The three primary body cavities are (1) thoracic (pleural), (2) abdominal (peritoneal), and (3) pericardial. In health, these potential cavities contain a small amount of serous fluid that acts as a lubricant allowing the free motion of internal organs against one another and body cavity walls. The body cavities are lined by mesothelial cells, which are specialized cells that play an active role in the homeostasis of these potential spaces. The abnormal accumulation of fluid within a body cavity is called effusion. Effusion is not a disease in itself; rather, it results from alterations in fluid production, lymphatic drainage, or a combination of both. Some of the major factors that affect production and resorption of fluid include changes in capillary hydrostatic pressure, plasma osmotic pressure, and capillary permeability.
Fluid analysis, including cytological evaluation and classification, is a quick, inexpensive, and relatively safe way to obtain useful information in the diagnosis, prognosis, and treatment of diseases that cause thoracic, abdominal, and pericardial fluid accumulations. Common causes of body cavity effusions include trauma, neoplasia, cardiovascular compromise, metabolic disorders, altered Starling forces, ruptured urinary or gall bladder, ruptured vessels or lymphatics, and bleeding diathesis, as well as infectious and inflammatory diseases.
General mechanisms of thoracic, abdominal, and pericardial effusions will be categorized in the section titled “General Classification of Effusions” and explored in detail in the section titled “Specific Disorders Causing Effusions.”
Thoracic and Abdominal Effusions
Dogs and cats with thoracic effusions often exhibit dyspnea as the most common clinical sign.1, 2 Other clinical signs include a crouched, sternal recumbent position with extension of the head and neck; open-mouth breathing; tachypnea; and forceful abdominal respiration. Cyanosis may be present. With milder effusions, lethargy and lack of stamina may be the only clinical signs. Animals, especially cats, with mild to moderately severe effusions often adapt by decreasing their activity, thus concealing their illness until it is severe. With chronic pleural effusion, dogs and cats may present with coughing as the only clinical sign.1 Physical findings depend on the amount of fluid present but include muffled heart and lung sounds.
Patients with abdominal effusions may present for lethargy, weakness, and abdominal distension—the latter may be mistaken by pet owners as weight gain, gas, or ingesta. Physical findings include a fluid wave during ballottement in high-volume effusions or pain if peritonitis is present.
Pericardial Effusions
Pericardial effusion in cats is often secondary to congestive heart failure or feline infectious peritonitis but may be caused by primary cardiac neoplasia, such as lymphoma.3 The most common causes of pericardial effusion in the dog include cardiac neoplasia and idiopathic pericardial effusion.4, 5 Hemangiosarcoma is the most common cardiac neoplasm reported, but other reported neoplasms include lymphoma, chemodectoma, and thyroid carcinoma.4 Other less common causes include cardiac disease, inflammatory or infectious diseases, trauma, coagulopathy, and congenital defects.9
Clinical signs include weakness, lethargy, exercise intolerance, collapse, and coughing. Physical findings vary with the amount of fluid present but include muffled heart sounds, weak pulses, and pallor.
Collection Techniques
For all fluids collected, a portion of the fluid (2–3 milliliters [mL]) should be placed in an ethylenediaminetetraacetic acid (EDTA) lavender-top Vacutainer (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) tube for cell counts, protein analysis, and cytological evaluation. EDTA prevents clots from forming in the fluid in the event of iatrogenic blood contamination. A second portion (2–3 mL) should be placed in a sterile red-top Vacutainer tube without anticoagulant to assess for clot formation and to have on hand if aerobic or anaerobic bacterial cultures are required or biochemical analyses are desired (bilirubin, cholesterol, triglyceride, creatinine, etc.).
Thoracocentesis
Pleural effusions are typically abundant and bilateral but may be mild, unilateral, compartmentalized, or both. Radiography and ultrasonography help determine the extent and location of the effusion and guide thoracocentesis. If the fluid is not compartmentalized, thoracocentesis is performed approximately two-thirds down the chest, near the costochondral junction at the sixth, seventh, or eighth intercostal spaces.
The patient is restrained in the sternal recumbent or standing position. The site of needle insertion is shaved and aseptically prepared. Tranquilization and local anesthesia are generally not necessary for collecting a small sample for analysis but may be needed if a large amount of fluid must be drained from the chest. Large dogs may require a 1½-inch, 18- to 20-gauge needle or over-the-needle catheter, but a ⅞-inch, 19- or 21-gauge butterfly needle is preferred for cats and small dogs. The catheter unit allows the needle to be withdrawn after the catheter is introduced into the thoracic cavity, thus decreasing the chances of injury to intrathoracic organs.
The needle should be inserted next to the cranial surface of the rib to minimize the risk for lacerating the vessels on the rib’s caudal border. If the needle or catheter is below the fluid line, air will not be aspirated into the thoracic cavity. If only a single syringe of fluid is to be collected, the syringe may be attached to the catheter, the fluid aspirated, and the catheter withdrawn with the syringe attached. If a larger volume of fluid is to be removed or the syringe is to be repeatedly filled, extension tubing and a three-way stopcock should be attached (Fig. 15.1 ).
Fig. 15.1.

Basic equipment for thoracocentesis, abdominocentesis, or pericardiocentesis: syringe, three-way stopcock, extension tubing, butterfly catheter, and over-the-needle catheter.
Abdominocentesis
The ventral midline of the abdomen, 1 to 2 cm caudal to the umbilicus, is the usual site of needle insertion. This site avoids the falciform fat, which may block the needle barrel. The urinary bladder is emptied to help avoid accidental cystocentesis. The site of needle insertion is shaved and aseptically prepared. Neither local nor general anesthesia is usually needed. With the animal in lateral recumbency, a ventral midline puncture is made using a 1- to 1½-inch, 20- to 22-gauge needle, or a 16- to 20-gauge, 1½- to 2-inch plain or fenestrated over-the-needle catheter without the syringe attached. Free-flowing fluid should be collected into appropriate collection tubes. The needle may be rotated if fluid is not visible in the needle hub or a syringe may be attached and gentle negative pressure applied.6 If a scar from a previous surgical incision is present, the needle should be inserted at least 1.5 cm away from the site to avoid abdominal viscera that may have adhered to the abdominal wall in the area of the scar. To enhance fluid collection, abdominal compression may be applied when an over-the-needle catheter is used after the stylet has been removed, leaving only the catheter in the abdominal cavity. Although the catheter may kink, sufficient fluid can usually be collected for analysis.
If the technique described above fails to yield fluid, four-quadrant paracentesis or diagnostic peritoneal lavage (DPL) may be performed. In four-quadrant paracentesis, the umbilicus serves as a central point, and centesis, as previously described, is performed in the right and left cranial and caudal quadrants.7 If DPL is performed, the animal is placed in dorsal recumbency, the area is clipped and aseptically prepared, and a small 2-cm incision caudal to the umbilicus is made. A peritoneal lavage catheter without the trocar is inserted into the abdominal cavity and directed caudally into the pelvis. A syringe is attached and gentle suction applied. If no fluid is obtained, 20 mL/kg of warm sterile saline may be infused into the abdominal cavity. The patient is then rolled from side to side and the fluid collected via gravity drainage.6, 7
Pericardiocentesis
Pericardiocentesis may be performed with the animal in the standing position or in sternal or left lateral recumbency. Adequate restraint is needed to avoid cardiac puncture, coronary artery laceration, or pulmonary laceration. Sedation is used as necessary. Electrocardiography (ECG) monitoring is recommended but not essential. Cardiac contact with the catheter or needle usually causes an arrhythmia. A large area of the right hemithorax from the third rib to the eighth rib is shaved and aseptically prepared. Local anesthesia, including infiltration of the pleura with lidocaine, may be used to minimize discomfort associated with pleural penetration. Puncture is generally made between the fourth and fifth intercostal spaces at the costochondral junction. The needle is attached to a three-way stopcock, extension tubing, and syringe, and gentle negative pressure is applied.5, 8
Slide Preparation and Staining
Preparation of the sample for cytological evaluation depends on the character and quantity of the fluid, the type of stain used, and whether the cytological evaluation will be performed in the hospital or sent to a diagnostic laboratory.
Clear, colorless fluids are usually transudates (low protein content and low cellularity). Preparation of sedimented or cytocentrifuged concentrated slides from low cellularity fluids aid in cytological evaluation. Clear-amber and mildly opaque fluids are often effusions of low to moderate cellularity. Moderately to markedly opaque fluids, however, are usually exudates of moderate to very high cellularity. Slide preparation and staining techniques are detailed in Chapter 1.
Sediment smears should be made on all nonturbid fluid specimens. This is done by centrifuging the fluid for 5 minutes at 165 to 360 gravity (G). This may be achieved in a centrifuge with a radial arm length of 14.6 cm by centrifuging the fluid at 1000 to 1500 revolutions per minute (rpm). After centrifugation, most of the supernatant is poured off, leaving only about 0.5 mL of fluid with the pellet in the bottom of the tube. The supernatant may be used for total protein and chemical analysis (EDTA samples should be avoided because EDTA interferes with chemical analyses). The pellet is then resuspended in the remaining 0.5 mL of fluid by gentle agitation, a drop of the suspension is placed on a glass slide, and a routine pull smear or squash prep is made (see Chapter 1). The smear is air-dried and then stained with an appropriate hematological stain.
Opaque fluids may need only a direct smear because of high cellularity. Direct smears may be made by making either pull smears or squash preps on well-mixed, uncentrifuged fluid.
Laboratory Data
Cell Counts and Counting Techniques
Accurate nucleated cell counts may be determined at commercial laboratories on many modern automated cell counters or by manual methods with use of a hemacytometer; however, cell clumping, cell fragmentation, and noncellular debris may cause counting errors with automated and manual techniques.10 Determination of nucleated cell counts should be performed on EDTA-preserved fluid to prevent clot formation or clumping of the sample. Serum separator tubes may introduce artifact to the count and therefore should not be used. A nucleated cell differential may be made on cytology preparations and often aids in classification of the effusion type.
Automated red blood cell (RBC) counts may help determine the amount of blood in the effusion. If grossly bloody fluid is obtained, a packed cell volume (PCV) may be performed. The RBC count, together with cytological assessment (identification of platelets, erythrophagocytosis, and intracellular and extracellular heme pigments, e.g., hemosiderin and hematoidin), may aid in determination of the origin of the blood as far as iatrogenic blood contamination, per-acute hemorrhage, chronic hemorrhage, and increased capillary permeability with diapedesis of RBC into the cavity.
Total Protein Measurement and Techniques
Fluid total protein concentration is used with the nucleated cell count to classify effusions and to estimate the severity of inflammation, if present. The total protein content may be determined biochemically or estimated by using refractometry. For ease and accuracy, the method of choice for determining total protein concentrations in effusions is refractometry. If the fluid is opaque, it is best to determine the refractive index of the supernatant after centrifugation, as the refraction of light by suspended nonprotein particles (i.e., lipoproteins, urea, cholesterol, and glucose) may result in an erroneous total protein reading.10, 11 It must be kept in mind that chylous or lipemic fluids may not separate sufficiently to allow total protein to be estimated by using refractometry or chemical methods.
Biochemical Analysis
In conjunction with fluid analysis, measurement of abdominal effusion supernatant bilirubin and creatinine and comparison with serum values may be performed to diagnose bile peritonitis and uroperitoneum, respectively. Additionally, when a white, opaque effusion is obtained, measurement and comparison of effusion supernatant and serum triglyceride and cholesterol values may be used to distinguish between chylous and pseudochylous effusions (Table 15.1 ). Specific biochemical analyses performed on effusions will be highlighted in the section titled “Specific Disorders Causing Effusions.”
TABLE 15.1.
Ancillary Tests: Biochemical Analysis of Effusion Fluid
| Biochemical Test | Indications | Interpretation |
|---|---|---|
| Bilirubin | Bile peritonitis | Twofold or greater concentration in effusion fluid than serum supports bile peritonitis. (Mucocele rupture may not have concentration differences.) |
| Creatinine | Uroperitoneum | Twofold or greater concentration in effusion fluid versus serum supports uroperitoneum |
| Triglyceride | Chylous effusion | Triglyceride level greater than 100 milligrams per deciliter (mg/dL) in fluid supports chylous effusion. |
| Cholesterol | Nonchylous effusion | Higher concentration of cholesterol in effusion fluid versus serum supports nonchylous effusion (not common in veterinary species). |
Microbiological Cultures
Effusion fluid may be submitted for either bacterial or fungal cultures. It is best to contact the laboratory for specific information regarding submission protocol, types of transport containers, and media, as many laboratories will provide special transport tubes and, in the case of anaerobic bacterial culture, special anaerobic tubes or media. In general, if the cytological evaluation of an effusion suggests bacterial infection caused by the presence of large numbers of neutrophils or if definitive bacteria are identified, the fluid should be cultured for both aerobic and anaerobic bacteria. Fluid samples for aerobic and anaerobic cultures should be collected by using aseptic technique to avoid contamination. Fluid should be placed into a sterile tube (i.e., sterile red-top tube) without EDTA, which may be bacteriostatic or bactericidal. Note that some transport systems support both aerobic and anaerobic bacteria. Submission of fluid for fungal culture should be performed as in the case of fluid submitted for aerobic bacterial culture. Chapter 1 contains a detailed discussion of methods for collection and transportation of samples for microbiological culture.
Cells and Structures Seen in Effusions
Neutrophils
Neutrophils are present to some degree in most effusions and tend to predominate in effusions associated with inflammation. Cytologically, two general classes of neutrophils exist: degenerate and nondegenerate. Degenerate neutrophils are neutrophils that have undergone hydropic degeneration. This is a morphological change that occurs in tissue and effusions secondary to bacterial toxins that alter cell membrane permeability. The toxins allow water to diffuse into the cell and through the nuclear pores, causing the nucleus to swell, fill more of the cytoplasm, and stain homogeneously eosinophilic. This swollen, loose, homogeneous, eosinophilic nuclear chromatin pattern characterizes the degenerate neutrophil (Fig. 15.2 ). Although all cell types are exposed to the same toxin, degenerative change is evaluated only in neutrophils.
Fig. 15.2.

Numerous degenerate neutrophils with swollen nuclear chromatin. Phagocytized bacterial rods (arrowhead) and extracellular bacteria are in the background (arrows).
Nondegenerate neutrophils, such as peripheral blood neutrophils, are those with tightly clumped, basophilic nuclear chromatin (Fig. 15.3 ). Some neutrophils in effusions may be hypersegmented. Hypersegmentation is an age-related change; the nuclear chromatin condenses and eventually breaks into round, tightly clumped spheres (pyknosis) (Fig. 15.4 ). These aged neutrophils are often seen phagocytized by macrophages (cytophagia) (Fig. 15.5 ). The presence of nondegenerate neutrophils suggests that the fluid is not septic; however, bacteria that are not strong toxin producers, for example, Actinomyces spp., may be associated with nondegenerate neutrophils. Also, some infectious agents, such as Ehrlichia and Toxoplasma spp. and various fungi, may be associated with nondegenerate neutrophils.
Fig. 15.3.

Feline abdominal fluid. Numerous nondegenerate neutrophils. The chromatin is clumped and segmented. Also present are macrophages (arrows) and a small lymphocyte (arrowhead).
Fig. 15.4.

Feline abdominal fluid. Numerous nondegenerate neutrophils are present; some are hypersegmented with only thin chromatin strands connecting the segments (small arrows). Pyknotic nuclei (arrowheads) are also present.
Fig. 15.5.

Abdominal fluid from a cat. A large macrophage is present in the center, with a phagocytized remnant of cellular material. Numerous small lymphocytes, neutrophils, and macrophages are also present.
Effusions may also contain toxic neutrophils. Toxic changes (i.e., Döhle bodies, toxic granulation, diffuse cytoplasmic basophilia, foamy cytoplasm) develop in bone marrow in response to accelerated granulopoiesis caused by inflammation. Toxic neutrophils in peripheral blood migrate into the body cavity and are observed in effusions of the cavity. Although foamy cytoplasm is considered a toxic change, cytoplasmic vacuolation may be seen in neutrophils of peritoneal or thoracic fluid smears because of age-related change or EDTA-induced artifact.
Mesothelial Cells
Mesothelial cells line pleural, peritoneal, and pericardial cavities, as well as visceral surfaces, and are present in variable numbers in most effusions. Mesothelium easily becomes activated and reactive or hyperplastic in the face of inflammation or fluid accumulation of any type. In effusions, mesothelial cells are large, round, with moderate amounts of pale to dark basophilic cytoplasm and singular, round, central nuclei. Multinucleation can occur when cells are reactive (Fig. 15.6 ). The nuclear chromatin has a fine reticular pattern, and nucleoli may be present. Reactive mesothelial cells may have a pronounced, pink, cytoplasmic coronal fringe (Fig. 15.7 ). Mesothelial cells may be present in low or moderate numbers, as individual cells, in small clusters, and sometimes in large, three-dimensional aggregates (Fig. 15.8 ). Reactive mesothelial cells may have several morphological characteristics of malignancy and may be easily confused with neoplastic cells.
Fig. 15.6.

A binucleate mesothelial cell with deeply basophilic cytoplasm and an encircling eosinophilic fringe border.
Fig. 15.7.

A small cluster of mesothelial cells with prominent eosinophilic corona.
Fig. 15.8.

Pericardial fluid from a dog. In a concentrated cytocentrifuge specimen, a three-dimensional aggregate of mildly pleomorphic mesothelial cells is present and surrounded by small lymphocytes, monocytes, macrophages, few red blood cells and rare nondegenerate neutrophils. Pleomorphic mesothelial cells are often present in pericardial fluid.
Macrophages
Macrophages found in effusions generally have a single oval to bean-shaped nucleus and resemble tissue macrophages. The nuclear chromatin is lacy and cytoplasm is frequently vacuolated and may contain phagocytized debris or degenerate cells (Fig. 15.10, Fig. 15.9 ).
Fig. 15.10.

Numerous nondegenerate neutrophils and macrophages with variable nuclear morphology (arrows).
Fig. 15.9.

Several macrophages with varying morphology. In the upper right and left, the macrophages are vacuolated. Macrophages in the lower right area are monocyte-like. In addition, there are few mesothelial cells, including one that is binucleate and a few nondegenerate neutrophils.
Lymphocytes
Lymphocytes are commonly seen in effusions and are often the predominant cells in lymphocytic and chylous effusions (Fig. 15.11 ) and neoplastic effusions secondary to lymphoid malignancy (Fig. 15.12 ). Small to intermediate or medium-sized lymphocytes present in body cavity fluids will appear similar to peripheral lymphocytes, with a scant rim of blue cytoplasm, round nucleus with evenly clumped to coarse chromatin, and no apparent nucleoli. Normal fluids will have low numbers of lymphocytes. Reactive lymphocytes may be seen in inflammatory effusions and are usually larger than small lymphocytes, with deeply basophilic cytoplasm imparting a plasmacytoid appearance (Fig. 15.13 ).
Fig. 15.11.

Chylous pleural effusion from a cat. Small lymphocytes are the predominant cells. The small lymphocytes are typically smaller than neutrophils. They have round or indented nuclei and a small amount of cytoplasm.
Fig. 15.12.

Feline thoracic fluid with many lymphoblasts, which are large, with a scant rim of basophilic cytoplasm, eccentric round nuclei with smooth chromatin and visible nucleoli. The high cell yield and large numbers of lymphoblasts are compatible with a neoplastic effusion secondary to high-grade lymphosarcoma.
Fig. 15.13.

Reactive lymphocyte. This cell is slightly larger than a small lymphocyte with deeply basophilic cytoplasm and a perinuclear clearing (plasmacytoid features).
Eosinophils
Eosinophils are readily recognized by their rod-shaped (in cats) or variably sized, round (in dogs), pink granules (Fig. 15.14 ). When eosinophils are present in moderate to high numbers in effusions, differentials could include: Parasitical disease (heartworm infection), allergic or hypersensitivity disease, a paraneoplastic response to lymphoma or mast cell neoplasia, a foreign body, infectious etiologies or idiopathic hypereosinophilic syndrome.12
Fig. 15.14.

Canine pleural effusion with numerous eosinophils, neutrophils, several monocytes, and red blood cells.
Mast Cells
Mast cells (Fig. 15.15, Fig. 15.16 ) are readily identified by their numerous, round, red-purple cytoplasmic granules. Mast cells may be found in low numbers in effusions in dogs and cats with many different inflammatory disorders. Mast cell tumors within body cavities may be associated with effusions and may exfoliate large numbers of mast cells into the effusion. Visceral forms of mast cell neoplasia are rare in the dog.12, 13 Visceral forms of mast cell tumors in cats most often involve the spleen14 or the gastrointestinal (GI) tract.15
Fig. 15.15.

Many heavily granulated mast cells in an effusion secondary to mast cell neoplasia.
Fig. 15.16.

Canine abdominal effusion secondary to mast cell neoplasia. Note the large numbers of heavily granulated mast cells, with fewer admixed vacuolated macrophages, eosinophils and few mature lymphocytes.
Erythrocytes
Erythrocytes may be seen cytologically within effusions secondary to overt hemorrhage or contamination with peripheral blood. It is important to differentiate iatrogenic causes from true intracavity hemorrhage, on the basis of clinical signs, laboratory data, and the presence or absence of erythrophagia, hemosiderin or hematoidin pigments, and platelets in the effusion, as described in the discussion of hemorrhagic effusions later in this chapter.
Neoplastic Cells
Neoplastic cells may be observed in low to high numbers in effusions secondary to many different types of neoplasia. Various carcinomas and adenocarcinomas (epithelial cell tumors), lymphoma and mast cell tumors (discrete cell tumors), hemangiosarcoma (vascular endothelial–derived neoplasia), and mesothelioma may exfoliate neoplastic cells into the pleural, peritoneal, or pericardial cavity. Identification of the neoplastic cells depends on the viewer’s ability to recognize the cell type and signs of malignancy. (See the discussion of neoplasia later in this chapter and the general criteria of malignancy in Chapter 1.)
Miscellaneous Findings
Glove Powder
Cornstarch (glove powder) may be seen on slides made from effusion fluid (Fig. 15.17 ). Typically, it is a clear-staining, large, round to hexagonal structure with a central fissure and may be slightly refractile. Glove powder is a contaminant and should not be confused with an organism or cell.
Fig. 15.17.

Glove powder artifact (arrows).
Microfilariae
Microfilariae may occasionally be seen within hemorrhagic effusions. These are generally Dirofilaria or Acanthocheilonema (formerly Dipetalonema) larvae that have entered the cavity with the peripheral blood (see Fig. 15.42 later in the chapter).
Fig. 15.42.

Hemodilute background with two large, basophilic microfilaria and few scattered blood leukocytes and few small platelet clumps.
Courtesy Jennifer Neel, North Carolina State University.
Basket Cells (Broken Cells)
Basket cells are free cell nuclei from ruptured nucleated cells. When cells rupture, the nuclear chromatin spreads out and stains eosinophilic. Nucleated cells may rupture because of the stresses induced in slide preparation; however, certain effusions (i.e., chylous effusions and septic exudates) cause increased cell fragility and may result in increased numbers of ruptured cells. Neoplastic effusions may contain fragile neoplastic cells, and increased cellular rupture may be seen (especially true for neoplastic immature lymphocytes).
General Classification of Effusions
Traditionally, abdominal, thoracic, and pericardial fluid accumulations have been classified as transudates, modified transudates, or exudates, on the basis of the total nucleated cell count (TNCC) and total protein (TP) concentration (Fig. 15.18 ). If overlap occurs in these classifications (i.e., a fluid may have TNCC in the transudate range and TP in the modified transudate range), TP is the more important criterion in separating transudates from modified transudates, and cellularity is more important in separating modified transudates from exudates. Modified transudates often are the least specific categorization from a diagnostic standpoint. Various other classification schemes have been proposed, such as those based on etiology, septic versus nonseptic exudate, vascular or lymphatic disruption, internal organ rupture, or by the presence of particular cells exfoliated into the effusion.16 Additionally, some schemes classify transudative effusions as protein-poor or protein-rich, addressing the general pathological mechanism of the effusions.10
Fig. 15.18.

Traditional algorithm to classify effusions as transudates, modified transudates, or exudates, based on total protein content and nucleated cell count.
Classifying the effusion helps direct the clinician to the general mechanism of fluid accumulation and may provide a preliminary list of differential diagnoses. However, whatever schema is used, the effusion should always be interpreted in light of clinical signs, history, physical examination findings, laboratory data, imaging information, and importantly, results of cytological examination of the fluid. The results of the effusion fluid examination may be diagnostic for specific conditions, such as neoplasia or infection, or may simply indicate a process. The following sections will initially characterize effusions mechanistically as transudates, modified transudates, and exudates, followed by specific etiologies.
Transudates
Protein-poor transudative effusions are generally clear and colorless, with TP concentrations less than 2.0 g/dL, and often contain less than 1500 cells/μL. Cells found in transudative effusions consist primarily of mononuclear cells (macrophages and small lymphocytes), mesothelial cells, and low numbers of neutrophils. The general mechanism is fluid shifts caused by changes in oncotic and/or hydraulic pressure or impaired lymphatic drainage. Conditions associated with these mechanisms include severe hypoproteinemia (primarily hypoalbuminemia) (<1.0 g/dL) secondary to protein-losing nephropathy, hepatic insufficiency, protein-losing enteropathy, malnutrition, malabsorption, portal hypertension, and early myocardial insufficiency (more common in cats). Early bladder rupture may result in a fluid classified as a transudate; however, the nature of the effusion will change rapidly because uroperitoneum often induces a rapid chemical peritonitis. Intravenous fluid administration after marked blood loss may result in an acute and marked hypoproteinemia, which, in turn, may cause a transudative process triggered by an acute disruption in oncotic pressures and insufficient lymphatic drainage.10
Modified transudates (also known as protein-rich transudates) occur because of increased hydraulic pressure caused by venous congestion in the lungs or liver.10 TP concentration is greater than 2 g/dL, and the effusion often contains less than 5000 cells/μL. Effusion fluid color and turbidity may vary. Cells found in this effusion are reactive mesothelial cells, neutrophils, macrophages, and few small lymphocytes. Common conditions associated with modified transudates are congestive heart failure and portal hypertension.
Dogs develop abdominal effusion secondary to right-sided heart failure, and cats often develop pleural effusion. Effusions caused by congestive heart failure are multifactorial and result from changes in vascular pressure, poor cardiac output, and retention of excess water. With portal hypertension, there is increased intrahepatic pressure and decreased lymphatic drainage, causing congestion and subsequent leakage of high-protein hepatic lymph into the abdominal cavity. No cytological finding in these effusions is pathognomonic for these conditions. Physical examination findings and imaging studies often help confirm functional abnormalities.
Fig. 15.19 is a flow chart of the more common causes of transudative effusions.
Fig. 15.19.

Classification and causes of transudative effusions.
Exudates
Exudates typically have TP concentrations greater than 2 g/dL and contain greater than 5000 cells/μL. Exudates may vary in color but are often turbid to cloudy. A flow chart of the more common causes of exudative effusions is outlined in Fig. 15.20 . Exudates are inflammatory in nature and occur because of vascular permeability caused by the release of inflammatory mediators from the inflamed tissue. Neutrophils are typically the predominant cell type in most exudates, but macrophages and, to some extent, lymphocytes are also increased. Exudates may be infectious (septic) or not (nonseptic). Septic exudates are most often caused by bacteria but may be caused by fungi, protozoa, or parasites. Nonseptic exudates may be associated with a wide range of pathological conditions that elicit an inflammatory response, such as tumor necrosis; chemical irritants, such as urine and bile; or the presence of a sterile foreign body.
Fig. 15.20.

Classification and causes of exudative effusions.
In the case of a predominantly neutrophilic exudate, a thorough investigation for an infectious agent is warranted. Degenerate neutrophils may be present in cases of sepsis; however, the presence of nondegenerate neutrophils does not preclude the possibility of an infectious etiology, and neither does the absence of cytologically visible organisms. Previous or concurrent antibiotic use may reduce bacterial numbers. Whenever a significant neutrophilic inflammatory component is present, regardless of the cytological presence or absence of bacteria, bacterial culture should be considered.
Occasionally, because of abundant exfoliation of neoplastic cells or secondary to a chronic chylous effusion, an effusion that has not yet been cytologically examined may fit into the exudative category solely on the basis of high cellularity. Once examined, these effusions are named according to etiology.
Specific Disorders Causing Effusions
Septic Exudates
Inflammation is associated with the production of inflammatory mediators released from tissue causing increased neutrophil and monocyte or macrophage migration and the influx of protein-rich fluid as a result of increased vascular permeability. A septic effusion may result from hematogenous or lymphatic spread from systemic sepsis, from extension of pleuropneumonia or GI compromise or perforation, or by introduction of organisms via penetration of the body cavity (i.e., trauma, foreign body, surgery, and prior centesis).
Degenerate neutrophils may predominate in bacterial infections; organisms may be intracellular or extracellular (Fig. 15.21, Fig. 15.22 ). The presence of long, slender, filamentous rods in a fluid with “tomato soup–like” characteristics is highly suggestive of Actinomyces spp., Nocardia spp., Fusobacterium spp., or any combination of the three (Fig. 15.23, Fig. 15.24 ). Spirochetes are occasionally seen in association with bacterial peritonitis and pleuritis secondary to bite wounds. Although bacterial infections are the most common causes of septic exudates, mycotic infections associated with Histoplasma spp. (Fig. 15.25 ), Blastomyces spp. (Fig. 15.26 ), Coccidioides spp. (Fig. 15.27, Fig. 15.28 ), Candida spp. (Fig. 15.29, Fig. 15.30, Fig. 15.31 ), and other fungal infections (Fig. 15.32 ) may occur. Fungal culture can be used to further define the fungal infection when hyphae are found. Additionally, effusions secondary to protozoal infections (Neospora spp., Toxoplasma spp., Leishmania spp.) (Fig. 15.33 ) have been reported.17, 18
Fig. 15.21.

Septic exudate showing degenerate neutrophils and phagocytized bacteria (arrow).
Fig. 15.22.

Feline pyothorax. Large numbers of markedly degenerate neutrophils and abundant phagocytized and extracellular mixed bacteria consisting of cocci and long strands of bacilli.
Fig. 15.23.

Phagocytized filamentous bacterial rods (arrow).
Fig. 15.24.

Phagocytized filamentous bacterial rods.
Fig. 15.25.
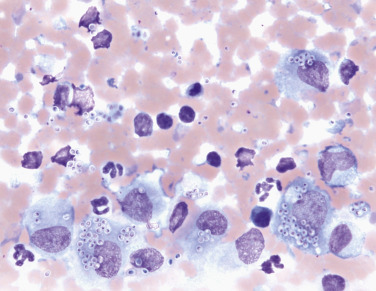
Numerous Histoplasma capsulatum organisms are both phagocytized by macrophages and present extracellularly.
Fig. 15.26.
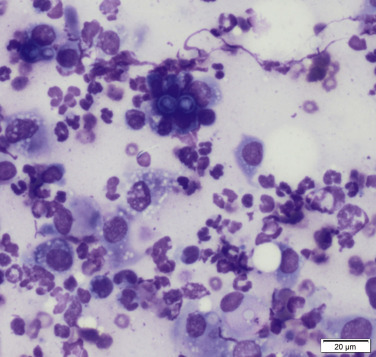
Pleural fluid from a dog. Several Blastomyces spp. organisms are surrounded by inflammatory cells (macrophages and degenerate neutrophils). The organisms exhibit broad-based budding.
Fig. 15.27.

Pleural fluid from a dog. In the center, a singular, large, round, pale blue, round yeast with a cell wall is present extracellularly, consistent with Coccidioides spp. The yeast is surrounded by macrophages nondegenerate neutrophils and fewer macrophages.
Fig. 15.28.
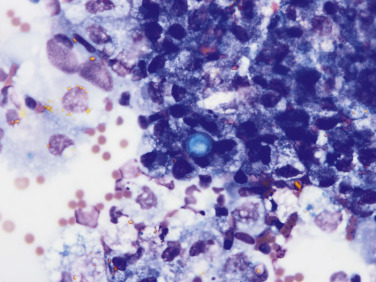
Pericardial fluid from a dog. In the center, a singular Coccidioides spp. yeast is present in the extracellular space. There are surrounding macrophages that contain intracellular hemosiderin and hematoidin. Extracellular rhomboid, golden hematoidin crystals are also evident.
Fig. 15.29.

Abdominal fluid from a dog. A large foamy macrophage with intracellular Candida spp. yeast.
Courtesy Dr. James Meinkoth, Oklahoma State University
Fig. 15.30.

Abdominal fluid from a dog with gastrointestinal compromise. Candida spp. yeast with narrow budding, phagocytized by a neutrophil in the center. Lower right, a degenerate neutrophil with phagocytized bacteria.
Fig. 15.31.

Abdominal fluid from a dog. Candida spp. pseudohyphae surrounded by degenerate inflammatory cells.
Courtesy Dr. James Meinkoth, Oklahoma State University
Fig. 15.32.

In the center, note the large, pale-staining, fungal hyphal structure surrounded by foamy macrophages, neutrophils, and eosinophils.
Fig. 15.33.

Extracellular Toxoplasma-like organisms from a cat.
Tissue Inflammation
Inflammation of an intracavity organ (liver, pancreas, lungs), or a walled-off abscess may cause an exudative effusion. Inflammatory mediators released from affected tissue results in increased vascular permeability, increased neutrophil and monocyte or macrophage migration, and the influx of protein-rich fluid. In effusions caused by tissue inflammation, nondegenerate neutrophils generally predominate, but macrophages, mesothelial cells, and some lymphocytes are also present. Macrophages, however, may become the predominant cell type in some chronic inflammatory processes. Cytological examination of these effusions readily identifies the inflammatory process but may not be able to determine a specific etiology.
Feline Infectious Peritonitis
Effusive FIP is the classic infectious exudate in the cat, caused by a virus. The virus is not detectable with microscopic examination of the fluid. Clinical FIP may occur in cats of all ages, but the proportion of cats with FIP between ages 6 months and 2 years is significantly higher compared with the control cats in similar age groups.19 In effusive FIP, fluid may accumulate in the abdomen, thorax, or pericardium or in all cavities. Evaluation of fluid may lend significant support to a diagnosis of FIP. The effusion is odorless, straw colored to golden, may contain flecks or fibrin strands, and foams upon agitation because of the high protein content, which is often greater than 4 g/dL. Cell counts may be variable but are typically 2000 to 6000 cells/μL, and typically hemodilution is minimal. Cytologically, the typical FIP effusion has a prominent stippled proteinaceous basophilic background (Fig. 15.34 ) and consists primarily (60%–80%) of nondegenerate to mildly degenerate neutrophils and lesser numbers of macrophages, small lymphocytes, and occasionally plasma cells. Effusions consisting primarily of neutrophils but with large numbers of macrophages are referred to as pyogranulomatous and are also common in effusions associated with FIP. Although these findings are not diagnostic of FIP, when correlated with clinical findings, a presumptive diagnosis of FIP may be made. Other diagnostic tests are often used collaboratively to diagnose FIP. These include determining the albumin-to-globulin (A:G) ratio in serum and fluid. A serum A:G ratio less than 0.8 g/dL and effusion A:G ratio less than 0.9 g/dL are often present with FIP. Anti–feline corona virus (FCoV) antibodies in serum should be interpreted with caution because many healthy cats are FCoV antibody positive.19, 20 Low to medium titers (1:25, 1:100, 1:400) of FCoV antibodies are of no diagnostic value in determining FIP infection; however, antibody titers of 1:1600 increased the probability of FIP.20 A negative test result does not rule out the possibility of FIP infection. In one study, the anti-FCoV antibody test was negative in 10% of the cats, which did, in fact, have FIP.20 Tests that show promise include reverse transcriptase–polymerase chain reaction (RT-PCR) performed on effusion fluids and an RT-PCR for the detection of FCoV messenger ribonucleic acid (mRNA) in peripheral blood mononuclear cells. Thus far, histological examination of affected tissue samples remains the gold standard for diagnosing FIP.
Fig. 15.34.

Abdominal fluid from a cat with effusive feline infectious peritonitis. Note the nondegenerate neutrophils, macrophages, and rare small lymphocytes within a granular, stippled, proteinaceous background.
Bile Peritonitis
Release of bile into the abdominal cavity secondary to gallbladder or bile duct rupture produces peritonitis. Rupture of the biliary system may occur secondary to bile duct obstruction, trauma, mucocele formation, biliary tract inflammation, and percutaneous biopsy of the liver. Bile in the peritoneal cavity causes a chemical peritonitis that is typically exudative. The effusion fluid color may be green tinged to yellow-orange. Amorphous to slightly spiculated, blue-green to yellow-green bile pigment may be present within macrophages and/or in the background fluid (Fig. 15.35, Fig. 15.36 ). These pigments may resemble hemosiderin seen in hemorrhagic effusions, and caution should be exercised during interpretation. If definitive differentiation is necessary, cytochemical staining may be used to highlight the iron in hemosiderin. Bilirubin concentration can be measured in the abdominal fluid and compared with the serum concentration: If the abdominal fluid bilirubin level is at least twofold greater than concurrent serum bilirubin levels, bile peritonitis is likely.
Fig. 15.35.

Abdominal fluid from a dog. Extracellular bile pigment (arrows) and nondegenerate neutrophils.
Fig. 15.36.

Abdominal fluid from a dog. Dark, yellow-green, amorphous extracellular and phagocytized bile pigment, with several mildly degenerate neutrophils and macrophages.
A mucocele (mucinous cystic hyperplasia) of biliary and gallbladder epithelial cells may occur secondary to inflammation and cholelithiasis. Mucoceles may result from dysfunction of mucus-secreting cells within the gallbladder mucosa, leading to accumulation of bile and potential rupture.21 Rupture of a biliary mucocele may cause atypical bile peritonitis, and the effusion fluid may be yellow or red in color. The cellularity is exudative and composed of predominantly nondegenerate to mildly degenerate neutrophils and low to moderate numbers of macrophages and reactive mesothelial cells. Varying amounts of mostly extracellular amorphous, homogeneous, mucinous, basophilic material is seen in small clumps and lakes. This material has been termed “white bile,” although this mucinous material does not contain bile constituents (Fig. 15.37, Fig. 15.38 ). In these cases, abdominal fluid bilirubin concentrations are typically, but not always, higher than serum bilirubin concentrations.22
Fig. 15.37.

Abdominal fluid from a dog. Extracellular homogeneous basophilic material, or “white bile” (arrows).
Fig. 15.38.

Abdominal fluid from a dog. “White bile” seen as a large accumulations of extracellular, homogeneous, pale basophilic, mucinous-type material, with many mildly degenerate neutrophils.
Uroperitoneum
Uroperitoneum may result from leakage of urine from the kidney, ureter, urinary bladder, or urethra. Urine released into the peritoneal cavity acts as a chemical irritant and causes inflammation that may lead to an exudative process. Uroperitoneum effusions will have varying numbers of inflammatory cells depending on the duration and dilutional effect of urine; however, nucleated cell counts are typically less than 6000 cells/μL and the total protein content is generally less than 3 g/dL as a result of the dilutional effect of urine volume. Neutrophils may be degenerate and ragged even in a nonseptic fluid because of the chemically irritating property of urine. Bacteria and urinary crystals may be found in the abdominal fluid if they were present in the bladder at the time of rupture. Comparing serum creatinine concentrations to the concentration of abdominal fluid creatinine will confirm uroperitoneum. Creatinine of the abdominal fluid will generally be higher than the creatinine level of serum because it equilibrates more slowly compared with blood urea nitrogen (BUN). Hyperkalemia and hyponatremia are often present.23
Chylous Effusions
Chylous effusions contain chylomicron-rich lymph fluid (chyle) that circulates in the lymphatic system. Chylomicrons are triglyceride-rich lipoproteins absorbed from the intestines after the ingestion of food containing lipids. Chylous effusions in dogs and cats occur most frequently as bilateral pleural effusions; chylous abdominal effusions occur less frequently.24
The classic description of a chylous effusion is a “milky” fluid that does not clear after centrifugation and cytologically consists primarily of small lymphocytes (Fig. 15.39 ). Macrophages may have small, punctate clear cytoplasmic vacuoles, and plasma cells may also present (Fig. 15.40 ). Chylous effusions are odorless and may vary in color from classic “milky white” to an opaque-yellow or pink, depending on diet (i.e., thin or anorectic patients may not have the characteristic opaque white fluid because of lack of dietary lipids) and the number of RBCs in the fluid. Although small lymphocytes are typically thought of as the predominant cell type, chylous effusions may occur with predominantly neutrophils, lipid-containing macrophages, or both.24 Increased neutrophils may occur secondary to inflammation induced by repeated thoracocentesis or merely the presence of chyle in the pleural cavity. Chyle is an irritant, and chronic chylous effusions may cause an inflammatory reaction that may eventually lead to pleural fibrosis.24 Bacterial infection in chylous effusions is uncommon because of the bacteriostatic effect of the fatty acids in chyle.2, 24, 25 However, bacteria may be introduced as a result of repeated thoracocentesis.
Fig. 15.39.

White, opaque chylous pleural effusion from a cat.
Fig. 15.40.
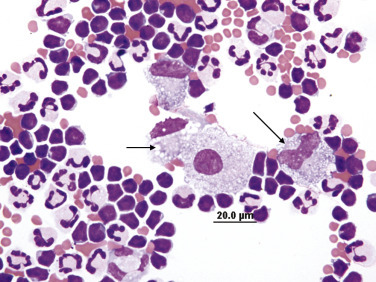
Chylous effusion. Numerous small lymphocytes and several macrophages containing small, distinct clear cytoplasmic vacuoles (arrows).
An effusion composed of mostly small lymphocytes but not exhibiting the typical physical characteristics (opaque) of a chylous effusion can be confirmed by measuring and comparing effusion and serum triglyceride concentrations.24, 25 The chylous effusion triglyceride concentration is higher than the serum concentration.24, 25 Chyle normally drains from the thoracic duct into the venous system. Chylous effusions form when there is an obstruction (physical or functional) of lymphatic flow resulting in increased pressure within lymphatics and dilation of the thoracic duct (lymphangiectasia). Rupture of the thoracic duct (i.e., after surgery or blunt trauma) is a rare cause of chylous effusion in veterinary medicine and is usually self-limiting.2, 24 Physical obstructions of the thoracic duct may result from neoplasms (thymoma, lymphoma, lymphangiosarcoma), granulomas, or inflammatory reactions in the mediastinum that compress the thoracic duct or the vessels into which it drains, or secondary to obstruction of intralymphatic flow with neoplastic cells. Functional obstructions may occur with cardiovascular disease from increased central venous pressure (right-sided heart failure) or increased lymphatic flow from increased hepatic lymph production that exceeds drainage capability.24, 25 Cardiovascular disease (i.e., cardiomyopathy, heartworm disease, pericardial effusions) resulting in poor venous flow may also lead to chylous effusion as a functional effect.
Many other miscellaneous causes of chylous effusion, including coughing and vomiting, diaphragmatic herniation, congenital defects, trauma, and thrombosis of the thoracic duct, have been reported, and often no underlying etiology can be determined despite extensive testing (idiopathic chylous effusion).2, 24, 25
Although most opaque effusions are true chylous effusions, they may rarely be pseudochylous. True pseudochylous effusions are a debated entity; however, they are opaque effusions that do not contain chyle. Instead, the white color is classically thought to be the result of cellular debris, lecithin globulin complex, cholesterol from cell membranes, or all of these. Pseudochylous effusions described in humans are most commonly the result of long-standing pleural effusions caused by tuberculosis, rheumatoid pleuritis, and malignant effusions, with resultant cell breakdown within the fluid. Despite much discussion about differentiating these two types of fluids, pseudochylous effusions are not well described in veterinary medicine and are rare in dogs and cats.2, 24 Cytologically, the presence of cellular breakdown material, such as cholesterol crystals, and the lack of a significant lymphocytic cellular component may suggest a pseudochylous effusion (Fig. 15.41 ). Additionally, pseudochylous effusions have high cholesterol and low triglyceride content compared with serum.
Fig. 15.41.
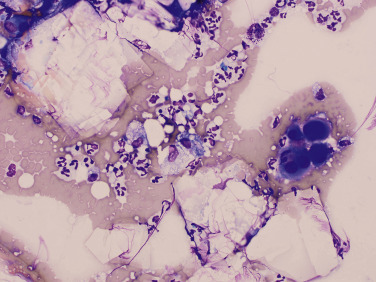
Abdominal effusion from a dog. Large numbers of extracellular, clear, flat, notched cholesterol crystals, with red blood cells, few reactive mesothelial cells (right center), and admixed nondegenerate neutrophils and macrophages.
Hemorrhagic Effusions
Hemorrhagic effusions may be seen with various primary disorders, such as hemostatic defects (congenital or acquired coagulopathies); trauma; neoplasia; and heartworm infection (Fig. 15.42 ). Hemorrhagic effusions secondary to neoplasia may not contain neoplastic cells, or neoplastic cells may be present in low, moderate, or high numbers. The presence or absence of neoplastic cells in a hemorrhagic effusion is often dependent on the type of neoplasm. For example, mesenchymal neoplasms such as hemangiosarcoma (splenic, hepatic, cardiac) often lack neoplastic cells or contain low numbers of neoplastic cells within the hemorrhagic effusion. In comparison, if mesothelioma is associated with a hemorrhagic effusion, moderate to high numbers of atypical mesothelial cells may be evident. However, it should be kept in mind that reactive mesothelial cells may have significant atypia and that differentiating a reactive population from a malignant population is often difficult, even for experienced cytopathologists. Determining the etiology of a hemorrhagic effusion, just as in the case of other effusion categories, requires not only cytological assessment of the fluid but also correlation with clinical signs, history, laboratory data, imaging studies, and often fine-needle aspiration (FNA) of abnormalities found in the respective body cavity. Distinguishing hemorrhagic effusions from iatrogenic blood contamination or inadvertent aspiration of an organ (i.e., liver, spleen) is of diagnostic importance. Differentiating blood contamination from per-acute or acute hemorrhage may be difficult; however, assessment of clinical signs, physical examination findings, and laboratory data are helpful. Hemorrhage of greater than 24 hours’ duration may be differentiated from blood contamination by identifying erythrophagocytosis in the sample (Fig. 15.43 ) and by noting the presence or absence of platelets and the RBC breakdown products, hemosiderin, and hematoidin (Fig. 15.44 ). When blood enters a body cavity, the platelets quickly aggregate, degranulate, and disappear. Also, RBCs are phagocytized and digested by macrophages. Therefore the presence of platelets and lack of erythrophagocytosis or heme breakdown products suggests either per-acute hemorrhage or iatrogenic blood contamination. Concurrent identification of platelets and erythrophagocytosis, with or without heme breakdown products, suggests either chronic hemorrhage or previous hemorrhage with iatrogenic contamination. The absence of platelets, with evidence of erythrophagocytosis, heme breakdown products, or a combination of both, supports chronic or previous hemorrhage.
Fig. 15.43.

Erythrophagocytosis. Note macrophage containing several intact phagocytized red blood cells.
Fig. 15.44.

Hemorrhagic pericardial effusion, with many hemosiderin and hematoidin-laden macrophages.
In cases of inadvertent organ aspiration, inadvertent major vessel puncture, or frank intracavity hemorrhage, the fluid obtained will be grossly bloody. With a major vessel or splenic aspirate, the PCV of the fluid is generally equal to (vessel puncture) or greater than (splenic aspirate) the peripheral blood PCV. With severe intracavity hemorrhage, clinical signs of hemorrhagic shock are expected.
Neoplastic Effusions
Effusions may occur secondary to many forms of neoplasia (lymphoma, mast cell neoplasia, sarcoma, mesothelioma, carcinoma or adenocarcinoma, etc.) and may often be diagnosed with cytological examination of the fluid. In one study, the sensitivity of cytological examination of effusions to detect malignant neoplasms was 64% in dogs and 61% in cats.26 Poorly exfoliating tumors may have effusions in the modified transudate to exudative range if there is concurrent inflammation. Many tumors do not exfoliate neoplastic cells, and the absence of neoplastic cells within effusions does not rule out neoplasia. Similar to tissue aspirates, neoplastic cells in an effusion must be distinguished from dysplastic cells secondary to inflammation or reactive mesothelial cells. Thus the presence of concurrent inflammation in the fluid may confound the diagnosis of neoplasia, especially if neoplastic cells are not present in high numbers, do not exhibit significant cytological criteria of malignancy, or both. Distinguishing neoplastic epithelial cells (exfoliative carcinoma or adenocarcinoma) from mesothelioma, and mesothelioma from hyperplastic and reactive mesothelial cells, which are frequently found in both neoplastic and nonneoplastic fluids, is a particular challenge. This dilemma is discussed further in the section on mesothelioma below. Neoplastic effusions are inherently difficult samples to interpret for many of the reasons outlined above, and therefore in-house samples interpreted as neoplastic, or suspected of being neoplastic, should be confirmed by a veterinary clinical pathologist.
Lymphoma
A neoplastic effusion secondary to high-grade lymphoma may occur with lymphoma of the intracavitary lymph nodes, spleen, liver GI tract, kidneys, thymus, and mediastinum. Cytologically, low to high numbers of a monomorphic population of exfoliating large immature lymphocytes may be present (Fig. 15.45 ; and see Fig. 15.12). Immature lymphocytes are large cells, with a scant to moderate amount of basophilic cytoplasm, round to variably shape nuclei, finely stippled nuclear chromatin, and prominent nucleoli.
Fig. 15.45.

Pericardial fluid from a dog with lymphoma. Large immature lymphocytes predominate.
Courtesy James Meinkoth, Oklahoma State University.
Mast Cell Neoplasia
Mast cell tumors within body cavities (nodal, hepatic, splenic, and GI) may cause effusions and frequently exfoliate large numbers of mast cells into the effusion (see Fig. 15.15, Fig. 15.16). Mast cells are readily identified by large numbers of metachromatic (purple) cytoplasmic granules. In effusions, mast cells tend to have “packeted” granules, and because of the high affinity of granules for stain and stain exhaustion, the nucleus may stain poorly or not at all. Diff-Quik stain does not undergo the same metachromatic reaction as Wright-Giemsa or modified Wright stain and often does not stain mast cell granules well. Eosinophils are occasionally (but not reliably) present, as are few scattered nondegenerate neutrophils, mesothelial cells, and macrophages. It should be noted that pleomorphic (anisocytosis, anisokaryosis, prominent nucleoli, multinucleation) and poorly granular mast cell tumors may be found in an effusion.
Sarcoma
Sarcomas involving intracavity organs often do not exfoliate neoplastic mesenchymal cells into effusions and are rarely diagnosed on fluid analysis alone. Often, effusions secondary to mesenchymal tumors are hemorrhagic secondary to rupture of the tumor (i.e., splenic and hepatic hemangiosarcoma) and of low nucleated cellularity. In this case, making concentrated specimens or buffy coat preparations may aid in concentrating low numbers of neoplastic cells. In the rare event that neoplastic cells are identified, the cells have a characteristic spindle appearance and malignant features (see Chapter 2).
Mesothelioma
Mesotheliomas are uncommon tumors in domestic species and can be well differentiated or pleomorphic. Subtypes are based on histological evaluation of growth patterns (epithelioid, biphasic, sarcomatoid, and undifferentiated). Mesothelioma is often difficult to diagnose cytologically because of the moderate to marked pleomorphism exhibited by reactive mesothelial cells. Thus, when an effusion contains significant numbers of mesothelial cells where marked cytological criteria of malignancy is not evident, it is often impossible to differentiate mesothelial reactivity or hyperplasia from mesothelioma. When an effusion contains large numbers of mesothelial cells, and the cells exhibit marked criteria of malignancy—extreme macrocytosis, marked anisokaryosis, large variably shaped nucleoli, large numbers of mitotic figures, and aberrant mitoses—although diagnostic on cytology for a malignant exfoliative neoplasm, it may be nearly impossible to differentiate between mesothelioma, carcinoma, or adenocarcinoma (Fig. 15.46, Fig. 15.47 ).26 Identification of a primary carcinoma/adenocarcinoma is helpful. If a primary tumor is not identified, histopathology of the affected mesothelium may be necessary. Often, a combination of history, imaging findings, cytology, and histopathology is needed to diagnose mesothelioma or to distinguish mesothelioma from epithelial neoplasia. Histological differentiation of reactive or hyperplastic mesothelial cells from neoplastic mesothelial cells may also be challenging, particularly if the biopsy sample is small and not representative of the lesion. Histologically, no single defined criterion to diagnose mesothelioma exists; however, assessment for neoplastic invasion into the submesothelial tissues and immunohistochemistry may be helpful.27 Fig. 15.48 demonstrates the histopathology of mesothelioma.
Fig. 15.46.

Effusion fluid consistent with mesothelioma. Aggregates and individualized markedly pleomorphic, neoplastic mesothelial cells. Note the many features of malignancy, such as cell gigantism, multinucleation, macrokaryosis, macronucleoli, and multiple nucleoli.
Fig. 15.47.

Canine thoracic effusion secondary to mesothelioma. Histopathology was consistent with epithelioid subtype.
Fig. 15.48.

Histological section of mesothelioma. Papillary projections of multilayer neoplastic mesothelium with significant cellular pleomorphism and frequent mitoses.
Courtesy Luke Borst, North Carolina State University.
Carcinoma or Adenocarcinoma
Carcinomas and adenocarcinomas may often be diagnosed by cytological evaluation of effusions on the basis of significant numbers of exfoliating cells and numerous criteria of malignancy. Neoplastic effusions may be inflammatory or noninflammatory. Neoplastic epithelial cells often form aggregates, clusters, and sheets. Occasional glandular (acinar) arrangements may be found. Significant anisocytosis, anisokaryosis and anisonucleoliosis may exist, with cell gigantism and abundant basophilic cytoplasm (Fig. 15.49 ). Cytoplasm may also contain large clear vacuoles, which push the nucleus to the periphery of the cell, or numerous fine, foamy cytoplasmic vacuoles (Fig. 15.50, Fig. 15.51 ), and may also contain intracytoplasmic eosinophilic secretory material. Documenting strong nuclear criteria of malignancy is important, including anisokaryosis; nuclear gigantism; coarse nuclear chromatin; large, bizarre, or angular nucleoli; multiple nucleoli; nuclear molding; high nucleus-to-cytoplasm ratios; multinucleation (see Fig. 15.49); numerous mitotic figures; and aberrant mitoses (see Fig. 15.50). When an effusion is diagnostic for epithelial neoplasia, imaging studies of the respective body cavity may identify masses or organomegaly, prompting FNA or tissue biopsy for further characterization.
Fig. 15.49.

Pleural fluid from a dog with a neoplastic effusion secondary to epithelial neoplasia. Note the clusters of pleomorphic cells, with abundant basophilic cytoplasm, significant variation in the nuclear-to-cytoplasmic (N:C) ratios, occasional multinucleation, and marked anisocytosis.
Fig. 15.50.

Few neoplastic cells contain large clear vacuoles that push the nucleus to the periphery. Additionally, note the two mitotic figures. The bottom right mitotic figure is aberrant.
Fig. 15.51.

Feline abdominal effusion secondary to epithelial neoplasia. Note the numerous, clear, small cytoplasmic vacuoles.
Thymoma
Thymoma is a neoplasm of thymic epithelium and is a top consideration in the differential diagnosis for a cranial mediastinal mass. Thymomas may be benign or malignant, and both invasive and noninvasive forms exist. Additionally, thymomas may be heterogeneous, cystic, or inflamed. A detailed description of the cytological appearance of aspirates from thymomas is provided in Chapter 17. When thymomas are associated with a thoracic effusion, the effusion may be inconclusive or suggestive of thymoma. An effusion associated with thymoma may contain large numbers of small lymphocytes, which are often a significant and predominant nonneoplastic cell population in thymomas. The presence of a prominent population of small lymphocytes, together with low to moderate numbers of well-differentiated mast cells (also a prominent cell population in thymomas), helps lend support to a diagnosis of thymoma if a cranial mediastinal mass is evident. It is uncommon for the neoplastic epithelial component to exfoliate, and if epithelial cells are present, it may be difficult to differentiate them from reactive mesothelial cells. The neoplastic epithelial cells have somewhat ill-defined borders, are found in aggregates and sheets, and contain small to moderate amounts of pale blue cytoplasm, round central nuclei, and indistinct nucleoli. The cells often are minimally pleomorphic. Direct aspiration, tissue biopsy and histopathology, or flow cytometry of either the mass or effusion fluid may be used to differentiate thymoma from thymic small cell lymphoma. Lymphocyte coexpression of CD4 and CD8, which is characteristic of thymocytes, is suggestive of thymoma.28
Parasitic Effusions
Abdominal effusion caused by aberrant larval migration of the tapeworm Mesocestoides spp. is uncommon. Cases of canine infection are reported in northwestern United States, particularly in California, with fewer cases in Washington.29 Clinical signs may include anorexia, vomiting, weight loss, depression, and abdominal distension. In reported cases of parasitical effusions, the gross appearance of the fluid contains small opaque flecks, which are the metacestodes.29 Analysis of the aspirated fluid is in the exudative range. Cytological features include numerous inflammatory cells, partial to intact metacestodes, and numerous round to angular, clear to pink refractile calcareous corpuscles (Fig. 15.52, Fig. 15.53 ).
Fig. 15.52.

Metacestode remnant. Note the size of the red blood cells and inflammatory cells in the background compared with this structure. The clear, nonstaining structures are calcareous corpuscles (arrows).
Fig. 15.53.

Metacestode remnant. The clear, nonstaining structures are calcareous corpuscles (arrow).
References
- 1.Nelson O.L. Pleural effusion. In: Ettinger S.J., Feldman E.C., editors. Textbook of Veterinary Internal Medicine. Saunders; Philadelphia, PA: 2005. pp. 204–207. [Google Scholar]
- 2.Fossum T.W. Surgery of the lower respiratory system: pleural cavity and diaphragm. In: Fossum T.W., editor. Small Animal Surgery. Mosby; St. Louis, MO: 2005. pp. 788–820. [Google Scholar]
- 3.Zoia A., Hughes D., Connolly D.J. Pericardial effusion and cardiac tamponade in a cat with extranodal lymphoma. J Small Anim Pract. 2004;45:467–471. doi: 10.1111/j.1748-5827.2004.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 4.Tobias A.H. Pericardial disorders. In: Ettinger S.J., Feldman E.C., editors. Textbook of Veterinary Internal Medicine. Saunders; Philadelphia, PA: 2005. pp. 1107–1111. [Google Scholar]
- 5.Gidlewski J., Petrie J.P. Therapeutic pericardiocentesis in the dog and cat. Clin Tech Small Anim Pract. 2005;20:151–155. doi: 10.1053/j.ctsap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Fossum T.W. Surgery of the abdominal cavity. In: Fossum T.W., editor. Small Animal Surgery. Mosby; St. Louis, MO: 2002. pp. 271–272. [Google Scholar]
- 7.Walters J.M. Abdominal paracentesis and diagnostic peritoneal lavage. Clin Tech Small Anim Pract. 2003;18(1):32–38. doi: 10.1016/1096-2867(03)90023-5. [DOI] [PubMed] [Google Scholar]
- 8.D’Urso L. Thoracic and pericardial taps and drains. In: Ettinger S.J., Feldman E.C., editors. Textbook of Veterinary Internal Medicine. Saunders; Philadelphia, PA: 2005. pp. 380–831. [Google Scholar]
- 9.Johnson M. A retrospective study of clinical findings, treatment and outcome in 143 dogs with pericardial effusion. J Small Anim Pract. 2004;45:546–552. doi: 10.1111/j.1748-5827.2004.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 10.Stockham S.L., Scott M.A. Vol. 851. Blackwell Publishing; Ames, IA: 2008. Cavitary Effusions. (Fundamentals of Veterinary Clinical Pathology). 849, 841, 842. [Google Scholar]
- 11.George J.W. The usefulness and limitations of hand-held refractometers in veterinary laboratory medicine: an historical and technical review. Vet Clin Path. 2001;30(4):201–210. doi: 10.1111/j.1939-165x.2001.tb00432.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowgill E., Neel J. Pleural fluid from a dog with marked eosinophilia. Vet Clin Pathol. 2003;32(4):147–149. doi: 10.1111/j.1939-165x.2003.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T. Visceral mast cell tumors in dogs: 10 cases (1982-1997) J Am Vet Assoc. 2000;216(2):222–226. doi: 10.2460/javma.2000.216.222. [DOI] [PubMed] [Google Scholar]
- 14.Spangler W.L., Culbertson M.R. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991) J Am Vet Med Assoc. 1992;201:773–776. [PubMed] [Google Scholar]
- 15.Rissetto K., Villamil J.A., Selting K.A., Tyler J., Henry C.J. Recent trends in feline intestinal neoplasia: an epidemiologic study of 1129 cases in the veterinary medical database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28–36. doi: 10.5326/JAAHA-MS-5554. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien P.J., Lumsden J.H. The cytologic examination of body cavity fluids. Semin Vet Med Surg (Small Animal) 1988;3(2):140–156. [PubMed] [Google Scholar]
- 17.Arndt Holmberg T., Vernau W., Melli A.C., Conrad P.A. Neospora caninum associated with septic peritonitis in an adult dog. Vet Clin Pathol. 2006;35(2):235–238. doi: 10.1111/j.1939-165x.2006.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 18.Dell’Orco M., Bertazzolo W., Paccioretti F. What is your diagnosis? Peritoneal effusion from a dog. Vet Clin Pathol. 2009;38(3):367–369. doi: 10.1111/j.1939-165X.2009.00130.x. [DOI] [PubMed] [Google Scholar]
- 19.Rohrbach B.W. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J Am Vet Assoc. 2001;218(7):1111–1115. doi: 10.2460/javma.2001.218.1111. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann K. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Intern Med. 2003;17:781–790. doi: 10.1111/j.1939-1676.2003.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike F.S. Gallbladder mucocele in dogs: 30 cases (2000-2002) J Am Vet Assoc. 2004;224(10):1615–1622. doi: 10.2460/javma.2004.224.1615. [DOI] [PubMed] [Google Scholar]
- 22.Owens S.D. Three cases of canine bile peritonitis with mucinous material in abdominal fluid as the prominent cytologic finding. Vet Clin Pathol. 2003;32(3):114–120. doi: 10.1111/j.1939-165x.2003.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 23.Aumann M., Worth L.T., Drobatz K.J. Uroperitoneum in cats: 26 cases (1986-1995) J Am Anim Hosp Assoc. 1998;34(4):315–324. doi: 10.5326/15473317-34-4-315. [DOI] [PubMed] [Google Scholar]
- 24.Meadows R.L., MacWilliams P.S. Chylous effusions revisited. Vet Clin Pathol. 1994;23:54–62. doi: 10.1111/j.1939-165x.1994.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 25.Mertens M.M., Fossum T.W. Pleural and extrapleural diseases. In: Fossum T.W., editor. Small Animal Surgery. Mosby; St. Louis, MO: 2002. pp. 1281–1282. [Google Scholar]
- 26.Hirschberger J. Sensitivity and specificity of cytologic evaluation in the diagnosis of neoplasia in body fluids from dogs and cats. Vet Clin Path. 1999;28(4):142–146. doi: 10.1111/j.1939-165x.1999.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 27.Reggeti F., Brisson B., Ruotsalo K. Invasive epithelial mesothelioma in a dog. Vet Pathol. 2005;42:77–81. doi: 10.1354/vp.42-1-77. [DOI] [PubMed] [Google Scholar]
- 28.Lana S., Plaza S. Diagnosis of mediastinal masses in dogs by flow cytometry. JVIM. 2006;20:1161–1165. doi: 10.1892/0891-6640(2006)20[1161:dommid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Caruso K.J. Cytologic diagnosis of peritoneal cestodiasis in dogs caused by Mesocestoides sp. Vet Clin Pathol. 2003;32(2):50–60. doi: 10.1111/j.1939-165x.2003.tb00314.x. [DOI] [PubMed] [Google Scholar]


