appli Biosafety. 2011;16:139–157. [Google Scholar]
- Blancou J. History of disinfection from early times until then end of the 18th century. Rev Sci Tech. 1995;14:31–39. [PubMed] [Google Scholar]
- Boyce J.M. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65:50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- Boyce J.M. New approaches to decontamination of rooms after patients are discharged. Infect Control Hosp Epidemiol. 2009;30:515–517. doi: 10.1086/598999. [DOI] [PubMed] [Google Scholar]
- Boyce J.M., Potter-Bynoe G., Chenevert C., King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–627. [PubMed] [Google Scholar]
- Boyce J.M., Havill N.L., Mcdonald L.C., Adams N.M., Thompson A., Wiggs L., Noble-Wang J. Impact of hydrogen peroxide vapor room biodecontamination on environmental contamination and nosocomial transmission of Clostridium difficile; 16th Annual Meeting of the Society for Healthcare Epidemiology of America (SHEA), Chicago, Illinois (2006) Abstract 155; 2006. [Google Scholar]
- Boyce J.M., Havill N.L., Otter J.A., Mcdonald L.C., Adams N.M., Cooper T., Thompson A., Wiggs L., Killgore G., Tauman A., Noble-Wang J. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol. 2008;29:723–729. doi: 10.1086/589906. [DOI] [PubMed] [Google Scholar]
- Boyce J.M., Havill N.L., Dumigan D.G., Golebiewski M., Balogun O., Rizvani R. Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect Control Hosp Epidemiol. 2009;30:678–684. doi: 10.1086/598243. [DOI] [PubMed] [Google Scholar]
- Boyce J.M., Havill N.L., Moore B.A. Terminal decontamination of patient rooms using an automated mobile UV light unit. Infect Control Hosp Epidemiol. 2011;32:737–742. doi: 10.1086/661222. [DOI] [PubMed] [Google Scholar]
- Bryant L.R. Mechanical respirators. Their use and application in lung trauma. JAMA. 1967;199:149–154. doi: 10.1001/jama.199.3.149. [DOI] [PubMed] [Google Scholar]
- Byers K.E., Durbin L.J., Simonton B.M., Anglim A.M., Adal K.A., Farr B.M. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1998;19:261–264. doi: 10.1086/647806. [DOI] [PubMed] [Google Scholar]
- Byrns G., Fuller T.P. The risks and benefits of chemical fumigation in the health care environment. J Occup Environ Hyg. 2011;8:104–112. doi: 10.1080/15459624.2011.547453. [DOI] [PubMed] [Google Scholar]
- Callahan K.L., Beck N.K., Duffield E.A., Shin G., Meschke J.S. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) on various environmental surfaces by mist application of a stabilized chlorine dioxide and quaternary ammonium compound-based disinfectant. J Occup Environ Hyg. 2010;7:529–534. doi: 10.1080/15459624.2010.487806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling P.C., Briggs J.L., Perkins J., Highlander D. Improved cleaning of patient rooms using a new targeting method. Clin Infect Dis. 2006;42:385–388. doi: 10.1086/499361. [DOI] [PubMed] [Google Scholar]
- Carling P.C., Parry M.M., Rupp M.E., Po J.L., Dick B., Von Beheren S. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:1035–1041. doi: 10.1086/591940. [DOI] [PubMed] [Google Scholar]
- Chan H.T., White P., Sheorey H., Cocks J., Waters M.J. Evaluation of the biological efficacy of hydrogen peroxide vapour decontamination in wards of an Australian hospital. J Hosp Infect. 2011;79:125–128. doi: 10.1016/j.jhin.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Chmielarczyk A., Higgins P.G., Wojkowska-Mach J., Synowiec E., Zander E., Romaniszyn D., Gosiewski T., Seifert H., Heczko P., Bulanda M. Control of an outbreak of Acinetobacter baumannii infections using vaporized hydrogen peroxide. J Hosp Infect. 2012;81:239–245. doi: 10.1016/j.jhin.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59:587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- Clark J., Barrett S.P., Rogers M., Stapleton R. Efficacy of super-oxidized water fogging in environmental decontamination. J Hosp Infect. 2006;64:386–390. doi: 10.1016/j.jhin.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cooper T., O’Leary M., Yezli S., Otter J.A. Impact of environmental decontamination using hydrogen peroxide vapour on the incidence of Clostridium difficile infection in one hospital Trust. J Hosp Infect. 2011;78:238–240. doi: 10.1016/j.jhin.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Dancer S.J. Mopping up hospital infection. J Hosp Infect. 1999;43:85–100. doi: 10.1053/jhin.1999.0616. [DOI] [PubMed] [Google Scholar]
- Dancer S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect. 2004;56:10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer S.J. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73:378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Dancer S.J., White L.F., Lamb J., Girvan E.K., Robertson C. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC Med. 2009;7:28. doi: 10.1186/1741-7015-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R., Platt R., Yokoe D.S., Huang S.S. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011;171:491–494. doi: 10.1001/archinternmed.2011.64. [DOI] [PubMed] [Google Scholar]
- Davies A., Pottage T., Bennett A., Walker J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J Hosp Infect. 2011;77:199–203. doi: 10.1016/j.jhin.2010.08.012. [DOI] [PubMed] [Google Scholar]
- De Lorenzi S., Romanini L., Finzi G., Barrai I., Salvatorelli G. Reducing microbial contamination of surfaces using RelyOn Virkosept aerosol spray. J Hosp Infect. 2011;78:240–241. doi: 10.1016/j.jhin.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Department of Health HCAI Technology Innovation Programme. Showcase Hospitals report number 3. 2009. www.clean-safe-care.nhs.uk Available from.
- Department of Health and Health Protection Agency . Clostridium difficile: how to deal with the problem. 2009. [Google Scholar]
- Dettenkofer M., Block C. Hospital disinfection: efficacy and safety issues. Curr Opin Infect Dis. 2005;18:320–325. doi: 10.1097/01.qco.0000172701.75278.60. [DOI] [PubMed] [Google Scholar]
- Drees M., Snydman D., Schmid C., Barefoot L., Hansjosten K., Vue P., Cronin M., Nasraway S., Golan Y. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46:678–685. doi: 10.1086/527394. [DOI] [PubMed] [Google Scholar]
- Dryden M., Parnaby R., Dailly S., Lewis T., Davis-Blues K., Otter J.A., Kearns A.M. Hydrogen peroxide vapour decontamination in the control of a polyclonal meticillin-resistant Staphylococcus aureus outbreak on a surgical ward. J Hosp Infect. 2008;68:190–192. doi: 10.1016/j.jhin.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Dubberke E.R., Reske K.A., Noble-Wang J., Thompson A., Killgore G., Mayfield J., Camins B., Woeltje K., McDonald J.R., McDonald L.C., Fraser V.J. Prevalence of Clostridium difficile environmental contamination and strain variability in multiple health care facilities. Am J Infect Cont. 2007;35:315–318. doi: 10.1016/j.ajic.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Dumford D.M., III, Nerandzic M.M., Eckstein B.C., Donskey C.J. What is on that keyboard? Detecting hidden environmental reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains. Am J Infect Control. 2009;37:15–19. doi: 10.1016/j.ajic.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Eckstein B.C., Adams D.A., Eckstein E.C., Rao A., Sethi A.K., Yadavalli G.K., Donskey C.J. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECRI Enhanced environmental disinfection systems. Health Devices. 2011;40:150–162. [PubMed] [Google Scholar]
- EPA . Compatibility of material and electronic equipment with hydrogen peroxide and chlorine dioxide fumigation. 2010. (Assessment and evaluation report). [Google Scholar]
- EPA Standard operating procedure for AOAC use dilution method for testing disinfectants. 2010. http://www.epa.gov/pesticides/methods/atmpmethods/MB-05-08.pdf
- EPA . EPA Issues Order to NJ Hospital Group. 2011. [Google Scholar]
- Falagas M.E., Thomaidis P.C., Kotsantis I.K., Sgouros K., Samonis G., Karageorgopoulos D.E. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect. 2011;78:171–177. doi: 10.1016/j.jhin.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T., Sholtz L.A., Marion N., Turner P., Carling P.C., Rupp M.E. Maintenance of environmental services cleaning and disinfection in the ICU after a performance improvement project. Am J Infect Control. 2012;40:e159. [Google Scholar]
- Fraise A. Currently available sporicides for use in healthcare, and their limitations. J Hosp Infect. 2011;77:210–212. doi: 10.1016/j.jhin.2010.06.029. [DOI] [PubMed] [Google Scholar]
- French G.L., Otter J.A., Shannon K.P., Adams N.M., Watling D., Parks M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Friedman H., Volin E., Laumann D. Terminal disinfection in hospitals wihh quaternary ammonium compounds by use of a spray-fog technique. Appl Microbiol. 1968;16:223–227. doi: 10.1128/am.16.2.223-227.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T.Y., Gent P., Kumar V. Efficacy, efficiency and safety aspects of hydrogen peroixde vapour and aerosolized hydrogen peroixde room disinfection systems. J Hosp Infect. 2012;80:199–205. doi: 10.1016/j.jhin.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Galvin S., Boyle M., Russell R.J., Coleman D.C., Creamer E., O’Gara J.P., Fitzgerald-Hughes D., Humphreys H. Evaluation of vaporized hydrogen peroxide, Citrox and pH neutral Ecasol for decontamination of an enclosed area: a pilot study. J Hosp Infect. 2012;80:67–70. doi: 10.1016/j.jhin.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Goodman E.R., Platt R., Bass R., Onderdonk A.B., Yokoe D.S., Huang S.S. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol. 2008;29:593–599. doi: 10.1086/588566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.M., Chander Y., Yezli S., Otter J.A. Hydrogen peroxide vapor (HPV) inactivation of feline calicivirus, a surrogate for norovirus – an update. Infection Prevention Society Annual Meeting; 2011. [Google Scholar]
- Grare M., Dailloux M., Simon L., Dimajo P., Laurain C. Efficacy of dry mist of hydrogen peroxide (DMHP) against Mycobacterium tuberculosis and use of DMHP for routine decontamination of biosafety level 3 laboratories. J Clin. Microbiol. 2008;46:2955–2958. doi: 10.1128/JCM.00250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Otter J.A., Chewins J., Wengenack N.L. Use of hydrogen peroxide vapor for deactivation of Mycobacterium tuberculosis in a biological safety cabinet and a room. J Clin Microbiol. 2007;45:810–815. doi: 10.1128/JCM.01797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Otter J.A., Chewins J., Wengenack N.L. Deactivation of the dimorphic fungi Histoplasma capsulatum, Blastomyces dermatitidis and Coccidioides immitis using hydrogen peroxide vapor. Med Mycol. 2008;46:189–191. doi: 10.1080/13693780701744809. [DOI] [PubMed] [Google Scholar]
- Hardy K.J., Gossain S., Henderson N., Drugan C., Oppenheim B.A., Gao F., Hawkey P.M. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J Hosp Infect. 2007;66:360–368. doi: 10.1016/j.jhin.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Harrington B.J., Valigosky M. Monitoring ultraviolet lamps in biological safety cabinets with cultures of standard bacterial strains on TSA blood agar. Labmedicine. 2007;38 [Google Scholar]
- Havill N.L., Havill H.L., Mangione E., Dumigan D.G., Boyce J.M. Cleanliness of portable medical equipment disinfected by nursing staff. Am J Infect Control. 2011;39:602–604. doi: 10.1016/j.ajic.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Havill N.L., Moore B.A., Boyce J.M. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light processes for room decontamination. Infect Control Hosp Epidemiol. 2012;33:507–512. doi: 10.1086/665326. [DOI] [PubMed] [Google Scholar]
- Hayden M.K., Bonten M.J., Blom D.W., Lyle E.A., Van De Vijver D.A., Weinstein R.A. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis. 2006;42:1552–1560. doi: 10.1086/503845. [DOI] [PubMed] [Google Scholar]
- Hayden M.K., Blom D.W., Lyle E.A., Moore C.G., Weinstein R.A. Risk of hand or glove contamination after contact with patients colonized with vancomycin-resistant enterococcus or the colonized patients’ environment. Infect Control Hosp Epidemiol. 2008;29:149–154. doi: 10.1086/524331. [DOI] [PubMed] [Google Scholar]
- Health and Safety Executive . EH40/2005 Workplace exposure limits. 2005. [Google Scholar]
- Holmdahl T., Lanbeck P., Wullt M., Walder M.H. A head-to-head comparison of hydrogen peroxide vapor and aerosol room decontamination systems. Infect Control Hosp Epidemiol. 2011;32:831–836. doi: 10.1086/661104. [DOI] [PubMed] [Google Scholar]
- Howe R.D., Matsuoka Y. Robotics for surgery. Annu Rev Biomed Eng. 1999;1:211–240. doi: 10.1146/annurev.bioeng.1.1.211. [DOI] [PubMed] [Google Scholar]
- Huang S.S., Datta R., Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166:1945–1951. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]
- Humphreys P.N. Testing standards for sporicides. J Hosp Infect. 2011;77:193–198. doi: 10.1016/j.jhin.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Jeanes A., Rao G., Osman M., Merrick P. Eradication of persistent environmental MRSA. J Hosp Infect. 2005;61:85–86. doi: 10.1016/j.jhin.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Johnston M.D., Lawson S., Otter J.A. Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. J Microbiol Methods. 2005;60:403–411. doi: 10.1016/j.mimet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Kaatz G.W., Gitlin S.D., Schaberg D.R., Wilson K.H., Kauffman C.A., Seo S.M., Fekety R. Acquisition of Clostridium difficile from the hospital environment. Am J Epidemiol. 1988;127:1289–1294. doi: 10.1093/oxfordjournals.aje.a114921. [DOI] [PubMed] [Google Scholar]
- Kac G., Podglajen I., Si-Mohamed A., Rodi A., Grataloup C., Meyer G. Evaluation of ultraviolet C for disinfection of endocavitary ultrasound transducers persistently contaminated despite probe covers. Infect Control Hosp Epidemiol. 2010;31:165–170. doi: 10.1086/649794. [DOI] [PubMed] [Google Scholar]
- Kahnert A., Seiler P., Stein M., Aze B., McDonnell G., Kaufmann S.H. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett Appl Microbiol. 2005;40:448–452. doi: 10.1111/j.1472-765X.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- Kaiser M., Elemendorf S., Kent D., Evans A., Harrington S.M., McKenna D. IDSA; 2011. Management of a multi-year MDR Acinetobacter baumannii outbreak in the ICU setting. Abstract 394. [Google Scholar]
- Koburger T., Below H., Dornquast T., Kramer A. Decontamination of room air and adjoining wall surfaces by nebulizing hydrogen peroxide. GMS Krankenhhyg Interdiszip. 2011;6:Doc09. doi: 10.3205/dgkh000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H.E., Borriello S.P. Quantitative study of antibiotic-induced susceptibility to Clostridium difficile enterocecitis in hamsters. Antimicrob Agents Chemother. 1990;34:1348–1353. doi: 10.1128/aac.34.7.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T.D., Clare S., Deakin L.J., Goulding D., Yen J.L., Raisen C., Brandt C., Lovell J., Cooke F., Clark T.G., Dougan G. Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl Environ Microbiol. 2010;76:6895–6900. doi: 10.1128/AEM.00718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Wang Y.C. Surface germicidal effects of ozone for microorganisms. AIHA J (Fairfax, Va) 2003;64:533–537. doi: 10.1202/559.1. [DOI] [PubMed] [Google Scholar]
- Low A. Regulation of sporicides under the European Biocidal Products Directive. J Hosp Infect. 2011;77:189–192. doi: 10.1016/j.jhin.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Mahamat A., Mackenzie F.M., Brooker K., Monnet D.L., Daures J.P., Gould I.M. Impact of infection control interventions and antibiotic use on hospital MRSA: a multivariate interrupted time-series analysis. Int J Antimicrob Agents. 2007;30:169–176. doi: 10.1016/j.ijantimicag.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Maki D.G., Alvarado C.J., Hassemer C.A., Zilz M.A. Relation of the inanimate hospital environment to endemic nosocomial infection. N Engl J Med. 1982;307:1562–1566. doi: 10.1056/NEJM198212163072507. [DOI] [PubMed] [Google Scholar]
- Manian F.A., Griesenauer S., Senkel D., Setzer J.M., Doll S.A., Perry A.M., Wiechens M. Isolation of Acinetobacterbaumanniicomplex and methicillin-resistant Staphylococcus aureus from hospital rooms following terminal cleaning and disinfection: can we do better? Infect Control Hosp Epidemiol. 2011;32:667–672. doi: 10.1086/660357. [DOI] [PubMed] [Google Scholar]
- Manian F.A., Griesnauer S., Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41:537–541. doi: 10.1016/j.ajic.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Mayfield J.L., Leet T., Miller J., Mundy L.M. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31:995–1000. doi: 10.1086/318149. [DOI] [PubMed] [Google Scholar]
- McDonnell G. Hydrogen peroxide fogging/fumigation. J Hosp Infect. 2006;62:385–386. doi: 10.1016/j.jhin.2005.09.006. [DOI] [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J.E., Jr. Environmental factors in nosocomial infection – a selective focus. Rev Infect Dis. 1981;3:760–769. doi: 10.1093/clinids/3.4.760. [DOI] [PubMed] [Google Scholar]
- McGowan M.J., Shimoda L.M., Woolsey G.D. Effects of sodium hypochlorite on denture base metals during immersion for short-term sterilization. J Prosthet Dent. 1988;60:212–218. doi: 10.1016/0022-3913(88)90318-6. [DOI] [PubMed] [Google Scholar]
- Memarzadeh F., Olmsted R.N., Bartley J.M. Applications of ultraviolet germicidal irradiation disinfection in healthcare facilities: effective adjunct, but not stand-alone technology. Am J Infect Cont. 2010;38:S13–S24. doi: 10.1016/j.ajic.2010.04.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Cookson B. Does microbial resistance or adaptation to biocides create a hazard in infection prevention and control? J Hosp Infect. 2010;76:200–205. doi: 10.1016/j.jhin.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Mirabelli M.C., Zock J.P., Plana E., Anto J.M., Benke G., Blanc P.D., Dahlman-Hoglund A., Jarvis D.L., Kromhout H., Lillienberg L., Norback D., Olivieri M., Radon K., Sunyer J., Toren K., Van Sprundel M., Villani S., Kogevinas M. Occupational risk factors for asthma among nurses and related healthcare professionals in an international study. Occup Environ Med. 2007;64:474–479. doi: 10.1136/oem.2006.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moat J., Cargill J., Shone J., Upton M. Application of a novel decontamination process using gaseous ozone. Can J Microbiol. 2009;55:928–933. doi: 10.1139/w09-046. [DOI] [PubMed] [Google Scholar]
- Morter S., Bennet G., Fish J., Richards J., Allen D.J., Nawaz S., Iturriza-Gomara M., Brolly S., Gray J. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J Hosp Infect. 2011;77:106–112. doi: 10.1016/j.jhin.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Mulvey D., Redding P., Robertson C., Woodall C., Kingsmore P., Bedwell D., Dancer S.J. Finding a benchmark for monitoring hospital cleanliness. J Hosp Infect. 2011;77:25–30. doi: 10.1016/j.jhin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Munster A.M., Ostrander W.E. Terminal disinfection of contaminated patient care areas: to fog or not to fog? Am Surg. 1974;40:713–715. [PubMed] [Google Scholar]
- Nerandzic M.M., Cadnum J.L., Pultz M.J., Donskey C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. doi: 10.1186/1471-2334-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir S., Blazejewski C., Lubret R., Wallet F., Courcol R., Durocher A. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the ICU. Clin Microbiol Infect. 2011;17:1201–1208. doi: 10.1111/j.1469-0691.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- Orlando P., Cristina M.L., Dallera M., Ottria G., Vitale A., Badolati G. Surface disinfection: evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J Prev Med Hyg. 2008;49:116–119. [PubMed] [Google Scholar]
- OSHA . Occupational safety and health guideline for hydrogen peroxide. 2005. [Google Scholar]
- Ostrander W.E., Griffith L.J. Evaluation of disinfectants for hospital housekeeping use. Appl Microbiol. 1964;12:460–463. doi: 10.1128/am.12.6.460-463.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., French G.L. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2009;47:205–207. doi: 10.1128/JCM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Yezli S. Cycle times for hydrogen peroxide vapour decontamination. Can J Microbiol. 2010;56:356–357. doi: 10.1139/w10-017. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S. A call for clarity when discussing hydrogen peroxide vapour and aerosol systems. J Hosp Infect. 2011;77:83–84. doi: 10.1016/j.jhin.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S. Are commercially available Geobacillus stearothermophilus biological indicators an appropriate standard for hydrogen peroxide vapour systems in hospitals? J Hosp Infect. 2012;80:272–273. doi: 10.1016/j.jhin.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Otter J.A., French G.L., Adams N.M., Watling D., Parks M.J. Hydrogen peroxide vapour decontamination in an overcrowded tertiary care referral centre: some practical answers. J Hosp Infect. 2006;62:384–385. doi: 10.1016/j.jhin.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Cummins M., Ahmad F., Van Tonder C., Drabu Y.J. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J Hosp Infect. 2007;67:182–188. doi: 10.1016/j.jhin.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Davies B., Klein J., Watts T.L., Kearns A.M., French G.L. 2008. Identification and control of an outbreak of gentamicin-resistant, methicillin-susceptible Staphylococcus aureus on a neonatal unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Havill N.L., Adams N.M., Cooper T., Tauman A., Boyce J.M. Environmental sampling for Clostridium difficile: swabs or sponges? Am J Infect Control. 2009;37:517–518. doi: 10.1016/j.ajic.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Puchowicz M., Ryan D., Salkeld J.A., Cooper T.A., Havill N.L., Tuozzo K., Boyce J.M. Feasibility of routinely using hydrogen peroxide vapor to decontaminate rooms in a busy United States hospital. Infect Control Hosp Epidemiol. 2009;30:574–577. doi: 10.1086/597544. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Barnicoat M., Down J., Smyth D., Yezli S., Jeanes A. Hydrogen peroxide vapour decontamination of a critical care unit room used to treat a patient with Lassa fever. J Hosp Infect. 2010;75:335–337. doi: 10.1016/j.jhin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Havill N.L., Boyce J.M. Hydrogen peroxide vapor is not the same as aerosolized hydrogen peroxide. Infect Control Hosp Epidemiol. 2010;31:1201–1202. doi: 10.1086/657076. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S., Schouten M.A., Van Zanten A.R., Houmes-Zielman G., Nohlmans-Paulssen M.K. Hydrogen peroxide vapor decontamination of an intensive care unit to remove environmental reservoirs of multidrug-resistant gram-negative rods during an outbreak. Am J Infect Control. 2010;38:754–756. doi: 10.1016/j.ajic.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S., French G.L. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S., French G.L. Impact of the suspending medium on susceptibility of meticillin-resistant Staphylococcus aureus to hydrogen peroxide vapour decontamination. 2012;82:213–215. doi: 10.1016/j.jhin.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Otter J.A., Yezli S., Perl T.M., Barbut F., French G.L. The role for ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83:1–13. doi: 10.1016/j.jhin.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Passaretti C.L., Otter J.A., Reich N.G., Myers J., Shepard J., Ross T., Carroll K.C., Lipsett P., Perl T.M. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis. 2013;56:27–35. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- Peleg A.Y., Hooper D.C. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perencevich E.N., Stone P.W., Wright S.B., Carmeli Y., Fisman D.N., Cosgrove S.E. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133. doi: 10.1086/521852. [DOI] [PubMed] [Google Scholar]
- Piskin N., Celebi G., Kulah C., Mengeloglu Z., Yumusak M. Activity of a dry mist-generated hydrogen peroxide disinfection system against methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii. Am J Infect Control. 2011;39:757–762. doi: 10.1016/j.ajic.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Po J.L., Carling P.C. The need for additional investigation of room decontamination processes. Infect Control Hosp Epidemiol. 2010;31:776–777. doi: 10.1086/653819. [DOI] [PubMed] [Google Scholar]
- Pottage T., Richardson C., Parks S., Walker J.T., Bennett A.M. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Infect. 2010;74:55–61. doi: 10.1016/j.jhin.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Pottage T., Macken S., Walker J.T., Bennett A.M. Meticillin-resistant Staphylococcus aureus is more resistant to vaporized hydrogen peroxide than commercial Geobacillus stearothermophilus biological indicators. J Hosp Infect. 2012;80:41–45. doi: 10.1016/j.jhin.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Pyrek K. Infection Control Today Special Report. 2011. Area decontamination technology: vaporized hydrogen peroxide and germicidal UV light. [Google Scholar]
- Ray A., Perez F., Beltramini A.M., Jakubowycz M., Dimick P., Jacobs M.R., Roman K., Bonomo R.A., Salata R.A. Use of vaporized hydrogen peroxide decontamination during an outbreak of multidrug-resistant Acinetobacter baumannii infection at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2010;31:1236–1241. doi: 10.1086/657139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeda N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M.M. The disinfection of sick-rooms and their contents. Am J Nursing. 1901;1:568–573. [Google Scholar]
- Roberts C.G. Hydrogen peroxide vapor and aerosol room decontamination systems. Infect Control Hosp Epidemiol. 2012;33:312. doi: 10.1086/664043. [DOI] [PubMed] [Google Scholar]
- Rutala W.A. Disinfection and sterilization: from benchtop to bedside. Elaine L. Larson Lectureship. 2012. (APIC 2012). [Google Scholar]
- Rutala W.A., Weber D.J. Are room decontamination units needed to prevent transmission of environmental pathogens? Infect Control Hosp Epidemiol. 2011;32:743–747. doi: 10.1086/661226. [DOI] [PubMed] [Google Scholar]
- Rutala W.A., Weber D.J., HICPAC Guideline for disinfection and sterilization in healthcare facilities. 2008. http://www.cdc.gov/hicpac/pdf/guidelinesDisinfection_Nov_2008.pdf
- Rutala W.A., Gergen M.F., Weber D.J. Room decontamination with UV radiation. Infect Control Hosp Epidemiol. 2010;31:1025–1029. doi: 10.1086/656244. [DOI] [PubMed] [Google Scholar]
- Sexton T., Clarke P., O’Neill E., Dillane T., Humphreys H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect. 2006;62:187–194. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Shapey S., Machin K., Levi K., Boswell T.C. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J Hosp Infect. 2008;70:136–141. doi: 10.1016/j.jhin.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Sharma M., Hudson J.B. Ozone gas is an effective and practical antibacterial agent. Am J Infect Control. 2008;36:559–563. doi: 10.1016/j.ajic.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Shaughnessy M.K., Micielli R.L., Depestel D.D., Arndt J., Strachan C.L., Welch K.B., Chenoweth C.E. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–206. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- SHEA/APIC/AHE SHEA, APIC and AHE respond to EPA regarding fogging applications. 2011. http://www.she-online.org/View/Articled/Sq/SHEA-APIC-and-AHE_Respond-to-EPA-Regarding-Fogging-Applications.aspx
- Smith K., Hunter I.S. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966–973. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- Stibich M., Stachowiak J., Tanner B., Berkheiser M., Moore L., Raad I., Chemaly R.F. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on hospital operations and microbial reduction. Infect Control Hosp Epidemiol. 2011;32:286–288. doi: 10.1086/658329. [DOI] [PubMed] [Google Scholar]
- Stiefel U., Cadnum J.L., Eckstein B.C., Guerrero D.M., Tima M.A., Donskey C.J. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol. 2011;32:185–187. doi: 10.1086/657944. [DOI] [PubMed] [Google Scholar]
- Sweeney C.P., Dancer S.J. Can hospital computers be disinfected using a hand-held UV light source? J Hosp Infect. 2009;72:92–94. doi: 10.1016/j.jhin.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Taneja N., Biswal M., Emmanuel R., Singh M., Sharma M. Hydrogen peroxide fogging in an overcrowded tertiary care referral centre: some practical queries. J Hosp. Infect. 2005;60:85. doi: 10.1016/j.jhin.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Taneja N., Biswal M., Kumar A., Edwin A., Sunita T., Emmanuel R., Gupta A.K., Sharma M. Hydrogen peroxide vapour for decontaminating air-conditioning ducts and rooms of an emergency complex in northern India: time to move on. J Hosp Infect. 2011;78:200–203. doi: 10.1016/j.jhin.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Tuladhar E., Terpstra P., Koopmans M., Duizer E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J Hosp Infect. 2012;80:110–115. doi: 10.1016/j.jhin.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Tyan Y.C., Liao J.D., Klauser R., Wu I., Weng C.C. Assessment and characterization of degradation effect for the varied degrees of ultra-violet radiation onto the collagen-bonded polypropylene non-woven fabric surfaces. Biomaterials. 2002;23:65–76. doi: 10.1016/s0142-9612(01)00080-1. [DOI] [PubMed] [Google Scholar]
- van’t Veen A., Van Der Zee A., Nelson J., Speelberg B., Kluytmans J.A., Buiting A.G. Outbreak of infection with a multiresistant Klebsiella pneumoniae strain associated with contaminated roll boards in operating rooms. J Clin Microbiol. 2005;43:4961–4967. doi: 10.1128/JCM.43.10.4961-4967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity P., Wilcox M.H., Fawley W., Parnell P. Prospective evaluation of environmental contamination by Clostridium difficile in isolation side rooms. J Hosp Infect. 2001;49:204–209. doi: 10.1053/jhin.2001.1078. [DOI] [PubMed] [Google Scholar]
- Vickery K., Deva A., Jacombs A., Allan J., Valente P., Gosbell I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J Hosp Infect. 2012;80:52–55. doi: 10.1016/j.jhin.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Vonberg R.P., Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect. 2006;63:246–254. doi: 10.1016/j.jhin.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Walder M., Holmdahl T. Reply to Roberts. Infect Control Hosp Epidemiol. 2012;33:312–313. [Google Scholar]
- Weber D.J., Rutala W.A. Self-disinfecting surfaces. Infect Control Hosp Epidemiol. 2012;33:10–13. doi: 10.1086/663648. [DOI] [PubMed] [Google Scholar]
- Weber D.J., Rutala W.A., Sickbert-Bennett E.E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51:4217–4224. doi: 10.1128/AAC.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- Weinstein R.A. Nosocomial infection update. Emerg Infect Dis. 1998;4:416–420. doi: 10.3201/eid0403.980320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry C., Lawrence J.M., Sanderson P.J. Contamination of detergent cleaning solutions during hospital cleaning. J Hosp Infect. 1988;11:44–49. doi: 10.1016/0195-6701(88)90038-2. [DOI] [PubMed] [Google Scholar]
- Wilcox M.H., Fawley W.N., Wigglesworth N., Parnell P., Verity P., Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect. 2003;54:109–114. doi: 10.1016/s0195-6701(02)00400-0. [DOI] [PubMed] [Google Scholar]
- Yezli S., Otter J.A. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Microbiol. 2011;3:1–30. doi: 10.1007/s12560-011-9056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoutman D., Shannon M., Mandel A. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am J Infect Cont. 2011;39:873–879. doi: 10.1016/j.ajic.2011.01.012. [DOI] [PubMed] [Google Scholar]


