Abstract:
Immunoglobulin Y (IgY) is the major antibody in chickens, and is transferred in large quantities to the egg yolk to confer passive immunity to the developing embryo. IgY can easily be harvested from the yolk, and is an ideal alternative to using mammalian IgG antibodies. The following chapter discusses the production and purification of IgY, as well as its many advantages and applications, including the use of IgY as a diagnostic and analytical tool and for passive immunotherapy to treat numerous health conditions.
Key words: hen egg yolk antibodies (IgY), IgY purification, passive immunotherapy, diagnostics, immunoaffinity ligand
17.1. Introduction
It has been over 100 years since the existence of immunoglobulin (Ig) Y was first described by Klemperer (1893). It was found to be the major serum antibody in hens, and termed IgY due to its enrichment in egg yolk (Leslie and Clem, 1969). Since then, the avian immune system, and IgY, has been studied extensively, revealing many important characteristics and applications of IgY. The laying hen is an ethical alternative for the production of large quantities of specific antibodies at a low cost (Schade and Hlinak, 1996). Owing to its favorable immunochemical characteristics when compared to mammalian IgG, IgY has found many applications in the medical and research fields, including areas such as diagnostics and biomarker discovery. IgY has perhaps received the most attention for its immunotherapeutic potential. Passive immunotherapy, the administration of pre-formed antibodies to prevent infectious diseases or treat existing conditions, may be one of the most valuable applications of antibodies, and is currently driven by the need to find alternatives to traditional antibiotic therapy (Reilly et al., 1997, Carlander et al., 2000). Antibody therapy is, however, often limited by the significant amount of antibody required for treatment (Hatta et al., 1997b). Since IgY is transferred in large quantities to the egg yolk, and can easily be extracted and isolated, eggs are an ideal source of specific antibodies for passive immunotherapy.
The following chapter describes structural, physical and immunochemical properties of IgY, as well as an overview of IgY production and purification, and its applications in diagnostics, analytical applications and immunotherapy.
17.2. Overview of the avian immune system and IgY biosynthesis
The avian immune system is made up of the bursa of Fabricius, bone marrow, spleen, thymus, the Harderian gland, lymph nodes, circulating lymphocytes and various lymphoid tissues. The thymus serves as the primary lymphoid organ for T-cell differentiation, while the antibody-synthesizing B-cells are produced in the bursa of Fabricius (Sharma, 1997, Carlander et al., 1999a). The spleen is the centre for plasma cell proliferation and memory B cells (Carlander et al., 1999a). The vertebrate immune system has the ability to produce an exceedingly high number of different antibody molecules, through the existence of multiple variable (V), diversity (D) and joining (J) elements in the genome (the germ line repertoire), as well as existence of somatic recombination processes and point mutations (Reynaud et al., 1985, Parvari et al., 1988). In the avian immune system, however, this combinatorial diversity is restricted, as the rearrangement of immunoglobulin genes is not an ongoing process, but rather takes place as a single wave during early embryogenesis. The total number of rearrangements from which the chicken B-cell repertoire may be generated is limited to the number of B-cell precursors in the bursa (Reynaud et al., 1989, Reynaud et al., 1991), estimated to be around 2–3 × 104 cells (Pink et al., 1985). Despite the fact that chickens have an extremely limited number of immunoglobulin genes compared with mammals, they are capable of producing a wide range of immune responses and diverse antibody molecules (Sharma, 1997).
Three classes of antibody are found in the chicken, IgY, IgA and IgM, and are present in the serum at concentrations of 5.0, 1.25 and 0.61 mg/mL, respectively (Leslie and Martin, 1973). IgY, the functional equivalent of mammalian IgG, is the major antibody produced by chickens, and makes up approximately 75% of the total antibody population (Carlander et al., 2000). During egg formation, serum IgY is selectively transferred to the yolk via a receptor on the surface of the yolk membrane specific for IgY translocation, in order to confer passive immunity to the developing embryo (Fig. 17.1 ) (Loeken and Roth, 1983, Tressler and Roth, 1987, Morrison et al., 2002, West et al., 2004, Tesar et al., 2008). Egg yolk can contain between 5 to 25 mg/mL of IgY, whereas IgA and IgM are found in the egg white, at concentrations of around 0.15 and 0.7 mg/mL respectively (Rose et al., 1974, Schade et al., 1991, Li et al., 1997).
Fig. 17.1.
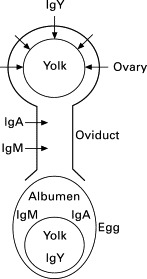
Distribution of antibodies in hen eggs.
Adapted from Hatta et al. (1997a).
17.3. Production and purification of IgY
17.3.1. Production
Several aspects of hen immunization, including the immunization route and vaccine adjuvant, have been studied in an effort to improve IgY production and yolk deposition (Levesque et al., 2007). Chang et al. (1999) demonstrated that intramuscular immunization resulted in higher levels of specific IgY when compared with immunization via the subcutaneous route. Oil-based adjuvants, such as Freund’s complete (FCA) and incomplete (FIA) adjuvant, continue to be the adjuvants of choice for IgY production (Trott et al, 2008); however, alternatives are continually being sought due to potentially severe injection site reactions (Wanke et al, 1996). The inclusion of bacterial components or immunostimulants in vaccine formulations has been shown to increase antigen-specific antibody levels in the yolk, and may require fewer injections and less antigen, and ultimately be more cost effective (Levesque et al., 2007, Trott et al., 2008, Herath et al., 2010). In fact, Levesque et al. (2007) found that adding synthetic oligonucleotides containing unmethylated CpG dinucleotides, characteristic of bacterial DNA (CpG ODNs), to FIA could increase specific IgY production by 480%. The use of DNA vaccines has also been described for IgY production (Romito et al., 2001, Cova, 2005, Witkowski et al., 2009). This process involves immunizing birds with plasmid DNA encoding the antigen of interest, and not only avoids the potentially costly and tedious preparation of purified antigens, but can also overcome interference by maternal antibodies (Elazab et al., 2009).
17.3.2. Purification
Egg yolk is a liquid emulsion of water-soluble protein and a dispersed phase of lipoprotein particles. One of the major challenges in IgY purification is separating the water-soluble IgY from the yolk lipoproteins (Hatta et al., 2008). IgY purification typically involves isolation of the IgY-containing water-soluble fraction (WSF), followed by additional purification steps. Simple dilution of the yolk with water, which results in the aggregation of yolk lipoproteins at low ionic strength (Jensenius et al., 1981), followed by centrifugation or ultrafiltration (UF), has been reported for the isolation of WSF (Akita and Nakai, 1992, Kim and Nakai, 1996, Kim and Nakai, 1998). Likewise, freezing and thawing of diluted yolk, producing lipid aggregates that are large enough to be removed by conventional low speed centrifugation (Jensenius and Koch, 1993), has also been employed, resulting in a purity of around 70% (Deignan et al., 2000). For these dilution methods, pH and extent of dilution are very important for optimal IgY recovery, and Nakai et al. (1994) found that the best results were obtained using a six-fold water dilution, at pH 5.0.
Other methods of removing lipoproteins prior to IgY purification include separation by ultracentrifugation (Mcbee and Cotterill, 1979, Akita and Nakai, 1992, Akita and Li-Chan, 1998), organic solvent delipidation (Polson, 1990, Kwan et al., 1991, Horikoshi et al., 1993, Mclaren et al., 1994), or use of lipoprotein coagulating agents such as polyethylene glycol (Akita and Nakai, 1993, Svendsen et al., 1995), dextran sodium sulfate (Jensenius et al., 1981), polyacrylic resin (Hamada et al., 1991) and dextran blue (Bizhanov and Vyshniauskis, 2000); however, application of these methods for large- scale IgY production is limited by problems related to food safety as well as cost constraints (Hatta et al., 2008). To address this problem, natural polysaccharides, which have been found to act as effective yolk lipoprotein coagulating agents (Hatta et al., 2008), have been employed, including sodium alginate (Hatta et al., 1988, Hatta et al., 1990), xanthan gum (Akita and Nakai, 1993) and α-carrageenan (Hatta et al., 1990). Chang et al. (2000) reported the precipitation of over 90% of lipoproteins from yolk using λ-carrageenan, sodium alginate, carboxymethyl cellulose and pectin.
A number of methods of purifying IgY following lipoprotein removal have been reported, and include ion exchange chromatography (Mccannel and Nakai, 1990, Akita and Nakai, 1992, Fichtali et al., 1992, Fichtali et al., 1993, Ko and Ahn, 2007), ammonium sulfate precipitation (Ko and Ahn, 2007), hydrophobic interaction chromatography (Hassl and Aspock, 1988), immobilized metal ion affinity chromatography (Mccannel and Nakai, 1990, Greene and Holt, 1997), thiophilic interaction chromatography (Hansen et al, 1998), affinity chromatography using alkaline conditions (Kuronen et al., 1997) and synthetic peptide ligands, designed specifically for immobilizing IgY (Fassina et al., 1998, Verdoliva et al., 2000) which resulted in 92% purity and 78% recovery (Dong et al., 2008). Hernandez-Campos et al. (2010) recently demonstrated that UF purification of WSF using polyethersulphone (PES) or modified PES membranes increased purity and resulted in greater than 90% IgY recovery.
Recent proteomic analysis and identification of the proteins in WSF prepared by the water dilution method revealed that preparations obtained by this method are highly reproducible with low levels of cholesterols and triglycerides, and provides information that may be valuable for the prediction of potential allergic reactions to WSF preparations (Nilsson et al., 2008).
17.4. Advantages of eggs as an alternative antibody source
The use of chickens for antibody production provides several advantages over the conventional method of IgG production in mammals. Antibody production in chickens is much less invasive, requiring only the collection of eggs, rather than the collection of blood, and therefore is less stressful on the animal (Fig. 17.2 ) (Karlsson et al., 2004). Owing to the genetic distance between chickens and mammals, it is possible to produce antibodies against highly conserved mammalian proteins that otherwise would not be possible in mammals. In contrast to mammalian systems, much less antigen is required to produce an efficient immune response, and sustained high antibody titers reduce the need for frequent injections (Larsson et al., 1998). In fact, it has been estimated that the productivity of antibodies in hens is nearly 18 times greater than that in rabbits, based on the weight of antibody produced per animal (Nakai et al., 1994), which translates to an overall reduction in animal use for the same level of antibody production. A laying hen typically lays around 240–280 eggs per year (Sim et al., 2000), and an egg yolk contains 100–150 mg of IgY. Therefore, around 28–42 g of IgY per year may be obtained from a single chicken (Karlsson et al., 2004). In contrast to mammalian serum, egg yolk contains only a single class of antibody (IgY), which can easily be isolated from the yolk by precipitation techniques (Gassmann et al., 1990), as discussed in the previous section. Once purified, IgY has been found to be stable for years when stored at 4 °C (Larsson et al., 1993).
Fig. 17.2.
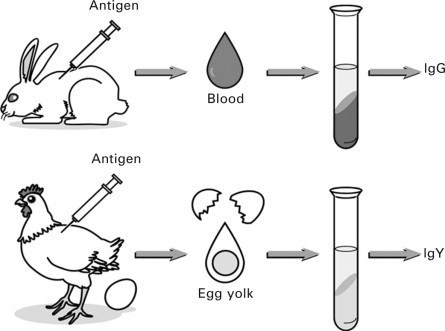
Comparison of preparation of specific antibody from hen (Hatta et al.,1997a).
17.4.1. Structure and stability of IgY
Although IgG and IgY share a similar biological function, there exist some profound differences in their chemical structures and resulting immunoreactivity (Camenisch et al., 1999, Zhang, 2003). In contrast to IgG, which has a molecular mass of 150 kDa, the IgY molecule has a molecular mass of 180 kDa, owing to its increased number of heavy-chain constant domains and an extra pair of carbohydrate chains (Hatta et al., 1993a, Sun et al., 2001). In addition, the hinge region of IgY is shorter and less flexible (Warr et al., 1995), and the content of β-sheet structure in the constant domains of IgY has been reported to be lower (Shimizu et al., 1992), which may account for the differences in stability observed between IgY and IgG.
Both IgG and IgY contain Asn-linked oligosaccharides; however, the structure of oligosaccharides in IgY differ from those of any mammalian IgG, containing instead unusual monoglucosylated oligomannose-type oligosaccharides with Glc1Man7–9GlcNAc2 structure (Ohta et al., 1991, Matsuura et al., 1993). The isoelectric point of IgY is lower than that of IgG (Polson et al., 1980), IgY does not associate with mammalian complement, and the binding of IgY with human and bacterial Fc-receptors on cell surfaces is less than that of IgG (Gardner and Kaye, 1982). Furthermore, it has also been suggested that IgY may be more hydrophobic than IgG, which matches the lipid-rich environment of the yolk (Davalos-Pantoja et al., 2000).
In general, when used as reagents for immunochemical or research purposes, where antibodies are stored and used under relatively mild conditions, stability does not warrant much attention. However, the stability of IgY under various processing and physiological conditions needs to be considered when they are to be used in industrial, food or medical applications (Shimizu et al., 1993b). IgY is stable in the pH range of 4–9, and up to temperatures of 65 °C (Shimizu et al., 1992; K. A. Lee et al., 2002), however this range may be extended by the addition of stabilizers such as sugars, complex carbohydrates, or polyols (Shimizu et al., 1994, Chang et al., 2000; K. A. Lee et al., 2002). IgY is relatively resistant to treatment with trypsin and chymotrypsin, but sensitive to pepsin digestion (Shimizu et al., 1988). Hatta et al. (1993b) found that almost all of the IgY activity was lost following digestion with pepsin; however, activity remained even after 8 hours incubation with trypsin or chymotrypsin.
Studies in humans have demonstrated that orally administered antibodies undergo a significant loss of activity upon passage through the stomach, and several reports have demonstrated around 4–20% survival of orally administered antibody, with similar results observed in both adults and infants (Blum et al., 1981, Hilpert et al., 1987, Roos et al., 1995). In order to circumvent the inactivation of IgY following oral administration, a number of encapsulation techniques have been examined. The microencapsulation of IgY using liposomes (Shimizu et al., 1993a) and multiple emulsions (Shimizu and Nakane, 1995) have been previously described; however, these have shown limited effectiveness, in some cases destroying antibody activity by the encapsulation procedure itself. The macroencapsulation of IgY using enteric-coated gelatin capsules has also been examined, and was found to significantly improve antibody stability (Akita and Nakai, 2000). The use of a pH-sensitive methacrylic acid copolymer and chitosan–alginate microcapsules (Kovacs-Nolan and Mine, 2005, Li et al., 2007, Li et al., 2009a, Li et al., 2009b) have also been shown to increase IgY stability to gastric conditions both in vitro as well as in vivo.
17.5. Applications of IgY
17.5.1. Use of IgY as an immunoaffinity ligand
Immunoaffinity chromatography, involving the specific interaction between an antigen and its corresponding antibody, has been widely used for the purification of proteins from complex starting materials. The use of this process on a large scale is limited by the high cost of the technique, and problems relating to the production of antibody and the efficiency of immobilization (Akita and Li-Chan, 1998). Specific IgY, which can easily be produced on a large scale required for industrial applications, would provide an ideal replacement for other polyclonal or monoclonal antibodies currently used in immunoaffinity chromatography (Li-Chan, 2000). Although IgY is more sensitive to low pH than IgG, it was found that an IgY immunoaffinity column was capable of retaining stability when subjected to standard affinity chromatography conditions, and could be reused over 50 times without a significant decrease in binding capacity (Akita and Li-Chan, 1998).
More recently, the unique features of IgY have made it advantageous for use in proteomic applications (Huang et al, 2005). The depletion of abundant proteins from complex biological samples containing a wide dynamic range of protein concentrations, such as plasma, serum and urine, prior to twodimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE) and mass spectrometry presents a significant challenge for proteomic analysis (Huang and Fang, 2008, Magagnotti et al., 2010). Immunoaffinity fractionation is one of the most effective methods used during sample preparation to improve the ability to detect low-abundance proteins (Huang and Fang, 2008). Owing to the ease of production of IgY and the ability to produce high affinity antibodies against conserved mammalian proteins, IgY is now widely used for immunoaffinity protein depletion in proteomic applications and biomarker discovery (Qian et al., 2008, Rajic et al., 2009, Polaskova et al., 2010).
17.5.2. Use of IgY in diagnostic and analytical applications
Antibodies serve as essential components in a variety of diagnostic assays used for the qualitative and quantitative determination of a wide range of substances (Schade and Hlinak, 1996). While the antigen-binding specificity of IgY is comparable to that of IgG (Hatta et al., 1997b), IgY presents several advantages that make it well suited for applications in medical diagnostics. A number of proteins exist whose amino acid sequences are well preserved among mammals, and when used as an antigen in mammals have limited or no antigenicity (Hatta et al., 2008). Evolutionary differences allow the production of high avidity IgY against conserved mammalian proteins, with a more diversified antibody repertoire than is possible in mammals (Olovsson and Larsson, 1993, Woolley and Landon, 1995, Carlander et al., 1999a). The use of IgY as a substitute for IgG in clinical testing can potentially eliminate false positives and often results in low background and less interference (Xiao and Gao, 2010). Human serum samples often contain an active complement system, which would be activated by mammalian antibodies. IgY, which does not activate the human complement system, eliminates the interference that would otherwise be caused by IgG (Larsson and Mellstedt, 1992). Human serum can also contain rheumatoid factor (RF) and human anti-mouse IgG antibodies (HAMA), which are well-known causes of false-positive reactions in immunological assays (Carlander et al., 1999a). RF reacts with the Fc portion of IgG, and HAMA, which can occur naturally in human serum, will bind to any mouse antibodies being used in an immunoassay (Carlander et al., 1999a). Since IgY does not react with RF (Larsson and Sjoquist, 1988, Larsson et al., 1991) or HAMA (Larsson and Mellstedt, 1992), it has been suggested as a replacement for IgG in immunological assays of human serum.
IgY has been used in numerous medical and veterinary diagnostic applications, including detection and quantitation of cancer biomarkers in breast and ovarian cancer and non-Hodgkin’s lymphoma patients (Grebenschikov et al., 1997, AL-Haddad et al., 1999, Lemamy et al., 1999, Pan et al., 2010, Xiao and Gao, 2010), the diagnosis of gastric cancer (Noack et al., 1999), as well as for the detection of African horsesickness virus (Du Plessis et al., 1999), Mycobacterium avium subsp. paratuberculosis (Shin et al., 2009), Leptospira spp. in human blood (Vasconcellos et al., 2010), bovine leukemia virus (Juliarena et al., 2007), serotyping of foot-and-mouth disease (Veerasami et al., 2008), and detection of opiate drugs in urine (Gandhi et al., 2009). IgY against the SARS-CoV nucleocapsid protein was incorporated into an immunoswab assay for point of care detection of SARS-infected individuals (Kammila et al., 2008).
Furthermore, IgY has been used in assays involving food safety concerns, including detection of Escherichia coli O157:H7 (Sunwoo et al., 2006), Listeria spp. (Kim et al., 2005), Campylobacter jejuni (Hochel et al., 2004), screening agricultural products for citrinin contamination (Duan et al., 2009), detection of ciguatoxin contamination in fish tissue (Empey Campora et al., 2008), the identification of allergens (Alessandro et al., 2009), and IgY against dichlorodiphenyltrichloroethane (DDT) was incorporated into a dipstick assay to test for pesticide contamination (Lisa et al., 2009).
17.6. Immunotherapeutic applications of IgY
Increases in antibiotic resistant bacteria and pathogens that do not respond to antibiotics, such as viral pathogens, along with an escalating number of immunocompromised individuals, has prompted much research into the administration of specific antibodies as an alternative to antibiotics and antimicrobial chemotherapy to treat infections (Casadevall and Scharff, 1995, Reilly et al., 1997). IgY is well suited to passive immunotherapy applications since it does not react with rheumatoid factors, human Fc- receptors, or HAMA, which are all well-known cell activators and mediators of inflammation (Larsson et al., 1993, Larsson and Carlander, 2003).
17.6.1. Oral administration of IgY
Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients Respiratory infection caused by colonization of the airways by Pseudomonas aeruginosa (PA) is the major cause of morbidity and mortality in cystic fibrosis (CF) patients (Shale and Elborn, 1996), and once a chronic infection has been established it is very difficult to eliminate, even with the use of antibiotics (Kollberg et al., 2003). An ongoing study in CF patients has found that an oral rinse containing anti-PA IgY given on a continuous basis could reduce or prevent PA colonization, thereby reducing the need for antibiotic treatment (Carlander et al., 2000, Kollberg et al., 2003, Nilsson et al., 2007b, Nilsson et al., 2008).
In vitro, these antibodies were found to inhibit adhesion of the bacteria to epithelial cells, but did not inhibit bacterial growth, suggesting that the IgY might be capable of interfering with the bacterial infection process and preventing colonization in CF patients (Carlander et al, 1999b). More recently, the immunoreactivity of these antibodies was examined in detail using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), and PA flagellin was identified as the major antigen. The flagellin is important for establishing infections in hosts as well as being involved in PA chemotaxis, motility, adhesion and inflammation (Nilsson et al., 2007a). These results further support the ability of anti-PA IgY to prevent infection and host invasion.
The stability of the anti-PA IgY in saliva from healthy individuals was also examined (Carlander et al., 2002), and antibody activity remained after 8 hours. After 24 hours, the antibody activity had decreased significantly, but was still detectable in some subjects, indicating that oral treatment with specific IgY for various local infections, including the common cold and tonsillitis, might also be possible.
Prevention of Helicobacter pylori infection and gastric symptoms Helicobacter pylori is a common cause of gastritis and gastric ulcers, and has been implicated in the development of gastric carcinomas (Dunn et al., 1997). The development of antibiotic resistance has prompted the investigation into alternative methods of treating and preventing H. pylori infection (DeLoney and Schiller, 2000). IgY produced against H. pylori reduced bacterial adhesion, growth and urease activity in vitro and decreased H. pylori-induced gastric mucosal injury and inflammation in an animal model (Shin et al., 2002). Since antibodies produced against whole-cell H. pylori might cross-react with other bacteria, including normal human flora (Shin et al., 2003), the production and efficacy of IgY against immunodominant H. pylori proteins or epitopes has also been described, including IgY against urease (Nomura et al., 2005), urease-derived peptides (Shin et al., 2004) and a 58 kD highly reactive H. pylori antigen (Hp58) (Attallah et al., 2009).
The effectiveness of anti-urease IgY has been demonstrated in humans. A functional drinking yogurt containing Lactobacillus acidophilus and Bifidobacterium spp. supplemented with 1% egg yolk IgY-urease was produced commercially, and given to volunteers testing positive for H. pylori (Horie et al., 2004). Volunteers consumed the IgY-containing yogurt three times daily for four weeks, following which urea breath test values and antigen detection in feces were significantly decreased, indicating suppression of H. pylori infection.
Prevention of dental caries and periodontitis
Porphyromonas gingivalis is one of the most important causative agents of periodontitis (Yokoyama et al., 2007b). In vitro, anti-P. gingivalis IgY dose- dependently decreased bacterial adhesion and hydrolytic activity (Yokoyama et al., 2007a), and reduced levels of P. gingivalis when applied as a gel to the teeth of periodontitis patients (Yokoyama et al., 2007b). The production of IgY against the P. gingivalis 40 kD outer membrane protein, which aggregates with other oral bacteria to form plaque (Hamajima et al., 2007), as well as P. gingivalis hemagglutinin (HagA),which allows the bacteria to adhere to gingival tissue cells (Tezuka et al., 2006), have also been described and found to inhibit aggregation and hemagglutination in vitro.
Streptococcus mutans is believed to be the principal causative bacterium of dental caries in humans (Hamada and Slade, 1980), and IgY against S. mutans has also been shown to exert anti-cariogenic properties. IgY against S. mutans was examined for the passive prevention of dental caries (Hamada et al., 1991, Otake et al., 1991, Chang et al., 1999), and it was found that supplementation of 2% IgY-containing yolk powder to a cariogenic diet significantly lowered caries scores. Likewise, levels of S. mutans were decreased in volunteers who gargled with a mouth rinse containing anti-S. mutans IgY (Hatta et al., 1997c). IgY produced against the S. mutans glucan binding protein B (GBP-B), which is believed to be involved in S. mutans biofilm development, has also been examined. Using a rat model of dental caries, Smith et al. (2001) observed a decrease in S. mutans accumulation in rats treated with anti-GBP-B IgY, as well as a decrease in the overall amount of dental caries, as compared with control rats.
Prevention of Escherichia coli infection
Diarrheal diseases continue to be a health problem worldwide, especially in developing countries, where they are estimated to be responsible for 2.5 million infant deaths per year (Estrada-Garcia et al., 2009), as well as causing economic losses in livestock, especially neonatal calves and piglets (Morris and Sojka, 1985). One of the most prominent causes of diarrheal diseases remains Escherichia coli, and a number of strategies to prevent E. coli infection using IgY have been examined.
The production of IgY against the fimbrial antigens K88, K99 and 987P of porcine enterotoxigenic E. coli (ETEC), has been described. Anti-K88, -K99 and -987P IgY inhibited the binding of E. coli K88 +, K99 + and 987P + strains to porcine epithelial cells (Yokoyama et al., 1992) and porcine intestinal mucus (Jin et al., 1998) in vitro. When given orally to piglets, these antibodies dose-dependently protected against E. coli infection (Yokoyama et al., 1992). Marquardt et al. (1999) also found that the oral administration of anti-K88 IgY resulted in 100% survival of ETEC-infected piglets, compared with a control group, and a reduction in the incidence and severity of diarrhea. The passive protective effect of anti-ETEC IgY, in neonatal calves has also been shown. Calves fed milk containing IgY had transient diarrhea, 100% survival and good body weight gain during the course of the study (Ikemori et al., 1992).
More recently, IgY against recombinant adherence-associated proteins of E. coli O157:H7 was shown to reduce adherence in vitro in cultured cells, suggesting they may have the potential to reduce E. coli O157:H7 colonization in hosts such as cattle and humans (Cook et al., 2007). Similarly, Girard et al. (2006) demonstrated that IgY produced against virulence factors of attaching and effacing E. coli (AEEC) reduced bacterial adherence of porcine enteropathogenic E. coli, as well as heterologous AEEC strains, including human, bovine and canine enteropathogenic E. coli and O157:H7, and found that oral administration of these antibodies reduced E. coli-induced lesions in pigs challenged with the porcine enteropathogenic E. coli strain ECL1001.
Furthermore, anti-E. coli O78:K80 IgY was found to reduce E. coli numbers and improve intestinal health in chickens challenged with E. coli (Mahdavi et al., 2010), and IgY against mastitis-causing E. coli (O111) dose-dependently inhibited E. coli growth and enhanced phagocytic activity in vitro (Zhen et al., 2008b). Amaral et al. (2008) demonstrated the in vitro anti-E. coli effects of IgY against EPEC O111, Shiga toxin-producing E. coli (STEC) O111 and STEC O157, suggesting possible future therapeutic use in humans.
Prevention of Salmonella infection
Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) are the main causes of salmonellosis outbreaks in humans and infections in chickens (E. N. Lee et al., 2002). Salmonella possesses several surface components which are virulence related, including outer membrane protein (OMP) (Isibasi et al., 1988), flagella (Fla) and, in some strains, fimbrial antigens (Thorns et al., 1990, Thorns et al., 1992). The ability of IgY specific for OMP, LPS or flagella (Fla), to passively protect against experimental salmonellosis in mice has been demonstrated. In vitro using a cultured human intestinal cell line (Caco-2), Chalghoumi et al. (2009) found that IgY against OMP of SE and ST reduced the Salmonella-induced decrease in transepithelial electrical resistance (TER) of the infected Caco-2 cell monolayers and blocked Salmonella sp. adhesion in a concentration-dependent manner, suggesting that passive immunization with Salmonella OMP-specific IgY could be useful to prevent Salmonella colonization in broiler chickens and the subsequent carcass contamination during processing. In vivo in mice challenged with SE or ST, treatment with IgY against OMP, LPS or Fla resulted in a significantly higher survival rate when compared to control mice (Yokoyama et al, 1998b). Salmonella infection in calves is also a significant problem, with ST and S. dublin accounting for most cases of salmonellosis. Passive protection against ST and S. dublin was investigated by challenging calves with SE or S. dublin, and then orally administering anti-SE or anti-S. dublin IgY. All control calves died within 7–10 days, whereas only fever and diarrhea were observed in calves given the high titer IgY (Yokoyama et al., 1998a).
Prevention and treatment of rotavirus gastroenteritis
Rotavirus is a major cause of acute gastroenteritis in infants in both developed and developing countries (Clark et al., 1999). In vivo, IgY produced against murine, human and simian strains of rotavirus prevented rotavirus-induced diarrhea in mice (Yolken et al., 1988, Ebina, 1996). The anti-rotavirus effect was seen both when administered before and after viral challenge, suggesting its use for both the prevention and treatment of rotavirus gastroenteritis (Hatta et al., 1993a). The production of IgY against recombinant rotavirus coat proteins, VP8 (Kovacs-Nolan et al., 2001) and VP7 (Zhang et al., 2009) has also been described, and anti-VP8 IgY exhibited significant neutralizing activity against human rotavirus (HRV) in vitro, suggesting its potential use for the prevention and treatment of rotavirus in humans.
Neonatal calf diarrhea, caused by bovine rotavirus (BRV), is a significant cause of mortality in cattle (Lee et al., 1995). Using a mouse model of BRV infection, Kuroki et al. (1993) observed the protection against two strains of BRV using orally administered anti-BRV IgY. The passive protection of calves against BRV infection, using anti-BRV IgY, has also been demonstrated (Kuroki et al., 1994).
Treatment of inflammatory bowel disease
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is characterized by chronic inflammation of the gastrointestinal tract (Podolsky, 2002). Treatment typically involves corticosteroids and immunosuppressive agents; however, these have shown limited therapeutic efficacy and have been associated with severe side effects and long-term toxicity (Atreya and Neurath, 2008). Tumor necrosis factor (TNF)-α is one of the key pro-inflammatory cytokines involved in the pathogenesis of IBD (Garside, 1999) and immunotherapy using monoclonal mouse antibodies against TNF-α has been approved for use; however, it can be costly and adverse side effects have been reported in patients receiving systemic anti-TNF therapy (Sandborn and Hanauer, 1999). Worledge et al. (2000) reported that orally administered anti-TNF-α IgY was capable of effectively treating acute and chronic phases of colitis in rats, as well as neutralizing human TNF-α in vitro, indicating its possible use for the treatment of IBD in humans.
Use of IgY in aquaculture
Yersinia ruckeri causes enteric redmouth disease, a systemic bacterial septicemia of salmonid fish (Stevenson et al., 1993), and the persistence of Y. ruckeri in carrier fish and shedding of bacteria in feces can present a continuing source of infection. Lower mortality rates and reduced infection rates were observed in rainbow trout fed anti-Y. ruckeri IgY when given before or after challenge with Y. ruckeri (Lee et al., 2000).
White spot syndrome virus (WSSV) causes high mortality and large economic losses in cultured shrimp (Lu et al, 2008). IgY produced against WSSV was shown to passively protect shrimp (Lu et al., 2008) and crayfish (Lu et al., 2009) from WSSV infection.
Edwardsiella tarda is a fish pathogen spread by infection through the intestinal mucosa, and Edwardsiellosis in Japanese eels is a serious problem for the eel farming industry, especially due to the appearance of antibiotic-resistant strains (Hatta et al., 1994). Eels challenged with E. tarda and then given anti-E. tarda IgY survived without any symptoms of infection, in contrast to the control eels which died within 15 days (Gutierrez et al., 1993, Hatta et al., 1994). This antibody is now in use commercially, following a field test on nearly 2,400,000 tails of cultured eels with confirmed disease prevention with anti-E. tarda IgY (Hatta et al., 2008).
Prevention and treatment of other diseases
Specific IgY has been shown to be effective at preventing and treating several other diseases, including for the passive protection of chicks against infectious bursal disease virus (IBDV) (Eterradossi et al., 1997, Yousif et al., 2006) and avian coccidiosis caused by Eimeria spp. (Lee et al., 2009a, Lee et al., 2009b), for the protection of piglets against porcine epidemic virus (PEDV) (Kweon et al., 2000), for the protection of calves against bovine coronavirus (BCV) (Ikemori et al., 1997), and for the protection of dogs against canine parvovirus-2 (CPV-2) (Van Nguyen et al., 2006). IgY has also been shown to be effective against Candida albicans, dose-dependently preventing C. albicans growth, adherence and biofilm formation in vitro (Wang et al., 2008, Fujibayashi et al., 2009) and preventing colonization in mice (Ibrahim el et al., 2008). Finally, the production and testing of IgY against influenza virus H5N1 and H1N1 has also been described. When administered before or after infection, the IgY prevented mice from infection and significantly reduced viral replication resulting in complete recovery from the disease, suggesting that IgY may be a safe and affordable alternative method for the control of influenza outbreaks (Nguyen et al., 2010).
17.6.2. Systemic administration of IgY
Neutralization of venom
Envenoming resulting from the bites of venomous snakes, scorpions or spiders is an important public health hazard in many regions, particularly in tropical and subtropical countries. The most widely used treatment is the direct injection of specific anti-venoms, often antibodies produced in horses or sheep, to neutralize the toxic and potentially lethal effects of the venom (Gutierrez et al, 2006). There have been several reports of the production and neutralization of venom by chicken anti-venom IgY (Paul et al., 2007, Meenatchisundaram et al., 2008a, Meenatchisundaram et al., 2008b, Araujo et al., 2010), including the production of polyvalent anti-African snake venom IgY to be used against bites of several different snakes (de Almeida et al., 2008). IgY was found to have a higher bioactivity than anti-venoms traditionally raised in horses (Thalley and Carroll, 1990, De Almeida et al., 2008), and also has a lower likelihood of producing side effects such as serum sickness and anaphylactic shock, which can occur upon administration of mammalian serum proteins (Thalley and Carroll, 1990).
Prevention of Staphylococcus aureus infection and toxic shock syndrome Staphylococcal enterotoxins are a family of bacterial superantigens produced by Staphylococcus aureus, and are associated with a number of serious diseases, including food poisoning and toxic shock syndrome (Fraser et al, 1976). Specific IgY has been shown to inhibit the production of S. aureus enterotoxin-A in vitro (Sugita-Konishi et al., 1996). It has also been reported that IgY against S. aureus enterotoxin-B (SEB) systemically administered both before and after challenge could protect rhesus monkeys from toxic shock syndrome (LeClaire et al., 2002), suggesting that it might be useful for both the prevention and treatment against lethal doses of S. aureus enterotoxins, and could be used to reduce or eliminate enterotoxin-mediated disorders.
Anti-S. aureus IgY has also been used in veterinary applications, where it was found to inhibit S. aureus growth in vitro (Zhen et al., 2008a, Guimaraes et al., 2009) and prevented bovine mastitis caused by S. aureus in cattle when administered by intramammary infusion (Zhen et al., 2009).
Prevention of rabies virus
Rabies remains a major public health problem in developing countries, claiming the lives of an estimated 55,000 people each year (Ertl, 2009). Infection with rabies virus causes encephalitis in humans that has a case fatality rate of almost 100% (Johnson et al., 2010). Motoi et al., 2005a, Motoi et al., 2005b reported the production of anti-rabies IgY, raised against a part of the G protein of rabies virus. In vitro, these antibodies bound virions and cells infected with the rabies virus, and neutralized rabies virus infectivity. Administration of anti-rabies IgY to mice infected with rabies virus reduced mortality caused by the virus, suggesting that IgY directed against the rabies virus G protein could serve as a possible alternative to currently available anti-rabies human or equine immunoglobulins (Motoi et al., 2005b).
IgY for the prevention of hyperacute rejection in xenotransplantation
Transgenic pigs are promising donor organisms for xenotransplantation as they share many anatomical and physiological characteristics with humans (Klymiuk et al., 2010); however one of the most important barriers with such xenografts is hyperacute rejection, mediated by natural antibodies in humans against pig antigens, complement fixation, and the rapid onset of intravascular coagulation (Sandrin and McKenzie, 1994). The major target of these natural antibodies is the carbohydrate epitope, Galα1-3Gal, which is expressed by all mammals except for humans, apes, and some monkeys. Besides humans and monkeys, chickens also lack Galα1-3Gal expression (Bouhours et al., 1998). Since IgY does not bind human complement or Fc-receptors, anti-α-Gal IgY may be a suitable candidate for use as blocking antibodies to inhibit the interactions that may contribute to xenograft rejection. Fryer et al. (1999) demonstrated that anti-α-Gal IgY blocked human xenoreactive antibody binding to both porcine and rat tissues in vitro, and inhibited lysis of porcine cells by human serum, suggesting that IgY could be of potential use in inhibiting pig-to-human xenograft rejection. Anti-α-Gal IgY was also found to significantly reduce the infectivity of porcine endogenous retrovirus (PERV), an α-Gal bearing virus which has emerged as a potential zoonotic agent, with possible pig-to-human transmission (Leventhal et al, 2001).
17.7. Conclusion and future trends
The production of IgY in chickens and the extraction of specific antibodies from egg yolk is increasingly attracting the interest of the scientific community, as evidenced by the significant growth in the amount of IgY-related literature (Schade et al, 2005). Indeed, the immunization of hens represents an ideal alternative to efficiently generate large quantities of polyclonal antibodies since housing is inexpensive, egg collection is non-stressful and non- invasive, and the isolation and purification of IgY is relatively simple and high-yielding (Zhang, 2003). Recently, it has also been suggested that the selective transport of IgY from the blood into the egg yolk may also provide a new strategy for delivering substances into the egg yolk in an attempt to produce designer eggs (Bae et al., 2010), and may further expand the application of IgY. Immunization protocols and adjuvant systems continue to be modified in order to increase specific antibody production, and the identification and production IgY against of new antigens will continue to lead to new applications of egg yolk antibodies for human health and research applications.
17.8 References
- Akita E.M., Li-Chan E.C. Isolation of bovine immunoglobulin G subclasses from milk, colostrum, and whey using immobilized egg yolk antibodies. J Dairy Sci. 1998;81:54–63. doi: 10.3168/jds.S0022-0302(98)75550-X. [DOI] [PubMed] [Google Scholar]
- Akita E.M., Nakai S. Immunoglobulins from egg yolk: isolation and purification. J Food Sci. 1992;57:629–634. [Google Scholar]
- Akita E.M., Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J Immunol Methods. 1993;160:207–214. doi: 10.1016/0022-1759(93)90179-b. [DOI] [PubMed] [Google Scholar]
- Akita E.M., Nakai S. Preparation of enteric-coated gelatin capsules of IgY with cellulose acetate phthalate. In: Sim J.S., Nakai S., Guenter W., Egg Nutrition Biotechnology, editors. CAB International; New York: 2000. pp. 301–310. [Google Scholar]
- AL-Haddad S., Zhang Z., Leygue E., Snell L., Huang A., Niu Y., Hiller-Hitchcock T., Hole K., Murphy L.C., Watson P.H. Psoriasin (S100A7) expression and invasive breast cancer. Am J Pathol. 1999;155:2057–2066. doi: 10.1016/S0002-9440(10)65524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandro R., Gallo A., Barranca M., Principe S., Taverna S., Duro G., Cassata G., Becchi M., Fontana S., De Leo G. Production of an egg yolk antibody against Parietaria judaica 2 allergen. Poult Sci. 2009;88:1773–1778. doi: 10.3382/ps.2009-00054. [DOI] [PubMed] [Google Scholar]
- Amaral J.A., De Franco M.T., Zapata-Quintanilla L., Carbonare S.B. In vitro reactivity and growth inhibition of EPEC serotype O111 and STEC serotypes O111 and O157 by homologous and heterologous chicken egg yolk antibody. Vet Res Commun. 2008;32:281–290. doi: 10.1007/s11259-007-9029-3. [DOI] [PubMed] [Google Scholar]
- Araujo A.S., Lobato Z.I., Chavez-Olortegui C., Velarde D.T. Brazilian IgY- Bothrops antivenom: studies on the development of a process in chicken egg yolk. Toxicon. 2010;55:739–744. doi: 10.1016/j.toxicon.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Atreya R., Neurath M.F. New therapeutic strategies for treatment of inflammatory bowel disease. Mucosal Immunol. 2008;1:175–182. doi: 10.1038/mi.2008.7. [DOI] [PubMed] [Google Scholar]
- Attallah A.M., Abbas A.T., Ismail H., Abdel-Raouf M., El-Dosoky I. Efficacy of passive immunization with IgY antibodies to a 58-kDa H. pylori antigen on severe gastritis in BALB/c mouse model. J Immunoassay Immunochem. 2009;30:359–377. doi: 10.1080/15321810903187922. [DOI] [PubMed] [Google Scholar]
- Bae H.D., Kobayashi M., Horio F., Murai A. Identification of the amino acid residues involved in human IgG transport into egg yolks of Japanese quail (Coturnix japonica) Mol Immunol. 2010;47:1404–1410. doi: 10.1016/j.molimm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Bizhanov G., Vyshniauskis G. A comparison of three methods for extracting IgY from the egg yolk of hens immunized with Sendai virus. Vet Res Commun. 2000;24:103–113. doi: 10.1023/a:1006460506303. [DOI] [PubMed] [Google Scholar]
- Blum P.M., Phelps D.L., Ank B.J., Krantman H.J., Stiehm E.R. Survival of oral human immune serum globulin in the gastrointestinal tract of low birth weight infants. Pediatr Res. 1981;15:1256–1260. doi: 10.1203/00006450-198109000-00006. [DOI] [PubMed] [Google Scholar]
- Bouhours J.F., Richard C., Ruvoen N., Barreau N., Naulet J., Bouhours D. Characterization of a polyclonal anti-Galalpha1-3Gal antibody from chicken. Glycoconj J. 1998;15:93–99. doi: 10.1023/a:1006903919461. [DOI] [PubMed] [Google Scholar]
- Camenisch G., Tini M., Chilov D., Kvietikova I., Srinivas V., Caro J., Spielmann P., Wenger R.H., Gassmann M. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. FASEB J. 1999;13:81–88. doi: 10.1096/fasebj.13.1.81. [DOI] [PubMed] [Google Scholar]
- Carlander D., Stalberg J., Larsson A. Chicken antibodies: a clinical chemistry perspective. Ups J Med Sci. 1999;104:179–189. doi: 10.3109/03009739909178961. [DOI] [PubMed] [Google Scholar]
- Carlander D., Sundstrom J., Berglund A., Larsson A., Wretlind B., Kollberg H. Immunoglobulin Y (IgY) – a new tool for the prophylaxis against Pseudomonas aeruginosa in cystic fibrosis patients. Pediatr Pulmonol. 1999;19:241. [Google Scholar]
- Carlander D., Kollberg H., Wejaker P.E., Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res. 2000;21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlander D., Kollberg H., Larsson A. Retention of specific yolk IgY in the human oral cavity. BioDrugs. 2002;16:433–437. doi: 10.2165/00063030-200216060-00004. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalghoumi R., Thewis A., Beckers Y., Marcq C., Portetelle D., Schneider Y.J. Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on Salmonella enterica serovars Enteritidis and Typhimurium in vitro. Foodborne Pathog Dis. 2009;6:593–604. doi: 10.1089/fpd.2008.0258. [DOI] [PubMed] [Google Scholar]
- Chang H.M., Ou-Yang R.F., Chen Y.T., Chen C.C. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY) J Agric Food Chem. 1999;47:61–66. doi: 10.1021/jf980153u. [DOI] [PubMed] [Google Scholar]
- Chang H.M., Lu T.C., Chen C.C., Tu Y.Y., Hwang J.Y. Isolation of immunoglobulin from egg yolk by anionic polysaccharides. J Agric Food Chem. 2000;48:995–999. doi: 10.1021/jf990539k. [DOI] [PubMed] [Google Scholar]
- Clark H.F., Glass R.I., Offit P.A. Vaccines; Philadelphia, Saunders: 1999. Rotavirus vaccines, in Plotkin SA and Orenstein WA; pp. 987–1005. [Google Scholar]
- Cook S.R., Maiti P.K., Devinney R., Allen-Vercoe E., Bach S.J., Mcallister T.A. Avian- and mammalian-derived antibodies against adherence-associated proteins inhibit host cell colonization by Escherichia coli O157:H7. J Appl Microbiol. 2007;103:1206–1219. doi: 10.1111/j.1365-2672.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- Cova L. DNA-designed avian IgY antibodies: novel tools for research, diagnostics and therapy. J Clin Virol. 2005;34(Suppl 1):S70–S74. doi: 10.1016/s1386-6532(05)80013-7. [DOI] [PubMed] [Google Scholar]
- Davalos-Pantoja L., Ortega-Vinuesa J.L., Bastos-Gonzalez D., Hidalgo-Alvarez R. A comparative study between the adsorption of IgY and IgG on latex particles. J Biomater Sci Polym Ed. 2000;11:657–673. doi: 10.1163/156856200743931. [DOI] [PubMed] [Google Scholar]
- De Almeida C.M., Da Silva C.L., Couto H.P., Escocard R.D.E.C., Da Rocha D.G., Sentinelli Lde P., Kipnis T.L., Da Silva W.D. Development of process to produce polyvalent IgY antibodies anti-African snake venom. Toxicon. 2008;52:293–301. doi: 10.1016/j.toxicon.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Deignan T., Kelly J., Alwan A., O’farrelly C. Comparative analysis of methods of purification of egg yolk immunoglobulin. Food Agric Immunol. 2000;12:77–85. [Google Scholar]
- Deloney C.R., Schiller N.L. Characterization of an in vitro-selected amoxicillin- resistant strain of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:3368–3373. doi: 10.1128/aac.44.12.3368-3373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Liu H., Xiao Q., Li R. Affinity purification of egg yolk immunoglobulins (IgY) with a stable synthetic ligand. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:51–54. doi: 10.1016/j.jchromb.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Du PLEssis D.H., Van Wyngaardt W., Romito M., Du PLEssis M., Maree S. The use of chicken IgY in a double antibody sandwich ELISA for detecting African horsesickness virus. Onderstepoort J Vet Res. 1999;66:25–28. [PubMed] [Google Scholar]
- Duan Z.H., Lin Z.S., Yao H.R., Gao Y.H., Zhang K., Zhao S.Q., Zhu Z.Y. Preparation of artificial antigen and egg yolk-derived immunoglobulin (IgY) of citrinin for enzyme- linked immunosorbent assay. Biomed Environ Sci. 2009;22:237–243. doi: 10.1016/S0895-3988(09)60051-9. [DOI] [PubMed] [Google Scholar]
- Dunn B.E., Cohen H., Blaser M.J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217–223. doi: 10.1007/978-3-7091-6553-9_23. [DOI] [PubMed] [Google Scholar]
- Elazab M.F., Fukushima Y., Horiuchi H., Matsuda H., Furusawa S. Prolonged suppression of chick humoral immune response by antigen specific maternal antibody. J Vet Med Sci. 2009;71:417–424. doi: 10.1292/jvms.71.417. [DOI] [PubMed] [Google Scholar]
- Empey Campora C., Hokama Y., Yabusaki K., Isobe M. Development of an enzyme- linked immunosorbent assay for the detection of ciguatoxin in fish tissue using chicken immunoglobulin Y. J Clin Lab Anal. 2008;22:239–245. doi: 10.1002/jcla.20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H.C. Novel vaccines to human rabies. PLoS Negl Trop Dis. 2009;3:e515. doi: 10.1371/journal.pntd.0000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Garcia T., Lopez-Saucedo C., Thompson-Bonilla R., Abonce M., Lopez-Hernandez D., Santos J.I., Rosado J.L., Dupont H.L., Long K.Z. Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among Mexican children and association of atypical enteropathogenic E. coli with acute diarrhea. J Clin Microbiol. 2009;47:93–98. doi: 10.1128/JCM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eterradossi N., Toquin D., Abbassi H., Rivallan G., Cotte J.P., Guittet M. Passive protection of specific pathogen free chicks against infectious bursal disease by in-ovo injection of semi-purified egg-yolk antiviral immunoglobulins. Zentralbl Veterinarmed B. 1997;44:371–383. doi: 10.1111/j.1439-0450.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Fassina G., Verdoliva A., Palombo G., Ruvo M., Cassani G. Immunoglobulin specificity of TG19318: a novel synthetic ligand for antibody affinity purification. J Mol Recognit. 1998;11:128–133. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<128::AID-JMR408>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fichtali J., Charter E.A., Lo K.V., Nakai S. Separation of egg yolk immunoglobulins using an automated liquid chromatography system. Biotechnol Bioeng. 1992;40:1388–1394. doi: 10.1002/bit.260401113. [DOI] [PubMed] [Google Scholar]
- Fichtali J., Charter E.A., Lo K.V., Nakai S. Purification of antibodies from industrially separated egg yolk. J Food Sci. 1993;58:1282–1285. [Google Scholar]
- Fraser J., Arcus V., Kong P., Baker E., Proft T. Superantigens-powerful modifiers of the immune system. Mol Med Today. 1976;6:125–132. doi: 10.1016/s1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- Fryer J., Firca J., Leventhal J., Blondin B., Malcolm A., Ivancic D., Gandhi R., Shah A., Pao W., Abecassis M., Kaufman D., Stuart F., Anderson B. IgY antiporcine endothelial cell antibodies effectively block human antiporcine xenoantibody binding. Xenotransplantation. 1999;6:98–109. doi: 10.1034/j.1399-3089.1999.00015.x. [DOI] [PubMed] [Google Scholar]
- Fujibayashi T., Nakamura M., Tominaga A., Satoh N., Kawarai T., Narisawa N., Shinozuka O., Watanabe H., Yamazaki T., Senpuku H. Effects of IgY against Candida albicans and Candida spp. adherence and biofilm formation. Jpn J Infect Dis. 2009;62:337–342. [PubMed] [Google Scholar]
- Gandhi S., Caplash N., Sharma P., Raman Suri C. Strip-based immunochromatographic assay using specific egg yolk antibodies for rapid detection of morphine in urine samples. Biosens Bioelectron. 2009;25:502–505. doi: 10.1016/j.bios.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Gardner P.S., Kaye S. Egg globulins in rapid virus diagnosis. J Virol Methods. 1982;4:257–262. doi: 10.1016/0166-0934(82)90072-6. [DOI] [PubMed] [Google Scholar]
- Garside P. Cytokines in experimental colitis. Clin Exp Immunol. 1999;118:337–339. doi: 10.1046/j.1365-2249.1999.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M., Thommes P., Weiser T., Hubscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990;4:2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Girard F., Batisson I., Martinez G., Breton C., Harel J., Fairbrot Her J.M. Use of virulence factor-specific egg yolk-derived immunoglobulins as a promising alternative to antibiotics for prevention of attaching and effacing Escherichia coli infections. FEMS Immunol Med Microbiol. 2006;46:340–350. doi: 10.1111/j.1574-695X.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- Grebenschikov N., Geurts-Moespot A., De Witte H., Heuvel J., Leake R., Sweep F., Benraad T. A sensitive and robust assay for urokinase and tissue-type plasminogen activators (uPA and tPA) and their inhibitor type I (PAI-1) in breast tumor cytosols. Int J Biol Markers. 1997;12:6–14. doi: 10.1177/172460089701200102. [DOI] [PubMed] [Google Scholar]
- Greene C.R., Holt P.S. An improved chromatographic method for the separation of egg yolk IgG into subpopulations utilizing immobilized metal ion (Fe3 +) affinity chromatography. J Immunol Methods. 1997;209:155–164. doi: 10.1016/s0022-1759(97)00155-5. [DOI] [PubMed] [Google Scholar]
- Guimaraes M.C., Amaral L.G., Rangel L.B., Silva I.Z., Matta C.G., Matta M.F. Growth inhibition of Staphylococcus aureus by chicken egg yolk antibodies. Arch Immunol Ther Exp (Warsz) 2009;57:377–382. doi: 10.1007/s00005-009-0041-x. [DOI] [PubMed] [Google Scholar]
- Gutierrez J.M., Theakston R.D., Warrell D.A. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 2006;3:e150. doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.A., Miyazaki T., Hatta H., Kim M. Protective properties of egg yolk IgY containing anti-Edwardsiella tarda antibody against paracolo disease in the Japanese eel, Anguilla japanica Temminck & Schlegel. J Fish Dis. 1993;16:122–133. [Google Scholar]
- Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Horikoshi T., Minami T., Kawabata S., Hiraoka J., Fujiwara T., Ooshima T. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect Immun. 1991;59:4161–4167. doi: 10.1128/iai.59.11.4161-4167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima S., Maruyama M., Hijiya T., Hatta H., Abiko Y. Egg yolk-derived immunoglobulin (IgY) against Porphyromonas gingivalis 40-kDa outer membrane protein inhibits coaggregation activity. Arch Oral Biol. 2007;52:697–704. doi: 10.1016/j.archoralbio.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hansen P., Scoble J.A., Hanson B., Hoogenraad N.J. Isolation and purification of immunoglobulins from chicken eggs using thiophilic interaction chromatography. J Immunol Methods. 1998;215:1–7. doi: 10.1016/s0022-1759(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Hassl A., Aspock H. Purification of egg yolk immunoglobulins. A two-step procedure using hydrophobic interaction chromatography and gel filtration. J Immunol Methods. 1988;110:225–228. doi: 10.1016/0022-1759(88)90107-x. [DOI] [PubMed] [Google Scholar]
- Hatta H., Sim J.S., Nakai S. Separation of phospholipids from egg yolk and recovery of water-soluble proteins. Journal of Food Science. 1988;53:425–427. [Google Scholar]
- Hatta H., Kim M., Yamamoto T. A novel isolation method for hen egg yolk antibody, ‘IgY’. Agric Biol Chem. 1990;54:2531–2535. [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T. Productivity and some properties of egg yolk antibody (IgY) against human rotavirus compared with rabbit IgG. Biosci Biotechnol Biochem. 1993;57:450–454. doi: 10.1271/bbb.57.450. [DOI] [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T., Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosci Biotechnol Biochem. 1993;57:1077–1081. doi: 10.1271/bbb.57.1077. [DOI] [PubMed] [Google Scholar]
- Hatta H., Mabe K., Kim M., Yamamoto T., Gutierrez M.A., Miyazaki T. Egg Uses and Processing Technologies; New Developments, Wallingford, UK, CAB International: 1994. Prevention of fish disease using egg yolk antibody, in Sim J S and Nakai S; pp. 241–249. [Google Scholar]
- Hatta H., Juneja L.R., Yamamoto T. Hen egg science: its basic and applied science. Kagaku to Seibutu. 1997;35:274–281. [Google Scholar]
- Hatta H., Ozeki M., Tsuda K. Egg yolk antibody IgG and its application. In: Yamamoto T., Juneja L.R., Hatta H., Kim M., editors. Hen Eggs: Their basic and applied science. CRC Press; New York: 1997. pp. 151–178. [Google Scholar]
- Hatta H., Tsuda K., Ozeki M., Kim M., Yamamoto T., Otake S., Hirasawa M., Katz J., Childers N.K., Michalek S.M. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31:268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- Hatta H., Kapoor M.P., Juneja L.R. Bioactive components in egg yolk. In: Mine Y., editor. Egg Bioscience and Biotechnology. John Wiley & Sons, Inc; Hoboken: 2008. pp. 185–237. [Google Scholar]
- Herath C., Kumar P., Singh M., Kumar D., Ramakrishnan S., Goswami T.K., Singh A., Ram G.C. Experimental iron-inactivated Pasteurella multocida A: 1 vaccine adjuvanted with bacterial DNA is safe and protects chickens from fowl cholera. Vaccine. 2010;28:2284–2289. doi: 10.1016/j.vaccine.2009.12.068. [DOI] [PubMed] [Google Scholar]
- Hernandez-Campos F.J., Brito-De La Fuente E., Torrestiana-Sanchez B. Purification of egg yolk immunoglobulin (IgY) by ultrafiltration: effect of pH, ionic strength, and membrane properties. J Agric Food Chem. 2010;58:187–193. doi: 10.1021/jf902964s. [DOI] [PubMed] [Google Scholar]
- Hilpert H., Brussow H., Mietens C., Sidoti J., Lerner L., Werchau H. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. J Infect Dis. 1987;156:158–166. doi: 10.1093/infdis/156.1.158. [DOI] [PubMed] [Google Scholar]
- Hochel I., Viochna D., Skvor J., MusiL M. Development of an indirect competitive ELISA for detection of Campylobacter jejuni subsp. jejuni O:23 in foods. Folia Microbiol (Praha) 2004;49:579–586. doi: 10.1007/BF02931537. [DOI] [PubMed] [Google Scholar]
- Horie K., Horie N., Abdou A.M., Yang J.O., Yun S.S., Chun H.N., Park C.K., Kim M., Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J Dairy Sci. 2004;87:4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- Horikoshi T., Hiraoka J., Saito M., Hamada S. IgG antibody from hen egg yolk: purification by ethanol fractionation. J Food Sci. 1993;58:739–742. [Google Scholar]
- Huang L., Fang X. Immunoaffinity fractionation of plasma proteins by chicken IgY antibodies. Methods Mol Biol. 2008;425:41–51. doi: 10.1007/978-1-60327-210-0_4. [DOI] [PubMed] [Google Scholar]
- Huang L., Harvie G., Feitelson J.S., Gramatikoff K., Herold D.A., Allen D.L., Amunngama R., Hagler R.A., Pisano M.R., Zhang W.W., Fang X. Immunoaffinity separation of plasma proteins by IgY microbeads: meeting the needs of proteomic sample preparation and analysis. Proteomics. 2005;5:3314–3328. doi: 10.1002/pmic.200401277. [DOI] [PubMed] [Google Scholar]
- Ibrahim El S.M., Rahman A.K., Isoda R., Umeda K., Van Sa N., Kodama Y. In vitro and in vivo effectiveness of egg yolk antibody against Candida albicans (anti-CA IgY) Vaccine. 2008;26:2073–2080. doi: 10.1016/j.vaccine.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Ikemori Y., Kuroki M., Peralta R.C., Yokoyama H., Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli. Am J Vet Res. 1992;53:2005–2008. [PubMed] [Google Scholar]
- Ikemori Y., Ohta M., Umeda K., Icatlo F.C., Jr., Kuroki M., Yokoyama H., Kodama Y. Passive protection of neonatal calves against bovine coronavirus-induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet Microbiol. 1997;58:105–111. doi: 10.1016/S0378-1135(97)00144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isibasi A., Ortiz V., Vargas M., Paniagua J., Gonzalez C., Moreno J., Kumate J. Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9,12,d, Vi. Infect Immun. 1988;56:2953–2959. doi: 10.1128/iai.56.11.2953-2959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius J.C., Koch C. On the purification of IgG from egg yolk. J Immunol Methods. 1993;164:141–142. doi: 10.1016/0022-1759(93)90285-f. [DOI] [PubMed] [Google Scholar]
- Jensenius J.C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies Methods for purification of yolk IgG. J Immunol Methods. 1981;46:63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Baidoo S.K., Marquardt R.R., Fröhlich A.A. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol Med Microbiol. 1998;21:313–321. doi: 10.1111/j.1574-695X.1998.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Johnson N., Cunningham A.F., Fooks A.R. The immune response to rabies virus infection and vaccination. Vaccine. 2010;28:3896–3901. doi: 10.1016/j.vaccine.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Juliarena M., Gutierrez S., Ceriani C. Chicken antibodies: a useful tool for antigen capture ELISA to detect bovine leukaemia virus without cross-reaction with other mammalian antibodies. Vet Res Commun. 2007;31:43–51. doi: 10.1007/s11259-006-3422-1. [DOI] [PubMed] [Google Scholar]
- Kammila S., Das D., Bhatnagar P.K., Sunwoo H.H., Zayas-Zamora G., King M., Suresh M.R. A rapid point of care immunoswab assay for SARS-CoV detection. J Virol Methods. 2008;152:77–84. doi: 10.1016/j.jviromet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Kollberg H., Larsson A. Chicken IgY: utilizing the evolutionary advantage. World’s Poult Sci J. 2004;60:341–347. [Google Scholar]
- Kim H., Nakai S. Immunoglobulin separation from egg yolk: a serial filtration system. J Food Sci. 1996;61:510–513. [Google Scholar]
- Kim H., Nakai S. Simple separation of immunolgobulin from egg yolk by ultrafiltration. J Food Sci. 1998;63:485–490. [Google Scholar]
- Kim S.H., Park M.K., Kim J.Y., Chuong P.D., Lee Y.S., Yoon B.S., Hwang K.K., Lim Y.K. Development of a sandwich ELISA for the detection of Listeria spp. using specific flagella antibodies. J Vet Sci. 2005;6:41–46. [PubMed] [Google Scholar]
- Klemperer F. Ueber naturliche Immunitat und ihre Verwertung fur die Immunisierungstherapie. Archiv fur Experimentelle Pathologie und Pharmakologie. 1893;31:356–382. [Google Scholar]
- Klymiuk N., Aigner B., Brem G., Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- Ko K.Y., Ahn D.U. Preparation of immunoglobulin Y from egg yolk using ammonium sulfate precipitation and ion exchange chromatography. Poult Sci. 2007;86:400–407. doi: 10.1093/ps/86.2.400. [DOI] [PubMed] [Google Scholar]
- Kollberg H., Carlander D., Olesen H., Wejaker P.E., Johannesson M., Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003;35:433–440. doi: 10.1002/ppul.10290. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J., Mine Y. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J Immunol Methods. 2005;296:199–209. doi: 10.1016/j.jim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J., Sasaki E., Yoo D., Mine Y. Cloning and expression of human rotavirus spike protein, VP8*. Escherichia coli. Biochem Biophys Res Commun. 2001;282:1183–1188. doi: 10.1006/bbrc.2001.4717. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Ikemori Y., Yokoyama H., Peralta R.C., Icatlo F.C., Jr., Kodama Y. Passive protection against bovine rotavirus-induced diarrhea in murine model by specific immunoglobulins from chicken egg yolk. Vet Microbiol. 1993;37:135–146. doi: 10.1016/0378-1135(93)90188-d. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Ohta M., Ikemori Y., Peralta R.C., Yokoyama H., Kodama Y. Passive protection against bovine rotavirus in calves by specific immunoglobulins from chicken egg yolk. Arch Virol. 1994;138:143–148. doi: 10.1007/BF01310045. [DOI] [PubMed] [Google Scholar]
- Kuronen I., Kokko H., Mononen I., Parviainen M. Hen egg yolk antibodies purified by antigen affinity under highly alkaline conditions provide new tools for diagnostics. Human intact parathyrin as a model antigen. Eur J Clin Chem Clin Biochem. 1997;35:435–440. doi: 10.1515/cclm.1997.35.6.435. [DOI] [PubMed] [Google Scholar]
- Kwan L., Li-Chan E.C., Helbig N., Nakai S. Fractionation of water-soluble and- insoluble components from egg yolk with minimum use of organic solvents. Journal of Food Science. 1991;56:1537–1541. [Google Scholar]
- Kweon C.H., Kwon B.J., Woo S.R., Kim J.M., Woo G.H., Son D.H., Hur W., Lee Y.S. Immunoprophylactic effect of chicken egg yolk immunoglobulin (Ig Y) against porcine epidemic diarrhea virus (PEDV) in piglets. J Vet Med Sci. 2000;62:961–964. doi: 10.1292/jvms.62.961. [DOI] [PubMed] [Google Scholar]
- Larsson A., Carlander D. Oral immunotherapy with yolk antibodies to prevent infections in humans and animals. Ups J Med Sci. 2003;108:129–140. [PubMed] [Google Scholar]
- Larsson A., Mellstedt H. Chicken antibodies: a tool to avoid interference by human anti-mouse antibodies in ELISA after in vivo treatment with murine monoclonal antibodies. Hybridoma. 1992;11:33–39. doi: 10.1089/hyb.1992.11.33. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sjoquist J. Chicken antibodies: a tool to avoid false positive results by rheumatoid factor in latex fixation tests. J Immunol Methods. 1988;108:205–208. doi: 10.1016/0022-1759(88)90420-6. [DOI] [PubMed] [Google Scholar]
- Larsson A., Karlsson-Parra A., Sjoquist J. Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clin Chem. 1991;37:411–414. [PubMed] [Google Scholar]
- Larsson A., Balow R., Lindahl T.L., Forsberg P. Chicken antibodies: taking advantage of evolution; a review. Poult Sci. 1993;72:1807–1812. doi: 10.3382/ps.0721807. [DOI] [PubMed] [Google Scholar]
- Larsson A., Carlander D., Wilhelmsson M. Antibody response in laying hens with small amounts of antigen. Food Agric Immunol. 1998;10:29–36. [Google Scholar]
- Leclaire R.D., Hunt R.E., Bavari S. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect Immun. 2002;70:2278–2281. doi: 10.1128/IAI.70.5.2278-2281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.N., Sunwoo H.H., Menninen K., Sim J.S. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult Sci. 2002;81:632–641. doi: 10.1093/ps/81.5.632. [DOI] [PubMed] [Google Scholar]
- Lee J., Babiuk L.A., Harland R., Gibbons E., Elazhary Y., Yoo D. Immunological response to recombinant VP8* subunit protein of bovine roravirus in pregnant cattle. J Gen Virol. 1995;76(Pt 10):2477–2483. doi: 10.1099/0022-1317-76-10-2477. [DOI] [PubMed] [Google Scholar]
- Lee K.A., Chang S.K., Lee Y.J., Lee J.H., Koo N.S. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J Biochem Mol Biol. 2002;35:488–493. doi: 10.5483/bmbrep.2002.35.5.488. [DOI] [PubMed] [Google Scholar]
- Lee S.B., Mine Y., Stevenson R.M. Effects of hen egg yolk immunoglobulin in passive protection of rainbow trout against Yersinia ruckeri. J Agric Food Chem. 2000;48:110–115. doi: 10.1021/jf9906073. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Park D.W., Jang S.I., Morales A., Garcia D., Lucio E., Larios R., Victoria G., Marrufo D., LillehoJ E.P. Induction of passive immunity in broiler chickens against Eimeria acervulina by hyperimmune egg yolk immunoglobulin Y. Poult Sci. 2009;88:562–566. doi: 10.3382/ps.2008-00340. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Park D.W., Jang S.I., Morales A., Garcia D., Lucio E., Larios R., Victoria G., Marrufo D., Lillehoj E.P. Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet Parasitol. 2009;163:123–126. doi: 10.1016/j.vetpar.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lemamy G.J., Roger P., Mani J.C., Robert M., Rochefort H., Brouillet J.P. High- affinity antibodies from hen’s-egg yolks against human mannose-6-phosphate/insulin- like growth-factor-II receptor (M6P/IGFII-R): characterization and potential use in clinical cancer studies. Int J Cancer. 1999;80:896–902. doi: 10.1002/(sici)1097-0215(19990315)80:6<896::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Leslie G.A., Clem L.W. Phylogeny of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J Exp Med. 1969;130:1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie G.A., Martin L.N. Studies on the secretory immunologic system of fowl. 3. Serum and secretory. IgA of the chicken. J Immunol. 1973;110:1–9. [PubMed] [Google Scholar]
- Leventhal J.R., Su A., Kaufman D.B., Abecassis M.I., Stuart F.P., Anderson B., Fryer J.P. Altered infectivity of porcine endogenous retrovirus by ‘protective’ avian antibodies: implications for pig-to-human xenotransplantation. Transplant Proc. 2001;33:690. doi: 10.1016/s0041-1345(00)02204-1. [DOI] [PubMed] [Google Scholar]
- Levesque S., Martinez G., Fairbrot Her J.M. Improvement of adjuvant systems to obtain a cost-effective production of high levels of specific IgY. Poult Sci. 2007;86:630–635. doi: 10.1093/ps/86.4.630. [DOI] [PubMed] [Google Scholar]
- Li X., Nakano T., Sunwoo H.H., Paek B.H., Chae H.S., Sim J.S. Effects of egg and yolk weights on yolk antibody (IgY) production in laying chickens. Poultry Science. 1997;77:266–270. doi: 10.1093/ps/77.2.266. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Jin L.J., Mcallister T.A., Stanford K., Xu J.Y., Lu Y.N., Zhen Y.H., Sun Y.X., Xu Y.P. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY) J Agric Food Chem. 2007;55:2911–2917. doi: 10.1021/jf062900q. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Jin L.J., Lu Y.N., Zhen Y.H., Li S.Y., Wang L.H., Xu Y.P. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY): effects of chitosan concentration. Appl Biochem Biotechnol. 2009;159:778–787. doi: 10.1007/s12010-009-8628-6. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Jin L.J., Uzonna J.E., Li S.Y., Liu J.J., Li H.Q., Lu Y.N., Zhen Y.H., Xu Y.P. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY): in vivo evaluation in a pig model of enteric colibacillosis. Vet Immunol Immunopathol. 2009;129:132–136. doi: 10.1016/j.vetimm.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Li-Chan E.Y.C. Applications of egg immunoglobulins in immunoaffinity chromatography. In: Sim J.S., Nakai S., Guenter W., editors. Egg Nutrition and Biotechnology. CAB International; New York: 2000. pp. 323–339. [Google Scholar]
- Lisa M., Chouhan R.S., Vinayaka A.C., Manonmani H.K., Thakur M.S. Gold nanoparticles based dipstick immunoassay for the rapid detection of dichlorodiphenyltrichloroethane: an organochlorine pesticide. Biosens Bioelectron. 2009;25:224–227. doi: 10.1016/j.bios.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Loeken M.R., Roth T.F. Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology. 1983;49:21–28. [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Liu J., Jin L., Li X., Zhen Y., Xue H., You J., Xu Y. Passive protection of shrimp against white spot syndrome virus (WSSV) using specific antibody from egg yolk of chickens immunized with inactivated virus or a WSSV-DNA vaccine. Fish Shellfish Immunol. 2008;25:604–610. doi: 10.1016/j.fsi.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Lu Y., Liu J., Jin L., Li X., Zhen Y., Xue H., Lin Q., Xu Y. Passive immunization of crayfish (Procambius clarkiaii) with chicken egg yolk immunoglobulin (IgY) against white spot syndrome virus (WSSV) Appl Biochem Biotechnol. 2009;159:750–758. doi: 10.1007/s12010-009-8555-6. [DOI] [PubMed] [Google Scholar]
- Magagnotti C., Fermo I., Carletti R.M., Ferrari M., Bachi A. Comparison of different depletion strategies for improving resolution of the human urine proteome. Clin Chem Lab Med. 2010;48:531–535. doi: 10.1515/CCLM.2010.109. [DOI] [PubMed] [Google Scholar]
- Mahdavi A.H., Rahmani H.R., Nili N., Samie A.H., Soleimanian-Zad S., Jahanian R. Effects of dietary egg yolk antibody powder on growth performance, intestinal Escherichia coli colonization, and immunocompetence of challenged broiler chicks. Poult Sci. 2010;89:484–494. doi: 10.3382/ps.2009-00541. [DOI] [PubMed] [Google Scholar]
- Marquardt R.R., Jin L.Z., Kim J.W., Fang L., Fröhlich A.A., Baidoo S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88 + infection in neonatal and early-weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Matsuura F., Ohta M., Murakami K., Matsuki Y. Structures of asparagine linked oligosaccharides of immunoglobulins (IgY) isolated from egg-yolk of Japanese quail. Glycoconj J. 1993;10:202–213. doi: 10.1007/BF00702201. [DOI] [PubMed] [Google Scholar]
- Mcbee L.E., Cotterill O.J. Ion-exchange chromatography and electrophoresis of egg yolk proteins. J Food Sci. 1979;44:656–667. [Google Scholar]
- Mccannel A.A., Nakai S. Separation of egg yolk immunoglobulins into subpopulations using DEAE-ion exchange chromatography. Can Inst Food Sci Technol J. 1990;23:42–46. [Google Scholar]
- Mclaren R.D., Prosser C.G., Grieve R.C., BorissenkO M. The use of caprylic acid for the extraction of the immunoglobulin fraction from egg yolk of chickens immunised with ovine alpha-lactalbumin. J Immunol Methods. 1994;177:175–184. doi: 10.1016/0022-1759(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Meenatchisundaram S., Parameswari G., Michael A., Ramalingam S. Neutralization of the pharmacological effects of Cobra and Krait venoms by chicken egg yolk antibodies. Toxicon. 2008;52:221–227. doi: 10.1016/j.toxicon.2008.04.179. [DOI] [PubMed] [Google Scholar]
- Meenatchisundaram S., Parameswari G., Michael A., Ramalingam S. Studies on pharmacological effects of Russell’s viper and Saw-scaled viper venom and its neutralization by chicken egg yolk antibodies. Int Immunopharmacol. 2008;8:1067–1073. doi: 10.1016/j.intimp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Morris J.A., Sojka W.J. Academic Press, Inc.; London: 1985. Escherichia coli as a pathogen in animals, in Sussman M, The Virulence of Escherichia coli. [Google Scholar]
- Morrison S.L., Mohammed M.S., Wims L.A., Trinh R., Etches R. Sequences in antibody molecules important for receptor-mediated transport into the chicken egg yolk. Mol Immunol. 2002;38:619–625. doi: 10.1016/s0161-5890(01)00095-5. [DOI] [PubMed] [Google Scholar]
- Motoi Y., Inoue S., Hatta H., Sato K., Morimoto K., Yamada A. Detection of rabies- specific antigens by egg yolk antibody (IgY) to the recombinant rabies virus proteins produced in Escherichia coli. Jpn J Infect Dis. 2005;58:115–118. [PubMed] [Google Scholar]
- Motoi Y., Sato K., Hatta H., Morimoto K., Inoue S., Yamada A. Production of rabies neutralizing antibody in hen’s eggs using a part of the G protein expressed in Escherichia coli. Vaccine. 2005;23:3026–3032. doi: 10.1016/j.vaccine.2004.11.071. [DOI] [PubMed] [Google Scholar]
- Nakai S., Li-Chan E., Lo K.V. Separation of immunoglobulin from egg yolk. In: Sim J.S., Nakai S., editors. Egg Uses and Processing Technologies - New Developments, Wallingford. CAB International; UK: 1994. pp. 94–105. [Google Scholar]
- Nguyen H.H., Tumpey T.M., Park H.J., Byun Y.H., Tran L.D., Nguyen V.D., Kilgore P.E., Czerkinsky C., Katz J.M., Seong B.L., Song J.M., Kim Y.B., Do H.T., Nguyen T., Nguyen C.V. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One. 2010;5:e10152. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E., Amini A., Wretlind B., Larsson A. Pseudomonas aeruginosa infections are prevented in cystic fibrosis patients by avian antibodies binding Pseudomonas aeruginosa flagellin. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:75–80. doi: 10.1016/j.jchromb.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Kollberg H., Johannesson M., Wejaker P.E., Carlander D., Larsson A. More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. J Med Food. 2007;10:375–378. doi: 10.1089/jmf.2006.214. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Larsson A., Olesen H.V., Wejaker P.E., Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008;43:892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- Noack F., Helmecke D., Rosenberg R., Thorban S., Nekarda H., Fink U., Lewald J., Stich M., Schütze K., Harbeck N., Magdolen V., Graeff H., Schmitt M. CD87-positive tumor cells in bone marrow aspirates identified by confocal laser scanning fluorescence microscopy. Int J Oncol. 1999;15:617–623. doi: 10.3892/ijo.15.4.617. [DOI] [PubMed] [Google Scholar]
- Nomura S., Suzuki H., Masaoka T., Kurabayashi K., Ishii H., Kitajima M., Nomoto K., Hibi T. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2005;10:43–52. doi: 10.1111/j.1523-5378.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Ohta M., Hamako J., Yamamoto S., Hatta H., Kim M., Yamamoto T., Oka S., Mizuochi T., Matsuura F. Structures of asparagine-linked oligosaccharides from hen egg-yolk antibody (IgY). Occurrence of unusual glucosylated oligo-mannose type oligosaccharides in a mature glycoprotein. Glycoconj J. 1991;8:400–413. doi: 10.1007/BF00731292. [DOI] [PubMed] [Google Scholar]
- Olovsson M., Larsson A. Biotin labelling of chicken antibodies and their subsequent use in ELISA and immunohistochemistry. Comp Immunol Microbiol Infect Dis. 1993;16:145–152. doi: 10.1016/0147-9571(93)90007-r. [DOI] [PubMed] [Google Scholar]
- Otake S., Nishihara Y., Makimura M., Hatta H., Kim M., Yamamoto T., Hirasawa M. Protection of rats against dental caries by passive immunization with hen-egg-yolk antibody (IgY) J Dent Res. 1991;70:162–166. doi: 10.1177/00220345910700030101. [DOI] [PubMed] [Google Scholar]
- Pan Z.L., Ji X.Y., Shi Y.M., Zhou J., He E., Skog S. Serum thymidine kinase 1 concentration as a prognostic factor of chemotherapy-treated non-Hodgkin’s lymphoma patients. J Cancer Res Clin Oncol. 2010;136:1193–1199. doi: 10.1007/s00432-010-0769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvari R., Avivi A., Lentner F., Ziv E., Tel-Or S., Burstein Y., Schechter I. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. Embo J. 1988;7:739–744. doi: 10.1002/j.1460-2075.1988.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K., Manjula J., Deep A.E.P., Selvanayagam Z.E., Ganesh K.A., Subba Rao P.V. Anti-Echis carinatus venom antibodies from chicken egg yolk: isolation, purification and neutralization efficacy. Toxicon. 2007;50:893–900. doi: 10.1016/j.toxicon.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Pink J.R., Vainio O., Rijnbeek A.M. Clones of B lymphocytes in individual follicles of the bursa of Fabricius. Eur J Immunol. 1985;15:83–87. doi: 10.1002/eji.1830150116. [DOI] [PubMed] [Google Scholar]
- Podolsky D.K. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Polaskova V., Kapur A., Khan A., Molloy M.P., Baker M.S. High-abundance protein depletion: comparison of methods for human plasma biomarker discovery. Electrophoresis. 2010;31:471–482. doi: 10.1002/elps.200900286. [DOI] [PubMed] [Google Scholar]
- Polson A., Von Wechmar M.B., Fazakerley G. Antibodies to proteins from yolk of immunized hens. Immunol Commun. 1980;9:495–514. doi: 10.3109/08820138009066011. [DOI] [PubMed] [Google Scholar]
- Polson A. Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol Invest. 1990;19:253–258. doi: 10.3109/08820139009041840. [DOI] [PubMed] [Google Scholar]
- Qian W.J., Kaleta D.T., Petritis B.O., Jiang H., Liu T., Zhang X., Mottaz H.M., Varnum S.M., Camp D.G., 2nd., Huang L., Fang X., Zhang W.W., Smith R.D. Enhanced detection of low abundance human plasma proteins using a tandem IgY12-SuperMix immunoaffinity separation strategy. Mol Cell Proteomics. 2008;7:1963–1973. doi: 10.1074/mcp.M800008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajic A., Stehmann C., Autelitano D.J., Vrkic A.K., Hosking C.G., Rice G.E., Ilag L.L. Protein depletion using IgY from chickens immunised with human protein cocktails. Prep Biochem Biotechnol. 2009;39:221–247. doi: 10.1080/10826060902952915. [DOI] [PubMed] [Google Scholar]
- Reilly R., Domingo R., Sandhu J. Oral delivery of antibodies; future pharmacokinetic trends. Clin Pharmacokinet. 1997;4:313–323. doi: 10.2165/00003088-199732040-00004. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Anquez V., Dahan A., Weill J.C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985;40:283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Dahan A., Anquez V., Weill J.C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Anquez V., Weill J.C. The chicken D locus and its contribution to the immunoglobulin heavy chain repertoire. Eur J Immunol. 1991;21:2661–2670. doi: 10.1002/eji.1830211104. [DOI] [PubMed] [Google Scholar]
- Romito M., Viljoen G.J., Du Plessis D.H. Eliciting antigen-specific egg-yolk IgY with naked DNA. Biotechniques. 2001;31:674–675. doi: 10.2144/01313dd05. 670, 672. [DOI] [PubMed] [Google Scholar]
- Roos N., Mähe S., Benamouzig R., Sick H., Rautureau J., Tome D. 15 N-labeled immunoglobulins from bovine colostrum are partially resistant to digestion in human intestine. J Nutr. 1995;125:1238–1244. doi: 10.1093/jn/125.5.1238. [DOI] [PubMed] [Google Scholar]
- Rose M.E., Orlans E., Buttress N. Immunoglobulin classes in the hen’s egg: their segregation in yolk and white. Eur J Immunol. 1974;4:521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Hanauer S.B. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Sandrin M.S., Mckenzie I.F. Gal alpha (1,3) Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol Rev. 1994;141:169–190. doi: 10.1111/j.1600-065x.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Schade R., Hlinak A. Egg yolk antibodies, state of the art and future prospects. Altex. 1996;13:5–9. [PubMed] [Google Scholar]
- Schade R., Pfister C., Halatsch R., Henklein P. Polyclonal IgY antibodies from chicken egg-an alternative to the production of mammalian IgG type antibodies in rabbits. ATLA. 1991;19:403–419. [Google Scholar]
- Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim. 2005;33:129–154. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- Shale D.J., Elborn J.S. Lung injury. In: Shale D.J., editor. Cystic Fibrosis. BMJ Publishing Group; London: 1996. pp. 62–78. [Google Scholar]
- Sharma J.M. The structure and function of the avian immune system. Acta Vet Hung. 1997;45:229–238. [PubMed] [Google Scholar]
- Shimizu M., Nakane Y. Encapsulation of biologically active proteins in a multiple emulsion. Biosci Biotechnol Biochem. 1995;59:492–496. doi: 10.1271/bbb.59.492. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Fitzsimmons R.C., Nakai S. Anti-E. coli immunoglobulin Y isolated from egg yolk of immunized chickens as a potential food ingredient. J Food Sci. 1988;53:1360–1368. [Google Scholar]
- Shimizu M., Nagashima H., Sano K., Hashimoto K., Ozeki M., Tsuda K., Hatta H. Molecular stability of chicken and rabbit immunoglobulin. G. Biosci Biotechnol Biochem. 1992;56:270–274. doi: 10.1271/bbb.56.270. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Miwa Y., Hashimoto K., Goto A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci Biotechnol Biochem. 1993;57:1445–1449. doi: 10.1271/bbb.57.1445. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Nagashima H., Hashimoto K. Comparative studies on molecular stability of immunoglobulin G from different species. Comp Biochem Physiol. 1993;106B:255–261. doi: 10.1016/0305-0491(93)90297-i. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Nagashima H., Hashimoto K., Suzuki T. Egg yolk antibody (IgY) stability in aqueous solution with high sugar concentrations. J Food Sci. 1994;59:763–772. [Google Scholar]
- Shin J.H., Yang M., Nam S.W., Kim J.T., Myung N.H., Bang W.G., Roe I.H. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2002;9:1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.H., Nam S.W., Kim J.T., Yoon J.B., Bang W.G., Roe I.H. Identification of immunodominant Helicobacter pylori proteins with reactivity to H. pylori-specific egg-yolk immunoglobulin. J Med Microbiol. 2003;52:217–222. doi: 10.1099/jmm.0.04978-0. [DOI] [PubMed] [Google Scholar]
- Shin J.H., Roe I.H., Kim H.G. Production of anti-Helicobacterpylori urease-specific immunoglobulin in egg yolk using an antigenic epitope of H. pylori urease. J Med Microbiol. 2004;53:31–34. doi: 10.1099/jmm.0.05327-0. [DOI] [PubMed] [Google Scholar]
- Shin S.J., Lee S.S., Manning E.J., Collins M.T. Production of and applications for a polyclonal IgY diagnostic reagent specific for Mycobacterium avium subsp. paratuberculosis. J Microbiol. 2009;47:600–609. doi: 10.1007/s12275-009-0052-7. [DOI] [PubMed] [Google Scholar]
- Sim J.S., Sunwoo H.H., Lee E.N. Ovoglobulin IgY. In: Naidu A.S., editor. Natural Food Antimicrobial Systems. CRC Press; New York: 2000. pp. 227–252. [Google Scholar]
- Smith D.J., King W.F., Godiska R. Passive transfer of immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infect Immun. 2001;69:3135–3142. doi: 10.1128/IAI.69.5.3135-3142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R.M.W., Flett D., Raymond B.T. Enteric redmouth (ERM) and other enterobacterial infections of fish. In: Inglis V., Roberts R.J., Bromage N.R., editors. Bacterial Diseases of Fish. Blackwell Scientific Publications; Oxford: 1993. pp. 80–105. [Google Scholar]
- Sugita-Konishi Y., Shibata K., Yun S.S., Hara-Kudo Y., Yamaguchi K., Kumagai S. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Biosci Biotechnol Biochem. 1996;60:886–888. doi: 10.1271/bbb.60.886. [DOI] [PubMed] [Google Scholar]
- Sun S., Mo W., Ji Y., Liu S. Preparation and mass spectrometric study of egg yolk antibody (IgY) against rabies virus. Rapid Commun Mass Spectrom. 2001;15:708–712. doi: 10.1002/rcm.271. [DOI] [PubMed] [Google Scholar]
- Sunwoo H.H., Wang W.W., Sim J.S. Detection of Escherichia coli O157:H7 using chicken immunoglobulin Y. Immunol Lett. 2006;106:191–193. doi: 10.1016/j.imlet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Svendsen L., Crowley A., Ostergaard L.H., Stodulski G., Hau J. Development and comparison of purification strategies for chicken antibodies from egg yolk. Lab Anim Sci. 1995;45:89–93. [PubMed] [Google Scholar]
- Tesar D.B., Cheung E.J., Bjorkman P.J. The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Mol Biol Cell. 2008;19:1587–1593. doi: 10.1091/mbc.E07-09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka A., Hamajima S., Hatta H., Abiko Y. Inhibition of Porphyromonas gingivalis hemagglutinating activity by IgY against a truncated HagA. J Oral Sci. 2006;48:227–232. doi: 10.2334/josnusd.48.227. [DOI] [PubMed] [Google Scholar]
- Thalley B.S., Carroll S.B. Rattlesnake and scorpion antivenoms from the egg yolks of immunized hens. Biotechnol (N Y) 1990;8:934–938. doi: 10.1038/nbt1090-934. [DOI] [PubMed] [Google Scholar]
- Thorns C.J., Sojka M.G., Chasey D. Detection of a novel fimbrial structure on the surface of Salmonella enteritidis by using a monoclonal antibody. J Clin Microbiol. 1990;28:2409–2414. doi: 10.1128/jcm.28.11.2409-2414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorns C.J., Sojka M.G., Mclaren I.M., Dibb-Fuller M. Characterisation of monoclonal antibodies against a fimbrial structure of Salmonella enteritidis and certain other serogroup D salmonellae and their application as serotyping reagents. Res Vet Sci. 1992;53:300–308. doi: 10.1016/0034-5288(92)90130-t. [DOI] [PubMed] [Google Scholar]
- Tressler R.L., Roth T.F. IgG receptors on the embryonic chick yolk sac. J Biol Chem. 1987;262:15406–15412. [PubMed] [Google Scholar]
- Trott D.L., Hellestad E.M., Yang M., Cook M.E. Additions of killed whole cell bacteria preparations to Freund complete adjuvant alter laying hen antibody response to soluble protein antigen. Poult Sci. 2008;87:912–917. doi: 10.3382/ps.2007-00481. [DOI] [PubMed] [Google Scholar]
- Van Nguyen S., Umeda K., Yokoyama H., Tohya Y., Kodama Y. Passive protection of dogs against clinical disease due to canine parvovirus-2 by specific antibody from chicken egg yolk. Can J Vet Res. 2006;70:62–64. [PMC free article] [PubMed] [Google Scholar]
- Vasconcellos F.A., Coutinho M.L., Da Silva E.F., Fernandes C.P., Monte L.G., Seyffert N., Dellagostin O.A., Aleixo J.A. Testing different antigen capture ELISA formats for detection of Leptospira spp. in human blood serum. Trans R Soc Trop Med Hyg. 2010;104:259–264. doi: 10.1016/j.trstmh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Veerasami M., Singanallur N.B., Thirumeni N., Rana S.K., Shanmugham R., Ponsekaran S., Muthukrishnan M., Villuppanoor S.A. Serotyping of foot-and-mouth disease virus by antigen capture-ELISA using monoclonal antibodies and chicken IgY. New Microbiol. 2008;31:549–554. [PubMed] [Google Scholar]
- Verdoliva A., Basile G., Fassina G. Affinity purification of immunoglobulins from chicken egg yolk using a new synthetic ligand. J Chromatogr B Biomed Sci Appl. 2000;749:233–242. doi: 10.1016/s0378-4347(00)00426-6. [DOI] [PubMed] [Google Scholar]
- Wang X.Z., Fan B., Liu L.G., Hu X.Y., Li R.Y., Wei Y., Wan Z., Deng X.L. In vitro inhibition of oral Candida albicans by chicken egg yolk antibody (IgY) Mycopathologia. 2008;165:381–387. doi: 10.1007/s11046-008-9097-0. [DOI] [PubMed] [Google Scholar]
- Wanke R., Schmidt P., Erhard M.H., Sprick-Sanjose Messing A., Stangassinger M., Schmahl W., Hermanns W. Freund’s complete adjuvant in the chicken: efficient immunostimulation with severe local inflammatory reaction. Zentralbl Veterinarmed A. 1996;43:243–253. [PubMed] [Google Scholar]
- Warr G.W., Magor K.E., Higgins D.A. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- West A.P., Jr., Herr A.B., Bjorkman P.J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]
- Witkowski P.T., Bourquain D.R., Höhn O., Schade R., Nitsche A. Gene gun-supported DNA immunisation of chicken for straightforward production of poxvirus-specific IgY antibodies. J Immunol Methods. 2009;341:146–153. doi: 10.1016/j.jim.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley J.A., Landon J. Comparison of antibody production to human interleukin-6 (IL-6) by sheep and chickens. J Immunol Methods. 1995;178:253–265. doi: 10.1016/0022-1759(94)00263-v. [DOI] [PubMed] [Google Scholar]
- Worledge K.L., Godiska R., Barrett T.A., Kink J.A. Oral administration of avian tumor necrosis factor antibodies effectively treats experimental colitis in rats. Dig Dis Sci. 2000;45:2298–2305. doi: 10.1023/a:1005554900286. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Gao X. Use of IgY antibodies and semiconductor nanocrystal detection in cancer biomarker quantitation. Biomark Med. 2010;4:227–239. doi: 10.2217/bmm.10.7. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Peralta R.C., Diaz R., Sendo S., Ikemori Y., Kodama Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect Immun. 1992;60:998–1007. doi: 10.1128/iai.60.3.998-1007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H., Peralta R.C., Umeda K., Hashi T., Icatlo F.C., Jr., Kuroki M., Ikemori Y., Kodama Y. Prevention of fatal salmonellosis in neonatal calves, using orally administered chicken egg yolk Salmonella-specific antibodies. Am J Vet Res. 1998;59:416–420. [PubMed] [Google Scholar]
- Yokoyama H., Umeda K., Peralta R.C., Hashi T., Icatlo F.C., Jr., Kuroki M., Ikemori Y., Kodama Y. Oral passive immunization against experimental salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella enteritidis and S. typhimurium. Vaccine. 1998;16:388–393. doi: 10.1016/s0264-410x(97)80916-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Sugano N., Rahman A.K., Oshikawa M., Ito K. Activity of anti- Porphyromonas gingivalis egg yolk antibody against gingipains in vitro. Oral Microbiol Immunol. 2007;22:352–355. doi: 10.1111/j.1399-302X.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Sugano N., Shimada T., Shofiqur R.A., Ibrahim el S.M., Isoda R., Umeda K., SA N.V., Kodama Y., Ito K. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J Oral Sci. 2007;49:201–206. doi: 10.2334/josnusd.49.201. [DOI] [PubMed] [Google Scholar]
- Yolken R.H., Leister F., Wee S.B., Miskuff R., Vonderfecht S. Antibodies to rotaviruses in chickens’ eggs: a potential source of antiviral immunoglobulins suitable for human consumption. Pediatrics. 1988;81:291–295. [PubMed] [Google Scholar]
- Yousif A.A., Mohammad W.A., Khodeir M.H., Zeid A.Z., El-Sanousi A.A., Saber M.S., Reda I.M. Oral administration of hyperimmune IgY: an immunoecological approach to curbing acute infectious bursal disease virus infection. Egypt J Immunol. 2006;13:85–94. [PubMed] [Google Scholar]
- Zhang S.H., Luo Q.L., Zhou Y.D., Li J., Xu Y.H., Shen J.L. Cloning and expression of rotavirus SA11 VP7 and preparation of IgY antibodies against recombinant VP7. Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43:526–530. [PubMed] [Google Scholar]
- Zhang W.W. The use of gene-specific IgY antibodies for drug target discovery. Drug Discov Today. 2003;8:364–371. doi: 10.1016/s1359-6446(03)02655-2. [DOI] [PubMed] [Google Scholar]
- Zhen Y.H., JIN L.J., Guo J., Li X.Y., Li Z., Fang R., Xu Y.P. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Staphylococcus aureus. J Appl Microbiol. 2008;105:1529–1535. doi: 10.1111/j.1365-2672.2008.03920.x. [DOI] [PubMed] [Google Scholar]
- Zhen Y.H., JIN L.J., Guo J., Li X.Y., Lu Y.N., Chen J., Xu Y.P. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Escherichia coli. Vet Microbiol. 2008;130:126–133. doi: 10.1016/j.vetmic.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Zhen Y.H., JIN L.J., Li X.Y., Guo J., Li Z., Zhang B.J., Fang R., Xu Y.P. Efficacy of specific egg yolk immunoglobulin (IgY) to bovine mastitis caused by Staphylococcus aureus. Vet Microbiol. 2009;133:317–322. doi: 10.1016/j.vetmic.2008.07.016. [DOI] [PubMed] [Google Scholar]


