Overview of Feline Immunodeficiency Virus Infection.
First Described: California, 1986 (Pedersen)1; serologic evidence of infection dates back to the 1960s2., 3.
Cause: Feline immunodeficiency virus (FIV) (family Retroviridae, subfamily Orthoretrovirinae, genus Lentivirus)
Affected Hosts: Domestic and wild cats; hyenas
Geographic Distribution: Worldwide
Mode of Transmission: Biting, and to a lesser extent transplacental, through milk, possibly venereal, blood transfusion
Major Clinical Signs: Lethargy, fever, pallor, stomatitis, diarrhea, muscle atrophy, neurologic signs, signs of underlying neoplastic or immune-mediated disorders or opportunistic infections
Differential Diagnoses: FeLV infection, bartonellosis, other causes of stomatitis such as feline calicivirus infection, immune-mediated disease, other chronic inflammatory and neoplastic diseases of cats that occur in the absence of detectable infection with FIV.
Human Health Significance: FIV does not infect humans.
Etiology and Epidemiology
Feline immunodeficiency virus (FIV) is an enveloped, RNA virus that belongs to the Lentivirus genus of the Retroviridae. It infects domestic and wild cats worldwide, as well as hyenas.4 Like HIV, FIV establishes a chronic, persistent infection that, in some cats, ultimately culminates in immunodeficiency. Because of its similarities to HIV, FIV infection in cats has been used as a research model for HIV infection and acquired immunodeficiency syndrome (AIDS),5 and relative to other viral infections of companion animals, much is known about its pathogenesis.
Knowledge of retroviral structure and replication is required in order to understand diagnostic, treatment, and prevention strategies that target these viruses. Like other retroviruses, FIV has a three-layered structure that is composed of an innermost genome-nucleocapsid complex with helical symmetry, an icosahedral capsid, and an envelope with glycoprotein spikes (Figure 21-1 ). The FIV genome contains three major genes: gag, which encodes the virion core proteins (capsid [p24], nucleocapsid, and matrix); pol, which encodes the reverse transcriptase, protease and integrase enzymes; and env, which encodes surface (gp120) and transmembrane virion (gp41) envelope glycoproteins. Several other accessory and regulatory genes are also present. FIV invades cells via the primary receptor CD134, which is expressed on feline CD4+ T cells, B cells, and activated macrophages,6., 7. and the secondary receptor CXCR4, which is normally a chemokine receptor. The viral envelope fuses with the cell membrane, and the capsid enters the cytoplasm, where reverse transcription occurs and a double-stranded DNA (dsDNA) copy of the retroviral genome is made. Additional sequences, known as long terminal repeats (LTRs), are added to either end of the viral genome. The virus then passes into the nucleus where the dsDNA copy integrates into the host genome; at this point the integrated dsDNA becomes a provirus. Transcription of this DNA, which is controlled by the LTRs, leads to synthesis of new virion components, and virus assembly and budding occur at the cell surface (Figure 21-2 ). Depending on the cellular environment, proviral DNA may either be latent, whereby transcription does not occur, or be transcriptionally active. Latency is one mechanism by which retroviruses can evade the host immune system. Because the reverse transcriptase enzyme is prone to error, the mutation rate of retroviruses is high, and a diversity of viral variants continuously emerges from infected hosts. Integration of retroviral DNA into host cell DNA can disrupt genes that are responsible for cell growth and differentiation (proto-oncogenes). Alternatively, cellular oncogenes captured and carried by retroviruses develop mutations during retroviral replication, and are then re-inserted into host cell DNA. The end result is abnormal cell growth and differentiation, which results in tumor formation by some retroviruses.
FIGURE 21-1.
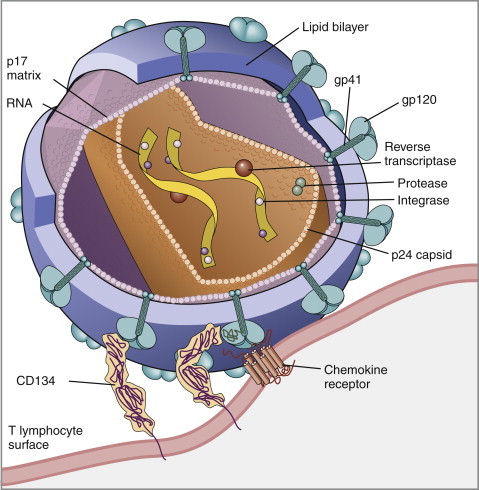
Structure of FIV. An FIV virion is shown adjacent to the surface of a CD4+ T cell. Lentiviruses contain two identical strands of RNA (the viral genome) and associated enzymes, which include reverse transcriptase, integrase, and protease, packaged into a core composed of the p24 capsid protein with a surrounding p17 protein matrix, all enclosed by a phospholipid membrane envelope that is derived from the host cell. Viral encoded membrane proteins (gp41 and gp120) are bound to the envelope. CD134 and CXCR4 (chemokine) receptors on the host cell surface function as the receptors for FIV.
FIGURE 21-2.

The life cycle of FIV. The sequential steps in FIV reproduction are shown, from initial infection of a host cell to release of a new virus particle. An infected cell produces many virions, each capable of infecting nearby cells, with subsequent spread of the infection.
Based on sequence diversity of the env gene, there are six different subtypes of FIV, A through F. Subtypes A and B are distributed most widely, followed by subtype C8., 9., 10.—although a recent study from the United States showed an equal distribution of subtypes A, B, and C (Table 21-1 ).11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31. In addition, a number of recombinant subtypes have been recognized, such as A/B, A/C, B/D, B/E, and A/B/C recombinants, and additional subtypes also likely exist.9., 11. The heterogeneity of the virus complicates the design of molecular diagnostic tests and vaccines for FIV. Whether differences in the clinical manifestations of disease relate to infection by different subtypes requires further study.9., 10., 31.
TABLE 21-1.
Distribution of FIV Subtypes Worldwide
| A | Australia, New Zealand, United States (especially western United States), South Africa, northwestern Europe, Japan, United Kingdom11., 12., 13., 14., 15., 16., 17., 18., 19. |
| B | Central and eastern United States, Caribbean, central and western Europe, Brazil, eastern Japan11., 14., 20., 21., 22., 23., 24., 25. |
| C | United States, Canada, New Zealand, southeast Asia11., 12., 26., 27., 28., 29. |
| D | Japan, Vietnam, rarely United States21., 27., 31. |
| E | Argentina10., 30. |
| F | United States10., 31. |
Retroviruses survive only minutes outside the host and are very susceptible to disinfection. FIV is shed in high concentrations in saliva, and the major mode of transmission is through bites. Transplacental transmission, transmission during parturition, and through milk have been documented experimentally, but these modes appear to be uncommon in naturally infected cats, and kittens infected by this route may not sustain productive infection.32., 33. Venereal transmission has not been demonstrated, but FIV can be recovered from semen, and experimental inoculation of FIV into the vaginas of queens results in transmission.34., 35. Transmission also has the potential to occur through blood donation.
Seropositivity to FIV (which is equivalent to infection because of viral persistence) is consistently associated with a history of bite wounds, older age, male sex, illness, and outdoor access (Table 21-2 ).12., 36., 37., 38., 39., 40., 41. Being of mixed breed has also been a risk factor in some studies. The mean age at diagnosis is around 6 to 8 years, and 80% to 90% of cats are more than 2 years of age. Male cats are up to 4.7 times more likely to be seropositive than female cats.40 Indoor housing decreases transmission but does not eliminate it.42 Worldwide, the seroprevalence of FIV in domestic pet cats ranges from around 1% to 12%.9., 40., 41. A study that included more than 18,000 North American cats estimated the overall prevalence of FeLV and FIV infections as around 2.3% and 2.5%, respectively.5 Higher prevalences are found in feral and free-ranging cats and sick cats. In the North American study, the prevalence of FIV infection in sick, feral cats was 18.2%, whereas that in healthy indoor cats was only 0.7%. A study from France showed a seroprevalence of 21% in unowned cats, compared with 10% in owned cats.43 In Japan, the overall seroprevalence is very high (23% in one study), and as many as one-third of male cats test positive.12 Occasionally, co-infections with FeLV occur, and infection with one retrovirus is a risk factor for infection with the other.36., 37.
TABLE 21-2.
Risk Factors for FIV Infection in Cats Seen at the UC Davis VMTH
| Variable | Number of FIV+ Cats∗ | Number of Control Cats† | Odds Ratio | P-value‡ |
|---|---|---|---|---|
| Sex Male neutered Male intact Female/female neutered |
100 10 17 |
117 5 88 |
— 2.729 0.24Åò |
0.08 <0.001 |
| Breed Mixed Purebred |
116 11 |
170 40 |
— 0.37 |
0.02 |
| Environment Indoor Outdoor |
15 85 |
57 107 |
— 2.58 |
0.01 |
| Stray history No Yes |
101 26 |
195 15 |
— 3.73 |
<0.001 |
| Cats in household <3 ≥3 |
34 39 |
70 61 |
— 1.25 |
0.53 |
All cats were >6 months of age and none had a history of FIV vaccination.
All control cats were negative for FIV antibody at their visit to the University of California, Davis.
P-values <0.05 were significant.
In other words, female cats were four times less likely to be FIV+ than male neutered cats.
Modified from Trott KA, Kass PH, Sparger EE, et al. A clinical case control study: clinical presentation of FIV-positive cats. University of California, Davis, STARS in Science Day. 2007; abstr.
Clinical Features
Signs and Their Pathogenesis
The main cellular target for FIV is the CD4+ T cell. However, FIV also infects CD8+ T cells, B cells, macrophages, and dendritic cells, microglia, and astrocytes. The subsequent effects of the virus on the immune system are complex, incompletely understood, and seem to result in both immune suppression and immune activation.
Three phases of disease have been delineated, acute (primary), subclinical, and terminal, although not all phases are recognized in many naturally infected cats. After inoculation, the virus replicates in lymphoid tissues, and high concentrations of virus are present in blood 2 weeks after infection. A peak of viremia occurs 8 to 12 weeks after infection (Figure 21-3 ). There is a decline in CD4+ and CD8+ T cells in peripheral blood. This may be associated with transient illness, which lasts 3 to 6 months and is often unrecognized by cat owners. Some cats show lethargy, fever, anorexia, diarrhea, stomatitis, weight loss, and/or lymphadenopathy during the acute phase. Lymphadenopathy results from lymphoid hyperplasia, and can persist for weeks to months. Neutropenia can also occur,44 possibly as a result of neutrophil apoptosis. CD4+/CD25+ T regulator (Treg) cells are infected and activated during the acute phase. These cells then inhibit the proliferation of activated CD4+ and CD8+ T cells, and cause them to undergo apoptosis. This may contribute to persistence of FIV and further immunosuppression.45., 46., 47. Altered dendritic cell function may also occur.48., 49. In general, impaired T cell function in acute infection is thought to result from cytokine dysregulation, immunologic anergy (failure to respond to specific antigens), and increased apoptosis.45 Nevertheless, most cats survive this phase because of a rebound in CD8+ T cell numbers and a strong humoral immune response.
FIGURE 21-3.

Changes in virus load and CD4+ T Cell numbers over the course of infection with HIV. A similar clinical course of infection occurs for FIV infection. Viremia is detected early after infection and may be accompanied by systemic signs (acute phase). Plasma viremia then falls to very low levels and remains this way for many years (subclinical phase). Although this period is referred to here as “clinical latency,” the virus itself is not latent during this time, because there is ongoing production of virus and a steady decline in CD4+ T cell counts. In the terminal phase of infection, signs of immunodeficiency develop and plasma viremia increases. Some infected cats never reach this phase. Antibody production declines, and antibody tests may be negative in cats with advanced terminal-phase disease, but PCR assays are more likely to be positive.
In the subclinical (or asymptomatic) phase, CD4+ T cell numbers rebound, and the plasma virus load declines to very low levels. Cats remain subclinically infected, often for years or even for life. This is not latent infection, because virus production continues at low levels; a slow, progressive decline in CD4+ T cell numbers; reduction in the CD4+:CD8+ T cell ratio; and in some cats, hyperglobulinemia, which results from B cell hyperactivation. Some studies also describe a sustained increase in CD8+ T cell numbers. Although activated, paradoxically, T cells have a reduced ability to respond to antigenic stimulation. Altered lymphocyte expression of cell surface molecules (including CD4 and cytokine receptors, and MHC II antigens), and continued alteration of dendritic cell and neutrophil function also contribute to immunosuppression. Dysregulation of cytokine production occurs. For example, cats that are chronically infected with FIV fail to produce Il-2, Il-6, and Il-12 in response to Toxoplasma gondii infection and instead produce elevated levels of the antiinflammatory cytokine Il-10.45., 50. There is a slow influx of immune cells into the brain, with gradual progression of central nervous system (CNS) disease.51 The rate of progression of the subclinical phase depends on factors such as the virus strain, co-infections with other agents that activate virus transcription, and host immunity.
In some cats, these changes ultimately lead to the terminal phase, which is characterized by clinical signs of opportunistic infections, neoplastic disease, myelosuppression, and neurologic disease. This is the phase most commonly recognized in naturally infected cats (Table 21-3 ). However, many infected cats never develop FIV-related clinical signs, even when CD4+ T cell counts are low, and instead die from other causes. The terminal phase of FIV infection is commonly associated with moderate to severe periodontal disease, lymphoplasmacytic stomatitis (Figure 21-4 ), gingivitis, and feline odontoclastic resorptive lesions,52 which may result from opportunistic bacterial and viral infections. Other opportunistic infections include chronic bacterial skin and ear infections, persistent viral upper respiratory tract infections, dermatophytosis, mycobacterial infections, fungal infections such as cryptococcosis and sporotrichosis, hemoplasmosis, toxoplasmosis, and/or parasitic infections such as demodecosis and severe flea burdens. With the exception of stomatitis, the relationship between many of these opportunistic infections and FIV infection is somewhat unclear, because the prevalence of many of these infections in cats with FIV infection is similar to that in cats without FIV infection. However, infections are often more severe and less responsive to treatment than the same infections in immunocompetent cats.
TABLE 21-3.
Disease Diagnoses in 127 Cats with FIV Infection and 210 Age-Matched Control Cats Seen over the Same Time Period at the UC Davis VMTH∗
| Disease | Cats Infected with FIV (n = 127)† | Controls (n = 210)‡ | P-value§ |
|---|---|---|---|
| Intraocular inflammation | 16 | 17 | 0.18 |
| Stomatitis | 21 | 11 | < 0.001 |
| Cardiomyopathy | 21 | 17 | 0.02 |
| Upper respiratory tract disease | 13 | 9 | 0.03 |
| Neurologic signs | 16 | 24 | 0.75 |
| Chronic kidney disease | 23 | 40 | 0.83 |
| Diabetes mellitus | 9 | 18 | 0.63 |
| Hyperthyroidism | 11 | 14 | 0.49 |
| Lymphoma | 23 | 20 | 0.02 |
| Squamous cell carcinoma (SCC) | 12 | 9 | 0.057 |
| Fibrosarcoma | 2 | 9 | 0.17 |
| Carcinomas other than SCC | 4 | 12 | 0.28 |
Results should be interpreted with caution because the data were retrospectively collected. Some cats may have had undiagnosed disease, such as subclinical chronic kidney or cardiac disease. The pathogenesis of a disease process in FIV-positive cats might also differ from that found in FIV-negative cats.
All cats were >6 months of age and none had a history of FIV vaccination.
All control cats were negative for FIV antibody at their visit to the University of California, Davis.
As determined by chi-square (univariate) analysis. P-values <0.05 were significant.
Modified from Trott KA, Kass PH, Sparger EE, et al. A clinical case control study: clinical presentation of FIV-positive cats. University of California, Davis, STARS in Science Day. 2007; abstr.
FIGURE 21-4.

severe lymphoplasmacytic stomatitis in a cat infected with feline immunodeficiency virus. A, There is marked hyperemia, ulceration, and proliferative lesions in the palatoglossal folds. (From Bonello D, Roy CG, Verstraete FJM. Non-neoplastic proliferative oral lesions. In: Verstraete FJM, Lommer MJ, eds. Oral and Maxillofacial Surgery in Dogs and Cats. Philadelphia, PA: Saunders; 2011.) B, Histopathology of a biopsy from a cat with severe stomatitis associated with FIV infection.
The development of neoplasia in FIV-infected cats is thought to result primarily from immune suppression, although there is serologic evidence that cats may be infected with a gammaherpesvirus similar to Epstein-Barr virus that might be reactivated in cats infected with FIV.53 Lymphomas are the most commonly reported FIV-associated tumor, especially B cell lymphomas, but also T cell and non-B, non-T cell lymphomas.54 FIV-infected cats are 5 times more likely to develop lymphoma than cats not infected with FIV and are more likely to develop it an earlier age. Leukemia and squamous cell carcinomas (SCCs) are also common tumors in cats with FIV, although the association with SCC may be confounded by outdoor exposure. Other tumors include mast cell tumors, fibrosarcomas, meningiomas, and metastatic carcinomas; some infected cats develop more than one type of neoplasia. Rarely, lymphoma may develop as a result of viral integration into the genome and disruption of proto-oncogenes.54., 55.
Immune dysregulation and increased circulating immune complexes in the terminal phase can lead to immune-mediated disorders, such as immune-mediated glomerulonephritis and uveitis.56 Myelodysplasia develops in some cats,57., 58. which may be manifested by clinical signs of lethargy, inappetence, pallor, or evidence of bleeding tendencies such as petechial hemorrhages.
The end result of infection by neurovirulent strains of FIV can be progressive behavioral changes such as increased aggression and cognitive disturbances, tremors, sleep disturbances, anisocoria, delayed reflexes, abnormal cranial nerve function, urinary and fecal incontinence, and seizures. Decreased nerve conduction velocities and abnormal electroencephalograms and brainstem evoked potentials have been documented.59 The virus does not infect neurons, but neuronal cell death occurs through multiple incompletely defined mechanisms.51
Inflammatory lesions can develop in a variety of organs in cats infected with FIV. An inflammatory myopathy has been described,60 and terminally, severe muscle atrophy can occur. Infection is associated with gut inflammation and intestinal epithelial cell damage (also known as AIDS enteropathy), which may result in chronic diarrhea and failure to gain weight.61 The extent to which chronic FIV infection contributes to inflammatory or degenerative changes in other organs, such as the myocardium and the kidneys, is not well understood, because the prevalence of cardiomyopathy and interstitial nephritis in geriatric cats not infected with FIV is high (see Table 21-3).
When transplacental transmission occurs in FIV-infected queens, only some kittens in a litter may be infected. In the first year of infection, the overall rate of such transmission is around 70%.62 Rates of mother-to-kitten transmission are highest in queens that have a CD4+ count less than 200 cells/µL, those with signs of immunodeficiency, and those infected within the past 15 months.63 In utero transmission can lead to arrested fetal development, abortion, stillbirth, low birth weights, and the birth of T cell deficient kittens.62., 64.
Physical Examination Findings
Many cats infected with FIV have no signs of illness, or clinical signs that are unrelated to FIV infection. Cats in the acute phase of FIV infection may be febrile or show generalized peripheral lymphadenopathy. Behavioral abnormalities such as obtundation, aggression, and cognitive impairment may be evident in cats with terminal neurologic disease; other neurologic abnormalities include ataxia, anisocoria, and abnormal segmental reflexes. Ocular abnormalities may be present as a result of the FIV infection itself, opportunistic infections, or lymphoma, and include anterior uveitis, hyphema, pars planitis, chorioretinitis, and/or glaucoma.65 Other common physical examination findings in cats with terminal disease are periodontal disease and chronic stomatitis, signs of chronic upper respiratory tract infection, otitis externa, pyoderma, cutaneous abscesses, and a diverse and variable spectrum of disease manifestations that relate to other underlying opportunistic infections or neoplasia.
Diagnosis
For many cats, infection with FIV is diagnosed during screening efforts. Screening for infection should be performed with tests that detect antibody against FIV, because these assays have the highest overall sensitivity and are rapid and widely available (Table 21-4 ). It has been recommended that the retrovirus status of all cats be known regardless of the presence of absence of illness.66 Indications for testing as recommended by the American Association of Feline Practitioners (AAFP) are shown in Box 21-1 . In practice, compliance with retrovirus testing is low. In a study that evaluated 967 cats with bite wounds or cutaneous abscesses that presented to veterinary practitioners from 134 practices in 30 states, the combined FeLV-FIV status of only 96 (9.9%) of the cats was known.38 In addition, despite the availability of a financial incentive for retesting, only 64 of 478 cat owners returned their cats for retesting after treatment.
TABLE 21-4.
Diagnostic Assays Available for Feline Immunodeficiency Virus Infection
| Assay | Specimen Type | Target | Performance |
|---|---|---|---|
| Serology (ELISA, Western blotting, IFA) | Blood, serum | Antibody to FIV | Positive test results in cats >6 months of age that have not been vaccinated for FIV equal infection, but confirmation is recommended in healthy cats using a second test from a different manufacturer or Western blot. False positives occur in cats <6 months of age (maternal antibody) and cats vaccinated with the FIV vaccine. False negatives can occur in the first 2 months of infection or with advanced disease and severe immunosuppression. |
| PCR | Blood | FIV proviral DNA or viral RNA | Sensitivity and specificity vary between laboratories. Even well-designed assays may be insensitive because of viral variability and low-level viremia. PCR assays are the only assay that can definitively diagnose infection in cats vaccinated for FIV. Never use without performing serology. |
| Virus isolation | Blood | FIV | Difficult, not widely available. Used primarily as a research tool. |
IFA, immunofluorescent antibody.
BOX 21-1. Indications for Serologic Testing for FIV and FeLV.
-
•
Sick cats, even if tested negative in the past
-
•
All newly acquired cats or kittens (2 tests, at least 60 days apart)
-
•
After exposure to a retrovirus-infected cat or a cat with unknown status, and especially after a bite wound (2 tests, at least 60 days apart)
-
•
Cats that live in a household with other retrovirus-infected cats (annual retesting unless isolated)
-
•
Before initial vaccination with FeLV or FIV vaccines
-
•
Before use as a blood donor (in conjunction with real-time PCR)
-
•
On entry to a shelter or before adoption (2 tests, at least 60 days apart); if financial resources are not available for this, cats should be held singly and postadoption testing (2 tests, at least 60 days apart) recommended before mingling with other cats occurs. The status of the other cats in the household should be known before new cats are introduced.
-
•
For group-housed cats, before introduction (2 tests, at least 60 days apart if possible) and on an annual basis
-
•
Testing is considered optional for feral cat trap-neuter-return programs
Modified from Levy J, Crawford C, Hartmann K, et al. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg 2008;10:300-316.
When positive test results occur in sick cats, the role that FIV plays as a cause of the signs may be unclear, although it is reasonable to assume that FIV may be playing a role in cats with severe stomatitis, unusual infections with intracellular pathogens such as mycobacteria, and lymphoma. With refinement and improved availability of test methodologies in the future, clinical assessment of CD4+ T cell counts, the CD4+/CD8+ ratio, and plasma viral loads may facilitate interpretation of the relationship between disease manifestations and infection and provide prognostic information, as in human patients infected with HIV.67
Laboratory Abnormalities
Complete Blood Count
Common abnormalities on the CBC in cats infected with FIV include mild anemia, lymphopenia, and neutropenia. Occasionally severe anemia, thrombocytopenia, thrombocytosis, monocytopenia, or leukocytosis occur. In one large European study, neutrophil counts of FIV-infected cats were lower than those of control cats, and lymphocyte counts were higher than those of control cats.68 Leukopenia and neutropenia were more likely to be present in FIV-infected cats.
Serum Biochemical Tests
The most common and significant abnormality on the chemistry panel in cats infected with FIV is hyperproteinemia, which results from increased γ-globulin concentrations and is a direct result of FIV infection (rather than the result of opportunistic infection). Total protein concentrations that ranged from 4.5 to 11 g/dL were reported in one study.68 Other findings are variable and relate to the presence of concurrent disease, neoplasia, or opportunistic infections.
Urinalysis
Urinalysis may reveal proteinuria in cats with glomerulonephritis.
Coagulation Testing
Cats infected with FIV can have mild prolongations in their APTT, thrombin time, and fibrinogen concentrations, although the PT and platelet function are generally normal.69
Cytologic Evaluation of Bone Marrow
Bone marrow cytologic evaluation in FIV-infected cats with cytopenias and nonregenerative anemias may show mild dysplasia (usually not as severe as in FeLV infections); erythroid hypoplasia; and/or myeloid hyperplasia despite peripheral leukopenia, sometimes with a left shift. The latter suggests ineffective hematopoiesis or maturation arrest.
Microbiologic Tests
Serologic Diagnosis
The initial assay of choice for diagnosis of, and screening for, FIV infection is an ELISA assay that detects antibody to FIV. Provided there has not been a history of vaccination for FIV and the tested cat is less than 6 months of age, positive antibody test results equate with infection, because the virus establishes a lifelong, persistent infection. Point-of-care, lateral-flow ELISA assays and diagnostic laboratory-based ELISA assays are in widespread use and have rapid turnaround times and high sensitivities and specificities.70 These assays usually detect antibodies to the FIV p24 core protein, and sometimes to the gp40 transmembrane protein. False positive test results occur rarely as a result of operator error or nonspecific reactivity against tissue culture components after vaccination.71 When Western immunoblotting was used as the gold standard, the sensitivities, specificities, and positive and negative predictive values of six different ELISA assays (Synbiotics Witness, Viracheck FIV, IDEXX SNAP Combo Plus, PetChek Plus Anti-FIV, MegaCor Fastest, and Bio Veto Duo Speed) in one U.S. study of 535 cats with an overall FIV seroprevalence of 10.3% ranged from 94.5% to 100%, 98.5% to 100%, 91.2% to 100%, and 99.2% to 100%, respectively.70 The Mapic FIV test had a high (23.1%) rate of invalid test results and was not recommended for clinical practice. When these assays are used to screen healthy cats for infection, confirmation of positive results is recommended because of the low prevalence of infection in this population of cats and the higher possibility that false-positive test results may occur. Confirmation can be done using an assay from a different manufacturer or Western blotting. However, Western blotting is technically demanding, subject to interoperator variability in interpretation, and it may be less sensitive than ELISA assays when performed by some laboratories.72
Positive ELISA assay results in the absence of FIV infection can occur in cats that have been vaccinated for FIV, or kittens less than 6 months of age that possess maternal antibody (because of infection or vaccination of the queen). No currently available serologic test, including Western immunoblotting, distinguishes between natural infection and vaccination or the presence of maternal antibody. Kittens that test positive should be retested after 6 months of age (the Advisory Bureau on Cat Diseases recommends retesting after 4 months of age).66., 73. Nevertheless, kittens less than 6 months of age should still be tested, because the vast majority of negative kittens will be declared free of infection. Molecular testing could be considered to confirm infection in kittens that test positive at less than 6 months of age (see later discussion). Cats with a history of FIV vaccination may remain antibody-positive for more than 4 years.66 Because infection can occur in the face of vaccination, positive test results in a vaccinated cat may represent infection and/or historical vaccination. Currently, molecular testing with PCR assays is required to identify infection in these cats, but some infected cats test PCR-negative. An ELISA assay has been developed that can distinguish naturally infected from vaccinated cats, but this assay is not commercially available. The assay was used to test blood samples from 73 uninfected, unvaccinated cats; 89 uninfected, FIV-vaccinated cats; and 102 FIV-infected cats, including 3 cats that had been vaccinated.9 The assay had a sensitivity of 97% and a specificity of 100% for detection of FIV infection in these cats.
False-negative ELISA assay results occur early in the course of illness, because cats may take up to 60 days to develop an antibody response.66 Rarely, antibody production is delayed for 6 months or longer. Thus when recent exposure is possible, testing should be repeated a minimum of 2 months later. False-negative test results also occur in cats in the terminal phase of disease, as a result of impaired antibody production, or in kittens with rapidly progressive infections. These cats often have high plasma viral loads. Thus, if advanced FIV infection is suspected, negative test results should be followed by virus detection using PCR. When serology is used as a screening test, negative results are considered to be highly reliable because of the high sensitivity of the test and the low prevalence of infection in most populations of healthy cats.66
Immunofluorescent antibody (IFA) assays that detect antibodies to FIV have also been described. When performed by experience laboratory personnel, these have sensitivities and specificities that range from 95% to 100% when compared with Western immunoblotting.71
Molecular Diagnosis Using the Polymerase Chain Reaction
A variety of PCR assays have been developed for diagnosis of FIV infection. Assays may detect viral RNA (reverse transcriptase [RT]-PCR), proviral DNA, or both RNA and proviral DNA in peripheral blood. Because the FIV vaccine is inactivated and does not replicate or integrate into the host genome, PCR assays should not detect vaccine virus in cats that have been vaccinated for FIV. Compared with serology, PCR can be insensitive (sensitivity <80%), because viral loads in healthy cats are often extremely low, and some strains may not be detected because of variability in the sequence of the viral genome among FIV isolates. Sensitivity is likely to be higher in cats in the acute and terminal phases of disease when viral loads are higher, but this requires further study. False-positive test results have the potential to occur as a result of laboratory contamination; in some commercial laboratories, unacceptable sensitivities and specificities have been reported (as low as 41% and 44%, respectively).74., 75. In advertising materials, IDEXX Laboratories report an assay sensitivity and specificity of 80.5% and 99.9%, respectively, among 36 FIV-infected cats, 96 uninfected vaccinated cats and 92 uninfected unvaccinated cats, with no difference in specificity in the latter two groups.76 A FRET real-time PCR assay directed at the gag gene has been described that differentiates between FIV subtypes (see Chapter 5 for an explanation of FRET PCR assays).11 The assay was positive in 60% of 101 cats with positive antibody tests for FIV. A history of vaccination with the FIV vaccine was present in 13 of the cats, and all these cats tested negative. The vaccination history of the remaining cats was unknown. Given their limitations, PCR assays should not be used for diagnosis of FIV infection in the absence of concurrent serologic testing, and the results of PCR assays should not be used to decide whether vaccination should be performed. Cats that are seronegative that test positive with PCR may be in the terminal phase of disease and unable to produce antibody, or the PCR assay result may be a false positive. Latently infected cats have rarely been described that test positive for proviral DNA but negative for antibody.76 These cats lack any immunologic or clinical abnormalities that occur with active infection.76 Whether these cats are likely to develop productive infection (as reported in humans with latent HIV infection) is unknown.77
Virus Isolation
FIV can be isolated from peripheral blood lymphocytes in primary feline T cell cultures, but this is technically demanding and expensive and can take 2 to 3 weeks, so it is not used for routine diagnostic purposes.
Pathologic Findings
Gross pathologic findings in cats with FIV infection include emaciation, stomatitis, lymphadenopathy, and evidence of secondary neoplasia or opportunistic infections. Evidence of disorders of aged cats, such as hyperthyroidism, cardiomyopathy, and interstitial nephritis, may also be present. Histopathologic changes in cats with FIV infection include follicular hyperplasia or, in the chronic phase, lymphoid depletion in the paracortical regions and plasma cell infiltration of lymph nodes (Figure 21-5 ); lymphoplasmacytic ulcerative stomatitis (see Figure 21-4, B); and bone marrow pathology as described previously in this chapter. Evidence of chronic interstitial nephritis, sometimes with membranous glomerulonephritis and glomerulosclerosis, may be present. Inflammatory changes may be seen within skeletal muscle and/or the gastrointestinal tract. Histopathology of the CNS in cats infected with neurovirulent FIV strains may show mild lymphoplasmacytic meningitis, perivascular lymphocytic infiltrates, diffuse gliosis, microglial nodules, and mild neuronal degeneration and apoptosis.59., 78. Neuronal dysfunction may be more important than neuronal loss.59
FIGURE 21-5.

A, Depletion of paracortical areas in the mesenteric lymph node of an 8-year-old female spayed domestic shorthair with FIV infection that was euthanized as a result of the development of progressive neurologic signs. B, Normal feline lymph node for comparison purposes.
Treatment and Prognosis
Supportive Care
Cats in the terminal phase of FIV infection may require fluid therapy, nutritional support, regular dental prophylaxis, dilute chlorhexidine-based mouth washes or oral gels, and dental extractions and antimicrobial drugs with activity against anaerobes for severe stomatitis (Table 21-5 ). Whole mouth extractions have been beneficial for some cats with severe stomatitis, but it is critical that all tooth roots be removed. Referral to a board-certified veterinary dentist should be considered. Topical glucocorticoids (e.g., 1% prednisolone acetate, every 4 to 12 hours depending on severity) and topical atropine are indicated for cats with anterior uveitis. Systemic glucocorticoids should only be used if absolutely necessary, because their use is associated with increased plasma viremia. Some cats with advanced neurologic signs show clinical improvement after treatment with glucocorticoids. Opportunistic infections may respond to appropriate antimicrobial treatment, but prolonged or lifelong treatment may be required. Griseofulvin should not be used to treat dermatophytosis, because it has been associated with bone marrow suppression in FIV-positive cats.79 Recombinant human erythropoietin or darbepoetin may be useful for some cats with nonregenerative anemia and does not appear to increase viral load through activation of transcription of latent virus.80 Although recombinant human granulocyte colony stimulating factor (G-CSF) can increase neutrophil counts in cats infected with FIV, antibodies can develop within a few weeks that cross-react with endogenous G-CSF. When recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) was used, an increase in virus load occurred, so the use of these drugs is not recommended.
TABLE 21-5.
Suggested Medications for Treatment of Cats in the Terminal Phase of Feline Immunodeficiency Virus Infection
| Drug | Dose | Route | Interval (hours) | Comments |
|---|---|---|---|---|
| Zidovudine (AZT) | 5 to 15 mg/kg | PO | 12 | May have benefit for cats with neurologic signs or stomatitis. Monitor CBC (see text). Toxicity may be more likely at doses above 5 mg/kg. |
| Human recombinant interferon alpha | 1 to 50 U/cat | PO | 24 | For stomatitis |
| 0.12% Chlorhexidine plus zinc gluconate oral gel | 1 mL | Topical | 12 to 48 | For stomatitis |
| Clavulanic acid–amoxicillin | 12.5 to 25 mg/kg | PO | 12 | For stomatitis |
| Lactoferrin | 200 mg powder or 40 mg/kg solution | Topical | 24 | For refractory gingivitis and stomatitis. Must be purchased from chemical suppliers. |
| Prednisolone | 5 mg | PO | 24 | For refractory stomatitis or advanced neurologic disease. May also be required for lymphoma or other CNS neoplasms. Use only if absolutely necessary. |
Antiviral and Immunomodulator Drugs
Topical lactoferrin administration has been associated with clinical improvement in some FIV-infected cats with stomatitis (see Chapter 7). Cats with stomatitis or neurologic disease may benefit from oral or subcutaneous treatment with zidovudine (AZT), but AZT can cause bone marrow suppression, so the CBC must be monitored weekly for the first month and monthly thereafter. Treatment is not recommended for cats with myelosuppressive disease.73 AZT should be discontinued if the hematocrit drops below 20%, after which anemia usually resolves in a few days.73 AZT-resistant FIV mutants can develop as early as 6 months after the start of treatment. Fozivudine (45 mg/kg PO q12h) reduces viremia in cats with acute FIV infection and may be less likely to produce hematologic adverse effects.81 Plerixafor (AMD3100), a selective antagonist of the CXCR4 receptor, is active against FIV in vitro.82 When used in 40 naturally infected cats for 6 weeks in a placebo-controlled double-blind study, a decreased proviral load was reported without evidence of adverse effects, but there was no improvement in clinical or immunologic variables.73., 83.
Results of treatment with human recombinant IFN-α have been mixed, but prolonged survival was reported in 24 FIV-infected cats after oral administration of a low dose of the drug when compared with 6 untreated control cats.83 Clinical scores and laboratory parameters improved in some sick, naturally infected cats that were treated with recombinant feline IFN-ω, despite no significant changes in viral load.84
Management of FIV-Infected Cats
Cats that test positive for FIV should be housed indoors to prevent transmission to other cats, as well as to protect them from other infections. The latter is important even in subclinically infected cats, as other infections have the potential to activate viral transcription and accelerate disease progression. The feeding of raw foods and hunting behavior should be avoided. Cats should be neutered to reduce the chance of roaming and aggressive interactions with other cats. Minimally, FIV-positive cats should be rechecked every 6 months in order to monitor body weight, assess for periodontal disease, and discuss the need for routine laboratory testing and vaccination with core vaccines. A complete physical examination, CBC, serum biochemistry panel, and urinalysis are recommended on an annual basis.66 At least during acute FIV infection, cats can mount an immune response to vaccines,85., 86. but because vaccination with core vaccines may activate viral transcription, vaccination should be performed only for FIV-positive cats that are likely to be exposed to other cats. The use of inactivated vaccines is recommended, because of the potential for vaccine-induced disease in immunosuppressed cats.73 When hospitalized, FIV-infected cats should be kept in separate cages away from isolation, where there may be other cats with transmissible infectious diseases. Perioperative antimicrobial drug treatment could be considered for cats that undergo invasive surgery or dental treatments.66., 73.
Prognosis
A limited number of studies have shown no significant difference in life span between FIV-infected cats and uninfected cats.42., 87. In one study, the median survival times of these two groups after testing for FIV infection were 3.9 (39 cats) and 5.9 (22 cats) years, respectively.87 In a larger study of more than 1000 FIV-infected cats and more than 8000 age- and sex-matched control cats, the median survival times of the two groups were 4.9 years and 6.0 years, respectively.66 The progression and severity of disease is related to virus strain and host immunity. Infection of geriatric and neonatal cats is associated with more rapid progression and severity of disease than infection of young adult cats.84., 88. For example, progression to terminal immunodeficiency can occur within 2 months in kittens.89 Because the life span of FIV-infected cats may not differ greatly from that of uninfected cats, no cat should be euthanized on the basis of a positive FIV test alone.66., 73. However, other factors, such as control of the infection in group-housed or breeding cats, may necessitate euthanasia or rehoming of some cats that test positive. Once terminal FIV-related disease occurs, life spans are typically less than 1 year.
Immunity and Vaccination
The FIV vaccine first became available in the United States in July 2002 (Fel-O-Vax FIV, Fort Dodge Animal Health) and is an inactivated, adjuvanted, whole-virus vaccine that contains FIV Petaluma (subtype A) and FIV Shizuoka (subtype D). The vaccine has been available in New Zealand and Australia since 2004. It is licensed for the vaccination of healthy cats that are 8 weeks of age or older as an aid in the prevention of FIV, and in experimental challenge studies, protected 60% to 80% of cats from infection. The introduction of the vaccine generated controversy, because (1) existing serologic assays cannot differentiate between natural infection and vaccination, (2) vaccination provides only partial protection from infection, and (3) PCR assay results cannot be relied upon in vaccinated cats. Although concerns exist that the vaccine may only protect against infection with strains from homologous subtypes, studies have shown moderate protection against infection with subtype B strains.90., 91. In one study, 10 of 14 cats were protected from subtype B infection when challenged 1 year after vaccination compared with none of 5 control cats.90 However, 13-week-old vaccinated cats were not protected from challenge with FIV subtype A strain Glasgow-8; all vaccinated and control cats were infected.92
Because of diagnostic test interference, uncertain efficacy in the field, and the increased risk of sarcoma formation associated with adjuvanted vaccines, the FIV vaccine is considered a noncore vaccine that should only be used for cats at high risk of infection, such as outdoor cats that fight with other cats. The initial vaccine should only be given to cats that test negative with FIV ELISA assays. Cat owners should be informed that protection is incomplete and that identification of subsequent infection may be impossible in some cats with the available diagnostic tests. The initial vaccine dose is followed by two booster doses at 3- to 4-week intervals, followed by annual boosters so long as risk persists. An identification microchip should be placed that links FIV vaccination to the microchip number, so should the cat escape or be relinquished to a shelter, a positive antibody test result is not interpreted as FIV infection.
Prevention
Transmission of FIV is reduced when cats are housed indoors. When one positive cat is identified in a household, all other cats should be tested and no new cats should be introduced, as this may lead to conflict and increased fighting behavior. Isolation of positive cats in a household should be considered. In breeding catteries, infection can be prevented by screening all new introductions, preferably with a 2-month quarantine period for introductions followed by retesting, and removal of all infected queens and toms from the cattery. Transmission in shelters can be reduced by testing on intake or housing cats singly in cages, and testing at or shortly after adoption, together with education of adopters in regard to the need to retest for infection.
Surgical needles, endotracheal tubes, breathing circuits, and instruments should never be shared between cats without proper cleaning and disinfection, and all cats used as blood donors should test negative for FIV antibodies with ELISA and, if possible, PCR assays offered by a diagnostic laboratory that has a high level of quality control. Fluid lines and multidose vials should not be shared between cats, because they can become contaminated with body fluids.
Public Health Aspects
Despite its similarities to HIV, no evidence of natural infection of humans with FIV exists.
CASE EXAMPLE.
Signalment
“Arthur” an 11-year-old male neutered domestic shorthair from northern California
History
Arthur was initially brought to his veterinarian for a 2-day history of lethargy and decreased appetite. Physical examination was unremarkable. Blood work showed a hematocrit of 28% (reference range, 29% to 38%), a white cell count of 6400 cells/µL with a normal differential, clumped platelets, mild hyperbilirubinemia (0.5 mg/dL), mild hypoalbuminemia (2.4 g/dL), and hyperglobulinemia (5.5 g/dL). He was treated with amoxicillin and mirtazapine, but lethargy and inappetence persisted. Serial hemograms performed over the subsequent 3 months showed progressive leukopenia due to a neutropenia and lymphopenia (Table 21-6 ), during which time Arthur was treated with orbifloxacin, with some improvement in his appetite and attitude. A bone marrow aspirate performed 5 weeks after the onset of illness showed myeloid hyperplasia with a left shift. Serology for FIV and FeLV was performed 11 weeks after onset of illness, and the cat was negative for FeLV antigen and had an equivocal test result for FIV antibody. Serum thyroxine concentration was low (0.7 mcg/dL, reference range 1.1 to 3.3 mcg/dL). The cat was referred for further evaluation.
TABLE 21-6.
Progression of Laboratory Abnormalities in an 11-Year-old Male Neutered Domestic Shorthair That Tested Positive for Antibodies to FIV 3 Years Earlier, at Which Time a CBC was Normal
| Days after Onset of Illness |
||||||
|---|---|---|---|---|---|---|
| 39 | 47 | 56 | 74 | 83 | Reference Range | |
| Hematocrit (%) | 29 | 29 | 33 | 27 | 22 | 30-50 |
| White blood cells/µL | 2000 | 2100 | 1000 | 1100 | 1100 | 4500-14,000 |
| Neutrophils/µL | 1180 | 1407 | 610 | 572 | 704 | 2000-9000 |
| Lymphocytes/µL | 700 | 588 | 340 | 462 | 220 | 1000-7000 |
| Platelets/µL | 191,000 | 196,000 | Clumped | Clumped | 32,000 | 180,000-500,000 |
| FIV/FeLV serology∗ | Equivocal/ negative |
Negative/ negative |
||||
SNAP FIV/FeLV Combo Test, IDEXX Laboratories.
Arthur was an indoor and outdoor cat and had a history of predation (mice and birds) and fighting with other cats in the neighborhood. His diet otherwise consisted of a commercial wet cat food. He was vaccinated regularly for rabies, FeLV, feline herpesvirus-1, feline calicivirus, and feline panleukopenia virus infections.
Current Medications
Orbifloxacin, 7 mg/kg PO q12h
Other Medical History
Routine blood work had been performed 3 years previously, which showed a normal CBC, a globulin of 5 g/dL, a urine specific gravity of 1.069, and a T4 of 1.3. At that time, Arthur tested negative for FeLV antigen and positive for FIV antibody.
Physical Examination
Body Weight
3.1 kg
General
Quiet, alert, responsive, estimated to be 5% to 7% dehydrated, T = 103.9° F (39.9° C), HR = 144 beats/min, RR = 50 breaths/min, mucous membranes pale pink, CRT < 2 s. A dry haircoat with moderate amounts of scale was present. There was no evidence of ectoparasites.
Eyes, Ears, Nose, and Throat (with Dilated Fundoscopic Examination)
Moderate gingivitis and absent mandibular canine teeth. No other clinically significant abnormalities were detected.
Musculoskeletal
Body condition score 2/9. Generalized muscle atrophy was noted. Ambulatory in all four limbs.
Cardiovascular
Aside from an intermittent gallop rhythm, no abnormalities were noted.
Respiratory
No abnormalities were detected.
Gastrointestinal and Urogenital
Abdomen soft and nonpainful on palpation, mild hepatomegaly. Urinary bladder was small and soft.
Lymph Nodes
All lymph nodes less than 1 cm in diameter.
Laboratory Findings
CBC
HCT 22.5% (30%-50%)
MCV 47.5 fL (42-53 fL)
MCHC 32.9 g/dL (30-33.5 g/dL)
Reticulocytes 7400 cells/µL
WBC 1100 cells/µL (4500-14,000 cells/µL)
Neutrophils 704 cells/µL (2000-9000 cells/µL)
Band neutrophils 99 cells/µL
Lymphocytes 220 cells/µL (1000-7000 cells/µL)
Monocytes 77 cells/µL (50-600 cells/µL)
32,000 platelets/µL (180,000-500,000 platelets/µL).
Neutrophils showed moderate toxicity with many Döhle bodies, and there were many macroplatelets.
Serum Chemistry Profile
Sodium 149 mmol/L (151-158 mmol/L)
Potassium 3.1 mmol/L (3.6-4.9 mmol/L)
Chloride 115 mmol/L (117-126 mmol/L)
Bicarbonate 17 mmol/L (15-21 mmol/L)
Phosphorus 4.3 mg/dL (3.2-6.3 mg/dL)
Calcium 8.8 mg/dL (9.0-10.9 mg/dl)
BUN 17 mg/dL (18-33 mg/dL)
Creatinine 1.1 mg/dL (1.1-2.2 mg/dL)
Glucose 158 mg/dL (63-118 mg/dL)
Total protein 8.1 g/dL (6.6-8.4 g/dL)
Albumin 3.0 g/dL (2.2-4.6 g/dL)
Globulin 5.1 g/dL (2.8-5.4 g/dL)
ALT 121 U/L (27-101 U/L)
AST 113 U/L (17-58 U/L)
ALP 10 U/L (14-71 U/L)
Creatine kinase 278 U/L (73-260 U/L)
Gamma GT <3 U/L (0-4 U/L)
Cholesterol 204 mg/dL (89-258 mg/dL)
Total bilirubin 0.8 mg/dL (0-0.2 mg/dL)
Magnesium 2.4 mg/dL (1.5-2.5 mg/dL).
Anti-nuclear Antibody Serology
Positive at 1:32
Microbiologic and Virologic Testing
Point-of-care ELISA serology for FeLV antigen and FIV antibody: negative
Feline coronavirus antibody serology: negative at 1:25
Aerobic and anaerobic blood cultures (three specimens): negative
PCR for FIV proviral DNA: positive (cycle threshold value = 30, values <40 are positive)
Imaging Findings
Abdominal Ultrasound
Hepatomegaly was identified and the spleen had a mottled echotexture. There was mild enlargement of the ileocecal lymph nodes and mild diffuse thickening of the small intestinal submucosal layer.
Thoracic Radiographs
Cardiopulmonary structures were within normal limits.
Cytologic Findings
Liver Aspiration Cytology
A moderately bloody background contained scattered, variably sized clusters of uniform hepatocytes and low numbers of nucleated cells. The nucleated cells consisted of a mixture of nondegenerate neutrophils, lymphocytes, macrophages, and rare myeloid precursors. Several macrophages were erythrophagic. Interpretation: Possible mild myeloid extramedullary hematopoiesis.
Spleen Aspiration Cytology
The specimen was highly cellular with a pink background that contained a moderate amount of blood and scattered clumps of hemosiderin. A mixed population of lymphocytes was admixed with variable numbers of hematopoietic precursor cells. Hematopoietic precursors were primarily granulocytic precursors, with many progranulocytes. A few erythroid precursors, including erythroblasts, were noted. Plasma cells were moderately increased in number, and both immature and mature forms were seen. Several binucleated and rare trinucleated plasma cells and scattered immature lymphocytes were noted. Vacuolated macrophages were increased in number and often contained phagocytized erythrocytes or blue-green pigment consistent with hemosiderin. Scattered mitotic figures were noted. Interpretation: Moderate plasmacytosis, extramedullary hematopoiesis, and histiocytic hyperplasia with erythrophagocytosis and increased iron. The large number of immature plasma cells raised the possibility of plasma cell neoplasia; however, reactive plasmacytosis could not be ruled out.
Bone Marrow Aspiration Cytology
Numerous hypercellular unit particles were present. A few mature megakaryocytes were noted. Hematopoietic cells consisted largely of myeloid precursors, with an estimated myeloid to erythroid ratio greater than 10. Myeloid cells showed orderly maturation to the band neutrophil stage, with few segmented neutrophils. Few erythroid precursors were noted, which included rare prorubricytes, rubricytes, and metarubricytes. Scattered well-differentiated plasma cells and a small amount of hemosiderin were noted. Interpretation: Marked myeloid hyperplasia, marked erythroid hypoplasia, and moderate megakaryocytic hypoplasia. The myeloid hyperplasia together with persistent neutropenia suggested myelodysplasia.
Serum Protein Electrophoresis
A mild increase in α2 globulins was present (1.34 g/dL, reference range, 0.4-0.9 g/dL) together with a broad-based gamma region. This was consistent with an acute-phase inflammatory response.
Diagnosis
Terminal phase of FIV infection, characterized by pancytopenia secondary to myelodysplasia; hepatopathy (open diagnosis).
Comments
The progression of FIV infection was apparent in this cat, which transitioned from a seropositive to a seronegative, PCR-positive state as a result of progressive decline in immune function and, ultimately, failure to produce antibodies. The cause of the hepatopathy was not identified. Blood cultures were negative, and spleen and liver aspiration cytology failed to reveal evidence of underlying neoplasia. Because of the plasmacytosis in the spleen, serum protein electrophoresis was performed to assist diagnosis of a plasma cell tumor (as supported by a monoclonal gammopathy), but this was not apparent. The cat was treated with clavulanic acid–amoxicillin without clinical improvement. Treatment with glucocorticoids was also offered because of the possibility of undiagnosed round cell neoplasia or immune-mediated neutropenia (in light of the myeloid hyperplasia combined with a positive antinuclear antibody test). The cat died at home 1 month later, and necropsy was not performed.
Suggested Readings
- Goldkamp C.E., Levy J.K., Edinboro C.H. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats with abscesses or bite wounds and rate of veterinarian compliance with current guidelines for retrovirus testing. J Am Vet Med Assoc. 2008;232:1152–1158. doi: 10.2460/javma.232.8.1152. [DOI] [PubMed] [Google Scholar]
- Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet Immunol Immunopathol. 2011;143(3-4):190–201. doi: 10.1016/j.vetimm.2011.06.003. (for a review of molecular pathogenetic mechanisms of disease) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Addie D., Belak S. Feline immunodeficiency. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:575–584. doi: 10.1016/j.jfms.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Crawford C., Hartmann K. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg. 2008;10:300–316. doi: 10.1016/j.jfms.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Pedersen N.C., Ho E.W., Brown M.L. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 2.Gruffydd-Jones T.J., Hopper C.D., Harbour D.A. Serological evidence of feline immunodeficiency virus infection in UK cats from 1975-76. Vet Rec. 1988;123:569–570. doi: 10.1136/vr.123.22.569. [DOI] [PubMed] [Google Scholar]
- 3.Reid R.W., Barr M.C., Scott F.W. Retrospective serologic survey for the presence of feline immunodeficiency virus antibody: a comparison of ELISA and IFA techniques. Cornell Vet. 1992;82:359–369. [PubMed] [Google Scholar]
- 4.Troyer J.L., Pecon-Slattery J., Roelke M.E. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elder J.H., Lin Y.C., Fink E. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Curr HIV Res. 2010;8:73–80. doi: 10.2174/157016210790416389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Parseval A., Chatterji U., Sun P. Feline immunodeficiency virus targets activated CD4+ T cells by using CD134 as a binding receptor. Proc Natl Acad Sci U S A. 2004;101:13044–13049. doi: 10.1073/pnas.0404006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimojima M., Miyazawa T., Ikeda Y. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- 8.Samman A., McMonagle E.L., Logan N. Phylogenetic characterisation of naturally occurring feline immunodeficiency virus in the United Kingdom. Vet Microbiol. 2011;150:239–247. doi: 10.1016/j.vetmic.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward J.J., Rodrigo A.G. Molecular epidemiology of feline immunodeficiency virus in the domestic cat (Felis catus) Vet Immunol Immunopathol. 2010;134:68–74. doi: 10.1016/j.vetimm.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver E.A. A detailed phylogenetic analysis of FIV in the United States. PLoS One. 2010;5:e12004. doi: 10.1371/journal.pone.0012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C., Johnson C.M., Ahluwalia S.K. Dual-emission fluorescence resonance energy transfer (FRET) real-time PCR differentiates feline immunodeficiency virus subtypes and discriminates infected from vaccinated cats. J Clin Microbiol. 2010;48:1667–1672. doi: 10.1128/JCM.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y., Ura A., Hirata M. An updated nation-wide epidemiological survey of feline immunodeficiency virus (FIV) infection in Japan. J Vet Med Sci. 2010;72:1051–1056. doi: 10.1292/jvms.09-0574. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann M.H., Mathiason-Dubard C., Learn G.H. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinrigl A., Klein D. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: a prerequisite for vaccination and molecular diagnostics. J Gen Virol. 2003;84:1301–1307. doi: 10.1099/vir.0.18736-0. [DOI] [PubMed] [Google Scholar]
- 15.Kann R., Seddon J., Kyaw-Tanner M. Phylogenetic analysis to define feline immunodeficiency virus subtypes in 31 domestic cats in South Africa. J S Afr Vet Assoc. 2006;77:108–113. doi: 10.4102/jsava.v77i3.356. [DOI] [PubMed] [Google Scholar]
- 16.Kann R.K., Kyaw-Tanner M.T., Seddon J.M. Molecular subtyping of feline immunodeficiency virus from domestic cats in Australia. Aust Vet J. 2006;84:112–116. doi: 10.1111/j.1751-0813.2006.tb13392.x. [DOI] [PubMed] [Google Scholar]
- 17.Kann R.K., Seddon J.M., Meers J. Feline immunodeficiency virus subtypes in domestic cats in New Zealand. N Z Vet J. 2007;55:358–360. doi: 10.1080/00480169.2007.36795. [DOI] [PubMed] [Google Scholar]
- 18.Kann R., Seddon J., Kyaw-Tanner M. Co-infection with different subtypes of feline immunodeficiency virus can complicate subtype assignment by phylogenetic analysis. Arch Virol. 2007;152:1187–1193. doi: 10.1007/s00705-007-0940-2. [DOI] [PubMed] [Google Scholar]
- 19.Iwata D., Holloway S.A. Molecular subtyping of feline immunodeficiency virus from cats in Melbourne. Aust Vet J. 2008;86:385–389. doi: 10.1111/j.1751-0813.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 20.Sodora D.L., Shpaer E.G., Kitchell B.E. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J Virol. 1994;68:2230–2238. doi: 10.1128/jvi.68.4.2230-2238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakinuma S., Motokawa K., Hohdatsu T. Nucleotide sequence of feline immunodeficiency virus: classification of Japanese isolates into two subtypes which are distinct from non-Japanese subtypes. J Virol. 1995;69:3639–3646. doi: 10.1128/jvi.69.6.3639-3646.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pistello M., Cammarota G., Nicoletti E. Analysis of the genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates a high prevalence and heterogeneity of subtype B. J Gen Virol. 1997;78(Pt 9):2247–2257. doi: 10.1099/0022-1317-78-9-2247. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y., Goto Y., Pang H. Genetic heterogeneity of env gene of feline immunodeficiency virus obtained from multiple districts in Japan. Virus Res. 1998;57:101–112. doi: 10.1016/s0168-1702(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 24.Martins A.N., Medeiros S.O., Simonetti J.P. Phylogenetic and genetic analysis of feline immunodeficiency virus gag, pol, and env genes from domestic cats undergoing nucleoside reverse transcriptase inhibitor treatment or treatment-naive cats in Rio de Janeiro, Brazil. J Virol. 2008;82:7863–7874. doi: 10.1128/JVI.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly P.J., Stocking R., Gao D. Identification of feline immunodeficiency virus subtype-B on St. Kitts, West Indies by quantitative PCR. J Infect Dev Ctries. 2011;5:480–483. doi: 10.3855/jidc.1844. [DOI] [PubMed] [Google Scholar]
- 26.Hayward J.J., Taylor J., Rodrigo A.G. Phylogenetic analysis of feline immunodeficiency virus in feral and companion domestic cats of New Zealand. J Virol. 2007;81:2999–3004. doi: 10.1128/JVI.02090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura K., Suzuki Y., Ikeo K. Phylogenetic analysis of Vietnamese isolates of feline immunodeficiency virus: genetic diversity of subtype C. Arch Virol. 2003;148:783–791. doi: 10.1007/s00705-002-0954-8. [DOI] [PubMed] [Google Scholar]
- 28.Uema M., Ikeda Y., Miyazawa T. Feline immunodeficiency virus subtype C is prevalent in northern part of Taiwan. J Vet Med Sci. 1999;61:197–199. doi: 10.1292/jvms.61.197. [DOI] [PubMed] [Google Scholar]
- 29.Reggeti F., Bienzle D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J Gen Virol. 2004;85:1843–1852. doi: 10.1099/vir.0.19743-0. [DOI] [PubMed] [Google Scholar]
- 30.Pecoraro M.R., Tomonaga K., Miyazawa T. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J Gen Virol. 1996;77(Pt 9):2031–2035. doi: 10.1099/0022-1317-77-9-2031. [DOI] [PubMed] [Google Scholar]
- 31.Nichols J., Litster A., Leutenegger C. Relationship between viral subgroup, viral load and health status in a population of cats naturally infected with feline immunodeficiency virus. Proceedings of the 2nd Symposium of the International Society for Companion Animal Infectious Diseases. 2012 Abstract 07. [Google Scholar]
- 32.Ueland K., Nesse L.L. No evidence of vertical transmission of naturally acquired feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1992;33:301–308. doi: 10.1016/0165-2427(92)90002-8. [DOI] [PubMed] [Google Scholar]
- 33.Allison R.W., Hoover E.A. Covert vertical transmission of feline immunodeficiency virus. AIDS Res Hum Retroviruses. 2003;19:421–434. doi: 10.1089/088922203765551764. [DOI] [PubMed] [Google Scholar]
- 34.Jordan H.L., Howard J., Barr M.C. Feline immunodeficiency virus is shed in semen from experimentally and naturally infected cats. AIDS Res Hum Retroviruses. 1998;14:1087–1092. doi: 10.1089/aid.1998.14.1087. [DOI] [PubMed] [Google Scholar]
- 35.Jordan H.L., Howard J.G., Bucci J.G. Horizontal transmission of feline immunodeficiency virus with semen from seropositive cats. J Reprod Immunol. 1998;41:341–357. doi: 10.1016/s0165-0378(98)00070-9. [DOI] [PubMed] [Google Scholar]
- 36.Little S., Sears W., Lachtara J. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can Vet J. 2009;50:644–648. [PMC free article] [PubMed] [Google Scholar]
- 37.Gleich S.E., Krieger S., Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg. 2009;11:985–992. doi: 10.1016/j.jfms.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldkamp C.E., Levy J.K., Edinboro C.H. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats with abscesses or bite wounds and rate of veterinarian compliance with current guidelines for retrovirus testing. J Am Vet Med Assoc. 2008;232:1152–1158. doi: 10.2460/javma.232.8.1152. [DOI] [PubMed] [Google Scholar]
- 39.Levy J.K., Edinboro C.H., Glotfelty C.S. Seroprevalence of Dirofilaria immitis, feline leukemia virus, and feline immunodeficiency virus infection among dogs and cats exported from the 2005 Gulf Coast hurricane disaster area. J Am Vet Med Assoc. 2007;231:218–225. doi: 10.2460/javma.231.2.218. [DOI] [PubMed] [Google Scholar]
- 40.Levy J.K., Scott H.M., Lachtara J.L. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc. 2006;228:371–376. doi: 10.2460/javma.228.3.371. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto J.K., Hansen H., Ho E.W. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- 42.Addie D.D., Dennis J.M., Toth S. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet Rec. 2000;146:419–424. doi: 10.1136/vr.146.15.419. [DOI] [PubMed] [Google Scholar]
- 43.Hellard E., Fouchet D., Santin-Janin H. When cats’ ways of life interact with their viruses: a study in 15 natural populations of owned and unowned cats (Felis silvestris catus) Prev Vet Med. 2011;101:250–264. doi: 10.1016/j.prevetmed.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto J.K., Sparger E., Ho E.W. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258. [PubMed] [Google Scholar]
- 45.Tompkins M.B., Tompkins W.A. Lentivirus-induced immune dysregulation. Vet Immunol Immunopathol. 2008;123:45–55. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mexas A.M., Fogle J.E., Tompkins W.A. CD4+CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet Immunol Immunopathol. 2008;126:263–272. doi: 10.1016/j.vetimm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vahlenkamp T.W., Tompkins M.B., Tompkins W.A. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J Immunol. 2004;172:4752–4761. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 48.Dean G.A., LaVoy A., Yearley J. Cytokine modulation of the innate immune response in feline immunodeficiency virus-infected cats. J Infect Dis. 2006;193:1520–1527. doi: 10.1086/503873. [DOI] [PubMed] [Google Scholar]
- 49.Lehman T.L., O’Halloran K.P., Hoover E.A. Utilizing the FIV model to understand dendritic cell dysfunction and the potential role of dendritic cell immunization in HIV infection. Vet Immunol Immunopathol. 2010;134:75–81. doi: 10.1016/j.vetimm.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy J.K., Liang Y., Ritchey J.W. Failure of FIV-infected cats to control Toxoplasma gondii correlates with reduced IL2, IL6, and IL12 and elevated IL10 expression by lymph node T cells. Vet Immunol Immunopathol. 2004;98:101–111. doi: 10.1016/j.vetimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher N.F., Meeker R.B., Hudson L.C. The neuropathogenesis of feline immunodeficiency virus infection: barriers to overcome. Vet J. 2011;188:260–269. doi: 10.1016/j.tvjl.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofmann-Lehmann R., Berger M., Sigrist B. Feline immunodeficiency virus (FIV) infection leads to increased incidence of feline odontoclastic resorptive lesions (FORL) Vet Immunol Immunopathol. 1998;65:299–308. doi: 10.1016/S0165-2427(98)00163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beatty J.A., Troyer R.M., Brewester C. Feline immunodeficiency virus (FIV)-associated lymphoma. Is a gammaherpesvirus involved? Proceedings of the 2nd Symposium of the International Society for Companion Animal Infectious Diseases. 2012 Abstract 03. [Google Scholar]
- 54.Magden E., Quackenbush S.L., VandeWoude S. FIV associated neoplasms—a mini-review. Vet Immunol Immunopathol. 2011;143:227–234. doi: 10.1016/j.vetimm.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Beatty J.A., Callanan J.J., Terry A. Molecular and immunophenotypical characterization of a feline immunodeficiency virus (FIV)-associated lymphoma: a direct role for FIV in B-lymphocyte transformation? J Virol. 1998;72:767–771. doi: 10.1128/jvi.72.1.767-771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto H., Takemura N., Sako T. Serum concentration of circulating immune complexes in cats infected with feline immunodeficiency virus detected by immune adherence hemagglutination method. J Vet Med Sci. 1997;59:395–396. doi: 10.1292/jvms.59.395. [DOI] [PubMed] [Google Scholar]
- 57.Fujino Y., Horiuchi H., Mizukoshi F. Prevalence of hematological abnormalities and detection of infected bone marrow cells in asymptomatic cats with feline immunodeficiency virus infection. Vet Microbiol. 2009;136:217–225. doi: 10.1016/j.vetmic.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Shelton G.H., Linenberger M.L., Grant C.K. Hematologic manifestations of feline immunodeficiency virus infection. Blood. 1990;76:1104–1109. [PubMed] [Google Scholar]
- 59.Meeker R.B. Feline immunodeficiency virus neuropathogenesis: from cats to calcium. J Neuroimmune Pharmacol. 2007;2:154–170. doi: 10.1007/s11481-006-9045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Podell M., Chen E., Shelton G.D. Feline immunodeficiency virus associated myopathy in the adult cat. Muscle Nerve. 1998;21:1680–1685. doi: 10.1002/(sici)1097-4598(199812)21:12<1680::aid-mus9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 61.Maingat F., Halloran B., Acharjee S. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25:2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neil L.L., Burkhard M.J., Diehl L.J. Vertical transmission of feline immunodeficiency virus. Semin Vet Med Surg (Small Anim) 1995;10:266–278. [PubMed] [Google Scholar]
- 63.O’Neil L.L., Burkhard M.J., Hoover E.A. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J Virol. 1996;70:2894–2901. doi: 10.1128/jvi.70.5.2894-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weaver C.C., Burgess S.C., Nelson P.D. Placental immunopathology and pregnancy failure in the FIV-infected cat. Placenta. 2005;26:138–147. doi: 10.1016/j.placenta.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 65.English R.V., Davidson M.G., Nasisse M.P. Intraocular disease associated with feline immunodeficiency virus infection in cats. J Am Vet Med Assoc. 1990;196:1116–1119. [PubMed] [Google Scholar]
- 66.Levy J., Crawford C., Hartmann K. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg. 2008;10:300–316. doi: 10.1016/j.jfms.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goto Y., Nishimura Y., Baba K. Association of plasma viral RNA load with prognosis in cats naturally infected with feline immunodeficiency virus. J Virol. 2002;76:10079–10083. doi: 10.1128/JVI.76.19.10079-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gleich S., Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats. J Vet Intern Med. 2009;23:552–558. doi: 10.1111/j.1939-1676.2009.0303.x. [DOI] [PubMed] [Google Scholar]
- 69.Hart S.W., Nolte I. Hemostatic disorders in feline immunodeficiency virus-seropositive cats. J Vet Intern Med. 1994;8:355–362. doi: 10.1111/j.1939-1676.1994.tb03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartmann K., Griessmayr P., Schulz B. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J Feline Med Surg. 2007;9:439–445. doi: 10.1016/j.jfms.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barr M.C., Pough M.B., Jacobson R.H. Comparison and interpretation of diagnostic tests for feline immunodeficiency virus infection. J Am Vet Med Assoc. 1991;199:1377–1381. [PubMed] [Google Scholar]
- 72.Levy J.K., Crawford P.C., Slater M.R. Effect of vaccination against feline immunodeficiency virus on results of serologic testing in cats. J Am Vet Med Assoc. 2004;225:1558–1561. doi: 10.2460/javma.2004.225.1558. [DOI] [PubMed] [Google Scholar]
- 73.Hosie M.J., Addie D., Belak S. Feline immunodeficiency. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:575–584. doi: 10.1016/j.jfms.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bienzle D., Reggeti F., Wen X. The variability of serological and molecular diagnosis of feline immunodeficiency virus infection. Can Vet J. 2004;45:753–757. [PMC free article] [PubMed] [Google Scholar]
- 75.Crawford P.C., Slater M.R., Levy J.K. Accuracy of polymerase chain reaction assays for diagnosis of feline immunodeficiency virus infection in cats. J Am Vet Med Assoc. 2005;226:1503–1507. doi: 10.2460/javma.2005.226.1503. [DOI] [PubMed] [Google Scholar]
- 76.Dandekar S., Beebe A.M., Barlough J. Detection of feline immunodeficiency virus (FIV) nucleic acids in FIV-seronegative cats. J Virol. 1992;66:4040–4049. doi: 10.1128/jvi.66.7.4040-4049.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.IDEXX Reference Laboratories Diagnostic Update. 2009. http://www.idexx.com/view/xhtml/en_us/smallanimal/reference-laboratories/testmenu/innovative-tests/real-pcr.jsf?SSOTOKEN=0. Last accessed May 18, 2012.
- 78.Maingat F., Vivithanaporn P., Zhu Y. Neurobehavioral performance in feline immunodeficiency virus infection: integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J Neurosci. 2009;29:8429–8437. doi: 10.1523/JNEUROSCI.5818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shelton G.H., Grant C.K., Linenberger M.L. Severe neutropenia associated with griseofulvin therapy in cats with feline immunodeficiency virus infection. J Vet Intern Med. 1990;4:317–319. doi: 10.1111/j.1939-1676.1990.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 80.Arai M., Darman J., Lewis A. The use of human hematopoietic growth factors (rhGM-CSF and rhEPO) as a supportive therapy for FIV-infected cats. Vet Immunol Immunopathol. 2000;77:71–92. doi: 10.1016/s0165-2427(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 81.Fogle J.E., Tompkins W.A., Campbell B. Fozivudine tidoxil as single-agent therapy decreases plasma and cell-associated viremia during acute feline immunodeficiency virus infection. J Vet Intern Med. 2011;25:413–418. doi: 10.1111/j.1939-1676.2011.0699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egberink H.F., De Clercq E., Van Vliet A.L. Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. J Virol. 1999;73:6346–6352. doi: 10.1128/jvi.73.8.6346-6352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hartmann K., Stengel C., Klein D. Efficacy and adverse effects of the antiviral compound pleraxifor in feline immunodeficiency virus-infected cats. J Vet Intern Med. 2012;26(3):483–490. doi: 10.1111/j.1939-1676.2012.00904.x. [DOI] [PubMed] [Google Scholar]
- 84.Pedersen N.C., Leutenegger C.M., Woo J. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats. Vet Immunol Immunopathol. 2001;79:53–67. doi: 10.1016/s0165-2427(01)00252-5. [DOI] [PubMed] [Google Scholar]
- 85.Doménech A., Miró G., Collado V.M. Use of recombinant interferon omega in feline retrovirosis: from theory to practice. Vet Immunol Immunopathol. 2011;143(3-4):301–306. doi: 10.1016/j.vetimm.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawson S., Smyth N.R., Bennett M. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. AIDS. 1991;5:747–750. doi: 10.1097/00002030-199106000-00016. [DOI] [PubMed] [Google Scholar]
- 87.Ravi M., Wobeser G.A., Taylor S.M. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: Prevalence, disease associations, and survival analysis. Can Vet J. 2010;51:271–276. [PMC free article] [PubMed] [Google Scholar]
- 88.George J.W., Pedersen N.C., Higgins J. The effect of age on the course of experimental feline immunodeficiency virus infection in cats. AIDS Res Hum Retroviruses. 1993;9:897–905. doi: 10.1089/aid.1993.9.897. [DOI] [PubMed] [Google Scholar]
- 89.O’Neil L.L., Burkhard M.J., Obert L.A. Regression of feline immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1997;13:713–718. doi: 10.1089/aid.1997.13.713. [DOI] [PubMed] [Google Scholar]
- 90.Huang C., Conlee D., Gill M. Dual-subtype feline immunodeficiency virus vaccine provides 12 months of protective immunity against heterologous challenge. J Feline Med Surg. 2010;12:451–457. doi: 10.1016/j.jfms.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusuhara H., Hohdatsu T., Okumura M. Dual-subtype vaccine (Fel-O-Vax FIV) protects cats against contact challenge with heterologous subtype B FIV infected cats. Vet Microbiol. 2005;108:155–165. doi: 10.1016/j.vetmic.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Dunham S.P., Bruce J., MacKay S. Limited efficacy of an inactivated feline immunodeficiency virus vaccine. Vet Rec. 2006;158:561–562. doi: 10.1136/vr.158.16.561. [DOI] [PubMed] [Google Scholar]


