Stress and Immunosuppression
Stress and immunosuppression are often underlying factors for many diseases affecting captive crocodilians. Stress has been defined as “a physiological answer to a perceived threat that includes, but is not restricted to, increased adrenal secretion.”1 Stress is also thought of as any event that challenges homeostasis, and likely the response to that challenge involves more than an adrenal response. The autonomic nervous system, the hypothalamic adrenal axis, neuropeptides, neurotransmitters, and neuroimmunologic mediators all have a role in the response of the immune system to stress.2 Studies in crocodilians have evaluated the stress associated with restraint, long-term corticosterone implants, cold shock, and stocking densities.3, 4, 5, 6 Lance et al. provide an overview of the physiology and endocrinology of stress in crocodilians.7 Catecholamines, glucocorticoids, glucose, and lactate have been implicated in the stress response of crocodilians. In addition, an argument is made for immunosuppression on the basis of changes observed in the white blood cells.3, 4, 5, 7 Factors that influence stress in crocodilian and reptile species are reviewed by Rooney and Guillette.1 Enough evidence exists to suggest that stress plays an important role in the physiology of crocodilians, and it may indeed predispose them to illness. Overcrowding, handling, excessive noise, diet changes, water and air quality, temperature irregularities, and more should all be considered as predisposing or confounding factors of disease.
Systemic Signs
Anorexia, Lethargy, and Acute Mortalities
The first signs of illness in captive crocodilians are usually nonspecific in nature. These include anorexia, lethargy, and death. In commercial operations, the workers may notice excess food remaining from the day before, which is sometimes followed by a perceived change in the behavior of the animals. One should not underestimate these observations because most workers are well tuned to the daily routine and behavior of the animals. A visit to the ranch or farm should be performed during feeding time to avoid additional stress to the animals. This also allows observation of the feeding and water change practices. At this time, a thorough history and a collection of animals for diagnostics and necropsy can be obtained. In addition to routine samples, tissues should be frozen for possible bacterial, fungal, or viral cultures.
The list of differentials for nonspecific clinical signs must be narrowed after diagnostic results are obtained. However, one must remember that husbandry and disease go hand in hand. Most systemic diseases of captive crocodilians are thought to be secondary in nature. Bacterial and fungal diseases are commonly encountered. The majority of data available on bacterial disease in crocodilians are from studies involving the American alligator (Alligator mississippiensis). Table 136.1 presents a large number of isolates from diseased and nondiseased animals, including a survey of fecal coliforms.8 Huchzermeyer et al. also have extensive data on enteric bacterial and fungal isolates from African Dwarf Crocodiles (Osteolaemus tetraspis).9 Important bacterial infections reported in crocodilians include Mycoplasma alligatoris, 10 Mycoplasma crocodyli, 11 and Chlamydia spp.
TABLE 136.1.
Bacteria Isolated From Alligator Mississippiensis With and Without Signs of Disease
| Isolate | Tissue | Clinical Signs/Lesions |
|---|---|---|
| Aeromonas hydrophila | Blood34 | Yes |
| Lungs, heart, liver, kidneys, intestines, oral cavity49 | Yes, No | |
| Lungs, blood50 | Yes | |
| Eye51, 52 | Yes | |
| Oral cavity, water53 | No | |
| Cloaca8 | No | |
| Aeromonas sp. | Lungs10 | Yes |
| Acinetobacter calcoaceticus | Oral cavity53 | No |
| Aerobacter radiobacter | Oral cavity53 | No |
| Bacteroides asaccharolyticus | Oral cavity53 | No |
| Bacteroides bivius | Oral cavity, water53 | No |
| Bacteroides loescheii/denticola | Oral cavity, water53 | No |
| Bacteroides oralis | Oral cavity53 | No |
| Bacteroides sordellii | Oral cavity53 | No |
| Bacteroides thetaiotamicron | Oral cavity53 | No |
| Bacteroides vulgatus | Oral cavity53 | No |
| Bacteroides sp. | Water53 | No |
| Citrobacter freundii | Blood55 | Yes |
| Oral cavity53 | No | |
| cloaca8 | No | |
| Citrobacter braakii | Cloaca8 | No |
| Clostridium bifermentans | Oral cavity, water53 | No |
| Lungs10 | Yes | |
| Clostridium clostridioforme | Oral cavity53 | No |
| Clostridium innoculum | Water53 | No |
| Clostridium limosum | Oral cavity53 | No |
| Clostridium sordellii | Oral cavity, water53 | No |
| Clostridium sporogenes | Blood10 | Yes |
| Clostridium tetani | Oral cavity53 | No |
| Clostridium sp. | Blood10 | Yes |
| Corynebacterium sp. | Tail abscess50 | Yes |
| Diphtheroid sp. | Oral cavity53 | No |
| Edwardsiella tarda | Kidney, feces54 | Yes |
| Fat body, pericardial fluid10 | Yes | |
| Cloaca8 | No | |
| Edwardsiella aerogenes | Cloaca8 | No |
| Enterobacter aggiomerans | Blood55 | Yes |
| Enterobacter cloacae | Oral cavity, water53 | No |
| Cloaca8 | No | |
| Enterobacillus sp. | Lungs50 | Yes |
| Escherichia coli | Cloaca8 | No |
| Fusobacterium nucleatum | Oral cavity53 | No |
| Fusobacterium varium | Oral cavity53 | No |
| Hafnia alvei | Cloaca8 | No |
| Klebsiella oxytoca | Skin55 | Yes |
| Oral cavity53 | No | |
| Klebsiella sp. | Lungs25 | Yes |
| Klebsiella pneumonia | Cloaca8 | No |
| Micrococcus kristinae | Blood34 | Yes |
| Moraxella sp. | Oral cavity, water53 | No |
| Morganella morganii | Blood55 | Yes |
| Oral cavity53 | No | |
| Lung10 | Yes | |
| Cloaca8 | No | |
| Mycoplasma alligatoris | Multiple tissues34 | Yes |
| Pantoea spp. | Cloaca8 | No |
| Pasteurella haemolytica | Oral cavity53 | No |
| Pasteurella multocida | Lungs56 | Yes |
| Pasteurella pneumotropica | Cloaca8 | No |
| Pasteurella sp. | Oral cavity, water53 | No |
| Peptococcus magnus | Oral cavity53 | No |
| Peptococcus prevotii | Oral cavity53 | No |
| Pleisomonas shigelloides | Cloaca8 | No |
| Proteus mirabilis | Blood34 | Yes |
| Proteus vulgaris | Oral cavity53 | No |
| Oviduct54 | Yes | |
| Blood34 | Yes | |
| Lung10 | Yes | |
| Proteus sp. | Blood55 | Yes |
| Providencia alcalifaciens | Cloaca8 | No |
| Providencia rettgeri | Cloaca8 | No |
| Pseudomonas cepacia | Oral cavity53 | No |
| Pseudomonas diminuta | Water53 | No |
| Pseudomonas fluorescens | Water53 | No |
| Pseudomonas pickettii | Oral cavity53 | No |
| Pseudomonas vesicularis | Water53 | No |
| Pseudomonas sp. | Lungs, pharynx50 | Yes |
| Water53 | No | |
| Salmonella typhimurium | Gastrointestinal tract50 | Yes |
| Salmonella braenderup, anatum Arizona spp. | Cloaca50 | No |
| Salmonella sp. (subgroup III) | Lung57 | Yes |
| Serratia marcescens | Skin55 | Yes |
| Cloaca8 | No | |
| Serratia odorifera | Oral cavity53 | No |
| Staphylococcus aureus | Lungs56 | Yes |
| Staphylococcus cohnii | Blood34 | Yes |
| Streptococcus sp., hemolytic | Lungs10 | Yes |
| Vibrio parahaemolyticus | Blood34 | Yes |
| Vibrio cholerae, putative | Cloaca8 | No |
| Vibrio fluvialis | Cloaca8 | No |
Fungal organisms are often opportunistic invaders of the integument and respiratory system, but primary fungal infections are also reported. Viral diseases are likely underdiagnosed in reptile medicine because of the challenges in diagnostic techniques and scarce information. Poxvirus, West Nile virus (WNV), and herpesvirus have been reported as pathogens in crocodilians. An adenovirus-like infection in captive Nile Crocodiles (Crocodylus niloticus) has also been reported.12 Coronavirus, influenza C virus, and paramyxovirus have been identified with transmission electron microscope in the feces of crocodilians, but their significance remains to be determined. Finally, evidence exists of seroconversion to paramyxovirus and eastern equine encephalitis virus in crocodilians.13
Toxicities are not common in captive crocodilians. Lead toxicity is reported from alligators fed lead-shot nutria. Clinical signs include weakness, lethargy, anorexia, and death. Feeding of nutria is no longer common practice.
For privately owned and zoo animals, there are additional differentials to be considered. A faulty or broken heater can lead to sudden death from electrocution. This can also present a risk for humans, therefore, it is important to isolate, protect, and inspect all equipment within the enclosure. A thermostat malfunction can lead to altered water temperatures that can affect the animal's behavior. In colder climates, animals may become lethargic if the heater is not working properly. Conversely, if a thermostat fails, allowing the heater to remain on, the animals’ behavior may change because of increased temperatures. At first they may appear agitated but eventually anorexia and lethargy will occur if overheated. Overheating may also occur as a result of inappropriately sized or placed heat lamps.
Foreign bodies should be a differential for anorexic crocodilians, especially those in outdoor exhibits where trash, coins, and other objects may be tossed in by the public. It is also important to pick up and secure all tools such as buckets and hoses, which may also be ingested by animals.
Physiological anorexia can be observed as part of the normal reproductive cycle, in particular during the later stages of egg development. However, dystocia (egg binding) can lead to a more prolonged anorexia that may be the first indication that the animal is ill. Unless the actual mating date is known or the reproductive cycle is monitored by ultrasonography, it can be very difficult to ascertain what constitutes true dystocia. In animals housed outdoors in their natural climate, dystocia should be considered if oviposition has not occurred for 30 days past the expected date. For those housed indoors or outside their natural climate, behavior together with plasma biochemistry, hematology, and diagnostic imaging can be used to aid in the diagnosis of dystocia.
Secondary nutritional hyperparathyroidism can also cause animals to be lethargic and anorexic. This is more common in juvenile animals. Older animals may succumb to secondary renal hyperparathyroidism.
Poor Growth and Runting
Another phenomenon seen in captive crocodilian operations is runting, the lack of growth and failure to thrive of some animals within a group. In some operations, animals are separated by size and some buildings contain only the runts. A clear size difference is observed in same-age animals between the runts and the otherwise healthy ones. These animals are not as hardy in the captive environment and are potentially more susceptible to disease. Dominance by other animals, environment, and even incubation factors14 contribute to the presence of runts.
Dermatological Signs
Integumentary disease is often secondary to poor water quality, poor enclosure design, stress, and immunosuppression or a combination of these factors. Lacerations, abscesses, and draining tracts can all be observed in captive and wild crocodilians. Consequently, these open wounds can serve as a nidus for bacteria and fungi. An additional factor in commercial operations or small enclosures is the accumulation of a fatty slime layer on the surface of the water when meat supplements, in particular chicken, are fed in addition to a dry commercial diet. This slime attaches to the skin of the animals and the enclosure, creating an environment for bacterial and fungal growth. The same is true for accumulation of biofilm on the surface of the enclosure. A soap or detergent can be used as a surfactant to help reduce the fatty layer on the water surface. Any product used must be nontoxic, or the animals must be removed from the enclosure while in use. Fungal dermatitis is also common because many fungi thrive in the water column and environment of captive alligators. In most instances, both bacteria and fungi are present in skin lesions. Culture and sensitivity testing can be frustrating in these cases because of the mixed flora found in the lesions. The first step in addressing integumentary abnormalities is to analyze water quality and make improvements as needed. In addition to improvements in water quality, antimicrobial or antifungal therapy may be necessary. Medicating a large group of crocodilians is challenging because it must usually be done orally by mixing the medication with feed. In zoos or private collections, individual crocodilians can be medicated by injecting the prey item or via injection. A pole syringe can be used in large or aggressive animals. There is a lack of pharmacokinetic data for oral antimicrobials in crocodilians, and it has been shown that oral tetracycline is not effectively absorbed in American alligators.15 These factors pose a challenge for effective treatment and the potential for promoting antimicrobial resistance. Therefore a combination of good hygiene, water treatments with disinfectants, and improved husbandry is recommended.
A number of parasites are known to affect the skin of crocodilians. Paratrichosoma spp. are capillaroid parasites that cause a zigzag lesion on the skin. These lesions appear to be purely cosmetic. This parasite is known to affect various crocodile species in the wild and is believed to have a stage of its lifecycle dependent on soil; therefore, it is not observed in captive scenarios where animals are kept on concrete.13 Regardless of the etiology, good hygiene is essential in the prevention and treatment of integumentary disease in crocodilians. The etiology itself is at times not as important as what conditions predisposed them to the disease. A herpesvirus was identified via TEM from saltwater crocodiles (Crocodylus porosus) with concurrent pox virus and bacterial infection of the skin.46
Dermatophilosis (Brown Spot Disease)
Dermatophilosis causes brown-red to ulcerative lesions most commonly located between scales on the ventral skin (Fig. 136.1 ). Most of the cultures appear to resemble Dermatophilus congolensis. 16, 17 These filamentous bacteria do not respond well to antibiotic therapy; therefore, intensive hygiene practices are necessary to prevent and control outbreaks.
FIG 136.1.

Brown lesions typical of dermatophilosis on the ventral skin of an American alligator (A. mississippiensis). Lesions usually arise at the junction of the scales and then spread outward.
(Courtesy of Javier G. Nevarez.)
Pox Virus
Parapoxvirus or poxlike viruses have been identified in five different crocodilian species: Spectacled caiman (Caiman crocodilus fuscus),18, 19 Brazilian caiman (Caiman crocodilus yacare),20 Nile crocodile,21, 22 saltwater crocodile (Crocodylus porosus),23 and freshwater crocodile (Crocodylus johnstoni).23 In caimans, poxvirus is characterized by 1- to 3-mm-diameter, gray to white, coalescing to macular skin lesions. These can be found on the head, palpebrae, maxilla, mandible, limbs, palate, tongue, and gingiva.18, 19, 20 Other signs observed include palpebral and generalized edema. Resolution of clinical signs was observed 6 weeks after improvement of husbandry in one case19 and after 5 months in another case, with no changes in husbandry.20
Lesions in crocodiles are described as 2- to 8-mm-diameter, yellow to brown, wartlike, sometimes firm, and unraised to raised nodules with occasional shallow ulcers. These lesions can be found on the head, palpebrae, nostrils, sides of the mouth, oral cavity, limbs, ventral neck, and coelom and at the root of the tail.21, 22 Resolution of lesions was reported to occur as early as 3 to 4 weeks.21 Light microscopy reveals epithelial hyperplasia, acanthosis, hyperkeratosis, and necrosis, and at times Borrel and Bollinger's bodies are also visible.18, 19, 20, 21, 22 Secondary bacterial and fungal infections may also be present. Horner reported the use of an autogenous vaccine to treat poxvirus in Nile crocodiles.21 No specific treatment recommendations exist. Maintaining appropriate husbandry is essential in the prevention and resolution of poxvirus in crocodilian farms.
West Nile Virus
West Nile virus (WNV) has been reported to cause lymphohistiocytic proliferative syndrome of alligators (LPSA).24, 25 LPSA skin lesions are a chronic manifestation of WNV infection and occur in animals that have survived a WNV outbreak.25 This production problem affects the quality of the hide and consequently decreases profit for alligator ranchers. Gross lesions can be seen as multifocal, 1 mm to 2 mm, gray to red foci on the ventral mandibular, abdominal, and sometimes tail scales (Fig. 136.2 ). They can be found on any section of a scale and do not appear concave or convex with respect to the scale's surface. Routine microscopic examination reveals dermal nodular lymphoid proliferation with perivascular cuffing. Similar lesions can be found in other tissues besides the skin. Identification of the lesions is complicated by the fact they are sometimes not seen until the hides are removed from the animal. Therefore antemortem identification alone is not very accurate.
FIG 136.2.

Gross lesions (arrows) of lymphohistiocytic proliferative syndrome of alligators on the ventral skin of an American alligator (A. mississippiensis). Lesions appear in animals that survived WNV infection. There are over 50 lesions in this image.
(Courtesy of Javier G. Nevarez.)
Neurological Signs
Neurological deficits are not commonly seen in crocodilians and deserve special attention if encountered. Clinical signs may include swimming in circles or on one side of the body, abnormal flotation, lethargy, ataxia, head tilt, and muscle tremors. Anorexia often accompanies the neurological signs. Thiamine deficiencies should be considered in animals fed a frozen fish diet. Signs of thiamine deficiency typically include severe lethargy that leads to coma.
Hypoglycemia has also been reported as a cause of neurological signs in alligators.26 These animals have muscle tremors, loss of the righting reflex, and mydriasis. Stress seems to be the main contributing factor. The author has also observed neurological signs associated with low oxygen levels inside alligator enclosures. Neurological signs may also be observed after ingestion of metal foreign bodies, including coins, containing lead or zinc.
West Nile Virus
West Nile virus causes neurological signs in captive-reared American alligators raised indoors.27, 28 Presence of antibodies, but no clinical signs, is reported from free-ranging and captive crocodilians housed outdoors.29, 30 WNV in crocodilians is transmitted by mosquitoes and horizontally via fecal shedding. This represents an opportunity for zoonosis in captive operations. Strict building quarantine and hygiene strategies should be implemented to prevent spread to other animals in the facility. WNV is a reportable disease, and one should contact the state veterinarian when diagnosis is confirmed.
WNV can affect an animal of any age, but hatchlings often experience severe per-acute mortalities of up to 50% to 60%.28 WNV has two disease stages in alligators, acute and chronic. Clinical signs during the acute stage include swimming in circles, head tilt (Fig. 136.3 ), muscle tremors, weakness, lethargy, anorexia, and death. Animals that survive the acute infection go on to develop LPSA skin lesions, the chronic stage of WNV. There is no treatment. Definitive diagnosis is accomplished with reverse transcriptase polymerase chain reaction (RT-PCR) of liver and brain. However, it must be noted that RT-PCR can detect viral RNA from a commercially available WNV vaccine for alligators (BIVI, St. Joseph, MO). Therefore vaccine status must be known before diagnosis. Maternal transfer of antibodies has also been observed in hatchlings.31 Veterinarians should take additional precautions during necropsies of suspect animals. Aggressive mosquito control and vaccination have proven extremely effective at controlling WNV infection in commercial operations.
FIG 136.3.

Young alligator (Alligator mississippiensis) with the head tilt typical of those affected with West Nile virus. Notice that it still maintains an aggressive stand with the mouth open.
Respiratory Signs
This is one of the most common presentations of captive crocodilians, second only to integumentary disease. Respiratory signs can often be confused with neurological signs, and a clear distinction must be established. However, respiratory disease may accompany neurological disease as a consequence of weakness leading to aspiration. Clinical signs associated with respiratory disease in crocodilians may include dyspnea, tachypnea, nasal discharge, excessive basking, abnormal swimming (either in circles or on one side of the body), and anorexia, among others. As the animals become weak, the basihyoid valve does not function properly and they can aspirate food or water. The pharyngeal anatomy of crocodilians makes aspiration unlikely in healthy animals. Therefore, if aspiration is suspected, evaluation for an underlying illness should be pursued. A number of rhinitis and pharyngitis syndromes have also been described in some crocodilian species.13 Most respiratory infections are either bacterial or fungal in origin. An early report showed a fungal pneumonia associated with Beauveria bassiana in two alligators,32 and Fusarium moniliforme was found in another case.33
Mycoplasmosis
One well-documented respiratory pathogen is Mycoplasma alligatoris. Clinical signs are nonspecific and include lethargy, weakness, anorexia, white ocular discharge, paresis, and edema (facial, periocular, cervical, limbs).10 Pneumonia, pericarditis, and polyarthritis are often diagnosed on necropsy. Pathogenicity of M. alligatoris has been documented for A. mississippiensis and for the broad-nosed caiman (Caiman latirostris).34, 35 Other crocodilian species closely related to alligators are also potentially susceptible. Helmick et al. reviewed antimicrobial susceptibility for M. alligatoris. 36 A second Mycoplasma species, M. crocodyli, is known to affect Nile crocodiles.11 Lesions are similar to those observed with M. alligatoris. Some studies have examined the use of an autogenous vaccine for M. crocodyli, but work is still needed to determine its true efficacy.37, 38 Currently antemortem diagnosis is difficult and requires a combination of serology and tissue biopsy for PCR. Therefore most cases are confirmed via histopathology and PCR postmortem.
Mycobacteriosis
Although not commonly reported from crocodilians, the author has worked on a number of alligator cases with acid-fast organisms consistent with Mycobacterium sp. in the lungs. These animals had evidence of pneumonia on necropsy as shown by multiple white foci, 1 to 4 mm in diameter, on the lung parenchyma. Other cases of pulmonary and enteric mycobacterial infections have been suggested.39 Difficulties of growing Mycobacterium sp. make its definitive diagnosis a challenge.
Musculoskeletal Signs
Musculoskeletal disease can be the result of alterations in the incubation temperature or environment (Fig. 136.4 ) or trauma from fighting (Figs. 136.5 ), transport, restraint, neoplasia, or secondary to nutritional disease. Limb fractures and partial amputations can be seen after altercations. It is possible for animals that have lost limbs after a fight to survive to the point of complete healing (Fig. 136.6 ). These lesions however may also lead to nerve or muscle damage and consequent paresis or paralysis. As mentioned in the respiratory signs section, M. alligatoris and M. crocodyli cause polyarthritis, which can be evident antemortem.
FIG 136.4.

Congenital abnormality in an American alligator (Alligator mississippiensis). This animal was hatched with this deformity and is able to eat and thrive among others in the group.
FIG 136.5.

Mandibular fracture in a wild alligator (A. mississippiensis). This type of lesion is not uncommon in wild animals, particulary in males during breeding season.
FIG 136.6.

(A) Rear limb open fracture with tissue necrosis in a wild alligator (A. mississippiensis). Wild animals can recover from these types of lesions, but they can lead to severe septicemia in captive ones. (B) Wild alligator with a healed lesion that resulted in amputation of the distal rear limb.
Nutritional secondary hyperparathyroidism can occur in young crocodilians not being fed whole prey or pelleted diets. Affected animals may also be runts of the group and have concurrent disease. The innate requirement of ultraviolet B light for the production of vitamin D3 in crocodilians is not known. They appear to be able to obtain appropriate levels from their diet, but further research in this area is needed.
Gout also occurs in crocodilians (Figs. 136.7A and B ). Clinical signs can be nonspecific in nature, but limb paresis/paralysis and joint enlargement may be observed. A case of gout with concurrent suspected hypovitaminosis A in crocodile hatchlings has been reported.40
FIG 136.7.


(A and B) Renal and visceral gout in an American alligator (A. mississippiensis). Oversupplementation with a vitamin and mineral mix was suspected in this case.
(Courtesy of Javier G. Nevarez.)
Deficiencies of vitamins A, B, C, or E can also lead to a variety of musculoskeletal disorders. These are not commonly seen when commercial diets are fed in addition to meat products.
Gastrointestinal Signs
Anorexia is likely the most common clinical sign associated with gastrointestinal disease in captive crocodilians. Foreign body ingestion, gastric ulcers, enteritis (Fig. 136.8 ), and trauma to the oral cavity can be observed in crocodilians. Ingestion of foreign bodies is more common in wild crocodilians. However, it must also be a differential in captive specimens. Malfunction of water pumps, water filters, or construction can lead to the presence of foreign bodies in the enclosures. In outdoor exhibits, the public is often responsible for the presence of foreign objects that are thrown into their enclosures. These can then be ingested by the animals and cause severe problems in the case of nails and other sharp objects (Fig. 136.9 ).
FIG 136.8.
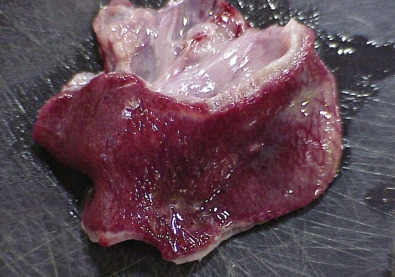
Enteritis in a captive-reared alligator (A. mississippiensis). Notice the red mucosa.
FIG 136.9.

Farmed alligator (A. mississippiensis) with a nail foreign body that was ingested and perforated its stomach.
(Courtesy of Javier G. Nevarez.)
Infectious enteritis may be difficult to assess grossly because crocodilians have very thick intestinal walls normally. However, crocodilians appear to have a characteristic response to insult of the gastrointestinal tract. Accumulations of fibrous material or necrotizing lesions are commonly seen on necropsy (Fig. 136.10A and B ). This reaction is aggressive and can even lead to obstructions caused by the fibrous material. Obstruction can also occur with fecal impactions and torsions. Gastric ulcerations and abnormalities of the gastric mucosa are also routinely observed on necropsy and may be associated with stress and diet. Finally, the intestinal tract contains a large amount of Peyer's patches and likely represents a site of aggressive inflammatory response to infectious agents. A novel herpesvirus was reported from an American alligator with lymphoid follicular inflammation of the cloaca.45
FIG 136.10.

(A and B) Fibrotic membrane on the intestinal mucosa of a captive-reared alligator (A. mississippiensis). This was an incidental finding for this animal.
Ocular Signs
Chlamydia
Chlamydiosis has been reported from C. niloticus (South Africa and Zimbabwe), C. porosus (Papua New Guinea), and C. siamensis (Thailand).41, 42, 43 The author has also diagnosed Chlamydiosis in A. mississippiensis (United States). Although it has been proposed as a Chlamydophila psittaci-like strain in C. niloticus,44 the Chlamydia species affecting crocodilians is likely a novel specie(s). Infection usually leads to hepatitis and/or conjunctivitis (Fig. 136.11 ), but lung and spleen can also be infected. Morbidity and mortality can exceed 50%, resulting in significant economic losses for farmed animals.42 Treatment with oxytetracycline is anecdotally reported to be effective in crocodiles. This creates significant concerns for possible antimicrobial resistance, zoonosis, and the safety of the meat from these animals.
FIG 136.11.
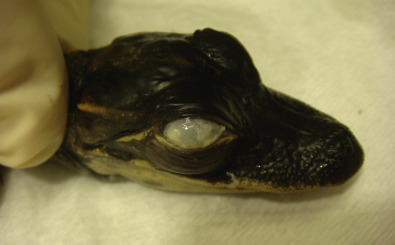
Keratitis and conjunctivitis because of chlamydiosis in a hatchling American alligator (A. mississippiensis).
(Courtesy of Javier G. Nevarez.)
Miscellaneous
A novel herpesvirus was reported from an American alligator with lymphoid follicular inflammation of the cloaca.45 A herpesvirus was identified via TEM from saltwater crocodiles (Crocodylus porosus) with concurrent pox virus and bacterial infection of the skin.46 In captive C. porosus and freshwater crocodiles (C. johnstoni) from Australia, three novel herpesviruses have been identfied.47 C. porosus exhibited conjunctivitis-pharyngitis, systemic lymphoid proliferation and encephalitis, or lymphonodular skin lesions.47, 48 C. johnstoni had systemic lymphoid proliferation.47 Chlamydiaceae was also identified from conjunctival and pharyngeal tissues of some of the C. porosus cases.48 Of interest is that these syndromes occur concurrently with other infectious agents and have been associated with significant morbidity and mortality of infected animals.
References
See www.expertconsult.com for a complete list of references.
References
- 1.Rooney AA, Guillette LJJ. Biotic and abiotic factors in crocodilian stress: the challenge of a modern environment. In: Grigg GC, Seebacher F, Franklin CE, editors. Crocodilian Biology and Evolution. Surrey Beatty and Sons; Chipping Norton: 2001. pp. 214–228. [Google Scholar]
- 2.Dohms JE, Metz A. Stress-mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991;30:89–109. doi: 10.1016/0165-2427(91)90011-z. [DOI] [PubMed] [Google Scholar]
- 3.Lance VA, Elsey RM. Plasma catecholamines and plasma corticosterone following restraint stress in juvenile alligators. J Exp Zool. 1999;283(6):559–565. doi: 10.1002/(sici)1097-010x(19990501)283:6<559::aid-jez7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Morici LA, Elsey RM, Lance VA. Effects of long-term corticosterone implants on growth and immune function in juvenile alligators, Alligator mississippiensis. J Exp Zool. 1997;279(2):156–162. [PubMed] [Google Scholar]
- 5.Lance VA, Elsey RM. Hormonal and metabolic response of juvenile alligators to cold shock. J Exp Zool. 1999;283:566–572. doi: 10.1002/(sici)1097-010x(19990501)283:6<559::aid-jez7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Elsey RM, Joanen T, McNease L. Growth rate and plasma corticosterone levels in juvenile alligators maintained at different stocking densities. J Exp Zool. 1990;255:30–36. [Google Scholar]
- 7.Lance VA, Morici LA, Elsey RM. Physiology and endocrinology of stress in crocodilians. In: Grigg GC, Seebacher F, Franklin CE, editors. Crocodilian Biology and Evolution. Surrey Beatty and Sons; Chipping Norton: 2001. [Google Scholar]
- 8.Johnston MA, Porter DE, Scott GI. Isolation of faecal coliform bacteria from the American alligator (Alligator mississippiensis) J Appl Microbiol. 2009;108:965–973. doi: 10.1111/j.1365-2672.2009.04498.x. [DOI] [PubMed] [Google Scholar]
- 9.Huchzermeyer FW, Henton MM, Riley J. Aerobic intestinal flora of wild-caught African dwarf crocodiles Osteolaemus tetraspis. Onderstepoort J Vet Res. 2000;67(3):201–204. [PubMed] [Google Scholar]
- 10.Clippinger TL, Bennett RA, Johnson CM. Morbidity and mortality associated with a new Mycoplasma species from captive American alligators (Alligator mississippiensis) J Zoo Wildl Med. 2000;31(3):303–314. doi: 10.1638/1042-7260(2000)031[0303:MAMAWA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Mohan K, Foggin CM, Muvavarirwa P. Mycoplasma-associated polyarthritis in farmed crocodiles (Crocodylus niloticus) in Zimbabwe. Onderstepoort J Vet Res. 1995;62(1):45–49. [PubMed] [Google Scholar]
- 12.Jacobson ER, Gardiner CH, Foggin CM. Adenovirus-like infection in two Nile crocodiles. J Am Vet Med Assoc. 1984;185(11):1421–1422. [PubMed] [Google Scholar]
- 13.Huchzermeyer FW. CABI Publishing; Cambridge: 2003. Crocodiles: Biology, Husbandry and Diseases. [Google Scholar]
- 14.Allsteadt J, Lang JW. Incubation temperature affects body size and energy reserves of hatchling American Alligators (Alligator mississippiensis) Physiol Zool. 1995;68(1):76–97. [Google Scholar]
- 15.Rivera S, Nevarez JG, Maxwell LK. Pharmacokinetics of tetracycline after single-dose oral administration in the American alligator (Alligator mississippiensis) J Zoo Wildl Med. 2012;43(4):858–863. doi: 10.1638/2012-0092R2.1. [DOI] [PubMed] [Google Scholar]
- 16.Buenviaje GN, Hirst RG, Ladds PW. Isolation of Dermatophilus sp. from skin lesions in farmed saltwater crocodiles (Crocodylus porosus) Aust Vet J. 1997;75(5):365–367. doi: 10.1111/j.1751-0813.1997.tb15720.x. [DOI] [PubMed] [Google Scholar]
- 17.Buenviaje GN, Ladds PW, Martin Y. Pathology of skin diseases in crocodiles. Aust Vet J. 1998;76(5):357–363. doi: 10.1111/j.1751-0813.1998.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson ER, Popp JA, Shields RP. Pox like skin lesions in captive caimans. J Am Vet Med Assoc. 1979;175(9):937–940. [PubMed] [Google Scholar]
- 19.Penrith ML, Nesbit JW, Huchzermeyer FW. Pox virus infection in captive juvenile caimans (Caiman crocodilus fuscus) in South Africa. J S Afr Vet Assoc. 1991;62(3):137–139. [PubMed] [Google Scholar]
- 20.Ramos MC, Coutinho SD, Matushima ER. Poxvirus dermatitis outbreak in farmed Brazilian caimans (Caiman crocodilus yacare) Aust Vet J. 2002;80(6):371–372. doi: 10.1111/j.1751-0813.2002.tb14792.x. [DOI] [PubMed] [Google Scholar]
- 21.Horner RF. Poxvirus in farmed Nile crocodiles. Vet Rec. 1990;122(19):459–462. doi: 10.1136/vr.122.19.459. [DOI] [PubMed] [Google Scholar]
- 22.Pandey GS, Inoue N, Ohshima K. Poxvirus infection in Nile crocodiles (Crocodylus niloticus) Res Vet Sci. 1990;49(2):171–176. [PubMed] [Google Scholar]
- 23.Buenviaje GN, Ladds PW, Melville L. Poxvirus infection in two crocodiles. Aust Vet J. 1992;69(1):15–16. doi: 10.1111/j.1751-0813.1992.tb09857.x. [DOI] [PubMed] [Google Scholar]
- 24.Nevarez JG, Mitchell MA, Johnson AJ. Establishing an association between West Nile virus exposure and the development of lymphohistiocytic proliferative syndrome of American alligators, Alligator mississippiensis. J Herp Med Surg. 2007;17(1):4–7. [Google Scholar]
- 25.Nevarez JG, Mitchell MA, Morgan T. Association of West Nile virus with lymphohistiocytic proliferative cutaneous lesions in American alligators (Alligator mississippiensis) detected by RT-PCR. J Zoo Wildl Med. 2008;39(4):562–566. doi: 10.1638/2007-0133.1. [DOI] [PubMed] [Google Scholar]
- 26.Wallach JD, Hoessle C, Bennett J. Hypoglycemic shock in captive alligators. J Am Vet Med Assoc. 1967;151(7):893–896. [PubMed] [Google Scholar]
- 27.Miller DL, Mauel MJ, Baldwin C. West Nile virus in farmed alligators. Emerg Infect Dis. 2003;9(7):794–799. doi: 10.3201/eid0907.030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevarez JG, Mitchell MA, Kim DY. West Nile virus in alligator ranches from Louisiana. J Herp Med Surg. 2005;15(3):4–9. [Google Scholar]
- 29.Steinman A, Banet-Noah C, Tal S. West Nile virus infection in crocodiles. Emerg Infect Dis. 2003;9(7):887–889. doi: 10.3201/eid0907.020816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loza-Rubio E, Rojas-Anaya E, López-Ramírez C. Prevalence of neutralizing antibodies against West Nile virus (WNV) in monkeys (Ateles geoffroyi and Alouatta pigra) and crocodiles (Crocodylus acutus and C. acutus-C. moreletti hybrids) in Mexico. Epidemiol Infect. 2016;21:1–3. doi: 10.1017/S0950268816000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nevarez JG. 2015. WNV vaccination of alligators, unpublished data. [Google Scholar]
- 32.Fromtling RA, Kosanke SD, Jensen JM. Fatal Beauveria bassiana infection in a captive American alligator. J Am Vet Med Assoc. 1979;175(9):934–936. [PubMed] [Google Scholar]
- 33.Frelier PF, Sigler L, Nelson PE. Mycotic pneumonia caused by Fusarium moniliforme in an alligator. Sabouraudia. 1985;23(6):399–402. [PubMed] [Google Scholar]
- 34.Brown DR, Nogueira MF, Schoeb TR. Pathology of experimental mycoplasmosis in American alligators. J Wildl Dis. 2001;37(4):671–679. doi: 10.7589/0090-3558-37.4.671. [DOI] [PubMed] [Google Scholar]
- 35.Pye GW, Brown DR, Nogueira MF. Experimental inoculation of Broad-Nosed Caimans (Caiman latirostris) and Siamese Crocodiles (Crocodylus siamensis) with Mycoplasma alligatoris. J Zoo Wildl Med. 2001;32(2):196–201. doi: 10.1638/1042-7260(2001)032[0196:EIOBNC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Helmick KE, Brown DR, Jacobson ER. In vitro susceptibility pattern of Mycoplasma alligatoris from symptomatic American Alligators (Alligator mississippiensis) J Zoo Wildl Med. 2002;33(2):108–111. doi: 10.1638/1042-7260(2002)033[0108:IVDSPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Mohan K, Foggin CM, Muvavarirwa P. Vaccination of farmed crocodiles (Crocodylus niloticus) against Mycoplasma crocodyli infection. Vet Rec. 1997;141(18):476. doi: 10.1136/vr.141.18.476. [DOI] [PubMed] [Google Scholar]
- 38.Mohan K, Foggin CM, Dziva F. Vaccination to control an outbreak of Mycoplasma crocodyli infection. Onderstepoort J Vet Res. 2001;68(2):149–150. [PubMed] [Google Scholar]
- 39.Youngprapakorn P, Ousavaplangchai L, Kanchanapangka S. Style Creative House; Thailand: 1994. A Color Atlas of Diseases of the Crocodile. [Google Scholar]
- 40.Ariel E, Ladds PW, Buenviaje GN. Concurrent gout and suspected hypovitaminosis A in crocodile hatchlings. Aust Vet J. 1997;75(4):247–249. doi: 10.1111/j.1751-0813.1997.tb10089.x. [DOI] [PubMed] [Google Scholar]
- 41.Huchzermeyer FW, Gerdes GH, Foggin CM. Hepatitis in farmed hatchling Nile crocodiles (Crocodylus niloticus) due to chlamydial infection. J S Afr Vet Assoc. 1994;65(1):20–22. [PubMed] [Google Scholar]
- 42.Huchzermeyer FW, Langelet E, Putterill JF. An outbreak of chlamydiosis in farmed Indopacific crocodiles (Crocodylus porosus) J S Afr Vet Assoc. 2008;79(2):99–100. doi: 10.4102/jsava.v79i2.253. [DOI] [PubMed] [Google Scholar]
- 43.Sariya L, Kladmanee K, Bhusri B. Molecular evidence for genetic distinctions between Chlamydiaceae detected in Siamese crocodiles (Crocodylus siamensis) and known Chlamydiaceae species. Jpn J Vet Res. 2015;63(1):5–14. [PubMed] [Google Scholar]
- 44.Mohan K, Dziva F, Mukarati NL. Possible new Chlamydophila species causing chlamydiosis in farmed Nile crocodiles (Crocodylus niloticus) Vet Rec. 2005;157:23–25. doi: 10.1136/vr.157.1.23. [DOI] [PubMed] [Google Scholar]
- 45.Govett PD, Harms CA, Johnson AJ. Lymphoid follicular cloacal inflammation associated with a novel herpesvirus in juvenile alligators (Alligator mississippiensis) J Vet Diagn Invest. 2005;14:474–479. doi: 10.1177/104063870501700513. [DOI] [PubMed] [Google Scholar]
- 46.McCowan C, Shepherdley C, Slocombe RF. Herpesvirus-like particles in the skin of a saltwater crocodile (Crocodylus porosus) Aust Vet J. 2004;82:375–377. doi: 10.1111/j.1751-0813.2004.tb11109.x. [DOI] [PubMed] [Google Scholar]
- 47.Hyndman TH, Shilton CM, Wellehan JFX. Molecular identification of three novel herpesviruses found in Australian farmed saltwater crocodiles (Crocodylus porosus) and Australian fresh water crocodiles (Crocodylus johnstoni) Vet Microbiol. 2015;18:183–189. doi: 10.1016/j.vetmic.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Hilton CM, Jerrett IV, Davis S. Diagnostic investigation of new disease syndromes in farmed Australian saltwater crocodiles (Crocodylus porosus) reveals association with herpesviral infection. J Vet Diagn Invest. 2016;28(3):279–290. doi: 10.1177/1040638716642268. [DOI] [PubMed] [Google Scholar]
- 49.Gorden RW, Hazen TC, Esch GW. Isolation of Aeromonas hydrophila from the American alligator, Alligator mississippiensis. J Wildl Dis. 1979;15(2):239–243. doi: 10.7589/0090-3558-15.2.239. [DOI] [PubMed] [Google Scholar]
- 50.Shotts EB, Jr, Gaines JL, Jr, Martin L. Aeromonas-induced deaths among fish and reptiles in an eutrophic inland lake. J Am Vet Med Assoc. 1972;161(6):603–607. [PubMed] [Google Scholar]
- 51.Millichamp NJ, Jacobson ER, Wolf ED. Diseases of the eye and ocular adnexae in reptiles. J Am Vet Med Assoc. 1983;183(11):1205–1212. [PubMed] [Google Scholar]
- 52.Jacobson ER. Immobilization, blood sampling, necropsy techniques and diseases of crocodilians: a review. J Zoo Anim Med. 1984;15:38–45. [Google Scholar]
- 53.Flandry F, Lisecki EJ, Domingue GJ. Initial antibiotic therapy for alligator bites: characterization of the oral flora of Alligator mississippiensis. South Med J. 1989;82(2):262–266. doi: 10.1097/00007611-198902000-00027. [DOI] [PubMed] [Google Scholar]
- 54.Wallace LJ, Gore WF. Isolation of Edwardsiella tarda from a sea lion and two alligators. J Am Vet Med Assoc. 1966;149(7):881–883. [PubMed] [Google Scholar]
- 55.Novak SS, Seigel RA. Gram-negative septicemia in American alligators (Alligator mississippiensis) J Wildl Dis. 1986;22(4):484–487. doi: 10.7589/0090-3558-22.4.484. [DOI] [PubMed] [Google Scholar]
- 56.Jacobson ER. Bacterial diseases of reptiles. In: Jacobson ER, editor. Infectious Diseases and Pathology of Reptiles. CRC Press; Boca Raton: 2007. pp. 461–526. [Google Scholar]
- 57.Brown DR, Farley JM, Zacher LA. Mycoplasma alligatoris sp. nov., from American alligators. Int J Syst Evol Microbiol. 2001;51(Pt 2):419–424. doi: 10.1099/00207713-51-2-419. [DOI] [PubMed] [Google Scholar]


