Natural History, Anatomy, and Physiology
Recent studies have revealed that the Musteloidea emerged approximately 32.4 to 30.9 million years ago in Asia. During the Oligocene, musteloids diversified into four primary divisions: Mephitidae, Ailuridae, Procyonidae, and Mustelidae. Mustelidae arose approximately 16.1 million years ago. The early offshoots largely evolved into the ecologic niches of badgers and martens, whereas later divergences have adapted to other niches, including those of weasels, polecats, minks, and otters.35
Extant mustelids are classified in the order Carnivora, suborder Caniformia, family Mustelidae, and subfamily Mustelinae and Mephitinae. The family Mustelidae currently includes 25 recent genera and approximately 67 species of terrestrial carnivores or piscivores inhabiting all continents except Australia and Antarctica and are also absent in New Guinea, Madagascar and Antarctica. They have been introduced into New Zealand. In the course of evolution, several behavioral adaptations and many physical features have developed, as some species live mainly in the ground (stoat, weasel, polecat) or even partially underground (badger), whereas others are active also above the ground in trees (pine marten). Some have selected marine or fresh water as their preferred habitats most or part of the time (mink, river otter, sea otter).
Included in this family are the smallest living carnivore, the common or least weasel, and the largest representatives, the giant and sea otters in water and the wolverine on land. Mustelid body weights range from under 70 grams (g) (least weasel at 19 centimeters [cm] long) to 45 kg (sea otter at 190 cm long).
The family Mustelidae includes five subfamilies. The weasel-like carnivores (Mustelinae) represent the group with the greatest number of species, comprising 10 genera with approximately 33 species including weasels (11 species), polecats (3 species), minks (2 species), grison (1 species), and wolverine (1 species). The subfamily Mellivorinae is represented by only a single species, the honey badger or ratel (Mellivora capensis). Subfamily Melinae includes five genera in eight species of badgers represented in Africa, Asia, South America, or wide ranges of northern Eurasia and North America. Skunks (subfamily Mephitinae, recently elevated to Family Mephitidae) are exclusively common in North America. Otters (subfamily Lutrinae) are small to large forms that show the most highly developed adaptations to marine life of all mustelids. They lead an amphibious life and feed mainly on fish or crustaceans. Most mammologists recognize four genera and 13 species.30
Most mustelids have a highly flexible spinal column; the limbs are comparatively short, ending in feet with five digits, and they walk either digitigrade or plantigrade. The claws are not (or only partly) retractable. Mustelids lack the clavicle and cecum. They present the typical carnivore dentition with number of teeth varying from 28 to 40. Developed canine (C) teeth are always present and the last premolar (P) in the upper jaw and the first molar (M) in the lower jaw jointly form the “crushing shears” for processing food. The dental formula of weasels is incisors (I) 3/3, C 1/1, P 3/3, M 1/2 on the upper and lower jaws. In the wolverine the formula is I 3/3, C 1/1, P 4/4, M 1/2 upper and lower. Eurasian badger formula is I 3/3, C 1/1, P 4/4, M 1/2 upper and lower, and in the members of the Lutra and Lontra genera the formula is I 3/3, C 1/1, P 3-4/3, M 1/2 upper and lower. The pine marten has a dental formula of I 3/3, C 2/1, P 4/4, M 1/2 upper and lower (40 teeth total), which is different from that of other mustelids. Glands may be located in various regions of the body surface. The paired anal glands produce odorous secretions characteristic of the species and used for marking their habitat, sometimes for generations. Some species may spray these secretions over long distances as a method to discourage or harm enemies.
In otters, the mandibular salivary glands and lymph nodes lie in the angle of the mandible, whereas the retropharyngeal nodes lie dorsolateral and slightly caudal to the larynx. The thyroid glands of otters are also different from those of other mustelids in that they are long, flat, and tapering, with no isthmus, and closely attached to the trachea. The heart of otters is usually globular with a thick-walled left ventricle and a thin-walled right ventricle. The shape of the heart and thickness of the ventricles should not be confused with ventricular hypertrophy. Otters have a seven-lobed liver. A common hepatic and cystic bile duct joins the duodenum adjacent to the pancreatic duct. The kidneys of otters, like those of cows and cetaceans, are multilobulated. The lungs of otters and badgers are composed of two lobes on the left, three lobes on the right, and an intermediate lobe where the right bronchus terminates.5, 15
Mustelids are predominantly solitary, sexually dimorphic mammals (males are 25% larger than females). Smaller mustelid species have high metabolic rates. Males and females come together only during the reproductive period, and social communities generally include the mother and offspring. Table 48-1 summarizes the biologic data of selected mustelids.
TABLE 48-1.
Biologic Information of Selected Species of Mustelids
| Scientific Name | Common Name | Weight | Geographic Distribution | Distinguishing Features | Life Span | Food |
|---|---|---|---|---|---|---|
| Mustela nivalis | Common weasel Least weasel | Female (F): 30–120 grams (g) Male (M):36–250 g |
North Africa, Western Europe, Eastern Siberia; Japan, Alaska, and Northeastern USA, (New Zealand) | Smallest species of family Body size and fur color highly varying |
About 1 year | Burrowing voles, true mice, birds, frogs, lizards |
| Mustela erminea | Ermine, Stoat, Short tail weasel | F: 85–200 g M: 200–310 g |
Europe-Eastern Siberia, Japan, Alaska, Northern Greenland, Northern USA, (New Zealand) | Summer fur cinnamon-brown or even yellow on back; underside white | About 1 year | Burrowing voles, true mice, hares, birds, eggs, lizards, frogs |
| Mustela putorius | European polecat | F: 650–820 g M: 1000–1500 g |
Europe | Ancestor of domestic ferret, M. putorius furo, facial mask | 5–6 years, 10 years and more in isolated cases | Small rodents, rabbits, hares, birds, eggs, frogs, snakes, insects |
| Mustela nigripes | Black footed ferret | F: 750–850 g M: 900–1000 g |
Alberta to northern Texas | Facial mask; black limbs | 12 years | Prairie dogs and other small rodents, birds |
| Mustela lutreola | European mink | 400–1200 g | Western Siberia, Eastern Europe, (Western Europe) | Polecat-like; long vibrissae on snout | 7–10 years | Mouselike rodents, fishes, crayfish, mollusks, birds, amphibians, reptiles |
| Mustela vison | American mink | 500–2300 g | Canada, USA, (Iceland, north and central Europe, Siberia) | Sparse white spots on chin and ventrum, otherwise very similar to European mink | 8–10 years | Same as European mink |
| Vormela peregusna | Marbled polecat | 370–715 g | Southeastern Europe to western China | Spotted back, large ears | 8 years | Gerbils, jumping mice, susliks, hamsters, and other rodents |
| Poecilogale albinucha | White-naped weasel | F: 230–290 g M: 280–380 g |
South Africa to Zaire, Uganda | White neck; stripes on back | 5 years | Small rodents, birds, snakes, grasshoppers and other insects |
| Ictonyx striatus | Zorrila or African striped polecat | 420–1400 g | Senegal, Ethiopia, and South Africa | Stripes on back; squirts secretion from anal glands for defense | 13 years | Small rodents, birds, eggs, insects |
| Martes martes | Pine marten | F: 800–1300 g M: 1200–1600 g |
Western Europe to Western Siberia | Summer fur thin and short, winter fur thick and long | 15 years | Mouselike rodents, squirrels, hares, rabbits, birds, eggs, reptiles, amphibians, insects, fruits, berries, nuts |
| Martes foina | Stone marten or beech marten | F: 1100–1500 g M: 1700–2400 g |
Western Europe to Himalayas, and Altai | Similar to pine marten, but heavier, shorter limbs, white throat spot | Unknown | Similar to pine marten |
| Martes americana | American marten | F: 600–775 g M: 700–1300 g |
Canada, north USA | Similar to pine marten; irregular cream to orange spots on throat and chest | 17 years | Similar to pine marten |
| Eira barbara | Tayra | 4–6 kilograms (kg) | Northeastern Mexico to Argentina | Dark brown to black body | 18 years | Guinea pigs, harelike rodents, birds, reptiles, insects, honey, fruits |
| Mellivora capensis | Ratel | 7–13 kg | Northern India to Arabia, Africa, and south of Sahara | Some animals completely black; forelimbs muscular, with strong claws | Unknown | Small rodents, birds, eggs, lizards, snakes, turtles, frogs, insects, honey, berries, fruits, roots |
| Meles meles | Badger | 7–13 kg in summer; 15–25 kg in fall | Europe, Japan, and southern China | Silvery gray back and flanks; throat, chest, belly and legs black or brown | 16 years | Mouselike rodents, small birds, eggs, frogs, lizards, insects, snails, earthworms, fruits, nuts, berries |
| Taxidea taxus | American badger | 6–8 kg in summer; 8–12 kg in fall |
Southwestern Canada to central Mexico | Thick dense fur; predominantly gray black with white stripe from nose to root of tail; dark, oblong cheek spot | 16 years | Small mammals, birds, eggs, reptiles, insects, invertebrates |
| Mephitis mephitis | Stripped skunk | 1.2–2.5 kg; in the fall up to 5.3 kg | Southern Canada to northern Mexico | Black, with mostly two white lateral stripes; spray secretion from anal glands up to 6 m with accurate aim into eyes of attacker | 10 years | Small rodents, birds, eggs, insects, worms, fruits, berries, corn |
| Lutra lutra | Eurasian otter | 5–12 kg | Eurasia, North Africa, Sri Lanka, Taiwan, Sumatra, Java | Shiny dark brown or chestnut brown back; fingers and toes joined by swimming membranes | 22 years | Fishes, crustaceans, clams, frogs, small rodents, worms |
| Lontra canadensis | Nearctic river otter | 3.4–15.4 kg | Canada, USA | Head blunt, small, flat bullous nose; small eyes; interdigital webs | 14 years in wild; 16–18 years in captivity | Fish (primarily), crustaceans (cray fish); amphibians; insects, birds, mammals |
| Pteronura brasiliensis | Giant otter | 22–32 kg | Venezuela to Argentina | Very dark fur; chin, throat, and chest have cream-colored spots; flattened tail; swimming membranes | 13 years | Fishes, crustaceans, other aquatic animals |
| Enhydra lutris | Sea otter | F: 36 kg M: 46 kg |
Bering Sea to California | Largest mustelid by weight; light brown to nearly black pelage; interdigital webs | In wild 22 years (females) 15 years (males) |
Generalist predator; decapod crustaceans, gastropod and bivalve mollusks, echinoderms |
| Gulo gulo | Wolverine | 10–20 kg | Scandinavia, Siberia, Alaska, Canada, Western USA | Bushy tail; long flowing fur; thick, strong paws; partly retractable claws | 18 years | Rodents, harelike rodents, reindeer, elk, carcasses, ground-nesting birds, berries |
Items in parenthesis refer to areas where a particular species as been introduced.
Members of the family range from the International Union for the Conservation of Nature (IUCN) status Endangered (black-footed ferret) to Near Threatened (wolverine) to IUCN status Least Concern (badger).
Unique Aquatic Adaptations
The family Mustelidae contains numerous fully terrestrial species, two that are semi-aquatic (minks), and a number that are amphibious to fully aquatic (the Lutrinae). The latter have adaptations for the aquatic habits that may be relevant for the clinical management. Underwater vision presents challenges for the mammalian eye: the need for increased sensitivity to light, accommodation of the spectral shift toward the blue-green wavelengths, and modification of the ocular focusing capacity because of refractive differences compared with those in air. Different adaptations for these challenges have been proposed, although visual acuity in water is somewhat reduced in some otter species (i.e., Asian small-clawed otter). Little is known of the importance, sensitivity, and mechanisms of hearing in otters, in the aquatic or the terrestrial environment. Olfaction has been retained as an important sense for aquatic mustelids, largely but not exclusively in support of their activities on land. However, evidence suggests that otters have less complex scent production capacities compared with terrestrial mustelids and that scent production capacity in sea otters may be more poorly developed and less important than in other otter species. These changes probably have resulted from the increased importance of vision and the reduced importance of olfaction in the aquatic environment. The long, lean body of Mustelinae species makes them vulnerable to rapid heat loss on land and in the water. Insulation in aquatic mustelids is achieved by means of a dense underfur that prevents water penetration to the skin while providing buoyancy. Because fur is an efficient insulator, furred aquatic mammals require some means of thermoregulation; in sea otters, thermoregulation is conducted through the enlarged rear flippers. In otters and minks, swimming is the primary means of locomotion. These species demonstrate many adaptations that enhance swimming performance and reduce energy expenditure while in the water: body streamlining, large, specialized plantar surfaces for propulsion, and the ability to remain submerged for extended periods. However, most otters, unlike most aquatic mammals, are capable of quadrupedal locomotion on land, and this is why they are considered morphologically intermediate between terrestrial and aquatic mammals.12
Outdoor and Indoor Environments
Most species tolerate a wide range of temperature ranges. Temperate and cold-adapted species held outdoors need protection from sunlight when the temperature exceeds 50° F (10° C). Tropical species require heated shelters when ambient temperatures drop below 69° F (20° C). Animals kept indoors should not be exposed to temperatures exceeding 78° F (25° C). It is important to be aware that required temperature ranges vary among individuals as well as between species, so individual animals should be given the opportunity to select a comfortable ambient temperature from a gradient provided in the enclosure. Humidity for indoor enclosures should range from 30% to 70% but may be higher for tropical species. The amount of time individuals held indoors are exposed to light should replicate the natural photoperiod of their native environment, particularly for those species that are expected to reproduce in captivity. Currently, data on the effects of varying light intensity or type of light (fluorescent versus natural) on reproductive behavior are not available; however, a correlation exists between the onset of estrus in northern mustelid species.4 Indoor exhibits should have a negative air pressure of five to eight air changes per hour (for odor control) of non-recirculated air; however, this is not necessarily a requirement and recirculated air may be used in some cases.
Fresh drinking water should be provided at all times. Nonfiltered water, contained in pools or moats and used for swimming, should be changed on a regular basis. Even if water is filtered, it should be completely changed periodically. Mustelids should not be given access to pools that have recently been treated with chlorine (levels should be <0.5 parts per million [ppm]). For otters that normally inhabit fresh or brackish water environments, dissolved nutrients should be monitored and water changes performed, as appropriate. It is suggested that the coliform level not exceed 400 colony forming units per milliliter (CFU/mL) (water with a level of 100 CFU/mL is reported to be safe for humans). Filtration should be used in closed pools for otter. Sand filters, pool pumps, charcoal filters, and ozone pressure sand filters have all been used effectively. Drain outlets and filter and skimmer inlets should be covered to prevent furnishings from obstructing them or from otters getting stuck in them. Natural flow-through systems work well in otter exhibits. Water flowing in must be clean and pollutant free. All uneaten food items should be removed from pools on a daily basis. Because minks are highly susceptible to methyl mercury toxicity, pools need to be maintained at a neutral or basic pH (acidic pH enhances methylation of mercury).
Controlling of sounds and vibrations that may be detected by mustelids is important to their well-being. Anecdotal reports of loud noises and vibrations of certain amplitude affecting parturition and early kit rearing in mustelids have been published.3, 4
Habitat Design and Containment
Exhibits should be designed to satisfy the physical, social, behavioral, and psychological needs of the species while closely replicating their habitat in the wild. Enclosure size for arboreal and terrestrial mustelids is based on species' and individual needs (e.g., juveniles versus adults versus geriatric animals). Exhibits that are provided extensive enrichment and are structurally varied may be smaller than exhibits lacking these characteristics. (Note that enrichment items must be chosen carefully, since many mustelid species are prone to chewing and ingesting enrichment parts, putting them at risk of gastrointestinal [GI] foreign body obstruction). Recommended exhibit sizes are based on species size, behavioral repertoire, home range size, daily movements, and activity patterns. Detailed information is given in the Mustelid (Mustelidae) and Otter (Lutrinae) Care Manuals provided by the Association of Zoos and Aquariums, Small Carnivore Taxon Advisory Group.
Animal and human safety must be kept in mind when designing and building mustelid exhibits. Additionally, mustelids are not well suited for free-ranging exhibits because of their uncanny ability to escape. Exhibits must be designed to prevent them from digging, jumping, climbing, or swimming out of enclosures. Outdoor exhibits should have containment perimeters, tops and hotwire 3 to 5 feet (ft.; 1–1.52 meters [m]) installed above ground level to prevent them from climbing and falling. For burrowing species (e.g., badgers), the bottom of the containment fence may need to be buried to a sufficient depth and angled toward the center of the exhibit to prevent escape. For amphibious species (e.g., otters), optimal land-to-water ratios are species dependent. These ratios may need to be changed as exhibit size increases or decreases (e.g., smaller exhibits will require a higher land area proportion within the ratio).3, 4
Feeding and Nutrition
Within the Mustelidae family, food habits vary significantly. Some are strict carnivores (ferrets, weasels, polecats, etc.), some are omnivorous (skunks, badgers or tayras), and some are piscivorous (fish and crustacean eaters such as otters) (see Table 48-1). Mustelids have a relatively simple stomach and a short GI tract and, as mentioned above, no cecum. The more omnivorous species have flattened molars. Captive mustelid species are fed on a great variety of items: commercial dry dog food, mink food, and cat food, and cereal diets mixed with meat, fresh or frozen fish, shellfish, crabs, and crayfish. Fruits, vegetables (carrots, lettuce, green beans, cucumber, collard greens, kale, potatoes, among others), eggs, and live or killed food items (crickets, mealworms, mice, prairie dogs) have also been incorporated into captive diets. Target dietary nutrient values for mustelids are based on several sources. The cat is typically the model species used to establish nutrient guidelines for strict carnivorous animals. The National Research Council (NRC, 2006, for dogs and cats), and Association of American Feed Control Officials (1994, for cats) have provided recommendations. A limited amount of information has been provided by the NRC publication on mink and foxes, which represents the requirements of another mustelid species (Table 48-2 ). The complete dietary requirements of domestic ferrets are still unknown, so no one particular diet is currently being recommended over another. In the ferret and mink diet, the protein should be of high quality and easily digestible because of their short GI transit time of 3 to 4 hours. Generally, most mustelids need a diet high in good-quality meat protein and fat and low in complex carbohydrates, inclusive of sugars, and fiber. High levels of protein from plant sources have been associated with urolithiasis in mustelids and are therefore undesirable. Food should be offered at least twice a day, and water must be available at all times. When developing appropriate dietary management plans for a specific mustelid species, the following should be considered: feeding ecology, target nutrient values, food items available at zoos, and information collected from diets offered by institutions successfully maintaining and breeding for the species.
TABLE 48-2.
Nutrient Requirements and Target Nutrient Ranges for Selected Carnivore Species
| Nutrient | Cat* (National Research Council [NRC], 1986) | Dog* (NRC, 1974) | Mink†Mustela vison | Artic Fox‡Vulpes vulpes (NRC, 1982) |
Asian Small-Clawed Otter§Aonyx cinerea |
|---|---|---|---|---|---|
| Protein % | 24 | 22 | 38 (23.9) | 24.7 | 24–32.5 |
| Fat % | — | 5 | — | — | 15–30 |
| Vitamin A, international unit per gram (IU/g) | 3.3 | 5.0 | 5.93 | 2.44 | 3.3–10 |
| Vitamin D (IU/g) | 0.5 | 0.5 | — | — | 0.5–1.0 |
| Vitamin E, milligram per kilogram (mg/kg) | 30 | 50 | 27 | — | 30–120 |
| Thiamin (mg/kg) | 5.0 | 1.0 | 1.3 | 1.0 | 1–5 |
| Riboflavin (mg/kg) | 4.0 | 2.2 | 1.6 | 3.7 | 3.7–4.0 |
| Pantothenic acid (mg/kg) | 5.0 | 10.0 | 8.0 | 7.4 | 5–7.4 |
| Niacin (mg/kg) | 40.0 | 11.4 | 20.0 | 9.6 | 9.6–40 |
| Pyridoxine (mg/kg) | 4.0 | 1.0 | 1.6 | 1.8 | 1.8–4 |
| Folacin (mg/kg) | 0.80 | 0.18 | 0.5 | 0.2 | 0.2–1.3 |
| Biotin (mg/kg) | 0.07 | 0.1 | 0.12 | — | 0.07–0.08 |
| Vitamin B12 (mg/kg) | — | 0.022 | — | — | 0.02–0.025 |
| Calcium % | 0.8 | 1.1 | 0.40 (0.3) | 0.6 | 0.6–0.8 |
| Phosphorous % | 0.6 | 0.9 | 0.40 (0.3) | 0.6 | 0.6 |
| Potassium % | 0.4 | 0.6 | — | — | 0.2–0.4 |
| Sodium % | 0.05 | — | — | — | 0.04–0.6 |
| Magnesium % | 0.04 | 0.04 | — | — | 0.04–0.07 |
| Iron (mg/kg) | 80 | 60 | — | — | 80–114 |
| Zinc (mg/kg) | 50 | 50 | — | — | 50–94 |
| Copper (mg/kg) | 5.0 | 7.3 | — | — | 5.0–6.25 |
| Iodine (mg/kg) | 0.35 | 1.54 | — | — | 1.4–4.0 |
| Selenium (mg/kg) | 0.1 | 0.11 | — | — | — |
National Research Council: Nutrient requirements of dogs and cats. Washington, DC, 2006, National Academy Press.
Growing and weaning to 13 weeks. Numbers between parentheses are for maintenance (from National Research Council: Nutrient requirements for minks and foxes. Washington, DC, 1982, National Academy Press).
National Research Council: Nutrient requirements for minks and foxes. Washington, DC, 1982, National Academy Press).
Maslanka CS: Asian small-clawed otters: Nutrition and dietary husbandry. In: Nutrition Advisory Group handbook, 1999.
Restraint and Handling
Even though some captive mustelids may be gentle with their keepers, all members of this family may be handled with nets, snares, or squeeze cages. Caution must be used while managing wild mustelids, as they have needle-sharp teeth and are agile and aggressive and may inflict severe bites. They are also potential vectors of rabies, so they should be handled with caution. Leather gloves should be used by operators when handling any kind of mustelid, whatever the size. The ferret is best restrained when grasped above the shoulders, with one hand gently squeezing the forelimbs together and the thumb under the animal's chin. Minks are grasped by the tail with one hand, while the other hand grasps the animal behind the neck, with the thumb and finger around the head. Polecats, ermines, weasels, and martens are better restrained initially with a net when an injection has to be administered by hand. Skunks defend themselves by spraying the secretions of the anal sacs, and they may bite as well. The defensive position assumed by a threatened skunk is hindquarters facing the enemy, feet planted firmly on the ground, and tail straight up in the air. They should be captured with a net from behind a shield of glass or plastic, or the handler should wear goggles and protective rain gear. Larger mustelids such as otters, badgers, and wolverine may be placed in a small squeeze cage for manual injection of a tranquilizer or directly injected by means of a pole syringe or a blowpipe.16
Mustelids are susceptible to stress caused by improper handling and transport. Fresh water and marine otters are particularly susceptible to stress-associated exertional myopathy. Different techniques have been developed for safe management of this species. Only trained personal should handle mustelids, and usually, a combination of physical restraint and chemical restraint is advocated to reduce stress and avoid capture myopathy. The duration of restraint should be brief, and care should be taken to avoid trauma to the oral cavity and limbs. As mentioned above, sea otters are extremely susceptible to stress caused by improper handling and transporting. Different techniques have been developed for the safe management of this species.19, 24
Chemical Restraint
Different drugs have been used extensively for the chemical immobilization of mustelids. In most species, dissociative-benzodiazepine– α2-agonists combinations have been used and are highly recommended for induction or short-term anesthesia. Ketamine in combination with midazolam, diazepam, xylazine, medetomidine, or acepromazine (caution: hyperthermia or hypothermia) to improve muscle relaxation. Xylazine, medetomidine, or dexmedetomidine combined with ketamine has been recommended to improve muscle relaxation, and both combinations may be reversed with atipamezole (2.5 milligram [mg] per 5 mg medetomidine, and 1 mg per 8–12 mg xylazine).2, 13, 27 Tiletamine–zolazepam is another option. Doses ranging from 2.2 to 22 mg/kg have been reported for numerous species of mustelids; higher doses result in prolonged recovery. In otters, the usage of a low dose of tiletamine–zolazepam to achieve anesthetic induction, and supplementation with isoflurane or ketamine (5 mg/kg) for maintenance, has been advocated. Flumazemil (0.05–0.1 mg/kg) may be used to antagonize the zolazepam portion of this combination to hasten recovery, but its usage has not been reported in mustelids other than the Nearctic river otter.38 Drugs and dosages commonly used to provide chemical restraint and sedation in selected mustelids are listed in Table 48-3 . These combinations usually provide short periods of chemical restraint (30–45 minutes). If longer periods of anesthesia are needed, inhalation anesthetics (isoflurane and sevoflurane) delivered via an induction chamber, mask, or endotracheal tube is efficient, although the results of chamber induction with inhalation agents may vary and cause excitement in some species. Otters hypoventilate during inhalation anesthesia and require assisted ventilation to prevent hypoxemia and hypercarbia.26
TABLE 48-3.
Drugs and Dosages Recommended for Immobilization of Selected Mustelids
| Species | Recommended Anesthetic Combination (milligram per kilogram [mg/kg]) | Comments/Alternative |
|---|---|---|
| American badger | Tiletamine-zolazepam (4.4) | Ketamine (15), xylazine (1) |
| American river otter | Ketamine (8–12) + midazolam (0.25–5) / Ketamine (3) + medetomidine (0.030) (atipamezole) | Ketamine (10–12) + diazepam (0.3–5) / Tiletamine–zolazepam (4) + flumazenil (0.08)Respiratory depression may occur |
| Asian small-clawed otter |
Ketamine (15–18) + midazolam (0.75–1) |
Ketamine (4–5) + medetomidine (0.1–0.12) (atipamezole) |
| Respiratory depression may occur | ||
| Black footed ferret | Ketamine (3) + medetomidine (0.075) (atipamezole) | Ketamine (15) + diazepam (0.1) |
| Ermine and weasel | Ketamine (5) + medetomidine (0.1) (atipamezole) | Ketamine (3)/ Tiletamine-zolazepam (11–22) |
| Eurasian badger | Ketamine (5–10) + medetomidine (0.05–0.1) (atipamezole)/ tiletamine–zolazepam (10) | Ketamine (10–16) + xylazine (2–6)/ medetomidine (0.04) + tiletamine–zolazepam (2.5) |
| Eurasian otter |
Ketamine (5) + medetomidine (0.5) (atipamezole) |
Ketamine (15) + diazepam (0.5) |
| Respiratory depression may occur | ||
| Ferret |
Ketamine (10–30) + xylazine (1–2) or diazepam (1–2) or acepromazine (0.05–0.3) |
Tiletamine-zolazepam (22) |
| Recovery time may be prolonged | ||
| Giant otter | Ketamine (8.5–10.6) + xylazine (1.5–2) | Prolonged recovery |
| Marten | Ketamine (10) + medetomidine (0.2) (atipamezole) | Ketamine (60) + xylazine (12) |
| Mink | Tiletamine–zolazepam (15) / Ketamine (40) + xylazine (1) | Ketamine (5) + medetomidine (0.1) (atipamezole) |
| Ratel (honey badger) | Tiletamine–zolazepam (2.2) | Ketamine (6) + xylazine (0.5) |
| Sea otter |
Butorphanol (0.5)/ oxymorphon (0.3) |
Fentanyl (0.3) + azaperone (0.25) |
| Caution: Numerous reports of fatal complications | ||
| Stripped skunk | Tiletamine–zolazepam (10) | Ketamine (15) + acepromazine (0.2) |
| Tayra | Tiletamine–zolazepam (3.3) | |
| Wolverine | Ketamine (5–8) + medetomidine (0.1–0.15) | Ketamine (20) + acepromazine (0.2) |
Whenever possible, the following parameters should be recorded when immobilizing or anesthetizing a mustelid: actual weight, relative oxyhemoglobin saturation (clamp located on tongue, lips, ears, toes), heart and respiratory rates, and rectal temperature. Possible anesthetic complications include respiratory depression (apnea, bradypnea, tachypnea, hypoxemia), hyperthermia, hypothermia, bradycardia, tachycardia, poor myorelaxation, and excitability during recovery. Hypoventilation has been reported to be a cause of mortality in otters with the use of inhalation anesthesia. During recovery from anesthesia, animals should be kept in a quiet, dark denning box or cage or in a confined area to facilitate smooth recovery from anesthesia.26
Diagnostics
Blood may be collected from various sites; the technique and site chosen depend on the species, how much blood is needed, and operator preference. Sites include the jugular vein, cranial vena cava, ventral coccygeal artery, median caudal vein, lateral saphenous vein, cephalic vein, and femoral vein. Published reference ranges for hematologic and serum biochemistry analyses for selected mustelids are listed in Tables 48-4 and 48-5 . Techniques for urine collection, urinary catheterization, splenic and bone marrow aspiration, placement of intravenous and intraosseous catheters, administration of fluids, and blood transfusion have been described for ferret and may be useful when treating other mustelids.33 A technique of mandibular salivary gland biopsy for rabies testing has been developed in Nearctic river otters.39 Other diagnostic techniques such as ultrasonography, electrocardiography, radiography, and auscultation are applicable but vary for each species.
TABLE 48-4.
Reference Range for Hematologic Parameters of Selected Mustelid Speciesa
| North American Parameter* | Nearctic river otter | Eurasian otter | Mink† | Striped skunk | Ferret | European polecat‡ | Striped skunk‡ |
|---|---|---|---|---|---|---|---|
| Erythrocytes ×106/microliter (µL) | 6.10–14.50 | 5.2–7.8 | 8.07 ± 0.67 | 6.8–12.2 | 6.35–11.2 | 8.39 ± 1.86 | 8.08 ± 0.68 |
| PCV (%) | 32.2–60.8 | 37.8–69.1 | 45.9 ± 3.1 | 42–61 | 36.7–54.9 | 43.6 ± 8.7 | 43.0 ± 6.5 |
| Hemoglobin, gram per deciliter (g/dL) | 10.4–19.0 | 11.0–19.9 | 15. 6 ± 1.1 | 15–18 | 11.1–17.1 | 14.3 ± 2.7 | 13.4 ± 1.1 |
| MCV, (fL) | 38.3–49.0 | 60.7–105.2 | 56.9 ± 1.9 | — | 45.6–54.7 | 52.1 ± 407 | 53.0 ± 2.6 |
| MCH, picogram (pg) | 11.3–15.8 | 16.3–26.9 | — | — | 14.0–17.6 | 17.3 ± 1.2 | 17.0 ± 0.4 |
| MCHC (%) | 27.8–39.2 | 24.6–30.9 | 34.0 ± 0.52 | — | 30.7–32.9 | 33.2 ± 1.9 | 31.8 ± 1.2 |
| WBC (103/µL) | 4.7–33.2 | 3.1–19.2 | 6.49 ± 2.02 | 4-0–19 | 2.0–9.8 | 6.20 ± 2.36 | 8.01 ± 3.12 |
| Neutrophils (103/µL) | 3.0–28.2 | 1.41–12.86 | 2.64 ± 1.27 | 0.62–3.33 | 2.88 ± 1.63 | 4.22 ± 2.43 | |
| Band neutrophils (103/µL) | 0–0.48 | 0–1.8 | 0.008 ± 0.020 | — | — | 0.09 ± 0.05 | 0.22 ± 0.38 |
| Lymphocytes (103/µL) | 0.12–4.95 | 0.58–3.84 | 3.12± 1.05 | — | — | 2.98 ± 1.73 | 3.08 ± 1.65 |
| Eosinophils (103/µL) | 0–1.83 | 0–1.39 | 0.47 ± 0.44 | — | — | 0.24 ± 0.19 | 0.18 ± 0.08 |
| Monocytes (103/µL) | 0–2.38 | 0–0.99 | 0.19 ± 0.13 | — | 0.18–0.90 | 0.15 ± 0.11 | 0.16 ± 0.07 |
| Basophils (103/µL) | 0–0.21 | 0–0.18 | 0.05 ± 0.54 | — | 0.01–0.10 | 0.10 ± 0.07 | 0.0 ± 0.0 |
| Platelets (103/µL) | 298–931 | 178–777 | 729.58 ± 125.40 | 277–882 | 303 ± 133 | 437 ± 0.0 | |
| Reticulocytes (%) | — | — | 2.1 ± 0.9 | — | 1–12 | — | — |
MCH, Mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume.
Values are presented as a range or mean plus-or-minus standard deviation.
Values for mink refer to although no statistical differences were determined between male and female minks.
International Species Information System: Physiological data reference values. Apple Valley, MN, 2002, ISIS.
TABLE 48-5.
Reference Ranges for Serum Biochemical Parameters for Selected Mustelid Species
| North American Parameter* | Nearctic River Otter | Eurasian Otter | Mink | Striped Skunk† | Ferret | Pine Marten | European Polecat† |
|---|---|---|---|---|---|---|---|
| Total protein, gram per deciliter (g/dL) | 5.7–9.0 | 6.0–7.7 | 5.94 ± 0.31 | 6.2 ± 1.2 | 5.1–7.4 | 6.1 ± 7 | 5.7 ± 8 |
| Albumin (g/dL) | 2.4–4.1 | 1.25–3.6 | 2.98 ± 0.14 | — | 2.6–4.1 | 3.0 ± 4 | 3.3 ± 0.4 |
| Globulin (g/dL) | 2.9–5.8 | 2.7–4.8 | 3.1 ± 4 | 2.4 ± 0.7 | |||
| Calcium (mg/dL) | 6.8–10.0 | 5.2–10.3 | 9.54 ± 0.39 | 2.43 ± 0.23 | 8.0–11.8 | 9.2 ± 1.6 | 9.12 ± 0.92 |
| Phosphorus (mg/dL) | 3.2–8.3 | 4.2–8.7 | 5.29 ± 0.79 | 1.74 ± 0.61 | 4.0–9.1 | 4.95 ± 0.92 | 6.19 ± 1.70 |
| Sodium, milliequivalent per liter (mEq/L) | 136–158 | 142–158 | 153.7 ± 1.3 | 149 ± 7 | 137–162 | 155 ± 3 | 152 ± 6 |
| Potassium (mEq/L) | 3.5–5.3 | 3.9–5.7 | 4.34 ± 0.23 | 4.8 ± 0.7 | 4.3–7.7 | 4.0 ± 0.2 | 4.7 ± 0.6 |
| Chloride (mEq/L) | 94–121 | 102–125 | 114.5 ± 1.7 | 110 ± 6 | 102–125 | 126 ± 1 | 116 ± 8 |
| Creatinine (mg/dL) | 0.4–0.8 | 0.7–1.0 | 0.71 ± 0.08 | 1.09 ± 0.80 | 0.2–0.9 | 0.79 ± 0.18 | 0.49 ± 0.20 |
| Urea nitrogen (mg/dL) | 17–56 | 17.3–68.1 | 15.2 ± 5.6 | 33.9 ± 32.9 | 10–45 | 31.64 ± 11.2 | 12.5 ± 3.99 |
| Cholesterol (mg/dL) | 63–279 | 95–220 | 172.4 ± 103.8 | 64–296 | 176.9 ± 23.0 | 191.9 ± 52.6 | |
| Glucose (mg/dL) | 56–225 | 51–400 | 125.8 ± 18.7 | 124.8 ± 62.9 | 62.5–207 | 314.5 ± 70.90 | 106.9 ± 28.9 |
| SERUM ENZYMES | |||||||
| Lactic acid dehydrogenase, international unit per liter (IU/L) | 36–10,820 | 555–3,620 | — | 581 ± 323 | — | 1,875 ± 520 | 474 ± 403 |
| Alkaline phosphatase (IU/L) | 29–282 | 9.0–199 | 71.6 ± 56.9 | 70 ± 57 | 9–120 | 77 ± 29 | 64 ± 79 |
| Gamma-glutamyl transferase (IU/L) | 8–38 | — | — | 2 ± 3 | — | — | 10 ± 8 |
| Creatine kinase (IU/L) | 67–1,300 | 26–1,794 | — | 895 ± 252 | — | 555 ± 234 | 379 ± 384 |
| Alanine aminotransferase (IU/L) | 46–990 | 34–307 | — | 120 ± 98 | 82–289 | 173 ± 44 | 102 ± 56 |
| Aspartate aminotransferase (IU/L) | 34–1,260 | 71–328 | 67.0 ± 13.7 | 75 ± 22 | 28–248 | 159 ± 18 | 74 ± 28 |
Values are presented as a range or mean plus-or-minus standard deviation.
International Species Information System: Physiological data reference values. Apple Valley, MN, 2002, ISIS.
Diseases
Viral and Bacterial Diseases
The following viral diseases have been reported in mustelids: Aleutian mink disease (plasmacytosis), influenza, canine distemper, rabies, rotavirus diarrhea, infectious canine hepatitis, pseudorabies (Aujeszky disease), transmissible mink encephalopathy, mink enteritis, epizootic catarrhal enteritis of ferret (coronavirus) feline panleukopenia, canine parvovirus, feline leukemia, Powassan virus disease (arbovirus), herpes, and necrotizing encephalitis (herpes simplex).18, 21, 22, 23
The following bacteria have been identified as pathogenic in mustelids: Helicobacter mustelae, Desulfovibrio spp., Campylobacter jejuni, C. coli, Salmonella spp., Clostridium perfringens type A, C. botulinum, C. welchii, Mycobacterium spp., Actinomyces spp., Pseudomonas aeruginosa, P. putrefaciens (also known as Shewanella putrefaciens), Streptococcus spp., Staphylococcus spp., Erysipelothrix rhusiopathiae, Escherichia coli, Klebsiella pneumoniae, K. oozaenae, Bordetella bronchiseptica, Listeria monocytogenes, Yersinia pestis, Y. ruckeri, Bacillus anthracis, Brucella abortus, Pasteurella multocida, P. pseudotuberculosis, Francisella tularensis, Leptospira spp., Bacteroides melanigenicus, Proteus vulgaris, P. mirabilis, and Plesiomonas shigelloides.
Fungal diseases are rarely reported in mustelids, but those cited include histoplasmosis, cryptococcosis, blastomycosis, coccidiomycosis, mucormycosis (Absidia corymbifera), adiaspiromycosis (Emmonsia crescens), and dermatomycosis (Microsporum sp. and Trichophyton sp.).
Table 48-6 contains information about some common infectious diseases reported in mustelids.
TABLE 48-6.
Selected Infectious Diseases of Mustelids
| Disease | Causative Agent | Epizootiology | Clinical Signs | Diagnosis | Management | Species Reported |
|---|---|---|---|---|---|---|
| Viral canine distemper | Canine distemper virus (Paramyxoviridae) | Transmission of the virus is most commonly accomplished by aerosol exposure or direct contact with conjunctival and nasal exudates, urine, feces, and skin | Weight loss, anorexia, hyperemia of the face and ears, hyperkeratosis of the nasal planum and footpads, and oculonasal discharge | Histopathology, immunofluorescent antibody (IFA) test on conjunctival smear | Vaccination with canary-pox recombinant canine distemper virus subunit vaccine (Purevax, Merial) | Domestic ferret, black footed ferret, American and Eurasian badger, weasel, striped skunk, Eurasian and American mink, sable, stone and pine marten, polecat, weasel, Nearctic river and Eurasian otter |
| Influenza | Orthomyxoviridae (several strains) | Transmission by inhalation of aerosol droplets | Sneezing, conjunctivitis, unilateral otitis, fever, photophobia | Clinical signs, presence of hemagglutination-inhibiting (HI) antibodies (hemagglutination inhibition test) | Prevention of exposure of susceptible animals to infected individuals (animals or caretakers) Antihistamine, antivirals, and antibiotics may be used |
Domestic ferret and mink |
| Aleutian disease and plasmacytosis | Parvoviridae | Infected animals may serve as potential source of infection | Weight loss, hypergammaglobulinemia, reproductive failure, hemorrhagic enteritis, and immune-mediated glomerulonephritis | Hypergammaglobulinemia usually greater than 20% of total serum protein IFA test, counter immunoelectrophoresis test |
No vaccine is available | Typically a disease of farm-raised mink, but has been found in feral mink, domestic ferret, and striped skunk |
| Ferret kit disease | Rotavirus | Affects kits May become enzootic in the facility |
Watery diarrhea, anorexia, and lethargy | Negatively stained virus particles identified in fresh feces | Subcutaneous electrolyte solutions and oral antibiotics (spectinomycin, amoxicillin, and trimethoprim-sulfa) | Ferret |
| Bacterial salmonellosis | Salmonella newport, Styphimurium, Scholerasuis, S. anatum, S. enteritidis, S. kentucky, S. hadar |
Salmonella spp. have been isolated in a number of clinically normal animals Associated to feeding with uncooked meat |
Hemorrhagic enteritis, dehydration, loss of body weight, fever, and lethargy | Fecal culture | Supportive care and antibiotics | Many mustelids |
| Tuberculosis | Mycobacterium spp. (M. bovis, M. avium-intracellulare, M. tuberculosis) | Usually infected by eating Mycobacterium-contaminated meat | Weight loss, enlarged lymph nodes, chronic respiratory disease, mastitis | Direct examination of tissue, culture | Evaluate zoonotic potential in case of treatment | Mink, ferret, otters, and Eurasian badger |
| Campylobacteriosis | Campylobacter jejuni, C. coli | Ferrets may be asymptomatic carriers Raw meat diets may predispose mink to infection |
Fever, leukocytosis, abortion, diarrhea | Fecal culture | Antimicrobials (erythromycin, amoxicillin and others) | Ferret, mink |
| Botulism | Type A, B, C, E Clostridium botulinum, and C. perfringens type A, C. welchii | Caused by eating uncooked or contaminated meat Associated with capture stress in wild otters |
Animals are found dead or with paralysis, and dyspnea before dying Enterotoxemia, acute gastric distention, cyanosis |
Fecal Gram stain, toxin assay | Prevention and treatment difficult Aggressive therapy |
Otters, black-footed ferret |
| Pneumonia | Pseudomonas aeruginosa, P. putrefaciens, S. zooepidemicus, S. pneumoniae, E. coli, Klebsiella pneumoniae, Bordetella bronchiseptica, Listeria monocytogenes | Concurrent infection with calicivirus or picornavirus may predispose animal to infection | Dyspnea, cyanotic mucous membranes, increased lung sounds, nasal discharge, fever, lethargy, and anorexia | Clinical signs, complete blood count results (leukocytosis), culture, and cytologic findings | Supportive care, antimicrobial therapy according to test results Antibiotics to consider include trimethoprim-sulfa, and cephalosporins |
Most mustelids |
| Anthrax | Bacillus anthracis | Acute death, with blood draining from body cavities | Staining smears of peripheral blood, postmortem lesions | Penicillin: streptomycin | Eurasian badger, honey badger, and mink | |
| Fungal dermatomycosis | Microsporum sp. and Trichophyton sp. | Transmitted by direct contact or via fomites and is associated with overcrowding and exposure to cats | Skin and hair lesions similar to those reported in other species | Clinical signs are suspicious but diagnosis is made on the basis of a mycotic culture | Topical treatment with keratolytic shampoos, povidone-iodine scrubs, and antifungal medications (itraconazole, ketoconazole) | Most species |
Parasitic Diseases
Although not generally associated with disease, numerous external and internal parasites have been identified in both wild and captive mustelids. Table 48-7 includes data on selected parasites reported to cause disease in mustelids. Parasitic diseases are also important for wild animals undergoing translocation because of the immune suppression possibly induced by stress.22, 25
TABLE 48-7.
Selected Parasitic Diseases of Mustelids
| Parasite | Location in Host | Clinical Signs | Diagnosis | Management | Species Reported |
|---|---|---|---|---|---|
| Toxoplasma gondii | Multiple organs (disseminated) | Elevated rectal temperature, lymphadenitis, splenomegaly, myocarditis, pneumonitis, hepatitis, encephalitis | Serologic | Prevention Avoid contact with feline species and feline feces Treatment with pyrimethamine and sulfamerazine, others |
Skunk, ferret, weasel, polecat, otters |
| Lung worms (Crenosoma spp., Perostrongylus spp., Filaroides spp., Skrjabingylus spp.) | Lung and sinus | Cachexia, anemia, coughing, dyspnea, depression, nasal discharge, and neurologic signs | Finding the ova or first stage infective larvae in fecal samples | Use of appropiate anthelmintic drug (ivermectin, fenbendazole, mebendazole) | Mink, skunk, sable, Eurasian badger, otter, ermine |
| Kidney worm (Dioctophyma renale) | Kidney (usually right kidney) | Weight loss, hematuria, polyuria, renal colic, and trembling | Finding of characteristic ova in urine, radiography or ultrasonography | Surgical treatment (removal of the parasitized kidney), fluid and antibiotic therapy | Mink, otter, weasel, ermine, marten, fisher, grison |
| Sarcoptic mange (Sarcoptes scabiei) | Skin (especially head and neck) | Scab formation around head and neck, tail, and feet In advanced cases, the entire body may be involved |
Finding the mites in skin scraping or biopsy Diagnostic treatment with ivermectin |
Ivermectin (0.3–0.4 milligram per kilogram [mg/kg]) as a single injection, or 0.2 mg/kg, orally (PO) every other day for 2 weeks if severe; antibiotics for secondary infection | Most mustelids |
| Fleas (most often Ctenocephalides sp.) | May be asymptomatic, pruritus and flea allergy dermatitis, with chronic scratching and rubbing Severe infestation may lead to debilitation by exsanguination |
Visualization of fleas or flea defecations | Affected animals and enclosures should be repeatedly treated with suitable insecticides (pyrethrins, fibronil, imidacloprid [use small cat/kitten vial/dose], lufenuron) | Most mustelids | |
Ectoparasites
External parasites reported to affect mustelids include the following: fleas (Ctenocephalides canis, C. felis, Pulex irritans, Nosopsyllus fasciatus, Ceratophyllus gallinae, Chaetopsylla globiceps, Parceras melis, Spilopsyllus cuniculi, Monopsyllus sciurorum), ticks (Ixodes ricinus, I. bansksi, Amblyomma americanum, Dermacentor variabilis), lice (orders Mallophaga, and Anoplura), demodectic mange (Demodex sp.), sarcoptic mange (Sarcoptes scabiei), ear mites (Otodectes cynotis), myiasis (Cuterebra spp., and Wohlfahrtia vigil), Guinea worm (Dracunculus insignis), filarial dermatitis (Filaria taxidae). Mite, tick, and flea treatments include concurrent treatments of the environment and the animals. Topical treatment should include those approved for use in cats (pyrethrin powders and sprays and others). Organophosphates and carbamates should be used with caution, as safe protocols for mustelids have not been established.
Internal Parasites
Protozoal infection include Giardia spp., Isospora spp., Eimeria spp., Sarcocystis spp., Toxoplasma gondii, Neospora caninum, Sarcosporidium sp., Besnoitia spp., Hepatozoon spp., Pneumocystis carinii, Trypanosomiasis cruzi, Cryptosporidium spp.40
Helminths reported from mustelids in both zoos and from the wild include: lung flukes (Paragonimus westermani and P. kellicotti), intestinal fluke (Nanophyetus salmincola, Troglotrema acutum), liver flukes (Fasciola hepatica), Acanthocephala (Corynsoma semerme, C. strumosum, Macracanthorhynchus ingens), tapeworms (Taenia sp., Monordotaenia sp., Oschmarenia sp.), trichinosis (Trichinella sp.), lung worms (Skrjabingylus spp., Crenosoma spp., Perostrongylus spp., and Filaroides spp.), heartworms (Dirofilaria spp.), ascariasis (Ascaris spp., Baylisascaris devosi, Toxocara canis), Dioctophyma renale, Dracunculus spp., Strongyloides spp., Capillaria hepatica, Uncinaria sp., Euyhelmis squamula, Aonthotheca putorii, Eucoleus sp., Pearsonema plica, Molineus patens, and Mastophorus muris.
Table 48-8 lists common drugs and doses used for controlling parasitic diseases in mustelids.
TABLE 48-8.
Parasiticides Recommended for Mustelids
| Generic Name | Dosage (milligram per kilogram [mg/kg]) | Route of Administration | Comments |
|---|---|---|---|
| Amprolium | 19, every 24 hours | Orally (PO) | Coccidia |
| Carbaryl (0.5%) shampoo | Weekly for 3 weeks | Mange | |
| Fenbendazole | 50, for 3–5 days | Oral | Alternatively 20 mg/kg for 5 days |
| Fipronil | 1 pump of spray or 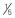 – –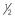 of cat dose every 60 days of cat dose every 60 days |
Topical | Flea adulticide |
| Ivermectin |
0.2–0.5, repeat every 2 weeks if needed |
Subcutaneous (SC) or PO |
0.006 mg/kg, PO, monthly for heartworm prevention |
| Ectoparasites and endoparasites | |||
| Levamisole | 10 | PO or SC | May be toxic at higher dosages |
| Mebendazole | 50 mg/kg q12h × 2 days | PO | Nematodes |
| Metronidazole | 15–20, every 12 hours for 2 weeks | PO | Protozoa: Clostridium spp. |
| Pyrethroids | — | Ectoparasites | |
| Praziquantel | 5–25, repeat in 2 weeks | PO or SC | Cestodes and trematodes |
| Propoxur | — | Topical | Ectoparasites |
| Pyantel pamoate | 5–60, repeat in 14 days OR 4.4 mg/kg q2 weeks | PO | Nematodes |
| Sulfadimethoxin | 20–50, every 12–24 hours | PO | Antiparasitic, coccidian antimicrobial |
| Thiacetarsemide | 2.2, every 12 hours for 2 days | Intravenously (IV) | Heartworm adulticide |
| Follow 3–4 weeks later with ivermectin | |||
| Caution must be used | |||
Noninfectious Diseases
The following have been reported to affect wild and domestic mustelids (Table 48-9 ). Renal calculi (calcium oxalate and urate calculi) were detected in 66.1% of the captive North American adult population of Asian small-clawed otters that had been imaged or necropsied, and prevalence in wild-born otters was 76.7%. The captive diet appears to be a contributing factor to urolith formation and progression.32 Other medical problems associated with nutrition and feeding practices in mustelids are hypovitaminosis A; vitamin E, thiamin, (Chastek disease), calcium, vitamin D, zinc, and biotin deficiencies; zinc toxicity; nutritional secondary hyperparathyroidism (NSH); fibrous osteodystrophy; gastric trichobezoars; dental disease (dental calculus, gingivitis, and periodontal disease); gastric and duodenal ulceration; and gastric dilatation and torsion.34, 36
TABLE 48-9.
Selected Noninfectious Diseases of Mustelids
| Disease | Etiology | Signs | Management | Prevention | Species Reported |
|---|---|---|---|---|---|
| Exertional myopathy | Often, associated to recently immobilized, captured, and transported wild animals | Vary with species Elevated body temperature, depression, lack of response to the environment, ataxia, weakness, dark colored urine, elevated renal and muscular serum enzymes |
Treatment is rarely successful Selenium or vitamin E preparations given subcutaneously or intramuscularly, sodium bicarbonate balanced electrolyte solution, and nonsteroidal anti-inflammatories |
Improve methods of capture or restraint Reduce stress and hyperthermia during animal handling |
Badger, otter, black footed ferret |
| Urolithiasis | Magnesium ammonium phosphate, calcium oxalate, calcium urate, calcium phosphate, and ammonium urate Primary cause unknown Diet (?) |
Normally unnoticeable Abdominal radiography and ultrasonography are the most important diagnostic tools Signs may be similar to those in dogs and cats |
In some cases, surgery or lithotripsy may be considered | — | Mink, ferret, Eurasian otter, small-clawed otter |
| Petroleum pollution | Spilled petroleum oils (crude or fuel) | Animals look wet and chilled Lethargy, dermatitis, conjunctivitis, respiratory distress, dehydration, malnutrition, anemia, thermoregulatory dysfunction, diarrhea, and neurologic signs |
Primarily symptomatic Warm intravenous, intraosseous, subcutaneous isotonic fluids, glucose, antibiotics, and glucocorticoids Good ventilation, flushing the eyes Hand or tube feeding may be required Monitor blood parameters |
— | Any aquatic mustelid may be affected |
| Polychlorinated biphenyls (PCBs) | Accumulation of high level of PCBs, especially by fish eating species | Anorexia, bloody stools, hepatic liver, kidney degeneration, gastric ulcers, decreased baculum weight, feminization of males Population declines, reproductive complications and kit mortality |
— | — | Effects diagnosed in mink, Eurasian otter, polecat; may affect any piscivore species |
| Amyloidosis | Deposition of amyloid (17 different proteins) either locally or systemically | Relate to the specific sites of amyloid deposition Histologic evaluation of tissues obtained by biopsy or at necropsy |
Usually progressive. Treatment unsuccessful In humans, some trials include antibiotics, colchicine, and dimethyl sulfoxide |
— | Beech marten, pine marten, mink, wolverine, Asian small-clawed otter |
| Thiamine deficiency | Thiaminase present in some fish (especially carp, bullhead, smelt, herring) | Anorexia, salivation, ataxia, incoordination, pupillary dilation, and sluggish reflexes | Parenteral thiamine administration | Supplement with thiamine in piscivores species | Mink and otter May be a problem in piscivores species |
| Self-mutilation | Agitation, cutaneous excoriation, hair loss, cutaneous hemorrhage, secondary bacterial infection | Observation, physical examination, skin scrapings for cytology, etc. | Buspirone 10 milligram per kilogram (mg/kg), orally (PO) twice daily for 18 months | Proper housing, diet, species pairings | American badger |
Metabolic Diseases
Urolithiasis (magnesium ammonium phosphate, calcium oxalate, calcium urate, calcium phosphate, and ammonium urate uroliths), hypocalcemia, pregnancy toxemia, agalactia, hyperestrogenism, hormonal alopecia, idiopathic hypersplenism, gastric dilatation and torsion (possibly associated with Clostridium welchii), dental and skeletal anomalies, periodontal disease, amyloidosis, hyperadrenocorticism (ferret), insulinoma (ferret), diabetes mellitus (ferret), fatty liver, cardiovascular calcification, osteomalacia, and degenerative joint disease.11, 37
Neoplasia
Over 50 different neoplasms have been reported in the domestic ferret. Although no current consensus exists on the cause of the high prevalence of neoplasia in ferrets, several theories have been proposed: genetic predisposition, early neutering of ferrets at 5 to 6 weeks of age, lack of natural photoperiod or exposure to natural sunlight, diet, and infectious agents. However, neoplasms are not common in species other than ferrets and include: seminoma, leiomyoma, adenocarcinoma, pheochromocytoma, teratoma, lymphosarcoma, anal sac carcinoma, lymphoreticular tumor, bronchoalveolar carcinoma, thyroid carcinoma, malignant melanoma, and a tumor resembling Hodgkin disease.6, 7
Miscellaneous Diseases
Reproductive toxicity (including decreased baculum weight, cryptorchidism, cystic vas deferens) in European otters exposed to polychlorinated biphenyls and polychlorinated dibenzo-p-dioxins; organophosphate and carbamate intoxication; mortality associated with melarsomine and petroleum residues; mercury toxicity; secondary exposure to rodenticide; shock; exertional myopathy (capture myopathy); trauma; intestinal volvulus; pneumoperitoneum; uterine torsion; interspecific aggression (especially following introductions); behavior problems (self-mutilation); cystic kidneys; dilated cardiomyopathy; cor pulmonale; intervertebral disk disease; osteoarthritis; tail alopecia syndrome; overgrowth of claws; oral, gastric, and intestinal foreign bodies; gastric and intestinal ulcers; pyometra; capture related injuries (mostly digit and tooth damage); pulmonary silicosis; fibrocartilaginous emboli; trauma (mostly associated with gunshots, vehicle encounters, and from traps); and hydrocephalus in European otter cubs have all been reported.9, 14, 17, 24, 31
Reproduction
Important variations exist in the reproductive cycles among mustelids. Some data for representative species are listed in Table 48-10 . Most mustelids are seasonal breeders, with the sea otter and the Eurasian otter being exceptions. The duration of the breeding season may vary from 1 month (African striped weasel) to 12 months (Eurasian badger). Some mustelids are polyestrous, and others are monoestrous. The duration of estrus ranges from 3 to 5 days to 5 to 8 weeks. Most males that have been studied have active spermatogenesis for only about 3 to 4 months in a year, although exceptions such as the Eurasian badger do exist. Mustelids may be either induced or spontaneous ovulators.
TABLE 48-10.
Some Reproductive Characteristics of Selected Mustelids
| Parameter | Badger (American/Eurasian) | Ferret, Black-Footed Ferret | Marten (Pine/Stone) | Mink (American; Eurasian) | Otter (Nearctic River; Eurasian) | Giant Otter | Skunk (Striped; Spotted) | Tayra | European Polecat | Common Weasel/Ermine | Wolverine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestation | 8 months; 9–12 months | 41–42 days; 42–43 days | 9 months | 40–70 days; 35–72 days | 245–365 days; 63–63 days | 65–70 days | In South, 59–77 days; in North, 230–350 days | 63–70 days | 40–42 days | 34–37 days; 10 months | 7–9 months |
| Delayed implantation | Yes | No | Yes | Yes | Yes/no | No | No/yes | No | No | No/yes | Yes |
| Litter size | 1–7; 1–6 | 1–18; 1–6 | 2–5/2–7 | 3–10; 2–7 | 2–5; 2–4 | 1–5 | 2–10/2–9 | 2 | 4–6 | 4–7; 4–8 | 2–3 |
| Mass at birth | 90–98 grams (g); 5–85 g | 8–10 g; unknown | 30 g | 6–12 g; unknown | 100–120 g | 170–230 g | 32–35;22 g | 75–95 g | 7–12 g | 0.9–2.3/2.6–4.2 g | 80–100 g |
| Weaning | 3 months | 6–8 weeks; unknown | 4 months | 3 months | 3–4 months | 3–4 months | 2 months | Unknown | 1 month | 60 days; unknown | 3 months |
| Sexual maturity | 1 year | 4–8 months in first year | 28 months | In first year | 23–27 months; in 2–3 years | Unknown | 10 months in first year | 1.5–2 years | In first year | 115–1150 days; unknown | In 2–3 years |
| Type estrus* | M; P | P; M | M; — | P; — | M; P | — | M; P | P | —; M | P | |
| Teats (pairs) | 4; 3 | 2 | 4 | —; 2–3 | — | 5–7; 5 | — | 3–5 | 5; 4–5 | 2 | |
M, Monoestrous; P, polyestrous.
Many mustelids exhibit delayed implantation: sea otters, Nearctic river otters, hog badgers, American and Eurasian badgers, ratels, striped skunks, western spotted skunks, wolverines, all martens, ermines, long tail weasels, minks, and marbled polecats. In those species, embryo development proceeds to the blastocyst stage and then ceases. This period of blastocyst dormancy is called diapause and varies from a few weeks in minks and striped skunks to almost a year in the Eurasian badgers. Extensive studies have been conducted on the mechanisms that control embryonic diapause in three species of mustelids: minks (Mustela vison), Eurasian badgers (Meles meles), and western spotted skunks (Spilogale gracilis). Numerous investigators have speculated on the ecologic significance and selective pressures that might have favored the development of delayed implantation.29
Changes in photoperiods are known to alter the secretion of pituitary hormones and thus the onset and duration of breeding, puberty, and timing of implantation. In this way, photomanipulation has been used in some species. Adequate numbers of animals should be maintained for mating, but compatibility does not ensure reproductive success. If copulation or gestation does not occur, different pairings should be tried, but in some cases, animals that are not compatible during most of the year will often breed if introduced during estrus. For this, determining when females are in estrous may be crucial. Various methods for estrus detection have been proposed in different species, including behavioral changes, vulvar swelling, vaginal cytology, and fecal and urinary hormone analyses. In males, the testes enlarge during the breeding season. Pregnancy may be determined by urinary progesterone and conjugated estrogen levels, palpation, radiography (end of gestation period), and ultrasonography.4
In ferrets, continued high levels of estradiol from persistent estrus may lead to alopecia and bone marrow suppression, resulting in pancytopenia and even death, so nonbreeding females should be neutered.
Contraception
No specific recommendations for contraception exist for mustelids, and ovariohysterectomy, vasectomy, and castration are currently the safest permanent sterilization procedures of birth control. Melengestrol acetate hormone implants have been used successfully to prevent conception in mustelids. These should be removed after 2 years for one pregnancy, if possible, and are not recommended for more than a total of 4 years. The human contraceptive implant Norplant contains levonorgestrel, a synthetic progestin, and has been used to prevent pregnancy in the striped skunk. Depo-Provera injection (5 mg/kg every 2 months) has also been used. Although no data exist for mustelids, progestin contraceptives may be associated to progressive endometrial hyperplasia, resulting in infertility, infections, and sometimes uterine cancer in other carnivores. Deslorelin implants (gonadotropin-releasing hormone [GnRH] analogue) have been used as an alternative to melengestrol acetate.1
Preventive Medicine
Many of the clinical and surgical procedures used in dogs and cats are applied to mustelids. Specialized surgical procedures have been developed for some mustelid species.20, 28, 38 Periodic examinations should include the following:
-
♦
Checking transponders and tattoos, and reapplication, if necessary
-
♦
Checking baseline physiologic parameters (weight, breeding status)
-
♦
Examination of the oral cavity
-
♦
Evaluation of the reproductive tract, whole body radiography
-
♦
Collecting blood for hematologic and biochemical evaluation
-
♦
Checking for heartworm in endemic areas using a heartworm enzyme-linked immunosorbent antigen assay test
-
♦
Serum banking
-
♦
Performing fecal examination for internal parasites (and administering anthelmintics, if necessary). Table 48-8 lists some of the antiparasitic drugs commonly used to treat mustelids. Other drugs (e.g., antibiotics) are dosed at rates for ferrets, dogs, and cats.8
-
♦
Updating vaccinations
Few viral diseases have been reported in mustelids, except ferrets, although they have been routinely vaccinated against a wide variety of viral diseases. Mustelids have varying susceptibility (species and exposure dependent) to feline panleukopenia, canine distemper, rabies, and leptospirosis.10 Most authors recommend vaccination of mustelids against rabies and canine distemper. Safety and efficacy of modified live canine distemper vaccinations in exotic species of carnivores has been historically problematic because vaccine-induced distemper has occurred (e.g., a modified-live virus derived from chick embryo cell culture caused the death of four female black-footed ferrets [Mustela nigripes], or protection was not achieved). In the past, killed distemper vaccines have not provided longstanding protection in most species. A recombinant canarypox-vectored canine distemper vaccine (Purevax, Merial, Athens, GA) has been shown to be safe and efficacious and is the best choice for general mustelid protection against canine distemper virus.41 If an alternative modified-live canine distemper vaccine is used, it should be given separately and not in multiple forms, since immunosuppression and other untoward vaccine interactions might lead to disease. Ferret or mink cell culture-derived modified-live vaccines should never be used in mustelids. A modified-live canine distemper vaccine of primate kidney tissue cell origin, Onderstepoort type, is available in the United States (Galaxy D; Schering-Plough Animal Health Corporation, Omaha, NE) and has been proven to be safe and efficacious in hybrid black-footed ferrets and Siberian polecats. The only vaccine approved by the U.S. Department of Agriculture (USDA) for ferrets, Fervac-D (United Vaccines, Madison, WI), which is an egg-adapted strain, has induced anaphylactoid and anaphylactic reactions in some mustelids, so its use is not recommended.
Vaccination schedules for nondomestic species are extrapolated from studies of the domestic dog. Neonates receiving colostrum should be vaccinated every 3 to 4 weeks between 6 and 16 weeks of age. Colostrum-deprived neonates should be given two vaccinations administered at a 3- to 4-weeks interval and starting at 2 weeks of age because maternal antibodies acquired in utero may be absent by 4 to 6 weeks of age. Data on maternal antibody interference with vaccination in ferrets suggest that a final canine distemper vaccine should be administered after 10 weeks of age.
If an animal has an adverse reaction to canine distemper vaccine, an antihistamine (e.g., diphenhydramine hydrochloride, 0.5–2 mg/kg, intravenously [IV] or intramuscularly [IM]) or, for severe reactions, epinephrine (20 microgram per kilogram [µg/kg], IV, IM, subcutaneously [SC], or intratracheally (IT]) should be administered and supportive care provided.
Mustelids are also vaccinated with a killed rabies vaccine (Imrab), although the efficacy of this vaccine has not been proven in exotic mustelids. Rabies should be given at 16 weeks of age to animals at risk of contracting rabies and given boosters annually thereafter.
Acknowledgment
The authors acknowledge Helena Marques, Elena Rafart, Hugo Fernandez, Carlos Feliu, Jon Arnemo, Marie-Pierre Ryser-Degiorgis, Lucy Spelman, Jordi Ruiz, Rafael Cebrian, Jose Domingo, Victor Bonet, Willem Schaftenaar, and Eric Miller for assistance with the preparation of this chapter.
References
- 1.Association of Zoo and Aquariums Wildlife Contraception Center www.aza.org/wildlife-contraception-center
- 2.Arnemo JM, Moe RO, Søli NE. Immobilization of captive pine martens (Martes martes) with medetomidine-ketamine and reversal with atipamezole. J Zoo Wildlife Med. 1994;25:548–554. [Google Scholar]
- 3.Association of Zoos and Aquariums . Association of Zoos and Aquariums; Silver Spring, MD: 2009. Association of Zoo and Aquarium, Small Carnivore Tag 2009. Otter (Lutrinae) care manual. pp 5–18. [Google Scholar]
- 4.Association of Zoos and Aquariums . Association of Zoos and Aquariums; Silver Spring, MD: 2009. Association of Zoo and Aquarium, Small Carnivore Tag 2010. Mustelid (Mustelidae) care manual. pp 5–20, 2010. [Google Scholar]
- 5.Baitchman E, Kollias GV. Clinical anatomy of the North American river otter (Lontra canadensis) J Zoo Wildl Med. 2000;32(4):473–483. doi: 10.1638/1042-7260(2000)031[0473:CAOTNA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett SL, Imai DM, Trupkiewicz JG. Intestinal lymphoma of granular lymphocytes in a fisher (Martes martes) J Zoo Wildl Med. 2010;41:309–315. doi: 10.1638/2009-0210R.1. [DOI] [PubMed] [Google Scholar]
- 7.Bunting EM, Garner MM, Abou-Madi N. Proliferative thyroid lesions and hyperthyroidism in captive fishers (Martes martes) J Zoo Wildl Med. 2010;41:296–308. doi: 10.1638/2009-0186R2.1. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JW, Marion CJ. Saunders; St. Louis, MO: 2013. Exotic animal formulary. pp 561–594. [Google Scholar]
- 9.Cooper JE. Other mustelids. In: Mullineaux E, Best D, Cooper JE, editors. British Small Animal Veterinary Association manual of wildlife casualties. British Small Animal Veterinary Association; Quedgeley, Gloucester: 2003. pp. 147–151. [Google Scholar]
- 10.Deem SL, Spelman LH, Yates RA, Montali RJ. Canine distemper in terrestrial carnivores: A review. J Zoo Wildl Med. 2000;31:441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Elhensheri M, Linke RP, Blankenburg R. Idiopathic systemic AA-amyloidosis in a skunk (Mephitis miphitis) J Zoo Wildl Med. 2012;43:181–185. doi: 10.1638/2011-0090.1. [DOI] [PubMed] [Google Scholar]
- 12.Estes JA. Adaptations for aquatic living by carnivores. In: Gittleman JL, editor. vol II. Cornell University Press; New York: 1996. pp. 242–282. (Carnivore behavior, ecology, and evolution). [Google Scholar]
- 13.Fahlman A, Arnemo JM, Persson J. Capture and medetomidine-ketamine anesthesia of free-ranging wolverines (Gulo gulo) J Wildl Dis. 2008;44:133–142. doi: 10.7589/0090-3558-44.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Fairbrother A, Locke LN, Hoff GL. Iowa State University Press; Ames, IA: 1996. Noninfectious diseases of wildlife. p 219. [Google Scholar]
- 15.Fernandez-Moran J. Mustelidae. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. ed 5. Saunders; Philadelphia, PA: 2003. pp. 501–516. [Google Scholar]
- 16.Fowler ME. ed 3. Wiley-Blackwell; Ames, IA: 2008. Restraint and handling of wild and domestic animals. pp 280–283. [Google Scholar]
- 17.Gage LJ. Use of buspirone and enrichment to manage aberrant behavior in an American badger (Taxidea taxus) J Zoo Wildl Med. 2005;36:520–522. doi: 10.1638/04-100.1. [DOI] [PubMed] [Google Scholar]
- 18.Graham E, Lamm C, Denk D. Systemic coronavirus-associated disease resembling feline infectious peritonitis in ferrets in the UK. Vet Rec. 2013;171:200–201. doi: 10.1136/vr.e5652. [DOI] [PubMed] [Google Scholar]
- 19.Hartup BK, Kollias GV, Jacobsen MC. Exertional myopathy in translocated river otters from New York. J Wild Dis. 1999;35(3):542–547. doi: 10.7589/0090-3558-35.3.542. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Divers SM, Kollias GV, Abou-Madi N. Surgical technique for intraabdominal radiotransmitter placement in North American river otters (Lontra canadensis) J Wildl Med. 2001;32(2):202–205. doi: 10.1638/1042-7260(2001)032[0202:STFIAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Keller SM, Gabriel M, Terio KA. Canine distemper in an isolated population of fishers (Martes pennanti) from California. J Wildl Dis. 2012;48:1034–1041. doi: 10.7589/2011-12-350. [DOI] [PubMed] [Google Scholar]
- 22.Kimber KR, Kollias GV. Infectious and parasitic diseases and contaminated-related problems of North American river otters (Lontra canadensis): A review. J Zoo Wildl Med. 2000;31:45–472. doi: 10.1638/1042-7260(2000)031[0452:IAPDAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Kimber KR, Kollias GV, Dubovi EJ. Serologic survey of selected viral agents in recently captured wild North American river otters. J Zoo Wildl Med. 2000;31(2):168–175. doi: 10.1638/1042-7260(2000)031[0168:SSOSVA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Kimber K, Kollias GV. Evaluation of injury, severity, and hematological and plasma biochemistry values for recently captured North American river otters (Lontra canadensis) J Zoo Wildl Med. 2005;3693:371–384. doi: 10.1638/03-125.1. [DOI] [PubMed] [Google Scholar]
- 25.Kollias GV. Health assessment, medical management, and prerelease conditioning of translocated North American river otters. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. Saunders; Philadelphia, PA: 1999. pp. 443–448. [Google Scholar]
- 26.Kollias GV, Abou-Madi N. Procyonids and mustelids. In: West G, Heard D, Caulkett N, editors. Zoo animal and wildlife immobilization and anesthesia. Blackwell Publishing; Ames, IA: 2007. pp. 419–427. [Google Scholar]
- 27.Kreeger TJ, Arnemo JM, Raath JP. Wildlife Pharmaceuticals, Inc; Ft. Collins, CO: 2002. Handbook of wildlife chemical immobilization. [Google Scholar]
- 28.McEwen MM, Moon-Masset PF, Butler FC. Polymerized bovine hemoglobin (oxyglobin solution) administration in two river otters (Lontra canadensis) Vet Anesth Anal. 2001;28:214–219. doi: 10.1046/j.1467-2987.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 29.Mead RA. The physiology and evolution of delayed implantation in carnivores. In: Gittleman JL, editor. vol II. Cornell University Press; New York: 1996. pp. 437–464. (Carnivore behavior, ecology, and evolution). [Google Scholar]
- 30.Melquist WE, Polechia PJ, Toweill D. River otter (Lontra canadensis) In: Feldman GA, Thompson BC, Champman JA, editors. Wild mammals of North America—biology, management and conservation. ed 2. The Johns Hopkins University Press; Baltimore, MD: 2003. pp. 708–734. [Google Scholar]
- 31.Neifer DL, Klein EC, Calle PP. Mortality associated with melarsomine dihydrochloride administration in two North American river otters (Lontra canadensis) and a red panda (Ailurus fulgens fulgens) J Zoo Wildl Med. 2002;33:242–248. doi: 10.1638/1042-7260(2002)033[0242:MAWMDA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Petrini KR, Lulich JP, Treschel L, Nachreiner RF. Evaluation of urinary and serum metabolites in Asian small-clawed otters (Aonyx cinerea) with calcium oxalate urolithiasis. J Zoo Wildlife Med. 1999;30:54–63. [PubMed] [Google Scholar]
- 33.Quesenberry K, Carpenter JW. ed 3. Elsevier; St. Louis, MO: 2012. Ferrets, rabbits, and rodents. [Google Scholar]
- 34.Righton AL, St. Leger JA, Schmitt T. Serum vitamin A concentrations in captive sea otters (Enhydra lutris) J Zoo Wildl Med. 2011;42:124–127. doi: 10.1638/2010-0011.1. [DOI] [PubMed] [Google Scholar]
- 35.Sato JJ, Welsan M, Prevosti F. Evolutionary and biogeographic history of weasel-like carnivorans (Musteloidea) Mol Phylogenet Evol. 2012;63:745–757. doi: 10.1016/j.ympev.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Simpson VR, King MA. Otters. In: Mullineaux E, Best D, Cooper JE, editors. British Small Animal Veterinary Association manual of wildlife casualties. British Small Animal Veterinary Association; Quedgeley, Gloucester: 2003. pp. 137–146. [Google Scholar]
- 37.Simpson VR, Tomlinson AJ, Molenaar FM. Renal calculi in wild Eurasian otters (Luta lutra) in England. Vet Rec. 2011;169:49. doi: 10.1136/vr.d1929. [DOI] [PubMed] [Google Scholar]
- 38.Spelman LH. Otter anesthesia. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine: Current therapy. ed 4. Saunders; Philadelphia, PA: 1999. pp. 436–443. [Google Scholar]
- 39.Tocidlowski ME, Harms CA, Summer PW, Summer PW. Technique of mandibular salivary gland biopsy in river otters (Lutra lutra) J Zoo Wildlife Med. 1999;30:252–255. [PubMed] [Google Scholar]
- 40.Van Der Hage MH, Dorrestein GM. Proceedings of the 4th scientific meeting, European association of Zoo and Wildlife Veterinarians. 2002. Neospora caninum: myocarditis in a European pine marten (Martes martes) p. 217. Heidelberg, Germany. [Google Scholar]
- 41.Wimsatt J, Biggins D, Innes K. Evaluation of oral and subcutaneous delivery of an experimental canary pox recombinant canine distemper vaccine in the Siberian polecat (Mustela exermanni) J Zoo Wildl Med. 2003;34:25–35. doi: 10.1638/1042-7260(2003)34[0025:EOOASD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


