Summary
Immune dysfunction is a hallmark of chronic HCV infection and viral clearance with direct antivirals recover some of these immune defects. TCRVγ9Vδ2 T cell dysfunction in treated HCV patients however is not well studied and was the subject of this investigation. Peripheral blood cells from patients who had achieved sustained virologic response (SVR) or those who had relapsed after interferon-free therapy were phenotyped using flow cytometry. Functional potential of Vγ9Vδ2-T cells was tested by measuring proliferation in response to aminobisphosphonate Zoledronic acid, and cytotoxicity against HepG2 hepatoma cell line. TCR sequencing was performed to analyze impact of HCV infection on Vδ2-T cell repertoire. Vγ9Vδ2 cells from patients were activated and therapy resulted in reduction of CD38 expression on these cells in SVR group. Relapsed patients had Vδ2 cells with persistently activated and terminally differentiated cytotoxic phenotype (CD38+CD45RA+CD27−CD107a+). Irrespective of outcome with therapy, majority of patients had persistently poor Vδ2-T cell proliferative response to Zoledronate along with lower expression of CD56, which identifies anti-tumor cytotoxic subset, relative to healthy controls. There was no association between the number of antigen reactive Vγ2-Jγ1.2 TCR rearrangements at baseline and levels of proliferation indicating non-response to Zoledronate is not due to depletion of phosphoantigen responding chains. Thus, HCV infection results in circulating Vγ9Vδ2-T cells with a phenotype equipped for immediate effector function but poor cytokine response and expansion in response to antigen, a functional defect that may have implications for susceptibility for carcinogenesis despite HCV cure.
Keywords: γδ T cells, HCV, DAA therapy, Zoledronic acid, Hepatocellular carcinoma
1. Introduction
Hepatitis C virus (HCV) infection develops chronicity in 50-90% of adults [1] where it eventually manifests as chronic liver disease including cirrhosis, liver failure and hepatocellular carcinoma. HCV is non-cytopathic virus and the pathogenesis is presumed to be mostly driven by activated intrahepatic host immunity that includes cellular as well as soluble effectors of immune response [2–4]. At the same time, persistence of chronic hepatitis C (CHC) infection is associated with exhaustion of virus specific CTL and CD4+ T cells [5]. While the dysfunction in αβ T cells and their role in HCV immunity is well defined, the impact of chronicity on another T cell population, one with γ- and δ-chain TCR containing T cells (γδ-T cell) less studied.
γδ-T cells are part of innate immune system and are classified into two major subsets based on their TCR expression: Vδ1 and Vδ2 γδ-T cells [6]. The Vδ2 subset expressing Vγ2-Jγ1.2Vδ2 (Vγ9Vδ2, alternate nomenclature) T cell receptor is the most abundant γδ-T cell population in peripheral blood, comprising up to 5% of T lymphocytes [7]. They are innate immune cells with potent effector function against diverse infectious agents (including bacteria, protozoan and viruses) [8] and cancers including hepatocellular carcinoma [9]. Human γδ-T cells activated by phosphoantigens or aminobisphosphonate drugs including zoledronic acid (zoledronate) provide a Th1-oriented immune response [10]. A potential antiviral role for Vδ2-T cells is inferred from studies showing inhibition of HCV replication mediated by zoledronate induced IFN-γ production [11, 12]. Use of zoledronate for large-scale expansion of functional Vδ2 T cells in cancer patients is being explored for its potential in adoptive immunotherapy [10, 13, 14]. Thus, a persistent adverse effect of HCV infection on this immune cell can compromise it’s antiviral and anticancer function and hinder it’s use in autologous immunotherapy. Conflicting reports in recent years have shown various outcomes for CHC patients treated with direct acting antivirals (DAA). While consensus is that SVR after DAA therapy reduces the incidence of HCC [15] or has no impact on HCC [16], some patients were reported to develop HCC significantly earlier than those treated with PEG-IFNg/RBV or to have earlier HCC recurrence [17, 18]. Whether the onset of HCC is related to suboptimal immune effector cells despite effective anti-viral treatment in patients has not been studied. The impact of HCV infection and therapy induced viral clearance on γδ-T cell is of importance in this context, because the γδ-T cells are important for natural tumor surveillance.
Phenotyping studies in untreated HCV patients showed that Vδ2-T cells have an activated and cytotoxic profile, although they produce less IFNγ. This functional profile is consistent with reduced antiviral activity and potentially contributing more to liver inflammation [19]. The impact of HIV, another chronic viral disease, on frequency and function of γδ-T cells is well characterized [20] and even long term effective viral suppression with antiretroviral therapy does not recover the frequency and function of Vδ2 T cells to normal levels [20, 21]. Unlike HIV, for CHC there is effective DAA therapy that results in cure rates over 90% particularly in genotype 1 infections [22], providing an opportunity to study immune reconstitution after a chronic viral pathogen has been eliminated. Effects of HCV clearance on HCV-specific T cells, NK cells, and MAIT cells indicate there is selective recovery within some of the immune cell subsets [23–25]. However, data on the composition, dysfunction and recovery status of Vδ2-T cells after DAA for HCV infection is scant.
The purpose of this study was to investigate the impact of DAA therapy on phenotypes and function of Vδ2-T cells in patients with chronic HCV infection and to evaluate the relationship to SVR. To delineate differences between SVR and relapsed groups we have used a cohort of HCV patients that was treated with multiple/selective DAAs for 4 weeks only, which effectively cleared virus at the end of therapy (EOT) in all treated patients but achieved SVR in only 30% of them by 12-week post therapy [26]. We then confirmed the most important findings in a cohort of patients treated with standard 12-week duration therapy. Our results show selective improvement in Vδ2-T cell phenotypes in those who cleared infection upon treatment. We discovered important defect in the Vδ2-T cell phenotype and proliferative response to zoledronate stimulation in CHC patients which persists despite viral cure with DAA therapy. The implications of this persisting defect long after viral clearance are discussed.
2. Methods
2.1. Patients, treatments and samples
We used peripheral blood mononuclear cells (PBMC) samples from three cohorts. For cohort 1, samples from a previous clinical trial conducted at the National Institute of Allergy and infectious Diseases (NIAID), National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland were available ( NCT01805882). Patient characteristics and clinical results of the trial have been published elsewhere [26]. In brief, CHC patients, predominantly African American, aged 18 years or older, having chronic HCV genotype 1 infection (serum HCV RNA level ≥2000 IU/mL) and stage F0 to F2 liver fibrosis with no prior treatment history were included in the study. Patients were treated with short duration DAA therapy (ledipasvir, sofosbuvir, and GS-9451/GS-9699) for 4 weeks. 96% patients had unquantifiable levels of HCV RNA at the end of treatment (week 4). Before therapy (Baseline) and end of treatment (EOT) samples from patients who achieved SVR (N=14) and those who relapsed (N=14) were available to us for this study. For cohort 2, PBMC samples at baseline and 12 weeks from end of treatment (SVR12) (N=5 pairs) from race, gender and age matched CHC patients treated with a standard 12-week DAA regimen (sofosbuvir, velpatasvir and voxilaprevir) were available. A third cohort comprised of race and age matched healthy blood donors (N= 15) and served as HCV negative controls. Supplementary table shows demographic profile of patients and controls. All patient samples used are from already existing collections. All participants signed informed consent approved by the National Institute of Allergy and Infectious Disease Institutional Review Board at the time of screening and enrollment and all samples were anonymized. All methods utilized for this study were performed in accordance with the relevant guidelines and regulations.
2.2. Antibody staining and Flow cytometry
Phenotypes of Vδ2 cells were analyzed using antibody staining for 30 min in 4°C and flow cytometry with following combinations of anti-human monoclonal antibodies for cell surface markers: anti-CD3-FITC/PE (UCHT-1), anti-Vδ2-FITC/PE (B6), anti-CD45RA-PE-Cy7 (HI100), anti-CD27-PE (M-T217), anti-CD38-PE-Cy7 (H-B7) purchased from Biolegend and anti-CD56-PE from Beckman Coulter. Cytokine production from Vγ9Vδ2-T-cells upon stimulation was used to assess their functional capacity. 2×105 cells were stimulated with 1 uM Zoledronate (zoledronate/Zol/Zometa; Novartis, Basel, Switzerland), 15 μM IPP (Sigma Chemical Co., St Louis, MO, USA) or 1 μg/ml anti-CD3 for 18 hours in presence of anti-CD107a antibody and Brefeldin and Monensin. Production of IFNγ, TNFα and CD107a was measured by intracellular antibody staining with anti-IFNγ-BV421 (4S.B3), anti-TNFα-PE (MAb11) from Biolegend and anti-CD107a-PE from BD bioscience followed by flow cytometry. Data were acquired on FACS Aria II and analyzed with FlowJo (TreeStar Incorporation).
2.3. Vδ2-T cell proliferation
To determine the proliferative capacity of Vδ2-T cells from HCV-infected patients, PBMCs were stimulated with Zoledronate plus IL-2 as described previously [27]. Briefly, stored cells were thawed and resuspended in RPMI-1640 medium supplemented with 10% FBS, 2mM L-glutamine, 1 U/ml penicillin streptomycin (Invitrogen) and 100 IU/ml recombinant human IL-2 (Tecin, Biological Resources Branch, NIH, Bethesda, MD, USA). Zoledronic acid or IPP was added at concentrations of 1 μg/mL and 15 μmol, respectively, to trigger Vγ2Vδ2 proliferation and cells were incubated at 37°C with 5% CO2. Fresh medium was added on days 3, 7 and 10 along with 100 IU/ml IL-2 supplementation. Cells were stained with antibodies against CD3, Vδ2, CD56, and proliferation of Vγ9Vδ2 T-cells was determined by the change in frequency of Vδ2 T cells among total CD3+ lymphocytes at day 14. Cultures stimulated with IL-2 alone were tested to measure non-specific proliferation.
2.4. Cytotoxicity of Vδ2-T cell
Cytotoxic potential of Vδ2-T cells was measured against hepatoma cell line HepG2 using PKH-26 and CFSE staining as described previously [28]. Briefly, HepG2 target cells were stained with cytoplasmic dye CFSE and membrane dye PHK-26 followed by incubation with zoledronate expanded PBMCs at different Effector:Target (starting from 50:1, 25:1 up to 0.4:1) ratios for 5 hours. At the end of these incubations, cells were analyzed by flowcytometry. HepG2 cells were identified by gating on PHK-26Hi, and CFSE loss on PHK26 positive cells was used to measure lysed targets. Three samples from HCV-infected patients that had Vδ2-T cells (frequency of lymphocytes) >85% at day 14 were compared with similarly expanded cultures from healthy individuals.
2.5. Vδ2 TCR sequencing
Baseline PBMCs of 5 individuals each with zoledronate response resulting in 75-85% Vδ2 expansion (“high-expanded”) or <25% Vδ2 expansion (“low-expanded”) were obtained. Sample preparation and DNA sequencing was performed as described previously [29] (details of the procedure are provided in supplementary methods). Sequences were loaded on an automated sequencer ABI3700 and analyzed using Sequencher and MacClade software.
2.6. Vδ2 TCR Repertoire analysis
In response to phosphoantigen stimulation, Vδ2 subset in most individuals have strong bias toward Vγ2-Jγ1.2 TCR rearrangement and have a high frequency of public Vγ2 chains [30]. DNA sequences surrounding and including the TCR Vγ2 chain CDR3 regions were aligned. We determined how many nucleotide sequences (nucleotypes) were repeated in the library from each donor and generated the corresponding amino acid sequences (clonotypes). Data were expressed as the proportion of Vγ2-Jγ1.2 rearranged nucleotypes and unique Vγ2-Jγ1.2 clonotypes among all Vγ2 clonotypes. Identical clonotypes of the CDR3 region of TCR of Vγ9Vδ2 cells found in more than one unrelated individual were defined as public clonotypes [30]. Since we had a small sample size, public clonotypes within the groups were identified and matched with a previously reported list of public clonotypes for the African-American control group [29]. Proportions of Vγ2-Jγ1.2 public clonotypes in each sample were assessed by the formula (total Vγ2-Jγ1.2 public clonotype rearrangements/ total Vγ2-Jγ1.2 clonotype rearrangements) × 100. We also verified the diversity of Vγ2-Jγ1.2 public clonotypes by measuring the proportion of unique Vγ2-Jγ1.2 public clonotypes among all unique Vγ2 clonotypes for each sample by the formula (number of unique Vγ2-Jγ1.2 public clonotypes / number of all unique Vγ2-Jγ1.2 clonotypes) × 100.
2.7. Statistical Analysis
Comparison of Vγ9Vδ2-T cells were made between chronic HCV-infected patients versus healthy donors, different time points (baseline vs EOT/SVR12) for DAA treated CHC patients and between patient groups (SVR vs relapsed) at baseline and EOT. Statistical analysis was performed using GraphPad prism software. Normal distribution for each parameter was tested with D’Agostino & Pearson omnibus test and Student’s t test or Mann-Whitney U test were employed for parametric and non-parametric data, respectively. For comparison between baseline and EOT for each sample Paired t test or Wilcoxon matched-pairs signed rank test were used for parametric and non-parametric data, respectively.
3. Results
3.1. Altered Vδ2-T cell memory phenotypes in CHC patients
The median frequencies for Vδ2-T cells among circulating lymphocytes were similar in healthy donors (HDs) and HCV-infected patients at pre-treatment (baseline) and at end of treatment (EOT) with DAA (Figure 1A, B). Based on the expression of the surface markers CD45RA and CD27, Vδ2-T cells can be classified into four functional subsets: naïve (Tnaïve; CD45RA+ CD27+), central memory (TCM; CD45RA- CD27+), effector memory (TEM; CD45RA- CD27-) and terminally differentiated effector memory (TEMRA; CD45RA+ CD27-) (Figure 1C) . Frequency of TEM cells in PBMCs is a vital measure of Vδ2-T cell immediate effector function. This subset is highly responsive to stimulation and comprises the subset of potent effector cells that includes cytotoxic effectors and cells that release proinflammatory cytokines. Reduced frequencies of Vδ2-T cell TEM subsets in peripheral blood was observed in CHC patients, while the Tnaïve subset was proportionally increased (Figure 1D). At the end of DAA treatment, no changes were detected in the TEM/naïve frequencies from baseline. The frequency of TCM cells was significantly lower in the relapsed group compared to SVR group (P=0.002), whereas TEMRA frequencies were significantly higher in this group (P=0.017) (Figure 1E). Thus, patients that relapsed with short duration DAA therapy had lower levels of CM and higher levels of terminally differentiated Vδ2-T cells relative to those that achieved SVR. This possibly reflects continuous antigenic stimulation leading to terminal differentiation of this cell in those who fail these therapies.
Figure 1: CHC patients who relapsed after short term DAA therapy show terminally differentiated effector Vδ2-T cell phenotype compared to those who achieved SVR.
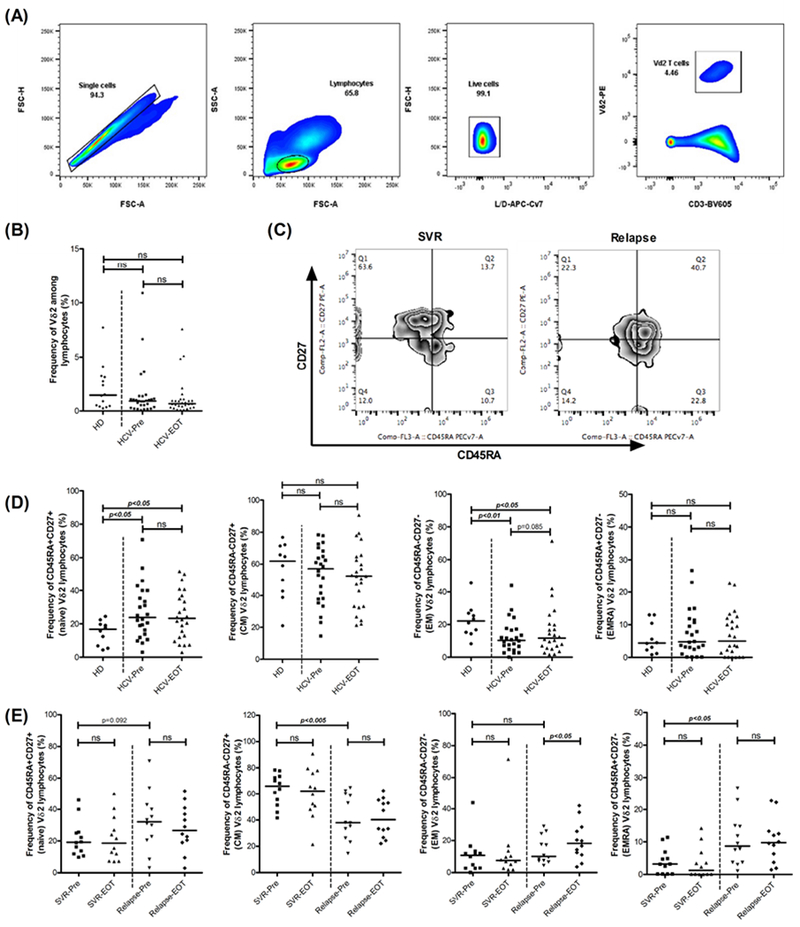
(A) Gating strategy for Vδ2-T cells. (B) Comparison of the proportions of circulating Vδ2-T cells among healthy donors (HD, N=14), HCV patients at baseline (HCV-Pre, N=29) and HCV patients at end of therapy (HCV-EOT, N=29). (C) Representative dot plots depicting memory differentiation status of the Vδ2-T cells based on the expression of CD27 and CD45RA. (D) Comparison of the proportions of naïve, CM, EM and EMRA Vδ2-T cells among HDs (N=10), HCV-Pre (N=24) and HCV-EOT (N=24). (E) Comparison of the proportions of naïve, CM, EM and EMRA Vδ2-T cells at baseline (Pre) and EOT between patients who achieved SVR (N=12) and who relapsed (N=12) after short term DAA therapy. Horizontal bar indicates median value. Paired t test or Wilcoxon matched-pairs signed rank test were used for comparisons between the groups HCV-Pre and HCV-EOT, SVR-Pre and SVR-EOT, Relapse-Pre and Relapsed-EOT and SVR12-Pre and SVR12 in (B), (D) and (E). Unpaired t test or Mann Whitney test was used for rest of the comparisons. ns= not significant.
3.2. HCV-associated activation of Vδ2-T cells reduces with DAA mediated viral clearance
Next, we investigated the effect of DAA mediated virus clearance on activation status of the Vδ2-T cells among CHC patients. CD38 expression identifies activated immune cells including CD8+, CD4+ and V-δ2 T cells in chronic viral infections like HIV and HCV [31, 32]. Frequency of CD38+ Vδ2-T cells was higher in all CHC patients relative to healthy controls (P=0.006). Also, patients in relapsed group had relatively higher frequency of CD38+ Vδ2-T cells at baseline compared to the SVR group (median 19.09% vs 26%). Interestingly, while CD38 expression on V δ2-T cells after 4 weeks therapy in SVR group was not different from healthy controls, relapsers had significantly higher CD38+ Vδ2-T cell frequencies at end of therapy relative to healthy controls (P=0.001) (Figure 2B).
Figure 2: Reduced activation of Vδ2-T cells with DAA mediated viral clearance in CHC patients who achieved SVR.

(A) Representative dot plots depicting Vδ2-T cells expressing CD38 activation marker and comparison of the proportion of CD38 expressing Vδ2-T cells among HDs (N=14), HCV-Pre (N=22) and HCV-EOT (N=22). (B) Frequency of CD38 expressing Vδ2-T cells in HDs (N=14) and at baseline and EOT in SVR (N=12) and Relapsed (N=12) groups. (C) Frequency of CD38 expressing Vδ2-T cells at baseline (SVR12-Pre, N=5) and 12-week follow up (SVR12, N=5) in HCV patients treated for 12 weeks with DAA. Proportion of CD56 (D) and CD107a (E) expressing Vδ2-T cells in HDs (N= 10 and N=6 for CD56 and CD107a, respectively) and at baseline and EOT in SVR (N=12 and N=6 for CD56 and CD107a, respectively) and relapsed (N=12 and N=7 for CD56 and CD107a, respectively) groups. (F) Expression of CD107a on Vδ2 T cell surface in response to Zol, IPP and anti-CD3/CD28 antibody stimulation in HCV patients and HD. (G) Comparison of the proportion of TNFα and IFNγ expressing Vδ2-T cells among HDs (N=7), HCV-Pre (N=10) and HCV-EOT (N=10) upon Zol stimulation. Horizontal bar indicates median value. ns= not significant. Expression of (H) IFNγ+TNFα+, (I) IFNγ+ and (J) TNFα+ on Vδ2 T cells in response to Zol, IPP and anti-CD3/CD28 antibody stimulation in HCV patients and HD. Paired t test or Wilcoxon matched-pairs signed rank test were used for comparisons between the groups HCV-Pre and HCV-EOT, SVR-Pre and SVR-EOT, Relapse-Pre and Relapsed-EOT and SVR12-Pre and SVR12 in (A), (B), (C), (D), (E) and (F). In (B), (D) and (E) Kruskal-Wallis non-parametric test and Dunn’s post-hoc test to control for multiple comparisons was performed for the comparison among the groups (SVR-Pre, Relapse-Pre and HD) and (SVR-EOT, Relapse-EOT and HD). Unpaired t test or Mann Whitney test was used for rest of the comparisons. ns= not significant.
We verified the frequencies of CD38+ Vδ2-T cells in a second cohort of CHC patients (who completed the standard 12-week therapy of DAA and achieved SVR; N=5). Here too CD38+ Vδ2-T cell frequencies were higher at baseline (SVR12-Pre) compared with HDs (P=0.018) and declined to levels comparable with HDs at 12 weeks (SVR12) after completion of therapy (Figure 2C).
Hyperactivation of Vδ2-T cells with increased differentiation towards effector phenotype in CHC patients led us to evaluate their functional abilities by measuring cytotoxic potential and cytokine production. Ex vivo expression of CD56 on Vδ2-T cells identifies the anti-tumor cytotoxic subset of these cells [33]. The proportion of CD56+ Vδ2-T cells was similar in CHC patients and HDs (Figure 2D). Also, we found no difference in frequency of this cytotoxic subset between SVR and relapse groups (Figure 2D). Next, we assessed the cytotoxic potential of Vδ2-T cells by measuring expression of degranulation marker CD107a in response to zoledronate stimulation. In over-night Zolderonate stimulated cultures, CD107a+ Vδ2-T cell frequencies were marginally higher at baseline in relapsed group compared to SVR group (P=0.051) (Figure 2E). Additional analysis of cytokine production upon Zolderonate stimulation revealed reduced IFNγ and TNFα production in HCV-infected patients (Figures 2F–I), which persisted with virus clearance. A similar functional dichotomy in Vδ2-T cells from HCV-infected patients characterized by reduced IFNγ and increased degranulation was reported recently, suggesting a phenotype that is less antiviral and more pathogenic [19].
Thus, activated Vδ2-T cells persist in the subset of patients that failed to respond to short-term DAA therapy. Responders to both short-term and standard duration therapy achieved a reduction of activated state of these cells showing this is a reversible defect, much like activation of CD4+ and CD8+ T cells which is normalized with DAA therapy [34].
3.3. Vδ2 proliferative response to Zoledronic acid is compromised in CHC and does not recover with DAA mediated viral clearance
One important measure of functional response of Vδ2-T cells is their proliferation in response to phosphoantigens. Vδ2-T cells from healthy individuals expanded to approximately 85% (median) of total PBMCs in 14-days culture with zoledronate and IL-2 (Figure 3A). CHC patients displayed an overall reduced Vδ2-T cell proliferation (Figure 3A, 3B, supplementary data). More than 50% (13 out of 24) of CHC patients had Vδ2-T expansion levels below any of the HDs. However, there were no significant differences in the proliferation of these cells between SVR and relapsed groups (Figure 3B) at either baseline or at EOT. Since this cohort of patients was treated for only 4 weeks, we validated this finding in a second cohort of five patients who had achieved SVR using standard therapy; proliferative response was tested at baseline and at 12 weeks after end of therapy (i.e. 3months after achieving cure). None of these patients recovered the proliferative response of Vδ2-T cells to Zoledronate stimulation at SVR12, reflecting persistence of this defect (Figure 3C).
Figure 3: Vδ2-T cell proliferative response to Zoledronate is impaired in CHC and persists after virus clearance.
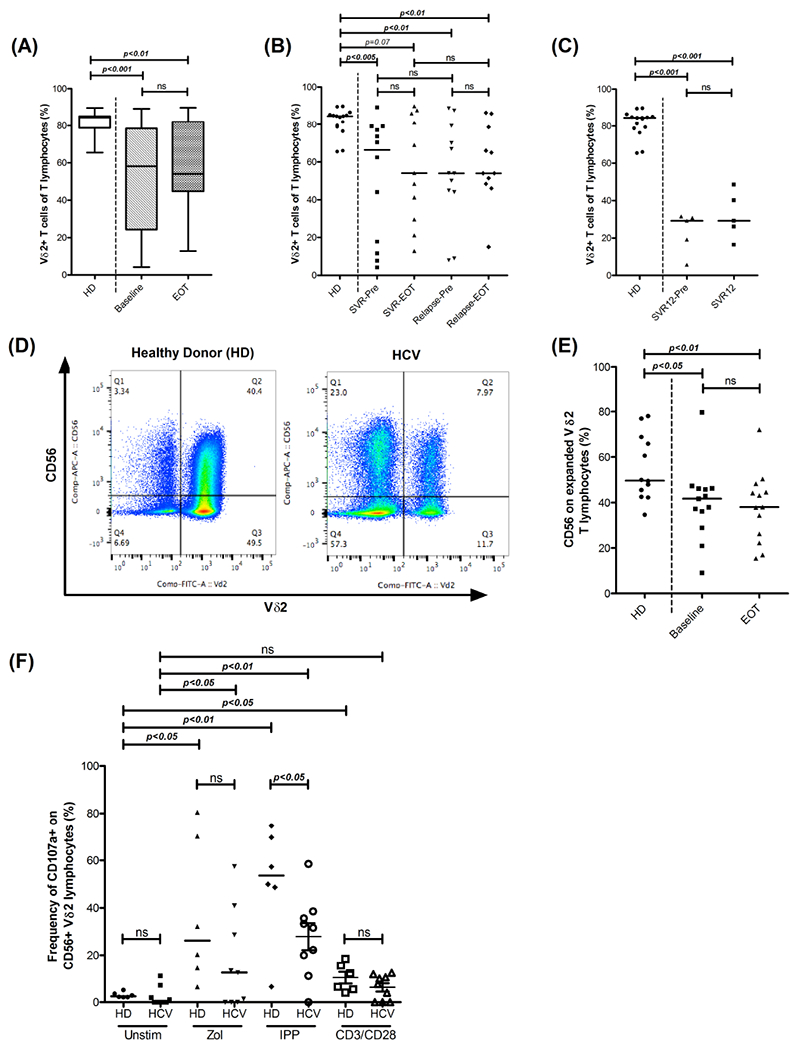
(A) Representative dot plots depicting CD56 expressing cytotoxic Vδ2-T cells after expansion in response to Zoledronic acid and IL2. (B) Box plot representing frequency of expanded Vδ2-T cells in HDs (N=15) and HCV-Pre (N=24) and HCV-EOT (N=22). Box encompasses interquartile range (IQR) and horizontal line dividing the box indicates median value. (C) Frequency of expanded Vδ2-T cells in HDs (N=15) and in SVR (N=12 and N=11 at baseline and EOT respectively) and Relapsed (N=12 and N=11 at baseline and EOT respectively) groups. (D) Frequency of expanded Vδ2-T cells in HDs (N=15) and at baseline (SVR12-Pre, N=5) and 12 weeks follow up (SVR12, N=5) in HCV patients treated for 12week with DAA. (E) Frequency of CD56 expressing expanded Vδ2-T cells in HDs (N= 12), HCV-Pre (N=13) and HCV-EOT (N=13). Horizontal bar indicates median value. Paired t test or Wilcoxon matched-pairs signed rank test were used for comparisons between the groups Baseline and EOT, SVR-Pre and SVR-EOT, Relapse-Pre and Relapsed-EOT and SVR12-Pre and SVR12 in (A), (B), (C) and (E). In (B), Kruskal-Wallis non-parametric test and Dunn’s post-hoc test to control for multiple comparisons was performed for the comparison among the groups (SVR-Pre, Relapse-Pre and HD) and (SVR-EOT, Relapse-EOT and HD). Wilcoxon matched-pairs signed rank test were used in (F) for comparisons among groups with different stimulant in HD and HCV groups. Mann Whitney test was used for rest of the comparisons. ns= not significant.
We further tested frequencies of CD56+Vδ2-T subset in zolderonate expanded cells in a subset of patients. Similar to Vδ2-T cell proliferation defect, patients had lower frequencies of CD56+ Vδ2-T cells compared to HDs at baseline (P=0.021) and EOT (P=0.008) (Figure 3D, 3E). In healthy individuals, CD56+ fraction of Vδ2-T cells is the cytotoxic subset. To confirm if this is the case in HCV infected patients, expression of cytotoxic molecules in Vδ2-T cell subsets was analyzed. Similar to healthy individuals, in ex vivo Vδ2-T cells, CD56+ subset expressed significantly higher levels of perforin and Granzyme B than CD56- subset (supplementary Fig S2). Further, in HCV patients, CD56+ Vδ2-T cells had lower degranulation upon phosphoantigen stimulation (Figure 3E). These data indicate that HCV infection adversely affects the expansion capacity and associated cytotoxic potential of peripheral Vδ2-T cells. These defects could potentially lower the effector response of these cells against HCV-infected hepatocytes or more importantly against tumor cells.
To determine cytotoxic potential of Vδ2-T cells from CHC patients against HepG2 hepatoma (HCC) cells, 14 days expanded cells (with >80% Vδ2+ lymphocyte frequency, N=5 HD and N=3 CHC) were used directly in the killing assays. Our goal here was to test whether a functional impairment at per cell basis exists in otherwise phosphoantigen responsive cells (those proliferating normally) in CHC patients. No significant difference in cytotoxicity mediated by Vδ2-T cell cultures were observed in CHC patients and HDs (data not shown). Here we only tested samples where normal proliferation was present. It will be important to test patients with poor proliferative response to test anti-CHC cytotoxic function, which could not be performed here due to technical difficulties in purifying Vδ2 subset from expanded cultures . HCC cell lines are not refractory to NK cell cytotoxicity, thus to test anti-HCC function of Vδ2 cells in patients with poor expansion of Vδ2 T cell, purification Vδ2+T cells is imperative. Commercially available negative selection kits do not allow purification of CD56+ fraction (which are the cytotoxic subset [35]), and use of antibody mediated sorting or positive selection kit leads to activation of TCR and confounds the readout. Nevertheless, majority of our CHC patients had a numerical defect in proliferative response to zoledronate (13/24 (54%) had day 14 Vδ2+ frequency <65% of lymphocytes while 6/24 (25%) had <20% expansion), and these patients are likely to have an impaired response to hepatocarcinogenesis even when HCV is cured.
3.4. Impaired proliferative response to Zoledronate stimulation in CHC is not associated with Vδ2-TCR repertoire
The finding of poor Vδ2-T cell proliferation in response to zoledronate for a large subset of CHC patients raised the question whether this dysfunction is related to altered TCR repertoire. Phosphoantigen responsive Vδ2 T cells are biased toward Vγ2-Jγ1.2 TCR rearrangement and a poor proliferative Vδ2-T response to phosphoantigens is related to profound depletion of these phosphoantigen reactive Vγ2-Jγ1.2 chains during chronic HIV infection [29]. We tested PBMC samples (ex vivo) from high-expanded and low-expanded CHC groups to determine the abundance of Vγ2-Jγ1.2 TCR chain rearrangement in the Vγ2-TCR repertoire. Similar proportions of Vγ2-Jγ1.2 TCR chains among all rearranged nucleotypes were present in the two groups (median High-expanded 49.4% vs low-expanded 47.2%) (Figure 4A). The diversity of Vγ2-Jγ1.2 clonotypes, measured by the proportion of unique Vγ2-Jγ1.2 clonotypes among all the clonotypes in the repertoire was also similar in both groups (Figure 4B).
Figure 4: Vδ2-TCR repertoire in CHC patients is not associated with proliferative response to Zoledronate stimulation.

(A) Comparison of the fraction of Vγ2-J1.2 nucleotype rearrangements among all cloned and sequenced rearrangements representing the Vγ2 TCR repertoire in high-expanded (N=5) and low-expanded (N=5) groups. (B) Proportion of the unique Vγ2-Jγ1.2 clonotypes in the representative Vγ2 TCR repertoire. (C) Fraction of Vγ2-Jγ1.2 chains expressing public Jγ1.2 clonotypes in the representative Vγ2 TCR repertoire. (D) Proportion of unique public Jγ1.2 clonotypes among all unique Vγ2-Jγ1.2 clonotypes representing the diversity of public Jγ1.2 clonotypes. (E) Clonotype abundance for the most common public Jγ1.2 chains in healthy African-American donors. Clonotypes marked by shaded color in bottom panel were public clonotypes identified in the CHC patients included for this study. Each column represents one patient. Dark box and white box indicate a public clonotype is present or absent, respectively, in the sample. Horizontal bar indicates median value. Mann Whitney test was used for the comparisons. ns= not significant.
Normal Vγ2 repertoire is dominated by public clonotypes, which are TCR chains dominating response to same antigenic epitope in multiple individuals. During chronic HIV infection significant decrease in frequency of Vγ2-Jγ1.2 chains expressing public Jγ1.2 clonotypes is present [29]. A similar reduction in proportion of public clonotypes in “low-expanded” samples can explain reduced proliferative response of Vδ2 T cells to zoledronate in this group. Majority (62%) of public Vγ2-Jγ1.2 clonotypes observed in our patients were same as previously published public clonotypes from African America donors [30]. In addition, we identified 7 more public Jγ1.2 clonotypes in our samples (Figure 4E). The fraction of Vγ2-Jγ1.2 chains expressing public Jγ1.2 clonotypes was similar in both groups (high-expanded 44.4% vs low-expanded 47.6%) (Figure 4C). A relatively higher diversity in public clonotypes (measured by number of unique clonotypes) was noted in low-expanded samples with proportion of unique public clonotypes at 40.9% compared to high-expanded samples with 30.0% (Figure 4D) however this difference did not reach statistical significance. These data indicate that proportions or composition of phosphoantigen reactive Vγ2-Jγ1.2 chains do not influence poor zoledronate response in CHC and factors other than shifts in the Vδ2-TCR repertoire are responsible for failure of Vδ2-T-cells proliferative response.
4. Discussion
In this study we demonstrated peripheral Vγ9Vδ2 gamma delta T cells in CHC patients have phenotypic and functional alterations despite cure with DAA therapy. Major defect in the circulating Vδ2-T cells associated with chronic HCV infection was the inability to proliferate and specifically to expand the CD56+ cytotoxic subset in response to aminobisphosphonate stimulation. Importantly, DAA mediated viral clearance failed to rescue this response even with standard 12-week duration therapy. We speculate that long term persistence of these functional defects could compromise immune surveillance against hepatocarcinogenesis after viral clearance in CHC patients.
Our results add to the list of immune cell dysfunctions that persist despite successful DAA therapy in CHC. Among the successes is NK cell functional recovery and partial rescue of exhausted HCV-specific CD8 T cells that is achieved with DAA mediated cure [23, 24]. On the other hand, virus clearance with these therapies failed to recover peripheral MAIT cell and Treg cell defects including reduced frequencies and function [25, 36]. Recently, Yin et al. showed Vδ2-T cells from untreated HCV-infected patients contained more effector subtypes (CD45RA+CD27-) compared to healthy individuals [19]. Using samples from a cohort treated for 4 weeks with DAA, here we had the opportunity to compare Vδ2-T cells between SVR and relapsed groups. SVR group had a similar phenotypic distribution to healthy donors based on the memory differentiation status, whereas, patients that relapsed had a shift from central memory phenotypes to terminally differentiated Vδ2 effector cells. Activation of Vδ2-T cells was present in all CHC patients and while CD38 expression reduced at end of treatment in patients who achieved SVR persistently activated Vδ2-T were present in those who relapsed. A similar reduction in CD38+ CD8+ or CD4+ T cells occurs with control of virus replication among patients that achieve SVR but not in relapsers [34] despite similar suppression in viral load at the end of treatment in patients of both groups. This could indicate a persistent immune activation among relapsed patients in response to antigen present below detection levels, as has been well described in chronic HIV-infected patients who are virologically suppressed on ART [37]. The increase in proportion of activated TEMRA Vδ2-T cells with high cytotoxic potential in peripheral blood of the relapsed CHC patients can be due to a lack of cell retention at the site of infection or excessive differentiation into TEMRA phenotype. Thus our observation of decreased levels of blood effector memory Vδ2-T cells in patients that cleared HCV with therapy may reflect better recruitment of effector cells to sites of infection where they can exert antiviral effect [12]. In our previous study [26], we characterized the outcomes of four-week DAA treatment on CHC patients where SVR was associated with baseline HCV viral load, younger age, and HCV genotype 1b. In the present study, we observed a strong association between dysfunctional Vδ2-T cells at baseline and viral relapse after short term DAA treatment. This study indicates that the subset of CHC patients marked by higher activation and terminally differentiated effector phenotype among circulating Vδ2-T cells are unlikely to control HCV virus with short term DAA therapy.
Circulating Vδ2 T cells exhibit robust expansion in response to aminobisphosphonates that reflects their normal response to infected or transformed cells. Our data demonstrate significantly reduced in vitro expansion of the Vδ2-T cells upon zoledronate stimulation in a substantial proportion of chronic HCV-infected patients. The TCR independent cytokine stimulation of Vδ2 T cells is not impacted during CHC [38]. Thus, unresponsiveness to zoledronate/IPP would suggest a selective TCR specific defect, similar to irreversible defect reported for MAIT cells during CHC [38]. In parallel with impaired proliferative potential, the proportion of CD56 expressing cytotoxic subset in expanded Vδ2-T cells was also reduced in CHC patients and therapy failed to rescue this subset. Chronic HIV is the most well studied viral infection regarding impact on Vδ2-T cells, and shows incomplete or no recovery upon viral suppression with effective ART [39]. Here, an incapability for in vitro expansion of Vδ2 T cells in response to zoledronate and IL2 [27], is due in part to depletion of phosphoantigen-responsive pool of Vδ2-T cells [20] during progressing disease [29]. HIV infection is known to significantly impact Vδ2-TCR repertoire in blood [21]. Vγ2-Jγ1.2 rearrangements in Vδ2 TCRs are the most abundant receptors that recognize stimulatory tumor cells [40], comprising up to 70% of all Vγ2 chains in peripheral blood of adults [41]. Normal Vγ2 repertoire is dominated by public clonotypes [42] due to the role for monomorphic butyrophilin molecules in mediating phosphoantigen responses. During chronic HIV infection effectively treated with ART, while there is a slow recovery of cells expressing the phosphoantigen-responsive Vγ2-Jγ1.2 chains, neither the frequencies nor in vitro proliferative responses of these cells return to the normal levels [27, 29]. Different from HIV disease, chronic HCV infection does not result in alteration of overall clonality and complexity of γδ-TCR repertoire [43]. We tested if depletion of Vγ2-Jγ1.2 chains in the Vγ2 TCR repertoire would explain the non-responsiveness of these cells to zoledronate. No difference in the abundance or diversity of Vγ2-Jγ1.2 chains between high-expanded and low-expanded samples was detected. Our results suggest impaired expansion of Vδ2-T cells with aminobisphosphonates is independent of the Vγ2 repertoire in CHC and emphasize need for further mechanistic investigation. Altered Vγ2 phenotypes observed in CHC patients could influence response to stimulation as was shown for certain cancers. Impaired response to zoledronate associated with an accumulation of TEM and TEMRA Vγ2 phenotypes in CLL patients [44], whereas in multiple myeloma low responders had decreased naïve and TCM Vδ2 subsets [45]. Other factors implicated in low response include adhesion molecules CD166 and ICAM-1[46, 47] or factors such as altered antigen presenting cells.
It is evident from many studies that γδ-T cells play a role in host defense against tumors and use of autologous bisphosphonate expanded Vδ2 T cells has promising immunotherapeutic potential against various cancers including HCC [48–50]. Our observation of functionally defective circulating Vδ2 T cells in a large proportion of CHC patients despite cure is important in this context. While several risk factors are associated with developing hepatocellular carcinoma both during chronic HCV infection and post-SVR [51], the impact of HCV infection on Vδ2 or other HCC immune effector cells is less studied in this context. Several relevant questions arise from this investigation including status of these cells during long term follow-up after successful clearance of virus and investigating Vδ2 defects in certain patient groups such as those with cirrhosis or with HIV co-infection. Importantly, liver Vδ2 during HCV infection were reported to be functionally impaired in cytokine production [12]. Whether Vδ2 dysfunction persists after successful treatment of CHC in the local liver environment, where these cells are critical for antiviral and anti-tumoral function remains to be seen.
Supplementary Material
Acknowledgements
The study was supported by intramural research funds from NIH (SK) and from IHV (BP, SK).
Footnotes
Conflict of interest
C.D. Pauza is employed by American Gene Technologies Inc. All other authors declare no competing interests.
References
- [1].Deterding K, Gruner N, Buggisch P, Wiegand J, Gale PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis 2013;13:497–506. [DOI] [PubMed] [Google Scholar]
- [2].Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural Killer Cells Are Polarized Toward Cytotoxicity in Chronic Hepatitis C in an Interferon-Alfa-Dependent Manner. Gastroenterology 2010;138:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jin YD, Fuller L, Carreno M, Zucker K, Roth D, Esquenazi V, et al. The immune reactivity role of HCV-induced liver infiltrating lymphocytes in hepatocellular damage. J Clin Immunol 1997;17:140–153. [DOI] [PubMed] [Google Scholar]
- [4].Pawlotsky JM. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol 2004;12:96–102. [DOI] [PubMed] [Google Scholar]
- [5].Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8(+) T cells associated with reversible immune dysfunction. J Virol 2007;81:9249–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jin Y, Xia MC, Saylor CM, Narayan K, Kang J, Wiest DL, et al. Cutting Edge: Intrinsic Programming of Thymic gamma delta T Cells for Specific Peripheral Tissue Localization. Journal of Immunology 2010;185:7156–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carding SR, Egan PJ. gamma delta T cells: Functional plasticity and heterogeneity. Nat Rev Immunol 2002;2:336–345. [DOI] [PubMed] [Google Scholar]
- [8].Qin G, Liu YP, Zheng J, Ng IHY, Xiang Z, Lam KT, et al. Type 1 Responses of Human V gamma 9V delta 2 T Cells to Influenza A Viruses. J Virol 2011;85:10109–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by V gamma 9V delta 2 T cells. Eur J Immunol 2009;39:1361–1368. [DOI] [PubMed] [Google Scholar]
- [10].Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gamma delta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008;10:842–856. [DOI] [PubMed] [Google Scholar]
- [11].Agrati C, Alonzi T, De Santis R, Castilletti C, Abbate I, Capobianchi MR, et al. Activation of V gamma 9V delta 2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int Immunol 2006;18:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cimini E, Bordoni V, Sacchi A, Visco-Comandini U, Montalbano M, Taibi C, et al. Intrahepatic Vgamma9Vdelta2 T-cells from HCV-infected patients show an exhausted phenotype but can inhibit HCV replication. Virus Res 2018;243:31–35. [DOI] [PubMed] [Google Scholar]
- [13].Silva-Santos B, Serre K, Norell H. gamma delta T cells in cancer. Nat Rev Immunol 2015;15:683–691. [DOI] [PubMed] [Google Scholar]
- [14].Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, et al. Zoledronate-activated V gamma 9 gamma delta T cell-based immunotherapy is feasible and restores the impairment of gamma delta T cells in patients with solid tumors. Cytotherapy 2011;13:92–97. [DOI] [PubMed] [Google Scholar]
- [15].Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- [16].Merchante N, Rodriguez-Arrondo F, Revollo B, Merino E, Ibarra S, Galindo MJ, et al. Hepatocellular carcinoma after sustained virological response with interferon-free regimens in HIV/hepatitis C virus-coinfected patients. Aids 2018;32:1423–1430. [DOI] [PubMed] [Google Scholar]
- [17].Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719–726. [DOI] [PubMed] [Google Scholar]
- [18].Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727–733. [DOI] [PubMed] [Google Scholar]
- [19].Yin WW, Tong SW, Zhang QF, Shao JY, Liu Q, Peng H, et al. Functional dichotomy of V delta 2 gamma delta T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-gamma production. Sci Rep-Uk 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li HS, Chaudhry S, Poonia B, Shao YM, Pauza CD. Depletion and dysfunction of V gamma 2V delta 2 T cells in HIV disease: mechanisms, impacts and therapeutic implications (vol 10, pg 42, 2013). Cell Mol Immunol 2013;10:283–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bordon J, Evans PS, Propp N, Davis CE, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. J Infect Dis 2004;189:1482–1486. [DOI] [PubMed] [Google Scholar]
- [22].Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. New Engl J Med 2014;370:1879–1888. [DOI] [PubMed] [Google Scholar]
- [23].Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015;149:190-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, et al. Restoration of HCV-specific CD8+T cell function by interferon-free therapy. J Hepatol 2014;61:538–543. [DOI] [PubMed] [Google Scholar]
- [25].Hengst J, Strunz B, Deterding K, Ljunggren HG, Leeansyah E, Manns MP, et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur J Immunol 2016;46:2204–2210. [DOI] [PubMed] [Google Scholar]
- [26].Kohli A, Kattakuzhy S, Sidharthan S, Nelson A, McLaughlin M, Seamon C, et al. Four-Week Direct-Acting Antiviral Regimens in Noncirrhotic Patients With Hepatitis C Virus Genotype 1 Infection. Ann Intern Med 2015;163:899-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy 2012;14:173–181. [DOI] [PubMed] [Google Scholar]
- [28].Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods 2001;249:99–110. [DOI] [PubMed] [Google Scholar]
- [29].Chaudhry S, Cairo C, Venturi V, Pauza CD. The gamma delta T-cell receptor repertoire is reconstituted in HIV patients after prolonged antiretroviral therapy. Aids 2013;27:1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pauza CD, Cairo C. Evolution and function of the TCR Vgamma9 chain repertoire: It’s good to be public. Cell Immunol 2015;296:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions? Aids 2000;14:1079–1089. [DOI] [PubMed] [Google Scholar]
- [32].Bofill M, Borthwick NJ. CD38 in health and disease. Chem Immunol 2000;75:218–234. [DOI] [PubMed] [Google Scholar]
- [33].Qin G, Liu YP, Zheng J, Xiang Z, Ng IHY, Peiris JSM, et al. Phenotypic and Functional Characterization of Human gamma delta T-Cell Subsets in Response to Influenza A Viruses. J Infect Dis 2012;205:1646–1653. [DOI] [PubMed] [Google Scholar]
- [34].Meissner EG, Kohli A, Higgins J, Lee YJ, Prokunina O, Wu D, et al. Rapid changes in peripheral lymphocyte concentrations during interferon-free treatment of chronic hepatitis C virus infection. Hepatol Commun 2017;1:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 2008;14:4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Langhans B, Nischalke HD, Kramer B, Hausen A, Dold L, van Heteren P, et al. Increased peripheral CD4(+) regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol 2017;66:888–896. [DOI] [PubMed] [Google Scholar]
- [37].Benito JM, Lopez M, Lozano S, Martinez P, Gonzalez-Lahoz J, Soriano V. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res Hum Retroviruses 2004;20:227–233. [DOI] [PubMed] [Google Scholar]
- [38].Provine NM, Binder B, FitzPatrick MEB, Schuch A, Garner LC, Williamson KD, et al. Unique and Common Features of Innate-Like Human Vdelta2(+) gammadeltaT Cells and Mucosal-Associated Invariant T Cells. Front Immunol 2018;9:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van den Heuvel D, Driessen GJA, Berkowska MA, van der Burg M, Langerak AW, Zhao D, et al. Persistent subclinical immune defects in HIV-1-infected children treated with antiretroviral therapy. Aids 2015;29:1745–1756. [DOI] [PubMed] [Google Scholar]
- [40].Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual V gamma 2-J gamma 1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immun 2007;56:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing V gamma 2-J gamma 1.2/V delta 2 T-cell receptors. Immunology 2001;104:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nat Rev Immunol 2008;8:231–238. [DOI] [PubMed] [Google Scholar]
- [43].Ravens S, Hengst J, Schlapphoff V, Deterding K, Dhingra A, Schultze-Florey C, et al. Human gammadelta T Cell Receptor Repertoires in Peripheral Blood Remain Stable Despite Clearance of Persistent Hepatitis C Virus Infection by Direct-Acting Antiviral Drug Therapy. Front Immunol 2018;9:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Coscia M, Vitale C, Peola S, Foglietta M, Rigoni M, Griggio V, et al. Dysfunctional Vgamma9Vdelta2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012;120:3271–3279. [DOI] [PubMed] [Google Scholar]
- [45].Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, et al. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia 2005;19:664–670. [DOI] [PubMed] [Google Scholar]
- [46].Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the activation of human gamma delta T cells by tumor cells sensitized with nonpeptide antigens. J Immunol 2006;177:877–884. [DOI] [PubMed] [Google Scholar]
- [47].Liu Z, Guo B, Lopez RD. Expression of intercellular adhesion molecule (ICAM)-1 or ICAM-2 is critical in determining sensitivity of pancreatic cancer cells to cytolysis by human gammadelta-T cells: implications in the design of gammadelta-T-cell-based immunotherapies for pancreatic cancer. J Gastroenterol Hepatol 2009;24:900–911. [DOI] [PubMed] [Google Scholar]
- [48].Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of V gamma 9V delta 2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010;161:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yoshikawa T, Takahara M, Tomiyama M, Nieda M, Maekawa R, Nakatsura T. Large-scale expansion of gammadelta T cells and peptide-specific cytotoxic T cells using zoledronate for adoptive immunotherapy. Int J Oncol 2014;45:1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sugai S, Yoshikawa T, Iwama T, Tsuchiya N, Ueda N, Fujinami N, et al. Hepatocellular carcinoma cell sensitivity to Vgamma9Vdelta2 T lymphocyte-mediated killing is increased by zoledronate. Int J Oncol 2016;48:1794–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baumert TF, Juhling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med 2017;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


