INTRODUCTION
Since the 1980s, immunohistochemistry (IHC) has dramatically transformed the approach to histopathologic diagnosis, specifically in the diagnosis and classification of tumors, and more recently in the diagnosis of infectious diseases in tissue samples.1
Pathologists play an important role in recognizing infectious agents in tissue samples from patients, providing a rapid morphologic diagnosis, and facilitating clinical decisions in patient treatment. When fresh tissue is not available for culture, pathologists can provide a rapid morphologic diagnosis and facilitate clinical decisions in patient treatment.2 In addition, pathologists have played a central role in identifying emerging and reemerging infectious agents, describing the pathogenetic processes of emerging diseases (e.g., hantavirus pulmonary syndrome, viral hemorrhagic fevers, leptospirosis, and rickettsial and ehrlichial infections), and diagnosing anthrax during the bioterrorist attack of 2001.3., 4., 5., 6., 7.
Cultures and serologic assays are usually used for microbial identification in infectious diseases. However, fresh tissue is not always available, and culturing fastidious pathogens can be difficult and may take weeks or months to yield results. Moreover, culture alone cannot distinguish colonization from tissue invasion. In addition, serologic results can be difficult to interpret in the setting of immunosuppression or when only a single sample is available for evaluation. Some microorganisms have distinctive morphologic characteristics that allow their identification in formalin-fixed tissues using routine and special stains. Nevertheless, in many instances it is difficult or even impossible to identify an infectious agent specifically by conventional morphologic methods.
Immunohistochemistry is one of the most powerful techniques in surgical pathology. There has been an increasing interest in the use of specific antibodies to viral, bacterial, fungal, and parasitic antigens in the detection and identification of the causative agents in many infectious diseases. Coons and associates were the first to use a specific antibody to detect a microbial antigen to detect pneumococcal antigen in tissues.8 The advantages of IHC over conventional staining methods (Table 3.1 ) and the contributions of IHC in infectious diseases (Table 3.2 ) are substantial. In many instances, IHC has shown high specificity, allowing the differentiation of morphologically similar microorganisms.9 Immunohistochemistry is especially useful when microorganisms are difficult to identify by routine or special stains, are fastidious to grow, or exhibit atypical morphology (Table 3.3 ).10., 11., 12., 13., 14. It is important to understand that there may be widespread occurrence of common antigens among bacteria and pathogenic fungi, and both monoclonal and polyclonal antibodies must be tested for possible cross-reactivity with other organisms.15 Finally, it is important to emphasize that IHC has several steps, and that all of them can affect the final result; however, in general the only limitations are the availability of specific antibodies and the preservation of epitopes.16
TABLE 3.1.
Advantages of IHC for the Diagnosis of Infectious Diseases
|
|
|
|
|
TABLE 3.2.
Contributions of IHC to the Diagnosis of Infectious Diseases
|
|
|
|
|
TABLE 3.3.
Applications of IHC in the Diagnosis of Infectious Diseases
|
|
|
|
|
Table 3.4 lists some commercially available antibodies for diagnostic use in surgical pathology.
TABLE 3.4.
Commercially Available Antibodies for Immunohistochemical Diagnosis of Infectious and Prion Diseases
| Microorganism | Antibody/Clone | Dilution | Pretreatment | Source |
|---|---|---|---|---|
| Adenovirus | Mab/20/11 and 2/6 | 1:2000 | Proteinase K | Chemicon |
| B. henselae | Mab | 1:100 | HIAR | Biocare Medical |
| BK virus | Mab/BK T.1 | 1:8000 | Trypsin | Chemicon |
| C. albicans | Mab/1B12 | 1:400 | HIAR | Chemicon |
| C. pneumoniae | Mab/RR402 | 1:200 | HIAR | Accurate |
| Cryptosporidium | Mab/Mabc1 | 1:100 | HIAR | Novocastra |
| CMV | Mab/DDG9/CCH2 | 1:50 | HIAR | Novocastra |
| Clostridium spp. | Rabbit polyclonal | 1:1000 | None | Biodesign |
| G. intestinalis | Mab/9D5.3.1 | 1:50 | HIAR | Novocastra |
| Hepatitis B core antigen | Rabbit polyclonal | 1:2000 | HIAR | Dako |
| Hepatitis B surface antigen | Mab/3E7 | 1:100 | HIAR | Dako |
| Herpes simplex 1 and 2 viruses | Rabbit polyclonal | 1:3200 | HIAR | Dako |
| H. pylori | Rabbit polyclonal | 1:40 | Proteinase K | Dako |
| HHV 8 | Mab/LNA-1 | 1:500 | HIAR | Novocastra |
| K. pneumoniae | Rabbit polyclonal | 1:200 | Proteinase K | Biogenex |
| L. monocytogenes | Rabbit polyclonal | 1:5000 | Proteinase K | Difco |
| M. pneumoniae | Mab/1.B.432 | 1:25 | HIAR | US Biological |
| Parvovirus B19 | Mab/R92F6 | 1:500 | HIAR | Novocastra |
| P. carinii | Mab/3F6 | 1:20 | HIAR | Novocastra |
| P. falciparum | Mab/BDI400 | 1:1000 | Proteinase K | Biodesign |
| Prion | Mab/3F4 | 1:200 | Antigen retrieval | Dako |
| Mab/12F10 | 1:1000 | Proteinase K | Cayman Chemical | |
| Mab/KG9 | 1:1000 | Proteinase K | TSE Resource Center | |
| Respiratory syncytial virus | Mab/5H5N | 1:200 | HIAR | Novocastra |
| S. aureus | Rabbit polyclonal | 1:500 | Proteinase K | Biodesign |
| T. pallidum | Rabbit polyclonal | HIAR | Biodesign | |
| T. gondii | Rabbit polyclonal | 1:320 | HIAR | Biogenex |
| West Nile virus | Mab/5H10 | 1:400 | Proteinase K | Bioreliance |
VIRAL INFECTIONS
Immunohistochemistry has played an important role not only in the diagnosis of a large number of viral infections but also in the study of their pathogenesis and epidemiology. Conventionally, the diagnosis of viral infections has relied on cytopathic changes observed by routine histopathologic examination. Several viral pathogens produce characteristic intracellular inclusions, which allow pathologists to make a presumptive diagnosis of viral infection. However, for some viral infections the characteristic cytopathic changes are subtle and sparse, requiring a meticulous search.17 Moreover, only 50% of known viral diseases are associated with characteristic intracellular inclusions.18 In addition, formalin, which is the most commonly used fixative in histopathology, is a poor fixative for demonstrating the morphologic and tinctorial features of viral inclusions.19 When viral inclusions are not detected in hematoxylin and eosin–stained sections or when the viral inclusions present cannot be differentiated from those of other viral diseases, immunohistochemical techniques offer a more reliable approach to reach a specific diagnosis.
Hepatitis B
Hepatitis B virus infection constitutes an important cause of chronic hepatitis in a significant proportion of patients. In many instances, the morphologic changes induced by hepatitis B virus in hepatocytes are not typical enough to render a presumptive diagnosis of hepatitis B viral infection. In other instances, there may be so little hepatitis B surface antigen (HBsAg) that it cannot be demonstrated by techniques such as orcein staining. In these cases, immunohistochemical techniques to detect HBsAg are more sensitive than histochemical methods and are helpful in reaching a diagnosis.20 Immunostaining for HBsAg has been used in the diagnosis of hepatitis B and in the study of carrier states.21., 22. Eighty percent or more of cases with positive serologic results for HBsAg demonstrate cytoplasmic HBsAg using IHC.23 By immunoperoxidase localization, hepatitis B core antigen (HBcAg) can be demonstrated within the nuclei or the cytoplasm of hepatocytes, or both. Cytoplasmic expression of HBcAg usually is associated with a higher grade of hepatitis activity,23 and diffuse immunostaining of nuclei for HBcAg generally suggests uncontrolled viral replication in the setting of immunosuppression.24 Immunostaining for HBsAg and HBcAg is useful in the diagnosis of recurrent hepatitis B infection in liver allografts, particularly when present with atypical histopathologic features.25
Herpesviruses
Histologically, the diagnosis of herpes simplex virus (HSV) infection involves the detection of multinucleated giant cells containing characteristic molded, ground glass–appearing nuclei and Cowdry’s type A intranuclear inclusions. When abundant viral inclusions exist within infected cells, the diagnosis is usually straightforward. However, diagnosis can be difficult when the characteristic intranuclear inclusions or multinucleated cells, or both, are absent or when the amount of tissue in a biopsy specimen is small.26 In these cases, IHC using either polyclonal or monoclonal antibodies against HSV antigens has proven to be a sensitive and specific technique used to diagnose HSV infections (Fig. 3.1 ).27., 28., 29., 30.
FIGURE 3.1.
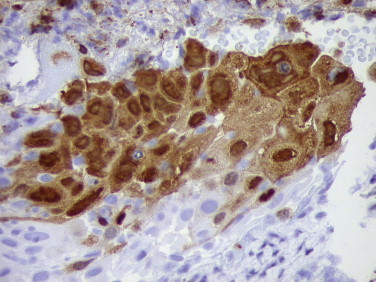
Photomicrograph of cervical biopsy from a patient with herpes simplex virus infection showing abundant nuclear and cytoplasmic antigen. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Although polyclonal antibodies against major HSV glycoprotein antigens are sensitive, they do not allow distinction between HSV-1 and HSV-2; this is because the two viruses are antigenically similar.31 In addition, the histologic features of HSV infection are not specific and can also occur in patients with varicella-zoster (VZV) infection. Monoclonal antibodies against the VZV envelope glycoprotein gp1 are sufficiently sensitive and specific to allow a clear-cut distinction between HSV and VZV infections.27., 32., 33.
Immunohistochemistry has also been useful in demonstrating the association of human herpes virus 8 (HHV-8) with Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease.34., 35., 36., 37., 38. Diagnosis of Kaposi’s sarcoma may be problematic because of its broad morphologic spectrum and similar appearance to other benign and malignant neoplastic vascular lesions. Immunostaining of latent associated nuclear antigen-1 (LANA-1) is useful to confirm the diagnosis of Kaposi’s sarcoma, particularly when difficult early lesions closely resemble the appearance of interstitial granuloma annulare and when the neoplasm presents in an unusual location. Immunostaining also allows distinction of Kaposi’s sarcoma from several morphologically similar vasoproliferative lesions.39., 40., 41. Immunostaining is restricted to the nuclei of spindle cells and endothelial cells of the slitlike vascular spaces (Fig. 3.2 ). Immunohistochemistry has also demonstrated expression of HHV-8 LANA-1 in mesothelial cells of HIV-associated recurrent pleural effusions.42
FIGURE 3.2.
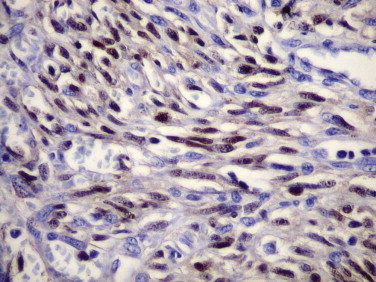
Lymph node biopsy from a patient with Kaposi’s sarcoma. The spindle cells show strong nuclear staining for HHV-8 LANA-1 antigen. Endothelial cells of well formed vascular spaces are negative. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Cytomegalovirus (CMV) continues to be an important opportunistic pathogen in immunocompromised patients; it is estimated that 30% of transplant recipients experience CMV disease.43 The range of organ involvement in post-transplant CMV disease is wide; hepatitis occurs in 40% of liver transplant recipients,44 and pneumonitis is more frequently seen in heart and heart-lung transplant patients.45 Other organs that are commonly affected are the gastrointestinal tract and the peripheral and central nervous systems. Histologic diagnosis of CMV in fixed tissues usually rests on identifying characteristic cytopathic effects including intranuclear inclusions, cytoplasmic inclusions, or both. However, histologic examination lacks sensitivity, and in some cases atypical cytopathic features can be confused with reactive or degenerative changes.46 Additionally, up to 38% of patients with gastrointestinal CMV disease fail to demonstrate any inclusions.47 In these cases, IHC using monoclonal antibodies against early and late CMV antigens allows the detection of CMV antigens in the nucleus and cytoplasm of infected cells (Fig. 3.3 ). The sensitivity of IHC for detecting CMV infection ranges from 78% to 93%.47., 48. In addition, IHC may allow detection of CMV antigens early in the course of the disease when cytopathic changes have not yet developed.49., 50., 51., 52., 53., 54. For example, CMV early nuclear antigen is expressed 9 to 96 hours after cellular infection and indicates early active viral replication. Immunohistochemistry has been used to detect CMV infection in patients with steroid refractory ulcerative colitis, and the routine use of IHC for the detection of CMV in the evaluation of these patients is now recommended.55., 56. CMV immunostaining has been used to detect occult CMV infection of the central nervous system in liver transplant patients who develop neurologic complications.57 It has also been used to demonstrate a high frequency of CMV antigens in tissues from first-trimester abortions.58 CMV is the most common opportunistic organism found in liver biopsies from transplant patients; nonetheless, the incidence of CMV hepatitis appears to be decreasing owing to better prophylactic treatments.59 Although CMV hepatitis presents with characteristic neutrophilic aggregates within the liver parenchyma, atypical features suggestive of acute rejection or changes indistinguishable from those of any other viral hepatitis are occasionally observed.60 In addition, parenchymal neutrophilic microabscesses have been described in cases with no evidence of CMV infection.61 In these cases, immunostaining for CMV antigens is most useful in determining the diagnosis of CMV infection.62
FIGURE 3.3.
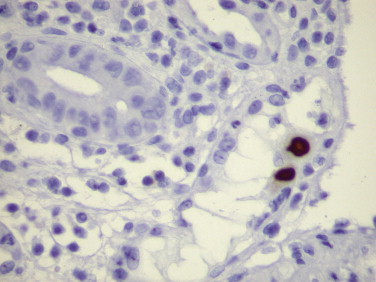
Colon biopsy of a patient with steroid-refractory ulcerative colitis. Rare epithelial cells show intranuclear CMV antigen. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
The sensitivity of IHC is better than light microscopic identification of viral inclusions and compares favorably with culture and in situ hybridization.49., 51., 52., 54., 63. Additionally, immunohistochemical assays can be completed faster than the shell vial culture technique, allowing for rapid results that are important for early anti-CMV therapy.54
Other herpesvirus infections that have been diagnosed using immunohistochemical methods include human herpesvirus 6 infection64 and Epstein-Barr viral infection.65 Immunohistochemistry has been used to identify EBV latent membrane protein-1 in cases of Hodgkin’s lymphoma and post-transplant lymphoproliferative disorder (Fig. 3.4 ).66
FIGURE 3.4.
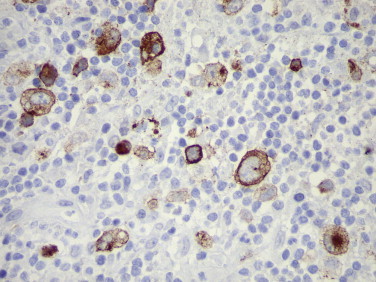
Epstein-Barr virus LMP-1 within cytoplasm of characteristic Reed-Sternberg cells in a case of Hodgkin’s lymphoma. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Adenoviruses
Adenovirus has been increasingly recognized as a cause of morbidity and mortality among immunocompromised patients owing to transplant and congenital immunodeficiency.67., 68. Rarely adenovirus infection has been described in HIV-infected patients.69., 70., 71. Characteristic adenovirus inclusions are amphophilic, intranuclear, homogeneous, and glassy. However, in some cases, the infection may contain only rare cells showing the characteristic cytopathic effect.70 In addition, other viral inclusions, including CMV, human papillomavirus (HPV), HSV, and VZV, can be mistaken for adenovirus inclusions and vice versa. In these circumstances, immunohistochemical assay may be necessary for a definitive diagnosis. A monoclonal antibody that is reactive with all 41 serotypes of adenovirus has been used in an immunohistochemical technique to demonstrate intranuclear adenoviral antigen in immunocompromised patients (Fig. 3.5 ).70., 71., 72., 73., 74. Histologic diagnosis of adenovirus colitis is difficult, and it is usually underdiagnosed. Moreover, in immunosuppressed patients, the incidence of coinfection with other viruses is high, and the presence of adenovirus tends to be overlooked. Immunohistochemical staining has been of value in differentiating adenovirus colitis from CMV colitis.70., 75.
FIGURE 3.5.
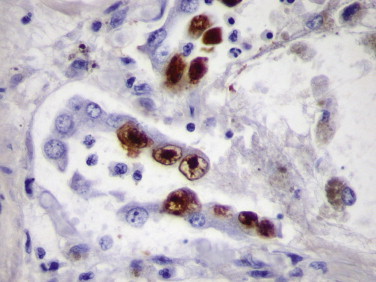
Adenovirus pneumonia in a heart transplant patient who developed ARDS and respiratory failure. Infected cells within necrotizing exudate show intranuclear reactivity with antibody to adenovirus antigen. Some cells show inclusions with a clear halo around them, making a differential diagnosis from CMV difficult on H&E stain. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Parvovirus B19 Infection
Parvovirus B19 has been associated with asymptomatic infections, erythema infectiosum, acute arthropathy, aplastic crisis, hydrops fetalis, and chronic anemia and red cell aplasia. In addition, parvovirus B19 infection has been recognized as an important cause of severe anemia in immunocompromised leukemic patients receiving chemotherapy.76
The diagnosis of parvovirus infection can be achieved by identifying typical findings in bone marrow specimens, including decreased or absent red cell precursors, giant pronormoblasts, and eosinophilic or amphophilic intranuclear inclusions in erythroid cells.77., 78. Because intravenous immunoglobulin therapy is effective, a rapid and accurate diagnostic method is important. Immunohistochemistry with a monoclonal antibody against VP1 and VP2 capsid proteins has been used as a rapid and sensitive method to establish the diagnosis of parvovirus B19 infection in formalin-fixed, paraffin-embedded tissues.79., 80., 81., 82. Immunohistochemistry is of particular help in detecting parvovirus B19 antigen in cases with sparse inclusions, to study cases not initially identified by examination of routinely stained tissue sections, or in cases of hydrops fetalis with advanced cytolysis (Fig. 3.6 ).79., 83., 84. Several studies have found a strong correlation among results obtained from morphologic, immunohistochemical, in situ hybridization (ISH), and polymerase chain reaction (PCR) methods.78., 79., 82., 84.
FIGURE 3.6.
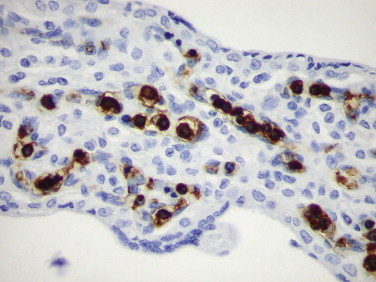
Hydrops fetalis caused by parvovirus B19 infection. Normoblasts within the villous capillaries show intranuclear viral antigen. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Viral Hemorrhagic Fevers
Since the 1980s, numerous emerging and reemerging agents of viral hemorrhagic fevers have attracted the attention of pathologists.3., 4., 5. Investigators have played an important role in identifying these agents and in supporting epidemiologic, clinical, and pathogenetic studies of emerging viral hemorrhagic fevers.4., 5., 7. Viral hemorrhagic fevers are often fatal. They are clinically difficult to diagnose (in the absence of bleeding or organ manifestations) and frequently require handling and testing of potentially dangerous biological specimens. In addition, histopathologic features are not pathognomonic, and they can resemble other viral, rickettsial, and bacterial (e.g., leptospirosis) infections. Immunohistochemistry is essential and has been successfully and safely applied to the diagnosis and study of the pathogenesis of these diseases.
Several studies have established the utility of IHC as a sensitive, safe, and rapid diagnostic method for the diagnosis of viral hemorrhagic fevers such as yellow fever (Fig. 3.7 ),85., 86., 87. dengue hemorrhagic fever,87., 88. Crimean-Congo hemorrhagic fever,89 Argentine hemorrhagic fever,90 Venezuelan hemorrhagic fever,91 and Marburg disease.92 Additionally, a sensitive, specific, and safe immunostaining method has been developed to diagnose Ebola hemorrhagic fever in formalin-fixed skin biopsies (Fig. 3.8 ).93 Immunohistochemistry demonstrated that Lassa virus targets primarily endothelial cells, mononuclear inflammatory cells, and hepatocytes (Fig. 3.9 ).93., 94., 95.
FIGURE 3.7.
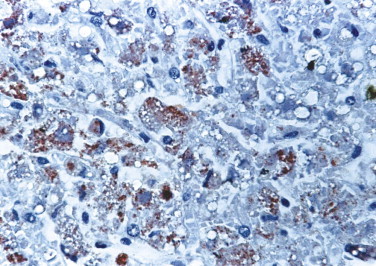
Yellow fever. Abundant yellow fever viral antigens are seen within hepatocytes and Kupffer cells. (Immunoperoxidase staining with AEC and hematoxylin counterstain; ×400.)
FIGURE 3.8.
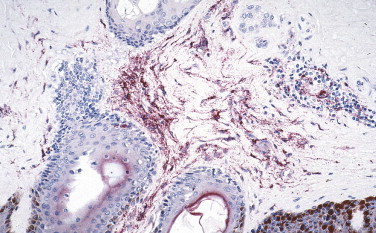
Extensive Ebola viral antigens are seen primarily within fibroblasts in the dermis of a skin specimen from a fatal case of Ebola hemorrhagic fever. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×20.)
FIGURE 3.9.
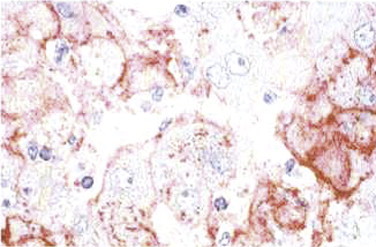
Lassa fever. Liver from a patient with Lassa fever. Scattered hepatocytes and reticuloendothelial cells show reactivity with monoclonal antibody to Lassa virus. (Naphthol fast red substrate and hematoxylin counterstain; original magnification ×100.)
Polyomaviruses
BK virus infections are frequent during infancy; in immunocompetent individuals the virus remains latent in the kidneys, central nervous system, and B lymphocytes. In immunocompromised patients, the infection reactivates and spreads to other organs. BK virus nephropathy is an important cause of graft failure in patients with a renal transplant,96 with a prevalence varying from 2% to 4.5% in different transplant centers.96., 97. Since specific clinical signs and symptoms are lacking in BK virus nephropathy, the diagnosis can only be made histologically in a graft biopsy.98 In the kidney, the infection is associated with mononuclear interstitial inflammatory infiltrates and tubular atrophy, findings that can be difficult to distinguish from acute rejection.98 The cytopathic changes observed in BK virus infection are not pathognomonic and can be observed in other viral infections. Moreover, in early BK virus infection there are minimal or no histologic changes, although IHC can identify viral antigen.99., 100. In this setting, IHC with an antibody against the large T antigen of SV40 virus has been effective in demonstrating BK virus infection (Fig. 3.10 ).96., 99., 101., 102., 103.
FIGURE 3.10.
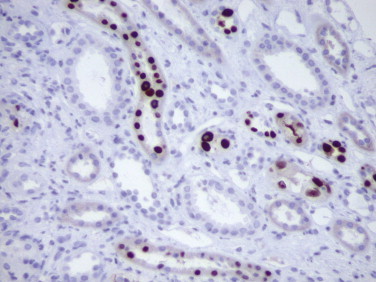
Immunohistochemical detection of SV40-T antigen in the nuclei of tubular cells in a renal transplant patient with BK virus–associated nephropathy. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
The human polyomavirus JC is a double-stranded DNA virus that causes progressive multifocal leukoencephalopathy (PML). This fatal demyelinating disease is characterized by cytopathic changes in oligodendrocytes and bizarre giant astrocytes. In addition to detection by antibodies to SV40-T antigen, IHC using a polyclonal rabbit antiserum against the protein VP1 is a specific, sensitive, and rapid method used to confirm the diagnosis of PML.104., 105., 106., 107. JC virus antigen is usually seen within oligodendrocytes (Fig. 3.11 ) and occasional astrocytes, and antigen-bearing cells are more commonly seen in early lesions.
FIGURE 3.11.
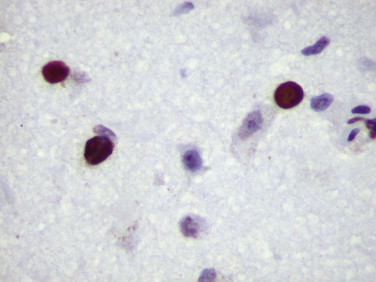
Progressive multifocal leukoencephalopathy: SV40-T antigen in the nuclei of enlarged oligodendrocytes in a patient with JC virus infection. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Other Viral Infections
Immunohistochemistry has also been used to confirm the diagnosis of respiratory viral diseases such as influenza A virus and respiratory syncytial virus infections (Fig. 3.12 ) when cultures were not available.108., 109., 110., 111.
FIGURE 3.12.
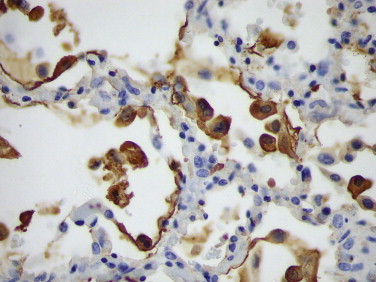
Immunostaining of RSV antigens in desquamated bronchial and alveolar lining cells using a monoclonal antibody. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
The diagnosis of rabies relies heavily on histopathologic examination of tissues to demonstrate its characteristic cytoplasmic inclusions (Negri bodies). In a significant percentage of cases, Negri bodies are inconspicuous and so few that confirming the diagnosis of rabies is extremely difficult.112 Furthermore, in non-endemic areas the diagnosis of rabies usually is not suspected clinically, or the patient may present with ascending paralysis. In these settings, immunohistochemical staining is a very sensitive, specific, and safe diagnostic tool for rabies (Fig. 3.13 ).112., 113., 114., 115., 116. Other viral agents that can be diagnosed using immunohistochemical methods include enteroviruses,117., 118., 119., 120. eastern equine encephalitis virus,121., 122., 123. and rotavirus.124., 125., 126.
FIGURE 3.13.
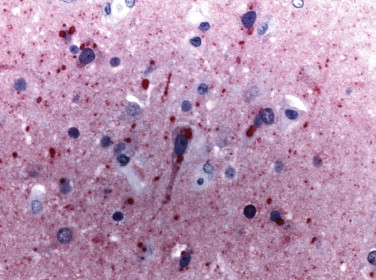
Immunostaining of rabies viral antigens in neurons of the CNS using a rabbit polyclonal antibody. Red precipitate corresponds to Negri inclusions by H&E staining. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×40.)
Immunohistochemical staining has been used in the histopathologic diagnosis of viral hepatitis C; however, IHC for this virus is not as effective as serologic assays and detection of HCV RNA in serum.
BACTERIAL INFECTIONS
Among bacterial infections, the greatest number of immunohistochemical studies has been performed in the investigation of Helicobacter pylori. A few studies have evaluated the use of IHC for other bacterial, mycobacterial, rickettsial, and spirochetal infections.
Antigen retrieval is generally not required for the immunohistochemical demonstration of bacteria in fixed tissue. However, interpreting the results can be complicated because many of these antibodies cross-react with other bacteria. Moreover, antibodies may react with only portions of the bacteria, and they may label remnants of bacteria or spirochetes when viable organisms are no longer present.
Helicobacter Pylori Infection
Gastric infection by H. pylori results in chronic active gastritis and is strongly associated with lymphoid hyperplasia, gastric lymphomas, and gastric adenocarcinoma. Heavy infections with numerous organisms are easily detected on routine hematoxylin and eosin–stained tissues; however, the detection rate is only 66% with many false-positive and false-negative results.127., 128. Conventional histochemical methods such as silver stains are more sensitive than hematoxylin and eosin in detecting H. pylori. Nonetheless, for detecting scant numbers of organisms, it has been proven that IHC has high specificity and sensitivity, is less expensive when all factors are considered, is superior to conventional histochemical methods, and has a low interobserver variation (Fig. 3.14 ).127 Treatment for chronic active gastritis and H. pylori infection can change the shape of the microorganism. This can make it difficult to identify and differentiate the organism from extracellular debris or mucin globules. In these cases IHC improves the rate of successful identification of the bacteria, even when histologic examination and cultures are falsely negative.129., 130., 131., 132.
FIGURE 3.14.
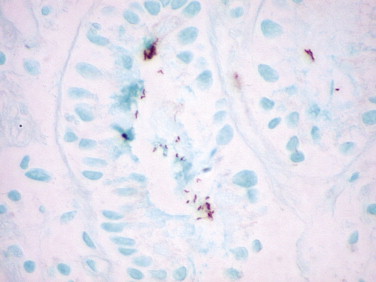
Numerous curved H. pylori in the superficial gastric mucus are clearly demonstrated by immunoperoxidase staining in this patient with chronic active gastritis. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Whipple’s Disease
Whipple’s disease affects primarily the small bowel and mesenteric lymph nodes and less commonly other organs such as the heart and central nervous system. Numerous foamy macrophages characterize the disease, and the diagnosis usually relies on the demonstration of PAS-positive intracytoplasmic bacteria. Nevertheless, the presence of PAS-positive macrophages is not pathognomonic; they can be observed in other diseases such as Mycobacterium avium infections, histoplasmosis, Rhodococcus equi infections, and macroglobulinemia. Tropheryma whipplei is a rare cause of endocarditis that shares many histologic features with other culture-negative endocarditides such as those caused by Coxiella burnetii and Bartonella sp.133 The development of specific antibodies against these microorganisms has significantly enhanced the ability to detect them in the heart valves of patients with culture-negative endocarditis.134 Immunohistochemical staining with rabbit polyclonal antibody provides a sensitive and specific method for the rapid diagnosis of intestinal and extraintestinal Whipple’s disease and for follow-up of treatment response.135., 136., 137.
Rocky Mountain Spotted Fever
Confirmation of Rocky Mountain spotted fever (RMSF) usually requires the use of serologic methods to detect antibodies to spotted fever group (SFG) Rickettsiae; however, most patients with RMSF lack diagnostic titers during the first week of disease. Immunohistochemistry has been successfully used to detect SFG Rickettsiae in formalin-fixed tissue sections, and it is superior to histochemical methods (Fig. 3.15 ).138., 139. Several studies illustrate the value of IHC in diagnosing suspected cases of RMSF using skin biopsies with high specificity and sensitivity, and in confirming fatal cases of seronegative RMSF.10., 140., 141., 142., 143., 144. R. rickettsii cannot be distinguished from other spotted fever group Rickettsiae such as R. parkeri or R. conorii because they cross-react.
FIGURE 3.15.
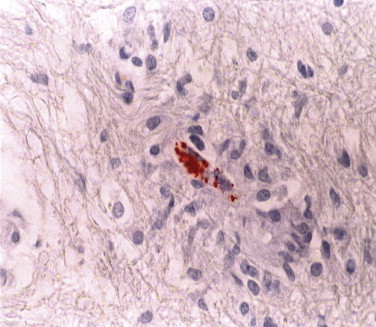
Immunohistologic demonstration of R. rickettsii within vascular endothelium in the pons of a patient with fatal Rocky Mountain spotted fever. (Immunoperoxidase staining with AEC and hematoxylin counterstain; ×600.)
Bartonella Infections
Bartonella are slow-growing, fastidious gram-negative, Warthin-Starry–stained bacteria associated with bacillary angiomatosis, peliosis hepatis, cat-scratch disease, trench fever, relapsing bacteremia, and disseminated granulomatous lesions of liver and spleen.145 Bartonella are important agents of blood culture–negative endocarditis. Traditional techniques such as histology, electron microscopy, and serology have been employed to identify the agents of culture-negative endocarditis. However, Bartonella sp., C. burnetii, and T. whipplei endocarditis share many morphologic features that do not allow for a specific histologic diagnosis.146 Besides, serologic tests for Bartonella sp. may show cross-reactivity with C. burnetii and Chlamydia sp.147 Immunostaining has been successfully used to identify Bartonella henselae and B. quintana in the heart valves of patients with blood culture–negative endocarditis and has significantly enhanced the ability to establish a specific diagnosis in these cases.148., 149. A polyclonal rabbit antibody that does not differentiate between B. henselae and B. quintana has also been used to detect these microorganisms in cat-scratch disease (Fig. 3.16 ), bacillary angiomatosis, and peliosis hepatis.150., 151. A commercially available monoclonal antibody specific for B. henselae is also available and has been used to demonstrate the organism in a case of spontaneous splenic rupture caused by this bacterium.152
FIGURE 3.16.
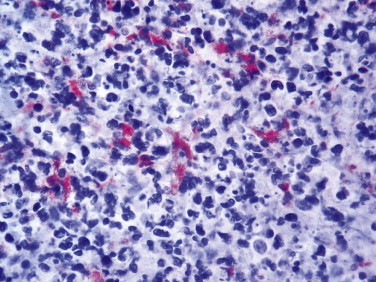
Photomicrograph of a lymph node biopsy from a patient with cat-scratch disease showing abundant extracellular, clumped coccobacilli of B. henselae in necrotic foci. (Immunoalkaline phosphatase with monoclonal antibody against B. henselae, naphthol fast red substrate and hematoxylin counterstain; ×200.)
Courtesy of Dr. Suimin Qiu, University of Texas Medical Branch.
Syphilis
Syphilis continues to be a public health problem caused by T. pallidum, a fastidious organism that has not been cultivated.153 The diagnosis of syphilis relies on serology and the identification of T. pallidum by dark-field microscopy. However, these methods have low sensitivity and specificity,154 and serologic methods can be negative in early stages of the disease and in immunosuppressed patients such as those coinfected with human immunodeficiency virus (HIV).155 In tissue sections, the usual method for detecting spirochetes is through silver impregnation stains (Warthin-Starry or Steiner). These stains, however, can be technically difficult to perform and interpret, are nonspecific, and frequently show marked background artifacts because silver stains also highlight melanin granules and reticulin fibers. Detection rates of spirochetes using silver stains vary from 33% to 71%.156 It has been shown that immunostaining of biopsy specimens with anti–T. pallidum polyclonal antibody (Fig. 3.17 ) is more sensitive and specific than silver staining methods, with sensitivities ranging from 71% to 94%.153., 156., 157.
FIGURE 3.17.
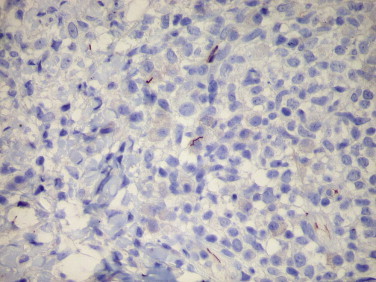
Syphilis. Skin biopsy from a patient with secondary syphilis. Scattered intact T. pallidum are easily visible with a rabbit polyclonal antibody against T. pallidum. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Mycobacterium Tuberculosis Infection
Identification of M. tuberculosis is routinely achieved by acid-fast bacilli (AFB) staining, culture of biopsy specimens, or both. Nevertheless, AFB staining has a low sensitivity, and it is not specific because it does not differentiate mycobacterial species.158 Furthermore, cultures may take several weeks, and sensitivity is low in paucibacillary lesions.159 In the histologic diagnosis of mycobacterial infections, IHC with anti-BCG polyclonal antibody has shown better sensitivity than AFB staining. However, in paucibacillary lesions it is inferior to AFB staining and cannot differentiate between M. tuberculosis and other mycobacteria.160 Recently a polyclonal antibody against the M. tuberculosis–secreted antigen MPT64 was used in cases of mycobacterial lymphadenitis. This method showed 90% sensitivity and 83% specificity and performed better than AFB staining in cases of paucibacillary disease and comparably to nested PCR.161
Other Bacterial Infections
Other bacterial diseases that can be identified by IHC in formalin-fixed tissue include leptospirosis, a zoonosis that usually presents as an acute febrile syndrome but occasionally can have unusual manifestations such as pulmonary hemorrhage with respiratory failure or abdominal pain.162., 163., 164. Rabbit polyclonal antibodies have been used in IHC to detect leptospiral antigens in the gallbladder and lungs from patients with unusual presentations (Fig. 3.18 ).162., 163., 164., 165.
FIGURE 3.18.
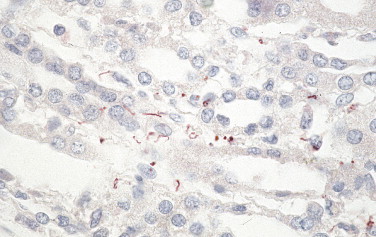
Leptospira. Immunostaining of intact leptospires and granular forms of leptospiral antigens in kidney of patient who died of pulmonary hemorrhage. (Immunoalkaline phosphatase with rabbit polyclonal antisera with naphthol fast red substrate and hematoxylin counterstain; original magnification ×63.)
Lyme disease has protean clinical manifestations, and Borrelia burgdoferi is difficult to culture from tissues and fluids. In addition, cultures are rarely positive before 2 to 4 weeks of incubation. Borrelia burgdorferi can be identified in tissues by immunostaining with polyclonal or monoclonal antibodies. Although IHC is more specific than silver impregnation staining, the sensitivity of immunostaining is poor, and the microorganisms are difficult to detect owing to the low numbers present in tissue sections.166., 167.
Q fever is a zoonosis caused by Coxiella burnetii and is characterized by protean and non-specific manifestations. Acute Q fever can manifest as atypical pneumonia or granulomatous hepatitis, frequently with characteristic fibrin ring granulomas. This microorganism is recognized as one agent that causes blood culture–negative chronic endocarditis.168 A monoclonal antibody has been used to specifically identify C. burnetii in cardiac valves of patients with chronic Q fever endocarditis.12., 169.
Recently IHC has been successfully used to identify Streptococcus pneumoniae in formalin-fixed organs with an overall sensitivity of 100% and a specificity of 71% when compared with cultures.170 Immunohistochemical assays are used to identify Clostridium sp., S. aureus, and S. pyogenes; 171., 172. Haemophilus influenzae; 173., 174., 175. Chlamydia species;176., 177., 178. Legionella pneumophila and L. dumoffii; 179., 180., 181. Listeria monocytogenes; 182., 183., 184. Salmonella; 185., 186. and rickettsial infections other than Rocky Mountain spotted fever such as boutonneuse fever, epidemic typhus, murine typhus,187 rickettsialpox,188., 189. African tick bite fever,138 and scrub typhus.190
FUNGAL INFECTIONS
The great majority of fungi are readily identified by hematoxylin and eosin staining alone or in combination with histochemical stains (e.g., periodic acid-Schiff [PAS], Gomori’s methenamine silver [GMS]). However, these stains cannot distinguish morphologically similar fungi with potential differences in susceptibility to antimycotic drugs. In addition, several factors may influence the appearance of fungal elements, which may appear atypical in tissue sections because of steric orientation, age of the fungal lesion, effects of antifungal chemotherapy, type of infected tissue, and host immune response.191 Currently the final identification of fungi relies on culture techniques; however, culture may take several days or longer to yield a definitive result, and surgical pathologists rarely have access to fresh tissue.
In past years, IHC has been used to identify various fungal elements in paraffin-embedded, formalin-fixed tissue.192., 193., 194. Immunohistochemical methods have the advantage of providing rapid and specific identification of several fungi and allowing pathologists to identify unusual filamentous hyphal and yeast infections and to accurately distinguish them from confounding artifacts.193., 195. In addition, IHC allows pathologists to correlate microbiological and histologic findings of fungal infections and to distinguish them from harmless colonization. Immunohistochemistry can also be helpful when more than one fungus is present; in these cases dual immunostaining techniques can highlight the different fungal species present in the tissue.196 An important limitation of IHC in the identification of fungi is the well-known, widespread occurrence of common antigens among pathogenic fungi that frequently results in cross-reactivity with polyclonal antibodies and even with some monoclonal antibodies.193., 195., 196., 197. Therefore, assessing cross-reactivity using a panel of fungi is a very important step in the evaluation of immunohistochemical methods.193., 194.
Candida species are often stained weakly with hematoxylin and eosin, and sometimes the yeast form may be difficult to differentiate from Histoplasma capsulatum, Cryptococcus neoformans, and even Pneumocystis carinii. Polyclonal and monoclonal antibodies against Candida genus antigens are sensitive and strongly reactive and do not show cross-reactivity with other fungi tested.193., 194., 198., 199. In particular, two monoclonal antibodies against Candida albicans mannoproteins show high sensitivity and specificity. Monoclonal antibody 3H8 recognizes primarily filamentous forms of C. albicans, whereas monoclonal antibody 1B12 highlights yeast forms.199., 200., 201., 202., 203.
Identification of Cryptococcus neoformans usually is not a problem when the fungus produces a mucicarmine-positive capsule. However, infections by capsule-negative strains are more difficult to diagnose, and the disease can be confused with histoplasmosis, blastomycosis, or torulopsis. Also, in long-standing infections the yeast often appear atypical and fragmented. Polyclonal antibodies raised against C. neoformans yeast cells are sensitive and specific.193., 194. More recently, monoclonal antibodies have been produced that allow identification and differentiation of varieties of C. neoformans in formalin-fixed tissue. The antibodies are highly sensitive (97%) and specific (100%) and can differentiate C. neoformans var. neoformans from C. neoformans var. gattii.204., 205.
Sporothrix schenckii may be confused in tissue sections with Blastomyces dermatitidis and fungal agents of phaeohyphomycosis. In addition, yeast cells of Sporothrix schenckii may be sparsely present in tissues. Antibodies against yeast cells of S. schenckii are sensitive but demonstrate cross-reactivity with Candida species; however, after specific adsorption of the antibody with Candida yeast cells, the cross-reactivity of the antibodies is eliminated.193., 194.
Invasive aspergillosis is a frequent cause of fungal infection with high morbidity and mortality rates in immunocompromised patients.206 The diagnosis is often difficult and relies heavily on histologic identification of invasive septate hyphae and culture confirmation. Nevertheless, several filamentous fungi such as Fusarium species, Pseudallescheria boydii, and Scedosporium species share similar morphology with Aspergillus species in hematoxylin and eosin–stained tissues.207 A precise and rapid diagnosis of invasive aspergillosis is important because early diagnosis is associated with improved clinical response, and it allows planning of the correct duration and choice of antimycotic therapy. Researchers have shown that the yield of cultures in histologically proven cases is low, ranging from 25% to 50%.206., 208., 209., 210., 211. Several polyclonal and monoclonal antibodies against Aspergillus antigens have been tested in formalin-fixed tissues with variable sensitivities, and most of them cross-react with other fungi.197., 212., 213. More recently, monoclonal antibodies (WF-AF-1, 164G, and 611F) against Aspergillus galactomannan have shown high sensitivity and specificity in identifying A. fumigatus, A. flavus, and A. niger in formalin-fixed tissues without cross-reactivity with other filamentous fungi.211., 214., 215.
Cysts and trophozoites of Pneumocystis jirovecii can be detected in bronchoalveolar lavage specimens using monoclonal antibodies that yield results that are slightly more sensitive than GMS, Giemsa, or Papanicolaou staining (Fig. 3.19 ).194., 216., 217. Antibodies are most helpful in cases of extrapulmonary pneumocystosis or in the diagnosis of P. jirovecii pneumonia when atypical pathologic features are present (e.g., hyaline membranes or granulomatous pneumocystosis where microorganisms are usually very sparse).
FIGURE 3.19.
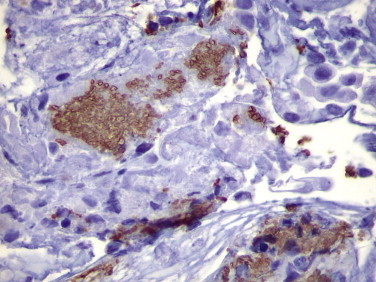
Immunodeficient patient with P. jirovecii pneumonia. Cohesive aggregates of cyst forms and trophozoites within alveolar spaces are demonstrated by a monoclonal antibody against pneumocystis with an immunoperoxidase technique. (Immunoperoxidase staining with DAB and hematoxylin counterstain; ×400.)
Penicillium marneffei can cause a disseminated infection in immunocompromised patients.218., 219. Morphologically the organisms must be differentiated from H. capsulatum, C. neoformans, and C. albicans. The monoclonal antibody EBA-1 against the galactomannan of Aspergillus species cross-reacts with and detects P. marneffei in tissue sections.209., 220. Immunohistochemistry has also been used to detect Blastomyces, Coccidioides, and Histoplasma.193., 194., 221. However, the antibodies have significant cross-reactivity with several other fungi.
PROTOZOAL INFECTIONS
Protozoa usually can be identified in tissue sections stained with hematoxylin and eosin or Giemsa stain; however, because of the small size of the organisms and the subtle distinguishing features, an unequivocal diagnosis cannot always be made. The role of IHC in the detection of protozoal infections has been particularly valuable in cases in which the morphology of the parasite is distorted by tissue necrosis or autolysis. In addition, in immunocompromised patients, toxoplasmosis can have an unusual disseminated presentation with numerous tachyzoites without bradyzoites (Fig. 3.20 ).222., 223. Immunohistochemistry has also been useful in cases with unusual presentation of the disease.224
FIGURE 3.20.
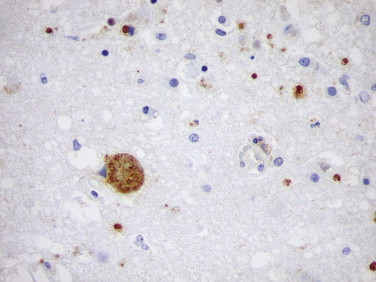
HIV-infected patient with toxoplasmic encephalitis. Immunoperoxidase staining highlights cyst forms and scattered tachyzoites. (DAB substrate with hematoxylin counterstain; ×400.)
The diagnosis of leishmaniasis in routine practice usually is not difficult; however, in certain circumstances pathologic diagnosis may be problematic, as is the case in chronic granulomatous leishmaniasis with small numbers of parasites, when the microorganism presents in unusual locations, or when necrosis distorts the morphologic appearance of the disease.225 In these cases, immunohistochemical staining has been a valuable diagnostic tool.225., 226., 227., 228. The highly sensitive and specific monoclonal antibody p19-11 recognizes different species of Leishmania and allows differentiation from morphologically similar microorganisms (Toxoplasma, Trypanosoma cruzi, and P. marneffei).225
Immunohistochemical assays using polyclonal antibodies specific for Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba sp. are used to demonstrate amebic trophozoites and cysts in areas of necrosis and can allow their differentiation from macrophages in cases of amebic meningoencephalitis.229
Immunohistochemistry has also been used to identify Cryptosporidium, 230 Entamoeba histolytica, 231 Trypanosoma cruzi, 232., 233., 234. babesia,235 Giardia lamblia, 236 Plasmodium falciparum, and P. vivax in fatal cases of malaria237 in formalin-fixed paraffin-embedded tissue samples.
EMERGING INFECTIOUS DISEASES
In 1992 the Institute of Medicine defined emerging infectious diseases (EIDs) as caused by new, previously unidentified microorganisms or those whose incidence in humans has increased within the previous two decades or threatens to increase in the near future.238 The list of pathogens newly recognized since 1973 is long and continues to increase. Recognizing emerging infections is a challenge, and many new infectious agents remain undetected for years before emerging as an identified public health problem.239 EIDs are a global phenomenon that requires a global response. The Centers for Disease Control and Prevention (CDC) has defined the strategy to prevent and detect EIDs.239 The anatomic pathology laboratory plays a critical role in the initial and rapid detection of EIDs.240., 241. Besides assisting in the identification of new infectious agents, IHC has contributed to the understanding of the pathogenesis and epidemiology of EIDs.
Hantavirus Pulmonary Syndrome
In 1993 in the southwestern United States, several previously healthy individuals died of rapidly progressive pulmonary edema, respiratory insufficiency, and shock.242., 243. Immunohistochemistry played a central role in identifying the viral antigens of an unknown hantavirus,244., 245. detecting the occurrence of unrecognized cases of hantavirus pulmonary syndrome prior to 1993, and showing the distribution of viral antigen in endothelial cells of the microcirculation, particularly in the lung (Fig. 3.21 ).244., 246.
FIGURE 3.21.
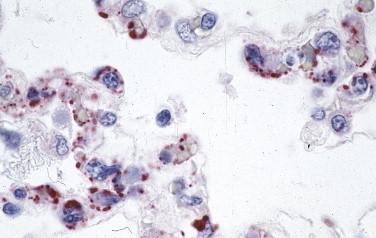
Hantavirus antigen–containing endothelial cells of the pulmonary microvasculature in the lung of an HPS patient as detected by immunohistochemistry using a mouse monoclonal antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×100.)
West Nile Virus Encephalitis
West Nile virus (WNV) was originally identified in Africa in 1937, and the first cases of WNV encephalitis in the United States were described in 1999. The clinical picture is variable and non-specific. It can range from subclinical infection to flaccid paralysis and encephalitis characterized morphologically by perivascular mononuclear cell inflammatory infiltrates, neuronal necrosis, edema, and microglial nodules, particularly prominent in the brainstem, cerebellum, and spinal cord.247., 248., 249., 250., 251. The diagnosis of WNV encephalitis is usually established by identifying virus-specific IgM in CSF and/or serum and by demonstrating viral RNA in serum, CSF, or other tissue.252 Immunostaining with monoclonal or polyclonal antibodies has been successfully used to diagnose WNV infection in immunocompromised patients with an inadequate antibody response (Fig. 3.22 ).248
FIGURE 3.22.
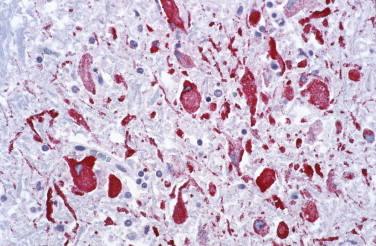
West Nile virus. Immunostaining of flaviviral antigens in neurons and neuronal processes in the central nervous system from an immunosuppressed patient who died of West Nile virus encephalitis. (Flavivirus-hyperimmune mouse ascitic fluid naphthol fast red substrate with hematoxylin counterstain; original magnification ×40.)
Enterovirus 71 Encephalomyelitis
Enterovirus 71 (EV71) has been associated with hand, foot, and mouth disease; herpangina; aseptic meningitis; and poliomyelitis-like flaccid paralysis. More recently EV71 has been associated with unusual cases of fulminant encephalitis, pulmonary edema and hemorrhage, and heart failure.253., 254. Severe and extensive encephalomyelitis of the cerebral cortex, brainstem, and spinal cord has been described. Immunohistochemical staining with monoclonal antibody against EV71 has played a pivotal role in the linking of EV71 infection to fulminant encephalitis (Fig. 3.23 ). Viral antigen is observed within neurons, neuronal processes, and mononuclear inflammatory cells.255., 256., 257.
FIGURE 3.23.
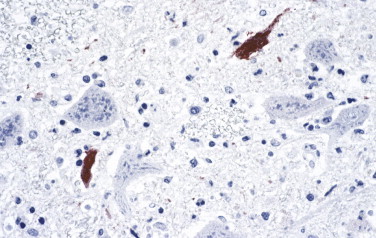
Enterovirus 71. Positive staining of EV71 viral antigens in neurons and neuronal processes of a fatal case of enterovirus encephalitis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×40.)
Nipah Virus Infection
Nipah virus is a recently described paramyxovirus that causes an acute febrile encephalitic syndrome with a high mortality rate.258., 259., 260. Pathology played a key role in identifying the causative agent. Histopathologic findings include vasculitis with thrombosis, microinfarctions, syncytial giant cells, and viral inclusions.258., 260. Although characteristic of this disease, syncytial giant endothelial cells are seen only in 25% of cases,258 and viral inclusions of similar morphology can been seen in other paramyxoviral infections. Immunostaining provides a useful tool for unequivocal diagnosis of the disease, demonstrating viral antigen within neurons and endothelial cells of most organs (Fig. 3.24 ).5., 258.
FIGURE 3.24.
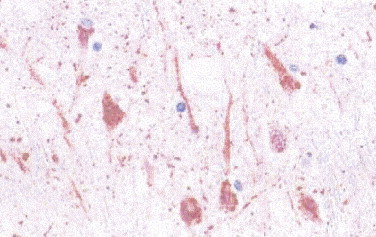
Nipah virus. Immunostaining of Nipah virus antigens in neurons and neuronal processes in CNS of a fatal case of Nipah virus encephalitis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×63.)
Ehrlichioses
Tick-transmitted intracellular gram-negative bacteria belonging to the genera Ehrlichia and Anaplasma are the agents of human monocytotropic ehrlichiosis and human granulocytotropic anaplasmosis, respectively. The acute febrile illnesses usually present with cytopenias, myalgias, and mild to moderate hepatitis.261., 262., 263., 264.
Diagnosis of ehrlichiosis depends upon finding the characteristic monocytic and/or granulocytic cytoplasmic inclusions (morulae), PCR analysis of blood, and detection of specific antibodies in blood. However, morulae are rare and often missed on initial evaluation; hematoxylin and eosin–stained sections often fail to show organisms even when IHC reveals abundant ehrlichial antigen; and antibody titers may take several weeks to rise to diagnostic levels.261., 265. Additionally, immunocompromised patients may not develop anti-ehrlichial antibodies prior to death.261., 263. In these cases, immunostaining for Ehrlichia or Anaplasma is a sensitive and specific diagnostic method.261., 263., 264., 266.
Immunohistochemistry has been a very valuable tool used to identify and study several other EIDs such as Ebola hemorrhagic fever;93., 94., 95. Hendra virus encephalitis;5., 267., 268. leptospirosis;163., 164., 165. emerging tick-borne rickettsioses such as R. parkeri 269 and R. africae; 270 and, recently, a new coronavirus associated with severe acute respiratory syndrome (SARS).271., 272. SARS was first recognized during a global outbreak of severe pneumonia that occurred in late 2002 in Guangdong Province, China, and then erupted in February 2003 with cases in more than two dozen countries in Asia, Europe, North America, and South America. Early in the investigation, clinical, pathologic, and laboratory studies focused on previously known agents of respiratory illness. Subsequently, a virus was isolated from the oropharynx of a SARS patient and identified by ultrastructural characteristics as belonging to the family Coronaviridae.271., 271. Various reports have described diffuse alveolar damage as the main histopathologic finding in SARS patients, and SARS-associated coronavirus (SARS-CoV) has been demonstrated in human and experimental animal tissues by immunohistochemical (Fig. 3.25 ) or ISH assays.273., 274., 275., 276., 277., 278., 279., 280., 281., 282.
FIGURE 3.25.
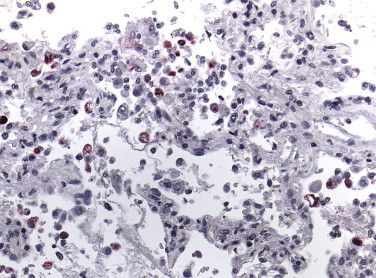
SARS. Coronavirus antigen–positive pneumocytes and macrophages in the lung of a SARS case. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×63.)
PATHOLOGISTS, IMMUNOHISTOCHEMISTRY, AND BIOTERRORISM
There is increasing concern about the use of infectious agents as potential biological weapons. Biological warfare agents vary from rare exotic viruses to common bacterial agents, and the intentional use of biologic agents to cause disease can simulate naturally occurring outbreaks or may have unusual characteristics.283 The CDC has issued recommendations for a complete public health response to a biological attack.284., 285., 286. Two important components of this response plan include the rapid diagnosis and characterization of biological agents. Pathologists using newer diagnostic techniques such as IHC, ISH, and PCR will have a direct impact on the rapid detection and control of emerging infectious diseases from natural or intentional causes.287 Immunohistochemistry provides a simple, safe, sensitive, and specific method for the rapid detection of biological threats (at the time of investigation or retrospectively), thereby facilitating the rapid implementation of effective public health responses.
Anthrax
Immunohistochemical staining of Bacillus anthracis with monoclonal antibodies against cell wall and capsule antigens has been successfully used in the recognition of bioterrorism-related anthrax cases and is an important step in early diagnosis and treatment (Fig. 3.26 A-C).5., 288., 289., 290., 291., 292. Gram staining and culture isolation of B. anthracis are usually used to diagnose anthrax; however, previous antibiotic treatment will affect culture yield and Gram stain identification of the bacteria.290 Immunohistochemistry has demonstrated high sensitivity and specificity for the detection of B. anthracis in skin biopsies, pleural biopsies, transbronchial biopsies, and pleural fluids (see Fig. 3.26).289., 290., 291., 293.
FIGURE 3.26.
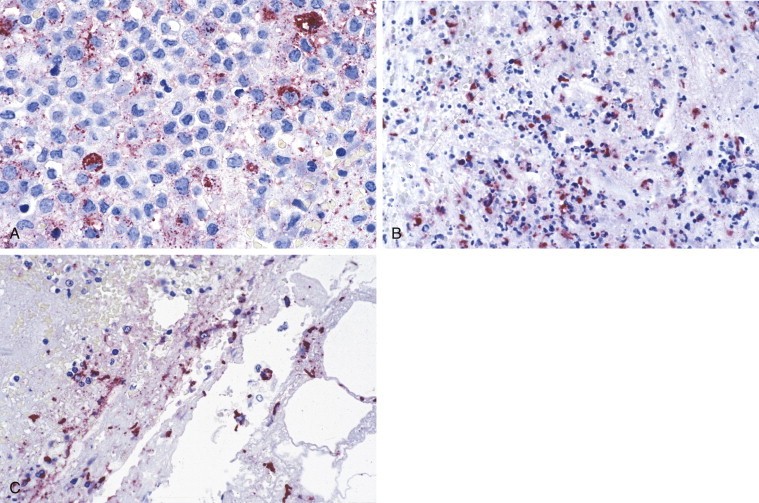
Anthrax. (A) Photomicrograph of a pleural effusion cell block from a patient with bioterrorism-associated inhalation anthrax showing bacillary fragments and granular antigen-staining using the anti–B. anthracis capsule antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×63.) (B) Skin biopsy from a patient with cutaneous anthrax showing abundant granular antigen and bacillary fragments using B. anthracis cell-wall antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×40.) (C) Photomicrograph of mediastinal lymph node from a patient with inhalational anthrax showing abundant granular antigen and bacillary fragments using anti–B. anthracis cell-wall antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×63.)
In addition, immunostaining has been very useful for determining the route of entry of the bacteria and identifying the mode of spread of the disease.290., 294.
Tularemia
Immunohistochemical staining is also valuable for rapid identification of Francisella tularensis in formalin-fixed tissue sections. Tularemia can have a variable clinical and pathologic presentation that can simulate other infectious diseases such as anthrax, plague, cat-scratch disease, or lymphogranuloma venereum. Moreover, the microorganisms are difficult to demonstrate in tissue sections, even with Gram stain or silver staining methods. A mouse monoclonal antibody against the lipopolysaccharide of F. tularensis has been used with high sensitivity and specificity to demonstrate intact bacteria and granular bacterial antigen in the lungs, spleen, lymph nodes, and liver (Fig. 3.27 ).295., 296.
FIGURE 3.27.
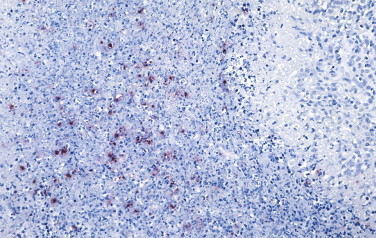
Tularemia. Immunohistochemistry of lymph node showing a stellate abscess with F. tularensis antigen-bearing macrophages in the central necrotic area using a mouse monoclonal antibody against the lipopolysaccharide of F. tularensis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×40.)
Plague
A mouse monoclonal antibody directed against the fraction 1 antigen of Yersinia pestis has been used to detect intracellular and extracellular bacteria in dermal blood vessels, lungs, lymph nodes, spleen, and liver (Fig. 3.28 ).297., 298., 299., 300., 301., 302. This technique is potentially useful for the rapid diagnosis of plague in formalin-fixed skin biopsies. In addition, IHC can differentiate primary and secondary pneumonic plague by identifying Y. pestis in different lung locations (e.g., alveoli vs. interstitium).297
FIGURE 3.28.
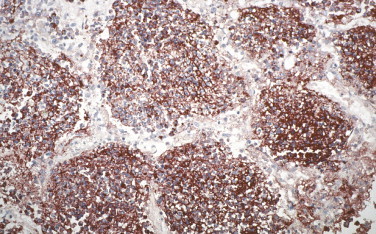
Immunohistochemical stain of lung containing abundant bacterial and granular Yersinia pestis antigen in the alveolar spaces using a mouse monoclonal antibody against F1 capsular antigen. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×20.)
Immunohistochemical methods using polyclonal or monoclonal antibodies have been used to identify several other potential biological terrorism agents, including the causative agents of brucellosis,5 Q fever,5., 138., 168., 169. viral encephalitides (eastern equine encephalitis) (Fig. 3.29 ),5., 121., 122., 123. rickettsioses (typhus and Rocky Mountain spotted fever),138., 139., 140., 141., 187. and viral hemorrhagic fevers (Ebola and Marburg hemorrhagic fever).5., 89., 90., 91., 92., 93., 94., 95.
FIGURE 3.29.
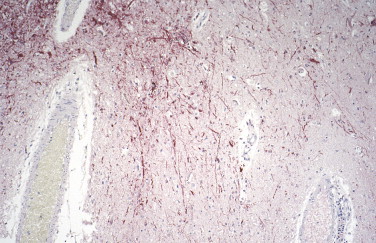
Immunostaining of viral antigens in neurons and neuronal processes in CNS using a mouse anti-eastern equine encephalitis virus antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×10.)
BEYOND IMMUNOHISTOLOGY: MOLECULAR DIAGNOSTIC APPLICATIONS
Since the 1980s, an enormous advancement in molecular technology has dramatically influenced the diagnosis and study of infectious diseases. The application of molecular probes to the study and diagnosis of infectious diseases is a great adjunct to IHC as a diagnostic method and often allows for even more rapid and specific identification of organisms.303., 304., 305., 306., 307., 308., 309. Along with rapid advances in molecular diagnostic techniques, there has been an increased interest in the use of paraffin-embedded specimens for nucleic acid hybridization assays. The two main hybridization formats used in the diagnostic pathology laboratory for the diagnosis of infectious diseases are ISH and PCR. In situ hybridization is analogous to IHC in that it allows the cellular identification and localization of microbial pathogens. Instead of microbial antigens, the targets of ISH are specific RNA or DNA sequences. Many viruses (FIGURE 3.30, FIGURE 3.31, FIGURE 3.32, FIGURE 3.33 ), bacteria (Fig. 3.34 ), and other microorganisms can be localized in tissues by ISH; these include Epstein-Barr virus, HPV (Fig. 3.35 ), polyomaviruses (Fig. 3.36 ), Mycobacterium leprae, Legionella, Haemophilus influenzae, zygomycetes, and Aspergillus. 108., 114., 207., 261., 282., 310., 311., 312., 313., 314., 315., 316., 317., 318., 319., 320., 321., 322., 323., 324., 325., 326., 327., 328., 329., 330., 331., 332. PCR has the advantage of increased sensitivity, minimal tissue requirements, and potential sequencing of the amplified product for specific identification of the microbial genotype or strain of the agent involved. There are PCR assays for most microorganisms that have been or can be adapted for use on formalin-fixed tissues.11., 124., 172., 293., 333., 334., 335., 336., 337., 338., 339., 340., 341., 342., 343., 344., 345.
FIGURE 3.30.
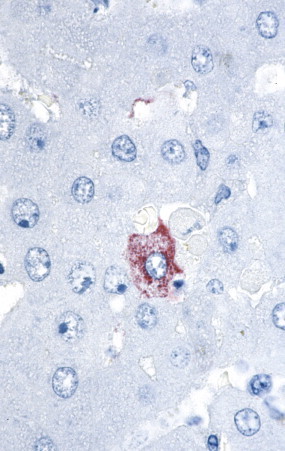
Crimean-Congo hemorrhagic fever (CCHF). Localization of CCHF viral RNA as seen in a single CCHF-infected hepatocyte. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×250.)
FIGURE 3.31.
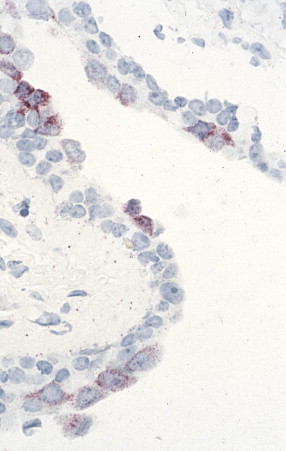
Influenza A. In situ hybridization showing localization of viral nucleic acids in bronchial epithelium using an influenza A hemagglutinin digoxigenin-labeled probe. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×158.)
FIGURE 3.32.
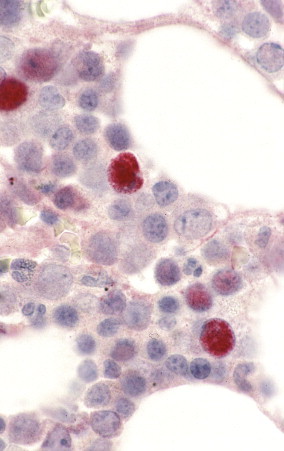
Parvovirus infection. Confirmation of B19-infected cells in bone marrow of an HIV-infected patient by using a digoxigenin-labeled B19 riboprobe and in situ hybridization. Staining is mainly nuclear and seen in multiple cells containing classic parvovirus inclusions. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×250.)
FIGURE 3.33.
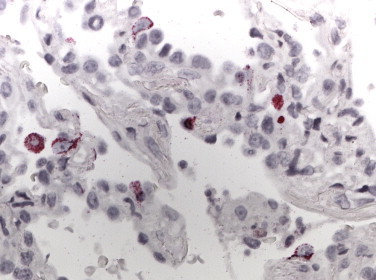
SARS. Lung showing diffuse alveolar damage and SARS-CoV nucleic acids primarily in pneumocytes as seen by colorimetric ISH. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×158.)
FIGURE 3.34.
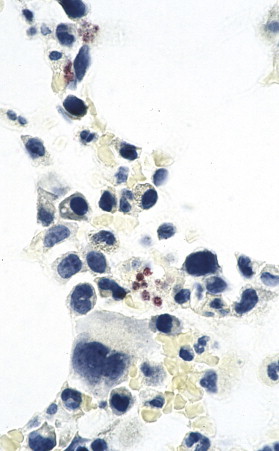
Ehrlichia chaffeensis. Organisms appear as red inclusions within monocytes by in situ hybridization. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×250.)
FIGURE 3.35.
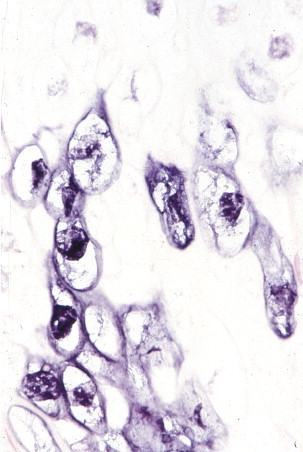
Human papillomavirus (HPV). In situ hybridization for HPV in a patient with a benign cervical lesion. HPV RNA is localized within nucleus and cytoplasm of koilocytotic cells. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×250.)
FIGURE 3.36.
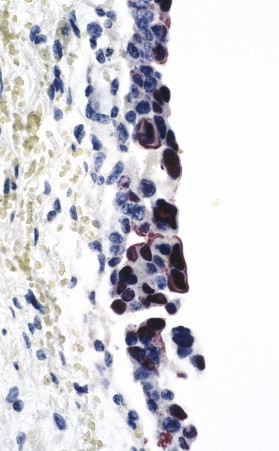
Polyomavirus (BK virus) infection. BK virus–infected cells in urothelium as seen by using a colorimetric ISH and a DNA probe. Staining is both nuclear and cytoplasmic and seen in multiple cells containing classic viral inclusions. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain; original magnification ×100.)
In summary, IHC, ISH, and PCR should be regarded as complementary diagnostic methods for use in the diagnostic pathology laboratory. The laboratory must consider the advantages and limitations of each method and how they apply to each case and the common needs of the laboratory. This ever-expanding field behooves all pathologists interested in the field of infectious diseases to keep abreast of the changing technology and its ever-increasing application in the arena of diagnosis.
REFERENCES
- 1.Cartun R.W. Use of immunohistochemistry in the surgical pathology laboratory for the diagnosis of infectious diseases. Pathol Case Rev. 1999;4:260–265. [Google Scholar]
- 2.Watts J.C. Surgical pathology in the diagnosis of infectious diseases. Am J Clin Pathol. 1994;102:711–712. doi: 10.1093/ajcp/102.6.711. (Editorial) [DOI] [PubMed] [Google Scholar]
- 3.Schwartz D.A., Bryan R.T. Infectious disease pathology and emerging infections: Are we prepared? Arch Pathol Lab Med. 1996;120:117–124. [PubMed] [Google Scholar]
- 4.Schwartz D.A. Emerging and reemerging infections: Progress and challenges in the subspecialty of infectious disease pathology. Arch Pathol Lab Med. 1997;121:776–784. [PubMed] [Google Scholar]
- 5.Zaki S.R., Paddock C.D. The emerging role of pathology in infectious diseases. In: Scheld W.M., Armonstrong D., Hughes J.M., editors. Emerging Infections 3. ASM Press; Washington, D.C: 1999. pp. 181–200. [Google Scholar]
- 6.Medical Examiners, Coroners, and Biologic Terrorism: A Guidebook for Surveillance and Case Management. MMWR Morbidity and Mortality Weekly Report. 2004;53(RR-8):1–53. [PubMed] [Google Scholar]
- 7.Zaki S.R., Peters C.J. Viral hemorrhagic fevers. In: Connor D.H., Chandler F.W., Schwartz D.A., Manz H.J., Lack E.E., editors. Pathology of Infectious Diseases. Appleton & Lange; Stamford, CT: 1997. pp. 347–364. [Google Scholar]
- 8.Coons A.H., Creech H.J., Jone R.N. The demonstration of pneumococcal antigen in tissues by use of fluorescent antibodies. J Immunol. 1942;45:159–170. [Google Scholar]
- 9.Cohen P.R. Tests for detecting herpes simplex virus and varicella-zoster virus infections. Dermatol Clin. 1994;12:51–68. [PubMed] [Google Scholar]
- 10.Procop G.W., Burchette J.L., Jr., Howell D.N. Immunoperoxidase and immunofluorescent staining of Rickettsia rickettsii in skin biopsies. Arch Pathol Lab Med. 1997;121:894–899. [PubMed] [Google Scholar]
- 11.Guarner J., Greer P.W., Whitney A. Pathogenesis and diagnosis of human meningococcal disease using immunohistochemical and PCR assay. Am J Clin Pathol. 2004;122:754–764. doi: 10.1309/A7M2-FN2T-YE6A-8UFX. [DOI] [PubMed] [Google Scholar]
- 12.Lepidi H., Houpikina P., Liang Z. Cardiac valves in patients with Q fever endocarditis: microbiological, molecular, and histologic studies. J Infect Dis. 2003;187:1097–1106. doi: 10.1086/368219. [DOI] [PubMed] [Google Scholar]
- 13.Eyzaguirre E.J., Haque A.K. Application of immunohistochemistry to infections. Arch Pathol Lab Med. 2008;132:424–431. doi: 10.5858/2008-132-424-AOITI. [DOI] [PubMed] [Google Scholar]
- 14.Lepidi H., Fenollar F., Dumler J.S. Cardiac valves in patients with Whipple endocarditis: microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. J Infect Dis. 2004;190:935–945. doi: 10.1086/422845. [DOI] [PubMed] [Google Scholar]
- 15.Jeavons L., Hunt L., Hamilton A. Immunochemical studies of heat-shock protein 80 of Histoplasma capsulatum. J Med Vet Mycol. 1994;32:47–57. doi: 10.1080/02681219480000071. [DOI] [PubMed] [Google Scholar]
- 16.Werner M., Chott A., Fabiano A. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24:1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Woods G.L., Walker D.H. Detection of infection or infectious agents by use of cytologic and histologic stains. Clin Microbiol Rev. 1996;9:382–404. doi: 10.1128/cmr.9.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandler F.W. Invasive microorganisms. In: Spicer S.S., editor. Histochemistry in Pathology Diagnosis. Marcel Dekker; New York, NY: 1987. pp. 77–101. [Google Scholar]
- 19.Clausen P.P., Thomsen P. Demonstration of hepatitis B surface antigen in liver biopsies. A comparative investigation of immunoperoxidase and orcein staining on identical sections on formalin-fixed, paraffin-embedded tissue. Acta Pathol Microbiol Scand [A] 1978;86A:383. [PubMed] [Google Scholar]
- 20.Thomsen P., Clausen P.P. Occurrence of hepatitis B-surface antigen in a consecutive material of 1539 liver biopsies. Acta Pathol Microbiol Immunol Scand [A] 1983;91:71. doi: 10.1111/j.1699-0463.1983.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 21.Al Adnani M.S., Ali S.M. Patterns of chronic liver disease in Kuwait with special reference to localization of hepatitis B surface antigen. J Clin Pathol. 1984;37:549. doi: 10.1136/jcp.37.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor C. Lung, pancreas, colon and rectum, stomach, liver. In: Taylor C.R., Cote R.J., editors. Immunomicroscopy: A Diagnostic Tool for the Surgical Pathologist. 2nd ed. Saunders; Philadelphia: 1994. pp. 292–317. [Google Scholar]
- 23.Park Y.N., Han K.H., Kim K.S. Cytoplasmic expression of hepatitis B core antigen in chronic hepatitis B virus infection: role of precore stop mutants. Liver. 1999;19:199–205. doi: 10.1111/j.1478-3231.1999.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 24.McDonald J.A., Harris S., Waters J.A. Effect of human immunodeficiency virus (HIV) infection on chronic hepatitis B hepatic viral display. J Hepatol. 1987;4:337–342. doi: 10.1016/s0168-8278(87)80543-3. [DOI] [PubMed] [Google Scholar]
- 25.Hubscher S.G., Portmann B.C. Transplantation pathology. In: Burt A.D., Portmann B.C., Ferrell L.D., editors. MacSween’s Pathology of the Liver. 5th ed. Elsevier; Philadelphia: 2007. pp. 815–879. [Google Scholar]
- 26.Feiden W., Borchard F., Burrig K.F. Herpes esophagitis: I. Light microscopical immunohistochemical investigations. Virchows Arch [A] 1984;404:167–176. doi: 10.1007/BF00704061. [DOI] [PubMed] [Google Scholar]
- 27.Nikkels A.F., Delvenne P., Sadzot-Delvaux C. Distribution of varicella zoster virus and herpes simplex virus in disseminated fatal infections. J Clin Pathol. 1996;49:243–248. doi: 10.1136/jcp.49.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenson J.K., Beschorner W.E., Boitnott J.K. Prominent mononuclear cell infiltrate is characteristic of herpes esophagitis. Hum Pathol. 1991;22:541–549. doi: 10.1016/0046-8177(91)90230-m. [DOI] [PubMed] [Google Scholar]
- 29.Wang J.Y., Montone K.T. A rapid simple in situ hybridization method for herpes simplex virus employing a synthetic biotin-labeled oligonucleotide probe: a comparison with immunohistochemical methods for HSV detection. J Clin Lab Anal. 1994;8:105–115. doi: 10.1002/jcla.1860080209. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T.K., Ueda M., Nishino T. Brush cytology of herpes simplex virus infection in oral mucosa: use of the ThinPrep processor. Diag Cytopath. 1998;18:71–75. doi: 10.1002/(sici)1097-0339(199801)18:1<71::aid-dc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll J.A.R., Love S., Burton P.A. Autopsy findings in two cases of neonatal herpes simplex virus infection: detection of virus by immunohistochemistry, in situ hybridization and the polymerase chain reaction. Histopathology. 1994;24:257–264. doi: 10.1111/j.1365-2559.1994.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 32.Nikkels A.F., Debrus S., Sadzot-Delvaux C. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Archiv A Pathol Anat. 1993;422:121–126. doi: 10.1007/BF01607163. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P.R. Tests for detecting herpes simplex virus and varicella-zoster virus infections. Dermat Clin. 1994;12:51–68. [PubMed] [Google Scholar]
- 34.Katano H., Sato Y., Kurata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 35.Katano H., Suda T., Morishita Y. Human herpesvirus 8-associated solid lymphomas that occur in AIDS patients takes anaplastic large cell morphology. Mod Pathol. 2000;13:77–85. doi: 10.1038/modpathol.3880012. [DOI] [PubMed] [Google Scholar]
- 36.Ely S.A., Powers J., Lewis D. Kaposi’s sarcoma-associated herpesvirus-positive primary effusion lymphoma arising in the subarachnoid space. Hum Pathol. 1999;30:981–984. doi: 10.1016/s0046-8177(99)90254-x. [DOI] [PubMed] [Google Scholar]
- 37.Katano H., Sato Y., Kurata T. High expression of HHV-8-encoded ORF73 protein in spindle-shape cells of Kaposi’s sarcoma. Am J Pathol. 1999;155:47–52. doi: 10.1016/S0002-9440(10)65097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said J.W., Shintaku I.P., Asou H. Herpesvirus 8 inclusions in primary effusion lymphoma: report of a unique case with T-cell phenotype. Archiv Pathol Lab Med. 1999;123:257–260. doi: 10.5858/1999-123-0257-HIIPEL. [DOI] [PubMed] [Google Scholar]
- 39.Cheuk W., Wong K.O., Wong C.S. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335–342. doi: 10.1309/B8TC-0LBV-H8XY-5MFV. [DOI] [PubMed] [Google Scholar]
- 40.Robin Y.M., Guillou L., Michels J.J. Human herpesvirus 8 immunostaining. A sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 41.Wada D.A., Perkins S.L., Tripp S. Human herpesvirus 8 and iron staining are useful in differentiating Kaposi sarcoma from interstitial granuloma annulare. Am J Clin Pathol. 2007;127:263–270. doi: 10.1309/GMH9CENH4909AWVB. [DOI] [PubMed] [Google Scholar]
- 42.Bryant-Greenwood P., Sorbara L., Filie A.C. Infection of mesothelial cells with human herpesvirus 8 in human immunodeficiency virus-infected patients with Kaposi sarcoma, Castleman disease, and recurrent pleural effusions. Mod Pathol. 2003;16:145–153. doi: 10.1097/01.MP.0000052374.61768.79. [DOI] [PubMed] [Google Scholar]
- 43.de la Hoz R.E., Stephens G., Sherlock C. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25(suppl 2):S1–S12. doi: 10.1016/s1386-6532(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 44.Bronsther O., Makowka L., Jaffe R. The occurrence of cytomegalovirus hepatitis in liver transplant patients. J Med Virol. 1988;24:423–434. doi: 10.1002/jmv.1890240409. [DOI] [PubMed] [Google Scholar]
- 45.Drummer J.S., White L.T., Ho M. Morbidity of cytomegalovirus infection in recipients of heart or heart-lung transplant who receive cyclosporine. J Infect Dis. 1985;152:1182–1191. doi: 10.1093/infdis/152.6.1182. [DOI] [PubMed] [Google Scholar]
- 46.Anwar F., Erice A., Jessurun J. Are there cytopathic features associated with cytomegalovirus infection predictive of resistance to antiviral therapy? Ann Diag Pathol. 1999;3:19–22. doi: 10.1016/s1092-9134(99)80005-7. [DOI] [PubMed] [Google Scholar]
- 47.Cote L., Drouet E., Bissuel F. Diagnostic value of amplification of human cytomegalovirus DNA from gastrointestinal biopsies from human immunodeficiency virus-infected patients. J Clin Microbiol. 1993;31:2066–2069. doi: 10.1128/jcm.31.8.2066-2069.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlinson W.D. Broadsheet number 50: Diagnosis of human cytomegalovirus infection and disease. Pathology. 1999;31:109–115. doi: 10.1080/003130299105287. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan M.M., Coker R., Coleman D.V. Detection of cytomegalovirus (CMV) in HIV+ patients: comparison of cytomorphology, immunohistochemistry and in situ hybridization. Cytopath. 1998;9:29–37. [PubMed] [Google Scholar]
- 50.Kutza A.S., Muhl E., Hackstein H. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 51.Saetta A., Agapitos E., Davaris P.S. Determination of CMV placentitis: Diagnostic application of the polymerase chain reaction. Virchows Arch. 1998;432:159–162. doi: 10.1007/s004280050150. [DOI] [PubMed] [Google Scholar]
- 52.Solans E.P., Yong S., Husain A.N. Bronchioloalveolar lavage in the diagnosis of CMV pneumonitis in lung transplant recipients: an immunocytochemical study. Diagn Cytopath. 1997;16:350–352. doi: 10.1002/(sici)1097-0339(199704)16:4<350::aid-dc9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Nebuloni M., Pellegrinelli A., Ferri A. Etiology of microglial nodules in brains of patients with acquired immunodeficiency syndrome. J Neurovirol. 2000;6:46–50. doi: 10.3109/13550280009006381. [DOI] [PubMed] [Google Scholar]
- 54.Rimsza L.M., Vela E.E., Frutiger Y.M. Rapid automated combined in situ hybridization and immunohistochemistry for sensitive detection of cytomegalovirus in paraffin-embedded tissue biopsies. Am J Clin Pathol. 1996;106:544–548. doi: 10.1093/ajcp/106.4.544. [DOI] [PubMed] [Google Scholar]
- 55.Kandiel A., Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 56.Kambham N., Vij R., Cartwright C.A. Cytomegalovirus infection in steroid-refractory ulcerative colitis. A case-control study. Am J Surg Pathol. 2004;28:365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Ribalta T., Martinez A.J., Jares P. Presence of occult cytomegalovirus infection in the brain after orthotopic liver transplantation. An autopsy study of 83 cases. Virchows Arch. 2002;440:166–171. doi: 10.1007/s004280100497. [DOI] [PubMed] [Google Scholar]
- 58.Cruz-Spano L., Lima-Pereira F.E., Gomes da Silva-Basso N. Human cytomegalovirus infection and abortion: an immunohistochemical study. Med Sci Monit. 2002;8:BR230–BR235. [PubMed] [Google Scholar]
- 59.Seehofer D., Rayes N., Tullius S.G. CMV hepatitis after liver transplantation: incidence, clinical course, and long term follow-up. Liver Transp. 2002;8:1138–1146. doi: 10.1053/jlts.2002.36732. [DOI] [PubMed] [Google Scholar]
- 60.Lautenschlager I., Hockerstedt K., Taskinen E. Histologic findings associated with CMV infection in liver transplantation. Transplant Proc. 2003;35:819. doi: 10.1016/s0041-1345(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 61.Lamps L.W., Pinson C.W., Raiford D.S. The significance of microabscesses in liver transplant biopsies: a clinicopathological study. Hepatology. 1998;28:1532–1537. doi: 10.1002/hep.510280613. [DOI] [PubMed] [Google Scholar]
- 62.Barkholt L.M., Ehrnst A., Veress B. Clinical use of immunohistopathologic methods for the diagnosis of cytomegalovirus hepatitis in human liver allograft biopsy specimens. Scand J Gastroenterol. 1994;29:553–560. doi: 10.3109/00365529409092472. [DOI] [PubMed] [Google Scholar]
- 63.Colina F., Jucá N.T., Moreno E. Histological diagnosis of cytomegalovirus hepatitis in liver allografts. J Clin Pathol. 1995;48:351–357. doi: 10.1136/jcp.48.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lones M.A., Shintaku I.P., Weiss L.M. Posttransplant lymphoproliferative disorder in liver allograft biopsies: a comparison of three methods for the demonstration of Epstein Barr virus. Hum Pathol. 1997;28:533–539. doi: 10.1016/s0046-8177(97)90074-5. [DOI] [PubMed] [Google Scholar]
- 65.Challoner P.B., Smith K.T., Parker J.D. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson J. Epstein-Barr virus and Hodgkin’s lymphoma. HERPES. 2006;13:12–16. [PubMed] [Google Scholar]
- 67.Flomenberg P., Babbitt J., Drobyski W.R. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 68.Strickler J.G., Singleton T.P., Copenhaver G.M. Adenovirus in the gastrointestinal tracts of immunosuppressed patients. Am J Clin Pathol. 1992;97:555–558. doi: 10.1093/ajcp/97.4.555. [DOI] [PubMed] [Google Scholar]
- 69.Yi E.S., Powell H.C. Adenovirus infection of the duodenum in an AIDS patient: an ultrastructural study. Ultrastruct Pathol. 1994;18:549–551. doi: 10.3109/01913129409021897. [DOI] [PubMed] [Google Scholar]
- 70.Yan Z., Nguyen S., Poles M. Adenovirus colitis in human immunodeficiency virus infection: an underdiagnosed entity. Am J Surg Pathol. 1998;22:1101–1106. doi: 10.1097/00000478-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Dombrowski F., Eis-Hubinger A.M., Ackermann T. Adenovirus-induced liver necrosis in a case of AIDS. Virchows Archiv. 1997;431:469–472. doi: 10.1007/s004280050125. [DOI] [PubMed] [Google Scholar]
- 72.Simsir A., Greenebaum E., Nuovo G. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation. 1998;65:592–594. doi: 10.1097/00007890-199802270-00027. [DOI] [PubMed] [Google Scholar]
- 73.Saad R.S., Demetris A.J., Lee R.G. Adenovirus hepatitis in the adult allograft liver. Transplantation. 1997;64:1483–1485. doi: 10.1097/00007890-199711270-00021. [DOI] [PubMed] [Google Scholar]
- 74.Ohori N.P., Michaels M.G., Jaffe R. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26:1073–1079. doi: 10.1016/0046-8177(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 75.Marc J., Dieterich D.T. The histopathology of 103 consecutive colonoscopy biopsies from 82 symptomatic patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2001;125:1042–1046. doi: 10.5858/2001-125-1042-THOCCB. [DOI] [PubMed] [Google Scholar]
- 76.El-Mahallawy H.A., Mansour T., El-Din S.E. Parvovirus B19 infection as a cause of anemia in pediatric acute lymphoblastic leukaemia during maintenance chemotherapy. J Pediatr Hematol Oncol. 2004;26:403–406. doi: 10.1097/00043426-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Brown K.E., Young N.S. Parvovirus B19 infection and hematopoiesis. Blood Rev. 1995;9:176–182. doi: 10.1016/0268-960x(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 78.Jordan J.A., Penchansky L. Diagnosis of human parvovirus B19-induced anemia: correlation of bone marrow morphology with molecular diagnosis using PCR and immunohistochemistry. Cell Vision. 1995;2:279–282. [Google Scholar]
- 79.Morey A.L., O’Neil H.J., Coyle P.V. Immunohistological detection of human parvovirus B19 in formalin-fixed, paraffin-embedded tissues. J Pathol. 1992;166:105–108. doi: 10.1002/path.1711660204. [DOI] [PubMed] [Google Scholar]
- 80.Puvion-Dutilleul F., Puvion E. Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Nat Acad Sci U S A. 1998;95:8227–8232. doi: 10.1073/pnas.95.14.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yufu Y., Matsumoto M., Miyamura T. Parvovirus B19-associated haemophagocytic syndrome with lymphadenopathy resembling histiocytic necrotizing lymphadenitis (Kikuchi’s disease) Br J Haematol. 1997;96:868–871. doi: 10.1046/j.1365-2141.1997.d01-2099.x. [DOI] [PubMed] [Google Scholar]
- 82.Vadlamudi G., Rezuke N., Ross J.W. The use of monoclonal antibody R92F6 and polymerase chain reaction to confirm the presence of parvovirus B19 in bone marrow specimens of patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123:768–773. doi: 10.5858/1999-123-0768-TUOMAR. [DOI] [PubMed] [Google Scholar]
- 83.Wright C., Hinchliffe S.A., Taylor C. Fetal pathology in intrauterine death due to parvovirus B19 infection. Br J Obstet Gynaecol. 1996;103:133–136. doi: 10.1111/j.1471-0528.1996.tb09664.x. [DOI] [PubMed] [Google Scholar]
- 84.Essary L.R., Vnencak-Jones C.L., Manning S.S. Frequency of parvovirus B19 infection in nonimmune hydrops fetalis and utility of three diagnostic methods. Hum Pathol. 1998;29:696–701. doi: 10.1016/s0046-8177(98)90278-7. [DOI] [PubMed] [Google Scholar]
- 85.Monath T.P., Ballinger M.E., Miller B.R. Detection of yellow fever viral RNA by nucleic acid hybridization and viral antigen by immunohistochemistry in fixed human liver. Am J Trop Med Hyg. 1989;40:663–668. doi: 10.4269/ajtmh.1989.40.663. [DOI] [PubMed] [Google Scholar]
- 86.De Brito T., Siqueira S.A., Santos R.T. Human fatal yellow fever. Immunohistochemical detection of viral antigens in the liver, kidney and heart. Pathol Res Pract. 1992;188:177–181. doi: 10.1016/S0344-0338(11)81176-3. [DOI] [PubMed] [Google Scholar]
- 87.Hall W.C., Crowell T.P., Watts D.M. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 88.Ramos C., Sanchez G., Pando R.H. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol. 1998;4:465–468. doi: 10.3109/13550289809114548. [DOI] [PubMed] [Google Scholar]
- 89.Burt F.J., Swanepoel R., Shieh W.-J. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 90.Maiztegui J.I., Laguens R.P., Cossio P.M. Ultrastructural and immunohistochemical studies in five cases of Argentine hemorrhagic fever. J Infect Dis. 1975;132:35–53. doi: 10.1093/infdis/132.1.35. [DOI] [PubMed] [Google Scholar]
- 91.Hall W.C., Geisbert T.W., Huggins J.W. Experimental infection of guinea pigs with Venezuelan hemorrhagic fever virus (Guanarito): a model of human disease. Am J Trop Med Hyg. 1996;55:81–88. doi: 10.4269/ajtmh.1996.55.81. [DOI] [PubMed] [Google Scholar]
- 92.Geisbert T.W., Jaax N.K. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 93.Zaki S.R., Shieh W.-J., Greer P.W. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. J Infect Dis. 1999;179(suppl 1):S36–S37. doi: 10.1086/514319. [DOI] [PubMed] [Google Scholar]
- 94.Ksiazek T.G., Rollin P.E., Williams A.J. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of Congo. J Infect Dis. 1999;179:S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 95.Wyers M., Formenty P., Cherel Y. Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J Infect Dis. 1999;179(suppl 1):S54–S59. doi: 10.1086/514300. [DOI] [PubMed] [Google Scholar]
- 96.Nickeleit V., Hirsch H.H., Zeiler M. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324–332. doi: 10.1093/ndt/15.3.324. [DOI] [PubMed] [Google Scholar]
- 97.White L.H., Casian A., Hilton R. BK virus nephropathy in renal transplant patients in London. Transplantation. 2008;85:1008–1015. doi: 10.1097/TP.0b013e31816a32fc. [DOI] [PubMed] [Google Scholar]
- 98.Drachenberg C.B., Papadimitriou J.C., Ramos E. Histologic versus molecular diagnosis of BK polyomavirus-associated nephropathy: a shifting paradigm? Clin J Am Soc Nephrol. 2006;1:374–379. doi: 10.2215/CJN.02021205. [DOI] [PubMed] [Google Scholar]
- 99.Vogler C., Wang Y., Brink D.S. Renal pathology in the pediatric transplant patient. Adv Anat Pathol. 2007;14:202–216. doi: 10.1097/PAP.0b013e3180504927. [DOI] [PubMed] [Google Scholar]
- 100.Dall A., Hariharan S. BK virus nephritis after renal transplantation. Clin J Am Soc Nephrol. 2008;3(suppl 2):S68–S75. doi: 10.2215/CJN.02770707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Latif S., Zaman F., Veeramachaneni R. BK polyomavirus in renal transplants: role of electron microscopy and immunostaining in detecting early infection. Ultrastruct Pathol. 2007;31:199–207. doi: 10.1080/01913120701376113. [DOI] [PubMed] [Google Scholar]
- 102.Nebuloni M., Tosoni A., Boldorini R. BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123:807–811. doi: 10.5858/1999-123-0807-BVRIIA. [DOI] [PubMed] [Google Scholar]
- 103.Elli A., Banfi G., Battista-Fogazzi G. BK polyomavirus interstitial nephritis in a renal transplant patient with no previous acute rejection episodes. J Nephrol. 2002;15:313–316. [PubMed] [Google Scholar]
- 104.Jochum W., Weber T., Frye S. Detection of JC virus by anti-VP1 immunohistochemistry in brains with progressive multifocal leukoencephalopathy. Acta Neuropathol. 1997;94:226–231. doi: 10.1007/s004010050697. [DOI] [PubMed] [Google Scholar]
- 105.Chima S.C., Agostini H.T., Ryschlkeewitsch C.F. Progressive multifocal leukoencephalopathy and JC virus genotypes in West African patients with acquired immunodeficiency syndrome. A pathologic and DNA sequence analysis of 4 cases. Arch Pathol Lab Med. 1999;123:395–403. doi: 10.5858/1999-123-0395-PMLAJV. [DOI] [PubMed] [Google Scholar]
- 106.Aoki N., Mori M., Kato K. Antibody against synthetic multiple antigen peptides (MAP) of JC virus capsid protein (VP1) without cross reaction to BK virus: a diagnostic tool for progressive multifocal leukoencephalopathy. Neurosci Lett. 1996;205:111–114. doi: 10.1016/0304-3940(96)12389-2. [DOI] [PubMed] [Google Scholar]
- 107.Silver S.A., Arthur R.R., Rozan Y.S. Diagnosis of progressive multifocal leukoencephalopathy by stereotactic brain biopsy utilizing immunohistochemistry and the polymerase chain reaction. Acta Cytol. 1995;39:35–44. [PubMed] [Google Scholar]
- 108.Guarner J., Shieh W.J., Dawson J. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- 109.Guarner J., Paddock C.D., Shieh W.J. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003-2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- 110.Nielson K.A., Yunis E.J. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- 111.Wright C., Oliver K.C., Fenwick F.I. A monoclonal antibody pool for routine immunohistochemical detection of human respiratory syncytial virus antigens in formalin-fixed, paraffin-embedded tissue. J Pathol. 1997;182:238–244. doi: 10.1002/(SICI)1096-9896(199706)182:2<238::AID-PATH822>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 112.Jogai S., Radotra B.D., Banerjee A.K. Immunohistochemical study of human rabies. Neuropathology. 2000;20:197–203. doi: 10.1046/j.1440-1789.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 113.Jogai S., Radotra B.D., Banerjee A.K. Rabies viral antigen in extracranial organs: a postmortem study. Neuropathol Appl Neurobiol. 2002;28:334. doi: 10.1046/j.1365-2990.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 114.Warner C.K., Zaki S.R., Shieh W.J. Laboratory investigation of humans from vampire bat rabies in Peru. Am J Trop Med Hyg. 1999;60:502–507. doi: 10.4269/ajtmh.1999.60.502. [DOI] [PubMed] [Google Scholar]
- 115.Sinchaisri T.A., Nagata T., Yoshikawa Y. Immunohistochemical and histopathological study of experimental rabies infection in mice. J Vet Med Sci. 1992;54:409–416. doi: 10.1292/jvms.54.409. [DOI] [PubMed] [Google Scholar]
- 116.Jackson A.C., Ye H., Phelan C.C. Extraneural organ involvement in human rabies. Lab Invest. 1999;79:945–951. [PubMed] [Google Scholar]
- 117.Yousef G.E., Mann G.F., Brown I.N. Clinical and research application of an enterovirus group-reactive monoclonal antibody. Intervirol. 1987;28:199–205. doi: 10.1159/000150017. [DOI] [PubMed] [Google Scholar]
- 118.Hohenadl C., Klingel K., Rieger P. Investigation of the coxsackievirus B3 nonstructural proteins 2B, 2C, and 3AB: generation of specific polyclonal antisera and detection of replicating virus in infected tissue. J Virol Methods. 1994;47:279–295. doi: 10.1016/0166-0934(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H., Li Y., Peng T. Localization of enteroviral antigen in myocardium and other tissues from patients with heart muscle disease by an improved immunohistochemical technique. J Histochem Cytochem. 2000;48:579–584. doi: 10.1177/002215540004800501. [DOI] [PubMed] [Google Scholar]
- 120.Li Y., Bourlet T., Andreoletti L. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation. 2000;101:231–234. doi: 10.1161/01.cir.101.3.231. [DOI] [PubMed] [Google Scholar]
- 121.Del Piero F., Wilkins P.A., Dubovi E.J. Clinical, pathologic, immunohistochemical, and virologic findings of eastern equine encephalitis in two horses. Vet Pathol. 2001;38:451–456. doi: 10.1354/vp.38-4-451. [DOI] [PubMed] [Google Scholar]
- 122.Patterson J.S., Maes R.K., Mullaney T.P. Immunohistochemical diagnosis of eastern equine encephalomyelitis. J Vet Diag Invest. 1996;8:156–160. doi: 10.1177/104063879600800203. [DOI] [PubMed] [Google Scholar]
- 123.Garen P.D., Tsai T.F., Powers J.M. Human eastern equine encephalitis: immunohistochemistry and ultrastructure. Mod Pathol. 1999;12:646–652. [PubMed] [Google Scholar]
- 124.Tatti K.M., Gentsch J., Shieh W.J. Molecular and immunological methods to detect rotavirus in formalin-fixed tissue. J Virol Methods. 2002;105:305–319. doi: 10.1016/s0166-0934(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 125.Morrison C., Gilson T., Nuovo G.J. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum Pathol. 2001;32:216–221. doi: 10.1053/hupa.2001.21565. [DOI] [PubMed] [Google Scholar]
- 126.Cioc A.M., Nuovo G.J. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod Pathol. 2001;15:914–922. doi: 10.1097/01.MP.0000024291.37651.CD. [DOI] [PubMed] [Google Scholar]
- 127.Toulaymant M., Marconi S., Garb J. Endoscopic biopsy pathology of Helicobacter pylori gastritis. Arch Pathol Lab Med. 1999;123:778–781. doi: 10.5858/1999-123-0778-EBPOHP. [DOI] [PubMed] [Google Scholar]
- 128.El-Zimaity H.M.T., Graham D.Y., Al-Assis M.T. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 129.Marcio L., Angelucci D., Grossi L. Anti-Helicobacter pylori specific antibody immunohistochemistry improves the diagnostic accuracy of Helicobacter pylori in biopsy specimen from patients treated with triple therapy. Am J Gastroenterol. 1998;93:223–226. doi: 10.1111/j.1572-0241.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 130.Rotimi O., Cairns A., Gray S. Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 2000;53:756–759. doi: 10.1136/jcp.53.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldstein N.S. Chronic inactive gastritis and coccoid Helicobacter pylori in patients treated for gastroesophageal reflux disease or with H. pylori eradication therapy. Am J Clin Pathol. 2002;118:719–726. doi: 10.1309/LJ4D-E2LX-7UMR-YMTH. [DOI] [PubMed] [Google Scholar]
- 132.Eshun J.K., Black D.D., Casteel H.B. Comparison of immunohistochemistry and silver stain for the diagnosis of pediatric Helicobacter pylori infection in urease-negative gastric biopsies. Ped Devel Pathol. 2001;4:82–88. doi: 10.1007/s100240010129. [DOI] [PubMed] [Google Scholar]
- 133.Lepidi H., Fenollar F., Dumler J.S. Cardiac valves in patients with Whipple endocarditis: microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. J Infect Dis. 2004;190:935–945. doi: 10.1086/422845. [DOI] [PubMed] [Google Scholar]
- 134.Houpikian P., Raoult D. Diagnostic methods: current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Infect Dis Clin N Am. 2007;16:377–392. doi: 10.1016/s0891-5520(01)00010-1. [DOI] [PubMed] [Google Scholar]
- 135.Baisden B.L., Lepidi H., Raoult D. Diagnosis of Whipple disease by immunohistochemical analysis. A sensitive and specific method for the detection of Tropheryma whipplei (the Whipple bacillus) in paraffin-embedded tissue. Am J Clin Pathol. 2002;118:742–748. doi: 10.1309/8YGR-FE7L-39LL-L37C. [DOI] [PubMed] [Google Scholar]
- 136.Lepidi H., Fenollar F., Gerolami R. Whipple disease: Immunospecific and quantitative immunohistochemical study of intestinal biopsy specimens. Hum Pathol. 2003;34:589–596. doi: 10.1016/s0046-8177(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 137.Lepidi H., Costedoat N., Piette J.C. Immunohistological detection of Tropheryma whipplei (Whipple bacillus) in lymph nodes. Am J Med. 2002;113:334–336. doi: 10.1016/s0002-9343(02)01174-9. [DOI] [PubMed] [Google Scholar]
- 138.Dumler J.S., Walker D.H. Diagnostic tests for Rocky Mountain spotted fever and other rickettsial diseases. Dermat Clin. 1994;12:25–36. [PubMed] [Google Scholar]
- 139.Parola P., Paddock C.D., Raoult D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walker D.H., Burday M.S., Folds J.D. Laboratory diagnosis of Rocky Mountain spotted fever. South Med J. 1980;73:1443–1447. doi: 10.1097/00007611-198011000-00007. [DOI] [PubMed] [Google Scholar]
- 141.Paddock C.D., Greer P.W., Ferebee T.L. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis. 1999;179:1469–1476. doi: 10.1086/314776. [DOI] [PubMed] [Google Scholar]
- 142.Kaplowitz L.G., Lange J.V., Fischer J.J., Walker D.H. Correlation of rickettsial titers, circulating endotoxin, and clinical features in Rocky Mountain spotted fever. Arch Intern Med. 1983;143:1149–1151. [PubMed] [Google Scholar]
- 143.Dumler J.S., Gage W.R., Pettis G.L. Rapid immunoperoxidase demonstration of Rickettsia rickettsii in fixed cutaneous specimens from patients with Rocky Mountain spotted fever. Am J Clin Pathol. 1990;93:410–414. doi: 10.1093/ajcp/93.3.410. [DOI] [PubMed] [Google Scholar]
- 144.Chapman A.S., Bakken J.S., Folk S.M. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis—United States: A practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 145.Spach D.H., Koehler J.E. Bartonella-associated infections. Infect Dis Clin North Am. 1998;12:137–155. doi: 10.1016/s0891-5520(05)70414-1. [DOI] [PubMed] [Google Scholar]
- 146.Lepidi H., Durack D.T., Raoult D. Diagnostic methods: Current best practices and guidelines for histologic evaluation in infective endocarditis. Infect Dis Clin North Am. 2002;16:339–361. doi: 10.1016/s0891-5520(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 147.Albrich W.C., Kraft C., Fisk T. A mechanic with a bad valve: Blood-culture-negative endocarditis. Lancet Infect Dis. 2004;4:777–784. doi: 10.1016/S1473-3099(04)01226-5. [DOI] [PubMed] [Google Scholar]
- 148.Lepidi H., Fournier P.E., Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–889. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 149.Baorto E., Payne R.M., Slater L.N. Culture-negative endocarditis caused by Bartonella henselae. J Pediatr. 1998;132:1051–1054. doi: 10.1016/s0022-3476(98)70410-x. [DOI] [PubMed] [Google Scholar]
- 150.Reed J.A., Brigati D.J., Flynn S.D. Immunohistochemical identification of Rochalimaea henselae in bacillary (epithelioid) angiomatosis, parenchymal bacillary peliosis, and persistent fever with bacteremia. Am J Surg Pathol. 1992;16:650–657. doi: 10.1097/00000478-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 151.Min K.W., Reed J.A., Welch D.F. Morphologically variable bacilli of cat scratch disease are identified by immunohistochemical labeling with antibodies to Rochalimaea henselae. Am J Clin Pathol. 1994;101:607–610. doi: 10.1093/ajcp/101.5.607. [DOI] [PubMed] [Google Scholar]
- 152.Daybell D., Paddock C.D., Zaki S.R. Disseminated infection with Bartonella henselae as a cause of spontaneous splenic rupture. Clin Infect Dis. 2004;39:21–24. doi: 10.1086/422001. [DOI] [PubMed] [Google Scholar]
- 153.Buffet M., Grange P.A., Gerhardt P. Diagnosing Treponema pallidum in secondary syphilis by PCR and immunohistochemistry. J Invest Dermatol. 2007;127:2345–2350. doi: 10.1038/sj.jid.5700888. [DOI] [PubMed] [Google Scholar]
- 154.Jethwa H.S., Schmitz J.L., Dallabetta G. Comparison of molecular and microscopic techniques for detection of Treponema pallidum in genital ulcers. J Clin Microbiol. 1995;33:180–183. doi: 10.1128/jcm.33.1.180-183.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kingston A.A., Vujevich J., Shapiro M. Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus. Arch Dermatol. 2005;141:431–433. doi: 10.1001/archderm.141.4.431. [DOI] [PubMed] [Google Scholar]
- 156.Hoang M.P., High W.A., Molberg K.H. Secondary syphilis: A histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595–599. doi: 10.1111/j.0303-6987.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 157.Guarner J., Greer P.W., Bartlett J. Congenital syphilis in a newborn: an immunopathologic study. Mod Pathol. 1999;12:82–87. [PubMed] [Google Scholar]
- 158.Ulrichs T., Lefmann K., Reich M. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissues. J Pathol. 2005;205:633–640. doi: 10.1002/path.1728. [DOI] [PubMed] [Google Scholar]
- 159.Daniel T.M. The rapid diagnosis of tuberculosis: A selective review. J Lab Clin Med. 1990;116:277–282. [PubMed] [Google Scholar]
- 160.Carabias E., Palenque R., Serrano J.M. Evaluation of an immunohistochemical test with polyclonal antibodies raised against mycobacteria used in formalin-fixed tissue compared with mycobacterial specific culture. APMIS. 1998;106:385–388. doi: 10.1111/j.1699-0463.1998.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 161.Mustafa T., Hg Wiker, Mfinanga S.G.M. Immunohistochemistry using a Mycobacterium tuberculosis complex specific antibody for improved diagnosis of tuberculosis lymphadenitis. Mod Pathol. 2006;19:1606–1614. doi: 10.1038/modpathol.3800697. [DOI] [PubMed] [Google Scholar]
- 162.Zaki S.R., Shieh W.J. the Epidemic Working Group. Leptospirosis associated with an outbreak of acute febrile illness and pulmonary hemorrhage, Nicaragua 1995. Lancet. 1996;347:535–536. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 163.Trevejo R.T., Rigau-Pérez J.G., Ashford D.A. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua. J Infect Dis. 1995;1998(178):1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 164.Guarner J., Shieh W.J., Morgan J. Leptospirosis mimicking acute cholecystitis among athletes participating in a triathlon. Hum Pathol. 2001;32:750–752. doi: 10.1053/hupa.2001.25599. [DOI] [PubMed] [Google Scholar]
- 165.Nicodemo A.C., Duarte M.I., Alves V.A. Lung lesions in human leptospirosis: microscopic, immunohistochemical, and ultrastructural features related to thrombocytopenia. Am J Trop Med Hyg. 1997;56:181–187. doi: 10.4269/ajtmh.1997.56.181. [DOI] [PubMed] [Google Scholar]
- 166.Lebech A.M., Clemmensen O., Hansen K. Comparison of in vitro, immunohistochemical staining, and PCR for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J Clin Microbiol. 1995;33:2328–2333. doi: 10.1128/jcm.33.9.2328-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aberer E., Kersten A., Klade H. Heterogeneity of Borrelia burgdorferi in the skin. Am J Dermatopathol. 1996;18:571–579. doi: 10.1097/00000372-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 168.Brouqui P., Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14:177–207. doi: 10.1128/CMR.14.1.177-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brouqui P., Dumler J.S., Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–458. doi: 10.1016/0002-9343(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 170.Guarner J., Packard M.M., Nolte K.B. Usefulness of immunohistochemical diagnosis of Streptococcus pneumoniae in formalin-fixed, paraffin-embedded specimens compared with culture and gram stain techniques. Am J Clin Pathol. 2007;127:617–618. doi: 10.1309/J3LD0RBP788W1TM8. [DOI] [PubMed] [Google Scholar]
- 171.Guarner J., Bartlett J., Reagan S. Immunohistochemical evidence of Clostridium sp, Staphylococcus aureus, and group A Streptococcus in severe soft tissue infections related to injection drug use. Hum Pathol. 2006;37:1482–1488. doi: 10.1016/j.humpath.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 172.Guarner J., Sumner J., Paddock C.D. Diagnosis of invasive group A streptococcal infections by using immunohistochemical and molecular assays. Am J Clin Pathol. 2006;126:148–155. doi: 10.1309/KHGV-R72C-BRM4-FQ58. [DOI] [PubMed] [Google Scholar]
- 173.Terpstra W.J., Groeneveld K., Eijk P.P. Comparison of two nonculture techniques for detection of Hemophilus influenzae in sputum: In situ hybridization and immunoperoxidase staining with monoclonal antibodies. Chest. 1988;94:126S. [PubMed] [Google Scholar]
- 174.Groeneveld K., van Alphen L., van Ketel R.J. Nonculture detection of Haemophilus influenzae in sputum with monoclonal antibodies specific for outer membrane lipoprotein P6. J Clin Microbiol. 1989;27:2263. doi: 10.1128/jcm.27.10.2263-2267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Forsgren J., Samuelson A., Borrelli S. Persistence of nontypeable Haemophilus influenzae in adenoid macrophages: a putative colonization mechanism. Act Oto-Laryngol. 1996;116:766–773. doi: 10.3109/00016489609137922. [DOI] [PubMed] [Google Scholar]
- 176.Shurbaji M.S., Dumler J.S., Gage W.R. Immunohistochemical detection of chlamydial antigens in association with cystitis. Am J Pathol. 1990;93:363. doi: 10.1093/ajcp/93.3.363. [DOI] [PubMed] [Google Scholar]
- 177.Paukku M., Puolakkainen M., Paavonen T. Plasma cell endometritis is associated with Chlamydia trachomatis infection. Am J Clin Pathol. 1999;112:211–215. doi: 10.1093/ajcp/112.2.211. [DOI] [PubMed] [Google Scholar]
- 178.Naas J., Gnarpe J.A. Demonstration of Chlamydia pneumoniae in tissue by immunohistochemistry. APMIS. 1999;107:882–886. [PubMed] [Google Scholar]
- 179.Suffin S.C., Kaufmann A.F., Whitaker B. Legionella pneumophila. Identification in tissue sections by a new immunoenzymatic procedure. Arch Pathol Lab Med. 1980;104:283–286. [PubMed] [Google Scholar]
- 180.Maruta K., Miyamoto H., Hamada T. Entry and intracellular growth of Legionella dumoffii in alveolar epithelial cells. Am J Respir Crit Care Med. 1998;157:1967–1974. doi: 10.1164/ajrccm.157.6.9710108. [DOI] [PubMed] [Google Scholar]
- 181.Fiore A.E., Nuorti J.P., Levine O.S. Epidemic Legionnaires’ disease two decades later: Old sources, new diagnostic methods. Clin Infect Dis. 1998;26:426–433. doi: 10.1086/516309. [DOI] [PubMed] [Google Scholar]
- 182.Parkash V., Morotti R.A., Joshi V. Immunohistochemical detection of Listeria antigens in the placenta in perinatal listeriosis. Int J Gynecol Pathol. 1998;17:343–350. doi: 10.1097/00004347-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 183.Chiba M., Fukushima T., Koganei K. Listeria monocytogenes in the colon in a case of fulminant ulcerative colitis. Scand J Gastroenterol. 1998;33:778–782. doi: 10.1080/00365529850171765. [DOI] [PubMed] [Google Scholar]
- 184.Weinstock D., Horton S.B., Rowland P.H. Rapid diagnosis of Listeria monocytogenes by immunohistochemistry in formalin-fixed brain tissue. Vet Pathol. 1995;32:193–195. doi: 10.1177/030098589503200215. [DOI] [PubMed] [Google Scholar]
- 185.Pospischil A., Wood R.L., Anderson T.D. Peroxidase-antiperoxidase and immunogold labeling of Salmonella typhimurium and Salmonella cholerasuis var kunzendorf in tissues of experimentally infected swine. Am J Vet Res. 1990;51:619–624. [PubMed] [Google Scholar]
- 186.Thygesen P., Martinsen C., Hougen H.P. Histologic, cytologic, and bacteriologic examination of experimentally induced Salmonella typhimurium infection in Lewis rats. Comp Med. 2000;50:124–132. [PubMed] [Google Scholar]
- 187.Walker D.H., Feng H.M., Ladner S. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod Pathol. 1997;10:1038–1042. [PubMed] [Google Scholar]
- 188.Koss T., Carter E.L., Grossman M.E. Increased detection of rickettsialpox in a New York City hospital following the anthrax outbreak of 2001: Use of immunohistochemistry for the rapid confirmation of cases in an era of bioterrorism. Arch Dermatol. 2003;139:1545–1552. doi: 10.1001/archderm.139.12.1545. [DOI] [PubMed] [Google Scholar]
- 189.Walker D.H., Hudnall S.D., Szaniawski W.K. Monoclonal antibody-based immunohistochemical diagnosis of rickettsialpox: the macrophage is the principal target. Mod Pathol. 1999;12:529–533. [PubMed] [Google Scholar]
- 190.Moron C.G., Popov V.L., Feng H.M. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–759. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 191.Schwarz J. The diagnosis of deep mycoses by morphological methods. Hum Pathol. 1982;13:519–533. doi: 10.1016/s0046-8177(82)80267-0. [DOI] [PubMed] [Google Scholar]
- 192.Marques M.E.A., Coelho K.I.R., Bacchi C.E. Comparison between histochemical and immunohistochemical methods for the diagnosis of sporotrichosis. J Clin Pathol. 1992;45:1089–1093. doi: 10.1136/jcp.45.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reed J.A., Hemaan B.A., Alexander J.L. Immunomycology: Rapid and specific immunocytochemical identification of fungi in formalin-fixed, paraffin-embedded material. J Histochem Cytochem. 1993;41:1217–1221. doi: 10.1177/41.8.8331285. [DOI] [PubMed] [Google Scholar]
- 194.Jensen H.E., Schonheyder H., Hotchi M. Diagnosis of systemic mycosis by specific immunohistochemical tests. APMIS. 1996;104:241–258. doi: 10.1111/j.1699-0463.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 195.Fukuzawa M., Inaba H., Hayama M. Improved detection of medically important fungi by immunoperoxidase staining with polyclonal antibodies. Virchows Arch. 1995;427:407–414. doi: 10.1007/BF00199390. [DOI] [PubMed] [Google Scholar]
- 196.Kauffman L. Immunohistologic diagnosis of systemic mycosis: An update. Eur J Epidemiol. 1992;8:377–382. doi: 10.1007/BF00158571. [DOI] [PubMed] [Google Scholar]
- 197.Verweij P.E., Smedts F., Poot T. Immunoperoxidase staining for identification of Aspergillus species in routinely processed tissue sections. J Clin Pathol. 1996;49:798–801. doi: 10.1136/jcp.49.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Breier F., Oesterreicher C., Brugger S. Immunohistochemistry with monoclonal antibody against Candida albicans mannan antigen demonstrates cutaneous Candida granulomas as evidence of Candida sepsis in an immunosuppressed host. Dermatology. 1997;194:293–296. doi: 10.1159/000246130. [DOI] [PubMed] [Google Scholar]
- 199.Marcilla A., Monteagudo C., Mormeneo S. Monoclonal antibody 3H8: A useful tool in the diagnosis of candidiasis. Microbiol. 1999;145:695–701. doi: 10.1099/13500872-145-3-695. [DOI] [PubMed] [Google Scholar]
- 200.Monteagudo C., Marcilla A., Mormeneo S. Specific immunohistochemical identification of Candida albicans in paraffin-embedded tissue with a new monoclonal antibody (1B12) Am J Clin Pathol. 1995;103:130–135. doi: 10.1093/ajcp/103.2.130. [DOI] [PubMed] [Google Scholar]
- 201.Jarvensivu A., Hietanen J., Rautemaa R. Candida yeast in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Diseases. 2004;10:106–112. doi: 10.1046/j.1354-523x.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 202.Williams D.W., Jones H.S., Allison R.T. Immunohistochemical detection of Candida albicans in formalin fixed, paraffin embedded material. J Clin Pathol. 1998;51:857–859. doi: 10.1136/jcp.51.11.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jarvensivu A., Rautemaa R., Sorsa T. Specificity of the monoclonal antibody 3H8 in the immunohistochemical identification of Candida species. Oral Diseases. 2006;12:428–433. doi: 10.1111/j.1601-0825.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 204.Kockenberger M.B., Canfield P.J., Kozel T.R. An immunohistochemical method that differentiates Cryptococcus neoformans varieties and serotypes in formalin-fixed paraffin-embedded tissues. Med Mycol. 2001;39:523–533. doi: 10.1080/mmy.39.6.523.533. [DOI] [PubMed] [Google Scholar]
- 205.Tsunemi T., Kamata T., Fumimura Y. Immunohistochemical diagnosis of Cryptococcus neoformans var. gatti infection in chronic meningoencephalitis: The first case in Japan. Intern Med. 2001;40:1241–1244. doi: 10.2169/internalmedicine.40.1241. [DOI] [PubMed] [Google Scholar]
- 206.Chamilos G., Luna M., Lewis R.E. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: An autopsy study over a 15-year period. Haematologica. 2006;91:986–989. [PubMed] [Google Scholar]
- 207.Hayden R.T., Isolato P.A., Parrett T. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue sections. Diagn Mol Pathol. 2003;12:21–26. doi: 10.1097/00019606-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 208.Rickerts V., Mousset S., Lambrecht E. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis. 2007;44:1078–1083. doi: 10.1086/512812. [DOI] [PubMed] [Google Scholar]
- 209.Hope W.W., Walsh T.J., Denning D.W. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–622. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 210.Tarrand J.J., Lichterfeld M., Warraich I. Diagnosis of invasive septated mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119:854–858. doi: 10.1309/EXBV-YAUP-ENBM-285Y. [DOI] [PubMed] [Google Scholar]
- 211.Choi J.K., Mauger J., McGowan K.L. Immunohistochemical detection of Aspergillus species in pediatric tissue samples. Am J Clin Pathol. 2004;121:18–25. doi: 10.1309/DK1C-G9MA-TKYY-BFMQ. [DOI] [PubMed] [Google Scholar]
- 212.Pierard G.E., Arrese-Estrada J., Pierard-Franchimont C. Immunohistochemical expression of galactomannan in the cytoplasm of phagocytic cells during invasive aspergillosis. Am J Clin Pathol. 1991;96:373–376. doi: 10.1093/ajcp/96.3.373. [DOI] [PubMed] [Google Scholar]
- 213.Phillips P., Weiner M.H. Invasive aspergillosis diagnosed by monoclonal and polyclonal reagents. Hum Pathol. 1987;18:1015–1024. doi: 10.1016/s0046-8177(87)80218-6. [DOI] [PubMed] [Google Scholar]
- 214.Fenelon L.E., Hamilton A.J., Figueroa J.I. Production of specific monoclonal antibodies to Aspergillus species and their use in immunohistochemical identification of aspergillosis. J Clin Microbiol. 1999;37:1221–1223. doi: 10.1128/jcm.37.4.1221-1223.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jensen H.E., Salonen J., Ekfors T.O. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J Pathol. 1997;181:100–105. doi: 10.1002/(SICI)1096-9896(199701)181:1<100::AID-PATH100>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 216.Wazir J.E., Brown I., Martin-Bates E. EB9, a new antibody for the detection of trophozoites of Pneumocystis carinii in bronchoalveolar lavage specimens in AIDS. J Clin Pathol. 1994;47:1108–1111. doi: 10.1136/jcp.47.12.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wazir J.E., Macrorie S.G., Coleman D.V. Evaluation of the sensitivity, specificity, and predictive value of monoclonal antibody 3F6 for the detection of Pneumocystis carinii pneumonia in bronchoalveolar lavage specimens and induced sputum. Cytopathol. 1994;5:82–89. doi: 10.1111/j.1365-2303.1994.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 218.Cooper C.R., McGinnis M.R. Pathology of Penicillium marneffei: An emerging acquired immunodeficiency syndrome-related pathogen. Arch Lab Pathol Med. 1997;121:798–804. [PubMed] [Google Scholar]
- 219.Chaiwun B., Khunamornpong S., Sirivanichai C. Lymphadenopathy due to Penicillium marneffei infection: Diagnosis by fine needle aspiration cytology. Mod Pathol. 2002;15:939–943. doi: 10.1097/01.MP.0000027203.44333.95. [DOI] [PubMed] [Google Scholar]
- 220.Arrese Estrada J., Stynen D., Van Cutsem J. Immunohistochemical identification of Penicillium marneffei by monoclonal antibody. Int J Dermatol. 1992;31:410–412. doi: 10.1111/j.1365-4362.1992.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 221.Burke D.G., Emancipator S.N., Smith M.C. Histoplasmosis and kidney disease in patients with AIDS. Clin Infect Dis. 1997;25:281–284. doi: 10.1086/514556. [DOI] [PubMed] [Google Scholar]
- 222.Arnold S.J., Kinney M.C., McCormick M.S. Disseminated toxoplasmosis: Unusual presentations in the immunocompromised host. Arch Pathol Lab Med. 1997;121:869–873. [PubMed] [Google Scholar]
- 223.Warnke C., Tuazon C.U., Kovacs A. Toxoplasma encephalitis in patients with acquired immunodeficiency syndrome: Diagnosis and response to therapy. Am J Trop Med Hyg. 1987;36:509. doi: 10.4269/ajtmh.1987.36.509. [DOI] [PubMed] [Google Scholar]
- 224.Ganji M., Tan A., Maitar M.L. Gastric toxoplasmosis in a patient with acquired immunodeficiency syndrome. A case report and review of the literature. Arch Pathol Lab Med. 2003;127:732–734. doi: 10.5858/2003-127-732-GTIAPW. [DOI] [PubMed] [Google Scholar]
- 225.Hofman V., Brousset P., Mougneau E. Immunostaining of visceral leishmaniasis caused by Leishmania infantum using monoclonal antibody (19-11) to the Leishmania homologue of receptors for activated C-kinase. Am J Clin Pathol. 2003;120:567–574. doi: 10.1309/R3DK-4MR3-W6E5-PH17. [DOI] [PubMed] [Google Scholar]
- 226.Azadeh B., Sells P.G., Ejeckman G.C. Localized Leishmania lymphadenitis immunohistochemical studies. Am J Clin Pathol. 1994;102:11–15. doi: 10.1093/ajcp/102.1.11. [DOI] [PubMed] [Google Scholar]
- 227.Kenner J.R., Aronson N.E., Bratthauer G.L. Immunohistochemistry to identify Leishmania parasites in fixed tissues. J Cutan Pathol. 1999;26:130–136. doi: 10.1111/j.1600-0560.1999.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 228.Amato V.S., Duarte M.I.S., Nicodemo A.C. An evaluation of clinical, serologic, anatomopathologic and immunohistochemical findings for fifteen patients with mucosal leishmaniasis before and after treatment. Rev Inst Med Trop S Paulo. 1998;40:23–30. doi: 10.1590/s0036-46651998000100006. [DOI] [PubMed] [Google Scholar]
- 229.Guarner J., Bartlett J., Shieh W.J. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol. 2007;20:1230–1237. doi: 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 230.Bonnin A., Petrella T., Dubremetz J.F. Histopathologic methods for diagnosis of cryptosporidiosis using monoclonal antibodies. Eur J Clin Microbiol Infect Dis. 1990;9:664–665. doi: 10.1007/BF01964268. [DOI] [PubMed] [Google Scholar]
- 231.Perez de Suarez E., Perez-Schael I., Perozo-Ruggeri G. Immunocytochemical detection of Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1987;81:624–626. doi: 10.1016/0035-9203(87)90435-4. [DOI] [PubMed] [Google Scholar]
- 232.Guarner J., Bartlett J., Zaki S.R. Mouse model for Chagas disease: Immunohistochemical distribution of different stages of Trypanosoma cruzi in tissues throughout infection. Am J Trop Med Hyg. 2001;65:152–158. doi: 10.4269/ajtmh.2001.65.152. [DOI] [PubMed] [Google Scholar]
- 233.Anez N., Carrasco H., Parada H. Myocardial parasite persistence in chronic chagasic patients. Am J Trop Med Hyg. 1999;60:726–732. doi: 10.4269/ajtmh.1999.60.726. [DOI] [PubMed] [Google Scholar]
- 234.Reis M.M., Higuchi Mde L., Benvenuti L.A. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 235.Torres-Velez F.J., Nace E.K., Won K.Y. Development of an immunohistochemical assay for the detection of babesiosis in formalin-fixed, paraffin-embedded tissue samples. Am J Clin Pathol. 2003;120:833–838. doi: 10.1309/A4RG-P4LF-12GG-H8MW. [DOI] [PubMed] [Google Scholar]
- 236.Sanad M.M., Darwish R.A., Nasr M.E. Giardia lamblia and chronic gastritis. J Egypt Soc Parasitol. 1996;26:481–495. [PubMed] [Google Scholar]
- 237.Genrich G.L., Guarner J., Paddock C.D. Fatal malaria infection in travelers: Novel immunohistochemical assays for the detection of Plasmodium falciparum in tissues and implications for pathogenesis. Am J Trop Med Hyg. 2007;76:251–259. [PubMed] [Google Scholar]
- 238.Institute of Medicine . National Academy Press; Washington, DC: 1992. Emerging infections: microbial threats to health in the United States. [PubMed] [Google Scholar]
- 239.CDC. Preventing Emerging Infectious Diseases: A Strategy for the 21st Century. Overview of the Updated CDC Plan. MMWR Recomm Rep. 1998;47:1–14. [PubMed] [Google Scholar]
- 240.Perkins B.A., Flood J.M., Danila R. Unexplained deaths due to possibly infectious causes in the United States: Defining the problem and designing surveillance and laboratory approaches. Emerg Infect Dis. 1998;2:47–53. doi: 10.3201/eid0201.960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Houpikina P., Raoult D. Traditional and molecular techniques for the study of emerging bacterial diseases: One laboratory’s perspective. Emerg Infect Dis. 2002;8:122–131. doi: 10.3201/eid0802.010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Khan A.S., Khabbaz R.F., Armstrong L.R. Hantavirus pulmonary syndrome: The first 100 US cases. J Infect Dis. 1996;173:1297–1303. doi: 10.1093/infdis/173.6.1297. [DOI] [PubMed] [Google Scholar]
- 243.Moolenaar R.L., Dalton C., Lipman H.B. Clinical features that differentiate hantavirus pulmonary syndrome from three other acute respiratory illnesses. Clin Infect Dis. 1995;21:643–649. doi: 10.1093/clinids/21.3.643. [DOI] [PubMed] [Google Scholar]
- 244.Nolte K.B., Feddersen R.M., Foucar K. Hantavirus pulmonary syndrome in the United States: A pathological description of a disease caused by a new agent. Hum Pathol. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 245.Zaki S.R., Greer P.W., Coffield L.M. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 246.Zaki S.R., Khan A.S., Goodman R.A. Retrospective diagnosis of hantavirus pulmonary syndrome, 1978-1993. Implications for emerging infectious diseases. Arch Pathol Lab Med. 1996;120:134–139. [PubMed] [Google Scholar]
- 247.Shieh W.J., Guarner J., Layton M. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg Infect Dis. 2000;6:370–372. doi: 10.3201/eid0604.000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cushing M.M., Brat D.J., Mosunjac M.I. Fatal West Nile virus encephalitis in a renal transplant recipient. Am J Clin Pathol. 2004;121:26–31. doi: 10.1309/G23C-P54D-AR1B-CY8L. [DOI] [PubMed] [Google Scholar]
- 249.Petersen R.L., Roehrig J.T., Hughes J.M. West Nile virus encephalitis. N Engl J Med. 2002;347:1225–1226. doi: 10.1056/NEJMo020128. [DOI] [PubMed] [Google Scholar]
- 250.Sampson B.A., Ambrosi C., Charlot A. The pathology of human West Nile virus infection. Hum Pathol. 2000;31:527–532. doi: 10.1053/hp.2000.8047. [DOI] [PubMed] [Google Scholar]
- 251.Guarner J., Shieh W.J., Hunter S. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35:983–990. doi: 10.1016/j.humpath.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 252.Lanciotti R.S., Kerst A.J., Nasci R.S. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ho M., Chen E.R., Hsu K.H. An epidemic of enterovirus 71 infection in Taiwan. Taiwan enterovirus epidemic working group. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 254.Chan L.G., Parashar U.D., Lye M.S. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: Clinical and pathological characteristics of the disease. Clin Infect Dis. 2000;31:678–683. doi: 10.1086/314032. [DOI] [PubMed] [Google Scholar]
- 255.Wong K.T., Chua K.B., Lam S.K. Immunohistochemical detection of infected neurons as a rapid diagnosis of enterovirus 71 encephalomyelitis. Ann Neurol. 1999;45:271–272. doi: 10.1002/1531-8249(199902)45:2<271::aid-ana23>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 256.Yan J.J., Wang J.R., Liu C.C. An outbreak of enterovirus 71 infection in Taiwan 1998: A comprehensive pathological, virological, and molecular study on a case of fulminant encephalitis. J Clin Virol. 2000;17:13–22. doi: 10.1016/s1386-6532(00)00067-6. [DOI] [PubMed] [Google Scholar]
- 257.Shieh W.J., Jung S.M., Hsueh C. Pathologic studies of fatal causes in outbreak of hand, foot, and mouth disease. Taiwan. Emerg Infect Dis. 2001;7:146–148. doi: 10.3201/eid0701.700146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wong K.T., Shieh W.J., Kumar S. Nipah virus infection. Pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Goh K.J., Tan C.T., Chew N.K. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342:1229–1235. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 260.Chua K.B., Bellini W.J., Rota P.A. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 261.Dawson J.E., Paddock C.D., Warner C.K. Tissue diagnosis of Ehrlichia chaffeensis in patients with fatal ehrlichiosis by use of immunohistochemistry, in situ hybridization, and polymerase chain reaction. Am J Trop Med Hyg. 2001;65:603–609. doi: 10.4269/ajtmh.2001.65.603. [DOI] [PubMed] [Google Scholar]
- 262.Walker D.H., Dumler J.S. Human monocytic and granulocytic ehrlichiosis. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 263.Paddock C.D., Suchard D.P., Grumbach K.L. Fatal seronegative ehrlichiosis in a patient with HIV infection. N Engl J Med. 1993;329:1164–1167. doi: 10.1056/NEJM199310143291605. [DOI] [PubMed] [Google Scholar]
- 264.Lepidi H., Bunnell J.E., Martin M.E. Comparative pathology and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am J Trop Med Hyg. 2000;62:29–37. doi: 10.4269/ajtmh.2000.62.29. [DOI] [PubMed] [Google Scholar]
- 265.Childs J.E., Sumner J.W., Nicholson W.L. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: Implications for surveillance. J Clin Microbiol. 1999;37:2997–3000. doi: 10.1128/jcm.37.9.2997-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sehdev A.E., Dumler J.S. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am J Clin Pathol. 2003;119:859–865. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 267.Hooper P.T., Russell G.M., Selleck P.W. Immunohistochemistry in the identification of a number of new diseases in Australia. Vet Microbiol. 1999;68:89–93. doi: 10.1016/s0378-1135(99)00064-4. [DOI] [PubMed] [Google Scholar]
- 268.Williamson M.M., Hooper P.T., Selleck P.W. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) J Comp Pathol. 2000;122:201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- 269.Paddock C.D., Summer J.W., Comer J.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 270.Lepidi H., Fournier P.E., Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332–1337. doi: 10.3201/eid1209.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 272.Peiris J.S., Lai S.T., Poon L.L. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nakajima N., Asahi-Ozaki Y., Nagata N. SARS coronavirus-infected cells in lung detected by new in situ hybridization technique. Jpn J Infect Dis. 2003;56:139–141. [PubMed] [Google Scholar]
- 274.Kuiken T., Fouchier R.A., Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chong P.Y., Chui P., Ling A.E. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: Challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.5858/2004-128-195-AODDTS. [DOI] [PubMed] [Google Scholar]
- 276.McAuliffe J., Vogel L., Roberts A. Replication of SARS coronavirus administered into the respiratory tract of African green, rhesus and cynomolgus monkeys. Virology. 2004;5:8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Roberts A., Vogel L., Guarner J. SARS coronavirus infection of golden Syrian hamsters. J Virol. 2005;79:503–511. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen P.C., Hsiao C.H., Re: To K.F., Tong J.H., Chan P.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: An in-situ hybridization study of fatal cases. J Pathol. 2004;203:729–731. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Re: To K.F., Tong J.H., Chan P.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: An in-situ hybridization study of fatal cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chow K.C., Hsiao C.H., Lin T.Y. Detection of severe acute respiratory syndrome-associated coronavirus in pneumocytes of the lung. Am J Clin Pathol. 2004;121:574–580. doi: 10.1309/C0EDU0RAQBTXBHCE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-Co-V) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sheih W.J., Cheng-Hsiang H., Paddock C.D. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ashford D.A., Kaiser R.B., Bales M.E. Planning against biological terrorism: Lessons from outbreak investigations. Emerg Infect Dis. 2003;9:515–519. doi: 10.3201/eid0905.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Inglesby T.V., O’Toole T., Henderson D.A. Preventing the use of biological weapons: Improving response should prevention fail. Clin Infect Dis. 2000;30:926–929. doi: 10.1086/313794. [DOI] [PubMed] [Google Scholar]
- 285.Lillibridge S.R., Bell A.J., Roman R.S. Thoughts for the new millennium: Bioterrorism. Centers for Disease Control and Prevention bioterrorism preparedness and response. Am J Infect Control. 1999;27:463–464. doi: 10.1016/s0196-6553(99)70020-9. [DOI] [PubMed] [Google Scholar]
- 286.Franz D.R., Zajtchuk R. Biological terrorism: Understanding the threat, preparation, and medical response. Dis Month. 2000;46:125–190. doi: 10.1016/s0011-5029(00)90017-8. [DOI] [PubMed] [Google Scholar]
- 287.Guarner J., Zaki S.R. Histopathology and immunohistochemistry in the diagnosis of bioterrorism agents. J Histochem Cytochem. 2006;54:3–11. doi: 10.1369/jhc.5R6756.2005. [DOI] [PubMed] [Google Scholar]
- 288.Ezzell J.W., Abshire T.G., Little S.F. Identification of Bacillus anthracis by using monoclonal antibodies to cell wall galactose-n-acetylglucosamine polysaccharide. J Clin Microbiol. 1990;28:223–231. doi: 10.1128/jcm.28.2.223-231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shieh W.J., Guarner J., Paddock C. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am J Pathol. 2003;163:1901–1910. doi: 10.1016/S0002-9440(10)63548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Guarner J., Jernigan J.A., Shieh W.J. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003;163:701–709. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jernigan J.A., Stephens D.S., Ashford D.A. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jernigan D.B., Raghunathan P.L., Bell B.P. Investigation of bioterrorism-related anthrax, United States, 2001: Epidemiologic findings. Emerg Infect Dis. 2002;8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tatti K.M., Greer P., White E. Morphologic, immunologic, and molecular methods to detect Bacillus anthracis in formalin-fixed tissues. Appl Immunohistochem Mol Morphol. 2006;14:234–243. doi: 10.1097/01.pai.0000178390.39047.78. [DOI] [PubMed] [Google Scholar]
- 294.Grinberg L.M., Abramova F.A., Yampolskaya O.V. Quantitative pathology of inhalational anthrax I: Quantitative microscopic findings. Mod Pathol. 2001;14:482–495. doi: 10.1038/modpathol.3880337. [DOI] [PubMed] [Google Scholar]
- 295.Guarner J., Greer P.W., Bartlett J. Immunohistochemical detection of Francisella tularensis in formalin-fixed paraffin-embedded tissue. Appl Immun Mol Morphol. 1999;7:122–126. [Google Scholar]
- 296.DeBey B.M., Andrews G.A., Chard-Bergstrom C. Immunohistochemical demonstration of Francisella tularensis in lesions of cats with tularaemia. J Vet Diagn Invest. 2002;14:162–164. doi: 10.1177/104063870201400213. [DOI] [PubMed] [Google Scholar]
- 297.Guarner J., Shieh W.J., Greer P.W. Immunohistochemical detection of Yersinia pestis in formalin-fixed paraffin-embedded tissue. Am J Clin Pathol. 2002;117:205–209. doi: 10.1309/HXMF-LDJB-HX1N-H60T. [DOI] [PubMed] [Google Scholar]
- 298.Davis K.J., Vogel P., Fritz D.L. Bacterial filamentation of Yersinia pestis by β-lactam antibiotics in experimentally infected mice. Arch Pathol Lab Med. 1997;121:865–868. [PubMed] [Google Scholar]
- 299.Davis K.J., Fritz D.L., Pitt M.L. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops) Arch Pathol Lab Med. 1996;120:156–163. [PubMed] [Google Scholar]
- 300.Williams E.S., Mills K., Kwiatkowski D.R. Plague in black-footed ferret (Mustela nigripes) J Wild Dis. 1994;30:581–585. doi: 10.7589/0090-3558-30.4.581. [DOI] [PubMed] [Google Scholar]
- 301.Gabastou J.M., Proaño J., Vimos A. An outbreak of plague including cases with probable pneumonic infection, Ecuador, 1998. Trans R Soc Tro Med Hyg. 2000;94:387–391. doi: 10.1016/s0035-9203(00)90114-7. [DOI] [PubMed] [Google Scholar]
- 302.Guarner J., Shieh W.J., Chu M. Persistent Yersinia pestis antigens in ischemic tissues of a patient with septicemic plague. Hum Pathol. 2005;36:850–853. doi: 10.1016/j.humpath.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 303.Figueroa M.E., Rasheed S. Molecular pathology and diagnosis of infectious diseases. Am J Clin Pathol. 1991;95:S8–S21. [PubMed] [Google Scholar]
- 304.Fredricks D.N., Relman D.A. Application of polymerase chain reaction to the diagnosis of infectious diseases. Clin Infect Dis. 1999;29:475–486. doi: 10.1086/598618. quiz 487-488. [DOI] [PubMed] [Google Scholar]
- 305.McNicol A.M., Farquharson M.A. In situ hybridization and its diagnostic applications in pathology. J Pathol. 1997;182:250–261. doi: 10.1002/(SICI)1096-9896(199707)182:3<250::AID-PATH837>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 306.Mothershed E.A., Whitney A.M. Nucleic acid-based methods for the detection of bacterial pathogens: Present and future considerations for the clinical laboratory. Clin Chim Acta. 2006;363:206–220. doi: 10.1016/j.cccn.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 307.Procop G.W., Wilson M. Infectious disease pathology. Clin Infect Dis. 2001;32:1589–1601. doi: 10.1086/320537. [DOI] [PubMed] [Google Scholar]
- 308.Sklar J. DNA hybridization in diagnostic pathology. Hum Pathol. 1985;16:654–658. doi: 10.1016/s0046-8177(85)80147-7. [DOI] [PubMed] [Google Scholar]
- 309.Tang Y.W., Procop G.W., Persing D.H. Molecular diagnostics of infectious diseases. Clin Chem. 1997;43:2021–2038. [PubMed] [Google Scholar]
- 310.Andrade Z.R., Garippo A.L., Saldiva P.H. Immunohistochemical and in situ detection of cytomegalovirus in lung autopsies of children immunocompromised by secondary interstitial pneumonia. Pathol Res Pract. 2004;200:25–32. doi: 10.1016/j.prp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 311.Burt F.J., Swanepoel R., Shieh W.J. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 312.Fredricks D.N., Relman D.A. Localization of Tropheryma whippelii rRNA in tissues from patients with Whipple’s disease. J Infect Dis. 2001;183:1229–1237. doi: 10.1086/319684. [DOI] [PubMed] [Google Scholar]
- 313.Gentilomi G., Musiani M., Zerbini M. Double in situ hybridization for detection of herpes simplex virus and cytomegalovirus DNA using non-radioactive probes. J Histochem Cytochem. 1992;40:421–425. doi: 10.1177/40.3.1313062. [DOI] [PubMed] [Google Scholar]
- 314.Gentilomi G., Zerbini M., Musiani M. In situ detection of B19 DNA in bone marrow of immunodeficient patients using a digoxigenin-labelled probe. Mol Cell Probes. 1993;7:19–24. doi: 10.1006/mcpr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 315.Hayden R.T., Qian X., Procop G.W. In situ hybridization for the identification of filamentous fungi in tissue section. Diagn Mol Pathol. 2002;11:119–126. doi: 10.1097/00019606-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 316.Hayden R.T., Qian X., Roberts G.D. In situ hybridization for the identification of yeastlike organisms in tissue section. Diagn Mol Pathol. 2001;10:15–23. doi: 10.1097/00019606-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 317.Hulette C.M., Downey B.T., Burger P.C. Progressive multifocal leukoencephalopathy. Diagnosis by in situ hybridization with a biotinylated JC virus DNA probe using an automated Histomatic Code-On slide stainer. Am J Surg Pathol. 1991;15:791–797. [PubMed] [Google Scholar]
- 318.Krimmer V., Merkert H., von Eiff C. Detection of Staphylococcus aureus and Staphylococcus epidermidis in clinical samples by 16S rRNA-directed in situ hybridization. J Clin Microbiol. 1999;37:2667–2673. doi: 10.1128/jcm.37.8.2667-2673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Matsuse T., Matsui H., Shu C.Y. Adenovirus pulmonary infections identified by PCR and in situ hybridisation in bone marrow transplant recipients. J Clin Pathol. 1994;47:973–977. doi: 10.1136/jcp.47.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Montone K.T., Litzky L.A. Rapid method for detection of Aspergillus 5S ribosomal RNA using a genus-specific oligonucleotide probe. Am J Clin Pathol. 1995;103:48–51. doi: 10.1093/ajcp/103.1.48. [DOI] [PubMed] [Google Scholar]
- 321.Morey A.L., Keeling J.W., Porter H.J. Clinical and histopathological features of parvovirus B19 infection in the human fetus. Br J Obstet Gynaecol. 1992;99:566–574. doi: 10.1111/j.1471-0528.1992.tb13822.x. [DOI] [PubMed] [Google Scholar]
- 322.Morey A.L., Porter H.J., Keeling J.W. Non-isotopic in situ hybridisation and immunophenotyping of infected cells in the investigation of human fetal parvovirus infection. J Clin Pathol. 1992;45:673–678. doi: 10.1136/jcp.45.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Moter A., Göbel U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 324.Musiani M., Zerbini M., Venturoli S. Rapid diagnosis of cytomegalovirus encephalitis in patients with AIDS using in situ hybridisation. J Clin Pathol. 1994;47:886–891. doi: 10.1136/jcp.47.10.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Naoumov N.V., Daniels H.M., Davison F. Identification of hepatitis B virus-DNA in the liver by in situ hybridization using a biotinylated probe. Relation to HBcAg expression and histology. J Hepatol. 1993;19:204–210. doi: 10.1016/s0168-8278(05)80572-0. [DOI] [PubMed] [Google Scholar]
- 326.Porter H.J., Padfield C.J., Peres L.C. Adenovirus and intranuclear inclusions in appendices in intussusception. J Clin Pathol. 1993;46:154–158. doi: 10.1136/jcp.46.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Schmidbauer M., Budka H., Ambros P. Herpes simplex virus (HSV) DNA in microglial nodular brainstem encephalitis. J Neuropathol Exp Neurol. 1989;48:645–652. doi: 10.1097/00005072-198911000-00006. [DOI] [PubMed] [Google Scholar]
- 328.Schmidbauer M., Budka H., Pilz P. Presence, distribution and spread of productive varicella zoster virus infection in nervous tissues. Brain. 1992;115(Pt 2):383–398. doi: 10.1093/brain/115.2.383. [DOI] [PubMed] [Google Scholar]
- 329.Thompson C.H., Biggs I.M., de Zwart-Steffe R.T. Detection of molluscum contagiosum virus DNA by in situ hybridization. Pathology. 1990;22:181–186. doi: 10.3109/00313029009086657. [DOI] [PubMed] [Google Scholar]
- 330.Unger E.R. In situ diagnosis of human papillomaviruses. Clin Lab Med. 2000;20:289–301. [PubMed] [Google Scholar]
- 331.Wu T.C., Mann R.B., Epstein J.I. Abundant expression of EBER1 small nuclear RNA in nasopharyngeal carcinoma. A morphologically distinctive target for detection of Epstein-Barr virus in formalin-fixed paraffin-embedded carcinoma specimens. Am J Pathol. 1991;138:1461–1469. [PMC free article] [PubMed] [Google Scholar]
- 332.Zaki S.R., Judd R., Coffield L.M. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1992;140:1345–1355. [PMC free article] [PubMed] [Google Scholar]
- 333.Akhtar N., Ni J., Langston C. PCR diagnosis of viral pneumonitis from fixed-lung tissue in children. Biochem Mol Med. 1996;58:66–76. doi: 10.1006/bmme.1996.0034. [DOI] [PubMed] [Google Scholar]
- 334.Amaker B.H., Chandler F.W., Jr., Huey L.O. Molecular detection of JC virus in embalmed, formalin-fixed, paraffin-embedded brain tissue. J Forensic Sci. 1997;42:1157–1159. [PubMed] [Google Scholar]
- 335.Beqaj S.H., Flesher R., Walker G.R. Use of the real-time PCR assay in conjunction with MagNA Pure for the detection of mycobacterial DNA from fixed specimens. Diagn Mol Pathol. 2007;16:169–173. doi: 10.1097/PDM.0b013e318037552e. [DOI] [PubMed] [Google Scholar]
- 336.Bhatnagar J., Guarner J., Paddock C.D. Detection of West Nile virus in formalin-fixed, paraffin-embedded human tissues by RT-PCR: A useful adjunct to conventional tissue-based diagnostic methods. J Clin Virol. 2007;38:106–111. doi: 10.1016/j.jcv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 337.Clavel C., Binninger I., Polette M. [Polymerase chain reaction (PCR) and pathology. Technical principles and application] Ann Pathol. 1993;13:88–96. [PubMed] [Google Scholar]
- 338.Guarner J., Bhatnagar J., Shieh W.J. Histopathologic, immunohistochemical, and polymerase chain reaction assays in the study of cases with fatal sporadic myocarditis. Hum Pathol. 2007;38:1412–1419. doi: 10.1016/j.humpath.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 339.Lamps L.W., Madhusudhan K.T., Greenson J.K. The role of Yersinia enterocolitica and Yersinia pseudotuberculosis in granulomatous appendicitis: A histologic and molecular study. Am J Surg Pathol. 2001;25:508–515. doi: 10.1097/00000478-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 340.Paddock C.D., Sanden G.N., Cherry J.D. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47:328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 341.Qian X., Jin L., Hayden R.T. Diagnosis of cat scratch disease with Bartonella henselae infection in formalin-fixed paraffin-embedded tissues by two different PCR assays. Diagn Mol Pathol. 2005;14:146–151. doi: 10.1097/01.pas.0000176771.64165.fb. [DOI] [PubMed] [Google Scholar]
- 342.Schild M., Gianinazzi C., Gottstein B. PCR-based diagnosis of Naegleria sp. infection in formalin-fixed and paraffin-embedded brain sections. J Clin Microbiol. 2007;45:564–567. doi: 10.1128/JCM.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Singh H.B., Katoch V.M., Natrajan M. Improved protocol for PCR detection of Mycobacterium leprae in buffered formalin-fixed skin biopsies. Int J Lepr Other Mycobact Dis. 2004;72:175–178. doi: 10.1489/1544-581X(2004)072<0175:IPFPDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 344.Tatti K.M., Wu K.H., Sanden G.N. Molecular diagnosis of Bordetella pertussis infection by evaluation of formalin-fixed tissue specimens. J Clin Microbiol. 2006;44:1074–1076. doi: 10.1128/JCM.44.3.1074-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wilson D.A., Reischl U., Hall G.S. Use of partial 16S rRNA gene sequencing for identification of Legionella pneumophila and non-pneumophila Legionella spp. J Clin Microbiol. 2007;45:257–258. doi: 10.1128/JCM.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


