Abstract
The unique characteristics of polyolefins make them the best candidates in single use healthcare products, medical textiles, and water filters. Most medical textiles and polymeric materials used in hospitals and hotels are good media for cross-transmission of diseases since most microorganisms can survive on fibrous materials for several hours to several months. Use of medical devices with antimicrobial functions has been considered as a major avenue to fight against microbial infections. In addition, suitable packaging can slow the deterioration rate, and hence, extend the shelf-life of food. Physical and chemical incorporation of antimicrobial agents into polyolefins can be implemented in common polyolefin processing. In this chapter, antimicrobial polyolefins are summarized based on processes that were studied to incorporate biocides into the polymers. These processes include blending, coating, and chemical modifications.
Key words: antimicrobial activity, heavy metals, polycationic agents, halamine precursors, rechargability
8.1. Introduction
Among all synthetic polymers, olefin polymers such as isotactic polypropylene (PP) and polyethylene (PE) are the most popular thermoplastic polymers which are widely applied in textiles, medical devices, food packaging, automobiles and many other products (Pasquini, 2005). The unique characteristics of polyolefins such as superior chemical resistance and robust mechanical properties, as well as low costs, make them the best candidates in single use healthcare products, medical textiles and water filters. For example, PP has found applications in medical syringes, lab-ware applications, diagnostic devices, and Petri dishes (Pasquini, 2005). These medical devices are usually sterilized by using sterilizing agent of irradiation before use. Thus, resistance to various chemical agents as well as to UV and other irradiation sources is necessary for medical devices. PP is the most popular polymer used in making medical use materials such as nonwoven fabrics which are employed in liquid proofing disposable surgical and isolation gowns, drapes, central supply room sterilization wraps, face masks and pharmaceutical filter media, etc. (Sen, 1997).
Most medical textiles and polymeric materials used in hospitals and hotels are conductive to cross-transmission of diseases since most microorganisms can survive on fibrous materials for several hours to several months (Zerr et al., 2005; Burke, 2003). Thus, polymeric surfaces and textile materials may be responsible for disease transmission and the spread of new strains of diseases from hospitals to elsewhere. In the last two decades there has been a growing concern on the emerging super infections such as MRSA (methicillin-resistant Staphylococcus aureus) and SARS (severe acute respiratory syndrome) which can easily spread through physical contact and aerosol transmission. Moreover, healthcare-associated infections (nosocomial effects) in hospitals are increasing, causing about 2 million cases and ranking as the fourth most common cause of death in the United States. This number accounts for up to 5% of hospitalized patients and results in $4.5 billion extra healthcare costs (Isakow et al., 2007). Use of medical devices with antimicrobial functions has been considered as a major avenue to fight against the nosocomial effects.
In addition, food products undergo several physical, chemical, and microbial changes during storage. The stability of foods is a function of changes occurring in the food components, such as food proteins, lipids, carbohydrates, and water, due to environmental and processing factors. The use of plastics in the packaging of foods as a protective coating or barrier to contaminants not only retards unfavorable deterioration of food, but may also enhance its quality (Goddard and Hotchkiss, 2007). Suitable packaging can slow the deterioration rate, and hence, extend the shelf-life of food. Such approaches, designed to perform some desirable function other than providing an inert barrier, has led to the concept of incorporating antimicrobial agents directly into package films, which is called active packaging. The active packaging may delay or even prevent the growth of microorganisms on the food surface and, hence, lead to an extension of the shelf-life and/or the improved safety of the food product (Brody et al., 2001).
8.2. Antimicrobial functions
Antimicrobial function in general is the ability to inhibit growth of a broad spectrum of microbes such as bacteria, molds, fungi, viruses and yeasts, which includes antibacterial, antibiotic, germicide, antiviral, and antifungal or antimycotic functions. Antimicrobial agents have different activities which affect microorganisms differently. Antimicrobial materials can be categorized into two groups: biocidal and biostatic materials, according to their functions (Sun and Worley, 2005). Biostatic functions refer to inhibiting growth of microorganisms on materials, and biostatic agents inhibit further growth of microorganisms without completely killing them. As long as the microorganism is exposed to the biostatic agent, the microorganism will not be able to reproduce rapidly. Biostatic agents can prevent the materials from biodegradation. Biocidal materials are able to kill microorganisms completely, thus eliminating their growth and possibly protecting users from biological contamination and transmission. If a biocidal agent cannot completely eradicate microorganisms because there is a limited amount, then it will only provide a biostatic effect.
Antimicrobial functions of polymeric materials can be attained by physically or chemically incorporating biocides into the polymers. In a physical method, the antimicrobial agent could be blended with, coated or sprayed on polymeric surfaces. The physical processes are relatively simple, easy, and inexpensive to implement, but are also limited in providing desired durability and stability. Chemical approaches are able to incorporate durable and permanent antimicrobial functions to polymers by covalently linking biocides to polymer backbones. This incorporation can be carried out by copolymerization or surface grafting polymerization of biocidal structures to the polymers (Kenawy et al., 2007).
Antimicrobial materials usually consume biocides in the polymers or biocidal structures on surfaces when providing the biocidal function. Generally speaking, to provide antimicrobial functions for a certain period of time the materials should release sufficient amount of biocides during this period. Thus, controlled release of the biocides from the materials is a method of providing durable antimicrobial functions. In recent years, the authors' research group has developed a regenerable process by incorporating precursor structures of biocides onto polymers and activating the biocidal function using certain chemicals such as chlorine or oxygen bleaches (Sun and Xu, 1998; Sun and Sun, 2001a, 2001b, 2001c, 2001d; Sun, 2001; Liu and Sun, 2006). Here, precursors or precursor structures of biocides are incorporated onto polymers with covalent bond connections. The precursor structures can be converted to biocidal sites after activation with chlorine or oxygen bleaches. The biocidal functions of the materials are regenerable after repeated usages, which is particularly important and useful for textiles.
The antimicrobial agents include antibiotics, alcohol, formaldehyde, heavy metal ions (silver, copper), cationic surfactants (quaternary ammonium salts with long hydrocarbon chains), phenols, oxidizing agents such as chlorine, chloramines, hydrogen peroxide, iodine, and ozone. These antimicrobial agents have different activities against microorganisms. Some antimicrobials, such as alcohols and quaternary ammonium compounds, act directly on microbial cells to dissolve them. Others may penetrate the cells and cause the release of amino acids, nuclear material, and other important chemical constituents. Some compounds penetrate microbial cell walls and inactivate essential membrane transport systems, so that the cells can no longer obtain the nutrients necessary for them to survive and reproduce. Others coagulate certain vital materials in cells, thereby destroying the microorganisms. A few agents disrupt the metabolism of the cells, so that they can no longer assimilate nutrients; as a result, the cells starve and die. However, all of them have limitations when applied onto polymeric materials such as textiles and consumer products (Sun and Worley, 2005).
8.3. Antimicrobial polyolefins
Antimicrobial polyolefins have been extensively investigated and developed. Two common processes of producing antimicrobial polyolefins are: direct incorporation of antimicrobial agents into polyolefins such as blending into polymers and coating or adsorbing antimicrobial agents onto polyolefin surfaces; and chemical incorporation using copolymerization grafting to immobilize or impart antimicrobial agents into or onto polyolefin by ionic or covalent linkages.
Physical incorporation of quaternary ammonium salt, inorganic metals and inherently antimicrobial polymers are the main additives which have been widely presented. Many antimicrobials are not easily incorporated into or not homogenously distributed in polyolefins (Appendini and Hotchkiss, 2002). The antimicrobial activity of polyolefins produced using this method relies on a slow releasing mechanism. It means first, the biocides from polymer body gradually migrate toward the surface and provide antimicrobial functions when they are in contact with germs. The rate of release and migration has a direct effect on the antimicrobial efficacy. Too fast release produces short-term biocidal activity but poor durability. For instance, in the case of antimicrobial textiles, laundering and cleaning are the common procedures in the maintenance of textile materials. Therefore, washing durability is an extremely important property for antimicrobial textile products. So, enhanced interactions between biocides and fibers to provide controlled release of the biocides is the key to sustain biocidal activities. In addition, incorporation of excessive amount of biocides into textile materials could also provide prolonged usage. However, the biocidal function eventually will be worn out without rejuvenation of the biocides. To prepare durable protective antimicrobial polymers, regenerable biocides and novel approaches should be explored.
In the following sections, antimicrobial polyolefins will be summarized based on processes that were studied to incorporate biocides onto the polymers. These processes include blending, coating, and chemical modifications.
8.3.1. Blending biocides into polyolefins
Heavy metals
Among many biocides that can be blended with polyolefins, silver is the most popular, as it is known to have strong antimicrobial effects against a broad spectrum of microorganisms, is stable and easy to apply, as well as being non-toxic to the human body (Jeong et al., 2005a, 2005b; Yeo and Jeong, 2003; Yeo et al., 2003). Metallic silver does not release the ion easily, compared with copper, and so its antimicrobial activity is not quite as strong as its metallic state. In fact, silver nitrate that forms silver ions, Ag+, in water solution has strong antimicrobial activity (Yamanaka et al., 2005). Polyolefins have poor miscibility with silver ions, thus use of micro or nano-sized metal particles would increase miscibility of the metal in polymers. In fact, nanosized silver particles have been introduced into polyolefins during the extrusion process (Jeong et al., 2005a and 2005b; Yeo and Jeong, 2003; Yeo et al., 2003). Accessibility of the silver particles inside the materials to microorganisms and low homogeneous dispersion of silver nano-sized particles are the main challenges in applications of this technique (Jeong et al., 2005a; Yeo et al., 2003). Moreover, the formation of Ag+ requires water uptake, so only hygroscopic polymers are good candidates in this context. Thus, addition of certain additives as a carrier for silver ions or a modifier to increase hydrophilicity of polyolefins has been proposed (Kumar and Münstedt, 2004; Radheshkumar and Münstedt, 2006). Silver substituted zeolites are the most widely used additives in this context for food packaging applications (Brody et al., 2001). Based on this technology, DuPont has introduced MicroFree™ brand silver salt antimicrobial powders to impart antimicrobial activity to plastic resin systems.
Another heavy metal ion, copper ion, also can destroy microorganisms and viruses, and is proven safe to humans (Borkow and Gabbay, 2005). Gavia and co-workers introduced copper oxide into the PP by adding a cupric oxide powder to the polymer during the master-batch preparation stage. Spunbonded nonwoven fabrics made of melt-blended polymer and copper presented excellent biocidal activities (Gavia et al., 2006). However, copper ion is more toxic to the aquatic environment, which is a limitation of the applications of copper ion.
Again, heavy metal biocides can be easily incorporated into polyolefins during extrusion processes and could provide good antimicrobial functions on the materials with a contact time of a few hours (Table 8.1 ). Silver is the most popular, being widely employed in many polymeric materials including textiles, household plastics, and medical devices because of its safety to humans. But due to relatively slow functions, the products containing the ions may have limited power to provide complete and quick kill against most pathogens. In addition, all heavy ions are toxic to aquatic life, which is a major concern in the use of heavy metal ions.
Table 8.1.
Incorporation of heavy metals into polyolefins
| Antimicrobial Activity |
|||
|---|---|---|---|
| Antimicrobial agent/polymer fabrication method | Minimum concentration contact time (method) bacteria reduction | Remarks | Ref. |
| Silver nano-sized particle/PP Melt blending & film casting |
|
Show activity for micro-sized when Conc. > 0.5 % | Jeong et al. (2005a, 2005b) |
| Silver nano-sized particle/PP Melt blending & sheath/core fiber spinning |
|
No activity when silver was in core | Yeo and Jeong (2003), Yeo et al. (2003) |
| Elementary silver/PP Melt blending & film casting |
|
Adding carrier improve activities | Radheshkumar and Münstedt (2006) |
| Copper oxide/PPMelt blending, fiber spinning & making spun-bonded fabric |
|
No difference due to layer thickness | Gabbay et al. (2006) |
Macromolecular antimicrobial agents
Poly (2-tert-butylaminoethyl) methacrylate, PTBAEMA (Fig. 8.1 ), is a typical representative water-insoluble biocide which can be blended with polyolefins to provide surface modification (Seyfriedsberger et al., 2006) (Table 8.2 ). The major role of PTBAEMA is believed to be the displacement of Ca2+ and/or Mg2+ ions from the outer membrane of the bacteria, which is accordingly disorganized and finally disrupted.
8.1.
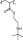
Structure of poly (2-ferf-butylaminoethyl) methacrylate (PTBAEMA).
Table 8.2.
Incorporation of high molecular weight cationic antimicrobial agents into polyolefins
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/polymer fabrication method | Minimum concentration contact time (method) bacteria reduction | Remarks | Ref. |
| PTBAEMA/LLDPEMelt blending and film casting |
|
Seyfriedsberger et al. (2006) | |
| PTBAEMA/LDPESynthesize PEB-b-PTBAEMA, melt blending with PE and film casting |
|
Diblock copolymer is a biocidal | Lenoir et al. (2006) |
| PTBAEMA/PPSynthesize PP-g-MAH-PTBAEMA through ATRP and melt blending with PP then fiber spinning |
|
No activity for PTBAEMA without amino end group | Thomassin et al. (2007) |
Physical entrapment of PTBAEMA into LLDPE by blending and film casting provides moderate antimicrobial activity for long contact time (Seyfriedsberger et al., 2006). Since the physical trapping of the antimicrobial macromolecule is the only binding mechanism to improve miscibility of these two polymers, Lenoir and co-workers have developed a block copolymer that combines a short polyolefin block miscible with LDPE and a PTBAEMA block which has been synthesized by controlled atom transfer radical polymerization (ATRP) (Lenoir et al., 2006). This diblock copolymer was synthesized by ATRP of TBAEMA onto poly(ethylene-co-butylene) (PEB) oligomer which has activated bromide as a macroinitiator. Morphological changes of E. coli bacteria in contact with melt-blended film indicated that the diblock copolymer is bactericide rather than bacteriostatic (Lenoir et al., 2006). A reasonable explanation for this claim is that some fibrous and granular material, most probably cell content, is released after 60 min contact time with modified LDPE which serves as evidence of the destruction of the bacteria membrane. Also, no bacteria have been observed sticking on the active surface of LDPE, which indicates the bacteria were killed by contact and released to the aqueous environment.
The same idea was employed in making antimicrobial PP recently (Table 8.2). To overcome the deleterious effects of immiscibility of two polymers, Thomassin and co-workers grafted PTBAEMA onto PP backbone by reactive blending of polypropylene-g-maleic anhydride (PP-g-MAH) with a primary amine-end-capped poly((2-tert-butylamino) ethyl methacrylate) (Thomassin et al., 2007). The antimicrobial activity of functional fibers showed that the functional PP fibers were able to provide long-lasting antibacterial activity because the PTBAEMA biocides anchored onto PP chains prevent themselves from being released from the surface of the fibers. Such incorporated biocides in polymers behave similarly to chemically incorporated ones, which could provide more durable functions.
Other antimicrobial agents
N-halamine antimicrobial agents has been employed in polyolefins by incorporating hindered amine light stabilizers (HALSs) as N-halamine precursor (Chen and Sun, 2005a, 2005b) (Fig. 8.2 ). HALSs are one of the most important light/thermal stabilizing agents of polymeric materials (Table 8.3 ).
8.2.
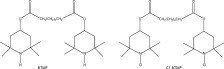
Structure of bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate (BTMP) and N-chlorine-BTMP.
Table 8.3.
Incorporation of other antimicrobial agents into polyolefins
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/polymer fabrication method | Minimum concentration contact time (method) bacteria reduction | Remarks | Ref. |
| Nisin/PE Melt blending & film casting |
|
Adding polyethylene oxide or EDTA significantly improves activity | Cutter et al. (2001) |
| BTMP/PP Synthesize Cl-BTMP and solvent casting |
|
Ability to recharge and durable | Chen and Sun (2005a, 2005b) |
| Benzoic anhydride/LDPE Solvent casting |
|
Released benzoic acid is active compound | Weng and Hotchkiss, (1993) |
The researchers found HALSs could be readily transformed into N-chloro-hindered amine (NCHAs) in diluted sodium hypochlorite bleach solution. Although they were able to fabricate PP by solvent casting, the solvent cast film showed excellent antimicrobial activities against E. coli and S. aureus. Also, they claimed strong storage stability of functional polymer as well as durability and rechargability of this functionality.
Using miscellaneous antimicrobial agents such as nisin or simple antifungal agents such as propionic, benzoic, and sorbic acids (in the form of anhydride for increased compatibility) to polymers such as LDPE to produce active packaging for food has been studied (Table 8.3). These materials may have limited washing durability since they are mostly soluble in water (Cutter et al., 2001; Weng and Hotchkiss, 1993).
8.3.2. Coating antimicrobial agents onto surfaces
Antimicrobial agents that cannot survive elevated processing temperatures can be coated onto polyolefins (Zhang et al., 2000). In this case, a processed polyolefin material such as nonwoven fabric or polymeric film is impregnated in a solution of antimicrobial agent, padded and dried for removal of water by heat treatment, and finally cured if necessary. Thus, only surface properties of the materials are changed while the bulk properties of the substrate are intact. Good mechanical properties of the coating and good adhesion to the substrate are critical points in the treatment of the polyolefins. In comparison to melt blending, functional coating has several advantages. For instance, after the melt blending process, most antimicrobial agents such as metal nano-sized particles may not stay on the surface and are unable to contribute to antimicrobial effects. If the agents and polyolefins are immiscible the products may not have acceptable transparency. For instance, silver nanoparticles and copper have been coated on the surface of polymer films or fabrics. To overcome low durability of the antimicrobial functions, plasma treatment with or without gas implantation was employed in coating of copper ions onto polyolefins (Table 8.4 ) (Zhang et al., 2006c, 2007a, 2007b).
Table 8.4.
Coating common antimicrobial agents onto polyolefin surfaces
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/substrate | Minimum concentration (thickness) contact time (method) bacteria reduction | Remarks | Ref. |
| Nano-particle silver/Spun-bonded PP |
|
Usage of magnetron sputter coating | Wang et al. (2007) |
| Silver-doped organic-inorganic hybrid/LDPE thin film |
|
Sol-gel coating of PE-PEG-Si/silica hybrid | Marini et al. (2007) |
| Nano-sized silver colloid/PE-PP spun-bonded non-woven |
|
Ethanol based colloid with (without) sulfur composite show 99.9% reduction for both bacteria | Jeong et al. (2005a) |
| Copper/PE film |
|
Using plasma immersion ion implantation technique (PIII) | W. Zhang et al. (2006c) |
| Gas co-implantation can regulate the copper release rate | Zhang et al. (2007b) | ||
| Gold or Gold-palladium (60–40)/PP mesh graft |
|
Zero and 30% infection for in-vivo Au-Pd and Au samples | Saygun et al. (2006) |
| MHPA and MDHP/PP fabric |
|
No change after 5 times laundering | Vigo and Danna (1995) |
| Chitosan oligomer/PP non-woven |
|
30% reduction (K. pneumoniae and P. aeruginosa) for more than 1.0% add-on chitosan level | Shin et al. (1999) |
| Triclosan/PE film |
|
No antimicrobial activity when bacteria conc. is 108 CFU/ml | Zhang et al. (2006a) |
| Bronopol/PE film |
|
No antimicrobial activity when bacteria conc. is 108 CFU/ml | Zhang et al. (2006b) |
| Polyhexamethylene biguanide hydrochloride/SMS non-woven PP |
|
No change in activity due to applying fluorochemical repellent finishing | Huang and Leonas (2000) |
In the case of silver nano-sized particles chemical vapor deposition (CVD), sol-gel technique or magnetron sputter coating was used to prepare nanostructured silver films (Wang et al., 2007; Marini et al., 2007; Jeong et al., 2005a). Sol-gel provides a simple technological approach, but such a coating was not uniform and compact. Also, weak bonding forces between film and polymer substrate may cause low durability of the coating. CVD does not pollute the environment, and sputter coating is quite expensive and not applicable to large and or complex item geometries (Table 8.4).
Preparatory gold coating for SEM imaging was used as antimicrobial coating on mesh grafts of PP (Agalar et al., 2006). The coated materials showed antimicrobial effects, with the gold-palladium coated one exhibiting better functions than the gold coated one at a contact time of 24 h. In in vivo tests of the samples for eight days after inoculation, the wound infection rate was 0% for gold-palladium coated PP grafts and 30% for gold coated PP grafts, whereas virgin PP grafts demonstrated 100% growth rate (Table 8.4).
In addition, magnesium hydroperoxyacetate (MHPA) and magnesium dihydroperoxide (MDHP) as antibacterial agents were applied onto PP fabrics through pad-dry cure, and the products exhibited good antibacterial activity (>99% reduction of growth) after zero and five launderings (Vigo and Danna, 1995). Coating other antimicrobial agents such as triclosan (2,4,4P-trichloro-2P-hydroxydiphenylether) (Zhang et al., 2006a), bronopol (2-bromo-2-nitropropane-1,3-diol) on plasma-modified PE (Zhang et al., 2006b), water-soluble chitosan oligomer (Shin et al., 1999), a deacetylated product of chitin, onto PP nonwoven, Reputex®, with polyhexamethylene biguanide hydrochloride (PHBM) as an active ingredient, onto spunbonded-meltblown-spunbonded (SMS) PP non-woven fabrics have been proposed (Huang and Leonas, 2000) (Table 8.4).
Recently, Han and co-workers used polyamide as a coating medium to incorporate active compounds on surfaces of LDPE film (Han et al., 2006, 2007). The polyamide resin was dissolved in alcohol and then various antimicrobial agents, such as sorbic acid, cymophenol, thymol, rosemary oleoresin, and trans-cinnamaldehyde were added to the prepared solution. Afterwards, the prepared coating medium was coated on LDPE film and irradiated with electron beams to increase covalent binding between the coating and the polymer film.
Coating processes, serving as an optional process to incorporate antimicrobial agents to polyolefins, have been successfully employed in different products. Since biocides on surfaces can be removed rapidly during usage, different polymeric binders, chemically or physically enhanced interactions between the biocide and polyolefins were adopted to increase durability of the antimicrobial functions. These methods do work for various products that may not be repeatedly used and cleaned. For certain polyolefin materials such as reusable textiles chemical incorporation of biocides onto polyolefins might be a better option.
8.3.3. Chemical incorporation of biocides to polyolefins
Various chemical modification methods have been developed, including ionic or covalent linkage, cross-linking, and graft copolymerization. Antimicrobial agents have been linked as side substitutes to polymer backbone by means of cleavable bonds. Release of the active agents occurs without significantly affecting properties of the original polymer. Therefore, durability of antimicrobial functions is much higher than with physical incorporation techniques, though cleavage of immobilized antimicrobial agent is the most critical point. This method of obtaining antimicrobial polymers requires the presence of suitable functional groups on both the antimicrobial and the polymer. Peptides, enzymes and polyamines are the functional-bearing antimicrobial agents. Few examples of immobilization of antimicrobial onto unmodified polyolefins have been published because of non-availability of suitable functional groups on the polyolefin polymers. Therefore, most research efforts have focused on introducing functional groups onto the polymers.
Cationic substances
Chitosan is a good polymeric biocide and can be immobilized onto PP films or fabrics. In order to provide proper functional groups on polyolefins that are interactive with NH2 groups in chitosan, carboxylic groups have been suggested to be grafted onto polypropylene. In this case, acrylic acid (AA), glycidal methacrylate (GMA), or methacrylic acid (MA) can be grafted onto polymer surfaces by using plasma or irradiation, and afterwards immobilization of chitosan is carried out (Vartiainen et al., 2005; Wafa et al., 2007; Wang and Chen, 2005; Yang et al., 2003; Chen et al., 2005). Polypropylene surfaces can be modified with amino groups by plasma treatment and glutaraldehyde can be used as a cross-linker to link both the polymer and chitosan (Scheme 8.1). The mechanism with glutaraldehyde is based on the formation of imine bonds between aldehyde groups of glutaraldehyde and amino groups of enzyme and amino plasma activated PP (Vartiainen, et al., 2005).
Scheme 8.1.

Schematic diagram of immobilization of chitosan onto amino-activated PP films
Other non-leaching antimicrobial agents such as poly 2-dimethylaminoethyl methacrylate (PDMAEMA) have been immobilized on the surface of PP, and the modified product also showed powerful biocidal activity (Huang et al., 2007) (Table 8.5 ). Leachable antimicrobial activity could also be achieved by applying a hydrolyzable ester linkage between the biocide and the polymer (Scheme 8.2). The gradually hydrolyzed ester bonds will provide slow release of the antimicrobial agents (McCubbin et al., 2006a, 2006b).
Table 8.5.
Chemical incorporation of cationic antimicrobial agents into polyolefins
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/substrate fabrication method | Minimum concentration contact time (method) (% or log) bacteria reduction | Remarks | Ref. |
| HTCC/PP non-woven Grafting GMA onto plasma-treated PP followed by immobilization | 29.7% GMA/2.6% HTCC
|
Wafa et al. (2007) | |
| β -cyclodexrin (CD)/PP non-woven Same as above |
|
Higher GMA/CD conc. show inverse effect on activities | Wafa et al. (2007) |
| Chitosan/PP non-woven Solution grafting with AA followed by immobilization in presence of collagen and glutaraldehyde |
|
Water-uptake and diffusion coefficient decrease at higher immobilization percentage | Wang and Chen (2005) |
| Chitosan/PP film Amino and carboxyl-plasma activation of PP followed by immobilization |
|
> 95% reduction in oxygen permeability, using glutaraldehyde as coupling agent | Vartiainen et al. (2005) |
| Chitosan/PP non-woven AA grafting under γ-ray irradiation followed by immobilization in presence of EDAC |
|
Higher water content after treatment | Yang et al. (2003) |
| Quaternary ammonium salt/PP fabricMA γ-irradiation grafting followed by quaternization with amine hydrochloride–epichlorhydrin condensate |
|
Same activity even without quaternization | Mosleh et al. (2003) |
| Quaternary ammonium salts/LLDPE film AA glow discharge grafting followed by quaternization |
|
No activity for C12 chain lengthNo activity when bacteria conc. > 105 CFU/ml | McCubbin et al. (2006b) |
| Quaternary ammonium salt/PP fabricMorpholine ethyl methacrylate (MEMA) E-beam irradiation grafting followed by quaternization |
|
Less activity when benzyl chloride used as alkylating agent | Mosleh et al. (2003) |
| Quaternary ammonium salt/PP fabricRadiation grafting of vinyl pyridine (4-VP) followed by quaternization by ethyl/butyl/hexadecyl or benzyl halide |
|
Benzyl bromide was the most effective Higher activity with more plied layers | Tan et al. (2000) |
| PQA/PP plateATRP of DMAEMA onto PP followed by quaternization |
|
Huang et al. (2007) |
Scheme 8.2.

Conversion of the grafted carboxylic acid to the quaternary salt
Metallic complexation
Several monomers such as acrylic acid (AA) (Park et al., 1998), sulfonated styrene (Nho et al., 1999), and 2N-morpholine ethyl methacrylate (MEMA) (Mosleh et al., 2003) have been grafted onto PP substrates and their metallic salt complexes showed antimicrobial properties. Among all applied metallic elements silver-complexed polymer showed a strong biocidal activity against all bacteria. Since the target site of the cationic biocides is the cytoplasmic membranes of bacteria, adsorption of metallic salt onto the bacterial cell surface is the first step for their action. It is expected that adsorption can be enhanced with increasing charge density of the cationic biocides (Table 8.6 ).
Table 8.6.
Chemical incorporation of heavy metals into polyolefins
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/substrate fabrication method | Minimum concentration contact time (method) bacteria reduction (% or log) | Remarks | Ref. |
| Metallic salt/PP fabric AA γ-irradiation grafting followed by metallic complexation |
|
No activity for Ni-complexed fabric | Park et al. (1998) |
| Metallic salt/PP fabric Styrene γ-irradiation grafting followed by sulfonation and then metallic complexation |
|
Nho et al. (1999) | |
| Metallic salt/PP fabric Morpholine ethyl methacrylate (MEMA) E-beam irradiation grafting followed by quaternization |
|
Total kill even without using alkylating agent | Mosleh et al. (2003) |
Halamines and halogens
Among the currently investigated antimicrobial agents, only N-halamines have shown the capability of providing fast and total kill against a wide range of microorganisms without causing resistance from the microorganisms. An N-halamine is a compound in which one halogen atom is attached to nitrogen and is formed by the halogenation of imide, amide, amino-azine or amino groups. N-halamine compounds provide the most durable and rechargeable biocidal properties compared to other antimicrobial agents. Several efforts have been devoted to incorporating them into polymeric materials in the last 10 years (Sun and Xu, 1998, 1999a, 1999b; Sun and Sun, 2001a, 2001b, 2001c, 2001d, 2002a, 2003, 2004; Sun, 2001; Liu and Sun, 2006). Blending an N-halamine agent with polymeric material and grafting on polymer backbone are the most common ways to introduce N-halamine agents into polymeric structures. The former one has been discussed before whereas the second method, somehow, is difficult to control, but seems to be the most facile approach.
Chemical incorporation of halamine structures to various polymers has experienced great development over the past decade but it has been applied more onto hydrophilic polymers. N-halamine structures were grafted onto PP fabrics by a conventional pad-dry-cure finishing process (Sun and Sun, 2002b). This technique provided powerful, durable and rechargeable biocidal activities on several synthetic polymers, but is limited to surface modification.
Recently, chemical incorporation of N-halamine precursor structure into polyolefins during melt extrusion was explored (Badrossamay and Sun, 2008). By using a reactive extrusion process and radical graft polymerization reaction, several cyclic and acyclic halamine precursor structures were successfully grafted onto polypropylene. The chlorinated grafted polymers provided fast, durable and rechargeable biocidal activity against bacteria and are prone to be applied in melt-blown nonwoven systems. Such an approach of chemical modification of polyolefins opens up new avenues of making antimicrobial polymers.
In addition, grafting of polyvinylpyrrolidone iodine (PVP-I) complex onto PP films to achieve antimicrobial activity has been proposed (Xing et al., 2005). The activated surfaces showed total kill after a minimum of 4 h contact time without propensity to recharge or refresh (Table 8.7 ).
Table 8.7.
Chemical incorporation of haloamine or halogen antimicrobial agents into polyolefins
| Antimicrobial activity |
|||
|---|---|---|---|
| Antimicrobial agent/substrate fabrication method | Minimum concentration contact time (method) bacteria reduction (% or log) | Remarks | Ref. |
| Polyvinylpyrrolidone-Iodine (PVP-I) complex/PP film UV grafting of NVP followed by Iodine complexation |
|
Xing et al. (2005) | |
| N-Halamine/PP fabric Pad-dry cure of hydantoin derivative followed by chlorine activation |
|
High durable and ability to recharge | Sun and Sun (2002b) |
| N-Halamine/PP polymer Melt-induced grafting melamine derivatives followed by fiber formation and chlorine activation |
|
Rechargeable and durable activity | Badrossamay and Sun (2008) |
8.4. Future trends
Current antimicrobial polyolefin products could provide actions against a broad spectrum of pathogens and have found applications in many areas. Undoubtedly, the use of antimicrobial polymers in hygienic products and medical devices can protect human health and improve quality of life. The range of applications of the polymers has been extended to many fields including artificial organs, drugs, healthcare products, implants, bone replacement and other prostheses, wound-healing, food, textile industry, and water treatment, etc.
The antimicrobial polymers are on the verge of rapid expansion, which is evidenced by emerging new classes of antimicrobial products in the past few years (Kenawy et al., 2007). However, current antimicrobial products still need several hours of contact time to provide substantial reductions of pathogens, which is still far below expectation. Furthermore, many current structural modifications on polyolefins could adversely affect their original properties, thus making the polymer lack antimicrobial applications. However, modification of polyolefins and their fibrous products with more powerful biocidal properties as well as with improved porosity, wettability, and biocompatibility could lead to applications in implants and biomedical devices as well as biological protective materials.
8.5 References
- Agalar F., Daphan C., Saygun M., Ceken S., Akkus A. Gold and Gold-Palladium Coated Polypropylene Grafts in a S. epidermidis Wound Infection Model. Journal of Surgical Research. 2006;131(1):73. doi: 10.1016/j.jss.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Appendini P., Hotchkiss J.H. Review of Antimicrobial Food Packaging. Innovative Food Science and Emerging Technologies. 2002;3(2):113. [Google Scholar]
- Badrossamay M.R., Sun G. Rechargeable Biocidal Polypropylene Prepared by Melt Radical Grafting of Polypropylene with Diallyl-Amino Triazine. European Polymer Journal. 2008;44:733. [Google Scholar]
- Borkow G., Gabbay J. Copper as a Biocidal Tool. Current Medicinal Chemistry. 2005;12(18):2163. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- Brody A.L., Strupinsky E.R., Kline L.R. Active Packaging for Food Applications. CRC Press; 2001. pp. 131–194. [Google Scholar]
- Burke J.P. Infection Control – A Problem for Patient Safety Source. The New England Journal of Medicine. 2003;348(7):651. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- Chen K.S., Ku Y.U., Lee C.H., Lin H.R., Lin F.H., Chen T.H. Immobilization of Chitosan Gel with Cross-linking Reagent on PNIPAAm gel/PP Non-woven Composites Surfaces. Materials Science & Engineering C. 2005;25(4):472. [Google Scholar]
- Chen Z., Sun Y. Antimicrobial Polymers Containing Melamine Derivatives. II. Biocidal Polymers derived from 2-vinyl-4,6-diamino-1,3,5-triazine. Journal Polymer Science, Part A: Polymer Chemistry. 2005;43:4089. [Google Scholar]
- Chen Z., Sun Y. N-Chloro-Hindered Amines as Multifunctional Polymer Additives. Macromolecules. 2005;38:8116. [Google Scholar]
- Cutter C.N., Willett J.L., Siragusa G.R. Improved Antimicrobial Activity of Nisin-incorporated Polymer Films by Formulation Change and Addition of Food Grade Chelator. Letters in Applied Microbiology. 2001;33(4):325. doi: 10.1046/j.1472-765x.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Gabbay J., Borkow G., Mishal J., Magan E., Zatcoff R., Sheme-Avni Y. Copper Oxide Impregnated Textiles with Potent Biocidal Activities. Journal of Industrial Textiles. 2006;35(4):323. [Google Scholar]
- Gavia J., Borkow G., Mishal J., Magen E., Zatcoff R. Copper Oxide Impregnated Textiles with Potent Biocidal Activities. Journal of Industrial Textiles. 2006;35(4):323. [Google Scholar]
- Goddard J.M., Hotchkiss J.H. Polymer Surface Modification for the Attachment of Bioactive Compounds. Progress in Polymer Science. 2007;32(7):698. [Google Scholar]
- Han J., Castell-Perez M.E., Moreira R.G. The Influence of Electron Beam Irradiation on the Effectiveness of Trans-cinnamaldehyde-coated LDPE/Polyamide Films. Journal of Food Science. 2006;71(5):E245. [Google Scholar]
- Han J., Castell-Perez M.E., Moreira R.G. The Influence of Electron Beam Irradiation of Antimicrobial-coated LDPE/Polyamide films on Antimicrobial Activity and Film Properties. LWT – Food Science and Technology. 2007;40(9):1545. [Google Scholar]
- Huang J., Murata H., Koepsel R.R., Russell A.J., Matyjaszewski K. Antibacterial Polypropylene via Surface-initiated Atom Transfer Radical Polymerization. Biomacromolecules. 2007;8(5):1396. doi: 10.1021/bm061236j. [DOI] [PubMed] [Google Scholar]
- Huang W., Leonas K.K. Evaluating a One-Bath Process for Imparting Antimicrobial Activity and Repellency to Non-woven Surgical Gown Fabrics. Textile Research Journal. 2000;70(9):774. [Google Scholar]
- Isakow W., Morrow L.E., Kollef M.H. Probiotics for Preventing and Treating Nosocomial Infections, Review of Current Evidence and Recommendations. Chest Journal. 2007;132(1):286. doi: 10.1378/chest.06-2156. [DOI] [PubMed] [Google Scholar]
- Jeong S.H., Yeo S.Y., Yi S.C. The Effect of Filler Particle Size on the Antibacterial Properties of Compounded Polymer/silver Fibers. Journal of Materials Science. 2005;40(20):5407. [Google Scholar]
- Jeong S.H., Hwang Y.H., Yi S.C. Antibacterial Properties of Padded PP/PE Non-woven Incorporating Nano-sized Silver Colloids. Journal of Materials Science. 2005;40(20):5413. [Google Scholar]
- Kenawy E., Worley S.D., Broughton R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art. Biomacromolecules. 2007;8(5):1359. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- Kumar R., Münstedt H. Silver Ion Release from Antimicrobial Polyamide/silver Composites. Biomaterials. 2004;26(14):2081. doi: 10.1016/j.biomaterials.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Lenoir S., Pagnoulle C., Galleni M., Compère P., Jérôme P., Detrembleur C. Polyolefin Matrixes with Permanent Antibacterial Activity: Preparation, Antibacterial Activity, and Action Mode of the Active Species. Biomacromolecules. 2006;7(8):2291. doi: 10.1021/bm050850c. [DOI] [PubMed] [Google Scholar]
- Liu S., Sun G. Durable and Regenerable Biocidal Polymers: Acyclic Nhalamine Cotton Cellulose. Industrial and Engineering Chemistry Research. 2006;45(19):6477. [Google Scholar]
- Marini M., Niederhausern S.D., Iseppi R., Bondi M., Sabia C., Toselli M., Pilati F. Antibacterial Activity of Plastics Coated with Silver-Doped Organic-Inorganic Hybrid Coatings Prepared by Sol-Gel Processes. Biomacromolecules. 2007;8(4):1246. doi: 10.1021/bm060721b. [DOI] [PubMed] [Google Scholar]
- McCubbin P.J., Forbes E., Gow M.M., Gorham S.D. Covalent Attachment of Quaternary Ammonium Compounds to a Polyethylene Surface via a Hydrolysable Ester Linkage: Basis for a Controlled-release System of Antiseptics from an Inert Surface. Journal of Applied Polymer Science. 2006;100(1):538. [Google Scholar]
- McCubbin P.J., Forbes E., Gow M.M., Gorham S.D. Novel Self-Disinfecting Surface. Journal of Applied Polymer Science. 2006;100(1):381. [Google Scholar]
- Mosleh S., Gawish S.M., Sun Y. Characteristic Properties of Polypropylene Cationic Fabrics and their Derivatives. Journal of Applied Polymer Science. 2003;89(11):2917. [Google Scholar]
- Mosleh S., Gawish S.M., Khalil F.H., Bieniek R.F. Properties and Application of Novel Amphoteric Polypropylene Fabrics. Journal of Applied Polymer Science. 2005;98(6):2373. [Google Scholar]
- Nho Y.C., Park J.S., Jin J.H., Kwon O.H. Antibacterial Activity of Sulfonated Styrene-grafted Polypropylene Fabric and its Metallic Salt. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry. 1999;A36:731. [Google Scholar]
- Park J.S., Kim J.H., Nho Y.C., Kwon O.H. Antibacterial Activities of Acrylic Acid-grafted Polypropylene Fabric and its Metallic Salt. Journal of Applied Polymer Science. 1998;69(11):2213. [Google Scholar]
- Pasquini N. Polypropylene – The Business. In: Pasquini N., editor. Polypropylene Handbook. Hanser; Munich: 2005. pp. 491–569. [Google Scholar]
- Radheshkumar C., Münstedt H. Antimicrobial Polymers from Polypropylene/silver Composites – Ag+Release Measured by Anode Stripping Voltammetry. Reactive and Functional Polymers. 2006;66(7):780. [Google Scholar]
- Saygun O., Avalar C., Aydinuraz K., Agalar F., Daphan C., Saygun M., Ceken S., Akkus A., Denkbas E.B. Gold and Gold-Palladium Coated Polypropylene Grafts in a S. epidermidis Wound Infection Model. Journal of Surgical Research. 2006;13(1):73. doi: 10.1016/j.jss.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Sen K. Polypropylene Fibers. In: Gupta V.B., Kothari V.K., editors. Manufactured Fiber Technology. Chapman & Hall; London: 1997. pp. 457–479. [Google Scholar]
- Seyfriedsberger G., Rametsteiner K., Kern W. Polyethylene Compounds with Antimicrobial Surface Properties. European Polymer Journal. 2006;42(12):3383. [Google Scholar]
- Shin Y., Yoo D.I., Min K. Antimicrobial Finishing of Polypropylene Nonwoven Fabric by Treatment with Chitosan Oligomer. Journal of Applied Polymer Science. 1999;74(12):2911. [Google Scholar]
- Sun G. In: Edwards J.V., Vigo T.L., editors. Durable and Regenerable Antimicrobial Textiles; American Chemical Society, Symposium Series No. 792. Bioactive Fibers and Polymers; 2001. pp. 243–252. [Google Scholar]
- Sun G., Worley S.D. Chemistry of Durable and Regenerable Biocidal Textiles. Journal Chemical Education. 2005;82(1):60. [Google Scholar]
- Sun G., Xu X. Durable and Regenerable Antibacterial Finishing of Fabrics. Textile Chemist and Colorist. 1998;30(6):26. [Google Scholar]
- Sun G., Xu X. Durable and Regenerable Antibacterial Finishing of Fabrics: Chemical Structure. Textile Chemist and Colorist. 1999;31(5):31. [Google Scholar]
- Sun G., Xu X. Durable and Regenerable Antibacterial Finishing of Fabrics: Fabric Properties. Textile Chemist and Colorist. 1999;31(1):21. [Google Scholar]
- Sun G., Xu X., Bickett J.R., Williams J.F. Durable and Regenerable Antimicrobial Finishing of Fabrics with a New Hydantoin Derivative. Industrial Engineering Chemistry Research. 2001;41:1016. [Google Scholar]
- Sun Y., Chen T.Y., Worley S.D., Sun G. Novel Refreshable N-Halamine Polymeric Biocides-Containing Imidazolidin-4-one Derivatives. Journal of Polymer Science, Polymer Chemistry. 2001;39(18):3073. [Google Scholar]
- Sun Y., Sun G. Novel Regenerable N-halamine Polymeric Biocides I: Synthesis, Characterization and Antimicrobial Activity of Hydantoin-containing Polymers. Journal of Applied Polymer Science. 2001;80(13):2460. [Google Scholar]
- Sun Y., Sun G. Novel Regenerable N-halamine Polymeric Biocides II: Grafting Hydantoin-containing Monomers onto Cotton Cellulose. Journal of Applied Polymer Science. 2001;81(3):617. [Google Scholar]
- Sun Y., Sun G. Novel Regenerable N-halamine Polymeric Biocides III: Grafting Hydantoin-containing Monomers onto Synthetic Fabrics. Journal of Applied Polymer Science. 2001;81(6):1517. [Google Scholar]
- Sun Y., Sun G. Durable and Refreshable Polymeric N-Halamine Biocides Containing 3-(4′-vinylbenzyl)-5,5-dimethylhydantoin. Journal of Polymer Science, Polymer Chemistry. 2001;39(19):3348. [Google Scholar]
- Sun Y., Sun G. Synthesis, Characterization and Antibacterial Activities of Novel N-halamine Polymer Beads Prepared by Suspension Copolymerization. Macromolecules. 2002;35:8909. [Google Scholar]
- Sun Y., Sun G. Durable and Regenerable Antimicrobial Textile Materials Prepared by a Continuous Grafting Process. Journal of Applied Polymer Science. 2002;84(8):1592. [Google Scholar]
- Sun Y., Sun G. Novel Refreshable N-Halamine Polymeric Biocides: Grafting Hydantoin-containing Monomers onto High-Performance Fibers by a Continuous Process. Journal of Applied Polymer Science. 2003;88(4):1032. [Google Scholar]
- Sun Y., Sun G. Novel Refreshable N-Halamine Polymeric Biocides: Nchlorination of Aromatic Polyamides. Industrial and Engineering Chemistry Research. 2004;43:5015. [Google Scholar]
- Tan S., Li G., Shen J., Liu Y., Zong M. Study of Modified Polypropylene Non-woven Cloth. II. Antibacterial Activity of Modified Polypropylene Non-woven Cloths. Journal of Applied Polymer Science. 2000;77:1869. [Google Scholar]
- Thomassin J.M., Lenoir S., Riga J., Jérôme R., Detrembleur C. Grafting of Poly [2-(tert-butylamino) ethyl methacrylate] onto Polypropylene by Reactive Blending and Antibacterial Activity of the Copolymer. Biomacromolecules. 2007;8:1171. doi: 10.1021/bm0611228. [DOI] [PubMed] [Google Scholar]
- Vartiainen J., Rättö M., Tapper U., Paulussen S., Hurme E. Surface Modification of Atmospheric Plasma Activated BOPP by Immobilizing Chitosan. Polymer Bulletin. 2005;54(4–5):343. [Google Scholar]
- Vigo T.L., Danna G.F. Magnesium Hydroperoxyacetate (MHPA) and Magnesium Dihydroperoxide (MDHP): New Antibacterial Agents for Fibrous Substrates. Polymers for Advanced Technologies. 1995;7(1):17. [Google Scholar]
- Wafa D.M., Breidt F., Gawish S.M., Matthews S.R., Donohue K.V., Roe R.M., Bourham M.A. Atmospheric Plasma-aided Biocidal Finishes for Non-woven Polypropylene Fabrics. II. Functionality of Synthesized Fabrics. Journal of Applied Polymer Science. 2007;103(3):911. [Google Scholar]
- Wang C.C., Chen C.C. Antibacterial and Swelling Properties of Acrylic Acid Grafted and Collagen/Chitosan Immobilized Polypropylene Non-woven Fabrics. Journal of Applied Polymer Science. 2005;98(1):391. [Google Scholar]
- Wang H., Wang J., Hong J., Wei Q., Gao W., Zhu Z. Preparation and Characterization of Silver Nanocomposite Textile. Journal of Coatings Technology and Research. 2007;4(1):101. [Google Scholar]
- Weng Y.H., Hotchkiss J.H. Anhydrides as Antimycotic Agents Added to Polyethylene Films for Food Packaging. Packaging Technology & Science. 1993;6(3):123. [Google Scholar]
- Xing C.M., Deng J.P., Yang W.T. Synthesis of Antibacterial Polypropylene Film with Surface Immobilized Polyvinylpyrrolidone-Iodine Complex. Journal of Applied Polymer Science. 2005;97(5):2026. [Google Scholar]
- Yamanaka M., Hara K., Kudo J. Bactericidal Actions of a Silver Ion Solution on Escherichia coli, Studied by Energy-Filtering Transmission Electron Microscopy and Proteomic Analysis. Applied & Environmental Microbiology. 2005;71(11):7589. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.M., Lin H.T., Wu T.H., Chen C.C. Wettability and Antibacterial Assessment of Chitosan Containing Radiation-induced Graft Non-woven Fabric of Polypropylene-g-Acrylic Acid. Journal of Applied Polymer Science. 2003;90(5):1331. [Google Scholar]
- Yeo S.Y., Jeong S.H. Preparation and Characterization of Polypropylene/silver Nanocomposite Fibers. Polymer International. 2003;52(7):1053. [Google Scholar]
- Yeo S.Y., Lee H.J., Jeong S.H. Preparation of Nanocomposite Fibers for Permanent Antibacterial Effect. Journal of Materials Science. 2003;38(10):2143. [Google Scholar]
- Zerr D.M., Garrison M.M., Allpress A.L., Heath J., Christakis D.A. Infection Control Policies and Hospital-associated Infections among Surgical Patients: Variability and Associations in a Multicenter Pediatric Setting. Pediatrics. 2005;115(4):e387. doi: 10.1542/peds.2004-2014. [DOI] [PubMed] [Google Scholar]
- Zhang W., Chu P.K., Ji J., Zhang Y., Fu R.K.Y., Yan Q. Antibacterial Properties of Plasma-modified and Triclosan or Bronopol Coated Polyethylene. Polymer. 2006;47(3):931. [Google Scholar]
- Zhang W., Chu P.K., Ji J., Zhang Y., Ng S.C., Yan Q. Surface Antibacterial Characteristics of Plasma-modified Polyethylene. Biopolymers. 2006;83(1):62. doi: 10.1002/bip.20527. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang Y.H., Ji J.H., Zhao J., Yan W., Chu P.K. Antimicrobial Properties of Copper Plasma-modified Polyethylene. Polymer. 2006;47(21):7441. [Google Scholar]
- Zhang W., Ji J., Zhang Y., Yan Q., Kurmaev E.Z., Moewes A., Zhao J., Chu P.K. Effects of NH3, O2, and N2 Co-implantation on Cu Out-diffusion and Antimicrobial Properties of Copper Plasma-implanted Polyethylene. Applied Surface Science. 2007;253(22):8981. [Google Scholar]
- Zhang W., Zhang Y., Ji J., Yan Q., Huang A., Chu P.K. Antimicrobial Polyethylene with Controlled Copper Release. Biomedical Material Research A. 2007;83(3):838. doi: 10.1002/jbm.a.31436. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jiang J., Chen Y. Migration of Antimicrobial Agents in the Polypropylene Fibers. Polymer-Plastics Technology and Engineering. 2000;39:223. [Google Scholar]


