Abstract
There is strong evidence linking changes in DNA methylation with cigarette smoking, and smoking has long been associated with cardiovascular disease; however not many studies have investigated the effects of smoking related DNA methylation changes on cardiovascular risk, especially in young adults. We explored this relationship in 480 African American and European American men and women aged 27.3±3.5. Out of the DNA methylation data obtained from Illumina 450k in peripheral leukocytes, 62 CpG sites that have been associated with smoking in multiple studies were selected. Of these, 48 were significantly related to smoking within our population. These CpG sites were then used to predict 2 subclinical markers of cardiovascular health: carotid intima media thickness and left ventricular mass (LVM). There was a significant association (FDR<0.05) between LVM and 13 of these CpG sites. We constructed a DNA methylation score using these CpG sites and found a significant association between this score and LVM (p<0.01). Mediation test showed that 36.5% of the effect of smoking on LVM could be explained by this methylation score. Our data suggests that, in young adult populations, cigarette smoking related DNA methylation changes are already associated with changes in subclinical markers of cardiovascular health.
Keywords: smoking, DNA methylation, cardiovascular risk, youth
INTRODUCTION
Cigarette smoking has long been associated with negative effects to cardiovascular health [1] and Epigenome Wide Association Studies (EWAS) have identified multiple DNA methylation changes associated with smoking in peripheral leukocytes [2]. These methylation biomarkers exhibit strong association with all-cause and cardiovascular mortality [3]. One recent study identified a link between smoking associated DNA methylation changes and subclinical atherosclerosis [4]. However, these studies were conducted in older populations and the role of these smoking related DNA methylation changes in the early development of subclinical markers of cardiovascular diseases (CVD) remains unknown. This study aims to test whether cigarette smoking related DNA methylation changes are linked with early signs of CVD risk in young adults using two indices: carotid intima media thickness (IMT) and left ventricular mass (LVM, corrected by body surface area). Because the pathogenesis of CVD starts in youth, understanding the potential molecular mechanisms for smoking related CVD risk at this age could provide evidence-based knowledge for early prevention.
METHODS
Subjects
This study was comprised of participants from a longitudinal cohort. It consisted of 396 European American (EA) and 349 African American (AA) children aged 7-16 at baseline with evaluations conducted every 1-3 years during the follow-up. All participants were recruited from the Southeastern United States and were overtly healthy. Study design and selection criteria have been described previously [5]. The Institutional Review Board at the Medical College of Georgia had given approval for this study. Informed consent was provided by all subjects and by parents if subjects were <18 years of age.
Genome-wide DNA methylation data from peripheral leukocytes were obtained from 480 participants (mean±SD age, 27.7±3.0 years; range, 20.5 to 35.9 years; 47.3% AAs and 54% males) on visit 15. Of the 480, subclinical measures of CVD including LVM and carotid IMT were available for 380 and 371 participants, respectively. No differences in the distribution of age, gender, or ethnicity were observed between the participants with or without missing values. Supplementary Table 1 lists the general characteristics of the participants.
Measurements
DNA methylation data were obtained using Illumina Infinium Human Methylation 450K Beadchip (Illumina Inc.). Quantification, data preprocessing, quality control, estimation and correction of cell composition have been outlined previously [6].
Cigarette smoking was assessed by the self-reported number of days smoked during the past 30 days and the number of cigarettes smoked per day. Individuals who smoked at least five cigarettes in the past 30 days were considered smokers.
Subclinical measures of CVD were obtained by methods outlined previously [7, 8]. Briefly, LVM was measured using echocardiograph and normalized to body surface area to obtain LVM index. The common carotid artery IMT was measured using Hewlett-Packard Sonos 5500 (Andover, MA). The mean carotid IMT for the far wall of both sides was used as the index of carotid IMT.
Statistical Analysis
All analyses were done using R and Stata. Limma [9] was used to identify differentially methylated CpG sites related to smoking with age, gender, ethnicity, and BMI as covariates. False discovery rate (FDR) was used to account for multiple testing with a FDR<0.05 set as the significance threshold. This list was compared to the 62 CpG sites that have been shown to be associated with smoking in multiple studies (≥ 3) [2] and the CpG sites that appeared on both lists (n=48) were selected for further analyses. The association between the 48 CpG sites and smoking was provided in Supplementary Table 2.
Next, we tested whether DNA methylation levels of these 48 CpG sites were associated with LVM and IMT using linear regression with age, gender, ethnicity, BMI, and systolic blood pressure (SBP) as covariates. A FDR<0.05 was set as the significance threshold. LASSO (Least Absolute Shrinkage and Selection Operator) approach [10] was then used to choose a list of the significant CpG sites to develop a methylation score for each subclinical measures of CVD. LASSO was run 100 times and if a CpG site was included in ≥ 90 models, it was included in the calculation of the score. The associations of the score with the subclinical measures of CVD were assessed using linear regression, adjusted for the covariates listed above.
We further tested whether the methylation score could mediate the effect of smoking on the subclinical measures of CVD using the Sobel test [11]. Mediation was considered to be present when smoking’s effect on subclinical measures of CVD decreased upon the addition of the methylation score to the model and the Sobel test gave P < 0.05.
RESULTS
As shown in Table 1, of the 48 CpG sites, 13 demonstrated a significant association with LVM at FDR<0.05. None of the 48 CpG sites demonstrated a significant association with IMT. The results were listed in Supplementary Table 3.
Table 1.
Associations between smoking related CpG sites and LVM index.
| CpG site | Chr. | Closest Gene | Direction* | P value | FDR |
|---|---|---|---|---|---|
| cg01940273 | 2 | ALPPL2 | − | 4.43E-05 | 2.13E-03 |
| cg12803068 | 7 | MYO1G | + | 2.21E-04 | 3.61E-03 |
| cg21566642 | 2 | ALPPL2 | − | 2.97E-04 | 3.61E-03 |
| cg05575921 | 5 | AHRR | − | 3.36E-04 | 3.61E-03 |
| cg05951221 | 2 | ALPPL2 | − | 3.96E-04 | 3.61E-03 |
| cg22132788 | 7 | MYO1G | + | 4.51E-04 | 3.61E-03 |
| cg26703534 | 5 | AHRR | − | 8.64E-04 | 5.92E-03 |
| cg27241845 | 2 | ECEL1P2 | − | 1.50E-03 | 9.02E-03 |
| cg21161138 | 5 | AHRR | − | 3.21E-03 | 1.71E-02 |
| cg19859270 | 3 | GPR15 | − | 4.07E-03 | 1.95E-02 |
| cg03329539 | 2 | ALPPL2 | − | 5.22E-03 | 2.28E-02 |
| cg25648203 | 5 | AHRR | − | 6.43E-03 | 2.57E-02 |
| cg24996979 | 14 | C14orf43 | − | 1.27E-02 | 4.67E-02 |
CpG sites used in the methylation score are highlighted in bold
“+” indicates positive associations and “-”indicates negative associations between CpG sites and LVM index.
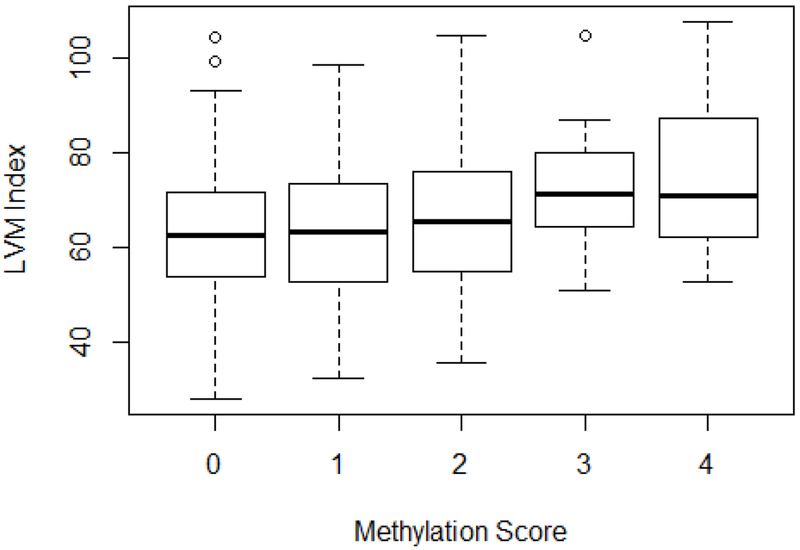
LASSO analysis identified 4 CpG sites (cg12803068, cg27241845, cg01940273 and cg24996979, highlighted in Table 1). To develop the score, one point was awarded if the methylation was in the lowest quartile for cg27241845, cg01940273 or cg24996979 and one point was awarded if methylation was in the highest quartile of cg12803068. This was determined according to whether the CpG site was more or less methylated in the smoking group. The score ranged from 0 to 4. As shown in Figure 1, the methylation score was significant associated with LVM (p = 4.79e-05).
Figure 1:
Left ventricular mass index by methylation score
Mediation analysis was conducted with smoking as the independent variable, LVM index as the dependent variable and the methylation score as the mediator. When controlling for covariates, smoking was a significant predictor of LVM index (p=7.94e-4). Smoking’s effect on LVM decreased upon addition of the methylation score to the model (β changes from 4.587 to 2.912) and the Sobel test was significant (p= 0.004), indicating 36.5% of smoking’s effect on LVM being explained by the methylation score.
DISCUSSION
Our study confirmed the EWAS findings that cigarette smoking can leave DNA methylation signatures in leukocytes [2]. We further established that these changes were already associated with increased LVM in young adults.
Increasing evidence indicates that smoking affects cardiac remodeling [12]. Increased LVM is an important cardiac remodeling trait that is an intermediate phenotype for heart failure [13] and is associated with increased risk for various CVD outcomes [13]. Cross-sectional and longitudinal studies have demonstrated an association between smoking and increased LVM in young adults and throughout adult life [12, 15]. However, the exact mechanisms underlying this association are not clear. Regardless of the underlying mechanisms, cigarette smoking related cardiac remodeling is often accompanied by systemic inflammation and immune cells infiltration into the myocardium [16]. Therefore, we hypothesized that smoking related changes in DNA methylation of leukocytes are involved in the pathogenesis of cardiac hypotrophy. In this study, we identified 13 smoking related CpG sites showing significant associations with LVM, providing potential molecular mechanisms by which smoking drives the cardiac remodeling. A methylation score using 4 of the 13 CpG sites demonstrated a clear dose-response relationship with LVM, and could mediate 36.5% of smoking’s effect on LVM. The top LVM associated CpG site, cg01940273, and 3 other CpG sites, cg21566642, cg05951221, and cg03329539, are located in chromosome 2 and in proximity to the alkaline phosphatases gene group, with ALPPL2 being the closest gene. Another LVM associated CpG site, cg27241845, is located in the same region, closer to the ECL1P2 (endothelin converting enzyme like 1 pseudogene 2) gene. A group of endothelin converting enzyme like 1 pseudogenes including ECEL1P1 and ECEL1P3 are also located in the same region. The LVM associated CpG sites also included 4 on AHRR gene, an aryl-hydrocarbon receptor repressor. DNA methylation levels of these sites have been associated with subclinical atherosclerosis in participants of a population study with average age of 70 [4]. Other LVM associated CpG sites can be found in the MYO1G (cg12803068), GPR15 (cg19859270), and C14orf43 genes (cg24996979). The potential involvement of these genes in cardiac remodeling has not been reported. Further experimental validation is warranted.
The role of cigarette smoking on atherosclerosis is well documented. In this study, we observed that smoking was significantly (p=0.04) associated with carotid IMT. However, we did not observe that any smoking related CpG site was associated with IMT, differing from the study by Reynolds et al. in which DNA methylation levels of AHRR CpG sites were significantly associated with carotid atherosclerosis [4]. The discrepancy may be explained by the age of the participants (young adult vs. elderly) and the index of carotid atherosclerosis (IMT vs. carotid plaque scores). Further studies in this aspect are needed.
Our study has limitations. First, the cross-sectional design limited our ability to discern the temporal relationship between LVM and DNA methylation. Second, mediation analysis does not distinguish confounding from mediating effects; therefore, although we observed the methylation score significantly mediated the effect of smoking on LVM, these results do not confirm biological mediation and should be interpreted with caution.
In conclusion, our study suggests that, in young adults, smoking related DNA methylation changes are already associated with increased LVM. The LVM associated CpG sites provide a potential molecular mechanism to explain the negative effect of smoking on cardiac remodeling.
Supplementary Material
HIGHLIGHTS.
Methylation level of 13 smoking-related CpG sites showed association with LVM.
A total score from these CpG sites explained 36.5% of smoking’s effect on LVM.
These smoking related changes were already evident in young adults.
ACKNOWLEDGEMENTS
The Georgia Stress and Heart study was supported by grant HL69999 from the National Heart Lung and Blood Institute (NHLBI). The DNA methylation data was obtained by grant HL125577 from NHLBI. C.S. was supported by Children’s Summer Scholars Program, Medical College of Georgia at Augusta University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors report no relationships that could be construed as a conflict of interest.
REFERENCES
- [1].U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. . In: Atlanta: U.S: Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor.2014. [Google Scholar]
- [2].Gao X, Jia M, Zhang Y, Breitling LP, Brenner H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin Epigenetics. 2015;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang Y, Schottker B, Florath I, Stock C, Butterbach K, Holleczek B, et al. Smoking-Associated DNA Methylation Biomarkers and Their Predictive Value for All-Cause and Cardiovascular Mortality. Environ Health Perspect. 2016;124:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reynolds LM, Wan M, Ding J, Taylor JR, Lohman K, Su D, et al. DNA Methylation of the Aryl Hydrocarbon Receptor Repressor Associations With Cigarette Smoking and Subclinical Atherosclerosis. Circ Cardiovasc Genet. 2015;8:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131:1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang X, Pan Y, Zhu H, Hao G, Huang Y, Barnes V, et al. An epigenome-wide study of obesity in African American youth and young adults: novel findings, replication in neutrophils, and relationship with gene expression. Clin Epigenetics. 2018;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood Pressure Trajectories From Childhood to Young Adulthood Associated With Cardiovascular Risk: Results From the 23-Year Longitudinal Georgia Stress and Heart Study. Hypertension. 2017;69:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–51. [DOI] [PubMed] [Google Scholar]
- [9].Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–75. [DOI] [PubMed] [Google Scholar]
- [10].Tibshirani R The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–95. [DOI] [PubMed] [Google Scholar]
- [11].Sober ME. Asymptotic confidence intervals for indirect effects in structural equation models Leinhardt Sociological Methodology. Washington, DC: American Sociological Association; 1982. [Google Scholar]
- [12].Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and longterm change in the framingham offspring study. Circulation. 2009;119:3085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. [DOI] [PubMed] [Google Scholar]
- [14].Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. Mmode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87:1051–7. [DOI] [PubMed] [Google Scholar]
- [15].Markus MR, Stritzke J, Baumeister SE, Siewert U, Baulmann J, Hannemann A, et al. Effects of smoking on arterial distensibility, central aortic pressures and left ventricular mass. Int J Cardiol. 2013;168:2593–601. [DOI] [PubMed] [Google Scholar]
- [16].Kaplan A, Abidi E, Ghali R, Booz GW, Kobeissy F, Zouein FA. Functional, cellular, and molecular remodeling of the heart under influence of oxidative cigarette tobacco smoke. Oxid Med Cell Longev. 2017; 2017:3759186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.