Abstract
Abiotic factors, climatic factors (such as temperature and rainfall) and biotic factors (such as population density and the structure of host communities and reservoirs) are essential variables in the transmission of infectious or parasitic agents.
Keywords: Biodiversity crisis, Community modification, Dilution effect, Emerging diseases, Habitat modification, Maastricht Globalization Index, Viruses and bacteria, Zoonotic infectious diseases
3.1. Introduction
Abiotic factors, climatic factors (such as temperature and rainfall) and biotic factors (such as population density and the structure of host communities and reservoirs) are essential variables in the transmission of infectious or parasitic agents [AND 91, MOR 08].
Current global changes (climate change, land use change, biological invasion) are shaking up the epidemiological environment [DAI 96]. These changes are responsible for new occurrences and distributions of infectious disease epidemics, as well as the emergence of new infections through the modification of biotic and abiotic factors [WIL 05a, JON 08, DE 08] (see Box 3.1 ).
Box 3.1. The concept of emerging diseases.
In the 1960s, the World Health Organization launched a major program for the global eradication of smallpox, which was successfully completed in 1980. The late 1960s marked the culmination of the triumph of Pasteurian medicine. The use of vaccines, antibiotics and insecticides suggested that all these infections, which had plagued human societies throughout history, could be eradicated. However, subsequent decades were marked by the appearance of new infectious diseases like Legionnaires' disease, Ebola hemorrhagic fever and AIDS. The 1989 Washington Conference on emerging viruses popularized a new term: “emerging diseases”. Doctors, microbiologists, virologists and epidemiologists, including many from military laboratories, were invited along by Stephen Morse, a virologist who co-organized the conference [MOR 90a]. Historians such as William McNeill, ecologists such as Thomas Lovejoy (creator of the term “biological diversity”) and even theorist Robert May were also present. Morse [MOR 95] defined an emerging infectious disease as an infection that has appeared recently within a population or that existed previously but whose incidence or geographic area is rapidly increasing. The notion of “emerging disease” had already been introduced by René Dubos in his book “Mirage of Health” in 1959. According to Dubos, emergence occurs due to the accumulation of mutations in the infectious microbe, which leads to new coevolutive ecologies that potentially bring about new health challenges. At a time when public health was trying to eradicate infections and prepare for a new epidemiological transition with the replacement of infectious diseases by modern non-communicable diseases (cancer, diabetes, infarction), Dubos rejected the possibility of eradication with the Darwinian theory of permanent co-evolution between resistance of the infectious agents and the struggle against them. As a precursor, Dubos inserted this co-evolution into the ecological context of a changing planet.
Alt-text: Box 3.1.
The 1989 Washington Conference and the 1991 United States Academy of Sciences “Committee on Emerging Microbial Threats to Health” [LED 92] recognized this coevolving nature as inscribed in the global ecology of emerging diseases and highlighted the difficulty of spatiotemporal prediction of new infectious diseases. Their conclusion was that we must prepare for the unpredictable. The United States' Center for Disease Control and Prevention (CDC) was set up and published the first issue of a new journal “Emerging Infectious Diseases” in 1995, with an article written by Stephen Morse.
In the early 2000s, the Central Intelligence Agency (CIA) published a report on the risks of infectious diseases and bioterrorism to the national security of the United States. This was followed by a Pentagon report that highlighted the need to take climate change and the resurgence of infectious diseases seriously as threats to national security [COO 06a]. The United States believes that preparing for emerging infectious diseases, just as they would for bioterrorism, in situations of maximum uncertainty is “preparedness”, in addition to worst-case scenarios developed by successive US administrations [ZYL 13].
Explanations for these new epidemiologies were associated with climate change and its impact on climate variability, increasing global trade, urbanization, overexploitation of biological resources, increasing population pressures and loss of biodiversity [CHI 04, WIL 05a]. The biodiversity crisis is a direct result of altering natural landscapes due to increased urbanization and intensification of agriculture [GIB 10]. These habitat changes appear to be associated with the emergence of new pathogens due to increased contact between wildlife, domestic animals and humans [LLO 09, LIN 15a, HAS 17].
3.2. Epidemiology of infectious diseases
A significant decline in parasitic and infectious diseases has been observed throughout the last century, particularly in developed countries [ARM 99]. This decline started significantly before the extended use of antibiotics or vaccinations came into play. In the United States, mortality from infectious diseases decreased from nearly 800 deaths in 1900 to fewer than 40 deaths per 100,000 people in 1980 (Figure 3.1 ). It is worth noting the exception of the 1918 influenza pandemic. The mortality rate then rose slightly from 1981 to 1995 to nearly 60 deaths per 100,000 people in the late 1990s. This increase in infectious disease mortality was mainly due to the emergence of AIDS [ARM 99] (Figure 3.1).
Figure 3.1.
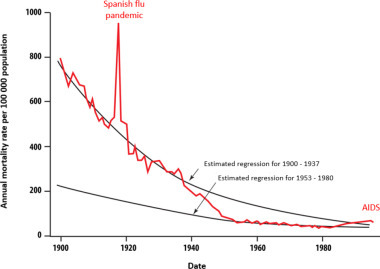
Decline of infectious diseases in the United States during the last century [ARM 99]. Between 1938 and 1952, the mortality rate due to infectious diseases decreased by 8.2% per year, and from 1953 to 1980 the decline was reduced to 2.3% per year. This was followed by an increase of 4.8% from 1980 to the end of the 1990s. For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
(taken from [ARM 99])
Similar trends in the decline of infectious diseases have also been seen in developing countries. The economic development associated with the integration of these countries into the world economy has improved the overall level of health of populations. Thus, Martens et al. [MAR 10] showed a positive correlation between the degree of integration of a country in the world economy as estimated by the Maastricht Globalization Index and the level of health of the population as estimated by the infant mortality rate. The Maastricht Globalization Index is relatively well correlated with the WHO's Health Development Index and these two indices are negatively associated with the diversity of infectious and parasitic diseases by country (Figure 3.2 ). The diversity of endemic infectious diseases is reduced as a result of economic growth and integration into the global economy and the associated increase in public health spending.
Figure 3.2.
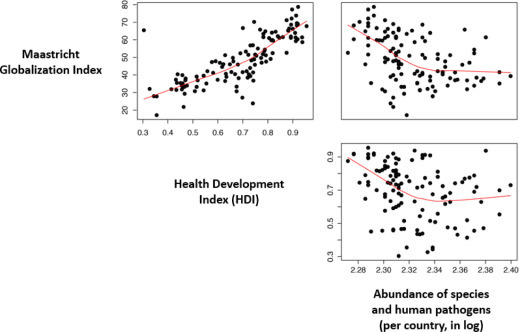
Relationships between the Maastricht Globalization Index, the Health Development Index and the abundance of infectious and parasitic diseases by country. Data on the Maastricht Globalization Index are from Figge & Martens [FIG 14] and data on the Health Development Index and the number of infectious and parasitic diseases are from Morand et al. [MOR 14b]. For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
Recent decades have also seen an increase in the number of newly emerging infectious diseases [JON 08, SMI 14] (Figure 3.3 ). These emerging infectious diseases are predominantly zoonotic viruses, bacteria and prions (Figure 3.3(a)), in other words, those for which the reservoirs are wild or domestic animals.
Figure 3.3.
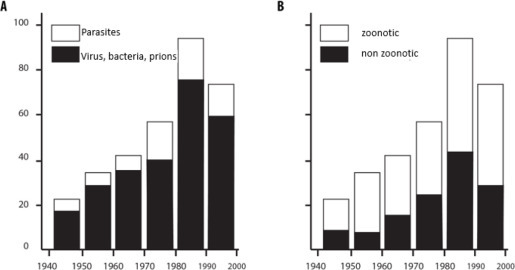
Characteristics of infectious diseases that have emerged in recent decades according to (A) the type of infectious or parasitic agent and (B) the zoonotic or non-zoonotic character
(taken from [JON 08])
The increase in emergence of infectious diseases affects domestic and wild animals and plants (Box 3.2 ).
Box 3.2. Emergences in wild animals.
Historically, rinderpest epidemics were known in Europe and India and it was only in the 1890s that this disease appeared in the Horn of Africa, subsequent to the introduction of contaminated cattle from India [BLA 00]. It caused 80-90% mortality in wild ungulates in Africa, and the current geographical distribution of many antelope species still reflects the effects of this terrible epidemic [SPI 86, DOB 95]. Rinderpest also caused significant human famine and affected pastoral breeding societies. In his well-known ethnographical study of the Nuers in Sudan, Evans-Pritchard showed that rinderpest profoundly affected the social organization of the Nuers as far as he was able to tell in the 1930s. The Nuers thus shifted from total nutritional and economic dependence on livestock to diversify their activities with the introduction of horticulture.
Marine mammals were not spared either with a significant increase in mortality in various pinniped colonies due to virus outbreaks [FRI 06] (Figure 1 ).
Figure 1.
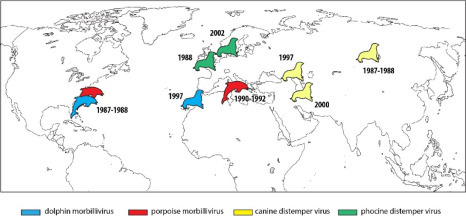
Examples of events of mass mortality of marine mammals caused by infection from morbilliviruses (according to [FRI 06]). For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
The last decades have shown an increasing number of infectious diseases in fungi-related animals and plants [FIS 12] (Figure 2 ).
Figure 2.
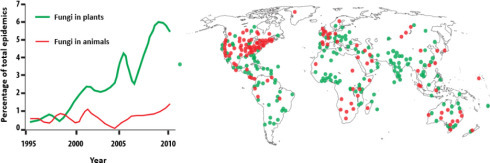
Increasing epidemics in fungal diseases in plants and animals: on the left, percentage of total number of cases recorded over time; on the right, spatial representation. For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
(taken from [FIS 12])
Alt-text: Box 3.2.
Some diseases have caused the extinction of certain wildlife species. The Batrachochytrium dendrobatidis fungus emerged as a global threat to amphibians in addition to several environmental stressors such as habitat destruction and climate change [FIS 09]. The invasive American frog, Rana catesbeiana, is known to spread the fungus to new habitats thus endangering many indigenous amphibian species [HAT 12].
At the same time, the number of infectious disease epidemics has also increased exponentially over the last 60 years [MOR 12a, MOR 14a] (Figure 3.4(A) ).
Figure 3.4.
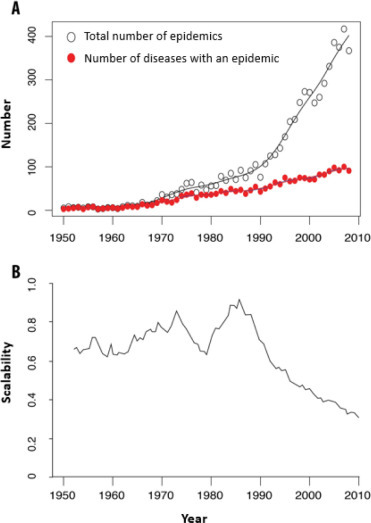
(A) Evolution of the total number of infectious disease epidemics and of the number of different infectious diseases that had at least one epidemic in a given year on a global scale over the last six decades (based on data from [MOR 14b]). (B) Analysis of the modularity of epidemic networks that are shared between countries showing that, per year, the number of countries that share epidemics for the same infectious diseases decreases from the 1980s onwards while the total number of epidemics increases (A). Increasingly, more countries are sharing epidemics. For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
(reprinted from [POI 15])
Recent decades have featured an increase in the number of infectious disease epidemics on a global scale, which mainly concern virus-type infectious agents (AIDS virus, Ebola virus, hantavirus, SARS-Coronavirus, etc.) or bacteria (Legionella pneumophila, Bartonella clarridgeiae, Borrelia burgdorferi, etc.) and many of which the origins are to be sought in domestic or wild animals. These epidemics are not accompanied by a significant increase in mortality rates (except AIDS) due to surveillance and public health systems.
An important aspect of these new emergences is their location. The vast majority of these agents were found in developed countries in North America (USA, Canada), Europe, Asia (Japan) and Australia (see map in Jones et al. [JON 08]). However, the risks of emergence of these new infectious diseases (especially those related to wildlife) concern countries with high biodiversity and high human density, and thus mainly those located in the intertropical regions [JON 08].
The emergence of new infectious diseases in countries in the intertropical zone followed by their identification (mainly in developed countries) is explained by another aspect of the model: the intensification of human and commercial exchanges.
In recent decades, an increasing number of global outbreaks of infectious diseases with a small and steady increase in new pathogens worldwide has been observed (Figure 3.4(A)) A second trend is the homogenization of the overall distribution of parasites. This homogenization seems to have begun in the 1960s, according to Smith et al. [SMI 07], and is characterized by a striking homogenization of global epidemiological patterns. Countries are looking increasingly similar in terms of characteristics of their epidemics in infectious diseases.
Using network analysis, one can also see a striking decrease in the modularity of country/infectious disease networks over the past decades [POI 15] (Figure 3.4(B)), which highlights that infectious disease epidemics are shared more and more by an increasing number of countries. An epidemic is more likely to reach a large number of countries because of trade links and human displacement.
The observations presented above strongly suggest that global changes affect the worldwide epidemiological environment by promoting the spread of infectious diseases and increasing the risk of pandemics.
3.3. Reservoirs of zoonotic infectious diseases
Woolhouse and Gowtage-Sequeria [WOO 05] characterized the reservoirs of agents that are responsible for new infectious diseases, mainly viruses and bacteria (Figure 3.5 ).
Figure 3.5.
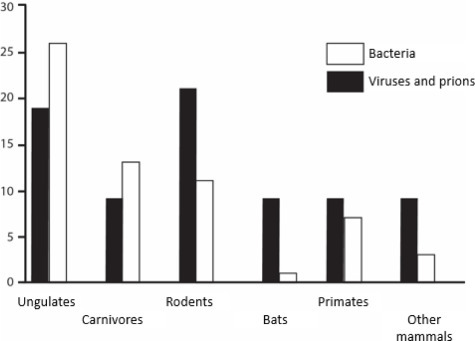
Reservoirs of emerging zoonoses
(taken from [WOO 05])
Ungulates (cattle, horses, goats and sheep) are major reservoirs of new emergences in recent decades, but carnivores (dogs, cats) also play an active role in the spread of emerging infectious agents. Thus, we can appreciate the importance of domestic animals in the diversity of human infectious diseases (Chapter 2).
For wildlife, rodents form the reservoir group that contributes the most to emergence of infectious diseases, followed by primates and then bats.
The incriminated rodents are mostly old commensals of humans (black rats, Norway rats, domestic mice) or “new pets” (prairie dogs, Gambian giant rats). Emerging rodent-related infectious diseases include leptospirosis and hemorrhagic fevers of viral origin (hantavirus, Lassa virus) [MEE 09].
Bats are a source of many emerging viral diseases like Ebola, Hendra, Nipah or SARS-Coronavirus (Box 3.3 ).
Box 3.3. What emerging viruses come from bats?
Although the majority of human rabies cases occur from a rabid dog bite, the lyssavirus responsible for this zoonotic disease originates from bats. Carnivores acquired this virus secondarily, which also infected many other animals. Thus, in the early 1900s in Brazil, 4,000 bovines and 1,000 horses and mules died of paralytic rabies. Bats had been seen around these animals trying to bite them and it was the bovines and horses that were actually infected with the rabies virus. This was the first causal link between bats and viral diseases [HAL 07].
Different Ebola viruses have been responsible for several epidemic outbreaks in Central Africa, with the most recent outbreak in West Africa in 2014. The first emergence dates back to 1976 (Ebola virus in Zaire), followed by the Sudan virus, Tai Forest virus and Bundibugyo virus. Transmission of the disease was often due to the consequence of handling bushmeat on markets, as is the case of primates infected in markets in the Democratic Republic of Congo. High mortalities and inter-human transmissions make Ebola a high-risk zoonotic disease. The reservoirs of these Ebola viruses are bats [LEE 15]. Reston virus (RESTV), which is also from the Ebola group, was discovered in macaques in the Hazleton laboratories in the United States in 1989. This virus is non-pathogenic for humans but dangerous for monkeys. It was found in macaques in Southeast Asia and then in bats [JAY 15]. The first infections of the Marburg virus (a German city) involved researchers from a pharmaceutical laboratory who became ill after handling kidney cells from green monkeys imported from Uganda. Epidemics were subsequently recorded in the Republic of Congo in 1998, in East Africa in 2000, in Angola in 2004 and 2005 and in Uganda in 2014. The reservoir was dogfish.
Some emerging viruses belong to the Paramyxoviridae family [WAN 08]. The viruses in this family include measles and mumps in humans, and Newcastle disease, rheumatoid arthritis and rinderpest in domestic animals. Four new bat-born paramyxoviruses have emerged since 1994 in Australia, South and Southeast Asia and the Arabian Peninsula. These include the Hendra virus (HeV) in horses and infected humans in Australia in 1996, Nipah virus (NiV) in humans and pigs in Malaysia in 1999, and Menangle virus (MenV) in pigs in Australia in 1997. The different Hendra virus epidemics in Australia have affected horses and some humans who were in direct contact with infected horses. Large frugivorous bats were the reservoirs of this virus. Nipah virus outbreaks occurred in Malaysia in 1998, when farmed pigs and humans became infected. In Singapore, human infestations occurred in slaughterhouse staff where pigs were imported from contaminated areas of Malaysia. Dogfish and small insectivorous bats were reservoirs of NiV. Other Nipah virus epidemics occurred in Bangladesh between 2001 and 2005, and in India in 2001. Infestations were reported to be directly from bats with proven human-to-human transmissions [LUB 09]. The Menangle virus emerged in Australia in 1997 in a large intensive pigsty near Sydney, where two human cases were associated with swine disease. Coronaviridae viruses cause innocuous human diseases for four of these viruses, but two viruses caused two major health crises: the SARS virus (Severe Acute Respiratory Syndrome) with more than 8,000 people infected in some 30 countries and the MERS virus (Middle East Respiratory Syndrome). In 2002, a coronavirus emerged in the province of Guangdong in China, causing the SARS epidemic to be linked to small carnivores like civets, which were sold in bushmeat markets in southern China. The wild reservoirs for this disease were bats [WAN 06]. In the Arabian Peninsula in 2012, the first human case of infection from a new coronavirus that was responsible for a respiratory syndrome was identified. This was the MERS-CoV. Human-to-human transmission was identified with cases being imported into Europe, Asia and the United States. The reservoirs were small insectivorous bats, but human infection was via dromedaries that were infected with the virus [CHU 14].
Alt-text: Box 3.3.
What should we remember about the emergence of viruses in bats? First of all, the diseases caused by these viruses have high levels of lethality and are the source of major health crises, such as SARS, Nipah, MERS-CoV and the recent Ebola in West Africa. However, direct viral contamination from a bat to a human is rare. This occurs through the intermediary of primates, carnivores, horses, pigs or dromedaries. These animals are close to us, either phylogenetically like primates or because they have been domesticated for millennia. Finally, two main geographical areas host these emergences: Central and Western Africa and the Asia-Pacific region.
3.4. Emerging infectious diseases and the biodiversity crisis
Anthropogenic changes have been repeatedly challenged as the explanatory factor for the emergence and re-emergence of infectious or parasitic diseases (Table 3.1 ) with deforestation, land-use change, food production systems and biodiversity change.
Table 3.1.
Infectious diseases: mechanisms of emergence in relation to anthropogenic factors
| Infectious diseases | Mechanisms of emergence | Anthropogenic factors |
|---|---|---|
| Malaria | Invasion, expansion of vectors | Deforestation, irrigation |
| Chagas disease | Expansion of vectors | Deforestation, spread of urbanization |
| Leishmaniasis | Expansion of vectors, change of host | Habitat alteration |
| Trypanosomiasis | Expansion of vectors | Deforestation |
| Cryptosporidiosis | Expansion of oocyte contamination | Poor management of watersheds and wastewater |
| Cryptosporidiosis | Soil disturbance | Climate variability |
| Filariasis | Expansion of vectors | Urbanization, irrigation |
| Bilharzia | Expansion of vectors | Irrigation |
| Onchocerciasis | Expansion of vectors | Irrigation |
| Lyme's disease | Loss of predators, expansion of reservoirs | Loss of biodiversity, habitat fragmentation |
| Cholera | Ocean surface temperature | Climate variability |
| Leptospirosis | Increased bacterial contact, expansion of reservoirs | Habitat alteration, agricultural development, precarious increase of habitat |
| Meningitis | Dust storms | Desertification |
| Dengue | Invasion, expansion of vectors | Urbanization, poor housing conditions |
| Hantavirus | Abundance of reservoirs, resource variation | Climate variability |
| Rabies | Change of host | Loss of biodiversity |
| Rift Valley Fever | Abundance of vectors, heavy rainfall | Climate variability |
| Encephalitis | Expansion of vectors | Irrigation |
| SARS-Coronavirus | Transfer of host | Bushmeat, wildlife/domestic animal contact |
| West Nile Fever | Expansion of vectors | International trade, climate variability |
| Ebola | Human/wildlife contact | Bushmeat, habitat alteration |
| Nipah/Hendra | Niche invasion, wildlife/ domestic animal contact | Agricultural intensity |
| H5N1, avian influenza | Extension of livestock production | Trade, agricultural intensification |
(adapted from [MOR 12])
The existence of a correlation between biological diversity and the diversity of human pathogens (see Chapter 2) makes it difficult to explain why a loss of biodiversity can increase emerging infectious risks.
Morand et al. [MOR 14a] showed that the number of endemic infectious diseases per country in the Asia-Pacific region was highly correlated with the number of mammalian and bird species. They also showed that the total number of zoonotic epidemics in recent decades is positively correlated with the number of endangered mammalian and bird species (according to IUCN criteria), while taking potential biases into account such as population size, economic wealth and health surveillance level of countries. It is interesting to note that the number of endangered human languages in Southeast Asia is also positively correlated with the number of endangered species of birds and mammals [MOR 17a].
If data are taken globally, there is a good correlation among the number of circulating pathogens and parasites, the number of birds and mammals in danger of extinction, and the number of endangered languages on a country scale (taking geographical and demographic size biases of countries into account, as well as economic wealth) (Figure 3.6 ). Declines in biodiversity and cultural diversity are not independent of the number of infectious diseases.
Figure 3.6.
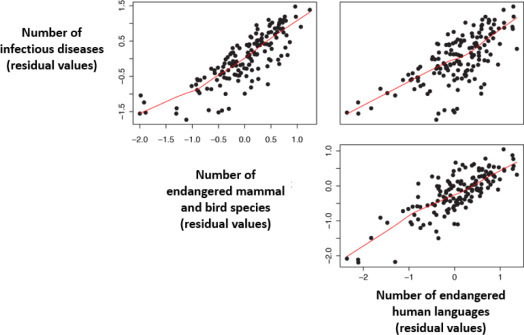
Correlations between the number of pathogens and parasites, the number of endangered mammals and birds (according to IUCN criteria) and the number of endangered languages (according to UNESCO criteria). The variables are corrected for geographical area, demographic size of the country and wealth (GDP per capita) (data supplemented by [MOR 14a]). For a color version of the figure, see www.iste.co.uk/morand/biodiversity.zip
These correlative and comparative approaches suggest that biodiversity is a source of diversity in pathogens, but endangered biodiversity is a source of epidemics. Biological diversity not only affects the diversity of pathogens, but also potentially their prevalence. Thus, Derne et al. [DER 11] showed a negative correlation between the number of mammalian species and annual incidence of human leptospirosis in island states and territories.
3.5. Mechanisms of emergence through habitat modification
Loss of habitat and habitat fragmentation usually result in a significant loss of species because the remaining habitats are too small or too isolated to persist or colonize [MOR 10a]. The effect of fragmentation on host-parasite interactions has been the subject of many studies on conservation medicine or emerging diseases [PAT 14a].
No comparative study has yet analyzed the potential links between abundance of zoonotic parasite/pathogen species and habitat changes. On the other hand, prevalence values appear to be affected by changes in fragmented habitats. Thus, Brearley et al. [BRE 13] analyzed prevalence changes reported in 19 studies to assess the influence of man-made landscapes. Half of the studies showed an increase in parasite or infectious prevalence with a landscape change. Gottdenker et al. [GOT 14] analyzed more studies and showed a similar trend with 56.9% of studies documenting increased pathogen transmission in response to anthropogenic habitat change. Studies on urban landscapes also tend to show an increase in prevalence of infectious agents [HAS 17].
Murray and Daszak [MUR 13] proposed two non-exclusive hypotheses for the emergence of infectious diseases due to habitat alterations. The “disturbance” hypothesis assumes that a change in land use disrupts transmission dynamics in multi-host systems by disrupting inter-species transmission. The “pool of pathogens” hypothesis assumes that land use change exposes new hosts to a greater diversity of pathogens favoring transmission between species.
Long-term studies from observatories on change in land use are particularly useful in understanding the effect of habitat changes on biodiversity. The framework developed by Haddad et al. [HAD 15] makes it possible to link consequences of habitat fragmentation to transmission of diseases through these effects on biodiversity (such as reservoirs and vectors) (Figure 3.7 ).
Figure 3.7.
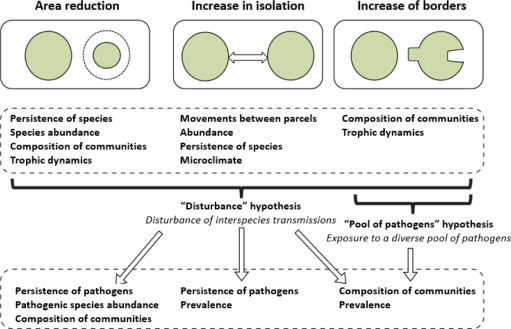
The framework by Haddad et al. [HAD 15] links various aspects of habitat fragmentation to biodiversity. It proposes landscape mechanisms that are responsible for the emergence of infectious diseases according to the hypotheses developed by Murray and Daszak [MUR 13]: the “disturbance” hypothesis and the “pool of pathogens” hypothesis
(taken from [MOR 17a])
In their review of observation and experimental devices, Haddad et al. [HAD 15] showed that fragmentation reduces species abundance and/or community composition. They also showed that a reduction in size of habitats and increase of borders with other habitat interfaces leads to major changes in the composition of plant and animal communities. Thus, regardless of the aspect of fragmentation (reduction of surface area of different habitats, increased isolation, increase of borders), there will be a change in the diversity and structure of plant and animal communities. The “disturbance” hypothesis can be applied to each aspect of fragmentation and should lead to a reduction in the diversity of pathogenic species and changes in their prevalence. The “pool of pathogens” hypothesis should mainly be applied to a mode of fragmentation that leads to an increase in borders favoring contact between different reservoir and vector communities.
3.6. Mechanisms of emergence through community modification
The “dilution effect” hypothesis has been proposed for zoonotic or vector-borne diseases [SCH 01] (taking up the “disease diversity hypothesis” proposed for plants by Elton in 1959). The dilution effect suggests that the abundance and diversity of host species play a protective role in the propagation of pathogens, which is very similar to the “biological resistance hypothesis” formulated for biological invasions.
Species-rich host communities contribute to reducing the transmission of parasites relative to host communities that are not very abundant but have a high proportion of competent hosts. The transmission of a pathogen to an incompetent host is lost, and this type of loss of transmission increases with species-specific abundance. This phenomenon has been observed in several vector-borne diseases such as Lyme's disease [LOG 03] and West Nile Valley disease [SWA 08], but also in rodent-borne diseases such as the hantavirus [CAR 11].
The first meta-analysis of 13 studies tested the importance of dilution in disease transmission [SAL 13]. It showed that biodiversity had a weak influence suggesting that transmission was dependent on local factors. A second analysis of 90 studies [JOH 15] showed the existence of a dilution effect for various diseases that affect humans, wildlife, livestock or plants. A third study on 61 species of parasites [CIV 15] showed that biodiversity reduces parasite abundance. The dilution effect is robust but its magnitude is related more to the frequency than the population density of the host species.
A change in the predator-prey relationships can affect the transmission of pathogens. Orrock et al. [ORR 11] showed that the prevalence of the Sin Nombre virus (SNV) in rodents was higher on islands that host fewer predators of these rodents. A study by Levi et al. [LEV 12a] suggested that the increase in recent years of Lyme's disease in the United States is correlated with the decline of the red fox, a key predator of small mammalian reservoirs of the bacteria; this decline can be explained by the competitive expansion of coyote populations.
Ultimately, the consequences of introduction of pathogens, vectors or reservoirs can have dramatic effects on species and local communities [MOR 17]. For example, 10 species of birds among 64 invasive species recorded in Europe have an impact on health [SHI 09]. An invasion may also decrease the transmission of a native parasite. Telfer et al. [TEL 05] observed a decrease in the prevalence of two intracellular bacteria, Bartonella birtlesii and Bartonella taylorii, in indigenous rodent populations, taking the density of another invading rodent into account in their model. This decline in prevalence can be explained by a reduction in transmission of the bacteria by vector fleas.
3.7. Genetic diversity of hosts and transmission of infectious diseases
Host individuals within a given species or population have a genetic variability that is based on heterogeneity in the susceptibility or competence of a given parasite. This intra-species or intra-population genetic diversity must be considered in the understanding of transmission. From a theoretical point of view, genetically diverse populations are susceptible to a wide spectrum of parasites (in terms of number of species) and therefore prove to be beneficial to parasites by harboring a high level of diversity of parasite and pathogen species. Empirical studies in plants, insects and vertebrates support this hypothesis. Reduced genetic diversity in the host favors parasitic transmission and aggravates the negative impacts of parasites by selecting for an increase in their virulence [KIN 12]. In other words, a high genetic diversity of hosts could slow down the spread of parasites and limit their impact.
3.8. Conclusion
This chapter examined the relationship between biodiversity change and transmission of infectious and parasitic diseases from a global to local scale with a brief overview of the mechanisms (Figure 3.8 ).
Figure 3.8.
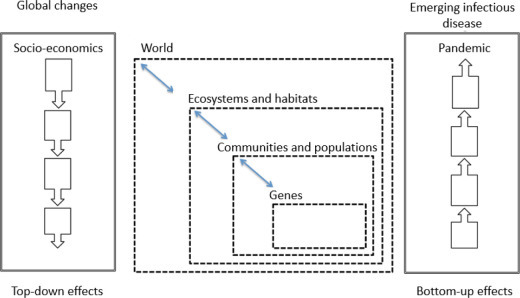
Top-down effects of global changes in biodiversity and bottom-up effects on infectious diseases
While human mortality from infectious diseases has declined dramatically over the past century, recent decades have seen an increase in epidemics of more infectious agents, mostly from wild or domestic animals. This increase in epidemics is accompanied by a greater extension to regional or even global distributions due to an increasingly connected world [TAT 06]. Epidemics are becoming increasingly globalized and frequent.
Global and local changes affect all components of biodiversity (ecosystems, habitats, communities, populations, genes) and the interactions between predators and prey, hosts and parasites, as well as human cultural diversity.
Empirical studies and often-correlative analyses show that biodiversity is a source of pathogens, but increases in epidemics and risks of emergence are associated with decreased biodiversity, which is itself associated with land use changes (deforestation, agronomic intensification) and thus favoring interfaces between wildlife, domestic animals and humans [KEE 10].
The decline in biodiversity also concerns parasites and pathogens and this loss of biodiversity does not always have positive consequences on health.


