Introduction
Animals may prove hazardous to humans through traumatic attacks (e.g. by large felines, bears, crocodiles, sharks, etc.), through poisoning after their flesh has been ingested (see Chapters 133.1, 133.2), through envenoming and allergic hypersensitization (this chapter and Chapter 136) and through transmission of zoonotic infections—see “Bats” (Chapter 134.6 and elsewhere in this book).
Venomous Bites and Stings, and Envenoming
Envenoming (American/Australian: “envenomation”), as distinct from poisoning, occurs when venoms secreted by specialized glands are injected through the victim's skin or applied to absorbent mucous membranes. To inject their venoms, venomous snakes and lizards have grooved or cannulated fangs or solid teeth; spiders have venom jaws (chelicerae); vampire bats, insectivorous mammals and leeches have solid teeth; male monotreme mammals (platypus and echidna) have venomous spurs; centipedes have modified legs (maxillipeds, forcipules or prehensors); insects, scorpions, ticks, fish and echinoderms (sea urchins etc.) have rigid, sharpened, barbed stings, spines, or hypostomes; echinoderms have venomous grapples (pedicellariae); cnidarians (jellyfish, coelenterates) have stinging hairs (cnidocytes, nematocysts); octopuses (cephalopods mollusks) have venomous beaks; and cone shells (gastropod mollusks) impale their prey with a venom-filled radular tooth mounted on a harpoon-like proboscis. Some elapid snakes, scorpions, “blister” beetles, millipedes and other arthropods can “spit” or squirt their venoms—a largely defensive ploy.
Venoms are noxious substances secreted by specialized glands. They vary in their complexity from simple organic acids and phenols in some arthropod venoms, to complex mixtures of hundreds of different proteins (polypeptide toxins, enzymes, etc.) and smaller, pharmacologically-active molecules in snake venoms. Evolution has selected venoms and venom-administering organs to immobilize and digest animals’ prey, to prevent blood clotting in the case of leeches, vampire bats and other “blood sucking” species, and for defense. A few venom toxins are modified salivary gland secretions, but most venom genes originated from other organs through repeated episodes of gene duplication and gene recruitment.
Here, the venomous animals of greatest medical importance in human and veterinary medicine are selected for discussion: snakes, lizards, fish, arthropods, leeches and the aquatic cnidarians, mollusks and echinoderms.
134.1. Venomous Marine Animals
Key features.
-
•
The many species of venomous fish that inhabit tropical and temperate oceans and rivers have stinging apparatus in their fins, gill covers or, in sting rays, at the base of the tail. Common causes of fish stings are weevers (Europe), sting rays (Americas and Amazon tributaries) and scorpion fish that are popular aquarium pets. Stone fish (Synanceja) are the most dangerous
-
•
Cnidarians (coelenterates)—including jellyfish, cubomedusoids, sea wasps, Portuguese-men-o’-war or bluebottles, stinging corals and sea anemones—envenom by firing numerous stinging hairs into the dermis, causing pain, weals and sometimes cardiovascular collapse
-
•
Echinoderms (starfish and sea urchins) have sharp venomous spines that can impale waders’ feet
-
•
Mollusks capable of causing fatal envenoming are cone shells and blue-ringed octopuses
-
•
The pain of marine stings is relieved by hot (45°C) water. Antivenoms are manufactured for envenoming by scorpion fish and box jellyfish
Venomous fish 1, 2, 3
Although more than 1200 species of fish are thought to be venomous, only about 200 species can inflict dangerous stings. Rarely, fatal stings are inflicted by cartilagenous fish (class Chondrichthyes), such as sharks and dogfish (order Squaliformes); stingrays and mantas (order Rajiformes); and bony fish (superclass Osteichthyes), such as ray-finned fish (class Actinopterygii) of the orders Siluriformes (catfish), Perciformes [families Trachinidae (weever fish), Uranoscopidae (stargazers or stone-lifters) and others] and Scorpaeniformes (scorpion fish, stonefish, lion fish—Synanceja/Synanceia spp.) (FIGURE 134.1.1, FIGURE 134.1.2 ). Tropical oceans have the richest venomous fish fauna, but dangerous species also occur in temperate northern waters. Large rivers in South America, West Africa and Southeast Asia are inhabited by freshwater stingrays (Potamotrygon spp.) (Fig. 134.1.3 ). Venom glands are embedded in grooves in the spines or, in the case of stingrays, lie beneath a membrane covering the long barbed precaudal spine.
FIGURE 134.1.1.

Lion fish (Pterois volitans- Scorpaenidae)
(© David A. Warrell).
FIGURE 134.1.2.

Weedy or pop-eyed scorpionfish Rhinopias frondosa—Scorpaenidae)
(© David A. Warrell).
FIGURE 134.1.3.

Freshwater stingray (Potamotrygon spp.—Potamotrygonidae)
(© David A. Warrell).
Incidence and Epidemiology
Some years, hundreds of weever fish stings occur around the British coast and along the Adriatic coast. An estimated 1500 stings by rays and 300 stings by scorpion fish occur in the USA each year. Stings by freshwater rays are common in the Amazon region. Ornate, but aggressive, Pterois and Dendrochirus spp. (lion, zebra, tiger, turkey or red fire fish) are popular aquarium pets. People wading near the shore or coral reefs may tread upon well-camouflaged stonefish (Synanceja spp.) lying partly covered by sea plants and anemones [1]. Stingrays lash their tails, usually impaling the ankle [2].
Prevention
Employing a shuffling gait when wading, avoiding the handling of living or dead fish, and keeping away from fish near tropical reefs all aid in prevention. Footwear protects against most species, except stingrays.
Venom Composition
Stingray and weeverfish venoms contain peptides, enzymes, vasoactive kinins, 5-hydroxytryptamine, histamine and catecholamines. Venoms cause local necrosis and target cardiac, skeletal and smooth muscle resulting in electrocardiogram changes, hypotension and paralysis.
Clinical Features [3]
Immediate sharp, agonizing pain is typical. Hot, painful, erythematous swelling extends up the stung limb and may persist for days and be complicated by necrosis and secondary infection by marine Vibrio spp. (such as Vibrio vulnificus) and freshwater Aeromonas hydrophila, particularly if the spine is left in the wound. Stingray spines (up to 30 cm long) can cause severe lacerating and penetrating wounds, sometimes with fatal results.
Stings by rays or Scorpaenidae (scorpion and stonefish) may cause nausea, vomiting, diarrhea, sweating and hypersalivation, cardiac arrhythmias, hypotension, respiratory distress, neurologic signs and generalized convulsions. However, fatalities are very rare.
Treatment
Treatment is common to all wounds caused by venomous fish and includes: (i) pain relief; (ii) neutralizing effects of venom; (iii) prevention of secondary infection; and (iv) supportive care of systemic symptoms. Immersion of the stung part in uncomfortably hot, but not scalding, water (less than 45°C) relieves pain and neutralizes venom. Alternatively, inject local anesthetic as a ring block or local nerve block. The barbed venomous spine, its covering membrane and other foreign material should be removed as soon as possible. Systemic effects are treated symptomatically. Cardiopulmonary resuscitation may be required on the beach. In Australia, CSL (Melbourne, Australia) manufacture an antivenom specific for Synanceja trachynis, Synanceja verrucosa, and Synanceja horridus which has paraspecific activity against venoms of North American scorpion fish (Scorpaena guttata) and some other Scorpaenidae. Doxycycline or co-trimoxazole covers Vibrio and Aeromonas spp.
Cnidarians (Coelenterates)
Cnidarian (Jellyfish, Cubomedusoids, Sea Wasps, Portuguese-Men-O’-War or Bluebottles, Hydroids, Stinging Corals, Sea Anemones, etc.) tentacles are armed with millions of stinging capsules (nematocysts) which, triggered by contact or chemicals, evert stinging hairs that penetrate the skin, producing lines of painful, irritant weals. Venoms contain peptides and other vasoactive compounds provoking pain, inflammation and urticaria.
Epidemiology
The northern Australian box jellyfish or sea wasp (Chironex fleckeri) is the most dangerous species, having killed more than 70 people since 1883. The peak season for stings is December through January. Fatal stings in the Indo-Pacific region are attributable to Chiropsalmus quadrumanus and Chiropsalmus quadrigatus and, elsewhere, Portuguese men-o’-war (Physalia spp.) and Chinese Stomolophus nomurai have caused a number of fatalities. Irukandji syndrome occurs in northern Queensland and the Florida-Caribbean area, and is caused by stings of tiny cubomedusoids like Carukia barnesi. Epidemics of “mauve stinger” Pelagia noctiluca stings occur in the northern Adriatic coast where stinging sea anemones (Anemonia sulcata) also occur.
Prevention
Avoid the sea when warning notices are displayed or bathe only in “stinger-resistant” enclosures. “Lycra” or wetsuits and nylon stockings protect against nematocyst stings.
Clinical Features
Stings may produce diagnostic patterns: C. fleckeri—striated brownish-purple weals (Fig. 134.1.4 ); Carukia barnesi—a transient erythematous macule; Portuguese man-o’-war (Physalia spp.)—chains of oval weals surrounded by erythema. Immediate severe pain is the commonest symptom. Chirodropids (Chironex and Chiropsalmus spp.) can cause respiratory arrest, generalized convulsions, pulmonary edema, and cardiac arrest within minutes of the sting. Symptoms include cough, nausea, vomiting, abdominal colic, diarrhea, rigors, severe musculoskeletal pains and profuse sweating. Irukandji syndrome comprises severe musculoskeletal pain, anxiety, trembling, headache, piloerection, sweating, tachycardia, hypertension, and pulmonary edema starting about 30 min after a sting by C. barnesi and other cubomedusoids) and persisting for hours. Envenoming by Physalia species may result in intravascular hemolysis, peripheral gangrene and renal failure.
FIGURE 134.1.4.
Lines of weals inflicted by a box jellyfish or “sea wasp” (Chironex fleckeri—Chirodropidae) sting in Darwin, Australia
(© Bart Currie).
Treatment
To prevent drowning, remove victims from the water as soon as possible. A slurry of baking soda and water [50% (w/v)] is recommended by American authorities for stings by the widely distributed Atlantic genus, Chrysaora. For stings by C. fleckeri and other cubozoans, including Irukandji, but not for Chrysaora, Physalia or Stomalophus spp., apply vinegar or 3–10% aqueous acetic acid solution, which inhibits nematocyst discharge. Pressure immobilization and verapamil are not recommended. Shave off adherent tentacles with a razor. Hot water treatment (see venomous fish above) relieves the pain of Physalia stings [4]. Specific “sea wasp” antivenom for C. fleckeri is manufactured in Australia, but its efficacy is questionable.
Echinodermata (Starfish and Sea Urchins) (Fig. 134.1.5)
FIGURE 134.1.5.

Long-spined sea urchin (Diadema setosum—Diadematidae) Papua New Guinea
(© David A. Warrell).
Echinoderm spines cause penetrating injuries and envenoming. There is severe pain and local swelling, but rarely systemic effects.
Treatment
Hot water (see above) may relieve the pain. Spines should be squeezed out or removed surgically, but this may prove impossible. There is a risk of marine bacterial infections (see above).
Mollusca (Cone Shells and Octopuses)
Cone shells (genus Conus) (Fig. 134.1.6 ) are carnivorous marine snails that harpoon their prey, implanting a radular tooth charged with venom containing a mixture of many small (10–30 amino acid) peptide toxins. Careless handling of these attractive shells may result in a potentially fatal sting. Symptoms of envenoming are nausea, vomiting, paraesthesia and numbness of the lips and site of sting, numbness, dizziness, ptosis, diplopia, dysarthria, dyspnea and loss of consciousness.
FIGURE 134.1.6.

Geography cone (Conus geographus—Conidae)
(© David A. Warrell).
Small, blue-ringed octopuses of the Australian and West Pacific region (Hapalochlaena spp.) (Fig. 134.1.7 ) can inject tetrodotoxin when they bite with their powerful beaks. These bites are painful and cause local bleeding, swelling and inflammation. Severe neurotoxic symptoms, and even fatal generalized paralysis, may develop within 15 minutes of the bite.
FIGURE 134.1.7.

Greater blue-ringed octopus (Hapalochlaena lunulata—Octopodidae) Papua New Guinea
(© David A. Warrell).
Treatment
No antivenoms are available. Cardiopulmonary resuscitation and mechanical ventilation may be required.
References
- 1.Bergbauer M, Myers RF, Kirschner M. A&C Black; London: 2009. Dangerous marine animals. [Google Scholar]
- 2.Sutherland SK, Tibballs J. 2nd edn. Oxford University Press; Melbourne: 2001. Australian animal toxins. The creatures, their toxins and care of the poisoned patient. [Google Scholar]
- 3.Williamson JA, Fenner PJ, Burnett JW, Rifkin JF, editors. Venomous and poisonous marine animals: a medical and biological handbook. University of New South Wales Press; Sydney: 1996. [Google Scholar]
- 4.Loten C, Stokes B, Worsley D. A randomised controlled trial of hot water (45 degrees C) immersion versus ice packs for pain relief in bluebottle stings. Med J Aust. 2006;184:329–333. doi: 10.5694/j.1326-5377.2006.tb00265.x. [DOI] [PubMed] [Google Scholar]
134.2. Leeches (Phylum Annelida, Class Hirudinea)
Key features.
-
•
Land and aquatic leeches are blood-sucking annelids, most common in tropical forests, rivers and lakes
-
•
Bleeding continues after the leech has fed and detached because of salivary anticoagulants
-
•
Prevention is by protective clothing and liberal use of diethyl toluamide (DEET) repellent
-
•
Attached leeches should be gently removed. Chemicals may encourage regurgitation into, and infection of, the wound (e.g. with their symbiotic Aeromonas hydrophila)
These blood-sucking, hermaphroditic, egg-laying annelids attach their elongated annulated bodies to leaves, rocks or the host by a posterior sucker. By standing on the posterior sucker and waving the anterior sucker, they can sense their prey with amazing efficiency. They drop on to the prey or pursue it with a looping or lashing motion. The anterior sucker contains the mouth, armed with three radially arranged jaws which make a Y-shaped incision and secrete saliva containing a histamine-like vasodilator and anticoagulants, such as hirudin [from the medicinal leech (Hirudo medicinalis), which inhibits thrombin and factor IXa]; hementin (from Haementeria ghilianii, which is directly fibrinolytic); and hementerin [from Haementeria depressa (Haementeria lutzi), a plasminogen activator]. Other enzymes include esterases, antitrypsin, antiplasmin and anti-elastase [1].
Land leeches, 1–8 cm long, infest rainforest vegetation and usually attach themselves to the lower legs or ankles after a bite that may be painless. They ingest about a milliliter of blood in one hour and then drop off, but the wound continues to bleed—sometimes for a week.
Aquatic leeches are swallowed when stagnant water is drunk and they invade the mouth, nostrils, eyes, vulva, vagina, urethra or anus of swimmers.
Blood sucking leeches have a global distribution but have the greatest impact in damp forests of the subtropical and tropical regions of the Indo-Pacific region. Pond, lake and stream water is often used for bathing, washing clothes, washing utensils and animals, and human consumption in villages. Leeches are commonly present in ponds, particularly in the rainy season.
Prevention
Apply repellents such as dibutyl phthalate and diethyl toluamide to clothing, skin and the inside and outside of footwear. Children should be discouraged from bathing in leech-infested waters and all drinking water should be boiled or filtered.
Clinical Features [2]
The main effect is blood loss, but other symptoms include local soreness, secondary infection, residual itching and phobia. Ingested aquatic leeches may penetrate the bronchi or esophagus but usually attach to the pharynx or nasal passages, causing a feeling of movement at the back of the throat with cough, hoarseness, stridor, breathlessness, epistaxis, hemoptysis, hematemesis and fatal upper airway obstruction. Leeches are no longer thought to be a cause of “halzoun” (Lebanon) or “marrara” (Sudan) (Chapter 135). Leeches penetrating the anus may reach the rectosigmoid junction causing perforation and peritonitis. Bleeding may persist for up to a week after the leech has dropped off. Transmission of pathogens has been suggested, but not proved—except in the case of wound infection by A. hydrophila, which lives symbiotically in the leeches gut.
Treatment [3]
Leeches will detach if salt, alcohol, turpentine or vinegar is applied, but these chemicals may make the leech regurgitate into the wound. Gentle mechanical removal is preferred, but avoid pulling off the leech so roughly that its mouth parts are left in the wound to cause chronic infection. A styptic, such as silver nitrate, or a firm dressing stops the bleeding. Invasive aquatic leeches must be removed by endoscope, aided by 30% cocaine, 10% tartaric acid or dilute (1 : 10 000) adrenaline (epinephrine) in the nasopharynx, larynx, trachea or esophagus, and concentrated salt solution in the genitourinary tract and rectum.
References
- 1.Sawyer RT. Oxford University Press; Oxford: 1986. Leech Biology and Behaviour. [Google Scholar]
- 2.Montazeri F, Bedayat A, Jamali L. Leech endoparasitism: report of a case and review of the literature. Eur J Pediatr. 2009;168:39–42. doi: 10.1007/s00431-008-0706-1. [DOI] [PubMed] [Google Scholar]
- 3.Keegan HL. Leeches as pests of man in the Pacific region. In: Keegan HL, McFarlane WR, editors. Venomous and Poisonous Animals and Noxious Plants of the Pacific Region. Pergamon Press; Oxford: 1963. pp. 99–104. [Google Scholar]
134.3. Fish Capable of Inflicting Serious Trauma
Key features.
-
•
Shark attacks can be devastating but only 70–100 occur each year with 5–15 fatalities. Florida, Australia and South Africa have the highest risk
-
•
Attacks are best prevented by taking local advice to avoid high risk locations, circumstances and behavior
-
•
Medical problems include extensive trauma, hemorrhagic shock and a high risk of bacterial contamination by unusual marine pathogens
-
•
First aid is securing the victim from drowning, resuscitation, control of bleeding and perforating injuries, intravenous fluid replacement and rapid evacuation to hospital for emergency surgery and treatment of infection
-
•
Other fish capable of causing severe and fatal trauma include barracuda, Moray eels, needle fish, sting rays, piranhas and candiru
Sharks
Sharks are most common in oceans between latitudes 47° south and 46° north, especially where water temperature is above 20°C. There are about 70–100 shark attacks with 5–15 fatalities each year (case fatality ~8%), mostly in North American (especially Florida), Australian and South African waters [1]. Great white (Carcharodon carcharias) (length ~16 m, weight 2250 kg), tiger (Galeocerdo cuvier) (5.5 m, 900 kg) (Fig. 134.3.1A–E ) and bull (Carcharhinus leucas) (3.5 m, 360 kg) sharks are the most dangerous, but attacks have been reported by 32 species; all 70 species exceeding 2 m in length are potentially lethal. Devastating deep wounds, especially of buttocks, thighs or shoulders result in massive bleeding from severed arteries causing shock and the risk of drowning. Shark wounds are characterized by sharp incisions without abrasions, serrated edges, a triangular or rectangular flap of skin, regular spacing corresponding to the shark's teeth, gouge marks on the bones and severing of body parts at the joints without fractures (Fig. 134.3.2A–C ) [2]. Bumping or rubbing against shark skin can inflict severe abrasions caused by their placoid scales.
FIGURE 134.3.1.

(A) Tiger shark (Galeocerdo cuvier). Specimen weighing 268 kg caught off Watamu, Kenya (copyright D. A. Warrell).(B–E) Specimen captured off Madang, Papua New Guinea in 2001 and responsible for the attack illustrated
(© Steve Allen).
FIGURE 134.3.2.
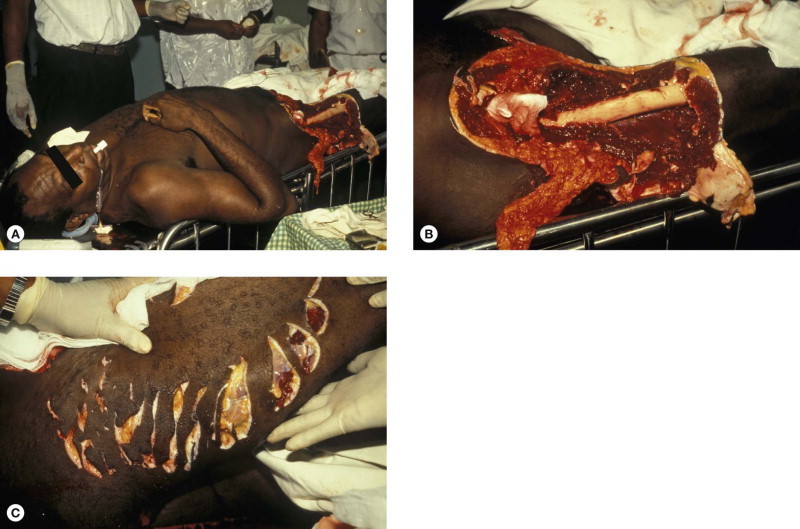
(A–C) Victim of a tiger shark attack off Madang, Papua New Guinea in 2001
(© Steve Allen).
Management
Vascular injury is a major determinant of mortality. Immediate medical care involves resuscitation (control of hemorrhage, fluid replacement and treatment of hypothermia), washout, debridement and follow-up for prevention of infection and closure of more complex wounds 3, 4, 5. A study of shark wounds in Recife, Brazil discovered more than 80 bacterial pathogens, mainly Enterobacteriaceae, all of which were covered by gentamicin, vancomycin and levofloxacin [6]. Isolates include Vibrio spp. such as Vibrio carchariae, Vibrio parahaemolyticus and Photobacterium (Vibrio) damsela and Aeromonas spp.
Prevention
Take local advice. Most attacks occur between 06.00 h and 20.00 h, but sharks come closer to the shore and are most active in twilight or darkness. Avoid bathing between sand bars and the ocean, far out to sea, where dead fish or sewage are being discharged and flocks of birds are feeding. Do not bathe if you are injured, bleeding, wearing jewelry or brightly patterned or colored clothes, or with a pet dog. Surfers and surface swimmers are targeted more often than divers. Reduce risk by bathing in groups, close to the shore and only in daylight. If attacked by a shark, fight back—hit it on the nose and claw at its eyes and gills. Chemical and electrical-field repellents and chainmail protective suits have been developed.
Other Dangerous Fish [4]
Most fish can inflict a painful and damaging bite if handled carelessly on a line or in a net, but the following deserve special mention.
Barracudas
The great barracuda (Sphyraena barracuda) of the tropical Atlantic can grow to almost two meters in length. Its powerful jaws and numerous long, razor-sharp, fang-like teeth can sever a digit or even a hand. Hands and ankles may be bitten when a landed fish is thrashing about still on the line or while the hook is being disengaged. Barracudas are attracted by shining objects, such as rings and bracelets on hands dangled in the water and may leap out of the water in pursuit of a fish that is being pulled in on an angler's line.
Moray Eels (Muraenidae)
The giant Moray (Gymnothorax javanicus) can reach a length of 3 meters and a weight of 36 kilograms; the Californian Moray (Gymnothorax mordax) reaches 1.5 meters in length (Fig. 133.2.1). Moray eels may be encountered by divers exploring coral reefs and wrecks. Attacks are unusual but the eels’ multiple rows of long, fang-like, backward-pointing teeth may cause deep puncture wounds with avulsion of tissue if the animal is forcefully removed (Fig. 134.3.3 ). Despite much speculation, they have no venom apparatus but a major threat is infection of the bite wounds with a variety of marine Vibrio, Aeromonas and Pseudomonas spp. [7].
FIGURE 134.3.3.
Moray eel injury
(© R. Sautter).
Needle Fish (Garfish) (Tylosurus spp. Belonidae) (Fig. 134.3.4A,B)
FIGURE 134.3.4.
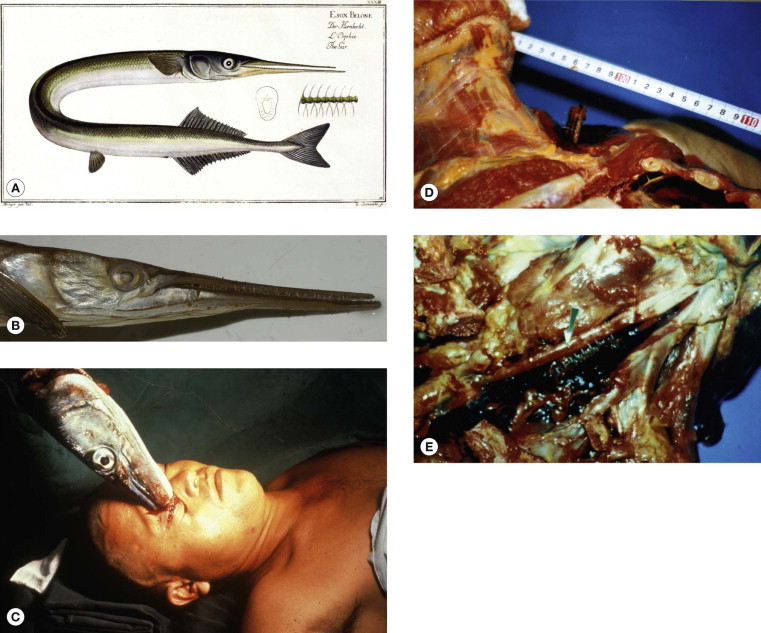
Needle fish (gar fish). (A) Illustration from Marcus Elieser Bloch's “Ichthyologie ou histoire naturelle generale et particuliere poisons” Berlin, 1785–1797 (© David A. Warrell). (B)Tylosurus graviloides (© David A. Warrell). (C) Japanese victims of attacks by crocodile needlefish (Tylosurus crocodilus): victim impaled in the orbit (courtesy of Dr Mashiro Kohama, Okinawa). (D–E) Victim fatally impaled in the supraclavicular region, rupturing the left subclavian artery and showing the needle like lower jaw with teeth in situ.
These Indo-Pacific fish can leap out the sea at speeds of 60 km/h, attracted by light. They have impaled fishermen and, rarely, surfers and divers, sometimes causing fatal injuries (Fig. 134.3.4C–E) 8, 9, 10.
Sting Rays (Dasyatidae)
Sting rays can inflict fatal penetrating trauma with their spines (see below).
Piranhas (Pirañas) (Characidae) (Fig. 134.3.5A)
FIGURE 134.3.5.
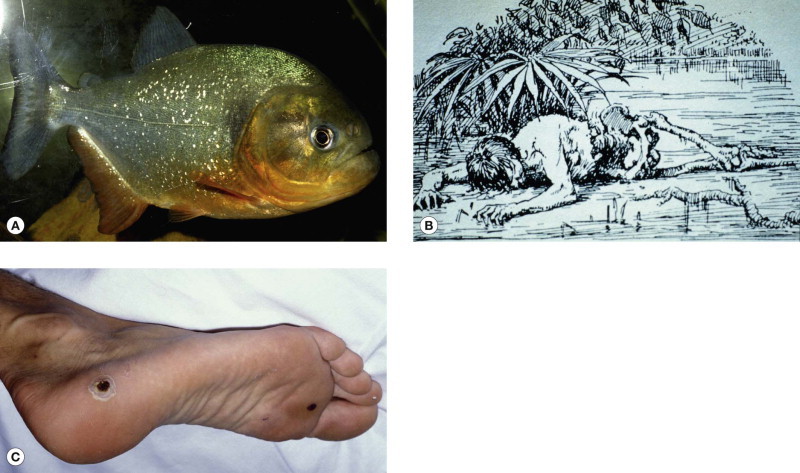
Piranha attack. (A) Red-bellied piranha (Pygocentrus-Serrasalmus-nattereri) (© David A. Warrell). (B) Mythical representation of a mass attack in the Amazon (© David A. Warrell). (C) Piranha bite Brazil
(© David A. Warrell).
These ferocious fish of the Amazon, Orinoco and other South American river systems are alleged to have stripped unwary swimmers to the bone (Fig. 134.3.5B). Such mass attacks on people or animals have rarely been reported, but piranhas sharp teeth can bite out chunks of flesh (Fig. 134.3.5C).
Candiru (Vampire, Tooth Pick or Penis Fish) (Vandellia cirrhosa Trichomycteridae)
In the Amazon region, these tiny parasitic catfish (Portuguese “candirú”, Spanish “canero”) (Fig. 134.3.6 ) are feared more than piranhas. Normally, they attach to the gills of large fish and feed off their blood, but these fish are apparently also attracted to bathers by detecting urine or blood. Once they have burrowed into the urethra, vagina or anus (especially in menstruating women), they erect spines on their gill covers which prevents removal. One hospital in Puerto Maldonado, Peru, admits more than 10 cases each year. Although several herbal concoctions are said to promote their elimination, surgery or cystoscopy is usually necessary 11, 12.
FIGURE 134.3.6.

Candiru (Vandellia cirrhosa) Brazil
(© David A. Warrell).
Management of Injuries by other Dangerous Fish
The same principles apply as with shark bites, although trauma is usually on a much smaller scale. Infection with aquatic microorganisms is a serous problem, especially in immunocompromised people; in salt water: Fusarium solani, Vibrio vulnificus, V. parahaemolyticus, Vibrio alginolyticus, Erysipelothrix rhusiopathiae (causing erysipeloid, “seal finger” and “whale finger”), Plesiomonas shigelloides, Acinetobacter spp., Chromobacterium violaceum, Flavobacterium spp., Pseudomonas aeruginosa, Mycobacterium marinum, Prototheca spp. and Staphylococcus aureus (off populous beeches such as Waikiki, Honolulu); and in freshwater, Aeromonas hydrophila and free-living amoebae.
In cases of severe, extensive injuries, especially if there has been delay in presentation, blind antibiotic treatment is appropriate with oral doxycycline or co-trimoxazole or parenteral tetracycline and an aminoglycoside combined with either cefotaxime or a fluoroquinolone. Results of cultures will direct specific therapy.
References
- 1.International Shark Attack File http://www.flmnh.ufl.edu/fish/sharks/statistics/2005attacksummary.htm Available at. (last accessed 28 December 2011)
- 2.Ihama Y, Ninomiya K, Noguchi M. Characteristic features of injuries due to shark attacks: a review of 12 cases. Leg Med (Tokyo) 2009;11:219–225. doi: 10.1016/j.legalmed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Caldicott DGE, Mahajani R, Kuhn M. The anatomy of shark attack: a case report and review of the literature. Injury Int J Care Injured. 2001;32:445–453. doi: 10.1016/s0020-1383(01)00041-9. [DOI] [PubMed] [Google Scholar]
- 4.Auerbach PS, Burgess GH. Injuries from nonvenomous aquatic animals. In: Auerbach PS, editor. Wilderness Medicine. 5th edn. Mosby Elsevier, Philadelphia; Philadelphia: 2007. pp. 1654–1691. [Google Scholar]
- 5.Lentz AK, Burgess GH, Perrin K. Mortality and management of 96 shark attacks and development of a shark bite severity scoring system. Am Surg. 2010;76:101–106. [PubMed] [Google Scholar]
- 6.Interaminense JA, Nascimento DC, Ventura RF. Recovery and screening for antibiotic susceptibility of potential bacterial pathogens from the oral cavity of shark species involved in attacks on humans in Recife, Brazil. J Med Microbiol. 2010;59:941–947. doi: 10.1099/jmm.0.020453-0. [DOI] [PubMed] [Google Scholar]
- 7.Erickson T, Vanden Hoek TL, Kuritza A, Leiken JB. The emergency management of Moray eel bites. Annals Emergency Med. 1992;21:212–216. doi: 10.1016/s0196-0644(05)80169-6. [DOI] [PubMed] [Google Scholar]
- 8.Barss PG. Penetrating wounds caused by needle-fish in Oceania. Med J Aust. 1985;143:617–618. doi: 10.5694/j.1326-5377.1985.tb119973.x. 621–2. [DOI] [PubMed] [Google Scholar]
- 9.McCabe MJ, Hammon MW, Halstead BW, Newton TH. A fatal brain injury caused by a needlefish. Neuroradiology. 1978;15:137–139. doi: 10.1007/BF00329055. [DOI] [PubMed] [Google Scholar]
- 10.Kerkhoffs GMMJ, op den Akker JW, Hammacher ER. Surfer wipe out by predator fish. Br J Sports Med. 2003;37:537–539. doi: 10.1136/bjsm.37.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudger EW. Bookshelf browsing on the alleged penetration of the human urethra by an Amazonian catfish called candiru. Am J Surgery. 1930;8:170–188. 443–57. [Google Scholar]
- 12.Spotte S. Creative Arts Book Company; Berkeley: 2002. Candiru: Life and Legend of the Bloodsucking Catfish. [Google Scholar]
134.4. Lizards
Key features.
-
•
Bites by venomous helodermid lizards (Gila monsters and Mexican beaded lizards of Middle America) are rare: envenoming causes severe pain, swelling, hypotension, nausea, vomiting, sweating and sometimes angioedema and evidence of myocardial effects
-
•
First aid involves urgent disengagement of the animal's jaws. As no antivenom is available, treatment is supportive and symptomatic
-
•
Varanid, iguanid and agamid lizards may be capable of envenoming—notably the Komodo dragon
Introduction: Venomous Lizards
Venomous salivary secretion have been demonstrated in Iguanas (Iguanidae), glass/alligator lizards (Anguidae) and monitors (Varanidae)—notably the Komodo dragon (Varanus komodoensis) which has been responsible for human fatalities that were attributed to trauma or infection of the bite wounds [1]. However, the only two lizards of proven medical importance are members of the family Helodermatidae that inhabit dry forests and deserts in western Middle America 2, 3, 4. The Mexican beaded lizard or escorpión (Heloderma horridum) (Fig. 134.4.1A, B ) is the larger (up to 1 m in total length and 2 kg in weight) with a relatively longer tail and a more arboreal habit. It is found in western Mexico, south to Guatemala. The Gila monster (Heloderma suspectum) (Fig. 134.4.1B–D), which reaches a total length of 55 cm and a weight of 1 kg, occurs in the south-western USA (south-east Nevada, south-west Utah, south-east California, south-west New Mexico, Arizona) and adjacent areas of Mexico (Sonora and northern Sonaloa). The two species overlap in Mexico (southern Sonora and northern Sinaloa).
FIGURE 134.4.1.
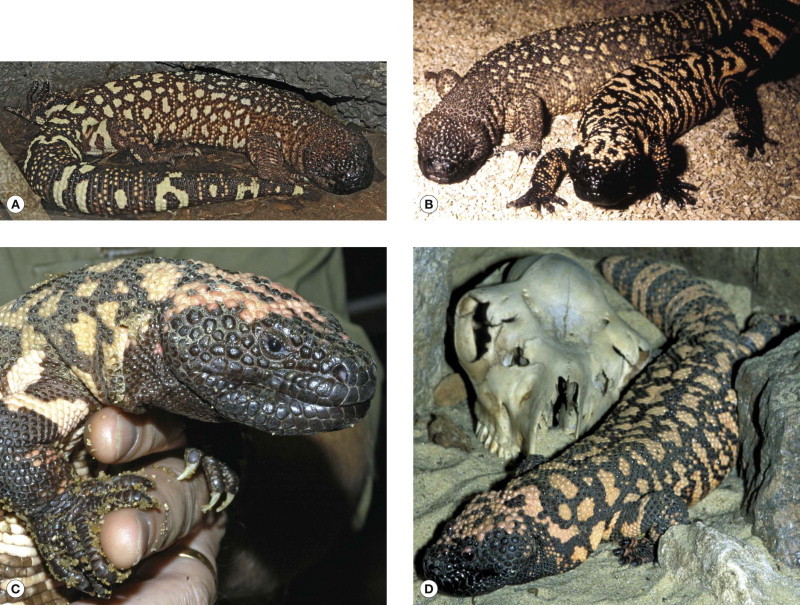
(A) Mexican beaded lizard (Heloderma horridum exasperatum) (© David A. Warrell). (B) Mexican beaded lizard (Heloderma horridum horridum) (left) compared with Gila monster (Heloderma suspectum) (right) (courtesy of D. Ball and Zoological Society of London). (C) Gila monster (H. suspectum) (© David A. Warrell). (D) Gila monster (H. suspectum)
(© David A. Warrell).
Venom Apparatus
Venom from bulging multi-lobed anterior submandibular glands pools in labial gutters in the lower jaw. When threatened, the lizards open and shut their mouths to promote drooling of venom-enriched saliva. When they bite and chew, venom is inoculated by the mandibular (dentary) teeth of which the fourth to the seventh are most prominently grooved, and the maxillary teeth, which are less grooved.
Venom Composition 4, 5
Heloderma venoms contain toxic proteases, hyaluronidase, phospholipase A2, horridum toxin (a glycoprotein tissue kallikrein-like enzyme that releases bradykinin and is probably responsible for hypotension in human victims of envenoming), bioactive peptides of great scientific interest, including helospectin (a vasoactive intestinal peptide analogue) and exendins -3 and -4, which are glucagon-like peptide-1 (GLP-1) homologues that stimulate insulin secretion and inhibit glucagon secretion. A synthetic homologue of exendin-4, exenatide, is a high affinity GLP-1 receptor agonist which has been developed for treatment of type 2 diabetes mellitus.
Heloderma Bites
These are virtually never accidental because helodermids are reclusive and non-aggressive animals that inhabit thinly-populated rural areas. Almost exclusively, bites are inflicted on the fingers, hands and forearms of young men who are handling or trying to catch the lizards. Alcohol consumption seems to have contributed on many occasions, as is the case with bites by pet exotic snakes. Bites may be “slashing” in type, in which the anterior maxillary teeth strike and can envenom but do not engage, and “gripping”, when the animal clings on and chews for up to 15 minutes before it can be removed [6]. Not all bites result in envenoming.
Symptoms of Envenoming 6, 7, 8, 9
Envenoming by H. suspectum and H. horridum causes identical clinical syndromes. Pain may start immediately and is described as throbbing or burning. It may radiate up the bitten limb to the shoulder, chest and epigastrium, and is often excruciating in intensity, persisting for 24 hours or more. Swelling also develops rapidly, extending, in some cases, to involve the whole limb, but although it may be tense, compartment syndrome has never been described. The bite site is erythematous or cyanosed with traumatic ecchymoses and persistent bleeding, but tissue necrosis does not develop. Red lymphangitic lines extend up the limb and regional lymph glands may become tender and enlarged. Local paresthesia, hyperesthesia and even paralysis have been described. The earliest systemic symptoms start within five minutes of the bite: dizziness, weakness, nausea, vomiting, profuse generalized sweating, breathlessness and weakness. Hypotension and tachycardia are commonly recorded. These symptoms may be transient or recurrent. Less commonly, there is angioedema (swelling of lips, tongue, throat and upper airway) [10], increased secretions, chills, fever and tinnitus.
Investigations
Neutrophil leukocytosis is common. In exceptional cases, thrombocytopenia and mild coagulopathy have been described. Electrocardiographic changes include T-wave abnormalities, conduction defects and, in one case, myocardial infarction and acute kidney injury were documented in a previously fit 23-year-old man 11, 12. Clearly, envenoming may be life-threatening but the few alleged fatalities, all reported before 1930, are difficult to attribute.
Treatment: First-Aid
The longer the lizard is allowed to retain its grip and to chew, the more venomous saliva will be inoculated into the wound. The priority is to disengage, but the powerful jaws make this difficult. Rejected dangerous and barbaric methods include application of a flame under the animal's jaw, instilling gasoline or chloroform into its mouth or severing its jaw muscles with a knife. Pulling the animal off by its tail is quick but risks extending the bite wounds and detaching teeth. Expert opinion currently favors levering the jaws apart with a screw driver, putting the attached lizard under the tap, placing its four feet on the ground or introducing some alcohol into its mouth.
Treatment: Medical
Severe pain is relieved by local anesthetic or systemic analgesia. Opiates may be required. The wound should be explored for shed teeth which are not detectable by radiography. The risk of infection has not been studied, but prophylactic antibiotics are not justified. However, tetanus toxoid is recommended and the wound should be observed for evidence of sepsis. Specific antivenoms have been raised experimentally in rabbits but they are not generally available. Hypotension can be treated with intravenous fluids and, if necessary, with epinephrine or dopamine. Angioedema responds to epinephrine, antihistamine and hydrocortisone. Otherwise, treatment is symptomatic and supportive and the patient may be expected to recover completely in less than 1 week.
References
- 1.Fry BG, Vidal N, Norman JA. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- 2.Bogert CM, Martín del Campo R. Society for the Study of Amphibians and Reptiles; Ohio: 1993. The Gila Monster and its Allies. [Google Scholar]
- 3.Brown DE, Carmony NB. High-Lonesome Books; Silver City: 1991. Gila Monster. Facts and Folklore of America's Aztec lizard. [Google Scholar]
- 4.Beck DD. University of California Press; Berkeley: 2005. Biology of Gila Monster and Beaded lLizards. [Google Scholar]
- 5.Russell FE, Bogert CM. Gila monster, venom and bite—a review. Toxicon. 1981;19:341–359. doi: 10.1016/0041-0101(81)90040-4. [DOI] [PubMed] [Google Scholar]
- 6.Strimple PD, Tomassoni AJ, Otten EJ, Bahner D. Report on envenomation by a Gila monster (Heloderma suspectum) with a discussion of venom apparatus, clinical findings, and treatment. Wilderness Environ Med. 1997;8:111–116. doi: 10.1580/1080-6032(1997)008[0111:roebag]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Albritton DC, Parrish HM, Allen ER. Venenation by the Mexican beaded lizard (Heloderma horridum): report of a case. S D J Med. 1970;23:9–11. [PubMed] [Google Scholar]
- 8.Ariano-Sánchez D. Envenomation by a wild Guatemalan Beaded Lizard Heloderma horridum charlesbogerti. Clin Toxicol (Phila) 2008;46:897–899. doi: 10.1080/15563650701733031. [DOI] [PubMed] [Google Scholar]
- 9.Hooker KR, Caravati EM, Hartsell SC. Gila monster envenomation. Ann Emerg Med. 1994;24:731–735. doi: 10.1016/s0196-0644(94)70285-3. [DOI] [PubMed] [Google Scholar]
- 10.Piacentine J, Curry SC, Ryan PJ. Life-threatening anaphylaxis following gila monster bite. Ann Emerg Med. 1986;15:959–961. doi: 10.1016/s0196-0644(86)80686-2. [DOI] [PubMed] [Google Scholar]
- 11.Bou-Abboud CF, Kardassakis DG. Acute myocardial infarction following a gila monster (Heloderma suspectum cinctum) bite. West J Med. 1988;148:577–579. [PMC free article] [PubMed] [Google Scholar]
- 12.Preston CA. Hypotension, myocardial infarction, and coagulopathy following gila monster bite. J Emerg Med. 1989;7:37–40. doi: 10.1016/0736-4679(89)90408-3. [DOI] [PubMed] [Google Scholar]
134.5. Snakes
Key features.
-
•
Venomous snake bites are largely an occupational/environmental hazard of agricultural workers and their children in rural areas of the tropics. Most bites could be prevented by wearing protective footwear, by using lights while walking at night and by sleeping off the ground or under a well-tucked-in mosquito net
-
•
Snake venoms are rich in toxic proteins that cause necrosis, shock, hemostatic disturbances, paralysis, rhabdomyolysis and acute kidney injury
-
•
Bites by Elapidae (cobras, kraits, mambas, coral snakes, Australasian snakes, and sea snakes) may cause descending flaccid paralysis. Some elapid venoms cause local necrosis, rhabdomyolysis and hemostatic disturbances
-
•
Bites by Viperidae (vipers, adders and pit vipers: rattlesnakes, moccasins, lanceheads) can cause severe local swelling, bruising, blistering and necrosis together with shock, consumptive coagulopathy, spontaneous systemic bleeding, acute kidney injury and, with some species, neurotoxicity
-
•
First aid involves reassurance, immobilization of the whole patient, especially the bitten limb, rapid evacuation to the nearest hospital and avoidance of dangerous traditional methods
-
•
Specific antivenom is given if there is evidence of systemic or severe local envenoming. Assisted ventilation, renal dialysis or cardiovascular support may be required
Introduction
The three families of venomous snakes—Atractaspididae, Elapidae and Viperidae—contain some 500 species, whereas the fourth—the Colubridae, once considered nonvenomous—contains at least 40 species venomous to humans. Less than 200 species have caused clinically severe envenoming ending in death or permanent disability.
Distribution of Venomous Snakes [1]
Venomous species are widely distributed, except at altitudes above 5000 meters, in polar regions and in most islands of the western Mediterranean, Atlantic, Caribbean and Pacific. There are no venomous snakes in Madagascar, New Zealand, Ireland, Iceland and Chile. The range of Vipera berus extends into the Arctic Circle. Sea snakes exist in the Indian and Pacific oceans and in estuaries, rivers (New Guinea) and lakes (Philippines, Cambodia, Solomon Islands).
Snake Classification
Medically-important snakes always possess one or more pairs of enlarged teeth in the upper jaw—the fangs—which penetrate the skin of their victim and conduct venom into the tissues along a groove or through a lumen.
Colubridae
The fangs of colubrids are relatively short, are situated at the posterior end of the maxilla and are capable of only restricted movement (Fig. 134.5.1 ). African species, the boomslang (Dispholidus typus) and the vine, twig or bird snakes (Thelotornis spp.) have killed a few people. The Japanese yamakagashi (Rhabdophis tigrinus) has caused coagulopathy and at least two deaths, whereas the related Southeast Asian red-necked keelback (Rhabdophis subminiatus) has been responsible for cases of severe envenoming.
FIGURE 134.5.1.

Short posterior maxillary fang of the African Boomslang (Dispholidus typus—Colubridae) specimen from Nigeria
(© David A. Warrell).
Atractaspidinae
The African and Middle Eastern burrowing asps, also known as burrowing or mole vipers, or adders and stiletto snakes, have long front fangs and strike sideways. Three species are known to have caused fatal envenoming.
Elapidae
This family includes African and Asian cobras, Asian kraits, African mambas, American coral snakes, Australasian terrestrial venomous snakes and sea snakes. The relatively short anterior fangs of these snakes are permanently erect and capable of little movement (Fig. 134.5.2 ). In the ringhals and African and Asian spitting cobras, the venom channel opens forward before it reaches the tip of the fang, allowing venom to be ejected as a fine spray for a distance of several meters into the eyes of an aggressor.
FIGURE 134.5.2.
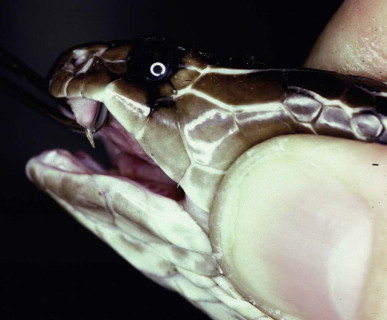
Short fixed front fang in a Sri Lankan cobra (Naja naja—Elapidae)
(© David A. Warrell).
Viperidae
The fangs are situated anteriorly, are up to 2.5 cm in length, curved and are capable of a wide range of movement (Fig. 134.5.3 ). Pit vipers (subfamily Crotalinae) comprise rattlesnakes, moccasins, South American lance-headed vipers and Asian pit vipers. They possess a heat-sensitive pit organ behind the nostril (Fig. 134.5.4 ). The Old World vipers, subfamily Viperinae include the European, African and Asian vipers and adders.
FIGURE 134.5.3.
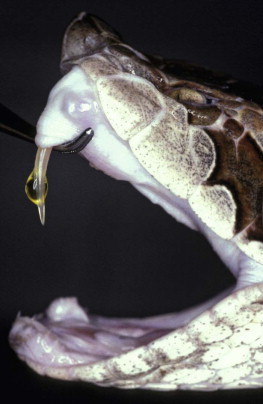
Long hinged front fang of Malayan pit viper (Calloselasma rhodostoma—Viperidae, Crotalinae) Thailand
(© David A. Warrell).
FIGURE 134.5.4.

Heat-sensitive pit organ of dark green pit viper [Cryptelytrops (Trimeresurus) macrops—Viperidae, Crotalinae] Thailand
(© David A. Warrell).
Medically Important Snakes [1]
Table 134.5.1 lists some of the species commonly responsible for human deaths and serious disability, usually resulting from local necrosis. Scientific and common English names are given.
TABLE 134.5.1.
Species responsible for most deaths and morbidity resulting from snakebite.
Specific antivenoms are manufactured for the treatment of envenoming by all these species (see Table 134.5.2 and reference [11])
| Area of distribution | Latin name* | English name |
|---|---|---|
| North America | Crotalus adamanteus | Eastern diamondback rattlesnake |
| Crotalus atrox | Western diamondback rattlesnake | |
| Crotalus oreganus | Pacific rattlesnake | |
| Central America | Crotalus simus | Central American rattlesnake |
| Bothrops asper | Terciopelo | |
| South America | Bothrops atrox | Common lancehead, fer-de-lance, barba amarilla |
| Bothrops asper | Terciopelo | |
| Bothrops jararaca | Jararaca | |
| Crotalus durissus | South American rattlesnake | |
| Europe | Vipera berus | Viper, adder |
| Vipera ammodytes | Long-nosed viper | |
| Africa | Echis spp. | Saw-scaled or carpet viper |
| Bitis arietans | Puff adder | |
| Naja nigricollis, Naja mossambica, etc. | African spitting cobras | |
| Naja haje | Egyptian cobra | |
| Dendroaspis spp. | Mambas | |
| Middle East | Echis spp. | Saw-scaled or carpet vipers |
| Daboia palaestinae | Palestine viper | |
| Macrovipera lebetina | Levantine viper | |
| Southeast Asia and India | Naja spp. | Asian cobras |
| Bungarus caeruleus | Common krait | |
| Daboia russelii | Western Russell's viper | |
| Daboia siamensis | Eastern Russell's viper | |
| Echis carinatus | Saw-scaled or carpet viper | |
| Calloselasma | Malayan pit viper | |
| (Agkistrodon) rhodostoma | ||
| Cryptelytrops (Trimeresurus) spp. | Green pit vipers | |
| Far East | Naja atra | Chinese cobra |
| Bungarus multicinctus | Chinese krait | |
| Gloydius spp. | Mamushis | |
| Protobothrops flavoviridis | Habu | |
| Protobothrops mucrosquamatus | Chinese habu | |
| Australasia | Acanthophis species | Death adders |
| Notechis scutatus | Tiger snake | |
| Oxyuranus scutellatus | Taipan | |
| Pseudonaja textilis | Eastern brown snake | |
Scientific (Latin) names are important because they are used internationally to describe the range of specificity of antivenoms.
Incidence and Importance of Snakebite [2]
Snake bite is an important medical emergency in some parts of the rural tropics but its incidence is usually underestimated because most victims seek the help of traditional healers rather than practitioners of Western-style medicine. In coastal Kenya, where snake bites cause 6.7 deaths per 100,000 per year (0.7% of all deaths), 68% of bitten people had sought treatment from traditional healers. In the Benue Valley of north-east Nigeria, Echis ocellatus causes some 500 bites per 100,000 population per year, with a 12% mortality. Recently, a study of snake bite mortality in India, based on verbal autopsy, estimated 46,000 deaths each year. In Burma, Russell's viper bite is a common cause of acute kidney injury and is responsible for most of the estimated 1000 snake-bite deaths each year. In the USA, there are about 45,000 bites and a few deaths each year. Rattlesnakes, especially (Crotalus adamanteus, Crotalus atrox, Crotalus horridus, Crotalus oreganus, Crotalus scutulatus, Crotalus viridis and Sistrurus miliaris), are the most dangerous species. In Britain, the adder or viper (Vipera berus) is the only venomous species, biting more than 200 people each year. Only 14 deaths have been reported since 1876—the last in 1975. This species is important in Scandinavian countries. Vipera aspis causes most bites in France, while Vipera ammodytes is important in eastern Europe.
Some hunter-gatherer tribes, such as the Yanomamo of Venezuela, the Waorani of Ecuador, the Kaxinawa of Brazil, the Hadza of Tanzania and some tribal groups in Papua New Guinea, suffer high mortality from snake bites. Global estimates of annual snake bite mortality vary from 20,000 to 125,000. Many survivors are left with permanent physical disability.
Epidemiology [3]
Most snake bites are inflicted on the lower limbs of farmers, plantation workers, herdsmen and hunters in rural areas of tropical developing countries. The incidence of bites by a particular species in a particular geographic area depends on the densities of human and snake populations, the snake's irritability (its inclination to bite when trodden on or disturbed) and diurnal rhythm, and the extent to which human activities encroach on its chosen habitat. The snake is usually trodden on at night or in undergrowth. Some species, such as the Asian kraits (Bungarus spp.) and African spitting cobras (Naja nigricollis, Naja mossambica, etc.), enter human dwellings at night and may bite people who roll over onto them while sleeping on the floor. Snakes do not bite without provocation, but may strike if inadvertently trodden upon or touched. In Europe, North America, and Australia, exotic snakes are increasingly popular pets. In these countries, bites are inflicted on the hands of (usually) males who are picking up their pets, often late at night while drunk. In the USA, 25% of bites result from snakes being attacked or handled. Serious bites by back-fanged (colubrid) snakes usually occur only under these circumstances. Seasonal peaks in the incidence of snake bite are associated with agricultural activities, such as ploughing before the annual rains in the West African Sahel and the rice harvest in Southeast Asia, or to fluctuations in the activity or population of venomous snakes. Severe flooding, by concentrating the human and snake populations, has given rise to epidemics of snake bite, notably in Colombia, Pakistan, India, Bangladesh, Nepal, Burma and Vietnam. Invasion of virgin jungle during construction of new highways and irrigation and hydroelectric schemes has led to an increased incidence of snake bite in Brazil and Sri Lanka. On rare occasions, snakebite or injection of snake venom has been used for suicide or murder.
Prevention of Snake Bite
To reduce the risk of bites, snakes should never be disturbed, attacked, cornered or handled—even if they are thought to be a harmless species or appear to be dead. Venomous species should not be kept as pets or as performing animals. In snake-infested areas, boots, socks and long trousers should be worn for walks in undergrowth or deep sand, and a light should always be carried at night. Collecting firewood, dislodging logs and boulders with bare hands, pushing sticks or fingers into burrows, holes and crevices, climbing rocks and trees covered with dense foliage, and swimming in overgrown lakes and rivers are particularly hazardous activities. Unlit paths and gutters are especially dangerous after heavy rains. Sleeping on the ground carries a risk of nocturnal krait bites in South Asia and of spitting cobra bites in Africa, but mosquito nets are protective. It is futile and ecologically undesirable to attempt to exterminate venomous snakes. Various substances toxic to snakes, such as insecticides and methylbromide, have been used to keep human dwellings free of these animals. However, no effective yet harmless snake repellent has been discovered.
Venom Apparatus
The venom glands are surrounded by compressor muscles and are situated behind or below the eye. The venom duct opens within a sheath at the base of the fang and venom is conducted toward the tip in a partially or completely closed groove or fang canal. Venomous snakes can inject doses of venom lethal to their natural prey at each of 10 or more consecutive strikes. Whether the dose can be adjusted according to the size of the prey or the intention of the snake is controversial. The high proportion of bites without envenoming (“dry bites”) reported for species such as Calloselasma rhodostoma (>50%) or Pseudonaja spp. (>80%) is more likely to reflect mechanical inefficiency than to voluntary control by the snake, and the concept of a defensive bite may not be valid. There is no support for the popular belief that snakes are less dangerous after they have eaten. The snake uses only a small fraction of the content of its venom gland at each strike.
Venom Composition
More than 90% of the dry weight of venom is protein and each venom may contain more than 100 different proteins: enzymes (80–90% of viperid; 25–70% of elapid venoms), non-enzymatic polypeptide toxins and non-toxic proteins such as nerve growth factor. These include digestive hydrolases, hyaluronidase and activators or inactivators of many physiologic processes. Viperid venoms have metalloproteinases, endopeptidase, arginine ester hydrolase, kininogenase, as well as thrombin-like factor X, and prothrombin-activating enzymes responsible for the anti-hemostatic effects of envenoming. Phospholipases A2 (lecithinase) are found in many venoms. They damage mitochondria, red blood cells, leukocytes, platelets, peripheral nerve endings, skeletal muscle, vascular endothelium and other membranes, and produce presynaptic neurotoxic activity, opiate-like sedative effects and the autopharmacologic release of histamine. Some are anti-coagulant. Hyaluronidase promotes the spread of venom through tissues.
Necrotoxins
A variety of venom myotoxic and cytolytic factors may contribute to local tissue necrosis at the site of the bite. Studies of terciopelo (Bothrops asper) venom induced necrosis implicate zinc-dependent metalloproteinases and myotoxic phospholipases A2. In other cases, other digestive hydrolases, hyaluronidase, polypeptide cytotoxins (Elapidae) and, perhaps, secondary effects of inflammation are involved.
Neurotoxins
Postsynaptic (α-)neurotoxins, such as α-bungarotoxin and cobrotoxin, are three-finger fold polypeptides that bind to acetylcholine receptors on the motor end-plate, like curare, competitively inhibiting acetylcholine. Presynaptic (β-)neurotoxins, such as β-bungarotoxin, crotoxin, taipoxin and notexin, are phospholipases A2 that prevent release of acetylcholine at the neuromuscular junction and damage the nerve endings irreparably. Myotoxic phospholipases A2 in venoms of Elapidae, notably sea snakes and some Australasian, American and Asian terrestrial elapids an Viperidae can cause generalized rhabdomyolysis.
Cardiovascular and autopharmacologic toxins
Some venoms release vasodilating autacoids such as histamine and kinins. Venom of the Brazilian jararaca (Bothrops jararaca) and other vipers contain bradykinin potentiating and angiotensin converting enzyme (ACE) inhibiting peptides that cause hypotension. Sarafotoxins from the venom of the Israeli burrowing asp (Atractaspis engaddensis) are similar to physiologic endothelins. They are potent vasoconstrictors of the coronary arteries and delay atrioventricular conduction.
Variation in Venom Composition
Venom composition varies enormously from species to species but also within a single species throughout its geographic range, at different seasons of the year and as the snake matures.
Pharmacology
When snakes bite humans, venom is usually introduced subcutaneously or intramuscularly. Intravenous injection is a rare possibility. Smaller Mw elapid neurotoxins are rapidly absorbed into the bloodstream, whereas larger Mw phospholipase A2 presynaptic toxins and viperid enzymes are taken up more slowly through the lymphatics, sometimes causing visible lymphangitis and enlarged, painful lymph nodes. Continuing absorption of venom from the depot at the site of bite may explain delayed or recurrent envenoming after an initial therapeutic response to antivenom. Redistribution of venom toxins into the vascular compartment may occur as a result of antivenom treatment. Envenoming after ingestion of snake venom has not been reported in humans. Most venoms are concentrated and bound in the kidney and some components are eliminated in the urine. Crotaline venoms are selectively bound in the lungs, concentrated in the liver and excreted in bile, while polypeptide neurotoxins, such as α-bungarotoxin, are tightly bound at neuromuscular junctions. Most venom components do not cross the intact blood brain barrier. Central nervous system effects of venoms remain controversial.
Clinical Effects [4]
The patient who has been bitten by a snake may present with symptoms resulting from fear, from prehospital treatment and from effects of the venom itself. Snake bite is usually a terrifying experience, especially for those who believe that all bites are rapidly fatal. Physiologic manifestations of anxiety, and even frank hysteria, may confuse the clinical picture. Thus, patients who are bitten, but not envenomed, may feel flushed and breathless, with constriction of the chest, and a thumping pulse, palpitations, sweating and effects of hyperventilation, such as faintness, acroparesthesia and even tetany, may be noticed. Such symptoms dominate many accounts of snake bites written by the victims and are falsely attributed to neurotoxicity. Misguided traditional prehospital treatments can result in swelling and ischemia of limbs whose circulation is occluded by tourniquets, bleeding or sensory loss resulting from local incisions, vomiting and other side effects caused by ingested herbal remedies, smarting eyes and conjunctivitis from instillation of plant juices, and bronchospasm from insufflation of oils. Rarely, snake bite may precipitate vaso-vagal collapse, angina pectoris, myocardial infarction or cardiac arrhythmia.
General symptoms and signs
The evolution of symptoms and signs of envenoming depends on the nature of the venom, the dose and the site of injection. The earliest symptom is usually pain—felt immediately. Local swelling may start within minutes and consumption coagulopathy with undetectable plasma concentrations of fibrinogen and other clotting factors can develop in half an hour (C. rhodostoma and Echis species). Rarely, death may occur as soon as 15 minutes after an elapid (e.g. Naja naja or Dendroaspis species) or viper (e.g. Daboia russelii, Daboia siamensis) bite. Usually, however, death comes hours after an elapid or sea snake bite and days after a viper bite.
Local effects
This is characteristic of bites by the Viperidae, including the pit vipers of the subfamily Crotalinae, African spitting cobras (e.g. N. mossambica, N. nigricollis) and Asian cobras (e.g., N. naja, Naja kaouthia and Naja siamensis). Tender swelling spreads from the site of the bite and there is early tender enlargement of lymph nodes draining the bitten area. Within a few hours, blood or fluid-filled bullae may appear under the epidermis (Fig. 134.5.5 ). With elapid bites, blistering is nearly always followed by tissue necrosis, usually superficial, which may extend up the fascial planes of the limb, sometimes as skip lesions separated by areas of unaffected skin (Fig. 134.5.6 ). Bullae caused by Viperidae bites frequently dry up and slough without the development of necrosis. A pale, anesthetic, demarcated area of skin with a characteristic odor of putrefaction signals the appearance of necrosis. This is an effect of the venom, but the necrotic tissue is vulnerable to secondary infection by bacteria, including anaerobes.
FIGURE 134.5.5.
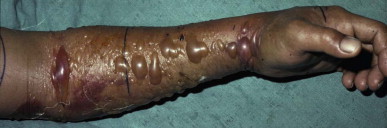
Intense edema, bruising and formation of bullae 25 hours after a bite on the forearm by a Malayan pit viper (Calloselasma rhodostoma) in Thailand
(© David A. Warrell).
FIGURE 134.5.6.
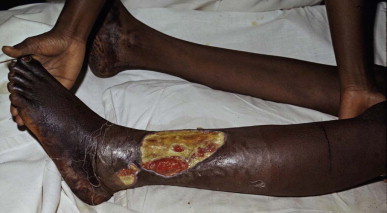
Blistering with necrosis of skin and subcutaneous tissue one week after a bite on the ankle by a black-necked spitting cobra (Naja nigricollis) in Nigeria
(© David A. Warrell).
Massive swelling of the bitten limb indicates increased permeability leading to extravasation of circulating volume; a swollen limb can accommodate several liters of blood. The result may be hypovolemia and hypotension. Envenoming by rattlesnakes (Crotalus) produces local pain with swelling that appears within 15 minutes of the bite and may spread rapidly. Bruising along the path of lymphatics and bullae, and local necrosis may develop. Paresthesias of the tongue and lips and an abnormal metallic taste are common early symptoms following bites by Pacific (C. oreganus), eastern diamondback (C. adamanteus), western diamondback (C. atrox) and timber (C. horridus) rattlesnakes. Other symptoms include weakness, rigors, sweating, fasciculation, spontaneous bleeding, neurotoxic effects (C. adamanteus, C. scutulatus) and gastrointestinal symptoms. Bites by the Mohave rattlesnake (C. scutulatus) and Crotalus durissus terrificus may produce little, or no, local swelling but severe systemic signs.
Bleeding and clotting disturbances [5]
This combination is characteristic of vipers, pit vipers and some Australasian elapids. Spontaneous bleeding is most frequently detected in the gingival sulci (Fig. 134.5.7 ). The most common sites of hemorrhage are intracerebral, gastrointestinal and retroperitoneal. Incoagulable blood is suggested by oozing from venipuncture sites or sites of recent trauma.
FIGURE 134.5.7.
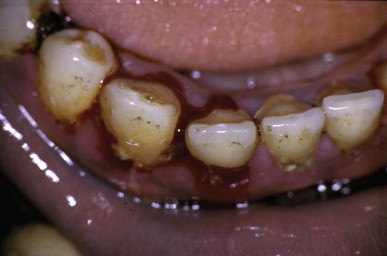
Spontaneous bleeding from gingival sulci after a bite by a West African saw-scaled viper (Echis ocellatus) in Nigeria
(© David A. Warrell).
Hypotension and shock
Early syncope can occur as part of the autopharmacologic syndrome after bites by vipers, Australasian elapids and burrowing asps. Shock is most commonly the result of hypovolemia (Fig. 134.5.8 ), vasodilatation or direct action of venom on the myocardium.
FIGURE 134.5.8.
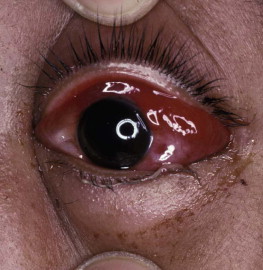
Clinical evidence of increased systemic vascular permeability: chemosis (edema of the conjunctiva) 48 hours after a bite by Russell's viper (Daboia siamensis) in Burma
(© David A. Warrell).
Neurotoxicity
This is a feature of envenoming by elapids. A few species of vipers also produce neurotoxic effects, for example C. durissus terrificus, Gloydius brevicaudus, Bitis atropos and D. russelii (especially in Sri Lanka). Typically, neurotoxic symptoms develop early, but after krait bite there may be a delay of 10 or more hours. Symptoms include vomiting, headache, paresthesia, drowsiness, apathy or euphoria, hyperacusis, diplopia, blurred vision, heaviness of the eyelids and difficulty in speaking. The levator palpebrae superioris and extraocular muscles are the most sensitive to neuromuscular blockade and, in some patients, the only feature of envenoming is ptosis and ophthalmoplegia. More serious effects are paralysis of the palate, jaws, tongue, vocal cords, neck muscles and muscles of deglutition and respiration (Fig. 134.5.9 ). The intercostal muscles are affected before the diaphragm and limbs. Paralyzed patients are fully conscious unless they are hypoxemic (respiratory failure) or hypotensive (circulatory failure). Neurotoxicity is usually completely reversible; in some cases there is a rapid response to specific antivenom or anticholinesterase, and in others there is slow, spontaneous resolution. With no specific antivenom, patients supported by artificial ventilation recover sufficient diaphragmatic movement to breathe adequately in 1–4 days. The ocular muscles recover in 2–4 days and there is usually full recovery of motor function in 3–7 days. Upper airway obstruction by the tongue or inhaled vomitus may precipitate respiratory arrest.
FIGURE 134.5.9.
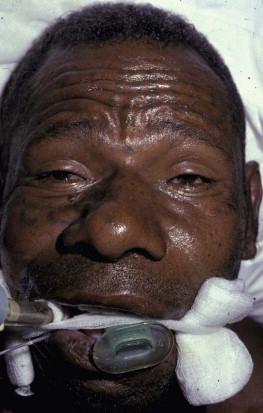
Neurotoxic envenoming after a bite by a Papuan taipan (Oxyuranus scutellatus canni). Note bilateral ptosis (the patient is attempting to open his eyes by contracting the frontalis muscle) and inability to breathe spontaneously (mechanical intubation via endotracheal tube)
(© David A. Warrell).
Generalized rhabdomyolysis
Envenoming by sea snakes [6], some Australasian elapids, D. russelii in Sri Lanka and C. durissus terrificus can cause systemic rhabdomyolysis. The symptoms are muscle pain and stiffness with trismus and respiratory muscle paralysis. Myoglobinemia, myoglobinuria, hyperkalemia and renal failure may result.
Acute kidney injury
Renal failure can complicate almost any severe case of snakebite, but it is the major cause of death in victims of sea snakes, some Australasian elapids, Russell's vipers and C. durissus terrificus. Mechanisms of renal damage include ischemia (from hypotension, renal vasoconstriction or disseminated intravascular coagulation) (Fig. 134.5.10 ), hemorrhage, direct nephrotoxicity or pigment nephropathy associated with massive intravascular hemolysis, microangiopathic hemolysis, generalized rhabdomyolysis and associated electrolyte disturbances.
FIGURE 134.5.10.
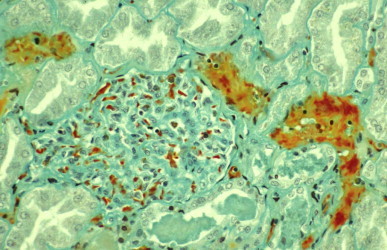
Kidney of a patient who died 15 hours after being bitten by Russell's viper (Daboia siamensis) in Burma, showing fibrin thrombi (staining red) in glomerular and peritubular vessels
(© Dr Nicholas Francis).
Venom ophthalmia caused by spitting cobras [7]
The ringhals (Hemachatus haemachatus) and African and Asian spitting cobras can eject venom in a fine stream from the tips of their fangs for a distance of a few meters. If venom enters the eye, there is intense local pain, leukorrhea, blepharospasm and palpebral edema. Because most patients make an uneventful recovery, these injuries used to be thought trivial, but slit lamp examination reveals corneal erosions in more than half of the cases. There is the same risk of secondary infection as with other corneal injuries (Fig. 134.5.11 ), leading to permanent blindness in some cases. The venom may be absorbed also into the anterior chamber, resulting in anterior uveitis with hypopyon.
FIGURE 134.5.11.
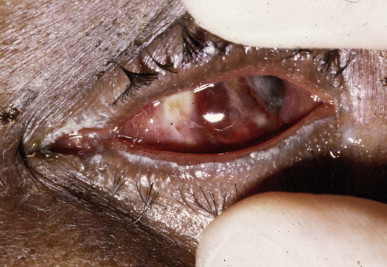
Severe venom ophthalmia that led to blindness in a patient “spat at” by a black-necked spitting cobra Naja nigricollis in Nigeria. Failure to treat with a local antimicrobial agent may allow secondary infection of corneal abrasions with these disastrous results (panophthalmitis requiring enucleation)
(© David A. Warrell).
Laboratory Investigations
The peripheral white blood cell count is raised in patients severely envenomed by many species of snakes. Anemia is the result of bleeding or, much more rarely, intravascular and microangiopathic hemolysis. Thrombocytopenia occurs with disseminated intravascular coagulation, together with a hemolytic uremic-like syndrome in victims of some viper and Australian elapid envenomings. Important simple tests for venom-induced defibrination are the 20-minute whole blood clotting test A few milliliters of blood are placed in a new, clean, dry glass test tube, left undisturbed for 20 minutes and then tipped once to check for clotting (Fig. 134.5.12 ). Serum potassium is elevated by the generalized rhabdomyolysis of sea snake envenoming. Serum enzymes, such as aspartate and alanine aminotransferases and creatine kinase, are mildly elevated in patients with local tissue damage but are grossly raised if there is generalized rhabdomyolysis. Electrocardiographic changes, such as inverted T waves, raised ST segments, prolonged Q-Tc intervals and arrhythmias, have been reported. The urine of snakebite victims commonly contains red and white blood cells and granular casts. Dark urine should be tested for hemoglobin and myoglobin.
FIGURE 134.5.12.
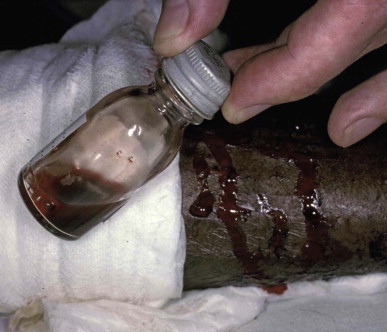
Twenty-minute whole blood clotting test. Blood taken from a patient envenomed by a Papuan taipan (Oxyuranus scutellatus) remains incoagulable after standing undisturbed in a glass tube for 20 minutes. The patient with venom-induced consumption coagulopathy continues to bleed from razor cuts made inadvisedly at the site of the bite
(© David A. Warrell).
Venom Immunodiagnosis
Specific snake venom antigens have been detected in wound swabs, aspirates or biopsies, serum, urine, cerebrospinal fluid and other body fluids. Enzyme immunoassay (EIA) has been the most widely used. Under ideal conditions, relatively high venom antigen concentrations (wound swabs or aspirates) may be detected quickly enough (15–30 min) to allow the selection of the appropriate monospecific antivenom. A commercial venom detection kit for Australian elapids is produced by CSL (Melbourne, Australia). For retrospective diagnosis, including forensic cases, tissue around the fang punctures, wound and blister aspirate, serum and urine should be stored for EIA immunodiagnosis.
Treatment of Snake Bite 3, 4, 8, 9, 10
Snakebite is a rare emergency in most parts of the world and because its management is thought to require specialized knowledge, many clinicians close their minds to the simple therapeutic principles that could prevent morbidity and mortality. Management starts with first aid by relatives, friends or fellow workers of the snakebite victim who happen to be present where and when the bite occurs. Therefore, first aid principles should be a priority subject for community health education in schools, at clinics and via the media.
First-Aid Treatment
Most snakebite victims are terrified and require reassurance. The bitten limb should be immobilized, if possible, with a splint or sling and the patient quickly moved to the nearest treatment facility. Pain can be treated with oral acetaminophen or codeine phosphate. Aspirin and nonsteroidal anti-inflammatory agents should be avoided, if possible, as they may lead to persistent gastric bleeding in patients with incoagulable blood. Local incisions and suction are more likely to introduce infection and cause persistent bleeding than to remove significant amounts of venom from the wound. In one study of viper bites in Jammu, India, 94% of patients who had received incisions developed local infection, compared with none in the group without incisions. Vacuum extractors, potassium permanganate instillation and ice packs can increase local necrosis. Electric shock treatment is potentially dangerous and of unproven value.
Tourniquets
Tight (arterial-occlusive) tourniquets must never be recommended for snake bite first-aid because they have been responsible for gangrenous limbs, peripheral nerve damage and other harmful effects.
Pressure-Immobilization (P-I) and Pressure Pad
These are the only two acceptably safe and promising first-aid methods currently available, but both require further clinical testing. In animal studies, compressing superficial veins and lymphatics in the bitten limb at about 55 mmHg reduced the spread of larger Mw toxins such as the presynaptic phospholipase A2 toxins of Australian elapid venoms. In practice, the entire bitten limb is bound firmly, using a 10-cm wide elasticated bandage, starting at the toes or fingers and finishing at the groin or axilla. A splint is incorporated to aid immobilization. Anecdotal experience supports the use of the method but it has proved difficult to train people to apply it correctly. However, it is the only known method for delaying the onset of potentially fatal respiratory paralysis after a neurotoxic elapid bite before the patient reaches medical care without incurring the dangers of a tight arterial tourniquet. If the necessary skills and equipment are available immediately, pressure-immobilization (P-I) should be applied, unless it is possible to eliminate the possibility of a neurotoxic elapid bite (e.g. by confident identification of the snake or exclusion on geographical grounds). A local pressure pad applied over the bite wound has been advocated for Russell's viper bites in Burma.
Transport to medical care
Patients should be transported to hospital as quickly, but as passively, as possible. They should be placed on their left side in the recovery position to prevent aspiration of vomit. Persistent vomiting can be treated with chlorpromazine by intramuscular injection (25–50 mg in adults, 1 mg/kg in children) [intravenous (IV) injection risks hypotension] or chlorpromazine or prochlorperazine by intrarectal suppository. Syncope, shock, angio-oedema and other anaphylactic symptoms can be treated with 0.1% adrenaline (epinephrine) by intramuscular injection (0.5 ml for adults, 0.01 ml/kg for children) and an antihistamine such as chlorphenamine maleate, by IV injection (10 mg for adults, 0.2 mg/kg for children). Respiratory distress and cyanosis should be treated by clearing the airway, preventing obstruction by the tongue by jaw-lifting, inserting an oropharyngeal airway and by positioning, giving oxygen and, if necessary, assisted ventilation. If the patient is unconscious and no femoral or carotid pulses can be detected, cardiopulmonary resuscitation must be started immediately.
If the snake has been killed it should be brought to the hospital for identification, but it must be handled cautiously as even snakes that appear dead and severed heads can cause envenoming. They should be carried on a stick or maneuvered into a container.
Treatment by Medically-Trained Personnel in Hospital or Dispensary
Because of the uncertainties about the type, quantity and quality of venom injected and the variable time course for development of signs of envenoming, all victims of snakebite should be hospitalized and observed for at least 24 hours. Frequent observations of the level of consciousness, blood pressure, and pulse and respiratory rate, and new signs, such as ptosis, should be made. Any ligatures should be released, preferably after starting administration of antivenom (see “Antivenom Treatment” below). Physical examination should include assessment of local swelling, tender enlargement of regional lymph nodes draining the bitten area, spontaneous bleeding [most often detected in the gingival sulci (Fig. 134.5.1), nose and gastrointestinal and genitourinary tracts, blood pressure, ptosis [the earliest sign of neurotoxic envenoming (Fig. 134.5.3)] and assessment of respiratory muscle power. If a coagulopathic venom is suspected, hemostasis should be checked at the bedside by the 20-minute whole blood clotting test or by rapid laboratory tests of hemostasis.
Antivenom Treatment
Antivenom (also known as antivenin, antivenene and anti-snakebite serum) is the only specific antidote to envenoming. It is the partially purified immunoglobulin (whole IgG, F(ab’)2, or Fab fragments) of horses or sheep that have been hyperimmunized with venoms of one species of snake (monovalent) or several species (polyvalent) of greatest medical importance in a particular region [11].
Indications
Because of their high cost and the inherent danger of reactions, antivenoms should not be used indiscriminately. Antivenom is indicated if there is systemic envenoming evidenced by hypotension or other signs of cardiovascular toxicity, signs of neurotoxicity or generalized myotoxicity, impaired consciousness, spontaneous systemic bleeding and incoagulable blood. Supporting evidence of severe envenoming is provided by a peripheral leukocytosis of more than 20 × 109/l, elevated serum enzymes, hemoglobinuria, myoglobinuria, severe anemia or hemoconcentration, uremia and oliguria. In the absence of systemic envenoming, massive local swelling (involving more than half of the bitten limb), bites on fingers or toes and rapidly progressive swelling following bites by species known to cause necrosis are indications for antivenom.
Contraindications
There is no absolute contraindication to antivenom; however, because of the increased danger of severe reactions, atopic individuals and those known to be hypersensitive to equine serum should be pretreated with epinephrine, hydrocortisone and antihistamine (doses above) and watched carefully for at least two hours after completion of antivenom administration.
Administration
Antivenom should be given as soon as indicated, but it may be effective as long as signs of systemic envenoming persist (seven days or more after the bite in the case of patients with viperid bite coagulopathy). To prevent local envenoming, antivenom must be given early, within a few hours of envenoming. Only specific antivenom—one whose range of specificity includes the biting species—should be used. Some antivenoms raised against the venom of one or two species have paraspecific activity against venoms of related species. Antivenom should be diluted in an appropriate volume of fluid and given by “push” injection over 10–15 minutes or by IV infusion over 30–60 minutes. Epinephrine 0.1% solution, 0.5 ml for adults or 0.01 ml/kg for children, must be ready to be given, by intramuscular injection, in case of early anaphylactic reactions during the infusion. The patient must be watched carefully while antivenom is being given and for at least two hours afterward. At the first sign of a reaction, administration of antivenom should be stopped and epinephrine given. Once the symptoms of the reaction have subsided, antivenom infusion can be completed. IV chlorphenamine maleate (10 mg in adults, 0.2 mg/kg in children) and hydrocortisone (100 mg in adults, 2 mg/kg in children) are given to combat released mediators and to calm the patient. Antivenom reactions are not predicted by conjunctival or intradermal “hypersensitivity tests” which can only detect specific IgE. Anti-H1 histamines blockers and corticosteroids have proved ineffective, singly or in combination, in preventing early anaphylactic antivenom reactions, but in a recent, powerful study, epinephrine (adult dose 0.25 ml of 0.1% solution) administered subcutaneously before antivenom was given reduced the frequency of severe early anaphylactic reactions [12]. Appropriate initial doses of antivenom have been established for some antivenoms (Table 134.5.2 ). Assessment of the antivenom dose will remain a matter of clinical judgment. The dose of antivenom for children and adults should be the same.
TABLE 134.5.2.
Guide to initial dosage of some important antivenoms
| Species |
Manufacturer, antivenom | Approximate initial dose | |
|---|---|---|---|
| Latin name | English name | ||
| Acanthophis spp. | Death adder | CSL*, monospecific | 3000–6000 units |
| Bitis arietans | Puff adder | Sanofi-Pasteur, Fav Afrique and Favi Rept, SAVP†; polyspecific | 80 ml |
| Bothrops asper | Terciopelo | Instituto Clodomiro Picado | 50–100 ml |
| Bothrops atrox | Common lance-head | Suero Antiofidico (Instituto Nacional de Higiene y Medicina Tropical “Leopoldo Izquieta Perez” Guayaquil, Ecuador); Soro Antibotropico (Instituto Butantan, San Paulo, Brazil) | 20 ml |
| Bothrops (Bothriopsis) bilineatus | As above | 20 ml | |
| Bothrops jararaca | Jararaca | Instituto Butantan and other Brazilian manufacturers, Bothrops polyspecific | 20 ml |
|
Bungarus caeruleus Bungarus candidus |
Common krait Malayan krait |
Indian manufacturers§, polyspecific Thai Red Cross, Bangkok monospecific |
100 ml 100 ml |
| Calloselasma (Agkistrodon) rhodostoma | Malayan pit viper | Thai Red Cross, Bangkok, monospecific or hemato-polyvalent | 100 ml |
| Crotalus adamanteus | Eastern diamondback rattlesnakes | Protherics “CroFab” | 7–15 vials |
| Crotalus atrox | Western diamondback rattlesnakes | Protherics “CroFab” | 7–15 vials |
| Crotalus oreganus and Crotalus viridis subspp. | Western rattlesnakes | Protherics “CroFab” | 7–15 vials |
| Cryptelytrops (Trimeresurus) albolabris | Green pit viper | Thai Red Cross, monospecific or hemato-polyvalent | 100 ml |
| Daboia (Vipera) palaestinae | Palestine viper | Rogoff Medical Research Institute, Tel Aviv, Palestine, viper-monospecific | 50–80 ml |
| Daboia (Vipera) russelii | Western Russell's viper | Indian manufacturers,§ polyspecific | 100 ml |
| Daboia siamensis | Eastern Russell's viper | Thai Red Cross, monospecific or hemato-polyvalent | 100 ml |
| Myanmar Pharmaceutical Factory monospecific | 50 ml | ||
| Echis ocellatus, Echis leucogaster, Echis pyramidum (Africa) | African saw-scaled or carpet vipers | SAIMR†, Echis, monospecific; Aventis-Pasteur “Fav Afrique” | 20 ml |
| Elapidae (Hydrophiinae) | Sea snakes | CSL*, sea snake | 1000 units |
| Naja kaouthia | Monocellate Thai cobra | Thai Red Cross, monospecific or neuro-polyvalent | 100 ml |
| Naja naja | Indian cobra | Indian manufacturers,§ polyspecific | 100 ml |
| Notechis scutatus | Tiger snake | CSL*, monospecific | 3000–6000 units |
| Oxyuranus scutellatus | Taipan | CSL*, polyspecific | 12,000 units |
| Pseudonaja textilis | Eastern brown snake | CSL*, monospecific | 3000–6000 units |
| Vipera berus | European adder | Immunoloski Zavod-Zagreb Vipera, polyspecific | 10–20 ml |
| Protherics Fab. Monospecific “ViperaTAb” | 100–200 mg | ||
Contacts for procurement of antivenoms: http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en/; http://www.searo.who.int/EN/Section10/Section17.htm; http://www.toxinfo.org/antivenoms/ (all websites last accessed 28 December 2011).
Commonwealth Serum Laboratories, Melbourne, Australia.
South African Vaccine Producers [formerly SAIMR (South African Institute for Medical Research)].
Haffkine, Kasauli, Serum Institute of India, Vins, Bharat, Biological Evans, King Institute, etc.
Response to antivenom
Spontaneous systemic bleeding usually stops within about 30 minutes of initiating antivenom therapy and blood clotting is usually restored within 6 hours if an adequate neutralizing dose of antivenom has been given. Improvement in neurotoxic symptoms attributable to postsynaptic toxins [e.g. Australian and Papuan death adder (genus Acanthophis) and Asian cobras (genus Naja)] may be observed within 30–60 minutes. However, severe, local envenoming nephrotoxicity following Russell's viper bites, and presynaptic neurotoxicity, are not reversed by antivenom. Further antivenom should be given if severe signs of envenoming persist after 1–2 hours or if blood coagulability is not restored within about six hours..
Antivenom Reactions
Early anaphylactic reactions usually develop within 10–60 minutes of starting IV administration of antivenom. Premonitory symptoms include restlessness, cough, itching of the scalp, nausea, vomiting, a feeling of heat or an increase in pulse rate followed by generalized pruritus and appearance of urticaria, fever, tachycardia, autonomic manifestations and, in a few patients, severe hypotension, airflow obstruction and angioedema. The incidence of early reactions is dose-dependent and also depends on the quality of manufacture. The cause of most reactions is complement activation by IgG aggregates, residual Fc fragments or osmotic effects rather than IgE-mediated type I hypersensitivity to equine serum, which is rare.
Pyrogenic reactions occur within 1–2 hours of antivenom treatment and can precipitate febrile convulsions in children. They are attributable to endotoxin-contamination during manufacture [11].
Late reactions of serum sickness type may develop 5–10 days after antivenom treatment. They are dose-related. Clinical features include fever, pruritus, urticaria, subcutaneous and periarticular swellings, polyarthritis, lymphadenopathy, mononeuritis multiplex and other neurologic symptoms, and proteinuria.
Supportive Treatment
Neurotoxic Envenoming
Patients unable to cough up or swallow their secretions aspirate and may develop fatal airway obstruction or pneumonia. Others die of respiratory paralysis. Endotracheal intubation should be performed at an early stage, when pooling of secretions in the pharynx becomes evident—before obstruction or respiratory arrest has developed. If respiratory muscle power is inadequate, ventilation must be assisted. Patients have recovered from respiratory paralysis after being manually ventilated by relays of relatives or nurses for up to 30 days and after mechanical ventilation for up to 10 weeks. Most effects of neurotoxic envenoming are fully reversible; therefore, artificial ventilation should always be attempted.
Anticholinesterases have a variable, but potentially useful, effect in patients with neurotoxic envenoming, especially when postsynaptic neurotoxins are involved. The “Tensilon test” can be performed, as with suspected myasthenia gravis. Atropine sulphate (0.6 mg for adults; 50 µg/kg for children) or glycopyrronium is given by IV injection followed by edrophonium chloride (Tensilon) by slow IV injection in an adult dose of 10 mg (0.25 mg/kg for children) or neostigmine bromide or methylsulphate (Prostigmin) by intramuscular injection 0.02 mg/kg for adults (0.04 mg/kg for children). Patients who respond convincingly can be maintained on neostigmine methylsulphate, 0.5–2.5 mg every 1–3 hours up to 10 mg/24 hours maximum for adults or 0.01–0.04 mg/kg every 2–4 hours for children by intramuscular, IV or subcutaneous injection.
Circulatory collapse
Antivenom should be given, as well as plasma expanders. Clinical observation of jugular venous pressure or measurement of central venous pressure or pulmonary wedge pressure (via a Swan-Ganz catheter) helps to prevent fluid overload and precipitation of pulmonary edema. If hypotension persists despite restoration of central venous pressure to +10 to 15 cm H2O, an infusion of dopamine should be started at an initial dose of 2 µg/kg/minute through the central catheter.
Local necrosis
Once definite signs of necrosis have appeared, surgical debridement is required, with antibiotic prophylaxis to cover anaerobic organisms.
Intracompartmental syndrome and fasciotomy
Increased pressure within tight fascial compartments, such as the digital pulp spaces and anterior tibial compartment, may cause ischemia. Necrosis of digits is especially common. The signs are excessive pain, weakness and tenderness of the compartmental muscles and pain when they are passively stretched, hypoesthesia of skin supplied by nerves running through the compartment, and obvious tenseness of the compartment. Detection of arterial pulses by palpation or Doppler does not exclude intracompartmental ischemia. Intracompartmental pressures exceeding 45 mmHg carry a high risk of ischemic necrosis. In these circumstances, fasciotomy may be justified, but it did not prove effective in saving envenomed muscle in experimental animals. Fasciotomy is contraindicated until blood coagulability has been restored (by adequate doses of antivenom followed by clotting factors) and must be justified by demonstration that the intracompartmental pressure is consistently raised (to less than 30 mmHg below mean arterial pressure). Early adequate antivenom treatment will prevent the development of intracompartmental syndromes in most cases.
Local Infection at the Site of the Bite
Local infections may result from unusual bacteria derived from the snake's venom or fangs or contamination of the wound from asterile incisions. A booster dose of tetanus toxoid should be given, but prophylactic antibiotics are not indicated unless the wound has been incised or tampered with in any way. If the wound is necrotic, Clostridium tetani should be eliminated with a large dose of benzyl penicillin or metronidazole and an aminoglycoside, such as gentamicin, given for 48 h. If a local abscess develops, it should be drained and the patient given a course of antibiotics, such as penicillin, chloramphenicol or erythromycin, which are usually available and affordable.
Acute kidney injury
Some snake bite victims admitted with oliguria and elevated blood urea nitrogen and creatinine levels are simply hypovolemic. Urine output, serum creatinine, urea and electrolytes should be measured each day in patients with severe envenoming and in those bitten by species known to cause acute kidney injury. If urine output drops below 400 ml in 24 h, urethral and central venous catheters should be inserted. If urine flow fails to increase after cautious rehydration, diuretics should be tried (e.g. furosemide by slow intravenous injection, 100 mg followed by 200 mg) and then mannitol. Dopamine (2.5 µg/kg per min by IV infusion) has proved effective in some cases. If these measures are ineffective, the patient should be placed on strict fluid balance. Peritoneal or hemodialysis will usually be required.
Other drugs
Heparin, antifibrinolytic agents (aprotinin, ε-aminocaproic acid), corticosteroids, antihistamines and a variety of herbal and other remedies have been advocated for treatment of snakebite. None has proved effective and many are harmful.
Treatment of snake venom ophthalmia [7]
When cobra venom is “spat” into the eyes, first-aid consists of irrigation with generous volumes of water or any other bland liquid, such as milk—or even urine, which may be available. Unless a corneal abrasion can be excluded by fluorescein staining or slit-lamp examination, treatment should be the same as for any corneal injury; a topical antimicrobial such as tetracycline or chloramphenicol should be applied. Instillation of antivenom is not recommended. Epinephrine (0.1%) or local anesthetic eye drops relieve the pain.
References
- 1.World Health Organization WHO guidelines for the production, control and regulation of snake antivenom immunoglobulins. http://apps.who.int/bloodproducts/snakeantivenoms/database/ Available at. (last accessed 28 December 2011) [DOI] [PubMed]
- 2.Warrell DA. Snake bite. Lancet. 2010;376:77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- 3.Warrell DA. Epidemiology, clinical features and management of snakebites in Central and South America. In: Campbell J, Lamar WW, Greene H, editors. Venomous Reptiles of the Americas. Cornell University Press; Ithaca: 2004. pp. 709–761. [Google Scholar]
- 4.Meier J, White J, editors. Handbook of Clinical Toxicology of Animal Venoms and Poisons. CRC Press; Boca Raton: 1995. [Google Scholar]
- 5.Reid HA, Thean PC, Chan KE, Baharou AR. Clinical effects of bites by Malayan viper (Ancistrodon rhodostoma) Lancet. 1963;i:617–621. doi: 10.1016/s0140-6736(63)91268-6. [DOI] [PubMed] [Google Scholar]
- 6.Gopalakrishnakone P, editor. Sea snake Toxinology. Singapore University Press; Singapore: 1994. pp. 1–36. [Google Scholar]
- 7.Chu ER, Weinstein SA, White J, Warrell DA. Venom ophthalmia caused by venoms of spitting elapid and other snakes: report of nine cases with review of epidemiology, clinical features, pathophysiology and management. Toxicon. 2010;56:259–272. doi: 10.1016/j.toxicon.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland SK, Tibballs J. 2nd edn. Oxford University Press; Melbourne: 2001. Australian Animal Toxins. The Creatures, Their Toxins and Care of The Poisoned Patient. [Google Scholar]
- 9.Warrell DA. Treatment of bites by adders and exotic venomous snakes. BMJ. 2005;331:1244–1247. doi: 10.1136/bmj.331.7527.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warrell DA. Bites by venomous snakes outside The Americas. In: Auerbach PA, editor. Wilderness Medicine. 5th edn. Elsevier; Philadelphia: 2007. pp. 1086–1123. [Google Scholar]
- 11.World Health Organization WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en/ Available at. (last accessed 28 December 2011)
- 12.de Silva HA, Pathmeswaran A, Ranasinha CD. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000435. e1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Useful Websites
- Snake bites in South and Southeast Asia http://www.searo.who.int/EN/Section10/Section17.htm (last accessed 28 December 2011)
- Snake bites in Africa http://www.afro.who.int/en/clusters-a-programmes/hss/essential-medicines/highlights/2731-guidelines-for-the-prevention-and-clinical-management-of-snakebite-in-africa.html
- Envenoming worldwide http://www.toxinology.com/ (last accessed 28 December 2011)
- Antivenoms http://www.toxinfo.org/antivenoms/ (last accessed 28 December 2011)
134.6. Bats
Key features.
-
•
Bats are increasingly recognized vectors and reservoirs of zoonotic infections
-
•
Lyssavirus infections transmissible to humans by bats include Genotypes 1 (classic rabies), 4 (Duvenhage), 5 and 6 (European bat lyssaviruses), and 7 (Australian bat lyssavirus)
-
•
Vampire bats (Desmodontinae) transmit classic rabies to humans and domestic animals in Latin America
-
•
Insectivorous and frugivorous bats are vectors or reservoirs of Lyssaviruses Genotypes 1, 4, 5, 6 and 7, Filoviruses (Ebola and Marburg), Henipaviruses (Hendra and Nipah) and some other viruses, bacteria and fungi
-
•
Bat-transmitted rabies infections can be prevented by vaccination
-
•
Vampire bat rabies can be controlled by vaccinating the bats or killing them with anticoagulants
Introduction 1, 2
The medical importance of bats (Order Chiroptera) to human health is increasingly recognized. They are proven, or potential, reservoirs of zoonotic human pathogens and vectors. Transmission may be direct, by bat bites, scratches or more subtle contact; or indirect by infecting other mammalian hosts or by creating in their accumulated feces (bat guano)—a culture medium for pathogenic fungi whose spores may be inhaled by people entering bat caves. Bats may damage fruit crops, and cattle and other domestic animals are vulnerable to bat-transmitted infections. In Latin America, vampire bat-transmitted bovine rabies (“derriengue”) and trypanosomiasis (Trypanosoma evansi infection causing “surra”) threatens meat production. Nipah virus causes fatal disease in pigs in Southeast Asia where epidemics have led to the culling of more than 1 million of these animals. Hendra, Menangle and Tioman viruses have been responsible for fatal diseases in horses and pigs in Australia and Malaysia. However, bats are much less important, medically and economically, than rodents. Rats, mice and voles are vectors and reservoirs of many prevalent zoonotic pathogens, such as Hanta- and Arena- viruses, plague bacillus, leptospires, rickettsiae and parastrongyloids. Rodents also consume crops and food stores and damage human dwellings and installations. In their favor, bats are fascinating and beautiful animals which are protected and conserved in many Western countries and which benefit the environment by controlling insects, pollinating fruit trees and distributing seeds. Bats are eaten medicinally in China and elsewhere and as a delicacy and valuable source of protein in West Africa, Southeast Asia, Indonesia, New Guinea and Australia (by native Australians). Catching and butchering bats and consuming inadequately cooked bats incurs the risk of infection.
Bat Biology
Bats have a number of special characteristics, some of which are unique. They show extreme diversity, constituting 20% of all mammalian species, and they are widely distributed in all continents except Antarctica and some oceanic islands. Some species breed within the Arctic circle. Bats are enormously abundant, sometimes occuring in colonies, flocks or clouds composed of as many as 20–30 million individual animals. In caves inhabited by Mexican free-tailed bats (Tadarida brasiliensis), there may be more than 3000 bats/m3 in their cave roosts. Bats are the only mammals capable of flying, as opposed to gliding. Some species migrate more than 1500 kilometers and North American hoary bats (Lasiurus cinereus—Vespertilionidae) have reached Iceland and the Orkney Islands (UK). The wing membrane is formed by the skin of the back and belly, supported by elongated fingers, externally rotated and adducted legs and, in some cases, the tail. Some bats, such as true vampires, are also capable of quadrupedal gait. Most bats are nocturnal. They are the only vertebrates capable of catching insects in complete darkness, achieved by the use of sophisticated echolocation. Bats are variously insectivorous, frugivorous, flower-feeding, hematophagous or carnivorous. Their weight ranges from 2 g (one of the tiniest vertebrates) to 1.2 kg. Metabolic flexibility allows them to be heterothermic (extreme variation in body core temperature 2–41°C) and to hibernate. They are the longest-living of tiny mammals (e.g. Myotis lucifugus, weighing only 7 g, has lived to 35 years). Bats roost, hanging upside down from their feet or clinging onto surfaces, in human constructions such as roof spaces, eaves and attics of dwellings (bringing them into close contact with people), tombs, temples, mines, pipes, irrigation tunnels and bridges and in caves, rock crevices, foliage, tree cavities, trees and bird and termite nests. Some species can fashion tent-like shelters out of leaves. Recently, an epidemic of white-nosed syndrome has killed more than a million bats in the north-east USA. A cold-growing fungus, Geomyces destructans, has been implicated (“WNS fungus”), but this may not be the primary cause. Bats are infested with numerous ectoparasites, including bat flies, fleas, mites, ticks and Chimex bugs.
Medically importance species are members of both suborders: Megachiroptera [flying foxes, fruit bats and rousettes (Pteropodidae)] (FIGURE 134.6.1, FIGURE 134.6.2 ) and Microchiroptera, which include sheath-tailed bats (Emballonuridae), free-tailed bats (Molossidae, e.g. the Mexican free-tailed bat Tadarida brasileinsis mexicana) (Fig. 134.6.3 ), moustache bats (Mormoopidae), slit-faced bats (Nycteridae), New World leaf-nosed bats (Phyllostomidae) (Fig. 134.6.4 )—containing the true vampire bats (sub-family Desmodontinae) (FIGURE 134.6.5, FIGURE 134.6.6, FIGURE 134.6.7 )—horseshoe bats (Rhinolophidae) and evening bats (Vespertilionidae).
FIGURE 134.6.1.

Indian fruit bats (Pteropus giganteus—Pteropodidae) roosting at Mawanella, Sri Lanka
(© David A. Warrell).
FIGURE 134.6.2.
Grey headed fruit bat/flying fox (Pteropus poliocephalus—Pteropodidae), as depicted by John Gould.
FIGURE 134.6.3.

Mexican free-tailed bat (Tadarida brasiliensis—Molossidea) in Peru
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.4.

Seba’a short-tailed bat (Carollia perspicillata—Phyllostomidae)
(© David A. Warrell).
FIGURE 134.6.5.

Common vampire bat (Desmodus robustus—Desmodontina) flying in a cave roost in Peru
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.6.

Common vampire bat (Desmodus robustus—Desmodontina) showing cleft in lower lip for sucking up the blood meal
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.7.

Common vampire bat (Desmodus robustus—Desmodontina) showing the enlarged thumb enabling quadrupedal gait
(© Dr Ivan Vargas Meneses).
Bat-Transmitted Infections (Table 134.6-1)
TABLE 134.6-1.
Viruses, bacteria, fungi and parasites that have been isolated from bats and are capable of causing human disease.
| Human pathogen | Bat vector/reservoir | Geographical area |
|---|---|---|
| Viruses | ||
| Orthoreoviruses | ||
| Melaka* Pulau, Kampar | Pteropodidae | Malaysia |
| Orthobunyaviruses | ||
| Kaeng Khoi | Molossidae | Thailand, Cambodia |
| Phleboviruses | ||
| Toscana | Vespertilionidae | Mediterranean |
| Coronaviruses | Pteropodidae, Rhinolophidae, Phyllostomidae, Vespertilionidae | China, Brazil |
| Filoviruses | ||
| Marburg, Ebola* | Pteropodidae, Rhinopholidae | Uganda, Gabon, Democratic Republic of Congo |
| Flaviviruses | ||
| Dengue Ilheus Japanese encephalitis Kyasanur Forest Rio Bravo virus St Louis encephalitis West Nile Yellow fever |
Pteropodidae, Phyllostomidae Mormoopidae, Phyllostomidae Rhinolophidae, Vespertilionidae Pteropodidae, Rhinolophidae Molossidae Molossidae, Mormoopidae, Phyllostomidae, Natalidae Pteropodidae, Vepertilionidae Molossidae, Mormoopidae |
Costa Rica, Ecuador, south China Latin America Japan, Taiwan India USA, Mexico, Trinidad, Guatemala, Brazil USA, Guatemala, Trinidad India, USA Ethiopia |
| Influenza viruses | Pteropodidae, Megadermatidae, Vespertilionidae | Kazakhstan |
| Paramyxoviruses | Pteropodidae | |
| Hendra* | Australia | |
| Nipah* | Southeast Asia | |
| Menangle* | Australia | |
| Lyssaviruses | ||
| Genotype I Classic rabies* Genotype 4 Duvenhage virus* Genotype 5 European bat lyssavirus-1* Genotype 6 European bat lyssavirus-2* Genotype 7 Australian bat lyssavirus* |
Ptropodidae, Molossidae, Phyllostomidae, Vespertilionidae Vespertilionidae Pteropodidae, Molossidae, Rhinolophidae Vestpertilionidae Pteropodidae, Emballonuridae |
India, Americas South Africa, Kenya Europe Europe Australia |
| Alphaviruses | ||
| Chikungunya Eastern equine encephalitis Venezuelan equine encephalomyelitis Western equine encephalitis |
Vespertilionidae Emballonuridae, Phyllostomidae PhyllostomidaePhyllostomidae, Vespertilionidae |
Senegal Latin America Mexico, Guatemala, Ecuador, Panama, USA, Panama, USA, Colombia |
| Bacteria | ||
|
Borrelia johnsonii Leptospira Brucella |
Vespertilionidae (Eptesicus fuscus) Phyllostomidae, Pteropodidae Desmodus rotundus |
Iowa, USA (in Carios kelleyi bat ticks) Brazil, Peru, Trinidad, Australia Brazil |
| Fungi | ||
| Histoplasma capsulatum |
Mormoopidae, Vespertilionidae, Natilidae Phyllostomidae |
Panama, Mexico, Colombia, Trinidad, USA |
| Scopulariopsis, Geomyces, Trichophyton, Aphanoascus, Myceliophthora, Chrysosporium spp. | Rhinolophidae, Vespertilionidae | Mexico, Colombia, France |
Evidence of direct or indirect bat to human transmission is documented in cases marked *, but varies with the other pathogens
Rabies and Rabies-Related Lyssaviruses
See Chapter 34.1 (Rabies) 3, 4
Genotype 1 (classic) rabies
In the Americas, this virus has been found in bats of the families Molossidae, Phyllostomidae (both in true vampire bats Desmodontinae and in non-vampire bats, such as Seba's short-tailed leaf-nosed bat (Carollia perspicillata; Fig. 134.6.4)] and Vespertilionidae. It has been transmitted to humans by members of these families. In India, Genotype 1 was found in Pteropodidae, but transmission to humans has not yet been documented; in China, a patient died of bat-transmitted rabies in Jilin Province in 2002 (bat species and virus type unknown).
Vampire bat rabies (Latin America) 5, 6
Vampire biology
The three species of true vampire bats (Desmodontinae) must be distinguished from non-hematophagous species that have been named “vampires”, such as the spectral vampire bat (Vampyrum spectrum—Phyllostomidae)—the largest bat of the New World—and the false vampire bats (Megadermatidae) of Africa, Asia and Australia. The common vampire (Desmodus rotundus) (FIGURE 134.6.5, FIGURE 134.6.6, FIGURE 134.6.7), hairy-legged vampire (Diphylla ecaudata) and white-winged vampire (Diaemus youngi) are confined to Central and South America (Fig. 134.6.8 ). Vampire bats feed at night on the blood of vertebrates (FIGURE 134.6.9, FIGURE 134.6.10 ): mammals, birds and reptiles. Diaemus and Diphylla feed largely on birds, while Desmodus prefers mammals, especially large domestic herbivores. Self-sharpening incisor and canine teeth are modified to allow painless venesection. The vampire's saliva flows down a groove on the dorsal surface of its tongue into the wound. Salivary toxins increase capillary permeability, inhibit platelet aggregation, inhibit activated factors X and IX (“draculin”) and activate plasminogen (“desmoteplase”—currently being developed as a thrombolytic drug, DSPA-alpha-1).
FIGURE 134.6.8.

World distribution of vampire bats
(© David A. Warrell).
FIGURE 134.6.9.

Bite sites of vampire bats on a cow in Peru
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.10.
Bite sites of vampire bats on a chicken in Peru
(© Dr Ivan Vargas Meneses).
These anti-hemostatic factors ensure blood flow from the wound that the bat sucks up through a cleft in its protruded lower lip (Fig. 134.6.6), underneath its tongue along paired ventral grooves. Predation is facilitated by vampires’ ability to clamber, hop and spring using their hind legs and enlarged thumb (Fig. 134.6.7). Vampires can regurgitate blood when they return to the roost, feeding not only their young and relatives, but also other members of the colony—altruistic behavior that is unique among animals. Vampires roost in jungle caves (Fig. 134.6.11 ), hollow trees and in man-made tunnels and drains (Fig. 134.6.12 ).
FIGURE 134.6.11.

Natural cave roost for vampire bats in Peru
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.12.

Irrigation drain used as a roost for vampire bats in Peru
(© Dr Ivan Vargas Meneses).
Rabies epizootics
Rabies (classic genotype 1) is enzootic in many, but not all, vampire bat populations. Since the late 14th century, European explorers of the Caribbean and South America reported the association between vampire bat attacks and fatal human illness. Ecologic changes, such as destruction of forest and introduction of cattle ranching, construction of trans-Amazonian highways, the invasion of newly-accessible jungle by gold miners and electric power failures, have led to small epidemics of human rabies transmitted by vampires. These have accounted for more than 500 deaths since 1975, usually affecting rural communities of indigenous Amerindians, such as the Warao in north-east Venezuela. There have been recent outbreaks in Colombia (Chocó, Santander), Peru (Condorcanqui, Bagna, Puerto Maldanado, Ayacucho), Brazil (Para, Maranhão) and Venezuela (Delta Amacuro). Humans are bitten at night on their extremities, ears and faces. Children appear particularly vulnerable. They may not wake up but discover bleeding wounds showing a double puncture made by the incisors (Fig. 134.6.12) or a depressed lesion with raised edge (FIGURE 134.6.13, FIGURE 134.6.14 ) in the morning. Mosquito nets are normally protective but accessible body parts may be attacked and the vampire's melena stool may be found staining the net (Fig. 134.6.15 ). Historical epidemics of vampire bat rabies, such as occurred in Trinidad from 1929, were characterized by predominantly paralytic disease, but a minority of patients develop hydrophobia and other features of furious rabies. Vampire bat-related strains of classic Genotype 1 rabies virus have been found in many South American wild and domestic mammals and have been transmitted to humans, for example by pet marmosets in Brazil.
FIGURE 134.6.13.
Bite inflicted by a vampire bat in Puerto Maldonado, Peru
(© Dr Ivan Vargas Meneses).
FIGURE 134.6.14.

Bite inflicted by a vampire bat in Tapirai, São Paulo State, Brazil
(© João Luiz Costa Cardoso).
FIGURE 134.6.15.

Mosquito net through which a man was bitten by a vampire in Puerto Maldonado, Peru. Note the bat's melena stool staining the net
(© Dr Ivan Vargas Meneses).
Annually, bovine rabies of vampire bat origin (“derriengue”) kills 30,000 cattle in Brazil and 100,000 cattle in the whole of South America, with considerable economic loss. “Derriengue” means limping illness, emphasizing its paralytic nature.
Prevention and control of vampire bat rabies [6]
People, especially children, living in areas where vampire bat bites are common should, ideally, be protected by rabies pre-exposure immunization, but this is rarely practicable. However, the high risk of fatal rabies in affected communities (e.g. 16 of 57 people bitten by vampires in one village in north-east Brazil) reflects the inaccessibility of post-exposure prophylaxis. Bat proofing of dwellings and sleeping under mosquito nets affords some protection and electric house lighting may deter vampires. Vampires are highly susceptible to anticoagulants, such as warfarin, which can be distributed to vampire colonies by capturing the bats and releasing them after plastering them with warfarin paste. Treatment of cattle with controlled doses of warfarin will kill vampires feeding on them. Cattle can be vaccinated using adjuvanted Pasteur Virus strain (PV) vaccine and vampires themselves with recombinant vaccinia-vaccine.
Non-vampire bat rabies
In the USA, 30 of the 40 cases of human rabies reported between 1995 and 2008 were attributed to bat strains of Genotype 1 rabies virus. Fifteen were associated with Lasionycteris noctivagans or Pipistrellus subflavus, 11 with Tadarida brasiliensis, and 1 each with Myotis spp. and Eptesicus fuscus. However, there was a clear history of a bat bite in only a few of these cases, although some had been in direct contact with bats and others had bats in their homes. This suggests the possibility of subtle contact such as the bat's licking an open wound or intact mucous membranes of a sleeping person 7, 8. Bat strains of Genotype 1 appear more tolerant of low temperatures and are more able to replicate in endothelial cells than canine strains of Genotype 1. The survival of three American children from bat-transmitted rabies may also suggest decreased virulence of bat strains. Aerosol transmission of rabies was claimed to explain infection of two visitors to Frio Caves in Texas inhabited by 20–30 million T. brasilensis, but it is far more likely that they were infected transdermally.
In Asia, evidence of serotype 1 infection was found in insectivorous and frugivorous bats in Cambodia and China.
Genotype 2 Lagos Bat Virus (LGV)
Lyssavirus Genotype 2 is grouped together with Genotype 3 Mokola virus (not found in bats) in Phylogroup II. Although human disease has not been reported, direct or serologic evidence of Lagos bat virus (LGV) infection has been found in several species of fruit bats (Pteropodidae) in Africa: the very widely distributed straw-coloured fruit bat (Eidolon helvum) that is often eaten as “bush meat”, epauletted fruit bats (Epomophorsu, Epomops and Micropteropus spp.), Egyptian rousettes (Rousettus aegyptiacus); and in vespertilionid species: long-winged bats (Miniopterus spp.) and Gambian slit-faced bats (Nycteris gambiensis). A new LGV-like lyssavirus (Shomoni virus) has been described in a Commerson's leaf-nosed bat (Hipposideros commersoni) in coastal Kenya. Seroprevalence of LGV is 30–70% in R. aegyptiacus and E. helvum. Serologic evidence of LGV-like infection was found in insectivorous and frugivorous bats in Cambodia.
Genotype 4 Duvenhage virus
Lyssavirus Genotype 4 has been found in insectivorous and frugivorous bats, such as the Egyptian slit-faced bat (Nycteris thebaica—Nycteridae), the common bent-wing bat (Miniopterus schreibersii) and other vespertilionid species in east and southern Africa. The three identified human cases in South Africa and Kenya showed clinical features indistinguishable from classic rabies encephalomyelitis.
Genotype 5 and 6 European Bat Lyssavirus (EBLV)
This genotype is the only zoonotic virus in European bats. European bat lyssaviurs (EBLV)-1 infection has been found in sorentine bats (Eptesicus sorentinus) and R. aegyptiacus and EBLV-2 in Daubenton's bats (Myotis daubentonii), pond bats (Myotis dasycneme) and other Myotis spp. and greater horseshoe bats (Rhinolophus ferrumequinum).
Genotype 7 Australian Bat Lyssavirus (ABLV)
Genotype 7 Australian bat lyssavirus (ABLV) was first isolated from a black flying fox (Pteropus alecto) in New South Wales in 1996 and subsequently from three other species of flying fox (Pteropus poliocephalus, Pteropus scapulatus, Pteropus conspicillatus) and one species of insectivorous bat, the yellow-bellied sheath tailed bat (Saccolaimus flaviventris—Emballonuridae). Two Queensland women died of diseases indistinguishable from classic rabies, one five weeks after scratches by a S. flaviventris and the other more than two years after bites by a flying fox. In the Philippines, ABLV has been found in Lyle's flying foxes (Pteropis lylei) obtained from restaurants, and in Miniopterus schreibersii and great round-leafed bats (Hipposideros armiger) in Cambodia and Thailand.
Other rabies-related viruses
Aravan (Kyrghistan), Khujand (Tajikistan), Irkut (Siberia) and West Caucasian bat lyssaviruses (Phylogroup I) have not yet been classified. They have been isolated in one African and one Eurasian bat. Serologic evidence of West Caucasian bat virus was found in long-winged bats (Miniopterus spp.—Vespertilionidae) in Kenya and of Aravan and Khujand bat viruses in Indian fruit bats (Pteropus giganteus—Pteropodidae) in Bangladesh, Irkut and in Thailand (Pteropus lylei). No transmission to humans has been described.
Filoviruses: Marburg virus and Ebola virus 9, 10
The environmental reservoirs and vectors of these deadly hemorrhagic fevers have long been debated, but, in Uganda, serologic and PCR evidence of Ebola and Marburg virus infections has been found in Egyptian fruit bats or rousettes (R. aegyptiacus), suggesting that this species may be a natural host of both viruses. Evidence of Lake Victoria Marburg virus has been found in R. aegyptiacus in Kitum Cave, Mount Elgon, Kenya, and Python and Kitaka Caves in Uganda—sites where humans have been infected. Touching bat feces or being hit by low-flying bats were identified as possible risk factors for acquisition of the infection. Insectivorous bats, such as greater long-fingered bats Miniopterus inflatus (Vespertilionidae), and horseshoe bats (Rhinolophus elocuens) and round leaf bats (Hipposideros spp. Rhinolophidae) have also been implicated in Gabon, Democratic Republic of Congo and Uganda. In Democratic Republic of Congo, hunting of bats, such as migratory hammer-headed or big-lipped bats (Hypsignathus monstrosus Pteropodidae) for human consumption was linked to the 2007 outbreak of Ebola virus. In the Philippines, Ebola virus Reston has been found in domestic pigs. Several asymptomatic human infections were reported.
Paramyxoviruses (Nipah, Hendra, Menangle) [11]
Three emerging paramyxovirus infections have been described for which bats are the likely natural reservoirs and domestic horses and pigs have proved to be the amplifying vectors for human infection. Hendra and Nipah viruses are Henipaviruses; Menangle is a rubulavirus.
Hendra virus [12]
In 1994, there was an outbreak of fatal respiratory disease in horses and humans in Australia, attributed to a new pathogen, Hendra virus, whose natural reservoir is Pteropus spp. (P. alecto, P. poliocephalus, P. scapulatus, P. conspicillatus). There have been 13 subsequent outbreaks. In 2008, 2009 and 2011, equine and human victims showed primarily encephalitic features. Among six human cases, three died.
Nipah virus [11]
In 1998, there was an epidemic of encephalitis in Malaysia and Singapore affecting pigs and pig-handlers in whom the case fatality was more than 40%. The causative virus, named Nipah after an affected village, is closely related to Hendra virus. Pteropus vampyrus and Pteropus hypomelanus are the natural reservoirs. There were outbreaks in Bangladesh in 2001, 2003 and 2004 (case fatality >74%) and in adjacent West Bengal in 2001. So far, more than 440 human cases of Nipah virus encephalitis have been diagnosed. The epidemics have been attributed to disruption of Pteropus ecology by deforestation (e.g. building the new Kuala Lumpur airport), which displaced the bats from their traditional roosts to agricultural areas where they have contact with domestic animals and humans. In Bangladesh, where there have been 130 deaths, human-to-human transmission within families has been inferred.
Menangle and Tioman viruses have been isolated from Pteropus spp. in Australia and Malaysia, and from sick pigs. Influenza-like illness in pig farmers with Menangle sero-conversion has been reported.
Other Viruses
Many of the other viruses that have been isolated from bats have not been proved to be either transmissible to humans, directly or indirectly, or to cause human disease. These include severe acute respiratory syndrome (SARS) viruses [13], the Arenavirus Tacaribe virus, Orthoreoviruse Nelson Bay virus [14], Mojuí dos Campos, Bimiti, Catu, Guama, Manzanilla, Nepuyo, Oriboca, Montana myotis leukoencephalitis, Tamana bat, Nepuyo, Catu, Mount Elgon bat, Entebbe and Astroviruses.
Bacterial Infections
Leptospira and Brucella have both been reported to occur naturally in bats. Leptospira-infected bats are found in Asia and Europe, as well as the Americas. Brucella infection has been found in vampire bats in Brazil. Bats can be infected with enteric bacteria such as Samonella spp. and Escherichia coli, but transmission to humans has not been reported. Transmission of Hansen's disease (Mycobacterium leprosum) by vampire bats has been suspected but not proven.
Histoplasmosis
Bat guano provides a rich medium for the growth of Histoplasma capsulatum; environment in the bat roost fosters this growth. Humans exposed to dried guano have suffered massive infection and death by inhalation.
Other Fungi
Many other fungi have been found in association with bat roosts, including Candida and Scopulariopsis and Geomyces spp. (including White-Nosed Syndrome (WNS) fungus of bats see above), which can infect humans. The involvement of bats seems limited to the provision of a rich environment in the roost as a culture medium for these organisms.
Protozoa
Trypanosoma evansi, the causative agent of surra in domestic animals, has been demonstrated in vampire bats. These bats can mechanically transmit the trypanosome from host to host. Trypanosoma cruzi has been reported also, but evidence for transmission to humans is lacking.
Prevention of Bat-Transmitted Infections
For measures against vampire bat rabies see above. Ideally, bats and humans should not share the same microenvironment, but bats are rightly protected in many countries and so expert advice should be sought if they are found roosting in attics. An aggressive bat is likely to be unwell, perhaps rabid. All case of scratches, bites and possible mucosal contact should be reviewed for possible post-exposure rabies prophylaxis. Even the handling of a dead bat has been associated with transmission. Grounded, trapped or sick bats should, ideally, be handled only by experts and never without gloves. The risk of inhaling aerosolized fungal spores and acquiring other bat-related diseases must be recognized by cave explorers (spelunkers) and prevented by wearing appropriate clothing and masks.
References
- 1.Calisher CH, Childs JE, Field HE. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong S, Lau S, Woo P, Yuen KY. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElhinney LM, Marston DA, Stankov S. Molecular epidemiology of lyssaviruses in Eurasia. Dev Biol (Basel) 2008;131:125–131. [PubMed] [Google Scholar]
- 4.Banyard AC, Hayman D, Johnson N. Bats and lyssaviruses. Adv Virus Res. 2011;79:239–289. doi: 10.1016/B978-0-12-387040-7.00012-3. [DOI] [PubMed] [Google Scholar]
- 5.Greenhall AM, Schmidt U, editors. Natural History of Vampire Bats. CRC Press; Boca Raton: 1988. [Google Scholar]
- 6.Schneider MC, Romijn PC, Uieda W. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev Panam Salud Publica. 2009;25:260–269. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 7.Warrell MJ. Human deaths from cryptic bat rabies in the USA. Lancet. 1995;346:65. doi: 10.1016/s0140-6736(95)92106-0. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med. 2002;39:528–536. doi: 10.1067/mem.2002.121521. [DOI] [PubMed] [Google Scholar]
- 9.Pourrut X, Souris M, Towner JS. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzmin IV, Niezgoda M, Franka R. Marburg virus in fruit bat, Kenya. Emerg Infect Dis. 2010;16:352–354. doi: 10.3201/eid1602.091269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wild TF. Henipaviruses: a new family of emerging Paramyxoviruses. Pathol Biol (Paris) 2009;57:188–196. doi: 10.1016/j.patbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Field H, Schaaf K, Kung N. Hendra virus outbreak with novel clinical features, Australia. Emerg Infect Dis. 2010;16:338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LF, Shi Z, Zhang S. Review of bats and SARS. Emerg Infect Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua KB, Voon K, Crameri G. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003803. e3803. [DOI] [PMC free article] [PubMed] [Google Scholar]


