Typically there is little fluid present in the peritoneal, pleural, and pericardial cavities, and thus they are considered potential spaces. Detailed physiologic descriptions of serous body cavity homeostasis are available (Bouvy et al., 1991; Forrester, 1988; Kirby, 2003). These serous body cavities are lined by specialized cells, termed mesothelial cells. Clinical signs of the presence of increased amounts of fluid include abdominal distension, abdominal pain, dyspnea, muffled heart sounds, and cardiac arrhythmia. Collection and evaluation of fluid from these sites may be therapeutic as well as diagnostic for the presence of inflammatory, hemorrhagic, neoplastic, lymphatic, or bilious conditions. Additionally, further diagnostic tests may be indicated by the cytologic characteristics. Removal and examination of fluid is highly recommended unless anesthetic risks or bleeding diathesis are present or further injury is likely.
COLLECTION TECHNIQUES
Abdominal Fluid
Place the patient in left lateral recumbency and restrain. Clip and surgically prepare an area (e.g., 4 to 6 inches square) with the umbilicus in the center. The urinary bladder should be emptied before performing paracentesis. Infiltrate a small area with local anesthetic, if desired. Use a 20-to 22-gauge needle or over-the-needle catheter to penetrate the abdomen. Attempt to obtain fluid in four quadrants, allowing the fluid to flow freely by gravity and capillary action. If needed, gentle suction with a 3- or 6-ml syringe can be employed. For a complete description of the technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006). Allow the animal to rest quietly while fluid is being removed. Moving the animal or allowing the patient to move while the needle is in the abdomen can result in laceration of the organs. Some investigators prefer to have the patient standing for fluid removal; however, it is more likely that the omentum will occlude the needle if the patient is in a standing position.
Pleural Fluid
For removal of fluid from the thorax, the patient should be in a standing or in ventral/sternal recumbency. Clip the hair and surgically prepare the thoracic wall from the 5th to the 11th intercostal space. Infiltrate a small area at the 7th to 8th intercostal space at the level of the costochondral junction with local anesthetic. It is best to attach extension tubing to the hub of the needle or over-the-needle catheter and a three-way stopcock for removal of pleural fluid. Insert the needle or catheter into the chest wall at the surgically prepared site taking care to avoid the intercostal vessels located just caudal to each rib. For a complete description of this technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006).
Pericardial Fluid
For removal of fluid from the pericardial sac, sedate the patient if necessary. Surgically prepare an area over the lower to mid 5th to 7th intercostal space bilaterally. Place the patient in lateral or sternal recumbency. Attach ECG leads to monitor for dysrhythmias during the procedure. Infiltrate an area at the costochondral junction, or approximately where the lower and mid thorax meet, with local anesthetic. Use an over-the-needle catheter or Intrafusor system with a three-way valve to which a 30-ml syringe is attached. Always maintain negative pressure on the syringe as the chest wall is punctured. Carefully advance the needle into the 4th intercostal space through a nick incision in the direction of the heart. Advance the needle until resistance is met (from the pericardium). A release will be felt as the needle enters the pericardial sac and a flash of blood is often seen. Thread the tubing or catheter so that it is securely within the pericardial sac. For a complete description of this technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006).
SAMPLE HANDLING
Note the color and character of the fluid initially upon removal (Fig. 6-1 ). If the fluid is clear initially then turns red, iatrogenic blood contamination is likely. The fluid should be collected into both a lavender-top tube (EDTA anticoagulant) for cytology and a red-top tube (or any sterile tube without additives) for potential bacterial culture. Also at collection, make both direct unconcentrated smears by a squash or blood smear technique and smears from spun samples. Romanowsky-type stains such as Wright stain or an aqueous-based Wright (quick) stain can be applied to a few slides for immediate in-house evaluation. The remaining unstained smears, as well as an EDTA and serum tube filled with fluid, should be submitted to the laboratory. This will allow the clinical pathologist evaluating the sample to compare the cellularity of the sample and the appearance of the cells at the time of collection with that which was submitted in the tube.
FIGURE 6-1.

Effusion color and character. Gross appearance of various effusions. From left to right these are: (a) clear and colorless—transudate; (b) yellow and slightly turbid—modified transudate; (c) red and slightly turbid (likely hemolyzed red blood cells)—hemorrhage; (d) orange and turbid—likely inflammatory fluid with blood; (e) sedimented fluid—note thick pellet of cells on the bottom of the tube; (f) red and turbid—bloody as a result of either hemorrhage or iatrogenic blood contamination; (g) brown and slightly turbid—possible bile or red blood cell breakdown.
LABORATORY EVALUATION
Protein Quantitation
Protein quantitation is typically done via refractometry; however, some institutions will determine protein via spectrophotometry or automated analysis. Both methods offer accurate readings in a wide range of protein concentrations (George, 2001; George and O'Neill, 2001). It has been shown with canine effusions that refractometry underestimates the protein content when < 2.0 g/dl and that the spectrophotometry using the biuret method is more accurate when there is high protein content (Braun et al., 2001). Others have found that refractometry can be used accurately down to 1.0 g/dl (George and O'Neill, 2001). A similar finding of underestimating protein content in feline effusions with refractometry may occur (Papasouliotis et al., 2002). In that same study, a dry chemistry analyzer produced increased globulin concentration and therefore lower Albumin:Globulin (A:G) ratios when compared with a reference wet analyzer using the same biuret and bromocresol green methodologies (Papasouliotis et al., 2002). This finding is particularly important since decreased A:G ratios support a diagnosis of feline infectious peritonitis (Hartmann et al., 2003).
For cloudy or turbid samples and bloody samples, the fluid should be centrifuged and the protein measured on the supernatant. Turbidity may interfere with evaluation of protein by either refractometry or spectrophotometry. The protein content is used with the nucleated cell count to classify the effusion and help formulate a list of possible causes.
Red Blood Cell and Total Nucleated Cell Count
Although an initial impression of the cellularity and amount of blood can usually be made by visual inspection of the sample (see Fig. 6-1), knowledge of the actual cell counts for erythrocytes and nucleated cells is important for further classification of the type of fluid. With this information, one can begin to narrow down the list of possible causes for the abnormal fluid accumulation. For samples being submitted to a reference laboratory, placing some of the sample in a lavender-top tube (EDTA) and some in a red-top tube is recommended. The lavender-top tube contains anticoagulant, which prevents the sample from clotting if there is a high protein content. EDTA is bactericidal, however, and is contraindicated if a sample is to be cultured (Songer and Post, 2005). Thus, some of the fluid should also be put into a red-top tube or sterile tube without additives. The cell counts will be done either with a hemocytometer or an automated cell-counting instrument. If the amount of fibrinogen in the fluid sample is high, then the sample in the red-top tube is likely to clot, producing erroneous results.
Note: Do not use gel-containing serum separator tubes (SST) for submission of fluid to a reference laboratory. Cells may bind to the gel in these tubes and result in an artifactually lowered cell count.
Nucleated Cell Differential
Standard procedures for performing a differential of the nucleated cells vary among laboratories. Some laboratories do no differential, others a three-part differential of 100 cells (large mononuclear cells, small mononuclear cells, and neutrophils), while others will provide a 100-cell differential of all cell types observed. The differential provides a relative picture of the types and numbers of cells and aids in establishing a list of potential causes for the fluid accumulation. A differential is not a substitute for a cytologic evaluation. The cytologic evaluation is performed in an attempt to determine a specific diagnosis.
NORMAL CYTOLOGY AND HYPERPLASIA
Normally, only a very small amount of fluid is found in the peritoneal, pleural, and pericardial spaces; thus cytologic evaluation is generally not typically performed unless an increased amount of fluid accumulates. Normal fluid is clear and colorless (see Fig. 6-1). Several types of cells may be found in body cavity effusions and their relative proportions vary depending on the cause of the fluid accumulation. Cells expected to be in normal fluid include mesothelial, mononuclear phagocytes, lymphocytes, and rare neutrophils. Mesothelium will easily become hyperplastic or reactive when increased body cavity fluid or inflammation is present.
Mesothelial Cells
In most cases, the cytologist will find reactive mesothelial cells in body cavity fluids. These are considered as large mononuclear cells for the purpose of the three-part cell differential. Mesothelial cells may be seen as individualized cells or in variably sized clusters. They contain a moderate amount of medium-blue cytoplasm (Fig. 6-2 ). Hyperplastic mesothelial cells are large (12 to 30 μm) with deep-blue cytoplasm and may display a pink to red “fringed” cytoplasmic border (Fig. 6-3A&B ). This feature helps identify these cells as mesothelial cells. These cells may contain one or more nuclei of equal size (see Fig. 6-3B). Nucleoli may be visible and occasional mitotic figures may be evident.
FIGURE 6-2.
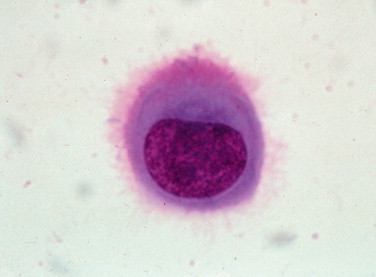
Normal mesothelial cell. Exfoliated cell in an effusion with its characteristic pink fringe along the cytoplasmic border. (Wright-Giemsa; HP oil.)
(From Meyer DJ, Franks PT: Classification and cytologic examination, Compend Contin Educ Pract Vet 9:123–29, 1987.)
© 2010
FIGURE 6-3.

Reactive mesothelial cell. A, Exfoliated binucleate mesothelial cell (upper right) and mildly vacuolated and basophilic macrophage in an effusion. The mesothelial cell has a characteristic pink fringe along the cytoplasmic border. (Modified Wright; HP oil.) B, A loose group of variably reactive mesothlelial cells at the feathered edge of a smear made from an effusion. These cells may contain one or more nuclei. Note the presence of the “fringe” (glycocalyx) on the mesothelial cells. Several cells contain paranuclear dark granules, the significance of which is unknown. (Modified Wright; HP oil.)
Macrophages
Macrophages are large mononuclear cells with abundant pale-gray to light-blue cytoplasm and a round to kidney bean–shaped nucleus (Figs. 6-3A and 6-4 ). The chromatin may be fine and nucleoli may be visible. Macrophages often contain vacuoles or previously phagocytosed cells and/or debris if there is inflammation or if the fluid has been present for a long time (Fig. 6-5 ). Macrophages are considered as large mononuclear cells for the purpose of the three-part cell differential.
FIGURE 6-4.

Macrophages. Three unremarkable to mildly basophilic and vacuolated macrophages from an effusion are present. (Modified Wright; HP oil.)
FIGURE 6-5.

Macrophage. Neutrophils. Shown are a moderately vacuolated and basophilic macrophage and two nondegenerate neutrophils. (Modified Wright; HP oil.)
Lymphoid Cells
Small and medium lymphocytes found in effusions appear similar to those found in peripheral blood. These nucleated cells often have a thin rim of lightly basophilic cytoplasm and a round nucleus. Lymphocytes are considered as small, mononuclear cells for the purpose of the three-part cell differential. In normal fluids they are present in higher proportions in cats and cattle than in dogs and horses. The nucleus nearly fills the cell, producing a uniformly high nuclear-to-cytoplasmic (N:C) ratio. The chromatin is finely stippled to evenly clumped; nucleoli are not visible (Fig. 6-6A&B ).
FIGURE 6-6.

A, Normal fluid/transudate. Note the macrophage and small lymphocyte with several erythrocytes. Normal fluid and transudates contain very low nucleated cell counts (<1000/μl) and low protein content (<2.5 g/dl). (Romanowsky; HP oil.) B, Modified transudate. Pleural. Cat. The effusion cells have been concentrated in this animal with cardiomyopathy. Note a large macrophage, many small lymphocytes, and nondegenerate neutrophils. This fluid had a protein of 2.5 g/dl and an increased nucleated cell count of 4000 cells/μl. The macrophage has phagocytized a red blood cell. (Romanowsky; HP oil.)
Neutrophils
Neutrophils appear similar to those found in peripheral blood. They are medium-sized cells with pale to clear cytoplasm and a segmented nucleus. Neutrophils should be absent or present in very low numbers in normal fluid but they will be found in increased numbers with chronic fluid accumulation or with inflammation (see Figs. 6-5 and 6-6B).
GENERAL CLASSIFICATION OF EFFUSIONS
Effusions are usually classified as transudates, modified transudates, and exudates, related to the protein concentration, nucleated cell count, and cell types present (Table 6-1 ).
TABLE 6-1.
Classification of Common Body Cavity Effusions Based on Fluid Characteristics
| Effusion Type | Color/Turbidity | Total Protein (g/dl) | Specific Gravity* | WBC (# per μl) | Predominant Cell Type (s) |
|---|---|---|---|---|---|
| General Conditions | |||||
| Transudate | Colorless/clear | <2.5 | <1.017 | <1000 |
|
| Modified transudate | Light yellow to apricot/clear to cloudy | ≥2.5 | 1.017–1.025 | >1000 | Mononuclear cells |
| Exudate | Apricot to tan/cloudy | >3.0 | >1.025 | >5000 |
|
| Specific Conditions | |||||
| Chylous | White/opaque | >2.5 | >1.017 | Variable |
|
| Neoplastic | Light yellow to apricot/clear to cloudy | >2.5 | >1.017 | Variable |
|
| Hemorrhagic | Pink to red/cloudy | >3.0 | >1.025 | >1000 |
|
| Bilious | Dark yellow or brown or green/opaque | >3.0 | >1.025 | >5000 | Mixed population with blue-green, brown, or yellow material phagocytized by macrophages |
Note that measurement of specific gravity using a standard refractometer has not been validated for use with body cavity fluids, only urine. Therefore, values should be regarded with caution (George, 2001).
Transudate
Fluids are classified as transudates when they have a low protein content and a low cell count (protein < 2.5 g/dl and cells < 1000/μl). These fluids increase in volume in response to physiologic mechanisms, such as increased hydrostatic vascular pressure or decreased colloidal osmotic pressure, that cause the normal homeostatic mechanisms of fluid production and resorption to be overwhelmed. Some causes for transudate accumulation include severe hypoalbuminemia, portal hypertension, hepatic insufficiency, portosystemic shunt, and early myocardial insufficiency. The cells commonly found in transudates are similar to those in normal fluid, which are mostly mononuclear cells consisting of macrophages, small lymphocytes, and mesothelial cells (see Fig. 6-6A). Neutrophils may compose a small proportion of the population.
Modified Transudate
A fluid is classified as a modified transudate when a transudate changes its physical features. The accumulation of transudative fluid in a body cavity causes increased pressure, which is irritating to the mesothelial cells lining the space. They respond by proliferating and sloughing into the effusion. With time, the sloughed mesothelial cells die and in so doing release chemoattractants that draw small numbers of phagocytes into the effusion to remove cellular debris. The result is a mild increase in both total protein (≥2.5 g/dl) and nucleated cell count (less than 5,000/μl). Thus, modified transudates are generally transudates that have been present long enough to elicit a mild inflammatory reaction (see Fig. 6-6B). It is most often associated with cardiovascular disease or neoplastic conditions.
In cases of extended duration, modified transudates can have a cloudy to almost milky gross appearance. Such fluids strongly resemble chyle and, in fact, have in the past been called “pseudochylous effusions” (a term no longer used). The gross appearance of these fluids is the result of high lipid content (due to higher cholesterol content than serum) but is in no way related to a true chylous effusion, as there are no triglycerides or chylomicrons present (Hillerdal, 1997; Tyler and Cowell, 1989). The phagocytes attracted to remove cellular debris from transudates are rich in enzymes that digest protein but are virtually devoid of enzymes that will break down complex lipids. Consequently, while most of the constituents of dying cells are removed by phagocytosis, lipid content of the cells simply accumulates in the effusion. Effusions formed in this manner are easily distinguished cytologically from true chylous effusions.
The principle cellular constituent of the modified transudate is the reactive mesothelial cell (see Fig. 6-3A). Because of the ability of mesothelial cells to respond to irritation by proliferation, the presence of increased numbers of mesothelial cell clusters and rafts is a common finding in reactivity (see Fig. 6-3B). Mitoses are increased and occasional multinucleated reactive mesothelial cells are seen. Reactive mesothelial cells in clusters are capable of imbibing lipid from the effusion fluid and when they do, they take on the characteristics of secretory cells. In this form they must be differentiated from metastatic adenocarcinoma or mesothelioma. This may be done by critically evaluating the cell populations for criteria of malignancy.
As modified transudates mature, the proportion of inflammatory cells they contain will increase. In most cases the principal inflammatory cell is the nondegenerate neutrophil, but neutrophils rarely account for more than 30% of the total cell population. Over time, modified transudates gradually become cytologically indistinguishable from nonspecific exudates. One method used to distinguish modified transudates from exudates or transudates from exudates is measurement of C-reactive protein concentration in canine effusions (Parra et al., 2006).
Exudate
Exudates are the result of either increased vascular permeability secondary to inflammation or vessel injury/leakage (hemorrhagic effusion, chylous effusion). An exudative fluid usually contains both increased protein and an increased nucleated cell count. The total protein concentration is usually greater than 3.0 g/dl along with cell counts greater than 5,000/μl. Infectious causes for exudates include bacteria (Fig. 6-7A&B ), fungi, viruses, protozoa such as Toxoplasma (Toomey et al., 1995), or helminthes such as Mesocestoides sp. (Caruso et al., 2003). Noninfectious causes involve organ inflammation such as pancreatitis, steatitis, inflammatory neoplasia and irritants such as bile or urine. Cytologic evaluation is useful to determine an underlying cause in cases of exudative effusions.
FIGURE 6-7.

A, Septic peritonitis, dog. Numerous degenerate neutrophils are noted with a large number of pleomorphic bacteria seen, both in the background and within neutrophils. This concentrated smear was made from a dog with a ruptured pancreatic abscess. (Modified Wright; HP oil.) B, Septic exudate. Pleural. Cat. Degenerate neutrophils are bloated, with foamy or vacuolated cytoplasm and also swollen lytic nuclei. Note presence of small gram-positive, pleomorphic bacterial rods. Aerobic and anaerobic cultures are recommended in cases of pyothorax. This sample contains many nucleated cells (>100,000 cells/μl) and >3.0 g/dl protein. (Gram; HP oil.)
Inflammatory effusions are classified according to the standard rules for inflammation as neutrophilic, mixed, or macrophagic. In neutrophilic reactions, neutrophils (either nondegenerate or degenerate) comprise >70% of the inflammatory cells seen. Mixed reactions are characterized by a mixture of neutrophils and macrophages. In histiocytic inflammation, macrophages are the prevalent cell seen.
Most inflammatory effusions are cytologically nonspecific in terms of etiologic diagnosis. However, as with inflammatory responses elsewhere, cytomorphology provides significant clues as to the underlying cause. Neutrophilic inflammatory effusions indicate severe active irritation (Fig. 6-8 ). If neutrophils are degenerate, an effort should be made to identify bacterial organisms within phagocytes (primarily neutrophils). This is generally easiest at the feathered edge of the smear. If organisms are not seen, the fluid should still be cultured. Mixed and macrophagic inflammatory effusions reflect less severe irritation and are found with resolving acute effusions or in association with less irritating etiologic agents than bacteria (e.g., fungal organisms or foreign bodies). Chemical evaluation of effusion fluid is also useful in recognizing sepsis.
FIGURE 6-8.

Nonseptic exudate. Peritoneal. Dog. Concentrated fluid from an animal with pancreatitis showing one cluster of reactive mesothelial cells and neutrophils. The protein content in this sample was 3.0 g/dl with a cell count of 8000 cells/μl. Most of the cells are neutrophils, suggesting an underlying inflammatory condition. Further testing (e.g., fluid and serum lipase or ultrasonography) is required to determine the specific cause for the fluid accumulation. (Romanowsky; HP oil.)
Septic effusions may be diagnosed by use of biochemical parameters in dogs and cats. It was shown that effusion fluid in dogs and cats with a pH <7.2, pCO2 >55 mm Hg, glucose concentration <50 mg/dl, and lactate concentration >5.5 mmol/L is highly likely to have bacterial infection (Swann et al., 1996). A study involving peritoneal fluid in dogs and cats evaluated blood and effusion fluid glucose concentrations. This study found that a difference of blood-effusion glucose >20 mg/dl provides a rapid and reliable means to differentiate septic and nonseptic effusion fluid (Bonczynski et al., 2003). In this study, a difference of blood-effusion lactate concentration in dogs <−2.0 mmol/L was 100% sensitive and 100% specific for the diagnosis of septic peritonitis.
SPECIFIC TYPES OF EFFUSIONS
While most inflammatory effusions are cytologically nonspecific, some etiologies cause reactions with characteristic diagnostic features. These effusions are discussed below.
Feline Infectious Peritonitis
Feline infectious peritonitis (FIP) is unique among the causes of inflammatory effusion in that the fluid that accumulates is usually high in protein, yet has a low cellularity (McReynolds et al., 1997). Classification as an inflammatory effusion is based primarily on the presence of high total protein (often >4.5 g/dl) which is a reflection of a similar elevation in serum protein. Electrophoresis of either the effusion fluid or the serum reveals a polyclonal gammopathy. An albumin-to-globulin ratio of less than 0.8 on the fluid is very suggestive for FIP. It has been reported that if gamma globulin is greater than 32% of the protein in the effusion fluid, this is suggestive of FIP (Shelly et al., 1988). An additional test that can help rule in or rule out FIP is the Rivalta test. To perform this relatively simple test, place one drop of 98% acetic acid into 5 ml distilled water and mix well in a reagent tube. Then add slowly one drop of the effusion fluid to the surface of the acetic acid solution. A positive test requires that the drop of effusion retain its form on the surface or slowly sink to the bottom as a droplet or jellyfish-like shape (Fig. 6-9A ). This test indicates a high protein content as well as fibrin and inflammatory mediators. This test has a positive predictive value of 86% and a negative predictive value of 97% (Hartmann et al., 2003). A more specific cytologic test for FIP involves immunofluorescence of intracellular feline coronavirus (FCo V) within effusion macrophages. When positive (Fig 6-9B), this test is diagnostic for FIP; however, the negative predictive value is 57%, so a negative test may miss cases of FIP (Hartmann et al., 2003).
FIGURE 6-9.

A-C, Abdominal effusion, cat. A, Rivalta test. Positive test results are indicated by a layer of gel on top of the acetic acid solution. Fluid is from a cat with PCR confirmed FIP. The cat was moderately icteric. Note the yellow streaks of gel in the middle of the tube from partially floating material. B, FCo V immunofluorescence test. A specific cytologic test for FIP involves immunofluorescence of intracellular feline coronavirus (FCo V). Shown are three infected and intact macrophages (green) present in abdominal fluid from a cat with FIP. (FCo V; HP oil.) C, This concentrated smear comes from a cat diagnosed with FIP. Note the moderately basophilic macrophages and nondegenerate neutrophils. The background contains basophilic, coarsely granular protein as well as basophilic protein crescents and strands of fibrin. (Modified Wright; HP oil.) D, Nonseptic exudate. Peritoneal. Cat. This animal was diagnosed with FIP. Fluid contains foamy, vacuolated macrophages, mildly degenerate neutrophils, and intermediate to large lymphoid cells. Lymphocytes may be intermediate in size and appear reactive in some cases of FIP. Also note the granular precipitated protein throughout the slide. (Romanowsky; HP oil.)
(A, Photo by Sam Royer, Purdue University. B, Courtesy of Jacqueline Norris, University of Sydney, Australia.)
Cytologically these fluids are usually relative low in cell number (1000 to 30,000/μl) and inconsistent with regard to the cell types present. In a majority of cases the predominant cell is the nondegenerate neutrophil (Fig. 6-9C). However, mixed to macrophagic reactions may be seen (Fig. 6-9D). In rare cases, the lymphocyte is prevalent. Regardless of the predominant cell type, slides in all cases have a purple granular background that results from the high protein content (see Fig. 6-9C).
Nocardial/Actinomycotic Effusions
Complex bacteria such as Nocardia asteroides and Actinomyces sp. are important causes of both peritoneal and pleural effusions in dogs and cats. Grossly, these effusions are turbid and yellow to blood-tinged “tomato soup.” Even when collected in EDTA they typically contain visible particulates or granules (the so-called “sulfur granules”) (Songer and Post, 2005).
On the basis of physical parameters, these effusions are typical exudates, with high total protein and markedly high cellularity. Because of the high cellularity, direct smears are generally adequate for cytologic examination. If particles are observed in the fluid, it is important to make squash preparations of these particles in addition to making smears of the fluid alone.
Microscopically, nocardial and actinomycotic infections are characterized by neutrophilic to mixed inflammation, probably dependent on the duration of the disease. In the more chronic reactions, there is generally a significant reactive mesothelial cell component to the response. A striking feature of the inflammatory response is the morphology of the neutrophils. Whereas most cases of septic pleuritis and peritonitis are signaled by the presence of predominantly degenerating neutrophils, in nocardial and actinomycotic effusions the majority of the neutrophils away from the organisms are nondegenerate. Degenerating neutrophils are only seen immediately in the vicinity of the bacterial organisms because these agents, in contrast to most other bacteria, produce only weak local toxins. The net effect of this phenomenon is that smears in these cases may be easily misinterpreted as noninfectious, particularly if the organisms are not widespread. Because the particles seen grossly often are composed of bacterial colonies, it is extremely important that squash preparations of these particles be examined to ensure that the diagnosis is not missed (Fig. 6-10A&B ).
FIGURE 6-10.

Septic exudate, actinomycosis. Pleural. Dog. Same case A-C. A, Direct smear from a pleural effusion demonstrates a dense colony (i.e., sulfur granule) along with many lysed cells and nuclear streaks. These granules often are dragged to the feathered edge, as was the case with this smear. (Modified Wright; IP.) B, Higher magnification of the colony in A. (Modified Wright; HP oil.) C, The fluid has marked suppurative inflammation with many degenerate neutrophils and several foamy macrophages. Short and long filamentous organisms and lysed cells are seen throughout the background. In areas of the smear lacking bacteria (not shown), the neutrophils were only mildly degenerate. (Modified Wright; HP oil.)
Morphology of the organisms microscopically is quite characteristic. Colonies are composed of delicate filamentous, often beaded, organisms and are often found at the feathered edge of smears (Fig. 6-10C). The most significant diagnostic feature of these organisms is that the filaments are branching. Using standard hematologic stains, Nocardia organisms cannot be differentiated from Actinomyces. However, Nocardia sp. is gram positive and are variably acid-fast positive whereas Actinomyces sp. are gram positive and acid-fast negative (Songer and Post, 2005).
Cytologic diagnosis should be confirmed by bacterial culture of the effusion and/or sulfur granules. Because these species have special culture requirements, it is important that the bacteriology laboratory be fully aware of the provisional diagnosis at the time of sample submission.
Systemic Histoplasmosis
Systemic histoplasmosis caused by Histoplasma capsulatum is a moderately frequent cause of peritoneal effusion in dogs living in the Ohio River Valley (United States). Because the fungus is ubiquitous in the area, serology cannot be relied upon for diagnosis. In many cases, cytologic identification of the organism is essential.
The effusion fluid of histoplasmosis is relatively unique. The fluid is usually clear and colorless. On the basis of physical characteristics, the fluid is a modified transudate; however, cytologically, it is clearly inflammatory with significantly more neutrophils than are seen in the typical canine modified transudate. Whenever inflammatory modified transudates are seen in dogs, the possibility of histoplasmosis should be considered in the differential diagnosis. If seen in an effusion, the disease is considered disseminated and can be found elsewhere such as the liver, spleen, bone marrow, rectal wall, and peripheral blood.
Demonstration of the organism is best done in macrophages (Fig. 6-11 ) at the feathered edge of sediment smears. Histoplasma organisms measure approximately 2 to 3 μm in diameter, are round to ovoid in shape, and have a single basophilic nucleus surrounded by a thick, colorless cell wall. In fluids, it is common to see individual organisms free in the background. The only other organisms of similar cytologic morphology are Leishmania. However, Leishmanial protozoans are distinguished by the presence of an internal kinetoplast, which gives them the appearance of having two nuclei.
FIGURE 6-11.

Histoplasmosis. Dog. A macrophage containing numerous oval 2–3 μm yeast organisms of Histoplasma capsulatum is seen at left center. There are numerous red cells in the background of the smear. (Modified Wright; HP oil.)
Bilious Effusion
Rupture of the gall bladder or common bile duct may occur in any species secondary to direct trauma or disease of the biliary tree. In addition, it is an infrequent accompaniment to diaphragmatic hernia from any cause in the dog and cat. When the results of direct trauma are mainly to the biliary system, leakage of bile is virtually always restricted to the peritoneal cavity, with a resulting peritonitis. When associated with diaphragmatic hernia, leakage of bile occurs when liver is trapped in the diaphragmatic rent and there is necrosis of the gall bladder or common bile duct. In this circumstance, both peritonitis and pleuritis can result. Bile is a very irritative substance; its presence quickly elicits an inflammatory response. Grossly, the fluid may be initially brown (see Fig. 6-1G) to yellow to greenish; however, as the response becomes more and more cellular, this discoloration may become masked. Large volumes of fluid can usually be obtained.
Cytologically, the striking feature of bilious effusion is the presence of bile on the slide. Frequently, bile is seen as yellow to green to blue-black granular material scattered in the slide background (Fig. 6-12A-C ) and in the cytoplasm of neutrophils, reactive mesothelial cells, and macrophages. In reactions of greater duration, bile granules may have all been converted to rhomboidal to amorphous golden crystals of bile pigment. When such crystals are found in the cytoplasm of effusion phagocytes in the absence of evidence of prior hemorrhage (e.g., erythrophagocytosis), the possibility of bilious effusion should be strongly considered. In addition to the typical appearance of bilious effusions, acellular, amorphous, fibrillar blue-grey mucinous material (Fig. 6-13A ) has been associated with biliary tree rupture, particularly of the common bile duct in dogs (Owens et al., 2003). Instead of the green or yellow bile granules present, lakes of extracellular material are the predominant cytologic finding. It is suspected that this bile-free material is produced by biliary and gallbladder epithelium as a consequence to extrahepatic biliary obstruction with regurgitation of normal bile into hepatic lymph and venous blood.
FIGURE 6-12.

Bilious effusion. Peritoneal. Dog. Same case A-B. A, Degenerate neutrophils are surrounded by dark yellow to black amorphous bile material that is free in the background. (Wright-Giemsa; HP oil.) B, Large numbers of mostly nondegenerate neutrophils accompany the presence of amorphous material. The basophilic bile material is coated by stain precipitate producing a pink, granular appearance. This greenish, flocculent fluid had a protein level of 3.0 g/dl and an estimated nucleated cell count of greater than 60,000/μl. (Wright-Giemsa; HP oil.) C, Note extracellular gold-brown crystalline material, vacuolated neutrophils, and macrophages. Some of the neutrophils contain pyknotic nuclei and others contain karyolytic nuclei. (Romanowsky; HP oil.)
(A and B, Courtesy of Rose Raskin, University of Florida.)
FIGURE 6-13.

Bile peritonitis. Dog. Same case A-C. A, This is a concentrated smear of fluid from a dog with a ruptured gall bladder. Note the numerous neutrophils and lakes of blue-grey amorphous mucinous material that are present throughout the background. (Modified Wright; IP.) B, Note the suppurative inflammation with variably degenerate neutrophils as well as basophilic and foamy macrophages. Amorphous blue-gray material, likely mucin, is seen in the background. (Modified Wright; HP oil.) C, Note the suppurative inflammation and foamy macrophages that contain blue-grey to dark blue granular material. The background contains mucinous and finely granular protein. (Modified Wright; HP oil.)
The cellular response to bile is generally mixed with large numbers of neutrophils nearly always present (Fig. 6-13B&C). The degree of degeneration of neutrophils is variable. In addition, bile causes severe irritation to the mesothelial lining of the body cavities, resulting in marked reactive mesothelial cell hyperplasia.
Fluid bilirubin levels that are markedly elevated and several times than levels in serum also give support for a diagnosis of bilious effusion (Owens et al., 2003).
Eosinophilic Effusion
Effusions with more than 10% eosinophils are termed eosinophilic effusions regardless of the protein content or cell count. This condition is uncommonly seen in veterinary medicine. With large numbers of eosinophils, the fluid grossly may have a green tint. The presence of eosinophils does not provide a specific diagnosis and the cause is often unknown in these cases. Neoplasia such as lymphoma or mastocytosis involved half of the cases in one study (Fossum et al., 1993). Heartworm disease, systemic mastocytosis, interstitial pneumonia, disseminated eosinophilic granulomatosis, and lymphomatoid granulomatosis are other possibilities in dogs (Mertens et al., 2005; Bounous et al., 2000).
Uroperitoneum
Urine in the peritoneal space results in chemical irritation. The protein content may be markedly low as a result of dilution from the urine but the cell count and predominant cell type is usually indicative of inflammation or an exudate (see Table 6-1). Early in the condition, a mononuclear cell population may predominate, suggestive of a modified transudate. Bacteria may or may not be present. Neutrophils exposed to the irritant material may show karyolysis with ragged nuclear borders (Fig. 6-14 ). In some cases, urinary crystals are found on cytologic examination, which leads to a diagnosis of uroperitoneum. In one study in cats, creatinine or potassium was increased in fluid and served as a useful predictor for uroperitoneum, generally in a ratio of 2:1 compared with that in serum (Aumann et al., 1998). Simultaneous evaluation of fluid and serum creatinine should show a higher creatinine in the fluid, as it equilibrates much slower than urea nitrogen (BUN). In addition, the serum Na:K also tends to depress in cases of uroabdomen (Aumann et al., 1998; Burrows and Bovee, 1974).
FIGURE 6-14.

Uroperitoneum. Dog. The fluid contained a high number of neutrophils, many of which appeared similar to this “ragged” cell. Urine acts as a chemical irritant causing karyolytic changes to cells. (Wright-Giemsa; HP oil.)
(Courtesy of Rose Raskin, University of Florida.)
Parasitic Ascites (Abdominal Cestodiasis)
In a small number of dogs with ascites, often from western North America, the etiology is aberrant cestodiasis from Mesocestoides infection (Stern et al., 1987; Crosbie et al., 1998; Caruso et al., 2003). Rare reports of infection involve cats. Peritoneal aspirates from anorexic, ascitic dogs are macrophagic with the appearance of tapioca pudding or cream of wheat (Fig. 6-15 ). Motile cestodes can be seen in fluid with the unaided eye. Microscopic examination may show acephalic metacestodes or acoelomic tissue with calcareous corpuscles (Fig. 6-16 ), which may be seen in nonspecific cestode infections. Less often seen microscopically are metacestodes with visible tetrathyridia, a unique larval form having four suckers that represents the asexual reproductive form of Mesocestoides spp. infection (Fig. 6-17 ). Cestode ova are not usually found in the feces (Crosbie et al., 1998).
FIGURE 6-15.

Cestodiasis. Peritoneal. Dog. The ascitic fluid had a tapioca pudding appearance grossly. Motility of these granules may be observed with the unaided eye.
(Courtesy of Jocelyn Johnsrude, IDEXX, West Sacramento, CA.)
FIGURE 6-16.

Cestodiasis. Acoelomic metacestode tissue with amorphous degenerate debris and calcareous corpuscles. Inflammatory cells are also present surrounding the structure. (Romanowsky; LP.)
FIGURE 6-17.

Cestodiasis. Tetrathyridia larval stage. Same case as in Figure 6-15. Note the oval structures at one end that represent suckers and identify the parasite as Mesocestoides spp. (Romanowsky; LP.)
Chylous Effusions
Chylous effusions contain chyle, which is a mixture of lymph and chylomicrons. Chylomicrons, derived from dietary lipids processed in the intestine and transported via lymphatics, are primarily composed of triglycerides. Historically, chylous effusions were thought to be primarily a result of thoracic duct rupture. It is now known that there are a variety of causes for chylous effusions and that rupture of the thoracic duct is uncommon. Causes for chylous effusions in the thoracic cavity include cardiovascular disease, neoplasia (e.g., lymphoma, thymoma, and lymphangiosarcoma), heartworm disease, diaphragmatic hernia, lung torsion, mediastinal fungal granulomas, chronic coughing, vomiting, or idiopathic (Fossum, 1993; Fossum et al., 1986a; Forrester et al., 1991; Waddle and Giger, 1990). In one study (Fossum et al., 1986a). Afghan hounds appeared to have a higher incidence of chylothorax compared to other breeds of dogs. It seems that chylous pleural effusions are more common in cats than dogs, presumably because their lymphatics are more easily damaged by trauma or obstruction.
Chylous ascites is less common. Causes for chyloperitoneum include intra-abdominal neoplasia, steatitis, biliary cirrhosis, lymphatic rupture or leakage, postoperative accumulation following ligation of the thoracic duct, congenital lymphatic abnormalities, and other causes (Fossum et al., 1992; Gores et al., 1994).
Grossly, chylous effusions are described as “milky” white to pink-white fluids, depending on dietary fat content and the presence or absence of hemorrhage (Fig. 6-18A&B ). Some cases of chylous effusion may have fluid that is clear to serosanguineous. Chylous effusions contain triglycerides at a level higher than that found in serum in a ratio often greater than 3:1 (Meadows and MacWilliams, 1994). A cholesterol-to-triglyceride (C/T) ratio of less than 1 is generally considered characteristic of a chylous effusion (Fossum et al., 1986b). Based on lipoprotein electrophoretic studies, pleural chylous effusions can be better identified by fluid triglyceride concentrations greater than 100 mg/dl and nonchylous effusions by concentrations less than 100 mg/dl (Waddle and Giger, 1990). In those same studies, C/T ratios were less reliable.
FIGURE 6-18.

Chylous effusion. Pleural. Cat. A, A pink tint is found in this chylous effusion, indicating some degree of hemorrhage is present. The fluid had 13,000/μl nucleated cell count, 267 mg/dl triglycerides, and 169 mg/dl cholesterol. B, Turbid “milky” fluid in most cases, due to the presence of chyle. Measurement of high fluid triglyceride levels confirms the diagnosis. The cell count of this sample is <10,000 cells/μl and the protein is 4.0 g/dl.
(A, Courtesy of Rose Raskin, University of Florida.)
Cell counts and protein concentrations are elevated over pure transudate levels and therefore chylous effusions generally fit into modified transudate or exudate (most commonly) categories depending on the degree of chronicity. The total protein evaluated by refractometry can be spurious due to interference by the lipid in the solution (George, 2001).
Cytologically, they are characterized by the presence of large numbers of morphologically normal small lymphocytes (Figs. 6-19 and 6-20A ). Lesser numbers of reactive lymphocytes are also usually present. Because these fluids are mildly irritating, long-standing chylous effusions also may contain moderate numbers of reactive mesothelial cells and other inflammatory cells that may contain phagocytized lipid. This lipid generally appears as multiple discrete, colorless vacuoles within the cytoplasm (Fig. 6-20B). Some cases of chronic chylous effusion present with significant numbers of eosinophils. The presence of lipid in the background of the slide, visualized as small, unstained droplets at the periphery of the nucleated cells, is variable.
FIGURE 6-19.

Chylous effusion. Peritoneum. Dog. This direct smear is characteristic of the entire smear. It is composed of essentially all small lymphocytes. Numerous lysed cells are present, as the lipid in the solution acts as a detergent, making the cells particularly fragile. (Modified Wright; HP oil.)
FIGURE 6-20.

Chylous effusion. Pleural. Cat. A-B same case as inFigure 6-18B. A, Note small lymphocytes, neutrophils, one eosinophil (arrow), and one large macrophage. Initially chylous effusions contain predominantly small lymphocytes and macrophages. As the duration of fluid presence increases, neutrophils and eosinophils will increase in number. (Romanowsky; HP oil.) B, Concentrated preparation. Note the punctate lipid vacuoles within macrophages along with small lymphocytes, neutrophils, and low number of red blood cells. (Romanowsky; HP oil.)
Hemorrhagic Effusions
True hemorrhagic effusions can occur in any of the major body cavities. Grossly, these effusions are red to serosanguineous depending on the age of the exudate and the extent of the hemorrhage. Physical evaluation reveals a protein level reflective of, but somewhat less than, that of peripheral blood. Both nucleated cell counts and red blood cell counts are usually elevated.
Cytology is needed to differentiate true hemorrhagic effusions from sample contamination at the time of collection. Hemorrhagic effusions contain predominantly red blood cells with lesser numbers of nucleated cells. The most significant indicator of true hemorrhage is the presence of macrophages containing phagocytized red cells (erythrophagocytosis) and/or hemosiderin (Figs 6-21 and 6-22A ). Hemosiderin in macrophages indicates that the hemorrhage occurred more than two days before collection. These cells are best observed at the feathered edge of sediment smears. Erythrophagocytosis is not seen if hemorrhage is strictly a collection artifact. A second significant observation is whether platelets are seen. True hemorrhagic exudates are devoid of platelets but they are commonly observed in contaminated samples. If uncertainty exists whether the pigment is hemosiderin, in contrast to bile, melanin, or carbon, Prussian blue staining can indicate the presence of iron in hemosiderin (Fig. 6-22B).
FIGURE 6-21.

Hemorrhagic effusion. Pleura. Dog. This direct smear shows numerous enlarged and vacuolated macrophages, a few nondegenerate neutrophils, and two deeply basophilic reactive mesothelial cells (left). Many macrophages contain varying amounts of hemosiderin and erythrophagia is occurring in several cells. (Modified Wright; HP oil.)
FIGURE 6-22.

Hemorrhagic effusion. Cat. Same case A-B. A, Several moderately foamy macrophages contain variable amounts of blue-grey, finely granular pigment, presumed to be hemosiderin. Also noted are two mildly basophilic and granular mesothelial cells, likely reactive. (Modified Wright; HP oil.) B, Two hemosiderophages are noted filled with Prussian blue positive material, confirming it as hemosiderin. (Prussian Blue; HP oil.)
Neoplastic Effusions
Neoplastic processes, both primary and metastatic, are relatively common causes of both abdominal and thoracic effusions in dogs and cats. Neoplastic effusions may be accompanied by significant hemorrhage and/or inflammation but generally they are noninflammatory. Grossly, the fluid may be clear to cloudy and hemorrhagic. Total protein levels are often elevated but nucleated cell counts are highly variable.
In dogs and cats, the common causes of neoplastic effusions are lymphoma (pleural), and adenocarcinoma or carcinoma (either pleural or peritoneal). Sarcomas may cause effusions but neoplastic cells of mesenchymal origin seldom shed cells into the effusion fluid. Mesothelioma can be a rare cause of effusion in any species.
Regardless of the type of neoplasia present, diagnostic cells may or may not be evident in an effusion. If a mass is known to be present, fluid analysis a well as fine-needle aspiration of the mass may be necessary for definitive diagnosis. In one study of the detection of malignant tumors in abdominal and thoracic fluids, sensitivity was low at 64% for dogs and 61% for cats; however, specificity was high at 99% for dogs and 100% for cats (Hirschberger et al., 1999). In the following paragraphs the cytologic features of the principal neoplastic effusions are summarized and illustrated.
Lymphoma
Lymphomatous effusions are generally highly cellular and contain a pleomorphic population of discrete round cells that are morphologically consistent with lymphocytes. The neoplastic cells have high nuclear-to-cytoplasmic ratios, scant to moderate amounts of cytoplasm, and often scattered, small cytoplasmic vacuoles. Occasionally granular lymphocytes are the predominant neoplastic cell (Fig. 6-23 ). Moderate numbers of mitoses are seen. Scattered among the neoplastic cells are red blood cells, reactive mesothelial cells, and inflammatory cells.
FIGURE 6-23.

Neoplastic effusion. Granular lymphoma. Pleural. Cat. This fluid was light yellow, hazy with a protein of 4.2 g/dl and WBC of 5600/μl. 63% of nucleated cells were granulated; two are shown. Granules varied from fine to coarse (as shown) and were frequently eccentrically placed to one side of the cell. Nondegenerate neutrophils (one shown), small lymphocytes, and occasional phagocytes were also present. (Wright-Giemsa; HP oil.)
(Courtesy of Rose Raskin, University of Florida.)
The principle differential diagnosis for lymphomatous effusion is chylous effusion. In truth, lymphomatous invasion of lymphatics often causes a concomitant chylous effusion. However, recognizing the presence of lymphoma is generally not a major problem. Most cases of lymphoma consist of malignant cells that are much larger and more pleomorphic than normal lymphocytes with nuclei containing prominent, often bizarre and angular nucleoli (Fig. 6-24A&B ). Only rare cases of lymphoma have cells that are morphologically the same as normal small lymphocytes. In these cases the principal differentiating feature of the neoplastic disease is a much greater cellularity than what is generally seen with chylous effusion alone.
FIGURE 6-24.
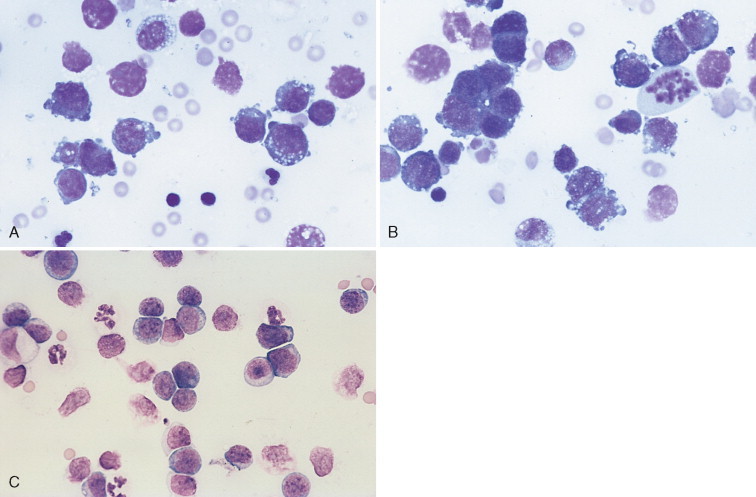
Neoplastic effusion. Lymphoma. Pleural. A, Dog. Note the individual large round cells with high nuclear-tocytoplasmic ratio and mildly vacuolated cytoplasm. Also evident are two small lymphocytes and one neutrophil. Light-purple, round structures are free nuclei from lysed cells. These cells cannot be evaluated. The cellularity of this sample is 15,000 cells/μl with a protein of 3.4 g/dl. (Romanowsky.) B, Dog. Note individual lymphoblasts, one mitotic figure, two intermediate-size lymphocytes, and one small lymphocyte. A few lysed cells and red blood cells are also present. (Romanowsky.) C, Cat. Note the monomorphic population of large lymphoblasts, few neutrophils, and few lysed cells. The lymphoblasts are larger than neutrophils and contain a small rim of cytoplasm with a large, round nucleus. The nuclear chromatin is fine and nucleoli are visible in many of the cells. The nucleated cell count of this fluid is 14,000 cells/μl with increased protein (4.0 g/dl). (Romanowsky; ×200.)
Evaluation of the types of lymphoid cells found within the fluid by immunocytochemistry or flow cytometry may provide significant information related to the origin of these lymphoid cells. A monotypic population of medium and/or large lymphocytes is more likely associated with neoplasia (Figs. 6-24C, 6-25 , and 6-26 ) than with a reactive process. Immunocytochemistry with antibodies against the molecules CD3 (T-cell) and CD79a (B-cell) is helpful in these cases.
FIGURE 6-25.

Neoplastic effusion. Pleural. T-cell lymphoma. Dog. Same case A-B. A, Cytocentrifugated specimen demonstrating a variably sized round cell population having scant to moderate amounts of basophilic cytoplasm, fine to moderately coarse chromatin with occasional prominent nucleoli, and variable N:C. (Modified Wright; HP oil.) B, Sediment smear displays positive cell surface staining in all lymphoid cells for the CD3 antigen, which supports a clonal population of T-lymphocytes. Note the small, likely normal, lymphocyte below center. Red cells are barely visible beneath the size bar. Rare small normal lymphocyte reacted with CD79a antibody (not shown). (CD3 antibody; HP oil.)
(A and B, Courtesy of Rose Raskin, Purdue University.)
FIGURE 6-26.

Neoplastic effusion. Pleural. B-cell lymphoma. Cat. Same case A-B. A, Cytocentrifuged specimen demonstrating marked pleomorphism of round cells. Some cells have multiple nuclei and irregularly shaped nuclei. Nucleoli are usually large and multiple. There is surface blebbing on several cells, which may be mesothelial in origin. (Modified Wright; HP oil.) B, All large round cells are positively stained for the CD79a antigen in this sediment smear of pleural fluid supporting a B-cell neoplasm. CD3 antigen was absent on all cells (not shown). (CD79a antibody; HP oil.)
(A and B, Courtesy of Rose Raskin, Purdue University.)
Carcinoma and Adenocarcinoma
Carcinomatous effusions in dogs and cats may be the result of either a primary or secondary neoplastic process. In the chest, the principal primary neoplasm is pulmonary adenocarcinoma. In order for neoplastic cells from this tumor to be present in pleural effusions, the neoplasm must have invaded either into pulmonary vessels and lymphatics or directly through the pleural surface of the lung and into the pleural cavity.
Pulmonary adenocarcinoma is a relatively uncommon tumor that rarely extends into the pleural cavity. As a consequence, most carcinomatous pleural effusions are the result of metastatic disease. In females, by far the most common cause of such effusions is metastatic mammary carcinoma; in males, the two most common metastatic carcinomas seen are prostatic carcinoma and transitional cell carcinoma.
In the peritoneal cavity the principal causes of carcinomatous effusions are those neoplasms which spread by implantation on the peritoneal surface. Significant among these are cholangiocarcinoma, pancreatic adenocarcinoma, ovarian adenocarcinoma in females, and prostatic carcinoma in males. Metastatic mammary carcinoma is also a common cause of peritoneal carcinomatosis.
Cytologically, all of these tumors are morphologically similar and cannot be readily differentiated (Clinkenbeard, 1992). Carcinomatous effusions are characterized by the presence of rafts and acinar arrays (adenocarcinoma) of round to polygonal cells with variable amounts of often extremely basophilic cytoplasm. Cytoplasmic basophilia may be so intense as to obscure nuclear detail. Inflammation may or may not be present, but reactive mesothelial cell hyperplasia is a constant feature (Figs. 6-27A&B and 6-28A&B ).
FIGURE 6-27.

Neoplastic effusion. Adenocarcinoma. Pleural. Dog. Same case A-B. A, Note the cohesive cluster of neoplastic epithelial cells. The large, distorting vacuoles suggest a secretory nature to the tissue of origin. Prominent nuclear molding is seen in the upper left side of the image. The background contains a mixture of inflammatory cells. (Modified Wright; HP oil.) B, Note the two neoplastic cells that exhibit marked pleomorphism of their nucleoli. Compare the neoplastic cells to the nearby macrophages. (Modified Wright; HP oil.)
FIGURE 6-28.

Neoplastic effusion. Adenocarcinoma. Pleural. Cat. Same case A-B. A, Note the presence of clusters and sheets of large cells. This fluid is highly cellular (23,000 cells/μl) and has an increased protein content (3.6 g/dl). Inflammatory cells are often found in effusions associated with neoplasia. (Romanowsky; IP.) B, Note that the cells are large with abundant basophilic, lightly vacuolated cytoplasm. The nuclei are round with granular coarse chromatin and a large prominent nucleolus. Neutrophils can be seen within the cytoplasm of some of the neoplastic cells. (Romanowsky; HP oil.)
Establishing a diagnosis of pleural or peritoneal carcinomatosis is probably the most difficult challenge in diagnostic fluid cytology. Carcinoma cells strongly resemble reactive mesothelial cells and cytoplasmic basophilia in both of these populations makes evaluation of nuclear criteria of malignancy particularly difficult. The problems become even more exaggerated when significant inflammation is present because reactive mesothelial cells can become quite dysplastic. For these reasons it is particularly important to search diligently for nuclear criteria of malignancy in suspect populations of cells. Areas where nuclear detail can be seen must be found (see Figs. 6-27B and 6-28B). Whereas in most circumstances four nuclear criteria are sufficient to allow a diagnosis of malignancy, in the case of pleural or peritoneal carcinomatosis, at least five distinct nuclear criteria should be demonstrated.
Once the diagnosis of malignancy has been established, differentiation of carcinoma from adenocarcinoma is much easier. Adenocarcinomas are secretory tumors; as such, most instances of these vacuoles are unstained. In some cells the amount of secretory product is sufficient to displace the nucleus peripherally. Simple carcinomas are devoid of secretory vacuoles.
Mesothelioma
Mesothelioma is a rare tumor that can occur in any species. The neoplasm arises from the mesothelial lining of the serous body cavities. Cytologically, the tumor is very difficult to differentiate from carcinoma and reactive mesothelial hyperplasia. Tumor cells are round to polygonal and are arranged primarily in clusters. Nuclei are hyperchromic and located centrally. Often nuclei of adjacent cells within a cluster appear to press against each other resulting in triangulation of nuclei (nuclear molding). Because of the difficulty of differentiating mesothelioma from reactive mesothelial hyperplasia, caution must be used when evaluating suspect populations for criteria of malignancy. As with carcinomatous effusions, at least five definite nuclear criteria of malignancy must be seen before a presumptive diagnosis is made (Fig. 6-29A–C ).
FIGURE 6-29.

Neoplastic effusion. Mesothelioma. Same case A-C. A, Pleural. Dog. The neoplastic cells are present as individual cells and in small clusters. The nucleoli are variably shaped and very prominent. (Romanowsky; HP oil.) B, These cells contain a variable amount of cytoplasm with one or more nuclei. The nuclei may be of odd number and variable size. (Romanowsky; HP oil.) C, The nuclear chromatin is coarsely granular and irregularly clumped with large prominent nucleoli. Many cells retain the fringed glycocalyx border. Also present are low numbers of small lymphocytes, neutrophils, and red blood cells. (Romanowsky; HP oil.)
Once the diagnosis of malignancy has been established, an attempt to differentiate mesothelioma from carcinoma can be made. There are no well-established morphologic criteria for differentiating these tumors; consequently, such attempts are often an exercise in futility. Based on relative frequency of occurrence, most of these effusions are simply considered to be carcinomatous until proven otherwise. A case of mesothelioma has been recognized by the use of the immunohistochemical marker calretinin in a horse (Stoica et al., 2004); it is possible that this marker can be used to differentiate mesothelioma and carcinoma in other species.
PERICARDIAL EFFUSIONS
Pericardial effusions may also be classified as transudates, modified transudates, or exudates. Because they are somewhat unique in their presentation, they are considered separately.
In many cases, pericardial effusion fluid is hemorrhagic (Figs. 6-30 and 6-31 ). Causes of pericardial effusions include neoplasia, which involves 41% (Kerstetter et al., 1997) to 58% (Dunning, 2001) of canine cases examined. Benign idiopathic pericardial hemorrhage accounted for up to 45% of cases in dogs in one study (Kerstetter et al., 1997). A variety of other infrequently seen causes, including infection, cardiac insufficiency, uremia, trauma, foreign body, coagulopathy, pericarditis, hernia, or left atrial rupture, have been reported (Bouvy and Bjorling, 1991; Petrus and Henik, 1999; Shubitz et al., 2001; Peterson et al., 2003). Pericardial effusions in cats are most often related to congestive heart failure (28%). FIP, which accounted for 17%, is the second most frequent disease causing pericardial effusion in cats (Bouvy and Bjorling, 1991).
FIGURE 6-30.

Hemorrhagic effusion. Pericardial. Dog. Buffy-coat preparation. Note the variety of cells, including neutrophils, lymphocytes, erythrophages, and reactive mesothelial cells. This fluid was red and turbid with 5,000,000 red blood cells/μl, 7000 nucleated cells/μl, and protein of 4.0 g/dl. (Romanowsky; HP oil.)
FIGURE 6-31.

Hemorrhagic effusion. Pericardial. Dog. Observe the erythrophagia, reactive mesothelial cells, macrophage, and small lymphocytes with many red blood cells. Also note that one macrophage contains brown hemosiderin pigment. One mesothelial cell contains two nuclei. Cytologic evaluation of pericardial fluid is important to rule out infection and some types of neoplasia. Often additional testing is required to determine the cause of hemorrhagic pericardial effusion. (Romanowsky; HP oil.)
Cytologic evaluation of pericardial effusion is challenging, but is most valuable in ruling out infection and inflammation. The pericardium is notorious for eliciting mesothelial hyperplasia. These mesothelial cells become very large and basophilic, often binucleate with prominent nucleoli (see Fig. 6-3B) and mitotic figures. It is often not possible to distinguish these reactive mesothelial cells from possible neoplastic cells. In addition, hemangiosarcoma may cause pericardial hemorrhage, but neoplasia of mesenchymal origin typically will not shed neoplastic cells into effusions. In one study (Sisson et al., 1984), 74% of 19 neoplastic effusions were not detected on the basis of cytologic findings and 13% of 31 non-neoplastic effusions were falsely reported as positive, leading the authors to conclude that pericardial fluid analysis did not reliably distinguish neoplastic from non-neoplastic disorders. More recently, cardiac troponins I (cTnI) and T (cTnT) have been evaluated in pericardial fluid. Results from this study involving 37 dogs suggest that cTNI may be useful in differentiating idiopathic pericardial effusions from those caused by hemangiosarcoma (Shaw et al., 2004).
Further testing is often required (e.g., ultrasonography, coagulation testing, pericardiectomy, finding other evidence of trauma) to determine the underlying cause for the effusion. In a study of 51 dogs with pericardial effusions, the pH of the fluid was examined and compared with a final diagnosis (Edwards, 1996). When measured by precise instrumentation, the pH indicated that a reading greater than 7.3 was likely due to noninflammatory conditions, usually neoplasia. Using a less accurate urinary dipstick, pericardial fluid pH = 7.0 was associated with neoplasia in 93% of the cases, while <7.0 suggested benign or non-neoplastic conditions. The pH of the fluid needs to be measured shortly after obtaining the sample. A recent study found similar results; however, considerable overlap was detected, thus limiting the usefulness of pH to differentiate the etiology of pericardial effusions (Fine et al., 2003).
ANCILLARY TESTS
In some instances, other laboratory tests on an effusion may be indicated to help determine a specific cause for the fluid accumulation. A summary of previously described tests is shown in Table 6-2 .
TABLE 6-2.
Biochemical and Electrophoretic Tests Used to Evaluate Effusions
| Test | Use/Expected Result | Effusion |
|---|---|---|
| Creatinine/potassium | Fluid values are higher than those of serum creatinine and/or potassium | Uroperitoneum |
| Triglycerides | Fluids often contain triglycerides that exceed 100 mg/dl | Chylous |
| Cholesterol | Fluid level that is higher than that of serum cholesterol | Nonchylous |
| Bilirubin | Fluid level that is higher than serum bilirubin | Bile peritonitis/pleuritis |
| Lipase/amylase | Fluid values are higher than those of serum lipase and/or amylase | Pancreatitis |
| Protein electrophoresis | A:G ratio of <0.8 on the fluid is very suggestive for FIP | FIP infection |
| Lipoprotein electrophoresis | Presence of chylomicrons in fluid when triglyceride levels are equivocal | Chylous |
| pH | pH <7.0 may suggest benign or non-neoplastic conditions | Pericardial |
| pH, pCO2, glucose, lactate | Fluids with pH <7.2, pCO2 >55 mm Hg, glucose <50 mg/dl, or lactate >5.5 mmol/L are likely to have bacterial infection | Septic |
| Serum-effusion glucose | A difference of serum-effusion glucose of >20 mg/dl indicates bacterial infection | Septic peritonitis |
| Serum-effusion lactate | A difference in serum-effusion lactate of <−2.0 mmol/L (in dogs) indicates bacterial infection | Septic peritonitis |
REFERENCES
- Aumann M, Worth LT, Drobatz KJ. Uroperitoneum in cats: 26 cases (1986–1995) J Am Anim Hosp Assoc. 1998;34:315–324. doi: 10.5326/15473317-34-4-315. [DOI] [PubMed] [Google Scholar]
- Bonczynski JJ. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentrations as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg. 2003;32:161. doi: 10.1053/jvet.2003.50005. [DOI] [PubMed] [Google Scholar]
- Bounous DI, Bienzle D, Miller-Liebl D. Pleural effusion in a dog. Vet Clin Pathol. 2000;29:55–58. doi: 10.1111/j.1939-165x.2000.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Bouvy BM, Bjorling DE. Pericardial effusion in dogs and cats. Part I. Normal pericardium and causes and pathophysiology of pericardial effusion. Compend Contin Educ Pract Vet. 1991;13:417–424. [Google Scholar]
- Braun JP. Comparison of four methods for determination of total protein concentrations in pleural and peritoneal fluid from dogs. Am J Vet Res. 2001;62:294. doi: 10.2460/ajvr.2001.62.294. [DOI] [PubMed] [Google Scholar]
- Burrows CF, Bovee KC. Metabolic changes due to experimentally induced rupture of the canine urinary bladder. Am J Vet Res. 1974;35(8):1083–1088. [PubMed] [Google Scholar]
- Caruso KJ, James MP, Fisher D. Cytologic diagnosis of peritoneal cestodiasis in dogs caused by Mesocestoides sp. Vet Clin Pathol. 2003;32(1):50–60. doi: 10.1111/j.1939-165x.2003.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Clinkenbeard KD. Diagnostic cytology: carcinomas in pleural effusions. Compend Contin Educ Pract Vet. 1992;14(2):187–194. [Google Scholar]
- Crosbie PR, Boyce WM, Platzer EG. Diagnostic procedures and treatment of eleven dogs with peritoneal infections caused by Mesocestoides spp. J Am Vet Med Assoc. 1998;213:1578–1583. [PubMed] [Google Scholar]
- Dunning D. Pericardial effusion. In: Wingfield WE, editor. Veterinary emergency medicine secrets. ed 2. Hanley and Belfus; Philadelphia: 2001. pp. 219–223. [Google Scholar]
- Edwards NJ. The diagnostic value of pericardial fluid pH determination. J Am Anim Hosp Assoc. 1996;32:63–67. doi: 10.5326/15473317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Fine DM, Tobias AH, Jacob KA. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17(4):525–529. doi: 10.1111/j.1939-1676.2003.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Ford RB, Mazzaferro EM. Handbook of veterinary procedures and emergency treatment. ed 8. Saunders; Philadelphia: 2006. pp. 6–7. 52-53, 132-33. [Google Scholar]
- Forrester SD, Fossum TW, Rogers KS. Diagnosis and treatment of chylothorax associated with lymphoblastic lymphosarcoma in four cats. J Am Vet Med Assoc. 1991;198:291–294. [PubMed] [Google Scholar]
- Forrester SD, Troy GC, Fossum TW. Pleural effusions: pathophysiology and diagnostic considerations. Compend Contin Educ Pract Vet. 1988;10:121–136. [Google Scholar]
- Fossum TW. Feline chylothorax. Compend Contin Educ Pract Vet. 1993;15:549–567. [Google Scholar]
- Fossum TW, Birchard SJ, Jacobs RM. Chylothorax in 34 dogs. J Am Vet Med Assoc. 1986;188:1315–1318. [PubMed] [Google Scholar]
- Fossum TW, Hay WH, Boothe HW. Chylous ascites in three dogs. J Am Vet Med Assoc. 1992;200:70–76. [PubMed] [Google Scholar]
- Fossum TW, Jacobs RM, Birchard SJ. Evaluation of cholesterol and triglyceride concentrations in differentiating chylous and nonchylous pleural effusions in dogs and cats. J Am Vet Med Assoc. 1986;188:49–51. [PubMed] [Google Scholar]
- Fossum TW, Wellman M, Relford RL. Eosinophilic pleural or peritoneal effusions in dogs and cats: 14 cases (1986–1992) J Am Vet Med Assoc. 1993;202:1873–1876. [PubMed] [Google Scholar]
- George JW. The usefulness and limitations of hand-held refractometers in veterinary laboratory medicine: an historical and technical review. Vet Clin Pathol. 2001;30(4):201–210. doi: 10.1111/j.1939-165x.2001.tb00432.x. [DOI] [PubMed] [Google Scholar]
- George JW, O'Neill SL. Comparison of refractometer and biuret methods for total protein measurement in body cavity fluids. Vet Clin Pathol. 2001;30(1):16–18. doi: 10.1111/j.1939-165x.2001.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Gores BR, Berg J, Carpenter JL. Chylous ascites in cats: Nine cases (1978–1993) J Am Vet Med Assoc. 1994;205:1161–1164. [PubMed] [Google Scholar]
- Hartmann K, Binder C, Hirschberger J. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Intern Med. 2003;17:781–790. doi: 10.1111/j.1939-1676.2003.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerdal G. Chylothorax and pseudochylothorax. Euro Resp J. 1997;10(5):1157–1162. doi: 10.1183/09031936.97.10051157. [DOI] [PubMed] [Google Scholar]
- Hirschberger J, DeNicola DB, Hermanns W. Sensitivity and specificity of cytologic evaluation in the diagnosis of neoplasia in body fluids from dogs and cats. Vet Clin Pathol. 1999;28:142–146. doi: 10.1111/j.1939-165x.1999.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Kerstetter KK, Krahwinkel DJ, Millis DL. Pericardiectomy in dogs: 22 cases (1978–1994) J Am Vet Med Assoc. 1997;211:736–740. [PubMed] [Google Scholar]
- Kirby BM. Peritoneum and Peritoneal Cavity. In: Slatter D, editor. Textbook of small animal surgery. ed 3. Saunders; Philadelphia: 2003. pp. 414–418. [Google Scholar]
- McReynolds C, Macy D. Feline infectious peritonitis. Part I: Etiology and diagnosis. Compend Contin Educ Pract Vet. 1997;19(9):1007–1016. [Google Scholar]
- Meadows RL, MacWilliams PS. Chylous effusions revisited. Vet Clin Pathol. 1994;23:54–62. doi: 10.1111/j.1939-165x.1994.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Mertens MM, Fossum TW, MacDonalds KA. Pleural and extrapleural diseases. In: Ettinger EJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 6. Saunders; Philadelphia: 2005. pp. 1272–1283. [Google Scholar]
- Meyer DJ, Franks PT. Effusion: classification and cytologic examination. Compend Contin Educ Pract Vet. 1987;9:123–129. [Google Scholar]
- Owens SD, Gossett R, McElhaney MR. Three cases of canine bile peritonitis with mucinous material in abdominal fluid as the prominent cytologic finding. Vet Clin Pathol. 2003;32:114–120. doi: 10.1111/j.1939-165x.2003.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Papasouliotis K. Use of the Vettest 8009 and refractometry for determination of total protein, albumin, and globulin concentrations in feline effusions. Vet Clin Pathol. 2002;31:162. doi: 10.1111/j.1939-165x.2002.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Parra MD, Papasouliotis K, Ceron JJ. Concentrations of C-reactive protein in effusions in dogs. Vet Rec. 2006;158:753–757. doi: 10.1136/vr.158.22.753. [DOI] [PubMed] [Google Scholar]
- Peterson PB, Miller MW, Hansen EK. Septic pericarditis, aortic endarteritis, and osteomyelitis in a dog. J Am Anim Hosp Assoc. 2003;39(6):528–532. doi: 10.5326/0390528. [DOI] [PubMed] [Google Scholar]
- Petrus DJ, Henik RA. Pericardial effusion and cardiac tamponade secondary to brodifacoum toxicosis in a dog. J Am Vet Med Assoc. 1999;215:647–648. [PubMed] [Google Scholar]
- Shaw SP, Rozanski EA, Rush JE. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med. 2004;18(3):322–324. doi: 10.1892/0891-6640(2004)18<322:ctiati>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shelly SM, Scarlett-Kranz J, Blue JT. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J Am Anim Hosp Assoc. 1988;24:495–500. [Google Scholar]
- Shubitz LF, Matz ME, Noon TH. Constrictive pericarditis secondary to Coccidioides immitis infection in a dog. J Am Vet Med Assoc. 2001;218(4):537–540. doi: 10.2460/javma.2001.218.537. [DOI] [PubMed] [Google Scholar]
- Sisson D, Thomas WP, Ruehl WW. Diagnostic value of pericardial fluid analysis in the dog. J Am Vet Med Assoc. 1984;184:51–55. [PubMed] [Google Scholar]
- Songer JG, Post KW. Veterinary microbiology: bacterial and fungal agents of animal disease. Saunders; St. Louis: 2005. pp. 10–12. 55-59, 83-86. [Google Scholar]
- Stern A, Walder EJ, Zontine WJ. Canine Mesocestoides infections. Compend Contin Educ Pract Vet. 1987;9:223–231. [Google Scholar]
- Stoica G, Cohen N, Mendes O. Use of immunohistochemical marker calretinin in the diagnosis of a diffuse malignant metastatic mesothelioma in an equine. J Vet Diagn Invest. 2004;16(3):240–243. doi: 10.1177/104063870401600313. [DOI] [PubMed] [Google Scholar]
- Swann H. Use of abdominal fluid pH, pCO2, [glucose] and [lactate] to differentiate bacterial peritonitis from non-bacterial causes of abdominal effusion in dogs and cats. J Vet Emerg Crit Care. 1996;6:114. [Google Scholar]
- Toomey JM, Carlisle-Nowak MM, Barr SC. Concurrent toxoplasmosis and feline infectious peritonitis in a cat. J Am Anim Hosp Assoc. 1995;31:425–428. doi: 10.5326/15473317-31-5-425. [DOI] [PubMed] [Google Scholar]
- Tyler RD, Cowell Rl. Evaluation of pleural and peritoneal effusions. Veterinary Clinics of North America: Small Animal Practice. 1989;19(4):743–768. doi: 10.1016/s0195-5616(89)50082-2. [DOI] [PubMed] [Google Scholar]
- Waddle JR, Giger U. Lipoprotein electrophoresis differentiation of chylous and nonchylous pleural effusions in dogs and cats and its correlation with pleural effusion triglyceride concentration. Vet Clin Pathol. 1990;19:80–85. doi: 10.1111/j.1939-165x.1990.tb00548.x. [DOI] [PubMed] [Google Scholar]


