Epidemiologic Study Methods
Epidemiology is the study of health-related events in defined human or animal populations. These events include specific diseases and conditions as well as the exposures and host factors that contribute to their occurrence. The science of epidemiology was originally derived from the study of epidemics and has now been broadened to encompass all phenomena related to health in populations.1 Simply stated, epidemiology involves the careful description of events within populations and the comparison of rates at which these events occur between groups within those populations. Similar concepts and methods of epidemiology apply to both infectious and noninfectious diseases.2
The strength and adaptability of epidemiologic methods come from their underlying simplicity. For example, John Snow's application of epidemiologic study methods led to the classic intervention of pulling the handle from the Broad Street pump during an outbreak of cholera in London in 1851. His work was based on a careful description of his observations and on a quantitative approach in analyzing the occurrence of cholera among the citizens of London. The influence of his work led to legislation mandating that all water companies in London filter their water. Of note, it was not until 1883 that Robert Koch discovered Vibrio cholerae. 3
Goals of Epidemiologic Analysis
As applied to infectious diseases, at least 10 goals of epidemiologic analysis can be listed:
-
1.
Describe patterns of infection and disease occurrence in populations.
-
2.
Identify outbreaks or unusual rates of disease occurrence.
-
3.
Facilitate laboratory-based efforts to identify infectious agents.
-
4.
Describe the occurrence of asymptomatic infection and the spectrum of disease associated with specific agents.
-
5.
Provide population-based descriptions of clinical illness to improve the specificity of diagnosis for individual diseases.
-
6.
Assist in the understanding of disease pathogenesis.
-
7.
Identify and characterize factors in the chain of infection that contribute to agent transmission and the development of disease.
-
8.
Develop and evaluate treatment protocols through clinical trials.
-
9.
Develop and evaluate primary, secondary, and tertiary prevention and control measures for individuals.
-
10.
Describe and assess the use of prevention measures on a community-wide basis.
These comprehensive goals far exceed the often-considered goal of epidemiologic analysis to investigate and control epidemics or outbreaks.
The goals of epidemiologic analysis can be illustrated by a historical review of the unfolding of the human immunodeficiency virus (HIV) epidemic. After the acquired immunodeficiency syndrome (AIDS) was initially described in 1981, a national epidemiologic surveillance case definition was developed. Disease surveillance was initiated to characterize the cases by standard measures of time, place, and person and to identify population groups at risk. Based on these efforts, an infectious etiology was hypothesized early in the epidemic, before the first laboratory evidence of an etiologic agent was presented. Combined clinical, epidemiologic, and laboratory studies led to the identification of HIV as the cause of AIDS and to the development of sensitive and specific serologic tests for infection. This progress in turn led to studies that characterized the spectrum of illness associated with HIV infection.
Epidemiologic studies of persons infected with HIV (with or without AIDS) have characterized the routes of HIV transmission, have shown that the occurrence of other sexually transmitted infections can increase the risk of HIV transmission, and have demonstrated that HIV infection can enhance the transmission of other agents, such as Mycobacterium tuberculosis. Longitudinal follow-up studies of HIV-infected persons have identified long-term survivors—individuals who have been infected for more than 10 years (and now more than 30 years) and have received no treatment yet remain without disease.4 Others have been studied who were exposed to HIV on numerous occasions but did not become infected. Collectively, these studies provided important observations leading to better understanding of the mechanisms of resistance to HIV infection and disease. Clinical trials were conducted to assess the efficacy of antiretroviral agents and combinations of drugs to increase the effectiveness of therapy and reduce the rate of resistance to individual drugs. Development of potential HIV vaccines progressed to the implementation and innovative design of phase III human trials.5, 6 Other trials were conducted to assess the efficacy of a range of antimicrobial agents aimed at preventing a variety of opportunistic infections. Finally, community-based programs were developed on the basis of epidemiologic data to promote behavior change aimed at reducing the risk of HIV transmission. Epidemiologic methods were also applied to evaluate these community-based programs and to establish a framework in which a disease-modifying HIV vaccine could be integrated into a comprehensive HIV prevention program. These examples illustrate the broad range of roles that epidemiologic methods have played in understanding and controlling the HIV epidemic.
During 2003, the global application of combined clinical, epidemiologic, and laboratory studies led to the rapid detection, characterization, and, ultimately, control of an epidemic of severe acute respiratory syndrome (SARS) caused by a novel coronavirus.7, 8, 9 Epidemiologic studies identified the original source of transmission from palm civets to humans through wild-animal markets in China and demonstrated the global spread of the epidemic through person-to-person transmission.10 The eradication of the epidemic strain from humans and the identification of the wildlife reservoir of SARS coronaviruses established a framework for preventing future SARS outbreaks. The global response to SARS serves as a model for the usefulness of epidemiologic methods.
In 2012, the emergence of human infections caused by another novel coronavirus in the Middle East, the Middle East respiratory syndrome coronavirus (MERS-CoV) again resulted in the combined rapid conduct of clinical, epidemiologic, and laboratory studies to reduce the risk of this virus causing another SARS-like epidemic.11, 12, 13, 14 As of August 1, 2013, there have been 94 laboratory-confirmed cases of infection with MERS-CoV, including 46 deaths. The lessons learned from similar studies conducted in 2003 have been invaluable in responding to the occurrence of MERS-CoV 2012 to 2013 (see Chapter 157).
On March 31, 2013, the public health authorities of China reported three cases of laboratory-confirmed human infection with a novel avian influenza A (H7N9) virus. By late May 2013, approximately 2 months after the initial report, the number of laboratory-confirmed H7N9 infections reached 132, with 37 deaths.15 The rapid conduct of clinical, epidemiologic, and laboratory studies were initiated by Chinese medical and public health officials with support from experts from around the world. It was determined that the novel H7N9 viruses were reassortants, comprising H7 HA, N9 NA, and the six internal genes of H9N2 influenza A viruses. This combination of influenza genes had not previously been identified among viruses obtained from birds, humans, or any other species, although individual genes are related to those of recent avian influenza viruses circulating in East Asia. H7N9 viruses obtained from human cases, poultry, and environmental samples were closely related and contained a number of genetic signatures previously associated with low pathogenicity in poultry, enhanced capacity for mammalian infection, and resistance to the adamantane class of antiviral drugs.16, 17 The detection of H7N9 virus in live poultry markets in the vicinity of human cases in Shanghai, the contact history with live poultry or live poultry markets in a substantial number of cases, and the major reduction in human cases after the closure of live poultry markets throughout eastern China, suggest exposure to live poultry as a key risk factor for human H7N9 infection18 (see Chapter 157).
Defining Infections, Diseases, and Populations
An essential aspect of any epidemiologic study is careful definition of the infection, disease, condition, or factor that is being studied. Specificity and sensitivity are concepts that are frequently used in reference to laboratory test performance, particularly with tests that are used for screening purposes.1 However, in the epidemiologic study of infectious diseases, it is important to also apply the concepts of specificity and sensitivity more broadly in terms of diagnosis of infection and disease. For example, the diagnosis of smallpox was both highly specific and sensitive. Few other diseases could be confused with smallpox (i.e., the diagnosis was specific), and clinical disease developed in most people who became infected with smallpox virus (i.e., the diagnosis was sensitive). These qualities, in addition to the facts that humans were the only important reservoir for the smallpox virus and that highly immunogenic vaccines had been developed, led to the successful eradication of smallpox.19
In contrast, many clinical conditions or syndromes, such as diarrhea, are caused by more than one etiologic agent. Epidemiologic studies of diarrheogenic Escherichia coli are complicated by the fact that diarrhea is not specific for E. coli, and the sensitivity of E. coli detection is limited due to an array of virulence factors that can result in disease yet are not detected by standard biochemical tests.20 Even the ability to detect specific agents and virulence factors by rapid, nonculture methods does not resolve these difficulties.21 E. coli O157:H7 and some other Shiga-toxin–producing E. coli (STECs) may lead to a broad spectrum of clinical illnesses, including uncomplicated diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome. However, not all STEC strains have the same disease-causing potential. Depending on whether the goals of a particular study address the clinical illness, the specific agent, or the public health implications of detecting the agent in clinical, food, or environmental samples, investigators may choose a case definition that casts a wide net or is more narrowly focused. The type of definition can have a substantial impact on study results and should be carefully considered before a specific study is undertaken.
Epidemiologic studies may be designed to evaluate outcome variables other than infection or disease occurrence. In these situations, how the outcome variables and study population are defined and measured can affect interpretation of the results and the validity of the conclusions. For example, in the development of recombinant vaccines for hepatitis B virus (HBV), two vaccine formulations, containing either 10 µg or 20 µg of hepatitis B surface antigen (HBsAg) in each dose, were evaluated in clinical trials. Higher antibody titers developed in subjects who were administered vaccine with the higher dose. Both vaccines produced sufficient levels of antibody to be considered protective against infection, and both were licensed by the U.S. Food and Drug Administration. However, when the vaccines were more broadly administered to Minnesota hospital employees, those who received vaccine with 20 µg HBsAg per dose were more likely than those given the lower-dose vaccine to have detectable antibody when tested within 6 months after completing the three-dose series.22 The results of this investigation suggested that sociodemographic factors of the community-vaccinated population, such as age, gender, weight, and smoking, affected the outcome of vaccination programs in ways that were not predicted by the clinical trials.23 In a study of children with inflammatory bowel disease, only 56% of children previously vaccinated against HBV had immunity to HBV, a mean of 13 years later.24 Older age, lower albumin levels, and the presence of pancolitis were associated with lack of immunity. The use of immunosuppressive therapy was not associated with a lack of immunity, but it was associated with a lack of response to an additional dose of HBV vaccine given to children who lacked immunity.24
Establishing specific enrollment criteria for cases of infection or disease in epidemiologic studies is critical to obtaining valid and biologically meaningful results. For example, large multistate outbreaks of E. coli O157:H7 have been documented with increasing frequency. However, without molecular subtyping of E. coli O157:H7 strains, population-based surveillance is limited in its ability to detect and determine when an unexpected number or temporal clustering of cases actually documents a common vehicle-associated outbreak. In September 2006, epidemiologists and public health laboratory workers in Wisconsin noticed a small cluster of E. coli O157:H7 cases with a common pulsed-field gel electrophoresis (PFGE) subtype pattern. The Wisconsin state laboratory posted the PFGE pattern on the national subtyping network for foodborne disease surveillance known as PulseNet. Within 1 week, multiple states reported matching cases, an epidemiologic study was conducted to evaluate case exposures, and fresh spinach was identified as the vehicle.25 Ultimately, 205 cases in 26 states were linked to the outbreak, and the results of the investigation stimulated important investments in research related to the safety of leafy-green produce items. Because of its discriminatory ability, the Centers for Disease Control and Prevention (CDC) has adopted PFGE as the standard molecular subtyping method for national surveillance.26 CDC-PulseNet now commonly plays an important role in identifying and investigating multistate outbreaks of foodborne disease.
A similar issue regarding the definition of cases and the population in which they occur confronts public health officials when they must consider intervention activities because of a possible outbreak of certain infectious diseases. It is common practice to define outbreaks as the occurrence of cases of disease at a frequency greater than expected.1 When an outbreak occurs, it is necessary to define the population at risk (i.e., the denominator) if an accurate measure of the rate of disease is to be calculated. For example, it is not unusual to recognize a cluster of cases of Neisseria meningitidis disease in the community in populations not previously vaccinated. Because outbreaks of invasive N. meningitidis disease are known to occur in closed populations, such as persons living in dormitories and barracks, and because a vaccine and antibiotic chemoprophylaxis are available to prevent or control these outbreaks, the occurrence of multiple cases of meningococcal disease inevitably prompts a rapid public health assessment.27 Cases of meningococcal disease tend to occur during well-described seasonal peak periods, so it is possible that a cluster of unrelated cases may occur in a defined population. Conversely, a common strain may be transmitted within social networks that form a population group that is not easy to define. During 2005 to 2006, 23 cases of serogroup C meningococcal disease occurred among illicit drug users and their contacts in Brooklyn, New York.28 From 2010 to 2012, 18 men who have sex with men (MSM) in New York City were infected by a closely related strain.29 The need for public health intervention is quite different for a cluster of cases representing an outbreak associated with a single strain, compared with a cluster in which each case is caused by a different group or strain of N. meningitidis. 30 However, in many situations, strains are not available for further subtyping, because laboratory capacity to distinguish strains is limited.
As illustrated above, a companion problem to the definition of cases is definition of the population at risk. To determine whether cases of disease are occurring at a frequency greater than expected, it is necessary to consider baseline incidence rates of disease. During the outbreak of meningococcal disease among illicit drug users, the population-based rate of illness in Brooklyn never approached the threshold for public health intervention of 10 cases per 100,000 population over a 3-month time period.27, 28 Although it was not possible to enumerate the actual population at risk, the ongoing occurrence of cases in persons with similar histories led to empirical judgments that an outbreak was occurring and a vaccination program targeting illicit drug users was needed.28 In the outbreak involving MSM, data from a community health survey was available to estimate the population of MSM and determine that the risk of meningococcal disease among MSM was 80 times the risk among non-MSM.29 Timely decisions regarding major community-based interventions after the observation of a cluster of meningococcal diseases often are made without adequate information regarding the status of a possible outbreak. Similar situations occur with other pathogens as well.
Two common measures of the occurrence of disease in populations are incidence and prevalence.1 Incidence represents the occurrence of new cases of infection or disease per unit of population per time period. It is common to express incidence rates in terms of person-years of exposure. Prevalence describes the number of current cases of disease per population unit at the time of observation. The relationship between incidence and prevalence depends on the duration of infection or disease. For example, the incidence of Lyme disease or hepatitis A virus (HAV) infections over a period of 1 year is always greater than its prevalence at a given point because the disease has a very short duration. In contrast, the prevalence of HIV or M. tuberculosis infections is always greater than its incidence because the infection is chronic, and infected persons may live for years after the initial infection.
Biology and Statistics
The results of epidemiologic studies to compare the risk of infection or disease and the presence or absence of specific risk factors are presented in terms of relative risk and odds ratios. Relative risk (RR) is the ratio of the rate of illness or infection among persons who were exposed to the rate among persons who were not exposed (Fig. 13-1 ). RRs may also be called rate ratios and are the products of cohort studies. In case-control studies, odds ratios (ORs) are determined and approximate the relative risk. ORs provide a valid estimate of the RR under conditions that prevail in most case-control studies: the cases of disease are newly diagnosed, prevalent cases are not included in the control group, and the selection of cases and controls is not based on exposure status.31 An increased RR or OR (i.e., >1.0) for an exposure variable indicates that the exposure is related to an increased risk of disease. Similarly, a decreased RR or OR (i.e., <1.0) indicates that the exposure variable is related to a decreased risk of disease. For example, the consumption of undercooked ground beef has been associated with an increased risk of E. coli O157:H7 infection in outbreak settings and of sporadic E. coli O157:H7 infections in the community.32
FIGURE 13-1.
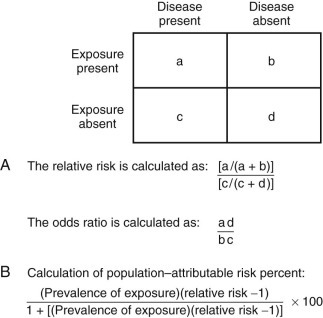
The calculation of and relationship among relative risk (RR), odds ratio (OR), and attributable risk.
A, The calculation of RR and OR from a two-by-two table. The OR provides a valid estimate of the RR under conditions that prevail in most case-control studies: the cases of disease are newly diagnosed, prevalent cases are not included in the control group, and the selection of cases and controls is not based on exposure status. B, The calculation of population–attributable risk percent. In a case-control study, attributable risk can be estimated from the prevalence of exposure among controls (b/b + d) and the OR. The validity of this approach is limited by how representative controls are of the population and how well the OR estimates the RR.
Although RRs and ORs do provide a measure of the risk of disease associated with a specific factor, they do not directly describe how much disease in the community can be attributed to that factor. Rather, the attributable risk or fraction considers both the RR for an exposure variable and the proportion of the population exposed to that variable. In a case-control study of sporadic E. coli O157:H7 infections conducted by the Foodborne Disease Active Surveillance Network (FoodNet) in 1996 to 1997, persons who ate undercooked hamburgers away from home had approximately a 6 times greater risk of E. coli O157:H7 than those who did not. For those who ate undercooked hamburger at home, the risk was only 2 times greater. However, eating undercooked hamburger at home was more common than eating it away from home. Therefore, eating undercooked hamburger at home accounted for an estimated 8% of cases, whereas the riskier (i.e., higher OR) practice of eating undercooked hamburger away from home accounted for 7% of cases. Furthermore, persons who ate at a table service restaurant were only 1.7 times as likely to have E. coli O157:H7 infection than those who did not. But, because eating at a table service restaurant is a very common practice and therefore represents more frequent exposure, it accounted for an estimated 20% of cases. Although it had the weakest statistical association, it accounted for the highest proportion of cases. Similarly, in a case-control study of sporadic listeriosis cases reported in FoodNet sites from 2000 to 2003, living on a cattle farm had the strongest association with illness (OR = 13.75), but, because it was an uncommon exposure, it had a population attributable fraction of only 1.6%. However, eating melons at a commercial establishment accounted for 10.6% of cases, even though the OR was only 2.6.33 The identification of melon as a risk factor for sporadic listeriosis in this study subsequently made it easier for investigators to identify cantaloupe as the source for a large multistate outbreak of listeriosis in 2011.34 As these examples show, both RR and attributable risk are important measures for describing the epidemiology of infectious diseases and determining public health priorities.
In the epidemiology of infectious diseases, many factors are evaluated to determine their relationship or association with a specific disease. Statistical associations, both positive and negative, may represent a true causal relationship, a confounding relationship with another factor, or a chance occurrence. If more than one factor is statistically associated with infection or disease status in univariate (single-variable) analyses, the relationship between individual factors and infection or disease status can be evaluated by multivariate regression analysis.35 These procedures allow the investigator to simultaneously control for a combination of factors in the analysis and to determine whether any of the risk factors are associated with infection or disease status independently of other factors. Another critical way of distinguishing causation from confounding or chance is by assessing the biologic plausibility of the association. An unexpected statistical association found in conjunction with an epidemiologic study may result in new understanding of how agent transmission or disease occurs. The temptation to stretch the plausibility of biology to provide meaning to statistical results is a constant danger. However, such results may be a useful guide to evaluate new hypotheses in future studies.
Furthermore, “statistically significant results” may be unimportant from a disease control or a practical perspective. Statistical significance, which has historically been considered to be an event that would happen less than 1 in every 20 instances by chance alone (i.e., P < .05), represents a combination of the sample size and the strength or degree of the association. Studies with a large number of persons enrolled can produce statistically significant results for weak associations (i.e., RRs or ORs greater than 1 but less than 2), whereas studies with a limited number of enrollees may not be able to produce statistically significant results even for moderately strong or increased associations (i.e., RRs or ORs > 5).
Determining Epidemiologic Methods Appropriate to the Study Setting
The clinical trial is cited as the gold standard of epidemiologic research. However, many epidemiologic studies cannot take place under such rigorously controlled conditions. Taking advantage of opportunities to study diseases in clinical and community settings is one of the strengths of epidemiology. In the setting of a clinical practice, epidemiology may involve studying a series of patients, participating in multicenter trials, or being a reporting source for cases of disease to public health officials. This last aspect of epidemiologic study may be a legal obligation, but it should also be viewed as an opportunity for all practicing clinicians to participate in the practice of community-based epidemiology. Academic-based research centers are often settings for clinical trials, studies requiring newly developed laboratory methods, or studies derived from referrals to clinical specialty groups. Public health departments typically do not have direct access to or contact with patients for clinical trials, but they are responsible for surveillance of reportable diseases and the investigation of outbreaks. There has been a debate about how to distinguish public health surveillance from research and the ethical considerations of that distinction.36 Each of the described settings provides opportunities for epidemiologic studies that can make major contributions to the understanding, prevention, and control of infectious diseases.
Several major constraints are confronted in the design of epidemiologic studies of infectious diseases. Time is frequently a problem in the investigation of outbreaks. The need to quickly design and conduct outbreak investigations has increased with the frequency of widespread foodborne outbreaks and concerns over the potential for intentional contamination of the food supply. This necessarily limits the investigator's ability to fully explore the outbreak setting and can result in the loss of information. In any study involving the retrospective collection of data, information may be lost because of difficulty in recalling exposure or in verifying information about the exposure.
For many infectious diseases, it may be difficult to identify sufficient numbers of cases in clinical settings to conduct meaningful epidemiologic studies. In such situations, multisite collaborative projects are often needed. For example, a CDC working group on prevention of invasive group A streptococcal (GAS) disease among household contacts concluded in 1995 that the data available from a single study conducted in Ontario, Canada, were inadequate to recommend chemoprophylaxis to household contacts.37, 38 Although the Canadian study suggested an increased risk of invasive disease among household contacts, this assessment was based on only four subsequent cases in households. Based on the recommendations of the work group, a multisite study coordinated by the CDC was initiated in multiple states or areas with active surveillance of invasive GAS disease. Additional surveillance studies supported the conclusion that outbreaks of invasive disease among household contacts are rare, so that recommendations for chemoprophylaxis need to be made on an individual basis.39, 40
Types of Epidemiologic Studies
Several schemes can be used to classify or define types of epidemiologic studies (Table 13-1 ). Studies can be classified as descriptive or analytic and as observational or experimental. A descriptive study is designed to describe only the existing distribution of case characteristics, without regard to causal or other hypotheses.41 For example, the results of community-based surveillance for Campylobacter infection may include a summary of all cases reported in a given year by date of onset, county of residence, age, gender, and race. An analytic study is one designed to examine associations, particularly hypothesized causal relationships. For example, a case-control study could be designed to examine whether consumption of ready-to-eat meat and poultry products is a risk factor for cases of invasive listeriosis infections identified through surveillance activities. In addition to case-control studies, cohort studies, clinical trials, and cross-sectional surveys are common types of analytic studies. In practice, most epidemiologic studies involve both descriptive and analytic elements. For example, surveillance for methicillin-resistant Staphylococcus aureus (MRSA) infections in nine surveillance sites in the United States permitted both the characterization and differentiation of community- and health care–associated infections.42 Results showed that MRSA affects certain populations disproportionately. They also demonstrated that the problem is primarily related to health care but no longer is confined to intensive care units, acute care hospitals, or any health care institution. Finally, this surveillance effort demonstrated a 26% decrease in MRSA infections in the nine surveillance areas between 2007 to 2008 and 2011.
TABLE 13-1.
Classification Schemes for Epidemiologic Studies
| OBSERVATIONAL | EXPERIMENTAL | |
|---|---|---|
| Descriptive | Surveillance | |
| Case series | ||
| Analytic | Case-control studies | Clinical trials |
| Cohort studies | Community interventions | |
| Seroincidence studies | ||
| Cross-sectional surveys | ||
| Seroprevalence surveys | ||
| Outbreak investigations |
A more relevant distinction can be made between observational and experimental studies. Observational studies are conducted in natural settings where changes in one characteristic are studied in relation to others without the intervention of the investigator.43 Observational studies represent the bulk of epidemiologic research because they focus on events, exposures, and diseases occurring in the population during the course of routine living conditions. In contrast, experimental studies are ones in which the study conditions are under the direct control of the investigator.43 Such studies may include randomization of subjects to treatment or placebo groups and blinding of subjects and investigators to placement status. Clinical trials are the prototypical experimental study. On a broader scale, community intervention trials can also be conducted.
Observational Studies
Disease Surveillance
Disease surveillance is an ongoing process that involves the systematic collection, analysis, interpretation, and dissemination of information regarding the occurrence of diseases in defined populations for public health action to reduce morbidity and mortality.44 Surveillance can be conducted in the community and in institutional settings, where it may form the basis for an infection-prevention program. For most infectious diseases, community-based surveillance is the domain of public health departments at the local or state level. All jurisdictions require licensed physicians to report the occurrence of selected diseases to the health department.45 Typically, such diseases include sexually transmitted infections, vaccine-preventable diseases, bloodborne pathogens, tuberculosis, certain invasive bacterial diseases, and enteric infections caused by Salmonella, Shigella, E. coli O175:H7, and Campylobacter. In addition to categorical reporting, most states require reporting of disease outbreaks, regardless of the cause, and have some provision to solicit reports of new and emerging diseases.
Increasingly, syndromic surveillance systems are being developed to take advantage of large data streams through electronic medical records and social media,46, 47 as opposed to surveillance based on isolation of a specific infectious agent. This type of surveillance has been very useful to supplement surveillance of influenza-like illness in sentinel physician practices, nursing homes, and schools to monitor influenza activity each influenza season. Surveillance for unexplained deaths from possible infectious causes, with characterization of such deaths based on the clinical syndrome at the initial evaluation, is a way to monitor the emergence of potential new infectious disease threats.48 Finally, syndromic surveillance has been established in several large cities to serve as an early warning system for the detection of bioterrorist events.49 Although useful for tracking community-wide spread of influenza-like illness, syndromic surveillance systems have shown very little sensitivity to the occurrence of infectious disease outbreaks in these cities.50, 51
Surveillance for certain pathogens has evolved to include surveillance for antimicrobial resistance. The worldwide emergence of extensively drug-resistant tuberculosis has made surveillance for drug-resistant tuberculosis routine in all jurisdictions.52 Successive waves of emergence and clonal dispersion of multidrug-resistant Salmonella Typhimurium DT 104 and multidrug-resistant Salmonella Newport among food animals and humans in the United States was detected through national surveillance to monitor resistant enteric infections.53, 54 The establishment of surveillance for drug-resistant pneumococcal infections was an important step in the evaluation of the impact of the introduction of pneumococcal conjugate vaccines.55 Rates of invasive bacterial infections caused by resistant strains dropped by more than 50% after the introduction of these vaccines. The importance of surveillance for drug-resistant infections will continue to grow in the 21st century, and data collected through public health surveillance can be extremely useful to clinical care providers.
Case reports for use in surveillance can be collected in an active or a passive manner. Active surveillance involves a regular, systematic effort to contact reporting sources or to review records within an institution to ascertain information on the occurrence of newly diagnosed diseases or infections. An example of an active surveillance system for foodborne illnesses is FoodNet, which operates as part of CDC's Emerging Infections Program.56 Active laboratory-based surveillance for confirmed cases of Campylobacter, Cryptosporidium, Cyclospora, E. coli O157:H7, Listeria, Salmonella, Shigella, and Vibrio increased from five sites, covering 5% of the U.S. population in 1996 to 10 sites covering approximately 15% of the population in 2007. Each clinical laboratory in the surveillance catchment areas is contacted weekly or monthly to ensure that all confirmed infections under surveillance have been reported. These data have been extremely useful in establishing national estimates for the burden of foodborne illness in the United States and for monitoring trends in the incidence of specific foodborne agents. Passive surveillance relies on the individual clinician or laboratory to initiate the report. For many diseases of public health importance, passive surveillance can be almost as comprehensive as active surveillance. Although surveillance systems are labeled as active or passive based on how cases are reported, all surveillance systems require an active review and analysis of reported cases, with dissemination of results to key stakeholders.44
Two key qualities of community-based surveillance for infectious diseases that must be considered when interpreting surveillance data are representativeness and timeliness. These qualities vary by disease and depend on multiple factors. The first factor of importance is that the patient must seek medical attention. It is not common for persons with mild or limited illnesses to seek medical attention. Second, the physician must seek laboratory testing of appropriate clinical specimens to confirm the diagnosis. Third, the laboratory must have the capability to identify the agent. Fourth, the physician and laboratory must report the clinical and laboratory findings to public health officials in a timely manner. Fifth, the availability of molecular subtyping techniques such as PFGE and the ability to compare PFGE patterns electronically through the national computer network PulseNet can greatly increase both the sensitivity and the specificity of pathogen-specific surveillance. Even in states where laboratory-based infectious disease reporting is required, there may be confusion among physicians and laboratory officials regarding who has the responsibility for reporting. Finally, public health agencies must have the resources to conduct timely and routine follow-up of such reports, to ascertain basic case demographic and other relevant data. Failure at any step of this process results in loss of information to the community-based surveillance system.
The efficiency of community-based surveillance systems varies greatly, depending on the disease, how the diagnosis is made, and the resources targeted toward the surveillance effort.44 Many emerging diseases require a diagnosis based on clinical findings, either because an etiologic agent has not been identified or because reliable diagnostic tests have not been developed. For example, for many years, the diagnosis of Lyme disease presented difficulties because many patients were not seen when the typical clinical manifestations of the disease were present, and laboratory testing was not adequate to establish the diagnosis. In contrast, the diagnosis of measles can be confirmed by specific serologic testing, regardless of whether the physician sees the patient or has the training and experience to recognize the pathognomonic clinical features of the disease. Surveillance for invasive bacterial diseases such as those caused by N. meningitidis is facilitated by the need for medical treatment because of the relative severity of the disease and the laboratory-supported diagnosis. For diseases such as these, active case ascertainment can greatly enhance the effectiveness of surveillance activities. However, active surveillance requires the commitment of personnel and other resources that are limited for many reportable diseases. Nonetheless, it may be critical to evaluating the impact of vaccines for invasive diseases such as Haemophilus influenzae type b, which declined by 95% within 6 years after the introduction of the conjugate vaccine in 1989. Active surveillance for invasive H. influenzae disease has confirmed the effective control of H. influenzae type b with no evidence of increased disease caused by non-B serotypes in young children in the United States.57
Typically, active surveillance may be conducted for a limited period when complete data are most critical. Examples include the characterization of emerging diseases such as AIDS or SARS and special surveillance projects aimed at assessing an intervention, such as evaluating whether the occurrence of intussusception was causally related to the use of rotavirus vaccine.58 Most infectious disease surveillance conducted by public health departments in the United States is passive in that it relies on the physician or the laboratory to initiate the report. Passive surveillance systems are subject to selection bias because disease reports are likely to come from a nonrepresentative sample of practicing physicians who may report specific diseases because of personal interest.44 In addition, some data (i.e., age and gender versus clinical and pathologic information) may be more readily reported because of ease of ascertainment.44
Active surveillance is relatively more common in the hospital setting. For example, surveillance of nosocomial infections is an important hospital infection-prevention activity.59 This highly specialized surveillance system has the operational advantage of a defined population, routine clinical observation of the patient population, and direct access to the laboratory. Hospital-based surveillance has been a primary epidemiologic tool in the study of drug-resistant organisms.
Case Series
A common type of descriptive study that is conducted in clinical settings is the case series. A case series describes the clinical features of a disease and the demographic profiles and other interesting features of patients with the disease. They are typically the domain of practicing clinicians and serve as a way of communicating significant clinical observations. For example, the SARS epidemic was first recognized outside of China as an unusual series of cases of patients with atypical pneumonia.60 As the case series grew, with evidence of transmission to hospital staff, it became apparent that an unusual outbreak was occurring. More recently, a series of 33 patients hospitalized in a medical intensive care unit during an outbreak of chikungunya virus on Reunion Island demonstrated that chikungunya virus infection can cause severe neurologic disease with the involvement of other organ systems.61
Case-Control Studies
In case-control studies, persons with infection or disease are compared with controls (i.e., persons without the infection or disease under study) with respect to prior exposures likely to be related to agent transmission.1 Case-control studies by nature are retrospective, because the outcome (i.e., case status) is known at the outset of the study. Case-control studies are the most widely conducted type of epidemiologic study because they are relatively cheap, powerful, and adaptable to many settings.35 For example, in a nationwide outbreak of Salmonella enteritidis infection, the results of a case-control study identified the ice cream made by a large national producer as the source of the outbreak 10 days before S. enteritidis could be isolated from samples of the implicated ice cream.62 When S. enteritidis was isolated from the ice cream, it was shown to be present at levels of less than one to six organisms per half-cup serving, levels that rendered microbiologic surveillance of ice cream insensitive. Furthermore, the case-control study established that contamination of the pasteurized ice cream premix occurred during transport in tanker trailers that had previously carried nonpasteurized liquid eggs, even though regulatory officials were not able to isolate S. enteritidis from any environmental samples.
The primary considerations in designing case-control studies are defining cases, establishing enrollment criteria, identifying suitable controls, and developing interview or other data collection processes that do not systematically result in different standards of data collection for cases versus controls. In the community setting, it is customary to select controls from the same area of residence as the cases. It is desirable for controls to resemble cases with respect to variables that are not being studied. Controls may also be matched by age, gender, or any other factor that the investigator considers necessary. For example, in studying risk factors for listeriosis, it has been important to select or match controls with a similar risk of illness based on the presence of an immunocompromising condition or treatment. This is necessary because healthy community control subjects who have exactly the same exposures are less likely to develop disease. Therefore, a case-control study of listeriosis using healthy community-based controls would require simultaneously trying to assess the risk for exposure as well as the risk for illness given exposure. However, overmatching, such as requiring the control to have the same birthday as the case, may make it difficult to identify and recruit controls. Also, once a variable is used as a matching criterion, it is no longer available for evaluation. In hospital settings, controls are frequently selected from patients with unrelated diagnoses who might otherwise be comparable to the cases.
Analysis of case-control studies involves comparing exposure differences between cases and controls. Such comparison allows associations between exposure and disease to be studied even when the disease is a rare outcome of the exposure. For example, a case-control study of Guillain-Barré syndrome demonstrated an association between Campylobacter infection and Guillain-Barré syndrome.63 This association could not have been easily evaluated in a prospective cohort study because of the population size necessary to identify a similar number of cases with this syndrome. The power of the case-control methodology comes from the fact that, although illness may be an uncommon outcome of a given exposure, the common history of exposure among cases may stand in stark contrast to that among controls.
Cohort Studies
In cohort studies, the development of infection or disease is observed in groups who are either exposed or not exposed to the previously defined risk factors.1 Cohort studies are traditionally considered prospective studies. However, this nomenclature is misleading because, in reality, cohort studies can be prospective or retrospective, depending on how the exposed and comparison groups were identified and monitored. Cohort studies provide the advantage of direct measurement of illness rates by exposure status, which allows direct measurement of relative risk. Furthermore, when conducted prospectively, cohort studies allow the investigator better control over data collection and identification of potential confounding variables. The use of cohort studies is limited to groups in which exposures can be defined and measured.
Cohort studies of homosexual men have helped evaluate risk factors for transmission of HIV, HBV, and hepatitis C virus (HCV).64, 65 These studies are also examples of seroincidence surveys, in which the appearance of antibody to an agent in the second of two sequentially collected specimens indicates infection with that agent during the interval between the two times of collection. Seroincidence surveys allow the investigator to (1) define total infection rates, (2) relate infection rates to prior antibody levels, and (3) identify risk factors for infection.66 Prospective cohort studies are limited because of the enrollment size and observation period requirements for diseases of low incidence. Retrospective cohort studies in which previous exposures can be identified offer the advantage of not requiring additional observation periods. However, they may be limited by the recall of study subjects or the adequacy of available medical records.
Cross-Sectional Surveys
Cross-sectional surveys provide a point-in-time assessment of the population or study group. These surveys may be conducted to determine the prevalence of a disease in the community, but a more common use is to establish the prevalence of risk factors or serologic markers of infection.31 For example, a cross-sectional survey of patients attending a sexually transmitted disease clinic demonstrated that HCV infection occurred infrequently; however, patients with a history of intravenous drug use had a significantly higher rate of serologic markers for HCV infection.67
An important type of cross-sectional survey is the seroprevalence survey. Serologic prevalence data reflect total infection rates and thus represent both clinical and subclinical (or asymptomatic) infections. Seroprevalence surveys can therefore provide information on patterns of infection or immunity to agents that could not be obtained by ordinary surveillance methods based on the reporting of clinical cases.66 For example, seroprevalence surveys have demonstrated that fewer than 1% of human West Nile virus infections result in serious neurologic illness, and about 20% cause systemic febrile illness.68 A serosurvey of pregnant women after a community-wide outbreak of West Nile disease in Colorado demonstrated a 4% infection rate with no increased risk of an adverse outcome at birth.69
Outbreak Investigations
A final category of observational study that integrates multiple epidemiologic methods is the outbreak investigation. A special feature of outbreak investigations is that they are frequently conducted with a sense of urgency because of the ongoing occurrence of cases, the need to rapidly implement control measures, or intense public and media interest in the outbreak. Investigations of the first documented outbreak of legionnaires' disease, the 1993 outbreak of hantavirus-associated respiratory illness in the southwestern United States, and the posting of anthrax-contaminated letters were lead stories for national news media. The importance of rapid investigation of outbreaks has increased with perceptions of the threats posed by such events, whether naturally emerging or intentional. Standard methods for conducting outbreak investigations are available.43
Specific surveillance systems have been established for outbreaks of foodborne and waterborne diseases, influenza, and a range of infections in institutional settings. At the local or state level, outbreaks may be reported because a physician or the public is aware of the health department's existence and desires some intervention. Once an outbreak has been recognized, it is necessary to determine its extent in terms of person, place, and time. For example, the nationwide outbreak associated with Schwan's ice cream initially appeared as an increased occurrence of cases in southeastern Minnesota.62 These cases served to index the larger outbreak occurring throughout the distribution area for the implicated product. Similarly, a cluster of four human Salmonella serotype I 4,5,12:i:− infections with an identical PFGE pattern was identified by the Pennsylvania Department of Health and reported to PulseNet in June 2007. As recognition of the outbreak increased and the case count soared to 401 cases across 41 states, a series of independent and coordinated studies was conducted by state and local health departments in collaboration with CDC. After a cluster of cases was linked to consumption of potpies by the Minnesota Department of Health in October, a multistate case-control study quickly confirmed the association, identified a specific product, and determined that most of the patients cooked the potpies in a microwave oven, without regard to cooking instructions.70 However, the potpies were not ready-to-eat foods. Undercooking of foods in microwave ovens has surfaced as an important risk factor in several recent outbreaks of salmonellosis.71
Molecular subtyping by PFGE and comparison of subtype patterns through PulseNet has become the primary method for detecting multistate foodborne outbreaks caused by E. coli O157:H7, Salmonella, and Listeria. 72 However, the recognition of these outbreaks requires the accumulation of multiple cases over time. The timeliness of outbreak investigations is further limited by the need to interview cases, formulate hypotheses, and subsequently recruit and interview controls. Currently, all listeriosis cases in the United States are interviewed as part of a national Listeria initiative.73 This has allowed the use of case-case comparisons, with the results of subtyping to distinguish outbreak cases from unrelated cases.74 Such methods led to the rapid identification of cantaloupe as the source of a multistate outbreak of listeriosis.73 If Salmonella and E. coli O157:H7 cases were similarly interviewed on a routine basis, the same approach could be applied to these outbreaks as well. A recent development that has been used to accelerate outbreak investigations has been to compare exposure histories among cases to an expected rate of exposure based on population surveys.75 This has the benefit of eliminating the labor-intensive and time-consuming process of recruiting and interviewing controls. However, it is limited by the availability of population exposure data. Most outbreaks using this method have used FoodNet population survey data, which is only available for FoodNet sites, includes a limited number of food items, and was last updated in 2006 to 2007.76 In addition, because the use of binomial statistics creates the functional equivalent of a case-control study with a very large control group, it increases the potential for type 1 error, in which an association is accepted when it should not be. The key step to prevent type 1 error with this method is to confirm the association through traceback of suspected food items to a common production or distribution source.75
The second major category of foodborne outbreaks consists of those that are recognized because of the occurrence of a similar illness among persons with a common exposure, such as eating at a restaurant or attending a banquet. Although many of these outbreaks may seem to be self-limited events unique to the establishment, they may serve to index much larger outbreaks. They also provide opportunities to identify emerging foodborne pathogens. For example, in both 1996 and 1997, the nationwide outbreaks of cyclosporiasis associated with raspberries imported from Guatemala were manifested as a large series of otherwise unrelated outbreaks associated with restaurants, banquets, and parties.77, 78 It was only through collective investigation and tracing the product back from these individual events that the nature of the outbreak was recognized. Similarly, the investigation of an outbreak of gastrointestinal illness with clinical and epidemiologic features of enterotoxigenic E. coli at a restaurant led to the identification of a novel strain of atypical enteropathogenic E. coli. 20 Nationwide surveillance efforts conducted by individual states, coordinated by the CDC, and facilitated by resources such as FoodNet and PulseNet offer great promise to enhance understanding of foodborne diseases in the coming years. A challenge to this promise is the rapid development and use of nonculture diagnostic tests that may reduce the ability of PulseNet to identify outbreaks if isolates are not submitted to public health laboratories for molecular subtyping.79 However, if these tests are cheaper and produce results that are faster and more clinically relevant, it may greatly increase the diagnosis of Salmonella, Shiga-toxin–producing E. coli, and other foodborne pathogens. This could lead to more sensitive outbreak detection and increase the usefulness of outbreak investigations to attribute foodborne diseases to specific food items and routes of exposure.
Experimental Studies
Clinical Trials
Clinical trials are research activities that involve the administration of a treatment or prevention regimen to humans to evaluate its safety and efficacy.1 In general, these trials involve a comparison of clinical outcomes of a group of patients receiving treatment with the outcomes of a comparable control group. Most clinical trials of interest in infectious disease epidemiology are trials of antimicrobial agents and vaccines. An early forerunner to the modern clinical trial was a U.S.-based smallpox trial conducted in 1800.80 During the 1950s, several multicenter trials were developed to evaluate chemotherapy in the treatment of tuberculosis.81 In 1953, the U.S. poliomyelitis vaccine trials were conducted in collaboration with the U.S. Public Health Service and state health departments.82
Many considerations are necessary when designing a clinical trial. First, should the trial be conducted at all? Is enough known about the safety and biologic activity of the treatment or vaccine to allow it to be administered to patients? This consideration requires some knowledge of the immunogenicity of candidate vaccines or the in vitro activity of an antibiotic against specific pathogens. Second, would patients be harmed by withholding the treatment or vaccine? These issues gained particular attention regarding trials of drugs and vaccines for the treatment of HIV infection. The initial double-blind, placebo-controlled study of oral azidothymidine (AZT) in AIDS patients was stopped early because of the marked effectiveness of the treatment.83 Over the past decade, multiple efficacy trials of an HIV-1 vaccine were terminated early when an interim analysis demonstrated that the vaccine did not protect against HIV infection or reduce viral loads after infection and may have increased susceptibility in some subjects.84, 85 An overriding concern in HIV-related clinical trials is the need to maintain behavior-related educational interventions for all participants in the trials. Although this may reduce the likelihood of demonstrating vaccine efficacy because of the lack of new infections among placebo recipients, to withhold such education would be unethical.
Other considerations include the specification of both test and control treatments, an outcome measure for evaluating the treatments, a bias-free method for assigning patients to treatment groups, and calculation of the necessary sample size.86 Sample size calculations are affected by the number of treatment groups to be studied, the desired significance level for rejecting the null hypothesis, the statistical power to detect a difference, and the desired detectable treatment difference.
Limitations of clinical trials relate largely to the size of the trial and how well the treatment groups reflect the larger target population for the vaccine or treatment.23 As noted earlier, the results of HBV vaccine trials did not adequately predict the performance of the vaccines among health care workers. In addition, sample size limitations may not allow for the full characterization of potentially rare complications, such as intussusception after administration of rotavirus vaccine.58 In these situations, postlicensure surveillance becomes a critical measure of the safety and effectiveness of the vaccine or treatment.
Community Intervention Trials
Community intervention trials are related to the clinical trial but are carried out on a larger scale. In these experiments, large groups or communities are selected to receive a therapeutic or preventive regimen.86 For example, the mass distribution of long-lasting insecticide-treated bed nets and distribution of antimalarial medications by community health workers rapidly reduced the incidence of malaria among children in Rwanda.87 Community trials are particularly well suited to broad-based interventions, such as changing physician antibiotic-prescribing practices through the promotion of judicious antibiotic use.88
Host-Agent Relationship
Although advances in medical science have made us less vulnerable to some infectious diseases, epidemics and pandemics continue to occur as they have throughout human history. During the 1960s and 1970s, there was a growing sense that infectious diseases were being “successfully” conquered on a global basis.89 However, in 2013, infectious diseases still remain the leading cause of death worldwide. The world's human and animal populations continue to struggle against an increasingly recognized number of viral, bacterial, protozoal, helminthic, and fungal agents.90
A 2003 National Academy of Sciences Institute of Medicine report detailed the combination of emerging social, political, and economic factors favoring infectious agents in humans and animals: “a transcendent moment nears upon the world for a microbial perfect storm.”91 Thirteen factors were identified in the report that favor the emergence of infectious agents: microbial adaptation and change, human susceptibility to infection, climate and weather, changing ecosystems, human demographics and behavior, economic development and land use, international travel and commerce, technology and industry, breakdown of public health measures, poverty and social inequality, war and famine, lack of political will, and intent to harm.
For the study of infectious disease epidemiology, it is important to consider both infection and disease because these may be different. Infection results from an encounter between a potentially pathogenic agent and a susceptible human host in conjunction with a suitable portal of entry. Because the source of most human infections lies outside the individual human host, exposure to the environment or to other infected hosts is a key factor. Disease is one of the possible outcomes of infection, and its development is related to factors of both the host and the agent.
Whereas the clinician is primarily concerned with disease, the epidemiologist is interested in both infection and disease. Because infection without disease occurs frequently for many agents, a study of only clinical illness may provide a misleading understanding of the epidemiology of a specific infectious disease in the community. For example, unvaccinated adults infected with HAV frequently experience clinical hepatitis, whereas similarly unvaccinated infants and toddlers with HAV infection are usually asymptomatic.92 Therefore, to determine the incidence of hepatitis A associated with child care facilities and subsequent transmission to family members and child care providers, investigators need to determine both the diagnosis of asymptomatic HAV infection and the level of HAV-related disease.
If the balance between agent and host favors the agent, infection (and in some instances, disease) will occur. This relationship among the agent, the route or mechanism of transmission, and the host is referred to as the chain of infection. Control and prevention of infection depend on sufficient understanding of the dynamics of these interrelating factors.
Characteristics of the agent or host are frequently seen as independent factors. However, it is necessary to consider both the host and the agent together in any discussion of the relationship resulting in infection and disease. For example, smallpox was a disease of dramatic human suffering; historically, it was one of the most feared of all infectious diseases. Yet, the ability of the smallpox virus (variola virus) to infect and cause disease only in humans and subhuman primates was an important consideration in approaches to control and prevention (i.e., vaccination of the human population).93 Consideration of the smallpox virus as highly virulent was tempered by the fact that inoculation of this virus into many animal species did not result in infection. In contrast, most Salmonella serotypes can cause mild-to-severe infection in humans and in a variety of animal species. A notable exception is Salmonella Typhi, which causes infections only in humans. Therefore, any description of the characteristics for either the agent or the host must be understood in the context of their interrelationship.
Agent
Any agent or microorganism is of epidemiologic importance if it can be transmitted through the environment, cause infection in a host (either human or animal), and produce clinical disease. These agents, regardless of their classification as bacterial, viral, protozoal, helminthic, or fungal, are considered the first necessary component in the chain of infection. Three characteristics of agents must be considered in terms of their epidemiologic importance: (1) those that are involved in spread or transport of the agent through the environment, (2) those that are involved in the production of infection, and (3) those that are involved in the production of disease.94
The characteristics of agents involved in spread through the environment vary with the method of transmission, but in any case, it is necessary for a minimum number of organisms to survive transport through the environment to reach and enter a susceptible host. Agents that are transmitted by direct person-to-person contact tend to have minimal ability to survive stressful environmental conditions (such as changes in temperature, humidity, or pH). In contrast, agents that are capable of multiplication within the environment (i.e., in food products, water, soil, and plants) have a unique advantage for survival. Some agents, such as Legionella pneumophila or Bacillus anthracis, do not necessarily multiply within the environment but can survive for months to many years in relatively hostile conditions, including distilled water or soil.95, 96 For those agents for which humans are the only known reservoir, the longer the time that is likely to elapse before contact with another susceptible host, the greater the resistance the agent must have to environmental conditions, such as heat, drying, ultraviolet light, or dilution by airflow. Finally, some agents have the capacity to infect nonhuman hosts, such as animals, birds, or an insect vector. Such nonhuman hosts may play an important role in maintenance of the agent in the environment.
The ability of an agent to cause infection or disease has to be considered in the context of host characteristics. For example, an agent is considered to colonize a host when its presence in that host does not cause a specific immune response or infection. However, should the relationship between the agent and the host change, such as by the introduction of certain normal microbial flora from the gastrointestinal tract into the bloodstream, infection can result. This type of infection is known as endogenous. If the agent is transported from an external source to the host (exogenous infection) and the balance between the agent and host favors the agent, infection usually develops.
Several aspects of the agent-host relationship can be related to the agent. Other aspects must be considered only in the context of both agent and host characteristics. For example, infectiousness is a characteristic of an agent that is concerned with the relative ease with which the agent is transmitted to other hosts. A droplet-spread agent, such as a respiratory virus, tends to be more infectious than one transmitted by direct contact, such as an organism causing a sexually transmitted disease. Characteristics of the portals of exit and entry are determinants of infectiousness, as is the agent's ability to survive away from the host. Some factors that are often ascribed to an agent are actually the result of both agent and host characteristics. These factors include infectivity, pathogenicity, virulence, and antigenicity or immunogenicity.
Infectivity is typically defined as the characteristic of the infectious agent that embodies its capability to enter, survive, and multiply in the host. A measure of infectivity is the secondary attack rate, or the probability that infection will occur in a susceptible individual after exposure to an infected individual. Infectivity is often expressed as the number of individuals infected divided by the number susceptible and exposed. A population with an increased number of individuals who have compromised specific or nonspecific immune responses may have a higher proportion of individuals who actually become infected after exposure. For example, individuals who have decreased gastric acidity because of antacid use are at a higher risk for the development of salmonellosis after a low infectious dose than are those with normal gastric pH.97
Pathogenicity is the property of an agent that determines the extent to which overt disease is produced in an infected population.1 The pathogenicity of an agent is measured by the ratio of the number of persons in whom clinical disease develops to the number infected. Although pathogenicity is frequently considered a sole property of the agent, host characteristics play an important role in defining it. For example, in HAV infection, the ratio of disease to total infections varies widely by host age.92 In general, those agents with the highest levels of pathogenicity possess characteristics that protect them against nonspecific host defenses. They may elaborate a number of enzymes or toxins or induce host-mediated disease associated with the immune response to the infection.
The gradient of infection, or the biologic gradient, is the range of manifestations of illness in the host resulting from infection with an agent. It extends from death at one extreme to inapparent or subclinical illness at the other. In this regard, virulence is frequently used as a quantitative expression of the disease-producing potential of a pathogenic agent. It is defined as the ratio of the number of cases of serious or disability-producing infection to the total number of people infected.1 If death is the only criterion for determining severity, this measure of virulence is referred to as the case-fatality rate.
From an epidemiologic perspective, the virulence of an organism must be viewed in light of the host. For example, the clinical outcome of HBV infection can range from limited, subclinical infection to acute fulminant hepatitis, depending on immune-mediated disease and important genetic factors of the host.98 Similarly, the severity of tuberculosis is increased among African Americans who have host characteristics similar to those of people of other races with tuberculosis.99 The development of drug resistance among organisms, regardless of the mechanism, is an important consideration related to virulence. Infection that is caused by agents sensitive to a variety of antimicrobial drugs is less likely to result in serious disease if treated in a timely and appropriate manner than is infection caused by a highly resistant organism. With rapidly increasing drug resistance among all groups of infectious agents, this virulence characteristic will become even more important in the future.100, 101
A final characteristic usually ascribed to an agent is antigenicity or immunogenicity. This is defined as the ability of an agent to produce a systemic or local immunologic reaction in the host.1 This characteristic also must be considered in the context of both agent and host. The antigenicity of an agent is important from a clinical perspective because it is a primary determinant in the host's ability to mount an initial immune response to infection and thus affects both pathogenicity and virulence. It also determines the host's development of long-term immunity to a specific agent. Therefore, it is a critical factor in the assessment and development of vaccines for human and animal use.
In general, the host immune system includes all physiologic mechanisms with the capacity to recognize materials foreign to itself and to neutralize, eliminate, or metabolize them with or without injury to its own tissues.102 The immune response may be classified into two categories: specific and nonspecific. Specific immune responses depend on exposure to a foreign configuration, such as an infectious agent, and the subsequent recognition of and reaction to that agent. An example of this type of response is the development of humoral and cell-mediated immunity related to a specific agent. A nonspecific response occurs after initial and subsequent exposure to a foreign antigen; although it is selective in differentiating “self” from “nonself,” it is not dependent on selective recognition. A number of factors modify the host's immune mechanisms, including genetic, age, metabolic, environmental, anatomic, physiologic, and microbial factors.
An example of the complex nature of the interaction between agent and host can be demonstrated by the relationship between H. influenzae type b and the age of the host. Children younger than 2 years do not mount an effective immune response to agents with capsular polysaccharide, such as H. influenzae type b, N. meningitidis, and Streptococcus pneumoniae.103 Polysaccharide antigens are T-cell–independent antigens, in contrast to protein antigens, which induce a T-cell effect. T-cell–independent antigens are poorly handled by very young children because of their immature immune system. Efforts were undertaken to develop vaccines for H. influenzae in younger children. This approach required that the H. influenzae type b polysaccharide be conjugated to various carrier proteins.103 The combination of polysaccharide and protein resulted in vaccines with enhanced immunogenicity that were able to induce a T-cell response in infants. Use of these second-generation H. influenzae conjugate vaccines has resulted in a dramatic decrease in the occurrence of invasive H. influenzae type b disease in infants in the United States.104
Similar efforts to develop and market conjugated polysaccharide vaccines for N. meningitidis and S. pneumoniae have resulted in similar dramatic impacts on these diseases in the United States.27, 35 Because the use of vaccines has proved to be one of the most cost-effective methods for preventing infectious diseases, the need to understand antigenicity in terms of both agent and host is a high priority.
Host
In addition to characteristics of the agent, those of the host also play an important role in the eventual outcome of an agent-host interaction. Host factors that influence exposure, infection, and disease are summarized in Table 13-2 . Factors can be classified into two categories: those that influence exposure and those that influence the likelihood of infection and the occurrence and severity of disease.
TABLE 13-2.
Host Factors That Influence Exposure, Infection, and Disease
| Factors That Influence Exposure |
| Animal exposure, including pets |
| Behavioral factors related to age, drug use, alcohol consumption |
| Blood or blood product receipt |
| Child daycare attendance |
| Closed living quarters: military barracks, dormitories, homeless shelters, facilities for the elderly and mentally handicapped, prisons |
| Food and water consumption |
| Familial exposure |
| Gender |
| Hospitalization or outpatient medical care |
| Hygienic practices, including toilet training and hand washing |
| Occupation |
| Recreational activities, including sports |
| Recreational injection drug use |
| Sexual activity: heterosexual and homosexual, type and number of partners |
| School attendance |
| Socioeconomic status |
| Travel, especially to developing countries |
| Vector exposure |
| Factors That Influence Infection and the Occurrence and Severity of Disease |
| Age at the time of infection |
| Alcoholism |
| Anatomic defect |
| Antibiotic resistance (agent) |
| Antibiotic use (host) |
| Coexisting noninfectious diseases, especially chronic |
| Coexisting infections |
| Dosage: amount and virulence of the organism to which the host is exposed |
| Duration of exposure to the organism |
| Entry portal of the organism and presence of trauma at the site of implantation |
| Gender |
| Genetic makeup, especially influences on the immune response |
| Immune state at the time of infection, including immunization status |
| Immunodeficiency (specific or nonspecific): natural, drug induced, or viral (HIV) |
| Mechanism of disease production: inflammatory, immunopathologic, or toxic |
| Nutritional status |
| Receptors for organism on cells needed for attachment or entry of the organism |
HIV, human immunodeficiency virus.
Modified from Evans AS, Brachman PS. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. New York: Plenum; 1998.
All of the factors that influence human exposure to an infectious agent depend on contact with sources of infection within the environment or promotion of person-to-person transmission.104 The importance of the factors that influence exposure tends to change with host age, culture, geographic residence, season, and family status.
Although most of the factors that influence infection and the occurrence and severity of disease are host characteristics, those that are related to both the agent and the host, as described by pathogenicity, virulence, and antigenicity, are important. Also, the agent infectious dose, mechanisms of disease production, antibiotic resistance of the infecting agent, and portal of entry contribute to infection and disease status. For most infections, two host factors play key roles in determining the likelihood of clinical illness and the severity of that illness: (1) the immune status of the host and (2) age at the time of infection. The highest levels of pathogenicity and virulence associated with the agent-host relationship tend to occur very early in life, when immune disease mechanisms are immature, or at an old age, when they may be deteriorating. Finally, genetic factors tend to influence both susceptibility and disease outcome, although they are primarily related to the host immune response to infection.
Routes of Transmission
Transmission of infectious agents is defined as any mechanism by which an infectious agent is spread through the environment or to another person.1, 105 These mechanisms can be classified as direct or indirect.
Of the three modes of direct agent transmission, the most common is direct and immediate transfer of an infectious agent to a receptive portal of entry through which human infection is established. This type of direct contact transmission occurs in association with touching, kissing, or sexual intercourse or by the direct projection (droplet spread) of droplet spray from an infected host onto the conjunctiva or the mucous membranes of the nose or mouth of another host. Typically, droplet spread is limited to a distance of approximately 1 m. The second type of direct transmission occurs when host-susceptible tissue is exposed to the agent, such as through the bite of a rabid animal or contact with soil or decaying matter in which the agent leads a saprophytic existence (e.g., systemic mycosis). As an example, direct human contact with infected pet prairie dogs led to an outbreak of monkeypox in the United States.106 Transplacental transmission is the third form of direct transmission.
The three primary mechanisms of indirect agent transmission are vehicle-borne, vector-borne, and airborne. Vehicle-borne transmission occurs when any material serves as an intermediate means by which an infectious agent is transported or introduced into the susceptible host through a suitable portal of entry. These materials may include water; food; biologic products such as blood, serum, plasma, tissues, and organs; and objects (fomites) such as toys, soiled clothing, bedding, or surgical instruments. It is not necessary that the agent multiply or develop in or on the vehicle before it is transmitted.
The second method of indirect transmission is vector-borne transmission, which may be mechanical or biologic. Mechanical transmission occurs when an insect carries an infectious agent through the soiling of its feet or proboscis or through carriage in its gastrointestinal tract. Mechanical transmission does not require multiplication or development of the organism. In contrast, biologic vector-borne transmission occurs when propagation (multiplication), cyclic development, or a combination of these events (cyclopropagative) is required before the arthropod can transmit the infected form of the agent to humans.
The third type of indirect transmission is airborne, involving the dissemination of aerosols with infectious agents to a suitable portal of entry into a host, usually the respiratory tract. These aerosols are suspensions of particles in the air that consist partially or wholly of infectious agents. The particles are in the range of 1 to 5 µm. Airborne transmission does not include droplets and other large particles that promptly settle out, which result in direct transmission. Some infections transmitted by the airborne route may be carried great distances from their sources, as documented by outbreaks of measles, legionnaires' disease, and anthrax.107, 108 For this reason, there is particular concern that agents such as B. anthracis and Yersinia pestis could be used as weapons of mass destruction in a civilian bioterrorism event.109, 110
There has been substantial debate about the efficacy of measures to reduce the risk of influenza transmission during the next pandemic in the absence of a vaccine. In particular, questions have been raised about the role of droplet versus aerosol transmission of the influenza virus. A study conducted in a hospital emergency room collected size-fractionated aerosol particles and tested for airborne influenza virus.111 In 53% of the samples, detectable influenza virus was found, supporting the conclusion that aerosols may play an important role in human-to-human transmission.
Disease Prevention and Control
Individual-, Institutional-, Community-, and Global-Based Strategies
Disease prevention and control activities for infectious agents occur at four levels. The first level is targeted to the individual and is predominantly the domain of the clinician. A variety of prevention activities can be targeted to individuals through their primary care provider. Use of chemoprophylaxis to prevent surgical wound infections is an example of a control measure targeted to the individual. The second level is that of the institution, which is predominantly the domain of the infection-control practitioner or the school health official. This level includes health care facilities, nursing homes, other residential facilities, and schools. Programs to prevent the spread of bloodborne pathogens or tuberculosis to health care workers in hospitals are examples of control strategies targeted at the institutional level. The third level is targeted to the community in general and is predominantly the domain of public health agencies (at the local, state, and national levels). Removal of a contaminated food product from the market is an example of a control measure targeted to the community. The fourth level is related to global strategies. For a number of important pathogens, it has become clear that global control strategies are critical to have an impact on disease occurrence within the United States. The growing proportion of tuberculosis cases among refugees and immigrants to the United States and ongoing episodes of importation of measles from abroad are two examples pertinent to U.S. disease control in the early 21st century.
Although some control measures are specific to these different levels, a substantial amount of overlap can occur. For example, immunization programs operate at all four levels. Clinicians play an important role in the health maintenance of their individual patients by providing immunizations against a variety of pathogens. Immunization programs are also an important activity at the institutional level, such as routine annual immunization against influenza in nursing homes and immunization of health care workers against HBV. Public health agencies monitor vaccination levels in the community and provide vaccination clinics open to the public. Finally, ensuring that foreign travelers from countries with selected endemic vaccine-preventable diseases are adequately vaccinated before travel is a critical control measure for the prevention of diseases such as measles.
When assessing or developing disease prevention and control activities targeted to infectious diseases, the weakest link in the chain of infection (agent, transmission, host) needs to be considered for each specific pathogen. In some situations, control of the agent in a specific reservoir may be the best way to reduce disease occurrence. Chlorination of water and pasteurization of milk are examples of destruction of an agent in its reservoir or elimination of a possible mode of transmission.
Strategies aimed at transmission need to be tailored to the type of transmission involved. For example, the use of condoms in prevention of sexually transmitted diseases is a control strategy targeted at preventing contact transmission. Transmission through common vehicles frequently involves food and water and may involve other vehicles, such as blood in the case of transfusions. Irradiation of food and screening of blood for infectious agents are control activities targeted to a common vehicle. An example of a control activity targeted to airborne transmission is the use of respirators to prevent transmission of tuberculosis in the health care setting. Control of vector-borne transmission can be targeted toward destroying the vector or its breeding sites or toward the use of protective clothing and repellents.
Methods aimed at limiting population mixing between infected or infectious cases and uninfected individuals can result in the application of isolation or quarantine. Often referred to as “police powers,” federal, state, and local governments, depending on specific legal authorities, can control the movement of individuals or populations to protect the general public from communicable diseases.112, 113 Isolation is the process and procedure used to prevent an infected individual from potentially transmitting a communicable disease to others. Traditionally, it has been applied to individuals with serious, life-threatening infections who have not or cannot be treated so as to be rendered noninfectious. The potential for bioterrorism-related illnesses, such as smallpox or pneumonic plague and the occurrence of SARS, have highlighted this issue. Quarantine, the separation or restriction of movement of persons who are not ill but are believed to have been exposed to infection, to prevent transmission of disease was developed in the 14th century but has been implemented rarely on a large scale during the past century. The SARS pandemic demonstrated that governments might use quarantine as a public health tool to treat infectious diseases, particularly if other preventive interventions (e.g., vaccines and antibiotics) are unavailable.113, 114
In some instances, the best mechanism to prevent disease occurrence is through modification of the host, such as developing or boosting immunity through active or passive immunization. Other examples of control activities targeted to the host include improving nutritional status and providing chemoprophylaxis against a variety of agents.
Assessment of Risk, Feasibility, Cost, and Effectiveness
When disease prevention and control strategies are being developed, several issues need to be considered, including risk, feasibility, cost, and effectiveness. Risk can be defined by the potential for exposure. Epidemiologic studies or analyses of surveillance data can serve to define persons or populations at risk and to quantify risk within different populations. At the individual level, risk can be evaluated by assessing host characteristics, such as the need for preventing opportunistic infections among hematopoietic stem cell transplant recipients.
An example of evaluating risk at the institutional level is assessing occupational exposure to infectious agents such as bloodborne pathogens. At the community level, groups at risk for a variety of conditions can be defined by demographic features such as age, race, country of origin, socioeconomic status, and geographic location. For example, persons born outside the United States are at increased risk for infectious diseases such as tuberculosis and for being chronic carriers of infections such as hepatitis B. Screening programs targeted to these populations, with subsequent interventions such as isoniazid prophylaxis for persons with M. tuberculosis infection or immunization of susceptible household contacts of HBV carriers, can serve as important community-based strategies to prevent infectious disease occurrence.115, 116, 117 Another example of defining risk at the community level is assessing behavior that increases the risk for specific diseases, such as injection drug use as a risk behavior for acquiring HIV or HCV infection. Education and drug treatment programs targeted to this population can serve as an important disease prevention and control strategy. Finally, assessing the population of species-specific mosquitoes and viability of local breeding sites can provide a risk assessment of the likelihood of vector-borne disease transmission (e.g., West Nile virus infection).118, 119
In developing control programs, the feasibility of a strategy also needs to be assessed. Feasibility is dependent on the sociodemographic factors of the population involved. For example, high immunization rates can clearly prevent the occurrence of infectious diseases. In the United States, immunizations should be readily available; however, in the late 1980s, numerous large outbreaks of measles occurred in U.S. inner-city populations because of low immunization rates.120 A variety of sociodemographic factors contributed to these low rates, such as inadequate access to medical care and other barriers to immunization. Until such barriers are removed and control strategies are developed to specifically target at-risk populations, adequate control of vaccine-preventable diseases in the United States cannot be accomplished.121
Cost and availability of resources also need to be considered when developing control strategies. Adequate water treatment facilities and distribution systems in developing countries would do much to eliminate the spread of cholera. However, in many countries, resources to build and develop such facilities are not available. Consequently, control strategies need to be focused on simpler, less expensive methods, such as boiling water or improving water storage in the home.
Finally, control strategies need to be evaluated for their effectiveness. For example, the effectiveness of the control strategy is a critical issue in evaluating ways to curb the HIV epidemic in the absence of vaccination. Evaluation of educational programs on preventing HIV infection or of HIV counseling and testing programs is essential in assessing the effectiveness of currently available strategies. Cost-effectiveness models are often used in making recommendations for population-based vaccination programs.122, 123
Primary, Secondary, and Tertiary Prevention
Prevention strategies for infectious diseases can be characterized by the traditional concepts of primary, secondary, or tertiary prevention.1 Primary prevention can be defined as the prevention of infection by personal and community-wide efforts. Secondary prevention includes measures available to individuals and the population for detection of early infection and effective intervention. Tertiary prevention consists of measures available to reduce or eliminate the long-term impairment and disabilities caused by infectious diseases.
Primary Prevention
A key example of primary prevention is immunoprophylaxis, which can be active or passive (see Chapter 321).117 Active immunoprophylaxis involves the administration of all or part of a microorganism (live or inactivated) or a product of that microorganism (such as a toxoid) to alter the host by stimulating an immunologic response aimed at protecting against infection. Live vaccines are often more immunogenic than inactivated vaccines and may require fewer booster doses. Live-attenuated vaccines contain weakened or avirulent viruses or bacteria. They are generally contraindicated in immunocompromised persons. Examples of live-attenuated vaccines include vaccines against measles, mumps, rubella, and yellow fever; oral polio vaccine; oral typhoid vaccine; and Calmette-Guérin bacillus vaccine. Examples of vaccines created from inactivated organisms include inactivated polio vaccine and vaccines against anthrax, influenza, HBV, cholera, pertussis, and rabies. H. influenzae type b, pneumococcal, and meningococcal conjugate vaccines are polysaccharide-protein conjugates. Examples of toxoid vaccines include tetanus, diphtheria, and botulinal toxoids.105
Currently, at least four types of active immunization programs are being conducted. The first is routine childhood immunization. Current practices include routine childhood immunization against measles, mumps, rubella, tetanus, diphtheria, pertussis, H. influenzae type b, pneumococcus, meningococcus, varicella, rotavirus, polio (inactivated vaccine), HAV, and HBV.117 In many parts of the world, Calmette-Guérin bacillus vaccine is also given routinely in early childhood. As the routine childhood immunization schedule becomes increasingly complex, new methods of vaccine delivery need to be developed. Of particular interest is the development of new multiple-antigen vaccines to simplify the routine schedule and maximize efficiency of vaccine delivery. The goals of routine childhood immunization are twofold: to protect the individual and to provide herd immunity, which can be effective in controlling certain diseases at the population level (e.g., measles, mumps, rubella, H. influenzae).124 Ongoing adequate surveillance for these diseases is essential to monitor the effectiveness of population-based immunization programs so that strategies can be adapted as needed. The expansion of measles immunization to a two-dose schedule in the United States in 1989 is an example of using surveillance data to revise immunization practices.125
A second type of immunization program is travel-related immunization. Examples include the administration of typhoid, yellow fever, Japanese encephalitis, and meningococcal vaccines for travel to areas endemic for these conditions.
The third type of immunization program is based on occupational exposure. Recommendations for immunization of health care workers have been made because of their special risk of exposure to a variety of vaccine-preventable diseases. Examples include immunization of laboratory workers against anthrax, rabies, smallpox, and botulism in settings in which these organisms are or may be handled; immunization of health care workers against measles, smallpox, and hepatitis B based on exposure to bloodborne pathogens; and immunization against anthrax and smallpox for those responding to bioterrorism-related exposures.126, 127
Active immunization is also used in certain postexposure situations, including immunization after exposure to N. meningitidis, HBV, measles, pertussis, or rabies. Some of these vaccines are given in conjunction with various types of immunoglobulins in the postexposure setting.
Passive immunization involves the administration of preformed antibodies, often to specific agents, after exposure. The broadest form of passive immunization is the use of normal human immune globulin (also referred to as gamma globulin). It is most often used after exposure to HAV and may be effective if given within 14 days after exposure. Normal human immune globulin is recommended before travel to countries endemic for hepatitis A. It may also be effective in reducing clinical disease in persons exposed to measles if provided within 6 days after exposure.117 For persons with hypogammaglobulinemia or agammaglobulinemia, it may be given as immunoglobulin G replacement therapy. Specific types of immune globulins are also used in postexposure settings to prevent infection; examples include immune globulins specific to HBV, cytomegalovirus, rabies, varicella-zoster virus, and tetanus.
A second type of primary prevention is antimicrobial prophylaxis, often referred to as chemoprophylaxis. Use of effective chemoprophylaxis requires that the infectious agent be susceptible to the antimicrobial drug used. As a primary prevention strategy, it may be used before or after exposure to prevent infection. Examples of chemoprophylaxis in the postexposure setting include erythromycin after exposure to pertussis; rifampin after N. meningitidis; amantadine, rimantadine, zanamir, or oseltamivir after influenza A virus; and zidovudine after HIV exposure. Use of antiretroviral drugs by HIV-infected pregnant women has been shown to substantially reduce the risk of perinatal HIV infection.128 Prophylaxis against surgical wound infections with broad-spectrum coverage before surgery and prophylaxis of neonates against ophthalmia neonatorum are examples of chemoprophylaxis used in the hospital setting. In such situations, chemoprophylaxis is used because a likelihood of exposure to pathogenic organisms is present, even if exposure is not clearly documented. Chemoprophylaxis is also used in anticipation of exposure during travel; examples include the use of chloroquine or mefloquine to prevent malaria or antimicrobials against enteric pathogens to prevent traveler's diarrhea.
In addition to immunoprophylaxis and chemoprophylaxis, other important primary prevention activities are focused on the individual, institutional, and community levels. Examples have been discussed in earlier sections of this chapter.
Secondary Prevention
Secondary prevention activities traditionally entail chemoprophylaxis and involve the identification of early or asymptomatic infection with subsequent treatment so that the infection is eradicated and sequelae are prevented. Although most secondary prevention programs involve intervention at the individual level through the use of chemoprophylaxis, such programs often operate within the context of a population-based or institutional-based screening effort. Routine screening programs for sexually transmitted diseases such as Chlamydia infection are examples of secondary prevention strategies.129, 130 Contact investigations for partners of persons with sexually transmitted diseases are also part of a secondary prevention strategy focused on those at highest risk of infection (i.e., those with known exposure). Another example of a secondary prevention program using chemoprophylaxis is screening of high-risk populations for tuberculosis infection and subsequent therapy with an antimicrobial drug, such as isoniazid, to prevent active disease.
Although most secondary prevention strategies involve chemoprophylaxis (and, rarely, immunoprophylaxis), the concept can be broadened to other prevention efforts aimed at intervention and correction of a recognized specific health hazard. Most such efforts occur at the community level. Examples of community-based secondary prevention efforts include the early identification of contaminated products through outbreak investigations and subsequent removal of such products from the market to prevent additional illnesses and restore “the community's health.” A boil-water order for a waterborne disease outbreak of cryptosporidiosis is another example of a secondary prevention strategy aimed at correcting an existing community-wide problem.
Tertiary Prevention
Tertiary prevention efforts are measures used to eliminate long-term impairment and disabilities resulting from an existing condition. Because most infectious diseases are treatable, tertiary prevention activities are less common than those found with chronic diseases such as hypertension, diabetes, and coronary artery disease. However, this concept is still applicable to the control of infectious diseases inasmuch as some viral infections are chronic and cannot be eradicated. Current treatment of HIV infection, including prophylaxis against other opportunistic agents, is an example of a tertiary prevention activity.
Key References
The complete reference list is available online at Expert Consult.
- 1.Porta M, editor. A Dictionary of Epidemiology. 5th ed. Oxford University Press; New York: 2009. [Google Scholar]
- 3.Snow J. On the mode of communication of cholera (1855) In: Frost WH, editor. Snow on Cholera. Commonwealth Fund; New York: 1936. Reprinted in: [Google Scholar]
- 5.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 8.Ksiazek TG, Erdman D, Goldsmith C. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 11.Zaki AM, van Boheemen S, Bestebroer TM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 12.Assiri A, McGeer A, Perl TM. Hosptial outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao R, Cao B, Hu Y. Human infection with novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization China—WHO Joint Mission on Human Infection with Avian Influenza A(H7N9) Virus, 18-24 April 2013 Mission Report. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ChinaH7N9JointMissionReport2013u.pdf Available at.
- 17.World Health Organization Overview of the Emergence and Characteristics of the Avian Influenza A(H7N9) Virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/WHO_H7N9_review_31May13.pdf Available at.
- 18.World Health Organization Influenza at the Human-Animal Interface. http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_03July13.pdf Summary and Assessment as of 4 July 2013. Available at.
- 19.World Health Organization Global eradication of smallpox. Bull World Health Organ. 1980;58:161–163. [Google Scholar]
- 23.Maldonado G, Greenland S. Estimating causal effects. Int J Epidemiol. 2002;31:422–429. [PubMed] [Google Scholar]
- 26.Swaminathan B, Barrett TJ, Hunter SB. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn AC, MacNeil JR, Clark TA. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62(RR-2):1–28. Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 28.Weiss D, Stern EJ, Zimmerman C. Epidemiologic investigation and targeted vaccination initiative in response to an outbreak of meningococcal disease among illicit drug users in Brooklyn, New York. Clin Infect Dis. 2009;48:894–901. doi: 10.1086/597257. New York City Meningococcal Investigation Team. [DOI] [PubMed] [Google Scholar]
- 30.Popovic T, Schmink S, Rosenstein N. Evaluation of pulsed-field gel electrophoresis in epidemiological investigations of meningococcal disease outbreaks caused by Neisseria meningitidis serogroup C. J Clin Microbiol. 2001;39:75–85. doi: 10.1128/JCM.39.1.75-85.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlesselman JJ, Stolley PD. Oxford University Press; New York: 1982. Case-Control Studies: Design, Conduct, Analysis. [Google Scholar]
- 36.Fairchild AL, Bayer R. Ethics and the conduct of public health surveillance. Science. 2004;303:631–632. doi: 10.1126/science.1094038. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Methicillin-Resistant Staphylococcus aureus. 2011. http://www.cdc.gov/abcs/reports-findings/survreports/mrsa11.pdf Available at.
- 43.Reingold AL. Outbreak investigations: a perspective. Emerg Infect Dis. 1998;4:21–27. doi: 10.3201/eid0401.980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chorba TL, Berkelman RL, Saffor SK. Mandatory reporting of infectious diseases by clinicians. JAMA. 1989;262:3018–3026. [PubMed] [Google Scholar]
- 49.Buehler JS, Berkelman RL, Hartley DM. Syndromic surveillance and bioterrorism-related epidemics. Emerg Infect Dis. 2003;9:1197–1204. doi: 10.3201/eid0910.030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996-2012. MMWR Morb Mortal Wkly Rep. 2013;62:283–744. [PMC free article] [PubMed] [Google Scholar]
- 57.MacNeil JR, Cohn AC, Farley M. Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 1989-2008. Clin Infect Dis. 2011;53:1230–1236. doi: 10.1093/cid/cir735. [DOI] [PubMed] [Google Scholar]
- 58.Murphy TV, Smith PJ, Gargiullo PM. The first rotavirus vaccine and intussusception: epidemiological studies and policy decisions. J Infect Dis. 2003;187:1309–1313. doi: 10.1086/374420. [DOI] [PubMed] [Google Scholar]
- 62.Hennessey TW, Hedberg CW, Slutsker L. A national outbreak of Salmonella enteritidis infections from ice cream. N Engl J Med. 1996;334:1281–1286. doi: 10.1056/NEJM199605163342001. [DOI] [PubMed] [Google Scholar]
- 72.Boxrud D, Monson T, Stiles T, Besser J. The role, challenges, and support of PulseNet laboratories in detecting foodborne disease outbreaks. Public Health Rep. 2010;125:57–62. doi: 10.1177/00333549101250S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2006-2007. Foodborne Active Surveillance Network (FoodNet) Population Survey Atlas of Exposures. [Google Scholar]
- 79.Jones TF, Gerner-Smidt P. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis. 2012;18:513–514. doi: 10.3201/eid1803.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barouch D. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–616. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The National Institute of Allergy and Infectious Diseases NIH Discontinues Immunizations in HIV Vaccine Study. http://www.niaid.nih.gov/news/newsreleases/2013/Pages/HVTN505April2013.aspx Available at.
- 89.Berkelman RL, Hughes JM. The conquest of infectious diseases: who are we kidding? Ann Intern Med. 1993;119:426–428. doi: 10.7326/0003-4819-119-5-199309010-00014. [DOI] [PubMed] [Google Scholar]
- 91.Smolinski MS, Hamburg MA, Lederberg J, editors. Microbial Threats to Health: Emergence, Detection, and Response. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 100.Swedish Strategic Programme against Antibiotic Resistance . Swedish Institute for Infectious Disease Control; Solna, Sweden: 2008. SWEDRES 2007: A Report on Swedish Antimicrobial Utilisation and Resistance in Human Medicine. [Google Scholar]
- 104.Murphy TV, White KE, Pastor P. Declining incidence of Haemophilus influenzae type b since introduction of vaccination. JAMA. 1993;269:246–248. [PubMed] [Google Scholar]
- 105.Heymann DL, Nunn M, editors. Control of Communicable Diseases Manual. 19th ed. American Public Health Association; Washington, DC: 2008. [Google Scholar]
- 106.Reed KD, Melski JW, Graham MB. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 107.Ehresmann KR, Hedberg CW, Grimm MB. An outbreak of measles at an international sporting event with airborne transmission in a domed stadium. J Infect Dis. 1995;171:679–683. doi: 10.1093/infdis/171.3.679. [DOI] [PubMed] [Google Scholar]
- 109.Inglesby TV, O'Toole T, Henderson DA. Anthrax as a biological weapon, 2002. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 110.Inglesby TV, Dennis DT, Henderson DA. Plague as a biological weapon; medical and public health management. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 111.Blachere FM, Lindsey WG, Pearce TA. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2008;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 112.Barbera J, Macintyre A, Gostin L. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286:2711–2717. doi: 10.1001/jama.286.21.2711. [DOI] [PubMed] [Google Scholar]
- 113.Centers for Disease Control and Prevention Use of quarantine to prevent transmission of severe acute respiratory syndrome—Taiwan, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:680–683. [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention General recommendations on immunizations: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 2011;60(RR02):1–60. [Google Scholar]
- 118.Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases . 3rd revision. U.S. Department of Health and Human Services, Public Health Service; Fort Collins, CO: 2003. Epidemic/epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. [Google Scholar]
- 119.Nash D, Mostashari F, Fine A. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 124.Fine PE. Herd immunity: History, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
References
- 1.Porta M, editor. A Dictionary of Epidemiology. 5th ed. Oxford University Press; New York: 2009. [Google Scholar]
- 2.Barrett-Connor E. Infectious and chronic disease epidemiology: separate and unequal? Am J Epidemiol. 1979;109:245–249. doi: 10.1093/oxfordjournals.aje.a112679. [DOI] [PubMed] [Google Scholar]
- 3.Snow J. On the mode of communication of cholera (1855) In: Frost WH, editor. Snow on Cholera. Commonwealth Fund; New York: 1936. Reprinted in. [Google Scholar]
- 4.Levy JA. 3rd ed. American Society for Microbiology Press; Washington, DC: 2007. HIV and the Pathogenesis of AIDS. [Google Scholar]
- 5.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 6.Corey L, Nabel GJ, Dieffenbach C. HIV-1 vaccines and adaptive trial designs. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 8.Ksiazek TG, Erdman D, Goldsmith C. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 9.Lipsitch M, Cohen T, Cooper B. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng VCC, Lau SKP, Woo PCY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaki AM, van Boheemen S, Bestebroer TM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 12.Assiri A, McGeer A, Perl TM. Hosptial outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Interim Surveillance Recommendations for Human Infection with Middle East Respiratory Syndrome Coronavirus. http://www.who.int/csr/disease/coronavirus_infections/InterimRevisedSurveillanceRecommendations_nCoVinfection_27Jun13.pdf Available at.
- 14.World Health Organization Infection Prevention and Control during Health Care for Probable or Confirmed Cases of Novel Coronavirus (nCoV) Infection. http://www.who.int/csr/disease/coronavirus_infections/InterimRevisedSurveillanceRecommendations_nCoVinfection_27Jun13.pdf Available at.
- 15.Gao R, Cao B, Hu Y. Human infection with novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization China—WHO Joint Mission on Human Infection with Avian Influenza A(H7N9) Virus, 18-24 April 2013 Mission Report. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ChinaH7N9JointMissionReport2013u.pdf Available at.
- 17.World Health Organization Overview of the Emergence and Characteristics of the Avian Influenza A(H7N9) Virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/WHO_H7N9_review_31May13.pdf Available at.
- 18.World Health Organization Influenza at the Human-Animal Interface. http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_03July13.pdf Summary and Assessment as of 4 July 2013. Available at.
- 19.World Health Organization Global eradication of smallpox. Bull World Health Organ. 1980;58:161–163. [Google Scholar]
- 20.Hedberg CW, Savarino SJ, Besser JM. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent not fitting into the existing scheme for classifying diarrheogenic E. coli. J Infect Dis. 1997;176:1625–1628. doi: 10.1086/517342. [DOI] [PubMed] [Google Scholar]
- 21.Gould LH, Mody RK, Ong KL. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis. 2013;10:453–460. doi: 10.1089/fpd.2012.1401. [DOI] [PubMed] [Google Scholar]
- 22.Wood RC, MacDonald KL, White KE. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935–2939. [PubMed] [Google Scholar]
- 23.Maldonado G, Greenland S. Estimating causal effects. Int J Epidemiol. 2002;31:422–429. [PubMed] [Google Scholar]
- 24.Moses J, Alkhouri N, Shannon A. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol. 2012;107:133–138. doi: 10.1038/ajg.2011.295. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach, United States, September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1045–1046. [PubMed] [Google Scholar]
- 26.Swaminathan B, Barrett TJ, Hunter SB. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn AC, MacNeil JR, Clark TA. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62(RR-2):1–28. Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 28.Weiss D, Stern EJ, Zimmerman C. Epidemiologic investigation and targeted vaccination initiative in response to an outbreak of meningococcal disease among illicit drug users in Brooklyn, New York. Clin Infect Dis. 2009;48:894–901. doi: 10.1086/597257. New York City Meningococcal Investigation Team. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Notes from the field: serogroup C invasive meningococcal disease among men who have sex with men—New York City, 2010-2012. MMWR Morb Mortal Wkly Rep. 2013;61:1048. [PubMed] [Google Scholar]
- 30.Popovic T, Schmink S, Rosenstein N. Evaluation of pulsed-field gel electrophoresis in epidemiological investigations of meningococcal disease outbreaks caused by Neisseria meningitidis serogroup C. J Clin Microbiol. 2001;39:75–85. doi: 10.1128/JCM.39.1.75-85.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennekens CH, Burning JE. Little, Brown; Boston: 1987. Epidemiology in Medicine. [Google Scholar]
- 32.Kassenborg HD, Hedberg CW, Hoekstra M. Farm visits and undercooked hamburgers as major risk factors for sporadic Escherichia coli O157:H7 infection: data from a case-control study in 5 FoodNet sites. Clin Infect Dis. 2004;38:S271–S278. doi: 10.1086/381596. [DOI] [PubMed] [Google Scholar]
- 33.Varma JK, Samuel MC, Marcus R. Listeria monocytogenes infection from foods prepared in a commercial establishment: a case-control study of potential sources of sporadic illness in the United States. Clin Infect Dis. 2007;44:521–528. doi: 10.1086/509920. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August-September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1357–1358. [PubMed] [Google Scholar]
- 35.Schlesselman JJ, Stolley PD. Oxford University Press; New York: 1982. Case-Control Studies: Design, Conduct, Analysis. [Google Scholar]
- 36.Fairchild AL, Bayer R. Ethics and the conduct of public health surveillance. Science. 2004;303:631–632. doi: 10.1126/science.1094038. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Prevention of invasive group A streptococcal disease among household contacts of case-patients and among postpartum post-surgical patients. Clin Infect Dis. 2002;35:950–959. doi: 10.1086/342692. [DOI] [PubMed] [Google Scholar]
- 38.Davies HD, McGeer A, Schwartz B. A prospective, population-based study of invasive group A streptococcal infections, including toxic shock syndrome and the risk of secondary invasive disease. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients. Clin Infect Dis. 2002;35:950–959. doi: 10.1086/342692. [DOI] [PubMed] [Google Scholar]
- 40.Robinson KA, Rothrock G, Phan Q. Risk for severe group A streptococcal disease among patients’ household contacts. Emerg Infect Dis. 2003;9:443–446. doi: 10.3201/eid0904.020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lilienfeld AM, Lilienfeld DE. 2nd ed. Oxford University Press; New York: 1980. Foundations of Epidemiology. [Google Scholar]
- 42.Centers for Disease Control and Prevention Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Methicillin-Resistant Staphylococcus aureus. 2011. http://www.cdc.gov/abcs/reports-findings/survreports/mrsa11.pdf Available at.
- 43.Reingold AL. Outbreak investigations: a perspective. Emerg Infect Dis. 1998;4:21–27. doi: 10.3201/eid0401.980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Morb Mortal Wkly Rep. 2001;50(RR-13):1–35. [PubMed] [Google Scholar]
- 45.Chorba TL, Berkelman RL, Saffor SK. Mandatory reporting of infectious diseases by clinicians. JAMA. 1989;262:3018–3026. [PubMed] [Google Scholar]
- 46.Westheimer E, Paladini M, Balter S. Evaluating the New York City Emergency Department syndromic surveillance for monitoring influenza activity during the 2009-2010 influenza season. PLoS Curr. 2012;4 doi: 10.1371/500563f3ea181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dugas AF, Jalalpour M, Gel Y. Influenza forecasting with Google Flu Trends. PLoS One. 2013;8:e56176. doi: 10.1371/journal.pone.0056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajjeh RA, Relman D, Cieslak PR. Surveillance for unexplained deaths and critical illnesses due to possibly infectious causes, United States, 1995-1998. Emerg Infect Dis. 2002;8:145–153. doi: 10.3201/eid0802.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buehler JS, Berkelman RL, Hartley DM. Syndromic surveillance and bioterrorism-related epidemics. Emerg Infect Dis. 2003;9:1197–1204. doi: 10.3201/eid0910.030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention Syndromic surveillance for bioterrorism following the attacks on the World Trade Center—New York City, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:13–15. [PubMed] [Google Scholar]
- 51.Balter S, Weiss D, Hanson H. Three years of emergency department gastrointestinal syndromic surveillance in New York City: what have we found? MMWR Morb Mortal Wkly Rep. 2005;54:175–180. [PubMed] [Google Scholar]
- 52.Shah NS, Pratt R, Armstrong L. Extensively drug-resistant tuberculosis in the United States, 1993-2007. JAMA. 2008;300:2153–2160. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 53.Glynn MK, Bopp C, Dewitt W. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Fontana J, Crowe C. Emergence of multi-drug resistant Salmonella enterica serotype Newport infections resistant to expanded spectrum cephalosporins in the United States. J Infect Dis. 2003;188:1707–1716. doi: 10.1086/379668. [DOI] [PubMed] [Google Scholar]
- 55.Kyaw MH, Lynfield R, Schaffner W. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996-2012. MMWR Morb Mortal Wkly Rep. 2013;62:283–744. [PMC free article] [PubMed] [Google Scholar]
- 57.MacNeil JR, Cohn AC, Farley M. Current epidemiology and trends in invasive Haemophilus influenzae disease—United States, 1989-2008. Clin Infect Dis. 2011;53:1230–1236. doi: 10.1093/cid/cir735. [DOI] [PubMed] [Google Scholar]
- 58.Murphy TV, Smith PJ, Gargiullo PM. The first rotavirus vaccine and intussusception: epidemiological studies and policy decisions. J Infect Dis. 2003;187:1309–1313. doi: 10.1086/374420. [DOI] [PubMed] [Google Scholar]
- 59.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 1659–1702. [Google Scholar]
- 60.Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 61.Lemant J, Boisson V, Winer A. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005-2006. Crit Care Med. 2008;36:2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. [DOI] [PubMed] [Google Scholar]
- 62.Hennessey TW, Hedberg CW, Slutsker L. A national outbreak of Salmonella enteritidis infections from ice cream. N Engl J Med. 1996;334:1281–1286. doi: 10.1056/NEJM199605163342001. [DOI] [PubMed] [Google Scholar]
- 63.Mishu B, Blaser MJ. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clin Infect Dis. 1993;17:104–108. doi: 10.1093/clinids/17.1.104. [DOI] [PubMed] [Google Scholar]
- 64.Moss AR, Osmond D, Bacchetti P. Risk factors for AIDS and HIV seropositivity in homosexual men. Am J Epidemiol. 1987;125:1035–1047. doi: 10.1093/oxfordjournals.aje.a114619. [DOI] [PubMed] [Google Scholar]
- 65.Osmond DH, Charlebois E, Sheppard HW. Comparison of risk factors for hepatitis C and hepatitis B infection in homosexual men. J Infect Dis. 1993;167:66–71. doi: 10.1093/infdis/167.1.66. [DOI] [PubMed] [Google Scholar]
- 66.Evans AS, editor. Viral Infections of Humans: Epidemiology and Control. 2nd ed. Plenum; New York: 1982. [Google Scholar]
- 67.Weinstock HS, Bolar G, Reingold AL. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA. 1993;269:392–394. [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention Serosurveys of West Nile virus infection—New York and Connecticut counties, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:31–39. [PubMed] [Google Scholar]
- 69.Paisley JE, Hinckley AF, O’Leary DR. West Nile virus infection among pregnant women in a northern Colorado community, 2003 to 2004. Pediatrics. 2006;117:814–820. doi: 10.1542/peds.2005-1187. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention Multistate outbreak of Salmonella infections associated with frozen pot pies—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1277–1280. [PubMed] [Google Scholar]
- 71.Smith KE, Medus C, Meyer SD. Outbreaks of salmonellosis in Minnesota (1998 through 2006) associated with frozen, microwaveable, breaded, stuffed chicken products. J Food Prot. 2008;71:2153–2160. doi: 10.4315/0362-028x-71.10.2153. [DOI] [PubMed] [Google Scholar]
- 72.Boxrud D, Monson T, Stiles T, Besser J. The role, challenges, and support of PulseNet laboratories in detecting foodborne disease outbreaks. Public Health Rep. 2010;125:57–62. doi: 10.1177/00333549101250S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009-2011. MMWR Morb Mortal Wkly Rep. 2013;62:448–452. [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy N, Giesecke J. Case-case comparisons to study causation of common infectious diseases. Int J Epidemiol. 1999;28:764–768. doi: 10.1093/ije/28.4.764. [DOI] [PubMed] [Google Scholar]
- 75.Miller BD, Rigdon CE, Ball J. Use of traceback methods to confirm the source of a multistate Escherichia coli O157:H7 outbreak due to in-shell hazelnuts. J Food Prot. 2012;75:320–327. doi: 10.4315/0362-028X.JFP-11-309. [DOI] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2006-2007. Foodborne Active Surveillance Network (FoodNet) Population Survey Atlas of Exposures. [Google Scholar]
- 77.Herwaldt BL, Ackers ML. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N Engl J Med. 1997;336:1548–1556. doi: 10.1056/NEJM199705293362202. [DOI] [PubMed] [Google Scholar]
- 78.Herwaldt BL, Beach MJ. The return of Cyclospora in 1997: another outbreak of cyclosporiasis in North America associated with imported raspberries. The Cyclospora Working Group. Ann Intern Med. 1999;130:210–220. doi: 10.7326/0003-4819-130-3-199902020-00006. [DOI] [PubMed] [Google Scholar]
- 79.Jones TF, Gerner-Smidt P. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis. 2012;18:513–514. doi: 10.3201/eid1803.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waterhouse B. Cambridge Press; Cambridge, England: 1800. A Prospect for Exterminating the Smallpox. [Google Scholar]
- 81.Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veteran's Administration and Armed Forces of the USA: an account of the evolving education of the physician in clinical pharmacology. Adv Tuber Res. 1960;10:1–68. [PubMed] [Google Scholar]
- 82.Francis T, Karns RF, Voight RB. An evaluation of the 1954 poliomyelitis vaccine trial: Summary report. Am J Public Health. 1955;45:S1–S51. [PMC free article] [PubMed] [Google Scholar]
- 83.Fischl MA, Richman DD, Grieco MH. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex: a double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 84.Barouch D. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–616. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The National Institute of Allergy and Infectious Diseases NIH Discontinues Immunizations in HIV Vaccine Study. http://www.niaid.nih.gov/news/newsreleases/2013/Pages/HVTN505April2013.aspx Available at.
- 86.Meinert CL. Oxford University Press; New York: 1986. Clinical Trials: Design, Conduct, and Analysis. vol. 8. Monographs in Epidemiology and Biostatistics. [Google Scholar]
- 87.Sievers AC, Lewey J, Musafiri P. Reduced paediatric hospitalizations for malaria and febrile illness patterns following implementation of community-based malaria control programme in rural Rwanda. Malar J. 2008;7:167. doi: 10.1186/1475-2875-7-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stille CJ, Rifas-Shiman SL, Kleinman K. Physician responses to a community-level trial promoting judicious antibiotic use. Ann Fam Med. 2008;3:206–212. doi: 10.1370/afm.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berkelman RL, Hughes JM. The conquest of infectious diseases: who are we kidding? Ann Intern Med. 1993;119:426–428. doi: 10.7326/0003-4819-119-5-199309010-00014. [DOI] [PubMed] [Google Scholar]
- 90.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 91.Smolinski MS, Hamburg MA, Lederberg J, editors. Microbial Threats to Health: Emergence, Detection, and Response. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 92.Hadler SC, Webster HM, Erben JJ. Hepatitis A in day care centers: a community-wide assessment. N Engl J Med. 1980;302:1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- 93.Benenson AS. Smallpox. In: Evans AS, editor. Viral Infections of Humans: Epidemiology and Control. Plenum; New York: 1982. pp. 541–568. [Google Scholar]
- 94.Evans AS, Brachman PS, editors. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum; New York: 1998. [Google Scholar]
- 95.Fox M, Kaufmann AF, Zendel SA. Anthrax in Louisiana, 1971: epizootiologic study. J Am Vet Med Assoc. 1973;163:446–451. [PubMed] [Google Scholar]
- 96.Skaliy P, McEachern HV. Survival of the legionnaires’ disease bacterium in water. Ann Intern Med. 1979;90:577–580. doi: 10.7326/0003-4819-90-4-662. [DOI] [PubMed] [Google Scholar]
- 97.Black PH, Kunz LJ, Swartz MN. Salmonellosis: a review of some unusual aspects. N Engl J Med. 1960;262:811–816. doi: 10.1056/NEJM196004212621606. [DOI] [PubMed] [Google Scholar]
- 98.Lau JYN, Wright TL. Molecular virology and pathogenesis of hepatitis B. Lancet. 1993;342:1335–1339. doi: 10.1016/0140-6736(93)92249-s. [DOI] [PubMed] [Google Scholar]
- 99.Stead WW, Senner JW, Reddick WT. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 100.Swedish Strategic Programme against Antibiotic Resistance . Swedish Institute for Infectious Disease Control; Solna, Sweden: 2008. SWEDRES 2007: A Report on Swedish Antimicrobial Utilisation and Resistance in Human Medicine. [Google Scholar]
- 101.Baba T, Takeuchi F, Kuroda M. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 102.Owen J, Punt J, Stranford S. 7th ed. WH Freeman; New York: 2013. Kuby Immunology. [Google Scholar]
- 103.Granoff DM, Munson RS., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986;153:448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- 104.Murphy TV, White KE, Pastor P. Declining incidence of Haemophilus influenzae type b since introduction of vaccination. JAMA. 1993;269:246–248. [PubMed] [Google Scholar]
- 105.Heymann DL, Nunn M, editors. Control of Communicable Diseases Manual. 19th ed. American Public Health Association; Washington, DC: 2008. [Google Scholar]
- 106.Reed KD, Melski JW, Graham MB. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 107.Ehresmann KR, Hedberg CW, Grimm MB. An outbreak of measles at an international sporting event with airborne transmission in a domed stadium. J Infect Dis. 1995;171:679–683. doi: 10.1093/infdis/171.3.679. [DOI] [PubMed] [Google Scholar]
- 108.Meselson M, Guillemin J, Hugh-Jones M. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266:1202–1208. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 109.Inglesby TV, O'Toole T, Henderson DA. Anthrax as a biological weapon, 2002. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 110.Inglesby TV, Dennis DT, Henderson DA. Plague as a biological weapon; medical and public health management. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 111.Blachere FM, Lindsey WG, Pearce TA. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2008;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 112.Barbera J, Macintyre A, Gostin L. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286:2711–2717. doi: 10.1001/jama.286.21.2711. [DOI] [PubMed] [Google Scholar]
- 113.Centers for Disease Control and Prevention Use of quarantine to prevent transmission of severe acute respiratory syndrome—Taiwan, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:680–683. [PubMed] [Google Scholar]
- 114.Misrahi JJ, Foster JA, Shaw FE. HHS/CDC legal response to SARS outbreak. Emerg Infect Dis. 2004;10:353–355. doi: 10.3201/eid1002.030721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Centers for Disease Control and Prevention Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: recommendations of the CDC, the Infectious Diseases Society of America, and the American Society of Blood and Marrow Transplantation. MMWR Morb Mortal Wkly Rep. 2000;49(RR-10):1–128. [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention General recommendations on immunizations: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 2011;60(RR02):1–60. [Google Scholar]
- 118.Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases . 3rd revision. U.S. Department of Health and Human Services, Public Health Service; Fort Collins, CO: 2003. Epidemic/epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. [Google Scholar]
- 119.Nash D, Mostashari F, Fine A. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 120.The National Vaccine Advisory Committee The measles epidemic: the problems, barriers, and recommendations. JAMA. 1991;266:1547–1552. [PubMed] [Google Scholar]
- 121.Shalala DE. Giving pediatric immunizations the priority they deserve. JAMA. 1993;269:1844–1845. [PubMed] [Google Scholar]
- 122.Tucker AW, Haddix AC, Bresee JS. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998;279:1371–1376. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 123.Sisk JE, Moskowitz AJ, Whang W. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA. 1997;278:1333–1339. [PubMed] [Google Scholar]
- 124.Fine PE. Herd immunity: History, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 125.Centers for Disease Control Measles prevention: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 1989;38:1–13. [Google Scholar]
- 126.Centers for Disease Control and Prevention Use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2002;51:1024–1026. [PubMed] [Google Scholar]
- 127.Wharton M, Strikas RA, Harpaz R. Recommendations for using smallpox vaccine in a pre-event vaccination program: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Morb Mortal Wkly Rep. 2003;52(RR07):1–16. [PubMed] [Google Scholar]
- 128.Centers for Disease Control and Prevention Public Health Services Task Force recommendations for the use of antiretroviral drugs in pregnant women infected with HIV-1 for maternal health and for reducing perinatal HIV-1 transmission in the United States. MMWR Morb Mortal Wkly Rep. 1998;47(RR-2):1–30. [PubMed] [Google Scholar]
- 129.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-12):1–116. [PubMed] [Google Scholar]
- 130.Centers for Disease Control and Prevention Recommendations for the prevention and management of Chlamydia trachomatis infections, 1993. MMWR Morb Mortal Wkly Rep. 1993;42(RR-12):1–39. [PubMed] [Google Scholar]


