In reptile virology, it may be difficult to recognize the true emergence of a pathogen, as detection of previously unknown organisms has occurred rapidly in recent years. This has been due both to the use of new technologies in reptile diagnostics and to the increased interest of researchers from various backgrounds in reptiles as virus hosts. The increasing use of next-generation sequencing (NGS) methods has led to many of these discoveries and appears likely to increase our knowledge of viruses in zoo, aquarium, and wildlife species significantly in the coming years. The development and commercial availability of sensitive diagnostic tests has increased, and changes in detection rates may not always reflect a true increase in viral prevalence among reptiles. This underlines the importance of additional studies using a wide range of disciplines and techniques to understand both the clinical significance of many viruses in various reptile species as well as their host ranges, the pathogenesis of associated disease, and epidemiology.
Numerous factors influence the emergence of viral infections in reptile populations. These include effects of climate change, which may influence the spread of viral infections in reptiles in multiple ways, including increasing the range of invertebrate vectors, especially for arboviruses (Table 39.1 ); influencing the reptile immune system; and the replication rates of viruses capable of infecting reptiles. Direct human interaction has been shown to affect the spread of multiple diseases in wild animals, and the pet trade plays a large role in the international spread of viruses (e.g., for ranaviruses,1, 2 herpesviruses,3 and reptarenaviruses) in pet reptiles.4 In addition, the prevalence of viruses in pet reptiles and possibly also wild reptiles appears to undergo a fluctuating pattern in many cases, so that study over multiple years or decades may be necessary in order to understand the natural dynamics of viral infection in these animals.5 This may be in part due to functions of reptile immunity as well as, in some cases, the long time span that may occur between infection and the development of disease.
TABLE 39.1.
Arboviruses Described in Reptiles
| Virus Family | Virus Name | Invertebrate Vectors | Reptilian Hosts | Geographic Distribution* | Associated Disease in Reptiles | Zoonotic Potential | Ref. |
|---|---|---|---|---|---|---|---|
|
Bunyaviridae |
Orthobunyvirus: Cache Valley virus, Tensaw virus, Kowanyama virus | Mosquitoes, Anopheles spp. | Texas soft-shelled turtle (Apalone [Trionyx] spinifera emoryi), Skink (Cryptoblepharus [Ablepharus boutonii] virgatus) | North America, Australia | None | Yes | 55, 56 |
| Nairovirus: Crimean-Congo hemorrhagic fever virus | Ticks (Hyalomma aegyptium) | Horsfield's tortoise (Testudo horsfieldii) | Middle East | None | Yes | 57 | |
| Togaviridae | Eastern equine encephalitis virus (EEEV), Venezuelan equine encephalitis virus (VEEV), western equine encephalitis virus (WEEV) | Mosquitoes | Various squamates, most often in various snake spp.; chelonians, crocodilians | North and South America | None | Yes | 58, 59, 60 |
| Flaviviridae | Japanese encephalitis virus, St. Louis encephalitis virus, Powasan virus, West Nile virus (WNV), Zika virus | Mosquitoes | Crocodilians, squamates, chelonians; WNV especially in crocodilians | Asia, North and South America | Neurologic and skin lesions in crocodilians | Yes | 59, 60, 61, 62 |
| Rhabdoviridae | Vesicular stomatitis virus, Charlesville virus, Almpiwar virus, Marco virus, Timbo virus, Chaco virus, Sena Madureira virus | Mosquitoes (e.g., Aedes aegypti), sandflies (Phlebotomus spp.), midges (Forcipomyia spp., Culicoides spp.) | Redbelly water snake (Nerodia [Natrix] erythrogaster), Australian house gecko (Gehyra australis), teiid lizards: giant ameiva (Ameiva ameiva ameiva), Striped forest whiptail (Kentropyx calcarata), Caiman lizard (Dracaena guianensis), skink (Cryptoblepharus virgatus) | Australia, North and South America | None reported | Yes | 55, 56, 63, 64 |
| Iridoviridae | Hemocytiviruses | Unknown | Various squamate and chelonian spp.; sequence data available from: Bearded dragon (Pogona vitticeps), peninsula ribbon snake (Thamnophis sauritus sackenii), Iberian mountain lizard (Iberolacerta [Lacerta] monticola) |
Africa, Asia, Europe, North America, | Anemia, systemic disease | Unlikely | 65, 66, 67, 68, 69 |
This table includes descriptions of detection of viruses by various methods as well as serologic evidence of previous infection (without proof of viremia).
Geographic areas in which detection in reptiles has been reported. The geographic range of individual viruses may be greater.
A wide range of viruses has been described in reptiles (Table 39.2 ). This chapter focuses on select viruses that have been recently described or for which there is evidence that their epidemiology has changed in recent years. Ranaviruses are important emerging pathogens in chelonians and squamates as well as in amphibians and fish; they are covered in Chapter 52.
TABLE 39.2.
Virus Families and Genera Described in Orders of Reptiles
| Virus Family | Virus Genus | Nonavian Reptile Host Order |
||
|---|---|---|---|---|
| Testudines | Squamata | Crocodylia | ||
|
Adenoviridae |
Atadenovirus | X | X | |
| Siadenovirus | X | |||
| “Testadenovirus” | X | |||
| Unclassified | X | |||
|
Herpesviridae |
Scutavirus | X | ||
| Unassigned | X | X | ||
|
Iridoviridae |
Ranavirus | X | X | |
| Iridovirus | X | |||
| “Hemocytivirus”* | X | |||
|
Papillomaviridae |
Dyozetapapillomavirus | X | ||
| Unclassified | X | X | ||
|
Poxviridae |
Crocodylipoxvirus | X | ||
| Unclassified | X | X | ||
| Circoviridae | Unclassified | X | X | |
| Parvoviridae | Dependoparvovirus | X | ||
| Hepadnaviridae | Unassigned | X | X | |
|
Retroviridae |
Gammaretrovirus | X | ||
| Unassigned | X | X | X | |
| Reoviridae | Orthoreovirus | X | X | |
| Bornaviridae | Unassigned | X | ||
| Paramyxoviridae | Ferlavirus | X | X | |
| Sunviridae | Sunshinevirus | X | ||
| Rhabdoviridae | Unassigned | X | X | |
| Orthomyxoviridae | * | |||
| Arenaviridae | Reptarenavirus | X | ||
|
Bunyaviridae |
Orthobunyavirus | X | ||
| Unassigned | X | |||
| Coronaviridae | Unassigned | X | ||
|
Picornaviridae |
Torchivirus | X | ||
| “Rafivirus” | X | |||
| Unclassified | X | |||
| Caliciviridae | Vesivirus | X | ||
| Flaviviridae | Flavivirus | X | X | X |
| Togaviridae | Alphavirus | X | X | X |
There is some evidence of infection with these viruses in this group of reptiles.
Adenoviridae
Adenoviruses are large, nonenveloped double-stranded (ds) DNA viruses. Members of three different genera have been described in reptiles: atadenoviruses, siadenoviruses, and the proposed “testadenoviruses.”6 The atadenoviruses have been hypothesized to have evolved in squamate reptiles,7 and some of the viruses found in these reptiles appear to be host species–specific. Atadenovirus infections in squamates are not always associated with disease, although they may be involved in multipathogen disease processes. There is increasing evidence that numerous squamate atadenoviruses are able to switch between various squamate hosts, with unknown clinical and epidemiologic consequences8 as well as ramifications for viral detection methods. Although atadenoviral infections in squamates are well documented, reports of adenoviral infections in chelonians are relatively rare and the genetic diversity of the detected viruses is relatively high, indicating that at least in some cases adenoviruses have recently switched to chelonians as hosts. The first report of a disease outbreak associated with adenoviral infection in chelonians was in a group of Sulawesi tortoises (Indotestudo forsteni) that were illegally imported into the United States.9 Affected animals developed severe multisystemic disease with clinical signs including anorexia, lethargy, mucosal ulcerations, nasal and ocular discharge, and diarrhea. The group experienced a mortality rate of 82%. An adenovirus was detected by polymerase chain reaction (PCR) as well as by electron microscopy in cells of affected tissues. The virus was determined to belong in the genus Siadenovirus. Siadenoviruses had previously been described in birds but are hypothesized to have evolved in amphibians.10 The same virus was later also found in impressed tortoises (Manouria impressa) and a Burmese star tortoise (Geochelone platynota) with systemic disease.11
Other adenoviruses found in chelonians have differed genetically from previously described genera. These viruses have been described in a wide range of terrestrial and aquatic species in the United States and Europe. They have been found in both diseased and clinically healthy animals. The viruses detected in these cases have been preliminarily named “testadenoviruses,” based on the hypothesis that they have coevolved in chelonians.6 In another case in a spur-thighed tortoise (Testudo graeca) with stomatitis and esophagitis, a virus in the genus Atadenovirus was detected by PCR and sequencing.12 There are therefore a multitude of different adenoviruses belonging to at least three different genera that have been discovered in chelonians within the past decade. The viruses hypothesized to have recently switched hosts into chelonian species (the siadenovirus and atadenovirus) appear to have been associated with more severe pathologies.
Herpesviridae
Herpesviruses are large, enveloped dsDNA viruses that are known to cause latent infections in hosts surviving acute infection. Herpesviruses have long been described as pathogens in a wide range of reptilian hosts, especially chelonians. Detections in various orders of reptiles have increased in recent years. In many cases, virus detection is based on histologic detection of intranuclear inclusion bodies in infected cells, followed by electron microscopy and on detection of viral DNA using a panherpesviral PCR targeting a portion of the DNA-dependent DNA polymerase.13
Crocodilian Herpesviruses
Recent studies in Australia in farmed saltwater crocodiles (Crocodylus porosus) have led to the description of three different crocodilian herpesviruses.14 Infections have been associated with three disease syndromes: conjunctivitis and/or pharyngitis (CP), lymphoid proliferation and nonsuppurative encephalitis (SLPE), and multifocal lymphohistiocytic infiltration of the dermis (LNS).5 CP was found primarily in hatchlings, SLPE in juveniles and growers, and LNS in harvest-sized animals. The causative nature of the herpesviruses for each disease syndrome has not been fully established, although herpesviruses were found significantly more often in diseased crocodiles than in controls.5
Squamate Herpesviruses
Herpesviruses have been described periodically in various squamate species. Genetically, little information is available on these viruses, but what is available indicates that they are diverse and not closely related to chelonian or crocodilian herpesviruses. In lizards, herpesviruses have been associated with stomatitis,15, 16 papillomas,17 hepatitis, and enteritis.18 In a disease outbreak associated with a herpesvirus infection in captive adult horned vipers (Vipera ammodytes ammodytes) in Europe, animals developed widespread hemorrhage, coelomic and pericardial effusion, and hepatitis. All of the horned vipers in the collection died, whereas common European vipers (Vipera berus) housed in the same facility remained unaffected.19
Chelonian Herpesviruses
Herpesviruses have long been described as important pathogens in various chelonian species, mostly in sea turtles, where they are considered the cause of fibropapillomatosis (see Chapter 57), as well as grey patch disease,20 lung, eye, and trachea disease,21 loggerhead genital-respiratory herpesvirus-associated disease, and loggerhead orocutaneous herpesvirus-associated disease,22 and in tortoises, in which they have mostly been associated with stomatitis, rhinitis, and conjunctivitis. The chelonid fibropapillomatosis–associated herpesvirus, officially known as Chelonid alpha herpesvirus 5, has been placed in a separate genus, Scutavirus, in the subfamily Alphaherpesvirinae.23 All reported herpesviruses of chelonians appear to cluster in this genus.24 Recent studies on herpesviruses in a wide range of chelonian species have led to the description of numerous new virus types and have also indicated shifts in prevalence of specific viruses in some pet chelonians.
There are four known genetically distinct herpesviruses that can infect tortoises, named testudinid herpesvirus 1 through 4 (TeHV1-4). TeHV1, 2, and 3 have all been associated with severe stomatitis and glossitis as well as rhinitis, conjunctivitis, and hepatitis. TeHV4 was originally detected in a clinically healthy tortoise during quarantine screening.25 TeHV3 has been associated with higher morbidity and mortality rates than TeHV1.26 Recent studies using molecular techniques to investigate genomic differences between TeHV3 strains as well as transmission studies have provided evidence that there may also be differences in pathogenicity between different isolates.24, 27 In a study in Europe almost 20 years ago in which samples from tortoises kept as pets were tested for the presence of herpesviruses, only TeHV1 and TeHV3 were detected, with TeHV3 making up greater than 80% of the detected herpesviruses.28 In a recent study screening samples from over 1000 chelonians in Europe for herpesviruses and other pathogens, approximately half of the herpesviruses detected were categorized as TeHV1, indicating that the prevalence of this virus in pet tortoises in Europe has increased over the past years, possibly due to changes in the pet trade.3 It might also reflect a change in the popularity of specific tortoise species in the pet trade. During that same study, a TeHV4 was detected for the first time in Europe in an African tortoise species, the leopard tortoise (Stigmochelys pardalis).29
Detections of herpesviruses in other families of chelonians has also increased rapidly in recent years.30, 31, 32, 33, 34, 35, 36 In the family Emydidae, herpesviruses have been detected in a freshwater turtle (Pseudemys concinna concinna), a northern map turtle (Graptemys geographica), wild bog turtles (Glyptemys muhlenbergii), wood turtles (G. insculpta), spotted turtles (Clemmy guttata), and box turtles (Terrapene sp.). Clinical signs associated with infection in these animals have ranged from detections in clinically healthy animals to stomatitis, papillomatous skin lesions, rhinitis, and sudden death. Histology has demonstrated hepatic lipidosis, pneumonia, and both hepatocellular and splenic necrosis. Intranuclear inclusions have been found in cells in the liver, lung, and spleen.31 Infected animals have included both captive and wild turtles.
In the order Pleurodira, herpesviruses have been described in a captive Krefft's river turtle (Emydura macquarii krefftii) (family Chelidae) in Australia with ulcerative lesions of the skin and shell associated with orthokeratotic hyperkeratosis with intranuclear inclusions in keratinocytes.35 In the family Pelomedusidae, a herpesvirus was detected in West African mud turtles (Pelusios castaneus) that were imported into Europe from Africa and were clinically healthy.36
Picornaviridae in Tortoises
Picorna-like viruses have been known to occur in tortoises in Europe since the mid-1990s. They were originally isolated in cell culture and, proving difficult to characterize, were sometimes called “virus x.” Viruses in this category have been sequenced and shown to represent a new genus in the family Picornaviridae, with the name Torchivirus.37, 38 This group of viruses have been detected mostly in Testudo spp. but also in several African tortoise species. They have been associated with softening of the plastron in juvenile tortoises and with rhinitis, stomatitis, and ascites in adult tortoises. They have also been isolated from clinically healthy animals.28, 39 Transmission studies with T. hermanni and T. graeca showed that the kidneys were most severely affected.40 Serologic testing has shown that some wild-caught tortoises in Europe and Africa have antibodies against these viruses. Epidemiology of these viruses in Europe appears to undergo some fluctuation over time. A recent study showed that torchiviruses were detected by PCR using mostly oral swabs as samples in 2.2% of over 1000 chelonians tested over a 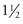 -year period in a commercial laboratory in Europe.3 Another picornavirus with the suggested genus name “Rafivirus” was described from Sulawesi tortoises that were also infected with an adenovirus.41 Animals died with severe systemic disease,9 but the role of the picornavirus in the disease is not known.
-year period in a commercial laboratory in Europe.3 Another picornavirus with the suggested genus name “Rafivirus” was described from Sulawesi tortoises that were also infected with an adenovirus.41 Animals died with severe systemic disease,9 but the role of the picornavirus in the disease is not known.
Nidovirales
Coronaviruses belong to the order Nidovirales, and viruses in this family have been among the most prominent emerging viruses in a wide variety of mammalian species in recent years, causing various respiratory disease syndromes including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). NGS of samples from reptiles displaying signs of respiratory disease has led to the description of viruses in the order Nidovirales, family Coronaviridae, subfamily Torovirinae in various python species and in boas.42, 43, 44, 45 A new genus name, “Barnivirus”, has been suggested for these viruses.42 Infections have been reported most commonly in ball pythons (Python regius) but also in Indian pythons (P. molurus), Burmese pythons (P. bivittatus), green tree pythons (Morelia viridis), carpet pythons (M. spilota), and boa constrictors (Boa constrictor). A genetically related but distinct virus has been described in shingleback lizards (“bobtails,” Tiliqua rugosa) in Australia.46 In ball pythons, the “Barnivirus” was associated with a proliferative interstitial pneumonia. In some cases, tracheitis, stomatitis, esophagitis, and/or rhinitis were also associated with infection. In individual cases, lesions were also observed in other parts of the body and included encephalitis, acute nephritis, salpingitis, hepatic lipidosis, keratitis, and colitis. The greatest viral load was detected in the lungs of affected snakes.42 A similar disease syndrome of unknown etiology was reported to have been observed in ball pythons since the 1990s.42
The shingleback lizards, in which a related virus was detected, were wild caught in western Australia and found to have a disease syndrome called “bobtail flu,” with clinical signs including mucopurulent discharge from the eyes and nose, lethargy, lack of appetite, pale mucous membranes, depression, and loss of body condition.46 Following detection of a barnivirus-like virus in affected animals, a real-time PCR was developed and used to detect virus in oral secretions. Virus was detected significantly more often in diseased than in healthy animals, although virus was also detected in some of the lizards that were considered healthy at the time of sampling.46
Arenaviridae in Snakes
Inclusion body disease (IBD) was originally described in the early 1980s and 1990s47 and was defined by the presence of characteristic eosinophilic to amphophilic intracytoplasmic inclusions in neurons and in epithelial cells of a wide range of tissues.48 The inclusions are made up of a specific protein known as inclusion body disease protein (IBDP).48 The disease affects various species of boas and pythons and is associated with a wide variety of clinical signs. Central nervous system (CNS) signs are most often described, but animals may develop anorexia, pneumonia, various skin lesions, mouth rot, and other problems. However, a large number of snakes (especially boa constrictors) may remain clinically healthy despite the presence of inclusions and/or virus. In 2012, NGS showed the probable cause of IBD to be newly discovered viruses in the family Arenaviridae in the new genus Reptarenavirus.49, 50, 51 These viruses are closely associated with the disease and can cause the development of similar inclusions in cell culture. Antibodies against reptarenaviruses isolated in cell culture bind to typical inclusions in the cells of affected animals.49, 50 IBDP is believed to be accumulated viral nucleocapsid protein.49 Additional studies on snakes infected with arenaviruses have demonstrated a huge amount of genetic diversity in these viruses, with multiple species and possibly different genera detected.4, 52 In many cases, snakes were infected with multiple viruses, and the two genomic segments of the reptarenaviruses, L and S, appeared to reassort readily.4 It has been hypothesized that snake importation and husbandry practices may have created this diversity among reptarenaviruses, with capture of wild snakes, importation into various countries, mixing of animals for breeding purposes, and overcrowding all being part of an “anthropogenic disruption of pathogen ecology.”4 It has been hypothesized that coinfection or superinfection with multiple reptarenaviruses could play a role in disease development.52
Other Newly Described Viruses of Reptiles
There are many other examples of recently described viruses in reptiles, with new reports coming out regularly. Recent developments include a description of the new family Sunviridae for Sunshinevirus in the Mononegavirales. Sunshinevirus is associated with CNS and respiratory disease in pythons, mostly in Australia.53 Bornaviruses have also recently been detected in snakes in several cases,54 and there is some indication that infections may be associated with neurologic disease (T. Hyndman, personal communication, March 30, 2016).
References
- 1.Duffus ALJ, Waltzek TB, Stöhr AC. Distribution and host range of ranaviruses. In: Gray MJ, Chinchar VG, editors. Ranaviruses: lethal pathogens of ectothermic vertebrates. SpringerOpen; 2015. pp. 9–58. [Google Scholar]
- 2.Stöhr AC, Blahak B, Heckers KO. Ranavirus infections associated with skin lesions in lizards. Vet Res. 2013;44:84. doi: 10.1186/1297-9716-44-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolesnik E, Obiegala A, Marschang RE. Detection of Mycoplasma spp., herpesviruses, topiviruses, and ferlaviruses in diagnostic samples from chelonians in Europe. J Vet Diagn Invest. 2017 doi: 10.1177/1040638717722387. [DOI] [PubMed] [Google Scholar]
- 4.Stenglein MD, Jacobson ER, Chang L-W. Widespread recombination, reassortment, and transmission of unbalanced compound viral genotypes in natural arenavirus infections. PLoS Pathog. 2015;11(5):e1004900. doi: 10.1371/journal.ppat.1004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shilton CM, Jerrett IV, Davis S. Diagnostic investigation of new disease syndromes in farmed Australian saltwater crocodiles (Crocodylus porosus) reveals associations with herpesvirus infection. J Vet Diagn Invest. 2016;28:279–290. doi: 10.1177/1040638716642268. [DOI] [PubMed] [Google Scholar]
- 6.Doszpoly A, Wellehan JF, Jr, Childress AL. Partial characterization of a new adenovirus lineage discovered in testudinoid turtles. Infect Genet Evol. 2013;17:106–112. doi: 10.1016/j.meegid.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Harrach B. Reptile adenoviruses in cattle? Acta Vet Acad Sci Hung. 2000;48:485–490. doi: 10.1556/004.48.2000.4.11. [DOI] [PubMed] [Google Scholar]
- 8.Ball I, Ofner S, Funk RS. Prevalence of neutralising antibodies against adenoviruses in lizards and snakes. Vet J. 2014;202:176–181. doi: 10.1016/j.tvjl.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Rivera S, Wellehan JF, Jr, McManamon R. Systemic adenovirus infection in Sulawesi tortoises (Indotestudo forsteni) caused by a novel siadenovirus. J Vet Diagn Invest. 2009;21:415–426. doi: 10.1177/104063870902100402. [DOI] [PubMed] [Google Scholar]
- 10.Davison AJ, Benkö M, Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol. 2003;84:2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher VL, Innis CJ, Garner MM. Sulawesi tortoise adenovirus-1 in two impressed tortoises (Manouria impressa) and a Burmese star tortoise (Geochelone platynota) J Zoo Wildl Med. 2012;43:501–510. doi: 10.1638/2011-0228R.1. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Morante B, Pénzes JJ, Costa T. Hyperplastic stomatitis and esophagitis in a tortoise (Testudo graeca) associated with an adenovirus infection. J Vet Diagn Invest. 2016;28:579–583. doi: 10.1177/1040638716659903. [DOI] [PubMed] [Google Scholar]
- 13.Van Devanter DR, Warrener P, Bennett L. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyndman TH, Shilton CM, Wellehan JF., Jr Molecular identification of three novel herpesviruses found in Australian farmed saltwater crocodiles (Crocodylus porosus) and Australian captive freshwater crocodiles (Crocodylus johnstoni) Vet Microbiol. 2015;181:183–189. doi: 10.1016/j.vetmic.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Wellehan JF, Johnson AJ, Latimer KS. Varanid herpesvirus 1: a novel herpesvirus associated with proliferative stomatitis in green tree monitors (Varanus prasinus) Vet Microbiol. 2005;105:83–92. doi: 10.1016/j.vetmic.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Wellehan JF, Nichols DK, Li LL. Three novel herpesviruses associated with stomatitis in Sudan plated lizards (Gerrhosaurus major) and a black-lined plated lizard (Gerrhosaurus nigrolineatus) J Zoo Wildl Med. 2004;35:50–54. doi: 10.1638/03-011. [DOI] [PubMed] [Google Scholar]
- 17.Literak I, Robesova B, Majlathova V. Herpesvirus-associated papillomatosis in a green lizard. J Wildl Dis. 2010;46:257–261. doi: 10.7589/0090-3558-46.1.257. [DOI] [PubMed] [Google Scholar]
- 18.Hughes-Hanks JM, Schommer SK, Mitchell WJ. Hepatitis and enteritis caused by a novel herpesvirus in two monitor lizards. J Vet Diagn Invest. 2010;22:295–299. doi: 10.1177/104063871002200224. [DOI] [PubMed] [Google Scholar]
- 19.Catoi C, Gal AF, Taulescu MA. Lethal herpesvirosis in 16 captive horned vipers (Vipera ammodytes ammodytes): pathological and ultrastructural findings. J Comp Pathol. 2014;150:341–344. doi: 10.1016/j.jcpa.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Rebel G, Rywlin A, Haines H. A herpesvirus agent associated with skin lesions of green sea turtles in aquaculture. Am J Vet Res. 1975;36:1221–1224. [PubMed] [Google Scholar]
- 21.Jacobson ER, Gaskin JM, Roelke M. Conjunctivitis, tracheitis, and pneumonia associated with herpesvirus infection in green sea turtles. J Am Vet Med Assoc. 1986;189:1020–1023. [PubMed] [Google Scholar]
- 22.Stacy BA, Wellehan JFX, Foley AM. Two herpesviruses associated with disease in wild Atlantic loggerhead sea turtles (Caretta caretta) Vet Microbiol. 2008;126:63–73. doi: 10.1016/j.vetmic.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Davison A, McGeoch D. Create genus Scutavirus (type species: the currently unassigned species Chelonid herpesvirus 5) in subfamily Alphaherpesvirinae, family Herpesviridae. 2010. https://talk.ictvonline.org/ICTV/proposals/2010.016a-eV.A.v2.Scutavirus.pdf Retrieved March 9, 2017 from.
- 24.Gandar F, Wilkie GS, Gatherer D. The genome of a tortoise herpesvirus (testudinid herpesvirus 3) has a novel structure and contains a large region that is not required for replication in vitro or virulence in vivo. J Virol. 2015 doi: 10.1128/JVI.01794-15. pii: JVI.01794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bicknese EJ, Childress AL, Wellehan JF., Jr A novel herpesvirus of the proposed genus Chelonivirus from an asymptomatic bowsprit tortoise (Chersina angulata) J Zoo Wildl Med. 2010;41:353–358. doi: 10.1638/2009-0214R.1. [DOI] [PubMed] [Google Scholar]
- 26.Marschang RE. Viruses infecting reptiles. Viruses. 2011;3:2087–2126. doi: 10.3390/v3112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Origgi FC, Tecilla M, Pilo P. A genomic approach to unravel host-pathogen interaction in chelonians: the example of testudinid herpesvirus 3. PLoS ONE. 2015;10:e0134897. doi: 10.1371/journal.pone.0134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marschang RE. 2001. Isolierung und Charakterisierung von Irido-, Herpes- und Reoviren aus Landschildkröten sowie Beschreibung eines nicht charakterisierten zytopathogenen Agens. Vet Med Diss, Justus-Liebig-Universität Giessen, Germany. [Google Scholar]
- 29.Kolesnik E, Mittenzwei F, Marschang RE. Detection of testudinid herpesvirus type 4 in a leopard tortoise (Stigmochelys pardalis) Tierärztl Praxi Ausg K Kleintiere Heimtiere. 2016;44:283–286. doi: 10.15654/TPK-150843. [DOI] [PubMed] [Google Scholar]
- 30.Jungwirth N, Bodewes R, Osterhaus AD. First report of a new alphaherpesvirus in a freshwater turtle (Pseudemys concinna concinna) kept in Germany. Vet Microbiol. 2014;170:403–407. doi: 10.1016/j.vetmic.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Ossiboff RJ, Newton AL, Seimon TA. Emydid herpesvirus 1 infection in northern map turtles (Graptemys geographica) and painted turtles (Chrysemys picta) J Vet Diagn Invest. 2015;27:392–395. doi: 10.1177/1040638715584793. [DOI] [PubMed] [Google Scholar]
- 32.Ossiboff RJ, Raphael BL, Ammazzalorso AD. Three novel herpesviruses of endangered Clemmys and Glyptemys turtles. PLoS ONE. 2015;10:e0122901. doi: 10.1371/journal.pone.0122901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim RR, Norton TM, Bronson E. Identification of a novel herpesvirus in captive Eastern box turtles (Terrapene carolina carolina) Vet Microbiol. 2015;175:218–223. doi: 10.1016/j.vetmic.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Yonkers SB, Schneider R, Reavill DR. Coinfection with a novel fibropapilloma-associated herpesvirus and a novel Spirorchis sp. In an eastern box turtle (Terrapene carolina) in Florida. J Vet Diagn Invest. 2015;27:408–413. doi: 10.1177/1040638715589612. [DOI] [PubMed] [Google Scholar]
- 35.Cowan ML, Raidal SR, Peters A. Herpesvirus in a captive Australian Krefft's river turtle (Emydura macquarii krefftii) Aust Vet J. 2015;93:46–49. doi: 10.1111/avj.12290. [DOI] [PubMed] [Google Scholar]
- 36.Marschang RE, Heckers KO, Heynol V. [Herpesvirus detection in clinically healthy West African mud turtles (Pelusios castaneus)] Tierarztl Prax Ausg K Kleintiere Heimtiere. 2015;43:166–169. doi: 10.15654/TPK-140575. [DOI] [PubMed] [Google Scholar]
- 37.Farkas SL, Ihász K, Fehér E. Sequencing and phylogenetic analysis identifies candidate members of a new picornavirus genus in terrestrial tortoise species. Arch Virol. 2015;160:811–816. doi: 10.1007/s00705-014-2292-z. [DOI] [PubMed] [Google Scholar]
- 38.Zell R, Delwart E, Gorbalenya AE. Create 1 new species (Torchivirus A) in a new genus (Torchivirus) 2016. https://data.ictvonline.org/proposals/2016.018a-dS.A.v1.Torchivirus.pdf Retrieved Feb. 6, 2018 from.
- 39.Heuser W, Pendl H, Knowles NJ. Soft plastron, soft carapace with skeletal abnormality in juvenile tortoises. Histopathology and isolation of a novel picornavirus from Testudo graeca and Geochelone elegans. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2014;42:310–320. [PubMed] [Google Scholar]
- 40.Paries S. 2015. The role of virus “x” (tortoise picornavirus nsp.) in kidney disease and shell weakness syndrome in various European tortoise species determined by experimental infection; p. 351. in Proceedings of the 2nd International Conference of Avian Herpetological and Exotic Mammal Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng TF, Wellehan JF, Coleman JK. A tortoise-infecting picornavirus expands the host range of the family Picornaviridae. Arch Virol. 2015;160:1319–1323. doi: 10.1007/s00705-015-2366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenglein MD, Jacobson ER, Wozniak EJ. Ball python nidovirus: a candidate etiologic agent for severe respiratory disease in Python regius. MBio. 2014;5(5) doi: 10.1128/mBio.01484-14. e01484-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodewes R, Lempp C, Schürch AC. Novel divergent nidovirus in a python with pneumonia. J Gen Virol. 2014;95:2480–2485. doi: 10.1099/vir.0.068700-0. [DOI] [PubMed] [Google Scholar]
- 44.Uccellini L, Ossiboff RJ, de Matos RE. Identification of a novel nidovirus in an outbreak of fatal respiratory disease in ball pythons (Python regius) Virol J. 2014;11:144. doi: 10.1186/1743-422X-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marschang RE, Kolesnik E. Detection of nidoviruses in live pythons and boas. Tierärztl Praxis Ausg K Kleintiere Heimtiere. 2016;44:22–26. doi: 10.15654/TPK-151067. [DOI] [PubMed] [Google Scholar]
- 46.O'Dea MA, Jackson B, Jackson C. Discovery and partial genomic characterisation of a novel nidovirus associated with respiratory disease in wild shingleback lizards (Tiliqua rugosa) PLoS ONE. 2016;11:e0165209. doi: 10.1371/journal.pone.0165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher J, Jacobson ER, Homer BL. Inclusion body disease in boid snakes. J Zoo Wildl Med. 1994;25:511–524. [Google Scholar]
- 48.Chang LW, Jacobson ER. Inclusion body disease, a worldwide infectious disease of boid snakes: a review. J Exot Pet Med. 2010;19:216–225. [Google Scholar]
- 49.Stenglein MD, Sanders C, Kistler AL. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. MBio. 2012;3(4) doi: 10.1128/mBio.00180-12. e00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hetzel U, Sironen T, Laurinmäki P. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J Virol. 2013;87:10918–10935. doi: 10.1128/JVI.01123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodewes R, Kik M, Raj VS. Detection of novel divergent arenaviruses in boid snakes with inclusion body disease in the Netherlands. J Gen Virol. 2013;94:1206–1210. doi: 10.1099/vir.0.051995-0. [DOI] [PubMed] [Google Scholar]
- 52.Hepojoki J, Salmenperä P, Sironen T. Arenavirus coinfections are common in snakes with boid inclusion body disease. J Virol. 2015;89:8657–8660. doi: 10.1128/JVI.01112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyndman TH, Marschang RE, Wellehan JFX. Isolation and molecular identification of Sunshine virus, a novel paramyxovirus found in Australian snakes. Infect Genet Evol. 2012;12:1436–1446. doi: 10.1016/j.meegid.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Stenglein MD, Leavitt EB, Abramovitch MA. Genome sequence of a bornavirus recovered from an African garter snake (Elapsoidea loveridgei) Genome Announc. 2014;2 doi: 10.1128/genomeA.00779-14. e00779-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoff G, Trainer O. Arboviruses in reptiles: isolation of a bunyamwera group virus from a naturally infected turtle. J Herpetol. 1973;7:55–62. [Google Scholar]
- 56.Doherty RL. Arboviruses of Australia. Aust Vet J. 1972;48:172–180. doi: 10.1111/j.1751-0813.1972.tb09267.x. [DOI] [PubMed] [Google Scholar]
- 57.Široký P, Bělohlávek T, Papoušek I. Hidden threat of tortoise ticks: high prevalence of Crimean-Congo haemorrhagic fever virus in ticks Hyalomma aegyptium in the Middle East. Parasit Vectors. 2014;7:101. doi: 10.1186/1756-3305-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobson ER. Viruses and viral diseases of reptiles. In: Jacobson ER, editor. Infectious diseases and pathology of reptiles. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2007. pp. 395–460. [Google Scholar]
- 59.Kuno G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts. Rev Med Virol. 2001;11:165–190. doi: 10.1002/rmv.314. [DOI] [PubMed] [Google Scholar]
- 60.Marschang RE: Virology. In Divers SJ, Stahl S, editors: Mader’s reptile and amphibian medicine and surgery, ed 3. In press, Elsevier.
- 61.Marka A, Diamantidis A, Papa A. West Nile Virus State of the Art Report of MALWEST Project. Int J Environ Res Public Health. 2013;10:6534–6610. doi: 10.3390/ijerph10126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bueno MG, Martinez N, Abdalla L. Animals in the Zika virus life cycle: what to expect from megadiverse Latin American countries. PLoS Negl Trop Dis. 2016;10(12):e0005073. doi: 10.1371/journal.pntd.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doherty RL, Carley JG, Standfast HA. Isolation of arboviruses from mosquitoes, biting midges, sandflies and vertebrates collected in Queensland, 1969 and 1970. Trans R Soc Trop Med Hyg. 1973;67:536–543. doi: 10.1016/0035-9203(73)90084-9. [DOI] [PubMed] [Google Scholar]
- 64.Wellehan JFX, Pessier AP, Archer LL. Initial sequence characterization of the rhabdoviruses of squamate reptiles, including a novel rhabdovirus from a caiman lizard (Dracaena guianensis) Vet Microbiol. 2012;158:274–279. doi: 10.1016/j.vetmic.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alves de Matos AP, Paperna I, Crespo E. Experimental infection of lacertids with lizard erythrocytic viruses. Intervirology. 2002;45:150–159. doi: 10.1159/000065868. [DOI] [PubMed] [Google Scholar]
- 66.Alves de Matos AP, Caeiro MF, Papp T. New viruses from Lacerta monticola (Serra da Estrela, Portugal): further evidence for a new group of nucleo-cytoplasmic large deoxyriboviruses (NCLDVs) Microsc Micoanal. 2011;17:101–108. doi: 10.1017/S143192761009433X. [DOI] [PubMed] [Google Scholar]
- 67.Eid R, Radad K, Al-Shraim M. Iridoviral infection consistent with lizard erythrocytic virus in Chamaelo calyptratus. Wien Tierärztl Monatsschr. 2011;98:82–86. [Google Scholar]
- 68.Grosset C, Wellehan JF, Jr, Owens SD. Intraerythrocytic iridovirus in central bearded dragons (Pogona vitticeps) J Vet Diagn Invest. 2014;26:354–364. doi: 10.1177/1040638714534851. [DOI] [PubMed] [Google Scholar]
- 69.Wellehan JF, Jr, Strik NI, Stacy BA. Characterization of an erythrocytic virus in the family Iridoviridae from a peninsula ribbon snake (Thamnophis sauritus sackenii) Vet Microbiol. 2008;131:115–122. doi: 10.1016/j.vetmic.2008.03.003. [DOI] [PubMed] [Google Scholar]


