TEACHING POINTS.
-
•
The majority of all childhood lower respiratory illnesses are caused by viral agents.
-
•
In healthy patients, the course of viral respiratory infections is usually relatively predictable in terms of both the nature of onset and the duration of the acute phase.
-
•
When abrupt deviations in the course of an acute viral illness occur, the possibility of bacterial superinfection must be considered.
-
•
Of the more recently recognized viral agents, human metapneumovirus stands out as an important cause of respiratory syncytial virus–like illness.
Worldwide, it is estimated that 3 to 5 million children die annually as a result of acute respiratory disease. In the United States, respiratory diseases account for 75% to 80% of all acute morbidity. Viral infections are the greatest contributors to this morbidity rate, causing approximately 80% of respiratory illness. Many of these infections produce mild, self-limited symptoms of the upper respiratory tract. Lower respiratory tract illness (LRI), particularly that associated with the presence of crackles or wheezes on physical examination or as parenchymal disease on a chest radiograph, accounts for the majority of severe disease. One third of children develop LRI in the first year of life.1 Childhood asthma may be initially difficult to discriminate from LRI in some patients; indeed, both conditions can be present simultaneously.
Although 60% or more of LRIs are primarily viral2 (Table 33-1 ), the concern often remains as to whether bacterial infection is present, as either a primary problem or a complication of viral infection. Of the bacteria, Streptococcus pneumoniae is by far the most common, followed by Haemophilus influenzae and Staphylococcus aureus. Clinical features that suggest these causes include an abrupt onset or a change in symptoms over a few to several hours, toxicity, and radiographic findings of parenchymal consolidation, pleural effusions, or both. White blood cell counts are of variable help, but extreme leukocytosis (>20,000 cells/mm3) or increased neutrophil band counts (>1500 cells/mm3) suggest possible bacterial involvement. However, such findings are not absolute; severe viral infections can produce leukocytosis and variable shifts to the left. Conversely, overwhelming bacterial pneumonia can present with ominous leukopenia. The magnitude of fever is thought by some to be helpful in determining the possible presence of a bacterial infection, but viral LRI can also provoke high fevers, which may persist for several days or longer. Serum levels of procalcitonin and C-reactive protein have shown the most promise to date in distinguishing serious bacterial infection from virus-caused disease, but these findings remain untested in respiratory diseases.3, 4
Table 33-1.
Major Causes of Acute Lower Respiratory Tract Illnesses
| Syndrome | Viruses | Nonviral Agents | Estimated Percentage Caused by Viruses |
|---|---|---|---|
| Epiglottitis | Rare | Haemophilus influenzae, Streptococcus pyogenes, Streptococcus pneumoniae, Neisseria meningitidis, Corynebacterium diphtheriae | 5-10 |
| Laryngitis and croup | Parainfluenza viruses, influenza viruses, adenoviruses, respiratory syncytical virus, human metapneumovirus, rhinoviruses, coronaviruses, echoviruses | Rare | 90 |
| Laryngotracheitis and laryngotracheobronchitis | Same as for laryngitis and croup | H. influenzae, S taphylococcus aureus | 90 |
| Bronchitis | Parainfluenza viruses, influenza viruses, respiratory syncytial virus, human metapneumovirus, adenoviruses, coronaviruses | Bordetella pertussis, Bordetella parapertussis, H. influenzae, Mycoplasma pneumoniae, Chlamydia pneumoniae | 80 |
| Bronchiolitis | Respiratory syncytial virus, parainfluenza viruses, human metapneumovirus, influenza viruses, adenoviruses | Chlamydia trachomatis, C. pneumoniae, M. pneumoniae | 90 |
| Pneumonia | Same as for bronchiolitis | M. pneumoniae, C. trachomatis, C. pneumoniae, S. pneumoniae, H. influenzae, S. aureus, Legionella species, N. meningitidis, mixed aerobic and anaerobic flora* | 70-80 |
Aspiration-related and lung abscess.
Other nonviral agents include Chlamydia trachomatis, Chlamydia pneumoniae, Mycoplasma pneumoniae, Mycobacterium tuberculosis, deep mycoses, and Pneumocystis jirovecii. C. trachomatis pneumonia is most common among infants between 2 weeks and 6 months. Characteristically, the onset of respiratory symptoms is insidious over several days, the infant is usually afebrile, and air trapping with interstitial infiltrates is often apparent on chest radiographs. Symptomatic M. pneumoniae infections are uncommon in children younger than 5 years; however, they frequently cause pneumonia among children in the 5- to 19-year age group.5 C. pneumoniae infections appear to follow clinical and age-specific patterns similar to those of M. pneumonia infections, but more data are needed before such a comparison can be confirmed.6 Tuberculosis and deep mycoses should be considered when the symptoms and radiographic abnormalities insidiously progress over days to weeks. Finally, P. jirovecii infections are suggested in patients who have progressive hypoxemia (often without significant hypercarbia), alveolar infiltrates, and significant risk factors such as congenital or acquired immunodeficiency, malignancy, and severe protein malnutrition.
A common clinical dilemma occurs in reliably discerning treatable causes, such as bacteria, from viral agents that may not be susceptible to specific therapies. Often, the choice is made to treat nearly all young patients who have an acute LRI with an antibiotic in case a bacterial agent is involved. Such therapy is useless in viral disease and has not been shown to alter the risk of bacterial superinfection; furthermore, such a practice can result in the selection of more resistant organisms if secondary infection does occur. With the emergence of molecular diagnostic methods, a timely, specific diagnosis of many viral LRIs is possible. In addition, specific antiviral therapy is available for some viral infections and may reduce both the morbidity and the mortality rates.
Despite the concern of bacterial coinfection, viral infections remain the most common causes of pediatric LRIs, especially among children younger than 5 years.7, 8 Of these, respiratory syncytial virus (RSV); human metapneumovirus (hMPV); parainfluenza virus types 1, 2, and 3; influenza virus types A and B; and adenoviruses comprise the majority.7 Rhinoviruses, human coronaviruses (HCVs), influenza virus type C, and parainfluenza virus type 4 are known to have roles in upper respiratory disease, but relatively little is known about their contribution to LRI. Other viruses, such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human herpesvirus-6 (HHV-6), have been associated occasionally with LRI, either as primary pathogens or as possible cofactors with other agents. All three increase in overall importance in the setting of immunocompromise; this is especially true for CMV.9 Measles virus has a long history as a significant cause of LRI. Although eradication of this virus seems possible, it remains a significant problem in underdeveloped nations.
RESPIRATORY SYNCYTIAL VIRUS
RSV is the most common cause of lower respiratory infection in infants and young children, although infants younger than 2 years are most frequently and severely affected. RSV causes a range of illnesses, including croup, tracheobronchitis, bronchiolitis, pneumonia, or some combination thereof, and is associated with such complications as progressive pulmonary failure, cor pulmonale, and a risk of bacterial superinfection. Some evidence suggests there may be a strong association between severe RSV LRI in infancy and asthma or allergic airways disease in childhood. RSV is discussed thoroughly in Chapter 34.
HUMAN METAPNEUMOVIRUS
Human metapneumovirus (hMPV) is an infectious agent that apparently has existed for a long time but only recently (2001) has been recognized as a major cause of LRI. Initially described in patients with LRI in the Netherlands,10 hMPV has now been identified in pediatric and adult patients with LRI worldwide. hMPV is in the same subfamily of Paramyxoviruses as RSV and displays the same syncytial formation in cell culture. Although comparative prevalence studies are difficult to evaluate due to differences in sensitivity of detection methods, hMPV certainly appears to cause a significant portion of LRI worldwide. In one prospective 25-year study, hMPV prevalence was 12% in nasopharyngeal samples from patients with LRI, compared with 15% RSV, 10% parainfluenza virus, and 5% influenza virus.11 Infection usually occurs during childhood, causing hMPV seropositivity in up to 52% of 24-month-old infants and 100% of 5-year-old children.12, 13 Like RSV, hMPV has a seasonal pattern, causing its greatest impact in the winter months. Coinfection with hMPV and other respiratory viruses occurs and appears to increase the severity of illness.14
Clinically, hMPV infection is similar to RSV disease and includes mild upper respiratory tract disease, influenza-like symptoms (including fever, myalgia, and vomiting), croup, pneumonia, and, most commonly, bronchiolitis.11, 13 Patients typically present with symptoms commonly seen with RSV but, in general, have less severe signs of respiratory distress (Table 33-2 ). However, patients with immunocompromise or extremes of age are at higher risk for more severe LRI. Patients hospitalized for hMPV-associated LRI require similar length of hospital stays to RSV patients (6 to 7 days15). The relationship of hMPV to asthma and reactive airways disease is unclear as studies have demonstrated both positive and negative impacts.
Table 33-2.
Clinical and Radiologic Findings in Patients with Human Metapneumovirus (hMPV) Compared with Those with Respiratory Syncytial Virus (RSV)
| Percent of Patients with hMPV | Percent of Patients with RSV | |
|---|---|---|
| Clinical findings | ||
| Cough | 72-90 | 76 |
| Rhinitis | 80-88 | 72 |
| Fever | 52-61 | 48 |
| Retractions | 60 | 64 |
| Hypoxemia | 47 | 82 |
| Anorexia | 33-36 | 76 |
| Wheezing | 22-24 | 32 |
| Chest radiograph findings | ||
| Atelectasis | 40 | 19 |
| Hyperinflation | 33 | 44 |
| Infiltrate | 33 | 31 |
| Bronchial thickening | 0 | 13 |
From Williams JV, Harris PA, Tollefson SJ, et al: Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350:443-450, 2004; Mejias A, Chavez-Bueno S, Ramilo O: Human metapneumovirus: A not so new virus. Pediatr Infect Dis J 23:1-10, 2004; and van den Hoogen BG, van Doornum GJJ, Fockens JC, et al: Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis 188:1571-1577, 2003.
© 2008
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
Diagnosis of hMPV is difficult because the virus does not grow readily on routine cell cultures. Reverse transcription–polymerase chain reaction (RT-PCR) assays are the currently available identification methods of choice. Serologic (immunofluorescence and enzyme-linked immunosorbent assays [ELISAs]) testing is not readily available and is compromised by near universal infection in childhood: a ≥4-fold rise in antibody titers must be demonstrated to confirm recent infection.16 Management of hMPV infection remains supportive, although studies evaluating the effect of ribavirin and intravenous immunoglobulin preparations are in progress. Bronchodilators such as albuterol may benefit those patients with wheezing as part of their symptom complex, but corticosteroids have no demonstrated impact. Prevention of hMPV infection remains the most desirable form of intervention, but no studies on hMPV vaccine products for humans have been reported.16
PARAINFLUENZA VIRUSES
The parainfluenza viruses represent the next most common cause of LRI in children younger than 5 years.8 Parainfluenza virus type 3 is the most common cause of LRI among this group. Such infections can be seen at any time during the year but are most common in the spring and summer.17 Localized outbreaks of upper respiratory illness, bronchitis, and croup caused by parainfluenza virus types 1 or 2 frequently occur in the autumn and early winter. Parainfluenza virus type 4 is rarely detected and has been associated primarily with upper respiratory symptoms.
The clinical course of parainfluenza virus infection is similar to that described for RSV as depicted in Figure 33-1 . Initial symptoms are mild nasal stuffiness and coryza, with variable progression over 1 to 3 days as the infection progresses downward in the respiratory tract causing cough as a predominant manifestation. Parainfluenza 3 often causes bronchitis, pneumonia, and croup in children younger than 1 year. Patients with immunocompromise are at increased risk for serious morbidity and mortality from parainfluenza 3–associated pneumonia.18 Duration of acute illness can vary from 4 to 21 days but is usually 7 to 10 days.
Figure 33-1.
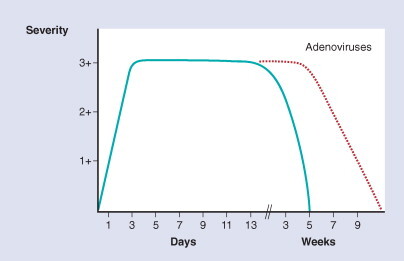
Natural history of lower respiratory tract illnesses caused by RSV and parainfluenza viruses, as measured by severity and duration in normal patients (blue line). The prodrome with upper respiratory symptoms usually progresses to lower tract involvement over 1 to 3 days. Maximal severity persists as a “plateau” phase for 7 to 21 days (average, 10 days), followed by progressive recovery in the succeeding few weeks. The hatched red line illustrates the similar but more protracted course often seen with severe adenovirus infections.
The basic principles of management for parainfluenza LRIs are the same as those described elsewhere for RSV. When eating and drinking become difficult for the patient, adequate fluids usually need to be provided via an intravenous route. Humidified oxygen is also given when hypoxemia is present, usually beginning at a concentration of 30% to 40%. In severe cases, the oxygen concentration is increased as guided by blood gas determinations. If there is clinical evidence of progressive respiratory failure, endotracheal intubation with mechanical ventilatory assistance is required.
Bronchodilators also may be tried but must be administered cautiously and discontinued if no clear-cut benefit can be demonstrated. Corticosteroids have no demonstrated role in treatment of lower respiratory infection. Their role in upper respiratory infection is discussed elsewhere. Parainfluenza types 1 and 3 are susceptible in vitro to ribavirin, but no controlled clinical trials yet support its use in these infections.
INFLUENZA VIRUSES
The epidemiology of influenza virus types A and B is generally well known: rapidly evolving outbreaks that usually occur during the cooler months of the year. Both types can cause serious, lethal LRI in infants and children.19 The clinical course contrasts with that of RSV and parainfluenza viruses in that fever, malaise, and often myalgia usually develop and rapidly become more severe over 12 to 24 hours (Fig. 33-2 ). Nasal congestion, cough, and subsequent respiratory distress often do not appear until a day or two after the onset of systemic symptoms. Although the mean overall duration of the acute illness (3 to 7 days) is somewhat shorter than with RSV or parainfluenza viruses, the convalescent phase often lasts several weeks, and rapid deterioration as a result of bacterial superinfection can occur at any time in the course. Rarely, patients may develop a rapidly progressive, overwhelming viral pneumonia resulting in death within 2 to 3 days after the initial onset (Fig. 33-3 ). Pandemic influenza has occurred three times in the past century, causing unusually severe, hemorrhagic pneumonia and millions of deaths. The pathogenicity of these pandemics appears related to a lack of neutralizing serum immunity against the pandemic strain, presumably due to antigenic drift of its hemagglutinin antigen.20, 21
Figure 33-2.

Natural history of influenza in normal patients, as measured by severity and duration. Initial symptoms of fever and malaise develop and reach maximal severity over the first 12 to 24 hours and are followed by additional respiratory symptoms. Maximal severity persists for 3 to 7 days (shorter than for RSV and parainfluenza), followed by convalescent period lasting several weeks.
Figure 33-3.

Photomicrograph demonstrating bronchiolar necrosis and hemorrhage in a case of overwhelming influenza (original magnification × 100).
Diagnosis and basic management are similar to those described for RSV and parainfluenza viruses. Oral amantadine hydrochloride is somewhat effective in treating influenza A but is ineffective against influenza B. If begun within 24 to 48 hours of onset and continued for 5 to 7 days, it may reduce the duration of fever and systemic symptoms and result in a more rapid improvement of peripheral airways function.22 It also has use as prophylaxis in high-risk children during influenza A outbreaks. A related drug, rimantadine, is also effective and appears to produce fewer adverse effects than amantadine.22, 23 The rapid development of viral resistance to both amantadine and rimantadine has been observed, particularly when either medication is used in households for the simultaneous treatment of symptomatic infection and contact prophylaxis.24
The neuraminidase inhibitors (zanamivir and oseltamivir) can also significantly reduce duration and symptom severity of influenza, if begun within 30 hours of onset. Importantly, both agents have efficacy against influenza A and B, are well tolerated, and appear to be associated with viral resistance infrequently.25
The combination vaccines for influenza virus types A and B are evaluated annually and reformulated as necessary to antigenically match the strains expected to circulate widely in a subsequent year. Their protective efficacy varies from year to year, ranging between 50% and 95% in immunologically normal individuals older than 6 months. Efficacy in the first 6 months of life is not known, and the influenza vaccine is not recommended for this group; otherwise, annual immunization commencing in the autumn can be used in persons of all ages, except those who have experienced anaphylactic reactions to chickens or eggs. Two doses administered 1 month apart are recommended for children receiving the vaccine for the first time. An intranasal, live attenuated influenza vaccine is also available for children and adults from 5 to 49 years old. This product is highly effective and well tolerated and provides an alternative to further injections. However, it is contraindicated for the high-risk, immunocompromised patient populations, for whom influenza vaccination is recommended.
Children who especially should be immunized annually against influenza include those with chronic pulmonary, cardiac, hematologic, immunologic, and metabolic conditions and those receiving long-term salicylate therapy. In addition, household contacts of these high-risk children and other caregivers (e.g., nurses, physicians, therapists) should be targeted. If an annual immunization is missed and a significant exposure is subsequently suspected (e.g., during a documented outbreak), immunization followed immediately by chemoprophylaxis with amantadine or rimantadine for 2 weeks after the vaccine schedule of one or two doses has been completed can provide “bridging” protection until there is a vaccine response.
Influenza virus type C has been reported to cause outbreaks of febrile respiratory illnesses, especially among pediatric clinic patients and children younger than 3 years. A survey in Los Angeles detected a 64% prevalence of antibodies to this virus among children 5 years and younger.26 However, longitudinal studies have not supported a significant role for influenza virus type C in LRI, at least among those from birth to 3 years.17
Avian influenza A (H5N1) recently has emerged as a highly pathogenic cause of poultry infection in Asian countries that now has gained the ability to occasionally cause human disease. Influenza A (H5N1) infection in humans is characterized by fever, severe respiratory symptoms, lymphopenia, and a high risk of death.27 In 2004, at least 44 persons were infected and 32 died; most had direct contact with infected poultry. However, a probable case of person-to-person transmission28 raises fears of an impending pandemic because few humans will have any immunity against this strain. Chemoprophylaxis with the neuraminidase inhibitors appears to have some benefit, but concerns about emerging viral resistance may limit their use. Thus, such preventive measures as vaccine discovery and prospective poultry screening and culling are critical. Unfortunately, only a few countries have developed influenza pandemic plans and even fewer have the economic and social resources to pursue vaccine development and antiviral stockpiling. Perhaps the greatest impact so far has been the financial reality of domestic poultry destruction in those countries hardest hit by this virus.29
ADENOVIRUSES
Adenoviruses are very common causes of fevers, upper respiratory illnesses, and conjunctivitis in young children. Fortunately, they produce LRI only occasionally and sporadically. Adenoviral pneumonia, most commonly caused by types 3, 5, and 7, initially progresses much like the pneumonia described for RSV and parainfluenza viruses (see Fig. 33-1), but the illnesses can be extremely severe and can last for several weeks. Adenovirus types 3 and 7 have been associated with epidemics of LRI.30 Risk factors for severe, potentially lethal disease include immunocompromise, congenital heart disease, and protein-calorie malnutrition. During the acute phase, chest radiographs often reveal extensive consolidation, particularly in perihilar areas (Fig. 33-4 ). These findings, along with a frequent occurrence of high fevers, leukocytosis, and multisystem involvement, can make it difficult to discern such infections from bacterial conditions. Other systemic manifestations that sometimes develop are hepatic dysfunction (including Reye's syndrome31), encephalopathy, coagulopathies, measles-like exanthems, and diarrhea.32
Figure 33-4.

Chest radiograph demonstrating extensive perihilar consolidation in a two-year-old patient with adenovirus 3 pneumonia.
The diagnosis is confirmed best by the detection of the virus in tissues such as lung aspirate or biopsy specimens. Because asymptomatic shedding from the throat orgastrointestinal tract is common in young children, isolation of the virus from the throat must be regarded as diagnostically supportive but not confirmatory, and isolation from stools or rectal swabs needs to be interpreted even more cautiously. Serologic studies of paired acute and convalescent sera obtained at least 2 weeks apart may further aid in confirming the diagnosis. Rapid detection has been used, including immunofluorescence and immunoenzyme assays. Such methods are generally quite specific, but sensitivity ranges between 30% and 60%.
Supportive management is all that can be offered at present. These patients must be followed closely because they are at high risk for bacterial superinfection for many weeks. They can also sustain permanent pulmonary sequelae, including pulmonary fibrosis, bronchiolitis obliterans, recurrent wheezing, and bronchiectasis.33, 34, 35 Young age and a previous measles-like illness, as well as nutritional or immunologic deficiencies, have been reported to be risk factors.34 The persistence of adenoviral antigens and the sustained expression of adenovirus genes in the airway epithelium have been proposed as causes.35, 36
HUMAN CORONAVIRUSES
Until recently, the role of human coronaviruses (HCoV) in LRIs was not well understood. The two strains most frequently associated with LRI were OC43 and 229E. HCoV OC43 infections produce cough and nasal symptoms in adults and sore throat, cough, coryza, and fever in children.37 HCoV infections have also been associated with acute attacks of wheezing in children with asthma.38 In a provocative study of pediatric HCoV LRI,39 ELISA detected HCoV antigens in 30% of 108 acute respiratory episodes experienced by 30 children younger than 6 years who had a history of at least 10 recurrent respiratory illnesses in the preceding year. In addition, 29% of 51 acute respiratory episodes experienced by the siblings of these patients were also associated with HCoV. Interestingly, 30% of the HCoV infections detected in the former group were associated with LRI symptoms, including wheezy bronchitis, whereas none of the siblings with HCoV had LRI findings. Most infections were due to HCoV 229E, and peaks occurred in the late autumn, early winter, and early summer. Family studies in Seattle have shown that increased levels of antibodies to HCoV strains OC43 and 229E occur more frequently during the winter40; children were apparently infected 3 times more often than adults, and serologic evidence suggesting reinfection was frequently observed over 3 years.
Severe Acute Respiratory Syndrome
The 2002 emergence of severe acute respiratory syndrome (SARS) precipitated a new era of international scientific and medical cooperation and led to the discovery of a third coronavirus with human pathogenicity (SARS-CoV).41, 42 The first cases of SARS occurred in China in November 2002 and were followed over the next year by 8098 cases in 26 countries, with 774 deaths.43 SARS-CoV–like viruses have been detected in raccoon-dogs and Himalayan palm civets in China and also were isolated from such human samples as nasal secretions, serum, feces, and bronchial washings.42, 43 There is good evidence that bats represent the primary reservoirs of these viruses.44 Because SARS-CoV has not been demonstrated previously in humans, it appears possible that the virus has crossed the species barrier from animals to humans.45
The SARS incubation period ranges from 2 to 10 days, followed by an influenza-like prodrome with such symptoms as fever, myalgias, headache, and watery diarrhea. Respiratory symptoms begin 2 to 7 days after the prodrome and initially include a dry, nonproductive cough and mild dyspnea, accompanied by lung high-resolution CT findings of ground-glass consolidations. Further progression occurs 8 to 12 days later and ranges from a “mild cough variant” with persistent intractable cough to the more common “moderate to severe variant” with hypoxia and dyspnea. Ten percent to 20% of hospitalized patients will require intubation and mechanical ventilation, often heralded by subtle, progressive decreases in oxygen saturation. Recovery begins approximately 14 to 18 days after onset of symptoms.43, 46, 47, 48
Diagnosis of SARS-CoV is available by use of culture and RT-PCR, although the sensitivity of culture is lower than RT-PCR. Specimens obtained 10 days from symptom onset are associated with the highest yield, correlating with the timing of peak virus load. Serologic assays for SARS-CoV include immunofluorescent assays, ELISA, and Western blot assays; however, none is sensitive early enough in the disease course to be useful for rapid diagnosis and thus should be used for paired acute and convalescent-phase serologic diagnosis.43
Treatment of SARS remains largely supportive and aimed at those patients who develop respiratory failure. Barotrauma appears to be a frequent complication of SARS-CoV infection, leading to pneumothorax and pneumomediastinum in 20% to 34% of ventilated patients.49 Thus, a “lung protective” ventilation strategy is recommended for those patients on mechanical ventilation. Other therapeutic interventions have included use of ribavirin, interferon a, lopinavir-ritonavir, and such immunomodulatory therapies as corticosteroids, intravenous immunoglobulin, and plasma exchange.43 However, no controlled trials have demonstrated benefit in outcome or mortality related to these. Despite the high mortality and devastating impact of SARS-CoV in 2002, the story of its emergence serves to describe a victory due to the worldwide public health measures used successfully to bring the outbreak under control.
A fourth HCoV, NL63, has also been identified and found to cause a significant proportion of acute respiratory disease in humans. Initially described in the Netherlands,50 HCoV NL63 now has been demonstrated globally, including Australia, Canada, Japan, Belgium, China, Switzerland, and the United States.51, 52, 53, 54 In the United States, the virus currently is named HCoV-New Haven (NH) and most likely represents the same or closely related species as the NL63 described elsewhere. Of 895 children less than 5 years old with acute respiratory illness, 8.8% tested positive for HCoV-NH and negative for other common viral respiratory pathogens.51 In addition to upper respiratory symptoms, children with HCoV NL63 infection can develop croup, asthma exacerbation, febrile seizures, and such lower respiratory tract findings as tachypnea, abnormal breath sounds, hypoxia, and abnormal chest radiographs.51, 52 HCoV NL63 may demonstrate a variable seasonality, ranging from fall and winter in temperate zones to spring and summer in tropical zones.
Most recently, a fifth HCoV, HKU1, has been described. Initially demonstrated in a 71-year-old man who returned to Hong Kong from an SARS-endemic area of China,55 a subsequent prospective study of nasopharyngeal aspirates from 418 patients with community-acquired pneumonia demonstrated 10 (2.4%) positive for HCoV-HKU1.56 All 10 cases occurred in spring and winter; 9 were adults, and 4 had underlying respiratory tract disease. All had symptoms that were indistinguishable from other study participants with community-acquired pneumonia. The global impact of this novel virus remains to be determined.
RHINOVIRUSES
The rhinovirus group is composed of at least 115 unique serotypes that are widely known as the agents responsible for many of the upper respiratory (“common cold”) symptoms in adults and older children. Rhinoviruses have been isolated from infants and children hospitalized with LRIs but not at rates that differ significantly from children without respiratory illnesses.57 The usual incubation period is 2 to 3 days, and the acute symptoms usually last 3 to 7 days. In one retrospective study, the clinical features of 44 rhinovirus culture-positive children with respiratory symptoms, who were either hospitalized or seen in an emergency department, included bronchiolitis or pneumonia; sometimes, both conditions were found (32 patients). The majority of patients were younger than 12 months. Although infrequent, LRIs in children infected with rhinoviruses were indistinguishable from those caused by RSV.58 Rhinoviruses appears to have a role in triggering episodes of acute asthma.59, 60
The routine diagnosis of rhinovirus infections depends on tissue culture, although a PCR assay exists that is several times more sensitive than culture. Technical difficulties of identifying rhinoviruses in diagnostic specimens may contribute to an underestimation of their role as causes of LRI. The treatment of rhinovirus infections consists of supportive care; no antiviral medications are recommended at this time. Great attention has been given to possible preventive and therapeutic benefit of such alternatives as zinc and Echinacea, but no controlled studies to date have demonstrated benefit of either.
HERPESVIRUSES
Although uncommon as pulmonary pathogens in healthy children, all members of the herpesvirus family (herpes simplex virus [HSV] types I and II, varicella-zoster virus [VZV], CMV, EBV, HHV-6, HHV-7, and HHV-8) can cause LRI in immunocompromised patients, generally hematogeneously spread as part of a systemic infection. CMV is primarily of concern in the recipients of allogeneic bone marrow transplants, but normal children with lower respiratory tract symptoms can also have positive cultures for CMV. Serologic studies do not generally support a primary role for CMV in LRI among healthy children17; however, pneumonia associated with acute, primary, systemic CMV infection in otherwise normal infants has been reported.61
CMV pneumonitis in recipients of bone marrow transplantation most frequently results from reactivation of latent virus and less often by transmission via blood products to seronegative patients. Graft-versus-host disease is a significant risk factor for the development of CMV pneumonitis; thus autologous and syngeneic recipients of bone marrow transplant are affected much less often. CMV pneumonitis commonly presents as a primary pulmonary process characterized by fever, tachypnea, and progressive pulmonary distress. Diffuse, bilateral pulmonary infiltrates are generally seen on chest radiographs. The diagnosis can be made by culture of the virus from bronchoalveolar lavage fluid or from lung tissue, although detection of increased viral load by nucleic acid detection methods in the setting of LRI is strongly suggestive. The advent of prophylaxis with intravenous ganciclovir or oral valacyclovir in high-risk patients has greatly reduced the incidence of CMV during the first 3 months after bone marrow transplantation.62 Ganciclovir is less effective in treating CMV pneumonitis in patients after disease is clinically apparent.
Herpes simplex pneumonitis is most commonly seen as part of perinatally acquired disseminated infections but can also be transmitted during resuscitative efforts. Immunocompromise of any type is also a risk factor for HSV pneumonitis at all ages.
Varicella pneumonitis is a life-threatening complication of primary VZV infection in neonates, immunocompromised patients, and, rarely, healthy children. VZV is spread by aerosolized respiratory secretions, and the initial round of viral replication occurs in the lungs. A primary wave of viremia then occurs and is followed by further viral replication in lymphatic tissue. Characteristic cutaneous lesions erupt after a secondary viremia. Varicella pneumonitis generally develops 2 to 5 days after the outbreak of a rash.63 The clinical and radiographic manifestations vary. Asymptomatic miliary lung lesions (especially in adult varicella) may later become apparent as calcified foci. In others, a mild interstitial pneumonia is present, which may be overshadowed by a severe bacterial pneumonia caused by such organisms as group A streptococci or S. aureus. These are often accompanied by effusions and empyemas and can be extremely difficult to treat. Varicella alone can cause a progressive, lethal pneumonia in immunocompromised patients.
The transmission of VZV to neonates occurs when primary maternal chickenpox occurs within 3 weeks of parturition. Transplacental transfer of maternal antibody specific for VZV is minimal if the onset of illness occurs fewer than 5 days before birth. Thus, if maternal varicella develops within 5 days before to 2 days after delivery, systemic disease and pneumonitis can develop in the neonate, with an estimated case-fatality rate of 5%.64
The diagnosis of varicella in normal hosts is generally made by clinical observation. Culture or immunofluorescence staining of vesicular lesions is important in immunocompromised hosts to rule out disseminated herpes simplex infections. Serologic antibody tests are useful for assessing immune status. g-Globulin preparations containing high titers of anti-VZV activity are effective in preventing varicella if given soon after exposure. Neonates and immunocompromised children are candidates for this treatment.64 Acyclovir and other related agents are the drugs of choice in treating high-risk patients and reduce the severity of illness if administered within 2 days of the outbreak of a rash. Fortunately, a live VZV vaccine has resulted in a significant reduction of primary varicella and its complications.65
Like the other herpesviruses, EBV produces life-long infections and causes a variety of clinical syndromes, including infectious mononucleosis, central nervous system illnesses, malignant lymphoproliferative diseases, and nasopharyngeal carcinoma. Pneumonitis, pneumonia and pleural effusion can occur as part of primary EBV infection but are uncommon, although perhaps underdiagnosed. In a group of 113 normal children with documented EBV-induced mononucleosis, 6 children, all younger than 4 years, developed pneumonia during the illness.66 Pneumonia was self-limited in all cases. The diagnosis of primary EBV infection is made on clinical grounds in conjunction with serologic tests for heterophil antibody or IgM anti-VCA antibody.
HHV-6, the usual causative agent of roseola infantum, has been suggested as a possible cause of some cases of interstitial pneumonitis in recipients of bone marrow transplants.67 This virus is one of the most ubiquitous of human viruses: More than 90% of the population becomes infected by 2 years of age. Detection of HHV-6 by culture is not routinely available, but the diagnosis of primary infection can be made by evidence of serologic conversion. Reactivation is difficult to document because a majority of healthy individuals have evidence of continuous viral replication in the salivary glands and blood. HHV-7, more recently described, appears to have similar epidemiology and pathogenicity as HHV-6, whereas HHV-8 has a well-known association with Kaposi sarcoma in immunocompromised and, rarely, immunocompetent patients. Both HHV-7 and HHV-8 appear to cause interstitial pneumonitis in bone marrow transplant patients.68, 69 HHV-6, HHV-7, and HHV-8 are not thought to cause pneumonia in normal persons, and no specific therapy is available.
MEASLES VIRUS
Otitis media and pharyngitis are normal components of the early phase of measles infection. Bronchitis is common, and severe laryngitis may occur occasionally. Pneumonia caused by the measles virus probably occurs in at least 50% of children. In most cases, it is a mild bronchopneumonia and is recognized only by nonspecific lower respiratory signs and hyperinflation on chest radiograph. In other cases the inflammation is more extensive, leading to diffuse infiltrates or even segmental or lobar consolidation on chest radiographs.
Children with measles are prone to secondary bacterial infection with organisms such as pneumococci, H. influenzae, S. aureus, and Streptococcus pyogenes. This is particularly important in developing countries, where infection secondary to measles is a major cause of death in young children. Measles may also have a deleterious effect on the course of tuberculosis in malnourished infants.
The immunodeficient child is prone to develop a progressive and fatal infection that evolves about 3 weeks after exposure. The clinical manifestations start with a fever, and there may be an atypical rash. Nonspecific respiratory symptoms and signs evolve over 2 to 3 days. The chest radiograph generally shows coarse nodular infiltrates, and air leaks are common.
The diagnosis is usually made on culture from nasopharyngeal secretions or bronchoalveolar lavage fluid. Occasionally, a lung biopsy may be necessary. The histopathologic picture is most often that of giant cell pneumonia with inflammatory cell exudate and thickening of the alveolar walls with inflammatory cells. The alveolar lining cells are transformed and contain intranuclear and intracytoplasmic inclusions.70 Rapid diagnosis by immunofluorescence and serologic diagnosis are also possible.
Treatment of measles involves supportive measures and close observation for signs of bacterial superinfection. Prevention is available via a live, attenuated vaccine that is highly immunogenic but contraindicated in pregnant and immunocompromised individuals. Measles pneumonia can be prevented in the immunodeficient host by active community immunization. Those exposed should have immunoglobulin as soon as possible after exposure.
Modified measles does occur in the partially immune host and presents with fever and an itchy maculopapular rash, particularly over the wrists and ankles. Respiratory involvement is relatively common in this type of measles, presenting with dyspnea and widespread crackles heard on auscultation. The chest radiograph shows hilar adenopathy with nodular infiltrates and frequently a pleural effusion.71
HANTAVIRUS
In 1993, an outbreak of acute febrile illness progressing within 3 to 5 days to respiratory failure and shock was noted in the southwestern United States. The hallmark of this syndrome, the hantavirus pulmonary syndrome, is unexplained severe noncardiogenic pulmonary edema occurring in previously healthy persons. Subsequent studies have implicated at least three different hantaviruses, which are primarily maintained as zoonotic agents in rodent reservoirs.72 As of November 2004, more than 379 cases of hantavirus pulmonary syndrome had been reported from 31 states in the United States, with a case-fatality rate of greater than 50%.73, 74, 75 Hantavirus pulmonary syndrome has also been documented in Canada, Brazil, Argentina, and Paraguay. Few cases have been reported in people 16 years or younger, but the disease spectrum and mortality are similar to those reported in adults.75, 76 The diagnosis can be made by serologic tests, PCR study of frozen tissues, immunochemistry, or paraffin-embedded tissues. No specific therapy has proved efficacious; however, intravenous ribavirin has been suggested based on in vitro data and experience with other hantaviruses.
SIMULTANEOUS INVOLVEMENT BY MULTIPLE PATHOGENS
Other viruses, C. trachomatis, M. pneumoniae, and B. pertussis, can also be detected in a significant number of young children with infections caused by RSV. In the Tucson Childrens' Respiratory Study,77 10.9% of previously healthy patients with RSV infection were coinfected with another potential pathogen as documented by culture or antigen detection, and the proportion became even greater when serologic results were also extensively used for diagnosis. The clinical diagnosis and outcomes were no different among patients with RSV alone compared with those already coinfected with additional agents. Routine searches for coinfecting agents when a primary pathogen such as RSV is identified are not recommended for otherwise healthy infants and children. Such extensions of a diagnostic workup are best reserved for patients who are known to have significant underlying illnesses or patients whose clinical course is not congruent with that expected for the pathogen detected.
PROSPECTS FOR PREVENTION AND TREATMENT
Current knowledge concerning the major causes of viral LRIs and their epidemiology and diagnosis has matured remarkably. Exciting advances are now being made in the critical areas of immunopathogenesis, molecular virology, and antiviral therapy. Future discoveries will surely provide even more rational approaches to prevention and treatment, including specifically designed peptide vaccines that can more appropriately recruit specific T-cell populations as allies in long-lasting protection. Other emerging strategies include enhancement of host defenses by cytokine manipulation and novel applications of older concepts, such as specific antibodies for prophylaxis and treatment. There is considerable cause for optimism, in contrast to the state of affairs described by Andrewes more than 4 decades ago, when he suggested that clinicians should perhaps accept these infections as “one of the stimulating risks of being mortal.78”
SUGGESTED READINGS
- Berman S, McIntosh D. Selective primary health care: Strategies for control of disease in the developing world. XXI. Acute respiratory infections. Rev Infect Dis. 1985;7:674–691. doi: 10.1093/clinids/7.5.674. [DOI] [PubMed] [Google Scholar]
- Ray CG. Influenza, respiratory syncytial virus, adenovirus, and other respiratory viruses. In: Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. An Introduction to Infectious Diseases. McGraw-Hill; New York: 2004. pp. 495–512. [Google Scholar]
- Ray CG, Holberg CJ, Minnich LL. Acute lower respiratory illnesses during the first three years of life: Potential roles for various etiologic agents. Pediatr Infect Dis J. 1993;12:10–14. doi: 10.1097/00006454-199301000-00004. [DOI] [PubMed] [Google Scholar]
Metapneumovirus
- Williams JV, Harris PA, Tollefson SJ. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Influenza
- Kobasa D, Takada A, Shinya K. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- Perez DR, Sorrell EM, Donis RO. Avian influenza: An omnipresent pandemic threat. Pediatr Infect Dis J. 2005;24:S208–S216. doi: 10.1097/01.inf.0000188160.83709.b7. [DOI] [PubMed] [Google Scholar]
Human Coronavirus
- Christian MD, Poutanen SM, Loutfy MR. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F, Weibel C, Ferguson D. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hantavirus
- Peters CJ, Khan AS. Hantavirus pulmonary syndrome: The new American hemorrhagic fever. Clin Infect Dis. 2002;34:1224–1231. doi: 10.1086/339864. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Henrickson KJ. Advances in the laboratory diagnosis of viral respiratory disease. Pediatr Infect Dis J. 2004;23:S6–S10. doi: 10.1097/01.inf.0000108187.63151.ea. [DOI] [PubMed] [Google Scholar]
- 2.Wright AL, Taussig LM, Ray CG. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 3.Gendrel D, Raymond J, Coste J. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Galetto-Lacour A, Zamora SA, Gervaix A. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referral center. Pediatrics. 2003;112:1054–1060. doi: 10.1542/peds.112.5.1054. [DOI] [PubMed] [Google Scholar]
- 5.Denny FW, Clyde WA. Acute lower respiratory tract infection in nonhospitalized children. J Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 6.Grayston JT. Infections caused by C. pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–763. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 7.Ray CG. Influenza, respiratory syncytial virus, adenovirus, and other respiratory viruses. In: Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. An Introduction to Infectious Diseases. McGraw-Hill; New York: 2004. pp. 495–512. [Google Scholar]
- 8.Berman S, McIntosh D. Selective primary health care: strategies for control of disease in the developing world. XXI. Acute respiratory infections. Rev Infect Dis. 1985;7:674–691. doi: 10.1093/clinids/7.5.674. [DOI] [PubMed] [Google Scholar]
- 9.Pollard RB. Cytomegalovirus infections in renal, heart-lung and liver transplantation. Pediatr Infect Dis J. 1988;7:S97–S102. [PubMed] [Google Scholar]
- 10.van den Hoogen BG, de Jong JC, Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JV, Harris PA, Tollefson SJ. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf DG, Zakay-Rones Z, Fadeela A. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188:1865–1867. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- 13.Mejias A, Chavez-Bueno S, Ramilo O. Human metapneumovirus: A not so new virus. Pediatr Infect Dis J. 2004;23:1–10. doi: 10.1097/01.inf.0000105103.60288.0e. [DOI] [PubMed] [Google Scholar]
- 14.Semple MG, Cowell A, Dove W. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hoogen BG, van Doornum GJJ, Fockens JC. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 16.Hamelin M-E, Boivin G. Human metapneumovirus: A ubiquitous and long-standing respiratory pathogen. Pediatr Infect Dis J. 2005;24:S203–S207. doi: 10.1097/01.inf.0000188158.27840.7c. [DOI] [PubMed] [Google Scholar]
- 17.Ray CG, Holberg CJ, Minnich LL. Acute lower respiratory illnesses during the first three years of life: potential roles for various etiologic agents. Pediatr Infect Dis J. 1993;12:10–14. doi: 10.1097/00006454-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Elizaga J, Olavarria E, Apperley JF. Parainfluenza virus 3 Infection after stem cell transplant: Relevance to outcome of rapid diagnosis and ribavirin treatment. Clin Infect Dis. 2001;32:413–418. doi: 10.1086/318498. [DOI] [PubMed] [Google Scholar]
- 19.Troendle JF, Demmler GJ, Glezen WP. Fatal influenza B virus pneumonia in pediatric patients. Pediatr Infect Dis J. 1992;11:117–121. doi: 10.1097/00006454-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kobasa D, Takada A, Shinya K. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 21.Hoft DF, Belshe RB. The genetic archaeology of influenza. N Engl J Med. 2004;351:2550–2551. doi: 10.1056/NEJMcibr043708. [DOI] [PubMed] [Google Scholar]
- 22.Tominack RL, Hayden FG. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A infection. Infect Dis Clin North Am. 1987;1:459–478. [PubMed] [Google Scholar]
- 23.Hall CB, Dolin R, Gala CL. Children with influenza A infection: Treatment with rimantadine. Pediatrics. 1987;80:275–282. [PubMed] [Google Scholar]
- 24.Hayden FG, Belshe RB, Clover RD. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med. 1989;321:1696–1702. doi: 10.1056/NEJM198912213212502. [DOI] [PubMed] [Google Scholar]
- 25.Monto AS, Fleming DM, Henry D. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 26.Dykes AC, Cherry JD, Nolan CE. A clinical, epidemiologic serologic, and virologic study of influenza C virus infection. Arch Intern Med. 1980;140:1295–1298. [PubMed] [Google Scholar]
- 27.Hien TT, Liem NT, Dung NT. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 28.Ungchusak K, Auewarakul P, Dowell SF. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 29.Perez DR, Sorrell EM, Donis RO. Avian influenza: An omnipresent pandemic threat. Pediatr Infect Dis J. 2005;24:S208–S216. doi: 10.1097/01.inf.0000188160.83709.b7. [DOI] [PubMed] [Google Scholar]
- 30.Hong JY, Lee HJ, Piedra PA. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: Epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32:1423–1429. doi: 10.1086/320146. [DOI] [PubMed] [Google Scholar]
- 31.Edwards KM, Bennett SR, Garner WL. Reye's syndrome associated with adenovirus infections in infants. Am J Dis Child. 1985;139:343–346. doi: 10.1001/archpedi.1985.02140060025019. [DOI] [PubMed] [Google Scholar]
- 32.Murtagh P, Cerqueiro C, Halac A. Adenovirus type 7 respiratory infections: A report of 29 cases of acute lower respiratory disease. Acta Paediatr. 1993;82:557–561. doi: 10.1111/j.1651-2227.1993.tb12753.x. [DOI] [PubMed] [Google Scholar]
- 33.Simil S, Linna O, Lanning P. Chronic lung damage caused by adenovirus type 7: A ten-year follow-up study. Chest. 1981;80:127–131. doi: 10.1378/chest.80.2.127. [DOI] [PubMed] [Google Scholar]
- 34.Sly PD, Soto-Quiros ME, Landau LI. Factors predisposing to abnormal pulmonary function after adenovirus type 7 pneumonia. Arch Dis Child. 1984;59:935–939. doi: 10.1136/adc.59.10.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macek V, Sorli J, Kopriva S. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med. 1994;150:7–10. doi: 10.1164/ajrccm.150.1.8025775. [DOI] [PubMed] [Google Scholar]
- 36.Matsuse T, Hayachi S, Kuwano K. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1991;146:177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 37.Hendley JO, Fishburne HB, Gwaltney JM. Coronavirus infections in working adults: Eight-year study with 229E and OC43. Am Rev Respir Dis. 1972;105:805–811. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 38.Thumerelle C, Deschildre A, Bouquillon C. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: A prospective study in the Nord-Pas de Calais region (France) Pediatr Pulmonol. 2003;35:75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaacs D, Flowers D, Clarke JR. Epidemiology of coronavirus respiratory infections. Arch Dis Child. 1983;58:500–503. doi: 10.1136/adc.58.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt OW, Allan ID, Cooney MK. Rises in titers of antibody to human coronaviruses OC43 and 229E in Seattle families during 1975-1979. Am J Epidemiol. 1986;123:862–868. doi: 10.1093/oxfordjournals.aje.a114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ksiazek TG, Erdman E, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 42.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 43.Christian MD, Poutanen SM, Loutfy MR. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 45.Peiris JSM, Yuen KY, Osterhaus ADME. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 46.Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 47.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 48.Poutanen SM, Low DE, Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 49.Fowler RA, Lapinsky SE, Hallett D. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 50.van der Hoek L, Pyrc K, Jebbink MF. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esper F, Weibel C, Ferguson D. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiu SS, Chan KH, Chu KW. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bastien N, Anderson K, Hart L. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser L, Regamey N, Roiha H. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24:1015–1017. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- 55.Woo PC, Lau SK, Chu CM. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–889. doi: 10.1128/JVI.79.2.884-895.2005. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo PC, Lau SK, Tsoi H-w. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portnoy B, Eckert HL, Salvatore MA. Rhinovirus infection in children with acute lower respiratory disease: Evidence against etiological importance. Pediatrics. 1965;35:899–905. [PubMed] [Google Scholar]
- 58.McMillan JA, Weiner LB, Higgins AM. Rhinovirus infection associated with serious illness among pediatric patients. Pediatr Infect Dis J. 1993;12:321–325. doi: 10.1097/00006454-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mertsola J, Ziegler T, Ruuskanen O. Recurrent wheezy bronchitis and viral respiratory infections. Arch Dis Child. 1991;66:124–129. doi: 10.1136/adc.66.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stagno S, Brasfield DM, Brown MB. Infant pneumonitis associated with cytomegalovirus, Chlamydia, Pneumocystis, and Ureaplasma: A prospective study. Pediatrics. 1981;68:322–329. [PubMed] [Google Scholar]
- 62.Winston DJ, Yeager AM, Chandrasekar PH. Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis. 2003;36:749–758. doi: 10.1086/367836. [DOI] [PubMed] [Google Scholar]
- 63.Feldman S, Lott L. Varicella in children with cancer: Impact of antiviral therapy and prophylaxis. Pediatrics. 1987;80:465–472. [PubMed] [Google Scholar]
- 64.Miller E, Cradock-Watson JE, Ridehalgh MKS. Outcome in newborn babies given antivaricella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster Virus. Lancet. 1986;2:371–374. doi: 10.1016/s0140-6736(89)90547-3. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005;352:450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- 66.Andiman WA, McCarthy P, Markowitz RI. Clinical, virologic, and serologic evidence of Epstein-Barr virus infection in association with childhood pneumonia. J Pediatr. 1981;99:880–886. doi: 10.1016/S0022-3476(81)80010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cone RW, Hackman RC, Huang MLW. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med. 1993;329:156–161. doi: 10.1056/NEJM199307153290302. [DOI] [PubMed] [Google Scholar]
- 68.David H. Dockrell CVP: Human herpesvirus-6 and -7 in transplantation. Rev Med Virol. 2001;11:23–36. doi: 10.1002/rmv.299. [DOI] [PubMed] [Google Scholar]
- 69.Taplitz RA, Jordan MC. Pneumonia caused by herpesviruses in recipients of hematopoietic cell transplants. Semin Respir Infect. 2002;17:121–129. doi: 10.1053/srin.2002.33447. [DOI] [PubMed] [Google Scholar]
- 70.Lewis MJ, Cameron AH, Shah KJ. Giant cell pneumonia caused by measles and methotrexate in childhood leukaemia in remission. BMJ. 1978;1:330–331. doi: 10.1136/bmj.1.6109.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laptook A, Wind E, Nussbaum M. Pulmonary lesions in atypical measles. Pediatrics. 1978;62:42–46. [PubMed] [Google Scholar]
- 72.Butler JC, Peters CJ. Hantaviruses and hantavirus pulmonary syndrome. Clin Infect Dis. 1994;19:387–395. doi: 10.1093/clinids/19.3.387. [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention Two cases of hantavirus pulmonary syndrome—Randolph County, West Virginia, July 2004. MMWR CDC Surveill Summ. 2004;53:1086–1089. [PubMed] [Google Scholar]
- 74.Peters CJ, Khan AS. Hantavirus pulmonary syndrome: The new American hemorrhagic fever. Clin Infect Dis. 2002;34:1224–1231. doi: 10.1086/339864. [DOI] [PubMed] [Google Scholar]
- 75.Ramos MM, Overturf GD, Crowley MR. Infection with sin nombre hantavirus: Clinical presentation and outcome in children and adolescents. Pediatrics. 2001;108:e2. doi: 10.1542/peds.108.2.e27. [DOI] [PubMed] [Google Scholar]
- 76.Overturf GD. Clinical sin nombre hantaviral infections in children. Pediatr Infect Dis J. 2005;24:373–374. doi: 10.1097/01.inf.0000159892.32202.60. [DOI] [PubMed] [Google Scholar]
- 77.Ray CG, Minnich LL, Holberg CJ. Respiratory syncytial virus associated lower respiratory illnesses: Possible influence of other agents. Pediatr Infect Dis J. 1993;12:15–19. [PubMed] [Google Scholar]
- 78.Andrewes CH. The complex epidemiology of respiratory virus infections. Science. 1964;146:1274–1277. doi: 10.1126/science.146.3649.1274. [DOI] [PubMed] [Google Scholar]


