Abstract
Lung disease is common in the tropics; lower respiratory tract infections are a major cause of mortality, especially in children under 5 years of age. The World Health Organization has launched strategies to tackle this killer of children. Infections, including tuberculosis and drug-resistant tuberculosis, find vulnerable hosts in the tropics where human immunodeficiency virus co-infection is widespread, especially in India and sub-Saharan Africa. Parasitic infections can cause pulmonary manifestations (e.g., pleural effusion and cavitary lesions). Blood and pulmonary eosinophilia are common in such infections. Non-communicable lung disease such as chronic obstructive pulmonary disease and lung cancer associated with tobacco use, contribute to mortality, particularly where there is poor access to health structures.
Keywords: pneumonia, tuberculosis, HIV co-infection, parasitic infections, tropical pulmonary eosinophilia, non-communicable lung diseases
Key Features
-
•
Pneumonia is a major cause of death in the tropics, especially in children under 5 years old.
-
•
Symptoms and physical examination determine care-seeking behaviors and clinical management.
-
•
Most new tuberculosis cases occur in the tropics, often with human immunodeficiency virus (HIV) co-infection.
-
•
Parasitic infections can manifest as wheezing, eosinophilic pneumonia, pleural effusion, and cavitary lesions.
-
•
The impact of non-communicable disease (e.g., chronic obstructive pulmonary disease [COPD] and lung cancer) on mortality is projected to rise in low- and middle-income countries.
Introduction
The term tropics refers to the region of earth lying between the Tropic of Cancer and the Tropic of Capricorn. In the tropics, warm climate, poverty, lack of education, and poor sanitation provide an ideal environment for pathogens, vectors, and intermediate hosts to flourish.1 In this vast landmass, respiratory infections are a major cause of morbidity and mortality. Respiratory infections may be due to common pathogens encountered worldwide, as well as mycobacterial, parasitic, and fungal organisms. Infections are more prominent in immunocompromised hosts. On the other hand, non-communicable diseases (chronic obstructive pulmonary disease [COPD], chronic respiratory diseases, lung cancer, occupational lung diseases) are increasingly recognized in the tropics, where low socio-economic, educational, and nutritional status favor worse prognosis. A reasonable approach to the patient with lung disease in the tropics starts with age, occupational exposure, physical examination, HIV status, chest x-ray, and blood tests.
Pneumonia
World Health Organization (WHO) data showed that pneumonia is the leading cause of death in children under 5 years of age, and is responsible for 16% of deaths (Fig. 1.1 ). The incidence and mortality in the tropics are particularly increased, especially in South Asia and sub-Saharan Africa (Fig. 1.2 ). In Tanzania, 85% of children with suspected pneumonia are taken for care; however, proper care depends on family income, with the richest families seeking care 9.5 times more often. In Ethiopia only 30% of children with pneumonia were taken for care in a facility or in the community.2 WHO data reveal that as few as 39% of newborns are breastfed, 60% of children with suspected pneumonia access care, and 31% receive antibiotics. Poor vaccination, sanitation, and nutrition; crowded homes; indoor air pollution; and smoking all adversely affect pneumonia outcomes in the tropics.
Fig. 1.1.

Child causes of death, 2000-2015
(Global health estimates technical paper: WHO/HIS/IER/GHE/2016.1)
Fig. 1.2.

Ten countries with the highest mortality from pneumonia.
(Source: WHO Pneumonia and Diarrhea Report 2012; 2004 global burden of disease sub-analysis.)
Common pathogens include bacteria, viruses, and fungi. Streptococcus pneumoniae is the most common bacterial cause of pneumonia followed by Haemophilus influenzae type b. Staphylococcus aureus pneumonia accounts for 2% to 10% of acute community-acquired pneumonias.
Atypical pneumonias caused by Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella spp., viruses, fungi, and parasites occur. Respiratory syncytial virus (RSV) is the most common etiology of viral pneumonia. In HIV-infected infants, fungal pneumonia due to Pneumocystis jirovecii (formerly P. carinii) accounts for 25% of all pneumonia deaths. M. pneumoniae infections occur worldwide, affecting mostly school-aged children and young adults. The tropical physician should be aware of the non-respiratory manifestations of mycoplasma infection, including anemia, myringitis, Stevens–Johnson syndrome, hepatitis, and neuritis.
In 2002 to 2003, a coronavirus was responsible for more than 8000 cases of a severe acute respiratory syndrome (SARS) that spread via international travel across continents from its origin in Guangdong Province, China. After droplet inhalation of the virus, there was an incubation period of 2 to 7 days, then fever, cough, malaise, and headache. Pulmonary inflammation was characterized by desquamation of pneumocytes, hyaline membrane formation, and acute respiratory distress syndrome (ARDS). Recovery could be slow, and some patients developed fibrosis. Mortality was 10% to 20%, with the elderly and those with cardiovascular problems being especially at risk.
In 2012 another novel coronavirus appeared in Saudi Arabia, named Middle East Respiratory Syndrome coronavirus (MERS-CoV). Between 2012 and 2017, 2040 laboratory-confirmed cases have been reported to WHO, with small outbreaks outside of the Middle East. Transmission has occurred from human to human, but requires close contact and has particularly been seen in health care facilities between patients or between patients and health care providers. A dromedary camel reservoir is postulated. Initial symptoms are fever, cough, and shortness of breath, with disease severity ranging from asymptomatic or mild common cold symptoms to SARS. Thirty-five percent of confirmed cases have died.
Influenza viruses with pandemic potential are a risk for global disease spread.3 East Asia has seen continued outbreaks of avian influenza (e.g., H5N1, H7N9, and H9N2) with occasional spread to humans. In 2009–2010, a swine influenza virus (H1N1) originating in Mexico led to a global pandemic.
Investigations and Management
Sputum examination is an important aid in the diagnosis of pneumonia: color, amount, consistency, and odor. Mucopurulent sputum is commonly found in bacterial pneumonia or bronchitis. Scanty, watery sputum is often noted in atypical pneumonia; “rusty” sputum is seen in pneumococcal pneumonia; and currant-jelly or dark-red sputum suggests Klebsiella pneumoniae. Foul-smelling sputum is associated with anaerobic infections due to aspiration, lung abscess, and necrotizing pneumonia. A blood count usually reveals leukocytosis in bacterial pneumonia, a normal white cell count or leukopenia in viral infection, and eosinophilia in parasitic infection. When available, chest x-ray, serum procalcitonin, and C-reactive protein can be obtained (Fig. 1.3 ). Naso-pharyngeal swabs for polymerase chain reaction (PCR) can help establish the diagnosis of specific viral infections if available.
Fig. 1.3.
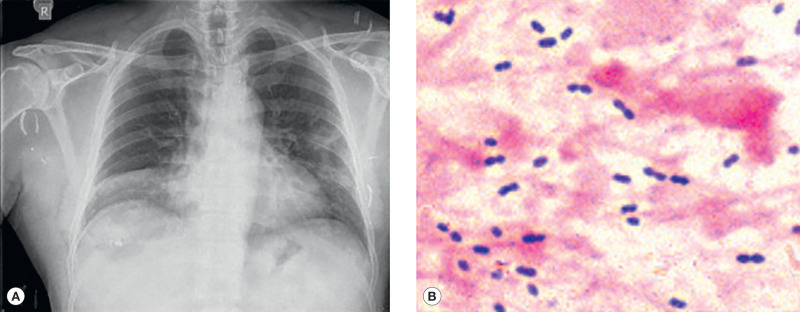
(A) Pneumococcal pneumonia, right middle lobe. (B) Gram-positive diplococci (sputum).
In children, the Integrated Management of Childhood Illness guidelines for treating pneumonia are recommended (Table 1.1 ).4 Childhood pneumonia is now classified in two classes: tachypnea and chest in-drawing indicate treatment with oral antibiotics, whereas severe pneumonia with danger signs (hypothermia, unconsciousness, convulsions) requires intravenous treatment in hospital.
TABLE 1.1.
Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities
|
|
|
|
|
WHO's strategy to reduce pneumonia burden has three components: protection, by promoting breastfeeding and vitamin A supplementation; prevention, using vaccines for measles, H. influenzae, pertussis, rotavirus, and S. pneumoniae and promoting handwashing, sanitation, HIV prevention, and cotrimoxazole prophylaxis in HIV-infected patients; and treatment, securing care seeking, proper case management, and antibiotic administration.
Tuberculosis
Tuberculosis (TB) is the most common infectious cause of death worldwide, surpassing both HIV and malaria.5 In 2015, 10.4 million incident cases were reported worldwide—480,000 with multi-drug resistant tuberculosis (MDR-TB).6 About half of these cases come from the tropics (India, Indonesia, Pakistan, South Africa, Nigeria) (Fig. 1.4 ). MDR-TB as well as extensively drug-resistant TB (XDR-TB) are increasing in African and Asian regions.7
Fig. 1.4.

Estimated TB incidence rates, 2015.
(Source: WHO: 2016 Global Tuberculosis Report.)
Morbidity and mortality from TB increase with HIV co-infection and concurrence of diabetes mellitus. Refugee and migrant camps are hot-spots for TB transmission and spread. In Africa, the Ebola virus epidemic reduced TB as well as measles vaccination and favored disease spread. In Africa, 31% of TB cases have HIV co-infection, rising to 50% in regions of southern Africa.
Strategies to control TB are to monitor children under 5 years of age with exposure to TB cases, diagnose TB in HIV-infected subjects, implement modern diagnostic tools such as Xpert MTB/RIF that identifies the presence of TB and rifampin resistance, and make available anti-tuberculous drugs.
Parasitic and Other Pulmonary Infections in the Tropics
These infections include malaria, pulmonary schistosomiasis, amebiasis, melioidosis, paragonimiasis, echinococcal cysts, Chagas disease, ascariasis, strongyloidiasis, filariasis, and tropical eosinophilia (Table 1.2 ).8, 9, 10 Individuals who come in contact with birds or animals may develop zoonoses such as tularemia, psittacosis, Q fever, and leptospirosis.
TABLE 1.2.
Key Pulmonary Manifestations of Parasitic Infections With or Without Eosinophilic Pneumonia
| Schistosomiasis | Portal-pulmonary hypertension, arterio-venous fistulas |
| Amebiasis | Direct lung invasion through liver, pleuro-pulmonary involvement, anchovy sauce content |
| Malaria | Adult respiratory distress syndrome |
| Trypanosoma cruzi | Chagas disease |
| Ascariasis | Bronchopneumonia |
| Strongyloides stercoralis | Hyper-infection |
| Filariasis | Pulmonary infarction, milky pleural fluid |
| Echinococcus | Cyst, empyema, pneumothorax, hemoptysis, anaphylaxis |
| Paragonimus westermani | Eosinophilic pneumonia/pleural effusion |
| Melioidosis | Upper lobe cavity, acute/chronic infection, hemoptysis |
| Wuchereria bancrofti, Brugia malayi | Tropical pulmonary eosinophilia |
Leptospirosis is common in tropical areas where sanitation is poor and the water not adequately treated. Epidemics of leptospirosis occur after high rainfall in monsoon seasons when the water supply is contaminated by sewage or animal urine. About half of the patients with leptospirosis have fever, cough, hemoptysis, and pneumonitis.11 Other features are jaundice, conjunctivitis, and impaired renal function.
Melioidosis, caused by Burkholderia pseudomallei, is endemic in Southeast Asia (Vietnam, Cambodia, Myanmar), northern Australia, and West Africa. Melioidosis is hyperendemic in northern Australia and in parts of northeastern Thailand and is an important cause of fatal community-acquired pneumonia. Chronic infection in patients with cystic fibrosis can occur.12 The radiologic picture of upper lobe infiltration and cavity formation can be indistinguishable from TB. The mortality rate ranges from 20% to 50% but is higher in HIV-infected and immunocompromised hosts.
Respiratory symptoms of cough and chest pain in typhoid are present in up to 50% of cases at the onset of the disease. Pulmonary infiltrates may be associated with positive sputum cultures for Salmonella typhi. A fever chart showing continuous fever is highly suggestive of enteric fever. Diagnosis may be difficult without the ability to culture blood and stool.
In brucellosis, the lungs are involved in about 5% to 10% of cases, usually after inhalation of organisms. Abnormalities include bronchopneumonia, solitary or multiple lung nodes, miliary interstitial lung disease, lung abscess, and pleural effusion. Organisms can be identified on stains or sputum cultures.
Tularemia is a generalized infection caused by Francisella tularensis and occurs after skin or mucous membrane contact with infected mammals or through the bite of an arthropod, usually a tick or biting fly. Diagnosis should be considered when there is a skin ulcer associated with fever, generalized lymphadenopathy, cough, and signs of pneumonia. Pneumonia, either primary from inhalation of an infected aerosol or secondary to systemic infection, occurs in about 20% of cases.
Pneumonic plague is less common than either bubonic or septicemic disease. Nevertheless, fatal bronchopneumonia can occur without lymphadenopathy and is characterized by watery, bloody sputum. A sputum Gram stain can show bipolar stunted rods. Pneumonic plague and tularemic pneumonia should be considered when a severe, rapidly progressive bronchopneumonia is reported in an endemic area and “typical” bacterial pneumonias have been ruled out.
In slaughterhouses, meat-processing plants, and areas with sheep and goat husbandry, Q fever (Coxiella burnetii) can cause epidemics of atypical pneumonia. Inhalation of dried infected material is the chief source; fever, headache, and dry cough are the main symptoms. Occasionally, the sputum is blood-streaked.
Bornholm's disease (caused by coxsackie viruses and occasionally other enteroviruses), also known as epidemic pleurodynia or devil's grip, causes chest pain and cough. Widespread epidemics of Bornholm's disease occur in the Pacific islands and South Africa.
Kawasaki's disease occurs in children under 5 years of age. This acute multi-system disease of unknown cause is characterized by fever of 5 days' duration and four of five clinical features: non-purulent conjunctivitis; fissured lips, strawberry tongue; cervical lymphadenopathy; a maculopapular rash; and changes in the extremities with erythema and edema of the hands and feet. Pneumonitis occurs in 10% of the children and coronary artery dilatation and aneurysms in 20% to 25% of untreated cases. In Brazil, there has been a seasonal rise of the condition at the beginning and end of the monsoon season.13
Cryptococcus neoformans and C. gatti are saprophytic fungi distributed worldwide and are particularly abundant in soil in the tropics as well as in temperate countries. Pulmonary infection results from inhalation of the organisms from environmental sources. C. neoformans is the most common cause of meningoencephalitis in AIDS patients.
Eosinophilic Pneumonias
Systemic helminth infection usually elicits eosinophilia and increased levels of IgE. Although eosinophilia can be a clue to a pulmonary helminth infestation, the definitive diagnosis requires demonstration of ova or larvae in sputum, bronchial alveolar lavage fluid, pleural fluid, or lung biopsy.14 Loeffler's syndrome refers to “simple” pulmonary eosinophilia with no or minimal systemic and pulmonary symptoms. In many helminth infections (Ascaris, strongyloidiasis, hookworm), the larvae migrate through the lung and in heavy infections can cause fever, cough, dyspnea, wheezing, hemoptysis, and lung infiltrate.
Schistosomes cause two clinical syndromes. In acute disease, immature schistosomula pass through the lung and can lead to fever, eosinophilia, and pulmonary infiltrate. In chronic schistosomiasis, especially when portal hypertension has led to venous shunts, eggs can bypass the liver and plug pulmonary capillaries and arterioles, producing granuloma and pulmonary hypertension. Radiographs may show dilated pulmonary arteries and arterio-venous abnormalities (Fig. 1.5 ).15
Fig. 1.5.

Bilateral pulmonary artery dilatation in schistosomiasis.
In paragonimiasis, the lung is the predominantly involved organ. The diagnosis must be considered in a patient from Southeast Asia with cough, hemoptysis (which is recurrent in >80% of cases), a pulmonary cavity, and pleural effusion.
Tropical pulmonary eosinophilia, typically in India and other South Asian countries, is caused by immunologic hyperresponsiveness to Wuchereria bancrofti, Brugia malayi, or other microfilariae. Clinical presentation consists of nocturnal cough, wheezing, fever, and weight loss. Chest radiographs show diffuse interstitial miliary infiltrates (Fig. 1.6 ); there is a high eosinophil count. In developed countries, serum IgE and anti-filarial antibodies can be used to confirm the diagnosis.
Fig. 1.6.

Bilateral interstitial opacities affecting all lung fields in a patient with tropical pulmonary eosinophilia.
Non-Communicable Lung Diseases
Non-communicable diseases (NCD) of the lung refer to non-infectious chronic respiratory diseases, such as COPD, bronchial asthma, lung cancer, and bronchiectasis.
NCDs now represent an increasing portion of global morbidity and mortality burden in low- and middle-income countries. This is due to increased tobacco use and to low overall health and nutritional status, resulting in excess probability of mortality from NCD as shown in Fig. 1.7 . In 2015 mortality in low-income countries accounted for 75% of overall global mortality. Most of the deaths occurred in South Asia and sub-Saharan Africa where mortality from NCDs and injuries often exceeded traditional infectious causes.16, 17
Fig. 1.7.
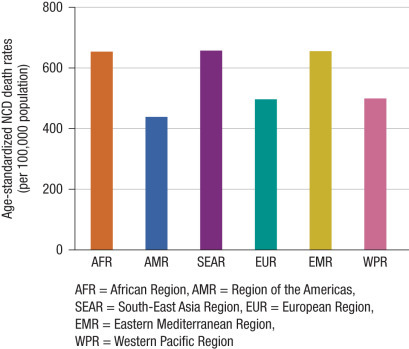
High and increasing probability of mortality from non-communicable diseases in 2012.
(Source: WHO: Global status report on non-communicable diseases 2014.)
The incidence of asthma in the tropics is low for unclear reasons; however, “all that wheezes is not asthma” is a dictum that is true in the tropics, as there are many entities that cause wheezing and difficulty in breathing, including tropical eosinophilia and mitral stenosis. Asthma monitoring in the tropics can be achieved by using an inexpensive peak flow meter.
COPD is a progressive disease characterized by fixed airway obstruction. Causes of COPD in the tropics are increasing tobacco use and the widespread use of dung and biomass for indoor cooking and heating, demonstrated in one study in Indian males to decrease FEV1 by 70 mL per year.18 The most common symptoms are dyspnea and chronic cough. The onset of dyspnea is insidious. With progression of airway obstruction, patients become short of breath at rest. When chest x-ray and pulmonary function testing are not available, a peak flow meter is an inexpensive device to assess severity of airway obstruction and monitor the response to treatment.
Bronchiectasis is a chronic, debilitating condition. Dilatation and distortion of the airways lead to impaired mucociliary clearance, which encourages bacterial colonization and bronchial inflammation. The diagnosis of bronchiectasis in developed countries is confirmed by computed tomography of the chest (Fig. 1.8 ), whereas in the tropics, the diagnosis is mainly clinical and depends on a compatible history, presence of finger clubbing, sputum that settles into three layers (mucoid or frothy, mucopurulent, and purulent), and a chest x-ray, if available.
Fig. 1.8.

Computed tomography of the chest: cylindrical and cystic bronchiectasis.
Pleural Effusion
Pleural effusion is frequent and has variable clinical signs and symptoms. Large effusions are associated with dyspnea and diminished chest movements on the affected side.
If possible, all but the smallest effusions should be tapped determining whether the fluid is serous, bloody, chylous, or contains pus. The effusion can be further divided into transudative and exudative, according to pleural fluid characterization. Laboratory tests that guide the management of a pleural effusion are macroscopic appearance, pleural fluid cell counts, biochemistry, pH, and Gram stain. A simple test is centrifugation of the fluid. If an originally “milky” fluid clears with that process, it is presumably an empyema. If not, it is either a chylothorax (pleural fluid triglycerides >110 mg/dL) or a cholesterol effusion (pleural fluid cholesterol >200 mg/dL).
Transudative pleural effusions occur in heart failure, liver disease, endomyocardial fibrosis, hypoproteinemia/malnutrition, and hypothyroidism. The pleural fluid white blood cell count is typically <1000 cells/mm3, the pH >7.2, protein <3.0 g/L, the lactate dehydrogenase (LDH) <200 IU/L, and the glucose ≥60 mg/dL. A bloody effusion is caused by hemothorax, trauma, malignancy, and pulmonary embolism.
Exudative effusions typically have cell counts, protein, and biochemical markers opposite to those of transudates. Exudates can be further classified into neutrophilic, lymphocytic, and eosinophilic. A neutrophilic exudate occurs commonly in bacterial pneumonia cases and can progress to a complicated effusion or to an empyema, both necessitating pleural fluid drainage with a chest tube thoracostomy in addition to antibiotic treatment.
The disease presenting with the highest pleural fluid lymphocytosis is tuberculous pleuritis. A large volume of pleural fluid should be obtained for examination for acid-fast bacilli. In about one-third of cases, the tuberculin skin test is negative initially and converts to positive after 2 to 4 weeks.
An eosinophilic exudate is more common in the tropics. Endemic parasitic and fungal infections are major causes of such an effusion. Ascariasis, echinococcosis, and paragonimiasis are some of the parasitic infections. Paragonimiasis is associated with low pleural fluid glucose and low pH. Fungal diseases responsible for such an effusion are histoplasmosis, cryptococcosis, and coccidioidomycosis.
Non-Tuberculous Granulomatous Lung Disease
In the absence of chest x-ray or biopsy evidence, it is not possible to diagnose pulmonary involvement due to sarcoidosis and other granulomatous diseases. Consequently, in the tropics, these disorders usually remain undiagnosed. The possibility of sarcoidosis should be considered in a patient with dyspnea, uveitis, hepatosplenomegaly, peripheral lymphadenopathy, chronic skin lesions, and a chest x-ray film showing bilateral hilar adenopathy.14
Occupational and Dust Lung Diseases
The occupational disorders result from human social activity, and as such are preventable. The dusts that provoke occupational disorders can be classified into those that induce granulomatous reaction (e.g., beryllium, talc and organic antigens); those that cause fibrosis (e.g., silica, asbestos, and coal); and those that cause neither inflammation nor fibrosis, thus remaining inert (e.g., iron, barium, and tin). Poorly recognized occupational diseases in the tropics are byssinosis (due to cotton dust), mostly in Asia and Africa; bagassosis (due to sugar cane), mostly in Americas, Cuba, and India; and hypersensitivity pneumonitis.
Podoconiosis is an endemic non-filarial elephantiasis occurring in individuals exposed to red clay soil derived from alkaline rock. The silica particles are found in the skin, lymph nodes, and lymphatics of affected and unaffected individuals. These individuals have reduced lung function compared with adults living in areas of low silica concentration.19
References
- 1.Zumla A, James D. Immunological aspects of tropical lung disease. Clin Chest Med. 2002;23:283–308. doi: 10.1016/s0272-5231(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 2.Noordam AC, Carvajal-Velez L, Sharkey AB. Care seeking behavior for children with suspected pneumonia in countries in sub-Saharan Africa with high pneumonia mortality. PLOSONE. 2015;10(2):e0117919. doi: 10.1371/journal.pone.0117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Avian and other zoonotic influenza. http://www.who.int/influenza/human_animal_interface/en/
- 4.World Health Organization . 2012. Revised WHO classification and treatment of childhood pneumonia at health facilities. Evidence Summaries. Recommendations for management of common childhood conditions, Evidence for technical update of pocket book recommendations. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 5.Petersen E, Maeurer M, Marais B. World TB day 2017: advances, challenges and opportunities in the end-TB era. Int J Infect Dis. 2017;56:1–5. doi: 10.1016/j.ijid.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Global Tuberculosis Report 2016. WHO.
- 7.Dheda K, Gumbo T, Maartens G. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017 doi: 10.1016/S2213-2600(17)30079-6. pii: S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 8.Vijayan V. Parasitic lung infections. Curr Opin Pulm Med. 2009;15:274–282. doi: 10.1097/MCP.0b013e328326f3f8. [DOI] [PubMed] [Google Scholar]
- 9.Martinez S, Restrepo S, Carrillo JA. Thoracic manifestations of tropical parasitic infections: a pictorial review. Radiographics. 2005;25:135–155. doi: 10.1148/rg.251045043. [DOI] [PubMed] [Google Scholar]
- 10.2015. Investing to overcome the global impact of neglected tropical diseases. 3rd WHO report on neglected tropical diseases. [PubMed] [Google Scholar]
- 11.Carvalho CRR, Bethlem EP. Pulmonary complications of leptospirosis. Clin Chest Med. 2002;23:469–478. doi: 10.1016/s0272-5231(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 12.Viberg LT, Sarovich DS, Kidd TJ. Within-host evolution of Burkholderia pseudomallei during chronic infection of seven Australasian cystic fibrosis patients. MBio. 2017;8(2) doi: 10.1128/mBio.00356-17. pii: e00356-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magalhaes C, Vasconcelos P, Pereira M. Kawasaki disease: a clinical and epidemiological study of 70 children in Brazil. Trop Doct. 2009;39:99–101. doi: 10.1258/td.2008.080124. [DOI] [PubMed] [Google Scholar]
- 14.Mihailovic-Vucinic V, Sharma O. Tropical granulomas: diagnosis. In: Sharma OP, editor. Lung biology in health and disease: tropical lung disease. 2nd ed. Taylor & Francis; New York: 2006. pp. 173–193. 211. [Google Scholar]
- 15.Goncalves-Macedo L, Coutinho Domingues A, Pessoa Lopes E. Pulmonary shunts in severe hepatosplenic schistosomiasis: diagnosis by contrast echocardiography and their relationship with abdominal ultrasound findings. PLoS Negl Trop Dis. 2017;11(4):e0005417. doi: 10.1371/journal.pntd.0005417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global status report on noncommunicable diseases 2014. WHO. [DOI] [PubMed]
- 17.Kassebaum N, Kyu HH, Zoeckler L. Child and adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA Pediatr. 2017;171(6):573–592. doi: 10.1001/jamapediatrics.2017.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dave M, Ahankari AS, Myles PR. Household air pollution and lung function in Indian adults: a cross-sectional study. Int J Tuberc Lung Dis. 2017;21(6):702–704. doi: 10.5588/ijtld.16.0615. [DOI] [PubMed] [Google Scholar]
- 19.Morrison C, Davey G. Assessment of respiratory function in patients with podoconiosis. Trans R Soc Trop Med Hyg. 2009;103:315–317. doi: 10.1016/j.trstmh.2008.10.021. [DOI] [PubMed] [Google Scholar]


