Abstract
Receptors for the Fc portion of immunoglobulins (FcRs) account for most cell-mediated biological activities of antibodies. The majority of FcRs are encoded by a set of genes, clustered in the fcr locus, on chromosome 1 in humans and on chromosome 1 and 3 in mice. Eight (in humans) and six (in mice) new genes were found, intermixed with FcR genes in corresponding fcr loci, which encode FcR-like molecules (FcRLs). FcRs and FcRLs are genetically, phylogenetically, structurally, and functionally related. FcRs and FcRLs, however, markedly differ by their ligands, their tissue distribution, and, therefore, by the biological functions they control. A systematic comparison of their biological properties leads to the conclusion that FcRLs are not like FcRs. They altogether form a single family within the immunoreceptor family, whose members fulfill distinct but complementary roles in immunity by differentially controlling innate and adaptive responses.
Keywords: Adaptive immunity, Antibodies, Cell activation, Fc receptors, Fc receptor-like molecules, Immunoglobulin superfamily, Immunoreceptors, Immunoreceptor tyrosine-based activation motifs, Immunoreceptor tyrosine-based inhibition motifs, Innate immunity, Lymphoid cells, Myeloid cells, Negative regulation, Positive regulation
From Fc Receptors to Fc Receptor-Like Molecules
The concept of receptors for the Fc portion of immunoglobulins arose in the 1960s to explain cell-mediated biological activities of antibodies. ‘Opsonins’ indeed enabled antigen to enter phagocytic cells (Berken and Benacerraf, 1966); ‘cytophilic’ antibodies sensitized tissues that released histamine upon antigen challenge (Bloch, 1967); distinct classes of antibodies differentially regulated secondary antibody responses (Henry and Jerne, 1968). These biological effects requiring the Fc portion of antibodies, the name ‘Fc receptor’ (FcR) was coined (Paraskevas et al., 1972). FcRs for various antibody classes were identified as binding sites on a variety of cells (Vaughan and Boyden, 1964; Kulczycki and Metzger, 1974; Unkeless et al., 1988). FcRs were characterized functionally and biochemically (Holowka et al., 1980; Ernst et al., 1993; Pfefferkorn and Yeaman, 1994). Murine and human cDNAs encoding FcRs were cloned, sequenced, and expressed by transfection (Ravetch and Kinet, 1991); corresponding genes were located on chromosomes and their exon/intron organization was elucidated (Qiu et al., 1990). The extracellular domains of FcRs were recognized as members of the immunoglobulin superfamily (IgSF) (Williams and Barclay, 1988); amino acid sequences enabling them to interact with antibodies, extracellularly (Hulett and Hogarth, 1994), and to signal, intracellularly (Daëron, 1997), were dissected; the 3D-structure of their extracellular domains in complex with immunoglobulin Fc portions was solved (Garman et al., 1998; Maxwell et al., 1999). Finally, a collection of genetically modified FcR knock out (KO), knock in (KI), and transgenic mice was generated that enabled FcR functions to be delineated in vivo (Smith et al., 2012). FcRs thus appeared as a family of functionally, structurally, and genetically related molecules that play major roles in antibody-dependent processes in physiology, in pathology, and, with the advent of passive immunotherapy, in therapeutics.
Genes encoding FcR-related molecules were unexpectedly discovered, clustered with human FCR genes (according to the usual typographic convention, protein names are in roman type, whereas gene names are in italics; names of human genes are in upper case, whereas names of murine genes are in lower case), in the early 1990s (Imboden et al., 1989; Seaman et al., 1991). Similar genes were found in the same clusters as mouse fcr genes (Figure 1 ). A whole family of putative Fc receptor-like molecules (FcRLs – the abbreviation ‘FcRL’ is used instead of ‘FCRL’ for consistency with ‘FcR’) thus emerged (Davis et al., 2001; Hatzivassiliou et al., 2001), whose existence was progressively confirmed (Li et al., 2014). As FcRLs originated from genetic studies, much less is known of their biological functions, compared to FcRs. A syntenic chromosomal linkage, a similar genetic organization, a common membership of the IgSF suggest that FcRLs may be functionally related to FcRs. Supporting this assumption, both FcRs and FcRLs possess immunoreceptor tyrosine-based activation motifs (ITAMs), like B cell and T cell receptors (BCR and TCR) for antigen (Reth, 1989), and/or immunoreceptor tyrosine-based inhibition motifs (ITIMs), like inhibitory receptors expressed by natural killer (NK) cells (Vivier and Daëron, 1997). FcRs and FcRLs therefore belong to the immunoreceptor family. Differences in their structure, ligands, and pattern of expression, however, indicate that FcRs and FcRLs play distinct, complementary roles.
Figure 1.
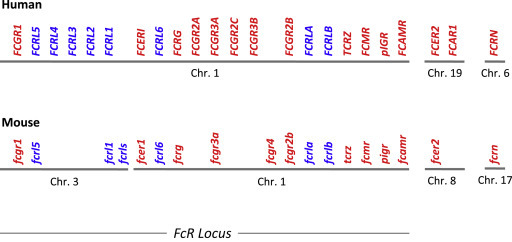
Human and murine Fc receptor (FcR) and Fc receptor-like molecule (FcRL) genes. Organization of the genes encoding FcRs (red) and FcRLs (blue) in humans and mice, on their respective chromosomes (Chr.) (Davis et al., 2002; Akula et al., 2014). The figure was not drawn at scale.
I will discuss the genetic and phylogenetic relationships between FcRs and FcRLs; the structure and biological properties of FcRs and FcRLs; the tissue distribution and biological functions of FcRs and FcRLs; the roles of FcRs and FcRLs in health and disease; and the biological significance of FcRs and FcRLs within the immunoreceptor family.
From Genes Encoding FcRLs to Genes Encoding FcRs
Human (h) FcRs comprise ‘classical FcRs,’ a receptor for IgA (FcαRI) (Pfefferkorn and Yeaman, 1994), an MHC-related receptor (FcRn) (Simister and Rees, 1985), and a lectin-like receptor (FcεRII) (Conrad, 1990). Genes that encode classical hFcRs are within the FCR locus on chromosome 1, whereas the FCAR1 gene is in the leukocyte receptor complex (LRC) locus on chromosome 19 (Akula et al., 2014). The LRC locus contains genes that encode the natural killer receptors (KIRs), the leukocyte Ig-like receptors (LILRs), and the leukocyte-associated Ig-like receptors (LAIRs), with which FcαRI shows a higher sequence homology than with classical FcRs. FCER2, the gene that encodes hFcεRII, is also located on chromosome 19. Noticeably, genes encoding signaling homodimers shared by FcRs, NK receptors, and T cell receptors also lie in the same two loci. Genes encoding FcRγ and TCRξ are in the FCR locus, whereas genes encoding DAP10 and DAP12 are in the LRC locus. FcRn stands for neonatal FcR because this IgG receptor was first observed in newborn mice. FcRn is related neither structurally nor genetically with classical FcRs. It is an MHC class I molecule encoded by a gene of the MHC complex on chromosome 6 (Ghetie and Ward, 2000).
Mice have no equivalent of hFcαRI. Indeed, mouse genes encoding KIR-like molecules moved from the lrc complex to chromosome X, and the fcarl gene is thought to have been lost during translocation (Woof and Kerr, 2006). Mouse (m) FcRs therefore comprise classical FcRs encoded by genes of the fcr locus, FcRn and FcεRII. The fcr locus, however, was split into two fragments. The gene encoding mouse high-affinity IgG receptors (mFcγRI) is on chromosome 3, while other classical fcr genes are on chromosome 1 (Akula et al., 2014). fcer2, the gene that encodes mFcεRII, is on chromosome 8 (Conrad et al., 1993). The gene that encodes mFcRn is among other MHC-I genes, on chromosome 17.
Genes that encode FcRLs are in the same loci as genes encoding classical FcRs in both species (Figure 1). All human FCRL genes are in the single human FCR locus on chromosome 1. Murine fcrl genes are distributed in the two murine fcr loci, on chromosomes 1 (fcrla and b) and 3 (fcrl1, fcrl5, fcrl6, and fcrls) (Davis et al., 2002; Akula et al., 2014).
Bioinformatic, genetic, and phylogenic analyses in mammals, birds, reptiles, amphibians, bony fishes, cartilaginous fishes, and lampreys unraveled that classical FcRs and FcRLs first appeared together and remained closely linked during evolution, as their complexity increased in parallel with that of immunoglobulins. Genes encoding the IgA/IgM poly-immunoglobulin receptor (pIgR), FcRLs, and FcRγ appeared first within the fcr locus, as well as genes homologous to mammalian genes of the LRC locus in early bony fishes. Noticeably, genes encoding FcRLs were the ancestors of genes encoding FcRs for IgG (FcγRI, II, III, and IV) and IgE (FcεRI), while duplicated sequences from the pigr gene provided sequences for genes encoding receptors for IgA/IgM (FcαμR) and for IgM (FcμR) during early mammalian evolution (Akula et al., 2014). The majority of classical FcRs therefore derive from FcRLs.
Structure and Biological Properties of FcRs and FcRLs
Most FcRs and FcRLs are transmembrane molecules that generate intracellular signals when engaged by extracellular ligands. These biological properties depend (1) on the structure of their extracellular domains and their interactions with extracellular ligands and (2) on the signaling motifs in their intracytoplasmic domains and their ability to transduce signals across the plasma membrane and to generate productive signalosomes. The properties of nontransmembrane FcRLs are not well characterized.
FcR and FcRL Structure
Some FcRs are single-chain immunoglobulin-binding molecules. These include IgG receptors (FcγRIIA, FcγRIIB, FcγRIIC, and FcγRIIIB), IgE receptors (FcεRII), IgM receptors (FcμR), and IgA/IgM receptors (pIgR and FcαμR). Other FcRs are multichain receptors. They include IgA (FcαRI), IgE (FcεRI), and IgG (FcγRI, FcγRIIIA, FcγRIV, and FcRn) receptors (Daëron, 2014). Multichain FcRs are composed of a specific immunoglobulin-binding subunit named FcRα and one or two common subunits. FcRγ is a disulfide-bonded homodimer shared by most multichain FcRs (Orloff et al., 1990). FcRβ is a tetraspanin that associates with multichain FcRs in mast cells and basophils (Kurosaki et al., 1992). Like other MHC-I molecules, FcRn associates with β-2 microglobulin (Israel et al., 1995). FcRγ and β-2 microglobulin are mandatory for the expression of multichain FcRs and FcRn, respectively. FcRβ is mandatory for the expression of FcεRI in mice. All FcRLs are single-chain receptors. They comprise six transmembrane molecules in humans (hFcRL1, 2, 3, 4, 5, 6) and three in mice (mFcRL1, 5, 6), two intracellular molecules (FcRLA and B) in both species, and one soluble molecule (mFcRLs) in mice (Li et al., 2014).
Except FcεRII whose extracellular domain is a C-type lectin, transmembrane FcRs and FcRLs have extracellular domains made of variable numbers of IgSF domains. Three receptors have IgSF domains of the V-type. There are five such domains in the pIgR and one in FcμR and FcαμR. Other mouse and human FcRs have IgSF domains of the C2-type. All have two such domains except FcγRI that has three. Human FcRL1 has three, FcRL2 and FcRL4 have four, FcRL3 has six, and FcRL5 has nine C2-IgSF domains. Mouse FcRL1 and FcRL6 have two and FcRL5 has five C2-IgSF domains. In both mice and humans, FcRLA and FcRLB also have IgSF domains, but these are intracellular, as well as a unique C-terminal mucin-like region (Li et al., 2014).
Most FcRs and FcRLs contain or are associated with subunits that contain tyrosine-based signaling motifs. In both humans and mice, one FcR only (FcγRIIB) contains an ITIM (Daëron et al., 1995a). Except three low-affinity IgG receptors that are unique to humans (FcγRIIA and FcγRIIC, which contain an ITAM in their own intracytoplasmic domain, and FcγRIIIB, which has no intracytoplasmic domain), most other human and murine FcRs are constitutively associated with the ITAM-containing FcRγ subunit. The FcRβ subunit also contains an ITAM. The more distant FcRs pIgR, FcμR, and FcαμR, as well as FcRn, have no known activation or inhibition motif. All transmembrane FcRLs contain ITIMs and/or ITAMs in their intracytoplasmic domain. Human and mouse FcRL1 contain two ITAMs, whereas hFcRL4 contains two ITIMs. Human and mouse FcRL6 contain one ITIM only. Human and mouse FcRL5, as well as hFcRL2, contain two ITIMs and one ITAM. hFcRL3 contains one ITAM and one ITIM (Akula et al., 2014; Li et al., 2014).
FcR and FcRL Ligands
An ability to bind immunoglobulins defines FcRs. Due to their structural and genetic parenthood with FcRs, FcRLs were expected to bind immunoglobulins too. Three FcRLs, hFcRL4, hFcRL5, and the intracellular hFcRLA, do but, in spite of extensive search, other FcRLs do not. Instead, mFcRL5 and hFcRL6 bind MHC molecules. The remaining FcRLs are orphan receptors.
Fc Receptors
The affinity with which antibodies bind to FcRs depends both on the receptors and ligands. The binding of antibodies to FcRs is reversible and it obeys the mass action law. It is characterized by an affinity constant (Ka), calculated by dividing the association constant by the dissociation constant. The affinity constant is a characteristic of FcRs. One distinguishes two classes of FcRs. High-affinity FcRs have a Ka between 107 and 1010 M− 1 (Kulczycki and Metzger, 1974; Unkeless and Eisen, 1975). They can bind immunoglobulins as monomers, that is, not in complex with antigen. Low-affinity FcRs have a Ka between 105 and 107 M− 1 (Bruhns et al., 2009). They cannot bind monomeric immunoglobulins. Both high- and low-affinity FcRs, however, bind immune complexes with a high avidity. A proportion of high-affinity FcRs are occupied in vivo, whereas low-affinity FcRs are not in spite of the high concentration of circulating immunoglobulins. They are therefore available for binding immune complexes. Occupied high-affinity FcRs, however, can be freed as bound antibodies dissociate (Mancardi et al., 2008). The dissociation constant therefore critically determines the availability of high-affinity FcRs.
High-affinity FcRs include IgA (FcαRI, in humans, and pIgR, in humans and mice), IgE (FcεRI, in humans and mice), and IgG receptors (FcγRI and FcRn, in humans and mice, and FcγRIV, in mice only). Low-affinity FcRs include IgE (FcεRII, in humans and mice) and IgG receptors (FcγRII and III, in humans and mice). Humans have three FcγRII (FcγRIIA, B, and C), and two FcγRIII (FcγRIIIA and B), whereas mice have one receptor of each type (FcγRIIB and FcγRIIIA) only. The diversity of hFcγRII and III is further increased by polymorphisms in their extracellular domains (H131R in hFcγRIIA (Warmerdam et al., 1990), F158V in hFcγRIIIA (Ravetch and Perussia, 1989), N65S, A78D, D82N, and V106I in FcγRIIIB (Ory et al., 1989)). Altogether, 10 hFcγRs were described.
FcRs are not isotype-specific. Antibodies of several isotypes can bind to one FcR. Vice versa, several FcRs can bind antibodies of one isotype. Thus, every FcγR can bind several subclasses of IgG, especially in humans where IgG1, IgG2, IgG3, and IgG4 bind similarly to hFcγRI; hFcγRIIA, B, and C; and hFcγRIIIA and B (Bruhns et al., 2009). Also, mouse IgE can bind to mFcγRIIB and mFcγRIIIA (Takizawa et al., 1992) and to FcγRIV (Mancardi et al., 2008).
The ability of immunoglobulins to bind to FcRs also depends on the glycosylation of their Fc portion (Arnold et al., 2007). Each heavy chain contains a covalently attached N-glycan at the highly conserved N297 residue in its CH2 domain. Point mutations of this glycosylation site abrogate the ability of IgG antibodies to bind to FcγRs, but not to FcRn (Veri et al., 2007). Other mutations that remove fucose residues from the glycan chain enhance the binding of antibodies to FcγRIIIA (Natsume et al., 2005; Niwa et al., 2005). Recently, the Fc portion of immunoglobulins was found to oscillate between a ‘closed’ and an ‘open’ conformation which also determines their affinity for FcRs (Ahmed et al., 2014). Thus, when having a closed conformation, IgE bind preferentially to FcεRI, whereas when having a closed conformation, they bind preferentially to FcεRII (Pincetic et al., 2014).
FcR-Like Molecules
The majority of FcRLs have no known ligand. These include hFcRLl, hFcRL2, hFcRL3, hFcRLB, mFcRLl, mFcRL6, mFcRLA, and mFcRLB. The two types of ligands identified are immunoglobulins and MHC molecules. Only hFcRLs were found to have an affinity for immunoglobulins. hFcRL4 binds heat-aggregated IgA, and hFcRL5 binds IgG of the different subclasses (Li et al., 2014). Noticeably, IgG binds to hFcRL5 and to hFcγRs by different mechanisms. Binding indeed requires not only the Fc portion, but also the F(ab')2 moiety of intact IgG through two independent binding events. Like binding to FcRs, binding to hFcRL5 requires glycosylated IgG (Franco et al., 2013). Although intracellular, hFcRLA was also reported to have an affinity for IgA, IgM, and IgG. One human and one murine FcRL interact with MHC molecules. hFcRL6 has an affinity for MHC class II molecules and this affinity varies with the MHC-II haplotype (Schreeder et al., 2010). mFcRL5 has an affinity for an MHC-related viral protein. This MHC class I–like molecule encoded by the cowpox virus also binds to NKG2D on NK cells (Campbell et al., 2010).
FcR and FcRL Signaling
FcRs trigger signals when aggregated on cell membranes by antibodies and plurivalent antigens (Maeyama et al., 1986; Metzger, 1992). Although the result is the same, the sequence of events leading to receptor aggregation is different for high-affinity and low-affinity FcRs. Monomeric antibodies bind first to high-affinity FcRs that are aggregated later, when a plurivalent antigen binds to receptor-bound antibodies. Antibodies bind first to antigen, generating immune complexes that can bind to and, therefore, simultaneously aggregate low-affinity FcRs. FcRL signaling is not well documented, due to the paucity of natural ligands known. It was mostly investigated using anti-FcRL antibodies expected to mimic FcRL natural ligands, sometimes on FCRLs expressed by transfection in a murine B cell line.
Fc Receptors
FcRs can trigger activation signals and/or inhibition signals. The nature of signals primarily depends on molecular motifs contained in the intracytoplasmic domains of FcRs or of receptor subunits with which FcRs associate. ITAMs consist of two YxxL motifs separated by a 6-8 variable amino acid sequence (Reth, 1989). ITIMs consist of a single YxxL motif preceded by a loosely conserved often hydrophobic residue at position Y-2 (Vivier and Daëron, 1997). Internalization motifs enable FcRn and pIgR to transcytose IgG and/or IgA across polarized cells.
Activating FcRs are FcαRI, FcεRI, FcγRI, FcγRIIA, FcγRIIC, FcγRIIIA, and FcγRIV. Upon receptor aggregation, ITAMs are phosphorylated by src family tyrosine kinases (Pribluda et al., 1994), which initiates the constitution of dynamic intracellular signalosomes (Kent et al., 1994). Not only activation signals are generated by activating FcRs, however. These, indeed, generate a mixture of positive and negative signals (Malbec et al., 2004), the dominant effect of which is activation under physiological conditions. Under other conditions, though, such as an excess of antigen that leads to a hyperaggregation of FcRs, negative signals overcome positive signals and, paradoxically, activating FcRs prevent cell activation (Gimborn et al., 2005).
Inhibitory FcRs are FcγRIIB (Daëron, 1997; Ravetch and Bolland, 2001). FcγRIIB generates inhibition signals only. Their inhibitory properties depend on the ITIM present in all murine and human FcγRIIB isoforms (Daëron et al., 1995a). Unlike activating receptors, FcγRIIB does not signal upon aggregation. They trigger negative signals when they are coaggregated with activating receptors by immune complexes (Daëron et al., 1995b). Under these conditions, the ITIM of FcγRIIB is phosphorylated by the same src family tyrosine kinase that phosphorylates ITAMs in activating receptors (Malbec et al., 1998). Phosphorylated FcγRIIB recruits inhibitory molecules that are brought into signalosomes. This renders inhibition signals dominant over activation signals (Lesourne et al., 2005; Daëron and Lesourne, 2006).
The aggregation of identical FcRs only (homoaggregation) is a rare situation. Different FcRs are coaggregated when IgG immune complexes interact with cells that coexpress different FcγRs or when pluri-isotypic immune complexes bind to cells that coexpress FcRs for several classes of antibodies. Even when cells express one type of FcR only (e.g., FcγRIIB in murine B cells or FcγRIIIA in murine NK cells), immune complexes can coengage FcRs with other immunoreceptors (BCR in B cells or NKR on NK cells). Heteroaggregation, that is, the coaggregation of different types of FcR or the coaggregation of FcRs with other immunoreceptors, is actually a rule, rather than an exception, under physiological conditions. Because there are FcRs for all antibody classes, because immune complexes contain more than one class of antibody, and because most cells express more than one type of FcR, various combinations of FcRs can be engaged at the cell surface to form heteroaggregates with a nonpredetermined composition. FcRs can thus generate a variety of signaling complexes, depending on the relative proportion of ITAM-containing and ITIM-containing receptors that are coengaged by immune complexes on any given cell (Daëron, 2014).
FcR-Like Molecules
Using specific antibodies that mimic FcRL ligands, FcRL signaling was found to obey similar rules as immunoreceptor signaling (Ehrhardt and Cooper, 2011). The engagement of hFcRL1 or mFcRL1, which contains two ITAMs, generates activation signals. Like the BCR and the TCR, but unlike FcRs, FcRLs trigger both activation and proliferation signals. The engagement of the two-ITIM- and one-ITAM-containing hFcRL2, hFcRL5, and mFcRL5 generates a mixture of effects, the dominant effect of which is inhibition. Although it contains both activation and inhibition motifs, hFcRL5 does not signal upon aggregation. It requires to be coengaged with activating receptors for triggering negative signals. When hFcRL5 is coligated with BCR, the N-terminal hFcRL5 ITAM recruits the src kinase Lyn, which phosphorylates the ITIM, which in turn recruits the tyrosine phosphatase(s) SHP-1/2, which inhibits BCR signaling (Zhu et al., 2013). Unlike hFcRL5, when expressed in Ramos B cells, the two-ITIM-containing hFcRL4 was constitutively phosphorylated and associated with SHP-1/2, suggesting that it could exert a constitutive negative effect (Sohn et al., 2011).
Tissue Distribution and Biological Functions of FcRs and FcRLs
FcRs and FcRLs have no specific function per se. They transduce signals that trigger, inhibit, or generally speaking, control the functions of FcR- and FcRL-expressing cells. Responding cells are selected by the ligands their receptors interact with. Biological functions induced via FcRs and FcRLs therefore primarily depend on the tissue distribution of these receptors. Ultimately, they depend on the functional repertoires of FcR- and FcRL-expressing cells.
Tissue Distribution of FcRs and FcRLs
Except FcRn and pIgR, both FcRs and FcRLs are primarily expressed by cells of the hematopoietic lineage. FcRs, however, are expressed mostly, though not only, by myeloid cells, whereas FcRLs are expressed mostly, if not only, by lymphoid cells, especially B lymphocytes.
Fc Receptors
Activating FcRs are expressed by myeloid cells of all types, that is, monocytes, macrophages, dendritic cells, polymorphonuclear cells of the three types, mast cells, etc. They are also expressed by NK cells (Perussia et al., 1989), NKT cells, and intraepithelial γ/δ T cells (Deusch et al., 1991; Sandor et al., 1992; Woodward and Jenkinson, 2001). FcγRIIIA were also reported on a subset of murine CD8 T cells (Dhanji et al., 2005). Inhibitory FcRs are expressed by most myeloid cells and by B lymphocytes. Noticeably, human basophils express much higher levels of FcγRIIB than any other blood cells (Cassard et al., 2012). A few nonhematopoietic cells, such as some endothelial cells and some tumor cells (Cassard et al., 2002), also express FcRs. FcRn are expressed by many cells including epithelial cells, myeloid cells, and hepatocytes (Ghetie and Ward, 2000). The pIgR is expressed by polarized epithelial cells, especially of the mammary gland and the gut (Kaetzel et al., 1991).
FcR-Like Molecules
FcRLs have a much more restricted distribution in both humans and mice (Li et al., 2014). FcRL1–5 and FcRLA/B are expressed by B cells: FcRL1 by all B cells, FcRL2–5 by B cell subsets; hFcRLA/B by subsets of germinal center B cells, mFcRLA by peripheral B cells; the expression of mFcRLB is not known. hFcRL6 is not expressed by B cells, but by T cells and NK cells. hFcRL3 is also expressed by T and NK cells, besides by B cells. Finally mFcRLs and hFcRLs are expressed by melanocytes.
Biological Functions of FcRs and FcRLs
Biological responses induced by antibodies depend on the functional repertoire of FcR-expressing cells. The wide tissue distribution of FcRs therefore endows antibodies with a wide spectrum of biological functions. Antibodies, however, do not necessarily activate, they can as well inhibit those responses of cells that coexpress activating and inhibitory FcRs. FcRLs essentially regulate B cell functions. Noticeably, they appear to control differentially BCR- and TLR-dependent activation, proliferation, and differentiation of various B cell subsets.
Fc Receptors
FcRs control the internalization of immune complexes. All cell types pinocytose and endocytose, some phagocytose, and others can transcytose. Specific cells can exocytose. They release granules that contain cytotoxic, vasoactive, or proinflammatory mediators and proteases. Many cells can synthesize and secrete cytokines, chemokines, or growth factors. Immune responses being pluri-isotypic and cells of different types sharing FcRs for the same isotypes, antibodies select heterogeneous, rather than homogeneous cell populations, when in complex with antigen. These populations comprise a mixture of FcR-expressing cells that are present, were recruited, and/or proliferated locally. Biological processes in which FcRs are involved are therefore a result of those of many cells.
FcR-Like Molecules
FcRLs differentially control B cell functions. Activation signals generated by the two ITAM-containing human and murine FcRL1 stimulate B cell proliferation, like signals generated by the BCR. Conversely, the ITAM + ITIM-containing FcRL2–5 generally negatively regulate BCR signaling. However, when coligated with BCR, FcRL3 inhibited activation signals, whereas it enhanced B cell activation, proliferation, and survival when coligated with TLR9 (Li et al., 2013). Likewise, the constitutive negative regulation of BCR signaling by FcRL4 was accompanied by a positive regulation of TLR9 signaling (Sohn et al., 2011). Noticeably, while enhancing proliferation, the coligation of FcRL3 and TLR9 inhibited plasma cell differentiation and antibody production (Li et al., 2014). When coengaged with BCR, mFcRL5 had antagonistic effects on Ca2+ responses and on MAPK activation, which differentially controlled BCR-dependent signals in B1 B cells and in marginal zone B cells (Zhu et al., 2013). These results altogether indicate that FcRLs which contain both ITAMs and ITIMs can differentially regulate (1) BCR- and TLR-dependent, that is, adaptive and innate signals, (2) activation versus proliferation and differentiation signals, and (3) B cell subsets.
FcRs and FcRLs in Health and Disease
In Physiology
Due to their cellular expression, FcRs control the many biological functions of myeloid cells, while FcRLs primarily regulate B cell activation and antibody responses.
Fc Receptors
FcRs mediate most biological activities induced by antibodies. They are not readily accessible to investigation in physiology. FcRs were, however, shown to protect and transport immunoglobulins and to control adaptive immune responses.
FcRn protects IgG from degradation (Huber et al., 1993; Raghavan et al., 1993; Junghans and Anderson, 1996). It also transports IgG across the gut (Yoshida et al., 2004; He et al., 2008) and maternal IgG across the placenta (Palmeira et al., 2012). The pIgR transcytoses IgA and IgM, especially through the mammary gland (Johansen et al., 1999).
Activating FcRs enhance MHC-I and II presentation of tumor antigens (Desai et al., 2007), while FcγRIIB dampens dendritic cell maturation and antigen presentation (Wernersson et al., 1999; Kalergis and Ravetch, 2002). FcγRIIB therefore contribute to peripheral T cell tolerance (Desai et al., 2007). Conversely, FcγRIIB expressed by follicular dendritic cells can ‘present’ T-independent antigens to B cells (Szakal et al., 1985; Mond et al., 1995). Follicular dendritic cell FcγRIIB also prevent the Fc portions of IgG immune complexes from coengaging FcγRIIB with BCR and inhibit B cell activation (Tew et al., 2001; El Shikh et al., 2006; Wu et al., 2008).
Unlike immune responses to soluble antigen that are enhanced by IgG antibodies (Hjelm et al., 2006), immune responses to particulate antigens such as erythrocytes are suppressed by minute amounts of IgG antibodies. This observation has provided the rationale for injecting Rh− mothers of Rh+ babies with anti-RhD antibodies to prevent hemolytic disease of the newborn. FcγRIIB-dependent negative regulation, however, does not account for feedback regulation by antibodies, which was altered neither in FcγRIIB-deficient mice (Heyman et al., 2001), nor in mice lacking all FcγRs (Karlsson et al., 1999).
IgE antibodies are potent adjuvants (Getahun et al., 2005). When interacting with FcεRII on B cells, IgE immune complexes present antigen to T cells and enhance antibody responses of all classes (Westman et al., 1997). This enhancement is antigen-specific because only FcεRII-expressing B cells that possess the specific BCR receive cognate T cell help (Hjelm et al., 2006).
FcR-Like Molecules
Little is known of the roles played by FcRLs in physiology. Reasons are the limited knowledge on FcRL ligands, but also the small number of genetically engineered mice with altered fcrl genes available. Only transgenic mice with a targeted disruption of the fcrla and fcrlb genes, which encode the intracytoplasmic FcRLs with no known ligand, were published. FcRLA-deficient mice displayed an enhanced secondary (but not primary) IgG1 antibody response to a T-dependent particulate antigen like sheep erythrocytes. Responses to T-independent antigens or to soluble T-dependent antigens were unaffected (Wilson et al., 2010). FcRLB-deficient mice displayed an enhanced IgG1 response to nitrophenylated chicken γ-globulins. However, due to unexpected deletions of regulatory sequences, fcrlb −/− mice also had a reduced FcγRIIB expression that could account for the observed hyperresponsiveness (Masuda et al., 2010).
In Pathology
Fc Receptors
FcRs can both protect, as in infectious diseases, and be pathogenic, as in inflammatory diseases. FcRs are involved in protection against infections. Legionella (Joller et al., 2010), Salmonella (Tobar et al., 2004), and Toxoplasma (Joiner et al., 1990) are phagocytosed via FcRs. The neutralization of Bacillus anthracis toxin depends on FcRs (Abboud et al., 2010). FcRγ-deficient mice fail to control Leishmania major (Padigel and Farrell, 2005) or Mycobacterium tuberculosis (Maglione et al., 2008) infection. Conversely, FcγRIIB-deficient mice display an enhanced resistance to these bacteria. FcγRIIIB polymorphisms are associated with clinical malaria (Adu et al., 2012), and FcγRI protected from plasmodium in mouse models (McIntosh et al., 2007). Instead of being protective, antibodies may favor infection. If anti-Spike antibodies can prevent the severe acute respiratory syndrome (SARS) coronavirus from entering epithelial cells, they enable FcγR-expressing cells to be infected (Jaume et al., 2011). Likewise, anti-HIV antibodies can use FcRs to infect monocytes (Jouault et al., 1991; Fust, 1997).
The role of mast cell and basophil FcεRI is well known in allergy. FcεRI-deficient mice are resistant to IgE-induced passive systemic anaphylaxis (PSA) (Dombrowicz et al., 1993); hIgE induce PSA in hFcεRI-expressing transgenic mice (Dombrowicz et al., 1996; Fung-Leung et al., 1996). IgG1 antibodies can also trigger passive cutaneous anaphylaxis (PCA) when engaging mFcγRIIIA (Hazenbos et al., 1996), and FcγRIV expressed by neutrophils accounted for active systemic anaphylaxis (ASA), together with FcγRIIIA (Jonsson et al., 2011). FcγRIIB-deficient mice display enhanced anaphylaxis (Takai et al., 1996; Ujike et al., 1999). Both hFcγRI and hFcγRIIA triggered IgG-induced PSA and ASA in transgenic mice (Jonsson et al., 2012; Mancardi et al., 2013). Human mast cell FcγRIIA account for IgG-induced PCA (Zhao et al., 2006). When coengaged on human basophils, FcγRIIA and FcγRIIB inhibit cell activation. Consequently, basophils failed to be activated by IgG immune complexes, and IgG immune complexes that coengaged FcγRs with FcεRI inhibited IgE-dependent basophil activation (Cassard et al., 2012).
FcγRIIB-deficient C57BL/6 mice develop a systemic lupus erythematosus (SLE)-like disease when aging (Ravetch and Bolland, 2001). Anti-platelet antibody-induced thrombocytopenia was prevented in FcRγ-deficient mice (Fossati-Jimack et al., 1999). mFcγRI, IIIA, and IV were found to contribute to platelet depletion (Fossati-Jimack et al., 1999; Nimmerjahn et al., 2005; Nimmerjahn and Ravetch, 2005), SLE (Seres et al., 1998), hemolytic anemia (Meyer et al., 1998; Syed et al., 2009), glomerulonephritis (Fujii et al., 2003), and arthritis (Ioan-Facsinay et al., 2002; Bruhns et al., 2003; Mancardi et al., 2011). hFcγRIIA induced thrombocytopenia purpura (Reilly et al., 1994) or arthritis (Pietersz et al., 2009) in transgenic mice. Antimyelin antibodies found in multiple sclerosis and anti-dopaminergic neurons antibodies found in Parkinson disease (McRae-Degueurce et al., 1988) are thought to activate FcR-expressing phagocytic cells. FcRγ-deficient mice indeed displayed less or milder lesions in murine models of Alzheimer (Das et al., 2003), Parkinson (He et al., 2002), multiple sclerosis (Robbie-Ryan et al., 2003), and ischemic stroke (Komine-Kobayashi et al., 2004). Inversely, FcγRIIB-deficient mice had an enhanced disease susceptibility.
FcR-Like Molecules
FcRLs have been involved in three types of diseases, infectious diseases, autoimmune diseases, and proliferative diseases, which are linked to B cell abnormalities.
When binding to integrins on B cells, the HIV envelope protein gp120 upregulates FcRL4 expression, which inhibits B cell proliferation (Jelicic et al., 2013). The expression of FcRL4 is also upregulated in chronic infection by viruses such as HIV and hepatitis C virus (Charles et al., 2008; Moir et al., 2008).
SNPs in FcRL1-5 have been associated with several autoimmune disorders including rheumatoid arthritis, SLE, and Graves' disease. One SNP, the T169C variant, which affects an NF-κB-binding site in the FCRL3 promoter, enhances FcRL3 expression (Kochi et al., 2005), making FCRL3 an autoimmune susceptibility candidate gene (Chistiakov and Chistiakov, 2007).
FcRL1–5 are upregulated in most B cell proliferative disorders including lymphoid leukemias, Burkitt, follicular, diffuse B cell, and mantle cell lymphomas (Li et al., 2014). FcRL4, which is normally expressed by marginal zone B cells, is expressed in marginal zone leukemias. FcRL2 was associated with IGHV-unmutated aggressive chronic lymphoid leukemias.
In Therapeutics
Fc Receptors
Therapeutic antibodies against cancer use FcRs as tools. The antitumor activities of Rituximab, a humanized anti-CD20 antibody that has been approved for B cell malignancies, and of Trastuzumab, an anti-HER2 antibody used in breast, ovary, and lung cancer, depend on FcγRs (Clynes et al., 2000; Manches et al., 2003). The therapeutic effects of these mAbs were increased by enhancing their affinity for FcRn, which enhances their half-life (Ward and Ober, 2009), and by removing fucose residues from their Fc portion, which increases their affinity for activating hFcγRIIIA (Natsume et al., 2005; Niwa et al., 2005).
Therapeutic antibodies against autoimmune or allergic inflammation use FcRs either as tools or as targets. Therapeutic strategies have been developed, aiming at coengaging FcεRI or FcεRI-bound IgE with mast cell or basophil FcγRII to prevent allergy (Zhu et al., 2002; Tam et al., 2004). FcγRIIB indeed exerts a dominant inhibitory effect on FcγRIIA and FcεRI in human basophils (Cassard et al., 2012). Anti-FcγRI (Ericson et al., 1996) and anti-FcγRIIIA antibodies (Clarkson et al., 1986) reduced symptoms in idiopathic thrombocytopenia. Anti-IgE antibodies (Omalizumab) used in asthma (Busse et al., 2001), rhinitis (Casale et al., 2001), and chronic urticaria (Kaplan et al., 2008) deplete plasma IgE (Djukanovic et al., 2004) and downregulate FcεRI on basophils and mast cells. Their efficacy was markedly enhanced, by increasing their affinity for FcγRIIB (Chu et al., 2012).
Initially conceived as a substitutive treatment of immunodeficiencies, intravenous Immunoglobulins (IVIG) proved efficient in arthritis, idiopathic thrombocytopenia, or SLE (Bayary et al., 2006). IVIG Fc had similar effects as intact IVIG, suggesting a role of FcRs (Anthony and Ravetch, 2010). The therapeutic effect of IVIG was enhanced by increasing their concentration in sialic acid–rich immunoglobulins (Kaneko et al., 2006). The mechanism underlying this phenomenon remains unclear.
FcR-Like Molecules
FcRLs are potential therapeutic targets in B cell malignancies. Toxin-conjugated anti-FcRL1 mAbs have been used as an anti-pan-B cell (BCR and TCR) depleting reagent (Du et al., 2008), while FcRL5, which is expressed by plasma cells, has been specifically targeted in multiple myeloma (Elkins et al., 2012). FcRLs are also potential therapeutic tools in infectious diseases. Knocking-down FcRL4 (as well as other inhibitory receptors) in chronic viral infections indeed restored BCR-dependent B cell proliferation and HIV-specific antibody responses (Kardava et al., 2011).
FcRs and FcRLs among Immunoreceptors
FcRLs are more than Fc receptor-like molecules. FcRs and FcRLs indeed form a single family that shares genetic, structural, and functional properties. Genes encoding hFcRLs all lie in the FCR locus that contains the vast majority of genes encoding FcRs on chromosome 1. Likewise, genes encoding mFcRLs all lie in the fcr locus, even though one segment of this locus was translocated to chromosome 3. Importantly, fcrl genes were the ancestors of genes encoding FcγRI, FcγRII, FcγRIII, FcγRIV, and FcεRI, that is, the majority of classical FcRs, which appeared with early mammalians during evolution. These receptors account for most properties of IgG and IgE antibodies in humans and mice. FcRs and FcRLs, however, differ by their ligands. Most FcRLs do not bind immunoglobulins whereas, by definition, all FcRs do.
All FcRLs, some single-chain FcRs, and the subunits with which multisubunit FcRs associate contain tyrosine-based signaling motifs. This makes the FcR/FcRL family a member of the wider immunoreceptor family which, itself, belongs to the IgSF. The immunoreceptor family, defined as gathering receptors that use ITAMs and/or ITIMs for signaling, contains also B cell and T cell receptors for antigens, as well as an increasing number of activating and inhibitory receptors (Daëron et al., 2008). The majority of FcRs are ITAM-containing activating receptors; only one is an ITIM-containing inhibitory receptor. FcRLs contain ITAMs only, ITIMs only, or ITAMs and ITIMs. FcRLs may therefore have more subtle regulatory effects than FcRs. When engaged by immune complexes, however, FcRs form heteroaggregates in which variable numbers of ITIM- and ITAM-containing receptors generate mixtures of positive and negative signals (Daëron, 2014), as FcRLs that contain both ITAMs and ITIMs do, when engaged by their ligands.
FcRs and FcRLs have markedly different tissue distributions. FcRs are expressed by myeloid cells and by some lymphoid cells, including B cells and NK cells. FcRLs are expressed by lymphoid cells, primarily B cells, but also T and NK cells. Myeloid cells thus express a variety of activating and inhibitory FcRs, but no FcRLs. B lymphocytes express a variety of activating and inhibitory FcRLs, as well as inhibitory FcRs, but no activating FcRs. NK cells and some T cells express activating and inhibitory FcRLs, as well as activating FcRs but no inhibitory FcRs. FcRs and FcRLs therefore control different functions of different cell types. When engaged by antigen–antibody complexes, FcRs use the many cells of the innate immune system for adaptive immune responses (Daëron, 2014), whereas FcRLs differentially control responses of cells of the adaptive immune system (but also of NK cells) to adaptive and innate signalings (Li et al., 2014).
Finally, FcRs and FcRLs are also the relatives of other members of the immunoreceptor family encoded by genes of the LRC locus. These include LILRs A and B, ILTs, KIRs and KIRL, and NCR1, whose genes are all on chromosome 19 with FCAR1 in humans, and LIRA, PIRA/B, NCR1, whose genes are on chromosome 7, and KIRL genes on chromosome X in mice (Akula et al., 2014). The vast majority of these receptors contain ITIMs, some contain ITAMs, and a minority contain both. Being expressed by myeloid cells, B cells, T cells, and NK cells, but also a variety of nonhematopoietic cells, these receptors are involved in a multitude of immune and nonimmune responses (Daëron et al., 2008). It follows that altogether, receptors of the immunoreceptor family, among which FcRs and FcRLs, are major, complementary, regulators of innate and adaptive responses.
See also
B CELL ACTIVATION | T Cell–Dependent B Cell Activation; SIGNAL TRANSDUCTION | Signal Transduction by the B Cell Antigen Receptor; SIGNAL TRANSDUCTION | Signaling Pathways Downstream of TLRs and IL-1 Family Receptors; SIGNAL TRANSDUCTION | TCR Signaling: Proximal Signaling; STRUCTURE AND FUNCTION OF DIVERSIFYING RECEPTORS | Structure, Function, and Spatial Organization of the B Cell Receptor.
References
- Abboud N., Chow S.K., Saylor C. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu B., Dodoo D., Adukpo S. Fc gamma receptor IIIB (FcgammaRIIIB) polymorphisms are associated with clinical malaria in Ghanaian children. PLoS One. 2012;7:e46197. doi: 10.1371/journal.pone.0046197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.A., Giddens J., Pincetic A. Structural characterization of antiinflammatory immunoglobulin G Fc proteins. J. Mol. Biol. 2014;426:3166–3179. doi: 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula S., Mohammadamin S., Hellman L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS One. 2014;9:e96903. doi: 10.1371/journal.pone.0096903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R.M., Ravetch J.V. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol. 2010;30(Suppl. 1):S9–S14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- Arnold J.N., Wormald M.R., Sim R.B., Rudd P.M., Dwek R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Bayary J., Dasgupta S., Misra N. Intravenous immunoglobulin in autoimmune disorders: an insight into the immunoregulatory mechanisms. Int. Immunopharmacol. 2006;6:528–534. doi: 10.1016/j.intimp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J. Exp. Med. 1966;123:119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K.J. The anaphylactic antibodies of mammals including man. Prog. Allergy. 1967;10:84–150. [PubMed] [Google Scholar]
- Bruhns P., Iannascoli B., England P. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- Bruhns P., Samuelsson A., Pollard J.W., Ravetch J.V. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- Busse W., Corren J., Lanier B.Q. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- Campbell J.A., Davis R.S., Lilly L.M. Cutting edge: FcR-like 5 on innate B cells is targeted by a poxvirus MHC class I-like immunoevasin. J. Immunol. 2010;185:28–32. doi: 10.4049/jimmunol.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale T.B., Condemi J., LaForce C. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–2967. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- Cassard L., Cohen-Solal J.F., Galinha A. Modulation of tumor growth by inhibitory Fc(gamma) receptor expressed by human melanoma cells. J. Clin. Invest. 2002;110:1549–1557. doi: 10.1172/JCI15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassard L., Jonsson F., Arnaud S., Daëron M. Fcgamma receptors inhibit mouse and human basophil activation. J. Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- Charles E.D., Green R.M., Marukian S. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–1356. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov D.A., Chistiakov A.P. Is FCRL3 a new general autoimmunity gene? Hum. Immunol. 2007;68:375–383. doi: 10.1016/j.humimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Chu S.Y., Horton H.M., Pong E. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcgammaRIIb with Fc-engineered antibody. J. Allergy Clin. Immunol. 2012;129:1102–1115. doi: 10.1016/j.jaci.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Clarkson S.B., Bussel J.B., Kimberly R.P. Treatment of refractory immune thrombocytopenic purpura with an anti-Fc gamma-receptor antibody. N. Engl. J. Med. 1986;314:1236–1239. doi: 10.1056/NEJM198605083141907. [DOI] [PubMed] [Google Scholar]
- Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Conrad D.H. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu. Rev. Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- Conrad D.H., Kozak C.A., Vernachio J. Chromosomal location and isoform analysis of mouse Fc epsilon RII/CD23. Mol. Immunol. 1993;30:27–33. doi: 10.1016/0161-5890(93)90423-9. [DOI] [PubMed] [Google Scholar]
- Daëron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Daëron M. Fc receptors as adaptive immunoreceptors. Curr. Top. Microbiol. Immunol. 2014;382:131–164. doi: 10.1007/978-3-319-07911-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M., Jaeger S., Du Pasquier L., Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol. Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- Daëron M., Latour S., Malbec O. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of FcgRIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- Daëron M., Malbec O., Latour S., Arock M., Fridman W.H. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J. Clin. Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M., Lesourne R. Negative signaling in Fc receptor complexes. Adv. Immunol. 2006;89:39–86. doi: 10.1016/S0065-2776(05)89002-9. [DOI] [PubMed] [Google Scholar]
- Das P., Howard V., Loosbrock N. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/-knock-out mice. J. Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.S., Dennis G., Jr., Odom M.R. Fc receptor homologs: newest members of a remarkably diverse Fc receptor gene family. Immunol. Rev. 2002;190:123–136. doi: 10.1034/j.1600-065x.2002.19009.x. [DOI] [PubMed] [Google Scholar]
- Davis R.S., Wang Y.H., Kubagawa H., Cooper M.D. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D.D., Harbers S.O., Flores M. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J. Immunol. 2007;178:6217–6226. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- Deusch K., Pfeffer K., Reich K. Phenotypic and functional characterization of human TCR gamma delta+ intestinal intraepithelial lymphocytes. Curr. Top. Microbiol. Immunol. 1991;173:279–283. [PubMed] [Google Scholar]
- Dhanji S., Tse K., Teh H.S. The low affinity Fc receptor for IgG functions as an effective cytolytic receptor for self-specific CD8 T cells. J. Immunol. 2005;174:1253–1258. doi: 10.4049/jimmunol.174.3.1253. [DOI] [PubMed] [Google Scholar]
- Djukanovic R., Wilson S.J., Kraft M. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- Dombrowicz D., Brini A.T., Flamand V. Anaphylaxis mediated through a humanized high affinity IgE receptor. J. Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- Dombrowicz D., Flamand V., Brigman K.K., Koller B.H., Kinet J.P. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- Du X., Nagata S., Ise T., Stetler-Stevenson M., Pastan I. FCRL1 on chronic lymphocytic leukemia, hairy cell leukemia, and B-cell non-Hodgkin lymphoma as a target of immunotoxins. Blood. 2008;111:338–343. doi: 10.1182/blood-2007-07-102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt G.R., Cooper M.D. Immunoregulatory roles for fc receptor-like molecules. Curr. Top. Microbiol. Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- El Shikh M.E., El Sayed R., Szakal A.K., Tew J.G. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur. J. Immunol. 2006;36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]
- Elkins K., Zheng B., Go M. FcRL5 as a target of antibody-drug conjugates for the treatment of multiple myeloma. Mol. Cancer Ther. 2012;11:2222–2232. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- Ericson S.G., Coleman K.D., Wardwell K. Monoclonal antibody 197 (anti-Fc gamma RI) infusion in a patient with immune thrombocytopenia purpura (ITP) results in down-modulation of Fc gamma RI on circulating monocytes. Br. J. Haematol. 1996;92:718–724. doi: 10.1046/j.1365-2141.1996.393931.x. [DOI] [PubMed] [Google Scholar]
- Ernst L.K., Duchemin A.M., Anderson C.L. Association of the high-affinity receptor for IgG (Fc gamma RI) with the gamma subunit of the IgE receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6023–6027. doi: 10.1073/pnas.90.13.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati-Jimack L., Reininger L., Chicheportiche Y. High pathogenic potential of low-affinity autoantibodies in experimental autoimmune hemolytic anemia. J. Exp. Med. 1999;190:1689–1696. doi: 10.1084/jem.190.11.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A., Damdinsuren B., Ise T. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J. Immunol. 2013;190:5739–5746. doi: 10.4049/jimmunol.1202860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Hamano Y., Ueda S. Predominant role of FcgammaRIII in the induction of accelerated nephrotoxic glomerulonephritis. Kidney Int. 2003;64:1406–1416. doi: 10.1046/j.1523-1755.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W.P., De Sousa-Hitzler J., Ishaque A. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J. Exp. Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fust G. Enhancing antibodies in HIV infection. Parasitology. 1997;115(Suppl.):S127–S140. doi: 10.1017/s0031182097001819. [DOI] [PubMed] [Google Scholar]
- Garman S.C., Kinet J.P., Jardetzky T.S. Crystal structure of the human high-affinity IgE receptor. Cell. 1998;95:951–961. doi: 10.1016/s0092-8674(00)81719-5. [DOI] [PubMed] [Google Scholar]
- Getahun A., Hjelm F., Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J. Immunol. 2005;175:1473–1482. doi: 10.4049/jimmunol.175.3.1473. [DOI] [PubMed] [Google Scholar]
- Ghetie V., Ward E.S. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- Gimborn K., Lessmann E., Kuppig S., Krystal G., Huber M. SHIP down-regulates FcepsilonR1-induced degranulation at supraoptimal IgE or antigen levels. J. Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G., Miller I., Takizawa J. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- Hazenbos W.L., Gessner J.E., Hofhuis F.M. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- He W., Ladinsky M.S., Huey-Tubman K.E. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Le W.D., Appel S.H. Role of Fcgamma receptors in nigral cell injury induced by Parkinson disease immunoglobulin injection into mouse substantia nigra. Exp. Neurol. 2002;176:322–327. doi: 10.1006/exnr.2002.7946. [DOI] [PubMed] [Google Scholar]
- Henry C., Jerne N.K. Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J. Exp. Med. 1968;128:133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman B., Dahlstrom J., Diaz De Stahl T. No evidence for a role of FcgammaRIIB in suppression of in vivo antibody responses to erythrocytes by passively administered IgG. Scand. J. Immunol. 2001;53:331–334. doi: 10.1046/j.1365-3083.2001.00890.x. discussion 339–345. [DOI] [PubMed] [Google Scholar]
- Hjelm F., Carlsson F., Getahun A., Heyman B. Antibody-mediated regulation of the immune response. Scand. J. Immunol. 2006;64:177–184. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- Holowka D., Hartmann H., Kanellopoulos J., Metzger H. Association of the receptor for immunoglobulin E with an endogenous polypeptide on rat basophilic leukemia cells. J. Recept. Res. 1980;1:41–68. doi: 10.3109/10799898009039254. [DOI] [PubMed] [Google Scholar]
- Huber A.H., Kelley R.F., Gastinel L.N., Bjorkman P.J. Crystallization and stoichiometry of binding of a complex between a rat intestinal Fc receptor and Fc. J. Mol. Biol. 1993;230:1077–1083. doi: 10.1006/jmbi.1993.1220. [DOI] [PubMed] [Google Scholar]
- Hulett M.D., Hogarth P.M. Molecular basis of Fc receptor function. Adv. Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- Imboden J.B., Eriksson E.C., McCutcheon M., Reynolds C.W., Seaman W.E. Identification and characterization of a cell-surface molecule that is selectively induced on rat lymphokine-activated killer cells. J. Immunol. 1989;143:3100–3103. [PubMed] [Google Scholar]
- Ioan-Facsinay A., de Kimpe S.J., Hellwig S.M. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- Israel E.J., Patel V.K., Taylor S.F., Marshak-Rothstein A., Simister N.E. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J. Immunol. 1995;154:6246–6251. [PubMed] [Google Scholar]
- Jaume M., Yip M.S., Cheung C.Y. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH-and cysteine protease-independent FcgammaR pathway. J. Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic K., Cimbro R., Nawaz F. The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-beta1 production and FcRL4 expression. Nat. Immunol. 2013;14:1256–1265. doi: 10.1038/ni.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen F.E., Pekna M., Norderhaug I.N. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K.A., Fuhrman S.A., Miettinen H.M., Kasper L.H., Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Joller N., Weber S.S., Muller A.J. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20441–20446. doi: 10.1073/pnas.1013827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson F., Mancardi D.A., Kita Y. Mouse and human neutrophils induce anaphylaxis. J. Clin. Invest. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson F., Mancardi D.A., Zhao W. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood. 2012;119:2533–2544. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouault T., Chapuis F., Bahraoui E., Gluckman J.C. Infection of monocytic cells by HIV1: combined role of FcR and CD4. Res. Virol. 1991;142:183–188. doi: 10.1016/0923-2516(91)90055-8. [DOI] [PubMed] [Google Scholar]
- Junghans R.P., Anderson C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel C.S., Robinson J.K., Chintalacharuvu K.R., Vaerman J.P., Lamm M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalergis A.M., Ravetch J.V. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J. Exp. Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Nimmerjahn F., Ravetch J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kaplan A.P., Joseph K., Maykut R.J., Geba G.P., Zeldin R.K. Treatment of chronic autoimmune urticaria with omalizumab. J. Allergy Clin. Immunol. 2008;122:569–573. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Kardava L., Moir S., Wang W. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Invest. 2011;121:2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M.C., Wernersson S., Diaz de Stahl T., Gustavsson S., Heyman B. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2244–2249. doi: 10.1073/pnas.96.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent U.M., Mao S.Y., Wofsy C. Dynamics of signal transduction after aggregation of cell-surface receptors: studies on the type I receptor for IgE. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3087–3091. doi: 10.1073/pnas.91.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y., Yamada R., Suzuki A. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat. Genet. 2005;37:478–485. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine-Kobayashi M., Chou N., Mochizuki H. Dual role of Fcgamma receptor in transient focal cerebral ischemia in mice. Stroke. 2004;35:958–963. doi: 10.1161/01.STR.0000120321.30916.8E. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr., Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J. Exp. Med. 1974;140:1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Gander I., Wirthmueller U., Ravetch J.V. The beta subunit of the Fc epsilon RI is associated with the Fc gamma RIII on mast cells. J. Exp. Med. 1992;175:447–451. doi: 10.1084/jem.175.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourne R., Fridman W.H., Daëron M. Dynamic interactions of Fc gamma receptor IIB with filamin-bound SHIP1 amplify filamentous actin-dependent negative regulation of Fc epsilon receptor I signaling. J. Immunol. 2005;174:1365–1373. doi: 10.4049/jimmunol.174.3.1365. [DOI] [PubMed] [Google Scholar]
- Li F.J., Schreeder D.M., Li R., Wu J., Davis R.S. FCRL3 promotes TLR9-induced B-cell activation and suppresses plasma cell differentiation. Eur. J. Immunol. 2013;43:2980–2992. doi: 10.1002/eji.201243068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.J., Won W.J., Becker E.J., Jr. Emerging roles for the FCRL family members in lymphocyte biology and disease. Curr. Top. Microbiol. Immunol. 2014;382:29–50. doi: 10.1007/978-3-319-07911-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeyama K., Hohman R.J., Metzger H., Beaven M.A. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J. Biol. Chem. 1986;261:2583–2592. [PubMed] [Google Scholar]
- Maglione P.J., Xu J., Casadevall A., Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J. Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- Malbec O., Fong D.C., Turner M. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J. Immunol. 1998;160:1647–1658. [PubMed] [Google Scholar]
- Malbec O., Malissen M., Isnardi I. Linker for activation of T cells integrates positive and negative signaling in mast cells. J. Immunol. 2004;173:5086–5094. doi: 10.4049/jimmunol.173.8.5086. [DOI] [PubMed] [Google Scholar]
- Mancardi D.A., Albanesi M., Jonsson F. The high-affinity human IgG receptor FcgammaRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121:1563–1573. doi: 10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- Mancardi D.A., Iannascoli B., Hoos S. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancardi D.A., Jonsson F., Iannascoli B. Cutting Edge: the murine high-affinity IgG receptor FcgammaRIV is sufficient for autoantibody-induced arthritis. J. Immunol. 2011;186:1899–1903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- Manches O., Lui G., Chaperot L. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–954. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- Masuda K., Mori H., Ohara O. Defining the immunological phenotype of Fc receptor-like B (FCRLB) deficient mice: confounding role of the inhibitory FcgammaRIIb. Cell. Immunol. 2010;266:24–31. doi: 10.1016/j.cellimm.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K.F., Powell M.S., Hulett M.D. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat. Struct. Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- McIntosh R.S., Shi J., Jennings R.M. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 2007;3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Degueurce A., Rosengren L., Haglid K. Immunocytochemical investigations on the presence of neuron-specific antibodies in the CSF of Parkinson's disease cases. Neurochem. Res. 1988;13:679–684. doi: 10.1007/BF00973287. [DOI] [PubMed] [Google Scholar]
- Metzger H. Transmembrane signaling: the joy of aggregation. J. Immunol. 1992;149:1477–1487. [PubMed] [Google Scholar]
- Meyer D., Schiller C., Westermann J. FcgammaRIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood. 1998;92:3997–4002. [PubMed] [Google Scholar]
- Moir S., Ho J., Malaspina A. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J.J., Lees A., Snapper C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Natsume A., Wakitani M., Yamane-Ohnuki N. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded antibody comprising a single-chain antibody linked the antibody constant region. J. Immunol. Methods. 2005;306:93–103. doi: 10.1016/j.jim.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Niwa R., Sakurada M., Kobayashi Y. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin. Cancer Res. 2005;11:2327–2336. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- Orloff D.G., Ra C.S., Frank S.J., Klausner R.D., Kinet J.P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990;347:189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Ory P.A., Goldstein I.M., Kwoh E.E., Clarkson S.B. Characterization of polymorphic forms of Fc receptor III on human neutrophils. J. Clin. Invest. 1989;83:1676–1681. doi: 10.1172/JCI114067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padigel U.M., Farrell J.P. Control of infection with Leishmania major in susceptible BALB/c mice lacking the common gamma-chain for FcR is associated with reduced production of IL-10 and TGF-beta by parasitized cells. J. Immunol. 2005;174:6340–6345. doi: 10.4049/jimmunol.174.10.6340. [DOI] [PubMed] [Google Scholar]
- Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas F., Lee S.T., Orr K.B., Israels L.G. A receptor for Fc on mouse B-lymphocytes. J. Immunol. 1972;108:1319–1327. [PubMed] [Google Scholar]
- Perussia B., Tutt M.M., Qui W.Q. Murine natural killer cells express functional Fcg receptor II encoded by the FcgRa gene. J. Exp. Med. 1989;170:73–86. doi: 10.1084/jem.170.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn L.C., Yeaman G.R. Association of IgA-Fc receptors (Fc alpha R) with Fc epsilon RI gamma 2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of gamma 2. J. Immunol. 1994;153:3228–3236. [PubMed] [Google Scholar]
- Pietersz G.A., Mottram P.L., van de Velde N.C. Inhibition of destructive autoimmune arthritis in FcgammaRIIa transgenic mice by small chemical entities. Immunol. Cell Biol. 2009;87:3–12. doi: 10.1038/icb.2008.82. [DOI] [PubMed] [Google Scholar]
- Pincetic A., Bournazos S., DiLillo D.J. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda V.S., Pribluda C., Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W.Q., de Bruin D., Brownstein B.H., Pearse R., Ravetch J.V. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science. 1990;248:732–735. doi: 10.1126/science.2139735. [DOI] [PubMed] [Google Scholar]
- Raghavan M., Gastinel L.N., Bjorkman P.J. The class I major histocompatibility complex related Fc receptor shows pH-dependent stability differences correlating with immunoglobulin binding and release. Biochemistry. 1993;32:8654–8660. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Bolland S. IgG fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Kinet J.P. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J. Exp. Med. 1989;170:481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly A.F., Norris C.F., Surrey S. Genetic diversity in human Fc receptor II for immunoglobulin G: fc gamma receptor IIA ligand-binding polymorphism. Clin. Diagn. Lab. Immunol. 1994;1:640–644. doi: 10.1128/cdli.1.6.640-644.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Robbie-Ryan M., Tanzola M.B., Secor V.H., Brown M.A. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J. Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- Sandor M., Houlden B., Bluestone J. In vitro and in vivo activation of murine gamma/delta T cells induces the expression of IgA, IgM, and IgG Fc receptors. J. Immunol. 1992;148:2363–2369. [PubMed] [Google Scholar]
- Schreeder D.M., Cannon J.P., Wu J. Cutting edge: FcR-like 6 is an MHC class II receptor. J. Immunol. 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W.E., Niemi E.C., Stark M.R. Molecular cloning of gp42, a cell-surface molecule that is selectively induced on rat natural killer cells by interleukin 2: glycolipid membrane anchoring and capacity for transmembrane signaling. J. Exp. Med. 1991;173:251–260. doi: 10.1084/jem.173.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seres T., Csipo I., Kiss E., Szegedi G., Kavai M. Correlation of Fc gamma receptor expression of monocytes with clearance function by macrophages in systemic lupus erythematosus. Scand. J. Immunol. 1998;48:307–311. doi: 10.1046/j.1365-3083.1998.00383.x. [DOI] [PubMed] [Google Scholar]
- Simister N.E., Rees A.R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur. J. Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Smith P., DiLillo D.J., Bournazos S., Li F., Ravetch J.V. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn H.W., Krueger P.D., Davis R.S., Pierce S.K. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118:6332–6341. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S.N., Konrad S., Wiege K. Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a. Eur. J. Immunol. 2009;39:3343–3356. doi: 10.1002/eji.200939884. [DOI] [PubMed] [Google Scholar]
- Szakal A.K., Gieringer R.L., Kosco M.H., Tew J.G. Isolated follicular dendritic cells: cytochemical antigen localization, Nomarski, SEM, and TEM morphology. J. Immunol. 1985;134:1349–1359. [PubMed] [Google Scholar]
- Takai T., Ono M., Hikida M., Ohmori H., Ravetch J.V. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- Takizawa F., Adamczewski M., Kinet J.P. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as Fc gamma RII and Fc gamma RIII. J. Exp. Med. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S.W., Demissie S., Thomas D., Daëron M. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–780. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- Tew J.G., Wu J., Fakher M., Szakal A.K., Qin D. Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol. 2001;22:361–367. doi: 10.1016/s1471-4906(01)01942-1. [DOI] [PubMed] [Google Scholar]
- Tobar J.A., Gonzalez P.A., Kalergis A.M. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J. Immunol. 2004;173:4058–4065. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- Ujike A., Ishikawa Y., Ono M. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J. Exp. Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J.C., Eisen H.N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J. Exp. Med. 1975;142:1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J.C., Scigliano E., Freedman V.H. Structure and function of human and murine receptors for IgG. Annu. Rev. Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Vaughan R.B., Boyden S.V. Interactions of macrophages and erythrocytes. Immunology. 1964;7:118–126. [PMC free article] [PubMed] [Google Scholar]
- Veri M.C., Gorlatov S., Li H. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121:392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Daëron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- Ward E.S., Ober R.J. Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Adv. Immunol. 2009;103:77–115. doi: 10.1016/S0065-2776(09)03004-1. Chapter 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmerdam P.A., van de Winkel J.G., Gosselin E.J., Capel P.J. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J. Exp. Med. 1990;172:19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S., Karlsson M.C., Dahlstrom J. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J. Immunol. 1999;163:618–622. [PubMed] [Google Scholar]
- Westman S., Gustavsson S., Heyman B. Early expansion of secondary B cells after primary immunization with antigen complexed with IgE. Scand. J. Immunol. 1997;46:10–15. doi: 10.1046/j.1365-3083.1997.d01-89.x. [DOI] [PubMed] [Google Scholar]
- Williams A.F., Barclay A.N. The immunoglobulin superfamily-domains for cell surface recognition. Annu. Rev. Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wilson T.J., Gilfillan S., Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. J. Immunol. 2010;185:2960–2967. doi: 10.4049/jimmunol.1001428. [DOI] [PubMed] [Google Scholar]
- Woodward J., Jenkinson E. Identification and characterization of lymphoid precursors in the murine intestinal epithelium. Eur. J. Immunol. 2001;31:3329–3338. doi: 10.1002/1521-4141(200111)31:11<3329::aid-immu3329>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Woof J.M., Kerr M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- Wu Y., Sukumar S., El Shikh M.E. Immune complex-bearing follicular dendritic cells deliver a late antigenic signal that promotes somatic hypermutation. J. Immunol. 2008;180:281–290. doi: 10.4049/jimmunol.180.1.281. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Claypool S.M., Wagner J.S. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Zhao W., Kepley C.L., Morel P.A. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J. Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Kepley C.L., Zhang M., Zhang K., Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat. Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Li R., Li H., Zhou T., Davis R.S. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E1282–E1290. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]


