To unravel molecular mechanisms with the ultimate goal to achieve improved stress resilience or increased yield, plants are often studied under highly controlled conditions in which stresses are applied and in which growth‐ or architecture‐related traits are meticulously recorded. Over the past decades, this has led to a boost in our understanding of key molecular players and in strategies to improve yield stability. However, many single‐gene traits fail to translate into applications (Nuccio et al., 2018). One example of a single‐gene modification identified in the growth chamber as leaf growth enhancing through maintaining cells in an undifferentiated state for a longer period of time that translated into biomass and seed yield increases under agronomical conditions in the field is the ectopic expression of PLASTOCHRON1 (PLA1) (Sun et al., 2017). These data suggest that the growth components studied in the 4th leaf in the growth chamber may hold true for other organs and under agronomic field conditions. The aim of this research was to examine the transcriptional differences in growing leaf tissue between the growth chamber and the field to gain insights into the molecular differences between maize plants cultivated in a growth chamber and the field.
Both transgenic and non‐transgenic plants ectopically expressing PLA1 were cultivated in the growth chamber and the field during two consecutive growing seasons (2015 and 2016) and the basal 1 cm of the growing 4th leaf in the field and the growth chamber was sampled for RNA‐seq. For every sample, three pools were collected, randomly across the growth chamber or across each of the three plot replicates. In 2015, only one time point (2 days after leaf appearance) was sampled (2 genotypes × 2 conditions × 1 time point × 3 replicates) while in 2016 two time points (2 days and 6 days after leaf appearance) were considered (2 genotypes × 2 conditions × 2 time points × 3 replicates). 2016 was characterized by a rainy spring, while 2015 was closer to the ten‐year‐average. Library preparation was done using the TruSeq RNA Sample Preparation Kit v2 (Illumina), followed by sequencing on an Illumina HiSeq2500 with the TruSeq SBS Kit v3 in paired‐end mode with 150 nt read length. RNA‐seq data were deposited in the ArrayExpress database at EMBL‐EBI under accession numbers E‐MTAB‐8094 and E‐MTAB‐8095. The processing of the raw reads and the differential expression analysis for genes with an expression value higher than 1 count per million in at least three samples was performed as described in Sun et al. (2017). To identify the robustly differentially expressed genes that are independent of genotype, growing season or time point during leaf development, the contrast of interest was the difference between the growth conditions (growth chamber versus field). 634 genes were robustly differentially expressed (fold change > 2 and false discovery rate < 0.05) in all conditions with 213 genes higher and 421 genes lower expressed in the field than in the growth chamber. GO term analysis, performed with PLAZA 3.0 (Monocots) using default settings (Proost et al., 2015), showed that genes up‐regulated in the field have an enriched GO term for response to heat, temperature, high light intensity, hydrogen peroxide and abiotic stress. The robustly down‐regulated genes were enriched in auxin‐mediated signalling, lignin metabolism, oxidation–reduction reactions, gibberellin metabolic process and gene expression.
Several stress tolerance genes that confer abiotic stress tolerance in the laboratory when overexpressed were massively up‐regulated in the field compared to the growth chamber. The two highest, robustly up‐regulated genes were two valine‐glutamine (VQ)‐motif‐containing proteins, VQ43 and VQ45, while VQ1, a member of the same phylogenetic clade, was also in the top ten of highly up‐regulated genes (Figure 1). VQ43 could be highly induced by severe drought and VQ1, and to a lesser extend VQ45, by salt stress (Song et al., 2015). The remaining two members of this phylogenetic clade, VQ3 and VQ56, were not differentially expressed in any condition in our study. In addition, transcription factors homologous to the heat shock factor (HSF) A6b in Arabidopsis, were highly up‐regulated in all field conditions (Figure 1). In Arabidopsis, HSFA6b has been shown to be highly up‐regulated under salt, osmotic and cold stress, whereas its overexpression confers drought and salt tolerance (Huang et al., 2016). Because HSFs are known to regulate the transcription of stress‐related genes (Huang et al., 2016), it was not surprising that among the robustly up‐regulated genes many genes encoding chaperonins and heat shock proteins were found. In such a tsunami of extremely abundantly expressed genes, the levels of overexpression obtained in the transgenic lines might not be sufficient to make a difference and result in drought tolerance.
Figure 1.
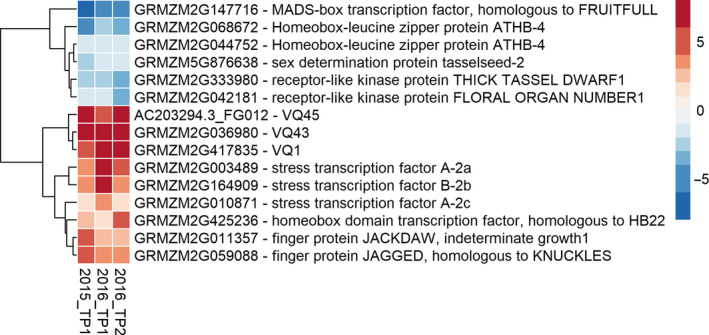
Heatmap showing the log2‐fold change of selected robustly differentially expressed genes between the growth chamber and the field. Samples from growth chamber were compared to field trials during two consecutive growing seasons (2015 and 2016) for one or two time points (TP). The gene order is based on complete linkage clustering of the euclidean distance (pheatmap package in R).
Besides the high differential expression of genes involved in abiotic stress tolerance, the transcriptome study also provided lines of evidence that the plants cultivated in the growth chamber and the field differed in a shade avoidance response, such as genes involved in hormonal regulation, light and flowering. Several genes affecting regulation of hormones were significantly down‐regulated between the field and the growth chamber, including SAURs, IAAs, ABA‐induced proteins, ethylene‐responsive element‐binding proteins, genes involved in GA metabolism and response (GA2‐OXIDASEs, MAGPIE, DELLA) and cytokinin response (KISS ME DEADLY). Two homologs of ATHB4, thought to be an integrator of shade perception and hormone‐mediated growth (Sorin et al., 2009), were among the down‐regulated genes.
The high number of genes encoding chloroplast located proteins, proteins involved in phototropism and proteins with a role in anthocyanin biosynthesis points towards light as a major factor in the comparison between the growth chamber and the field. In addition, genes involved in floral identity are known to be light‐regulated and several of them were present among the genes that were differentially expressed: INDETERMINATE1, HOMEOBOX PROTEIN22 and KNUCKLES were significantly up‐regulated in the field versus the growth chamber. Conversely, several genes involved in floral identity were significantly lower expressed in the field compared with the growth chamber: THICK TASSEL DWARF1 and FLORAL ORGAN NUMBER1, TASSELSHEET2, a CYCLING DOF FACTOR and FRUITFULL (Figure 1).
The shade avoidance response is characterized by red:far‐red ratio of the light experienced by the plants, resulting in changes in flowering time, plant growth and architecture, driven by auxin, GA and brassinosteroids responses (Carriedo et al., 2016) of which many genes were differentially expressed in our study. Using a hypergeometric test (phyper() function in R), we showed statistically significant (P = 2.25e‐07) overlap between the genes that were robustly differentially expressed in our study and the early shade avoidance responses in maize triggered by a low red:far‐red ratio (Wang et al., 2016).
In our conditions, plants grown in the growth chamber experienced typical phenotypes of shade avoidance, with longer leaves. The space between neighbouring plants was comparable in the growth chamber and the field (13–15 cm), but there was no spacing or organization into rows in the growth chamber. Both light intensity and light quality differed between the field and the growth chamber. The average light intensity in Belgium during May is 143.4 kWh/m2, which is eighteen times higher than what is achieved in the growth chamber. High‐pressure sodium lamps in the growth chamber have an red:far‐red ratio of about 4.8, while sunlight emits almost as much far‐red radiation as red light. Because increasing planting density was one of the major maize yield improvements over the past decades, it is believed that domestication and genetic improvement have attenuated the shade avoidance response (Carriedo et al., 2016; Wang et al., 2016). This rendered current commercial varieties very different from the inbreds that are typically used for research and were used in this study, for a response that is very pronounced in laboratory‐to‐field translation.
Our data thus show that the characteristics of both the modern varieties, as well as the conditions that the plants experience in the field hamper proper translation from the laboratory to the field. Therefore, an important step in translating basic knowledge towards applications will lie in studying plants in iterative cycles between the field and the laboratory, by not only using inbreds but also modern varieties. Our study now shows that some of the genes, are often considered as candidates for improving stress tolerance in the field because they are induced by stress in laboratory conditions, are already much higher expressed in field conditions than in the laboratory, and even higher than is typically achieved by overexpression. Therefore, transcriptomes of field‐grown plants could be applied as an additional filter to select genes for engineering stress tolerance, to increase the chance that we identify genes with a high penetrance and translatability to improve stress tolerance.
Funding
The research was funded by the European Research Council under the European Community's Seventh Framework Programme [FP7/2007‐2013] under ERC grant agreement n° [339341‐AMAIZE]11 and by the Hercules Foundation (ZW1101).
Conflict of interest
The authors declare no conflict of interest.
Nelissen H, Sprenger H, Demuynck K, De Block J, Van Hautegem T, De Vliegher A and Inzé D (2020). From laboratory to field: yield stability and shade avoidance genes are massively differentially expressed in the field. Plant Biotechnol. J., 10.1111/pbi.13269
References
- Carriedo, L.G. , Maloof, J.N. and Brady, S.M. (2016) Molecular control of crop shade avoidance. Curr. Opin. Plant Biol. 30, 151–158. [DOI] [PubMed] [Google Scholar]
- Huang, Y.‐C. , Niu, C.‐Y. , Yang, C.‐R. and Jinn, T.‐L. (2016) The heat stress factor HSFA6b connects ABA signaling and ABA‐mediated heat responses. Plant Physiol. 172, 1182–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio, M.L. , Paul, M. , Bate, N.J. , Cohen, J. and Cutler, S.R. (2018) Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Sci. 273, 110–119. [DOI] [PubMed] [Google Scholar]
- Proost, S. , Van Bel, M. , Vaneechoutte, D. , Van de Peer, Y. , Inzé, D. , Mueller‐Roeber, B. and Vandepoele, K. (2015) PLAZA 3.0: an access point for plant comparative genomics. Nucleic Acids Res. 43, D974–D981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W. , Zhao, H. , Zhang, X. , Lei, L. and Lai, J. (2015) Genome‐wide identification of VQ motif‐containing proteins and their expression profiles under abiotic stresses in maize. Front. Plant Sci. 6, 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin, C. , Salla‐Martret, M. , Bou‐Torrent, J. , Roig‐Villanova, I. and Martínez‐García, J.F. (2009) ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J. 59, 266–277. [DOI] [PubMed] [Google Scholar]
- Sun, X. , Cahill, J. , Van Hautegem, T. , Feys, K. , Whipple, C. , Novak, O. , Delbare, S. et al. (2017) Altered expression of maize PLASTOCHRON1 enhances biomass and seed yield by extending cell division duration. Nat. Commun. 8, 14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wu, G. , Zhao, B. , Wang, B. , Lang, Z. , Zhang, C. and Wang, H. (2016) Regulatory modules controlling early shade avoidance response in maize seedlings. BMC Genom. 17, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]


