Publisher Summary
This chapter provides an overview of the biology and medical care of rabbits. There are about 50 different breeds of domestic rabbits. Rabbits have very thin and delicate skin that is covered with fine fur comprised of both a soft undercoat and stiff guard hairs. Care must be taken when clipping fur because the skin is prone to tearing. A rabbit enclosure should be large enough to provide a sleeping space, eating space, and latrine. Animals housed for long periods of time should also have ample room to exercise. The enclosure should be tall enough to allow the rabbit to sit up and not have its ears touch the top of the cage. Because rabbits are prone to heatstroke, they should be housed in temperatures ranging from 60°F to 75°F. If rabbits are housed outdoors, they must have access to shade and clean water, and the shelter should protect them from the elements as well as from predators. The ideal substrate for rabbits is grass hay; however, for indoor, caged rabbits, a foam rubber pad or a towel covered with newspaper and a thick layer of timothy hay can also be used.
Domestic rabbits, Oryctolagus cuniculus, belong to the order Lagomorpha, and their ancestors are from Western Europe and northwestern Africa.1, 2 Unlike rodents, lagomorphs have a second set of maxillary incisors directly caudal to the first set. Pet rabbits have unique, lively, and affectionate personalities that make them ideal pets for mature children and adults. Handling pet rabbits when they are young will likely make them more comfortable with humans later in life. In contrast, wild rabbits, no matter their age, do not become comfortable with human interaction. Rabbit owners commonly seek medical attention for their rabbits and husbandry guidance from veterinarians.
COMMON BREEDSKEPT IN CAPTIVITY
There are as many as 50 different breeds of domestic rabbits.3 However, many of the rabbits presented to private practice are mix breeds. Breed classification can be divided by size or fur type. The size classification is more helpful to the practicing veterinarian and can be divided into small breeds, less than 2 kg (e.g., Netherland dwarfs, lion head, mini-lop, and Dutch breeds); medium breeds, 2 to 5 kg (e.g., Rex, English, Angora, and Belgium hares), and large to giant breeds, more than 5 kg (e.g., New Zealand whites, English lops, British Giants, and Flemish Giants). The American Rabbit Breeders Association publishes information on the different breeds and various standards, and is an excellent resource for veterinarians (http://www.arba.net/).
Certain breeds are predisposed to health problems. For example, dwarf breeds, such as the Netherland rabbits, have short maxillae that make them more prone to nasolacrimal duct blockage and incisor malocclusion. In addition, dwarf breeds appear more susceptible to Encephalocytozoon cuniculi infections.2 Dutch, Havana, tan, and French silver breeds are more likely to develop uterine neoplasia.4 Because Rex rabbits have thin fur on the plantar surface of their feet, they are more susceptible to developing hock sores. Large and giant breeds are prone to developing arthritis and cardiomyopathies. British Giant and French lops are more likely to develop skin fold pyoderma under their dewlaps and in the perineum area, in addition to developing entropion.2
BIOLOGY AND PHYSIOLOGY
See Box 14-1 .
BOX 14-1. Physiologic Parameters for Captive Rabbits.
| Life span | 6-13 yr |
| Gestation | 30-32 days |
| Ages of weaning | 4-6 wk |
| Age of puberty | Small breeds 4-5 mo |
| Large breeds 5-8 mo | |
| Age of recommended neutering | Castration >3 mo, spay >5 mo |
| Temperature (rectal) | 37.8°-39.4° F (100°–103° F) |
| Heart rate | 130-325 beats/min |
| Respiratory rate | 32-60 |
Integumentary System
Rabbits have very thin and delicate skin that is covered with fine fur comprised of both a soft undercoat and stiff guard hairs. Care must be taken when clipping fur because the skin is prone to tearing. Unlike dogs and cats, rabbits lack footpads; instead, their feet are covered with thick coarse fur that protects the plantar and palmar surfaces of the feet. Because they lack footpads, rabbits should be provided soft padded areas within their enclosures. Rabbits have nonretractable claws, making declawing an inappropriate procedure. Certain breeds of female rabbits have a dewlap, which is analogous to a second chin. Female rabbits may pluck fur from the dewlap during the breeding season to build a nest. Male and female rabbits may have three types of scent glands, including a single chin gland, paired inguinal glands, and paired anal glands. The glands are used to mark the rabbit's territory.
Sense Organs
Rabbits’ ears represent a large amount of the animals’ surface area. Because rabbits are low in the food web, these large ears serve an important function in assisting the animal with identifying potential predators. In addition, rabbits’ ears are highly vascularized and serve an important role in thermoregulation via vasoconstriction and vasodilatation.5 Rabbit ears are very sensitive and delicate and should never be used for restraint.
Rabbits have a wide field of vision, which is ideal for a prey species. With the lateral positioning of their eyes and their large corneas, rabbits have an overlapping field of vision of 190 degrees.3 Although rabbits are visually acute within this region, they cannot visualize items below the horizon, including the area below their nose. Instead, rabbits use their highly sensitive vibrissae and lips as tactile structures to distinguish food items. Rabbits possess good night vision and some color vision. Rabbits have functional third eyelids that partially close with sleep or while under anesthesia.5 A harderian gland is found at the base of the third eyelid. If this gland prolapses, then it may present as a mass under the third eyelid. Surgical intervention is recommended to replace the gland. Tear drainage from the eyes occurs via the nasolacrimal gland. There is a single lacrimal punctum located in the craniomedial aspect of the lower eyelid. The punctum empties into a cuniculus, leading to a lacrimal sac and continuing to the base of the maxillary incisor. At this point, the duct sharply changes direction, runs along the incisor root, and emerges in the nasal cavity in the ventromedial aspect of the alar fold.2 Blockage of this duct is common in captive rabbits. Affected animals may have unilateral or bilateral epiphora. Rabbits have a large retrobulbar orbital venous plexus. When an enucleation is performed, this venous plexus must be ligated to minimize hemorrhage.
Musculoskeletal System
Rabbits have a light skeleton surrounded by a very well-developed muscular system, which makes the vertebrae and long bones susceptible to fractures. To minimize the likelihood of injury, the hindlegs should always be supported when transporting the rabbit. The number of vertebrae varies between breeds, from 12 to 13 thoracic and 6 to 7 lumbar vertebrae.6 The epaxial and large muscles of the hindlimbs can be used for intramuscular injections.
Reproductive System
Female rabbits are classified as does, males as bucks, and neonates as kits. The onset of puberty occurs at approximately 4½ months of age; however, reproductive activity is generally dependent upon animal size, not age. In the dwarf breeds, sexual maturity occurs earlier than in larger breeds. The reproductive life of a rabbit is approximately 4 years.1 Rabbits are induced ovulators. Their receptivity periods last approximately 14 to 16 days, followed by 1 to 2 days of nonreceptivity.2 When females are ready to ovulate, they become restless, rub their chins, and assume a lordosis-like posture. Gestation lasts 31 to 32 days, and litters average 4 to 10 kits. As gestation or pseudopregnancy reaches the last 3 to 4 days, does initiate nesting behavior and line their nests with hair from the hip and dewlap.3 During this period, does consume less food, which can cause an increased risk for pregnancy toxemia. Parturition usually occurs in the early morning and lasts less than 30 minutes. Kits generally only suckle 1 or 2 times a day and are not usually seen with the doe, especially in the wild. Weaning occurs at approximately 4-6 weeks of age.
Does have two separate uterine horns and two cervices. Rabbits do not have a uterine body. The cervices open into the vagina, along with the urethra, to form one common urogenital opening. Bucks have a hairless scrotum. The inguinal rings of the buck are open, which allows the testicles to ascend and descend into and out of the abdomen. Male rabbits do not have an os penis or nipples.
Urinary System
The kidneys of rabbits are located in the retroperitoneal cavity, and their position in the body cavity is similar to that in cats. The kidneys are generally covered in fat and can be palpated on the physical examination. The urinary bladder is located in the ventral body cavity, and it is ventral to the colon. Rabbits, like rodents, have a unique system for calcium metabolism. Serum calcium is reflective of dietary calcium, and the urinary system is the primary site of calcium and magnesium excretion. Rabbits that consume a high calcium diet will often have thick and creamy urine due to the formation of calcium carbonate precipitate. On average, rabbits produce 30 to 35 ml of urine daily. Rabbit urine may be straw-colored, yellow, or red. It is not uncommon for rabbit urine to have a turbid appearance. When a rabbit presents with “red” urine, it is important to differentiate whether the urine has blood or porphyrins. Porphyrins can be produced as a result of the ingestion of various vegetables, such as beet root, cabbage, broccoli, and dandelions, or as a result of stress.2
Gastrointestinal System
Rabbits have open-rooted teeth that grow continuously throughout their lives at a rate of approximately 10 to 12 cm a year.1 The dental formula of the rabbit is 2 × (2/1 I, 0/0 C, 3/2 P, 3/3 M). Directly caudal to the maxillary incisors is a second pair of small incisors called peg teeth, whose function is to protect the palate from injury against the sharp surfaces of the lower incisors. The buccal surface of the upper incisors’ enamel is thicker and wears more slowly than the rest of the tooth, forming a chisel-like appearance. Caudal to the incisors is a space called the diasterna.7 The cheek teeth are located caudal to the diasterna and are responsible for macerating and grinding food.
Rabbits have simple, glandular stomachs. Due to the positioning and well-developed nature of the cardiac sphincter, rabbits are unable to vomit.8 Adult rabbits have a gastric pH of 1.5 to 2.2, whereas suckling rabbits have a higher pH of 5.0 to 6.5. The higher gastric pH in neonates is necessary for bacterial colonization of the intestinal tract. This elevated pH also makes suckling rabbits more susceptible to gastrointestinal disease. Dietary absences, especially during weaning, should be gradual, and foods high in sugars and starch should be avoided. Rabbits have a large cecum that acts as an anaerobic fermentation vat. The cecum has a complex and delicate population of microbes. The most common organisms found in the rabbit cecum are the Gram-positive Bacillus spp. In the cecum, fiber is broken down and separated. Rabbits are highly susceptible to antibiotic-induced enterotoxemia, especially when there is Clostridium spiriforme contamination in the environment. The rabbit's gastrointestinal tract is designed to eliminate fiber from the gut rapidly, in the form of hard feces, and digest the nonfiber portion of the diet, creating caecotrophs, which are reingested at a later time. Cecotrophs are covered with a thin mucus, which protects the volatile fatty acids, vitamins, and amino acids from the low pH of the stomach. Cecotrophs differ from feces in that they are soft, moist, usually stuck together, and are eaten directly from the anus.8 The gastrointestinal transit time in rabbits is approximately 20 hours. Rabbits offered a diet deficient in fiber usually have increased gastrointestinal transit times.
The pancreas is found within the mesentery along the lesser curvature of the stomach and extends to the right side of the abdominal cavity to the level of the duodenum. The rabbit pancreas has both exocrine and endocrine functions.
The rabbit liver has a caudate lobe with a narrow stalk, which can displace on occasion. Rabbits have a gall bladder, and the primary function of this structure is to store bile. The rabbit liver performs many of the same functions that the liver plays in the dog and cat.
Respiratory System
Rabbits are obligate nasal breathers. A rabbit that presents for open-mouth breathing generally has a guarded prognosis. The respiratory rate of the rabbit is approximately 30 to 60 breaths per minute. In a healthy animal, the nose will move up and down approximately 20 to 120 times a minute. The thoracic cavity of the rabbit is small in comparison to that of nonlagomorph or rodent mammals. Rabbit lungs have four right and two left lung lobes.3 Rabbits have an extremely narrow and long oral cavity. Because of these features, it can be difficult to visualize the glottis for intubation. Endoscopic examination provides the best visualization of the glottis for intubation. Inflammation of the upper respiratory tract increases the risk of anesthetic mortality in nonintubated animals.4
Cardiovascular System
Rabbits have a relatively small heart, and the average heart rate of the rabbit is 130 to 325 beats per minute.9 Systolic blood pressure is approximately 90 to 120 mmHg. The right atrioventricular valve has only two cusps, and the aorta rhythmically contracts.4 Rabbit veins are thin and susceptible to hematomas, so care must be taken with venipuncture.
HUSBANDRY
Environmental Considerations
ENCLOSURE SIZE
A rabbit enclosure should be large enough to provide a sleeping space, eating space, and latrine. Animals housed for long periods of time should also have ample room to exercise. The enclosure should be tall enough to allow the rabbit to sit up and not have its ears touch the top of the cage. In general, 3 square feet (sq ft) should be a minimum cage size for rabbits 2 to 4 kg, 4 sq ft for rabbits 4 to 4.5 kg, and larger rabbits require at least 5 sq ft.5 Rabbits housed in hutches and cages should be allowed a minimum of 4 hours of exercise daily.2 Indoor rabbits should be caged when they cannot be supervised, to decrease the likelihood of inappropriate chewing of carpet, electrical wire, or furniture. Cages should be cleaned daily to remove feces and urine. Gentle soap and hot water or a dilute bleach solution (1 : 32) should be used for cleaning. If bleach is used, then the cage should be cleaned in a well-ventilated area. Organic material should be removed before applying the bleach, to minimize the degradation of the bleach. The bleach or soap should have a minimum of 3 to 5 minutes of contact time on the cage to increase the killing potential of these products. It is important to clean, rinse, and dry off all detergents.
TEMPERATURE/HUMIDITY
Because rabbits are prone to heatstroke, they should be housed in temperatures ranging from 60° F to 75° F.2 If rabbits are housed outdoors, they must have access to shade and clean water, and the shelter should protect them from the elements as well as from predators. Rabbits are prone to developing fur and skin problems when housed in environments with high humidity; therefore, these animals should be provided environments with low to moderate humidity (30%-60%).
LIGHTING
Rabbits show a diurnal rhythm for eating, activity, and even hematologic variation. Most feeding takes place from after noon to evening. During foraging, rabbits commonly produce fecal pellets; in times of decreased intake, rabbits will actually ingest cecotrophs. In addition, hematologic parameters, such as the total white blood cell count, are lowest in the late afternoons and evenings. Although the eosinophils peak in early afternoon, the heterophils are highest in the late afternoon and early evening. Other changes can be seen with diurnal patterns, including changes in blood urea nitrogen, body temperature, and bile acid production.2 Because much of the rabbits’ behavior and physiology is regulated by diurnal patterns, it is important to provide a consistent light cycle. Although there are no specific lighting recommendations for rabbits, nonreproductive rabbits should be provided a 12-hour photoperiod, and breeding females should be provided 14 to 16 hours of light.10
SUBSTRATE
The ideal substrate for rabbits is grass hay; however, for indoor, caged rabbits, a foam rubber pad or a towel covered with newspaper and a thick layer of timothy hay can also be used.2 Avoid wood shavings, which contain oils that can lead to hepatotoxicity.2 Rabbits can easily be trained to use a litter box filled with straw, hay, or newspaper litter. It is important to keep any substrate clean and dry. If a rabbit is housed on a wire mesh floor, it is important that the mesh openings are no larger than 1 × 2.5 inches to prevent the feet from getting trapped and injured. In addition, it is also important to offer a solid, nonslip surface to provide rest off the mesh and prevent pododermatitis.4
ACCESSORIES
Most cat and bird toys can be used for rabbits. Because rabbits can chew these toys, it is important to be sure that they do not contain any toxins or small materials that can be ingested. Sticks or blocks of wood provide an excellent material for rabbits to file their teeth.
HOSPITALIZATION
In the veterinary hospital, rabbits should be housed in a cage with a nonslip surface on the bottom, such as a towel or thick bedding of fresh straw or hay. Rabbits should be hospitalized in a quiet, stress-free area within the practice, and protected from noises associated with dogs, cats, and ferrets. While in the hospital, rabbits should be offered fresh food and water, preferably what is being fed to them in their home environment. It is important to disinfect and dry the cage and accessories in contact with sick rabbits, using a dilute bleach, Roccal (Pfizer Animal Health, Kalamazoo, MI), or other hospital cleaner.
Nutrition
An average adult house rabbit should be offered ad lib grass hay, such as timothy, prairie, or oat brome; approximately 1 cup of leafy green vegetables; and, at most, ¼ cup of high-fiber (18%-22% to prevent obesity), low-protein (<18%) pellets per 2.2 kg (5 lbs) of body weight daily.4, 8 Examples of dark leafy greens include romaine lettuce, dandelion greens, Swiss chard, parsley, endive, kale, mustard greens, and carrot, beet, and turnip tops. Diets low in fiber, such as commercial, pelleted rations, may be associated with hypomotility, changes in the gastrointestinal pH and microflora, wool block from increased hair consumption, and cheek tooth overgrowth.7 Alfalfa hay should be avoided in adult animals, because of its high protein and calcium content, and instead, reserved for animals less than 6 months of age and for pregnant and lactating does with increased nutritional demands. When offering alfalfa hay to lactating does, slowly increase the amount over the first 5 days, as this will reduce the likelihood of milk overproduction and decrease the chance for the development of mastitis.4 Any diet change should be gradual.
Rabbits ingest cecotrophs formed from fermentation of nonfiber ingesta; these cecotrophs contain volatile fatty acids, vitamins, amino acids, and microorganisms. Cecotrophs are ingested straight from the anus; consuming them allows for absorption of these nutrients that would be lost otherwise. They are covered with a mucus that protects them from breakdown from stomach acids.
Chlorinated water should always be in fresh and ample supply. Rabbits are very sensitive to water deprivation and therefore should experience no restriction. Rabbits generally drink 50 to 100 ml/kg/day.2 Water should be offered in a nonleaking sipper bottle or in a heavy crock that cannot be tipped over. Rabbits that drink from crocks may develop “blue fur,” which is a moist dermatitis of the dewlap that is associated with Pseudomonas infections.
PREVENTIVE MEDICINE
There are no U.S.-approved vaccines for domestic rabbits. It is recommended that all house rabbits receive an annual physical examination, and as they become geriatric (>4 years), a biannual examination is recommended. In addition to the annual examination, a serum chemistry panel, complete blood counts (CBCs), and fecal exams for parasites are also recommended annually. Ovariohysterectomy or orchiectomy should also be recommended for all nonbreeding rabbits to decrease aggression, decrease the incidence of scent marking with urine and feces, and avoid unwanted pregnancy, pseudopregnancy, and neoplasia.
When adding new rabbits into an existing population, it is always recommended to quarantine the new additions for a minimum of 90 days. Separating new arrivals can decrease the likelihood of introducing infectious diseases and parasites to a standing population of rabbits. Before the rabbits are released from quarantine, they should have a CBC and chemistry profile done, and have 3 to 5 negative (2 weeks apart) fecal examinations consecutively. The client should be made aware that even a 90-day quarantine may be insufficient to detect some infectious diseases.
Rabbits' enclosures should be cleaned on a daily basis. Organic material should be removed before disinfectant is applied. Fresh substrate should be offered either daily or as it is soiled. The cage may be disinfected with mild soap and water or a dilute bleach solution (1 : 32). The disinfectant should have a minimum of 3 to 5 minutes of contact time to increase the effectiveness of the compound. The solutions should be thoroughly rinsed away, and the surface of the cage dried completely. Roccal D or chlorhexidine (Fort Dodge Inc., Fort Dodge, IA) can also be used to disinfect rabbit enclosures.
RESTRAINT
Manual Restraint
Rabbit physical restraint needs to be carefully performed to avoid injury to the animal. Because rabbits have a well-developed muscular system and thin cortical bone, they are subject to vertebral and long bone fractures if restrained incorrectly. Because most skeletal injuries associated with incorrect restraint occur in the lumbar vertebrae, it is important to firmly restrain the hindlegs. Rabbits should be handled in a manner similar to cats; place one hand on under the forelimbs, and use the other hand to hold the rear legs against the body. Always place the rabbit onto a nonslip surface to ensure that it has good footing. To restrain the animal, lightly scruff the animal and support its dorsum with the same arm. The opposite arm is used to support the body and rear legs (Figure 14-1 ). It can be helpful to tuck the head of the rabbit into the crook of one's elbow to decrease the rabbit's vision, which can decrease the stress on the animal. Placing a rabbit in dorsal recumbency evokes an immobility response, which can be helpful during simple procedures. However, this response is performed by prey species under stressful or threatening conditions and may not be welcomed by all rabbits (Figure 14-2 ). The immobility response is characterized by a lack of spontaneous movement and a failure to respond to external stimuli. This restraint technique should only be used in those cases where chemical sedation is not desired.2 A towel or blanket is usually necessary during the physical examination to cover the stainless steel exam table and to minimize slipping or kicking, which can lead to injury. In addition, a towel may be used to wrap the rabbit into a “bunny burrito” (Figure 14-3 ). The bunny burrito can be used to hold a rabbit for transport or to facilitate the examination of the head. Cat bags can also be used to restrain rabbits. In addition to using these techniques for examination, they can also be used to administer medication, collect blood samples, or perform nail trims.
Figure 14-1.

Proper restraint while transporting a rabbit. Notice the head is tucked in the crook of the elbow and the dorsum and feet are supported.
Figure 14-2.

Rabbits placed in dorsal recumbency will enter a trance-like state that may facilitate simple procedures, such as taking radiographs.
Figure 14-3.

Rabbits can be wrapped firmly in a towel making a “bunny burrito.” This technique can be used to facilitate an examination, nail trim, or other noninvasive procedure.
Chemical Restraint
Chemical restraint in rabbits should be used to decrease anxiety, and to provide sedation or immobilization for surgery. (The most common drugs used for premedication, sedation, and anesthesia are described later, in Anesthesia.) Isoflurane (Abbott Laboratories, Chicago, IL) or sevoflurane (Abbott Laboratories, Chicago, IL) can be used to anesthetize rabbits; however, in high stress animals, a premedication is usually recommended.
PERFORMING A PHYSICAL EXAMINATION
Before beginning the “hands-on” examination, observe the rabbit at rest, paying close attention to the animal's mentation, how the rabbit is holding its body, if it has signs of respiratory distress, its nose movements, its gait, and fecal and urine output. Very relaxed and very ill rabbits’ noses will be still. Note body condition for obesity, as well as signs of weight loss. When placing a rabbit on an exam table, be sure to offer it a nonslip surface such as a rubber mat or a towel. Routine physical exams should follow a thorough and consistent pattern, examining by system or from the head to the toes, being sure to examine everything in a similar manner to avoid overlooking a potential problem.
A thorough oral examination is an important component to any rabbit examination. Rabbits commonly present to the veterinarian with malocclusions of both incisors and cheek teeth. To examine the incisors, gently lift the lips for inspection. Be sure buccal surfaces are smooth (no horizontal ridges are present) and proper occlusion is met with mandibular incisors slightly caudal to the maxillary ones. A common noninvasive technique for examining the cheek teeth, which can be performed during every examination, is inserting an otoscope or vaginal speculum gently into the diasterna. Position the instrument caudally to visualize molars and premolars from both buccal and lingual surfaces (Figure 14-4 ). This technique does not provide the most thorough examination; however, it is relatively stress-free to the rabbit. If the rabbit is too stressed or uncooperative or a more thorough examination is necessary, lightly sedate the animal with an injectable sedative, or use isoflurane to get a better view. Once sedated, use a mouth speculum, mouth gag, or an endoscope to examine the molars (Figure 14-5 ). The most common locations for molar lesions are on the lingual surface of the mandibular cheek teeth and the buccal aspect of the maxillary teeth. The gingiva surrounding the molars should be at the level of the crowns except for the first mandibular cheek teeth in which the cranial aspect of the crowns will be more exposed. In addition to examining the teeth for overgrowth and malocclusion, it is important to inspect the oral mucosa and tongue for ulcerations and lacerations that may occur as a result of sharp points on teeth. The cheeks and jaws should be palpated for any swellings (e.g., abscesses) and the chin and dewlap examined for signs of drooling or slobbering. Abnormalities may be indicative of dental disease.
Figure 14-4.

An oral examination can be performed using an otoscope.
Figure 14-5.

An oral examination performed under sedation enables the veterinarian to perform a more thorough exam.
Rabbits should be given a thorough ophthalmic examination. Eyes should be closely examined for evidence of discharge, as ocular discharge is one of the most common ocular problems seen in veterinary practice. Purulent epiphora can be due to a variety of problems, including bacterial infection, corneal ulceration, a blocked nasolacrimal duct, or dental disease. The corneas should be clear and free from ulceration. A fluorescein stain should be done if ulceration is suspected. The anterior chamber should be examined closely. Hypopyon is a common finding in bacterial septicemia. The lens should be examined for clarity. Cataracts can occur as a result of congenital or traumatic causes. A fundic examination should be done to rule out retinal defects. Encephalitozoon cuniculi can cause retinal disease.
In addition to ocular discharge, it is important to evaluate the rabbit patient for nasal discharge. Rabbits are meticulous groomers and routinely use their front legs to clean their faces. Animals with nasal discharge routinely have secretions on their forelimbs. In rabbits, nasal discharge has been associated with upper respiratory infections, hypersensitivity (e.g., allergies), foreign bodies, and neoplasia.
An otoscope should be used to examine the ears on both sides of the tragus. Closely inspect the ears for evidence of pus, ceruminous discharge, and mites. With manipulation of the pinna and otoscope, it is possible to visualize deeper in the canal; however, it can still be difficult to visualize the tympanic membrane.
The legs should be palpated from a proximal to distal position. Limb fractures are common sequelae to inappropriate handling. Rabbits do not have footpads. Closely examine the plantar surface of feet for pododermatitis. Pododermatitis is a common problem in rabbits housed in metal-grate caging.
A rectal temperature should be taken during the examination. The body temperature of rabbits is generally between 100° F and 103° F (37.8° C to 39.4° C). To minimize the likelihood of measuring a falsely elevated temperature associated with stress, the body temperature should be collected early in the examination.
The integument should be thoroughly evaluated. Closely inspect the rabbit for alopecia or dandruff, especially near the shoulder blades. Palpate the mammary glands for abnormal masses. Mammary adenocarcinoma and mastitis are common findings in intact female rabbits. Evaluate the perianal area for urine scalding or fecal staining. This is very common in obese rabbits with redundant perianal tissue.
During the abdominal palpation, pay particular attention to the cranial left quadrant (e.g., stomach) and caudal lower quadrant (e.g., uterus, urinary bladder). A firm mass in the cranial abdomen may indicate a trichobezoar or delayed gastric emptying. A swelling or enlargement in the caudal quadrant of an intact doe may suggest pregnancy, pseudopregnancy, pyometra, or uterine adenocarcinoma. Cystic calculi can also occur and be palpated in the caudal abdominal quadrants.
Rabbits are obligate closed-mouth breathers. Open-mouth breathing in rabbits indicates an emergency situation. The heart and lungs should be ausculted. It is normal to hear the rapid movement of the air during inhalation and exhalation. These are usually referred to as sounds from the upper respiratory tract. An absence of these sounds can be associated with consolidation of the lungs. The respiratory rates of rabbits are generally more than 40 breaths per minute. The heart rate should be more than 180 beats per minute. To auscult the heart, place the stethoscope on the chest wall at the point of the elbow.
DIAGNOSTIC TESTING
Rabbits are prey species and have evolved to mask their illnesses. It is imperative that veterinarians working with these animals develop a thorough diagnostic plan to ensure success with diagnosing and treating a case. The following is a review of common diagnostic tests performed on rabbits.
Clinical Pathology
Venipuncture should be done with care, as rabbit vessels are delicate and prone to hematoma formation. The maximum volume of blood that should be collected at one time is 1 ml/100 g body weight.9 Common sites for venipuncture include the jugular, lateral saphenous, cephalic and marginal ear veins. The jugular vein is the preferred site when a large sample is needed. To collect a jugular sample, the animal needs to be properly restrained. For restraint, a rabbit can be placed into a towel or a cat bag, and the neck gently extended in a manner similar to a cat; however, care should be taken not to extend the head too far. In some female rabbits, a large dewlap can reduce access to the jugular vein. Marginal ear veins can be used to collect blood samples with a 25- or 26-gauge needle. Generally, this site can only be used to collect small blood samples. This vein can also be used to place a catheter; however, catheterization can lead to vascular necrosis, thrombosis, and sloughing of the ear and should be avoided in breeds with small ears (e.g., dwarf breeds). The lateral saphenous vein is suitable for small- to moderate-sized blood samples (Figure 14-6 ). Placing alcohol over the venipuncture site or clipping the fur in that area can facilitate visualization of the vein. The cephalic vein can also be used to collect samples; however, this site is often reserved for intravenous catheter placement.
Figure 14-6.

Shaving the lateral hindlimb will facilitate visualization of, and access to, the lateral saphenous vein.
Hematology/Chemistry Panels
CBCs and serum chemistry panels should be performed in rabbits presenting for any disease process and as part of an annual exam. Rabbits have some unique hematologic differences in their CBC compared to other mammals. Healthy rabbits are primarily lymphocytic. Both small and large lymphocytes can be found in circulation. Rabbit lymphocytes are similar in appearance to this cell type in other vertebrates and are characterized by a deep blue cytoplasm, acentric nucleus, and high nuclear-to-cytoplasmic ratio. The acute inflammatory cell in rabbits is the heterophil. Although the function is similar to the neutrophil of other mammals, the staining characteristic of this cell type is different. Monocytes are the largest circulating white blood cell in rabbits and are characterized by diffuse nuclear chromatin, blue cytoplasm, and large, dark red granules in cytoplasm.9, 11 Eosinophils have large granules and a bilobed or horseshoe-shaped nucleus. Low-grade eosinophilia can be seen with chronic parasitism. Basophils are relatively common in rabbits and can represent up to 30% of the differential in healthy animals. Infections rarely cause elevated white blood cell counts (>15,000 cells/ml) in rabbits; however, several changes may be associated with an acute infection, including a low total number of white blood cells (<5000 cells/ml) with a normal differential or normal cell counts with a shift to heterophilia, thrombocytopenia, and nucleated red blood cells. Other reasons nucleated red blood cells may be found are endothelial changes and regenerative responses.
Rabbits, like rodents, have control over calcium excretion at the level of the kidney. Elevations in serum calcium can be attributed to an increase in dietary intake of the mineral or to renal disease; a reduction in serum calcium may be indicative of hypoalbuminemia, diarrhea, chronic renal failure, or hyperparathyroidism. Phosphorous can also increase with renal disease, but reduced levels can be seen with calcium excretion in the urine.
As with other mammals, creatinine levels can become elevated when reduced blood flow to the kidney occurs accompanied by a 50% to 70% loss of renal function. Blood urea nitrogen may also rise due to renal disease when 50% to 70% of kidney function is lost. There are a number of potential causes of renal failure in rabbits, including neoplasia, glomerular nephritis, and pyelonephritis. Elevations in blood urea nitrogen and creatinine can also occur with postrenal diseases (e.g., urethral calculi) and prerenal diseases (e.g., dehydration).
There are no liver-specific enzymes in rabbits. In many mammals, alanine aminotransferase is liver specific; however, in rabbits, this enzyme is found in both the liver and cardiac muscles. Aspartate aminotransferase is found in a number of tissues, including muscle (cardiac, skeletal, smooth), liver, kidneys, and pancreas. Elevations of both enzymes, however, should be considered suspicious and other testing done to rule out liver disease (e.g., liver biopsy). (See Table 14-1 for rabbit hematologic and serum chemistry reference ranges.)
TABLE 14-1.
Reference Hematologic and Serum Chemistry Values for Rabbits
| Parameter | Range | Comments |
|---|---|---|
| Erythrocytes (×106/μl) | 4.9-7.8 | Anisocytosis, polychromasia, low numbers of NRBCs, and Howell-Jolly bodies can be normal on rabbit blood smears for pet rabbits. |
| Hematocrit (%) | 31-50 | |
| Hemoglobin (g/dl) | 10-17.4 | |
| MCV (fl) | 57.5-75 | |
| MCH (pg) | 17.1-23.9 | |
| MCHC (%) | 28.2-37 | |
| Platelets (×103/μl) | 250-650 | |
| Leukocytes (×103/μl) | 5.2-12.5 | |
| Heterophils (%) | 20-75 | In healthy rabbits, heterophil-to-lymphocyte ratio is usually 1 : 1 or slightly lymphocytic. |
| Lymphocytes (%) | 30-85 | |
| Monocytes (%) | 2-10 | |
| Eosinophils (%) | 0-5 | |
| Basophils (%) | 0-8 | |
| ALP (U/L) | 4-16 | |
| ALT (U/L) | 14-80 | |
| AST (U/L) | 14-113 | |
| Bicarbonate (mEq/L) | 16.2-38 | |
| Bilirubin, total (mg/dl) | 0-0.75 | |
| BUN (mg/dl) | 13-30 | |
| Calcium (mg/dl) | 5.6-14 | |
| Chloride (mEq/L) | 92-112 | |
| Cholesterol (mg/dl) | 10-80 | |
| Creatinine (mg/dl) | 0.5-2.5 | |
| Glucose (g/dl) | 75-155+ | |
| LDH (U/L) | 43-129 | |
| Lipids, total (mg/dl) | 243-390 | |
| Phosphorous (mg/dl) | 2.3-6.9 | |
| Potassium (mEq/L) | 3.6-6.9 | |
| Protein, total (g/dl) | 5.4-8.3 | |
| Albumin (g/dl) | 2.4-4.6 | |
| Globulin (g/dl) | 1.5-2.8 | |
| Sodium (mEq/L) | 131-155 | |
| Triglycerides (mg/dl) | 124-156 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NRBCs, nucleated red blood cells.
Combined data from Carpenter JW, Mashima TY, Gentz EJ et al: Caring for rabbits: an overview and formulary, Vet Med Rabbit Symposium: Rabbits Are Not Small Cats, pp 348-380, April 1995; Harcourt-Brown F: Textbook of Rabbit Medicine, London, 2002, Elsevier; Mader DR: Basic approach to veterinary care. In Queensberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, St Louis, 2004, WB Saunders; Fudge AM: Laboratory Medicine: Avian and Exotic Pets, Philadelphia, 2000, WB Saunders.
The electrolytes reference ranges for rabbits are similar to those described for other mammalian vertebrates (see Table 14-1). Elevations in sodium and chloride can occur with dehydration or excess salts in the diet, whereas losses are generally associated with diarrhea or low dietary salt. Potassium levels may be low in animals with diets low in potassium or in animals that are starved. Total protein levels in rabbits can be higher compared to those levels found in other vertebrates.
Urinalysis
A urinalysis can provide a significant amount of information regarding a rabbit's health. Either a free-catch or cystocentesis urine sample can be used, but the cystocentesis sample is preferred. To perform a cystocentesis in a rabbit, a 22- to 25-gauge needle fastened to a 6-ml syringe is needed. The rabbit should be placed in dorsal recumbency. The bladder can be difficult to palpate; however, with care, it can be found cranial to the pelvis. The cystocentesis site should be disinfected with 70% isopropyl alcohol. The rabbit bladder should be stabilized between the index finger and the thumb, and the needle inserted along the ventral midline into the bladder. The consistency of the urine should be noted.
Urinary catheterization can be performed with a well-lubricated 9-French catheter inserted into the urethra.11 Rabbit urine is generally straw-colored and clear to cloudy. If the urine has a red appearance, then it is important to rule out blood in the urine from porphyrins. To differentiate hematuria from porphyrin pigmentation, use a urine dipstick or a woods lamp, as porphyrins will fluoresce. If a urinary tract infection is suspected, obtain a cystocentesis and submit a culture and sensitivity in addition to performing a complete urinalysis. Cloudy urine in rabbits is usually due to calcium excretion and the presence of crystals. The presence of crystals in the urine can make it difficult to identify bacteria on a cytologic test, and a culture should be performed if a bacterial cystitis is suspected. (See Box 14-2 for normal urinalysis values.)
BOX 14-2. Reference Ranges for a Rabbit Urinalysis.
| Specific gravity | 1.003-1.036 |
| pH | 8.2 |
| Crystals | Calcium carbonate monohydrate, anhydrous calcium carbonate, ammonium magnesium phosphate |
| Casts, cells, bacteria | None |
| WBC | Occasional |
| RBC | Occasional |
| Albumin | In young occasionally |
| Average urine production | 130 ml/kg/day |
From Mader DR: Basic approach to veterinary care. In Queensberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, St Louis, 2004, WB Saunders.
Fine needle aspirates can be done to ascertain the histologic response of a mass. Although abscesses are a common finding in dermal and subcutaneous masses, neoplasia, granulomas (e.g., fungal), and hematomas can also occur. Rabbit abscesses are generally inspissated, so it is difficult to obtain much of a sample from a fine needle aspirate. The procedure for fine needle aspiration in a rabbit is similar to the technique described for domestic mammals. Using a local anesthetic can minimize the pain associated with the procedure. Applying topical EMLA cream (AstraZeneca LP, Wilmington, DE) over the fine needle aspirate site 20 to 30 minutes before performing the procedure can minimize the discomfort associated with the procedure.
Bone marrow aspirates should be performed when blood cell production problems are suspected. The proximal femur is the preferred site for sample collection. This procedure should be done with general anesthesia. A standard sterile preparation should be done to minimize the likelihood of introducing opportunistic skin pathogens. A spinal needle with a stylet should be used to perform the procedure. The needle should be inserted in the trochanteric ridge.
Diagnostic Imaging
Radiography is a useful diagnostic test in rabbit medicine. It is important to always take at least two radiographs (e.g., lateral and dorsoventral/ventrodorsal). Many times radiographs of the abdomen or thorax can be taken with little or no sedation if the handlers are patient and careful. If more restraint is necessary, isoflurane or midazolam can be used to sedate or immobilize the patient. Skull radiographs are required to evaluate the roots of molars and incisors and should be taken in cases of suspected or known malocclusions. The most useful radiographic views for examining the teeth are rostrocaudal, dorsoventral, and lateral (Figure 14-7 ). In cases of ocular discharge, contrast media can be injected into the lacrimal duct to determine if any of the molar roots are impinging on the duct (Figure 14-8 ). Chronic cases of otitis or neurologic disease warrant evaluation of the tympanic bullae for radiographic changes. In cases of facial abscess, radiology can be used as a prognostic indicator; periosteal bone reaction decreases the prognosis for full recovery.
Figure 14-7.

Normal lateral skull radiograph of a domestic rabbit.
Figure 14-8.

Contrast media can be instilled into the nasolacrimal duct to determine if the incisors are impinging on the duct.
Abdominal radiographs can be used to assess the gastrointestinal, reproductive, and excretory systems. Survey radio graphs can be useful for diagnosing ileus or gastric foreign bodies. Rabbits with severe generalized ileus carry a grave prognosis. Rabbits with trichobezoars are frequently anorectic. Therefore, the presence of a soft tissue mass in the stomach of an anorectic rabbit is highly suggestive of the presence of a gastric foreign body (e.g., trichobezoar). In cases where a gastrointestinal foreign body is suspected but not conclusive from survey radiographs, the veterinarian should consider doing a contrast study. Uterine adenocarcinoma should be considered in a differential list for any intact doe with a suspicious soft tissue mass in the caudoventral abdomen. Renal, urethral, and cystic calculi can generally be diagnosed from survey radiographs, although contrast studies are also needed in some cases.
Thoracic radiography is an important diagnostic test for ruling out respiratory and cardiac disease. The thoracic field of a rabbit is small in comparison with that of other mammalian vertebrates (Figure 14-9 ). However, radiograph interpretation is similar. Pneumonia, metastatic neoplasia, and trauma are common disease problems that can be diagnosed radiographically.
Figure 14-9.

Normal thoracic radiograph. Note the small size of the thorax.
In addition to radiography, the other commonly used diagnostic imaging techniques can also be useful for assessing the rabbit patient. Ultrasound can provide excellent images of the abdomen and thorax and has been used to diagnose abdominal pathology, uterine disease, foreign bodies, excretory calculi, and cardiac disease. Success with ultrasound is based on the sonographer having a good knowledge of anatomy, so that he or she can interpret an image based on the position of the probe. A 7.5- to 10-mHz transducer is recommended for rabbit ultrasonography. Clipping the fur of the rabbit in the area of interest can also help improve the image by providing the contact between the skin and the probe; however, caution must be used when clipping the fur, as the skin tears easily. Echocardiography is an appropriate diagnostic aid in cases of suspected heart disease or in patients with audible murmurs. Echocardiographs should be conducted using a high-frequency transducer (7.5-12 MHz) and high frame rate ultrasound machine.4 Magnetic resonance imaging and computed tomography (CT) can prove invaluable in diagnosing certain conditions, including diseases associated with the sinuses, inner ear, cranium, and teeth. Although these tests are becoming more available, they still remain cost prohibitive for some clients.
Microbiology
Bacterial and fungal cultures are routinely collected to assist in the diagnosis of specific pathogens. Interpretation of the results, however, can sometimes require more art than science. It is important for veterinarians to recognize that the results they receive from their laboratories are based on what happens at the level of the culture plate. For example, although Pasteurella multocida is often considered a primary differential in many cases based on the animal's presentation, the culture results may suggest otherwise. P. multocida is a fastidious organism and may be outcompeted on a plate by other organisms (e.g., Staphylococcus spp.). Therefore, the interpretation of the results should be reviewed cautiously.
Rabbits can become bacteremic and develop an infection in practically any tissue. The most common sites of infection, however, are the urinary bladder, respiratory system, and integument. Urine cultures obtained from cystocentesis are preferred to those collected via free-catch. Rabbit urine should have a basic pH. If the urine pH becomes acidic, it will promote the growth of potential opportunistic pathogens. Most pathogens of the urinary tract are Gram-negative bacteria. Female rabbits are more susceptible because of their stouter urethras. In addition to urine cultures, deep nasal cultures for chronic respiratory disease and cultures of abscesses are considered routine. Deep nasal cultures usually require sedation, as the microculturette is advanced into the nasal passage up to the level of the medial canthus of the eye. When culturing an abscess, the best results are often obtained from sampling the abscess wall.11 Samples collected from the nasal sinuses and abscesses often have a significant number of inflammatory cells associated with them. These cells can affect the results of a culture by reducing the number of organisms to the point where analytical sensitivity of culture cannot detect the bacteria. In some cases, serial samples may be needed to determine if an organism is a trace pathogen. Culturing microorganisms from rabbits is otherwise routine. If anaerobes are suspected, then the veterinarian should contact the laboratory to obtain the appropriate media for collection and transport.
Parasitology
Although rabbits are captive bred, and the expectation is that parasites would be absent or low in these animals, parasites remain a problem because few preventive health programs are instituted at the breeding population level. Endoparasites can be diagnosed using routine direct saline smears or fecal flotations. Direct saline smears are preferred for identifying protozoa, whereas a fecal flotation is preferred for finding nematodes, trematodes, and cestodes. Annual parasitological examinations include both techniques. Because parasites can be shed transiently, serial samples should be performed. Pooling fecal samples produced over the course of a week may reduce the likelihood of misclassifying a rabbit's parasitic status. For systemic parasitologic disease, serologic testing or histopathology is required. E. cuniculi, a microsporidian, is generally diagnosed antemortem via serology or postmortem via histopathology. E. cuniculi is shed in the urine and may also be diagnosed from direct visualization in a urine sample, but this is not analytically sensitive. Eimeria steidae, a hepatic coccidian, can be diagnosed antemortem via a fecal direct smear or postmortem via histopathology.
Ectoparasites are also common in captive rabbits. If ectoparasites are suspected, a routine dermatologic examination should be performed, including a skin scrape and mineral oil preparation of the fur. Microscopic examination of these samples can provide confirmation of the presence of these parasites. Multiple samples should be collected to prevent misclassification as negative, and samples should be collected along the edge of the lesion. A flea comb can be used to confirm the presence of adult fleas or flea dirt.
Miscellaneous Diagnostics
SEROLOGY
Serologic testing in rabbits is limited. The two most common serologic tests being used today are for E. cuniculi and P. multocida. As with any test, it is important to know the sensitivity and specificity of the assay to be able to be able to determine the value of the result. The interpretation of a single titer should be done cautiously. If a rabbit has a single positive titer, it suggests that the animal has been exposed to the infectious disease. To confirm that the animal has a current infection, a paired sample would need to be collected 2 to 3 weeks later. A two- to fourfold rise in the titer should be observed before concluding an animal is actively infected. However, in cases where the animal's first titer was already significantly elevated, a similarly high second titer may be used to confirm infection. If the titer is negative, then the animal may not have been exposed to the disease, or the result may have been a false negative. False negative results can occur during a peracute disease when a titer has not developed or when the immunoglobulin being measured (e.g., IgM or IgG) either has not been developed or has already passed. Serologic results should always be interpreted in combination with the animal's physical examination findings.
POLYMERASE CHAIN REACTION
Polymerase chain reaction (PCR) assays have improved veterinarians’ ability to diagnose diseases. The PCR test provides a more sensitive method of characterizing the presence of an organism than culture. One shortcoming of PCR is that a specific assay needs to be developed for each disease in a species. If there generally is not a high demand for a test, it will not be developed. PCR tests are available for E. cuniculi and P. multocida.
COMMON DISEASE PRESENTATIONS
Infectious Disease
BACTERIAL DISEASE
Pasteurella multocida
Snuffles is a generic term used to describe upper respiratory tract infection in rabbits. Although a variety of opportunistic pathogens can cause this “disease,” it is generally associated with P. multocida. This is unfortunate because it often leads to misdiagnosis. Snuffles is characterized by purulent nasal discharge, sneezing, and coughing or “snuffling.” Affected animals may be weak, lethargic, and anorectic. Ideally, a deep nasal culture should be performed to determine which species of bacteria is responsible for the infection. Primary dental disease must be ruled out as a differential for purulent nasal discharge.
P. multocida is a Gram-negative organism. This bacterium is relatively fastidious and can be difficult to culture. P. multocida has been associated with multiple clinical diseases, including pneumonia, upper respiratory tract disease, abscesses, pleuritis, pericarditis, pyometra, orchitis, otitis media, neurologic disease, and ocular disease.
Both acute and chronic disease states can occur. In cases of acute pneumonia, animals can succumb within hours, whereas in chronic disease states, there may be limited or no overt clinical signs. Transmission of P. multocida can occur through direct contact with acutely infected animals, fomites, and via airborne bacteria. The pathogen generally is thought to enter the rabbit through the respiratory tract. P. multocida can be isolated from a healthy rabbit's respiratory tract; however, when epithelium and local defenses are disrupted, bacterial numbers can increase to levels at which an infection can occur. Conditions that predispose a patient to P. multocida infections include temperature change, presence of carrier rabbits, poor sanitation (causing increased ammonia fumes), reproduction status, and age.13
A thorough diagnostic work-up should be done because rabbits with other bacteria can exhibit the same clinical signs. Preferably, a diagnosis is made using PCR and paired serology; however, a single serologic sample and positive deep nasal culture may suffice.
A CBC may show an inflammatory leukogram, characterized by heterophils/neutrophils and monocytes, or may be within reference levels. Plasma biochemistry panels are generally within acceptable reference limits; however, if the animal is dehydrated, the panels may show electrolyte and protein disturbances. Radiographs often reveal soft tissue changes in the sinuses and lungs. Ideally, treatment should be based upon culture and antibiotic sensitivity results. Most cases of respiratory P. multocida infections can be treated with enrofloxacin (5 mg/kg PO BID × 14 days), ciprofloxacin (20 mg/kg PO SID × 10 days), injectable penicillin (24,000 U/kg SC/IM), or chloramphenicol (50 mg/kg PO BID).4 For chronically infected animals, these treatments need to be extended until the clinical signs are no longer present and the serologic titers decrease. Reproductive disease can be managed by performing an orchiectomy or ovariohysterectomy and providing appropriate antibiotics. P. multocida abscesses can occur anywhere on the body and can be difficult to treat. An extensive review of abscess treatment can be found later in this chapter (see Abscesses).
Bacterial dysbiosis
The microflora of rabbits is comprised of a variety of aerobic and anaerobic bacteria. This dynamic flora is what enables the rabbit to thrive on an herbivorous diet. Unfortunately, this microflora is extremely sensitive to several groups of orally administered antibiotics, including clindamycin, lincomycin, ampicillin, amoxicillin, amoxicillin-clavulanic acid, cephalosporin, penicillin, and erythromycin.2 These antibiotics can lead to alterations in the microflora, resulting in fluctuations in the intestinal pH, an increase in volatile fatty acids, and proliferation of Clostridium spiriforme. The endotoxemia produced by this organism is responsible for the peracute death of rabbits. If there is no environmental exposure to endotoxin-forming bacteria, this condition will not occur. In general, oral antibiotics are also more likely to cause diarrhea than are parenterally administered antibiotics. Trimethoprim sulfa and enrofloxacin are considered two of the safest oral antibiotics and can be administered orally over extended periods of time. In comparison with oral penicillin, injectable penicillin can be used to treat disease with minimal risk of dysbiosis. Many veterinarians prescribe probiotics to minimize the risk for dysbiosis. Probiotics are comprised of bacteria considered to represent indigenous microflora. These anaerobic microbes are thought to compete for colonization sites within the intestines with pathogens, alter the microflora environment (e.g., pH fluctuations), and produce their own natural “antibiotics” to kill pathogenic bacteria. The true value of these products remains controversial; however, they do not appear to cause disease. Treatment for antibiotic dysbiosis is primarily focused on the provision of supportive care. Fluids should be given to correct losses associated with diarrhea. Calories should be provided to ensure gut motility. Offending antibiotics must be discontinued. Additional safe antibiotics (e.g., enrofloxacin) may be considered to minimize the likelihood of other opportunistic pathogens infecting the compromised epithelium.
Clostridium spp.
Clostridium piliforme, the causative agent of Tyzzer's disease, is a motile, Gram-positive, obligate intracellular bacterium. Infections caused by C. piliforme are commonly reported in compromised rabbits. Overcrowding, unsanitary conditions, inappropriate breeding conditions, and high ambient temperatures have all been associated with C. piliforme outbreaks. Transmission is via the ingestion of spores. Clinical signs shown by rabbits with acute disease can include watery diarrhea, depression, and sudden death. Weanlings (6-12 weeks) are most severely affected; however, this pathogen can cause disease in rabbits of any age. Adult rabbits usually develop a chronic disease course, which can lead to intestinal fibrosis, liver necrosis and stenosis, and myocardial disease.2 Tissue culture and serologic testing are available for diagnosis. Treatment is usually not successful but consists of providing supportive care.
Endotoxemia can be caused by C. spiriforme, C. difficile, and C. perfringes. These organisms can cause significant enteric pathology, and are often associated with a hign number of mortalities. Weanling rabbits are most likely to be affected and may be more predisposed to disease development. Adults can develop clostridial enterotoxemia from inappropriate antibiotic administration, which was described previously.2
Escherichia coli
In neonatal rabbits, colibacillosis is associated with high morbidity and mortality. E. coli is a Gram-negative bacterium from the family Enterobacteriaceae. Members of this family are frequently associated with disease in vertebrates. The most severe disease occurs in rabbits that are 1 to 14 days of age. Affected animals show signs of profuse, watery diarrhea and staining of the abdominal and perineal areas. Dehydration is a common sequela. Intestinal coccidiosis may enhance E. coli proliferation by increasing the severity of the clinical signs.2 A diagnosis can be made from a fecal culture of a pure isolate. A CBC and biochemistry panel should be done, and generally support a diagnosis of an inflammatory disease with dehydration. Treatment should be based on an antibiotic sensitivity profile from the culture. Supportive care, including fluids and calories, is required because of the poor reserves of neonatal rabbits. Generally, the goal of therapy is to quell the spread of disease and prevent additional losses, as clinically affected animals often succumb to the disease.
Miscellaneous bacterial diseases
Lawsonia intracellularis, Salmonella spp., and Pseudomonas spp. are also common pathogens in rabbits. Lawsonia intracellularis is a Gram-negative, spiral-shaped, intracellular bacterium that commonly infects weanling rabbits between 2 and 4 months of age. Affected animals present with profuse, watery diarrhea (e.g., wet tail). Salmonella spp. and Pseudomonas spp. can also cause enteritis in rabbits and are usually disseminated through contaminated food or water sources. Affected animals may present with diarrhea, pyrexia, dehydration, and weight loss. Some animals can become asymptomatic reservoirs. With virulent organisms, peracute death is common. Diagnosis is via culture and antibiotic sensitivity profile. A CBC can provide information regarding the animal's response to the infection, and a radiograph can provide information regarding intestinal motility. Ileus is a common sequela. Treatment should include supportive care and antibiotics.
Treponematosis (Treponema cuniculi), otherwise known as rabbit syphilis, is a sexually transmitted disease that is spread during birth from a doe to a kit. Affected animals often present with crusts on the mucocutaneous junction of the nose, lips, eyelids, and/or external genitalia. Does may abort kits, retain placentas, or develop metritis.14 A diagnosis can be made from a scraping or biopsy. Serologic tests are also available to assist with diagnosis. Silver stain or dark field microscopy is necessary to identify the organisms. Injectable penicillin (40,000 U/kg IM SID × 3-5 days;14 40,000 U/kg IM q7days × 3 treatments)15 is the treatment of choice.
Staphylococcus aureus is considered a component of the indigenous microflora of the nasal cavity, conjunctiva, and skin of rabbits; however, when the epithelial barrier of these structures is compromised, the pathogen can invade. Mammary glands, respiratory and ocular tissues, and the integument are common sites for S. aureus infections. S. aureus infections can lead to fatal septicemia. Diagnosis is based on culture and cytologic and histopathologic testing. Treatment should be based on the antibiotic sensitivity profile. Enrofloxacin (5 mg/kg BID) or trimethoprim sulfa (15 mg/kg BID) are both broad-spectrum antibiotics that can be used to manage the case while the sensitivity profile is pending.
Bordetella bronchiseptica is a Gram-negative bacterium that generally infects the upper respiratory tract of rodents and lagomorphs. Infection caused by B. bronchiseptica strikes primarily older animals. Clinical signs include sneezing and nasal discharge; rabbits can also serve as asymptomatic carriers. Culture is used to confirm diagnosis. Antibiotics should be given based on antibiotic sensitivity profile, and, again, enrofloxacin can be used for initially treating the animal.
VIRAL DISEASE
Rotavirus is a disease of neonatal rabbits. Affected animals are generally 4 to 6 weeks of age. In populations where this virus is endemic, there is generally a low morbidity in weanlings; however, if naive animals are exposed to the virus, then the disease course can be severe.16 Rotavirus can cause severe changes to the intestinal villi, including blunting and fusing. Severe damage to the villi can result in inability to absorb nutrients and in loss of fluids.17, 18 There is no effective treatment. Affected animals should be given supportive care and broad-spectrum antibiotics. Quarantine is recommended to prevent introduction of the virus.
Enteric coronavirus causes disease in 3- to 10-week-old rabbits; it is characterized by the loss of the brush border of the enterocytes. Clinical signs in affected rabbits include lethargy, watery diarrhea, and abdominal swelling. Morbidity can reach between 40% and 60%. Death usually occurs within 24 hours of the onset of signs. Corona or corona-like virus can lead to pleural effusion disease or infectious cardiomyopathy. Infected rabbits may present for anorexia, weight loss, tachypnea, or sudden death. Mortality can exceed 75% of affected animals. Virus isolation from the feces and histopathology consistent with the disease are the two common methods of diagnosis. Affected animals should be provided supportive care and broad-spectrum antibiotics to combat opportunistic bacterial infections. This virus can be isolated from healthy adult rabbits. Because of this, quarantine and prescreening animals are recommended to prevent introduction of the virus into rabbitry.
Myxoma virus is a member of the poxvirus family. The virus group has two distinct geographic origins: South America and California. Myxoma virus is associated with a benign cutaneous fibroma in its natural hosts: Sylvilagus bachmani (wild brush rabbits in the western United States—the Californian type), and S. brasiliensis (the South American type). Both of the myxoma strains can cause lethal disease in the European rabbit.3, 16 Although rare, this virus has been found to cause high mortality in domestic rabbits from Oregon and California.19 This virus is transmitted via mosquitoes and fleas. The clinical signs associated with myxomatosis in natural hosts include swelling of the eyelids, face, head and testes/scrotum, the production of a milky ocular discharge, 1-cm mucoid cutaneous nodules, pyrexia, lethargy, depression, and anorexia. Infected domestic rabbits may become lethargic, pyrexic, anorectic, and develop skin hemorrhages and seizures.4 Death usually occurs within 14 days of clinical onset. Although the exact mechanism leading to the animal's demise is not well understood, it has been suggested that it is due to the proliferation of Gram-negative bacteria secondary to the immunosuppression caused by the virus. No effective therapy is known, and euthanasia is recommended.15 Cross-immunity can be achieved using a live, attenuated, Shope fibroma virus vaccine. However, the vaccine does not provide full coverage and is safe only in healthy nonpregnant animals. Boosters should be administered at 6- to 12-month intervals in susceptible animals.15
Shope fibroma virus is a Leporipoxvirus and is related to the myxoma virus. Cross-immunity can be inferred between the viruses. Shope fibroma virus can cause localized, benign fibromas and generalized disease in newborn, wild rabbits. Outbreaks have been reported in rabbitries. Clinical signs include subcutaneous, nonattached, wart-like fibromas on the legs or feet and around the muzzle and eyes. In young rabbits, this virus has been associated with lethargy, reduced body condition, skin lesions, and edema.20 Diagnosis is made from histologic samples. No treatment is available.
Shope papillomavirus is the causative agent in papillomatosis. This disease is generally found in wild cottontails from the midwestern United States. Domestic rabbits are susceptible to the disease, and outbreaks have been reported in rabbitries. Horny warts are the most common clinical finding, and they are generally found on the shoulders, abdomen, and neck. Oral papillomavirus can also lead to the development of papillomas on the ventral surface of the tongue; however, cross-immunity develops when the virus causes dermal lesions. Diagnosis is made based on histopathologic findings and viral isolation. There is no treatment for this virus. The virus can induce malignant neoplasia that is similar to squamous cell carcinoma.
Rabbit pox is a rare but highly contagious disease. Clinical signs include lymphadenopathy, fever, and dermatologic changes (e.g., crusts, nodules, papules). Death can occur in severe cases. Edematous changes may also be seen on the face, oral cavity, scrotum, and vulva. Diagnosis is via viral isolation and histopathology.4 There is no treatment for this virus.
Rabbit calicivirus, formerly known as rabbit viral hemorrhagic disease, is a viral-induced hemorrhagic disease. This virus is primarily found in the eastern hemisphere. An outbreak in Mexico occurred in 1988; however, the virus has since been eradicated from that country. A single outbreak has been reported in the United States; the event occurred in Iowa. Morbidity from this disease can be as high as is 70% to 80%, and mortality can approach 100%. The virus is spread directly from infected to noninfected rabbits. Infected rabbits may present for frank hemorrhage around the face and muzzle, tachypnea, cyanosis, abdominal distension, constipation, or diarrhea.20
FUNGAL DISEASE
The most common fungal infections of rabbits are associated with Trichophyton mentagrophytes and Microsporum canis. Clinical signs in infected rabbits are similar to those reported in infected domestic pets and include crusty erythematous alopecia that may or may not be pruritic. Lesions can occur anywhere on the body but are often found on the head, shoulders, and legs. Ringworm is most often diagnosed in young animals that have received inappropriate husbandry. A diagnosis can be made with a KOH preparation from a skin scrape or fungal culture.21 Infected rabbits can be treated with weekly lime-sulfur dips, a topical povidone-iodine cleansing agent, and, in severe cases, griseofulvin (25 mg/kg PO BID × 30 days).21 Because this disease is potentially zoonotic, clients should be advised to minimize handling during treatment. Rabbits can acquire ringworm from humans too, so clients should be advised to consult their clinician if they are the source of infection.
Parasitic Disease
ENDOPARASITES
Coccidial infections are common when rabbits are held at high densities under inappropriate husbandry conditions. Infections are more problematic in weanling rabbits. Eimeria spp. can infect both the intestinal tract and the liver (E. steidae). The transmission of these protozoal parasites is via the fecal-oral route. Sporulated oocysts represent the infectious stage. Clinical signs associated with intestinal coccidiosis include inappetence, weight loss, dehydration, diarrhea (± hemorrhagic), and, in chronic cases, intussusception. Hepatic coccidiosis is considered a more serious disease. Rabbits infected with hepatic coccidiosis may have limited growth, diarrhea, weight loss, dehydration, ascites, hepatomegaly, icterus, and hepatic encephalopathy. Severe cases can result in death. A fecal flotation and direct smear or histopathology can be useful to confirm a diagnosis. Treatment may be accomplished using trimethoprim sulfadiazine (30 mg/kg PS or SC BID × 7-10 days), sulfadimethoxine (50 mg/kg PO once, then 25 mg/kg SID × 21 days), or sulfaquinoxaline (0.025% in feed or pellets).14 Extended treatments may be required for some populations. If routine treatment is practiced, then the anticoccidial drugs should be rotated to minimize the likelihood of inducing resistance.
E. cuniculi is an obligate, intracellular, microsporidian protozoan.22 The parasite is disseminated via contaminated urine. E. cuniculi is shed transiently and can live for 4 weeks at room temperature. Clinical signs can vary depending upon the organ or system affected and the severity and duration of the disease. This protozoan affects primarily the kidneys, eyes, and neurologic system. Most infected rabbits present for vestibular disease. Clinical signs may include head tilt, hemiparesis, an inability to right itself, tumbling/rolling, seizures, ataxia, and paralysis (Figure 14-10 ). When E. cuniculi affects the neurologic system, the prognosis for recovery is grave. In some cases, rabbits show a reduction in vestibular signs but require life-long treatment. Without treatment, the quality of life is usually poor, and euthanasia is recommended. When the kidneys are affected, clinical signs may be absent because renal function is not usually altered. Cataracts and uveitis are common sequelae to ocular infections (Figure 14-11 ). Phacoemulsification is the treatment of choice for E. cuniculi–induced cataracts. Without treatment, the lens can rupture and induce a phacoelastic uveitis. Diagnosing E. cuniculi antemortem is usually done from the physical examination, hematology, and serology. Serology is generally only used to rule out E. cuniculi infection; however, a positive titer does not conclusively rule in disease. Albendazole, fenbendazole, or oxibendazole is generally used to minimize the inflammatory response associated with the infection.4 Antibiotics that penetrate the blood-brain barrier (e.g., chloramphenicol) can be used to combat opportunistic infections. Diazepam can be used to control seizures. Although some corticosteroids have been used for treating E. cuniculi, they can cause immunosuppression and should be used with caution. The steroid's primary benefit is to increase the uptake of the respective antiparasitics. Life-long treatment is required to minimize the clinical signs associated with the disease.23
Figure 14-10.
Head tilt in rabbit caused by E. cuniculi.
Figure 14-11.

Lenticular changes associated with an E. cuniculi infection.
(Courtesy Dr. David Guzman.)
Baylisascaris procyonis, the raccoon roundworm, has been documented in rabbits and can be associated with fatal neurologic disease. Affected animals may present for torticollis, ataxia, and tremors.24 Rabbits become infected from eating food or drinking water contaminated with raccoon feces. A diagnosis can be made from a serologic titer (exposure) or postmortem. Albendazole can be used to minimize the clinical disease associated with the parasites.
ECTOPARASITES
Cuterebra larvae, or bot flies, are common finding in rabbits housed outdoors. The larvae can be diagnosed easily by the presence of a small breathing hole. Infected rabbits generally have 1 to 5 bot larvae. The animal's fur may be matted around the breathing hole as a result of the rabbit licking in reaction to the pain associated with the larvae. Treatment requires surgical removal of the larvae. An incision should be made through the air hole, and the larvae extracted. The larval cyst should be removed to prevent secondary opportunistic infection. Care should be taken not to crush the larvae during extraction, as this can lead to anaphylaxis. Rabbits housed outdoors should be protected against flies by means of appropriate fly traps and cage screening.
Fly strike, or myiasis, is a common presentation for rabbits housed outdoors during the summer. Obesity, underlying dermatitis, and unsanitary conditions can predispose a rabbit to this condition. Aggressive wound debridement and maggot removal are required to treat affected rabbits. Ivermectin (0.2 mg/kg SC once) can be used to kill the maggots. Antibiotics may be used if the wound is large or there is a concern for systemic disease. Analgesics should be used during wound treatment to control pain. The dog flea (Ctenocephalides canis) and cat flea (C. felis) are the most common fleas found on rabbits. The fleas are found primarily along the dorsum between the shoulders and pelvis. Flea powder that is safe for kittens can be used for rabbits. Although not labeled for use in rabbits, lufenuron (Program, Novartis Animal Health Canada, Mississauga, Ontario) and Advantage (Bayer Animal Health, Agriculture Division, Shawnee Mission, KS) for cats have been used safely for rabbits. For rabbits that are 10 weeks of age and less than 4 kg, 0.4 ml of topical solution can be applied to the skin at the base of neck, as recommended for dogs and cats. Rabbits that are ≥4 kg can be treated with 0.8 ml.4 When treating the animal's environment, it is important to remove the rabbit and only return it once the chemicals are dry. Frontline (Merial Limited, Duluth, GA) should not be used on rabbits, as it can cause liver impairment.
Cheyletiella parasitovorax, an obligate, nonburrowing mite, is commonly referred to as “walking dandruff” (Figure 14-12 ). Clinical signs associated with an infection include mild pruritus, large flakes of white scales on limbs and neck, alopecia, and oily dermatitis. Infestations occur more commonly in young, obese, or otherwise immunosuppressed animals. A diagnosis can be confirmed from the microscopic examination of a skin scrape or scotch tape prep. Ivermectin (0.2-0.4 mg/kg SC × 3 treatments) is the treatment of choice.4 Lime-sulfur dips and flea powder can also be used to treat affected rabbits. Cleaning the environment and treating other affected animals are also required to eliminate this parasite. This parasite can be zoonotic, so it is important to advise clients to take precautions when handling/treating affected animals.
Figure 14-12.

Cheyletiella parasitovorax is often described as “walking dandruff.”
Psoroptes cuniculi is the common rabbit ear mite. Affected animals can develop severe otitis externa. The external canal and pinnae can also have significant quantities of crusty exudates. The crusty exudate is the result of a hypersensitivity reaction to the mite and can cause a significant discharge and pruritus. One or both ears can be infested. Ear mites are transmitted by direct contact or contact with fomites. Mites can survive off the host for 21 days. Psoroptes’ life cycle is 21 days. Ivermectin (0.4 mg/kg SC, ½ done in each ear) or selamectin (Revolution; Pfizer Animal Health, New York, NY) (6 or 18 mg/kg topically 1-2 times) can be used to eliminate mites.25 Do not remove the crusts or clean the ears, as the skin under the crusts is ulcerated and painful. Prednisone (0.5 mg/kg PO BID × 5 days) or meloxicam (0.2 mg/kg PO BID × 5 days) may be used to reduce the otitis and provide pain relief.
Sarcoptes scabiei, the causative agent of scabies, can induce a crusty, pruritic dermatitis of the face, nose, lips, and external genitalia.2 Diagnosis is via a deep skin scrape of the lesion. Ivermectin (0.4 mg/kg SC q14days × 3 treatments) or weekly lime-sulfur dips (1 : 40) and environmental clean-up can be used to eliminate the mites. The mite is zoonotic, so clients should be instructed to wear gloves when handling/treating an infected animal.
Passalurus ambiguous, a pinworm, is considered by some to be commensal. This nematode is thought to play a role in the digestion of plant material. Clinical disease has been attributed to heavy burdens. Affected animals may be anorectic, lose weight, develop impaction, and be in poor condition. A diagnosis can be made from a fecal flotation. Fenbendazole (20 mg/kg PO SID × 5 days), thiabendazole (50 mg/kg PO q2 wk-q3 wk), or Piperazine (200-500 mg/kg/day PO × 2 days) can be used to treat affected animals.3, 5, 15
Haemodipsus ventricosus, the sucking rabbit louse, is rare in pet rabbits. Most cases occur in rabbits from poorly managed rabbitries. Affected animals can develop intense pruritus and anemia. Ivermectin (0.4 mg/kg SC q14days × 3 treatments) is the treatment of choice.4
Nutritional Disease
Hairballs, or trichobezoars, are most common in rabbits fed diets with inadequate fiber. Stress, barbering, and nest building have also been associated with the formation of trichobezoars. Anorexia, lethargy, diarrhea, small dry feces or an absence of feces, hair loss, and a palpable firm mass in the cranial abdomen are common findings in affected rabbits. A thorough history, physical examination, and survey radiographs (± contrast) are generally required to make a diagnosis. Endoscopy can be used to confirm a diagnosis. A history of anorexia in combination with a soft tissue density in the stomach is highly suggestive of gastric trichobezoars (Figure 14-13 ). Positive or negative contrast radiography can be used to help outline a trichobezoar. In most cases, medically managing a trichobezoar yields a higher treatment success than does surgery. However, there are cases where surgical intervention is the only method that can be used to treat a trichobezoar. Treatment should focus on rehydrating the gastric contents through fluid administration and providing motility stimulants, analgesics, antibiotics, and nutritional support. Fresh greens and hay should be offered to animals that are still eating. Rabbits that are anorectic should be force-fed a critical care diet (Oxbow Pet Products, Murdock, NE) to prevent hepatic lipidosis. Fresh pineapple juice contains the enzyme bromelain, and this enzyme is thought to have ceratolytic properties (5-10 ml BID).7 Papaya and prozyme may also be used to assist in the digestion of the trichobezoar. Metoclopramide or cisapride can be used to stimulate gastric motility; however, this should not be used if impaction is suspected. A broad-spectrum antibiotic, such as enrofloxacin or trimethoprim sulfa, is generally used to limit the overgrowth of bacterial pathogens. Analgesics, such as buprenorphine, flunixin meglumine, metacam, or carprofen, can be used to minimize the pain associated with the alterations in gastrointestinal motility. Without treatment, trichobezoars can lead to malnutrition and death or gastric rupture causing fatal peritonitis.26 If the ingested material is synthetic (e.g., carpeting), surgery is usually required. The prognosis for cases going to surgery is generally considered guarded to grave; however, if the animal is stabilized before the procedure, then the likelihood for success is higher.
Figure 14-13.
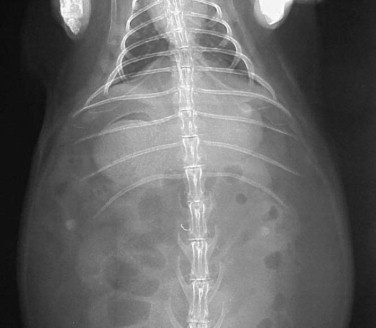
A history of anorexia and a radiograph showing soft tissue density in the stomach suggest the presence of trichobezoars in rabbits.
Obesity is a common problem in house rabbits and has been associated with high protein/fat pellets, free choice pellets, and restricted exercise. Obesity can increase the incidence of skin fold pyoderma, fecal staining of the perineum due to grooming difficulty, sore hocks, and an inability to acquire night feces. To prevent obesity, a rabbit should be provided a diet that is less than 18% protein, less than 10% fat, and more than 18% fiber. It is also important for clients to ensure their rabbits are given daily exercise.
Hepatic lipidosis in rabbits follows the same pathophysiologic response seen in other species. Obese or overweight animals are at higher risk of developing hepatic lipidosis than are those in good physical condition. If a rabbit becomes anorectic for any reason, such as dental disease, stress, illness, pregnancy, or postoperative complications, it can develop hepatic lipidosis. To minimize the likelihood of hepatic lipidosis from occurring in an anorectic rabbit, the veterinarian should provide a high-quality critical care diet (Oxbow). The underlying cause of hepatic lipidosis needs to be determined to break the cycle of anorexia and pathology.
Abnormal tooth wear can lead to laceration and ulceration of the tongue and buccal surfaces, which can lead to discomfort and difficulty chewing (Figure 14-14 ). There are a number of suspected etiologies attributed to the development of abnormal tooth wear, including genetics, anatomy, and nutrition. Feeding rabbits a pelleted diet can actually alter the natural movements associated with mastication by reducing the lateral, and increasing the vertical, jaw action.7 The most common sites to find dental pathology (e.g., sharp points) are the lingual side of the mandibular teeth and the buccal surface of the maxillary teeth. Correcting these abnormalities is generally sufficient to get the rabbit eating again. With sedation, these sharp points can be floated or drilled down to appropriate length and angle to allow for a more natural bite (Figure 14-15 ). When evaluating the teeth of a rabbit, it is important to take radiographs to evaluate the tooth roots for any pathology (e.g., abscesses) (Figure 14-16 ). For chronic incisor overgrowth, removal is recommended. For cheek teeth abnormalities, removal of nondiseased teeth that oppose missing teeth or normal teeth left behind after the conspecific tooth is removed is not recommended unless essential, because they can be difficult to remove and possibly result in the fracture of an attached bone.
Figure 14-14.
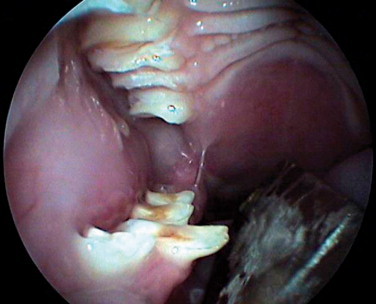
Lacerations on the tongue are a common sequela to cheek tooth malocclusion.
(Courtesy of Dr. David Guzman.)
Figure 14-15.

Rabbit dental instruments can be used to trim (a) and float (b) rabbit cheek teeth; however, care should be taken to limit secondary complications (e.g., tooth fracture, gingival hemorrhage).
Figure 14-16.

Dental disease as seen on radiographs. Note the cheek teeth elongation and the root pathology.
Neoplasia
Uterine adenocarcinoma is the most common neoplasia reported in female rabbits over 4 years of age. There is an increased incidence of this neoplasia (50%-80%) in Dutch, Havana, tan, French, and silver breeds.27 Uterine adenocarcinoma is a slow-developing neoplasia; it can take 10 to 12 months for those tumors to metastasize from the uterine wall to surrounding peritoneal structures or lungs. The most common sites for metastasis include the liver, lungs, mammary glands, brain, and bones. Rabbits with uterine adenocarcinoma can present with a range of clinical signs, including hematuria, vaginal discharge, decreased fertility, increased kit mortality, depression, anorexia, dyspnea, and cystic mammary glands. A diagnosis can be made from a thorough history (e.g., age, intact), physical examination, CBC, chemistry panel, whole body radiographs, and ultrasound. It is often possible to palpate an enlarged uterus in rabbits with uterine adenocarcinoma. The neoplastic organ will palpate as a tubular structure in the caudoventral abdomen. An increased soft tissue density in the same area may be seen radiographically. Thoracic radiographs should be taken to assess the patient for metastasis. If metastasis to the lungs has occurred, then the prognosis is grave. If metastasis has not occurred, then an ovariohysterectomy is recommended. During the surgical procedure, the abdomen should be inspected for localized metastasis. Any suspect tissues should be biopsied.4
Mammary adenocarcinoma is another common neoplasia of adult (>3-4 years of age) rabbits.4 These cases should be managed using the diagnostic plan described for uterine adenocarcinomas. Removing the affected mammary gland (local or radical) and the reproductive tract provides the best prognosis. Metastasis can be found in regional lymph nodes, lungs, or other organs. The incidence of mammary adenocarcinoma can be reduced significantly by performing ovariohysterectomies in rabbits before they are sexually mature. Some clinicians use different chemotherapeutics as adjunctive therapy to surgical treatment. Tamoxifen is a nonsteroidal, antiestrogen drug with antitumor effects that works by binding estrogen receptors.28 The overall value of this drug in rabbits is unknown for spayed animals. However, clinicians using the protocol believe, subjectively, that survival is extended. If tamoxifen is used, the patient should be routinely monitored for changes in behavior, tumor size, and liver or CBC changes.
Although adenocarcinoma is the most common reported reproductive neoplastic condition in captive rabbits, uterine leiomyoma and leiomyosarcoma, vaginal squamous cell carcinoma, ovarian hemangioma, testicular seminoma, interstitial cell carcinoma, testicular adenocarcinoma, and teratoma have also been reported.
Lymphosarcoma, as in other mammalian species, is a commonly diagnosed neoplasia in rabbits. Urinary neoplasia rarely occurs; however, nephroma, lymphosarcoma, renal carcinoma, and urinary bladder leiomyoma have been reported. Primary neoplasia of the lungs is very rare. Carcinomas have been found to be associated with the nasal turbinates, causing an unresponsive nasal discharge. Thymomas have been reported in both juvenile and adult rabbits. Affected animals often present with tachypnea, dyspnea, and, occasionally, bilateral exophthalmos resulting from the interference of the blood flow to the heart. A thymomectomy can be performed via a median sternotomy.4 Metastasis to the lungs is a common sequela for many different malignant neoplasias. Both teratoma and neurinoma affect the central nervous system of rabbits. Neoplastic conditions found to affect the digestive tract include adenocarcinoma, leiomyosarcoma, papilloma, and metastatic uterine adenocarcinoma. Surgical resection is the treatment of choice for those conditions. Bile duct carcinoma has been reported in rabbits; however, in these cases, surgery is not an option and metastatic disease carries a grave prognosis. Cutaneous neoplasias that have been reported in rabbits include the following: cutaneous lymphosarcoma, nonviral papilloma, basal cell carcinoma, squamous cell carcinoma, and sebaceous gland carcinoma. Malignant melanoma has been associated with ocular, abdominal, thoracic, and vertebral disease.31
Miscellaneous Diseases
DERMATOLOGY
Many different diseases or conditions, such as obesity, sore hocks, arthritis, poor diet, dental disease, urinary disease, and neurologic disease, can lead to secondary dermatitis. Moist dermatitis around the oral cavity is a common sequela to dental disease. Discomfort caused by tooth malocclusion can lead to drooling, and the chronic wetness associated with the drooling predisposes the animal to bacterial dermatitis. Skin fold pyoderma is a common problem in obese animals; the sites most commonly affected are the dewlap, perianal, and genital areas. Treatment for those conditions requires aggressive wound debridement. Fur from the affected area should be clipped to provide adequate exposure. The wound should be cleaned with a topical disinfectant (e.g., Betadine or chlorhexidine). To control bacterial infections, we recommend using a hyperosmotic solution (e.g., 50% dextrose). The wound should be liberally irrigated with the 50% dextrose solution. After 1 to 2 minutes, the wound should be irrigated with isotonic saline to remove the dextrose. Extended contact with the dextrose can dry out the healing tissue. The procedure should be repeated at least three times, twice daily. In addition to this topical treatment, obese animals should be placed on a calorie-restrictive diet. This is best achieved by reducing the amount of pellets and providing (ad libitum) grass hay (e.g., timothy hay). Surgery may be required to remove excess skin folds. In cases of tooth malocclusion, the primary problem should be identified and corrected.
Dermal abscesses are a common presentation in rabbits and can occur anywhere on the body. Abscesses in rabbits are generally more caseous in nature than in other mammals. The thick, caseous nature of rabbit abscesses limits their drainage, and the use of standard Penrose drains is usually futile. Treating abscesses with (only) systemic antibiotics is ineffective, as the nucleus of the abscess in unaffected by the treatment. To effectively treat rabbit abscesses, the entire abscess (including the capsule) must be removed. In cases where the abscess and capsule are removed, the prognosis is good; however, in most cases the capsule is inherently attached to vital organs or bone and is difficult to remove. When it is impossible to extract the entire capsule, as much of the capsule should be removed as possible to avoid contact between caseous material and unaffected tissue. Culture and antibiotic sensitivity testing of the purulent material or capsule is recommended. Abscesses that cannot be completely removed should be left open for daily cleansing and debridement. When choosing a topical antibiotic therapy, be sure to select antibiotics that will not induce bacterial dysbiosis when the rabbit grooms itself. Penicillin (80,000 IU/kg) or ampicillin (20 mg/kg), although not to be given orally because of the potential for dysbiosis, can be used parenterally to treat local skin abscesses. In our experience, the application of honey to the abscess as a hyperosmotic has produced moderate success. The honey, in addition to acting as an antimicrobial, encourages a rabbit to groom or lick at the area and promotes drainage.2 The honey also acidifies the area and promotes healing. Systemic oral antimicrobials and analgesics should also be considered when treating rabbit abscesses.29 Antibiotic-impregnated polymethylmethacrylate (PMMA) beads can also be used to manage rabbit abscesses. Heat-stable antibiotics must be used to make PMMA beads. Amikacin 1.25 g/20 g methylmethacrylate, cefazolin 2 g/20 g methylmethacrylate, cephalothin 2 g/20 g methylmethacrylate, gentamicin 1 g/20 g methylmethacrylate, or tobramycin 1 g/20 g methylmethacrylate are all appropriate for PMMA beads.30 Treating abscesses in rabbits can be frustrating and may require multiple procedures. In some cases, treatment is not successful, and amputation may be the only option. Owners should always be made aware of their pet's prognosis before treatment is initiated. Systemic antibiotics are appropriate for generalized abscesses. Antimicrobial treatment is ideally based on culture and antibiotic sensitivity testing. While testing is pending, abscesses can be treated with benzathine/penicillin G procaine (40,000 IU/kg SC SID × 14 days, then q48h × 2 weeks); enrofloxacin (5 mg/kg PO BID); or metronidazole (30 mg/kg PO BID).30, 35 The use of trime thoprim sulfa-dimethoxine should be avoided when there is caseous material present because the antibiotic is inactivated by pus.
“Sore hocks,” or ulcerative pododermatitis, is caused by an avascular necrosis of the plantar surface of the rear feet. This is a painful, progressive disease that can be difficult to treat. Predisposing factors for this disease include breed (e.g., Rex), housing (e.g., metal grate flooring, concrete, carpet), lack of exercise, and obesity. Rabbits housed on an inappropriate flooring surface alter their weight distribution and bear the brunt of their weight on their metatarsus and hock instead of on the claws and plantar surface of the feet.2 This shift in weight leads to the medical displacement of the superficial flexor tendon, which can exacerbate the condition.2 Treatment is aimed at relieving the pressure on the affected area. A nonabrasive, dry surface should be provided. Providing an affected animal time during the day on a grass lawn or deep bed of hay is helpful. A thick foam rubber pad, towels, or newspaper substrate can also be used to minimize the likelihood of exacerbating this disease. Skin wounds can be managed using the technique described previously. Surgical skin glue can be used to protect the wounds. A padded splint can be used to alleviate any pressure on the lesions. Opportunistic infection should be managed with appropriate antibiotics. Analgesics (e.g., nonsteroidal antiinflammatories, opioids) should be used to alleviate discomfort. Underlying problems, such as obesity and inappropriate substrate, should be corrected. Surgery is usually not performed because there is no skin for closure.
Hypersensitivity reactions can occur immediately or days after an injection is given. Affected animals present with erythema and swelling around the face and extremities. Most animals heal without complication or with a need for topical therapy. Giving injections subcutaneously may reduce the occurrence of these reactions.2 In severe cases, such as may occur with intramuscular injections, self-mutilation may occur.21 Severe reactions can be managed with steroidal (prednisone, 0.5-1.0 mg/kg) or nonsteroidal (meloxicam, 0.3 mg/kg) antiinflammatories.
Alopecia is a common problem in captive rabbits and may result from self-mutilation, barbering, parasites, endocrine disease, or infectious causes. Self-mutilation is most common in rabbits after a traumatic experience or surgery. Barbering is the result of aggression between cagemates. If barbering is suspected, animals should be separated. Diagnosis of parasitic, infectious, or endocrine disease requires a complete diagnostic work-up. Treatment is dependent on the specific diagnosis.
Rectal papillomas can present as cauliflower-like masses extending from the rectum. Most of these masses do not cause a problem; however, occasionally these masses can cause frank hemorrhage or mild diarrhea. Surgical resection of the mass is required for treatment.
Rare dermatologic disorders of rabbits include sebaceous adenitis, nonpruritic scaling and alopecia, Ehlers-Danlos syndrome, and neoplasia (see Neoplasia). Infectious and parasitic diseases are frequently associated with dermatologic disease in rabbits (see Infectious Disease; Parasitic Disease).
DISEASES OF THE SENSE ORGANS
Ears represent a significant proportion of the surface area of a rabbit. Otitis externa is a common problem, and it is most often associated with a parasitic or bacterial predisposition. Parasitic infections were described previously (see Parasitic Disease).32 Lop breeds are predisposed to developing bacterial otitis because of the flexion of the cartilage at the base of their ears. Rabbits with otitis externa may present for shaking, pruritus, erythematous pinna, malodor, and possible auricular discharge. Because otitis externa can be painful, care should be taken when manipulating the ears; we prefer to treat affected rabbits with systemic antibiotics and antiinflammatories rather than treating the ears topically. Bacterial cultures should be collected before treatment. Otitis externa can advance to otitis media or otitis interna. Otitis interna should be ruled out in rabbits with a head tilt. Radiographs and/or CT scans can be used to assess the tympanic bullae. In severe otitis cases, a permanent osteotomy may be required.32 The most common causes of conjunctivitis in rabbits are bacterial infections (e.g., Pasteurella), trauma, and entropion. When evaluating rabbit eyes, the approach to the examination should be similar to that for other mammals. Unless trauma has been acknowledged in the history, a thorough work-up should be pursued, including a cytologic scraping and bacterial culture. For confirmed bacterial infections, topical antibiotics can be used. Treatment regimens may require three to four doses daily. Application of topical antibiotics three to four times a day in addition to removing the initial cause of conjunctivitis is recommended. Entropion is a common presentation in large breed and obese rabbits, and surgical resection is necessary for resolution.21
Epiphora can be associated with early signs of dental disease, causing the blockage of the nasolacrimal ducts, or it can occur secondarily to conjunctivitis or rhinitis. Any rabbit presenting with dacryocystitis, conjunctivitis, or epiphora should receive a thorough ophthalmic examination, in addition to skull radiographs, to evaluate the roots of the teeth. Because many cases of epiphora are the direct result of blocked or inflamed lacrimal ducts, the lacrimal punctum should be flushed with sterile saline to facilitate the removal of debris (Figure 14-17 ). The ducts can be flushed with antibiotics too if a bacterial infection is suspected.
Figure 14-17.

Flushing the nasolacrimal duct.
Anterior uveitis can occur as a result of trauma, bacterial disease, or E. cuniculi–induced lens disease. Corneal ulcers are also somewhat common in rabbits housed with other animals. A fluorescein stain should be used to confirm the presence of an ulcer. Retrobulbar abscesses can be problematic; diagnosis and treatment should be based on culture and antibiotic sensitivity testing and aggressive antibiotic therapy instituted. In many cases, enucleation is required. Because of the large retroorbital venous sinus, enucleation can be difficult.
NEUROLOGIC DISEASES
Vertebral luxation or fracture is a common finding in rabbits with a history of hind end paresis or paralysis. Rabbits have a highly developed muscular system and a relatively delicate vertebral column, making them more prone to fractures. The most common cause of these injuries is associated with inappropriate restraint (Figure 14-18 ). A diagnosis can be confirmed from a history, physical examination, and vertebral radiographs. The prognosis for these cases depends upon the extent of the lesions. With vertebral luxation, medical management may be attempted; however, it is usually not successful. Prednisolone sodium succinate (0.5 mg/kg IV) can be used to control edema in the spinal canal. In addition to the steroid treatment, cage rest and the manual expression of the bladder can be done.21 Surgery can be attempted, but only by experienced surgeons.
Figure 14-18.

Vertebral injuries are common in rabbits that are not restrained properly.
(Courtesy Dr. David Guzman.)
Rabbits with torticollis, difficulty righting, or abnormal locomotion (e.g., flipping over) generally have a central nervous system disorder. The three most common causes of head tilt in rabbits are E. cuniculi infection, trauma, and P. multocida infection. Although less common, listeriosis and aberrant larval migrans can also cause torticollis. Diagnosis is generally based on a thorough history, physical examination, radiographs, CBC, serology, and CT scans. Treatment can be difficult and prolonged. Enrofloxacin can be used to manage P. multocida and fenbendazole/albendazole to manage E. cuniculi infections.21
DISEASES OF THE MUSCULOSKELETAL SYSTEM
Splay leg or hip dysplasia is an apparent congenital condition seen in young rabbits. Affected rabbits present with an inability to abduct their legs, which prevents ambulation. Amputation is recommended if only one leg is affected.14 Although this condition is thought to be genetic, environmental factors may also have some effect. In a recent study, rabbits housed on slippery surfaces had an increased incidence of splay leg compared with those housed with appropriate traction.33 In severe cases, humane euthanasia is warranted.
DISEASES OF THE REPRODUCTIVE SYSTEM
Pyometra occasionally occurs in rabbits and is usually reported shortly after parturition. Affected rabbits may be asymptomatic or may show signs of anorexia, weight loss, vaginal discharge, hematuria, or infertility. Diagnosis is made based on history, physical exam, CBC, radiology, and ultrasound. Inflammatory leukograms are common. Survey radiographs and ultrasound often reveal a fluid-filled soft tissue structure in the caudoventral abdomen. Affected animals should be provided supportive care (e.g., fluids, calories) to correct any deficiencies. Systemic antibiotics are warranted. Ovariohysterectomy is the treatment of choice.
Orchitis is relatively uncommon in captive rabbits because many animals are neutered at a young age. The disease is spread venereally, and can be caused by a bacterial infection. Clinical signs generally include enlarged testicles or epididymis and discomfort. Orchiectomy can be done in pet animals. Antibiotics may be used to treat breeding rabbits. The animal should be reevaluated before it is placed back into the breeding rotation.
Pregnancy toxemia is a problem that occurs in does at the end of gestation during nesting; these animals, especially obese Dutch, Polish, and English breeds, fast or have reduced caloric intake.14 Clinical signs include weakness, depression, incoordination, coma, convulsions, or death. Fasting leads to ketoacidosis and shock. Treatment should be aimed at reversing ketoacidosis and providing both shock therapy and supportive care. Supportive care may include the provision of calcium gluconate, fluids, and nutritional support. Treatment is usually unsuccessful, so prevention is the key. Clients should attempt to reduce obesity in breeding does and monitor their caloric intake carefully during pregnancy.
Cystic uterine hyperplasia is a problem in aged, intact does. Clinical signs include hematuria, anemia, reduced activity, a firm irregular uterus, cystic mammary glands, and cystic ovaries. Diagnosis is via clinical signs, radiology, and ultrasound. Ovariohysterectomy should be recommended to treat this condition.4
Pseudopregnancy can occur in does and lasts, on average, 16 to 18 days. Toward the end of the pseudopregnancy, does will go through normal nesting behavior (e.g., pulling out hair to line nests) and will have mammary development. Pseudopregnancy can resolve spontaneously or lead to pyometra or hydrometra.4 Ovariohysterectomy is the treatment of choice.
Dystocia and retained fetuses occur commonly in obese does, in cases where the fetus is too large for the doe's pelvic canal, or in does with uterine inertia. Clinical signs include nonproductive contractions and bloody to greenish-brown vaginal discharge. In nonobstructive dystocia, calcium gluconate and oxytocin may aid in the delivery of fetuses. In obstructive dystocia, a cesarean section is required. For prolonged dystocia events, the prognosis is guarded for both the doe and fetus.
Lactating or pseudopregnant does are prone to developing mastitis. Clinical signs in affected animals are similar to those observed in other animals and include pyrexia, swelling, blue coloration of teats, inappetence, and depression. Bacteria, such as Staphylococcus spp. and Streptococcus spp., are usually the causative agent. Without treatment, mastitis can progress to septicemia and death. Treatment includes surgical drainage or mastectomy, warm compresses, and appropriate antibiotics. Penicillin (40,000 U/kg IM BID × 5 days) has been used with success.14
Other reproductive problems that occur in rabbits are abortion-reabsorption, decreased fertility, prolapsed vagina, endometrial venous aneurysm, hydrometra, uterine torsion, cryptorchidism, testicular neoplasia, venereal spirochetosis, and cystic mastitis.
DISEASES OF THE EXCRETORY SYSTEM
The exact cause of urolithiasis in rabbits is unknown; however, diet, vitamin and mineral oversupplementation, and infections can be predisposing factors. Clinical signs are often nonspecific and include lethargy, inappetence, abdominal distension, urine scalding, perineal debris, and hematuria. A thickened bladder can sometimes be palpated on physical examination. A diagnosis can be made from radiographs and a urinalysis (e.g., crystals). A cystotomy is generally needed to treat urolithiasis. Reducing calcium in the diet may help decrease the likelihood of urolithiasis formation.
Cystitis is a common problem in does. Diagnosis is generally made from a cytologic examination (e.g., inflammatory cells and bacteria) and bacterial culture and sensitivity. Opportunistic Gram-negative bacteria are the most common isolates. Treatment is ideally based on culture and antibiotic sensitivity results.
Renal failure can occur in rabbits as a result of hypercalciuria (e.g., mineralization) or infectious agents (e.g., E. cuniculi). Clinical signs include polyuria and polydypsia, weight loss, inappetence, anemia, and lethargy. Diagnosis can be made based on radiology, chemistry panel, CBC, and urinalysis. An inverse calcium-to-phosphorus (Ca:P) ratio is generally indicative of renal failure. Survey radiographs may reveal mineral densities in the kidneys. Isosthenuria, in the face of an inverse Ca:P ratio, on a urinalysis can be used to confirm a diagnosis. Confirmation can be made via renal biopsy. Treatment should focus on monitoring fluid levels.
Renal cysts, or renal polycystic kidney syndrome, have been reported in New Zealand white rabbits. Most affected rabbits have nonspecific clinical signs. Animals with clinical disease generally are lethargic and anorectic. Polycystic disease can be associated with hypercalcemia, hypercreatinemia, and arterial mineralization.34
E. cuniculi can cause renal disease in rabbits. Early in the disease course, the parasite causes segmental, granulomatous, interstitial nephritis, which can be seen as irregular depressions on the surface of the kidneys. However, interstitial fibrosis can occur late in the disease. Many times these changes do not cause a reduction in kidney function. (For more information about E. cuniculi, see Parasitic Disease.)
“Red urine” is a common problem in rabbits. In these cases, veterinarians must determine whether a sample is red because of the presence of blood or porphyrin pigment. Blood indicates a breakdown in the epithelial barrier and is common with cystitis, uroliths, and neoplasia. The presence of porphyrin pigment is associated most with stress, dietary changes, or both. A thorough history will often provide insight into whether a recent stress event or dietary change occurred. A urinalysis should be done to determine if a color change in the urine is based on blood or pigment. The presence of red blood cells is diagnostic. Disease rule-outs for animals with blood in their urine include neoplasia (e.g., adenocarcinoma), endo metrial aneurysms, urinary or reproductive tract polyps, cystitis, pyelonephritis, urolithiasis, disseminated intravascular coagulopathy, and lead toxicosis.
DISEASES OF THE GASTROINTESTINAL SYSTEM
Dental disease, as previously mentioned, is common in pet rabbits. Clinical signs can vary, but generally include difficulty with prehension of food, anorexia, cachexia, dacryocystitis, abnormal facial swelling, and excessive salivation. Incisor malocclusion has been attributed to congenital factors, such as mandibular prognathism, and is seen at an increased rate in rabbits less than 1.5 kg or certain breeds (e.g., Netherland dwarf rabbits).2 Animals that suffer traumatic injuries to the jaws are also more susceptible to developing tooth malocclusion. Abnormal incisor growth can lead to the blockage of the nasolacrimal duct, resulting in epiphora. Incisor malocclusion can also lead to molar malocclusion, causing further dental complications. Trimming incisors is the primary method for correcting malocclusion, and this can be done using a high-speed Dremel tool (Dremel, Racine, WI) or dental drill (Figure 14-19 ). Incisors grow at a rate of 3 mm per week when the opposing incisor is present and 1 mm per day when there is no tooth opposition. Therefore, trimming should be done at approximately 3-week intervals when there is a malocclusion or no opposing incisor. Toenail clippers have been recommended for trimming incisors; we do not recommend this, as toenail clippers have been associated with tooth fracture. Where there is severe malocclusion, complete incisor extraction is recommended. This is a relatively easy procedure; however, it does require patience. Aggressive, rough manipulation of the teeth can lead to tooth fracture and, again, pain and discomfort. (This procedure is discussed in detail in Surgery.) If the entire root and germinal tissue are not removed, the teeth can regenerate, requiring additional surgery.
Figure 14-19.

High-speed dental tools are recommended for trimming rabbit teeth.
Cheek teeth or molar malocclusion is characterized by crown elongation and the development of hooks and points. Coronal overgrowth (due to incisor overgrowth or poor occlusion) requires not only that the spikes be removed but also that the crowns be reduced and the angulation be corrected for ideal occlusion. Maxillary cheek teeth and all but the first mandibular cheek teeth can be reduced to the level of the gingiva; however, this is not always necessary. In some cases, removing the points is sufficient. The gingiva naturally exposes the cranial aspect of the first mandibular cheek tooth. Ideally, when trimming or floating the cheek teeth, the procedure should be done with power equipment (e.g., flat fissure bur) to ensure rapid tooth removal without damaging the surrounding tissue. The use of rasps or clippers can create friction and rock the teeth, causing periapical and periodontal damage. For this reason, these tools are not recommended or should be used with extreme caution. Extraction of the cheek teeth is much more complicated than removal of the incisor and more traumatic to the rabbit. (Crossley DA, personal communication) When several extractions are required, it is best to perform multiple procedures to minimize the pain and discomfort to the animal. For example, extracting the teeth on one side of the jaw will allow the animal to still use the opposite side to process its food. (Cheek tooth extraction is discussed in detail in Surgery.)
Incisor and cheek tooth root elongation can result in an overall deterioration of the tooth quality. Affected animals often present with epiphora, mandibular swelling, or nasal discharge. If left undiagnosed and untreated, root elongation can lead to abscess formation and osteomyelitis. Dental or skull radiographs can be used to evaluate the roots of the teeth, and if any are diseased, extraction should be considered.
Mucoid enteropathy is a poorly understood condition that causes both constipation and diarrhea, especially in young rabbits. The etiology of this disease is unknown, but it may be associated with a reduction in cecal pH. Feeding rabbits a diet that is high in fiber appears to control cecal pH and reduce the likelihood of this disease. Because the etiology of this disease is unknown, treatment is based on providing supportive care, antibiotics against opportunistic bacteria, and probiotics.
Rabbits are susceptible to a number of different infectious and parasitic diseases, many of which affect the gastrointestinal system. (A review of those diseases can be found in Infectious Disease.)
DISEASES OF THE RESPIRATORY SYSTEM
Upper respiratory tract disease (URTD) is a common presentation in rabbits. Affected rabbits often present with rhinitis and sinusitis. There are numerous potential causes of URTD in rabbits, including bacterial infections (e.g., P. multocida, B. bronchiseptica, and Staphylococcus spp.), foreign bodies, dental abscesses, and myxomatosis. Rabbits with URTD are often anorectic, as they cannot smell or locate their food. Serous to purulent nasal discharge is common. Epiphora is also a common finding. Many rabbits with clinical disease have snuffles. This is a generic term used to describe clinical signs, but it is attributed primarily to pasteurellosis. It is important to recognize that snuffles is nonspecific and may be associated with any of the previously mentioned causes. Rabbits with URTD often have “wet” forelegs as a result of the chronic nasal discharge. In severe cases of URTD, when a rabbit cannot move air through its nasal passages, it becomes dyspneic and breathes with an open mouth. Hematologic testing (e.g., CBC), culture and antibiotic sensitivity testing, radiographs, and endoscopy may be required to diagnose a specific cause of URTD. Treatment should be based on a specific diagnosis. Animals that are dehydrated and anorectic should be provided fluids and caloric support.
In rabbits, lower respiratory tract disease is primarily associated with bacterial disease. Gram-negative bacteria (e.g., P. multocida, B. bronchiseptica, Klebsiella spp.) are the most common cause of pneumonia in rabbits. Although not as common, Gram-positive bacteria (Staphylococcus aureus) have also been associated with pneumonia. Viral diseases are not well-studied but likely play a limited role. Primary lung neoplasia can occur, but it is not common. Rabbits are more likely to develop secondary metastasis. Rabbits with pneumonia may present in either an acute or chronic disease state. Rabbits with acute pneumonia are often acutely depressed, lethargic, and anorectic. The rabbits may or may not have audible respiratory sounds (e.g., wheezing, crackling). On physical examination, the rabbit may be febrile, have nasal discharge, and have auscultable respiratory wheezing or crackling. With chronic pneumonic presentations, the rabbit may appear clinically normal (e.g., walking pneumonia) but may have abnormal lung sounds. Beyond the physical examination, a CBC, survey radiographs, tracheal wash with cytologic examination and culture and antibiotic sensitivity profile, and endoscopic examination can be used to diagnose the pneumonic condition and establish a prognosis. Pneumonia in rabbits generally carries a poor to grave prognosis. Treatment should be based on the diagnostic results. Fluoroquinolones and potentiated sulfonamides are excellent first-choice antibiotics, as they have excellent distribution to the lungs.
CARDIAC DISEASE
Cardiac disease is a problem being encountered with increased frequency in captive rabbits. The primary reason for this is improved husbandry and the resultant increased longevity. Rabbits with cardiac disease may be dyspneic, tachypneic, exercise intolerant, lethargic, and depressed and have edematous extremities. A complete diagnostic work-up is required to characterize the status of the patient and provide the client a prognosis. Beyond a physical examination and careful cardiac auscultation, suspect cardiac cases should have an electrocardiogram done to determine if there are any abnormal arrhythmias, survey radiographs to evaluate heart and great vessel size, and an echocardiogram to assess the valves, muscle thickness, and cardiac function. A CT scan or MRI can also be done to evaluate the heart in more detail.
Cardiomyopathy can occur in all breeds, but it occurs at a higher incidence in giant breeds.2 Affected animals often develop myocardial fibrosis. A number of different causal factors have been associated with cardiomyopathy in rabbits, including bacterial and corona-virus infections and vitamin E deficiency. Arteriosclerosis is also a common finding in rabbits fed a vitamin D and calcium-rich diet. Although a diagnosis of arteriosclerosis is most often made at necropsy, in some cases, a diagnosis can be made from survey radiographs. An increased mineralized density in the aorta is the most common radiographic finding.
Congestive heart failure is common in middle-aged to geriatric rabbits. Rabbits with congestive heart failure should be stabilized and the excess burden on the heart controlled. Furosemide (1-4 mg/kg IV or IM) and 2% nitroglycerine ointment (⅛ strip on the ear) can be used to eliminate excess fluid and improve contractility.4 In cases of pleural effusion, a thoracocentesis may be performed. After the animal's immediate condition is stabilized, further work should focus on diagnosing the cause of the congestive heart failure. Because rabbit cardiac drug doses are empirical and frequently inaccessible, doses for cats or ferrets can be used.4 It is important to monitor rabbits for adverse effects to cardiac drugs, as would be done in domestic pets.
HEAT STROKE
Rabbits are extremely sensitive to elevated environmental temperature, especially when combined with high humidity. Because rabbits are unable to sweat, and panting is inefficient for them, they rely on the large surface area of the ears to serve as the site of evaporative cooling. Rabbits with heat stroke may present for lethargy, lateral recumbency, dyspnea, cyanosis, diarrhea, hyperthermia (>104° F), and seizures. Heat stroke carries a grave prognosis. Cooling a hyperthermic rabbit too quickly can lead to iatrogenic hypothermia, which can exacerbate the physiologic shock the animal experiences. In addition to cool water baths, 70% isopropyl alcohol can be applied to the ears and extremities to facilitate body cooling. An intravenous catheter should be placed, and fluids should be provided to maintain the intracellular and extracellular fluid balance. Antiinflammatories (e.g., meloxicam and carprofen) should be administered to minimize the catastrophic effects of the inflammatory response. Parenteral antibiotics are warranted to minimize the impact of opportunistic infections. Because some of the deleterious effects associated with heat stroke are not immediately apparent (e.g., cell death resulting from fluid shift), veterinarians should monitor animals closely for 7 to 14 days after the presentation.
THERAPEUTICS
Many of the therapeutics used to treat dogs and cats can also be used to manage rabbit patients, which reduces the burden of maintaining an appropriate rabbit pharmacy for the veterinarian. However, in some cases, it may be necessary for the veterinarian to obtain rabbit-specific formulations, that are flavored (e.g., fruity) or more specific to the needs of the rabbit. Fortunately, there are a number of compounding pharmacies that can assist with designing specific formulations for these animals. The following section is an introduction to the therapeutics commonly used to treat rabbits in captivity. (For a more extensive list, see Carpenter, JW: Exotic Animal Formulary, St. Louis, 2005, WB Saunders.)
Fluid Therapy
Rabbits that are dehydrated require immediate attention. The redistribution of fluids that results from dehydration can lead to a number of internal changes. One of these changes, occurring when fluid is resorbed from the intestinal tract, can lead to reduced intestinal motility and catastrophic changes to the microflora.
The maintenance fluid rate for rabbits is 80 to 100 ml/kg/day. For rehydration the rate can be increased to 10 to 20 ml/kg/hr over the first few hours.9 In addition to providing the maintenance fluids for an anorectic rabbit, the veterinarian should also estimate and correct the animal's fluid deficit. Skin elasticity, mucous membrane moisture, capillary refill time, femoral pulse, positioning of the globe, and packed cell volume and total solids concentration are useful indicators for assessing dehydration. Slowed skin elasticity and tacky mucous membranes generally suggest an animal is 3% to 5% dehydrated. Those findings, in combination with a decreased capillary refill time, increased packed cell volume and total solids, and increased “thready” pulse, suggest 5% to 8% dehydration. All of these findings, in combination with the presence of sunken eyes, suggest an animal is more than 8% dehydrated. It is essential that the fluid deficit be replaced promptly (e.g., over 24 hours) to reestablish the fluid balance between the extra- and intracellular spaces.
When selecting fluids for replacement, it is important to consider the rabbit's physiologic status. Animals with elevated osmolalities should be provided hypotonic fluids to prevent worsening the fluid dynamics. Normasol (293 mOsm/L), lactated Ringer's (273 mOsm/L), and 0.9% saline (308 mOsm/L) represent isotonic crystalloids that can be used for fluid replacement. Dextrose (253 mOsm/L) can also be used, especially if the animal is hypoglycemic or if there is a need to rapidly replace the intracellular space. Animals that are hypoproteinemic may require colloid fluid replacement. Colloids can be used to offset the loss of oncotic pressure that can occur with hypoalbuminemia. Rabbits that are anemic may also require a blood transfusion or oxyglobin, a synthetic hemoglobin replacement. All rabbit patients should be monitored closely during fluid replacement so that neither fluid overload nor insufficient fluid replacement occurs. Pulmonary edema, ascites, and/or anasarca may occur in animals provided excess fluids. Animals provided insufficient fluids will remain clinically dehydrated. In these cases, the fluid deficit should be reevaluated and the appropriate fluid volume provided.
Fluids can be delivered per os (PO), subcutaneously (SC), intravenously (IV), intraosseously (IO), or intraperitoneally (IP). The route of administration should be based on the needs of the rabbit. If the animals is mildly dehydrated and has a functional gastrointestinal tract (e.g., no diarrhea), then fluids should be provided PO. This represents the natural route of fluid administration. For mildly dehydrated animals that will not tolerate PO fluids or have diarrhea, SC fluids are recommended. SC fluids can be administered on the dorsum between the scapulae or over the lateral body wall. Animals that are moderately to severely dehydrated should be given fluids IV or IO. The primary IV sites we use are the cephalic, lateral saphenous, or auricular veins. The site for catheter placement should be sterilely prepared before catheter placement. The largest bore catheter that can be fitted comfortably into the vein should be used. For most large breeds, a 20- or 22-gauge catheter can be used, whereas a 24- or 26-gauge catheter may be required for smaller breeds. A smaller bore catheter should be used for the auricular vein. The peripheral site for IO catheter placement is the femur. A surgical preparation using a disinfectant (e.g., Betadine or chlorhexidine) should be done to minimize contamination at the catheter site. A local anesthetic should be used to minimize the discomfort associated with the procedure. The trochanteric fossa is the landmark for catheter insertion. A spinal needle should be used for the procedure, as it has a stylet and will prevent the needle from being clogged with a bone core. The IO route is preferred in cases where the animal is severely dehydrated and the veins are collapsed. IP fluids are generally reserved for moderately dehydrated rabbits when IO or IV catheters are not available or for juvenile rabbits. To administer these fluids, place the rabbit in dorsal recumbency and insert the needle in the caudal abdomen. The needle should be inserted at a 20- to 30-degree angle to the body. Aspirating before delivering the fluids should ensure that the needle is not in the viscera.
Antimicrobial Therapy
ANTIBACTERIAL AND ANTIFUNGAL AGENTS
Antimicrobial therapy is ideally based on culture and antibiotic sensitivity testing. When selecting an antimicrobial for a rabbit, it is important to avoid those compounds that may predispose the rabbit to dysbiosis, such as lincomycin, ampicillin, amoxicillin, amoxicillin-clavulanic acid, cephalosporins, clindamycin, penicillins, and erythromycin. Because most infections in rabbits are associated with opportunistic Gram-negative bacteria, initial antibiotic selection should be based on broad coverage against these bacteria. Enrofloxacin and trimethoprim-sulfadiazine are two excellent first-choice antibiotics. For a complete list of antibiotic compounds, see Box 14-3 . A list of common antifungal compounds can be found in Box 14-4 .
BOX 14-3. Antibiotic Agents Commonly Used to Treat Bacterial Infections in Rabbits.
| Chloramphenicol | 30 mg/kg PO BID |
| Ciprofloxacin | 10-20 mg/kg PO BID |
| Ophthalmic 0.3%: 1 drop BID-TID | |
| Enrofloxacin | 5 mg/kg PO, SC, IM, IV BID35 |
| Metronidazole | 20 mg/kg PO BID |
| 40 mg/kg PO SID × 3 days | |
| Penicillin | 40,000-60,000 IU/kg SC, IM SID × 5-7 days |
| Penicillin G, benzathine | 42,000-60,000 IU/kg SC, IM q48h |
| Penicillin G procaine | 42,000-84,000 IU/kg SC, IM SID |
| Rifampin/clarithromycin | R-40 mg/kg; C-80 mg/kg PO BID |
| Silver sulfadiazine | Topical SID; safe if ingested |
| Sulfadimethoxine | 10-15 mg/kg PO BID |
| Trimethoprim sulfadimethoxine | 30 mg/kg PO BID |
BID, twice a day; IM, intramuscular; IU, international unit; IV, intravenous; PO, per os; SC, subcutaneous; SID, once a day; TID, three times a day.
From Carpenter JW, Mashima TY, Rupiper DJ: Exotic Animal Formulary, ed 2, Philadelphia, 2001, WB Saunders.
BOX 14-4. Antifungal Drugs Commonly Used to Treat Rabbits.
| Clotrimazole | Topical |
| Fluconazole | 25-43 mg/kg IV slowly BID |
| Ketoconazole | 10-40 mg/kg PO SID × 14 days |
| Lime-sulfur dip (2%-3%) | Topical q 5-7 days × 4 wk |
| Miconazole | Topical SID × 2-4 wk |
BID, twice a day; PO, per os; SC, subcutaneous; SID, once a day.
Antiparasitic Therapy
Rabbits are susceptible to a number of endo- and ectoparasites. The most common endoparasites are coccidia and nematodes. The drugs and doses commonly used to treat these parasites are found in Box 14-5 . Ectoparasites, including mites and lice, are also common.
BOX 14-5. Antiparasitic Drugs Commonly Used to Eliminate Parasites in Rabbits.
| Drug | Dosage | Parasite |
|---|---|---|
| Albendazole | 7.5-20 mg/kg PO SID | Nematodes; E. cuniculi |
| Ivermectin | 0.4 mg/kg SC q7days × 2-3 wk | Nematodes, ectoparasites |
| Fenbendazole | 20 mg/kg SID | E. cuniculi |
| 10 mg/kg PO, repeat in 14 days | Nematodes | |
| Lime-sulfur dip (2%-3%) | Topically q7days × 4 wk | Ectoparasites |
| Sulfadimethoxine | 10-15 mg/kg PO BID | Coccidia |
BID, twice a day; PO, per os; SC, subcutaneous; SID, once a day.
Nonsteroidal Antiinflammatories
Nonsteroidal antiinflammatories (NSAIDs) are routinely used to manage inflammation and pain in rabbits. Some of their most common uses include treating musculoskeletal pain associated with trauma, arthritis, soft tissue pain from dental disease, and gastric stasis. Historically, flunixin meglumine was the only compound available for rabbits. This drug serves an important purpose in managing pain, but it has to be used judiciously because of the potential for gastric ulcer formation. The newer generation NSAIDs (e.g., carprofen and meloxicam) are more selective and less likely to cause complications. A list of commonly used NSAIDs and their doses can be found in Box 14-6 .
BOX 14-6. Nonsteroidal Antiinflammatory Drugs Commonly Used to Manage Inflammation and Pain in Rabbits.
| Meloxicam | 0.3 mg/kg PO SID |
| 0.2 mg/kg IM or SC SID | |
| Flunixin meglumine | 1-2 mg/kg IM or SC BID; limit to 3 days to minimize gastric ulcer formation |
| Carprofen | 2-4 mg/kg SC SID |
| 1.5 mg/kg PO BID | |
| Ketoprofen | 3 mg/kg IM or SC BID |
BID, twice a day; IM, intramuscular; PO, per os; SC, subcutaneous; SID, once a day.
Steroids
The use of steroidal compounds is controversial in rabbits. In the past, steroids have been recommended for managing inflammation associated with neurologic disease (e.g., trauma or E. cuniculi infection) or shock. However, hydrocortisone has been found to cause significant immunosuppression in rabbits.36 Because of the high likelihood for immunosuppression, steroids should be used judiciously. We generally reserve their use for the treatment of spinal disease (prednisone sodium succinate, 1 mg/kg IV) and dermatologic conditions (e.g., flea bite allergy or extreme hypersensitivity) (prednisone, 0.5-1 mg/kg SID to BID, taper dose).
Cardiac Drugs
Long-term therapy for heart disease may require treatment with diuretics (furosemide, 1-4 mg/kg IM) and other compounds required for a particular cardiac disease presentation. The use of cat and ferret dosages for cardiac drugs is advocated. Monitoring for drug side effects should be done as in feline medicine.
Emergency Drugs
Rabbits that develop respiratory or cardiac arrest should be treated immediately with the appropriate emergency drugs. Dopram may be used for respiratory arrest, whereas epinephrine should be administered during cardiac arrest. A list of commonly used emergency drugs and their doses is listed in Box 14-7 . Rabbits have atropinases, so the use of atropine is not commonly recommended. Glycopyrrolate can be used to help manage bradycardia; however, it is important to consider that this compound can increase the viscosity of tracheal secretions and impede the airflow to the lungs. Intubating and ventilating animals can minimize this risk.
BOX 14-7. Emergency Drugs Commonly Used to Treat Rabbits.
| Epinephrine | 0.2 mg/kg IV or IT |
| Furosemide | 1-4 mg/kg IM |
| Hetastarch | 20 ml/kg IV |
| Lactated Ringer's solution | 10-20 ml/kg/hr |
| Lidocaine | 1-2 mg/kg IV |
IM, intramuscular; IT, intratracheal; IV, intravenous.
Transfusions
In cases of severe anemia (PCV < 10%) or acute blood loss (20%-25%), a blood transfusion is recommended. The blood volume of a rabbit is estimated to be between 55 and 65 ml/kg.37 Cross-matching for transfusion in rabbits is not necessary for a first-time recipient.37 The donor must be healthy and must not have a history of infectious disease or neoplasia. A healthy blood donor can have up to 10 ml/kg of blood collected. The jugular vein is the recommended site for blood collection from the donor rabbit. The blood should be collected with a citrate anticoagulant that is mixed with blood at a rate of 1 : 3.5 (citrate:donor blood). The blood transfusion should be done within 4 to 6 hours of collection at a rate of 6 to 12 ml/kg/hr.
Nutritional Support
Anorexia in rabbits should be treated as an emergency. When rabbits stop eating, physiologic changes can occur in the gastrointestinal tract (e.g., mucosal atrophy) that alter the gastrointestinal permeability. From this, a cascade of events can occur (e.g., reduced motility, decreased water absorption, pH alterations, microbial flora changes) that lead the animal into a life-threatening situation. The first step in managing these cases should be to restimulate the gastrointestinal tract, and the second step is to diagnose the cause of the anorexia. Providing nutritional support is the best method for restimulating the gastrointestinal tract. Motility modifiers can also be used, but these should be given only when there is no concern for a gastric or small intestine foreign body (e.g., trichobezoar).
The preferred method for administering nutritional support to mild or moderately ill animals is syringe feeding. In more severe cases, an orogastric tube or nasogastric tube should be placed. A 3.5- to 5.0-French pediatric feeding tube can be used as a nasogastric tube. Before placing the tube, it should be marked to estimate the distance between the nare and the stomach. Placement can be achieved by inserting the tube through the ventral medial nasal meatus and advancing it ventromedially. The rabbit's head should be maintained in a natural position during the procedure to facilitate passage of the tube. Placement of the tube should be evaluated using survey radiographs. Once placed, the tube can be secured to the skin covering the nasal bones using suture. A pharyngostomy or gastrotomy tube can also be placed to facilitate feeding.
Enteral diets used for dogs or cats are not appropriate for use in rabbits. The domestic pet enterals are based on the nutritional needs of omnivores (dogs) and carnivores (cats) and will not meet the needs of an herbivore (rabbit). Oxbow critical care for herbivores (Oxbow Pet Products, Murdock, NE) is our preferred enteral. High-fiber Ensure or Ensure plus (Ross Laboratories, Columbus, OH) can also be used, but, again, these are not considered ideal. Although less complete, a variety of other pelleted rations, leafy greens, and vegetable baby foods can also be used as enterals.9 Label directions should be followed closely to ensure that the rabbit receives the appropriate quantity of calories. In cases where homemade diets are used, calorie content of the enteral constituents should be estimated or determined using available resources in the literature or on the Internet. The provision of nutritional support should continue until the rabbit is free-feeding on its own.
SURGERY
Preoperative and Postoperative Considerations
Although fasting is not necessary in rabbits because they cannot vomit, eliminating access to food 1 to 2 hours before inducing anesthesia minimizes the amount of food in the oral cavity and improves the direct visualization of the larynx for intubation. We recommend performing presurgical blood work to determine the physiologic status of the rabbit patient. This information can be used to provide the client with a prognosis related to the anesthetic procedure. Any rabbit with preexisting respiratory disease should be thoroughly evaluated before it undergoes a procedure. Rabbits have a small lung capacity, and any pulmonary disease can have a negative impact on the anesthesia event.
Postoperative considerations for rabbits include minimizing the likelihood for anorexia, providing analgesia and antibiotics (when necessary), and monitoring fluid therapy. Thermal support should be provided until the rabbit has recovered and can maintain a normal body temperature. Rabbits should be recovered in a quiet area. Excessive noise or commotion can lead to increased stress (e.g., increased cortisol), immunosuppression, and decreased healing. Animals should be offered their normal food for a prolonged stay and a comfortable substrate in their cages.
Anesthesia
Anesthetic selection should be based on the procedure being performed. For example, in a case where a localized abscess needs to be lanced, a local anesthetic (e.g., lidocaine, prilocaine) and an opioid can be used to perform the procedure. Many veterinarians anesthetize rabbits using only inhalant anesthesia. Rabbits can be induced using a face mask or induction chamber. We prefer to induce rabbits with additional compounds before we provide inhalants, because it more effectively relaxes the animal for intubation. A benzodiazepam (e.g., diazepam, midazolam) and opioid are routinely used to provide relaxation and analgesia before inducing the animal. Other commonly used veterinary anesthetics (e.g., ketamine HCl, medetomidine, acepromazine) and analgesics can also be used for induction or as maintenance anesthetics. Doses for these compounds can be found in TABLE 14-2, TABLE 14-3, TABLE 14-4 . One compound that should be avoided is Telazol (Fort Dodge Animal Health, Fort Dodge, IA), as it has been shown to cause severe renal tubular necrosis in rabbits.38
TABLE 14-2.
Induction and General Anesthetics for Rabbits
| Agent | Dose (mg/kg) | Route | Sedation | Analgesia |
|---|---|---|---|---|
| Acepromazine | 1 mg/kg | IM | ++ | * |
| Diazepam/midazolam | 0.5-2 mg/kg | IV, IM | + to ++ | * |
| Fentanyl (can cause respiratory depression; can be partially reversed with buprenorphine) | 0.2-0.5 mg/kg | IM | + to +++ | + to +++ |
| Glycopyrrolate | 0.01 mg/kg | IV | ||
| 0.1 mg/kg | SC, IM | |||
| Ketamine | 10-50 mg/kg | IM | ++ to +++ | + |
| Medetomidine (reversible with atipamizole) | 0.1-0.5 mg/kg | IM | + to +++ | + |
| Xylazine (reversible with atipamizole 1 mg/kg or yohimbine 0.2 mg/kg IV) | 2-5 mg/kg | IM | + to ++ | + |
| Propofol (light anesthesia; can cause apnea, use with caution in un-intubated patients) | 3-6 mg/kg | IV |
IM, intramuscular; IV, intravenous; SC, subcutaneous.
, none; +, minimal; ++, moderate; +++, heavy.
From Harcourt-Brown F: Textbook of Rabbit Medicine, London, 2002, Elsevier; Flecknell P: Laboratory Animal Anaesthesia, ed 2, San Diego, 1996, Academic Press.
TABLE 14-3.
Analgesics Commonly Used for Captive Rabbits
| Agents | Dose (mg/kg) | Route | Comments |
|---|---|---|---|
| Buprenorphine | 0.01-0.05 | IV, SC | Analgesia for 6-12 h; for acute soft tissue, GI, and urogenital pain |
| Oxymorphone | 0.05-0.2 | IM, SC | q8-12h |
| Butorphanol | 0.1-0.5 | IM, SC, IV | Analgesia lasts 2-4 h; can be used with acepromazine to deliver sedation |
| Fentanyl | 0.2-0.3 | IM | |
| Carprofen | 2-4 | SC | q24h |
| 1.5 | PO | q12h | |
| Meloxicam | 0.2 | IM, SC | q12h |
| 0.3 | PO | ||
| Flunixin meglumine | 1-2 | IM, SC | q12h |
| Ketoprofen | 3 | IM, SC | q12h |
IM, intramuscular; IV, intravenous; PO, per os; SC, subcutaneous.
From Harcourt-Brown F: Textbook of Rabbit Medicine, London, 2002, Elsevier; Heard DJ: Anesthesia, analgesia, and sedation of small mammals. In Queensberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, St Louis, 2004, WB Saunders; Paul-Murphy J, Ramer JC: Urgent care of the pet rabbit, Vet Clin North Am Exot Anim Pract 1(1):125-152, 1998.
TABLE 14-4.
Common Anesthetic Combinations Used to Anesthetize Rabbits
| Agents | Dose (mg/kg) | Route | Duration/anesthesia |
|---|---|---|---|
| Medetomidine | 0.2 mg/kg | SC, IM | Anesthesia 30-40 min; recovery 1-4 h |
| Ketamine | 10 mg/kg | ||
| Butorphanol | 0.05 mg/kg | ||
| Fentanyl | 0.3 ml/kg | IM | Anesthesia 20-40 min with good muscle relaxation; recovery 1-2 h |
| Midazolam | 0.5-2 mg/kg | IV | |
| Ketamine | 20-35 mg/kg | IM/IV | Anesthesia 25-35 min; recovery 1-2 h |
| Xylazine | 3-5 mg/kg | ||
| Ketamine | 20-35 mg/kg | IM/IV | Anesthesia 20-30 min; recovery 1½ h |
| Diazepam | 1-5 mg/kg |
From Harcourt-Brown F: Textbook of Rabbit Medicine, London, 2002, Elsevier; Flecknell P: Laboratory Animal Anesthesia, ed 2, San Diego, 1996, Academic Press. IM, intramuscular; IV, intravenous; PO, per os; SC, subcutaneous.
We recommend intubating rabbits for any procedure that requires general anesthesia. When an animal is intubated, it gives the anesthetist more control over the patient. Rabbits that are not intubated and experience respiratory arrest are rarely recovered. Intubating rabbits can be difficult. These animals have long, narrow oral cavities, which restrict direct visualization of the larynx. Several techniques have been described for intubating rabbits. Blind intubation using a 2.0 to 2.5 outside diameter (OD) endotracheal tube can be done, but it can be associated with laryngeal trauma.9 To perform the procedure, the head should be elevated and neck stretched. The endotracheal tube should be gently lowered into the trachea. This technique requires patience and skill. Practicing this technique on recently expired rabbits is one way of obtaining experience with this procedure. Our preferred method for intubating rabbits is via an endoscope.39 A 2.7-mm rigid endoscope can be used to directly visualize the larynx and ensure passage of the tube into the trachea. In those cases where laryngospasms limit access to the trachea, lidocaine can be applied topically.
Rabbits should be monitored closely during a surgical procedure. An ultrasonic Doppler, ECG, and/or pulse oximeter can be used to monitor the heart rate and rhythm during the procedure. A respiratory monitor can be used to monitor respirations. We prefer to use a ventilator during rabbit procedures to ensure that the rabbit is receiving an appropriate dose of anesthesia and oxygen. The ventilator also ensures that the animal is respiring. A capnograph can be added in line to measure carbon dioxide levels and determine the rabbit's respiratory efficiency. In addition to the capnograph, the pulse oximeter can provide additional blood gas information by providing an estimate of oxygen saturation levels. Rabbits should be provided thermal support during a procedure, and its body temperature should be measured throughout the procedure. Routine anesthetic monitoring during a procedure is essential to a veterinarian's success and will limit the occurrence of anesthetic deaths.
Surgical Procedures
The majority of the surgical procedures performed on rabbits are associated with the reproductive tract, gastrointestinal tract, and integument. Of course, a rabbit can develop a need for surgical procedures on other systems as well, but, in our experience, they are less common. When faced with the need to perform a surgery on a rabbit, the veterinarian must identify the specific anatomic features that will be affected by the procedures and familiarize himself or herself with any specific indications on tissue handling methods. In cases where there are no specific descriptions for a surgical procedure in rabbits, veterinarians may rely on those surgical descriptions reported in canine and feline surgery.
When preparing a rabbit for surgery, it is important to adhere to strict aseptic techniques. Because rabbit skin is thin and tears easily, care should be taken when clipping the fur. We generally clip the fur with a small, battery-powered clipper or shave the fur with a razor. Holding the skin tight during fur removal can help reduce the likelihood of tearing the skin (Figure 14-20 ). There are a variety of methods that can be used to aseptically prepare a surgical site. Chlorhexidine and Betadine are excellent disinfectants. We generally use physiologic saline instead of alcohol during the preparation because it does not act as a heat sink, lowering the rabbit's body temperature.
Figure 14-20.

When shaving rabbit fur for a surgical procedure, be careful to avoid tearing the delicate skin.
The surgical instruments used for domestic pet surgery can also be used for rabbit surgery. Because of the small size of some dwarf rabbit breeds, ophthalmic instruments can also be beneficial. Microscopic loupes are helpful when manipulating fine tissues. Radiosurgery can be used to provide hemostasis during a procedure and minimize tissue trauma.
REPRODUCTIVE SURGERY
Orchiectomy or ovariohysterectomy should be recommended for rabbits when the pet owner has no interest in reproducing the animal. This prevents unwanted pregnancies in mixed gender populations, reduces the risk for certain neoplastic conditions, and can reduce the likelihood for certain behaviors (e.g., aggression in males). It is generally recommended that males be castrated after 3 months of age and females be spayed after 5 months. The procedure can be performed at an earlier age if deemed appropriate, such as may be the case in large, mixed colonies, but this may affect the growth of the animal (e.g., smaller size).
Orchiectomy
The inguinal canals of a rabbit are open throughout the animal's life. Therefore, a male rabbit can actively move its testicles between the scrotum and body cavity. Because the inguinal canals remain open, it is important to either perform a closed orchiectomy, or to close the inguinal canal after performing an open orchiectomy. If the inguinal canals are left open, it is possible for the animal to herniate omentum or bowel into the scrotal area.
There are two approaches that can be taken when making an initial incision. Either a prescrotal incision can be made, similar to that made for the dog, or scrotal incisions can be made, similar to those recommended for the cat. When performing the scrotal approach, each testicle is removed through a separate incision. If the tunic is not incised, a closed procedure can be done. At least two sutures should be placed on the cord in case a suture fails. Once ligated, the cords can be replaced into the scrotum, and the scrotum closed with an absorbable suture using a subcuticular pattern. Tissue glue can also be used to complete the skin closure. For the prescrotal incision, each testicle must be retrograded out of the incision. The same technique described earlier can be used for the orchiectomy, or a suture can be placed around the tunic at the cranial aspect of the incision, an open castration done, the remnant cord directed cranially past the suture placement, and the suture ligature tied down to close the canal. Again, a subcuticular closure or tissue glue can be used to close the skin incision. The animal should be monitored postoperatively for any swelling or discharge in the area of the incision or scrotum.
Ovariohysterectomy
An ovariohysterectomy should be recommended for any female rabbit not intended for breeding. It is recommended that the procedure be done when the animal is young (<5 months of age) because the amount of intraabdominal fat can increase dramatically with age and will reduce the surgeon's visibility. The incision for a rabbit ovariohysterectomy should be made from cranial to the pelvis to the umbilicus. The incision may need to be extended in some cases. Care should be taken when entering the peritoneal cavity, as the urinary bladder and/or cecum may be directly under the body wall. The ovaries are located in the same location as would be expected for a cat or dog. The ovaries can generally be grasped by hand, but a spay hook may be needed for some large breeds. Once the ovary is in hand, the ovarian pedicle should be identified. Circumferential sutures (at least two) should be placed around the pedicle, and the pedicle incised. The ovary and uterine horns should be gently grasped and elevated from the body cavity. Rabbits have two separate uterine horns, each with its own cervix (Figure 14-21 ). Each uterine horn should be ligated just cranial to its cervix. A circumferential and transfixation suture can be used to ligate the uterine horn. The large uterine vessels running parallel to the uterine horns should also be ligated at this time. Closure for an ovariohysterectomy is routine. The body wall, subcutaneous space, and subcuticular space can be closed with absorbable suture in a continuous pattern.
Figure 14-21.

A rabbit reproductive tract. Note the two cervices.
TEETH EXTRACTIONS
Incisor removal in rabbits should be done under general anesthesia. The gingival surfaces surrounding the base of the incisors should be thoroughly cleaned and disinfected. The peg teeth (second upper incisors) should be removed first. A dental elevator can be inserted in a 360-degree manner around the base of the tooth to separate the periodontal ligament. The tooth, when loose, can be gently extracted. For the large primary incisors, the elevator or incisor luxator blade can be used to perform the same procedure (Figure 14-22 ). Care should be taken when manipulating the tooth to prevent fracture. Once the tooth is loosened, gently rock it and pull it in the direction of the natural curvature. If the pulp tissue is not removed with the tooth, curette the socket with a sterile probe to destroy any remaining germinal tissue. If there is evidence of infection, treatment should include flushing with a disinfectant and the provision of systemic antibiotics.
Figure 14-22.

Incisor tooth elevators can be used to facilitate extraction.
Cheek teeth extractions can be done in a similar manner to incisor extractions. Anesthetize the patient, clean and disinfect the teeth and gingiva, and use a molar luxator to break down the periodontal fibers that secure the tooth to the bone. Molar forceps can be used to extract the cheek teeth. Mandibular cheek teeth extraction can also be performed via surgical access from the ventrolateral border of the mandible. In this case, the teeth are elevated from the apical end.
GASTROINTESTINAL SURGERY
In cases where gastric foreign bodies (e.g., carpet or hair) cannot be successfully treated medically, surgery should be attempted. However, it is important that owners be informed that the prognosis for gastrotomies in rabbits is poor. The prognosis can be improved if the surgical procedure is performed before the animal's condition worsens (e.g., hepatic lipidosis, bowel rupture). Before the surgical procedure is attempted, it is important to correct any fluid deficits or nutritional anomalies (e.g., hepatic lipidosis).
The approach to a gastrotomy in rabbits is similar to that described for domestic animals. An incision should be made that extends from the umbilicus to just caudal to the xiphoid process. The entire abdominal cavity should be explored, and any abnormalities noted. Biopsies of the liver may be collected to assess the status of the organ. The stomach should be isolated and surrounded with moist lap pads. Two stay sutures should be placed in the greater curvature of the stomach and an incision made in the avascular region between the greater and lesser curvature. The gastric contents should be removed using a sterile spoon. Once emptied, the stomach should be lavaged. The pylorus should be palpated to confirm its patency. The stomach should be closed using a two-layer inverting pattern with absorbable monofilament suture (3-0 or 4-0). The abdomen should be lavaged with warm saline before being closed. Closure of the body wall, subcutaneous space, and subcuticular space are routine. Intestinal resection and anastomosis and enterotomies are similar to other small animals.
INTEGUMENTARY SURGERY
Lateral ear canal resection
Otitis can be a chronic, frustrating disease in rabbits. Pet owners often become disillusioned with the constant medical treatments required to care for their pets. In cases where medical management is not successful, surgery should be considered. Lateral ear resection has been found to provide relief to both the rabbit patient and the pet owner. The approach to the lateral ear resection is similar to that described for dogs. For the procedure, the rabbit should be placed in lateral recumbency. Two parallel incisions should be made at the base of the ear that follow into the line of the pinna. The skin and subcutaneous tissue should be dissected free and reflected rostrally to reveal the vertical canal. An additional two parallel incisions are made into the lateral wall of the vertical canal. The cartilage should be carefully dissected to avoid the auricular veins. The dissection should continue to the level of the horizontal ear canal. Once exposed, the flap of cartilage is removed and the cartilage of the horizontal and vertical canals is sutured to the surrounding subcutaneous and skin tissues using absorbable suture. Rabbits should be maintained in the hospital for a minimum of 5 to 7 days to ensure that the surgical site is disinfected daily and does not dehisce.32
In severe, chronic cases of otitis interna, when rabbits have nystagmus, head tilt, and radiographic abnormalities in the bulla(e), a total ear canal ablation and tympanic bulla(e) osteoectomy can be performed. For the procedure, the rabbit should be placed in lateral recumbency and the area around the base of the ear clipped and prepped. The skin at the base of the ear should be incised, and the lateral wall of the ear canal dissected away from the subcutaneous tissue without entering the canal. During the dissection process, it is important to remain close to the auricular cartilage to avoid damaging the rostral and caudal auricular veins. Next, the horizontal tract of the ear canal should be dissected down to the junction of the tympanic bulla. At that point, the canal should be transected. The opening of the tympanic bulla should be widened (using needle drivers), debrided, and flushed. The subcutaneous tissue surrounding the ablated vertical canal should be closed with 3-0 absorbable suture. After closing the subcutaneous sites, the surgeon should close the tissue surrounding the bulla by marsupializing the opening to the bulla. This will allow for postoperative flushing.32
ZOONOSES
Zoonotic diseases are not commonly associated with rabbits. Although not generally considered an important disease of domestic or wild rabbits, rabies has been diagnosed in several pet rabbits. The clinical course of rabies in rabbits is nonspecific, but affected animals are generally depressed and weak and exhibit paralytic signs. In areas where rabies is endemic and prevalent, rabbits housed outdoors need to be protected from exposure to mesopredators (e.g., raccoons, foxes, coyotes) that may serve as rabies vectors.40 Additionally, E. cuniculi, Cheyletiella, ringworm, Pasteurella sp., Salmonella sp., tularemia, and Mycobacterium spp. have zoonotic potential to humans, especially in immunocompromised individuals.
REFERENCES
- 1.Carpenter JW, Mashima TY, Gentz EJ. Caring for rabbits: an overview and formulary. Vet Med Rabbit Symposium: Rabbits Are Not Small Cats. April 1995:348–380. [Google Scholar]
- 2.Harcourt-Brown F. Textbook of Rabbit Medicine. Elsevier; London: 2002. [Google Scholar]
- 3.Hillyer EV. Pet rabbits. Vet Clin North Am Small Anim Pract. 1994;24(1):25–64. doi: 10.1016/S0195-5616(94)50002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paré JA, Paul-Murphy J. Disorders of the reproductive and urinary systems. In: Queensberry KE, Carpenter JW, editors. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery. WB Saunders; St Louis: 2004. [Google Scholar]
- 5.Harkness JE. Rabbit husbandry and medicine. Vet Clin North Am Small Anim Pract. 1987;17(5):1019–1044. doi: 10.1016/s0195-5616(87)50103-6. [DOI] [PubMed] [Google Scholar]
- 6.Greenway JG, Partlow GD, Gonsholt NL. Anatomy of the lumbosacral spinal cord in rabbits. JAAHA. 2001;37:27–34. doi: 10.5326/15473317-37-1-27. [DOI] [PubMed] [Google Scholar]
- 7.Crossley DA. Oral biology and disorders of lagomorphs. Vet Clin North Am Exot Anim Pract. 2003;6:629–659. doi: 10.1016/s1094-9194(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins JR. Feeding recommendations for the house rabbit. Vet Clin North Am Exot Anim Pract. 1999;2(1):143–151. doi: 10.1016/s1094-9194(17)30144-5. [DOI] [PubMed] [Google Scholar]
- 9.Paul-Murphy J, Ramer JC. Urgent care of the pet rabbit. Vet Clin North Am Exot Anim Pract. 1998;1(1):125–152. doi: 10.1016/s1094-9194(17)30158-5. [DOI] [PubMed] [Google Scholar]
- 10.Suckow MA, Brammer DW, Rush HG. Biology and diseases of rabbits. In: Fox JG, Anderson LC, Loew FM, Chrisp CE, editors. Laboratory Animal Medicine. ed 2. Academic Press; San Diego: 2002. [Google Scholar]
- 11.Benson KG, Paul-Murphy J. Clinical pathology of the domestic rabbit. Vet Clin North Am Exot Anim Pract. 1999;2(3):539–551. doi: 10.1016/s1094-9194(17)30109-3. [DOI] [PubMed] [Google Scholar]
- 12.Fudge AM. Laboratory Medicine: Avian and Exotic Pets. WB Saunders; Philadelphia: 2000. [Google Scholar]
- 13.Johnson JH, Wolf AM. Ovarian abscesses and pyometra in a domestic rabbit. JAVMA. 1993;203(5):667–669. [PubMed] [Google Scholar]
- 14.Gentz EJ, Harrenstien LA, Carpenter JW. Dealing with gastrointestinal genitourinary and musculoskeletal problems in rabbits. Vet Med. 1995:365–372. April 1995: [Google Scholar]
- 15.Meredith A. Skin diseases of rabbits. Irish Vet J. 2003;56(1):52–56. [Google Scholar]
- 16.Thouless ME, DiGiacomo RF, Deeb BJ. Pathogenicity of rotavirus in rabbits. J Clin Microbiol. 1988:943–947. doi: 10.1128/jcm.26.5.943-947.1988. May 1988: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeb TR, Casebolt DB, Walker VE. Rotavirus-associated diarrhea in a commercial rabbitry. Lab Anim Sci. 1986;36(2):149–152. [PubMed] [Google Scholar]
- 18.Harris IE. Rabbits: medicine and other veterinary aspects. Aust Vet Prac. 1988;18(1):6–10. [Google Scholar]
- 19.Kerr PJ, Best SM. Myxoma virus in rabbits. Rev Sci Tech. 1998;17(1):256–268. doi: 10.20506/rst.17.1.1081. [DOI] [PubMed] [Google Scholar]
- 20.DiGiacomo RF, Mare CF. Viral diseases. In: Manning PJ, Ringler DH, Newcomer CE, editors. The Biology of the Laboratory Rabbit. ed 2. Academic Press; San Diego: 1994. [Google Scholar]
- 21.Harrenstien L, Gentz EJ, Carpenter JW. How to handle respiratory ophthalmic, neurologic and dermatologic problems in rabbits. Vet Med. 1995:373–380. April 1995: [Google Scholar]
- 22.Harcourt-Brown FM, Holloway HKR. Encephalocytozoon cuniculi in pet rabbits. Vet Rec. 2003;152:427–431. doi: 10.1136/vr.152.14.427. [DOI] [PubMed] [Google Scholar]
- 23.Pilny AA. Encephalitozoon cuniculi-associated phacoclastic uveitis in a dwarf rabbit. Exot Health. 2004;3:2. [PubMed] [Google Scholar]
- 24.Kazacos KR, Reed WM, Kazacos EA. Fatal cerebrospinal disease caused by Baylisascaris procyonis in domestic rabbits. JAVMA. 1983;183(9):967–971. [PubMed] [Google Scholar]
- 25.McTier TL, Hair JA. Efficacy and safety of topical administration of selamectin for treatment of ear mite infestation in rabbits. JAVMA. 2003;223(2):322–332. doi: 10.2460/javma.2003.223.322. [DOI] [PubMed] [Google Scholar]
- 26.Lee KJ, Johnson WD, Lang CM. Acute peritonitis in the rabbit (Oryctolagus cuniculus) resulting from a gastric trichobezoar. Lab Anim Sci. 1978;28(2):202–204. [PubMed] [Google Scholar]
- 27.Queensberry K. Nutritional and gastrointestinal diseases of rabbits. Intern Med: Small Companion Anim Proc. 1998;306:61. [Google Scholar]
- 28.Physicians’ Desk Reference, ed 58, Montvale, NJ, 2004.
- 29.Taylor M. A wound packing technique for rabbit dental abscesses. Exot DVM. 2003;5(pt 3):28. [Google Scholar]
- 30.Carpenter JW, Mashima TY, Rupiper DJ. Exotic Animal Formulary. ed 2. WB Saunders; Philadelphia: 2001. [Google Scholar]
- 31.Weisbroth SH. Neoplastic disease. In: Weisbroth SH, Flatt RE, Kraus AL, editors. The Biology of the Laboratory Rabbit. Academic Press; New York: 1974. [Google Scholar]
- 32.Capello E. Surgical treatment of otitis external and media in pet rabbits. Exot DVM. 2004;6(pt 3):15. [Google Scholar]
- 33.Owiny JR, Vandewoude S, Painter JT. Hip dysplasia in rabbits. Comp Med. 2001;51(1):85–88. [PubMed] [Google Scholar]
- 34.Maurer KJ, Marini RP, Fox JG. Polycystic kidney syndrome in New Zealand White rabbits resembling human polycystic kidney disease. Kidney Int. 2004;65:482–489. doi: 10.1111/j.1523-1755.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 35.Broome RL, Brooks DL, Babish JG. Pharmacokinetic properties of enrofloxacin in rabbits. Am J Vet Res. 1991;52(11):1835–1841. [PubMed] [Google Scholar]
- 36.Nagornev VA, Zubzhiskii IuN, Pigarevskii PV. [Effect of hydrocortisone on the development of experimental atherosclerosis in rabbits] [Article in Russian] Biull Eksp Biol Med. 1980;90(10):407–409. [PubMed] [Google Scholar]
- 37.Flecknell P. Laboratory Animal Anaesthesia. ed 2. Academic Press; San Diego: 1996. [Google Scholar]
- 38.Doerning BJ, Brammer DW, Chrisp CE. Nephrotoxicity of Tiletamine in New Zealand White rabbits. Lab Anim Sci. 1992;42(3):267–269. [PubMed] [Google Scholar]
- 39.Worthley SG, Roque M, Helft G. Rapid oral endotracheal intubation with a fibre-optic scope in rabbits: a simple and reliable technique. Lab Anim. 2000;34:199–201. doi: 10.1258/002367700780457554. [DOI] [PubMed] [Google Scholar]
- 40.Karp BE, Ball NE, Scott CR. Rabies in two privately owned domestic rabbits. JAVMA. 1999;215(12):1824–1827. [PubMed] [Google Scholar]


