INTRODUCTION
Bioterrorism can be broadly defined as the deliberate use of microbial agents or their toxins as weapons against noncombatants outside the setting of armed conflict. The concept is analogous to biologic warfare in a combat theater. The broad scope and mounting boldness of worldwide terrorism was impressively demonstrated by the massive attacks on New York City and Washington DC on September 11, 2001; the multifocal anthrax attacks that followed shortly thereafter, while not known to be directly related to 9/11, awakened civilized society to the threats posed by these ‘weapons of mass terror’. This realization, in concert with recent revelations regarding the apparent willingness of terrorist organizations to acquire and deploy biologic weapons, constitutes ample evidence that the specter of bioterrorism poses a persistent global threat.
Biologic weapons have been used against both military and civilian targets throughout history. It has been variously speculated that at least some of the plagues visited upon ancient Egypt, as documented in the biblical book of Exodus, represented natural outbreaks of endemic infectious diseases that were recast as supreme forms of bioterrorism. In the 14th century Tatars attempted to use epidemic disease against the defenders of Kaffa by catapulting plague-infected corpses into the city.1 British forces gave Native American tribespeople blankets from a smallpox hospital in an attempt to affect the balance of power in the 18th century Ohio River Valley.1 In addition to their well-described use of chemical weapons, Axis forces purportedly infected livestock with anthrax and glanders to weaken Allied initiatives during the First World War. Perhaps the most egregious period in the history of biologic weaponry involved the Japanese program in Manchuria from 1932 to 1945. Based on survivor accounts and confessions of Japanese participants, thousands died as a result of experimental infection with a multitude of virulent pathogens at Unit 731, the code name for the biologic weapons facility there.2
The USA maintained an offensive biologic weapons program from 1942 until 1969, when the program was terminated by President Nixon. The Convention on the Prohibition of the Development, Production, and Stockpiling of Biological and Toxin Weapons and on Their Destruction (BWC) was ratified in 1972 and formally banned the development or use of biologic weapons, with enforcement the responsibility of the United Nations.1 Unfortunately, the BWC has not been effective in its stated goals; multiple signatories, including the former Soviet Union and Iraq, have violated the terms and spirit of the agreement. The accidental release of aerosolized anthrax spores from a military plant in Sverdlovsk in 1979, resulting in at least 68 human deaths from inhalational anthrax, verifies the existence of an active Soviet offensive biologic weapons program.
THREAT ASSESSMENT
Biologic agents are considered weapons of mass destruction (WMD) because, as with certain conventional, chemical and nuclear weapons, their use may result in large-scale morbidity and mortality. In a World Health Organization (WHO) assessment model of the hypothetical casualty estimates from the intentional release of 50 g of aerosolized anthrax spores upwind from a population center of 500 000 (analogous to Providence, Rhode Island, USA), nearly 200 000 people might be killed or incapacitated by the event.3 Biologic weapons possess unique properties among all WMD. Unlike other forms, biologic agents are associated with a clinical latency period of days to weeks in most cases, during which time exposed individuals are asymptomatic and early detection is quite difficult with currently available technology. Additionally, specific antimicrobial therapy and, in select circumstances, vaccines are available for the treatment and prevention of illness caused by biologic weapons; casualties from other forms of WMD can generally only be treated by decontamination, trauma mitigation and supportive care.
Nations adhering to democratic principles are vulnerable to bioterrorism because of the inherent freedoms that their citizens and visitors enjoy. This freedom of movement and access to public and private institutions can be exploited by rogue nations, terrorist organizations or malicious individuals intent on untoward acts. When coupled with worldwide cultural tensions, geopolitical conflicts and economic instability, open societies provide fertile ground for terrorism.
Recent events have established bioterrorism as a credible and ubiquitous threat and, in some quarters, as a potential tool for political coercion. The intentional contamination of restaurant salad bars with Salmonella by a religious cult trying to influence a local election in The Dalles, Oregon, in 1984;4 the revelations that Aum Shinrikyo, the Japanese cult responsible for the sarin gas attack in the Tokyo subway system in 1995, experimented on multiple occasions with spraying anthrax from downtown Tokyo rooftops; and the findings of the United Nations weapons inspectors of massive quantities of weaponized biologic weapons in Iraq during the first Gulf War and its aftermath5 served as sentinel warnings of a shift in terrorism trends. The anthrax attacks in the USA in October and November 2001, following the catastrophic events of September 11th, elevated bioterrorism to the fore of the international dialogue.
The aims of bioterrorism are those of terrorism in general: morbidity and mortality among civilian populations, disruption of societal fabric, and exhaustion or diversion of resources.6 A ‘successful’ outcome, from a terrorist standpoint, may be achieved without furthering all of these aims and, in fact, may be accomplished simply by the credible threat of action or by a small-scale agent deployment. The anthrax attacks in 2001 evoked fear and anxiety and diverted public health and health-care resources away from other critical activities despite the limited number of casualties associated with the event.
Biologic weapons offer other, significant advantages to terrorists:
-
•
they are relatively inexpensive to acquire as compared with conventional or nuclear weaponry;
-
•
they can be deployed in a stealth fashion due to a variable clinical latency period, thus allowing the perpetrator opportunity to escape if desired; and
-
•
they clearly evoke anxiety and panic in a population that is, in some instances, out of proportion to their physical effects.
From a rogue government's standpoint, the technology for bioterrorism is ‘dual use’, i.e. it can serve legitimate functions such as vaccine or pharmaceutical production as readily as biologic weapons production, thus making detection by inspectors all the more difficult.
To be employed in large-scale bioterrorism, biologic agents must undergo complex processes of production, cultivation, chemical modification and weaponization. For these reasons state sponsorship or direct support from governments or organizations with significant resources, contacts and infrastructure would predictably be required in large-scale events.6 However, some agents may be acquired by terrorist groups on the black market and in other illicit settings.7 Although an efficient mode of delivery has traditionally been felt to be necessary, the anthrax attacks in the USA in late 2001 illustrated the devastating results that can be achieved with relatively primitive delivery methods, e.g. high-speed mail sorting equipment and mailed letters.
Numerous attributes contribute to the effectiveness of a biologic weapon:
-
•
availability or ease of large-scale production;
-
•
ease of dissemination, especially by the aerosol route;
-
•
stability in storage and delivery;
-
•
cost; and
-
•
clinical virulence.
The last refers to the reliability with which the pathogen causes high mortality, morbidity or social disruption. The Centers for Disease Control and Prevention (CDC) have prioritized biologic agent threats based upon the aforementioned characteristics,8, 9 and this has influenced current preparedness strategies (Table 71.1 ). Category A agents, considered the highest priority, are associated with high mortality and the greatest potential for major impact on public health. Category B agents are considered ‘incapacitating’ because of their potential for moderate morbidity but relatively low mortality. Most of the category A and B agents have been experimentally weaponized in the past. Category C agents include emerging threats and pathogens that may be available for development in the future.
Table 71.1.
Agents of concern for use in bioterrorism
| Highest priority: category A (based upon potential mortality, morbidity, virulence, transmissibility, aerosol feasibility and psychosocial implications of an attack) | |
|---|---|
| Microbe/toxin | Disease |
| Bacillus anthracis | Anthrax: inhalational, cutaneous |
| Variola virus | Smallpox and its variants |
| Yersinia pestis | Plague: pneumonic, bubonic, septicemic |
| Clostridium botulinum toxin | Botulism |
| Francisella tularensis | Tularemia: pneumonic, typhoidal |
| Viral hemorrhagic fevers | |
| Filoviruses | Ebola, Marburg |
| Arenaviruses | Lassa fever, South American hemorrhagic fevers |
| Bunyaviruses | Rift Valley fever, Congo–Crimeanhemorrhagic fever |
| Flaviviruses | Dengue |
| Moderately high priority: category B (based upon potential morbidity, aerosol feasibility, dissemination characteristics, and diagnostic difficulty) | |
|---|---|
| Microbe/toxin | Disease |
| Coxiella burnetti | Q fever |
| Brucella spp. | Brucellosis |
| Burkholderia mallei | Glanders |
| Burkholderia pseudomallei | Melioidosis |
| Alphaviruses (e.g. EEE, VEE) | Viral encephalitides |
| Ricinus communis toxin | Ricin intoxication |
| Staphylococcal enterotoxin B | Staphylococcal toxin illness |
| Salmonella spp., Shigella dysenteriae, Escherichia coli O157:H7, Vibrio cholerae,Cryptosporidiumparvum, Listeria monocytogenes, Campylobacter, jejuni, Yersinia enterocolitica | Food- and waterborne gastroenteritis |
| Rickettsia prowazekii | Epidemic typhus |
| Chlamydiapsittaci | Psittacosis |
| Epsilon toxin of Clostridium perfringens | C. perfringens intoxication |
| Emerging threat agents: category C (based upon potential for production and dissemination, availability, morbidity/mortality) | |
|---|---|
| Microbe/toxin | Disease |
| Hantaviruses | Viral hemorrhagic fevers |
| Flaviviruses | Yellow fever, West Nile virus |
| Mycobacterium tuberculosis | Multidrug-resistant tuberculosis |
| Nipah virus | Systemic flu-like illness |
| Miscellaneous (other examples of candidate threat agents that possess some elements of bioterrorism concern) | |
|---|---|
| Genetically engineered vaccine- and/or antimicrobial-resistant category A or B agents | |
| HIV-1 | |
| Adenoviruses | |
| Influenza | |
| Rotaviruses | |
| Molecular hybrid pathogens (e.g. smallpox–plague, smallpox–ebola) | |
| Severe acute respiratory syndrome coronavirus |
EEE, eastern equine encephalomyelitis; VEE, Venezuelan equine encephalomyelitis.
Adapted from Patrozou & Artenstein.26
Another factor that must be addressed in assessing future bioterrorism risk and predicted agents is the historical track record of experimentation with specific pathogens, an area that has been informed from the corroborated claims of various high-level Soviet defectors and data released from the former offensive weapons programs of the USA and the UK.1, 7, 10 It is apparent from these sources, combined with the burgeoning fields of molecular biology and genomics, that future risk scenarios may have to contend with genetically altered and ‘designer’ pathogens that may be equipped with enhancements in virulence, such as antimicrobial resistance or augmented toxin production, or modifications that enhance dissemination, such as prolonged aerosol stability. To this end the author has added a miscellaneous grouping of potential threat agents to the extant CDC categories (see Table 71.1). The most cautious approach to assessing risk may be to remain open to additional, novel possibilities.
BIOTERRORISM RECOGNITION
By definition bioterrorism is insidious; absent advance warning or specific intelligence information, clinical illness will be manifest before the circumstances of a release event are known. For this reason health-care providers are likely to be the first responders to this form of terrorism. This is in contrast to the more familiar scenarios in which police, firefighters, paramedics and other emergency services personnel are deployed to the scene of an attack with conventional weaponry or a natural disaster. Physicians and other health-care workers must therefore maintain a high index of suspicion of bioterrorism and recognize suggestive epidemiologic clues and clinical features to enhance early recognition, disseminate information rapidly and guide initial management of casualties. This remains the most effective way to minimize the deleterious effects of bioterrorism on individual patients and on the public health.
Early recognition is hampered for multiple reasons. Terrorists have an unlimited number of targets in most open, democratic societies; it is unrealistic to expect that without detailed intelligence data, all of these can be secured at all times. Certain sites, such as government institutions, historic landmarks or large events may be predictable targets, but there are other, less predictable possibilities. Metropolitan areas are considered vulnerable, but owing to the expansion of suburbs, commuters, and the clinical latency period between exposure and symptoms inherent with biologic agents, casualties of bioterrorism are likely to present for medical attention in diverse locations and at varying times following a common exposure. An event in New York City on a Wednesday morning may result in clinically ill individuals presenting over the ensuing weekend to a variety of emergency rooms within a 60-mile radius. Additionally, our mobile society ensures that affected individuals will likely present for medical care thousands of miles away from the original release point of the bioterrorist's weapon. This adds layers of complexity to managing a bioterrorism event and illustrates the critical importance of surveillance, cooperation and real-time communication in this setting.
Further hindering the early recognition of bioterrorism is that initial symptoms may be nondiagnostic. In the absence of a known exposure, many symptomatic individuals may not seek medical attention early on or may be misdiagnosed with a flu-like illness if they do. Once beyond the early stages many of these illnesses progress quite rapidly and treatment may be less successful. Most of the diseases caused by agents of bioterrorism are rarely, if ever, seen in clinical practice; physicians are, therefore, likely to be inexperienced with their clinical presentation. Additionally, these agents, by definition, will have been laboratory-manipulated and may not present with the classic clinical features of naturally occurring infection. This was dramatically illustrated by differences in the clinical presentations of some of the inhalational anthrax cases in the USA in October 2001 as compared with those described in earlier outbreaks.11
Early identification of bioterrorism is facilitated by the recognition of epidemiologic and clinical clues. Clustering of patients with common symptoms and signs, especially if these are unusual or known to be associated with bioterrorism agents, should prompt expeditious notification of local public health authorities. This approach may not only detect malicious events but will also lead to the recognition of outbreaks of naturally occurring disease or novel, emerging pathogens. The recognition of a single case of a rare or nonendemic infection, in the absence of a travel history or other potential natural exposure, should raise the specter of bioterrorism and should prompt notification of public health authorities. Finally, unusual patterns of disease, such as unusual age distributions, more severe clinical forms of infection or concurrent illness in human and animal populations, should raise suspicions for bioterrorism or another form of emerging infection. In fact for some category A, B or C agents of bioterrorism, available evidence supports the potential role of animals as early warning sentinels of an attack or as markers of persistent exposure risks to humans.12
Infectious diseases specialists are uniquely suited to play pivotal roles in the recognition, investigation and mitigation of bioterrorism, based on:
-
•
an understanding of epidemiologic principles and risk assessment;
-
•
expertise in specific threat agents, their clinical presentations and diagnostic approaches;
-
•
knowledge of communicability and infection control principles; and
-
•
an understanding of the tenets of treatment and prophylaxis of infectious diseases.
Nonetheless, an effective response to bioterrorism requires coordination of the medical system at all levels, from the community physician to the tertiary care center, with active engagement of public health, emergency management and law enforcement infrastructures.
THREAT AGENTS
This section will cover the biologic threat agents felt to be of major current concern, largely the CDC category A agents. Extensive coverage of specific pathogens can be found in related chapters in this text (cross-referenced in Table 71.2 ) and in other sources.13, 14 Data concerning clinical incubation periods, transmission characteristics and infection control procedures for agents of bioterrorism are provided in Table 71.2. Syndromic differential diagnoses for select clinical presentations are detailed in Table 71.3 .
Table 71.2.
Epidemiologic characteristics for selected category A and B bioterrorism-associated diseases
| Disease | Incubation period range (days) | Person-to-person transmission | Infection control precautions for patients | Case fatality rate |
|---|---|---|---|---|
| Inhalational anthrax (see Chapter 128) | 2–43* | No | Standard |
|
| Cutaneous anthrax (see Chapter 128) | 1–12 | No | Standard |
|
| Botulism (see Chapter 21) | 12–72 hours | No | Standard | 6% |
| Primary pneumonic plague (see Chapter 120) | 1–6 | Yes | Droplet |
|
| Bubonic plague (see Chapter 120) | 2–8 | No | Standard |
|
| Smallpox | 7–19 | Yes | Contact and airborne |
|
| Tularemia pneumonia (see Chapter 121) | 1–21 | No | Standard |
|
| Viral hemorrhagic fevers (see Chapter 126) | 2–21 | Yes | Contact and airborne |
|
| Viral encephalitides (see Chapter 19) | 1–14 | No | Standard | 10–35% |
| Q fever (see Chapter 176) | 2–41 | No | Standard | 3% |
| Brucellosis (see Chapter 123) | 5–60 | No | Standard | Untreated 5% |
| Glanders | 1–21 | Yes | Contact and droplet |
|
Based on limited data from human outbreaks; experimental animal data support clinical latency periods of up to 100 days.
Adapted from Patrozou & Artenstein.26
Table 71.3.
Syndromic differential diagnoses and clinical clues for category A agents of bioterrorism
| Syndrome | Clinical presentation | Differential diagnosis | Bioterrorism-associated disease | Disease-specific clues |
|---|---|---|---|---|
| Influenza-like illness | Nonspecific constitutional and upper respiratory symptoms: malaise, myalgias, nausea, emesis, dyspnea, cough with or without chest discomfort, without coryza or rhinorrhea, leading to abrupt onset of respiratory distress, with or without shock, mental status changes, with chest radiograph abnormalities (wide mediastinum or infiltrates or pleural effusions) | Influenza, community-acquired bacterial pneumonia, viral pneumonia, Legionella, Q fever, psittacosis, Mycoplasma, Pneumocystis pneumonia, tularemia, dissecting aortic aneurysm, bacterial mediastinitis, SVC syndrome, histoplasmosis, coccidioidomycosis, sarcoidosis, ricin and staphylococcal enterotoxin B (pulmonary edema/ARDS), Nipah virus | Inhalational anthrax |
|
| Skin lesion(s) | Pruritic, painless papule on exposed areas leading to vesicle(s), ulcer, then edematous black eschar, with or without massive local edema and regional adenopathy and fever, evolving over 3–7 days | Recluse spider bite, staphylococcal lesion, atypical Lyme disease, orf, glanders, tularemia, plague, rat-bite fever, ecthyma gangrenosum, rickettsialpox, atypical Mycobacteria, cutaneous diphtheria, cutaneous leishmaniasis | Cutaneous anthrax |
|
| Fulminant pneumonia | Abrupt onset of constitutional symptoms and rapidly progressive respiratory illness with cough, fever, rigors, headache, sore throat, myalgias, dyspnea, pleuritic chest pain, GI symptoms, lung consolidation, with or without hemoptysis, shock; variable progression to respiratory failure | Severe community-acquired bacterial anthrax, pulmonary infarct, pulmonary hemorrhage, influenza, Mycoplasma pneumonia, Legionella, Q fever, bacterial pneumonia, SARS, tuberculosis, melioidosis |
|
|
| Sepsis with bleeding diathesis and capillary leak | Sepsis syndrome, GI symptoms, mucosal hemorrhage, altered vascular permeability, DIC, purpura, acral gangrene, hepatitis, hypotension, with or without CNS findings, multiorgan system failure | Meningococcemia; Gram-negative sepsis; streptococcal, pneumococcal or staphylococcal bacteremia with shock; malaria; leptospirosis; typhoid fever; borreliosis; typhoidal tularemia; overwhelming postsplenectomy sepsis; acute leukemia; Rocky Mountain spotted fever; fulminant hepatitis; TTP; hemolytic uremic syndrome; SLE; hemorrhagic smallpox; hemorrhagic varicella (in immunocompromised); dengue. |
|
|
| Febrile prodrome with generalized exanthem | Fever, malaise, prostration, headache, myalgias and enanthema followed by development of synchronous, progressive, centrifugal papular, leading to vesicular/pustular rash on face, mucous membranes, extremities more than trunk, leading to generalization with or without hemorrhagic component, with systemic toxicity | Varicella, drug eruption, Stevens–Johnson syndrome, measles, secondary syphilis, erythema multiforme, severe acne, disseminated herpes zoster or simplex, meningococcemia, monkeypox, generalized vaccinia related to smallpox vaccination, insect bites, coxsackievirus, vaccine reaction | Smallpox |
|
| Progressive weakness | Acute onset of afebrile, symmetric, descending flaccid paralysis that begins in bulbar muscles, dilated pupils, diplopia or blurred vision, dysphagia, dysarthria, ptosis, dry mucous membranes leading to airway obstruction and respiratory muscle paralysis. Clear sensorium and absence of sensory changes | Myasthenia gravis, brain stem CVA, polio, Guillain-Barré syndrome variant, tick paralysis, chemical intoxication | Botulism |
|
ARDS, acute respiratory distress syndrome; CVA, cerebrovascular accident; DIC, disseminated intravascular coagulation; GI, gastrointestinal; SLE, systemic lupus erythematosus; SVC syndrome, superior vena cava syndrome; TTP, thrombotic thrombocytopenic purpura; VHF, viral hemorrhagic fever.
Anthrax
(See healthmap.org for outbreaks of Anthrax)
Anthrax results from infection with Bacillus anthracis, a Gram-positive, spore-forming, rod-shaped organism that exists in its host as a vegetative bacillus and in the environment as a spore. Details of the microbiology and pathogenesis of anthrax are found in Chapter 128 of this text. In nature anthrax is a zoonotic disease of herbivores that is prevalent in many geographic regions; sporadic human disease results from environmental or occupational contact with endospore-contaminated animal products.15 Anthrax is uncommon in developed countries. In developing areas the cutaneous form of anthrax is the most common presentation; gastrointestinal and inhalational forms are exceedingly rare in naturally acquired disease. Cutaneous anthrax is rarely seen in current-day industrialized countries due to importation restrictions. The last known case of naturally occurring inhalational anthrax in the USA occurred in 1976.16
The recent attacks in the USA were on a relatively small scale, and nearly 40% of the confirmed cases were of the cutaneous variety.17 The serious morbidity and mortality, however, were related to inhalational disease, as was noted in the Sverdlovsk outbreak in 1979. Therefore, planning for larger-scale events with aerosolized agent continues to be warranted.
The clinical presentations and differential diagnoses of cutaneous and inhalational anthrax are described in Table 71.3. The lesion of cutaneous anthrax may be similar in appearance to other lesions, including cutaneous forms of other agents of bioterrorism; however, it may be distinguished by epidemiologic as well as certain clinical features. Anthrax is traditionally a painless lesion, unless secondarily infected, and associated with significant local edema (Fig. 71.1 ). The bite of Loxosceles reclusa, the brown recluse spider, shares many of the local and systemic features of anthrax but is typically painful from the outset and lacks significant edema.18 Cutaneous anthrax is associated with systemic disease and its attendant mortality in up to 20% of untreated cases, although with appropriate antimicrobial therapy, mortality is less than 1%.16
Fig. 71.1.

Lesion of cutaneous anthrax. © Diepgen TL, Yihune G, et al. Dermatology Online Atlas (http://www.dermis.net).
Rights were not granted to include this figure in electronic media. Please refer to the printed book.
Reprinted with permission.
© 2010 Diepgen TL, Yihune G
Once the inhaled endospores reach the terminal alveoli of the lungs, generally requiring particle sizes of 1–5 µm, they are phagocytosed by macrophages and transported to regional lymph nodes, where they germinate into vegetative bacteria and, subsequently, disseminate hematogenously.15 The bacteria generate potent exotoxins, lethal toxin and edema toxin, which lead to hemorrhagic mediastinitis, systemic illness and death. Spores may remain latent for extended periods of time in the host, up to 100 days in some experimental animal exposures17 This has translated into prolonged clinical incubation periods following exposure to endospores; cases of inhalational anthrax occurred up to 43 days after exposure in the Sverdlovsk experience, although the average incubation period is 2–10 days, perhaps influenced by exposure inoculum.15, 17
Prior to the anthrax attacks in the USA in October 2001, most of the clinical data concerning inhalational anthrax derived from Sverdlovsk, the largest outbreak recorded in humans. While the clinical experience derived from the US anthrax attacks in 2001 had much in common with the clinical manifestations of inhalational anthrax noted in the Sverdlovsk cases, more detailed data are available from the recent US experience and some novel findings were noted. There were 11 confirmed cases of inhalational anthrax, 5 (45%) of whom died. Although this contrasts with a case fatality rate of greater than 85% reported from Sverdlovsk, the reliability of reported data from the latter outbreak is questionable17 and, perhaps more importantly, patients in the 2001 outbreak were more likely to receive appropriate treatment at an earlier stage. Patients with inhalational anthrax almost uniformly present for medical attention an average of 3.3 days after symptom onset with fevers, chills, malaise, myalgias, nonproductive cough, chest discomfort, dyspnea, nausea or vomiting, tachycardia, peripheral neutrophilia and liver enzyme elevations.11, 19
Many of these findings are nondiagnostic and overlap considerably with those of influenza or other common viral respiratory tract infections. Data suggest that discrimination between inhalational anthrax and benign, influenza-like illnesses may be possible on the basis of presenting symptoms; shortness of breath, nausea, and vomiting are significantly more common in anthrax while rhinorrhea is uncommonly seen in anthrax but noted in the majority of community-acquired viral respiratory infections.20
Other common clinical manifestations of inhalational anthrax as informed by the recent attacks include abdominal pain, headache, mental status abnormalities and hypoxemia. Abnormalities on chest radiography appear to be universally present, although these may only be identified retrospectively in some cases. Pleural effusions are the most common abnormality; infiltrates, consolidation and/or mediastinal adenopathy/widening are noted in the majority (Fig. 71.2a ). The latter is felt to be an early indicator of disease, but CT scan appears to provide greater sensitivity than chest radiographs for this finding (Fig. 71.2b). In the recent outbreak of inhalational anthrax, more than 80% of cases were noted to have mediastinal widening with or without pleural effusions or infiltrates.
Fig. 71.2.


(a) Chest X-ray, inhalational anthrax, United States, 2001 demonstrating mediastinal widening (arrows). (b) Chest CT scan demonstrating mediastinal widening (arrows) and bilateral pleural effusions.
From Jernigan et al.11
The clinical manifestations of inhalational anthrax generally evolve to a fulminant septic picture with progressive respiratory failure. B. anthracis is routinely isolated in blood cultures if obtained prior to the initiation of antimicrobials (Fig. 71.3 ). Pleural fluid is typically hemorrhagic; the bacteria can either be isolated in culture or documented by antigen-specific immunohistochemical stains of this material (Fig. 71.4 ) in the majority of patients.11 The average time from hospitalization until death was 3 days (range 1–5 days) in the five recent US fatalities, consistent with other reports related to the clinical virulence of this infection. Autopsy data typically reveal hemorrhagic mediastinal lymphadenitis and disseminated metastatic infection. Pathology data from the Sverdlovsk outbreak confirm meningeal involvement, typically hemorrhagic meningitis, in 50%21 and, in fact, meningoencephalitis was the presenting diagnosis (Fig. 71.5 ) in the index case of the 2001 attacks.22
Fig. 71.3.

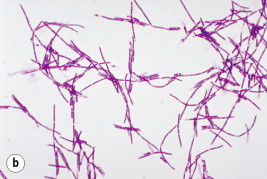
Bacillus anthracis. (a) Bacillus anthracis appearing as Gram-positive bacilli. (b) The typical ‘jointed bamboo-rod’ appearance of the organism from blood cultures.
Courtesy of CDC and Dr William A Clark.
Fig. 71.4.

Pleural fluid cell block immunohistochemical stain demonstrating Bacillus anthracis antigen (red) within a mononuclear inflammatory cell infiltrate.
From Jernigan et al.11
Fig. 71.5.

Cerebrospinal fluid Gram stain from anthrax index case, United States, 2001, demonstrating numerous Gram-positive rods and neutrophils.
From Jernigan et al.11
The diagnosis of inhalational anthrax should be entertained in the setting of a consistent clinical presentation in the context of a known exposure, a possible exposure or epidemiologic factors suggesting bioterrorism, e.g. clustered cases of a rapidly progressive systemic illness. The diagnosis should also be considered in a single individual with a consistent or suggestive clinical illness in the absence of another etiology. Table 71.3 delineates a detailed differential diagnosis with specific discriminating features. It should be noted that multiple bioterrorism threat agents are included in the differential diagnosis of inhalational anthrax.
The early recognition and treatment of inhalational anthrax appear to be associated with a survival advantage;11 in the US experience patients who received appropriate antimicrobials within 4.7 days of symptom onset had a mortality rate of 40% as compared with a mortality rate of 75% for those treated after that period.23 Therefore, prompt empiric antimicrobial therapy should be initiated if infection is clinically suspected. Combination parenteral therapy is appropriate in the ill individual for a number of reasons:11
-
•
to cover the possibility of antimicrobial resistance;
-
•
to target specific bacterial virulence properties, e.g. the theoretical effect of clindamycin on toxin production;
-
•
to ensure adequate drug penetration into the central nervous system; and
-
•
to favorably impact survival.
In order to optimize the outcome in inhalational anthrax it is likely that novel therapies, such as toxin inhibitors or receptor antagonists, will need to be developed and deployed.24 A variety of such strategies, guided by the pathogenesis of the organism and its disease-producing toxins, has shown promise in animal studies to date and will likely be components of effective therapeutic regimens in the future.25
Detailed therapeutic and postexposure prophylaxis recommendations for adults, children and special groups have been recently reviewed elsewhere.17, 26 Anthrax vaccine adsorbed (AVA), the current product in use for select indications, has been proven to be effective in preventing cutaneous anthrax in human clinical trials and in preventing inhalational disease after aerosol challenge in nonhuman primates.27 The vaccine has generally been found to be safe but requires six doses over 18 months with the need for frequent boosting. Because of the aforementioned dosing issues and the limited availability of AVA, second-generation anthrax vaccines employing recombinant protective antigen and humanized antiprotective antigen monoclonal antibodies are in production.
Smallpox
(See healthmap.org for outbreaks of Smallpox)
The last known naturally acquired case of smallpox occurred in Somalia in 1977; in 1980, as the culmination of a 12-year, intensive campaign by the World Health Organization (WHO), the disease became the first in history to be officially certified as ‘eradicated’ as a scourge of humans.28 However, because of concerns that variola virus stocks may have either been removed from or sequestered outside of their WHO-designated repositories, smallpox is considered to be a potential agent of bioterrorism. Smallpox is an attractive biologic weapon as its re-introduction into human populations would be a global public health catastrophe. It is stable in aerosol form with a low infective dose; case fatality rates approach 30%; secondary attack rates among unvaccinated close contacts are 37–88% and are amplified, especially in health-care settings; and much of the world's population is susceptible. Routine civilian vaccination was terminated more than two decades ago and vaccine-induced immunity appears to wane over time in vaccinees.29 Vaccine supplies remain limited, and there are currently no antiviral therapies of proven clinical effectiveness against this pathogen.
Following an average incubation period of 10–12 days (range 7–19 days), patients experience the acute onset of a 2- to 3-day prostrating prodrome consisting of fever, rigors, malaise, vomiting, headache and backache. They subsequently develop a centrifugally distributed eruption that initially involves the face and extremities and then generalizes as it evolves through macular, papular, vesicular and pustular stages in synchronous (i.e. lesions progress concurrently and have similar appearances diffusely) fashion over approximately 8 days, with umbilication in the latter stages (Fig. 71.6 ). Enanthema in the oropharynx typically precede the exanthem by a day or two; this represents high titer viral replication in the upper respiratory tract and correlates with high infectivity. The rash generally remains denser peripherally and typically involves the palms and soles early on, a potentially useful clue in narrowing the differential diagnosis (Fig. 71.7 ). The umbilicated pustules begin crusting during the second week of the eruption; separation of scabs is usually complete by the end of the third week, but the course of the systemic illness may be attenuated and the appearance of the exanthem milder in those with partial, pre-existing immunity or more progressive and virulent in those with immunodeficient states.
Fig. 71.6.


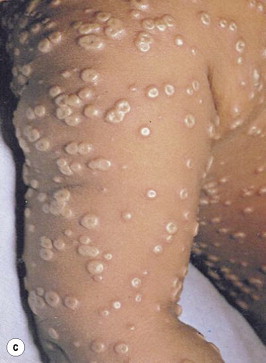

Smallpox. (a) Third day of rash in smallpox. Additional lesions continue to appear and some of the papules are becoming obviously vesicular. (b) Fifth day of rash in smallpox. Almost all the papules have now become vesicular or pustular, the truly ‘vesicular’ stage usually being very brief. Some of the lesions on the upper arm show early umbilication. (c) Eighth day of rash in smallpox. This case is now clearly classified as discrete ordinary-type smallpox. In the confluent subtype of ordinary-type smallpox the lesions would have been confluent on the face and forearms: in the semiconfluent subtype they would have been confluent on the face but not on the forearms. (d) Twentieth day of rash in smallpox. The scabs have separated except on the palms of the hands and the soles of the feet, leaving depigmented areas.
From Fenner et al.28
Fig. 71.7.


(a) Typical centrifugal distribution of the rash in smallpox. (b) Patient with smallpox, Kosovo, Yugoslavia epidemic, March and April 1972. The scabs will eventually fall off leaving marks on the skin that will become pitted scars. The infection is transmissible until all scabs have fallen off.
(a) Courtesy of CDC and Dr Paul B Dean; (b) Courtesy of CDC and Dr William Foege.
The differential diagnosis of smallpox is extensive (see Table 71.3) but may be aided by a number of features of the disease: synchronous lesions, umbilicated appearance in the pustular stage, early involvement of palms and soles, and the centrifugal distribution of the eruption. Historically, varicella and drug reactions posed the most diagnostic dilemmas,29 although the recent importation of monkeypox to the USA from its animal reservoir in Africa elevates this entity to a loftier position on the differential diagnosis list.30 While the diagnosis of smallpox is suggested by clinical features, definitive diagnosis is accomplished by vaccinated clinicians acquiring samples of blood and lesional contents or scrapings from crusts for analysis by electron microscopy, viral antigen immunohistochemistry, polymerase chain reaction and viral isolation. Because processing and evaluation of specimens from a suspected case of smallpox requires high-level biocontainment facilities, collaboration with public health authorities is necessary.
Smallpox is transmitted from person-to-person by respiratory droplet nuclei and, less commonly, by contact with lesions or contaminated fomites. Airborne transmission by fine-particle aerosols has, under certain conditions, been documented31 and should be assumed as a potential mode of spread in a bioterrorism event. The virus is communicable from the onset of the enanthem, generally one or two days prior to the rash, until all of the scabs have separated, although patients are felt to be most contagious during the first week of the rash due to high titers of replicating virus in the oropharynx. Household members, other face-to-face contacts and health-care workers have traditionally been at highest risk for secondary transmission; the last group is obviously of greatest concern with regards to amplification of infection, especially among medically vulnerable populations. Thus, hospitalized cases of suspected smallpox must immediately be placed in negative-pressure rooms with contact and airborne precautions; those not requiring hospital-level care should remain isolated at home in order to avoid infecting others.
The suspicion of a single smallpox case should prompt immediate notification of local public health authorities and the hospital epidemiologist. Containment of the disease is predicated on the ‘ring vaccination’ strategy, which was successfully deployed in the WHO global eradication campaign and which mandates the identification and immunization of all directly exposed persons or those at high risk of exposure, including close contacts, health-care workers and laboratory personnel. Vaccination of infected individuals, if deployed within 4 days of infection during the early incubation period, can significantly attenuate or prevent disease and may favorably impact secondary transmission.29 Because the disease does not exist in nature, the occurrence of even a single case of smallpox would be tantamount to bioterrorism and would warrant an epidemiologic investigation to ascertain the perimeter of the initial release, so that tracing of those initially exposed can be accomplished.32
Botulism
(See healthmap.org for outbreaks of Botulism)
Botulism is an acute neurologic disease resulting from intoxication with Clostridium botulinum that occurs sporadically and in focal outbreaks throughout the world. Generally, the illness is associated with wound contamination by the bacterial form or ingestion of preformed, food-borne toxin. A detailed discussion of botulism is found in Chapter 21. Aerosol forms of the toxin, a rare mode of acquisition in nature, have been weaponized for use in bioterrorism although their actual use has never been documented.5 Botulinum toxin is considered to be the most toxic molecule known; it is lethal to humans in minute quantities and acts by blocking the release of the neurotransmitter acetylcholine from presynaptic vesicles, thereby inhibiting muscle contraction.33
Botulism presents with the clinical features of an acute, afebrile, symmetric, descending, flaccid paralysis without mental status or sensory changes. The disease manifests initially in the bulbar musculature; fatigue, dizziness, dysphagia, dysarthria, diplopia, dry mouth, dyspnea, ptosis, ophthalmoparesis, tongue weakness and facial muscle paresis are early findings seen in more than 75% of cases. Progressive muscular involvement leads to respiratory failure in untreated cases. The clinical presentations of food-borne and inhalational botulism are indistinguishable in experimental animals.33
The diagnosis of botulism is based largely on epidemiologic and clinical features and the exclusion of other possible differential diagnoses (see Table 71.3); there is no commercial assay currently available to confirm intoxication. While sporadic or clustered cases occur regularly, albeit infrequently in developed countries, it must be recognized that any single case of botulism could be the result of bioterorism or could herald a larger scale ‘event’. Certainly, large numbers of epidemiologically unrelated, geographically dispersed or multifocal cases should raise the specter of an intentional release of the agent, either in food/water supplies or as an aerosol.
The mortality from food-borne botulism has declined from 60% to 6% over the last four decades, probably as a result of improvements in intensive and supportive care. Because the need for mechanical ventilation may be prolonged in these patients, the finite resource of ventilators would be rapidly overwhelmed in the event of a large-scale bioterrorism event using botulism toxin, even though these devices are part of the Strategic National Stockpile in the USA for such incidents. New developments in ventilator technology may mitigate some of the predicted shortfalls. Treatment with an equine antitoxin is available in limited supply from the CDC and may ameliorate disease if given early.
Plague
(See healthmap.org for outbreaks of Plague)
Plague, a systemic disease caused by the Gram-negative pathogen Yersinia pestis, presents in a variety of clinical forms in nature as detailed in Chapter 120. Plague is endemic in parts of South East Asia, Africa and the western USA. While naturally acquired disease results from a variety of exposure modes, bioterrorism carried out using aerosolized preparations of the agent would likely result in cases of primary pneumonic plague occurring outside of endemic areas. As was the case with the anthrax attacks in the USA in 2001, however, unexpected forms of the disease, such as bubonic and septicemic plague, might also occur in an event.
Primary pneumonic plague classically presents as an acute, febrile, pneumonic illness with prominent respiratory and systemic symptoms; gastrointestinal symptoms, purulent sputum production or hemoptysis occur variably.34 Chest roentgenogram typically shows patchy, bilateral, multilobar infiltrates or consolidations (Fig. 71.8 ). Untreated or inappropriately treated patients progress rapidly to develop respiratory failure, vascular collapse, purpuric skin lesions, necrotic digits and death. The differential diagnosis is essentially one involving etiologies of rapidly progressive pneumonia and includes clinical syndromes caused by a number of other agents of bioterrorism (see Table 71.3). The diagnosis may be suggested by observing the characteristic small, Gram-negative, coccobacillary forms in sputum specimens with bipolar or ‘safety pin’ uptake of Giemsa or Wright stain (Fig. 71.9 ).35 Culture confirmation is necessary to confirm the diagnosis; the microbiology laboratory should be notified in advance if plague is suspected, as special techniques and precautions must be employed to prevent inadvertent transmission to laboratory personnel.
Fig. 71.8.

Chest X-ray, pneumonic plague, demonstrating multilobar infiltrates.
Courtesy of CDC and Dr Jack Poland.
Fig. 71.9.

Peripheral blood smear demonstrating bipolar uptake of stain, the so-called ‘safety pin’ appearance of Yersinia pestis.
Courtesy of CDC and Dr Jack Poland.
Treatment recommendations for plague have been reviewed elsewhere.26, 35 Pneumonic plague can be transmitted from person-to-person by respiratory droplet nuclei, thus placing close contacts, such as patients and health-care workers in the health-care setting, at risk. Domestic cats may participate in maintaining a transmission chain during a bioterrorism event.12 Prompt recognition and treatment of plague cases, appropriate deployment of postexposure prophylaxis, and early institution of droplet precautions for infected individuals will interrupt secondary transmission.
Tularemia
The causative agent of tularemia, Francisella tularensis, is another small Gram-negative coccobacillus that would be predicted to cause a primary pneumonic illness if delivered as an aerosol in a bioterrorism event. Once again, however, vigilance is necessary as naturally occurring disease can be acquired by a variety of routes and present in many clinical forms; therefore an intentional release of bacteria may also result in more than one form of tularemia. Pulmonic tularemia presents with the abrupt onset of a febrile systemic illness with prominent upper respiratory symptoms, pleuritic chest pain, and the variable development of pneumonia, hilar adenopathy and progression to respiratory failure and death in approximately 30% of inappropriately treated patients.36 The diagnosis is generally established on clinical features, based on the differential diagnosis (see Table 71.3) and microbiologic data; laboratory personnel should be notified in advance if tularemia is suspected, as the organism can be very infectious when manipulated in laboratory conditions. This agent is discussed in depth in Chapter 121.
Viral hemorrhagic fevers
(See healthmap.org for outbreaks of hemorrhagic fevers)
Pathogenic members of four distinct families of RNA viruses are potential agents of viral hemorrhagic fevers (VHF): the agents of Ebola, Marburg, Lassa fever, Rift Valley fever and Congo–Crimean hemorrhagic fever. These syndromes are discussed in detail in Chapter 126. VHF cause clinical syndromes with many common features: fever, malaise, headache, myalgias, prostration, mucosal hemorrhage and other signs of increased vascular permeability, leading to shock and multiorgan system failure in advanced cases.37 Additionally, specific pathogens are associated with specific target organ effects.
Hemorrhagic fever viruses have generally been viewed as emerging infections in nature due to their sporadic occurrence in focal outbreaks throughout the world and environmental disruption by expanding human populations. These viruses are also potential weapons of bioterrorism for a number of reasons:10
-
•
they are highly infectious in aerosol form;
-
•
they are transmissible in health-care settings;
-
•
they cause high morbidity and mortality; and
-
•
they are purported to have been successfully weaponized.
Blood and other body fluids from infected patients are infectious. As such, person-to-person airborne transmission may occur and strict contact and airborne precautions should be instituted in these cases.37 Transmission in health-care settings is a well-described risk with these agents. Treatment is largely supportive and includes the early use of vasopressors as needed. Ribavirin is effective against some forms of VHF but not those caused by Ebola and Marburg viruses. Nonetheless, this drug should be initiated empirically in patients presenting with a consistent clinical syndrome until an alternate etiology is confirmed.
ASSOCIATED ISSUES AND SEQUELAE OF BIOTERRORISM
Surveillance
Surveillance is perhaps the most critical element in the early recognition and identification of bioterrorism events. In the context of the individual clinician surveillance is analogous to clinical vigilance; in the broader context of communities and larger populations, it involves a public health system and infrastructure designed to detect perturbations in the baseline occurrence of either symptoms, as is the case with syndromic surveillance systems, or diseases, as is the case with a standard public health system of reportable diseases. Syndromic surveillance systems have been used recently for monitoring influenza activity and other emerging infectious diseases, and various real-time, electronic platforms are currently in use by a number of organizations to detect early, sensitive indicators of disease activity.
Quarantine
Quarantine, the physical separation and geographic restriction of groups of uninfected individuals potentially exposed to a communicable illness, has been variably considered to be one management strategy following bioterrorism. The potential effectiveness, feasibility, legality and consequences of quarantine have recently been reviewed.38 The logistics of this approach are complex and impractical, and it can be associated with adverse consequences, such as increased risk of disease transmission among a quarantined group or riots. It seems clear that there are only limited scenarios in which the potential public health benefits of the imposition of quarantine may outweigh the potential problems engendered by this approach; these largely revolve around highly transmissible, lethal agents. In most situations a disease-specific containment strategy, based on transmission epidemiology and disease prevention approaches, is preferable.
Management of special patient populations
The approach to the management of diseases of bioterrorism must include provisions for children, pregnant women and immunocompromised individuals. Specific recommendations for treatment and prophylaxis of these special patient groups for selected bioterrorism agents have recently been reviewed.16, 26, 35, 36 A general approach requires an assessment of the risk of using certain drugs or products in select populations versus the potential risk of the infection in question, accounting for the extent of exposure and agent involved. Live virus immunizations such as the smallpox vaccine pose higher risk to these special groups than to others. This consideration will impact mass vaccination decisions and, like most other aspects of medicine, will require an assessment of risk versus benefit.
Psychosocial morbidity
An often overlooked but vitally important issue is that of psychosocial morbidity related to bioterrorism. These sequelae may take the form of acute anxiety reactions and exacerbations of chronic psychiatric illness during the stress of the event, or post-traumatic stress disorder (PTSD) in its aftermath, and may involve clinical victims of bioterrorism as well as health-care workers and other first responders. Nearly half of the emergency department visits during the Gulf War missile attacks in Israel in 1991 were related to acute psychological illness or exacerbations of underlying problems.39 Data from recent acts of terrorism in the USA suggest that PTSD and/or depression may develop in more than 35% of those impacted by the events.40, 41 Although close proximity to an event and personal loss appear to be directly correlated with PTSD and depression, respectively, those indirectly involved also experience substantial morbidity.41 The long-term psychosocial impact of these events and of the persistent threat of terrorism in general remains to be determined.
CONCLUSION
The response to bioterrorism is unique among weapons of mass destruction because it necessitates management strategies common to all disasters as well as the application of basic infectious diseases principles: disease surveillance, infection control, antimicrobial therapy and prophylaxis, and vaccine prevention. For these reasons, we, as physicians (and specifically infectious diseases specialists), are likely first responders to bioterrorism and must keep our diagnostic and clinical skills current and our clinical vigilance active regarding potential threat agents. We are expected to be reliable sources of information for our patients, colleagues and public health authorities.42 As a group we must guard against the inexorable ‘bioterrorism fatigue’ that may otherwise result from a persistent state of heightened readiness without an actual event taking place.6
REFERENCES
- 1.Christopher G.W., Cieslak T.J., Pavlin J.A. Biological warfare: a historical perspective. JAMA. 1997;278:412–417. [PubMed] [Google Scholar]
- 2.Harris S.H. Factories of death: Japanese biological warfare, 1932–45, and the American cover-up. Routledge; New York: 1994. [Google Scholar]
- 3.World Health Organization . Health aspects of chemical and biological weapons: report of a WHO group of consultants. Geneva; WHO: 1970. pp. 98–99. [Google Scholar]
- 4.Torok T.J., Tauxe R.V., Wise R.P. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA. 1997;278:389–395. doi: 10.1001/jama.1997.03550050051033. [DOI] [PubMed] [Google Scholar]
- 5.Zilinskas R.A. Iraq's biological weapons: The past as future? JAMA. 1997;278:418–424. [PubMed] [Google Scholar]
- 6.Martin T.M., Artenstein A.W. Bioterrorism. In: Mayer K.H., Pizer H.F., editors. The social ecology of infectious diseases. Academic Press; Boston: 2007. pp. 316–350. [Google Scholar]
- 7.Miller J., Engelberg S., Broad W. Germs: biological weapons and America's secret war. Simon and Schuster; New York: 2001. [Google Scholar]
- 8.Centers for Disease Control and Prevention http://www.bt.cdc.gov/agent/agentlist-category.asp Emergency preparedness and response: bioterrorism agents/diseases. Online. Available:
- 9.National Institutes of Health http://www3.niaid.nih.gov/topics/BiodefenseRelated/Biodefense/research/CatA.htm NIAID category A, B and C priority pathogens. Online. Available:
- 10.Alibek K. Biohazard. Random House; New York: 1999. [Google Scholar]
- 11.Jernigan J., Stephens D.S., Ashford D.A. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinowitz P., Gordon Z., Chudnov D. Animals as sentinels of bioterrorism agents. Emerg Infect Dis. 2006;12:647–652. doi: 10.3201/eid1204.051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidell F.R., Takafuji E.T., Franz D.R., editors. Medical aspects of chemical and biological warfare. Textbook of Military Medicine series. Part I, Warfare, weaponry and the casualty. Office of the Surgeon General, Department of the Army; Washington DC: 1997. USA. [Google Scholar]
- 14.Artenstein A.W. Biologic attack. In: Ciottone G., editor. Disaster medicine. 3rd ed. Philadelphia; Mosby: 2006. pp. 415–423. [Google Scholar]
- 15.Dixon T.C., Meselson M., Guillemin J. Anthrax. N Engl J Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 16.Inglesby T.V., Henderson D.A., Bartlett J.G. Anthrax as a biological weapon: medical and public health management. JAMA. 1999;281:1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 17.Inglesby T.V., O'Toole T., Henderson D.A. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 18.Freedman A., Afonja O., Chang M.W. Cutaneous anthrax associated with microangiopathic hemolytic anemia and coagulopathy in a 7-month-old infant. JAMA. 2002;287:869–874. doi: 10.1001/jama.287.7.869. [DOI] [PubMed] [Google Scholar]
- 19.Barakat L.A., Quentzel H.L., Jernigan J.A. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287:863–868. doi: 10.1001/jama.287.7.863. [DOI] [PubMed] [Google Scholar]
- 20.Hupert N., Bearman G.M.L., Mushlin A.I., Callahan M.A. Accuracy of screening for inhalational anthrax after a bioterrorist attack. Ann Intern Med. 2003;139:337–345. doi: 10.7326/0003-4819-139-5_part_1-200309020-00009. [DOI] [PubMed] [Google Scholar]
- 21.Abramova F.A., Grinberg L.M., Yampolskaya O. Pathology of inhalational anthrax in forty-two cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci USA. 1993;90:2291–2294. doi: 10.1073/pnas.90.6.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush L.M., Abrams B.H., Beall A., Johnson C.C. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N Engl J Med. 2001;345:1607–1610. doi: 10.1056/NEJMoa012948. [DOI] [PubMed] [Google Scholar]
- 23.Holty J-EC, Bravata D.M., Liu H. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 24.Friedlander A.M. Tackling anthrax. Nature. 2001;414:160–161. doi: 10.1038/35102660. [DOI] [PubMed] [Google Scholar]
- 25.Artenstein A.W. Anthrax: from antiquity to answers. J Infect Dis. 2007;195:471–473. doi: 10.1086/510859. [DOI] [PubMed] [Google Scholar]
- 26.Patrozou E., Artenstein A. Bioterrorism. In: Schlossberg D., editor. Clinical infectious diseases. 3rd ed. Cambridge University Press; New York: 2008. pp. 865–877. [Google Scholar]
- 27.Friedlander A.M., Pittman P.R., Parker G.W. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA. 1999;282:2104–2106. doi: 10.1001/jama.282.22.2104. [DOI] [PubMed] [Google Scholar]
- 28.Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 29.Breman J.G., Henderson D.A. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 30.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrle P.F., Posch J., Richter K.H. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull WHO. 1970;43:669–679. [PMC free article] [PubMed] [Google Scholar]
- 32.Artenstein A.W. Initial management of a suspected outbreak of smallpox. In: Cohen J., Powderly W.G., editors. Infectious diseases. 2nd ed. Mosby; London: 2003. pp. 1022–1024. [Google Scholar]
- 33.Arnon S.S., Schechter R., Inglesby T.V. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 34.Artenstein A.W., Lucey D.R. Occupational plague. In: Couturier A.J., editor. Occupational and environmental infectious diseases. OEM Press; Beverly Farms, MA: 2000. pp. 329–335. [Google Scholar]
- 35.Inglesby T.V., Dennis D.T., Henderson D.A. Plague as a biological weapon: medical and public health management. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 36.Dennis D.T., Inglesby T.V., Henderson D.A. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 37.Borio L., Inglesby T., Peters C.J. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 38.Barbera J., Macintyre A., Gostin L. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286:2711–2717. doi: 10.1001/jama.286.21.2711. [DOI] [PubMed] [Google Scholar]
- 39.Karsenty E., Shemer J., Alshech I. Medical aspects of the Iraqi missile attacks on Israel. Isr J Med Sci. 1991;27:603–607. [PubMed] [Google Scholar]
- 40.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 41.Galea S., Ahern J., Resnick H. Psychological sequelae of the September 11 terrorist attacks in New York City. N Engl J Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- 42.Artenstein A.W., Neill M.A., Opal S.M. Bioterrorism and physicians. Ann Intern Med. 2002;137:626. doi: 10.7326/0003-4819-137-7-200210010-00031. [DOI] [PubMed] [Google Scholar]


