Viruses are small entities (20 to 300 nm) whose genomes replicate inside cells using host cellular machinery to create progeny virions (virus particles). On its own, a virus may be considered an inert biochemical complex of macromolecules because it cannot replicate outside a living cell. However, viruses are known to infect all living organisms, and a broad variety of viruses contribute to human disease.
Key Points about Viruses.
-
▪
Viruses are entities whose genomes replicate inside cells using host cellular machinery to create progeny virions (virus particles) that can transfer their genome to other cells.
-
▪
A broad variety of viruses are of high medical significance and contribute to manifestations of human disease.
Viral Classification and Structure
Historically, viruses were named according to common pathogenic properties, organ tropism, and modes of transmission. Viral groups are classified based on viral host range, particle morphology, and genome type. Viral hosts represent species from all classes of cellular organisms; prokaryotes (including the Archaea and Bacteria), eukaryotes (including algae, plants, protozoa, and fungi), and complex invertebrates and vertebrates. Indeed, viruses can cross phyla; for example, different members of Poxviridae can infect vertebrates and insects.
Virion structure varies among different viral groups, yet all virus particles are enclosed by a capsid structure that surrounds the viral genome. Icosahedral symmetry is the preferred capsid morphology (Fig. 13-1 ). The capsid is a protein shell composed of repeating subunits, or protomers (also referred to as capsomeres). The capsid together with the enclosed nucleic acid is called the nucleocapsid. The term virion denotes the complete infective virus particle. Many viruses demonstrate the classic capsid polyhedron structure of 20 equilateral triangular faces and 12 vertices, which define axes of fivefold rotational symmetry. However, alternative virion morphologies exist. Some viruses have a helical nucleocapsid, consisting of a helical array of capsid proteins composed of identical protomers wrapped around a filament of nucleic acid. Thus, for these viruses, such as myxoviruses (e.g., tobacco mosaic virus), the length of the helical nucleocapsid is determined by the length of the nucleic acid.
Figure 13-1.
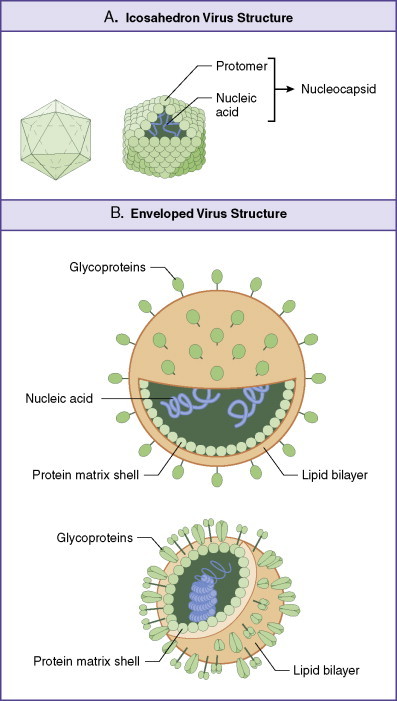
Basic virus structure.
The basic virus structure is an icosahedron having 20 equilateral triangular faces and 12 vertices. Lines through opposite vertices define axes of fivefold rotational symmetry with structural features repeating five times within each complete rotation about any axis (A). A nucleocapsid contains DNA or RNA encapsulated within a capsid composed of protomer subunits. Enveloped virions (B) support an outer lipid bilayer studded with transmembrane glycoprotein spikes. Other forms of viral structure exist, such as those with helical nucleocapsid symmetry in which size of the virus is dictated by the length of nucleic acid core (not shown).
Other virus families have an outer envelope consisting of a lipid bilayer surrounding the viral capsid. Such viral envelopes are derived in part from modified host cell membranes during particle formation and release (budding) from the infected cell. The exterior of the bilayer is studded with transmembrane proteins, revealed as glycoprotein spikes or knobs. Both the outer capsid and envelope proteins of viruses are glycosylated and are important in determining the host range and antigenic composition of the virion.
Key Points About Viral Classification and Structure.
-
▪
Icosahedral symmetry is the preferred status for organization of virus structure subunits (protomers) making up the capsid protein shell.
-
▪
Other structural forms exist, yet they all share commonality in packaging of genetic material (DNA or RNA) for delivery to host cells upon infection.
Viral Genetic Material: RNA or DNA
Each virus carries within the protective capsid a nucleic acid–based blueprint for replication of infectious virus particles (virions). Once a virus has invaded a cell, it is able to direct the host cell machinery to synthesize new progeny. The viral genome may be composed of RNA or DNA, single or double stranded. Encoded proteins may be nonstructural, such as nucleic acid polymerases required for replication of genetic material, or structural (those proteins necessary for assembly of new infectious virions). However, all viruses lack the genetic information encoding proteins necessary to generate metabolic energy or protein synthesis. The viral genome (DNA or RNA) rarely codes for more than the few proteins necessary for replication or physical structure.
Viruses as a group are the only class of organisms with subspecies that keep RNA as their sole genetic material. Likewise, they are the only group of self-replicating organisms with subspecies that use single-stranded DNA as genomic content. Multiple forms of virus genomes are found in virions infecting human cells (Fig. 13-2 ).
Figure 13-2.
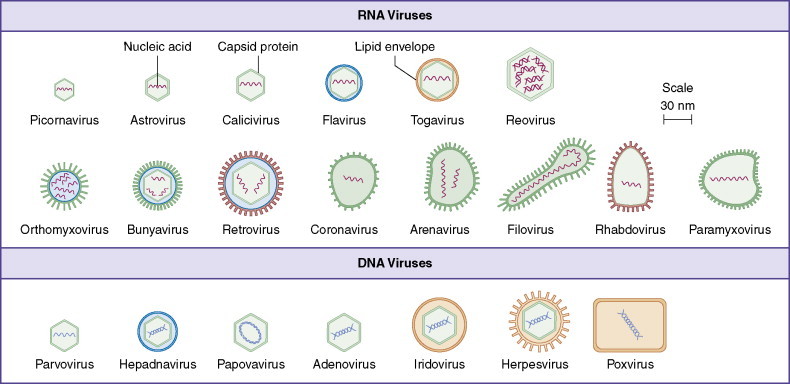
At least 21 families of viruses are capable of infecting the human host and are distinguished by the presence of an envelope or characteristic capsid and by internal nucleic acid genomic content.
Strategies for infectivity and replication
The first stage of viral infection and subsequent replication involves entry into the host cell (Fig. 13-3 ). The host cell phenotype has a great deal of influence on the strategy the virus uses to gain access; in turn, specific virus types may use different strategies to gain access to the same cell type. In general, the steps involve attachment and penetration, uncoating of the virus genome, and synthesis of early proteins (enzymes involved in viral replication), followed by synthesis of late proteins or structural components required for assembly and release of the infectious virion.
Figure 13-3.
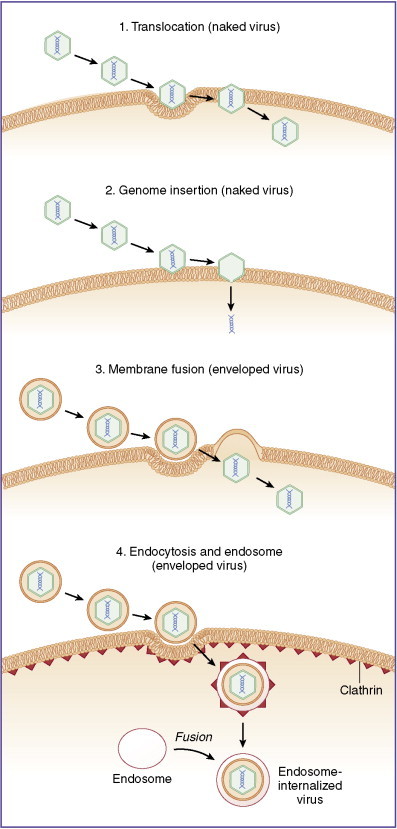
Viruses enter host cells following attachment by multiple means including (1) translocation, in which a virus crosses membranes intact; (2) genome insertion, in which attached viruses inject genetic material directly into cytoplasm; (3) membrane fusion, in which genomic contents of a virus are dumped into the host cell cytoplasm; and (4) endocytosis dictated by surface receptor binding and clathrin-mediated transport, sometimes leading to fusion into intracellular endosomes.
Viral entry into the cell is usually a passive reaction that does not require energy on the part of the virus. Naked viral particles may enter by membrane translocation, in which the entire virus crosses the cell border intact (pinocytosis). Alternatively, the naked particle binds to cell surface receptors and subsequent invagination occurs by either clathrin-mediated endocytosis (e.g., Adenoviridae) or other endocytic mechanisms such as interaction with caveolae or lipid rafts. A naked viral particle may also bind to the cell surface and inject genomic material into the host cell without complete cellular penetration of the invading virion. Enveloped viruses must enter host cells by using mechanisms of membrane fusion, either by receptor-mediated endocytosis or through fusion of viral and host membranes followed by injection of genomic material into the host cell cytoplasm (e.g., HIV-1 and HIV-2). In all cases, the critical component is the release of the viral genome from its protective capsid so that it can be transcribed to form new progeny virions.
In many cases, viral genetic material undergoes translation using host cellular machinery before viral genome replication (the exception being retroviruses and negative-sense RNA viruses). The first proteins generated are usually nonstructural DNA or RNA polymerases. Nucleic acid replication produces new viral genomes for incorporation into progeny virions. In general, DNA viruses replicate mainly in the nucleus and RNA viruses mainly in the cytoplasm, but there are exceptions (e.g., poxviruses contain DNA but replicate in the cytoplasm). Retroviruses are a special category of RNA viruses that require reverse transcription of their single-stranded RNA genome to a double-stranded DNA intermediate, which is then integrated into the host cell genome before viral replication can take place. Retroviruses not only encode a reverse transcriptase enzyme as part of the virion but also package it into newly formed virions.
The next set of proteins to be transcribed are structural in nature, including capsid protomers and scaffolding proteins that are required for assembly of the virion together with the newly replicated viral nucleic acid. Assembly of viral nucleocapsids can take place in either the nucleus (herpesvirus, adenovirus) or cytoplasm (poliovirus) or on the cell surface (influenza). Diagnostic inclusions, sometimes visible by light microscopy, are the result of virions accumulating at the sites of assembly. The final stage of replication results in the release of newly formed virions from the host cell. This may occur by budding from the cell surface (enveloped viruses) or via host cellular secretory pathways in which Golgi-derived vesicles are transported to the cell surface. In nonenveloped viruses, host cells are destroyed with consequent release of infectious virions upon cell lysis.
The Baltimore classification of viruses establishes seven groupings according to genome types and replication strategies (Table 13-1 ).
Biochemistry.
Clathrin-Mediated Endocytosis
Receptor-mediated endocytosis is a complex phenomenon in which binding of large extracellular molecules, such as viruses, to receptors on the cell membrane triggers assembly of clathrin triskelions. Specific clathrin adapter complexes are involved in transport across the membrane, resulting in endosomal compartment formation.
Key Points About Strategies for Infectivity and Replication.
-
▪
Initiation of viral replication begins with attachment and entry of viral particles into host cells, followed by replication of genetic material and production of assisting proteins (polymerases and structural proteins) required for assembly of virions with mature nucleocapsids.
-
▪
Newly formed virions are released from the host cells by assembly at the cell surface, via budding, or by lysis of the host cell.
-
▪
Multiple and diverse replication strategies are used depending on the type and class of genetic material contained within the virus entity.
Table 13-1.
Different Classes of Viruses Grouped According to Replication Strategy (Baltimore Classification)
| Class | Genome Type | Replication Strategy | Examples of Genus Infecting Human Cells |
|---|---|---|---|
| I | dsDNA | Semiconservative method using bidirectional replication forks from a single origin | Poxvirus Herpesvirus Adenovirus Papovavirus |
| II | ssDNA | Formation of a replicative from double-stranded DNA intermediate | Parvovirus |
| III | dsRNA replicating via (+) RNA | Conservative mechanism in which input RNA is transcribed to mRNA | Reovirus |
| IV | ssRNA (+) sense genomes | Synthesis of (–) sense RNA on a (+) sense template | Coronavirus Flavivirus Astrovirus Picornavirus |
| V | ssRNA (–) sense genomes | Begins with transcription by virion-associated RNA-dependent RNA polymerase | Arenavirus Orthomyxovirus Paramyxovirus Rhabdovirus |
| VI | Diploid ssRNA | Uses a dsDNA, longer than genome length intermediate (provirus), which is integrated covalently into host cell chromosomal DNA | Retrovirus |
| VII | dsDNA | Uses gapped or nicked circular dsDNA genomes to replicate via longer than genome length messenger-sense ssRNA intermediates | Hepadnavirus |
(+), Sense strand; (–), antisense strand.
Viral disease patterns and pathogenesis
Virus-induced pathology is the result of direct viral action leading to host cell death and tissue damage with subsequent sequelae. Almost all naked (nonenveloped) viruses produce acute infections in this manner as a result of cell lysis during replication and spread of infection to surrounding host cells. However, pathologic damage often is a result of an active immune response to viral antigens and epitopes presented on the surface of infected cells (Fig. 13-4 ). This becomes especially apparent in chronic infections when persistent virus production allows vigorous development of cellular (T helper cell [TH] and cytotoxic T lymphocyte generation) and humoral (B cells with specific antiviral antibodies) responses. In the case of latent infections, short periods of active viral replication are kept in check by active immune responses, only to reemerge when immune system surveillance wanes.
Key Points About Viral Disease Patterns and Pathogenesis.
-
▪
Pathology associated with viral infections is directly linked to viral cell tropism and mechanism of associated replication.
-
▪
Damage to surrounding tissue may be a direct result of the immune response and attempts to limit viral replication and spread.
Figure 13-4.
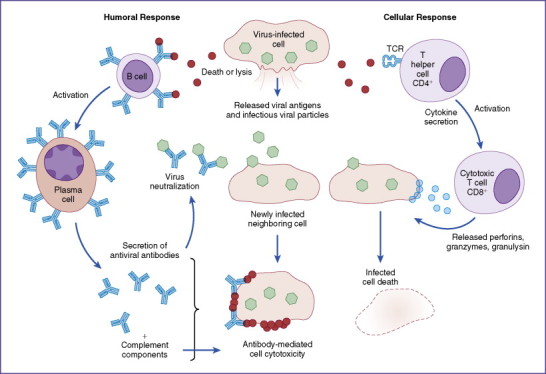
Damage to tissue may be initiated by response to released viral antigens and to viral antigens presented by major histocompatability complex molecules on the surface of infected host cells. Released antigens allow development of antibody responses (left side), leading to deposition on the surface of infected targets and cellular destruction by complement mediation. T helper cells release cytokines that assist cytotoxic lymphocytes to induce killing of target cells (right side). During both processes, bystander killing of surrounding tissue may occur, leading to manifestation of pathology. TCR, T-cell receptor.
Diagnostic virology
The large number of possible viral agents that produce a given disease or pathology precludes the use of one simple test as diagnostic for a specific viral infection. Rather, laboratory diagnosis is usually performed under the assumption of clinical disease spectrum, relying on symptoms and epidemiologic data (Fig. 13-5 ). Clinical observations alone are at times sufficient for diagnosis, allowing for clear therapeutic intervention prior to verification of virus identity. The identification of a virus from a clinical specimen relies on general characteristics such as the ability to replicate or produce certain phenotypes in cell culture. In vitro propagation in tissue culture can determine the level of infection. Typically, viral growth in tissue culture produces a cytopathogenic effect, which may be visualized as a plaque or vacancy within a monolayer of cells. Infected cells may be further fixed and stained; immunohistochemical methods can be used to determine the presence of characteristic and diagnostic inclusions (or inclusion bodies) in cytoplasm or in the nucleus. Along with these diagnostic tools, specificity may be obtained by using antibodies to neutralize the viral agent and confirm its identification. Incubation of infected cells with neutralizing antibodies will result in reduction of growth of the virus and subsequent reduction in the number of plaques formed.
Figure 13-5.
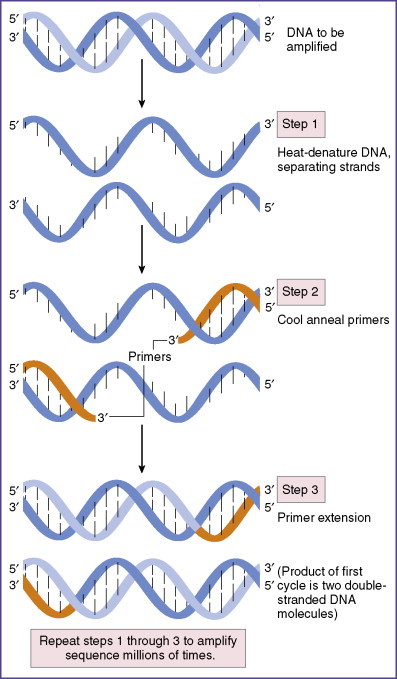
Viral identification can be accomplished by multiple means, including cell culture, microscopic identification, serologic methods to detect antiviral antibodies, direct detection of viral antigens, or detection of nucleic acids by polymerase chain reaction. The method of polymerase chain reaction amplification of viral nucleic acids is depicted here.
Serologic tests are useful to confirm induced responses to viral antigens. Serum collected from infected individuals may be assessed for antibody response by the enzyme-linked immunosorbent assay. Enzyme immunoassays, also known as solid-phase immunoassays, are designed to detect antibodies through secondary production of an enzyme-triggered color change. Enzyme immunoassays are useful in detecting and quantifying the presence of viral agents in clinical specimens (blood, vaginal swabs, and feces). Use of specific antibodies immobilized to a solid phase also allows capture of pathogens for diagnostic quantitation to known standards.
Identification of viral agents in clinical specimens also may be performed by using highly sensitive genetic tools. RNA or DNA can be extracted and sequences probed by hybridization techniques. If the agent is already identified, analysis can also be accomplished by use of polymerase chain reaction or reverse-transcriptase polymerase chain reaction to specifically amplify sequences unique to the viral agent under consideration. However, in some instances, this task is difficult or impossible because of atypical clinical presentation or histopathologic features. Molecular diagnostics using DNA microarrays, or gene chip arrays, offer the promise of precise, objective, and systematic virus classification from clinically obtained specimens. In addition, diagnostic gene chip arrays that carry sequences of major clinically relevant viral pathogens allow extremely rapid screening of small diagnostic samples.
Therapy and prophylaxis for viral infections
Therapy against viral infection makes use of chemotherapeutic agents that are effective to control infection and disease. Virucides, such as detergents, chloroform, and ultraviolet light, use general mechanisms to limit environmental spread of viruses. This is especially effective at reducing levels of enveloped organisms that are sensitive because of their bilipid envelope. Antiviral agents are more specific and include molecules that target receptors for cell attachment or that inhibit viral penetration, uncoating of the viral genome, or viral replication. Inhibition of replication may occur at multiple stages; agents may be directed against macromolecular synthesis and inhibit transcription, translation, or posttranslational modification (e.g., protease inhibitors). The majority of clinically available antiviral agents target nucleic acid synthesis and limit viral replication rather than completely eliminating organisms (virustatic vs. virucidal). Problems associated with host toxicity limit the use of virucidal agents in many instances. An additional concern is the selective pressure associated with prolonged use of antiviral agents, which allows mutants to arise that are no longer susceptible to drug action.
Another class of antiviral agents includes those that function as immunomodulators to improve host response to combat infection. These antivirals do not directly attack the specific pathogen, but rather they globally stimulate host immune responses. Interferons (IFNs) were first defined as glycoproteins that interfere with viral replication through degradation of viral mRNA. Most nucleated cells make IFN-α and IFN-β, secreted molecules that bind to specific receptors on adjacent cells to protect them against subsequent infection by progeny viruses. There are at least 17 different subtypes of IFN-α but only one subtype of IFN-β. In addition to direct antiviral effects, IFN-α and -β enhance expression of class I and class II major histocompatability complex molecules on infected cells, in effect increasing viral antigen presentation to specific TH and cytotoxic T cells. A functionally related molecule, IFN-γ, produced by TH1 cells, cytotoxic lymphocytes, and natural killer cells, is a potent activator of macrophages and a powerful antiviral immunomodulating agent.
A more specific approach is to synthesize antibodies that bind to viral pathogens to mark them for attack and clearance by other elements of the immune system. Vaccination (immunoprophylaxis) induces a primed state so that secondary exposure to a pathogen generates a rapid immune response, leading to accelerated elimination of the organism and protection against onset of clinical disease. Success depends on the generation of memory T and B cells and the presence in the serum of antivirus-specific neutralizing antibody. Neutralizing antibodies work to inhibit viral attachment, penetration, uncoating, and even viral replication. Alternatively, nonneutralizing antibodies can be quite therapeutically functional, assisting in viral clearance by marking the virus for phagocytosis by monocytes. Antiviral vaccines are effective when presented to the host in a manner that is similar to natural exposure to viral antigens, thus generating protective immunity. This may be accomplished through active immunization with live modified viral strains, with inactivated virus particles, and with subunit vaccines (Table 13-2 ). In general, active immunization leads to long-lasting immunity. Alternatively, passive antiviral treatment is possible by administration of high-titer, specific antivirus antibodies (hyperimmune globulin) that confer host resistance. Passive administration leads to fast-acting but temporary immunity to imminent or ongoing exposure.
Pharmacology.
Antiviral Agents: Highly Active Antiretroviral Therapy
Highly active antiretroviral therapy holds great promise to limit HIV infection by means of agents that are terminal nucleoside analogs, non-nucleoside reverse transcriptase inhibitors, and viral protease inhibitors. Zidovudine was the first Food and Drug Administration approved antiretroviral and is an analog of thymidine that works as a nucleoside analog reverse transcriptase inhibitor.
Key Points About Therapy and Prophylaxis for Viral Infections.
-
▪
INFs are a natural part of the immediate protective host response, which is elicited upon invasion by viruses.
-
▪
Among the few successful chemotherapeutic agents that inhibit viral replication are nucleoside analogs that compete with normal nucleotides for incorporation into viral DNA or RNA and protease inhibitors that interfere with virus assembly.
-
▪
Immunization with subunit vaccines or live attenuated viruses increases both antibody production and long-term protective cell-mediated responses directed at viruses upon subsequent infection.
Key Points.
-
▪
Viruses replicate by using host cellular machinery to create progeny virions. All viruses share commonality in packaging of genetic material (DNA or RNA) for delivery to host cells.
-
▪
Viruses are organized according to icosahedral symmetry, with protomer subunits comprising a capsid protein shell.
-
▪
Viral replication begins with attachment and entry into host cells, replication of genetic material, and production of polymerases and structural proteins required for production and subsequent assembly of virions with mature nucleocapsids.
-
▪
Newly formed virions are released from the host cells by assembly at the cell surface, via budding, or by lysis of the host cell.
-
▪
Viral cell tropism and mechanism of replication play a major role in development of associated pathology. Subsequent damage to tissue may also result from immune recognition and targeted responses to limit further infection of neighboring cells.
-
▪
Antiviral chemotherapeutic agents that inhibit viral replication include nucleoside analogs to compete with nucleotides for incorporation into viral particles, and protease inhibitors that interfere with virus assembly.
-
▪
All nucleated cells are capable of producing a subclass of interferons for immediate protective host responses; additional help is provided by antibody, natural killer cells, and adaptive T lymphocytes. Preimmunization increases both antibody production and long-term protective cell-mediated responses, allowing quicker and more effective responsiveness upon infection.
Table 13-2.
Virus Vaccines
| Vaccine Class | Virus | Comments |
|---|---|---|
| Live virus vaccines | Adenovirus Chickenpox Measles Mumps Poliovirus (Sabin vaccine) Rotavirus Rubella Smallpox (variola) Yellow fever |
Active immunization using avirulent attenuated strains Effective at inducing antibodies and cytotoxic lymphocyte responses |
| Killed virus vaccines | Hepatitis A Influenza Poliovirus (Salk vaccine) Rabies |
Active immunization using heat or chemically inactive virus particles Vaccination may be combined with other viruses (polyvalent) |
| Virus-like particles | Human papillomavirus | Immunization using particles assembled from recombinant coat proteins |
| Subunit vaccines | Adenovirus | Active immunization using purified proteins |
| Polypeptide vaccines | Hepatitis B | Active immunization using synthesized polypeptide protein sequences |
| DNA vaccines (evaluation only) | HIV Influenza |
Experimental Useful for induction of cytotoxic T-lymphocyte response |
| Passive antibodies | Hepatitis A Hepatitis B Measles Mumps Rabies Respiratory syncytial virus Rubella Varicella-zoster |
Injected purified antibodies obtained from another source Short-lived function with little value when given after disease onset |
HIV, human immunodeficiency virus.
Self-assessment questions can be accessed at www.StudentConsult.com.


