Part I. Cardiovascular Diseases
Cardiac Disease
Cardiac disease has become increasingly recognized in domestic rabbits. Despite their frequent use as laboratory models for cardiac disease in humans, little is known about the pathogenesis and treatment of naturally occurring heart disease in rabbits. Reports of spontaneous heart disease are sporadic and case numbers are often low. Despite this, a complete cardiac evaluation and systematic approach can lead to a correct diagnosis and successful treatment of rabbits with cardiovascular disease.
Normal Cardiovascular Structure
The rabbit heart is unique in several ways: the tricuspid valve is composed of two rather than three cusps; the aortic nerve is not associated with chemoreceptors but only with baroreceptors; the rabbit pulmonary artery and its branches are heavily muscular6; and a persistent left cranial vena cava is also normally found and drains into the coronary sinus.25 Additionally, the myocardium has limited collateral circulation and is therefore predisposed to ischemia mediated by coronary vasoconstriction.36
Examination of the Rabbit with Cardiovascular Disease
Information gathered from a thoughtful history and complete physical examination comprises the most important part of the cardiac evaluation.
History
A thorough general history, including husbandry, diet, and past or present illnesses, should be obtained for rabbits suspected of having cardiac disease. Tachypnea, dyspnea, syncope, anorexia, weight loss, and malaise may be signs of heart disease in rabbits.
Physical Examination
Take care to minimize the handling of a rabbit in cardiac distress; a complete physical examination and further diagnostic testing must sometimes be delayed until the rabbit is clinically stable. Stress can be minimized by examining the rabbit in a quiet room and moving slowly. Rabbits are a prey species and thereby very sensitive to external stimuli. If necessary, sedatives may also be given to minimize the risk of self-injury. Sedation can be achieved with midazolam 0.5 to 1 mg/kg intramuscularly45 or, for heavier sedation, a combination of ketamine 5 to 20 mg/kg and midazolam 1 to 2 mg/kg subcutaneously13 with minimal cardiovascular depression (see Chapters 31 and AppendixChapter 31Appendix). Provide flow-by oxygen as needed if a rabbit is dyspneic or becomes tachypneic with handling. In rabbits suspected of having cardiac disease or respiratory distress, focus the initial examination on observing the respiratory rate and pattern, obtaining the heart rate and rhythm, auscultating the thorax, examining the mucous membranes, and palpating the pulses. Normal heart rate is 180 to 250 beats per minute and normal respiratory rate is 30 to 60 breaths per minute. Peripheral pulses can be palpated at the central artery of the ear and should be strong and synchronous with the heartbeat. Two heart sounds (S1 and S2) are normally heard. Rabbits are obligate nose breathers; therefore do not occlude the nares.
Rabbits with heart disease may have cyanotic or pale mucous membranes, arrhythmias, or heart murmurs. Rabbits with congestive heart failure often have tachycardia, tachypnea, and labored breathing. Pulses may be irregular or weak. Systematic auscultation of the thorax is the most important part of the cardiac examination. The entire thorax should be auscultated to localize heart murmurs and detect extra heart sounds (gallop sounds), arrhythmias, and abnormal lung sounds. A pediatric stethoscope allows better localization of heart sounds in rabbits and is preferred for cardiac auscultation; a larger-diaphragm stethoscope enhances auscultation of the lungs. Auscultatory findings vary among rabbits with congestive heart failure, and these findings are not pathognomonic. The examiner may hear muffled heart and lung sounds with pleural effusion and increased bronchial sounds or crackles with pulmonary edema.
Diagnostic Methods
Diagnosis of cardiovascular disease is based on a complete history and physical examination findings complemented by appropriate diagnostic tests. Thoracic radiographs, electrocardiography, echocardiography, and routine blood tests are useful in reaching a definitive diagnosis and treatment plan.
Radiography
Thoracic radiography provides critical information in the patient with cardiopulmonary disease—namely cardiac shape and size, pulmonary pattern, vascular pattern, and other thoracic lesions. Congestive heart failure and respiratory disease can be differentiated by evaluating thoracic radiographs. Radiographic findings supporting a diagnosis of cardiac disease are similar to those in other species and include cardiac enlargement, pulmonary vascular enlargement, pulmonary interstitial and alveolar pulmonary pattern of pulmonary edema, and pleural effusion. Fig. 20-1, Fig. 20-2 show thoracic radiographs of a normal adult rabbit and a rabbit with heart disease, respectively.
Fig. 20-1.
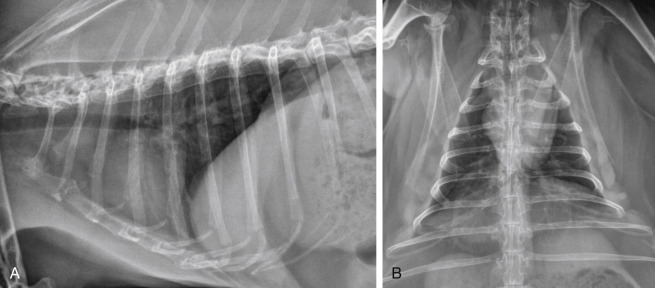
Normal thoracic radiographs of a 6-year-old rabbit. A, Right lateral projection. B, Ventrodorsal projection.
Fig. 20-2.

Severe generalized cardiomegaly with congestive heart failure in a 5-year-old male castrated angora rabbit. A, In the right lateral projection, the cardiac silhouette is generally enlarged. The carina is elevated owing to left atrial and ventricular enlargement. A marked bronchointerstitial pattern is present in the caudal dorsal lung lobes compatible with pulmonary edema. The lung lobes are mildly retracted and the ventral border of the cardiac silhouette is obscured, indicating mild pleural effusion. B, In the ventrodorsal projection, a markedly enlarged left auricle is present (black arrowheads). An enlarged right atrium is also present (white arrowhead). An increased bronchointerstitial pattern is present in the caudal lung lobes, worse on the right than the left. Retraction of the lung lobe indicates pleural effusion (black arrows).
Electrocardiography
Electrocardiography (ECG) is a simple and practical diagnostic test in rabbits with suspected or confirmed cardiac disease. An ECG is critical to diagnose and manage arrhythmias or syncope. The ECG may also be a helpful addition to the cardiac database. However, one should not use an ECG to assess or detect chamber enlargement or hypertrophy. Record the ECG with the rabbit in sternal recumbency. File the alligator-style ECG clips to minimize skin trauma, and place medical-grade alcohol or electrode gel on the clips to enhance conduction. Record tracings with a vertical calibration of 2 cm/mV and a horizontal paper speed of 50 mm/s. Normal rabbit rhythm is sinus and does not include respiratory sinus arrhythmia.44 Spontaneous changes in the QRS complex have been observed in normal rabbits in serial ECG recordings. Normal ECG values for a variety of pet rabbit breeds have been reported.34., 45. In the authors’ experience, R-wave amplitudes in lead II of 0.1 to 0.2 mV seem most common. Reference ranges for ECGs are summarized in Table 20-1 .
Table 20-1.
Electrocardiographic Values in Clinically Normal Pet Rabbits
| ECG Parameter | Values34 | Values45 |
|---|---|---|
| Heart rate | 198-330 (mean 264) beats/minute | 240 beats/minute (mean) |
| Measurements (lead II) | ||
| P wave | ||
| Amplitude (height) | 0.04-0.12 mV | 0.04-0.07 mV |
| Duration (width) | 0.01-0.05 s | 0.02-0.04 s |
| P-R interval | ||
| Duration | 0.04-0.08 s | 0.05-0.07 s |
| QRS complex | ||
| R-wave amplitude | 0.03-0.039 mV | 0.12-0.2 mV |
| Duration | 0.02-0.06 s | 0.03-0.04 s |
| Q-T interval | ||
| Duration | 0.08-0.16 s | — |
| T wave | ||
| Amplitude | 0.05-0.17 mV | — |
| Electrical axis (frontal plane) | 43-80 degrees | — |
| Body weight | 1.1-7.9 (mean 2.57) kg | — |
Echocardiography
Echocardiography provides a sensitive, accurate, noninvasive means of assessing the heart. Most rabbits tolerate echocardiography easily, making it a practical diagnostic tool. However, in rabbits that are tachypneic or dyspneic, give oxygen by face mask during restraint. The echocardiographic exam may be performed in lateral or sternal recumbency. Standard views used to evaluate dogs and cats can also be obtained with the rabbit. Because of the rabbit’s rapid heart rate and small size, optimal evaluation requires a high-frequency transducer and high-frame-rate ultrasound machine. Two-dimensional and M-mode echocardiography assess cardiac structure, chamber size, wall thickness, and motion as well as extracardiac structures, masses, and pleural effusion. Color-flow, spectral, and tissue Doppler echocardiography assess direction and velocity of blood flow, further defining cardiac conditions with more insight on systolic and diastolic function. Normal echocardiographic values have been published for several breeds of rabbits (Table 20-2 ). Sedative drugs used for restraint may affect cardiac measurements, especially alpha2-agonists such as dexmedetomidine and xylazine. Reduced percent fractional shortening, E/A reversal, decreased heart rate, and other changes have been documented with intramuscular ketamine at dose of 50 mg/kg and xylazine at 4 mg/kg.54 Sedation with ketamine 20 mg/kg and midazolam 2 mg/kg given subcutaneously has been shown to be less cardiodepressive.13
Table 20-2.
Echocardiographic Values in Clinically Normal Rabbitsa
| Parameter | Dutch Belted Rabbits36 (n = 6) | Japanese White Rabbits50 (n = 4) | New Zealand White Rabbits54 (n = 20) | New Zealand White Rabbits13 (n = 26) |
|---|---|---|---|---|
| Body weight (kg) | 2.32 ± 0.36 | 3.0 (mean) | 2.92 (mean) | 2.3 ± 0.4 |
| Age (months) | 7 (mean) | >13 | 4 | 4-5 |
| LVEDD (cm) | 1.17 ± 0.19 | 1.69 ± 0.05 | 1.54 ± 0.112 | 1.351 ± 0.105 |
| LVESD (cm) | 0.70 ± 0.09 | 1.15 ± 0.05 | 1.009 ±0.091 | 0.864 ± 0.082 |
| %FS | 39.50 ± 5.39 | — | 34.5 ± 4.9 | 36.01 ± 4.31 |
| IVSD (cm) | 0.25 ± 0.05 | 0.33 ± 0.03 | 0.217 ± 0.056 | 0.265 ± 0.031 |
| LVPWD (cm) | 0.31 ± 0.08 | 0.33 ± 0.03 | 0.274 ± 0.041 | 0.225 ± 0.029 |
| LA (cm) | — | 1.05 ± 0.25 | — | 0.749 ± 0.114 |
| Ao (cm) | 0.67 ± 0.10 | 1.07 ± 0.12 | — | 0.657 ± 0.046 |
| LA/Ao | 1.38 ± 0.32 | — | — | 1.15 ± 0.19 |
| RADs (cm) | 0.61 ± 0.08 | — | — | — |
| RA/Ao | 0.88 ± 0.17 | — | — | — |
| EPSS (cm) | 0.05 ± 0.05 | — | — | 0.141 ± 0.025 |
| MVEFS (mm/s) | 70.17 ± 31.82 | — | — | — |
| RVOT velocity (m/s) | 0.83 ± 0.10 | — | — | 0.78 ± 0.12 |
| LVOT velocity (m/s) | 0.65 ± 0.14 | — | 0.749 ± 0.195 | 0.86 ± 0.12 |
| LVET (s) | 0.08 ± 0.01 | — | 0.126 ± 0.014 | 0.096 ± 0.010 |
| VCF (circumference/s) | 4.74 ± 0.45 | — | — | — |
| MV E (m/s) | — | 0.44 ± 0.12 | 0.715 ± 0.138 | 0.78 ± 0.15 |
| MV A (m/s) | — | 0.46 ± 0.17 | 0.514 ± 0.145 | 0.55 ± 0.11 |
| MV E/A | — | 1.0 ± 0.2 | 1.44 ± 0.28 | 1.44 ± 0.16 |
| MV DT (m/s) | — | 41.3 ± 2.5 | — | — |
| TDI E LW (m/s) | — | — | 0.067 ± 0.019 | 0.16 ± 0.05 |
| TDI A LW (m/s) | — | — | 0.039 ± 0.007 | 0.09 ± 0.03 |
Ao, aorta; EPSS, E point to septal separation; IVSD, intraventricular septal thickness at end-diastole; IVSS, intraventricular septal thickness at end-systole; LA, left atrium; LA/Ao, left atrium-to-aorta ratio; LVEDD, left ventricular end-diastolic dimension; LVESD, LV end-systolic dimension; LVET, left ventricular ejection time; LVOT, left ventricular outflow tract; LVPWD, LV posterior wall thickness at end-diastole; MV A, mitral valve A wave; MV E/A, mitral valve E-to-A ratio; MV DT, mitral valve deceleration time; MV E, mitral valve E wave; MVEFS, mitral valve E-F slope; %FS, percent LV fractional shortening; RA/Ao, right atrium-to-aorta ratio; RADs, right atrial dimension in systole; RVOT, right ventricular outflow tract; TDI A LW, tissue Doppler imaging A’ wave from the left ventricular free wall; TDI E LW, tissue Doppler imaging E’ wave from the left ventricular free wall; VCF, velocity of circumferential fiber shortening.
Values are mean ± SD.
Diseases and Management
Congestive Heart Failure
In rabbits, congestive heart failure (CHF) is the clinical condition in which pulmonary edema, pleural effusion, or hepatomegaly develops as a result of structural or functional cardiac disease. The goal of therapy is to relieve congestion, control future retention of sodium and fluids, and improve cardiac performance. To this end, numerous management strategies are used during the acute stage. Place the patient in a quiet cage with supplemental oxygen. Administer parenteral furosemide (1-4 mg/kg IV or IM q4-12h) and nitroglycerin 2% ointment (1⁄8 inch applied transdermally q6-12h). In a rabbit with pleural effusion, perform therapeutic pleurocentesis if the rabbit is dyspneic.
Long-term therapy of CHF should include a diuretic (furosemide 1-2 mg/kg PO q8-24h) combined with treatment directed at the underlying precipitating cause. Knowledge of the cardiac disease process is the basis of specific treatment of the underlying condition. No cardiac drugs are approved for use in rabbits by the U.S. Food and Drug Administration. Drug dosages for rabbits are not available for all cardiac medications, but drugs and dosages published for cats or ferrets may be successfully used on a milligram-per-kilogram basis. Angiotensin-converting enzyme inhibitors such as enalapril maleate (0.25-0.5 mg/kg PO q24-48h, begun q24h) may be beneficial in treating rabbits with congestive heart failure. Pimobendan (0.1-0.3 mg/kg PO q12-24h) may also be used for treatment of systolic dysfunction and myocardial failure.45 During acute and chronic management, it is critical that clinical and radiographic signs, hydration status, appetite, and body weight as well as serum or plasma blood urea nitrogen, creatinine, and electrolyte concentrations be monitored. For drugs and dosages, refer to Chapter 41.
Congenital Heart Disease
Congenital heart disease in rabbits is rarely reported. Ventricular septal defect, diagnosed with echocardiography, has been described.47 A ventricular septal defect, pulmonary hypertension, and valvular cyst identified at necropsy have been described in a New Zealand white rabbit.31
Arrhythmia
Arrhythmias such as atrial fibrillation and ventricular premature complexes have been identified in pet rabbits with underlying cardiomyopathies and congestive heart failure.35 We have also diagnosed arrhythmias in several rabbits that exhibited syncope and an irregular heartbeat. In a rabbit with an arrhythmia, the treatment protocol should be based on ECG findings and clinical signs. For syncope associated with bradycardia, oral theophylline or pacemaker implantation may be indicated. Glycopyrrolate may be more effective than atropine sulfate in increasing heart rate.43 Atropine may not be as effective because some rabbits produce atropinesterases. Supraventricular tachycardias may be treated with oral digoxin or diltiazem. Ventricular tachyarrhythmias may respond to intravenous lidocaine. Dosages are published for lidocaine, verapamil, atropine, and glycopyrrolate. Other antiarrhythmic drugs may be used at dosages published for cats or ferrets.
Myocardial Disease
Numerous myocardial diseases have been reported in rabbits, and cardiomyopathy is a common postmortem finding in older rabbits.47 Idiopathic hypertrophic cardiomyopathy and dilated cardiomyopathy have been diagnosed by echocardiography (Fig. 20-3 ).44 Vitamin E deficiency produces a muscular dystrophy in which the myocardium may be affected.3 In experimental studies, myocardial disease has been created through inoculation of Trypanosoma cruzi 49 and administration of doxorubicin.59 Infectious myocardial diseases are rare in pet rabbits. Known infectious organisms include Pasteurella multocida, Salmonella species, Encephalitozoon cuniculi, 36 and coronavirus.8 The alpha-agonist drug detomidine has been associated with myocardial necrosis and fibrosis in New Zealand white rabbits.23 A similar ischemia-mediated process is suggested in association with ketamine/xylazine administration.36
Fig. 20-3.
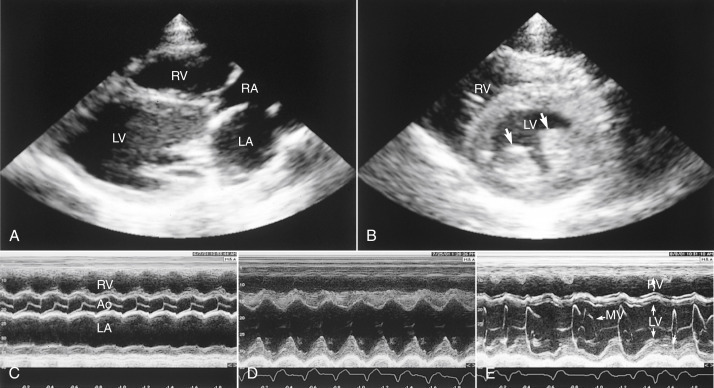
Standard echocardiographic views in rabbits with cardiac disease. A, Right parasternal long-axis four-chamber view. Notice the left and right ventricular dilation in this rabbit with mitral and tricuspid insufficiency. B, Right parasternal short-axis left ventricle papillary muscles view. Notice the prominence of the left ventricular papillary muscles (arrows) in this rabbit with left ventricular hypertrophy. C, Parasternal short-axis M-mode echocardiogram in which the M-mode beam is directed across the right ventricle, aortic valve, and left atrium. Note the dilation of the left atrium. D, Parasternal short-axis M-mode view of the left ventricle in which the M-mode beam is directed across the right ventricle (top) and left ventricle (bottom). Notice the dilated left ventricle and the decreased excursion of the septum compared with the posterior wall in this rabbit with cardiomyopathy and ventricular tachycardia. E, Parasternal short-axis M-mode echocardiogram of the left ventricle and mitral valve. Note the irregular rhythm. LV, Left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; Ao, aorta; MV, mitral valve.
Valvular Disease
Mitral and tricuspid insufficiencies are not uncommon and have been identified in pet rabbits.44 Valvular insufficiencies may be associated with primary valve degeneration, cardiomyopathy, or infection. Progression of the condition leads to volume overload and potential congestive heart failure. A focal murmur is the most common clinical finding. Valvular disease is diagnosed with two-dimensional and Doppler echocardiography (see Fig. 20-3). Echocardiographic findings most often include thickening of one or both atrioventricular valves, dilation of cardiac chambers, and turbulent regurgitation of blood detected by Doppler.
Valvular endocarditis, caused by Staphylococcus aureus, has been reported in rabbits.53
Vascular Disease
Spontaneous arteriosclerosis of the aorta and other arteries has been observed in nearly all rabbit breeds. Clinical signs, if present, may include lethargy, anorexia, and weight loss. The cause is unknown. In rabbits with spontaneous arteriosclerosis, arterial walls and other soft tissues mineralize; the aortic arch and descending thoracic aorta are most commonly affected. Radiopaque vessels, caused by calcification, may be visible on radiographs.32., 51.
Pulmonary hypertension associated with high altitude has been reported in a rabbit.20 Lesions included right ventricular hypertrophy and pulmonary artery proliferation from hypoxia. Based on basic research, rabbits with pulmonary hypertension may respond to phosphodiesterase type-5 inhibitors, particularly vardenafil.55
Part II. Lymphoproliferative Disorders and Thymomas
Lymphoproliferative disorders are uncommonly seen in pet rabbits, but clinicians treating rabbits should be able to properly recognize and diagnose these diseases when presented and offer treatment options when appropriate. Before the 1960s, rare cases of lymphoproliferative disease in domestic rabbits had been reported in the European literature, and lymphoma (malignant lymphoma or lymphosarcoma) was first reported in a domestic rabbit in the United States in 1968. In that report, a New Zealand white rabbit from a research colony had generalized lymphoid neoplasia involving the lymph nodes, liver, spleen, lungs, and gastrointestinal tract.58 Since then, lymphoproliferative diseases have been reported in both laboratory and pet rabbits, whereas most of these cases were diagnosed as generalized or multicentric lymphoma; however, cutaneous lymphoma, and lymphoid leukemia, thymoma, and thymic lymphoma have also been described.
Etiology
The cause of lymphoid neoplasia is largely unknown and likely multifactorial in rabbits. Owing to their use in biomedical research, much is known about disease pathogenesis of induced lymphoid neoplasia in rabbits; however, few studies have investigated the cause of naturally occurring lymphoid disorders.
Genetic Factors
In one of the first reports, lymphoma was described in a series of rabbits from a breeding farm,33 leading to speculation that a breed or strain susceptibility exists or an infectious agent is associated with disease.14., 33., 58. A strain susceptibility has been shown in Wirehair (WH) rabbits associated with a single autosomal recessive gene, termed Is. In the WH strain, affected rabbits typically die at 5 to 13 months of age, usually with generalized lymphoma involving visceral organs and lymph nodes similar to the distribution of organ involvement seen in other domestic animals.14
Infectious Factors
Generalized lymphoma in strains of rabbits has been speculated as compatible with vertical transmission of an oncogenic virus, as occurs with feline leukemia virus. Some have speculated that lymphoma in rabbits may be caused by an oncogenic C-type tumor virus, similar to viruses causing this disease in rodents. In an early study attempting to evaluate this theory, tissues samples from an adult New Zealand white rabbit with generalized lymphoma were examined. Results of electron microscopic examination, tissue culture, immunodiffusion studies with FeLV antiserum, and immunofluorescent tests were negative for a virus.57 However, virus-like particles were demonstrated by electron microscopy in the kidneys of a 7-month-old New Zealand white rabbit with generalized lymphoma.18 In this case, the significance of the virus-like particles was not determined. To date, the hypothesis that a retrovirus (oncogenic virus) may be involved in the pathogenesis of lymphoid disease in rabbits has not been confirmed.
Types of Lymphoproliferative Disorders
Multicentric Lymphoma
Multicentric lymphoma is the most common type of lymphoproliferative disease in rabbits and has been reported in several rabbit breeds, including New Zealand white, Japanese white, Dutch, and Netherland dwarf rabbits.5., 19., 52., 56., 57. Clinically, lymphoma has been observed in a variety of rabbit breeds, including satin, mini lop, and tan breeds. Lymphoma can occur in rabbits of all ages, from animals less than 1 year of age to geriatric animals.∗ In pet rabbits with lymphoma seen at the Animal Medical Center in New York City, ages have ranged from 2 to 9 years, with most averaging 4 to 5 years.
Lymphoma of both T- and B-cell origin has been documented in rabbits. In a domestic rabbit with lymphoma and lymphocytic leukemia, the neoplasia was of T-cell origin.56 In a pet Dutch dwarf rabbit, T- and B-cell infiltrates were observed in the skin, lung, kidneys, liver, intestine, and lymph nodes. In this rabbit, the diagnosis was multicentric, T-cell–rich, B-cell lymphoma with cutaneous involvement.16 A 22-month-old rabbit that presented with acute unilateral exophthalmos was diagnosed with retrobulbar lymphoma.61 In this rabbit, histopathologic examination identified the mass as a B-cell lymphoma of the Harder’s gland, and the mesenteric lymph nodes, cecum, and both kidneys were also affected. In a rabbit that presented with pelvic paralysis, the diagnosis was spinal lymphoma of the sixth lumbar vertebra and concurrent pulmonary filariasis.48 In this rabbit, the neoplasm was immunophenotyped as B-cell lymphoma and the filariasis was considered an incidental finding. In another case, the authors described a rare lymphoma that developed in the cecum of a 6-year-old pet rabbit.24
Rabbits with multicentric lymphoma may exhibit nonspecific general signs, such as anorexia, lethargy, emaciation, pallor, diarrhea, and rhinitis, depending on organ involvement and location. In a 2-year-old rabbit seen at The Animal Medical Center in New York City, the presenting clinical sign was severe upper respiratory stridor. At necropsy, lymphoma was present in the nasal turbinates and sinus, stomach, liver, spleen, kidneys, lymph nodes, and bone marrow. Another rabbit with multicentric lymphoma presented with an acute onset of hind-limb paresis and gastrointestinal stasis. At necropsy, neoplastic cells compatible with large-cell lymphoma (immunoblastic) were found in the spleen, kidneys, lungs, cecum, intestines, lymph nodes, and adrenal glands; no lesions were found in the spinal cord.
Laboratory findings often depend on the organs involved. Results of plasma biochemical analysis may be unremarkable or reveal increases in concentrations of aspartate aminotransferase, creatine phosphokinase, blood urea nitrogen, and creatinine. Rabbits may be moderately to severely anemic14., 65.; in young rabbits, fluctuating and depressed hematocrit values were considered the best diagnostic tool for early identification of lymphoma.14 In rabbits with multicentric lymphoma seen at The Animal Medical Center in New York City, most had hematocrit levels in the low normal range (30%-33%; reference interval, 30%-50%).2 The white blood cell (WBC) count is often within reference intervals; however, some rabbits with lymphoma have shown a leukemic phase (see below). In young rabbits with multicentric lymphoma, high WBC counts were less frequent than a relative predominance of lymphoid cells, including immature and atypical cells, representing 80% to 90% of total WBCs. In an 18-month-old rabbit seen at the Animal Medical Center, the WBC count was 10,000/mL, with 63% lymphocytes. Lymphoma was present in the bone marrow of this rabbit.
At necropsy, neoplastic lesions are commonly found in the lymph nodes, gastrointestinal tract, kidneys, liver, spleen, adrenal glands, gonads, and bone marrow.∗ Less common sites of neoplastic infiltrates are the auditory meatus, vertebrate, eye, and heart.5., 48.
Cutaneous Lymphoma
Cutaneous lymphoma is usually primary but can be secondary to multicentric involvement. Two forms have been distinguished histologically and immunohistochemically: the epitheliotrophic form (mycosis fungoides) is composed of T lymphocytes, whereas the nonepitheliotropic form is composed of B lymphocytes (sparing the epidermis affecting the middle and deep portions of the dermis) (see Chapter 18, Fig. 18-5). Cutaneous lymphomas have the potential to metastasize to visceral organs, or cases may be diagnosed as visceral lymphomas with cutaneous involvement.
Several cases of cutaneous lymphoma have been reported in rabbits.21., 62., 65. In an 18-month-old Netherland dwarf rabbit with multiple subcutaneous swellings over the shoulders, cutaneous lymphoma was diagnosed by histologic examination of biopsy samples.21 At necropsy, no gross or histologic evidence of lymphoma was found in any other organ system, and no viral particles were seen on electron microscopic examination of neoplastic tissue. In another report, three domestic rabbits were diagnosed with cutaneous lymphoma.65 Two rabbits were young (7 months and 1 year) and the third was 9 years old. One young rabbit had erythematous alopecia and hemorrhagic crusts of the chin and ventral neck. At necropsy, neoplastic lymphocytes were found in the skin, lymph nodes, and lungs. In skin sections, the lymphocytes infiltrated the entire dermis and into the epidermis. The second rabbit had bilateral blepharitis that was unresponsive to treatment. At necropsy, superficial and deep lymph nodes were markedly enlarged, and lungs had reddened areas. Lymphocytic infiltrates were found in the skin, lungs, liver, kidneys, and heart. In the skin sections, lymphoid infiltrates were primarily in the subcutis and deep dermis. The third rabbit had nonpruritic alopecia of the left lateral thorax. Cutaneous lymphoma was diagnosed by biopsy of a skin sample; in this rabbit, lymphocytes infiltrated the superficial dermis and epidermis. The rabbit lived for an additional year after diagnosis, with no response to treatment with interferon alpha-2b (see “Treatment,” below). A necropsy was not performed. In all three rabbits, immunologic staining of tissue sections confirmed the lymphoma to be of T-cell origin. In a large retrospective study of cutaneous neoplasms in pet rabbits over 16 years, the authors reported a single case of lymphoma in the flank of a 4-year-old female rabbit. In this case, epitheliotropism was not noted.62
In three clinical cases seen by one of the present authors (KQ), rabbits presented because of one or more subcutaneous masses. In a 7-year-old rabbit, cutaneous lymphoblastic lymphoma was diagnosed by excisional biopsy of a subcutaneous nodule on its dorsal neck. Three more masses developed within 1 month and were excised, but the rabbit died 2 months after the first biopsy. At necropsy, lymphoma was found in the cervical, mesenteric, and thoracic nodes. In another rabbit, a large, hemorrhagic mass was present on the ventral thorax. This rabbit was euthanatized. On histologic examination, lymphoma involving the skin mass and spleen was diagnosed. A 9-year-old rabbit presented with multifocal subcutaneous masses on the dorsum and ventral abdomen. Fine-needle aspirates of the masses revealed large-round-cell neoplasia. At necropsy, lymphoma was widely disseminated to the skin and subcutis, all abdominal organs, lungs, diaphragm, heart, adipose tissues, mandible, and incisor pulp cavity with marked intratumoral necrosis. Because of the extensive involvement of multiple sites, the primary source was unclear.
Leukemia
Lymphoid leukemia is the proliferation of neoplastic lymphocytes that typically originate in the bone marrow and occasionally in the spleen. The neoplastic cells may or may not be found circulating in peripheral blood. Three cases of lymphoblastic leukemia and one case of myeloid leukemia have been documented in rabbits.5., 10., 37., 56. In rabbits with lymphoid leukemia, WBCs have ranged from 30,000 to more than 100,000/μL. In all rabbits with lymphoblastic leukemia, neoplastic cells were present in bone marrow, lymph nodes, and other organs typical of stage V lymphoma.
Thymic Masses: Thymoma/Thymic Lymphoma/Thymic Carcinoma
Rabbits normally have a persistent large thymus that lies cranioventral to the heart and extends into the thoracic inlet (Fig. 20-4 ).29 In adult rabbits, the thymus can become hyperplastic and enlarge up to three or four times normal size, grossly resembling a tumor but with no neoplastic characteristics on histologic examination.64 A thymoma is a tumor derived from the epithelial components of the thymus and is composed of a mix of lymphoid and reticuloepithelial cells. Thymic lymphoma denotes that the neoplasm is of T-lymphocytic origin, possibly with other organ and systemic involvement. In some cases the lymphoid cells are small mature cells; in others, lymphocytes are pleomorphic with prominent nucleoli. Thymic lymphomas are unique because they reflect the function of the thymus gland as an organ involved in two-cell generation and differentiation. Diagnosis is made from cytologic examination of fine-needle aspirates or histopathologic examination of biopsy specimens. Clinically and cytologically, distinguishing thymoma from thymic lymphoma can be difficult. Tissue samples are often needed, since these tumors are classified based on the cells undergoing neoplastic transformation (epithelial and/or lymphocytic). Because of the difficulty in distinguishing hyperplasia, thymoma, thymic lymphoma, and carcinoma on the basis of gross appearance or imaging results, histologic examination is important.
Fig. 20-4.
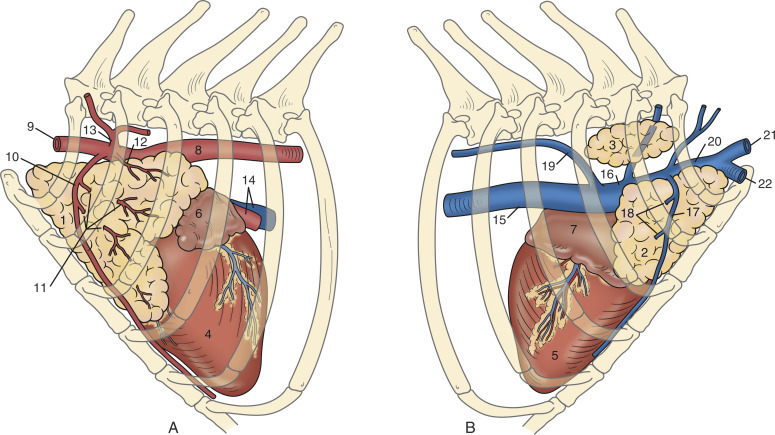
Anatomy of the thymus of a normal adult rabbit. A, left lateral view; B, right lateral view. The thymus remains large in adult rabbits and is located cranioventral to the heart; it extends into the thoracic inlet. 1, Left thoracic lobe, thymus; 2, right ventral thoracic lobe, thymus; 3, right dorsal thoracic lobe, thymus; 4, left ventricle, heart; 5, right ventricle, heart; 6, left auricle, heart; 7, right auricle, heart; 8, thoracic aorta; 9, left subclavian artery; 10, left internal thoracic artery; 11, thymic branches; 12, thymic branch of the left subclavian artery; 13, costocervical trunk; 14, left pulmonary artery, pulmonary vein; 15, caudal vena cava; 16, right cranial vena cava; 17, right internal thoracic vein; 18, thymic veins; 19, right azygos vein; 20, right subclavian vein; 21, right external jugular vein; 22, right axillary vein.
(Adapted from Popesko P, Rajtova V, Horak J, eds. Colour atlas of the anatomy of small laboratory animals. Vol I: Rabbit and guinea pig. St. Louis: Elsevier, 1990/1992.)
Thymoma, thymic lymphoma, and thymic carcinoma have been reported in rabbits.∗ Thymic lymphoma and thymic carcinoma are rare. Although thymomas are seen more frequently clinically, the incidence of thymoma appears to be low. In an early study of 1,100 female rabbits more than 2 years of age submitted for necropsy over a 17-year period, 234 rabbits had tumors. Of those 234 rabbits, 55 had multiple primary tumors, and 4 of these had thymomas.17 In a study of thymomas in 19 rabbits, incidence in males (9) and females (10) was similar, and mean age at presentation was 6.7 years.1
The presence of the cranial mediastinal mass usually causes difficulty breathing (hyperpnea, open-mouth breathing), which is commonly the presenting complaint. Physical examination findings typically relate to respiratory symptoms with nasal flaring evident, secondary to increased respiratory rate and effort.28,46 Auscultation of the thoracic cavity may reveal decreased or muffled lung sounds over the anterior mediastinum.46 Although judged subjectively, decreased compressibility of the thoracic cavity in smaller breeds of rabbits may be detected as well. On thoracic radiographs, a mediastinal mass is identified (Fig. 20-5 , A) and pleural effusion may be present. In a 6-year-old Netherland dwarf rabbit with pleural effusion, chylothorax was diagnosed and considered secondary to the presence of the mediastinal mass.46
Fig. 20-5.

A, Right lateral thoracic radiographs of a lop rabbit with a thymic mass. Ultrasound-guided fine-needle aspirates were diagnostic of thymoma. At presentation, preoperative radiographs show loss of cardiac silhouette, with tracheal elevation on the lateral radiograph. B, Postoperative lateral radiograph with surgical staple present show a more defined cardiac silhouette and return of the trachea to its normal anatomic position.
Uniquely, rabbits with thymic masses may have exophthalmos or present with prolapse of the third eyelids.28., 60., 63. In rabbits with exophthalmos, the eyes can be retropulsed without evidence of pain, suggesting that the exophthalmos is not caused by the presence of a space-occupying retrobulbar mass. These signs are consistent with a diagnosis of cranial vena caval syndrome (precaval syndrome) caused by the space-occupying mass compressing the vessels of the anterior thorax and impeding vascular return to the heart.4
The signs of paraneoplastic syndrome have also been described in rabbits and include hypercalcemia and exfoliative dermatitis (see Chapter 18).12., 26., 60. In one rabbit, the hypercalcemia (14.7 mg/dL) was described as a paraneoplastic syndrome similar to that seen in dogs with thymoma.60 However, because of the unique calcium homeostasis in rabbits and the fact that the influence of diet and calcium metabolism on the plasma calcium concentration was not considered in this case, this conclusion is likely erroneous. Rabbits with thymoma may present with generalized scaling and sebaceous adenitis associated with thymoma. In one case, a paraneoplastic syndrome of thymoma-associated exfoliative dermatitis similar to the syndrome in cats was described.12 In another rabbit that responded to treatment with cyclosporine and oral tryglycerides, an autoimmune reaction directed at the sebaceous glands and a defect in lipid metabolism was suspected.26 Metastasis of thymic carcinoma in rabbits has been reported.63
Rabbits with thymic masses may have a normal complete blood count (CBC), a high WBC count, or anemia. Heterophilia with monocytosis, eosinophilia, basophilia, and thrombocyto-sis was described in one rabbit.1 In two clinical cases of thymoma, the WBC counts were 42,000 and 18,000/μL, with more than 70% lymphocytes. On microscopic interpretation of a blood smear from the first rabbit, the cells were characterized as small lymphocytes with cleaved nuclei and scant cytoplasm. A bone marrow aspirate showed no marrow infiltration. Both rabbits were eventually euthanized and no bone marrow involvement was found on postmortem examination in either one. On histopathologic examination, the masses in both rabbits were identified as thymoma.
Diagnosis
The diagnostic workup of a rabbit with a suspected lymphoproliferative disease is similar to that of other small animals. If the CBC results reflect either a high WBC count with mature lymphocytosis or a normal WBC count with an inverse lymphocyte/heterophil ratio, repeat the test to confirm the findings. If anemia is found on a routine CBC, consider ruling out possible lymphoma and perform additional tests. Submit a blood sample for a plasma biochemical analysis to look for evidence of multiorgan involvement. Obtain both thoracic and abdominal radiographs and an abdominal ultrasound to identify abdominal masses or enlarged lymph nodes. Obtain fine-needle aspirates or do a biopsy of any enlarged peripheral lymph nodes or subcutaneous masses and submit a bone marrow sample for evaluation if appropriate. Submit full-thickness skin biopsy samples to a dermatohistopathologist if cutaneous lymphoma is suspected.
If a thoracic mass is present, perform ultrasound of the thorax to determine the architecture of the mass. Do an ultrasound-guided fine-needle aspirate of the mass if possible and submit the sample for cytologic examination. Because thymomas are frequently cystic and do not exfoliate well, aspiration results may be nondiagnostic. Cytologic evaluation of cases with thymomas usually yields mostly mature lymphocytes as opposed to lymphoblasts, which would support a diagnosis of lymphoma. Thoracic needle core biopsy can be a diagnostic option but may yield necrotic and/or cystic material that might prohibit a definitive diagnosis.
Computed tomography with contrast is indicated to determine the location and extent of a thoracic mass, particularly if surgery or radiation therapy are treatment options (see Chapter 35).
Treatment
In general, little information is available on treating rabbits with lymphoproliferative diseases. Much information is anecdotal, with protocols based on those used in other small animals. At best, the long-term prognosis with treatment is guarded to poor. Consultation with an oncologist is indicated when clients are interested in pursuing chemotherapy or radiation treatments. Advise clients of the risks, benefits, and potential side effects of treatment options.
Chemotherapy
Although much information has been published about chemotherapeutic agents in experimental studies in rabbits, few reports describe the use of chemotherapy in clinical cases of lymphoproliferative disease. As described above, a 9.5-year-old rabbit with cutaneous lymphoma was treated with recombinant human interferon alpha-2b at 1.5 million U/m2 administered subcutaneously three times weekly.65 After 2 months, no response was seen, and isotretinoin (4 mg/kg q24h on food) was added to the treatment for 2.5 weeks. No change was seen in the lesions and all treatments were discontinued; the rabbit died suddenly 1 year after diagnosis.
Anecdotal information is available on the use of chemotherapeutic agents in pet rabbits. The CVP/COP (cyclophosphamide, vincristine, prednisolone) protocol, with or without doxorubicin and l-asparaginase, has also been recommended.42 Because of the lack of information available on the benefits of chemotherapy in rabbits, the potential risks must be considered before treatment. In one study, rabbits that were intraperitoneally infected with spores of E. cuniculi were treated with cyclophosphamide (50 mg/kg first dose, then 15 mg/kg weekly during the 12-week experimental period).22 In these rabbits, clinical signs of encephalitozoonosis developed between weeks 4 and 6, and all died during week 6. No signs of infection were seen in control rabbits. These results indicate that immunosuppression induced by cyclophosphamide gave rise to lethal encephalitozoonosis. Therefore consider serologic testing to determine if a rabbit is seropositive for E. cuniculi before beginning treatment with immunosuppressive drugs (see below).
Other side effects of chemotherapy can include severe anemia, enteritis, typhlitis, and nephrotoxicity. In an experimental study of rabbits given daunorubicin or doxorubicin at 3 mg/kg weekly for 10 weeks, cardiotoxicity was documented with daunorubicin but not with doxorubicin.27 Both drugs produced hemotoxicosis manifesting as aplastic anemia. Rabbits treated with doxorubicin exhibited more weight loss and had higher mortality rates than those treated with daunorubicin. A single dose of l-asparaginase at 10,000 IU/kg when given intravenously can induce a hyperinsulinemic, insulin-resistant, diabetic syndrome in rabbits.30 However, in small animals, l-asparaginase is currently given intramuscularly or subcutaneously; therefore route of administration may be a factor in toxicity. The neurotoxic effects of vincristine have been well studied in rabbits.11., 40., 41.
Rabbits with thymomas have been treated with immunosuppressive therapy.1., 39. Prednisone (0.5-2.0 mg/kg q12h) has been used successfully as adjuvant therapy in rabbits with thymoma undergoing radiation therapy.39 Prednisone is used both for its antineoplastic and anti-inflammatory effects, potentially helping to ameliorate the side effects of radiating thoracic structures, in particular radiation pneumonitis.39 However, in one clinical case of a rabbit treated with radiation therapy and prednisone (0.5 mg/kg q12h for 28 days), although the tumor regressed, the rabbit was euthanized 3 months after diagnosis because of lethargy and severe pleural edema. On histologic examination, the mediastinum was markedly fibrotic, with sterile granulomatous inflammation and thrombi found in mediastinal vessels. Chronic active pyelonephritis was also found, with numerous E. cuniculi organisms in renal tubular cells and lumens. In another clinical case of thymoma, the rabbit was treated with a low dose of prednisone (0.5 mg/kg q24h), with no other therapy. After 1 month, the tumor had not changed in size. The rabbit was euthanized 5 months later because of labored breathing and poor clinical condition.
In one rabbit treated with radiation therapy and cyclophosphamide, severe bilateral renal fibrosis was found at necropsy, with death attributed to renal failure. The renal changes were attributed to repeated cyclophosphamide administration.1
Treatment Options for Thymomas
Radiation
Radiation therapy (RT) appears to be a good treatment option in rabbits with thymomas. In a recent study of 19 rabbits with thymomas that were treated with radiation, median overall survival was 313 days; when 3 rabbits that died acutely during the first 14 days of treatment were excluded, median survival was 727 days.1 Total radiation dose ranged from 28.8 to 48 Gy in rabbits receiving definitive fractionated protocols (≤4 Gy delivered three or more times weekly) and 24 to 32 Gy in rabbits receiving coarsely fractionated protocols (defined as any other RT protocol). Complications associated with radiation were uncommon and included radiation-induced myocardial failure, radiation pneumonitis, and alopecia. Tumors may be considerably smaller after only a few treatments (see Chapter 35, Fig. 35-9). Rabbits have been used in research studies regarding effects of irradiation on soft tissues.7., 9. Risk factors to consider with radiation therapy include the number of anesthetic episodes and radiation-associated side effects, including late-term side effects. Cost is also a factor, with definitive radiation therapy being the most expensive because of the number of treatments necessary.
Surgical Excision
Surgery is the treatment of choice for thymoma in all species when there is a solitary mass, as it provides the best chance of a cure by removal of the entire tumor (Fig. 20-5, B). Surgical excision of thymomas is done successfully in rabbits, but the risk of surgical or anesthetic-related complications and death is high. In three rabbits treated with surgery alone, postoperative survival ranged from 8 months to 3 years.1 Two rabbits treated with radiation therapy were also treated with surgical cytoreduction after completion of radiation.1 In a series of rabbits with mediastinal masses treated surgically by median sternotomy and mass excision, 7 of 14 rabbits survived 6 months or longer. One rabbit died during surgery, 6 died within 10 days after surgery, 1 survived 6 to 12 months, 4 survived 12 to 24 months, and 2 survived more than 24 months (F. Harcourt-Brown, personal communication, 2010). In a clinical report, a right fourth intercostal thoracotomy was performed in one rabbit with thymoma and the mass was excised. A chest drain was placed, but pneumothorax persisted after surgery and the rabbit was euthanized.60 In another rabbit, the mass was removed by median sternotomy.4 A chest drain was kept in place for 24 hours, after which it was removed and the rabbit recovered uneventfully. When the rabbit was euthanized 9 months later because of recurrent appendicular neurofibrosarcoma, no evidence of thymoma recurrence was present. In a clinical case seen by one of the present authors (AP), the mass was excised successfully but the patient went into cardiac arrest and died approximately 28 hours after surgery.
Tumor-related cardiac dysfunction, anesthesia-related perfusion abnormalities, stress, and analgesia are important considerations in these cases. When a rabbit is positioned in dorsal recumbency for surgery, the mass may impinge on normal lung tissue and decrease ventilation and perfusion. Therefore intubation and a short anesthesia time are extremely important.
Therapeutic Aspiration of Cystic Thymomas
Thymomas characteristically are cystic. In rabbits with thymomas that have large cystic components, removal of cystic fluid may help in relieving clinical signs of dyspnea and improving quality of life. In four clinical cases seen by one of the authors (KQ), periodic aspiration of the fluid by ultrasound-guided aspirate was done at varying intervals over 8 to 12 months. In three rabbits, the owners declined surgery or radiation therapy. Serial ultrasound-guided aspiration of cystic fluid was the only treatment done, and all rabbits improved clinically immediately after each aspiration. The procedure was repeated at varying intervals, usually at 3 to 6 months, as clinical signs of dyspnea or tachypnea recurred. In one rabbit treated with radiation therapy, the rabbit continued to develop large amounts of cystic fluid after treatment. Cystic fluid was aspirated periodically over a 12-month period to provide symptomatic relief. The rabbit subsequently died during surgery to excise the remaining thymoma.
Footnotes
References
- 1.Andres K., Kent M., Seidlecki C. The use of megavoltage radiation therapy in the treatment of thymomas in rabbits: 19 cases. Vet Comp Oncol. 2011 doi: 10.1111/j.1476-5829.2011.00273.x. In press. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter J.W., Mashima T.Y., Rupiper D.J. Exotic animal formulary. 2nd ed. WB Saunders; Philadelphia: 2001. [Google Scholar]
- 3.Cheeke P.R. Nutrition and nutritional diseases. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The biology of the laboratory rabbit. 2nd ed. Academic Press; San Diego: 1994. pp. 321–333. [Google Scholar]
- 4.Clippinger T.L., Bennett R.A., Alleman A.R. Removal of a thymoma via median sternotomy in a rabbit with recurrent appendicular neurofibrosarcoma. J Am Vet Med Assoc. 1998;213(1131):1140–1143. [PubMed] [Google Scholar]
- 5.Cloyd G.G., Johnson G.R. Lymphosarcoma with lymphoblastic leukemia in a New Zealand white rabbit. Lab Anim Sci. 1978;28:66–69. [PubMed] [Google Scholar]
- 6.Cruise L.J., Brewer N.R. Anatomy. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The biology of the laboratory rabbit. 2nd ed. Academic Press; San Diego: 1994. pp. 47–51. [Google Scholar]
- 7.Danielsson M., Engfeldt B., Larsson B. Effects of therapeutic proton doses on healthy organs in the neck, chest, and upper abdomen of the rabbit. Acta Radiol Ther Phys Biol. 1971;10:215–224. doi: 10.3109/02841867109129758. [DOI] [PubMed] [Google Scholar]
- 8.DiGiacoma R.F., Maré C.J. Viral diseases. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The biology of the laboratory rabbit. 2nd ed. Academic Press; San Diego: 1994. pp. 171–204. [Google Scholar]
- 9.Engfeldt B., Larsson B., Naeslund C. Effect of single dose or fractionated proton irradiation on pulmonary tissue and Vx2 carcinoma in lung of rabbit. Acta Radiol Ther Phys Biol. 1971;10:298–310. doi: 10.3109/02841867109130794. [DOI] [PubMed] [Google Scholar]
- 10.Finnie J.W., Bostock D.E., Walden N.B. Lymphoblastic leukaemia in a rabbit: a case report. Lab Anim. 1980;14:49–51. doi: 10.1258/002367780780943169. [DOI] [PubMed] [Google Scholar]
- 11.Fiori M.G., Schiavinato A., Lini E. Peripheral neuropathy induced by intravenous administration of vincristine sulfate in the rabbit. An ultrastructural study. Toxicol Pathol. 1995;23:248–255. doi: 10.1177/019262339502300302. [DOI] [PubMed] [Google Scholar]
- 12.Florizoone K. Thymoma-associated exfoliative dermatitis in a rabbit. Vet Dermatol. 2005;16:281–284. doi: 10.1111/j.1365-3164.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 13.Fontes-Sousa A.P., Moura C., Carneiro C.S. Echocardiographic evaluation including tissue Doppler imaging in New Zealand white rabbits sedated with ketamine and midazolam. Vet J. 2009;181:326–331. doi: 10.1016/j.tvjl.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Fox R.R., Meier H., Crary D.D. Lymphosarcoma in the rabbit: genetics and pathology. J Nat Cancer Inst. 1970;45:719–729. [PubMed] [Google Scholar]
- 15.Fox R.R., Norberg R.F., Meier H. Clinical hematological progression of hereditary lymphosarcoma in rabbits. J Hered. 1976;67:376–380. doi: 10.1093/oxfordjournals.jhered.a108756. [DOI] [PubMed] [Google Scholar]
- 16.Gómez L., Gázquez A., Roncero V. Lymphoma in a rabbit: histopathological and immunohistochemical findings. J Small Anim Pract. 2002;43:224–226. doi: 10.1111/j.1748-5827.2002.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 17.Greene H.S.N., Strauss J.S. Multiple primary tumors in the rabbit. Cancer. 1949;2:673–691. doi: 10.1002/1097-0142(194907)2:4<673::aid-cncr2820020414>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Gupta B.N. Lymphosarcoma in a rabbit. Am J Vet Res. 1976;37:841–843. [PubMed] [Google Scholar]
- 19.Hayden D.W. Generalized lymphosarcoma in a juvenile rabbit: a case report. Cornell Vet. 1970;60:73–82. [PubMed] [Google Scholar]
- 20.Heath D., Williams D., Rios-Dalenz J. Pulmonary vascular disease in a rabbit at high altitude. Int J Biometeorol. 1990;34:20–23. doi: 10.1007/BF01045815. [DOI] [PubMed] [Google Scholar]
- 21.Hinton H., Regan M. Cutaneous lymphoma in a rabbit. Vet Rec. 1978;103:140–141. doi: 10.1136/vr.103.7.140. [DOI] [PubMed] [Google Scholar]
- 22.Horváth M., Leng L., Stefkovic M. Lethal encephalitozoonosis in cyclophosphamide-treated rabbits. Acta Vet Hung. 1999;47:85–93. doi: 10.1556/AVet.47.1999.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Hurley R.J., Marini R.P., Avison D.L. Evaluation of detomidine anesthetic combinations in the rabbit. Lab Anim Sci. 1994;44:472–478. [PubMed] [Google Scholar]
- 24.Ishikawa M., Maeda H., Kondo H. A case of lymphoma developing in the rabbit cecum. J Vet Med Sci. 2007;69:1183–1185. doi: 10.1292/jvms.69.1183. [DOI] [PubMed] [Google Scholar]
- 25.James T.N. Anatomy of the cardiac conduction system in the rabbit. Circ Res. 1967;20:638–648. doi: 10.1161/01.res.20.6.638. [DOI] [PubMed] [Google Scholar]
- 26.Jassles-van der Lee A., van Zeeland Y., Kik M. Successful treatment of sebaceous adenitis in a rabbit with ciclsproin and triglycerides. Vet Dermatol. 2009;20:67–71. doi: 10.1111/j.1365-3164.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 27.Klimtová I., Simunek T., Mazurová Y. Comparative study of chronic toxic effects of daunorubicin and doxorubicin in rabbits. Hum Exp Toxicol. 2002;21:649–657. doi: 10.1191/0960327102ht311oa. [DOI] [PubMed] [Google Scholar]
- 28.Kostolich M., Panciera R.J. Thymoma in a domestic rabbit. Cornell Vet. 1992;82:125–129. [PubMed] [Google Scholar]
- 29.Kozma C., Macklin W., Cummins L.M. Anatomy. In: Weisbroth S.H., Flatt R.E., Krause S.E., editors. The biology of the laboratory rabbit. Academic Press; New York: 1974. pp. 50–72. [Google Scholar]
- 30.Lavine R.L., Dicintio D.M. l-Asparaginase-induced diabetes mellitus in rabbits. Diabetes. 1980;29:528–531. doi: 10.2337/diab.29.7.528. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Murphy J.C., Lipman N.S. Eisenmenger’s syndrome in a New Zealand white rabbit. Lab Anim Sci. 1995;45:618–620. [PubMed] [Google Scholar]
- 32.Lindsey J.R., Fox R.R. Inherited diseases and variations. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The biology of the laboratory rabbit. 2nd ed. Academic Press; San Diego: 1994. pp. 293–319. [Google Scholar]
- 33.Loliger H. Ueber das vorkommen von leukosen beim kaninchen. Berlin u Munchen Tierarztl Wchnschr. 1966;79:192. [PubMed] [Google Scholar]
- 34.Lord B., Boswood A., Petrie A. Electrocardiography of the normal domestic pet rabbit. Vet Rec. 2010;167:961–965. doi: 10.1136/vr.c3212. [DOI] [PubMed] [Google Scholar]
- 35.Lord B., Devine C., Smith S. Congestive heart failure in two pet rabbits. J Small Anim Prac. 2011;52:46–50. doi: 10.1111/j.1748-5827.2010.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marini R.P., Li X., Harpster N.K. Cardiovascular pathology possibly associated with ketamine/xylazine anesthesia in Dutch belted rabbits. Lab Anim Sci. 1999;49:153–160. [PubMed] [Google Scholar]
- 37.Meier H., Fox R.R., Crary D.D. Myeloid leukemia in the rabbit (Oryctolagus cuniculus) Cancer Res. 1972;32:1785–1787. [PubMed] [Google Scholar]
- 38.Meier H., Fox R.R. Hereditary lymphosarcoma in WH rabbits and hereditary hemolytic anemia associated with thymoma in strain X rabbits. Bibl Haematol. 1973;39:72–92. doi: 10.1159/000427803. [DOI] [PubMed] [Google Scholar]
- 39.Morrisey J.K., McEntee M. Therapeutic options for thymoma in the rabbit. Sem Avian Exot Pet Med. 2005;14:175–181. [Google Scholar]
- 40.Muzylak M., Maslinska D. Neurotoxic effect of vincristine on ultrastructure of hypothalamus in rabbits. Folia Histochem Cytobiol. 1992;30:113–117. [PubMed] [Google Scholar]
- 41.Ogawa T., Mimura Y., Kato H. The usefulness of rabbits as an animal model for the neuropathological assessment of neurotoxicity following the administration of vincristine. Neurotoxicology. 2000;21:501–511. [PubMed] [Google Scholar]
- 42.Ogilvie G., Bennett A., Bergman P. Cutaneous lymphoma in rabbits. http://www.vin.com/Members/SearchDB/Boards/B0047500/B0046942.htm Retrieved December 30, 2009, from.
- 43.Olson M.E., Vizzutti D., Morck D.W. The parasympathetic effects of atropine sulfate and glycopyrrolate in rats and rabbits. Can J Vet Res. 1994;58:254–258. [PMC free article] [PubMed] [Google Scholar]
- 44.Orcutt C.J. Cardiac and respiratory disease in rabbits. Autumn Meet Brit Vet Zoo Soc. 2000:68–73. Proceedings. [Google Scholar]
- 45.Pariaut R. Cardiovascular physiology and diseases of the rabbit. Vet Clin North Am Exot Anim Pract. 2009;12:135–144. doi: 10.1016/j.cvex.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Pilny A.A., Reavill D.R. Chylothorax and thymic lymphoma in a pet rabbit (Oryctolagus cuniculus) J Exot Pet Med. 2008;17:295–299. [Google Scholar]
- 47.Redrobe S. Imaging techniques in small mammals. Sem Avian Exot Pet Med. 2001;10:195. [Google Scholar]
- 48.Reed S.D., Shaw S., Evans D.E. Spinal lymphoma and pulmonary filariasis in a pet domestic rabbit (Oryctolagus cuniculus domesticus) J Vet Diag Invest. 2009;21:253–256. doi: 10.1177/104063870902100215. [DOI] [PubMed] [Google Scholar]
- 49.Rossi M.A. Microvascular changes as a cause of chronic cardiomyopathy in Chagas’ disease. Am Heart J. 1990;120:233–236. doi: 10.1016/0002-8703(90)90191-y. [DOI] [PubMed] [Google Scholar]
- 50.Saku K., Fujino M., Yamamoto K. Cardiac function of WHHL rabbit, an animal model of familial hypercholesterolemia. Artery. 1990;17:271–280. [PubMed] [Google Scholar]
- 51.Shell L.G., Saunders G. Arteriosclerosis in a rabbit. J Am Vet Med Assoc. 1989;194:679–680. [PubMed] [Google Scholar]
- 52.Shibuya K., Tajima M., Kanai K. Spontaneous lymphoma in a Japanese white rabbit. J Vet Med Sci. 1999;61:1327–1329. doi: 10.1292/jvms.61.1327. [DOI] [PubMed] [Google Scholar]
- 53.Snyder S.B., Fox J.G., Campbell L.H. Disseminated staphylococcal disease in laboratory rabbits (Oryctolagus cuniculus) Lab Anim Sci. 1976;26:86–88. [PubMed] [Google Scholar]
- 54.Stypmann J., Engelen M.A., Breithardt A.K. Doppler echocardiography and tissue Doppler imaging in the healthy rabbit: differences of cardiac function during awake and anaesthetised examination. Int J Cardiol. 2007;115:164–170. doi: 10.1016/j.ijcard.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Toque H.A., Teixeira C.E., Priviero F.B. Varendafil, but not sildenafil or tadalafil, has calcium-channel blocking activity in rabbit isolated pulmonary artery and human washed platelets. Br J Pharmacol. 2008;154:787–796. doi: 10.1038/bjp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toth L.A., Olson G.A., Wilson E. Lymphocytic leukemia and lymphosarcoma in a rabbit. J Am Vet Med Assoc. 1990;197:627–629. [PubMed] [Google Scholar]
- 57.Ubertini T.R. Brief communication: etiological study of a lymphosarcoma in a domestic rabbit. J Natl Cancer Inst. 1972;48:1507–1511. [PubMed] [Google Scholar]
- 58.Van Kampen K.R. Lymphosarcoma in the rabbit. A case report and general review. Cornell Vet. 1968;58:121–128. [PubMed] [Google Scholar]
- 59.Van Vleet J.F., Ferrans V.J. Clinical and pathologic features of chronic adriamycin toxicosis in rabbits. Am J Vet Res. 1980;41:1462–1469. [PubMed] [Google Scholar]
- 60.Vernau K.M., Grahn B.H., Clarke-Scott H.A. Thymoma in a geriatric rabbit with hypercalcemia and periodic exophthalmos. J Am Vet Med Assoc. 1995;206:820–822. [PubMed] [Google Scholar]
- 61.Volopich S., Gruber A., Hassan J. Malignant B-cell lymphoma of the Harder’s gland in a rabbit. Vet Ophthalmol. 2005;8:259–263. doi: 10.1111/j.1463-5224.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- 62.von Bomhard W., Goldschmidt M.H., Shofer F.S. Cutaneous neoplasms in pet rabbits: a retrospective study. Vet Pathol. 2007;44:579–588. doi: 10.1354/vp.44-5-579. [DOI] [PubMed] [Google Scholar]
- 63.Wagner F., Beinecke A., Fehr M. Recurrent bilateral exophthalmos associated with metastatic thymic carcinoma in a pet rabbit. J Small Anim Pract. 2005;46:393–397. doi: 10.1111/j.1748-5827.2005.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 64.Weisbroth S.H. Neoplastic diseases. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The biology of the laboratory rabbit. 2nd ed. Academic Press; New York: 1994. pp. 259–292. [Google Scholar]
- 65.White S.D., Campbell T., Logan A. Lymphoma with cutaneous involvement in three domestic rabbits (Oryctolagus cuniculus) Vet Dermatol. 2000;11:61–67. doi: 10.1046/j.1365-3164.2000.00159.x. [DOI] [PubMed] [Google Scholar]


