Coronaviridae are enveloped nonsegmented, single-stranded, positive-sense RNA viruses named after their corona- or crown-like surface projections seen on electron microscopy that correspond to large surface spike proteins (Figure 222-1, Figure 222-2 ). Coronaviruses are host-specific and can infect humans as well as a variety of different animals, causing diverse clinical syndromes.1 Three serologically and genetically distinct groups of coronaviruses have been described. Human coronaviruses (HCoVs) are part of groups 1 and 2 and primarily cause a variety of respiratory tract infections1 (Table 222-1 ).
Figure 222-1.

Electron micrograph of a typical coronavirus. Negative-contrast electron micrograph of severe acute respiratory syndrome coronaviruses (SARS-CoV). The typical crown-like spike proteins on the surface of the coronavirus particles are shown. Bar = 100 nm.
(From Kuiken T, Fouchier RA, Schutten M, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003;362:263–270.)
Figure 222-2.
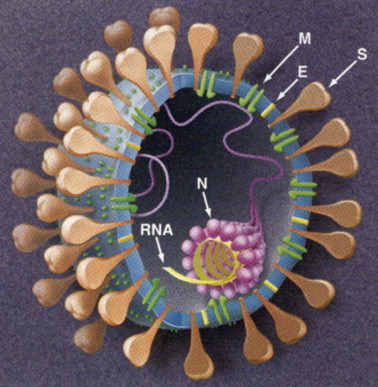
Pictorial illustration of a typical coronavirus. The organization of the spike (S), membrane (M), and envelope (E) glycoproteins is shown. The RNA is protected by the nucleocapsid proteins (N).
(From Holmes KV, Enjuanes L. The SARS coronavirus: a postgenomic era. Science 2003;300:1377–1378.)
TABLE 222-1.
Human and Representative Animal Coronaviruses (CoV)
| Group | Common Name of Virus | Acronym | Host | Associated Diseases |
|---|---|---|---|---|
| 1 | Human CoV-229E | HCoV-229E | Human | Respiratory tract infection |
| Human CoV-NL63 | HCoV-NL63 | Human | Respiratory tract infection | |
| Feline infectious peritonitis virus | FIPV | Cat | Hepatitis, respiratory tract, enteric, and neurologic infection | |
| 2 | Human CoV-OC43 | HCoV-OC43 | Human | Respiratory tract infection |
| Human CoV-HKU1 | HCoV-HKU1 | Human | Respiratory tract infection and possibly gastroenteritis | |
| Severe acute respiratory syndrome-CoVa | SARS-CoVa | Human | Severe acute respiratory syndrome (SARS) | |
| Mouse hepatitis virus | MHV | Mouse | Hepatitis, encephalitis, and enteric infection | |
| 3 | Infectious bronchitis virus | IBV | Chicken | Respiratory tract and enteric infection |
SARS-CoV appears to be an outlier of group 2 but some phylogenetic analyses suggest it is the first member of a fourth group of coronaviruses.131
Epidemiology
In the 1930s, coronaviruses were recognized as disease agents in animals.2 Thirty years later, coronaviruses were identified as agents of respiratory tract infections in humans. The first recognized HCoVs included 229E and OC43. Less well recognized strains, such as B814, OC16, OC37, and OC48, also were described but were not investigated further and to date little is known regarding their prevalence and associated clinical illnesses.3, 4, 5 Coronavirus-like particles also have been detected in stool as possible enteric pathogens, primarily in infants with gastroenteritis and necrotizing enterocolitis, but further characterization has been possible because viruses have not been culturable from these specimens.6, 7, 8 Dramatically, in 2003, severe acute respiratory syndrome (SARS)-CoV was identified as a novel respiratory pathogen responsible for a global outbreak of SARS. This outbreak lasted 9 months and ultimately resulted in 8098 people infected and 774 deaths.9, 10, 11, 12, 13 Most experts believe SARS-CoV evolved from a natural reservoir of SARS-CoV-like viruses in horseshoe bats, with civet cats serving as intermediate hosts.14, 15, 16, 17, 18 Finding a novel HCoV initiated a renewed interest in CoV research and 2 years later, NL63 (referred to in various publications as NL and NH) and HKU1 were identified as newly recognized HCoVs.19, 20, 21 HCoV-NL63 has since been shown to have been present in human respiratory samples as early as 1981.22 How HCoV-NL63 and HCoV-HKU1 relate to HCoVs originally described in the 1960s, such as B814, OC16, OC37, and OC48, or the enteric coronavirus-like particles detected in stool, is unclear.23
HCoVs other than SARS-CoV are found worldwide and cause disease predominantly in winter and spring months in temperate climates.22, 24 Seroprevalence data suggest that exposure is common in early childhood.25 Approximately 90% of adults are seropositive for HCoV-229E, HCoV-OC43, and HCoV-NL63 and 60% for HCoV-HKU1.26 SARS-CoV, on the other hand, has not been identified since December 2003/January 2004 when 4 sporadic cases of SARS with no associated transmission were identified in China27, community-acquired and 13 cases of laboratory-acquired SARS (2 isolated cases and a cluster of 11 cases with 1 death) were identified in Southeast Asia related to breaches in biosafety practices in different laboratories cultivating SARS-CoV.28, 29, 30 Modes of transmission for HCoV other than SARS-CoV have not been well studied. However, based on studies of other respiratory viruses, it is likely that transmission occurs primarily via a combination of droplet and direct and indirect contact spread.31 Which mode is most important remains to be determined, and the possible role of aerosol spread needs further study. Droplet and direct contact spread are likely the most common modes of transmission for SARS-CoV, although evidence for indirect contact spread and aerosol spread also exists.32, 33, 34, 35, 36, 37, 38, 39 There is no evidence of vertical transmission of SARS-CoV.40, 41
Based on data for HCoV-229E and HCoV-OC43, HCoVs other than SARS-CoV are most likely to be transmitted during the first few days of illness when symptoms and viral load in the respiratory tract is highest.42, 43 Further study is needed to confirm if this also is the case for the more recently identified HCoV-NL63 and HCoV-HKU1. SARS-CoV, on the other hand, is most likely to be transmitted during the second week of illness when both symptoms and viral load in the respiratory tract peak.44, 45, 46
The incubation period for HCoV-229E is 2 to 5 days (median 3 days).43, 47 Further study is needed to confirm the incubation periods for the other non-SARS-HCoVs. The incubation period for SARS-CoV is 2 to 10 days (median 4 days).44
Pathogenesis and Immunity
The pathogenesis of HCoV has been best described for 229E and SARS-CoV. For SARS-CoV, most evidence is from infections in adults given that few children were affected by the 2002–2003 outbreak.48 More study is needed to further understand the pathogenesis of other HCoVs.
HCoV-229E infections are initiated through inoculation of mucosal surfaces of the respiratory tract. HCoV-229E infection is associated with nasal mucosal plasma exudation and increased levels of interferon-γ (IFN-γ) in nasal lavage specimens, which correlate with symptom severity.49, 50 Viral load in respiratory tract specimens peaks within the first 3 days after infection and drops off dramatically at 1 week, correlating with development and subsequent improvement in symptoms.42, 51 Antibodies can be detected starting at 1 week, correlating with the drop in viral load, and reach a maximum levels approximately 1 week later.52 Thereafter, antibody titers decline slowly. Immunity is not complete and reinfection is common.52, 53 Higher circulating antibody levels and especially levels of specific IgA anti-HCoV correlate with reduced virus shedding and reduced symptoms upon re-exposure.52, 54
SARS-CoV infection most likely is initiated through inoculation of the respiratory tract mucosa. Subsequent viremia is followed by predominant replication in the lung and gastrointestinal tract.55, 56 Replication at other sites also likely occurs given the wide distribution of SARS-CoV in tissues examined at autopsy.57, 58 Peak viral loads in nasopharyngeal specimens are noted during the second week of symptoms.45, 56 A rise in SARS-CoV specific antibodies typically is seen starting at week 2 after infection. Increasing antibody titers and symptomatic improvement during the second and third week are associated with a fall in the quantity of SARS-CoV, as measured by reverse transcriptase polymerase chain reaction (RT-PCR).45, 59 Paradoxically, despite a fall in SARS-CoV viral load and a rise in SARS-specific antibodies, clinical deterioration is observed in some patients. This suggests that host immune responses likely are responsible for clinical deterioration.45 Indeed, SARS is associated with an elevation of IFN-γ, inflammatory cytokines interleukin (IL)-1, IL-6, and IL-12 as well as elevations in neutrophil chemokine IL-8, monocyte chemoattractant protein 1, and IFN-γ-inducible protein-10. Levels of IL-6 correlate with severity of disease.60, 61
Clinical Manifestations
HCoVs 229E, OC43, NL63, and HKU1, are commonly associated with the common cold, typically characterized by rhinorrhea, nasal congestion, sore throat, sneezing, and cough that may be associated with fever.20, 22, 51, 62, 63, 64, 65 Together, they are the next most common cause of the common cold after rhinoviruses.66, 67 Based on data for HCoV-229E, symptoms typically peak on day 3 or 4 of illness and are self-limiting.51, 68 These HCoVs also may be associated with acute otitis media or exacerbations of asthma.22, 63, 65, 69, 70 Less frequently, these viruses are associated with lower respiratory tract infections including bronchiolitis and pneumonia, primarily in infants and immunocompromised children and adults.21, 21, 63, 65, 71, 72, 73, 74, 75, 76, 77, 78, 79 Compared with other HCoVs, HCoV-NL63 more frequently is associated with croup, being the next most common isolate after parainfluenza virus type 1.80, 81 A possible association of HCoV-NL63 with Kawasaki disease was not substantiated.82, 83 HCoV-HKU1 has been associated with symptoms of gastroenteritis, including vomiting and diarrhea, that typically occur along with respiratory symptoms.65, 70, 84 HCoV-HKU1 also appears to be more frequently associated with febrile seizures compared with other HCoVs.65, 70
Compared with other HCoVs, SARS-CoV is associated with more severe symptoms.85, 86, 87 SARS-CoV disproportionately affects adults, who typically manifest fever, myalgia, headache, malaise, and chills followed by a nonproductive cough and dyspnea 3 to 5 days later. Approximately 25% develop watery diarrhea. Respiratory distress progresses to require intubation and ventilation in 25% of cases. The overall associated mortality rate is approximately 10%, most deaths occurring in the third week of illness.86 The case-fatality rate in persons over the age of 60 approaches 50%.88 Typical laboratory abnormalities include lymphopenia and increased serum lactate dehydrogenase and creatine kinase levels.89, 90 The majority have progressive unilateral or bilateral ill-defined air-space infiltrates on chest imaging.89, 91, 92, 93 Pneumothoraces and other signs of barotrauma are common in critically ill patients receiving mechanical ventilation.86
Infants and children appear to be protected against SARS-CoV infection, and clinical manifestations in infected children are less severe. Notably no infants or children died due to SARS-CoV infection in the 2002–2003 outbreak.48, 94, 95, 96, 97 Infants and children <12 years of age who develop SARS typically manifest fever, cough, and rhinorrhea. Associated lymphopenia is less severe and radiographic changes are milder and generally resolve more quickly than in adolescents and adults. Adolescents who developed SARS had clinical courses more closely resembling that of adults, manifesting fever, myalgia, headache, and chills. Adolescents are more likely to develop dyspnea, hypoxemia, and worsening chest radiographic findings. Laboratory abnormalities are comparable with those in adults.
Women infected with SARS-CoV during pregnancy who survive have an increased risk of spontaneous miscarriage, preterm delivery, and intrauterine growth restriction 40, 41, 98 Two neonates born to mothers with SARS in the 2002–2003 outbreak developed gastrointestinal complications (jejunal perforation, necrotizing enterocolitis with ileal perforation) shortly after birth but neither had clinical evidence of SARS-CoV infection.41 It is unclear whether these findings were related to complications of maternal SARS-CoV infection or treatments used during pregnancy, such as ribavirin and corticosteroids.
Diagnosis
In the past, the diagnosis of infections due to HCoVs typically was not attempted in clinical settings outside of outbreak situations or epidemiologic surveys. However, the 2002–2003 SARS outbreak renewed interest in identifying the etiology of respiratory tract infections and some specialized laboratories now offer comprehensive diagnostic testing for respiratory tract specimens primarily based on RT-PCR; some panels include detection of HCoV.65, 99 Antibody tests also are available for SARS-CoV.100
Upper and lower respiratory tract specimens are the most appropriate samples for viral detection when testing is available.42, 56, 63, 65, 101 Stool samples frequently are positive in patients with SARS and have been positive in some children with HCoV-HKU1 infection.56, 70, 84, 101 Serum samples may be positive in patients with SARS-CoV. For HCoV-299E and HCoV-OC43, specimens are most likely to be positive during the first few days of illness;42 whether this also is true for HCoV-NL63 and HCoV-HKU1 needs further study. For SARS-CoV, serum samples for RT-PCR testing are most likely to be positive in the first week of illness,55, 102 but respiratory and stool specimens may not be positive until the second week of illness when symptoms and viral loads peak.45, 56 Compared with adults, infants and children with SARS-CoV infections are less likely to have positive specimens. This is consistent with the milder symptoms and presumed correspondingly lower viral loads in children.94, 95
Laboratory guidance for SARS-CoV diagnostic testing is available on the Centers for Disease Control and Prevention website.103 Given the potential for false-positive results and the associated public health implications, testing for SARS-CoV in the absence of known person-to-person transmission of SARS-CoV only should be done with caution, preferably in consultation with regional public health departments, and when there is a high degree of clinical suspicion with no alternative diagnosis.
Treatment
Because of mild symptoms and the self-limited nature of HCoV infections other than SARS-CoV, few treatment studies have been performed. Generally, care is supportive. SARS-CoV infections are more serious. Corticosteroids, type 1 IFN agents, convalescent plasma, ribavirin, and lopinavir/ritonavir all have been used to treat SARS.89, 104, 105, 106, 107 For most of these treatments, anecdotal reports suggest benefit, and in vitro assays and animal models are supportive.89, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 Despite some reports of anecdotal clinical improvement with ribavirin, however, in vitro studies do not support likely efficacy.113, 117, 118 Since the SARS outbreak, other agents have been tested in vitro and appear promising. These include viral entry and protease inhibiting agents, RNA interfering agents, and glycyrrhizin.119 However, no definitive conclusions can be drawn regarding efficacy of these treatments. This is noteworthy because controlled studies have not been performed for any of these agents, and there are reports of uneventful recovery for patients given supportive care alone. In the event that SARS-CoV re-emerges, clarification of the effectiveness of these treatments through controlled clinical trials will be needed.
Prevention
Meticulous hand and respiratory hygiene is the most useful and easily implemented control measure to curb the spread of all respiratory viruses including HCoVs.120, 121 Other preventive measures have been assessed. Prophylactic intranasal IFN-α has been shown to reduce the duration and severity of 229E infection in research settings but has not been used clinically.122, 123 IFN-α has not been studied for prevention of other HCoVs. A proprietary extract of the roots of North American ginseng (Panax quinquefolium) has been shown to reduce the number of colds as well as the severity and duration of cold symptoms in adults when taken daily, presumably due to immune stimulation.124, 125, 126, 127 Efficacy for decrease in colds specifically due to HCoVs has not been studied.
Healthcare personnel should wear a mask when evaluating persons with a cough illness and should use a gown, gloves, mask, and eye protection for the duration of illness when caring for children hospitalized with signs and symptoms of a respiratory tract infection.128 The same precautions, with the replacement of the mask with a respirator, if available, plus negative-pressure isolation are recommended for patients with SARS-CoV infection for the duration of illness or 10 days after resolution of fever, provided respiratory symptoms are absent or improving.128 Cleaning and disinfection of environmental surfaces that are frequently touched by infected persons, using standard disinfectants, should decrease the potential for indirect transmission of HCoVs via fomites.129
The control of the 2002–2003 SARS outbreak is credited to the rapid identification of cases and early implementation of infection control and public health measures including contact tracing and quarantine. If SARS-CoV re-emerges, all measures should be implemented quickly in an attempt to prevent a recurrent worldwide outbreak.130, 131
Key Points.: Epidemiology, Clinical Manifestations, Diagnosis, and Treatment of Human Coronavirus (HCoV) Infections.
Epidemiology
-
•
HCoVs 229E, OC43, NL63, and HKU1 – found worldwide; exposure common in early childhood; in temperate climates, primarily causes infections in winter and spring months
-
•
SARS-CoV – not identified in the world since January 2004 (soon after the 2002–2003 global outbreak of SARS); possibility/probability of a large-scale re-emergence of SARS is unknown
-
•
Most common modes of transmission are through droplet and direct and indirect contact
Clinical Manifestations
-
•
HCoVs 229E, OC43, NL63, and HKU1 – associated with the common cold, acute otitis media, asthma exacerbations, and less frequently, bronchiolitis and pneumonia; HCoV-NL63 – also associated with croup; HCoV-HKU1 – also associated with vomiting and diarrhea frequently with respiratory tract symptoms; appears to be associated more frequently with febrile seizures compared with other HCoVs
-
•
SARS-CoV – associated with SARS with an attendant mortality rate of 10% which primarily affects adults and adolescents; children <12 years of age who develop SARS typically have less severe manifestations (fever, cough, and rhinorrhea)
Diagnosis
-
•
Upper and lower respiratory tract specimens can be tested by HCoV RT-PCR
-
•
Stool samples frequently are positive in patients with SARS-CoV and have been positive in some children with HCoV-HKU1 infection; antibody tests also are available for SARS-CoV
Treatment
-
•
HCoVs 229E, OC43, NL63, and HKU1 – supportive care
-
•
SARS-CoV – no definitive treatment can be recommended because of lack of controlled trials
References
- 1.Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn JS. The widening scope of coronaviruses. Curr Opin Pediatr. 2006;18:42–47. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh K. Coronaviruses in the limelight. J Infect Dis. 2005;191:489–491. doi: 10.1086/428510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;5448:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntosh K, Dees JH, Becker WB. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen ML, Ray CG, Payne CM. Coronaviruslike particles in human gastrointestinal disease. Epidemiologic, clinical, and laboratory observations. Am J Dis Child. 1985;139:928–934. doi: 10.1001/archpedi.1985.02140110082036. [DOI] [PubMed] [Google Scholar]
- 7.Resta S, Luby JP, Rosenfeld CR. Isolation and propagation of a human enteric coronavirus. Science. 1985;229:978–981. doi: 10.1126/science.2992091. [DOI] [PubMed] [Google Scholar]
- 8.Gerna G, Passarani N, Battaglia M. Human enteric coronaviruses: antigenic relatedness to human coronavirus OC43 and possible etiologic role in viral gastroenteritis. J Infect Dis. 1985;151:796–803. doi: 10.1093/infdis/151.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 10.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 11.Peiris JS, Lai ST, Poon LL. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christian MD, Poutanen SM, Loutfy MR. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SARS: breaking the chains of transmission. http://www.who.int/features/2003/07/en/ World Health Organization. Available at. Accessed May 3, 2011.
- 14.Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 15.Guan Y, Zheng BJ, He YQ. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 16.Lau SK, Woo PC, Li KS. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 18.Cheng VC, Lau SK, Woo PC. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der HL, Pyrc K, Jebbink MF. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouchier RA, Hartwig NG, Bestebroer TM. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo PC, Lau SK, Chu CM. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot HK, Shepherd BE, Crowe JE., Jr The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28:682–687. doi: 10.1097/INF.0b013e31819d0d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esper F, Weibel C, Ferguson D. Coronavirus HKU1 infection in the United States. Emerg Infect Dis. 2006;12:775–779. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vabret A, Dina J, Gouarin S. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health. 2008;44:176–181. doi: 10.1111/j.1440-1754.2007.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dijkman R, Jebbink MF, El Idrissi NB. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46:2368–2373. doi: 10.1128/JCM.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severance EG, Bossis I, Dickerson FB. Development of a nucleocapsid-based human coronavirus immunoassay and estimates of individuals exposed to coronavirus in a U.S. metropolitan population. Clin Vaccine Immunol. 2008;15:1805–1810. doi: 10.1128/CVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang G, Chen Q, Xu J. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis. 2004;10:1774–1781. doi: 10.3201/eid1010.040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim PL, Kurup A, Gopalakrishna G. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350:1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 29.SARS case in laboratory worker in Taiwan, China. http://www.who.int/mediacentre/releases/2003/np26/en World Health Organization. Available at. Accessed May 3, 2011.
- 30.ProMED-mail. SARS – Worldwide (30): China, Cases. http://www.promedmail.org ProMED-mail. Available at. (archive number 20040703.1774). Accessed May 3, 2011.
- 31.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19:S97–102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lau JT, Lau M, Kim JH. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10:235–243. doi: 10.3201/eid1002.030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeb M, McGeer A, Henry B. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scales DC, Green K, Chan AK. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong TW, Lee CK, Tam W. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu IT, Li Y, Wong TW. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 37.Olsen SJ, Chang HL, Cheung TY. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 38.Chen YC, Huang LM, Chan CC. SARS in hospital emergency room. Emerg Infect Dis. 2004;10:782–788. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowell SF, Simmerman JM, Erdman DD. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong SF, Chow KM, Leung TN. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shek CC, Ng PC, Fung GP. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 42.van Elden LJ, van Loon AM, van AF. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradburne AF, Bynoe ML, Tyrrell DA. Effects of a “new” human respiratory virus in volunteers. Br Med J. 1967;3:767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) http://www.who.int/csr/sars/en/WHOconsensus.pdf World Health Organization. Available at. Accessed May 3, 2011.
- 45.Peiris JS, Chu CM, Cheng VC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng PK, Wong DA, Tong LK. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lessler J, Reich NG, Brookmeyer R. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denison MR. Severe acute respiratory syndrome coronavirus pathogenesis, disease and vaccines: an update. Pediatr Infect Dis J. 2004;23:S207–S214. doi: 10.1097/01.inf.0000144666.95284.05. [DOI] [PubMed] [Google Scholar]
- 49.Akerlund A, Greiff L, Andersson M. Mucosal exudation of fibrinogen in coronavirus-induced common colds. Acta Otolaryngol. 1993;113:642–648. doi: 10.3109/00016489309135878. [DOI] [PubMed] [Google Scholar]
- 50.Linden M, Greiff L, Andersson M. Nasal cytokines in common cold and allergic rhinitis. Clin Exp Allergy. 1995;25:166–172. doi: 10.1111/j.1365-2222.1995.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiol Infect. 1993;111:143–156. doi: 10.1017/s0950268800056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callow KA, Parry HF, Sergeant M. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monto AS, Lim SK. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129:271–276. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callow KA. Effect of specific humoral immunity and some non-specific factors on resistance of volunteers to respiratory coronavirus infection. J Hyg (Lond) 1985;95:173–189. doi: 10.1017/s0022172400062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng EK, Hui DS, Chan KC. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49:1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan KH, Poon LL, Cheng VC. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding Y, He L, Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farcas GA, Poutanen SM, Mazzulli T. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Shi Y, Li P. Profile of antibodies to the nucleocapsid protein of the severe acute respiratory syndrome (SARS)-associated coronavirus in probable SARS patients. Clin Diagn Lab Immunol. 2004;11:227–228. doi: 10.1128/CDLI.11.1.227-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong CK, Lam CW, Wu AK. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Li J, Zhan Y. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendley JO, Fishburne HB, Gwaltney JM., Jr Coronavirus infections in working adults. Eight-year study with 229 E and OC 43. Am Rev Respir Dis. 1972;105:805–811. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 63.Bastien N, Robinson JL, Tse A. Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol. 2005;43:4567–4573. doi: 10.1128/JCM.43.9.4567-4573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bastien N, Anderson K, Hart L. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau SK, Woo PC, Yip CC. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makela MJ, Puhakka T, Ruuskanen O. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Zalm MM, van Ewijk BE, Wilbrink B. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396–400. doi: 10.1016/j.jpeds.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pappas DE, Hendley JO, Hayden FG. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27:8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- 69.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vabret A, Dina J, Gouarin S. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis. 2006;42:634–639. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McIntosh K, Chao RK, Krause HE. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974;130:502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pene F, Merlat A, Vabret A. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37:929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riski H, Hovi T. Coronavirus infections of man associated with diseases other than the common cold. J Med Virol. 1980;6:259–265. doi: 10.1002/jmv.1890060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arden KE, Nissen MD, Sloots TP. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esper F, Weibel C, Ferguson D. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiu SS, Chan KH, Chu KW. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebihara T, Endo R, Ma X. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75:463–465. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moes E, Vijgen L, Keyaerts E. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect Dis. 2005;5:6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sung JY, Lee HJ, Eun BW. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. 2010;29:822–826. doi: 10.1097/INF.0b013e3181e7c18d. [DOI] [PubMed] [Google Scholar]
- 81.van der HL, Sure K, Ihorst G. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2:e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang LY, Chiang BL, Kao CL. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193:283–286. doi: 10.1086/498875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esper F, Shapiro ED, Weibel C. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esper F, Ou Z, Huang YT. Human coronaviruses are uncommon in patients with gastrointestinal illness. J Clin Virol. 2010;48:131–133. doi: 10.1016/j.jcv.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsui PT, Kwok ML, Yuen H. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fowler RA, Lapinsky SE, Hallett D. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 87.Lew TW, Kwek TK, Tai D. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 88.Donnelly CA, Ghani AC, Leung GM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 90.Wang JT, Sheng WH, Fang CT. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong KT, Antonio GE, Hui DS. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 92.Muller NL, Ooi GC, Khong PL. Severe acute respiratory syndrome: radiographic and CT findings. AJR Am J Roentgenol. 2003;181:3–8. doi: 10.2214/ajr.181.1.1810003. [DOI] [PubMed] [Google Scholar]
- 93.Wong KT, Antonio GE, Hui DS. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 94.Bitnun A, Allen U, Heurter H. Children hospitalized with severe acute respiratory syndrome-related illness in Toronto. Pediatrics. 2003;112:e261. doi: 10.1542/peds.112.4.e261. [DOI] [PubMed] [Google Scholar]
- 95.Hon KL, Leung CW, Cheng WT. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang GG, Lin SZ, Liao KW. SARS-associated coronavirus infection in teenagers. Emerg Infect Dis. 2004;10:382–383. doi: 10.3201/eid1002.030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu WK, Cheung PC, Ng KL. Severe acute respiratory syndrome in children: experience in a regional hospital in Hong Kong. Pediatr Crit Care Med. 2003;4:279–283. doi: 10.1097/01.PCC.0000077079.42302.81. [DOI] [PubMed] [Google Scholar]
- 98.Ng PC, Leung CW, Chiu WK. SARS in newborns and children. Biol Neonate. 2004;85:293–298. doi: 10.1159/000078174. [DOI] [PubMed] [Google Scholar]
- 99.Poon LL, Chan KH, Wong OK. Early diagnosis of SARS coronavirus infection by real time RT-PCR. J Clin Virol. 2003;28:233–238. doi: 10.1016/j.jcv.2003.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan PK, Ng KC, Chan RC. Immunofluorescence assay for serologic diagnosis of SARS. Emerg Infect Dis. 2004;10:530–532. doi: 10.3201/eid1003.030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang P, Louie M, Richardson SE. Interpretation of diagnostic laboratory tests for severe acute respiratory syndrome: the Toronto experience. CMAJ. 2004;170:47–54. [PMC free article] [PubMed] [Google Scholar]
- 102.Grant PR, Garson JA, Tedder RS. Detection of SARS coronavirus in plasma by real-time RT-PCR. N Engl J Med. 2003;349:2468–2469. doi: 10.1056/NEJM200312183492522. [DOI] [PubMed] [Google Scholar]
- 103.Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS) (Supplement F: Laboratory Guidance) http://www.cdc.gov/ncidod/sars/guidance/index.htm Centers for Disease Control and Prevention. Available at. Accessed May 3, 2011.
- 104.Chan KS, Lai ST, Chu CM. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 105.Loutfy MR, Blatt LM, Siminovitch KA. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 106.Wong VW, Dai D, Wu AK. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 107.Zhao Z, Zhang F, Xu M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- 108.Barnard DL, Hubbard VD, Burton J. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-D-N4-hydroxycytidine. Antivir Chem Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. [DOI] [PubMed] [Google Scholar]
- 109.Chu CM, Cheng VC, Hung IF. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cinatl J, Jr, Michaelis M, Scholz M. Role of interferons in the treatment of severe acute respiratory syndrome. Expert Opin Biol Ther. 2004;4:827–836. doi: 10.1517/14712598.4.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haagmans BL, Kuiken T, Martina BE. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hensley LE, Fritz LE, Jahrling PB. Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stroher U, DiCaro A, Li Y. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon- alpha. J Infect Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tan EL, Ooi EE, Lin CY. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamamoto N, Yang R, Yoshinaka Y. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng B, He ML, Wong KL. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma) J Interferon Cytokine Res. 2004;24:388–390. doi: 10.1089/1079990041535610. [DOI] [PubMed] [Google Scholar]
- 117.Cinatl J, Morgenstern B, Bauer G. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhaori G. Antiviral treatment of SARS: can we draw any conclusions? CMAJ. 2003;169:1165–1166. [PMC free article] [PubMed] [Google Scholar]
- 119.Groneberg DA, Poutanen SM, Low DE. Treatment and vaccines for severe acute respiratory syndrome. Lancet Infect Dis. 2005;5:147–155. doi: 10.1016/S1473-3099(05)01307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts L, Smith W, Jorm L. Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized, controlled trial. Pediatrics. 2000;105:738–742. doi: 10.1542/peds.105.4.738. [DOI] [PubMed] [Google Scholar]
- 121.Apisarnthanarak A, Apisarnthanarak P, Cheevakumjorn B. Implementation of an infection control bundle in a school to reduce transmission of influenza-like illness during the novel influenza A 2009 H1N1 pandemic. Infect Control Hosp Epidemiol. 2010;31:310–311. doi: 10.1086/651063. [DOI] [PubMed] [Google Scholar]
- 122.Turner RB, Felton A, Kosak K. Prevention of experimental coronavirus colds with intranasal alpha-2b interferon. J Infect Dis. 1986;154:443–447. doi: 10.1093/infdis/154.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Higgins PG, Phillpotts RJ, Scott GM. Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers. Antimicrob Agents Chemother. 1983;24:713–715. doi: 10.1128/aac.24.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McElhaney JE, Goel V, Toane B. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. J Altern Complement Med. 2006;12:153–157. doi: 10.1089/acm.2006.12.153. [DOI] [PubMed] [Google Scholar]
- 125.Predy GN, Goel V, Lovlin R. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173:1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang M, Guilbert LJ, Ling L. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium) J Pharm Pharmacol. 2001;53:1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 127.Wang M, Guilbert LJ, Li J. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4:311–315. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 128.Siegel JD, Rhinehart E, Jackson M. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf Centers for Disease Control and Prevention. Available at. Accessed May 3, 2011. [DOI] [PMC free article] [PubMed]
- 129.Rabenau HF, Cinatl J, Morgenstern B. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roper RL, Rehm KE. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8:887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stadler K, Masignani V, Eickmann M. SARS – beginning to understand a new virus. Nat Rev Microbiol. 2003;1:209–218. doi: 10.1038/nrmicro775. [DOI] [PMC free article] [PubMed] [Google Scholar]


