This chapter discusses vaccines for military and biodefense research personnel as well as some vaccines that are in development for uncommon or geographically limited diseases. Military personnel have the potential to be exposed to many infectious agents as endemic diseases and in their unnatural form as biological weapons. Increasingly, civilian populations may be targets for terrorist attacks using microorganisms (or their toxins), as was demonstrated by the purposeful dissemination of anthrax spores following ballistic attacks on the World Trade Center and the Pentagon in 2001.
The U.S. Army has had a longstanding program to develop vaccines to combat these threats. Within the U.S. Department of Defense (DOD), the U.S. Army Medical Research and Materiel Command (USAMRMC), located at Fort Detrick, Maryland, is the principal organization responsible for vaccines and other medical countermeasures against biological warfare agents. Within USAMRMC, the unit with direct responsibility for this defensive mission is the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), also at Fort Detrick. Before 1969, when the United States maintained an offensive biological weapons program, the U.S. Army Medical Unit within the Walter Reed Army Institute of Research in Washington, DC, served in this capacity.
Many of the biowarfare vaccines currently available at USAMRIID and elsewhere to protect laboratory researchers studying these agents were conceived at the U.S. Army Medical Unit and were further developed at the now closed National Drug Laboratories (also known as The Salk Institute's Government Services Division [TSI-GSD]). Most of the vaccines developed at Fort Detrick, for a variety of reasons, remain investigational new drugs (INDs). However, all these vaccines have undergone extensive preclinical testing and have progressed to Phase II trials that have continued for many years. Meanwhile, USAMRIID researchers and others continue to apply new technology to develop improved vaccines.
Table 12.1 presents all IND vaccine products of military interest developed at USAMRMC/USAMRIID; most of these vaccines are currently available for administration only through USAMRIID's Special Immunizations Program (SIP).1 One of the vaccines, the Candid #1 Junin (Argentine hemorrhagic fever [AHF]) vaccine, shown to be efficacious in a Phase III trial2 in Argentina, has been incorporated into that country's public health program and administered to thousands of individuals. Several other vaccines, including Venezuelan equine encephalitis (VEE) TC-83, eastern equine encephalitis (EEE) vaccine, western equine encephalitis (WEE) vaccine, tularemia live vaccine strain (LVS), Rift Valley fever (RVF) inactivated vaccine, and the whole-cell Q fever vaccine, have also been administered to more than 1000 volunteers, primarily through the SIP. In contrast, the chikungunya (CHIK) vaccine strain 181/clone 25, live attenuated RVF vaccine MP-12, Q fever chloroform-methanol residue (CMR) vaccine, and vaccinia/Hantaan vaccine have been administered to no more than a few hundred volunteers each.
TABLE 12.1.
Investigational New Drug, Limited-Use Vaccines in Inventory at USAMRIID: Characteristics and Administration
| Name | IND Number | Type | Dosage (mL) | Route | Schedule (days) | Boosters |
|---|---|---|---|---|---|---|
| VEE TC-83 (NDBR 102) |
BB-IND 142 | Live attenuated | 0.5 | SC | 0 | Boost with C84 per titer |
| VEE C-84 (TSI-GSD 205) |
BB-IND 914 | Inactivated | 0.5 | SC | 0a | Yes; based on titer |
| WEE (TSI-GSD 210) |
BB-IND 2013 | Inactivated | 0.5 | SC | 0, 7, 28 | Mandatory boost at month 6b |
| EEE (TSI-GSD 104) |
BB-IND 266 | Inactivated | 0.5 | SC | 0, 28 | Mandatory boost at month 6c |
| Chikungunya 181/clone 25 (TSI-GSD 218) |
BB-IND 2426 | Live attenuated | 0.5 | SC | 0 | Not determined |
| RVF (TSI-GSD 200) |
BB-IND 365 | Inactivated | 1.0 | SC | 0, 7, 28 | Yes; based on titerd |
| RVF MP-12, ZH548 (TSI-GSD 223)e |
BB-IND 4307 | Live attenuated | 1.0 | IM | 0 | No |
| Junin, or Candid #1e | BB-IND 2257 | Live attenuated | 0.5 | SC | 0 | No |
| Q fever (NDBR 105)e, f |
BB-IND 610 | Inactivated | 0.5 | SC | 0 | No |
| Q fever CMRe (TSI-GSD 217) |
BB-IND 3516 | Inactivated | 0.5 | SC | 0 | Not determined |
| Tularemia LVS (NDBR 101, TSI-GSD 213) |
BB-IND 157 | Live attenuated | 0.06 | Scarification | 0 | No |
CMR, chloroform-methanol residue; EEE, eastern equine encephalitis; IM, intramuscular; IND, investigational new drug; LVS, live vaccine strain; RVF, Rift Valley fever; SC, subcutaneous; TSI-GSD, The Salk Institute's Government Services Division; USAMRIID, U.S. Army Medical Research Institute of Infectious Diseases; VEE, Venezuelan equine encephalitis; WEE, western equine encephalitis.
C-84 is given only after TC-83 and titer <1:20.
After month 6, boosts of the WEE vaccine are given as needed per titer.
Boosts of the EEE vaccine before and after month 6 are given as needed per titer (as 0.1 mL intradermally).
Initial responders to RVF (inactivated) vaccine: mandatory boost at month 6, then as needed per titer. Initial nonresponders: boost within 90 days of low titer.
These vaccines are currently not in active use at USAMRIID.
A skin test (using Q fever skin test antigen, MNLBR 110, a dilution of the NDBR 105) is conducted 1 week prior to vaccination with Q fever vaccine (NDBR 105).
Each of the vaccines in active use in USAMRIID's SIP is administered under an approved human use protocol, and all volunteers provide written informed consent before vaccination. These IND vaccines are administered on a voluntary basis to at-risk laboratory and field workers. Most SIP participants are scientists and technicians from USAMRIID, but scientists from academia, other federal and state agencies, and private drug companies also participate.1 Extramural scientists who wish to receive one of these vaccines must come to USAMRIID for vaccine administration and safety monitoring. The protocols and consent forms are reviewed by the USAMRIID Scientific Review Committee, the Headquarters USAMRMC Institutional Review Board, and the Office of Research Protections (within USAMRMC Headquarters at Fort Detrick) before submission to the U.S. Food and Drug Administration (FDA). Protocols are conducted in accordance with the Belmont Principles, DOD Instruction 3216.02 (“Protection of Human Subjects and Adherence to Ethical Standards in DOD-Supported Research”), and other applicable DOD and FDA regulations and guidelines. Many of these vaccines have been in use since the 1960s and 1970s, and all are subject to periodic potency and lot-release testing as required by FDA.
Continuous monitoring for safety and immunogenicity is conducted through the IND vaccine managing authority: the U.S. Army Medical Materiel Development Activity and the Non-Clinical Studies Division of USAMRIID. Funding for testing of these products is provided through the Medical Countermeasure Systems, Fort Belvoir, Virginia. To date, all vaccines have demonstrated an acceptable safety profile and reasonable immunogenicity (Table 12.2 ), except the botulinum toxoid pentavalent (ABCDE) vaccine, which is no longer administered.3 The most reactogenic product is the live attenuated VEE TC-83 vaccine, which frequently induces a short-term systemic reaction.4 The inactivated vaccines, except the whole-cell Q fever vaccine, require multiple doses for priming and frequent periodic boosting to maintain acceptable levels of neutralizing antibody. All vaccines have been administered to men and nonpregnant women within a broad range of ages, ethnicities, and races. With the exception of Junin vaccine, efficacy of these products is inferred by the absence of laboratory-acquired infection among recipients. However, because laboratory practices and engineering controls have evolved in concert with use of the vaccines, quantifying efficacy on a continuous basis is difficult.
TABLE 12.2.
Assessment of Efficacy and Safety of Selected Limited-Use Vaccines
| Name | Tests of Effectiveness | Effective? | Relative Severity and Frequency of Vaccine-Related Reactions |
|
|---|---|---|---|---|
| Systemic Severity/Frequency | Local Severity/Frequency | |||
| VEE TC-83, live | Reduction in laboratory-associated infections | Yes | +++/+++ | +/+ |
| VEE C-84, inactivated | Insufficient data | Unknown | +/+ | +/+ |
| WEE, inactivated | Reduction in laboratory-associated infections | Probably | +/++ | +/+ |
| EEE, inactivated | Reduction in laboratory-associated infections | Probably | +/++ | +/++ |
| Chikungunya, live | Insufficient data | Unknown | +/++ | +/++ |
| RVF, inactivated | Reduction in laboratory-associated infections | Yes | +/+ | +/+ |
| RVF MP-12, live | Insufficient data | Unknown | +/++ | +/+ |
| Junin, live | Formal Phase III field trial | Yes | +/+ | +/+ |
| Q fever, inactivated | Reduction in laboratory-associated infections | Probably | +/+++ | +/+++ |
| Q fever CMR, inactivated | Insufficient data | Unknown | +/++ | +/+++ |
| Tularemia LVS, live | Reduction in laboratory-associated infections | Yes | +/+++ | +/+++ |
EEE, eastern equine encephalitis; LVS, live vaccine strain; RVF, Rift Valley fever; VEE, Venezuelan equine encephalitis; WEE, western equine encephalitis.
Severity of reactions: +, most reactions are mild, <5% are severe; ++, most reactions are mild or moderate, <15% are severe; +++, a majority of reactions are mild or moderate, ≥15% are severe.
Frequency of reactions: +, typically occur after <10% of doses; ++, typically occur after ≥10% but <30% of doses; +++, typically occur after ≥30% of doses.
USAMRIID has been proactive in evaluating, not only the short-term reactogenicity, but also the long-term medical safety of its vaccines and other vaccines administered to at-risk laboratory workers. One study5 was aimed at detecting any long-term medical effects from repeated injections with multiple vaccines; a second study6 evaluated volunteers who participated in biomedical research as part of Operation Whitecoat.
The first study consisted of 155 volunteers who had participated in a multiple immunizations program (MIP cohort) and 265 community control volunteers who had not participated in a MIP, matched by age, race, and sex.5 The majority of study volunteers were male (83%) and older in age (average age: 69.4 years). This study did not link any disease or medical condition to repetitive immunization with multiple antigens or any single antigen. Fatigue was noted more commonly among subjects who had received multiple immunizations, but this finding was not associated with number of shots, number of antigens, or time in the MIP. Although statistically significant increases and decreases in several clinical laboratory tests were observed, none of these findings appeared to be clinically significant.
The single most important finding of this study was the greater frequency of serum monoclonal proteins observed among MIP subjects compared with control subjects. However, no associations between the presence of monoclonal proteins and specific diseases or medical conditions were seen.5 The significance and implications of this finding are unclear, warranting further investigation. A larger study with more than 1100 volunteers who participated in the SIP has been completed and should, once the results are analyzed, elucidate these findings further. The results of this study are expected to be published in 2017.
The second study involved men entering military service in the early days of the Cold War and the Vietnam War who expressed a conscientious objection to combat. These men were offered the opportunity to serve as medical research volunteers in Fort Detrick's biological warfare defense program. More than 2000 subjects participated in what became known as Operation Whitecoat. A study to assess the current health status of Operation Whitecoat medical research volunteers who served between 1954 and 1973 was developed as a joint effort between DOD and the Seventh-Day Adventist Church.6
Many of the volunteers enrolled in this study had been exposed to an infectious agent, vaccine, or other biological product as part of Operation Whitecoat (study group; n = 358); others had participated in research studies as unexposed control subjects or chose not to participate at all (control group; n = 164). Volunteers completed a self-administered questionnaire regarding their health status, clinical signs and symptoms, reproductive outcomes, and diseases or conditions diagnosed by medical providers. Attempts to assess frequencies of clinical signs and symptoms or diseases by individual vaccine, individual virulent agent, and individual antibiotic/inert substance exposure yielded numbers too small for meaningful analysis for all but recipients of VEE vaccine(s) and tularemia vaccine(s) as well as individuals exposed to virulent Coxiella burnetii. Among these exposures, a possible association between developing asthma and receipt of tularemia vaccine(s) (but not VEE vaccine or virulent C. burnetii) was observed (13.3% of vaccinees vs 2.4% of control subjects). In this same cohort, an association between receipt of a tularemia vaccine and a greater frequency of chronic headaches was suggested (with 35.6% of vaccinees vs 18.3% of control subjects reporting that headaches were occasionally or more frequently a problem as opposed to never or rarely a problem); however, this association did not reach statistical significance. No adverse impact on the overall health of Operation Whitecoat volunteers could be conclusively attributed to participation in research studies at Fort Detrick.6 Loma Linda University will continue with intermittent follow-up studies.
Limited-Use Vaccines Against Viral Diseases
Alphaviruses
The alphaviruses are a group of mosquito-borne, lipid-enveloped, positive-sense, single-stranded RNA viruses belonging to the family Togaviridae. Alphaviruses are responsible for two distinct clinical syndromes: fever, chills, headache, myalgias, vomiting, and encephalitis (e.g., VEE, EEE, and WEE) and fever, rash, and polyarthralgias/arthritis (e.g., CHIK, Ross River virus disease [RRVD, formerly called epidemic polyarthritis], and o'nyong-nyong). Several excellent reviews are available on the classification, epidemiology, and clinical features of these agents.7, 8, 9 Although the DOD has developed vaccines against VEE virus (VEEV), WEE virus (WEEV), EEE virus (EEEV), and CHIK virus (CHIKV), these vaccines remain investigational. Ongoing research seeks to develop improved vaccines against these pathogens.10 For example, a recent study using replicon technology showed that individual replicon vaccine candidates for VEE and EEE or a combined VEEV/WEEV/EEEV replicon particle vaccine elicited strong neutralizing antibodies and protection against aerosol challenge with VEEV subtype I-AB (Trinidad donkey strain) and EEEV. However, the individual WEEV replicon and the combined VEEV/WEEV/EEEV replicon vaccine elicited low levels of neutralizing antibodies to WEEV and conferred poor protection in macaques.11 In particular, Wolfe and colleagues12 argue that an FDA-licensed trivalent VEE/WEE/EEE vaccine—one that overcomes the safety, immunogenicity, and immune interference issues of the existing IND vaccines—is required to meet the requirements of the biodefense community. Such a vaccine also would be of great value to the public health and agricultural communities.
Experience with sequential administration of alphavirus vaccines at USAMRIID has led to a number of interesting observations relating to immunologic interference. Prior immunization with inactivated EEE and/or WEE vaccines decreases one's ability to mount a neutralizing antibody response following receipt of live attenuated VEE TC-83 vaccine. However, no interference is observed when the order of administration is reversed, such that VEE TC-83 vaccine is given first.13 Similarly, interference occurs when two live attenuated alphavirus vaccines, VEE TC-83 and a CHIK vaccine (CHIK 181/clone 25), are administered sequentially.14 Volunteers initially vaccinated with VEE TC-83 exhibited poor neutralizing antibody responses to live attenuated CHIK vaccine (46% response rate). Among persons initially inoculated with the CHIK vaccine or placebo who then received the live attenuated VEE TC-83 vaccine, geometric mean antibody titers to VEEV, as measured by 80% plaque reduction neutralization test (PRNT80), were uniformly depressed in CHIK vaccine recipients compared with placebo recipients.14 Interference may be an important consideration for the development of next-generation alphavirus vaccines, and particularly for the development of multiagent alphavirus vaccines.
Venezuelan Equine Encephalitis Virus
The two VEE vaccines available for human use as INDs (vaccines)—the live attenuated product, TC-83, and a formalin-inactivated product, C-84—both derive from the same lineage. The live attenuated TC-83 virus, a subtype I-AB strain, was isolated from a donkey brain in Trinidad and was passaged 13 times in embryonated eggs.15 The virus was attenuated by 78 passages in fetal guinea pig heart (FGPH) cell cultures, plaque-picked in chick embryo fibroblasts (CEFs), and passaged four additional times in FGPH cell cultures.16 The VEE TC-83 virus designation is a direct reference to the 83 passages in cell culture. VEE C-84 vaccine is formalin-inactivated and is made from the TC-83 production seed, TC-82, that has undergone one additional passage in CEFs.17 This C-84 production seed is passaged once more in CEFs to derive the C-84 vaccine, which is inactivated with 0.1% formalin and then freeze-dried. The inactivation procedure is based on that used by Salk and colleagues18 to inactivate the poliovirus. TC-83 and C-84 contain streptomycin and neomycin, each at a concentration of 50 µg/mL. Laboratory infections with epizootic VEEV strains closely related to the parent strain have essentially been eliminated since the introduction of these vaccines and improvement in personal protective equipment.
At USAMRIID, immunologically naïve people at risk for exposure to VEE receive a single dose of the live attenuated TC-83 vaccine. Those who seroconvert (PRNT80 titer ≥1 : 20) receive a single booster of C-84 as needed based on their titer (PRNT80 <1 : 20); nonresponders to TC-83 receive a booster with C-84. The response rate to TC-83 alone is 82%.4 When TC-83 is followed by a single boost with C-84, a combined response rate of well over 90% is observed. Female responders to TC-83 tend to have titers similar to those of male responders, but the frequency of nonresponders tends to be higher among women than men; in one study, the initial response rate among recipients of a single dose of TC-83 was 74% and 85%, respectively, for women and men. The nature of this sex difference is not understood.
Approximately 23% of persons receiving the live attenuated TC-83 sustain adverse reactions, including headache, sore throat, malaise, fatigue, myalgias, arthralgias, chills, and fever—a suite of symptoms similar to those seen following natural VEEV infection but less severe.4 The local reaction rate is less than 5%. The inactivated vaccine C-84 has a local reaction rate of approximately 5%, but essentially no systemic reactions are associated with its administration. Diabetes mellitus, abortion, and teratogenesis have been associated—epidemiologically and/or in animal studies—with natural wild-type VEEV infection.19, 20, 21 Before pregnancy testing prior to vaccination became available, three cases of spontaneous abortion or stillbirth were temporally related to the administration of TC-83.22 However, VEEV was not recovered from culture of tissues in either case. (Though they were reported to FDA, these cases were never published as case reports.) Since the advent of pregnancy testing, great care has been used to ensure that women are not pregnant before administration of TC-83. Out of an abundance of caution, persons with a family history of diabetes mellitus are considered ineligible for vaccination with TC-83, despite the lack of evidence for a causal association between diabetes mellitus and VEEV infection or vaccination with TC-83.
The ideal VEE vaccine would have a high seroconversion rate (>95%) and a low reaction rate (<5%). By these standards, TC-83 is reactogenic and has a moderate response rate, as measured by neutralizing antibody. In addition, TC-83 does not protect adequately against distantly related VEEV subtype I-AB variants or the other enzootic VEEV subtypes II through VI. Finally, the manufacturing process for TC-83 requires the manipulation of infectious viral particles in a biosafety level 3 containment laboratory.10
A new-generation, live attenuated vaccine candidate, V3526, uses twin site-directed mutagenesis of the full-length complementary DNA clone of the virulent virus RNA.23 V3526 has two deletion mutations—a lethal deletion at the PE-2 cleavage signal site and a suppressor mutation at site 253 of the E1 glycoprotein—that should prevent reversion to wild-type VEEV. This vaccine candidate also has limited potential for transmission by mosquitoes and elicits cross-protection against different viral strains.10 After showing promise in preclinical studies,24 V3526 elicited the development of impressive neutralizing antibody levels in human volunteers during Phase I clinical trials. However, the vaccine was associated with a high frequency of fever and other flu-like symptoms; thus, further development was discontinued.25, 26
Because of the long history of frequent adverse reactions related to live attenuated VEE vaccines, the manufacturer decided to inactivate V3526 and further develop it as an inactivated vaccine to be used as a priming vaccine that would replace C-84. Testing has shown reduced infectivity by both formalin inactivation (fV3526) and gamma-irradiation (gV3526). For example, both inactivated vaccine candidates showed a loss of neurovirulence after intracerebral inoculation of suckling BALB/c mice, suggesting that the vaccines were completely inactivated. Both fV3526 and gV3526 elicited robust immune responses; furthermore, protection was demonstrated by both vaccine candidates against subcutaneous challenge with VEEV I-AB Trinidad donkey strain following two doses of either vaccine. Recently, researchers have been testing these vaccine candidates, using various adjuvants and routes of administration, against subcutaneous and aerosol challenge.24, 27, 28
In addition to fV3526 and gV3526, other technologies are being evaluated for the production of vaccines against VEE. For example, Sharma and colleagues29 found that the hydrophobic alkylating compound 1,5-iodonaphthylazide can effectively inactivate virulent VEEV strain V3000. The resulting inactivated vaccine candidate was efficacious in protecting mice from virulent VEEV challenge, and its efficacy was enhanced by the use of adjuvants. Rossi and colleagues30, 31 developed a live attenuated vaccine using an encephalomyocarditis virus internal ribosome entry site (IRES)—a construct that inhibits translation of viral proteins in mosquito cells, thus preventing transmission by the natural VEEV vector. This IRES-based VEE vaccine fully protected mice and macaques from clinical disease after aerosol challenge with virulent VEEV.
DNA vaccines are also under development and, in protective efficacy studies, have shown promise in both mice and guinea pigs.32 One DNA vaccine candidate has also demonstrated protection against aerosol challenge with wild-type VEEV in nonhuman primates.33, 34 A candidate based on a novel approach to DNA vaccine development, infectious DNA (iDNA), was protective in one BALB/c mouse challenge study.35
Western Equine Encephalitis Virus
An inactivated WEE vaccine, TSI-GSD 210, has been used at Fort Detrick since the 1970s to immunize at-risk laboratory personnel. The WEE vaccine is a lyophilized product derived from supernatant fluids of primary CEF cell cultures infected with the attenuated CM4884 strain of WEEV.36, 37 The supernatant fluid is harvested and filtered, the virus is inactivated with formalin, and the final product is lyophilized for storage at −20°C. The vaccine contains 50 µg/mL of neomycin. The primary end point used to measure immunogenicity of the WEE vaccine is the PRNT80, with a titer of at least 1 : 40 considered indicative of a response.
In an analysis of data from 363 volunteers who received 0.5 mL of inactivated TSI-GSD 210 vaccine subcutaneously at days 0, 7, and 28, 151 subjects (41.6%) responded with a PRNT80 titer of 1 : 40 or greater, whereas 212 subjects (58.4%) failed to achieve this neutralizing antibody titer. Of 115 initial nonresponders, 76 (66.1%) converted to responder status after a single booster. Kaplan–Meier plots showed that a regimen consisting of three initial doses and one booster induced a PRNT80 titer of at least 1 : 40 lasting 1.6 years in 50% of initial responders. Local and systemic adverse events are uncommon with this vaccine. Among 363 vaccinees receiving three initial injections of the WEE vaccine, only 5 reported local or systemic reactions (P.R. Pittman, P.H. Gibbs, and T.L. Cannon, unpublished data).
No instances of occupational WEE have been documented among laboratory workers who develop neutralizing antibodies following vaccination. WEE vaccine continues to be administered as part of Phase II clinical trials; however, no efficacy trial has been conducted.
A new lot of the WEE vaccine, Western Equine Encephalitis Vaccine, Inactivated, TSI-GSD 210, Lot 3-1-92, was found to be safe and immunogenic in a recent Phase I clinical trial (Dr. Robert Rivard, USAMRIID, personal communication, 2015). In this study, WEE vaccine was administered in 0.5-mL doses subcutaneously in the upper outer aspect of the triceps in a three-dose primary series (days 0, 7, and 28) with a mandatory boost (day 180). All 10 subjects were classified as responders at day 56. For 4 of 10 subjects, the titers had waned below the acceptable level by month 6. Following the month 6 boost, all 10 subjects developed titers above 1 : 40, and all remained above this level for at least 1 year.
DNA-based vaccines against WEE have shown promise in challenge models in mice. One such study evaluated a DNA vaccine, pVHX-6, expressing the 26 S structural gene of WEEV strain 71 V-1658. All mice receiving four intraepidermal doses of pVHX-6 survived challenge with a homologous strain, but only 62% and 50% survived challenge with Fleming and CBA87 strains, respectively.38 Other studies have demonstrated, in a murine model, the efficacy of DNA vaccines expressing the capsid and envelope proteins of WEEV in challenge models. In two studies, a replication-defective human adenovirus serotype-5 (HAd5) was used as a vector for vaccine delivery. In the first study, a HAd5 vector encoding E2 and E1, administered as a single dose, conferred protection against challenge with homologous and heterologous WEEV strains.39, 40 In the second study, a HAd5 vector encoding E1 alone, designated adenovirus (Ad) serotype-5-E1 (Ad5-E1), provided total protection against homologous and heterologous strains of WEEV in mice.41 A potential drawback of this approach in humans is the widespread prevalence of antibodies against adenovirus.
Eastern Equine Encephalitis Virus
The EEE vaccine (TSI-GSD 104) is a lyophilized product originating in primary CEF cell cultures infected with the attenuated PE-6 strain of EEEV. The seed for the EEE vaccine is passaged twice in adult mice and twice in guinea pigs, then passaged nine times in embryonated eggs, followed by three passages in CEFs.42 The supernatant fluid, which is harvested and filtered, contains 50 µg each of neomycin and streptomycin and 0.25% w/v (weight per volume) of human serum albumin, USP (U.S. Pharmacopoeia). The virus is then inactivated with 0.05% formalin. When inactivation is completed, the residual formalin is neutralized by treatment with sodium bisulfite. The final product is lyophilized for storage at −20°C.
Among 255 volunteers who received the two-dose primary series of EEE vaccine (administered subcutaneously) between 1992 and 1998, 197 (77.3%) responded with a PRNT80 titer of 1 : 40 or greater. Of initial nonresponders, 66% subsequently seroconverted following receipt of an EEE vaccine booster (which is administered intradermally). Among initial responders whose titers waned over time, 98.6% responded to a booster dose of EEE vaccine. Local and systemic side effects are infrequent, occurring in less than 1% of vaccinees after the primary vaccine series and in 3.7% after the first booster. Kaplan–Meier plots show that two primary doses and one intradermal booster of TSI-GSD 104 provide satisfactory neutralizing anti-EEEV antibodies in 50% of initial responders for up to 2.2 years.43
Among recent findings regarding next-generation EEE vaccines, a single subcutaneous dose of an EEE vaccine candidate, attenuated via an IRES, protected 100% of vaccinated mice against intraperitoneal challenge.44 In addition, an EEEV replicon and a combined VEEV/WEEV/EEEV replicon protected macaques from aerosol challenge.11
Chikungunya Virus
CHIKV can cause an acute viral syndrome in humans characterized by fever, rash, and arthritis.45 Some people, especially those who are human leukocyte antigen (HLA)-B27–positive, may develop long-term joint involvement.46 Although documented epidemics have occurred since the late 18th century, the virus was first isolated during the 1952–1953 Tanzanian epidemic.47 During 2004–2006, an epidemic ravaged the Indian Ocean islands east of Madagascar—La Réunion, Mauritius–Seychelles, and Mayotte.48 More than 2 million people have been affected since the start of the epidemic, which has since spread to the Caribbean and India, with cases also occurring in persons from the United States, Europe, and elsewhere who traveled to the Caribbean.49, 50, 51, 52 In December 2014, Kendrick and colleagues53 reported on the first 11 cases of CHIK originating in the United States (Florida). Genomic sequencing of six isolates from the Indian Ocean outbreak suggests that the strain responsible for the outbreak is related to East African isolates.49 In addition, evidence from the sequence data suggests that the CHIKV strain responsible for the Indian Ocean outbreak evolved, during the course of the outbreak, into several distinct variants. One mutation may have allowed the CHIKV to be efficiently transmitted by the most abundant mosquito species on La Réunion (Aedes albopictus); this may help explain the noted robustness of the epidemic.49, 52, 54
Several attempts were made, with variable success, to develop an efficacious inactivated vaccine using chick embryo cell cultures, suckling mouse brain, and African green monkey kidney.55 A live attenuated vaccine was made from the seed of the African green monkey kidney vaccine, using CHIKV strain 15561, which had originally been isolated from an infected patient during a 1962 CHIK epidemic in Thailand.55, 56 The 11th African green monkey kidney passage of CHIKV strain 15561, made at the Walter Reed Army Institute of Research, was transferred to USAMRIID and passaged in Medical Research Council (MRC)–5 cells. After 18 passages in MRC-5 cells, CHIKV 181/clone 25 was selected as a vaccine seed strain based on biomarkers. CHIKV 181/clone 25 was efficacious in challenge models in suckling mice and in nonhuman primates.55
A randomized, double-blind, placebo-controlled trial of the CHIK 181/clone 25 vaccine documented a seroconversion rate of 98% among alphavirus-naïve volunteers.56 One year after immunization, 85% of vaccinees remained seropositive. Injection site and systemic symptoms, including flu-like symptoms, were similar in vaccine and placebo recipients. However, the vaccine was temporally associated with arthralgia in 8% of vaccinees. One volunteer in the vaccine group developed a pruritic, eczema-like rash at the injection site. Interference between the CHIK 181/clone 25 vaccine and the live attenuated VEE TC-83 vaccine is discussed above. This live attenuated CHIK vaccine requires additional Phase II and Phase III clinical testing. Current epidemics in the Caribbean, Indian Ocean, and India offer unique prospects for Phase III clinical testing of this vaccine.
Additional strategies for the development of a safe and efficacious vaccine for CHIK are being pursued. These include chimeric alphavirus vaccine candidates using VEEV attenuated vaccine strain TC-83, a naturally attenuated strain of EEEV, or Sindbis virus as a backbone with the structural protein genes of CHIKV. Wang and colleagues57 found that each of these chimeras produced robust neutralizing antibody responses, and vaccinated mice were fully protected against disease and viremia after CHIKV challenge. One DNA vaccine candidate expressing a component of the CHIKV envelope glycoprotein produced neutralizing antibodies in mice and macaques.58 A second DNA vaccine candidate uses a plasmid coding for the CHIKV-capsid, E1 and E2. When injected into mice, this construct induced broad cellular immunity and produced antibodies, detectable by enzyme-linked immunosorbent assay (ELISA), that recognized native antigen.59 Another group is evaluating a formalin-inactivated Vero cell–adapted vaccine candidate prepared using a strain from the India epidemic.60 Most recently, a promising virus-like particle (VLP) vaccine containing the viral envelope has elicited high titers of neutralizing antibodies in nonhuman primates and in human volunteers.61, 62, 63 In addition, a single immunization with a measles virus–vectored CHIK vaccine candidate induced high CHIKV antibody titers and protected mice from a lethal CHIKV challenge.64
Ross River Virus
RRVD, or epidemic polyarthritis, was first recognized in Australia in 1928; Ross River virus (RRV), its causative agent, was first isolated in 1963 from a pool of mosquitoes.65, 66, 67 RRVD is essentially limited to Australia, Fiji, and the surrounding islands, including American Samoa and the Cook Islands, where several thousand cases occur per year.68, 69 In humans, polyarthritis may be followed by fever, rash, and lethargy; symptoms generally resolve after 3 to 6 months.69, 70 In 1997, U.S. marines participating in Operation Tandem Thrust in Queensland, Australia, had an infection rate of 1.5%; RRVD developed in nine individuals.71
A vaccine candidate was derived from a virus isolated from a human case of classical RRVD using the C6-36 cell line (A. albopictus).72 The candidate underwent four serial passages in MRC-5 human fetal lung cells followed by two passages in Vero cells. Cell cultures contained penicillin (100 U/mL) and streptomycin (100 µg/mL). The virus was inactivated using binary ethyleneimine. In mice, the vaccine induced neutralizing antibodies and conferred protection against viremia after intravenous challenge with live RRV.73
More recently, a Vero cell–grown, formalin-inactivated, benzonase-digested (to digest host cell DNA), sucrose gradient–purified vaccine candidate induced neutralizing antibodies in mice.74 Immunized mice and guinea pigs failed to develop viremia following intravenous challenge with the prototype strain of RRV (T48). In a Phase I/II dose escalation study75 in 382 healthy, RRV-naïve adults, this vaccine was safe and immunogenic when administered as three immunizations (at days 0 and 21 and month 6) at four dose levels, with or without aluminum hydroxide added as an adjuvant; the adjuvanted 2.5-µg dose stimulated the highest immune response. This group recently completed a Phase III study in 1755 healthy younger adults (ages 16–59 years) and 209 healthy older adults (ages 60 and older).76 The vaccine was well tolerated. Neutralizing antibody titers (≥1 : 10) were achieved by 91.5% of the younger adults and 76.0% of the older adults after the third vaccination. The potential for antibody-dependent enhancement of disease, which has been described for RRV in vitro, will need to be considered during the development of vaccines against RRV.69, 77
Bunyaviruses
The Bunyaviridae are a large family of segmented, lipid-enveloped, negative-stranded RNA viruses. Vaccines against human pathogens representing two of the five genera, RVF virus (Phlebovirus genus) and Hantaan virus (Hantavirus genus), have been developed at Fort Detrick.
Rift Valley Fever Virus
RVF is a mosquito-borne infection endemic to sub-Saharan Africa and primarily affecting ruminant animals. Under appropriate climatologic conditions, however, explosive epizootics among animals and epidemics in humans occur with considerable morbidity and mortality. In recent years, RVF has demonstrated its ability to spread northward to Egypt and into Yemen and Saudi Arabia on the Asian continent. The consequences of further spread into naïve animal and human populations are potentially devastating.78 The spread of West Nile virus (WNV) has demonstrated that viruses once bounded by the Atlantic Ocean have the potential to overcome that barrier. Thus, the development of a vaccine or other countermeasures against the RVF virus is important for the protection of human and animal populations in Africa and the Middle East, U.S. military personnel involved in campaigns in those regions, and livestock and people in the United States and elsewhere.
The U.S. Army has developed two vaccines to combat this threat: an inactivated RVF vaccine (TSI-GSD 200) and, more recently, a live attenuated product (RVF MP-12; TSI-GSD 223). The Entebbe strain of RVF, isolated from a mosquito pool in Bwamba County, Uganda, is the source for the inactivated vaccine.79 The virus was passaged 184 times in adult mice, followed by two passages in fetal rhesus lung (FRhL) cells to form the production seed. Although the original vaccine was produced in primary African green monkey kidney cells, the current vaccine lots are produced in FRhL cells.80, 81 The vaccine is inactivated in 0.05% formalin. Following verification of viral inactivation, the residual formaldehyde is neutralized with sodium bisulfite to less than 0.01%. A study of the immunogenicity and safety of inactivated RVF vaccine in humans during a 12-year period showed that the vaccine was safe and immunogenic when the three-dose primary series and one booster are administered.82, 83 In particular, 540 (90%) of 598 volunteers given three 1.0-mL doses of TSI-GSD 200 subcutaneously on days 0, 7, and 28 responded with a PRNT80 titer of 1 : 40 or greater. Three-fourths of the initial nonresponders developed a PRNT80 titer of 1 : 40 or greater after a single booster. However, approximately 10% of recipients of the inactivated RVF vaccine require repeated boosting. In these individuals, a booster typically results in an adequate titer, which wanes over the next year to <1 : 40, prompting another booster (P.R. Pittman, unpublished data).
An isolated RVF strain recovered from a nonfatal human case that occurred during the first Egyptian epidemic in 1977 was used to derive the live attenuated RVF vaccine. The virus (ZH548) was passaged twice in suckling mouse brain, then once in FRhL cells. It was then attenuated by 12 serial alternating passages in human lung cell cultures (MRC-5 cells, certified for vaccine use) by previously described methods84 in the presence or absence of 5-fluorouracil. The resulting RVF MP-12 vaccine is a lyophilized product originating from supernatant fluids harvested from the final mutagenesis passage. The vaccine has undergone extensive safety testing and challenge studies in several animal species: rodents, sheep (including pregnant ewes and naïve neonatal lambs), cattle (including in utero–vaccinated bovids), and monkeys.85, 86, 87, 88, 89 Furthermore, Miller and colleagues90 showed that RVF MP-12 vaccine, administered to sheep, results in long-lasting immunity and has limited potential to be transmitted to mosquitoes feeding on vaccinated animals.
RVF MP-12 has undergone clinical evaluation in human volunteers at USAMRIID. In a Phase I randomized, double-blind, dose-escalation/route-seeking study, 56 healthy, nonpregnant subjects were randomly selected to receive RVF MP-12 (104.7 plaque-forming units [PFU] subcutaneously, n = 10; 103.4 PFU intramuscularly, n = 6; or 104.4 PFU intramuscularly, n = 27) or placebo (n = 13).91 Only infrequent and minor side effects were seen among placebo and MP-12 recipients. One volunteer had a titer of 1.3 log by direct plaquing in cell culture. Six vaccinees had transient low-titer viremia detected by amplification only; all six of these volunteers were from the group receiving 104.4 PFU intramuscularly. Neutralizing antibodies (measured by PRNT80 titer), as well as RVF-specific immunoglobulin (Ig) M and IgG, were observed in 40 (93%) of 43 vaccine recipients. The highest peak geometric mean antibody titers were observed in the group receiving 104.4 PFU intramuscularly. Overall, 28 (82%) of 34 RVF MP-12 recipients available for testing remained seropositive (PRNT80 = 1 : 20) at 1 year following inoculation.91
A joint program between the University of Texas Medical Branch and USAMRIID for further development of the RVF MP-12 vaccine has been completed. This study, funded by the National Institute of Allergy and Infectious Diseases, involved the administration of a single intramuscular dose of RVF MP-12 vaccine to 19 naïve male and nonpregnant female subjects. The vaccine was safe and immunogenic in this vaccine trial. A total of 18 (95%) of 19 subjects developed neutralizing antibodies against RVF virus, as determined by a PRNT80 titer of 1 : 20 or more. Results suggested that the vaccine resulted in, at most, only low-level viremia. No virus was detected by direct plaque assay; however, during the first 14 days after vaccination, nine MP-12 isolates were recovered from five subjects with the use of amplification by blind, double passage in Vero cells. No single-nucleotide polymorphisms or reversions were observed in the attenuating mutations of the parent virus.92
Another RVF live attenuated vaccine candidate is Clone 13. This vaccine candidate is a plaque-purified clone of RVF virus that contains a large deletion in the small (S) genome segment that disrupts the biological functions of the nonstructural proteins NSs. Clone 13 has proven highly immunogenic in mice, sheep, and goats, although it has demonstrated only moderate immunogenicity in cattle.93, 94
Other approaches to the development of vaccines against RVF virus, all of which are at the preclinical stage, include vaccines based on viral vectors95, 96, 97; DNA vaccines with molecular adjuvants98; subunit vaccines based on purified proteins99, 100, 101, 102, 103; and vaccines based on single-cycle replicable vaccine mutants.104
Hantaviruses
Hantaviruses causing hemorrhagic fever with renal syndrome (HFRS) have been, and continue to be, significant endemic disease threats to U.S. military forces on the Korean peninsula and throughout Europe.105 At least 14 distinct viral strains of HFRS-causing viruses are distributed worldwide.106 Three viral proteins are able to induce protection: two surface glycoproteins, G1 and G2 (also called Gn and Gc), and the N nucleocapsid protein. Neutralizing antibodies are protective, although T-cell responses may also be useful.107 In addition to HFRS, hantaviruses are responsible for hantavirus pulmonary syndrome.106, 108 The hantavirus causing this syndrome in the southwest United States is called Sin Nombre virus (SNV). Humans become infected with hantaviruses via inhalation of aerosolized rodent excreta.109 The prototype hantavirus, Hantaan, was first isolated in Korea, and the first vaccine also was developed there to protect against HFRS.108, 110, 111 This inactivated vaccine, the ROK84/105 strain, harvested from suckling mouse brains, is concentrated by protamine sulfate precipitation and centrifugation. The concentrate is then exposed to formalin inactivation and purified by ultrafiltration and sucrose gradient ultracentrifugation. Aluminum hydroxide is added to the vaccine as an adjuvant, thimerosal as a preservative, and gelatin as a stabilizer. The final product, produced by Rhein Biotech, reportedly has less than 0.01 ng/mL of myelin basic protein. The recommended regimen for this product, Hantavax, is two doses of 5120 ELISA units (0.5 mL) given 1 month apart by the subcutaneous or intramuscular route.
Little has been published on the vaccine, but the manufacturer reports that tolerance is good, although allergic reactions occur, presumably as a result of mouse brain antigens. A serologic response measurable by indirect fluorescence is seen in nearly all vaccinees. Neutralizing antibodies are usually absent after one dose, but are measured in approximately 75% of subjects after the second dose.112 They decrease to 16% at 12 months, at which time a booster is recommended. Although placebo-controlled data on the efficacy of the vaccine are not available, a similar vaccine made in North Korea reportedly showed 88% to 100% efficacy, and Hantavax itself has been effective in uncontrolled epidemiologic studies done in South Korea and Yugoslavia.113, 114 Although neutralizing antibody responses were not persistent and not stimulated by a booster dose,107, 115 a case-control study conducted in the Republic of Korea estimated efficacies of 46% for two doses and 75% for three doses, but with wide confidence limits.116
Vaccines made in cell culture against hantavirus and Seoul virus strains are licensed in China. The substrate is cell cultures of golden hamster kidney or Mongolian gerbil kidney. The efficacy of these bivalent vaccines is reportedly better than 90%, with fewer reactions than after the use of mouse brain–derived vaccine.113, 114
Another Korean company has developed a hantavirus vaccine made in Vero cells that protected challenged animals by eliciting strong responses to the G1, G2, and N proteins of the virus.117
At USAMRIID, advances in technology fueled the development of bioengineered hantavirus vaccines. Two experimental approaches included recombinant vaccinia-vectored Hantaan virus vaccine carrying envelope and nucleocapsid genes118, 119 and the same genes inserted into bacterial plasmids as DNA vaccines. The recombinant vaccinia-vectored Hantaan virus vaccine was efficacious in the hamster infectivity model; even if preexisting immunity to vaccinia virus was present, it could be overcome by a second intramuscular injection of the vaccine candidate.118 A double-blind, placebo-controlled clinical trial involving 142 volunteers using two subcutaneous injections 4 weeks apart showed that, for vaccinia-naïve volunteers, neutralizing antibodies to Hantaan virus or vaccinia virus were detected in 72% or 98%, respectively, whereas neutralizing antibodies to Hantaan virus were detected in only 26% of vaccinia-immune volunteers, showing the effect of prior vector-directed immunity.119 This vaccine has been abandoned.
DNA plasmids coding for the G1 and G2 proteins protected monkeys against challenge with a South American hantavirus and elicited antibodies that exerted passive protection in hamsters.120 Boosting the monkeys 1 to 2 years later showed that immunological memory had been induced.121 Alphavirus replicons, baculovirus-produced proteins, and chimeric hepatitis B VLPs are all under study as additional hantavirus vaccine candidates.113, 122, 123
Boudreau and colleagues124 report on a Phase I clinical trial that evaluated Hantaan virus and Puumala virus M-segment DNA vaccines for preventing HFRS. Each volunteer received either Hantaan DNA vaccine (n = 9), Puumala DNA vaccine (n = 9), or both vaccines (n = 9). Three doses (containing 8 µg DNA/4 mg gold per dose), administered 4 weeks apart, were provided to volunteers by particle-mediated epidermal delivery. The single vaccines elicited neutralizing antibodies to Hantaan virus or Puumala virus, respectively, in 30% and 44% of vaccinees. Neutralizing antibodies to one or both viruses were detected in 56% of volunteers who received the combined vaccine. In an attempt to increase seroconversion, Hooper and colleagues125 conducted a Phase I clinical trial of these two DNA vaccines delivered by intramuscular electroporation, similar to the Boudreau study. Volunteers (three groups of nine individuals each) received three doses, 28 days apart, of either the Hantaan DNA vaccine, the Puumala DNA vaccine, or a mixture of both vaccines. Each dose of vaccine contained 2 mg DNA in a total volume of 1 mL saline; the combined vaccine contained 1 mg of each DNA vaccine. Neutralizing antibodies were found in 56% and 78% of volunteers who received the Hantaan or Puumala DNA vaccines, respectively. Results of the combined vaccine showed that 78% of vaccinees developed neutralizing antibodies against Puumala virus. The three volunteers with the highest antibody levels against Puumala also developed neutralizing antibodies against Hantaan. No serious adverse events resulting from the vaccines were noted in either study.
Hooper and colleagues126, 127 also found that DNA vaccines encoding the virus envelope glycoproteins of SNV or Andes virus (both of which cause hantavirus pulmonary syndrome) elicit high-titer neutralizing antibodies in animal models. Hamsters vaccinated with three doses of the SNV DNA vaccine were fully protected from lethal challenge with SNV. Further, a pan-hantavirus vaccine using Andes virus, SNV, Hantaan virus, and Puumala virus plasmids elicited neutralizing antibodies against all four viruses.126
West Nile Virus
The spread of the flavivirus WNV from its introduction into New York City in 1999 across the United States has been a dramatic example of an emerging infection. WNV is an arbovirus of birds, for which it is often lethal, and is transmitted by multiple mosquito species. Although the incidence of human infections has recently decreased, WNV frequently causes febrile illness, with the complication of meningoencephalitis in about 1% of infections.128
WN-VAX, a formalin-inactivated WNV vaccine derived from a strain isolated in New York City in 1999, has been tested in a number of animal models. This vaccine, administered intraperitoneally in two doses, protected 100% of 4-week-old mice against a lethal challenge with WNV.129 More recently, Muraki and colleagues130 found that mice passively immunized with serum from WN-VAX–immunized mice were also protected from lethal WNV infection. When administered to macaques, WN-VAX elicited neutralizing antibodies.130 Clinical trials on this vaccine are planned.
Several Phase I and Phase II clinical trials have been completed on WNV vaccine candidates. The ChimeriVax West Nile vaccine candidate, containing a yellow fever virus backbone and expressing the premembrane and envelope proteins of WNV, was reported to be safe and induced neutralizing antibodies in a Phase I clinical trial.131, 132 In a Phase II clinical trial, ChimeriVax was well tolerated, and more than 96% of vaccinees seroconverted.133
In a Phase I clinical trial, a DNA vaccine candidate elicited neutralizing antibodies against WNV.134 A recombinant subunit vaccine candidate, consisting of an envelope protein truncated at the C-terminal end and containing 80% of the N-terminal amino acids of the native WNV protein (WN-80E) mixed with adjuvant, produced antibodies detectable by ELISA and neutralizing antibody assays. In the challenge experiment, all control rhesus macaques had detectable viremia for at least 3 days after challenge, whereas none of the vaccinated animals showed viremia.135 A Phase I trial assessing the safety of this vaccine was recently completed.
Arenaviruses
Several members of the Arenaviridae family of segmented, negative-stranded RNA viruses are recognized as causative agents for viral hemorrhagic fever syndromes in humans. To date, efforts to develop protective immunogens against arenaviruses have met with limited success.136 During the 1980s, the first successful vaccine against an arenavirus, Junin virus, was developed through a collaboration between the government of Argentina and the U.S. Army. Efforts to develop vaccines against other pathogenic arenaviruses have resulted in a number of promising candidates, but none of these has progressed to clinical trials.137
Junin Virus
Junin virus is the causative agent of AHF, which is endemic to the pampas of north-central Argentina. Humans become infected with the Junin virus by inhalation of infected rodent secretions and excretions.138, 139, 140 Death occurs in 15% to 30% of untreated patients afflicted with AHF.
For several decades, attempts were made in Argentina to develop an efficacious vaccine against AHF. The resulting inactivated and live attenuated vaccine candidates all failed for various reasons.136 The product developed at USAMRIID, Candid #1, is a descendant of the prototype XJ strain Junin virus, isolated in guinea pig from a fatal AHF case. Following another passage in the guinea pig, the virus underwent 44 newborn mouse brain passages, then was cloned and passaged 19 times in certified FRhL cells.136, 141, 142 Candid #1 proved effective in preventing disease in guinea pigs and rhesus macaques after lethal Junin virus challenge.140, 143, 144 In Phase I and Phase II clinical testing in humans, Candid #1 was safe and immunogenic.145, 146 More than 90% of volunteers developed antibodies against Junin virus, although at lower levels than those seen after mild natural infection,147 and 99% developed a Junin virus–specific cellular immune response. In a pivotal efficacy study, 3255 volunteers were randomized to receive the vaccine and 3245 were randomized to receive a placebo. During the trial, 23 volunteers developed an illness that met the clinical case definition for AHF.148, 149 Of these, 22 had received a placebo and 1 had received the vaccine; vaccine efficacy by intent-to-treat analysis was 95% (95% confidence interval, 82%–99%; P < .001). Argentina's National Institute of Human Viral Diseases produced its own Junin vaccine, also called Candid #1, against AHF and tested it in 946 human volunteers to compare its safety and immunogenicity with that of the Candid #1 vaccine produced in the United States and used in previous studies.150 The vaccine was comparable to the U.S.-produced vaccine in terms of safety and efficacy. No severe adverse events were related to the vaccine. As a result of this study, Argentina's national regulatory authority (ANMAT) licensed the locally produced Candid #1 vaccine.151, 152
Vaccination with Candid #1 is recommended for persons at risk of occupational (agricultural or laboratory) exposure to the Junin virus. The development of this vaccine represents a successful collaboration between USAMRIID and the Argentine Ministry of Health and Social Action, under the auspices of the United Nations Development Programme and the Pan American Health Organization. Over the years, hundreds of thousands of people in Argentina and other countries have been inoculated, with an excellent record of safety and effectiveness.153
Lassa Virus
Another important Arenavirus is Lassa virus, transmitted by rodents in West Africa, where there has been a recent large epidemic. Several candidate vaccines have shown protection in nonhuman primate models, including reassortants, vesicular stomatitis virus-vectored recombinants, and alphavirus replicons.153a
Middle East Respiratory Syndrome Virus
The Middle East respiratory syndrome (MERS) virus—like the severe acute respiratory syndrome (SARS) virus that spread from China and caused a worldwide outbreak in 2002–2003—is a coronavirus.154, 155 The reservoir for MERS is camels, particularly young animals, which acquire a respiratory illness that can be transmitted to humans.156, 157 In addition, human-to-human transmission has been repeatedly observed in Saudi Arabia and in South Korea.158 All coronaviruses possess a glycoprotein spike that induces neutralizing antibodies; both monoclonal antibodies and vaccines based on the spike have shown good protection in experimental challenge models, including macaques.159 Development of vaccines for camels and for humans is proceeding.
Novavax has developed 0.2-µm nanoparticles consisting of MERS spike protein which, together with ISCOMATRIX adjuvant, have been shown to induce neutralizing antibodies in mice.160 German workers have used the MVA vaccinia virus as a vector for the MERS spike protein and induced neutralizing antibodies and protection in a mouse challenge model.161 The Vaccine Research Center at the National Institutes of Health has developed plasmids and recombinant proteins representing the full-length MERS spike or the S1 domain of the spike. When both antigens were administered to mice or macaques, neutralizing antibodies were induced and animals were protected from challenge with the virus.162 The further development of these candidate vaccines will depend on epidemiology and market size.
Limited-Use Vaccines Against Bacterial Diseases
Q Fever
Q fever is a highly infectious zoonotic disease of humans usually caused by aerosol transmission of C. burnetii from infected sheep or goats.163 The organism was thought to be a Rickettsia but is now considered to be related to Legionella bacteria. Fever and pneumonia are the most frequent clinical manifestations of Q fever, although hepatitis, endocarditis, and a variety of other complications may develop.164 Antibiotics are effective but may act slowly, and chronic fatigue syndrome after Q fever has been reported, presumably as a result of cytokine dysregulation.165
Mechanisms of immunity to Q fever are complex. Although antibodies are important to clear extracellular organisms, it is sensitization of T-cell lymphocytes to Q fever antigen and secretion of lymphokines that clear intracellular infection and provide immunologic memory.166
In the development of a Q fever vaccine, C. burnetii was adapted to grow in the chick embryo yolk sac, and an early vaccine was made by formalin inactivation of organisms produced in eggs.167 This vaccine was effective in humans, including in those subjected to experimental challenge, but it also occasionally resulted in severe local reactions that sometimes progressed to abscess formation. Eventually it was shown that reactions were associated with preexisting immunity to Q fever. Accordingly, the practice of screening prospective vaccinees was adopted—using serology for Q fever antibodies and observation for local induration after inoculation with a skin test antigen made from diluted vaccine.
Advances in Coxiella biology and vaccinology led to a new generation of vaccines. For example, evidence showed that only Phase I Q fever organisms, analogous to smooth forms of bacteria, were protective, and that a transition to Phase II (rough) would occur if the organism was passaged too many times in chick embryo. Therefore, Q fever vaccines are based on Phase I organisms only.168 In addition, researchers recognized that purification to remove chicken protein and lipid and isolation of whole inactivated Coxiella by extraction, filtration, or centrifugation would result in a cleaner, less reactogenic, and highly immunogenic product. Two whole-cell vaccines came into use in high-risk subjects: an Australian vaccine made and licensed by Commonwealth Serum Laboratories166, 169 and a vaccine (IND 610) made and tested by the U.S. Army.170 Both are administered as a single 30-µg subcutaneous injection. The Australian vaccine, Q-Vax, is purified using high concentrations of NaCl to remove nonprotective antigens. The U.S. Army vaccine, Whole-Cell, Inactivated, Q-Fever Vaccine NDBR 105, IND 610, is also extracted with NaCl, then subjected to ethanol–Freon 113 extraction; finally, it is purified further on a CaHPO4 2H2O (brushite) column. Despite these purification processes, however, skin testing of prospective recipients remains necessary to prevent serious reactions in persons who have experienced prior infection.
Whole-cell vaccines have undergone considerable clinical testing. In Australia, a placebo-controlled trial171 and an open trial in abattoir workers172, 173 showed efficacy approaching 100% (Table 12.3 ). From 2002 to 2006, during a vaccination program funded by the Australian government, the incidence of Q fever in Australia declined by more than 50%.166, 174 The U.S. Army vaccine was subjected to a controlled challenge in volunteers; this trial also proved highly successful.175 Two meta-analyses found that Q-Vax was, respectively, 97% and 83% to 100% effective, but the data were considered to be of only moderate quality.176, 177 A Q fever outbreak, apparently caused by dairy goats, occurred in The Netherlands between 2007 and 2011, affecting more than 4000 individuals.178 Control measures that included Q-Vax vaccination of individuals at high risk for developing chronic Q fever were effective in containing the outbreak despite limited coverage of the vaccination campaign.179, 180, 181
TABLE 12.3.
Protection by Q Fever Vaccines
| Vaccine | Type of Study | No. of Cases of Q Fever |
||
|---|---|---|---|---|
| Vaccinated | Unvaccinated | Protection (%) | ||
| United States | Random, challenge | 2/32 | 5/6 | 92 |
| Australian | Random, placebo-controlled | 0/98 | 7/102 | 100 |
| Australian | Observational | 3a/2716 | 52/2012 | 96–100 |
Vaccinated during incubation period of Q fever.
Data from Ormsbee RA, Marmion BP. Prevention of Coxiella burnetii infection: vaccines and guidelines for those at risk. In: Marne TJ, ed. Q Fever: The Disease, vol 1. Boca Raton, FL: CRC Press; 1990:225–248.
To eliminate the problem of reactions, third-generation acellular Q fever vaccines have been prepared in the United States182, 183 and in the former Czechoslovakia.184 These vaccines, extracted with chloroform–methanol or other lipid solvents to remove lipid A (thought to be the chief offending substance in C. burnetii), have not yet undergone sufficient clinical testing to determine whether they are equivalent to whole-cell Q fever vaccine in protection of humans. In addition, it is not yet clear if these vaccines overcome the problem of serious reactions in preexposed individuals.185, 186
A live attenuated Q fever vaccine produced in chick embryo yolk sac has been developed in Russia, but its safety has been questioned. Other vaccine approaches under investigation are based on the P1 29-kDa protein, a 67-kDa antigen, and DNA plasmids coding for various proteins.187, 188, 189
Better Q fever vaccines are needed that induce antibodies and T-helper type 1 (Th1) cell responses and yet are well tolerated by individuals with preexisting immunity. Elucidation of the key protective antigens is required.185, 189
Tularemia
Tularemia, a bacterial bioterrorism threat, can be a significant endemic and epidemic human disease.190 The causative bacterium of tularemia, Francisella tularensis, was isolated in 1912; in 1919, Edward Francis made the association with the human disease then known as “deer fly fever.”191
Andersson and colleagues192 describe the transcriptional response in peripheral blood following ulceroglandular tularemia in humans. The authors identified seven genes whose changes in expression predict the early phase of tularemia. In addition to identifying how the host defense develops or is turned off by F. tularensis invasion, these data are important in identifying potential diagnostic markers for tularemia infection.
Many of the more than 200 cases of F. tularensis documented in the American medical literature resulting from laboratory exposure occurred in laboratory personnel who had received one or more injections of a phenol-killed vaccine and/or acetone-prepared vaccine.193, 194, 195, 196 One of these early inactivated products, the Foshay vaccine, showed incomplete protective efficacy against tularemia organisms introduced by the respiratory and intracutaneous routes.197, 198 Although circulating antibodies could be demonstrated following administration of these vaccines, the antibodies generated were not protective. It was concluded that the protective antigen of F. tularensis was destroyed in the inactivation procedures used to prepare the vaccines, although now antibodies alone are considered insufficient.
Partly as a result of the experience with killed vaccines, an effort to develop a live vaccine against F. tularensis was undertaken. By avoiding destruction of the protective antigen, live vaccines, which cause actual infection, are thought to produce an immunity closer to that caused by the disease itself, in particular by generating persistent antibodies and CD8-mediated cellular immunity, both of which are required for protection against disease.
Soviet investigators initially developed a live tularemia vaccine in 1942.199 Ampoules of this “viable” tularemia vaccine were brought to the United States from the Russian Institute of Epidemiology and Microbiology (Gamaleia Institute) by Shope200 in 1956. From an ampoule of this product, Eigelsbach and Downs201 derived a vaccine strain, which they designated LVS.
Studies done at Fort Detrick in the early 1960s showed that LVS was protective for mice and guinea pigs after a challenge.201 This protection was subsequently found to be the result of cell-mediated immunity when passively transferred spleen cells from immunized mice provided protection to nonimmune recipients.202 Similar immunization studies were done in monkeys, which also demonstrated a significant immune response to vaccination with LVS.203
LVS initially was given by scarification to volunteers at Fort Detrick in 1958.204 The vaccination procedure and adverse events after vaccination were evaluated in 29 subjects.198 Only a pink scar remained 1 month after vaccination. Transient axillary lymphadenitis was observed in approximately half of the subjects, but none of the 29 men exhibited fever or other systemic reactions following immunization with LVS. All persons showed bacterial agglutinin antibodies; peak titers were observed 29 to 59 days after vaccination and were sustained.205
After challenge doses of up to 2500 organisms, a significant protective effect was seen in the volunteers vaccinated with LVS, with only 20% of vaccinated persons showing clinical illness compared with 85% of the unvaccinated control subjects. None of the vaccinated persons developed symptoms severe enough to require treatment. At higher challenge doses, however, immunity was overcome; after challenge with 25,000 organisms, 90% of vaccinated persons showed some symptomatology compared with 85% of the control subjects. However, LVS modulated illness severity such that only 60% of vaccinated volunteers had symptoms severe enough to require treatment, compared with 100% of those in the control group.205
The efficacy of the live tularemia vaccine, when administered by aerosol, has also been evaluated.206, 207 In one study,207 aerosol vaccination with LVS protected 6 of 16 subjects against challenge with the virulent SCHU-S4 strain of F. tularensis. All protected subjects had measurable circulating antibodies, while only one of the 10 subjects who developed disease had circulating antibodies.
LVS (also referred to as NDBR 101) has been used since the mid-1960s and has been associated with a significant decline in the rate of laboratory-acquired infections at Fort Detrick.208 In the absence of firm knowledge concerning the attenuating mutations in LVS and its residual virulence, the vaccine remains investigational and is administered only under protocol and with written informed consent. The lots undergo lot-release and potency tests as required by FDA for IND products.
Efforts are underway to develop new-generation vaccines against tularemia using modern technologies. Lipopolysaccharide from F. tularensis gives partial protection through the induction of antibodies, but clearly, cell-mediated responses to other antigens will be necessary for an optimal vaccine against highly virulent strains.209, 210 In addition, aerosol delivery may give better protection than peripheral inoculation.211 Much work is underway to create new live attenuated mutants211, 212, 213, 214, 215, 216, 217 and novel subunit vaccines.218 In one promising approach, the oxidant-sensitive emrA1 mutant of F. tularensis LVS elicited a strong humoral immune response in mice without causing adverse effects. Mice vaccinated with a single intranasal dose of the emrA1 mutant of F. tularensis LVS were protected against a lethal respiratory LVS challenge; this vaccine also provided partial protection against lethal challenge with the virulent F. tularensis SchuS4 strain.219
Brucellosis
Primarily a disease of animals, brucellosis first was described in humans in 1859 as “Mediterranean gastric remittent fever” or “Malta fever.”220 The consumption of unpasteurized goat milk was found to be the source in the Malta epidemic.182 Bruce221 isolated the etiologic agent in 1886 from a fatal case of brucellosis. Initially named Micrococcus melitensis, the genus later was named for its discoverer and is currently known as Brucella melitensis. Brucella species are non–spore-forming, Gram-negative coccobacilli that exist worldwide. Several animal species become infected with Brucella species. Humans become infected on contact with infected animals, by consuming unpasteurized infected milk or other dairy products, and by working with the organism under laboratory conditions. Brucellosis manifests with nonspecific flu-like symptoms that are often much more prominent than the gastrointestinal symptoms.222 The feasibility of infection by aerosol makes Brucella a possible agent of biowarfare.
Effective vaccines, both live and killed, are available for animals. Use of these vaccines has led to decreases in human disease where animal infection is enzootic. Nevertheless, an estimated 500,000 annual human cases still occur worldwide.223 A live vaccine, Brucella abortus S19, was extensively used in Russia and gave more than 50% protection, although it was somewhat reactogenic.224
Ongoing research seeks to further attenuate live vaccines used in animals and to develop subunit vaccines based on membrane and ribosomal proteins or DNA plasmids coding for those proteins.225, 226, 227, 228, 229, 230, 231, 232, 233, 234 For example, protection in animals has been achieved with an outer membrane protein and its gene,235, 236 with periplasmic protein bp26,237 by intranasal administration of Brucella lipopolysaccharide complexed with Neisseria meningitidis outer membrane protein,238, 239 and a recombinant glucokinase protein Brucella vaccine candidate.240
Pseudomonas
Pseudomonas aeruginosa is a common pathogen in ventilated patients and in those with burns; however, it also causes life-threatening infections in people with cystic fibrosis owing to its production of a thick, mucoid capsule. Although antibodies to the lipopolysaccharide develop in people with cystic fibrosis after infection, they are of low affinity and do not protect. In contrast, artificial immunization with lipopolysaccharide from eight different strains coupled chemically with the Pseudomonas exotoxin A elicited high-affinity IgG antibodies.241, 242 Studies of cellular immune responses showed induction of antigen-specific lymphocyte proliferation and a Th1-like cytokine secretion.243 During a 10-year period, people with cystic fibrosis immunized with O polysaccharide conjugated to toxin A had fewer Pseudomonas infections and a tendency toward less colonization with mucoid strains (Herzog C. Personal communication to S. Plotkin, November 26, 2002).244, 245 Importantly, lung function was also better in vaccinees.244, 246, 247 In a Phase III trial, patients with cystic fibrosis who received four doses of a bivalent P. aeruginosa flagella vaccine were significantly less likely to have one or more acute P. aeruginosa infections than were those who received placebo (19.6% vs 30.7% among vaccinees and placebo recipients, respectively).248 However, additional antigens are expected to provide better protection.249 A vaccine containing formalin-inactivated whole Pseudomonas bacteria has been administered orally to healthy volunteers without adverse reactions and with significant serum IgA responses.250
Many other experimental vaccines are under development, including ones based on flagellar antigens, outer membrane proteins such as OprF and OprI, and exotoxin A.251, 252, 253, 254 For example, one DNA vaccine encoding Pseudomonas exotoxin A and PcrV has shown promise in mice,255 and a P. aeruginosa hybrid outer membrane protein OprE/I (IC43) vaccine was well tolerated and immunogenic in a recent randomized, placebo-controlled Phase I study.256 The mucoid exopolysaccharide of P. aeruginosa has been conjugated with keyhole limpet hemocyanin and found to elicit opsonophagocytic antibodies in mice.257 An attenuated salmonella vector expressing the O antigen of P. aeruginosa lipopolysaccharide given intranasally protected mice against challenge.258 Interestingly, Th17 responses, as in the case of other mucosal bacteria, may be necessary for protection,259 and mucosal routes of administration may improve efficacy.260, 261, 262
The virulence factor alginate is produced by mucoid forms of P. aeruginosa. One strategy in Pseudomonas vaccine development is to raise opsonic antibodies against alginate. One group conjugated alginate to the outer membrane vesicle of N. meningitides. Immunization of mice with this conjugate provided protection against intranasal challenge with P. aeruginosa,263 thus validating the concept.
Helicobacter pylori
Helicobacter pylori, which colonizes the stomach early in life but exerts its effects in adults, is the cause of gastritis, peptic ulcers, and low-grade gastric lymphomas.264, 265 The mechanisms of disease involve stimulation of Th1, regulatory T, and possibly Th17 cells; antibody and cellular mechanisms seem to be involved in the protection demonstrated in animal models. Clinical trials have tested urease, which is secreted by H. pylori; the cagA pathogenicity island; the VacA cytotoxin; and the neutrophil-activating protein.266 The results in the past have not been promising; more recently, however, an aluminum-adjuvanted combination of the latter three antigens stimulated strong antibody and cellular responses in volunteers and will undergo further clinical development.267 Other approaches, such as VLPs,268 epitope-based vaccines,269, 270, 271 alkyl hydroperoxide reductase and mannosylated AhpC,272 and thiolperoxidase,273 have shown promise in single- and multicomponent vaccine studies using murine models.
Botulism
Botulism is a neurologic intoxication caused by botulinum neurotoxin, the most poisonous agent known. It is produced by the bacterium Clostridium botulinum. Seven toxin types (A through G) have been recognized; all but G cause human disease.274 Botulinum neurotoxin is a significant biowarfare and bioterrorism threat. The neurotoxin binds to the presynaptic membrane and prevents acetylcholine release, resulting in a symmetric, descending flaccid paralysis with classical bulbar palsies characterized by diplopia, dysphonia, dysarthria, and dysphagia.275 As paralysis progresses, generalized weakness occurs; if untreated, death occurs from airway obstruction and diaphragmatic muscle paralysis. Interestingly, this toxin has many therapeutic uses as well—notably, but not limited to, treatment of blepharospasm, strabismus, cervical torticollis, and various dystonias.276, 277, 278
Pentavalent (ABCDE) botulinum toxoid (PBT) was available as a prophylactic countermeasure to botulism from 1959 until November 30, 2011, when the Centers for Disease Control and Prevention stopped providing the vaccine under IND 161 because of reduced potency and increased reactogenicity.3 Although the old methodology for producing toxoids (i.e., the methods used to produce PBT) can be improved by reducing formalin concentrations and increasing the purity of the botulinum proteins, the most advanced candidates are recombinant nontoxic proteins. The Hc subunits of toxin types A and B were immunogenic in a clinical trial and are proceeding in development.279, 280, 281, 282 Other peptides of the toxin, including the light chain and the N terminal of the heavy chain, are also being tested283 (Fig. 12.1 ). A nonreplicating adenovirus-vectored monovalent (C) vaccine, given intranasally or orally, has also been shown to induce serum and secretory antibodies and to protect animals.284, 285 A recombinant bivalent vaccine (rBV A/B) has completed Phase II safety and immunogenicity testing in humans.286 Inevitably, new vaccines against botulism will have to be licensed through a demonstration of efficacy in animals. Although a titer of antibodies correlating with efficacy is not known for humans, studies in guinea pigs suggest that very low levels (<0.1 U) will be protective.287
Figure 12.1.
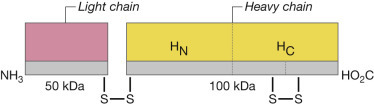
Structure of botulinum toxin.
In March 2013, the FDA approved a heptavalent equine (ABCDEFG) botulism antitoxin to treat individuals with symptoms of botulism after exposure or suspected exposure to botulinum neurotoxin.288, 289 BabyBIG has been available in California to treat infant botulism since 2003.290 However, no vaccines for prophylactic protection against botulism are currently available in the United States.
Clostridium difficile
Clostridium difficile is an inhabitant of the human gastrointestinal tract that, under certain conditions, produces toxins that cause diarrhea. The precipitating cause of C. difficile infection (CDI) seems to be the elimination of other intestinal flora by antibiotic treatment. Incidence of the disease has increased considerably in recent years.291, 292, 293, 294 Antibodies against both of the toxins produced by C. difficile, A and B, are needed to prevent illness.295, 296
Much work is in progress to develop and use monoclonal antibodies against the toxins for prophylaxis and treatment.297, 298, 299, 300 Vaccines against CDI are currently under clinical development at Sanofi Pasteur301 and Pfizer.301a Both candidate C. difficile toxoid vaccines are composed of a highly purified formalin or genetically inactivated toxoids together with aluminum adjuvants delivered by intramuscular injection. Early clinical data suggest a relationship between an immune response to toxins A and B with protection against a primary or recurrent case of CDI.297, 302, 303, 304, 305 After colonization, a systemic anamnestic response to toxin A, as evidenced by increased serum IgG antitoxin A levels, is associated with asymptomatic carriage of C. difficile. Serum IgG antitoxin A levels have also been significantly correlated with IgG levels against toxin B antigens, which were also higher in asymptomatic carriers.303 In patients with CDI, higher IgG antibodies to toxin A are associated with protection against recurrence.303 In an initial Phase I study, the candidate vaccine induced IgG antibodies to toxins A and B with resolution of recurrent diarrhea.304 In a more recent Phase II study, the Sanofi candidate, given in a high dose, elicited functional antibody responses in adult and elderly volunteers for at least 180 days without safety issues.306
In addition, work has begun on the use of colonization factors as vaccine antigens. These include a protease (Cwp84) that is an adhesion molecule307, 308 and a cell wall polysaccharide conjugated to the diphtheria toxoid variant CRM.309 In another approach to vaccine development, Baliban and colleagues310 created a synthetic DNA vaccine encoding receptor binding domains from C. difficile toxin A and toxin B. In mice and nonhuman primates, this vaccine demonstrated robust immunogenicity, and immunized mice were protected from a challenge with C. difficile spores from homologous and heterologous strains.
Acknowledgments
We thank Elizabeth S. Brown, PhD, for critical review of this manuscript. George R. French contributed to this chapter in prior editions.
![]() References for this chapter are available at ExpertConsult.com.
References for this chapter are available at ExpertConsult.com.
References
- 1.National Research Council . National Academies Press; Washington, DC: 2011. Protecting the frontline in biodefense research: the Special Immunizations Program. [PubMed] [Google Scholar]
- 2.Maitzegui JI, McKee KT, Jr, Barrera Oro JG. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J Infect Dis. 1998;177:277–283. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Notice of CDC's discontinuation of investigational pentavalent (ABCDE) botulinum toxoid vaccine for workers at risk for occupational exposure to botulinum toxins. MMWR Morb Mortal Wkly Rep. 2011;60(42):1454–1455. [PubMed] [Google Scholar]
- 4.Pittman PR, Makuch RS, Mangiafico JA. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14:337–343. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 5.Pittman PR, Coonan KM, Gibbs PH. Long-term health effects of repeated exposure to multiple vaccines. Vaccine. 2004;23:525–536. doi: 10.1016/j.vaccine.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Pittman PR, Norris SL, Coonan KM. An assessment of health status among medical research volunteers who served in the Project Whitecoat program at Fort Detrick, Maryland. Mil Med. 2005;170:183–187. doi: 10.7205/milmed.170.3.183. [DOI] [PubMed] [Google Scholar]
- 7.Weaver SC, Powers AM, Brault AC. Molecular epidemiological studies of veterinary arboviral encephalitides. Vet J. 1999;157:123–138. doi: 10.1053/tvjl.1998.0289. [DOI] [PubMed] [Google Scholar]
- 8.Gould EA, Coutard B, Malet H. Understanding the alphaviruses: recent research on important emerging pathogens and progress towards their control. Antiviral Res. 2010;87:111–124. doi: 10.1016/j.antiviral.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol. 2010;140:281–286. doi: 10.1016/j.vetmic.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carossino M, Thiry E, de la Grandière A. Novel vaccination approaches against equine alphavirus encephalitides. Vaccine. 2014;32:311–319. doi: 10.1016/j.vaccine.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 11.Reed DS, Glass PJ, Bakken RR. Combined alphavirus replicon particle vaccine induces durable and cross-protective immune responses against equine encephalitis viruses. J Virol. 2014;88(20):12077–12086. doi: 10.1128/JVI.01406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe DN, Heppner DG, Gardner SN. Perspective piece: current strategic thinking for the development of a trivalent alphavirus vaccine for human use. Am J Trop Med Hyg. 2014;91(3):442–450. doi: 10.4269/ajtmh.14-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittman PR, Liu CT, Cannon CL. Immune interference following sequential alphavirus vaccine immunizations. Vaccine. 2009;27:4879–4882. doi: 10.1016/j.vaccine.2009.02.090. [DOI] [PubMed] [Google Scholar]
- 14.De Madrid AT, Porterfield JS. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol. 1974;23:91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- 15.Calisher CH, Karabatsos N, Dalrymple JM. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70(Pt 1):37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 16.Clarke DH. Further studies on antigenic relationships among the viruses of the group B tick-borne complex. Bull World Health Organ. 1964;31:45–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Cole FW, May SW, Eddy GA. Inactivated Venezuelan equine encephalomyelitis virus prepared from attenuated (TC-83) virus. Appl Microbiol. 1974;27:150–153. doi: 10.1128/am.27.1.150-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salk JE, Krech U, Youngner JS. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am J Public Health. 1954;44:563–570. doi: 10.2105/ajph.44.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger F. Venezuelan equine encephalitis. Teratology. 1977;16(3):359–362. doi: 10.1002/tera.1420160317. [DOI] [PubMed] [Google Scholar]
- 20.Bowen GS, Rayfield EJ, Monath TP. Studies of glucose metabolism in rhesus monkeys after Venezuelan equine encephalitis virus infection. J Med Virol. 1980;6(3):227–234. doi: 10.1002/jmv.1890060306. [DOI] [PubMed] [Google Scholar]
- 21.Rayfield EJ, Gorelkin L, Curnow RT. Virus induced pancreatic disease by Venezuelan encephalitis virus alteration in glucose tolerance and insulin release. Diabetes. 1975;25(7):623–631. doi: 10.2337/diab.25.7.623. [DOI] [PubMed] [Google Scholar]
- 22.Casamassima AC, Hess LW, Marty A. TC-83 Venezuelan equine encephalitis vaccine exposure during pregnancy. Teratology. 1987;36(3):287–289. doi: 10.1002/tera.1420360303. [DOI] [PubMed] [Google Scholar]
- 23.Davis NL, Brown KW, Greenwald GF. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212:102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 24.Martin SS, Bakken RR, Lind CM. Evaluation of formalin inactivated V3526 virus with adjuvant as a next generation vaccine candidate for Venezuelan equine encephalitis virus. Vaccine. 2010;28:3143–3151. doi: 10.1016/j.vaccine.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holley P, Fine DL, Terpening SJ. 2008. Safety of an attenuated Venezuelan equine encephalitis virus (VEEV) vaccine in humans. Presented at the 48th ICAAC/IDSA meeting, Oct 25. [Google Scholar]
- 26.Martin SS, Bakken RR, Lind CM. Telemetric analysis to detect febrile responses in mice following vaccination with a live-attenuated virus vaccine. Vaccine. 2009;27:6814–6823. doi: 10.1016/j.vaccine.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine DL, Jenkins E, Martin SS. A multisystem approach for development and evaluation of inactivated vaccines for Venezuelan equine encephalitis virus (VEEV) J Virol Methods. 2010;163:424–432. doi: 10.1016/j.jviromet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin SS, Bakken RR, Lind CM. Comparison of the immunological responses and efficacy of gamma-irradiated V3526 vaccine formulations against subcutaneous and aerosol challenge with Venezuelan equine encephalitis virus subtype IAB. Vaccine. 2010;28:1031–1040. doi: 10.1016/j.vaccine.2009.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma A, Gupta P, Glass PJ. Safety and protective efficacy of INA-inactivated Venezuelan equine encephalitis virus: implication in vaccine development. Vaccine. 2011;29:953–959. doi: 10.1016/j.vaccine.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Rossi SL, Guerbois M, Gorchakov R. IRES-based Venezuelan equine encephalitis vaccine candidate elicits protective immunity in mice. Virology. 2013;437:81–88. doi: 10.1016/j.virol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi SL, Russell-Lodrigue KE, Killeen SZ. IRES-containing VEEV vaccine protects cynomolgus macaques from IE Venezuelan equine encephalitis virus aerosol challenge. PLoS Negl Trop Dis. 2015;9(5):e0003797. doi: 10.1371/journal.pntd.0003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riemenschneider J, Garrison A, Geisbert J. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 33.Dupuy LC, Richards MJ, Reed DS. Immunogenicity and protective efficacy of a DNA vaccine against Venezuelan equine encephalitis virus aerosol challenge in nonhuman primates. Vaccine. 2010;28:7345–7350. doi: 10.1016/j.vaccine.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Dupuy LC, Richards MJ, Ellefsen B. A DNA vaccine for Venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin Vaccine Immunol. 2011;18(5):707–716. doi: 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tretyakova I, Lukashevich IS, Glass P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine. 2013;31:1019–1025. doi: 10.1016/j.vaccine.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson DM, Berman S, Lowenthal JP. Western equine encephalomyelitis vaccine produced in chick embryo cell cultures. Appl Microbiol. 1966;14:1011–1014. doi: 10.1128/am.14.6.1011-1014.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartelloni PJ, McKinney RW, Calia FM. Inactivated western equine encephalomyelitis vaccine propagated in chick embryo cell culture: clinical and serological evaluation in man. Am J Trop Med Hyg. 1971;20:146–149. doi: 10.4269/ajtmh.1971.20.146. [DOI] [PubMed] [Google Scholar]
- 38.Nagata LP, Hu WG, Masri SA. Efficacy of DNA vaccination against western equine encephalitis virus infection. Vaccine. 2005;23:2280–2283. doi: 10.1016/j.vaccine.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 39.Wu JQ, Barabé ND, Chau D. Complete protection of mice against a lethal dose challenge of western equine encephalitis virus after immunization with an adenovirus-vectored vaccine. Vaccine. 2007;25:4368–4375. doi: 10.1016/j.vaccine.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Barabé ND, Rayner GA, Christopher ME. Single-dose, fast-acting vaccine candidate against western equine encephalitis virus completely protects mice from intranasal challenge with different strains of the virus. Vaccine. 2007;25:6271–6276. doi: 10.1016/j.vaccine.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 41.Swayze RD, Bhogal HS, Barabé ND. Envelope protein E1 as vaccine target for western equine encephalitis virus. Vaccine. 2011;29:813–820. doi: 10.1016/j.vaccine.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Maire LF, III, McKinney RW, Cole FE., Jr An inactivated eastern equine encephalomyelitis vaccine propagated in chick-embryo cell culture, I: production and testing. Am J Trop Med Hyg. 1970;19:119–122. doi: 10.4269/ajtmh.1970.19.119. [DOI] [PubMed] [Google Scholar]
- 43.Pittman PR. US Army Medical Research Institute of Infectious Diseases; Ft. Detrick, MD: 1999. Immunization of persons at risk of exposure to eastern equine encephalitis virus: continued assessment of the safety and effectiveness of eastern equine encephalitis vaccine, inactivated, TSI-GSD 104 (IND 266; Protocol No FY99-11) [Google Scholar]
- 44.Pandya J, Gorchakov R, Wang E. A vaccine candidate for eastern equine encephalitis virus based on IRES-mediated attenuation. Vaccine. 2012;30:1276–1282. doi: 10.1016/j.vaccine.2011.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deller JJ, Jr, Russell PK. Chikungunya disease. Am J Trop Med Hyg. 1968;17:107–111. doi: 10.4269/ajtmh.1968.17.107. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy AC, Fleming J, Solomon L. Chikungunya viral arthropathy: a clinical description. J Rheumatol. 1980;7:231–236. [PubMed] [Google Scholar]
- 47.Johnston RE, Peters CJ. Alphaviruses. In: Knipe DM, Howley PM, editors. Fields' Virology. 3rd ed. Lippincott-Raven; Philadelphia, PA: 1996. pp. 843–898. [Google Scholar]
- 48.Paquet C, Quatresous I, Solet JL. Chikungunya outbreak in Reunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill. 2006;11:E060202. doi: 10.2807/esw.11.05.02891-en. [DOI] [PubMed] [Google Scholar]
- 49.Schuffenecker I, Iteman I, Michault A. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.10-Day action plan to contain chikungunya. 2009. http://www.thehindu.com/2006/09/07/stories/100810020100.htm The Hindu. September 7.
- 51.Thiboutot MM, Kannan S, Kawalekar OU. Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4:e623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers AM. Risks to the Americas associated with the continued expansion of chikungunya virus. J Gen Virol. 2015;96:1–5. doi: 10.1099/vir.0.070136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kendrick K, Stanek D, Blackmore C. Notes from the field: transmission of chikungunya virus in the continental United States—Florida. MMWR Morb Mortal Wkly Rep. 2014;63(48):1137–2014. [PMC free article] [PubMed] [Google Scholar]
- 54.Vazeille M, Moutailler S, Coudrier D. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2(11):e1169. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitt NH, Ramsburg HH, Hasty SE. Development of an attenuated strain of Chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 56.Edelman R, Tacket CO, Wasserman SS. Phase II safety and immunogenicity study of live Chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 57.Wang E, Volkova E, Adams AP. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallilankaraman K, Shedlock DJ, Bao H. A DNA vaccine against Chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muthumani K, Lankaraman KM, Laddy DJ. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiwari M, Parida M, Santhosh SR. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27:2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 61.Akahata W, Young ZY, Anderson H. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akahata W, Nabel GJ. A specific domain of the chikungunya virus E2 protein regulates particle formation in human cells: implications for alphavirus vaccine design. J Virol. 2012;86(16):8879–8883. doi: 10.1128/JVI.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang L-J, Dowd KA, Mendoza FH. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384:2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- 64.Brandler S, Ruffié C, Combredet C. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 65.Nimmo JR. An unusual epidemic. Med J Aust. 1928;1:549–550. [Google Scholar]
- 66.Doherty RL, Whitehead RH, Gorman BM. The isolation of a third group-A arbovirus in Australia with preliminary observations on its relationship to epidemic polyarthritis. Aust J Sci. 1963;26:183–184. [Google Scholar]
- 67.Doherty RL, Whitehead RH, Wetters EJ. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland, II: arbovirus infections of mosquitoes, man and domestic fowls, 1963–1966. Trans R Soc Trop Med Hyg. 1968;62:430–438. doi: 10.1016/0035-9203(68)90095-3. [DOI] [PubMed] [Google Scholar]
- 68.Marshall ID, Miles JAR. Ross River virus and epidemic polyarthritis. In: Harris KF, editor. Vol. 2. Praeger; New York, NY: 1984. pp. 31–56. (Current Topics in Vector Research). [Google Scholar]
- 69.Rulli NE, Suhrbier A, Hueston L. Ross River virus: molecular and cellular aspects of disease pathogenesis. Pharmacol Ther. 2005;107:329–342. doi: 10.1016/j.pharmthera.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Harley D, Bossingham D, Purdie DM. Ross River virus disease in tropical Queensland: evolution of rheumatic manifestations in an inception cohort followed for six months. Med J Aust. 2002;177:352–355. doi: 10.5694/j.1326-5377.2002.tb04836.x. [DOI] [PubMed] [Google Scholar]
- 71.Russel RC, Cope SE, Yung AJ. Combating the enemy-mosquitoes and Ross River virus in a joint military exercises in tropical Australia. Am J Trop Med Hyg. 1998;59:S307. [Google Scholar]
- 72.Yu S, Aaskov JG. Development of a candidate vaccine against Ross River virus infection. Vaccine. 1994;12:1118–1124. doi: 10.1016/0264-410x(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 73.Aaskov J, Williams L, Yu S. A candidate Ross River virus vaccine: preclinical evaluation. Vaccine. 1997;15:1396–1404. doi: 10.1016/s0264-410x(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 74.Kistner O, Barrett N, Bruhmann A. The preclinical testing of a formaldehyde inactivated Ross River virus vaccine designed for use in humans. Vaccine. 2007;25:4845–4852. doi: 10.1016/j.vaccine.2007.01.103. [DOI] [PubMed] [Google Scholar]
- 75.Aichinger G, Ehrlich HJ, Aaskov JG. Safety and immunogenicity of an inactivated whole virus Vero cell-derived Ross River virus vaccine: a randomized trial. Vaccine. 2011;29:9376–9384. doi: 10.1016/j.vaccine.2011.09.125. [DOI] [PubMed] [Google Scholar]
- 76.Wressnigg N, van der Velden VW, Portsmouth D. An inactivated Ross River virus vaccine is well tolerated and immunogenic in an adult population in a randomized phase 3 trial. Clin Vaccine Immunol. 2015;22(3):267–273. doi: 10.1128/CVI.00546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Linn ML, Aaskov JG, Suhrbier A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77(Pt 3):407–411. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- 78.Mandell RB, Flick R. Rift Valley fever virus: an unrecognized emerging threat? Hum Vaccin. 2010;6(7):597–601. doi: 10.4161/hv.6.7.11761. [DOI] [PubMed] [Google Scholar]
- 79.Randall R, Gibbs CJ, Jr, Aulisio CG. The development of a formalin-killed Rift Valley fever virus vaccine for use in man. J Immunol. 1962;89:660–671. [PubMed] [Google Scholar]
- 80.Eddy GA, Peters CJ, Meadors G. Rift Valley fever vaccine for humans. In: Swartz TA, Klingberg MA, Goldblum N, editors. Vol. 3. S. Karger; Rift Valley Fever, Basel, Switzerland: 1981. pp. 124–141. (Contributions to Epidemiology and Biostatistics). [Google Scholar]
- 81.Meadors GF, Gibbs PH, Peters CJ. Evaluation of a new Rift Valley fever vaccine: safety and immunogenicity trial. Vaccine. 1986;4:179–183. doi: 10.1016/0264-410x(86)90007-1. [DOI] [PubMed] [Google Scholar]
- 82.Pittman PR, Liu CT, Cannon TL. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine. 2000;18:181–189. doi: 10.1016/s0264-410x(99)00218-2. [DOI] [PubMed] [Google Scholar]
- 83.Rusnak JM, Gibbs P, Boudreau E. Immunogenicity and safety of an inactivated Rift Valley fever vaccine in a 19-year study. Vaccine. 2011;29:3222–3229. doi: 10.1016/j.vaccine.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 84.Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66(Pt 10):2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 85.Morrill JC, Carpenter L, Taylor D. Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine. 1991;9:35–41. doi: 10.1016/0264-410x(91)90314-v. [DOI] [PubMed] [Google Scholar]
- 86.Baskerville A, Hubbard KA, Stephenson JR. Comparison of the pathogenicity for pregnant sheep of Rift Valley fever virus and a live attenuated vaccine. Res Vet Sci. 1992;52:307–311. doi: 10.1016/0034-5288(92)90029-2. [DOI] [PubMed] [Google Scholar]
- 87.Morrill JC, Mebus CA, Peters CJ. Safety of a mutagen-attenuated Rift Valley fever virus vaccine in fetal and neonatal bovids. Am J Vet Res. 1997;58:1110–1114. [PubMed] [Google Scholar]
- 88.Morrill JC, Mebus CA, Peters CJ. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am J Vet Res. 1997;58:1104–1109. [PubMed] [Google Scholar]
- 89.Morrill JC, Peters CJ. Protection of MP-12–vaccinated rhesus macaques against parenteral and aerosol challenge with virulent Rift Valley fever virus. J Infect Dis. 2011;204:229–236. doi: 10.1093/infdis/jir249. [DOI] [PubMed] [Google Scholar]
- 90.Miller MM, Bennett KE, Drolet BS. Evaluation of the efficacy, potential for vector transmission, and duration of immunity of MP-12, an attenuated Rift Valley fever virus vaccine candidate, in sheep. Clin Vaccine Immunol. 2015;22(8):930–937. doi: 10.1128/CVI.00114-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pittman PR, McClain D, Quinn X. Safety and immunogenicity of a mutagenized, live attenuated Rift Valley fever vaccine, MP-12, in a Phase 1 dose escalation and route comparison study in humans. Vaccine. 2016;34(4):424–429. doi: 10.1016/j.vaccine.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 92.Pittman PR, Norris SL, Brown ES. Rift Valley fever MP-12 vaccine phase 2 clinical trial: safety, immunogenicity, and genetic characterization of virus isolates. Vaccine. 2016;34(4):523–530. doi: 10.1016/j.vaccine.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller R, Saluzzo JF, Lopez N. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995;53:405–411. doi: 10.4269/ajtmh.1995.53.405. [DOI] [PubMed] [Google Scholar]
- 94.Njenga MK, Njagi L, Thumbi SM. Randomized controlled field trial to assess the immunogenicity and safety of Rift Valley fever clone 13 vaccine in livestock. PLoS Negl Trop Dis. 2015;9(3):e0003550. doi: 10.1371/journal.pntd.0003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmaljohn CS, Parker MD, Ennis WH. Baculovirus expression of the M genome segment of Rift Valley fever virus and examination of antigenic and immunogenic properties of the expressed proteins. Virology. 1989;170:184–192. doi: 10.1016/0042-6822(89)90365-6. [DOI] [PubMed] [Google Scholar]
- 96.Mandell RB, Koukuntla R, Mogler LJ. A replication-incompetent Rift Valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology. 2009;397:187–198. doi: 10.1016/j.virol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heise MT, Whitmore A, Thompson J. An alphavirus replicon-derived candidate vaccine against Rift Valley fever virus. Epidemiol Infect. 2009;137:1309–1318. doi: 10.1017/S0950268808001696. [DOI] [PubMed] [Google Scholar]
- 98.Dempsey PW, Allison ME, Akkaraju S. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 99.Habjan M, Penski N, Wagner V. Efficient production of Rift Valley fever virus-like particles: the anti-viral protein MxA can inhibit primary transcription of bunyaviruses. Virology. 2009;385:400–408. doi: 10.1016/j.virol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 100.Naslund J, Lagerqvist N, Habjan M. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley fever virus. Virology. 2009;385:409–415. doi: 10.1016/j.virol.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 101.de Boer SM, Kortekaas J, Antonis AF. Rift Valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine. 2010;28:2330–2339. doi: 10.1016/j.vaccine.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 102.Faburay B, Wilson W, McVey DS. Rift Valley fever virus structural and nonstructural proteins: recombinant protein expression and immunoreactivity against antisera from sheep. Vector Borne Zoonotic Dis. 2013;13(9):619–629. doi: 10.1089/vbz.2012.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Faburay B, Lebedev M, McVey DS. A glycoprotein subunit vaccine elicits a strong Rift Valley fever virus neutralizing antibody response in sheep. Vector Borne Zoonotic Dis. 2014;14(10):746–756. doi: 10.1089/vbz.2014.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terasaki K, Tercero BR, Makino S. Single-cycle replicable Rift Valley fever virus mutants as safe vaccine candidates. Virus Res. 2016;216:55–65. doi: 10.1016/j.virusres.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee HW. Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis. 1989;11(suppl 4):S864–S876. [PubMed] [Google Scholar]
- 106.Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3(2):95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hjelle B. Vaccines against hantaviruses. Expert Rev Vaccines. 2002;1:373–384. doi: 10.1586/14760584.1.3.373. [DOI] [PubMed] [Google Scholar]
- 108.Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 109.Nichol ST, Spiropoulou CF, Morzunov S. Genetic identification of a Hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 110.Lee HWACN. Field trial of an inactivated vaccine against HFRS in humans. Arch Virol Suppl. 1990;1:35–47. [Google Scholar]
- 111.A major breakthrough in preventive medicine: Hantavirus (HFRS vaccine, KGCC) Korea Green Cross Organization; Seoul, Korea: 1997. [Google Scholar]
- 112.Cho HW, Howard CR. Antibody responses in humans to an inactivated Hantavirus vaccine (Hantavax) Vaccine. 1999;17:2569–2575. doi: 10.1016/s0264-410x(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 113.Hooper JW, Li D. Vaccines against Hantaviruses. Curr Top Microbiol Immunol. 2001;256:171–191. doi: 10.1007/978-3-642-56753-7_10. [DOI] [PubMed] [Google Scholar]
- 114.Kruger DH, Ulrich R, Lundkvist AA. Hantavirus infections and their prevention. Microbes Infect. 2001;3:1129–1144. doi: 10.1016/s1286-4579(01)01474-5. [DOI] [PubMed] [Google Scholar]
- 115.Cho HW, Howard CR, Lee HW. Review of an inactivated vaccine against Hantaviruses. Intervirology. 2002;45:328–333. doi: 10.1159/000067925. [DOI] [PubMed] [Google Scholar]
- 116.Park K, Kim CS, Moon KT. Protective effectiveness of Hantavirus vaccine. Emerg Infect Dis. 2004;10:2218–2220. doi: 10.3201/eid1012.040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi Y, Ahn CJ, Seong KM. Inactivated Hantaan virus vaccine derived from suspension culture of Vero cells. Vaccine. 2003;21:1867–1873. doi: 10.1016/s0264-410x(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 118.Schmaljohn CS, Hasty SE, Dalrymple JM. Preparation of candidate vaccinia-vectored vaccines for haemorrhagic fever with renal syndrome. Vaccine. 1992;10:10–13. doi: 10.1016/0264-410x(92)90412-d. [DOI] [PubMed] [Google Scholar]
- 119.McClain DJ, Summers PL, Harrison SA. Clinical evaluation of a vaccinia-vectored Hantaan virus vaccine. J Med Virol. 2000;60:77–85. doi: 10.1002/(sici)1096-9071(200001)60:1<77::aid-jmv13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 120.Custer DM, Thompson E, Schmaljohn CS. Active and passive vaccination against Hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J Virol. 2003;77:9894–9905. doi: 10.1128/JVI.77.18.9894-9905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hooper JW, Custer DM, Smith J. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology. 2006;347:208–216. doi: 10.1016/j.virol.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 122.Ulrich R, Koletzki D, Lachmann S. New chimaeric hepatitis B virus core particles carrying Hantavirus (serotype Puumala) epitopes: immunogenicity and protection against virus challenge. J Biotechnol. 1999;73:141–153. doi: 10.1016/s0168-1656(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 123.Maes P, Clement J, Gavrilovskaya I. Hantaviruses: immunology, treatment and prevention. Viral Immunol. 2004;17:481–497. doi: 10.1089/vim.2004.17.481. [DOI] [PubMed] [Google Scholar]
- 124.Boudreau EF, Josleyn M, Ullman D. A phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for hemorrhagic fever with renal syndrome. Vaccine. 2012;30:1951–1958. doi: 10.1016/j.vaccine.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 125.Hooper JW, Moon JE, Paolino KM. A phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin Microbiol Infect. 2014;20:110–117. doi: 10.1111/1469-0691.12553. [DOI] [PubMed] [Google Scholar]
- 126.Hooper JW, Josleyn M, Ballantyne J. A novel Sin Nombre virus DNA vaccine and its inclusion in a candidate pan-hantavirus vaccine against hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS) Vaccine. 2013;31:4314–4321. doi: 10.1016/j.vaccine.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kwilas S, Kishimori JM, Josleyn M. A hantavirus pulmonary syndrome (HPS) DNA vaccine delivered using a spring-powered jet injector elicits a potent neutralizing antibody response in rabbits and nonhuman primates. Curr Gene Ther. 2014;14:200–210. doi: 10.2174/1566523214666140522122633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayes EB, Sejvar JJ, Zaki SR. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Posadas-Herrera G, Inoue S, Fuke I. Development and evaluation of a formalin-inactivated West Nile virus vaccine (WN-VAX) for a human vaccine candidate. Vaccine. 2010;28:7939–7946. doi: 10.1016/j.vaccine.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 130.Muraki Y, Fujita T, Matsuura M. The efficacy of inactivated West Nile vaccine (WN-VAX) in mice and monkeys. Virol J. 2015;12:54. doi: 10.1186/s12985-015-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monath TP, Liu J, Kanesa-Thasan N. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guy G, Guirakhoo F, Barban V. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 133.Biedenbender R, Bevilacqua J, Gregg AM. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infect Dis. 2011;203:75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin JE, Pierson TC, Hubka S. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lieberman MM, Nerurkar VR, Luo H. Immunogenicity and protective efficacy of a recombinant subunit West Nile Virus vaccine in Rhesus monkeys. Clin Vaccine Immunol. 2009;16:1332–1337. doi: 10.1128/CVI.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barrera Oro JG, McKee KT, Jr, Spisso J. A refined complement-enhanced neutralization test for detecting antibodies to Junin virus. J Virol Methods. 1990;29:71–80. doi: 10.1016/0166-0934(90)90009-5. [DOI] [PubMed] [Google Scholar]
- 137.Ölschläger S, Flatz L. Vaccination strategies against highly pathogenic arenaviruses: the next steps toward clinical trials. PLoS Pathog. 2013;9(4):e1003212. doi: 10.1371/journal.ppat.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maiztegui JI. Clinical and epidemiological patterns of Argentine haemorrhagic fever. Bull World Health Organ. 1975;52:567–575. [PMC free article] [PubMed] [Google Scholar]
- 139.Mills JN, Ellis BA, McKee KT., Jr Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am J Trop Med Hyg. 1991;44:589–597. doi: 10.4269/ajtmh.1991.44.589. [DOI] [PubMed] [Google Scholar]
- 140.Mills JN, Ellis BA, McKee KT., Jr A longitudinal study of Junin virus activity in the rodent reservoir of Argentine hemorrhagic fever. Am J Trop Med Hyg. 1992;47:749–763. doi: 10.4269/ajtmh.1992.47.749. [DOI] [PubMed] [Google Scholar]
- 141.Barren OHG, Eddy G. 1982. Characteristics of candidate live attenuated Junin virus vaccine. In: Abstracts of the 4th International Conference on Comparative Virology. Banff, Canada; Oct 17–22. Abstract S4-S10. [Google Scholar]
- 142.Walen KH. Demonstration of inapparent heterogeneity in a population of an animal virus by single-burst analyses. Virology. 1963;20:230–234. doi: 10.1016/0042-6822(63)90110-7. [DOI] [PubMed] [Google Scholar]
- 143.McKee KT, Jr, Oro JG, Kuehne AI. Candid no. 1 Argentine hemorrhagic fever vaccine protects against lethal Junin virus challenge in rhesus macaques. Intervirology. 1992;34:154–163. doi: 10.1159/000150276. [DOI] [PubMed] [Google Scholar]
- 144.McKee KT, Jr, Oro JG, Kuehne AI. Safety and immunogenicity of a live-attenuated Junin (Argentine hemorrhagic fever) vaccine in rhesus macaques. Am J Trop Med Hyg. 1993;48:403–411. doi: 10.4269/ajtmh.1993.48.403. [DOI] [PubMed] [Google Scholar]
- 145.MacDonald C, McKee K, Peters C. 1987. Initial assessment of humans inoculated with a live-attenuated Junin virus vaccine. In: Abstracts of the 7th International Congress of Virology. Edmonton, Canada; Aug 9–14. [Google Scholar]
- 146.Maiztegui JI, McKee KT., Jr . 1989. Inoculation of human volunteers with a vaccine against Argentine hemorrhagic fever. In: Abstracts of the 6th International Conference on Comparative and Applied Virology. Banff, Canada; Oct 15–21. [Google Scholar]
- 147.Ambrosio AM, Riera LM, Saavedra MC. Immune response to vaccination against Argentine hemorrhagic fever in an area where different arenaviruses coexist. Viral Immunol. 2006;19:196–201. doi: 10.1089/vim.2006.19.196. [DOI] [PubMed] [Google Scholar]
- 148.Maiztegui JI, McKee KT, Jr, Barrera Oro JG. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J Infect Dis. 1998;177:277–283. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 149.Harrison LH, Halsey NA, McKee KT., Jr Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis. 1999;28:1091–1094. doi: 10.1086/514749. [DOI] [PubMed] [Google Scholar]
- 150.Enria DA, Ambrosio AM, Briggiler AM. Candid#1 vaccine against Argentine hemorrhagic fever produced in Argentina. Immunogenicity and safety. Medicina (B Aires) 2010;70(3):215–222. [PubMed] [Google Scholar]
- 151.Regulatory Agency of Argentina. ANMAT, Disposicion No. 4812, August 29, 2006.
- 152.McKee KT, Enria DA, Barrera Oro JG. Junin (Argentine hemorrhagic fever) In: Stanberry LR, Barrett Alan DT, editors. Vaccines for Biodefense and Emerging and Neglected Diseases. Academic Press; London, UK: 2009. pp. 538–547. [Google Scholar]
- 153.Enria DA, Barrera Oro JG. Junin virus vaccines. Curr Top Microbiol Immunol. 2002;263:239–261. doi: 10.1007/978-3-642-56055-2_12. [DOI] [PubMed] [Google Scholar]
- Lukashevich IS, Pushko P. Vaccine platforms to control Lassa fever. Expert Rev Vaccines. 2016 doi: 10.1080/14760584.2016.1184575. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 154.Zaki AM, van Boheemen S, Bestebroer TM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 155.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 156.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Memish ZA, Cotten M, Meyer B. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382(9893):694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Coleman CM, Liu YV, Mu H. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Volz A, Kupke A, Song F. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol. 2015;89(16):8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang L, Shi W, Joyce MG. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Centers for Disease Control and Prevention Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(3) [PubMed] [Google Scholar]
- 164.Gikas A, Kokkini S. Tsioutis C. Q fever: clinical manifestations and treatment. Expert Rev Anti Infect Ther. 2010;8(5):529–539. doi: 10.1586/eri.10.29. [DOI] [PubMed] [Google Scholar]
- 165.Penttila IA, Harris RJ, Storm P. Cytokine dysregulation in the post-Q-fever fatigue syndrome. QJM. 1998;91:549–560. doi: 10.1093/qjmed/91.8.549. [DOI] [PubMed] [Google Scholar]
- 166.Ormsbee RA, Marmion BP. Prevention of Coxiella burnetii infection: vaccines and guidelines for those at risk. In: Marne TJ, editor. Vol. 1. CRC Press; Boca Raton, FL: 1990. pp. 225–248. (Q Fever: The Disease). [Google Scholar]
- 167.Smadel JE, Snyder MJ. Vaccination against Q fever. Am J Hyg. 1948;47:71–81. doi: 10.1093/oxfordjournals.aje.a119187. [DOI] [PubMed] [Google Scholar]
- 168.Spicer DS, DeSanctis AN. Preparation of phase 1Q fever antigen suitable for vaccine use. Appl Environ Microbiol. 1976;32:85–88. doi: 10.1128/aem.32.1.85-88.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marmion BP. Development of Q fever vaccines, 1937 to 1967. Med J Aust. 1967;2:1074–1078. doi: 10.5694/j.1326-5377.1967.tb27293.x. [DOI] [PubMed] [Google Scholar]
- 170.Luoto L, Bell JF, Casey M. Q fever vaccination of human volunteers, I: the serologic and skin-test response following subcutaneous injections. Am J Hyg. 1963;78:1–15. [PubMed] [Google Scholar]
- 171.Shapiro RA, Siskind V, Schofield FD. A randomized, controlled, double-blind, cross-over, clinical trial of Q fever vaccine in selected Queensland abattoirs. Epidemiol Infect. 1990;104:267–273. doi: 10.1017/s0950268800059446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marmion BP, Ormsbee RA, Kyrkou M. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984;2:1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 173.Ackland JR, Worswick DA, Marmion BP. Vaccine prophylaxis of Q fever: a follow-up study of the efficacy of Q-Vax (CSL) 1985-1990. Med J Aust. 1994;160:704–708. [PubMed] [Google Scholar]
- 174.Gidding HF, Wallace C, Lawrence GL. Australia's national Q fever vaccination program. Vaccine. 2009;27:2037–2041. doi: 10.1016/j.vaccine.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 175.Beneson AS. Q fever vaccine: efficacy and present status. In: Smadel JE, editor. Symposium on Q Fever, vol. 6. Walter Reed Army Institute of Medicine Science; Washington, DC: 1959. p. 47. [Google Scholar]
- 176.Gefenaite G, Munster JM, van Houdt R. Effectiveness of the Q fever vaccine: a meta-analysis. Vaccine. 2011;29:395–398. doi: 10.1016/j.vaccine.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 177.Chiu CK, Durrheim DN. A review of the efficacy of human Q fever vaccine registered in Australia. N S W Public Health Bull. 2007;18:133–136. doi: 10.1071/nb07057. [DOI] [PubMed] [Google Scholar]
- 178.Isken LD, Kraaij-Dirkzwager M, Vermeer de Bondt PE. Implementation of a Q fever vaccination program for high-risk patients in the Netherlands. Vaccine. 2013;31(23):2617–2622. doi: 10.1016/j.vaccine.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 179.Schoffelen T, Herremans T, Sprong T. Limited humoral and cellular responses to Q fever vaccination in older adults with risk factors for chronic Q fever. J Infect. 2013;67(6):565–573. doi: 10.1016/j.jinf.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 180.Schoffelen T, Wong A, Rumke HC. Adverse events and association with age, sex and immunological parameters of Q fever vaccination in patients at risk for chronic Q fever in the Netherlands 2011. Vaccine. 2014;32(49):6622–6630. doi: 10.1016/j.vaccine.2014.09.061. [DOI] [PubMed] [Google Scholar]
- 181.Vermeer-de Bondt PE, Schoffelen T, Vanrolleghem AM. Coverage of the 2011 Q fever vaccination campaign in the Netherlands, using retrospective population-based prevalence estimation of cardiovascular risk-conditions for chronic Q fever. PLoS ONE. 2015;10(4):e0123570. doi: 10.1371/journal.pone.0123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Williams E. The Mediterranean Fever Commission: its origin and achievements. In: Young EJ, Corbek MJ, editors. Brucellosis: Clinical Laboratory Aspects. CRC Press; Boca Raton, FL: 1989. pp. 11–23. [Google Scholar]
- 183.Fries LF, Waag DM, Williams JC. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect Immun. 1993;61:1251–1258. doi: 10.1128/iai.61.4.1251-1258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kazar J, Brezina R, Palanova A. Immunogenicity and reactogenicity of a Q fever chemovaccine in persons professionally exposed to Q fever in Czechoslovakia. Bull World Health Organ. 1982;60:389–394. [PMC free article] [PubMed] [Google Scholar]
- 185.Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006;367:679–688. doi: 10.1016/S0140-6736(06)68266-4. [DOI] [PubMed] [Google Scholar]
- 186.Ruiz S, Wolfe DN. Vaccination against Q fever for biodefense and public health indications. Front Microbiol. 2014;5:726. doi: 10.3389/fmicb.2014.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Q, Niu D, Wen B. Protective immunity against Q fever induced with a recombinant P1 antigen fused with HspB of Coxiella burnetii. Ann N Y Acad Sci. 2005;1063:130–142. doi: 10.1196/annals.1355.021. [DOI] [PubMed] [Google Scholar]
- 188.Tyczka J, Eberling S, Baljer G. Immunization experiments with recombinant Coxiella burnetii proteins in a murine infection model. Ann N Y Acad Sci. 2005;1063:143–148. doi: 10.1196/annals.1355.022. [DOI] [PubMed] [Google Scholar]
- 189.Zhang G, Samuel JE. Vaccines against Coxiella infection. Expert Rev Vaccines. 2004;3:577–584. doi: 10.1586/14760584.3.5.577. [DOI] [PubMed] [Google Scholar]
- 190.Hepburn MJ, Simpson AJH. Tularemia: current diagnosis and treatment options. Expert Rev Anti Infect Ther. 2008;6(2):231–240. doi: 10.1586/14787210.6.2.231. [DOI] [PubMed] [Google Scholar]
- 191.Francis E. Deer-fly or Pahvant Valley Plague: a disease of man of hitherto unknown etiology. Public Health Prev. 1919;34:2061–2062. [Google Scholar]
- 192.Andersson H, Hartmanova B, Back E. Transcriptional profiling of the peripheral blood response during tularemia. Genes Immun. 2006;7:503–513. doi: 10.1038/sj.gene.6364321. [DOI] [PubMed] [Google Scholar]
- 193.Overholt EL, Tigertt WD, Kadull PJ. An analysis of forty-two cases of laboratory-acquired tularemia: treatment with broad spectrum antibiotics. Am J Med. 1961;30:785–806. doi: 10.1016/0002-9343(61)90214-5. [DOI] [PubMed] [Google Scholar]
- 194.Van MT, Jr, Kadull PJ. Laboratory-acquired tularemia in vaccinated individuals: a report of 62 cases. Ann Intern Med. 1959;50:621–632. doi: 10.7326/0003-4819-50-3-621. [DOI] [PubMed] [Google Scholar]
- 195.Foshay L, Hesselbrock WH, Wittenberg HJ. Vaccine prophylaxis against tularemia in man. Am J Public Health. 1942;21:1131–1145. doi: 10.2105/ajph.32.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kadull PJ, Reames HR, Coriell LL. Studies on tularemia, V: immunization of man. J Immunol. 1950;65:425–435. [PubMed] [Google Scholar]
- 197.Saslaw S, Eigelsbach HT, Wilson HE. Tularemia vaccine study, I: intracutaneous challenge. Arch Intern Med. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 198.Saslaw S, Eigelsbach HT, Prior JA. Tularemia vaccine study, II: respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 199.Tigertt WD. Soviet viable Pasteurella tularensis vaccines: a review of selected articles. Bacteriol Rev. 1962;26:354–373. doi: 10.1128/br.26.3.354-373.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shope RE. Yale University; New Haven, CT: 1956. An account of the observations made by the United States Medical Mission to the USSR. [Google Scholar]
- 201.Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines, I: production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–425. [PubMed] [Google Scholar]
- 202.Eigelsbach HT, Hunter DH, Janssen WA. Murine model for study of cell-mediated immunity: protection against death from fully virulent Francisella tularensis infection. Infect Immun. 1975;12:999–1005. doi: 10.1128/iai.12.5.999-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Eigelsbach HT, Tulis JJ, McGavran MH. Live tularemia vaccine, I: host-parasite relationship in monkeys vaccinated intracutaneously or aerogenically. J Bacteriol. 1962;84:1020–1027. doi: 10.1128/jb.84.5.1020-1027.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hornick RB. Annual Report; Ft. Detrick, MD: 1958. Studies on Pasteurella tularensis: evaluation of a living vaccine for tularemia; pp. 1–5. US Army Medical Unit; Section II. [Google Scholar]
- 205.Saslaw S, Carhart S. Studies with tularemia vaccines in volunteers, III: serologic aspects following intracutaneous or respiratory challenge in both vaccinated and nonvaccinated volunteers. Am J Med Sci. 1961;241:689–699. [PubMed] [Google Scholar]
- 206.McCrumb FRJ. US Army, Armed Forces Epidemiological Board, Office of the Surgeon General, Annual Report; Washington, DC: 1962. Commission on epidemiological survey; pp. 81–86. [Google Scholar]
- 207.Sawyer WD, Tigertt WD, Crozier D. U.S. Army Medical Unit; Ft. Detrick, MD: 1962. Annual Progress Report. [Google Scholar]
- 208.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 209.Griffin KF, Oyston PC, Titball RW. Francisella tularensis vaccines. FEMS Immunol Med Microbiol. 2007;49:315–323. doi: 10.1111/j.1574-695X.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 210.Oyston PC. Francisella tularensis vaccines. Vaccine. 2009;27(suppl 4):D48–D51. doi: 10.1016/j.vaccine.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 211.Pechous RD, McCarthy TR, Zahrt TC. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev. 2009;73:684–711. doi: 10.1128/MMBR.00028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Barry EM, Cole LE, Santiago AE. Vaccines against tularemia. Hum Vaccin. 2009;5:832–838. doi: 10.4161/hv.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jia QB, Lee BY, Bowen R. A Francisella tularensis live vaccine strain (LVS) mutant with a deletion in capB, encoding a putative capsular biosynthesis protein, is significantly more attenuated than LVS yet induces potent protective immunity in mice against F. tularensis challenge. Infect Immun. 2010;78:4341–4355. doi: 10.1128/IAI.00192-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim T-H, Pinkham JT, Heninger SJ. Genetic modification of the O-polysaccharide of Francisella tularensis results in an avirulent live attenuated vaccine. J Infect Dis. 2012;205(7):1056–1065. doi: 10.1093/infdis/jir620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kaur R, Chen S, Arevalo MT. Protective immunity against tularemia provided by an adenovirus-vectored vaccine expressing Tul4 of Francisella tularensis. Clin Vaccine Immunol. 2012;19(3):359–364. doi: 10.1128/CVI.05384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jia Q, Bowen R, Sahakian J. A heterologous prime-boost vaccination strategy comprising the Francisella tularensis live vaccine strain capB mutant and recombinant attenuated Listeria monocytogenes expressing F. tularensis Ig1C induces potent protective immunity in mice against virulent F. tularensis aerosol challenge. Infect Immun. 2013;81(5):1550–1561. doi: 10.1128/IAI.01013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mahawar M, Rabadi SM, Banik S. Identification of a live attenuated vaccine candidate for tularemia prophylaxis. PLoS ONE. 2013;8(4):e61539. doi: 10.1371/journal.pone.0061539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Richard K, Mann BJ, Stocker L. Novel catanionic surfactant vesicle vaccines protect against Francisella tularensis LVS and confer significant partial protection against F. tularensis Schu S4 strain. Clin Vaccine Immunol. 2014;21(2):212–226. doi: 10.1128/CVI.00738-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Suresh RV, Ma Z, Sunagar R. Preclinical testing of a vaccine candidate against tularemia. PLoS ONE. 2015;10(4):e0124326. doi: 10.1371/journal.pone.0124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marston JA. Report on fever (Malta) (Br) Army Med Report for 1861. 1863;3:486–498. [Google Scholar]
- 221.Bruce D. Note on the recovery of a microorganism in Malta fever. Practitioner. 1887;39:161. [Google Scholar]
- 222.Dean AS, Crump L, Greter H. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(12):e1929. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pappas G, Papadimitriou P, Akritidis N. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 224.Vershilova PA. The use of live vaccine for vaccination of human beings against brucellosis in the USSR. Bull World Health Organ. 1961;24:85–89. [PMC free article] [PubMed] [Google Scholar]
- 225.Cutler SJ, Whatmore AM, Commander NJ. Brucellosis: new aspects of an old disease. J Appl Microbiol. 2005;98:1270–1281. doi: 10.1111/j.1365-2672.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 226.Luo D, Ni B, Li P. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp 16 genes of Brucella abortus in BALB/c mice. Infect Immun. 2006;74:2734–2741. doi: 10.1128/IAI.74.5.2734-2741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bhattacharjee AK, Izadjoo MJ, Zollinger WD. Comparison of protective efficacy of subcutaneous versus intranasal immunization of mice with a Brucella melitensis lipopolysaccharide subunit vaccine. Infect Immun. 2006;74:5820–5825. doi: 10.1128/IAI.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM. Brucellosis: the case for live, attenuated vaccines. Vaccine. 2009;27(suppl 4):D40–D43. doi: 10.1016/j.vaccine.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wolfram JH, Kokanov SK, Verkhovsky OA. Diagnostic and vaccine chapter. Vaccine. 2010;28(suppl 5):F49–F53. doi: 10.1016/j.vaccine.2010.04.051. [DOI] [PubMed] [Google Scholar]
- 230.Clapp B, Skyberg JA, Yang X. Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect Immun. 2011;79(10):4165–4174. doi: 10.1128/IAI.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Al-Mariri A, Mahmoud NH, Hammoud R. Efficacy evaluation of live Escherichia coli expression Brucella p39 protein combined with CpG oligodeoxynucleotides vaccine against Brucella melitensis 16M, in BALB/c mice. Biologicals. 2012;40(2):140–145. doi: 10.1016/j.biologicals.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 232.Al-Mariri A, Akel R, Abbady AQ. A DNA vaccine encoding p39 and sp41 of Brucella melitensis induces protective immunity in BALB/c mice. Arch Med Vet. 2014;46(1):53–62. [Google Scholar]
- 233.Jain S, Afley P, Dohre SK. Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine. 2014;32(35):4537–4542. doi: 10.1016/j.vaccine.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 234.Cherwonogrodzky JW, Barabe ND, Grigat ML. Thermostable cross-protective subunit vaccine against Brucella species. Clin Vaccine Immunol. 2014;21(12):1681–1688. doi: 10.1128/CVI.00447-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cassataro J, Estein SM, Pasquevich KA. Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4T helper 1 response that protects against Brucella melitensis infection. Infect Immun. 2005;73:8079–8088. doi: 10.1128/IAI.73.12.8079-8088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cassataro J, Velikovsky CA, de la Barrero S. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun. 2005;73:6537–6546. doi: 10.1128/IAI.73.10.6537-6546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang X, Hudson M, Walters N. Selection of protective epitopes for Brucella melitensis by DNA vaccination. Infect Immun. 2005;73:7297–7303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bhattacharjee AK, Van de Verg L, Izadjoo MJ. Protection of mice against brucellosis by intranasal immunization with Brucella melitensis lipopolysaccharide as a noncovalent complex with Neisseria meningitidis group B outer membrane protein. Infect Immun. 2002;70:3324–3329. doi: 10.1128/IAI.70.7.3324-3329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Siadat SD, Vaziri F, Eftekhary M. Preparation and evaluation of a new lipopolysaccharide-based conjugate as a vaccine candidate for brucellosis. Osong Public Health Res Perspect. 2015;6(1):9–13. doi: 10.1016/j.phrp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vrushabhendrappa S, Amit K, Balakrishna K. Studies on recombinant glucokinase (r-glk) protein of Brucella abortus as a candidate vaccine molecule for brucellosis. Vaccine. 2014;32(43):5600–5606. doi: 10.1016/j.vaccine.2014.07.106. [DOI] [PubMed] [Google Scholar]
- 241.Schaad UB, Lang AB, Wedgwood J. Safety and immunogenicity of Pseudomonas aeruginosa conjugate A vaccine in cystic fibrosis. Lancet. 1991;338:1236–1237. doi: 10.1016/0140-6736(91)92103-9. [DOI] [PubMed] [Google Scholar]
- 242.Zuercher AW, Horn MP, Que JU. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol Med Microbiol. 2006;47:302–308. doi: 10.1111/j.1574-695X.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 243.Zuercher AW, Imboden MA, Jampen S. Cellular immunity in healthy volunteers treated with an octavalent conjugate Pseudomonas aeruginosa vaccine. Clin Exp Immunol. 2006;143:132–138. doi: 10.1111/j.1365-2249.2005.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lang AB, Schaad UB, Rudeberg A. Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Pediatr. 1995;127:711–717. doi: 10.1016/s0022-3476(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 245.Lang AB, Rudeberg A, Schoni MH. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J. 2004;23:504–510. doi: 10.1097/01.inf.0000129688.50588.ac. [DOI] [PubMed] [Google Scholar]
- 246.Lang AB, Metcalfe IC. Proceedings of 25th European Cystic Fibrosis Conference. Monduzzi Editore; Genova, Italy: 2002. Vaccination against Pseudomonas aeruginosa: clinical trial results. June 20–23. [Google Scholar]
- 247.Lang AB, Horn MP, Imboden MA. Prophylaxis and therapy of Pseudomonas aeruginosa infection in cystic fibrosis and immunocompromised patients. Vaccine. 2004;22(suppl 1):S44–S48. doi: 10.1016/j.vaccine.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 248.Doring G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci USA. 2007;104:11020–11025. doi: 10.1073/pnas.0702403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Campodonico VL, Llosa NJ, Grout M. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect Immun. 2010;78:746–755. doi: 10.1128/IAI.00806-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cripps AW, Peek K, Dunkley M. Safety and immunogenicity of an oral inactivated whole-cell Pseudomonas aeruginosa vaccine administered to healthy human subjects. Infect Immun. 2006;74:968–974. doi: 10.1128/IAI.74.2.968-974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pier G. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev Vaccines. 2005;4:645–656. doi: 10.1586/14760584.4.5.645. [DOI] [PubMed] [Google Scholar]
- 252.Sedlak-Weinstein E, Cripps AW, Kyd JM. Pseudomonas aeruginosa: the potential to immunise against infection. Expert Opin Biol Ther. 2005;5:967–982. doi: 10.1517/14712598.5.7.967. [DOI] [PubMed] [Google Scholar]
- 253.Digiandomenico A, Rag J, Harcher K. Intranasal immunization with heterologously expressed polysaccharide protects against multiple Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA. 2007;104:4624–4629. doi: 10.1073/pnas.0608657104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Döring G, Pier GB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine. 2008;26:1011–1024. doi: 10.1016/j.vaccine.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 255.Jiang M, Yao J, Feng G. Protective effect of DNA vaccine encoding Pseudomonas exotoxin A and PcrV against acute pulmonary P. aeruginosa infection. PLoS ONE. 2014;9(5):e96609. doi: 10.1371/journal.pone.0096609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Westritschnig K, Hochreiter R, Wallner G. A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprE/I vaccine (IC43) in healthy volunteers. Hum Vaccin Immunother. 2014;10(1):170–183. doi: 10.4161/hv.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Theilacker C, Coleman FT, Mueschenborn S. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect Immun. 2003;71:3875–3884. doi: 10.1128/IAI.71.7.3875-3884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zaid TS, Priebe GP, Pier GB. A live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect Immun. 2006;74:975–983. doi: 10.1128/IAI.74.2.975-983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Priebe GP, Walsh RL, Cederroth TA. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181:4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bumann D, Behre C, Behre K. Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers. Vaccine. 2010;28:707–713. doi: 10.1016/j.vaccine.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 261.Kamei A, Coutinho-Sledge YS, Goldberg JB. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multi-factorial immunity against Pseudomonas aeruginosa acute lung infection. Infect Immun. 2011;79:1289–1299. doi: 10.1128/IAI.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Grimwood K, Kyd JM, Owen SJ. Vaccination against respiratory Pseudomonas aeruginosa infection. Hum Vaccin Immunother. 2015;11(1):14–20. doi: 10.4161/hv.34296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Farjah A, Owlia P, Siadat SD. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against Pseudomonas aeruginosa. APMIS. 2015;123(2):175–183. doi: 10.1111/apm.12337. [DOI] [PubMed] [Google Scholar]
- 264.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 265.D'Elios MM, Anderson LP. Inflammation, immunity, and vaccines for Helicobacter pylori. Helicobacter. 2009;14(suppl 1):21–28. doi: 10.1111/j.1523-5378.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 266.Del Giudice G, Malfertheiner P, Rappuoli R. Development of vaccines against Helicobacter pylori. Expert Rev Vaccines. 2009;8:1037–1049. doi: 10.1586/erv.09.62. [DOI] [PubMed] [Google Scholar]
- 267.Malfertheiner P, Schultze V, Rosenkranz SH. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135:787–795. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 268.Kotiw M, Johnson M, Pandey M. Immunological response to parenteral vaccination with recombinant hepatitis B virus surface antigen virus-like particles expressing Helicobacter pylori KatA epitopes in a murine H. pylori challenge model. Clin Vaccine Immunol. 2012;19(2):268–276. doi: 10.1128/CVI.05295-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li H-B, Zhang J-Y, He Y-F. Systemic immunization with an epitope-based vaccine elicits a Th1-based response and provides protection against Helicobacter pylori in mice. Vaccine. 2012;31(1):120–126. doi: 10.1016/j.vaccine.2012.10.091. [DOI] [PubMed] [Google Scholar]
- 270.Lv X, Yang J, Song H. Therapeutic efficacy of the multi-epitope vaccine CTB-UE against Helicobacter pylori infection in a Mongolian gerbil model and its microRNA-155-associated immune-protective mechanism. Vaccine. 2014;32(41):5343–5352. doi: 10.1016/j.vaccine.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 271.Rashidi N, Moghim S, Fagheri J. Catalase epitopes vaccine design for Helicobacter pylori: a bioinformatics approach. Afr J Biotechnol. 2011;10(44):8895–8901. [Google Scholar]
- 272.O'Riordan AA, Morales VA, Mulligan L. Alkyl hydroperoxide reductase: a candidate Helicobacter pylori vaccine. Vaccine. 2012;30(26):3876–3884. doi: 10.1016/j.vaccine.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 273.Stent A, Every AL, Ng GZ. Helicobacter pylori thiolperoxidase as a protective antigen in single- and multi-component vaccines. Vaccine. 2012;30(50):7214–7220. doi: 10.1016/j.vaccine.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 274.Arnon SS. Botulism as an intestinal toxemia. In: Blaser MJ, Smith PD, Ravdin JI, editors. Infections of the Intestinal Tract. Raven Press; New York, NY: 1995. pp. 257–271. [Google Scholar]
- 275.Zhang JC, Sun L, Nie QH. Botulism: where are we now? Clin Toxicol (Phila) 2010;48:867–879. doi: 10.3109/15563650.2010.535003. [DOI] [PubMed] [Google Scholar]
- 276.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J Pediatr Ophthalmol Strabismus. 1980;17:21–25. doi: 10.3928/0191-3913-19800101-06. [DOI] [PubMed] [Google Scholar]
- 277.Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev. 1992;56:80–99. doi: 10.1128/mr.56.1.80-99.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jakovic J, Hallet M, editors. Therapy with Botulinum Toxin. Marcel Dekker; New York, NY: 1994. [Google Scholar]
- 279.Baldwin MR, Tepp WH, Przedpelski A. Subunit vaccine against the seven serotypes of botulism. Infect Immun. 2008;76:1314–1318. doi: 10.1128/IAI.01025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shone C, Agostini H, Clancy J. Bivalent recombinant vaccine for botulinum neurotoxin types A and B based on a polypeptide comprising their effector and translocation domains that is protective against the predominant A and B subtypes. Infect Immun. 2009;77:2795–2801. doi: 10.1128/IAI.01252-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu YZ, Li N, Zhu HQ. The recombinant Hc subunit of Clostridium botulinum neurotoxin serotype A is an effective botulism vaccine candidate. Vaccine. 2009;27:2816–2822. doi: 10.1016/j.vaccine.2009.02.091. [DOI] [PubMed] [Google Scholar]
- 282.Zichel R, Mimran A, Keren A. Efficacy of a potential trivalent vaccine based on Hc fragments of botulinum toxins A, B, and E produced in a cell-free expression system. Clin Vaccine Immunol. 2010;17:784–792. doi: 10.1128/CVI.00496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Smith LA. Botulism and vaccines for its prevention. Vaccine. 2009;27(suppl 4):D33–D39. doi: 10.1016/j.vaccine.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 284.Xu Q, Pichichero ME, Simpson LL. An adenoviral vector-based mucosal vaccine is effective in protection against botulism. Gene Ther. 2009;16:367–375. doi: 10.1038/gt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen S, Xu Q, Zeng M. Oral vaccination with an adenovirus-vectored vaccine protects against botulism. Vaccine. 2013;31(7):1009–1011. doi: 10.1016/j.vaccine.2012.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Webb RP, Smith LA. What next for botulism vaccine development? Expert Rev Vaccines. 2013;12(5):481–492. doi: 10.1586/erv.13.37. [DOI] [PubMed] [Google Scholar]
- 287.Smith LA, Rusnak JM. Botulinum neurotoxin vaccines: past, present, and future. Crit Rev Immunol. 2007;27:303–318. doi: 10.1615/critrevimmunol.v27.i4.20. [DOI] [PubMed] [Google Scholar]
- 288.US Food and Drug Administration FDA approves first botulism antitoxin for use in neutralizing all seven known botulinum nerve toxin serotypes: product to be stored in Strategic National Stockpile for emergency preparedness and response [news release] 2013. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345128.htm 22 March.
- 289.Cangene Corporation . Cangene Corporation; Winnipe.g., MB, Canada: revised Mar 2013. BAT, botulism antitoxin heptavalent (A, B, C, D, E, F, G)–(equine) [package insert] [Google Scholar]
- 290.Arnon SS, Schechter R, Maslanka SE. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354:462–471. doi: 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 291.Kim J, Smathers SA, Prasad P. Epidemiological features of Clostridium difficile-associated disease among inpatients of children's hospitals in the United States, 2001–2006. Pediatrics. 2008;122:1266–1270. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 292.Bouza E, Martin A, Van den Berg RJ. Laboratory-acquired clostridium difficile polymerase chain reaction ribotype 027: a new risk. Clin Infect Dis. 2008;47(11):1493–1494. doi: 10.1086/593109. [DOI] [PubMed] [Google Scholar]
- 293.Carroll KC, Bartlett JB. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501–521. doi: 10.1146/annurev-micro-090110-102824. [DOI] [PubMed] [Google Scholar]
- 294.Kaslow DC, Shiver JW. Clostridium difficile and methicillin-resistant Staphylococcus aureus: emerging concepts in vaccine development. Annu Rev Med. 2011;62:201–215. doi: 10.1146/annurev-med-051109-101544. [DOI] [PubMed] [Google Scholar]
- 295.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Leav BA, Blair B, Leney M. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–969. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 297.Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine. 2004;22:848–856. doi: 10.1016/j.vaccine.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 298.Babcock GJ, Broering TJ, Hernandez HJ. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile–induced mortality in hamsters. Infect Immun. 2006;67:527–538. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lowy I, Molrine DC, Leav BA. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 300.Aboudola S, Kotloff KL, Kyne L. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003;71:1608–1610. doi: 10.1128/IAI.71.3.1608-1610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Foglia G, Shah S, Luxemburger C. 2011. Clostridium difficile: development of a novel candidate vaccine. Presented at 5th Semmering Vaccine Symposium; Vienna, Austria; April, 28–30. [DOI] [PubMed] [Google Scholar]
- Sheldon E, Kitchin N, Peng Y. A phase 1, placebo-controlled, randomized study of the safety, tolerability and immunogenicity of a Clostridium difficile vaccine administered with or without aluminum hydroxide in healthy adults. Vaccine. 2016;34:2082–2091. doi: 10.1016/j.vaccine.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 302.Kyne L, Warny M, Qamar A. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;346:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 303.Kyne L, Warny M, Qamar A. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 304.Sougioultzis S, Kotloff KL, Drudy D. Clostridium difficile toxoid vaccine in recurrent C. difficile–associated diarrhea. Gastroenterology. 2005;128:764–770. doi: 10.1053/j.gastro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 305.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 306.de Bruyn G, Saleh J, Workman D. Defining the optimal formulation and schedule of a candidate toxoid vaccine against Clostridium difficile infection: a randomized Phase 2 clinical trial. Vaccine. 2016;34:2170–2178. doi: 10.1016/j.vaccine.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 307.Péchiné S, Denève C, Le Monnier A. Immunization of hamsters against Clostridium difficile infection using the Cwp84 protease as an antigen. FEMS Immunol Med Microbiol. 2011;63:73–81. doi: 10.1111/j.1574-695X.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 308.Janoir C, Pechine S, Grosdidier C. Cwp84, a surface-associated protein of Clostridium difficile, is a cysteine protease with degrading activity on extracellular matrix proteins. J Bacteriol. 2007;189(20):7174–7180. doi: 10.1128/JB.00578-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Oberli MA, Hecht ML, Bindschadler P. A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem Biol. 2011;18:580–588. doi: 10.1016/j.chembiol.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 310.Baliban SM, Michael A, Shammassian B. An optimized, synthetic DNA vaccine encoding the toxin A and toxin B receptor binding domains of Clostridium difficile induces protective antibody responses in vivo. Infect Immun. 2014;82(10):4080–4091. doi: 10.1128/IAI.01950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.McClain DJ, Pittman PR, Ramsburg HH. Immunologic interference from sequential administration of live attenuated alphavirus vaccines. J Infect Dis. 1998;177:634–641. doi: 10.1086/514240. [DOI] [PubMed] [Google Scholar]
- 312.Waag DM, England MJ, Bolt CR. Low-dose priming before vaccination with the phase I chloroform-methanol residue vaccine against Q fever enhances humoral and cellular immune responses to Coxiella burnetii. Clin Vaccine Immunol. 2008;15(10):1505–1512. doi: 10.1128/CVI.00119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ambrosio A, Saavedra M, Mariana M. Argentine hemorrhagic fever vaccines. Hum Vaccin. 2011;7(6):694–700. doi: 10.4161/hv.7.6.15198. [DOI] [PubMed] [Google Scholar]
- 314.Hoke CH, Jr, Pace-Templeton J, Pittman P. US military contributions to the global response to pandemic chikungunya. Vaccine. 2012;30:6713–6720. doi: 10.1016/j.vaccine.2012.08.025. [DOI] [PubMed] [Google Scholar]


