Introduction
The major function of the lung is to facilitate gas exchange between the air and blood compartments, which takes place in the alveolar region of the lung. In the adult lung, the alveolar-capillary barrier that permits efficient gas exchange is formed by thin cytoplasmic extensions of alveolar type I (TI) cells and capillary endothelial cells, separated by a common fused basement membrane.1
The alveolar surface area comprises more than 99.5% of the large internal surface area of the lung,2 estimated in the adult human to be approximately 100 to 150 m2. Efficient diffusion of gases between the air and blood is dependent on the thin aqueous/lipid lining layer and the cellular compartments (epithelial, interstitial, and endothelial). Capillaries are located in the interalveolar septum that bridges different alveolar surfaces.3 On the air side, the alveolus is lined by two morphologically distinct epithelial cells, TI and alveolar type II (TII) cells. The morphologic and morphometric characteristics of both TI and TII cells are remarkably constant over an approximately 10,000-fold range in mammalian lung size,4 from which one may infer that there is a conservation of functions of both cell types. Although the distinctive anatomic features of alveolar cells permit the efficient diffusion of gases by forming a thin cellular barrier, alveolar epithelial cells have also evolved to perform other necessary functions, including producing pulmonary surfactant, maintaining an optimal alveolar liquid layer by regulating ion and water transport, protecting against inhaled toxins and infecting agents, and repairing the alveolar epithelium following lung injury or inflammation.4, 5
Pulmonary surface-active material (surfactant) is critical for normal lung function. Without surfactant, the work of breathing increases markedly and respiratory distress develops rapidly. In the absence of surfactant the work of breathing may increase from less than 2% to more than 10% of total oxygen consumption. Surfactant provides the low surface tension at the air-liquid interface that is necessary to prevent atelectasis, alveolar flooding, and severe hypoxia. A complex but highly regulated process has evolved for the synthesis, secretion, and reutilization of surface-active material. In addition to its well-recognized property of providing alveolar stability, surfactant is also important for maintaining the patency of small airways and preventing alveolar flooding.
This chapter reviews the functions of TI cells and TII cells, the physiologic properties of pulmonary surfactant, the use of surfactant replacement therapy in the treatment of hypoxic respiratory failure (newborn respiratory distress syndrome [NRDS]), and the role of the alveolar epithelium and surfactant in selected lung diseases. Additional discussion of the alveolar epithelium can be found in Chapter 1, and comprehensive reviews of acute respiratory distress syndrome (ARDS) can be found in Chapter 100.
Alveoli
The number of alveoli in a lung is largely dependent on the size of the lungs, with larger lungs containing more alveoli. On average, Ochs and colleagues6 have estimated that the adult human lung contains approximately 480 million alveoli, each approximately 4.2 × 106 cu µ in size. Recently, the number of the alveoli in the mouse lung has been estimated to be 560 in young mice (12 weeks) and 880 in old mice (91 weeks); these estimates were derived by use of computed tomography imaging, a technique that is quite different from traditional stereology, and may allow quantitative measurements in disease and lung injury models.7 In the adult lung, the average alveolar fluid depth is estimated to be 0.14 to 0.20 µm.8
Alveolar Epithelial Intercellular Junctions
Early physiologic studies9 demonstrated that transport of water and low-molecular-weight solutes out of the pulmonary capillaries is rapid, whereas transport of solutes out of the alveoli is extremely slow. The anatomic basis for the barrier function of the alveolar epithelium became clear from freeze-fracture studies of the lung10, 11 that demonstrated that epithelial intercellular junctions had morphologic characteristics of “tight junctions,” in contrast to endothelial intercellular junctions that had morphologic characteristics of gap or “leaky” junctions.
Epithelial cells are attached to one another at their lateral membranes by a series of different junctional complexes, including tight junctions, adherens junctions, and gap junctions. These junctional complexes are each composed of different proteins that convey specific functions. Although both tight junctions and adherens junctions associate with the actin cytoskeleton, they have somewhat different functions; tight junctions regulate paracellular movement of ions and solutes, whereas adherens junctions mediate cell-cell adhesion and participate in cell signaling.
The most apical of the intercellular junctions is the tight junction or zona occludens (e.g., “closing belt”). By ultrastructural analyses, tight junctions appear as a series of anatomic appositions between the lateral membranes of adjacent cells that extend in a beltlike network surrounding cells, attaching them to adjacent cells and forming a continuous seal. The tight junction is the major factor in determining paracellular permeability. In addition to the “barrier” functions that regulate the passage of water, ion, and various molecules through paracellular spaces, tight junctions also have a “fence” function, which prevents intermixing of proteins and lipids in the apical and lateral membranes, establishing cell polarity. The tight junction is composed of a dynamic complex of proteins that include transmembrane proteins, cytoplasmic scaffolding proteins, signaling proteins, and adaptors that link to the cytoskeleton. Claudins play key roles in forming tight junction strands and determining paracellular permeability.12 Permeability can be further modulated by various other factors, including Rho-associated protein kinase, protein kinase C, and other kinases, as well as various cytokines, growth factors, and hormones.13
A second type of intercellular junction, the adherens junction, is located more basally. Adherens junctions are composed of clusters of adherens molecules, such as vascular endothelial-cadherin, β-catenin, and plakoglobin, linked to the actin cytoskeleton. There are also associations with kinases, phosphatases, and growth factor receptors. Complex intermolecular interactions mediate cell-cell adhesion and modulate cell signaling from growth factors and from mechanical forces such as sheer stress. Adherens junctions play important roles in both development and wound healing. The complex field of adherences junctions has been well reviewed.14, 15, 16
A third type of junction, the gap junction, is ubiquitous in most mammalian tissues. Gap junctions are composed of connexins, proteins that form intercellular channels, enabling the diffusion of molecules throughout interconnected cells, enabling cells to function in a coordinated measure. In the alveolar epithelium, gap junctions mediate intercellular calcium fluxes, which play a role in surfactant secretion.17
Alveolar Type I Cells
In the mammalian lung, TI cells comprise only approximately 10% of the cells in the alveolar region but cover more than 95% of the internal alveolar surface area; TII cells (≈18% of alveolar cells) cover the remaining less than 5% of the surface area.4 TI cells are large (surface area ≈7 × 103 sq µm) squamous cells with thin cytoplasmic extensions that form the epithelial component of the air-blood barrier. The cytoplasmic extensions of TI cells are only 50 to 100 nm thick, below the resolution of conventional light microscopy. Prior to the development of the electron microscope, there was intense debate about whether the lining of the lung was cellular or acellular. Electron microscopic studies definitively demonstrated that the alveolar surface was lined by a continuous epithelium.18, 19 TII cells are much smaller cells (≈30% of the volume and 3% of the surface area of TI cells) that are often, but not always, cuboidal in shape (Fig. 8-1 ).4 Both TI cells and TII cells are not necessarily confined to one side of the alveolar septum but can bridge two or three different alveoli19 (see Fig. 8-3B). These complex three-dimensional morphologic relationships may complicate simple interpretations of cellular function that assume distinct apical, basal, and lateral membrane domains.3
Figure 8-1.

Immunofluorescent TI and TII cells in the mouse lung.
The majority of the alveolar epithelial surface area is composed of TI cells. Transgenic mouse with TII cells expressing enhanced green fluorescent protein (green) and TI cells expressing rat podoplanin (secondary immunofluorescence, red).
(J. Vanderbilt, L. Allen, and L. Dobbs, unpublished micrograph.)
Figure 8-3.

Alveolar epithelial TII cells.
A, A portion of a TII cell, showing lamellar bodies, a portion of a tight junction (TJ, arrow) between TI and TII cells, and a lamellar body undergoing exocytosis. B, Alveolar epithelium showing a TII cell bridging two different alveolar surfaces (arrows) and the perinuclear region of a TI cell. C, A portion of a TII cell and secreted lamellar bodies and tubular myelin (TM) in the alveolar space. D, A portion of a TII cell with a lamellar body (LB) and a composite body (CB).
(Lennell Allen and L. Dobbs, unpublished micrographs.)
We have learned a considerable amount about the biology of TII cells from the study of isolated and cultured TII cells and the development of transgenic mouse models. In contrast, techniques to isolate and study TI cells took many years to develop because of the lack of biochemical or molecular reagents to isolate and evaluate the cells and the fragility of isolated TI cells. Until fairly recently, the accepted concept was that the TI cell was a biologically inactive, terminally differentiated cell. However, recent evidence based on studies of isolated TI cells and gene expression profiling suggests that TI cells play important roles in numerous alveolar functions.
Many published images show only small portions of TI cells because of the large surface area of the cell and limited sampling area of electron microscopy. These images have given rise to an incorrect impression that TI cells do not contain either microvilli or subcellular organelles necessary for biosynthetic and other pathways. In fact, TI cells contain microvilli, abundant mitochondria, Golgi, rough and smooth endoplasmic reticulum, small intracellular vesicles, and caveolae, subcellular domains consistent with both metabolic and endocytic functions (Fig. 8-2 ).
Figure 8-2.





Alveolar septum and TI cells.
A, Low magnification electron micrograph showing the alveolar area of the lung. A TI cell is in the center of the image; from the perinuclear area (N), thin cytoplasmic extensions (arrows) cover the alveolar surface. TI cells contain mitochondria (M) and abundant Golgi apparatus (G). Erythrocytes (R) can be seen in capillaries. Opposing arrowheads delineate the width of an extension of a different TI cell. B, The cytoplasmic extensions (arrows) of two different TI cells form a tight junction (boxed area). C, Higher magnification of the boxed area in panel B showing more detailed ultrastructure of the tight junction (TJ). D, Higher magnification view of a different TI cell showing mitochondria (M), flasklike membrane invaginations typical of caveolae (arrow, C) and other endocytic structures (arrowheads). E, Anatomic detail of the air-blood barrier, with thin extensions of a TI cell (TI, arrows) and an endothelial cell (endo, arrow) and the common basement membrane (BM) between the endothelial and epithelial compartments. The capillary contains erythrocytes (RBC).
(Lennell Allen and L. Dobbs, unpublished micrographs.)
More detailed analyses of the differences in TI cells and TII cells from both specific studies and gene expression profiling have provided some insight into the functional differences between the two cell types.20, 21 For example, there are differences in the expression of intercellular junctional proteins. Both TI and TII cells express primarily three claudins (claudin-3, claudin-4, and claudin-18.1).22, 23 Claudin-3 is associated with decreased barrier function, whereas claudins 4 and 18 are associated with increased barrier function. The proportion of each claudin is different between the two cell types.12 In TI cells, the lung-specific claudin-18.1 constitutes more than 50% of claudin mRNA, whereas in TII cells, claudin-3 is the dominant claudin. Because TII cells contain approximately 20-fold more claudin-3 protein than do TI cells, it has been suggested that claudin-3 is localized mainly to TI-TII cell junctions and that TI-TII and TI-TI cell junctions may have different paracellular permeability characteristics; for example, it has been proposed that TI-TII cell junctions are more permeable than the TI-TI cell junctions that constitute the majority of the alveolar epithelial surface.12, 22 There may be other differences between TI-TI and TI-TII junctions. For example, in models of lung injury, neutrophils appear to migrate across the epithelial barrier selectively between TI and TII cells, although the molecular mechanisms responsible for this apparent selectivity are unknown.24, 25
Our concepts about alveolar fluid homeostasis have evolved over the past 10 years. Studies of clearance of exogenously added lung liquid demonstrate rapid clearance of liquid from the alveolar space, suggesting that clearance is mediated by Na+ channels with a high conductance and/or large numbers of channels with a lower conductance. Ions are transported actively, with water following passively. The osmotic water permeability of TI cells is one of the highest found in mammalian cell membranes, suggesting that TI cells play a major role in passive water transport.26 Presumably aquaporin 5, which is localized in the apical plasma membrane of TI cells,27 is responsible for the high osmotic water permeability; the molecular basis for water transport across the basal surface remains unknown.14 TI and TII cells also differ in the content of certain ion channels. Studies with either freshly isolated TI and TII cells28 or TII cells cultured under special conditions29 demonstrated that both cell types contain high-conductance sodium channels. TI cells, unlike TII cells, contain cyclic nucleotide gated (CNG) channels and K+ channels, and TII cells contain more cystic fibrosis transmembrane conductance regulator (CFTR) than do TI cells per unit area.28 By immunohistochemical methods and Western blotting, TI cells contain the epithelial sodium channel (ENaC) and various subunits of the Na+-, K+-ATPase.30 In addition to high-conductance sodium channels, both cell types contain less selective Na+ channels, although these are far less abundant.28 In most species, less than half of fluid transport in the lung is inhibitable by amiloride31, 32; thus, it has been postulated that the amiloride-insensitive CNG channels are responsible for the bulk of transport. The importance of amiloride-insensitive sodium transport was recently reviewed by O'Brodovich and associates.33 Although patch clamping data are consistent with the presence of CNG channels in TI cells but not TII cells, the presence of CNG channels in TII cells cannot be definitively excluded.
Whereas, in the adult, the epithelium from the alveolus to the nose is, in general, a sodium absorbing epithelium, in utero the alveolar epithelium actively secretes chloride into the developing airspace.8, 34 Chloride secretion in the adult alveolar epithelium has not been clearly established and probably varies depending on microenvironmental conditions. However, human alveolar epithelial cells in vitro can be stimulated to secrete chloride.35 Both TI cells (CFTR, Cl−/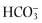 anion exchanger, and voltage-gated Cl− channels) and TII cells (CFTR) contain Cl− channels, and TI cells have been shown to transport Cl−.36 In addition, the TII cell has a proton sodium exchanger that is likely important in acidifying alveolar fluid.8, 37
anion exchanger, and voltage-gated Cl− channels) and TII cells (CFTR) contain Cl− channels, and TI cells have been shown to transport Cl−.36 In addition, the TII cell has a proton sodium exchanger that is likely important in acidifying alveolar fluid.8, 37
The pump linked to active sodium resorption is the Na+-, K+-ATPase. It has been estimated that approximately 60% of unstimulated fluid transport in the lung is mediated through the α2 subunit of Na+-, K+-ATPase, which is expressed in TI cells but not in TII cells.38 Data at present support the hypothesis that ions and water are transported across the entire alveolar epithelium. Taken together, the large discrepancy in surface area between the cell types, the channel density per unit area (Table 8-1 ), the likely central role for CNG channels, and the relative importance of the α2 subunit of Na+-, K+-ATPase all suggest that the TI cell, rather than the TII cell, plays the major role in bulk fluid transport in the alveolar region.
Table 8-1.
Ratios of Various Transport Parameters in TI and TII Cells
| TI : TII Cell Ratio | References | |
|---|---|---|
| Cell surface area | 43 | 4 |
| Osmotic water permeability | 7 | 26 |
| Na+ and K+ uptake/µg protein | ≈3 | 30 |
| Apical Na+ channels/cell | ≈40 | 28 |
| CNG and K+ channels | * | 28 |
| CFTR | 6 | 28 |
CFTR, cystic fibrosis transmembrane conductance regulator; CNG, cyclic nucleotide gated.
Present in TI cells, not TII cells.
The innate immune functions of TII cells have been well documented, but it is only recently that TI cells have also been shown to have potential immunomodulatory functions.39 TI cells produce proinflammatory cytokines such as TNF-α, IL-6, and IL-1β after treatment with lipopolysaccharide (LPS).40 The production of cytokines in TI cells appears to be modulated by the renin-angiotensin system.40
TI cells appear to be more susceptible to acute lung injury than are TII cells,41 and their cellular repair processes are relevant to restoring lung function after injury. TI cells contain abundant caveolae (see Fig. 8-2D) and express caveolin-1.42 Caveolae have diverse functions including endocytosis and membrane trafficking,43 and, in addition, they are believed to play an important protective role by providing a source of membrane to protect against cellular lysis. Recent studies of TI cells injured in vitro have confirmed that membrane repair by lipid recruitment is facilitated by the caveolar endocytic pathway and by the remodeling of the actin cytoskeleton close to the site of wounding.44, 45
On the basis of studies in animal models of lung injury, it has been believed that the injured alveolar epithelium is repaired by proliferation and transdifferentiation of TII cells to TI cells, with TI cells regarded as terminally differentiated cells without proliferative potential.46, 47 However, on the basis of recent in vitro studies, cultured TI cells have been shown to have a high proliferative potential.48, 49 Both the potential proliferative capacity and phenotypic plasticity of the TI cell in vitro raise the possibility that TI cells in vivo may participate in lung repair after injury.
Some researchers have stressed the concept that pulmonary epithelium in the conducting airways can sense the environment (e.g., oxidant gases, cigarette smoke, nanoparticles) (see Chapter 10). If this is also true in the alveolus, then it likely that the TI cells, which cover nearly all the alveolar surface, will be shown to be important sensors of the environment.
Alveolar Type II Cells
TII cells were first comprehensively described by C. C. Macklin before the advent of electron microscopy.50 Many of his initial predictions about the function of these cells have been verified over the past 60 years. In electron micrographs, TII cells are readily identified by the presence of lamellar bodies, the intracellular storage form of pulmonary surfactant (Fig. 8-3 ). The major functions of TII cells include: producing pulmonary surfactant, serving as the progenitor cells for maintaining the alveolar epithelium under normal circumstances and after mild injury, transporting fluid to keep the alveoli from flooding, and playing an important role in innate immunity. The high density of mitochondria and high glucose and oxygen consumption indicate that TII cells are highly metabolic. Because TII cells are the only source of surfactant, which is rich in phospholipids, lipid metabolism of the TII cells has been studied extensively.51 TII cells are highly enriched in genes involved in lipid synthesis.52, 53 For example, TII cells can be identified in the lung by expression of two enzymes of lipid metabolism, fatty acid synthase and stearoyl CoA desaturase-1, as well as by expression of the traditional markers such as surfactant protein (SP) C.52 Lipogenesis is upregulated at the end of gestation and is regulated by the transcription factors sterol regulatory element binding protein-1c and CCAAT/enhancer binding protein (C/EBP) alpha, similar to other lipogenic cells.52, 54 TII cells also are unique in expression of one of the surfactant proteins, SP-C, and the promoter for SP-C has been extremely useful for expressing or deleting genes in murine TII cells. However, while the TII cells express other surfactant proteins, SP-A, SP-B, and SP-D, these proteins are also expressed in nonciliated bronchiolar cells (club cells [Clara]) and at low levels in some extrapulmonary issues.55, 56
The turnover of TII cells in the normal, uninflamed lung is relatively slow. However, in response to injury to TI cells, TII cells proliferate rapidly to restore the epithelium. These TII cells become a transit-amplifying population.57 The initial studies to demonstrate this relationship used oxidant injuries in rodents to identify the proliferating cells with titrated thymidine.58, 59, 60, 61, 62 In these studies, there was an initial labeling of TII cells and, with time, the labeled cells differentiated into TI cells. More recently, the ability of TII cells to proliferate and transdifferentiate into TI cells was documented by lineage tracing techniques in mouse lung.46 This recent report demonstrated that TII cells are the source of new TI cells and TII cells under normal conditions and under conditions of mild injury. Hence, TII cells can serve as a transient proliferating cell population to maintain the alveolar epithelium under normal circumstances. However, in response to severe injury such as after influenza or toxins specific to TII cells, other epithelial cells from the bronchioalveolar junction or terminal airways that express keratin V, α6/β4 integrin, or club cell secretory protein (Clara), can proliferate, migrate, and, in some circumstances, express SP-C.63 These additional pathways have been reported in mice and, although they are not fully defined, may be important in ARDS. Although the precise regulation of the transition of the TII cell to TI cell phenotype is not known, bone morphogenic protein 2 and transforming growth factor-β 1 (TGF-β1) have been suggested as regulators of the TII cell to TI cell transdifferentiation in vitro.64 Keratinocyte growth factor and hepatocyte growth factor are also important mitogens for TII cells in vivo and in vitro.65, 66, 67, 68, 69, 70, 71, 71a
As stated earlier, alveolar TII cells can also transport sodium and chloride to help maintain alveolar fluid volume.72, 73 This apical to basal surface fluid transport was first shown in vitro by the formation of domes, collections of fluid under the cells that lifted them up into a domelike structure.72, 73 Under normal circumstances, TII cells resorb fluid by transporting sodium through amiloride-sensitive and amiloride-insensitive sodium channels, thereby keeping the alveolus relatively free of fluid.33 However, under certain in vitro conditions, TII cells can also secrete chloride.35 Unfortunately, defining the precise transport functions of alveolar TII cells in vitro is compromised by the fact that the culture conditions employed to study transport do not maintain the TII cell phenotype, as determined by the loss of expression of cell differentiation–specific markers, such as the surfactant proteins.
Alveolar TII cells are also important in the initial innate immune response to environmental insults, such as air pollutants, toxins, bacteria, and viruses. Alveolar TII cells are part of the first responders when microbes and other toxic agents enter the alveolus. Surfactant proteins A and D (SP-A and SP-D) are important components of the innate immune system and are discussed in detail later in this chapter. SP-A and SP-D are multivalent lectins (i.e., proteins that bind to carbohydrates and can play numerous roles in biologic recognition). Indeed, SP-A and SP-D can bind to a variety of viruses, bacteria, and fungi. However, TII cells can also be the target of specific respiratory viruses such as SARS-CoV and influenza.74, 75 For example, if influenza is instilled into excised human lung, it mainly infects TII cells and does not infect TI cells.76 In response to viral infection, TII cells secrete a variety of cytokines to activate macrophages, recruit monocytes, and trigger the adaptive immune system.75 The dominant interferon produced by TII cells is interferon-λ (IL29), a type III interferon. The overall magnitude of the cytokine response is robust and similar to the response in alveolar macrophages. The major cytokines produced in response to influenza include CXCL10, IL6, RANTES, and IL29.75 Microbes can be recognized by pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs). At the mRNA level, human TII cells have significant expression of TLR2, TLR3, and TLR5 but low expression of TLR4 and TLR7, similar to that in bronchial epithelial cells.75 TII cells can also respond to danger-associated molecular patterns (DAMPs).77
TII cells are thought to be important in various lung diseases.78 In ARDS and models of acute lung injury, TII cells can repair the alveolar epithelium, although it is possible that other cell populations also participate in this process during severe injury.79 TII cells are necessary for restoring the surfactant system, which is critical for gas exchange and for minimizing transudation of fluid into the alveolar space. In interstitial lung disease, TII cell hyperplasia is a common pathologic feature. One of the current hypotheses on the pathogenesis of idiopathic pulmonary fibrosis (IPF) is that the fibrotic response is caused by TII cell dysfunction due to protein misfolding and endoplasmic reticulum (ER) stress, which ultimately leads to secretion of TGF-β and other profibrogenic factors.80, 81 Although some have considered an epithelial mesenchymal transition (EMT) to be an important part of the fibrotic response, this is unlikely to be important on the basis of recent lineage tracing studies.47 Mutations of SP-A and SP-C are thought to cause some types of familial pulmonary fibrosis, and this is thought to be due to protein misfolding and endoplasmic reticular stress.82, 83, 84, 85, 86 Potentially more will be learned about the role of TII cells in interstitial lung disease when these cells are isolated from diseased lung. Finally, TII cells can be a source of adenocarcinomas.87 Bronchioalveolar carcinomas and some adenocarcinomas likely arise in TII cells. Surfactant proteins and the transcription factor TTF1 have been used to differentiate lung adenocarcinomas from extrapulmonary sources and the presence of micrometastases in regional lung nodes in staging adenocarcinomas. In the mouse, urethane adenomas all express SP-C, which indicates that they are of TII cell origin88 and adenocarcinomas induced by KRAS mutations are also of TII cell origin.89
Physiologic Functions of Pulmonary Surfactant
The discovery of pulmonary surfactant came from direct physiologic observation and an understanding of the Laplace relationship for calculating surface tension. In 1929, von Neergaard90 discovered that there was a difference in the elastic recoil properties of the lung depending on whether the lung was inflated with air or saline so that it took more pressure to inflate a lung with air than with saline (Fig. 8-4 ). Even though it takes more pressure to inflate the lungs with air than with saline, von Neergaard90 calculated that the pressure needed to inflate the lungs with air was much less than expected at low lung volumes. From these observations, von Neergaard deduced that the surface tension in the lung was low.90 By studying extracts of lung, Clements and Pattle91, 92 independently demonstrated that surfactant, specifically the phospholipids of surfactant, was responsible for lowering the surface tension. The low surface tension, produced by pulmonary surfactant, markedly decreases the work of breathing, prevents alveolar collapse and atelectasis, allows for alveoli of different size (radius of curvature) to be stable, and prevents alveolar flooding. Soon after these discoveries, Avery and Mead demonstrated that a deficiency in surface-active material caused NRDS.93 The critical component of surfactant that provides the low surface tension is dipalmitoylphosphatidylcholine (DPPC). This is an unusual species of phosphatidylcholine in that both fatty acids are saturated. DPPC molecules can pack closely together and allow the surface monolayer to withstand the high film pressures required to produce a low surface tension at low lung volumes.93a
Figure 8-4.

Air and saline volume-pressure curves.
This figure depicts the classic physiologic observation that led to the discovery of surfactant. It takes more pressure to inflate the lung with air than with saline. However, the pressure difference is much less than would be expected if the alveolar lining had the same surface tension as other biologic fluids, which indicates the presence of a surface active material. The descending limb of the air volume-pressure curve is very reproducible and is used to estimate the surface tension of the lung and static compliance.
(Adapted from Radford EP Jr: Recent studies of mechanical properties of mammalian lungs. In Remington JW, editor: Tissue elasticity. Washington, DC, 1957, American Physiological Society, pp 177–190.)
Film pressure is the pressure required to maintain a surface film and is easiest measured and conceptualized in a Langmuir-Whilhemy balance in which a moveable barrier is used to compress a surface monolayer. Compression of the surface balance reduces the area of the surface film similar to exhalation. Surfactant produces a stable film and is able to withstand high film pressures without collapsing. During inflation, surfactant must be absorbed into the surface. During deflation, as the surface area decreases, the film pressure rises and the surface tension falls. Phospholipid molecules already present in the air-liquid interface get packed closely together. High film pressures squeeze some components of the film, such as unsaturated phospholipids and the surfactant proteins, out of the monolayer. The surface film generated at low lung volumes is thought to be composed of nearly pure (95%) DPPC.94 Epifluorescence studies with hydrophilic markers have shown that, as the film is compressed, there are areas from which aqueous markers are excluded. Fixation of the lung with osmium vapors has shown that some of the alveolar surface is covered with multilayers of phospholipids, which serve as a reservoir of surfactant to enter the film at high lung volumes. The surfactant system provides a low surface tension in the alveoli and small airways but not in the large airways or trachea.95, 96
There are four surfactant-associated proteins, each of which has different functions. From in vitro studies on film formation, it is clear that SP-A, SP-B, and SP-C can increase the rate of delivery of phospholipids to the air-liquid interface; by itself, DPPC has an extremely low rate of adsorption to the surface. In addition, SP-A and SP-B were found to be necessary for the formation of tubular myelin (Fig. 8-5 ).97 Tubular myelin is a unique extracellular form of surfactant, in which an organized lattice of surfactant bilayers is formed. These bilayers appear to form at right angles in both transmission electron micrographs and freeze fracture images.11 Tubular myelin is the form of surfactant intermediate between the lamellar body, which is secreted, and the surface monolayer. Tubular myelin is a major component of large-aggregate surfactant, the form that sediments rapidly on centrifugation and is surface active. However, because SP-A–deficient mice lack tubular myelin and have normal respiratory mechanics, the formation of tubular myelin is not absolutely necessary for surfactant function.98
Figure 8-5.

Tubular myelin.
Pulmonary surfactant forms a unique three-dimensional structure composed of phospholipids and the surfactant proteins SP-A, SP-B, and presumably SP-C. Tubular myelin is found only extracellularly and is thought to represent a reservoir of surfactant that can rapidly adsorb into the air-liquid interface. Tubular myelin is isolated as a component of large aggregate surfactant, which is the fraction that sediments readily and is most surface active.
(Electron micrograph courtesy Mary Williams, Boston University.)
The major physiologic function of surfactant is to lower surface tension and thereby provide alveolar stability, but surfactant has other functions as well.99 Surfactant is important for maintaining the patency and stabilization of small airways.100, 101 Studies in narrow fluid-filled tubes have demonstrated the critical importance of low surface tension and the need for added surfactant to reduce opening pressures. For this reason, surfactant is important in asthma, constrictive bronchiolitis, and other diseases of small airways. Finally, there is compelling evidence that SP-A and SP-D play important roles in host defense (see later).102, 103
Composition and Pool Sizes
The critical components of purified surfactant are DPPC, unsaturated phosphatidylcholine, phosphatidylglycerol, and the surfactant proteins (Table 8-2 ).104 In a variety of species, the quantity of saturated phosphatidylcholine is related to the alveolar surface area.105 Phosphatidylglycerol is an anionic phospholipid that is thought to be important in the electrostatic and calcium-dependent interactions with the surfactant proteins. In lung injury, a reduction in the percentage of phosphatidylglycerol is the earliest and most sensitive alteration in the composition of surfactant. Recently, pure phosphatidylglycerol liposomes have been reported to have antiviral properties.106 However, the phosphatidylglycerol found in mixed micelles with other phospholipids in natural surfactant likely does not have significant antiviral properties. The mechanism by which pool sizes of surfactant are regulated is not understood. However, recently it has been suggested that the TII cell senses the pool of surfactant via SP-D and the orphan receptor Hepta/GPR116.107, 108, 109 A major physiologic regulator of surfactant pool size is clearance by alveolar macrophages as discussed later in this chapter and in Chapter 70.
Table 8-2.
Composition of Pulmonary Surfactant
| PHOSPHOLIPIDS: 85%* | % OF PHOSPHOLIPIDS |
| Phosphatidylcholine | 76.3 |
| Dipalmitoylphosphatidylcholine | 47.0 |
| Unsaturated phosphatidylcholine | 29.3 |
| Phosphatidylglycerol | 11.6 |
| Phosphatidylinositol | 3.9 |
| Phosphatidylethanolamine | 3.3 |
| Sphingomyelin | 1.5 |
| Other | 3.4 |
| NEUTRAL LIPIDS: 5%† | |
| Cholesterol, free fatty acids | |
| PROTEINS: 10%‡ | |
| SP-A | ++++ |
| SP-B | + |
| SP-C | + |
| SP-D | ++ |
| Other |
The phospholipid composition is constant in most mammalian species. Disaturated phosphatidylcholine represents about two thirds of the total phosphatidylcholine. Dipalmitoylphosphatidylcholine makes up the majority species of the disaturated phosphatidylcholine fraction and is the critical molecule for providing the low surface tension.
There is about 5% neutral lipid, most of which is cholesterol and free fatty acids. There is relatively little triglyceride and cholesterol ester.
The composition of the surfactant proteins is not known precisely, but on a mass basis there appears to be more SP-A than SP-D and more SP-A than SP-B and SP-C. However, there is significant uncertainty about the exact values for SP-B and SP-C.
Surfactant Protein A
SP-A was the first surfactant protein identified by its association with surfactant phospholipids (Fig. 8-6 ). SP-A is a large octadecameric protein with a molecular mass of about 650 kDa.110, 111 SP-A is a collagenous glycoprotein with a complex, highly ordered tertiary structure. The overall organizational structure of SP-A is similar to serum mannose-binding lectin and the complement component C1q. These molecules form a polarized bouquet-like structure composed of 18 monomers that are organized as six trimeric units. The C-terminal carbohydrate recognition domain (CRD) is critical for most SP-A functions and consists of a globular domain that binds carbohydrate and other ligands recognized by SP-A. The structure of the CRD is highly conserved in this class of calcium-dependent lectins. The macromolecular structure of SP-A is 20 nm from the N-terminal to the C-terminal CRD unit and across the array of CRD units. The crystal structure has revealed a hydrophobic binding pocket in the CRD that likely accounts for the lipid binding properties of SP-A.112
Figure 8-6.

Structural organization of surfactant proteins A and D (SP-A and SP-D).
SP-A (top) and SP-D (bottom) are collagenous glycoproteins with four important domains. The amino-terminal region contains cysteines for intermolecular disulfide bonding to form covalent oligomeric units. The collagen-like domain imparts structural rigidity and elongated molecular structure to both proteins. In SP-A, the collagen region has a kink, which accounts for the bend in the collagen region and the bouquet-like structure of the octadecamer. In SP-D, the collagen domain is straight and allows formation of a cross-shaped dodecamer. The neck contributes to the trimeric assembly of polypeptide subunits and spacing for the terminal carbohydrate recognition domain (CRD). The CRD is a globular region of the molecule that plays a major role in the recognition of multiple ligands. In SP-A, the CRD accounts for most of the binding to dipalmitoylphosphatidylcholine vesicles, TII cells, macrophages, and inhaled organisms. In SP-D, the CRD unit is responsible for all the reported interactions with viruses and bacteria.
(Adapted from Kuroki Y, Voelker DR: Pulmonary surfactant proteins. J Biol Chem 269:25943–25946, 1994.)
SP-A is localized primarily in the gas-exchange units of the lung and small airways. In rodents, SP-A is found in alveolar TII cells and also in nonciliated bronchiolar cells (club cells) that line the conducting airways. In humans almost all the SP-A is found in the alveoli, and little is found in the respiratory pseudostratified epithelium that lines the conducting airways. However, there is SP-A in human tracheal submucosal glands.113
The gene for SP-A is located on chromosome 10 near the closely related proteins, SP-D and mannose-binding lectin. Humans have two genes for SP-A, which code for proteins with minor amino acid alterations in the collagen domain.114 Newly synthesized SP-A undergoes a variety of posttranslational modifications that include proteolytic removal of the signal peptide, addition of N-linked carbohydrates, sialylation, acetylation, and sulfation. Secreted SP-A is highly glycosylated, whereas most of the intracellular form of SP-A is not. A number of factors have been reported to increase the synthesis of SP-A, including cyclic adenosine monophosphate (AMP), keratinocyte growth factor, and interleukin (IL)-1. Corticosteroids produce a biphasic dose response with stimulation at low concentrations and inhibition at high concentrations.
SP-A is secreted from TII cells by two different routes. The dominant route is by direct constitutive secretion independent of exocytosis of the lamellar bodies.115, 116 In addition, there is secretion of SP-A contained within lamellar bodies. Directly secreted SP-A is newly synthesized, whereas the SP-A found in lamellar bodies is likely derived from recycled SP-A.
In vitro the functions of SP-A include lipid binding and formation of tubular myelin, acceleration of the adsorption of surfactant to the air-liquid interface, and inhibition of surfactant secretion. However, the physiologic importance of these in vitro observations on surfactant regulation has been seriously questioned because the SP-A–deficient mouse has normal surfactant function.98, 117 More than 99% of SP-A in lavage fluid is bound to phospholipid.118, 119 SP-A may also be important for maintaining the function of surfactant during acute lung injury, when a variety of serum factors can inhibit surfactant function; SP-A partially inhibits these effects.
However, the major function of SP-A appears to be in innate immunity; SP-A binds to a variety of microorganisms, promotes their clearance by phagocytic cells, and directly alters the function of immune effector cells.102, 120, 121 SP-A, like SP-D, is a multivalent pattern recognition molecule and binds to a wide variety of glycoproteins and other ligands. Because the binding is somewhat promiscuous with a low affinity, the physiologic effects of SP-A are likely due to its polyvalent structure and multiple binding sites on cells and organisms. It has been proposed that SP-A associated with tubular myelin might be one of the first barriers for host protection; multivalent SP-A could bind both surfactant lipid in alveolar fluid and inhaled organisms or particles.122
SP-A binds to both gram-positive and gram-negative bacteria and is thought to be an important component of host defense. SP-A binds gram-negative bacteria with the rough form of LPS, aggregates these bacteria, and increases phagocytosis and killing.123, 124 SP-A binds poorly to the smooth variants of Escherichia coli. Gram-negative bacteria that colonize the respiratory tract usually display the smooth form of LPS, whereas those that colonize the gastrointestinal tract display rough forms of LPS. SP-A also enhances the adherence and subsequent phagocytosis of mycobacteria by macrophages. Lipoglycans, especially mannosylated lipoarabinomannan, are important ligands for SP-A on mycobacteria. SP-A also binds to a variety of viruses including influenza and respiratory syncytial virus and likely aggregates extracellular virus, which may inhibit infection.
SP-A has been reported to bind to several receptors on macrophages and to modulate the expression of microcidal factors, TLR2 and 4, and the clearance of apoptotic cells.102, 125, 126 The binding of SP-A to alveolar macrophages appears to be specific and not seen to other mononuclear phagocytes as Kupffer cells, resident peritoneal macrophages, or peritoneal macrophages.127 The studies on direct activation of inflammatory cells are complex and depend on the method of SP-A isolation and the state of the inflammatory cells, especially if they have been primed by a cytokine such as interferon-γ. In addition, for in vitro studies to simulate the in vivo situation, it is important that the effect of SP-A is assessed in the presence of surfactant phospholipids because SP-A may bind surfactant phospholipids with a higher affinity than microbes or inflammatory cells. Finally, some preparations of SP-A may have contained TGF-β, which could account for some anti-inflammatory properties.128
SP-A appears to suppress the secretion of inflammatory cytokines by macrophages in the normal lung but enhances cytokine production during infection or lung injury. This is sometimes referred to as the inflammatory paradox of SP-A. Gardai and colleagues129 formulated an interesting mechanism for these observations. In the normal setting, SP-A interacts with macrophages via its CRD domain and binds to signal-inhibitory regulatory protein α, which suppresses cytokine production. However, during infection, SP-A binds the invading organism with its CRD domain and instead interacts with macrophages via its N-terminal domain and binds to the calreticulin/CD91 complex to stimulate the production of inflammatory cytokines. Hence, SP-A can both stimulate and inhibit inflammatory cytokine production in vitro. It is important to recall that in vivo there is a complex interaction among SP-A, the phospholipids of surfactant, inhaled microbes, and receptors on inflammatory cells. Each has a different binding affinity for SP-A and independent regulation. Finally, SP-A has also been shown to bind to apoptotic cells and increase their uptake and removal by macrophages,125 although SP-A appears to be less important than SP-D in the clearance of apoptotic cells in vivo (see Chapter 12).
Identifying functional cell receptors for SP-A and SP-D has been challenging because both are polyvalent lectins and can bind to a variety of glycoproteins and glycolipids.111, 130 Receptors for SP-A are presumably on TII cells for surfactant recycling and on macrophages for surfactant clearance and modulation of the innate immune response. There have been several putative receptors on macrophages, including the calreticulin/CD91 complex, signal-inhibitory regulatory protein α, and SP-R210.131, 132 The major functional receptor for SP-A on TII cells is P63 (CKAP4).130, 133 Currently, we do not know the precise role of these receptors in normal surfactant metabolism or in disease.
SP-A also serves as a fetal hormone of parturition. Mendelson and colleagues134 have suggested that the fetal lung secretes SP-A, which activates fetal macrophages that migrate to the uterine wall, where they produce IL-1β and initiate labor.
SP-A–deficient mice have demonstrated the importance of SP-A in host defense for viral and bacterial pathogens.135, 136, 137 SP-A–deficient mice are more susceptible to infection by a variety of organisms. There are no reported genetic diseases due to the deficiency of SP-A in humans. However, there is a suggestion that mutation in SP-A may contribute to a familial form of pulmonary fibrosis.85
In summary, SP-A is bound to pulmonary surfactant phospholipids in vivo and functions primarily in innate immunity and host defense.
Surfactant Protein B
SP-B is the only surfactant protein that has been demonstrated to be crucial for surfactant function and is thought to be critical for the adsorption and surface spreading of phospholipids.138, 139, 140 Mature SP-B is a homodimer composed of two 79 amino acid polypeptide chains linked by disulfide bonds. The monomer has an expected mass of 8.7 kDa and the homodimer 17.4 kDa. Each monomer has five amphipathic helices that interact with the surface of the monolayer but do not span the monolayer. There are three internal disulfide bridges within the monomer (C8–C17, C11–C71, and C35–C46) and an interchain disulfide at C48.
There is one gene for SP-B that is located on human chromosome 2. The synthesis of SP-B is markedly stimulated by corticosteroids in vitro. This protein undergoes extensive intracellular processing before the mature homodimer is formed. Within the ER, the signal peptide is cleaved and a precursor 42-kd species of SP-B is formed. In multivesicular bodies, additional proteolysis removes a 16-kd amino terminal peptide and a 30-kd carboxy terminal peptide. The final processing of SP-B probably takes place within the multivesicular bodies, and the mature SP-B homodimer is formed by the time it arrives in the lamellar body.
SP-B is required for organizing phospholipids in lamellar bodies, for the formation of tubular myelin, and for delivering phospholipids to the alveolar air-liquid interface. SP-B is thought to be important in film formation and in the entry of phospholipids into the surface monolayer when the film is expanded during inspiration. The five amphipathic helices of SP-B are envisioned to lie along the lipid bilayer or surface monolayer.138 SP-B interacts with phospholipid bilayers by electrostatic interactions between the polar head groups of the phospholipids and its positively charged amino acids and through the nonpolar faces of the amphipathic helices that interact with the phospholipid acyl chains. SP-B is positively charged and has a preference for interacting with negatively charged phospholipids, such as phosphatidylglycerol. This surfactant protein is squeezed out of the monolayer at moderate film pressures (40 to 45 mN/m) and does not insert directly into the monolayer or lipid bilayers. SP-B can convert lipid vesicles into phospholipid sheets and is likely to be important in moving lipid into the monolayer with each respiratory cycle.
Mice with homozygous null alleles for SP-B die of respiratory insufficiency shortly after birth, and heterozygotes have impaired surfactant function.139, 141, 142, 143 In genetically targeted mice with conditional expression of SP-B, reduction of SP-B below 25% of the wild-type level results in respiratory failure.139 As demonstrated in congenital SP-B deficiency in infants and in genetically altered mice, SP-B is absolutely required for the formation of highly structured lamellar bodies.142, 143, 144, 145 SP-B is secreted exclusively as a component of lamellar bodies, and lamellar body formation may depend on the ability of SP-B to organize and bind two adjacent lipid bilayers. SP-B is found in natural surfactant preparations, although the content may vary from batch to batch and may differ from that found in surfactant isolated from normal animals. SP-B is also found in club cells (Clara), but the function of SP-B associated with club cells is not known. In SP-B deficiency, there is also aberrant processing of SP-C, which results in the secretion of a partially processed 12 kDa form of SP-C.
In summary, SP-B is a hydrophobic protein critical for the function of surfactant, and a total deficiency in SP-B is a cause of respiratory distress in the newborn. This form of respiratory distress is not responsive to surfactant replacement therapy and requires lung transplantation.
Surfactant Protein C
SP-C is an extremely hydrophobic protein that is unique to surfactant and alveolar TII cells.146, 147, 148 SP-C is expressed only in the lung and is a highly specific marker for identifying TII cells. However, the precise physiologic function of this protein is not completely understood. The fully processed form of SP-C is a 35–amino acid lipopeptide that has two palmitates attached as thioesters at amino acids C5 and C6. The segment between residues 13 and 28 forms a hydrophobic α-helix containing aliphatic amino acids, mainly valine. This α-helix is the membrane-spanning portion of SP-C and is extremely stable. In most species, there are two positively charged amino acids, lysine at position 11 and arginine at position 12, which appear to be necessary to move the protein from the ER into the Golgi network, where it is palmitoylated. The precise orientation of the palmitates relative to the valine-rich α-helix is not fully resolved, but both regions are probably important for interactions with phospholipid bilayers or monolayers. SP-C can mix with phospholipids and promote spreading and fusion of phospholipids. SP-C inserts into the monolayer and is squeezed out of the surface monolayer only at relatively high film pressures (>55 mN/m). Presumably, SP-C is important in organizing the phospholipids during the respiratory cycle. SP-C is thought to stabilize the surface film and minimize film collapse. Unlike SP-B, SP-C does not appear to interact with SP-A and is not critical for the formation of tubular myelin. SP-C is found in all preparations of natural surfactant, and a recombinant form of SP-C is used in one type of surfactant replacement therapy.
In humans, there is one gene for SP-C that is located on human chromosome 8. Like SP-B, SP-C is synthesized as a larger precursor form of 21 kDa and processed by proteolytic cleavage of both N-terminal and C-terminal fragments. Most of the posttranslational processing has been completed before SP-C arrives at the lamellar body. There is apparently some interaction between the processing of SP-B and SP-C because, in hereditary deficiency of SP-B, an aberrantly processed 12 kDa form of a precursor SP-C accumulates in TII cells and in alveolar fluid. The SP-C promoter has been an important tool for overexpressing a variety of transgenes in TII cells in mice.
The physiologic role of SP-C is still not completely known. In one strain, SP-C null mice appear normal until about 6 months of age, at which time they develop chronic pneumonitis and air space enlargement.149 This demonstrates that SP-C is not absolutely necessary for surfactant function. However, in another strain, mice develop chronic pneumonitis sooner, within 3 months.150 The genetic background and disease-modifying genes are, therefore, likely involved in clinical manifestations of SP-C deficiency and mutations. Some SPC mutations in humans also produce a misfolded protein that accumulates and causes ER stress in TII cells and a chronic interstitial lung disease.151, 152 These observations provide the background for investigating genetic variants in SP-C as a cause of idiopathic interstitial lung diseases, which is discussed later.
In summary, SP-C is a hydrophobic protein that inserts into the surfactant monolayer but does not appear to be critical for surfactant function. However, mutations in this protein and total absence can result in interstitial lung disease. This is the one surfactant protein that is restricted to TII cells.
Surfactant Protein D
SP-D is a calcium-dependent lectin and an important component of innate immunity.102, 153, 154, 155 Recombinant SP-D has even been proposed as a therapeutic agent in the treatment of pulmonary infections. Serum SP-D is increased in interstitial lung diseases and may predict outcome in lung transplant recipients.156 In humans, the gene for SP-D is located near the locus of SP-A on chromosome 10. SP-D is a collagenous glycoprotein with a complex but highly ordered tertiary structure (see Fig. 8-6). The primary structure consists of four domains. The N-terminal segment contains cysteines that form interchain disulfide bonds that cross-link subunits to form covalent oligomeric structures. The adjacent collagen-like region promotes the formation of noncovalent trimers, imparts a rigid longitudinal structure to the molecules, and organizes the spatial distribution of the C-terminal CRDs. The coiled-coil motif of the neck contributes to the spatial organization of the CRD domains. The C-terminal CRD domain contains the calcium and carbohydrate binding sites. The crystal structure of the CRD and neck region of SP-D has shown a distinct spatial distribution of the three CRDs, has defined the carbohydrate binding pocket, and has allowed for computer docking studies to demonstrate the importance of specific vinyl hydroxyl groups in sugars for binding.157, 158 Identical monomers of SP-D assemble as trimers and then combine to form the final dodecamer, a large symmetric cruciform-shaped molecule with a distance of about 100 nm between the terminal CRDs, about five times larger than SP-A. The CRD unit is primarily responsible for the multivalent binding to surface ligands on microorganisms. As in the case of SP-A, the full oligomerization of SP-D appears to play an important role in maintaining biologic potency that may result from specific spatial cross-linking of ligands, as well as amplification of relatively weak interactions through multiple binding sites.
Conceptually, SP-D should be thought of as a protein distinct from the surfactant system. SP-D binds the phospholipids of surface-active material weakly and is mostly soluble in alveolar fluid. However, SP-D does bind two lipids with carbohydrate motifs, phosphatidylinositol and glucosylceramide, but not phosphatidylcholine. The location of SP-D in the lung also suggests that it is not directly involved with the surfactant system. SP-D is found in ER of TII cells and in the secretory granules of club cells or nonciliated bronchiolar cells but not in lamellar bodies of TII cells or in tubular myelin. SP-D is highly expressed in the hyperplastic TII cells present in interstitial lung diseases. Like SP-A, SP-D is highly expressed in conducting airways of rodents but sparsely in the major conducting airways in humans. SP-D is also present on many other mucosal surfaces.56, 159
SP-D is an important host defense molecule and binds a variety of organisms, usually through its CRD domain.160 In terms of monosaccharide binding, SP-D has a preference for maltose, glucose, and mannose. The binding of SP-D to organisms can be altered by physiologic glucose concentrations, and impaired binding in the presence of glucose may be important in diabetics.161 SP-D binds influenza A, inhibits hemagglutination, and inhibits infection.162, 163, 163a, 163b It is interesting to note that the annual severity of influenza infections is related to the ability of SP-D to bind to the circulating strains of influenza.163, 164 Strains with less SP-D binding such as the 1918 H1N1 strain and the pandemic 2009 strain are clinically more virulent. Although studies on the interaction of SP-D with viruses have been reported for only a few viruses, it is likely that SP-D binds to many respiratory viruses. SP-D also binds to bacteria and should be considered an important molecule in host defense. SP-D binds both major components of gram-positive cell walls, peptidoglycan, and lipoteichoic acid.154, 165 SP-D also binds to LPS and gram-negative bacteria with the rough form of LPS and aggregates these bacteria. SP-D also is likely important in defense against fungal infections. SP-D binds to Aspergillus fumigatus conidia and histoplasma and enhances their phagocytosis and killing. SP-D also binds to and agglutinates Saccharomyces cerevisiae, and the cell wall ligand for binding is β (1→6) glucan.166 This binding is inhibited by pustulan, an extremely effective competitive inhibitor of SP-D. SP-D also binds Alternaria, a common mold and aeroallergen, and SP-D may be important in clearing a variety of inhaled fungal spores. Finally, SP-D agglutinates Mycobacterium tuberculosis but inhibits its phagocytosis by human macrophages. Once in macrophages, SP-D promotes phagosome-lysosome fusion and thereby intracellular killing of mycobacteria.167
SP-D has been reported to bind to several different receptors on macrophages and to stimulate microbicidal metabolism, as indicated by the production of reactive oxygen species.102 Rat SP-D enhances oxygen radical production by alveolar macrophages but not peritoneal macrophages.168 However, there remain concerns that, in the early studies, the stimulation of macrophages by SP-D could be due to endotoxin contamination.169 SP-D is also involved in the clearance of apoptotic cells. SP-D binds to apoptotic cells and facilitates their ingestion by macrophages through the calreticulin and CD91 complex.125, 170
The phenotype of the SP-D knockout mouse was unexpected from prior in vitro studies. The knockout mouse shows an increase in the extracellular pools of surfactant and an accumulation of large foamy macrophages with excess metalloprotease activity, which results in alveolar wall destruction and subsequent air space enlargement.171 The implications are that SP-D assists in clearance of surfactant and regulates macrophage function. In addition, a large number of apoptotic cells are found in the air spaces and lavage fluid of SP-D null mice, indicating that SP-D has an important role in the clearance of apoptotic cells. SP-D-deficient mice are also susceptible to infection with various microbes, including influenza A virus and Aspergillus. 136, 172
The interactions of SP-D and SP-A with organisms and phagocytic cells are complex, and the clear identification of the receptors for these proteins is awaited in order to sort out apparently conflicting observations. The interaction will be affected by the organism, growth cycle of the organism, phagocytic cell, and state of activation of the phagocytic cell, as well as the source of SP-A or SP-D and the presence or absence of contaminating endotoxin or TGF-β in the surfactant protein preparation.
In summary, SP-D is a host defense protein like mannose binding lectin and should not be considered as part of the surfactant system. The major functions of SP-D are the clearance of heavily glycosylated viruses such as influenza and the removal of apoptotic cells.
Secretion and Extracellular Processing of Surfactant
Secretion of the phospholipid components of surfactant has been studied extensively (Fig. 8-7 ).173 Turnover studies have demonstrated that the surfactant system is dynamic; 10% to 20% of the surfactant pool is secreted each hour.174, 175 Secretion requires active extrusion of the lamellar body contents,176 which involves fusion of the lamellar body limiting membrane with the plasma membrane.97 Several different independent pathways for stimulating secretion work through different receptors and signaling mechanisms. In vivo secretion is stimulated by hyperventilation or even a single deep breath or sigh. Stretch is an important physiologic stimulation, which signals exocytosis through an elevation of intracellular calcium that may in part be stimulated by waves of calcium increases in neighboring cells transmitted through gap junctions.12, 177 In vitro, tetradecanoyl acetate and adenosine triphosphate (ATP) greatly stimulate basal secretion. Agents that stimulate secretion via cyclic AMP–dependent pathways such as β-agonists and cholera toxin stimulate secretion more modestly. In vivo secretion is likely to be highly regulated, and multiple checks and balances may be present so that alteration in one pathway does not alter turnover.
Figure 8-7.

Metabolic trafficking of surfactant phospholipids.
The phospholipids are synthesized in the rough endoplasmic reticulum (RER) of the alveolar TII cell (1). They are transported to the multivesicular bodies (MVB) (2), where the first lamellae are formed. These lamellae increase to form lamellar bodies (LB), which are subsequently secreted by exocytosis (3). The secreted lamellar body unfolds to form tubular myelin (TM) (4) and other large aggregates (LA). These forms adsorb into the expanded surface monolayer (5). This is a critical step for producing a low surface tension in the alveolus. During the respiratory cycle, as the film is compressed during exhalation, the film pressure rises, and a compressed, closely packed monolayer of nearly pure dipalmitoylphosphatidylcholine is formed (6). Material is excluded from the monolayer (7) and forms small aggregates (SA). Some of these aggregates are ingested by macrophages (8), but most are endocytosed for reprocessing by alveolar type II cells (9 and 10).
After exocytosis by alveolar TII cells, the secreted lamellar bodies undergo physical rearrangements extracellularly (see Fig. 8-5). The initial change is the conversion from the multilamellar state to tubular myelin (see Fig. 8-7). This requires SP-A, SP-B, and calcium and can be reproduced in vitro.97 In lavage samples, large aggregate (LA) surfactant can be isolated by differential centrifugation. These aggregates contain SP-A and SP-B and are composed of tubular myelin, multilamellar structures, and other loose lipid arrays, which are the expected forms of secreted and unraveling lamellar bodies. LA adsorbs rapidly to the air-liquid interface and can be considered an extracellular reservoir of surfactant. LA can be converted into smaller aggregates (SAs), which are much less surface active. These SAs are thought to represent surfactant that has left the air-liquid interface and are available to be taken up by TII cells and reprocessed. The two dominant routes for the catabolism of surface-active material are uptake by TII cells and catabolism by alveolar macrophages.174 The current estimates are that about 85% of secreted surfactant is recycled by adult TII cells and about 15% is catabolized by macrophages.178, 179 Relatively little pulmonary surfactant goes up the mucociliary escalator, and very little enters the bloodstream or lymphatics. Catabolism of extracellular surface-active material is regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) and its ability to activate macrophages.180 The autoimmune alveolar proteinosis syndrome is caused by an autoantibody to GM-CSF181, 182; some pediatric forms of alveolar proteinosis are due to mutations in the receptor for GM-CSF.183, 184 The extracellular pool of surfactant appears to be regulated predominately by alveolar macrophages. Deficiency in macrophages or inhibition of their state of activation impairs surfactant clearance and leads to the accumulation of surfactant in the alveolar spaces (see Chapter 70 for additional details).
Surfactant Abnormalities in Lung Disease
Primary Surfactant Deficiency of the Newborn
The importance of the pulmonary surfactant system in the pathophysiology of the NRDS was first reported by Avery and Mead in the late 1950s.93 The observation that NRDS was due to a primary surfactant deficiency has been confirmed by the tremendous impact that exogenous surfactant administration has had on infant mortality.185, 186 The incidence of NRDS varies with gestational age and birth weight. Approximately 15% of infants born between 34 and 37 weeks (<1700 g) develop NRDS, whereas 50% of infants between 30 and 34 weeks (<1500 g) and 70% of those born at less than 30 weeks (<1250 g) develop this disease. These rates may also be influenced by other factors, including race, gender, socioeconomic status, and maternal health.
Even though artificial ventilation and exogenous surfactant have decreased infant mortality, there still exists a major disability rate among the survivors, particularly in the infants of very low birth weight. Amniotic fluid surfactant analysis reliably predicts the risk of NRDS. Pathologic changes typical of NRDS include widespread atelectasis, capillary congestion, micro-hemorrhage, edema, and the presence of hyaline membranes.93
Fujiwara and colleagues187 reported the first successful use of exogenous surfactant in neonates with NRDS in 1980. Several large clinical trials demonstrated significant improvement in gas exchange, decreased barotrauma, and decreased infant mortality in response to this therapy. Results of these trials have also shown that surfactant administered prophylactically (i.e., before the first breath) is superior to surfactant given after a period of ventilation. These differences were particularly notable in the infants of very low birth weight (<28 weeks of gestational age) and thus, prophylactic treatment is reserved for infants in this group. Otherwise, surfactant should be administered once the diagnosis of NRDS is established, usually within 30 minutes after birth. The usual dose of surfactant administered to these infants is approximately 100 mg phospholipid/kg body weight. Although surfactant therapy is effective for NRDS, approximately 30% of affected infants do not respond to this therapy.
Hereditary Surfactant Protein B Deficiency
Although most cases of NRDS result from immaturity, there are inherited forms specifically due to SP-B deficiency.188, 189, 190, 191, 192 Associated abnormalities include aberrantly structured tubular myelin, a decreased number of lamellar bodies, and an abnormal processing of the SP-C protein. Regardless of gestational age, affected patients develop respiratory distress in the first few days of life that is refractory to all therapy, including surfactant supplementation. These infants usually die in the first few months of life, unless they receive a lung transplant. Although the most common abnormality involves a 1-bp deletion and 3-bp insertion in codon 121 in exon 4, which results in a premature stop codon, other mutations can also lead to respiratory failure. The heterozygotes, representing about 1 in 3000 individuals in the general population, would be predicted to have about half the SP-B level and might be more susceptible to ARDS. In mice about 20% of wild-type SP-B is required for normal ventilatory function, but more than 50% of SP-B may be necessary to protect against susceptibility to acute lung injury. NRDS also results from other deficiencies in surfactant synthesis and assembly. The most common deficiency is of the ATP-binding cassette family member A3 (ABCA3), a lamellar body phospholipid transport protein.190, 191, 193, 194, 195 Thyroid transcription factor 1 (TTF-1) deficiency also produces respiratory failure in infancy accompanied by thyroid and neurologic abnormalities.196, 197
Hereditary SP-C Deficiency and Mutations
Deficiency of SP-C would not be expected to produce acute respiratory failure. In mice SP-C deficiency causes diffuse nonspecific chronic interstitial pneumonitis over months to years and depends on the genetic strain. A splice site mutation in intron 4 of the SP-C gene produces chronic interstitial lung disease with an autosomal dominant inheritance pattern.189, 198, 199 Recently additional cases of nonspecific interstitial pneumonitis and usual interstitial pneumonitis have been reported with a single gene mutation.200 This kindred also showed an autosomal dominant inheritance pattern. The hypothesis is that the mutations cause a misfolded SP-C protein, which accumulates and produces ER stress and chronic lung disease. The same genetic abnormality may have a variable phenotype, which indicates the importance of other disease-modifying genes.
Acute Respiratory Distress Syndrome
Early descriptions of ARDS suggested that surfactant abnormalities play an important role in the lung dysfunction. Petty and Ashbaugh201 recorded abnormal pressure-volume curves in lungs isolated from patients dying of ARDS. Morphologic changes noted in the lung tissue of these patients at autopsy were similar to those of preterm infants suffering from NRDS. The clinical criteria used to diagnose ARDS, including severe hypoxemia, decreased lung compliance, and bilateral infiltrates on chest radiograph, are also similar to those used to diagnose NRDS. As opposed to a primary deficiency of surfactant, however, ARDS is much more complex, with many different etiologies and an inflammatory cascade resulting in lung injury and alveolar edema. The alveolar epithelium is severely damaged in ARDS, whereas the alveolar epithelium is relatively intact at the onset of NRDS.
Extensive animal studies and several human reports characterizing surfactant alterations in ARDS and acute lung injury have shown a decrease in the percentage of phosphatidylcholine, phosphatidylglycerol, and DPPC and a corresponding increase in phosphatidylinositol, sphingomyelin and, in some cases, lysophosphatidylcholine.202, 203, 204 Decreased levels of SP-A and SP-B have also been demonstrated in bronchoalveolar lavage (BAL) samples from patients with ARDS, and the surface tension–lowering properties of the isolated surfactant are abnormal.203 These abnormalities correlated with the severity of ARDS. BAL samples isolated from patients with severe ARDS also show a decrease in LA surfactant and an increase in poorly surface-active SA. In animal models of acute lung injury, there is a severe reduction of surfactant protein gene expression at the onset of respiratory insufficiency.205 With increased alveolar permeability in patients with ARDS, a number of proteins enter the air space and bind surfactant phospholipids and proteins or directly compete with surfactant molecules for space at the air-liquid interface. Interestingly, this nonstoichiometric inhibition of surfactant by plasma proteins can be overcome in vitro by increasing the concentration of surfactant. This observation provides rationale for administering large doses of exogenous surfactant to patients with ARDS. Thrombogenic components leaking into the airspace can also coagulate and incorporate surfactant phospholipids into fibrin clots, thereby rendering the surfactant inaccessible to the air-liquid interface. Surfactant inactivation has also been described due to an increase in the neutral lipids, primarily cholesterol. Another mechanism contributing to surfactant dysfunction in ARDS involves the increased conversion of LA into SA. In vivo studies have shown that increased tidal volumes, but not respiratory rates or positive end-expiratory pressure (PEEP) levels, correlated with an increased conversion of LA into SA206 and with physiologic deterioration. Minimizing these phasic changes in surface area by means of smaller tidal volumes allows surfactant to be maintained in LA forms and results in improved physiologic outcomes.206 These findings are supported by clinical trials demonstrating improved patient outcomes when lower tidal volumes are used in treating patients with ARDS.207, 208
Exogenous surfactant administration has been shown to improve oxygenation but not mortality in patients with ARDS.209, 209a A few large multicenter clinical trials have evaluated this therapy and have shown disappointing results.210, 211, 212 Presumably, the complexity of ARDS is not addressed by administering exogenous surfactant alone.
Interstitial Lung Diseases
In addition to diseases involving acute alveolar inflammation, alterations in surfactant have also been characterized in various interstitial lung diseases. Decreased levels of DPPC and phosphatidylglycerol, increased levels of phosphatidylinositol, and reduced levels of SP-A have been observed in BAL fluid from patients with IPF.213, 214, 215 The reduction in SP-A in BAL fluid is found in a variety of diffuse interstitial lung diseases. The ratio of SP-A to phospholipid, used to correct for recovery of total surfactant, is reduced in patients with IPF. In addition, Günther and colleagues216 demonstrated that biophysical properties of surfactant are impaired in IPF.
SP-A and SP-D have also been measured in serum and may serve as biomarkers of lung disease, especially when alveolar epithelial integrity is compromised. Serum concentrations of SP-A and SP-D are increased in patients with alveolar proteinosis, ARDS, and IPF.214, 217, 218 Measuring SP-A and SP-D in serum might be useful in the diagnosis of alveolar proteinosis because, together with a highly specific image on high-resolution computed tomographic scanning, high levels of SP-A and D might obviate the need for a biopsy. A small subset of patients with familial pulmonary fibrosis has been attributed to SP-C mutations. Most of the cases have a histopathologic diagnosis of nonspecific interstitial pneumonia, but a few have the morphologic features of usual interstitial pneumonia.200 Mutations in the gene coding for SP-A2 have been also reported to be associated with interstitial lung disease and lung cancer.219
Obstructive Lung Diseases
The role of surfactant as a down-regulator of inflammation and the importance of surfactant in maintaining the openness of conducting airways suggest that surfactant alterations may be important in obstructive lung diseases.101, 220 BAL samples from patients with asthma showed decreased SP-A levels and a normal phospholipid composition.119 No surfactant alterations have been demonstrated in patients with chronic bronchitis or emphysema.
Cystic fibrosis is characterized by colonization of both gram-negative and gram-positive bacteria and early airway inflammation. Both SP-A and SP-D are reduced in BAL from patients with cystic fibrosis, and the relative deficiency could contribute to bacteria colonization.221
Other Lung Diseases
Autoimmune alveolar proteinosis has been shown to be a disease produced by an antibody to GM-CSF, which results in decreased macrophage clearance of surfactant (see Chapter 70 for more information).181, 182 Alveolar phospholipids and surfactant proteins SP-A, SP-B, and SP-D are markedly increased in the BAL from affected patients. Serum SP-A and SP-D are also increased in these patients.214, 218 Decreased catabolism of surfactant results in an excess accumulation of multilamellated structures within the alveoli. Effective treatment involves removing this material through whole lung lavage and/or treatment with exogenous GM-CSF. Pediatric forms of alveolar proteinosis can be due to alterations in GM-CSF signaling, usually due to alterations in the GM-CSF receptor.183, 184 Other forms of genetic impairment of macrophage function in mice lead to an alveolar proteinosis syndrome.222
The ischemia-reperfusion injury associated with lung transplantation results in alterations in pulmonary surfactant similar to those observed in patients with severe ARDS. Surfactant administered shortly after transplantation and reperfusion results in improved gas exchange in comparison with non–surfactant-treated lungs. Moreover, when surfactant is administered to donor lungs before storage, longer storage times are tolerated (up to 38 hours) with relatively better physiologic function after reperfusion.
Measurement of SP-A and SP-D in pleural fluid might also be useful to distinguish metastatic adenocarcinoma of the lung from other adenocarcinomas or mesothelioma.223 In addition, surfactant protein gene expression can be used to screen for micrometastases in lymph nodes.224 In mice with pulmonary adenomas, serum SP-D correlates extremely well with tumor size.225
Acknowledgment
The authors thank Lennell Allen for Figure 8-1, Figure 8-2, Figure 8-3; Dennis Voelker for the figure on SP-A and SP-D; Mary Williams for the figure of tubular myelin; and Sarah Murrell for administrative assistance.
Key Points.
-
▪
Alveolar type I epithelial cells cover about 95% of the alveolar surface of the normal lung, are critical for effective gas exchange, participate in transepithelial fluid movement, and play a role in innate immunity.
-
▪
Alveolar type II epithelial cells cover about 5% of the alveolar surface in the normal lung, produce pulmonary surfactant, participate in transepithelial fluid movement, are the precursors of type I cells in normal injury and repair, and play a role in innate immunity.
-
▪
Pulmonary surfactant allows for the low surface tension in the gas-exchange portions of the lung and prevents atelectasis, decreases the work of breathing, improves oxygenation, and helps maintain patency of small airways.
-
▪
Surfactant protein B is the only surfactant protein critical to surfactant formation in vivo.
-
▪
Mutations in the genes encoding SP-A, SP-B, and SP-C can lead to parenchymal lung disease.
-
▪
SP-A and SP-D are polyvalent lectins (i.e., proteins that bind to carbohydrates) and are thought to be important components of innate immunity. They bind to both microorganisms and inflammatory cells.
-
▪
Surfactant replacement therapy in neonatal respiratory distress syndrome improves survival, whereas replacement therapy in acute respiratory distress syndrome improves oxygenation but not survival.
Complete reference list available at ExpertConsult.
Key Readings
- Ballard PL, Lee JW, Fang X. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol. 2010;299:L36–L50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. Dependence of pressure-volume characteristics of lungs on intrinsic surface active material. Am J Physiol. 1956;187:592. [Google Scholar]
- Dobbs LG, Johnson MD, Vanderbilt J. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Yang YH, Griffin C. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L179–L189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez RF, Dobbs LG. Isolation and culture of alveolar epithelial type I and type II cells from rat lungs. Methods Mol Biol. 2013;945:145–159. doi: 10.1007/978-1-62703-125-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss V, Hunt AN, Postle AD. Regulation of lung surfactant phospholipid synthesis and metabolism. Biochim Biophys Acta. 1831;448–458:2013. doi: 10.1016/j.bbalip.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Widdicombe JH, Allen L. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci U S A. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y, Yoneda K. The type II epithelial cells of the lung. I. Method of isolation. Lab Invest. 1974;30:76–84. [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin CC. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954:1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- Mason RJ, Williams MC, Widdicombe JH. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A. 1982;79:6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
References
- 1.Burri PH. Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald JA, editor. Lung growth and development. Marcel Dekker; New York, Basel, Hong Kong: 1997. pp. 1–35. [Google Scholar]
- 2.Mercer RR, Russell ML, Roggli VL. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 3.Burri PH. Structural aspects of postnatal lung development—alveolar formation and growth. Biol Neonate. 2006;89:313–322. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 4.Stone KC, Mercer RR, Gehr P. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochs M, Nyengaard JR, Jung A. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 7.Vasilescu DM, Gao Z, Saha PK. Assessment of morphometry of pulmonary acini in mouse lungs by nondestructive imaging using multiscale microcomputed tomography. Proc Natl Acad Sci U S A. 2012;109:17105–17110. doi: 10.1073/pnas.1215112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson SM, Olver RE, Walters DV. Developmental regulation of lumenal lung fluid and electrolyte transport. Respir Physiol Neurobiol. 2007;159:247–255. doi: 10.1016/j.resp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AE, Guyton AC, Bishop VS. Permeability of the alveolar membrane to solutes. Circ Res. 1965;16:353–362. doi: 10.1161/01.res.16.4.353. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger EE, Karnovsky MJ. Substructure of intercellular junctions in freeze-fractured alveolar-capillary membranes of mouse lung. Circ Res. 1976;38:404–411. doi: 10.1161/01.res.38.5.404. [DOI] [PubMed] [Google Scholar]
- 11.Williams MC. Freeze-fracture studies of tubular myelin and lamellar bodies in fetal and adult rat lungs. J Ultrastruct Res. 1978;64:352–361. doi: 10.1016/s0022-5320(78)90043-6. [DOI] [PubMed] [Google Scholar]
- 12.Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol. 2013;75:551–567. doi: 10.1146/annurev-physiol-030212-183809. [DOI] [PubMed] [Google Scholar]
- 13.Hu YJ, Wang YD, Tan FQ. Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep. 2013;40:6123–6142. doi: 10.1007/s11033-013-2724-y. [DOI] [PubMed] [Google Scholar]
- 14.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Lampugnani MG. Endothelial cell-to-cell junctions: adhesion and signaling in physiology and pathology. Cold Spring Harb Perspect Med. 2012;2(10) doi: 10.1101/cshperspect.a006528. pii:a006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collinet C, Lecuit T. Stability and dynamics of cell-cell junctions. Prog Mol Biol Transl Sci. 2013;116:25–47. doi: 10.1016/B978-0-12-394311-8.00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LN, Koval M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid Redox Signal. 2009;11:355–367. doi: 10.1089/ars.2008.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low FN. Electron microscopy of the rat lung. Anat Rec. 1952;113:437–449. doi: 10.1002/ar.1091130406. [DOI] [PubMed] [Google Scholar]
- 19.Weibel ER. The mystery of “non-nucleated plates” in the alveolar epithelium of the lung explained. Acta Anat (Basel) 1971;78:425–443. doi: 10.1159/000143605. [DOI] [PubMed] [Google Scholar]
- 20.Dahlin K, Mager EM, Allen L. Identification of genes differentially expressed in rat alveolar type i cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Yang YH, Griffin C. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L179–L189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- 22.Frank JA. Claudins and alveolar epithelial barrier function in the lung. Ann N Y Acad Sci. 2012;1257:175–183. doi: 10.1111/j.1749-6632.2012.06533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaFemina MJ, Rokkam D, Chandrasena A. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L724–L734. doi: 10.1152/ajplung.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damiano VV, Cohen A, Tsang AL. A morphologic study of the influx of neutrophils into dog lung alveoli after lavage with sterile saline. Am J Pathol. 1980;100:349–364. [PMC free article] [PubMed] [Google Scholar]
- 25.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res. 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 26.Dobbs LG, Gonzalez R, Matthay MA. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci U S A. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen S, King LS, Christensen BM. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MD, Bao HF, Helms MN. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci U S A. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain L, Chen XJ, Ramosevac S. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol. 2001;280:L646–L658. doi: 10.1152/ajplung.2001.280.4.L646. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MD, Widdicombe JH, Allen L. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci U S A. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 32.Norlin A, Lu LN, Guggino SE. Contribution of amiloride-insensitive pathways to alveolar fluid clearance in adult rats. J Appl Physiol (1985) 2001;90:1489–1496. doi: 10.1152/jappl.2001.90.4.1489. [DOI] [PubMed] [Google Scholar]
- 33.O’Brodovich H, Yang P, Gandhi S. Amiloride-insensitive na+ and fluid absorption in the mammalian distal lung. Am J Physiol Lung Cell Mol Physiol. 2008;294:L401–L408. doi: 10.1152/ajplung.00431.2007. [DOI] [PubMed] [Google Scholar]
- 34.Olver RE, Walters DV, Wilson MS. Developmental regulation of lung liquid transport. Annu Rev Physiol. 2004;66:77–101. doi: 10.1146/annurev.physiol.66.071702.145229. [DOI] [PubMed] [Google Scholar]
- 35.Bove PF, Grubb BR, Okada SF. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem. 2010;285:34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson M, Allen L, Dobbs L. Characteristics of cl- uptake in rat alveolar type I cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L816–L827. doi: 10.1152/ajplung.90466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielson DW, Goerke J, Clements JA. Alveolar subphase ph in the lungs of anesthetized rabbits. Proc Natl Acad Sci USA. 1981;78:7119–7123. doi: 10.1073/pnas.78.11.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridge KM, Olivera WG, Saldias F. Alveolar type 1 cells express the alpha2 na,k-atpase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Ferrari JD, Cao Y. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol. 2012;189:2450–2459. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong MH, Chapin OC, Johnson MD. Lps-stimulated cytokine production in type I cells is modulated by the renin-angiotensin system. Am J Respir Cell Mol Biol. 2012;46:641–650. doi: 10.1165/rcmb.2011-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 42.Campbell L, Hollins AJ, Al-Eid A. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem Biophys Res Commun. 1999;262:744–751. doi: 10.1006/bbrc.1999.1280. [DOI] [PubMed] [Google Scholar]
- 43.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 44.Godin LM, Vergen J, Prakash YS. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol Lung Cell Mol Physiol. 2011;300:L615–L623. doi: 10.1152/ajplung.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, Singh RD, Godin L. Endocytic response of type I alveolar epithelial cells to hypertonic stress. Am J Physiol Lung Cell Mol Physiol. 2011;300:L560–L568. doi: 10.1152/ajplung.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkauskas CE, Cronce MJ, Rackley CR. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rock JR, Barkauskas CE, Cronce MJ. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type i cells proliferate, express oct-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1045–L1055. doi: 10.1152/ajplung.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Hubmayr RD. Type I alveolar epithelial phenotype in primary culture. Am J Respir Cell Mol Biol. 2011;44:692–699. doi: 10.1165/rcmb.2009-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macklin CC. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954:1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- 51.Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol. 1992;262:L367–L385. doi: 10.1152/ajplung.1992.262.4.L367. [DOI] [PubMed] [Google Scholar]
- 52.Mason RJ, Pan T, Edeen KE. Keratinocyte growth factor and the transcription factors c/ebpα, c/ebpδ, and srebp-1c regulate fatty acid synthesis in alveolar type ii cells. J Clin Invest. 2003;112:244–255. doi: 10.1172/JCI16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballard PL, Lee JW, Fang X. Regulated gene expression in cultured type ii cells of adult human lung. Am J Physiol Lung Cell Mol Physiol. 2010;299:L36–L50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang Y, Edeen K, Lu X. Keratinocyte growth factor induces lipogenesis in alveolar type ii cells through a sterol regulatory element binding protein-1c-dependent pathway. Am J Respir Cell Mol Biol. 2006;35:268–274. doi: 10.1165/rcmb.2006-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akiyama J, Hoffman A, Brown C. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50:993–996. doi: 10.1177/002215540205000713. [DOI] [PubMed] [Google Scholar]
- 56.Stahlman MT, Gray ME, Hull WM. Immunolocalization of surfactant protein-d (sp-d) in human fetal, newborn, and adult tissues. J Histochem Cytochem. 2002;50:651–660. doi: 10.1177/002215540205000506. [DOI] [PubMed] [Google Scholar]
- 57.Weiss DJ, Bates JH, Gilbert T. Stem cells and cell therapies in lung biology and diseases: conference report. Ann Am Thorac Soc. 2013;10:S25–S44. doi: 10.1513/AnnalsATS.201304-089AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans MJ, Cabral LJ, Stephens RJ. Renewal of alveolar epithelium in the rat following exposure to no2. Am J Pathol. 1973;70:175–198. [PMC free article] [PubMed] [Google Scholar]
- 59.Evans MJ, Cabral LJ, Stephens RJ. Transformation of alveolar type ii cells to type i cells following exposure to nitrogen dioxide. Exp Mol Pathol. 1975;22:145–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 60.Adamson IYR, Bakowska J. Relationship of keratinocyte growth factor and hepatocyte growth factor levels in rat lung lavage fluid to epithelial cell regeneration after bleomycin. Am J Pathol. 1999;155:949–954. doi: 10.1016/S0002-9440(10)65194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adamson IYR, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. Lab Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 62.Adamson IYR, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol. 1990;137:385–392. [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng D, Limmon GV, Yin L. A cellular pathway involved in Clara cell to alveolar type II cell differentiation after severe lung injury. PLoS ONE. 2013;8:e71028. doi: 10.1371/journal.pone.0071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L, Yee M, O’Reilly MA. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-beta and bmp signaling. Am J Physiol Lung Cell Mol Physiol. 2013;305:L409–L418. doi: 10.1152/ajplung.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panos RJ, Rubin JS, Aaronson SA. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993;92:969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panos RJ, Bak PM, Simonet WS. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verghese GM, McCormick-Shannon K, Mason RJ. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Am J Resp Crit Care Med. 1998;158:386–394. doi: 10.1164/ajrccm.158.2.9711111. [DOI] [PubMed] [Google Scholar]
- 68.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 69.Yanagita K, Matsumoto K, Sekiguchi K. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:21212–21217. [PubMed] [Google Scholar]
- 70.Gazdhar A, Temuri A, Knudsen L. Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats. Hum Gene Ther. 2013;24:105–116. doi: 10.1089/hum.2012.098. [DOI] [PubMed] [Google Scholar]
- 71.Crestani B, Marchand-Adam S, Quesnel C. Hepatocyte growth factor and lung fibrosis. Proc Am Thorac Soc. 2012;9:158–163. doi: 10.1513/pats.201202-018AW. [DOI] [PubMed] [Google Scholar]
- Shyamsundar M, McAuley DF, Ingram RJ. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189(12):1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mason RJ, Williams MC, Widdicombe JH. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci USA. 1982;79:6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugahara K, Caldwell JH, Mason RJ. Electrical currents flow out of domes formed by cultured epithelial cells. J Cell Biol. 1984;99:1541–1546. doi: 10.1083/jcb.99.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian Z, Travanty EA, Oko L. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Nikrad MP, Phang T. Innate immune response to influenza a virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol. 2011;45:582–591. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinheimer VK, Becher A, Tonnies M. Influenza a viruses target type II pneumocytes in the human lung. J Infect Dis. 2012;206:1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolle LB, Standiford TJ. Danger-associated molecular patterns (damps) in acute lung injury. J Pathol. 2013;229:145–156. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 78.Kasper M, Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol. 1996;11:463–483. [PubMed] [Google Scholar]
- 79.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 80.Haschek WM, Witschi H. Pulmonary fibrosis—a possible mechanism. Toxicol Appl Pharm. 1979;51:475–487. doi: 10.1016/0041-008x(79)90372-7. [DOI] [PubMed] [Google Scholar]
- 81.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 82.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L721–L729. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawson WE, Cheng DS, Degryse AL. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong Q, Zhou B, Ann DK. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol. 2011;45:498–509. doi: 10.1165/rcmb.2010-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maitra M, Wang Y, Gerard RD. Surfactant protein a2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem. 2010;285:22103–22113. doi: 10.1074/jbc.M110.121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korfei M, Ruppert C, Mahavadi P. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ten Have-Opbroek AA, Benfield JR, van Krieken JH. The alveolar type ii cell is a pluripotential stem cell in the genesis of human adenocarcinomas and squamous cell carcinomas. Histol Histopathol. 1997;12:319–336. [PubMed] [Google Scholar]
- 88.Mason RJ, Kalina M, Nielsen LD. Surfactant protein c expression in urethane-induced murine pulmonary tumors. Am J Pathol. 2000;156:175–182. doi: 10.1016/S0002-9440(10)64717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu X, Rock JR, Lu Y. Evidence for type II cells as cells of origin of k-ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neergaard K. New interpretations of basic concepts of respiratory mechanisms. Z Gesamte Exp Med. 1929;66:373–394. [Google Scholar]
- 91.Clements J. Dependence of pressure-volume characteristics of lungs on intrinsic surface active material. Am J Physiol. 1956;187:592. [Google Scholar]
- 92.Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- 93.Avery M, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez E, Pérez-Gil J. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim Biophys Acta. 2014;1838(6):1568–1585. doi: 10.1016/j.bbamem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 94.Hildebrandt JN, Goerke J, Clements JA. Pulmonary surface film stability and composition. J Appl Physiol. 1979;47:604–611. doi: 10.1152/jappl.1979.47.3.604. [DOI] [PubMed] [Google Scholar]
- 95.Im Hof V, Gehr P, Gerber V. In vivo determination of surface tension in the horse trachea and in vitro model studies. Respiration Physiol. 1997;109:81–93. doi: 10.1016/s0034-5687(97)84032-7. [DOI] [PubMed] [Google Scholar]
- 96.Schürch S, Goerke J, Clements JA. Direct determination of surface tension in the lung. Proc Natl Acad Sci U S A. 1976;73:4698–4702. doi: 10.1073/pnas.73.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams MC, Hawgood S, Hamilton RL. Changes in lipid structure produced by surfactant proteins SP-A, SP-B, and SP-C. Am J Respir Cell Mol Biol. 1991;5:41–50. doi: 10.1165/ajrcmb/5.1.41. [DOI] [PubMed] [Google Scholar]
- 98.Ikegami M, Korfhagen TR, Whitsett JA. Characteristics of surfactant from SP-A-deficient mice. Am J Physiol. 1998;275:L247–L254. doi: 10.1152/ajplung.1998.275.2.L247. [DOI] [PubMed] [Google Scholar]
- 99.Hamm H, Fabel H, Bartsch W. The surfactant system of the adult lung: physiology and clinical perspectives. [review] Clin Investigator. 1992;70:637–657. doi: 10.1007/BF00180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Enhorning G. Surfactant in airway disease. Chest. 2008;133:975–980. doi: 10.1378/chest.07-2404. [DOI] [PubMed] [Google Scholar]
- 101.Enhorning G, Duffy LC, Welliver RC. Pulmonary surfactant maintains potency of conducting airways in the rat. Am J Respir Crit Care Med. 1995;151:534–556. doi: 10.1164/ajrccm.151.2.7842219. [DOI] [PubMed] [Google Scholar]
- 102.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 103.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 104.Veldhuizen R, Nag K, Orgeig S. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 105.Clements JA, Nellenbogen J, Traham HJ. Pulmonary surfactant and evolution of the lungs. Science. 1970;169:603–604. doi: 10.1126/science.169.3945.603. [DOI] [PubMed] [Google Scholar]
- 106.Numata M, Kandasamy P, Nagashima Y. Phosphatidylglycerol suppresses influenza a virus infection. Am J Respir Cell Mol Biol. 2012;46:479–487. doi: 10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fukuzawa T, Ishida J, Kato A. Lung surfactant levels are regulated by IG-hepta/GPR116 by monitoring surfactant protein D. PLoS ONE. 2013;8:e69451. doi: 10.1371/journal.pone.0069451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bridges JP, Ludwig MG, Mueller M. Orphan G protein-coupled receptor GPR116 regulates pulmonary surfactant pool size. Am J Respir Cell Mol Biol. 2013;49:348–357. doi: 10.1165/rcmb.2012-0439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang MY, Hilton MB, Seaman S. Essential regulation of lung surfactant homeostasis by the orphan G protein-coupled receptor GPR116. Cell Rep. 2013;3:1457–1464. doi: 10.1016/j.celrep.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heinrich S, Hartl D, Griese M. Surfactant protein A—from genes to human lung diseases. Curr Med Chem. 2006;13:3239–3252. doi: 10.2174/092986706778773112. [DOI] [PubMed] [Google Scholar]
- 111.Kishore U, Greenhough TJ, Waters P. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Seaton BA, Crouch EC, McCormack FX. Review: structural determinants of pattern recognition by lung collectins. Innate Immun. 2010;16:143–150. doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- 113.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol. 1998;275:L1–L13. doi: 10.1152/ajplung.1998.275.1.L1. [DOI] [PubMed] [Google Scholar]
- 114.Scavo LM, Ertsey R, Gao BQ. Human surfactant proteins A1 and A2 are differentially regulated during development and by soluble factors. Am J Physiol. 1998;275:L653–L669. doi: 10.1152/ajplung.1998.275.4.L653. [DOI] [PubMed] [Google Scholar]
- 115.Ochs M, Johnen G, Muller KM. Intracellular and intraalveolar localization of surfactant protein a (SP-A) in the parenchymal region of the human lung. Am J Respir Cell Mol Biol. 2002;26:91–98. doi: 10.1165/ajrcmb.26.1.4570. [DOI] [PubMed] [Google Scholar]
- 116.Osanai K, Mason RJ, Voelker DR. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am J Respir Cell Mol Biol. 1998;19:929–935. doi: 10.1165/ajrcmb.19.6.3292. [DOI] [PubMed] [Google Scholar]
- 117.Ikegami M, Korfhagen TR, Bruno MD. Surfactant metabolism in surfactant protein A-deficient mice. Am J Physiol. 1997;272:L479–L485. doi: 10.1152/ajplung.1997.272.3.L479. [DOI] [PubMed] [Google Scholar]
- 118.Tino MJ, Wright JR. Surfactant protein a stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–L688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 119.van de Graaf EA, Jansen HM, Lutter R. Surfactant protein A in bronchoalveolar lavage fluid. J Lab Clin Med. 1992;120:252–263. [PubMed] [Google Scholar]
- 120.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haagsman HP, Hogenkamp A, van Eijk M. Surfactant collectins and innate immunity. Neonatology. 2008;93:288–294. doi: 10.1159/000121454. [DOI] [PubMed] [Google Scholar]
- 122.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu H, Kuzmenko A, Wan S. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kabha K, Schmegner J, Keisari Y. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 125.Vandivier RW, Ogden CA, Fadok VA. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: Calreticulin and cd91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 126.Henning LN, Azad AK, Parsa KV. Pulmonary surfactant protein a regulates tlr expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Iwaarden F, Welmers B, Verhoef J. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 128.Kunzmann S, Wright JR, Steinhilber W. TGF-beta1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ t lymphocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L747–L756. doi: 10.1152/ajplung.00401.2005. [DOI] [PubMed] [Google Scholar]
- 129.Gardai SJ, Xiao YQ, Dickinson M. By binding sirpalpha or calreticulin/cd91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 130.Bates SR. P63 (ckap4) as an SP-A receptor: implications for surfactant turnover. Cell Physiol Biochem. 2010;25:41–54. doi: 10.1159/000272062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang CH, Szeliga J, Jordan J. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18a. J Biol Chem. 2005;280:34447–34457. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sever-Chroneos Z, Krupa A, Davis J. Surfactant protein a (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class a. J Biol Chem. 2011;286:4854–4870. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bates SR, Kazi AS, Tao JQ. Role of p63 (ckap4) in binding of surfactant protein-a to type ii pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2008;295:L658–L669. doi: 10.1152/ajplung.90233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Condon JC, Jeyasuria P, Faust JM. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.LeVine AM, Hartshorn K, Elliott J. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 136.LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect / Institut Pasteur. 2001;3:161–166. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- 137.LeVine AM, Bruno MD, Huelsman KM. Surfactant protein A-deficient mice are susceptible to group b streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 138.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 139.Melton KR, Nesslein LL, Ikegami M. SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L543–L549. doi: 10.1152/ajplung.00011.2003. [DOI] [PubMed] [Google Scholar]
- 140.Serrano AG, Perez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids. 2006;141:105–118. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 141.Nesslein LL, Melton KR, Ikegami M. B deficiency perturbs lung function and causes air space abnormalities. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1154–L1161. doi: 10.1152/ajplung.00392.2004. [DOI] [PubMed] [Google Scholar]
- 142.Tokieda K, Whitsett JA, Clark JC. Pulmonary dysfunction in neonatal SP-B deficient mice. Am J Physiol. 1997;273:L875–L882. doi: 10.1152/ajplung.1997.273.4.L875. [DOI] [PubMed] [Google Scholar]
- 143.Clark JC, Weaver TE, Iwamoto HS. Decreased lung compliance and air trapping in heterozygous SP-B deficient mice. Am J Respir Cell Mol Biol. 1997;16:46–52. doi: 10.1165/ajrcmb.16.1.8998078. [DOI] [PubMed] [Google Scholar]
- 144.Nogee LM, Garnier G, Dietz HC. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nogee LM, DeMello DE, Dehner LP. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. New Engl J Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 146.Mulugeta S, Beers MF. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006;8:2317–2323. doi: 10.1016/j.micinf.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 147.Beers MF, Mulugeta S. Surfactant protein c biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- 148.Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992;6:595–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- 149.Glasser SW, Burhans MS, Korfhagen TR. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci U S A. 2001;98:6366–6371. doi: 10.1073/pnas.101500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Glasser SW, Detmer EA, Ikegami M. Pneumonitis and emphysema in SP-C gene targeted mice. J Biol Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 151.Ono S, Tanaka T, Ishida M. Surfactant protein c g100s mutation causes familial pulmonary fibrosis in japanese kindred. Eur Respir J. 2011;38:861–869. doi: 10.1183/09031936.00143610. [DOI] [PubMed] [Google Scholar]
- 152.Thomas AQ, Lane K, Phillips J., 3rd Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 153.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006;36:423–435. doi: 10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 154.Palaniyar N, Nadesalingam J, Reid KB. Pulmonary innate immune proteins and receptors that interact with gram-positive bacterial ligands. Immunobiology. 2002;205:575–594. doi: 10.1078/0171-2985-00156. [DOI] [PubMed] [Google Scholar]
- 155.Hansen S, Holmskov U. Lung surfactant protein d (SP-D) and the molecular diverted descendants: Conglutinin, cl-43 and cl-46. Immunobiology. 2002;205:498–517. doi: 10.1078/0171-2985-00150. [DOI] [PubMed] [Google Scholar]
- 156.Bejvl I, Weseslindtner L, Strassl R. Analysis of plasma surfactant protein D levels in lung transplant recipients. Transpl Infect Dis. 2013;15(6):645–651. doi: 10.1111/tid.12132. [DOI] [PubMed] [Google Scholar]
- 157.Allen MJ, Laederach A, Reilly PJ. Polysaccharide recognition by surfactant protein d: novel interactions of a c-type lectin with nonterminal glucosyl residues. Biochemistry. 2001;40:7789–7798. doi: 10.1021/bi002901q. [DOI] [PubMed] [Google Scholar]
- 158.Shrive AK, Tharia HA, Strong P. High-resolution structural insights into ligand binding and immune cell recognition by human lung surfactant protein d. J Mol Biol. 2003;331:509–523. doi: 10.1016/s0022-2836(03)00761-7. [DOI] [PubMed] [Google Scholar]
- 159.Akiyama J, Hoffman A, Brown C. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50:993–996. doi: 10.1177/002215540205000713. [DOI] [PubMed] [Google Scholar]
- 160.Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev. 2000;173:66–78. doi: 10.1034/j.1600-065x.2000.917308.x. [DOI] [PubMed] [Google Scholar]
- 161.Reading PC, Allison J, Crouch EC. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol. 1998;72:6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.White M, Kingma P, Tecle T. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. J Immunol. 2008;181:7936–7943. doi: 10.4049/jimmunol.181.11.7936. [DOI] [PubMed] [Google Scholar]
- 163.Hillaire ML, Haagsman HP, Osterhaus AD. Pulmonary surfactant protein D in first-line innate defence against influenza a virus infections. J Innate Immun. 2013;5:197–208. doi: 10.1159/000346374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaire ML, Haagsman HP, Osterhaus AD. Pulmonary surfactant protein D in first-line innate defence against influenza A virus infections. J Innate Immun. 2013;5(3):197–208. doi: 10.1159/000346374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaire ML, van Eijk M, Vogelzang-van Trierum SE. Recombinant porcine surfactant protein D inhibits influenza A virus replication ex vivo. Virus Res. 2014;181:22–26. doi: 10.1016/j.virusres.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 164.Reading PC, Morey LS, Crouce EC. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van de Wetering JK, van Eijk M, van Golde LM. Characteristics of surfactant protein a and d binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J Infect Dis. 2001;184:1143–1151. doi: 10.1086/323746. [DOI] [PubMed] [Google Scholar]
- 166.Allen MJ, Voelker DR, Mason RJ. Interactions of surfactant proteins A and D with saccharomyces cerevisiae and aspergillus fumigatus. Infect Immun. 2001;69:2037–2044. doi: 10.1128/IAI.69.4.2037-2044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferguson JS, Martin JL, Azad AK. Surfactant protein D increases fusion of mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Iwaarden JF, Shimizu H, van Golde PHM. Rat surfactant protein D enhances the production of oxygen radicals by rat alveolar macrophages. Biochem J. 1992;286:5–8. doi: 10.1042/bj2860005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wright JR, Zlogar DF, Taylor JC. Effects of endotoxin on surfactant protein A and D stimulation of no production by alveolar macrophages. Am J Physiol. 1999;276:L650–L658. doi: 10.1152/ajplung.1999.276.4.L650. [DOI] [PubMed] [Google Scholar]
- 170.Clark H, Reid KB. Structural requirements for SP-D function in vitro and in vivo: therapeutic potential of recombinant SP-D. Immunobiology. 2002;205:619–631. doi: 10.1078/0171-2985-00159. [DOI] [PubMed] [Google Scholar]
- 171.Wert SE, Yoshida M, LeVine AM. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci U S A. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madan T, Reid KB, Clark H. Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol. 2010;47:1923–1930. doi: 10.1016/j.molimm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 173.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259–L271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 174.Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]
- 175.Ueda T, Ikegami M, Henry M. Clearance of surfactant protein B from rabbit lungs. Am J Physiol. 1995;268:L636–L641. doi: 10.1152/ajplung.1995.268.4.L636. [DOI] [PubMed] [Google Scholar]
- 176.Frick M, Bertocchi C, Jennings P. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2004;286:L210–L220. doi: 10.1152/ajplung.00332.2003. [DOI] [PubMed] [Google Scholar]
- 177.Wirtz HRW, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 178.Jacobs HC, Ikegami M, Jobe AH. Reutilization of surfactant phosphatidylcholine in adult rabbits. Biochim Biophys Acta. 1985;837:77–84. doi: 10.1016/0005-2760(85)90087-6. [DOI] [PubMed] [Google Scholar]
- 179.Magoon MW, Wright JR, Baritussio A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983;750:18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- 180.Ikegami M, Ueda T, Hull W. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol. 1996;270:L–650-L658. doi: 10.1152/ajplung.1996.270.4.L650. [DOI] [PubMed] [Google Scholar]
- 181.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 182.Kitamura T, Tanaka N, Watanabe J. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suzuki T, Sakagami T, Rubin BK. Familial pulmonary alveolar proteinosis caused by mutations in csf2ra. J Exp Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martinez-Moczygemba M, Doan ML, Elidemir O. Pulmonary alveolar proteinosis caused by deletion of the gm-csfralpha gene in the x chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–163. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 186.Sweet DG, Carnielli V, Greisen G. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants–2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 187.Fujiwara T, Maeta H, Chida S. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;79:31–37. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 188.Cole FS, Hamvas A, Nogee LM. Genetic disorders of neonatal respiratory function. Pediatr Res. 2001;50:157–162. doi: 10.1203/00006450-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 189.Hamvas A. Inherited surfactant protein-b deficiency and surfactant protein-c associated disease: clinical features and evaluation. Semin Perinatol. 2006;30:316–326. doi: 10.1053/j.semperi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 190.Hamvas A, Cole FS, Nogee LM. Genetic disorders of surfactant proteins. Neonatology. 2007;91:311–317. doi: 10.1159/000101347. [DOI] [PubMed] [Google Scholar]
- 191.Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr. 2006;18:287–292. doi: 10.1097/01.mop.0000193310.22462.1f. [DOI] [PubMed] [Google Scholar]
- 192.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12:253–274. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Henderson LB, Melton K, Wert S. Large abca3 and sftpc deletions resulting in lung disease. Ann Am Thorac Soc. 2013;10:602–607. doi: 10.1513/AnnalsATS.201306-170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thavagnanam S, Cutz E, Manson D. Variable clinical outcome of abca3 deficiency in two siblings. Pediatr Pulmonol. 2013;48:1035–1038. doi: 10.1002/ppul.22698. [DOI] [PubMed] [Google Scholar]
- 195.Besnard V, Matsuzaki Y, Clark J. Conditional deletion of abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L646–L659. doi: 10.1152/ajplung.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Galambos C, Levy H, Cannon CL. Pulmonary pathology in thyroid transcription factor-1 deficiency syndrome. Am J Respir Crit Care Med. 2010;182:549–554. doi: 10.1164/rccm.201002-0167CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hamvas A, Deterding RR, Wert SE. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene nkx2-1. Chest. 2013;144:794–804. doi: 10.1378/chest.12-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nogee LM, Dunbar AE, 3rd, Wert SE. A mutation in the surfactant protein c gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 199.Hartl D, Griese M. Interstitial lung disease in children—genetic background and associated phenotypes. Respir Res. 2005;6:32. doi: 10.1186/1465-9921-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thomas AQ, Lane K, Phillips J., 3rd Heterozygosity for a surfactant protein c gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 201.Petty TL, Ashbaugh DG. The adult respiratory distress syndrome: clinical features factors influencing prognosis and principles of management. Chest. 1971;60:233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- 202.Gregory TJ, Longmore WJ, Moxley MA. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gunther A, Siebert C, Schmidt R. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153:176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 204.Markart P, Ruppert C, Wygrecka M. Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: putative role of neutral lipids. Thorax. 2007;62:588–594. doi: 10.1136/thx.2006.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Savani RC, Godinez RI, Godinez MH. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. Am J Physiol Lung Cell Mol Physiol. 2001;281:L685–L696. doi: 10.1152/ajplung.2001.281.3.L685. [DOI] [PubMed] [Google Scholar]
- 206.Veldhuizen RA, Marcou J, Yao LJ. Alveolar surfactant aggregate conversion in ventilated normal and injured rabbits. Am J Physiol. 1996;270:L152–L158. doi: 10.1152/ajplung.1996.270.1.L152. [DOI] [PubMed] [Google Scholar]
- 207.Amato MBP, Barbas LV, Medeiros DM. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;339:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 208.Eisner MD, Thompson T, Hudson LD. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 209.Lewis JF, Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu Rev Physiol. 2003;65:613–642. doi: 10.1146/annurev.physiol.65.092101.142434. [DOI] [PubMed] [Google Scholar]
- El-Gendy N, Kaviratna A, Berkland C, Dhar P. Delivery and performance of surfactant replacement therapies to treat pulmonary disorders. Ther Deliv. 2013;4(8):951–980. doi: 10.4155/tde.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dushianthan A, Cusack R, Goss V. Clinical review: exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome—where do we go from here? Crit Care. 2012;16:238. doi: 10.1186/cc11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011;27:525–559. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Taut FJ, Rippin G, Schenk P. A search for subgroups of patients with ards who may benefit from surfactant replacement therapy: a pooled analysis of five studies with recombinant surfactant protein-C surfactant (venticute) Chest. 2008;134:724–732. doi: 10.1378/chest.08-0362. [DOI] [PubMed] [Google Scholar]
- 213.McCormack FX, King TEJ, Voelker DR. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis. 1991;144:160–166. doi: 10.1164/ajrccm/144.1.160. [DOI] [PubMed] [Google Scholar]
- 214.Honda Y, Kuroki Y, Matsuura E. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med. 1995;152:1860–1866. doi: 10.1164/ajrccm.152.6.8520747. [DOI] [PubMed] [Google Scholar]
- 215.McCormack FX, King TE, Jr, Bucher BL. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995;152:751–759. doi: 10.1164/ajrccm.152.2.7633738. [DOI] [PubMed] [Google Scholar]
- 216.Gunther A, Schmidt R, Nix F. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur Respir J. 1999;14:565–573. doi: 10.1034/j.1399-3003.1999.14c14.x. [DOI] [PubMed] [Google Scholar]
- 217.Greene KE, Bucher-Bartelson BL, Akino T. and SP-D levels are elevated in patients with IPF. Am J Respir Crit Care Med. 1997;155:A216. [Google Scholar]
- 218.Kuroki Y, Takahashi H, Chiba H. Surfactant proteins A and D: disease markers. Biochim Biophys Acta. 1998;1408:334–345. doi: 10.1016/s0925-4439(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Kuan PJ, Xing C. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hohlfeld J, Fabel H, Hamm H. The role of pulmonary surfactant in obstructive airway disease. Eur Respir J. 1997;10:482–891. doi: 10.1183/09031936.97.10020482. [DOI] [PubMed] [Google Scholar]
- 221.Postle AD, Mander A, Reid KB. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- 222.Nakamura A, Ebina-Shibuya R, Itoh-Nakadai A. Transcription repressor bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function. J Exp Med. 2013;210:2191–2204. doi: 10.1084/jem.20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shijubo N, Tsutahara S, Hirasawa M. Pulmonary surfactant protein A in pleural effusions. Cancer. 1992;69:2905–2909. doi: 10.1002/1097-0142(19920615)69:12<2905::aid-cncr2820691207>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 224.Betz C, Papadopoulos T, Buchwald J. Surfactant protein gene expression in metastatic and micrometastatic pulmonary adenocarcinomas and other non-small cell lung carcinomas: detection by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995;55:4283–4286. [PubMed] [Google Scholar]
- 225.Zhang F, Pao W, Umphress SM. Serum levels of surfactant protein D are increased in mice with lung tumors. Cancer Res. 2003;63:5889–5894. [PubMed] [Google Scholar]


