Abstract
“COVID-19” is the word that certainly isn’t forgotten by everybody who lives in the first half of the twenty-first century. COVID-19, as a pandemic, has led many researchers from different biomedical fields to find solutions or treatments to manage the pandemic. However, no standard treatment for this disease has been discovered to date. Probably, preventing the severe acute respiratory infection form of COVID-19 as the most dangerous phase of this disease can be helpful for the treatment and reduction of the death rate. In this regard, mesenchymal stem cells (MSCs)-based immunomodulation treatment has been proposed as a suitable therapeutic approach and several clinical trials have begun. Recently, MSCs according to their immunomodulatory and regenerative properties attract attention in clinical trials. After the intravenous transplantation of MSCs, a significant population of cells accumulates in the lung, which they alongside immunomodulatory effect could protect alveolar epithelial cells, reclaim the pulmonary microenvironment, prevent pulmonary fibrosis, and cure lung dysfunction. Given the uncertainties in this area, we reviewed reported clinical trials and hypotheses to provide useful information to researchers and those interested in stem cell therapy. In this study, we considered this new approach to improve patient’s immunological responses to COVID-19 using MSCs and discussed the aspects of this proposed treatment. However, currently, there are no approved MSC-based approaches for the prevention and/or treatment of COVID-19 patients but clinical trials ongoing.
Keywords: COVID-19, Coronavirus, Mesenchymal stem cell, Stem cell therapy, Immunomodulatory, Clinical trials
Introduction
At the 2019 year’s end, numerous cases of severe respiratory infections were reported in Wuhan, China, and were initially thought to be a seasonal flu disease, given that some patients had a history of attending or working in the wholesale market for fish and seafood. The market was immediately shut down on January 1, and environmental sanitation and sanitation were fully implemented. A few days later, after rejecting the diagnosis of seasonal influenza, avian influenza, adenovirus, coronavirus, SARS, coronavirus, and other pathogens, on Jan. 1, the virus was declared a causative agent of the disease in four of the nine hospitalized patients: A new coronavirus that has a 5% genetic association with SARS and is a subset of Sarbecovirus [1]. Currently, the virus has been briefly named SARS-CoV-2 virus for further information and COVID-19, the name was given by the World Health Organization (WHO) to the SARS-CoV-2 virus-associated disease.
This disease has resulted in that clinicians and researchers from different branches of biomedicine were mobilized to find a solution or treatment for the management of this pandemic. According to a recent announcement of the International Society for Stem Cell Research (ISSCR), currently, there are no approved stem cell-based approaches for the prevention and treatment of COVID-19 infection. However, recently, mesenchymal stem cells (MSCs) have introduced one of the therapeutic approaches for using in the treatment of COVID-19 [2]. As we know, MSCs opposes viral infection due to the presence of specific cytokines improved qualities. These features are present in MSCs in the intrinsic niche before their separation process happens. Therefore, MSCs can be expected to survive even if they are transplanted into a patient with a confirmed COVID-19. Due to there is disagreement in MSCs therapy to treat COVID-19, we reviewed reported clinical trials and news to present helpful information to researchers and enthusiasts of the stem cell-based therapy field. In this study, we considered this proposed approach to improve patient’s immunological responses to COVID-19 using MSCs and discussed the aspects of this therapeutic approach.
SARS-CoV-2 and COVID-19
“COVID-19” the word that certainly it isn’t forgotten by everybody who lived in the first half of the twenty-first century. Coronavirus disease 2019 which known as COVID-19 is the result of one coronavirus infection in the name of SARS-CoV-2. Coronaviruses (CoV) are a large family of viruses that some of them are more known such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV), but some of them are not more known like Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 that previously known by the 2019 novel coronavirus (2019-nCoV), is a new strain of coronavirus that hasn’t been identified in humans up to late December of 2019. However, there are reports that demonstrate the SARS-CoV-2 virus originated from bats and then moving into camels, but its exact dynamics are currently unknown. Moreover, much of the pathogenesis information regards to SARS-CoV-2 is not fully known.
SARS-CoV-2 is from the Nidovirales order, a member of the genus ß-coronavirus (ß-CoV) [3]. ß-CoV includes five subgenus including in embecovirus (including HCoV-OC43 and HCoV-HKU1) [4], nobecovirus (including BtCoV-HKU9) [4], hibecovirus (including Bat Hp-betacoronavirus Zhejiang2013) [4], sarbecovirus (including SARSr-CoV and its strains such as SARS-CoV, SARS-CoV-2, and Bat SL-CoV-WIV1), merbecovirus (including Middle East respiratory syndrome (MERS)-CoV, BtCoV-HKU4, and BtCoV-HKU5) (https://talk.ictvonline.org/taxonomy/) [4, 5].
SARS-CoV-2 is enveloped, positive-sense, single-stranded RNA virus (with nucleocapsid) by 79.6% sequence identity the same to SARS-CoV [6] that that known as the largest discovered RNA viruses by approximately 30 kb in length genome structure. The genome sequence of SARS-CoV-2 has been submitted to GISAID (https://www.gisaid.org/; accession number: EPI_ISL_402124). It was first isolated using human airway epithelial cells [7] but can be isolated from the bronchoalveolar lavage fluid from a COVID-19 patient [6, 7]. Generally, both of the SARS-CoV and the SARS-CoV-2 are isolated and grew readily in Vero cells [7, 8] (a lineage of cells that was isolated from kidney epithelial cells of an African green monkey [9]). Also, this virus as SARS-CoV enters its host cell by binding to the angiotensin-converting enzyme 2 (ACE2) receptor [10, 11].
On March 11, 2020, the World Health Organization (WHO) characterized the spread of COVID-19 as a pandemic that it has caused unreasonable fear and led to unnecessary suffering and death [12]. To date (29 March 2020), according to the Worldomete site (https://www.worldometers.info/coronavirus/) report more than 199 countries and territories around the world have been affected, with major outbreaks respectively in the USA, Italy, central China, Spain, Germany, and Iran. The mortality rate of COVID-19 has been reported from 0.7% [13] to 15·2% [14] according to different studies in diverse territories and countries. As well, its maximum incubation period has been assumed 2 weeks [15] to 8 weeks [14, 16]. COVID-19 has resulted in that many researchers from different branches of biomedicine were attracted to find a solution or treatment for the management of this pandemic. However, to date haven’t been discovered the standard cure for this disease.
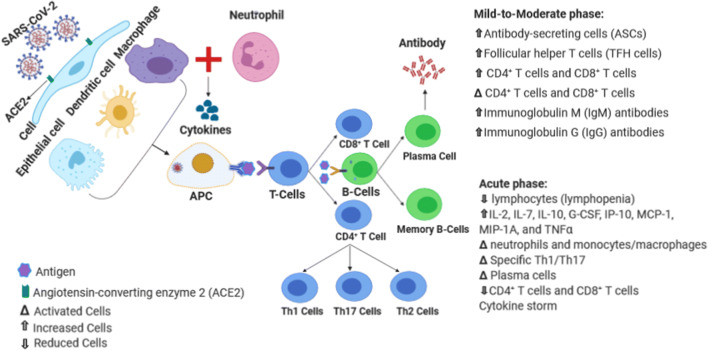
Several studies have shown that the first stage of the pathogenesis of this type of virus is the identification of angiotensin-converting enzyme-2 (ACE2) receptor by its spike protein [17]. For this reason, ACE2-positive cells are infected by this virus [17]. Another study has shown that the cellular protease TMRRSS2 is also required to allow the entry of coronavirus into host cells [18]. It is conceivable that the ACE2 receptor is widely distributed on the surface of human cells, especially alveolar type 2 (AT2) and capillary epithelium, and AT2 cells largely express TMPRSS2 [18]. On the other hand, interestingly, bone marrow, lymph nodes, thymus, spleen, and immune cells, such as T and B lymphocytes and macrophages are always negative for ACE2 (Fig. 1) [19]. These findings suggest that immunoglobulin therapy can help treat patients with the virus infection. Therefore, it should be noted that the capacity of the virus is greatly diminished by the cytokine-induced storm of the virus. The current hallmark of SARS-CoV-2 pathogenesis is the cytokine storm in the lung. Virally-triggered acute cytokine release of GSCF, IP10, MCP1, MIP1A, IL-2, IL-6, IL-7, and TNF results in pulmonary edema, dysfunction of air-exchange, acute respiratory distress syndrome (ARDS), and acute cardiac injury, and leading to death [2].
Fig. 1.
Schematic of host immune system responses during SARS-CoV-2 infection; Data obtained from [31, 32] (Figure is made with biorender: https://biorender.com/)
To date, there is no specific cure for Covid-19, although clinical management of these patients currently includes prevention or control of the infection and supportive care, including supplemental oxygen and mechanical ventilation support when needed. Recently, in the viral surface glycoprotein, several epitopes, including in 5 CTL epitopes, 3 sequential B cell epitopes, 5 discontinuous B cell epitopes of immune cells [20], and 13 MHC-I and 3 MHC-II antigenic epitopes [21], have been reported via immuno-informatics approach that some of these epitopes maybe had potential candidates for the development of 2019-nCoV vaccines.
A series of approved drugs for other symptoms is currently underway in clinical trials for these patients, including Chloroquine, Hydroxychloroquine, and Remdesivir worldwide. This because, safe, timely and effective supportive treatments are the inevitable principle in patients who develop severe manifestations of COVID19.
Mesenchymal Stem Cell Therapy
Currently, cell-based therapy and especially stem cell therapy has become a promising therapeutic field, in which many see opportunities to cure incurable diseases [22]. Despite the significant development of the stem cell-based therapy field, immunogenicity, limited cell source and ethical issue as the main limitations of this therapeutic approach have not been solved yet. Among these, MSCs has attracted attention due to source potential, a high proliferation rate, low invasive procedure, and free of ethical issues. There is much superiority in using MSC therapy in comparison with other treatments [23], including in I) They are easily accessible and can be isolated from various tissues such as bone marrow and adipose tissues, including in umbilical cord, dental pulp, menstrual-blood, buccal fat pad, fetal liver, etc.; II) They are multipotent stem cells; III) MSCs can easily expand to clinical volume in a suitable period of time; IV) MSCs can be stored for repetitive therapeutic usage; V) Clinical trials of MSCs so far haven’t shown adverse reactions to allogeneic MSC; VI) Safety and effectiveness of MSCs have been obviously documented in several clinical trials [23].
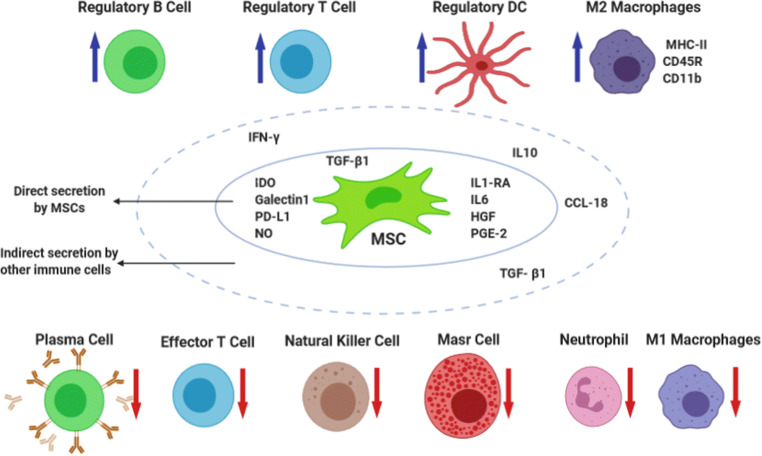
As mentioned, Following the COVID-19, may trigger a destroying immune overreaction in the body. In COVID-19 patients, the immune system produces large amounts of inflammatory factors, causing a cytokine storm including, in an overproduction of immune cells and cytokines [24]. Here, it is the beginning of the MSC therapy idea in the treatment of COVID-19 patients. Probably, MSC therapy can prevent the storm release of cytokines by the immune system and promote endogenous repair by reparative properties of the stem cells (Fig. 2).
Fig. 2.
Proposed interaction of MSCs with host immune cells and released cytocines; Data obtained from [33, 34], The figure is made with biorender (https://biorender.com/)
After intravenous injection, part of the MSC population entraps in the lung, which often in systemic infusion it is remembered as a limitation. But here these MSCs could recover the pulmonary microenvironment, protect alveolar epithelial cells, intercept pulmonary fibrosis, and cure lung dysfunction and COVID-19 pneumonia [25]. However, one of the main restrictions in this approach is the suppling source of clinical-grade MSCs and subsequently the speed of preparation for clinical usage that here stem cell banks can play an important role. Also, MSCs can be isolated from different adult tissues, including preferably bone marrow (BM), peripheral blood (PB) and adipose tissues (AT) (such as abdominal fat, infrapatellar fat pad, and buccal fat pad) and neonatal birth-associated tissues, including placenta (PL), umbilical cord (UC), Warton jelly (WJ), amniotic fluid (AF), and cord blood (CB), and then stored for future possible applications. Therefore, it seems MSCs-based therapy may possibly be an ideal candidate for clinical trials or at least the combination of treatment to treat COVID-19 patients.
MSC Clinical Trials for COVID-19
Recently, China, USA, Jordon, Iran, and several other countries have begun cell-based therapy clinical studies and some reports have been published (Table 1). Interestingly, one of the available methods to evaluate its efficacy in the maintenance or repair of damaged vital organs is the use of mesenchymal stem cell (MSCs) therapy that widely used in the treatment of type 2 diabetes, autoimmune disease, spinal cord injury, GVHD and several other diseases specially with high immunity rates have been used [23, 26–28]. MSCs, using their immunomodulatory properties and their differentiation ability, can prevent lung tissue death by counteracting the cytokine storm and regeneration and reconstruction of damaged tissues (Fig. 2). Recently, the use of these cells in the clinical treatment of H5N1 viral infections that have similar effects on the lung has also been suggested [29]. In addition, recently a case study was reported in China on a female patient with an acute COVID19 syndrome that the results of laboratory tests and CT images provided extremely effective results after 21 days of treatment with umbilical cord MSCs. A recent case study of a case report of a 65-year-old female patient diagnosed in critical condition with COVID-19, then identified the exact 2019nCoV variant now called SARS-CoV-2 [30]. The patient had a neutrophil increase of 87% and a lymphocyte decrease of 9.8% and was treated with antiviral drugs such as lopinavir / ritonavir, IFN-α and oseltamivir as well as intravenous injection of moxifloxacin, Xuebijing, methylprednisolone and immunoglobulin. The patient was also subjected to non-invasive mechanical ventilation to facilitate breathing and relieve muscle fatigue due to poor oxygenation. As the vital signs worsened, the patient was treated with cord MSCs alone and with α1 thymosin 5 × 107 cells each three times. The results of the study showed that after the second injection, serum albumin, CRP, and ALT / AST gradually decreased, as well as other vital signs improved. Thereafter, the patient was removed from the ventilator and able to walk, and the number of white blood cells and neutrophils in the patient decreased to a normal level, while the number of lymphocytes increased to their normal level. Most importantly, CD3+ T cell, CD4+ T cell and CD8+ T cell numbers were significantly increased. Also, the qualitative results obtained from CT images after the second and third injections of cord stem cells showed that the pneumonia was very relieved, 2 days after the third injection the patient was discharged from the ICU ward and most of the vital signs and clinical laboratory parameters in the They were normal. The results suggested that umbilical cord mesenchymal stem cells could be an ideal treatment option alone or in combination with other immune modulators for acute COVID-19 patients [30]. In another study released recently in China and in collaboration with the United States, 7 patients with COVID19 pneumonia in Beijing YouAn Hospital from January 23 to February 16 underwent mesenchymal stem cell transplantation and clinical manifestations, changes in immune function levels [25]. Also, inflammation was assessed within 14 days after transplantation. The results showed that the clinical symptoms of all patients improved significantly 2 days after stem cell transplantation. Among the patients studied, one was very acute and two patients with milder conditions were discharged from the hospital 10 days after transplantation. Their results also showed that peripheral lymphocyte levels increased, activated cytokine-secreting immune cells, such as CXCR3+ CD4+ T cells, CXCR3+ CD8+ T cells and NK CXCR3+ cells disappeared on day 6–6. A group of CD14+ CD11c+ CD11bmid regulatory DC cell populations also increased dramatically. At the same time, TNF-α levels were significantly decreased, whereas IL-10 was increased in patients treated with MSCs compared with patients treated with conventional therapy. In addition, gene expression profiling of mesenchymal stem cells showed that these cells are ACE2- and TMPRSS2-, which showed that mesenchymal stem cells are free of COVID19 infection [25]. Therefore, they concluded that MSCs would be safe and effective for treating patients with COVID19 pneumonia, especially for patients with very acute conditions.
Table 1.
List of registered Cell-based clinical trials for treating COVID-19
| Clinical trial No | Cell Source | Ref |
|---|---|---|
| ChiCTR2000031319 | Allogeneic Human Dental Pulp- MSC | http://www.chictr.org.cn |
| ChiCTR2000031139 | Human embryonic stem cell-derived M cells (CAStem) | |
| ChiCTR2000030944 | human NK cells and MSCs transplantation | |
| ChiCTR2000030509 | NK Cells | |
| ChiCTR2000030329 | Umbilical cord blood CIK and NK cells | |
| ChiCTR2000030300 | human umbilical cord-MSCs (huc-MSCs) | |
| ChiCTR2000030224 | MSCs | |
| ChiCTR2000030173 | hUC-MSCs | |
| ChiCTR2000031319 | Human MSCs | |
| ChiCTR2000030088 | Umbilical cord Wharton’s Jelly derived-MSC | |
| ChiCTR2000030020 | MSCs | |
| ChiCTR2000029816 | hUCB-MSCs | |
| ChiCTR2000029812 | Umbilical cord blood mononuclear cells | |
| ChiCTR2000029606 | human Menstrual blood-derived stem cells | |
| ChiCTR2000029580 | Ruxolitinib in combination with MSCs | |
| ChiCTR2000029572 | Umbilical cord blood mononuclear cells | |
| ChiCTR2000029569 | Umbilical cord blood mononuclear cells | |
| CTR2000030116 | hUC-MSCs | |
| ChiCTR2000030484 | HU-MSCs and Exosomes | |
| ChiCTR2000030866 | hUC-MSCs | |
| ChiCTR2000030835 | hUC-MSCs | |
| ChiCTR2000030138 | hUC-MSCs | |
| NCT04313322 | Wharton’s Jelly derived-MSC | https://clinicaltrials.gov |
| NCT04315987 | NestCell®-MSC | |
| NCT04302519 | Dental Pulp- MSCs | |
| NCT04288102 | MSCs | |
| NCT04273646 | UC-MSC | |
| NCT04252118 | MSCs | |
| NCT04299152 | MSCs | |
| NCT04269525 | UC-MSCs | |
| NCT04276987 | MSCs-derived exosomes |
Conclusion
Immunomodulatory and anti-inflammatory properties of MSCs in the treatment of respiratory diseases were confirmed by 17 completed clinical studies, and also more than 70 trials are registered in this regard (https://clinicaltrials.gov). To date, 20 clinical trials have been registered in the Chinese clinical trial registry site (http://www.chictr.org.cn). In addition, 9 clinical trials have been registered in Clinicaltrial.gov. Umbilical cord, umbilical cord blood, Wharton’s jelly, menstrual blood, dental pulp, and the company produced-MSCs are the important MSC sources that will be used in these trials. However, the process of developing new therapeutic and bringing it to clinical application has important practical implications and not over for MSC therapy of COVID-19. However, the cost-effective and speed of therapeutic preparation are the capable discussed topic for MSC-based therapy for COVID-19, but certainly, the life of a human is more worthy and COVID-19 is so dangers. Therefore, the clinical use of MSCs therapy to treat COVID-19 is still some time away, but there are some promising reports to apply. Stem cell therapy and especially MSCs may possibly be is one of the most ideal therapeutics, or a combination of treatment to treat COVID-19 patients. However, scientists are trying incessantly to develop a vaccine for COVID-19, as well as therapeutics to treat this disease.
Authors’ Contributions
A.G. conceptualized the outline and topic of the article. All authors participated in designing the study, drafting, writing and editing the manuscript, and approving it for submission.
Compliance with Ethical Standards
Conflict of Interest
We have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Golchin, Email: agolchin.vet10@yahoo.com.
Abdolreza Ardeshirylajimi, Email: r.ardeshiry.62@gmail.com.
References
- 1.Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang Y-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalfe Su M. Mesenchymal stem cells and management of COVID-19 pneumonia. Medicine in Drug Discovery. 2020;5:100019. doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nidovirales. (2012). In Virus taxonomy (pp. 784–794). Elsevier. 10.1016/b978-0-12-384684-6.00066-5.
- 4.Wong Antonio, Li Xin, Lau Susanna, Woo Patrick. Global Epidemiology of Bat Coronaviruses. Viruses. 2019;11(2):174. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo Patrick C. Y., Wang Ming, Lau Susanna K. P., Xu Huifang, Poon Rosana W. S., Guo Rongtong, Wong Beatrice H. L., Gao Kai, Tsoi Hoi-wah, Huang Yi, Li Kenneth S. M., Lam Carol S. F., Chan Kwok-hung, Zheng Bo-jian, Yuen Kwok-yung. Comparative Analysis of Twelve Genomes of Three Novel Group 2c and Group 2d Coronaviruses Reveals Unique Group and Subgroup Features. Journal of Virology. 2006;81(4):1574–1585. doi: 10.1128/JVI.02182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F., Tan Wenjie. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park, W. B., Kwon, N. J., Choi, S. J., Kang, C. K., Choe, P. G., Kim, J. Y., … Oh, M. D. (2020). Virus isolation from the first patient with SARS-CoV-2 in Korea. J Korean Med Sci, 35(7 PG-84–84), e84–e84. 10.3346/jkms.2020.35.e84. [DOI] [PMC free article] [PubMed]
- 9.Govorkova EA, Murti G, Meignier B, de Taisne C, Webster RG. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. Journal of Virology. 1996;70(8):5519–5524. doi: 10.1128/jvi.70.8.5519-5524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann, M., Kleine-Weber, H., Krüger, N., Müller, M., Drosten, C., & Pöhlmann, S. (2020). The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv, 2020.01.31.929042. 10.1101/2020.01.31.929042.
- 11.Li F, Li W, Farzan M, Harrison SC. Structural biology: Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 12.WHO. (2020). WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Retrieved March 14, 2020, from https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020.
- 13.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. The Lancet Global Health. 2020;0(C):30068. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baud, D., Qi, X., Nielsen-Saines, K., Musso, D., Pomar, L., & Favre, G. (2020). Real estimates of mortality following COVID-19 infection. The Lancet Infectious Diseases, 0(0). 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed]
- 15.(January 2020). Backer, J. A., Klinkenberg, D., & Wallinga, J. (2020). Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 25(5). 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed]
- 16.WHO. (2020). Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed Feb 2020.
- 17.Rothan Hussin A., Byrareddy Siddappa N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of Autoimmunity. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann Markus, Kleine-Weber Hannah, Schroeder Simon, Krüger Nadine, Herrler Tanja, Erichsen Sandra, Schiergens Tobias S., Herrler Georg, Wu Nai-Huei, Nitsche Andreas, Müller Marcel A., Drosten Christian, Pöhlmann Stefan. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. Journal of Medical Virology. 2020;92(5):495–500. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya Manojit, Sharma Ashish R., Patra Prasanta, Ghosh Pratik, Sharma Garima, Patra Bidhan C., Lee Sang‐Soo, Chakraborty Chiranjib. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): Immunoinformatics approach. Journal of Medical Virology. 2020;92(6):618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golchin A, Farahany TZ. Biological products: Cellular therapy and FDA approved products. Stem Cell Reviews and Reports. 2019;15(2):1–10. doi: 10.1007/s12015-018-9866-1. [DOI] [PubMed] [Google Scholar]
- 23.Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A. The clinical trials of Mesenchymal stem cell therapy in skin diseases: An update and concise review. Current Stem Cell Research & Therapy. 2018;14(1):22–33. doi: 10.2174/1574888x13666180913123424. [DOI] [PubMed] [Google Scholar]
- 24.Mehta Puja, McAuley Daniel F, Brown Michael, Sanchez Emilie, Tattersall Rachel S, Manson Jessica J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng Zikuan, Zhu Rongjia, Hou Wei, Feng Yingmei, Yang Yanlei, Han Qin, Shan Guangliang, Meng Fanyan, Du Dongshu, Wang Shihua, Fan Junfen, Wang Wenjing, Deng Luchan, Shi Hongbo, Li Hongjun, Hu Zhongjie, Zhang Fengchun, Gao Jinming, Liu Hongjian, Li Xiaoxia, Zhao Yangyang, Yin Kan, He Xijing, Gao Zhengchao, Wang Yibin, Yang Bo, Jin Ronghua, Stambler Ilia, Lim Lee Wei, Su Huanxing, Moskalev Alexey, Cano Antonio, Chakrabarti Sasanka, Min Kyung-Jin, Ellison-Hughes Georgina, Caruso Calogero, Jin Kunlin, Zhao Robert Chunhua. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and disease. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golchin Ali, Shams Forough, Karami Farshid. Advances in Experimental Medicine and Biology. New York, NY: Springer US; 2019. Advancing Mesenchymal Stem Cell Therapy with CRISPR/Cas9 for Clinical Trial Studies. [DOI] [PubMed] [Google Scholar]
- 27.Novello S, Debouche A, Philippe M, Naudet F, Jeanne S. Clinical application of mesenchymal stem cells in periodontal regeneration: A systematic review and meta-analysis. Journal of Periodontal Research. 2020;55(1):1–12. doi: 10.1111/jre.12684. [DOI] [PubMed] [Google Scholar]
- 28.Zhao, K., & Liu, Q. (2016, May 18). The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. Journal of Hematology and Oncology. BioMed Central Ltd.10.1186/s13045-016-0276-z. [DOI] [PMC free article] [PubMed]
- 29.Chen, J., Hu, C., Chen, L., Tang, L., Zhu, Y., Xu, X., et al. (2020). Clinical study of Mesenchymal stem cell treatment for acute respiratory distress syndrome induced by Epidemic Influenza A (H7N9) infection: A hint for COVID-19 treatment. Engineering.10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed]
- 30.Bing Liang, Junhui Chen, Tao Li, Haiying Wu, Wenjie Yang, Yanjiao Li, J., Li, Congtao Yu, Fangang Nie, Zhaoxia Ma, Mingxi Yang, Panrong Nie, Y. G., & Chuanyun Qian, M. H. (2020). Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord. chinaXiv, 10.12074/202002.00084. [DOI] [PMC free article] [PubMed]
- 31.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific journal of allergy and immunology. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 32.Huang Chaolin, Wang Yeming, Li Xingwang, Ren Lili, Zhao Jianping, Hu Yi, Zhang Li, Fan Guohui, Xu Jiuyang, Gu Xiaoying, Cheng Zhenshun, Yu Ting, Xia Jiaan, Wei Yuan, Wu Wenjuan, Xie Xuelei, Yin Wen, Li Hui, Liu Min, Xiao Yan, Gao Hong, Guo Li, Xie Jungang, Wang Guangfa, Jiang Rongmeng, Gao Zhancheng, Jin Qi, Wang Jianwei, Cao Bin. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss, A. R. R., & Dahlke, M. H. (2019). Immunomodulation by Mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Frontiers in Immunology, 10(JUN), 1–10. 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed]
- 34.Glenn JD. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World Journal of Stem Cells. 2014;6(5):526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]