WHY BE CONCERNED?
The risk of emerging, reemerging, foreign, and intentionally introduced animal disease is real, and many perceive this as a growing problem. The understanding of animal-human pathogen relationships relies on scientific information about various species and populations of animals. National regulatory statutes that provide disease protection between animals and humans must be current and must utilize up-to-date scientific data to protect human health and our food supply while not jeopardizing or overregulating any one animal.
In the United States, the U.S. Department of Agriculture (USDA), the Department of the Interior (DOI), and the Department of Homeland Security (DHS) are the federal agencies tasked with protecting the nation's wildlife, livestock industries, companion animals, human population, and the food supply from disease. The need for regulations has become more acute with the advent of modern transportation systems that allow movement of animals from any part of the world in a matter of hours. Also, our animal and human populations, along with the U.S. food supply, will now and always need to be protected against the threat of intentionally introduced animal and human disease because bioterrorism will remain a threat.
In a late December 2003 news conference, then–Secretary of Agriculture Ann Veneman made the following statement while talking about a case of bovine spongiform encephalopathy that had been diagnosed Dec. 23, 2003, in a cow in the state of Washington: “The USDA has a primary goal of using science as the basis for decisions involving livestock health matters.”
It is not clear that all federal regulatory officials always use known science to draft regulations and apply them to risk situations. Zoo veterinarians ask only that camelids, which are classified as domestic animals, and other species of captive and free-ranging wild animals be treated fairly, using science-based consensus of understanding that may be incorporated into laws, regulations, policies, and programs. Current scientific information is the least that these populations of unique animals deserve.
CLASSIFICATION AND EVOLUTION
Camelids are not ruminants taxonomically, physiologically, or behaviorally.7, 8 Most importantly, from a veterinary standpoint, camelids and ruminants differ in susceptibility to infectious and parasitic diseases. The differences between camelids and ruminants should exclude camelids from being classified as ruminants. Nonetheless, camelids have been placed in various categories, such as “exotic animals,” “wild animals,” “other livestock species,” and “ruminants,” by state and federal regulators. Camelids have consistently been subjected to sudden, adverse regulations (some inappropriate) when an emerging disease of livestock appears on the scene.
The closing of the Canadian border to camelids when bovine spongiform encephalitis was diagnosed in a cow in Alberta, Canada, is a case in point. Camelids were classified as ruminants and subjected to all restrictions placed on ruminants. The fact that camelids have never been diagnosed with any of the transmissible spongiform encephalopathies anywhere in the world (and are not ruminants) was not given proper consideration.
When questioned about that action, the response was that ruminants are defined by an “encyclopedia” as animals that chew a cud, are cloven hoofed, and have three- or four-chambered stomachs. Regulators completely disregarded the scientific literature that clearly shows that foregut fermentation, complex multicompartmentalized stomachs, food regurgitation, and rechewing are not limited to “ruminants” but are found in species as diverse as kangaroos and nonhuman primates.13 In kangaroos, regurgitation and rechewing is referred to as merycism (Greek, “chewing the cud”). Foregut fermentation and multicompartmented stomachs are also seen in many species, including the hippopotamus, kangaroo, colobus monkey, and peccary.4
Modern paleontologic and taxonomic scientists clearly state that camelids belong in a separate suborder Tylopoda (Latin, “padded foot”) in the order Artiodactyla, which is distinct from the suborder Ruminantia* (Box 46-1 ).
Box 46-1. Classification of the Artiodactyla.
Class—Mammalia
- Order—Artiodactyla
- Suborder—Suiformes
- Family—Hippopotamidae-Hippopotamuses
- Family—Suidae—Pigs
- Family—Tayassuidae—Peccaries
- Suborder—Tylopoda (L., “padded foot”)
- Family—Camelidae
- Camelus bactrianus ferus—Wild Bactrian camel
- C. bactrianu—Bactrian camel (two humps)
- C. dromedarius—romedary camel (one hump)
- Lama guanacoe—Guanaco
- L. glama—Llama
- L. (Vicugna) pacos—Alpaca
- Vicugna vicugna—Vicuña
- Suborder—Ruminantia—Ruminants
- Family—Tragulidae—Chevrotain, mouse deer
- Family—Moschinae—Musk deer
- Family—Giraffidae—Giraffe
- Family—Cervidae—Deer, elk, caribou
- Family—Antilocapridae—Pronghorn
- Family—Bovidae—Cattle, bison, antelope, sheep, goats
Camelid evolution began in North America 40 to 50 million years ago in the early Eocene epoch.6, 7 Separation of the Tylopoda and Ruminantia occurred early in the evolutionary process, when the progenitors of both groups were small goat-sized animals with simple stomachs.33
Tylopods and ruminants continued to evolve by what is known as parallel evolution, which is the development of similarities in separate but related evolutionary lineages through the operation of similar selective factors acting on both lines6, 7, 33 (Figure 46-1 ).
Fig 46-1.
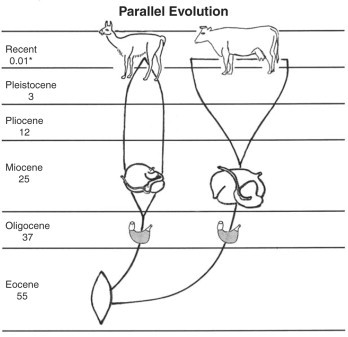
Diagram of parallel evolution of Camelidae and Ruminantia.
The Pleistocene epoch was characterized by a series of periods of extreme cold and glaciations in northern North America and Europe. The last glacial retreat occurred about 10,000 years ago, marking the beginning of the Recent epoch.
Asia and Alaska are now separated by the 90-km (56-mile)–wide Bering Strait. However, during the height of one of the early Pleistocene glacial periods, the sea level was lowered sufficiently to expose a wide land bridge. Plant and animal species moved back and forth across this bridge; the camel line of Camelidae migrated from North America into Asia, where the evolutionary process continued and domestication took place.
Progenitors of the South American camelids (SACs) (guanaco, vicu-a, llama, and alpaca) migrated to South America at the beginning of the Pleistocene epoch (∼3 million years ago), when an open land connection between North and South America developed.6 Evolution continued in South America, where llama and alpaca were domesticated.9, 10, 35
DIFFERENCES BETWEEN CAMELIDS AND RUMINANTS
Anatomic and physiologic differences between camelids and ruminants abound (Table 46-1 ).
Table 46-1.
Differences Between Camelids and Ruminants
| South American Camelids | Ruminants | |
|---|---|---|
| Evolutionary pathways | Diverged 40 million years ago. | Diverged 40 million years ago. |
| Blood | ||
| Red blood cells | Elliptic and small (6.5 μm). | Round and large (10 μm). |
| Predominant white blood cell | Neutrophil. | Lymphocyte. |
| Leukocytes | Up to 22,000. | Up to 12,000. |
| Blood glucose levels | Higher than ruminants (73-121 mg/dL). | 18-65 mg/dL. |
| Integument | ||
| Horns or antlers | None. | Usually present in male. |
| Foot | Triangular-shaped toenails and fat pad covered by soft, flexible slipper. | Has hooves and sole. |
| Upper lip | Split and prehensile. | Not split. |
| Flank fold | None. | Pronounced. |
| Musculoskeletal system | ||
| Stance | Modified digitigrades. | Unguligrade ending in a hoof. |
| Second and third phalanges | Horizontal. | Almost vertical. |
| Foot | Not cloven. | Cloven. |
| Dewclaws | None. | Many have dewclaws. |
| Digestive system | ||
| Foregut fermenter, with regurgitation, rechewing, and reswallowing. | Same (parallel evolution). | |
| Stomach | Three compartments not homologous with rumen, reticulum, omasum, and abomasum; all compartments have glandular epithelium; stomach motility from caudad to craniad; resistant to bloat. | Four compartments; susceptible to bloat. |
| Dental formula* | I 1/3, C 1/1, PM 1-2/1-2, M 3/3 × 2 = 28-32 | I 0/3, C 0/1, PM 3/3, M 3/3 × 2 =32 |
| Vicuña has incisors that continue to erupt. | ||
| Reproduction | ||
| Ovulation | Induced. | Spontaneous. |
| Estrous cycle | No. | Yes. |
| Follicular wave cycle | Yes. | No. |
| Copulation | In prone position. | In standing position. |
| Placenta | Diffuse and noninvasive. | Cotyledonary. |
| Epidermal membrane | Surrounding fetus. | None on fetus. |
| Cartilaginous projection on tip of penis | Yes. | No. |
| Ejaculation | Prolonged. | Short and intense. |
| Respiratory system | ||
| Soft palate | Elongated; primarily a nasal breather. | Short; nasal or oral breather. |
| Urinary system | ||
| Kidney | Smooth and elliptic. | Smooth or lobed. |
| Suburethral diverticulum | In female at external urethral orifice | None |
| Dorsal urethral recess | In male at junction of pelvic and penile urethra. | In some species. |
| Parasites | ||
| Lice | Unique biting and sucking lice. | Lice species different. |
| Coccidia | Eimeria species (coccidia) are different. | Unique species of coccidia. |
| Gastrointestinal nematodes | Share some with cattle, sheep, and goats. | Share with camelids. |
| Infectious diseases | ||
| Tuberculosis | Minimally susceptible. | Highly susceptible. |
| Bovine brucellosis | No known natural. | Highly susceptible. |
| Foot-and-mouth disease | Mild susceptibility. | Highly susceptible. |
| Rare clinical disease with other bovine and ovine viral diseases. | ||
| Behavior | ||
| Females do not lick their offspring | Females lick offspring. | |
| Females do not touch/lick aborted fetuses. | Females investigate dead fetuses. | |
| Females do not consume the placenta. | Females may consume the placenta. |
I, incisors; C, canines; PM, premolars; M, molars.
Susceptibility to infectious and parasitic agents is of greater concern. The USDA Animal and Plant Health Inspection Service (APHIS) has stated that camelids should be classified as ruminants because, “regardless of their taxonomic classification, camelids meet the definition of ruminants and are regulated as ruminants based on their susceptibility to ruminant diseases such as foot and mouth disease, tuberculosis (Mycobacterium bovis, M. tuberculosis, and M. avium), brucellosis, Johne's disease, etc.”11
It is true that there are diseases that camelids, cattle, sheep, and goats all acquire, but a careful appraisal of Table 46-2, Table 46-3, Table 46-4, Table 46-5, Table 46-6, Table 46-7, Table 46-8, Table 46-9 should dispel the myth that “llamas and alpacas are susceptible to all cattle and sheep diseases.”34 In fact, they are quite resistant to many regulated ruminant diseases.
Table 46-2.
Clinical Infectious Diseases of Camelids and Ruminants
| Camelids and Ruminants | Ruminants (Not Seen in Camelids) | Camelids (Not Seen in Ruminants) |
|---|---|---|
| Contagious ecthyma | Malignant catarrhal fever | Camelpox |
| Rabies (common to many mammals) | Bovine leukemia | Camel papillomatosis |
| Foot-and-mouth disease (FMD; occurs in many nonruminants) | Cowpox | Mycoplasma hemolama (Eperythrozoonosis) |
| Rinderpest (camels) | Pseudorabies | Lama adenoviruses, serotypes 1-6 |
| West Nile virus (WNV) encephalopathy (seen in many mammals and birds) | Bovine papillomatosis | |
| Fungal diseases (ringworm) (common to many mammals) | Ovine progressive pneumonia | |
| Tetanus and other clostridial diseases | Sheeppox or goatpox | |
| Bovine tuberculosis (seen in many nonruminant species) | Balanoposthitis | |
| Johne's disease | Sheep or goat papillomatosis | |
| Necrobacillosis | Scrapie | |
| Streptococcosis (common to many nonruminant species) | Bovine spongiform encephalopathy (BSE) | |
| Staphylococcosis (common to many nonruminant species) | Chronic wasting disease (CWD) of cervids | |
| Caprine/ovine brucellosis | Bovine brucellosis | |
| Anaplasmosis |
Table 46-3.
Infectious Disease Agents Producing Antibody Response, but Rare or No Clinical Disease in Camelids
| Agent | Disease |
|---|---|
| Bovine herpesvirus type 1 | Infectious bovine rhinotracheitis |
| Equine herpesvirus type 1 | Equine rhinopneumonitis |
| Retinal degeneration in SACs | |
| Bluetongue/epizootic hemorrhagic disease virus | Bluetongue, epizootic hemorrhagic disease of deer |
| Rift Valley fever virus (camels) | Rift Valley fever |
| Rotavirus | Enteritis (diarrhea) |
| Coronavirus | Enteritis (diarrhea) |
| Adenovirus | Enteritis (diarrhea) |
| Encephalomyocarditis virus | Encephalomyocarditis (EMC) |
| Brucella abortus | Bovine brucellosis |
| Borna disease virus | Viral encephalitis |
| Vesicular stomatitis virus | Vesicular stomatitis |
SACs, South American camelids.
Table 46-4.
Programmed Diseases of Ruminants in United States Compared with Camelids and Other Species
| CLINICAL DISEASES IN CAMELIDS | ||||
|---|---|---|---|---|
| Programmed Diseases of Cattle, Sheep, and Goats | From Natural Transmission | From Experimental Inoculation | Antibody Response in Camelids | Nonruminant Hosts Developing Natural Disease |
| Bovine brucellosis Brucella abortus | None | Yes | Yes | Humans, horses (fistula of withers), carnivores, marine mammals |
| Bovine tuberculosis Mycobacterium bovis | Yes (rare) | Yes | Yes | Humans, European badger, brush-tailed possum |
| Chronic wasting disease (CWD) of cervids | None | None | None | None |
| Scrapie | None | None | Not applicable | None |
Table 46-5.
Comparison of Ruminant Emergency Conditions, Compared with Camelid and Other Species
| CLINICAL DISEASES IN CAMELIDS |
|||||
|---|---|---|---|---|---|
| Emergency Conditions of Cattle, Sheep, and Goats | From Natural Transmission | From Experimental Inoculation | Antibody Response in Camelids | Nonruminant Hosts Developing Natural Disease | Nonruminant Hosts, Experimental Disease |
| Anthrax | Yes | Not reported | Not reported | Humans, numerous species of mammals | Many |
| Bovine spongiform encephalopathy (BSE) | No | No | Not applicable | Human, cat, cheetah, lion, tiger, puma | Brain extracts from infected cattle have produced disease in cattle, sheep, pigs, and mice. |
| Contagious bovine pleuropneumonia (mycoplasmosis) | No | Not reported | Not reported | None | Not reported |
| Foot-and-mouth disease (FMD) | Yes (rare) | Yes | Yes | Hedgehogs, pigs, peccaries, insectivores, xenarthra, rabbits, squirrel, hyrax, elephant, bears, marsupials | |
| Hemorrhagic septicemia | None reported | Not reported | None reported | Broad range of mammals | Wide variety |
| Pasteurella multocida + other agents | |||||
| Malignant catarrhal fever (African) | None reported | Not reported | One llama | Pigs (Norway) | Rabbit |
| Rift Valley fever | Yes, camel | Not reported | None reported | Human, dog, cat, rodents | Unknown |
| Rinderpest | Yes, camel | Not reported | Yes | Pig, peccary | Pig, peccary, dog, elephant, hyena, jackal, tiger, vulture, zebra |
| Vesicular stomatitis | Yes (rare) | Yes | Yes | Horse, pig | Unknown |
| Contagious agalactia (mycoplasmosis) | None reported | Not reported | Not reported | None | Unknown |
| Contagious caprine pleuropneumonia (mycoplasmosis) | None reported | Not reported | Not reported | None | Unknown |
| Heartwater | None reported | Not reported | Not reported | Numerous vertebrates may be intermediate hosts for Ehrlichia | Unknown |
| Ehrlichia (formerly Cowdria) ruminantium | |||||
| Nairobi sheep disease (tick borne, viral) | None reported | Not reported | Not reported | African field rat | Not successful at experimental transmission |
| Peste des petits ruminants | None reported | Not reported | Not reported | Not reported | Unknown |
| Pulmonary adenomatosis | None reported | Not reported | Not reported | Not reported | Unknown |
Table 46-6.
Comparison of Regulated Infectious and Parasitic Diseases of Ruminants Compared with Camelids and Other Species
|
CLINICAL DISEASES IN CAMELIDS |
||||
|---|---|---|---|---|
| Regulated Diseases of Cattle, Sheep, and Goats | From Natural Transmission | From Experimental Inoculation | Antibody Response in Camelids | Nonruminant Hosts Developing Natural Disease |
| Rabies | Yes | Not reported | Yes | Most species of mammals |
| Bovine brucellosis | None | Yes | Yes | Human, horse, carnivore, marine mammals |
| Brucella abortus | ||||
| Bovine tuberculosis | Yes (rare) | Yes | Yes | Human, European badger, brush-tailed possum |
| Mycobacterium bovis | ||||
| Bovine scabies (mange) | Yes | Not applicable | Not applicable | Many mammal species |
| Sarcoptes scabiei, Psoroptes ovis | ||||
| Trichomoniasis | None reported | Not reported | Not applicable | Unknown |
| Tritrichomonas fetus | ||||
| Caprine/ovine brucellosis | Yes | Not reported | Yes, may cross react with bovine brucellosis | Human |
| Brucella melitensis | ||||
| Scrapie | None | Not reported | Not applicable | None |
| Sheep/goat scabies | Yes | Not applicable | Not applicable | Unknowns |
| Psoroptes ovis | ||||
Table 46-7.
Monitored Diseases of Ruminants in United States Compared with Camelids and Other Species
|
CLINICAL DISEASES IN CAMELIDS |
|||||
|---|---|---|---|---|---|
| Monitored Diseases of Cattle, Sheep, and Goats | From Natural Transmission | From Experimental Inoculation | Antibody Response in Camelids | Nonruminant Hosts, Developing Natural Disease | Nonruminant Hosts, Experimental Disease |
| Avian tuberculosis | Yes | None reported | Yes | Many species of birds and mammals; swine; humans | Unknown |
| Anaplasmosis | No | Yes | Yes | None | Unsuccessful attempts |
| Bluetongue | Yes, but with questions | Not reported | Yes | None | Raccoon, opossum, hares |
| Bovine leukosis, viral | None reported | Not reported | Not reported | None | Unknown |
| Johne's disease | Yes | Not reported | Yes | Rabbits, nonhuman primates | Unknown |
| Malignant catarrhal fever (North America) | None reported | Not reported | Not reported | None | Unknown |
| Bovine cysticercosis | None reported | Not reported | Not applicable | Human Taenia saginata | None reported |
| Infectious bovine rhinotracheitis | None reported | Not reported | Yes | None reported | Unknown |
| Bovine genital campylobacteriosis (vibriosis) | None reported | Not reported | Not reported | None reported | None reported |
| Echinococcosis | Yes | Not reported | Not applicable | Humans, carnivores | Unknown |
| Leptospirosis | Yes | Not reported | Yes | Numerous mammals | Rodents and rabbits |
| Ovine progressive pneumonia (Maedi-Visna) | None reported | Not reported | Not reported | None reported | None reported |
| Q fever | None reported | Not reported | Not reported | Humans and many other species | None reported |
| Caprine arthritis/encephalitis | None reported | Not reported | Not reported | None reported | Unknown |
| Ovine chlamydiosis Chlamydia psittaci | None reported | Not reported | Not reported | Birds, humans, koala | Numerous species |
| Ovine epididymitis Brucella ovis | None reported | Not reported | Not reported | None | None |
Table 46-8.
Comparison of Regulated Parasitic Diseases of Cattle, Sheep, and Goats with Camelids
| Regulated Parasitic Diseases of Cattle and Sheep | Etiology | Status in Camelids | Intermediate Hosts | Location in Host |
|---|---|---|---|---|
| Screwworm myiasis | Cochliomyia hominivorax or Chrysomyia bezziana | All animals, including camelids, may become infested with screwworms. | None | Wounds, necrotic tissue |
| African trypanosomiasis (surra) | Trypanosoma evansi | Important disease of camels; may involve other species of Trypanosoma. | Blood-sucking flies (tabanids, Stomoxys), tsetse flies, and other | Blood |
| SACs also infected. | ||||
| Bovine babesiosis (piroplasmosis) | Babesia bovis | No verified reports in either camels or SACs | Ticks | Blood |
| Theileriosis (East Coast fever, corridor disease) | Theileria spp. | No verified reports in either camels or SACs | Ticks | Blood |
| Cattle scabies (multiple types) | Sarcoptes scabiei, Psoroptes ovis | Both may infest camelids. | None, direct contact | Skin |
| Sheep scabies | Psoroptes ovis | Yes | None, direct contact | Skin |
| Echinococcosis (hydatid disease) | Echinococcus granulosum | Many species, including camelids | Carnivore is primary host; herbivores are intermediate host. | Variable, but liver and lungs common |
SACs, South American camelids.
Table 46-9.
Comparison of Selected Parasitic Diseases of Ruminants with Camelids
| Parasitic Diseases of Ruminants | Etiology in Ruminants | Status in Camelids | Etiology in Camelids | Location in Host | Comments |
|---|---|---|---|---|---|
| Pediculosis (lice) | Biting lice Damalinia bovis (cattle) | None of the lice of ruminants infect camelids, or vice versa. | Biting louse of SACs: Damalinia breviceps; none in camels | Skin | Biting lice do not readily respond to ivermectin therapy. |
| Damalinia ovis (sheep) | |||||
| Sucking lice: Microthoracis spp. (M. cameli, M. mazzai, M. minor, M. praelongiceps) | |||||
| Sucking lice in ruminants (Haematopinus, Linognathus, and Solenopotes) | |||||
| Coccidiosis | Eimeria bovis, E. zuernii, and many other Eimeria spp. | Not a common parasite, and usually only in young animals | Eimeria lamae, E. alpacae, E. punoensis, E. macusaniensis, E bactriani, E. cameli, E. dromedarii, E. pellerdyi | Small intestine | It is common to find coccidia in feces, but animals should not be treated unless clinical syndrome is severe. |
| Trichuriasis (whipworms) | Trichuris ovis | Common | Trichuris tenuis | Large intestine | Serious parasite of camelids |
| Nematodiriasis | Nematodirus spp. | May be a significant parasitism | Nematodirus battus, N. lamae | Small intestine | |
| Spiculopteragiasis | Does not affect cattle, sheep, or goats in South America | Found only in South America | Spiculopteragia peruviana | Small intestine | |
| Unique to SACs | |||||
| Graphinemiasis | Does not affect cattle, sheep, or goats in South America | Found only in South America | Graphinema aucheniae | Small intestine | |
| Unique to SACs | |||||
| Lamanemiasis | Does not affect cattle, sheep, or goats in South America | Llama is secondary host. | Lamanema chavezii | Small intestine | Serious parasite of young alpacas |
| Primary host is a rodent (viscacha). | Affects the liver |
Data from Fowler ME: Medicine and surgery of South American camelids, ed 2, Ames, 1998, Iowa State University Press; Wernery U, Kaaden OR: Infectious diseases in camelids, ed 2, Boston, 2002, Blackwell Science; and Bowman DD: Georgis' parasitology for veterinarians, ed 8, Philadelphia, 2003, Saunders SACs, South American camelids.
Foot-and-mouth disease (FMD) virus is highly contagious in cattle and sheep. When llamas and alpacas were first imported from South America to the United States for the blossoming private llama industry, government officials expressed concern that llamas and alpacas might pose a risk for the introduction of FMD to the United States. The USDA expended considerable experimental effort to determine the risk. It was concluded that llamas and alpacas could be infected by inoculation but did not acquire FMD when cohabiting with infected swine, in contrast with almost 100% of cattle that acquired the infection.6, 25, 30 The virus could not be detected after 14 days postinoculation.
The same could be said for vesicular stomatitis. Only one animal has been diagnosed with the natural disease.2 Llamas may be infected experimentally.14
Bovine tuberculosis (TB) caused by Mycobacterium bovis is another concern of government officials. Llamas and alpacas have developed the disease under natural conditions, when cohabiting with infected elk, but have shown resistance to acquiring TB, in contrast to ruminants.31
Llamas and alpacas have been experimentally infected with Brucella abortus, but the natural disease does not occur in these species.12, 13
There are no reports of the transmission of any regulated ruminant disease from camelids to ruminants.
CONCLUSION
Camelids are not ruminants taxonomically, anatomically, physiologically, or behaviorally. Camelids also are not a threat to the livestock industry because they either have total resistance to infection or have minimal susceptibility to the infectious and parasitic diseases of ruminants.
Footnotes
References
- 1.Bohlken H. Remarks on the stomach and the systematic position of the tylopoda. Proc Zool Soc Lond. 1960;134:207–315. [Google Scholar]
- 2.Bridges VE, McCuskey BJ, Salman MD. Review of the 1995 vesicular stomatitis outbreak in the western United States. J Am Vet Med Assoc. 1997;211(5):556–560. [PubMed] [Google Scholar]
- 3.Colbert EH. Evolution of the vertebrates: a history of the backboned animals through time. ed 3. John Wiley & Sons; New York: 1980. Artiodactyls. [Google Scholar]
- 4.Clutton-Brock J. Camels and llamas. In: Clutton-Brock J, editor. A natural history of domesticated mammals. University of Texas Press; Austin: 1987. pp. 121–129. [Google Scholar]
- 5.Feldhamer GA, Dickamer LC, Vessey SH, Merritt JF. Mammalogy, adaptation, diversity and ecology. McGraw-Hill; Boston: 1999. [Google Scholar]
- 6.Fondevila NA, Marcoveccio FJ, Bianco-Viera J. Susceptibility of llamas Lama glama to infection with foot and mouth disease virus. J Vet Med Series B. 1995;42(10):595–599. doi: 10.1111/j.1439-0450.1995.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 7.Fowler ME. Evolutionary history and differences between camelids and ruminants. J Camel Pract Res. 1997;4(2):99–105. [Google Scholar]
- 8.Fowler ME. Medicine and surgery of South American camelids. ed 2. Iowa State University Press; Ames: 1998. pp. 1–7. [Google Scholar]
- 9.Franklin WL. Mammalian biology in South America, Pymatuning Laboratory of Ecology, vol 6, Special Publication Series. University of Pittsburgh; Pittsburgh: 1982. Biology, ecology, and relationship to man of the South American camelids; pp. 457–489. [Google Scholar]
- 10.Franklin WL. vol 5. McGraw-Hill; New York: 1990. South American tylopods; pp. 96–111. (Grzimek's encyclopedia of mammals). [Google Scholar]
- 11.Frost R: Personal communication, Lincoln, Calif, 2005.
- 12.Gidlewski T, Cheville NF, Rhyan JC. Experimental Brucella abortus induced abortion in a llama: pathologic effects. Vet Pathol. 2000;37(1):77–82. doi: 10.1354/vp.37-1-77. [DOI] [PubMed] [Google Scholar]
- 13.Gilsdorf MJ, Thoen CO, Temple RMS. Experimental exposure of llamas Lama glama to Brucella abortus, humoral antibody response. Vet Microbiol. 2001;81(1):85–91. doi: 10.1016/s0378-1135(01)00346-7. [DOI] [PubMed] [Google Scholar]
- 14.Gomez UD: [Tests on the sensitivity of South American camelids to vesicular stomatitis], Segunda Congr Vet Zootec, Lima, Peru, 1964, pp 403-406.
- 15.Grubb P. Artiodactyla. In: Wilson DE, Reeder DA, editors. Mammal species of the world. ed 2. Smithsonian Institution Press; Washington, DC: 1993. pp. 377–382. [Google Scholar]
- 16.Havesson YI, Schmidt GA. [The classification of Tylopoda in the system of placental mammals] Gegenbaurs Morph Jahrb. 1978;124(5):680–684. (Leipzig) [PubMed] [Google Scholar]
- 17.Honey JG, Harrison JA, Prothero DR, Stevens MS. Camelidae. In: Janis CM, editor. Evolution of tertiary mammals of North America. Cambridge University Press; Cambridge, UK: 1998. [Google Scholar]
- 18.Hume ID. Digestive physiology and nutrition of marsupials. Cambridge University Press; Cambridge: 1982. pp. 112–119. [Google Scholar]
- 19.Klingel H. vol 5. McGraw-Hill; New York: 1990. Camels; pp. 82–96. (Grzimek's encyclopedia of mammals). [Google Scholar]
- 20.Kurten B, Anderson E. Pleistocene mammals of North America. Columbia University Press; New York: 1980. Family Camelidae: camels and llamas; pp. 300–401. [Google Scholar]
- 21.Mason IL. Evolution of domesticated animals. Longman; New York: 1984. Camels; pp. 106–115. [Google Scholar]
- 22.Matthew WP. Reclassification of the artiodactyl families. Bull Geol Soc Am. 1929;40:403–409. [Google Scholar]
- 23.Novoa C, Wheeler JC. Evolution of domesticated animals. Longman; New York: 1984. Llama and alpaca; pp. 116–128. [Google Scholar]
- 24.Nowak RM. ed 6. vol 2. Johns Hopkins University Press; Baltimore: 1999. pp. 1051–1083. (Walker's mammals of the world). [Google Scholar]
- 25.Puntel M, Fondevila NA, Viera JB. Serological survey of viral antibodies in llamas Lama glama in Argentina. J Vet Med Series B. 1999;46(3):157–161. doi: 10.1046/j.1439-0450.1999.00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Romer AS. Vertebrate paleontology. ed 3. University of Chicago Press; Chicago: 1966. Artiodactyls; pp. 273–290. [Google Scholar]
- 27.Shmidt GA. Differences in the embryogenesis of Tylopods and ruminants. Soviet J Dev Biol. 1971;2(1):31–40. [PubMed] [Google Scholar]
- 28.Simpson CD. Artiodactyls. In: Anderson S, Jones JK Jr, editors. Orders and families of recent mammals of the world. John Wiley & Sons; New York: 1984. pp. 563–588. [Google Scholar]
- 29.Simpson GG. The principles of classification of mammals. Bull Am Museum Nat Hist. 1945;85:1–350. [Google Scholar]
- 30.Sutmoller P. Risk of disease transmission by llama embryos. Rev Sci Tech. 1999;18(3):719–728. doi: 10.20506/rst.18.3.1197. [DOI] [PubMed] [Google Scholar]
- 31.Thoen CO, Richards WD, Jamagin JL. Mycobacteria isolated from exotic animals. J Am Vet Med Assoc. 1977;170:987–990. [PubMed] [Google Scholar]
- 32.Webb SD. Osteology of camelops. Bull Los Angeles County Museum Sci. 1965;1:1–54. [Google Scholar]
- 33.Webb SD. Pleistocene mammals of Florida. University of Florida Press; Gainesville: 1974. pp. 170–259. [Google Scholar]
- 34.Wernery U, Kaaden OR. Infectious diseases in camelids. ed 2. Blackwell Science; Boston: 2002. [Google Scholar]
- 35.Wheeler JC. Evolution and present situation of the South American Camelidae. Biol J Linnean Soc. 1995;54:271–295. [Google Scholar]
- 36.Zeuner FE. A history of domestic animals. Harper & Row; New York: 1963. New world species; pp. 436–439. [Google Scholar]


