Abstract
BACKGROUND
Intrahepatic cholangiocarcinoma (ICC) is a heterogeneous hepatobiliary cancer with limited treatment options. A number of studies have illuminated the relationship between inflammation-based prognostic scores and outcomes in patients with ICC. However, the use of reliable and personalized prognostic algorithms in ICC after resection is pending.
AIM
To assess the prognostic value of the gamma-glutamyltransferase to lymphocyte ratio (GLR) in ICC patients following curative resection.
METHODS
ICC patients following curative resection (2009-2017) were divided into two cohorts: The derivation cohort and validation cohort. The derivation cohort was used to explore an optimal cut-off value, and the validation cohort was used to further evaluate the score. Overall survival (OS) and recurrence-free survival (RFS) were analyzed, and predictors of OS and RFS were determined.
RESULTS
A total of 527 ICC patients were included and randomly divided into the derivation cohort (264 patients) and the validation cohort (263 patients). The two patient cohorts had comparable baseline characteristics. The optimal cut-off value for the GLR was 33.7. Kaplan-Meier curves showed worse OS and RFS in the GLR > 33.7 group compared with GLR ≤ 33.7 group in both cohorts. After univariate and multivariate analysis, the results indicated that GLR was an independent prognostic factor of OS [derivation cohort: hazard ratio (HR) = 1.620, 95% confidence interval (CI): 1.066-2.462, P = 0.024; validation cohort: HR = 1.466, 95%CI: 1.033-2.142, P = 0.048] and RFS [derivation cohort: HR = 1.471, 95%CI: 1.029-2.103, P = 0.034; validation cohort: HR = 1.480, 95%CI: 1.057-2.070, P = 0.022].
CONCLUSION
The preoperative GLR is an independent prognostic factor for ICC patients following hepatectomy. A high preoperative GLR is associated with worse OS and RFS.
Keywords: Gamma-glutamyltransferase, Lymphocyte ratio, Gamma-glutamyltransferase to lymphocyte ratio, Intrahepatic cholangiocarcinoma, Prognosis, Survival analysis
Core tip: This study investigated the clinical significance of preoperative gamma-glutamyltransferase to lymphocyte ratio (GLR) levels in intrahepatic cholangiocarcinoma (ICC) patients following hepatectomy. We retrospectively enrolled 527 ICC patients underwent curative hepatectomy at our center. The results showed that a higher GLR is associated with worse overall survival and recurrence-free survival in ICC patients after hepatectomy. Thus, the preoperative GLR is an independent prognostic factor for ICC patients following curative resection.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC), the second most type of common biliary malignancy, is a rare epithelial malignancy that results in poor prognosis[1]. An increasing incidence of ICC has been reported worldwide over the last few decades[2]. Surgical resection and liver transplantation may be the potentially curative method for patients with ICC. However, contemporary studies do not support the choice of liver transplantation for ICC patients as the preferred treatment. Despite advances in early detection and surgical techniques, the 5-year survival rate of ICC after curative resection is approximately 30%[3]. This poor outcome is mainly caused by tumor recurrence and metastasis[4-6]. Furthermore, the discovery of effective blood biomarkers or prognostic models for recurrence and survival in patients with ICC following curative resection remains an unmet need.
Accumulating evidence has demonstrated that a disordered inflammatory response plays an important role in carcinogenesis or tumor recurrence[7,8]. Recently, studies have suggested that inflammation-based prognostic indexes such as the platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR) or aspartate transaminase to lymphocyte ratio index can predict the risk and prognosis of various solid tumors; the gamma-glutamyltransferase (GGT) to lymphocyte ratio (GLR) is one of these prognostic indexes[9-12]. Previous studies have reported that the preoperative GLR has significant prognostic value for patients with nonfunctional pancreatic neuroendocrine tumor after curative resection[11]. However, the relationship between GLR and the prognosis of ICC patients following curative resection has not been reported.
Clinical practice needs to enhance the understanding of factors that underlie differences in prognosis observed among patients and to distinguish patients with a risk of recurrence for the development of personalized therapeutic approaches. Thus, we performed a retrospective study to assess the prognostic value of the GLR for ICC patients after curative resection.
MATERIALS AND METHODS
Patients
The study enrolled patients who underwent hepatic resection for ICC, between January 2009 and September 2017, at the West China Hospital of Sichuan University. This study was approved by the Medical Ethics Committee of the West China Hospital of Sichuan University. The included patients in this study met the following criteria: (1) Histologically confirmed ICC; and (2) Received curative hepatectomy. The exclusion criteria were as follows: (1) ICC accompanied by other lethal diseases or cancers; (2) Patients who received chemotherapy and/or radiotherapy; and (3) Metastasis. The patients were divided into a cohort and validation cohort. The derivation group was used to generate an optimal cut-off value, and the validation group was used to evaluate this cut-off value.
Follow up and data extraction
The follow-up time ended in October 2018. Informed consent was obtained from all patients. For each patient enrolled in our analysis, the following data were collected using electronic patient medical records: Age, sex, histological features, hepatitis virus infection, Child-Pugh levels, tumor-node-metastasis (TNM) stage, Barcelona Clinic Liver Cancer (BCLC) stage, preoperative serum GGT levels and neutrophil, platelet, and lymphocyte counts.
Statistical analysis
The primary and secondary endpoints were overall survival (OS) and recurrence-free survival (RFS), respectively. OS was defined as the time in months from resection to death or to the last follow-up. RFS was defined as the time in months from resection to recurrence or to the last follow-up. The NLR, PLR, and GLR were calculated by relevant laboratory parameters. The cut-off points for the inflammation-based prognostic scores were identified using receiver operating characteristic curve analysis, which was performed in the derivation cohort. Based on the optimal cut-off points, including the GLR, we divided the derivation and validation cohorts into two subgroups. Continuous variables were analyzed using the Student’s t-test or Kruskal-Wallis test, and categorical variables were analyzed using Fisher’s exact test or the χ2 test. Univariate and multivariate analyses were conducted using logistic regression models. Variables with P values less than 0.10 in univariate regression analyses were selected for multivariate regression models. P < 0.05 was considered statistically significant. All analyses were performed using SPSS® software (version 23.0; Chicago, IL, United States) and Medcalc software (version 15.2.2.0; Ostend, Belgium).
RESULTS
Patient population
Of the 527 patients enrolled, 254 (48.2%) were men and 277 (51.8%) were women (Supplementary Table 1). These patients were diagnosed at a mean age of 57.26 ± 10.71 years and underwent a mean follow-up of 25 mo. Serum hepatitis B surface antigen (HBsAg) was positive in 151 patients (28.8%), hepatitis C virus infected 3 patients (0.6%) and hepatolithiasis existed in 88 patients (16.7%). Among them, 24 (4.6%) patients had Child-Pugh grade B liver function. Ascites existed in 50 patients (9.5%). Serum carbohydrate antigen 19-9 (CA19-9) < 22 U/mL were observed in 149 patients (28.3%). The mean value of NLR, PLR, and GLR were 2.73, 113, and 44.7, respectively. The numbers of patients classified into TNM stage IA, IB, II, IIIA, and IIIB were 63 (12%), 37 (7%), 55 (10.4%), 241 (45.7%), and 131 (24.9%), respectively. The numbers of patients classified into BCLC stage 0, A, B and C were 23 (4.4%), 141 (26.8%), 240 (45.5%), and 123 (23.3%), respectively. These patients were divided into a derivation cohort (n = 264) and a validation cohort (n = 263). There were no differences in sex, age, hepatitis virus infection, Child-Pugh grade, presence of ascites or serum CA19-9 levels (all P > 0.05). In addition, there were also no differences in histological features or the number of patients classified into TNM stages or BCLC stages (all P > 0.05).
Determination of optimal cut-off value
Using the 2-year overall survival rate as an endpoint, the optimal cut-off values were confirmed using receiver operating characteristic curve analyses. For all 264 ICC patients in the derivation cohort, a GLR cut-off value equal to 33.7 provided the best separation of the survival curves between the two groups. In addition, the cut-off values of the NLR and PLR were 2.62 and 103, respectively.
Relationships between GLR and patient characteristics
According to the cut-off value, 264 patients in the derivation cohort and 263 patients in the validation cohort were divided into the GLR > 33.7 group and the GLR ≤ 33.7 group, respectively. In the derivation cohort, the preoperative GLR was correlated with sex (P = 0.024), CA19-9 level (P = 0.005), tumor size (P < 0.001), solitary tumor (P = 0.001), macrovascular (P = 0.011) and microvascular invasion (MVI) (P = 0.026), node-positive (P = 0.005), perineural invasion (P = 0.044), TNM stage (P = 0.002), and BCLC stage (P < 0.001) (Table 1). Somewhat differently, in the validation cohort, the preoperative GLR was correlated with sex (P = 0.03), CA 19-9 level (P = 0.043), tumor size (P = 0.007), macrovascular invasion (P < 0.001), perineural invasion (P = 0.009), and BCLC stage (P < 0.001). In addition, no significant differences were observed between preoperative GLR and other clinicopathological variables such as age, HBsAg, hepatolithiasis, Child-Pugh grade B, ascites, well-differentiated tumors, MVI, liver capsule invasion, and cirrhosis (all P > 0.05).
Table 1.
Correlation between gamma-glutamyltransferase to lymphocyte ratio and clinicopathological characteristics in intrahepatic cholangiocarcinoma
| Variables |
Derivation |
P value |
Validation |
P value | ||
| GLR ≤ 33.7 | GLR > 33.7 | GLR ≤ 33.7 | GLR > 33.7 | |||
| Total patients | 107 | 157 | 97 | 166 | ||
| Age, yr | 57.96 (11.48) | 57.80 (10.35) | 0.902 | 56.97 (10.69) | 56.48 (10.56) | 0.716 |
| Male gender, n (%) | 42 (39.3) | 85 (54.1) | 0.024a | 38 (39.2) | 89 (53.6) | 0.030a |
| HBsAg, n (%) | 36 (33.6) | 37 (23.6) | 0.073 | 31 (32.3) | 47 (28.5) | 0.575 |
| Hepatolithiasis, n (%) | 14 (13.1) | 27 (17.2) | 0.396 | 15 (15.5) | 32 (19.3) | 0.511 |
| Child-Pugh grade B, n (%) | 3 (2.8) | 8 (5.1) | 0.533 | 2 (2.1) | 11 (6.6) | 0.141 |
| Ascites, n (%) | 6 (5.6) | 21 (13.4) | 0.061 | 4 (4.1) | 19 (11.4) | 0.068 |
| CA-199 < 22, n (%) | 38 (36.5) | 42 (27.5) | 0.005a | 29 (31.2) | 40 (24.2) | 0.043a |
| Tumor size, cm | 5.14 (2.29) | 6.51 (2.78) | < 0.001a | 5.40 (2.22) | 6.36 (2.97) | 0.007a |
| Solitary tumor, n (%) | 89 (83.2) | 102 (65.0) | 0.001a | 67 (69.1) | 113 (68.1) | 0.895 |
| Well tumor differentiation, n (%) | 6 (5.6) | 4 (2.5) | 0.331 | 5 (5.2) | 7 (4.2) | 0.767 |
| Macrovascular invasion, n (%) | 17 (15.9) | 45 (28.7) | 0.011a | 8 (8.2) | 53 (31.9) | < 0.001a |
| Microvascular invasion, n (%) | 6 (5.6) | 23 (14.6) | 0.026a | 7 (7.2) | 17 (10.2) | 0.514 |
| Liver capsule invasion, n (%) | 67 (62.6) | 107 (68.2) | 0.362 | 64 (66.0) | 97 (58.4) | 0.248 |
| Node-positive, n (%) | 16 (15.0) | 48 (30.6) | 0.005a | 18 (18.6) | 47 (28.3) | 0.107 |
| Perineural invasion, n (%) | 9 (8.4) | 27 (17.2) | 0.044a | 8 (8.2) | 35 (21.1) | 0.009a |
| Cirrhosis, n (%) | 27 (25.2) | 53 (33.8) | 0.174 | 19 (19.6) | 46 (27.7) | 0.182 |
| TNM stage, n (%) | 0.002a | 0.107 | ||||
| IA | 23 (21.5) | 12 (7.6) | 13 (13.4) | 15 (9.0) | ||
| IB | 6 (5.6) | 12 (7.6) | 6 (6.2) | 13 (7.8) | ||
| II | 6 (5.6) | 13 (8.3) | 10 (10.3) | 26 (15.7) | ||
| IIIA | 55 (51.4) | 72 (45.9) | 50 (51.5) | 64 (38.6) | ||
| IIIB | 17 (15.9) | 48 (30.6) | 18 (18.6) | 48 (28.9) | ||
| BCLC stage, n (%) | < 0.001a | < 0.001a | ||||
| 0 | 6 (5.6) | 9 (5.7) | 3 (3.1) | 5 (3.0) | ||
| A | 44 (41.1) | 25 (15.9) | 34 (35.1) | 38 (22.9) | ||
| B | 40 (37.4) | 78 (49.7) | 52 (53.6) | 70 (42.2) | ||
| C | 17 (15.9) | 45 (28.7) | 8 (8.2) | 53 (31.9) | ||
P < 0.05. HBV: Hepatitis B virus; CA-199: Carbohydrate antigen-199; TNM: Tumor-node-metastasis; BCLC: BARCELONA Clinic Liver Cancer; GLR: Gamma-glutamyltransferase to lymphocyte ratio; ICC: Intrahepatic cholangiocarcinoma; HR: Hazard ratio; CI: Confidence interval.
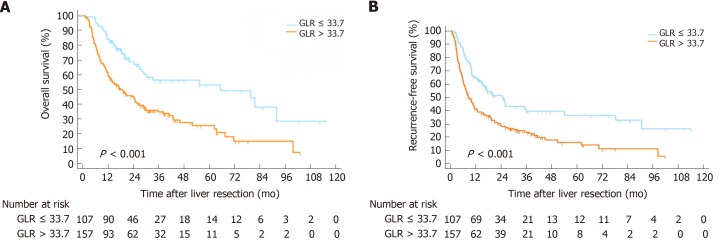
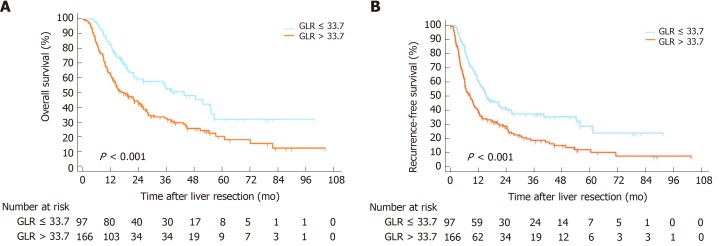
Kaplan-Meier curves
The Kaplan-Meier curves suggested that the OS rates of the GLR > 33.7 group were markedly shorter than those of the GLR ≤ 33.7 group (P < 0.001) (Figure 1A), while the RFS rates of the GLR > 33.7 group were also shorter than those of the GLR ≤ 33.7 group in the derivation cohort (P < 0.001) (Figure 1B). The poorer outcome of those with GLR > 33.7 was demonstrated in the validation cohort, as those patients had shorter OS (P < 0.001) and RFS (P < 0.001) (Figure 2).
Figure 1.
Kaplan-Meier survival curves and risk tables for overall survival and recurrence-free survival in the derivation cohort. A: Gamma-glutamyltransferase to lymphocyte ratio > 33.7 was correlated with shorter overall survival; B: Recurrence-free survival in intrahepatic cholangiocarcinoma patients following curative resection. OS: Overall survival; RFS: Recurrence-free survival; GLR: Gamma-glutamyltransferase to lymphocyte ratio; ICC: Intrahepatic cholangiocarcinoma.
Figure 2.
Kaplan-Meier survival curves and risk tables for overall survival and recurrence-free survival in the validation cohort. A: Gamma-glutamyltransferase to lymphocyte ratio > 33.7 was correlated with shorter overall survival; B: Recurrence-free survival in intrahepatic cholangiocarcinoma patients undergoing curative resection. OS: Overall survival; RFS: Recurrence-free survival; GLR: Gamma-glutamyltransferase to lymphocyte ratio; ICC: Intrahepatic cholangiocarcinoma.
Previous studies have reported on the prognostic significance of NLR and PLR in ICC patients[13,14]. Therefore, based on the cutoff value of NLR, two cohorts were divided into the NLR ≤ 2.62 group and NLR > 2.62 group, respectively. The Kaplan-Meier curves showed that the OS rates of the NLR > 2.62 group were shorter than those of the NLR ≤ 2.62 group (derivation cohort: P = 0.002; validation cohort: P = 0.002) (Supplementary Figure 1), while the RFS rate of the NLR > 2.62 group was also shorter than that of the NLR ≤ 2.62 group (derivation cohort: P = 0.026; validation cohort: P < 0.001) (Supplementary Figure 2) in both cohorts. In the same way, two cohorts were divided into the PLR ≤ 103 group and PLR > 103 groups. However, PLR > 103 was associated with worse OS in the validation cohort but not in the derivation cohort (derivation cohort: P = 0.063; validation cohort: P = 0.028) (Supplementary Figure 3). Meanwhile, PLR > 103 was associated with a worse RFS in the derivation cohort but not in the validation cohort (derivation cohort: P = 0.043; validation cohort: P = 0.661) (Supplementary Figure 4).
Prognostic significance of GLR
Univariate analysis for OS revealed that preoperative GLR, CA19-9, tumor size, tumor number, MVI, node-positive, NLR, and PLR had P values of ≤ 0.10 in both cohorts (Tables 2 and 3). Similarly, univariate analysis for RFS identified that GLR, ascites, CA19-9 level, tumor size, tumor number, tumor differentiation, MVI, node-positive, TNM stage, BCLC stage, and NLR had P values of ≤ 0.10 in both cohorts (Tables 2 and 3).
Table 2.
Univariate analysis in the derivation cohort
| Variables |
Overall survival |
Recurrence-free survival |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 1.002 | 0.987-1.017 | 0.819 | 0.998 | 0.985-1.011 | 0.763 |
| Gender, F/M | 0.752 | 0.546-1.035 | 0.080a | 0.846 | 0.633-1.132 | 0.262 |
| HBsAg | 1.210 | 0.853-1.717 | 0.285 | 1.274 | 0.924-1.756 | 0.139 |
| Hepatolithiasis | 1.593 | 1.061-2.393 | 0.025a | 1.176 | 0.785-1.761 | 0.433 |
| Child-Pugh grade, A /B | 0.614 | 0.323-1.167 | 0.136 | 0.732 | 0.381-1.367 | 0.317 |
| Ascites | 1.639 | 1.011-2.658 | 0.045a | 1.532 | 0.988-2.376 | 0.056a |
| CA-199 (≥ 22/< 22) | 0.953 | 0.926-0.981 | 0.001a | 1.546 | 1.259-1.899 | < 0.001a |
| Tumor size | 1.075 | 1.012-1.141 | 0.019a | 1.123 | 1.063-1.187 | < 0.001a |
| Tumor number, Multiple/Single | 1.655 | 1.172-2.328 | 0.004a | 1.893 | 1.388-2.582 | < 0.001a |
| Tumor differentiation, Moderate-Poor/Well | 1.891 | 0.699-5.115 | 0.209 | 3.277 | 1.045-10.274 | 0.042a |
| Macrovascular invasion | 1.371 | 0.953-1.972 | 0.089a | 1.277 | 0.913-1.787 | 0.153 |
| Microvascular invasion | 1.619 | 1.029-2.548 | 0.037a | 1.983 | 1.306-3.012 | 0.001a |
| Liver capsule invasion | 0.815 | 0.586-1.134 | 0.225 | 1.140 | 0.833-1.561 | 0.412 |
| Node-positive | 2.846 | 2.030-3.989 | < 0.001a | 2.484 | 1.810-3.409 | < 0.001a |
| Perineural invasion | 1.737 | 1.129-2.673 | 0.012a | 1.245 | 0.821-1.887 | 0.302 |
| Cirrhosis | 1.526 | 1.094-2.130 | 0.013a | 1.182 | 0.863-1.617 | 0.297 |
| TNM stage, III/I-II | 1.067 | 0.745-1.529 | 0.722 | 1.431 | 1.010-2.028 | 0.044a |
| BCLC, B-C/0-A | 1.295 | 0.906-1.850 | 0.156 | 1.607 | 1.150-2.246 | 0.005a |
| NLR, > 2.62/≤ 2.62 | 1.701 | 1.221-2.371 | 0.002a | 1.422 | 1.057-1.912 | 0.020a |
| PLR, > 103/≤ 103 | 1.366 | 0.983-1.897 | 0.063a | 1.360 | 1.009-1.833 | 0.043a |
| GLR, > 33.7/≤ 33.7 | 2.316 | 1.617-3.316 | < 0.001a | 1.931 | 1.413-2.639 | < 0.001a |
P < 0.10. M: male; F: Female; HBV: Hepatitis B virus; CA-199: Carbohydrate antigen-199; TNM: Tumor-node-metastasis; BCLC: Barcelona Clinic Liver Cancer; NLR: Neutrophil to lymphocyte ratio; PLR: Platelet to lymphocyte ratio; GLR: Gamma-glutamyltransferase to lymphocyte ratio; HR: Hazard ratio; CI: Confidence interval.
Table 3.
Univariate analysis in the validation cohort
| Variables |
Overall survival |
Recurrence-free survival |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 0.996 | 0.981-1.011 | 0.594 | 0.990 | 0.976-1.004 | 0.148 |
| Gender, F/M | 0.907 | 0.667-1.233 | 0.534 | 0.920 | 0.695-1.218 | 0.560 |
| HBsAg | 1.004 | 0.717-1.405 | 0.982 | 1.110 | 0.819-1.505 | 0.500 |
| Hepatolithiasis | 1.092 | 0.744-1.602 | 0.654 | 0.799 | 0.551-1.158 | 0.236 |
| Child-Pugh grade, A /B | 0.852 | 0.435-1.671 | 0.642 | 0.751 | 0.418-1.350 | 0.339 |
| Ascites | 1.440 | 0.871-2.382 | 0.155 | 1.580 | 1.004-2.487 | 0.048a |
| CA-199, ≥ 22/< 22 | 1.744 | 1.390-2.188 | < 0.001a | 1.402 | 1.140-1.722 | 0.001a |
| Tumor size | 1.054 | 0.997-1.115 | 0.064a | 1.084 | 1.030-1.141 | 0.002a |
| Tumor number, Multiple/Single | 1.845 | 1.344-2.531 | < 0.001a | 1.874 | 1.397-2.513 | < 0.001a |
| Tumor differentiation, Moderate-Poor/Well | 13.449 | 1.881-96.150 | 0.010 | 6.053 | 1.930-18.982 | 0.002a |
| Macrovascular invasion | 1.050 | 0.730-1.511 | 0.792 | 1.009 | 0.718-1.418 | 0.959 |
| Microvascular invasion | 1.995 | 1.228-3.242 | 0.005a | 2.023 | 1.290-3.172 | 0.002a |
| Liver capsule invasion | 1.553 | 1.118-2.159 | 0.009a | 1.280 | 0.955-1.716 | 0.099a |
| Node-positive | 2.009 | 1.438-2.808 | < 0.001a | 1.525 | 1.115-2.085 | 0.008a |
| Perineural invasion | 1.366 | 0.906-2.059 | 0.136 | 1.262 | 0.867-1.838 | 0.225 |
| Cirrhosis | 0.984 | 0.686-1.412 | 0.932 | 1.054 | 0.762-1.459 | 0.749 |
| TNM stage, III/I-II | 1.774 | 1.242-2.533 | 0.002a | 1.413 | 1.035-1.929 | 0.029 |
| BCLC, B-C/0-A | 1.619 | 1.136-2.308 | 0.008a | 1.849 | 1.335-2.562 | < 0.001a |
| NLR, > 2.62/≤ 2.62 | 1.649 | 1.202-2.261 | 0.002a | 1.635 | 1.226-2.180 | 0.001a |
| PLR, > 103/≤ 103 | 1.417 | 1.036-1.938 | 0.029a | 1.065 | 0.803-1.414 | 0.661 |
| GLR, > 33.7/≤ 33.7 | 1.826 | 1.300-2.565 | 0.001a | 1.780 | 1.315-2.408 | < 0.001a |
P < 0.10. M: Male; F: Female; HBV: Hepatitis B virus; CA-199: Carbohydrate antigen-199; TNM: Tumor-node-metastasis; BCLC: Barcelona Clinic Liver Cancer; NLR: Neutrophil to lymphocyte ratio; PLR: Platelet to lymphocyte ratio; GLR: Gamma-glutamyltransferase to lymphocyte ratio; HR: Hazard ratio; CI: Confidence interval.
The results of multivariate analysis are shown in Table 4. The results indicated that the GLR was an independent predictor of OS [derivation cohort: hazard ratio (HR) = 1.620, 95% confidence interval (CI): 1.066-2.462, P = 0.024; validation cohort: HR = 1.466, 95%CI: 1.033-2.142, P = 0.048] and RFS (derivation cohort: HR = 1.471, 95%CI: 1.029-2.103, P = 0.048; validation cohort: HR = 1.480, 95%CI: 1.057-2.070, P = 0.022). In addition, CA19-9 was also demonstrated to be an independent predictor of OS and RFS in both cohorts (all P < 0.05).
Table 4.
Multivariate analysis of prognostic factors for overall survival and recurrence-free survival
| OS |
Derivation cohort (n = 264) |
Validation cohort (n = 263) |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender, F/M | 0.847 | 0.58-1.216 | 0.367 | - | ||
| Hepatolithiasis | 1.073 | 0.677-1.699 | 0.765 | - | ||
| Child-Pugh grade, A /B | 0.775 | 0.38-1.583 | 0.485 | - | ||
| Ascites | 0.758 | 0.428-1.339 | 0.340 | 0.640 | 0.376-1.090 | 0.100 |
| CA-199, ≥ 22/< 22 | 1.731 | 1.331-2.252 | < 0.001a | 1.612 | 1.252-2.075 | < 0.001a |
| Tumor size | 1.053 | 0.967-1.146 | 0.234 | 0.931 | 0.857-1.011 | 0.088 |
| Tumor number, Multiple/Single | 1.662 | 1.086-2.543 | 0.019a | 1.677 | 1.189-2.366 | 0.003a |
| Tumor differentiation, Moderate-Poor/Well | - | 7.927 | 1.083-58.02 | 0.042a | ||
| Macrovascular invasion | 1.199 | 0.781-1.843 | 0.407 | - | ||
| Microvascular invasion | 1.082 | 0.635-1.842 | 0.773 | 1.349 | 0.801-2.274 | 0.261 |
| Liver capsule invasion | - | 1.036 | 0.533-2.013 | 0.917 | ||
| Node- positive | 2.038 | 1.365-3.042 | < 0.001a | 1.269 | 0.835-1.928 | 0.265 |
| Perineural invasion | 1.252 | 0.773-1.116 | 0.169 | 0.973 | 0.591-1.603 | 0.914 |
| Cirrhosis | 1.589 | 1.074-2.351 | 0.020a | - | ||
| TNM stage, III/I-II | - | 1.294 | 0.607-2.761 | 0.505 | ||
| BCLC, B-C/0-A | 0.627 | 0.364-1.078 | 0.091 | 1.307 | 0.813-2.101 | 0.269 |
| NLR, > 2.62/≤ 2.62 | 1.357 | 0.912-2.017 | 0.132 | 1.287 | 0.897-1.846 | 0.170 |
| PLR, > 103/≤ 103 | 1.141 | 0.776-1.679 | 0.502 | 1.094 | 0.778-1.539 | 0.604 |
| GLR, > 33.7/≤ 33.7 | 1.620 | 1.066-2.462 | 0.024a | 1.466 | 1.033-2.142 | 0.048a |
| RFS | ||||||
| Age | - | 0.991 | 0.976-1.006 | 0.236 | ||
| HBsAg | 1.367 | 0.974-1.919 | 0.071 | - | ||
| Ascites | 0.763 | 0.473-1.230 | 0.266 | 0.621 | 0.382-1.010 | 0.055 |
| CA-199, ≥ 22/< 22 | 1.406 | 1.128-1.752 | 0.002a | 1.319 | 1.050-1.656 | 0.017a |
| Tumor size | 1.073 | 0.994-1.158 | 0.072 | 0.975 | 0.906-1.050 | 0.501 |
| Tumor number, Multiple/Single | 1.434 | 0.998-2.060 | 0.051 | 1.613 | 1.172-2.219 | 0.003a |
| Tumor differentiation, Moderate-Poor/Well | 2.068 | 0.635-6.734 | 0.228 | 3.617 | 1.114-11.741 | 0.032a |
| Macrovascular invasion | 1.149 | 0.785-1.682 | 0.475 | - | ||
| Microvascular invasion | 1.643 | 1.031-2.618 | 0.037a | 1.607 | 0.986-2.618 | 0.057 |
| Liver capsule invasion | - | 0.778 | 0.428-1.417 | 0.413 | ||
| Node-positive | 1.859 | 1.272-2.716 | 0.001a | 0.972 | 0.664-1.421 | 0.882 |
| TNM stage, III/I-II | 0.892 | 0.559-1.328 | 0.572 | 1.524 | 0.778-2.988 | 0.220 |
| BCLC, B-C/0-A | 0.784 | 0.480-1.280 | 0.331 | 1.339 | 0.859-2.087 | 0.198 |
| NLR, > 2.62/≤ 2.62 | 1.128 | 0.811-1.571 | 0.474 | 1.352 | 0.967-1.891 | 0.077 |
| PLR, > 103/≤ 103 | 1.125 | 0.809-1.565 | 0.483 | 0.805 | 0.589-1.100 | 0.173 |
| GLR, > 33.7/≤ 33.7 | 1.471 | 1.029-2.103 | 0.034a | 1.480 | 1.057-2.070 | 0.022a |
P < 0.05. OS: Overall survival; RFS: Recurrence-free survival; HBV: Hepatitis B virus; CA-199: Carbohydrate antigen-199; TNM: Tumor-node-metastasis; BCLC: Barcelona Clinic Liver Cancer; GLR: Gamma-glutamyltransferase to lymphocyte ratio; HR: Hazard ratio; CI: Confidence interval.
Prognostic values of GLR in different ICC subgroups
We further investigated the prognostic value of the GLR in various subgroups of ICC patients. The results suggested that the GLR was a prognostic factor for OS and RFS in patients age > 60 or ≤ 60, male or female, with or without cirrhosis, without ascites, with solitary tumor, with tumor size ≥ 5 cm or < 5 cm, with MVI-negative, with or without macrovascular invasion, with node-positive or node-negative, with or without perineural invasion, with BCLC stage 0-A or B-C and TNM stage I-II or III (all P < 0.05) (Figures 5 and 6). However, preoperative GLR was not a prognostic marker for OS and RFS in patients with ascites, multiple tumor and MVI-positive (all P > 0.05) (Figures 5 and 6).
DISCUSSION
It is clear that the poor prognosis and limited effective treatment options for ICC are common obstacles faced by clinicians[1,6]. Thus, a prognostic model to screen surgical patients with a high risk of recurrence or metastasis is of great value for developing additional personalized therapeutic approaches. The main finding of the present study relates to the identification of the GLR as a novel biomarker of prognosis in ICC patients undergoing curative resection. High preoperative GLR is associated with poor outcomes in patients with ICC after curative resection. In addition, we observed that a GLR > 33.7 was associated with the highly aggressive features of tumors, such as high CA19-9 levels and the presence of macrovascular invasion and perineural invasion.
GGT is a glycoprotein that is known as a marker of cardiovascular disease or bibulosity[15,16]. In addition, previous studies have confirmed the association of GGT with ICC and hepatocellular carcinoma[17-19]. It was previously reported that GGT plays a prooxidant role and is be associated with inflammation in carcinogenesis[20-22]. Similarly, lymphocytes can reflect systemic inflammation in various primary malignancies; thus, the lymphocyte count has been considered a prognostic predictor in patients with cancer. Accumulating evidence has highlighted the important role of systemic inflammation in tumor progression and aggressiveness[22,23]. In recent years, various inflammation-based scores have been considered as prognostic indicators in different solid cancers.
In 125 patients with nonfunctional pancreatic neuroendocrine tumor, the GLR was identified as an independent predictor for outcomes in multivariate analyses, with patients with preoperative a GLR > 10.3 demonstrating worse OS and disease-free survival compared with those with a GLR ≤ 10.3[11]. To the best of our knowledge, this is the first study to assess the prognostic significance of the GLR for ICC. The GLR index was developed using a cohort of 264 ICC patients and was validated in a validation cohort of 263 patients who underwent resection. There were no significant differences in the baseline characteristics. In this study, we first confirmed the optimal cut-off value of the preoperative GLR according to the receiver operating characteristic curve. We noticed that the elevated GLR was correlated with tumor size, the presence of macrovascular and perineural invasion and BCLC stage in both cohorts. Notably, all of these clinicopathological features indicated that the GLR might implicate the tumor burden. After further analysis, we identified that the GLR was a prognostic factor for OS and RFS in ICC patients after resection. Patients with a high GLR tended to have a poorer outcome. In addition, a high preoperative GLR could also predict worse OS and RFS in various subgroups. Hence, the preoperative GLR can be considered an independent prognostic factor for ICC patients after resection. Additionally, a high CA19-9 level could also act as an independent predictor of worse outcomes in ICC patients undergoing resection.
Previous studies have investigated the prognostic effects of the NLR and PLR in various cancers, including ICC[24-26]. These studies suggested that increased preoperative NLR and PLR values were independent risk factors for long-term outcomes. However, our results showed that NLR and PLR were not independent predictors of OS or RFS in ICC patients in our center.
There were several limitations in our study. First, the main limitation of this study is its retrospective nature. Second, the present study involved a single institution. Moreover, it identified the prognostic value of the GLR only in ICC patients who received curative resection. Given these limitations, future studies should include more centers and additional patients with various treatment modalities.
In conclusion, our study demonstrates that the preoperative GLR is an independent predictor of worse OS and RFS for ICC patients after resection. Therefore, as a readily available inflammatory marker, the preoperative GLR should be considered for incorporation into guiding selection of treatment methods by surgeons for ICC patients.
ARTICLE HIGHLIGHTS
Research background
Intrahepatic cholangiocarcinoma (ICC) is a heterogeneous hepatobiliary cancer with limited treatment options and has a high mortality. Therefore, it is important to probe effective biomarkers or prognostic models for ICC patients following hepatic resection at risk of recurrence or metastasis. Accumulating studies has found that a system inflammatory response is important in tumor progression and recurrence. However, it is not yet clear whether neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) or gamma-glutamyltransferase to lymphocyte ratio (GLR), can be used as a novel prognostic factor for ICC patients following hepatic resection.
Research motivation
Timely and effective establishment of prognostic models for ICC patients following curative resection is of great value for the long-term outcomes of these patients.
Research objectives
The main aim of our study was to examine the role of inflammation markers in ICC patients and evaluate the prognostic value of GLR in ICC patients following curative resection.
Research methods
We retrospectively enrolled ICC patients following curative resection between January 2009 and September 2017 at the West China Hospital of Sichuan University. The ICC patients were divided into a derivation cohort and a validation cohort. The derivation cohort was used to explore an optimal cut-off value, and the validation cohort was used to further evaluate the score.
Research results
In all, 527 ICC patients were included and divided into the derivation cohort (264 patients) and the validation cohort (263 patients). The two cohorts had comparable baseline characteristics. The optimal cut-off values for the NLR, PLR and GLR were 2.62, 103 and 33.7, respectively. The overall survival (OS) and recurrence-free survival (RFS) were shorter in the GLR > 33.7 group than GLR ≤ 33.7 group in both derivation cohort and validation cohort. Multivariate analysis revealed that the GLR was an independent predictor of OS [derivation cohort: hazard ratio (HR) = 1.620, 95% confidence interval (CI): 1.066-2.462, P = 0.024; validation cohort: HR = 1.466, 95%CI: 1.033-2.142, P = 0.048] and RFS (derivation cohort: HR = 1.471, 95%CI: 1.029-2.103, P = 0.048; validation cohort: HR = 1.480, 95%CI: 1.057-2.070, P = 0.022). Besides, CA19-9 also be demonstrated as an independent predictor of OS and RFS in both cohorts (all P < 0.05). However, our results showed that NLR and PLR were not independent predictors of OS or RFS in ICC patients in our center.
Research conclusions
The OS and RFS of ICC patients following curative resection are shorter in the GLR > 33.7 group than GLR ≤ 33.7 group. The preoperative GLR is an independent prognostic factor for ICC patients following hepatectomy. A high preoperative GLR is associated with worse OS and RFS.
Research perspectives
Because our study used a single-center retrospective design and enrolled limited patients. Future studies which included more centers and patients are needed to further verify our results.
Footnotes
Institutional review board statement: This work was reviewed and approved by the Ethics Committee of the West China Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare no conflicts of interest.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: November 30, 2019
First decision: January 16, 2020
Article in press: March 5, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farshadpour F, Talabnin C S-Editor: Zhang L L-Editor: Filipodia E-Editor: Ma YJ
Contributor Information
Jin-Ju Wang, Department of Liver Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Hui Li, Department of Liver Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Jia-Xin Li, Department of Liver Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Lin Xu, Laboratory of Liver Surgery, West China Hospital, Sichuan University, Chengdu 610065, Sichuan Province, China.
Hong Wu, Department of Liver Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Yong Zeng, Department of Liver Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China. zengyong@medmail.com.cn.
References
- 1.Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res. 2016;22:291–300. doi: 10.1158/1078-0432.CCR-14-3296. [DOI] [PubMed] [Google Scholar]
- 2.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90:817–837. doi: 10.1016/j.suc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Marusawa H, Endo Y, Chiba T. Inflammation-mediated genomic instability: roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–1206. doi: 10.1111/j.1349-7006.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Liu X, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin J Cancer. 2016;35:57. doi: 10.1186/s40880-016-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative gamma-glutamyltransferase to lymphocyte ratio index in nonfunctional pancreatic neuroendocrine tumors after curative resection. Sci Rep. 2017;7:13372. doi: 10.1038/s41598-017-13847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DS, Ren C, Qiu MZ, Luo HY, Wang ZQ, Zhang DS, Wang FH, Li YH, Xu RH. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol. 2012;33:749–756. doi: 10.1007/s13277-011-0285-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Wang H, Ning Z, Xu L, Zhuang L, Wang P, Meng Z. Prognostic nutritional index serves as a predictive marker of survival and associates with systemic inflammatory response in metastatic intrahepatic cholangiocarcinoma. Onco Targets Ther. 2016;9:6417–6423. doi: 10.2147/OTT.S112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Dai Z, Yin D, Yang LX, Wang Z, Xiao YS, Fan J, Zhou J. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore) 2015;94:e574. doi: 10.1097/MD.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 16.Dillon JF, Miller MH. Gamma glutamyl transferase 'To be or not to be' a liver function test? Ann Clin Biochem. 2016;53:629–631. doi: 10.1177/0004563216659887. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Liu S, Yi Y, Ni X, Wang J, Huang J, Fu Y, Cao Y, Zhou J, Fan J, Qiu S. Serum gamma-glutamyl transferase levels affect the prognosis of patients with intrahepatic cholangiocarcinoma who receive postoperative adjuvant transcatheter arterial chemoembolization: A propensity score matching study. Int J Surg. 2017;37:24–28. doi: 10.1016/j.ijsu.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Song P, Inagaki Y, Wang Z, Hasegawa K, Sakamoto Y, Arita J, Tang W, Kokudo N. High Levels of Gamma-Glutamyl Transferase and Indocyanine Green Retention Rate at 15 min as Preoperative Predictors of Tumor Recurrence in Patients With Hepatocellular Carcinoma. Medicine (Baltimore) 2015;94:e810. doi: 10.1097/MD.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, Meng X. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88:761–769. [PubMed] [Google Scholar]
- 20.Stark AA, Russell JJ, Langenbach R, Pagano DA, Zeiger E, Huberman E. Localization of oxidative damage by a glutathione-gamma-glutamyl transpeptidase system in preneoplastic lesions in sections of livers from carcinogen-treated rats. Carcinogenesis. 1994;15:343–348. doi: 10.1093/carcin/15.2.343. [DOI] [PubMed] [Google Scholar]
- 21.Everhart JE, Wright EC. Association of γ-glutamyl transferase (GGT) activity with treatment and clinical outcomes in chronic hepatitis C (HCV) Hepatology. 2013;57:1725–1733. doi: 10.1002/hep.26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin X, Zheng SS, Zhang BH, Zhou Y, Chen XH, Ren ZG, Qiu SJ, Fan J. Elevation of serum γ-glutamyltransferase as a predictor of aggressive tumor behaviors and unfavorable prognosis in patients with intrahepatic cholangiocarcinoma: analysis of a large monocenter study. Eur J Gastroenterol Hepatol. 2013;25:1408–1414. doi: 10.1097/MEG.0b013e328364130f. [DOI] [PubMed] [Google Scholar]
- 23.Ma H, Zhang L, Tang B, Wang Y, Chen R, Zhang B, Chen Y, Ge N, Wang Y, Gan Y, Ye S, Ren Z. γ-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol. 2014;21:3084–3089. doi: 10.1245/s10434-014-3724-4. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Liao Y, Suo L, Zhu P, Chen X, Dang W, Liao M, Qin L, Liao W. A novel prognostic index-neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio (NγLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep. 2017;7:9229. doi: 10.1038/s41598-017-09696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Association of Preoperative Platelet-to-Lymphocyte Ratio with Poor Outcome in Patients with Distal Cholangiocarcinoma. Oncology. 2019;96:290–298. doi: 10.1159/000499050. [DOI] [PubMed] [Google Scholar]
- 26.Beal EW, Wei L, Ethun CG, Black SM, Dillhoff M, Salem A, Weber SM, Tran T, Poultsides G, Son AY, Hatzaras I, Jin L, Fields RC, Buettner S, Pawlik TM, Scoggins C, Martin RC, Isom CA, Idrees K, Mogal HD, Shen P, Maithel SK, Schmidt CR. Elevated NLR in gallbladder cancer and cholangiocarcinoma - making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:950–957. doi: 10.1016/j.hpb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]