Abstract
Although it has been used for centuries, bismuth remains one of the least understood elements in the periodic table. Metallic bismuth and bismuth compounds have been widely used in the manufacture of alloys, pigments, cosmetics, and pharmaceuticals. As a “green” heavy metal, the substitution of lead with bismuth in some industries may partially resolve the environmental problems related to heavy metal pollution. In health care, as bismuth has low toxicity to humans, bismuth-based drugs such as colloidal bismuth subcitrate (CBS), ranitidine bismuth citrate (RBC), bismuth subsalicylate (BSS), bismuth iodoform and radioactive bismuth (212Bi/213Bi) complexes have been developed and used in clinics to treat various diseases. In most cases, bismuth therapies exhibit high therapeutic efficacies and little side effects; nevertheless, there are still reported cases of bismuth toxicity caused by bismuth over-dosage.
Keywords: Anticancer, Antimicrobial, Antivirus, Biomolecule, Bismuth, Environment, Health, Medicine, Pollution, Side effects, Toxicity
Introduction
Bismuth is a metallic chemical element, symbolled as Bi, with atomic number of 83. Although it has been known since ancient times, it became familiar to the Greeks and Romans during the Middle Ages, before that bismuth was often confused with other metals, such as lead and tin. It is considered to be a metal in the periodic table but has more similarity to semimetals. Bismuth is categorized among the group of elements conventionally known as “poor elements” due to its rarity. It has been used quite extensively for various purposes including cosmetic, industrial, laboratory, and pharmaceutical. Bismuth serves as a leading nontoxic replacement for lead in brass plumbing fixtures, fishing sinkers, free machining steels, and solders, as well as a metallurgical additive in the foundry. The manufacture applications of bismuth also include ceramic glazes, pearlescent pigments, lubricating greases, and crystal ware.
Major applications of bismuth in medicine and health care are related to its high effectiveness in treating burns, intestinal disorders, and stomach ulcers as well as its potential activities against microorganisms, viruses and malignant tumors. With the development of new therapies and better understanding of the mechanism of action of bismuth, the applications of bismuth-based agents in health care will be further extended.
Consumption and Distribution of Bismuth in the World
Bismuth is a relatively rare element in the Earth and ranked 69th in terms of its natural abundance in the Earth’s crust, estimated at about 2 ppb by atoms. The world reserves of bismuth is usually estimated based on the content of lead resources since bismuth is always a by-product of the processing of other metal ores such as lead, silver, tin, copper, and zinc. Only the Tasna Mine in Bolivia and a mine in China produce bismuth as a major product. World reserves of bismuth are estimated at around 320,000 tons.
Bismuth has been consumed rapidly over the last decade. In 2018 approximately 16,000 tons of refinery bismuth were produced worldwide. Among all the countries, China is the leading producer of refined bismuth with ca. 79.9% of the world’s total production, followed by Laos, Japan, and Mexico with ca. 12.3%, 3.6%, and 2.1% respectively. The estimated global reserve of bismuth is shown in Fig. 1 .
Fig. 1.
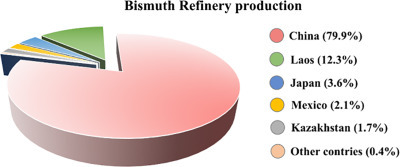
Estimated global bismuth reserve base.
Adapted with permission from the USGS 2019 minerals yearbook.
Bismuth in the Manufactory Industry
Comparison to majority of other heavy metals, bismuth has low toxicity, which marks it as a “green metal” for the environment. Bismuth and its alloys have widespread commercial applications such as in the production of lubricating grease, chemicals, catalysts, shot bullets, cosmetics, fire sprinkler systems, solders, thermoelectric materials, pigments, fishing sinkers, 235U/233U carriers, medicines, malleable steels, etc.
The semimetal crystal structure of bismuth along with its other physical–chemical properties such as expansion on solidification, the widest range between melting and boiling points among all metals, and the lowest thermal and heat conductivity make bismuth an ideal substitute for lead analogs in extreme-pressure additives (EP additives). Substitution of the formerly widely used lead-based EP additives by bismuth-sulfur additives not only provides a higher lubricant efficacy, but also reduces the amount of heavy metal used in extreme-pressure lubricants by a half, in accordance with the new ecological and environmental philosophy of the world. In recent decades, the substitution of lead with bismuth in glass production may lead to potential application for bismuth in the manufacturing of automobile glass, the finest tableware, and art objects, with the purpose of environmental protection. In North America, Bi—Sn has been used to replace lead in shotshells for the hunting of wetland birds. Although the bismuth-containing shotshells cannot be approved as “nontoxic,” in comparison to the high toxicity of lead (e.g. a toxic intake level of 1 mg for a 70 kg human), the higher tolerance to bismuth among humans (e.g. a toxic intake level of 15 × 103 mg for a 70 kg human) renders it a “relatively” safe substitute for preventing environmental problems caused by lead accumulation. Owing to their satiny luster and low absorption properties, some bismuth compounds such as bismuth oxychloride and bismuth vanadate have also been used in cosmetics including nail polishes, lipsticks, and eye shadows. Recently in Europe, the Restriction of Hazardous Substances Directive (RoHS-2002/95/EC) has stated that lead must be eliminated in the manufacturing of various types of electronic and electrical equipment. This restriction, which has been supported by commercial and research organizations in Japan and the North America, may further increase the demand for bismuth in industry in future, and accelerate the replacement of lead and other heavy metals with bismuth in the manufacturing industry.
Bismuth in Pharmaceuticals
The medical use of bismuth can be traced back to 200 years ago, and bismuth-related agents have been involved in the treatment of a wide range of diseases including syphilis (sodium/potassium bismuth tartrate, bismuth quinine iodide, iododbismitol, bismuth chloride, etc.), colitis (bismuth subnitrate and bismuth citrate), wound infection (bismuth oxide) and parasite infections (sodium bismuth thioglycolate). The massive usage of bismuth compounds for the treatment of syphilis and wound infections in early 20th century was dramatically dropped owing to the discovery of antibiotics, thus, contemporary use of bismuth in medicine principally lies in the treatment of gastrointestinal disorders such as gastric and duodenal ulcers (e.g. colloidal bismuth subcitrate (CBS), bismuth subsalicylate, and bismuth subnitrate), dyspepsia (bismuth subsalicylate, bismuth subnitrate, etc.), and diarrhea (bismuth subsalicylate, bismuth nitrate, etc.).
Bismuth subsalicylate (BSS, PeptoBismols; the Procter & Gamble Company, Cincinnati, Ohio, United States) is one of the most commercially successful bismuth medicines with annual sales of 82.6 million USD in United States in 2013 (statistics from The Statistics Portal). It has been widely used for rapid relief of heartburn, nausea, indigestion and diarrhea. On the basis of crystallographic analysis, BSS possesses a complex cluster including Bi(III) ions, mono-deprotonated salicylate, deprotonated H2O and other oxo ligands. Upon interaction with gastric acid in stomach, this medicine releases Bi(III) ions, which is known to inhibit the growth of Helicobacter pylori (H. pylori, Fig. 2 ), and salicylate, which ameliorates gastritis and antagonizes inflammation.
Fig. 2.
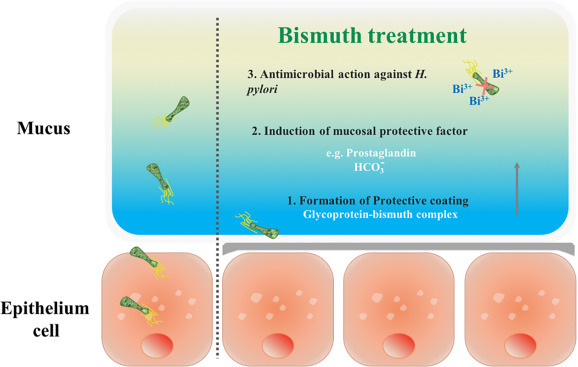
Bismuth exerts multi-anti-H. pylori properties.
Bismuth Compounds as Anti-Helicobacter pylori (H. pylori) Agents
Bismuth citrate-based compounds, represented by CBS (De-Nol; Gist Brocades and Yamanouchi), are the most typical anti-H. pylori bismuth drugs and have been widely utilized in “cocktail” therapy to treat a variety of H. pylori-associated infections. The therapeutic efficacy of bismuth citrate-based drugs against the H. pylori-associated infections may be attributed to (1) preferential formation of a “protective coating” on the ulcer craters owing to the fact that CBS can form polymeric structures (Fig. 3 ); (2) induction of the secretion of increasing mucosal protective factors; (3) antimicrobial activity of Bi(III), which is attributable to its ability to disrupt multiple biological pathways via binding to key proteins andenzymes. Ranitidine bismuth citrate (RBC, Tritec and Pylorid, GlaxoSmithKline plc.) was later found to be a more efficient antiulcer agent, since it incorporates an H2-receptor antagonist, ranitidine, to suppress secretion of excess stomach acid.
Fig. 3.
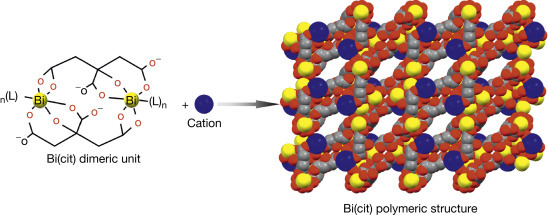
Formation of bismuth citrate ([Bi2(cit)2]22n−) or Bi(cit)) polymeric structure by the dimeric unit ([Bi2(cit)2]22n−). The polymeric framework probably coats on the ulcer craters to prevent the erosion of gastric acid. The negatively charged ([Bi2(cit)2]22n−) framework is balanced by cations. Color code: Bi, yellow; C, gray; O, red; cation, blue.
The newly developed bismuth-based triple or quadruple regimens, which combine the bismuth citrate-based drugs with antibiotics such as amoxicillin, tetracycline, clarithromycin, or nitroimidazole, and proton-pump inhibitors (PPIs) have been often recommended in clinics for treating H. pylori-associated infections. In comparison to the non-bismuth therapies, bismuth-based regimens significantly increase the eradication rate of antibiotic resistant H. pylori (Table 1 ) even for antibiotic resistant strains. A new drug of bismuth with d-poly galacturonic acid, colloidal bismuth pectin, was approved for clinical use in China in the treatment of peptic ulcers and is as effective as CBS.
Table 1.
The efficacy of first-, second-, and third-line H. pylori eradication therapies
| Therapy | Regimen | Number treated | Number successful | Success (%) | Bacteriolytic in regimen |
|---|---|---|---|---|---|
| First-line theraphy | PPI-based regimens | 364 | 243 | 66.8 | Amoxicillin, nitromidazole, clarithromycin, erythromycin, and tetracycline |
| Bi-based regimens | 105 | 100 | 95.2 | Amoxicillin, nitroimidazole, and clarithromycin | |
| Second-line therapy | PPI-based regimens | 16 | 6 | 37.5 | Nitroimidazole, amoxicillin, and clarithromycin |
| Bi-based regimens | 50 | 36 | 72.0 | Nitroimidazole, tetracycline, amoxicillin, and clarithromycin | |
| Third-line therapy | Other regimens | 14 | 8 | 57.1 | Omeprazole, rifabutin, and amoxicillin |
| Bi-based regimens | 6 | 5 | 83.3 | Clarithromycin, tetracycline, and tinidazole |
Apart from over-the-counter bismuth drugs, numbers of novel bismuth compounds with remarkable anti-H. pylori activity have been synthesized in recent decades. Bi(III) complexes with hydroxamic acids, indole-carboxylic acids, α-amino acids, nonsteroidal antiinflammatories, acetylsalicylic acid, glycosaminoglycan, polysaccharide, and some other organic ligands exhibit higher or comparable anti-H. pylori activity to BSS and CBS. Since nanotechnology has been extensively explored, bismuth-based nanomaterials have also received more attention in pharmaceuticals. For example, bismuth subcarbonate ((BiO)2CO3) nanotubes exhibit slightly higher activities against H. pylori than the clinically used colloidal bismuth subcitrate under similar conditions, and importantly, bismuth nanotubes could be used as “capsules” in bismuth-based triple or quadruple therapies for the treatment of H. pylori infection, or as drug “carriers” for sustained-release of other ingredients in the human body, when used in combination with other drugs for the treatment of other diseases.
Bismuth is an effective anti-H. pylori agent though it appears more bacteriostatic than bactericidal. What differentiates bismuth from organic antibiotics is that bismuth drugs have multiple cellular targets. Thus, the anti-H. pylori actions of bismuth drugs are resulted from comprehensive factors including but not limited to, hindrance of synthesis of bacterial cell wall, impediment of bacterial adhesion, inhibition of ATP synthesis, abolishment of defense of oxidative stress and pH buffering ability, interference of metal homeostasis (e.g. Ni2 + homeostasis), inhibition of activity of key enzymes such as alcohol dehydrogenase, urease, DnaK. A recent report showed that bismuth inhibits urease activity by disruption of the cellular maturation of urease via functional perturbation of one of its accessory proteins, UreG, rather than directly targets the metal center of urease. Despite that extensive studies have been made at molecular levels, the exact mode of action of bismuth drug in H. pylori still remains to be unclear.
Bismuth Compounds as Antimicrobial Agents Other Than H. pylori
Bismuth also exhibits potential activities against microorganisms beyond H. pylori. Bismuth thiolates are one of the most representative antimicrobial agents among those newly developed bismuth compounds. The antimicrobial activities of bismuth are greatly enhanced when coordinated with lipophilic thiol-containing ligands such as 1,3-propanedithiol, 3-mer-capto-2-butanol, β-mercaptoethanol, dimercaprol, and dithiothreitol. These complexes show antimicrobial activity against some Gram-negative bacterial strains such as Escherichia coli, Burkholderia cepacia complex, Klebsiella pneumoniae, as well as some Gram-positive bacterial strains such as Staphylococcus aureus (S. aureus) and Streptococcus pyogenes, with minimum inhibitory concentration (MIC) at micromolar levels. Subsequent studies show that Bi(III) thiolates exhibit remarkable anti-biofilm activity against Pseudomonas aeruginosa and methicillin-resistant S. aureus biofilms. Recent research indicates that the antimicrobial spectrum of bismuth thiolates could be further broadened when prepared in nanoparticle form (exemplified as BisBAL NPs). The enhanced antimicrobial potency of bismuth thiolates is possibly in part due to the chelation effect of thiols that significantly improves the solubility and lipophilicity of Bi(III) compounds, as well as the rapid exchange of those kinetically labile thiolate ligands inside bacterial cells.
The antimicrobial activity of bismuth can be synergistically enhanced by coordination with other antimicrobial agents. For example, bismuth conjugates with fluoroquinolone, i.e., norfloxacin and ciprofloxacin, show notable activity with MIC at micromolar levels against both Gram-positive and-negative bacterial strains such as E. coli, S. aureus, S. epidermideis, E. facecalis, B. cereus and B. pumilus.
Recent research reveals that bismuth compounds or drugs could be potentially used as a novel class of antibiotic adjuvant in the treatment of infections caused by metallo-β-lactamase (MBL) producing superbugs (Fig. 4 ). Bismuth drugs, i.e., colloidal bismuth subcitrate (CBS), exhibit potent inhibition on MBL activity through displacement of zinc ions (as essential cofactors) from enzyme’s active site. Bismuth-based antibiotic combination therapy greatly boosted the antimicrobial effectiveness of existing antibiotic, by which the survival of mice infected by MBL superbugs was significantly increased.
Fig. 4.
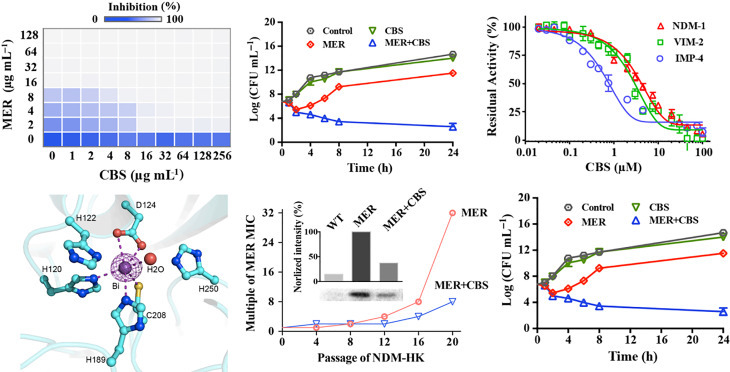
Bismuth serving as novel MBL inhibitor boosts antimicrobial activity of existing β-lactam antibiotics. Combined use of bismuth (as CBS) reduced β-lactam (as meropenem, MER) MIC and lowered the MBL producing bacterial loads (top left and top middle). CBS effectively inactivate MBLs through metal displacement (top right and bottom left). Bismuth-based antibiotic therapy slowed down MBL development and showed boosted therapeutic effectiveness in MBL superbugs peritoneal infection mouse model.
Adapted from Wang R, Lai TP, et al. (2018) Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nature Communications 9: 439.
It shows that bismuth-based compounds and related metallodrugs could become a novel class of MBL inhibitors, and they have great potential to be used as effective antibiotic adjuvants to treat infections caused by MBL superbugs in a wider clinical context.
Bismuth Compounds as Anticancer Agents
Bismuth compounds have been widely found to exhibit potential antineoplastic activity against malignant tumor cell lines. The most promising candidates with potent chemotherapeutic activity are those bismuth compounds with bismuth coordinated to the ligands including thiosemicarbazone/thiocarbonohydrazone, hydrazine, dithiocarbamate and halide, as well as some radiosensitive bismuth-based nanomaterials. For example, a 9-coordinated Bi(III) complex of [Bi(H2L)(NO3)2]NO3 (H2L = 2,6-diacetylpyridine bis((4)N-methylthiosemicarbazone)) has an IC50 value of 26.8 μM against K562 leukemia cells and an inhibitory rate of 61.6% in a hepatocarcinoma (H22 cell line) xenograft mouse model. Bi(III) dithiocarbamate complexes with a general formula of Bi(S2CNR2)3 exhibit powerful anticancer activity against seven cancer cell lines including breast cancer cell line (MCF-7) and ovarian cancer cell line (IGROV) with IC50 values of no more than 0.22 μM. A water-soluble bismuth cyclen based compound, abbreviated as Bi-TPC, exhibits great anticancer activity that is 100-time more potent than cisplatin, probably via interactions with DNA under physiologically relevant conditions.
It has been shown that radioactive bismuth (212Bi and 213Bi) exhibits promising potential as a novel therapeutic agent for mall volume tumors. Both 212Bi and 213Bi have a series of branched decays, resulting in the emission of α −/β-particles. Although the half-life of 212Bi is short (t1/2 1 h), and radioactive lead (212Pb, t1/2 10.6 h) can be used as an in vivo generator of 212Bi. With their short-ranged penetration (50–80 mm), bismuth radionuclides can reduce the nonspecific irradiation to normal tissues around the target cells. Furthermore, in order to deliver bismuth to the site of action effectively, a chelate ligand can be used together with bismuth radionuclides. In this type of combination treatment, the strong chelate ligand is conjugated to a monoclonal antibody or a fusion protein, a standard treatment for tumors, via modification of the ligand to eventually produce a bismuth-radiolabeled “complex.” Once the metal radiolabeled “complex” is introduced into the host, it targets specific cell types or sites of diseases and releases the α-particles only at or near the tumor tissues, thereby minimizing damage to the surrounding normal tissues.
Recently, a chemical conjugation, abbreviated as Tam-Bi2S3@mPS NPs, incorporating bismuth sulfide@mesoporous silica core-shell nanoparticles and trastuzumab, an antibody targeting HER-2 overexpressed breast cancer cells, is rationally designed to exert good anticancer activity with good biocompatibility and drug loading ability. This conjugate reaches certain degree of directed targeting with 16-fold higher bismuth content in targeted tumor group compared with non-targeted group. This type of Bi-based nanomaterial may pioneer a new direction for the development of target-directed therapeutic agents for cancer treatment.
The antitumor mechanism of action of Bi(III) compounds is principally correlated to the generation of ROS, reduction in mitochondrial membrane potential, induction of apoptosis via caspase-3 mediated or G2/M arrest mechanisms, rather than DNA binding as what platinum compounds carry out, though the bismuth-DNA binding was sporadically reported. These unique modes of action of Bi(III)-based anticancer agents display a great potential to circumvent the therapeutic stress placed upward by cisplatin resistance. But the detailed mechanism of action of Bi-based anticancer agents still remains a mystery. Further exploration at the mechanistic and therapeutic levels on bismuth compounds as anticancer agents might serve as a new direction in future.
Other Potential Medicinal Applications of Bismuth
There have not been reports about antiviral applications of bismuth compounds with the exception of bismuth sodium triglycollamate, historically used to treat warts. Recently, bismuth was discovered to be effective in inhibiting severe acute respiratory syndrome coronavirus (SARS-CoV) with an IC50 less than 1 μM. The potential target of bismuth is the SCV NTPase/helicase, a zinc-containing enzyme that has RNA capping activity and controls the virus reproduction. Binding of bismuth may induce conformational changes in the enzyme, which subsequently affects the helicase RNA/DNA unwinding activity, thereby inhibiting the virus proliferation (Fig. 5 ).
Fig. 5.
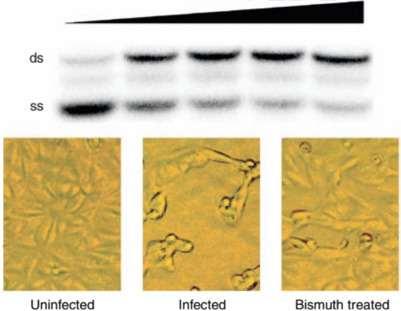
Inhibition of the DNA unwinding activity of SARS helicase by bismuth (top); uninfected cell (bottom left); SARS infected cell (bottom middle); bismuth treated SARS infected cell (bottom right). ds, double-stranded DNA; ss, single-stranded DNA.
Adapted from Yang N, Tanner JA, Zheng BJ, et al. (2007) Bismuth complexes inhibit the SARS coronavirus. Angewandte Chemie International Edition 46: 6464–6468: with permission from Wiley-VCH.
Another potential application of bismuth compounds is in the amelioration of side effects induced by the anticancer drug, cisplatin. Cisplatin and its analogs (e.g. carboplatin and oxaliplatin) have been used in clinics worldwide to treat various solid cancers. However, a major obstacle to the more widespread use of cisplatin-based drugs resides in their severe side effects. The major dose-limiting factor is nephrotoxicity, including tubular degeneration, loss of brush border, necrosis, and mineralization of the tubular epithelial cells. These renal damages are caused by platinum (Pt). The mechanism of platinum nephrotoxicity may be similar to that of mercury (Hg) and likely relates to the depletion of thiolate groups in the renal tubes. Bismuth compounds, such as bismuth (sub)nitrate, have been reported to alleviate cisplatin-induced nephrotoxicity without interfering its antitumor response.
Recent studies show that a bismuth-citrate based complex, BiZn, significantly preserved the renal function of mice receiving overdose of cisplatin treatment. The compound could potentially simulate the production of antioxidative biomolecules, metallothionein and glutathione from kidney cells and minimize cisplatin-induced apoptosis. In vivo experiments displayed that when compared with the cisplatin-alone group, the group of mice receiving pretreatment of bismuth compounds can greatly reduce the blood urea nitrogen (BUN) and creatinine level, defined as an indicator of renal damage and the survival rate was significantly increased (Fig. 6 ).
Fig. 6.
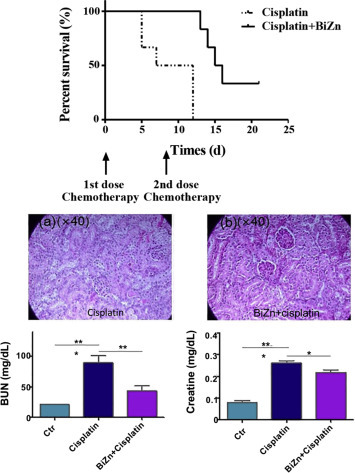
Protection effect of bismuth on mice exposed to overdosed cisplatin treatment. Survival curve of cisplatin-treated SCID Beige mice with or without treatment of BiZn (Top). Renal histological differentiation (middle) and Renal functions i.e. Blood BUN and creatinine (bottom) between mice that untreated or pretreated with BiZn after days of cisplatin administration.
Adapted from Chan C, Wang R, et al. (2019) A novel synthetic compound, bismuth zinc citrate, could potentially reduce cisplatin-induced toxicity without compromising the anticancer effect through enhanced expression of antioxidant protein. Translational Oncology 12: 788–799.with permission from Elsevier.
Potential Physiological Fate of Bismuth in Biological Systems
Unlike other heavy metals, e.g. lead and mercury, bismuth and its related drugs have been involved in clinic treatment of H. pylori-associated infections for decades, and seldom reported to have acute toxicity in humans even being administered at extreme dosages in some cases. Over a long period of time, it remains a mystery that why bismuth(III) is selectively toxic to certain human pathogens rather than human beings. One possible reason is the limited gastrointestinal tract (GIT) absorption of bismuth after its oral administration. Recent studies indicated that glutathione (GSH) may play a vital role for bismuth metabolism and detoxification in mammalian cells. Bismuth ions were found to be passively absorbed, conjugated to GSH and then transported into vesicles via MRP transporter; the sequestration of absorbed bismuth consumed cytosolic glutathione and activated was in de novo biosynthesis, which in turn facilitated passive uptake of bismuth (Fig. 7 ). The self-propelled positive feedback cycle actively eliminated bismuth from both intra- and extracellular space, thus protecting critical systems of human body from acute toxicity. Considering GSH is ubiquitous in most living cells, but only absent in certain anaerobic or microanaerobic bacteria such as H. pylori, the GSH and MRP mediated self-propelled disposal of bismuth in host cells might be accountable for the selective toxicity of bismuth drugs.
Fig. 7.
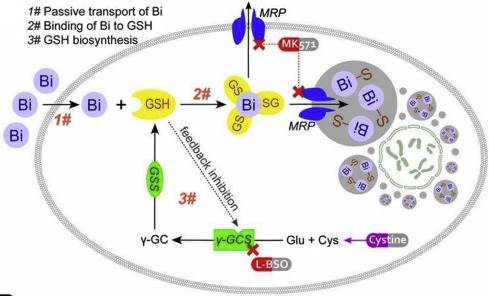
Schematic diagram depicting SAPT model of bismuth disposal in a human cell.
Hong Y, Lai YT, Chan GC, and Sun H (2015) Glutathione and multidrug resistance protein transporter mediate a self-propelled disposal of bismuth in human cells. Proceedings of the National Academy of Sciences 112: 3211–3216, with permission from PNAS.
In biological systems, proteins and enzymes have been regarded as potential targets for bismuth. Investigations of bismuth-protein interactions will not only improve understanding of the mechanism of action of bismuth but will also provide a basis for the design of more effective bismuth agents. Bismuth was found to be binding to transferrin (an iron transport protein) preferentially to the C-lobe with carbonate (CO3 2 −) as a synergistic anion. Transferrin is likely to act as a bismuth transport “vehicle,” as it is only 30% saturated with iron in blood plasma. Importantly, the metal-bound transferrin can be rapidly recognized by the transferrin receptor because of the higher affinity of the receptor to metal-bound transferrin than to its apo-form (Fig. 8 ). Although human serum albumin is the most abundant protein in serum (0.63 mM) and has been hypothesized to be the target of bismuth in plasma. Around 70% bismuth associates to transferrin and the rest to increasing bismuth concentration serum albumin, indicating a higher selectivity of bismuth to transferrin.
Fig. 8.
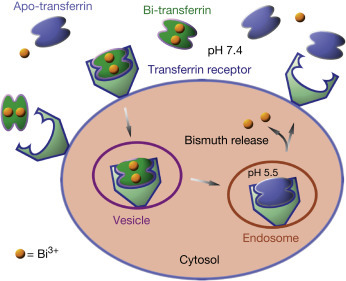
The proposed transport of bismuth ions by transferrin mediated endocytosis.
Lactoferrin is another type of major iron binding protein in the transferrin family, which can also strongly bind to bismuth ions. The bismuth-bound lactoferrin is able to compete with the iron-bound lactoferrin in both the membrane and intracellular, providing another route for bismuth transport. Histidine-rich proteins, such as Hpn and Hpn-like (46.7% and 25% histidine residues, respectively) in H. pylori are also potential targets of bismuth ions. Mutagenesis experiments have shown that H. pylori with hpn gene knock-out are fourfold susceptible to bismuth antiulcer drugs than that of the wild-type, indicating a protective role of Hpn in H. pylori responses to bismuth-based therapies. The interaction of bismuth with enzymes is related to the high affinity to cysteine residues. For example, bismuth inhibits urease activity probably by blocking the enzyme active site by coordinating to a cysteine residue at the entrance of the active site.
Side Effects of Bismuth
Although bismuth is considered to be nontoxic as stated previously, the long-term use of bismuth may result in certain degree of side effects on human subjects. Besides the few cases caused by occupational exposure to bismuth in the manufacturing industry, most of the poisoning incidents occur in the form of accidental or deliberate over-dosage of bismuth drugs. The extent of bismuth toxicity depends on individual cases, i.e., the types of bismuth compounds and the amounts absorbed. It is still not clear why only selected individuals develop bismuth toxicity.
Patients suffer toxicity at different bismuth levels in blood but the syndrome is rare when bismuth levels are below 50 μg/L. Among the bismuth-based regimens, the use of insoluble bismuth compounds such as bismuth oxychloride and bismuth subcarbonate are related to low toxicity, whereas the use of soluble bismuth organic compounds such as bismuth sodium tartrate and tripotassium dicitratobismuthate, or the combined use of bismuth with thiolate-containing ligands, are associated with high toxicity, such as neurotoxicity and nephrotoxicity. This is probably due to the enhanced uptake of soluble bismuth salts in human bodies. It has also been suggested that the oral bismuth drugs need to undergo methylation by intestinal microbes to enable them to be absorbed. Absorbed bismuth will accumulate in the kidneys, lungs, spleen, liver, brain, and muscles, and will be eliminated in urine and feces via bile and intestinal secretions. In the clinic, depending on the administration time of bismuth, its toxicity can be roughly divided into acute and chronic exposures. Both exposure doses can cause neurotoxicity, gastrointestinal toxicity, nephrotoxicity, hepatotoxicity, and increased bismuth concentration in blood. In spite of the toxicity, most of these side effects can be alleviated after the discontinuation of bismuth therapies. Bismuth iodoform paraffin paste (BIPP), which reduces the risk of bacterial infection, renders a deep necrotic wound cavity clean and promotes the development of granulation tissue, and is widely used in oral, maxillofacial, and ENT surgery (ear, nose, and throat surgery) as an antiseptic dressing. However, a few examples of serious adverse effects of BIPP were observed in the clinic when some patients were treated with BIPP. In one case, the patient became acutely confused and the gait became unsteady, indicative of an encephalopathy caused by over-dosage of bismuth. This was confirmed by the observation of a toxic level of bismuth in the patient’s serum. Another case involved using BIPP to cover the dura mater in a wound after removal of a large basal cell carcinoma. The patient became confused and then comatose. An encephalopathy was confirmed by the observation of diffuse cerebral edema in a tomographic scan. However, upon removal of the BIPP, the patient recovered, and deteriorated if the pack was applied again. The mechanism of intoxication has not been well understood till now. It was probably caused by the interference of bismuth with the oxidative metabolism of the central nervous system by binding to essential enzymes and reducing cerebral blood flow.
There are only few cases on nephrotoxicity after over-dosage of CBS. In one case, even the gastric lavage was performed initially, a 2-year-old boy suffered from an acute renal failure (ARF) associated with uremia and oliguria after ingestion of 28 De-Nol tablets (CBS, 8.4 g) for 2 days. After ingestion of CBS for 10 days, bismuth levels in both the blood and urine were 739 and 693 μg/L respectively. With the treatment of peritoneal dialysis, his urine volumes increased and plasma BUN and creatinine levels decreased gradually. After 100 days of admission, the patient recovered and his bismuth level in blood went back to normal. To cure some serious side effects caused by bismuth over-dosage, some antidotes such as d-penicillamine, 2,3-dimercapto-1-propane-sulfonic acid (DMPS), dimercapto-succinic acid (DMSA), and dimercaprol have been tested in animals and limited clinical trials. It was shown that DMPS and DMSA effectively alleviate bismuth poisoning due to their strong chelating ability to bismuth ions.
See also
Beryllium: Environmental Geochemistry and Health Effects; Chromium: Environmental Pollution, Health Effects and Mode of Action; Lithium: Environmental Pollution and Health Effects; Manganese: Environmental Pollution and Health Effects; Tungsten: Environmental Pollution and Health Effects.
Abbreviations
- AAS
Atomic absorption spectrometry
- AFS
Atomic-fluorescent spectrometry
- ARF
Acute renal failure
- BIPP
Bismuth iodoform paraffin paste
- BSS
Bismuth subsalicylate
- BUN
Blood urea nitrogen
- C. difficile
Clostridium difficile
- CBS
Colloidal bismuth citrate
- CDDP
Cis-diamminedichloroplatinum
- DL
Detection limit
- DMPS
2,3-dimercapto-1-propanesulfonic acid
- DMSA
Dimercaptosuccinic acid
- ENT
Surgery ear, nose and throat surgery
- EP
Additives extreme-pressure additives
- GSH
Glutathione
- H. pylori
Helicobacter pylori
- ICP-MS
Inductively coupled plasma mass spectrometry
- MBL
Metallo-β-lactamase
- MIC
Minimum inhibitory concentration
- MT
Metallothionein
- PPI-based
Proton pump inhibitor-based
- RBC
Ranitidine bismuth citrate
- S. aureus
Staphylococcus aureus
- SARS-CoV
Severe acute respiratory syndrome coronavirus
Further Reading
- Beales I.L.P. Efficacy of Helicobacter pylori eradication therapies: A single centre observational study. BMC Gastroenterology. 2001;1:7–15. doi: 10.1186/1471-230X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder D. Oxford University Press; Oxford: 2014. Bioinorganic Chemistry. [Google Scholar]
- Briand G.G., Burford N. Bismuth compounds and preparations with biological or medicinal relevance. Chemical Reviews. 1999;99:2601–2658. doi: 10.1021/cr980425s. [DOI] [PubMed] [Google Scholar]
- Chan C., Wang R. A novel synthetic compound, bismuth zinc citrate, could potentially reduce cisplatin-induced toxicity without compromising the anticancer effect through enhanced expression of antioxidant protein. Translational Oncology. 2019;12:788–799. doi: 10.1016/j.tranon.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Sun H. Bioinorganic chemistry of bismuth and antimony: Target sites of metallodrugs. Accounts of Chemical Research. 2007;40:267–274. doi: 10.1021/ar600001b. [DOI] [PubMed] [Google Scholar]
- Ge R., Sun X., Gu Q. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. Journal of Biological Inorganic Chemistry. 2007;12:831–842. doi: 10.1007/s00775-007-0237-7. [DOI] [PubMed] [Google Scholar]
- Guo Z., Sadler P.J. Metals in medicine. Angewandte Chemie International Edition. 1999;38:1512–1531. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hong Y., Lai Y.T., Chan G.C., Sun H. Glutathione and multidrug resistance protein transporter mediate a self-propelled disposal of bismuth in human cells. Proceedings of the National Academy of Sciences. 2015;112:3211–3216. doi: 10.1073/pnas.1421002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Işlek I., Uysal S., Gök F., Dündaröz R., Küçüködük S. Reversible nephrotoxicity after overdose of colloidal bismuth subcitrate. Pediatric Nephrology. 2001;16:510–514. doi: 10.1007/s004670100584. [DOI] [PubMed] [Google Scholar]
- Jayasinghe R., Tsuji L.J.S., Gough W.A. Determining the background levels of bismuth in tissues of wild game birds: A first step in addressing the environmental consequences of using bismuth shotshells. Environmental Pollution. 2004;132:13–20. doi: 10.1016/j.envpol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Li H., Wang R., Sun H. Systems approaches for unveiling the mechanism of action of bismuth drugs: New medicinal applications beyond Helicobacter pylori infection. Accounts of Chemical Research. 2019;52:216–227. doi: 10.1021/acs.accounts.8b00439. [DOI] [PubMed] [Google Scholar]
- Li H., Sun H. Recent advances in bioinorganic chemistry of bismuth. Current Opinion in Chemical Biology. 2012;16:74–83. doi: 10.1016/j.cbpa.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Sadler P.J., Sun H. Unexpectedly strong binding of a large metal ion (Bi3 +) to human serum transferrin. Journal of Biological Chemistry. 1996;271:9483–9489. [PubMed] [Google Scholar]
- Schramel P., Wendler I., Angerer J. The determination of metals (antimony, bismuth, lead, cadmium, mercury, palladium, platinum, tellurium, thallium, tin and tungsten) in urine samples by inductively coupled plasma-mass spectrometry. International Archives of Occupational and Environmental Health. 1997;69:219–223. doi: 10.1007/s004200050140. [DOI] [PubMed] [Google Scholar]
- Gisbert J.P. Helicobacter pylori eradication: A new, single-capsule bismuth-containing quadruple therapy. Nature Review Gastroenterology Hepatology. 2011;8:307–309. doi: 10.1038/nrgastro.2011.84. [DOI] [PubMed] [Google Scholar]
- Wang R., Lai T.P. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nature Communications. 2018;9:439. doi: 10.1038/s41467-018-02828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hu L. Integrative approach for the analysis of the proteome-wide response to bismuth drugs in Helicobacter pylori. Chemical Science. 2017;8:4626–4633. doi: 10.1039/c7sc00766c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Koohi-Moghadam M. Metallochaperone UreG serves as a new target for design of urease inhibitor: A novel strategy for development of antimicrobials. PLoS Biology. 2018;16:e2003887. doi: 10.1371/journal.pbio.2003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Sun H. Biocoordination chemistry of bismuth: Recent advances. Coordination Chemistry Reviews. 2007;251:2354–2366. [Google Scholar]
- Yang N., Tanner J.A., Zheng B.J. Bismuth complexes inhibit the SARS coronavirus. Angewandte Chemie International Edition. 2007;46:6464–6468. doi: 10.1002/anie.200701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman L., Harris S. BIPP madness; an iatrogenic cause of acute confusion. Age and Ageing. 2004;33:406–407. doi: 10.1093/ageing/afh103. [DOI] [PubMed] [Google Scholar]


