Abstract
Background
Iron deficiency anemia (IDA) is a prevalent yet underdiagnosed condition with a significant impact on quality of life. Oral iron supplementation is often poorly tolerated or yields inadequate response, requiring the use of intravenous iron (IVI) in some patients. Administration of certain IVI preparations has been associated with decreases in serum phosphate levels and clinically significant hypophosphatemia, which has been reported to lead to adverse events including serious fatigue and osteomalacia.
Objective
The purpose of this study was to systematically assess the prevalence, clinical consequences, and reporting of treatment-emergent hypophosphatemia within literature investigating IVI therapies marketed in the United States (US).
Methods
A systematic literature review (SLR) was conducted using the PubMed database to identify publications reporting serum phosphate levels or rates of hypophosphatemia within adult IDA patient populations receiving current US-marketed IVIs.
Results
The SLR yielded 511 unique publications, with 40 records meeting the final inclusion criteria. Most studies did not report phosphate monitoring methodology or an explicit definition of hypophosphatemia. Hypophosphatemia rates ranged from 0.0% to 92.1% for ferric carboxymaltose (FCM), 0.0% to 40.0% for iron sucrose, 0.4% for ferumoxytol, and 0.0% for low-molecular-weight (LMW) iron dextran. Randomized controlled studies described hypophosphatemia as “asymptomatic” or did not report on other associated sequelae. Eleven case reports detailed treatment-emergent hypophosphatemia in patients treated with FCM. Patients with acute hypophosphatemia primarily developed severe fatigue; those with repeated FCM dosing developed chronic hypophosphatemia associated with osteomalacia and bone deformities.
Conclusion
Studies analyzed in this SLR reported a range of hypophosphatemia rates, with the highest consistently seen in patients treated with FCM. Across the clinical literature, there appeared to be minimal standardization of phosphate monitoring and definitions of hypophosphatemia. Although multiple cases have documented serious clinical consequences of hypophosphatemia associated with certain IVIs, current trials neither consistently nor adequately assess the frequency and severity of treatment-emergent hypophosphatemia and may underestimate its prevalence.
Keywords: IDA, phosphate, hypophosphatemia, iron supplementation
Plain Language Summary
Iron deficiency anemia (IDA) is a common and debilitating condition that may negatively impact a patient’s quality of life. Although oral iron supplementation is the most common treatment for IDA, some patients may have poor tolerance, response, or compliance with oral iron. Intravenous iron (IVI) therapies have thus become increasingly common and are becoming preferred treatments for many patients with iron deficiency anemia (IDA). Although newer generation IVIs are broadly effective and safe, an increasing number of case reports and clinical trials have recently associated certain IVIs with treatment-emergent hypophosphatemia (abnormally low serum phosphate). This literature review analyzed the prevalence, clinical consequences, and reporting of treatment-emergent hypophosphatemia within the clinical literature investigating US-marketed IVI therapies. This review identifies significant inconsistency across the clinical literature in the reporting of serum phosphate monitoring, definitions and rates of treatment-emergent hypophosphatemia. Current IVI studies likely significantly underestimate the prevalence and consequences of hypophosphatemia due to lack of trials designed to systematically identify and monitor changes in serum phosphate.
Introduction
Iron deficiency anemia (IDA) is a prevalent, yet underdiagnosed condition with significant clinical and quality of life impact on patients.1 Symptoms of IDA include chronic weakness, fatigue, difficulty concentrating and exercise intolerance, resulting in decreased productivity.2 IDA affects an estimated five million adults in the United States (US), and globally represents the fifth leading cause of years lived with disability.3,4 Common etiologies of IDA include chronic inflammatory diseases or conditions leading to blood loss or malabsorption of iron such as hypermenorrhea or abnormal uterine bleeding (AUB), pregnancy, inflammatory bowel disease (IBD), chronic kidney disease (CKD), chronic heart failure, cancer, and bariatric surgery.2
Due to poor tolerability, efficacy and compliance with oral iron supplementation, intravenous iron (IVI) has become an increasingly utilized therapeutic option in the treatment of IDA.5–8 Research suggests that IVIs are better tolerated than oral iron and provide a faster onset of action due to the much more rapid correction of body iron stores. Certain IV formulations also allow the administration of an entire therapeutic iron dose in a single sitting, which may be more convenient and improve patient compliance.9–11 Although older IVI preparations such as high-molecular-weight (HMW) iron dextran have been associated with potentially serious adverse events such as hypersensitivity reactions, newer IVI formulations including iron sucrose, ferric gluconate, low-molecular-weight (LMW) iron dextran, ferric carboxymaltose (FCM), and ferumoxytol have been generally shown to be safer.12–15
A growing number of case reports have described treatment-emergent hypophosphatemia (abnormally low serum phosphate) following IVI administration as a safety consideration.16–18 In the acute setting, hypophosphatemia may cause rhabdomyolysis, arrhythmias, and respiratory failure; chronic hypophosphatemia has been associated with osteomalacia, bone deformities, and increased inpatient mortality.19,20 Although hypophosphatemia may have serious clinical consequences, its diagnosis may be commonly missed in the clinic due to initial nonspecific symptomatic presentation as generalized weakness and fatigue.21
To the best of our knowledge, a systematic investigation of hypophosphatemia and its downstream clinical consequences across all US-marketed intravenous iron therapies has not been conducted to date.2,22 This systematic literature review (SLR) was hence designed to analyze the prevalence, clinical consequences, and reporting of treatment-emergent hypophosphatemia within the clinical literature investigating IVI therapies marketed in the US.
Methods
Literature Search Methodology
An SLR was conducted in PubMed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify publications reporting on current US-marketed IVIs within adult IDA patient populations of any etiology. English-language articles published within 10 years of the search date (2/28/2019) were included in the literature review. Search terms (Appendix A) for IDA, as well as generic and trade names of all US-marketed IVIs, were used to index all possible literature for subsequent screening by reviewers. At the time of the literature search, US-marketed IVIs included LMW iron dextran (INFeD®, CosmoFer®), ferric gluconate (Ferrlecit®), ferric carboxymaltose (Injectafer®, Ferinject®), ferumoxytol (Feraheme®), and iron sucrose (Venofer®).
Study Selection
Two reviewers independently screened all studies identified in the initial literature search based on previously established inclusion and exclusion criteria. Studies were included if they represented original research, investigated US-marketed IVI therapies, and reported serum phosphate levels (even if not classified as hypophosphatemia explicitly) and/or hypophosphatemia rates (including literature citing 0.0% rates, if reported). If studies met these requirements, they were included, regardless of the frequency with which phosphate levels were collected. Studies that did not report either of these endpoints were excluded. Studies were also excluded if they met any of the following criteria: (a) duplicates, letters, commentaries, or reviews; (b) investigated non-US marketed IVI therapies exclusively; (c) focused on pediatric populations; or (d) included only hemodialysis patients (excluded due to these patients’ inability to excrete phosphate and potential confounding use of phosphate binders). Study subgroups or treatment arms that fulfilled the screening criteria were included even if other treatment arms or subgroups within that same study did not. For example, studies reporting separately on patients with non-dialysis and dialysis-dependent chronic kidney disease (CKD) were broken out to only include the non-dialysis dependent CKD (NDD-CKD) patient arm of the study.
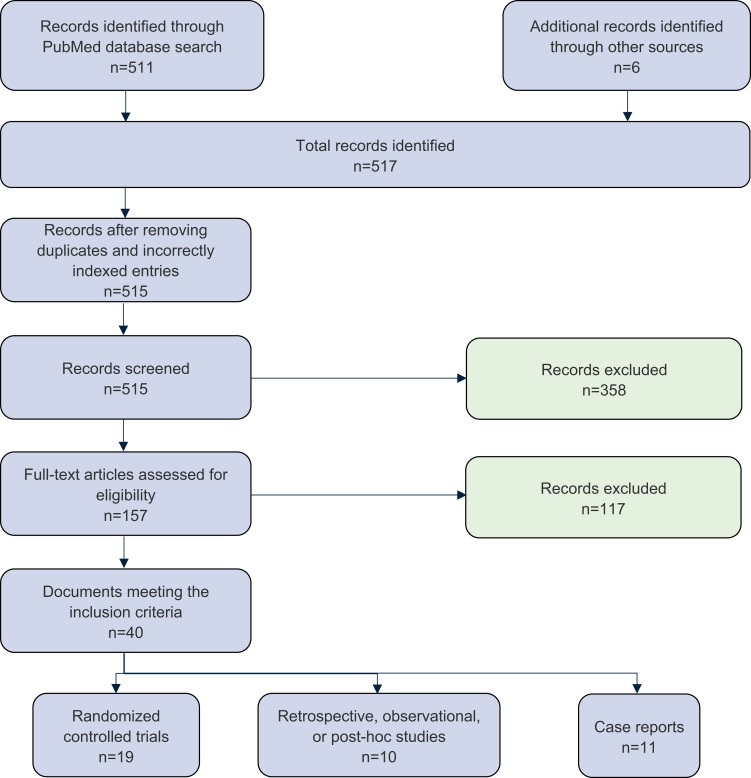
Publications meeting all screening criteria were stratified by level of evidence (Figure 1). Level I evidence included randomized controlled trials (RCTs), Level II evidence included observational, retrospective, or post hoc studies, and Level III evidence included case reports.
Figure 1.
PRISMA flow diagram detailing SLR record screening for hypophosphatemia in adult IDA patients receiving US-marketed IVI therapies.
Abbreviations: SLR, systematic literature review; IDA, iron deficiency anemia; US, United States; IVI, intravenous iron.
Data Extraction
Two reviewers independently screened and extracted data from each publication that met the inclusion criteria. Data included study sample demographics, methodology (including dosing schedule, serum phosphate measurement, and follow-up protocol), as well as reported rates and clinical consequences of hypophosphatemia. Relevant reporting specifications, including definition of hypophosphatemia (when indicated) and serum phosphate reference ranges used were collected. We relied solely upon the publications cited in our analysis; we did not contact the authors or journals to seek unpublished information. Rates of hypophosphatemia were recorded using definitions of hypophosphatemia presented within the individual study. All data analysis was performed using Microsoft Excel. This review was not considered appropriate for a meta-analysis due to the heterogeneity of patient populations, IVI dosing regimens, phosphate measurement methodologies, and inconsistent definitions of hypophosphatemia reported across studies.
Results
The PubMed literature search yielded 511 unique publications. Additional searches in PubMed and bibliographies of review articles yielded 6 case reports which were added to our literature pool. After removing duplicates and mis-indexed publications, 515 records remained for screening. Abstract screening removed 358 records. Two independent reviewers screened 157 full-text publications, excluding 117 additional records. Reasons for exclusion included sole evaluation of IVI therapies marketed outside of the US exclusively, lack of reporting of hypophosphatemia or serum phosphate levels as an endpoint, and focus on pediatric, non-hemodialysis or non-IDA patient populations (Appendix B presents a complete summary of the excluded publications). A total of 40 records across the 3 evidence levels met the final inclusion criteria. A PRISMA-compliant summary of the literature search and screening process is included in Figure 1.
Definitions and Reporting of Hypophosphatemia
Within the literature reviewed, 13 of the 19 Level I and 4 of the 10 Level II studies did not report a definition of hypophosphatemia.9,23-38 Methodologies for serum phosphate measurement and clinical definition of hypophosphatemia varied within and across Level I and Level II studies. Of the 19 Level I studies, 15 did not explicitly report the methodology or timing of serum phosphate measurement. Three of the 10 Level II studies simply used any serum phosphate measurement pre- and post-administration.17,36,38 Serum phosphate measurement in RCTs likely occurred during hematological evaluations; however, only 5 studies explicitly reported serum phosphate measurement.26,30,39-41 In 2 studies, only laboratory abnormalities deemed “clinically significant” were recorded by study investigators.25,26 One such study by Breymann et al (2017) reported phosphate levels below 0.6 mmol/L (2 mg/dL)—the Common Terminology Criteria for Adverse Events (CTCAE) threshold for “severe” hypophosphatemia—at week 3 in 11 women (10 from the FCM arm and 1 from the comparator oral ferrous sulphate arm).31 However, this study did not consider these depressed phosphate levels to be clinically relevant as phosphate levels in patients recovered by the end of the study. More importantly, this study did not record this finding as a hypophosphatemic treatment-emergent adverse event. In contrast, Prats et al (2013) classified NDD-CKD patients enrolled in their study as either hypophosphatemic or non-hypophosphatemic if phosphate levels decreased or remained unchanged or even increased compared to baseline levels at week 3 post-treatment.42 In others, the timing of hematological evaluation was not explicitly reported by investigators.9,30
Standard guidelines advocated by the CTCAE designate hypophosphatemia as Grade 1 (mild) (<LLN–2.5 mg/dl; <LLN–0.8 mmol/l), Grade 2 (moderate) (<2.5–2.0 mg/dl; <0.8–0.6 mmol/l), Grade 3 (severe) (<2.0–1.0 mg/dl; <0.6–0.3 mmol/l), Grade 4 (Life-threatening consequences; urgent intervention indicated) (<1.0 mg/dl; <0.3 mmol/l) and Grade 5 (Death).43 One RCT adopted the CTCAE “moderate” hypophosphatemia definition.29 Five RCTs used the CTCAE definition for “severe” hypophosphatemia.16,39-41,44 An additional study defined hypophosphatemia simply as any decrease in serum phosphate.42
Level I Evidence
Literature screening identified 19 RCTs reporting on serum phosphate or hypophosphatemia.9,16,23-34,39–41,44,45 Key characteristics of the identified studies are presented in Table 1. Of the 19 studies, 18 evaluated ferric carboxymaltose (FCM), 5 evaluated iron sucrose, and 1 each evaluated LMW iron dextran and ferumoxytol, respectively. Fourteen studies had sample sizes greater than 100 patients.9,16,23-27,30–34,41,44,45 Study patient populations spanned a variety of IDA etiologies and study timelines ranged in duration from 2 to 52 weeks. In most studies, FCM was administered as 1 or 2 doses of 750 mg or 1000 mg; ferumoxytol was dosed in 2 doses of 510 mg each; iron sucrose was dosed in multiple doses of 200 mg, and iron dextran was dosed via the Ganzoni formula: weight in kg × (15-current hemoglobin g/dL) × 2.4 + 500 = total iron requirement in mg.
Table 1.
Summary of RCT Studies
| Study | Treatment Evaluated | Population | Treatment Arm Sample Size | Mean Dose per Patient (g) | Study Duration (Weeks) | Hypophosphatemia Rate (%) | Definition of Hypophosphatemia | Serum Phosphate Measurement Methodology |
|---|---|---|---|---|---|---|---|---|
| Onken et al9,a | FCM | IDA refractory to oral iron (poor toleration or response to oral iron) | 489 | 1.435 | 5 | 4.6% | Not defined, likely serum phosphate < 2.0 mg/dL | Not explicitly reported |
| Van Wyck et al16,b | FCM | Women with IDA and history of heavy uterine bleeding | 228 | 1.568 | 6 | 68.9% | Serum phosphate < 2.0 mg/dL | Phosphate appears to be measured at baseline, week 1, 2, 4, and 6 |
| Qunibi et al23,c | FCM | IDA patients with NDDCKD | 147 | 1.218 | 8 | 2.7% | Not defined | Lab tests at baseline, weeks 2, 4, 6 and 8 or early termination visit |
| Charytan et al24 | FCM | IDA patients 18–85 with CKD | 204 | 1 (exact dose not specified) | 4 | 4.3% | Not defined | Lab and safety data collected “before and after study drug administration” |
| Bailie et al25,d | FCM | IDA of any etiology & intolerance/unsatisfactory response to oral iron | 559 | 0.962 | 2 | 16.0% | Not defined | Phosphate appears to be measured at weeks 1 & 2 |
| Onken et al (REPAIR-IDA)26,e | FCM | IDA patients with non-dialysis-dependent CKD | 1276 | 1.5 (exact dose not specified) | 8 | 18.5% | Not defined | Serum phosphate measured at “multiple study visits” for “development of potentially clinically significant changes in serum phosphate” |
| Iron Sucrose | 1285 | 1(exact dose not specified) | 0.8% | |||||
| Hussain et al39,f | FCM | IDA of any etiology & intolerance/unsatisfactory response to oral iron | 82 | 1.45 | 6 | 8.5% | Serum phosphate < 2.0 mg/dL | Clinical evaluation of phosphate at baseline, week 1, 2, 4, 6 |
| Iron Dextran | 5 | 1.34 | 0.0% | |||||
| Barish et al44,g | FCM | IDA of any etiology & intolerance/unsatisfactory response to oral iron | 343 | 0.75 | 5 | 7.3% | Serum phosphate < 2.0 mg/dL | Labs measured on days 0, 7 and 30 or end-of-treatment visit for single-dose study and days 0, 7, 14, 28, and 42 or end-of-study for multidose study |
| Seid et al27,h | FCM | Women with postpartum IDA or IDA due to heavy uterine bleeding | 996 | 0.944 | 5 | 0.6% | Not defined, likely serum phosphate < 2.0 mg/dL | Lab values determined from blood samples obtained at days 0 and 30 |
| Wolf et al40,i | FCM | Women with IDA and history of heavy uterine bleeding | 17 | 0.91 | 5 | 58.8% | Serum phosphate < 2.0 mg/dL | Labs at baseline, days 1, 7, 14, and 35. If phosphate was below normal range after day 0, patients had testing at 14 day intervals until return to normal reference range |
| Jose et al28 | FCM | Pregnancy-related IDA | 50 | 1.7396 ± 0.1055 | 12 | 4.0% | Not defined | Phosphate likely measured at baseline, week 3, 6, 12 |
| Iron Sucrose | 50 | 1.7304 ± 0.1219 | 6.0% | |||||
| Ikuta et al29,j | FCM | Digestive disease-related IDA | 39 | 1.1836 ± 0.340 | 12 | 92.1% | Phosphate below 2.5 mg/dL at least once during study | General labs at weeks 1, 2, 4, 6, 8, and 12 |
| Ikuta et al45,k | FCM | Women with AUB IDA | 119 | 0.9882 or 1.4582 (dependent on dose assignment) | 12 | 18.5% | Not defined, described only as “phosphorus decreased” | General labs at baseline and weeks 1, 2, 4, 6, 8, and 12 |
| Adkinson et al (FIRM)41,l | FCM | IDA refractory to oral iron(any etiology besides dialysis dependent CKD) | 992 | 1.458 | 5 | 38.7% | Serum phosphate <2.0 mg/dL at 2 weeks, (CTCAE grade 3 “severe”) | Phosphate and fractional excretion of phosphate evaluated at baseline, week 2, and week 5 |
| Ferumoxytol | 994 | 0.994 | 0.4% | |||||
| Derman et al30,m | Iron Sucrose | IDA of various etiology | 168 | 1.128 | 5 | 0.0% | Not defined | Serum phosphate measured as a secondary safety endpoint; however timing not reported |
| Breymann et al (FER-ASAP)31,n | FCM | Pregnancy-related IDA | 123 | 1-1.5 (exact dose not specified) | 12 | 8.1% | Not defined | General labs at baseline, weeks 3, 6, 9, and 12 and/or prior to delivery |
| Mahey et al32 | FCM | AUB IDA | 30 | 1.524 ± 0.261 | 12 | 50.0% | Not defined | General labs at baseline, weeks 2, 4, 6, 12 |
| Iron Sucrose | 30 | 1.463 ± 0.196 | 40.0% | |||||
| Macdougall et al (FIND-CKD)33,o | FCM | CKD IDA | 154 | 2.685 | 52 | 0.0% | Not defined | Appendix Figure S4 shows change in serum phosphate from baseline at weeks 4, 8, 12, 24, 36, & 52 |
| Evstatiev etal (FERGICOR)34,p | FCM | IBD IDA | 244 | 1.377 ± 0.381 | 12 | 2.5% | Not defined | General labs at weeks 1, 2, 4, 8, and 12 |
| Iron Sucrose | 239 | 1.160 ± 0.316 | 0.0% |
Notes: aPotentially clinically significant low phosphorus in 53.1% of Group A and 40.7% of Group C. bLowest value of phosphate recorded was 0.9 mg/dL on Day 21 after IV FCM in patient whose baseline phosphate was 2.6 mg/dL. cLowest serum phosphorus level during the study was 1.7 mg/dL. dInvestigators made a judgment as to the clinical significance of any laboratory abnormality to decide lab AEs. eProportion of subjects with potentially clinically significant decreases in phosphate was higher in FCM group compared to iron sucrose group. fAnalysis only considered the 5 patients on LMW iron dextran for iron dextran subgroup. In the FCM group, “the mean [phosphate] value reached its nadir (2.05 mg/dL) at Day 14 and was within the normal range (2.5–4.5 mg/dL) by Day 42”. gOnly reported hypophosphatemia rates within multi-dose subgroup; a significant decrease in phosphorus levels (< 2 mg/dL) was observed in both FCM groups. hPostpartum IDA and HUB groups were combined for analysis. 21.3% of subjects with heavy menstrual bleeding in the FCM group had a transient decrease in serum phosphorus compared to only 0.7% of subjects with postpartum anemia. iIn FCM group, phosphate decreased significantly by 0.6 mg/dL by day 7, and by 0.7 mg/dL by day 14 with recovery by day 35. Among 6 participants whose phosphate remained below normal range at day 35, levels normalized by day 80. jMean serum phosphorus levels reached their lowest values at week 2, and the mean value (± SD) was 1.57 ± 0.48 mg/dL. Phosphorus levels gradually increased after week 4, and the mean level (±SD) recovered to 2.86 ± 0.67 mg/dL at week 12. k65% of subjects in the FCM group had serum phosphate values below the lower limit of normal at week 1, and at week 4, 9.3% of subjects had values < 1.0 mg/dL. One subject in the FCM group had a value <1.0 mg/dL at week 12, achieving recovery on Day 138. lAmong those who received FCM, the mean baseline serum phosphate decreased by 40% by week 2. mThis study reported a 0% hypophosphatemia rate among adverse drug reactions. nPhosphate levels below 2.0 mg/dL (CTCAE severe hypophosphatemia) were recorded in 10 patients; however no hypophosphatemia was reported as TEAEs. oNo data presented on number or percentage of patients with hypophosphatemia; however, mean decrease in serum phosphate was ~0.175mmol/L at 4 weeks and remained ~0.15 mmol/dL or ~0.5mg/dL decreased from baseline at 8 weeks. pOnly reported hypophosphatemia if considered an AE. Mean serum phosphate levels in the FCM group decreased from baseline (1.12 ± 0.22 mmol/L) to week 2 (0.69 ± 0.24 mmol/L) and returned to normal between week 4 and week 12 (1.11 ± 0.23 mmol/L).
Abbreviations: NDDCKD, non-dialysis-dependent chronic kidney disease; CKD, chronic kidney disease; AUB, abnormal uterine bleeding; HUB, heavy uterine bleeding; IBD, inflammatory bowel disease.
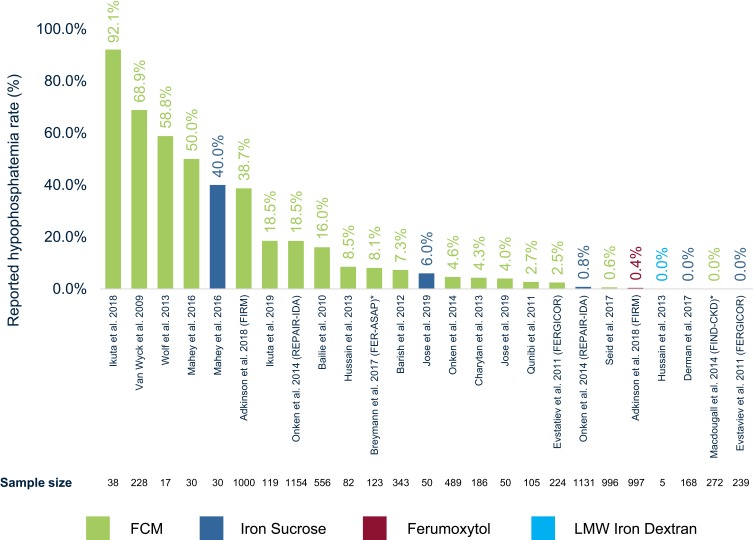
Rates of treatment-emergent hypophosphatemia varied significantly across RCTs (Figure 2). Hypophosphatemia rates ranged from 0.0% to 92.1% for FCM, 0.0% to 40.0% for iron sucrose, 0.4% for ferumoxytol, and 0.0% for LMW iron dextran. All studies either described hypophosphatemia as “asymptomatic” or did not report on other symptoms caused by hypophosphatemia.
Figure 2.
Hypophosphatemia rate (%) reported per RCT.
Notes: Reported hypophosphatemia rates are given as noted in Table 1. Corresponding sample size of study treatment arm is shown below each study title. *Breymann et al31 report 0% hypophosphatemia treatment-emergent adverse events in patients; however, this study reports serum phosphate levels below the threshold of CTCAE grade 3 hypophosphatemia in 8.1% of patients. Macdougall et al33 report no “clinically significant hypophosphatemia”; however this study reports phosphate decrease in supplemental figures but without detailed information on number or % of patients falling below CTCAE threshold.
Abbreviations: RCT, randomized controlled trial; IVI, intravenous iron; FCM, ferric carboxymaltose; LMW, low-molecular-weight.
Across all studies reviewed, the highest hypophosphatemia rates were observed in patients with IDA due to abnormal uterine bleeding (AUB). Five studies of FCM within AUB patients reported hypophosphatemia ranging from 0.6% to 68.9% and a single study of iron sucrose with IDA secondary to AUB reported a rate of 40.0%.16,27,29,32,40 The lowest rates of hypophosphatemia were observed in pregnancy-related IDA (range 0.0–6.0%).27,28,31
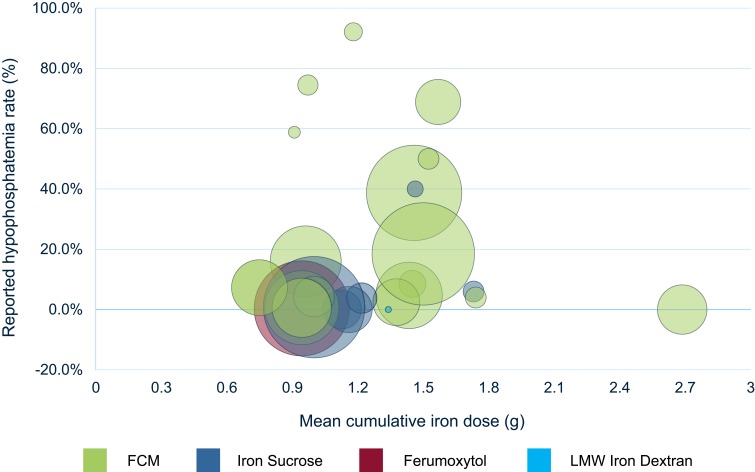
Reported mean cumulative IVI dosing (MCID) ranged from 750 mg to 2.685 g across RCTs. Two studies did not explicitly report MCID, giving only ranges.31,45 Out of 19 RCTs, 18 reported MCID below 1.75 g. Studies with hypophosphatemia rates greater than 10.0% reported MCID between 910 mg and 1.568 g. Cumulative dosing across studies appeared largely determined by FDA-approved labeling. Studies did not report a clear relationship between cumulative IVI dose and treatment-emergent hypophosphatemia. Figure 3 plots hypophosphatemia rates against MCID by IVI treatment within each RCT study.
Figure 3.
Hypophosphatemia rate (%) reported per RCT vs mean cumulative iron dose (g) by IVI treatment within each RCT.
Notes: Reported hypophosphatemia rates are given as noted in Table 1. Bubble size shown is proportional to the sample size of treatment arm within the study; cumulative iron doses shown are derived from study summary statistics of cumulative dosing.
Abbreviations: RCT, randomized controlled trial; IVI, intravenous iron; FCM, ferric carboxymaltose; LMW, low-molecular-weight.
Level II Evidence
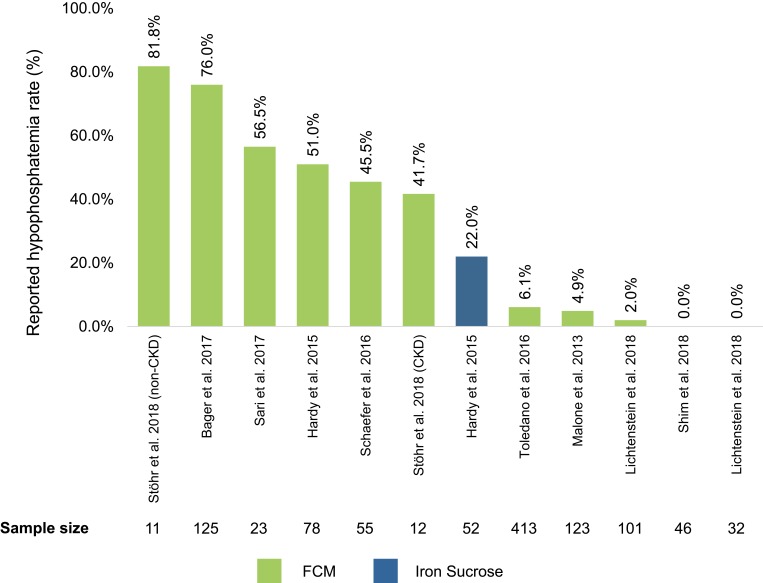
Ten observational, retrospective, or post hoc studies were identified as a supplemental data source to RCT articles (Figure 4).17,35-38,42,46-49 All ten studies investigated FCM; two also reported on iron sucrose treatment arms.17,38 Level II studies reported hypophosphatemia rates ranging 0.0–82.0% with FCM and 0.0–22.0% with iron sucrose. Many Level II studies lacked detailed summary statistics for the analyzed sample, including 4 of ten that did not explicitly report dosing of administered IVIs.17,36,38,47 Seven of ten observational studies described hypophosphatemia as “asymptomatic” or “transient” due to spontaneous resolution after intervention cessation and no need for further interventions.35–38,47–49 A retrospective review of patient medical records by Hardy et al (2015) reported that some FCM-treated patients experienced persistent fatigue despite anemia correction as a clinical consequence of hypophosphatemia.17 Two other studies proposed further investigation of potential clinical consequences of hypophosphatemia such as bone disease development, osteomalacia and other long-term outcomes associated with repeat IVI treatments.42,46
Figure 4.
Hypophosphatemia rate (%) reported by individual non-RCT publication.
Notes: Reported hypophosphatemia rates given as noted in Table 1. The rate of hypophosphatemia reported by study treatment arm was taken using definitions for hypophosphatemia included within each study.
Abbreviation: Non-RCT, non-randomized controlled trial.
Level III Evidence
Eleven case reports evaluating treatment-emergent hypophosphatemia after IVI administration in patients receiving FCM were identified as Level III evidence (Appendix B).18,49-58 Five cases reported treatment-emergent hypophosphatemia with severe muscle weakness and fatigue.50,52,54,57,58 Four reported hypophosphatemic osteomalacia.18,53,55,56 The remaining two case reports described asymptomatic hypophosphatemia.49,51
Severe weakness and fatigue were reported in 5 patients who received two doses of 500 mg FCM separated by a 1-week interval.50,52,54,57,58 IDA etiology varied among these cases: 2 patients presented with AUB,50,57 1 each with IDA secondary to IBD58 and renal transplant,54 and 1 with severe iron deficiency without anemia.52 Patients reported either no improvement of fatigue or complained about worsening weakness after treatment with FCM. In addition to generalized weakness, headache and respiratory distress were also reported in 1 patient each.57,58
Hypophosphatemic osteomalacia was detailed in 4 cases of patients receiving repeated FCM doses of either 750 or 1000 mg.18,53,55,56 The duration of patients’ repeated FCM therapy regimen ranged from 8 months to 15 years. All patients had experienced severe bone pain and recurrent fractures that persisted for months to years.
Clinical sequelae due to IVI-induced hypophosphatemia were eventually reversed in all but 1 case.55 In patients with severe weakness and fatigue, symptoms resolved on normalization of serum phosphate (range 2 weeks to 2 months). In patients with hypophosphatemic osteomalacia, full resolution of osteomalacia took 5 to 12 months. In 1 case, hypophosphatemic osteomalacia resolved, but severe bone deformities were reported, which were considered likely to be permanent.55 A search conducted in August 2019 identified 5 additional articles published since the inception of this manuscript, but these were not included in the detailed analysis due to publication after the original search was performed.
Discussion
Phosphate plays an essential role in the management of metabolism, enzymatic function, bone mineralization, and cellular structure.59 Depression of phosphate homeostasis may therefore lead to serious short- and long-term clinical consequences including fatigue, osteomalacia, rhabdomyolysis, and even death depending on its severity and duration. Although the precise mechanism by which IVIs may cause hypophosphatemia remains incompletely understood, recent literature suggests that certain parenteral iron preparations may increase the urinary fractional excretion of phosphate via modulation of the intracellular metabolism of fibroblast growth factor 23 (FGF23).40,60,61 Despite the suggestion of older studies that hypophosphatemia may be due simply to higher intracellular phosphate uptake coincident with increased erythropoiesis, recent studies have demonstrated no significant correlation between erythropoiesis (as measured by increase in Hgb) or total iron dose and rates of hypophosphatemia.16,60
Although hypophosphatemia has been recognized as a potential adverse event associated with IVIs, only a small proportion of studies indexed in this literature review reported hypophosphatemia rates or changes in serum phosphate levels. Out of 62 US-based or global RCTs investigating US-marketed IVIs for IDA treatment included in this study, only 19 presented data on rates of treatment-emergent hypophosphatemia or serum phosphate changes. When reported, rates of treatment-emergent hypophosphatemia within the literature varied extensively across RCTs, observational, retrospective, and post hoc studies. Differences in study designs and data analysis may account for this inconsistency. Within the literature reviewed, our analysis found (1) inconsistent inclusion of serum phosphate and hypophosphatemia as end-points of interest in studies of IVIs within IDA, (2) a lack of a standard approach and timeline for measurement of phosphate levels, and (3) significant variability in the reporting, definitions and follow-up of any hypophosphatemia observed. These findings indicate that there is a clear need for additional rigorous and standardized research into hypophosphatemia as a clinical consequence of IVI administration.
Furthermore, the majority of studies reviewed did not explicitly report serum phosphate measurement methodology or definitions of hypophosphatemia used for analysis. Although serum phosphate may have been analyzed alongside other hematological parameters recorded within studies, only a minority of publications actively described phosphate measurement when reporting study methodologies. Importantly, timepoints of serum phosphate measurement were not explicitly reported in most Level I and II studies. Within the Level II cohort in particular, timepoints of serum phosphate measurement were neither reported nor consistent among patients, even within the same study. The reported rates of hypophosphatemia may therefore vary significantly as a result of the timing of post-treatment measurement of serum phosphate levels in these studies.
In addition to inconsistent serum phosphate measurement methodologies, reported definitions of hypophosphatemia were either absent or highly variable across studies. Within the minority of studies that did report hypophosphatemia definitions, serum phosphate reference ranges and other qualifying criteria differed significantly. Although CTCAE guidelines for the classification of hypophosphatemia exist, only a few publications explicitly stated the use of this reference range in designating hypophosphatemia within study populations.43 Moreover, even if the CTCAE suggested reference ranges were used, an investigator’s choice of units may have contributed to variations in reported rates of hypophosphatemia. CTCAE guidelines suggest the upper limit of Grade 3 (“severe”) hypophosphatemia to be either 0.6 mmol/L or 2.0 mg/dL; however, these figures are not precisely equal, with 0.6 mmol/L actually corresponding to slightly less than 1.9 mg/dL (requiring a lower serum phosphate level to be considered hypophosphatemic compared to 2.0 mg/dL). Therefore, it is also plausible that the use of differing reference ranges and classification criteria for hypophosphatemia could have contributed to the inconsistent reporting.
Our review also identified several studies in which hypophosphatemia rates appeared to be reported inconsistently across trial arms or appeared to diverge from reported changes in serum phosphate. For example, Barish et al (2012) only reported rates of hypophosphatemia in 1 study arm (multi-dose patients) despite citing statistically significant changes in phosphorus levels within all study subgroups.44 Seid et al (2017) reported a cumulative FCM hypophosphatemia rate of 0.6% despite reporting a statistically significant transient phosphate decrease in 9.0% of all patients dosed with FCM versus 0.0% of patients given standard medical care (no explicit definition of hypophosphatemia was mentioned). A subgroup analysis within this study additionally cited a transient decrease in serum phosphorus in 21.3% of AUB and 0.7% of pregnancy-related IDA patients, respectively. Ikuta et al (2019) reported hypophosphatemia in 18.5% of patients despite acknowledging that 65.0% of subjects dosed with FCM had serum phosphorus levels “below the lower limit of normal” at week 1.45 Until definitions and consistent measurement of hypophosphatemia as well as detailed reporting of serum phosphate are systematically included in all trials and clinical practice, it may continue to be difficult to fully understand the severity and magnitude of treatment-emergent hypophosphatemia.
The studies analyzed within this review may have underestimated the occurrence of hypophosphatemia and its hypothesized clinical consequences due to the short duration of dosing regimens and follow-up evaluation used within study protocols. Only 1 trial among the Level I evidence cohort, FIND-CKD, reported data on hypophosphatemia rates in patients dosed for over 12 weeks.33 Consequently, the long-term effects of hypophosphatemia or repeat IVI dosing may not have been taken into consideration in most RCTs.
Although trials reporting hypophosphatemia noted the condition to be “asymptomatic” or “transient” in patients, it is important to note that certain symptoms of hypophosphatemia—namely fatigue—may present identically to those of IDA and thus may not have been recognized by trial investigators as treatment-emergent adverse events. In contrast to the “asymptomatic” hypophosphatemia reported in trials, case reports demonstrate that IVI–induced hypophosphatemia may be a serious clinical consideration. Patient cases in the literature detail short-term consequences of severe muscle weakness and fatigue and long-term concerns of fractures and bone deformities due to hypophosphatemic osteomalacia in patients undergoing repeat IVI dosing. Although hypophosphatemia was reversed in all cases reviewed, patients were forced to discontinue IVI therapy and required weeks or months-long courses of phosphate and calcitriol supplementation. These patients additionally needed to undergo long-term evaluation by their care teams to prevent further complications.
Similarly, although hypophosphatemia was reported as “transient” in many of the Level I and II studies, evidence of adverse events secondary to chronic hypophosphatemia in case reports of patients undergoing repeat dosing with FCM indicates that there may be long-term health effects not captured in current trial designs. For instance, studies have indicated that nadir serum phosphate in patients given certain IVIs may occur approximately 2 to 3 weeks after IVI administration; however, research has shown that a significant percentage of patients continue to manifest severe hypophosphatemia at 5 weeks.62 In addition, although serum phosphate typically recovered to baseline by the end of the study period for many patients described within trials, there may be many patients in the real-world setting who regularly receive repeat dosing of IVIs as often as once every 4 weeks. In these patients, as with those detailed in the case reports, there may be a potential “stacking” effect of hypophosphatemia—with patients unable to recover serum phosphate to baseline by the time of their next infusion. Although these populations have not been systematically studied within the literature, they may be at risk of chronic hypophosphatemia and thus susceptible to the serious consequences of persistently depressed serum phosphate levels such as hypophosphatemic osteomalacia.
Findings of this literature review suggest that patients with normal renal function and higher iron dose per kg are at highest risk of developing hypophosphatemia.60,62 These findings are supported by a post hoc analysis of the FIRM trial population conducted by Wolf et al (2018) demonstrating an increased risk of hypophosphatemia in patients without CKD.62 Another clinical risk factor for the development of hypophosphatemia reported in the literature was the presence of IDA due to AUB; CKD and pregnancy-related IDA appeared to be associated with lower risk.16,62 The AUB population may be a particularly at-risk subgroup of patients who could potentially benefit from frequent and timely evaluations for hypophosphatemia symptoms.
Lastly, among all IVI therapies investigated within this review, FCM displayed the highest rates of hypophosphatemia. This finding did not appear to differ for any subgroup of IDA patients examined. Recent head-to-head studies of treatment-emergent hypophosphatemia in IDA patients have indicated that of all risk factors for the development of hypophosphatemia, the use of FCM as opposed to another treatment was the most predictive.62 There did not appear to be a class-wide correlation between hypophosphatemia and iron dose administered with IVI therapies. Furthermore, the efficacy observed in IVI patients did not appear to be correlated with hypophosphatemia development.
Limitations
This SLR had several limitations owing to the inherent nature of literature review methodology, restricted structure of our search, and the heterogeneity of available data sources. This study was focused on US-marketed IVIs and thus products not marketed in the US at the time of the literature review were excluded from our analysis. Since the time of our literature search, iron isomaltoside has been approved by the US Food and Drug Administration (as ferric derisomaltose). A newly published article by Wolf et al (2020) has reported rates of hypophosphatemia from 2 randomized controlled trials comparing iron isomaltoside and ferric carboxymaltose (7.9–8.1% and 73.7–75.0%, respectively).63 Patient populations’ IDA etiology and baseline anemia status differed significantly across the included studies and case reports. The observed variability in IVI dosing regimens, timing of phosphate measurement, serum phosphate reference ranges and definitions of hypophosphatemia may additionally confound reported hypophosphatemia rates. This heterogeneity of available sources precluded a robust quantitative and statistical analysis of hypophosphatemia and its associated clinical sequelae. Findings of this study should thus be interpreted with these caveats in mind. Despite these limitations, this study’s findings suggest that there may be value in additional scientific and real-world studies on this topic, designed to control for underlying patient conditions and etiologies which consistently define, measure and report hypophosphatemia rates.
Conclusion
In summary, our analysis suggests that there is a significant need for further research to elucidate the mechanism of IVI–induced hypophosphatemia as well as for more consistent inclusion, measurement, and reporting of serum phosphate levels as an endpoint in clinical trials. Moving forward, research and guidelines should aim to standardize the definitions of hypophosphatemia and suggest specific timing for serum phosphate measurement within clinical trials as well as real-world studies. Current CTCAE guidelines for hypophosphatemia may be a good reference point for future studies. Given the potential clinical impact of IVI-induced hypophosphatemia, particularly with repeated dosing of certain IVIs, further research, will also be needed to assess the effect of various dosing regimens on long-term serum phosphate levels.
Current studies may be significantly underestimating rates of treatment-emergent hypophosphatemia due to inconsistent and infrequent measurement of serum phosphate, inconsistent definitions of hypophosphatemia, and similarity between the symptoms of hypophosphatemia and anemia. Across all studies, the highest rates of hypophosphatemia were seen with FCM. All case reports profiled severe weakness and fatigue or hypophosphatemic osteomalacia in FCM patients who developed treatment-induced hypophosphatemia.
Hypophosphatemia may not only impact patients’ clinical outcomes and quality of life in the short- and long term, but may also create a burden on the healthcare system due to the need for additional medications and close monitoring of patients. Until the true prevalence and clinical impact of IVI–induced hypophosphatemia is more fully characterized and quantified within the literature, findings of this SLR suggest that physicians and researchers actively consider the possibility of hypophosphatemia in all patients receiving IVIs, particularly in those receiving therapies such as FCM, which has been associated with elevated rates of treatment-emergent hypophosphatemia in various clinical studies. Given that the symptoms of hypophosphatemia may appear almost identical to those of IDA, healthcare professionals should remain aware of the possibility of hypophosphatemia in their IDA patients, and carefully and consistently monitor pre- and post-treatment serum phosphate levels in all patients receiving IVIs.
Of note, during the finalization of this manuscript, the US FDA revised the prescribing information for Injectafer (ferric carboxymaltose), adding a warning that severe symptomatic episodes of hypophosphatemia requiring clinical intervention have occurred following Injectafer administrations and recommending serum phosphate monitoring in patients at risk for hypophosphatemia who require a repeat course of treatment. These labeling changes highlight both the relevance of the conclusions of this analysis and the need for heightened awareness of this phenomenon.
Funding Statement
This study was sponsored by AMAG Pharmaceuticals, Inc. Editorial support in the preparation of this manuscript was provided by Trinity LifeSciences, funded by AMAG Pharmaceuticals, Inc.
Author Contributions
The authors were responsible for all content and editorial decisions related to the development of this manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MZ Lim-Watson was an employee of AMAG Pharmaceuticals, Inc at the time the study was conducted. A. Bajic-Lucas, NV Dahl, and WE Strauss are full-time employees of AMAG Pharmaceuticals, Inc. and hold equity in AMAG Pharmaceuticals, Inc. MA Libre, SS Karkare, and N Hadker are employees of Trinity LifeSciences, which has conducted research for AMAG Pharmaceuticals, Inc. JA Glaspy is a consultant for AMAG Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907–916. doi: 10.1016/S0140-6736(15)60865-0 [DOI] [PubMed] [Google Scholar]
- 2.Camaschella C. Iron-deficiency anemia. Longo DL, ed. N Engl J Med. 2015;372(19):1832–1843. doi: 10.1056/NEJMra1401038 [DOI] [PubMed] [Google Scholar]
- 3.Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013;3(7):a011866–a011866. doi: 10.1101/cshperspect.a011866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38. doi: 10.1002/ajh.24201 [DOI] [PubMed] [Google Scholar]
- 6.Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. Strnad Ped. PLoS One. 2015;10(2):e0117383. doi: 10.1371/journal.pone.0117383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88(2):97–101. doi: 10.1002/ajh.23354 [DOI] [PubMed] [Google Scholar]
- 8.Reinisch W, Chowers Y, Danese S, et al. The management of iron deficiency in inflammatory bowel disease - an online tool developed by the RAND/UCLA appropriateness method. Aliment Pharmacol Ther. 2013;38(9):1109–1118. doi: 10.1111/apt.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2013;54(2):n/a–n/a. doi: 10.1111/trf.12289 [DOI] [PubMed] [Google Scholar]
- 10.Vadhan-Raj S, Strauss W, Ford D, et al. Efficacy and safety of IV ferumoxytol for adults with iron deficiency anemia previously unresponsive to or unable to tolerate oral iron. Am J Hematol. 2014;89(1):7–12. doi: 10.1002/ajh.23582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematol. 2010;2010(1):338–347. doi: 10.1182/asheducation-2010.1.338 [DOI] [PubMed] [Google Scholar]
- 12.Ehlken B, Nathell L, Gohlke A, Bocuk D, Toussi M, Wohlfeil S. Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron (III) isomaltoside 1000 in Europe based on data from EudraVigilance and VigiBaseTM between 2014 and 2017. Drug Saf. 2019;42(3):463–471. doi: 10.1007/s40264-018-0769-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314(19):2062. doi: 10.1001/jama.2015.15572 [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines the European Medicines Agency’s Committee for Medicinal Products for Human Use; 2013. Available from: www.ema.europa.eu. Accessed June26, 2019.
- 15.Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99(11):1671–1676. doi: 10.3324/haematol.2014.111492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Blood management: large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719–2728. doi: 10.1111/j.1537-2995.2009.02327.x [DOI] [PubMed] [Google Scholar]
- 17.Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol. 2015;2015:1–6. doi: 10.1155/2015/468675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartko J, Roschger P, Zandieh S, Brehm A, Zwerina J, Klaushofer K. Hypophosphatemia, severe bone pain, gait disturbance, and fatigue fractures after iron substitution in inflammatory bowel disease: a case report. J Bone Miner Res. 2018;33(3):534–539. doi: 10.1002/jbmr.3319 [DOI] [PubMed] [Google Scholar]
- 19.Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. 2010;103(7):449–459. doi: 10.1093/qjmed/hcq039 [DOI] [PubMed] [Google Scholar]
- 20.Brunelli SM, Goldfarb S. Hypophosphatemia: clinical consequences and management. J Am Soc Nephrol. 2007;18(7):1999–2003. doi: 10.1681/ASN.2007020143 [DOI] [PubMed] [Google Scholar]
- 21.Imel EA, Econs MJ. Approach to the hypophosphatemic patient. J Clin Endocrinol Metab. 2012;97(3):696–706. doi: 10.1210/jc.2011-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations. Mayo Clin Proc. 2015;90(1):12–23. doi: 10.1016/j.mayocp.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 23.Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607. doi: 10.1093/ndt/gfq613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charytan C, Bernardo MV, Koch TA, Butcher A, Morris D, Bregman DB. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: a randomized, active-controlled, multi-center study. Nephrol Dial Transplant. 2013;28(4):953–964. doi: 10.1093/ndt/gfs528 [DOI] [PubMed] [Google Scholar]
- 25.Bailie GR, Mason NA, Valaoras TG. Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial Int. 2010;14(1):47–54. doi: 10.1111/j.1542-4758.2009.00409.x [DOI] [PubMed] [Google Scholar]
- 26.Onken JE, Bregman DB, Harrington RA, et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29(4):833–842. doi: 10.1093/ndt/gft251 [DOI] [PubMed] [Google Scholar]
- 27.Seid MH, Butcher AD, Chatwani A. Ferric carboxymaltose as treatment in women with iron-deficiency anemia. Anemia. 2017;2017:1–9. doi: 10.1155/2017/9642027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose A, Mahey R, Sharma JB, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy-randomised controlled trial. BMC Pregnancy Childbirth. 2019;19(1):54. doi: 10.1186/s12884-019-2200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikuta K, Ito H, Takahashi K, Masaki S, Terauchi M, Suzuki Y. Safety and efficacy of intravenous ferric carboxymaltose in Japanese patients with iron-deficiency anemia caused by digestive diseases: an open-label, single-arm study. Int J Hematol. 2019;109(1):50–58. doi: 10.1007/s12185-018-2529-9 [DOI] [PubMed] [Google Scholar]
- 30.Derman R, Roman E, Modiano MR, Achebe MM, Thomsen LL, Auerbach M. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286–291. doi: 10.1002/ajh.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J. FER-ASAP investigators. Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med. 2017;45(4):443–453. doi: 10.1515/jpm-2016-0050 [DOI] [PubMed] [Google Scholar]
- 32.Mahey R, Kriplani A, Mogili KD, Bhatla N, Kachhawa G, Saxena R. Randomized controlled trial comparing ferric carboxymaltose and iron sucrose for treatment of iron deficiency anemia due to abnormal uterine bleeding. Int J Gynecol Obstet. 2016;133(1):43–48. doi: 10.1016/j.ijgo.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Macdougall IC, Bock AH, Carrera F, et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29(11):2075–2084. doi: 10.1093/ndt/gfu201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterol. 2011;141(3):846–853.e2. doi: 10.1053/j.gastro.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 35.Shim J-Y, Kim MY, Kim YJ, et al. Efficacy and safety of ferric carboxymaltose versus ferrous sulfate for iron deficiency anemia during pregnancy: subgroup analysis of Korean women. BMC Pregnancy Childbirth. 2018;18(1):349. doi: 10.1186/s12884-018-1817-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledano A, Luporsi E, Morere JF, et al. Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support Care Cancer. 2016;24(1):67–75. doi: 10.1007/s00520-015-2728-3 [DOI] [PubMed] [Google Scholar]
- 37.Malone M, Barish C, He A, Bregman D. Comparative review of the safety and efficacy of ferric carboxymaltose versus standard medical care for the treatment of iron deficiency anemia in bariatric and gastric surgery patients. Obes Surg. 2013;23(9):1413–1420. doi: 10.1007/s11695-013-0939-6 [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein GR, Onken JE. Improved hemoglobin response with ferric carboxymaltose in patients with gastrointestinal-related iron-deficiency anemia versus oral iron. Dig Dis Sci. 2018;63(11):3009–3019. doi: 10.1007/s10620-018-5204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain I, Bhoyroo J, Butcher A, Koch TA, He A, Bregman DB. Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia. 2013;2013:169107. doi: 10.1155/2013/169107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–1803. doi: 10.1002/jbmr.1923 [DOI] [PubMed] [Google Scholar]
- 41.Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol. 2018;93(5):683–690. doi: 10.1002/ajh.25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prats M, Font R, García C, Cabré C, Jariod M, Vea AM. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: post-hoc analysis of a prospective study. BMC Nephrol. 2013;14(1):167. doi: 10.1186/1471-2369-14-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; 2009. Available from: http://www.meddramsso.com. Accessed June19, 2019.
- 44.Barish CF, Koch T, Butcher A, Morris D, Bregman DB. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: two randomized, controlled trials. Anemia. 2012;2012:1–9. doi: 10.1155/2012/172104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikuta K, Hanashi H, Hirai K, et al. Comparison of efficacy and safety between intravenous ferric carboxymaltose and saccharated ferric oxide in Japanese patients with iron-deficiency anemia due to hypermenorrhea: a multi-center, randomized, open-label noninferiority study. Int J Hematol. 2019;109(1):41–49. doi: 10.1007/s12185-018-2501-8 [DOI] [PubMed] [Google Scholar]
- 46.Schaefer B, Würtinger P, Finkenstedt A, et al. Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. Pantopoulos K ed. PLoS One. 2016;11(12):e0167146. doi: 10.1371/journal.pone.0167146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bager P, Hvas CL, Dahlerup JF. Drug specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol. 2017;83(5):1118–1125. doi: 10.1111/bcp.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stöhr R, Sandstede L, Heine GH, Marx N, Brandenburg V. High-dose ferric carboxymaltose in patients with HFrEF induces significant hypophosphatemia. J Am Coll Cardiol. 2018;71(19):2270–2271. doi: 10.1016/j.jacc.2018.03.448 [DOI] [PubMed] [Google Scholar]
- 49.Sari V, Atiqi R, Hoorn EJ, Heijboer AC, van Gelder T, Hesselink DA. Ferric carboxymaltose-induced hypophosphataemia after kidney transplantation. Neth J Med. 2017;75(2):65–73. [PubMed] [Google Scholar]
- 50.Anand G, Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep. 2017;2017:bcr2016219160. doi: 10.1136/bcr-2016-219160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blazevic A, Hunze J, Boots JMM. Severe hypophosphataemia after intravenous iron administration. Neth J Med. 2014;72(1):49–53. [PubMed] [Google Scholar]
- 52.Fierz YC, Kenmeni R, Gonthier A, Lier F, Pralong F, Coti Bertrand P. Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur J Clin Nutr. 2014;68(4):531–533. doi: 10.1038/ejcn.2014.20 [DOI] [PubMed] [Google Scholar]
- 53.Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018:bcr-2017-222851. doi: 10.1136/bcr-2017-222851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mani L-Y, Nseir G, Venetz J-P, Pascual M. Severe hypophosphatemia after intravenous administration of iron carboxymaltose in a stable renal transplant recipient. Transplant. 2010;90(7):804–805. doi: 10.1097/TP.0b013e3181f00a18 [DOI] [PubMed] [Google Scholar]
- 55.Moore KLF, Kildahl-Andersen O, Kildahl-Andersen R, Tjønnfjord GE. Uncommon adverse effect of a common medication. Tidsskr nor Laegeforen. 2013;133(2):165. doi: 10.4045/tidsskr.12.0494 [DOI] [PubMed] [Google Scholar]
- 56.Urbina T, Belkhir R, Rossi G, et al. Iron supplementation-induced phosphaturic osteomalacia: FGF23 is the culprit. J Bone Miner Res. 2018;33(3):540–542. doi: 10.1002/jbmr.3369 [DOI] [PubMed] [Google Scholar]
- 57.Vandemergel X, Vandergheynst F. Potentially life-threatening phosphate diabetes induced by ferric carboxymaltose injection: a case report and review of the literature. Case Rep Endocrinol. 2014;2014:843689. doi: 10.1155/2014/843689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasquez-Rios G, Marin E. Harder to breathe: an unusual case of severe hyperphosphaturic hypophosphatemia and normal FGF-23 levels in a young female patient. Am J Kidney Dis. 2018;71:594. [Google Scholar]
- 59.Bansal VK. Serum Inorganic Phosphorus. Butterworths; 1990. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21250152. Accessed July10, 2019. [PubMed] [Google Scholar]
- 60.Huang LL, Lee D, Troster SM, et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dialysis transplant. 2018;33(9):1628–1635. doi: 10.1093/ndt/gfx310 [DOI] [PubMed] [Google Scholar]
- 61.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–2337. doi: 10.1210/jc.2008-2396 [DOI] [PubMed] [Google Scholar]
- 62.Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3:23. doi: 10.1172/JCI.INSIGHT.124486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–443. doi: 10.1001/jama.2019.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines the European Medicines Agency’s Committee for Medicinal Products for Human Use; 2013. Available from: www.ema.europa.eu. Accessed June26, 2019.
- Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; 2009. Available from: http://www.meddramsso.com. Accessed June19, 2019.