Introduction
Cough is an important defense mechanism of the lungs and is a common symptom, particularly during winter months. In most patients, it is self-limited. However, cough can be ominous, indicating serious underlying disease, because of accompanying problems (hemoptysis) or because of serious consequences of the cough itself (e.g., syncope and hemorrhage).
(See Nelson Textbook of Pediatrics, p. 2027)
 Pathophysiology
Pathophysiology
The cough reflex serves to prevent the entry of harmful substances into the tracheobronchial tree and to expel excess secretions and retained material from the tracheobronchial tree. Cough begins with stimulation of cough receptors, located in the upper and lower airways, and in many other sites such as the ear canal, tympanic membrane, sinuses, nose, pericardium, pleura, and diaphragm. Receptors send messages via vagal, phrenic, glossopharyngeal, or trigeminal nerves to the “cough center,” which is in the medulla. Because cough is not only an involuntary reflex activity but also one that can be initiated or suppressed voluntarily, “higher centers” must also be involved in the afferent limb of the responsible pathway. The neural impulses go from the medulla to the appropriate efferent pathways to the larynx, tracheobronchial tree, and expiratory muscles.
The act of coughing (Fig. 2.1 ) begins with an inspiration, followed by expiration against a closed glottis (compressive phase), resulting in the buildup of impressive intrathoracic pressures (50-300 cm H2O). These pressures may be transmitted to vascular, cerebrospinal, and intraocular spaces. Finally, the glottis opens, allowing for explosive expiratory airflow (300 m/sec) and expulsion of mucus, particularly from the larger, central airways. The inability to seal the upper airway (tracheostomy) impairs, but does not abolish, the effectiveness of cough. Weak ventilatory muscles (muscular dystrophy) impair both the inspiratory and the compressive phase.
FIGURE 2.1.

Cough mechanics, showing changes in expiratory flow rate, air volume, subglottic pressure, and sound recording during cough.
(Modified from Yanagihara N, et al. The physical parameters of cough: the larynx in a normal single cough. Acta Otolaryngol. 1996;61: 495-510.)
 History
History
The patient history often provides the most important body of information about a child's cough. A diagnosis can often be discerned with relative certainty from the family history, the environmental and exposure history, and the acute nature and characterization of the cough.
Demographics
The patient's age (Table 2.1 ) helps to focus the diagnostic possibilities. Congenital anatomic abnormalities may be symptomatic from birth, whereas toddlers, who may have incomplete neurologic control over swallowing and often put small objects in their mouths, are at risk for foreign body aspiration; adolescents may experiment with smoking or inhaled drugs. Socioeconomic factors must be considered; a family that cannot afford central heating may use a smoky wood-burning stove; spending time at a daycare center may expose an infant to respiratory viruses; and several adult smokers in a small home expose children to a high concentration of respiratory irritants.
TABLE 2.1.
Causes of Cough
| Age Group | Acute | Recurrent | Chronic (>4 wk) |
|---|---|---|---|
| Infants | Infection1* Aspiration2 Foreign body3 |
Asthma1 CF1 GER1 Aspiration2 Anatomic abnormality3† Passive smoking3 |
Asthma1 CF1 GER1 Aspiration2 Pertussis2 Anatomic abnormality3† Passive smoking3 |
| Toddlers | Infection1 Foreign body2 Aspiration3 |
Asthma1 CF1 GER1 Aspiration2 Anatomic abnormality3 Passive smoking3 |
Asthma1 CF1 GER1 Aspiration2 Pertussis2 Anatomic abnormality3 Passive smoking3 |
| Children | Infection1 Foreign body3 |
Asthma1 CF1 GER1 Passive smoking3 |
Asthma1 CF1 GER2 Pertussis2 Mycoplasma3 Psychogenic3 Anatomic abnormality3 Passive smoking3 |
| Adolescents | Infection1 | Asthma1 CF1 GER1 Aspiration2 Anatomic abnormality3 |
Asthma1 CF1 GER2 Smoking2 Tuberculosis3 Psychogenic2 Pertussis3 Aspiration3 Anatomic abnormality3 Tumor3 |
CF, cystic fibrosis; GER, gastroesophageal reflux.
Infections include upper (pharyngitis, sinusitis, tracheitis, rhinitis, otitis) and lower (pneumonia, abscess, empyema) respiratory tract disease.
Anatomic abnormality includes tracheobronchomalacia, tracheoesophageal fistula, vascular ring, abnormal position or take-off of large bronchi.
Common;
less common;
much less common.
Characteristics of the Cough
The various cough characteristics can help determine the cause of cough. The causes of acute, recurrent, and chronic coughs may be quite different from each other (Fig. 2.2 ; see also Table 2.1). A cough can be paroxysmal, brassy, productive, weak, volitional, and “throat-clearing,” and it may occur at different times of the day (Tables 2.2 and 2.3 ).
FIGURE 2.2.

Algorithm for differential diagnosis of cough. HIV, human immunodeficiency virus.
TABLE 2.2.
Clinical Clues About Cough
| Characteristic | Think of |
|---|---|
| Staccato, paroxysmal | Pertussis, cystic fibrosis, foreign body, Chlamydia species, Mycoplasma species |
| Followed by “whoop” | Pertussis |
| All day, never during sleep | Psychogenic, habit |
| Barking, brassy | Croup, psychogenic, tracheomalacia, tracheitis, epiglottitis |
| Hoarseness | Laryngeal involvement (croup, recurrent laryngeal nerve involvement) |
| Abrupt onset | Foreign body, pulmonary embolism |
| Follows exercise | Asthma |
| Accompanies eating, drinking | Aspiration, gastroesophageal reflux, tracheoesophageal fistula |
| Throat clearing | Postnasal drip |
| Productive (sputum) | Infection |
| Night cough | Sinusitis, asthma |
| Seasonal | Allergic rhinitis, asthma |
| Immunosuppressed patient | Bacterial pneumonia, Pneumocystis jiroveci, Mycobacterium tuberculosis, Mycobacterium avium–intracellulare, cytomegalovirus |
| Dyspnea | Hypoxia, hypercarbia |
| Animal exposure | Chlamydia psittaci (birds), Yersinia pestis (rodents), Francisella tularensis (rabbits), Q fever (sheep, cattle), hantavirus (rodents), histoplasmosis (pigeons) |
| Geographic | Histoplasmosis (Mississippi, Missouri, Ohio River Valley), coccidioidomycosis (Southwest), blastomycosis (North and Midwest) |
| Workdays with clearing on days off | Occupational exposure |
TABLE 2.3.
Cough: Some Aspects of Differential Diagnosis
| Cause | Abrupt Onset | Only When Awake | Yellow Sputum | Responds to Inhaled Bronchodilator (by History) | Responds to Antibiotics (by History) | Responds to Steroids (by History) | Failure to Thrive | Wheeze | Digital Clubbing |
|---|---|---|---|---|---|---|---|---|---|
| Asthma | + | ++ | ++ | +++ | + | +++ | + | +++ | – |
| Cystic fibrosis | + | ++ | ++ | + | +++ | + | ++ | ++ | +++ |
| Infection | + | + | ++ | – | ++ | – | + | + | – |
| Aspiration | + | + | + | + | + | + | ++ | ++ | + |
| Gastroesophageal reflux | + | ++ | – | – | – | + | ++ | ++ | – |
| Foreign body | +++ | + | ++ | + | ++ | + | + | ++ | + |
| Habit | – | +++ | – | – | – | – | – | – | – |
+++, very common and suggests the diagnosis; ++, common; +, uncommon; –, almost never and makes examiner question the diagnosis.
The previous response or lack of response to some therapies for recurrent and chronic cough can provide important information (see Table 2.3). Furthermore, some coughs may be caused or worsened by medications (Table 2.4 ).
TABLE 2.4.
Drugs Causing Cough
| Drug | Mechanism |
|---|---|
| Tobacco, marijuana | Direct irritants |
| β-Adrenergic blockers | Potentiate asthma |
| ACE inhibitors | (?) Possibly potentiate asthma |
| Bethanechol | Potentiates asthma |
| Nitrofurantoin | (?) Via oxygen radicals vs via autoimmunity |
| Antineoplastic agents | Various (including pneumonitis/fibrosis, hypersensitivity, noncardiogenic pulmonary edema) |
| Sulfasalazine | (?) Causes bronchiolitis obliterans |
| Penicillamine | (?) Causes bronchiolitis obliterans |
| Diphenylhydantoin | Hypersensitivity pneumonitis |
| Gold | (?) Causes interstitial fibrosis |
| Aspirin, NSAIDs | Potentiate asthma |
| Nebulized antibiotics | (?) Direct irritant |
| Inhaled/nebulized bronchodilators | Increases tracheal/bronchial wall instability in airway malacia; or via reaction to vehicle |
| Theophylline, caffeine | Indirect, via worsened gastroesophageal reflux (relaxation of lower esophageal sphincter) |
| Metabisulfite | Induces allergic asthma |
| Cholinesterase inhibitors | Induce mucus production (bronchorrhea) |
ACE, angiotensin-converting enzyme; NSAIDs, nonsteroidal anti-inflammatory drugs.
Associated Symptoms
A history of accompanying signs or symptoms, whether localized to the respiratory tract (wheeze, stridor) or elsewhere (failure to thrive, frequent malodorous stools) can give important clues (Table 2.5 ; see also Tables 2.2 and 2.3). It is essential to remember that the daily language of the physician is full of jargon that may be adopted by parents but with a different meaning from that understood by physicians. If a parent says that a child “wheezes” or “croups” or is “short of breath,” it is important to find out what the parent means by that term.
TABLE 2.5.
Nonpulmonary History Suggesting Cystic Fibrosis
| Maldigestion, malabsorption, steatorrhea (in 80–90%) |
| Poor weight gain |
| Family history of cystic fibrosis |
| Salty taste to skin |
| Rectal prolapse (up to 20% of patients) |
| Digital clubbing |
| Meconium ileus (in 10–15%) |
| Intestinal atresia |
| Neonatal cholestatic jaundice |
| Male sterility |
Family and Patient's Medical History
Because many disorders of childhood have genetic or nongenetic familial components, the family history can provide helpful information:
-
•
Are there older siblings with cystic fibrosis (CF) or asthma?
-
•
Is there a coughing sibling whose kindergarten class has been closed because of pertussis?
-
•
Is there an adolescent or adult with chronic cough (bronchitis) who may have pertussis or tuberculosis?
-
•
Was the child premature, and, if so, did he or she spend a month on the ventilator, and does he or she now have chronic lung disease (bronchopulmonary dysplasia)?
-
•
Did the toddler choke on a carrot or other food 3 months ago?
-
•
Did the child have RSV, bronchiolitis, or rhinovirus infection as an infant?
-
•
Did the child receive a bone marrow transplant a year ago?
-
•
Is the child immunized?
-
•
Did the infant have a tracheoesophageal fistula repaired in the neonatal period?
 Physical Examination
Physical Examination
Inspection
Initial inspection often reveals the seriousness of an illness:
-
•
Is the child struggling to breathe (dyspnea)?
-
•
Does the child have an anxious look?
-
•
Can the child be calmed or engaged in play?
-
•
Is the child's skin blue (representing cyanosis) or ashen?
-
•
Does the child appear wasted, with poor growth that may indicate a chronic illness?
The respiratory rate is often elevated with parenchymal lung disease or extrathoracic obstruction. Respiratory rates vary with the age of the child (Fig. 2.3 ) and with pulmonary infection, airway obstruction, activity, wakefulness and sleep, fever, metabolic acidosis, and anxiety.
FIGURE 2.3.

Mean values (blue line) ±2 standard deviations (red and yellow lines) of the normal respiratory rate at rest (during sleep in children younger than 3 years). There is no significant difference between the genders.
(Data from Pasterkamp H. The history and physical examination. In: Chernick V, ed. Kendig's Disorders of the Respiratory Tract in Children. 6th ed. Philadelphia: WB Saunders; 1998:88.)
Odors may also give helpful clues. Does the examining room or the clothing smell of stale cigarette smoke? Is there a foul odor from a diaper with a fatty stool, which may suggest pancreatic insufficiency and CF? Is the child's breath malodorous, as can be noticed in sinusitis, nasal foreign body, lung abscess, or bronchiectasis?
Fingers.
Cyanotic nail beds suggest hypoxemia, poor peripheral circulation, or both. The examiner looks for the presence of digital clubbing (Fig. 2.4 ), which makes asthma or acute pneumonia extremely unlikely. The absence of digital clubbing but a history of severe chronic cough in an older child makes CF unlikely.
FIGURE 2.4.
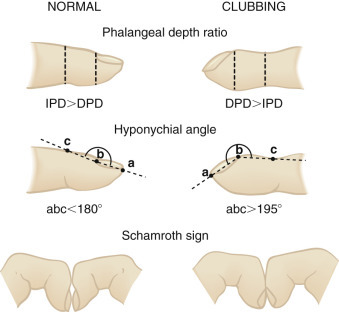
Measurement of digital clubbing. The ratio of the distal phalangeal depth (DPD) to the interphalangeal depth (IPD), or the phalangeal depth ratio, is normally less than 1 but increases to more than 1 with finger clubbing. The DPD/IPD ratio can be measured with calipers or, more accurately, with finger casts. The hyponychial angle is measured from lateral projections of the finger contour on a magnifying screen and is normally less than 180 degrees but greater than 195 degrees with finger clubbing. Schamroth sign is useful for bedside assessment. The dorsal surfaces of the terminal phalanges of similar fingers are placed together. With clubbing, the normal diamond-shaped aperture or “window” at the bases of the nail beds disappears, and a prominent distal angle forms between the end of the nails. In normal subjects, this angle is minimal or nonexistent.
(From Pasterkamp H. The history and physical examination. In: Chernick V, ed. Kendig's Disorders of the Respiratory Tract in Children. 6th ed. Philadelphia: WB Saunders; 1998.)
Chest, abdomen, and spine.
The shape of the chest gives information. Is the anteroposterior (AP) diameter increased, which indicates hyperinflation of the lungs from obstruction of small airways (asthma, bronchiolitis, CF)? Is this diameter small, as can be seen with some restrictive lung diseases with small lung volumes (muscular dystrophy, spinal muscular atrophy)? The normal infant has a “round” chest configuration, with the AP diameter of the chest about 84% of the transverse (lateral) diameter. With growth, the chest becomes more flattened in the AP dimension, and the AP-to-transverse ratio is between 70% and 75%. Although obstetric calipers can be used to give an objective assessment of the AP diameter of the chest, most clinicians rely on their subjective assessment of whether the diameter is increased: Does the patient look “barrel-chested”?
Intercostal, subcostal, suprasternal, and supraclavicular retractions (inspiratory sinking in of the soft tissues) indicate increased effort of breathing and reflect both the contraction of the accessory muscles of respiration and the resulting difference between intrapleural and extrathoracic pressure. Retractions occur most commonly with obstructed airways (upper or lower), but they may occur with any condition leading to the use of the accessory muscles. Any retractions other than the mild normal depressions seen between an infant's lower ribs indicate a greater than normal work of breathing.
Less easy to notice than intercostal retractions is their bulging out with expiration in a child with expiratory obstruction (asthma). Contraction of the abdominal muscles with expiration is easier to notice and is another indication that a child is working harder than normal to push air out through obstructed airways.
Inspection of the spine may reveal kyphosis or scoliosis. There is a risk of restrictive lung disease if the curvature is severe.
Palpation
Palpating the trachea, particularly in infants, may reveal a shift to one side, which suggests loss of volume of the lung on that side or extrapulmonary gas (pneumothorax) on the other side. Placing one hand on each side of the chest while the patient breathes may enable the examiner to detect asymmetry of chest wall movement, either in timing or in degree of expansion. The former indicates a partial bronchial obstruction, and the latter suggests a smaller lung volume, voluntary guarding, or diminished muscle function on one side. Palpating the abdomen gently during expiration may allow the examiner to feel the contraction of the abdominal muscles in cases of expiratory obstruction. Hyperinflation may push the liver down making it palpable below the costal margin.
Palpation for tactile fremitus, the transmitted vibrations of the spoken word (“ninety-nine” is the word often used to accentuate these vibrations), helps determine areas of increased parenchymal density and hence increased fremitus (as in pneumonic consolidation) or decreased fremitus (as in pneumothorax or pleural effusion).
Percussion
The percussion note determined by the examiner's tapping of one middle finger on the middle finger of the other hand, which is firmly placed over the patient's thorax, may be dull over an area of consolidation or effusion and hyperresonant with air trapping. Percussion can also be used to determine diaphragmatic excursion. The lowest level of resonance at inspiration and expiration determines diaphragmatic motion.
Auscultation
Because lung sounds tend to be higher-pitched than heart sounds, the diaphragm of the stethoscope is better suited to pulmonary auscultation than is the bell, whose target is primarily the lower-pitched heart sounds (Table 2.6 ). The adult-sized stethoscope generally is superior to the smaller pediatric or neonatal diaphragms, even for listening to small chests, because its acoustics are better (Figs. 2.5 and 2.6 ).
TABLE 2.6.
Physical Signs of Pulmonary Disease
| Disease Process | Mediastinal Deviation | Chest Motion | Fremitus | Percussion | Breath Sounds | Adventitious Sounds | Voice Signs |
|---|---|---|---|---|---|---|---|
| Consolidation (pneumonia) | No | Reduced over area, splinting | Increased | Dull | Bronchial or reduced | Crackles | Egophony,* whispering pectoriloquy increased† |
| Bronchospasm | No | Hyperexpansion with limited motion | Normal or decreased | Hyperresonant | Normal to decreased | Wheezes, crackles | Normal to decreased |
| Atelectasis | Shift toward lesion | Reduced over area | Decreased | Dull | Reduced or absent | None or crackles | None |
| Pneumothorax | Tension deviates trachea and PMI to opposite side | Reduced over area | None | Resonant, tympanitic | None | None | None |
| Pleural effusion | Deviation to opposite side | Reduced over area | None | Dull | None | Friction rub; splash, if hemopneumothorax | None |
PMI, point of maximal impulse.
Egophony is present when e sounds like a.
Whispering pectoriloquy produces clearer sounding whispered words (e.g., “ninety-nine”).
Modified from Dantzker D, Tobin M, Whatley R. Respiratory diseases. In: Andreoli TE, Carpenter CJ, Plum F, Smith LH, eds. Cecil Essentials of Medicine. Philadelphia: WB Saunders; 1986:126-180.
FIGURE 2.5.

Projections of the pulmonary lobes on the chest surface. The upper lobes are white, the right-middle lobe is black, and the lower lobes are purple.
(From Pasterkamp H. The history and physical examination. In: Chernick V, ed. Kendig's Disorders of the Respiratory Tract in Children. 6th ed. Philadelphia: WB Saunders; 1998.)
FIGURE 2.6.

Characteristics of breath sounds. Tracheal breath sounds are very harsh, loud, and high-pitched; they are heard over the extrathoracic portion of the trachea. Bronchial breath sounds are loud and high-pitched; normally, they are heard over the lower sternum and sound like air rushing through a tube. The expiratory component is louder and longer than the inspiratory component; a definite pause is heard between the two phases. Bronchovesicular breath sounds are a mixture of bronchial and vesicular sounds. The inspiratory (I) and expiratory (E) components are equal in length. They are usually heard only in the first and second interspaces anteriorly and between the scapulae posteriorly, near the carina and mainstem bronchi. Vesicular breath sounds are soft and low-pitched; they are heard over most of the lung fields. The inspiratory component is much longer than the expiratory component; the latter is softer and often inaudible.
(From Swartz MH, ed. The chest. In: Textbook of Physical Diagnosis: History and Examination. Philadelphia: WB Saunders; 1989.)
Adventitious sounds come in a few varieties, namely, stridor, crackles, rhonchi, and wheezes. Other sounds should be described in clear, everyday language.
-
•
Stridor is a continuous musical sound usually heard on inspiration and is caused by narrowing in the extrathoracic airway, as with croup or laryngomalacia.
-
•
Crackles are discontinuous, representing the popping open of air-fluid menisci as the airways dilate with inspiration. Fluid in larger airways causes crackles early in inspiration (congestive heart failure). Crackles that tend to be a bit lower in pitch (“coarse” crackles) than the early, higher-pitched (“fine”) crackles are associated with fluid in small airways (pneumonia). Although crackles usually signal the presence of excess airway fluid (pneumonia, pulmonary edema), they may also be produced by the popping open of noninfected fibrotic or atelectatic airways. Fine crackles are not audible at the mouth, whereas coarse crackles may be. Crackles is the preferred term, rather than the previously popular “rales.”
-
•
Rhonchi, or “large airway sounds,” are continuous gurgling or bubbling sounds typically heard during both inhalation and exhalation. These sounds are caused by movement of fluid and secretions in larger airways (asthma, viral URI). Rhonchi, unlike other sounds, may clear with coughing.
-
•
Wheezes are continuous musical sounds (lasting longer than 200 msec), caused by vibration of narrowed airway walls, as with asthma, and perhaps vibration of material within airway lumens. These sounds are much more commonly heard during expiration than inspiration.
 Diagnostic Studies
Diagnostic Studies
Radiography
The chest radiograph is often the most useful diagnostic test in the evaluation of the child with cough. Table 2.7 highlights some of the radiographic features of the most common causes of cough in pediatric patients. Radiographic findings are often similar for a number of disorders, and thus these studies may not indicate a definitive diagnosis. Chest radiographs are normal in children with psychogenic (habit) cough and in children with sinusitis or gastroesophageal reflux (GER) as the primary cause of cough. A normal chest radiograph indicates the unlikelihood of pneumonia caused by respiratory syncytial virus (RSV), influenza, parainfluenza, adenovirus, Chlamydia species, or bacteria. Although children with cough resulting from cystic fibrosis (CF), Mycoplasma species, tuberculosis, aspiration, a bronchial foreign body, or an anatomic abnormality usually have abnormal chest radiographs, a normal radiograph does not exclude these diagnoses. Hyperinflation of the lungs is commonly seen on chest radiographs of infants with RSV bronchiolitis or Chlamydia pneumonia, and a lobar or round (coin lesion) infiltrate is the radiographic hallmark of bacterial pneumonia. The diagnosis of sinusitis cannot be sustained with normal sinuses on radiograph or computed tomography (CT) scan.
TABLE 2.7.
Cough: Laboratory Evaluation
| CHEST RADIOGRAPH |
Abnormal Sinus Radiograph | COMPLETE BLOOD COUNT |
↑IgG | ↑IgM | ↑IgE | + NP PCR | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Hyper | Lobar Infil | Diff Infil | Other | ↑WBC | ↑LY | ↑EOS | ↑PMN | |||||||
| Asthma | + | ++ | – | – | – | + | + | + | ++ | – | + | + | ++ | +bdilator1 | |
| Cystic fibrosis | + | ++ | + | + | ++ | +++ | ++ | + | + | ++ | ++ | + | + | See Table 2.8 | |
| Other infection | |||||||||||||||
| Croup | ++ | + | + | + | ++2 | – | – | + | + | – | Paraflu +++ |
||||
| Epiglottitis | ++ | + | + | + | ++3 | – | +++ | + | + | +++ | Direct look | ||||
| Sinusitis | +++ | – | – | – | – | +++ | ++ | – | + | +++ | ++ | + | |||
| Bronchiolitis | – | +++ | + | ++ | + | – | + | + | + | + | RSV, metapneumovirus +++ |
||||
| Pneumonia | |||||||||||||||
| Influenza | – | ++ | + | ++ | + | – | ++ | + | +++ | ||||||
| Paraflu | – | + | ++ | + | – | ++ | + | +++ | |||||||
| Adenovirus | – | + | ++ | + | – | ++ | + | + | ++ | +++ | |||||
| Pertussis | ++ | + | – | + | + | – | ++ | +++ | + | + | ++ | + | – | ++4 | |
| Chlamydia | – | +++ | + | +++ | + | – | + | + | ++ | + | +++ | +++ | – | +++ | |
| Mycoplasma | + | + | + | + | ++5 | + | + | + | + | + | – | ++ | – | ++ | +Cold agglutinin |
| TB | + | – | ++ | + | ++ | – | + | + | + | + | +PPD, Quantiferon | ||||
| Bacterial | – | + | +++ | + | ++5 | – | +++ | + | + | +++ | ++ | + | + | + | +Bld cult6 |
| Foreign body | – | ++7 | ++ | – | ++7 | – | ++ | + | + | ++ | Bronch | ||||
| GE reflux | +++ | + | – | – | – | + | – | – | – | – | – | – | + | – | Esoph pH8 |
| Aspiration | + | + | + | + | ++9 | + | + | – | + | + | – | – | + | – | 10 |
| Anatomic | + | + | + | – | ++11 | – | + | – | – | + | – | – | – | – | 12 |
| Habit | +++ | – | – | – | – | – | – | – | – | – | – | – | – | – | |
+++, almost always—if not present, must question diagnosis; ++, common; +, less common; –, seldom—if present, must question diagnosis.
+Bld cult, blood culture may be positive; Bronch, bronchoscopy can reveal the foreign body; Diff, diffuse or scattered; ↑EOS, increased eosinophil count; Esoph pH, prolonged esophageal pH probe monitoring; GE, gastroesophageal; Hyper, hyperinflated; Ig, immunoglobulin; Infil, infiltrates; ↑LY, increased lymphocyte count; +NP aspirate PCR, nasopharyngeal positive for specific organism; Paraflu, parainfluenza virus; PCR, polymerase chain reaction; ↑PMN, increased polymorphonuclear neutrophil count; PPD, purified protein derivative (TB); RAD, reactive airways disease; RSV, respiratory syncytial virus; TB, tuberculosis; ↑WBC, increased white blood cell count.
Positive response to bronchodilators, either as a home therapeutic trial or in a pulmonary function test in the laboratory.
“Steeple” sign: narrowing of upper tracheal air column.
“Swollen thumb”: sign of thickened epiglottis.
Low yield in paroxysmal stage.
Pleural effusion relatively common.
Blood culture positive in 10%; needle aspiration of pleural fluid or lung fluid may yield organism; bacterial antigen in urine. In older infants and children, common pathogens include pneumococci and group A streptococci; Staphylococcus aureus is rare and may be associated with pneumatoceles or empyema.
Localized hyperinflation is common; localized atelectasis is common; inspiratory-expiratory radiographs may show ball-valve obstruction.
Esophageal biopsy specimen shows esophagitis.
Multilobular or multisegmental, dependent lobes.
(?) Lipid-laden macrophages from bronchoscopy or gastric washings; barium swallow or radionuclide study showing aspiration.
Right-sided arch, mass effect on airways, mass identified; magnetic resonance imaging (MRI).
Bronchoscopy; computed tomography; MRI.
Hematology/Immunology
The white blood cell (WBC) count may help exclude or include certain entities for a differential diagnosis. For example, a WBC count of 35,000 with 85% lymphocytes strongly suggests pertussis, but not every child with pertussis presents such a clear hematologic picture. The presence of a high number or large proportions of immature forms of WBCs suggests an acute process, such as a bacterial infection. Immunoglobulins provide supportive evidence for a few diagnoses, such as chlamydial infection, which rarely occurs without elevated serum concentrations of immunoglobulins G and M.
Bacteriology/Virology
Specific bacteriologic or virologic diagnoses can be made in a number of disorders causing cough, including RSV, influenza, parainfluenza, adenovirus, and Chlamydia pneumonia. In most cases, the viruses can be rapidly identified with amplification of the viral genome through polymerase chain reaction (PCR). In bacterial pneumonia, the offending organism can be cultured from the blood in a small proportion (10%) of patients. A positive culture provides definitive diagnosis, but a negative culture specimen is not helpful. Throat cultures are seldom helpful (except in CF) in identifying lower respiratory tract bacterial organisms. Sputum cultures and Gram stains may help guide initial empirical therapy in older children with pneumonia or purulent bronchitis, but their ability to identify specific causative organisms with certainty (with the exception of CF) has not been shown clearly.
Infants and young children usually do not expectorate but rather swallow their sputum. Specimens obtained via bronchoscopy may be contaminated by mouth flora, but heavy growth of a single organism in the presence of polymorphonuclear neutrophils certainly supports the organism's role in disease. If pleural fluid or fluid obtained directly from the lung via needle aspiration is cultured, the same rules apply: Positive cultures are definitive, but negative cultures are not.
Other Tests
A number of specific tests can help to establish diagnoses in a child with cough (see Table 2.7). These include a positive response to bronchodilators in a child with asthma; visualizing the red, swollen epiglottis in epiglottitis (to be done only under very controlled conditions); the bronchoscopic visualization of the peanut, plastic toy, or other offender in foreign body aspiration; a positive purified protein derivative (PPD) or Quantiferon assay in tuberculosis; and several studies of the esophagus in GER. Several imaging techniques, such as CT or magnetic resonance imaging (MRI), can help to delineate various intrathoracic anatomic abnormalities, pulmonary embolism, and bronchiectasis. Multiple tests can be employed to confirm the diagnosis of CF (Table 2.8 ).
TABLE 2.8.
Laboratory Tests for Cystic Fibrosis
| Usefulness | Test | Sensitivity | Specificity | Cost |
|---|---|---|---|---|
| Definitive |
Sweat chloride test | .99+ | .95+ | $96 |
| DNA analysis | .85–.90 | .99 | $962 | |
| Suggestive |
Throat or sputum culture* positive for mucoid Pseudomonas aeruginosa | .70-.80 | .85 | $183 |
| Sinus radiographs | $179 | |||
| Pansinusitis | .95 | .90 | ||
| Positive IRT newborn screen | .98 | .25 | $1 | |
| Supportive | Fecal elastase | $587 | ||
| Pulmonary function tests: | $100–$800 | |||
| Obstructive pattern, especially small airways and especially if patient is poorly responsive to bronchodilator | .70+ | ? | ||
| Chest radiograph: | $160 | |||
| Hyperinflation, ± other findings; especially with right upper lobe infiltrate/atelectasis | .70+ | ? | ||
| Throat or sputum culture*: | $183 | |||
| Positive for Staphylococcus aureus | .20 | .20 | ||
| Positive for Haemophilus influenzae | .05-.20 | .15 | ||
IRT, immunoreactive trypsinogen.
Throat is usually deep pharyngeal culture.
 Differential Diagnosis and Treatment
Differential Diagnosis and Treatment
Infection
Infections are the most common cause of acute cough in all age groups and are responsible for some chronic coughs. The age of the patient has a large impact on the frequency of the type of infection.
Infections in infants.
Viral upper respiratory infections (common cold); croup (laryngotracheobronchitis); viral bronchiolitis, particularly with RSV or human metapneumovirus; and viral pneumonia are the most frequently encountered respiratory tract infections and hence the most common causes of cough in infancy. Viral illness may predispose to bacterial superinfection (croup and Staphylococcus aureus tracheitis or influenza and H. influenzae or S. aureus pneumonia).
Viral upper respiratory infections (URI).
Viral URI symptoms and signs usually include stuffy nose with nasal discharge, sore throat, and sneezing. There may be fever, constitutional signs (irritability, myalgias, and headache), or both. Cough is common and may persist for 5-7 days. The mechanism by which URIs cause cough in children is undetermined. In adults, it is generally thought that “postnasal drip”—that is, nasal or sinus secretions draining into the posterior nasopharynx—causes cough and, in fact, may be one of the most frequent causes of cough. Indeed, sinus CT in older patients with URIs often reveals unexpected involvement of the sinus mucosa. Other authorities believe that cough in a child with a URI indicates involvement (inflammation or bronchospasm) of the lower respiratory tract. Over-the-counter cough and cold medications are commonly used. Evidence of efficacy of these medications for children with URI is lacking. Because of the known risk for unintentional overdose from these medications, their use is not recommended in children under age 4 years.
Common viral pathogens include rhinovirus, RSV, coronaviruses, and parainfluenza viruses. The differential diagnosis includes allergic rhinitis, which often demonstrates clear nasal secretions with eosinophils and pale nasal mucosa, and sinusitis, which presents with mucopurulent nasal secretions containing neutrophils and erythematous mucosa.
Croup (laryngotracheobronchitis).
Infectious croup (see Chapter 3) is most common in the first 2 years of life. Its most dramatic components are the barking (“croupy”) cough and inspiratory stridor, which appear a few days after the onset of a cold. In most cases, the patient has a low-grade fever, and the disease resolves within a day or two. In severe cases, the child can be extremely ill and is at risk for complete laryngeal obstruction. There may be marked intercostal and suprasternal retractions and cyanosis. Stridor at rest signifies significant obstruction. Diminishing stridor in a child who is becoming more comfortable is a good sign, but diminishing stridor in and of itself is not necessarily good: If the child becomes fatigued because of the tremendous work of breathing through an obstructed airway and can no longer breathe effectively, smaller-than-needed tidal volumes make less noise.
(See Nelson Textbook of Pediatrics, p. 2032.)
It is important to distinguish croup from epiglottitis in the child with harsh, barking cough and inspiratory stridor because the natural histories of the two diseases are quite different (see Table 2.7). Epiglottitis occurs more commonly in unimmunized toddlers than in infants (see Chapter 3).
Treatment of mild croup is usually not needed. For decades, pediatricians have recommended putting a child with croup in a steamy bathroom or driving to the office or emergency department with the car windows rolled down. It is likely that these remedies are effective because of the heat exchange properties of the upper airway; air that is cooler or more humid than the airway mucosa will serve to cool the mucosa, thus causing local vasoconstriction and probably decreasing local edema.
In a child who has stridor at rest, evaluation is indicated. Symptomatic, often dramatic relief through decreased laryngeal edema can usually be achieved with aerosolized racemic epinephrine (2.25% solution, 0.25 to 0.5 mL/dose). It is essential to remember that the effects of the epinephrine are transient, lasting only a few hours, although the course of the illness is often longer. The result is that when the racemic epinephrine's effect has worn off, the child's cough and stridor will probably be as bad or even worse than before the aerosol was administered. This is not a “rebound” effect: The symptoms are not worse because of the treatment but, rather, because of the natural progression of the viral illness. Repeating the aerosol will probably again have a beneficial effect. A child who responds favorably to such an aerosol needs to be observed for several hours because further treatment may be needed. A single dose of dexamethasone (0.6 mg/kg orally, intramuscularly, or intravenously) reduces the severity and hastens recovery. Aerosolized steroids (budesonide) may also be effective in patients with mild to moderately severe croup.
Bronchiolitis.
Bronchiolitis is a common and potentially serious lower respiratory tract disorder in infants (see Chapter 3). It is caused usually by RSV but on occasion by parainfluenza, influenza, human metapneumovirus, adenovirus, enterovirus, and human rhinovirus. It mostly occurs in the winter months, often in epidemics. RSV bronchiolitis is seen uncommonly in children older than 4 years. Typically, “cold-like” symptoms of rhinorrhea precede the harsh cough, increased respiratory rate, and retractions. Respiratory distress and cyanosis can be severe. The child's temperature is seldom elevated above 38°C.
The chest is hyperinflated, widespread crackles are audible on inspiration, and wheezing marks expiration. The chest radiograph invariably reveals hyperinflation, as depicted by a depressed diaphragm, with an enlarged retrosternal air space in as many as 60% of patients, peribronchial thickening in approximately 50%, and consolidation and/or atelectasis in 10-25%.
The diagnosis is confirmed with demonstration of RSV by PCR of nasopharyngeal secretions. In most cases, no treatment is needed because the disease does not interfere with the infant's eating or breathing. Apnea is a common complication of RSV bronchiolitis in neonates and may necessitate close monitoring. In severe cases, often those in which there is underlying chronic heart, lung, or immunodeficiency disease, RSV can be life-threatening. In severe cases, hospital care with supplemental oxygen and intravenous fluids is indicated. Suctioning of secretions is an essential part of the treatment. Many other treatment modalities have been tried for hospitalized infants with bronchiolitis. Aerosolized bronchodilators and systemic glucocorticoids do not seem to alter clinical outcome and are not recommended in most patients. Nebulized saline may reduce the length of hospitalization. Use of high-flow nasal cannula may reduce the need for more invasive forms of respiratory support in infants with impending respiratory failure.
Viral pneumonia.
Viral pneumonia can be similar to bronchiolitis in its manifestation, with cough and tachypnea, after a few days of apparent URI. There can be variable degrees of fever and of overall illness. Infants and children with viral pneumonia may appear relatively well or, particularly with adenovirus or influenza, may have a rapidly progressive course. Frequent symptoms include poor feeding, cough, cyanosis, fever (some patients may be afebrile), apnea, and rhinorrhea. Frequent signs include tachypnea, retractions, crackles, and cough. Cyanosis is less common.
(See Nelson Textbook of Pediatrics, p. 2091.)
The most common agents causing viral pneumonia in infancy and childhood are RSV, influenza, and parainfluenza. Adenovirus is less common, but it is important because it can be severe and leave residua, including bronchiectasis and bronchiolitis obliterans. Adenovirus pneumonia is often accompanied by conjunctivitis and pharyngitis, in addition to leukocytosis and an elevated erythrocyte sedimentation rate (ESR); the ESR and leukocyte count are usually not elevated in other types of viral pneumonia. Additional viral agents include enteroviruses, human metapneumovirus and rhinovirus. Radiographs most often reveal diffuse, bilateral peribronchial infiltrates, with a predilection for the perihilar regions, but occasionally lobar infiltrates are present. Pleural effusions are not common. On occasion, if an infant is extremely ill, bronchoscopy with bronchoalveolar lavage may be indicated to isolate the virus responsible for the pneumonia.
Treatment is largely supportive, with oxygen and intravenous fluids. Mechanical ventilation may be necessary in a small minority of infants.
In young infants, the afebrile pneumonia syndrome may be caused by Chlamydia, Ureaplasma, or Mycoplasma species; cytomegalovirus; or Pneumocystis jiroveci. In this syndrome, cough and tachypnea are common. Severe pneumonia may develop in neonates as a result of herpes simplex.
Pertussis (whooping cough).
Pertussis is a relatively common cause of lower respiratory tract infection in infants, children, adolescents, and adults, especially in those who are underimmunized or not immunized. The causative organism, Bordetella pertussis, has a tropism for tracheal and bronchial ciliated epithelial cells; thus the disease is primarily bronchitis, but spread of the organism to alveoli, or secondary invasion by other bacteria, can cause pneumonia. The disease can occur at any age, from early infancy onward, although its manifestations in young infants and in those who have been partially immunized may be atypical.
Most commonly, pertussis has three stages:
-
•
Catarrhal, in which symptoms are indistinguishable from a viral URI
-
•
Paroxysmal, dominated by repeated forceful, paroxysmal coughing spells; spells may be punctuated by an inspiratory “whoop,” post-tussive emesis, or both
-
•
Convalescent, in which the intensity and frequency of coughing spells gradually diminish
Each stage typically lasts 1-2 weeks, except the paroxysmal stage, which lasts many weeks. (Pertussis is known as the “100 day cough” in China.) Most children are entirely well between coughing spells, when physical findings are remarkably benign. Infants younger than 6 months of age are at highest risk for complications. The majority of infants with pertussis need to be hospitalized.
Diagnosis can be difficult because the definitive result—namely, culturing the organism from nasopharyngeal secretions—requires special culture medium (Bordet-Gengou, which must be prepared fresh for each collection). Culture specimens are much less likely to be positive during the paroxysmal stage than during the catarrhal stage, when the diagnosis is not being considered. PCR assay of an adequate nasopharyngeal (NP) specimen is the most commonly used test because of improved sensitivity and faster turnaround time compared to culture. An elevated WBC count, as high as 20,000-50,000, with lymphocytes predominating is suggestive of pertussis in infants and children but often absent in adolescents. Chest radiographic findings are nonspecific. Infants with severe disease may require hospitalization.
Treatment is largely supportive, with oxygen, fluids, and small frequent feedings for patients who do not tolerate their normal feedings. Treatment with azithromycin decreases infectivity and may ameliorate the course of the disease if given during the catarrhal stage.
Complications include those related to severe coughing (Table 2.9 ) and those specific to pertussis, such as seizures and encephalopathy. The risk of acquiring pertussis is markedly reduced by immunizations (three primary immunizations and regular booster immunizations). Neither pertussis infection nor immunization produces lifelong immunity.
TABLE 2.9.
Potential Complications of Cough
| Musculoskeletal | Rib fractures Vertebral fractures Rupture of rectus abdominis muscle Asymptomatic elevation of serum creatine phosphokinase |
| Pulmonary | Chest wall pain* Bronchoconstriction Pneumomediastinum Pneumothorax Mild hemoptysis Subcutaneous emphysema Irritation of larynx and trachea |
| Cardiovascular | Rupture of subconjunctival,* nasal,* and anal veins Bradycardia, heart block Transient hypertension |
| Central nervous system | Cough syncope Headache Subarachnoid hemorrhage |
| Gastrointestinal | Hernias (ventral, inguinal) Emesis Rectal prolapse Pneumoperitoneum |
| Miscellaneous | Anorexia* Malnutrition Sleep loss* Urinary incontinence Disruption of surgical wounds Vaginal prolapse Displacement of intravenous catheters |
Common.
Chlamydial infection.
Chlamydia trachomatis can cause pneumonia in young infants, particularly those aged 3-12 weeks. Cough, nasal congestion, low-grade or no fever, and tachypnea are common. Conjunctivitis is an important clue to chlamydial disease but is present in only 50% of infants with chlamydial pneumonia at the time of presentation. Affected infants may have a paroxysmal cough similar to that of pertussis, but post-tussive emesis is less common. Crackles are commonly heard on auscultation, but wheezing is much less common than the overinflated appearance of the lungs on radiographs would suggest. The organism may be recovered from the nasopharynx by culture or antigen testing. The complete blood cell count may reveal eosinophilia. Chlamydial infection responds to oral erythromycin therapy.
Ureaplasmal infection.
Ureaplasma urealyticum pneumonia is difficult to diagnose but causes cough in some infants. There are no particularly outstanding features to distinguish this relatively uncommon infection from viral pneumonias.
Bacterial pneumonia.
Bacterial pneumonia is relatively less common in infants than is viral pneumonia but can cause severe illness, with cough, respiratory distress, and fever. Chest radiographs are abnormal, and the WBC count is elevated.
Treatment is with antibiotics effective against pneumococci, group A streptococci, and, if illness is severe, S. aureus.
Infections in toddlers and children
Viral URIs.
In early childhood, as children attend daycare and nursery schools, they are constantly exposed to respiratory viruses to which they have little or no immunity (e.g., RSV, rhinoviruses, adenoviruses, parainfluenza, and enteroviruses). Young children may have as many as 6-8 or even more URIs in a year. The remarks concerning colds and cough in infants (see previous discussion) apply to this older age group. The differential diagnosis of rhinorrhea is noted in Table 2.10 .
TABLE 2.10.
Differential Diagnosis of Rhinorrhea
| Etiology | Frequency | Duration* | Discharge | Comment |
|---|---|---|---|---|
| Viral | Common | Acute | Purulent | Polymorphonuclear neutrophils in smear |
| Allergic | Common | Acute/Chronic | Clear | Eosinophils in smear, seasonal |
| Vasomotor | Common | Chronic | Variable | ? Environmental triggers |
| Sinusitis | Common | Chronic | Purulent | Sinus tenderness |
| Rhinitis medicamentosusa | Common | Chronic | Variable | Medication use |
| Response to stimuli | Common | Acute | Clear | Odors, exercise, cold air, pollution |
| Nasal polyps | Uncommon | Chronic | Variable | Consider cystic fibrosis |
| Granulomatous disease | Uncommon | Chronic | Bloody | Sarcoid, Granulomatosis with polyangiitis, midline granuloma |
| Cerebrospinal fluid fistula | Uncommon | Chronic | Watery | Trauma, encephalocele |
| Foreign body | Uncommon | Chronic | Purulent | Often malodorous |
| Tumor | Uncommon | Chronic | Clear to bloody | Angiofibroma, hemangioma, rhabdomyosarcoma, lymphoma, nasopharyngeal carcinoma, neuroblastoma |
| Choanal atresia, stenosis | Uncommon | Chronic | Clear to purulent | Congenital |
| Nonallergic eosinophilic rhinitis syndrome | Uncommon | Chronic | Clear | Eosinophils in smear |
| Septal deviation | Unknown | Chronic | Clear | Congenital, trauma |
| Drugs | Uncommon | Chronic | Variable | Cocaine, glue and organic solvents, angiotensin-converting enzyme inhibitors, β blockers |
| Hypothyroidism | Uncommon | Chronic | Clear | |
| Cluster headache | Uncommon | Intermittent | Clear | Associated tearing, headache |
| Horner syndrome | Uncommon | Chronic | Clear | Ptosis, miosis, anhidrosis |
Less than 1 week is considered acute.
Sinusitis.
The sinuses may become the site for viral and subsequent secondary bacterial infection spreading from the nasopharynx (Fig. 2.7 ). The signs and symptoms are usually localized, including nasal congestion, a feeling of “fullness” or pain in the face (Fig. 2.8 ), headache, sinus tenderness, day or night cough, and fever. Maxillary toothache, purulent nasal discharge for more than 10 days, and a positive transillumination (opacification) are important clues. Sinus radiographs or (more accurate) CT scan may facilitate the diagnosis of sinusitis by demonstrating opacification of the sinus with mucosal thickening. Sinusitis is thought to be a cause of cough in adults and can probably be listed, with lower certainty, as a cause of cough in children.
FIGURE 2.7.

The paranasal sinuses. 1, Frontal. 2, Ethmoid. 3, Maxillary. 4, Sphenoid.
(From Smith RP. Common upper respiratory tract infections. In: Reilly B, ed. Practical Strategies in Outpatient Medicine. 2nd ed. Philadelphia: WB Saunders; 1991.)
FIGURE 2.8.

Typical pain locations in patients with various anatomic sites of acute sinusitis.
(From Smith RP. Common upper respiratory tract infections. In: Reilly B, ed. Practical Strategies in Outpatient Medicine. 2nd ed. Philadelphia: WB Saunders; 1991.)
(See Nelson Textbook of Pediatrics, p. 2014.)
Sinusitis is frequently seen in other conditions known to cause cough, especially CF, asthma, ciliary dyskinesia, and granulomatosis with polyangiitis with or without eosinophilia. It may be difficult to ascertain whether the cough is a direct result of the sinus infection or the underlying problem (purulent bronchitis in the child with CF or ciliary dyskinesia, exacerbation of asthma). In the first two situations, it may not matter because treatment is the same. In the case of the child with asthma, it is important to treat the asthma with bronchodilating and antiinflammatory agents, as well as to treat the infected sinuses with antibiotics.
The treatment of sinusitis involves the use of oral antibiotics active against the common pathogens (i.e., Streptococcus pneumoniae, nontypable H. influenzae, Moraxella catarrhalis, and, in rare cases, anaerobic bacteria or Streptococcus pyogenes). Treatment regimens include the use of amoxicillin, amoxicillin-clavulanate, cefuroxime, cefpodoxime, or cefdinir. Amoxicillin is considered the initial agent of choice. Oral (pseudoephedrine, phenylephrine) or topical (phenylephrine, oxymetazoline) decongestants may be of benefit by increasing the patency of the sinus ostia, which permits drainage of the infected and obstructed sinuses. Oral antihistamines may benefit patients with an allergic history. Treatment with antimicrobial agents should continue for at least 7 days after the patient has responded. This may require 14-21 days of therapy. Many patients with presumed sinusitis recover without antibiotic therapy.
Complications of acute sinusitis include orbital cellulitis, abscesses (orbital, cerebral), cranial (frontal) osteomyelitis (Pott puffy tumor), empyema (subdural, epidural), and thrombosis (sagittal or cavernous sinus).
Pneumonia.
The features discussed for viral pneumonia in infants are relevant for viral pneumonia in older children. The differentiation of viral or atypical pneumonia from classical bacterial pneumonia is noted in Table 2.11 . Adenovirus and influenza pneumonia may present similar to bacterial pneumonia in severity and acuteness.
TABLE 2.11.
Differentiation of Classical Bacterial Pneumonia from Viral and Atypical Pneumonias*
| Bacterial | Viral/Atypical | |
|---|---|---|
| History | Precedent URI | Headache, malaise, URI, myalgias |
| Course | Often biphasic illness | Often monophasic |
| Onset | Sudden | Gradual |
| Temperature | High fever | Low-grade fever |
| Rigors | Common | Uncommon |
| Vital signs | Tachypnea, tachycardia | Usually normal |
| Pain | Pleuritic | Unusual |
| Chest examination | Crackles, signs of consolidation | Consolidation unusual |
| Pleural effusion | Common | Uncommon |
| Sputum | Productive, purulent, many PMNs, one dominant organism on Gram stain | Scant, no organisms; PMNs or mononuclear cells |
| ESR | Elevated | Usually normal |
| WBC count | Elevated; left shift | Often normal; predominant lymphocytes |
| Chest radiography | Lobar consolidation, round infiltrate, parapneumonic effusion; may be “bronchopneumonia” | Diffuse, bilateral, patchy, interstitial or bronchopneumonia; lower lobe involvement common; chest radiograph may look worse than patient's condition |
| Progression | May be rapid | Rapid if Legionella species, hantavirus, SARS, herpesvirus, adenovirus |
| Diagnosis | Blood, sputum, and pleural fluid specimens for culture; antigen detection possible; BAL if progressive | Viral, chlamydial culture or PCR detection; acute and convalescent titers; BAL if progressive |
BAL, bronchoalveolar lavage; ESR, erythrocyte sedimentation rate; PMNs, polymorphonuclear neutrophils; SARS, severe acute respiratory syndrome; URI, upper respiratory tract infection; WBC, white blood cell.
Atypical pneumonias include Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella species (L. pneumophila, L. micdadei), Q fever, psittacosis.
Bacterial pneumonia is more common in toddlers and older children than in infants. The most common pathogen is S. pneumoniae. (Table 2.12 ). Cough may not be as prominent a presenting symptom or sign as tachypnea and grunting. Raised respiratory rates (>50 in infants 2-12 months old, >40 in children 1-5 years old) plus retractions and grunting with or without hypoxia (oxygen saturation <90%) have a high specificity and sensitivity for pneumonia. Chest pain, abdominal pain, headache, or any combination of these symptoms may occur. Upper lobe pneumonia may produce meningeal signs, and lower lobe involvement may cause abdominal pain and an ileus.
TABLE 2.12.
Causes of Infectious Pneumonia
| Bacterial | |
| Common | |
| Streptococcus pneumoniae | See Table 2.11 |
| Group B streptococci | Neonates |
| Group A streptococci | See Table 2.11 |
| Mycoplasma pneumoniae* | Adolescents; summer-fall epidemics |
| Chlamydia pneumoniae* | Adolescents (see Table 2.11) |
| Chlamydia trachomatis | Infants |
| Mixed anaerobes | Aspiration pneumonia |
| Gram-negative enteric | Nosocomial pneumonia |
| Uncommon | |
| Haemophilus influenzae type B | See Table 2.11 |
| Staphylococcus aureus | Pneumatoceles; infants |
| Moraxella catarrhalis | |
| Neisseria meningitidis | |
| Francisella tularensis | Animal, tick, fly contact |
| Nocardia species | Immunosuppressed persons |
| Chlamydia psittaci* | Bird contact |
| Yersinia pestis | Plague |
| Legionella species* | Exposure to contaminated water; nosocomial |
| Viral | |
| Common | |
| Respiratory syncytial virus | See Table 2.11 |
| Parainfluenza types 1-4 | Croup, Type 3 and 4 seen in the summer |
| Influenza A, B | High fever; winter months |
| Adenovirus | Can be severe; occurs all year round |
| Human metapneumovirus | Similar to RSV |
| Rhinovirus | Rhinorrhea |
| Uncommon | |
| Enterovirus | Neonates |
| Herpes simplex | Neonates |
| Cytomegalovirus | Infants, immunosuppressed persons |
| Measles | Rash, coryza, conjunctivitis |
| Varicella | Adolescents |
| Hantavirus | Southwestern United States |
| SARS agent | Asia |
| Fungal | |
| Histoplasma capsulatum | Geographic region; bird, bat contact |
| Cryptococcus neoformans | Bird contact |
| Aspergillus species | Immunosuppressed |
| Mucormycosis | Immunosuppressed |
| Coccidioides immitis | Geographic region |
| Blastomyces dermatitidis | Geographic region |
| Rickettsial | |
| Coxiella burnetii* | Q fever, animal (goat, sheep, cattle) exposure |
| Rickettsia rickettsiae | Tick bite |
| Mycobacterial | |
| Mycobacterium tuberculosis | See Table 2.14 |
| Mycobacterim avium–intracellulare | Immunosuppressed persons |
| Parasitic | |
| Pneumocystis jiroveci | Immunosuppressed, steroids |
| Eosinophilic | Various parasites (e.g., Ascaris, Strongyloides species) |
SARS, severe acute respiratory syndrome.
Atypical pneumonia syndrome (see Table 2.11); atypical in terms of extrapulmonary manifestations, low-grade fever, patchy diffuse infiltrates, poor response to penicillin-type antibiotics, and negative sputum Gram stain.
Examination of the chest shows tachypnea but may be otherwise surprisingly normal. In older children, there may be localized dullness to percussion, with crackles or amphoric (bronchial) breath sounds over a consolidated lobe. The chest radiograph may be normal in the first hours of the illness, inasmuch as the radiographic findings often lag behind the clinical manifestations. Nonetheless, both anterior-posterior and lateral views are the main diagnostic tools; lobar consolidation is usual, with or without pleural effusion. In infants, the pattern may be more diffuse and extensive.
Some clinical and radiographic features may be suggestive of the bacterial cause of pneumonia. Children (especially infants) with staphylococcal pneumonia are more likely to have a rapid overwhelming course. Staphylococcal pneumonia may be accompanied by more extensive radiographic abnormalities, including multilobar consolidation, pneumatocele formation, and extensive pleural (empyema) fluid. The presence of a pleural effusion is not helpful in indicating the specific bacterial diagnosis because other bacterial pneumonias may be accompanied by pleural effusion. Pleural effusions may represent a reactive parapneumonic effusion or an empyema. Pleural fluid may be characterized as transudate, exudate, or empyema (Table 2.13 ). If the effusion is of sufficient size, as demonstrated by a lateral decubitus radiograph or ultrasonography, a thoracentesis may be indicated to differentiate the nature of the effusion and to identify possible pathogens. For young children who require sedation for thoracentesis and who have an effusion needing drainage, a primary chest tube placement is preferred over thoracentesis to decrease the risks from multiple procedures with sedation.
TABLE 2.13.
Differentiation of Pleural Fluid
| Transudate | Exudate | Complicated Empyema | |
|---|---|---|---|
| Appearance | Clear | Cloudy | Purulent |
| Cell count | <1000 | >1000 | >5000 |
| Cell type | Lymphocytes, monocytes | PMNs | PMNs |
| LDH | <200 U/L | >200 U/L | >1000 U/L |
| Pleural/serum LDH ratio | <0.6 | >0.6 | >0.6 |
| Protein >3 g | Unusual | Common | Common |
| Pleural/serum protein ratio | <0.5 | >0.5 | >0.5 |
| Glucose* | Normal | Low | Very low* (<40 mg/dL) |
| pH* | Normal (7.40–7.60) | 7.20–7.40 | <7.20 |
| Gram stain | Negative | Usually positive | >85% positive unless patient received prior antibiotics |
LDH, lactate dehydrogenase; PMNs, polymorphonuclear neutrophils.
Low glucose or pH may be seen in malignant effusion, tuberculosis, esophageal rupture, pancreatitis (positive pleural amylase), and rheumatologic diseases (e.g., systemic lupus erythematosus).
Differentiating among the causes of bacterial pneumonia can be done with certainty only with positive cultures from blood, pleural fluid, fluid obtained by direct lung tap, or, in rare cases, sputum. Current or previous antibiotic treatment diminishes the yield of such cultures. Bronchoscopy with or without lavage may yield helpful specimens from the progressively ill child or the child who has not responded promptly to empirical antibiotics.
Treatment of uncomplicated presumed bacterial pneumonia is with antibiotics. Ampicillin is the drug of choice for the previously healthy child who requires hospitalization with lobar pneumonia who is fully immunized. If the child is not fully immunized, either cefotaxime or ceftriaxone is indicated. For the critically ill child, vancomycin and cefotaxime/ceftriaxone may be considered for possible drug-resistant S. pneumoniae and methicillin-resistant S aureus (MRSA). Many children with pneumonia do well with oral antibiotics and respond within hours to the first dose. Repeated or follow-up chest radiographs may remain abnormal for 4-6 weeks after appropriate treatment and are not indicated for a single episode of uncomplicated pneumonia (i.e., no effusion, no abscess, and good response to treatment). Mycoplasma pneumoniae is a common cause of pneumonia among school-aged children. The disease often occurs in community outbreaks in the fall. The illness typically begins with extrapulmonary symptoms (i.e., sore throat, myalgias, headache, fever), which then progress to include cough, which can be paroxysmal at times. Patients do not often appear acutely ill, but cough may persist for weeks. There may be no specific abnormalities on the chest examination, although a few crackles may be heard, and about one third of younger patients wheeze.
The radiographic findings in mycoplasmal pneumonia can mimic almost any intrathoracic disease; scattered infiltrates with nonspecific “dirty” lung fields, predominantly perihilar or lower lobes, are common, and lobar infiltrates and pleural effusion are occasionally seen. Laboratory data (complete blood cell count, ESR, sputum culture) may not be helpful. A rise in antimycoplasma immunoglobulin G over 1-2 weeks may be demonstrated but is seldom helpful in guiding therapy. A positive immunoglobulin M response may be useful, although it can persist in serum for several months and, consequently, may not indicate current infection. PCR is helpful. The cold agglutinin test yields positive results in about 70% of patients with mycoplasmal pneumonia, but they are also positive in other conditions, including adenovirus infection. The more severe the illness is, the greater is the frequency of positive cold agglutinins. The diagnosis is often made from the history of an older child who has a lingering coughing illness in the setting of a community outbreak, unresponsive to most (nonerythromycin) antibiotic regimens.
Treatment with azithromycin, clarithromycin, or erythromycin in children <8 years old or tetracycline or doxycycline in children ≥8 years old usually shortens the course of illness. Extrapulmonary complications of mycoplasmal infection include aseptic meningitis, transverse myelitis, peripheral neuropathy, erythema multiforme, myocarditis, pericarditis, hemolytic anemia, and bullous otitis media (myringitis). In patients with sickle cell anemia, severe respiratory failure and acute chest syndrome may develop. Infection with Chlamydia pneumoniae mimics respiratory disease resulting from M. pneumoniae, inasmuch as it occurs in epidemics, is seen in older children, and produces an atypical pneumonia syndrome and pharyngitis.
Tuberculosis.
Tuberculosis is uncommon in developed countries; 95% of the disease burden worldwide is in developing countries. Tuberculosis must be considered in the child with chest disease that is not easily explained by other diagnoses, especially if the child lives in or has migrated from an endemic area of the world or has been exposed to an adult with active tuberculosis. Nonetheless, tuberculosis is an infrequent cause of cough in children, even in those with active disease.
The diagnosis is made primarily by skin testing (purified protein derivative [PPD]) or a positive Quantiferon test; a history of contact with a person who has tuberculosis; and recovery of the organism from sputum, bronchoalveolar lavage, pleural fluid or biopsy, or morning gastric aspirates (Table 2.14 ). The yield from these procedures is relatively low, even from children with active pulmonary tuberculosis.
TABLE 2.14.
Definitions of Positive Tuberculosis (TB) by Mantoux Skin Test (5 TU)*
| Cutaneous Induration ≥5 mm |
|
| Cutaneous Induration ≥10 mm |
| Children at increased risk |
|
| Children with likelihood of increased exposure |
|
| Cutaneous Induration ≥15 mm |
|
BCG, bacille Calmette-Guérin; HIV, human immunodeficiency virus; TU, tuberculin units.
BCG vaccination status not relevant.
Data from American Academy of Pediatrics. Tuberculosis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. 2015 Red Book: Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:806.
The patterns of disease in normal hosts include primary pulmonary tuberculosis, with subsequent inactivation usually noted in young children and reactivation pulmonary disease among adolescents. Primary pulmonary disease is often noted as a lower or middle lobe infiltrate during the period of T lymphocyte reaction to the initial infection. Before resolution, the Mycobacterium tuberculosis infection may disseminate to the better oxygenated upper lobes and extrathoracic sites, such as bone, or the central nervous system. If the immune response contains the initial infection, the radiographic findings may be indistinguishable from those of any other pneumonic process. With altered immune function, however, there may be progressive local disease, dissemination to miliary pulmonary disease, or early reactivation (months to 5 years) at distal sites, which produces tuberculous meningitis or osteomyelitis. Reactivation of upper lobe pulmonary disease may produce cavities that are similar to the disease among adults. Cavitary and endobronchial lymph node involvement are highly infectious, in contrast to the much less contagious nature of the hypersensitivity reaction noted in primary pulmonary disease.
Aspiration
Inhaling food, mouth or gastric secretions, or foreign bodies into the tracheobronchial tree causes acute, recurrent, or chronic cough. Interference with normal swallowing disrupts the coordination of swallowing and breathing that prevents aspiration. Structural causes of disordered swallowing include esophageal atresia (in neonates), strictures, webs, or congenital stenoses. Mediastinal lesions (tumors, lymph nodes), including vascular rings, may compromise the esophageal lumen and esophageal peristalsis, increasing the likelihood of aspiration. Functional disorders include central nervous system dysfunction or immaturity, dysautonomia, achalasia, and diffuse esophageal spasm. Prior neck surgery, including tracheostomy, may alter normal swallowing. Tracheoesophageal fistula and laryngeal clefts are congenital malformations with direct physical connections between the tracheobronchial tree and the upper gastrointestinal tract; thus oral contents enter the lungs directly.
Making the diagnosis of aspiration as the cause of cough may be difficult. Barium contrast studies during swallowing may help characterize these disorders if barium enters the trachea. Because most patients aspirate sporadically, a normal barium swallow does not rule out aspiration. Radionuclide studies can be helpful if ingested radiolabeled milk or formula is demonstrated over the lung fields at several-hour intervals after the meal. Bronchoscopy and bronchoalveolar lavage that recover large numbers of lipid-laden macrophages suggest that aspiration has taken place; however, the finding is neither sensitive nor specific for aspiration.
Treatment depends largely on the cause of aspiration. Because many patients who aspirate do so because of lack of neurologic control of swallowing and breathing, it is often difficult to prevent. Even gastrostomy feedings cannot prevent aspiration of oral secretions. In extreme cases, tracheostomy with ligation of the proximal trachea has been employed. This not only prevents aspiration but also prevents phonation, and it must be considered only in unusual situations. Aspiration pneumonia is often treated with intravenous ampicillin-sulbactam or clindamycin to cover mouth flora of predominant anaerobes. Additional coverage against gram-negative organisms may be indicated if the aspiration is nosocomial.
Foreign Body
Any child with cough of abrupt onset should be suspected of having inhaled a foreign body into the airway. Toddlers, who by nature put all types of things into their mouths and who have incompletely matured swallowing and airway protective mechanisms, are at high risk. Infants with toddlers or young children in the household who may “feed” the baby are also at risk. In older children, it is usually possible to obtain an accurate history of the aspiration event. These events are described as choking, gagging, and coughing while something (e.g., peanuts, popcorn, small toys, sunflower seeds) is in the mouth. The child may come to the physician with cough and wheeze immediately after the event, with a clear history and a straightforward diagnosis. In many children with a tracheobronchial foreign body, however, the initial episode is not recognized; these children may not come to medical attention for days, weeks, or even months. The initial episode may be followed by a relatively symptom-free period lasting days or even weeks, until infection develops behind an obstructed segmental or lobar bronchus. At this point, cough, perhaps with hemoptysis, with or without wheeze, recurs.
On physical examination early after an aspiration episode, there is cough, wheeze, or both, often with asymmetry of auscultatory findings. There may be locally diminished breath sounds. Later, localized wheeze or crackles may be detected. The triad of wheezing, coughing, and decreased breath sounds is present in fewer than 50% of patients. The presence of laryngotracheal foreign bodies often manifests with stridor, retractions, aphonia, cough, and normal radiographs.
Chest radiographs may be normal in 15% of patients with intrathoracic foreign bodies but should be obtained in both inspiration and expiration because in some cases the only abnormality is unilateral or unilobar air trapping, which is occasionally more clearly identified with an expiratory radiograph. In this view, an overdistended lung that had appeared normal on the inspiratory view does not empty, but the normal, unobstructed lung empties normally. This phenomenon causes a shift of the mediastinum toward the emptying lung, away from the side with the obstructing foreign body (Fig. 2.9 ). In other patients, localized infiltrate or atelectasis may be present behind the obstructing object. In a few patients, it may be possible to identify the foreign body itself; nonetheless, most inhaled food particles are not radiopaque and cannot be seen on radiographs. Aspiration is usually unilateral (80%); 50-60% of the objects are in the right lung (the lobe depends on body position—supine versus standing—but is often the right middle lobe). The definitive diagnostic and therapeutic maneuver is bronchoscopy; either the flexible or rigid open-tube bronchoscope enables direct visualization of the object; the rigid instrument also enables its removal.
FIGURE 2.9.

A, Normal inspiratory chest radiograph in a toddler with a peanut fragment in the left main bronchus. B, Expiratory radiograph of the same child showing the classic air trapping on the involved side.
(From Schroeder JW Jr, Holinger LD. Foreign bodies in the airway. In: Kliegman RM, Stanton BF, St Geme JW III, Schor N, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia: Elsevier; 2016:2040, Fig. 387.2.)
Gastroesophageal Reflux
(See Nelson Textbook of Pediatrics, p. 1787.)
GER is a common cause of cough in all age groups (see Chapter 12). The typical patient is an infant in the first 6 months of life who spits up small amounts of milk frequently after feedings. This “regurgitant reflux” most commonly resolves by 1 year of age. However, many toddlers and children continue to have reflux, although it may be “silent” or nonregurgitant (without spitting up).
In most people with GER, it is merely a nuisance or not noticed. In some there are sequelae, and this condition is designated gastroesophageal reflux disease (GERD). One manifestation is cough; the mechanisms for the cough are not fully understood. Aspiration of refluxed material is one mechanism for cough but is probably not very common in neurologically intact children. A major mechanism for GERD with cough is mediated by vagal esophagobronchial reflexes (bronchoconstriction), stimulated by acid in the esophagus. Whether acid in the esophagus is sufficient stimulus to cause bronchoconstriction by itself or whether it merely heightens bronchial reactivity to other stimuli is not yet clear. Many children with reactive airways disease have cough or wheeze that is difficult to control until their concurrent GER is also treated. Many episodes of cough caused by GERD occur in children with asthma that is difficult to control.
The diagnosis of GERD must also be considered in the child with chronic or recurrent cough with no other obvious explanation. The child who coughs after meals or at night, when the supine position may provoke GER, should be evaluated for GER. If GER is confirmed, the next step is a therapeutic trial of antireflux therapy.
Treatment in a child whose cough is related to GER may be accomplished by treating the reflux (see Chapter 12) or by a combination of antireflux and antiasthma treatment (see Chapter 3). On occasion, the cough may be abolished by stopping all antiasthma medications. In such cases, the cough was a manifestation of reactive airways with esophageal acidification as the trigger for bronchospasm; the esophageal acidification was caused by the bronchodilator effects on the lower esophageal sphincter.
Asthma
Cough is frequently the sole or most prominent manifestation of asthma; wheezing may be entirely absent. In fact, asthma is almost certainly the most common cause of recurrent and chronic cough in childhood (see Chapter 3). Some of the features that characterize the cough of a child with asthma are listed in Table 2.15 . Treatment for asthma manifesting as cough is the same as the treatment for asthma.
TABLE 2.15.
Asthma as a Cause of Cough: History
| Any age (even infants) |
| Coexistence of allergy increases likelihood, but absence of allergy does not decrease likelihood |
| Wheeze need not be present |
| ↑Cough with upper respiratory infections |
| ↑Cough with (and especially after) exercise |
| ↑Cough with hard laughing or crying |
| ↑Cough with exposure to cold |
| ↑Cough with exposure to cigarette smoke |
| Usually a history of dramatic response to inhaled β-agonists |
Cystic Fibrosis
Cystic fibrosis (CF) is a common cause of recurrent or chronic cough in infancy and childhood. CF occurs in 1 in 2000-3000 live births among white persons, is far less common among African Americans (1 in 15,000), and is rare among Native Americans and Asians. Early diagnosis improves the prognosis for untreated CF; if untreated, many patients die in infancy or early childhood. With current state-of-the-art care, median length of survival is upper 30s.
CF is a genetic disorder, inherited as an autosomal recessive trait. The CF gene is on the long arm of chromosome 7; more than 1900 mutations have been identified at the CF locus. Of these mutations, one (ΔF508, indicating a deletion, Δ, of a single phenylalanine, F, at position 508 of the protein product) is the most common, responsible for 70-75% of all CF chromosomes. The mutation affects the gene's protein product, termed cystic fibrosis transmembrane regulator (CFTR), which acts as a chloride channel and affects other aspects of membrane transport of ions and water. Not all the consequences of the defective gene and protein have been determined. In general, however, the defective gene product results in the long-observed clinical manifestations of the disease, including thick, viscid mucus in the tracheobronchial tree, leading to purulent bronchiolitis and bronchitis with subsequent bronchiectasis, pulmonary fibrosis, and respiratory failure; pancreatic duct obstruction, leading to pancreatic insufficiency with steatorrhea and failure to thrive; and abnormally high sweat chloride and sodium concentrations. The airway disease in CF is characterized by infection, inflammation, and endobronchial obstruction. The infection begins with S. aureus, H. influenzae, Escherichia coli, Klebsiella species, or combinations of these organisms but eventually is dominated by nonmucoid or mucoid Pseudomonas aeruginosa. Other organisms, such as Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, Aspergillus fumigatus, or nontuberculous mycobacteria may also appear; their significance remains undetermined. In some patients, B. cepacia has been associated with rapid deterioration and death, and in others, Aspergillus species has caused allergic bronchopulmonary aspergillosis (ABPA). The airway inflammation in all patients with CF appears to be the result of toxic substances, including elastase, released by neutrophils as they respond to the endobronchial infection and by similar enzymes released by the invading organisms.
CF may manifest at birth with meconium ileus (10-15% of patients), or later, with steatorrhea and failure to thrive despite a voracious appetite, in an apparent effort to make up for the calories that are lost in the stool (see Chapter 11). The most common presenting symptom is cough, which may appear within the first weeks of life or may be delayed for decades. The cough can be dry, productive, or paroxysmal. Cough may respond to antibiotics or perhaps steroids, but it is less likely to improve with bronchodilators (see Tables 2.3 and 2.5). Although CF is a genetic disease, there is often no family history. Furthermore, in atypical cases, patients may not have pancreatic insufficiency (~10% of patients) and thus may not demonstrate steatorrhea and failure to thrive. In addition, malabsorption may not be evident in the neonatal period.
There is no such thing as a child who looks “too good” to have CF; common abnormalities found on physical examination are noted in Table 2.16 . One of the most important physical findings is digital clubbing. In most patients with CF, clubbing develops within the first few years of life. Although the list of conditions associated with digital clubbing (Table 2.17 ) is long, they are less common than CF, or the incidence of digital clubbing with these conditions is low. There is some relationship between the degree of pulmonary disease severity and the degree of digital clubbing. A child who has had years of severe respiratory symptoms without digital clubbing is not likely to have CF.
TABLE 2.16.
Physical Examination Features of Cystic Fibrosis
| General |
| Low weight for height (>50% of patients) |
| Head, eyes, ears, nose, and throat |
| Nasal polyps (20%) |
| Chest |
| Cough |
| Barrel chest (↑anteroposterior diameter) |
| Intercostal, suprasternal retractions |
| Crackles, especially upper lobes |
| Wheeze |
| Abdomen |
| Hepatomegaly (10%) |
| Right lower quadrant fecal mass (5-10%) |
| Extremities |
| Digital clubbing (80%) |
| Reproductive tract |
| Bilateral atresia or absence of vas deferens (>95% of males) |
TABLE 2.17.
Causes of Digital Clubbing in Children
| Pulmonary |
|
| Cardiac |
|
| Gastrointestinal |
|
| Hepatic |
|
| Hematologic |
|
| Miscellaneous |
|
++, very common cause of digital clubbing; +, common cause of digital clubbing.
The diagnosis is confirmed by a positive sweat test or confirming the presence of two CF mutations on chromosome 7. The sweat test, if not performed correctly in a laboratory with extensive experience with the technique (as, for example, in an accredited CF center), yields many false-positive and false-negative results. The proper technique is to use quantitative analysis of the concentration of chloride in the sweat produced after pilocarpine iontophoretic stimulation. Chloride concentrations higher than 60 mmol/L are considered positive, and those lower than 40 mmol/L are negative (normal). Healthy adults have slightly higher sweat chloride concentrations than do children, but the same guidelines hold for positive tests in adults. The non-CF conditions yielding elevated sweat chloride concentrations are listed in Table 2.18 . False-negative results of sweat tests can be seen in CF children presenting with edema or hypoproteinemia and in samples from children with an inadequate sweat rate. Sweat testing can be performed at any age. Newborns within the first few weeks of life may not produce a large enough volume of sweat to analyze (75 mg minimum), but in those who do (the majority), the results are accurate. Indications for sweat testing are noted in Table 2.19 .
TABLE 2.18.
Conditions Other Than Cystic Fibrosis with Elevated Sweat Chloride
| Adrenal insufficiency (untreated) |
| Ectodermal dysplasia |
| Autonomic dysfunction |
| Hypothyroidism |
| Malnutrition, including psychosocial dwarfism |
| Mucopolysaccharidosis |
| Glycogen storage disease (type I) |
| Fucosidosis |
| Hereditary nephrogenic diabetes insipidus |
| Mauriac syndrome |
| Pseudohypoaldosteronism |
| Familial cholestasis |
| Nephrosis with edema |
TABLE 2.19.
Indications for Sweat Testing
| Pulmonary Indications |
|
| Gastrointestinal Indications |
|
| Miscellaneous Indications |
|
RUL, right upper lobe.
From Kercsmar CM. The respiratory system. In: Behrman RE, Kliegman RM, eds. Nelson Essentials of Pediatrics. 2nd ed. Philadelphia: WB Saunders; 1994:451.
In patients for whom sweat testing is difficult (e.g., because of distance from an experienced laboratory, small infants who have not produced enough sweat, patients with extreme dermatitis, or patients with intermediate-range sweat chloride concentrations), DNA mutation testing can be useful. Demonstration of two known CF mutations confirms the diagnosis. Finding one or no known mutation makes the diagnosis less likely but is not exclusive, inasmuch as there are patients with not-yet-characterized mutations. Furthermore, commercial laboratories do not identify all of the mutations.
Recovery of mucoid Pseudomonas aeruginosa from respiratory tract secretions is strongly suggestive of CF. Similarly pansinusitis is nearly universal among CF patients but is quite uncommon in other children. All 50 states are using a neonatal screen for CF. The CF screen assays include measuring serum immunoreactive trypsinogen (IRT) levels, which are elevated in most infants with CF for the first several weeks of life, and DNA analysis for CFTR mutations. The main drawback of the IRT assay is that it has relatively poor specificity; as many as 90% of the positive results on the initial screen are false-positive results. If an infant's IRT screen is positive, the test should be repeated, or DNA analysis for the 23 most common CFTR mutations should be performed. At 2-3 weeks of age, which is when the IRT is repeated, the false-positive rate has fallen dramatically but is still quite high (25%). Definitive testing with the sweat chloride test needs to be carried out on infants with positive screening results.
Laboratory data that may support the diagnosis of CF include low levels of fecal elastase. This suggests pancreatic insufficiency, which occurs most commonly in CF but can be seen in other diseases. The test is not perfect for confirming CF as some CF patients have sufficient pancreatic function. Pulmonary function test findings with an obstructive pattern, incompletely responsive to bronchodilators, are consistent with CF but, of course, can be seen in other conditions. Conversely, some patients with CF also have asthma and may show a marked response to a bronchodilator. Complications of CF that should suggest the diagnosis are noted in Table 2.20 .
TABLE 2.20.
Complications of Cystic Fibrosis
| Pulmonary Complications |
|
| Gastrointestinal Complications |
|
| Other Complications |
|
From Kercsmar CM. The respiratory system. In: Behrman RE, Kliegman RM, eds. Nelson Essentials of Pediatrics. 2nd ed. Philadelphia: WB Saunders; 1994:451.
The treatment of patients with CF requires a comprehensive approach, best performed in, or in conjunction with, an approved CF center. Several studies have shown survival to be significantly better in center-based care than in non–center-based care.
Anatomic Abnormalities
Table 2.21 lists the main anatomic abnormalities that cause cough.
TABLE 2.21.
Anatomic Abnormalities Causing Cough
| Condition | Other Symptoms | Diagnostic Evidence | Treatment |
|---|---|---|---|
| Vascular ring/sling | Stridor; dysphagia, emesis | Radiographic: deviated trachea, right-sided arch Barium swallow: esophageal indentation Bronchoscopy: extrinsic compression MRI/angiography: definitive |
Surgical |
| Pulmonary sequestration | Fever, dyspnea; may be asymptomatic | Radiographic: left lower lobe density, usually with cysts Angiography: blood supply from aorta |
Surgical |
| Congenital pulmonary airway malformation | Respiratory distress; recurrent infection | Radiographic: multiple cysts alternating with solid areas | Surgical |
| Congenital lobar emphysema | Respiratory distress; wheeze; may be asymptomatic | Radiographic: localized overinflation, other lobes (even other lung) collapsed Bronchoscopy to rule out foreign body |
Surgical (if symptomatic) |
| Tracheoesophageal fistula, cleft | Gagging, choking with feeds; respiratory distress (especially with esophageal atresia) | Barium esophagram: barium in tracheobronchial tree Bronchoscopy: direct visualization |
Surgical |
| Airway hemangioma | Stridor; wheeze; dysphagia; hemoptysis | Bronchoscopy | Propranolol, steroids; laser; occasionally tracheostomy required |
| Mediastinal lymph nodes | Stridor | Radiographic: hilar nodes; compressed tracheal air column | Treat cause |
| Bronchial stenosis | Wheeze; recurrent pneumonia | Bronchoscopy | Balloon dilatation; surgery |
| Bronchogenic cysts | Wheeze, stridor | Radiographic: hyperinflation of one lung; visible mass (carina, posterior mediastinum) Bronchoscopy: extrinsic compression CT: often definitive |
Surgical |
CT, computed tomography; MRI, magnetic resonance imaging.
Vascular rings and slings.
Vascular rings and slings are often associated with inspiratory stridor because the abnormal vessels compress central airways, most commonly the trachea (see Chapter 3). The patient may also have difficulty swallowing if the esophagus is compressed.
The diagnosis may be suspected from plain radiographs of the chest, especially those showing tracheal deviation and a right-sided aortic arch. Further support for the diagnosis can be found at bronchoscopy (which shows extrinsic compression of the trachea or a main stem bronchus), barium esophagram (which shows esophageal compression), or both. The definitive diagnosis is made with computed tomographic angiography or magnetic resonance angiography. Treatment is surgical.
Pulmonary sequestration.
Pulmonary sequestration is relatively unusual, occurring in 1 in 60,000 children. It occurs most commonly in the left lower lobe and can manifest in several ways (Fig. 2.10 ; see also Table 2.21). The chest radiograph usually shows a density in the left lower lobe; this density often appears to contain cysts (Fig. 2.11 ). The feature distinguishing a sequestered lobe from a complicated pneumonia is that the blood supply arises from the aorta and not the pulmonary circulation. Doppler ultrasonography and CT angiography provide the definitive diagnosis. The treatment is surgical removal.
FIGURE 2.10.

Different modes of presentation of sequestered lobe and number of children with each problem.
(From Phelan PD, Olinsky A, eds. Respiratory Illness in Children. Oxford: Wiley-Blackwell; 1994.)
FIGURE 2.11.

Anterior-posterior and lateral chest radiographs showing a left lower lobe pulmonary sequestration (bold arrow) in a 15-year-old girl.
Congenital pulmonary airway malformation (CPAM).
Congenital pulmonary airway malformations (formerly known as congenital cystadenomatoid malformations or CCAMs) are rare. They manifest in infancy with respiratory distress in nearly 50% of cases; the other half may manifest as cough with recurrent infection later in childhood or even adulthood. The chest radiograph reveals multiple cysts, separated by dense areas. Chest CT scans can help make the diagnosis with near certainty. Surgical removal is the treatment of choice if the lesion is symptomatic.
Congenital lobar emphysema.
Congenital lobar emphysema occurs in one of 50,000 live births. It can manifest dramatically with respiratory distress in the neonatal period or later (Fig. 2.12 ), with cough or wheeze, or as an incidental finding on a chest radiograph. Radiography shows localized overinflation, often dramatic, with compression of adjacent lung tissue and occasionally atelectasis of the contralateral lung because of mediastinal shift away from the involved side. The appearance on chest CT scan is typical, with widely spaced blood vessels (as opposed to congenital cysts, for example, which have no blood vessels within the overinflated area). Bronchoscopy can document patent bronchi and should probably be performed in older children in whom congenital lobar emphysema can be confused with acquired overinflation of a lobe as the result of bronchial obstruction, as with a foreign body. If the disease is symptomatic, treatment is surgical.
FIGURE 2.12.

Different modes of presentation of congenital lobar emphysema. A, Age at presentation. B, Symptoms at presentation. C, Involved lobe. Numbers refer to the number of children in each category.
(From Phelan PD, Olinsky A, eds. Respiratory Illness in Children. Oxford: Wiley-Blackwell; 1994.)
Tracheoesophageal fistula.
Tracheoesophageal fistula is common, with an incidence of about one in 5000 live births. Of these fistulas, the large majority (85%) are associated with esophageal atresia; only 3% are the isolated, H-type fistula (a patent esophagus with fistulous tract connecting the esophagus and trachea). A neonate with esophageal atresia experiences respiratory distress, excessive drooling, and choking and gagging with feeding. The H-type fistula causes more subtle signs and may be undiagnosed for months or even years. The child may have only intermittent feeding trouble, especially with liquids. There may be recurrent lower respiratory tract infections.
The diagnosis is not challenging in the infant with esophageal atresia; a nasogastric tube cannot be passed, and swallowed barium outlines the trachea. In the older child with H-type fistula, a barium esophagogram may or may not reveal the fistula. Bronchoscopy and esophagoscopy should permit direct visualization of the fistula; however, the opening may be hidden in mucosal folds.
Treatment is surgical. Many children born with tracheoesophageal fistula have recurrent cough and lower respiratory tract infection for many years, even after successful surgical correction. The cough is characteristically the harsh cough of tracheomalacia, which is present at the site of the fistula. The infections result from several causes, including GER, with or without aspiration, and altered mucociliary transport. Treatment involves regular chest physiotherapy and early and aggressive use of antibiotics whenever there is evidence of increased pulmonary symptoms.
Hemangiomas.
Hemangiomas may be present within the airway and can cause cough, rarely with hemoptysis. Stridor (if the hemangioma is high in the airway) and respiratory distress (if the hemangioma is large) may also occur. In rare cases, with very large airway hemangiomas, there may even be dysphagia from extrinsic compression. Children with cutaneous hemangiomas in the mandibular or neck region (“beard” distribution) are at risk for an airway hemangioma.
The diagnosis is made with bronchoscopy. These lesions may resolve spontaneously over the first year or so. However, if they cause symptoms, it may not be advisable or possible to wait for them to resolve.
Many airway hemangiomas regress with steroid treatment; however, due to the side effect profile, propranolol is considered the treatment of choice. Asthma is a contraindication for propranolol treatment due to its beta-blocking effect and potential to worsen asthma. Laser ablation may be indicated in some refractory cases that do not respond to first-line treatment. In the case of a large subglottic hemangioma, a tracheostomy is performed and maintained until the mass regresses.
Enlarged lymph nodes.
Enlarged mediastinal lymph nodes, such as those resulting from tuberculosis, leukemia, other hematologic malignancies, or other infections, are occasionally a cause of cough in children (Table 2.22 ; see also Table 2.21). These nodes are usually seen on plain radiographs of the chest. The x-ray study or bronchoscopy may show extrinsic compression of the trachea. Treatment is directed at the underlying cause.
TABLE 2.22.
Intrathoracic Mass Lesions
| Anterior Mediastinum |
|
| Hilar–Middle Mediastinum |
|
| Posterior Mediastinum |
|
Bronchial stenosis.
Occasionally bronchial stenosis, either congenital or acquired, may cause cough. The diagnosis is made with bronchoscopy, after suspicion has been raised by the child having recurrent infiltrates in the same lobe, especially with localized wheeze.
Treatment may be difficult. In some cases, endoscopic balloon dilatation or airway stent placement is successful; in others, surgical resection of stenotic areas may be necessary.
Bronchogenic cysts.
Bronchogenic cysts are uncommon, but they can cause cough, wheeze, stridor, or any combination of these. They may also cause recurrent or persistent pneumonia if they block a bronchus sufficiently to interfere with normal drainage of the segment or lobe. Radiography may show localized overinflation if the cyst causes a ball-valve–type obstruction. The cyst itself may or may not be seen on plain radiographs. Bronchoscopy reveals extrinsic compression of the airway. CT studies often definitively show the lesion. Surgical removal is indicated.
Habit (Psychogenic) Cough
On occasion, a school-aged child may develop a cough that lasts for weeks, often after a fairly typical cold. This cough occurs only during wakefulness, never during sleep. In many cases, the cough is harsh and foghorn-like. It often disrupts the classroom, and the child is asked to leave. The child is otherwise well and may seem rather unbothered by the spectacle created. There is no response to medications. It seems that this type of cough, previously termed “psychogenic,” or “psychogenic cough tic,” but now called habit cough, has given the child valuable attention. This attention then serves as the sustaining force, and the cough persists beyond the original airway inflammation. In the small minority of cases, there may be deep-seated emotional problems of which the cough is the physical expression.
During the history or physical examination, the child appears completely well and may cough when attention is drawn to the child or when the word “cough” is uttered. The physical examination findings are otherwise completely normal, as are laboratory values. Because this may occur in any child, evidence of mild reactive airways disease (history or pulmonary function testing) does not rule out the diagnosis. Once a physician has seen a child with this problem, it is usually possible to make the diagnosis with certainty on entering the examining room or, indeed, from the hallway outside the room.
Treatment can prove more difficult. The child and family should be reassured that the child is well. Suggestion therapy empowers and encourages the patient to suppress the cough for short increments of time. The goal is for them to gradually lengthen the cough-free intervals. Speech therapy may be helpful (Table 2.23 ).
TABLE 2.23.
Speech Therapy Techniques for Treating Habit Cough
| Increase abdominal breathing |
| Reduce muscle tension in neck, chest, and shoulders |
| Interrupt early cough sensation by swallowing |
| Substitute gentle cough for racking cough |
| Interrupt cough sequence with diaphragm breathing and tightly pursed lips |
| Increase the patient's awareness of initial sensations that would trigger cough |
Other Causes of Cough
Table 2.24 lists several miscellaneous causes of cough in children.
TABLE 2.24.
Miscellaneous Causes of Cough in Children
| Postnasal drip (?) Bronchiectasis Ciliary dyskinesia Interstitial lung disease Heart failure/pulmonary edema Pulmonary hemosiderosis Drug-induced (see Table 2.4) α1-Antitrypsin deficiency Graft-versus-host disease Bronchopulmonary dysplasia Tumor (bronchial adenoma, carcinoid; mediastinal) Alveolar proteinosis Tracheomalacia, bronchomalacia Spasmodic croup |
Cerumen impaction Irritation of external auditory canal Obliterative bronchiolitis Follicular bronchiolitis Mediastinal disease (nodes, pneumomediastinum) Nasal polyps Hypersensitivity pneumonitis (extrinsic allergic alveolitis) Thyroid lesions Subphrenic abscess Sarcoidosis Anaphylaxis Pulmonary embolism Lung contusion |
Bronchiectasis.
Bronchiectasis is defined as an abnormal dilation of the subsegmental bronchi and is usually associated with chronic cough and purulent sputum production. It occasionally occurs after severe pneumonias (bacterial or viral); it eventually develops in nearly all patients with CF. Diagnosis may, on occasion, be made with plain-chest radiographs, but high-resolution CT scanning is the diagnostic procedure of choice. Treatment of bronchiectasis consists of airway clearance with chest physiotherapy with postural drainage or high-frequency chest wall oscillation, occasionally bronchodilators and mucolytic agents, and antibiotic therapy during exacerbations. Surgical resection may be indicated in cases that are progressive and localized when medical therapy has failed. The prognosis of bronchiectasis depends on the underlying cause. CF-associated bronchiectasis is a major cause of CF-related morbidity and mortality; whereas, non-CF bronchiectasis may remain stable or even regress with therapy.
Ciliary dyskinesia.
Conditions in which the cilia do not function properly (immotile cilia or ciliary dyskinesia) lead to cough, usually because infection (and bronchiectasis) occurs in the absence of normal mucociliary transport. Treatment is similar to that for CF, with regular chest physiotherapy and frequent and aggressive use of antibiotics at the first sign of airways infection, most commonly increased cough.
Interstitial lung disease.
Interstitial lung diseases are now classified based on those that occur during the neonatal period and those that are not as prevalent in infancy. Interstitial lung diseases that manifest with cough include aspiration (chronic and recurrent) pneumonitis, hypersensitivity pneumonitis, bronchiolitis obliterans, and cryptogenic organizing pneumonia (formerly known as bronchiolitis obliterans organizing pneumonia or BOOP). Lung biopsy may be required for a diagnosis.
Pulmonary hemosiderosis.
Pulmonary hemosiderosis is a rare, and often fatal, condition of bleeding into the lung that can manifest with cough. If sputum is produced, it is often frothy and blood-tinged. There may be frank hemoptysis. However, the cough may be nonproductive, or the sputum may be swallowed. Some cases are associated with milk hypersensitivity (Heiner syndrome), and affected children may have upper airway obstruction. Some cases are associated with collagen vascular disorders. Radiographs usually show diffuse fluffy infiltrates, and there is invariably iron deficiency anemia. The diagnosis is based on lung biopsy findings.
Tumors.
Tumors causing cough are rare in childhood. Cough occurs because of bronchial blockage, either extrinsic or endobronchial (see Table 2.22). The diagnosis is usually made from bronchoscopy, chest CT, or both. Treatment depends on the cell type, but it usually involves at least some surgical removal. Chemotherapy or radiation may be used in some cases.
Tracheomalacia and bronchomalacia.
Isolated tracheomalacia or bronchomalacia is uncommon but can cause cough in some children. The cough of tracheomalacia is typically harsh and brassy. Treatment is difficult but, fortunately, is seldom needed.
Spasmodic croup.
Some children, usually preschoolers, may episodically awaken at night with stridor and a harsh, barking cough indistinguishable from that of viral croup. This entity is termed spasmodic croup and is of unclear origin. Viral and allergic causes have been postulated. GER may be the cause in some patients.
Treatment with cool mist or racemic epinephrine is effective in most patients. If GER is the underlying cause, antireflux treatment is beneficial.
Obliterative bronchiolitis.
Obliterative bronchiolitis is very rare except in lung transplant recipients. In other instances, it may arise after adenovirus, measles, or influenza pneumonia; after exposure to certain toxins; or in other rare circumstances. Children may exhibit cough, respiratory distress, and exercise intolerance.
The diagnosis is suggested by the pulmonary function test or radiographic evidence of small airways obstruction; however, these findings are not always present. Not all chest radiographs show overinflated lungs, and not all pulmonary function tests show decreased small airways function.
The definitive diagnosis is histologic via open or transbronchial biopsy. No specific treatment is available. Most children with obliterative bronchiolitis recover, but many progress to chronic disability or death.
Hemoptysis
The child who coughs out blood or bloody mucus presents special diagnostic and therapeutic challenges. Although hemoptysis is relatively uncommon in children, particularly among those without CF, many conditions can cause it (Table 2.25 ). It is important (and not always easy) to distinguish cases in which blood has originated in the tracheobronchial tree (true hemoptysis), the nose (epistaxis), and the gastrointestinal tract (hematemesis). Table 2.26 gives some guidelines to help localize sites of origin of blood that has been reported or suspected as hemoptysis. None of these guidelines is foolproof, partly because blood that has originated in one of these sites might well end up in another before being expelled from the body; for instance, blood from the nose can be swallowed and vomited or aspirated and expectorated.
TABLE 2.25.
Hemoptysis: Differential Diagnosis
| Infection | Lung abscess Pneumonia* Tuberculosis Bronchiectasis* (cystic fibrosis, ciliary dyskinesia) Necrotizing pneumonia Fungus (especially allergic bronchopulmonary aspergillosis or mucormycosis) Parasite Herpes simplex |
| Foreign body | Retained |
| Congenital defect | Heart (various) Primary pulmonary hypertension Eisenmenger syndrome Arteriovenous malformation Telangiectasia (Osler-Weber-Rendu) Pulmonary sequestration Bronchogenic cyst |
| Autoimmune-inflammatory | Henoch-Schönlein purpura Goodpasture syndrome Systemic lupus erythematosus Sarcoidosis Granulomatosis with polyangiitis |
| Pulmonary hemosiderosis | Idiopathic or with milk allergy (Heiner syndrome) |
| Trauma | Contusion* Fractured trachea, bronchus |
| Iatrogenic | After surgery After transbronchial lung biopsy* After diagnostic lung puncture* |
| Tumors | Benign (neurogenic, hamartoma, hemangioma, carcinoid) Malignant (adenoma, bronchogenic carcinoma) Metastatic (Wilms tumor, osteosarcoma, sarcoma) |
| Pulmonary embolus | Cardiogenic Deep vein thrombosis |
| Other | Factitious Endometriosis Coagulopathy* Congestive heart failure After surfactant therapy in neonates Kernicterus Hyperammonemia Intracranial hemorrhage Epistaxis* Idiopathic |
A common cause of hemoptysis.
TABLE 2.26.
Hemoptysis: Differentiating Sites of Origin of Blood
| Pulmonary | Gastrointestinal Tract | Nose | |
|---|---|---|---|
| History | Cough, with or without gurgling in lung before episode | Nausea, vomiting, abdominal pain | With or without nosebleed dripping in back of throat |
| Physical | Cough; localized crackles or decreased breath sounds; digital clubbing | ↑Liver, spleen, epigastric tenderness | Blood in nose |
Infection is among the most common causes of hemoptysis. Lung abscess and tuberculosis need to be considered. Bronchiectasis can readily cause erosion into bronchial vessels, often made tortuous by years of local inflammation, and produce hemoptysis. Other infectious causes are less common and include necrotizing pneumonias and fungal and parasitic lung invasion.
Foreign bodies in the airway can cause hemoptysis by direct irritation, by erosion of airway mucosa, or by secondary infection.
Pulmonary embolus is uncommon in children and adolescents, but it needs to be considered in the differential diagnosis of an adolescent with hemoptysis of unclear origin. Clues to the diagnosis of pulmonary embolus include a positive family history, severe dyspnea, chest pain, hypoxia, a normal chest radiograph, an accentuated second heart sound, an abnormal compression ultrasonographic study of the leg veins, a positive Homans sign, a positive helical CT scan, and a high-probability lung ventilation-perfusion scan.
The diagnosis of several causes of hemoptysis is straightforward. For example, hemoptysis that occurs immediately after a surgical or invasive diagnostic procedure in the chest should suggest an iatrogenic problem. The chest radiograph can help suggest lung abscess, pulmonary sequestration, bronchogenic cyst, or tumor. Chest CT can help with cases of arteriovenous malformations, and additional laboratory values can support the diagnosis of collagen vascular disease. Bronchoscopy can sometimes localize a bleeding site, identify a cause (e.g., a foreign body or endobronchial tumor), or recover an offending bacterial, fungal, or parasitic pathogen. In many instances, bronchoscopy does not help except by excluding some possibilities, because either no blood or blood throughout the tracheobronchial tree is found. Bronchial artery angiography may help to identify the involved vessel or vessels.
Treatment of hemoptysis depends on the underlying cause. It can be a terrifying symptom to children and their parents, and a calm, reassuring approach is essential. Because hemoptysis is seldom fatal in children, reassurance is usually warranted. Furthermore, hemoptysis most often resolves, and treatment of the bleeding itself is not often needed. What is required is treatment of the underlying cause of the hemoptysis, such as therapy for infection, removal of a foreign body, or control of collagen vascular disease. When death occurs from hemoptysis, it is more likely to be from suffocation than from exsanguination. In cases of massive bleeding, the rigid open-tube bronchoscope may help suction large amounts of blood while ventilating and keeping unaffected portions of lung clear of blood. Interventional radiologists treat as well as localize a bleeding site by injecting the offending vessel with occlusive substances (embolization). In extremely rare instances, emergency lobectomy may be indicated.
When Cough Itself Is a Problem
Cough itself seldom necessitates specific treatment. Nonetheless, cough is not always completely benign (see Table 2.9). Most complications are uncommon, and most accompany only very severe cough, but some are serious enough to justify treatment of the cough itself.
Cough suppressants include codeine and hydrocodone (two narcotics) and dextromethorphan (a nonnarcotic D-isomer of the codeine analog of levorphanol). Such agents should be used only for severe cough that may produce significant complications (see Table 2.9). For most diseases, suppressing the cough offers no advantage. Disadvantages include narcotic addiction and loss of the protective cough reflex with subsequent mucous retention and possible superinfection. Demulcent preparations (sugar-containing, bland soothing agents or honey) temporarily suppress the cough response from pharyngeal sources, and decongestant-antihistamine combinations may reduce postnasal drip.
Summary and Red Flags
Cough is important because it is a symptom and sign of underlying disease that frequently merits treatment. In the acute setting, severe disease, including massive hemoptysis or profound dyspnea or hypoxemia, warrants immediate attention, rapid diagnosis, and rapid management. Certain chronic conditions, including those that suggest CF and those in which symptoms have persisted and interfere with a child's daily activities and quality of life, warrant further evaluation and treatment. Finally, a child whose cough fails to respond to what should have been reasonable treatment should be referred to a pulmonary specialist (Table 2.27 ).
TABLE 2.27.
Red Flags for Cough
| If associated with severe, acute |
| Hemoptysis |
| Dyspnea |
| Hypoxemia |
| If associated with chronic |
| Failure to thrive |
| Steatorrhea |
| Decreased exercise tolerance |
| Digital clubbing |
| Persistence of |
| Cough for 6 weeks or more |
| Radiographic abnormality, especially if asymmetric |
| Failure to respond to empirical therapy |
| Antibiotics for presumed infection |
| Bronchodilators for presumed reactive airways |
References
A bibliography is available at ExpertConsult.com.
References
Cough
- Black P. Evaluation of chronic or recurrent cough. In: Hilman BC, editor. Pediatric Respiratory Disease: Diagnosis and Treatment. WB Saunders; Philadelphia: 1993. pp. 143–154. [Google Scholar]
- Chang A, Glomb WB. Guidelines for evaluating chronic cough in pediatrics. ACCP Evidence-based Clinical Practice Guidelines. Chest. 2006;129:260S–283S. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- Shields MD, Bush A, Everard ML. Recommendations for the assessment and management of cough in children. Thorax. 2008;63(suppl III):iii1–iii15. doi: 10.1136/thx.2007.077370. [DOI] [PubMed] [Google Scholar]
Upper Respiratory Infection
- Donnelly BW, McMillan JA, Weiner LB. Bacterial tracheitis: Report of eight new cases and review. Rev Infect Dis. 1990;12:729–735. doi: 10.1093/clinids/164.5.729. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer MK, Shehab N, Cohen AL. Adverse events from cough and cold medication in children. Pediatrics [Internet] 2008;121:783–787. doi: 10.1542/peds.2007-3638. [DOI] [PubMed] [Google Scholar]
- Williams JW, Simel DL. Does this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA. 1993;270:1242–1246. [PubMed] [Google Scholar]
Pneumonia
- American Academy of Pediatrics . Pertussis (whooping cough) In: Kimberlin DW, Brady MT, Jackson MA, editors. 2015 Red Book: Report of the Committee on Infectious Diseases. 30th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2015. pp. 608–621. [Google Scholar]
- American Academy of Pediatrics . Tuberculosis. In: Kimberlin DW, Brady MT, Jackson MA, editors. 2015 Red Book: Report of the Committee on Infectious Diseases. 30th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2015. pp. 805–831. [Google Scholar]
- Balfour-Lynn IM, Abrahamson E, Cohen G. BTS guidelines for the management of pleural infection in children. Thorax. 2005;60:i1–i21. doi: 10.1136/thx.2004.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke SJ. Chlamydial respiratory infections. BMJ. 1993;306:1219–1220. doi: 10.1136/bmj.306.6887.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JS, Byington CL, Shah SS. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasfield DM, Stagno S, Whitley RJ. Infant pneumonitis associated with cytomegalovirus, Chlamydia, Pneumocystis, and Ureaplasma: Follow-up. Pediatrics. 1987;79:76–83. [PubMed] [Google Scholar]
- Gibson NA, Hollman AS, Paton JY. Value of radiological follow-up of childhood pneumonia. BMJ. 1993;307:117. doi: 10.1136/bmj.307.6912.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Clark J, Coote N. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax. 2011;66:ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler LA, Starke JR. Tuberculosis (Myconbacterium tuberculosis) In: Kliegman RM, editor. Nelson Textbook of Pediatrics. 20th ed. Elsevier; Philadelphia: 2016. pp. 1445–1461. [Google Scholar]
- McCracken GH., Jr Etiology and treatment of pneumonia. Pediatr Infect Dis J. 2000;19:373–377. doi: 10.1097/00006454-200004000-00032. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Stein DS. Pulmonary manifestations of acquired immunodeficiency syndrome. Clin Infect Dis. 1992;14:98–113. doi: 10.1093/clinids/14.1.98. [DOI] [PubMed] [Google Scholar]
- Mulholland EK, Simoes EAF, Costales MOD. Standardized diagnosis of pneumonia in developing countries. Pediatr Infect Dis. 1992;11:77–81. doi: 10.1097/00006454-199202000-00004. [DOI] [PubMed] [Google Scholar]
- Onyango FE, Steinhoff MC, Wafula EM. Hypoxaemia in young Kenyan children with acute lower respiratory infection. BMJ. 1993;306:612–614. doi: 10.1136/bmj.306.6878.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348–361. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- Ralston SL, Lieverthal AS, Meissner C. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- Rubin BK. The evaluation of the child with recurrent chest infections. Pediatr Infect Dis. 1985;4:88–98. doi: 10.1097/00006454-198501000-00022. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mendoza-Sassi RA, Wainwright C. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2013;(31) doi: 10.1002/14651858.CD006458.pub3. CD006458. [DOI] [PubMed] [Google Scholar]
Foreign Bodies
- Black RE, Choi K-J, Syme WC. Bronchoscopic removal of aspirated foreign bodies in children. Am J Surg. 1984;148:778–781. doi: 10.1016/0002-9610(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Friedman EM. Tracheobronchial foreign bodies. Otolaryngol Clin North Am. 2000;33:179–185. doi: 10.1016/s0030-6665(05)70214-0. [DOI] [PubMed] [Google Scholar]
- Gay BB, Atkinson GO, Vanderzalm T. Subglottic foreign bodies in pediatric patients. Am J Dis Child. 1986;140:165–168. doi: 10.1001/archpedi.1986.02140160083041. [DOI] [PubMed] [Google Scholar]
- Puhakka H, Svedstrom E, Kero P. Tracheobronchial foreign bodies. Am J Dis Child. 1989;143:543–545. [PubMed] [Google Scholar]
Cystic Fibrosis
- Hamos A, Corey M. The cystic fibrosis genotype-phenotype consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1993;329:1308–1316. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics. The road ahead. Chest. 2013;143:207–213. doi: 10.1378/chest.12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem E, Reisman J, Corey M. Wheezing in infants with cystic fibrosis: Clinical course, pulmonary function, and survival analysis. Pediatrics. 1992;90:703–706. [PubMed] [Google Scholar]
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- Prasad SA, Tannenbaum EL, Mikelsons C. Physiotherapy in cystic fibrosis. J R Soc Med. 2000;93:27–36. [PMC free article] [PubMed] [Google Scholar]
- Ranasinha C, Assoufi B, Shak S. Efficacy and safety of short-term administration of aerosolised recombinant human DNase I in adults with stable stage cystic fibrosis. Lancet. 1993;342:199–202. doi: 10.1016/0140-6736(93)92297-7. [DOI] [PubMed] [Google Scholar]
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132:589–595. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- Rubin BK. Emerging therapies for cystic fibrosis. Chest. 1999;115:1120–1126. doi: 10.1378/chest.115.4.1120. [DOI] [PubMed] [Google Scholar]
- Taylor RFH, Gaya H, Hodson ME. Pseudomonas cepacia: Pulmonary infection in patients with cystic fibrosis. Respir Med. 1993;87:187–192. doi: 10.1016/0954-6111(93)90090-m. [DOI] [PubMed] [Google Scholar]
Asthma
- Agertoft L, Pederson S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064–1069. doi: 10.1056/NEJM200010123431502. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez JA, Holberg CJ, Wright AL. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Rye PJ, Gibson NA. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- Reddel HK, Taylor DR, Bateman ED. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations. Standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Suissa S, Ernst T, Benayoun S. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
Hemoptysis
- David M, Andrew M. Venous thromboembolic complications in children. J Pediatr. 1993;123:337–346. doi: 10.1016/s0022-3476(05)81730-5. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28:1642–1647. doi: 10.1097/00003246-200005000-00066. [DOI] [PubMed] [Google Scholar]
- Jones DK, Davies RJ. Massive haemoptysis: Medical management will usually arrest the bleeding. BMJ. 1990;300:889–890. doi: 10.1136/bmj.300.6729.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch HB, Schidlow DV. Pathogenesis and management of hemoptysis in children. Int Pediatr. 1989;4:241–244. [Google Scholar]


