Introduction
Influenza viruses remain the most frequently identified causes of viral infection in the lung, and respiratory syncytial virus (RSV) remains a major cause of severe pneumonia in children worldwide and is an increasingly recognized cause of illness in adults and elderly patients. Other agents, such as rhinovirus (RV), have been increasingly detected mirroring advances in molecular and serologic diagnostic techniques and increased surveillance programs. Nonetheless, the diversity of viral agents that cause pulmonary disease is extremely broad and continues to expand (Table 13.1 ). Several newly recognized viral pathogens have been identified in the past few decades that are among the most feared and lethal of all emerging infections, including those caused by hantaviruses, Nipah virus, pandemic influenza A (H1N1), and severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses. Conversely, certain viral infections, particularly those that occur in vulnerable patient cohorts, have diminished during this same interval. For example, the U.S. incidence of varicella pneumonia has declined more than 65% since universal childhood vaccination for varicella was implemented in 1995, and advances in the clinical management of transplant recipients have reduced the incidence of cytomegalovirus (CMV) pneumonia. This chapter captures and describes select viral diseases in which the pulmonary system is a main target of infection, although it is important to note that other viral agents have been detected in pulmonary tissue, such as chikungunya virus, West Nile virus, rabies virus, and Heartland virus, typically in the context of systemic disease.
TABLE 13.1.
Viral Infections in the Lung
| Family/Agents | Virus |
|---|---|
| Adenoviridae | Adenovirus |
| Bunyaviridae | Hantavirus |
| Coronaviridae | SARS coronavirus MERS coronavirus 229E, NL63 |
| Herpesviridae | Cytomegalovirus Herpes simplex Varicella zoster |
| Orthomyxoviridae | Influenza |
| Paramyxoviridae | Measles virus Parainfluenza virus Respiratory syncytial virus Human metapneumovirus Nipah virus Hendra virus |
| Picornaviridae | EnterovirusesRhinoviruses |
| Viral hemorrhagic fevers | Arenaviruses, bunyaviruses, flaviviruses, and filoviruses |
Adenoviruses
Adenovirus Pneumonia—Fact Sheet.
Definition
-
▪
Pulmonary infections caused by nonenveloped double-stranded DNA viruses in the family Adenoviridae
Incidence
-
▪
Approximately 5% to 10% of all pneumonias in infants and young children
-
▪
Periodic epidemics of adenovirus pneumonia in young adults occur, particularly among military recruits
-
▪
Immunocompromised adults, especially transplant recipients, are vulnerable to severe and sometimes fatal pneumonias caused by adenoviruses
Morbidity and Mortality
-
▪
In immunocompromised patients, the case fatality rate of adenoviral pneumonia approaches 60%, versus an approximately 15% mortality in immunocompetent patients
-
▪
Immunocompromised and young patients can develop disseminated disease
-
▪
Obliterative bronchiolitis is a potential cause of long-term morbidity in some survivors of the infection; SJMS is rare complication
Gender, Race, and Age Distribution
-
▪
Most pediatric cases of adenovirus pneumonia occur between 6 months and 5 years of age and are caused by serotypes 3, 7, and 21
-
▪
Causes severe disease in neonates that may lead to long-term complications
-
▪
In adults, pneumonia is generally associated with serotypes 3, 4, and 7 with reemergence of serotypes 14 and 55 in certain cohorts
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Acute upper respiratory tract disease is manifested by tracheobronchitis
-
▪
Adenovirus pneumonia presents with signs and symptoms similar to other types of pneumonia, including fever, cough, and chest pain
Radiologic Features
-
▪
Bilateral, multifocal, lobar, or segmental consolidations; bronchial wall thickening; hyperaeration; and lobar atelectasis
-
▪
Pleural effusions and pneumatoceles are reported less frequently
Prognosis and Therapy
-
▪
No proven effective antiviral therapy
-
▪
Most patients receive only supportive care for symptoms of the disease
-
▪
Severe infections may progress to death in 2 to 3 weeks
Adenovirus Pneumonia—Pathologic Features.
Gross Findings
-
▪
Lungs are typically heavy and edematous with consolidation and regions of hemorrhage
-
▪
Bronchi are generally filled with mucoid, fibrinous, or purulent exudates and have hemorrhagic and congested mucosae
-
▪
Necrotic and inflammatory foci in the pulmonary parenchyma may be represented by palpable yellow nodules
Microscopic Findings
-
▪
Necrotizing tracheobronchitis and bronchiolitis with extensive denudation of the surface epithelium, particularly in medium-sized (1–2 mm in diameter) bronchi
-
▪
Affected airways may be occluded by homogeneous eosinophilic material, mixed inflammatory cells, detached epithelium, and cellular debris
-
▪
Tracheal and bronchial serous and mucous glands are also often involved and show necrosis and mixed inflammatory infiltrates
-
▪
Bronchocentric parenchymal necrosis with hemorrhage, neutrophilic and mononuclear cell infiltrates, against a background of exudative diffuse alveolar damage
-
▪Intranuclear inclusions in respiratory epithelial cells and alveolar pneumocytes, generally most abundant at the viable edges of necrotic foci
-
–Early inclusions appear as small, dense, amphophilic structures surrounded by a cleared zone and peripherally marginated chromatin, similar to herpetic inclusions
-
–Mature inclusions are larger and more basophilic, and the margins of the nuclear membrane become blurred to form the characteristic “smudge cell”
-
–
Immunohistochemical Features
-
▪
IHC stains the intranuclear accumulations of viral antigen and commercial antibodies detect multiple serotypes
Ultrastructural Features
-
▪
Intranuclear paracrystalline array of virions represented by icosahedral capsids that measure 70 to 90 nm in diameter
Pathologic Differential Diagnosis
-
▪
Herpes simplex viruses
-
▪
VZV
-
▪
CMV
Adenoviruses are nonenveloped double-stranded DNA viruses represented by a ubiquitous and diverse group of at least 51 serotypes that are found naturally in the upper respiratory tracts and gastrointestinal systems of humans, other mammals, and birds. More than 50% of the known adenovirus serotypes are associated with human diseases and encompass a wide range of presentations, with infections targeting a wide variety of organ systems. The others are rarely encountered and may or may not cause recognizable disease.
Clinical Features
It is estimated that approximately 5% to 10% of all pneumonias in infants and young children are caused by adenoviruses. Most pediatric cases of adenovirus pneumonia occur between 6 months and 5 years of age and are caused by serotypes 3, 7, and 21, with serotypes 3 and 7 being particularly pathogenic adenoviruses with the ability to result in disseminated and often fatal disease in previously healthy children. The majority of infections in pediatric populations spontaneously resolve and often do not result in clinically apparent illness. In adults, pneumonia is generally associated with serotypes 3, 4, and 7. Periodic epidemics of adenovirus pneumonia in immunocompetent adults have been identified, such as with the reemergence of adenovirus subtypes 14 and 55 that have been associated with severe disease in military camps, hospitals, and schools in the United States, Europe, and Asia. In a manner similar to other pathogens, adenoviruses take advantage of impaired or destroyed immune systems to establish persistent and disseminated infections in immunocompromised hosts, a population that is susceptible to a broader range of different serotypes. Because some adenoviruses establish latency in lymphoid tissues and the kidneys of their host, it is believed that many, possibly most, cases of clinical disease caused by adenoviruses in immunocompromised patients are reactivated infections.
Radiologic Features
Chest films typically show bilateral, multifocal, lobar, or segmental consolidations; bronchial wall thickening; hyperaeration; and lobar atelectasis. Pleural effusions and pneumatoceles are reported less frequently.
Pathologic Features
Gross Findings
The lungs of patients with adenovirus pneumonia are heavy and edematous with areas of consolidation, hemorrhage, and well-demarcated yellow foci representing necrotic and inflamed pulmonary parenchyma. The mucosae of the large airways are generally hemorrhagic and congested, and bronchi are filled with mucoid or fibrinopurulent exudates.
Microscopic Findings
The primary histopathologic findings include necrotizing tracheobronchitis and bronchiolitis with extensively denuded epithelium, particularly in medium-sized (1–2 mm in diameter) intrapulmonary bronchi (Fig. 13.1A ). Affected airways may be occluded by homogeneous eosinophilic material, mixed inflammatory cells, detached epithelium, and cellular debris. The lamina propria of bronchi and bronchioles is typically congested and infiltrated by predominantly mononuclear inflammatory cell infiltrates. Tracheal and bronchial serous and mucous glands are also often involved and show necrosis and mixed inflammatory infiltrates (Fig. 13.1C). As the infection progresses, there is involvement of the more distal pulmonary parenchyma, forming foci of bronchocentric necrosis with hemorrhage, neutrophilic and mononuclear cell infiltrates, and karyorrhexis (Fig. 13.1B). These findings occur against a background of diffuse alveolar damage. Adenoviruses form intranuclear inclusions in respiratory epithelial cells of the trachea, bronchi, and bronchioles; in the acinar cells of bronchial glands; and in alveolar pneumocytes and are generally most abundant at the viable edges of necrotic foci. On hematoxylin-eosin stain, early inclusions appear as small, dense, amphophilic structures surrounded by a cleared zone and peripherally marginated chromatin, similar to herpetic inclusions. As the cellular infection progresses, the inclusion becomes larger (as large as 14 microns in some cells) and more basophilic, and the margins of the nuclear membrane become blurred to form the characteristic “smudge cell” (Fig. 13.1C and D). Concurrent bacterial, fungal, and viral infections are common.
FIG. 13.1.

Adenovirus pneumonia. (A and B) Characteristic necrotizing bronchiolitis, characterized by necrosis of respiratory epithelium and filling of conducting ways by necrotic cell debris, fibrin, and mixed inflammatory cells. Note generalized congestion and filling of adjacent alveolar spaces by necrotic and inflammatory cell debris. (C) Necrosis of tracheal mucous glands and numerous large, basophilic, intranuclear inclusions (arrows). (D) Numerous intranuclear inclusions (arrows) in alveolar pneumocytes, forming characteristic “smudge cells” that can be observed in advanced adenovirus infections. (E) Immunohistochemical localization of adenovirus-infected cells in the pulmonary parenchyma of patient with fatal adenovirus pneumonia. (F) Paracrystalline arrays of 70- to 90-nm adenovirus particles in the nucleus of an infected pneumocyte.
(F, courtesy of C.S. Goldsmith.)
Ancillary Studies
Various methods can be used to diagnose adenovirus infections, including antigen detection (fluorescence antibody assays and enzyme immunoassays [EIAs]), cell culture, electron microscopy, molecular assays, and serologic testing for group-specific or type-specific antibodies. Immunohistochemical (IHC) staining methods can detect adenovirus-infected cells in formalin-fixed, paraffin-embedded tissues using various commercially available adenovirus group–specific antibodies (Fig. 13.1E). Electron microscopy of adenovirus-infected tissues reveals a paracrystalline array of virions represented by icosahedral capsids that measure 70 to 90 nm in diameter (Fig. 13.1F). Most adenoviruses can be isolated in cell culture from bronchial washings, tracheal aspirates, or lung biopsy specimens during the early stage of the illness. Molecular assays, particularly gene amplification using polymerase chain reaction (PCR) and in situ hybridization (ISH) methods, have been developed to detect adenovirus nucleic acid in respiratory secretions and in formalin-fixed, paraffin-embedded tissues.
Differential Diagnosis
The differential diagnosis includes those agents that cause necrotizing bronchiolitis, pneumonia, and intranuclear viral inclusions, particularly herpes simplex viruses (HSV), varicella zoster virus (VZV), and CMV. Histologic clues to distinguish these agents from adenovirus include the presence (HSV and VZV) or absence (adenovirus) of multinucleated cells, cytoplasmic inclusions (CMV), or distinctive smudge cells (adenovirus); however, ancillary studies are generally required for confirmation.
Prognosis and Therapy
In immunocompromised patients, the case fatality rate of adenoviral pneumonia approaches 60%, compared with an approximately 15% mortality in immunocompetent patients. There is no proven effective antiviral therapy for adenovirus infections, though some studies report positive outcomes with treatment with cidofovir. Most patients receive only supportive care for symptoms of the disease, which includes cessation of immune-suppressing drugs in those patients with iatrogenic immunosuppression. Chronic lung disease, such as bronchiectasis and organizing pneumonia, are reported long-term sequelae. A rare complication, Swyer-James-Macleod, or single-sided clear lung, syndrome (SJMS), frequently occurs in infancy and in childhood, developing secondarily to bronchiolitis obliterans after adenovirus infections.
Hantaviruses
Hantaviruses—Fact Sheet.
Definition
-
▪
Hantaviral diseases are caused by closely related, trisegmented, negative-sense RNA viruses of the genus Hantavirus, of the family Bunyaviridae
-
▪
Two classes of hantavirus-associated illnesses have been described: HFRS for disease in which the kidneys are primarily involved and HPS for disease in which the lungs are primarily affected
Incidence and Location
-
▪
Zoonotic viruses maintained in nature by asymptomatic infection of rodents
-
▪
Transmission to humans is usually associated with exposure to rodents in and around the home, performing agricultural activities, cleaning animal sheds, sleeping on the ground, and with certain occupations
-
▪
Serotypes are distributed throughout the world
-
▪
HFRS is more common in Europe and Asia, whereas HPS is almost exclusively seen in the Americas
-
▪
Rare cause of pneumonia
Morbidity and Mortality
-
▪
In HFRS, mortality rates range from 1% to 15%
-
▪
In HPS, mortality rates may exceed 50%
-
▪
In survivors of HFRS, recovery is usually complete, with no long-term sequelae
Gender, Race, and Age Distribution
-
▪
No specific gender, race, or age distribution is generally seen
Clinical Features
-
▪
Prodrome of fever, myalgias, headache, vomiting, weakness, and cough is common in both HFRS and HPS
-
▪
Renal involvement is seen in all cases of HFRS, and the clinical presentation ranges from a mild illness with minimal renal dysfunction to a more severe form with acute renal failure and shock
-
▪
In HPS, the prodrome is followed by rapidly progressive pulmonary edema, respiratory insufficiency, and shock
Radiologic Features
-
▪
Interstitial edema without consolidation in the majority of cases within 48 hours of hospitalization
-
▪
Pleural effusions are very common
Prognosis and Therapy
-
▪
Supportive therapy, such as dialysis and circulatory and respiratory support
-
▪
Ribavirin is effective in treatment of HFRS but not HPS
Hantaviruses—Pathologic Features.
Gross Findings
-
▪
Severe HFRS: gelatinous retroperitoneal fluid accumulation and a distinctive triad of hemorrhagic necrosis of the junctional zone of the renal medulla, right atrium of the heart, and anterior pituitary
-
▪
HPS: large bilateral pleural effusions and heavy edematous lungs
Microscopic Findings
-
▪
HFRS: the most severe and characteristic microscopic lesions involve the kidney
-
▪HPS:
-
–Lungs: an interstitial pneumonitis is seen in most cases, characterized by edema and an interstitial mononuclear cell infiltrate, with variable hyaline membranes and diffuse alveolar damage with chronicity
-
–Extrapulmonary: immunoblasts in spleen, lymph nodes, and peripheral blood
-
–
Immunohistochemical Features
-
▪
IHC testing of formalin-fixed tissues is a sensitive method to confirm infection
-
▪
Hantaviral antigens are most commonly detected in endothelial cells of involved organs in HPS and HFRS
Ultrastructural Features
-
▪
Virus particles are 70 to 120 nm in diameter and generally appear spherical to oval in shape
-
▪
A lipid envelope containing glycoprotein spikes surrounds a core consisting of the genome nucleocapsids arranged in delicate tangles of filaments
-
▪
Granulofilamentous viral inclusions can be seen in endothelial cells
-
▪
Viral particles can be extremely difficult to visualize in tissues
Pathologic Differential Diagnosis
-
▪
Histopathologic and hematologic findings suggest the diagnosis in HPS and HFRS; however, laboratory confirmation is essential for confirmation of the diagnosis
-
▪
Differential diagnosis includes a large number of viral, rickettsial, and bacterial infections, as well as noninfectious diseases
Hantaviral diseases are zoonotic diseases caused by a group of closely related, trisegmented, single-stranded, negative-sense RNA viruses of the genus Hantavirus, of the family Bunyaviridae. Several genotypes have been identified and are associated with different host-adapted rodent reservoirs, and the primary means of infection is through inhalation of rodent secretions or excreta. Two classes of hantavirus-associated illnesses have been described: hemorrhagic fever renal syndrome (HFRS), for disease in which the kidneys are primarily involved, and hantavirus pulmonary syndrome (HPS), also known as hantavirus cardiopulmonary syndrome, for disease in which the lungs are primarily affected. In the United States infection primarily occurs in May to July and primarily in the southwestern states.
Clinical Features
The initial symptoms of HFRS and HPS are similar and resemble those seen in early phases of many other viral diseases. Fever, myalgia, headache, vomiting, weakness, and cough are common symptoms in early phases of both HFRS and HPS. Renal involvement is seen in all cases of HFRS, and the clinical presentation ranges from a mild illness with minimal renal dysfunction to a more severe form with acute renal failure and shock. Only HFRS patients who die during the later phases of renal failure typically show significant pulmonary edema. The clinical picture for HPS is quite different from that for HFRS. The initial prodrome is followed by rapidly progressive pulmonary edema, respiratory insufficiency, and shock. In fatal cases, the majority of deaths occur within 6.4 days of hospitalization. Hemorrhages and peripheral signs of vasomotor instability, such as flushing, conjunctival injection, and periorbital edema as seen in HFRS, are extremely rare. Hematologic features on peripheral blood smears, including neutrophils without significant toxic change, myelocytosis, increased circulating immunoblastic lymphocytes, thrombocytopenia, and hemoconcentration, in combination with acute pulmonary edema, is very suggestive of a presumptive diagnosis of hantavirus infection.
Radiologic Features
Chest radiographs may be normal early in the course of HPS, but evidence of bilateral interstitial edema can be observed in the majority of cases within 48 hours of hospitalization and may resembled acute respiratory distress syndrome (ARDS).
Pathologic Features
Gross Findings
Large quantities of protein-rich, gelatinous retroperitoneal edema fluid are found in the hypotensive phase of severe HFRS, whereas all HPS patients have large bilateral pleural effusions and heavy, edematous lungs. In fatal Far Eastern HFRS, a distinctive triad of hemorrhagic necrosis of the junctional zone of the renal medulla, right atrium of the heart, and anterior pituitary can be seen. In patients with HPS, hemorrhages are exceedingly rare, and ischemic necrotic lesions, except those attributed to shock, are not seen.
Microscopic Findings
Histologically, morphologic changes of the endothelium are uncommon but, when seen, consist of prominent and swollen endothelial cells. Vascular thrombi and endothelial cell necrosis are rare. In HFRS, the most severe and characteristic microscopic lesions involve the kidney and include microvascular inflammation, medullary interstitial hemorrhages, and acute tubular necrosis; however, an interstitial pneumonitis can also be seen in some fatal cases. In contrast, the microscopic changes in HPS are principally seen in the lung and spleen. The lungs show a mild-to-moderate interstitial pneumonitis with interstitial mononuclear cells composed of a mixture of small and enlarged mononuclear cells with the appearance of immunoblasts and variable degrees of edema (Fig. 13.2A ). Focal hyaline membranes composed of condensed proteinaceous intraalveolar edema fluid, fibrin, and variable numbers of inflammatory cells are observed. Typically, neutrophils are scant, and the alveolar pneumocytes are intact with no evidence of cellular debris, nuclear fragmentation, or hyperplasia. In fatal cases, with a prolonged survival interval, tissues show features more characteristic of the exudative and proliferative stages of diffuse alveolar damage (Fig. 13.2B). Other characteristic microscopic findings in HPS cases include variable numbers of immunoblasts within the splenic red pulp and periarteriolar white pulp (Fig. 13.2E), lymph nodal paracortical zones, hepatic portal triads, and peripheral blood.
FIG. 13.2.

Hantavirus pulmonary syndrome (HPS). (A) Lung showing mononuclear interstitial pneumonitis and intraalveolar edema in a typical case of HPS. (B) Type II pneumocyte hyperplasia in the alveoli of an HPS patient as seen in patients with a prolonged clinical course. (C) Widespread immunostaining of hantaviral antigens in pulmonary microvasculature of an HPS patient. (D) Ultrastructural appearance of a typical granulofilamentous hantavirus inclusion within pulmonary capillary endothelium. (E) Spleen from a fatal case in which immunoblasts are seen in the periarteriolar sheath. Note prominent nucleoli and high nuclear-to-cytoplasmic ratio.
(D, courtesy of C.S. Goldsmith.)
Ancillary Studies
Virus-specific diagnosis and confirmation can be achieved through serology, PCR for hantavirus RNA, or IHC for hantaviral antigens. Serologic testing can detect hantavirus-specific immunoglobulin M or rising titers of immunoglobulin G in patient sera and is considered the method of choice for laboratory confirmation of HPS. PCR detects viral RNA in blood and tissues and is extremely useful for diagnostic and epidemiologic purposes. Hantaviral RNA can also be detected in formalin-fixed, paraffin-embedded archival tissues by reverse transcription (RT) PCR. IHC testing of formalin-fixed tissues is a sensitive method to confirm hantaviral infections, and viral antigens are found primarily within capillary endothelium throughout various tissues in both HPS and HFRS (Fig. 13.2C). In HPS, marked accumulations of hantaviral antigens are found in the pulmonary microvasculature and in splenic and lymph nodal follicular dendritic cells. Electron microscopic studies of HPS lung tissue demonstrate infection of endothelial cells and macrophages. The virus or viruslike particles observed are infrequent and extremely difficult to identify in autopsy tissues; in contrast, typical endothelial granulofilamentous inclusions are seen more frequently (Fig. 13.2D).
Differential Diagnosis
HPS should be suspected in cases of ARDS without a known precipitating cause among previously healthy individuals. The level of suspicion should be particularly high when patients have a known exposure to rodents in areas where deer mice (Peromyscus maniculatus) or other reservoirs of hantavirus are found. Physicians need to differentiate HPS from other common acute respiratory diseases, such as pneumococcal pneumonia, influenza virus, and unexplained ARDS. Diseases that need to be distinguished pathologically from HPS include a relatively large number of different viral, rickettsial, and bacterial infections, as well as various noninfectious disease processes.
Prognosis and Therapy
Recovery in HFRS is usually complete, with no apparent long-term sequelae. Mortality rates for HFRS range from 1% to 15%, with shock and uremia being the main contributing causes of death, although pulmonary edema has been implicated in some patients. In one study accounting for confirmed HPS cases in the United States from 1993 to 2009 the case-fatality rate was 35% though mortality rates may exceed 50% depending on the serotype involved. Though similar biochemical alterations occur, those patients with the lowest thrombocytopenia (median 33,500 cells/mL), as well as increased hematocrits, creatinine levels, and leukocyte counts, occurred more often in fatal HPS cases. The need for supplementary oxygen and intubation has also been associated with a poor outcome. Management of patients with HPS or HFRS is often complex and phase specific. Supportive therapy, such as dialysis and circulatory and respiratory support, is the basis of treatment.
Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome Coronaviruses
SARS and MERS Coronaviruses—Fact Sheet.
Definition
-
▪
SARS and MERS coronaviruses are enveloped, positive-stranded RNA viruses that are members of the genus Coronavirus, of the family Coronaviridae
Incidence and Location
-
▪
SARS was first reported in Guangdong Province in southern China in 2002, but rapidly spread to become a worldwide illness in 2003
-
▪
MERS was first reported in Saudi Arabia in 2012, with the majority of cases occurring throughout the Arabian Peninsula
Morbidity and Mortality
-
▪
SARS is fatal in about 5% to 10% of patients, and MERS is fatal in about 35% of patients
-
▪
In patients who survive the illness, the recovery is usually complete
-
▪
Mortality and risk of complications are higher among elderly persons and persons of any age with certain underlying health conditions
-
▪
Secondary bacterial pneumonias with organisms may occur as a complication
Gender, Race, and Age distribution
-
▪
People of all ages are vulnerable
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Spread is primarily person to person through the coughing and sneezing of infected persons
-
▪
Estimated incubation period is 2 to 14 days
-
▪
Uncomplicated illness is an influenza-like illness characterized by an abrupt onset of fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis
Radiologic Features
-
▪
Peripheral lung opacities with lower lobe predominance and a mixture of ground-glass opacities, interstitial thickening, and bronchiectasis or air bronchograms
-
▪
CT at the time of diagnosis shows multifocal peripheral subpleural ground-glass opacities and consolidation
-
▪
Pneumomediastinum can be seen later in the disease
Prognosis and Therapy
-
▪
Supportive respiratory and intensive care therapy
-
▪
Inconclusive studies regarding the efficacy of antivirals
-
▪
Specific antibiotic therapy in cases with secondary bacterial infection
-
▪
Prevention of nosocomial transmission is an important strategy for management of cases in a hospital setting
SARS and MERS Coronaviruses—Pathologic Features.
Gross Findings
-
▪
Lungs are usually heavy and edematous with varying degrees of red and gray hepatization and consolidation
-
▪
Multiple bilateral pulmonary hemorrhagic infarcts and subpleural hemorrhages can be seen with SARS
Microscopic Findings
-
▪
Diffuse alveolar damage
-
▪
Hemorrhage
-
▪
Edema
-
▪
Multinucleated cells in about 10% of cases
-
▪
Viral inclusions cannot be identified by light microscopy
Immunohistochemical Features
-
▪
IHC reveals SARS antigens primarily in the respiratory epithelial cells of airways and pneumocytes, particularly in patients who die within the first 2 weeks of onset of the illness
-
▪
IHC reveals MERS antigens primarily in pneumocytes, syncytial cells, and bronchial glands
Ultrastructural Features
-
▪
In SARS virions form by alignment of the helical nucleocapsids along the membranes of the endoplasmic reticulum or Golgi complex and acquire an envelope by budding into the cisternae
-
▪
Cellular vesicles become filled with virions and progress to the cell surface for release of viral particles
-
▪
Negative stains reveal particles averaging 80 to 100 nm in size with a characteristic crownlike fringe on the surface
Pathologic Differential Diagnosis
-
▪
Other causes of diffuse alveolar damage, including many viral, rickettsial, and bacterial infections, as well as noninfectious diseases (trauma, drugs, and toxins)
Coronaviruses are enveloped, positive-stranded RNA viruses belonging to the Coronaviridae family. Six coronaviruses are known to infect people and include alpha coronaviruses 229E and NL63 and beta coronaviruses OC43, HKU1, SARS-CoV, and MERS-CoV. Until the emergence of SARS and MERS, coronaviral infection was typically associated with mild self-limiting upper respiratory tract illness, though rare reported cases of severe pneumonia associated with coronavirus exist, particularly in immunocompromised patients.
SARS was recognized during a global outbreak of severe pneumonia that began in late 2002 in Guangdong Province, China, and gained prominence in early 2003 as cases were identified in Asia, Europe, and in North and South America. SARS was contained in 2003, with the last reported cases occurring as laboratory-acquired cases in 2004. MERS was first detected in Saudi Arabia in 2012 with most cases reported in the Arabian Peninsula, and numerous travel-associated cases and health care–associated outbreaks reported, the largest being in the Republic of Korea in 2015. The animal reservoir of SARS and MERS is uncertain, though bats have been suspected to play a role. It appears likely that civet cats and other small mammals play a role as amplifier hosts within animal markets for transmission of SARS, and there is very strong evidence linking dromedary camels and camel-related products to transmission of MERS to humans.
Clinical Features
SARS and MERS cause a spectrum of illness ranging from asymptomatic infection, to an influenza-like illness that typically presents with acute onset of fever, myalgia, malaise, and chills, to respiratory failure, shock, multiorgan failure, and death. Other symptoms can include sore throat, nausea and vomiting, and diarrhea. Infection can occur in people of all ages. In SARS children tend to have a much milder clinical course than adults, and in MERS severe disease and death occur most frequently in patients with chronic comorbidities. The estimated incubation period is 2 to 14 days, and transmission is thought to primarily occur by person-to-person contact, particularly with respiratory secretions.
Radiologic Features
The radiologic features of SARS include the peripheral appearance of lung opacities; lower lobe predominance; and a mixture of ground-glass opacities, interstitial thickening, and bronchiectasis. Pneumomediastinum without preceding positive-pressure ventilation or intubation can be seen later in the disease. Multifocal peripheral subpleural ground-glass opacification or consolidation has been the most commonly observed computed tomographic (CT) feature at the time of diagnosis in patients with SARS. In MERS radiologic features include progressive bilateral pulmonary opacities, including ground-glass and alveolar-interstitial infiltrates with consolidation and air bronchograms, and peripheral interstitial infiltrates and lower lobe consolidation on CT.
Pathologic Features
Gross Findings
In fatal cases of SARS, the lungs are usually heavy and edematous with varying degrees of red and gray hepatization. Multiple bilateral hemorrhagic infarcts are commonly seen in association with subpleural hemorrhages. Autopsy results have only been reported in a single case with fatal MERS infection. In that case gross examination revealed abundant pleural effusion and significant pericardial and abdominal effusion, edematous and consolidate pulmonary parenchyma, and congestion.
Microscopic Findings
The main histopathologic pattern is diffuse alveolar damage (Fig. 13.4A and B). Increased mononuclear cell infiltrates in the interstitium can be seen in some cases of SARS (Fig. 13.3A ), and mixed inflammation edema was noted within the septa from a fatal MERS case. Other findings identified in some patients include focal intraalveolar hemorrhage, necrotic inflammatory debris in small airways, and organizing pneumonia, as well as type II pneumocyte hyperplasia, intraalveolar fibrin, and necrosis of alveoli and submucosal glands of conducting airways (Fig. 13.4C ). In addition, multinucleated syncytial cells without inclusion formation may be seen in the alveolar spaces of some fatal SARS cases in patients who die 14 days or more after onset of illness, and were also noted in pulmonary tissues from the MERS fatality (Fig. 13.3B).
FIG. 13.4.

Middle East respiratory syndrome coronavirus (MERS). (A and B) Extensive edema (A) and exudative-phase diffuse alveolar damage with hyaline membrane formation, fibrin, type II pneumocyte hyperplasia, and variable septal expansion by mixed inflammatory cells in pulmonary tissue from a fatal MERS case. (C) Lymphocytic inflammation in the submucosal glands of a bronchus with a large region of glandular necrosis. (D) Immunohistochemistry of the same bronchial gland highlights numerous viral antigens within the necrotic focus. (E and F) Viral antigen within pneumocytes (E) and a multinucleated epithelial syncytial cell (F). (G) Pleomorphic spherical viral particles are seen in this thin section electron microscopic image.
(G, courtesy of M. Metcalfe.)
FIG. 13.3.

Severe acute respiratory distress syndrome coronavirus (SARS). (A) Lung showing interstitial pneumonitis, patchy hyaline membranes and prominent intraalveolar edema. (B) Multinucleated syncytial giant cells can be seen in some cases of fatal SARS. Note absence of discernable viral inclusions. (C) Abundant immunostaining of coronavirus antigen in alveolar pneumocytes. (D) Ciliated epithelial cell in upper airway epithelium containing viral antigens. (E) Electron micrograph showing coronavirus particle. These 80- to 100-nm viral particles are named for the characteristic crownlike fringe on the surface.
(E, courtesy of C.D. Humphrey.)
Ancillary Studies
ISH and IHC studies of tissues from SARS patients have identified coronavirus in ciliated columnar epithelial cells in the trachea, bronchi, and bronchioles (Fig. 13.3D) and in pneumocytes (Fig. 13.3C) and occasional macrophages in some patients. Antigens are more readily identified in patients who die within the first 2 weeks of onset of illness. In MERS, immunoreactivity is seen in pneumocytes and multinucleated syncytial cells and necrotic and histologically unremarkable bronchial submucosal glands (Fig. 13.4D–F). Electron microscopic examination can show coronavirus particles in cytoplasmic membrane-bound vesicles with both viruses (Fig. 13.4G). In SARS, particles and nucleocapsid inclusions can also be along the cell membranes of pneumocytes, in phagosomes of macrophages, and associated with fibrin in alveolar spaces. Negative stains reveal particles averaging 80 to 100 nm in size with a characteristic crownlike fringe on the surface (Fig. 13.3E).
FIG. 13.14.

Human metapneumovirus pneumonia. (A) Diffuse alveolar damage in fatal HMPV infection showing prominent type II pneumocyte hyperplasia and hyaline membrane formation, as well as intraalveolar hemorrhage, fibrin, and edema. (B) Prominent mononuclear interstitial pneumonitis with hyaline membrane formation and intraalveolar hemorrhage from the same case. (C) IHC highlights viral antigen in the respiratory epithelium of a small bronchiole. (D) Negative stain of pleomorphic viral particle with prominent surface glycoproteins.
(D, courtesy of C.D. Humphrey.)
Differential Diagnosis
The histopathologic findings seen in the lungs of patients who die of MERS and SARS are somewhat nonspecific and can also be seen in acute lung injury cases caused by infectious agents, trauma, drugs, or toxic chemicals. Multinucleated syncytial cells can also be found in many viral infections, including measles, parainfluenza viruses, RSV, and Nipah virus infections. An unequivocal diagnosis can be made only by laboratory tests such as viral culture, direct fluorescent antibody, serology, PCR, or IHC.
Prognosis and Therapy
Patients can undergo complete recovery; however, the disease can progress to acute respiratory failure and death with about a 5% to 10% case fatality for SARS and an approximately 35% case fatality with MERS. No specific antiviral therapy exists for SARS and MERS, and case management is based on providing supportive care of complications and following infection prevention and control measures. Patients with underlying medical conditions may have an increased risk of developing severe illness, and these patients should be monitored closely. High-dose systemic corticosteroid administration can result in severe adverse effects and should be avoided unless indicated for another reason. Experimental and clinical trials are needed to evaluate the efficacy of various treatments, and development of targeted treatment is needed.
Cytomegalovirus
Cytomegalovirus Pneumonia—Fact Sheet.
Definition
-
▪
Human CMV is a β-herpesvirus with the largest genome (230 kbp) of all the herpesviruses known to infect humans
Incidence and Location
-
▪
Common cause of pneumonia in immunocompromised patients
-
▪
Worldwide distribution
-
▪
CMV is a ubiquitous human pathogen, and in North America infects approximately 50% to 90% of the population
-
▪
Patients with advanced HIV disease and recipients of hematopoietic stem cell and lung transplants are particularly at risk of developing CMV pneumonia
Mortality
-
▪
Mortality attributable to CMV pneumonia is approximately 50%
Gender, Race, and Age Distribution
-
▪
Can develop in patients of any age
-
▪
No apparent gender or racial predilection
Clinical Features
-
▪
Fever, nonproductive cough, rales, and hypoxemia
-
▪
Disseminated infection may also cause adrenalitis, hepatitis, or encephalitis
Radiologic Features
-
▪
Common findings are bilateral nodular or reticular opacities
-
▪
Pleural effusions are identified in approximately 10% to 30% of patients
-
▪
Some patients with documented infection have normal radiographs and CT studies
Therapy
-
▪
Ganciclovir, valganciclovir, foscarnet, cidofovir, and intravenous CMV immune globulin remain important lines of treatment
Cytomegalovirus Pneumonia—Pathologic Features.
Gross Findings
-
▪
Lungs are typically heavy and may appear diffusely consolidated or show scattered nodular foci of hemorrhage and necrosis
-
▪
Rarely, the infection manifests as a single pulmonary nodule
Microscopic Findings
-
▪
Multiple histopathologic patterns have been reported, including extensive intraalveolar hemorrhage, diffuse interstitial pneumonitis, and miliary inflammatory foci with necrosis
-
▪Virally induced cytopathic changes include cytomegaly (25–40 microns) and amphophilic to deeply basophilic intranuclear and intracytoplasmic inclusions in various cell types, including macrophages, pneumocytes, glandular epithelium, endothelium, and fibroblasts
-
–The single intranuclear inclusion is a large (up to 20 microns), round-to-ovoid body with a smoothly contoured border that is generally surrounded by a clear halo
-
–Cytoplasmic inclusions are small (1–3 microns), stain with periodic acid–Schiff stain, and are deeply argyrophilic with methenamine silver stains
-
–
Immunohistochemical Features
-
▪
Commercially available antibodies can assist in the diagnosis of CMV
Ultrastructural Features
-
▪
Mature enveloped virions from 150 to 200 nm
Pathologic Differential Diagnosis
-
▪
Herpes simplex viruses, VZV, and adenoviruses
-
▪
Reactive pneumocytes
CMV, a large, double-stranded DNA virus, is a ubiquitous human pathogen, and in North America infects approximately 50% to 90% of the population. Like all herpesviruses, CMV remains with its host for life after primary infection and establishes latency in various cell types, including vascular endothelial cells, monocytes and macrophages, neutrophils, and renal and pulmonary epithelial cells. Activation of viral replication occurs in persons with severely compromised immunity.
Clinical Features
Most CMV infections are inapparent, although cases of primary infection in otherwise healthy individuals can result in a self-limited mononucleosis syndrome resembling the illness caused by Epstein-Barr virus. Pulmonary involvement in CMV mononucleosis occurs in approximately 6% of these cases. Adults and children with advanced HIV disease, recipients of hematopoietic stem cell and lung transplants, and those with inflammatory bowel disease are at increased risk for developing CMV pneumonia. Before the use of CMV screening and effective antiviral prophylaxis regimens, 10% to 30% of all patients undergoing allogeneic bone marrow transplantation for leukemia, and 15% to 55% of solid organ transplants, developed CMV pneumonia, with case-fatality rates greater than 80% in some series. Neonates are also at risk. Symptomatology includes fever, dyspnea, cough, rales, and hypoxemia. Systemic dissemination and extrapulmonary involvement can occur in some patients.
Radiologic Features
Pulmonary CMV disease typically appears as bilateral nodular or reticular opacities on chest radiographs. Pleural effusions are identified in approximately 10% to 30% of patients. Because some patients may be coinfected with other pulmonary pathogens, radiologic findings may be confusing. Some patients with documented infection have normal radiographs and CT studies.
Pathologic Features
Gross Findings
There are several general patterns of pulmonary CMV infection. The lungs are typically heavy and may appear diffusely consolidated or show scattered nodular foci of hemorrhage and necrosis. Rarely, CMV infection of the lungs manifests as a single pulmonary nodule.
Microscopic Findings
Multiple histopathologic patterns have been reported for CMV pneumonia. Extensive intraalveolar hemorrhage with scattered cytomegalic cells and relatively scant inflammatory cell infiltrates may occur. In a similar manner, extensive involvement of the alveolar epithelium with minimal inflammation or overt evidence of parenchymal injury has also been described. Other patterns include multifocal or miliary lesions with mixed inflammatory cell infiltrates, hemorrhage, necrosis, and cytomegalic cells or a diffuse, predominantly mononuclear cell infiltrate, interstitial pneumonitis with intraalveolar edema and fibrin deposition, and diffusely distributed cytomegalic cells. The cytomegalic changes of CMV-infected cells are evident on standard hematoxylin-eosin staining and are virtually pathognomonic of active CMV infection. The cells are enlarged (25–40 microns) and contain amphophilic to deeply basophilic intranuclear and intracytoplasmic inclusions (Fig. 13.5A ). The single intranuclear inclusion is composed of viral nucleoprotein and assembled capsids, and is a large (up to 20 microns), round-to-ovoid body with a smoothly contoured border that is generally surrounded by a clear halo that gives the inclusion a distinctive “owl’s eye” appearance. Cytoplasmic inclusions are small (1–3 microns), granular bodies that appear after the intranuclear inclusion is well developed and are not uniformly present in all CMV-infected cells. These inclusions represent a mixture of virions and various cellular organelles and increase in size and number as the infection progresses. Unlike the intranuclear inclusions, the cytoplasmic inclusions stain with periodic acid–Schiff stain and are deeply argyrophilic with methenamine silver stains.
FIG. 13.5.

Cytomegalovirus (CMV) pneumonia. (A) CMV-infected cell showing a large, basophilic “owl’s eye” intranuclear inclusion and smaller, amphophilic cytoplasmic inclusions. (B) Immunohistochemical localization of CMV-infected cells in the pulmonary parenchyma. (C) Intranuclear viral capsids with (arrows) and without (arrowhead) a nuclear core. Capsids are partially bordered by electron dense chromatin.
(C, courtesy of M. Metcalfe.)
Ancillary Studies
CMV pneumonia is defined by the presence of signs or symptoms of pulmonary disease combined with the detection of CMV in bronchoalveolar lavage (BAL) fluid or lung tissue samples. Detection methods that support this definition include virus isolation, histopathologic observation of cytomegalic cells, ISH, or IHC stains (Fig. 13.5B). Detection by PCR alone is considered too sensitive for the diagnosis of CMV pneumonia and is insufficient for this purpose. CMV is most often cultured in human diploid fibroblasts using a shell vial method to enhance infectivity and can usually yield diagnostic results within 48 hours. Electron microscopic examination can show slightly pleomorphic spherical particles, with and without a nuclear core, that range from 150 to 200 nm (Fig. 13.5C).
Differential Diagnosis
Because the histopathologic features of CMV pneumonia are varied, the differential diagnosis depends on the predominant pattern of histologic pattern (hemorrhage, miliary inflammatory lesions, or diffuse interstitial pneumonitis). The cytopathologic changes of CMV-infected cells are generally sufficient to establish a diagnosis. CMV inclusions may on occasion, however, be confused with those of other herpesviruses, adenoviruses, or measles, but none of these pathogens collectively shows cytomegaly—a single large nuclear inclusion with a prominent halo—and multiple small cytoplasmic inclusions. Reactive pneumocytes can occasionally show enlarged nuclei, but the nuclei will be immunonegative with IHC for CMV.
Prognosis and Therapy
Ganciclovir, valganciclovir, foscarnet, cidofovir, and intravenous CMV immune globulin remain important lines of treatment for CMV pneumonia and have diminished mortality in immunosuppressed patients with this disease. Nonetheless, mortality attributable to CMV pneumonia is approximately 50%. Candidate vaccines are under development, but none are available for typical clinical use. Several viral mutations have been identified that are linked to drug resistance.
Herpes Simplex Viruses
Herpes Simplex Viruses—Fact Sheet.
Definition
-
▪Human HSVs are large, enveloped, double-stranded DNA viruses that exist in two serologic types
-
–HSV-1 is the serotype most commonly associated with adult HSV pneumonia
-
–HSV-2 is the serotype associated with approximately 80% of disseminated disease and pulmonary infections in newborn infants
-
–
Incidence and Location
-
▪
Worldwide distribution
-
▪
Newborn infants, severely immunosuppressed or burned patients, and patients with severe trauma are at greatest risk of developing HSV pneumonia
-
▪
Most cases of neonatal disease represent primary HSV infections and are acquired during parturition from HSV-infected mothers; the incidence of neonatal HSV infection is approximately 1 in 3,200 deliveries
Mortality
-
▪
Before the discovery and use of antiviral therapies, 85% of neonates with disseminated HSV disease died from the infection
-
▪
With early diagnosis and high-dose acyclovir therapy, mortality has been reduced to approximately 30%
Gender, Race, and Age Distribution
-
▪
People of all ages are susceptible
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
In adults, infection of the respiratory tract with HSV may be associated with disseminated herpetic infection but is more commonly identified as an isolated disease manifestation resulting from reactivation of latent herpetic infections in the oropharynx
-
▪
Disseminated disease develops in approximately 25% of infected neonates; approximately 40% to 50% of these patients develop pneumonia
-
▪
Infants with disseminated neonatal HSV infections first show signs and symptoms a mean of 5 days after birth (range, 0–12 days). As the disease progresses, the clinical picture often resembles bacterial sepsis, evolving rapidly to pneumonia, shock, and disseminated vascular coagulopathy
Radiologic Features
-
▪
Ill-defined nodular or reticular densities of various sizes scattered in both lung fields
-
▪
During the early stages of disease, these nodules measure 2 to 5 mm and are best seen in the periphery of the lungs; as the disease progresses, these lesions coalesce and enlarge to form more extensive segmental and subsegmental infiltrates
-
▪
Pleural effusions are commonly identified
-
▪
CT studies show patchy or diffuse opacities, multifocal peribronchial consolidations, or a mixture of both patterns
Therapy
-
▪
Antiviral therapies include acyclovir, valacyclovir, and famciclovir
-
▪
Foscarnet has been used effectively in some acyclovir-resistant patients
Herpes Simplex Viruses—Pathologic Features.
Gross Findings
-
▪
HSV tracheobronchitis: 5- to 15-mm ulcers covered by fibrinopurulent exudate on the mucous membranes of the large airways
-
▪
HSV pneumonia acquired through the airways: lungs are heavy and show nodular hemorrhagic foci that are generally distributed around bronchi and bronchioles
-
▪
Hematogenously acquired HSV pneumonia: hemorrhagic foci have a random or miliary distribution
Microscopic Findings
-
▪
HSV tracheobronchitis: large areas of denuded epithelium and exudate containing necrotic cells; cells with intranuclear inclusions may be sparse and are found most often at the margins of the ulcerated epithelium or occasionally in the mucous glands below the ulcerated mucosa
-
▪
HSV pneumonia: lesions show hemorrhage and necrosis with karyorrhectic debris; intranuclear inclusions are best appreciated in cells at the edge of necrotic foci
-
▪
Inclusions appear as homogeneous, amphophilic, and glassy or as eosinophilic with a halo separating the inclusion from the nuclear membrane
-
▪
Multinucleation and nuclear molding, ground-glass nuclear chromatin, and ballooning degeneration of the cytoplasm are more frequently associated with squamous epithelia and less often encountered in the lung
Immunohistochemical Features
-
▪
IHC testing of formalin-fixed tissues is a sensitive method to confirm HSV infections; antibodies reactive with both HSV-1 and HSV-2 are commercially available
Ultrastructural Features
-
▪
Virus particles are encapsulated and approximately 100 to 110 nm in diameter
-
▪
Individual particles demonstrate a targetoid appearance and are arranged in a latticelike pattern
Pathologic Differential Diagnosis
-
▪
VZV pneumonia
-
▪
Adenovirus pneumonia
-
▪
Measles pneumonia
-
▪
CMV pneumonia
Human HSVs are large, enveloped, double-stranded DNA viruses approximately 100 to 110 nm in diameter. Two serologic types are recognized, and each is most frequently associated with particular disease syndromes; however, either serotype may cause any of the associated clinical syndromes. HSV-1 causes gingivostomatitis, pharyngitis, esophagitis, keratoconjunctivitis, and encephalitis, and is the serotype most commonly associated with adult HSV pneumonia. HSV-2 typically infects genital sites and is the serotype associated with approximately 80% of disseminated disease and pulmonary infections in newborn infants.
Clinical Features
HSV, like all herpesviruses, has the ability to persist in an inactive state for varying periods and then recur spontaneously after undefined stimuli associated with physical or emotional stress, trauma to nerve roots or ganglia, fever, immunosuppression, or exposure to ultraviolet radiation. Tracheobronchitis and pneumonia are the primary respiratory tract manifestations of HSV infection. In adults, infection of the respiratory tract with HSV may be associated with disseminated herpetic infection but is more commonly identified as an isolated disease manifestation resulting from reactivation of latent herpetic infections in the oropharynx. Mucocutaneous herpetic infection generally precedes HSV pneumonia, and aspiration of virus-containing secretions into the lower respiratory tract is believed to be the most frequent cause of pulmonary infection with HSV; however, oral lesions may be absent in patients with herpetic laryngotracheitis and bronchopneumonia. Disease can also be associated with airway trauma caused by tracheal intubation or from hematogenous dissemination of HSV. Newborn infants, severely immunosuppressed or burned patients, and patients with severe trauma are at greatest risk of developing HSV pneumonia. Lower respiratory tract disease in neonates is most commonly associated with disseminated herpetic infections. Most cases of neonatal disease represent primary HSV infections and are acquired during parturition from HSV-infected mothers. The incidence of neonatal HSV infection is approximately 1 in 3,200 deliveries, and disseminated disease develops in approximately 25% of infected neonates. In disseminated infections, signs and symptoms appear a mean of 5 days after birth (range, 0–12 days), and approximately 40% to 50% of these patients develop pneumonia.
Radiologic Features
Chest radiographs of patients with HSV pneumonia show ill-defined nodular or reticular densities of various sizes scattered in both lung fields. During the early stages of disease, these nodules measure 2 to 5 mm and are best seen in the periphery of the lungs. As the disease progresses, these lesions coalesce and enlarge to form more extensive segmental and subsegmental infiltrates. CT shows patchy or diffuse areas of ground-glass opacities, multifocal peribronchial consolidations, or a mixture of both patterns. Pleural effusions are common.
Pathologic Features
Gross Findings
HSV tracheobronchitis appears as 5- to 15-mm ulcers covered by fibrinopurulent exudate on the mucous membranes. In HSV pneumonia acquired through the airways, the lungs are heavy and show nodular hemorrhagic foci that are generally distributed around bronchi and bronchioles. In hematogenously acquired HSV pneumonia, hemorrhagic foci usually have a random or miliary distribution.
Microscopic Findings
Herpetic tracheobronchitis is an ulcerative process characterized by large areas of denuded mucosal epithelium and fibrinopurulent exudate containing necrotic cells. Despite extensive tissue damage, cells with intranuclear inclusions may be sparse and are found most often at the margins of the ulcerated epithelium or occasionally in the mucous glands below the ulcerated mucosa. In the lung, herpetic lesions show extensive necrosis and karyorrhectic debris, and are associated with hemorrhage and a sparse-to-moderate neutrophilic infiltrate (Fig. 13.6A and B ). Intranuclear inclusions are best appreciated in cells at the leading edge of necrotic foci. Inclusions appear either as homogeneous, amphophilic, and glassy, typical of Cowdry type B inclusions, or as eosinophilic with a halo separating the inclusion from the nuclear membrane typical of Cowdry type A inclusions (Fig. 13.6C). Other changes associated with HSV, including multinucleation and nuclear molding, ground-glass nuclear chromatin, and ballooning degeneration of the cytoplasm (Fig. 13.6C), are more frequently associated with squamous epithelium and less often encountered in the lung.
FIG. 13.6.

Herpes simplex virus (HSV) pneumonia. (A and B) Extensive necrosis and hemorrhage associated with herpes simplex pneumonia. (C) Glassy, amphophilic, intranuclear inclusions in HSV-infected syncytial cells. (D and E) Immunohistochemical localization of HSV antigen in the lung of a patient with fatal HSV pneumonia. (F) Viral capsids within the nucleus of an HSV-infected cell.
(F, courtesy of C.S. Goldsmith.)
Ancillary Studies
Virus isolation remains an important diagnostic method; however, because HSV can be isolated from oropharyngeal secretions and occasionally from the lower respiratory tract of patients who lack overt pulmonary disease, virologic cultures must be interpreted in the context of complementary clinical, radiographic, and histopathologic findings as much as possible. PCR methods that amplify HSV DNA from clinical specimens, including tissue and blood, can be particularly useful for distinguishing between HSV-1 and HSV-2 infections. Commercially available antibodies exist for IHC detection of HSV in tissues (Fig. 13.6D and E). Electron microscopy can also be used to demonstrate encapsulated viral particles with a targetoid appearance arranged in a latticelike pattern (Fig. 13.6F).
Differential Diagnosis
HSV, VZV, adenoviruses, measles virus, and CMV can cause necrotizing hemorrhagic pneumonias, and each produces intranuclear inclusions that may be difficult to differentiate. The viral inclusions of HSV are identical to those of VZV; separation can be accomplished by IHC, molecular methods, or culture. The presence of smudge cells is supportive of a diagnosis of adenovirus, and HSV does not produce cytoplasmic inclusions, which should be seen in CMV and in measles.
Prognosis and Therapy
Before the discovery and use of antiviral therapies, 85% of neonates with disseminated HSV disease died from the infection. With early diagnosis and high-dose acyclovir therapy, however, mortality has been reduced to approximately 30%. Acyclovir is the treatment of choice, and foscarnet has been used effectively in some acyclovir-resistant patients. Concomitant bacterial pneumonia is common.
Varicella Zoster Virus
Varicella Zoster Virus—Fact Sheet.
Definition
-
▪
Primary infection causes varicella (chickenpox) and reactivation of latent virus causes herpes zoster (shingles)
Incidence and Location
-
▪
Ubiquitous worldwide pathogen, and humans are the only known host
-
▪
Highly contagious virus; the attack rate for previously uninfected household contacts exposed to varicella is approximately 90%
-
▪
The U.S. incidence of varicella pneumonia has dropped by two-thirds since universal childhood vaccination for varicella was implemented in 1995
Mortality
-
▪
Untreated adult varicella pneumonia is fatal in approximately 10% of cases
-
▪
Mortality is as high as 25% to 40% in certain high-risk cohorts, including pregnant women, transplant recipients, and neonates
Gender, Race, and Age Distribution
-
▪
Adult patients with varicella are approximately 25 times more likely than children to develop pneumonia; pneumonia occurs in approximately 10% to 15% of adults infected with VZV
-
▪
The greatest risk of severe disease and pneumonia occurs in those patients with chronic lung disease, immune-suppressing conditions, neonates, and pregnant women
-
▪
The incidence of pneumonia in bone marrow transplant recipients and acute leukemia patients infected with varicella may be as high as 30% to 45%
-
▪
No apparent gender or racial predilection
Clinical Features
-
▪
Primary infection occurs by inoculation of respiratory mucosa with infectious aerosols or by direct contact with skin lesions of patients with varicella or herpes zoster
-
▪
VZV pneumonia generally develops within 2 to 7 days after the onset of rash and may be characterized by fever, cough, tachypnea, chest pain, and hemoptysis
Radiologic Features
-
▪
Multifocal, bilateral, poorly defined nodular densities that measure 5 to 10 mm in greatest dimension and may coalesce to form more extensive areas of consolidation
-
▪
Pleural effusions are uncommon
-
▪
Some survivors of VZV pneumonia show persistent parenchymal nodules that mineralize and persist as small (2–3 mm) calcifications, predominantly in the lower zones of the lungs
Prognosis and Therapy
-
▪
Intravenous acyclovir is recommended for use in all patients for whom the risk of disseminated disease is particularly likely or unpredictable, including patients with leukemia, bone marrow transplant recipients, and severely immune-suppressed persons
-
▪
Other treatments include valacyclovir, penciclovir, famciclovir, and varicella-zoster immune globulin.
Varicella Zoster Virus—Pathologic Features.
Gross Findings
-
▪
Trachea and bronchi are generally edematous and erythematous with occasional vesicles or ulcers on the mucosal surfaces
-
▪
The lungs are generally two to three times heavier than normal, firm, and “plum colored”
-
▪
There are often multiple necrotic and hemorrhagic lesions on the pleura and in the lung parenchyma that resemble the pox lesions of skin
Microscopic Findings
-
▪
Interstitial pneumonitis and diffuse miliary foci of necrosis and hemorrhage in the pulmonary parenchyma
-
▪
Other findings may include alveolar collections of edema, fibrin, or hemorrhage, diffuse alveolar damage, and septal edema
-
▪
Virally infected cells with intranuclear inclusions may be identified in respiratory epithelial cells of the trachea and bronchi, pneumocytes, interstitial fibroblasts, or capillary endothelium
-
▪
Eosinophilic intranuclear inclusions and multinucleated syncytial cells may be difficult to locate but are best identified at the edges of necrotic foci
-
▪
In disseminated disease, similar necrotizing hemorrhagic lesions and occasional viral cytopathic changes are observed in other tissues and organs
Immunohistochemical Features
-
▪
IHC testing of formalin-fixed tissues is a sensitive method to confirm VZV infection and distinguish it from other viral infections, particularly HSV
Ultrastructural Features
-
▪
The enveloped viral particle is pleomorphic to spherical and 180 to 200 nm in diameter
-
▪
Viral particles are located within the nuclei of infected cells
Pathologic Differential Diagnosis
-
▪
HSV (histology is identical)
-
▪
Adenoviruses, measles, and CMV
VZV is a double-stranded DNA, human alpha herpesvirus closely related to HSV. Primary infection causes varicella (chickenpox), and reactivation of latent virus causes herpes zoster (shingles). VZV is ubiquitous in human populations around the world, and humans are the only known natural reservoir. During the prevaccine era in the United States, approximately 4 million cases, 4,000 to 9,000 hospitalizations, and 50 to 140 deaths were reported annually. VZV-related deaths have declined sharply in the United States, however, since universal childhood vaccination was implemented in 1995.
Clinical Features
Primary infection with VZV occurs by inoculation of respiratory mucosa with infectious aerosols or by direct contact with skin lesions of patients with varicella or herpes zoster. A pruritic vesicular rash (chickenpox) develops after an incubation period of 10 to 21 days. The attack rate for previously uninfected household contacts exposed to varicella is approximately 90%. VZV also establishes latent infection within satellite cells and neurons of the trigeminal and dorsal root ganglia and can reactivate under various conditions to cause herpes zoster, a painful unilateral vesicular eruption distributed in a dermatomal distribution. Although chickenpox is usually a relatively benign infection in children, adult patients are approximately 25 times more likely than children to develop pneumonia. Pneumonia occurs in approximately 10% to 15% of adults primarily infected with VZV; however, the incidence of pneumonia in bone marrow transplant recipients and acute leukemia patients may be as high as 30% to 45%. The greatest risk of severe disease and pneumonia occurs in those patients with chronic lung disease, immune-suppressing conditions, neonates, and pregnant women. The occurrence of pneumonia during herpes zoster is rare and limited primarily to profoundly immunosuppressed patients, particularly bone marrow transplant recipients. VZV pneumonia develops 2 to 7 days after the onset of rash and is characterized by fever, cough, tachypnea, chest pain, and hemoptysis. Massive pulmonary hemorrhage and pulmonary infarcts are frequent terminal events. Hematopoietic cell transplant recipients may present with signs of visceral dissemination and pneumonia 1 to 4 days before the localized cutaneous eruption of herpes zoster appears, and lower respiratory tract disease has been described in the absence of skin lesions, particularly in neonates and bone marrow transplant recipients.
Radiologic Features
The lungs show multifocal, bilateral, poorly defined nodular densities that measure 5 to 10 mm in greatest dimension. These opacities may coalesce to form more extensive areas of consolidation. Hilar adenopathy may also occur, but pleural effusions are uncommon. Some patients who survive VZV pneumonia show persistent parenchymal nodules that may mineralize and persist as small (2–3 mm) calcifications, predominantly in the lower zones of the lungs.
Pathologic Features
Gross Findings
The lungs of patients with fatal VZV pneumonia are two to three times heavier than normal, firm, and plum colored. There are often multiple necrotic and hemorrhagic lesions on the visceral and parietal pleura that resemble the pox lesions of skin. The trachea and bronchi are generally edematous and erythematous with occasional vesicles on the mucosal surfaces, and there may be lobular consolidation of the lungs, as well as randomly distributed hemorrhagic lesions.
Microscopic Findings
The lungs show interstitial pneumonitis and diffuse miliary foci of necrosis and hemorrhage in the pulmonary parenchyma involving alveolar walls, blood vessels, and bronchioles (Fig. 13.7A and B ). Other findings can include intraalveolar collections of edema, fibrin, or hemorrhage; diffuse alveolar damage; and septal edema. Virally infected cells with intranuclear inclusions may be identified in respiratory epithelial cells of the trachea and bronchi, pneumocytes, interstitial fibroblasts, or capillary endothelium. Eosinophilic intranuclear inclusions and multinucleated syncytial cells may be difficult to locate, but are best identified at the edges of necrotic foci (Fig. 13.7B). In cases of disseminated disease, similar necrotizing hemorrhagic lesions and occasional viral cytopathic changes in epithelial cells or fibroblasts may be observed in many other tissues and organs.
FIG. 13.7.

Varicella zoster virus (VZV) pneumonia. (A) Extensive necrosis and hemorrhage in a patient with fatal VZV pneumonia. (B) Amphophilic intranuclear inclusions (arrow) in VZV-infected cells at the edge of a necrotic focus. (C) Immunohistochemical staining of VZV antigens in the pulmonary parenchyma of patient with fatal VZV pneumonia. In the lungs, VZV infects many cell types, including respiratory epithelium, pneumocytes, endothelial cells, and fibroblasts.
Ancillary Studies
Because pulmonary symptoms most often occur several days after the onset of the characteristic rash of varicella, a pathologic diagnosis is seldom required for a real-time diagnosis of VZV pneumonia. Antigen detection kits using fluorescein-conjugated VZV monoclonal antibodies can be helpful for rapid diagnosis of cutaneous VZV infection. Antibodies are also commercially available for IHC detection of VZV in tissue specimens (Fig. 13.7C). Some commercial laboratories offer PCR amplification to detect viral nucleic acid in clinical specimens. Isolation of the virus in cell culture remains the reference standard for the diagnosis of VZV. Infectious VZV is usually recoverable from the clear fluid of cutaneous vesicles of varicella for approximately 3 days after the appearance of these lesions and for approximately 1 week from herpes zoster lesions. Under electron microscopy, VZV has an icosahedral nucleocapsid that is indistinguishable in appearance from other herpesviruses. The enveloped viral particle is pleomorphic to spherical in shape and 180 to 200 nm in diameter.
Differential Diagnosis
The histopathologic appearance of VZV pneumonia most closely resembles disease caused by HSV with respect to the general pattern of lung injury (eg, multicentric, necrotizing, and hemorrhagic lesions) and to the appearance of the glassy intranuclear inclusions.
Prognosis and Therapy
The U.S. incidence of varicella pneumonia declined markedly since universal childhood vaccination for varicella was implemented in 1995. Vaccine efficacy at preventing severe disease is approximately 97%. Untreated adult varicella pneumonia is fatal in approximately 10% of cases, but mortality is as high as 25% to 40% in certain high-risk cohorts, including pregnant women, transplant recipients, and neonates. Intravenous acyclovir is recommended for use in all patients for whom the risk of disseminated disease is particularly high or unpredictable, including patients with leukemia, bone marrow transplant recipients, and severely immune-suppressed persons. Other treatments include valacyclovir, penciclovir, famciclovir, and varicella zoster immune globulin.
Influenza Viruses
Influenza—Fact Sheet.
Definition
-
▪
Influenza viruses belong to the Orthomyxoviridae family and include the two important influenza virus types, A and B, which are associated with significant human disease
-
▪
All influenza viruses have a segmented, negative-sense RNA core surrounded by a lipid envelope
-
▪
Influenza A viruses are further classified into subtypes based on the antigenicity of their HA and NA surface glycoproteins
-
▪
There are 16 recognized HA subtypes and 9 NA subtypes of influenza A virus
-
▪
Only one type of HA and one type of NA are recognized for influenza B
Incidence and Location
-
▪
Worldwide distributions
-
▪
Influenza A occurs in both pandemic and interpandemic forms
Morbidity and Mortality
-
▪
Seldom fatal in the immunocompetent host, and recovery is usually complete
-
▪
Mortality and risk for complications from influenza are higher among persons aged 65 years or older, young children, and persons of any age with certain underlying health conditions
-
▪
Secondary bacterial pneumonias with organisms such as Streptococcus pneumoniae, Group A streptococcus, S. aureus, and Haemophilus influenzae may occur as a complication
Gender, Race, and Age Distribution
-
▪
People of all ages are vulnerable to influenza pneumonia
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Spread primarily through the coughing and sneezing of infected persons
-
▪
Typical incubation period is 1 to 4 days
-
▪
Uncomplicated influenza illness is characterized by the abrupt onset of fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis
Radiologic Features
-
▪
Unilateral or bilateral consolidation of the lungs
-
▪
Rarely associated with pleural effusions
Therapy and Prevention
-
▪
Vaccination is an important preventive strategy
-
▪
Supportive therapy, such as bed rest, oral hydration, and antipyretics
-
▪
Antivirals such as oseltamivir, zanamivir, and amantadine may be helpful early in the course of infection
-
▪
Specific antibiotic therapy in cases with secondary bacterial infection
Influenza—Pathologic Features.
Gross Findings
-
▪
Airways show hyperemia, hemorrhage, and edema and may be filled with exudate
-
▪
Cross-sections of the lungs have a granular appearance, in which the lower lobes are more affected than the upper lobes
-
▪
Gross pathologic features in secondary infections depend largely on the specific microbial (usually bacterial) pathogen involved and include consolidation, abscess formation, hemorrhage, and empyema
Microscopic Findings
-
▪
Necrotizing bronchitis and tracheitis
-
▪
Diffuse alveolar damage
-
▪
Thrombi
-
▪
Hemorrhage
-
▪
Edema
-
▪
Viral inclusions cannot be identified by light microscopy
Immunohistochemical Features
-
▪
IHC is extremely valuable for confirming infection
-
▪
Influenzaviral antigens are usually sparse and are primarily seen in the epithelial cells of larger airways
-
▪
In H1N1 influenza antigens are frequently noted in the lower airways in type I and type II pneumocytes
-
▪
Antigens are more readily identified in patients who die within 3 to 4 days of onset of illness
Ultrastructural Features
-
▪
Viral particles are pleomorphic (filamentous and spherical)
-
▪
A 10- to 12-nm layer of HA (rod-shaped) and NA (mushroom-shaped) spikes project radially from the surfaces of the influenza A and B viruses
Pathologic Differential Diagnosis
-
▪
A large number of viral, rickettsial, and bacterial infections, as well as noninfectious diseases, may have similar histologic features
-
▪
Unequivocal diagnosis can be made by laboratory tests such as viral culture, direct fluorescent antibody and rapid antigen assays, serology and IHC
Influenza viruses belong to the Orthomyxoviridae family and include the two important influenza viruses, types A and B, which are associated with significant human disease. All influenza viruses have a segmented, negative-sense RNA core surrounded by a lipid envelope. Influenza A viruses are further classified into subtypes based on the antigenicity of their hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins. Only one type of HA and one type of NA are recognized for influenza B. Influenza A occurs in both pandemic and interpandemic forms. The epidemiologic pattern of influenza in humans is related to two types of antigenic variation of its envelope glycoproteins, namely, antigenic drift and antigenic shift. Fortunately, pandemics, defined as worldwide outbreaks of severe disease, occur infrequently and result from antigenic shift and emergence of new potentially pandemic influenza A viruses that possess a novel HA alone or in combination with a novel NA. Since 1900, there have been four pandemic events, the most recent of which occurred in 2009 with influenza A H1N1 that emerged in North America and spread globally. However, since late 2010 this virus is considered to be in the post-pandemic phase with disease activity at seasonal levels. Interpandemic influenza occurs virtually every year as a result of antigenic drift resulting from point mutations in the surface glycoproteins and emergence of new strains related to those circulating in previous epidemics. This enables the virus to evade the immune system, leading to repeated outbreaks during interpandemic years.
Clinical Features
Influenza viruses are spread person to person primarily through the coughing and sneezing of infected persons. The typical incubation period is 1 to 4 days. Adults can be infectious from the day before symptoms begin through approximately 5 days after illness onset. Children can be infectious for 10 or more days, and young children can shed virus for several days before their illness onset. Severely immunocompromised persons can shed virus for weeks or months. Respiratory illness caused by influenza is difficult to distinguish from illnesses caused by other respiratory pathogens on the basis of symptoms alone. Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms, including fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis. Among children, otitis media, nausea, and vomiting are also commonly reported. Influenza typically resolves after 3 to 7 days in most patients, although cough and malaise can persist for more than 2 weeks. Pandemic 2009 H1N1 resulted in illness ranging from mild to severe and fatal, with over 12,000 deaths and estimates of over 60 million cases in the United States from 2009 to 2010. Complications include secondary bacterial pneumonias, febrile seizures, and, uncommonly, encephalopathy, transverse myelitis, Reye syndrome, myositis, myocarditis, and pericarditis. The risks for complications, hospitalizations, and deaths from influenza are higher among persons aged 65 years or older, young children, and persons of any age with certain underlying health conditions than among healthy older children and younger adults.
Radiologic Features
The main findings include unilateral or bilateral patchy consolidation of the lungs, which may progress to confluent lung disease. Pleural effusions are uncommon.
Pathologic Features
Gross Findings
Lungs in influenza virus pneumonia not associated with a bacterial infection can have different degrees of hemorrhage and edema. Airways can be filled with varying amounts of exudate, and the mucosae of the trachea and large bronchi are hyperemic and swollen. Cross-sections of the lungs show a more or less granular appearance, in which the lower lobes are more affected than the upper lobes. The gross pathologic features in secondary infections depend largely on the specific microbial (usually bacterial) pathogen involved. The mucosae of the large airways can demonstrate hyperemia, hemorrhages, or purulent necrotic debris. In the lungs, the extent of the pathologic process in the lower lobes is generally greater than the upper and may include consolidation, abscesses, hemorrhages, and empyema. Secondary inflammation in the regional lymph nodes may be present. Purulent mediastinitis and pericarditis may also be found in some cases.
Microscopic Findings
The histopathologic features of nonfatal and fatal influenza include necrotizing bronchitis, diffuse alveolar damage, hemorrhage, edema, and thrombi. The pathology is more prominent in larger bronchi, and inflammation may vary in intensity (Fig. 13.8A–C ). Viral inclusions cannot be identified by light microscopy (Fig. 13.8F). Secondary bacterial infections with organisms such as Streptococcus pneumoniae, Group A streptococcus, S. aureus, and Haemophilus influenzae may occur as a complication in about 50% to 75% of fatal cases and make it difficult to recognize the pathologic changes associated with the primary viral infection (Fig. 13.10C ). The histopathologic features in other organs may include myocarditis, cerebral edema, rhabdomyolysis, and hemophagocytosis (Fig. 13.9A and B).
FIG. 13.8.

Influenza. (A) Trachea from a child with fatal influenza B showing extensive vascular congestion and a predominantly mononuclear inflammatory cell infiltrate in the lamina propria and submucosa. (B) Interstitial pneumonitis in a patient with fatal influenza B. (C) Extensive ulceration of respiratory epithelium in a large airway of a patient with fatal influenza B. The lamina propria shows necrosis and mixed inflammatory cell infiltrates. (D and E) Immunohistochemical staining of influenza B virus in the respiratory epithelium of bronchioles. (F and G) Extensive infection of respiratory epithelial cells of a small airway by influenza A virus. This same focus of extensively infected respiratory epithelium, readily apparent by an immunohistochemical stain for hemagglutinin antigens (G) demonstrates that, unlike many other viral respiratory pathogens, influenza viruses do not elicit specific cytopathic effects in infected cells.
FIG. 13.10.

Influenza–pandemic influenza H1N1. (A and B) Histopathologic changes associated with the various phases of diffuse alveolar damage are commonly observed in lungs of patients with H1N1 influenza and relate to interval between onset of disease and time of tissue sampling. The changes include fibrin and acute inflammation with intraalveolar hemorrhage (A) and extensive hyaline membrane formation with congestion and edema (B). (C) Bacterial coinfections are common, and indicated by the presence of a neutrophilic bronchopneumonia, such as in this fatal case with Streptococcus species coinfection. (D) IHC highlighting viral antigens in airway epithelium and within sloughed and necrotic luminal contents. (E and F) IHC highlighting influenza virus A H1N1 nucleoprotein antigens localize to the nuclei and cytoplasm of type I and type II pneumocytes (E) and submucosal glands of large conducting airways (F).
Unlike mammalian influenza viruses, avian virus H5N1 preferentially infects cells in the lower respiratory tract of humans, resulting in extensive damage of the lungs with minimal pathology in the upper respiratory tract (Fig. 13.9C). This may explain why the H5N1 avian influenza virus is so lethal to humans but so difficult to spread from person to person. Studies show that the avian virus preferentially binds to the α-2,3 galactose receptors, which are found only in and around the alveoli. This is in contrast to the mammalian influenza viruses, which preferentially bind to the α-2,6 receptors, which are found throughout the respiratory tract from the nose to the lungs.
FIG. 13.9.

Influenza. (A) Hemophagocytosis in a peribronchial lymph node in a patient with fatal influenza B. (B) Focus of myocyte necrosis accompanied by a mixed inflammatory cell infiltrate in the heart of a patient with fatal influenza B. (C) Diffuse alveolar damage in a patient with fatal avian influenza (H5N1) showing extensive hyaline membrane formation and congestion. (D) Influenza virus A (H5N1) antigens in the nucleus of a pneumocyte of a patient with fatal avian influenza. Unlike other influenza viruses that cause disease in humans, H5N1 preferentially infects alveolar epithelial cells, and causes relatively minimal pathology in the upper respiratory tract.
Ancillary Studies and Differential Diagnosis
Because of the absence of any characteristic viral inclusions and because the overall pathologic features of influenza may resemble other viral, rickettsial, and certain bacterial infections, an unequivocal diagnosis can be made only by laboratory tests such as viral culture, direct fluorescent antibody and rapid antigen assays, serology, IHC, and ISH. IHC and ISH assays can demonstrate viral antigens and nucleic acids in epithelial cells of small airways (Fig. 13.8D–G) and in type I and II pneumocytes and glands of large conducting airways with H1N1 (Fig. 13.10E and F). Antigens are more readily identified in patients who die within 3 to 4 days of onset of illness.
Prognosis and Therapy
Vaccination is an important strategy in the prevention of influenza virus infections. Seasonal influenza is seldom fatal in the immunocompetent host, and recovery is usually complete. Supportive management with bed rest, hydration, and antipyretics is the basis of treatment. Antiviral agents may be helpful early in the course of illness. NA, a major antigenic determinant of influenza viruses, catalyzes the cleavage of glycosidic linkages to sialic acid and the release of progeny virions from infected cells. Accordingly, it has become an important target for drug inhibitors such as oseltamivir and zanamivir. The M2 surface component and channel of influenza A (not present in influenza B virus) regulates the internal pH of the virus and is blocked by the antiviral drug amantadine.
Measles Virus
Measles—Fact Sheet.
Definition
-
▪
Measles virus is a single-stranded RNA virus and a member of the genus Morbillivirus, of the family Paramyxoviridae
Incidence and Location
-
▪
Highly communicable disease of worldwide distribution
-
▪
A significant problem in underdeveloped countries
-
▪
Uncommon infection in the United States after the widespread use of the vaccine, but recent recrudescence particularly among unvaccinated populations
Morbidity and Mortality
-
▪
The most common complications are secondary bacterial pneumonia and otitis media
-
▪
Death occurs in about 1 of every 1,000 patients with measles, and about 1 out of every 1,000 patients develops acute encephalitis that can result in permanent brain damage
-
▪
The risk of death and other complications is substantially increased in infants, malnourished and immunocompromised individuals, persons with underlying illnesses, and nonimmunized populations
Gender, Race, and Age Distribution
-
▪
Infection in children younger than 9 months of age is uncommon because of passive protection from immune mothers
-
▪
Children (nonvaccinated) are usually infected by 6 years of age
-
▪
Natural infection results in lifelong immunity, and almost all adults are immune either due to exposure or vaccination
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Typical incubation period is 1 to 2 weeks
-
▪
Brief prodrome characterized by fever, rhinorrhea, cough, and conjunctivitis
-
▪
Koplik spots can be seen in the buccal mucosa shortly before rash onset
-
▪
An erythematous maculopapular rash begins on the face 3 to 4 days after prodromal symptoms and usually spreads to the trunk and extremities
-
▪
The rash lasts for approximately 6 days, fading in the same order as it appeared
Radiologic Features
-
▪
Fine reticular and ground-glass opacities in the lungs
-
▪
Nodules and patchy consolidation throughout the lungs may be seen
-
▪
Rarely associated with pleural effusions
Prognosis and Therapy
-
▪
Recovery is rapid and complete in most cases
-
▪
Supportive therapy is administered, such as bed rest, oral hydration, and antipyretics
-
▪
Immune globulins can be useful if treatment is given early in the infection
-
▪
Vaccination can also be helpful if given within 3 days of exposure
Measles—Pathologic Features.
Gross Findings
-
▪
Lungs are typically heavy, congested, hemorrhagic, and edematous
-
▪
Gross findings in cases with secondary infections depend largely on the specific microbial (usually bacterial) pathogen involved and may include consolidation, abscess formation, hemorrhage, and empyema
Microscopic Findings
-
▪
Interstitial pneumonitis with mononuclear cell infiltrates
-
▪
Diffuse alveolar damage
-
▪
Multinucleated giant cells with characteristic nuclear and cytoplasmic inclusions
Immunohistochemical Features
-
▪
Measles antigens can be detected in giant cells and alveolar lining cells
Ultrastructural Features
-
▪
Measles virions are pleomorphic, generally spherical, enveloped particles from 120 to 250 nm in diameter
-
▪
A lipid envelope surrounds a helical nucleocapsid composed of RNA and protein
Pathologic Differential Diagnosis
-
▪
Other viral pathogens that can cause giant cell pneumonia, such as RSV, parainfluenza, metapneumovirus, VZV, and henipaviruses
-
▪
Unequivocal diagnosis can be made by laboratory tests such as viral culture, direct fluorescent antibody and rapid antigen assays, serology, and IHC
Measles (rubeola) is an infectious, acute febrile viral illness characterized by upper respiratory tract symptoms, fever, and a maculopapular rash. The causative agent, a member of the genus Morbillivirus, of the family Paramyxoviridae, is an enveloped virus that contains a negative-sense, single-stranded RNA genome. Measles has a worldwide distribution. Although still a significant problem in underdeveloped countries, measles infection became uncommon in the United States after the development and widespread use of an effective measles vaccine. However, a recrudescence of measles infection occurred in several large U.S. urban centers in recent years, associated with reduced use of the vaccine among children and young adults. This trend resulted in over 600 confirmed cases in 2014, a record number since 2000 when measles elimination was documented in the United States.
Clinical Features
Measles virus is highly contagious and is spread by aerosols and droplets from respiratory secretions of acute cases. Children are usually infected by 6 years of age, resulting in lifelong immunity, and almost all adults are immune either from vaccination or exposure. Clinical infection in children younger than 9 months of age is generally uncommon because of passive protection afforded the infant by the transfer of maternal antibodies, although occasional infections have occurred in this age group. A person with acute measles is infective from just before the onset of symptoms to the end of fever. After an incubation period of about 1 to 2 weeks, the prodromal phase of measles begins with fever, rhinorrhea, cough, and conjunctivitis. Koplik spots, which are small, irregular, red spots with a bluish-white speck in the center, appear on the buccal mucosa in 50% to 90% of cases shortly before rash onset. An erythematous maculopapular rash begins on the face 3 to 4 days after prodromal symptoms and usually spreads to the trunk and extremities. The symptoms gradually resolve, with the rash lasting for approximately 6 days, fading in the same order as it appeared.
Radiologic Features
Chest radiographs typically show fine reticular and ground-glass opacities, as well as nodules and patchy consolidation. Bronchial thickening and peribronchial opacities may also be observed in some patients. Pleural effusions are rare.
Pathologic Features
Gross Findings
In fatal cases, the lungs are heavy and show congestion, hemorrhage, and edema. The gross pathologic features in secondary infections depend largely on the specific microbial (usually bacterial) pathogen involved.
Microscopic Findings
A focal or generalized interstitial pneumonitis, similar to that seen in many other viral infections, is seen in the lungs of measles patients. Histopathologic features include various degrees of peribronchial and interstitial mononuclear cell infiltrates, squamous metaplasia of bronchial endothelium, proliferation of type II pneumocytes, and intraalveolar edema, with or without mononuclear cell exudates and hyaline membranes. Secondary changes created by bacterial or viral superinfection or organizational changes may alter the original pathology. The hallmark of the disease is the formation of multinucleated epithelial giant cells. These cells, which are often numerous, are formed by fusion of bronchiolar or alveolar lining cells (Fig. 13.11A ). These cells generally contain characteristic nuclear and cytoplasmic inclusions. The intranuclear inclusions are homogenous, eosinophilic, and surrounded by a slight indistinct halo (Fig. 13.11B). The cytoplasmic inclusions are deeply eosinophilic and may form large masses with a “melted tallow” appearance (Fig. 13.11B). These giant cells may undergo degenerative changes with progressive loss of cytoplasm, increasing basophilia, and shrinkage of nuclei. The presence of measles virus in these giant cells may be demonstrated by immunofluorescence, IHC, and ISH techniques (Fig. 13.11C). These giant cells can also be seen in extrapulmonary tissues.
FIG. 13.11.

Measles pneumonia. (A) Generalized interstitial pneumonitis with hemorrhage, edema and fibrin from a fatal measles pneumonia case. Numerous multinucleated giant cells are seen and frequently line the alveoli, although some are found free within alveolar spaces. (B) High-power view of multinucleated epithelial syncytial cells showing numerous eosinophilic cytoplasmic (arrows) and intranuclear inclusions (arrowheads). Intranuclear inclusions are surrounded by a slight clear halo. (C) Viral antigens in cytoplasm of syncytial cells as seen by immunohistochemical staining. The multinucleated giant cells can be seen to originate by fusion of infected alveolar lining epithelial cells.
Ancillary Studies
Laboratory confirmation is useful to avoid possible confusion with other rash-causing illnesses. Diagnostic laboratory procedures consist of either direct detection of the virus or viral antigens, usually by indirect immunofluorescence or by serologic methods using hemagglutination inhibition, neutralization, or EIA. Specimens for serologic testing consist of acute- and convalescent-phase serum pairs. The presence of specific IgM antibody can be used to diagnose recent infection. IHC and ISH can be performed on tissue specimens. Ultrastructurally, measles virions are pleomorphic, generally spherical, enveloped particles from 120 to 250 nm in diameter, with a lipid envelope surrounding a helical nucleocapsid composed of RNA and protein.
Differential Diagnosis
In typical cases, the diagnosis of measles can usually be made on the basis of clinical signs and symptoms. Other causes of a similar rash, but without other features of measles, include rubella, dengue virus, enteroviruses (EVs), and drug reactions, especially to ampicillin. The histologic diagnosis is facilitated by the identification of the characteristic giant cells in a setting of interstitial pneumonitis. These giant cells are not seen in all cases of measles pneumonia, however, and their absence should not exclude the diagnosis. Furthermore, other viral pathogens, such as RSV, parainfluenza, metapneumovirus, VZV, and the recently discovered henipaviruses, may also give rise to pneumonias with giant cells and should be considered in the differential diagnosis. IHC or ISH testing can demonstrate viral antigens or nucleic acids in the majority of cases, assisting in the histologic diagnosis.
Prognosis and Therapy
Supportive therapy, such as bed rest, oral hydration, and antipyretics, usually produces rapid and complete recovery. Immune globulins can be useful if treatment is given early in the infection. Vaccination can also be helpful in the treatment regimen if given within 3 days of exposure. In a smaller number of patients, complications can arise as a result of continued and progressive virus replication, bacterial or viral superinfections, or abnormal host immune response. The most common complications are secondary bacterial pneumonia and otitis media. In these settings, specific antibiotic therapy is administered. Other complications include febrile convulsions, encephalitis, liver function abnormalities, chronic diarrhea, and sinusitis. Several pulmonary and central nervous system syndromes that are often fatal have been described. Death occurs in about 1 of every 1,000 measles cases; however, the risk of death and other complications is substantially increased in infants, malnourished and immunocompromised individuals, persons with underlying illnesses, and nonimmunized populations in underdeveloped countries.
Human Parainfluenza Viruses
Human Parainfluenza Viruses—Fact Sheet.
Definition
-
▪
The causative agents are negative-sense, nonsegmented, single-stranded, enveloped RNA viruses of the family Paramyxoviridae
-
▪
Four serotypes exist: HPIV-1 and 3 are included in the genus Respirovirus, and HPIV-2 and through HPIV-4 are included in the genus Rubulavirus
Incidence and Location
-
▪
Highly communicable disease of worldwide distribution
-
▪
Activity varies with serotype: HPIV-1 and 2 peak in the fall, HPIV-3 peaks in the spring and summer, and HPIV-4 is infrequently detected (less likely to cause severe illness)
-
▪
Common cause of upper respiratory illness, but an uncommon cause of lower respiratory tract disease
Morbidity and Mortality
-
▪
The highest rates of serious HPIV respiratory illnesses occur among young children
-
▪
HPIV infections are also an important cause of severe morbidity and mortality in immunocompromised adults
Gender, Race, and Age Distribution
-
▪
HPIVs infect most people by 5 years of age and can cause repeated infections throughout life
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Usually manifests as an upper respiratory illness (cold, sore throat, and croup) with a typical incubation period of 1 to 7 days
-
▪
Less commonly presents with symptoms of a lower respiratory illness (pneumonia, bronchitis, and bronchiolitis) in elderly or immunosuppressed patients
Radiologic Features
-
▪
Interstitial opacities, bronchial wall thickening, and peribronchial consolidation
-
▪
Rarely associated with pleural effusions
Prognosis and Therapy
-
▪
Recovery is rapid and complete in most cases
-
▪
Supportive management with bed rest, oral hydration, and antipyretics
-
▪
Aerosolized ribavirin can be used in immunosuppressed patients with severe illness
Human Parainfluenza Viruses—Pathologic Features.
Gross Findings
-
▪
Lungs can appear heavy, congested, hemorrhagic, and edematous
Microscopic Findings
-
▪
Interstitial pneumonitis with mononuclear cell infiltrates
-
▪
Diffuse alveolar damage
-
▪
Bronchiolitis
-
▪
Organizing pneumonia
-
▪
Multinucleated giant cells with characteristic cytoplasmic inclusions
Immunohistochemical Features
-
▪
HPIV antigens can be detected in giant cells and alveolar lining cells
Ultrastructural Features
-
▪
The virions are variable in shape and size, ranging from 150 to 300 nm
-
▪
A lipid envelope surrounds a helical nucleocapsid composed of RNA and protein
-
▪
The virus is morphologically indistinguishable from other members of the Paramyxoviridae family when viewed by negative contrast electron microscopy
Pathologic Differential Diagnosis
-
▪
Other viral pathogens that can cause giant cell pneumonia, such as measles, RSV, metapneumovirus, VZV, and henipaviruses
-
▪
Unequivocal diagnosis can be made by laboratory tests such as viral culture, direct fluorescent antibody and rapid antigen assays, serology, and IHC
Human parainfluenza viruses (HPIVs) are second only to RSV as a cause of lower respiratory tract disease in young children. HPIVs are negative-sense, nonsegmented, single-stranded, enveloped RNA viruses that possess fusion and hemagglutinin-neuraminidase glycoprotein “spikes” on their surface. The four serotypes of HPIV belong in the family Paramyxoviridae, subfamily Paramyxovirinae, and genera Respirovirus (HPIV-1 and -3) and Rubulavirus (HPIV-2 and -4).
Clinical Features
HPIVs are spread from respiratory secretions through close contact with infected persons or contact with contaminated surfaces or objects. Infection can occur when infectious material contacts mucous membranes of the eyes, mouth, or nose, and possibly through the inhalation of droplets generated by a sneeze or cough. HPIVs are ubiquitous and infect most people during childhood. Serologic surveys have shown that 90% to 100% of children aged 5 years and older have antibodies to HPIV-3, and about 75% have antibodies to HPIV-1 and -2. The different HPIV serotypes differ in clinical presentations, with HPIV-1 and HPIV-2 most frequently associated with outbreaks of croup and HPIV-3 more often associated with bronchiolitis and pneumonia. HPIV-4 is infrequently detected, possibly because it is less likely to cause severe disease. The incubation period is generally from 1 to 7 days. The HPIVs can also cause repeated infections throughout life, usually manifested by an upper respiratory tract illness (cold and sore throat). Serious lower respiratory tract disease (eg, pneumonia, bronchitis, and bronchiolitis) can also occur with repeat infection, especially among the elderly and among patients with compromised immunity. Nosocomial acquisition can occur in high rates in hospital settings, and thus strict prevention and control measures are advisable.
Radiologic Features
The main findings associated with HPIV pneumonia include diffuse interstitial opacities, bronchial wall thickening, and peribronchial consolidation. Infection is rarely associated with pleural effusions.
Pathologic Features
Gross Findings
In fatal cases, the lungs are typically heavy and display congestion, hemorrhage, and edema.
Microscopic Findings
In patients with severe HPIV infection, multinucleated giant cells derived from the respiratory epithelium may be seen in association with an interstitial pneumonitis, diffuse alveolar damage, bronchiolitis, and organizing changes (Fig. 13.12A–C ). These giant cells, which may contain intracytoplasmic eosinophilic inclusions (Fig. 13.12B), have also been reported in extrapulmonary tissues such as kidney, bladder, and pancreas.
FIG. 13.12.

Parainfluenza virus pneumonia. (A) Giant cell pneumonia showing numerous syncytial cells, type II pneumocyte hyperplasia, congestion, and intraalveolar rafts of sloughed and necrotic pneumocytes, fibrin, and mixed inflammatory infiltrates. (B) Epithelial syncytial cells containing multiple, prominent eosinophilic cytoplasmic inclusions. (C and D) Bronchiolitis is common and often characterized by mild mononuclear inflammation and edema. The corresponding IHC image shows parainfluenza virus antigens within respiratory epithelium. (E) IHC showing abundant viral antigen within epithelial syncytial cells. (F) Negative stain of filamentous nucleocapsids with typical herringbone appearance extruding from a viral particle. Surface glycoproteins are visible.
(F, courtesy of E. L. Palmer.)
Ancillary Studies
Diagnosis of infection with HPIVs can be made by virus isolation, direct detection of viral antigens by EIA or indirect fluorescent antibody (IFA) in clinical specimens, detection of viral RNA by RT-PCR, demonstration of a rise in specific serum antibodies, or a combination of these approaches. HPIV infections of lung can be confirmed by IHC testing of formalin-fixed tissues (Fig. 13.12D and E); viral antigens can be detected in giant cells, pneumocytes, and respiratory epithelial cells. Ultrastructural studies demonstrate variably shaped virions of varying size (ranging from 150–300 nm), with a lipid envelope surrounding a helical nucleocapsid composed of RNA and protein (Fig. 13.12F).
Differential Diagnosis
Other viral causes of giant cell pneumonia, including measles and RSV, should be considered in the histopathologic differential diagnosis, and laboratory testing, including IHC, can be useful in determining the correct diagnosis.
Prognosis and Therapy
Most HPIV infections cause a mild, self-limited illness. The highest rates of serious HPIV illnesses occur among young children. HPIV infections are also being increasingly recognized as an important cause of severe morbidity and mortality in immunocompromised adults. The mortality of bone marrow transplant patients with HPIV-3 infection has been reported to be as high as 60%. Supportive management with bed rest, oral hydration, and antipyretics is the basis of treatment. Aerosolized ribavirin has shown some efficacy in the treatment of severe cases of HPIV infection. There is no approved vaccine for HPIV, though several vaccinations are being evaluated in clinical trials.
Respiratory Syncytial Virus
Respiratory Syncytial Virus Pneumonia—Fact Sheet.
Definition
-
▪
RSV is a negative-sense, nonsegmented, single-stranded, enveloped RNA virus that is a member of the family Paramyxoviridae
Incidence and Location
-
▪
Worldwide distribution
-
▪
RSV spreads efficiently among children during annual outbreaks, infecting as many as 50% of children in their first year of life
-
▪
Most children will have serologic evidence of RSV infection by 2 years of age
-
▪
An estimated 0.5% to 2% of infants and young children infected with RSV are hospitalized
-
▪
Most children hospitalized for RSV infection are under 6 months of age or have cyanotic congenital heart disease, cystic fibrosis, bronchopulmonary dysplasia, or immunosuppression
Mortality
-
▪
Mortality in otherwise healthy children hospitalized for RSV pneumonia is less than 1%
-
▪
RSV pneumonia is fatal in as many as 15% to 40% of patients with immune suppression or underlying disease
-
▪
Mortality is greatest in infants with congenital heart disease associated with pulmonary hypertension, where it approaches 70%
Gender, Race, and Age Distribution
-
▪
RSV is the most common cause of bronchiolitis and pneumonia among infants and children less than 1 year of age
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Fever, runny nose, cough, sometimes wheezing
-
▪
During their first RSV infection, between 25% and 40% of infants and young children have signs or symptoms of bronchiolitis or pneumonia
-
▪
RSV can cause repeated infections throughout life, usually associated with moderate-to-severe coldlike symptoms
-
▪
Severe lower respiratory tract disease may occur at any age, especially among the elderly or among those with compromised cardiac, pulmonary, or immune systems
Radiologic Features
-
▪
Children most commonly show multifocal airspace consolidation and peribronchial thickening
-
▪
Bilateral interstitial opacities and multifocal consolidations are usually seen in adults
Prognosis and Therapy
-
▪
Otherwise healthy children usually recover completely
-
▪
Higher mortality in specific patient groups (see earlier)
-
▪
The only antiviral drug with in vitro efficacy against RSV is ribavirin
Respiratory Syncytial Virus Pneumonia—Pathologic Features.
Gross Findings
-
▪
Large and small airways can contain necrotic debris and mucus and may show ulceration
-
▪
The lungs may be heavy and firm and show areas of hyperexpansion or atelectasis
Microscopic Findings
-
▪
Necrotizing bronchiolitis
-
▪
Interstitial pneumonia
-
▪
Hyperplastic airway epithelium
-
▪
Multinucleated giant cells (in some cases) in bronchi, bronchioles, and alveoli that contain irregular, intracytoplasmic, eosinophilic inclusions surrounded by a clear halo
Immunohistochemical Features
-
▪
Viral antigens in multinucleated cells and respiratory epithelial cells
Ultrastructural Features
-
▪
The virion is variable in shape and size and ranges from 120 to 300 nm
-
▪
Particles show numerous 12-nm glycoprotein spikes
Pathologic Differential Diagnosis
-
▪
Other viral causes of giant cell pneumonia should be considered, primarily parainfluenza viruses and measles viruses
-
▪
Herpes simplex and VZV rarely produce multinucleated cytologic changes in the lung
-
▪
Confirmation of diagnosis is by clinical laboratory tests on IHC
RSV is a negative-sense, nonsegmented, single-stranded, enveloped RNA virus. RSV is a member of the family Paramyxoviridae and can be further distinguished genetically and antigenically into two subgroups, A and B. The subgroup A strains are usually associated with more severe infections.
Clinical Features
RSV is the most common cause of bronchiolitis and pneumonia among infants and children under 1 year of age. In temperate climates, RSV infections usually occur during annual community outbreaks, often lasting several months, during the late fall, winter, or early spring months. The timing and severity of outbreaks in a community vary from year to year. RSV spreads efficiently during the annual outbreaks, infecting as many as 50% of children in their first year of life. Most children will have serologic evidence of RSV infection by 2 years of age. Illness begins most frequently with fever, runny nose, cough, and sometimes wheezing. During their first RSV infection, between 25% and 40% of infants and young children have signs or symptoms of bronchiolitis or pneumonia, and 0.5% to 2% require hospitalization. The majority of children hospitalized for RSV infection are less than 6 months of age or are children with cyanotic congenital heart disease, cystic fibrosis, bronchopulmonary dysplasia, or immunosuppression. RSV can also cause repeated infections throughout life, and severe lower respiratory tract disease may occur at any age, especially among the elderly or among those with compromised cardiac, pulmonary, or immune systems.
Radiologic Features
Children with RSV infection most commonly show multifocal airspace consolidation and peribronchial thickening. In adults, disease is characterized by bilateral interstitial opacities and multifocal consolidations.
Pathologic Features
Gross Findings
Large and small airways can contain necrotic debris and mucus and may show ulceration. The lungs may be heavy and diffusely firm and may show areas of hyperexpansion or atelectasis.
Microscopic Findings
The major histopathologic changes described in fatal RSV infections are necrotizing bronchiolitis and interstitial pneumonia. Bronchiolar lumens and airways are usually filled with necrotic debris and inflammatory cells (Fig. 13.13C ). Airways show mixed or predominantly mononuclear infiltrates with hyperplastic epithelial changes (Fig. 13.13A). These findings may be accompanied by diffuse alveolar damage and secondary bacterial superinfection. Giant cell pneumonia is seen in some cases (Fig. 13.13D). The multinucleated giant cells represent epithelial cells in bronchi, bronchioles, and alveoli and sometimes contain irregular, intracytoplasmic, eosinophilic inclusions surrounded by a clear halo (Fig. 13.13E).
FIG. 13.13.
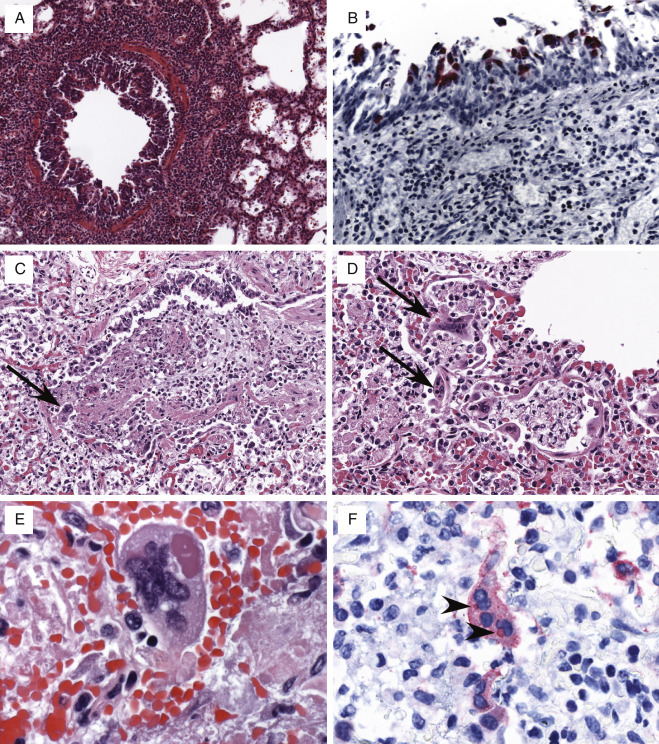
Respiratory syncytial virus pneumonia. (A) Bronchiolar and peribronchiolar inflammation in RSV infection. (B) Viral antigens highlighted in respiratory epithelial cells from the same case. (C) The respiratory epithelium of a small bronchiole is multifocally necrotic, sloughs into the lumen and is accompanied by fibrin, mucus, necrotic cell debris and mixed inflammatory cells. Multiple epithelial syncytial cells are noted along the periphery (arrow). (D) Diffuse alveolar damage with multiple epithelial syncytial cells (arrows). (E) Epithelial syncytial cell with large cytoplasmic inclusion. (F) IHC highlights RSV antigens in a syncytial cell. Note cytoplasmic inclusion bodies (arrowheads).
Ancillary Studies
Diagnosis of RSV infection can be made by virus isolation; direct detection of viral antigens in clinical specimens by EIA, IFA, or IHC (Fig. 13.13B and F); detection of viral RNA by RT-PCR; or demonstration of a rise in RSV-specific serum antibodies. The virus is labile, and attempts at culture isolation are often unsuccessful if there is delay or mishandling of the clinical specimen. Ultrastructural studies reveal virions of variable shape and size that range from 120 to 300 nm, with numerous 12-nm glycoprotein spikes.
Differential Diagnosis
Other viral causes of giant cell pneumonia should be considered in the histopathologic differential diagnosis, primarily parainfluenza viruses and measles viruses. Herpes simplex and VZVs less commonly produce multinucleated giant cells in the lung.
Prognosis and Therapy
Mortality in otherwise healthy children hospitalized for RSV pneumonia is less than 1%. However, the disease is fatal in as many as 15% to 40% of patients with immune suppression or underlying disease. Mortality is greatest in infants with congenital heart disease and pulmonary hypertension, where it approaches 70%. Palivizumab and ribavirin are the only two therapeutic treatments. There are no approved vaccines available for human use.
Human Metapneumovirus
Human Metapneumovirus—Fact Sheet.
Definition
-
▪
HMPV is a negative-sense, nonsegmented, single-stranded, enveloped RNA virus that is a member of the family Paramyxoviridae, subfamily Pneumovirinae, genus Metapneumovirus
-
▪
HMPV can be distinguished genetically and antigenically into two subgroups, A and B
Incidence and Location
-
▪
Worldwide distribution
-
▪
About 25% of all children aged 6 to 12 months and all children by age 5 years will have serologic evidence of HMPV infection
-
▪
Annual epidemic peaks occur late in the winter and spring months in temperate regions, often overlapping in part or in whole with RSV epidemics
-
▪
About 4% of all infant hospitalizations for acute respiratory illness or fever are associated with HMPV; the majority of children hospitalized for HMPV infection are under 6 months of age or children with underlying pulmonary disease, especially asthma
-
▪
May also contribute to severe respiratory disease in elderly or immunocompromised adults
Mortality
-
▪
Mortality in otherwise healthy children hospitalized for HMPV pneumonia is less than 1%
-
▪
Disease may be fatal in as many as 30% to 40% of patients with immune suppression or underlying disease
Gender, Race, and Age Distribution
-
▪
Common cause of bronchiolitis and pneumonia among infants under 1 year of age
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Most infections cause a mild, self-limited upper respiratory tract illness
-
▪
During their first HMPV infection, about 10% to 15% of outpatient infants and young children have signs or symptoms of bronchiolitis or pneumonia
-
▪
Severe lower respiratory tract disease (bronchiolitis, pneumonia) may occur at any age, especially among the elderly or among those with compromised cardiac, pulmonary, or immune systems
Radiologic Features
-
▪
Bilateral multifocal airspace consolidation and interstitial infiltrates, diffuse alveolar infiltrates, and emphysema without infiltrate
Prognosis and Therapy
-
▪
With supportive care, most children recover from illness in 1 to 2 weeks
-
▪
No licensed therapies or prophylactic treatments for HMPV
-
▪
The only antiviral drug shown to have in vitro efficacy against HMPV is ribavirin
Human Metapneumovirus—Pathologic Features.
Gross Findings
-
▪
In fatal cases, the lungs are heavy and display congestion, hemorrhage, and edema
Microscopic Findings
-
▪
Necrotizing bronchiolitis that evolves to chronic bronchiolitis has been described, as well as interstitial pneumonitis, acute or organizing diffuse alveolar damage, and increased intraalveolar macrophages and alveolar hemorrhage
-
▪
Organizing diffuse airway disease and chronic airway disease in patients who die later in the course of the illness
-
▪
BAL specimens may show multinucleated giant cells with cytoplasmic inclusions
Ultrastructural Features
-
▪
The virion is variable in shape and size, ranging from 150 to 300 nm, and is morphologically indistinguishable from other members of the Paramyxoviridae family when viewed by negative-stain electron microscopy
Pathologic Differential Diagnosis
-
▪
Other viral causes of giant cell pneumonia, necrotizing bronchiolitis, and interstitial pneumonitis and diffuse alveolar damage
-
▪
Noninfectious causes of diffuse alveolar damage
Human metapneumovirus (HMPV), first identified in 2001 from clinical specimens obtained from patients with acute respiratory illnesses, is a negative-sense, nonsegmented, single-stranded, enveloped RNA virus. HMPV has been categorized in the family Paramyxoviridae, subfamily Pneumovirinae, genus Metapneumovirus. HMPV can be further distinguished genetically and antigenically into two subgroups, A and B.
Clinical Features
HMPV infection is ubiquitous and occurs during infancy and early childhood, with annual epidemic peaks occurring late in the winter and spring months in temperate regions, often overlapping in part or in whole with the annual RSV epidemic. Seroprevalence studies reveal that 25% of all children aged 6 to 12 months have antibodies to HMPV; by age 5 years, 100% of patients have evidence of past infection. The incubation period is generally from 2 to 8 days. Although HMPV has been associated with a spectrum of respiratory disease, most infections cause a mild, self-limited illness, though severe disease can occur, particularly in immunocompromised patients. The patient may be asymptomatic, or symptoms may range from mild upper respiratory tract illness to severe bronchiolitis and pneumonia with hypotension and septic shock. During their first HMPV infection, about 10% to 15% of infants and young children have signs or symptoms of bronchiolitis or pneumonia. About one-half of the cases of lower respiratory illness in children occur in the first 6 months of life, suggesting that young age is a major risk factor for severe disease. Underlying pulmonary disease, especially asthma, may increase the risk of hospitalization for HMPV pneumonia, and persistent asymptomatic infection has been reported.
Radiologic Features
Radiographic findings vary and include diffuse bilateral alveolar infiltrates, interstitial infiltrates with focal consolidation commonly involving the lower lobes of the lung, and emphysema without infiltrate.
Pathologic Features
Gross Findings
In fatal cases, the lungs are typically heavy and display congestion, hemorrhage, and edema.
Microscopic Findings
Histopathologic descriptions are few, and assessment of the validity of some is complicated by the uncertainty, in some cases, of the clinical significance of detecting this ubiquitous virus. Nonetheless, BAL specimens collected from patients within a few days of a positive HMPV assay show degenerative changes and cytoplasmic inclusions within epithelial cells, multinucleated giant cells, and histiocytes. The intracytoplasmic inclusions are ill-defined, eosinophilic structures that measure 3 to 4 μm. Histologic changes include necrotizing bronchiolitis, diffuse alveolar damage with hyaline membrane formation, chronic airway inflammation, intraalveolar foamy and hemosiderin-laden macrophages, alveolar hemorrhage, acute and organizing lung injury, and organizing pneumonia (Fig. 13.14A and B ). In such cases, typical multinucleated giant cells or viral inclusions cannot be identified. ISH studies on a limited number of human cases suggest infection of alveolar and bronchial epithelial cells.
Ancillary Studies
HMPV is difficult to identify with commonly used viral diagnostic procedures. The virus replicates slowly in primary and tertiary monkey kidney cell lines, and cytopathic effects can be difficult to discern. Most HMPV studies have been conducted using RT-PCR assays or by demonstration of a rise in HMPV-specific serum antibodies. IHC and IFA assays also exist (Fig. 13.14C). The enveloped virion is variable in shape and size and ranges from 150 to 300 nm (Fig. 13.14D).
Differential Diagnosis
Other viral causes of giant cell pneumonia and diffuse alveolar damage, including measles, RSV, HPIV, measles, VZV, and HSV, may be considered, as well as noninfectious causes of diffuse alveolar damage. Laboratory testing, including IHC and ISH, can be useful in making this differentiation possible.
Prognosis and Therapy
Supportive management with bed rest, oral hydration, and antipyretics is the basis of treatment and usually leads to complete recovery. There are no licensed vaccines, therapies, or prophylactic treatments for HMPV at this time. Ribavirin and intravenous immunoglobulin, which have activity against RSV, were tested against HMPV in vitro and were found to have equivalent activity against HMPV and RSV.
Enteroviruses
Enteroviruses—Fact Sheet.
Definition
-
▪
EVs are single-stranded, nonenveloped RNA viruses that are members of the family Picornaviridae
-
▪
EV-D68 and RV are most commonly associated with respiratory disease
Incidence and Location
-
▪
Worldwide distribution
-
▪
Annual epidemic peaks occur in summer and fall months in temperate regions
-
▪
May also contribute to respiratory disease in healthy or immunocompromised including children, elderly and adults
Mortality
-
▪
Mortality is typically low, as most cases are mild and self-limited
-
▪
In some outbreaks mortality rate has reached nearly 10%
Gender, Race, and Age Distribution
-
▪
Infection can occur in all ages, though neonates and infants and patients with chronic respiratory disease are overrepresented in some outbreaks
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Most infections cause a mild, self-limited upper respiratory tract illness
-
▪
Severe lower respiratory tract disease, bronchiolitis, and pneumonia may occur at any age
Radiologic Features
-
▪
Alveolar and interstitial infiltrates and patchy consolidation
Prognosis and Therapy
-
▪
With supportive care, most cases are self-limiting
-
▪
No licensed therapies or prophylactic treatments exist for respiratory EVs
Enteroviruses—Pathologic Features.
Gross Findings
-
▪
Congestion, edema, and hemorrhage
Microscopic Findings
-
▪
Mononuclear interstitial pneumonitis and bronchiolitis have been described
Ultrastructural Features
-
▪
The virion is spherical to polyhedral, ranging from 24 to 30 nm; particles may aggregate and form crystalline arrays
Pathologic Differential Diagnosis
-
▪
Other viral causes of interstitial pneumonitis and bronchiolitis
The Enterovirus genus within the family Picornaviridae includes EVs and RVs, which are nonenveloped, single-stranded RNA viruses. Molecular and serologic characteristics have further defined the viruses into species, each of which contain many types. Seven of the species are human pathogens and include four EV species: EV-A, EV-B, EV-C, and EV-D and three RV species: RV-A, RV-B, and RV-C. Some important pathogenic EV members include EV-A71 and several Coxsackievirus group A (CVA) viruses within EV-A, Coxsackievirus group B (CVB) viruses and echoviruses within EV-B, polioviruses 1 to 3 and several CVA viruses within EV-C, and EV-D68 and EV-D70 within EV-D.
Clinical Features
EVs are one of the leading causes of upper respiratory tract infection and the common cold and are associated with diverse clinical syndromes spanning mild illness to severe and potentially fatal conditions. In the United States EV- and RV-associated respiratory infections occur most frequently in summer and fall months and can affect both healthy and immunocompromised patients, children and the elderly, though many reported infections occur in children. EV-D68 and RVs are the most common viruses associated with respiratory disease though other viruses, such as echovirus 30, CVB, EV-A71, and several EV-C viruses, are increasingly reported. Infections can be asymptomatic, mild with both upper and lower respiratory tract disease, or severe and life threatening. An increase in EV-D68 cases has been reported since 2008, including an outbreak in 2014 affecting more than 1,000 individuals across the United States. It is not entirely clear whether this increase is due to improved molecular identification and/or an increasing incidence. In addition, association of virus with illness can be complicated by the high prevalence of EVs, particularly RVs, in asymptomatic individuals. Symptoms include dyspnea, pharyngitis, cough, bronchiolitis, sinusitis, asthma exacerbation, wheezing, and pneumonia. Fever is not seen in all cases. Preexisting respiratory illness may be a risk factor for severe disease, because in some reports a large number of the patients have underlying respiratory illness, such as asthma or chronic obstructive pulmonary disease.
Radiologic Features
Radiographic findings include perihilar and alveolar infiltrates, patchy consolidation, pneumothorax, and atelectasis. Extensive-to-patchy ground-glass opacities have been reported with CT scans.
Pathologic Features
Gross Findings
Extensive gross pathology findings from fatal cases have not been reported. Findings in a few cases are similar to other cases of viral pneumonia, with congestion, hemorrhage, and edema. Hyperinflation and mucus plugging has also been noted.
Microscopic Findings
Similar to HMPV, histopathologic descriptions are few. Mononuclear bronchiolitis and interstitial pneumonitis are the main reported histologic findings (Fig. 13.15A–C ). Bronchiolitis obliterans with organizing pneumonia, acute alveolitis, and tracheobronchitis have also been reported. Comorbidities such as asthma and coinfections with other viral or bacterial pathogens can occur, and histologic changes are influenced by the specific agent or condition.
FIG. 13.15.

Respiratory enteroviruses. (A and B) Bronchiolitis and interstitial pneumonitis with scant intraalveolar edema, fibrin, and mixed inflammatory cells from a fatal rhinovirus case and corresponding high-magnification image showing abundant immunostaining within the same bronchiole. (C) Prominent mononuclear bronchitis from a fatal EV-D68 case. Note the mural eosinophilia (arrow), basement membrane thickening (arrowhead), and luminal mucus plugging consistent with asthma. (D) Extensive immunostaining of cells of intact and sloughed respiratory epithelium from the same case.
Ancillary Studies
Diagnosis can be made by virus isolation, ISH, direct detection of viral antigens by IFA or IHC (Fig. 13.15C and D); detection of viral RNA by PCR assays in fresh clinical samples or formalin-fixed, paraffin-embedded tissues; or a demonstrated rise in serum antibodies. Serology is not regarded as a reliable diagnostic tool, and PCR is often relied upon for a definitive diagnosis and specific virus identification. Systematic studies evaluating localization of EV antigens in tissue samples are needed. In one experimental inoculation study, viral nucleic acids were noted within bronchiolar epithelium by ISH. Antigen has been noted in airway epithelium, cells within alveoli and within alveolar macrophages, and bronchiolar glands in a small number of cases.
Differential Diagnosis
Other viral causes of interstitial pneumonitis and bronchiolitis, including RSV, HPIV, HMPV, and influenza virus, should be considered. Laboratory testing, including PCR and IHC, can be useful in making this differentiation possible.
Prognosis and Therapy
Supportive management is the basis of treatment, because there are no specific preventative or therapeutic treatments for respiratory EV infections commercially available at this time. Ribavirin and intravenous immunoglobulin therapies without substantial clinical benefit have been reported. Disease management should be related to infection control and prevention. Many infections are mild, though severe and possibly fatal lower respiratory tract infection has been reported in patients, particularly those with chronic or preexisting respiratory disease or hematopoietic stem cell transplant recipients.
Henipaviruses
Henipaviruses—Fact Sheet.
Definition
-
▪
Hendra and Nipah viruses are nonsegmented, negative-stranded RNA viruses, members of the family Paramyxoviridae and subfamily Paramyxovirinae
Incidence and Location
-
▪
Rare cases have occurred in Australia and Asia throughout the distribution of the animal reservoir, the fruit bat of the Pteropus genus
-
▪
Infection has occurred primarily in patients who have contact with sick horses and pigs
-
▪
Food-borne transmission has also been reported in an individual who consumed fruit contaminated by Pteropus bats
Morbidity and Mortality
-
▪
Death occurs in about 30% to 40% of Nipah cases associated with rapidly developing severe neurologic signs
-
▪
Four of seven of the known Hendra cases died
Gender, Race, and Age Distribution
-
▪
Patient demographics depend largely on the mode of exposure; in most cases occupational
-
▪
No apparent gender or racial predilection
Clinical Features
-
▪
Acute febrile encephalitis and influenza-like illness
Radiologic Features
-
▪
Bilateral infiltrates consistent with the acute respiratory distress syndrome
Therapy
-
▪
Treatment is supportive, including mechanical ventilation when needed
-
▪
Equivocal data regarding the efficacy of ribavirin in treatment
Henipaviruses—Pathologic Features.
Gross Findings
-
▪
In fatal cases, the lungs are heavy and display congestion, hemorrhage, and edema
Microscopic Findings
-
▪
Histopathologic involvement of the central nervous system and respiratory system
-
▪
Vasculitis, thrombosis, endothelial cell damage, and syncytial cell formation
-
▪
Multinucleated giant cells with intranuclear and cytoplasmic inclusions in the brain, lung, and other organs
-
▪
Organizing diffuse alveolar damage in patients who die later in the course of the illness
Immunohistochemical Features
-
▪
Widespread presence of henipavirus antigens can be seen by IHC in endothelial and smooth muscle cells of blood vessels, as well as in various parenchymal cells
Ultrastructural Features
-
▪
Pleomorphic viral particles composed of helical nucleocapsids enclosed within an envelope
Pathologic Differential Diagnosis
-
▪
Other viral causes of encephalitis, giant cell pneumonia, and diffuse alveolar damage, as well as noninfectious causes of diffuse alveolar damage
Hendra and Nipah viruses belong to the recently designated genus Henipavirus within the family Paramyxoviridae, subfamily Paramyxovirinae, and are nonsegmented, negative-stranded RNA viruses. These zoonotic pathogens were first identified in Australia and Malaysia and have been associated with acute febrile encephalitis and respiratory tract disease.
Clinical Features
Hendra was identified in 1994 when patients who came in close contact with sick horses developed an influenza-like illness with fever, myalgia, headache, lethargy, sore throat, nausea, and vomiting. Two patients died with pneumonitis and multiorgan failure. The closely related Nipah virus was identified during an outbreak in Malaysia and Singapore during 1998 to 1999 that included more than 250 patients. The incubation period ranged from 2 days to 1 month, but in most cases lasted between 1 and 2 weeks. Patients presented with a severe acute encephalitic syndrome, but some also had significant pulmonary manifestations. In Bangladesh in 2001 and 2003, outbreaks of Nipah encephalitis occurred. Similar to the Malaysian outbreak, the most prominent symptoms were fever, headache, vomiting, and an altered level of consciousness. Respiratory illness was much more common in the Bangladesh cases, however, with 64% having cough and dyspnea. Epidemiologic and laboratory investigations identified pteropid bats as asymptomatic carriers of Hendra and Nipah viruses and the natural animal reservoirs. Food-borne transmission has also been reported in an individual who consumed fruit contaminated by Pteropus bats.
Radiologic Features
In patients with respiratory illness, chest radiographs reveal bilateral infiltrates consistent with ARDS.
Pathologic Features
Gross Findings
In fatal infections, the lungs are heavy, congested, edematous, and hemorrhagic.
Microscopic Findings
Histopathologic findings in fatal cases of Hendra and Nipah infections are similar, with varying degrees of central nervous system and respiratory tract involvement. Findings include a systemic vasculitis with extensive thrombosis, endothelial cell damage, necrosis, and syncytial giant cell formation in affected vessels (Fig. 13.16D ). Multinucleated giant cells with intranuclear inclusions can occasionally be seen in lung, spleen, lymph nodes, and kidneys. In the lung, vasculitis and fibrinoid necrosis can be seen in the majority of cases, and interstitial pneumonitis, edema, and congestion with epithelial syncytial cells can be seen (Fig. 13.16A). Multinucleated giant cells with intranuclear inclusions are usually noted in alveolar spaces adjacent to necrotic areas (Fig. 13.16C).
FIG. 13.16.

Nipah virus pneumonia. (A) Interstitial pneumonitis, edema, and congestion with intraalveolar epithelial syncytial cells. (B) IHC highlights viral antigens within respiratory epithelium and syncytial cells. (C) Intraalveolar multinucleated giant cell with intranuclear inclusions (arrow). (D) Pulmonary vessel showing a multinucleated endothelial syncytium. (E) Large accumulation of extracellular pleomorphic viral particles.
(E, courtesy of C.S. Goldsmith.)
Ancillary Studies and Differential Diagnosis
The diagnosis of Nipah virus infection, suspected by patient history and clinical manifestations, can be supported by characteristic histopathologic findings. The most unique histopathologic finding is the presence of syncytial and parenchymal multinucleated endothelial cells. However, this feature occurs in only about one-fourth of the cases and cannot be used as a sensitive criterion for the diagnosis of henipavirus infections; furthermore, similar cells can also be seen in measles virus, RSV, HPIV, herpesviruses, and other infections. In addition to these viral infections, other noninfectious causes of diffuse alveolar damage may be considered in the differential diagnosis. Unequivocal diagnosis can be made only by laboratory tests such as IHC, cell culture isolation, PCR, or serology. IHC can reveal widespread presence of Nipah virus antigens in endothelial and smooth muscle cells of blood vessels, as well as in various parenchymal cells (Fig. 13.16B). Ultrastructural studies can also demonstrate the pleomorphic viral particles, which are composed of helical nucleocapsids enclosed within an envelope (Fig. 13.16E).
Prognosis and Therapy
Only seven persons are known to have been infected with Hendra virus. Death occurs in about 30% to 40% of patients infected with Nipah virus and is more frequent in patients with rapidly developing severe neurologic signs. Residual neurologic signs are common among survivors. Treatment is supportive, including mechanical ventilation for patients in a deep coma who are unable to maintain airways. Ribavirin was used in humans during the Nipah outbreak in Malaysia, with equivocal results, and passive immunization targeting the Nipah G glycoprotein has been evaluated as postexposure therapy in a ferret model and found to be of benefit.
Hemorrhagic Fever Viruses
Viral Hemorrhagic Fevers—Fact Sheet.
Definition
-
▪
VHFs are systemic infections characterized by fever and hemorrhage, caused by a group of viruses transmitted to humans by arthropods and rodents
-
▪
Viruses belong to four different families that differ in their genomic structure, replication strategy, and morphologic features (Arenaviridae, Bunyaviridae, Flaviviridae, and Filoviridae)
-
▪
Arenaviruses, bunyaviruses, and filoviruses are negative-stranded, whereas flaviviruses are positive-stranded RNA viruses
Incidence and Location
-
▪
Uncommon diseases
-
▪
Hemorrhagic fever viruses are distributed worldwide, and the diseases they cause are traditionally named according to the location where they were first described
-
▪
The distributions of particular VHFs are related to the distributions of their specific arthropod, rodent, and bat vectors
Mortality
-
▪
Case mortality ranges from about 15% with infections such as Lassa fever up to 90% with filovirus infections such as Ebola
Gender, Race, and Age Distribution
-
▪
Patient demographics depend largely on the agent and mode of exposure
-
▪
People of all ages are vulnerable to VHFs
-
▪
No recognized gender or racial predilection
Clinical Features
-
▪
Fever, myalgia, asthenia, and prostration
-
▪
Loss of vascular regulation, shock, and central nervous system dysfunction
-
▪
Hemorrhage
Radiologic Features
-
▪
Bilateral interstitial or alveolar edema and infiltrates
Prognosis and Therapy
-
▪
Supportive therapy
-
▪
Passive antibodies
-
▪
Antivirals (ribavirin)
-
▪
An experimental vaccine for Ebola is under development
Viral Hemorrhagic Fevers—Pathologic Features.
Gross Findings
-
▪
In fatal cases, the lungs display congestion, hemorrhage, and edema
-
▪
Widespread petechial hemorrhages and ecchymoses are present systemically
Microscopic Findings
-
▪
Histopathologic changes in the lung include varying degrees of hemorrhage, edema, interstitial pneumonitis, and diffuse alveolar damage
-
▪
Other systemic pathology as described in the text
Immunohistochemical Features
-
▪
Viral antigens can commonly be detected by IHC in the mononuclear phagocytic system and endothelium, as well as in parenchymal cells, depending on the particular VHF agent
Ultrastructural Features
-
▪
Features vary for the four families of viruses that cause VHF syndromes
-
▪
All viruses have a lipid envelope that is acquired by budding at either the cell surface or the internal membranes
-
▪
The sizes and shape of these viruses vary from relatively small (35–50 nm), uniform round particles, as seen with flaviviruses, to more pleomorphic, rod-shaped particles (occasionally measuring up to 15,000 nm) in the case of filoviruses
Pathologic Differential Diagnosis
-
▪
Other causes of hemorrhage, edema, and diffuse alveolar damage
-
▪
Unequivocal diagnosis can be made only by laboratory tests such as cell culture isolation, serology, PCR, and IHC
The combination of fever and hemorrhage can be caused by different viruses, rickettsiae, bacteria, protozoa, and fungi. However, the term viral hemorrhagic fever (VHF) is usually reserved for systemic infections characterized by fever and hemorrhage caused by a special group of viruses transmitted to humans by arthropods and rodents. VHFs are febrile illnesses characterized by abnormal vascular regulation and vascular damage and are caused by small, lipid-enveloped RNA viruses. This syndrome can be caused by RNA viruses belonging to four different families that differ in their genomic structure, replication strategy, and morphologic features (Arenaviridae, Bunyaviridae, Flaviviridae, and Filoviridae). Arenaviruses, bunyaviruses, and filoviruses are negative-stranded, whereas flaviviruses are positive-stranded RNA viruses. Hemorrhagic fever viruses are distributed worldwide, and the diseases they cause are traditionally named according to the location where they were first described. The oldest and best known is yellow fever virus; others include Lassa fever, lymphocytic choriomeningitis, Ebola, and dengue viruses. The distributions of the individual VHFs are related to the distributions of their specific arthropod and rodent vectors.
Clinical Features
VHF is characterized clinically by its disproportionate effect on the vascular system. Typical manifestations are related to a loss of vascular regulation (vasodilatation and hypotension), vascular damage (leakage of protein into the urine, edema in soft tissues of the face and other loose connective tissues, and petechial hemorrhage in the skin and internal organs), and severe systemic derangement that presents as fever, myalgia, and asthenia proceeding to a state of prostration. Hemorrhage is common with most of these diseases and usually originates from mucosal surfaces. Patients with severe hemorrhagic fever generally develop shock, diffuse bleeding, and central nervous system dysfunction.
Radiologic Features
In patients with respiratory illness, chest radiographs may reveal bilateral interstitial and alveolar edema and hemorrhage.
Pathologic Features
Gross Findings
In fatal VHF, the lungs show congestion, hemorrhage, and edema. Pleural effusion may be found with certain infections.
Microscopic Findings
At autopsy, common findings include widespread petechial hemorrhages and ecchymoses involving skin, mucous membranes, and internal organs. However, in many VHF patients manifestations of bleeding may be minimal or absent. Effusions, occasionally hemorrhagic, are also frequently seen. Widespread, focal, and sometimes massive necrosis is commonly observed in all organ systems and is often ischemic in nature. Necrosis is usually most prominent in the liver and lymphoid tissues. The most consistent microscopic feature is found in the liver and consists of multifocal hepatocellular necrosis with cytoplasmic eosinophilia, Councilman bodies, nuclear pyknosis, and cytolysis. Inflammatory cell infiltrates and necrotic areas are usually mild and, when present, consist of neutrophils and mononuclear cells. Commonly observed histopathologic changes in the lung include various degrees of hemorrhage, intraalveolar edema, interstitial pneumonitis, and diffuse alveolar damage (Fig. 13.17A and E ).
FIG. 13.17.

Viral hemorrhagic fevers. (A) Lung from a fatal Ebola case showing pulmonary congestion and lack of significant inflammatory infiltrates, edema or hemorrhage. Within the center of the image is an alveolar macrophage (arrow) containing a cytoplasmic eosinophilic inclusion that is highlighted in the magnified inset. (B and C) Immunohistochemistry from the same patient shows viral antigen in alveolar macrophages and pulmonary interstitium. (D) Filamentous Ebola viral particles are seen within an alveolar space. Rod-shaped nucleocapsids are visible within viral particles, and have a characteristic “bull’s-eye” appearance on cross-section (arrow). (E) Extensive intraalveolar hemorrhage from a fatal dengue case. (F) Dengue viral antigen-containing mononuclear cell.
(D, courtesy of C.S. Goldsmith.)
Ancillary Studies and Differential Diagnosis
The diagnosis of VHF should be suspected in patients with appropriate clinical manifestations returning from an endemic area, particularly if there is travel to rural areas during seasonal or epidemic disease activity. The diagnosis suspected by history and clinical manifestations can also be supported histopathologically. However, because of similar pathologic features seen in VHF and a variety of other viral, rickettsial, and bacterial infections, as well as noninfectious causes of hemorrhage, edema, and diffuse alveolar damage, unequivocal diagnosis can be made only by laboratory tests such as cell culture isolation, serology, PCR, and IHC (Fig. 13.17B, C, and F). Ultrastructural studies can also demonstrate the presence of virions. All viruses have a lipid envelope that is acquired by budding at either the cell surface or the internal membranes. The size and shape of these viruses vary from relatively small (35–50 nm), uniform, round particles, as seen with flaviviruses, to more pleomorphic, rod-shaped particles (measuring occasionally up to 15,000 nm) in the case of filoviruses (Fig. 13.17D).
Prognosis and Therapy
Case mortality ranges from about 15% with infections such as Lassa fever up to 90% with filovirus infections such as Ebola. Over 28,000 total suspected and confirmed cases were reported with over 11,000 deaths during the 2014 outbreak of Ebola in West Africa. Treatment depends on the particular agent and may include the use of passive antibodies, antiviral drugs such as ribavirin, or supportive therapy. Supportive therapy should include the reasonable measures that would be employed in any very ill patient with a fragile vascular bed. Volume replacement may be particularly important in some patients, especially with dengue hemorrhagic fever. Experimental vaccines and treatments for Ebola are under clinical trial.
Suggested Readings
General
- 1.Connor D.H., Chandler F.W., Schwartz D.A., Manz H.J., Lack E.E., editors. Pathology of Infectious Disease. Appleton & Lange; Connecticut: 1997. [Google Scholar]
- 2.de Roux A., Marcos M.A., Garcia E. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 3.Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott-Williams & Williams; Philadelphia: 2001. [Google Scholar]
- 4.Katzenstein AL., Infection unusual pneumonias . In: Katzenstein and Askin’s Surgical Pathology of Non-neoplastic Lung Diseases. 4th ed. Katzenstein A.L., editor. Saunders Co.; Philadelphia: 2006. pp. 261–304. [Google Scholar]
- 5.Mahy B.W.J., Collier L., editors. Microbiology and Microbial Infections: Virology. 9th ed. Arnold; London: 1998. [Google Scholar]
- 6.Palmer E.L., Martin M.L. CRC Press, Inc.; Boca Raton, FL: 1988. Electron Microscopy in Viral Diagnosis. [Google Scholar]
- 7.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis W.D., Colby T.V., Koss M.N. Lung infections. In: King D.W., editor. Non-Neoplastic Disorders of the Lower Respiratory Tract. American Registry of Pathology and the Armed Forces Institute of Pathology; Washington, DC: 2002. [Google Scholar]
- 9.Winn W.C., Walker D.H. Viral infections. In: Dail D.H., Hammar S.P., editors. Pulmonary Pathology. 2nd ed. Springer-Verlag; New York: 1994. pp. 429–464. [Google Scholar]
Adenoviruses
- 10.Becroft D.M. Histopathology of fatal adenovirus infection of the respiratory tract in young children. J Clin Pathol. 1967;20:561–569. doi: 10.1136/jcp.20.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudding B.A., Wagner S.C., Zeller J.A., Gmelich J.T., French G.R., Top F.H., Jr. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med. 1972;286:1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 12.Hierholzer J.C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5:262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajon A.E., Lu X., Erdman D.D. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J Infect Dis. 2010;202(1):93–103. doi: 10.1086/653083. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.J., Kim K., Park S.B., Hong D.J., Jhun B.W. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One. 2015;10(4):e0122642. doi: 10.1371/journal.pone.0122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham T.T., Burchette J.L., Jr., Hale L.P. Fatal disseminated adenovirus infections in immunocompromised patients. Am J Clin Pathol. 2003;120:575–583. doi: 10.1309/AWXD-GNC5-D70E-N7YT. [DOI] [PubMed] [Google Scholar]
- 16.Sun B., He H., Wang Z. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18(4):456. doi: 10.1186/s13054-014-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahradnik J.M. Adenovirus pneumonia. Semin Respir Infect. 1987;2:104–111. [PubMed] [Google Scholar]
Hantaviruses
- 18.Abel Borges A., Figueiredo L.T. Mechanisms of shock in hantavirus pulmonary syndrome. Curr Opin Infect Dis. 2008;21(3):293–297. doi: 10.1097/QCO.0b013e3282f88b6f. [DOI] [PubMed] [Google Scholar]
- 19.Duchin J.S., Koster F.T., Peters C.J. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 20.Gnemmi V., Verine J., Vrigneaud L. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavirus nephropathy. Hum Pathol. 2015;46(6):827–835. doi: 10.1016/j.humpath.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Koster F., Foucar K., Hjelle B. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;166:665–672. doi: 10.1309/CNWF-DC72-QYMR-M8DA. [DOI] [PubMed] [Google Scholar]
- 22.Lukes R.J. The pathology of thirty-nine fatal cases of epidemic hemorrhagic fever. Am J Med. 1954;16:639–650. doi: 10.1016/0002-9343(54)90270-3. [DOI] [PubMed] [Google Scholar]
- 23.MacNeil A., Ksiazek T.G., Rollin P.E. Hantavirus pulmonary syndrome, United States, 1993-2009. Emerg Infect Dis. 2011;17(7):1195–1201. doi: 10.3201/eid1707.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertz G.J., Brian H., Crowley M. Diagnosis and treatment of new world hantavirus infections. Curr Opin Infect Dis. 2006;19(5):437–442. doi: 10.1097/01.qco.0000244048.38758.1f. [DOI] [PubMed] [Google Scholar]
- 25.Nolte K.B., Feddersen R.M., Foucar K. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 26.Zaki S.R., Greer P.W., Coffield L.M. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 27.Zaki S.R., Khan A.S., Goodman R.A. Retrospective diagnosis of hantavirus pulmonary syndrome, 1978-1993: implications for emerging infectious diseases. Arch Pathol Lab Med. 1996;120:134–139. [PubMed] [Google Scholar]
Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome
- 28.Chong P.Y., Chui P., Ling A.E. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.5858/2004-128-195-AODDTS. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franks T.J., Chong P.Y., Chui P. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldsmith C.S., Tatti K.M., Ksiazek T.G. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10:320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guery B., Poissy J., el Mansouf L. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls J.M., Butany J., Poon L.L. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3:e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls J.M., Poon L.L., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng D.L., Al Hosani F.A., Keating M.K. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186(3):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pene F., Merlat A., Vabret A. Coronavirus 229E-related pneumonia in immunocompromised patients. CID. 2003;37:929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shieh W.J., Hsiao C.H., Paddock C.D. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.To K.F., Tong J.H., Chan P.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization guidance for clinical management of severe acute respiratory infections when novel coronavirus is suspected: what to do and what not to do. http://www.who.int/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13u.pdf Available at. Accessed August 15, 2016.
- 41.Zaki A.M., van Boheemen S., Bestebroer T.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. NEJM. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
Cytomegalovirus
- 42.Beschorner W.E., Hutchins G.M., Burns W.H., Saral R., Tutschka P.J., Santos G.W. Cytomegalovirus pneumonia in bone marrow transplant recipients: miliary and diffuse patterns. Am Rev Respir Dis. 1980;122:107–114. doi: 10.1164/arrd.1980.122.1.107. [DOI] [PubMed] [Google Scholar]
- 43.Cascia A., Iaria C., Ruggeri P. Cytomegalovirus pneumonia in patients with inflammatory bowel disease: a systematic review. Int J Infect Dis. 2012;16(7):e474–e479. doi: 10.1016/j.ijid.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Herry I., Cadranel J., Antoine M. Cytomegalovirus-induced alveolar hemorrhage in patients with AIDS: a new clinical entity? Clin Infect Dis. 1996;22:616–620. doi: 10.1093/clinids/22.4.616. [DOI] [PubMed] [Google Scholar]
- 45.Ison M.G., Fishman J.A. Cytomegalovirus pneumonia in transplant recipients. Clin Chest Med. 2005;26:691–705. doi: 10.1016/j.ccm.2005.06.013. viii. [DOI] [PubMed] [Google Scholar]
- 46.Ljungman P., Griffiths P., Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 47.Lurain N.S., Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace J.M., Hannah J. Cytomegalovirus pneumonitis in patients with AIDS: findings in an autopsy series. Chest. 1987;92:198–203. doi: 10.1378/chest.92.2.198. [DOI] [PubMed] [Google Scholar]
Herpes Simplex Viruses
- 49.Brodofel H., Vogel M., Spira D. Herpes-simplex-virus 1 pneumonia in the immunocompromised host: high-resolution CT patterns in correlation to outcome and follow-up. Eur J Radiol. 2012;81(4):e415–e420. doi: 10.1016/j.ejrad.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Chong S., Kim T.S., Cho E.Y. Herpes simplex virus pneumonia: high-resolution CT findings. Br J Radiol. 2010;83(991):585–589. doi: 10.1259/bjr/51409455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feldman S., Stokes D.C. Varicella zoster and herpes simplex virus pneumonias. Semin Respir Infect. 1987;2:84–94. [PubMed] [Google Scholar]
- 52.Kimberlin D.W. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1–13. doi: 10.1128/CMR.17.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nash G. Necrotizing tracheobronchitis and bronchopneumonia consistent with herpetic infection. Hum Pathol. 1972;3:2832–2891. doi: 10.1016/s0046-8177(72)80082-0. [DOI] [PubMed] [Google Scholar]
- 54.Ramsey P.G., Fife K.H., Hackman R.C., Meyers J.D., Corey L. Herpes simplex virus pneumonia: clinical, virologic, and pathologic features in 20 patients. Ann Intern Med. 1982;97:813–820. doi: 10.7326/0003-4819-97-6-813. [DOI] [PubMed] [Google Scholar]
- 55.Singer D.B. Pathology of neonatal herpes simplex virus infection. Perspect Pediatr Pathol. 1981;6:243–278. [PubMed] [Google Scholar]
Varicella Zoster Virus
- 56.Feldman S. Varicella-zoster virus pneumonitis. Chest. 1994;106:22S–27S. doi: 10.1378/chest.106.1_supplement.22s. [DOI] [PubMed] [Google Scholar]
- 57.Harger J.H., Ernest J.M., Thurnau G.R. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis. 2002;185:422–427. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- 58.Marin M., Bialek S.R., Seward J.F. Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013 Jul 19;62(28):574–576. [PMC free article] [PubMed] [Google Scholar]
- 59.Mermelstein R.H., Freireich A.W. Varicella pneumonia. Ann Intern Med. 1961;55:456–463. doi: 10.7326/0003-4819-55-3-456. [DOI] [PubMed] [Google Scholar]
- 60.Triebwasser J.H., Harris R.E., Bryant R.E., Rhoades E.R. Varicella pneumonia in adults: report of seven cases and a review of literature. Medicine (Baltimore) 1967;46:409–423. [PubMed] [Google Scholar]
- 61.Waring J.J., Neubuerger K., Geever E.F. Severe forms of chickenpox in adults. Arch Intern Med. 1942;69:384–408. [Google Scholar]
- 62.Zerboni L., Sen N., Oliver S.L. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12(3):197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Influenza Viruses
- 63.Guarner J., Paddock C.D., Shieh W.J. Histopathologic and immunohistochemical features of fatal influenza virus infections in children during the 2003-2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- 64.Guarner J., Shieh W.J., Dawson J. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- 65.Hers J.F., Masurel N., Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 66.Hers J.F.P. Changes in the respiratory mucosa resulting from infection with influenza virus B. J Pathol Bacteriol. 1957;73:565–568. [Google Scholar]
- 67.Martin C.M., Kunin C.M., Gottlieb L.S., Barnes M.W., Liu C., Finland M. Asian influenza A in Boston, 1957-1958: I. Observations in thirty-two influenza-associated fatal cases. AMA Arch Intern Med. 1959;103:515–531. doi: 10.1001/archinte.1959.00270040001001. [DOI] [PubMed] [Google Scholar]
- 68.Nolte K.B., Alakija P., Oty G. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000;21:375–379. doi: 10.1097/00000433-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 69.Paddock C.D., Liu L., Denison A.M. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205(6):895–905. doi: 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 70.Shieh W.J., Blau D.M., Denison A.M. 2009 Pandemic influenza A (H1N1) Am J Pathol. 2010;177(1):166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinya K., Ebina M., Yamada S., Ono M., Kasai N., Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 72.Shrestha S.S., Swerdlow D.L., Borse R.H. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010) Clin Infect Dis. 2011;52(Suppl 1):S75–S82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 73.Ungchusak K., Auewarakul P., Dowell S.F. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 74.Van Riel D., Munster V.J., de Wit E. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 75.Yeldandi A.V., Colby T.V. Pathologic features of lung biopsy specimens from influenza pneumonia cases. Hum Pathol. 1994;25:47–53. doi: 10.1016/0046-8177(94)90170-8. [DOI] [PubMed] [Google Scholar]
Measles Virus
- 76.Akhtar M., Young I. Measles giant cell pneumonia in an adult following long-term chemotherapy. Arch Pathol. 1973;96:145–148. [PubMed] [Google Scholar]
- 77.Archibald R.W., Weller R.O., Meadow S.R. Measles pneumonia and the nature of the inclusion-bearing giant cells: a light- and electron-microscope study. J Pathol. 1971;103:27–34. doi: 10.1002/path.1711030104. [DOI] [PubMed] [Google Scholar]
- 78.Breitfeld V., Hashida Y., Sherman F.E., Odagiri K., Yunis E.J. Fatal measles infection in children with leukemia. Lab Invest. 1973;28:279–291. [PubMed] [Google Scholar]
- 79.Kipps A., Kaschula R.O. Virus pneumonia following measles: a virological and histological study of autopsy material. S Afr Med J. 1976;50:1083–1088. [PubMed] [Google Scholar]
- 80.Measles Cases and Outbreaks. http://www.cdc.gov/measles/cases-outbreaks.html Available at. Accessed May 2016.
- 81.Monafo W.J., Haslam D.B., Roberts R.L., Zaki S.R., Bellini W.J., Coffin C.M. Disseminated measles infection after vaccination in a child with a congenital immunodeficiency. J Pediatr. 1994;124:273–276. doi: 10.1016/s0022-3476(94)70318-3. [DOI] [PubMed] [Google Scholar]
- 82.Sata T., Kurata T., Aoyama Y., Sakaguchi M., Yamanouchi K., Takeda K. Analysis of viral antigens in giant cells of measles pneumonia by immunoperoxidase method. Virchows Arch A Pathol Anat Histopathol. 1986;410:133–138. doi: 10.1007/BF00713517. [DOI] [PubMed] [Google Scholar]
- 83.Sobonya R.E., Hiller F.C., Pingleton W., Watanabe I. Fatal measles (rubeola) pneumonia in adults. Arch Pathol Lab Med. 1978;102:366–371. [PubMed] [Google Scholar]
Human Parainfluenza Virus
- 84.Akizuki S., Nasu N., Setoguchi M., Yoshida S., Higuchi Y., Yamamoto S. Parainfluenza virus pneumonitis in an adult. Arch Pathol Lab Med. 1991;115:824–826. [PubMed] [Google Scholar]
- 85.Butnor K.J., Sporn T.A. Human parainfluenza virus giant cell pneumonia following cord blood transplant associated with pulmonary alveolar proteinosis. Arch Pathol Lab Med. 2003;127:235–238. doi: 10.5858/2003-127-235-HPVGCP. [DOI] [PubMed] [Google Scholar]
- 86.Jarvis W.R., Middleton P.J., Gelfand E.W. Parainfluenza pneumonia in severe combined immunodeficiency disease. J Pediatr. 1979;94:423–425. doi: 10.1016/s0022-3476(79)80590-9. [DOI] [PubMed] [Google Scholar]
- 87.Little B.W., Tihen W.S., Dickerman J.D., Craighead J.E. Giant cell pneumonia associated with parainfluenza virus type 3 infection. Hum Pathol. 1981;12:478–481. doi: 10.1016/s0046-8177(81)80032-9. [DOI] [PubMed] [Google Scholar]
- 88.Madden J.F., Burchette J.L., Jr., Hale L.P. Pathology of parainfluenza virus infection in patients with congenital immunodeficiency syndromes. Hum Pathol. 2004;35:594–603. doi: 10.1016/j.humpath.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Weintrub P.S., Sullender W.M., Lombard C., Link M.P., Arvin A. Giant cell pneumonia caused by parainfluenza type 3 in a patient with acute myelomonocytic leukemia. Arch Pathol Lab Med. 1987;111:569–570. [PubMed] [Google Scholar]
- 90.Wendt C.H., Weisdorf D.J., Jordan M.C., Balfour H.H., Jr., Hertz M.I. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326:921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
Respiratory Syncytial Virus
- 91.Delage G., Brochu P., Robillard L., Jasmin G., Joncas J.H., Lapointe N. Giant cell pneumonia due to respiratory syncytial virus. Occurrence in severe combined immunodeficiency syndrome. Arch Pathol Lab Med. 1984;108:623–625. [PubMed] [Google Scholar]
- 92.Englund J.A., Sullivan C.J., Jordan M.C., Dehner L.P., Vercellotti G.M., Balfour H.H., Jr. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 93.Gomez R.S., Guisle-Marsollier I., Bohmwald K. Respiratory syncytial virus: pathology, therapeutic drugs and prophylaxis. Immunol Lett. 2014;162:237–247. doi: 10.1016/j.imlet.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Neilson K.A., Yunis E.J. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
Human Metapneumovirus
- 95.Cane P.A., van den Hoogen B.G., Chakrabarti S., Fegan C.D., Osterhaus A.D. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 96.Debiaggi M., Canducci F., Sampaolo M. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 97.Englund J., Boeckh M., Kuypers J. Fatal human metapneumovirus infection in stem cell transplant recipients. Ann Inter Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 98.Haynes A.K., Fowlkes A.L., Schneider E. Human metapneumovirus circulation in the United States, 2008-2014. Pediatrics. 2016;137(5):1–7. doi: 10.1542/peds.2015-2927. [DOI] [PubMed] [Google Scholar]
- 99.Kim Y.J., Boeckh M., Englund J.A. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cells and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 100.Kuiken T., van den Hoogen B.G., van Riel D.A. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol. 2004;164:1893–1900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sumino K.C., Agapov E., Pierce R.A. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis. 2005;192:1052–1060. doi: 10.1086/432728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enteroviruses
- 103.Abedi G.R., Watson J.T., Pham H. Enterovirus and human parechovirus surveillance—United States, 2009–2013. MMWR. 2015;64(34):940–943. doi: 10.15585/mmwr.mm6434a3. [DOI] [PubMed] [Google Scholar]
- 104.Cox D.W., Bizzintino J., Ferrari G. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Resp Crit Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gutman J.A., Peck A.J., Kuypers J., Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 2007;40:809–811. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacobson L.M., Redd J.T., Schneider E. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J. 2012;31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- 107.Jartti T., Lehtinen P., Vuorinen T. Respiratory picornaviruses and respiratory syncytial virus as causative agents of expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauinger I.L., Bible J.M., Halligan E.P. Patient characteristics and severity of human rhinovirus infections in children. J Clin Virol. 2013;58(1):216–220. doi: 10.1016/j.jcv.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lupo J., Schuffenecker I., Morel-Baccard C. Disseminated rhinovirus C8 Infection with infectious virus in blood and fatal outcome in a child with repeated episodes of bronchiolitis. J Clin Microbiol. 2015;53(5):1775–1777. doi: 10.1128/JCM.03484-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Midgley C.M., Jackson M.A., Selvarangan R. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR. 2014;63(36):798–799. [PMC free article] [PubMed] [Google Scholar]
- 111.Muehlenbachs A., Bhatnagar J., Zaki S.R. Tissue tropism, pathology and pathogenesis of enterovirus infection. J Pathol. 2015;235:217–228. doi: 10.1002/path.4438. [DOI] [PubMed] [Google Scholar]
- 112.Oermann C.M., Schuster J.E., Conners G.P. A focused review and clinical highlights from the 2014 U.S. outbreak. Ann Am Thorac Soc. 2015;12(5):775–781. doi: 10.1513/AnnalsATS.201412-592FR. [DOI] [PubMed] [Google Scholar]
- 113.Papadopoulos N.G., Bates P.J., Bardin P.G. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 114.Royston L., Tapparel C. Rhinoviruses and respiratory enteroviruses: not as simple as ABC. Viruses. 2016;8(1):16. doi: 10.3390/v8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henipahviruses
- 115.Aljofan M. Hendra and Nipah infection: emerging paramyxoviruses. Virus Res. 2013;177:119–126. doi: 10.1016/j.virusres.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Chua K.B., Bellini W.J., Rota P.A. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 117.Halpin K., Young P.L., Field H.E., Mackenzie J.S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 118.Hooper P., Zaki S., Daniels P., Middleton D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect. 2001;3:315–322. doi: 10.1016/s1286-4579(01)01385-5. [DOI] [PubMed] [Google Scholar]
- 119.Ksiazek T.G., Rota P.A., Rollin P.E. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 2011;162:173–183. doi: 10.1016/j.virusres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 120.Murray K., Selleck P., Hooper P. A Morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 121.O’Sullivan J.D., Allworth A.M., Paterson D.L. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349:93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 122.Paton N.I., Leo Y.S., Zaki S.R. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 123.Wong K.T., Shieh W.J., Kumar S. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemorrhagic Fever Viruses
- 124.Bhamarapravati N., Tuchinda P., Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 125.Burke T. Dengue haemorrhagic fever: a pathological study. Trans R Soc Trop Med Hyg. 1968;62:682–692. doi: 10.1016/0035-9203(68)90120-x. [DOI] [PubMed] [Google Scholar]
- 126.Burt F.J., Swanepoel R., Shieh W.J. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 127.Martines R.B., Ng D.L., Greer P.W. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol. 2015;235(2):153–174. doi: 10.1002/path.4456. [DOI] [PubMed] [Google Scholar]
- 128.Walker D.H., McCormick J.B., Johnson K.M. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982;107:349–356. [PMC free article] [PubMed] [Google Scholar]
- 129.Zaki S.R., Peters C.J. Viral hemorrhagic fevers. In: Connor D.H., Chandler F.W., Schwartz D.A., Manz H.J., Lack E.E., editors. Pathology of Infectious Diseases. Appleton and Lange; Stamford, CT: 1997. pp. 347–364. [Google Scholar]


