INTRODUCTION
Since the 1980s, surgical pathologists in general and infectious disease pathologists in particular have dealt with an increasing number of surgical specimens from patients in whom one or multiple infectious agents may be responsible for disease.1 In this context, pathologists have played an important role in recognizing infectious agents. In many cases, when fresh tissue is not available for culture, pathologists can provide a rapid morphologic diagnosis and facilitate clinical decisions in patient treatment.2 In addition, pathologists have played a central role in the identification of emerging and reemerging infectious agents and describing the pathogenetic processes of emerging diseases, such as hantavirus pulmonary syndrome and other viral hemorrhagic fevers, leptospirosis, and rickettsial and ehrlichial infections as well as the diagnosis of anthrax during the bioterrorist attack of 2001.3, 4, 5, 6, 7
Conventionally, microbial identification in infectious diseases has been made primarily by using serologic assays and culture techniques. However, serologic results can be difficult to interpret in the setting of immunosuppression or when only a single sample is available for evaluation. In addition, fresh tissue is not always available for culture, and culture of fastidious pathogens can be difficult and may take weeks or months to yield results. Moreover, culture alone cannot distinguish colonization from tissue invasion. Some microorganisms have distinctive morphologic characteristics that allow their identification in formalinfixed tissues using routine and special stains. Nevertheless, in several instances it is difficult or even impossible to identify an infectious agent specifically by conventional morphologic methods.
Immunohistochemistry is one of the most powerful techniques in surgical pathology. There has been an increasing interest in the use of specific antibodies to viral, bacterial, fungal and parasitic antigens in the detection and identification of the causative agents in many infectious diseases. The use of a specific antibody to detect a microbial antigen was first performed by Coons and associates8 to detect pneumococcal antigen in tissues. The advantages of immunohistochemistry over conventional staining methods (Table 2.1 ) and the contributions of immunohistochemistry in infectious diseases (Table 2.2 ) are substantial. It is important to emphasize that both monoclonal and polyclonal antibodies must be tested for possible cross-reactivities with other organisms. The widespread occurrence of common antigens among bacteria and pathogenic fungi is well established.1, 9 Finally it is important to understand that immunohistochemistry has several steps and all of them can affect the final result; however, in general the only limitations are the availability of specific antibodies and the preservation of epitopes.1, 10 It is well known that for larger microorganisms such as protozoa, fungi and some bacteria, pretreatment of formalinfixed, paraffin-embedded tissue is not required. In contrast, for smaller infectious agents, for example, microorganisms such as viruses and chlamydiae, pretreatment of the tissue with proteolytic enzymes or heat-induced epitope retrieval is necessary in order to enhance immunoreactivity.
Table 2.1.
Advantages of IHC for the diagnosis of infectious diseases
| Allows rapid results |
| Can be performed on formalinfixed, paraffin-embedded tissue, reducing the risk of exposure to serious infectious diseases |
| High sensitivity allowing identification of infectious agents even before morphologic changes occur |
| Useful for retrospective diagnosis of individual patients and for in-depth study of the disease |
| Specificity: monoclonal antibodies and some polyclonal antibodies allow for specific identification of infectious agents |
Table 2.2.
Contributions of IHC to the diagnosis of infectious diseases
| Allows identification of new human pathogens |
| Allows microbiologic/morphologic correlation establishing the pathogenic significance of microbiological results |
| Provides a rapid morphologic diagnosis allowing early treatment of serious infectious diseases |
| Contributes to understanding of the pathogenesis of infectious diseases |
| Provides a diagnosis when fresh tissue is not available or when culture methods do not exist |
Table 2.3 lists currently available antibodies for diagnostic use in surgical pathology.
Table 2.3.
Some commercially available antibodies for immunohistochemical diagnosis of infectious diseases
| Microorganism | Antibody/clone | Dilution | Pretreatment | Source |
|---|---|---|---|---|
| Adenovirus | Mab/20/11 and 2/6 | 1:2000 | Proteinase K | Chemicon |
| Aspergillus | Mab/WF-AF-1 | 1:200 | HIAR* | Dako |
| B. henselae | Mab | 1:100 | HIAR | Biocare Medical |
| BK virus | Mab/BK T. 1 | 1:8000 | Trypsin | Chemicon |
| C. albicans | Mab/1B12 | 1:400 | HIAR | Chemicon |
| C. pneumoniae | Mab/RR402 | 1:200 | HIAR | Accurate |
| Cryptosporidium | Mab/Mabc1 | 1:100 | HIAR | Novocastra |
| CMV | Mab/DDG9/CCH2 | 1:50 | HIAR | Novocastra |
| Giardia intestinalis | Mab/9D5.3.1 | 1:50 | HIAR | Novocastra |
| Hepatitis B core Ag. | Rabbit polyclonal | 1:2000 | HIAR | Dako |
| Hepatitis B surface Ag. | Mab/3E7 | 1:100 | HIAR | Dako |
| Herpes simplex 1 and 2 | Rabbit polyclonal | 1:3200 | HIAR | Dako |
| H. pylori | Rabbit polyclonal | 1:40 | Protase 1 | Dako |
| HHV 8 | Mab/LNA-1 | 1:500 | HIAR | Novocastra |
| L. monocytogenes | Rabbit polyclonal | 1:5000 | Proteinase K | Difco |
| Parvovirus B19 | Mab/R92F6 | 1:500 | HIAR | Novocastra |
| P. carinii | Mab/3F6 | 1:20 | HIAR | Novocastra |
| Respiratory syncytial virus | Mab/5H5N | 1:200 | HIAR | Novocastra |
| T. gondii | Rabbit polyclonal | 1:320 | HIAR | Biogenex |
| West Nile virus | Mab/5H10 | 1:400 | Proteinase K | Bioreliance |
Heat-induced antigen retrieval.
VIRAL INFECTIONS
Immunohistochemistry has played an important role not only in the diagnosis of a large number of viral infections but also in the study of their pathogenesis and epidemiology. Traditionally, the diagnosis of viral infections has relied on cytopathic changes observed on routine histopathology. Several viral pathogens produce characteristic intracellular inclusions, which allow pathologists to make a presumptive diagnosis of viral infection. However, for some viral infections the characteristic cytopathic changes are often subtle and sparse, requiring a meticulous search.11 Moreover, only 50% of the known viral diseases are associated with characteristic intracellular inclusions.12 In addition, formalin, which is the most commonly used fixative in histopathology, is a poor fixative for demonstrating the morphologic and tinctorial features of viral inclusions.12 When viral inclusions are not detected in hematoxylin-eosin stained sections, or when the viral inclusions present cannot be differentiated from those of other viral diseases, immunohistochemical techniques offer a more reliable alternative to reach a specific diagnosis.
Hepatitis B virus
Hepatitis B virus infection constitutes an important cause of chronic hepatitis in a significant proportion of patients. In many instances, the morphologic changes induced by hepatitis B virus on hepatocytes are not typical enough to render a presumptive diagnosis of hepatitis B viral infection. In other instances, there may be so little hepatitis B surface antigen (HBsAg) that it cannot be demonstrated by techniques such as orcein staining. In these cases, immunohistochemical techniques to detect HBsAg are more sensitive than histochemical methods and are helpful in reaching the diagnosis.13 Immunostaining for HBsAg has been used in the diagnosis of hepatitis B and in the study of carrier states.14, 15 Eighty percent or more of cases with positive serologic results for HBsAg demonstrate cytoplasmic HBsAg using immunohistochemistry (Fig. 2.1 ).16 By immunoperoxidase localization, hepatitis B core antigen (HBcAg) can be demonstrated within the nuclei or the cytoplasm of hepatocytes, or both (Fig. 2.2 ). Predominantly cytoplasmic expression of HBcAg is associated with a higher grade of hepatitis activity.17
Fig. 2.1.

Liver biopsy specimen from a patient with chronic hepatitis B. Scattered hepatocytes show cytoplasmic reactivity with monoclonal antibody to HBsAg. (Immunoperoxidase staining with diaminobenzidine [DAB] and hematoxylin counterstain, ×400)
Fig. 2.2.

Chronic active hepatitis B. Numerous hepatocytes display intranuclear reactivity with polyclonal antibody to hepatitis B core antigen (HBcAg). (Immunoperoxidase staining with DAB and hematoxylin counterstain, ×400)
Hepatitis C virus
The clinical diagnosis of hepatitis C virus (HCV) infection is based on serological demonstration of antibodies against HCV and detection of HCV RNA in serum. However, anti-HCV antibodies may be not detectable in sera of immunocompromised patients.18 Several polyclonal and monoclonal antibodies directed against HCV nonstructural proteins have been produced for use in immunohistochemistry. Nevertheless, most of the antibodies are not clinically useful because of low sensitivities compared with HCV RNA detection by RT-PCR.18, 19, 20, 21 Moreover, cross-reactivity with non-HCV epitopes has been found with the monoclonal antibody TORDJI-22.21, 22 Diffuse or coarse granular cytoplasmic staining is usually seen in a variable number of hepatocytes in patients with chronic HCV hepatitis,19, 23, 24 and within rare biliary epithelial cells, lymphocytes, and sinusoidal endothelial cells.
More recently, a monoclonal antibody against HCV E2 envelope glycoprotein has been demonstrated to be a highly sensitive antibody for the diagnosis and clinical follow-up of chronic HCV hepatitis with an overall accuracy of 95% when used with the EnVision technique.25 This antibody is useful for the early detection of graft reinfection in patients with liver transplant for HCV-related cirrhosis, and to differentiate reinfection from graft rejection.25
Herpesviruses
Histologically, the diagnosis of herpes simplex virus (HSV) infection involves the detection of multinucleated giant cells containing characteristic molded, ground glass-appearing nuclei and Cowdry's type A intranuclear inclusions. When there are abundant viral inclusions within infected cells, the diagnosis is usually straightforward. However, the diagnosis of HSV infection can be difficult when the characteristic intranuclear inclusions or multinucleated cells, or both, are absent or when the amount of tissue in a biopsy specimen is small.26 In these cases, the use of immunohistochemistry to detect HSV antigens is advantageous.27, 28
Immunohistochemistry using either polyclonal or monoclonal antibodies against HSV antigens has proven to be a sensitive and specific technique to diagnose HSV infections (Fig. 2.3 ).29, 30 Although polyclonal antibodies against the major HSV glycoprotein antigens are sensitive, they do not allow distinction between HSV-1 and HSV-2 because these two viruses are antigenically similar.31 In addition, the histologic features of HSV infection are not specific and can also occur in patients with varicella-zoster (VZV) infection. Monoclonal antibodies against the VZV envelope glycoprotein gp1 are sufficiently sensitive and specific to allow a clear-cut distinction between HSV and VZV infections.27, 32, 33
Fig. 2.3.

Herpes simplex hepatitis. The nuclei and cytoplasm of many hepatocytes and Kupffer cells are strongly immunostained for herpes simplex antigen. (Immunoperoxidase staining with DAB and hematoxylin counterstain, ×400)
Immunohistochemistry has also been useful in demonstrating the association of human herpesvirus 8 (HHV-8) with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease.34, 35, 36, 37, 38 The diagnosis of Kaposi's sarcoma may be problematic due to its broad morphologic spectrum and similar appearance to other benign and malignant neoplastic vascular lesions. Immunostaining of Kaposi's sarcoma latent associated nuclear antigen-1 (LANA-1) is useful to confirm the diagnosis of Kaposi's sarcoma, particularly in difficult early lesions or when the neoplasm presents in an unusual location, and allows distinction of Kaposi's sarcoma from several morphologically similar vasoproliferative lesions (see Chapter 12).39, 40 Immunostaining is restricted to the nuclei of spindle cells and endothelial cells of the slit-like vascular spaces. Immunohistochemistry has also demonstrated expression of HHV-8 LANA-1 in mesothelial cells of HIV-associated recurrent pleural effusions41 and in the cells of the plexiform lesions of primary pulmonary hypertension.42
Cytomegalovirus (CMV) is an important opportunistic pathogen in immunocompromised patients. Histologic diagnosis of CMV in fixed tissues usually rests on the identification of characteristic cytopathic effects, including intranuclear or cytoplasmic inclusions, or both. However, histologic examination lacks sensitivity, and in some cases atypical cytopathic features can be confused with reactive or degenerative changes.43 In these cases, immunohistochemistry using monoclonal antibodies against early and late CMV antigens allows the detection of CMV antigens in the nucleus and cytoplasm of infected cells (Fig. 2.4 ). In addition, immunohistochemistry may allow detection of CMV antigens early in the course of the disease when cytopathic changes have not yet developed.44, 45, 46, 47, 48, 49 For example, CMV early nuclear antigen is expressed 9 to 96 hours after cellular infection and indicates early active viral replication. Immunohistochemistry has been useful in the detection of CMV infection in patients with steroid refractory ulcerative colitis, and in detecting occult CMV infection of the central nervous system in liver transplant patients who develop neurological complications.50, 51 It has also been used to demonstrate a high frequency of CMV antigens in tissues from first trimester abortions.52 The sensitivity of immunohistochemistry is better than light microscopic identification of viral inclusions and compares favorably with culture and in situ hybridization.44, 46, 47, 49, 53 Additionally, immunohistochemical assays can be completed faster than the shell vial technique, with immunofluorescence, or culture allowing for rapid results that are important for early anti-CMV therapy.49
Fig. 2.4.

Cytomegalovirus (CMV) villitis in a case of congenital CMV infection. Stromal cells and Hofbauer cells show intranuclear and cytoplasmic CMV antigen. (Immunoperoxidase staining with diaminobenzidine [DAB] and hematoxylin counterstain, ×400)
Adenoviruses
Adenovirus is increasingly recognized as a cause of morbidity and mortality among immunocompromised patients owing to transplant and congenital immunodeficiency.54, 55 Rarely, adenovirus infection has been described in HIV-infected patients.56, 57, 58 Characteristic adenovirus inclusions are amphophilic, intranuclear, homogeneous, and glassy. However, in some cases, the infection may contain only rare cells showing the characteristic cytopathic effect.57 In addition, other viral inclusions, including CMV, human papillomavirus, HSV, and VZV, can be mistaken for adenovirus inclusions and vice versa. Moreover, in immunosuppressed patients the incidence of coinfection with other viruses is high. In these circumstances immunohistochemical assay may be necessary for a definitive diagnosis. A monoclonal antibody that is reactive with all 41 serotypes of adenovirus has been used in an immunohistochemical technique to demonstrate intranuclear adenoviral antigen in immunocompromised patients (Fig. 2.5 ).57, 58, 59, 60, 61 Histologic diagnosis of adenovirus colitis is difficult, and it is usually underdiagnosed. Immunohistochemical staining has been of value in differentiating adenovirus colitis from CMV colitis.57, 62
Fig. 2.5.

Adenovirus pneumonia. Infected cells within a necrotizing exudate show intranuclear reactivity with antibody to adenovirus antigen. (Immunoperoxidase staining with aminoethylcarbazole [AEC] and hematoxylin counterstain, ×400)
Other herpesviruses infections that have been diagnosed using immunohistochemical methods include Epstein-Barr viral infection63 and human herpesvirusus 6 infection.64
Parvovirus B19
Parvovirus B19 has been associated with asymptomatic infections, erythema infectiosum, acute arthropathy, aplastic crisis, hydrops fetalis, and chronic anemia and red cell aplasia. The diagnosis of parvovirus infection can be achieved by identifying typical findings in bone marrow specimens, including decreased or absent red cell precursors, giant pronormoblasts, and eosinophilic or amphophilic intranuclear inclusions in erythroid cells.65, 66 Because intravenous immunoglobulin therapy is effective, a rapid and accurate diagnostic method is important. Immunohistochemistry with a monoclonal antibody against VP1 and VP2 capsid proteins has been used as a rapid and sensitive method to establish the diagnosis of parvovirus B19 infection in formalinfixed, paraffin-embedded tissues.67, 68, 69, 70 Immunohistochemistry is of particular help in detecting parvovirus B19 antigen in cases with sparse inclusions, to study cases not initially identified by examination of routinely stained tissue sections, or in cases of hydrops fetalis where there is advanced cytolysis (Fig. 2.6 ).67, 71, 72 Several studies have found a good correlation between morphologic, immunohistochemical, in situ hybridization and polymerase chain reaction (PCR).66, 67, 70, 72
Fig. 2.6.

Hydrops fetalis caused by parvovirus B19 infection. Normoblasts within the villous capillaries show intranuclear viral antigen. (Immunoperoxidase staining with DAB and hematoxylin counterstain, ×600)
Viral hemorrhagic fevers
Since the 1980s, numerous emerging and reemerging agents of viral hemorrhagic fevers have attracted the attention of pathologists.3, 4, 5 These investigators have played an important role in the identification of these agents and supporting epidemiologic, clinical, and pathogenetic studies of the emerging viral hemorrhagic fevers.4, 5, 7 Viral hemorrhagic fevers are often fatal, and in the absence of bleeding or organ manifestations these diseases are clinically difficult to diagnose and frequently require handling and testing of potentially dangerous biological specimens. In addition, histopathologic features are not pathognomonic, and they can resemble other viral, rickettsial and bacterial (e.g., leptospirosis) infections. Immunohistochemistry is essential and has been successfully and safely applied to the diagnosis and study of the pathogenesis of these diseases.
Several studies have established the utility of immunohistochemistry as a sensitive, safe, and rapid diagnostic method for the diagnosis of viral hemorrhagic fevers such as yellow fever (Fig. 2.7 ),73, 74, 75 dengue hemorrhagic fever,75, 76 Crimean-Congo hemorrhagic fever,77 Argentine hemorrhagic fever,78 Venezuelan hemorrhagic fever,79 and Marburg disease.80 Additionally, a sensitive, specific, and safe immunostaining method has been developed to diagnose Ebola hemorrhagic fever in formalinfixed skin biopsies (Fig. 2.8 ).81 Immunohistochemistry demonstrated that Lassa virus targets primarily endothelial cells, mononuclear inflammatory cells, and hepatocytes (Fig. 2.9 ).81, 82, 83
Fig. 2.7.

Yellow fever. Abundant yellow fever viral antigen is seen within hepatocytes and Kupffer cells. (Immunoperoxidase staining with AEC and hematoxylin counterstain, ×400)
(Courtesy of Dr. JF Aronson, University of Texas Medical Branch.)
Fig. 2.8.

Ebola virus. Extensive Ebola viral antigens are seen primarily within fibroblasts in dermis of a skin specimen from a fatal case of Ebola hemorrhagic fever. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×20)
Fig. 2.9.

Lassa fever. Liver from a patient with Lassa fever. Scattered hepatocytes and reticuloendothelial cells show reactivity with monoclonal antibody to Lassa virus. (Naphthol fast red substrate and hematoxylin counterstain, original magnification ×100)
Papovaviruses
Immunohistochemistry for the detection of human papillomavirus in formalinfixed tissue has been replaced by more sensitive diagnostic molecular techniques such in situ hybridization.84, 85, 86, 87 In addition to low sensitivity compared with molecular techniques, immunohistochemistry detects only productive and not latent infections and cannot be used to determine the type of virus present (Fig. 2.10 ).
Fig. 2.10.

Immunoperoxidase staining for human papillomavirus (HPV) in a patient with mild squamous dysplasia. HPV viral antigen localizes within the nuclei of koilocytotic cells. (DAB with hematoxylin counterstain, ×600)
BK virus infections are frequent during infancy; in immunocompetent individuals the virus remains latent in the kidneys, central nervous system and B-lymphocytes. In immunocompromised patients, the infection reactivates and spreads to other organs. In the kidney, the infection is associated with mononuclear interstitial inflammatory infiltrates and tubular atrophy, findings that can be difficult to distinguish from acute transplant rejection. Besides, the cytopathic changes observed in BK virus infection are not pathognomonic and can be observed in other viral infections. In this setting, immunohistochemistry has been useful to demonstrate BK virus infection.88, 89, 90, 91
The human polyomavirus JC is a double-stranded DNA virus that causes progressive multifocal leukoencephalopathy (PML). This fatal demyelinating disease is characterized by cytopathic changes in oligodendrocytes and bizarre giant astrocytes. Immunohistochemical technique using a polyclonal rabbit antiserum against the protein VP1 is a specific, sensitive, and rapid method for confirming the diagnosis of PML.92, 93, 94, 95 JC virus antigen is usually seen within oligodendrocytes and occasional astrocytes, and antigen-bearing cells are more commonly seen in early lesions.
Other viruses
Immunohistochemistry has also been used to confirm the diagnosis of respiratory viral diseases such as influenza A virus and respiratory syncytial virus infections when cultures were not available.96, 97, 98, 99
The diagnosis of rabies relies heavily on histopathologic examination of tissues to demonstrate the characteristic cytoplasmic inclusions (Negri bodies). In an important percentage of cases, Negri bodies may be inconspicuous and so few that confirming the diagnosis of rabies may be extremely difficult.100 Furthermore, in nonendemic areas the diagnosis of rabies is usually not suspected clinically or the patient can present with an ascending type of paralysis. In these settings, immunohistochemical staining is a very sensitive, safe, and specific diagnostic tool for rabies (Fig. 2.11 ).100, 101, 102, 103, 104 Other viral agents that can be diagnosed using immunohistochemical methods include enterovirus,105, 106, 107, 108 Eastern equine encephalitis,109, 110, 111 and rotavirus.112, 113, 114
Fig. 2.11.

Rabies. Immunostaining of rabies viral antigens in neurons of CNS using a rabbit polyclonal antibody. Red precipitate corresponds to Negri inclusions on H&E. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×40)
BACTERIAL INFECTIONS
Among bacterial infections, the greatest number of immunohistochemical studies have been performed in the investigation of Helicobacter pylori. A few studies have evaluated the use of immunohistochemistry in other bacterial, mycobacterial, rickettsial, and spirochetal infections.
Antigen retrieval is generally not required for the immunohistochemical demonstration of bacteria in fixed tissue. However, interpretation of the results can be complicated by the fact that many of these antibodies will cross-react with other bacteria. Moreover, antibodies may react with only portions of the bacteria, and they may label remnants of bacteria or spirochetes when viable organisms are no longer present.
Helicobacter pylori infection
Gastric infection by H. pylori results in chronic active gastritis and is strongly associated with lymphoid hyperplasia, gastric lymphomas, and gastric adeno-carcinoma. Heavy infections with numerous organisms are easily detected on routine hematoxylin and eosin-stained tissues; however, the detection rate is only 66% with many false-positive and false-negative results.115, 116, 117 Conventional histochemical methods such as silver stains are more sensitive than hematoxylin and eosin in detecting H. pylori. Nonetheless, for the detection of scant numbers of organisms, immunohistochemistry has proved to be highly specific and sensitive, less expensive when all factors are considered, and superior to conventional histochemical methods (Fig. 2.12 ).116, 117 Treatment for chronic active gastritis and H. pylori infection can change the shape of the microorganism, making difficult its identification and differentiation from extracellular debris or mucin globules. In these cases, immunohistochemistry improves the rate of successful identification of the bacteria even when histologic examination and cultures are falsely negative.118, 119, 120, 121
Fig. 2.12.

Numerous curved Helicobacter pylori in the superficial mucus are clearly demonstrated by immunoperoxidase staining in this patient with chronic active gastritis. (DAB with hematoxylin counterstain, ×600)
Whipple's disease
Whipple's disease affects primarily the small bowel and mesenteric lymph nodes and less commonly other organs such as heart and central nervous system. Numerous foamy macrophages characterize the disease, and the diagnosis usually relies on the demonstration of PAS-positive intracytoplasmic bacteria. Nevertheless, the presence of PAS-positive macrophages is not pathognomonic; they can be observed in other diseases such as Mycobacterium avium complex infections, histoplasmosis, infections due to Rhodococcus equi, and macroglobulinemia. Immunohistochemical staining with a rabbit polyclonal antibody provides a sensitive and specific method for the rapid diagnosis of intestinal and extraintestinal Whipple's disease and for follow-up of treatment response.122, 123, 124
Rocky Mountain spotted fever
Confirmation of Rocky Mountain spotted fever (RMSF) usually requires the use of serologic methods to detect antibodies to spotted fever group (SFG) rickettsiae; yet a significant percentage of patients with RMSF lack diagnostic titers during the first week of disease. Immunohistochemistry has been successfully used to detect SFG rickettsiae in formalinfixed tissue sections (Fig. 2.13 ).125, 126 Several studies have illustrated the value of immunohistochemistry in the diagnosis of suspected cases of RMSF using skin biopsies, and in confirming fatal cases of seronegative RMSF.127, 128
Fig. 2.13.

Immunohistologic demonstration of Rickettsia rickettsii within endothelial cells surrounded by a small glial nodule in the brainstem of this patient with fatal Rocky Mountain spotted fever. (Immunoperoxidase staining with AEC and hematoxylin counterstain, ×600)
Bartonella infections
Bartonella are slow growing, fastidious Gram-negative, Warthin-Starry-stained bacteria associated with bacillary angiomatosis, peliosis hepatis, cat-scratch disease, and blood culture-negative endocarditis. Immunostaining has been successfully used to identify Bartonella henselae and B. quintana in heart valves from patients with blood culture-negative endocarditis (Fig. 2.14 ).129, 130 This polyclonal rabbit antibody that does not allow differentiation between B. henselae and B. quintana has also been used in the detection of these microorganisms in cat-scratch disease, bacillary angiomatosis, and peliosis hepatis.131, 132 A commercially available monoclonal antibody specific for B. henselae is also available and has been used to demonstrate the organism in a case of spontaneous splenic rupture caused by this bacterium.133
Fig. 2.14.

Bartonella. Immunohistologic demonstration of Bartonella henselae within heart valve of patient with culture-negative endocarditis. Mouse monoclonal anti-B. henselae antibody. (Naphthol fast red substrate and hematoxylin counterstain, original magnification ×40)
Other bacterial infections
Other bacterial diseases that can be identified by immunohistochemistry in formalinfixed tissue including leptospirosis, which is a zoonosis that usually presents as an acute febrile syndrome but occasionally can have unusual manifestations such as pulmonary hemorrhage with respiratory failure or abdominal pain.134, 135, 136 Rabbit polyclonal antibodies have been used in immunohistochemistry to detect leptospiral antigens in the gallbladder and lungs from patients with unusual presentations (Fig. 2.15 ).134, 135, 136, 137
Fig. 2.15.
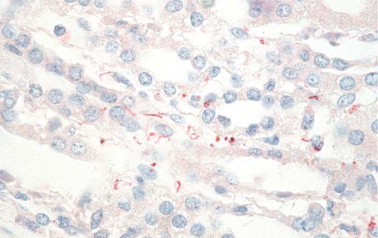
Leptospira. Immunostaining of intact leptospires and granular forms of leptospiral antigens in kidney of patient who died of pulmonary hemorrhage. (Immunoalkaline phosphatase with rabbit polyclonal antisera with naphthol fast red substrate and hematoxylin counterstain, original magnification ×63)
Lyme disease has protean clinical manifestations, and Borrelia burgdoferi is difficult to culture from tissues and fluids. In addition, cultures are rarely positive before 2–4 weeks of incubation. Borrelia burgdoferi can be identified in tissues by immunostaining with polyclonal or monoclonal antibodies. Although immunohistochemistry is more specific than silver impregnation stains, the sensitivity of immunostaining is poor, and the microorganisms are difficult to detect due to the low numbers present in tissue sections.138, 139 Immunohistochemistry is useful in identifying Haemophilus influenzae,140, 141, 142 Chlamydia species,143, 144, 145 Legionella pneumophila and L. dumoffii,146, 147, 148 Listeria monocytogenes,149, 150, 151 Salmonella,152, 153 mycobacteria,154, 155, 156, 157, 158, 159 rickettsial infections other than Rocky Mountain spotted fever such as boutonneuse fever, typhus fever,160 rickettsialpox,161, 162 African tick bite fever,125 scrub typhus,163 and spirochetes in patients with syphilis.164, 165, 166
FUNGAL INFECTIONS
The great majority of fungi are readily identified by hematoxylin and eosin staining alone or in combination with histochemical stains (periodic acid–Schiff [PAS], and Gomori's methenamine silver [GMS]). However, these stains cannot distinguish morphologically similar fungi with potential differences in susceptibility to antimycotic drugs. In addition, fungal elements may appear atypical in tissue sections because of several factors including steric orientation, age of the fungal lesion, effects of antifungal chemotherapy, type of infected tissue, and host immune response.167 Currently, the final identification of fungi relies on culture techniques; however, culture may take several days or longer to yield a definitive result, and often surgical pathologists have no access to fresh tissue.
In past years, immunohistochemistry has been used to identify various fungal elements in paraffin-embedded, formalinfixed tissue.168, 169, 170 Immunohistochemical methods have the advantage of providing rapid and specific identification of several fungi and allowing pathologists to be able to identify unusual filamentous hyphal and yeast infections and accurately distinguish them from confounding artifacts.169, 172 In addition, immunohistochemistry allows pathologists to correlate microbiological and histological findings of fungal infections and to distinguish them from harmless colonization. Immunohistochemistry can also be helpful when more than one fungus is present; in these cases dual immunostaining techniques can highlight the different fungal species present in the tissue.173 An important limitation of immunohistochemistry in the identification of fungi is the well-known, widespread occurrence of common antigens among pathogenic fungi that frequently results in cross-reactivity with polyclonal antibodies and even with some monoclonal antibodies.169, 171, 172, 173, 174 Therefore, assessment of cross-reactivity using a panel of fungi is a very important step in the evaluation of immunohistochemical methods.169, 170
Candida species are often stained weakly with hematoxylin and eosin, and sometimes the yeast form may be difficult to differentiate from Histoplasma capsulatum, Cryptococcus neoformans, and even Pneumocystis carinii. Polyclonal and monoclonal antibodies against Candida genus antigens are sensitive and strongly reactive and do not show cross-reactivity with other fungi tested.169, 170, 175, 176 In particular, two monoclonal antibodies against Candida albicans mannoproteins show high sensitivity and specificity. Monoclonal antibody 3H8 recognizes primarily filamentous forms of C. albicans, whereas monoclonal antibody 1B12 highlights yeast forms.176, 177
Identification of Cryptococcus neoformans usually is not a problem when the fungus produces a mucicarmine-positive capsule. However, infections by capsule-negative strains are more difficult to diagnose, and the disease can be confused with histoplasmosis, blastomycosis, or torulopsis. Also, in longstanding infections the yeast often appear atypical and fragmented. Polyclonal antibodies raised against C. neoformans yeast cells are sensitive and specific.169, 170 More recently, monoclonal antibodies have been produced that allow identification and differentiation of varieties of C. neoformans in formalinfixed tissue. The antibodies are highly sensitive (97%) and specific (100%) to differentiate C. neoformans var. neoformans from C. neoformans var. gattii.178, 179
Sporothrix schenckii may be confused in tissue sections with Blastomyces dermatitidis and fungal agents of pheohyphomycosis. In addition, yeast cells of S. schenckii may be sparsely present in tissues. Specific antibodies against yeast cells of S. schenckii are sensitive but demonstrate cross-reactivity with Candida species; however, after specific adsorption of the antibody with Candida yeast cells, the cross-reactivity of the antibodies is eliminated.169, 170
Invasive aspergillosis is a frequent cause of fungal infection with high morbidity and mortality rates in immunocompromised patients. The diagnosis is often difficult and relies heavily on histologic identification of invasive septate hyphae and culture confirmation. Nevertheless, several filamentous fungi such as Fusarium species, Pseudallescheria boydii, and Scedosporium species share similar morphology with Aspergillus species in hematoxylin and eosin-stained tissues. In addition, the yield of cultures in histologically proven cases is low, ranging from 30% to 50%.180, 181 Several polyclonal and monoclonal antibodies against Aspergillus antigens have been tested in formalinfixed tissues with variable sensitivities, and most of them cross-react with other fungi.174, 182, 183 More recently, monoclonal antibodies (WF-AF-1, 164G and 611F) against Aspergillus galactomannan have shown high sensitivity and specificity in identifying A. fumigatus, A. flavus, and A. niger in formalinfixed tissues without cross-reactivity with other filamentous fungi.181, 184
Cysts and trophozoites of Pneumocystis carinii can be detected in bronchoalveolar lavage specimens using monoclonal antibodies that yield results that are slightly more sensitive than GMS, Giemsa or Papanicolaou staining for detecting cysts (Fig. 2.16 ).170, 185, 186 Antibodies are most helpful in the diagnosis of P. carinii pneumonia (PCP) when atypical pathologic features are present such as granulomatous PCP or the presence of hyaline membranes or in cases of extrapulmonary pneumocystosis.
Fig. 2.16.

Human immunodeficiency virus (HIV)-infected immunodeficient patient with Pneumocystis carinii pneumonia. Cohesive aggregates of cyst forms and trophozoites within alveolar spaces are demonstrated with a monoclonal antibody against P. carinii in an immunoperoxidase technique. (DAB with hematoxylin counterstain, ×400)
Penicillium marneffei usually causes a disseminated infection in immunocompromised patients that clinically resembles histoplasmosis or leishmaniasis.171, 187 Morphologically, the organisms must be differentiated from H. capsulatum, C. neoformans, and C. albicans. The monoclonal antibody EBA-1 against the galactomannan of Aspergillus species cross-reacts with and detects P. marneffei in tissue sections.182, 188 Immunohistochemistry has also been used to detect Blastomyces, Coccidioides, and Histoplasma.169, 170, 189 However, the antibodies have significant cross-reactivity with several other fungi.
PROTOZOAL INFECTIONS
Protozoa usually can be identified in tissue sections stained with hematoxylin and eosin or Giemsa stain; however, because of the small size of the organisms and the subtle distinguishing features, an unequivocal diagnosis cannot always be made. The role of immunohistochemistry in the detection of protozoal infections has been limited to cases in which the morphology of the parasite is distorted by tissue necrosis or autolysis. In addition, in immunocompromised patients, toxoplasmosis can have an unusual disseminated presentation with numerous tachyzoites without bradyzoites (Fig. 2.17 ).190, 191 Immunohistochemistry has also been useful in cases with unusual presentation of the disease.192
Fig. 2.17.

HIV-infected patient with toxoplasmic encephalitis. Immunoperoxidase highlights pseudocysts and scattered tachyzoites. (DAB with hematoxylin counterstain, ×400).
The diagnosis of leishmaniasis in routine practice usually is not difficult; however, in certain circumstances the pathologic diagnosis may be more problematic as is the case in chronic granulomatous leishmaniasis with small numbers of parasites, when the microorganism presents in unusual locations, or when necrosis distorts the morphologic appearance of the disease.193 In these cases, immunohistochemical staining has been a valuable diagnostic tool.193, 194, 195, 196 The highly sensitive and specific monoclonal antibody p19-11 recognizes different species of Leishmania and allows differentiation from morphologically similar microorganisms (Toxoplasma, Trypanosoma cruzi, and P. marneffei).193
Immunohistochemistry has also been used to identify Cryptosporidium,197 Entamoeba histolytica,198 Trypanosoma cruzi,199, 200, 201 babesia,202 and Giardia lamblia 203 in formalinfixed, paraffin-embedded tissue samples.
EMERGING INFECTIOUS DISEASES
In 1992, the Institute of Medicine defined emerging infectious diseases (EID) as caused by new, previously unidentified microorganisms or those whose incidence in humans has increased within the past two decades or threatens to increase in the near future.204 The list of pathogens newly recognized since 1973 is long and continues to increase, and recognizing emerging infections is a challenge with many new infectious agents remaining undetected for years before emerging as identified public health problems.205 EID are global phenomena that require a global response. The Centers for Disease Control (CDC) has defined the strategy to prevent and detect EID.205 The anatomic pathology laboratory plays a critical role in the initial and rapid detection of EID.206, 207 Immunohistochemistry, besides assisting in the identification of new infectious agents, has contributed to the understanding of the pathogenesis and epidemiology of EID.
Hantavirus pulmonary syndrome
In 1993, several previously healthy individuals died of rapidly progressive pulmonary edema, respiratory insufficiency and shock in southwestern United States.208, 209 Immunohistochemistry was central in the identification of viral antigens of a previously unknown hantavirus.210, 211 Immunohistochemical analysis was also important in identifying the occurrence of unrecognized cases of hantavirus pulmonary syndrome prior to 1993 and in showing the distribution of viral antigen in endothelial cells of the microcirculation, particularly in the lung (Fig. 2.18 ).210, 212
Fig. 2.18.

Hantavirus antigen-positive endothelial cells of pulmonary microvasculature in lung of an HPS patient as determined by immunohistochemistry using a mouse monoclonal antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×100)
West Nile virus encephalitis
West Nile virus (WNV) was originally identified in Africa in 1937, and the first cases of WNV encephalitis in the US were described in 1999. The clinical picture is variable and non-specific ranging from subclinical to flaccid paralysis and encephalitis characterized morphologically by perivascular mononuclear cell inflammatory infiltrates, neuronal necrosis, edema, and microglial nodules, particularly prominent in the brainstem, cerebellum, and spinal cord.213, 214, 215, 216, 217 The diagnosis of WNV is usually established by identification of virus-specific IgM in CSF and/or serum, and demonstration of viral RNA in serum, CSF, or other tissue.218 Immunostaining with either monoclonal or polyclonal antibodies has been successfully employed to diagnose WNV infection in immunocompromised patients who lacked an adequate antibody response (Fig. 2.19 ).214
Fig. 2.19.

West Nile virus. Immunostaining of flaviviral antigens in neurons and neuronal processes in CNS tissue from an immunosuppressed patient who died of WNV encephalitis. (Flavivirus-hyperimmune mouse ascitic fluid, naphthol fast red substrate and hematoxylin counterstain, original magnification ×40)
Enterovirus 71 encephalomyelitis
Enterovirus 71 (EV71) has been associated with hand, foot, and mouth disease, herpangina, aseptic meningitis, and poliomyelitis-like flaccid paralysis. More recently, EV71 has been associated with unusual cases of fulminant encephalitis, pulmonary edema and hemorrhage, and heart failure.219, 220 Severe and extensive encephalomyelitis of the cerebral cortex, brainstem, and spinal cord has been described. Immunohistochemical staining with monoclonal antibody against EV71 has played a pivotal role in the linking of EV71 infection to fulminant encephalitis (Fig. 2.20 ). Viral antigen is observed within neurons, neuronal processes, and mononuclear inflammatory cells.221, 222, 223
Fig. 2.20.

Enterovirus 71. Positive staining of EV71 viral antigens in neurons and neuronal processes of a fatal case of enterovirus encephalitis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×40)
Nipah virus infection
Nipah virus is a recently described paramyxovirus that causes an acute febrile encephalitic syndrome with high mortality rates.224, 225, 226 Pathology played a key role in identifying the causative agent. Histopathologic findings include vasculitis with thrombosis, microinfarctions, syncytial giant cells, and viral inclusions.224, 226 Syncytial giant endothelial cells, albeit characteristic of this disease, are seen only in 25% of cases,224 and viral inclusions of similar morphology can be seen in other paramyxoviral infections. Immunostaining provides a useful tool for unequivocal diagnosis of the disease, demonstrating viral antigen within neurons and endothelial cells of most organs (Fig. 2.21 ).5, 224
Fig. 2.21.

Nipah virus. Immunostaining of Nipah virus antigens in neurons and neuronal processes in CNS of a fatal case of Nipah virus encephalitis. (Naphthol fast red substrate and hematoxylin counterstain, original magnification ×63)
Ehrlichioses
Bacteria belonging to the genera Ehrlichia and Anaplasma are the agents of human monocytotropic ehrlichiosis, and human granulocytotropic anaplasmosis, respectively. The acute febrile illnesses usually present with cytopenias, myalgias, and mild to moderate hepatitis.227, 228, 229, 230
Diagnosis of ehrlichiosis depends upon finding the characteristic monocytic and/or granulocytic cytoplasmic inclusions (morulae), PCR analysis of blood, and detection of specific antibodies in blood. However, morulae are rare and often missed on initial evaluation, hematoxylin and eosin-stained sections often fail to show organisms even when immunohistochemistry reveals abundant ehrlichial antigen, and antibody titers may take several weeks to rise to diagnostic levels.227 Additionally, immunocompromised patients may not develop anti-ehrlichial antibodies prior to death.227, 229 In these cases, immunostaining for Ehrlichia or Anaplasma has been demonstrated to be a sensitive and specific diagnostic method.227, 229, 230, 231
Immunohistochemistry has been a very valuable approach for the identification and study of several other EID such as Ebola hemorrhagic fever,81, 82, 83 hendra virus encephalitis,5, 232, 233 leptospirosis,135, 136, 137 and more recently to identify a new coronavirus associated with severe acute respiratory syndrome (SARS).234, 235 SARS was first recognized during a global outbreak of severe pneumonia that first occurred in late 2002 in Guangdong Province, China, and then erupted in February 2003 with cases in more than two dozen countries in Asia, Europe, North America, and South America. Early in the investigation the clinical, pathologic, and laboratory studies focused on previously known agents of respiratory illness. Subsequently, a virus was isolated from the oropharynx of a SARS patient and identified by ultrastructural characteristics as belonging to the family Coronaviridae.234, 235 Various reports have described diffuse alveolar damage as the main histopathologic findings in SARS patients, and SARS-associated coronavirus (SARS-CoV) has been demonstrated in human and experimental animal tissues by immunohistochemical (Fig. 2.22 ) or in situ hybridization (ISH) assays.236, 237, 238, 239, 240, 241, 242, 243, 244, 245
Fig. 2.22.
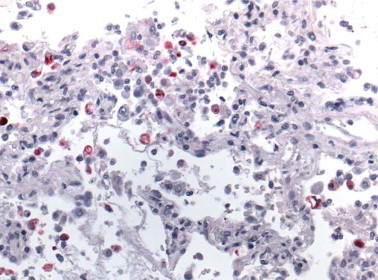
SARS. Coronavirus antigen-positive pneumocytes and macrophages in lung of a SARS case. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×63)
PATHOLOGISTS, IMMUNOHISTOCHEMISTRY, AND BIOTERRORISM
Currently, there is increasing concern about the use of infectious agents as potential biological weapons. Biological warfare agents vary from rare, exotic viruses to common bacterial agents, and the intentional use of biologic agents to cause disease can simulate naturally occurring outbreaks or may have unusual characteristics.246 The CDC has issued the recommendations for a complete public health response to a biological attack.247, 248, 249 Two important components of this response plan include the rapid diagnosis and characterization of biological agents. Pathologists using newer diagnostic techniques such as immunohistochemistry, in situ hybridization, and PCR will have a direct impact on the rapid detection and control of emerging infectious diseases from natural or intentional causes. Immunohistochemistry provides a simple, safe, sensitive, and specific method for the rapid detection, either at the time of investigation or retrospectively, of biological threats, facilitating the rapid implementation of effective public health responses.
Anthrax
Immunohistochemical staining of Bacillus anthracis with monoclonal antibodies against cell wall and capsule antigens has been successfully used in the identification of bioterrorism-related anthrax cases, being an important step in the early diagnosis and treatment of these cases.5, 250, 251, 252, 253, 254 Gram's staining and culture isolation of B. anthracis are the usual methods to diagnose anthrax; nevertheless, previous antibiotic treatment affects culture yield and Gram's staining identification of the bacteria.252 Immunohistochemistry has demonstrated high sensitivity and specificity for the detection of B. anthracis in skin biopsies, pleural biopsies, transbronchial biopsies, and pleural fluids (Fig. 2.23 ).251, 252, 253
Fig. 2.23.

Anthrax. (A) Photomicrograph of pleural effusion cell block showing bacillary fragments and granular antigen-staining using the B. anthracis capsule antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×63) (B) Skin biopsy from a patient with cutaneous anthrax showing abundant granular antigen-staining and bacillary fragments using B. anthracis cell wall antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×40) (C) Photomicrograph of mediastinal lymph node showing abundant granular antigen-staining and bacillary fragments using B. anthracis cell wall antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×63)
In addition, immunostaining has been very useful for determining the route of entry of the bacteria and identification of the mode of spread of the disease.252, 255
Tularemia
Immunohistochemical staining is also valuable in the rapid identification of Francisella tularensis in formalinfixed tissue sections. Tularemia can have a variable clinical and pathologic presentation that can simulate other infectious diseases such as anthrax, plague, cat-scratch disease, or lymphogranuloma venereum. Moreover, the microorganisms are difficult to demonstrate in tissue sections even with Gram's stain or silver staining methods. A mouse monoclonal antibody against the lipopolysaccharide of F. tularensis has been used to demonstrate intact bacteria and granular bacterial antigen in the lungs, spleen, lymph nodes, and liver with high sensitivity and specificity (Fig. 2.24 ).256, 257
Fig. 2.24.

Tularemia. Immunohistochemistry of lymph node showing a stellate abscess with F. tularensis antigen bearing macrophages in the central necrotic area using a mouse monoclonal antibody against the lipopolysaccharide of F. tularensis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×10)
Plague
A mouse monoclonal antibody directed against the fraction 1 antigen of Yersinia pestis has been used to detect intracellular and extracellular bacteria in dermal blood vessels, lungs, lymph nodes, spleen, and liver (Fig. 2.25 ).258, 259, 260, 261, 262 This technique is potentially useful for the rapid diagnosis of plague in formalinfixed skin biopsies. In addition, immunohistochemistry may allow distinction of primary and secondary pneumonic plague by identifying Y. pestis in different lung locations (i.e., alveolar versus interstitial).258
Fig. 2.25.

Plague. Immunohistochemical stain of a lung demonstrating abundant bacterial and granular antigen staining in the alveolar spaces using a mouse monoclonal antibody against F1 of Y. pestis. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×20)
Immunohistochemical methods using polyclonal or monoclonal antibodies have been applied to the identification of several other potential biological terrorism agents, including antibodies to the causative agents of brucellosis,5 Q fever,5, 125, 263, 264 viral encephalitides (Eastern equine encephalitis) (Fig. 2.26 ),5, 109, 110, 111 rickettsioses (typhus and Rocky Mountain spotted fever),125, 126, 127, 128, 160 and viral hemorrhagic fevers (Ebola, Marburg).5, 77, 78, 79, 80, 81, 82, 83
Fig. 2.26.

Immunostaining of viral antigens in neurons and neuronal processes in CNS using a mouse anti-EEE antibody. (Immunoalkaline phosphatase with naphthol fast red substrate and hematoxylin counterstain, original magnification ×10)
REFERENCES
- 1.Cartun RW. Use of immunohistochemistry in the surgical pathology laboratory for the diagnosis of infectious diseases. Pathol Case Rev. 1999;4:260–265. [Google Scholar]
- 2.Watts JC. Surgical pathology in the diagnosis of infectious diseases (Editorial) Am J Clin Pathol. 1994;102:711–712. doi: 10.1093/ajcp/102.6.711. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Bryan RT. Infectious disease pathology and emerging infections: Are we prepared? Arch Pathol Lab Med. 1996;120:117–124. [PubMed] [Google Scholar]
- 4.Schwartz DA. Emerging and reemerging infections: Progress and challenges in the subspecialty of infectious disease pathology. Arch Pathol Lab Med. 1997;121:776–784. [PubMed] [Google Scholar]
- 5.Zaki SR, Paddock CD. The emerging role of pathology in infectious diseases. In: Scheld WM, Armstrong D, Hughes JM, editors. Emerging infections 3. ASM Press; Washington, DC: 1999. pp. 181–200. [Google Scholar]
- 6.Medical Examiners, Coroners, and Biologic Terrorism A Guidebook for Surveillance and Case Management. MMWR Morbidity and Mortality Weekly Report. 2004;53(RR-8):1–53. [PubMed] [Google Scholar]
- 7.Zaki SR, Peters CJ. Viral hemorrhagic fevers. In: Connor DH, Chandler FW, Schwartz DA, editors. Pathology of infectious diseases. Appleton and Lange; Stamford, CT: 1997. pp. 347–364. [Google Scholar]
- 8.Coons AH, Creech HJ, Jones RN. The demonstration of pneumococcal antigen in tissues by use of fluorescent antibodies. J Immunol. 1942;45:159–170. [Google Scholar]
- 9.Jeavons L, Hunt L, Hamilton A. Immunochemical studies of heat-shock protein 80 of Histoplasma capsulatum. J Med Vet Mycol. 1994;32:47–57. doi: 10.1080/02681219480000071. [DOI] [PubMed] [Google Scholar]
- 10.Werner M, Chott A, Fabiano A. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24:1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Woods GL, Walker DH. Detection of infection or infectious agents by use of cytologic and histologic stains. Clin Microbiol Rev. 1996;9:382–404. doi: 10.1128/cmr.9.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler FW. Invasive microorganisms. In: Spicer SS, editor. Histochemistry in pathology diagnosis. Marcel Dekker; New York, NY: 1987. pp. 77–101. [Google Scholar]
- 13.Clausen PP, Thomsen P. Demonstration of hepatitis B surface antigen in liver biopsies. A comparative investigation of immunoperoxidase and orcein staining on identical sections on formalinfixed, paraffin-embedded tissue. Acta Pathol Microbiol Scand [A] 1978;86A:383. [PubMed] [Google Scholar]
- 14.Thomsen P, Clausen PP. Occurrence of hepatitis B-surface antigen in consecutive material of 1539 liver biopsies. Acta Pathol Microbiol Immunol Scand. [A] 1983;91:71. doi: 10.1111/j.1699-0463.1983.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 15.Al Adnani MS, Ali SM. Patterns of chronic liver disease in Kuwait with special reference to localization of hepatitis B surface antigen. J Clin Pathol. 1984;37:549. doi: 10.1136/jcp.37.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor C. Lung, pancreas, colon and rectum, stomach, liver. In: Taylor CR, Cote RJ, editors. Immunomicroscopy: a diagnostic tool for the surgical pathologist. 2nd edn. WB Saunders; Philadelphia, PA: 1994. pp. 292–317. [Google Scholar]
- 17.Park YN, Han KH, Kim KS. Cytoplasmic expression of hepatitis B core antigen in chronic hepatitis B virus infection: role of precore stop mutants. Liver. 1999;19:199–205. doi: 10.1111/j.1478-3231.1999.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 18.Dries V, von Both I, Müller M. Detection of hepatitis C virus in paraffin-embedded liver biopsies of patients negative for viral RNA in serum. Hepatology. 1999;29:223–229. doi: 10.1002/hep.510290118. [DOI] [PubMed] [Google Scholar]
- 19.Brody RI, Eng S, Melamed J. Immunohistochemical detection of hepatitis C antigen by monoclonal antibody TORDJI-22 compared with PCR viral detection. Am J Clin Pathol. 1998;110:32–37. doi: 10.1093/ajcp/110.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Nuovo GJ, Holly A, Wakely P. Correlation of histology, viral load, and in situ viral detection in hepatic biopsies from patients with liver transplants secondary to hepatitis C infection. Hum Pathol. 2002;33:277–284. doi: 10.1053/hupa.2002.32211. [DOI] [PubMed] [Google Scholar]
- 21.Komminoth P, Adams V, Long AA. Evaluation of methods for hepatitis C virus detection in archival liver biopsies. Comparison of histology, immunohistochemistry, in-situ hybridization, reverse transcriptase polymerase chain reaction (RT-PCR) and in-situ RT-PCR. Pathol Res Pract. 1994;190:1017–1025. doi: 10.1016/s0344-0338(11)80896-4. [DOI] [PubMed] [Google Scholar]
- 22.Doughty AL, Painter DM, McCaughan GW. Nonspecificity of monoclonal antibody TORDJI-22 for the detection of hepatitis C virus in liver transplant recipients with cholestatic hepatitis. Liver Transpl Surg. 1999;5:40–45. doi: 10.1002/lt.500050104. [DOI] [PubMed] [Google Scholar]
- 23.Sansonno D, Iacobelli AR, Cornacchiulo V. Immunohistochemical detection of hepatitis C virus-related proteins in liver tissue. Clin Exp Rheumatol. 1995;13(Suppl 13):S29–S32. [PubMed] [Google Scholar]
- 24.Blight K, Rowland R, Hall PD. Immunohistochemical detection of the NS4 antigen of hepatitis C virus and its relation to histopathology. Am J Pathol. 1993;143:1568–1573. [PMC free article] [PubMed] [Google Scholar]
- 25.Verslype C, Nevens F, Sinelli N. Hepatic immunohistochemical staining with a monoclonal antibody against HCV-E2 to evaluate antiviral therapy and reinfection of liver grafts in hepatitis C viral infection. J Hepatol. 2003;38:208–214. doi: 10.1016/s0168-8278(02)00389-6. [DOI] [PubMed] [Google Scholar]
- 26.Feiden W, Borchard F, Burrig KF. Herpes esophagitis: I. Light microscopical immunohistochemical investigations. Virchows Arch [A] 1984;404:167–176. doi: 10.1007/BF00704061. [DOI] [PubMed] [Google Scholar]
- 27.Nikkels AF, Delvenne P, Sadzot-Delvaux C. Distribution of varicella zoster virus and herpes simplex virus in disseminated fatal infections. J Clin Pathol. 1996;49:243–248. doi: 10.1136/jcp.49.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenson JK, Beschorner WE, Boitnott JK. Prominent mononuclear cell infiltrate is characteristic of herpes esophagitis. Hum Pathol. 1991;22:541–549. doi: 10.1016/0046-8177(91)90230-m. [DOI] [PubMed] [Google Scholar]
- 29.Wang JY, Montone KT. A rapid simple in situ hybridization method for herpes simplex virus employing a synthetic biotin-labeled oligonucleotide probe: a comparison with immunohistochemical methods for HSV detection. J Clin Lab Anal. 1994;8:105–115. doi: 10.1002/jcla.1860080209. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi TK, Ueda M, Nishino T. Brush cytology of herpes simplex virus infection in oral mucosa: use of the ThinPrep processor. Diag Cytopath. 1998;18:71–75. doi: 10.1002/(sici)1097-0339(199801)18:1<71::aid-dc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll JAR, Love S, Burton PA. Autopsy findings in two cases of neonatal herpes simplex virus infection: detection of virus by immunohistochemistry, in situ hybridization and the polymerase chain reaction. Histopathology. 1994;24:257–264. doi: 10.1111/j.1365-2559.1994.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 32.Nikkels AF, Debrus S, Sadzot-Delvaux C. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Archiv A Pathol Anat. 1993;422:121–126. doi: 10.1007/BF01607163. [DOI] [PubMed] [Google Scholar]
- 33.Cohen PR. Tests for detecting herpes simplex virus and varicella-zoster virus infections. Dermat Clin. 1994;12:51–68. [PubMed] [Google Scholar]
- 34.Katano H, Sato Y, Kurata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology. 2000;269:335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 35.Katano H, Suda T, Morishita Y. Human herpesvirus 8-associated solid lymphomas that occur in AIDS patients takes anaplastic large cell morphology. Mod Pathol. 2000;13:77–85. doi: 10.1038/modpathol.3880012. [DOI] [PubMed] [Google Scholar]
- 36.Ely SA, Powers J, Lewis D. Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma arising in the subarachnoid space. Hum Pathol. 1999;30:981–984. doi: 10.1016/s0046-8177(99)90254-x. [DOI] [PubMed] [Google Scholar]
- 37.Katano H, Sato Y, Kurata T. High expression of HHV-8-encoded ORF73 protein in spindle-shape cells of Kaposi's sarcoma. Am J Pathol. 1999;155:47–52. doi: 10.1016/S0002-9440(10)65097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said JW, Shintaku IP, Asou H. Herpesvirus 8 inclusions in primary effusion lymphoma: report of a unique case with T-cell phenotype. Archiv Pathol Lab Med. 1999;123:257–260. doi: 10.5858/1999-123-0257-HIIPEL. [DOI] [PubMed] [Google Scholar]
- 39.Cheuk W, Wong KOY, Wong CSC. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguishing Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335–342. doi: 10.1309/B8TC-0LBV-H8XY-5MFV. [DOI] [PubMed] [Google Scholar]
- 40.Robin YM, Guillou L, Michels JJ. Human herpesvirus 8 immunostaining. A sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 41.Bryant-Greenwood P, Sorbara L, Filie AC. Infection of mesothelial cells with human herpesvirus 8 in human immunodeficiency virus-infected patients with Kaposi sarcoma, Castleman disease, and recurrent pleural effusions. Mod Pathol. 2003;16:145–153. doi: 10.1097/01.MP.0000052374.61768.79. [DOI] [PubMed] [Google Scholar]
- 42.Cool CD, Pradeep RR, Yeager ME. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113–1122. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 43.Anwar F, Erice A, Jessurun J. Are there cytopathic features associated with cytomegalovirus infection predictive of resistance to antiviral therapy? Ann Diag Pathol. 1999;3:19–22. doi: 10.1016/s1092-9134(99)80005-7. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan MM, Coker R, Coleman DV. Detection of cytomegalovirus (CMV) in HIV+ patients: comparison of cytomorphology, immunohistochemistry and in situ hybridization. Cytopathology. 1998;9:29–37. [PubMed] [Google Scholar]
- 45.Kutza AS, Muhl E, Hackstein H. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 46.Saetta A, Agapitos E, Davaris PS. Determination of CMV placentitis: Diagnostic application of the polymerase chain reaction. Virchows Arch. 1998;432:159–162. doi: 10.1007/s004280050150. [DOI] [PubMed] [Google Scholar]
- 47.Solans EP, Yong S, Husain AN. Bronchioloalveolar lavage in the diagnosis of CMV pneumonitis in lung transplant recipients: an immunocytochemical study. Diagn Cytopath. 1997;16:350–352. doi: 10.1002/(sici)1097-0339(199704)16:4<350::aid-dc9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Nebuloni M, Pellegrinelli A, Ferri A. Etiology of microglial nodules in brains of patients with acquired immunodeficiency syndrome. J Neurovirol. 2000;6:46–50. doi: 10.3109/13550280009006381. [DOI] [PubMed] [Google Scholar]
- 49.Rimsza LM, Vela EE, Frutiger YM. Rapid automated combined in situ hybridization and immunohistochemistry for sensitive detection of cytomegalovirus in paraffin-embedded tissue biopsies. Am J Clin Pathol. 1996;106:544–548. doi: 10.1093/ajcp/106.4.544. [DOI] [PubMed] [Google Scholar]
- 50.Kambhan N, Vij R, Cartwright CA. Cytomegalovirus infection in steroid-refractory ulcerative colitis. A case-control study. Am J Surg Pathol. 2004;28:365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Ribalta T, Martinez AJ, Jares P. Presence of occult cytomegalovirus infection in the brain after orthotopic liver transplantation. An autopsy study of 83 cases. Virchows Arch. 2002;440:166–171. doi: 10.1007/s004280100497. [DOI] [PubMed] [Google Scholar]
- 52.Cruz-Spano L, Lima-Pereira FE, Gomes da Silva-Basso N. Human cytomegalovirus infection and abortion: an immunohistochemical study. Med Sci Monit. 2002;8:BR230–235. [PubMed] [Google Scholar]
- 53.Colina F, Juca NT, Moreno E. Histological diagnosis of cytomegalovirus hepatitis in liver allografts. J Clin Pathol. 1995;48:351–357. doi: 10.1136/jcp.48.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flomenberg P, Babbitt J, Drobyski WR. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 55.Strickler JG, Singleton TP, Copenhaver GM. Adenovirus in the gastrointestinal tracts of immunosuppressed patients. Am J Clin Pathol. 1992;97:555–558. doi: 10.1093/ajcp/97.4.555. [DOI] [PubMed] [Google Scholar]
- 56.Yi ES, Powell HC. Adenovirus infection of the duodenum in an AIDS patient: an ultrastructural study. Ultrastruct Pathol. 1994;18:549–551. doi: 10.3109/01913129409021897. [DOI] [PubMed] [Google Scholar]
- 57.Yan Z, Nguyen S, Poles M. Adenovirus colitis in human immunodeficiency virus infection: an underdiagnosed entity. Am J Surg Pathol. 1998;22:1101–1106. doi: 10.1097/00000478-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Dombrowski F, Eis-Hubinger AM, Ackermann T. Adenovirus-induced liver necrosis in a case of AIDS. Virchows Archiv. 1997;431:469–472. doi: 10.1007/s004280050125. [DOI] [PubMed] [Google Scholar]
- 59.Simsir A, Greenebaum E, Nuovo G. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation. 1998;65:592–594. doi: 10.1097/00007890-199802270-00027. [DOI] [PubMed] [Google Scholar]
- 60.Saad RS, Demetris AJ, Lee RG. Adenovirus hepatitis in the adult allograft liver. Transplantation. 1997;64:1483–1485. doi: 10.1097/00007890-199711270-00021. [DOI] [PubMed] [Google Scholar]
- 61.Ohori NP, Michaels MG, Jaffe R. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26:1073–1079. doi: 10.1016/0046-8177(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 62.Wang WH, Wang HL. Fulminant adenovirus hepatitis following bone marrow transplantation. A case report and brief review of the literature. Arch Pathol Lab Med. 2003;127:e246–e248. doi: 10.5858/2003-127-e246-FAHFBM. [DOI] [PubMed] [Google Scholar]
- 63.Lones MA, Shintaku IP, Weiss LM. Posttransplant lymphoproliferative disorder in liver allograft biopsies: a comparison of three methods for the demonstration of Epstein-Barr virus. Hum Pathol. 1997;28:533–539. doi: 10.1016/s0046-8177(97)90074-5. [DOI] [PubMed] [Google Scholar]
- 64.Challoner PB, Smith KT, Parker JD. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown KE, Young NS. Parvovirus B19 infection and hematopoiesis. Blood Rev. 1995;9:176–182. doi: 10.1016/0268-960x(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 66.Jordan JA, Penchansky L. Diagnosis of human parvovirus B19-induced anemia: correlation of bone marrow morphology with molecular diagnosis using PCR and immunohistochemistry. Cell Vision. 1995;2:279–282. [Google Scholar]
- 67.Morey AL, O'Neil HJ, Coyle PV. Immunohistological detection of human parvovirus B19 in formalinfixed, paraffin-embedded tissues. J Pathol. 1992;166:105–108. doi: 10.1002/path.1711660204. [DOI] [PubMed] [Google Scholar]
- 68.Puvion-Dutilleul F, Puvion E. Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci USA. 1998;95:8227–8232. doi: 10.1073/pnas.95.14.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yufu Y, Matsumoto M, Miyamura T. Parvovirus B19-associated haemophagocytic syndrome with lymphadenopathy resembling histiocytic necrotizing lymphadenitis (Kikuchi's diease) Br J Haematol. 1997;96:868–871. doi: 10.1046/j.1365-2141.1997.d01-2099.x. [DOI] [PubMed] [Google Scholar]
- 70.Vadlamudi G, Rezuke N, Ross JW. The use of monoclonal antibody R92F6 and polymerase chain reaction to confirm the presence of parvovirus B19 in bone marrow specimens of patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123:768–773. doi: 10.5858/1999-123-0768-TUOMAR. [DOI] [PubMed] [Google Scholar]
- 71.Wright C, Hinchliffe SA, Taylor C. Fetal pathology in intrauterine death due to parvovirus B19 infection. Br J Obstet Gynaecol. 1996;103:133–136. doi: 10.1111/j.1471-0528.1996.tb09664.x. [DOI] [PubMed] [Google Scholar]
- 72.Essary LR, Vnencak-Jones CL, Manning SS. Frequency of parvovirus B19 infection in nonimmune hydrops fetalis and utility of three diagnostic methods. Hum Pathol. 1998;29:696–701. doi: 10.1016/s0046-8177(98)90278-7. [DOI] [PubMed] [Google Scholar]
- 73.Monath TP, Ballinger ME, Miller BR. Detection of yellow fever viral RNA by nucleic acid hybridization and viral antigen by immunohistochemistry in fixed human liver. Am J Trop Med Hyg. 1989;40:663–668. doi: 10.4269/ajtmh.1989.40.663. [DOI] [PubMed] [Google Scholar]
- 74.De Brito T, Siqueira SA, Santos RT. Human fatal yellow fever. Immunohistochemical detection of viral antigens in the liver, kidney, and heart. Pathol Res Practice. 1992;188:177–181. doi: 10.1016/S0344-0338(11)81176-3. [DOI] [PubMed] [Google Scholar]
- 75.Hall WC, Crowell TP, Watts DM. Demonstration of yellow fever and dengue antigens in formalinfixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 76.Ramos C, Sanchez G, Pando RH. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol. 1998;4:465–468. doi: 10.3109/13550289809114548. [DOI] [PubMed] [Google Scholar]
- 77.Burt FJ, Swanepoel R, Shieh W-J. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 78.Maiztegui JI, Laguens RP, Cossio PM. Ultrastructural and immunohistochemical studies in five cases of Argentine hemorrhagic fever. J Infect Dis. 1975;132:35–53. doi: 10.1093/infdis/132.1.35. [DOI] [PubMed] [Google Scholar]
- 79.Hall WC, Geisbert TW, Huggins JW. Experimental infection of guinea pigs with Venezuelan hemorrhagic fever virus (Guanarito): a model of human disease. Am J Trop Med Hyg. 1996;55:81–88. doi: 10.4269/ajtmh.1996.55.81. [DOI] [PubMed] [Google Scholar]
- 80.Geisbert TW, Jaax NK. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 81.Zaki SR, Shieh W-J, Greer PW. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. J Infect Dis. 1999;179(Suppl 1):S36–S37. doi: 10.1086/514319. [DOI] [PubMed] [Google Scholar]
- 82.Ksiazek TG, Rollin PE, Williams AJ. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of Congo. J Infect Dis. 1999;179:S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 83.Wyers M, Formenty P, Cherel Y. Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J Infect Dis. 1999;179(Suppl 1):S54–S59. doi: 10.1086/514300. [DOI] [PubMed] [Google Scholar]
- 84.Delvenne P, Fontaine M-A, Delvenne C. Detection of human papillomaviruses in paraffin-embedded biopsies of cervical intraepithelial lesions: analysis by immunohistochemistry, in situ hybridization, and the polymerase chain reaction. Mod Pathol. 1994;7:113–119. [PubMed] [Google Scholar]
- 85.Lopez-Beltran A, Escudero AL, Carrasco-Aznar JC. Human papillomavirus infection and transitional cell carcinoma of the bladder. Immunohistochemistry and in situ hybridization. Pathol Res Pract. 1996;192:154–159. doi: 10.1016/S0344-0338(96)80210-X. [DOI] [PubMed] [Google Scholar]
- 86.Meyer MP, Markiw CA, Matuscak RR. Detection of human papillomavirus DNA in genital lesions by using a modified commercially available in situ hybridization assay. J Clin Microbiol. 1991;29:1308. doi: 10.1128/jcm.29.7.1308-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilbur DC, Reichman RC, Stoler MH. Detection of infection by human papillomavirus in genital condyloma. A comparison study using immunohistochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988;89:505. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]
- 88.Pappo O, Demetris AI, Raikow RB. Human polyomavirus infection of renal allografts: histopathologic diagnosis, clinical significance and literature review. Mod Pathol. 1996;9:105–109. [PubMed] [Google Scholar]
- 89.Nebuloni M, Tosoni A, Boldorini R. BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123:807–811. doi: 10.5858/1999-123-0807-BVRIIA. [DOI] [PubMed] [Google Scholar]
- 90.Elli A, Banfi G, Battista-Fogazzi G. BK polyomavirus interstitial nephritis in a renal transplant patient with no previous acute rejection episodes. J Nephrol. 2002;15:313–316. [PubMed] [Google Scholar]
- 91.Boldorini R, Omodeo-Zorini E, Sunno A. Molecular characterization and sequence analysis of polyomavirus strains isolated from needle biopsy specimens of kidney allograft recipients. Am J Clin Pathol. 2001;116:489–494. doi: 10.1309/GAUE-92W7-ACDV-X46M. [DOI] [PubMed] [Google Scholar]
- 92.Jochum W, Weber T, Frye S. Detection of JC virus by anti-VP1 immunohistochemistry in brains with progressive multifocal leukoencephalopathy. Acta Neuropathol. 1997;94:226–231. doi: 10.1007/s004010050697. [DOI] [PubMed] [Google Scholar]
- 93.Chima SC, Agostini HT, Ryschlkeewitsch CF. Progressive multifocal leukoencephalopathy and JC virus genotypes in West African patients with acquired immunodeficiency syndrome. A pathologic and DNA sequence analysis of 4 cases. Arch Pathol Lab Med. 1999;123:395–403. doi: 10.5858/1999-123-0395-PMLAJV. [DOI] [PubMed] [Google Scholar]
- 94.Aoki N, Mori M, Kato K. Antibody against synthetic multiple antigen peptides (MAP) of JC virus capsid protein (VP1) without cross reaction to BK virus: a diagnostic tool for progressive multifocal leukoencephalopathy. Neurosci Letts. 1996;205:111–114. doi: 10.1016/0304-3940(96)12389-2. [DOI] [PubMed] [Google Scholar]
- 95.Silver SA, Arthur RR, Rozan YS. Diagnosis of progressive multifocal leukoencephalopathy by stereotactic brain biopsy utilizing immunohistochemistry and the polymerase chain reaction. Acta Cytolog. 1995;39:35–44. [PubMed] [Google Scholar]
- 96.Guarner J, Shieh W-J, Dawson J. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- 97.Cartun RW, Tahhan HR, Knibbs DR. Immunocytochemical identification of respiratory syncytial virus (RVS) in formalinfixed, paraffin-embedded tissue from immunocompromised hosts. Mod Pathol. 1989;2:15. [Google Scholar]
- 98.Nielson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- 99.Wright C, Oliver KC, Fenwick FI. A monoclonal antibody pool for routine immunohistochemical detection of human respiratory syncytial virus antigens in formalinfixed, paraffin-embedded tissue. J Pathol. 1997;182:238–244. doi: 10.1002/(SICI)1096-9896(199706)182:2<238::AID-PATH822>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 100.Jogai S, Radotra BD, Banerjee AK. Immunohistochemical study of human rabies. Neuropathology. 2000;20:197–203. doi: 10.1046/j.1440-1789.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 101.Jogai S, Radotra BD, Banerjee AK. Rabies viral antigen in extracranial organs: a postmortem study. Neuropathol Appl Neurobiol. 2002;28:334. doi: 10.1046/j.1365-2990.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 102.Warner CK, Zaki SR, Shieh WJ. Laboratory investigation of humans from vampire bat rabies in Peru. Am J Trop Med Hyg. 1999;60:502–507. doi: 10.4269/ajtmh.1999.60.502. [DOI] [PubMed] [Google Scholar]
- 103.Sinchaisri TA, Nagata T, Yoshikawa Y. Immunohistochemical and histopathological study of experimental rabies infection in mice. J Vet Med Sc. 1992;54:409–416. doi: 10.1292/jvms.54.409. [DOI] [PubMed] [Google Scholar]
- 104.Jackson AC, Ye H, Phelan CC. Extraneural organ involvement in human rabies. Lab Invest. 1999;79:945–951. [PubMed] [Google Scholar]
- 105.Yousef GE, Mann GF, Brown IN. Clinical and research application of an enterovirus group-reactive monoclonal antibody. Intervirol. 1987;28:199–205. doi: 10.1159/000150017. [DOI] [PubMed] [Google Scholar]
- 106.Hohenadl C, Klingel K, Rieger P. Investigation of the coxsackievirus B3 nonstructural proteins 2B, 2C, and 3AB: generation of specific polyclonal antisera and detection of replicating virus in infected tissue. J Virol Methods. 1994;47:279–295. doi: 10.1016/0166-0934(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Li Y, Peng T. Localization of enteroviral antigen in myocardium and other tissues from patients with heart muscle disease by an improved immunohistochemical technique. J Histochem Cytochem. 2000;48:579–584. doi: 10.1177/002215540004800501. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Bourlet T, Andreoletti L. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation. 2000;101:231–234. doi: 10.1161/01.cir.101.3.231. [DOI] [PubMed] [Google Scholar]
- 109.Del Piero F, Wilkins PA, Dubovi EJ. Clinical, pathologic, immunohistochemical, and virologic findings of Eastern equine encephalitis in two horses. Vet Pathol. 2001;38:451–456. doi: 10.1354/vp.38-4-451. [DOI] [PubMed] [Google Scholar]
- 110.Patterson JS, Maes RK, Mullaney TP. Immunohistochemical diagnosis of Eastern equine encephalomyelitis. J Vet Diag Invest. 1996;8:156–160. doi: 10.1177/104063879600800203. [DOI] [PubMed] [Google Scholar]
- 111.Garen PD, Tsai TF, Powers JM. Human Eastern equine encephalitis: immunohistochemistry and ultrastructure. Mod Pathol. 1999;12:646–652. [PubMed] [Google Scholar]
- 112.Tatti KM, Gentsch J, Shieh WJ. Molecular and immunological methods to detect rotavirus in formalinfixed tissue. J Virol. 2002;105:305–319. doi: 10.1016/s0166-0934(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 113.Morrison C, Gilson T, Nuovo GJ. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum Pathol. 2001;32:216–221. doi: 10.1053/hupa.2001.21565. [DOI] [PubMed] [Google Scholar]
- 114.Cioc AM, Nuovo GJ. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod Pathol. 2001;15:914–922. doi: 10.1097/01.MP.0000024291.37651.CD. [DOI] [PubMed] [Google Scholar]
- 115.El-Zimaity HMT, Graham DY, Al-Assis MT. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 116.Barbosa AJ, Queiros DMM, Mendes EN. Immunocytochemical identification of Campylobacter pylori in gastritis and correlation with culture. Arch Pathol Lab Med. 1988;11:288–291. [PubMed] [Google Scholar]
- 117.Toulaymant M, Marconi S, Garb J. Endoscopic biopsy pathology of Helicobacter pylori gastritis. Arch Pathol Lab Med. 1999;123:778–781. doi: 10.5858/1999-123-0778-EBPOHP. [DOI] [PubMed] [Google Scholar]
- 118.Marcio L, Angelucci D, Grossi L. Anti-Helicobacter pylori specific antibody immunohistochemistry improves the diagnostic accuracy of Helicobacter pylori in biopsy specimen from patients treated with triple therapy. Am J Gastroenterol. 1998;93:223–226. doi: 10.1111/j.1572-0241.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 119.Tokunaga Y, Shirahase H, Yamamoto E. Semiquantitative evaluation for diagnosis of Helicobacter pylori infection in relation to histological changes. Am J Gastroenterol. 1998;93:26–29. doi: 10.1111/j.1572-0241.1998.026_c.x. [DOI] [PubMed] [Google Scholar]
- 120.Chan WY, Hui PK, Leung KM. Coccoid forms of Helicobacter pylori in the human stomach. Am J Clin Pathol. 1994;102:503–507. doi: 10.1093/ajcp/102.4.503. [DOI] [PubMed] [Google Scholar]
- 121.Goldstein NS. Chronic inactive gastritis and coccoid Helicobacter pylori in patients treated for gastroesophageal reflux disease or with H. pylori eradication therapy. Am J Clin Pathol. 2002;118:719–726. doi: 10.1309/LJ4D-E2LX-7UMR-YMTH. [DOI] [PubMed] [Google Scholar]
- 122.Baisden BL, Lepidi H, Raoult D. Diagnosis of Whipple disease by immunohistochemical analysis. A sensitive and specific method for the detection of Tropheryma whipplei (the Whipple bacillus) in paraffin-embedded tissue. Am J Clin Pathol. 2002;118:742–748. doi: 10.1309/8YGR-FE7L-39LL-L37C. [DOI] [PubMed] [Google Scholar]
- 123.Lepidi H, Fenollar F, Gerolami R. Whipple disease: immunospecific and quantitative immunohistochemical study of intestinal biopsy specimens. Hum Pathol. 2003;34:589–596. doi: 10.1016/s0046-8177(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 124.Lepidi H, Costedoat N, Piette JC. Immunohistological detection of Tropheryma whipplei (Whipple bacillus) in lymph nodes. Am J Med. 2002;113:334–336. doi: 10.1016/s0002-9343(02)01174-9. [DOI] [PubMed] [Google Scholar]
- 125.Dumler JS, Walker DH. Diagnostic tests for Rocky Mountain spotted fever and other rickettsial diseases. Dermat Clin. 1994;12:25–36. [PubMed] [Google Scholar]
- 126.White WL, Patrick JD, Miller LR. Evaluation of immunoperoxidase techniques to detect Rickettsia rickettsii in fixed tissue sections. Am J Clin Pathol. 1994;101:747–752. doi: 10.1093/ajcp/101.6.747. [DOI] [PubMed] [Google Scholar]
- 127.Procop GW, Burchette JL, Jr, Howell DN. Immunoperoxidase and immunofluorescent staining of Rickettsia rickettsii in skin biopsies. Arch Pathol Lab Med. 1997;121:894–899. [PubMed] [Google Scholar]
- 128.Paddock CD, Greer PW, Ferebee TL. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis. 1999;179:1469–1476. doi: 10.1086/314776. [DOI] [PubMed] [Google Scholar]
- 129.Lepidi H, Fornier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–889. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 130.Baorto L, Payne RM, Slater LN. Culture-negative endocarditis caused by Bartonella henselae. J Pediatr. 1998;132:1051–1054. doi: 10.1016/s0022-3476(98)70410-x. [DOI] [PubMed] [Google Scholar]
- 131.Reed JA, Brigati DJ, Flynn SD. Immunohistochemical identification of Rochalimaea henselae in bacillary (epithelioid) angiomatosis, parenchymal bacillary peliosis, and persistent fever with bacteremia. Am J Surg Pathol. 1992;16:650–657. doi: 10.1097/00000478-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 132.Min KW, Reed JA, Welch DF. Morphologically variable bacilli of cat scratch disease are identified by immunohistochemical labeling with antibodies to Rochalimaea henselae. Am J Clin Pathol. 1994;101:607–610. doi: 10.1093/ajcp/101.5.607. [DOI] [PubMed] [Google Scholar]
- 133.Daybell D, Paddock CD, Zaki SR. Disseminated infection with Bartonella henselae as a cause of spontaneous splenic rupture. Clin Infect Dis. 2004;39:21–24. doi: 10.1086/422001. [DOI] [PubMed] [Google Scholar]
- 134.Zaki SR, Shieh W-J, Epidemic Working Group Leptospirosis associated with an outbreak of acute febrile illness and pulmonary hemorrhage, Nicaragua 1995. Lancet. 1996;347:535–536. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 135.Trevejo RT, Rigau-Perez JG, Ashford DA. Epidemic leptospirosis associated with pulmonary hemorrhage – Nicaragua, 1995. J Infect Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 136.Guarner J, Shieh WJ, Morgan J. Leptospirosis mimicking acute cholecystitis among athletes participating in a triathlon. Hum Pathol. 2001;32:750–752. doi: 10.1053/hupa.2001.25599. [DOI] [PubMed] [Google Scholar]
- 137.Nicodemo AC, Duarte MI, Alves VA. Lung lesions in human leptospirosis: microscopic, immunohistochemical, and ultrastructural features related to thrombocytopenia. Am J Trop Med Hyg. 1997;56:181–187. doi: 10.4269/ajtmh.1997.56.181. [DOI] [PubMed] [Google Scholar]
- 138.Lebech AM, Clemmensen O, Hansen K. Comparison of in vitro, immunohistochemical staining, and PCR for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J Clin Microbiol. 1995;33:2328–2333. doi: 10.1128/jcm.33.9.2328-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aberer E, Kersten A, Klade H. Heterogeneity of Borrelia burgdorferi in the skin. Am J Dermpath. 1996;18:571–579. doi: 10.1097/00000372-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 140.Terpstra WJ, Groeneveld K, Eijk PP. Comparison of two nonculture techniques for detection of Haemophilus influenzae in sputum: In situ hybridization and immunoperoxidase staining with monoclonal antibodies. Chest. 1988;94:126S. [PubMed] [Google Scholar]
- 141.Groeneveld K, van Alphen L, van Ketel RJ. Nonculture detection of Haemophilus influenzae in sputum with monoclonal antibodies specific for outer membrane lipoprotein P6. J Clin Microbiol. 1989;27:2263. doi: 10.1128/jcm.27.10.2263-2267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Forsgren J, Samuelson A, Borrelli S. Persistence of nontypeable Haemophilus influenzae in adenoid macrophages: a putative colonization mechanism. Act Oto-Laryngol. 1996;116:766–773. doi: 10.3109/00016489609137922. [DOI] [PubMed] [Google Scholar]
- 143.Shurbaji MS, Dumler JS, Gage WR. Immunohistochemical detection of chlamydial antigens in association with cystitis. Am J Pathol. 1990;93:363. doi: 10.1093/ajcp/93.3.363. [DOI] [PubMed] [Google Scholar]
- 144.Paukku M, Puolakkainen M, Paavonen T. Plasma cell endometritis is associated with Chlamydia trachomatis infection. Am J Clin Pathol. 1999;112:211–215. doi: 10.1093/ajcp/112.2.211. [DOI] [PubMed] [Google Scholar]
- 145.Naas J, Gnarpe JA. Demonstration of Chlamydia pneumoniae in tissue by immunohistochemistry. APMIS. 1999;107:882–886. [PubMed] [Google Scholar]
- 146.Suffin SC, Kaufmann AF, Whitaker B. Legionella pneumophila. Identification in tissue sections by a new immuno-enzymatic procedure. Arch Pathol Lab Med. 1980;104:283–286. [PubMed] [Google Scholar]
- 147.Maruta K, Miyamoto H, Hamada T. Entry and intracellular growth of Legionella dumoffii in alveolar epithelial cells. Am J Respir Crit Care Med. 1998;157:1967–1974. doi: 10.1164/ajrccm.157.6.9710108. [DOI] [PubMed] [Google Scholar]
- 148.Fiore AE, Nuorti JP, Levine OS. Epidemic legionaire's disease two decades later: Old sources, new diagnostic methods. Clin Infect Dis. 1998;26:426–433. doi: 10.1086/516309. [DOI] [PubMed] [Google Scholar]
- 149.Parkash V, Morotti RA, Joshi V. Immunohistochemical detection of Listeria antigens in the placenta in peri-natal listeriosis. Int J Gynecol Pathol. 1998;17:343–350. doi: 10.1097/00004347-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 150.Chiba M, Fukushima T, Koganei K. Listeria monocytogenes in the colon in a case of fulminant ulcerative colitis. Scan J Gastroenterol. 1998;33:778–782. doi: 10.1080/00365529850171765. [DOI] [PubMed] [Google Scholar]
- 151.Weinstock D, Horton SB, Rowland PH. Rapid diagnosis of Listeria monocytogenes by immunohistochemistry in formalinfixed brain tissue. Vet Pathol. 1995;32:193–195. doi: 10.1177/030098589503200215. [DOI] [PubMed] [Google Scholar]
- 152.Pospischil A, Wood RL, Anderson TD. Peroxidase-antiperoxidase and immunogold labeling of Salmonella typhimurium and Salmonella cholerasuis var kunzendorf in tissues of experimentally infected swine. Am J Vet Res. 1990;51:619–624. [PubMed] [Google Scholar]
- 153.Thygesen P, Martinsen C, Hougen HP. Histologic, cytologic, and bacteriologic examination of experimentally induced Salmonella typhimurium infection in Lewis rats. Comp Med. 2000;50:124–132. [PubMed] [Google Scholar]
- 154.Carabias E, Palenque R, Serrano JM. Evaluation of an immunohistochemical test with polyclonal antibodies raised against mycobacteria used in formalinfixed tissue compared with mycobacterial specific culture. APMIS. 1998;106:385–388. doi: 10.1111/j.1699-0463.1998.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 155.Kim KM, Lee A, Choi KY. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998;93:606–609. doi: 10.1111/j.1572-0241.1998.173_b.x. [DOI] [PubMed] [Google Scholar]
- 156.Osaki M, Adachi H, Gomyo Y. Detection of mycobacterial DNA in formalinfixed, paraffin-embedded tissue specimens by duplex polymerase chain reaction: application to histopathologic diagnosis. Mod Pathol. 1997;10:78–83. [PubMed] [Google Scholar]
- 157.Barbolini G, Bisetti A, Colizzi V. Immunohistologic analysis of mycobacterial antigens by monoclonal antibodies in tuberculosis and mycobacteriosis. Hum Pathol. 1989;20:1078–1083. doi: 10.1016/0046-8177(89)90226-8. [DOI] [PubMed] [Google Scholar]
- 158.Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbo-hydrate antigens in skin and nerve from leprosy patients with type 1 (reversal) reactions. Am J Trop Med Hyg. 2002;66:409–415. doi: 10.4269/ajtmh.2002.66.409. [DOI] [PubMed] [Google Scholar]
- 159.Verhagen C, Faber W, Klatser P. Immunohistological analysis of in situ expression of mycobacterial antigens in skin lesions of leprosy patients across the histopathological spectrum. Association of mycobacterial lipoarabinomannan (LAM) and Mycobacterium leprae phenolic glycolipid-I (PGL-I) with leprosy reactions. Am J Pathol. 1999;154:1793–1804. doi: 10.1016/S0002-9440(10)65435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Walker DH, Feng HM, Ladner S. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod Pathol. 1997;10:1038–1042. [PubMed] [Google Scholar]
- 161.Koss T, Carter EL, Grossman ME. Increased detection of rickettsialpox in a New York city hospital following the anthrax outbreak of 2001: use of immunohistochemistry for the rapid confirmation of cases in an era of bioterrorism. Arch Dermatol. 2003;139:1545–1552. doi: 10.1001/archderm.139.12.1545. [DOI] [PubMed] [Google Scholar]
- 162.Walker DH, Hudnall SD, Szaniawski WK. Monoclonal antibody-based immunohistochemical diagnosis of rickettsialpox: the macrophage is the principal target. Mod Pathol. 1999;12:529–533. [PubMed] [Google Scholar]
- 163.Moron CG, Popov VL, Feng HM. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–759. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 164.Guarner J, Southwick K, Greer P. Testing umbilical cords for funisitis due to Treponema pallidum infection, Bolivia. Emerg Infect Dis. 2000;6:487–492. doi: 10.3201/eid0605.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chung KY, Lee MG, Chon CY. Syphilitic gastritis: demonstration of Treponema pallidum with the use of fluorescent treponemal antibody absorption complement and immunoperoxidase stains. J Am Acad Derm. 1989;21:183–185. doi: 10.1016/s0190-9622(89)70158-4. [DOI] [PubMed] [Google Scholar]
- 166.Guarner J, Greer PW, Bartlett J. Congenital syphilis in a newborn: an immunopathologic study. Mod Pathol. 1999;12:82–87. [PubMed] [Google Scholar]
- 167.Schwarz J. The diagnosis of deep mycoses by morphological methods. Hum Pathol. 1982;13:519–533. doi: 10.1016/s0046-8177(82)80267-0. [DOI] [PubMed] [Google Scholar]
- 168.Marques MEA, Coelho KIR, Bacchi CE. Comparison between histochemical and immunohistochemical methods for the diagnosis of sporotrichosis. J Clin Pathol. 1992;45:1089–1093. doi: 10.1136/jcp.45.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reed JA, Hemaan BA, Alexander JL. Immunomycology: rapid and specific immunocytochemical identification of fungi in formalinfixed, paraffin-embedded material. J Histochem Cytochem. 1993;41:1217–1221. doi: 10.1177/41.8.8331285. [DOI] [PubMed] [Google Scholar]
- 170.Jensen HE, Schonheyder H, Hotchi M. Diagnosis of systemic mycosis by specific immunohistochemical tests. APMIS. 1996;104:241–258. doi: 10.1111/j.1699-0463.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 171.Cooper CR, McGinnis MR. Pathology of Penicillium marneffei: an emerging acquired immunodeficiency syndrome-related pathogen. Arch Lab Pathol Med. 1997;121:798–804. [PubMed] [Google Scholar]
- 172.Fukuzawa M, Inaba H, Hayama M. Improved detection of medically important fungi by immunoperoxidase staining with polyclonal antibodies. Virchows Arch. 1995;427:407–414. doi: 10.1007/BF00199390. [DOI] [PubMed] [Google Scholar]
- 173.Kauffman L. Immunohistologic diagnosis of systemic mycosis: an update. Eur J Epidemiol. 1992;8:377–382. doi: 10.1007/BF00158571. [DOI] [PubMed] [Google Scholar]
- 174.Verweij PE, Smedts F, Poot T. Immunoperoxidase staining for identification of Aspergillus species in routinely pro-cessed tissue sections. J Clin Pathol. 1996;49:798–801. doi: 10.1136/jcp.49.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Breier F, Oesterreicher C, Brugger S. Immunohistochemistry with monoclonal antibody against Candida albicans mannan antigen demonstrates cutaneous Candida granulomas as evidence of Candida sepsis in an immunosuppressed host. Dermatology. 1997;194:293–296. doi: 10.1159/000246130. [DOI] [PubMed] [Google Scholar]
- 176.Marcilla A, Monteagudo C, Mormeneo S. Monoclonal antibody 3H8: a useful tool in the diagnosis of candidiasis. Microbiol. 1999;145:695–701. doi: 10.1099/13500872-145-3-695. [DOI] [PubMed] [Google Scholar]
- 177.Monteagudo C, Marcilla A, Mormeneo S. Specific immunohistochemical identification of Candida albicans in paraffin-embedded tissue with a new monoclonal antibody (1B12) Am J Clin Pathol. 1995;103:130–135. doi: 10.1093/ajcp/103.2.130. [DOI] [PubMed] [Google Scholar]
- 178.Kockenberger MB, Canfield PJ, Kozel TR. An immunohistochemical method that differentiates Cryptococcus neoformans varieties and serotypes in formalinfixed paraffin-embedded tissues. Med Mycol. 2001;39:523–533. doi: 10.1080/mmy.39.6.523.533. [DOI] [PubMed] [Google Scholar]
- 179.Tsunemi T, Kamata T, Fumimura Y. Immunohistochemical diagnosis of Cryptococcus neoformans var. gatti infection in chronic meningoencephailitis: the first case in Japan. Intern Med. 2001;40:1241–1244. doi: 10.2169/internalmedicine.40.1241. [DOI] [PubMed] [Google Scholar]
- 180.Tarrand JJ, Lichterfeld M, Warraich I. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119:854–858. doi: 10.1309/EXBV-YAUP-ENBM-285Y. [DOI] [PubMed] [Google Scholar]
- 181.Choi JK, Mauger J, McGowan KL. Immunohistochemical detection of Aspergillus species in pediatric tissue samples. Am J Clin Pathol. 2004;121:18–25. doi: 10.1309/DK1C-G9MA-TKYY-BFMQ. [DOI] [PubMed] [Google Scholar]
- 182.Pierard GE, Arrese-Estrada J, Pierard-Franchimont C. Immunohistochemical expression of galactomannan in the cytoplasm of phagocytic cells during invasive aspergillosis. Am J Clin Pathol. 1991;96:373–376. doi: 10.1093/ajcp/96.3.373. [DOI] [PubMed] [Google Scholar]
- 183.Phillips P, Weiner MH. Invasive aspergillosis diagnosed by monoclonal and polyclonal reagents. Hum Pathol. 1987;18:1015–1024. doi: 10.1016/s0046-8177(87)80218-6. [DOI] [PubMed] [Google Scholar]
- 184.Fenelon LE, Hamilton AJ, Figueroa JI. Production of specific monoclonal antibodies to Aspergillus species and their use in immunohistochemical identification of aspergillosis. J Clin Microbiol. 1999;37:1221–1223. doi: 10.1128/jcm.37.4.1221-1223.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wazir JE, Brown I, Martin-Bates E. EB9, a new antibody for the detection of trophozoites of Pneumocystis carinii in bronchoalveolar lavage specimens in AIDS. J Clin Pathol. 1994;47:1108–1111. doi: 10.1136/jcp.47.12.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wazir JE, Macrorie SG, Coleman DV. Evaluation of the sensitivity, specificity, and predictive value of monoclonal antibody 3F6 for the detection of Pneumocystis carinii pneumonia in bronchoalveolar lavage specimens and induced sputum. Cytopathol. 1994;5:82–89. doi: 10.1111/j.1365-2303.1994.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 187.Chaiwun B, Khunamornpong S, Sirivanichai C. Lymphadenopathy due to Penicillium marnerffei infection: Diagnosis by fine needle aspiration cytology. Mod Pathol. 2002;15:939–943. doi: 10.1097/01.MP.0000027203.44333.95. [DOI] [PubMed] [Google Scholar]
- 188.Arrese EJ, Stynen D, Pierard-Franchimont C. Immunohistochemical identification of Penicillium marneffei by monoclonal antibodies. Int J Dermatol. 1992;31:410–412. doi: 10.1111/j.1365-4362.1992.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 189.Burke DG, Emancipator SN, Smith MC. Histoplasmosis and kidney disease in patients with AIDS. Clin Infect Dis. 1997;25:281–284. doi: 10.1086/514556. [DOI] [PubMed] [Google Scholar]
- 190.Arnold SJ, Kinney MC, McCormick MS. Disseminated toxoplasmosis: unusual presentations in the immunocompromised host. Arch Pathol Lab Med. 1997;121:869–873. [PubMed] [Google Scholar]
- 191.Warnke C, Tuazon CU, Kovacs A. Toxoplasma encephalitis in patients with acquired immunodeficiency syndrome: diagnosis and response to therapy. Am J Trop Med Hyg. 1987;36:509. doi: 10.4269/ajtmh.1987.36.509. [DOI] [PubMed] [Google Scholar]
- 192.Ganji M, Tan A, Maitar ML. Gastric toxoplasmosis in a patient with acquired immunodeficiency syndrome. A case report and review of the literature. Arch Pathol Lab Med. 2003;127:732–734. doi: 10.5858/2003-127-732-GTIAPW. [DOI] [PubMed] [Google Scholar]
- 193.Hofman V, Brousset P, Mougneau E. Immunostaining of visceral leishmaniasis caused by Leishmania infantum using monoclonal antibody (19-11) to the leishmania homologue of receptors for activated c-kinase. Am J Clin Pathol. 2003;120:567–574. doi: 10.1309/R3DK-4MR3-W6E5-PH17. [DOI] [PubMed] [Google Scholar]
- 194.Azadeh B, Sells PG, Ejeckman GC. Localized Leishmania lymphadenitis immunohistochemical studies. Am J Clin Pathol. 1994;102:11–15. doi: 10.1093/ajcp/102.1.11. [DOI] [PubMed] [Google Scholar]
- 195.Kenner JR, Aronson NE, Bratthauer GL. Immunohistochemistry to identify Leishmania parasites in fixed tissues. J Cutan Pathol. 1999;26:130–136. doi: 10.1111/j.1600-0560.1999.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 196.Amato VS, Duarte MIS, Nicodemo AC. An evaluation of clinical, serologic, anatomopathologic and immunohistochemical findings for fifteen patients with mucosal leishmaniasis before and after treatment. Rev Inst Med Trop S Paulo. 1998;40:23–30. doi: 10.1590/s0036-46651998000100006. [DOI] [PubMed] [Google Scholar]
- 197.Bonnin A, Petrella T, Dubremetz JF. Histopathologic methods for diagnosis of cryptosporidiosis using monoclonal antibodies. Eur J Clin Microbiol Infect Dis. 1990;9:664–665. doi: 10.1007/BF01964268. [DOI] [PubMed] [Google Scholar]
- 198.Perez de Suarez E, Perez-Schael I, Perozo-Ruggeri G. Immunocytochemical detection of Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1987;81:624–626. doi: 10.1016/0035-9203(87)90435-4. [DOI] [PubMed] [Google Scholar]
- 199.Guarner J, Bartlett J, Zaki SR. Mouse model for Chagas disease: immunohistochemical distribution of different stages of Trypanosoma cruzi in tissues throughout infection. Am J Trop Med Hyg. 2001;65:152–158. doi: 10.4269/ajtmh.2001.65.152. [DOI] [PubMed] [Google Scholar]
- 200.Anez N, Carrasco H, Parada H. Myocardial parasite persistence in chronic chagasic patients. Am J Trop Med Hyg. 1999;60:726–732. doi: 10.4269/ajtmh.1999.60.726. [DOI] [PubMed] [Google Scholar]
- 201.Reis MM, Higuchi M de L, Benvenuti LA. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immun Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 202.Torres-Velez FJ, Nace EK, Won KY. Development of an immunohistochemical assay for the detection of babesiosis in formalinfixed, paraffin-embedded tissue samples. Am J Clin Pathol. 2003;120:833–838. doi: 10.1309/A4RG-P4LF-12GG-H8MW. [DOI] [PubMed] [Google Scholar]
- 203.Sanad MM, Darwish RA, Nasr ME. Giardia lamblia and chronic gastritis. J Egypt Soc Parasitol. 1996;26:481–495. [PubMed] [Google Scholar]
- 204.Institute of Medicine . Emerging infections: microbial threats to health in the United States. National Academy Press; Washington, DC: 1992. [PubMed] [Google Scholar]
- 205.CDC Preventing emerging infectious diseases: a strategy for the 21st century. MMWR. 1998;(47 No):RR-15. [PubMed] [Google Scholar]
- 206.Perkins BA, Flood JM, Danila R. Unexplained deaths due to possibly infectious causes in the United States: defining the problem and designing surveillance and laboratory approaches. Emerg Infect Dis. 1998;2:47–53. doi: 10.3201/eid0201.960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Houpikina P, Raoult D. Traditional and molecular techniques for the study of emerging bacterial diseases: one laboratory's perspective. Emerg Infect Dis. 2002;8:122–131. doi: 10.3201/eid0802.010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Khan AS, Khabbaz RF, Armstrong RC. Hantavirus pulmonary syndrome: the first 100 US cases. J Infect Dis. 1996;173:1297–1303. doi: 10.1093/infdis/173.6.1297. [DOI] [PubMed] [Google Scholar]
- 209.Moolenaar RL, Dalton C, Lipman HB. Clinical features that differentiate hantavirus pulmonary syndrome from three other acute respiratory illnesses. Clin Infect Dis. 1995;21:643–649. doi: 10.1093/clinids/21.3.643. [DOI] [PubMed] [Google Scholar]
- 210.Nolte KB, Feddersen RM, Foucar K. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 211.Zaki SR, Greer PW, Coffield LM. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 212.Zaki SR, Khan AS, Goodman RA. Retrospective diagnosis of hantavirus pulmonary syndrome, 1978–1993. Implications for emerging infectious diseases. Arch Pathol Lab Med. 1996;120:134–139. [PubMed] [Google Scholar]
- 213.Shieh WJ, Guarner J, Layton M. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg Infect Dis. 2000;6:370–372. doi: 10.3201/eid0604.000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cushing MM, Brat DJ, Mosunjac MI. Fatal West Nile virus encephalitis in an renal transplant recipient. Am J Clin Pathol. 2004;121:26–31. doi: 10.1309/G23C-P54D-AR1B-CY8L. [DOI] [PubMed] [Google Scholar]
- 215.Petersen RL, Roehrig JT, Hughes JM. West Nile virus encephalitis. N Engl J Med. 2002;347:1225–1226. doi: 10.1056/NEJMo020128. [DOI] [PubMed] [Google Scholar]
- 216.Sampson BA, Ambrosi C, Charlot A. The pathology of human West Nile virus infection. Hum Pathol. 2000;31:527–532. doi: 10.1053/hp.2000.8047. [DOI] [PubMed] [Google Scholar]
- 217.Guarner J, Shieh WJ, Hunter S. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35:983–990. doi: 10.1016/j.humpath.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 218.Lanciotti RS, Kerst AJ, Nasci RS. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ho M, Chen ER, Hsu KH. An epidemic of enterovirus 71 infection in Taiwan. Taiwan enterovirus epidemic working group. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 220.Chan LG, Parashar UD, Lye MS. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 2000;31:678–683. doi: 10.1086/314032. [DOI] [PubMed] [Google Scholar]
- 221.Wong KT, Chua KB, Lam SK. Immunohistochemical detection of infected neurons as a rapid diagnosis of enterovirus 71 encephalomyelitis. Ann Neurol. 1999;45:271–272. doi: 10.1002/1531-8249(199902)45:2<271::aid-ana23>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 222.Yan JJ, Wang JR, Liu CC. An outbreak of enterovirus 71 infection in Taiwan 1998: a comprehensive pathological, virological, and molecular study on a case of fulminant encephalitis. J Clin Virol. 2000;17:13–22. doi: 10.1016/s1386-6532(00)00067-6. [DOI] [PubMed] [Google Scholar]
- 223.Shieh WJ, Jung SM, Hsueh C. Pathologic studies of fatal causes in outbreak of hand, foot, and mouth disease, Taiwan. Emerg Infect Dis. 2001;7:146–148. doi: 10.3201/eid0701.700146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wong KT, Shieh WJ, Kumar S. Nipah virus infection. Pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Goh KJ, Tan CT, Chew NK. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342:1229–1235. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 226.Chua KB, Bellini WJ, Rota PA. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 227.Dawson JE, Paddock CD, Warner CK. Tissue diagnosis of Ehrlichia chaffeensis in patients with fatal ehrlichiosis by use of immunohistochemistry, in situ hybridization, and polymerase chain reaction. Am J Trop Med Hyg. 2001;65:603–609. doi: 10.4269/ajtmh.2001.65.603. [DOI] [PubMed] [Google Scholar]
- 228.Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichiosis. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 229.Paddock CD, Suchard DP, Grumbach KL. Fatal seronegative ehrlichiosis in a patient with HIV infection. N Engl J Med. 1993;329:1164–1167. doi: 10.1056/NEJM199310143291605. [DOI] [PubMed] [Google Scholar]
- 230.Lepidi H, Bunnell JE, Martin ME. Comparative pathology and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am J Trop Med Hyg. 2000;62:29–37. doi: 10.4269/ajtmh.2000.62.29. [DOI] [PubMed] [Google Scholar]
- 231.Sehdev AE, Dumler JS. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am J Clin Pathol. 2003;119:859–865. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 232.Hooper PT, Russell GM, Selleck PW. Immunohistochemistry in the identification of a number of new diseases in Australia. Vet Microbiol. 1999;68:89–93. doi: 10.1016/s0378-1135(99)00064-4. [DOI] [PubMed] [Google Scholar]
- 233.Williamson MM, Hooper PT, Selleck PW. Experimental hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) J Comp Pathol. 2000;122:201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- 234.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 235.Peiris JS, Lai ST, Poon LL. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nakajima N, Asahi-Ozaki Y, Nagata N. SARS coronavirus-infected cells in lung detected by new in situ hybridization technique. Jpn J Infect Dis. 2003;56:139–141. [PubMed] [Google Scholar]
- 237.Kuiken T, Fouchier RA, Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chong PY, Chui P, Ling AE. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.5858/2004-128-195-AODDTS. [DOI] [PubMed] [Google Scholar]
- 239.McAuliffe J, Vogel L, Roberts A. Replication of SARS coronavirus administered into the respiratory tract of African green, rhesus and cynomolgus monkeys. Virology. 2004;330:8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Roberts A, Vogel L, Guarner J. SARS coronavirus infection of golden Syrian hamsters. J Virol. 2005;79:503–511. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen PC, Hsiao CH. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol. 2004;203:729–730. doi: 10.1002/path.1510. author reply 730–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.To KF, Tong JH, Chan PK. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chow KC, Hsiao CH, Lin TY. Detection of severe acute respiratory syndrome-associated coronavirus in pneumocytes of the lung. Am J Clin Pathol. 2004;121:574–580. doi: 10.1309/C0EDU0RAQBTXBHCE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ding Y, He L, Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sheih W-J, Cheng-Hsiang H, Paddock CD. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ashford DA, Kaiser RB, Bales ME. Planning against biological terrorism: lessons from outbreak investigations. Emerg Infect Dis. 2003;9:515–519. doi: 10.3201/eid0905.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Inglesby TV, O'Toole T, Henderson DA. Preventing the use of biological weapons: improving response should prevention fail. Clin Infect Dis. 2000;30:926–929. doi: 10.1086/313794. [DOI] [PubMed] [Google Scholar]
- 248.Lillibridge SR, Bell AJ, Roman RS. Thoughts for the new millennium: Bioterrorism. Centers for Disease Control and prevention, bioterrorism preparedness and response. Am J Infect Control. 1999;27:463–464. doi: 10.1016/s0196-6553(99)70020-9. [DOI] [PubMed] [Google Scholar]
- 249.Franz DR, Zajtchuk R. Biological terrorism: understanding the threat, preparation, and medical response. Dis Month. 2000;46:125–190. doi: 10.1016/s0011-5029(00)90017-8. [DOI] [PubMed] [Google Scholar]
- 250.Ezzell JW, Abshire TG, Little SF. Identification of Bacillus anthracis by using monoclonal antibodies to cell wall galactose-N-acetylglucosamine polysaccharide. J Clin Microbiol. 1990;28:223–231. doi: 10.1128/jcm.28.2.223-231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shieh WJ, Guarner J, Paddock C. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am J Pathol. 2003;163:1901–1910. doi: 10.1016/S0002-9440(10)63548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guarner J, Jernigan JA, Shieh WJ. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003;163:701–709. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jernigan JA, Stephens DS, Ashford DA. Bioter-rorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jernigan DB, Raghunathan PL, Bell BP. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Grinberg LM, Abramova FA, Yampolskaya OV. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod Pathol. 2001;14:482–495. doi: 10.1038/modpathol.3880337. [DOI] [PubMed] [Google Scholar]
- 256.Guarner J, Greer PW, Bartlett J. Immunohistochemical detection of Francisella tularensis in formalinfixed paraffin-embedded tissue. Appl Immun Mol Morphol. 1999;7:122–126. [Google Scholar]
- 257.DeBey BM, Andrews GA, Chard-Bergstrom C. Immunohistochemical demonstration of Francisella tularensis in lesions of cats with tularaemia. J Vet Diagn Invest. 2002;14:162–164. doi: 10.1177/104063870201400213. [DOI] [PubMed] [Google Scholar]
- 258.Guarner J, Shieh WJ, Greer PW. Immunohistochemical detection of Yersinia pestis in formalinfixed paraffin-embedded tissue. Am J Clin Pathol. 2002;117:205–209. doi: 10.1309/HXMF-LDJB-HX1N-H60T. [DOI] [PubMed] [Google Scholar]
- 259.Davis KJ, Vogel P, Fritz DL. Bacterial filamentation of Yersinia pestis by β-lactam antibiotics in experimentally infected mice. Arch Pathol Lab Med. 1997;121:865–868. [PubMed] [Google Scholar]
- 260.Davis KJ, Fritz DL, Pitt ML. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops) Arch Pathol Lab Med. 1996;120:156–163. [PubMed] [Google Scholar]
- 261.Williams ES, Mills K, Kwiatkowski DR. Plague in black-footed ferret (Mustela nigripes) J Wild Dis. 1994;30:581–585. doi: 10.7589/0090-3558-30.4.581. [DOI] [PubMed] [Google Scholar]
- 262.Gabastou JM, Proaño J, Vimos A. An outbreak of plague including cases with probable pneumonic infection, Ecuador, 1998. Trans R Soc Trop Med Hyg. 2000;94:387–391. doi: 10.1016/s0035-9203(00)90114-7. [DOI] [PubMed] [Google Scholar]
- 263.van Moll P, Baumgartner W, Eskens U. Immunocytochemical demonstration of Coxiella burnetii antigen in the fetal placenta of naturally infected sheep and cattle. J Comp Pathol. 1993;109:295–301. doi: 10.1016/s0021-9975(08)80254-x. [DOI] [PubMed] [Google Scholar]
- 264.Brouqui P, Dumler JS, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–458. doi: 10.1016/0002-9343(94)90325-5. [DOI] [PubMed] [Google Scholar]


